Abstract
The partitioning of organic matter (OM) between dissolved and particulate phases is an important factor in determining the fate of organic carbon in the ocean. Dissolved organic matter (DOM) release by phytoplankton is a ubiquitous process, resulting in 2–50% of the carbon fixed by photosynthesis leaving the cell. This loss can be divided into two components: passive leakage by diffusion across the cell membrane and the active exudation of DOM into the surrounding environment. At present there is no method to distinguish whether DOM is released via leakage or exudation. Most explanations for exudation remain hypothetical; as while DOM release has been measured extensively, there has been relatively little work to determine why DOM is released. Further research is needed to determine the composition of the DOM released by phytoplankton and to link composition to phytoplankton physiological status and environmental conditions. For example, the causes and physiology of phytoplankton cell death are poorly understood, though cell death increases membrane permeability and presumably DOM release. Recent work has shown that phytoplankton interactions with bacteria are important in determining both the amount and composition of the DOM released. In response to increasing CO2 in the atmosphere, climate change is creating increasingly stressful conditions for phytoplankton in the surface ocean, including relatively warm water, low pH, low nutrient supply and high light. As ocean physics and chemistry change, it is hypothesized that a greater proportion of primary production will be released directly by phytoplankton into the water as DOM. Changes in the partitioning of primary production between the dissolved and particulate phases will have bottom-up effects on ecosystem structure and function. There is a need for research to determine how these changes affect the fate of organic matter in the ocean, particularly the efficiency of the biological carbon pump.
Introduction
The ocean covers 71% of the Earth’s surface, with photosynthesis in sunlit surface waters directly or indirectly supporting the majority of food webs in marine ecosystems. Based on satellite observations, net primary productivity of the global ocean is estimated to be 45–55 Pg C yr−1 (1 Pg = 1 Gt = 1015 g) (Longhurst et al., Citation1995; Field et al., Citation1998; Falkowski et al., Citation2000; Carr et al., Citation2006; Westberry et al., Citation2008). This estimate is 40–50% of the primary productivity of the total biosphere. There is an estimated 1–2 Pg C associated with living organisms in the ocean (Falkowski et al., Citation2000), of which between 0.25 and 0.65 Pg C are phytoplankton (Falkowski & Raven, Citation2007).The pool of non-living organic carbon in the ocean is estimated to be much greater than the living component at 1000 Pg C (Falkowski & Raven, Citation2007) and most of this is in the form of dissolved organic matter (DOM) rather than particulate organic matter (POM). Hansell et al. (Citation2009) estimated that the oceans contain 662 Pg C as dissolved organic carbon (DOC), which is within the range of the amount of carbon in CO2 in the atmosphere over recent history (612 Pg C in 1850 and 784 Pg C in 2000: Emerson & Hedges, Citation2008). The division between dissolved and particulate organic matter is operationally defined by the pore size used by researchers to separate the two fractions. Marine chemists generally use GF/F filters (Whatman, Maidstone, U.K.), as these glass fibre filters may be combusted to remove organic contamination (Wurl & Sin, Citation2009). The average pore size of a GF/F filter is 0.7 µm, which is significantly larger than many abundant particles in the ocean; viruses, for example, are generally 0.1 µm in diameter and found at concentrations of 109 to 1011 L−1 (Suttle, Citation2005). Despite the limitations of an operational definition, marine DOM is nonetheless a major reservoir of carbon, and understanding the factors affecting the production, loss and turnover of DOM are essential to understand the dynamics of the global carbon cycle. DOM is the major carbon source for heterotrophic prokaryotes in the water column. Some DOM binds trace elements in the ocean and this is particularly important in the case of iron, which limits phytoplankton growth in a significant proportion of the open ocean (Emerson & Hedges Citation2008). DOM also attenuates UV radiation in the surface ocean and serves as a catalyst for photochemical reactions (Emerson & Hedges, Citation2008). The partitioning of organic carbon between the dissolved and particulate pools has implications for the export of organic matter from the photic zone and its ultimate fate, as while particulate organic carbon (POC) sinks, DOC does not (Borchard & Engel, Citation2012).
A number of processes cause the contents of phytoplankton cells to be released into surrounding seawater as DOM. These processes include sloppy feeding by predators (Møller et al., Citation2003; Møller, Citation2007), lytic viral infections (Bratbak et al., Citation1993; Gobler et al., Citation1997, Bettarel et al., Citation2005), and cell death (Veldhuis et al., Citation2001). Moreover, living phytoplankton cells exude a significant proportion, and under some circumstances the majority, of their photosynthate into the surrounding medium (Fogg, Citation1983; Wood & Van Valen, Citation1990). There are major gaps in our understanding of the physiology and biogeochemical significance of DOM release by phytoplankton (Flynn et al., Citation2008).
The objective of this review is to synthesize what is currently known about DOM release by phytoplankton, building on previous reviews (Hellebust, Citation1974; Fogg, Citation1983; Williams, Citation1990; Myklestad, Citation2000; Nagata, Citation2000). I will focus on the physiological processes that affect DOM production by the phytoplankton themselves, rather than physical disruption processes (i.e. sloppy feeding and viral lysis). Within this framework I will emphasize the interaction between phytoplankton DOM production and global climate change. The increase in CO2 in the atmosphere as a result of human activity (IPCC, Citation2013) is causing the ocean to become both warmer (Levitus et al., Citation2000) and more acidic (Caldeira & Wickett, Citation2003; Royal Society, Citation2005) and there is an urgent need to understand how ecosystems and global biogeochemical cycles will respond. In this review I will bring together what little we know about the effects of ocean temperature and pH on DOM release by phytoplankton and suggest future research directions.
Production rates of DOM by phytoplankton
DOM release by phytoplankton has been measured in laboratory culture for specific organisms under highly controlled conditions, and in natural populations in the field, and it has been constrained in ecosystem models. Production rates of DOM by phytoplankton are usually expressed as Percentage Extracellular Release (PER). Total primary production (TPP) can be divided into particulate primary production (PPP) and dissolved primary production (DPP). PER is simply DPP/TPP × 100 (Marañón et al., Citation2005; Morán et al., Citation2006). While PER is a useful parameter to understand the physiology of phytoplankton, it is not as useful as the absolute measures of carbon flux for understanding the biogeochemistry of ecosystems. For example, in situations where PER is relatively constant, the amount of carbon exuded into the environment as DOM may be highly variable because primary productivity varies in response to environmental factors such as light availability. For this reason, some researchers have argued that the concept of PER should be abandoned (Smith & Wiebe, Citation1976; Mague et al., Citation1980).
DPP and PPP have been measured by essentially the same method over the last 50 years, which is an extension of Steemann-Nielsen’s (1952) revolutionary method for measuring primary productivity using 14C as a tracer. Samples of culture or natural waters are incubated in a bottle with a radioactive inorganic carbon source (NaH14CO3). During photosynthesis the radioactive inorganic carbon is fixed into organic carbon, which may be incorporated into the cell as PPP, exuded into the surrounding medium as DPP, or respired back to inorganic carbon. At the end of the timed incubation, PPP is separated from DPP by filtration. Unassimilated radioactive inorganic carbon is driven off the samples by acidification and the amount of radioactive carbon incorporated into the DPP and PPP is counted and used to calculate PER (Hellebust, Citation1965; Morán et al., Citation2006). Sources of error in this technique include the transformation of PPP to DPP through cell lysis during filtration (Sharp, Citation1977) and the binding of DOM to the filters (Karl et al., Citation1998; Morán et al., Citation1999).
High temperature catalytic oxidation (HTCO) instruments for the measurement of dissolved organic carbon (DOC) were developed in the late 1980s (Sugimura & Suzuki, Citation1988). The interpretation of the data produced from this method remained controversial for a number of years as it was often unclear what was being measured, mainly due to issues associated with preparing DOM-free water for blanks and the lack of standardized reference water samples for inter-laboratory comparisons (Hedges et al., Citation1993; Benner & Strom, Citation1993; Suzuki, Citation1993). However, these issues were resolved and the HTCO technique enabled DOM release by phytoplankton to be determined by simply measuring how much of the total organic carbon was allocated to the dissolved and particulate fractions over time, either in phytoplankton cultures (Chen & Wangersky, Citation1996; Biddanda & Benner, Citation1997) or in incubations of natural waters (Wetz & Wheeler, Citation2003). An advantage of this technique is that it avoids the safety issues and permissions involved with handling radioisotopes. Dissolved and particulate organic carbon are usually separated by filtration and are therefore prone to the same filtration artefacts as 14C tracer methods.
PER in phytoplankton cultures
Hellebust (Citation1965) made the first extensive measurements of PER by 22 species in laboratory grown cultures and concluded that phytoplankton release 3–6% of their photoassimilated carbon during exponential growth. Nagata (Citation2000) summarized PER measurements from laboratory experiments with 37 species, including the work of Hellebust (Citation1965). Nagata concluded that mean PER in cultures is 5% (range 2–10%), with higher rates in stressed cultures (nutrient limitation or suboptimal conditions of light and temperature) or when cells were in the stationary and senescent stages of growth. Wetz & Wheeler (Citation2007) also found PER rates of approximately 5% in batch cultures of four coastal diatom species: Cylindrotheca closterium, Odontella longicruris, Bellerochea sp., and Skeletonema sp. However, PER for Chaetoceros decipiens was higher, at 21%. López-Sandoval et al. (Citation2013) measured DOC release using 14C as a tracer in two-hour incubations using samples from non-axenic batch cultures. Their comprehensive survey of 22 species ranged in size from the small cyanobacterium Prochlorococcus sp. (0.12 µm3 cell volume) to the large diatom Coscinodiscus wailesii (2 500 000 µm3). Mean PER was approximately 2% with a range of 0.3–10%. Unlike other studies, they found that both cell size and growth phase had no effect on DOC release. The chemical composition of extracellular DOM was shown to be different from intracellular composition in the diatom Skeletonema costatum (Granum et al., Citation2002; Puskaric & Mortain-Bertrand, Citation2003), confirming earlier work by Mague et al. (Citation1980). These observations suggest that DOM release is not simply the passive leakage of intracellular metabolites into the surrounding medium.
Many phytoplankton are mixotrophic, meaning that they are able to supplement the organic carbon fixed via photosynthesis with the heterotrophic consumption and assimilation of organic matter (reviewed by Caron, Citation2000). Organic matter may be obtained by phagotrophy or through the uptake and assimilation of DOM. For example, some diatoms are able to grow in the dark on simple organic substrates such as glucose and acetate (Lewin & Lewin, Citation1960; White, Citation1974) and the cyanobacterium Prochlorococcus takes up amino acids in situ (Zubkov et al., Citation2004). The uptake of DOM by phytoplankton has not been accounted for during laboratory culture experiments to determine PER. Measured PER may represent ‘net PER’, being the sum of ‘gross PER’ and the subsequent re-uptake of a proportion of the PER by the phytoplankton (Kamjunke & Tittel, Citation2009). This may seem a very inefficient way to retain resources; however, it may cost the organism less to take up a proportion of the DOM that has leaked from the cell rather than to prevent the leakage in the first place. While differentiating between gross and net PER may be important for understanding the physiology of phytoplankton, it could be argued that only net PER matters ecologically as it is only net PER that is available to other organisms in the surrounding water.
PER in mesocosm experiments and natural waters
Baines & Pace (Citation1991) determined PER based on data from both marine and freshwater systems in 16 papers. The range of measured PER was < 1 to 75%, with the means for individual systems ranging from 3 to 40%. They concluded that PER averages 13% and extracellular release increases linearly with particulate primary productivity. Nagata (Citation2000) also reviewed the literature on DOM exudation in the field and concluded that there is a great variation in rates, with mean PER usually in the range 10–20%. These conclusions broadly agree with Baines & Pace (Citation1991). While there has been limited work on phytoplankton in monocultures since Nagata (Citation2000), researchers have continued to measure PER in situ or in large-scale manipulative experiments with natural communities in mesocosms (). Most of the research to date has focused on coastal waters; the lack of data from oceanic biomes means that it is difficult to make global generalizations concerning PER from these analyses. There has been a geographical bias towards certain oceans that largely reflects the locations of the scientists studying DOM production by phytoplankton. Thus, there is a significant body of work on the Atlantic Ocean and Mediterranean Sea, while relatively little work has been done on the Arctic, Pacific and Indian Oceans.
Table 1. Field measurements of DOM release by phytoplankton, expressed as percentage extracellular release (PER). Measurements of PER are means unless stated otherwise under notes. Data are taken from papers published since 2000 and grouped according to which ocean the measurements were made in.
PER rates in the field are consistently higher than rates measured with laboratory monocultures of phytoplankton. This may reflect physiological constraints. For example, the early stages of batch cultures are likely to be nutrient replete, whereas natural waters are often nutrient limited. However, this is not the only factor, as PER is also generally higher in situ at upwelling locations, where nutrients are available, compared with laboratory monocultures. Short-term incubations using water collected in situ include protozoan grazing and viral lysis, processes that are usually absent in phytoplankton cultures (Nagata, Citation2000; Teira et al., Citation2001a). In low biomass locations, such as the oligotrophic ocean, methodological constraints may lead to an overestimation of PER based on incubations using 14C, as the signal is relatively low compared with the noise, and this may be a concern in the interpretation of some of the early measurements (Williams, Citation1990). It is difficult to compare the in situ studies presented in due to the wide range of protocols used to obtain the data. For example, Wetz & Wheeler (Citation2003) conducted on-deck incubations of 20 litres, which were sampled each day for 7 days, whereas Teira et al. (Citation2001a) measured DOC release in 30-ml samples after an on-deck incubation time of 2 hours. These differences suggest that an inter-comparison of methods commonly used to measure PER would be a valuable exercise, potentially leading to a standard protocol and enabling direct comparisons between datasets collected by different groups at different times and locations.
In summary, PER is generally relatively low (10–20%) in nutrient-rich conditions, such as coastal upwelling, and frequently higher in warm, nutrient-poor conditions such as the North Atlantic subtropical gyre during summer. The phytoplankton that grow under these different conditions are also very different; coastal upwelling zones are dominated by large taxa and subtropical gyres are dominated by small taxa with large surface area to volume ratios. This is consistent with Fogg (Citation1983), who concluded that phytoplankton exude 5% of the products of photosynthesis in eutrophic waters and up to 40% in oligotrophic waters.
PER in biogeochemical models
DOM release by phytoplankton is a potentially important component of biogeochemical models on a variety of spatial and temporal scales, from ecophysiological models of individual phytoplankton cells, to ecosystem models and models of global biogeochemical cycles. Empirical and mechanistic methods have been used to incorporate phytoplankton DOM exudation into models.
The simple empirical approach is to assume a fixed value for PER, based on averaging data from measurements in the field or laboratory. The value for PER is then multiplied by a measure of primary production to estimate the amount of DOM produced. This approach has been used in multiple studies, as tabulated by Christian & Anderson (Citation2002), with values of PER generally around 5%. Aumont et al. (Citation2003) incorporated fixed values of DOC release into their ecosystem model of the global ocean containing two functional phytoplankton groups. DOC release was fixed at 5 and 3% for nanophytoplankton and diatoms, respectively. Recently, PER in the upper Chesapeake Bay was assumed to be a constant 26% (Keller & Hood, Citation2011), a relatively high value that seems to be based on a misinterpretation of Anderson & Williams (Citation1998).
Using the mechanistic approach, DOC release by phytoplankton is incorporated into models as a dynamic term that changes in response to environmental conditions. PER has been modelled using cell quotas (Baklouti et al., Citation2006) or N : C ratios (Flynn et al., Citation2008) as a measure of nutrient status. Kriest & Oschlies (Citation2007) modelled the effect of cell size on DOC release based on Bjørnsen’s (1988) model, which assumes that DOC release by phytoplankton is a passive process driven by concentration gradients across the cell membrane. In this model, small cells leak more than large cells and therefore the size spectrum of the phytoplankton population was a driver of DOC release (Kriest & Oschlies, Citation2007). The advantage of the mechanistic approach is that it implies an understanding of the physiological factors that affect DOC exudation by phytoplankton and therefore it can be used predictively to model situations where PER measurements are lacking.
Anderson & Williams (Citation1998) combined the empirical and mechanistic approaches to estimate PER in their model of DOM cycling in the English Channel. They divided DOC lost from phytoplankton cells into two components. Firstly, a constant 5% of carbon fixed by the phytoplankton was lost by leakage from the cells. Leakage was defined as the passive loss of ‘small molecules’ across the cell membrane. Secondly, they distinguished ‘leakage’ from ‘exudation’, which was defined as the loss of excess carbon due to changes in environmental conditions such as light and nutrient availability. The exudation contribution to extracellular DOC was calculated using two different methods: it was either simply directly proportional to primary production or it was calculated as a photosynthetic overflow under conditions of nutrient limitation. Total PER was calculated accounting for both leakage and exudation. Mongin et al. (Citation2003) used a similar approach, assuming that 10% of the phytoplankton biomass was passively leaked from the cells each day, with DOC and DON released in Redfield stoichiometry, plus a further leakage of DOC and DON released in Redfield stoichiometry equivalent to 5% of the inorganic nitrogen uptake each day. In addition, when C : N > 10, 30% of the photosynthetically fixed carbon was exuded as DOC to simulate exudation under nitrogen limitation.
The success of DOM release models will depend on accurate predictions over a wide range of naturally occurring conditions, enabling them to be incorporated into larger models of global primary production and biogeochemical cycles. Currently, the models reflect our lack of understanding of the basic physiology of DOM release by phytoplankton and associated physiological processes, such as cell death (see below). Mechanistic models at least enable us to identify what probably drives DOM release by phytoplankton, even if the relative importance of the different processes is currently difficult to determine. Drivers of DOM release in mechanistic models include photosynthesis rates, cell quotas and elemental stoichiometry, concentration gradients across the plasma membrane, cell size, growth rates, and cell death (Baklouti et al., Citation2006; Kriest & Oschlies, Citation2007; Flynn et al., Citation2008). However, because the drivers of DOM release by phytoplankton are poorly characterized, it is possible to tune models to fit data without realizing that the resulting model is unrealistic. Flynn et al. (Citation2008) concluded that suitable data to model DOM release limit our mechanistic understanding of the process. The ideal data, which Flynn et al. (Citation2008) were unable to find in the literature, should demonstrate mass balance of carbon (or nitrogen). In the case of carbon, this would mean simultaneous measurements of dissolved inorganic, dissolved organic and particulate carbon fractions under constrained axenic conditions, to enable a budget of carbon to be constructed with no assumed pools (Flynn et al., Citation2008). There is a need for integrated research, specifically designed to combine modelling with experimental work on phytoplankton physiology.
