ABSTRACT
The study evaluated the effect of betaine and ascorbic acid (AA) administration on the erythrocyte osmotic fragility (EOF) and malondialdehyde (MDA) concentration of broiler chickens during the hot-dry season. Eighty day-old broilers were divided into four groups: Group I (control) given sterile water; Group II, betaine (250 mg/kg); Group III, AA (50 mg/kg); and Group IV, betaine (250 mg/kg) + AA (50 mg/kg) orally for 42 days. Blood samples were collected from each bird with and without anticoagulant, sodium ethylenediaminetetraacetate, on days 21 and 42, for the determination of EOF and serum MDA concentrations. The dry-bulb temperature (DBT), relative humidity and temperature-humidity index (THI) recorded were 28.33–35.67°C, 69.00–93.00% and 28.18–34.82, respectively. The results showed that betaine + AA (7.78 ± 1.66%) significantly (P < .05) reduced EOF, compared to control birds (16.27 ± 9.35%) at 0.7% on day 21. MDA concentrations of broiler chickens in the betaine (1.37 ± 0.038 nmol/L), AA (1.41 ± 0.039 nmol/L) and betaine + AA (1.41 ± 0.040 nmol/L) groups during the experimental period were significantly (P < .05) lower when compared with that of the control group (1.54 ± 0.043 nmol/L). It is concluded that the co-administration of betaine and AA to broiler chickens decreased EOF and MDA during the hot-dry season.
1. Introduction
Broiler chicken production under high ambient temperature (AT) and high relative humidity (RH) has been shown to induce heat stress (Ali et al. Citation2010). Heat stress results in the excess production of reactive oxygen species (ROS) and, consequently, oxidative damage (Zhang et al. Citation2014). Under such adverse conditions, the body is not able to synthesize the enzymes required to destroy ROS or repair the damage. The hot-dry season, characterized by high AT and high RH, in the Northern Guinea Savannah zone has been shown to be thermally stressful to poultry (Sinkalu et al. Citation2015).
Erythrocyte osmotic fragility (EOF) is influenced by temperature (Oyewale et al. Citation2011). It has been demonstrated to be a biomarker of oxidative stress (Adenkola & Ayo Citation2009), and a diagnostic tool for evaluating transport-induced oxidative stress in quail (Minka & Ayo Citation2013) and rabbits (Ayo et al. Citation2015). Erythrocyte membrane could be damaged by lipid peroxidation, resulting from increased generation of ROS (Azeez et al. Citation2012), and measured by the malondialdehyde (MDA) concentration (Roberts & Sindhu Citation2009). Increased MDA concentration is involved in the pathogenesis of certain conditions in broiler chickens such as aflatoxicosis (Chen et al. Citation2013), structural injury and decreased immune function of the spleen due to low selenium intake (Peng et al. Citation2012).
Betaine is a powerful osmostress protectant (Bremer Citation2014) and a metabolite of choline (Lever & Slow Citation2010; Mitsuya et al. Citation2013). It is synthesized from choline by choline oxidase in the liver (Igwe et al. Citation2015). It plays a vital role in the integrity of cell membranes, methylation reactions and memory development (Hogeveen et al. Citation2013). Ascorbic acid (AA, vitamin C) is primarily synthesized in the chicken by the kidneys, but during heat stress, endogenous AA becomes insufficient to meet the bird’s requirements (Abidin & Khatoon Citation2013). AA supplementation to poultry, including broiler chickens, improves feed intake, feed efficiency, weight gain, nutrient digestibility, immune response and antioxidant status (Khan et al. Citation2012). However, Jena et al. (Citation2013) demonstrated that combined administration of two antioxidants, namely, vitamins C and E, enhanced erythrocytic antioxidant status more than their individual administrations in coloured broiler breeder hens under the stressful hot-humid conditions. Although studies have been conducted on the single effects of betaine and, especially, AA in broiler production, there is a paucity of information on their combined effects following heat stress on broiler chicken.
The aim of the study was to evaluate the ameliorative effects of the co-administration of betaine and AA on EOF and MDA concentration in broiler chickens during the hot-dry season.
