Abstract
Purpose
To explore quantitative and qualitative features of anomia in participants with left-hemisphere stroke, Parkinson’s disease, or multiple sclerosis.
Materials and methods
This descriptive cross-sectional study compares signs of anomia within and across participants (n = 87), divided into four groups; moderate to severe anomia after stroke (MSAS, n = 19), mild anomia after stroke (MAS, n = 22), PD (n = 19) and MS (n = 27). Aspects analysed include naming accuracy and speed, the nature of incorrect responses, semantic and phonemic verbal fluency, information content in re-telling, and the relationship between test results and self-reports on word-finding difficulties and communicative participation.
Results
All groups had impaired verbal fluency, prolonged response times and reduced information content in re-telling. The MSAS group had significantly more signs of anomia than the other groups. Results from the other groups overlapped on a MAS—PD—MS continuum. Both semantically and phonologically incorrect responses were common in the stroke groups, while semantically incorrect ones predominated in the PD and MS groups. All four groups reported a similar negative impact on self-perceived communicative participation. Correlations between self-reports and test results were inconsistent.
Conclusions
Anomia features have quantitative and qualitative similarities and differences across neurological conditions.
IMPLICATIONS FOR REHABILITATION
People with moderate or severe anomia after stroke not only exhibit more severe symptoms of word-finding difficulties but also manifest a wide variety of such symptoms, compared to people with Parkinson’s disease or multiple sclerosis.
The present findings underscore the need to ask patients about their self-perceived word-finding difficulties.
Regardless of the degree of difficulties or the underlying condition, self-perceived word-finding difficulties can have a negative impact on communicative participation and should therefore be appropriately addressed.
An assessment comprising aspects such as verbal fluency, connected-speech tasks and the measurement of response times in naming tasks may serve to affirm the self-reported word-finding difficulties.
Introduction
Being able to communicate effectively is indisputably important to people. In everyday life, most situations require some kind of information exchange or social interaction, and even seemingly mild communication deficits can interfere with the interaction between human beings [Citation1–5]. An impaired ability to communicate can have major consequences such as reduced communicative participation and quality of life [Citation6–9]. In relation to the components within the biopsychological International Classification of Functioning, Disability and Health (ICF) framework [Citation10,Citation11], a health condition such as left hemisphere stroke, Parkinson’s disease or multiple sclerosis inducing structural damage to the brain, may cause difficulties retrieving words (body functions and structures). Difficulty finding the right word, anomia, is very common [Citation3,Citation12–14] and may interfere with activities and participation in various everyday communicative situations [Citation8,Citation15,Citation16]. Environmental and personal factors may additionally hinder or facilitate everyday communication.
The process of word retrieval relies on the interaction of multiple elements, including general cognition and executive functions, and may be vulnerable to various disorders. According to the interactive-activation model of speech production [Citation17], when a person is searching for a word, activation spreads through a network of interconnected nodes containing semantic or phonological features and the word reaching the highest level of activation is retrieved. However, insufficiently strong activation may cause failure to retrieve any word at all. Moreover, the constant interaction between the activated semantic, lexical and phonological information sometimes results in the retrieval of a word different from the intended target word or in phonological substitutions [Citation17].
One common cause of anomia is brain damage affecting areas important for language processing [Citation12,Citation14]. Brain damage can result from various neurological conditions such as infarction, trauma and tumours; the nature and degree of symptoms can vary considerably between people with the same neurological condition. Three of the most common acquired neurological conditions in the world are stroke, Parkinson’s disease (PD) and multiple sclerosis (MS).
Stroke–i.e. acute infarction or haemorrhage affecting the brain, retina or spinal cord–is a leading cause of death and disability worldwide [Citation11], with an annual incidence ranging from 171.98 to 198.75 per 100, 000 and prevalence from 1002.23 to 1167.80 per 100, 000 population [Citation18]. In Europe in 2017, it had a prevalence of 9.53 million and an incidence of over 1.1 million [Citation19]. According to the British Heart Foundation [Citation20], 38% of those suffering a stroke are middle-aged (40–69 years), although the mean age is 73 years for women and 68 years for men. Survivors are typically affected by persistent symptoms such as weakness or paralysis of limbs, apraxia and visual-perception deficits as well as cognitive impairments such as problems with memory, planning, concentration, executive functions and attention. About 64% of stroke survivors experience communication disorders, most often (28%) a combination of dysarthria and aphasia [Citation21]. Commonly, aphasia is associated with left hemisphere stroke. To this can be added a somewhat uncertain but probably substantial prevalence of communication disorders associated with right-hemisphere stroke [Citation22]. As lesion size and location vary greatly, so do the severity and nature of communication difficulties, potentially affecting any or all language abilities, as manifested in verbal-production, comprehension, reading and writing deficits. The extent to which symptoms regress during recovery varies [Citation23]; word-finding difficulties constitute the most persistent symptom of language problems in left-hemisphere stroke [Citation12].
In PD, aggregations of misfolded α-synuclein protein form Lewy bodies in neuron somas and Lewy neurites in axons and dendrites. The pathology starts in the enteric or peripheral nervous system (olfactory bulbs) and spreads to medullary and midbrain nuclei, including the substantia nigra. Later on, limbic and higher-order cortical areas become affected by Lewy pathology [Citation24]. Onset typically occurs in midlife or late in life, most commonly above the age of 50 years. In Europe, the estimated prevalence rate of PD is 108–257 per 100,000 population while the annual incidence rate is estimated at 11–19 per 100,000 population [Citation25]. There are various motor and non-motor symptoms associated with PD, such as rigidity, tremor, bradykinesia, postural instability, olfactory loss and autonomic dysfunction [Citation25]. About 40% of all people with PD have some kind of cognitive impairment [Citation26] affecting attention, visuospatial and executive functions or language. Over 92% of the respondents in a study by Schalling, Johansson [Citation13] reported some kind of speech and communication problem; word-finding difficulties were among the most common ones. Motor-speech disorders and word-finding difficulties combine to make it hard to understand the speech of persons with PD [Citation27]. Further, language difficulties in PD may involve impairments to the processing and production of grammar and syntax [Citation28,Citation29], to the understanding of implicit language [Citation30] and to the production of well-formed sentences [Citation30,Citation31], all of which may reduce the information content of utterances [Citation32].
MS is a neurodegenerative demyelinating disease causing inflammatory activity which leaves scar tissue, or plaques, in the grey and white matter of the brain and the spinal cord. Most people affected by MS are of working age since the mean age at symptom onset is 20–40 years. The reported European MS prevalence rates and annual-incidence rates vary greatly: 20–200 per 100,000 population and 1–10 per 100,000 population, respectively [Citation33]. The Nordic countries have high estimated prevalence and annual incidence rates: 150 per 100,000 population and 4.9–11.6 per 100,000 population, respectively [Citation33]. MS can be categorised as relapsing-remitting (RR) or progressive [Citation34] and as active or not active. Motor, sensory and cognitive symptoms can appear at any stage of the disease [Citation35,Citation36] and may subside, persist or worsen. Motor symptoms typically include weakness, tremor or spasticity in the limbs. Motor-speech deficits are common (45%); they usually result in ataxic or spastic dysarthria and affect respiration, voice quality, articulation and prosody [Citation37]. Sensory symptoms can include pain, visual problems and abnormal sensations. The estimated prevalence of cognitive impairment in MS is 47–70% [Citation38]. Cognitive deficits can affect memory, attention, processing speed and executive functions [Citation39,Citation40]. In addition, impaired word retrieval and verbal fluency are common in MS [Citation41,Citation42]. Language difficulties in MS may involve impairments in the processing and production of grammar and syntax, the making of inferences [Citation43,Citation44] and the structuring of connected speech, for instance in a narrative [Citation45]. In a study by Johansson, Schalling [Citation3], 78% of respondents with MS reported at least one communication-related symptom and word-finding difficulties were the most common such symptom.
Language ability is linked to other cognitive abilities, including executive functions, and language difficulties in PD and MS are still often viewed as signs of a general cognitive deficit [Citation42,Citation43,Citation46,Citation47] or considered part of dementia syndromes associated with those conditions. Extensive cognitive deficits in MS are sometimes classified as subcortical dementia or white-matter dementia [Citation48]. People with PD are at high risk of developing dementia at the later stages of the disease, especially if they have presented with mild cognitive deficits at an early stage [Citation26]. Cognitive decline can result in word-finding difficulties, possibly due to fading semantic or lexical concepts, as in dementia [Citation49]. By contrast, people with cortical lesions due to traumatic brain injury or stroke, despite exhibiting similar problems with cognition and executive functions as people with PD or MS, are commonly not considered to have dementia. Instead, it is assumed that people with post-stroke anomia have preserved semantic and lexical information but fail to access and retrieve it. In fact, it has been proposed that anomia in MS is likewise due to difficulty accessing intact semantic and lexical information, rather than to vague representations of concepts [Citation50–52]. However, regardless of whether word-finding difficulties in PD or MS are a sign of a general cognitive decline, a specific language deficit, or both [Citation53], we still lack knowledge about whether the consequences for the individual are similar to those in people with post-stroke anomia [Citation9]. Despite the fact that these self-perceived language difficulties are common in stroke, PD and MS alike, SLT assessment and treatment in MS and PD often focus solely on motor speech and swallowing difficulties. Although speech and language difficulties are known to co-occur and combine to worsen communication difficulties [Citation27], the word-finding difficulties are insufficiently addressed, evidence-based treatment for mild anomia is lacking, and access to SLTs is inadequately supplied [Citation3,Citation13,Citation54].
Communication difficulties often obstruct participation in social and leisure activities [Citation6,Citation9]. Having a satisfactory social network, and belonging to and participating in a group, is closely associated with a person’s quality of life [Citation9]. Moreover, leading an active and psychosocially fulfilling life may make a person more resilient to cognitive decline and hence counteract cognitive impairment caused by neurodegenerative conditions [Citation55]. This means that reduced participation due to communication difficulties can lower a person’s quality of life [Citation6,Citation56] and impose a heavy burden on his or her cognitive reserves [Citation55], making the issue of communication difficulties very important to address.
Anomia can manifest itself in a variety of ways, for example as failure to respond at all, as inaccuracy or latency in naming tasks and as problems staying on topic in conversations [Citation2,Citation3,Citation13,Citation42]. However, it is only recently that language deficits in people with PD or MS have received any greater attention. Such deficits can be masked by more prominent motor-speech problems, meaning that they can be overlooked in assessment and thus underdiagnosed [Citation3,Citation13] and untreated. Further, there is a lack of studies comparing the quantitative and qualitative signs of word-finding difficulties in various language tasks between participants with different neurological conditions.
