ABSTRACT
Algae and bacteria establish complex relationships reciprocally influencing their growth. Associated bacterial communities alter microalgae metabolism and physiology, affecting biomass yield and quality. In this study, 203 bacterial isolates (33 different genera), recovered from Tetraselmis suecica F&M-M33 cultures, were identified and phenotypically characterized in relation to the microalgal growth-promoting (MGP) features of indol-3-acetic acid (IAA) and siderophore production. IAA production was observed in 30 isolates belonging to 10 genera, whilst siderophore production was observed in 12 isolates belonging to nine genera, indicating that bacteria which are phylogenetically different within the phycosphere community may contribute in similar ways to boosting algal growth. Moreover, strains belonging to a single genus (e.g. Bacillus, Muricauda, Labrenzia and Amorphus) showed different MGP features, but none of these isolates were capable of producing both growth-promoting factors. Twenty-two selected strains were further tested in co-culture assays to evaluate their effect on T. suecica F&M-M33 growth; four induced an increase in microalgal productivity. The exo-metabolome of T. suecica F&M-M33 cultures, either axenically or when co-cultured with either Vitellibacter strain AAD2 or Sphingopyxis flavimaris strain AG5, was determined using a non-targeted metabolomics approach using an Ultra High Performance Liquid Chromatography (UHPLC) system. This allowed for the detection of 133 entities (small chemical molecules, e.g. metabolites), of which 84 were identified. Most of the entities were common in all three cultures, showing that the T. suecica F&M-M33 phycosphere was rich in small peptides and organic acids, and a high number of terpenes were also observed. Seventeen entities were culture-specific, suggesting that they were directly related to microalgae–bacteria interactions.
Introduction
The study of microalgae–bacteria interactions has gained attention, especially in relation to the possible role of associated bacteria in modulating algal growth (Subashchandrabose et al., Citation2011; Fouilland, Citation2012). Microalgae are important for several applications, ranging from their use within the food industry to animal feed, cosmetics, energy and phycoremediation (Tredici et al., Citation2009; Borowitzka, Citation2013). In large-scale cultures, the maintenance of axenic conditions is challenging, so microalgae are usually cultivated with their associated bacterial community (Molina Grima et al., Citation2003; Spolaore et al., Citation2006; Park et al., Citation2010; Piampiano et al., Citation2019). Associated bacteria may have a strong influence on the productivity and cell density of different microalgal species (Biondi et al., Citation2018; Lian et al., Citation2018). Furthermore, bacteria may affect the microalgal cell size, pigment, lipid content and fatty acid composition (De-Bashan et al., Citation2002; Do Nascimento et al., Citation2013).
The effect of bacteria on microalgal growth is mediated by a broad array of compounds, such as vitamins, phytohormones (e.g. indole-3-acetic acid), amino acids, fatty acids, antibiotics and siderophores, which may act as algal growth-promoting molecules (Ramanan et al., Citation2016; Tandon et al., Citation2017; Patidar et al., Citation2018). The microscale area immediately surrounding the algal cell – where interactions between bacteria and microalgal cells take place – has been termed the ‘phycosphere’ in analogy to the plant rhizosphere (Bell & Mitchell, Citation1972). Modification of the phycosphere, such as through the addition of selected bacterial strains, may represent a strategy for improving the microalgal production and may lead to a cost reduction in the production of microalgae (Fukami et al., Citation1997). Therefore, the characterization of bacteria associated with microalgae for microalgal growth-promoting (MGP) features is gaining importance for microalgal biotechnological applications (Lian et al., Citation2018).
Tetraselmis suecica has various market applications, including aquaculture, where it is employed in the diet of bivalve molluscs and larval stages of crustaceans, food and cosmetic applications (Tredici et al., Citation2009; Sansone et al., Citation2017). Therefore, studying Tetraselmis suecica–bacteria interactions to improve microalgal biomass production potentially has great economic value. The aim of this study was to identify and analyse phycospheric bacteria able to promote T. suecica growth. Bacterial isolates from T. suecica F&M-M33 cultures were phenotypically characterized for MGP traits (i.e. indol-3-acetic acid and siderophore production), and some selected strains were tested in co-culture to evaluate their promoting effects on T. suecica F&M-M33 growth. The exo-metabolome of T. suecica F&M-M33 cultures, axenically and in co-culture with two selected bacterial strains, was determined using a non-targeted metabolomics approach.
Materials and methods
Bacterial strains
Bacterial strains were isolated from different T. suecica F&M-M33 cultures following the procedure described in Biondi et al. (Citation2017). Colonies were picked and streaked on Marine Agar plates (Laboratorios CONDA, Madrid, Spain) incubated at 27°C. Strains were identified by 16S rDNA sequencing, as described in Piampiano et al. (Citation2019). Sequence chromatograms were checked and edited to verify the absence of ambiguous peaks. The 16S rRNA gene amplicon sequence data are available in the GenBank database: accession numbers MK991145–MK991286. Sixty-one strains previously isolated from T. suecica F&M-M33 cultures in different seasons and growing conditions (indoors and outdoors) were also included (Biondi et al., Citation2017; Piampiano et al., Citation2019).
