Abstract
The history of expansion of bloom-forming cold water dinoflagellates in the Northern Baltic Sea was studied using 100-year-old sediment archives of their resting cysts. Vertical cyst distributions of Biecheleria baltica and Apocalathium malmogiense, two dinoflagellates indistinguishable by light microscopy and not recognized as distinct species in monitoring, and chain-forming Peridiniella catenata were analysed in Pb210 and Cs137 dated layers of a sediment core from deep, hypoxic accumulation bottoms of the Gulf of Finland. Cyst profiles showed that B. baltica and A. malmogiense were already present in the Baltic spring phytoplankton community at the beginning of the 20th century. This confirms that B. baltica, which was only recognized in the late 1980s, is a native species in the area. A drastic increase in B. baltica cyst concentrations in the 1930s to 1960s coincided with the acceleration of anthropogenic eutrophication. Large cyst deposits accumulated over several decades in the sediment which, by the 1980s, amounted to the seed stock necessary to inoculate dominant blooms. In the cyst records A. malmogiense always contributed a minor fraction of the two species. P. catenata had a relatively short cyst record in Gulf of Finland sediments despite demonstrated long-term presence in the plankton, which emphasizes that cyst-based historic surveys are not suitable for all cyst-forming dinoflagellates. This was corroborated by correspondence analyses of long-term plankton and cyst records which validated the trends from the sediment archive for B. baltica and A. malmogiense, but failed to do so for P. catenata. Germination experiments with 100-year-old cysts revealed a remarkable long-term survival capacity of A. malmogiense, making this species a suitable model for resurrection studies testing adaptation in heavily impacted systems such as the Baltic Sea.
Introduction
Phototrophic dinoflagellates are important constituents of phytoplankton in temperate marine and freshwater systems. Under favourable conditions they often form dense blooms which can have toxic or otherwise harmful effects on humans and co-occurring biota. When dinoflagellates dominate primary production, they can play an important role as drivers of carbon fixation and biogeochemical cycles. Many dinoflagellate species form resistant resting cysts as a part of their life cycle (Dale, Citation1983). Dormant cysts are primarily thought to ensure survival through periods of unfavourable conditions. However, various other biological and ecological functions of these life cycle stages have been recognized: in the majority of dinoflagellate species with known life cycles, cyst formation is part of the sexual reproduction process (von Stosch, Citation1973; Figueroa & Bravo, Citation2005) and supports genetic diversity (Garcés et al., Citation2002). The important role of cysts in dinoflagellate bloom dynamics and seasonal succession has been extensively documented (Anderson, Citation1998; Rengefors, Citation1998; Anglès et al., Citation2012). Pools of resting cysts in lake and marine sediments form ‘seed banks’ that anchor dinoflagellate populations in an area (Tahvanainen et al., Citation2012) and, being diverse genetic reservoirs (Lundholm et al., Citation2011), support their persistence through changing environmental conditions.
The walls of dinoflagellate resting cysts typically consist of resistant materials that effectively protect the cells from degradation (Zonneveld et al., Citation1997). When buried in sediment deposits, cysts can remain intact and alive for a long time (Lewis et al., Citation1999; McQuoid et al., Citation2002; Lundholm et al., Citation2011). The revival potential of some species is remarkable even after decades of burial, suggesting that these propagules are effective means to ensure persistence of populations through prolonged periods of unfavourable conditions (Ribeiro et al., Citation2011). Resurrected individuals or populations of planktonic organisms can provide valuable information on past environmental conditions and species responses to long-term changes in a water body. The fossilizable cyst walls of some dinoflagellates remain intact over geological time spans, making cysts useful proxies for environmental change. Cyst records have been extensively used in geology to reconstruct past marine and freshwater environments (de Vernal et al., Citation2013). Cyst profiles from more recent sediments were found to reflect effects of industrialization and eutrophication (Harland et al., Citation2006; McCarthy et al., Citation2011). Moreover, cyst distributions in dated sediment cores have helped to explain recent expansions of harmful dinoflagellate blooms by relating vertical cyst dynamics to decadal scale environmental fluctuations (Feifel et al., Citation2012) or reconstructing invasion histories (Ribeiro et al., Citation2012). It has been suggested that dinoflagellate sediment records could replace or extend plankton monitoring time-series (Klouch et al., Citation2016).
In the Baltic Sea, cold-water adapted dinoflagellates regularly dominate the spring phytoplankton community and may in some years contribute up to 80% of the annual new production (Lignell et al., Citation1993). The species involved – Peridiniella catenata (Levander) Balech, Biecheleria baltica Moestrup, Lindberg & Daugbjerg, Apocalathium malmogiense (G.Sjöstedt) Craveiro, Daugbjerg, Moestrup & Calado comb. nov. and Gymnodinium corollarium A.M.Sundström, Kremp & Daugbjerg – are phylogenetically diverse. While the chain-forming P. catenata is morphologically distinct, the other three species cannot be distinguished from one another by means of light microscopy. They were only recently recognized as individual species (Kremp et al., Citation2005; Sundström et al., Citation2009). In Baltic phytoplankton monitoring programmes the three species have not been differentiated but pooled in the ‘Scrippsiella complex’, referring to the long-used genus name of the only species of the three that had been properly identified from the spring bloom, Scrippsiella hangoei (Larsen et al., Citation1995), now named A. malmogiense (Craveiro et al., Citation2016). All four spring bloom dinoflagellates produce distinct dormant resting cysts (Kremp, Citation2000; Kremp et al., Citation2005; Sundström et al., Citation2009). Except for G. corollarium, which has an exceptionally small cyst stage that cannot be captured by standard sediment processing methods, these have been identified from Northern Baltic surface sediment samples.
