ABSTRACT
Analysis of trait trade-offs, through which physiological traits requiring common resources are ‘traded’ to optimize competitive advantage, provides a route to simplify and more readily understand the complexities of ecology. The concept of trait trade-offs has found favour in plankton research, especially directed at phytoplankton, defined here as phototrophs incapable of phagotrophy. Mixoplankton, defined as protists that combine phototrophy and phagotrophy, are now recognized as being widespread and significant members of the protist plankton community; many photoflagellate ‘phytoplankton’ are actually mixoplankton, as are many ‘(microbial) zooplankton’. Mixoplankton might be expected to be dominant, being able to exploit different trophic strategies while simultaneously eliminating competitors. That mixoplankton are not dominant suggests that physiological trait trade-offs erode their apparent competitive edge. We present a systematic analysis of potential trait trade-offs in phototrophic protists focused on mixoplankton. We find no clear evidence to support trait trade-off arguments in plankton research, except perhaps for acquired phototrophy in mixoplanktonic ciliates versus zooplanktonic ciliates. Our findings suggest that the presence of various mixoplankton throughout the surface ocean waters is most likely explained by factors other than trait trade-offs. Diversities in mixoplankton form and function thus reflect that evolution of these organisms from very different lineages, provide them with advantages to function competitively in mature ecosystems with complex trophic interplay. Indeed, the complexity of those lineages is inconsistent with core trait trade-off definitions; there is no single ancestral mixoplankton nor a common environment supporting trait-trade-off-directed evolution.
Highlights
Trait trade-offs do not explain the breadth of mixoplankton ecophysiological capabilities.
Diversity of mixoplankton form and function reflects phylogenetic diversity.
Only one potential trait trade-off was identified which was for ciliates that steal chloroplasts.
Introduction
The concept of trait trade-offs in biology probably emerged from Charles Darwin’s theory of variation (Garland, Citation2014) and has traditionally focused on terrestrial plants or animals for which trade-offs are defined as ‘costs paid in the currency of fitness when a beneficial change in one trait [within a given organism] is linked to a detrimental change in another’ (Stearns, Citation1989). Identification of trait trade-offs is typically supported by an empirical analysis of the co-occurrence of physiological traits. Negative relationships are looked for that may signal mutual exclusivity between those traits which require common resources; these traits are argued to have been ‘traded’ to optimize competitive advantage in a given environmental setting. The environment in this context applies to common spatial and temporal settings subjected to constant change (Snell‐Rood et al., Citation2015). Accordingly, trait trade-off analyses should only be made in reference to organisms from the same ecological setting (Litchman & Klausmeier, Citation2008), and between organisms with sufficient similarity in evolutionary lineage that trade-offs could provide a plausible mechanism (Garland, Citation2014).
The trait trade-off concept has proven to be a rich research strand in plankton research (e.g. Dolan & Pérez, Citation2000; Finkel et al., Citation2010; Kiørboe et al., Citation2018; Serra-Pompei et al., Citation2020; Litchman et al., Citation2021). Assumptions from such analyses have then been employed to inform configuration of global plankton models to predict oceanic carbon fixation (e.g. Ward & Follows, Citation2016). These efforts have typically been directed at phytoplankton (e.g. Litchman & Klausmeier, Citation2008; Finkel et al., Citation2010), with less emphasis on zooplankton (e.g. Kiørboe, Citation2011). This emphasis on phytoplankton aligns with the dichotomy of plankton between phototrophic phytoplankton and heterotrophic zooplankton that forms the bedrock of traditional marine ecology and biological oceanography.
The last decade has seen a growing appreciation that this perceived plant-animal dichotomy within the plankton community is at least overly simplistic, if not flawed (Flynn et al., Citation2013; Stoecker et al., Citation2017; Glibert & Mitra, Citation2022). It transpires that the marine protistan plankton community is not dominated by just ‘plant-like’ phytoplankton and ‘animal-like’ zooplankton but also includes organisms that engage in both phototrophy and phagotrophy (Mitra et al., Citation2016). These organisms have been termed ‘mixoplankton’ (Flynn et al., Citation2019; ), and their members include many organisms referred to, or ecologically considered, as ‘microalgae’, such as phototrophic members of the dinoflagellate genus Dinophysis, and the ciliate genus Myrionecta/Mesodinium. Indeed, various protist species traditionally labelled as ‘phytoplankton’ or ‘zooplankton’ are actually mixoplankton (Leles et al., Citation2017, Citation2019), including such iconic ‘phytoplankton’ as Tripos furca (Smalley & Coats, Citation2002), Emiliania huxleyii (Avrahami & Frada, Citation2020) and Phaeocystis globosa (Koppelle et al., Citation2022).
Fig. 1. Schematic of protist plankton functional types. Shown here are schematics for protozoan zooplankton (with no phototrophy), the generalist, plastidic-specialist and endosymbiotic-specialist non-constitutive mixoplankton (GNCM, pSNCM, eSNCM, respectively; note their acquired phototrophy), constitutive mixoplankton (CM), non-diatom and diatom protist phytoplankton (with no phagotrophy). The schematic for the eSNCM (such as the Rhizaria) shows the interplay between the phytoplankton-like symbionts (of which there may be hundreds or thousands of cells) growing within the zooplankton-like host cell. All protist types can use dissolved organic matter (DOM); phytoplankton (including diatoms) are thus mixotrophs by combining phototrophy with osmotrophy. Schematics are not to scale; eSNCM can be as large as mm to cm in cell size while all the other functional groups are typically in the size range of c. 3–200 µm (Mitra et al., Citation2023).
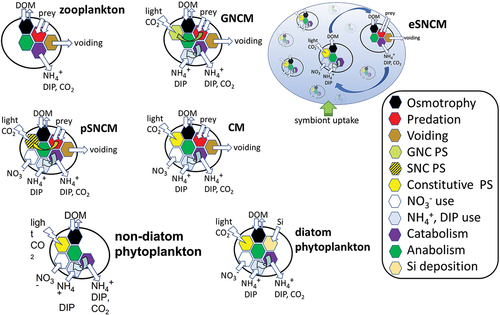
The term mixoplankton was coined specifically to delineate phagotrophic-microalgae from the other microalgae, namely phytoplankton, which cannot eat but are nonetheless mixotrophic, by virtue of the well-known coupling of phototrophy with osmotrophy (an ancestral feeding mode – Bremer et al., Citation2022 – exploiting dissolved organic resources; e.g. Lewin & Hellebust, Citation1970; Antia et al., Citation1981; Flynn & Butler, Citation1986; Burkholder et al., Citation2008; Meyer et al., Citation2022). This capability of microalgae for photo-osmo-mixotrophy has been exploited in biotechnology to boost algal production at high organic substrate levels under light-limitation (e.g. Cupo et al., Citation2021). Following the emergence of the mixoplankton paradigm, here we undertake an assessment of trait trade-offs that may have been employed by the different protist plankton functional groups – phytoplankton, mixoplankton and zooplankton – to succeed in a given environment.
Mixoplankton functional types and physiologies
Collectively, mixoplankton have a global significance (Leles et al., Citation2017, Citation2019; Faure et al., Citation2019) and contribute in various ways to ecosystem functioning (Mitra et al., Citation2014; Leles et al., Citation2021; Li et al., Citation2022). Mixoplankton include representatives across a diverse range of protists and a schematic showing core trait differences between different protist plankton functional groups is given in . Mixoplankton can be divided broadly into two groups based on (i) whether they possess an innate, constitutive ability to perform photosynthesis (constitutive mixoplankton; CM), or (ii) whether they acquire phototrophy from their prey or from symbionts (non-constitutive mixoplankton; NCM). The NCM can be further divided into (a) generalist non-constitutive mixoplankton (GNCM, e.g. Strombidium rassoulzadegani, Laboea strobila) that acquire phototrophy from a range of different prey items, (b) plastidic specialist NCM (pSNCM, e.g. Mesodinium rubrum, Dinophysis acuta) that can acquire phototrophy only from specific prey taxonomic groups. and (c) endosymbiotic specialist NCM (eSNCM, e.g. green Noctiluca scintillans, Globigerinoides sacculifer) that maintain prey symbionts for acquired phototrophy (Mitra et al., Citation2016; Flynn et al., Citation2019).
While CM appear simply as ‘phytoplankton that eat’, and NCM as ‘(microbial) zooplankton that photosynthesize’, the contributions of photosynthesis and eating for growth are very variable within members of both groups (Caron, Citation2000; Stoecker et al., Citation2009, Citation2017; Jeong et al., Citation2010; Gomes et al., Citation2018; Wilken et al., Citation2020). Furthermore, while photosynthesis is inevitably associated with provision of carbon (C) and energy, eating may additionally or perhaps primarily be associated with the acquisition of nitrogen (N), phosphorus (P) or other nutrients. Further, feeding in mixoplankton may not necessarily align with strict interpretations of ‘phagotrophy’ which would require a significant size difference between consumer and the engulfed prey. Rather, feeding may involve, after initial capture, engulfment (Tillmann, Citation1998; Jeong et al., Citation2005), semi-extracellular phagocytosis (Kamennaya et al., Citation2018), use of a peduncle (akin to a feeding straw inserted into the prey to suck out material; Larsen, Citation1988; Nagai et al., Citation2008) or the use of mucus nets to entrap potential prey (e.g. Blossom et al., Citation2017; Larsson et al., Citation2022). Alternatively, mixoplankton may release toxins that lyse the prey (and, also potentially other non-prey organisms), releasing particulate and dissolved organics which can then be consumed through a combination of phagotrophy and osmotrophy (Tillmann, Citation2003; Granéli et al., Citation2012).
Trait trade-offs in context
The origins of protist plankton saw repeated cycles of gains and losses of functionality traits (de Castro et al., Citation2009; Keeling et al., Citation2014; Bremer et al., Citation2022; ). Many organisms appearing to be closely related are actually products of different evolutionary paths played out in different ecological settings at different times (Mansour & Anestis, Citation2021; Bremer et al., Citation2022). Put simply, extant protist microalgae did not live and compete together such that evolution could select for different traits by ‘trading options’ against each other. While it may be tempting to consider the oceans as one environment, for microbes that is certainly not so (Zehr et al., Citation2017) and there are many additional drivers that select for competitive advantage other than resource demand and allocation (notably resilience against disease and predation). Application of trait trade-offs for plankton are, therefore, problematic (Flynn et al., Citation2015). Even ignoring the ‘same-environment’ and the ‘evolution from same lineage’ caveats for trait trade-offs (cf. Litchman & Klausmeier, Citation2008; Garland, Citation2014), there is also the question of significance in resource costs to which the trade-offs may be applied.
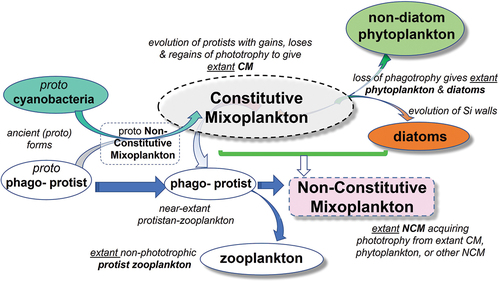
Fig. 2. An interpretation of the evolution of protist functional types. Multiple gains and losses of phototrophy have occurred within what we now term constitutive mixoplankton. Some of these have resulted in extant zooplankton and extant phytoplankton groups. See for schematics of the physiological traits of these organism types.