Screening for indole-3-acetic acid (IAA) and siderophore production
IAA production was determined using a modified protocol of the method described by Bric et al. (Citation1991). Bacteria were streaked on Marine Agar plates with added tryptophan (0.5 mM) and incubated for 24 h at 27°C. Each inoculated plate was overlain with a sterilized nylon membrane (Merck KGAaA, Darmstadt, Germany), and the plates were then incubated for 24 h at 27°C. Nylon membranes were then removed from agar plates and placed on Whatman paper discs (Merck KGAaA, Darmstadt, Germany) saturated with 2.5 ml Salkowski reagent (0.01 M FeCl3 in 35% HClO4) for 30 min at room temperature. The presence of IAA was determined by the development of a pink colour on nylon membranes.
Siderophore production was assessed by modifying the O-CAS assay (Perez-Miranda et al., Citation2007). Iron-deprived medium was prepared as follows: iron was removed from a peptone (75 g l−1)/yeast extract (15 g l−1) solution using 3% 8-hydroxyquinoline in chloroform; the peptone/yeast extract solution was sterilized by filtration (0.2 µm, Sarstedt Srl, Verona, Italy); and 100 ml was mixed with 900 ml of a solution containing 36 g of sea salts (Merck KGAaA, Darmstadt, Germany) and 16 g of agar. Cultures were grown at 27°C for 72 h, and then each inoculated plate was overlain with the CAS-agar overlay gel (Perez-Miranda et al., Citation2007). Strains were considered positive for siderophore production if a change in colour was observed in the overlay gel (from blue to orange) after 2 h of incubation.
Co-culture assays
Axenic cultures of the marine green alga T. suecica F&M-M33 were used as inoculum in co-culture assays. These cultures were grown in 100 ml flasks (50 ml of culture) capped with SILICOSEN stoppers (VWR International, Milan, Italy) using F medium (Guillard & Ryther, Citation1962) without vitamins and under continuous illumination (80 µmol photons m−2 s−1; metal halide lamps) at 27°C in static conditions. Axenic cultures were verified by microscopy observation (ECLIPSE E200 phase contrast microscope – Nikon) and by inoculating samples in Marine Broth (Difco-BD, Milan, Italy) and in a saline solution containing glucose (2 g l−1) and Yeast Extract (2 g l−1) for 72 h at 27°C. Co-culture assays were set up in 6-well plates, using a single plate for each bacterial strain to be tested. In each 6-well plate, three wells were inoculated with 4.5 ml of the T. suecica F&M-M33 axenic culture prepared at the density of 6.0 × 105 cells ml−1 (~0.2 g l−1) with F medium without vitamin addition and 0.5 ml of the bacterial suspension grown in Marine Broth for 24 h at 27°C, washed twice in F medium, and diluted in F medium at OD600 = 0.05. The other wells were inoculated with Marine Broth to monitor for possible contamination during the experiment.
In addition to the co-culture plates, a plate was prepared as a control. In this case, 4.5 ml of of T. suecica F&M-M33 axenic culture (at the same density as for co-culture experiments) were inoculated in three wells and 0.5 ml of F medium was added to reach the final test volume. The other wells contained uninoculated Marine Broth to allow for monitoring of contamination. In both co-cultures and control plates, the 5 ml inoculated in each well was the maximum possible volume to avoid leakage when on the orbital plate shaker (85 rpm). The 6-well plates were incubated for 10 days at 27°C under continuous illumination (90 µmol photons m−2 s−1) provided by daylight tubes (Ledbar-T5, Intec, F.A.N. Europe Lighting, Nola, Italy).
Algal growth at the end of the experiment was assessed by biomass dry weight (DW) determination. The DW of the inoculum and the final concentration of cultures at the end of the experiment were determined by filtration on pre-weighed glass-fibre membranes (1.2 µm nominal pore size, MFV3, Filter Lab Filtros Anoia, Barcelona, Spain), and successive drying following the modalities reported in Guccione et al. (Citation2014). Biomass productivity (g l−1 day−1) was calculated as follows: the DW at the start of the experiment was subtracted from DW at the end of the experiment and the difference was divided by the duration of the experiment (days). Statistical analyses of productivity, including a Shapiro–Wilk test for normality, ANOVA and Kruskal–Wallis test, were conducted in R version 3.5.1 (R Development Core Team, Citation2010).
Metabolomic analysis of Tetraselmis suecica F&M-M33 cultures
Metabolomic analysis was performed for co-cultures prepared in Erlenmeyer flasks (250 ml) with 47.5 ml of a T. suecica F&M-M33 axenic culture and 2.5 ml (OD600 = 0.05) of Vitellibacter strain AAD2 (homotypic synonym Aequorivita) or Sphingopyxis flavimaris strain AG5 liquid cultures grown in agitation for 24 h at 27°C in Marine Broth. The same T. suecica F&M-M33 axenic cultures used to prepare the co-cultures, also served as control. Co-cultures and control cultures were incubated for 60 days at 27°C under continuous illumination (60 µmol photons m−2 s−1) provided by metal halide lamps (Osram HQI-TS 150 W). After 60 days, each culture was centrifuged for 15 min at 938 × g (PK121R, ALC international, Pévy, France). The supernatant was aliquoted and concentrated 1.2× at 45°C for 90 min (Concentrator 5301, Eppendorf, Hamburg, Germany). Samples were sterilized by filtration (0.2 µm) and stored at −80°C.
