Abstract
Snow algae inhabit most of the cold regions worldwide, where long-lasting snow fields are common. The ecophysiology of snow algae has been studied intensively in North America and occasionally in polar regions. In the European Alps, the systematics of snow algae have been studied mainly by light microscopy. We studied temperature and light-dependence of photosynthesis, and plastid and extraplastid red pigment composition of red snow algae (Chlamydomonas nivalis) from snow patches in the high Alps of Austria. Both photosynthetic and respiratory data support the cryophilic adaptation of snow algal cells, but C. nivalis produced oxygen without any inhibition at temperatures up to 20°C and maintained this for 1 h, at irradiances up to 1800 µmol m−2s−1. Chlorophyll and primary carotenoid pigment composition was similar to that found in most other Chlorophyta. Additionally, large amounts of free and esterified astaxanthin were located in cytoplasmic lipid globules. Light and electron microscopy showed that the cell walls were frequently covered with tightly bound inorganic particles. Occasionally fungus- or bacteria-like structures were attached to the wall. The typical adult cell contained a single central chloroplast. Cytoplasmic structures were often difficult to resolve optically, as densely packed peripheral lipid globules, containing secondary carotenoids, occupied most of the cell volume. These pigments may shield the chloroplast from high irradiation (thus reducing the risk of photoinhibition) and may also be a potential carbon source during unfavourable climate conditions or the formation of daughter cells.
Introduction
‘Red snow’, the macroscopic expression of a massive growth of unicellular algae on alpine and polar snow fields, has been a well-known phenomenon for more than 2000 years. However, the development of microscopy in the nineteenth century was necessary before unicellular red-coloured algae could be identified as the cause. During the 20th century, systematic studies on these algae have been undertaken to identify the different species growing on or in the snow and to describe the species causing not only ‘red snow’ but also green, yellow, orange, or even grey snow (a comprehensive summary of the current state of knowledge of systematics, occurrence and physiology of snow algae is given by Hoham & Duval, Citation2001). The organisms causing these various snow colourations proved to be mostly green algae, but the exact taxonomic identity is often unclear and further investigation using electron microscopy and molecular methods is required (Müller et al., Citation1998; Hoham et al., Citation2002; Novis, Citation2002).
Significant progress in our understanding of algae has come from a number of combined studies undertaken in the high arctic of Spitsbergen. These studies covered conductivity of living cells, electron microscopy (Müller et al., Citation1998; Müller et al., Citation2001), the viability of snow algae in the field (Müller et al., Citation2001) and distribution, taxonomic, and pigment studies throughout the Spitsbergen archipelago based on previously published studies (Müller et al., Citation1998; Leya, Citation2004). These studies also produced a culture collection of cryophilic algae (Leya, Citation2004), which is now the basis for systematic strain identification by molecular methods (Leya, Citation2004).
Most snow algae belong to the genera Chlamydomonas and Chloromonas (Chlorophyta, Volvocales). It is important to consider their habitat when undertaking physiological measurements (Hoham & Duval, Citation2001; Müller et al., Citation2001). Snow algae frequently occur in persistent or permanent snow ecosystems in the Alps or the Arctic. During summer they have to adapt to extreme temperature regimes, high irradiance and low nutrient levels. Snow algae live in a unique microhabitat, namely the liquid water between snow crystals. For cells to be physiologically active, the snow has to be neither too cold nor too dry (as with freshly fallen snow). Flagellated stages enable these organisms to change their position within the snow layer to reach the optimal depth for their light and temperature requirements. Most of the life-cycle occurs as the immotile hypnoblast stage because this form is the most resistant to environmental changes, such as daily freeze–thaw cycles.
The transformation into hypnoblasts is characterized by a massive incorporation of reserve material, including sugars and lipids, and by formation of esterified extraplastidal secondary carotenoids. These pigments also occur in other algae from less extreme ecosystems, sometimes as a consequence of nitrogen deficiency. The older literature describes such algae as the ‘haematochrom’ (Kol, Citation1968; Ettl et al., Citation1983; Czygan, Citation1968, Citation1970). More recent studies (Bidigare et al., Citation1993; Müller et al., Citation1998; Hoham & Duval, Citation2001 and references therein) have shown that the cells mainly form oxycarotenoids, in particular astaxanthin, and traces of canthaxanthin and echinenone.
In the European Alps, snow algae cause red snow fields as they accumulate on the melting surface from May onwards at altitudes usually higher than 1800 m (Kol, Citation1968; personal observations). This cryophilic bloom can be observed on many high altitude mountainsides worldwide as well as in polar regions. The latter show the most intense algal development in coastal regions next to bird colonies.
Painter et al. (Citation2001) measured the distribution and abundance of snow algae over a 5.5 km2 region with an airborne imaging spectrometer. Using this method larger snow fields can be characterized and compared. Recently, snow algae were described from Africa (Atlas mountains of Morocco, Duval et al., Citation1999).
The material that we collected in the Austrian Alps had the typical appearance of red, unicellular, cyst-like, thick-walled spherical resting stages, which are associated with Chlamydomonas cf. nivalis. These hypnoblasts are difficult to study by light microscopy due to their similarity in appearance and because the structure of the chloroplast is almost totally covered by astaxanthin pigment.
