ABSTRACT
Algal turfs are ecosystem engineers receiving steadily growing attention in recent years in relation to their expansion on temperate reefs due to global change. However, their species diversity and taxonomy are still poorly understood, mostly based solely on morphological information. Algal turfs are the dominant type of assemblage in Macaronesia, but molecular diversity surveys have barely been applied in this region. We aim to show how molecular tools can assist us to improve our understanding of the diversity and taxonomy of natural algal turfs by studying the turf-forming rhodomelacean genus Stichothamnion which has only one recognized species in Macaronesia, the generitype S. cymatophilum. Stichothamnion resembles other members of the tribe Streblocladieae, but it has unbranched trichoblasts, an unusual character in the family. Here, we show using phylogenetic analyses of the rbcL gene that specimens collected in the Canary Islands and the Azores identifiable morphologically as S. cymatophilum exhibit cryptic diversity, and these analyses position Stichothamnion in the Vertebrata clade with full support. We place Stichothamnion in synonymy with Vertebrata, transfer the existing species, and describe a newly discovered cryptic species as V. barbarae Muñoz-Luque & Díaz-Tapia, sp. nov., found exclusively in the Azores. The new species was morphologically indistinguishable from V. cymatophila but diverged by 2.1–2.3% in the rbcL gene. This study illustrates the work required to better understand the extraordinary species diversity in algal turfs such as those in Macaronesia which remain taxonomically under-studied.
Introduction
Global change is triggering profound changes in shallow benthic ecosystems in temperate areas worldwide – kelp forests and canopy algae are being replaced by algal turfs (Filbee-Dexter & Wernberg, Citation2018; O’Brien & Scheibling, Citation2018; Filbee-Dexter et al., Citation2020). Whereas brown algae form kelp and large canopies, turfs are composed of small, often filamentous, densely entangled red, brown and green algae (Connell et al., Citation2014). Observed distribution shifts on temperate coasts have led to the current view that temperate algal turfs are a consequence of the degradation of reef assemblages (Connell et al., Citation2008; Filbee-Dexter & Wernberg, Citation2018; O’Brien & Scheibling, Citation2018; Filbee-Dexter et al., Citation2020). However, in temperate regions, algal turfs are dominant in habitats subject to a wide variety of natural stresses such as desiccation, wave-exposure or high sediment loads including sand scour and burial (Littler, Citation1980; Stewart, Citation1982; Akioka et al., Citation1999; Kelaher et al., Citation2001; Mei & Schiel, Citation2007; Díaz-Tapia et al., Citation2013a, b). Despite the significance of algal turfs as ecosystem engineers, their species diversity is still poorly understood, mostly characterized by morpho-anatomical identifications without molecular studies.
Molecular data have been successfully used in seaweed diversity surveys for the last 20 years leading to the discovery of cryptic diversity (Zuccarello & West, Citation2003; Saunders & Lehmkuhl, Citation2005; Jesus et al., Citation2019). This approach has rarely been applied to turf-forming species, even when they are good candidates for cryptic diversity. Morphological identifications are challenging because the small thalli are easily overlooked and numerous species have convergent habits, prostrate systems bearing erect axes (Price & Scott, Citation1992; Díaz-Tapia & Bárbara, Citation2013, Citation2014). Consequently, many species can only be distinguished, if at all, by detailed microscopic study of anatomical characters. Molecular tools are therefore essential for assessing species diversity. The few recent molecular studies that included algal turfs have led to the discovery of new regional records and cryptic or pseudo-cryptic diversity (Díaz-Tapia & Bárbara, Citation2013; Savoie & Saunders, Citation2016; Bustamante et al., Citation2017; Freshwater et al., Citation2017; Piñeiro-Corbeira et al., Citation2020a, b; Díaz-Tapia et al., Citation2020a, b).
Macaronesia comprises a group of Atlantic archipelagos in which algal turfs (Supplementary fig. S1) are the most common type of assemblage (Tuya & Haroun, Citation2006; Wallenstein et al., Citation2009). These archipelagos host an exceptional algal diversity (949 species; Freitas et al., Citation2019) characterized by early phycologists (e.g. Bory de Saint-Vincent, Citation1803; Børgesen, Citation1930; Schmidt, Citation1929) and updated in checklists or floras (e.g. Sansón, Citation1991; Neto, Citation1994; Rojas-González, Citation1997; Haroun et al., Citation2002). The Rhodomelaceae is a major component of algal turfs in these archipelagos and it has been morphologically studied in detail (Rojas-González, Citation1997). Thus, the rhodomelacean flora of this region offers an exceptional opportunity to analyse the link between morphological and molecular identification of turf-forming species.
We selected Stichothamnion as an example of turf-forming algae in Macaronesia. It was described by Børgesen (Citation1930) based on S. cymatophilum, a turf-forming species from the Canary Islands that has also been recorded in the Mediterranean (Sartoni, Citation1992). This species has exogenous branches, eight pericentral cells without cortication and one tetrasporangium per segment, characters that agree with Vertebrata (Díaz-Tapia et al. Citation2017b). Conversely, it is markedly dorsiventral, with erect and prostrate axes, leading Børgesen (Citation1930) to discuss its similarities with other genera. Moreover, S. cymatophilum has unbranched trichoblasts, a very unusual character that contrasts with the typical dichotomously branched trichoblasts of most Rhodomelaceae, leading Børgesen (Citation1930) to propose a new genus. A second species, S. antillarum, is only known from Saint Eustatius, Lesser Antilles (Vroman, Citation1967). The two species share the key character of unbranched trichoblasts, but differ in the number of pericentral cells (8 in S. cymatophilum vs. 10–11 in S. antillarum) and the diameter of the axes (70 vs. 85–110 µm).