The dominance of dinoflagellates during spring in the northern Baltic Sea is a recent phenomenon which has developed over the past four decades (Klais et al., Citation2011). It has been proposed that climate-mediated effects on life cycle transformations of B. baltica are a major driver of the bloom expansion (Klais et al., Citation2013; Warns et al., Citation2013). B. baltica has caused massive cyst sedimentation events since the late 1980s (Heiskanen, Citation1993; Kremp et al., Citation2005) suggesting that this species plays an important role in this development. However, it remains unclear whether the new B. baltica blooms are caused by an expanding native population or whether the rapid expansion of the species might be a result of a recent invasion. It is also unclear to what extent the other ‘look-alike’ species, A. malmogiense and G. corollarium, are involved.
The present study investigates the distribution of distinct resting cysts of spring bloom dinoflagellates in a hundred-year-old sediment archive from the Gulf of Finland to unravel the history of their recent expansion and resolve the role of different species therein. We also examine to what extent the patterns found in the sediment reflect trends inferred from time-series of phytoplankton monitoring data and test the viability of cysts retrieved from old sediment layers to assess their potential for future resurrection studies.
Materials and methods
Sample collection
Sediment cores were collected in February 2015 onboard RV Aranda at HELCOM monitoring station LL7 (latitude 59°50.79’, longitude 24°50.27’, water depth 100 m) located in the Gulf of Finland, Baltic Sea (). Due to severe eutrophication and the inflow of anoxic deep water from the Baltic Proper, this area is characterized by hypoxic bottom water that prevents bioturbation (Conley et al., Citation2002; Vallius et al., Citation2006) resulting in a vertically undisturbed sediment. For this study, two replicate core samples of 32–33 cm length were taken simultaneously using a GEMAX gravity corer. Samples were stored in their tubes under cold and dark conditions until further processing. In the laboratory cores were extruded from sampling tubes using a piston and sliced into 1 cm layers. To avoid contamination by smear from other sediment layers, the outer 5 mm of each slice were removed using a cutting device designed for this purpose. Individual slices were transferred immediately to plastic bags submersed in water to remove air. Tightly closed bags were stored at 4°C in the dark.
Sediment dating
Slices of one core were used for gamma-spectrometric dating of the sediment layers. Wet sediment slices were first weighed and then freeze-dried for 3 days in a Christ Beta 2-8 LD plus freeze dryer at 1.0 mbar and −20°C. Dry material was weighed again. Aliquots of dry sediment (3 g) were measured using gamma-spectrometry at the STUK Regional Laboratory of Northern Finland, using electrically cooled high purity germanium detectors (HPGE) with thin carbon fibre windows (Canberra and Ortec). Detector efficiencies were calibrated with certified National Physical Laboratory reference samples containing multiple nuclides with gamma energies ranging from 46.5 keV to 1836.1 keV, including Pb-210 and Cs-137. Activity concentrations were calculated using Gamma-99 software of STUK. The 46.5 and 661.7 keV gamma peaks were used for determination of Pb-210 and Cs-137, and for Ra-226 the 609 keV peak of Bi-214 and/or the 352 keV peak of Pb-214 were used. The water content and porosity of each sediment slice were calculated from dry and wet weights. Dry bulk density and mass depth of sediment slices were calculated using a constant sediment dry density of 2.3 g cm–3.
The measured Pb-210 concentration in the sediment consists of supported and unsupported (or excess) Pb-210 components. Supported Pb-210 is in radioactive equilibrium with the decay chain of Ra-226 in the sediment matter, and therefore represented directly by the measured Ra-226 concentration. Unsupported Pb-210 consists of atmospheric deposition and is calculated by subtracting the supported Pb-210 (Ra-226) from measured total Pb-210 for each sediment slice. The measurement uncertainties of total Pb-210 and Ra-226 are propagated into the uncertainty of unsupported Pb-210. The Pb-210 dating of sediments is based on tracking the record of unsupported Pb-210 in the sediment as a function of depth. After burial the unsupported Pb-210 will decay with a half-life of 22.23 years. However, assumptions have to be made about the supply rate of unsupported Pb-210 and the sedimentation rate, which may change over time or experience significant post-depositional changes through sediment mixing or diffusion. Pb-210 dating was validated by comparison with Cs-137. Cs-137 has been released into the atmosphere by nuclear weapons testing and nuclear accidents and can be used as a marker. Atmospheric nuclear weapons testing occurred mainly in the 1950s and 1960s, with a peak concentration of deposited Cs-137 around 1963. The Chernobyl accident caused a sudden release of Cs-137 into the environment, which produced a second marker in the sediment record during 1986.
The Pb-210 chronology of the LL7 sediment core was calculated using the well-known models of constant initial concentration (CIC) and constant rate of supply (CRS), using the methodology described in Appleby (Citation2001) and Lima et al. (Citation2005) and comparing it with Cs-137 data.
Determination of vertical cyst concentrations
To quantify concentrations of dinoflagellate resting cysts in vertical sediment layers, sediment slurries containing the cyst fraction were prepared from stored wet sediment samples of the second core in October 2016, c. 20 months after initial slicing. Three 2 ml subsamples were processed from each sediment layer down to 16 cm, the deepest layer where dinoflagellate cysts were found during initial screening. Replicate subsamples taken from well-mixed sediment samples were suspended in 20 ml of 0.2 µm-filtered local seawater (FSW, salinity 6) and sonicated for 30 s using a Bandelin Sonoplus Ultrasonication probe to disaggregate particles and remove fine organic material from the surface of resting cysts. Sonicated samples were rinsed through a 70 µm onto a 20 µm sieve using FSW to clean the slurries and concentrate the 20–70 µm size fraction that contained the dinoflagellate cysts of interest. The concentrated samples containing cysts of 2 ml wet sediment were resuspended in 15 ml FSW and transferred to 15 ml centrifuge tubes. These were closed tightly and wrapped immediately in aluminium foil to minimize exposure to light. Cyst slurries were stored in the cold and dark until microscopic examination and setting up of germination experiments. Cysts of B. baltica, P. catenata and A. malmogiense were counted microscopically using an inverted Leica DMI 3000B microscope (Leica Microsystems, Wetzlar, Germany), in Utermöhl chambers containing 1 ml of the processed sample. In this study, only intact cysts with visible cell contents were enumerated, assuming that permanently dark and hypoxic conditions at the sampling site consistently prevented germination of deposited cysts. The record of content-bearing cysts is thus considered to represent deposition from the plankton. Cyst counts were made in triplicate for each slice and wet sample concentrations were converted to cysts g–1 dry weight based on initial wet and dry weight measurements for respective layers.