One aim of our investigation was to describe the photosynthetic performance of C. nivalis taken from fresh field samples. We measured photosynthesis at different temperatures and light levels to obtain information on the adaptation strategy of this organism. The physiological data were correlated with ultrastructure and pigment content. These observations may be the first TEM (transmission electron microscopy) and HPLC (high pressure liquid chromatography) studies on red snow algae from European alpine environments.
Materials and methods
Collection sites and isolation
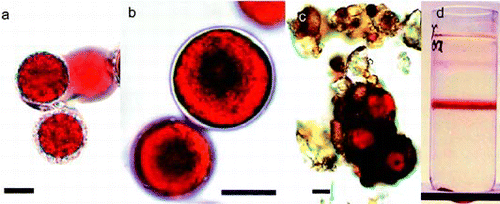
Patches of clearly visible red snow down to a depth of 10 cm were collected at five sites in Tyrol, Austrian Alps. Samples were transferred to the laboratory within 2–3 h for immediate study. In the laboratory, the red snow was melted slowly at 4°C and the material concentrated by filtration. The filtration reduced 3 l of snow to a final volume of about 50 ml. In some cases, the collected material contained numerous impurities (e.g. pollen and particles) and, therefore, C. nivalis was purified using sucrose density gradient centrifugation. The gradient consisted of 60%/40%/20% sucrose (w/v) in 5 mM KH2PO4/10 mM NaHCO3 buffer at pH 6. Bands developed after 10 min centrifugation at 500 g (). Samples were observed by light microscopy (Zeiss, FRG, Axiovert 200 M). Species type and abundance were identified according to Kol (Citation1968), Ettl et al. (Citation1983) and Komárek & Komárek (Citation2001).
Fig. 1. (a) Light micrograph of Chlamydomonas nivalis cells with adhering material or mucilage sheet. (b) C. nivalis sample with smooth cell wall. (c) Snow algae cells, found in dry rock surface dust. These hypnoblasts were kept for 6 months in darkness and appeared viable after resuspension in water. (d) Sucrose density-gradient purification of C. nivalis concentrated from melting snow. The band formed in the middle of the gradient was used for most analyses. Scale bar: 10 µm
Photosynthetic oxygen evolution
Oxygen evolution was monitored with a Clark-type suspension electrode (Delieu & Walker, Citation1972). Irradiances from 50 to 1800 µmol m−2 s−1 were obtained with a set of neutral density filters (Hansatech A5, Kings Lynn, GB), and temperature was controlled by a thermostat (DC 10, Therma Haake, Karlsruhe, FRG). The analogue signal output of the control box was digitally transformed by an A/D232-interface and analysed with ‘Oxygen’-software (Lütz, Citation1996).
Concentrated red snow in melting water (0.25 ml) was combined with 1.25 ml of 0.1 M Na2CO3/NaHCO3 as a CO2-source and loaded into the measurement chamber. Constant use of a magnetic stirrer ensured stable conditions. Before starting the measurement, the sample was allowed to acclimatise in darkness for 15 min at the experimental temperature. The cycle of analysis from dark respiration up to the highest irradiance took about 40 min. All P vs. E curves were measured in triplicate.
The amount of oxygen evolved was expressed per unit of chlorophyll a in the sample. For chlorophyll determination, the cells were collected by centrifugation (5 min at 5000g). To break the thick cell wall, the pellet was frozen and homogenized twice in liquid nitrogen (Micro-Dismembrator type S, Sartorius, Göttingen, FRG) with a 1-cm quartz ball for 5 min at 2000 rpm. The frozen cell material was immediately suspended in 2 ml dimethylformamide (DMF, Scharlau, Spain). For improved extraction, the sample was sonicated for 5 min with a Sonopuls GM2200 sonotrode combined with a microtip MS 72 (Bandelin eletronics, Berlin, FRG), set at 25% output. Cell debris was separated from the solvent by centrifugation (10 min at 10 000g). Chlorophyll a was assayed with a Lambda 20 Photometer (Perkin Elmer instruments, USA), using the formula of Porra et al. (Citation1989).
Pigment analyses (HPLC)
HPLC analysis used an Agilent Technologies 1100 system (Waldbronn, FRG), with a DAD-detector set at 440 nm for carotenoids, 648 nm for chlorophyll b, and 662 nm for chlorophyll a, and an FLD-detector set at 285 nm excitation and 325 nm emission for α-tocopherol quantification. The column was an RP-C18 (5 µm) type (Agilent LiChroCart 125 × 4 mm). A binary pump set at 1 ml min−1 provided a solvent gradient from 0 to 4 min of 100% A (acetonitrile:methanol:0.2 M Tris buffer pH 8.0 = 74:6:1), increasing linearly from 4 to 9 min to 100% B (methanol:hexane = 5:1) until the end of the run at 25 min. All solvents were of HPLC gradient grade quality. Pigment calibration and quantification was established for β-carotene, canthaxanthin, echinenone, lutein, neoxanthin, violaxanthin and zeaxanthin with standards from Carbon 14 Centralen, Hørsholm, DK. Astaxanthin and chlorophylls a and b were obtained from Sigma-Aldrich. Antheraxanthin and α-carotene were identified by comparing spectra and retention time with published HPLC results (Siefermann-Harms, Citation1988). Before injection, the DMF-extract was diluted 2:1 with 50% methanol, centrifuged (10 min at 10 000g) and filtered through 0.45 µm PTFE-membrane filters into HPLC vials. All pigment handling steps were carried out at low irradiances and low temperatures.
