ABSTRACT
Benthic diatoms are dominant primary producers in intertidal marine sediments, which are characterized by widely fluctuating and often extreme light conditions. To cope with sudden increases in light intensity, benthic diatoms display both behavioural and physiological photoprotection mechanisms. Behavioural photoprotection is restricted to raphid pennate diatoms, which possess a raphe system that enables motility and hence positioning in sediment light gradients (e.g. via vertical migration into the sediment). The main physiological photoprotection mechanism is to dissipate excess light energy as heat, measured as Non-Photochemical Quenching (NPQ) of chlorophyll fluorescence. A trade-off between vertical migration and physiological photoprotection (NPQ) in benthic diatoms has been hypothesized before, but this has never been formally tested. We exposed five epipelic diatom species (which move in between sediment particles) and four epipsammic diatom species (which live in close association with individual sand grains) to high light conditions, and characterized both NPQ and the relative magnitude of the migratory response to high light. Our results reveal the absence of a significant downward migratory response in an araphid diatom, but also in several raphid epipsammic diatoms, while all epipelic species showed a significant migratory response upon high light exposure. In all epipsammic species the upregulation of NPQ was rapid and pronounced; NPQ relaxation in low light conditions, however, occurred faster in the araphid diatom, compared with the raphid epipsammic species. In contrast, all epipelic species lacked a strong and flexible NPQ response and showed higher susceptibility to photodamage when not able to migrate. While overall our results support the vertical migration-NPQ trade-off, the lack of strong relationships between the capacity for vertical migration and NPQ within the epipsammic and epipelic groups suggests that other factors as well, such as cell size, substrate type and photoacclimation, may influence photoprotective strategies.
Introduction
Light is an indispensable but often highly variable resource for microalgae. While traits associated with light utilization are plastic, they can also differ between taxa, often in relation to the specific light climate in their respective habitats (Litchman & Klausmeier, Citation2008). For example, rapid physiological photoprotection mechanisms, such as excess energy dissipation as heat through Non-Photochemical Quenching of chlorophyll fluorescence (NPQ, related to de-epoxidation of xanthophyll pigments in the so-called xanthophyll cycle (XC); Lavaud & Goss, Citation2014) are more strongly developed in diatom species which inhabit strongly mixed and turbid coastal environments than in those inhabiting open ocean environments with a more stable light climate (Lavaud et al., Citation2007; Dimier et al., Citation2009; Bailleul et al., Citation2010).
At low tide, benthic diatoms living in intertidal sediments experience a light climate similar to the terrestrial environment, with often fast and unpredictable fluctuations in light (Lavaud & Goss, 2014), and display a high NPQ capacity (Perkins et al., Citation2010a). Large differences in NPQ capacity have been observed between benthic growth forms (Jesus et al., Citation2009; Cartaxana et al., Citation2011, Citation2016b; Barnett et al., Citation2015; Pniewski et al., Citation2015). Dense biofilms composed of epipelic diatoms form on fine-grained sediments (Sabbe, Citation1993; Ribeiro et al., Citation2013). Epipelic diatoms live freely on and in sediments and possess a raphe structure through which mucilage is secreted allowing movement (Round et al., Citation1990; Aumeier & Menzel, Citation2012). In addition to endogenous vertical migration rhythms in response to diurnal and tidal cycles (Consalvey et al., Citation2004), epipelic diatoms can also actively position themselves within sediment light gradients in order to maximize photosynthesis and/or avoid overexposure (Admiraal, Citation1984; Serôdio et al., Citation2006; Cartaxana et al., Citation2016a). While in situ epipelic diatom communities also activate the XC as a response to high light (Chevalier et al., Citation2010), downward vertical migration (VM) into the sediment is considered to be their prime response to high light stress (Perkins et al., Citation2010b). This behavioural response could as such minimize the need for physiological photoprotection (Serôdio et al., Citation2001; Raven, Citation2011).
In more sandy sediments, epipelic communities are largely replaced by communities of epipsammic diatoms. These diatoms are either araphid (and hence non-motile) and firmly attached to sand grains (either adnate or via a mucilage stalk), or raphid. In the latter case, it is hypothesized that their movement is largely restricted to the sphere of individual sand grains (Sabbe, Citation1997; Ribeiro et al., Citation2013). As in situ communities living on sandy sediments showed no migratory behaviour and exhibited higher diatoxanthin/diadinoxanthin (Dtx/Ddx) ratios than communities living on silt, a trade-off between behavioural (VM) and physiological photoprotection (NPQ) was proposed (van Leeuwe et al., Citation2008; Jesus et al., Citation2009). Exposing both silt- and sand-inhabiting communities to high light in controlled laboratory conditions supported this hypothesis as the sand-inhabiting communities showed higher Dtx/Ddx ratios while not migrating downward in response to high light (Cartaxana et al., Citation2011). These observations were confirmed by Barnett et al. (Citation2015) who used unialgal cultures to show that epipsammic diatoms indeed have a higher capacity for NPQ and XC than epipelic species. In addition, non-motile epipsammic species show a stronger coupling between NPQ development and the light saturation point (Ek) than motile epipsammic species. Finally, Laviale et al. (Citation2016) showed that in epipelic communities light induction of VM occurs at a similar rate as NPQ induction, an essential condition for a migration–physiology trade-off.
While all studies mentioned above support a trade-off between NPQ and VM, combined measurements of both traits are limited to natural communities (Perkins et al., Citation2010b; Serôdio et al., Citation2012; Laviale et al., Citation2015, Citation2016). Natural communities however usually contain a mix of growth forms and species (Hamels et al., Citation1998), which can hamper the interpretation of NPQ and XC measurements as both growth form and species responses can be quite specific (Underwood et al., Citation2005; Barnett et al., Citation2015; Cohn et al., Citation2015). The photoprotection capacity of raphid and araphid epipsammic growth forms has rarely been investigated in unialgal cultures (Barnett et al., Citation2015; Blommaert et al., Citation2017), while the capacity for vertical migration in epipsammic species has, to our knowledge, never been investigated in cultures.