2. Materials and methods
2.1. Experimental site and meteorological conditions
The experiment was performed at a poultry house in Samaru, Zaria (11°10′N, 07°38′E), located in the Northern Guinea Savannah zone of Nigeria. It was carried out from April to May 2013, during the hot-dry season (Dzenda et al. Citation2013). The AT was obtained using the dry- and wet-bulb thermometer (Esal Scientific Industries, Delhi, India). RH was obtained using Osmon’s hydrometric table (Narindra Scientific Industries, Haryana, India). The THI was determined using the following formula (Tao & Xin Citation2003):where THIbroiler = temperature-humidity index for broilers, Tdb = dry-bulb temperature and Twb = wet-bulb temperature.
The DBT, RH and THI ranges during the study period were 28.33–35.67°C, 69.00–93.00% and 28.18–34.82, respectively.
2.2. Experimental animals, grouping and management
Eighty birds, purchased at day-old from a commercial farm, were used for the experiment. The broiler chickens were fed with commercial feed (broiler pre-starter (days 0–14), broiler starter (days 15–28) and broiler finisher (days 29–42)) (). They were vaccinated against infectious bursal disease (at days 7 and 14) using Gumboro vaccine, and against Newcastle disease (at day 21) using Lasota vaccine. All vaccines were administered via drinking water. The birds were housed in the same pen, littered with wood shavings on a concrete floor and having a zinc roof with cardboard ceiling. The dimension of the pen was 8.4 m × 5.6 m × 1.91 m and the birds were stocked at 0.35 m2 per bird. The pen was partitioned using plywood into four cubicles to house each group. It was provided with biosecurity measures, including a footbath and non-accessibility to non-essential persons, animals, birds and rodents. Farm wears such as footwear and farm clothing were provided for persons assisting in carrying out the experiments. The birds were divided into four groups (Groups I–IV). Group I, which served as the control group, was given only sterile water. Group II was administered with betaine hydrochloride only at 250 mg/kg (Pillai et al. Citation2006), Group III was given AA only at 50 mg/kg (Sinkalu et al. Citation2008), while Group IV (betaine + AA) was co-administered with betaine and AA at 250 mg/kg and 50 mg/kg, respectively. Both betaine hydrochloride and AA were given daily by oral route, using gavage, to each bird, starting from day-old for 42 days, immediately after which the study was terminated.
Table 1. Proximate analysis of broiler diet during the study period.
2.3. Experimental measurements
2.3.1. Experimental drugs and their preparation
The AA (Sigma Chemical, St. Louis, MO, USA) was dissolved in 5 mL of distilled water and administered at the dose of 50 mg/kg, while the content of betaine hydrochloride capsules (Twinlab, Isi Brands Incorporated, American Fork, UT, USA), after carefully removing the capsules, was dissolved in 5 mL of distilled water and administered at 250 mg/kg. Betaine and AA were administered during the morning period at 06:00 h and 07:00 h, to avoid excessive stress on the birds. Four trained persons administered the agents to each group, and it took 15 s to administer each of the agents to the birds in each experimental group.
2.3.2. Collection of blood and serum samples
Blood sample (3 mL) was collected on days 21 and 42, from the wing vein of each bird into a test tube containing an anticoagulant, sodium ethylenediaminetetraacetate, and without an anticoagulant (Maini et al. Citation2007). For determining EOF, blood samples collected in sample bottles containing anticoagulant Ethylenediaminetetraacetic acid at concentration of 1.5 mg/mL (Robertson & Maxwell Citation1993) were used, while serum samples (0.5 mL) harvested from 1.5 mL of blood samples in sample bottles without anticoagulant were used to evaluate serum MDA. Blood samples for evaluating MDA levels were centrifuged at 3000g for 10 min and the serum harvested and stored at 4°C until assay (Ramnath et al. Citation2008).
2.3.3. Determination of EOF
The samples were transported to the laboratory for the determination of EOF and MDA concentration. The EOF test was performed as described by Oyewale (Citation1992), during the morning period at 08:00 h and 12:00 h. Briefly, 0.02 mL of blood was added to tubes containing increasing concentrations (0%, 0.1%, 0.3%, 0.5%, 0.7% and 0.9%) of phosphate-buffered sodium chloride (NaCl) solution at pH 7.4. The tubes were gently mixed and incubated at room temperature (26–28°C) for 30 min. The content of each tube was centrifuged at 150×g for 10 min and the supernatant decanted. Optical density (OD) of the supernatant was determined using a spectrophotometer (Spectronic-20, Philip Harris Limited, Shenstone, England) at the wavelength of 540 nm. Haemolysis in each tube was expressed in percentage, taking haemolysis in distilled water (0% NaCl) as 100%where OD = optical density.