Transmission electron microscopy
Snow algae were concentrated by centrifugation (5 min at 500g), and the pellet was suspended in 0.01 M cacodylate buffer (pH 6.8), containing 2% glutaraldehyde, and fixed for 1 h at 4°C. The suspension was centrifuged again, the supernatant removed and the pellet of fixed algae was slowly brought to 30°C and immediately mixed with 4% agar dissolved in the cacodylate buffer. The mixture was immediately cooled to 4°C. Pieces of ∼1 mm3 were cut from the solidified agar and washed for 1 h in cacodylate buffer. Osmium fixation (2% in cacodylate buffer) lasted for 2 h at 4°C; thereafter the samples were rinsed in cacodylate buffer, dehydrated gradually in ethanol and finally embedded in epoxy resin. Sections were viewed under a Philips EM 400T transmission electron microscope.
Results
Species of snow algae
All examined snow fields were fully exposed to the sun and had only a slight slope. Coloured snow was found most frequently at spots with wet and soft but still granular snow. Close observation revealed that the red cell mass was mainly situated in the liquid film which coats the icy granules. As shown in , C. nivalis was the dominant species (>98%) at all collecting sites. Some other cryophilic species like Chloromonas nivalis, Chloromonas alpina, Scotiella cryophila and Raphidonema nivale were present but had very low abundance (<1%). The spherical cells of C. nivalis had a diameter of 14.9 µm ± 5.7 µm (SD) and either a smooth cell wall (), occasionally surrounded by a mucilage layer, or a cell wall surface coated with organic and inorganic particles (). Particle attachment was very common at all sampling sites with the exception of the early collection survey (May 2002) in Kühtai (see ). Neither motile flagellates nor reproductive zygotes of C. nivalis, as reported by Müller et al. (Citation1998; fig. 4(b)), were detected.
Table 1. Collection sites of ‘red snow’ algae in the Austrian Alps, province Tyrol. Chlamydomonas nivalis was always the dominant form. The snow from Sellrain/Kühtai, sampled in May, contained younger C. nivalis cell stages showing less accumulation of secondary carotenoids, and surface coating with organic and inorganic particles.
Many of the algal-inhabited snow fields in the Austrian Alps melt at the end of summer, exposing the hypnoblasts to much warmer temperatures for many weeks, e.g., on sun-exposed soil or rock surfaces. We collected dry rock dust at several locations not far from red snow fields and kept this fine debris in darkness at room temperature for 6 months (data not shown). After resuspension of this grey, sandy material in water, scattered hypnoblasts of C. nivalis could be observed, which always looked intact (), indicating they can tolerate dryness as well as non-cryophilic temperatures for over 6 months. Most of them had a thick cell wall and considerable amounts of attached material. In the field, this species survives such mesophilic conditions, because the cells return to the warm soil after the snow fields melt away during summer, and remain there for weeks until fresh snow covers them again.
Photosynthesis
The samples used came from snow fields next to the Rettenbach (Ötztal valley) and the Schaufelferner glaciers (Stubai valley, ). As observed by light microscopy, cells from both sites were similar in size, morphology, colour and secondary pigment composition (data acquired by HPLC, not shown) and were considered, therefore, to be taxonomically identical.
Light and temperature dependence of photosynthesis is shown in . At 1.5°C, a temperature typical of a snow layer, dark respiration was moderate (0.040 µmol O2 h−1 µg chl a−1). At 4°C, consumption of oxygen more than doubled (0.092 µmol O2 h−1 µg chl a−1) and at 20°C there was nearly a four-fold increase. Thus there was a strong increase in dark respiration of C. nivalis when temperatures became mesophilic.
Fig. 2. Net photosynthesis vs. irradiance curves for snow algae at different temperatures. Values are means of three independent records, and error bars are standard deviations.
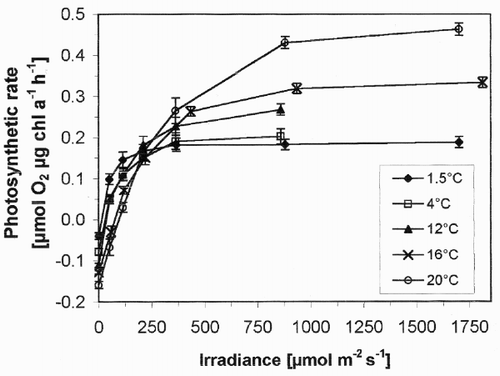
When exposed to light, the snow algae quickly showed a positive oxygen exchange. At low irradiances (<100 µmol m−2 s−1), however, net photosynthesis was positive only in samples between 1.5 and 12°C, and remained negative at 16 and 20°C. At 1.5 and 4°C, the photosynthesis of C. nivalis did not increase substantially with irradiance above 300–400 µmol m−2 s−1. In contrast, samples at 12, 16 and 20°C showed a significant increase in photosynthesis up to 900 µmol m−2 s−1. During the experiment (which took up to 40 min), no photoinhibition occurred. At high irradiances (1800 µmol m−2 s−1), net photosynthesis of the snow algae was about 2.5 times higher at 20°C than at 1.5°C. Surprisingly for a cryophilic organism, there was no decrease in net photosynthesis at higher temperatures.
