ABSTRACT
Vanadium-dependent haloperoxidases (V-HPO) are specific enzymes involved in the metabolism of reactive oxygen species in marine algae. These enzymes contribute to early embryogenesis of algae in the order Fucales, catalyzing the reactions which result in phlorotannin incorporation into the newly forming zygote cell wall and adhesive material. In this study we showed that early embryogenesis in Fucus vesiculosus is accompanied by synchronous changes of V-HPO activity, hydrogen peroxide content and content of cell-wall-associated phlorotannins. Phlorotannin incorporation into the cell wall of Fucus zygotes had already begun 1 h after fertilization, and this process was accompanied by rapid increase of H2O2 content and V-HPO activity. We suggest that altogether this initiates polymerization of phenolics and their cross-linking with the major cell wall constituent, alginic acid. Presumably, de novo phlorotannin biosynthesis is involved in this process, as GC-MS analysis showed dramatic changes in content of intracellular phloroglucinol and low-molecular-weight phlorotannins during the first 9 days of F. vesiculosus embryogenesis.
Introduction
Vanadium-dependent haloperoxidases (V-HPO) represent a specific group of enzymes contributing to the metabolism of reactive oxygen species (ROS) in marine algae. Lacking haeme in their structure, these enzymes contain vanadate as a prosthetic group (Wever, Citation2012). The reaction catalyzed by V-HPO is the two-electron oxidation of halides by hydrogen peroxide via a two-substrate mechanism in which H2O2 first binds to the active site followed by halide oxidation. This process results in the formation of active intermediates (e.g. HOBr, I+) non-specifically reacting with nucleophilic acceptors, thus leading to the synthesis of a variety of halogenated organic compounds. Algal V-HPOs possess bromo- and iodoperoxidase activity, while some fungal enzymes are also capable of oxidizing chloride (Butler & Carter-Franklin, Citation2004; Wever, Citation2012).
V-HPOs have several unique chemical and physical features, making them suitable for industrial applications. These enzymes are relatively thermostable, and they retain their activity in the presence of detergents, organic solvents and high concentrations of ROS (Wever et al., Citation1985; Everett et al., Citation1990; Almeida et al., Citation2000). These characteristics drew attention to this group of enzymes in the context of both ecology (Laturnus, Citation2001; Wever & van der Horst, Citation2013; Nitschke et al., Citation2018; Punitha et al., Citation2018) and biotechnology (Sheffield et al., Citation1994; Prodanovic et al., Citation2012). At the same time, our understanding of physiological functions of V-HPOs in algae is still limited. V-HPOs occur both in the cytosol and the apoplast of algal cells (Wever Citation2012). Apparently, these enzymes contribute significantly to the regulation of H2O2 content in the cell. Thus, V-HPOs of brown algae of the order Fucales are no less effective H2O2 scavengers than ascorbate-peroxidase (the principal algal intracellular peroxidase), and much more effective than catalase (Tarakhovskaya et al., Citation2015). V-HPO activity leads to the production of halocarbons with antibiotic and antifungal properties involved in the chemical defence of marine algae against colonization of their thalli by microorganisms (Fenical, Citation1975; Butler & Carter-Franklin, Citation2004; Punitha et al., Citation2018). Apoplastic V-HPOs supposedly contribute to cell wall rigidification and consequently, growth regulation. Intra-thallus profiles of V-HPO activity in several fucalean algae revealed the minimum halogenation level in young, actively growing tissues (Tarakhovskaya et al., Citation2015).
One of the most interesting functions of V-HPO is its contribution to early embryogenesis of brown algae (Berglin et al., Citation2004; Potin & Leblanc, Citation2006; Tarakhovskaya, Citation2014). Knowledge of this process came from studies of zygotes and embryos of the representative Fucales: Fucus, Pelvetia and Hormosira. Embryos of these algae develop independently of maternal tissues. Gametes are released from conceptacles into the water, where eggs are fertilized. Fucoid eggs are spherical and initially lack cell walls. From 14–16 h after fertilization the zygote germinates with a rhizoid protuberance appearing on its surface according to the developed polarity axis. Then, 20–22 h after fertilization, the first cell division occurs (Quatrano, Citation1974; Kropf et al., Citation1999; Homblé & Léonetti, Citation2007). Zygote germination is preceded by a series of crucial physiological and biochemical processes (Quatrano, Citation1974; Bisgrove et al., Citation2003; Tarakhovskaya et al., Citation2017), and at least two of these, cell wall formation and zygote attachment to the substratum, are considered V-HPO-dependent (Potin & Leblanc, Citation2006; Tarakhovskaya, Citation2014). Natural substrates for V-HPO-mediated halogenation in brown algal cells are specific phenolic compounds, phlorotannins, which are among the principal constituents of both the fucoid cell wall matrix and adhesive material (Schoenwaelder & Wiencke, Citation2000; Potin & Leblanc, Citation2006). Brown algal cell walls contain alginates and sulphated fucans as the main components (up to 45% of dry weight, DW) and smaller fractions of cellulose (1–8% DW), proteins (5–9% DW), phlorotannins and halide compounds (Mabeau & Kloareg, Citation1987; Verhaeghe et al., Citation2008b; Deniaud-Bouët et al., Citation2014).