Here we investigated the relationship between NPQ activation and relaxation and vertical migration capacity for a set of common epipelic (5) and epipsammic (4) diatom species. As it is important that trade-offs are studied with all else being equal (Litchman & Klausmeier, Citation2008), we quantified both traits under identical conditions, with all strains acclimated to the same light climate.
Materials and methods
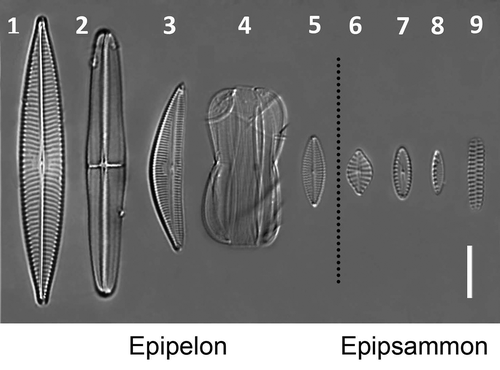
Epipelic and epipsammic diatom strains were obtained from the diatom culture collection (BCCM/DCG) of the Belgian Coordinated Collection of Micro-organisms (http:/BCCM.belspo.be) and the Nantes Culture Collection-France (NCC) (http://ncc.univ-nantes.fr/). Accession numbers are given in . Photographs of all species were taken with an Axiophot2 microscope (Carl Zeiss AG, Oberkochen, Germany), equipped with a monochrome digital camera, AxioCam MRm (Carl Zeiss AG, Oberkochen, Germany) (–). Species were grown at 20°C in batch cultures in a day/night regime of 16/8 h with a light intensity of 20 µmol photons m−2 s−1 using two L58W/840 Lumilux cool white tubes and one L58W/865 cool daylight fluorescent tube (Osram, Munich, Germany). Cells were cultured in Provasoli’s enriched f/2 seawater medium using Tropic Marin artificial sea salt (Dr. Beiner GmbH, Wartenberg, Germany) (34.5 g l−1) enriched with NaHCO3 (80 mg l−1 final concentration). Cultures were acclimated to these culturing conditions for at least 2 weeks prior to the experiments.
Table 1. Species information.
Figs 1–9. Light micrographs of the species used in this study. , Navicula arenaria var. rostellata; , Craspedostauros britannicus; , Seminavis robusta; , Entomoneis paludosa; , Navicula phyllepta; , Planothidium delicatulum; , Biremis lucens; , Nitzschia cf. frustulum; , Opephora guenter-grassii. Scale bar = 10 µm.
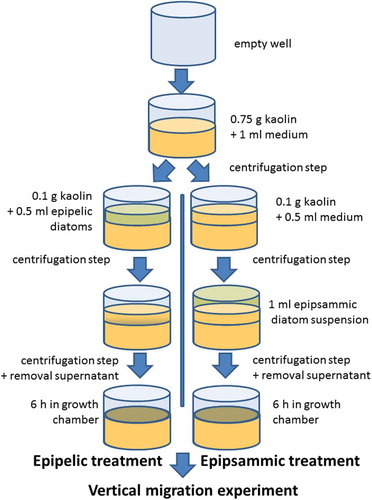
Preparation of monospecific biofilms
Light brown kaolin (Carl Roth GmbH, Karlsruhe, Germany) was used as a standard test substrate for diatom motility as it has similar properties to mudflat sediment (Hay et al., Citation1993) and is commercially available. Differences in sediment light climate between natural sediments and kaolin, however, could not be excluded. A schematic overview of artificial biofilm preparation is shown in . Twenty-four-well plates were filled with 0.75 g kaolin in each well and mixed with 1 ml of medium to obtain a homogeneous suspension. The sediment was pelleted by centrifugation at 1000 RCF for 5 min. For epipelic diatoms, monospecific suspensions (0.5 ml, 6–10 µg Chl a ml−1) were mixed with 0.1 g kaolin, pipetted on top of the kaolin within the wells and centrifuged at 50 RCF for 1 min. The diatom suspensions were mixed with kaolin because centrifugation without kaolin resulted in an uneven distribution of the diatom layer on the sediment. For epipelic species 10 µg Chl a ml−1 suspensions were used, except for both Navicula species (6 µg Chl a ml−1). After a second centrifugation step (to conform with the epipsammic treatment, see below), the supernatant medium was removed and the diatoms were allowed to migrate to the surface for 6 h in 20 µmol photons m−2 s−1 at 20°C. Higher light intensities were not used for upward migration to avoid a photophobic response or a change in photophysiology. For epipsammic diatoms, 0.1 g kaolin, mixed with 0.5 ml artificial seawater but without diatoms, was first added to the wells already containing 0.75 g centrifuged kaolin, as described above, and then centrifuged at 50 RCF for 1 min. Afterwards, 1 ml suspension of epipsammic diatoms (2 µg Chl a ml−1) was added and centrifuged at 50 RCF for 1 min after which the supernatant medium was removed. The epipsammic treatment was slightly different from the epipelic one because the cells were either non-motile or did not migrate to the sediment surface within the same time frame used for the epipelic diatoms. By analogy with the epipelic treatment, the epipsammic species were then placed in 20 µmol photons m−2 s−1 at 20°C for 6 h before high light exposure. Surface biomass (expressed as Normalized Difference Vegetation Index (NDVI), see below), measured before the start of the experiments, ranged between 0.1 and 0.25 for all species. To quantify the extent of vertical migration, relative values were calculated, thus standardizing for differences in initial surface biomass.
NDVI and chlorophyll fluorescence measurements
Both pulse amplitude modulated (PAM) fluorescence imaging and NDVI were measured with a standard MAXI Imaging PAM M-series (Heinz Walz GmbH, Effeltrich, Germany), equipped with an IMAG-K4 camera and mounted with an IMAG-MAX/F filter. The illumination unit of the imaging system contains red (660 nm) and near-infrared (NIR, 780 nm) LEDs, providing monochromatic pulse modulated light. The reflectance images of the monochromatically illuminated samples were captured by the same CCD-chip that captures chlorophyll fluorescence.