A trait trade-off must be associated with a significant saving in energy and/or material resources. The question arises as to how can significance be judged. One of the most expensive biochemical processes for phototrophic plankton is the assimilation of N supplied as nitrate versus that as ammonium. Using nitrate costs c. 20% more than when using ammonium in total photo-reductant production, with additional significant costs in iron (Fe) allocation (Flynn & Hipkin, Citation1999). Despite this very significant overhead cost, phototrophic plankton grown on nitrate typically grow at rates similar to those using ammonium (e.g. Thompson et al., Citation1989; Wood & Flynn, Citation1995). This is perhaps a salutary note of warning with respect to the level of cost differential needed to mark a putative trait trade-off as being of significance. We must also be careful not to confuse changes in trait expression within a species, in response to changes in environmental conditions (e.g. Blossom & Hansen, Citation2021), as evidence of metabolic trait trade-offs rather than as simply the consequence of a series of (de)repression feedback processes (Flynn et al., Citation2015).
Evaluation of trait trade-offs within protist plankton
A key driver for a need to reappraise plankton trait trade-offs, in consequence of the mixoplankton paradigm (Glibert & Mitra, Citation2022), is the formulation of plankton functional type models. Describing a modelled plankton as mixotrophic just by combining phototrophic and heterotrophic features could produce an all-conquering configuration (e.g. Thingstad et al., Citation1996; Hammer & Pitchford, Citation2005; Troost et al., Citation2005). To prevent mixoplankton from always dominating, modellers have applied assumed trait trade-offs, such that the phototrophic and phagotrophic activities in the mixoplankton are configured as individually less competitive than those exhibited by the ‘pure’ phytoplankton or the ‘pure’ zooplankton (e.g. Ward et al., Citation2011). The motivation for this current work grew from investigating and questioning approaches where trait trade-offs for photo-phagotrophs have been configured and implemented for applications from theoretical biology through to considering global plankton productivity studies (e.g. Ward et al., Citation2011; Andersen et al., Citation2016; Ward & Follows, Citation2016; Cadier et al., Citation2020). Here, we expand on such suggestions, to present an extensive critique of possible trait trade-offs that could affect competitiveness of mixoplankton versus their non-phagotrophic phytoplankton and non-phototrophic zooplankton counterparts.
Phagotrophic protists were the ancestral form from whence all protists evolved, while phytoplankton evolved from mixoplanktonic lineages (; Raven, Citation1997; Raven et al., Citation2009; Ponce-Toledo et al., Citation2017; Sánchez-Baracaldo et al., Citation2017; Bremer et al., Citation2022; Mitra et al., Citation2023). Thus, we first examine the different traits and potential trait trade-offs in mixoplankton versus zooplankton (), and then in mixoplankton versus phytoplankton (). As there are several fundamentally different mixoplankton functional groups, each of which contain organisms of very different evolutionary lineages (Mansour & Anestis, Citation2021), we have also undertaken an evaluation of advantages and disadvantages of traits within the mixoplankton themselves (). Following from Flynn et al. (Citation2019), we reserve the term ‘phytoplankton’ specifically for phototrophic protists that cannot feed, ‘zooplankton’ for protists that have no ability for phototrophy, and ‘mixoplankton’ for protists that engage in photosynthesis and phagotrophy (). As all phytoplankton are assumed mixotrophs by virtue of their capability for photo-osmo-mixotrophy (Flynn et al., Citation2019), we explore putative trait trade-offs in mixoplankton and not trait trade-offs for mixotrophy in phytoplankton (e.g. Litchman & Klausmeier, Citation2008) or zooplankton (e.g. Litchman et al., Citation2013).
Table 1. Trait trade-off hypotheses for mixoplankton compared with protist zooplankton. SDA, specific dynamic action; GNCM, generalist non-constitutive mixoplankton; SNCM, specialist non-constitutive mixoplankton.
Table 2. Trait trade-off hypotheses for mixoplankton compared with protist phytoplankton. SA, surface area; SDA, specific dynamic action.
Table 3. Mixoplankton traits that could be considered as providing competitive advantages over protist zooplankton and/or phytoplankton, and possible trait trade-offs. SDA, specific dynamic action.
Mixoplankton versus protist zooplankton
Allometry
Hypotheses H1.1 and H1.2 in consider trait trade-offs due to perceived conflicts when housing two contrasting nutritional strategies within the mixoplankton cell (). There is no evidence of competition at the cell surface for nutrient uptake required for phototrophy versus that required for phagotrophy to underpin a trait trade-off between nutrient uptake to support phototrophy and prey ingestion (H1.1; ; Li et al., Citation1999; Hausmann, Citation2002; Gavelis et al., Citation2017). Analysis of experimental data did not provide any clear evidence of a relationship between surface area or cell size with growth rate potential amongst mixoplanktonic versus zooplanktonic dinoflagellates (). Cell size variations of the scale in question, to accommodate both feeding vacuoles and chloroplasts, are common features of protist plankton (Flynn et al., Citation1996; Li et al., Citation1999; Lee et al., Citation2014). The trait trade-off concept of space sharing (H1.2; ) also does not take into account that mixoplankton do not necessarily ingest whole prey items (Tillmann, Citation2003; Park et al., Citation2006); rather they can use a peduncle (feeding tube), a mucus trap, etc. and therefore, do not need to allocate significant cell volume to digestive vacuoles. We thus find no evidence to support the hypotheses H1.1 or H1.2, that mixoplankton are compromised relative to zooplankton due to their need for more space to maintain two trophic modalities.
Fig. 3. Example putative trait trade-offs for mixoplankton versus zooplankton and phytoplankton. The diagram shows different protist cell configurations with cell-surface allocations to nutrient transport (solid line) or ingestion (gaps), and resource allocation within the cell for prey digestion (pink) or photosynthesis (green). a, zooplankton cell; b & d, mixoplankton cell; c, phytoplankton cell. b, shows presumptive trait trade-offs for mixoplankton due to the need to house two nutritional pathways. d, portrays the reality where ingestion occurs over a very small proportion of the cell surface, and cell volume and physiology are not constrained by space; it also shows the synergism between the phagotrophic and photosynthetic processes (yellow arrows). See also .
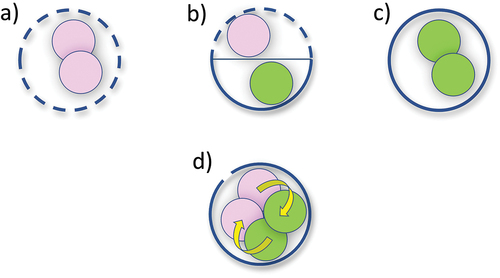
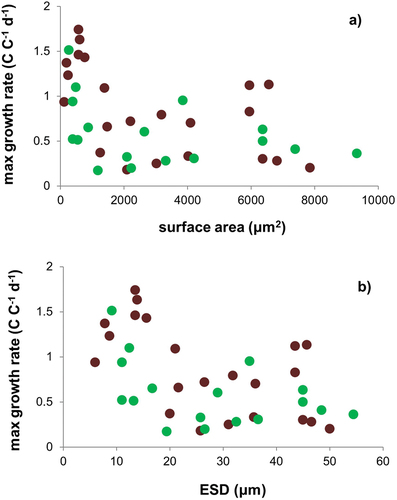
Fig. 4. Comparisons between maximum growth rates for mixoplanktonic dinoflagellates (green) and zooplanktonic dinoflagellates (brown) for different protist cell surface areas (a) and equivalent spherical diameter (ESD, b). Source data from Jeong et al. (Citation2010).
Physiology
Hypotheses H1.3 and H1.4 () consider potential conflicts in resource allocation and in light dependency, respectively. The primary drivers for mixoplankton evolution from zooplankton are likely to have been:
a mechanism provided by phototrophy as a back-up supply of C and energy, and
the retention of nutrients through phototrophy that would otherwise be lost during digestion and assimilation. This can comprise as much as 30% of prey being assimilated into new biomass through specific dynamic action (SDA; McCue, Citation2006).
Aspect (ii) alone would cover the nutrient resource demands of phototrophy, dismissing H1.3; in reality, additional nutrients would likely be taken up as well. There is an additional line of evidence indicating that resources for phototrophy are not restrictive. Both GNCM and SNCM may void, rather than digest, failing chloroplasts (Stoecker & Silver, Citation1990; Schoener & McManus, Citation2012; Kim et al., Citation2016), although there are examples of SNCM digesting sequestered chloroplasts (e.g. Elphidium crispum, Lee et al., Citation1988) similar to events observed in zooplankton (e.g. Oxyrrhis marina, Öpik & Flynn, Citation1989). We must assume that the net gain over the period of operating the acquired chloroplasts, and then voiding defunct plastids, exceeds the nutritional gain in simply directly digesting the chloroplasts else the trait of non-constitutive mixoplanktonic activity would not have survived in evolution.
Both the above-mentioned drivers require light, and this could be seen as a potential trait trade-off for those species that have an obligatory requirement for photosynthate (H1.4). However, while there are indeed examples where predation is coupled with phototrophy in mixoplankton (Adolf et al., Citation2003; Stoecker et al., Citation2017), there are examples where that coupling is not strong, or indeed where mixoplankton growth may continue in darkness (Caron et al., Citation1990; Hansen et al., Citation2000: McManus et al., Citation2012, Citation2018; Rottberger et al., Citation2013; McKie-Krisberg et al., Citation2015; Millette et al., Citation2017). Thus, there is no overwhelming evidence to support the absolute need for light as a generic trait trade-off (H1.4) for mixoplankton versus zooplankton.
Hypothesis H1.5 suggests a trait trade-off restricting the success of NCM to situations where their prey from whence they acquire phototrophy are available. It could be argued that the non-constitutive mixoplankton (NCM; e.g. plastidic ciliates, HAB forming Dinophysis sp., bloom forming green Noctiluca scintillans) which depend on coupled photo-phago-trophy for their nutritional needs and thence have to acquire their phototrophic potential from prey organisms are at a disadvantage compared with zooplankton (H1.5). The plausibility of such a trait trade-off especially between GNCM ciliates and their zooplanktonic counterparts () is raised not least because these organisms can inhabit the same environment, thus meeting the critical criterion for considerations of trait trade-offs (Litchman & Klausmeier, Citation2008), but also because, as ciliates, they share similar evolutionary lineages (Mansour & Anestis, Citation2021). As many as 50% of ciliates in the euphotic zone may be GNCM (Stoecker et al., Citation2017) and their presence could be attributed to their ability to negate SDA loss through phototrophy compared with their zooplankton counterparts (Anschütz & Flynn, Citation2020). It is, however, worth noting that a trait trade-off relating consumer success to the presence of appropriate food applies to all consumers, and not just to NCM.
It could also be argued that a physiological conflict may occur between phototrophy and digestion (H1.6), leading to down-regulations of these individual processes resulting in a decrease in growth rate. However, that would only be a real conflict if the physiologies were viewed as combative rather than providing synergy in support of growth; it is difficult to see why such a conflicting trait combination would be to competitive advantage under any circumstance. The data of Jeong et al. (Citation2010) indicate that mixoplanktonic dinoflagellates grow at rates similar to their zooplanktonic counterparts ().
Ecology
Hypothesis H1.7 () presents an argument that leakage of organics from phototrophic processes of a mixoplankton would attract predators employing chemo-receptors (Verity, Citation1991); a photo-pigmented mixoplankton could also be more obvious to visual predators. These two factors could lead to a greater level of loss of mixoplankton to predation as a trait trade-off. However, a zooplankton containing phytoplankton prey would also be pigmented, and they also leak organics (Flynn & Davidson, Citation1993) which would leave a scent trail for other protist grazers (Spero, Citation1985; Martel, Citation2006).
In total, while we see some support for H1.5, applied for ciliate zooplankton versus ciliate GNCM, there are no generic grounds to support trait trade-offs between zooplankton and mixoplankton.
Mixoplankton versus phytoplankton
For a comparison of mixoplankton versus phytoplankton, we turn to the argument that the demands for the support of phagotrophy may compromise the demands for phototrophy (). Many of the hypotheses have parallels with those in , though they are now viewed from a different perspective, i.e. addition of phagotrophy to a phototroph, rather than phototrophy added to a phagotroph, noting that while the latter occurred through evolution, the former did not.