Chloroplast pigments and secondary carotenoids
HPLC analyses showed the presence of chlorophylls a and b and the primary carotenoids, as usually found in thylakoids of green algae and higher plants. However, the pigment responsible for the red colour of adult C. nivalis cells is the secondary carotenoid astaxanthin and its fatty acid ester derivatives. Echinenone was detected only in trace amounts. The content of other pigments and α-tocopherol was expressed relative to the content of chlorophyll a (w/w; ). The sum of the xanthophyll cycle pigment amounts (violaxanthin, antheraxanthin, zeaxanthin) was on average about one-third of the amount of lutein. Only one sample contained either antheraxanthin or zeaxanthin and the only sample with a measurable epoxidation state was that from Kühtai with reddish-green cells instead of dark red cysts (AZ/VAZ = 0.27, ).
Table 2. Content of main pigments of snow algae sampled as in Table 1. Pigment contents are expressed in µg per µg chlorophyll a. Mean values calculated only from samples containing mostly adult cells; row 1 (spring harvest) not included. Replicate runs of each sample showed data variation of less than 1%. The description of sample plots is given in .
The content of astaxanthin was generally about 20 times that of chlorophyll a (), again with the exception of the sample from Kühtai, which was collected about 6 weeks earlier (20 May). Under the light microscope, these cells appeared less red, and some green chloroplast patches remained visible. In contrast, the primary carotenoid and α-tocopherol content was higher in these cells than in those collected later in the season. Only the ratio of neoxanthin to chlorophyll a stayed relatively constant. Despite the early stage of development, there was no visible correlation between the content of thylakoid membrane located pigments and the extraplastidic carotenoid astaxanthin.
Cytological observations
A density gradient enrichment of red cells (Fig 1(d)) did not result in a homogenous population of only one cell stage. Light microscope and TEM observations showed that non-algal impurities can be removed, but C. nivalis cells in the isolated fraction exhibited different levels of secondary carotenoid accumulation and attachment of extracellular structures to the cell wall (, , ). In most cases, inorganic impurities, rich in Si, Fe and Al (detected by parallel EELS transmission electron microscopy, unpublished data), were connected to the outer layer, probably supported by a mucilage sheet (). These occasionally exhibited prokaryote-like enclosures and sometimes fungus-like attachments were found (, probably Chionaster nivalis, see Kol, Citation1968).
Figs 3–4. Ultrastructure of hypnoblasts of Chlamydomonas nivalis. Fig. 3. Typical older hypnoblast. The grey droplets are astaxanthin esters. In the central region, some plastid structures appear. Irregular structures of unknown composition, which were not removed by gradient separation, cover the outer cell wall. Scale bar: 2 µm. Fig. 4. Part of an older hypnoblast with adhering fungus-like structure. Scale bar: 2 µm.
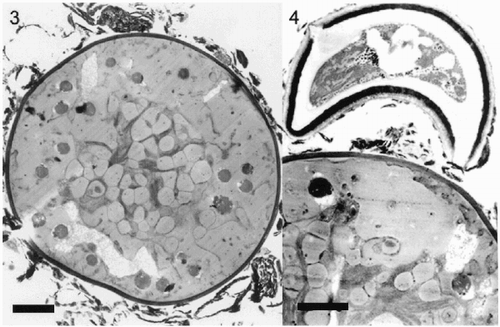
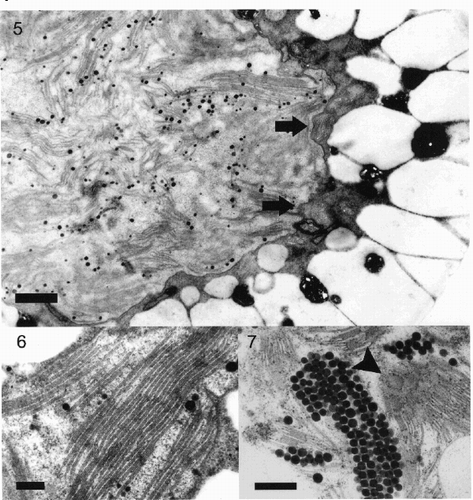
The figures show that cells of snow algae are occupied mostly by cytoplasmic lipid droplets of different sizes, representing extraplastidic carotenoid accumulation. As this carotenoid accumulation obviously depends on cell age, several cells with less developed lipid droplets were examined to resolve plastid structures. The chloroplast was situated in the central part of the cell with a lobe-like envelope membrane (). The thylakoid membranes occurred in grana-like parallel packages () in some parts of the plastid, and in shorter and sometimes undulating sets of membranes closer to the periphery of the plastid. Grana stacks were mostly composed of 3–7 thylakoids. In many cells the plastids contained a high number of plastoglobules, sometimes in regular arrangements ().