Correspondence analysis of sediment and plankton records
To estimate how historic cyst records in the sediment correspond to long-term bloom data in the water, quantitative phytoplankton monitoring data available from sampling locations within 30 km radius of LL7 (most of the samples located north of the sediment station) since 1966 were selected from the database established by Olli et al. (Citation2013). Annual means of log-transformed biovolume (μg l–1) were calculated from surface layer samples (0 to 4–10 m depth) for the ‘Scrippsiella complex’ and P. catenata. The ‘Scrippsiella complex’ represents both B. baltica and A. malmogiense. The correlation between cysts accumulated in sediment layers and respective annual biovolume means from plankton samples was estimated with Kendall’s rank correlation and Pearson’s linear correlation; in the latter case cyst abundances were log-transformed. Statistical analyses were performed in R.
Assessment of cyst viability
Viability of dinoflagellate cysts through time was assessed in germination experiments. For this purpose, cyst slurries (1 ml) were prepared from sediment slices obtained in February 2015. Subsamples of 1 ml containing intact cysts were incubated in triplicate wells of a 24 well tissue culture plate for each tested layer and species. Based on cyst distributions through the core, different layers were selected to represent cysts of different ages: 1, 6, 12 and 16 cm (= 3, 19, 50 and 106 years at the time of experiment) for B. baltica and A. malmogiense, and 1, 4, 5 and 6 cm (= 3, 11, 14 and 19 years, respectively) for P. catenata. Tissue culture plates were kept in an incubator for 4 weeks at 4°C, 50 µmol photons m–2 s–1 under a 14:10 h light/dark cycle. After 2 and 4 weeks, samples were screened microscopically for the appearance of empty cysts and swimming vegetative cells.
Results
Sediment chronology
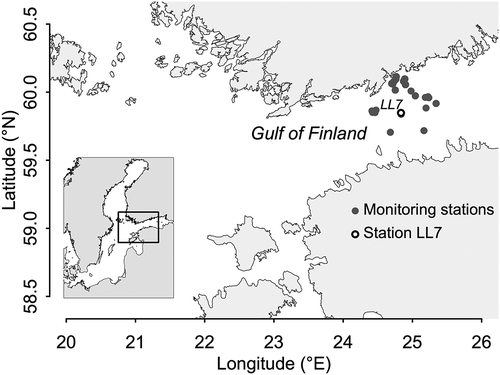
Calculated sediment properties, results of gamma spectrometry and results of CRS model calculations are listed in the Supplementary Material. Unsupported Pb-210 concentration ranged from ~500 Bq kg–1 at the top of the core to practically zero at 18 cm depth (). The variability of unsupported Pb-210 was too complex to fit the assumption of exponential decrease of the basic CIC dating model () and the fit became only marginally better when using the CIC model with compaction correction (not shown). For that reason the CRS model, which assumes a constant flux of unsupported Pb-210 but allows for a variable sedimentation rate, was applied for age calculation. Because of the very small amount of unsupported Pb-210 in the 16–18 cm region () the uncertainties of CRS ages and sedimentation rates were too high below 16 cm depth preventing reliable age estimation. The CRS age for 16 cm depth was 105±8 years, which corresponds to a date of 1910±8 ().
Figs 2–5. Gamma spectrometric results. Fig. 2. Pb-210 and Ra-226 in the LL7 sediment core: supported Pb-210 is represented by Ra-226, Pb-210 from atmospheric deposition (unsupported) is the difference between measured total Pb-210 and supported Pb-210. Fig. 3. Gamma spectrometric results of Cs-137: horizontal lines represent depths where peaks of Cs-137 are expected based on Pb-210 calculations. Fig. 4. Age-depth relationships of different Pb-210 dating models. Fig. 5. Dry mass sedimentation rate as a function of age, as calculated from the Pb-210 CRS model.
The Cs-137 data did not exhibit clear sharp peaks that could be definitively identified with Chernobyl or nuclear weapons testing depositional maxima (). However, the comparison of CRS model Pb-210 ages with Cs-137 shows good agreement with the key dates of 1963 and 1986 and the largest steps in the Cs-137 concentration profile (), giving confidence in the CRS model ages. The somewhat atypical Cs-137 profiles could result from the thickness of the slices (1 cm) that may have prevented resolution of the Cs-137 record into distinct peaks. Moreover, the concentration of Cs-137 remained high for several sediment slices above the 1986 level in the 6–9 cm depth which is in accordance with the high dry mass sedimentation rates calculated using the CRS model () for the period between 1985±2 and 1998±2 compared with other times. This corresponds to the first 15 years after the Chernobyl accident, a period of increased deposition of Cs-137-containing source materials which were transported to and deposited in the area. Similar patterns were found in other studies from Finnish coastal waters (Katajisto, Citation1996).
