ABSTRACT
An eight-week study was conducted on silvery-black porgy (Sparidentex hasta) juveniles to evaluate four isoproteic, isolipidic and isoenergetic different diets (50% crude protein, 20% crude lipids, 18.5 MJ kg−1) containing graded levels of soybean lecithin (SBL) (0, 30, 60 and 90 g kg−1 diet) at the expense of fish oil (FO). Fish fed the 60 g SBL kg−1 diet had significantly higher weight gain (32.4%) and feed intake (8.8 g fish−1) than the control group (SBL 0) (P < 0.05). The fillet fatty acid (FA) profiles were correlated with the FA profile of the experimental diets. Fish fed with SBL-supplemented diets had higher fillet phosphatidylcholine levels than the control group (P < 0.05). Plasma total immunoglobulin was higher in fish fed 60 and 90 g SBL kg−1 diets than in the other groups (P < 0.05). Total protease activity was higher in fish fed the 90 g SLB kg−1 diet than other treatments (P < 0.05). Results indicated that substitution of dietary FO with SBL diet up to 67% (60 g SLB kg−1 diet) improved somatic growth performance and profoundly affected the fillet fatty acid profile in silvery-black porgy juveniles.
Introduction
Phospholipids (PLs) play a major role in maintaining the structure, integrity, fluidity and function of cellular membranes (Tocher et al. Citation2008). Dietary PLs have been reported to improve growth performance, survival rates, stress resistance and digestive functions in different fish species, both in larvae and early juveniles, and can decrease the incidence of skeletal deformities at larval stages (see reviews by Coutteau et al. Citation1997; Tocher et al. Citation2008; Cahu et al. Citation2009). In addition, PLs by stimulating lipoprotein synthesis in enterocytes can enhance lipid transport, improve the intestinal absorption of long-chain polyunsaturated fatty acids (LC-PUFA) and reduce intestinal steatosis (Fontagné et al. Citation2000; Gisbert et al. Citation2005; Tocher et al. Citation2008). In this regard, soybean lecithin (SBL) because of its high availability and reasonable price in comparison to marine PL sources has been commercially used as a ubiquitous source of PLs in aquafeeds (Tocher et al. Citation2008). From a nutritional point of view, SBL may also serve as a feed attractant, providing vitamins and EFAs that are vital for fish growth (see reviews by Coutteau et al. Citation1997; Tocher et al. Citation2008; Cahu et al. Citation2009). Several studies conducted in different fish species have reported positive effects of dietary SBL supplementation on growth performance (Kenari et al. Citation2011; Kumar et al. Citation2012; Taylor et al. Citation2015), digestive processes (Hamza et al. Citation2008; Kenari et al. Citation2011; Adel et al. Citation2017) and antioxidant enzyme activities (Gao et al. Citation2014; Kumar et al. Citation2014; Adel et al. Citation2017), as well as stress and disease resistance (Kumar et al. Citation2012, Citation2014; Adel et al. Citation2017).
Silvery-black porgy S. hasta is recognized as one of the most promising candidates for promoting mariculture activities in the south of Iran. Thus, considerable research has been focused on establishing the nutritional requirements of this species in order to optimize its diet formulation (Mozanzadeh et al. Citation2017). Thus, in order to continue improving the formulation of compound diets for this fish species, the current study was designed to evaluate the effects of dietary SBL inclusion on growth performance, humoral immune responses as well as digestive and antioxidant enzymes activities of S. hasta juveniles.
Materials and methods
Experimental design
For evaluating the effects of dietary SBL inclusion on S. hasta juveniles performance, an eight-week feeding trial was conducted using four isonitrogenous (ca. 500 g kg−1 crude protein), isoenergetic (ca. 18.5 MJ kg−1) and isolipidic (ca. 200 g kg−1 crude lipids) diets containing graded levels of SBL (0, 30, 60 and 90 g kg−1 diet) (–) at the expense of fish oil (FO) as the main lipid source. Experimental diets were prepared as described in Mozanzadeh et al. (Citation2015). Diets were prepared by mixing all dry ingredients including fish meal, wheat meal, gluten meal, beef gelatin and premixes for 30 min. Then, FO, SBL and sufficient distilled water were added to form a soft dough and mechanically extruded to obtain pellets (3 mm). Pellets were dried in a convection oven at 25°C and stored in re-sealable plastic bags at −20°C until use.
Table 1. Ingredient and proximate composition of the experimental diets.
Table 2. Fatty acid composition of experimental diets (mg g−1 total fatty acids).
Table 3. Lipid classes of experimental diets (%).