Figs 5–7. Fig. 5. Part of a Chlamydomonas nivalis cell showing the centrally located plastid surrounded by a thick layer of carotenoid globules with only a small cytoplasmic area left between both structures. The envelope membrane (arrows) of the plastid is undulating, the plastid contains numerous small grana stacks. Scale bar: 1 µm. Fig. 6. High magnification detail of thylakoid structure in Chlamydomonas nivalis cells showing a well-preserved ultrastructure. Such large stacks of thylakoids occur only occasionally. Scale bar: 0.2 µm. Fig. 7. Detail of plastid of Chlamydomonas nivalis with large numbers of plastoglobules, packed in regular arrangement. Some of the surrounding small grana stacks are visible in surface-view (arrowhead). Scale bar: 0.5 µm.
Discussion
As far as we are aware, these are the first measurements of pigments in snow algae from alpine localities and the first use of O2-evolution measurements to follow photosynthesis in snow algae. Earlier studies used different methods (14C incubation or CO2 gas exchange) and, with one exception (a study of a few green snow species in the former Czechoslovakia, Komárek et al., Citation1973), were carried out on algae from other continents, such as North America (Thomas, Citation1972; Mosser et al., Citation1977; Thomas & Duval, Citation1995; Williams et al., Citation2003).
As our photosynthetic measurements suggest, snow algae are not strictly cryophilic. This is in contrast to earlier reports, where snow algae were observed to die at temperatures >4°C (Kawecka, Citation1978) and a photosynthetic optimum was reported at −3°C to +4°C in culture (Hoham, Citation1975). However, our measurements were short-term. The snow algae were kept at 4°C in the laboratory and were exposed to other temperatures for not more than one hour during observation with the Clark electrode. Thus we cannot say how long C. nivalis could actually tolerate those higher temperatures and maintain a positive rate of net photosynthesis in non-cryophilic conditions. Weiss et al. (Citation1999) showed that the photosynthetic optima measured in the short-term for other green algae (Micrasterias, Desmidiaceae) can also shift, depending on the culture temperatures.
The strong rise in dark respiration of this snow algae from 4 to 20°C may indicate that the cells are easily temperature-stressed in mesophilic environments. Increased dark respiration might result in the consumption of reserve carbon pools, including the protective secondary carotenoids. Nevertheless, the alpine red snow algae are well adapted to their naturally cold snow-layer habitat. A significant part of the cell population does not live directly at the surface. While surface irradiance frequently reaches 3000 µmol m−2 s−1 (Gorton et al., Citation2001) the irradiation decreases rapidly and logarithmically inside the snow layer (Komárek et al., Citation1973; Gorton & Vogelmann, Citation2003). Consequently, a large part of the population is exposed to low temperatures combined with low light conditions. Therefore, cells exposed both to lower temperatures and to low irradiation may have an advantage when compared to those growing in a warmer environment. In the latter situation, cells need a higher irradiance to achieve positive O2-exchange. shows that C. nivalis performs well photosynthetically in this typical environment and can thus be considered to be a ‘true’ snow algae.
Our samples showed no photoinhibition up to 1800 µmol m−2 s−1. This can be regarded as an accommodation of the resting cells, which are frequently exposed to full irradiation at the snow surface. The red hypnoblasts are not affected by excessive irradiance because no de-epoxidation in the xanthophyll-cycle could be measured. Only the younger cells from Kühtai, which still contained green parts of the chloroplast, not shaded by astaxanthin, indicated that some light dissipation took place (AZ/VAZ = 0.27). Similar results relating to the light-dependence of photosynthesis in red snow algae was reported by Williams et al. (Citation2003) for North America. Rae et al. (Citation2000) described photosynthesis in Antarctic phytoplankton as temperature-dependent and showed similar kinetics to the data in . Moreover, red cell stages of Haematococcus showed a similar light–response curve to C. nivalis without any sign of high-light photoinhibition (Wang et al., Citation2003). However, the mesophilic C. reinhardtii showed significant photoinhibition in high light (Leverenz et al., Citation1990).
Comparable data on the temperature-dependence of physiological processes are available for the green snow algae Chloromonas sp., which also forms secondary carotenoids and is widely distributed in the Antarctic Peninsula (Ling & Seppelt, Citation1993). Loppes et al. (Citation1996) compared nitrate reductase and argininosuccinate lyase in Chloromonas from Petrel Island with enzymes measured in C. reinhardtii, a mesophilic reference. The optimum temperature in the Antarctic species was 20°C lower. However, Devos et al. (Citation1998) found that the CO2-fixing enzyme Rubisco was not more cold stable than in C. reinhardtii, but the relative amount of the enzyme was twice as high. This was discussed as a novel adaptation strategy.
