Abstract
A three-factor, three-level, face-centred cubic design was adopted to investigate the effect of three pre-treatment parameters, namely sodium hydroxide-to-dry biomass ratio [0.00, 0.05, and 0.10 (w/w)], lime-to-dry biomass ratio [0.00, 0.05, and 0.10 (w/w)] and residence reaction time (24, 96, and 168 h), at moderate temperature (21°C) on 24-h cumulative gas production (GP24) and delignification, as the response variables. Under the optimal pre-treatment conditions of NaOH-to-dry biomass ratio of 0.09 (w/w), lime-to-dry biomass ratio of 0.09 (w/w) and residence time of 160.3 h, the model predicted 151.6 mL gas/g organic matter (OM) versus the experimental value of 147.2 mL gas/g OM. Under the optimal conditions of NaOH-to-dry biomass ratio of 0.10 (w/w), lime-to-dry biomass ratio of 0.08 (w/w) and residence time of 156.8 h, sugarcane bagasse (SCB) was maximally delignified by 41.2%, whereas the model predicted 43.6% delignification. Under the optimal conditions determined for GP24, the neutral detergent fibre degradability of the pre-treated biomass after 24 and 48 h was 37.2 and 56.3%, respectively, a 1.90-fold and 1.58-fold over the untreated biomass, respectively. Overall, it was found that combined alkali treatment at optimal conditions effectively enhanced the ruminal degradability of SCB.
1. Introduction
It is projected that with an increasing world population (a 65% increase by ~2050), the global demand for food to feed the future generation will grow constantly (Wallace Citation2000). Ruminal microbial communities specialize in degrading the cellulosic biomass (Hess et al. Citation2011), enabling the ruminant animal to utilize lignocellulose, the most plentiful renewable biomass in nature (Alvira et al. Citation2010). Sugarcane bagasse (SCB), a crushed fibrous residue of sugarcane stalks remaining after juice extraction (Ramli et al. Citation2005), is an abundant lignocellulosic biomass with an annual production of approximately 54 million tons throughout the world (Samariha & Khakifirooz Citation2011).
Effective utilization of SCB as animal feedstuff is advantageous first for lessening the food crisis by presenting an inexpensive, readily available and abundant alternative feed, and second for alleviating the waste disposal problems facing the sugar industry (Ramli et al. Citation2005). Because of its high lignin content, SCB cannot efficiently be digested in the rumen (Okano et al. Citation2007); therefore, an effective pre-treatment is needed to increase the digestibility of SCB for ruminants.
Alkaline pre-treatment is a highly effective pre-treatment which makes lignocellulose swollen and porous (Hendriks & Zeeman Citation2009), mainly through hemicellulose solubilization, and solubilization and/or redistribution of lignin (Lynd et al. Citation2002). These changes enhance the enzymatic hydrolysis during cellulosic-ethanol production process, which is highly correlated with improved ruminal degradability, because many fibrolytic enzymes of ruminal microorganisms are similar to the commercial enzyme cocktails, which are used during the enzymatic hydrolysis in lignocellulosic-ethanol production process (Anderson et al. Citation2010).
Although high-temperature alkali pre-treatment is fast and effective (Rezende et al. Citation2011; Ahmadi et al. Citation2013), but it is a high-energy demanding process, requiring heat- and corrosion-resistant equipment. Moreover, harsh pre-treatment conditions may lead to reactions that form carbon–carbon bonds between the lignin subunits; this can limit delignification and, subsequently decrease the enzymatic hydrolysis of carbohydrates (Pan et al. Citation2004). Therefore, pre-treatment at moderate temperatures may hold a promise for a low-cost and efficient method for conversion of SCB into a more digestible ruminant feed.
Lime [Ca(OH)2] pre-treatment at mild temperatures requires a long period of time to considerably enhance the digestibility of lignocellulose (Kim & Holtzapple Citation2005). Effective pre-treatment with NaOH can be achieved at faster rates using moderate temperatures (Wu et al. Citation2011), but is more costly [market price of $450 and $89.8 per ton for NaOH and lime, respectively (Kumar & Murthy Citation2011; Tao et al. Citation2011)]. However, by mixing NaOH and lime loadings, it may be possible to achieve a less expensive and at the same time an effective mixed alkaline pre-treatment at moderate temperatures (Xu & Cheng Citation2011).
