Abstract
Purpose
This study aimed to reveal information that can be used for composing a prehabilitation program tailored to elderly gynecological oncological patients and is applicable to healthcare professionals. We investigated possible content and indications for prehabilitation, and what potential barriers might exist.
Materials and methods
Because of the primary exploratory study aim, inductive thematic template analysis on semi-structured interviews with gynecologic oncological patients aged ≥60 years and healthcare professionals were used.
Results
16 patients and 20 healthcare professionals were interviewed. Three themes important for prehabilitation were found: (1) “Motivation,” (2) “Practical issues and facilitators,” and (3) “Patient-related factors.” A short time interval between diagnosis and surgery was reported as a potential barrier for prehabilitation. Given components for a tailor-made prehabilitation program are: (1) The first contact with a nurse who screens the patients, gives tailor-made advice on prehabilitation and keeps patients motivated and supports them mentally; (2) If patients are referred to a more extensive/supervised program, this should preferably be arranged close to a patients’ home.
Conclusion
Based on our findings, an outline of a patient-tailored prehabilitation program was developed. The main important themes for prehabilitation were “Motivation,” “Practical issues and facilitators,” and “Patient-related factors.”
Patients and healthcare professionals are positive about prehabilitation.
Main themes for designing a prehabilitation program are “Motivation,” “Practical issues and facilitators,” and “Patient-related factors.”
Nursing staff can play a key role in prehabilitation.
It is important to screen patients for specific impairments to obtain a tailor-made prehabilitation program.
For some patients, general advice on prehabilitation might be sufficient, while others may need more supervision.
The time interval between diagnosis and surgery is often short and is perceived as a potentially significant barrier for an effective prehabilitation program.
IMPLICATIONS FOR REHABILITATION
Introduction
Optimizing a patient’s preoperative condition by prehabilitation might improve postoperative results [Citation1,Citation2]. Prehabilitation can consist of an exercise program, treatment of anemia, nutritional support, smoking cessation, psychological support and/or a medication review. However, the most effective and suitable program for the appropriate patient has not been established yet. It is necessary to tailor optimal prehabilitation protocols for specific target groups, since different populations may need a specific approach [Citation1–3].
Especially elderly patients, whose functional reserves are limited, are expected to benefit from prehabilitation [Citation4,Citation5]. Frail patients are at higher risk for severe postoperative complications, mortality within 30 days after surgery, postoperative functional impairment, loss of independence, and lower quality of life [Citation6–10]. So there are gains to be made in this population. Frailty assessment has a crucial role in predicting postoperative complications and may indicate the group of people that could benefit from prehabilitation [Citation11]. The current literature shows promising effects of prehabilitation in the elderly and oncological surgeons believe that prehabilitation can be beneficial [Citation5,Citation12]. However, good quality evidence on prehabilitation for these older patients is limited [Citation5].
The gynecological oncological population consists of a large part of frail elderly (around 25% of the patients aged 65 years and older [Citation9]. It would be interesting to investigate if prehabilitation is effective in improving postoperative recovery in this population, since no previous research has been done specifically in older and frail patients undergoing gynecological oncologic surgery, except from one case report [Citation13,Citation14].
Since no specific prehabilitation program is designed for this population yet, a suitable program, that is tailored to the elderly gynecologic oncological patient, should be constructed to be able to investigate its effectiveness. In order to develop a prehabilitation program endorsing the demands and abilities of the elderly patients, we decided to investigate the possibilities, barriers, and wishes of these patients are. It can be challenging to motivate patients to participate in a prehabilitation program and to adhere to lifestyle interventions [Citation15]. How people acquire therapy compliance is determined individually, particularly in the elderly, customization of care is important to endorse their needs [Citation16].
Besides the importance of knowing the opinions and needs of patients, it is also crucial to know what healthcare professionals, who will be involved in the program, think about prehabilitation and if they are willing to support it. Their opinion, expectations, and mission statement are relevant to design a program that is feasible for use in clinical practice [Citation17].
This study aims to reveal information that can be used for composing a prehabilitation program that will be tailored to elderly gynecological oncological patients and their healthcare professionals. This study will investigate opinions, thoughts, and desires of elderly gynecological oncological patients and healthcare professionals with regard to feasibility, the possible content and indications for prehabilitation, and what potential barriers might exist for both groups upon designing a prehabilitation program.
Material and methods
Study design and setting
This qualitative, multicenter study was performed using thematic analysis on semi-structured interviews of healthcare professionals and patients. The study was conducted from a biopsychosocial [Citation18] perspective on health. In all participating hospitals, preoperative and postoperative care is given by a multidisciplinary team, mostly consisting of at least a gynecologist, an oncologist, a radiotherapist, and often also an oncology nurse. If necessary, a dietician, physical therapist, social worker, spiritual counselor, and/or psychologist were involved as well. Analysis was done from a limited realist position, combining realist ontology with a constructivist epistemology [Citation19]. Inductive thematic analysis was used since our study aim was primarily exploratory. We also examined how themes were interconnected using mind mapping. This made it possible to create an outline for a prehabilitation program.
The study was undertaken in compliance with the Helsinki Declaration, Good Clinical Practice Guidelines, and the COnsolidated criteria for REporting Qualitative research (COREQ) [Citation20] and was approved by the Local Ethics Committee of Gelre Hospitals, the Netherlands. Written informed consent was obtained from all participants. All data were analyzed anonymously.