Williams et al. (Citation2003) described CO2 fixation at snow temperatures of 1–3°C in a snow patch. They assumed that photosynthesis was related to the red snow covering the patch. Their mean values were about 50% of our values (assuming a photosynthetic quotient of 1; ), and were obtained with impure red snow, which may interfere with the estimation of algal metabolism due to the activity of bacteria and fungi in their samples. However, at the saturating irradiance of approximately 1000 µmol m−2 s−1, their photosynthetic rates were similar to ours.
The high photostability of snow algae is presently explained either by carotenoids acting as a filter to reduce irradiance by more than 50% (Gorton et al., Citation2001), or, especially for the UV region, by phenolics (measured as total phenolics, Duval et al., Citation2000). The esterification of astaxanthin with fatty acids, which was highly expressed in our C. nivalis samples, is regarded as a mechanism for efficient pigment concentration in cytoplasmic globules (Bidigare et al., Citation1993). The ultrastructure of our samples shows that massive globular structures fill a considerable part of the cell, with the plastid mainly centrally located. On the other hand, many species of snow algae do not accumulate secondary carotenoids (Kol, Citation1968; Hoham & Duval, Citation2001; Leya, Citation2004) and show a similar photostability in vivo. It remains to be determined whether or not algal cell walls contain UV-protective secondary metabolites, as described for higher plants (Schnitzler et al., Citation1996).
In several samples, mature hypnoblasts contained about 20 times more astaxanthin, which causes the red colour of Alpine red snow, than chlorophyll a. Similar results were obtained from the Arctic (ratio of 34.1:1 for dark-red cysts; Müller et al., Citation1998) and Rocky Mountains, USA (ratio of 20.76:1 for C. nivalis; Bidigare et al., Citation1993). However, some minor peaks in our HPLC separation had an additional absorption shoulder at 375 nm when compared to the spectra for the astaxanthin calibration standard. Further investigations on this putative UV-screening compound are ongoing.
Although α-tocopherol (vitamin E) is a well-known and important antioxidant within plastids (Trebst, Citation2003), there are no previous reports of the vitamin E content of snow algae. Young cells of C. nivalis cells from the Kühtai sample (see ) had about twice the α-tocopherol content of older stages (), suggesting that the increased accumulation of secondary carotenoids reduces light stress thus reduces the need for this antioxidant. It is likely that the sometimes considerable formation of plastoglobules in the plastid is a sign of the high tocopherol content (cf. ).
An additional feature of astaxanthin and related secondary carotenoids is their antioxidant activity. This is described for the green alga Haematococcus pluvialis, which forms similar amounts of secondary carotenoids to C. nivalis, but grows at mesophilic temperatures (Kobayashi & Okada, Citation2000; Boussiba et al., Citation2000). High-light can transform green H. pluvialis cells to red stages (Torzillo et al., Citation2005). However, antioxidant activity of astaxanthin located outside the chloroplast will not remove radicals formed during photosynthesis, as occurs with the intraplastidal α-tocopherol. Other authors describe culturing H. pluvialis for industrial production of astaxanthin, which can be used for food, feed and cosmetic purposes (Guerin et al., Citation2003).
Algae found in the Alps (personal observations) or the mountains of North America (cf. Weiss, Citation1983), have numerous structures such as dust particles (, ), fungi () or bacteria (not shown here) attached to the outer cell wall. Biota found close to the cells may represent an unknown form of symbiosis or even parasitism. The cell wall of C. nivalis, as the outer boundary to an extreme environment, is very rigid and difficult to destroy mechanically. The visible mucilage sheet, which occasionally covers larger cells in particular, will attract such organisms.
Sugar and mucilage excretion requires active photosynthesis, as is reported in other studies with high alpine freshwater green algae from the genus Micrasterias; cells well known for their mucilage production (Meindl & Lütz, Citation1996; Lütz et al., Citation1997; Weiss et al., Citation1999). The carbohydrates of the mucilage surrounding the cells may be utilised by bacteria and fungi. Moreover, mucilage and the outer cell wall structure (Müller et al., 1998a) support the adherence of organic and inorganic particles, which are transported into snow by winds, finally forming cryoconites (Takeuchi, Citation2002). These adhering particles may shade and thus protect C. nivalis against high irradiance. Not all snow algae species use this strategy, however; hypnoblasts of Chloromonas nivalis, for example, never show such attached structures (personal observations).
Chlamydomonas cf. nivalis has been reported from almost all arctic and alpine regions (Kol, Citation1968) and, consequently, can be regarded as a cosmopolitan cryophilic species. However, the difficulties of taxonomic identification remain (Leya, Citation2004) and molecular research is required to prove whether algae of coloured snow in the Alps have the same identity as algae found in similar habitats on other continents. Ultrastructural investigations can provide such identification and classification only for certain species and life-cycle stages. Consequently, the species we investigated might be transferred to a new species of Chlamydomonas in future.
Snow and ice cover large areas of our planet but to date have not been as intensively investigated as temperate or some tropical ecosystems. Williams et al. (Citation2003) claim that, world-wide, snow algae might play an important role in balancing the atmospheric carbon budget.