Affinities of the genus Stichothamnion within the Rhodomelaceae were discussed by Hommersand (Citation1963), who placed it in the Lophosiphonia group, erected by Falkenberg (Citation1901) for four dorsiventral genera not easily assignable to established tribes. The Lophosiphonia group is now known to include a morphologically diverse group of genera with divergent phylogenetic affinities (Díaz-Tapia et al., Citation2017b). Some of the genera are recognized as independent tribes (Ophidocladus, Pleurostichidium) or are members of other tribes (Lophosiphonia), while others were placed in synonymy with the genus Vertebrata in the tribe Streblocladieae (Ctenosiphonia, some species assigned to Lophosiphonia) (Phillips, Citation2000; Díaz-Tapia et al., Citation2017a, Citation2017b; Pasella et al., Citation2019). In the absence of molecular data for Stichothamnion, its phylogenetic relationships are enigmatic.
Our surveys of turf-forming species of the Rhodomelaceae in the Canary Islands and Azores in 2018 enabled us to obtain and sequence ~400 specimens. Among them, we found six samples morphologically assignable to Stichothamnion. The objective of this work is to reassess the species diversity of the genus Stichothamnion in Macaronesia and analyse its phylogenetic relationships with other members of the Rhodomelaceae.
Materials and methods
Algal turfs were collected during sampling surveys in 2018 from Lanzarote (Canary Islands, Spain; 10 sites) and São Miguel (Azores, Portugal; 12 sites) (Supplementary fig. S2). Rhodomelacean species of the turfs were morphologically recognized and carefully isolated using a stereomicroscope. As turf-forming species in Macaronesia are very small in size (<1 cm), each sample of each species consisted of several specimens. Part of each sample was preserved in silica gel for DNA extraction, while the remaining material was preserved for morphological study in 4% formalin seawater at 4°C and stored in the dark. For morphological observations, specimens were mounted in 20% Karo® Syrup (ACH Foods, Memphis, TN, USA) and 80% distilled water. Sections for microscopic observations were made by hand using a razor blade. Morpho-anatomical characters were studied by light microscopy. Voucher specimens were deposited in the herbarium of the University of Santiago de Compostela (SANT).
DNA was extracted from silica gel-dried material using an adapted cetyltrimethylammonium bromide (CTAB) protocol (Doyle & Doyle,Citation1987). PCR amplification was carried out for rbcL using the primers F2 or F8/R1462 (Díaz-Tapia et al., Citation2018). Reactions were performed in a total volume of 25 µl, consisting of 5 µl 5× MyTaqTM reaction buffer, 0.7 µl 10 µM of forward and reverse primers, 0.125 µl 1U/µl My TaqTM DNA Polymerase (Bioline), 17.475 µl MilliQ® water and 1 µl template DNA. The PCR profile consisted of initial denaturation (93°C for 3 min), 35 cycles of denaturation (94°C for 30 s), primer annealing (45°C for 30 s), and extension (74°C for 90 s) and final extension (74°C for 5 min). The PCR products were purified and sequenced commercially by Macrogen Inc. (Madrid, Spain).
Six new rbcL gene sequences were generated in this study for the target group. We also included in our phylogenetic analyses 64 sequences representative of all Vertebrata spp. for which molecular data were available, and representative species of the major lineages in the Streblocladieae according to previous phylogenetic studies (Díaz-Tapia et al., Citation2017a). To test the effect of species selection in the topology of the phylogenetic trees, we analysed a second dataset using the same selection of sequences but excluding three randomly chosen species (V. reptabunda, V. fruticulosa and Polysiphonia paniculata). Sequence data and GenBank accession numbers are listed in Supplementary table S1. Sequences were aligned using Muscle in Geneious 6.1.8 (Kearse et al., Citation2012). We included in our analyses a single sequence per species, except for the focal species of this study, for which all haplotypes were represented. The sequences included in the final alignment were selected considering their quality in terms of both length (the longest sequences) and excluding those with ambiguous bases. Phylogenetic trees for rbcL were estimated with Maximum likelihood (ML) using RAxML 8.1.X (Stamatakis, Citation2014). GTR-Gamma was used as the nucleotide model; branch support was estimated with 1000 bootstrap replicates. Four species of the tribe Polysiphonieae were selected as the outgroup based on our phylogenomic analyses of the major lineages of the Rhodomelaceae which resolve this tribe as sister to the Streblocladieae (Díaz-Tapia et al., Citation2017a).
Results
Molecular identification and phylogeny
Of the six rbcL sequences newly determined for specimens morphologically identified as Stichothamnion, four were from the Azores and two from the Canary Islands. They corresponded to three haplotypes that were resolved in a monophyletic, fully supported clade in our phylogeny (). Only one haplotype was found in the Canary Islands, the type locality of S. cymatophilum, while two haplotypes were found in the Azores. One of the Azorean haplotypes was resolved as sister to the Canarian haplotype with full support and divergence among them was only 0.5% (6 bp). Therefore, we assigned the Azorean specimens of this haplotype to S. cymatophilum. The second haplotype found in the Azores diverged by 2.1–2.3% (28–30 bp) from S. cymatophilum and we propose below a new species for these specimens.
Fig. 1. Maximum likelihood phylogeny of the tribe Streblocladieae based on rbcL sequences. Lineage that consists of samples originally identified as Stichothamnion is shaded. Values at the nodes represent bootstrap support, only shown if ≥ 70. Distribution of species of the genus Vertebrata is indicated: AUS = Australasia, AZ = Azores, CI = Canary Islands, At = Atlantic, NAt = North Atlantic, NEAt = North-eastern Atlantic, WAt = Western Atlantic, NEPa = North-eastern Pacific, NWPa = North-western Pacific; SEPa = South-eastern Pacific
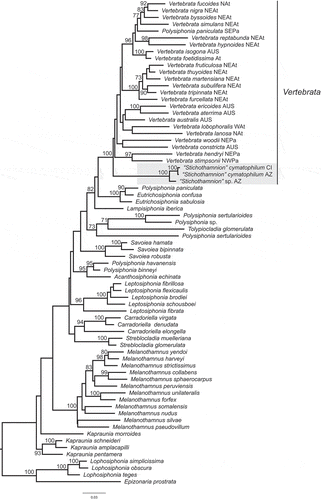
The species morphologically assigned to Stichothamnion were resolved in a fully supported clade containing the species of Vertebrata (), leading us to propose placing the genus Stichothamnion in synonymy with Vertebrata. Within Vertebrata, relationships of the Stichothamnion lineage with other members of the clade were unresolved. The current tree topology positioned the Stichothamnion lineage as sister of other Vertebrata species, but this placement was unsupported (). Trees constructed using different species selections of the tribe Streblocladieae (e.g. Supplementary fig. S3) placed the Stichothamnion lineage among other Vertebrata spp., again without support.
