Abstract
Purpose: To evaluate the impact of multiple potential sociodemographic and clinical stroke-related determinants on self-perceived manual ability in an unselected sample of individuals 12 months after first-ever stroke.
Methods: A cross-sectional sample of 68 participants (mean age 66) with UE impairments were followed up at 12 months post stroke. Stroke severity at onset was moderate for the majority. Manual ability was assessed by the patient-reported outcome measure ABILHAND Questionnaire. Determinants included in the multivariate regression analysis were age, gender, living situation, vocational situation, affected hand, stroke severity at onset and UE disability (motor function, sensory function, joint motion, pain, grip strength, spasticity and activity capacity) at 12 months post stroke.
Results: The strongest associated determinants with self-perceived manual ability were UE motor function and UE activity capacity at 12 months post-stroke. UE motor function together with age and grip strength explained 65% of the variance in one final multivariate model. UE activity capacity and grip strength explained 62% of the variance in a second final model.
Conclusion: In order to understand self-perceived difficulties in manual ability in daily activities in persons with stroke, assessments of UE motor function and activity capacity are recommended.
The ultimate goal of the upper extremity rehabilitation after stroke is to regain ability to use the UE in daily activities that are important to the individual in his or her own environment.
This requires a good understanding of factors that are associated with self-perceived manual ability in order to tailor effective rehabilitation interventions.
Upper extremity motor function and activity capacity are the strongest determinants associated with self-perceived manual ability one year after stroke.
These factors are recommended to be included in the assessment battery in stroke to fully understand the disability in daily life.
Implications for rehabilitation
Introduction
After a stroke, disability of the upper extremity (UE) is common. About 40% of the individuals have remaining UE impairments and activity limitations [Citation1–3]. UE recovery occurs mainly during the first month after stroke [Citation4,Citation5] but improvements can also continue for a longer time [Citation2,Citation6]. Early intensive repetitive, task-specific training has shown to be beneficial for long-term recovery after stroke [Citation7–9]. Rehabilitation interventions especially following these principles can also improve the functioning of the UE in later phases after stroke [Citation7]. However, nonuse phenomenon [Citation10] commonly observed after stroke and lack of participation can result in decreased functioning over time [Citation7].
In order to follow recovery and evaluate the effectiveness of rehabilitation interventions, reliable, valid and responsive outcome measures should be used. Activity can be measured as capacity assessed in a standardized environment and as performance assessed in real life [Citation11]. In addition to observational measures of activity, patient-reported outcome measures (PROM) can provide important piece of information of recovery and rehabilitation as perceived by the patient. Information from PROM is essential to fully understand the treatment impact, to support clinical decision-making and tailor rehabilitation interventions [Citation12]. Motor Activity Log [Citation13] and ABILHAND Questionnaire [Citation14] are PROMs that are commonly used in stroke for assessing UE activity performance. ABILHAND is developed using the Rasch measurement model [Citation14] and recommended for assessment of self-perceived ability to manage daily activities that require the use of the upper extremities after stroke [Citation15].
An increased use of PROM in patient evaluations requires good understanding of how these measures are associated with sociodemographic and clinical stroke-related factors. Previous studies have shown that initial stroke severity is strongly associated with the long-term outcome of activities in daily living post-stroke [Citation16]. Singe factors such as UE motor function, UE muscle strength, UE spasticity, UE sensory function and UE activity capacity have been proven to be associated with self-perceived manual ability in daily life [Citation17–21]. Age, gender, the dominance of the affected upper extremity, and living and vocational situation can also influence the overall outcome after stroke [Citation22–26]. To our knowledge, only two previous cross-sectional studies [Citation19,Citation20] have analyzed the impact of multiple factors on self-perceived manual ability in daily activities in a stable phase after stroke. In one of the studies [Citation19] the multivariate association of UE muscle strength, UE spasticity, UE somatosensation and UE pain were evaluated using the Motor Activity Log as the main outcome measure in a convenience sample 1–27 years after stroke. UE muscle strength was shown to be the strongest contributor and the only variable retained in the final model. In the other study [Citation20], additional determinants were included in the multivariate analysis such as age, gender, social and vocational situation, affected hand, UE activity capacity, perceived participation and life satisfaction. A convenience sample 4–116 months after stroke was used. UE activity capacity was the factor strongest associated with perceived manual ability measured by the ABILHAND Questionnaire and was together with perceived participation and grip strength included in the final multivariate model. However, these previous multivariate studies [Citation19,Citation20] used different determinants and outcomes and included participants with a large dispersion of time since stroke. As far as we know, no study has investigated how multiple factors influence self-perceived manual ability in a well-defined time point in chronic stroke.
