Abstract
We report on taxonomically distinguishing morphological features of six diatom taxa from the marine, nanoplanktonic genus, Minidiscus, found in Eastern Canada and neighbouring waters. Four of the six were also investigated using genetic approaches. We found that the culture deposited in the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (CCMP) as CCMP 496 was morphologically closest to the published type image of M. trioculatus, whereas other isolates were morphologically and/or genetically divergent. Consequently, we propose three new taxa. One monoclonal line isolated from the Bay of Fundy is proposed as a new variety, M. trioculatus var. monoculatus. The well-studied nanoplanktonic diatom strain, CCMP 495, currently considered to be ‘M. trioculatus’ was morphologically and genetically divergent from CCMP 496 and we propose specific status for it, M. variabilis. We isolated a new strain from the Gulf of St Lawrence that was nearly genetically identical in 18S and ITS regions to CCMP 495, but showed distinct morphological differences and we propose a new form for this strain. Culture-based data were supplemented by those from natural seawater whenever possible. Two species (M. comicus, M. chilensis) known from our region, but unavailable in culture, are presented for comparison.
Introduction
Minidiscus Hasle, a relatively recently described genus (Hasle, Citation1973 a, Citation b ), contains some of the smallest diatoms known, rarely exceeding 5 µm in diameter (Tomas, Citation1997), with most of the individuals from the natural environment ranging from 2 to 4 µm in diameter. The discovery of fultoportulae and the internal position of the areolar velum in the species previously classified as Coscinodiscus trioculatus Taylor (Citation1967) warranted its transfer to the family Thalassiosiraceae (Hasle, Citation1973 a) and established a new genus Minidiscus (Hasle, Citation1973 b). Generic diagnosis specifies the absence of a marginal circlet of fultoportulae (present in most other members of the thalassiosiroid lineage); instead the fultoportulae are irregularly scattered more or less around the centre of the valve face. Each fultoportula has a wide ring of silica at the base of an external tube. A single sub-central rimoportula is also present. The valve face of the generitype, M. trioculatus (Taylor) Hasle, is rimmed by a wide hyaline flange, but this character is not present in all species.
Minidiscus currently comprises seven species (Cheng et al., Citation1993; Tomas, Citation1997; Aké-Castillo et al., Citation2001; Quiroga & Chrétiennot-Dinet, Citation2004), some known only from the sites of their original description. However, more, yet undescribed, diversity has been postulated (Quiroga & Chrétiennot-Dinet, Citation2004). Due to their extremely small size, Minidiscus spp. are not readily detectable or routinely identifiable using classical light microscopy (Hasle, Citation1978). In addition, fluorescence, flow cytometry or pigment-based methods used to detect smaller nanoplankton (2–6 µm in diameter) and picoplankton (ca. 0.2–3 µm) community members do not allow identification below broad taxonomic groupings, rendering the distribution and autecology of small species ill defined. Nonetheless, some members of the genus have been reported in a number of studies, and at least two species, M. trioculatus and M. chilensis Rivera, seem to have cosmopolitan distributions and may occur at high cell densities (Tomas, Citation1997; Bérard-Therriault et al., Citation1999; Buck et al., Citation2008).
Here we present morphological and genetic analysis of six taxa of these easily overlooked nanoplanktonic diatoms. Our analysis indicates that more cryptic diversity exists within this genus and we propose a new species, a new variety and a new form. We augment the circumscription of M. trioculatus with observations from monoclonal cultures, strains from algal culture collections, and natural samples from the Canadian Maritime Provinces. Molecular tags are provided for cultured taxa (two species, one variety and one form), as a step towards molecular taxonomic (barcoding) and ecosystem functioning investigations of very small eukaryotes such as these diatoms.
Materials and methods
Field collection and cultures
Opportunistic samples were collected from a variety of planktonic and intertidal sites along the Atlantic coast of Canada between 1998 and 2005. Collection of samples occurred in the Bay of Fundy at Wolves Islands (44°59′61″N; 66°44′ 36″W) and Passamaquoddy Bay (45°04′00″N; 66°58′00″W), in the Gulf of St Lawrence at Cape Tormentine (46°13′25″N; 63°78′10″W) in the province of New Brunswick, and the Atlantic coast of the province of Nova Scotia at Ship Harbour (44°48′25″N; 62°52′56″W), near the port of Halifax. Vertical net hauls or bottle samples were collected following standard UNESCO protocols (Sournia, Citation1978). Samples intended for cell counts and biometrics were fixed on site with acidified Lugol's. Culture isolates were established using micropipette and agar-streak methods (Andersen, Citation2005). Our own isolates and two strains of Minidiscus from the Center for Culture of Marine Phytoplankton (CCMP), CCMP 495 and 496, were grown in f/2 (Guillard, Citation1975) or L1+ (Andersen, Citation2005) media, at temperatures ranging between 12 and 22°C, at an irradiance of 20–50 µmol m−2 s−1.
Two new monoclonal cultures were established, one from the waters near Wolves Islands in the Bay of Fundy and the other from Cape Tormentine in the Gulf of St Lawrence. These two clones were subjected to the same biometric and genetic analyses as the two strains from CCMP. CCMP 495 and CCMP 496 were isolated some 30 years ago from the Gulf of Maine and an undisclosed location, respectively (). In a reconnaissance examination, CCMP 495 demonstrated considerable morphological variability. Because of this, and because it has been in culture for years, we tested for divergence that might have occurred within the strain over this time. To do so, we established new sub-clones using flow-cytometry with a cell sorting module (Andersen, Citation2005). Using this instrument, cells of the CCMP 495 ‘whole strain’ were sorted one by one and individual cells were placed into separate mini-wells to start new monoclonal (single-cell derived) isolates. These isolates will be referred to as sub-clones and were examined individually with respect to the range of morphological variability in order to compare them to the ‘whole strain’. The first 10 valves encountered from each of 10 sub-clonal cultures were imaged and measured as described below.
Table 1. Summary of Minidiscus culture codes, isolation dates and locations, GenBank and BOLD accession numbers.
Acquisition of morphometric data
Species identification was carried out using scanning electron microscopy (SEM). Samples were cleaned and prepared for SEM as described in Kaczmarska et al. (Citation2005) and observed using a JEOL JSM-5600 SEM (JEOL USA, Peabody, MA, USA) at the Digital Microscopy Facility, Mount Allison University, operating at 10 kV and 8 mm working distance. Valve diameter, number of areolae, pores on the valve face and the valvocopulae, and the number of fultoportulae and rimoportulae per valve were determined for at least 30 valves using SEM images from each of four cultured isolates; CCMP 495 (prior to sorting sub-clones), CCMP 496, and two newly established cultures in our laboratory referred to hereafter as M. trioculatus var. monoculatus and M. variabilis f. inornata. The same characters were quantified from specimens of CCMP 495 subclones and of natural samples, whenever a species of Minidiscus was encountered.
Valve silicification was examined with energy dispersive X-ray spectroscopy (EDS) and was performed on the same SEM equipped with an Oxford Inca Energy 200 EDS system (Oxford Instruments, High Wycombe, UK) at 20 mm working distance. Ten consecutive valves were selected for collection of spectra. Since the only element of interest in this study was silicon (Si-Kα, X-ray energy 1.74 kV), an accelerating voltage of 10 kV provided sufficient overvoltage for efficient X-ray excitation, and gold coating did not present any problematic X-ray peak overlaps. Spectra were acquired for 100 s (dead time corrected) at 0.1 nA beam current with an energy range of 0–10 kV in 2048 channels. The small, thin valves approximate thin-film-on-substrate specimens (Pouchou & Pichoir, Citation1991), in that the interaction volume of the incident electron beam is sufficiently large to excite both the valve and the underlying polycarbonate filter. More heavily silicified valves will produce more silicon X-rays compared to thinly silicified valves. Peak-to-background ratios of the characteristic peak for silica were calculated to effectively remove the effect of fine-scale morphological details (Statham, Citation1979). While this method does not provide a quantitative determination of silicon, it does produce a relative assessment of the degree of silicification.