Optimization of pre-treatment conditions is a key step in development of an efficient and cost-effective pre-treatment (Karunanithy & Muthukumarappan Citation2011). Response surface methodology (RSM), with successful application to lignocellulose pre-treatment (Velmurugan & Muthukumar Citation2012; Rawat et al. Citation2013), is an effective optimization tool with which many operating variables along with their interactive effects on a desired response and/or responses can be identified with few observations (Karunanithy & Muthukumarappan Citation2011). In comparison with conventional methods such as classical one-at-a-time, RSM offers a time-saving, cost-effective approach because many variables are studied at the same time with a low number of experimental trials (Deepak et al. Citation2008).
The present study was aimed at determining the optimal pre-treatment conditions for maximum GP24 and delignification, as response variables, using RSM and proposing mathematical equations to predict these responses. Amongst the various incubation times, GP24 is an important incubation time with an in vivo correlation, which is widely used to predict metabolizable energy of feed (Menke & Steingass Citation1988). Therefore, GP24 was chosen as a response for evaluating the pre-treatment efficiency. Because of the very high positive correlation of ethanol yield during simultaneous saccharification with fermentation and GP24 (Weimer et al. Citation2005), the data of this experiment might provide a valuable predictor of ethanol production from the pre-treated substrates.
2. Material and methods
2.1. Feedstock preparation
Dried unmilled SCB was obtained from Khuzestan Sugarcane Factory in southwestern Iran, and its particle size distribution was determined by using the Penn State Particle Separator (Heinrichs & Kononoff Citation1996), as follows: >1.91 cm (9.1% of particles), 1.91–0.79 cm (37.8%), 0.79–0.18 cm (37.7%) and <0.18 cm (15.4%).
2.2. Pre-treatment procedure
Alkaline solutions were prepared by dissolving alkali reagents (lime or NaOH) with 300 mL distilled water. A 30-g sample of SCB [dry matter (DM) basis] was mixed thoroughly with the solution at a ratio of 1-g solid to 10 mL of solution in a 500-mL bottle. Because carbon dioxide in headspace may react with lime, the bottle was purged with a stream of N2 for 60 s, after which the bottle was tightly screw-capped and stirred at 500 rpm for 10 min. Because lime is poorly soluble in water [about 1.5–1.6% at ambient conditions (Tao et al. Citation2011)], it requires shaking during the pre-treatment, which was provided by horizontal shaking of bottles inside a shaking water-bath (Memmert, Model WNE 10, Schwabach, Germany) set at 30 back-and-forth movements per minute. Each pre-treatment run was replicated three times.
2.3. Determination of insoluble solid contents
At the end of each pre-treatment time, the slurry was harvested, pH was adjusted to around 7.0 by adding 1-N HCl and then vacuum-filtered (Whatman No. 41). The remaining solid was washed with distilled water and then filtered. The washing-filtration process was repeated three times or until the filtrate became colourless. The solid mass was dried in an air-forced oven for 48 h at 45°C. The solid recovery rate was determined gravimetrically (Kim Citation2004). A portion of each pre-treated sample was then used to determine chemical composition and in vitro gas production potential.
2.4. Compositional analysis
The oven-dried pre-treated or untreated bagasse samples were milled to pass through a 1-mm diameter screen using a Wiley mill (Arthur H. Thomas Co., Philadelphia, PA). DM (method ID 930.15), crude protein (CP, method ID 984.13) and ash (method ID 942.05) were determined according to AOAC (Citation2000). Ash-excluded neutral detergent fibre (NDF), without heat stable α-amylase, and acid detergent fibre were determined by an ANKOM200 Fiber Analyzer (Ankom Technology Corp., Fairport, NY, USA) according to Van Soest et al. (Citation1991). Acid detergent lignin was measured using an ANKOM200/220 DaisyII Incubator (ANKOM Technology, Fairport, NY, USA) with 72% (w/w) sulfuric acid according to Ankom protocol.
Delignification (D) was defined according to the following equation:
where L and L 0 are lignin content (%) in pre-treated and untreated biomass, respectively, and YT is the pre-treatment yield of the total insoluble solids determined after the pre-treatment (Kim & Holtzapple Citation2006).