Allometry
As in (H1.1, H1.2), the hypotheses here (, H2.1–H2.4) are based on assumptions of trait trade-offs due to sharing of cell surface area and volume between phototrophy and phagotrophy (). Housing two sets of trophic machinery may be expected to demand a larger cell size which, by biophysical arguments (Andersen et al., Citation2016), may suggest that mixoplankton fulfil an intermediate point on the allometric scale between smaller phytoplankton and larger zooplankton (H2.1). In reality, the smallest protist plankton size class spectrum includes mixoplankton, as well as similar sized phytoplankton (Finkel et al., Citation2010; Unrein et al., Citation2014; Flynn et al., Citation2019; Leles et al., Citation2019; Visintini et al., Citation2021). As there is evidence that viruses can be ingested by protist plankton (González & Suttle, Citation1993), it is possible that even the very smallest phototrophic protist could eat. There are thus no grounds to support H2.1.
Larger cells have thicker boundary layers and if mixoplankton were larger as a consequence of containing food vacuoles then this could be argued as deleterious for nutrient uptake (H2.2). However, mixoplankton would be acquiring nutrients from other sources (their prey), and this may be expected to mitigate against any shortfall in nutrient uptake (Tittel et al., Citation2003), including from thickening of the boundary layer. In addition, any increase in cell size due to the presence of food vacuoles would also increase the effective ratio of surface area (SA) to growing cell biomass (SA:biomass; i.e. ignoring digestive vacuoles containing ingested prey). It is this SA:biomass ratio, and not the SA:volume ratio, which is important in this regard as seen in competitive advantages shown by larger and more vacuolated diatom species against smaller diatom species (Flynn et al., Citation2018). Net leakage of metabolites (loss exceeding recovery) per cell may also be expected to be greater from larger cells (Flynn & Berry, Citation1999), though there is no simple size-relationship for the leakage or uptake of dissolved free amino acids (Flynn, Citation1990). However, even if the net leakage from mixoplankton was greater compared with similar-sized phytoplankton, such a loss of organics could well be advantageous for mixoplankton as these would attract microbial ‘prey’ (Martel, Citation2006; Wilken et al., Citation2014; Smriga et al., Citation2016). Through feeding on bacteria and cyanobacteria (e.g. Yoo et al., Citation2017), which are well adapted to acquire N, P and Fe from extremely low concentrations and from recalcitrant forms (Zehr et al., Citation2017), mixoplankton can access nutrients that are limiting for the growth of other planktonic primary producers (Zubkov & Tarran, Citation2008; Hartmann et al., Citation2012; Mitra & Flynn, Citation2023).
If prey ingestion required a significant part of the surface area of the mixoplankton cell, then the absence of that area for locating nutrient transporters could be expected to decrease scope for nutrient uptake (H2.3, ; Ward et al., Citation2011). We have already considered this above (, H1.1); processes associated with feeding do not occupy much surface area (c. <5%, ). On the plus side, a feeding mixoplankton will acquire nutrients (N, P, Fe, etc.) from its prey, with further saving of energy through the internal production of ammonium during prey digestion negating the need and use of nitrate (Anschütz & Flynn, Citation2020) with its allied costs in terms of Fe and reductant (see also section on ‘Trait Trade-Offs in context’, above). We have also considered whether the presence of food vacuoles could be argued to compete with space for chloroplasts within the mixoplankton cell (H2.4, cf. , H1.2); there is no evidence to support this hypothesis as protist cell volume for a given species is highly variable in response to nutrient status (Flynn et al., Citation1996; John & Flynn, Citation2002) as well as during the halving and doubling of cell volume over the cell cycle (e.g. for the mixoplankton Chattonella, Demura et al., Citation2009).
Physiology
Photoacclimation is required to maximize productivity without risking photodamage (Richardson et al., Citation1983). The situation is complicated by the high variability of light over the day which generates a trait trade-off, especially for those phototrophs growing in high-light summer waters, i.e. too little Chl:C and the cell is outcompeted in low light, while too much Chl:C with too much light causes photodamage. This modulation is more problematic if nutrient supply fluctuates and then becomes sub-optimal as this restricts the D1 repair cycle (Li et al., Citation2021). From hypothesis H2.4, H2.5 suggests a trait trade-off with the phototrophic potential of mixoplankton, as reflected by their low Chl:C in comparison with diatoms (Leles et al., Citation2021). Aside from the rejection of H2.4 which is required for H2.5, a low Chl:C itself does not evidence a trait trade-off. Mixoplankton can obtain C and energy from sources other than phototrophy, often growing in environments with high surface light in which they can migrate to optimize light incident on the cell surface. In consequence they do not require a high photopigment content (i.e. large chloroplast content). Mixoplankton thus have lowered risks of producing damaging oxidizing radicals. In mono-species blooms, a low Chl:C is advantageous to the collective (Flynn & Hansen, Citation2013). Phototrophic energetic costs for N assimilation are also lowered in mixoplankton through the previously noted decreased need for nitrate enabled by directly assimilating reduced prey-N (amino acids, nucleic acids), and through the internal recycling of ammonium released during anabolic prey assimilation. Mixoplankton exploiting those N-sources will save the 20% extra photoreductant cost for nitrate assimilation (Flynn & Hipkin, Citation1999) and, all else being equal, could have pro rata decreased Chl:C ratio in comparison to phytoplankton using nitrate-N. In contrast, diatoms in high light conditions with high Chl:C may need to vent excess photoreductant by superfluous nitrate reduction (Glibert et al., Citation2016), an opportunity unavailable in low-nitrate waters.
To add to the structural demands required with phototrophy, mixoplankton also have to resource the means to kill prey (e.g. with toxins), capture, ingest and then digest them. This could be argued as a trait trade-off for resource allocation and in physiology (, H2.6). The main expenses for capture and ingestion are for energy and especially for C. Phytoplankton release a large proportion of C-fixation as dissolved organic carbon (c. 10%; Biddanda & Benner, Citation1997; Wetz & Wheeler, Citation2007; Flynn et al., Citation2008) as do mixoplankton (Aaronson et al., Citation1971) and heterotrophic protists (Pelegri et al., Citation1999; Strom et al., Citation2003). Such releases are indicative of over-production (phototrophy), voiding (incomplete digestion with phagotrophy) and/or a lack of demand to recover losses. There is no specific reason to suspect that a re-direction of C and energy towards the synthesis of prey capture and processing apparatus should present a physiological challenge (Tillmann, Citation2003; Lee et al., Citation2014; Larsson et al., Citation2022) such that it would comprise a trait trade-off in mixoplankton. In addition, the time scales for synthesis and dissolution (recycling) of membranes for prey capture is in the scale of tens of minutes (Li et al., Citation1999). All protist plankton auto-digest and recycle cell components; the physiological machinery exists in them all. The (re)direction of resources for toxin production in protist plankton appears to be minor (John & Flynn, Citation2002), and there is no evidence that the toxin production represents a trade-off (Pančić & Kiørboe, Citation2018). In addition, phototrophy in mixoplankton supports additional routes for production of secondary metabolites providing offensive, defensive or allelopathic capabilities (Granéli & Flynn, Citation2006).
Physiological conflicts could be envisaged through resources flowing from phototrophy and phagotrophy that lead to down-regulations of these processes (H2.7; cf. , H1.6), resulting in a decrease in growth rate. The coupling of phototrophy and phagotrophy in the light phase of the diel cycle, although not ubiquitous (Caron et al., Citation1990; Rottberger et al., Citation2013; McKie-Krisberg et al., Citation2015; cf. , H1.4), may be seen as particularly likely to promote conflicts. However, in reality, these processes are more likely complementary as they use waste products produced by each other; most obviously the nutrients lost with phagotrophy-related SDA are recycled with phototrophy, and excess organics from phototrophy also counters other catabolic and anabolic demands (including those associated with motility, prey capture and digestion). Where it could possibly be argued for a trait trade-off is that the growth rate of the mixoplankton is not a sum of phagotrophy+phototrophy but is capped at the whole-cell level; this may give the impression that the individual processes are incapable of functioning at the rates seen in the zooplankton and phytoplankton comparators and hence for trait trade-off between these different organism types. This is addressed further below.
Ecology
Ecological-facing trait trade-offs of being a mixoplankton rather than a phytoplankton include the size of the cell (, H2.8) and motility required to enhance predation (H2.9), and therefore the possibility of increased encounter rate with their own predators. We have already shown that there is no evidence for the former (i.e. H2.8; cf. H2.1, H2.2). Motility is common across protist plankton including phytoplankton, either self-propelled or through a combination of buoyancy and turbulence; all of these increase encounter rates. For example, non-phagotrophic phytoplankton flagellates are self-propelled, and the turbulence required to maintain phytoplanktonic diatoms in suspension (Raven & Beardall, Citation2022) equally brings them into contact with predators. Further, diatoms Ethmodiscus and Rhizosolenium exhibit vertical migration within a cell division cycle by changing their cell density relative to seawater, thus enhancing nutrient acquisition at the nutricline and photon acquisition nearer the surface (Kemp & Villareal, Citation2013, Citation2018). Large eSNCM Rhizaria (Acantharia, Polystine Radiolaria) do not swim (they float) and capture motile and non-motile prey in webs of pseudopodia (Caron & Swanberg, Citation1990; Anderson, Citation1993; Caron, Citation2016). Rapid jumping motions in ciliates, including mixoplanktonic species, helps them to escape predation (Jonsson & Tiselius, Citation1990; Jiang & Johnson, Citation2017; Jiang et al., Citation2018).
In summary, there is little if any evidence to support generic trait trade-off arguments for mixoplankton versus phytoplankton (). For most putative aspects, much of the biochemical machinery is common between mixoplankton and phytoplankton. In mixoplankton, phototrophy enables the retention of nutrients that are otherwise lost during phagotrophy. While there is a possible trade-off if a potential synchronized linkage is considered between photosynthesis and phagotrophy (as that may restrict feeding to the light phase; H2.7), there are also sufficient exceptions to detract from this being a trait trade-off rule (Caron et al., Citation1990; Rottberger et al., Citation2013; McKie-Krisberg et al., Citation2015).
Potential advantages and trait trade-offs in mixoplankton physiology
We now review mixoplankton traits that could be considered to be of advantage for these organisms compared with the phytoplankton or zooplankton ().
Zooplankton, and indeed all consumers, inevitably lose a significant proportion of assimilated resources through biochemical conversions and the synthesis of their own biomass. This loss associated with anabolic respiration, as specific dynamic action (SDA), can cost a consumer c. 30% of nutrients. A mixoplankton, through photosynthesis, has scope to directly recover this loss of N, P and Fe (). In the case of N, there is an ancillary advantage (over phytoplankton) in that the internally recycled nitrogen as NH4+ is far cheaper than using externally sourced NO3– in terms of both photo-reductant and Fe (both required for nitrate reduction; Flynn & Hipkin, Citation1999). While, as noted above, the theoretical significant saving in resources does not often equate to differences in growth rate between ammonium versus nitrate grown phytoplankton, this trait would be of especial advantage under nutrient-limiting conditions. There is no evidence for how interactions between phagotrophy and ammonium versus nitrate consumption may affect mixoplankton growth at low light; for phytoplankton, there is, counterintuitively, no difference in growth using these DIN sources at low light, but there can be at high light (Thompson et al., Citation1989). The only caveat to the perceived advantage of internal nutrient recycling with photo-phago-synergism in mixoplankton, and one that is important in modelling, is that just because mixoplankton are able to be more efficient at retaining ingested prey-nutrient, they are not necessarily more efficient all the time; all consumers exhibit lower efficiency when resources are in abundance (Mitra & Flynn, Citation2007) and high growth efficiencies when prey abundance is limiting (Schoener & McManus, Citation2017).