DNA extraction, amplification and sequencing
Inter-clonal and intra-clonal sequence variability was tested by repeated extractions and amplifications in two independent laboratories, as indicated in . Sequence reliability was evaluated following recommendation of Feliner and Rosselló (Citation2007) and reasoning of Thornhill et al. (Citation2007).
At Laval University, aliquots of each culture were centrifuged to obtain a pellet; once the medium was removed, the pellet was frozen at–20°C for subsequent DNA extraction. DNA was extracted with a QIAGEN DNeasy plant mini kit (QIAGEN Inc., Mississauga, ON, Canada), following manufacturer's instructions. The rRNA genes were amplified by PCR with NSF4/18 (Handriks et al., 1989) and NLR/204 (Van der Auwera et al., 1994) primers. PCR reactions contained 5 µl of DNA extract, 1X ThermoPol reaction Buffer (NE Biolabs, Ipswich, MA, USA), 0.2 mM dNTP (Fermentas, Burlington, ON, Canada), 0.5 mM of each primer, 20 µg of BSA (Fermentas) and 1.25 units of Taq DNA polymerase (NE Biolabs) in a final volume of 50 µl. Reactions were performed with a BIORAD iCycler thermocycler (BIORAD Laboratories, Hercules, CA, USA) under the following conditions: a denaturation cycle of 94°C for 3 minutes followed by 30 cycles comprising a denaturation step at 94°C for 45 seconds, an annealing step at 55°C for 1 minute and an extension step at 72°C for 3 minutes with a final extension step at 72°C for 5 minutes. PCR products were purified and sequenced at the Centre Hospitalier de l’Université Laval (CHUL) with an ABI 3730xl system (Applied Biosystems, Foster City, CA, USA) using NSF4/18, 528f (Elwood et al., Citation1985), NLR204/21 (Van der Auwera et al., Citation1994) and 1055f primers adapted from F1337 (McCallum & Maden, Citation1985). NSF and NLR primers were from the European Ribosomal RNA database (http://bioinformatics. psb.ugent.be/webtools/rRNA/).
At Mount Allison University, cultured, concentrated cells were subjected to an UltraClean Soil DNA Kit (MoBio Laboratories, Carlsbad, CA, USA) to obtain DNA, following manufacturer's recommendations. The nuclear internal transcribed spacers, (ITS1 and ITS2) and the 5.8S rDNA gene (hereafter collectively referred to as the ITS region) were amplified using ITS1 and ITS4 primers (White et al., Citation1990) following Kaczmarska et al. (Citation2008). Sequencing was conducted at Nanuq (Genome Québec) following their standard procedure with the ITS1 and ITS4 primers.
Results
Taxonomic observations
We found three out of seven species attributed to the genus Minidiscus in the Canadian Maritimes: Minidiscus trioculatus, M. comicus Takano, and M. chilensis. In addition, for the specimens that did not conform fully to descriptions of existing species, new taxa are described; one new species, a new variety and a new form. These taxa are characterized morphologically and genetically (when cultures were available), in alphabetical order below.
Minidiscus chilensis Rivera,
In natural samples cells were always solitary. When planktonic (), the specimens were either free living, or sessile on debris, other diatoms or on aggregates of marine snow. This diatom has also been regularly found on sediments on intertidal mudflats, and was normally coated with clay in a manner that suggests it was growing in that environment ().
Figs 1–6. Figs 1–2. Minidiscus chilensis. . Valve of a planktonic specimen with a thread (presumably chitin) extruded from a central fultoportula (short arrows) with a small external tube, a small external rimoportula opening (long arrow), and short, radial rows of simple pores (arrowhead). . Specimen from the benthos, sessile on a grain of sediment and covered with particles of clay; note the external fultoportula tubes (arrowheads). . Minidiscus comicus, all planktonic forms. . One individual with a thread (presumably chitin) extruding from one fultoportula with a large, wide open external tube (short arrows). . Recently divided cells with collapsed cingulum. Note the wide openings of the external fultoportula tubes (short arrows), one with a thread emerging. . A strongly silicified specimen with small areola openings (short arrows), wide open external fultoportula tubes and centrally located external rimoportula opening (long arrow). . Lightly (incompletely) silicified valve showing radial areola arrangement and the outlines of the bases of the fultoportula external tubes (arrowheads). Scale bars: 1 µm.

Frustules were in the shape of a flat drum, the pervalvar axis shorter than the cell diameter (3.1–5.1 µm), with 2–3 open ligulate copulae. One row of large pores, corresponding to every second to third row of valve face pores, was present on the valvocopula. Valve mantles were shallow, but overlapped the valvocopulae. Ornamentation of both the external and the internal valve surfaces was similar and consisted of a peripheral ring of short, radial rows of simple pores, which occupied approximately one-fifth to one-quarter of the valve radius; 9–10 rows of pores in 1 µm (; ). Two fultoportulae and one rimoportula were located on the elevation and one fultoportula in the valve depression (). In addition, a small pore leading to a cavity was present between the rimoportula and the solitary fultoportula. All external endings of the processes were similar, with small and short thickened rings surrounding their openings. Internally rimoportulae ended in an ellipsoidal ring of thickened silica, and fultoportulae were subtended by two satellite pores. Valves showed very little variability in ornamentation.
Table 2. Morphometric summary of four strains of Minidiscusa .
DISTRIBUTION: This is the most common of the Minidiscus species in the Bay of Fundy, reaching frequencies of up to 260 000 cells l−1 in the four Wolves Islands samples (May 2004; counted using SEM). In Canada, this diatom has been found from coastal Quebec (Bérard-Therriault et al., Citation1986) and the Gulf of St Lawrence (Bérard-Therriault et al., Citation1999). It is widely distributed and abundant worldwide (Rivera & Koch, Citation1984; Lange, Citation1985; Sancetta, Citation1990; Kang et al., Citation2003; Buck et al., Citation2008). Although this is the first report of this species being sessile in intertidal sediments, Buck et al. (Citation2008) reported it as being commonly associated with mineral particles in an upwelling system of central California. This may afford the cells just enough extra mass to settle on the mudflats and wait for later re-suspension. If this speculation is correct, the cell mineral-particle association in this species may carry an adaptive advantage.
Minidiscus comicus Takano,
Cells were mostly solitary but capable of forming 2–3 cell colonies with cells connected via threads extruded from fultoportulae (). Frustules were spherical to ellipsoidal (2.1–3.2 µm in diameter), commonly with no discernible copulae, except during cell division (). Valves had no obviously separated mantle, were strongly convex and covered with a net of loculate and/or pseudoloculate areolae. External foramina of areolae may be very small in more heavily silicified valves (). Valve margins were very narrow, with marginal areolae terminating close to the valve edge (). There were 6–8 areolae in 1 µm. There were normally four processes, three fultoportulae and one rimoportula, located close to the valve centre. The position of the rimoportula was mostly central, with respect to the fultoportulae. Processes were usually separated by more than one areola (). When fully developed, the external fultoportula tubes were well developed, wide open tall cylinders (). Internal views of the valves were not found in our samples.
