ABSTRACT
The present study was conducted to evaluate the effect of egg yolk concentration and washing on sperm quality in cryopreserved Barbari buck semen at higher dilution (100 × 106 cells/ml). Five healthy Barbari bucks of similar age and weight were selected as semen donor. Six ejaculates were collected from each buck during the experiment. Collected semen samples were pooled and diluted with Tris-based semen extender containing 6% glycerol and egg yolk according to the treatments – T-1, that is, 3% egg yolk, T-2, that is, 20% egg yolk and T-3, that is, 20% egg yolk after washing and cryopreserved using the slow freezing technique. Semen was evaluated at three different steps, namely, after dilution, after equilibration and after freeze–thawing. Significantly (p ≤ .01) higher values for motility and viability were observed in T-3 just after dilution while significantly (p ≤ .01) higher values for acrosomal integrity and pattern F (uncapacitated sperm) was observed in T-2. Significantly (p ≤ .01) higher values were observed in T-2 after equilibration and thawing. It may be concluded that incorporation of 20% egg yolk compared to 3% egg yolk or 20% egg yolk after washing in extender confer better cryoprotection to Barbari buck spermatozoa as reflected in semen quality parameters studied.
1. Introduction
During the process of cryopreservation sperm cells are subjected to variable ranges of temperature that make them liable to cryoinjuries. The sperm plasma membrane is the primary site of damage during the freezing and thawing process (Januskauskas et al. Citation2003). Temperature fluctuations and cell dehydration induce changes in the lateral phase separation of lipids that leads to reordering of membrane components (Drobnis et al. Citation1993), loss of poly-unsaturated fatty acids and cholesterol that induces cryocapacitation and acrosomal damage (Watson Citation1995). Cryocapacitation involves cholesterol efflux and alteration in the sperm membrane proteins (Kadirvel et al. Citation2011), segregation of membrane proteins (Srivastava et al. Citation2013), inactivation of membrane-bound enzymes, decreased lateral protein diffusion within the membrane (Watson Citation1995) and changes in membrane architecture (Talukdar Citation2014). Capacitation-like changes accompanied by premature spontaneous acrosome reaction result in short life span of frozen–thawed spermatozoa (Bailey et al. Citation2000). Cryocapacitation is one of the major factors associated with reduced longevity and poor survivability of cryopreserved spermatozoa in the female reproductive tract (Talukdar et al. Citation2015).
The extender used for semen dilution maintains the biological environment necessary for survival of spermatozoa and prevents membrane damage during cryopreservation. Egg yolk in the semen extender regulates the efflux of integral protein, phospholipids and cholesterol, and thus protects the plasma membrane against temperature-related injury (Forouzanfar et al. Citation2010). Egg yolk is generally used at a concentration of 20% (vol/vol) in the semen extender (Hafez and Hafez Citation2000). In contrast to other farm species, semen dilution in goats with extender containing egg yolk results in poor post-thaw semen quality. Egg yolk reacts with glycoprotein-60 (BUSgp60) in bulbourethral secretion that has a triacylglycerol hydrolase activity that decreases sperm motility by disruption of the cell membrane (Pellicer-Rubio and Combarnous Citation1998) while phospholipase A2 catalyses the hydrolysis of egg yolk phosphatidylcholine into fatty acids and lysophophatidylcholine (LPC). LPC acts like a detergent on biomembrane. Both these interactions stimulate cryocapacitation, acrosomal damage, loss of motility and viability of sperm (Upreti et al. Citation1999). Therefore, to reduce these interactive losses, protocols that include sperm washing (Roca et al. Citation1997) and use of low or high egg yolk concentration (Bispo et al. Citation2011; Naing et al. Citation2011; Ranjan et al. Citation2015) with variable sperm count have been proposed. Sperm washing removes the protective proteins and antioxidative enzymes while the concentration of egg yolk affects the cryoprotective capacity of the extender (Ferreiral et al. Citation2014), indicating a cumulative effect of different extrinsic and intrinsic factors in regulating the semen quality at different steps during cryopreservation. The study was designed to evaluate the effect of egg yolk concentration and sperm washing on sperm quality following cryopreservation in Barbari buck semen.