Fish maintenance and feeding
This study was carried out at the Mariculture Research Station of the South Iranian Aquaculture Research Center (SIARC), Sarbandar, Iran. Fish were randomly distributed into 12 cylindrical polyethylene tanks (functional volume = 250 L), and each tank was stocked with 15 fish (BWi = 38.0 ± 0.1 g, mean ± standard error). Before beginning of the nutritional trial, fish were adapted to the experimental condition for two weeks. Tanks were supplied with running sea water (1 L min−1) in a flow-through system and the mean values for salinity, tempreture, pH and dissolved oxygen were 48.2 ± 0.2 ppt, 25.1 ± 1.6°C, 7.7 ± 0.1 and 6.8 ± 0.4 mg L−1, respectively. The photoperiod condition during experiment was 16L:8D (light:darkness). Each diet was tested by triplicate and fish were fed one of the above-mentioned diets by hand to visual satiation two times per day (0800 and 1500 h) for 56 days. Uneaten feed was removed from the bottom of the tank by siphoning 1 h after feeding, dried in an oven (60°C for 24 h) and weighed to determine feed intake values. All fish from each replicate were measured to the nearest 0.1 g for their body weight (BWf) and their standard length (SL) was measured to the nearest 1 mm. Four specimens from each replicate were sacrificed with an overdose 2-phenoxyethanol for evaluateing the weight of the liver, intraperitoneal fat and viscera. Sample collection for blood (n = 2 fish per replicate) and plasma (n = 2 fish per replicate), digestive (n = 2 fish per replicate) and antioxidant enzymes (n = 2 fish per replicate) was done as previously reported by Pagheh et al. (Citation2017).
Lipid classes and fatty acid (FA) analyses
Total lipids from diets and fish fillets were extracted by sample homogenization in chloroform/methanol (2:1, v/v) (Folch et al. Citation1957). Lipid class separation was performed by high-performance thin-layer chromatography (HPTLC) (Olsen and Henderson Citation1989). The HPTLC plates (10 × 10, Nano-sil 20, 0.2 mm of Nano-silica gel 60, Fiers, Kuurne, Belgium) were used for the separation of lipid classes. In this regard, plates were placed in chloroform:methanol (2:1) for 24 h, then they were transferred in the oven at 110°C for 30 min and let them cool in a desicator. A volume of 10 μl of samples was transferred to plates and developed using a mixture of methylacetate: isopropanol: chloroform: methanol: KCl (2:2:2:1:1). Then, plates were dried in a desicator for 15 min, and placed in a second solvent (29.75 ml of hexan + 5.25 ml of diethylether + 0.35 ml glacial acetic acid) for 15 min. Fewster mix (3% copper acetate in 8% orthophosphoric acid) was pulverized on the plates. Finally, plates were placed in the oven at 160°C for 20 min, and after cooling the lipid classes were quantified by densitometry (BioRad, GS-900, USA).
For determining the diet and fillet FA’s profiles, FA methyl esters were prepared by acidic methanolysis of lipid extracts using sulphuric acid in methanol (Christie Citation1993). In this regard, the lipid sample (up to 50 mg) is dissolved in 2.5% sulphuric acid in methanol (2 mL) in a test tube. The mixture was left for 1 h at 80°C, then samples were cooled down at room temperature. After that, water (1.5 mL) containing sodium chloride (0.9%) was added and the required esters extracted with hexane (2 × 1 mL) using Pasteur pipettes to separate the layers. The solution centrifuged (4000 g, 50 min, 4°C) and the upper layer, which contained FA methyl esters was separated and evaporated under a stream of nitrogen. Finally, the remained dry FA methyl esters were dissolved in isooctane (1 mL) and determined by gas chromatography. The FA composition of diet (n = 1) and fish fillet (n = 3) were determined by an auto sampler gas chromatography (GC, Agilent technologies 7890 N, USA), equipped with aflame ionization detector (FID) and a cyanopropyl–phenyl capillary column (DB-225MS, 30 m × 0.250 mm ID × 0.25 μm Film thickness, USA). Carrier gas was ultra-high purity nitrogen at a flow rate of 1 mL min−1. The column temperature was programmed as follows: holding at 100°C for 2 min, raising to 182°C at a rate of 30°C min−1, and again raising to 220°C at a rate of 2°C min−1, holding for 5 min, and finally column heating at a rate of 3°C min−1–230°C, then holding at this temperature for 3 min. The injector and detector temperatures were set at 230°C and 300°C, respectively. The split ratio was 30:1 and the sample volume injected for each analysis was 1 μL (total run time = 40 min per sample). The identification of fatty acids was performed by comparing their retention time with those of an external commercial standard mixture (GLC-68d, NuChek Prep., MN, USA) (Agh et al. Citation2014).