Vertical cyst distributions and long-term trends
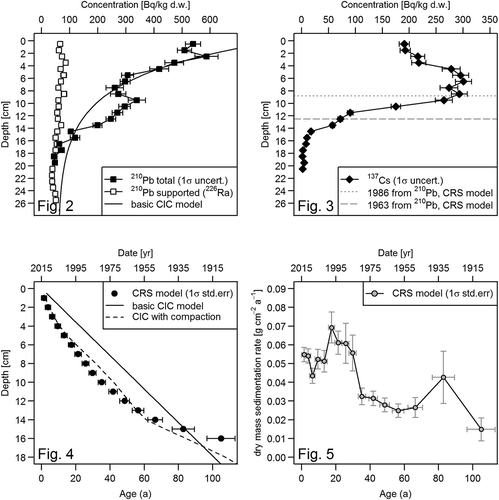
The distinct resting cysts of B. baltica (), A. malmogiense () and P. catenata () were the most prominent cyst types of phototrophic dinoflagellates found in the surface layer and throughout the LL7 sediment core. Intact B. baltica and A. malmogiense cysts were still detected at 15–16 cm depth in sediment more than 100 years old (, ). Here, as in younger sediment layers, newly germinated A. malmogiense cells often appeared in the samples while these were being examined for cyst abundances ().
Figs 6–11. Light micrographs of B. baltica (Figs 6, 9), A. malmogiense (Figs 7, 10) and P. catenata (Fig. 8) resting cysts. Cysts from surface sediment representing 2–4 years old deposits (Figs 6–8) and from the 15–16 cm sediment layer estimated to be c. 100 years old (Figs 9–10). Fig. 11. Vegetative cell of A. malmogiense germinated from the 15–16 cm layer.
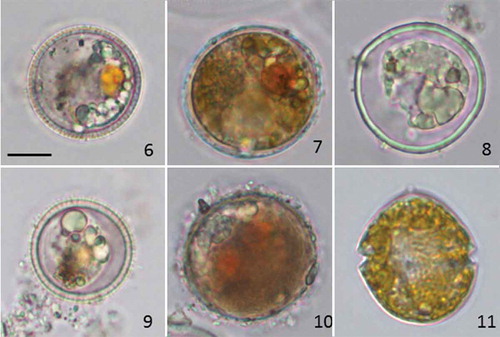
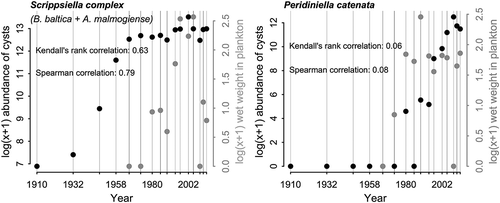
Patterns of vertical distribution differed among the three species (). Biecheleria baltica was the most abundant species in all examined layers of the sediment core. Highest cyst concentrations of this species (740 000 cysts g–1 dry weight) were measured at 5 cm depth, corresponding approximately to the year 2002. Though lower before and after this peak, cyst concentrations remained at the same order of magnitude (105 g–1 dry weight) between 1958 and 2013. At the beginning of the last century, B. baltica cyst concentrations (800 cysts g–1 dry weight) were only 0.1–0.2% of concentrations measured a century later. A substantial increase occurred between 1932 and 1958. The distinct cysts of A. malmogiense were much less abundant than cysts of the two other species, but occurred consistently in all 16 investigated sediment layers. Their vertical distribution followed a similar pattern to B. baltica, with highest cyst concentrations (1700–3800 cysts g–1 dry weight) in the most recent sediment layers (1–5 cm) and low numbers of cysts in the layers representing the first half of the past century. Nevertheless, cyst abundances of A. malmogiense did not change as dramatically as those of the other species through the century. Minimum abundances of this species, measured in the 1932 layer, were more than 1% of the maximum. Peridiniella catenata cysts were only detected down to 10 cm core depth, which corresponds approximately to the year 1980. Continuous cyst records of this species were only found in the uppermost 8 cm, i.e. since c. 1989. In the late 1990s and early 2000s cyst abundances increased exponentially, reaching peak concentrations of >250 000 cysts g–1 dry weight in the year 2009 (3 cm downcore). Since then P. catenata cyst concentrations have decreased; approximately 100 000 cysts g–1 dry weight were found in the uppermost layer of the core.
Fig. 12. Concentrations of intact B. baltica, P. catenata and A. malmogiense resting cysts in dated layers of a sediment core collected at station LL7 in the Gulf of Finland, Baltic Sea.
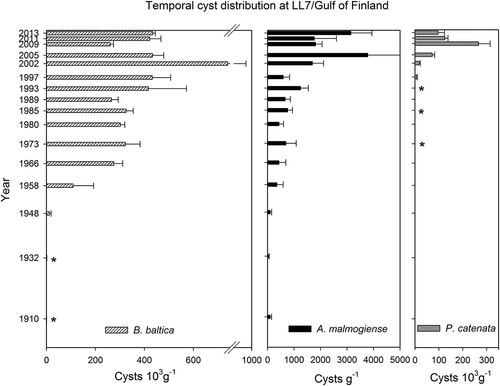
When comparing bloom intensities of the investigated spring dinoflagellates to the cyst accumulations shown in through the study period, the respective trends were closely correlated for B. baltica and A. malmogiense constituting the Scrippsiella complex (), both when using Kendall’s rank correlation (τ=0.68) as well as Pearson’s linear correlation coefficient (r=0.75). Cyst densities of P. catenata, in contrast, were not correlated with the abundance of this species in the plankton (τ=0.06, r=0.17).
Fig. 13. Correspondence between long-term sediment (resting cyst abundances, black circles) and plankton (grey circles) records of B. baltica and A. malmogiense (= ‘Scrippsiella complex’ in phytoplankton monitoring programmes), and P. catenata. Quantitative plankton data have been available since 1966 and originate from surface water samples collected by various monitoring programmes. Data points for plankton represent annual means of log-transformed biovolumes (μg l–1) from the samples collected within 30 km radius of LL7. Correlation was estimated with Kendall’s rank correlation, and Pearson’s linear correlation.