DISTRIBUTION: This species was rarely encountered; single specimens were only found in samples from Ship Harbour and the Bay of Fundy. Elsewhere in Canada it has been reported from the Gulf of St Lawrence (Bérard-Therriault et al., Citation1999). Worldwide, M. comicus is known from the Pacific and the Atlantic coasts, as well as from the Adriatic Sea (Takano, Citation1981 a; Lange, Citation1985; Tomas, Citation1997) suggesting a cosmopolitan distribution and low abundance.
Minidiscus trioculatus (Taylor) Hasle var. trioculatus (sensu Taylor, Citation1967, plate 5, fig. 43), ,
Cultured specimens were obtained from CCMP (strain 496), which was established by Taylor from an undisclosed location. In culture, cells were often solitary in exponentially growing cultures, but became aggregated in compact clusters in stationary phase. Each cell contained one parietal chloroplast appearing as a crescent in valve face orientation. Prior to cell division, the chloroplast divided and one sibling chloroplast migrated underneath each valve face. Cells were cylindrical in girdle view, the pervalvar axis 1–2 × the cell diameter (2.5–4.2 µm; ). Auxospore-like spherical cells were observed in the culture, but no other signs of sexual reproduction were ever found, suggesting the capacity for vegetative cell enlargement (). The valve face was circular, relatively flat with a tangential–linear arrangement of loculate areolae, mean 4.4–6.5 in 1 µm throughout, except for a wide hyaline flange (). Valves had no obvious mantle. The advalvar margin of a valvocopula folded underneath the valve face and subtended the valve margin (). Most often, only two fultoportulae were present, but three to five and very rarely up to seven, were seen on some valves. Internally, a cluster of pores corresponded to areola vela and there were 16–25 pores in 1 µm (). There was one small, sub-central rimoportula on the valve face. Copulae were in the form of open rings with at least two rows of pores, with larger pores in the advalvar position (). This strain showed very little morphological variability in culture or in natural samples.
Figs 7–10. Minidiscus trioculatus var. trioculatus, cultured material, CCMP strain 496 and a natural plankton specimen. . Larger, partially silicified, dome-like shaped valve, which possibly originated from an auxospore-like structure (see text for details); note characteristic wide basal part of the fultoportula external tube (short arrow). . Specimen from the natural environment, note tangential–linear valve face ornamentation and several copulae, one folded underneath the marginal flange of the valve (arrowhead). . An external view of a partially silicified valve with a tangential–linear areola pattern and a valvocopula with two rows of pores (arrowheads). Note the two satellite pores of the fultoportula (short arrows). . Internal valve view. Note the clusters of pores below the areolae (arrowheads), and the internal openings of two fultoportulae, each with two satellite pores (short arrows), and a rimoportula (long arrow). Scale bars: 1 µm.
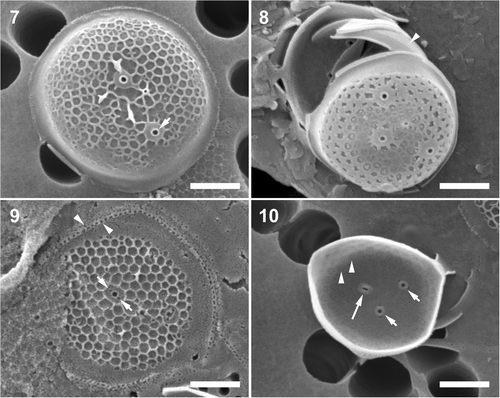
DISTRIBUTION: This diatom was quite rare in the Bay of Fundy samples. It is more common in plankton of the Gulf of St Lawrence and (at times) the Western coast of Canada (Sancetta, Citation1990; Bérard-Therriault et al., Citation1999). Minidiscus trioculatus is a cosmopolitan diatom reported from the eastern coast of Europe (Tomas, Citation1997), southwestern coast of Africa (Taylor, Citation1967), Australian waters (Hallegraeff, Citation1984), Japanese coasts (Takano, Citation1981 b, Citation1990) and the Gulf of Mexico (Hasle, Citation1973 b; Aké-Castillo et al., Citation2001).
Minidiscus trioculatus var. monoculatus Kaczmarska var. nov., ,
Figs 11–14. Minidiscus trioculatus var. monoculatus. . A typical specimen from a natural sample. Note one margin of the valvocopula is tucked underneath the marginal flange of the valve (short arrows). . Two valves from culture, showing a varying degree of areola development and the external openings of the fultoportula (arrowhead) and the rimoportula (long arrow). . A partially silicified valve illustrating tangential–linear areola organization and valvocopula with rows of pores (short arrow). . An internal view of a valve with a sub-central fultoportula (arrowhead), and a rimoportula (long arrow). Scale bars: 1 µm.
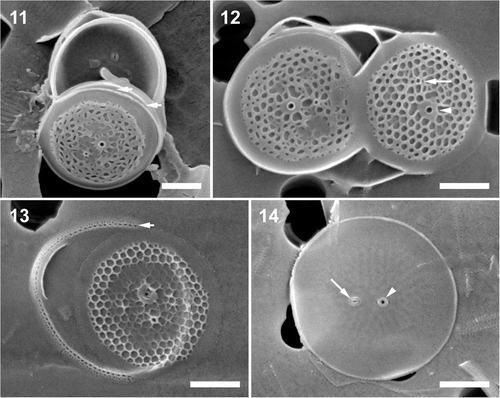
HOLOTYPE: SEM preparation #WV031999 () ex culture, deposited at the Botanic Garden and Botanical Museum Berlin-Dahlem, Germany B40 0040655. SSU and ITS-region sequences of the nuclear encoded rDNA of the type have been deposited in GenBank, while the molecular barcode was deposited in the Barcoding of Life Data (BOLD) System (accession numbers in ).
LOCUS TYPICUS: In sub-surface plankton off the Wolves Islands in the Bay of Fundy, Canada, from which a culture was started after the isolation of a single colony growing on agar.
HABITAT: In coastal plankton in the Quoddy Region of the Bay of Fundy, and the Atlantic Coast of Nova Scotia at Ship Harbour, often the most common Minidiscus species. In culture, our isolate grew best in an equal mixture of f/2 and L1+ media (Andersen, Citation2005) at 12–22°C, and an irradiance of 20–50 µmol m−2 s−1.
DIAGNOSIS: Valvae ferentes fultoportulam singularem subcentralem.
ENGLISH TRANSLATION OF DIAGNOSIS: Valves carry only one sub-centrally located fultoportula.
DIFFERENTIAL DIAGNOSIS: This diatom is closely related to M. trioculatus var. trioculatus (as in CCMP 496, not CCMP 495) in valve ornamentation, but carries only one fultoportula near the valve face centre.
DETAILED DESCRIPTION: Valves rather flat, circular, mean diameter 2.4–3.7 µm when in culture (). Specimens in natural samples are close to the lower end of the size range, 2.3–3.0 µm. Externally, the valve face of lightly silicified specimens is covered with a net of tangential–linear pseudolocular areolae; 4.8–6.8 areolae in 1 µm. In more heavily silicified individuals areolar foramina are narrow and irregular. Areolae are best developed at the valve periphery, with many valves showing little areolation throughout most of the centre of the valve face. Internally, the basal siliceous layer is perforated by clusters of minute pores, with a mean of 18–24 pores in 1 µm. One rimoportula and one fultoportula are located near the valve face centre. Cell shape, rimoportula and fultoportula structure are the same as in the nominate form (above). The morphology of this clone was invariable in culture over the four years of observation. Among hundreds of valves examined by SEM from cultured material, only one valve was found with two fultoportula.