According to the phylogenetic analyses, S. cymatophilum and a new species are respectively transferred or assigned to Vertebrata. In the absence of molecular data for S. antillarum, we propose its transfer to Vertebrata, as its unusual unbranched trichoblasts suggest that it is highly likely to be closely related to S. cymatophilum and the new species.
Vertebrata barbarae Muñoz-Luque & Díaz-Tapia, sp. nov. (, )
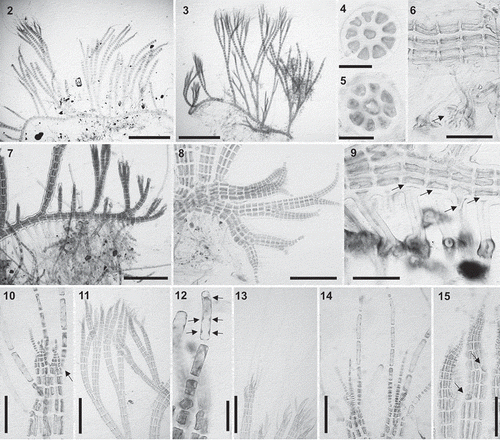
Figs 2–15. Vertebrata cymatophila (2, 4, 7, 10, 12–14) and V. barbarae sp. nov. (3, 5, 6, 8, 9, 11, 15): Vegetative morphology. Figs 2–3. Habit of a specimen with prostrate and erect axes. Figs 4–5. Cross section of an axis with nine (Fig. 4) and eight (Fig. 5) pericentral cells. Fig. 6. Prostrate axis with a rhizoid terminated in a digitate discoid pad (arrow) and a thick wall. Fig. 7. Prostrate axis with abundant rhizoids on every segment. Fig. 8. Apices of prostrate axes lacking trichoblasts. Fig. 9. Rhizoids cut off from pericentral cells (arrows). Fig. 10. Branch formed in the axil of trichoblasts (arrow). Fig. 11. Erect axes pseudodichotomously branched. Fig. 12. Trichoblast cell with multiple nuclei (arrows). Fig. 13. Apices of erect axes with long unbranched trichoblasts. Fig. 14. Apices of erect axes with spirally arranged trichoblasts. Fig. 15. Apices of erect axes with scar cells of trichoblasts (arrows). Scale bars: Figs 2–3 = 700 µm; Figs 4–5, 12 = 30 µm; Figs 6, 9, 10, 14, 15 = 80 µm; Figs 7, 11, 13 = 300 µm; Fig. 8 = 160 µm
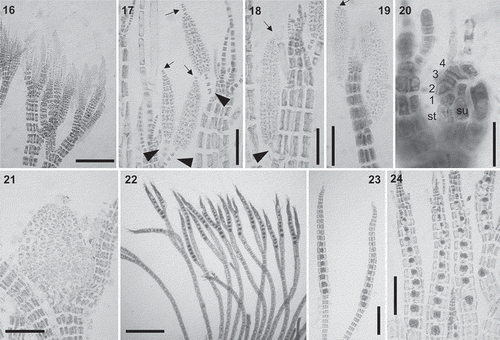
Figs 16–24. Vertebrata cymatophila (17, 18, 22, 24) and V. barbarae sp. nov. (16, 19–21, 23): Reproductive morphology. Fig. 16. Apical branches bearing abundant spermatangial branches. Figs 17–19. Spermatangial branches with 1–5 basal (arrowheads) and 0–4 apical (arrows) sterile cells. Fig. 20. Procarp showing the supporting cell (su), the sterile basal cell (st) and the four-celled carpogonial branch (1–4). Fig. 21. Cystocarp. Figs 22–24. Apical branches with tetrasporangia. Scale bars: Figs 16, 21, 24 = 150 µm; Figs 17–19 = 70 µm; Fig. 20 = 20 µm; Figs 22–23 = 200 µm
DIAGNOSIS: Thalli forming turfs, decumbent, composed of an extensive system of prostrate axes that ventrally produce rhizoids and dorsally bear erect axes, up to 3 mm in length. Rhizoids unicellular, cut off from the pericentral cells. Axes ecorticate, with 8–9 pericentral cells. Erect axes 60–125 µm in diameter, pseudodichotomously branched, branches arising in the axils of trichoblasts. Trichoblasts unbranched, spirally arranged on every segment or several segments apart. Spermatangial branches replacing trichoblasts. Procarps with 4-celled carpogonial branches. Cystocarps globose. Tetra-sporangia forming slightly spiral series, one per segment. rbcL sequence of the holotype: MW246788.
HOLOTYPE: SANT-Algae 33461, 14.iv.2018, leg. P. Díaz-Tapia.
TYPE LOCALITY: Lagoa, São Miguel, Azores (37°44’31”N, 25°34’23”W).
ETYMOLOGY: Named in honour of Dr Ignacio Bárbara for his significant contributions to the knowledge of the seaweed flora of Spain and Portugal.
HABITAT: Intertidal algal turfs.
DISTRIBUTION: São Miguel, Azores, Portugal (Supplementary fig. S2).
Vertebrata cymatophila (Børgesen) Muñoz-Luque & Díaz-Tapia, comb. nov. (, )
BASIONYM: Stichothamnion cymatophilum Børgesen (Citation1930: 119, Marine algae from the Canary Islands especially from Tenerife and Gran Canaria III. Rhodophyceae. Part III. Ceramiales. Kongelige Danske Videnskabernes Selskab, Biologiske Meddelelser 9: 1–159).
TYPE: Herbarium of the University of Copenhagen (Afonso Carrillo & Sansón, Citation1999).