Participants
Patients
People with (high risk for) a gynecological malignancy were called patients and were eligible if they were 60 years or older (World Health Organization’s definition of older age [Citation21]), and if elective oncological gynecological surgery had been planned or performed (less than a year ago).
At first, eligible patients from the PREsurgery study (a currently running, multicenter prospective cohort study on preoperative physical status in gynecological oncological patients) were recruited for the current study (convenience sampling). To acquire maximum variability within our data, we aimed to include a heterogeneous sample with variation in age, educational level, diagnosis, and physical condition. To obtain this, purposive sampling was used to augment the cohort with extra patients. For purposive sampling, patients were recruited in Gelre Hospitals, a general teaching hospital, and the University Medical Center Groningen, all situated in the Netherlands.
Healthcare professionals
The samples of healthcare professionals consisted of gynecologists operating oncological patients, residents in gynecology, physical therapists, dieticians, and oncological nurses. Gynecologists and residents were recruited from different hospitals in the Netherlands (academic and non-academic). Physical therapists, dieticians, and oncology nurses were recruited from Gelre Hospitals. An e-mail was sent to the head of the departments, to inquire which healthcare professionals were available for an interview. Some gynecologists were asked personally via the network of one of the researchers.
Research team
The research team consisted of a multidisciplinary group of six researchers. One internist-geriatrician, three gynecologists, of whom one is a gynecological oncologist, one epidemiologist, all with extensive research experience, and one Ph.D. student. The Ph.D. student is a medical doctor (MD) since 2018 with experience in patient contact and research. She followed a course on qualitative research at the Radboud University, Nijmegen, The Netherlands.
Procedures and data assessment
The interview guide for the semi-structured interviews (shown in ) was developed by the research team in consultation with an elderly care physician with extensive experience in qualitative research. If new themes were found during an interview, these themes were added to the themes discussed in the following interviews.
Table 1. Interview themes.
Eligible participants were recruited by phone (patients) or e-mail (healthcare professionals) by one of the researchers. After informed consent was given, semi-structured interviews were performed by the Ph.D. student. The only thing all participants knew of the researcher was that she was an MD and PhD student with an interest in gynecology, doing research on perioperative care in elderly gynecological patients. The interviewer did not have a relationship with the patients or healthcare professionals she interviewed, with the exception of two gynecologists who had been her supervisors during clinical work in the year preceding the interviews (2019).
The location of the interviews was determined based on practical reasons. Interviews were performed face-to-face in the hospital, at patients’ homes, by using videoconferencing or by telephone. All interviews were in Dutch. Patients were permitted to have someone accompanying them during the interview. After a standardized explanation about prehabilitation, the themes of the interview guide were discussed ().
All interviews were recorded and transcribed verbatim. Transcripts were not returned to participants, because we wanted to obtain their first thoughts, without corrections. Field notes were made during the whole process of data collection and analysis [Citation22]. After every interview, a systematic note was made about the context of the interview and the interviewee. Notes on thoughts about the data and notes of discussions with different researchers were taken as well.
Participants’ characteristics were collected. For patients we collected demographic data (age, gender, education level, living situation), the medical information of the patient (medical history, kind of malignancy, kind of surgery, if the patient received neoadjuvant or adjuvant treatment, and in which phase of their treatment they were) and information on health literacy (using the Dutch version of the “Brief Health Literacy Screening Tool” (BRIEF) with average scores ranges from 1–5. An average score >2 indicates adequate health literacy [Citation23,Citation24]). For healthcare professionals, we collected data on age, gender, the clinic they work in, how long they were working in their profession, and if they had been working in another specialty before because all these factors could influence their point of view.
Every participant was asked about how they judged their own health in general and to rate it with a number ranging from zero to ten (0 indicates worst health thinkable, 10 indicates the best health possible) [Citation25]. They were also asked to rate (from zero to ten) how important their health was for them, if they thought they moved enough and if they thought they ate healthily.
The number of interviews we intended to perform was based on theoretical and inductive thematic saturation. Saturation was considered when additional data did not lead to new themes or ideas in a diverse group of participants [Citation26]. Saturation was assessed per study group, for example, patients and healthcare professionals. When saturation was reached in each group (patients and healthcare professionals, respectively), we performed two more interviews to confirm this. Saturation was discussed by two researchers.
Analysis
All statistical analyses (demographic data) were performed using SPSS, version 25.0 (Chicago, IL). Data analysis of the interviews was done with template analysis during data collection following an iterative/open methodology [Citation27,Citation28]. This made it possible to make amendments to our interview protocol during our study, to gain clearer understandings of the key concepts. The transcripts were read and reviewed several times. Data were analyzed using Atlas.ti software, version 8.
All data were inductively coded by one researcher, who also performed the interviews and was mostly involved with the data collection. The code tree was checked by another researcher, and doubts on coding were discussed. The codes summarize ideas or concepts that were collected in the interviews. All codes are summarized in a hierarchic system. The first interviews were openly coded, creating a codebook. After three interviews (with two gynecologists and one patient), axial coding was used. During the coding process, codes were checked for adequacy and the codebook was modified if necessary. The final codebook was discussed with the coauthors and a hierarchic code tree for facilitators and barriers for prehabilitation was developed through discussion.
An outline of a prehabilitation program was developed based on the facilitators and barriers and the other findings of the study. The other findings were summarized per the main theme (e.g., duration of prehabilitation, coordination, target group, etc.) by the first author. She selected the most relevant (based on the frequency of appearance) and outstanding findings by reading and reviewing the quotations belonging to these themes and associated subthemes several times. The selection was discussed with the coauthors, looking at relevancy for answering the research questions. Minor, but relevant details (e.g., time people could spend on exercising, kind of advice they would prefer considering protein-rich food) were left out of the main text, but can be found in the Supplemental material D (For the best interactive experience of Supplement D, please save and open the file with adobe.).