The aim of this study was to evaluate the impact of multiple potential sociodemographic and clinical stroke-related determinants on self-perceived manual ability in an unselected sample of individuals 12 months after first-ever stroke.
Methods
Participants
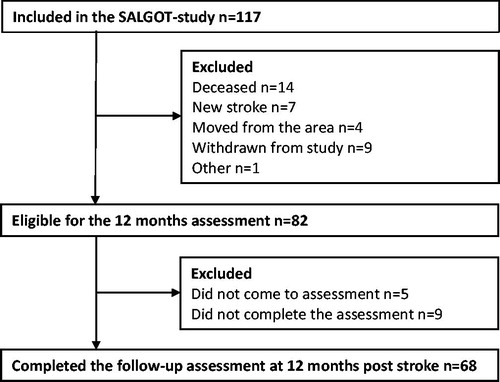
This cross-sectional study of potential determinants’ association with self-perceived manual ability after stroke is part of the Stroke Arm Longitudinal Study at the University of Gothenburg (SALGOT). SALGOT included a non-selected cohort of 117 adults with first-ever stroke admitted to the largest stroke unit at Sahlgrenska University Hospital during an 18 month period between 2009 to 2010 [Citation27]. Inclusion criteria were: ischemic or hemorrhagic stroke confirmed by clinical neuroimaging, presence of upper extremity disability at 3 days after stroke onset (Action Research Arm Test, ARAT <57 points), residency in the Gothenburg urban area, and age 18 or older. Exclusion criteria were: upper extremity condition prior to stroke that limits the functional use of arm and hand, severe multi-impairment or diminished physical condition prior to stroke and short life expectancy due to other chronic or terminal illness, and non-Swedish speaking. The full protocol of the SALGOT contained a battery of assessments at 8-time points: 3 and 10 days; 3, 4, and 6 weeks; 3, 6 and 12 months post stroke. A sample size estimation was made for the SALGOT study based on clinically important mean change of 6 points (10%) on ARAT, with a power of 0.8 and significance level of 0.05. From a total cohort of 763 persons, 117 were included in SALGOT and the current study comprised 68 participants, i.e. the individuals who completed the follow-up assessments at 12 months post-stroke. The flowchart of the inclusion process is shown in . The study protocol was approved by the Regional Ethical Review Board in Gothenburg, Sweden (225/08), and written informed consent was obtained from each participant or next of kin prior to their participation in the study. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting observational data were followed [Citation28].
Outcome measures
Stroke severity
Initial stroke severity was measured by the National Institutes of Health Stoke Scale (NIHSS) [Citation29] in terms of level of consciousness, neglect, visual-field loss, cognitive, language, motor and sensory impairments. The NIHSS score ranges from 0 (no deficit) to 42 (severe deficit) [Citation30]. Item 5 includes the assessment of paresis of the arm and was used as a measure of UE stroke severity (range 0–4). The NIHSS has shown high validity and reliability [Citation29].