Spoken word-retrieval ability is often assessed using word-fluency or confrontation-naming tasks. Confrontation naming of a specific single target word has been described as requiring a so-called convergent semantic processing skill, while word fluency tasks require a divergent semantic processing skill, as many, semantically or phonologically, similar words are requested [Citation57,Citation58]. The number of restrictions on what type of words are allowed in a fluency task has been considered to affect the task’s cognitive complexity [Citation59].
Performance on single word-retrieval tasks is known to be affected by certain features of the words used, such as their frequency of occurrence, word class, imageability, typicality, age of acquisition and phonological complexity [Citation49,Citation52,Citation60–62]. Specifically, abstract, low-frequency words are typically harder to retrieve than concrete, familiar, early-acquired, high-frequency words with high imageability. In terms of word class, nouns are typically more concrete and have higher imageability than verbs and thus tend to be retrieved and produced more easily and quickly, as has indeed been reported for some people with neurological conditions [Citation31,Citation60,Citation63,Citation64]. However, particularly in mild anomia, the symptoms can be very hard to detect or to distinguish from normal variation, in formal assessment [Citation30,Citation43,Citation65–69]. The use of insufficiently sensitive assessment materials is a possible reason why language difficulties go undetected when the symptoms involved are subtle.
Various ways to capture even mild symptoms of anomia in people with stroke, PD or MS have been suggested, including the use of tasks that place high demands on both semantic access and executive or other functions, such as verbal-fluency tasks with multiple restrictions [Citation59], and the measurement of response time in addition to accuracy in confrontation-naming tasks. It is often considered that a response time exceeding two seconds indicates the presence of word-finding difficulties [Citation42,Citation70]. Previous studies have found that compared with healthy subjects, people with anomia make significantly more and/or longer pauses in their speech, whether they have had a stroke [Citation70,Citation71] or have PD [Citation31] or MS [Citation42,Citation72]. A further method used to try to capture mild symptoms of anomia is to ask participants to produce connected speech rather than single words, for example by having them describe pictures showing several events or re-tell stories [Citation31,Citation73,Citation74]. Compared to single-word retrieval, connected speech tasks additionally require abilities to organize the words into well-formed phrases and sentences, and to choose the appropriate words for a particular context [Citation45,Citation75]. Thus, assessment tasks targeting both single-word retrieval and connected speech may reveal a greater range of word-finding difficulties. Moreover, the use of moving pictures may facilitate the elicitation of verbs and connected speech [Citation76], as the dynamic aspects of actions may be difficult to depict in still pictures.
It is also increasingly recognised that it is important to obtain information about communicative ability from the participants themselves [Citation8,Citation56,Citation77–79]. There can be a considerable discrepancy between the self-reported level of word-finding difficulties and the level detected through formal testing of confrontation naming or word fluency [Citation2,Citation31,Citation69,Citation80], indicating that, in order to obtain a comprehensive picture of a person’s communication deficit, it is a good idea to combine formal assessment tasks and self-reports.
There are a number of similarities in the neuropathology of stroke, PD and MS. For example, brain lesions in post-stroke anomia commonly affect cortical and sub-cortical areas, including the basal ganglia, like in PD. White-matter pathways may be disrupted in both stroke and MS, and additionally, grey-matter atrophy is present in MS [Citation81]. There may also be some similarities in the course of the conditions. Although PD and MS are both progressive neurological conditions while stroke is chronic or remitting, there may be acute relapses followed by remitting periods in MS. Finally, comparable cognitive deficits with regard to concentration, flexibility and processing speed occur in all three conditions. Even so, language difficulties are recognised as such to a much lesser extent in PD and MS than in left-hemisphere stroke–although people with all three conditions often report experiencing word-finding difficulties. We still lack knowledge about the quantitative and qualitative symptoms of word-finding difficulties in PD and MS as compared with left-hemisphere stroke for various tasks. To the best of our knowledge, no comparative study has yet been performed using an identical, comprehensive test battery for participants with all three conditions, including confrontation single word naming of both nouns and verb, oral verbal fluency, re-telling connected speech, and self-report measures pertaining to word-finding difficulties and communicative participation. The present study aims to explore quantitative and qualitative features of anomia in participants with self-perceived word-finding difficulties as well as stroke, MS or PD in order to address the following research questions:
When comparing groups of participants with stroke, MS or PD, what quantitative similarities and differences are there in
accuracy in confrontation-naming tasks involving nouns and verbs?
accuracy in phonemic and semantic oral verbal-fluency tasks?
accuracy in a re-telling task?
self-reported communicative participation?
What qualitative similarities and differences are there in
speed in confrontation-naming tasks involving nouns and verbs?
the nature of the inadequate responses in confrontation naming?
the proportion of correctly named nouns versus verbs in confrontation naming?
the proportion of phonemic versus semantic oral verbal fluency?
the proportion of accurate and complete versus incorrect or absent information as provided in a re-telling task?
What relationships are there between self-reports on word-finding difficulties and communicative participation, on the one hand, and formal-assessment scores for confrontation naming, verbal fluency and re-telling, on the other?
Materials and methods
Study design
This is a descriptive cross-sectional study comparing signs of anomia within and across groups of participants with stroke, PD or MS as well as self-perceived word-finding difficulties.
Ethics
Ethical approval for this study was received from the Regional Ethical Review Board of Gothenburg, Sweden (case numbers 622-12 and 506-16). All participants gave their written consent to participate. When needed, special efforts were made to ensure that the participants understood the information about the study, test instructions, and the content of the questionnaires, through the provision of picture support as well as both spoken and written information.
Participants
A total of 87 people aged 30–88 years who had between 6 and 25 years of formal education participated in this study. Of those, 41 had a unilateral left-hemisphere stroke (n = 29 ischemic; n = 12 haemorrhagic), 19 had PD and 27 had MS (n = 10 relapsing-remitting MS; n = 17 progressive MS). See for demographics and additional background variables.
Table 1. Participants.
Recruitment took place at clinics and through patient organisations across the south-western region of Sweden. Inclusion criteria were: (1) self-experienced anomia, (2) post-stroke (since at least six months) or PD or MS, (3) unimpaired or corrected hearing and vision, (4) sufficient auditory comprehension and stamina to participate in the assessment and (5) Swedish as a native language. Exclusion criteria were any other known neurological disease, injury or condition, drug abuse, untreated neuropsychiatric conditions, or severe dysarthria or apraxia of speech obstructing the assessment of language ability. However, mild motor speech difficulties were common in all groups, well reflecting the clinical reality.
There were significantly more women (n = 51) than men (n = 36), which reflects the higher prevalence of MS in women. The stroke group, unlike the PD and MS ones, included several participants with severe difficulties in verbal communication. Results on tasks requiring verbal responses inevitably depend heavily on the amount of speech produced. For this reason, the stroke group was divided into two in the analyses: one consisting of participants with moderate to severe anomia and one consisting of participants with mild anomia; the cut-off between the groups was set to 83% correct answers in a confrontation-naming task involving items from the Swedish version of the Object and Action Naming Battery (OANB) [Citation88]. This choice was based on previous studies, where the cut-off for mild anomia was about 72–83% accuracy in the BNT full version or BNT short forms [Citation89–94]. This cut-off yielded a “moderate to severe anomia after stroke” (MSAS) group with 19 members and a “mild anomia after stroke” (MAS) group with 22 members. In the MSAS group, the lesion location was frontal for one participant, middle for fourteen, posterior for three and unspecified for one. In the MAS group, it was frontal for five, middle for fourteen and multiple for three. Most participants (n = 21 out of 22) in the MAS group had fluent aphasia while all but four in the MSAS group had non-fluent aphasia. All participants with stroke underwent a comprehensive aphasia assessment prior to inclusion to ensure that they had sufficient auditory comprehension and stamina to participate in the upcoming assessments.
The MS group had significantly longer disease duration and significantly lower age than the other groups, reflecting that the age of symptom onset is lower in MS than in stroke or PD. There were no significant differences between the two stroke groups or between the stroke groups and the PD group with regard to either age or disease duration. Finally, there was no significant difference between any of the groups with regard to the level of education.
The participants rated the perceived frequency of word-finding difficulties in their everyday life on a scale from 0 = “not at all” to 7 = “every time I try to say something”; this was adapted from an item in the Sahlgrenska Academy Self-Reported Cognitive Impairment Questionnaire (SASCI-Q) [Citation87]. Mean ratings in all groups indicated anomia occurring daily (above 5) or several times a week (above 3). There were no significant differences between the groups regarding self-perceived anomia, with one exception: the MSAS group reported more frequent anomia than the PD group (p = 0.019). See for descriptive data on the participants.
As previously mentioned, word-finding difficulties are known to influence and be influenced by other factors. For this reason, several tasks were administered to explore background factors that might affect word retrieval, such as executive functions, or that might reflect the consequences of difficulties as mentioned in various self-reports. All results on the tasks exploring background variables are presented in .
First, since language ability is interconnected with other cognitive functions, and dependent on a general ability to plan, initiate and execute tasks, all participants were assessed for executive functions. All tasks were administered and scored in accordance with the original instructions. Note that all tasks include practice examples intended to ensure that the participants understood what they were asked to do.
The design-fluency task from the Delis–Kaplan Executive Function System (DKEF-S) [Citation85] and the Trail Making Test (TMT) [Citation95] both demand attention, concentration, psychomotor speed and flexibility in visually perceived tasks. The number-repetition task in the Clinical Evaluation of Language Fundamentals, fourth edition (CELF-4) [Citation84], targets attention, concentration and working memory in auditory tasks. The design-fluency task is seen as a non-verbal divergent fluency task, assessing the participants’ ability to generate different geometric patterns, without reflecting the impact of semantic language abilities such as in the verbal fluency tasks. The TMT requires some perceptive language skills, since letters and numbers are used, and the number-repetition task obviously requires expressive language skills.
Overall, the MSAS group exhibited the most difficulties in these tasks, especially–and unsurprisingly–in the number-repetition task, which requires a verbal response. In general, the results for many participants with stroke or PD indicated some executive dysfunction, while the MS group exhibited the least difficulties in the cognitive/executive-function tasks, often scoring within the normal range for healthy subjects.
Additionally, the Montreal Cognitive Assessment (MoCA) [Citation86] was administered to the participants with PD or MS. The MoCA was not administered to the participants with stroke, as the results would likely be affected by an inability to give verbal responses. The MoCA screens for general cognitive decline in tasks targeting executive functions, conceptual thinking, orientation, language perception and production, memory, calculations and visuospatial abilities. The PD group had significantly lower MoCA scores (p = 0.005) than the MS group, which implies that there were larger cognitive deficits in the PD group. Of the 19 participants with PD, 7 (37%) obtained scores indicating normal cognitive function while 9 (47%) had scores suggesting mild problems and 2 (10%) had scores indicating moderate problems. In the MS group, the scores of 19 out of 27 participants (70%) indicated normal cognitive function while 6 (22%) had scores suggesting mild problems and 2 (7%) had scores indicating moderate problems. All participants scoring below test norms had problems with the delayed-recall task; this may indicate a failing memory function. Some also had problems with verbal fluency and/or executive functions, visuospatial functions, and conceptual thinking.
