Abstract
Objective
We investigated the effectiveness of graded exercise therapy (GET) delivered to patients with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in a routine, specialist clinic by measuring patient-reported outcome data collected prospectively over several timepoints alongside therapy. Benchmarking analyses were used to compare our results with those found in randomised controlled trials (RCTs).
Methods
Data were collected from patients, with a diagnosis of CFS/ME, who had been referred to a specialist clinical service in South London. Measures included Chalder Fatigue Questionnaire, Physical Functioning Subscale of the Short-Form Health Questionnaire, and the Work and Social Adjustment Scale. Change on each measure was calculated over time using linear mixed-model analyses. Within group effect sizes were calculated and compared with previous RCTs.
Results
Fatigue scores were significantly reduced by session 4 (–5.18, 95%CIs −7.90, −2.45) and at follow-up (–4.73, 95%CIs −7.60, −1.85). Work and social adjustment and physical functioning progressively improved over the course of therapy, reaching significance at discharge and maintained at follow-up (WSAS −4.97, 95%CIs −7.97, −1.97; SF-36 10.75, 95%CIs 2.19, 19.31).
Conclusions
GET is an effective treatment for CFS/ME within clinical practice. However, effect sizes were smaller in routine clinical practice than RCTs suggesting that avenues for augmentation need to be considered.
It is important to assess whether patient reported outcomes of treatments that have been evaluated in the context of clinical trials are similar in routine clinical practice.
This study shows fatigue severity, physical functioning, and work and social adjustment can significantly improve after graded exercise therapy for patients with chronic fatigue syndrome within a specialist service.
Benchmarking methods showed clinical outcomes obtained smaller effect sizes than randomised controlled trials – techniques to maximise patient outcomes should be considered.
Implications for rehabilitation
Introduction
In the UK, the prevalence of clinically confirmed chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been estimated to affect over 500 000 people (0.76% of the population) [Citation1]. CFS/ME is categorised by disabling fatigue that is not assuaged by rest or explained by another medical condition. CFS/ME places an incredibly large burden on patients, carers, and their families as symptoms tend to persist for years and prognosis is poor if left untreated [Citation2]. In the UK, adult specialist services have estimated around half of patients with CFS/ME have stopped working [Citation3].
The diagnosis of CFS/ME requires fatigue to last between four and six months depending on the criteria used. The Fukuda criteria [Citation4] has been utilised extensively within research compared to the more recent Canadian consensus criteria (CDC) [Citation5], both of which require six months duration. In the UK, the National Institute of Health and Care Excellence (NICE) requires fatigue to be present for at least four months [Citation6] and is therefore more inclusive. Patients must report a substantial reduction in activity as well as report other symptoms such as pain and sleep disturbance. Clinical assessment and investigations should rule out any primary physical or mental explanations for the fatigue [Citation6].
There is good evidence from randomised controlled trials (RCTs) to suggest the symptoms and consequences of CFS/ME can be improved for some patients by graded exercise therapy (GET), one of the most widely used treatments for CFS/ME. A Cochrane systematic review of eight RCTs found that GET for CFS/ME was associated with reductions in fatigue and improved sleep, physical functioning, and overall health [Citation7]. There is some evidence to suggest that GET can safely be delivered alongside specialist medical care to moderately improve outcomes such as fatigue and disability for CFS/ME [Citation8]. While there are several RCTs evaluating GET for CFS/ME, there is a shortage of evidence for how these therapies fare when conducted in real-life clinical settings. There is also a need for better reporting of long-term follow-up outcomes [Citation7]. Treatment units can be a potential source for this type of evaluation.