Hematological and antioxidant status
Complete blood count was assessed according to Blaxhall and Daisley (Citation1973). Haemolytic and lysozyme activities, as well as total immunoglobulin (Ig) levels in the plasma were determined according to Andani et al. (Citation2012), Ellis (Citation1990) and Siwicki et al. (Citation1994), respectively. Superoxide dismutase (SOD) and catalase (CAT) activities, as well as total antioxidant capacity (TAC) in liver samples were assayed according to Kono (Citation1978), Koroluk et al. (Citation1988) and Benzie and Strain (Citation1996), respectively.
Activity of pancreatic digestive enzymes
Samples were processed and handled following the indications of Solovyev and Gisbert (Citation2016) regarding the time of sample storage and process. Dissected digestive tracts from the same tank were pooled and homogenized (1–2 min at 0–4°C; 3 volumes v/w of 50 mM 2 mM Tris–HCl buffer, pH 7.0) (Chong et al. Citation2002). Total alkaline proteases were assayed according to method described by García-Carreño and Haard (Citation1993). Bile salt-activated lipase activity was assayed according to the method described by Iijima et al. (Citation1998). The soluble protein of crude enzyme extracts was quantified by means of the Bradford’s method (Citation1976). All the assays were made in triplicate (methodological replicates). All oxidative stress condition parameters and digestive enzymes activities were measured in triplicate with a microplate scanning spectrophotometer (PowerWave HT, BioTek®, USA).
Statistical analyses
Data were analysed using SPSS ver.19.0 (Chicago, Illinois, USA). All data are presented as mean ± standard error of the mean calculated from three replicates (tanks). Arcsine transformations were conducted on data expressed as percentage. One-way ANOVA was performed at a significance level of 0.05 following confirmation of normality and homogeneity of the variance. Duncan’s procedure was used for multiple comparisons when statistical differences were found among groups by the one-way ANOVA.
Results
Fatty profile and lipid classes of experimental diets
As presented in , the levels of dietary polyunsaturated FA, mainly (linoleic and linolenic acids), increased; wheareas the content of monounsaturated fatty acids (oleic acid, 18:1n-9), as well as that of n-3 LC-PUFA (especially EPA and DHA) decreased with the progressive replacement of dietary FO with SBL. As expected, PL levels, including phosphatidyl choline, ethnolamine, serin and inositol in diets increased, and the level of tryacyglycerols decreased with the progressive replacement of dietary FO with SBL ().
Growth performance
In the present study, no mortality occurred throughout the experiment (). Growth performance of fish fed SBL-supplemented diets was improved in comparison with the control group. In this context, fish fed with the control (SBL 0) and 60 g SBL kg−1 diets had the lowest and highest WG (96.0 vs. 128.0%) and SGR (1.2 vs. 1.5% day−1) values, respectively, whereas the other groups showed intermediate values (). Values of the HSI were higher in fish fed the control diet than those fed SBL-supplemented diets, whereas there were no differences in other somatic indices including VSI, PFI and K among experimental groups (P > 0.05).
Table 4. Growth response and survival of S. hasta juvenile fed experimental diets differing in their content in soybean lecithin (SBL) (mean ± SEM, n = 3).
Fillet lipid classes and FA profiles
Lipid classes and FA composition of fillets significantly changed depending on lipid classes and FA composition of experimental diets ( and ). Fillets of fish fed the control diet had the highest content in saturated fatty acids [mainly palmitic (16:0) and stearic (18:0) acids] (P < 0.05). The levels of monounsaturated fatty acids (MUFA), especially oleic acid (18:1n-9, OA), significantly decreased in the fillet of fish fed the 90 g SLB kg−1 diet (P < 0.05). The amount of polyunsaturated fatty acids (PUFA), especially linoleic acid (18:2n-6, LA) and α-linolenic acid (18:3n-3, α-LNA), significantly increased with increasing dietary SBL levels; however, the concentrations of LC-PUFA including ARA, EPA and DHA as well as the n−3/n−6 ratio in the fillet significantly decreased with increasing SBL in diets (P < 0.05). Fish fed with SBL-supplemented diets had higher fillet phosphatidylcholine than the control group, whereas the fillet of fish fed the 90 g SLB kg−1 diet had the highest phosphatidylethanolamine levels (P < 0.05). However, levels of triacylglycerides were almost similar each other among different dietary groups (P > 0.05).