Results
Recruitment, sampling, and saturation
Patients
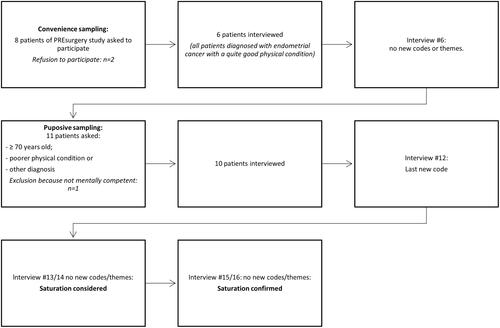
shows a flowchart of the recruitment, sampling, and saturation of patients. In total 19 patients were asked for an interview of whom 16 participated. At first, with convenience sampling, we asked eight patients of the PREsurgery study to participate in this study. Two of them refused to participate. In the sixth patient interview, no new themes or codes were retrieved anymore. Since these patients were all diagnosed with endometrial carcinoma and were all in quite a good physical condition, we started purposive sampling to include patients with other diagnoses, older patients, and patients with a poorer physical condition as well. The physical condition was estimated based on the clinical appearance of a patient as seen by the gynecologist and/or researcher.
At first, this resulted in five more patients participating (four with ovarian cancer and one patient with endometrial cancer). In the interviews with these five patients, no new themes or codes were generated either, but since we still missed older patients with a poorer physical condition, we continued with purposive sampling: Six more patients were asked to participate by their gynecologist on the basis of age (≥70 years old) and a lower physical condition. One patient was not mentally competent, the other five participated. In the first of these five interviews, one new code was generated. In the following two interviews, we did not find new themes, so saturation was reached. We did two more interviews to confirm saturation.
Healthcare professionals
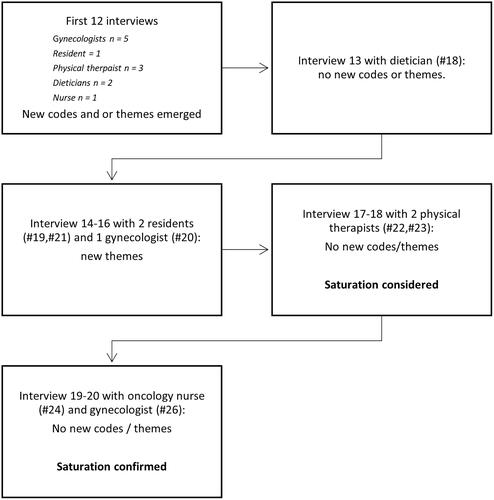
Supplemental material A shows the recruitment strategy of healthcare professionals. Reasons for not willing to participate were not given. In total, 20 healthcare professionals were interviewed. shows a flowchart on saturation. In the first 12 interviews, new themes were found in each interview. During the 13th interview with a caregiver (dietician #18) no new themes were discovered. This interview was followed by three interviews (with two residents and one gynecologist) with new themes, followed by four interviews without new themes (two physical therapists, one oncology nurse, and one gynecologist). After the interview with this second physical therapist, we considered the data-saturated, and performed two interviews that confirmed saturation in two other types of healthcare professionals (oncology nurse and gynecologist).
Participants
In total, we interviewed 36 participants, 16 patients, and 20 healthcare professionals. Interviews took between nine and 48 min (median 20 min). One interview with a patient was not recorded due to technical problems. After that particular interview, we directly made notes of this interview and transcribed it immediately afterward. The participant checked the interview afterward and approved it.
The median age of the included patients was 70 years (range 62–85 years). Seven patients had ovarian cancer, seven endometrial cancer, one vulvar cancer, and one cervical cancer. Fifteen patients underwent surgery, one rejected surgery because of her physical status. Most patients (n = 15) lived independently at home, one patient lived in sheltered housing. Educational level ranged from elementary school to university of applied science. Numeric Rating Scale (NRS) for general health ranged from 6 to 10 (median 8).
The median age of healthcare professionals was 47 years (range 27–63 years), 17 healthcare professionals were female and three were men. NRS for general health ranged from 7 to 10 (median 8). Seven gynecologists, three residents in gynecology, five physical therapists, three dieticians, one oncology nurse, and one medical secretary/assistant in gynecology were interviewed. Supplemental material B presents the characteristics of the individual participants.
Insights from the interviews
Thoughts on prehabilitation
All the participants, patients as well as health care providers, reacted positively to the idea of prehabilitation. Some healthcare professionals saw prehabilitation as an opportunity to talk about lifestyle in general and to motivate patients to live healthier, also after surgery. Participants suggested that patients should have an active role and be responsible for their own health and that prehabilitation can help them in feeling better.
Well, I think it is very good. Because, as I said, some people are going to sit and feel bad for themselves and I think that has a very negative impact. If you can do something, work on something, that works for me, and I think for everyone. (Participant #28, patient with ovarian cancer, 68 years old)
Most participants assumed that it is important to undergo surgery in good physical condition. But some participants suggested that if you only start to be physically active just before surgery, it might be too late. Most healthcare professionals, especially gynecologists and residents, mentioned that it was important for them that a prehabilitation program is evidence-based with a significant improvement of surgical outcomes.