2. Materials and methods
2.1. Experimental animals and feeding
Five healthy adult male of Barbari goat breed (aged > 2 years, weighing 25–35 kg) were selected according to their reproductive history and andrological examination. Bucks were reared and maintained at the experimental goat sheds, Department of Physiology, U.P. Pandit Deen Dayal Upadhayaya Pashu Chikitsa Vigyan Vishwavidyalaya Evam Go Anusandhan Sansthan, Mathura (UP), India. They were fed on concentrate mixture @ 250 g having DCP 13% and TDN 69% and 1 kg of green fodder (Berseem/Lucerne/Oat) per animal per day, besides 4–6 h of grazing at a nearby field that met the nutritional requirements of breeding buck. The bucks were maintained in a healthy and good body condition throughout the experiment. The work related to cryopreservation and thawing was conducted at the Hi-Tech laboratory Department of Physiology. All the chemicals used were of analytical grade (Sigma Aldrich, Germany)
2.2. Semen collection, initial evaluation and pooling
Semen was collected from each animal twice a week, in different and non-consecutive days, using an artificial vagina (length = 20 cm and diameter = 4.5 cm) according to the method of Memon et al. (Citation1986). A total of six ejaculates were collected from each buck. Immediately after collection the samples were kept in an insulated beaker maintained at 37°C and transported to the laboratory. Semen collected from each buck was initially evaluated for volume, sperm concentration, wave motion and percentage of motile spermatozoa. Only semen samples with live spermatozoa (>80%) progressive motility (>70%) and sperm concentration (>3 × 109 sperm/ml) were selected. Semen was evacuated from the collecting cup using a pipette and pooled in a separate cup maintained at 37°C.
2.3. Semen cryopreservation and thawing
A Tris-based extender (Tris 4.45 g/100 ml, citric acid 2.60 g/100 ml, glucose 0.75 g/100 ml, glycerol 6% (v/v): pH 6.8) was used as the base extender (freezing extender). The pooled sample was divided into three parts. Using a Neubauer chamber, the sperm concentration of the semen sample was determined and extended to the volume required for a final concentration of 100 × 106 sperm/ml. All the procedures were completed within 15 min of semen collection. During the experiment an attempt was made to evaluate the effect of egg yolk concentration and washing at higher dilution in caprine. The lower egg yolk concentration in the semen extender can minimize the lethal interaction and can prevent spermatozoa damage. Therefore Tris glucose extender with 3% egg yolk (3 ml of yolk/100 ml) was taken as T-1 treatment. But low egg yolk concentration in the extender increases the susceptibility of sperm to cryoinjuries. Therefore Tris glucose extender with 20% egg yolk at lower sperm concentration was taken as T-2 treatment. The higher egg yolk concentration (20% egg yolk) can increase the lethal interaction. So washing was attempted to reduce the seminal proteins responsible for lethal interactions in T-3 treatment prior to dilution with the extender containing 20% egg yolk. Thus T-3 had washing with 20% egg yolk, T-2 had 20% egg yolk without washing and T-1 had 3% egg yolk without washing. For the washing process, the semen was diluted with a sodium Ringer lactate solution and subjected to the centrifugation process for removal of the seminal plasma. The semen centrifugation was performed at 1500 rpm for 15 min at 5°C. Later, the semen was diluted with 20% egg yolk Tris glucose diluter. Diluted semen samples were equilibrated at 5°C for 4 h. Equilibrated samples were aspirated into 0.25-ml (medium-sized) French straws sealed with polyvinyl alcohol powder. The straws were frozen in liquid nitrogen vapour, 4 cm above the liquid nitrogen for 15 min and plunged into liquid nitrogen for storage. After being stored for seven days, the frozen straws were thawed individually at 37°C for 40 s in a water bath for microscopic evaluation. The percentage of live spermatozoa, progressive motility, acrosome integrity and capacitation status were evaluated just after dilution, after equilibration and after thawing during the experiment.