In two separate experiments, NPQ and VM (see below) were measured immediately before (0 min) and after 2.5, 5, 10, 15, 20, 25 and 30 min HL illumination (1900 µmol photons m−2 s−1, photosynthetically available radiation), provided by the MAXI Imaging PAM Blue LED-panel. The outer wells of the 24-well plates were not included to avoid heterogeneity in light intensity.
The intensities of both red and NIR illumination sources were calibrated with an 18% grey standard (Neutral Grey Card 4963, FOTOWAND-Technic Dietmar Meisel, Sudwalde, Germany), placed in the middle of the camera field of view as during HL exposure the NIR reflectance decreased while red reflectance increased. Red and NIR reflectance images were captured automatically using the script function in the ImagingWin software. Areas of interest (AOI) were placed in the middle of each well to avoid edge-effects using the ImagingWin (v2.41a) software (Heinz Walz GmbH, Effeltrich, Germany). Sediment temperature was maintained at 20°C by working in an air-conditioned room, removing the Perspex eye-protection hood and providing additional cooling with a fan, and keeping the 24-well plate, which was perforated in between the wells, in a water bath on a stirring plate.
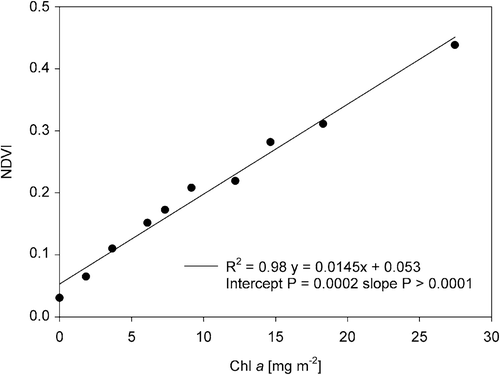
For NDVI measurements, a saturating pulse (0.8 s, INT 8) was fired at the end of each HL interval to create a new file in which only one red and NIR image could be saved. Red and NIR were captured after 10 s of darkness after this saturating pulse to avoid interference. NDVI was calculated as (R780 – R660)/(R780 + R660) using the AOI averages (Rouse et al., Citation1974). An NDVI vs. chlorophyll a (Chl a) calibration curve was constructed by creating artificial biofilms by centrifuging (cf. epipsammic treatment above) suspensions of known Chl a content (determined spectrophotometrically; Jeffrey & Humphrey, Citation1975) of the diatom Phaeodactylum tricornutum K. Bohlin (). The extent of vertical migration was calculated as the relative (percentage) decline in initial NDVI values (Laviale et al., Citation2016) after subtraction of the NDVI value of kaolin without diatoms, which was recorded simultaneously. Even though we controlled for changes in red and NIR illumination, we observed a decline in recorded NDVI of a sheet of green paper (absorbing in the red spectrum) under the same light conditions, possibly due to a shift in LED-spectrum resulting from the heating up of the LED panel during HL illumination. We corrected for this artifact (see Supplementary fig. S1) by adding the average decline of 15 observations (three independent measurements of 5 AIOs in the green sheet) to the recorded data for diatoms.
Fig. 11. The linear regression of the Normalized Difference Vegetation Index (NDVI) with chlorophyll a content, determined spectrophotometrically, on a Phaeodactylum tricornutum dilution series centrifuged on kaolin.
NPQ was measured on diatom suspensions in 24-well plates (1 ml containing 1 µg Chl a, measured as above) using the same light conditions as the vertical migration assay. As no sediment was present, a behavioural photoprotection response was not possible. NPQ reversal was measured during an additional 30 min low light (LL) recovery period (15 µmol photons m−2 s−1). Saturating pulses (0.8 s, INT 8) were fired automatically each 5 min. A fluorescence standard (Heinz Walz GmbH, Effeltrich, Germany) was measured simultaneously to correct for deviations in measuring light intensity during HL. NPQ was calculated as (Fm-Fm’)/Fm’, where Fm is the Maximum PSII chlorophyll fluorescence yield and Fm’ is maximum PSII Chl fluorescence yield (Fm) during illumination. As Fm’ values recorded during HL were lower than the minimum PSII Chl fluorescence yield F0 (as observed in diatoms by Lavaud et al., Citation2002), qN (as used by Laviale et al., Citation2016) was not determined. Photosynthetic efficiency of PSII (ΔF/Fm’) was calculated as (Fm’-F’)/Fm’ and expressed as a percentage, taking the maximal photosynthetic efficiency (Fv/Fm), measured immediately before HL onset as 100%.
The NPQ induction (1) and recovery (2) rates (k) were calculated by fitting an exponential decay function (non-linear regression), derived from Olaizola & Yamamoto (Citation1994):
(1) NPQ(t) = NPQmax + [NPQ0 – NPQmax]e−kt
where t represents time during HL and NPQmax and NPQ0 represent NPQ after 30 min HL and before HL onset, respectively.
(2) NPQ(t) = NPQr + [NPQmax − NPQr]e−kt
where t represents time during recovery and NPQmax and NPQr represent NPQ at the start of the recovery period and after 30 min of recovery in LL, respectively. Statistical analyses were conducted using the statistical software package SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Exponential decay functions were fitted using the non-linear regression procedure (PROC NLIN). Measured and fitted parameters (three biological replicates per species) were compared between species using ANOVA, followed by a Tukey’s test, using the General Linear Model procedure (PROC GLM). P-values of 0.05 or less were considered statistically significant. A decrease in surface biomass was evaluated as a one-sided t-test (only a decrease was considered).