UE motor function, sensory function, joint motion and pain
UE motor function together with sensory function, passive joint motion and pain were assessed with the Fugl-Meyer Assessment for Upper Extremity (FMA-UE) [Citation31]. FMA-UE is an observational rating scale to assess the sensorimotor impairment in individuals with stroke. The motor function (FMA-UE motor) has a maximum score of 66 points, the sensory function (FMA-UE sensory) 12 points, the passive range of joint motion (FMA-UE ROM) 24 points, and pain (FMA-UE pain) 24 points. A higher score of each part indicates better function. The FMA-UE has shown good validity and excellent reliability and is recommended to be included in clinical trials for assessment of sensorimotor function after stroke [Citation31–34].
Grip strength
Grip strength was measured with the hydraulic dynamometer JAMAR (Sammons Preston, Chicago) [Citation35]. The participants were seated with their arm resting at a table and verbal encouragement was given during the measurement. The mean of three measurements was used as the maximal grip strength value (in Pound force; 0–200) [Citation36]. Grip strength measurement with the JAMAR dynamometer has proven to have very high validity and reliability [Citation35].
UE spasticity
UE spasticity was measured by the response to resistance of passive movement according to the Modified Ashworth Scale (MAS) [Citation37]. MAS ranges from 0 (no increase in muscle tone) to 5 (rigid). The spasticity was assessed in the flexor and extensor muscles of the elbow and wrist and the score was dichotomized (not present vs present) and classified as present if larger or equal to 1 in any of measured muscle groups. The MAS has shown fair reliability but is yet considered as the best alternative for clinical spasticity assessment [Citation37,Citation38].
UE activity capacity
UE activity capacity was assessed by the Action Research Arm Test (ARAT) [Citation39]. ARAT is an observational rating scale that mainly assesses the ability to handle objects in different size, weight and shape. It includes 19 items divided into four hierarchical subtests: grasp, grip, pinch and gross movement. The time to complete the item and the quality of the movement is assessed on a scale from 0 to 3 points. The sum score ranges from 0 to 57, and a higher score indicates better UE activity capacity. The ARAT has been found to have good validity, responsiveness and high reliability after stroke [Citation39–43].
Self-perceived manual ability
Self-perceived ability to use the upper extremities in daily activities (manual ability) was assessed by the ABILHAND Questionnaire (stroke version) [Citation14]. ABILHAND is a performance-based activity measure that includes 23 common bimanual tasks. The tasks are rated during an interview as impossible (0 point), difficult (1 points) or easy (2 points) with a maximal ordinal score of 46 pints. The ordinal data are converted into an interval scale according to the Rasch measurement model [Citation14]. The interval scale is presented in logits (i.e. log-odds units) and ranges from plus to minus around zero as the center of the scale [Citation44]. The higher the logits values the better the self-perceived manual ability. The ABILHAND has proven to have acceptable test-retest reliability for individuals with stroke [Citation45].
Procedures
The clinical admission and discharge data were gathered from medical charts. Stroke severity was assessed at onset and UE disability at 12 months. Data on the current living and vocational situation were also registered at 12 months post-stroke. All assessments were performed according to a standardized protocol [Citation27] and predominantly carried out by two experienced physiotherapists after a joint training period. The majority of the assessments were performed at the hospital and if the participants were unable to travel, assessments were carried out at the participants’ home.
Statistics
Data were analyzed with the IBM SPSS Statistics version 23 (IBM Corporation, Armonk, New York, United States). Probability values less than 0.05 were defined as statistically significant.
Descriptive statistics, such as frequencies, means and standard deviations (SD) and medians and interquartile ranges were calculated and presented for demographic data and clinical characteristics of the participants.
The impact of potential determinants on self-perceived manual ability (continuous: logits) at 12 months post stroke was assessed by linear regression models. The potentially associated determinants were: age, gender (male vs female); living situation (living alone vs living together), vocational situation (working vs not working), affected hand (dominant vs non-dominant), stroke severity (NIHSS total) at onset, UE stroke severity (NIHSS item 5) at onset, UE motor function (FMA-UE motor) at 12 months, UE sensory function (FMA-UE sensory) at 12 months, UE passive range of joint motion (FMA-UE ROM) at 12 months, UE pain (FMA-UE pain) at 12 months, grip strength at 12 months, UE spasticity (not present vs present according to MAS) at 12 months, and UE activity capacity (ARAT) at 12 months ().