TYPE LOCALITY: Las Palmas de Gran Canaria (Børgesen, Citation1930).
HABITAT: Intertidal algal turfs.
DISTRIBUTION: Canary Islands, Spain; São Miguel, Azores, Portugal (Supplementary fig. S2).
Vertebrata antillarum (Vroman) Muñoz-Luque & Díaz-Tapia, comb. nov.
BASIONYM: Stichothamnion antillarum Vroman (Citation1967: 557, A new species of Stichothamnion (Rhodophyta) from the West Indies. Acta Botanica Neerlandica 15: 557–561).
HOLOTYPE: Herbarium of the Vrije Universiteit (Vroman, Citation1967).
TYPE LOCALITY: St. Eustatius, Back off Bay (Vroman, Citation1967).
DISTRIBUTION: Only known from its type locality.
Morphological observations of Macaronesian specimens
Morphological observations revealed that Vertebrata cymatophila and V. barbarae were indistinguishable. A description of their characters is presented with the measurements for each species in .
Table 1. Measurements of morphological characters for Vertebrata cymatophila and V. barbarae sp. nov
Vegetative morphology
Specimens forming densely interwoven turfs, mixed with other species with similar habit. Thalli decumbent, consisting of extensive prostrate systems that attach to the substratum by dorsally formed rhizoids and produce branches laterally that extend the prostrate axes (). Erect axes irregularly arranged throughout prostrate axes, pseudodichotomously branched up to five orders. Thalli pink to dark red in colour, with a fairly rigid texture.
Prostrate and erect axes with an axial cell surrounded by 8–9 pericentral cells, all similar in size; ecorticate (). Walls of the axes often thick in prostrate axes and basal parts of erect axes (). Prostrate axes expanding as erect axes produce rhizoids at their basal parts (), also producing lateral branches at irregular intervals with apical cells that lack trichoblasts (). Rhizoids abundant, one or two per segment and often formed on every segment (), unicellular, cut off from the pericentral cells, terminating in a rounded or digitate discoid pad ().
Erect axes with dome-shaped apical cells. Branches arising exogenously at the apices, in the axils of trichoblasts (), at intervals mostly of 4–8 segments (); adventitious branches occasional. Trichoblasts almost always present, scarce to abundant, short to well-developed at the apices of erect axes, spirally arranged on every segment or several segments apart, unbranched and with multinucleate basal cells, deciduous and leaving conspicuous scar cells when shed ().
Reproductive morphology
Spermatangial branches formed at the apices, on the second, third or fourth basal cells of fertile trichoblasts, cylindrical, with 0–2 sterile apical cells and, in V. cymatophila, sometimes with an apical filament ().
Female reproductive structures observed only in V. barbarae sp. nov. Procarps formed on modified trichoblasts at the apices of erect axes, consisting of a supporting cell, a four-celled carpogonial branch and two groups of sterile cells (). Cystocarps globose () and carpospores clavate. Tetrasporangia subspherical, one per segment, forming slightly spiral series in apical branches ().
Discussion
Cryptic diversity in the ‘Stichothamnion’ lineage
Our molecular and morphological study of algal turfs in Azores and the Canary Islands revealed the existence of two closely related species that matched the morphological characters proposed for delineating Stichothamnion cymatophilum (Børgesen, Citation1930), the only member of the genus recognized in the North-eastern Atlantic. The two species detected in our study were morphologically indistinguishable and, among the characters provided in the detailed original description of S. cymatophilum, it is particularly relevant that both are markedly dorsiventral, have 8–9 pericentral cells without cortication and unbranched trichoblasts. Other morphological characters in our specimens also agree with the description of S. cymatophilum (Børgesen, Citation1930), i.e. abundance of rhizoids, presence of thick prostrate axis walls, diameter of axes and reproductive structures, erect axes pseudodichotomously branched and trichoblasts growing on every segment or several segments apart. We observed a considerable variation in the anatomy of spermatangial branches in the Canary Islands specimens, as we found in a single specimen spermatangial branches with different numbers of sterile basal and apical cells. The original description of S. cymatophilum also shows some variability in spermatangial branch anatomy, as some of them lack or have a sterile apical cell, but there was uniformly one sterile basal cell (fig. 54b in Børgesen, Citation1930). The presence/absence of sterile apical cells is often considered a key character for species delineation in the Polysiphonieae and Streblocladieae as it is uniform in many species, but can vary within some species (Maggs & Hommersand, Citation1993; Stuercke & Freshwater, Citation2008; Díaz-Tapia & Bárbara, Citation2013; Díaz-Tapia et al., Citation2017b). The presence of several basal sterile cells in spermatangial branches is rarer among the Streblocladieae and Polysiphonieae. Thus, our observations suggest that variability in the anatomy of spermatangial branches has no diagnostic value at the species level in the Stichothamnion lineage.
Only one of the two species detected in this study was found in the Canary Islands, the type locality of S. cymatophilum (Børgesen, Citation1930), and consequently we concluded that the Canarian specimens belong to this species that is reported here for the first time in the Azores. The second species, proposed here as V. barbarae sp. nov., was exclusively found in the Azores and can be clearly distinguished from the only other member of the genus, S. antillarum from the Caribbean, by the number of pericentral cells (10–11 vs. 8–9) (Vroman, Citation1967). Stichothamnion cymatophilum has also been reported from Alborán Island, in the Mediterranean (Sartoni, Citation1992), but whether this record actually corresponds to V. cymatophila, V. barbarae, or might represent another cryptic species, cannot be ascertained in the absence of molecular data.
The two species identified in our study differed by 2.1–2.3% sequence divergence in the rbcL gene, evidence of the existence of cryptic diversity. Diversity surveys using molecular tools often lead to the discovery of new species that have remained hidden under a single taxon name (Savoie & Saunders, Citation2016, Citation2019; Jesus et al., Citation2019; Díaz-Tapia et al., Citation2020a). In most cases, detailed morphological studies reveal that newly discovered species can be morphologically distinguished and therefore represent pseudo-cryptic species (Walker et al., Citation2009; Piñeiro-Corbeira et al., Citation2020a). True cryptic diversity is less common in the red algae, but recognition of new species that cannot be distinguished morphologically at the resolution of light microscopy is becoming more frequent (Schneider et al., Citation2017; Díaz-Tapia et al., Citation2020b; Soares et al., Citation2020).