Further, at the beginning of the assessment session, all participants rated their current state of health on a study-specific rating scale (from 0, representing the worst possible state, to 10, representing the best possible one). These ratings were used to detect any influence of “having a bad day” on participants’ assessment performance. No significant differences were found between any of the groups with regard to their current state of health (p = 0.499). No participants reported any ongoing relapses or any disease worsening. Many participants with PD reported having taken their medication soon before the assessment session, but such data are not available for all participants with PD.
Second, since word-finding difficulties are known to potentially affect health-related quality of life, the participants with stroke were asked to complete the Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39) [Citation79]. The SAQOL-39 contains four sub-scales (physique, communication, psychosocial and energy) where the degree of difficulties experienced during the past week is rated on a scale from 1 (“could not do”) to 5 (“no trouble at all”). The SAQOL-39 is specially designed for assessing HQoL in people with stroke and aphasia [Citation79].
Participants with PD or MS instead completed the Sahlgrenska Academy Self-Reported Cognitive Impairment Questionnaire (SASCI-Q) [Citation87], which contains questions about self-perceived cognitive function in everyday life; including communication, memory, concentration, planning and executing activities. The first questions concern the present state, where 0 = “No or rare difficulties” and 1 = “Frequent difficulties”, while the following questions compare the present level of functioning to that of earlier in life, where a positive difference corresponds to a reduction in the level of functioning. In addition, there are 20 general questions, some of which relate to physique, psychosocial aspects and energy, similarly to three of SAQOL-39 sub-scales [Citation79]. In the present study, the responses to 8 of those 20 questions were dichotomised into positive (1) or negative (0) reports, in line with Ellbin, Jonsdottir [Citation96] p 7. Of those questions, two concerned physique (“Walking at least three days a week for at least 15 min” and “Engaging in a physically demanding activity at least once a week”), four concerned psychosocial aspects (“Engaging in any form of leisure activity outside the home at least once a week”, “Gone to the movies, theatre, musical concerts, sport events or similar kinds of events, at least once a week”, “Met with friends, acquaintances or relatives at least once a week”, and “Experienced any strain or stress”), and two concerned energy (“Felt tired during the day, for half of the days or more” and” Cancelling planned activities owing to a lack of energy, once a week or more”) [Citation96].
The SAQOL-39 and SASCI-Q self-report questionnaires were sent in advance by post to the participants so that they could complete them at their own pace. At the assessment session, the forms were jointly reviewed by the participant and the administrator, and it was checked that they had been completed as intended. All participants in the MSAS and MAS groups, 94% in the PD group and 96% in the MS group gave a negative report in at least one of the sub-categories. Moreover, 95% of the participants in the MSAS group, 14% in the MAS group, 18% in the PD group and 32% in the MS group gave a negative report in each sub-category. There were no significant differences between the groups completing the same questionnaire (MSAS vs. MAS and PD vs. MS). See for demographics and the additional background variables.
Assessment materials and procedures
To enable comparison across groups, all participants performed the same word-retrieval tasks. The assessment aimed to capture quantitative and qualitative aspects of spoken word-finding difficulties in tasks targeting single words and connected speech. Note that all data are quantitative measures. However, some are considered to reflect qualitative aspects of communication, such as the ability to use a diverse vocabulary in an adequate and timely manner.
The quantitative aspects evaluated in this study include test scores for accuracy as well as total scores from questionnaires. The qualitative aspects concern the manifestations of the word-finding difficulties in terms of quantitative data on speed and on the qualitative content of the verbal output in a re-telling task. Moreover, analyses of inadequate responses, or any skewness between word classes used, are considered to reflect the quality of the verbal output. All materials and procedures are described below. Most participants carried out all tasks, but CPIB scores are lacking for one participant with MS and one with PD, while the OANB response time is lacking for one participant with MS, owing to technical problems.
Quantitative aspects
Accuracy in confrontation naming
The proportion of correctly named items (accuracy) of different word classes (nouns and verbs) and of various frequencies of occurrence was assessed in single-word confrontation-naming tasks. Black and white line drawings depicting 60 nouns and 60 verbs were selected from the Object and Action Naming Battery (OANB) [Citation88] based on being easily perceived, culturally appropriate, unambiguous representations of the target words, as pre-tested on a group of native speakers of Swedish without known brain damage (n = 104) [Citation97]. The mean accuracy score for those items in the OANB was 98.8% (range: 91–100%) in a reference group (n = 115) of neurotypical native speakers of Swedish [Citation98], with significantly more incorrect responses for verbs than for nouns.
The pictures were selected to cover a wide range of semantic and phonological features, and both nouns and verbs, with the aspects of word length and imageability taken into account. However, all of the OANB words had a high frequency of occurrence. For this reason, they were supplemented with a selection of 20 low-frequency nouns from the Boston Naming Test (BNT) [Citation99], based on the highest level of difficulty presented in the standardised Swedish version of the BNT [Citation100]. This means that the 20-item short form of the BNT used in the present study is not identical to any of the previously used BNT short forms [Citation92,Citation94]. Hence there are no reference norms for it; however, the individual items were correctly named by 17–88% of the 111 reference persons used to establish the Swedish norms. The norm for the full Swedish version of the BNT is 47.58 ± 4.15, or 79% accuracy [Citation100].
Accuracy in phonemic and semantic oral verbal fluency
The number of correct responses with regard to phonemic or semantic oral verbal fluency was assessed in three tasks. First, a phonological task involved asking participants to name as many words as possible beginning with the letters F, A and S, respectively, in one minute per letter [Citation101]. Second, in a semantic task, they were asked to name as many animals [Citation102] and as many verbs [Citation57] as possible, in one minute per category. Third, a semantically and cognitively more demanding verbal-fluency task, the Complex Oral Semantic Fluency (COSEF) test [Citation59], was administered to those participants deemed to have the sufficient naming ability (>75% accuracy on the OANB): n = 2 in the MSAS group, n = 20 in the MAS group, n = 18 with PD and all 27 participants with MS. The task in COSEF is to name, in two minutes, as many words as possible that share two semantic features. Two pairs of semantic features are used: “long and sharp” and “round and flat”.
Information-conveying in a re-telling task
The re-telling task used video clips of Lineman cartoons (La linea, courtesy of Quipos®) [Citation103] to collect connected speech in a task resembling an everyday communicative situation: telling a story or sharing an experience with someone. The cartoons are simple stick-figure drawings without busy backgrounds or distracting sub-events, where the Lineman character comes across different objects and performs various activities, speaking mostly nonsense language. For each participant, one out of three sets (A, B and C) was used. The three sets are similar in semantic content, length, level of difficulty and imageability. The gist of the plot in these events, as expressed in terms of a number of main concepts (MC), was extracted by the first and second authors from recordings of a reference group of 36 native speakers of Swedish without known brain damage, collected and explored in yet unpublished works [Citation104,Citation105]. Set A was found to contain 30 MCs while sets B and C contained 28 MCs each. An overall MC score (expressed as a percentage to enable comparison across sets) reflecting the participants’ ability to convey accurate and complete information about those pre-determined MCs was used as a quantitative measurement of their re-telling ability.
Self-reports regarding communicative participation
The participants were asked to complete the General Short Form of the Communicative Participation Item Bank (CPIB) [Citation7], where they rated how much their condition interfered with communicative participation in ten everyday situations, on a scale from 3 (“not at all”) to 0 (“very much”).
Qualitative aspects
Speed, inadequate responses and proportions of nouns and verbs in confrontation naming
The response time (RT) was measured for all correctly named items in the confrontation-naming tasks. Additionally, the nature of the inadequate responses in the confrontation-naming tasks was explored to understand how the word-finding difficulties manifested themselves. The proportions of correctly named OANB items for nouns and verbs were compared to explore any differences related to word class.
Phonemic versus semantic oral verbal fluency
The results on the phonemic verbal-fluency tasks (FAS) were compared with those on the semantic verbal-fluency tasks (animals and verbs), to explore any predominance of either over the other.
Accurate and complete information in re-telling
The proportion of MCs that were accurate and complete was compared with the proportion of them that were incorrect or absent. More information about this is found below in the “Assessment procedures and scoring” section.
Assessment procedures and scoring
The assessments took place in a quiet room at the University of Gothenburg, at speech and language therapy clinics in Gothenburg and Skövde, in the participants’ homes or on the premises of their patient organisation–always in the south-west of Sweden. In order to avoid the effects of fatigue, the assessment sessions did not exceed three hours in one day, with breaks every 45 min. Most participants finished in one or two sessions, i.e. 2—6 h. All participants had the opportunity to choose the time and date of the assessment sessions at their convenience, to take additional breaks and to end a session whenever they so wished. The assessments were video- and audio-recorded to enable later analysis. All assessment tasks were administered and scored in accordance with specified guidelines by experienced speech and language therapists (SLTs) within the research team, who prior to the assessments trained together to reach a consensus.
Confrontation-naming ability
The pictures in the confrontation-naming tasks (OANB and BNT) were presented as slide shows on a computer screen (with the requisite permissions of the copyright owners), and the participants were asked to name them. For pictures of nouns, the participants were asked “What is this?”, and for pictures of verbs they were asked “What is she/he doing?” or “What is happening in this picture?” In accordance with the original instructions for the administration of the BNT, each slide was shown for a maximum of 20 s. Then the software automatically changed to the next slide. If a definite response (correct or incorrect) was given within 20 s, the administrator manually changed to the next slide. Each transition (automatic or manual) to the following slide was marked by a click sound, which was used as a starting point when measuring the response time. To begin with, a set of 20 consecutive nouns and 20 consecutive verbs from the OANB was shown to each participant. If the participant correctly named 15 nouns and 15 verbs, a second set of 20 nouns and 20 verbs was presented, and if the participant was again successful, a final set was shown. The three sets used (A, B and C) were matched and comparable with regard to frequency of occurrence, visual complexity, word length and age of acquisition [Citation98]. This method of administration ensured that at least 20 items of each word class were presented to each participant and that participants with a severely impaired naming ability were not subjected to an unnecessary excess of items while participants with only mild anomia were shown a sufficiently large number of items.
For a response to be deemed correct, it had to be either identical to the target word, a correct hyponym or one of several predetermined synonyms. Moreover, a response was deemed correct if the participant corrected him- or herself within 20 s or if the response contained a single phonological error but was otherwise fully understandable. For all correct responses, the response time was assessed using Audacity®. It was measured in tenths of a second, from the click sound (occurring at the transition to each new slide) to the beginning of the correct word.