Concurrent phototrophy and phagotrophy in mixoplankton provides scope for optimizing both physiologies (e.g. Wilken et al., Citation2014). There will be benefits through internal production and consumptions of O2 and CO2 () and perhaps the stabilization of cell surface pH. Consumption of O2 is of especial importance in optimizing C-fixation through RuBisCO (as O2 uptake competes with CO2 uptake). This also alleviates the need for carbon-concentrating mechanisms (CCMs; most dissolved inorganic C is as bicarbonate, while CO2 is the substrate for RuBisCO), though a role for CCMs in mixoplankton is unclear (Raven et al., Citation2009, Citation2020). During phototrophy pH increases and conversely during heterotrophy pH falls; at extremes, these changes can be deleterious and even lethal (Hansen, Citation2002), a situation that is more problematic with ocean acidification as the buffering capacity of seawater is weakened (Hofmann et al., Citation2010). Mixoplankton have scope to modulate near-cell and thence bulk-water pH levels, in the same way that calcification in coccolithophorids may stabilize external pH (Flynn et al., Citation2016). Caveats include that other plankton will also benefit from any such modulation in bulk water pH, and we do not know how temperature changes may affect the balance of phototrophy and phagotrophy (Ferreira et al., Citation2022).
Organism growth can only be maximal when internal nutrient conditions are optimal. This state of optimal stoichiometry may be considered to be more likely in a mixoplankton than in a phytoplankton or a zooplankton (; Stoecker et al., Citation1988; Adolf et al., Citation2006; Flynn & Mitra, Citation2009). The need for a balanced diet, for provision of different lipids for example (Wickham & Wimmer, Citation2019; Sato, Citation2020), is also more easily met with an internal phototrophic potential. The caveat in the advantage of this healthier cell status is that the organisms may also then provide a good (perhaps more attractive) food source for predators. The production of toxins and allelopathic compounds are also often associated with low nutrient stress (John & Flynn, Citation2002; Granéli & Flynn, Citation2006), and may thus be expected to be depressed in mixoplankton rather than in phytoplankton if the former are less stressed.
Of critical ecological importance, and a factor that will be missing from any autecological analysis of trait trade-offs focused on phytoplankton versus mixoplankton, is the role of predation. Predation offers mixoplankton scope to remove their competitors and even to kill potential grazers (; Thingstad et al., Citation1996; Tillmann, Citation2003). That activity is of value even though death of other organisms need not be directly linked to phagotrophy by the mixoplankton (Olli & Teeveer, Citation2007). Killing and exploiting competitors can occur at low mixoplankton abundances, but to control a much faster growing competitor this action requires high mixoplankton numbers as this is a density-dependent process. Likewise, the collective action of many mixoplankton cells against larger competitors or predators is dependent on high cell abundances. The complexities of interactions between organisms which display allelopathic and toxic potential makes predicting the winner extremely difficult (Flynn, Citation2008). Further, identifying generic trait trade-offs becomes even more problematic as the winner may not be the organism that we may think it is from autecological considerations. There are reasons to suspect that mixoplankton could ingest viruses, given evidence that viruses can be ingested by protist plankton (González & Suttle, Citation1993). There is, however, no reason to also suspect that such phagotrophy in mixoplankton would make them any more prone to viral infections than are zooplankton. If phagotrophy did provide a route for entry of viruses into protists resulting in infections, then this would represent a trait trade-off to the advantage of phytoplankton. There are many factors that impact on the success of viruses that would impact the assessment of such a putative trait trade-off (Flynn et al., Citation2022).
Research on mixoplankton has been complicated by the sensitivity of these organisms to conditions and difficulties in maintaining them in culture, especially in axenic cultures. In large measure such problems probably reflect ignorance over the need for specific abiotic and biotic growth conditions for the growth of these niche specialists (). Some CM can be grown as de facto ‘phytoplankton’ (as in most plankton culture collections), while some may be grown heterotrophically in the dark (Lie et al., Citation2018; Abreu et al., Citation2022), and others require feeding with specific prey (notably pSNCM). This cultivation problem itself flags how little we know of the ecophysiology of these organisms (e.g. Blossom et al., Citation2017; Larsson et al., Citation2022). Indeed, there are suspicions that the ability to feed is lost on prolonged maintenance of cultures without prey (Blossom & Hansen, Citation2021). Unless we understand the required conditions for optimal growth, we cannot formulate meaningful trait trade-off tests of general applicability. Perhaps the breadth of mixoplankton functionality prevents such a formulation.
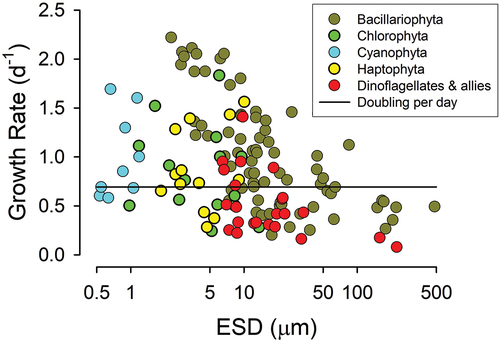
Finally, we return to the issue of additive growth support from phagotrophy+phototrophy (associated with , H2.7) and the perception that mixoplankton are slow growers. Growth rate is the most readily used benchmark of trait trade-off discussions, making the (not necessarily correct) assumption that this relates to fitness. If a mixoplankton has a lower maximum growth rate than a similar sized (purely phototrophic) phytoplankton or (purely phagotrophic) zooplankton, then this may be argued to evidence a trait trade-off in being mixoplanktonic. However, the great variation in growth rate potential within a given phototrophic plankton size (, with r2 = 0.15, see Finkel et al., Citation2010) clearly flags that a simple trait trade-off is not at play. Similarly, we see such variation within dinoflagellates, be they mixoplankton or heterotrophic zooplankton (). Furthermore, while many mixoplankton are slow growing (), there are exceptions (Dolan & Pérez, Citation2000; Adolf et al., Citation2006), suggesting that being mixoplanktonic per se is not the explanation. Combining niche specialization () with the proposed universal trait trade-off of matching the maximum growth rate potential to the environmental conditions (Flynn & Skibinski, Citation2020) provides an explanation; this is revisited in detail below. Also important is that mixoplanktonic activity is synergistic or cooperative (Mitra & Flynn, Citation2010, Citation2023; Wilken et al., Citation2014). Only in some instances does the activity appear to be additive (e.g. Jeong et al., Citation2010). Any perceived weakness in a particular physiological trait could reflect a trait trade-off but it could equally, if not more likely, reflect a balance between supply and demand for resource acquisition and handling through different complementary mechanisms.
Fig. 5. Maximum growth rates for phototrophic plankton of different sizes. Many of the dinoflagellates and haptophytes are known, or are prospective, mixoplankton (cf. the mixoplankton database, Mitra et al., Citation2023). Note the great variety in growth rate potential within a given size range. The horizontal line indicates growth at 0.693 day–1 (a doubling per day) as an exemplar of rates expected of cells with division cycles synchronized to the diel light-dark cycle (Nelson & Brand, Citation1979). Source data from Finkel et al. (Citation2010).
Discussion
Why are mixoplankton not all-conquering?
Our analysis finds no compelling evidence for trait trade-offs between mixoplankton and their non-mixoplankton competitors except for one instance, associated with GNCM versus non-mixoplanktonic ciliates (, H1.5). The balance of advantage and disadvantage for GNCM ciliates is consistent with the repeated gain and loss of photosynthesis by protists over evolution (; Raven et al., Citation2009). The assumptions required to support the generality of the other potential trait trade-off arguments () have too many exceptions and too little (if any) lines of evidence. Considerations of trait trade-offs within mixoplankton functional types are greatly tempered by the great taxonomic range across these organisms. The analysis in Mitra et al. (Citation2023; their ) shows that while GNCM are confined to Ciliophora, pSNCM are in Foraminifera, Ciliophora and Dinoflagellata, eSNCM are in Radiolaria, Foraminifera, Ciliophora and Dinoflagellata, while CM are found in Cercozoa, Dinoflagellate, Ochrophyta, Haptophyta, Cryptophyta and Chlorophyta. Taken with other views that ecological factors can readily overturn autecology arguments (Sommer et al., Citation2017), we conclude that the complexity of the ecosystems in which different mixoplankton live identifies the trophic interactions that are key to the success or failure of different members of each functional type in any given time and space. This raises the question as to why mixoplankton are not dominant everywhere.
Mixoplankton proliferate in mature (K-selecting) ecosystems, which are characterized neither by non-limiting inorganic nutrient concentrations, nor by abundant prey species supportive of the growth of specialist phytoplankton and zooplankton, respectively. Phytoplankton and protistan zooplankton dominate as r-select species in immature ecosystems (Mitra et al., Citation2014). Flynn & Skibinski (Citation2020) suggest that the maximum growth rate evolves to match the potential of the environmental conditions to support that growth rate (consistent with Droop, Citation1974); a high growth rate potential leads to deleterious stresses in an organism growing in an environment that can only support low growth rates. On the contrary, growth in optimal conditions selects for faster growth rates in microbes (Lenski et al., Citation1998). Such a concept helps explain the variety of growth rates for a given size group of organisms, as seen in , when we consider that the organisms tested were isolated from very different environments. A key emergent trait, the potential maximum growth rate, then becomes unavailable for mixoplankton trait trade-off arguments.
Phytoplankton evolved from mixoplanktonic lineages (); the more appropriate trade-off question concerns the loss of phagotrophy in phytoplankton. In this context the diatoms stand out as being the primary comparator against which to consider mixoplankton. Diatoms are an extremely successful and relatively recently evolved group (Behrenfeld et al., Citation2021); they are not known to be capable of phagotrophy (akin to feeding), though mixotrophy via osmotrophy is well documented (see Introduction). There is, however, evidence in diatoms of: endocytosis in vegetative diatom cells in the form of siderophore uptake (Kazania et al., Citation2018); intracellular bacteria in diatoms within chloroplast invaginations of Pinnularia (Schmid, Citation2003a, Citation2003b); and symbiotic diazotrophic cyanobacterium Richelia intracellularis within the diatoms Hemiaulus and Rhizosolenia (Tuo et al., Citation2021). Presumably, phagocytosis was involved in (cyano)bacteria entering the diatom protoplast; in view of the much greater size of these (cyano)bacteria than of pores in the diatom frustules, (cyano)bacterial entry to the protoplast of Hemiaulus and Pinnularia, and association with the plasmalemma surface in Rhizosolenia, entry is most likely during sexual reproduction when cell walls are temporarily absent.
While it is tempting to try and consolidate different plankton species into a few simple groups, in reality, the variation between organisms reflects selective pressures for evolution in (especially considered on a microbial scale) very different environments. By comparing the schematics in , it can be readily appreciated that the variety amongst mixoplankton functional types exceeds that within the non-diatom and diatom phytoplankton. The mixoplankton are far from a single functional group that could be amenable to a single set of trait trade-off arguments, or to a sliding scale of physiological constraints (e.g. Ward & Follows, Citation2016). The model analysis by Anschütz & Flynn (Citation2020) shows how physiological differences may affect the success of protist plankton groups but there are clearly many avenues that remain to be explored. For example, the sensitivity of success for each organism to ecosystem nutrient loading affects the balance of competitors, predators and potential prey (Anschütz et al., Citation2022). The varieties in mixoplankton ecophysiology are consequences of the food web structure in which these organisms live (Leles et al., Citation2021) and ultimately evolve. It is important to note that net growth rate set against gains and losses is the critical issue in evolution (Flynn & Skibinski, Citation2020), and not the gross growth rate of the individual which most readily forms the base of trait trade-off considerations. The high connectivity between organisms and resources in mature systems is probably one of the factors affecting the sensitivity of mixoplankton to specific conditions ().