2.5. In vitro gas production assay
Prior to morning feeding, rumen fluid was harvested from two non-lactating fistulated Holstein cows fed a diet of wheat straw (30%), concentrate mix (40%) containing mineral/vitamin supplements and alfalfa hay (30%). Details for preparation of rumen fluid and artificial saliva were as described earlier (Ahmadi et al. Citation2013). Gas volume in each volume-calibrated serum bottle, sealed with flanged butyl rubber stopper and aluminium crimp seal, was measured using a water displacement apparatus (Fedorak & Hrudey Citation1983) after 2, 4, 6, 8, 12, 16, 24, 48 and 72 h of incubation. Net gas production of each bottle at each incubation time was calculated after subtraction of the average gas production from four blank bottles (containing buffered rumen fluid but no substrate). Gas data were fitted using a first-order exponential model (Ørskov & McDonald Citation1979). Average fermentation rate (AFR) was calculated according to the equation proposed by Hervás et al. (Citation2005). In vitro dry matter degradability (IVDMD) was determined gravimetrically by centrifuging the contents of each bottle (after 72 h of incubation) at 3000 × g for 10 min, discarding the supernatant and drying the precipitate at 65°C to a constant weight (Mellenberger et al. Citation1970). Data on gas production were recorded from two separate runs, with three replicate bottles per treatment for each run.
2.6. Determination of NDF degradability
Degradability of NDF (at 24 and 48 h) for both untreated and optimum pre-treated SCB was determined by filling F-57 ANKOM bags (25-µm pore size) with approximately 500-mg substrate, transferring the bags to a loose mesh sack (2-mm pore size) and then incubating in the rumen. The bags were removed from the rumen after 24 or 48 h, hand-washed with cold water until the rinsing water became colourless and then dried at 60°C for 48 h (Ghasemi, Ghorbani et al. Citation2014). Ruminal degradability of NDF was determined gravimetrically by an ANKOM200 Fiber Analyzer (Van Soest et al. Citation1991).
2.7. Experimental design
The influence of three independent variables, namely sodium hydroxide-to-dry biomass ratio (w/w), lime-to-dry biomass ratio (w/w) and residence reaction time (h), on GP24 and delignification (as response variables), was determined using RSM. The range of each pre-treatment variable was determined based on economic feasibility and preliminary study, and each independent variable at three levels was coded according to: −1, 0 and +1 (). A three-factor, three-level, face-centred design with 20 experimental runs and 6 replicates at the centre points () was developed using Design-Expert® software (Version 7.0.0, Stat-Ease Inc., Minneapolis, USA). The experimental data were fitted to following second-order polynomial model (Equation Equation2):
where Y is the dependent variable (GP24 or delignification); β 0 is the intercept coefficient; β 1, β 2 and β 3 are linear coefficients; β 11, β 22 and β 33 are quadratic coefficients; β 12, β 13 and β 23 are interaction coefficients; A, B and C represent the sodium hydroxide-to-dry biomass ratio (w/w), lime-to-dry biomass (w/w) and pre-treatment time (h), respectively; and ε is the error term. The effects of variables and their interactive effects were determined by analysis of variance (ANOVA). The data were presented as means, and the level of significance was set at P < 0.05.
Table 1. Codes and levels of the independent variables used for optimization.
Table 2. Experimental design of both coded and actual values for GP24 and delignification, observed and predicted values.
2.8. Data analysis and model verification
Using the predictive equations of RSM, the optimal alkaline pre-treatment conditions were determined, and output variables, GP24 and delignification, were again measured under the proposed optimal conditions to verify the validity of the models. Under the optimal conditions determined for GP24, SCB was pre-treated and subjected to some supplementary tests to gain a better insight into the influence of the pre-treatment on chemical composition and in vitro degradability of SCB. Data obtained from the supplementary tests were analysed using one-way ANOVA (SPSS software, Ver. 16.0.0, 2007, SPSS Inc., Chicago, IL, USA). The level of significance was set at P < 0.05 and mean comparison was performed by the Duncan's multiple range test.
2.9. Fourier transform infrared–attenuated total reflection (FT-IR–ATR) spectroscopy
FT-IR spectroscopic analysis was performed to detect changes in the functional groups of the pre-treated biomass. FT-IR spectra were recorded by averaging 16 scans per sample from 4000 to 600 cm−1 at a resolution of 4 cm−1 using FT-IR–ATR spectroscopy (Tensor 27 FT-IR spectrometer, Bruker, Billerica, MA, USA) at the central laboratory in Isfahan University of Technology, Isfahan, Iran.
3. Results
3.1. Effect of pre-treatment on GP24
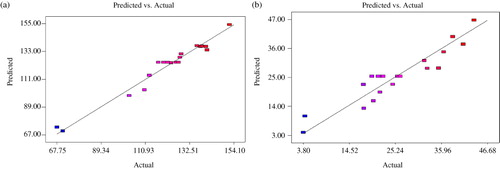
The results on GP24 data () showed that the model used to fit GP24 data was highly significant (P < 0.0001), suggesting that the model adequately represented the relationship between the response and independent variables (Hossain et al. Citation2012). Determination coefficient (R
2) was determined to be 0.9661, indicating that some 96.6% of variability in the response variable could be explained by the model. The predicted R
2 (0.8255) was in close agreement with (0.9443; ). The precision and reliability of the experiments conducted for GP24 were verified by a low value of 4.46% for the coefficient of variation (CV). The variation of the data around the fitted model is described by lack of fit; non-significant (P value = 0.1159) lack of fit indicated that the model is a good predictor of the response.