Facilitators and barriers for prehabilitation
presents an overview of factors that patients and healthcare professionals mentioned that could have a positive or negative effect on prehabilitation. Supplemental material C presents illustrative quotations per factor.
Table 2. Factors that could have a positive or negative effect on different aspects of prehabilitation.
The most positive influencing factors on prehabilitation that were mentioned by our participants belonged to two main themes: the first one was “motivation” and the second “practical facilitators.”
The motivation theme is divided into two sub-themes: “Motivational reasons” and “motivational support.” One of the motivational reasons that were called was “patient sees the benefit.”
And mainly, I think the most important is, that people see the need for it. Because if they don’t see that, then they won’t do it. Then they will listen obediently to all that information, but at home, nobody watches them. (Participant #17, Gynecologist, female, 39 years old.)
Besides the need for motivational reasons, participants also mentioned things that could help patients in getting and keeping motivated (“motivational support”). This is divided into things that could be done individually and in support of others. Participants mentioned the following things that could help patients in getting and keeping motivated without the need for others: setting goals, seeing good results, and using an activity tracker. Patients also believed it could motivate them if prehabilitation is tailor-made for a patient.
Yes, you know, you have to like it. I can tell someone to go for a walk, but if you don’t like that, then you will not keep it up. You have to do something you like. When you cannot do something you don’t like, you won’t keep it up. (Participant #27, patient with ovarian cancer, 62 years old)
Participants referred to professionals and family and friends as possible motivators. Participants also described motivation by phone as an option. It was pointed out to be helpful if patients have a “big stick,” like going somewhere for prehabilitation, having a physical therapist visiting them, or doing it together with others.
Considering the second main positive theme “practical facilitators,” participants thought it probably works best for healthcare professionals if prehabilitation is part of a routine. According to healthcare professionals, a motivated team is necessary to start a successful prehabilitation program.
The two main negatively influencing factors on prehabilitation were “practical barriers” and “patient related factors.” Some patient characteristics, like physical condition, living alone, or being dependent on care can make it harder to fulfill a prehabilitation program for patients, participants said. It was mentioned by the participants, however also rejected by some patients, that the preoperative period can be stressful for patients. Patients who did not experience the preoperative period as stressful said for example that they did not have a lot of appointments and they had a level-headed attitude. Experiencing a stressful period can make it harder for patients to follow an extensive program.
There is a lot coming your way already. And if, besides that, you have to fit in all those other things that you aren’t familiar with. Because in my experience, when I sit down, then I really have to push myself to start doing something, because somehow you are a little paralyzed so to say… (Participant #29, patient with ovarian cancer, aged 63 years)
Practical barriers were divided into “practical barriers for patients” and “practical barriers in organization.” A possible organizational barrier that was called was “financial objections.” Some participants stressed that it should be clear how prehabilitation is being financed. Additionally, there should be enough personnel and space available, they said. Gynecologists stated they do not have enough time to have an active role in prehabilitation. Hence, healthcare professionals said someone else should coordinate the program and make sure there is good communication between healthcare providers, also if patients are referred to another hospital. Also, healthcare providers stated the importance of an evidence-based program.
Practical barriers for patients that were called were, for example, going to the hospital for prehabilitation. Therefore, participants said prehabilitation should be easily accessible for patients and the program should not be too complicated and demanding (e.g., if patients need to go for several appointments for more days a week, or if they have to do a lot of different things). To make it accessible, some thought that prehabilitation should take place in primary care as much as possible, close to a patient’s home. Patients and physicians also described that for most patients the time before surgery is short. Most gynecologists said they strive to operate on patients within 3–6 weeks after diagnosis, but sometimes patients are even operated on within 1 week. Healthcare professionals and patients described the short time period as the main barrier to prehabilitation.
Duration of prehabilitation
Since the short time period was called the main barrier for prehabilitation, participants were asked if they thought it would be desirable to postpone surgery for prehabilitation. Most patients and healthcare professionals said that postponing surgery would be stressful for patients. A lot of patients said that they would prefer to be operated on as soon as possible. Also, one gynecologist said that postponing surgery was difficult for himself as well:
In oncology, convincing someone to wait, and if after the operation the cancer stadium appears to be one stadium higher, yes, then you have to explain that it is not because of postponing surgery. That makes it difficult. (Participant #12, Gynecologist, male, 57 years old)
One gynecologist and one resident also said that it would be hard to postpone surgery if a patient has a lot of symptoms. It was also mentioned that for some tumors, postponement is not possible. Some gynecologists said that postponing surgery for some tumors is technically not a big problem, and if you can motivate people with evidence, it could be possible. Most patients also agreed that if they got good information, it could be possible to postpone surgery.
I think you should have a good reason then. If you say: “if you postpone it for three weeks, then you can work on your energy, or whatever.” If you have a clear picture, then I think I would accept it. But it has to be clear why. Not without a reason. But if they can explain that it is better for you, for your recovery, than I think I would accept it. (Participant #6, patient with endometrial cancer, 64 years old)
One patient, one resident, and the oncology nurse thought that it might be better not to tell the patient about the postponement, but just tell them about the operation date and prehabilitation program as if it is standard care. That might be less stressful for patients. On the other hand, the oncology nurse also mentioned that the patient should take that kind of decision for herself.
Future prehabilitation program
shows an outline of a prehabilitation program according to our participants. Supplemental material D shows an interactive version of providing extra information on ideas per discipline within a prehabilitation program.
Figure 3. Outline of the process of a prehabilitation program according to our participants. An interactive version of this figure with extra information on ideas per discipline within a prehabilitation program is available, see Supplemental material D (For the best interactive experience of Supplement D, please save and open the file with adobe.).