Viability of cysts
When resting cysts of the three species from different sediment layers were incubated under suitable temperature, oxygen and light conditions ~20 months after initial core processing, only A. malmogiense could be revived (). Biecheleria baltica cysts did not germinate in any well within 4 weeks. For P. catenata one new empty cyst was found after 4 weeks in one of three replicate wells containing material from 5 cm depth (14 year old sediment), but no swimming cells were observed. Germination of A. malmogiense occurred in material from all tested sediment layers, including sediment >100 years old. After 2 weeks, new empty cysts had appeared in at least 2 of the 3 replicate wells representing each tested sediment layer. In both, germination had occurred in all replicates of layers aged 19 and 106 years. Swimming cells were found in all wells that contained germinated cysts. The result was unchanged after 4 weeks.
Table 1. Germination and viability of germinated cells inferred from appearance of empty cysts and swimming cells in sediment slurries (three replicates) representing different sediment layers and age.
Discussion
Gamma-spectrometric radionuclide analyses of sediment layers showed that the sediment cores collected from the 100 m deep central Gulf of Finland consisted of chronologically arranged vertical layers that allowed us to estimate the age of resting cysts retrieved from different depths of the core. Pb-210 and Cs-137 values established for the LL7 core were in agreement with the model assumptions (Appleby et al., Citation2001) and confirmed that the investigated depth interval corresponded to ~100 years and thus represents nearly the entire 20th century.
The observation of B. baltica and A. malmogiense cysts in the oldest investigated sediment layers confirms that both species were already present in the Northern Baltic phytoplankton community a century ago, long before the expansion of cold water dinoflagellates began. For A. malmogiense the presence of cysts in sediment layers >100 years old is consistent with observations in the plankton. The species, then named Peridinium gracile, was identified by Lindemann (Citation1924) from a sample collected in 1905 on the SW coast of Finland. The B. baltica cysts from the 1910 sediment layer found in this study represent the oldest record of the species, which was only recognized and described a decade ago. Cyst abundances determined from the 1910 layer suggest that 100 years ago B. baltica was much more abundant than A. malmogiense, which only amounted to c. 10% of the total number. It can be assumed that this reflects the actual ratio of the two species at the time, since both have comparable cyst formation strategies with similar triggers and cyst yields (Kremp et al., Citation2009) allowing direct comparison of cyst abundances.
Cyst concentrations of B. baltica and A. malmogiense were several orders of magnitude lower in the deep layers compared with the sediment surface, indicating that at the beginning of the 20th century the two species were much less abundant in the water than today. Analyses of historic data (Hällfors et al., Citation2013) as well as qualitative historic surveys (Levander, Citation1901) indicate that dinoflagellates were a minor component of the diatom–dominated spring phytoplankton community before anthropogenic impact began to affect phytoplankton productivity and composition in the Northern Baltic Sea. The dramatic accumulation of B. baltica cyst deposits in the sediment layers representing the 1930s to 1960s coincided with significant anthropogenic nutrient loading to the Baltic Sea (Struck et al., Citation2000). Within 35 years, B. baltica cyst abundances had increased nearly 200-fold. Similar developments have been documented for a number of dinoflagellate species from coastal and lake sediments worldwide using palynological cyst walls as well as whole cyst extraction methods (McCarthy et al., Citation2011; Miyazano et al., 2012; Ribeiro et al., Citation2012) and linked to the acceleration of eutrophication in the 1920s to 1960s. The increase of B. baltica probably reflects the general increase of phytoplankton biomass, fuelled by the ever-increasing availability of nutrients in the Gulf of Finland during that period. The eutrophication signal was also apparent in the cyst dynamics of A. malmogiense, although cyst abundances of this species only tripled through the respective time interval. Interestingly, the expansion of B. baltica in the sediment preceded the community shift from diatom to dinoflagellate dominance in the plankton by several decades (Klais et al., Citation2011). This lag might be related to the low survival rate of B. baltica cysts, particularly in anoxic sediments (Kremp & Anderson, Citation2000). A favourable effect of the species’ large ‘seed beds’ as suggested by Klais et al. (Citation2011) would only be provided at very high cyst concentrations, which had apparently accumulated by the 1980s.
The sediment archive allowed us to trace the history of two species that cannot be distinguished from one another in the plankton monitoring data of the past century, using the patterns of their distinct cysts. As indicated by the cyst record, A. malmogiense has remained a minor component of the ‘Scrippsiella complex’ throughout eutrophication-induced phytoplankton productivity changes and the expansion of spring dinoflagellates in the Gulf of Finland. The cyst records show that A. malmogiense has increased proportionally since the late 1990s compared with B. baltica, now making up nearly 1% of the total again after having lost proportionally in the 1960 to 1990s.
To what extent the actual ratio of the two species in the plankton at a given time point is biased by differences in their preservation capacities remains unclear. Compared with the most recent sediment layers, B. baltica cyst concentrations decreased by a factor of 500 downcore, compared with A. malmogiense which was only 30 times less abundant in the 100-year-old sediment layer than at the sediment surface. Lundholm et al. (Citation2011) detected large differences in the depth distribution of cysts with cell content among different dinoflagellate species, suggesting that resistance to degradation can vary considerably. In fact, a relatively low preservation potential has been indicated for members of the Suessiales, Biecheleria sp. and Polarella glaciales (Heikkilä et al., Citation2016), suggesting that mineralization of cysts happens rapidly, leading to substantial loss of cysts within a year after sedimentation. The data presented here show that this is certainly not the case for B. baltica from the Gulf of Finland. So far, palynological techniques have not been applied to B. baltica or A. malmogiense and it remains unclear whether their cyst walls are preserved in the fossil and sub-fossil records.