COMPARISON WITH OTHER SPECIES: This diatom is clearly closely related to M. trioculatus var. trioculatus (CCMP 496, not CCMP 495), except for the number of fultoportulae, the finer areolation and the somewhat smaller and less well silicified valves (when grown under the same temperature, nutrient and light conditions; ). The means of the latter three characters were significantly different between varieties (t-test, p < 0.05).
DISTRIBUTION: In natural samples from the Bay of Fundy this form was the second most abundant Minidiscus, with up to 250 000 cells l−1 in the four Wolves Islands samples collected in May 2004. The nominate variety of M. trioculatus was generally infrequent, but nearly always absent in those samples where the new variety was present. Minidiscus species are so small that they are normally documented only by electron microscopy, yet a large number of studies illustrate them. However, only one study (Fukuyo et al., Citation1990), from the coast of Japan, mentions (but does not illustrate) M. trioculatus with one fultoportula, which may represent our new variety. We suggest that it is unlikely that the distinct valves of the new variety would remain unmentioned or undocumented if the taxon was common, and that the new variety has a more restricted distribution than the nominate form.
Minidiscus variabilis Kaczmarska, sp. nov. (in CCMP 495 as ‘M. trioculatus’), ,
Figs 15–18. Minidiscus variabilis var. variabilis, cultured material, CCMP strain 495. . External valve view showing the typical, radial areola arrangement. . A frustule showing the areola distribution and the ornamentation of the valvocopula (short arrow). . An incompletely silicified valve showing the number and distribution of portulae. Note a number of radial ridges running from the valve near-centre to the marginal flange. . Internal view of radial rows of minute pores. Scale bars: 1 µm.
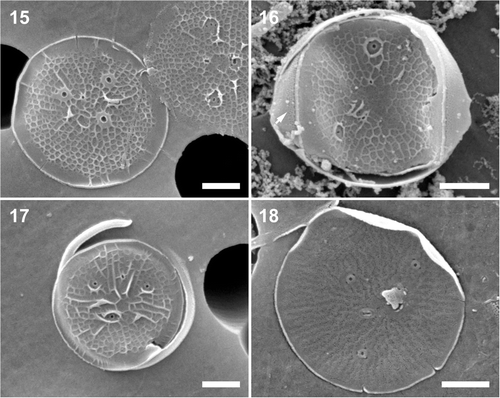
HOLOTYPE: SEM preparation #58-7 () ex culture, deposited at the Botanic Garden and Botanical Museum Berlin-Dahlem, Germany B40 0040656. The live culture of this strain, from which the holotype is designated, is held in CCMP as ‘CCMP 495 M. trioculatus’. The SSU and ITS-region sequences of the nuclear encoded rDNA of the taxonomic type has been deposited in GenBank, while the molecular barcode is available at BOLD (accession numbers in ).
LOCUS TYPICUS: Gulf of Maine, North Atlantic; collected and isolated by K. Haines in May 1976.
HABITAT: In coastal plankton.
DIAGNOSIS: Cellulae solitariae, 3.2–7.5 µm in diametro. Valvae ferentes fultoportulas 2–6 centrales et unam rimoportulam centralem. Areolae radialiter dispositae. Valvocopula serie una pororum majorum et seriebus 2–3 parallelis pororum minorum ornata.
Cells solitary, 3.2–7.5 µm in diameter. Valves with 2–6 fultoportulae and one rimoportula, all centrally located. Areolae are radially arranged. Valvocopula is ornamented by one ring of larger pores and 2–3 parallel rows of smaller pores.
DIFFERENTIAL DIAGNOSIS: This diatom differs from M. trioculatus (Taylor) Hasle by having pseudolocular and radial, rather than locular and tangential–linear, organization of valve areolae and a highly variable number of fultoportulae. Genetically, it differs from M. trioculatus by 2 bp in the SSU gene and 4–6 bp in ITS2 sequences.
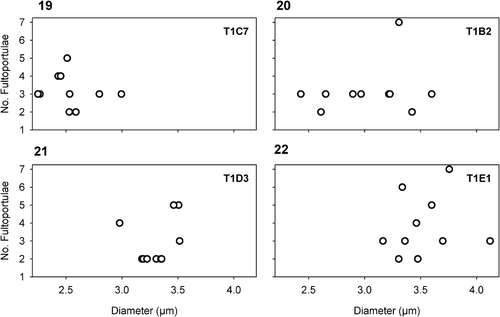
DETAILED DESCRIPTION: Morphometrics of the ‘whole strain’ (CCMP 495 culture) and the monoclonal sub-clones are included in the description below. Cells are solitary and drum-shaped, with a pervalvar axis 1–1.5 × the cell diameter. Cells contain one parietal, crescent-shaped chloroplast oriented toward the valve face. The valve face is fairly flat, 3.2–5.5 µm in diameter, covered with a net of pseudoloculate, radially-organized areolae, 5.4–7.2 areolae in 1 µm, encircled by a wide flange. The basal siliceous layer is perforated by radial rows of clusters of minute pores; 12–26 pores in 1 µm. Peripheral areolae are more or less even-sided, polygonal, but become more elongated (rectangular) towards the valve face centre. Fultoportulae are dispersed throughout the valve face, most commonly two to five, but in rare cases one, six or seven were also observed. The fultoportulae are subtended by two to three satellite pores. The number of fultoportulae varies greatly, even in newly isolated monoclonal sub-clones () and does not correlate with valve diameter. Normally only one sub-central rimoportula is present, but in one individual in cultured material two such portulae were found. In addition, a small rimoportula is generally centrally located with respect to the fultoportulae. Two open copulae per valve are frequently observed. The valvocopulae carry one row of larger pores, flanked by parallel rows of 3–4 minute pores.
Figs 19–22. Scatter plots of the diameter and the number of fultoportulae in four, representative, sub-clones. There is no relationship between valve diameter and number of fultoportulae in CCMP 495. T1C7, T1B2, T1D3 and T1E1 indicate the individual subclones.
COMPARISON WITH OTHER SPECIES: Compared with M. trioculatus, M. variabilis valve areola distribution is radial (not tangential–linear), and they are normally pseudolocular (not locular). Minidiscus variabilis is somewhat larger and has finer areolation compared to M. trioculatus (); the difference in the two characters is statistically significant (t-test, p < 0.05). Minidiscus variabilis also demonstrates a wider range in fultoportula number, even in a monoclonal culture.
Our specimens of M. variabilis are very similar to a valve illustrated as the larger of two valves of M. trioculatus by Takano (Citation1981 b, ). This valve was 4.8 µm in diameter, had radial areolae, three fultoportulae, one rimoportula and 5–6 rows of areolae in 1 µm, but differed from the other, smaller valve, which is of the same type as shown in (Takano, Citation1981 b) and we believe represents M. trioculatus proper (compare to Taylor, Citation1967).