Composition of DOM released by phytoplankton
Phytoplankton release a broad range of organic compounds. Lancelot (Citation1984) summarized the early literature on the composition of DOM released by phytoplankton, concluding that they were dominated by carbohydrates (monosaccharides, oligosaccharides and polysaccharides), nitrogenous compounds (amino acids, polypeptides and proteins), lipids (fatty acids) and organic acids (glycolate, tricarboxylic acids, hydroxamate and vitamins). As one might expect, this broadly matches the composition of phytoplankton cells. A typical phytoplankter is composed of 25–50% protein, 5–50% polysaccharide, 5–20% lipids, 3–20% pigments and 20% nucleic acids (Emerson & Hedges, Citation2008). Hellebust (Citation1974) grouped the extracellular products of algae (both phytoplankton and macroalgae) into the following categories: carbohydrates, nitrogenous substances, organic acids (mainly glycolate), phenolic substances, organic phosphates, volatile substances, enzymes, vitamins, sex factors, growth inhibitors and stimulators, and toxins. This list includes categories determined on the basis of their chemical composition (e.g. carbohydrates and nitrogenous substances), their chemical properties (volatile substances) and their biological function (e.g. enzymes and sex factors). Grouping extracellular products in this way is confusing, as there is overlap between categories; for example, enzymes are nitrogenous substances as well. I have grouped the extracellular products based on chemical composition, either in broad encompassing categories (e.g. carbohydrates) or by specific compounds (e.g. isoprene). The list is not exhaustive, but rather represents categories or compounds which are either extremely abundant or biogeochemically significant.
Amino acids and proteins
The extracellular release of amino acids, peptides and proteins by phytoplankton has not been extensively studied. Field (Bronk et al., Citation1994; Glibert & Bronk, Citation1994; Hu & Smith, Citation1998) and culture (Suratman et al., Citation2008) studies have shown that a range of phytoplankton taxa release a significant amount of dissolved organic nitrogen (DON). Many phytoplankton are also consumers of DON as a nitrogen source; it is often the largest pool of dissolved nitrogen in marine waters and it is less refractory than once thought (Bronk et al., Citation2007). Given that protein is a major component of phytoplankton, it is likely that amino acids and protein form a significant proportion of the DON released.
Work with cultures of diatoms has shown that Chaetoceros spp. (Poulet & Martin-Jézéquel, Citation1983; Myklestad et al., Citation1989) and Skeletonema costatum (Mague et al., Citation1980; Granum et al., Citation2002) release amino acids into the growth medium. Myklestad (Citation2000) concluded, based on the literature, that the following amino acids are commonly released by diatoms: serine, glycine, lysine, alanine, glutamic acid, aspartic acid, ornithine and histidine. Larger amino acid-containing molecules may be released by phytoplankton. For example, Thalassiosira weissflogii grown at several copper concentrations released the tripeptide glutathione (GSH) in response to copper-induced cell membrane damage (Tang et al., Citation2005). Emiliana huxleyi released thiols composed of the amino acids arginine, cysteine and glutamine in response to heavy metals (Dupont et al., Citation2004). Amino acids containing R-groups with aromatic carbon rings, and consequently delocalized electrons, are fluorescent. Common amino acids containing such R-groups are tryptophan, phenylalanine, and tyrosine and so these amino acids, and proteins that contain them, contribute to the fluorescent dissolved organic matter (FDOM) pool in the ocean (Coble, Citation1996). The concentration of ‘proteinaceous DOM’, determined using fluorescence, increased in mesocosms during phytoplankton exponential growth (Stedmon & Markager, Citation2005). Chaetoceros, Skeletonema, Prorocentrum and Micromonas all released ‘protein-like’ FDOM during culture experiments (Romera-Castillo et al., Citation2010). In addition, small protein-containing exopolymer particles are abundant in the ocean. These particles are known as Coomassie staining particles (CSP) after Coomassie Brilliant Blue G-250 dye, which is the protein stain used to detect them (Long & Azam, Citation1996). Data from my laboratory has shown that CSP are a ubiquitous component of the exopolymers produced in diatom cultures (, ) and the ocean ().
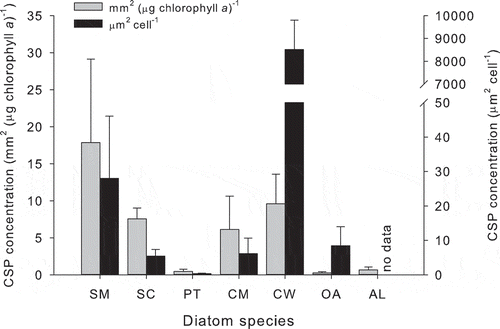
Fig. 1. Coomassie staining particle (CSP) concentration in diatom batch cultures at the end of the exponential growth phase after 7 days of growth in artificial seawater. The diatoms were Skeletonema marinoi (SM), Skeletonema costatum (SC), Phaeodactylum tricornutum (PT), Chaetoceros muelleri (CM), Coscinodiscus wailesii (CW), Odontella aurita (OA) and Achnanthes longipes (AL). Bars show mean + SD (n = 3 replicate cultures). Data collected by Michael Pohlen. CSP stained according to Long & Azam (Citation1996).
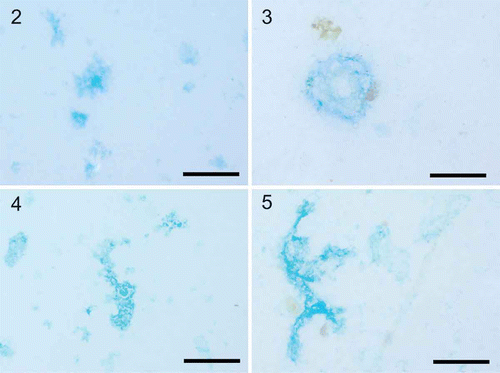
Figs 2–5. Exopolymer particles observed in diatom cultures and the Pacific Ocean. Exopolymer particles were collected using gentle filtration onto 0.4 µm polycarbonate filters and stained. 2. Coomassie brilliant blue staining particles (CSP) in a culture of Thalassiosira weissflogii. 3. CSP particles from the surface Pacific Ocean collected off the coast of Oregon during July 2011. 4. Transparent exopolymer particles (TEP) in a culture of Thalassiosira weissflogii. 5. TEP particles from the surface Pacific Ocean collected off the coast of Oregon during July 2011. TEP were stained with Alcian blue according to Alldredge et al. (Citation1993) and CSP were stained according to Long & Azam (Citation1996). Scale bars = 100 µm.
Carbohydrates
Carbohydrates are used in the structural components of the cell, such as cell walls, and as storage compounds for fixed carbon and energy. The composition and amount of carbohydrate that an individual phytoplankton cell contains are dependent on the physiological status of the cell and its taxonomic affiliation. Carbohydrates are a significant component of the DOM in the ocean (Aluwihare et al., Citation1997; Repeta & Aluwihare, Citation2006; Hansell et al., Citation2009).
Data indicate that different species produce different extracellular carbohydrates, in terms of monosaccharide composition and their relative proportions. It is probable that an individual phytoplankton strain or species is capable of producing multiple extracellular carbohydrates at the same time. This has been shown for benthic diatoms, whose extracellular carbohydrate production has been extensively studied over the last few years (Underwood & Paterson, Citation2003; Bellinger et al., Citation2005, 2009; Abdullahi et al., Citation2006). Monosaccharides commonly found in extracellular carbohydrates released by marine phytoplankton include aldohexoses (glucose, galactose and mannose), aldopentoses (arabinose and xylose), deoxysugars (fucose and rhamnose), uronic acids (glucuronic acid) and amino sugars (Aluwihare et al., Citation1997; Biersmith & Benner, Citation1998; Aluwihare & Repeta, Citation1999; Magaletti et al., Citation2004; Kragh & Søndergaard, Citation2009). The monosaccharide composition of the surface ocean is similar to the monosaccharide composition of phytoplankton extracellular release, indicating that phytoplankton are a major source of carbohydrates to the ocean (Aluwihare et al., Citation1997; Biersmith & Benner, Citation1998).
Over the last two decades there has been considerable interest in the role of large polymers in the ocean (see reviews by Passow, Citation2002a; Verdugo et al., Citation2004; Verdugo, Citation2012). There has been the realization that there is a continuum of organic matter from DOM to POM, with gels and exopolymer particles sharing some of the properties of both POM and DOM. Verdugo et al. (Citation2004) estimated that 10% of the DOM pool, or approximately 70 Pg C, is in the form of biopolymers that are assembled into gels such as transparent exopolymer particles (TEP). TEP are operationally defined as particles retained on 0.4 µm (or sometimes 0.2 µm) pore sized polycarbonate filters that stain with Alcian blue (, ) (Alldredge et al., Citation1993; Passow & Alldredge, Citation1995; Passow, Citation2002a). TEP are invisible using bright-field light microscopy, but become visible after staining, indicating that TEP contain acid polysaccharides (Ramus, Citation1977; Passow & Alldredge, Citation1995). TEP are ubiquitous and play an important role in particle dynamics and carbon cycling in the ocean (see reviews by Passow, Citation2002a; Burd & Jackson, Citation2009; Verdugo, Citation2012). For example, TEP are sticky and affect the further aggregation of particles into larger aggregates that sink rapidly though the water column, affecting the biological carbon pump (Engel, Citation2000; Passow, Citation2002a; Thornton, Citation2002). Exudation by phytoplankton plays a significant role in the formation of TEP (Passow, Citation2002b; Gärdes et al., Citation2011). Numerous studies have shown that exopolymer particles accumulate in phytoplankton cultures (Passow, Citation2002b; Claquin et al., Citation2008; Fukao et al., Citation2010) and during blooms (Passow et al., Citation1994; Engel et al., Citation2002; Engel, Citation2004; Harlay et al., Citation2009) (, ). Generally, TEP are not exuded directly by phytoplankton cells; they form abiotically from precursors released by cells. TEP accumulate in 0.2 µm filtrate from diatom cultures that is bubbled with air (Mopper et al., Citation1995; Mari, Citation1999) or exposed to fluid shear (Passow, Citation2000); therefore processes that bring the polymeric precursors together affect the formation of TEP.
Fatty acids and lipids
Myklestad (Citation2000) noted that there was little information on the exudation of fatty acids and lipids by phytoplankton, and there has been little further research on this issue over the last decade. Parrish (Citation1987) hypothesized that dissolved lipids he measured in Bedford Basin (Canada) were exuded by phytoplankton during the spring bloom. There has been some work on extracellular lipid production by marine diatoms grown in continuous culture (Parrish & Wangersky, Citation1987; Lombardi & Wangersky, Citation1991). Chaetoceros gracilis produced extracellular lipids under nutrient-replete conditions and also under conditions of phosphorus and silicon limitation (Lombardi & Wangersky, Citation1991). Similarly, Phaeodactylum tricornutum produced extracellular lipids under both nitrogen-limited and nitrogen-replete conditions (Parrish & Wangersky, Citation1987). Extracellular free fatty acids, triacylglycerols, sterols, phospholipids and glycolipids were measured in cultures of the dinoflagellate Gymnodinium cf. nagasakiense and were proposed to be exuded from the cells (Parrish et al., Citation1994).
Nucleic acids
There is little information on dissolved nucleic acids in the ocean and no specific information on the potential for phytoplankton to exude nucleic acids. The viral lysis of bacterioplankton is known to be a significant source of dissolved DNA (Brum, Citation2005; Riemann et al., Citation2009). Most of the nucleic acids in eukaryotic cells are large molecules contained within membrane-bound organelles, which will reduce the potential for leakage as each membrane will act as a barrier. Nucleic acids are rich in phosphorus and nitrogen and therefore the loss of significant amounts of nucleic acid would be costly to phytoplankton cells, which inhabit environments where nitrogen and phosphorus often limit growth. Based on these arguments, it seems unlikely that nucleic acids are a significant source of DOM produced by healthy phytoplankton cells. However, recent work has shown that extracellular DNA (eDNA) plays a significant structural role in the exopolymer matrix of many bacterial biofilms (reviewed by Flemming & Wingender, Citation2010). This raises the possibility that eDNA may be exuded by phytoplankton that produce large amounts of exopolymers, such as diatoms and the colonial microalga Phaeocystis. Dissolved DNA supplied 70% of bacterioplankton P demand in a P-limited estuary, indicating that eDNA may play a significant role in some ecosystems (Riemann et al., Citation2009).
Glycolate
Glycolate (C2H4O3) is produced during photorespiration (Falkowski & Raven, Citation2007). Much of the early work on DOM release by phytoplankton was focused on glycolate (also known as glycollate, glycolic acid or 2-hydroxyethanoic acid), mainly in freshwater model taxa such as Chlorella (Tolbert & Zill, Citation1956; Whittingham & Pritchard, Citation1963). Leboulanger et al. (Citation1998a) proposed that glycolate may represent 10% or more of the DOC in the ocean, though data to support this estimate were not presented. Concentrations varying between 0.20 and 0.80 µM were measured in coastal waters of the Irish Sea (Liverpool Bay) (Al-Hasan & Fogg, Citation1987). Leboulanger et al. measured glycolate concentrations up to 0.97 µM in mesotrophic waters and 0.22 µM in oligotrophic waters of the tropical Atlantic Ocean (Leboulanger et al., Citation1997), and 0.32–1.17 µM during a phytoplankton bloom in coastal Mediterranean waters (Leboulanger et al., Citation1994). Relatively high glycolate concentrations (0.89–4.50 µM) have been measured in coastal waters off Belgium (Billen et al., Citation1980). It is likely that there is rapid turnover of the glycolate pool in the ocean due to production associated with photosynthesis and consumption by heterotrophic prokaryotes. This is shown by the diurnal cycle in glycolate concentrations in the upper ocean, indicating net production during daylight and net consumption during the night (Leboulanger et al., Citation1997, 1998b).
Dimethylsulfoniopropionate (DMSP) and dimethysulfide (DMS)
The production of DMS (C2H6S) and its precursor compound DMSP (C5H10O2S) by phytoplankton has been studied extensively over the last 25 years. Early research focused on the production of DMS, as it is the major source of non-sea salt aerosol over areas of ocean remote from the continents (Stefels & van Boekel, Citation1993; Gondwe et al., Citation2003), it affects cloud formation and has a hypothetical role in climate regulation (Charlson et al., Citation1987; Ayers & Cainey, Citation2007; Quinn & Bates, Citation2011). However, recent research has focused on DMSP, which is often a significant component of the carbon quota of phytoplankton cells and may play a significant role in prokaryote production and the microbial loop (Kiene et al., Citation2000). Major determinants of DMSP and DMS concentrations in the ocean are phytoplankton biomass and community composition (Malin et al., Citation1998). Representatives from most of the major phylogenetic groups of eukaryote phytoplankton are known to produce DMSP (Malin & Kirst, Citation1997). Simó et al. (Citation2002) found that DMSP synthesis was proportional to photosynthesis in the North Atlantic and that phytoplankton invested 7% of net primary production into DMSP production. Compiling data from a range of in situ experiments and culture work, Kiene et al. (Citation2000) concluded that DMSP forms <1 to 39% of the cell carbon quota of phytoplankton, with most values lying in the range of 1–5%. Dissolved DMSP concentrations in surface waters were in the range 0.1–25 nM, with a turnover rate of 0.3–129 nM day–1 (Kiene et al., Citation2000). DMS production was associated with high in situ phytoplankton productivity during the Southern Ocean Iron enrichment Experiment (SOFeX). In a patch of water in which productivity was stimulated by the addition of the limiting nutrient iron, the mean (± SD) DMS concentration was 7600 ± 480 pptv, compared with 1560 ± 90 pptv outside the fertilized patch (Wingenter et al., Citation2004).
The physiological functions of DMSP and DMS are uncertain, though they may be components of an intracellular antioxidant system (Sunda et al., Citation2002). The release of DMSP and DMS generally depends on processes that disrupt cell integrity, rather than exudation by healthy cells (Kiene et al., Citation2000). Examples include senescence, grazing (Wolfe & Steinke, Citation1996; Wolfe et al., Citation1997) and viral lysis (Malin et al., Citation1998). However, a modelling study estimated that Prorocentrum minimum (a dinoflagellate) exudes 1% day−1 of its cell quota of DMSP, whereas Phaeocystis sp. (a prymnesiophyte) exudes from 3 to 11% day−1 of its cell quota of DMSP (Laroche et al., Citation1999). Given that the DMSP concentration in Phaeocystis sp. cells is 71–150 mM (Stefels & van Boekel, Citation1993) and that DMSP may form a significant component of the carbon cell quota, the exudation or leakage of even a small proportion of cell DMSP may be a significant source of DOM during phytoplankton blooms of high DMSP producing species.
Isoprene
Shaw et al. (Citation2003) grew a range of phytoplankton species and found that they all produced isoprene (2-methyl-1,3-butadiene, an alkene with the formula C5H8) during exponential growth in laboratory cultures. The range of isoprene production rates were between 1 × 10−21 and 4 × 10−19 mol cell–1 day−1. Shaw et al. (Citation2003) also measured the production of a range of light non-methane hydrocarbons (C2 to C6); however, only isoprene was consistently produced by the five phytoplankton species studied. Bonsang et al. (Citation2010) grew a range of phytoplankton species, representing different major phylogenetic groups (cyanobacteria, diatoms, coccolithophores and chlorophytes), in laboratory cultures. All the phytoplankton investigated emitted isoprene, with the highest production rates (per unit chlorophyll a) occurring in the cyanobacteria (Trichodesmium and Synechococcus) and the lowest in the chlorophyte Dunaliella tertiolecta. Isoprene production was also shown to be elevated in waters fertilized with iron during the SOFeX experiment (Wingenter et al., Citation2004). Mean (± SD) isoprene concentration was 560 ± 13 pptv in the fertilized patch, compared with 139 ± 6 pptv outside the patch in unfertilized water (Wingenter et al., Citation2004). Recent estimates of isoprene flux from ocean to atmosphere are 0.11–1.9 Tg isoprene year−1 (Palmer & Shaw, Citation2005; Arnold et al., Citation2009).