Any response that was not completely accurate was categorised as one or more of the following: (1) No response or abandoned attempt, (2) Single phonological paraphasia, (3) Multiple phonological paraphasia or neologism, (4) Related (single word) semantic paraphasia, (5) Unrelated (single word) semantic paraphasia, (6) Adequate (multiple words) circumlocution or association, (7) Inadequate (multiple words) circumlocution or association and (8) Visual misinterpretation.
Oral verbal fluency
All verbal-fluency tasks were administered and scored for accuracy (one point for each correct response) in accordance with the Swedish guidelines [Citation59,Citation106].
Re-telling ability
In the re-telling task, the participants were asked to account for the plot in video-clip Lineman cartoons shown to them on a computer screen. Each participant was presented with one of three sets of cartoons (A, B and C). The instructions were to give as much information as possible so that a listener might understand what was happening without seeing the clip. The clips (approximately 2.5 min long) were divided into 10-s sequences to ensure that any memory difficulties would not interfere with the performance of the task, and the administrator could pause the playback whenever the participants so wished. The time limit for this task was set to 15 min.
The recordings were scored for semantic correctness and completeness in relation to the pre-determined main concepts (MCs) in a main-concept analysis (MCA) [Citation73,Citation107]. Each MC was rated as either “Accurate and complete” (AC–3 points), “Accurate but incomplete” (AI–2 points), “Incorrect” (IN–1 point) or “Absent” (AB–0 points). Then a total MC score was calculated for each participant. To ensure comparability across the three cartoon sets (which did not all have the same number of MCs), the results are presented as percentages of the maximum possible score.
Self-reports on communicative participation
The CPIB self-report questionnaire was sent in advance by post to the participants so that they could complete it at their own pace. At the assessment session, the forms were jointly reviewed by the participant and the administrator, and it was checked that they had been completed as intended.
Statistical analyses
Owing to the limited number of participants, the unequal size of the groups and the non-normal distribution of the data, only non-parametric statistics were used. Independent-samples Kruskal–Wallis tests (two-sided) were used for all between-groups comparisons. Related-samples Wilcoxon signed-rank tests (two-sided) were used for analyses within the groups. All significant results were adjusted by Bonferroni correction for multiple comparisons. Two-tailed Spearman’s rank correlation was used to detect associations within the groups between test results, self-reports and demographic factors (age and level of formal education for all groups, and disease severity for the PD and MS groups). Eta correlation coefficients were used to detect associations between test results and lesion location for the stroke groups. All calculations were made in IBM SPSS Statistics for Windows, version 26.0.
Results
In this section, results regarding quantitative aspects of accuracy will be presented first, followed by results regarding the qualitative aspects of how the word-finding difficulties manifested themselves in terms of the speed and nature of the responses. Most results are presented as box plots showing the median, range and quartiles for each group; all statistically significant differences found are discussed in the text. All results are shown in .
Table 2. Scores on assessments.
Table 3. Group comparisons on quantitative aspects of word-finding difficulties.
Table 4. Group comparisons on qualitative aspects of word-finding difficulties.
Reliability
Agreement by two-way mixed, average-measures, absolute-agreement intraclass-correlation coefficient (ICC), as calculated on 30% of randomly selected recordings, was excellent [Citation108] for naming accuracy in the OANB: ICC = 0.97; 95% CI [0.96–0.97] and in the BNT: ICC = 0.93; 95% CI [0.92–0.93] as well as for the response time within 0.1 s (ICC = 0.98; 95% CI [.98–.98]). Likewise, it was excellent for oral verbal fluency (FAS: ICC = 0.99; 95% CI [0.99–1.0], Animals: ICC = 0.99; 95% CI [0.99–1.0], Verbs: ICC = 0.99; 95% CI [0.99–1.0], COSEF: ICC = 0.99; 95% CI [0.99–1.0]). For the main-concept analysis (MCA), agreement was good: ICC = 0.89; 95% CI [0.87–0.90].
Findings with regard to quantitative aspects of word-finding difficulties
In summary, all four groups had impaired verbal fluency, compared with test norms, and they all had difficulties providing accurate and complete information in the re-telling task. Both stroke groups had an impaired confrontation-naming ability, while the groups with Parkinson’s disease (PD) and multiple sclerosis (MS) had higher accuracy scores. On most assessment tasks, the group of participants with moderate to severe anomia after stroke (the MSAS group) obtained significantly lower scores than the other three groups. The PD group’s scores were often very similar to those of the group of participants with mild anomia after stroke (MAS) and to those of the MS group. The MS group obtained high scores on almost all formal-assessment tasks. However, it should be noted that all four groups reported similar negative consequences on their self-perceived communicative participation.
Accuracy in confrontation-naming tasks involving nouns and verbs
The accuracy of naming ability was assessed for a selection of high-frequency nouns and verbs from the Object and Action Naming Battery (OANB) [Citation88] and a selection of low-frequency nouns from the Boston Naming Test (BNT) [Citation99]. Since participants completed varying numbers of items, the results (see and ) are presented as percentages to enable comparison.
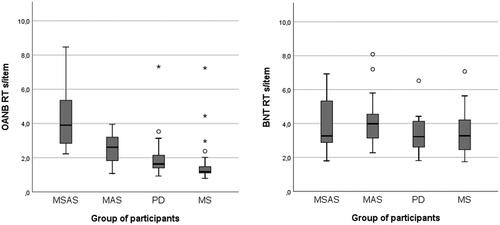
Figure 1. Confrontation-naming accuracy (in per cent) for high-frequency nouns and verbs (OANB, top left) and low-frequency nouns (BNT, top right). OANB: Object and Action Naming Battery; BNT: Boston Naming Test; MSAS: Moderate to severe anomia in stroke; MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis.
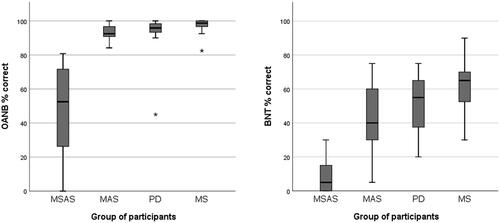
Using the OANB cut-off value of 83% accuracy, all of the participants with MS (n = 27) and all but one of the participants in the PD group (n = 19) either had mild anomia or unimpaired confrontation naming ability. The remaining participant with PD scored below the cut-off and so had moderate to severe anomia. However, since there were no indications that this extreme value was due to a measurement error or to any known deviancy from the inclusion criteria, this participant was not excluded from the sample. Instead, it was considered that this value reflected the variation in word-finding ability seen among people with PD. Even so, as recommended by Stevens [Citation109], additional results with this participant excluded are presented for comparison in the Appendix. When it comes to whether differences between groups were statistically significant, only one result (for animal fluency) was affected by the exclusion of this participant.
The MSAS group differed very strongly from all the other groups, with significantly lower accuracy for the OANB nouns and verbs (p < 0.001). The MAS group scored significantly lower than the MS group (p = 0.016), but other than that there were no statistically significant differences between the groups, see and .
With regard to accuracy for the low-frequency BNT nouns, the results were similar: the MSAS group had significantly lower scores than the other groups (p < 0.001) and the MAS group was significantly outscored by the MS group (p = 0.022) but there were no other significant differences between groups, see and .
Within the groups, a significant difference (p < 0.001 in all four cases) was found between naming accuracy for low-frequency items and naming accuracy for high-frequency items. The MAS, PD and MS groups’ mean scores are considerably below the 72–83% BNT cut-off for mild anomia used in previous studies [Citation89–94]. However, it should be noted that the items selected and used in the present study were restricted to those with a relatively high level of difficulty.
Accuracy in phonemic and semantic oral verbal fluency
Phonemic and semantic oral verbal fluency was assessed in terms of the number of correct responses; results are presented in and , and in .
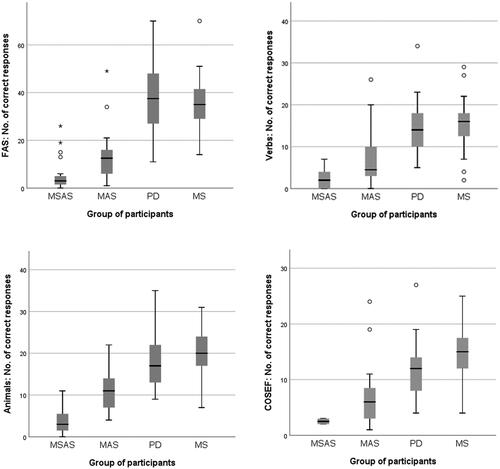
Figure 2. Scores on the oral verbal-fluency tasks. MSAS: Moderate to severe anomia in stroke; MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis; COSEF: complex oral semantic verbal fluency.
Phonemic oral verbal fluency (FAS)
All groups scored below the Swedish norms, which (for those with more than 12 years of formal education) are 49.0 ± 13.3 for 30–64-year-olds and 45.2 ± 10.1 for those aged 65 or above [Citation106]. Both groups of participants with stroke produced significantly fewer correct responses in the phonemic verbal-fluency task than the other two groups (p < 0.001). There were no other significant differences in FAS scores between groups.
Semantic oral verbal fluency
Animals
All groups scored below the relevant norms, which are 27.1 ± 5.4 (below 65 years of age) and 20.6 ± 5.7 (65 or older) [Citation106]. The MSAS group produced significantly fewer correct responses than the MAS group (p = 0.029) and than the PD and MS groups (p < 0.001 in both cases). Once again, the MAS group produced significantly fewer correct responses than the MS group (p < 0.001). No other significant differences between groups were found.
Verbs
Again, all groups had impaired fluency relative to test norms for people with 12 years or more of formal education: 22.3 ± 6.4 for those below 65 years of age and 19.4 ± 5.6 for those 65 or older. Like in the FAS task, both groups of participants with stroke produced significantly fewer correct responses in the verb task than the PD and MS groups (p < 0.001 in all cases except MAS vs. PD, where p = 0.015). There were no other significant differences between the groups.
COSEF (complex oral semantic verbal fluency)
Again, all groups scored below the reference value (16.95; SD = 5.58 [59]), both stroke groups had significantly fewer correct responses than the MS group (MSAS: p = 0.033; MAS: p < 0.001) and there were no other significant differences between groups. However, it should be noted that only two participants in the MSAS group performed the COSEF task, meaning that the result for that group should be viewed with due caution.
Accuracy in re-telling
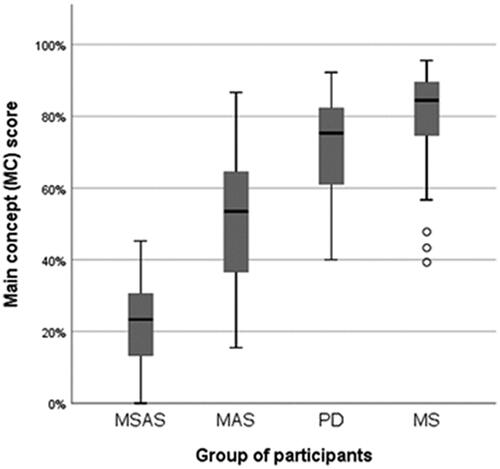
All groups scored below the reference mean MC score (87.2%) [Citation104]. Like for many other tasks, the MC score was significantly lower in the MSAS group than in the MAS group (p = 0.008) and in the groups of PD and MS participants (p < 0.001 in both cases). Again, like often before, the only other significant difference in total MC score was found between the MAS group and the MS group (p = 0.003). See and and .