The multivariate regression model building was made with a forward stepwise selection strategy that was evaluated at each step. This strategy was chosen as it increases the understanding of the different determinants significance. First, the association with self-perceived manual ability was evaluated for one determinant at the time in univariate regression analyses. A generous inclusion criterion (p ≤ 0.20) was used so that no potential determinant was excluded in the early stages. Secondly, the determinant with the lowest p values (if ≤0.20) from the univariate analyses was included and thereafter the other determinants were tentatively added, one at a time. Thirdly, the model of two determinants that had the lowest p values (if both determinants p ≤ 0.20) was kept and after that the remaining determinants were tentatively added, one at a time. Thus, in each step, an additional determinant was added to the model and the model with the lowest p values was kept. This procedure was continued as long as the p values for all included determinants were ≤0.20. However, in the final multivariate model determinants were only kept with a p value <0.05. Adjusted explained variances (R2) are given together with p values, unstandardized regression coefficients (B) and Confidence Intervals (CI). For the final model, the adjusted explained variance is given after successive addition of the included determinants. To ensure linearity, scatterplots were visually inspected for the bivariate associations. In addition, model assumptions were checked by means of residual analysis. Multicollinearity among determinants in the regression model building was examined by the variance inflation factor (VIF) [Citation46]. Determinants with a VIF that exceeded 4 were not included in the same model [Citation47].
Results
Participants
In , the characteristics of the 68 included participants are presented. The stroke severity at onset (NIHSS [Citation29]) and disability at discharge (modified Rankin Scale, mRS [Citation48]) was moderate for the majority of participants. At 12 months post-stroke 74% of the participants had motor deficits, 18% had sensory deficits, 47% decreased passive range of joint motion and 35% pain according to FMA-UE. Activity capacity limitations according to ARAT at 12 months were observed in 51% of the participants. The data of the main outcome measure ABILHAND Questionnaire showed that a majority of the participants reported difficulties to perform some of the attempted bimanual activities at 12 months.
Table 1. Demographic and clinical characteristics in 68 participants at one year post stroke.
Univariate regression analysis
presents the univariate regression analyses between self-perceived manual ability (ABILHAND logits) at 12 months post stroke and the potential determinants. The strongest associated determinants were UE motor function (FMA-UE motor) and UE activity capacity (ARAT) at 12 months with explained variances of 59% (p < 0.001) and 56% (p < 0.001), respectively. The regression coefficient (B) of UE motor function was 0.12 logits (i.e. one unit increase in UE motor function will give an improvement of 0.12 logits in manual ability) and the corresponding B for UE activity capacity was 0.10 logits. The determinants age, vocational situation, stroke severity, UE stroke severity, UE sensory function, UE joint motion, UE pain, grip strength, UE spasticity and UE activity capacity also had an association to self-perceived manual ability that fulfilled the criteria (p ≤ 0.20) for being included in the multivariate analyses.
Table 2. Associations between self-perceived manual ability (ABILHAND) and determinants obtained from the univariate linear regression models in 68 participants at one year post stroke.
Multivariate regression analyses
In the multivariate regression building, UE motor function and UE activity showed high multicollinearity (VIF >4). Two regression models were built based on each of these determinants. The final model with UE motor function (p < 0.001) included age (p = 0.045) and grip strength (p = 0.049) and explained 65% of the variance of manual ability (). Motor function explained 59% and when age was added to the model that increased the explained variance to 63% and when grip strength was added to 65%. The B for motor function changed from 0.12 to 0.09 in the multivariate model and the corresponding change for age was −0.07 to −0.04 and for grip strength 0.06 to 0.02. The other multivariate model included UE activity capacity (p < 0.001) and grip strength (p = 0.001) and explained 62% of the variance (). UE activity capacity explained 56% and grip strength added another 6%. The B for UE activity capacity changed from 0.10 to 0.07 in this multivariate model and the corresponding change for grip strength was 0.06 to 0.03.