Results
NDVI measured with the MAXI Imaging PAM M-series
A dilution series of Phaeodactylum tricornutum suspensions of known Chl a content was centrifuged on kaolin sediment to create artificial biofilms. NDVI of these biofilms correlated very well (R2 = 0.98, P < 0.0001) with Chl a content (). As the y-intercept was larger than zero (P = 0.0002), a blank with bare sediment was included and its NDVI measurement subtracted from all samples in all VM experiments.
Behavioural photoprotection – vertical migration (VM)
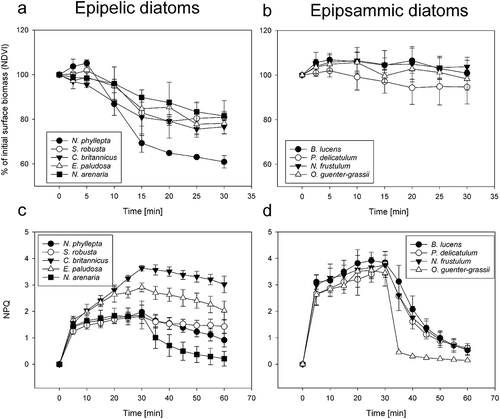
All epipelic species showed a significant (one-sided t-test, P < 0.05) decrease in surface biomass (20–40%) by the end of the HL illumination period (VM30) (, ; significant P-values listed in Supplementary table S1). As only two replicates were included for Entomoneis paludosa, this species was excluded from statistical analysis. None of the epipsammic species, including the motile ones, showed a significant surface biomass decline during the HL period (, ; P-values listed in Supplementary table S1). Of the epipelic species, only Craspedostauros britannicus showed a small but significant decrease in surface biomass after 2.5 min (P = 0.001). After 15 min of HL, C. britannicus, Navicula phyllepta and N. arenaria had significantly lower surface biomass than at the start of the experiment (one-sided t-test). The smallest epipelic species N. phyllepta exhibited the most pronounced vertical migration (); decreasing its surface biomass significantly more than any other tested epipelic species within the 30 min HL exposure period (ANOVA and Tukey’s post hoc pairwise comparison, P-values reported in Supplementary table S2). No significant differences in surface biomass decrease were found between the other epipelic diatoms after 30 min of HL (Supplementary table S2). A control experiment, using only the largest (N. arenaria) and smallest (N. phyllepta) epipelic diatoms (two technical replicates each), showed no VM in growth light conditions (20 µmol photons m−2 s−1, Supplementary fig. S2).
Fig. 12. The decrease in surface biomass, measured as NDVI, of epipelic diatoms (a) and epipsammic diatoms (b) on kaolin during 30 min of HL. Note that the y-axis in these plots starts at 50%. Non-photochemical quenching (NPQ) for epipelic diatoms (c) and epipsammic diatoms (d), measured during 30 min HL and 30 min LL recovery. Values represent averages of three independent measurements ± standard deviations.
Physiological photoprotection – NPQ
All investigated epipsammic species displayed a strong NPQ induction, resulting in significantly higher NPQ values than epipelic species after 5 min HL (, ) (ANOVA and Tukey’s post hoc pairwise comparison, P-values reported in Supplementary table S3). No significant differences in NPQ were observed between diatoms of the same growth form at this time point (Supplementary table S3). The NPQ induction rate was lowest in C. britannicus (rate constant k = 0.08 min−1; SD = 0.02), which was significantly lower than in B. lucens, E. paludosa, O. guenter-grassii and P. delicatulum (ANOVA and Tukey’s post hoc pairwise comparison, P-values reported in Supplementary table S4). The epipsammic species showed a comparable but lower further increase in NPQ during the rest of the HL period. In the epipelic species, NPQ diverged by the end of the HL exposure period. Both C. britannicus and E. paludosa showed a strong NPQ increase during the HL period, resulting in significantly higher NPQ values than the other tested epipelic species (except for the difference between E. paludosa and N. phyllepta which was not significant, ANOVA and Tukey’s post hoc pairwise comparison, p-values reported in Supplementary table S5).
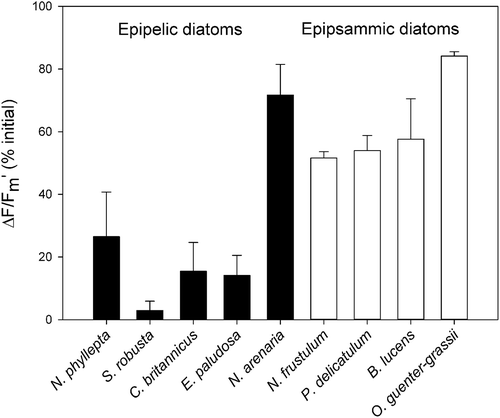
During the 30 min low light recovery period NPQ relaxed rapidly in all epipsammic species, whereas only one epipelic species, N. arenaria, showed clear NPQ relaxation. Sustained quenching (NPQs) after 30 min recovery was highest for C. britannicus, followed by E. paludosa. Fitting NPQ relaxation with exponential decay functions revealed significantly faster NPQ relaxation in the araphid epipsammic diatom Opephora guenter-grassii (rate constant k = 0.52 min−1; SD = 0.03), whereas no significant differences were observed between the raphid epipsammic diatoms and N. arenaria (rate constant k = 0.13 min−1; SD = 0.06) (ANOVA and Tukey’s post hoc pairwise comparison, P-values reported in Supplementary table S4). The recovery of the quantum yield of PSII (ΔF/Fm’) after 30 min of LL () was higher in all epipsammic species compared with the investigated epipelic species, with the notable exception of the epipelic species N. arenaria which showed an equally high recovery (ANOVA and Tukey’s post hoc pairwise comparison, P-values reported in Supplementary table S6).
Fig. 13. The quantum yield of PSII (ΔF/Fm’), after 30 min of HL and 30 min of LL recovery, expressed in percentage of the maximal photosynthetic efficiency of PSII (Fv/Fm) before HL exposure for epipelic (black bars) and epipsammic diatoms (white bars). Values represent averages of three independent measurements ± standard deviations.