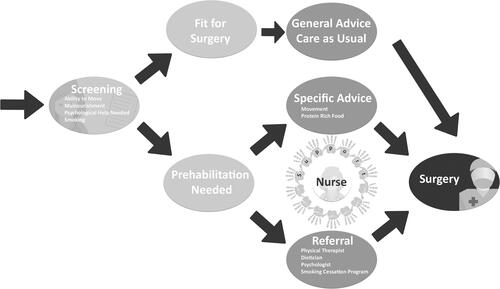
By formulating positively and negatively influencing factors, participants already said a lot about a future prehabilitation program. Besides the already mentioned factors, participants also mentioned the following things.
Target group
Defining the right target group for prehabilitation seems important. Some physical therapists said that they thought prehabilitation should be only given for patients who need it, the frailest group. That would be the most effective. Some patients said that they would not participate, because they were already in a good enough condition. A gynecologist said that everyone should at least have the opportunity to participate, because one cannot see directly if a patient needs specialized/supervised prehabilitation.
Next to functional limitations, the kind of treatment can also define the target group. Some gynecologists said that patients who receive neoadjuvant chemotherapy are a good target group, because they have more time preoperatively, and are in a poor condition more often. One gynecologist thought that patients who undergo minimally invasive surgery would benefit less from prehabilitation because they often recover quickly.
Tailor-made program and screening
Many participants stressed the importance of a tailor-made program. Each patient may have different impairments and needs. One patient might need psychological support, while the other needs physical exercises with a physical therapist the most, and yet another one is supported by some verbal and/or written advice only, according to our participants. Screening can help to reveal the needs of an individual patient, according to our participants.
Advice and information
According to our participants, advice and information (combined with motivation and support by an oncology nurse) will be sufficient to be able to prehabilitate for some. Face-to-face information is preferred.
I got a lot of information material, but I didn’t do a lot with that. You’re not reading it, orally is… I like it more face-to-face, because then you remember things you want to talk about, you are reminded of things by others. (Participant #29, patient with ovarian cancer, 62 years old)
Patients and healthcare professionals stressed that it is important for patients to have clear information. Receiving contradictory information from different professionals and changing plans are perceived to be confusing for patients.
Coordination
Who should perform screening and coordinate the program? One gynecologist said the following about coordination:
I think you should have one captain on the ship. If a lot of physicians are involved with a patient, nobody is responsible and everyone has his/her own part. Helicopter view, (…) One person should coordinate it. (…) Some sort of coach, who can accompany someone and the input from the different disciplines can come there. That could be done by an oncology nurse or physician assistant, something like that. (Participant #11, gynecologist, female, 48 years old)
One gynecologist said that they could do coordination themselves because the population of oncology patients is small enough. Also, one patient preferred to receive advice from her gynecologist instead of someone else. Some gynecologists thought that professionals from primary care, like a General Practitioner, should coordinate the program. But most participants thought an oncology nurse, a general nurse, or a medical secretary/assistant should do coordination. The gynecologist should only refer and inform patients about the importance of the program. Some came up with the idea of the oncology nurse themselves, and some were asked directly what they thought about that and responded in a positive way. A dietician and resident said the following on coordination:
And sometimes I think, there actually needs to be someone to make it organized. Because what you need to avoid is that a patient gets a diagnosis, and gets called by everybody and there’s not some kind of overview. So, that there’s somebody who’s kind of in charge maybe. … Maybe a nurse or something … a sort of umbrella person. But also a point of contact, so that if they don’t understand something, they have somewhere to turn to. (Participant #15, dietician, female, 28 years old)
I think, of course as a physician you do have some sort of signaling role, but I ultimately think that doesn’t necessarily have to be our job. Look, most oncology patients, of course, have an assigned nurse, who’s all over them, where she also provides low-threshold contact between the doctor and patient. And I think that’s someone who can take stock of that very well. …Kind of a nurse specialist…and that he or she just coordinates it, because I don’t think that really fits in with our tasks, in terms of time as well. (Participant #16, resident, female, 32 years old)
Many participants said that a nurse or oncology nurse could give lifestyle advice to patients and also screen for the need for extra care, like physical therapy, nutritional support, or psychological help, and refer the patient if that is indicated. A gynecologist said the following about this:
I think that the oncology nurse could really have the pivotal role. She’s much more into patient education and often calls these people again, and then she can ask them if they’re doing well. So I think that plays a role. … I think our role is limited. Because we actually see people quite briefly. You see people once and then you have a suspicion of, then you see them again one more time and then you say of it’s cancer. And then you either schedule the surgery here or you refer them. Uhm, so I really do see the role for the oncology nurse. … I think that if you leave it to the oncology nurses, I do know that as a very enthusiastic, good group, they can do something with that. (Participant #20, gynecologist, female, 54 years old)
An oncology nurse herself also said that oncology nurses could coordinate the program and play an important role in prehabilitation. Participants who are going to screen/coordinate the program desired clear guidelines or screening instruments.