Large-scale multi-centre studies collecting routine clinical service data have shown that evidence-based treatments including GET and cognitive behavioural therapy (CBT) improved fatigue symptoms, general functioning, depression, sleep and concentration and motivation. However, improvements in physical functioning, pain, and anxiety were weaker and less consistent [Citation9,Citation10]. More specifically, improvements in fatigue following treatment of CBT and GET were comparable to RCTs, while physical functioning improvements were somewhat smaller [Citation10]. In an earlier study, Quarmby et al. found that patients receiving CBT as part of a RCT had significantly better improvements in fatigue and social adjustment at 6-month follow-up compared to patients who received routine CBT at the same clinic [Citation11]. In conclusion, most RCT and routine clinic comparisons have been with CBT. We wish to expand on this evidence by establishing whether these trends are found in GET.
Benchmarking is a technique that has been used as a successful tool to evaluate the efficacy of therapies for CFS/ME delivered in routine clinical practice as compared to therapy delivered in the context of a RCT [Citation11]. Benchmarking is a statistical procedure that allows a direct statistical comparison of pre-treatment vs. post‐treatment scores across studies [Citation12], providing the treatment location meets the recommended requirements for a clinically representative setting [Citation12,Citation13]. Our study sample meets the majority of proposed requirements [Citation13], and is therefore, suitable for benchmarking against RCTs.
Objectives
We aimed to investigate the effectiveness of GET delivered to patients with CFS/ME in a routine, specialist clinical environment by measuring fatigue, social adjustment, physical functioning, depression, and anxiety over the course of treatment. We also compared effect sizes with those from previous RCTs.
We hypothesised that GET would result in a significant decrease in fatigue and work and social adjustment symptoms and a significant improvement in physical functioning scores over the course of therapy. However, we predicted that our effect sizes would be smaller than that of previous RCTs, since patients in clinical settings tend to be more heterogenous in their personal characteristics and therapy is often provided for one main diagnosis in research, compared with more complex presentations in clinical settings [Citation14].
Methods
Participants, design, and setting
The study used data collected prospectively from patients who were first seen in a specialist CFS/ME service based within a NHS Trust in south London. The unit assesses and treats CFS/ME as well as other long-term persistent physical symptoms. Patients were referred by their GP or by a consultant in secondary care. The unit has collected audit data from patients since 2002 resulting in a large longitudinal database. The data collection for this study was approved as a clinical audit evaluation by the Psychological Medicine Clinical Academic Group within South London and Maudsley Trust (ID number PPF191115) and includes patients who were seen in the unit from 2002 to 2017. This unit was a recruitment site for the PACE trial from March 2005 until November 2008 [Citation8]; however, these data do not include anyone who participated in the trial.
GET treatment
Rationale
Graded exercise therapy aims to increase physical activity through guided exercise. Fatigue symptoms experienced by patients with CFS/ME lead to a general decrease in activity which over time causes the body to become weaker and deconditioned. The graded exercise programme aims to reverse this, so the body becomes stronger and reconditioned, thereby reducing symptoms. Core aims of GET are to increase muscle flexibility, muscle strength, and fitness levels so that activities that may be difficult at the start of treatment can be carried out more comfortably in the future. This rationale combined with support from RCTs suggests that patients with CFS can respond favourably to GET [Citation7,Citation8]. Indeed, in our clinic experience, anecdotally, patients who prefer not to have a psychological component to their treatment, appreciate having GET as an option.
Procedure
First, an exercise baseline was established. This was defined as a level of exercise or physical activity that the individual felt they could cope with completing five days a week, even on more challenging days [Citation15]. This was whilst managing daily tasks and without substantially increasing CFS/ME symptoms. It was negotiated with each individual patient and tailored to their ability. For example, an initial baseline activity schedule could involve as little as walking to the garden gate and back five times a week. Longer term goals were discussed; specifically, patients stated what they wished to accomplish physically at the end of treatment, such as: to walk 30 min, five times a week or swim 20 lengths, twice a week. Once patients had defined longer term goals they were broken down into manageable smaller steps. Negotiating goals is a very important aspect of GET. Having personal, realistic goals minimise the risk for potential adverse reactions. The type of exercise carried out was agreed and specific according to patient preference.