Why do phytoplankton produce extracellular DOM?
John Sharp (Citation1977) originally posed the question ‘Excretion of organic matter by marine phytoplankton – do healthy cells do it?’ as the title of a well-cited paper. It was his contention that the observed release (which he called ‘excretion’) of DOM by phytoplankton was a methodological artefact arising from the 14C tracing methods used to determine photosynthesis rates and DOM production. He identified several potential problems that could lead to overestimates of DOM release by phytoplankton, including residual inorganic 14C contamination in the medium and the rupture of cells during filtration. Data over the last 35 years have convincingly answered Sharp’s (1977) question and demonstrated that healthy phytoplankton do release DOM. Considerable progress has been made in determining the composition and amount of DOM released by phytoplankton. However, the next logical question – Why do healthy phytoplankton release dissolved organic matter? (Bjornsen, 1988) – has not been adequately addressed. Generally, DOM released by phytoplankton can be divided into two categories: compounds that have specific functions (e.g. extracellular enzymes, siderophores and toxins) and compounds that have no known function. It is the production of the latter that dominates phytoplankton DOM release (Nagata, Citation2000). The release of DOM has a cost in terms of resources lost (fixed carbon and energy), which implies that the release of the majority of DOM must either have a negligible impact on the phytoplankton or have an unknown function. summarizes the major mechanisms that may account for the loss of fixed carbon by phytoplankton through DOM release. These mechanisms are not mutually exclusive; it is likely that an individual organism will lose fixed carbon through multiple mechanisms simultaneously. However, not all mechanisms will apply to all phytoplankton all of the time.
Fig. 6. Mechanisms affecting the release of dissolved organic matter (DOM) from phytoplankton cells via leakage and exudation. Leakage is passive loss, while exudation is the active transport of DOM to the environment outside the cell. A. Passive diffusion losses driven by the concentration gradient across the cell membrane and membrane permeability. B. Removal of excess carbon fixed during photosynthesis by photosynthetic overflow. C. Loss of cell membrane integrity during autocatalytic cell death. D. Loss of glycolate from the cell during photorespiration. E. Exudation of exoenzymes and siderophores used in the acquisition of nutrients. F. Exudation of compounds as chemical defense against predators or infectious agents. G. Exudation of molecules that carry information between organisms. H. Exudation of polymers which stick to the surface of the cell and reduce sinking rates by density reduction or increasing frictional resistance.
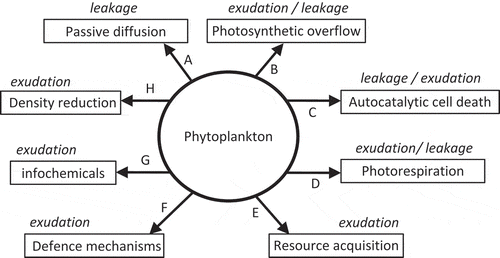
DOM release by phytoplankton is the sum of leakage across the cell membrane and active exudation, with the relative balance of these two processes depending on the physiological status of the cell. In , leakage is defined as any passive loss of DOM from the cell, including losses associated with defined processes (such as the diffusive loss of recent photosynthates) and a general loss by passive diffusion. While passive diffusion is constant, other processes associated with leakage and all active exudation processes will vary in response to environmental factors such as nutrient availability. For example, one could hypothesize that the loss rate of DOM across the cell membrane via passive diffusion is relatively constant in a healthy cell, whereas the loss of organic carbon associated with photorespiration will depend on photosynthetic activity, which in turn will depend on environmental factors such as light availability and temperature. Under such a scenario, the proportion of total DOM lost due to passive diffusion would be greater during the night than during daylight. The balance of low molecular weight and high molecular weight DOM released by the cell will depend on the relative proportions of DOM released through leakage or exudation. The DOM released through leakage will tend to be dominated by small, uncharged molecules that can easily pass through cell membranes, whereas exudates are often dominated by large polymers that do not diffuse across the cell membrane (Baines & Pace, Citation1991; Myklestad, Citation2000). At present, there is no method to directly measure the contributions of leakage and exudation to total DOM release. Consequently, the relative contribution of these processes is inferred, often on the basis of indirect evidence, such as the size of the molecules released.
DOM release by passive diffusion across the cell membrane
There is a 106 times increase in DOM concentration moving across the cell membrane from the external environment into the cell (Flynn et al., Citation2008). This concentration gradient is the major factor that drives the passive leakage of DOM from phytoplankton cells. Factors affecting the rate of DOM leakage by diffusion across the cell membrane are cell permeability, surface area to volume ratio of the cell, and the size of the intracellular pool of the compound in question (Bjørnsen, Citation1988):
Subsequent work has both supported and contradicted the passive diffusion model of DOM release. For example, Baines & Pace (Citation1991) concluded that DOM release is constrained by the total availability of photosynthates, rather than phytoplankton biomass, and therefore active processes are more important than diffusion. However, constant PER throughout the water column, which only increased at very low light conditions, led to the conclusion that DOC release was the result of passive diffusion rather than photosynthetic overflow (Marañón et al., Citation2004, 2005). The passive diffusion model implies that a population dominated by small cells should have a higher PER than populations of larger cells. Measurements of DON release using 15NH4+ as a tracer in two size fractions (< 94 µm and < 20 µm) of natural phytoplankton showed that the smallest size fraction had the greatest PER with respect to organic nitrogen, though the amount of DON released was greatest in the fraction containing large cells (Hasegawa et al., Citation2000). This reiterates that while PER is a useful concept, it does not give any information about the magnitude of the DOM release (Mague et al., Citation1980; Williams, Citation1990).
Overflow hypothesis
DOM exudation as a consequence of excess carbon being fixed by photosynthesis () is frequently invoked as the major mechanism to explain DOM release by phytoplankton. Like passive diffusion, the loss of DOM from photosynthetic overflow is generally regarded as a consequence of inefficiencies in cell physiology with the extracellular products serving no function outside the cells. The release of photosynthate under conditions of high light (Hellebust, Citation1965; Zlotnik & Dubinsky, Citation1989) and nutrient scarcity may be an overflow mechanism when photosynthesis occurs more rapidly than is required for growth (Fogg, Citation1983; Wood & Van Valen, Citation1990). For example, if a cell is growing under conditions of nitrogen limitation there will be insufficient nitrogen to support growth of the cell, which requires the synthesis of proteins, nucleic acids and other nitrogen-containing compounds. Equally, there may not be enough available nitrogen to ‘switch off’ photosynthesis, as this would require the synthesis of proteins and nucleic acids to reorganize the photosynthetic apparatus. After the carbohydrate storage capacity of the cell is exceeded, the excess organic carbon is exuded from the cell. As light and usable inorganic carbon sources are readily available under this scenario, the strategy has little or no cost to the phytoplankter.
Batch culture experiments have shown that phytoplankton release more DOM under conditions of nutrient limitation (Guillard & Wangersky, Citation1958; Marker, Citation1965; Myklestad, Citation1974, 1977; Obernosterer & Herndl, Citation1995). Myklestad (Citation1995) concluded that phytoplankton release carbohydrates into the surrounding medium under conditions of severe N or P limitation, as carbohydrates do not contain these potentially limiting nutrients. Myklestad et al. (Citation1989) found that the rates of release of carbohydrates and amino acids by Chaetoceros affinis were highest in the growth phase compared with stationary phase batch cultures. However, the photosynthesis rate of nutrient-limited cells was lower and therefore DOM release accounted for 58% of productivity in stationary phase cells, compared with 10% during exponential growth. It is assumed that the accumulation of DOM in phytoplankton cultures indicates the uncoupling of growth and photosynthesis. This is supported by the observation that the greatest release of DOM occurs during the transition between different phases of phytoplankton growth (Granum et al. Citation2002; Wetz & Wheeler, Citation2007), which was explained by Williams (Citation1990) as a ‘temporary loss of control’ of the organic matter pools within the cell as growth rate slows down. It is rarely considered that other processes, such as phytoplankton cell death resulting in lysis (Franklin et al., Citation2006), may also affect the release of DOM in batch cultures. In addition, there may be taxonomic differences: Skeletonema costatum and Phaeocystis sp. have relatively constant DOC release rates (which fits the passive diffusion model of DOM release), whereas Synechococcus bacillaris and Emiliania huxleyi release more DOC during the stationary phase (which fits the overflow hypothesis) (Biddanda & Benner, Citation1997).
If the overflow hypothesis is applicable, then it is probable that once the cell content (the cell quota: Droop, Citation1968, 1983) of key nutrients (such as N or P) drops below a threshold, the excess organic carbon is exuded from the cell. Alternatively, exudation may not be dependent on a quota or absolute amount of limiting nutrient, but rather the stoichiometric ratio of carbon to potentially limiting nutrients. Most models of phytoplankton growth use ratios, as nutrient quotas are normalized to carbon (e.g. Geider et al., Citation1998; Sunda et al., Citation2009). Determining the degree to which C : N : P stoichiometry of phytoplankton deviates from the canonical Redfield ratio of 106 : 16 : 1 is seen as an important step in understanding of the role of phytoplankton in biogeochemical cycling (Falkowski, Citation2000; Geider & La Roche, Citation2002). However, the Redfield ratio of 106 : 16 : 1 is a mean C : N : P ratio for marine organic matter and there are significant differences in the C : N and C : P ratios in different phyla of phytoplankton (Geider & La Roche, Citation2002; Quigg et al., Citation2003). Variation in the C : N : P ratios (and indeed the C : X ratios of other nutrients) is a combination of phylogenetics and the physiological status of the cells (Geider & La Roche, Citation2002; Arrigo, Citation2005). Consequently, it is unlikely that there is a single threshold value of C : N or C : P ratio, which if exceeded, would predict exudation as a result of photosynthetic overflow. This is logical when considered in the context of the variation in threshold stoichiometric ratios indicating limitation. For example, the critical N : P ratio indicating the transition between nitrogen and phosphorus limited growth is generally higher than the Redfield ratio and varies between 20 and 50 (Geider & La Roche, Citation2002).
β-1,3-glucan is a carbohydrate storage product of many diatoms and is used as a carbon source to produce exopolymers (EPS) by epipelic diatoms (Smith & Underwood, Citation1998, 2000). The glucan content of Chaetoceros affinis increases rapidly during the stationary phase in nitrate-depleted batch cultures (Myklestad & Haug, Citation1972). Granum et al. (Citation2002) dismissed the overflow hypothesis as a significant carbon sink and proposed that excess carbon in nitrogen limited Skeletonema costatum was stored within the cell as β-1,3-glucan. However, the storage of excess carbon within the cell and overflow are not necessarily mutually exclusive; it may be that overflow occurs once the storage capacity of the cell has been exceeded.
Photorespiration and glycolate production
The stressful conditions (particularly high light) that affect photosynthetic overflow may also lead to photorespiration. Glycolate is produced by phytoplankton during photorespiration (Tolbert, Citation1974; Falkowski & Raven, Citation2007), and while the majority of glycolate is metabolized, a proportion is released into the surrounding environment (Falkowski & Raven, Citation2007).
Carbon fixation occurs via a carboxylase reaction affected by the enzyme ribulose-1,5-bisphosphate carboxylase–oxygenase (RuBisCO). Whether RuBisCO acts as an oxygenase or carboxylase depends on the kinetics of RuBisCO within the organism and the steady state concentration of CO2 and O2 (Falkowski & Raven, Citation2007). Photorespiration is affected by the oxygenase activity of the enzyme, leading to the production of phosphoglycolate, which is a sink for phosphorus and an inhibitor of photosynthesis (Falkowski & Raven, Citation2007). Hydrolysis of phosphoglycolate produces inorganic phosphate and glycolate, thereby reducing the concentration of phosphoglycolate and recycling valuable phosphorus.
Allelochemicals
Allelopathy was a term first used by Molisch (1937; cited by Rice, Citation1979) to describe ‘biochemical interactions between all types of plants including microorganisms’. The consequences of those interactions could be either beneficial or detrimental to the organisms involved. Allelochemicals, according to the original definition, can have stimulatory effects; however, the vast majority of work has focused on the inhibitory effect of allelochemicals (Gross, Citation2003). Indeed, Legrand et al. (Citation2003) defined allelochemicals associated with phytoplankton as secondary metabolites that are released into the surrounding medium where they act as infochemicals or injurious agents. They concluded that allelopathic interactions between phytoplankton are an important aspect of competition. For example, cell-free filtrate from the prymnesiophyte Prymnesium parvum inhibits the growth of the diatom Thalassiosira weissflogii and the nutrient status of the diatom affects its response to the filtrate (Fistarol et al., Citation2005). The most common effects of allelochemicals are cell lysis and growth inhibition (Legrand et al., Citation2003). Being defined by function, allelochemicals have diverse chemical structures and molecular weights (Leão et al., Citation2009). Karenia brevis produces multiple small (500 to 1000 Da) allelochemicals containing aromatic groups that inhibit the growth of the diatom Asterionellopsis glacialis (Prince et al., Citation2010). In contrast, the raphidophyte Heterosigma akashiwo releases a high molecular weight polysaccharide-protein complex (> 1 000 000 Da) that inhibits the growth of the diatom Skeletonema costatum by binding to the surface of the cells (Yamasaki et al., Citation2009). There is an extensive and rapidly growing literature on allelopathy associated with phytoplankton, which is beyond the scope of this review (see reviews by Gross, Citation2003; Legrand et al., Citation2003; Leão et al., Citation2009).
Resource acquisition
Phytoplankton, like all microorganisms, produce extracellular proteins in the form of enzymes. Extracellular enzymes are used by phytoplankton to acquire inorganic nutrients. Extracellular enzymes may be closely associated with the cell, such as located in the periplasmic space, or adhered to the cell surface. They may also be released into the surrounding medium, where they will contribute to DOM. Examples of extracellular enzymes produced by phytoplankton include carbonic anhydrases (CA) (see reviews by Raven, Citation1997; Giordano et al., Citation2005) and extracellular alkaline phosphatase (AP) (Xu et al., Citation2010). Extracellular DOM may play other roles in resource acquisition. Exopolymers released by microorganisms may act to trap exoenzymes in the proximity of the cell, thereby ensuring that the cell that released the enzymes benefits most from the resources created by the enzyme activity (Decho, Citation1990). Extracellular saccharides enhance the bioavailability of iron to eukaryotic phytoplankton in the Southern Ocean (Hassler et al., Citation2011). This may be a significant finding, given that iron is the primary limiting nutrient over a significant area of the world ocean and carbohydrates are a major component of the DOM released by phytoplankton.
Autocatalytic cell death
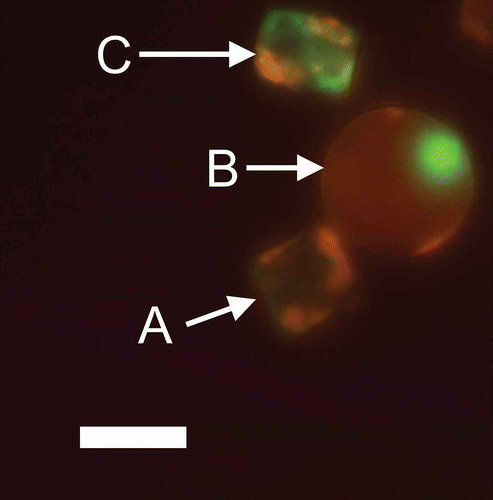
The process of death in phytoplankton has been overlooked until the last decade. It was generally assumed that individual phytoplankton cell lines were immortal unless an external factor (predation, viral infection or sinking into prolonged darkness) caused their death (Kirchman, Citation1999; Bidle & Falkowski, Citation2004; Franklin et al., Citation2006). Recent research has shown that phytoplankton also undergo the physiological process of autocatalytic cell-suicide, which can lead to lysis of the cells; this process is analogous to programmed cell death (PCD) in metazoans (Bidle & Falkowski, Citation2004; Franklin et al., Citation2006). Autocatalytic cell death is a process that is indicated by multiple changes within the phytoplankton cell and it is often difficult to characterize such cells as clearly dead or alive (Franklin et al., Citation2006, 2012). Cell membrane integrity is used as an early indicator of cell death (Veldhuis et al., Citation2001). Veldhuis et al. (Citation2001) showed that a significant proportion of phytoplankton populations grown in culture and in the field (North Atlantic during March) had compromised cell membranes, as indicated by SYTOX Green staining (). Veldhuis et al. (Citation2001) hypothesized that ‘automortality’ is a significant source of DOM leakage, particularly as cells with compromised cell membranes are often still capable of photosynthesis. Subsequent work has shown that stresses such as low irradiance or nutrient limitation affect compromised plasma membranes and autocatalytic cell death in phytoplankton (Berman-Frank et al., Citation2007; Timmermans et al., Citation2007; Franklin et al., Citation2012).
Fig. 7. Cell permeability in the diatom Thalassiosira weissflogii (CCMP 1051) visualized by epifluorescence microscopy. Cells were stained with SYTOX Green (Invitrogen, Life Technologies, Grand Island, U.S.A.), a membrane-impermeable nucleic acid stain. Chlorophyll autofluorescence is shown in red and SYTOX Green stained nucleic acids are shown in green. A. Intact cell containing chlorophyll but no SYTOX Green staining and therefore intact cell membranes. B. Intact cell containing chlorophyll with compromised cell membranes revealed by the staining of an intact nucleus with SYTOX Green. C. Dying cell with low chlorophyll autofluorescence, a disrupted nucleus and compromised cell membranes. Image courtesy of Jie Chen. Scale bar = 10 µm.