Samples were thawed, distributed in 15 ml tubes, and mixed. One millilitre of each sample was filtered through a 0.22 μm polyvinylidene fluoride (PVDF) filter. Separation was performed on a UHPLC system (Agilent 1290 Infinity, Agilent Technologies, Waldbronn, Germany) coupled to a mass quadrupole time of flight (6550 i-Funnel QTOF) with electrospray ionisation via Jet Stream Technology and a C18 column (Poroshell 120, 3 mm × 100 mm, 2.7 μm pore size) (UPLC–ESI–QTOF-MS). An aliquot of 200 μl of each supernatant was injected and separated on the column with a flow rate of 0.4 ml min−1 and mobile phases of water with 0.1% formic acid (v/v) (phase A) and acetonitrile (ACN) with 0.1% formic acid (v/v) (phase B). Compounds were separated using the following gradient conditions: 0–10 min, 1–18% phase B; 10–16 min, 18–38% phase B; 16–22 min, 38–95% phase B (the remaining solvent up to 100% was aqueous phase, phase A). Finally, the phase B content was returned to the initial conditions (1%) for 1 min and the column was re-equilibrated for 5 min. Nitrogen was used as a nebulizer (35 psi, 9 l min−1) and drying gas (280°C, 9 l min−1, sheath gas temperature 400°C, and sheath gas flow 12 l min−1). Spectra were acquired in the range of m z−1 100−1100 in negative mode, with a fragmentor voltage of 100 V and an acquisition rate of 1.5 spectra/s. Samples were also analysed in the positive mode, applying the same conditions. To assure mass accuracy during the mass spectrometry (MS) analysis, external calibration of the instrument was performed at the beginning of the batch, introducing a mixture of reference compounds using Tuning Mix (Agilent Technologies, Santa Clara, California, USA). Continuous internal calibration was performed during analyses with the use of signals m z−1 112.9855 and m z−1 1033.9881 in negative polarity, and m z−1 121.0508 and m z−1 922.0097 in positive polarity.
Metabolomic data treatment
The metabolomic data acquisition system stored the raw data set of chromatographic and mass spectral data in a profile and centroid data format to guarantee the complete collection of necessary information. Agilent metabolomics workstation software was used to process the raw data. MassHunter Qualitative Analysis (version B.06.00 Agilent Technologies, Santa Clara, California, USA) and ProFinder (version B.06.00 Agilent Technologies, Santa Clara, California, USA) were used to perform the metabolomic treatment analysis. MassHunter Qualitative Analysis software was used to examine the chromatographic profile and the mass spectrum of the samples. Profinder software was used for data processing. Data processing was performed using the total ions detected in the raw data. The molecular feature extraction (MFE) algorithm for small molecules and peptides was used to create the entity data matrix. Data processing included the following specific features: (i) peak picking, where the peaks were detected and extracted by retention time (RT) restriction of 1 000–25 000 min, m z−1 restriction of 100 0000–1 100 0000 m z−1, a filter for peak counts with a height of 1000 counts and peak spacing tolerance; (ii) compound binning and alignment, where the parameters were set with an RT tolerance of 0.30 min and mass tolerance of 20.00 ppm and 2.00 mDa; (iii) post-processing filtering by the score of the molecular feature extraction (MFE), where the score limit used was ≥80%. A total of 1631 entities were retained and aligned across all the samples after applying all the filters. After that, the most relevant entities were launched against databases for identification. Entities were all the ions extracted during the data acquisition step by the equipment (UPLC–ESI–QTOF-MS), which formed the data matrix after data processing and which may have the metabolite condition if they were successfully identified.
Results
Identification and phenotypic characterization of phycospheric bacterial strains
A total of 203 bacterial strains isolated from T. suecica F&M-M33 cultures were characterized: 142 were isolated and identified through the 16S rDNA sequencing performed in this work (Supplementary table 1), and 61 strains (Supplementary table 2) were previously isolated and identified (Biondi et al. Citation2017; Piampiano et al. Citation2019).
The 142 isolates identified in this work belong to at least 18 different genera (; it was not possible to assign the correct genus for 10 isolates) falling into three phyla: Firmicutes, Bacteroidetes and Proteobacteria. Most of the isolates (49%) belonged to the family Rhodobacteraceae, which accounted for the highest number of detected genera (nine out of 18). Seventeen isolates (11.9% of the total) affiliated with the class Gammaproteobacteria, and 13 of them were identified as members of the genus Marinobacter. Forty-six isolates affiliated with the phylum Bacteroidetes, and the majority of these (37 isolates) affiliated with the genus Muricauda, which represented 26% of the total isolates.
Table 1. Phylogenetic attribution of isolates from cultures of Tetraselmis suecica F&M-M33, based on 16S rDNA sequencing
All isolates were screened for their ability to produce the MGP molecules indol-3-acetic acid (IAA) and siderophores. Out of the 203 isolates tested, 30 were able to produce IAA (), with 17 isolates belonging to the genus Marinobacter (). Twelve isolates were able to produce siderophores; two of them were identified as Bacillus, six as Proteobacteria (genera Stappia, Roseivivax, Labrenzia and Amorphus), and four as Bacteroidetes (genera Vitellibacter, Muricauda, Cyclobacterium and Algoriphagus) (). None of the strains producing IAA also produced siderophores and vice versa.