Once a baseline level of exercise was established, it was reviewed and increased in duration every week by a maximum of 20%, if the patient agreed. The 20% rule was to minimise the effects of booming and busting with patients for whom this was a problem. During the initial stage of treatment, the intensity of exercise was not increased. Increasing exercise intensity too quickly could have resulted in the patient doing too much too soon, leading to an adverse reaction, and subsequent spike in fatigue symptoms. Once exercise had been gradually built up to 30 min, five days per week, the intensity of the exercise was gradually increased subjectively or objectively. For example, if the chosen exercise was walking, the speed was slightly increased for a small proportion of the walk or walking up a small hill introduced. Resources including the Borg Scale of Perceived Exertion [Citation16] or a heart rate monitor [Citation15] could be used by the patient throughout treatment when increasing duration or intensity.
To help patient understanding and progress, each patient was given a manual containing information on topics discussed during therapy and resources used throughout the course of treatment. Resources included activity diaries, sleep diaries, exercise record sheets, and homework sheets. Stretches were included in the treatment program to increase the flexibility of muscles, allowing patients to move more freely, along with strengthening exercises to make muscles stronger and to allow patients to carry out everyday activities more comfortably. An individual might begin with holding individualised stretches for two seconds and gradually increased them each week until a 20 s hold was achieved. Additionally, objective measures were utilised by therapist and patient to monitor progress. These included the One-minute Sit to Stand Test [Citation17], which was carried out at the start of sessions: at the beginning of therapy, mid-way, at discharge and during every follow-up. Where patients were unable to complete the sit-to-stand test it was replaced by the two-minute walk test [Citation18] recommended by White et al. [Citation8]. Performance on these measures was not recorded in this audit.
Initially, participants received an assessment from a consultant or other specialist to establish their diagnosis according to the NICE guidelines [Citation6]. After assessment, patients were asked to sign a consent form which described the routine collection of outcome measures which would be used to evaluate treatment effectiveness of the treatment provided in the clinic. Each consenting patient who fulfilled criteria for CFS/ME was offered between 12 and 16, individual, one-hour sessions of GET, followed-by the opportunity of four follow-up sessions at three-monthly intervals. Therapy was delivered by two physiotherapists experienced in treating CFS/ME and persistent physical symptoms with sessions scheduled to take place generally once every two weeks. Therapists received individual, monthly supervision. The intervention was largely unchanged since data collection began. However, it was officially manualised in 2011 [Citation8].
Primary outcomes
Participant characteristics
Demographic characteristics such as age, gender, and ethnicity were recorded. Patients were also asked questions regarding the duration and intensity of their symptoms. Participants were diagnosed with CFS/ME according to NICE criteria, but they were also asked questions that would indicate whether they fulfilled criteria according to the CDC criteria and Oxford criteria for CFS/ME [Citation4,Citation19].
Fatigue
Fatigue was measured using the Chalder Fatigue Questionnaire (CFQ). It has 11 items with Likert scoring 0–3, giving a total score ranging from 0 to 33, with the lowest score representing the least fatigue. Participants were asked to rate the frequency of a symptom, with four answer options, ranging from “less than usual” to “much more than usual”. The CFQ is reliable and valid in CFS/ME populations (Cronbach’s alpha: 0.88) [Citation20,Citation21].
Work and social adjustment
The Work and Social Adjustment Scale (WSAS) was used to measure participation in life. It comprises five items using a 0–8 scale; higher scores indicate poorer social adjustment. WSAS is reliable and has been validated in patients with CFS/ME [Citation22,Citation23].
Physical functioning
The Short-Form Health Survey (SF-36) subscale was used to measure physical functioning [Citation24]. This contains 10 self-reported items summed to give a total out of 100. A higher score indicates better physical functioning. This scale is valid and reliable in CFS/ME patient populations (Cronbach’s alpha: 0.90) [Citation25].
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS) [Citation26], was designed to indicate the potential presence of anxiety and depression within medical out-patient clinic settings. It contains two 7-item scales, with each item ranked on a scale from 0 to 3, giving a total score out of 21 for each condition. A score of 11 or more is a strong indicator that the target condition is present. It is reliable and valid [Citation27].