Despite these uncertainties, the established cyst record of B. baltica and A. malmogiense correlates well with the plankton record of the ‘Scrippsiella complex’ for the investigated period between 1966 and 2013 when plankton monitoring data were available. Though there must have been some bias due to the fact that the cyst concentrations represent a cumulative value whereas cell concentrations in the plankton are a current value, we are confident that the sediment archive of the ‘Scrippsiella complex’ represents a realistic trend through the past century and thus extends the plankton time-series data by 50 years to the beginning of the past century. The availability of a plankton monitoring time-series spanning nearly 50 years for the study area allowed us to validate the ‘time series’ represented by the cyst record. Typically, sediment archives are studied where plankton records do not exist, making it difficult to assess how realistically the cyst dynamics in the sediment core reflect trends in the plankton. So far, only quantitative DNA measurements from sediment archives have been systematically validated by plankton data (Klouch et al., Citation2016), though for a much shorter time span than the present study. Sometimes, indirect evidence, such as toxin monitoring data for a toxic dinoflagellate species, might provide orientation (Cox et al., Citation2008).
However, as the case of P. catenata presented here demonstrates, the cyst archive approach is not universally applicable for the reconstruction of dinoflagellate species histories in an area of interest. For this species trend correlation of plankton and cyst data was not successful. Cyst records of P. catenata could only be traced back 30–40 years, although historic reports refer to the presence of the species in the Gulf of Finland as early as 1894 (Levander, Citation1894). Peridiniella catenata (then Peridinium catenatum) was already referred to then as a prominent species of the spring phytoplankton community (Levander, Citation1901). In the Gulf of Finland it has been highly abundant and sometimes even dominant during spring (Niemi, Citation1975), but in the past 40 years the proportion of the species in the phytoplankton community has decreased steadily (Klais et al., Citation2013). The cyst profile of the LL7 core suggests a reverse pattern. The rapid decline of cyst abundances downcore is almost certainly related to the delicate nature of the cysts and the resulting low preservation capacity. As described in Kremp (Citation2000), the cyst wall of P. catenata is thin, resembling a temporary cyst rather than a resistant resting cyst. Nevertheless, these cysts have the demonstrated function of a dormant resting stage – preserving the cell from degradation at least through a mandatory dormancy period of 6 months. Low preservation potential of cysts of a number of dinoflagellate species was indicated by Lundholm et al. (Citation2011), who reported relatively short-term records of intact cysts (less than 30 years) in a sediment core from the Swedish West Coast e.g. for Alexandrium margalefii, Cochlodinium polykrikoides and several Diplopelta and Protoperidinium cyst types.
Of the three investigated species only A. malmogiense cysts germinated readily from old sediment layers. When examined under the light microscope, even the 100-year-old cysts of this species quickly showed signs of impending germination such as Brownian motion or the greenish colour of developing chloroplasts. Often, germinated cells would even appear in the slide within the 30–60 minutes of observation. Such high revival potential has also been shown for the peridinioid dinoflagellate Pentapharsodinium dalei, which has been extensively used in resurrection studies (Ribeiro et al., Citation2011, Citation2013; Lundholm et al., Citation2011). We established several clonal cultures from 100-year-old cysts and compared temperature-related traits to isolates from recent sediment layers (Hinners et al., Citation2017). This shows that A. malmogiense can be used as a model organism for evolutionary adaptation studies, particularly since it has experienced significant environmental changes during the past century in the heavily impacted Baltic Sea.
In contrast, B. baltica and P. catenata could not be revived from any sediment layer, not even from the surface which, at the time of the study, was 1.5–3 years old. It is likely that the long storage time after initial slicing of the sediment core (18 months), before germination experiments were performed, affected the results of the germination experiments as described by Lundholm et al. (Citation2011) who found that storage after core processing reduces cyst viability of some dinoflagellate groups significantly. Interestingly, in that study the tested peridinioid taxa were not affected, which is consistent with our results. However, for B. baltica lack of excystment in any sediment layer is consistent with a steep decrease in germination potential within the first year of burial, and a negative effect of anoxic conditions on cyst survival (Kremp & Anderson, Citation2000). Although healthy-looking B. baltica resting cysts were observed in deeper layers of the sediment cores, the amount of cysts lacking granular storage products – an indicator of cyst viability (Feifel et al., Citation2015; ) – increased significantly downcore. Published information on cyst survival is not available for P. catenata. Here, excystment, but no germling survival, was observed on one occasion in a slurry of 14-year-old cysts. This indicates that P. catenata might have shown a different germination behaviour, implying longer survival times, had experiments been set up immediately after sediment processing.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2017.1386330
Supplementary table 1: Calculated sediment properties, gamma-spectrometric results of Pb210, Ra226 and Cs137, and the results of CRS model Pb210 dating.
Supplementary_table_1.xps
Download (196.6 KB)Acknowledgements
We thank Juha Flinkmann for collecting the sediment core onboard Aranda and Anu Vehma and Miia Mannerla for assistance in core processing.
Additional information
Funding
Notes on contributors
Anke Kremp
A. Kremp: Original concept, cyst sample analyses and germination experiments, lead author of manuscript; J. Hinners: Participated in sediment processing and cyst analyses, contributed to manuscript writing; R. Klais: Statistical analyses of long-term plankton and cyst data, contributed to manuscript; A. Kallio and A.-P. Leppänen: Sediment dating, contributed to manuscript.
References
- Anderson, D.M. (1998). Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In Physiological Ecology of Harmful Algal Blooms (Anderson, D.M., Cembella, A.D. & Hallegraeff, G.M., editors), 29–48. NATO ASI series, Springer-Verlag, Berlin. Vol. G 41.
- Anglès, S., Garcés, E., Reñé, A. & Sampedro, N. (2012). Life-cycle alternations in Alexandrium minutum natural populations from the NW Mediterranean Sea. Harmful Algae, 16: 1–11.
- Appleby, P.G. (2001). Chronostratigraphic techniques in recent sediments. In Tracking Environmental Change Using Lake Sediments, Vol 1 (Last, W.M. & Smol, J.P., editors), 171–203. Kluwer, Dordrecht.