Table 3. ANOVA for response surface quadratic model for response variables, GP24 and delignification.
The second-order polynomial equation for GP24 was as follows:
The maximum GP24 was determined to be 147.2 mL/g OM at NaOH-to-dry biomass ratio of 0.09 (w/w), lime-to-dry biomass ratio of 0.09 (w/w) and pre-treatment duration of 160.3 h. Under these conditions, the model predicted GP24 of 151.6 mL/g OM, which was in a close agreement with the experimental value. depicts the close agreement between the observed GP24 (the response variable) values and those predicted from the model Equation (Equation3).
3.2. Effect of pre-treatment on delignification
Delignification data on SCB pre-treated under different conditions ranged from 3.8% to 43.6% (). All three independent variables linearly influenced the degree of delignification (P < 0.05), but there were no significant interaction between these variables (P > 0.05). Under the optimal pre-treatment conditions (NaOH-to-dry biomass ratio of 0.10, lime-to-dry biomass ratio of 0.08 and pre-treatment duration of 156.8 min), the model predicted 43.6% delignification. The degree of delignification obtained after using the proposed optimal conditions was 41.2%, a value very close to the predicted value. A high correlation was recorded between the predicted and experimental values ().
3.3. Changes in chemical composition of bagasse
shows the compositional analysis of untreated, washed-only and optimally pre-treated SCB. A slight change was observed in chemical composition of washed-only bagasse (in comparison with untreated one), whereas the pre-treatment at optimum conditions caused the cellulose concentration to increase by 8.9%, while it substantially solubilized that of hemicellulose by 30.4%. Protein degradation was a negative aspect of the pre-treatment, because it significantly decreased the crude protein content of bagasse (a reduction from 1.28% to 0.45%, for washed-only and optimum pre-treated biomass, respectively). Solid loss after washing alone was 5.1% compared with 14.7% for pre-treated materials under optimum processing conditions ().
Table 4. Chemical composition of SCB: untreated, washed-only and pre-treated under optimized conditions.
3.4. Effect of pre-treatment on NDF degradability and gas production kinetics
To gain a better insight into the impact of the pre-treatment on ruminal degradability of SCB, ruminal degradability of NDF (NDFd) was determined. Untreated bagasse had a 24-h NDFd of 19.6% (19.6 g NDF digested/100 g NDF incubated) and 48-h NDFd of 35.7%, while the pre-treatment (optimum pre-treatment) improved both 24- and 48-h NDFd by 90.0% and 57.7%, respectively ().
Table 5. NDF degradability and fermentation kinetic of SCB: untreated and pre-treated under optimal conditions.
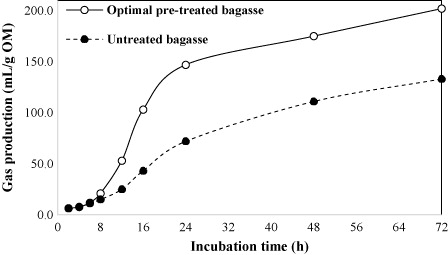
Under the optimal pre-treatment conditions, gas production rate constant (1/h) increased significantly (P = 0.001) from 0.021 to 0.043 for the untreated and optimally pre-treated substrate, respectively (). The AFR of optimally pre-treated bagasse was 2.33 times that of untreated bagasse. illustrates the cumulative gas production profile (during an incubation period of 72 h) of untreated bagasse and bagasse pre-treated under the optimum conditions. The pre-treatment substantially improved the gas production profile of the treated bagasse; both untreated and treated bagasse had a similar profile of gas production during the first 8 h of fermentation, followed by an increasing rate of gas production for the pre-treated biomass, and about 72.8% of its total gas (202 mL/g OM) was produced within the first 24 h of fermentation.