Missing data
We adjusted for incomplete measures. If a patient was missing 25% or less of the measure data, a prorated score was calculated using the mean of the remaining items. To maximise the number of patients analysed for benchmarking (pre-post comparison), if a patient did not complete a discharge questionnaire, the 3 months follow-up questionnaire was used if available. HADS scores were not combined in this way. Linear mixed-model analysis inherently accounts for missing data.
Inclusion/exclusion criteria
Patients were included in analyses if they were treated at the unit, over the age of 18, completed a pre-treatment questionnaire, and had a confirmed diagnosis of CFS according to NICE [Citation6]. Patients were excluded if they had a comorbid problem such as bipolar disorder or an eating disorder; had fatigue in the context of alcohol dependence; had cancer; had an organic disorder which had fatigue as a symptom (e.g., a cardiac problem); if they had received a treatment other than GET at the same time or in the past; or if they had not completed pre-treatment questionnaires. We did not exclude patients with other comorbid conditions associated with CFS such as postural tachycardia syndrome, ehlers danlos syndromes, hypermobility syndrome or hypermobility spectrum.
Data analysis
All patients who met inclusion criteria and after applying exclusion criteria were analysed. Statistical analyses were conducted using SPSS (SPSS Inc., Chicago, IL) [Citation28]. Characteristics collected included age, gender, ethnicity, and marital status, as well as illness duration and total number of sessions.
To assess if there was any significant change in outcome scores over the course of treatment and follow-up, linear mixed models (compound symmetry model) with time as the main predictor were applied using all available scores from the CFQ, WSAS, SF36, HADS-Anxiety, and HADS-Depression. This included pre-treatment, 4th session, 7th session, discharge, and 3 months follow-up. Following this, multiple post hoc, Bonferroni’s corrected [Citation29], paired t-tests were conducted between all treatment timepoints, to evaluate the direction and significance of change within each outcome measure.
Lastly, for benchmarking analyses, pre-treatment scores for each measure were compared with available posttreatment scores using paired sample t-test. A suitable “post-treatment score” was defined as the discharge score, or the next available follow-up score (in this case 3 months) if the discharge measure was unavailable. Manual calculations of Cohen’s d effect sizes (d) [Citation30] were calculated for each outcome measure.
Results
Number of sessions and dropout rate
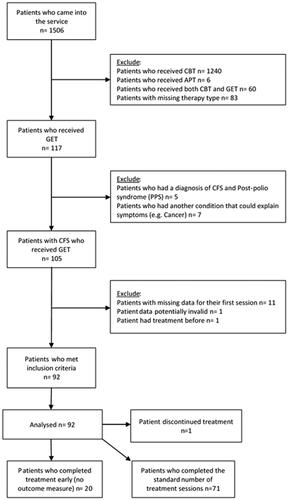
summarises the participant recruitment upon entering the service to inclusion in analyses. After applying exclusion criteria, data were available for 92 patients. To capture the routine delivery of GET within the service, outcome data from all patients who met our criteria regardless of whether they dropped out, fully adhered to treatment, or completed treatment early, were included in analyses.
The standard number of treatment sessions was 12; see appendix 2 for more detail. Twenty-one patients did not complete the optimal number of therapy sessions; see appendix 1 for a summary. With regard to adverse events, one patient discontinued treatment after 11 sessions because they felt GET was “not suitable for them”; 11 sessions is nearly a full course of treatment; however, unfortunately, with no outcome data around this time we cannot say whether the patient’s symptoms were improving or getting worse. No other adverse events were reported by patients. Some patients completed or stopped treatment early due to situational factors, such as, only being funded for a certain number of sessions, moving away, or becoming asymptomatic and therefore, not requiring further treatment. It should be noted that some patients gave no reason for stopping treatment.