Table 2. Identification of bacteria isolates producing at least a microalgal growth promoting compounds (MGP compounds): indole-3-acetic acid (IAA) and/or siderophores
Effect of individual bacterial strains on the productivity of Tetraselmis suecica F&M-M33
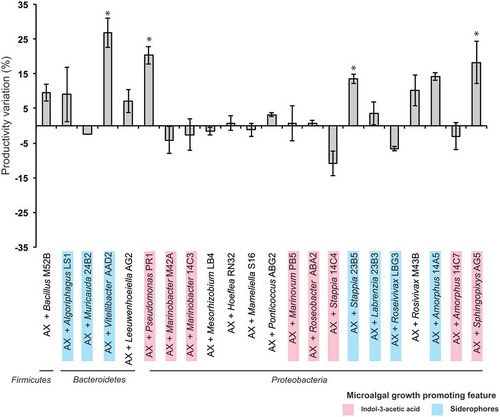
Twenty-two strains were further analysed to test their effect on T. suecica F&M-M33 growth (Supplementary table 3). The strain choice was based on bacterial phylogeny (18 different genera) and MGP features: eight strains were IAA producers, eight strains were siderophore producers, and six strains did not show any of the tested MGP traits ( and Supplementary table 3). None of the co-cultures exceeded a final concentration of 2 g l−1 biomass dry weight (average value 1.71 ± 0.19 g l−1). However, a high level of variability was observed (). Vitellibacter strain AAD2, Sphingopyxis flavimaris strain AG5, Pseudomonas strain PR1 and Stappia strain 23B5 were able to enhance (p < 0.05) the productivity in terms of biomass production compared with that of the axenic microalgal culture (). The highest value of biomass increase (more than 25%) was achieved with Vitellibacter strain AAD2. None of the tested strains were able to significantly enhance (p < 0.05) the calculated productivity based on the number of algal cells, and in co-culture with Stappia strain 23B5, algal cells even decreased by 14.3 ± 2.6% (p < 0.05).
Fig. 1. Tetraselmis suecica F&M-M33 biomass productivity. Co-cultures were obtained using an axenic culture of T. suecica F&M-M33 (AX) inoculated with a selected bacterial strain (AX + bacterial strain name). Strains with microalgal growth-promoting features are highlighted in pink (indol-3-acetic acid producers) or blue (siderophore producers). Co-cultures and axenic cultures were grown for 10 days at 27°C and under continuous illumination of 90 µmol photons m−2 s−1 and shaking (85 rpm). Bars show the relative percentage difference of productivity between co-cultures and axenic culture. * indicates statistically significant differences (ANOVA, p < 0.05)
Metabolomic analysis of Tetraselmis suecica F&M-M33 cultures
Metabolomic analysis was performed on T. suecica F&M-M33 axenic culture and on co-cultures with Vitellibacter AAD2 and S. flavimaris AG5, two of the four strains that had induced a significant (p < 0.05) increase in T. suecica F&M-M33 biomass. After 60 days of growth, samples of the supernatants of T. suecica F&M-M33 axenic culture (AX) and co-cultures with Vitellibacter AAD2 (AX+AAD2) or S. flavimaris AG5 (AX+AG5) were collected and analysed through UPLC–ESI–QTOF-MS. Analyses were performed in positive and negative mode to allow for determination of metabolites with different chemical properties. Complex UPLC–ESI–QTOF-MS metabolic profiles were obtained, and in total 133 entities were retrieved, considering both ionization modes (, Supplementary tables 4 and 5) from the two co-cultures and the axenic culture. A similar number of entities was retrieved for AX cultures and for the co-cultures in both ionization modes (). For AX, 61 and 59 entities were obtained for the positive and negative mode, respectively. In the co-culture AX+AAD2, 52 and 58 entities were detected, and in AX+AG5, 59 and 54 were detected for positive and negative mode, respectively (Supplementary tables 4 and 5). Out of the 133 entities obtained, it was possible to identify 103 entities for a total of 84 different compounds (Supplementary tables 4 and 5). Among the identified compounds, tri- and tetra-peptides accounted for 14.3% of the total. Terpenes were the second main category of detected molecules (13.1%). Six entities similar to prostaglandins (PGE1 and 2) were identified, which were named prostaglandin-like compounds (PG-like). PG-like were identified in both positive and negative mode analysis for all cultures. Compounds involved in tryptophan/IAA metabolism and in the metabolism of various vitamins of the B group, such as pantothenic acid (or vitamin B5), 5-piridoxolactone (vitamin B6 metabolism) and 8-amino-7-oxononanoate (vitamin B1 metabolism), were identified (Supplementary tables 4 and 5).
Figs 2–3. Venn diagrams showing unique entities, and entities shared by all or by two culture supernatants of axenic Tetraselmis suecica F&M-M33 (AX) and co-cultures of the axenic T. suecica F&M-M33 with either Sphingopyxis flavimaris strain AG5 (AX+AG5) or Vitellibacter strain AAD2 (AX+AAD2). Fig. 2. Positive ionization mode. Fig. 3. Negative ionization mode
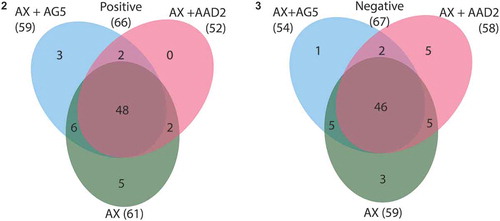
The number of entities in common between the two co-cultures was four (two for each ionization mode). KAPA (8-amino-7-oxononanoate) was among those compounds whose presence was observed in co-culture only (Supplementary table 4). There were 11 entities in common between the AX culture and the two co-cultures with AG5 (six in positive and five in negative mode, and Supplementary table 4) and seven with AAD2 (five in positive and two in negative mode, and Supplementary table 4).
In each culture, it was possible to identify culture-specific entities ( and ). A total of eight entities were present in AX samples only, six of which were identified as di-tert-butyl pentasulphide, succinic acid, 4-hydroxy-L-threonine, guanosine, L-phenylalanine and 1 PG-like compound (). In AX+AAD2 and AX+AG5 co-cultures, five and four culture-characteristic entities were observed, respectively (). Entities identified as Ser–Met–Arg, Asp–Asn–Ar–Ser and Phe–Phe–Ile–Ala were only retrieved in the AX+AAD2 co-culture. N-alpha-acetyl lysine and indole-3-acetic acid were only found in the supernatant of the AX+AG5 co-culture ().