DOM release and microbial food webs
In the ‘classic food web’ organic carbon fixed by photosynthesis is passed as POM from phytoplankton to herbivorous grazers and then to fish, whereas the microbial loop is driven by DOM (Azam et al., Citation1983; Ducklow, Citation1983). The release of DOM by phytoplankton is a significant driver of secondary production by heterotrophic prokaryotes. Generally, DOM release by phytoplankton is considered insufficient to support the observed heterotrophic bacterial growth (Baines & Pace, Citation1991; Nagata, Citation2000; Morán et al., Citation2002b; Teira et al., Citation2003b). In addition, processes such as viral lysis and sloppy feeding contribute a significant amount of the phytoplankton DOM that is utilized by bacteria. Baines & Pace (Citation1991) concluded that the DOC released by phytoplankton supports less than 50% of bacterial carbon requirements, based on a review of data from freshwater, estuarine and coastal environments, and modelling. Recent work has challenged this general assumption: DOC released by phytoplankton is sufficient to support the observed bacterial growth in the Southern Ocean (Morán et al., Citation2001, 2002b; Morán & Estrada, Citation2002) and in an area of coastal upwelling off the Iberian Peninsula in the Atlantic (Teira et al., Citation2003b).
Bacterial growth efficiency (BGE) is a key factor in determining whether sufficient DOC is released from phytoplankton to support bacterial growth. BGE is simply the proportion of the total bacterial carbon demand that is used for bacterial production:
The interactions between heterotrophic prokaryotes and phytoplankton are poorly understood. An early concept used to describe the relationship between phytoplankton and bacterioplankton was the ‘phycosphere’ (Bell & Mitchell, Citation1972; Stocker, Citation2012), which described a zone surrounding a phytoplankton cell in which bacterial growth is stimulated by DOM released from the cell. Bell et al. (Citation1974) grew two strains of bacteria with the diatom Skeletonema costatum and found that the growth of one bacterial strain was stimulated by the presence of the diatom while the growth of the second was inhibited. They concluded that the release of DOM by phytoplankton is important in determining bacterial community structure. Modern culture-independent molecular methods enable detailed investigations of the bacteria associated with phytoplankton. For example, there was a succession of bacterial species attached to cells of the dinoflagellate Lingulodinium polyedrum during a bloom off the Californian coast (Mayali et al., Citation2011).
Interactions between bacteria and phytoplankton may be both ‘top down’ and ‘bottom up’ at the same time. While the composition of the DOM released by phytoplankton may affect the growth and community structure of the bacterial community (Sapp et al., Citation2007; Pete et al., Citation2010), the bacteria will also affect the amount and composition of DOM released by the phytoplankton (Bruckner et al., Citation2011; Gärdes et al., Citation2011, 2012). Marinobacter adhaerens was isolated from marine particles in the German Wadden Sea and its complete genome sequenced (Gärdes et al., Citation2010). Subsequently, it has been grown with the diatom Thalassiosira weissflogii in a model system (Gärdes et al., Citation2012) in experiments showing that the interaction between the diatom and the bacterium depend on the nutrient status of the culture. Under ‘balanced’ nutrient conditions (N : P of 16 : 1), the bacterium enhanced exudation and TEP formation in batch cultures compared with axenic controls. However, if the cultures were grown under conditions resulting in nutrient limitation, M. adhaerens did not enhance TEP production. Aggregate formation is an important process, as it changes the size distribution of particles in the water column and affects the efficiency of the biological carbon pump, because larger particles sink faster than small particles. Diatoms, particularly under nutrient-limiting conditions, exude sticky polymers (such as TEP precursors) that affect aggregation (Thornton, Citation2002). Gärdes et al. (Citation2011) showed that bacteria are important in the aggregation of Thalassiosira weissflogii; aggregation did not occur in axenic cultures of the diatom, whereas bacteria attached to T. weissflogii cells enhanced aggregation.
The interactions between phytoplankton and heterotrophic prokaryotes that affect DOM release by phytoplankton are complex and largely unpredictable with our current knowledge. Bacterial community composition affects the composition of the phytoplankton community and vice versa. Environmental factors, such as nutrient limitation, moderate these interactions by affecting the physiology of the organisms and their allocation of resources. Consequently, the concentration and amount of DOM released by phytoplankton in natural waters are an emergent properties of the interactions between organisms and the surrounding environment.
Phytoplankton DOM release and global climate change
A major research challenge over the coming decades will be to evaluate the impact of global climate change on ecosystems and the ecosystem services that sustain human populations. The carbon dioxide mixing ratio in the atmosphere has risen from a pre-industrial value of 280 ppm (Caldeira & Wickett, Citation2003) to a current value of 394 ppm as measured at the Mauna Loa Observatory, Hawaii (NOAA, October 2013 value). The increasing amount of CO2 in the atmosphere is both trapping more heat in the troposphere and significantly altering the carbonate chemistry of the upper ocean. These changes have resulted in a mean warming of the Earth’s surface by 0.85°C over the period from 1880 to 2012 (IPCC, Citation2013) and a decrease in the pH of the surface ocean from a pre-industrial value of 8.2 to approximately 8.1 today (Royal Society, Citation2005). Key questions are: How will a warmer and more acidic ocean affect the structure and function of marine ecosystems? Will the ability of these altered ecosystems to sequester carbon change, and how will this feedback to the amount of carbon dioxide in the atmosphere?
Processes affecting the partitioning of organic carbon between the dissolved and particulate phases will play a significant role in determining the fate of carbon in the ocean as the climate changes. It is predicted that, as the Earth warms, the surface ocean will become both warmer and more stratified (Sarmiento et al., Citation2004; Behrenfeld et al., Citation2006). An increasingly stratified surface ocean will be increasingly nutrient limited, as there will be less mixing between the nutrient-depleted surface waters and the nutrient-rich deep waters. Thus, the photic zone will support a lower biomass of primary producers. In addition, the pH of the surface ocean will continue to decline, with a pH of 7.9 predicted for the end of the century and 7.4 predicted for the end of the millennium (Caldeira & Wickett, Citation2003). Potentially, therefore, phytoplankton living in large areas of the future ocean will be growing in an environment that is warmer than today, with a lower pH and lower availability of inorganic nutrients. These predicted changes will result in a more stressful environment for phytoplankton growth and, as already discussed, stresses such as high temperature and nutrient limitation affect DOM release by phytoplankton. Moreover, conditions of increased stratification and nutrient limitation would be expected to favour small-celled taxa (Finkel et al., Citation2010) with relatively large surface area to volume ratios and therefore greater potential for DOM loss via leakage. Successful integration of processes that occur over a range of temporal and spatial scales, from the physiology of individual phytoplankton cells to ocean circulation, is required to meet the challenge of understanding the effect of climate change on carbon cycling in the ocean. Over the next few paragraphs I will explore the evidence to support the hypothesis that phytoplankton PER will be greater as a result of climate change induced by increasing CO2 in the atmosphere.
Temperature and stratification
There is convincing evidence that the upper ocean is becoming warmer (Levitus et al., Citation2000; Barnett et al., Citation2005; Lyman et al., Citation2010). Environmental temperature has a profound effect on the physiology of phytoplankton metabolism and there are several reviews that address these effects in relation to climate change (Beardall & Raven, Citation2004; Finkel et al., Citation2010; Winder & Sommer, Citation2012). However, the effect of temperature on DOM release has generally been overlooked. Diatoms become more ‘sticky’ at elevated temperatures and prone to forming aggregates (Thornton & Thake, Citation1998; Piontek et al., Citation2009; Rzadkowolski & Thornton, Citation2012), which may be affected by the enhanced release of DOM by phytoplankton at elevated temperatures. Aggregate formation at elevated temperature has been hypothesized to be due to sticky polymers on the surface of the diatoms (Thornton & Thake, Citation1998) or the production of TEP (Piontek et al., Citation2009). Seven of eight phytoplankton species studied by Claquin et al. (Citation2008) produced more TEP with increasing temperature when grown in batch culture. The trend continued until a maximum TEP production rate was reached, with higher temperatures resulting in lower TEP production rates. The exception to this trend was the prymnesiophyte Emiliana huxleyi, which showed no relationship between TEP production rate and temperature. There is evidence from mesocosm experiments for increased DOM release by natural phytoplankton grown at elevated temperature (Engel et al., Citation2011; Wohlers et al., Citation2009). The C : N ratio of the DOM increased at a higher temperature due to increased production of dissolved carbohydrates (Engel et al., Citation2011). Short-term (6 h) warming experiments with natural phytoplankton communities from the Southern Ocean showed that increasing temperature from ambient (−1.4 to 0.4°C) to 2°C resulted in an increase in PER from 35 to 54% (Morán et al., Citation2006). While PPP remained relatively constant (0.7 mg C m−3 h−1), there was an increase in DPP from 0.5 to 0.9 mg C m−3 h−1 (Morán et al., Citation2006).
An increase in PER with climate change may not result in an increase in the total amount of DOM released by phytoplankton if the ocean supports a lower phytoplankton biomass. Long-term field datasets with sufficient coverage and resolution to enable scientists to determine whether global warming affects changes in phytoplankton distribution and abundance are lacking. Boyce et al. (Citation2010a) concluded that global phytoplankton concentration is declining rapidly in the ocean at a rate of approximately 1% year−1 in response to warming temperatures, using pigment and Secchi depth data going back to 1899. However, this conclusion was immediately challenged by several groups (Mackas, Citation2010; McQuatters-Gollop et al., Citation2010; Rykaczewski & Dunne, Citation2010). More work has been done at the regional scale. In the North Sea and North Atlantic, the Continuous Plankton Recorder (CPR) survey has produced data since 1931 on the distribution of larger phytoplankton (Leterme et al., Citation2005). CPR data is generally collected from latitudes higher than 40° N and is biased towards shelf waters (Boyce et al., Citation2010b). The plankton colour index (PCI), a crude estimate of phytoplankton biomass, has increased since 1958, with a decline in the relative abundance of diatoms compared with dinoflagellates (Leterme et al., Citation2005). Based on satellite-derived ocean colour data, the area of waters with the lowest chlorophyll concentrations (< 0.07 mg chl m−3) associated with the subtropical gyres (waters from 5° N to 45° N, and 5° S to 45° S) has expanded globally by 6.6 million km2, or 15%, from 1998 through to 2006 (Polovina et al., Citation2008). The expansion correlated with an increase in mean monthly sea surface temperature and was hypothesized to be the result of increased stratification causing an expansion of oligotrophic waters. Modelling studies have also shown an expansion of the subtropical gyres and increased stratification of the ocean since the industrial revolution, in response to warming of the surface ocean (Sarmiento et al., Citation2004; Bopp et al., Citation2005). Behrenfeld et al. (Citation2006) used satellite-derived ocean colour data to relate the annual productivity of the ocean to climate variability. Annual global productivity was low in years in which there was enhanced stratification, as this resulted in a larger area of low latitude, low productivity waters. Phytoplankton productivity is predicted to increase at high latitudes in a warmer ocean due to an extended growing season caused by reduced mixing; however, this will be insufficient to balance the reduction observed at lower latitudes (Bopp et al., Citation2005; Doney, Citation2006). These examples illustrate that the effect of global warming on phytoplankton biomass is regional and there is currently no robust global integration of these observations (Polovina et al., Citation2008). Lower biomass would be predicted to result in a lower release of DOM if the phytoplankton community does not change. However, it is unlikely to be this simple as the changes that affect phytoplankton biomass also affect community composition.
Coupled climate and biogeochemical models indicate that the biomass of diatoms will decrease with predicted climate change, relative to small phytoplankton, as a result of increased stratification and consequent decreased nutrient supply to surface waters at mid latitudes (Bopp et al., Citation2005; Marinov et al., Citation2010). The prediction of smaller cells in a warmer ocean is also supported by the geological record (Falkowski & Oliver, Citation2007). Falkowski & Oliver (Citation2007) hypothesized that the negative correlation between the surface area of diatom frustules and ocean temperature over the last 65 million years was not a direct function of temperature, but rather the result of lower nutrient supply to the surface ocean caused by reduced vertical mixing in warmer oceans. Similarly, resource availability is key to explaining the success of different algal size classes at different temperatures in the contemporary ocean (Marañón et al., Citation2012). Data from the Atlantic Ocean show water temperature affects phytoplankton cell size, with picophytoplankton becoming more dominant at warmer temperatures (Morán et al., Citation2010). Communities dominated by picophytoplankton in the Atlantic Ocean have higher PER than phytoplankton communities dominated by larger cells (Teira et al., Citation2001a, 2001b). However, in the field it is difficult to uncouple nutrient limitation and temperature effects. In the laboratory, Montagnes & Franklin (Citation2001) grew five species of diatom and two flagellates and found that growth rate increased with temperature, but cell volume decreased at a rate of 4% °C−1. A meta-analysis of the literature also found that protists decreased in cell volume with temperature at a similar rate (2.5% °C−1; Atkinson et al., Citation2003). Results from short-term laboratory experiments, during which natural phytoplankton communities from the Baltic Sea were exposed to varying degrees of nutrient stress at different temperatures, support the hypothesis that nutrient limitation mediates temperature effects on cell size (Peter & Sommer, Citation2013).
Ocean acidification
The dissolved inorganic carbon (DIC) concentration of seawater is approximately 2000 µM (Falkowski & Raven, Citation1997; Morel et al., Citation2002); however, the concentration of dissolved CO2 is only 10–20 µM (Falkowski & Raven, Citation1997; Morel et al., Citation2002). This value is below the half saturation constant of 30–40 µM for diatom RuBisCO (Badger et al., Citation1998; Morel et al., Citation2002), which suggests that phytoplankton photosynthesis could be CO2-limited in the contemporary ocean. Culture work has shown that the growth rate of diatoms is limited by CO2 supply under optimal conditions of light and nutrients (Riebesell et al., Citation1993). Increasing concentrations of carbon dioxide in the atmosphere will result not only in higher DIC concentrations in the surface ocean, but also in a shift in the equilibrium of the different components of the DIC systems as the ocean acidifies, resulting in higher dissolved CO2 concentrations (Royal Society, Citation2005). Carbon fixation rates may increase if more CO2 is available, resulting in photosynthetic overflow if the increased availability of organic carbon is not matched by an increased availability of other nutrients within the phytoplankton cell. There is evidence that elevated dissolved CO2 concentrations facilitate photosynthesis and affect the release of DOM by phytoplankton (Riebesell, Citation2004). However, phytoplankton response to increasing dissolved CO2 concentrations is unlikely to be straightforward as most have carbon concentrating mechanisms (Giordano et al., Citation2005) and, therefore, are not limited by the current concentration of dissolved CO2 in seawater. Consequently, photosynthesis may not be significantly stimulated under ocean acidification conditions (Beardall & Raven, Citation2004; Royal Society, Citation2005) and there may be shifts in phytoplankton community structure as the ocean acidifies based on the competitive costs and benefits of different inorganic carbon acquisition strategies.
Low pH may affect the rate of processes transforming DOM, such as the formation of exopolymer particles from dissolved precursors and the enzymatic hydrolysis of organic matter in the ocean. Research has shown increased abiotic TEP formation with decreasing pH (Mari, Citation2008) and increased production of extracellular carbohydrates by a diatom with decreasing pH (Thornton, Citation2009). However, as pointed out by Passow (Citation2012), the work of Mari (Citation2008) and Thornton (Citation2009) is not representative of the future ocean, as both researchers added acid to titrate the seawater to a lower pH under current atmospheric CO2 mixing ratios, resulting in a change in total alkalinity. In the future ocean the low pH will be associated with higher DIC concentrations, but no change in total alkalinity (Passow, Citation2012). Passow (Citation2012) showed that the abiotic formation of TEP from precursors is not affected by ocean acidification. These examples illustrate that many of the apparent discrepancies between results from ocean acidification experiments arise from the experimental design.
The degradation of phytoplankton-derived DOM in the future ocean will depend on complex interactions between microorganisms as ocean acidification will affect the composition and concentration of the DOM produced by individual taxa, the composition of the microbial community and potentially the chemistry of extracellular enzymes (Arnosti et al., Citation2011). The complexity of these relationships is shown by recent research. For example, extracellular glucosidase activity was enhanced under conditions simulating ocean acidification, leading to a greater degradation of algal-derived polysaccharides (Piontek et al., Citation2010). Other work has found that acidification had negligible effects on some enzymes and reduces the activity of others (Yamada & Suzumura, Citation2010).
Several experiments have been conducted in which natural populations of phytoplankton were grown in experimental systems with elevated atmospheric carbon dioxide mixing ratios to simulate future climate change scenarios. Kim et al. (Citation2011) grew natural populations of microorganisms from Korean coastal waters under control (ambient) conditions, acidification (900 ppm CO2), and acidification with warming (3°C warmer than ambient), in 3000 l mesocosms. At the end of the batch experiment, during nitrogen depletion, DOC concentrations were lowest in the control treatment and highest in the acidification-with-warming treatment. The ratio of DOC to POC was lowest in the control and highest in the acidification-with-warming treatment. This suggests that PER was greater than the control under conditions of acidification, and greatest under conditions of acidification with warming. However, Kim et al. (Citation2011) did not measure the photosynthetic allocation of carbon into PPP and DPP and therefore their measurements are not equivalent to PER. Riebesell et al. (Citation2007) conducted mesocosm experiments with natural phytoplankton assemblages grown under atmospheres containing three different CO2 mixing ratios (350, 700 and 1050 ppm) and found that the drawdown of DIC increased with increasing DIC concentrations, though the drawdown of other nutrients was not enhanced. There was an increase in the loss of organic carbon from the upper mixed layers of the mesocosms grown under acidified conditions. Riebesell et al. speculated that TEP may have played a significant role in the transformation of DOC to POC and the sinking of POC from the upper layer of the mesocosms with high pCO2. However, analysis of the TEP concentrations from the same experiment by Egge et al. (Citation2009) showed no difference in TEP production between the different pCO2 treatments. Recently, experiments using mesocosms in Arctic waters showed enhanced production of DOC by phytoplankton grown under elevated CO2 (Engel et al., Citation2013).
Future research
DOM release by phytoplankton has been a subject of research for more than 50 years. Despite this sustained interest, there remain many unresolved questions regarding the physiological processes that affect DOM release by marine phytoplankton and its biogeochemical significance. Many of our current explanations for DOM release are hypotheses that require further testing. Fortunately, new instrumentation for analytical geochemistry, the ‘omics’ methods of biology, and the data handling tools of bioinformatics will make it feasible to open the ‘black box’ of DOM biogeochemistry over the next few decades. In the following section, I will highlight some of the gaps in our current knowledge and suggest how future research can fill those gaps.