Plotting the NPQ values after 5 min HL versus VM30 (% NDVI decrease after 30 min) () revealed a clear VM-NPQ trade-off between the epipelic and epipsammic diatom groups, with epipelic diatoms displaying high VM30 and low NPQ, and epipsammic diatoms showing no clear VM and high NPQ. However, no VM30/NPQ trade-offs were observed within both functional groups.
Fig. 14. The extent of vertical migration (measured as the decrease in surface biomass in percentage, cf. )], measured after 30 min of HL in function of the NPQ capacity, measured after 5 min of HL to avoid the effect of photoinhibition for epipelic (black symbols) and epipsammic diatoms (white symbols). Three replicates per species are plotted.
![Fig. 14. The extent of vertical migration (measured as the decrease in surface biomass in percentage, cf. Fig. 12a, b)], measured after 30 min of HL in function of the NPQ capacity, measured after 5 min of HL to avoid the effect of photoinhibition for epipelic (black symbols) and epipsammic diatoms (white symbols). Three replicates per species are plotted.](/cms/asset/5d79eb04-be08-4d6f-9fbb-e321b419f857/tejp_a_1397197_f0006_b.gif)
Discussion
As the ability to vertically migrate (VM) away from high light to avoid photoinhibition might minimize the costs associated with high and flexible NPQ (Raven, Citation2011), we compared NPQ and VM capacity as photoprotection mechanisms in a set of five epipelic and four epipsammic species and confirm a general NPQ-VM trade-off between both functional groups.
The fact that no vertical movement was observed in the epipsammic species shows that the migration observed in the epipelic species was not caused by a passive process such as water percolation. To further test whether the response was indeed caused by high light, we performed a control experiment with the largest (N. arenaria) and smallest (N. phyllepta) epipelic diatoms in which we exposed them to growth light conditions (i.e. low light) instead of high light conditions. No migratory response was observed (see Supplementary fig. S2), clearly demonstrating that the migratory response was caused by high light.
While all epipelic species showed significant VM, they migrated less fast compared with natural epipelic communities, where surface biomass decreased up to 30% within the first 2.5 min of high light (Laviale et al., Citation2016). In our experiments, where VM was prevented under HL conditions, NPQ was initially low for all epipelic species, but for some species, it increased considerably during the course of the HL exposure. Most of this NPQ, however, coincided with high sustained NPQ (NPQs, see below). Epipsammic species compensated for the absence of significant VM with a strong NPQ response during HL onset. Consistent differences in NPQ induction rate between epipelic and epipsammic diatoms were not observed, possibly due to the fact that we have no data for the first five minutes of high light, during which most of the NPQ induction, due to Ddx de-epoxidation, takes place (Serôdio et al., Citation2005).
Besides a strong NPQ after HL onset, epipsammic diatoms were also able to relax NPQ rapidly during low light conditions. Within both growth forms, trade-offs between both photoprotective strategies were not observed. However, the only araphid (and hence by definition non-motile) epipsammic diatom included here showed considerably faster relaxation of NPQ after high light exposure than the raphid epipsammic diatoms and is therefore able to more efficiently track rapid changes in irradiance intensity (Lavaud et al., Citation2007; Lavaud & Lepetit, Citation2013). Taken together, our results confirm that epipelic and epipsammic growth forms can be seen as different functional groups exhibiting contrasting primary photoprotection strategies.
NPQ differences between epipelic and epipsammic growth forms were studied by Barnett et al. (Citation2015), and agree with our observations after 5 min HL. Higher NPQ values in epipsammic diatoms were attributed to higher Dtx production, originating from a larger Ddx + Dtx pool rather than a higher Ddx de-epoxidation state. The measured NPQ values in two epipelic species (C. britannicus and E. paludosa), however, increased to similar levels as in the epipsammic diatoms after 30 min HL, a feature not observed by Barnett et al. (Citation2015) as only 5 min illumination periods were used. During the low light recovery period, however, all epipelic species except N. arenaria showed high NPQs and a low recovery of PSII quantum yield. High NPQs has been observed after exposure to high light conditions or a combination of high light and elevated temperatures (Zhu et al., Citation2010; Lavaud & Lepetit, Citation2013; Laviale et al., Citation2015), and has been attributed to a slow epoxidation of Dtx back to Ddx (Lavaud & Lepetit, Citation2013; Lavaud & Goss, Citation2014; Blommaert et al., Citation2017) and/or photoinhibition (qI). In contrast with our observations, epipelic communities freshly obtained from the field are able to withstand high light doses (up to 1200 μmol photons m−2 s−1) for up to 3 hours, even when VM is inhibited (Serôdio et al., Citation2012; Laviale et al., Citation2015). Moreover, they show higher NPQ values, while relaxing their NPQ more in low light conditions (Serôdio et al., Citation2005, Citation2008, Citation2012), suggesting that these field communities are less sensitive to photoinhibition than monospecific epipelic cultures. This could be due to the fact that the diatom cultures used in our study were acclimated to rather low light intensities (20 μmol photons m−2 s−1) and exposed to relatively high light intensities. Acclimation to higher irradiances increased the NPQ capacity of epipelic diatoms (Cruz & Serôdio, Citation2008; Ezequiel et al., Citation2015; Barnett et al., Citation2015) and caused them to accumulate at higher light intensities in a light gradient (Ezequiel et al., Citation2015). As a result, the VM/NPQ trade-off between epipsammic and epipelic diatoms observed in the field may not be as pronounced as observed in our experiments.