2.3.4. Evaluation of serum MDA concentration
Serum MDA concentration was evaluated as an index of lipid peroxidation as described by Janero (Citation1990). Briefly, serum sample (100 µL) was mixed with sodium dodecyl sulphate-acetate buffer (pH 3.5) and an aqueous solution of thiobarbituric acid. After heating at 95°C for 60 min, the red pigment produced was extracted with an n-butanol–pyridine mixture and estimated by measuring the absorbance at 532 nm. Tetramethoxy-propane was used as an external standard and MDA concentration was expressed in nmol/L.
2.4. Statistical analysis
The data obtained were expressed as mean ± standard error of the mean, using Graphpad 4.0 for windows (San Diego, CA, USA). The values obtained were subjected to statistical analysis using repeated-measures analysis of variance, followed by Tukey’s post hoc test. Values of P < .05 were considered significant (Snedecor & Cochran Citation1994).
3. Results and discussion
3.1. Determination of EOF
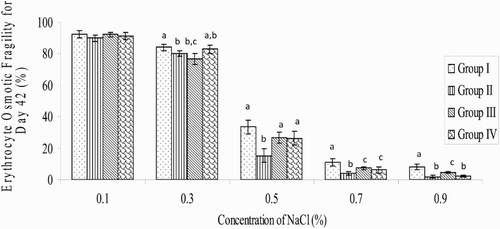
On day 21 and at 0.7% NaCl concentration, the decrease in EOF in all the treated groups (9.35 ± 2.71%; 6.98 ± 2.47% and 7.78 ± 1.66% for betaine, AA and betaine + AA groups, respectively), when compared respectively with the control group (16.27 ± 9.35%), was significant (P < .05). There were no significant decreases in percentage EOF at 0.5% NaCl concentration in the experimental groups, when compared with that of the control (). On day 42, at 0.7% NaCl concentration the percentage of EOF (3.97 ± 1.05%) decreased significantly (P < .05) in the betaine group, relative to the value in the control group (11.04 ± 2.09%). The EOF values of 6.23 ± 2.01% and 7.36 ± 0.97% obtained in betaine + AA and AA-treated groups, respectively, did not differ, when compared to the value in the control group (11.04 ± 2.09%). At 0.5% NaCl concentration, the EOF value (15.14 ± 4.38%) recorded in the betaine group decreased significantly (P < .01) when compared to the value (33.58 ± 4.48%) obtained in the control group ().
Figure 1. Variations in EOF of broiler chickens on day 21 of the study period (n = 20) a,b,cMeans with different superscript letters are significantly (P < .05) different; Group I = control; Group II = betaine administration; Group III = AA administration; Group IV = co-administration with betaine and AA.
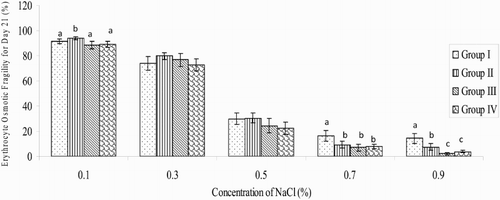
Figure 2. Variation in EOF of broiler chickens on day 42 during the study period (n = 20) a,b,cMeans with different superscript letters are significantly (P < .05) different; Group I = control; Group II = betaine administration; Group III = AA administration; Group IV = co-administration with betaine and AA.
3.2. Variations in MDA concentration
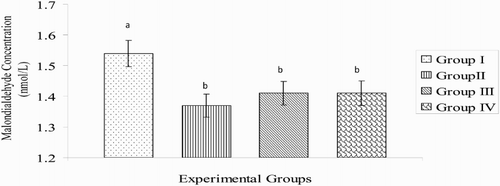
The MDA concentration in broiler chickens administered with AA (1.31 ± 0.04 nmol/L) was significantly (P < .05) lower than that recorded in the control group (1.50 ± 0.066 nmol/L). However, the decreases in MDA concentrations in the betaine (1.43 ± 0.036 nmol/L) or betaine + AA (1.40 ± 0.062 nmol/L) groups were not significant (P > .05) on day 21 of the experimental period (). On day 42, the MDA concentration was lower (P < .05) in the betaine (1.31 ± 0.068 nmol/L) group than in the control (1.59 ± 0.055 nmol/L) and in any of the treatment groups. The MDA concentration in betaine + AA group (1.41 ± 0.055 nmol/L) was significantly (P < .05) lower than that of the control (1.59 ± 0.055 nmol/L) or AA-treated group (1.51 ± 0.040 nmol/L) (). Overall, the MDA concentration of broiler chickens in any of the treatment groups during the experimental period was significantly (P < .05) lower when compared with that of the control group ().