Physiology of phytoplankton DOM release
The physiological status of the cell affects both the amount and composition of the DOM released into the surrounding medium. Flynn et al. (Citation2008) considered a lack of understanding of DOM release by phytoplankton to be a significant gap in our knowledge of phytoplankton physiology and a limitation for current biogeochemical models of primary production. If exudation is an active process, then presumably it is of some benefit to the phytoplankton. In some examples, such as extracellular enzymes, it is easy to envision the potential return on the investment of synthesizing the DOM and transporting it across the cell membrane into the external environment. However, with other processes, such as photosynthetic overflow, the costs and benefits are harder to discern. If exudation of carbohydrates in the stationary phase is really photosynthetic overflow when growth is limited, then why is it often exuded in the form of complex heteropolymers (high molecular weight [HMW] DOM) rather than low molecular weight (LMW) DOM? For example, the mean proportion of HMW (> 12 000 Da) carbohydrates produced by Skeletonema costatum increased from 20 ± 11% (mean ± SD) in the exponential phase to 72 ± 18% in the stationary phase (Malej & Harris, Citation1993). Complex heteropolymers should have a relatively high cost to synthesize and transport out of the cell, in contrast to, say, glucose, which is a direct product of photosynthesis and more likely to diffuse out of the cell. Photosynthetic overflow is invoked as a mechanism for coping with excess fixed carbon during nutrient limitation. However, the cellular machinery (RNA, proteins and vesicles) necessary for the synthesis of polymers requires likely limiting nutrients (e.g. N and P). These inconsistencies suggest that photosynthetic overflow is more complex than we currently appreciate, or that photosynthetic overflow is not the reason for this form of exudation.
Little is known of the physiology of DOM release by eukaryotic phytoplankton and there is a need for more research on the intracellular assembly, transport and subsequent extracellular release of DOM by phytoplankton. The term ‘exudation’ is poorly defined, reflecting our limited understanding of the physiological mechanisms affecting the release of DOM by phytoplankton (Chin et al., Citation2004). The secretion of polymers via exocytosis was observed in the prymnesiophyte Phaeocystis globosa, which is known to produce copious amounts of polymeric gels (Chin et al., Citation2004). The secretory vesicles inside cells of P. antarctica were shown to contain DMSP and DMS in addition to polymers (Orellana et al. (Citation2011), suggesting that these small molecules may also be secreted via exocytosis. The relative contributions of exudation and leakage to DOM release, and how the physiological status of the cells affects them, remain to be determined. Novel methods will be required to distinguish between the DOM released by passive leakage across the cell membrane and the DOM that is actively exuded.
The ecological significance of autocatalytic cell death of phytoplankton is unknown and there is much to be learnt about the physiology of the process (Bidle & Falkowski, Citation2004, Franklin et al., Citation2006). It is well established that cell membranes become more ‘leaky’ during cell death (; Veldhuis et al., Citation2001), but how much DOM this produces is unknown. It may be that the few dying cells in a population determine the amount and composition of the DOM released, as they have such high rates of leakage compared with the general population. There is a need to investigate the effect of phytoplankton cell death on the partitioning of the organic matter released between the dissolved and particulate pools. Factors affecting the permeability of phytoplankton cell membranes and the subsequent leakage of organic matter from the cells are largely unknown. Stress is predicted to make cell membranes more leaky, but there is little information on how stress affects the composition and amount of DOM passing through the cell membrane, or how the cell membrane itself changes. It may be that different stresses affect DOM production in different ways. For example, does P limitation limit the ability of phytoplankton to repair their cell membranes, resulting in significant leakage? Dyhrman et al. (Citation2012) showed that Thalassiosira pseudonana changes the composition of its lipids in response to P limitation, though they did not investigate whether these changes affected DOM release by the diatom.
Rhythms of phytoplankton activity in response to the light–dark cycle and other stimuli may affect the balance between exudation and leakage and the composition of the DOM released. Such effects have largely been overlooked, though it is well established that EPS production by benthic diatoms is affected by light–dark cycles (Staats et al., Citation2000; Smith & Underwood, Citation2000). Crocosphaera watsonii, an ecologically significant N-fixing cyanobacterium, has diel cycles of carbohydrate accumulation and release, with the internal carbohydrate pool decreasing as the cells release EPS and TEP into the surrounding water (Dron et al., Citation2012).
Finally, the new ‘omic’ approaches have great potential to contribute significantly to understanding the physiology of DOM release by phytoplankton. The draft genomes of several important phytoplankton species have been published over the last decade (Rynearson & Palenik, Citation2011). Publication of the annotated genomes of the diatoms Thalassiosira pseudonana (Armbrust et al., Citation2004) and Phaeodactylum tricornutum (Bowler et al., Citation2008) produced a vast amount of data on the potential physiology of these organisms, including information on nutrient transport and metabolic pathways. A new understanding of the diatom genome has led to research on gene expression in stressed diatoms using transcriptomic and proteomic approaches. For example, recent work with T. pseudonana has shown that it has a sophisticated suite of biochemical strategies to deal with phosphorus deficiency (Dyhrman et al., Citation2012) and putative death-related genes play a role in acclimation to low iron conditions (Thamatrakoln et al., Citation2012). Similar approaches could be used to investigate gene expression associated with metabolic pathways and transport proteins associated with exudation and leakage. Genomics provides information on which genes are present in an organism and transcriptomics tells us under what conditions those genes are expressed. However, neither of these approaches tells us anything about rates of processes. Consequently, ‘omic’ approaches should be used in conjunction with traditional physiological measurements of rates for the greatest insight into DOM release.
Chemical characterization of DOM released by phytoplankton
The chemical characterization of marine DOM is an on-going challenge for geochemists (Kujawinski, Citation2011). It is sobering to consider that even measuring the concentration of DOC in seawater, let alone its composition, was a challenge until the 1990s (Sharp, Citation2002). Marine DOM is basically treated as a ‘black box’ in biogeochemical models or crudely characterized by molecule size (LMW or HMW) or reactivity (labile or refractory) (Christian & Anderson, Citation2002). Better characterization of the DOM released by phytoplankton will contribute to continuing efforts to understand the composition of marine DOM in relation to its sources, reactivity, transformations and sinks. The complexity and chemical diversity of DOM mean that opening the ‘black box’ of DOM composition will remain a significant challenge over the coming decades. However, new analytical tools are being developed that may provide significant insights into this problem. For example, electrospray ionization coupled to mass spectrometry is being used to explore the composition of the largely uncharacterized LMW fraction of DOM (Kujawinski, Citation2011). HMW DOM has been better characterized than LMW DOM (< 1000 Da), even though small molecules make up the bulk (approximately 70%) of the DOM pool (Kujawinski, Citation2011). Characterization of the composition of LMW DOM, both in phytoplankton cultures and in situ, is the first step in determining its sources and biogeochemical significance. At the other end of the size spectrum, the HMW DOM that contributes to exopolymer particles has been poorly characterized. It is likely that both TEP and CSP contain chemical components other than those that stain with the dyes that define them (–). TEP plays an important role in the biogeochemistry of the biological carbon pump (Passow & Carlson, Citation2012) and consequently there is a need to better define its composition to determine its origin and biogeochemical significance. Much of the recent detailed work on the composition of EPS produced by diatoms has been conducted with laboratory-grown cultures of benthic epipelic diatoms (Underwood et al., Citation2004; Bellinger et al., Citation2005; Abdullahi et al., Citation2006). There is no reason why the techniques used in this research could not be applied to ecologically relevant taxa of phytoplankton.
Climate change and phytoplankton DOM release
Determining how phytoplankton will respond to a warming and acidifying ocean is going to be a key research theme over the coming years. While much progress has been made, there are as yet too many unknowns to enable scientists to make reliable predictions on how phytoplankton will respond to climate change. Understanding phytoplankton DOM release is one component of this significant problem.
Creating experimental conditions that realistically simulate ocean acidification remains a challenge (Rost et al., Citation2008; Hurd et al., Citation2009). Acidification is generally achieved by titrating the culture to the desired pH (using HCl and NaOH) or by bubbling the system with air containing an elevated mixing ratio of CO2 (Rost et al., Citation2008; Hurd et al., Citation2009). Manipulating the pH and DIC system in seawater is complex, as CO2 both dissolves in and reacts with seawater (Dring, Citation1982). Furthermore, phytoplankton physiology affects the pH of seawater, as photosynthesis increases pH and respiration decreases it. Koeve & Oschlies (Citation2012) suggested that DOM release by phytoplankton (e.g. organic acids) may be a further source of error in the characterization of the DIC system during acidification experiments. These effects are pronounced in phytoplankton cultures where cell concentrations are generally high. Poor control and characterization of the DIC system during culture experiments may explain some of the inconsistencies in experiments conducted with single species, such as Emiliania huxleyi (Hurd et al., Citation2009; Hoppe et al., Citation2011). Flynn et al. (Citation2012), in a recent modelling study, showed that the pH and DIC system may be significantly different at the cell surface than in the bulk medium. This difference will become more pronounced as the ocean acidifies and the buffering capacity of the ocean is reduced. Differences were greatest between the surface of the cell and the bulk medium in cells that were large with a greater metabolic activity. These results suggest that research considering the effects of climate change on phytoplankton permeability and DOM release should consider the microenvironment immediately surrounding the cell in addition to the bulk medium. Collectively, these results suggest that researchers need to pay more attention to the design of ocean acidification experiments with phytoplankton cultures, in terms of how to control conditions within the experiments and accurately monitor DIC (Rost et al., Citation2008; Hurd et al., Citation2009; Hoppe et al., Citation2012).
Most experiments investigating the effect of climate change have been relatively short term. Laboratory grown cultures are generally acclimated to experimental conditions for 7–10 generations before the experiment begins and the experiment is completed within a few days or weeks (Hurd et al., Citation2009). These experiments show how individuals change their patterns of gene expression, but the genetic composition of the population does not significantly change over the course of the experiment (Collins, Citation2012). The ocean is warming and acidifying rapidly; nonetheless, significant climate change in the surface ocean will encompass a time span of thousands to tens of thousands of generations of phytoplankton and so phytoplankton will be evolving as well as acclimating in response to the observed rate of ocean change. Microevolution may be occurring through the selection of particular clones or genotypes within a species and from de novo mutations (Collins, Citation2012). Researchers have started to investigate the evolutionary adaptation of phytoplankton to climate change and indeed, phytoplankton make good model organisms for investigating evolution in eukaryotes, as they are small and have generation times of one or two days. Significant phenotypic changes in some strains of the freshwater green alga Chlamydomonas occurred when they were grown under elevated CO2 for more than 1000 generations (Collins & Bell, Citation2004). Lohbeck et al. (Citation2012) conducted evolution experiments that lasted at least 500 generations with Emiliania huxleyi grown under control and elevated CO2 conditions. When both control and elevated-CO2 strains were grown under ocean acidification conditions, the elevated-CO2 strains were better adapted to acidified conditions, as they had faster growth rates and were able to calcify better than the controls. In contrast, there was little evidence of adaptation after 100 generations in the diatom Thalassiosira pseudonana grown under CO2 and pH predicted for the end of the century, compared with contemporary conditions (Crawfurd et al., Citation2011). Temperature has also been used as a selection pressure in phytoplankton growth experiments, with significant adaptation to elevated temperature observed over multiple generations (Huertas et al., Citation2011). Similar experiments could be conducted to determine how the amount and composition of DOM released from different phytoplankton taxa will change as they adapt in response to the selection pressure of climate change.
Perhaps the greatest challenge to determine the effect of climate change on DOM release by phytoplankton is accounting for interactions between different abiotic and biotic components of marine ecosystems (Rost et al., Citation2008). Much of the work that has been carried out so far has looked at either warming or acidification in isolation, whereas in reality the ocean’s pH is decreasing as it warms. Gao et al. (Citation2012) found that high CO2 and high light interact synergistically to reduce the primary productivity of natural assemblages in the South China Sea. Interactions between organisms also affect the composition and amount of DOM produced by phytoplankton (Gärdes et al., Citation2011, 2012). The complexity of this challenge is illustrated by the evolution experiments discussed above: in situ selection pressures will be a product of warming, elevated CO2 and the resulting interactions between organisms. To date, evolution experiments have used acidification or warming as a selection pressure in isolation, as it is too complex to set up manageable experiments accounting for interactions. A good place to start to examine this complexity would be to determine the effect of both warming and acidification on phytoplankton growth and DOM release in simple laboratory monoculture experiments.
There is now sufficient work on the effect of climate change on phytoplankton ecology to enable some broad generalizations that can be used to generate hypotheses for future work. Overall, the future ocean will be warmer, more acidic and more stratified than it is at present. Increased stratification will result in a lower supply of nutrients to the photic zone. These stresses (elevated temperature, acidification, high light and low nutrients) all cause phytoplankton to release more DOM into the surrounding water, through a combination of processes associated with leakage and exudation (). In addition, low nutrient, stratified conditions will select for small phytoplankton, which tend to leak a greater proportion of their organic carbon to the surrounding water. Therefore, I hypothesize that a greater proportion of total phytoplankton primary production will be released into the surrounding water as DOM as the surface ocean warms and becomes more acidic. This will have implications for the entire marine carbon cycle: potentially less organic matter will be available for metazoan grazers and their predators, more organic matter will pass through microbial food webs, and the efficiency of the biological carbon pump will be reduced, as less POC will be exported from the euphotic and mesopelagic zones into the deep ocean.
Acknowledgements
This research was supported by the National Science Foundation (U.S.A.) under Grant No. OCE 0726369 from the Division of Ocean Sciences. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation. The author would like to thank two anonymous reviewers, Julie M. Rose and the students who helped him develop his ideas (particularly Jie Chen and Michael Pohlen, who generously shared their data).
References
- Abdullahi, A.S., Underwood, G.J.C. & Gretz, M.R. (2006). Extracellular matrix assembly in diatoms (Bacillariophyceae). V. Environmental effects on polysaccharide synthesis in the model diatom, Phaeodactylum tricornutum. Journal of Phycology, 42: 363–378.
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. & Walter P. (2008). Molecular Biology of the Cell. 5th ed. Garland Science, New York.
- Al-Hasan, R.H. & Fogg, G.E. (1987). Glycolate concentrations in relation to hydrography in Liverpool Bay. Marine Ecology Progress Series, 37: 305–307.
- Alldredge, A.L., Passow, U. & Logan B.E. (1993). The abundance and significance of a class of large, transparent organic particles in the ocean. Deep-Sea Research Part I, 40: 1131–1140.
- Aluwihare, L.I. & Repeta, D.J. (1999). A comparison of the chemical characteristics of oceanic DOM and extracellular DOM produced by marine algae. Marine Ecology Progress Series, 186: 105–117.
- Aluwihare, L.I., Repeta, D.J. & Chen, R.F. (1997). A major biopolymeric component to dissolved organic carbon in surface sea water. Nature, 387: 166–169.
- Anderson, T.R. & Williams, P.J.L. (1998). Modelling the seasonal cycle of dissolved organic carbon at station E-1 in the English Channel. Estuarine Coastal and Shelf Science, 46: 93–109.
- Armbrust, E.V., Berges, J.A., Bowler, C., Green, B.R., Martinez, D., Putnam, N.H., Zhou, S.G., Allen, A.E., Apt, K.E., Bechner, M., Brzezinski, M.A., Chaal, B.K., Chiovitti, A., Davis, A.K., Demarest, M.S., Detter, J.C., Glavina, T., Goodstein, D., Hadi, M.Z., Hellsten, U., Hildebrand, M., Jenkins, B.D., Jurka, J., Kapitonov, V.V., Kroger, N., Lau, W.W.Y., Lane, T.W., Larimer, F.W., Lippmeier, J.C., Lucas, S., Medina, M., Montsant, A., Obornik, M., Parker, M.S., Palenik, B., Pazour, G.J., Richardson, P.M., Rynearson, T.A., Saito, M.A., Schwartz, D.C., Thamatrakoln, K., Valentin, K., Vardi, A., Wilkerson, F.P. & Rokhsar, D.S. (2004). The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science, 306: 79–86.
- Arnold, S.R., Spracklen, D.V., Williams, J., Yassaa, N., Sciare, J., Bonsang, B., Gros, V., Peeken, I., Lewis, A.C., Alvain, S. & Moulin, C. (2009). Evaluation of the global oceanic isoprene source and its impacts on marine organic carbon aerosol. Atmospheric Chemistry and Physics, 9: 1253–1262.
- Arnosti, C., Grossart, H.P., Mühling, M., Joint, I. & Passow, U. (2011). Dynamics of extracellular enzyme activities in seawater under changed atmospheric pCO2: a mesocosm investigation. Aquatic Microbial Ecology, 64: 285–298.
- Arrigo, K.R. (2005). Marine microorganisms and global nutrient cycles. Nature, 437: 349–355.
- Atkinson, D., Ciotti, B.J. & Montagnes, D.J.S. (2003). Protists decrease in size linearly with temperature: ca. 2.5% degrees °C–1. Proceedings of the Royal Society B, 270: 2605–2611.
- Aumont, O., Maier-Reimer, E., Blain, S. & Monfray, P. (2003). An ecosystem model of the global ocean including Fe, Si, P colimitations. Global Biogeochemical Cycles, 17: 1060.
- Ayers, G.P. & Cainey, J.M. (2007). The CLAW hypothesis: a review of the major developments. Environmental Chemistry, 4: 366–374.
- Azam, F., Fenchel, T., Field, J.G., Gray, J.S., Meyerreil, L.A. & Thingstad, F. (1983). The ecological role of water-column microbes in the sea. Marine Ecology Progress Series, 10: 257–263.
- Badger, M.R., Andrews, T.J., Whitney, S.M., Ludwig, M., Yellowlees, D.C., Leggat, W. & Price, D. (1998). The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany, 76: 1052–1071.
- Baines, S.B. & Pace, M.L. (1991). The production of dissolved organic-matter by phytoplankton and its importance to bacteria – patterns across marine and fresh-water systems. Limnology and Oceanography, 36: 1078–1090.
- Baklouti, M., Diaz, F., Pinazo, C., Faure, V. & Queguiner, B. (2006). Investigation of mechanistic formulations depicting phytoplankton dynamics for models of marine pelagic ecosystems and description of a new model. Progress in Oceanography, 71: 1–33.