Compared to patients who completed a standard course of therapy, patients who stopped treatment early, had a significantly lower mean score on the WSAS and CFQ at the beginning of treatment: −6.26, t(–3.06), df = 90, p = .003, −3.36, t(2.15), df = 89, p = .035, respectively. Additionally, patients who stopped treatment earlier had a significantly higher score on the SF-36 at pre-treatment, mean difference: 11.31, t(–2.10), df = 88, p = .038.
Participant characteristics
We analysed data from 92 CFS/ME patients who were offered GET. Participant characteristics are presented in . One therapist delivered 66.3% of treatment and the other 33.7% (neither therapist was involved in the analysis). All patients met NICE diagnostic criteria for CFS/ME [Citation6], with a large proportion also meeting Oxford (70.7%) and CDC criteria (60.9%).
Table 1. Participant demographics and characteristics.
Linear mixed effects: main outcomes
Fatigue
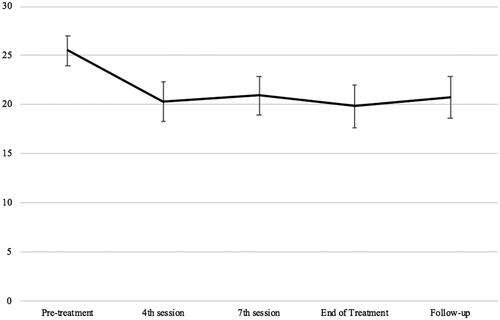
Linear mixed-effect analyses showed an overall decrease in fatigue scores over time, F (4, 168.089) = 13.792, p<.001. Post hoc paired t-tests showed that, compared with pre-treatment (M = 25.49) fatigue scores were reduced by session 4 (–5.18, p<.001, 95%CIs −7.90, −2.45) and then maintained until follow-up (–4.73, p<.001, 95%CIs −7.60, −1.85). Participant’s scores on the CFQ across time points are displayed in .
Work and social adjustment
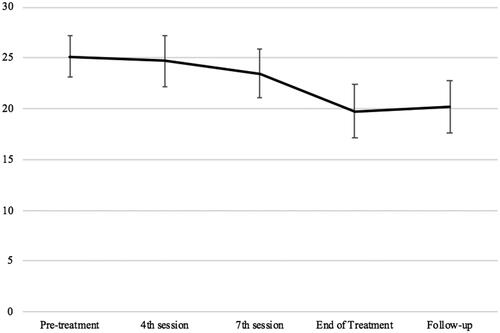
Similarly, linear mixed-effect analyses demonstrated an overall decrease in WSAS over time, F (4, 160.606) = 9.76, p<.001. Post hoc paired t-tests between timepoints showed that, compared with pre-treatment (M = 25.15) the mean WSAS score was reduced by discharge (–5.42, p<.001, 95%CIs −8.49, −2.35) and this was maintained at follow-up (–4.97, p<.001, 95%CIs −7.97, −1.97). Participant’s scores on the WSAS across therapy timepoints are displayed in .
Physical functioning
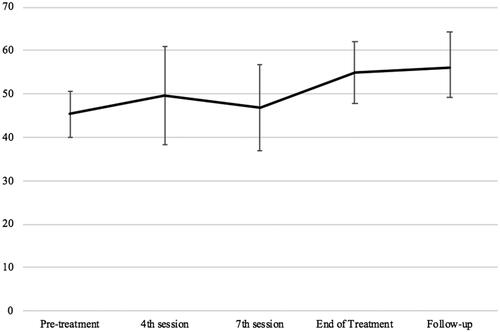
There was an overall increase in physical functioning over time, F (4, 96.841) = 4.41, p = .003. Post hoc t-tests showed that, compared to pre-treatment discharge scores were improved (9.63, p = .024, 95%CIs 0.74, 18.52), and maintained at follow-up (10.75, p = .005, 95%CIs 2.19, 19.31). Participant’s performance on the SF-36 physical functioning subscale across therapy timepoints is displayed in .