Figure 3. Total scores (percentages) on the re-telling task, based on main-concept analysis (MCA). MSAS: Moderate to severe anomia in stroke. MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis.
Scores for self-reported communicative participation
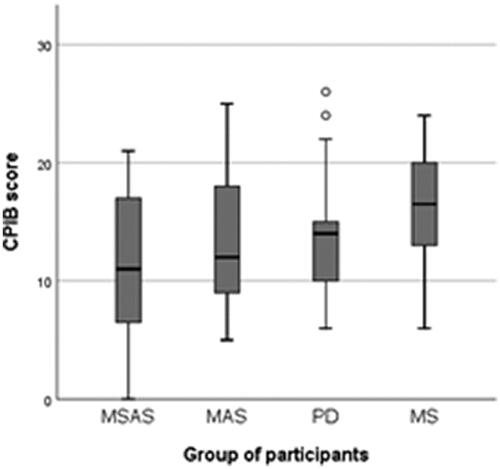
There were no statistically significant differences between any groups (p = 0.099) with regard to their self-perceived communicative participation as measured by the Communicative Participation Item Bank (CPIB) [Citation7], see . As can be seen in , several participants in each of the four groups experienced that their condition had a profound negative impact on their communicative participation in everyday situations. By contrast, there were also participants in each group who experienced only minor interference with their communicative participation.
Figure 4. Self-perceived communicative participation as reported in the 10 –item short-form Communicative Participation Item Bank (CPIB), where interference with communicative participation were rated from 3 (“not at all”) to 0 (“very much”). MSAS: Moderate to severe anomia in stroke; MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis.
Findings with regard to qualitative aspects of word-finding difficulties
In summary, all four groups had fairly long response times in the confrontation-naming tasks. However, in the PD and MS groups, this was more common for low-frequency than high-frequency items, while long response times were more or less equally common in the stroke groups for both categories of items. A further finding is that the word-finding difficulties observed in confrontation-naming and word-fluency tasks were both semantic and phonological in nature for the two stroke groups but predominantly semantic in nature for the PD and MS groups. Results are presented in and .
Speed in confrontation-naming tasks involving nouns and verbs
The MSAS group had a significantly longer mean response time for correctly named items in the OANB than the PD group (p = 0.002) and the MS group (p = 0.004). Further, the MAS group had a significantly longer response time (p < 0.001) for such items than the MS group. There were no other significant differences between groups with regard to the response time for correct OANB items, see .
There were no significant differences between any of the groups with regard to the response time for correctly named items in the BNT (p = 0.367), see . Even so, it is clear from the two graphs in , first, that there were participants in all four groups who had a clearly prolonged mean response time (> 2 s) and, second, that the participants with PD or MS were more likely to have a prolonged response time for more difficult items.
Figure 5. Response time (RT) in seconds per item in confrontation naming of high-frequency nouns and verbs (OANB) and low-frequency nouns (BNT). OANB: Object and Action Naming Battery (high-frequency items); BNT: Boston Naming Test (low-frequency items); MSAS: Moderate to severe anomia in stroke; MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis.
Nature of inadequate responses in confrontation naming
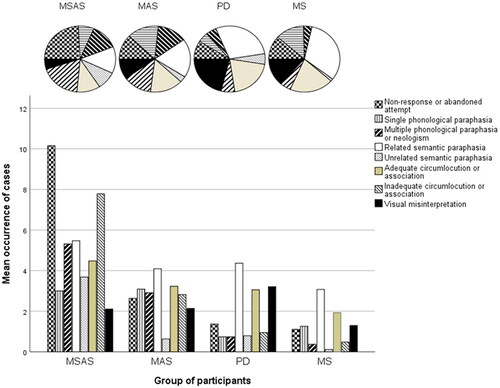
All inadequate responses in the confrontation-naming tasks were analysed and categorised as described above. Results are shown in and in and . The proportion of “no response or abandoned attempt” was significantly higher in the MSAS group than in the PD group (p = 0.018) and the MS group (p = 0.006). The proportion of “multiple phonological paraphasias or neologisms” was significantly higher in the MSAS group than in the MS group (p = 0.025), and in the MAS group than in the PD group (p = 0.035) and the MS group (p = 0.014). The proportion of “related semantic paraphasias” was lowest in the MSAS group and highest in the MS group. By contrast, the proportion of “unrelated semantic paraphasias” was lowest in the MS group and highest in the MSAS group–although this category was rare in all four groups. When it comes to “related semantic paraphasias”, there was a significant difference between the MSAS and MS groups (p = 0.001) and between the MSAS and PD groups (p = 0.007). For “unrelated semantic paraphasias”, there were significant differences between the MSAS and MS groups (p < 0.001), between the MSAS and MAS groups (p = 0.010) and between the PD and MS groups (p = 0.048). Finally, the proportion of “visual misinterpretation” was significantly higher in the PD group than in the MSAS group (p = 0.010). No other significant differences between groups regarding the nature of the inadequate responses were found.
Figure 6. Categories of inadequate responses in confrontation naming, as proportions (top) and as mean numbers of cases (bottom). MSAS: Moderate to severe anomia in stroke. MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis.
Proportion of correctly named nouns vs. verbs in confrontation naming
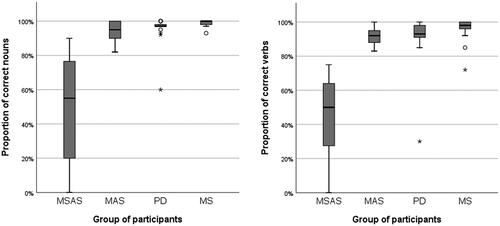
Yet again, the MSAS group named a significantly lower proportion of OANB verbs correctly than the other three groups (p < 0.001 in all cases). Further, there was a significant difference in verb-naming accuracy between the MAS group and the MS group (p = 0.031) but not between any other groups. Similarly, the proportion of correctly named nouns was significantly lower in the MSAS group than in the other groups (p < 0.001 in all cases), and there was a significant difference in noun-naming accuracy between the MAS group and the MS group (p = 0.035) but not between any other groups. Within-group comparison of word-class accuracy showed that producing verbs was significantly more difficult than producing nouns for the members of the MSAS (p = 0.031), PD (p = 0.004) and MS (p = 0.005) groups but not for those of the MAS group (p = 0.084). See and and .
Figure 7. Percentages of correctly named nouns and verbs in OANB confrontation naming. MSAS: Moderate to severe anomia in stroke. MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis.
Phonemic vs. semantic oral verbal fluency
For within-group comparison between semantic and phonemic fluency, scores for animals and scores for verbs were compared with a composite measure reflecting the mean score on the three sub-tasks of the phonemic FAS task (relating to each of the letters F, A and S). All four groups scored higher on the animal task than on the FAS task: p = 0.044 in the MSAS group, p < 0.001 in both the MAS group and the MS group, and p = 0.009 in the PD group. However, a significant difference between scores on the FAS task and the verb task was found only in the MAS group (p = 0.007) and the MS group (p = 0.001), not in the MSAS group (p = 0.255) and in the PD group (p = 0.214). See and .
Proportion of accurate and complete information vs. incorrect or absent information provided in the re-telling task
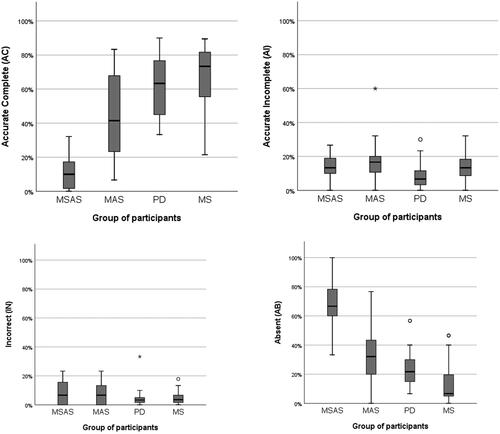
Compared with the reference group of healthy subjects, all groups had smaller proportions of accurate and complete main concepts (AC) but larger proportions of accurate but incomplete (AI), incorrect (IN) and absent (AB) main concepts. The mean MC score in the reference group was 87.2%, with 82.7% ACs, 5.7% AIs, 2.0% INs and 9.6% ABs on average [Citation104]. Further, in line with the results reported for other measures, the MSAS group had a significantly lower percentage of ACs than the MAS group (p = 0.002) and the PD and MS groups (p < 0.001 in both cases). Also in line with previous results, the MAS group had significantly fewer ACs than the MS group (p = 0.020); no other significant differences between groups were found. See results in and .
The percentage of AIs was low in all groups. The only significant difference (p = 0.004) with regard to this was between the PD group, which had the smallest proportion of AIs, and the MAS group. INs were also rare in all groups; there were no significant differences between any of them.
Finally, the percentage of ABs was significantly higher in the MSAS group than in the MAS group (p = 0.003) and the PD and MS groups (p < 0.001). As often, there was a significant difference between the MAS group and the MS group (p = 0.015) but not between any other groups. See .
Figure 8. Results from the main-concept analysis (MCA) of the re-telling task: percentages of accurate and compete (AC), accurate but incomplete (AI), incorrect (IN) and absent (AB) main concepts. MSAS: Moderate to severe anomia in stroke. MAS: Mild anomia in stroke; PD: Parkinson’s disease; MS: Multiple sclerosis. The corresponding proportions in a reference group were 82.7% AC, 5.7% AI, 2.0% IN and 9.6% AB.
Correlation between self-reports on word-finding difficulties and communicative participation, on the one hand, and formal assessment of confrontation naming, verbal fluency and re-telling, on the other
The frequency of anomia and communicative participation (“Anomia” and “CPIB” in ) as reported by participants did not consistently correspond to their accuracy in confrontation naming, their verbal fluency or their re-telling ability. This was particularly evident in the MS group, where the CPIB ratings did not correlate with any of the assessment results and where the self-reported occurrence of anomia correlated only with a single measure: the OANB response time (positive correlation). A similarly weak relationship between self-perceived difficulties and reduced naming speed was also found in the other groups, where CPIB scores correlated positively with scores on at least one of the verbal-fluency tasks, or negatively with the response time in confrontation naming, or both. Note that a high CPIB score represents less interference with communicative participation while a high Anomia score represents the more frequent occurrence of word-finding difficulties.
Table 5. Correlations between self-reports and formal assessments.