Table 3. Entities (small chemical molecules, e.g. metabolites) retrieved in the supernatant of Tetraselmis suecica F&M-M33 axenic culture (AX) only and entities exclusively present in the supernatants of T. suecica F&M-M33 co-cultured with Vitellibacter strain AAD2 (AX+AAD2) or with Sphingopyxis flavimaris strain AG5 (AX+AG5). Data are referring to the analysis performed in positive (pos) and negative (neg) ionization modes
Discussion
Tetraselmis suecica in all its commercial applications is usually grown with its associated bacterial community. It is therefore important to elucidate the role of the different bacteria present in its phycosphere and to identify the strains which have the potential to increase productivity.
Bacteria can enhance algal growth in different ways: by producing molecules that act as phytohormones, providing vitamins, and increasing the bioavailability of phosphate or iron (Fuentes et al., Citation2016). A range of different bacteria produces IAA, a plant hormone of the auxin class that can influence microalgal growth in terms of cell dimension and number (De-Bashan et al., Citation2008; Do Nascimento et al., Citation2013; Meza et al., Citation2015; Labeeuw et al., Citation2016). Microbial siderophores are often produced by marine bacteria, which have been found in association with microalgal species (Amin et al., Citation2009a; Lupette et al., Citation2016). Siderophores, such as Fe-catechol, did stimulate the growth of some diatom species and play an important role in controlling the uptake of iron complexed with organic materials (Naito et al., Citation2008; Amin et al., Citation2009b; Moejes et al., Citation2017).
Most of the bacterial strains isolated from T. suecica F&M-M33 and investigated in this study belong to the phyla Bacteroidetes and Proteobacteria. Out of the 203 bacterial strains tested, at least 23% are able to produce MGP molecules (either IAA or siderophores) with siderophore production observed in nine different genera, and IAA production in 11 different genera. This widespread capacity to produce MGP molecules among different phylogenetic groups points to a functional redundancy of these features and that different bacteria can occupy the respective role in a microalga-bacteria interaction. Indeed, the abundance of different bacterial groups fluctuates, so the presence of different groups/strains performing the same function may compensate for the variation of single phylogenetic groups. Previous studies have shown shifts in the bacterial relative abundance depending on the seasonality and growing conditions (Biondi et al., Citation2017; Piampiano et al., Citation2019). Therefore, bacteria belonging to different phylogenetic groups were selected for co-culture experiments. The selection of bacteria also took into account the ability to produce MGP compounds. Co-cultures were set up in non-limiting iron conditions (in the growing conditions generally used for Tetraselmis biomass production), and siderophore producers were also included because these bacteria may be considered as generally positively associated with the microalga.
Out of the 22 strains tested, four strains were able to significantly increase T. suecica F&M-M33 productivity: Sphingopyxis flavimaris strain AG5, Pseudomonas strain PR1 (both producing IAA), Vitellibacter strain AAD2 and Stappia strain 23B5 (both producing siderophores). Members of the genus Sphingopyxis have already been described in association with microalgae, i.e. with Gymnodinium catenatum and Scenedesmus quadricauda (Green et al., Citation2004; Krohn-Molt et al., Citation2017) and Stappia strains have been isolated from the phycosphere of Tetraselmis striata (Park et al., Citation2017). Our results did not permit us to correlate MGP production with an increase in biomass production, but they indicated that the in vitro production of siderophore and IAA is not a gold standard for the selection of bacterial strains capable of exerting a positive effect on microalgal growth. However, it is important to remember that the siderophore and IAA production is strongly dependent on the environmental conditions, and that co-cultures were set up under non-limiting iron conditions, so siderophore-producing strains may be able to boost microalgal growth through a different mechanism.
To deepen knowledge of the interactions between microalga and bacteria, we studied the exo-metabolome of an axenic T. suecica F&M-M33 culture and that of Tetraselmis-bacterial co-cultures with either of the two strains that induced the highest increase in Tetraselmis growth in co-cultures (S. flavimaris strain AG5 and Vitellibacter strain AAD2) (). Most of the retrieved compounds were found under all of the three tested conditions, axenic and in both co-cultures (72 compounds identified out of 94 shared entities). However, it was possible to identify culture-specific compounds. Eight entities were not found in the co-culture exo-metabolomes, because they were presumably used by bacteria. IAA was among the compounds found exclusively in the supernatant of T. suecica F&M-M33 co-cultured with S. flavimaris strain AG5, demonstrating that this strain was able to produce IAA when grown with the microalga. The metabolomic analysis of the supernatant of the AX+AAD2 (Vitellibacter) revealed the presence of several polypeptides (tri- and tetra-peptides).