Table 3. Determinants associated with self-perceived manual ability (ABILHAND) in 68 participants at one year post stroke.
Table 4. Determinants associated with self-perceived manual ability (ABILHAND) in 68 participants at one year post stroke.
Discussion
The main findings of this current study were that self-perceived manual ability 12 months post stroke was to large extent influenced by UE motor function and UE activity capacity. The two multivariate regression models that were built explained about two thirds (62% and 65%) of the variance in the final models.
This cross-sectional study shows novel data regarding the impact of sociodemographic and clinical stroke-related determinants on self-perceived manual ability in a well-defined unselected cohort at a recommended follow-up time-point [Citation49] after stroke. The follow-up assessments were performed with valid, reliable and recommended measures for UE stroke studies [Citation49,Citation50]. As the ultimate goal of the UE rehabilitation after stroke is to regain the ability to use the UE in daily activities in the individual’s own environment we used a PROM of manual ability as the dependent variable in the multivariate regression analysis. PROMs of manual activity have proven to be sensitive in the long term, especially in milder strokes, and they also provide important information of functional outcome that is relevant from the individual’s perspective [Citation12].
Functional outcome after stroke is a combination of “true” recovery and compensation. “True” recovery relies on neural repair and reflects the extent to which body structures, functions and activities have returned to their pre-stroke state [Citation51]. When a movement or task is accomplished in a new alternative manner (body function level) or by using alternative body parts (activity level) it is entitled as compensation [Citation52]. Findings from this study show that motor function assessed by the FMA-UE was the strongest determinant associated with self-perceived manual ability. This is in line with previous studies that have shown high univariate associations between FMA-UE and self-perceived manual ability [Citation12,Citation17,Citation21]. The motor part of FMA-UE is recommended to use to quantify motor impairment and proposed to be an important prognostic factor for UE outcome following stroke [Citation49]. This study shows that at one year after stroke the UE motor function is important for the self-perceived manual ability in daily activities.
In the univariate analysis in our study, UE activity capacity assessed by ARAT had the second strongest association to self-perceived manual ability and was also highly associated with UE motor function. Therefore, a second multivariate model was built with UE activity capacity as the strongest determinant. The final model with activity capacity and grip strength explained 62% of the variance, about the same as the final model including motor function, age and grip strength. A previous multivariate study also found that UE activity capacity is a strong determinant [Citation20] and together with the level of perceived participation their final model explained 48% of the variance of self-perceived manual ability measured by the ABILHAND. The UE activity capacity in that study [Citation20] was assessed with the mini Sollerman Hand Function Test [Citation53] and had a lower association to ABILHAND (R2 = 39%) compared to ARAT (R2 = 56%) in the present study. Furthermore, the other study had no determinant of motor function, such as FMA-UE, which makes it difficult to compare the results. Nevertheless, this proposes that UE activity capacity measured by ARAT is an important determinant for self-perceived manual ability after stroke. The results of this present study also suggest that even when the prognostic value of stroke severity at onset is one of the strongest predictors for long-term UE functioning [Citation16,Citation22], the current motor function and activity capacity might provide better values for understanding the self-perceived manual ability in daily activities in later phases after stroke.
Age was also included as the second strongest determinant in the final model together with UE motor function and grip strength. However, age as a determinant may also comprise age-associated factors and comorbidities that can influence functioning. As age is proposed to be an important prognostic factor for outcome after stroke [Citation16] there may be a tendency to offer older individuals less intensive rehabilitation. Older individuals may also have less support from partners that can result in lower performance in daily life after stroke [Citation54]. Thus, these factors may partly explain the association between age and self-perceived manual ability after stroke. However, age explained only a small part (4%) of the variance suggesting that at one year after stroke age is of lesser importance for self-perceived ability to perform manual activities.