3.5. FT-IR–ATR spectroscopic analysis
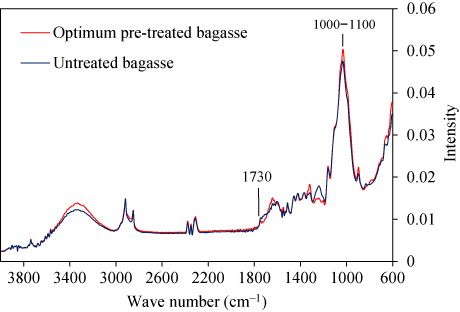
Chemical changes in the pre-treated SCB were characterized with FT-IR spectroscopy, which is a widely used technique for detecting the chemical changes in lignocellulosic materials (Xu et al. Citation2007). illustrates the FT-IR spectra of untreated and optimally pre-treated SCB. The spectrum of optimally pre-treated SCB showed slight differences in some functional groups, as compared with the untreated SCB, suggesting that pre-treatment chemically transformed SCB. The saponifying impact of the mixed alkaline pre-treatment on SCB was verified by a 12% reduction in a band at 1730 cm−1, which is attributed to saponification of the ester bonds, such as acetyl and uronic ester groups (Peng et al. Citation2012). An increase in absorptions peaks at 1000−1100 cm−1 in the spectrum of the pre-treated SCB solids demonstrated an increased concentration of cellulose in the pre-treated solid (Sun et al. Citation2008), as already shown by compositional analysis ().
4. Discussion
An ideal pre-treatment procedure aims at maximizing the substrate delignification while minimizing modification of polysaccharide, thereby enabling rapid digestion of pre-treated substrate by enhancing the accessibility of enzymes to polysaccharides (Ding et al. Citation2012). The effective delignifying power of both NaOH and lime has been demonstrated in several studies (Chen et al. Citation2007; Silverstein et al. Citation2007; Rezende et al. Citation2011; Shiroma et al. Citation2011; Xu & Cheng Citation2011). For example, Silverstein et al. (Citation2007), comparing the delignification effect of four chemical reagents (NaOH, H2SO4, H2O2 and ozone) on cotton stalks, showed that NaOH pre-treatment resulted in the highest lignin removal (65.6% at 2.0% NaOH, 90 min, 121°C). In another study, combination of sodium hydroxide [NaOH-to-raw biomass ratio of 0.1 (w/w) and lime-to-dry biomass ratio of 0.02 (w/w)] for 6 h at room temperature delignified switchgrass by 37.8% (Xu & Cheng Citation2011).
Of the three main components of SCB (i.e. hemicellulose, cellulose and lignin), the cellulosic fraction was not significantly affected by pre-treatment with alkali, whereas hemicellulose was significantly solubilized. In a recent study, where corn stover was pre-treated with NaOH to increase its enzymatic hydrolysis, more than 95% of the cellulosic fraction was preserved (Chen et al. Citation2013); it was also shown that when NaOH loading increased from 0.04 to 0.1 g/g corn stover, degradation of hemicellulose (xylan) was increased by 20%. Rezende et al. (Citation2011) pre-treated SCB with various loadings of NaOH to enhance its enzymatic digestibility; in general, compositional analysis of SCB pre-treated by various loading of NaOH showed that alkaline pre-treatment resulted in a significant increase in hemicellulose and lignin solubilization, whereas cellulosic fraction was well preserved and its concentration increased in retained solids. However, NaOH loadings above 2% did not further increase lignin and hemicellulose solubilization, and at these concentrations, cellulose loss began to increase. Overall, the results of that study indicated that NaOH pre-treatment with concentrations above 1% might not be effective (Rezende et al. Citation2011).
High recalcitrance and crystallinity of cellulose are the main factors reducing the sensitivity of cellulose with alkaline pre-treatment (Lai Citation1991; Gupta & Lee Citation2010). The reason why the pre-treatment significantly solubilized hemicellulosic fraction of bagasse results mainly from the fact that hemicellulose has an amorphous and branched structure, conferring little strength against alkali solubilization (Xu & Cheng Citation2011).
The high solid recovery after pre-treatment was expected, because sugarcane is extensively washed in sugar mill during sugar extraction process; for this reason, bagasse fibre becomes quite resistant to solubilization during the pre-treatment (Gandi et al. Citation1997). This is of great significance, because the economic viability of the pre-treatment process is highly dependent on high solid yield.
The enhancement in NDF degradability of the pre-treated SCB (both 24 and 48 h) () is of paramount importance, since the main obstacle facing the development of a digestible lignocellulosic ruminant feed is overcoming its high NDF content, which has generally poor ruminal digestibility (Falls Citation2011).