- Barnett, T.P., Pierce, D.W., Achutarao, K.M., Gleckler, P.J., Santer, B.D., Gregory, J.M. & Washington, W.M. (2005). Penetration of human-induced warming into the world's oceans. Science, 309: 284–287.
- Beardall, J. & Raven, J.A. (2004). The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia, 43: 26–40.
- Behrenfeld, M.J., O'Malley, R.T., Siegel, D.A., McClain, C.R., Sarmiento, J.L., Feldman, G.C., Milligan, A.J., Falkowski, P.G., Letelier, R.M. & Boss, E.S. (2006). Climate-driven trends in contemporary ocean productivity. Nature, 444: 752–755.
- Bell, W. & Mitchell, R. (1972). Chemotactic and growth responses of marine bacteria to algal extracellular products. Biological Bulletin, 143: 265–277.
- Bell, W.H., Lang, J.M. & Mitchell, R. (1974). Selective stimulation of marine bacteria by algal extracellular products. Limnology and Oceanography, 19: 833–839.
- Bellinger, B.J., Abdullahi, A.S., Gretz, M.R. & Underwood, G.J.C. (2005). Biofilm polymers: relationship between carbohydrate biopolymers from estuarine mudflats and unialgal cultures of benthic diatoms. Aquatic Microbial Ecology, 38: 169–180.
- Bellinger, B.J., Underwood, G.J.C., Ziegler, S.E. & Gretz, M.R. (2009). Significance of diatom-derived polymers in carbon flow dynamics within estuarine biofilms determined through isotopic enrichment. Aquatic Microbial Ecology, 55: 169–187.
- Benner, R. & Strom, M. (1993). A critical-evaluation of the analytical blank associated with DOC measurements by high-temperature catalytic-oxidation. Marine Chemistry, 41: 153–160.
- Berman-Frank, I., Rosenberg, G., Levitan, O., Haramaty, L. & Mari, X. (2007). Coupling between autocatalytic cell death and transparent exopolymeric particle production in the marine cyanobacterium Trichodesmium. Environmental Microbiology, 9: 1415–1422.
- Bettarel, Y., Kan, J., Wang, K., Williamson, K.E., Cooney, S., Ribblett, S., Chen, F., Wommack, K.E. & Coats, D.W. (2005). Isolation and preliminary characterisation of a small nuclear inclusion virus infecting the diatom Chaetoceros cf. gracilis. Aquatic Microbial Ecology, 40: 103–114.
- Bidle, K.D. & Falkowski, P.G. (2004). Cell death in planktonic, photosynthetic microorganisms. Nature Reviews Microbiology, 2: 643–655.
- Biddanda, B. & Benner, R. (1997). Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnology and Oceanography, 42: 506–518.
- Biersmith, A. & Benner, R. (1998). Carbohydrates in phytoplankton and freshly produced dissolved organic matter. Marine Chemistry, 63: 131–144.
- Billen, G., Joiris, C., Wijnant, J. & Gillain, G. (1980). Concentration and microbiological utilization of small organic-molecules in the Scheldt estuary, the Belgian coastal zone of the North Sea and the English Channel. Estuarine and Coastal Marine Science, 11: 279–294.
- Bjørnsen, P.K. (1988). Phytoplankton exudation of organic-matter – why do healthy cells do it. Limnology and Oceanography, 33: 151–154.
- Bonsang, B., Gros, V., Peeken, I., Yassaa, N., Bluhm, K., Zoellner, E., Sarda-Esteve, R. & Williams, J. (2010). Isoprene emission from phytoplankton monocultures: the relationship with chlorophyll-a, cell volume and carbon content. Environmental Chemistry, 7: 554–563.
- Bopp, L., Aumont, O., Cadule, P., Alvain, S. & Gehlen, M. (2005). Response of diatoms distribution to global warming and potential implications: a global model study. Geophysical Research Letters, 32: L19606.
- Borchard, C. & Engel, A. (2012). Organic matter exudation by Emiliania huxleyi under simulated future ocean conditions. Biogeosciences, 9: 3405–3423.
- Bowler, C., Allen, A.E., Badger, J.H., Grimwood, J., Jabbari, K., Kuo, A., Maheswari, U., Martens, C., Maumus, F., Otillar, R.P., Rayko, E., Salamov, A., Vandepoele, K., Beszteri, B., Gruber, A., Heijde, M., Katinka, M., Mock, T., Valentin, K., Verret, F., Berges, J.A., Brownlee, C., Cadoret, J.P., Chiovitti, A., Choi, C.J., Coesel, S., De Martino, A., Detter, J.C., Durkin, C., Falciatore, A., Fournet, J., Haruta, M., Huysman, M.J.J., Jenkins, B.D., Jiroutova, K., Jorgensen, R.E., Joubert, Y., Kaplan, A., Kroger, N., Kroth, P.G., La Roche, J., Lindquist, E., Lommer, M., Martin-Jezequel, V., Lopez, P.J., Lucas, S., Mangogna, M., McGinnis, K., Medlin, L.K., Montsant, A., Oudot-Le Secq, M.P., Napoli, C., Obornik, M., Parker, M.S., Petit, J.L., Porcel, B.M., Poulsen, N., Robison, M., Rychlewski, L., Rynearson, T.A., Schmutz, J., Shapiro, H., Siaut, M., Stanley, M., Sussman, M.R., Taylor, A.R., Vardi, A., von Dassow, P., Vyverman, W., Willis, A., Wyrwicz, L.S., Rokhsar, D.S., Weissenbach, J., Armbrust, E.V., Green, B.R., van de Peer, Y. & Grigoriev, I.V. (2008). The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature, 456: 239–244.
- Boyce, D.G., Lewis, M.R. & Worm, B. (2010a). Global phytoplankton decline over the past century. Nature, 466: 591–596.
- Boyce, D.G., Lewis, M.R. & Worm, B. (2010b). Boyce et al. reply. Nature, 472: E8–E9.
- Bratbak, G., Egge, J.K. & Heldal, M. (1993). Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Marine Ecology Progress Series, 93: 39–48.
- Bronk, D.A., Glibert, P.M. & Ward, B.B. (1994). Nitrogen uptake, dissolved organic nitrogen release, and new production. Science, 265: 1843–1846.
- Bronk, D.A., See, J.H., Bradley, P. & Killberg, L. (2007). DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences, 4: 283–296.
- Bruckner, C.G., Rehm, C., Grossart, H.P. & Kroth, P.G. (2011). Growth and release of extracellular organic compounds by benthic diatoms depend on interactions with bacteria. Environmental Microbiology, 13: 1052–1063.
- Brum, J.R. (2005). Concentration, production and turnover of viruses and dissolved DNA pools at Stn ALOHA, North Pacific Subtropical Gyre. Aquatic Microbial Ecology, 41: 103–113.
- Burd, A.B. & Jackson, G.A. (2009). Particle aggregation. Annual Review of Marine Science, 1: 65–90.
- Caldeira, K. & Wickett, M.E. (2003). Anthropogenic carbon and ocean pH. Nature, 425: 365.
- Caron, D.A. (2000). Symbiosis and mixotrophy among pelagic organisms. In Microbial Ecology of the Oceans (Kirchman, D.L., editor), 495–523. Wiley–Liss, New York.
- Carr, M. E., Friedrichs, M. A. M., Schmeltz, M., Aita, M. N., Antoine, D., Arrigo, K. R., Asanuma, I., Aumont, O., Barber, R., Behrenfeld, M., Bidigare, R., Buitenhuis, E. T., Campbell, J., Ciotti, A., Dierssen, H., Dowell, M., Dunne, J., Esaias, W., Gentili, B., Gregg, W., Groom, S., Hoepffner, N., Ishizaka, J., Kameda, T., Le Quere, C., Lohrenz, S., Marra, J., Melin, F., Moore, K., Morel, A., Reddy, T. E., Ryan, J., Scardi, M., Smyth, T., Turpie, K., Tilstone, G., Waters, K. & Yamanaka, Y. (2006). A comparison of global estimates of marine primary production from ocean color. Deep-Sea Research II, 53: 741–770.
- Charlson, R.J., Lovelock, J.E., Andreae, M.O. & Warren, S.G. (1987). Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate. Nature, 326: 655–661.
- Chen, W.H. & Wangersky, P.J. (1996). Production of dissolved organic carbon in phytoplankton cultures as measured by high-temperature catalytic oxidation and ultraviolet photo-oxidation methods. Journal of Plankton Research, 18: 1201–1211.
- Chin, W.C., Orellana, M.V., Quesada, I. & Verdugo, P. (2004). Secretion in unicellular marine phytoplankton: demonstration of regulated exocytosis in Phaeocystis globosa. Plant and Cell Physiology 45: 535–542.
- Christian, J. R. & Anderson, T.R. (2002). Modeling DOM biogeochemistry. In Biogeochemistry of marine dissolved organic matter (Hansell, D.A. & Carlson, C.A., editors), 717–755. Academic Press, San Diego, CA.
- Claquin, P., Probert, I., Lefebvre, S. & Veron, B. (2008). Effects of temperature on photosynthetic parameters and TEP production in eight species of marine microalgae. Aquatic Microbial Ecology, 51: 1–11.
- Coble, P.G. (1996). Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Marine Chemistry, 51: 325–346.
- Collins, S. (2012). Evolution on acid. Nature Geoscience, 5: 310–311.
- Collins, S. & Bell, G. (2004). Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature, 431: 566–569.
- Crawfurd, K.J., Raven, J.A., Wheeler, G.L., Baxter, E.J. & Joint, I. (2011). The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLOS One, 6: e26695.
- Decho, A.W. (1990). Microbial exopolymer secretions in ocean environments – their role(s) in food webs and marine processes. Oceanography and Marine Biology, 28: 73–153.
- Doney, S.C. (2006). Plankton in a warmer world. Nature, 444: 695–696.
- Dring, M. J. (1982). The biology of marine plants. Edward Arnold, London.
- Dron, A., Rabouille, S., Claquin, P., Chang, P., Raimbault, V., Talec, A. & Sciandra, A. (2012). Light:dark (12:12 h) quantification of carbohydrate fluxes in Crocosphaera watsonii. Aquatic Microbial Ecology, 68: 43–55.
- Droop, M.R. (1968). Vitamin B12 and marine ecology. IV. Kinetics of uptake growth and inhibition in Monochrysis lutheri. Journal of the Marine Biological Association of the United Kingdom, 48: 689–733.
- Droop, M.R. (1983). 25 years of algal growth-kinetics – a personal view. Botanica Marina, 26: 99–112.
- Ducklow, H.W. (1983). Production and fate of bacteria in the oceans. Bioscience, 33: 494–501.
- Dupont, C.L., Nelson, R.K., Bashir, S., Moffett, J.W. & Ahner, B.A. (2004). Novel copper-binding and nitrogen-rich thiols produced and exuded by Emiliania huxleyi. Limnology and Oceanography, 49: 1754–1762.
- Dyhrman, S.T., Jenkins, B.D., Rynearson, T.A., Saito, M.A., Mercier, M.L., Alexander, H., Whitney, L.P., Drzewianowski, A., Bulygin, V.V., Bertrand, E.M., Wu, Z.J., Benitez-Nelson, C. & Heithoff, A. (2012). The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLOS One, 7: e33768.
- Egge, J.K., Thingstad, T.F., Larsen, A., Engel, A., Wohlers, J., Bellerby, R.G.J. & Riebesell, U. (2009). Primary production during nutrient-induced blooms at elevated CO2 concentrations. Biogeosciences, 6: 877–885.
- Emerson, S.R. & Hedges, J.I. (2008). Chemical oceanography and the marine carbon cycle. Cambridge University Press, New York, NY.
- Engel, A. (2000). The role of transparent exopolymer particles (TEP) in the increase in apparent particle stickiness (α) during the decline of a diatom bloom. Journal of Plankton Research, 22: 485–497.
- Engel, A. (2004). Distribution of transparent exopolymer particles (TEP) in the northeast Atlantic Ocean and their potential significance for aggregation processes. Deep-Sea Research Part I – Oceanographic Research Papers, 51: 83–92.
- Engel, A., Goldthwait, S., Passow, U. & Alldredge, A. (2002). Temporal decoupling of carbon and nitrogen dynamics in a mesocosm diatom bloom. Limnology and Oceanography, 47: 753–761.
- Engel, A., Händel, N., Wohlers, J., Lunau, M., Grossart, H.P., Sommer, U. & Riebesell, U. (2011). Effects of sea surface warming on the production and composition of dissolved organic matter during phytoplankton blooms: results from a mesocosm study. Journal of Plankton Research, 33: 357–372.
- Engel, A., Borchard, C., Piontek, J., Schulz, K.G., Riebesell, U. & Bellerby, R. (2013). CO2 increases 14C primary production in an Arctic plankton community. Biogeosciences, 10: 1291–1308.
- Falkowski, P.G. (2000). Rationalizing elemental ratios in unicellular algae. Journal of Phycology, 36: 3–6.
- Falkowski, P.G. & Oliver, M.J. (2007). Mix and match: how climate selects phytoplankton. Nature Reviews Microbiology, 5: 813–819.
- Falkowski, P.G. & Raven, J.A. (1997). Aquatic photosynthesis. Blackwell Science, Oxford.
- Falkowski, P.G. & Raven J.A. (2007) Aquatic photosynthesis. 2nd ed. Princeton University Press, Princeton, NJ.
- Falkowski, P., Scholes, R. J., Boyle, E., Canadell, J., Canfield, D., Elser, J., Gruber, N., Hibbard, K., Hogberg, P., Linder, S., Mackenzie, F. T., Moore, B., Pedersen, T., Rosenthal, Y., Seitzinger, S., Smetacek, V. & Steffen, W. (2000). The global carbon cycle: a test of our knowledge of Earth as a system. Science, 290: 291–296.
- Field, C.B., Behrenfeld, M.J., Randerson, J.T. & Falkowski, P. (1998). Primary production of the biosphere: integrating terrestrial and oceanic components. Science, 281: 237–240.
- Finkel, Z.V., Beardall, J., Flynn, K.J., Quigg, A., Rees, T.A.V. & Raven, J.A. (2010). Phytoplankton in a changing world: cell size and elemental stoichiometry. Journal of Plankton Research, 32: 119–137.
- Fistarol, G.O., Legrand, C. & Graneli, E. (2005). Allelopathic effect on a nutrient-limited phytoplankton species. Aquatic Microbial Ecology, 41: 153–161.
- Flemming, H.C. & Wingender, J. (2010). The biofilm matrix. Nature Reviews Microbiology, 8: 623–633.
- Flynn, K.J., Clark, D.R. & Xue, Y. (2008). Modeling the release of dissolved organic matter by phytoplankton. Journal of Phycology, 44: 1171–1187.
- Flynn, K.J., Blackford, J.C., Baird, M.E., Raven, J.A., Clark, D.R., Beardall, J., Brownlee, C., Fabian, H. & Wheeler, G.L. (2012). Changes in pH at the exterior surface of plankton with ocean acidification. Nature Climate Change, 2: 510–513.
- Fogg, G.E. (1983). The ecological significance of extracellular products of phytoplankton photosynthesis. Botanica Marina, 26: 3–14.
- Franklin, D.J., Brussaard, C.P.D. & Berges, J.A. (2006). What is the role and nature of programmed cell death in phytoplankton ecology? European Journal of Phycology, 41: 1–14.
- Franklin, D.J., Airs, R.L., Fernandes, M., Bell, T.G., Bongaerts, R.J., Berges, J.A. & Malin, G. (2012). Identification of senescence and death in Emiliania huxleyi and Thalassiosira pseudonana: cell staining, chlorophyll alterations, and dimethylsulfoniopropionate (DMSP) metabolism. Limnology and Oceanography, 57: 305–317.
- Fukao, T., Kimoto, K. & Kotani, Y. (2010). Production of transparent exopolymer particles by four diatom species. Fisheries Science, 76: 755–760.
- Gao, K.S., Helbling, E.W., Häder, D.-P. & Hutchins, D.A. (2012). Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming. Marine Ecology Progress Series, 470: 167–189.
- Gärdes, A., Kaeppel, E., Shehzad, A., Seebah, S., Teeling, H., Yarza, P., Glöckner, F.O., Grossart, H.P. & Ullrich, M.S. (2010). Complete genome sequence of Marinobacter adhaerens type strain (HP15), a diatom-interacting marine microorganism. Standards in Genomic Sciences, 3: 97–107.
- Gärdes, A., Iversen, M.H., Grossart, H.P., Passow, U. & Ullrich, M.S. (2011). Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME Journal, 5: 436–445.
- Gärdes, A., Ramaye, Y., Grossart, H.P., Passow, U. & Ullrich, M.S. (2012). Effects of Marinobacter adhaerens HP15 on polymer exudation by Thalassiosira weissflogii at different N:P ratios. Marine Ecology Progress Series, 461: 1–14.
- Geider, R.J. & La Roche, J. (2002). Redfield revisited: variability of C : N : P in marine microalgae and its biochemical basis. European Journal of Phycology, 37: 1–17.
- Geider, R.J., Macintyre, H.L. & Kana, T.M. (1998). A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnology and Oceanography, 43: 679–694.
- Giordano, M., Bardall, J. & Raven, J.A. (2005). CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology, 56: 99–131.
- Glibert, P.M. & Bronk, D.A. (1994). Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp. Applied and Environmental Microbiology, 60: 3996–4000.
- Gobler, C.J., Hutchins, D.A., Fisher, N.S., Cosper, E.M. & Sanudo-Wilhelmy, S.A. (1997). Release and bioavailability of C, N, P, Se, and Fe following viral lysis of a marine chrysophyte. Limnology and Oceanography, 42: 1492–1504.
- Gondwe, M., Krol, M., Gieskes, W., Klaassen, W. & de Baar, H. (2003). The contribution of ocean-leaving DMS to the global atmospheric burdens of DMS, MSA, SO2, and NSS SO4=. Global Biogeochemical Cycles, 17: 1056.
- Granum, E., Kirkvold, S. & Myklestad, S.M. (2002). Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: diel variations and effects of N depletion. Marine Ecology Progress Series, 242: 83–94.
- Gross, E.M. (2003). Allelopathy of aquatic autotrophs. Critical Reviews in Plant Sciences, 22: 313–339.