Grip strength was included in both final multivariate models but only added 2–6% of the variance of self-perceived manual ability. This is in accordance with a previous multivariate study [Citation20] where grip strength was included in the final model but only explained 1% of the variance of manual ability measured with the ABILHAND. However, in another multivariate study, [Citation19] UE strength (composite score of shoulder, elbow and wrist strength) alone explained 78% of the variance in the final model. The sample in that study included individuals with more severe strokes compared to the current study and the Motor Activity Log [Citation13] was used as outcome measure for self-perceived manual ability. Compared to the ABILHAND [Citation14] the Motor Activity Log contains more unilateral and simpler tasks [Citation50]. This may imply that UE strength can be more valuable in tasks where strength can be used to compensate for lack of motor skills. Consequently, this present study indicates that in the performance of complex bimanual tasks motor function and activity capacity are of more importance than strength in the more affected UE.
ABILHAND is a recommended outcome measure that captures self-perceived manual ability in daily activities after stroke [Citation15]. In the current study, several potential determinants included in the linear regression analyses explained large part but not all of the variance in manual ability. Information from the ABILHAND is useful in the collaboration between clinicians and patients to assist goal setting and treatment planning. ABILHAND reflects the patient perspective and therefore complements information gained from assessments of motor function and activity capacity.
Strengths and limitations
The current study used an unselected sample of individuals with stroke that were assessed with stroke-specific outcome measures recommended for UE [Citation15,Citation49]. A well-defined and recommended follow-up time point, 12 months post-stroke, was used [Citation49]. Care was taken to standardize the test situation and trained examiners performed the assessments. The multivariate model included multiple important determinants in order to evaluate their association with self-perceived manual ability after stroke.
The sample size in the present study was based on a number of individuals sufficiently large to evaluate the associations of interest. However, for weak associations and further subgroup analyses, a larger study sample would have been required. Moreover, it cannot be excluded that other determinants may be of importance for the self-perceived manual ability after stroke, for example, cognitive functions, vision, fatigue, self-efficacy, aids and caregiver support.
In conclusion, this study showed that one year after stroke UE motor function and activity capacity together with age and grip strength are important for the self-perceived manual ability. These factors should, therefore, be considered and assessed after stroke to better understand manual difficulties perceived in everyday life.
Acknowledgements
The authors are grateful to the individuals who volunteered to participate and Eva-Lena Burstén for help with data collection and the Riks-Stroke Collaboration for its help with the demographic data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Persson HC, Parziali M, Danielsson A, et al. Outcome and upper extremity function within 72 hours after first occasion of stroke in an unselected population at a stroke unit. A part of the SALGOT study. BMC Neurol 2012;12:162.
- Broeks JG, Lankhorst GJ, Rumping K, et al. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21:357–364.
- Nakayama H, Jorgensen HS, Raaschou HO, et al. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–398.
- Hendricks HT, van Limbeek J, Geurts AC, et al. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–1637.
- Verheyden G, Nieuwboer A, De Wit L, et al. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:173–179.
- Mirbagheri MM, Rymer WZ. Time-course of changes in arm impairment after stroke: variables predicting motor recovery over 12 months. Arch Phys Med Rehabil. 2008;89:1507–1513.
- Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev 2014;11:CD010820.
- Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9:e87987.
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702.
- Taub E, Uswatte G, King DK, et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049.
- World Health Organization. International Classification of Functioning, Disability and Health 2001. Available from: http://www.who.int/classifications/icf/en. Accessed March 26, 2018.
- Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke 2013;44:1111–1116.
- van der Lee JH, Beckerman H, Knol DL, et al. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410–1414.
- Penta M, Tesio L, Arnould C, et al. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke. 2001;32:1627–1634.
- Alt Murphy M, Resteghini C, Feys P, et al. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015;15:29.
- Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? Int J Stroke. 2013;8:25–32.
- Pereira ND, Ovando AC, Michaelsen SM, et al. Motor Activity Log-Brazil: reliability and relationships with motor impairments in individuals with chronic stroke. Arq Neuro-Psiquiatr. 2012;70:196–201. Neuropsiquiatr.