Phlorotannins are oligomeric or polymeric compounds, of which the structural unit is phloroglucinol (1,3,5-trihydroxybenzene). These multifunctional molecules are usually divided into several structural classes, e.g. fucols, phlorethols, fuhalols, eckols. They are found in all taxonomic groups of brown algae and are especially abundant in fucoids (Ragan & Glombitza, Citation1986; Connan et al., Citation2006; Imbs & Zvyagintseva, Citation2018; Lemesheva & Tarakhovskaya, Citation2018). There are two subcellular phlorotannin fractions in brown algal cells: a water-soluble, intracellular fraction located in specific organelles, physodes; and the cell wall fraction, where phlorotannins are covalently linked to alginates (Schoenwaelder & Wiencke, Citation2000; Salgado et al., Citation2009; Deniaud-Bouët et al., Citation2014). The cell wall phlorotannin fraction has been virtually unstudied. We found few papers reporting phenolic content in cell walls of fucoid thalli (e.g. Koivikko et al., Citation2005). Apparently, polymerization of phlorotannins and cross-linking between phenolic residues and alginates proceed in the reactions catalyzed by V-HPO. Halogenation activates phenoxy radicals and thus stimulates the cross-linking process incorporating phlorotannin molecules into the cell wall (Eickhoff et al., Citation2001; Potin & Leblanc, Citation2006; Salgado et al., Citation2009). The same mechanism causes irreversible hardening of the adhesive material enabling fucoid zygote attachment to the substratum (Tarakhovskaya, Citation2014). In vitro experiments showed that the mixture containing phlorotannins, alginic acid, V-HPO, H2O2 and KI or KBr is spontaneously transformed into a stiff water-insoluble adhesive (Berglin et al., Citation2004; Bitton et al., Citation2006, Citation2007).
Key physiological processes during the early embryogenesis of Fucus require strict coordination of synthesis and localization of all involved metabolites. The objective of this study was to track the dynamics of V-HPO activity, H2O2 content, total content of intracellular and cell wall-associated phlorotannins, and also the relative content of phloroglucinol and several low molecular weight phlorotannins during early embryogenesis of Fucus vesiculosus L. The first nine days of embryo development are studied here with a particular attention to the zygote stage.
Materials and methods
Plant material collection and culture
Mature receptacles of the dioecious Fucus vesiculosus were collected in the Keret Archipelago (Kandalaksha Bay, White Sea), washed with seawater, dried with filter paper and stored in the dark at 4°C for up to 2 weeks. Collection of gametes and fertilization followed published protocols (Jaffe & Neuscheler, Citation1969; Quatrano, Citation1974). Fertilization was defined as 15 min after mixing suspensions of eggs and antherozoids. After fertilization, zygotes were divided into groups and placed into 60-mm plastic Petri dishes in Millipore (pore size 0.45 µm) filtered seawater. Later, developing zygotes and embryos were kept in Petri dishes with seawater at 15°C under continuous light provided by cool-white fluorescent bulbs at an irradiance of 20–25 μmol photons m–2 s–1. Germination rate for all samples was more than 90%.
Samples for all analyses were taken within 2 h of oogonial release (i.e. unfertilized eggs), at 1, 3, 6 and 12 h after fertilization (zygotes), and then at 1, 3, 6 and 9 days after fertilization (embryos).
Vanadium-dependent haloperoxidase activity assay
Extraction and purification of V-HPO from Fucus eggs, zygotes and embryos were carried out using the aqueous salt/polymer two-phase protocol (Vilter, Citation1994; Verhaeghe et al., Citation2008a). Protein concentration in purified samples was determined using the Bradford assay (Bradford, Citation1976).
Haloperoxidase activity was assessed via the reaction of thymolsulfonephthalein (thymol blue) halogenation (Verhaeghe et al., Citation2008a; Tarakhovskaya et al., Citation2015). The change in absorbance at 620 nm due to the formation of diiodothymolsulfonephthalein (40.3 mM–1 cm–1) or dibromothymolsulfonephthalein (37.2 mM–1 cm–1) from thymol blue was monitored for 15 min using a SPEKOL 1300 spectrophotometer (Analytik Jena AG, Germany). All assays were performed at 20 ± 1°C.
Hydrogen peroxide analysis
Measurement of H2O2 content in Fucus eggs, zygotes and embryos was carried out with the ferrous–xylenol orange (FOX) assay (Wolff, Citation1994; Gay & Gebicki, Citation2000). From 100–150 mg fresh weight (FW) of algal material was homogenized in 0.2 M perchloric acid and the extract was centrifuged (10 000 g, 10 min). The supernatant was neutralized with KOH and purified using the ion exchange Bio-Rad AG 1-X8 resin (200–400 mesh, chloride form). Each extract was divided into two aliquots, one of which was treated with catalase (Sigma-Aldrich C9322). Equal volumes of FOX reagent (0.2 mM xylenol orange, 200 mM sorbitol, 50 mM H2SO4, 0.5 mM (NH4)2SO3 and 0.5 mM FeSO4) was added to both solutions. After 45 min the extinction of the solutions was measured at 560 nm using a SPEKOL 1300 spectrophotometer. H2O2 concentration in the reaction mixture was calculated as the difference in absorbance between catalase-free and catalase-treated aliquots according to the calibration curve.
Phlorotannin content measurement
Extraction of intracellular and cell-wall-bound phlorotannins was performed according to Koivikko et al. (Citation2005), with modifications. Briefly, 10 mg FW of algal material was added to acetone:water (70:30, v/v), homogenized with a glass tissue grinder and left soaking in 1 ml of aqueous acetone for 1 hour. The extract was then centrifuged (5000 g, 10 min), the supernatant was transferred into a clean tube and the pellet was re-extracted with another 1 ml of aqueous acetone. Supernatants of five extractions were combined. The insoluble (cell wall) phlorotannin fraction was extracted from the precipitate of the remaining algal material after extraction of soluble phlorotannins. Precipitate was resuspended in 0.5 ml of 1 M aqueous NaOH solution (80°C) and then incubated for 2.5 h at room temperature with continuous shaking. After centrifugation (5000 g, 10 min), supernatant was transferred into a clean tube. The alkaline extraction was repeated three times. Combined supernatants were neutralized with concentrated H3PO4 to pH 6.8–7.0.
A modification of the Folin–Ciocalteu micro-method was used to measure total phlorotannin content in the samples with phloroglucinol (Sigma-Aldrich 79330) as standard (Cicco et al., Citation2009). The reaction mixture, containing 0.3 ml of sample, 0.3 ml of Folin reagent and 2.4 ml of 5% Na2CO3, was incubated at 45°C for 20 min, and then absorbance was measured at 750 nm using a SPEKOL 1300 spectrophotometer.