Table 5. Fatty acid (mg g−1 total fatty acids) composition of fillet of S. hasta juvenile fed experimental diets differing in their content in soybean lecithin (SBL) (mean ± SEM, n = 3).
Table 6. Lipid classes (mg g−1 extracted lipid) of fillet of S. hasta juvenile fed experimental diets differing in their content in soybean lecithin (SBL) (mean ± SEM, n = 3).
Hematological and antioxidant parameters
In the present study, hematological parameters, as well as hematological indices, were not affected by the inclusion of SBL in the basal diet (, P > 0.05). Regarding, non-specific serological parameters, fish fed the control diet had the highest plasma lysozyme activity than other experimental groups ((a); P < 0.05). However, plasma haemolytic activity ((b)) was not affected in different experimental groups (P > 0.05). Plasma total Ig ((c)) level was higher in fish fed diets supplemented with 60 and 90 g SBL kg−1 diets than in the other groups (P < 0.05). There were not significant differences in liver antioxidant parameters including SOD ((a)), CAT ((b)) and TAC ((c)) among experimental groups (P > 0.05).
Figure 1. Plasma humoral immune parameters including lysozyme level (a) haemolytic activity (b) total Ig (c), and in S. hasta fed different experimental diets.
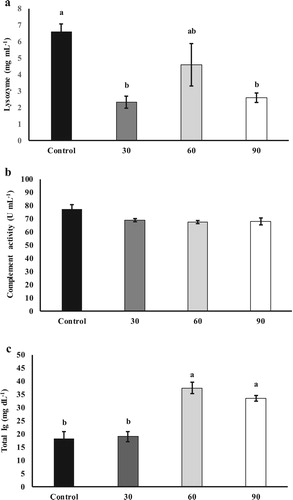
Figure 2. Liver SOD (a) and catalase (b) activities and total antioxidant capacity (c) in S. hasta fed different experimental diets.
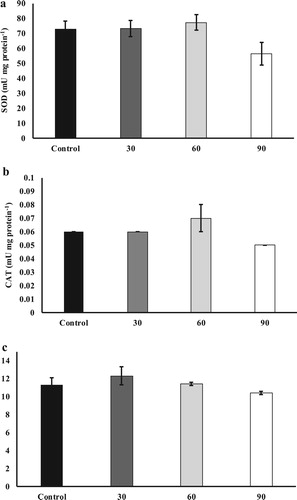
Table 7. Hematological profile of S. hasta juveniles fed experimental diets differing in their content in soybean lecithin (SBL) (mean ± SEM, n = 3).
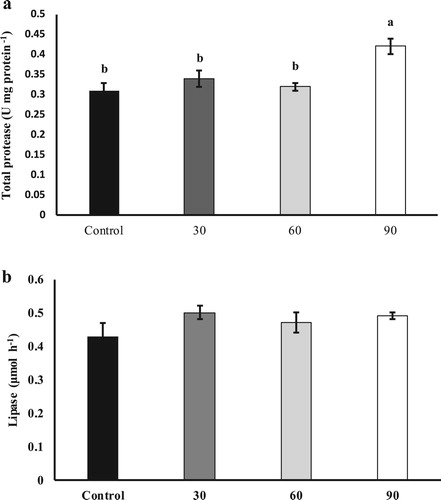
Digestive enzyme activity
In the present study, fish fed the 90 g SBL kg−1 diet had the highest total protease activity in comparison to the other groups ((a); P < 0.05); however, bile salt-activated lipase activity was not affected by dietary SBL supplementation ((b); P > 0.05).