Figure 3. Variations in MDA concentrations of broiler chickens on days 21 and 42 of the study period (n = 7). a,b,cMeans with different superscript letters are significantly (P < .05) different; Group I = control; Group II = betaine administration; Group III = AA administration; Group IV = co-administration with betaine and AA.
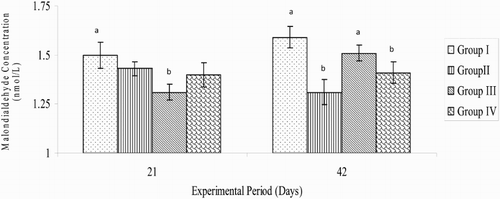
Figure 4. Fluctuations in MDA concentrations of broiler chickens during the study period (n = 7). a,bMeans with different superscript letters are significantly (P < .05) different; Group I = control; Group II = betaine administration; Group III = AA administration; Group IV = co-administration with betaine and AA.
4. Discussion
From the results of the present study, it can be observed that the DBT values obtained were outside the thermoneutral zone for broiler chickens above four weeks old, which is 18–21°C (Aengwanich & Simaraks Citation2004). The RH values obtained were predominantly outside the optimal range of 65–70% for broilers between the ages of three and seven weeks (Akyuz & Boyaci Citation2010). The findings of the present study agree with that of de Olivieira et al. (Citation2013), who showed that the optimum AT and RH values for broiler chickens are 21°C and 74%, respectively. The high THI recorded in the present study, with values exceeding 20.8 (Purswell et al. Citation2012), showed that the thermal environmental conditions were stressful to the broiler chickens. This thermally stressful condition is classical of the hot-dry season in the zone, and increases the risk of adverse effects of heat stress on the well-being of the birds (Sinkalu et al. Citation2008). This agrees with the finding of Wahab et al. (Citation2010), who showed that heat stress increases the risk of haemolysis in rats during the hot-dry season. The results of the present study show that heat stress induced significant haemolysis in broiler chickens. The findings of the present study also agree with those of Sinkalu et al. (Citation2015), who showed that high thermal environmental conditions in poultry housing adversely affect the EOF of broiler chickens during the hot-dry season in the zone. THI is an index of thermal comfort. It integrates the effects of temperature and humidity and may be used as a tool to predict the effects of thermal conditions on the performance of broiler chickens (Purswell et al. Citation2012).
The damage of the erythrocyte membrane may be due to the established finding that heat stress increases ROS generation (Ramnath et al. Citation2008), resulting in lipid peroxidation. Haemolysis may also be due to the denaturing of plasma membrane proteins during heat stress (Jóźwiak & Leyko Citation1992). Furthermore, in birds, including broiler chickens, heat stress induces loss of carbon dioxide through respiration, bicarbonate ions due to panting and monovalent cations (especially sodium and potassium) through urine, altering the acid–base balance (Ahmad & Sarwar Citation2006). The imbalance in pH of the erythrocyte environment adversely affects EOF (Oyewale et al. Citation2011). Erythrocytes of heat-stressed broiler chickens are longer and thinner than those not subjected to heat stress (Maxwell et al. Citation1992). These may necessitate the administration of antioxidants such as betaine and/or AA to broiler chickens under heat stress conditions.