Alternatively, the high NPQs/qI in epipelic species, as observed in this study, could be related to the origin of the strains. Diatom communities originating from Portuguese mudflats, as used in the above studies, tend to have overall higher NPQ and less NPQs and/or photoinhibition after high light exposure than communities sampled at higher latitudes along the Atlantic Coast (Laviale et al., Citation2015). Pniewski et al. (Citation2015) also observed photoinhibition (measured as a decline in oxygen evolution-irradiance curves) in epipelic communities from Aiguillon Bay (Atlantic coast, France). The observed absence or low amount of NPQs/qI and, in general, higher recovery of PSII quantum yield in epipsammic diatoms in this study confirm that the energy-dissipating mechanisms of these diatoms are capable of tracking light fluctuations (cf. Blommaert et al., Citation2017) and optimizing photosynthesis in rapidly fluctuating and high light conditions, as photosynthesis can be forgone if energy-dissipating mechanisms fail to relax in light-limiting conditions (Raven, Citation2011). Finally, sustained NPQ in MPB diatoms may be advantageous to keep the antenna system in a basal dissipative state, allowing the cells to cope with a sudden increase in light intensity after a long dark period as may happen during immersion and night emersion (Lavaud & Goss, Citation2014).
Differences in motility of epipelic diatoms (here mainly between N. phyllepta and larger species) were not reflected in NPQ capacity and thus do not point to a VM-NPQ trade-off within the epipelic group. Navicula phyllepta is much smaller (~13 µm long) than the other studied epipelic species (>30 µm long) but exhibited the strongest migratory response, whereas no differences in VM were observed between the larger species. The difference in VM between N. phyllepta and the larger species may be due to the fact that because of its smaller size (and assuming comparable pigment concentration per unit biovolume) pigment self-shading may be lower, rendering the cells more vulnerable to photoinactivation (Key et al., Citation2010) and therefore requiring higher photoprotection (i.c. VM). In this respect it is interesting to note that in a field study small naviculoid diatoms were mainly observed at the sediment surface in early morning when light intensity was still relatively low whereas larger species dominated the intertidal surface biofilm at noon (Underwood et al., Citation2005). It should also be noted that while VM was observed in all epipelic species, most diatom biomass stayed at the sediment surface as observed by Laviale et al. (Citation2015, Citation2016). Therefore, epipelic diatoms might have used alternative photoprotection strategies or displayed within-population cyclical micromigration at the sediment surface (Kromkamp et al., Citation1998), as such obscuring differences in VM as a photoprotection strategy.
An NPQ-VM capacity trade-off was also not observed within the epipsammic group: no significant NPQ differences were detected during the high light period and no significant VM was observed. However, all epipsammic species we tested, with the exception of O. guenter-grasssii, were raphid and therefore in principle capable of movement. However, despite the fact that some of them were in the same size range (see –) as N. phyllepta (which displayed the strongest migratory response), they did not migrate down in response to high light. The absence of vertical migration is in accordance with the lack of endogenous migratory rhythms in epipsammic communities (Jesus et al., Citation2009) and the observation that epipsammic diatoms in the field do not seem to migrate down in response to high light (Cartaxana et al., Citation2011). Barnett et al. (Citation2015) did observe differences in NPQ capacity between motile and non-motile epipsammic species, but not at the highest light intensity (2000 μmol photons m−2 s−1). The most notable difference between the epipsammic species in our study was the faster NPQ relaxation in the araphid species O. guenter-grassii, which is due to fast Dtx epoxidation in low light conditions (Blommaert et al., Citation2017). The slower relaxation in raphid epipsammic species may suggest that they do use motility, not to perform VM but to move to slightly more shaded areas on the sand grain surface, such as depressions, where epipsammic diatoms are often seen to aggregate (Miller et al., Citation1987; Jewson et al., Citation2006; Sabbe, unpubl. obs.). While it has been hypothesized that cell accumulations in depressions can represent a strategy to protect against abrasion (Miller et al., Citation1987), it could also be a way to reduce high light stress through increased cell shading caused by cell accumulation and the microtopography of the sand grains. This may slightly reduce the need for the very rapid NPQ relaxation observed in the araphid species. An alternative explanation for the absence of significant VM in raphid epipsammic species in sandy sediments is that because light is scattered and penetrates deeper than in silty sediments (Kühl et al., Citation1994; Cartaxana et al., Citation2016b), downward VM would probably not drastically change the experienced light climate (Cartaxana et al., Citation2016b). A third potential explanation for the observed difference in NPQ relaxation between the araphid and raphid epipsammic species could be that slower NPQ relaxation represents a phylogenetic signal typical for raphid, motile diatoms that was retained in raphid taxa which adopted an epipsammic growth form.
Finally, it needs to be pointed out that the distinction between araphid and raphid epipsammon does not necessarily coincide with a difference in motility. Some raphid species, such as Biremis lucens, are usually observed as small colonies which are attached to the sand grain surface via their girdle side (Sabbe et al., Citation1995). For this reason, this species was classified as non-motile in Barnett et al. (Citation2015). In the present study, we focused on the distinction between araphid and raphid, as raphid diatoms are at least potentially motile as they possess a raphe. Motility in most of these forms, however, has not yet been properly characterized.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2017.1397197
Supplementary table S1. Significance of surface biomass (NDVI) decline.
Supplementary table S2. Comparison of surface biomass differences between species after 30 min of HL.
Supplementary table S3. Comparison of NPQ, measured after 5 min HL, between species.
Supplementary table S4. Values (a) and comparison of the NPQ induction rate k per species (b).
Supplementary table S5. Comparison of NPQ, measured after 30 min HL, between species.
Supplementary table S6. Comparison of ΔF/Fm’, measured after 30 min LL recovery, between species.
Supplementary fig. S1. Decline in NDVI, expressed in percentage, during the HL period.
Supplementary fig. S2. Absence of vertical migration in the epipelic species Navicula phyllepta and N. arenaria at 20 µmol photons m−2 s−1.
Supplementary_material.docx
Download MS Word (92.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Wim Vyverman
L. Blommaert: experimental design, experimental work, drafting manuscript; J. Lavaud: revising the manuscript; W. Vyverman: original concept, revising the manuscript; K. Sabbe: Experimental design, editing and revising the manuscript.
References
- Admiraal, W. (1984). The ecology of estuarine sediment-inhabiting diatoms. Progress in Phycological Research, 3: 269–322.