Acknowledgements
This work was supported by the Austrian Science Foundation (FWF), Project P 17455 to C. Lütz. We thank R.M.M. Crawford for critical reading of the manuscript.
References
References
- Bidigare , RR , Ondrusek , ME , Kennicutt , MC , Iturriaga , R , Harvey , HR , Hoham , RW and Macko , SA . 1993 . Evidence for a photoprotective function for secondary carotenoids of snow algae . J. Phycol. , 29 : 427 – 34 .
- Boussiba , S . 2000 . Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response (Minireview) . Physiol. Plantarum , 108 : 111 – 117 .
- Czygan , FC . 1968 . Sekundär-Carotinoide in Grünalgen. I. Chemie, Vorkommen und Faktoren, welche die Bildung dieser Polyene beeinflussen . Arch. Mikrobiol. , 61 : 69 – 76 .
- Czygan , FC . 1970 . Blutregen und Blutschnee: Stickstoffmangelzellen von Haematococcus pluvialis und Chlamydomonas nivalis . Arch. Microbiol. , 74 : 69 – 76 .
- Delieu , T and Walker , DA . 1972 . An improved cathode for the measurement of photosynthetic oxygen evolution by isolated chloroplasts . New Phytol. , 71 : 201 – 225 .
- Devos , N , Ingouff , M , Loppes , R and Matange , R . 1998 . Rubisco adaptation to low temperatures: a comparative study in psychrophilic and mesophilic unicellular algae . J. Phycol. , 34 : 655 – 660 .
- Duval , B , Duval , W and Hoham , RW . 1999 . Snow algae of the Sierra Nevada, Spain and High Atlas Mountains of Morocco . Int. Microbiol. , 2 : 39 – 42 .
- Duval , B , Shetty , K and Thomas , WH . 2000 . Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light . J. Appl. Phycol. , 11 : 559 – 566 .
- Ettl H Gerloff J Heynig H Mollenhauer D 1983 Süßwasserflora von Mitteleuropa, Chlorophyta I: Phytomonadina, Spektrum-Fischer Stuttgart
- Gorton , HL and Vogelmann , TC . 2003 . Ultraviolet radiation and the snow alga Chlamydomonas nivalis (Bauer) Wille . Photochem. Photobiol. , 77 : 608 – 615 .
- Gorton , HL , Williams , WE and Vogelmann , TC . 2001 . The light environment and cellular optics of the Snow algae Chlamydomonas nivalis (Bauer) Wille . Photochem. Photobiol. , 73 : 611– 620
- Guerin , M , Huntley , ME and Olaizola , M . 2003 . Haematococcus astaxanthin: applications for human health and nutrition . Trends Biotechnol. , 21 : 210 – 216 .
- Hoham , RW . 1975 . Optimum temperatures and temperature ranges for growth of snow algae . Arct. Antarct. Alp. Res. , 7 : 13 – 24 .
- Hoham RW Duval B 2001 Microbial ecology of snow and freshwater ice with emphasis on snow algae Snow Ecology: An Interdisciplinary Examination of Snow-covered Ecosystems (Jones, H.G., Pomeroy, J.W., Walker, D.A. & Hoham, R.W. editors) 168 228 Cambridge University Press Cambridge
- Hoham , RW , Bonome , TA , Martin , CW and Leebens-Mack , JH . 2002 . A combined 18S rbcL phylogenetic analysis of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats . J. Phycol. , 38 : 1051 – 1064 .
- Kawecka , B . 1978 . Biology and ecology of snow algae. 1. The sexual reproduction of Chlamydomonas nivalis (Bauer) Wille (Chlorophyta, Volvocales) . Acta Hydrochem. Hydrobiol. , 20 : 111 – 116 .
- Kobayashi , M and Okada , T . 2000 . Protective role of astaxanthin against U.V.-B irradiation in the green alga Haematococcus pluvialis . Biotechnol. Lett. , 22 : 177 – 181 .
- Kol E 1968 Kryobiologie. Biologie und Limnologie des Schnees und Eises. I. Kryovegetation, Schweizerbart'sche Verlagsbuchhandlung Stuttgart
- Kómarek , O and Kómarek , J . 2001 . Contribution to the taxonomy and ecology of green cryosestic algae in the summer season 1995–96 at King George Island, S. Shetland Islands . Nova Hedwigia Beiheft , 123 : 121 – 140 .
- Komárek , J , Hindák , F and Javornický , P . 1973 . Ecology of the green kryophilic algae from Belankské Tatry Mountains (Czechoslovakia) . Algol. Studies , 9 : 427 – 449 .
- Leverenz , JW , Falk , S , Pilström , CM and Samuelsson , G . 1990 . The effects of photoinhibition on the photosynthesis light-response curve of green plant cells (Chlamydomonas reinhardtii) . Planta , 182 : 161 – 168 .
- Leya T 2004 Feldstudien und genetische Untersuchungen zur Kryophilie der Schneealgen Nordwestspitzbergens, Shaker Aachen
- Ling , H and Seppelt , RD . 1993 . Snow algae of the Windmill Islands, continental Antarctica-3-Chloromonas polyptera (Volvocales, Chlorophyta) . Polar Biol. , 20 : 320 – 324 .