Among our collection of 400 turf-forming red algae in the Macaronesia, only six specimens corresponded to V. cymatophila or V. barbarae, found at six sampling sites. This low collection rate shows that both species are of low abundance but must be relatively frequent in the study area. Both species were found growing intermixed with other species and a detailed inspection of turfs under the stereomicroscope was required to detect them. This finding is unsurprising, as rare species are commonly encountered during molecular diversity surveys that analyse large specimen numbers (Pardo et al., Citation2014; Savoie & Saunders, Citation2019; Díaz-Tapia et al., Citation2020a).
Our data suggest that at least one of the species studied here is endemic to Macaronesia. The archipelagos that compose this bioregion are of recent volcanic origin and host a rich flora and fauna including a large number of endemic species in both the terrestrial and marine realms (Kim et al., Citation2008; Freitas et al., Citation2019). Unlike many plant and animal groups, most seaweeds reported in Macaronesia have their type localities in mainland Europe and only 17 species are considered endemic (Freitas et al., Citation2019; Guiry & Guiry, Citation2020). Our study showed that more detailed seaweed diversity surveys in this bioregion using molecular tools reveal a more diverse endemic seaweed flora, especially relevant for algal turfs. A detailed study of species composition of algal turfs from the Azores based on morphological identifications found 139 species (Wallenstein et al., Citation2009) corresponding to 34% of the 405 macroalgal species known in the Azores (Freitas et al., Citation2019). To our knowledge no detailed reports of species composition of algal turfs in the Canary Islands are available, but this assemblage is mostly composed of members of the Ceramiales (Tuya & Haroun, Citation2006), which represents 38% of the 488 red algae known in this archipelago. Thus, algal turfs in Macaronesia are composed of an extraordinarily diverse set of species, and our finding of cryptic diversity in turf-forming species shows that much work is still needed to understand their diversity and taxonomy.
Phylogenetic relationships of ‘Stichothamnion’
The placement of Stichothamnion in the Vertebrata clade is in agreement with the morphological characters proposed for the delineation of the genus Vertebrata, including the synapomorphic character of multinucleate trichoblasts (Díaz-Tapia et al., Citation2017b). Having more than five pericentral cells is another uniform character in Vertebrata and is also observed in the Stichothamnion lineage (Choi et al., Citation2001; Díaz-Tapia et al., Citation2017b). By contrast, spermatangial branches replacing trichoblasts in the Stichothamnion lineage is unusual in Vertebrata, and most species form spermatangia on one of the trichoblast branches (Díaz-Tapia et al., Citation2017b). The only other known exception is the morphologically unusual obligate epiphyte V. lanosa that lacks vegetative trichoblasts (Maggs & Hommersand, Citation1993; Díaz-Tapia et al., Citation2017b). Trichoblasts in the Stichothamnion lineage are unbranched and consequently it is not surprising that spermatangial branches replace them.
The new placement of these species formerly assigned to Stichothamnion expands known morphological diversity in Vertebrata. This is the first time that unbranched trichoblasts have been reported in this genus, differing from the typical dichotomously branched trichoblasts in other species of Vertebrata and the family Rhodomelaceae (Hommersand, Citation1963; Maggs & Hommersand, Citation1993; Stuercke & Freshwater, Citation2008). Likewise, phylogenetic studies showed that five genera (Boergeseniella, Brongniartella, Ctenosiphonia, Enelitto-siphonia, Pterochondria) distinguished in the past by peculiar morphological characters, including having two tetrasporangia per segment, disc-like spermatangial branches or pigmented trichoblasts at maturity, are actually members of Vertebrata (Díaz-Tapia et al., Citation2017b; Savoie & Saunders, Citation2019). Moreover, placement of the Stichothamnion lineage in Vertebrata provides further examples in Vertebrata of species with clear differentiation between erect axes and an extensive prostrate system. The only dorsiventrally branched species previously included in Vertebrata are V. reptabunda and V. hypnoides (Díaz-Tapia & Bárbara, Citation2013, as Lophosiphonia and Ctenosiphonia, respectively, Díaz-Tapia et al., Citation2017b), while the radial branching pattern is more common (Díaz-Tapia et al., Citation2017b). These five dorsiventral species (three of the Stichothamnion lineage, V. reptabunda and V. hypnoides) all form part of low-growing (< 20 mm in height) intertidal algal turfs and their dorsiventral branching pattern is probably an adaptation to this type of growth. Similarly, the habit of many other turf-forming species typically consists of prostrate and erect axes (Price & Scott, Citation1992; Díaz-Tapia & Bárbara, Citation2013, Citation2014; Díaz-Tapia et al., Citation2013b; Connell et al., Citation2014).
The synonymy of Stichothamnion and Vertebrata contributes to clarifying the phylogenetic relationships of the seven genera that have been placed at one time or another in the Lophosiphonia group in Falkenberg’s (Citation1901) and Hommersand’s (Citation1963) classifications of the Rhodomelaceae. At present, Oligocladella and Falkenbergiella are the only genera of this group whose affinities with other Rhodomelaceae have not yet been studied using molecular data. Both genera are characterized by having four pericentral cells, endogenous branches and unicellular rhizoids (Weber-van Bosse, Citation1911, Citation1913; as Oligocladus; Stegenga et al., Citation1997). Considering the number of pericentral cells in these genera, it is very unlikely that they belong to Vertebrata. By contrast, these species resemble other members of the tribe Polysiphonieae, but molecular data are required to confirm their taxonomic placement.