- Conley, D.J., Humborg, C., Rahm, L., Savchuk, O.P. & Wulff, F. (2002). Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environmental Science & Technology, 36: 5315–5320.
- Cox, A.M., Shull, D.H. & Horner, R.A. (2008). Profiles of Alexandrium catenella cysts in Puget Sound sediments and the relationship to paralytic shellfish poisoning events. Harmful Algae, 7: 379–388.
- Craveiro, S.C., Daugbjerg, N., Moestrup, Ø. & Calado, A.J. (2016). Studies on Peridinium aciculiferum and Peridinium malmogiense (= Scrippsiella hangoei): comparison with Chimonodinium lomnickii and description of Apocalathium gen. nov. (Dinophyceae). Phycologia, 56: 21–35.
- Dale, B. (1983). Dinoflagellate resting cysts: “benthic plankton”. In Survival Strategies of the Algae (Fryxell, G.A., editor), 69–136. Cambridge University Press, Cambridge.
- de Vernal, A., Rochon, A., Fréchette, B., Henry, M., Radi, T. & Solignac, S. (2013). Reconstructing past sea ice cover of the northern hemisphere from dinocyst assemblages: status of the approach. Quaternary Science Reviews, 79: 122–134.
- Feifel, K.M., Fletcher, S.J., Watson, L.R., Moore, S.K. & Lessard, E.J. (2015). Alexandrium and Scrippsiella cyst viability and cytoplasmic fullness in a 60-cm sediment core from Sequim Bay, WA. Harmful Algae, 47: 56–65.
- Feifel, K.M., Moore, S.K. & Horner, R.A. (2012). An Alexandrium spp. cyst record from Sequim Bay, Washington State, USA, and its relation to past climate variability. Journal of Phycology, 48: 550–558.
- Figueroa, R.I. & Bravo, I. (2005). Sexual reproduction and two different encystment strategies of Lingulodinium polyedrum (Dinophyceae) in culture. Journal of Phycology, 41: 370–379.
- Garcés, E., Zingone, A., Montresor, M., Reguera, B. & Dale, B. (2002). LIFEHAB: Life histories of microalgal species causing harmful blooms. Office for the Official Publications of the European Communities, Luxembourg, 1–189.
- Hällfors, H., Backer, H., Leppänen, J.M., Hällfors, S., Hällfors, G. & Kuosa, H. (2013). The northern Baltic Sea phytoplankton communities in 1903–1911 and 1993–2005: a comparison of historical and modern species data. Hydrobiologia, 707: 109–133.
- Hällfors, H., Backer, H., Leppänen, J.M., Hällfors, S., Hällfors, G. & Kuosa, H. (2013). The northern Baltic Sea phytoplankton communities in 1903–1911 and 1993–2005: a comparison of historical and modern species data. Hydrobiologia, 707: 109–133.
- Harland, R., Nordberg, K. & Filipsson, H.L. (2006). Dinoflagellate cysts and hydrographical change in Gullmar Fjord, west coast of Sweden. Science of the Total Environment, 355: 204–231.
- Heikkilä, M., Pospelova, V., Forest, A., Stern, G.A., Fortier, L. & Macdonald, R.W. (2016). Dinoflagellate cyst production over an annual cycle in seasonally ice-covered Hudson Bay. Marine Micropaleontology, 125: 1–24.
- Heiskanen, A.-S. (1993). Mass encystment and sinking of dinoflagellates during a spring bloom. Marine Biology, 116: 161–167.
- Hinners, J., Kremp, A. & Hense, I. (2017). Evolution in temperature-dependent phytoplankton traits revealed from a sediment archive: Do reaction norms tell the whole story? Proceedings of the Royal Society B: Biological Sciences. 284: 20171888.
- Katajisto, T. (1996). Copepod eggs survive a decade in the sediments of the Baltic Sea. Hydrobiologia, 320: 153–159.
- Klais, R., Tamminen, T., Kremp, A., Spilling, K., An, B.W., Hajdu, S. & Olli, K. (2013). Spring phytoplankton communities shaped by interannual weather variability and dispersal limitation: mechanisms of climate change effects on key coastal primary producers. Limnology and Oceanography, 58: 753–762.
- Klais, R., Tamminen, T., Kremp, A., Spilling, K. & Olli, K. (2011). Decadal-scale changes of dinoflagellates and diatoms in the anomalous Baltic Sea spring bloom. PLoS ONE, 6: e21567.
- Klouch, K.Z., Schmidt, S., Andrieux-Loyer, F., Le Gac, M., Hervio-Heath, D., Qui-Minet, Z. N. & Siano, R. (2016). Historical records from dated sediment cores reveal the multidecadal dynamic of the toxic dinoflagellate Alexandrium minutum in the Bay of Brest (France). FEMS Microbiology Ecology, 92: fiw101.
- Kremp, A. 2000. Morphology and germination pattern of the resting cyst of Peridiniella catenata (Dinophyceae) from the Baltic Sea. Phycologia, 39: 183–186.
- Kremp, A. & Anderson, D.M. (2000). Factors regulating germination of resting cysts of the spring bloom dinoflagellate Scrippsiella hangoei from the northern Baltic Sea. Journal of Plankton Research, 22: 1311–1327.
- Kremp, A., Elbrächter, M., Schweikert, M., Wolny, J. & Gottschling, M. (2005). Woloszynskia halophila (Biecheler) comb. nov. – a bloom forming cold-water dinoflagellate co-occurring with Scrippsiella hangoei (Dinophyceae) in the Baltic Sea. Journal of Phycology, 41: 629–642.
- Kremp, A., Rengefors, K. & Montresor, M. (2009). Species-specific encystment patterns in three Baltic cold-water dinoflagellates: the role of multiple cues in resting cyst formation. Limnology and Oceanography, 54: 1125–1138.