Mixoplankton rarely thrive in the high nutrient and turbulent conditions that favour most diatoms, fully consistent with Margalef’s mandala relating these conditions to phytoplankton succession (Margalef, Citation1978; Glibert, Citation2016). Whether that is typically a consequence of direct physical damage to the mixoplankton cells, or acts through disruption of their food web, is unclear. The diatoms Azpeltia, Coscinodiscus, Rhizosolenia, Stephanopyxis and Thalassiosira occur at the deep chlorophyll maximum in stratified ocean waters (Kemp & Villareal, Citation2013, Citation2018) where the nutrient concentrations are significantly higher than near the surface waters. These are environments where mixoplankton may also dominate, and it is within such a restricted context that trait trade-offs may perhaps be sought. The bottom line, however, is that considerations of generic trait trade-offs between diatoms and mixoplankton are fraught with problems (), and conflict with the prime trait trade-off caveats to consider organisms from the same environment and of a similar evolutionary lineage that could have traded traits during their evolution. Furthermore, just as some diatoms thrive in calm conditions, so some mixoplankton thrive in turbulent conditions (e.g. coccolithophorids; Avrahami & Frada, Citation2020). Reports of bacterivory in the ‘phytoplankton’ Emiliania huxleyii (Avrahami & Frada, Citation2020) and Phaeocystis globosa (Koppelle et al., Citation2022) will surely not be the last revelations. The organisms that break the rules may be the winners in a given situation.
When ”trait trade-offs” are not trade-offs
We must be careful not to align what could be a series of disparate evolutionary events (Mansour & Anestis, Citation2021; Bremer et al., Citation2022) in organisms far separated in spatial and temporal scales, to generic trait trade-offs. An organism with a coupled phagotrophic and phototrophic metabolism may not have been subjected to evolutionary pressures to develop high affinity acquisition mechanisms as may organisms with only one of these trophic routes. At the extreme, prolonged lack of a need to express a particular trait probably results in (deleterious) mutations that are not selected against; this is seen in cultures of CM maintained solely as phytoplankton which lose the ability to eat (Blossom & Hansen, Citation2021). Such situations are indicative of the outcome of different evolutionary lineages developed in different environments and are not evidence of the existence of a trait trade-off. While some may argue that this is a matter of semantics, the net result being the same (mixoplankton perhaps being less well equipped than the ‘pure’ functional types for each resource route when grown in nutrient or prey replete conditions), we must only apply the trait trade-off label to an event that is indeed a plausible scientifically established trait trade-off. For other instances, we must use appropriate concepts and terminologies.
For organisms dominant in immature systems, from whence coincidentally most cultured plankton are isolated, considerations of trait trade-offs through primarily autecological aspects may possibly be appropriate. The mature ecosystem in which mixoplankton are more common, typified by the temperate summer, is inherently more complex than the immature temperate spring. We suggest that the exploitation of trait trade-offs as a meaningful route to drive research, is not applicable for mixoplankton. We need to search elsewhere for reasons as to why different mixoplankton succeed where and when they do. More appropriate marine plankton ecology models are required that are specifically developed to reflect diversity in physiological functionality, rather than the biogeochemical models which employ gross simplifications built around perceived generic rules, such as trait trade-offs (Flynn et al., Citation2015). After all, biogeochemistry is ultimately an emergent function of ecology.
Author contributions
A. Mitra: conceptualization, investigation and analysis, visualization, writing – original draft, review and editing. K.J. Flynn: conceptualization, investigation and analysis, visualization, writing – original draft, review and editing. D.K. Stoecker: investigation and analysis, writing – original draft, review and editing. J.A. Raven: investigation and analysis, writing – original draft, review and editing.
Acknowledgements
This is a contribution to SCOR WG #165 MixONET which is supported by grant OCE-214035 from the National Science Foundation to the Scientific Committee on Oceanic Research (SCOR) and contributions from SCOR National Committees.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data relating to this work are presented herein or referenced.
Additional information
Funding
References
- Aaronson, S., DeAngelis, B., Frank, O. & Baker, H. (1971). Secretion of vitamins and amino acids into the environment by Ochromonas danica. Journal of Phycology, 7: 215–218.
- Abreu, A.P., Morais, R.C., Teixeira, J.A. & Nunes, J. (2022). A comparison between microalgal autotrophic growth and metabolic accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renewable and Sustainable Energy Review, 159: 112247.
- Adolf, J.E., Stoecker, D.K. & Harding, L.W., Jr. (2003). Autotrophic growth and photoacclimation in Karlodi-nium micrum (Dinophyceae) and Storeatula major (Cryptophyceae). Journal of Phycology, 39: 1101–1108. doi:10.1111/j.0022-3646.2003.02-086.x
- Adolf, J.E., Stoecker, D.K. & Harding, L.W., Jr. (2006). The balance of autotrophy and heterotrophy during mixotrophic growth of Karlodinium micrum (Dinophyceae). Journal of Plankton Research, 28: 737–751. doi:10.1093/plankt/fbl007
- Andersen, K.H., Berge, T., Gonçalves, R.J., Hartvig, M., Heuschele, J., Hylander, S., Jacobsen, N.S., Lindemann, C., Martens, E.A., Neuheimer, A.B. & Olsson, K. (2016). Characteristic sizes of life in the oceans, from bacteria to whales. Annual Review of Marine Science, 8: 217–241. doi:10.1146/annurev-marine-122414-034144
- Anderson, O.R. (1993). The trophic role of planktonic Foraminifera and Radiolaria. Marine Microbial Food Webs, 7: 31–51.
- Anschütz, A.A. & Flynn, K.J. (2020). Niche separation between different functional types of mixoplankton: results from NPZ-style N-based model simulations. Marine Biology, 167: 1–21. doi:10.1007/s00227-019-3612-3
- Anschütz, A.A., Flynn, K.J. & Mitra, A. (2022). Acquired phototrophy and its implications for bloom dynamics of the Teleaulax-Mesodinium-Dinophysis-complex. Frontiers in Marine Science, 8. doi:10.3389/fmars.2021.799358
- Antia, N.J., Harrison, P.J. & Oliveira, L. (1981). The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia, 30: 1–89. doi:10.2216/i0031-8884-30-1-1.1
- Avrahami, Y. & Frada, M.J. (2020). Detection of phagotrophy in the marine phytoplankton group of the coccolithophores (Calcihaptophycidae, Haptophyta) during nutrient‐replete and phosphate‐limited growth. Journal of Phycology, 56: 1103–1108. doi:10.1111/jpy.12997
- Behrenfeld, M.J., Halsey, K.H., Boss, E., Karp-Boss, L., Milligan, A.J. & Peers, G. (2021). Thoughts on the evolution and ecological niche of diatoms. Ecological Monographs, 91: e01457. doi:10.1002/ecm.1457
- Biddanda, B. & Benner, R. (1997). Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnology and Oceanography, 42: 506–518. doi:10.4319/lo.1997.42.3.0506
- Blossom, H.E., Bædkel, T.D., Tillmann, U. & Hansen, P.J. (2017). A search for mixotrophy and mucus trap production in Alexandrium spp. and the dynamics of mucus trap formation in Alexandrium pseudogonyaulax. Harmful Algae, 64: 51–62. doi:10.1016/j.hal.2017.03.004
- Blossom, H.E. & Hansen, P.J. (2021). The loss of mixotrophy in Alexandrium pseudogonyaulax: implications for trade‐offs between toxicity, mucus trap production, and phagotrophy. Limnology and Oceanography, 66: 528–542. doi:10.1002/lno.11621
- Bremer, N., Tria, F.D., Skejo, J., Garg, S.G. & Martin, W.F. (2022). Ancestral state reconstructions trace mitochondria but not phagocytosis to the last eukaryotic common ancestor. Genome Biology and Evolution, 14: evac079. doi:10.1093/gbe/evac079
- Burkholder, J.M., Glibert, P.M. & Skelton, H.M. (2008). Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae, 8: 77–93. doi:10.1016/j.hal.2008.08.010
- Cadier, M., Hansen, A.N., Andersen, K.H. & Visser, A.W. (2020). Competition between vacuolated and mixotrophic unicellular plankton. Journal of Plankton Research, 42: 425–439. doi:10.1093/plankt/fbaa025
- Caron, D.A. (2000). Symbiosis and Mixotrophy Among Pelagic Microorganisms. In Microbial Ecology of the Oceans ( Kirchman, D.L. ed.), 495–523. Wiley-Liss, Inc., New York, USA.
- Caron, D.A. (2016). The rise of Rhizaria. Nature, 532: 444–445. doi:10.1038/nature17892
- Caron, D.A., Porter, K.G. & Sanders, R.W. (1990). Carbon, nitrogen, and phosphorus budgets for the mixotrophic phytoflagellate Poterioochromonas malhamensis (Chrysophyceae) during bacterial ingestion. Limnology and Oceanography, 35: 433–443. doi:10.4319/lo.1990.35.2.0433
- Caron, D.A. & Swanberg, N.R. (1990). The ecology of planktonic sarcodines. Aquatic Science, 3: 147–180.
- Cupo, A., Landi, S., Morra, S., Nuzzo, G., Gallo, C., Manzo, E., Fontana, A. & d’Ippolito, G. (2021). Autotrophic vs. heterotrophic cultivation of the marine diatom Cyclotella cryptica for EPA production. Marine Drugs, 19: 355. doi:10.3390/md19070355
- de Castro, F., Gaedke, U. & Boenigk, J. (2009). Reverse evolution: driving forces behind the loss of acquired photosynthetic traits. Plos One, 4: e8465. doi:10.1371/journal.pone.0008465
- Decelle, J., Probert, I., Bittner, L., Desdevises, Y., Colin, S., de Vargas, C., Galí, M., Simó, R. & Not, F. (2012). An original mode of symbiosis in open ocean plankton. Proceedings of the National Academy of Sciences, 109: 18000–18005. doi:10.1073/pnas.1212303109
- Demura, M., Noël, M.H., Kasai, F., Watanabe, M.M. & Kawachi, M. (2009). Taxonomic revision of Chattonella antiqua, C. marina and C. ovata (Raphidophyceae) based on their morphological characteristics and genetic diversity. Phycologia, 48: 518–535. doi:10.2216/08-98.1
- Dolan, J.R. & Pérez, M.T. (2000). Costs, benefits and characteristics of mixotrophy in marine oligotrichs. Freshwater Biology, 45: 227–238. doi:10.1046/j.1365-2427.2000.00659.x
- Droop, M.R. (1974). The nutrient status of algal cells in continuous culture. Journal of the Marine Biological Association of the UK, 54: 825–855. doi:10.1017/S002531540005760X
- Faure, E., Not, F., Benoiston, A.S., Labadie, K., Bittner, L. & Ayata, S.D. (2019). Mixotrophic protists display contrasted biogeographies in the global ocean. ISME Journal, 13: 1072–1083. doi:10.1038/s41396-018-0340-5
- Fenchel, T. & Finlay, B.J. (1983). Respiration rates in heterotrophic, free-living protozoa. Microbial Ecology, 9: 99–122.