The Stichothamnion lineage forms, within Vertebrata, a distinct clade with respect to other Atlantic clades of this genus (). This lineage has an amphi-Atlantic distribution including the Caribbean (V. antillarum), the western Mediterranean and Macaronesia (V. barbarae, V. cymatophila). Such distributions are often interpreted as indicative of lineages that are potential remnants of the former Tethys Sea (Haroun & Prud´homme van Reine, Citation1993; Bauzà-Ribot et al., Citation2012; Machín-Sánchez et al., Citation2018). Phylogeographic analyses suggest that the opening of the Atlantic 90 Mya promoted vicariant speciation processes (Bauzà-Ribot et al., Citation2012; Hou & Li,Citation2018) that might have led to the diversification of the Stichothamnion lineage in the eastern and western Atlantic. Alternatively, the diversification of this red algal lineage could be more recent, as the result of occasional dispersal events and subsequent speciation. Similar hypotheses have been proposed to explain the distribution of amphi-Atlantic lineages (Botello et al., Citation2013; Hou & Li, Citation2018). Further studies including molecular data for V. antillarum are required to test these hypotheses.
Supplementary fig. S1. Intertidal algal turfs from the Canary Islands (Spain).
Supplementary fig. S2. Sampling sites in São Miguel and Lanzarote. Distribution of Vertebrata barbarae sp. nov. and V. cymatophila are indicated by green and brown circles, respectively.
Supplementary fig. S3. Maximum likelihood phylogeny of the tribe Streblocladieae based on the alignment of 61 rbcL sequences (excluding three species of the Vertebrata lineage compared with ). Lineage that consists of samples originally identified as Stichothamnion indicated in bold. Values at the nodes represent bootstrap support, only shown if ≥70.
Supplementary table S1. GenBank accession numbers and collection information of the sequences used in phylogenetic analyses.
Author contributions
P. Díaz-Tapia: original concept, data collection, drafting and editing manuscript; L. Muñoz-Luque: data collection and curation, phylogenetic analyses; C. Piñeiro-Corbeira: conceptualization, editing manuscript; C.A. Maggs: conceptualization, editing manuscript.
Supplemental Material
Download PDF (2.3 MB)Acknowledgements
We warmly thank Ana Neto for providing assistance during fieldwork. This research was supported by computational facilities of Centro de Supercomputación de Galicia (CESGA).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2021.1871969
Additional information
Funding
References
- Afonso Carrillo, J. & Sansón, M. (1999). Algas, hongos y fanerógamas marinas de las Islas Canarias. Clave analítica. Servicio de Publicaciones Universidad de la Laguna, La Laguna.
- Akioka, H., Baba, M., Masaki, T. & Johansen, H.W. (1999). Rocky shore turfs dominated by Corallina (Corallinales Rhodophyta) in northern Japan. Phycological Research, 47: 199–206.
- Bauzà-Ribot, M.M., Francesco, Nardi C.J., Oromí, P., Pons, J. & Jaume, D. (2012). Mitogenomic phylogenetic analysis supports continental-scale vicariance in subterranean thalassoid crustaceans. Current Biology, 22: 2069–2074.
- Børgesen, F. (1930). Marine algae from the Canary Islands especially from Tenerife and Gran Canaria III. Rhodophyceae. Part III. Ceramiales. Kongelige Danske Videnskabernes Selskab, Biologiske Meddelelser, 9: 1–159.
- Bory de Saint-Vincent, J.B.G.M. (1803). Essais sur les Isles Fortunées et l’antique Atlantide, ou precis de l’histoire générale de l’Archipel des Canaries. Baudouin, Imprimeur de l’Institut National, Paris.
- Botello, A., Iliffe, T.M., Alvarez, F., Juan, C., Pons, J. & Jaume, D. (2013). Historical biogeography and phylogeny of Typhlatya cave shrimps (Decapoda: Atyidae) based on mitochondrial and nuclear data. Journal of Biogeography, 40: 594–607.
- Bustamante, D.E., Won, B.Y., Miller, K.A. & Cho, T.O. (2017). Wilsonosiphonia gen. nov. (Rhodomelaceae, Rhodophyta) based on molecular and morpho-anatomical characters. Journal of Phycology, 53: 368–380.
- Choi, H.-G., Kim, M.-S., Guiry, M.D. & Saunders, G.W. (2001). Phylogenetic relationships of Polysiphonia (Rhodomelaceae, Rhodophyta) and its relatives based on anatomical and nuclear small-subunit rDNA sequence data. Canadian Journal of Botany, 79: 1465–1476.
- Connell, S.D., Foster, M.S. & Airoldi, L. (2014). What are algal turfs? Towards a better description of turfs. Marine Ecology Progress Series, 495: 299–307.
- Connell, S.D., Russell, B.D., Turner, D.J., Shepherd, S.A. Kildea, T., Miller, D., Airoldi, L. & Cheshire, A. (2008). Recovering a lost baseline: missing kelp forests from a metropolitan coast. Marine Ecology Progress Series, 360: 63–72.
- Díaz-Tapia, P. & Bárbara, I. (2013). Seaweeds from sand-covered rocks of the Atlantic Iberian Peninsula. Part 1. The Rhodomelaceae (Ceramiales, Rhodophyta). Cryptogamie, Algologie, 34: 325–422.
- Díaz-Tapia, P. & Bárbara, I. (2014). Seaweeds from sand-covered rocks of the Atlantic Iberian Peninsula. Part 2. Palmariales, Ceramiales (excluding Rhodomelaceae), Gelidiales, Gigartinales, Plocamiales, Rhodymeniales and Scytothamniales. Cryptogamie Algologie, 35: 157–199.
- Díaz-Tapia, P., Bárbara, I. & Díez, I. (2013a). Multi-scale spatial variability in intertidal benthic assemblages: differences between sand-free and sand-covered rocky habitats. Estuarine, Coastal and Shelf Science, 133: 97–108,
- Díaz-Tapia, P., Boo, S.M., Geraldino, P.J.L., Maneiro, I., Bárbara, I. & Hommersand, M.H. (2013b). Morphology and systematics of Calliblepharis hypneoides, sp. nov. (Cystocloniaceae, Rhodophyta) from the Atlantic Iberian Peninsula. European Journal of Phycology, 48: 380–397.