Depression and anxiety
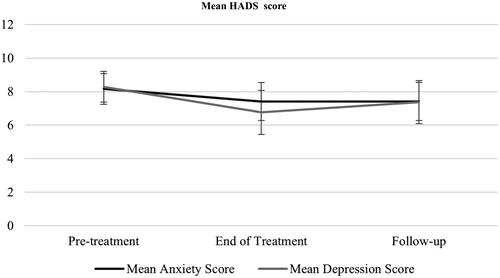
Results showed there was a significant decrease in depression score over time, F (2, 81.46) = 3.19, p = .047. However, paired t-tests showed there were no differences between pre-treatment and discharge (–1.52, p = .055, 95%CIs −0.02, −3.07) or follow-up (–0.92, p = .43, 95%CIs −2.42, −0.59). shows the change in HADS score over time. There was no effect of time over the course of therapy in anxiety scores, F (2, 69.23) = 2.15, p = .124. There were no differences between timepoints.
Benchmarking and comparison of effects
Paired pre-treatment and combined post-treatment data for the outcome fatigue, were available for 67 participants (n = 34 from 3 months follow-up). Uncontrolled effect sizes and averages comparing patient outcomes after GET, within the routine clinic against the outcomes of RCTs [Citation15,Citation31–33] are provided in . Results showed that mean fatigue score decreased between pre-treatment and post-treatment t(66) = 5.79, p < 0.001, d = 0.678. Data were available from 66 participants for the WSAS effect score post-treatment (n = 33 from 3 months follow-up). There was a mean decrease in WSAS between pre-treatment and post-treatment t(65) = 3.66, p = 0.001, d = 0.370. Data were available from 49 participants for the SF-36 physical functioning subscale post-treatment (n = 18 from 3 months follow-up). There was a mean increase in score between pre-treatment and post-treatment t(48)= −3.02, p = 0.004, d = 0.340. Discharge data were available from 32 patients for HADS-Depression score. There was a mean decrease in score between pre-treatment and post-treatment t(31) = 2.82, p = 0.008, d = 0.361. Discharge data were available from 32 patients for HADS-Anxiety scores. There was no difference between pre-treatment and discharge according to paired t-tests, t(31) = 1.384, p = 0.176, d = 0.155.
Table 2. Means and standard deviations at pre-treatment and post-treatment and within group effect sizes for GET clinic versus RCTs.
Discussion
Overall, there was an improvement in fatigue, work and social adjustment, physical functioning, and depression for patients with CFS/ME over the course of GET and at follow-up. This supports past research findings which showed that GET can be an effective treatment for CFS/ME within specialist clinics [Citation7,Citation9,Citation10].
Patients reported an overall reduction in fatigue after GET. Interestingly, a significant reduction in symptoms occurred between sessions 1 and 4 which were maintained until follow-up. This early change seems to mirror patterns seen in CBT for CFS/ME with some researchers finding about a quarter of patients displaying fatigue symptoms within normal limits within 6 weeks of their therapy starting [Citation35]. This pattern has not been reported on in GET before, although there is an apparent sharp reduction in fatigue symptoms earlier in treatment in a past RCT [Citation8]. In the CBT literature, during therapy, an improvement in cognitive responses including self-perceived level of general activity and sense of control over fatigue are associated with lower levels of fatigue at the end of treatment [Citation35]. Additionally, change in fear avoidance beliefs has been suggested to mediate the outcome of patients in GET [Citation36]. Improvements in these cognitive factors may have worked in tandem with the procedures employed early in GET. Our physiotherapists tended to schedule sessions 1–4 at weekly intervals; this concentrated approach may have provided patients with a rationale for taking control of their activity along with the opportunity to implement behaviour changes, such as developing a consistent approach to exercise and then gradually increasing. This may have improved patient’s perception of their control over their symptoms and activity levels as well as reducing fear avoidance beliefs, resulting in rapid reductions in fatigue symptoms. The closely spaced sessions would also help to build momentum within therapy and allow any beneficial effects on outcomes to be maintained over the course of therapy.