It was only in the group with the most severe anomia (the MSAS group) that the CPIB score correlated (positively) with scores on the re-telling task.
Known confounding factors such as age and level of education did not correlate strongly with the assessment results. Age correlated positively only with OANB accuracy in the MAS group and negatively only with the main-concept (MC) score in the PD group. Level of education correlated negatively with BNT response time in the MSAS and PD groups, and positively with verbal fluency in the PD and MS groups. Finally, the level of education correlated positively with the MC score in the MS group.
Additionally, group-specific analyses were performed to investigate the relationship between assessment-task scores and, first, disease severity in the PD group (H&Y scale) and the MS group (EDSS scale) and, second, lesion location in the stroke groups. No significant correlations were found in the PD group. In the MS group, disease severity only correlated (positively) with the proportion of “inadequate circumlocutions and associations” in the re-telling task, rs = 0.480, p = 0.023. In the stroke groups, there were only weak relationships between lesion location and assessment-task scores: ƞ = 0.001–0.050 (ƞ2 < 25%). Only the proportion of “single phonological paraphasias” in the naming task correlated with a more anterior lesion location: ƞ = 0.628 for both stroke groups combined, ƞ = 0.448 for the MSAS group and ƞ = 0.720 for the MAS group.
Discussion
This study aimed to describe and compare quantitative and qualitative features of word-finding difficulties within and across groups of participants with either left-hemisphere stroke, multiple sclerosis (MS) or Parkinson’s disease (PD) and self-reported anomia; the group of participants with stroke was split into two groups, based on the severity of anomia. Overall, there were found to be both quantitative and qualitative similarities and differences in confrontation naming, verbal fluency, re-telling ability and self-reported difficulties between the groups. Based on this, it was generally possible to place the conditions along an overlapping continuum with regard to quantitative and qualitative signs of anomia, with the participants with stroke and moderate to severe anomia (MSAS) at one end, followed first by the participants with stroke and mild anomia (MAS) and then by the participants with PD, and with the participants with MS at the other end. However, some clear and statistically significant differences were identified.
Quantitatively, the main findings from the present study were that all groups had impaired verbal fluency as well as difficulties providing sufficient information in re-telling. Within-group comparisons revealed a greater range of results in stroke, as the scores typically were disparate within both stroke groups. Contrary, most results were similar among participants with PD or MS. As expected, the MSAS group had significantly lower test scores than the other groups. The accuracy scores of the PD group most often overlapped with those of the MAS and MS groups. However, despite these disparate test results, the self-perceived negative consequences on communicative participation were similar in all four groups.
Qualitatively, the main findings were that all groups had prolonged response times in confrontation-naming tasks and that, while both semantic and phonological word-finding difficulties were common in the stroke groups, such difficulties were predominantly semantic in nature in the PD and MS groups. Further, there were discrepancies between test results and self-reports in all groups.
In single-word confrontation-naming tasks involving nouns and verbs, both accuracy and speed (response time) were affected in the MSAS group. By contrast, most participants in the PD and MS groups obtained high scores for accuracy, although some individuals had observable difficulties that were possible to capture with commonly used naming tasks. One noteworthy finding is that confrontation-naming accuracy in the PD group was comparable to that in the MAS group. This supports the claim that word-finding difficulties are probably underdiagnosed in PD [Citation13].
Regarding word class, the MSAS, PD and MS groups all found verbs to be more difficult to retrieve than nouns in confrontation naming. That the MS and PD groups, despite having only minor confrontation-naming difficulties in general, still had significantly greater difficulty naming verbs than nouns might indicate that verb-retrieval ability is more sensitive even to mild disturbances. Previous studies have shown verb deficits to be more severe than nouns deficits in stroke, PD and MS [Citation31,Citation63,Citation64]. Other studies have found that the noun–verb dissociation in aphasia is relative and that there is great individual variability [Citation93]. Such variability was in fact evident in the MAS group in the present study. While verb fluency was markedly impaired in that group, scores on the other fluency tasks were also low, and verb naming was not statistically significantly more difficult than noun naming. However, to the MSAS and PD groups, the verb fluency task was as demanding as the phonemic task while the animal fluency task was less challenging. Hence noun–verb dissociation was found to occur in confrontation naming and verbal fluency regardless of aetiology, although not consistently.
Accuracy in confrontation naming was not a sufficient measure to capture relatively mild word-finding difficulties, as evidenced by the fact that several participants had only a few inadequate responses in those tasks. However, most of them had several instances of prolonged response times well in excess of the two-second limit that is considered to mark the end of the normal range [Citation70]. It should also be noted that response time was measured only for correctly named items. Response times over 20 s were not included in the response-time analysis but counted as inadequate responses. This means that, particularly in participants with low accuracy scores, the “real” average response time could be even longer. For participants with high accuracy, long response times were the most common for the low-frequency BNT items. This was unsurprising, since the frequency of occurrence is a factor known to impose on word retrieval. Even so, the response times for those items were remarkably long in all groups, both at the group level and for specific individuals. These findings are well in line with previous research stating that response time is a more sensitive measure than accuracy and so can be used to detect even mild anomia [Citation42,Citation70]. The importance of the temporal aspect was evident in the fluency tasks as well. It should be noted that most of the participants in the present study had impaired oral verbal fluency according to Swedish norms [Citation59,Citation106]; this suggests that word fluency is a fairly sensitive measure when it comes to detecting anomia. The correlations found between self-reported difficulties, on the one hand, and response times in naming tasks and verbal fluency scores on the other, could indicate that the latency of response is highly noticeable to those who experience it. This would mean that both accuracy and speed may be important targets for SLT intervention, as suggested by De Dios Pérez, Cordova Luna [Citation42].
All groups found the phonemic word-fluency task harder than the semantic task. This difference in difficulty is in accordance with the norms, where the (semantic) animal task typically generates the most correct responses and the (phonemic) FAS tasks the fewest [Citation106]. However, variability was great, especially in the PD group. In fact, in the animal task, the PD group as a whole exhibited fluency deficits to the same extent as the MAS group. This could be an indication that the word-finding difficulties seen in PD are mainly semantic in nature. Further, both stroke groups had pronounced difficulties (scores far below test norms) in both types of fluency tasks, which supports the claim that there are concurrent disturbances in both the phonological and the semantic language-processing sub-systems. In fact, recent findings suggest that both phonological and semantic fluency involve shared cognitive processing. Using multivariate lesion-symptom mapping in 55 participants with left-hemisphere stroke, Thye, Szaflarski [Citation110] found that both types of fluency largely rely on the same neural network, including frontal and temporal regions of the brain and white-matter connections.
Similarly, both phonological and semantic difficulties were evident in the stroke groups with regard to signs of anomia resulting in inadequate responses in the confrontation-naming tasks. The most common such sign in the MSAS group was non-response or abandoned attempt. Although there is of course an inherent uncertainty about the cause of non-responses (due to the lack of an overt, analysable response), it has been proposed that they are most likely semantic rather than phonological in nature [Citation111]. Using multivariate lesion-symptom mapping, Chen, Middleton [Citation111] found that omissions arose from lexical-semantic difficulties associated with left-hemisphere frontal-lobe and mid-anterior temporal-lobe lesions.
In the present study, the overt inadequate responses were both semantic and phonological in nature in the stroke groups but predominantly semantic in the PD and MS groups. Unsurprisingly, the qualitative differences in inadequate responses were less clear where the degree of anomia was lower, indicating that word-finding difficulties are reflected as an increase in both frequency and types of inadequate responses where the anomia is more severe.
It should also be noted that any influence from dysarthria or apraxia of speech would increase the proportion of phonological errors. Motor-speech difficulties are more likely to be present in severe than mild strokes and could partly explain why phonological errors are more common in severe strokes. On the other hand, motor-speech difficulties are very common in PD and MS as well, and the PD and MS groups mainly made semantic errors. Further, all participants in the present study were screened for dysarthria and apraxia of speech severely affecting intelligibility and the more lenient scoring of naming accuracy that was used allowed a single phonological error. Thus, it is unlikely that motor-speech difficulties would account for all phonological errors. Rather, a possible reason might be that the damage to the brain (in terms of lesion size and locations) tends to concern a larger proportion of the brain and so affect language processing to a greater extent in stroke than in PD or MS. Disruptions to the spread of information in networks may cause both phonological and semantic difficulties [Citation17], and greater disturbances to the system are likely to cause greater vulnerability in the process of word retrieval. Hence the phonological and semantic sub-systems alike may be more affected in stroke than in PD and MS, and more affected in severe than mild stroke-related anomia.
In all groups, semantic inaccuracies most often were related to the target words; the proportion of unrelated paraphasias was low. Thus, the proportion of conflicting or opposing information given was small. However, it should be noted that, since words that are semantically related but still off target may be equally (or more) confusing to the listener, any occurrence of paraphasias risks complicating everyday communication. This is in line with previous findings on the presence of semantic errors in PD and MS in confrontation-naming tasks [Citation44,Citation52,Citation112], and may be part of the reason why individuals with PD and MS are reported to have difficulties staying on the topic [Citation3,Citation13] and to have reduced information content [Citation32].
The information content was also reduced in the re-telling task, where none of the four groups provided as much accurate and complete information as the reference group did. In line with the results from most other assessment tasks, the MSAS group conveyed the least information. Some MSAS participants did not convey any information at all, and none conveyed more than one-third of the contents in an accurate and complete manner. Against this background, the unexpected positive correlation with reduced communicative participation is no longer surprising. Any conversation would be severely affected by one party’s failure to convey more than, at best, one-third of the message intended. Further, variability was greatest in the MAS group, showing that even relatively mild anomia may interfere strongly with information conveyance in re-telling. The PD group, although it had a moderately high proportion of accurate main concepts, also had a fairly high proportion of absent main concepts, indicating that the members of this group also had problems conveying all the necessary information in a narrative. This finding corroborates previous reports that people with PD tend to leave out important information [Citation32]. The MS group had very few absent main concepts and a very high proportion (80.3%) of accurate (either complete or incomplete) main concepts, indicating that they conveyed the gist of the story successfully. Still, even they were not as informative as the reference group, which had 88.4% accuracy [Citation104]. These findings indicate that the main-concept analysis used in the re-telling task is a more sensitive measure than accuracy in confrontation naming, and that connected speech should be collected and analysed alongside tests of single-word naming–well in line with the recommendations of Stark and Fukuyama [Citation74]. Finally, in line with the low proportion of unrelated paraphasias found for the confrontation-naming tasks, the proportion of incorrect main concepts in the re-telling task was quite low in all groups. In other words, while important information could be missing, only a small proportion of the information actually conveyed was potentially misleading.