- Ferreira, G.D., Grigoropoulou, A., Saiz, E. & Calbet, A. (2022). The effect of short-term temperature exposure on vital physiological processes of mixoplankton and protozooplankton. Marine Environmental Research, 179: 105693. doi:10.1016/j.marenvres.2022.105693
- Finkel, Z.V., Beardall, J., Flynn, K.J., Quigg, A., Rees, T.A.V. & Raven, J.A. (2010). Phytoplankton in a changing world: cell size and elemental stoichiometry. Journal of Plankton Research, 32: 119–137. doi:10.1093/plankt/fbp098
- Flynn, K.J. (1990). Composition of intracellular and extracellular pools of amino acids, and amino acid utilization of microalgae of different sizes. Journal of Experimental Marine Biology and Ecology, 139: 151–166. doi:10.1016/0022-0981(90)90143-Z
- Flynn, K.J. (2008). Attack is not the best form of defense; lessons from harmful algal bloom dynamics. Harmful Algae, 8: 129–139. doi:10.1016/j.hal.2008.08.007
- Flynn, K.J. & Berry, L.S. (1999). The loss of organic nitrogen during marine primary production may be overestimated significantly when estimated using 15N substrates. Proceedings of the Royal Society B: Biological Sciences, 266: 641–647. doi:10.1098/rspb.1999.0684
- Flynn, K.J. & Butler, I. (1986). Nitrogen sources for the growth of marine microalgae; role of dissolved free amino acids. Marine Ecology Progress Series, 34: 281–304. doi:10.3354/meps034281
- Flynn, K.J., Clark, D.R. & Wheeler, G. (2016). The role of coccolithophore calcification in bioengineering their environment. Proceedings of the Royal Society B: Biological Sciences, 283: 20161099. doi:10.1098/rspb.2016.1099
- Flynn, K.J., Clark, D.R. & Xue, Y. (2008). Modelling the release of dissolved organic matter by phytoplankton. Journal of Phycology, 44: 1171–1187. doi:10.1111/j.1529-8817.2008.00562.x
- Flynn, K.J. & Davidson, K. (1993). Predator-prey interactions between Isochrysis galbana and Oxyrrhis marina. II. Release of non-protein amines and faeces during predation of Isochrysis. Journal of Plankton Research, 15: 893–905. doi:10.1093/plankt/15.8.893
- Flynn, K.J., Davidson, K. & Cunningham, A. (1996). Prey selection and rejection by a microflagellate; implications for the study and operation of microbial food webs. Journal of Experimental Marine Biology and Ecology, 196: 357–372. doi:10.1016/0022-0981(95)00140-9
- Flynn, K.J. & Hansen, P.J. (2013). Cutting the canopy to defeat the “selfish gene”; conflicting selection pressures for the integration of phototrophy in mixotrophic protists. Protist, 164: 811–823. doi:10.1016/j.protis.2013.09.002
- Flynn, K.J. & Hipkin, C.R. (1999). Interactions between iron, light, ammonium, and nitrate: insights from the construction of a dynamic model of algal physiology. Journal of Phycology, 35: 1171–1190. doi:10.1046/j.1529-8817.1999.3561171.x
- Flynn, K.J. & Mitra, A. (2009). Building the “perfect beast”: modelling mixotrophic plankton. Journal of Plankton Research, 31: 965–992. doi:10.1093/plankt/fbp044
- Flynn, K.J., Mitra, A., Anestis, K., Anschütz, A.A., Calbet, A., Ferreira, G.D., Gypens, N., Hansen, P.J., John, U., Martin, J.L. & Mansour, J.S. (2019). Mixotrophic protists and a new paradigm for marine ecology where does plankton research go now? Journal of Plankton Research, 41: 375–391.
- Flynn, K.J., Mitra, A., Wilson, W.H., Kimmance, S.A., Clark, D.R., Pelusi, A. & Polimene, L. (2022). “Boom-and-busted-dynamics” of phytoplankton-virus interactions explain the paradox of the plankton. New Phytologist, 234: 990–1002. doi:10.1111/nph.18042
- Flynn, K.J. & Skibinski, D.O.F. (2020). Exploring evolution of maximum growth rates in plankton. Journal of Plankton Research, 42: 497–513. doi:10.1093/plankt/fbaa038
- Flynn, K.J., Skibinski, D.O.F. & Lindemann, C. (2018). Effects of growth rate, cell size, motion, and elemental stoichiometry on nutrient transport kinetics. Plos Computational Biology, 14: e1006118. doi:10.1371/journal.pcbi.1006118
- Flynn, K.J., St. John, M., Raven, J.A., Skibinski, D.O.F., Allen, J.I., Mitra, A. & Hofmann, E.E. (2015). Acclimation, adaptation, traits and trade-offs in plankton functional type models: reconciling terminology for biology and modelling. Journal of Plankton Research, 37: 683–691. doi:10.1093/plankt/fbv036
- Flynn, K.J., Stoecker, D.K., Mitra, A., Raven, J.A., Glibert, P.M., Hansen, P.J., Granéli, E. & Burkholder, J.M. (2013). Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. Journal of Plankton Research, 35: 3–11. doi:10.1093/plankt/fbs062
- Garland, T. (2014). Trade-offs. Current Biology, 24: R60–R61. doi:10.1016/j.cub.2013.11.036
- Gavelis, G.S., Wakeman, K.C., Tillmann, U., Ripken, C., Mitarai, S., Herranz, M., Özbek, S., Holstein, T., Keeling, P.J. & Leander, B.S. (2017). Microbial arms race: ballistic “nematocysts” in dinoflagellates represent a new extreme in organelle complexity. Science Advances, 3: e.1602552. doi:10.1126/sciadv.1602552
- Glibert, P.M. (2016). Margalef revisited: a new phytoplankton mandala incorporating twelve dimensions, including nutritional physiology. Harmful Algae, 55: 25–30. doi:10.1016/j.hal.2016.01.008
- Glibert, P.M. & Mitra, A. (2022). From webs, loops, shunts, and pumps to microbial multitasking: evolving concepts of marine microbial ecology, the mixoplankton paradigm, and implications for a future ocean. Limnology and Oceanography, 67: 585–597. doi:10.1002/lno.12018
- Glibert, P.M., Wilkerson, F.P., Dugdale, R.C., Raven, J.A., Dupont, C.L., Leavitt, P.R., Parker, A.E., Burkholder, J.M. & Kana, T.M. (2016). Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen‐enriched conditions. Limnology and Oceanography, 61: 165–197. doi:10.1002/lno.10203
- Gomes, H.D.R., Goes, J.I., Matondar, S.G.P., Buskey, E.J., Basu, S., Parab, S. & Thoppil, P. (2014). Massive outbreaks of Noctiluca scintillans blooms in the Arabian Sea due to spread of hypoxia. Nature Communications, 5: 1–8.
- Gomes, H.D.R., McKee, K., Mile, A., Thandapu, S., Al-Hashmi, K., Jiang, X. & Goes, J.I. (2018). Influence of light availability and prey type on the growth and photo-physiological rates of the mixotroph Noctiluca scintillans. Frontiers in Marine Science, 5: 374. doi:10.3389/fmars.2018.00374
- González, J.M. & Suttle, C.A. (1993). Grazing by marine nanoflagellates on viruses and virus-sized particles: ingestion and digestion. Marine Ecology Progress Series, 94: 1–10. doi:10.3354/meps094001
- Granéli, E., Evardsen, B., Roelke, D.L. & Hagström, J.A. (2012). The ecophysiology and bloom dynamics of Prymnesium spp. Harmful Algae, 14: 260–270. doi:10.1016/j.hal.2011.10.024
- Granéli, E. & Flynn, K.J. (2006). Chemical and physical factors influencing toxin production. In Ecology of Harmful Algae, ( Granéli, E. and Turner, J.T., eds.), Vol. 189, 229–241. Ecological Studies, Springer-Verlag, Berlin.
- Hammer, A.C. & Pitchford, J.W. (2005). The role of mixotrophy in plankton bloom dynamics, and the consequences for productivity. ICES Journal of Marine Science, 62: 833–840. doi:10.1016/j.icesjms.2005.03.001
- Hansen, P.J. (2002). The effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquatic Microbial Ecology, 28: 279–288. doi:10.3354/ame028279
- Hansen, P.J., Skovgaard, A., Glud, R.N. & Stoecker, D.K. (2000). Physiology of the mixotrophic dinoflagellate Fragilidium subglobosum. II. Effects of time scale and prey concentration on photosynthetic performance. Marine Ecology Progress Series, 201: 137–146. doi:10.3354/meps201137
- Hartmann, M., Grob, C., Tarran, G.A., Martin, A.P., Burkill, P.H., Scanlan, D.J. & Zubkov, M.V. (2012). Mixotrophic basis of Atlantic oligotrophic ecosystems. Proceedings of the National Academy of Sciences, 109: 5756–5760. doi:10.1073/pnas.1118179109
- Hausmann, K. (2002). Food acquisition, food ingestion and food digestion by protists. Japanese Journal of Protozoology, 35: 85–95.
- Hofmann, A.F., Middleburg, J.J., Soetaert, K., Wolf-Gladrow, D.A. & Meysman, K.J.R. (2010). Proto cycling, buffering, and reaction stoichiometry in natural waters. Marine Chemistry, 121: 246–255. doi:10.1016/j.marchem.2010.05.004
- Jeong, H.J., Park, J.Y., Nho, J.H., Park, M.O., Ha, J.H., Seong, K.A., Jeng, C., Seong, C.N., Lee, K.Y. & Yih, W.H. (2005). Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquatic Microbial Ecology, 41: 131–143. doi:10.3354/ame041131
- Jeong, H.J., Yoo, Y.D., Kim, J.S., Seong, K.A., Kang, N.S. & Kim, T.H. (2010). Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Science Journal, 45: 65–91. doi:10.1007/s12601-010-0007-2
- Jiang, H. & Johnson, M.D. (2017). Jumping and overcoming diffusion limitation of nutrient uptake in the photosynthetic ciliate Mesodinium rubrum. Limnology and Oceanography, 62: 421–436. doi:10.1002/lno.10432
- Jiang, H., Kulis, D.M., Brosnahan, M.L. & Anderson, D.M. (2018). Behavioral and mechanistic characteristics of the predator-prey interaction between the dinoflagellate Dinophysis acuminata and the ciliate Mesodinum rubrum. Harmful Algae, 77: 43–54. doi:10.1016/j.hal.2018.06.007
- John, E.H. & Flynn, K.J. (2002). Modelling changes in paralytic shellfish toxin content of dinoflagellates in response to nitrogen and phosphorus supply. Marine Ecology Progress Series, 225: 147–160. doi:10.3354/meps225147
- Johnson, M.D. (2011). The acquisition of phototrophy: adaptive strategies of hosting endosymbionts and organelles. Photosynthesis Research, 107: 117–132. doi:10.1007/s11120-010-9546-8
- Jonsson, P.R. & Tiselius, P. (1990). Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Marine Ecology Progress Series, 60: 35–44. doi:10.3354/meps060035
- Kamennaya, N.A., Kennaway, G., Fucha, B.M. & Zubkov, M.V. (2018). “Pomacytosis” - Semi-extracellular phagocytosis of cyanobacteria by the smallest marine algae. Plos Biology, 16: e2003502. doi:10.1371/journal.pbio.2003502
- Kazania, E., Sutak, R., Paz-Yepes, J., Dorrell, R.G., Viera, F.R.J., Mach, J., Morrissey, J., Leon, S., Lam, F., Pelletier, E. & Camadro, J.M. (2018). Endocytosis-mediated siderophore uptake as a strategy for Fe acquisition in diatoms. Science Advances, 4: eaar4536. doi:10.1126/sciadv.aar4536
- Keeling, P.J., Burki, F., Wilcox, H.M., Allam, B., Allen, E.E., Amaral-Zettler, L.A., Armbrust, E.V., Archibald, J.M., Bharti, A.K., Bell, C.J. & Beszteri, B. (2014). The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP): illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. Plos Biology, 12: e.1001889. doi:10.1371/journal.pbio.1001889
- Kemp, A.E.S. & Villareal, T.A. (2013). High diatom production is stratified waters – potential negative feedback to global warming. Progress in Oceanography, 119: 4–23. doi:10.1016/j.pocean.2013.06.004
- Kemp, A.E. & Villareal, T.A. (2018). The case of the diatoms and the muddled mandalas: time to recognize diatom adaptations to stratified waters. Progress in Oceanography, 167: 138–149. doi:10.1016/j.pocean.2018.08.002
- Kim, G.H., Han, J.H., Kim, B., Han, J.W., Nam, S.W., Shin, W., Park, J.W. & Yih, W. (2016). Cryptophyte gene regulation in the kleptoplastidic, karyokleptic ciliate Mesodinium rubrum. Harmful Algae, 52: 23–33. doi:10.1016/j.hal.2015.12.004
- Kim, M., Nam, S.W., Shin, W., Coats, D.W. & Park, M.G. (2012). Dinophysis caudata (Dinophyceae) sequesters and retains plastids from the mixotrophic ciliate prey Mesodinium rubrum. Journal of Phycology, 48: 569–579. doi:10.1111/j.1529-8817.2012.01150.x
- Kiørboe, T. (2011). How zooplankton feed: mechanisms, traits and trade-offs. Biological Reviews of the Cambridge Philosophical Society, 86: 311–339. doi:10.1111/j.1469-185X.2010.00148.x
- Kiørboe, T., Visser, A. & Andersen, K.H. (2018). A trait-based approach to ocean ecology. ICES Journal of Marine Science, 75(6): 1849–1863. doi:10.1093/icesjms/fsy090
- Koppelle, S., López-Escardó, D., Brussaard, C.P., Huisman, J., Philippart, C.J., Massana, R. & Wilken, S. (2022). Mixotrophy in the bloom-forming genus Phaeocystis and other haptophytes. Harmful Algae, 117: 102292. doi:10.1016/j.hal.2022.102292
- Larsen, J. (1988). An ultrastructural study of Amphidinium poecilochroum (Dinophyceae), a phagotrophic dinoflagellate feeding on small species of cryptophytes. Phycologia, 27: 366–377. doi:10.2216/i0031-8884-27-3-366.1
- Larsson, M.E., Bramucci, A.R., Collins, S., Hallegraeff, G., Kahlke, T., Raina, J.-B., Seymour, J.R. & Doblin, M.A. (2022). Mucospheres produced by a mixotrophic protist impact ocean carbon cycling. Nature Communications, 13: 1–15. doi:10.1038/s41467-022-28867-8
- Lee, K.H., Jeong, H.J., Jang, T.Y., Lim, A.S., Kang, N.S., Kim, J.H., Kim, K.Y., Park, K.T. & Lee, K. (2014). Feeding by the newly described mixotrophic dinoflagellate Gymnodinium smaydae: feeding mechanism, prey species, and effect of prey concentration. Journal of Experimental Marine Biology and Ecology, 459: 114–125. doi:10.1016/j.jembe.2014.05.011
- Lee, J.J., Lanners, E. & Ter Kuile, B. (1988). The retention of chloroplasts by the foraminifer Elphidiurn crispum. Symbiosis, 5: 545–560.