In addition to morphology, the eco-physiology of the two species, M. trioculatus and M. variabilis, is different. Their valve formation and degree of silicification suggest different growth patterns and requirements. Differences in the degree of silicification between valves of these two species when grown under the same growth conditions suggest higher silica requirements for M. trioculatus. In addition, post-mitotic valve morphogenesis allows for greater variability in the number and location of fultoportulae in M. variabilis (suggesting non-interactive mitotic division, sensu Mann, Citation1994), while specimens of M. trioculatus form their fultoportulae with great fidelity, at least in culture. Three new Minidiscus species described by Cheng et al. (Citation1993) are all morphologically distinct from M. variabilis. Minidiscus ocellatus Gao has no fultoportulae and, assuming it is not aberrant or a case of heterovalvy, does not belong in the Thalassiosirales. Minidiscus spinulosus Gao has spinulae on the valve surface and lacks the marginal flange, while M. subtilis Gao is much more similar to M. chilensis than to any other Minidiscus. Valves of M. variabilis with partially developed areolation () resemble the elegant marginal ring of areolae in the recently described species M. decoratus (Quiroga & Chrétiennot-Dinet, Citation2004), but the marginal ring of areolae (if present) was a stage in valve morphogenesis in our material.
Minidiscus variabilis f. inornata, Kaczmarska forma nova ,
Figs 23–26. Minidiscus variabilis f. inornata. . A typically non-ornamented external view of a completely silicified valve showing a faint radial pattern with fultoportulae (arrowhead) and rimoportula (long arrow). Note that the fully formed external fultoportula tubes show that valve morphogenesis is complete. . Poroid areolae on copulae (short arrows). . External view of a valve with marginal ridges (short arrow) and a small external rimoportula opening (long arrow). . Internal view of a valve showing radiating rows of minute pores and the internal portula openings. Scale bars: 1 µm.
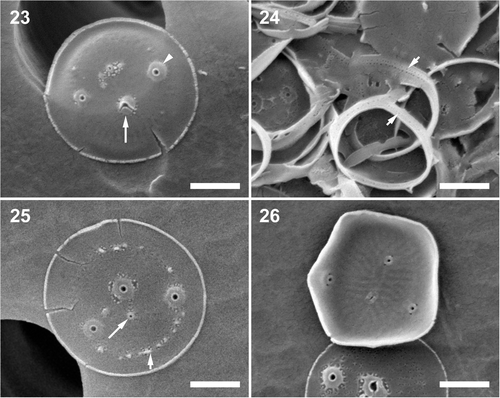
HOLOTYPE: SEM preparation #90-8 () ex culture, deposited at the Botanic Garden and Botanical Museum Berlin-Dahlem, Germany B40 0040657. The SSU and ITS-region sequences of the nuclear encoded rDNA of the type has been deposited in GenBank and the molecular barcode is available at BOLD (accession numbers are in ).
LOCUS TYPICUS: Isolated into culture from intertidal seawater collected on 17 January 2006, at Cape Tormentine, New Brunswick from the shore of the Gulf of St Lawrence, Canada.
HABITAT: In coastal plankton of the Gulf of St Lawrence.
DIAGNOSIS: Cellulae solitariae 2.0–3.7 µm in diametro. Valvae ferentes fultoportulas 2–4 centrales et unam rimoportulam centralem. Areolae loculatae nullae vel infirmiter evolutae; in loco earum series radialis pororum stratum silicae basalem perforantium interdum visa. Valvae, etiam infirmiter ornatae, valde silicificatae.
Cells solitary, 2.0–3.7 µm in diameter. Valves with two to four fultoportulae and one rimoportula, all centrally located. Loculate areolae are absent or poorly developed; instead a radial row of pores perforating the basal siliceous layer may be visible in mature valves. Regardless of the degree of development of areolation, valves are nonetheless strongly silicified (). Valvocopula is ornamented by parallel rows of smaller pores.
DIFFERENTIAL DIAGNOSIS: The form differs from the nominate variety by the near absence of areolae.
DETAILED DESCRIPTION. Cells are predominantly solitary when growing actively in culture, or aggregated into clumps in stationary phase. Pervalvar axis is 1–2 × longer than the diameter. Chloroplasts as described for M. variabilis above. Valves are circular, relatively flat, 2.0–3.7 µm in diameter with one sub-central rimoportula and two to four fultoportulae dispersed over the valve face with no obvious relationship to the rimoportula. In the majority of our specimens, the valve face showed no areolar ornamentation (). Faint radial rows of pores correspond to the internal pattern; 17–20 pores in 1 µm (), but absent on the marginal flange. A few valves showed faint areolation, covering the valve face, 5.7–7.5 faint areolae (when present) in 1 µm (, bottom valve). Copula ornamentation and interaction with the valve as in the nominate variety (). Despite its poorly expressed valve areolation, this form showed a stronger EDS silicon peak to background ratio than the nominate variety, suggesting that the basal siliceous layer is well developed and silicified, even though areolation is not evident.
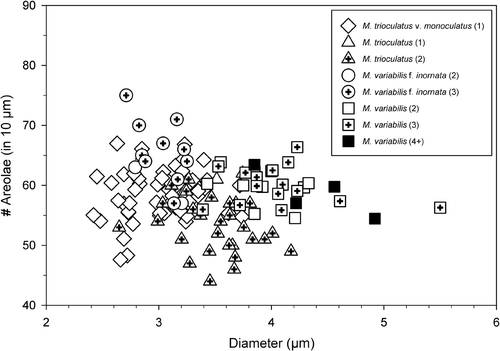
COMPARISON WITH OTHER SPECIES: This form differs from M. variabilis var. variabilis by the near absence of areolation on the valve, even when grown under the same conditions, and by stronger silicification of the basal siliceous layer than the nominate variety. It differs from M. decoratus Quiroga & Chrétiennot-Dinot by having non-hexagonal areolae (if areolae are present at all) and by the absence of an elegant ring of marginal areolation. Morphometric characteristics of the four taxa from cultures are illustrated in and summarized in .
Fig. 27. A plot illustrating the relationship between valve diameter, number of areolae and fultoportula. Due to the frequent lack of perforations in M. variabilis f. inornata, it was only possible to quantify all characters in a small number of cells. Numbers in parentheses correspond to the number of fultoportulae per valve.
Sequence comparison
We were able to assemble 2410-bp fragments of rDNA from our clones and CCMP strains 495 and 496. The assembled fragment contained almost the entire 18S rRNA and 5.8S genes, and the entire ITS1 and ITS2 regions. Repeated extraction and sequencing of PCR products with different primers produced 13 sequences, which were nearly identical, differing at various positions, with one site as an ambiguity and another as an indel (one case). There was little interspecific divergence in the 18S RNA gene, but M. trioculatus and M. variabilis differed at two variable sites within this gene. There was no difference between species in the 5.8 rRNA gene sequences. The ITS1 and ITS2 regions of the two species and varieties differed by 4–6 bp, depending on clone or variety (). In the ITS1 region the sole nucleotide substitution (T to C transition) occurred in M. variabilis f. inornata compared to all other taxa.
Table 3. Comparison matrix of nucleotide differences within the ITS region of various Minidiscus species a .
The ITS2 region of the four Minidiscus species were all 275 bp long, which is similar to other thalassiosiroids, but shorter than other eukaryotes whose average is 325 bp (Coleman, Citation2007). Minidiscus trioculatus sensu lato differed consistently from M. variabilis (s.l.) by 4 bp substitutions; one purine–purine substitution at position 196, beginning after the ITS2 conserved motif -ACCCC at the 5′ end (modelled from Stephanodiscus neoastraea Håkansson & Hickel; Coleman, Citation2007) of the Minidiscus sequences, and three pyrimidine-pyrimidine substitutions at positions 216, 233, and 240. The calculated distance between the two species was 0.0143–0.0146. Minidiscus variabilis and its form differed by just one pyrimidine–pyrimidine substitution at ITS2 position 237. Intraspecific calculated distances varied between 0–0.0014. Minidiscus trioculatus var. monoculatus also differed from the nominate variety, but only by an unresolved sequence trace showing adenine and thymine at position 219, whereas the trace from M. trioculatus var. trioculatus indicated adenine only.