The results of the present study demonstrate that the co-administration of betaine and AA exerted antioxidant effects on the erythrocyte membrane of heat-stressed broiler chickens during the hot-dry season in the zone. Betaine has been described to show antioxidant potentials on the carcass of broiler chickens reared under heat stress conditions (Alirezaei et al. Citation2012). It has been demonstrated to have a dipolar zwitterion potential, resulting in its osmoprotective properties at the cellular level. It is also involved in transmethylation reactions in organisms. Betaine as a methyl donor provides its labile methyl groups for the synthesis of several metabolically active substances such as creatine and carnitine (Eklund et al. Citation2005). The physiological and biochemical potentials of AA are due to its action as an electron donor. The ability to donate one or two electrons makes it an excellent reducing agent and antioxidant (Du et al. Citation2012). AA has also been demonstrated to exert antioxidant effects in pullets (Minka & Ayo Citation2010) and ostrich chicks (Minka & Ayo Citation2012) transported under thermally stressful hot-dry season. However, the effects of the co-administration of betaine and AA, which was the emphasis of the present study, indicate that both agents (betaine and AA) exerted their antioxidant effect on day 21 of the experimental period. This was evidenced by a significantly lower EOF observed in the broiler chickens administered with betaine + AA, as well as a decrease in the MDA value obtained in the same combination group. This finding is in agreement with those of Bai et al. (Citation2014), who showed that the combination of antioxidants had better results than their individual administrations.
Furthermore, the antioxidant effect of the combination of betaine and AA was apparent in the broiler chickens on day 42 of the experimental period, evidenced by a lower EOF at 0.7% and decreased MDA concentration in the birds treated with betaine + AA. This further demonstrates that the combined administration of betaine and AA to heat-stressed broiler chickens may confer some protection on the birds against the adverse effects of the thermally stressful hot-dry season in the zone. This finding is in agreement with the results obtained by Azeez et al. (Citation2011), who demonstrated that AA and/or vitamin E decreased the adverse effects of oxidative stress on the erythrocyte membrane of domestic chickens. The ability of both betaine and AA to reduce the adverse effects of heat stress-induced oxidative damage on the erythrocyte membrane may be due to their ability to scavenge ROS, generated in excess during thermally stressful conditions in the Northern Guinea Savannah zone. The antioxidant effect of both agents (betaine + AA) is beneficial in reducing haemolysis, thereby increasing the oxygen-carrying capacity of the erythrocytes in broiler chickens, reared under heat stress conditions.
5. Conclusion
In conclusion, the co-administration of betaine and AA decreased EOF and MDA in broiler chickens reared during the hot-dry season.
Acknowledgements
The contribution of the staff of the Research Laboratory of the Department of Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria, is acknowledged with thanks.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abidin Z, Khatoon A. 2013. Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. World’s Poult Sci J. 69(1):135–152. doi: 10.1017/S0043933913000123
- Adenkola AY, Ayo JO. 2009. Effect of road transportation on erythrocyte osmotic fragility of pigs administered ascorbic acid during the harmattan season in Zaria, Nigeria. J Cell Anim Biol. 3:4–8.
- Aengwanich W, Simaraks S. 2004. Pathology of heart, lung, liver and kidney in broiler under chronic heat stress. Songklanakarin J Sci Technol. 26:417–424.
- Ahmad T, Sarwar M. 2006. Dietary electrolyte balance: implications in heat stressed broilers: review article. World’s Poult Sci J. 62(4):638–653.
- Akyuz A, Boyaci S. 2010. Determination of heat and moisture balance for broiler house. J Anim Vet Adv. 9(14):1899–1901. doi: 10.3923/javaa.2010.1899.1901
- Ali MN, Qota EMA, Hassan RA. 2010. Recovery from adverse effects of heat stress on slow-growing chicks using natural antioxidants without or with sulphate. Int J Poult Sci. 9(2):109–117. doi: 10.3923/ijps.2010.109.117
- Alirezaei M, Gheisari HR, Ranjbar VR, Hajibemani A. 2012. Betaine a promising antioxidant agent for enhancement of broiler meat quality. Br Poult Sci. 53(5):699–707. doi: 10.1080/00071668.2012.728283
- Ayo JO, Minka NS, Hussein KA. 2015. Effect of ascorbic acid administration on erythrocyte osmotic fragility in rabbits (Oryctolagus cuniculus) subjected to road transportation. J Appl Anim Res. 43(1):26–32. doi: 10.1080/09712119.2014.887009
- Azeez OA, Oyagbemi AA, Iji OT. 2012. Haematology and erythrocyte osmotic fragility indices in domestic chicken following exposure to a polyvalent iodophorous disinfectant. Jordan J Biol Sci. 5(2):99–103.