2.4. Sperm viability
To ascertain the percentage of live or dead sperma in a semen sample the eosin-nigrosin staining technique was used as per the method described by Hancock (Citation1952). At the time of estimation, one small drop of semen was mixed with 5–6 drops of stain in a cavity block maintained at 37°C. Smears were made on a clean, grease-free slide (at 37°C) and dried in air. At least 200 sperm were counted and identified as live or dead under the oil immersion objective (100x). The stained spermatozoa (eosionophilic) obtained a pinkish colour and were categorized as dead and the unstained ones against a dark background of nigrosin were counted as live.
2.5. Sperm motility
Sperm motility was assessed by placing a drop of semen on a pre-warmed (37°C) glass slide, by phase contrast microscopy (400x).
2.6. Acrosomal integrity
Acrosome integrity was judged by the Giemsa staining technique as per the methodology described by Watson (Citation1975). A thin smear of diluted semen drop was made on a clean, grease-free slide. The smear was air-dried and kept in 5% formaldehyde solution for 30 min at 37°C. The slides were washed in double distilled water and air-dried. In a staining jar, 3 ml of Giemsa solution was added drop by drop in 2 ml Sorenson Phosphate buffer solution and 35 ml of double distilled water was added and the resulting mixture was mixed thoroughly. The slides were kept in the staining jar solution for 4 h at 37°C in an incubator. The stained slides were washed with distilled water, air-dried and 200 spermatozoa per slide were examined under high power of a phase contrast microscope.
2.7. Capacitation status and acrosome reaction status: chlortetracycline (CTC) assay
Capacitation status and acrosome reaction were assessed using CTC staining (Collin et al. Citation2000). The semen sample was washed in phosphate buffered saline (PBS), pH 7.4, and centrifuged (1500 rpm, 15 min) with 100 × 106 spermatozoa/ml. The CTC stock solution containing 750 µM CTC-HCl (Sigma), 130 mM NaCl, 5 mM l-cysteine, 20 mM Tris acid (pH 7.8) was prepared daily, wrapped in foil to protect against light and stored at 4°C until required. Ten microlitres of sperm suspension were mixed with 15 µL of CTC solution on a slide at room temperature. Then, 0.3 µL of 12.5% glutaraldehyde in 2.5 M Tris base was added as a fixative. Samples (in duplicate) were covered with coverslips and stored in the dark at 4°C. A total of 200 sperm per slide were observed within 24 h using a Nikon Eclipse TE 2000-S microscope with phase contrast and epifluorescence optics under blue-violet illumination (excitation at 400–440 nm and emission at 470 nm). Three different patterns, namely, pattern F (uncapacitated sperm) – even distribution of fluorescence over the entire head, pattern B (capacitated, acrosome intact sperm) – fluorescence-free band in the post acrosomal region, fluorescence in anterior portion of the head and pattern AR (acrosome-reacted cells) – fluorescent-free head except for a thin bright fluorescent band along the equatorial segment were observed.
2.8. Data analysis
The data recorded in the experiment were analysed as per the standard statistical procedure and the difference between the mean values were tested by Tukey’s post hoc test standard procedure using SPSS version 14.0.
3. Results
3.1. Fresh ejaculated semen
The seminal attributes of fresh ejaculated Barbari buck semen were studied. The average volume (ml) ranged between 0.62 ± 0.05 and 0.73 ± 0.05, the average mass motility ranged between 3.75 ± 0.11 and 3.83 ± 0.10, average sperm concentration (× 106/ml) ranged between 3670 ± 131.30 and 4121 ± 67.53. The average per cent live spermatozoa ranged between 84.17 ± 1.11 and 86.83 ± 0.70 and per cent intact acrosome ranged between 89.33 ± 0.42 and 91.50 ± 1.39. However, no significant differences were observed for the average values of volume, mass motility, sperm concentration, per cent live spermatozoa and per cent intact acrosome. The semen quality of all the five bucks was statistically similar, and hence pooled for further studies.