Fig. 4. Schematic overview of the Tetraselmis suecica F&M-M33 phycosphere and its possible interactions with Vitellibacter strain AAD2 and Sphingopyxis flavimaris strain AG5
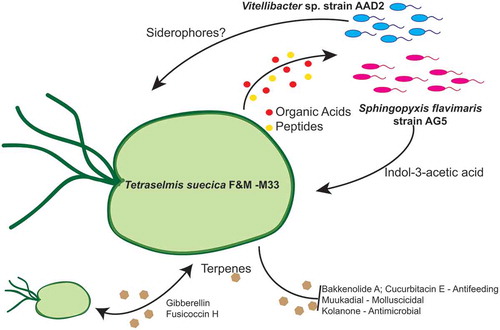
The metabolomic analysis allowed us to describe the general exo-metabolome of T. suecica F&M-M33 (both in axenic and co-cultures). Several amino acids and polypeptides (tri- and tetra-peptides) were observed, suggesting that they could serve as a carbon and/or nitrogen source for bacteria. Many carboxylic acids were also present, highlighting the phycosphere as a zone particularly rich in nutrients as the rhizosphere. The role of polypeptides is not limited to their nutritive function, and peptides may interact with many different macromolecules and biochemical compounds, modulating several functions and conditions in microalgae and bacteria, such as chemotaxis and conjugation (Möller et al., Citation2008; Apone et al., Citation2019). Some peptides may also have antimicrobial activity, and it has been demonstrated that Tetraselmis suecica extracts contain bioactive peptides (Guzmán et al., Citation2019); however, there is no evidence that these could be released in the phycosphere.
The high amount of nutrients released by T. suecica F&M-M33 may attract a plethora of different organisms that could be either beneficial or harmful for microalgal growth; thus T. suecica F&M-M33 may secrete bioactive molecules, such as terpenes, as a defence mechanism. In the T. suecica F&M-M33 exo-metabolome, several terpenes were observed: bakkenolide A, cinncassiol D1 glucoside, cofaryloside, confertifoline, cucurbitacin E, fusicoccin H, gibberellin, kolanone, mukaadial, phytuberin and rishitin. Terpenes have antioxidant and antibacterial activities (Huang & Osbourn, Citation2019): kolanone, a polyisoprenylated benzophenone, is well known for its antimicrobial properties (Hussain et al., Citation1982; Madubunyi, Citation1995). Bakkenolide A, a sesquiterpene originally isolated from the wild butterbur Petasites japonicus, has antifeedant effects (Reddy, Citation2004). Cucurbitacin E, which is usually found in Cucurbitaceae plants, where it works as a defence against herbivores (Chen et al., Citation2005), was also present. T. suecica F&M-M33 also produces mukaadial, a sesquiterpene that has molluscicidal activity (Kubo et al., Citation1983); however, we may hypothesize that it has a limited effect on marine molluscs as T. suecica is often used in aquaculture for bivalve mollusc feeding (Tredici et al., Citation2009). Terpenes, such as gibberellin and fusicoccin H, are also important for signal transduction. Among the compounds found in the T. suecica F&M-M33 exo-metabolome, which could be involved in signal transduction, there are also the PG-like compounds. These molecules are usually not associated with microalgae; however, animal-like prostaglandins were recently found in a microalga, the diatom Skeletonema marinoi, where they may act in cell-to-cell signalling (Di Dato et al., Citation2017).
In conclusion, only four strains (out of 16 putative MGP strains) could elicit a gain in microalgal biomass production. In vitro testing showed potential MGP features; but it was only possible to identify which strains may have a potential biotechnological application testing them in co-culture. The exo-metabolome of the three cultures (axenic and the two co-cultures) showed the presence of many common compounds, depicting the T. suecica F&M-M33 phycosphere as a zone particularly rich in small peptides and organic acids. Moreover, it has been demonstrated that T. suecica F&M-M33 produced a wide range of bioactive molecules potentially targeting different organisms, so it would be of interest to test the activity of its supernatant/extract as an additive for agricultural applications.
Author contributions
E. Piampiano: drafted the manuscript, analysed the samples and compiled the data, commented on and contributed to the final manuscript. F. Pini: drafted the manuscript, analysed the samples and compiled the data, commented on and contributed to the final manuscript. N. Biondi: collected the samples, analysed the samples and compiled the data, commented on and contributed to the final manuscript. C.J. Garcia: analysed the samples and compiled the data, commented on and contributed to the final manuscript. F. Decorosi: analysed the samples and compiled the data, commented on and contributed to the final manuscript. F.A. Tomàs-Barberàn: provided material support and funding for this project, commented on and contributed to the final manuscript. L. Giovannetti: conceived the project, drafted the manuscript, provided material support and funding for this project, commented on and contributed to the final manuscript. C. Viti: conceived the project, drafted the manuscript, provided material support and funding for this project, commented on and contributed to the final manuscript.
TEJP-2019-0069-File008.docx
Download MS Word (156.9 KB)Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://doi.org/10.1080/09670262.2020.1765024
Supplementary table 1. Identification of bacterial isolates by 16S rDNA sequencing. Strains were isolated from different cultures of Tetraselmis suecica F&M-M33.
Supplementary table 2. Bacterial strains, previously isolated from Tetraselmis suecica F&M-M33 cultures, used in this work.
Supplementary table 3. Bacterial strains used for co-culture assays with Tetraselmis suecica F&M-M33.
Supplementary table 4. Entities identified in the supernatant of Tetraselmis suecica F&M-M33 cultures. The supernatants of cultures of T. suecica F&M-M33 axenic (AX), T. suecica F&M-M33 co-cultured with Vitellibacter strain AAD2 (AX+AAD2) and T. suecica F&M-M33 co-cultured with Sphingopyxis flavimaris strain AG5 (AX+AG5) were analysed through a non-targeted metabolomics approach. Data refer to the analysis conducted in positive ionization mode. Entities were identified using METLIN metabolite and lipid databases.