In line with our hypothesis, effect sizes for outcomes ranged from moderate (0.7) for fatigue but small-moderate for work and social adjustment, physical functioning, and depression (0.4, 0.3, and 0.4, respectively). Comparing these with the effect sizes found in previous RCTs, our cohort’s effect sizes were smaller, possibly due to sampling bias in RCTs. Our naturalistic cohort may have included more complicated patient presentations than participants in RCTs. Motivational factors such as feeling unable to commit to the regimented nature of an RCT is likely to affect sampling. Our effect sizes are similar to trends found in other studies in clinical settings; fatigue symptoms improved as a result of treatment more so than other outcomes such as physical functioning [Citation9,Citation10]. This suggests there may be scope to modify treatments such as CBT and GET to better target physical functioning.
There were limitations with the current study. First, the lack of a controlled comparison group limits our ability to propose causal explanations about our patient outcomes. Our study was designed to be a pragmatic way of assessing outcomes of GET in a routine clinic versus research environments; this meant our study was not randomised or blind-assessed. However, our study had a pragmatic design, and we attempted to counteract this issue by incorporating benchmarking techniques.
Second, 20 of our patients did not complete a standard number of treatment sessions, and therefore lack a discharge measure. Reflecting on an individual case by case basis – patients may have done well with treatment and reached asymptomatic status faster. This is possible given they were significantly less symptomatic and less physically and socially impaired before treatment than those who had a standard course of therapy. Our effect sizes may have been an under-estimate because our effect size calculations were based on pre and post comparisons.
Third, our participants did not reach the cut-off to indicate the presence of an anxiety or depressive disorder at any point. Given past research indicates these conditions are often comorbid in CFS/ME, this is more likely to reflect a bias in our sample. In our service, assessors have an option to recommend a patient for CBT or GET. If a patient presents with comorbid depression or anxiety, it is likely that clinicians will have recommended CBT as opposed to GET. However, ultimately, patients are also able to choose the therapy they prefer.
Finally, our study utilised self-report measures which can be problematic because they are dependent on a patient’s perception of their own illness. However, previous studies have validated the outcome measures we used in CFS/ME populations [Citation21,Citation22,Citation25]. In addition, given that fatigue is a subjective experience and cannot be assessed objectively, patient reported outcome measures are important in assessing treatment objectives and progress with treatment. We will also consider auditing objective measures like the sit-to-stand test in the future.
Conclusions
In conclusion, GET therapy is a safe and efficacious treatment for patients with CFS/ME in a clinical specialist environment. Patients showed early gains in fatigue symptom reduction whereas improvements in physical functioning and reductions in work and social adjustment impairment were gradual; all improvements were maintained at follow-up to 3 months. Overall, as expected, we found small to moderate effect sizes, slightly smaller than those in RCTs, suggesting findings of effectiveness of GET in RCTs can be translated to routine clinical settings. However, care must be taken though to encourage the use of strategies that will bring about more pronounced change.
Appendix.tiff
Download TIFF Image (1.3 MB)TIDS-04-2020-117-R2-appendices.docx
Download MS Word (16.2 KB)Acknowledgements
We would like to thank all our students who have helped us maintain our data over the years.
Disclosure statement
T.C. is the author of several self-help books on chronic fatigue for which she has received royalties. T.C. (King’s College London, KCL) has received ad hoc payments for workshops carried out in long-term conditions. KCL have received payments for T.C.’s editor role in the Journal of Mental Health. T.C. acknowledges financial support from NIHR, Health Technology Assessment, Guy’s & St Thomas’ Charity, Muscle Disease UK. She has a patent background IP with a software company. The other authors have no competing interests to report.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes
* This article is referred to by: Patients with CFS remain severely disabled after treatment with graded exercise therapy in a specialist clinic in the UK Response to Smakowski et al. (https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2048911). Patients with chronic fatigue syndrome can improve with graded exercise therapy: Response to Vink et al. 2022 (https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2059112)
References
- Johnston S, Brenu EW, Staines D, et al. The prevalence of chronic fatigue syndrome/myalgic encephalomyelitis: a meta-analysis. Clin Epidemiol. 2013;5:105–110.
- Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med. 2005;55(1):20–31.
- Stevelink SAM, Fear NT, Hotopf M, et al. Factors associated with work status in chronic fatigue syndrome. Occup Med. 2019;69(6):453–458.
- Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959.
- Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–338.
- NICE. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management. National Institute of Clinical Excellence; 2007. https://www.nice.org.uk/guidance/cg53/chapter/1-Guidance#diagnosis.
- Larun L, Brurberg KG, Odgaard-Jensen J, et al. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2019;10(10):CD003200.
- White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377(9768):823–836.
- Collin SM, Crawley E. Specialist treatment of chronic fatigue syndrome/ME: a cohort study among adult patients in England. BMC Health Serv Res. 2017;17(1):488.
- Crawley E, Collin SM, White PD, et al. Treatment outcome in adults with chronic fatigue syndrome: a prospective study in England based on the CFS/ME National Outcomes Database. QJM. 2013;106(6):555–565.
- Quarmby L, Rimes KA, Deale A, et al. Cognitive-behaviour therapy for chronic fatigue syndrome: comparison of outcomes within and outside the confines of a randomised controlled trial. Behav Res Ther. 2007;45(6):1085–1094.
- Minami T, Serlin RC, Wampold BE, et al. Using clinical trials to benchmark effects produced in clinical practice. Qual Quant. 2008;42(4):513–525.
- Shadish WR, Matt GE, Navarro AM, et al. Evidence that therapy works in clinically representative conditions. J Consult Clin Psychol. 1997;65(3):355–365.
- Weisz JR, Weiss B, Donenberg GR. The lab versus the clinic: effects of child and adolescent psychotherapy. Am Psychol. 1992;47(12):1578–1585.
- Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ. 1997;314(7095):1647–1652.
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98.
- Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78(1):77–81.
- Solway S, Brooks D, Lacasse Y, et al. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270.
- Sharpe MC. A report – chronic fatigue syndrome: guidelines for research. J R Soc Med. 1991;84(2):118–121.
- Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res. 2010;69(1):17–22.
- Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a Fatigue Scale. J Psychosom Res. 1993;37(2):147–153.
- Cella M, Sharpe M, Chalder T. Measuring disability in patients with chronic fatigue syndrome: reliability and validity of the Work and Social Adjustment Scale. J Psychosom Res. 2011;71(3):124–128.
- Mundt JC, Marks IM, Shear MK, et al. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180(5):461–464.
- Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483.
- Buchwald D, Pearlman T, Umali J, et al. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med. 1996;101(4):364–370.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370.
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77.
- IBM Corp. Released 2019. IBM SPSS statistics for windows, Version 26.0. Armonk (NY): IBM Corp.
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates; 1988.
- Powell P. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ. 2001;322(7283):387–387.
- Moss-Morris R, Sharon C, Tobin R, et al. A randomized controlled graded exercise trial for chronic fatigue syndrome: outcomes and mechanisms of change. J Health Psychol. 2005;10(2):245–259.
- Clark LV, Pesola F, Thomas JM, et al. Guided graded exercise self-help plus specialist medical care versus specialist medical care alone for chronic fatigue syndrome (GETSET): a pragmatic randomised controlled trial. Lancet. 2017;390(10092):363–373.
- Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.1, in Chapter 6 Choosing effect measures and computing estimates of effect. 6.5.2.2 obtaining standard deviations from standard errors and confidence intervals for group means. Available from www.training.cochrane.org/handbook; Cochrane; 2020.
- Heins MJ, Knoop H, Burk WJ, et al. The process of cognitive behaviour therapy for chronic fatigue syndrome: which changes in perpetuating cognitions and behaviour are related to a reduction in fatigue? J Psychosom Res. 2013;75(3):235–241.
- Chalder T, Goldsmith KA, White PD, et al. Rehabilitative therapies for chronic fatigue syndrome: a secondary mediation analysis of the PACE trial. Lancet Psychiatry. 2015;2(2):141–152.