3.2. Cryopreserved semen
Viability, motility and acrosomal integrity (as shown in (a) and 1(b)) observed during different stages of cryoprocessing in all the three trial groups have been presented in –. Different pattern, namely, pattern F, pattern B and pattern AR, exhibited by spermatozoa has been presented in . A significant (p ≤ .01) decrease in number of the viable and motile sperm with uncapacitated and intact acrosomal was observed after dilution followed by equilibration and freeze thawing. On comparison of the three treatment groups, significantly (p ≤ .01) higher values for motility and viability were observed in T-3 just after dilution while significantly (p ≤ .01) higher values for acrosomal integrity and pattern F (uncapacitated sperm) were observed in T-2. The recorded values of sperm viability, motility acrosomal integrity and uncapacitated sperm (Pattern F) were significantly (p ≤ .01) higher in T-2 after equilibration and freeze–thawing.
Figure 1. (a, b): Photograph showing normal (intact) and abnormal acrosome (Giemsa stain, Magnification 100x). N: intact acrosome; I: first degree of acrosomal damage showing different degree of ruffled acrosome, II: second degree of acrosomal damage (process of denudation), III: third degree of acrosomal damage (release of acrosomal cap).
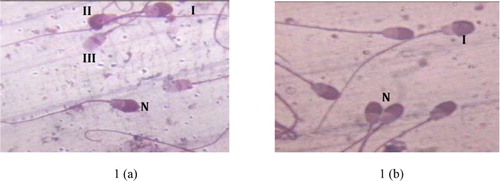
Figure 2 . Effect of different levels of egg yolk, washing and stage of processing on progressive motility of spermatozoa in Barbari Buck semen (100 million/ml).
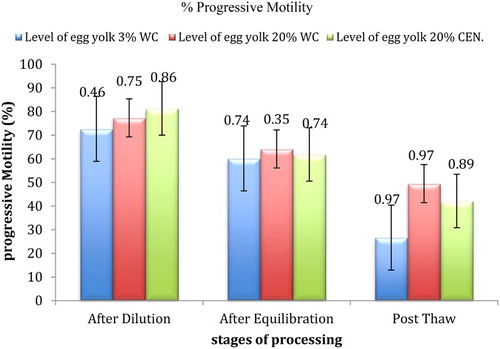
Figure 3. Effect of different levels of egg yolk, washing and stage of processing on per cent live spermatozoa in Barbari Buck semen (100 million/ml).
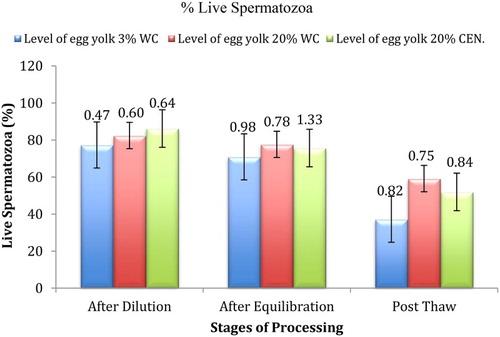
Figure 4. Effect of different levels of egg yolk, washing and stage of processing on spermatozoa with % intact acrosome spermatozoa in Barbari Buck semen (100 million/ml).
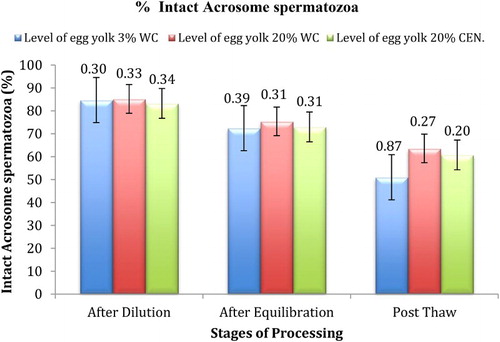
Table 1. Patterns observed (capacitation-like changes) during different stages of processing and addition of different levels of egg yolk in Barbari buck spermatozoa.