Interestingly, despite the considerable differences seen in results on all of the formal assessment tasks discussed above, the results with regard to self-reported communicative participation did not differ significantly between the groups. The lack of consistent correlations between test scores and self-reports on the occurrence of anomia and on communicative participation further underscores the need to obtain self-reports on the consequences of individuals’ health conditions for their everyday communication. Indeed, previous studies have also found that test results and subjective experience can be poorly related, suggesting that a combination of self-reports and test results may be necessary to make the best clinical decisions [Citation2,Citation3,Citation6,Citation8,Citation13,Citation69,Citation80]. Further, the vast range of the self-reported difficulties and their poor correlation with accuracy scores on the confrontation-naming tasks indicate that even objectively “mild” anomia can lead to a major negative impact on communicative participation. The participants’ reports on their quality of life emphasise the importance of addressing these issues.
Limitations
First, even though the participant sample in this study was reasonably representative of the respective populations, for example with regard to gender (more men in the PD group and more women in the MS group) and age (younger in the MS group), a larger group of participants would have been desirable. Considering the relatively small group sizes, and the fact that all participants perceived themselves to have word-finding difficulties, it is not possible to generalise the study findings to all people with left-hemisphere stroke, PD or MS. Second, since the aim of this study was to describe and compare features of word-finding difficulties between participants with different underlying neurological conditions, causality is beyond its scope and no causal inferences can be made. That would have required even more extensive examination, including stricter control of the medical conditions and medications as well as an extended neuropsychological test battery. Nevertheless, the comprehensive test battery used in the present study still allows far-reaching comparisons to be made. Third, while most assessments were performed in all four groups, different quality-of-life questionnaires were used, and although those questionnaires are similar in their content, the ratings based on them are not entirely comparable, as could be the case for the different Lineman cartoon sets. Further, although many established assessment materials were used, the rating scales for the occurrence of anomia and for the current state of health were study-specific, and their reliability and validity have not been tested. The related measures should be viewed with caution. Also, since the full version of the BNT was not used, reference values were lacking, meaning that comparisons with BNT test norms could not be made. All of these concerns should be considered and addressed in future studies.
Summary
In summary, as expected, the group of participants with stroke and moderate to severe anomia manifested significantly more quantitative signs of anomia than the other groups in most tasks. By contrast, there were several quantitative and qualitative overlaps between the other three groups of participants: those with stroke and mild anomia, those with Parkinson’s disease and those with multiple sclerosis. All four groups were similar in that they displayed impaired verbal fluency, prolonged response times, impaired re-telling ability and a discrepancy between test results and self-reported communicative participation. The clinical implications of the present findings are that people with moderate or severe anomia after stroke not only exhibit more severe symptoms of word-finding difficulties but also manifest a wide variety of such symptoms that obviously demand extensive attention from speech and language therapists (SLTs) and other clinicians. Further, and equally importantly, these findings underscore the need to ask patients about their self-perceived word-finding difficulties and the need to ensure that all those who report having such difficulties are assessed by an SLT. An assessment comprising aspects such as verbal fluency, connected-speech tasks and the measurement of response times in naming tasks may serve to affirm their experiences. Moreover, regardless of the degree of difficulties or the underlying condition, self-perceived word-finding difficulties can have a negative impact on communicative participation and should therefore be appropriately addressed. Apart from appropriate assessments, there is a need to develop evidence-based treatment methods targeting mild anomia and improving communicative participation.
Acknowledgements
The authors thank SLTs Johanna Moreno, Malin Bauer, Eli Hedman, Clara Helenius and Siri De Geer for their invaluable contributions to data collection as well as Professor Lena Hartelius for collecting data and for sharing valuable knowledge.
Disclosure statement
The authors have no conflicts of interest to report.
Additional information
Funding
References
- Armstrong E, Fox S, Wilkinson R. Mild aphasia: is this the place for an argument? Am J Speech Lang Pathol. 2013;22(2):S268–S278.
- Klugman TM, Ross E. Perceptions of the impact of speech, language, swallowing, and hearing difficulties on quality of life of a group of South African persons with multiple sclerosis. Folia Phoniatr Logop. 2002;54(4):201–221.
- Johansson K, Schalling E, Hartelius L. Self-Reported changes in cognition, communication and swallowing in multiple sclerosis: data from the Swedish multiple sclerosis registry and from a national survey [article. Folia Phoniatr Logop. 2021;73(1):50–62.
- Yorkston KM, Baylor C, Amtmann D. Communicative participation restrictions in multiple sclerosis: associated variables and correlation with social functioning. J Commun Disord. 2014;52:196–206.
- Cavanaugh R, Haley KL. Subjective communication difficulties in very mild aphasia. Am J Speech Lang Pathol. 2020;29(1S):437–448.
- Hilari K, Cruice M, Sorin-Peters R, et al. Quality of life in aphasia: state of the art. Folia Phoniatr Logop. 2015;67(3):114–118.
- Baylor C, Yorkston K, Eadie T, et al. The communicative participation item bank (CPIB): item bank calibration and development of a disorder-generic short form [article. J Speech Lang Hear Res. 2013;56(4):1190–1208.
- El-Wahsh S, Ballard K, Kumfor F, et al. Prevalence of self-reported language impairment in multiple sclerosis and the association with health-related quality of life: an international survey study. Mult Scler Relat Disord. 2019 Dec 16;39:101896.
- El-Wahsh S, Heard R, Bogaardt H, et al. Variables associated with self-reported language impairment in multiple sclerosis: a regression analysis. Int J MS Care. 2021;23(2):85–92.
- WHO. International Classification of Functining, Disability and Health (ICF) 2001. 2021. https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health https://icfeducation.org/what-is-icf
- WHO. The top 10 causes of death World Health Organization. 2021. https://www.who.int/data/global-health-estimates.
- Goodglass H, Wingfield A. Anomia: neuroanatomical and cognitive correlates. San Diego: Academic Press; 1997.
- Schalling E, Johansson K, Hartelius L. Speech and communication changes reported by people with Parkinson’s disease. Folia Phoniatr Logop. 2017;69(3):131–141.
- Laine M, Martin N. Anomia: theoretical and clinical aspects. London: Taylor & Francis Group; 2006.
- Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil. 2012 Jan;93(1 Suppl):S86–S95.
- Cruice M, Worrall L, Hickson L. Perspectives of quality of life by people with aphasia and their family: suggestions for successful living. Top Stroke Rehabil. 2006;13(1):14–24.
- Dell GS. A spreading-activation theory of retrieval in sentence production. Psychological Review. 1986;93(3):283–321.
- Lindsay MP, Norrving B, Sacco RL, et al. World stroke organization (WSO): global stroke fact sheet 2019. Int J Stroke. 2019;14(8):806–817.
- Wafa HA, Wolfe CDA, Emmett E, et al. Burden of stroke in Europe: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke. 2020;51(8):2418–2427.
- British Heart Foundation. 2021. https://www.bhf.org.uk.
- Mitchell C, Gittins M, Tyson S, et al. Prevalence of aphasia and dysarthria among inpatient stroke survivors: describing the population, therapy provision and outcomes on discharge. Aphasiology. 2021;35(7):950–960.
- Blake ML. The right hemisphere and disorders of cognition and communication: theory and clinical practice. San Diego: Plural Publishing, Incorporated; 2017.
- Plowman E, Hentz B, Ellis CJ. Post-stroke aphasia prognosis: a review of patient-related and stroke-related factors. J Eval Clin Pract. 2012;18(3):689–694.
- Del Tredici K, Braak H. Review: sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathol Appl Neurobiol. 2016;42(1):33–50.
- Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42.
- Monastero R, Cicero CE, Baschi R, et al. Mild cognitive impairment in Parkinson’s disease: the Parkinson’s disease cognitive study (PACOS). J Neurol. 2018;265(5):1050–1058.
- Saldert C, Bauer M. Multifaceted communication problems in everyday conversations involving people with Parkinson’s disease. Brain Sciences. 2017 Sep 25;7(10):123.
- Colman KSF, Koerts J, Stowe LA, et al. Sentence comprehension and its association with executive functions in patients with Parkinson’s disease. Parkinsons Dis. 2011;2011:213983.
- Murray LL. Language and Parkinson’s disease. Ann Rev Appl Linguist. 2008;28:113–127.
- Berg E, Bjornram C, Hartelius L, et al. High-level language difficulties in Parkinson’s disease. Clin Linguist Phon. 2003;17(1):63–80.
- Smith KM, Ash S, Xie SX, et al. Evaluation of linguistic markers of word-finding difficulty and cognition in Parkinson’s disease. J Speech Lang Hear Res. 2018;61(7):1691–1699.
- Altmann LJP, Troche MS. High-Level language production in Parkinson’s disease: a review. Parkinsons Dis. 2011;2011:238956–238912.
- Kingwell E, Marriott JJ, Jette N, et al. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013;13(1):128.
- Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5.
- Maloni H. Cognitive impairment in multiple sclerosis. J Nurse Practition. 2018;14(3):172–177.
- Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180.
- Noffs G, Perera T, Kolbe SC, et al. What speech can tell us: a systematic review of dysarthria characteristics in multiple sclerosis. Autoimmun Rev. 2018;17(12):1202–1209.
- Ozakbas S, Turkoglu R, Tamam Y, et al. Prevalence of and risk factors for cognitive impairment in patients with relapsing-remitting multiple sclerosis: multi-center, controlled trial. Mult Scler Relat Disord. 2018;22:70–76.
- Benedict RHB, DeLuca J, Enzinger C, et al. Neuropsychology of multiple sclerosis: looking back and moving forward. J Int Neuropsychol Soc. 2017;23(9-10):832–842.
- Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16(3):283–288.
- Renauld S, Mohamed-Saïd L, Macoir J. Language disorders in multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2016;10:103–111.
- De Dios Pérez B, Cordova Luna E, Cloutman L, et al. Anomia in people with rapidly evolving severe relapsing-remitting multiple sclerosis: both word retrieval inaccuracy and delay are common symptoms. Aphasiology. 2020;34(2):195–213.
- Laakso K, Brunnegård K, Hartelius L, et al. Assessing high-level language in individuals with multiple sclerosis: a pilot study. Clin Linguist Phonet. 2000;14(5):329–349.
- Lethlean JB, Murdoch BE. Naming errors in multiple sclerosis: support for a combined semantic/perceptual deficit. J Neurolinguist. 1994;8(3):207–223.
- Arnott WL, Jordan FM, Murdoch BE, et al. Narrative discourse in multiple sclerosis: an investigation of conceptual structure. Aphasiology. 1997;11(10):969–991.
- Friend KB, Rabin BM, Groninger L, et al. Language functions in patients with multiple sclerosis. Clin Neuropsychol. 1999;13(1):78–94.
- Lethlean JB, Murdoch BE. Performance of subjects with multiple sclerosis on tests of high-level language. Aphasiology. 1997;11(1):39–57.
- Filley CM. White matter dementia. Ther Adv Neurol Disord. 2012;5(5):267–277.