GC-MS analysis of phloroglucinol and low molecular weight phlorotannins
Twenty milligrams FW of algal material was added to cold methanol (−25°C), quickly ground in a pre-cooled glass tissue grinder and left soaking in 1 ml of cold methanol for extraction. A 500-μl aliquot of methanol extract was transferred into a clean 1.5 ml polypropylene Eppendorf tube (VWR, Dresden, Germany), and vacuum-dried for subsequent analysis.
GC-MS analysis was carried out according to Hutschenreuther et al. (Citation2012). Briefly, vacuum-dried extracts were incubated by shaking in a methoxyamine hydrochloride (Sigma-Aldrich, Taufkirchen, Germany) solution in pyridine and N,O-bis(trimethylsilyl)-trifluoroacetamide (Macherey–Nagel, Düren, Germany). After derivatization, samples were transferred into glass vial micro-inserts and subjected to GC–MS analysis on a Trace GC Ultra equipped with an A200S autosampler (CTC Analytics, Zwingen, Switzerland) and coupled with a MAT95 XP double focusing sector field mass spectrometer (Thermo Electron, Bremen, Germany) with standard electron impact ionization. Within each sequence, a mixture of alkanes (C10–C32) in hexane was measured for the calculation of Kovats retention indices (RIs) (Kovàts, Citation1958). Phloroglucinol was co-spiked to confirm the identity of the compound.
Peak deconvolution was accomplished using AMDIS 2.65 (Stein, Citation1999). Retention indices were automatically calculated using an AMDIS calibration file containing the batch retention times of each alkane. Golm metabolome database (GMD_ 20100614_VAR5_ALK, 24.09.2010; Kopka et al., Citation2005) and NIST14 (National Institute of Standards and Technology, Gaithersburg, Maryland, USA) were used for peak identification based on spectra comparison. Quantitation of compounds was performed by peak integration of the corresponding extracted ion chromatograms (m/z ± 0.5) for representative intense signals at specific retention times using Xcalibur 2.0.7.
Data analysis
Measurements were performed using 3–10 biological replicates. Excel 2016 (Microsoft, Redmond, Washin-gton, USA) was used for data processing, normalization procedures and creation of figures. The GC-MS data processing included peak area normalization to the median of all areas within a chromatogram. All values are expressed as means and standard deviations.
Results
Vanadium-dependent haloperoxidase activity
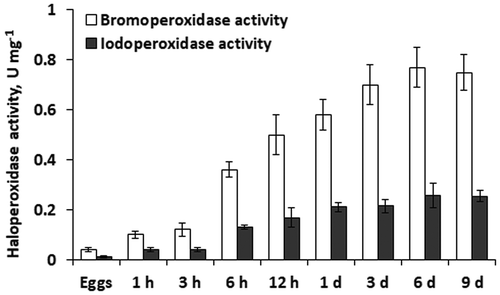
Unfertilized eggs of F. vesiculosus exhibited the lowest V-haloperoxidase activity with both Br− and I−. During the first 3 days after fertilization the level of halogenation increased gradually up to 0.7 U mg–1 for Br− and 0.21 U mg–1 for I− (). The most rapid increases of V-haloperoxidase activity took place during the first hour of zygote development, and between 3 and 6 h after fertilization. After 3 days of embryogenesis, haloperoxidase activity stabilized. In all studied stages of embryo development in F. vesiculosus, iodoperoxidase activity was ~3 times lower than bromoperoxidase activity ().
Hydrogen peroxide content
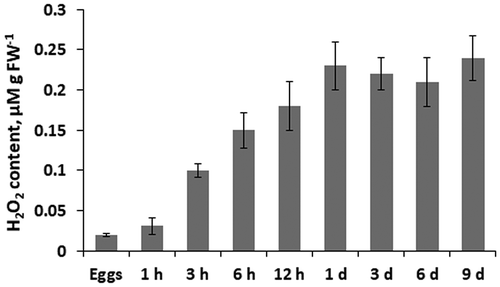
Hydrogen peroxide content in egg cells of F. vesiculosus was relatively low (0.02 μmol g–1 FW), but increased dramatically between 1 and 3 h after fertilization (). This rise of H2O2 lasted throughout day 1 of embryogenesis, so that 1-day old embryos contained 10 times more H2O2 than the eggs. During the next 8 days of development, there was no significant change in hydrogen peroxide content in F. vesiculosus embryos ().
Fig. 2. Hydrogen peroxide content in eggs, zygotes (1 h to 12 h) and embryos (1 day to 9 days) of Fucus vesiculosus during 9 days after fertilization. h, hours; d, days. Bars represent means ± SD.
Total phlorotannin content
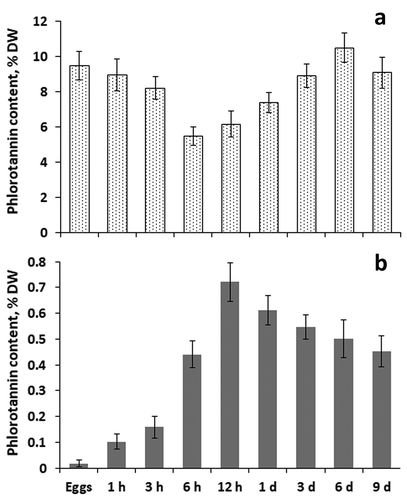
Total intracellular phlorotannins content of different age eggs, zygotes and embryos of F. vesiculosus varied from 5.5% DW (6-h old zygotes) to 10.5% DW (6-day old embryos; ). The content of cell wall phlorotannins in all analysed samples was much less than the content of intracellular phenolics, varying from 0.02% DW (unfertilized eggs) to 0.7% DW (12-h old zygotes) (). Content of intracellular phlorotannins decreased during the first 6 h after fertilization, and then returned gradually to the level in unfertilized eggs (). The dynamics of cell wall phlorotannin level was the opposite, i.e. phenolic content increased sharply during the first 12 h of zygote development and then gradually decreased, reaching values similar to those in 6-h zygotes ().