4. Discussion
During the process of semen cryopreservation, sperm are exposed to a variable range of temperatures that promotes structural (De Leeuw et al. Citation1990) or functional alterations (Hammerstedt et al. Citation1990). The changes are reflected through mitochondrial and membrane dysfunction. This affects the permeability of the sperm surface to water, ions and cryoprotectant (Hagiwara et al. Citation2009; Oldenhof et al. Citation2010), reducing the number of viable and progressive motile sperm with uncapacitated and intact acrosomal cap prior to artificial insemination (Cormier and Bailey Citation2003). The cryocapacitation and acrosomal cap damage are cellular exocytic-like events that influence the fertilizing ability of sperm, a fundamental prerequisite for successful conception after insemination (Lindsay et al. Citation2005). Egg yolk is an integral component of semen extender that acts as a non-penetrating cryoprotectant and prevents membrane damage. The action of egg yolk may be attributed to phospholipids (Lanz et al. Citation1965), cholesterol (Moce et al. Citation2010) and low-density lipoprotein (LDL) content (Bergeron and Manjunath Citation2006), which afford successful protection to the sperm plasma membrane against cold shock and cryoinjuries (Moussa et al. Citation2002). During the experiment, acrosomal status and capacitation-like changes were evaluated at different egg yolk concentrations and after sperm washing. Although egg yolk imparts cryoprotection, it simultaneously reacts with seminal plasma Glycoprotein-60 and Phospholipase A2 to form lethal compounds that lead to sperm death (Leboeuf et al. Citation2000). Impedance to sperm respiration by egg yolk increases with concentration and may lead to decreased motility and viability (Bencharif et al. Citation2008; Najafi et al. Citation2013). Furthermore, bulbourethral secretions contain Glycoprotein-60 (BUSgp60) with triacylglycerol hydrolase activity that decrease sperm motility and movement quality (Pellicer-Rubio and Combarnous Citation1998). Sperm washing is considered to be beneficial in goats as it eliminates the reactive proteins but the process simultaneously removes the protective proteins and antioxidative enzymes. The process of centrifugation accompanied by reduced antioxidative capacity makes sperm liable to oxidative stress. The exposure of sperm to low and ultra-low temperature results in ultrastructural, biochemical and functional damages to sperm through movement of cholesterol and membrane protein (Purdy Citation2006), thus altering plasma membrane integrity, leading to damaged acrosome and cryocapacitation accompanied by reduced progressive motion and viability. During the experiment a significant (p ≤ .01) decrease in number of the viable and motile sperm with uncapacitated and intact acrosomae was observed after dilution followed by equilibration and freeze–thawing. The decrease may be attributed to temperature variation during cryopreservation that adversely affects the physiology and membrane integrity of sperm (Drobnis et al. Citation1993). Among the three treatment groups, significantly (p ≤ .01) higher values for motility and viability were observed in T-3 just after dilution. It may be the result of sperm washing that removes the reactive seminal proteins preventing cell membrane disruption lethal to sperm. The sperm plasma membrane actively participates in the process of capacitation, mainly through the loss of cholesterol (Talukdar et al. Citation2015). Significantly (p ≤ .01) higher values for acrosomal integrity and pattern F (uncapacitated sperm) in T-2 after dilution are indicative of the protective role of seminal plasma in regulating the level of reactive oxygen species that prevent cholesterol efflux and plasma membrane damage.