- Lambon Ralph MA, Graham KS, Ellis AW, et al. Naming in semantic dementia–what matters? Neuropsychologia. 1998;36(8):775–784.
- Murdoch BE, Theodoros DG. Speech and language disorders in multiple sclerosis. London: Whurr; 2000.
- Sepulcre J, Peraita H, Goni J, et al. Lexical access changes in patients with multiple sclerosis: a two-year follow-up study. J Clin Exp Neuropsychol. 2011;33(2):169–175.
- Tallberg IM, Bergendal G. Strategies of lexical substitution and retrieval in multiple sclerosis. Aphasiology. 2009;23(9):1184–1195.
- Miller N. Communication changes in Parkinson’s disease. Pract Neurol. 2017;17(4):266–274.
- Kristensson J, Longoni F, Östberg P, et al. Evaluation of treatment effects of semantic feature analysis on mild anomia in multiple sclerosis. Aphasiology. 2022;36(6):706–731.
- Montemurro S, Mondini S, Signorini M, et al. Pragmatic language disorder in Parkinson’s disease and the potential effect of cognitive reserve. Front Psychol. 2019;10:1220.
- Baylor C, Burns M, Eadie T, et al. A qualitative study of interference with communicative participation across communication disorders in adults. Am J Speech Lang Pathol. 2011;20(4):269–287.
- Piatt AL, Fields JA, Paolo AM, et al. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia. 1999;37(13):1499–1503.
- Guilford JP. The nature of human intelligence. New York (NY): McGraw-Hill Book Company; 1967.
- Bauer M, Saldert C. Complex oral semantic verbal fluency in non-brain-damaged adults and individuals with multiple sclerosis and subjective anomia. Aphasiology. 2020;34(12):1471–1486.
- Masterson J, Druks J. Description of a set of 164 nouns and 102 verbs matched for printed word frequency, familiarity and age-of-acquisition. J Neuroling. 1998;11(4):331–354.
- Gilmore N, Meier EL, Johnson JP, et al. Typicality-based semantic treatment for anomia results in multiple levels of generalisation. Neuropsychol Rehabil. 2020;30(5):802–828.
- Ladanyi E, Lukacs A. Word retrieval difficulties and cognitive control in specific language impairment. J Speech Lang Hear Res. 2019;62(4):918–931.
- Benetello A, Finocchiaro C, Capasso R, et al. The dissociability of lexical retrieval and morphosyntactic processes for nouns and verbs: a functional and anatomoclinical study. Brain Lang. 2016;159:11–22.
- Kambanaros M, Messinis L, Nasios G, et al. Verb–noun dissociations in relapsing-remitting multiple sclerosis: verb effects of semantic complexity and phonological relatedness. Aphasiology. 2017;31(1):49–66.
- De Dios Pérez B. Anomia in people with relapsing-remitting multiple sclerosis [Masters of philosophy]. ProQuest Dissertations Publishing: University of Manchester; 2017.
- Saldert C. Pragmatic assessment and intervention in adults, chapter 20. In: Cummings L, editor. Research in clinical pragmatics. (Series: Perspectives in pragmatics, philosophy & psychology. Vol. 11). Wien: Springer; 2017. p. 527–558.
- Body R, Perkins MR. Terminology and methodology in the assessment of cognitive-linguistic disorders. Brain Impairment. 2006;7(3):212–222.
- Crosson B. Assessment of subtle language deficits in neuropsychological batteries: strategies and implications. In: Sburdone RJ, Long CJ, editors. Ecolog valid neuropsycholog testing. Delray, FL: GR Press/St Lucie Press Inc.; 1996. p. 243–259.
- Akbar N, Finlayson M. A mixed-methods study of cognitive performance in persons with multiple sclerosis: association between neuropsychological test performance and interviews about daily cognitive functioning. Mult Scler Relat Disord. 2021;52:102911–102911.
- Galletta EE, Goral M. Response time inconsistencies in object and action naming in anomic aphasia. Am J Speech Lang Pathol. 2018;27(1S):477–484.
- Sotiropoulou Drosopoulou C. Speed of word retrieval across neurotypical and aphasic participants: an investigation of novel assessment and treatment methods. ProQuest Dissertations Publishing, University of Manchester. 2016.
- De Geer S, Hartelius L, Kristensson J. Finding the right words in connected speech: an examination of word-finding difficulties in persons with multiple sclerosis. Manuscript in preparation.
- Nicholas LE, Brookshire RH. Presence, completeness, and accuracy of main concepts in the connected speech of non-brain-damaged adults and adults with aphasia. J Speech Hear Res. 1995;38(1):145–156.
- Stark BC, Fukuyama J. Leveraging big data to understand the interaction of task and language during monologic spoken discourse in speakers with and without aphasia. Lang Cognit Neurosci. 2021;36(5):562–585.
- Fergadiotis G, Wright HH. Modelling confrontation naming and discourse performance in aphasia. Aphasiology. 2016;30(4):364–380.
- Blankestijn-Wilmsen J, Damen I, Voorbraak-Timmerman V, et al. The effect of static versus dynamic depictions of actions in verb and sentence production in aphasia. Aphasiology. 2017;31(10):1166–1182.
- El-Wahsh S, Heard R, Bogaardt H. Development and validation of a speech pathology-specific questionnaire for persons with multiple sclerosis (SMS). Int J Speech Lang Pathol. 2019;21(6):553–563.
- Yorkston KM, Baylor CR, Klasner ER, et al. Satisfaction with communicative participation as defined by adults with multiple sclerosis: a qualitative study. J Commun Disord. 2007;40(6):433–451.
- Hilari K, Byng S, Lamping DL, et al. Stroke and aphasia quality of life scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke. 2003;34(8):1944–1950.
- van der Stelt CM, Fama ME, McCall JD, et al. Intellectual awareness of naming abilities in people with chronic post-stroke aphasia. Neuropsychologia. 2021;160:107961.
- Singh S, Tench CR, Tanasescu R, et al. Localised grey matter atrophy in multiple sclerosis and clinically isolated syndrome-a coordinate-based meta-analysis, meta-analysis of networks, and meta-regression of voxel-based morphometry studies. Brain Sciences. 2020;10(11):798.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1452.
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 4th ed. Toronto: The Psychological Corporation/A Harcourt Assessment Company; 2003.
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system. 2001.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
- Eckerström M, Skoogh J, Rolstad S, et al. Sahlgrenska academy self-reported cognitive impairment questionnaire (SASCI-Q) – a research tool discriminating between subjectively cognitively impaired patients and healthy controls. Int Psychogeriatr. 2013;25(3):420–430.
- Druks J, Masterson J. Object and action naming battery. Psychology Press; 2000.
- Hunting-Pompon R, Kendall D, Bacon Moore A. Examining attention and cognitive processing in participants with self-reported mild anomia. Aphasiology. 2011;25(6-7):800–812.
- Lambon Ralph MA, Snell C, Fillingham JK, et al. Predicting the outcome of anomia therapy for people with aphasia post CVA: both language and cognitive status are key predictors. Neuropsychol Rehabil. 2010;20(2):289–305.
- Neumann Y. A case series comparison of semantically focused vs. phonologically focused cued naming treatment in aphasia. Clin Linguist Phon. 2018;32(1):1–27.
- del Toro CM, Bislick LP, Comer M, et al. Development of a short form of the Boston naming test for individuals with aphasia. J Speech Lang Hear Res. 2011;54(4):1089–1100.
- Conroy P, Sage K, Lambon Ralph MA. Errorless and errorful therapy for verb and noun naming in aphasia. Aphasiology. 2009;23(11):1311–1337.
- Graves RE, Bezeau SC, Fogarty J, et al. Boston naming test short forms: a comparison of previous forms with new item response theory based forms. J Clin Exp Neuropsychol. 2004;26(7):891–902.
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Arizona: Neuropshycology press; 1985.
- Ellbin S, Jonsdottir IH, Eckerstrom C, et al. Self-reported cognitive impairment and daily life functioning 7–12 years after seeking care for stress-related exhaustion. Scand J Psychol. 2021;62(4):484–492.
- Hellberg M, Kroon M. Konfrontationsbenämning av substantiv och verb hos svensktalande vuxna utan anomi [Confrontation naming of nouns and verbs] Swedish. [Master’s thesis]: University of Gothenburg; 2015.
- Torinsson M, Åke S. Konfrontationsbenämning av aktiviteter och objekt hos vuxna svensktalande personer utan känd hjärnskada – en standardiseringsstudie [Confrontation naming of actions and objects in non-brain damaged native Swedish-speaking adults – a standardization study] Swedish. [Master’s thesis]: University of Gothenburg; 2017.
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger. Philadelphia, PA Digital pictures courtesy of Pro-Ed. 1983.
- Tallberg IM. The Boston naming test in Swedish: normative data. Brain Lang. 2005;94(1):19–31.
- Benton A, Hamsher K. Multilingual aphasia examination manual. Iowa City: University of Iowa. 1978.
- Lezak M, Howieson D, Loring D. Neuropsychological assessment. New York: Oxford University 1995.
- Cavandoli O. La linea [DVD]. Italy: Quipos©, Just4Kids©; 2007. DVD.
- Front V, Johansson von Braun A. Bedömning av anomi i sammanhängande tal till rörlig bild med ”main concept”-analys [Assessment of anomia through connected speech to a dynamic depiction using main concept analysis] Swedish. [Master’s thesis]: University of Gothenburg; 2021.
- Fleischer S, Lindström M. Bedömning av anomi genom återberättande av rörlig bild - En pilotstudie [Assessing anomia through video narration - a pilot study] Swedish. [Master’s thesis]: University of Gothenburg; 2018.
- Tallberg IM, Ivachova E, Jones Tinghag K, et al. Swedish norms for word fluency tests: FAS, animals and verbs. Scand J Psychol. 2008;49(5):479–485.
- Kong AP-H. The use of main concept analysis to measure discourse production in cantonese-speaking persons with aphasia: a preliminary report. J Commun Disord. 2009;42(6):442–464.
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Ass. 1994;6(4):284–290.
- Stevens JP. Applied multivariate statistics for the social sciences. Fifth Edition. London: Taylor & Francis Group; 2009.
- Thye M, Szaflarski JP, Mirman D. Shared lesion correlates of semantic and letter fluency in post-stroke aphasia. J Neuropsychol. 2021;15(1):143–150.
- Chen Q, Middleton E, Mirman D. Words fail: lesion-symptom mapping of errors of omission in post-stroke aphasia. J Neuropsychol. 2019;13(2):183–197.
- Cotelli M, Borroni B, Manenti R, et al. Action and object naming in Parkinson’s disease without dementia. Eur J Neurol. 2007;14(6):632–637.
Appendix
Table Appendix 1. Results when the PD participant with extreme value in the confrontation naming task was excluded.