Patients thought it could be helpful to have contact with a nurse to talk about prehabilitation, to support them along the way, and to help them to keep motivated. Patients who already had contact with an oncology nurse appreciated the positive low-key contact they experienced with them. One patient said for example:
It was nice to talk to the nurse, because you feel supported by someone that is also directing you in what is the best to do. … If you’ve had the first contact, you will call sooner to ask something. You will not call the gynecologist, there is something in between. You don’t want to bother him, and that aren’t questions he has to answer. (Participant #4, patient with endometrial cancer, 68 years old)
Discussion
This study aimed to reveal information that can be used for composing a prehabilitation program that will be tailored to elderly gynecological oncological patients and their healthcare professionals. The interviewed healthcare professionals stressed the importance of an evidence-based program before general implementation is possible. Although promising positive results of prehabilitation have been shown [Citation1,Citation2,Citation5], good quality evidence for prehabilitation in the elderly gynecological oncological population is still lacking [Citation13]. Our findings can be used for further research on the effectiveness of prehabilitation.
Patients and healthcare professionals were positive about the concept of prehabilitation. The main themes revealed from the interviews which have to be kept in mind when designing a prehabilitation program were “Motivation,” “Practical issues and facilitators.” and “Patient-related factors.” Interestingly, patients and healthcare professionals were of one mind about most themes. With the data from our study, we were able to construct an outline of a prehabilitation program ().
First of all, motivation and the patient seeing the benefit of prehabilitation were mentioned to be important. Healthcare professionals can play a role in motivating patients, as suggested by our participants. In a recent qualitative study by Polen-De et al., most women with advanced ovarian cancer (mean age 64 years old) did not exercise, but demonstrated high motivation and willingness to exercise during chemotherapy, particularly if it would have been recommended by their healthcare professionals and if they believe there will be a benefit on their treatment options or cancer cure [Citation29]. In line with our study, women in the study of Polen-De et al. also stated that the ability to contribute to their own health and distraction from negative thoughts was encouraging them [Citation29].
Furthermore, it seems essential to have a tailor-made program for each patient, because for every patient issues to work on might vary (i.e., physically, nutritionally, psychologically, and/or smoking cessation). Patients stated that it was important for them to have a patient-centered program and that they can do what suits their individual situation. This was also found in another qualitative study on prehabilitation among abdominal cancer patients [Citation30] and corresponds to the concept of a biopsychosocial health care model in which psychological, social, and medical issues interact [Citation18].
Participants stated that for some patients, who are already in quite a good condition, general advice might be enough to make them aware of the importance of preoperative preparation and keeping active. This statement is supported by a qualitative study among patients who were scheduled for major abdominal surgery (including for ovarian cancer). In that study, all patients received a leaflet with preoperative recommendations about preparation for surgery (among others about exercising, nutrition, relaxation, intoxications) [Citation30]. Patients actually did put an extra effort into preparing themselves, as they became aware of the small actions they could do that could be beneficial. They were able to increase their weekly exercise significantly [Citation31]. However, not feeling well or being in bad physical and/or psychological shape was a limitation for following the advice, which was also mentioned as a potential barrier in our study and by Polen-De et al. [Citation29,Citation30]. It is expected that for more frail patients, who are often also sedentary, general advice will not be enough to prehabilitate, whereas they probably belong to the group who will benefit the most from prehabilitation [Citation32–34]. Patients in bad physical shape could be referred to a physical therapist to do exercises under the supervision and/or to a dietician to receive specific advice. Patients with psychological problems could be referred to a psychologist. In order to be able to know which patients should be referred, screening was mentioned to be necessary. Our participants did not specifically mention screening with a comprehensive geriatric assessment (CGA). However, CGA can be of great value for identifying specific modifiable risk factors in frail patients, possibly as part of prehabilitation, as recommended by the International Society of Geriatric Oncology (SIOG) [Citation35]. Although participants were not specifically asked questions on geriatric involvement/CGA, it was clear to the participants that the study was about elderly patients. Therefore, there seems to be a gap in the existing knowledge on geriatric care by our participants.
We found that there seems to be an opportunity for screening and coordination of multidisciplinary prehabilitation by oncology nurses. A recent review article described the important role an oncology nurse could play in prehabilitation since nurses in particular are often the most consistent caregiver the patient sees throughout the whole treatment process [Citation36]. Patients and health care professionals were positive about coordination by a nurse and thought it could be beneficial if every patient has at least one contact with a nurse. Nurses could give general information on prehabilitation, as was given in the study of Beck et al. [Citation30], screen patients for impairments, malnutrition, and depression or anxiety, coordinate referrals and keep contact with the team of healthcare professionals, evaluate the overall effect and keep contact with patients for support and motivation. The involvement of the gynecologist was said to be important, to inform and motivate patients. The importance of empowerment by a physician as a facilitator for prehabilitation was also mentioned in other qualitative studies in abdominal cancer patients [Citation29,Citation37,Citation38]. But the major role suggested by our participants is for the oncology nurses and paramedics.
Since the preoperative period can be stressful for patients, which can make it harder to follow an extensive program, it seems desirable to organize prehabilitation close to a patient’s home and make the program easily accessible. The importance of easy access and the preference for a home-based or close-to-home program was also found in other qualitative studies with cancer patients and healthcare professionals [Citation29,Citation30,Citation37,Citation39]. They also stated the importance of a tailor-made approach [Citation30,Citation37,Citation39].
For most patients time before surgery is short, and postponing surgery was often said not to be desirable. So prehabilitation has to take place in a short period of time. A recent Canadian RCT in frail patients undergoing colorectal cancer surgery found that a 4-week prehabilitation program had no effect on postoperative outcomes. They also suggested that this time period was too short to find relevant results [Citation40]. Patients and healthcare professionals said that if postponement of surgery does not have an influence on the prognosis, postponement could be possible. There are few studies evaluating the maximum waiting time for surgery. For endometrial carcinoma, the safe maximum waiting time seems to be six weeks, especially for low stage tumors [Citation41]. For low stage cervical cancer, a waiting time longer than eight weeks was associated with poorer long-term survival after 5 years [Citation42]. However, these waiting times were not used for prehabilitation, which could possibly diminish the negative effects of long waiting. It is also not known if it is medically possible to postpone surgery for other gynecological cancer types. It could be interesting to investigate this, to find out if a longer prehabilitation period can be achieved and what minimal period is needed to achieve the effect of prehabilitation.