- Díaz-Tapia, P., Ly, M. & Verbruggen, H. (2020a). Extensive cryptic diversity in the widely distributed Polysiphonia scopulorum (Rhodomelaceae, Rhodophyta): molecular species delimitation and morphometric analyses. Molecular Phylogenetics and Evolution, 152: 106909.
- Díaz-Tapia, P., Maggs, C.A., Macaya, E.C. & Verbruggen, H. (2018). Widely distributed red algae often represent hidden introductions, complexes of cryptic species or species with strong phylogeographic structure. Journal of Phycology, 54: 829–839.
- Díaz-Tapia, P., Maggs, C.A., Nelson, W., Macaya, E.C. & Verbruggen, H. (2020b). Reassessment of the genus Lophurella (Rhodomelaceae, Rhodophyta) from Australia and New Zealand reveals four cryptic species. European Journal of Phycology, 55: 113–128.
- Díaz-Tapia, P., Maggs, C.A., West, J.A. & Verbruggen, H. (2017a). Analysis of chloroplast genomes and a supermatrix inform reclassification of the Rhodomelaceae (Rhodophyta). Journal of Phycology, 53: 920–937.
- Díaz-Tapia, P., McIvor, L., Freshwater, D.W., Verbruggen, H., Wynne, M.J. & Maggs, C.A. (2017b). The genera Melanothamnus Bornet & Falkenberg and Vertebrata S.F. Gray constitute well-defined clades of the red algal tribe Polysiphonieae (Rhodomelaceae, Ceramiales). European Journal of Phycology, 52: 1–20.
- Doyle, J.J., & Doyle, J.L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, 19: 11–15.
- Falkenberg, P. (1901). Die Rhodomelaceen des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel, Monographie 26. Berlin.
- Filbee-Dexter, K. & Wernberg, T. (2018). Rise of turfs: a new battlefront for globally declining kelp forests. BioScience, 68: 64–76.
- Filbee-Dexter, K., Wernberg, T., Grace, S.P., Thormar, J., Fredriksen, S., Narvaez, C.N., Feehan, C.J. & Norderhaug, K.M. (2020). Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Scientific Reports, 10: 13388.
- Freitas, R., Romeiras, M., Silva, L., Cordeiro, R., Madeira, P., González, J.A., Wirtz, P., Falcón, J.M., Brito, A., Floeter, S.R., Afonso, P., Porteiro, F., Viera-Rodríguez, M.A., Neto, A.I., Haroun, R., Farminhao, J.N.M., Reblelo, A.C., Baptista, L., Melo, C.S., Martínez, A., Núñez, J., Berning, B., Johnson, M.E. & Ávila, S.P. (2019). Restructuring of the ‘Macaronesia’ biogeographic unit: a marine multi-taxon biogeographical approach. Scientific Reports, 9: 15792.
- Freshwater, D.W., Idol, J.N., Parham, S.L., Fernández-García, C., León, N., Gabrielson, P.W. & Wysor, B. (2017). Molecular assisted identification reveals hidden red algae diversity from the Burica Peninsula, Pacific Panama. Diversity, 9: 19.
- Guiry, M.D. & Guiry, G.M. (2020). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
- Haroun, R.J., Gil-Rodríguez, M.C., Díaz de Castro, J. & Prud’homme van Reine, W.F. (2002). A checklist of the marine plants from the Canary Islands (central eastern Atlantic Ocean). Botanica Marina, 45: 139–169.
- Haroun, R.J. & Prud’homme van Reine, W.F. (1993). A biogeographical study of Laurencia and Hypnea species of the Macaronesian region. Courier Forschunsinstitut Senckenberg, 159: 119–125.
- Hommersand, M.H. (1963). The morphology and classification of some Ceramiaceae and Rhodomelaceae. University of California Publications in Botany, 35: 165–366.
- Hou, Z. & Li, S. (2018). Tethyan changes shaped aquatic diversification. Biological Reviews, 93: 874–896.
- Jesus, P.B., Costa, A.L., Nunes, J.M.C., Manghisi, A., Genovese, G., Morabito, M. & Schnadelbach, S. (2019). Species delimitation methods reveal cryptic diversity in the Hypnea cornuta complex (Cystocloniaceae, Rhodophyta). European Journal of Phycology, 54: 135–153.
- Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P. & Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647–1649.
- Kelaher, B.P., Chapman, M.G. & Underwood, A.J. (2001). Spatial patterns of diverse macrofaunal assemblages in coralline turf and their associations with environmental variables. Journal of the Marine Biological Association of the United Kingdom, 81: 917e930.
- Kim, S.-C., McGowen, M.R., Lubinsky, P., Barber, J.C., Mort, M.E. & Santos-Guerra, A. (2008). Timing and tempo of early and successive adaptive radiations in Macaronesia. PLoS ONE, 3: e2139.
- Littler, M.M. (1980). Overview of the rocky intertidal systems of southern California. In The California Islands: Proceedings of a Multidisciplinary Symposium (Power, D.M., editor), 265–306. Santa Barbara Museum of Natural History, Santa Barbara.
- Machín-Sánchez, M., Gil-Rodríguez, M.C. & Haroun, R. (2018). Phylogeography of the red algal Laurencia complex in the Macaronesia region and nearby coastal areas: recent advances and future perspectives. Diversity, 10: 10.
- Maggs, C.A. & Hommersand, M.H. (1993). Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3A. Ceramiales. HMSO, London.
- Mei, J. & Schiel, D.R. (2007). Survival strategies in Polysiphonia adamsiae and P. strictissima (Rhodophyta, Rhodomelaceae) subjected to sediment deposition and grazing pressure. New Zealand Journal of Marine and Freshwater Research, 41: 325–334.
- Neto, A.I. (1994). Checklist of the benthic marine macroalgae of the Azores. Arquipélago, 12A: 15–34.
- O'Brien, J.M. & Scheibling, R.E. (2018). Turf wars: competition between foundation and turf-forming species on temperate and tropical reefs and its role in regime shifts. Marine Ecology Progress Series, 590: 1–17.