- Azeez OI, Oyagbemi AA, Oyewale JO. 2011. Erythrocyte membrane stability after transportation stress in the domestic chicken as modulated by pretreatment with vitamins C and E. J Anim Vet Adv. 10(10):1273–1277. doi: 10.3923/javaa.2011.1273.1277
- Bai H, Liu R, Chen H, Zhang W, Wang X, Zhang X, Li W. 2014. Enhanced antioxidant effect of caffeic acid phenethyl ester and trolox in combination against radiation induced-oxidative stress. Chemico-Biol Interact. 207:7–15. doi: 10.1016/j.cbi.2013.10.022
- Bremer E. 2014. Liberate and grab it, ingest and digest it: the gbdr regulon of the pathogen pseudomonas aeruginosa. J Bacteriol. 196(1):3–6. doi: 10.1128/JB.01243-13
- Chen J, Chen K, Yuan S, Peng X, Fang J, Wang F, Cui H, Chen Z, Yuan J, Geng Y. 2013. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicol Ind Health. doi:10.1177/0748233713500819
- de Olivieira WP, de Oliveira RF, Donzele J, Neto AR, Gomes PC, Maia AP, Campos PHR, Gasparino E. 2013. Dietary crude protein reduction on growth and carcass performance of 22 to 42-day-old broilers reared under different temperatures. Revista Brasileira de Zootecnia. 42(8):599–604. doi: 10.1590/S1516-35982013000800010
- Du J, Cullen JJ, Buettnera GR. 2012. Ascorbic acid: chemistry, biology and the treatment of cancer: review. Biochim Biophys Acta – Rev Cancer. 1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003
- Dzenda T, Ayo JO, Lakpini CAM, Adelaiye AB. 2013. Seasonal, sex and liveweight variations in feed and water consumptions of adult captive african giant rat (Cricetomys gambianus, waterhouse-1840) kept individually in cages. J Anim Physiol Anim Nutr. 97:465–474. doi: 10.1111/j.1439-0396.2012.01287.x
- Eklund M, Bauer E, Wamatu J, Mosenthin R. 2005. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 18:31–48. doi: 10.1079/NRR200493
- Hogeveen M, Heijer M, Semmekrot BA, Sporken JM, Ueland PM, Blom HJ. 2013. Umbilical choline and related methylamines betaine and dimethylglycine in relation to birth weight. Pediatr Res. 73:783–787. doi: 10.1038/pr.2013.54
- Igwe IR, Okonkwo CJ, Uzoukwu UG, Onyenegecha CO. 2015. The effect of choline chloride on the performance of broiler chickens. Annu Res Rev Biol. 8(3):1–8. doi: 10.9734/ARRB/2015/19372
- Janero DR. 1990. Malondialdehyde and thiobarbituric acid reactivity as diagnositic indexes of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 9:515–540. doi: 10.1016/0891-5849(90)90131-2
- Jena BP, Panda N, Patra RC, Mishra PK, Behura NC, Panigrahi B. 2013. Supplementation of vitamin E and C reduces oxidative stress in broiler breeder hens during summer. Food Nutr Sci. 4:33–37. doi: 10.4236/fns.2013.48A004
- Jóźwiak Z, Leyko W. 1992. Role of membrane components in thermal injury of cells and development of thermotolerance: review. Int J Radiat Biol. 62(6):743–756. doi: 10.1080/09553009214552701
- Khan RU, Naz S, Nikousefai Z, Selvaggi M, Laudadio V, Tufarell V. 2012. Effect of ascorbic acid in heat stressed poultry. World Poult Sci J. 68:477–490. doi: 10.1017/S004393391200058X
- Lever M, Slow S. 2010. The clinical significance of betaine, an osmolyte with a key-role in methyl group metabolism. Clin Biochem. 43:732–744. doi: 10.1016/j.clinbiochem.2010.03.009
- Maini S, Rastogi SK, Korde JP, Madan AK, Shukla SK. 2007. Evaluation of oxidative stress and its amelioration through certain antioxidants in broilers during summer. J Poult Sci. 44:339–347. doi: 10.2141/jpsa.44.339
- Maxwell MH, Robertson GW, Mitchell MA, Carlisle AJ. 1992. The fine structure of broiler chicken blood cells, with particular reference to basophils, after severe heat stress. Comp Haematol Int. 2(4):190–200. doi: 10.1007/BF00216094
- Minka NS, Ayo JO. 2010. Behavioral and rectal temperature responses of black harco pullets administered vitamins C and E and transported by road during the hot-dry season. J Vet Behav: Clin Appl Res. 5(3):134–144. doi: 10.1016/j.jveb.2009.12.022
- Minka NS, Ayo JO. 2012. Modulatory effect of ascorbic acid on physiological responses of transported ostrich chicks. Onderstepoort J Vet Res. 79(1):1–7.