Strengths and limitations
Strengths: Firstly, this is the first study that qualitatively examined views of older people with a (high risk for) a gynecological malignancy and healthcare professionals on a possible prehabilitation program in gynecological oncological patients. It can be a starting point for further research on prehabilitation in this population since the evidence is lacking. Secondly, credibility[Citation43] was ensured by data triangulation, investigator triangulation, and prolonged engagement. We interviewed a wide range of people, from patients aged 62–85 years old to different types of healthcare professionals (both from general hospitals as well as university hospitals), revealing a comprehensive overview of thoughts on the subject from different angles. This makes our results generalizable to other Dutch healthcare settings and probably also to healthcare settings in other Western countries. We believe that most concepts we discuss are not context-dependent (for Western countries), however, some aspects could be different, such as waiting times. Several researchers were involved in the organizational aspects of the study and the process of analysis, and data analysis was done by two different researchers, attempting to increase objectivity. Our background in the field of gynecologic oncology enabled us to build trust with the participants and to obtain rich data. Third, confirmability[Citation43] was ensured by making field notes, including an audit trail of all research steps taken from the start of a research project to the development and reporting of the findings. Fourth, to make sure the reader can make a good transferability judgment[Citation43] for his/her specific situation, we give a thick description of our study, including several quotes, and we describe our research process carefully, transparently, and systematically using the COREQ[Citation20] standards. This adds to the validity and reliability of our study.
Limitations: The interviewer was inexperienced in performing qualitative research. However, as a medical doctor, she is experienced in patient contact, and along the way, she obtained more experience, which may have improved the quality of the interviews. Second, we did not analyze the differences between relatively older and younger patients, while older patients may have different needs than younger ones. However, the only new theme that was found in the older patients was the extra importance of psychological support, while younger participants did not specifically mention that. Nevertheless, this finding is in line with the need for a patient-centered approach, which was called by younger patients as well. Therefore, we do not think that this would alter our main findings and conclusion. Lastly, we chose not to interview geriatricians, because they are not involved in the care for these patients. We wanted to obtain the ideas and opinions of the healthcare providers that are working with this target group the most. However, geriatricians might have given us extra insights. We think that a comprehensive geriatric assessment could be part of prehabilitation for frail patients and could help in shared decision-making around surgery.
Further research
Based on previous research [Citation1,Citation2,Citation13], it is necessary to develop an evidence-based prehabilitation program targeted on the elderly gynecologic oncological patients, since current evidence on prehabilitation is lacking in this population. As recommended by Daniels et al., future studies should use methodologies designed for evaluating complex interventions, since prehabilitation programs are complex multi-component interventions [Citation5]. Complex interventions are defined as interventions that comprise multiple interacting components, are influenced by behaviors required by those delivering or receiving the intervention, have a number of groups or organizational levels targeted by the intervention, and require a degree of flexibility or tailoring of the intervention [Citation44]. We made the first step in the process from development through implementation of a complex intervention with this qualitative study. A second step could be a pilot and feasibility study of our proposed prehabilitation program in frail patients. If positive results (e.g., adequate feasibility) are found, the next step can be a randomized controlled trial.
The greatest barrier for effective prehabilitation seems to be the short time period prior to surgery. Further research should also clarify whether improved physical condition and improved postoperative outcomes can be achieved in this time period for prehabilitation or whether it would be better to postpone surgery.
Conclusion
In this study, we argued that prehabilitation, aimed to optimize postoperative outcomes, should be patient-tailored. Screening and coordination of a prehabilitation program by a nurse can ensure patient-centeredness. The main important facilitator for prehabilitation seemed to be “motivation.” Practical issues, such as a short time period and a too demanding program, may be barriers. Our findings can be used to further investigate the effectiveness of a tailored prehabilitation program for elderly gynecological oncological patients.
Supplemental Material
Download MS Word (27.8 KB)Supplemental_material_D_-_Figure_3_as_interactive_PFD.pdf
Download PDF (1.3 MB)Supplemental_material_B.pdf
Download PDF (133 KB)Supplemental_material_A.pdf
Download PDF (106.2 KB)Acknowledgements
The authors wish to thank Esther Helmich for her help with study design and methodology, Wouter Rijke for his help in using Atlas.ti, Mira Rasing for her language editing support, and Niek Teurlings for his help with the transcription of the interviews. The authors would like to sincerely thank the participants of the study for openly sharing their thoughts and insights.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol. 2018;44(7):919–926.
- Hughes MJ, Hackney RJ, Lamb PJ, et al. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg. 2019;43(7):1661–1668.
- Scheede-Bergdahl C, Minnella EM, Carli F. Multi-modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia. 2019;74:20–26.
- Wischmeyer PE, Carli F, Evans DC, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg. 2018;126(6):1883–1895.
- Daniels SL, Lee MJ, George J, et al. Prehabilitation in elective abdominal cancer surgery in older patients: systematic review and meta‐analysis. BJS Open. 2020;4(6):1022–1041.
- Courtney-Brooks M, Tellawi AR, Scalici J, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol. 2012;126(1):20–24.