Supplementary table 5. Entities identified in the supernatant of Tetraselmis suecica F&M-M33 cultures. The supernatants of cultures of T. suecica F&M-M33 axenic (AX), T. suecica F&M-M33 co-cultured with Vitellibacter strain AAD2 (AX+AAD2), and T. suecica F&M-M33 co-cultured with Sphingopyxis flavimaris strain AG5 (AX+AG5) were analysed through a non-targeted metabolomics approach. Data refer to the analysis conducted in negative ionization mode. Entities were identified using MELTIN metabolite and lipid databases.
References
- Amin, S.A., Green, D.H., Kupper, F.C. & Carrano, C.J. (2009a). Vibrioferrin, an unusual marine siderophore: iron binding, photochemistry, and biological implications. Inorganic Chemistry, 48: 11451–11458.
- Amin, S.A., Green, D.H., Hart, M.C., Kupper, F.C., Sunda, W.G. & Carrano, C.J. (2009b). Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proceedings of the National Academy of Sciences USA, 106: 17071–17076.
- Apone, F., Barbulova, A., & Colucci, M.G. (2019). Plant and microalgae derived peptides are advantageously employed as bioactive compounds in cosmetics. Frontiers in Plant Science, 10: 756.
- Bell, W. & Mitchell, R. (1972). Chemotactic and growth response of marine bacteria to algal extracellular products. The Biological Bulletin, 143: 265–277.
- Biondi, N., Cheloni, G., Rodolfi, L., Viti, C., Giovannetti, L. & Tredici, M. R. (2018). Tetraselmis suecica F&M-M33 growth is influenced by its associated bacteria. Microbial Biotechnology, 11: 211–223.
- Biondi, N., Cheloni, G., Tatti, E., Decorosi, F., Rodolfi, L., Giovannetti, L., Viti, C. & Tredici, M.R. (2017). The bacterial community associated with Tetraselmis suecica outdoor mass cultures. Journal of Applied Phycology, 29: 67–78.
- Borowitzka, M.A. (2013). High-value products from microalgae – their development and commercialisation. Journal of Applied Phycology, 25: 743–756.
- Bric, J.M., Bostock, R.M. & Silverstone, S.E. (1991). Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Applied and Environmental Microbiology, 57: 535–538.
- Chen, J.C., Chiu, M.H., Nie, R.L., Cordell, G.A. & Qiu, S.X. (2005). Cucurbitacins and cucurbitane glycosides: structures and biological activities. Natural Product Reports, 22: 386–399.
- De-Bashan, L.E., Antoun, H. & Bashan, Y. (2008). Involvement of indole-3-acetic acid produced by the growth-promoting bacterium Azospirillum spp. in promoting growth of Chlorella vulgaris. Journal of Phycology, 44: 938–947.
- De-Bashan, L.E., Bashan, Y., Moreno, M., Lebsky, V.K. & Bustillos, J.J. (2002). Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Canadian Journal of Microbiology, 48: 514–521.
- Di Dato, V., Orefice, I., Amato, A., Fontanarosa, C., Amoresano, A., Cutignano, A., Ianora, A. & Romano, G. (2017). Animal-like prostaglandins in marine microalgae. The ISME Journal, 11: 1722–1726.
- Do Nascimento, M., Dublan, M.L.A., Ortiz-Marquez, J.C.F. & Curatti, L. (2013). High lipid productivity of an Ankistrodesmus–Rhizobium artificial consortium. Bioresource Technology, 146: 400–407.
- Fouilland, E. (2012). Biodiversity as a tool for waste phycoremediation and biomass production. Reviews in Environmental Science and Bio/Technology, 11: 1–4.
- Fuentes, J.L., Garbayo, I., Cuaresma, M., Montero, Z., Gonzalez-Del-Valle, M. & Vilchez, C. (2016). Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Marine Drugs, 14: 5. doi:10.3390/Md14050100.
- Fukami, K., Nishijima, T. & Ishida, Y. (1997). Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia, 358: 185–191.
- Green, D.H., Llewellyn, L.E., Negri, A.P., Blackburn, S.I. & Bolch, C.J.S. (2004). Phylogenetic and functional diversity of the cultivable bacterial community associated with the paralytic shellfish poisoning dinoflagellate Gymnodinium catenatum. FEMS Microbiology Ecology, 47: 345–357.
- Guccione, A., Biondi, N., Sampietro, G., Rodolfi, L., Bassi, N. & Tredici, M.R. (2014). Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a green wall panel photobioreactor. Biotechnology Biofuels, 7: 84. doi: 10.1186/1754-6834-7-84.
- Guillard, R.R. & Ryther, J.H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8: 229–239.
- Guzmán, F., Wong, G., Román, T., Cárdenas, C., Alvárez, C., Schmitt, P., Albericio, F., & Rojas, V. (2019). Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) Butcher and bactericidal activity improvement. Marine Drugs, 17: 453.
- Huang, A.C. & Osbourn, A. (2019). Plant terpenes that mediate below-ground interactions: prospects for bioengineering terpenoids for plant protection. Pest Management Science. doi: 10.1002/ps.5410.
- Hussain, R.A., Owegby, A.G., Parimoo, P. & Waterman, P.G. (1982). Kolanone, a novel polyisoprenylated benzophenone with antimicrobial properties from the fruit of Garcinia kola. Planta Medica, 44: 78–81.
- Krohn-Molt, I., Alawi, M., Forstner, K.U., Wiegandt, A., Burkhardt, L., Indenbirken, D., Thiess, M., Grundhoff, A., Kehr, J. & Streit, W.R. (2017). Insights into microalga and bacteria interactions of selected phycosphere biofilms using metagenomic, transcriptomic, and proteomic approaches. Frontiers in Microbiology, 8: 1941. doi: 10.3389/Fmicb.2017.01941.