Phloroglucinol and low molecular weight phlorotannins content
GC-MS analysis allowed detection of the phlorotannin monomer (phloroglucinol), two phloroglucinol dimers (difucol and diphlorethol) and two phloroglucinol trimers (RI 3502 and 3530) in eggs, zygotes and embryos of F. vesiculosus.
Content of phloroglucinol and its dimers and trimers changed dramatically during early embryogenesis of F. vesiculosus (). During the first 12 h after fertilization, phloroglucinol gradually decreased, but during the next 12 h it rose sharply to an order of magnitude higher than the initial content in eggs. In the next 2 days, phloroglucinol content fell to a level close to that in the eggs and was stable up to day 9 of embryogenesis ().
Fig. 4. Scheme of putative metabolic links between low molecular weight phlorotannins in eggs, zygotes (1 h to 12 h) and embryos (1 day to 9 days) of Fucus vesiculosus. h, hours; d, days. Bars represent means ± SD. Arbitrary units are normalized peak areas. Trimers may be triphlorethols, trifucols or trifuhalols.
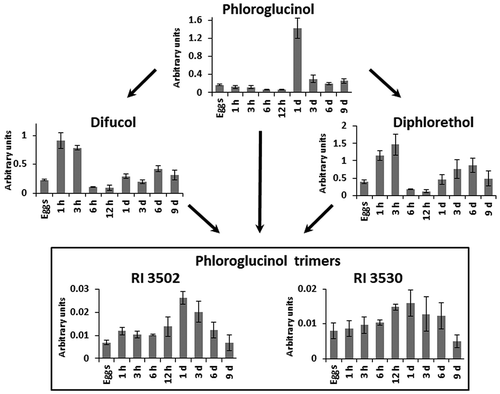
Both difucol and diphlorethol showed a dynamic different from their monomer, phloroglucinol. Amounts increased three- to four-fold during the first 3 h of zygote development and then declined, reaching their lowest levels at 6–12 h after fertilization. During the next 8 days, phloroglucinol dimers gradually returned to values close to those of unfertilized eggs ().
Dynamics of phloroglucinol trimers in developing zygotes and embryos principally resembled that of phloroglucinol but with smaller amplitudes. Like their monomer, trimers accumulated to the highest level in 1-day old embryos and then gradually decreased during the next 8 days. Compared with phloroglucinol, the changes in trimer levels were less prominent, especially for RI 3530 ().
Discussion
Our data demonstrate that the development of zygotes in F. vesiculosus is characterized by a dramatic increase in V-HPO activity (). Initial halogenation level in unfertilized eggs was very low, and bromoperoxidase activity, measured with the same assay in adult thalli of F. vesiculosus, was two orders of magnitude higher than in eggs. However, 1-day old embryos already had bromination levels close to that of meristematic tissues of adult thalli (; Tarakhovskaya et al., Citation2015). Activity of iodination in zygotes and embryos was ~3 times lower than that of bromination, corresponding to values from adult thalli of different Fucales (Tarakhovskaya et al., Citation2015). Notably, the dynamics of V-HPO activity during embryogenesis of F. vesiculosus was highly synchronized with changes in hydrogen peroxide content (, ). As H2O2 is a substrate of V-HPO, the increased level of this ROS may be a prerequisite for the rise of enzyme activity. Being a relatively stable ROS, and capable of migrating through cell membranes via both simple and aquaporin-facilitated diffusion, H2O2 has multiple signalling roles in plant cells (Bienert et al., Citation2006). It contributes to the regulation of physiological processes, such as cell cycle progression, elongation growth and cell wall strengthening (Apel & Hirt, Citation2004; Petrov & Van Breusegem, Citation2012). Clearly, H2O2 content in living cells is strictly controlled. In embryogenesis of Fucus, fertilization may be a trigger for activating H2O2 production.
There are two principal sources of hydrogen peroxide in plant cells: intracellular H2O2 originates mostly from photosynthetic processes; and an apoplastic pool of this ROS is maintained by cell wall and plasmalemma-located oxidases and oxidative activity of secretory peroxidases (Apel & Hirt, Citation2004). Eggs of Fucus are photosynthetic, and after fertilization photosynthesis activity increases (McLachlan & Bidwell, Citation1978; Kim et al., Citation2006). However, considerable changes in relative electron transport rate of Photosystem II, pigments and Rubisco content take place only after several days of embryo development (Lamote et al., Citation2003; Tarakhovskaya & Maslov, Citation2005; Tarakhovskaya et al., Citation2013), and can hardly account for the dramatic increase of H2O2 content during the first hours after fertilization (). Among extracellular sources of H2O2, plasma membrane NADPH oxidase is understood to be especially important in seaweeds, as they possess no secretory peroxidases (Passardi et al., Citation2007; Bischof & Rautenberger, Citation2012). Coelho et al. (Citation2002) reported a very fast (within minutes) increase of H2O2 content in embryo rhizoids of F. serratus subjected to hyperosmotic stress, and inhibitor analysis implied that H2O2 originated from NADPH oxidase activity. Potin & Leblanc (Citation2006) reported that developing Fucus zygotes constantly secreted H2O2 into ambient medium. Since the H2O2-consuming enzyme V-HPO occurs not only in algal cytoplasm, but also in the apoplast (Wever, Citation2012), we suggest that the newly forming cell wall of Fucus zygotes is the compartment undergoing the greatest changes of H2O2 metabolism.