During equilibration and freeze–thawing, sperm are exposed to lower temperature that stimulates the efflux of membrane constituents, especially cholesterol, together with increased production reactive oxygen species (Desai et al. Citation2010). Cholesterol efflux represents an integral part of the intrinsic regulatory property of sperm to undergo capacitation-like changes during cryopreservation (Talukdar et al. Citation2016). Loss of cholesterol and integral proteins make the plasma membrane liable to cryoinjury, stimulating acrosomal damage and capacitation-like change in the sperm head (Muller et al. Citation2008). The egg yolk and antioxidative enzymes in extended semen prevent the loss of selective permeability and integrity of the plasma membrane (Ortman and Rodriguez Citation1994), a release of intracellular enzymes (Harrison and White Citation1972) and lipids (Darin-bennett et al. Citation1973), a redistribution of ions (Quinn and White Citation1968), a change in the membranes of the acrosome (Jones and Martin Citation1973) and mitochondria, (Watson, Citation1995) and imparts protection against temperature-related injuries during the freeze–thawing process. After equilibration and freeze–thawing the average values pertaining to sperm viability, motility, acrosomal integrity and uncapacitated sperm (Pattern F) were significantly (p ≤ .01) higher in T-2. Ferreiral et al. (Citation2014) also reported that presence of seminal plasma and higher concentration of egg yolk in extender provides a higher viability in cryopreserved goat semen. The result recorded during the study may attributed to better counter-balance between the different factors that influence the semen quality during the different stages of cryopreservation, reducing oxidative stress and movement of cholesterol and hence maintained plasma membrane integrity, preventing acrosomal damage and capacitation-like changes in the sperm head with improved viability and motility.
5. Conclusion
It may be concluded that incorporation of 20% egg yolk compared to 3% egg yolk or 20% egg yolk after washing in the extender confers better cryoprotection to Barbari buck spermatozoa as reflected in semen quality parameters studied.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bailey JL, Bilodeau JF, Cormier N. 2000. Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon. J Androl. 21:1–8.
- Bencharif D, Amirat L, Anton M, Schmitt E, Desherces S, Delhomme G, Langlois ML, Barriere P, Larrat M, Tainturier D. 2008. The advantages of LDL (low density lipoproteins) in the cryopreservation of canine semen. Theriogenology. 70:1478–1488. doi: 10.1016/j.theriogenology.2008.06.095
- Bergeron A, Manjunath P. 2006. New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Mol Reprod Dev. 73:1338–1344. doi: 10.1002/mrd.20565
- Bispo CAS, Pugliesi G, Galvao P, Rodrigues MT, Ker PG, Filgueiras B, Carvalho GR. 2011. Effect of low and high egg yolk concentrations in the semen extender for goat semen cryopreservation. Small Ruminant Res. 100:54–58. doi: 10.1016/j.smallrumres.2011.05.003
- Collin S, Sirard M, Dufour M, Bailey J. 2000. Sperm calcium levels and chlortetracycline fluorescence patterns are related to the in vivo fertility of cryopreserved bovine semen. J Androl. 21:938–943.
- Cormier N, Bailey JL. 2003. A differential mechanism is involved during heparin and cryopreservation induced capacitation of bovine spermatozoa. Biol Reprod. 69:177–185. doi: 10.1095/biolreprod.102.011056
- Darin-bennett A, Poulos A, White IG. 1973. The effect of cold shock and freeze-thawing on release of phosphiolipids by ram, bull, and boar spermatozoa. Aust J Biol Sci. 26:1409–1420. doi: 10.1071/BI9731409
- De Leeuw FE, Chen HC, Colenbrander B, Verkleij AJ. 1990. Cold-induced ultra structural changes in bull and boar sperm plasma membranes. Cryobiology. 27:171–183. doi: 10.1016/0011-2240(90)90009-S
- Desai NR, Mahfouz R, Sharma R, Gupta S, Agarwal A. 2010. Reactive oxygen species levels are independent of sperm concentration, motility, and abstinence in a normal, healthy, proven fertile man: a longitudinal study. Fertil Steril. 94:1541–1543. doi: 10.1016/j.fertnstert.2009.12.041
- Drobnis EZ, Crowe LM, Berger T, Anchordoguy TJ, Overstreet W, Crowe JH. 1993. Cold shock damage is due to lipid phase transitions in cell membranes, a demonstration using sperm as a model. J Exp Zoo. 265:432–437. doi: 10.1002/jez.1402650413
- Ferreiral VS, Mello MRB, da Fonseca CEM, Dias ACF, Cardoso JM, Silva RB, Martins WP. 2014. Effect of seminal plasma and egg yolk concentration on freezability of goat semen. R Bras Zootec. 43:513–518. doi: 10.1590/S1516-35982014001000001
- Forouzanfar M, Sharafi M, Hosseini SM, Ostadhosseini S, Hajian M, Hosseini L, Abedi P, Nili N, Rahmani HR, Nasr-Esfahani MH. 2010. In vitro comparison of egg yolk-based and soybean lecithin-based extenders for cryopreservation of ram semen. Theriogenology. 73:480–487. doi: 10.1016/j.theriogenology.2009.10.005
- Hafez B, Hafez ESE. 2000. Reproduction in farm animals. 7th ed. New York: Lippincott Williams and Wilkins.