- Pardo, C., Lopez, L., Peña, V., Hernández-Kantún, J., Le Gall, L., Bárbara, I. & Barreiro, R. (2014). A multilocus species delimitation reveals a striking number of species of coralline algae forming maerl in the OSPAR maritime area. PLoS ONE, 9: e104073.
- Pasella, M.M., Verbruggen, H., Nelson, W.A. & Díaz-Tapia, P. (2019) The phylogenetic position of the morphologically unusual Pleurostichidium falkenbergii (Rhodomelaceae, Rhodophyta) based on plastid phylogenomics. Phycologia, 58: 319–325.
- Phillips, L.E. (2000). Taxonomy of the New Zealand-endemic genus Pleurostichidium (Rhodomelaceae, Rhodophyta). Journal of Phycology, 36: 773–786.
- Piñeiro-Corbeira, C., Maggs, C.A., Rindi, F., Bunker, F., Baldock, L. & Díaz-Tapia, P. (2020a). Molecular assessment of the tribes Streblocladieae and Polysiphonieae (Rhodomelaceae, Rhodophyta) in the British Isles reveals new records and species that require taxonomic revision. Cryptogamie, Algologie, 41: 55–72.
- Piñeiro-Corbeira, C., Verbruggen, H. & Díaz-Tapia, P. (2020b). Molecular survey of the red algal family Rhodomelaceae (Ceramiales, Rhodophyta) in Australia reveals new introduced species. Journal of Applied Phycology, 32: 2535–2547.
- Price, I.R. & Scott, F.J. (1992). The turf algal flora of the Great Barrier Reef. Part I. Rhodophyta. Botany Department, James Cook University, Townsville.
- Rojas-González, B. (1997). Estudio de las especies de la familia Rhodomelaceae (Rhodophyta), con exclusión de las tribus Chondrieae y Laurencieae, en las Islas Canarias. Universidad de La Laguna, La Laguna.
- Sansón, M. (1991). Estudio de las especies de la familia Ceramiaceae (Rhodophyta) en las Islas Canarias. Universidad de La Laguna, La Laguna.
- Sartoni, G. (1992). Stichothamnion cymatophilum (Rhodomelaceae, Rhodophyta) a new record for Mediterranean algal flora. Cryptogamie, Algologie, 13: 39–43.
- Saunders, G.W. & Lehmkuhl, K.V. (2005). Molecular divergence and morphological diversity among four cryptic species of Plocamium (Plocamiales, Florideophyceae) in northern Europe. European Journal of Phycology, 40: 293–312.
- Savoie, A.M. & Saunders, G.W. (2016). A molecular phylogenetic and DNA barcode assessment of the tribe Pterosiphonieae (Ceramiales, Rhodophyta) emphasizing the Northeast Pacific. Botany, 94: 917–939.
- Savoie, A.M. & Saunders, G.W. (2019). A molecular assessment of species diversity and generic boundaries in the red algal tribes Polysiphonieae and Streblocladieae (Rhodomelaceae, Rhodophyta) in Canada. European Journal of Phycology, 54: 1–25.
- Schmidt, O.C. (1929). Beiträge zur Kenntnis der Meeresalgen der Azoren. II. Hedwigia, 69: 165–72.
- Schneider, C.W., Quach, P.K. & Lane, C.E. (2017). A case for true morphological crypsis: Pacific Dasya anastomosans and Atlantic D. cryptica sp. nov. (Dasyaceae, Rhodophyta). Phycologia, 56: 359–368.
- Soares, L.P., Guimarães S.M.P. de B., Toyota Fujii, M., Yoneshigue-Valentin, Y., Sousa Batista, M.G. & Yokoya, N.S. (2020). Rhodachlya westii sp. nov. (Rhodachlyales, Rhodophyta), a new species from Brazil, revealed by an integrative taxonomic approach. Phycologia, 59: 346–354.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Stegenga, H., Bolton, J.J. & Anderson, R.J. (1997). Seaweeds of the South African West Coast. Bolus Herbarium, University of Cape Town, Cape Town.
- Stewart, J.G. (1982). Anchor species and epiphytes in intertidal algal turf. Pacific Science, 36: 45−59.
- Stuercke, B. & Freshwater, D.W. (2008). Consistency of morphological characters used to delimit Polysiphonia sensu lato species (Ceramiales, Florideophyceae): analyses of North Carolina, USA specimens. Phycologia, 47: 541–559.
- Tuya, F. & Haroun, R. (2006). Spatial patterns and response to wave exposure of shallow water algal assemblages across the Canarian Archipelago: a multi-scaled approach. Marine Ecology Progress Series, 311: 15–28.
- Vroman, M. (1967). A new species of Stichothamnion (Rhodophyta) from the West Indies. Acta Botanica Neerlandica, 15: 557–561.
- Walker, R.H., Brodie, J., Russell, S., Irvine, L.M. & Orfanidis, S. (2009). Biodiversity of coralline algae in the northeastern Atlantic including Corallina caespitosa sp. nov. (Corallinoideae, Rhodophyta). Journal of Phycology, 45: 287–297.
- Wallenstein, F.M., Terra, M.R., Pombo, J. & Neto, A.I. (2009). Macroalgal turfs in the Azores. Marine Ecology, 30: 113–117.
- Weber-van Bosse, A. (1911). Notice sur quelques genres nouveaux d’algues de l’Archipel Malaisien. Annales du Jardin Botanique de Buitenzorg, 24: 25–33.
- Weber-van Bosse, A. (1913). Marine algae. Rhodophyceae, of the “Sealark” Expedition, collected by Mr. J. Stanley Gardiner, M.A. Transactions of the Linnean Society of London, Second Series, Botany, 8: 105–142.
- Zuccarello, G.C. & West, J.A. (2003). Multiple cryptic species: molecular diversity and reproductive isolation in the Bostrychia radicans / B. moritziana complex (Rhodomelaceae, Rhodophyta) with focus on North American isolates. Journal of Phycology, 39: 948–959.