Cell wall formation in zygotes of Fucales starts within minutes of fertilization (Bisgrove & Kropf, Citation2001). Cellulose and alginates are considered to be the first wall constituents that appear on the cell surface (Quatrano & Stevens, Citation1976). Immunofluorescence labelling of cellulose and different types of alginates in developing zygotes of F. serratus showed cellulose on the cell surface within 2 h of fertilization. This was ahead of alginates, which were clearly detected only from 6 h of zygote development (Torode et al., Citation2016). These components are followed within 2 hours by incorporation of sulphated fucans (Torode et al., Citation2016) and phlorotannins (Schoenwaelder & Wiencke, Citation2000). Phlorotannins are supposed to be among the last constituents incorporated into the forming wall, because their V-HPO-catalyzed polymerization and cross-linking with alginates confer stiffness to the wall matrix (Schoenwaelder & Wiencke, Citation2000; Salgado et al., Citation2009; Deniaud-Bouët et al., Citation2014). Our results show that phlorotannin content increases rapidly in the cell walls of developing zygotes of F. vesiculosus during the first 12 h after fertilization, and particularly during the first 6 h (). This is in line with literature data showing stabilization of fucoid cell wall composition after 6 h of zygote development (Quatrano & Stevens, Citation1976). The detection of a very low, but still permanently present, level of cell-wall-associated phlorotannins in unfertilized Fucus eggs () looks counterintuitive, as eggs of Fucales possess no cell wall (Quatrano, Citation1974). Most probably, it is an artefact resulting from the difficulty in removing 100% of intracellular phlorotannins to obtain the cell wall phlorotannin fraction using a reasonable number of sequential acetone extractions (see Materials and methods). Thus, we should accept that the cell wall phlorotannin fraction contains traces of intracellular phlorotannins, and they contribute no more than 4% of the phlorotannins detected in fully developed cell walls of Fucus.
The increase of phlorotannin content in cell walls of F. vesiculosus zygotes was accompanied by a decrease of intracellular phlorotannins (). This result corresponds with the literature describing the origin of cell wall phlorotannin fraction in brown algal cells. Apparently, phlorotannin precursors are synthesized inside the cell, in the endoplasmic reticulum, and then transferred to the Golgi apparatus for further processing. Both these structures (endoplasmic reticulum and Golgi apparatus) give rise to physodes, membrane-bound organelles specific to brown algae, which are abundant in eggs of Fucales (Schoenwaelder & Wiencke, Citation2000). Physodes accumulate at the periphery of fucoid zygotes within an hour after fertilization and secrete their content into the forming cell wall via exocytosis (Brawley et al., Citation1976; Schoenwaelder & Clayton, Citation1998; Schoenwaelder & Wiencke, Citation2000). We hypothesize that each event of de novo cell wall formation is accompanied by a temporary decrease of intracellular phlorotannin content. These fluctuations might be more conspicuous in fucoid zygotes because of the relatively large area of newly formed cell walls, and also because this model allows simultaneous analysis of many cells synchronized in their development. The meristodermal cell walls of another fucalean species, Ascophyllum nodosum, with its repeated monthly sequence of wall deposition and shedding (Halat et al., Citation2015), would be an excellent model for future study of cell wall morphogenesis. The shed walls from vegetative branches of A. nodosum can be easily collected and evaluated in the context of changing cell wall chemistry during wall regeneration. Accordingly, they provide a developmental system for wall chemistry not associated with zygote development.
Our data show that the decrease in intracellular phlorotannin content during the first 12 h of Fucus zygote development () was ~4 times higher than simultaneous increase of cell wall phlorotannin level (). We suggest two explanations for this discrepancy. First, it may be the result of partial intracellular degradation of phlorotannins – a process which is difficult to discuss adequately because the metabolism of these compounds, besides phloroglucinol biosynthesis, is still poorly understood (Meslet-Cladière et al., Citation2013). A second explanation is that not all phlorotannins secreted outside the cell are then incorporated into the cell wall. Exudation of phenolic compounds from undamaged brown algal cells has been reported previously (Ragan & Jensen, Citation1979; Swanson & Druehl, Citation2002; Koivikko et al., Citation2005). In zygotes of Fucales, phlorotannin secretion may have several functions. Thus, the first massive release of phlorotannins by the zygote starts immediately after fertilization (presumably, before the start of cell wall formation) and provides a block to polyspermy. Phlorotannins reduce the motility of antherozoids and can damage their cell membrane, killing them (Schoenwaelder, Citation2002). As chemical defence is among the principal functions of phenolic compounds in brown algal cells (Lemesheva & Tarakhovskaya, Citation2018), enhanced phlorotannin secretion may protect zygotes from infection while the cell wall is incomplete. In addition, the last steps of cell wall formation in fucoid zygotes coincide with another extracellular event requiring phlorotannins (together with V-HPO and hydrogen peroxide), i.e. formation and irreversible rigidification of adhesive material by which zygotes attach to the substratum (Tarakhovskaya, Citation2014).
Altogether, our data show consistent positive correlation between changes in activity of V-HPO and accumulation of both its substrates, hydrogen peroxide and apoplastic phlorotannins during early embryogenesis of Fucus. Moreover, this coincides with alginate appearance on the cell surface (Torode et al., Citation2016). It was previously demonstrated by in vitro studies, that simultaneous presence of all these components (together with halide ions) is a prerequisite for the interaction between phlorotannins and alginates. This interaction leads to the formation of high-molecular-weight complexes which enable stiffening of the brown algal cell wall and adhesive material (Berglin et al., Citation2004; Bitton et al., Citation2006, Citation2007; Salgado et al., Citation2009). Thus, our data support the hypothesis that V-HPO plays a key role in the process of phlorotannin incorporation into the cell wall of Fucales.