Discussion
Taxonomy
The extremely small cell size of Minidiscus species makes it difficult to investigate their morphology with the same ease as larger phytoplankton; they can be investigated with the same rigour, but electron microscopy is obligatory for identification, as noted in earlier taxonomic (Cheng et al., Citation1993; Aké-Castillo et al., Citation2001; Quiroga & Chrétiennot-Dinet, Citation2004) and ecological studies (Aizawa et al., Citation2005). Undiscovered diversity among these nearly-picoplanktonic diatoms was anticipated (Quiroga & Chrétiennot-Dinet, Citation2004), but nonetheless it was surprising to find a new species hidden as M. trioculatus in an excellent culture collection, underscoring the importance of combined morphological and molecular analyses in microbial taxonomic investigations.
Minidiscus trioculatus and M. variabilis also differ genetically. By way of comparison, we found that intraspecific SSU sequence divergence for 18 morphological species, from three centric genera (Skeletonema Greville, Thalassiosira Cleve and Cyclotella Kützing) that are closely related to Minidiscus, varied between 0 and 0.8% and overlapped with the interspecific divergence (0.12–5.2%), based on 69 complete SSU sequences in GenBank. However, within another diatom group, the Sellaphora pupula-complex (some of which have now been elevated to species), the divergence between six syngens was smaller but also overlapping; 0.17% intrasyngenetically and 0.11–1.7% between sexually incompatible clones (Behnke et al., Citation2004). Thus, the SSU sequence divergence level recovered for the two species of Minidiscus (0.12%) is close to the lower end of the spectrum, but similar to other thalassiosiroids and to raphid pennate diatom species.
The ITS region normally provides a greater level of divergence when sequences are compared directly (uncorrected distances). Among morphologically defined species, sequence divergence varies greatly; from divergence that makes pair-wise alignments difficult, to as little as a 2 bp difference between Stephanodiscus yellowstonensis Theriot & Stoermer and S. niagarae Ehrenberg. Furthermore, some species of the Skeletonema costatum species complex (Sarno et al., Citation2005), as well as the morphologically distinct species Fragilariopsis nana (Steemann Nielsen) Paasche and F. cylindrus (Grunow ex Cleve) Frenguelli (Lundholm & Hasle, Citation2008), have fewer interspecific base pair differences than were recovered between two species of Minidiscus.
As in other microalgae (Coleman, Citation2007), Amato et al. (Citation2007) found that the ITS2 region was the sole genetic marker that differentiated closely related, sexually isolated species of the genus Pseudo-nitzschia Peragallo. The interspecific ITS sequence differences between two different syngens of the Pseudo-nitzschia calliantha-complex consisted of 4 bp, of which two were hemi-compensatory base changes and two were break pair bonds. Other syngens harbour a greater degree of divergence. For example, between some members of the S. pupula species complex (Behnke et al., Citation2004) divergence is different enough to make the inter-syngen alignment problematic. Compared with these examples, ITS2 sequence divergence was low between M. trioculatus and M. variabilis, but similar to closely related, semi-cryptic species of Pseudo-nitzschia (Amato et al., Citation2007), a pattern seen among other morphologically delineated species (see above).
Even without genetic data, the distinct morphology of M. variabilis f. inornata could be sufficient justification for raising it to species level. Quiroga & Chrétiennot-Dinet (Citation2004) described M. decoratus as a new species on the basis of similar morphological criteria. However, given the small genetic differences between the nominate and new forms, we have opted to be conservative.
Together with earlier work (Takano Citation1981 a, Citation b ; Rivera & Koch, Citation1984; Cheng et al., Citation1993; Quiroga & Chrétiennot-Dinet, Citation2004), our study suggests that the morphological and genetic diversity within Minidiscus has not yet been fully explored, and that the taxonomic affiliation of some of these extremely small diatoms continues to require critical re-evaluation and further sequencing of taxonomically informative genes in described species. Quiroga & Chrétiennot-Dinet (Citation2004) pointed out the particular problem of morphological similarities between M. spinulosus and Thalassiosira spinulata Takano (Takano, Citation1981a) and suggested a number of cases where species/specimen identity is in need of re-evaluation. The extent of this problem is illustrated by these two studies; each places the same morphotype in a different genus, emphasising the need for combined morphological and molecular taxonomic investigations.
Ecology
The ecological importance of specific pico- and nanoplanktonic diatoms is not well understood, even though some of these species occur at high densities. For example, Komuro et al. (Citation2005) and Buck et al. (Citation2008) reported M. trioculatus and M. chilensis at densities of up to 107 cells l−1, likely making them major contributors to the local seasonal diatom maxima. Interestingly, several recent publications clearly indicate that zooplankton are able to select food items and can enhance their reproductive success by choosing, avoiding or selecting certain species (Irigoien et al., Citation2002; Wyckmans et al., Citation2007). Pico- and nanoplanktonic diatoms are found in zooplankton fecal pellets (Urban et al., Citation1993; Kang et al., Citation2003). Diatoms such as Minidiscus are also ingested by mixotrophic flagellates (Martin-Cereceda et al., Citation2003), and are prey items of heterotrophic dinoflagellates (Sherr & Sherr, Citation2007). Parasites and viruses may be very specific in infecting host diatoms (Kühn, Citation1998), acting to ensure that competition does not drive rare species to extinction, thus maintaining diversity. Diversity among microbes and protists is thought to be important under specific environmental conditions (Duffy & Stachowicz, Citation2006) and may act as a buffer under changing climatic regimes.
Much has been learned about bacterial and archaeal picoplankton in the last two decades, including their identity, diversity and some hints of their function in the marine environment (Rippka et al., Citation2000). Until recently, eukaryotic picoplankton received much less scientific attention (López-García et al., Citation2001 a, Citation b ; Biegala et al., Citation2003). A multitude of flagellates, including prasinophytes, dinoflagellates and non-cultured groups dominate eukaryotic picoplankton communities when characterized molecularly (Moon van der Staay et al., 2001; Vaulot, Citation2008). Diatoms, though globally important contributors to the primary productivity of micro- and nano-plankton, are scarce in reports on picoplankton communities, even though a number of diatom species fall within the picoplankton size range in at least one dimension (Hargraves et al., Citation1989; Kaczmarska, unpublished data) and could pass through the 2 or 3 µm filters normally used to separate picoplankton from larger plankton. Non-culture-based environmental surveys are subject to PCR bias, such as competition for primers, and are not readily amplified in some mixed communities (Potvin & Lovejoy, Citation2008). However, diatom sequences have been reported from offshore Arctic waters (Lovejoy et al., Citation2006) and several of these diatoms had closest matches to known Arctic diatoms such as Fragilariopsis cylindrus (Grunow ex Cleve) Frenguelli. However, not all sequences were matched to known species, highlighting the need for reference sequences (barcodes) of known, correctly and consistently identified species to refine our understanding of microbial plankton community functioning.
Although environmental molecular approaches yield valuable information on taxon distributions at defined nucleotide differences, such survey results are not easily related to classical studies and interpretation of species diversity and ecology is limited. A mix of classical taxonomic and molecular approaches, involving wide-range sampling and use of barcodes, is needed to assess changing conditions in the marine biosphere. We found that combining culturing, electron microscopy and sequencing approaches provided the basis for linking old and new taxonomic reports. SEM records of Minidiscus provided a solid historical basis, and were reliable indicators of interspecific genetic differences. In the light of a growing number of reports demonstrating that even subtle variations in frustule morphology and sequence divergence may have taxonomic and/or ecological significance (Sarno et al., 2006; Amato et al., Citation2007; Evans et al., Citation2008), we attempted to document both. We believe that it is easier to combine taxa in the light of new evidence than to recover taxonomic, biogeographic and ecological signals if taxa are not adequately discriminated before the significance of morphological and genetic differences is sufficiently understood.