The higher amount of gas produced after 8 h of fermentation in the pre-treated bagasse may be explained by an enhanced reactivity of NDF portion of pre-treated bagasse to ruminal fibrolytic microorganisms. The volume of gas produced between 6 and 24 h of incubation is largely attributable to NDF digestion in cattle fed at high production levels, while the volume produced between 6 and 72 h of fermentation would be attributable to the amount of NDF which will be digested in cattle fed at maintenance intake levels (Robinson & Getachew Citation2002). Thus according to , the gas production profile of untreated and treated bagasse during an incubation period of 72 h, it can be concluded that high-producing dairy cattle may not efficiently benefit the pre-treated biomass, because they have a fast ruminal passage rate (Gressley et al. Citation2011), which gives the ruminal microorganisms a limited time for digestion of the pre-treated SCB. Hence, it would be more appropriate to consider its inclusion in the diet of cattle fed at the maintenance level, which have a slower ruminal passage rate.
Alkaline pre-treatment (using either lime or sodium hydroxide, or combination of them) for enhancing either ruminal digestibility or enzymatic digestibility in cellulosic-ethanol production process has been widely attempted (Haddad et al. Citation1998; Xu & Cheng Citation2011; Ahmadi et al. Citation2013; Chen et al. Citation2013). For example, combination of sodium hydroxide and lime to enhance the enzymatic hydrolysis in lignocellulosic-ethanol production process was reported by Xu and Cheng (Citation2011) in which, at the recommended conditions [6 h, 21°C, 1.0% (w/w) NaOH, and 0.2% (w/w) Ca(OH)2], the glucose and xylose yields of switchgrass reached 59.4% and 57.3% of their theoretical yields, respectively. Haddad et al. (Citation1998) investigated the efficacy of treating wheat straw with various combinations of sodium hydroxide and lime using the spraying (dry) technique on the performance of lactating Holstein cattle and determined the optimum processing conditions as 3% NaOH + 3% Ca(OH)2 (wt/wt, DM basis). Inclusion of the pre-treated wheat straw at 20% of diet of Holstein cows had minor impact on ruminal function and lactational performance (both short term and long term), in comparison with control diet which contained pre-bloom alfalfa hay. More recently, various combinations of sodium hydroxide and lime were used to improve ruminal degradability of date palm leaf; under the optimum processing conditions [71.4 h, 40°C, 0.6% (w/w) NaOH and 0.9% (w/w) Ca(OH)2], gas production at 24 h was 2.46-fold over the untreated biomass (Rajaee Rad et al. Citation2014). Enhanced ruminal degradability of the pre-treated SCB is most likely due to its lignin removal, since lignin, a highly oxygenated complex aromatic polymer which firmly complexes cellulose fibres (Lee et al. Citation2014), is an important impediment affecting the rate and extent of rumen digestion of fibre (Waghorn & McNabb Citation2003). By mitigating this barrier (), the accessibility of ruminal microorganisms to carbohydrate portion of the biomass may enhance, which consequently leads to an improved rumen degradability.
Supplementing lime with NaOH can be advantageous for animal feed purposes, because diets which are mainly composed of grains and mature forages are usually deficient in calcium (NRC Citation1984), thus mixing the calcium-enriched biomass with these diets can be beneficial for the ruminant animal (Gandi et al. Citation1997). After NaOH pre-treatment, high Na concentration may also exist in pre-treated biomass, but fortunately no health problem or impaired performance has been reported in ruminants consuming NaOH-pre-treated biomass; excess sodium ingested is excreted in the urine leading to an increase in water intake and urinary volume (Ghasemi, Khorvash et al. Citation2014).
5. Conclusions
Pre-treatment using the optimal conditions significantly improved the ruminal degradability of SCB, which may be suitable for inclusion in the ruminant diet. However, in vivo studies are needed to evaluate the effect of dietary inclusion of the pre-treated substrate on the performance of ruminants. A comprehensive cost–benefit analysis is also needed to select the most cost-effective pre-treatment procedure.
Acknowledgements
The authors gratefully acknowledge the Students’ Scientific Committee of Shiraz University for providing financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmadi F, Rajaee Rad A, Holtzapple MT, Zamiri MJ. 2013. Short-term oxidative lime pretreatment of palm pruning waste for use as animal feedstuff. J Sci Food Agric. 93:2061–2070.
- Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ. 2010. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol. 101:4851–4861.
- Anderson WF, Dien BS, Jung H-JG, Vogel KP, Weimer PJ. 2010. Effects of forage quality and cell wall constituents of Bermuda grass on biochemical conversion to ethanol. Bioenerg Res. 3:225–237.
- AOAC. 2000. Official methods of analysis. 17th ed. Gaithersburg, MD: Association of Official Analytical Chemists.