- Hagiwara M, Choi JH, Devireddy RV, Roberts KP, Wolkers WF, Makhlouf A, Bischof JC. 2009. Cellular biophysics during freezing of rat and mouse sperm predicts post-thaw motility. Biol Reprod. 81:700–706. doi: 10.1095/biolreprod.109.076075
- Hammerstedt RH, Graham JK, Nolan JP. 1990. Cryopreservation of mammalian sperm: what we ask them to survive. J Androl. 11:73–88.
- Hancock JL. 1952. The morphology of bull spermatozoa. J Exp Biol. 29:445–453.
- Harrison RAP, White IG. 1972. Glycolytic enzymes in the spermatozoa and cytopalsmic droplets of bull, boar and ram, and their leakage after cold shock. J Reprod Fertil. 30:105–115. doi: 10.1530/jrf.0.0300105
- Januskauskas A, Johannisson A, Rodriguez-Martinez H. 2003. Subtle membrane changes in cryopreserved bull semen in relation with sperm viability, chromatin structure, and field fertility. Theriogenology. 60:743–758. doi: 10.1016/S0093-691X(03)00050-5
- Jones RC, Martin ICA. 1973. The effects of dilution, egg yolk and cooling to 5–8°c in the ultrastructure of ram spermatozoa. J Reprod Fertil. 35:311–320. doi: 10.1530/jrf.0.0350311
- Kadirvel G, Kathiravan P, Kumar S. 2011. Protein tyrosine phosphorylation and zona binding ability of in vitro capacitated and cryopreserved buffalo spermatozoa. Theriogenology. 75:1630–1639. doi: 10.1016/j.theriogenology.2011.01.003
- Lanz RN, Pickett BW, Komarek RJ. 1965. Effect of lipid additives on pre and post-freeze survival of bovine spermatozoa. J Dairy Sci. 48:1692–1697. doi: 10.3168/jds.S0022-0302(65)88553-8
- Leboeuf B, Restall B, Salomon S. 2000. Production and storage of goat semen for artificial insemination. Anim Reprod Sci. 62:113–141. doi: 10.1016/S0378-4320(00)00156-1
- Lindsay G, Evans G, Maxwell WMC. 2005. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology. 63:445–457.
- Memon MA, Bretzlaff KN, Ott RS. 1986. Comparison of semen collection techniques in goats. Theriogenology. 26:823–827. doi: 10.1016/0093-691X(86)90011-7
- Moce E, Blanch E, Tomas C, Graham JK. 2010. Use of cholesterol in sperm cryopreservation: present moment and future prospects. Reprod Domestic Anim. 45:57–66. doi: 10.1111/j.1439-0531.2010.01635.x
- Moussa M, Martinet V, Trimeche A, Tainturier D, Anton M. 2002. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen–thawed bull semen. Theriogenology. 57:1695–1706. doi: 10.1016/S0093-691X(02)00682-9
- Muller K, Muller P, Pincemy G, Kurz A, Labbe C. 2008. Characterization of sperm plasma membrane properties after cholesterol modification: consequences for cryopreservation of rainbow trout spermatozoa. Biol Reprod. 78:390–399. doi: 10.1095/biolreprod.107.064253
- Naing SW, Haron AW, Goriman MAK, Yusoff R, Bakar MZA, Sarsaifi K, Bukar MM, Thein M, Kyaw T, San MM. 2011. Effect of Seminal Plasma Removal, Washing Solutions, and Centrifugation Regimes on Boer Goat Semen Cryopreservation. Pertanika J Trop Agri Sci. 34:271–279.