- Leles, S.G., Bruggeman, J., Polimene, L., Blackford, J., Flynn, K.J. & Mitra, A. (2021). Differences in physiology explain succession of mixoplankton functional types and affect carbon fluxes in temperate seas. Progress in Oceanography, 190: 102481. doi:10.1016/j.pocean.2020.102481
- Leles, S.G., Mitra, A., Flynn, K.J., Stoecker, D.K., Hansen, P.J., Calbet, A., McManus, G.B., Sanders, R.W., Caron, D.A., Not, F. & Hallegraeff, G.M. (2017). Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance. Proceedings of the Royal Society B: Biological Sciences, 284: 20170664. doi:10.1098/rspb.2017.0664
- Leles, S.G., Mitra, A., Flynn, K.J., Tillmann, U., Stoecker, D.K., Jeong, H.J., Burkholder, J., Hansen, P.J., Caron, D.A., Glibert, P.M. & Hallegraeff, G. (2019). Sampling bias misrepresents the biogeographic significance of constitutive mixotrophs across global oceans. Global Ecology and Biogeography, 28: 418–428. doi:10.1111/geb.12853
- Lenski, R.E., Mongold, J.A., Sniegowski, P.D., Travisano, M., Vasi, F., Gerrish, P.J. & Schmidt, T.M. (1998). Evolution of competitive fitness in experimental populations of E. coli: What Makes One Genotype A Better Competitor Than Another? Antonie Van Leeuwenhoek, 73: 35–47.
- Lewin, J. & Hellebust, J.A. (1970). Heterotrophic nutrition of the marine pennate diatom Cylindrotheca fusiformis. Canadian Journal of Microbiology, 16: 1123–1129. doi:10.1139/m70-188
- Li, M., Chen, Y., Zhang, F., Song, Y., Glibert, P.M. & Stoecker, D.K. (2022). A three-dimensional mixotrophic model of Karlodinium veneficum blooms for a eutrophic estuary. Harmful Algae, 113: 102203. doi:10.1016/j.hal.2022.102203
- Lie, A.A.Y., Liu, Z.F., Terrado, R., Tatters, A.O., Heidelberg, K.B. & Caron, D.A. (2018). A tale of two mixotrophic chrysophytes: insights into the metabolisms of two Ochromonas species (Chrysophyceae) through a comparison of gene expression. Plos One, 13: e0192439. doi:10.1371/journal.pone.0192439
- Li, Z., Lan, T., Zhang, J., Gao, K., Beardall, J. & Wu, Y. (2021). Nitrogen limitation decreases the repair capacity and enhances photoinhibition of photosystem II in a diatom. Photochemistry and Photobiology, 97: 745–752. doi:10.1111/php.13386
- Li, A., Stoecker, D.K. & Adolf, J.E. (1999). Feeding, pigmentation, photosynthesis and growth of the mixotrophic dinoflagellate Gyrodinium galatheanum. Aquatic Microbial Ecology, 19: 163–176. doi:10.3354/ame019163
- Litchman, E., Edwards, K.F. & Boyd, P.W. (2021). Toward trait-based food webs: universal traits and trait matching in planktonic predator–prey and host–parasite relationships. Limnology and Oceanography, 66: 3857–3872. doi:10.1002/lno.11924
- Litchman, E. & Klausmeier, C.A. (2008). Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution, and Systematics, 39: 615–639. doi:10.1146/annurev.ecolsys.39.110707.173549
- Litchman, E., Ohman, M.D. & Kiørboe, T. (2013). Trait-based approaches to zooplankton communities. Journal of Plankton Research, 35: 473–484. doi:10.1093/plankt/fbt019
- Mansour, J.S. & Anestis, K. (2021). Eco-evolutionary perspectives on mixoplankton. Frontiers in Marine Science, 8: 666160. doi:10.3389/fmars.2021.666160
- Margalef, R. (1978). Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanologis Acta, 1: 493–509.
- Martel, C.M. (2006). Prey location, recognition and ingestion by the phagotrophic marine dinoflagellate Oxyrrhis marina. Journal of Experimental Marine Biology and Ecology, 335: 210–220. doi:10.1016/j.jembe.2006.03.006
- McCue, M.D. (2006). Specific dynamic action: a century of investigation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 144: 381–394. doi:10.1016/j.cbpa.2006.03.011
- McKie-Krisberg, Z.M., Gast, R.J. & Sanders, R.W. (2015). Physiological responses of three species of Antarctic mixotrophic phytoflagellates to changes in light and dissolved nutrients. Microbial Ecology, 70: 21–29. doi:10.1007/s00248-014-0543-x
- McManus, G.B., Liu, W., Cole, R.A., Biemesderfer, D. & Mydosh, J.L. (2018). Strombidium rassoulzadegani: a model species for chloroplast retention in Oligotrich ciliates. Frontiers in Marine Science, 5: 205. doi:10.3389/fmars.2018.00205
- McManus, G.B., Schoener, D.M. & Haberlandt, K. (2012). Chloroplast symbiosis in a marine ciliate: ecophysiology and the risks and rewards of hosting foreign organelles. Frontiers in Microbiology, 3: 321. doi:10.3389/fmicb.2012.00321
- Meyer, N., Rydzyk, A. & Pohnert, G. (2022). Pronounced uptake and metabolism of organic substrates by diatoms revealed by pulse-labeling metabolomics. Frontiers in Marine Science, 9. doi:10.3389/fmars.2022.821167
- Millette, N.C., Pierson, J.J., Aceves, A. & Stoecker, D.K. (2017). Mixotrophy in Heterocapsa rotundata: a mechanism for dominating the winter phytoplankton. Limnology and Oceanography, 62: 836–845. doi:10.1002/lno.10470
- Mitra, A., Caron, D.A., Faure, E., Flynn, K.J., Leles, S.G., Hansen, P.J., McManus, G.B., Not, F., Gomes, H.R., Santoferrara, L., Stoecker, D.K. & Tillmann, U. (2023). The mixoplankton database – diversity of photo-phago-trophic plankton in form, function and distribution across the global ocean. Journal or Eukaryote Microbiology: e12972. doi:10.1111/jeu.12972
- Mitra, A. & Flynn, K.J. (2007). Importance of interactions between food quality, quantity, and gut transit time on consumer feeding, growth, and trophic dynamics. The American Naturalist, 169: 632–646. doi:10.1086/513187
- Mitra, A. & Flynn, K.J. (2010). Modelling mixotrophy in harmful algal blooms: more or less the sum of the parts? Journal of Marine Systems, 83: 158–169. doi:10.1016/j.jmarsys.2010.04.006
- Mitra, A. & Flynn, K.J. (2023). Low rates of bacterivory enhances phototrophy and competitive advantage for mixoplankton growing in oligotrophic waters. Scientific Reports, 13: 6900. doi:10.1038/s41598-023-33962-x
- Mitra, A., Flynn, K.J., Burkholder, J.M., Berge, T., Calbet, A., Raven, J.A., Granéli, E., Glibert, P.M., Hansen, P.J., Stoecker, D.K. & Thingstad, F. (2014). The role of mixotrophic protists in the biological carbon pump. Biogeosciences, 11: 995–1005. doi:10.5194/bg-11-995-2014
- Mitra, A., Flynn, K.J., Tillmann, U., Raven, J.A., Caron, D., Stoecker, D.K., Not, F., Hansen, P.J., Hallegraeff, G., Sanders, R. & Wilken, S. (2016). Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition; incorporation of diverse mixotrophic strategies. Protist, 167: 106–120. doi:10.1016/j.protis.2016.01.003
- Moeller, H.V. & Johnson, M.D. (2017). Preferential plastid retention by the acquired phototroph Mesodinium chamaeleon. Eukaryotic Microbiology, 65: 148–158. doi:10.1111/jeu.12446
- Nagai, S., Nitshitani, G., Tomaru, Y., Sakiyama, S. & Kamiyama, T. (2008). Predation by the toxic dinoflagellate Dinophysis fortii on the ciliate Myrionecta rubra and observation of sequestration of ciliate chloroplasts. Journal of Phycology, 44: 909–922. doi:10.1111/j.1529-8817.2008.00544.x
- Nelson, D.M. & Brand, L.E. (1979). Cell division periodicity in 13 species of marine phytoplankton on a light: darkcycle. Journal of Phycology, 15: 67–75. doi:10.1111/j.1529-8817.1979.tb02964.x
- Olli, K. & Teeveer, K. (2007). Self-toxicity of Prymnesium parvum (Prymnesiophyceae). Phycologia, 46: 109–112. doi:10.2216/06-32.1
- Öpik, H. & Flynn, K.J. (1989). The digestive process of the dinoflagellate, Oxyrrhis marina Dujardin, feeding on the chlorophyte, Dunaliella primolecta Butcher: a combined study of ultrastructure and free amino acids. New Phytologist, 113: 143–151. doi:10.1111/j.1469-8137.1989.tb04700.x
- Pančić, M. & Kiørboe, T. (2018). Phytoplankton defence mechanisms: traits and trade-offs. Biological Reviews, 93: 1269–1303. doi:10.1111/brv.12395
- Park, M.G., Kim, S., Kim, H.S., Myung, G., Kang, Y.G. & Yih, W. (2006). First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquatic Microbial Ecology, 45: 101–106. doi:10.3354/ame045101
- Pelegri, S.P., Christaki, U., Doland, J. & Rassoulzadegan, F. (1999). Particulate and dissolved organic carbon production by the heterotrophic nanoflagellate Pteridomonas danica Patterson and Fenchel. Microbial Ecology, 37: 276–284. doi:10.1007/s002489900150
- Ponce-Toledo, R.I., Deschamps, P., López-García, P., Zivanovic, Y., Benzenava, K. & Moreira, D. (2017). An early-branching freshwater cyanobacterium at the origin of plastids. Current Biology, 27: 368–391. doi:10.1016/j.cub.2016.11.056
- Raven, J.A. (1997). Phagotrophy in phototrophs. Limnology and Oceanography, 42: 198–205. doi:10.4319/lo.1997.42.1.0198
- Raven, J.A. & Beardall, J. (2022). Evolution of phytoplankton in relation to their physiological traits. Journal of Marine Science and Engineering, 10: 194. doi:10.3390/jmse10020194
- Raven, J.A., Beardall, J., Flynn, K.J. & Maberly, S.C. (2009). Phagotrophy in the origins of photosynthesis in eukaryotes and as a complementary mode of nutrition in phototrophs: relation to Darwin’s insectivorous plants. Journal of Experimental Botany, 60: 3975–3987. doi:10.1093/jxb/erp282