- Loppes , R , Devos , N , Willem , S , Barthélemy , P and Matagne , RF . 1996 . Effect of temperature on two enzymes from a psychrophilic Chloromonas (Chlorophyta) . J. Phycol. , 32 : 276 – 278 .
- Lütz , C . 1996 . Avoidance of photoinhibition and examples of photodestruction in high alpine Eriophorum . J. Plant Physiol. , 148 : 120 – 128 .
- Lütz , C , Seidlitz , HK and Meindl , U . 1997 . Physiological and structural changes in the chloroplast of the green alga Micrasterias denticulata induced by UV-B simulation . Plant Ecol. , 128 : 54 – 64 .
- Meindl , U and Lütz , C . 1996 . Effects of UV irradiation on cell development and ultrastructure of the green alga Micrasterias . J. Photochem. Photobiol. , 36 : 285 – 292 .
- Mosser , JL , Mosser , AG and Broeck , TD . 1977 . Photosynthesis in the snow: the alga Chlamydomonas nivalis (Chlorophyceae) . J. Phycol. , 13 : 22 – 27 .
- Müller , T , Bleiss , W , Martin , CD , Rogaschewski , S and Fuhr , G . 1998 . Snow algae from northwest Svalbard: their identification, distribution, pigment and nutrient content . Polar Biol. , 20 : 14 – 32 .
- Müller , T , Leya , T and Fuhr , G . 2001 . Persistent snow algal fields in Spitsbergen: field observations and a hypothesis about the annual cell circulation . Arct. Antarct. Alp. Res. , 33 : 42 – 51 .
- Novis , PM . 2002 . New records of snow algae for New Zealand, from Mt. Philistine, Arthur's Pass National Park . New Zeal. J. Bot. , 40 : 297 – 312 .
- Painter , TH , Duval , B , Thomas , WH , Mendez , M , Heintzelman , S and Dozier , J . 2001 . Detection and quantification of snow algae with an airborne imaging spectrometer . Appl. Environ. Microb. , 67 : 5267 – 5272 .
- Porra , RJ , Thompson , WA and Kreidemann , PE . 1989 . Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by absorption spectroscopy . Biochem. Biophys. Acta , 975 : 384 – 394 .
- Rae R Howard-Williams C Hawes I Vincent WF 2000 Temperature dependence of photosynthetic recovery from solar damage in Antarctic phytoplankton Antarctic Ecosystems: Models for Wider Ecological Understanding; Proceedings of the VII SCAR Biology Symposium (Davidson, W. Howard-Williams, C. & Broady, P. editors) 182 189 Caxton Press Christchurch New Zealand
- Schnitzler , JP , Jungblut , TP , Heller , W , Köfferlein , M , Hutzler , P , Heinzmann , U , Schmelzer , E , Ernst , D , Langebartels , C and Sandermann , H . 1996 . Tissue localization of U.V.-screening pigments and chalcone synthase mRNA in needles of Scots pine seedlings . New Phytol. , 32 : 247 – 258 .
- Siefermann-Harms , D . 1988 . High-performance liquid chromatography of chloroplast pigments . J. Chromatogr. , 448 : 411 – 416 .
- Takeuchi , N . 2002 . Optical characteristics of cryoconite (surface dust) on glaciers: the relationship between light absorbency and the property of organic matter contained in the cryoconite . Ann. Glaciol. , 34 : 409 – 414 .
- Thomas , WH . 1972 . Observations on snow algae in California . J. Phycol. , 8 : 1 – 9 .
- Thomas , WH and Duval , B . 1995 . Sierra Nevada, California, U.S.A., Snow algae: snow albedo changes, algal–bacterial interrelationships, and ultraviolet radiation effects . Arct. Antarct. Alp. Res. , 27 : 389 – 399 .
- Torzillo , G , Goskan , T , Kik , O and Gökpinar , S . 2005 . Photon irradiance required to support optimal growth & interrelations between irradiance & pigment composition in the green alga Haematococcus pluvialis . Eur. J. Phycol. , 40 : 233 – 240 .
- Trebst , A . 2003 . Function of β-Carotene and Tocopherol in Photosystem II . Z. Naturforschung , 58c : 609 – 620 .
- Wang , B , Zarka , A , Trebst , A and Boussiba , S . 2003 . Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance . J. Phycol. , 39 : 1116 – 1124 .
- Weiss , RL . 1983 . Fine structure of the snow alga Chlamydomonas nivalis and associated bacteria . J. Phycol. , 19 : 200 – 204 .
- Weiss , D , Lütz , C and Lütz-Meindl , U . 1999 . Photosynthesis and heat response of the green alga Micrasterias denticulata (Desmidiaceae) . Z. Naturforschung , 54c : 508 – 516 .
- Williams , WE , Gorton , HL and Vogelmann , TC . 2003 . Surface gas-exchange processes of snow algae . Proc. Nat. Acad. Sci. , 100 : 652 – 566 .