Phlorotannins comprise a large and diverse group of molecules with degrees of polymerization varying from one (phloroglucinol) to hundreds (Ragan & Glombitza, Citation1986). The literature implies that molecules differing in size and structure have specific roles in brown algal metabolism (Iken et al., Citation2007; Jormalainen et al., Citation2008; Gómez & Huovinen, Citation2010; Kirke et al., Citation2019). Thus, algal responses to intrinsic and environmental factors may be reflected not only in the changes of total phlorotannin content, but also in variation of phlorotannin molecular profiles (Lemesheva & Tarakhovskaya, Citation2018). Moreover, information about phlorotannin biosynthesis in brown algal cells is still limited. Phloroglucinol is synthesized from malonyl-CoA through the acetate-malonate (polyketide) pathway by polyketide synthase type III (Meslet-Cladière et al., Citation2013). Further, the diversity of phloro-tannin molecules is considered to be a consequence of V-HPO-dependent phloroglucinol polymerization, although detailed mechanisms are still unclear (Berglin et al., Citation2004; Potin & Leblanc, Citation2006; Bitton et al., Citation2006, Citation2007; Salgado et al., Citation2009). Our results show that content of phloroglucinol and several low-molecular-weight phlorotannins changes considerably during the first 9 days of embryogenesis in F. vesiculosus (), thus implying that not only is the pool of phlorotannins in egg cells mobilized to maintain embryo development, but that de novo biosynthesis of phenolic compounds occurs. Gradual decrease of phlorotannin monomer, and increase of dimer, content during the first 3 h of zygote development () may be explained by phloroglucinol consumption for difucol and diphlorethol synthesis. These dimers do not accumulate in zygotes but are transformed to molecules with higher degrees of polymerization. This transformation was confirmed by a rapid decrease of dimer content 3–6 h after fertilization, followed by a gradual increase in phloroglucinol trimers 6–24 h after fertilization (). We suggest that phloroglucinol biosynthesis may be enhanced during the first 12 h of zygote development, to support cell wall formation. Twelve hours after fertilization zygote cell wall biosynthesis is complete, then ~20 h after fertilization the first cell division takes place which also involves cell wall formation and a requirement for phlorotannins. Thus, a spike of phloroglucinol content in 1-day old embryos may result from temporary accumulation of ‘extra’ phlorotannin monomers due to a decline in consumption.
Our results contribute to a better understanding of the mechanisms of fucoid zygote development, in particular in relation to initial cell wall formation. We conclude that phlorotannin incorporation into the cell wall has already started 1 h after fertilization, accompanied by a rapid increase of H2O2 content and V-HPO activity, which together may provide phenolic polymerization and cross-linking with the major cell wall constituent, alginic acid. Presumably, de novo phlorotannin biosynthesis is involved in this process. Further characterization of changes in molecular profiles of intracellular and cell wall phlorotannin fractions during early embryogenesis of algae of the order Fucales would be a promising field for future research.
Author contributions
V. Lemesheva: practical work, data analysis, manuscript writing; C. Birkemeyer: GC-MS analysis, tentative compound identification, manuscript editing; D. Garbary: data analysis, manuscript editing; E. Tarakhovskaya: original concept, practical work, manuscript drafting and editing.
Acknowledgements
We thank Marine Biological Station ‘UNB Belomorskaya’ of St. Petersburg State University for providing facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Almeida, M.G., Humanes, M., Melo, R., Silva, J.A., Fraústo da Silva, J.J.R. & Wever, R. (2000). Purification and characterization of vanadium haloperoxidases from the brown alga Pelvetia canaliculata. Phytochemistry, 54: 5–11.
- Apel, K. & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55: 373–399.
- Berglin, M., Delage, L., Potin, P., Vilter, H. & Elwing, H. (2004). Enzymatic cross-linking of a phenolic polymer extracted from the marine alga Fucus serratus. Biomacromolecules, 5: 2376–2383.
- Bienert, G.P., Schjoerring, J.K. & Jahn, T.P. (2006). Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta – Biomembranes, 1758: 994–1003.
- Bischof, K. & Rautenberger R. (2012). Seaweed responses to environmental stress: reactive oxygen and antioxidative strategies. In Seaweed Biology (Wiencke, C. & Bischof, K., editors), 109–132. Springer-Verlag, Berlin.
- Bisgrove, S.R., Henderson, D.C. & Kropf, D.L. (2003). Asymmetric division in fucoid zygotes is positioned by telophase nuclei. Plant Cell, 15: 854–862.
- Bisgrove, S.R. & Kropf, D.L. (2001). Cell wall deposition during morphogenesis in fucoid algae. Planta, 212: 648–658.
- Bitton, R., Ben-Yehuda, M., Davidovich, M., Balazs, Y., Potin, P., Delage, L., Colin, C. & Bianco-Peled, H. (2006). Structure of algal-born phenolic polymeric adhesives. Macromolecular Bioscience, 6: 737–746.
- Bitton, R., Berglin, M., Elwing, H., Colin, C., Delage, L., Potin, P. & Bianco-Peled, H. (2007). The influence of halide-mediated oxidation on algae-born adhesives. Macromolecular Bioscience, 7: 1280–1289.
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry, 72: 248–254.
- Brawley, S.H., Wetherbee, R. & Quatrano, R.S. (1976). Fine structural studies of the gametes and embryos of Fucus vesiculosus L. (Phaeophyta). II. The cytoplasm of the egg and the young zygote. Journal of Cell Science, 20: 255–271.
- Butler, A. & Carter-Franklin, J.N. (2004). The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Natural Product Reports, 21: 180–188.
- Cicco, N., Lanorte, M.T., Paraggio, M., Viggiano, M. & Lattanzio V. (2009). A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchemical Journal, 91: 107–110.
- Coelho, S.M., Taylor, A.R., Ryan, K.P., Sousa-Pinto, I., Brown, M.T. & Brownlee C. (2002). Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. The Plant Cell, 14: 2369–2381.
- Connan, S., Delisle, F., Deslandes, E. & Ar Gall, E. (2006). Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Botanica Marina, 49: 39–46.