- Kubo, I., Matsumoto, T., Kakooko, A.B. & Mubiru, N.K. (1983). Structure of mukaadial, a molluscicide from the Warburgia plants. Chemistry Letters, 7: 979–980.
- Labeeuw, L., Khey, J., Bramucci, A.R., Atwal, H., De La Mata, A.P., Harynuk, J. & Case, R.J. (2016). Indole-3-acetic acid is produced by Emiliania huxleyi coccolith-bearing cells and triggers a physiological response in bald cells. Frontiers in Microbiology, 7: 828. doi: 10.3389/fmicb.2016.00828.
- Lian, J., Wijffels, R.H., Smidt, H. & Sipkema, D. (2018). The effect of the algal microbiome on industrial production of microalgae. Microbial Biotechnology, 11: 806–818.
- Lupette, J., Lami, R., Krasovec, M., Grimsley, N., Moreau, H., Piganeau, G. & Sanchez-Ferandin, S. (2016). Marinobacter dominates the bacterial community of the Ostreococcus tauri phycosphere in culture. Frontiers in Microbiology, 7: 1414. doi: 10.3389/Fmich.2010.01414
- Madubunyi, I.I. (1995). Antimicrobial activities of the constituents of Garcinia kola seeds. International Journal of Pharmacognosy, 33: 232–237.
- Meza, B., De-Bashan, L.E., Hernandez, J.P. & Bashan, Y. (2015). Accumulation of intra-cellular polyphosphate in Chlorella vulgaris cells is related to indole-3-acetic acid produced by Azospirillum brasilense. Research in Microbiology, 166: 399–407.
- Moejes, W.F., Succurro, A., Popa, O., Maguire, J. & Ebenhöh, O. (2017). Dynamics of the bacterial community associated with Phaeodactylum tricornutum cultures. Processes, 5. doi: 10.3390/pr5040077.
- Molina Grima, E., Belarbi, E.H., Acién-Fernández, F.G., Medina, A.R. & Chisti, Y. (2003). Recovery of microalgal biomass and metabolites: process options and economics. Biotechnology Advance, 20: 491–515.
- Möller, N.P., Scholz-Ahrens, K.E., Roos, N., & Schrezenmeir, J. (2008). Bioactive peptides and proteins from foods: indication for health effects. European Journal of Nutrition, 47: 171–182.
- Naito, K., Imai, I. & Nakahara, H. (2008). Complexation of iron by microbial siderophores and effects of iron chelates on the growth of marine microalgae causing red tides. Phycological Research, 56: 58–67.
- Park, J., Jin, H.F., Lim, B.R., Park, K.Y. & Lee, K. (2010). Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresource Technology, 101: 8649–8657.
- Park, J., Park, B.S., Wang, P., Patidar, S.K., Kim, J.H., Kim, S.-H., et al. (2017). Phycospheric native bacteria Pelagibaca bermudensis and Stappia sp. ameliorate biomass productivity of Tetraselmis striata (KCTC1432BP) in co-cultivation system through mutualistic interaction. Frontiers in Plant Science, 8. doi: 10.3389/fpls.2017.00289.
- Patidar, S.K., Kim, S.H., Kim, J.H., Park, J., Park, B.S. & Han, M.S. (2018). Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: dynamics of quorum-sensing precursors and strategic improvement in lipid productivity. Biotechnoogy Biofuels, 11. doi: 10.1186/S13068-018-1097-9.
- Perez-Miranda, S., Cabirol, N., George-Tellez, R., Zamudio-Rivera, L.S. & Fernandez, F.J. (2007). O-CAS, a fast and universal method for siderophore detection. Journal of Microbiological Methods, 70: 127–131.
- Piampiano, E., Pini, F., Biondi, N., Pastorelli, R., Giovannetti, L. & Viti, C. (2019). Analysis of microbiota in cultures of the green microalga Tetraselmis suecica. European Journal of Phycology, 54: 497–508.
- R Development Core Team (2010). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Ramanan, R., Kim, B.H., Cho, D.H., Oh, H.M. & Kim, H.S. (2016). Algae-bacteria interactions: evolution, ecology and emerging applications. Biotechnology Advance, 34: 14–29.
- Reddy, D.S. (2004). A general approach toward bakkanes: short synthesis of (±)-bakkenolide-A (fukinanolide). Organic Letters, 6: 3345–3347.
- Sansone, C., Galasso, C., Orefice, I., Nuzzo, G., Luongo, E., Cutignano, A., Romano, G., Brunet, C., Fontana, A., Esposito, F. & Ianora, A. (2017). The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Scientific Report, 7: 41215–41215.
- Spolaore, P., Joannis-Cassan, C., Duran, E. & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101: 87–96.
- Subashchandrabose, S.R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K. & Naidu, R. (2011). Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnology Advance, 29: 896–907.
- Tandon, P., Jin, Q. & Huang, L.M. (2017). A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microbial Cell Factories, 16. doi: 10.1186/S12934-017-0834-2.
- Tredici, M.R., Biondi, N., Ponis, E., Rodolfi, L. & Chini Zittelli, G. (2009). Advances in microalgal culture for aquaculture feed and other uses. In New Technologies in Aquaculture: Improving Production Efficiency, Quality and Environmental Management (Burnell, G. & Allan, G., editors), 610–686. Woodhead Publishing, Cambridge.