- Larsen, J., Kuosa, H., Ikävalko, J., Kivi, K. & Hällfors, S. (1995). A redescription of Scrippsiella hangoei (Schiller) comb. nov. – a ‘red tide’ forming dinoflagellate from the northern Baltic. Phycologia, 34: 135–144.
- Levander (1894). Peridinium catenatum n. sp. – eine kettenbildende Peridinee im Finnischen Meerbusen. Acta Societatis pro Fauna et Flora Fennica, 9: 1–18+1 Platte.
- Levander, K.M. (1901). Zur Kenntnis des Planktons und der Bodenfauna einiger seichten Brackwasserbuchten. Acta Societatis pro Fauna et Flora Fennica, 20: 1–34.
- Lewis, J., Harris, A.S.D., Jones, K.J. & Edmonds, R.L. (1999). Long-term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. Journal of Plankton Research, 21: 343–345.
- Lignell, R., Heiskanen, A.-S., Kuosa, H., Gundersen, K., Kuuppo-Leinikki, P., Pajuniemi, R. & Uitto, A. (1993). Fate of a phytoplankton spring bloom: sedimentation and carbon flow in the planktonic food web in the northern Baltic. Marine Ecology Progress Series, 94: 239–252.
- Lima, A.L., Hubeny, J.B., Reddy, C.M., King, J.W., Hughen, K.A. & Eglinton, T.I. (2005). High-resolution historical records from Pettaquamscutt River basin sediments: 1. 210Pb and varve chronologies validate record of 137Cs released by the Chernobyl accident. Geochimica et Cosmochimica Acta, 69: 1803–1812.
- Lindemann, E. (1924). Ueber finnische Peridineen. Archiv fuer Hydrobiologie, 15: 1–4.
- Lundholm, N., Ribeiro, S., Andersen, T.J., Koch, T., Godhe, A., Ekelund, F. & Ellegaard, M. (2011). Buried alive – germination of up to a century-old marine protist resting stages. Phycologia, 50: 629–640.
- McCarthy, F.M., Mertens, K.N., Ellegaard, M., Sherman, K., Pospelova, V., Ribeiro, S., Blasco, S., & Vercauteren, D. (2011). Resting cysts of freshwater dinoflagellates in southeastern Georgian Bay (Lake Huron) as proxies of cultural eutrophication. Review of Palaeobotany and Palynology, 166: 46–62.
- McQuoid, M.R., Godhe, A. & Nordberg, K. (2002). Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. European Journal of Phycology, 37: 191–201.
- Miyazono, A., Nagai, S., Kudo, I. & Tanizawa, K. (2012). Viability of Alexandrium tamarense cysts in the sediment of Funka Bay, Hokkaido, Japan: over a hundred year survival times for cysts. Harmful Algae, 16: 81–88.
- Niemi, Å. (1975). Ecology of phytoplankton in the Tvärminne area, SW coast of Finland. II. Primary production and environmental conditions in the archipelago and the sea zone. Acta Botanica Fennica, 105: 1–73.
- Olli, K., Trikk, O., Klais, R., Ptacnik, R., Andersen, T., Lehtinen, S. & Tamminen, T. (2013). Harmonizing large data sets reveals novel patterns in the Baltic Sea phytoplankton community structure. Marine Ecology Progress Series, 473: 53–66.
- Rengefors, K. 1998. Seasonal succession of dinoflagellates coupled to the benthic cyst dynamics in Lake Erken. Arch. Hydrobiol. Spec. Issues Advanc. Limnol., 51: 123–141.
- Ribeiro, S., Amorim, A., Andersen, T.J., Abrantes, F. & Ellegaard, M. (2012). Reconstructing the history of an invasion: the toxic phytoplankton species Gymnodinium catenatum in the Northeast Atlantic. Biological Invasions, 14: 969–985.
- Ribeiro, S., Berge, T., Lundholm, N. & Ellegaard, M. (2013). Hundred years of environmental change and phytoplankton ecophysiological variability archived in coastal sediments. PLOS ONE, 8: e61184.
- Ribeiro, S., Berje, T., Lundholm, N., Andersen, T.J., Abrantes, F. & Ellegaard, M. (2011). Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nature Communications, 2: 311.
- Struck, U., Emeis, K.C., Voss, M., Christiansen, C. & Kunzendorf, H. (2000). Records of southern and central Baltic Sea eutrophication in δ13C and δ15N of sedimentary organic matter. Marine Geology, 164: 157–171.
- Sundström, A., Kremp, A., Daugbjerg, N., Moestrup, Ø., Ellegaard, M., Hajdu, S. & Hansen, R. (2009). Gymnodinium corollarium sp. nov. (Dinophyceae) – a new cold-water dinoflagellate responsible for cyst sedimentation events in the Baltic Sea. Journal of Phycology, 45: 938–952.
- Tahvanainen, P., Alpermann, T.J., Figueroa, R.I., John, U., Hakanen, P., Nagai, S., Blomster, J. & Kremp, A. (2012). Patterns of post-glacial genetic differentiation in marginal populations of a marine micro-alga. PLoS ONE, 7: e53602.
- Vallius, H. (2006). Permanent seafloor anoxia in coastal basins of the northwestern Gulf of Finland, Baltic Sea. AMBIO: A Journal of the Human Environment, 35: 105–108.
- von Stosch, H.A. (1973). Observations on vegetative reproduction and sexual life cycles of two freshwater dinoflagellates, Gymnodinium pseudopalustre Schiller and Woloszynskia apiculata sp. nov. British Phycological Journal, 8: 105–134.
- Warns, A., Hense, I. & Kremp, A. (2013). Modeling the life cycle of dinoflagellates – a case study with Biecheleria baltica. Journal of Plankton Research, 35: 379–392.
- Zonneveld, K.A.F., Versteegh, G.J.M. & de Lange, G.J. (1997). Preservation of organic-walled dinoflagellate cysts in different oxygen regimes: a 10,000 year natural experiment. Marine Micropaleontology, 29: 393–405.