- Basilio ML, de Faria-Fortini I, Polese JC, et al. Handgrip strength deficits best explain limitations in performing bimanual activities after stroke. J Phys Ther Sci. 2016;28:1161–1165.
- Harris JE, Eng JJ. Paretic upper-limb strength best explains arm activity in people with stroke. Phys Ther. 2007;87:88–97.
- Ekstrand E, Rylander L, Lexell J, et al. Perceived ability to perform daily hand activities after stroke and associated factors: a cross-sectional study. BMC Neurol. 2016;16:208.
- Fleming MK, Newham DJ, Roberts-Lewis SF, et al. Self-perceived utilization of the paretic arm in chronic stroke requires high upper limb functional ability. Arch Phys Med Rehabil. 2014;95:918–924.
- Meyer MJ, Pereira S, McClure A, et al. A systematic review of studies reporting multivariable models to predict functional outcomes after post-stroke inpatient rehabilitation. Disabil Rehabil. 2015;37:1316–1323.
- Petrea RE, Beiser AS, Seshadri S, et al. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037.
- Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. 2006;20:380–389.
- Jellema S, van der Sande R, van Hees S, et al. Role of environmental factors on resuming valued activities poststroke: a systematic review of qualitative and quantitative findings. Arch Phys Med Rehabil. 2016;97:991–1002.
- Palmcrantz S, Widen Holmqvist L, Sommerfeld DK. Young individuals with stroke: a cross sectional study of long-term disability associated with self-rated global health. BMC Neurol. 2014;14:20.
- Alt Murphy M, Persson HC, Danielsson A, et al. SALGOT – Stroke Arm Longitudinal study at the University of Gothenburg, prospective cohort study protocol. BMC Neurol. 2011;11:56.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349.
- Brott T, Adams HP, Jr., Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870.
- Appelros P, Terent A. Characteristics of the National Institute of Health Stroke Scale: results from a population-based stroke cohort at baseline and after one year. Cerebrovasc Dis. 2004;17:21–27.
- Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31.
- van Wijck FM, Pandyan AD, Johnson GR, et al. Assessing motor deficits in neurological rehabilitation: patterns of instrument usage. Neurorehabil Neural Repair. 2001;15:23–30.
- Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610.
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798.
- Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226.
- Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74.
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207.
- Gregson JM, Leathley M, Moore AP, et al. Reliability of the Tone Assessment Scale and the modified Ashworth scale as clinical tools for assessing poststroke spasticity. Arch Phys Med Rehabil. 1999;80:1013–1016.
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492.
- Nordin A, Alt Murphy M, Danielsson A. Intra-rater and inter-rater reliability at the item level of the Action Research Arm Test for patients with stroke. J Rehabil Med 2014;46:738–745.
- Hsieh CL, Hsueh IP, Chiang FM, et al. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27:107–113.
- van der Lee JH, De Groot V, Beckerman H, et al. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19.
- van der Lee JH, Beckerman H, Lankhorst GJ, et al. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med. 2001;33:110–113.
- Rasch G. Probabilistic models for some intelligence and attainment tests. Expanded ed. Chicago: University of Chicago Press; 1980.
- Ekstrand E, Lindgren I, Lexell J, et al. Test-retest reliability of the ABILHAND questionnaire in persons with chronic stroke. Pm R. 2014;6:324–331.
- Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. New York: Springer Science & Business Media; 2011.
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406–416.
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607.
- Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12:451–461.
- Ashford S, Slade M, Malaprade F, et al. Evaluation of functional outcome measures for the hemiparetic upper limb: a systematic review. J Rehabil Med. 2008;40:787–795.
- Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Neurorehabil Neural Repair. 2017;31:793–799.
- Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319.
- Rosen B. Recovery of sensory and motor function after nerve repair. A rationale for evaluation. J Hand Ther 1996;9:315–327.
- Bagg S, Pombo AP, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke 2002;33:179–185.