- Guillard, R.R.L. & Wangersky, P.J. (1958). The production of extracellular carbohydrates by some marine flagellates. Limnology and Oceanography, 3: 449–454.
- Hansell, D.A., Carlson, C.A., Repeta, D.J. & Schlitzer, R. (2009). Dissolved organic matter in the ocean: a controversy stimulates new insights. Oceanography, 22: 190–201.
- Harlay, J., De Bodt, C., Engel, A., Jansen, S., D'Hoop, Q., Piontek, J., Van Oostende, N., Groom, S., Sabbe, K. & Chou, L. (2009). Abundance and size distribution of transparent exopolymer particles (TEP) in a coccolithophorid bloom in the northern Bay of Biscay. Deep-Sea Research I, 56: 1251–1265.
- Hassler, C.S., Schoemann, V., Nichols, C.M., Butler, E.C.V. & Boyd, P.W. (2011). Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proceedings of the National Academy of Sciences of the United States of America, 108: 1076–1081.
- Hasegawa, T., Koike, I. & Mukai, H. (2000). Release of dissolved organic nitrogen by size-fractionated natural planktonic assemblages in coastal waters. Marine Ecology Progress Series, 198: 43–49.
- Hedges, J.I., Bergamaschi, B.A. & Benner, R. (1993). Comparative analyses of DOC and DON in natural-waters. Marine Chemistry, 41: 121–134.
- Hellebust, J.A. (1965). Excretion of some organic compounds by marine phytoplankton. Limnology and Oceanography, 10: 192–206.
- Hellebust, J A. (1974). Extracellular products. In Algal physiology and biochemistry (Stewart, W.D.P., editor), 838–863. University of California Press, Berkeley, CA.
- Hoppe, C.J.M., Langer, G. & Rost, B. (2011). Emiliania huxleyi shows identical responses to elevated pCO2 in TA and DIC manipulations. Journal of Experimental Marine Biology and Ecology, 406: 54–62.
- Hoppe, C.J.M., Langer, G., Rokitta, S.D., Wolf-Gladrow, D.A. & Rost, B. (2012). Implications of observed inconsistencies in carbonate chemistry measurements for ocean acidification studies. Biogeosciences 9: 2401–2405.
- Hu, S.H. & Smith, W.O. (1998). The effects of irradiance on nitrate uptake and dissolved organic nitrogen release by phytoplankton in the Ross Sea. Continental Shelf Research, 18: 971–990.
- Huertas, I.E., Rouco, M., López-Rodas, V. & Costas, E. (2011). Warming will affect phytoplankton differently: evidence through a mechanistic approach. Proceedings of the Royal Society B, 278: 3534–3543.
- Hurd, C.L., Hepburn, C.D., Currie, K.I., Raven, J.A. & Hunter, K.A. (2009). Testing the effects of ocean acidification on algal metabolism: considerations for experimental designs. Journal of Phycology, 45: 1236–1251.
- IPCC (2013) Summary for policymakers. Climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V. & Midgley, P.M., editors). Cambridge University Press, Cambridge.
- Kamjunke, N. & Tittel, J. (2009). Mixotrophic algae constrain the loss of organic carbon by exudation. Journal of Phycology, 45: 807–811.
- Karl, D.M., Hebel, D.V., Björkman, K. & Letelier, R.M. (1998). The role of dissolved organic matter release in the productivity of the oligotrophic North Pacific Ocean. Limnology and Oceanography, 43: 1270–1286.
- Keller, D.P. & Hood, R.R. (2011). Modeling the seasonal autochthonous sources of dissolved organic carbon and nitrogen in the upper Chesapeake Bay. Ecological Modelling, 222: 1139–1162.
- Kiene, R.P., Linn, L.J. & Bruton, J.A. (2000). New and important roles for DMSP in marine microbial communities. Journal of Sea Research, 43: 209–224.
- Kim, J.M., Lee, K., Shin, K., Yang, E.J., Engel, A., Karl, D.M. & Kim, H.C. (2011). Shifts in biogenic carbon flow from particulate to dissolved forms under high carbon dioxide and warm ocean conditions. Geophysical Research Letters, 38: L08612.
- Kirchman, D.L. (1999). Oceanography – Phytoplankton death in the sea. Nature, 398: 293–294.
- Koeve, W. & Oschlies, A. (2012). Potential impact of DOM accumulation on fCO2 and carbonate ion computations in ocean acidification experiments. Biogeosciences, 9: 3787–3798.
- Kragh, T. & Søndergaard, M. (2009). Production and decomposition of new DOC by marine plankton communities: carbohydrates, refractory components and nutrient limitation. Biogeochemistry, 96: 177–187.
- Kriest, I. & Oschlies, A. (2007). Modelling the effect of cell-size-dependent nutrient uptake and exudation on phytoplankton size spectra. Deep-Sea Research Part I – Oceanographic Research Papers, 54: 1593–1618.
- Kujawinski, E.B. (2011). The impact of microbial metabolism on marine dissolved organic matter. Annual Review of Marine Science, 3: 567–599.
- Lagaria, A., Psarra, S., Lefèvre, D., Van Wambeke, F., Courties, C., Pujo-Pay, M., Oriol, L., Tanaka, T. & Christaki, U. (2011). The effects of nutrient additions on particulate and dissolved primary production and metabolic state in surface waters of three Mediterranean eddies. Biogeosciences, 8: 2595–2607.
- Lancelot, C. (1984). Extracellular release of small and large molecules by phytoplankton in the southern bight of the North Sea. Estuarine Coastal and Shelf Science, 18: 65–77.
- Laroche, D., Vézina, A.F., Levasseur, M., Gosselin, M., Stefels, J., Keller, M.D., Matrai, P.A. & Kwint, R.L.J. (1999). DMSP synthesis and exudation in phytoplankton: a modeling approach. Marine Ecology Progress Series, 180: 37–49.
- Leão, P.N., Vasconcelos, M. & Vasconcelos, V.M. (2009). Allelopathy in freshwater cyanobacteria. Critical Reviews in Microbiology, 35: 271–282.
- Leboulanger, C., Descolasgros, C. & Jupin, H. (1994). HPLC determination of glycolic acid in seawater – an estimation of phytoplankton photorespiration in the Gulf of Lions, western Mediterranean Sea. Journal of Plankton Research, 16: 897–903.
- Leboulanger, C., Oriol, L., Jupin, H. & Descolas-Gros, C. (1997). Diel variability of glycolate in the eastern tropical Atlantic Ocean. Deep-Sea Research I, 44: 2131–2139.
- Leboulanger, C., Serve, L., Comellas, L. & Jupin, H. (1998a). Determination of glycolic acid released from marine phytoplankton by post-derivatization gas chromatography mass spectrometry. Phytochemical Analysis, 9: 5–9.
- Leboulanger, C., Martin-Jézéquel, V., Descolas-Gros, C., Sciandra, A. & Jupin, H.J. (1998b). Photorespiration in continuous culture of Dunaliella tertiolecta (Chlorophyta): relationships between serine, glycine, and extracellular glycolate. Journal of Phycology, 34: 651–654.
- Legrand, C., Rengefors, K., Fistarol, G.O. & Graneli, E. (2003). Allelopathy in phytoplankton – biochemical, ecological and evolutionary aspects. Phycologia, 42: 406–419.
- Leterme, S.C., Edwards, M., Seuront, L., Attrill, M.J., Reid, P.C. & John, A.W.G. (2005). Decadal basin-scale changes in diatoms, dinoflagellates, and phytoplankton color across the North Atlantic. Limnology and Oceanography, 50: 1244–1253.
- Lewin, J.C. & Lewin, R.A. (1960). Auxotrophy and heterotrophy in marine littoral diatoms. Canadian Journal of Microbiology, 6: 127–133.
- Levitus, S., Antonov, J.I., Boyer, T.P. & Stephens, C. (2000). Warming of the world ocean. Science, 287: 2225–2229.
- Lohbeck, K.T., Riebesell, U. & Reusch, T.B.H. (2012). Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience, 5: 346–351.
- Lombardi, A.T. & Wangersky, P.J. (1991). Influence of phosphorus and silicon on lipid class production by the marine diatom Chaetoceros gracilis grown in turbidostat cage cultures. Marine Ecology Progress Series, 77: 39–47.
- Long, R.A. & Azam, F. (1996). Abundant protein-containing particles in the sea. Aquatic Microbial Ecology, 10: 213–221.
- Longhurst, A., Sathyendranath, S., Platt, T. & Caverhill, C. (1995). An estimate of global primary production in the ocean from satellite radiometer data, Journal of Plankton Research, 17: 1245–1271.
- López-Sandoval, D.C., Marañón, E., Fernández, A., González, J., Gasol, J.M., Lekunberri, I., Varela, M., Calvo-Díaz, A., Morán, X.A.G., Álvarez-Salgado, X.A. & Figueiras, F.G. (2010). Particulate and dissolved primary production by contrasting phytoplankton assemblages during mesocosm experiments in the Ria de Vigo (NW Spain). Journal of Plankton Research, 32: 1231–1240.
- López-Sandoval, D.C., Fernández, A. & Marañón, E. (2011). Dissolved and particulate primary production along a longitudinal gradient in the Mediterranean Sea. Biogeosciences, 8: 815–825.
- López-Sandoval, D.C., Rodríguez-Ramos, T., Cermeño, P. & Marañón, E. (2013). Exudation of organic carbon by marine phytoplankton: dependence on taxon and cell size. Marine Ecology Progress Series, 477: 53–60.
- Lyman, J.M., Good, S.A., Gouretski, V.V., Ishii, M., Johnson, G.C., Palmer, M.D., Smith, D.M. & Willis, J.K. (2010). Robust warming of the global upper ocean. Nature, 465: 334–337.
- Mackas, D.L. (2010). Does blending of chlorophyll data bias temporal trend? Nature, 472: E4–E5.
- Magaletti, E., Urbani, R., Sist, P., Ferrari, C.R. & Cicero, A.M. (2004). Abundance and chemical characterization of extracellular carbohydrates released by the marine diatom Cylindrotheca fusiformis under N- and P-limitation. European Journal of Phycology, 39: 133–142.
- Mague, T.H., Friberg, E., Hughes, D.J. & Morris, I. (1980). Extracellular release of carbon by marine-phytoplankton – a physiological approach. Limnology and Oceanography, 25: 262–79.
- Malej, A. & Harris, R.P. (1993). Inhibition of copepod grazing by diatom exudates – a factor in the development of mucus aggregates. Marine Ecology Progress Series, 96: 33–42.
- Malin, G. & Kirst, G.O. (1997). Algal production of dimethyl sulfide and its atmospheric role. Journal of Phycology, 33: 889–896.
- Malin, G., Wilson, W.H., Bratbak, G., Liss, P.S. & Mann, N.H. (1998). Elevated production of dimethylsulfide resulting from viral infection of cultures of Phaeocystis pouchetii. Limnology and Oceanography, 43: 1389–1393.
- Marañón, E., Cermeño, P., Fernández, E., Rodríguez, J. & Zabala, L. (2004). Significance and mechanisms of photosynthetic production of dissolved organic carbon in a coastal eutrophic ecosystem. Limnology and Oceanography, 49: 1652–1666.
- Marañón, E., Cermeño, P. & Pérez, V. (2005). Continuity in the photosynthetic production of dissolved organic carbon from eutrophic to oligotrophic waters. Marine Ecology Progress Series, 299: 7–17.
- Marañón, E., Cermeño, P., Latasa, M. & Tadonléké, R.D. (2012). Temperature, resources, and phytoplankton size structure in the ocean. Limnology and Oceanography, 57: 1266–1278.
- Mari, X. (1999). Carbon content and C : N ratio of transparent exopolymeric particles (TEP) produced by bubbling exudates of diatoms. Marine Ecology Progress Series, 183: 59–71.
- Mari, X. (2008). Does ocean acidification induce an upward flux of marine aggregates? Biogeosciences, 5: 1023–1031.
- Marinov, I., Doney, S.C. & Lima, I.D. (2010). Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature and light. Biogeosciences, 7: 3941–3959.
- Marker, A.F.H. (1965). Extracellular carbohydrate liberation in flagellates Isochrysis galbana and Prymnesium parvum. Journal of the Marine Biological Association of the United Kingdom, 45: 755–772.
- Mayali, X., Franks, P.J.S. & Burton, R.S. (2011). Temporal attachment dynamics by distinct bacterial taxa during a dinoflagellate bloom. Aquatic Microbial Ecology 63: 111–122.
- McQuatters-Gollop, A., Reid, P. C., Edwards, M., Burkill, P. H., Castellani, C., Batten, S., Gieskes, W., Beare, D., Bidigare, R. R., Head, E., Johnson, R., Kahru, M., Koslow, J.A. & Pena, A. (2010). Is there a decline in marine phytoplankton? Nature, 472: E6–E7.
- Møller, E.F. (2007). Production of dissolved organic carbon by sloppy feeding in the copepods Acartia tonsa, Centropages typicus, and Temora longicornis. Limnology and Oceanography, 52: 79–84.
- Møller, E.F., Thor, P. & Nielsen, T.G. (2003). Production of DOC by Calanus finmarchicus, C. glacialis and C. hyperboreus through sloppy feeding and leakage from fecal pellets. Marine Ecology Progress Series, 262: 185–191.
- Mongin, M., Nelson, D.M., Pondaven, P., Brzezinski, M.A. & Treguer, P. (2003). Simulation of upper-ocean biogeochemistry with a flexible-composition phytoplankton model: C, N and Si cycling in the western Sargasso Sea. Deep-Sea Research Part I – Oceanographic Research Papers, 50: 1445–1480.
- Montagnes, D.J.S. & Franklin, D.J. (2001). Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: reconsidering some paradigms. Limnology and Oceanography, 46: 2008–2018.
- Mopper, K., Zhou, J.A., Ramana, K.S., Passow, U., Dam, H.G. & Drapeau, D.T. (1995). The role of surface-active carbohydrates in the flocculation of a diatom bloom in a mesocosm. Deep-Sea Research II, 42: 47–73.
- Morán, X.A.G. & Estrada, M. (2001). Short-term variability of photosynthetic parameters and particulate and dissolved primary production in the Alboran Sea (SW Mediterranean). Marine Ecology Progress Series, 212: 53–67.
- Morán, X.A.G. & Estrada, M. (2002). Phytoplanktonic DOC and POC production in the Bransfield and Gerlache Straits as derived from kinetic experiments of C-14 incorporation. Deep-Sea Research II, 49: 769–786.
- Morán, X.A.G., Gasol, J.M., Arin, L. & Estrada, M. (1999). A comparison between glass fiber and membrane filters for the estimation of phytoplankton POC and DOC production. Marine Ecology Progress Series, 187: 31–41.
- Morán, X.A.G., Gasol, J.M., Pedrós-Alió, C. & Estrada, M. (2001). Dissolved and particulate primary production and bacterial production in offshore Antarctic waters during austral summer: coupled or uncoupled? Marine Ecology Progress Series, 222: 25–39.
- Morán, X.A.G., Gasol, J.M., Pedrós-Alió, C. & Estrada, M. (2002a). Partitioning of phytoplanktonic organic carbon production and bacterial production along a coastal-offshore gradient in the NE Atlantic during different hydrographic regimes. Aquatic Microbial Ecology, 29: 239–252.
- Morán, X.A.G., Estrada, M., Gasol, J.M. & Pedrós-Alió, C. (2002b). Dissolved primary production and the strength of phytoplankton bacterioplankton coupling in contrasting marine regions. Microbial Ecology, 44: 217–223.
- Morán, X.A.G., Sebástian, M., Pedrós-Alió, C. & Estrada, M. (2006). Response of Southern Ocean phytoplankton and bacterioplankton production to short-term experimental warming. Limnology and Oceanography, 51: 1791–1800.
- Morán, X.A.G., López-Urrutia, A., Calvo-Díaz, A. & Li, W.K.W. (2010). Increasing importance of small phytoplankton in a warmer ocean. Global Change Biology, 16: 1137–1144.
- Morel, F.M.M., Cox, E.H., Kraepiel, A.M.L., Lane, T.W., Milligan, A.J., Schaperdoth, I., Reinfelder, J.R. & Tortell, P.D. (2002). Acquisition of inorganic carbon by the marine diatom Thalassiosira weissflogii. Functional Plant Biology, 29: 301–308.
- Myklestad, S. (1974). Production of carbohydrates by marine planktonic diatoms. I. Comparison of nine different species in culture. Journal of Experimental Marine Biology and Ecology, 15: 261–274.
- Myklestad, S. (1977). Production of carbohydrates by marine planktonic diatoms. II. Influence of N/P ratio in growth medium on assimilation ratio, growth-rate, and production of cellular and extracellular carbohydrates by Chaetoceros affinis var. willei (Gran) Hustedt and Skeletonema costatum (Grev.) Cleve. Journal of Experimental Marine Biology and Ecology, 29: 161–179.
- Myklestad, S.M. (1995). Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Science of the Total Environment, 165: 155–164.
- Myklestad, S.M. (2000). Dissolved organic carbon from phytoplankton. In The handbook of environmental chemistry (D). Marine chemistry (Wangersky, P., editor), 111–148. Springer, Berlin.
- Myklestad, S. & Haug, A (1972). Production of carbohydrates by the marine diatom Chaetoceros affinis var. willei (Gran) Hustedt. I. Effect of the concentration of nutrients in the culture medium. Journal of Experimental Marine Biology and Ecology, 9:125–136.
- Myklestad, S., Holm-Hansen, O., Vårum, K.M. & Volcani, B.E. (1989). Rate of release of extracellular amino acids and carbohydrates from the marine diatom Chaetoceros affinis. Journal of Plankton Research, 11: 763–773.
- Nagata, T. (2000). Production mechanisms of dissolved organic matter. In Microbial ecology of the oceans (Kirchman, D.L., editor), 121–152. Wiley–Liss, New York.
- Obernosterer, I. & Herndl, G.J. (1995). Phytoplankton extracellular release and bacterial growth: dependence on the inorganic N : P ratio. Marine Ecology Progress Series, 116: 247–257.
- Orellana, M.V., Matrai, P.A., Janer, M. & Rauschenberg, C.D. (2011). Dimethylsulfoniopropionate storage in Phaeocystis (Prymnesiophyceae) secretory vesicles. Journal of Phycology, 47: 112–117.