- Deniaud-Bouët, E., Kervarec, N., Michel, G., Tonon, T., Kloareg, B. & Hervé, C. (2014). Chemical and enzymatic fractionation of cell walls from Fucales: insights into the structure of the extracellular matrix of brown algae. Annals of Botany, 114: 1203–1216.
- Eickhoff, H., Jung, G. & Rieker, A. (2001). Oxidative phenol coupling – tyrosine dimers and libraries containing tyrosyl peptide dimers. Tetrahedron, 57: 353–364.
- Everett, R.R., Kanofsky, J.R. & Butler, A. (1990). Mechanistic investigations of the novel non-heme vanadium bromoperoxidases: evidence for singlet oxygen production. Journal of Biological Chemistry, 265: 4908–4914.
- Fenical, W. (1975). Halogenation in the Rhodophyta: A review. Journal of Phycology, 11: 245–259.
- Gay, C. & Gebicki, J.M. (2000). A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Analytical Biochemistry, 284: 217–220.
- Gómez, I. & Huovinen, P. (2010). Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochemistry and Photobiology, 86: 1056–1063.
- Halat, L., Galway, M.E., Gitto, S. & Garbary, D.J. (2015). Epidermal shedding in Ascophyllum nodosum (Phaeophyceae): seasonality, productivity and relationship to harvesting. Phycologia, 54: 599–608.
- Homblé, F. & Léonetti, M. (2007). Emergence of symmetry breaking in fucoid zygotes. Trends in Plant Science, 12: 253–259.
- Hutschenreuther, A., Kiontke, A., Birkenmeier, G. & Birkemeyer, C. (2012). Comparison of extraction conditions and normalization approaches for cellular metabolomics of adherent growing cells with GCMS. Analytical Methods, 4: 1959–1963.
- Iken, K., Amsler, C.D., Hubbard, J.M., McClintock, J.B. & Baker, B.J. (2007). Allocation patterns of phlorotannins in Antarctic brown algae. Phycologia, 46: 386–395.
- Imbs, T.I. & Zvyagintseva, T.N. (2018). Phlorotannins are polyphenolic metabolites of brown algae. Russian Journal of Marine Biology, 44: 263–273.
- Jaffe, L.F. & Neuscheler, W. (1969). On the mutual polarization of nearby pairs of fucaceous eggs. Developmental Biology, 19: 549–565.
- Jormalainen, V., Koivikko, R., Eränen, J.K. & Loponen, J. (2008). Variation of phlorotannins among three populations of Fucus vesiculosus as revealed by HPLC and colorimetric quantification. Journal of Chemical Ecology, 34: 57–64.
- Kim, K.Y., Jeong, H.J., Main, H.P. & Garbary, D.J. (2006). Fluorescence and photosynthetic competency in single eggs and embryos of Ascophyllum nodosum (Phaeophyceae). Phycologia, 45: 331–336.
- Kirke, D.A., Rai, D.K., Smyth, T.J. & Stengel, D.B. (2019). An assessment of temporal variation in the low molecular weight phlorotannin profiles in four intertidal brown macroalgae. Algal Research, 41, 101550.
- Koivikko, R., Loponen, J., Honkanen, T. & Jormalainen, V. (2005). Contents of cytoplasmic, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. Journal of Chemical Ecology, 31: 195–209.
- Kopka, J., Schauer, N., Krueger, S., Birkemeyer, C., Usadel, B., Bergmüller, E., Dörmann, P., Weckwerth, W., Gibon, Y., Willmitzer, M.S.L., Fernie, A.R. & Steinhauser, D. (2005). [email protected]: the Golm Metabolome Database. Bioinformatics, 21: 1635–1638.
- Kovàts, E. (1958). Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helvetica Chimica Acta, 41: 1915–1932.
- Kropf, D.L., Bisgrove, S.R. & Hable, W.E. (1999). Establishing a growth axis in fucoid algae. Trends in Plant Science, 4: 490–494.
- Lamote, M., Darko, E., Schoefs, B. & Lemoine, Y. (2003). Assembly of the photosynthetic apparatus in embryos from Fucus serratus L. Photosynthesis Research, 77: 45–52.
- Laturnus, F. (2001). Marine macroalgae in polar regions as natural sources for volatile organohalogens. Environmental Science and Pollution Research, 8: 103–108.
- Lemesheva, V. & Tarakhovskaya, E. (2018). Physiological functions of phlorotannins. Biological Communication, 63: 70–76.
- Mabeau, S. & Kloareg, B. (1987). Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. Journal of Experimental Botany, 38: 1573–1580.
- McLachlan, J. & Bidwell, R.G.S. (1978). Photosynthesis of eggs, sperm, zygotes, and embryos of Fucus serratus. Canadian Journal of Botany, 56: 371–373.
- Meslet-Cladière, L., Delage, L., Leroux, C. J., Goulitquer, S., Leblanc, C., Creis, E., Gall E. A., Stiger-Pouvreau, V., Czjzek, M. & Potin, P. (2013). Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell, 25: 3089–3103.
- Nitschke, U., Walsh, P., McDaid, J. & Stengel D. B. (2018). Variability in iodine in temperate seaweeds and iodine accumulation kinetics of Fucus vesiculosus and Laminaria digitata (Phaeophyceae, Ochrophyta). Journal of Phycology, 54: 114–125.
- Passardi, F., Bakalovic, N., Teixeira, F.K., Margis-Pinheiro, M., Penel, C. & Dunand, C. (2007). Prokaryotic origins of the non-animal peroxidase superfamily and organelle-mediated transmission to eukaryotes. Genomics, 89: 567–579.
- Petrov, V.D. & Van Breusegem, F. (2012). Hydrogen peroxide – a central hub for information flow in plant cells. AoB PLANTS, pls014. doi:10.1093/aobpla/pls014
- Potin, P. & Leblanc, C. (2006). Phenolic-based adhesives of marine brown algae. In Biological Adhesives (Smith, A.M. & Callow, J.A., editors), 105–124. Springer-Verlag, Berlin.