- Aumeier, C. & Menzel, D. (2012). Secretion in the diatoms. In Secretions and Exudates in Biological Systems (Vivanco, J.M. & Baluška, F., editors), 221–250. Springer, Berlin.
- Bailleul, B., Rogato, A., De Martino, A., Coesel, S., Cardol, P., Bowler, C., Falciatore, A. & Finazzi, G. (2010). An atypical member of the light-harvesting complex stress-related protein family modulates diatom responses to light. Proceedings of the National Academy of Sciences of the USA, 107: 18214–18219.
- Barnett, A., Méléder, V., Blommaert, L., Lepetit, B., Gaudin, P., Vyverman, W., Sabbe, K., Dupuy, C. & Lavaud, J. (2015). Growth form defines physiological photoprotective capacity in intertidal benthic diatoms. The ISME Journal, 9: 32–45.
- Blommaert, L., Huysman, M.J.J., Vyverman, W., Lavaud, J. & Sabbe, K. (2017). Contrasting NPQ dynamics and xanthophyll cycling in a motile and a non motile intertidal benthic diatom. Limnology and Oceanography, 62: 1466–1479..
- Cartaxana, P., Ruivo, M., Hubas, C., Davidson, I., Serôdio, J. & Jesus, B. (2011). Physiological versus behavioral photoprotection in intertidal epipelic and epipsammic benthic diatom communities. Journal of Experimental Marine Biology and Ecology, 405: 120–127.
- Cartaxana, P., Cruz, S., Gameiro, C. & Kühl, M. (2016a). Regulation of intertidal microphytobenthos photosynthesis over a diel emersion period is strongly affected by diatom migration patterns. Frontiers in Microbiology, 7: 872.
- Cartaxana, P., Ribeiro, L., Goessling, J., Cruz, S. & Kühl., M. (2016b). Light and O2 microenvironments in two contrasting diatom-dominated coastal sediments. Marine Ecology Progress Series, 545: 35–47.
- Chevalier, E.M., Gévaert, F. & Créach, A. (2010). In situ photosynthetic activity and xanthophylls cycle development of undisturbed microphytobenthos in an intertidal mudflat. Journal of Experimental Marine Biology and Ecology, 385: 44–49.
- Cohn, S.A., Halpin, D., Hawley, N., Ismail, A., Kaplan, Z., Kordez, T., Kuhn, J., Macke, W., Marhaver, K., Ness, B., Olszewski, S., Rice, E., Sbarboro, J., Wolske, A. & Zapata, Y. (2015). Comparative analysis of light-stimulated motility responses in three diatom species. Diatom Research, 30: 213–225.
- Consalvey, M., Paterson, D.M. & Underwood, G.J.C. (2004). The ups and downs of life in a benthic biofilm: migration of benthic diatoms. Diatom Research, 19: 181–202.
- Cruz, S. & Serôdio, J. (2008). Relationship of rapid light curves of variable fluorescence to photoacclimation and non-photochemical quenching in a benthic diatom. Aquatic Botany, 88: 256–264.
- Dimier, C., Giovanni, S., Ferdinando, T. & Brunet, C. (2009). Comparative ecophysiology of the xanthophyll cycle in six marine phytoplanktonic species. Protist, 160: 397–411.
- Ezequiel, J., Laviale, M., Frankenbach, S., Cartaxana, P. & Serôdio, J. (2015). Photoacclimation state determines the photobehaviour of motile microalgae: the case of a benthic diatom. Journal of Experimental Marine Biology and Ecology, 468: 11–20.
- Hamels, I., Sabbe, K., Muylaert, K., Barranguet, C., Lucas, C., Herman, P. & Vyverman, W. (1998). Organisation of microbenthic communities in intertidal estuarine flats, a case study from the Molenplaat (Westerschelde estuary, the Netherlands). European Journal of Protistology, 34: 308–320.
- Hay, S.I., Maitland, T.C. & Paterson, D.M. (1993). The speed of diatom migration through natural and artificial substrata. Diatom Research, 8: 371–384.
- Jeffrey, S.W. & Humphrey, G.S. (1975). New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiology der Pflanzen, 167: 191–194.
- Jesus, B., Brotas, V., Ribeiro, L., Mendes, C.R., Cartaxana, P. & Paterson, D.M. (2009). Adaptations of microphytobenthos assemblages to sediment type and tidal position. Continental Shelf Research, 29: 1624–1634.
- Jewson, D.H., Lowry, S.F. & Bowen, R. (2006). Co-existence and survival of diatoms on sand grains. European Journal of Phycology, 41: 131–146.
- Key, T., McCarthy, A., Campbell, D.A., Six, C., Roy, S. & Finkel, Z.V. (2010). Cell size trade-offs govern light exploitation strategies in marine phytoplankton. Environmental Microbiology, 12: 95–104.
- Kromkamp, J.C., Barranguet, C. & Peene, J. (1998). Determination of microphytobenthos PSII quantum efficiency and photosynthetic activity by means of variable chlorophyll fluorescence. Marine Ecology Progress Series, 162: 45–55.
- Kühl, M., Lassen, C. & Jorgensen, B.B. (1994). Light penetration and light intensity in sandy marine sediments measured with irradiance and scalar irradiance fiber-optic microprobes. Marine Ecology Progress Series, 105: 139–148.
- Lavaud, J. & Goss, R. (2014). The peculiar features of the non-photochemical fluorescence quenching in diatoms and brown algae. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria (Demmig-Adams, B., Garab, G., Adams III, W. & Govindjee, editors), 421–443. Springer, Dordrecht.
- Lavaud, J. & Lepetit, B. (2013). An explanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms. Biochimica et Biophysica Acta, 1827: 294–302.
- Lavaud, J., Rousseau, B., Van Gorkom, H. J. & Etienne, A. (2002). Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiology, 129: 1398–1406.