Acknowledgements
We thank J.M. Ehrman for assistance with SEM and various stages of sample and data collection, the crew of the Pandalus III, M. LeGresley and J.L. Martin for sample collection, M.B.J. Moniz for unpublished sequences from the ITS region, and the Center for Culture of Marine Phytoplankton for two strains (CCMP 495 and CCMP 496). We thank two anonymous reviewers for constructive comments. We acknowledge funding from Mount Allison University (IK) and support, in part, through funding from the Canadian Barcode of Life Network from Genome Canada through the Ontario Genomics Institute, NSERC, and other sponsors listed at www.BOLNET.ca (IK).
References
- Aizawa , C , Tanimoto , M and Jordan , RW . 2005 . Living diatom assemblages from North Pacific and Bering Sea surface waters during summer 1999 . Deep-Sea Res. (2 Top. Stud. Oceanogr.) , 52 : 2186 – 2205 .
- Aké-Castillo , JA , Hernández-Becerril , ME , Del Castillo , MEM and Bravo-Sierra , E . 2001 . Species of Minidiscus (Bacillariophyceae) in Mexican Pacific Ocean . Cryptogam. Algol. , 22 : 101 – 107 .
- Amato , A , Kooistra , WHCF , Ghiron , JHL , Mann , DG , Pröschold , T and Montresor , M . 2007 . Reproductive isolation among sympatric cryptic species in marine diatoms . Protist , 158 : 193 – 207 .
- Andersen , RA . 2005 . Algal Culturing Techniques , Amsterdam, , The Netherlands : Elsevier .
- Behnke , A , Friedl , T , Chepurnov , VA and Mann , DG . 2004 . Reproductive compatibility and rDNA sequence analysis in the Sellaphora pupula species complex (Bacillariophyta) . J. Phycol. , 40 : 193 – 208 .
- Bérard-Therriault , L , Cardinal , A and Poulin , M . 1986 . Les Diatomées (Bacillariophyceae) benthiques de substrats durs des eaux marines et saumâtres du Québec . Nat. Can. , 114 : 81 – 103 .
- Bérard-Therriault , L , Poulin , M and Bossé , L . 1999 . Guide d’identification du phytoplancton marin de l’estuaire et du golfe du Saint-Laurent incluant également certains protozoaires . Publ. Spec. Can. Sci. Halieut. Aquat. , 128 : 1 – 387 .
- Biegala , IC , Not , F , Vaulot , D and Simon , N . 2003 . Quantitative assessment of picoeukaryotes in the natural environment by using taxon-specific oligonucleotide probes in association with pyrimidine signal amplification-fluorescence in situ hybridization and flow cytometry . Appl. Environ. Microbiol. , 69 : 5519 – 5529 .
- Buck , KR , Chavez , FP and Davis , AS . 2008 . Minidiscus trioculatus, a small diatom with a large presence in the upwelling system of central California . Nova Hedwig. Beih. , 133 : 1 – 6 .
- Cheng , Z , Gao , Y and Liu , S . 1993 . Nanodiatoms from Fujian Coast , Beijing, , China : China Ocean Press .
- Coleman , AW . 2007 . Pan–eukaryote ITS2 homologies revealed by RNA secondary structure . Nucl. Acids Res. , 35 : 3322 – 3329 .
- Duffy , JE and Stachowicz , JJ . 2006 . Why biodiversity is important to oceanography; potential roles of genetic, species and trophic diversity in pelagic ecosystem processes . Mar. Ecol. Prog. Ser. , 311 : 179 – 189 .
- Elwood , HJ , Olsen , GJ and Sogin , ML . 1985 . The small subunit ribosomal RNA gene sequences from the hypotrichous ciliate Oxytricha nova and Stylonychia pustulata . Mol. Biol. Evol. , 2 : 399 – 410 .
- Evans , KM , Wortley , AH , Simpson , GE , Chepurnov , VA and Mann , DG . 2008 . A molecular systematic approach to explore diversity within the Sellophora pupula species complex (Bacillariophyta) . J. Phycol. , 44 : 215 – 231 .
- Feliner , GN and Rosseló , JA . 2007 . Better the devil you know? Guidelines for insightful utilization of nrDNA ITS in species-level evolutionary studies in plants . Mol. Phylogenet. Evol. , 44 : 911 – 919 .
- Fukuyo , Y , Takano , H , Chihara , M and Matsuoka , K . 1990 . Red Tide Organisms in Japan–An Illustrated Guide , Tokyo, , Japan : Uchida Rokakuho .
- Guillard , RRL . 1975 . “ Culture of phytoplankton for feeding marine invertebrates ” . In Culture of Marine Invertebrate Animals , Edited by: Smith , WL and Chanley , MH . 26 – 60 . New York (NY), , USA : Plenum Press .
- Hallegraeff , GM . 1984 . Species of the diatom genus Thalassiosira in Australian waters . Bot. Mar. , 27 : 495 – 513 .
- Hargraves , PE , Vaillancourt , RD and Jolly , GA . 1989 . “ Autotrophic picoplankton in Narragansett Bay and their interaction with microplankton ” . In Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Tides and Other Unusual Blooms , Edited by: Casper , EM , Carpenter , EJ and Bricelj , M . 23 – 38 . New York (NY), , USA : Springer Verlag .
- Hasle , GR . 1973a . Thalassiosiraceae, a new diatom family . Nor. J. Bot. , 20 : 67 – 69 .
- Hasle , GR . 1973b . Some marine plankton genera of the diatom family Thalassiosiraceae . Nova Hedwig. Beih. , 45 : 1 – 49 .
- Hasle , GR . 1978 . “ Using the inverted microscope ” . In Phytoplankton Manual , Edited by: Sournia , A . 191 – 196 . Paris, , France : UNESCO .
- Hendriks , L , Goris , A , Neefs , JM , Vandepeer , Y , Hennebert , G and Dewatcher , R . 1989 . The nucleotide sequence of the small ribosomal-subunit RNA of the yeast Candida albicans and the evolutionary position of the fungi among the eukaryotes . Syst. Appl. Microbiol. , 12 : 223 – 229 .
- Irigoien , X , Harris , RP , Verheye , HM , Joly , P , Runge , J , Starr , M , Pond , D , Campbell , R , Shreeve , R , Ward , P , Smith , AN , Dam , HG , Peterson , W , Tirelli , V , Koski , M , Smith , T , Harbour , D and Davidson , R . 2002 . Copepod hatching success in marine ecosystems with high diatom concentrations . Nature (Lond.) , 419 : 387 – 389 .
- Kaczmarska , I , Legresley , MM , Martin , JL and Ehrman , JM . 2005 . Diversity of the diatom Pseudo-nitzschia Peragallo in the Quoddy Region of the Bay of Fundy, Canada . Harmful Algae , 4 : 1 – 19 .
- Kaczmarska , I , Reid , C , Martin , JL and Moniz , M . 2008 . Morphological, biological and molecular characteristics of Pseudo-nitzschia delicatissima from the Canadian Maritimes . Can. J. Bot. , 86 : 763 – 772 .