- Chen Y, Sharma-Shivappa RR, Keshwani D, Chen C. 2007. Potential of agricultural residues and hay for bioethanol production. Appl Biochem Biotechnol. 142:276–290.
- Chen Y, Stevens MA, Zhu Y, Holmes J, Xu, H. 2013. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol Biofuel. 6:1–10.
- Deepak V, Kalishwaralal K, Ramkumarpandian S, Babu SV, Senthilkumar SR, Sangiliyandi G. 2008. Optimization of media composition for Nattokinase production by Bacillus subtilis using response surface methodology. Bioresour Technol. 99:8170−8174.
- Ding S-Y, Liu Y-S, Zeng Y, Himmel ME, Baker JO, Bayer EA. 2012. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science. 338:1055–1060.
- Falls MD. 2011. Development of oxidative lime pretreatment and shock treatment to produce highly digestible lignocellulose for biofuel and ruminant feed applications [PhD Dissertation]. College Station (TX): Texas A& M University; p. 270.
- Fedorak PM, Hrudey SE. 1983. A simple apparatus for measuring gas production by methanogenic cultures in serum bottles. Environ Technol. 4:425–432.
- Gandi J, Holtzapple MT, Ferrer A, Byers FM, Turner ND, Nagwani M, Chang S. 1997. Lime treatment of agricultural residues to improve rumen digestibility. Anim Feed Sci Technol. 68:195–211.
- Ghasemi E, Ghorbani GR, Khorvash M, Emami MR. 2014. Adjustment of pH and enzymatic treatment of barley straw by dry processing method. J Appl Anim Res 42:400–405.
- Ghasemi E, Khorvash M, Ghorbani GR, Elmamouz F. 2014. Effects of straw treatment and nitrogen supplementation on digestibility, intake and physiological responses of water intake as well as urine and faecal characteristics. J Anim Physiol Anim Nutr. 98:100–106.
- Gressley TF, Hall MB, Armentano LE. 2011. Ruminant nutrition symposium: productivity, digestion, and health responses to hindgut acidosis in ruminants. J Anim Sci. 89:1120–1130.
- Gupta R, Lee Y. 2010. Investigation of biomass degradation mechanism in pretreatment of switchgrass by aqueous ammonia and sodium hydroxide. Bioresour Technol. 101:8185–8191.
- Haddad S, Grant R, Kachman S. 1998. Effect of wheat straw treated with alkali on ruminal function and lactational performance of dairy cows. J Dairy Sci. 81:1956–1965.
- Heinrichs J, Kononoff P. 1996. Evaluating particle size of forages and TMRs using the Penn State Particle Size Separator. Pennsylvania State University. Available from: http://extension.psu.edu/
- Hendriks A, Zeeman G. 2009. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol. 100:10–18.
- Hervás G, Frutos P, Giráldez FJ, Mora MJ, Fernández B, Mantecón AR. 2005. Effect of preservation on fermentative activity of rumen fluid inoculum for in vitro gas production techniques. Anim Feed Sci Technol. 123–124:107–118.
- Hess M, Sczyrba A, Egan R, Kim T-W, Chokhawala H, Schroth G, Luo S, Clark DS, Chen F, Zhang T. 2011. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science. 331:463–467.
- Hossain MB, Brunton NP, Patras A, Tiwari B, O’Donnell C, Martin-Diana AB, Barry-Ryan C. 2012. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason Sonochem. 19:582–590.
- Karunanithy C, Muthukumarappan K. 2011. Optimization of alkali soaking and extrusion pretreatment of prairie cord grass for maximum sugar recovery by enzymatic hydrolysis. Biochem Eng J. 54:71–82.
- Kim S, Holtzapple MT. 2005. Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour Technol. 96:1994–2006.
- Kim S, Holtzapple MT. 2006. Effect of structural features on enzyme digestibility of corn stover. Bioresour Technol. 97:583–591.
- Kim SH. 2004. Lime pretreatment and enzymatic hydrolysis of corn stover [ PhD Dissertation]. College Station (TX): Texas A& M University.
- Kumar D, Murthy GS. 2011. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol Biofuel. 4:1–19.
- Lai YZ. 1991. Chemical degradation. In: Hon DN-S, Shiraishi N, editors. Wood and cellulose chemistry. New York: Marcel Dekker; p. 455–473.
- Lee D-S, Wi SG, Lee SJ, Lee Y-G, Kim Y-S, Bae H-J. 2014. Rapid saccharification for production of cellulosic biofuels. Bioresour Technol. 158:239–247.
- Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 66:506–577.
- Mellenberger RW, Satter L, Millett M, Baker A. 1970. An in vitro technique for estimating digestibility of treated and untreated wood. J Anim Sci. 30:1005–1011.
- Menke KH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 23:103–116.
- NRC. 1984. Nutrient requirements of beef cattle. 6th rev ed. Washington (DC): National Academy Press.
- Okano K, Fukui S, Kitao R, Usagawa T. 2007. Effects of culture length of Pleurotus eryngii grown on sugarcane bagasse on in vitro digestibility and chemical composition. Anim Feed Sci Technol. 136:240–247.
- Ørskov E, McDonald I. 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agri Sci. 92:499–503.
- Pan X, Zhang X, Gregg DJ, Saddler JN. 2004. Enhanced enzymatic hydrolysis of steam-exploded Douglas fir wood by alkali-oxygen post-treatment. Appl Biochem Biotechnol. 115:1103–1114.
- Peng F, Bian J, Ren J-L, Peng P, Xu F, Sun R-C. 2012. Fractionation and characterization of alkali-extracted hemicelluloses from peashrub. Biomass Bioenerg. 39:20–30.
- Rajaee Rad A, Ahmadi F, Mohammadabadi T, Ziaee E, Polikarpov I. 2014. Combination of sodium hydroxide and lime as a pretreatment for conversion of date palm leaves into a promising ruminant feed: an optimization approach. Waste Biomass Valor. 6:243–252.
- Ramli M, Imura Y, Takayama K, Nakanishi Y. 2005. Bioconversion of sugarcane bagasse with Japanese Koji by solid-state fermentation and its effects on nutritive value and preference in goats. Asian Aust J Anim Sci. 18:1279–1284.
- Rawat R, Kumbhar B, Tewari L. 2013. Optimization of alkali pretreatment for bioconversion of poplar (Populus deltoides) biomass into fermentable sugars using response surface methodology. Ind Crops Prod. 44:220–226.
- Rezende CA, de Lima MA, Maziero P, Ribeiro deAzevedo E, Garcia W, Polikarpov I. 2011. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuel. 4:1–19.
- Robinson P, Getachew G. 2002. A practical gas production technique to determine the nutritive value of forages: the UC Davis approach. UC, Davis Cooperative Extension.
- Samariha A, Khakifirooz A. 2011. Application of NSSC pulping to sugarcane bagasse. BioResources. 6:3313−3323.
- Shiroma R, Park J-Y, Al-Haq MI, Arakane M, Ike M, Tokuyasu K. 2011. RT-CaCCO process: an improved CaCCO process for rice straw by its incorporation with a step of lime pretreatment at room temperature. Bioresour Technol. 102:2943–2949.
- Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne J. 2007 A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol. 98:3000–3011.
- Sun Y, Lin L, Deng H, Li J, He B, Sun R, Ouyang P. 2008. Structural changes of bamboo cellulose in formic acid. BioResources. 3:297–315.
- Tao L, Aden A, Elander RT, Pallapolu VR, Lee Y, Garlock RJ, Balan V, Dale BE, Kim Y, Mosier NS, et al. 2011. Process and technoeconomic analysis of leading pretreatment technologies for lignocellulosic ethanol production using switchgrass. Bioresour Technol. 102:11105–11114.
- Van Soest PJ, Robertson J, Lewis B. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Velmurugan R, Muthukumar K. 2012. Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: optimization through response surface methodology. Bioresour Technol. 112:293–299.
- Waghorn GC, McNabb WC. 2003. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc Nutr Soc. 62:383–392.
- Wallace J. 2000 Increasing agricultural water use efficiency to meet future food production. Agric Ecosyst Environ. 82:105–119.
- Weimer P, Dien B, Springer T, Vogel K. 2005. In vitro gas production as a surrogate measure of the fermentability of cellulosic biomass to ethanol. Appl Microb Biotechnol. 67:52–58.
- Wu L, Arakane M, Ike M, Wada M, Takai T, Gau M, Tokuyasu K. 2011. Low temperature alkali pretreatment for improving enzymatic digestibility of sweet sorghum bagasse for ethanol production. Bioresour Technol. 102:4793–4799.
- Xu J, Cheng JJ. 2011. Pretreatment of switchgrass for sugar production with the combination of sodium hydroxide and lime. Bioresour Technol. 102:3861–3868.
- Xu Z, Wang Q, Jiang ZH, Yang X, Ji Y. 2007. Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenerg. 31:162–167.