- Raven, J.A., Suggett, D.J. & Giordano, M. (2020). Inorganic carbon concentrating mechanisms in free-living and symbiotic dinoflagellates and chromerids. Journal of Phycology, 56: 1377–1397. doi:10.1111/jpy.13050
- Richardson, K., Beardall, J. & Raven, J.A. (1983). Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytologist, 93: 157–191. doi:10.1111/j.1469-8137.1983.tb03422.x
- Ross, O.N. (2006). Particles in motion: how turbulence affects plankton sedimentation from an oceanic mixed layer. Geophysical Research Letters, 33: L0609. doi:10.1029/2006GL026352
- Rottberger, J., Gruber, A., Boenigk, J. & Kroth, P.G. (2013). Influence of nutrients and light on autotrophic, mixotrophic and heterotrophic freshwater chrysophytes. Aquatic Microbial Ecology, 71: 179–191. doi:10.3354/ame01662
- Sánchez-Baracaldo, P., Raven, J.A., Pisari, D. & Knoll, A.H. (2017). Early photosynthetic eukaryotes inhabited low-salinity habitats. Proceedings of the National Academy of Sciences, 114: E7737–E7745. doi:10.1073/pnas.1620089114
- Sato, N. (2020). Complex origins of chloroplast membranes with photosynthetic machineries: multiple transfers of genes from divergent organisms at different times or a single endosymbiotic event? Journal of Plankton Research, 133: 15–33. doi:10.1007/s10265-019-01157-z
- Schmid, A.M.M. (2003a). Endobacteria in the diatom Pinnularia (Bacillariophyceae). I. “Scattered ct nucleoid” explained: DAPI-DNA complexes stem from exoplastidial bacteria boring into the chloroplasts. Journal of Phycology, 39: 122–138. doi:10.1046/j.1529-8817.2003.02084.x
- Schmid, A.M.M. (2003b). Endobacteria in the diatom Pinnularia (Bacillariophyceae). II. Host cell cycle-dependent translocation and transient chloroplast scars. Journal of Phycology, 39: 139–153. doi:10.1046/j.1529-8817.2003.02085.x
- Schoener, D.M. & McManus, G.B. (2012). Plastid retention, use and replacement in a kleptoplastidic ciliate. Aquatic Microbial Ecology, 67: 177–187. doi:10.3354/ame01601
- Schoener, D.M. & McManus, G.B. (2017). Growth, grazing, and inorganic C and N uptake in a mixotrophic and a heterotrophic ciliate. Journal of Plankton Research, 39: 379–391. doi:10.1093/plankt/fbx014
- Serra-Pompei, C., Soudijn, F., Visser, A.W., Kiørboe, T. & Andersen, K.H. (2020). A general size- and trait-based model of plankton communities. Progress in Oceanography, 189: 102473. doi:10.1016/j.pocean.2020.102473
- Sikes, C.S. & Wilbur, K.M. (1982). Functions of coccolith formation. Limnology and Oceanography, 22: 18–26. doi:10.4319/lo.1982.27.1.0018
- Smalley, G.W. & Coats, D.W. (2002). Ecology of the red‐tide dinoflagellate Ceratium furca: distribution, mixotrophy, and grazing impact on ciliate populations of Chesapeake Bay. Journal of Eukaryotic Microbiology, 49: 63–73. doi:10.1111/j.1550-7408.2002.tb00343.x
- Smriga, S., Fernandez, V.I., Mitchell, J.G. & Stocker, R. (2016). Chemotaxis toward phytoplankton drives organic matter partitioning among marine bacteria. Proceedings of the National Academy of Sciences, 113: 1576–1581. doi:10.1073/pnas.1512307113
- Snell‐Rood, E., Cothran, R., Espeset, A., Jeyasingh, P., Hobbie, S. & Morehouse, N.I. (2015). Life‐history evolution in the anthropocene: effects of increasing nutrients on traits and trade‐offs. Evolutionary Applications, 8: 635–649. doi:10.1111/eva.12272
- Sommer, U., Charalampous, E., Genitsaris, S. & Moustaka-Gouni, M. (2017). Benefits, costs and taxonomic distribution of marine phytoplankton body size. Journal of Plankton Research, 39: 494–508.
- Spero, H.J. (1985). Chemosensory capabilities in the phagotrophic dinoflagellate Gymnodinium fungiforme. Journal of Phycology, 21: 181–184. doi:10.1111/j.0022-3646.1985.00181.x
- Stearns, S.C. (1989). Trade-offs in life-history evolution. Functional Ecology, 3: 259–268. doi:10.2307/2389364
- Stoecker, D.K. (1999). Mixotrophy among dinoflagellates. Journal of Eukaryotic Microbiology, 46: 397–401. doi:10.1111/j.1550-7408.1999.tb04619.x
- Stoecker, D.K., Hansen, P.J., Caron, D.A. & Mitra, A. (2017). Mixotrophy in the marine plankton. Annual Reviews in Marine Science, 9: 311–335. doi:10.1146/annurev-marine-010816-060617
- Stoecker, D.K., Johnson, M.D., de Vargas, C. & Not, F. (2009). Acquired phototrophy in aquatic protists. Aquatic Microbial Ecology, 57: 279–310. doi:10.3354/ame01340
- Stoecker, D.K. & Silver, M.W. (1990). Replacement and aging of chloroplasts in Strombidium capitatum (Ciliata, Oligotrichida). Marine Biology, 107: 491–502. doi:10.1007/BF01313434
- Stoecker, D.K., Silver, M.W., Michaels, A.E. & Davis, L.H. (1988). Obligate mixotrophy in Laboea strobila, a ciliate which retains chloroplasts. Marine Biology, 99: 415–423. doi:10.1007/BF02112135
- Strom, S.L., Benner, R., Ziegler, S. & Dagg, M.J. (2003). Plankton grazers are potentially important source of marine dissolved organic carbon. Limnology and Oceanography, 42: 1364–1374. doi:10.4319/lo.1997.42.6.1364
- Thingstad, T.F., Havskum, H., Garde, K. & Riemann, B. (1996). On the strategy of “eating your competitor”: a mathematical analysis of algal mixotrophy. Ecology, 77: 2108–2118. doi:10.2307/2265705
- Thompson, P.A., Levessaur, M.E. & Harrison, P.J. (1989). Light-limited growth on ammonium vs nitrate: what is the advantage for marine phytoplankton? Limnology and Oceanography, 34: 1014–1024. doi:10.4319/lo.1989.34.6.1014
- Tillmann, U. (1998). Phagotrophy by a plastidic haptophyte Prymnesium patelliferum. Aquatic Microbial Ecology, 14: 155–160. doi:10.3354/ame014155
- Tillmann, U. (2003). Kill and eat your predator: a winning strategy of the planktonic flagellate Prymnesium parvum. Aquatic Microbial Ecology, 32: 73–84. doi:10.3354/ame032073
- Tittel, J., Bissinger, V., Zippel, B., Gaedke, U., Bell, E., Lorke, A. & Kamjunke, N. (2003). Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs. Proceedings of the National Academy of Science, 100: 12776–12781. doi:10.1073/pnas.2130696100
- Troost, T.A., Kooi, B.W. & Kooijman, S.A. (2005). Ecological specialization of mixotrophic plankton in a mixed water column. The American Naturalist, 166: E45–E61. doi:10.1086/432038
- Tuo, S.-H., Mulholland, M.R., Chen, Y.-L.-L., Chappell, R.D. & Chen, H.-Y. (2021). Patterns in Rhizosolenia- and Gionardia-associated Richelia abundances in the tropical marginal seas of the western North Pacific. Journal of Plankton Research, 43: 328–352. doi:10.1093/plankt/fbab022
- Unrein, F., Gasol, J.M., Not, F., Forn, I. & Massana, R. (2014). Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters. ISME Journal, 8: 164–176. doi:10.1038/ismej.2013.132
- Verity, P.G. (1991). Feeding in planktonic protozoans: evidence for non-random acquisition of prey. Journal of Eukaryote Microbiology, 38: 69–76.
- Visintini, N., Martiny, A.C. & Flombaum, P. (2021). Prochlorococcus, Synechococcus, and picoeukaryotic phytoplankton abundances in the global ocean. Limnology and Oceanography Letters, 6: 207–215. doi:10.1002/lol2.10188
- Ward, B.A., Dutkiewicz, S., Barton, A.D. & Follows, M.J. (2011). Biophysical aspects of resource acquisition and competition in algal mixotrophs. American Naturalist, 178: 98–112. doi:10.1086/660284
- Ward, B.A. & Follows, M.J. (2016). Marine mixotrophy increases trophic transfer efficiency, mean organism size, and vertical carbon flux. Proceedings of the National Academy of Sciences, 113: 2958–2963. doi:10.1073/pnas.1517118113
- Wetz, M.S. & Wheeler, P.A. (2007). Release of dissolved organic matter by coastal diatoms. Limnology and Oceanography, 52: 798–807. doi:10.4319/lo.2007.52.2.0798
- Wickham, S.A. & Wimmer, R. (2019). Does mixotrophy in ciliates compensate for poor-quality prey? Experiments with heterotrophic–mixotrophic species pairs. Journal of Plankton Research, 41: 583–593. doi:10.1093/plankt/fbz052
- Wilken, S., Choi, C.J. & Worden, A.Z. (2020). Contrasting mixotrophic lifestyles reveal different ecological niches in two closely related marine protists. Journal of Phycology, 56: 52–67. doi:10.1111/jpy.12920
- Wilken, S., Schuurmans, J.M. & Matthijs, H.C.P. (2014). Do mixotrophs grow as photoheterotrophs? Photophysiological acclimation of the chrysophyte Ochromonas danica after feeding. New Phytologist, 204: 882–889. doi:10.1111/nph.12975
- Wood, G. & Flynn, K.J. (1995). Growth of Heterosigma carterae (Raphidophyceae) on nitrate and ammonium at three photon flux densities: evidence for N-stress in nitrate-growing cells. Journal of Phycology, 31: 859–867. doi:10.1111/j.0022-3646.1995.00859.x
- Yoo, Y.D., Seong, K.A., Jeong, H.J., Yih, W., Rho, J.-R., Nam, S.W. & Kim, H.S. (2017). Mixotrophy in the marine red-tide cryptophyte Teleaulax amphioxeia and ingestion and grazing impact of cryptophytes on natural populations of bacteria in Korean coastal waters. Harmful Algae, 68: 105–117. doi:10.1016/j.hal.2017.07.012
- Zehr, J.P., Eitz, J.S. & Joint, I. (2017). Microbial life in the open ocean: a universe of tiny cells separated by empty space. Science, 357: 646–647. doi:10.1126/science.aan5764
- Zubkov, M.V. & Tarran, G.A. (2008). High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature, 455: 224–226. doi:10.1038/nature07236