- Minka NS, Ayo JO. 2013. Physiological and behavioural responses of goats to 12-hour road transportation, lairage and grazing periods, and the modulatory role of ascorbic acid. J Vet Behav. 8(5):349–356. doi: 10.1016/j.jveb.2013.01.001
- Mitsuya S, Kozaki K, Takabe T. 2013. Tissue localisation of the glycine betaine biosynthetic enzymes in Barley leaves. Plant Prod Sci. 16(2):117–122. doi: 10.1626/pps.16.117
- Oyewale JO. 1992. Effect of temperature and pH on osmotic fragility of erythrocytes of the domestic fowl (Gallus domesticus) and Guinea-fowl (Numida meleagris). Res Vet Sci. 52:1–4. doi: 10.1016/0034-5288(92)90049-8
- Oyewale J, Dzenda T, Yaqub L, Akanbi D, Ayo J, Owoyele O, Minka N, Dare T. 2011. Alterations in the osmotic fragility of camel and donkey erythrocytes caused by temperature, pH and blood storage. Vet Arhiv. 81(4):459–470.
- Peng X, Cui H, Fang J, Zuo Z, Deng J, Pan K, Lai W, Zhou Y. 2012. Low selenium diet alters cell cycle phase, apoptotic population and modifies oxidative stress markers of spleens in broilers. Biol Trace Element Res. 148(2):182–186. doi: 10.1007/s12011-012-9357-1
- Pillai PB, Fanatico AC, Beers KW, Blair ME, Emmert JL. 2006. Homocysteine remethylation in young broilers fed varying levels of methionine, choline, and betaine. Poult Sci. 85:90–95. doi: 10.1093/ps/85.1.90
- Purswell JL, Dozier CD, Olarenwaju AA, Davis JD, Yin H, Gates RS. 2012. Effect of temperature-humidity index on live performance in broiler chickesn grown from 49 to 63 days of age. ASAE conference presentation, 9th international livestock environment symposium; July 8–12; Valencia. p. 1–9.
- Ramnath V, Rekha PS, Sujatha KA. 2008. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by brahma rasayana. Evid Based Complement Altern Med. 5(1):77–84. doi: 10.1093/ecam/nel116
- Robertson GW, Maxwell MH. 1993. Importance of optimal mixtures of EDTA anticoagulant: blood for preparation of well-stained avian blood smears. Br Poult Sci. 34:615–617. doi: 10.1080/00071669308417617
- Roberts CK, Sindhu KK. 2009. Oxidative stress and metabolic syndrome. Life Sci. 84:705–712. doi: 10.1016/j.lfs.2009.02.026
- Sinkalu VO, Ayo JO, Abimbola AA, Ibrahim JE. 2015. Effects of melatonin on cloacal temperature and erythrocyte osmotic fragility in layer hens during the hot-dry season. J Appl Anim Res. 43(1):52–60. doi: 10.1080/09712119.2014.888003
- Sinkalu VO, Ayo JO, Adelaiye AB, Hambolu JO. 2008. Diurnal fluctuations in rectal temperature of black harco pullets administered ascorbic acid during the hot-dry season in Nigeria. Int J Meteorol. 33(334):327–333.
- Snedecor GW, Cochran WG. 1994. Statistical methods. Calcutta: Oxford and IBH Publishing Corporation. 509 pp.
- Tao X, Xin H. 2003. Acute synergistic effects of air temperature, humidity and velocity on homeostasis of market-size boilers. Trans Am Soc Agric Biol Eng. 46(2):491–497.
- Wahab AA, Mabrouk MA, Ayo JO, Ambali SF, Shittu M, Adenkola AY, Salawu EO. 2010. Effects of co-administrationof antioxidants on erythrocyte osmotic fragility of wistar rats during the hot-dry season. Eur J Sci Res. 46(1):073–079.
- Zhang B, Guo X, Chen J, Zhao Y, Cong X, Jiang Z, Cao R, Cui K, Gao S, Tian W. 2014. Saikosaponin-D attenuates heat stress-induced oxidative damage in llc-pk1 cells by increasing the expression of anti-oxidant enzymes and hsp72. Am J Chin Med. 42(5):1261–1277. doi: 10.1142/S0192415X14500797