- Driver JA, Viswanathan AN. Frailty measure is more predictive of outcomes after curative therapy for endometrial cancer than traditional risk factors in women 60 and older. Gynecol Oncol. 2017;145(3):526–530.
- George EM, Burke WM, Hou JY, et al. Measurement and validation of frailty as a predictor of outcomes in women undergoing major gynaecological surgery. BJOG. 2015;123:455–461.
- Kumar A, Langstraat CL, DeJong SR, et al. Functional not chronologic age: frailty index predicts outcomes in advanced ovarian cancer. Gynecol Oncol. 2017;147(1):104–109.
- Uppal S, Igwe E, Rice LW, et al. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137(1):98–101.
- Montroni I, Rostoft S, Spinelli A, et al. GOSAFE - Geriatric Oncology Surgical Assessment and Functional rEcovery after surgery: early analysis on 977 patients. J Geriatr Oncol. 2020;11(2):244–255.
- Saur NM, Montroni I, Ghignone F, et al. Attitudes of surgeons toward elderly cancer patients: a survey from the SIOG Surgical Task Force. Visc Med. 2017;33(4):262–266.
- Schneider S, Armbrust R, Spies C, et al. Prehabilitation programs and ERAS protocols in gynecological oncology: a comprehensive review. Arch Gynecol Obstet. 2020;301(2):315–326.
- Carli F, Brown R, Kennepohl S. Prehabilitation to enhance postoperative recovery for an octogenarian following robotic-assisted hysterectomy with endometrial cancer. Can J Anaesth. 2012;59(8):779–784.
- Essery R, Geraghty AW, Kirby S, et al. Predictors of adherence to home-based physical therapies: a systematic review. Disabil Rehabil. 2017;39(6):519–534.
- Jonkers CCM, Bakker CH. Artsen stellen behoeften van ouderen centraal. Ned Tijdschr Geneeskd. 2016;160:A8075.
- Fleuren MA, Paulussen TG, Van Dommelen P, et al. Towards a measurement instrument for determinants of innovations. Int J Qual Health Care. 2014;26(5):501–510.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136.
- King N, Brooks JM. Philosophical issues when using template analysis. Thousand Oaks (CA): SAGE Publications Ltd.; 2018. p. 13–23.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- WHO. Integrated care for older people: guidelines on community-level interventions to manage declines in intrinsic capacity. Geneva (Switzerland): World Health Organization; 2017.
- Phillippi J, Lauderdale J. A guide to field notes for qualitative research: context and conversation. Qual Health Res. 2018;28(3):381–388.
- Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–566.
- Fransen MP, Van Schaik TM, Twickler TB, et al. Applicability of internationally available health literacy measures in the Netherlands. J Health Commun. 2011;16(3):134–149.
- Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093.
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–1907.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
- Brooks J, McCluskey S, Turley E, et al. The utility of template analysis in qualitative psychology research. Qual Res Psychol. 2015;12(2):202–222.
- Polen-De C, Langstraat C, Asiedu GB, et al. Advanced ovarian cancer patients identify opportunities for prehabilitation: a qualitative study. Gynecol Oncol Reports. 2021;36:100731.
- Beck A, Thaysen HV, Soegaard CH, et al. Investigating the experiences, thoughts, and feelings underlying and influencing prehabilitation among cancer patients: a qualitative perspective on the what, when, where, who, and why. Disabil Rehabil. 2020;1–8.
- Beck A, Vind Thaysen H, Hasselholt Soegaard C, et al. Prehabilitation in cancer care: patients’ ability to prepare for major abdominal surgery. Scand J Caring Sci. 2021;35(1):143–155.
- Bruns ERJ, van den Heuvel B, Buskens CJ, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis. 2016;18(8):267–277.
- Carli F, Ferreira V. Prehabilitation: a new area of integration between geriatricians, anesthesiologists, and exercise therapists. Aging Clin Exp Res. 2018;30(3):241–244.
- Vigano A, Kasvis P, Di Tomasso J, et al. Pearls of optimizing nutrition and physical performance of older adults undergoing cancer therapy. J Geriatr Oncol. 2017;8(6):428–436.
- Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603.
- Lukez A, Baima J. The Role and scope of prehabilitation in cancer care. Semin Oncol Nurs. 2020;36(1):150976.
- Agasi-Idenburg CS, van Zuilen MK, Westerman MJ, et al. “I am busy surviving” - Views about physical exercise in older adults scheduled for colorectal cancer surgery. J Geriatr Oncol. 2020;11(3):444–450.
- Parker NH, Lee RE, O’Connor DP, et al. Supports and barriers to home-based physical activity during preoperative treatment of pancreatic cancer: a mixed-methods study. J Phys Act Health. 2019;16(12):1113–1122.
- Ferreira V, Agnihotram RV, Bergdahl A, et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer. 2018;26(8):2717–2723.
- Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer. JAMA Surg. 2020;155(3):233.
- AlHilli MM, Elson P, Rybicki L, et al. Time to surgery and its impact on survival in patients with endometrial cancer: a National Cancer Database study. Gynecol Oncol. 2019;153(3):511–516.
- Nanthamongkolkul K, Hanprasertpong J. Longer waiting times for early stage cervical cancer patients undergoing radical hysterectomy are associated with diminished long-term overall survival. J Gynecol Oncol. 2015;26(4):262–269.
- Korstjens I, Moser A. Practical guidance to qualitative research. Part 4: trustworthiness and publishing. Eur J Gen Pract. 2018;24(1):120–124.
- Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013;50(5):587–592.