- Lavaud, J., Strzepek, R.F. & Kroth, P.G. (2007). Photoprotection capacity differs among diatoms: possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnology and Oceanography, 52: 1188–1194.
- Laviale, M., Barnett, A., Ezequiel, J., Lepetit, B., Frankenbach, S., Méléder, V., Serôdio, J. & Lavaud, J. (2015). Response of intertidal benthic microalgal biofilms to a coupled light-temperature stress: evidence for latitudinal adaptation along the Atlantic coast of Southern Europe. Environmental Microbiology, 17: 3662–3677.
- Laviale, M., Frankenbach, S. & Serôdio, J. (2016). The importance of being fast: comparative kinetics of vertical migration and non-photochemical quenching of benthic diatoms under light stress. Marine Biology, 163: 10.
- van Leeuwe, M., Brotas, V., Consalvey, M., Forster, R., Gillespie, D., Jesus, B., Roggeveld, J. & Gieskes, W. (2008). Photoacclimation in microphytobenthos and the role of xanthophyll pigments. European Journal of Phycology, 43: 123–132.
- Litchman, E. & Klausmeier, C.A. (2008). Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution, and Systematics, 39: 615–639.
- Miller, A.R., Lowe, R.L. & Rotenberry, J.T. (1987). Succession of diatom communities on sand grains. Journal of Ecology, 75: 693–709.
- Olaizola, M. & Yamamoto, H.Y. (1994). Short-term response of the diadinoxanthin cycle and fluorescence yield to high irradiance in Chaetoceros muelleri (Bacillariophyceae). Journal of Phycology, 30: 606–612.
- Perkins, R.G., Kromkamp, J.C., Serôdio, J., Lavaud, J., Jesus, B., Mouget, J.L., Lefebvre, S. & Forster, R.M. (2010a). The application of variable chlorophyll fluorescence to microphytobenthic biofilms. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications (Suggett, D.J., Prášil, O. & Borowitzka, M.A., editors), 237–275. Springer, Dordrecht.
- Perkins, R.G., J. Lavaud, J. Serôdio, Mouget, J.L., Cartaxana, P., Rosa, P., Barille, L., Brotas, V. & Jesus, B. (2010b). Vertical cell movement is a primary response of intertidal benthic biofilms to increasing light dose. Marine Ecology Progress Series, 416: 93–103.
- Pniewski, F.F., Biskup, P., Bubak, I., Richard, P., Latała, A. & Blanchard, G. (2015). Photo-regulation in microphytobenthos from intertidal mudflats and non-tidal coastal shallows. Estuarine, Coastal and Shelf Science, 152: 153–161.
- Raven, J.A. (2011). The cost of photoinhibition. Physiologia Plantarum, 142: 87–104.
- Ribeiro, L., Brotas, V., Rincé, Y. & Jesus, B. (2013). Structure and diversity of intertidal benthic diatom assemblages in contrasting shores: a case study from the Tagus estuary. Journal of Phycology, 49: 258–270.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms – Biology and Morphology of the Genera. Cambridge University Press, Cambridge.
- Rouse, J.W., Jr, Haas, R.H., Schell, J.A. & Deering, D.W. (1974). Monitoring vegetation systems in the Great Plains with Erts. In Third Earth Resources Technology Satellite-1 Symposium – Volume I: Technical Presentations (Freden, S.C., Mercanti, E.P. & Becker, M. A., editors), 309–317. NASA, Washington, DC.
- Sabbe, K. (1993). Short-term fluctuations in benthic diatom numbers on an intertidal sandflat in the Westerschelde estuary (Zeeland, the Netherlands). Hydrobiologia, 269: 275–284.
- Sabbe, K. (1997). Systematics and ecology of intertidal benthic diatoms of the Westerschelde estuary (The Netherlands). Ghent University.
- Sabbe, K., Witkowski, A. & Vyverman, W. (1995). Taxonomy, morphology and ecology of Biremis lucens comb. nov. (Bacillariophyta): a brackish-marine, benthic diatom species comprising different morphological types. Botanica Marina, 38: 379–391.
- Serôdio, J., da Silva, J.M. & Catarino, F. (2001). Use of in vivo chlorophyll a fluorescence to quantify short-term variations in the productive biomass of intertidal microphytobenthos. Marine Ecology Progress Series, 218: 45–61.
- Serôdio, J., Cruz, S., Vieira, S. & Brotas, V. (2005). Non-photochemical quenching of chlorophyll fluorescence and operation of the xanthophyll cycle in estuarine microphytobenthos. Journal of Experimental Marine Biology and Ecology, 326: 157–169.
- Serôdio, J., Vieira, S., Cruz, S. & Coelho, H. (2006). Rapid light-response curves of chlorophyll fluorescence in microalgae: relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynthesis Research, 90: 29–43.
- Serôdio, J., Vieira, S. & Cruz, S. (2008). Photosynthetic activity, photoprotection and photoinhibition in intertidal microphytobenthos as studied in situ using variable chlorophyll fluorescence. Continental Shelf Research, 28: 1363–1375.
- Serôdio, J., Ezequiel, J., Barnett, A., Mouget, J.L., Méléder, V., Laviale, M. & Lavaud. J. (2012). Efficiency of photoprotection in microphytobenthos: role of vertical migration and the xanthophyll cycle against photoinhibition. Aquatic Microbial Ecology, 67: 161–175.
- Underwood, G.J.C., Perkins, R.G., Consalvey, M.C., Hanlon, A.R.M., Oxborough, K., Baker, N.R. & Paterson, D.M. (2005). Patterns in microphytobenthic primary productivity: species-specific variation in migratory rhythms and photosynthesis in mixed-species biofilms. Limnology and Oceanography, 50: 755–767.
- Zhu, S., Guo, J., Maldonado, M.T. & Green, B.R. (2010). Effects of iron and copper deficiency on the expression of members of the light-harvesting family in the diatom Thalassiosira pseudonana (Bacillariophyceae). Journal of Phycology, 46: 974–981.