- Najafi A, Zhandi M, Towhidi A, Sharafi M, Sharif AA, Motlagh MK, Martinez-Pastor F. 2013. Trehalose and glycerol have a dose-dependent synergistic effect on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology. 66:275–282. doi: 10.1016/j.cryobiol.2013.03.002
- Oldenhof H, Friedel K, Sieme H, Glasmacher B, Wolkers WF. 2010. Membrane permeability parameters for freezing of stallion sperm as determined by Fourier transform infrared spectroscopy. Cryobiology. 61:115–122. doi: 10.1016/j.cryobiol.2010.06.002
- Ortman K, Rodriguez, MH. 1994. Membrane damage during dilution, cooling and freezing-thawing of boar spermatozoa packaged in plastic bags. Vet Med A-Zbl Vet A – Physiol. 41:37–47.
- Pellicer-Rubio MT, Combarnous Y. 1998. Deterioration of goat spermatozoa in skimmed milk-based extenders as a result of oleic acid released by the bulbourethral lipase BUSgp60. J Reprod Fertil. 112:95–105. doi: 10.1530/jrf.0.1120095
- Purdy PH. 2006. A review on goat sperm cryopreservation. Small Ruminant Res. 63:215–225. doi: 10.1016/j.smallrumres.2005.02.015
- Quinn PJ, White IG. 1968. The effect of pH, cations and protective agents on the susceptibility of ram spermatozoa to cold shock. Exp Cell Res. 49:31–39. doi: 10.1016/0014-4827(68)90516-8
- Ranjan R, Goel, AK, Ramachandean N, Kharche SD, Jindal SK. 2015. Effect of egg yolk and equilibration period on freezability of jamunapari buck semen. Ind J Small Ruminant Res. 21:32–36. doi: 10.5958/0973-9718.2015.00027.6
- Roca J, Carrizosa JA, Campos I, Lafuente A, Vazquez JM, Martinez E. 1997. Viability and fertility of unwashed Murciano-Granadina goat spermatozoa diluted in Tris-egg yolk extender and stored at 5 °C. Small Ruminant Res. 25:147–153.
- Srivastava N, Jerome A, Srivastava SK, Ghosh SK, Amit K. 2013. Bovine seminal PDC–109 protein: a review of biochemical and functional properties. Anim Reprod Sci. 138:1–13. doi: 10.1016/j.anireprosci.2013.02.008
- Talukdar DJ. 2014. Morphological and functional characterization of in vitro capacitated and frozen thawed Swamp buffalo spermatozoa [Ph.D. thesis]. Guwahati, India: Assam Agricultural University.
- Talukdar DJ, Ahmed K, Deka BC, Sinha S, Deori S, Das GC. 2016. Cryo-capacitation changes during cryopreservation of swamp buffalo spermatozoa. Ind J Anim Sci. 86(4):397–400.
- Talukdar DJ, Ahmed K, Talukdar P. 2015. Cryocapacitation and fertility of cryopreserved semen. Int J Livestock Res. 5:11–18.
- Upreti GC, Hall EL, Koppens D, Oliver JE, Vishwanath R. 1999. Studies on the measurement of phospholipaseA2 (PL A2) and PL A2 inhibitor activities in ram semen. Anim Repord Sci. 56:107–121. doi: 10.1016/S0378-4320(99)00033-0
- Watson PF. 1975. Use of Geimsa stain to detect changes in acrosomes of frozen ram spermatozoa. Vet Res. 97:12–15.
- Watson PF. 1995. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fertil Dev. 7:871–891. doi: 10.1071/RD9950871