- Palmer, P.I. & Shaw, S.L. (2005). Quantifying global marine isoprene fluxes using MODIS chlorophyll observations. Geophysical Research Letters, 32: L09805.
- Parrish, C.C. (1987). Time series of particulate and dissolved lipid classes during spring phytoplankton blooms in Bedford Basin, a marine inlet. Marine Ecology Progress Series, 35: 129–139.
- Parrish, C.C. & Wangersky, P.J. (1987). Particulate and dissolved lipid classes in cultures of Phaeodactylum tricornutum grown in cage culture turbidostats with a range of nitrogen supply rates. Marine Ecology Progress Series, 35: 119–128.
- Parrish, C.C., Bodennec, G. & Gentien, P. (1994). Time courses of intracellular and extracellular lipid classes in batch cultures of the toxic dinoflagellate, Gymnodinium cf. nagasakiense. Marine Chemistry, 48: 71–82.
- Passow, U. (2000). Formation of transparent exopolymer particles, TEP, from dissolved precursor material. Marine Ecology Progress Series, 192: 1–11.
- Passow, U. (2002a). Transparent exopolymer particles (TEP) in aquatic environments. Progress in Oceanography, 55: 287–333.
- Passow, U. (2002b). Production of transparent exopolymer particles (TEP) by phyto- and bacterioplankton. Marine Ecology Progress Series, 236: 1–12.
- Passow, U. (2012). The abiotic formation of TEP under different ocean acidification scenarios. Marine Chemistry, 128: 72–80.
- Passow, U. & Alldredge, A.L. (1995). A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnology and Oceanography, 40: 1326–1335.
- Passow, U. & Carlson, C.A. (2012). The biological pump in a high CO2 world. Marine Ecology Progress Series, 470: 249–271.
- Passow, U., Alldredge, A.L. & Logan, B.E. (1994). The role of particulate carbohydrate exudates in the flocculation of diatom blooms. Deep-Sea Research I, 41: 335–357.
- Pete, R., Davidson, K., Hart, M.C., Gutierrez, T. & Miller, A.E.J. (2010). Diatom derived dissolved organic matter as a driver of bacterial productivity: the role of nutrient limitation. Journal of Experimental Marine Biology and Ecology, 391: 20–26.
- Peter, K.H. & Sommer, U (2013). Phytoplankton cell size reduction in response to warming mediated by nutrient limitation. PLoS ONE, 8: e71528.
- Piontek, J., Händel, N., Langer, G., Wohlers, J., Riebesell, U. & Engel, A. (2009). Effects of rising temperature on the formation and microbial degradation of marine diatom aggregates. Aquatic Microbial Ecology, 54: 305–318.
- Piontek, J., Lunau, M., Händel, N., Borchard, C., Wurst, M. & Engel, A. (2010). Acidification increases microbial polysaccharide degradation in the ocean. Biogeosciences, 7: 1615–1624.
- Polovina, J.J., Howell, E.A. & Abecassis, M. (2008). Ocean's least productive waters are expanding. Geophysical Research Letters, 35: L03618.
- Poulet, S.A. & Martin-Jézéquel, V. (1983). Relationships between dissolved free amino acids, chemical composition and growth of the marine diatom Chaetoceros debile. Marine Biology, 77: 93–100.
- Prince, E.K., Poulson, K.L., Myers, T.L., Sieg, R.D. & Kubanek, J. (2010). Characterization of allelopathic compounds from the red tide dinoflagellate Karenia brevis. Harmful Algae, 10: 39–48.
- Puskaric, S. & Mortain-Bertrand, A. (2003). Physiology of diatom Skeletonema costatum (Grev.) Cleve photosynthetic extracellular release: evidence for a novel coupling between marine bacteria and phytoplankton. Journal of Plankton Research, 25: 1227–1235.
- Quigg, A., Finkel, Z.V., Irwin, A.J., Rosenthal, Y., Ho, T.Y., Reinfelder, J.R., Schofield, O., Morel, F.M.M. & Falkowski, P.G. (2003). The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature, 425: 291–294.
- Quinn, P.K. & Bates, T.S. (2011). The case against climate regulation via oceanic phytoplankton sulphur emissions. Nature, 480: 51–56.
- Ramus, J. (1977). Alcian blue – quantitative aqueous assay for algal acid and sulfated polysaccharides. Journal of Phycology, 13: 345–348.
- Raven, J.A. (1997). Inorganic carbon acquisition by marine autotrophs. Advances in Botanical Research, 27: 85–209.
- Repeta, D.J. & Aluwihare, L.I. (2006). Radiocarbon analysis of neutral sugars in high-molecular-weight dissolved organic carbon: implications for organic carbon cycling. Limnology and Oceanography, 51: 1045–1053.
- Rice, E.L. (1979). Allelopathy – update. Botanical Review, 45: 15–109.
- Riebesell, U. (2004). Effects of CO2 enrichment on marine phytoplankton. Journal of Oceanography, 60: 719–729.
- Riebesell, U., Wolf-Gladrow, D.A. & Smetacek, V. (1993). Carbon dioxide limitation of marine phytoplankton growth rates. Nature, 361: 249–251.
- Riebesell, U., Schulz, K.G., Bellerby, R.G.J., Botros, M., Fritsche, P., Meyerhofer, M., Neill, C., Nondal, G., Oschlies, A., Wohlers, J. & Zöllner, E. (2007). Enhanced biological carbon consumption in a high CO2 ocean. Nature, 450: 545–548.
- Riemann, L., Holmfeldt, K. & Titelman, J. (2009). Importance of viral lysis and dissolved DNA for bacterioplankton activity in a P-limited estuary, Northern Baltic Sea. Microbial Ecology, 57: 286–294.
- Robinson, C. (2008). Heterotrophic bacterial respiration. In Microbial ecology of the oceans. 2nd ed. (Kirchman, D.L., editor), 289–325. Wiley-Blackwell, Hoboken, NJ.
- Romera-Castillo, C., Sarmento, H., Alvarez-Salgado, X.A., Gasol, J.M. & Marrasé, C. (2010). Production of chromophoric dissolved organic matter by marine phytoplankton. Limnology and Oceanography, 55: 446–454.
- Rost, B., Zondervan, I. & Wolf-Gladrow, D. (2008). Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Marine Ecology Progress Series, 373: 227–237.
- Royal Society (2005). Ocean acidification due to increasing atmospheric carbon dioxide. Royal Society, London.
- Rykaczewski, R.R. & Dunne, J.P. (2010). A measured look at ocean chlorophyll trends. Nature, 472: E5–E6.
- Rynearson, T.A. & Palenik, B. (2011). Learning to read the oceans: genomics of marine phytoplankton. Advances in Marine Biology, 60: 1–39.
- Rzadkowolski, C.E. & Thornton, D.C.O. (2012). Using laser scattering to identify diatoms and conduct aggregation experiments. European Journal of Phycology, 47: 30–41.
- Sapp, M., Schwaderer, A.S., Wiltshire, K.H., Hoppe, H.G., Gerdts, G. & Wichels, A. (2007). Species-specific bacterial communities in the phycosphere of microalgae? Microbial Ecology, 53: 683–699.
- Sarmiento, J.L., Slater, R., Barber, R., Bopp, L., Doney, S.C., Hirst, A.C., Kleypas, J., Matear, R., Mikolajewicz, U., Monfray, P., Soldatov, V., Spall, S.A. & Stouffer, R. (2004). Response of ocean ecosystems to climate warming. Global Biogeochemical Cycles, 18: GB3003.
- Sharp, J.H. (1977). Excretion of organic matter by marine-phytoplankton – do healthy cells do it? Limnology and Oceanography, 22: 381–399.
- Sharp, J.H. (2002). Analytical methods for total DOM pools. In Biogeochemistry of marine dissolved organic matter (Hansell, D.A. & Carlson, C.A., editors), 35–58. Academic Press, San Diego, CA.
- Shaw, S.L., Chisholm, S.W. & Prinn, R.G. (2003). Isoprene production by Prochlorococcus, a marine cyanobacterium, and other phytoplankton. Marine Chemistry, 80: 227–245.
- Simó, R., Archer, S.D., Pedrós-Alió, C., Gilpin, L. & Stelfox-Widdicombe, C.E. (2002). Coupled dynamics of dimethylsulfoniopropionate and dimethylsulfide cycling and the microbial food web in surface waters of the North Atlantic. Limnology and Oceanography, 47: 53–61.
- Smith, D.F. & Wiebe, W.J. (1976). Constant release of photosynthate from marine phytoplankton. Applied and Environmental Microbiology, 32: 75–79.
- Smith, D.J. & Underwood, G.J.C. (1998). Exopolymer production by intertidal epipelic diatoms. Limnology and Oceanography, 43: 1578–1591.
- Smith, D.J. & Underwood, G.J.C. (2000). The production of extracellular carbohydrates by estuarine benthic diatoms: the effects of growth phase and light and dark treatment. Journal of Phycology, 36: 321–333.
- Staats, N., Stal, L.J., De Winder, B. & Mur, L.R. (2000). Oxygenic photosynthesis as driving process in exopolysaccharide production of benthic diatoms. Marine Ecology Progress Series, 193: 261–269.
- Stedmon, C.A. & Markager, S. (2005). Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnology and Oceanography, 50: 1415–1426.
- Steeman-Nielsen, E. (1952). The use of radio-active carbon (C14) for measuring organic production in the sea. Journal du Conseil International pour l’Exploration de la Mer, 18: 117–140.
- Stefels, J. & van Boekel, W.H.M. (1993). Production of DMA from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp. Marine Ecology Progress Series, 97: 11–18.
- Stocker, R. (2012). Marine microbes see a sea of gradients. Science, 338: 628–633.
- Sugimura, Y. & Suzuki, Y. (1988). A high temperature catalytic oxidation method for the determination of non-volatile dissolved organic carbon in seawater by direct injection of a liquid sample. Marine Chemistry, 24: 105–131.
- Sunda, W., Kieber, D.J., Kiene, R.P. & Huntsman, S. (2002). An antioxidant function for DMSP and DMS in marine algae. Nature, 418: 317–320.
- Sunda, W.G., Shertzer, K.W. & Hardison, D.R. (2009). Ammonium uptake and growth models in marine diatoms: Monod and Droop revisited. Marine Ecology Progress Series, 386: 29–41.
- Suratman, S., Weston, K., Jickells, T., Chance, R. & Bell, T. (2008). Dissolved organic matter release by an axenic culture of Emiliania huxleyi. Journal of the Marine Biological Association of the United Kingdom, 88: 1343–1346.
- Suttle, C.A. (2005). Viruses in the sea. Nature 437: 356–361.
- Suzuki, Y. (1993). On the measurement of DOC and DON in seawater. Marine Chemistry, 41: 287–288.
- Tang, D., Shafer, M.M., Karner, D.A. & Armstrong, D.E. (2005). Response of nonprotein thiols to copper stress and extracellular release of glutathione in the diatom Thalassiosira weissflogii. Limnology and Oceanography, 50: 516–525.
- Teira, E., Pazó, M.J., Serret, P. & Fernández, E. (2001a). Dissolved organic carbon production by microbial populations in the Atlantic Ocean. Limnology and Oceanography, 46: 1370–1377.
- Teira, E., Serret, P. & Fernández, E. (2001b). Phytoplankton size-structure, particulate and dissolved organic carbon production and oxygen fluxes through microbial communities in the NW Iberian coastal transition zone. Marine Ecology Progress Series, 219: 65–83.
- Teira, E., Abalde, J., Álvarez-Ossorio, M.T., Bode, A., Cariño, C., Cid, A., Fernández, E., González, N., Lorenzo, J., Valencia, J. & Varela, M. (2003a). Plankton carbon budget in a coastal wind-driven upwelling station off A Coruna (NW Iberian Peninsula). Marine Ecology Progress Series, 265: 31–43.
- Teira, E., Pazó, M.J., Quevedo, M., Fuentes, M.V., Niell, F.X. & Fernández, E. (2003b). Rates of dissolved organic carbon production and bacterial activity in the eastern North Atlantic Subtropical Gyre during summer. Marine Ecology Progress Series, 249: 53–67.
- Thamatrakoln, K., Korenovska, O., Niheu, A.K. & Bidle, K.D. (2012). Whole-genome expression analysis reveals a role for death-related genes in stress acclimation of the diatom Thalassiosira pseudonana. Environmental Microbiology, 14: 67–81.
- Thornton, D.C.O. (2002). Diatom aggregation in the sea: mechanisms and ecological implications. European Journal of Phycology, 37: 149–161.
- Thornton, D.C.O. (2009). Effect of low pH on carbohydrate production by a marine planktonic diatom (Chaetoceros muelleri). Research Letters in Ecology, Article ID 105901.
- Thornton, D.C.O. & Thake, B. (1998). Effect of temperature on the aggregation of Skeletonema costatum (Bacillariophyceae) and the implication for carbon flux in coastal waters. Marine Ecology Progress Series, 174: 223–231.
- Timmermans, K.R., Veldhuis, M.J.W. & Brussaard, C.P.D. (2007). Cell death in three marine diatom species in response to different irradiance levels, silicate, or iron concentrations. Aquatic Microbial Ecology, 46: 253–261.
- Tolbert, N. E. (1974). Photorespiration. In Algal physiology and biochemistry (Stewart, W.D.P., editor), 474–504. University of California Press, Berkeley, CA.
- Tolbert, N.E. & Zill, L.P. (1956). Excretion of glycolic acid by algae during photosynthesis. Journal of Biological Chemistry, 222: 895–906.
- Underwood, G.J.C. & Paterson, D.M. (2003). The importance of extracellular carbohydrate production by marine epipelic diatoms. Advances in Botanical Research, 40: 183–240.
- Underwood, G.J.C., Boulcott, M., Raines, C.A. & Waldron, K. (2004). Environmental effects on exopolymer production by marine benthic diatoms: dynamics, changes in composition, and pathways of production. Journal of Phycology, 40: 293–304.
- Veldhuis, M.J.W., Kraay, G.W. & Timmermans, K.R. (2001). Cell death in phytoplankton: correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. European Journal of Phycology, 36: 167–177.
- Verdugo, P. (2012). Marine microgels. Annual Review of Marine Science, 4: 375–400.
- Verdugo, P., Alldredge, A.L., Azam, F., Kirchman, D.L., Passow, U. & Santschi, P.H. (2004). The oceanic gel phase: a bridge in the DOM-POM continuum. Marine Chemistry, 92: 67–85.
- Westberry, T., Behrenfeld, M.J., Siegel, D.A. & Boss, E. (2008). Carbon-based primary productivity modeling with vertically resolved photoacclimation. Global Biogeochemical Cycles, 22: GB2024.
- Wetz, M.S. & Wheeler, P.A. (2003). Production and partitioning of organic matter during simulated phytoplankton blooms. Limnology and Oceanography, 48: 1808–1817.
- Wetz, M.S. & Wheeler, P.A. (2007). Release of dissolved organic matter by coastal diatoms. Limnology and Oceanography, 52: 798–807.
- White, A.W. (1974). Growth of 2 facultatively heterotrophic marine centric diatoms. Journal of Phycology, 10: 292–300.
- Whittingham, C.P. & Pritchard, G.G. (1963). Production of glycollate during photosynthesis in Chlorella. Proceedings of the Royal Society B, 157: 366–380.
- Williams, P.J. Le B. (1990). The importance of losses during microbial growth: commentary on the physiology, measurement and ecology of the release of dissolved organic material. Marine Microbial Food Webs, 4: 175–206.
- Winder, M. & Sommer, U. (2012). Phytoplankton response to a changing climate. Hydrobiologia, 698: 5–16.
- Wingenter, O.W., Haase, K.B., Strutton, P., Friederich, G., Meinardi, S., Blake, D.R. & Rowland, F.S. (2004). Changing concentrations of CO, CH4, C5H8, CH3Br, CH3I, and dimethyl sulfide during the Southern ocean iron enrichment experiments. Proceedings of the National Academy of Sciences of the United States of America, 101: 8537–8541.
- Wohlers, J., Engel, A., Zollner, E., Breithaupt, P., Jürgens, K., Hoppe, H.G., Sommer, U. & Riebesell, U. (2009). Changes in biogenic carbon flow in response to sea surface warming. Proceedings of the National Academy of Sciences of the United States of America, 106: 7067–7072.
- Wood, A.M. & Van Valen, L.M. (1990). Paradox lost? On the release of energy-rich compounds by phytoplankton. Marine Microbial Food Webs, 4: 103–116.
- Wolfe, G.V. & Steinke, M. (1996). Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnology and Oceanography, 41: 1151–1160.
- Wolfe, G.V., Steinke, M. & Kirst, G.O. (1997). Grazing-activated chemical defence in a unicellular marine alga. Nature, 387: 894–897.
- Wurl, O. & Sin, T.M. (2009). Analysis of dissolved and particulate organic carbon with the HTCO technique. In Practical guidelines for the analysis of seawater (Wurl, O., editor), 33–48. CRC Press, Boca Raton, FL.
- Xu, Y., Boucher, J.M. & Morel, F.M.M. (2010). Expression and diversity of alkaline phosphatase EHAP1 in Emiliania huxleyi (Prymnesiophyceae). Journal of Phycology, 46: 85–92.
- Yamada, N. & Suzumura, M. (2010). Effects of seawater acidification on hydrolytic enzyme activities. Journal of Oceanography, 66: 233–241.
- Yamasaki, Y., Shikata, T., Nukata, A., Ichiki, S., Nagasoe, S., Matsubara, T., Shimasaki, Y., Nakao, M., Yamaguchi, K., Oshima, Y., Oda, T., Ito, M., Jenkinson, I.R., Asakawa, M. & Honjo, T. (2009). Extracellular polysaccharide-protein complexes of a harmful alga mediate the allelopathic control it exerts within the phytoplankton community. ISME Journal, 3: 808–817.
- Zlotnik, I. & Dubinsky, Z. (1989). The effect of light and temperature on DOC excretion by phytoplankton. Limnology and Oceanography, 34: 831–839.
- Zubkov, M.V., Tarran, G.A. & Fuchs, B.M. (2004). Depth related amino acid uptake by Prochlorococcus cyanobacteria in the Southern Atlantic tropical gyre. FEMS Microbiology Ecology, 50: 153–161.