- Kang , J-S , Kang , S-H , Kim , D and Kim , D-Y . 2003 . Planktonic centric diatom Minidiscus chilensis dominated sediment trap material in eastern Bransfield Strait, Antarctica . Mar. Ecol. Prog. Ser. , 255 : 93 – 99 .
- Komuro , C , Narita , H , Imani , K , Nojiri , Y and Jordan , RW . 2005 . Microplankton assemblages at Station KNOT in the subarctic western Pacific, 1999–2000 . Deep-Sea Res. (2 Top. Stud. Oceanogr.) , 52 : 2206 – 2217 .
- Kühn , SF . 1998 . Infection of Coscinodiscus spp. by the parasitoid nanoflagellate Pirsonia diadema: II. Selective infection behaviour for host species and individual host cells . J. Plankton Res. , 20 : 443 – 454 .
- Lange , KB . 1985 . Spatial and seasonal variations of diatom assemblages off the Argentinean coast (South Western Atlantic) . Oceanol. Acta , 8 : 361 – 369 .
- López-García , P , Rodrígues-Valera , F , Pedrós-Alió , C and Moreira , D . 2001a . Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton . Nature (Lond.) , 409 : 603 – 607 .
- López-García , P , López-López , A , Moreira , D and Rodríguez-Valera , F . 2001b . Diversity of free-living prokaryotes from a deep-sea site at the Antarctic Polar Front . FEMS Microbiol. Ecol. , 36 : 193 – 202 .
- Lovejoy , C , Massana , R and Pedrós-Alió , C . 2006 . Diversity and distribution of marine microbial eukaryotes in the Arctic Ocean and adjacent seas . Appl. Environ. Microbiol. , 72 : 3085 – 3095 .
- Lundholm , N and Hasle , GR . 2008 . Are Fragilariopsis cylindrus and Fragilariopsis nana bipolar diatoms?–Morphological and molecular analyses of two sympatric species . Nova Hedwig. Beih. , 133 : 231 – 250 .
- Mann , DG . 1994 . “ The origins of shape and form in diatoms: the interplay between morphogenetic studies and systematics ” . In Shape and Form in Plants and Fungi , Edited by: Fugraru , DS and Hudson , AJ . 17 – 38 . London, , UK : Academic Press .
- Martin-Cereceda , M , Novarino , G and Young , JR . 2003 . Grazing Prymnesium parvum on small planktonic diatoms . Aquat. Microb. Ecol. , 33 : 191 – 199 .
- McCallum , FS and Maden , BE . 1985 . Human 18S ribosomal-RNA sequence inferred from DNA sequence variations in 18S sequences and secondary modification patterns between vertebrates . Biochem. J. , 232 : 725 – 733 .
- Moon van der Staay , SY , De Wachter , R and Vaulot , D . 2001 . Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity . Nature (Lond.) , 409 : 607 – 610 .
- Pouchou , JL and Pichoir , F . 1991 . “ Quantitative analysis of homogeneous or stratified microvolumes applying the model “PAP” . In Electron Probe Quantitation , Edited by: Heinrich , KFJ and Newbury , DE . 31 – 75 . New York (NY), , USA : Plenum Press .
- Potvin , M and Lovejoy , C . 2009 . PCR-based diversity estimates of artificial and environmental 18S rRNA gene libraries . J. Eukaryot. Microbiol. , 56 : 174 – 181 .
- Quiroga , I and Chrétiennot-Dinet , MJ . 2004 . A new species of Minidiscus (Diatomophyceae, Thalassiosiraceae) from the eastern English Channel, France . Bot. Mar. , 47 : 341 – 348 .
- Rippka , R , Coursin , T , Hess , W , Lichtle , C , Scanlan , DJ , Palinska , KA , Iteman , I , Partensky , F , Houmard , J and Herdman , M . 2000 . Prochlorococcus marinus Chisholm et al. 1992 subsp. pastoris subsp. nov. strain PCC 9511, the first axenic chlorophyll a2/b2-containing cyanobacterium (Oxyphotobacteria) . Int. J. Syst. Evol. Microbiol. , 50 : 1833 – 1847 .
- Rivera , P and Koch , P . 1984 . “ Contribution to diatom flora of Chile II ” . In Proceedings of the 7th International Diatom Symposium , Edited by: Mann , DG . 279 – 298 . Koenigstein, , Germany : O. Koeltz .
- Sancetta , C . 1990 . Occurrence of Thalassiosiraceae (Bacillariophyceae) in two fjords of British Columbia . Nova Hedwig. Beih. , 100 : 199 – 215 .
- Sarno , D , Kooistra , WCHF , Medlin , LK , Percopo , I and Zingone , A . 2005 . Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species . J. Phycol. , 41 : 151 – 176 .
- Sherr , EB and Sherr , BF . 2007 . Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea . Mar. Ecol. Prog. Ser. , 352 : 187 – 197 .
- Sournia , A . 1978 . Phytoplankton Manual. Monographs on Oceanographic Methodology 6 , Paris, , France : UNESCO .
- Stathum , PJ . 1979 . Measurement and use of peak-to-background ratios in X-ray analysis . Microchim. Acta. (Suppl.) , 8 : 229 – 242 .
- Takano , H . 1981a . New and rare diatoms from Japanese marine waters. VI. Three new species in Thalassiosiraceae . Bull. Takai Reg. Fish. Res. Lab. , 106 : 31 – 43 .
- Takano , H . 1981b . New and rare diatoms from Japanese marine waters. VII. Ten species from neritic waters . Bull. Takai Reg. Fish. Res. Lab. , 105 : 45 – 57 .
- Takano , H . 1990 . “ Diatoms ” . In Red Tide Organisms in Japan–An Illustrated Taxonomic Guide , Edited by: Fukuyo , Y , Takano , H , Chihara , M and Matsouka , K . 162 – 331 . Tokyo, , Japan : Uchida Rokakuho .
- Taylor , JR . 1967 . Phytoplankton of the South Western Indian Ocean . Nova Hedwig. Beih. , 12 : 433 – 476 .
- Thornhill , DJ , Lajeunesse , TC and Santos , SR . 2007 . Measuring rDNA diversity in eukaryotic microbial systems: how intragenomic variation, pseudogenes, and PCR artifacts confound biodiversity estimates . Mol. Ecol. , 16 : 5326 – 5340 .
- Tomas , CR . 1997 . Identifying Marine Phytoplankton , San Diego : Academic Press .
- Urban , JL , Mckenzie , CH and Deibel , D . 1993 . Nanoplankton found in fecal pellets of macrozooplankton in Coastal Newfoundland waters . Bot. Mar. , 36 : 267 – 281 .
- Van der Auwera , G , Chapelle , S and De Wachter , R . 1994 . Structure of large ribosomal subunit RNA of Phytophthora megasperma and phylogeny of oomycetes . FEBS Lett. , 338 : 133 – 136 .
- White , TJ , Bruns , T , Lee , S and Taylor , J . 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols: A Guide to Methods and Application , Edited by: Innis , MA , Gelfand , DH , Sninsky , JJ and White , JT . 315 – 322 . San Diego (CA), , USA : Academic Press .
- Vaulot , D . 2008 . The diversity of small eukaryotic phytoplankton (<3 µm) in marine ecosystems . FEMS Microbiol. Rev. , 32 : 795 – 820 .
- Wyckmans , M , Chepurnov , VA , Vanreusel , A and De Troch , M . 2007 . Effects of food diversity on diatom selection by harpacticoid copepods . J. Exp. Mar. Biol. Ecol. , 345 : 119 – 128 .