- Prodanovic, R., Ostafe, R., Blanusa, M. & Schwaneberg, U. (2012). Vanadium bromoperoxidase-coupled fluorescent assay for flow cytometry sorting of glucose oxidase gene libraries in double emulsions. Analytical and Bioanalytical Chemistry, 404: 1439–1447.
- Punitha, T., Phang, S.-M., Juan, J.C. & Beardall, J. (2018). Environmental control of vanadium haloperoxidases and halocarbon emissions in macroalgae. Marine Biotechnology, 20: 282–303.
- Quatrano, R.S. (1974). Developmental biology: development in marine organisms. In Experimental Marine Biology ( Mariscal, R.N., editor), 303–346. Academic Press, New York.
- Quatrano, R.S. & Stevens, P.T. (1976). Cell wall assembly in Fucus zygotes: 1. Characterization of the polysaccharide components. Plant Physiology, 58: 224–231.
- Ragan, M.A. & Glombitza, K.W. (1986). Phlorotannins, brown algal polyphenols. Progress in Phycological Research, 4: 129–241.
- Ragan, M.A. & Jensen, A. (1979). Quantitative studies on brown algal phenols. III. Light-mediated exudation on polyphenols from Ascophyllum nodosum (L.) Le Jol. Journal of Experimental Marine Biology and Ecology, 36: 91–101.
- Salgado, L.T., Cinelli, L.P., Viana, N.B., de Carvalho, R.T., De Souza Mourão, P.A., Teixeira, V.L., Farina, M. & Filho, G.M.A. (2009). A vanadium bromoperoxidase catalyzes the formation of high‐molecular‐weight complexes between brown algal phenolic substances and alginates. Journal of Phycology, 45: 193–202.
- Schoenwaelder, M.E.A. (2002). The occurrence and cellular significance of physodes in brown algae. Phycologia, 41: 125–139.
- Schoenwaelder, M.E.A. & Clayton, M.N. (1998). Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpia (Fucales, Phaeophyceae). Journal of Phycology, 34: 969–980.
- Schoenwaelder, M.E.A. & Wiencke, C. (2000). Phenolic compounds in the embryo development of several northern hemisphere fucoids. Plant Biology, 2: 24–33.
- Sheffield, D.J., Harry, T.R., Smith, A.J. & Rogers, L.J. (1994). Immobilization of bromoperoxidase from Corallina officinalis. Biotechnology Techniques, 8: 579–582.
- Stein, S.E. (1999). An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. Journal of the American Society for Mass Spectrometry, 10: 770–781.
- Swanson, A.K. & Druehl, L.D. (2002). Induction, exudation and the UV protective role of kelp phlorotannins. Aquatic Botany, 73: 241–253.
- Tarakhovskaya, E., Lemesheva, V., Bilova, T. & Birkemeyer, C. (2017). Early embryogenesis of brown alga Fucus vesiculosus L. is characterized by significant changes in carbon and energy metabolism. Molecules, 22(9): 1509.
- Tarakhovskaya, E.R. (2014). Mechanisms of bioadhesion of macrophytic algae. Russian Journal of Plant Physiology, 61: 23–30.
- Tarakhovskaya, E.R., Bilova, T.E. & Maslov, Y.I. (2015). Hydrogen peroxide content and vanadium-dependent haloperoxidase activity in thalli of six species of Fucales (Phaeophyceae). Phycologia, 54: 417–424.
- Tarakhovskaya, E.R., Kang, E.J., Kim, K.Y. & Garbary, D.J. (2013). Influence of phytohormones on morphology and chlorophyll a fluorescence parameters in embryos of Fucus vesiculosus L. (Phaeophyceae). Russian Journal of Plant Physiology, 60: 176–183.
- Tarakhovskaya, E.R. & Maslov, Y.I. (2005). Description of the photosynthetic apparatus of Fucus vesiculosus L. in early embryogenesis. Biology Bulletin, 32: 456–460.
- Torode, T.A., Siméon, A., Marcus, S.E., Jam, M., Le Moigne, M.-A., Duffieux, D., Knox, J. P. & Hervé, C. (2016). Dynamics of cell wall assembly during early embryogenesis in the brown alga Fucus. Journal of Experimental Botany, 67: 6089–6100.
- Verhaeghe, E., Buisson, D., Zekri, E., Leblanc, C., Potin, P. & Ambroise, Y. (2008a). A colorimetric assay for steady-state analyses of iodo- and bromoperoxidase activities. Analytical Biochemistry, 379: 60–65.
- Verhaeghe, E.F., Fraysse, A., Guerquin-Kern, J.L., Wu, T.D., Devès, G., Mioskowski, C., Leblanc, C., Ortega, R., Ambroise, Y. & Potin, P. (2008b). Microchemical imaging of iodine distribution in the brown alga Laminaria digitata suggests a new mechanism for its accumulation. Journal of Biological Inorganic Chemistry, 13: 257–269.
- Vilter, H. (1994). Extraction of proteins from sources containing tannins and anionic mucilages. Methods in Enzymology, 228: 665–672.
- Wever, R. (2012). Structure and function of vanadium haloperoxidases. In Vanadium: Biochemical and Molecular Biological Approaches (Michibata, H., editor), 995–125. Springer, Dordrecht.
- Wever, R., Plat, H. & de Boer, E. (1985). Isolation procedure and some properties of the bromoperoxidase from the seaweed Ascophyllum nodosum. Biochimica et Biophysica Acta, 830: 181–186.
- Wever, R. & van der Horst, M.A. (2013). The role of vanadium haloperoxidases in the formation of volatile brominated compounds and their impact on the environment. Dalton Transactions, 42: 11778–11786.
- Wolff, S.P. (1994). Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods in Enzymology, 233: 182–189.