Discussion
Supplementing diets with functional feed additives (e.g. acidifiers, phospholipids, pro-, pre- and synbiotics) not only increases nutrient digestibility but also improves growth performance and general health in farmed aquatic animals (Hussain et al. Citation2017; Rabia et al. Citation2017; Wang et al. Citation2017). Regarding the importance of dietary PLs, several studies have revealed that juvenile fish may also need dietary PLs supplementation for optimal somatic growth performance (Uyan et al. Citation2007, Citation2009; Salini et al. Citation2016) as they have a limited synthesis capacity (Tocher et al. Citation2008). In the present study, the improvement in somatic growth of fish fed SBL-supplemented diets could be explained by different reasons. For instance, increasing feed intake (FI) without affecting FCR values in fish fed the SBL-supplemented diets in comparison with the control group might have resulted in better growth performance in these groups. In this sense, it has been proved that the trimethyl group of the choline base of phosphatidylcholine, as well as as well as inositol group of the phosphatidylinositol can stimulate the gustatory response of fish (Izquierdo and Koven Citation2010; La et al. Citation2018). Similar results were also reported in juveniles of other marine and freshwater fish species such as Japanese flounder (Paralichthys olivaceus, Uyan et al. Citation2007), rainbow trout (Oncorhynchus mykiss, Rinchard et al. Citation2007), amberjack (Seriola dumerilli, Uyan et al. Citation2009), Atlantic salmon (Salmo salar, De Santis et al. Citation2015) and yellowtail Seriola quinqueradiata (La et al. Citation2018). Secondly, dietary lecithin as the major source of phosphatidylcholine can be hydrolysed in the digestive tract to the form of lysophosphatidylcholine, an important precursor of PLs, which may save some energy for their biosynthesis (Tocher et al. Citation2008), energy that may be derived to other metabolic processes, including somatic growth. In addition, dietary lecithin can increase the digestibility of diets and stimulate the synthesis and secretion of lipoproteins, and the utilization of dietary lipids (Tocher et al. Citation2008), improving somatic growth (Seiliez et al. Citation2006). Under current experimental conditions, the increased HSI values in the control group may be due to a higher accumulation of fat stores in the liver, which may be attributed to an insufficient dietary PLs, thus, affecting the normal lipid transportation in the body as also reported in common carp (Cyprinus carpio L.) larvae fed PL deficient diets (Fontagné et al. Citation1998). In addition, the slight increase in HSI level with increasing dietary SBL inclusion might be as a result of lipid accumulation due to the high percentage of linoleic acid in SBL, which promoted lipid accumulation in the liver (Piedecausa et al. Citation2007).
In the current study, the FA profile and lipid classes of the fillet of fish generally reflected those of experimental diets. The concentrations of OA as well as linoleic acid (18:2n-6, LA) in the fillet from different experimental groups were significantly lower than their levels in respective diets, indicating that these FAs were mainly catabolized for energy purposes, which was in agreement with other studies in different fish species (Bell et al. Citation2003; Regost et al. Citation2003; Benedito-Palos et al. Citation2008; Wassef et al. Citation2009; Tocher et al. Citation2010; Mozanzadeh et al. Citation2015, Citation2016a). The amount of polyunsaturated fatty acids (PUFA), especially LA and α-linolenic acid (18:3n-3, α-LNA), significantly increased with increasing dietary SBL levels, as it has also been reported in other fish species fed diets supplemented with SBL (Benedito-Palos et al. Citation2008; Alves Martins et al. Citation2010; Sotoudeh et al. Citation2011; Azarm et al. Citation2013; Saleh et al. Citation2015; Salini et al. Citation2016). Fish oil is the main source of the LC-PUFA, including arachidonic (20:4n-6, ARA), eicosapentaenoic (20:5n-3, EPA) and docosahexaenoic (22:6n-3, DHA) (Glencross Citation2009; Turchini et al. Citation2009). Thus, the replacement of FO with SBL led to a decrease in LC-PUFA including ARA, EPA and DHA, as it has also been reported in different fish species such as gilthead seabream larvae (Sparus aurata, Alves Martins et al. Citation2010) and juveniles at commercial size (Benedito-Palos et al. Citation2008), Caspian brown trout (Salmo trutta caspius, Sotoudeh et al. Citation2011) and juvenile barramundi (Lates calcarifer; Salini et al. Citation2016). The selective retention of DHA in S. hasta tissues has been proved in previous studies (Mozanzadeh et al. Citation2015, Citation2016a, Citation2016b), and it has a similar pattern that was demonstrated in other sparid species (Benedito-Palos et al. Citation2008; Peng et al. Citation2008). In this study, the incorporation of gradient levels of SBL at the expense of FO, increased the PL concentration in the diet and modified its lipid classes. In this sense, fish fed with SBL-supplemented diets had higher fillet PC content than those fed other experimental diets. Similar to our results, Teshima et al. (Citation1986) reported that the concentrations of PLs such as PC slightly higher in the shrimp larvae (Penaeus japonicus) receiving SBL-PC than in those receiving other PL classes. In contrast to the result of the present study, Geurden et al. (Citation1998) reported higher deposition of neutral lipids in the whole body of turbot post-larvae (Scophthalmus maximus) fed with PL- supplemented diet in comparison with fish fed a PL-free one. These differences may be attributed to the differences in lipid metabolism between different developmental stages (juvenile vs. post-larvae) (Tocher et al. Citation2008).
Because of their potent antioxidant capacity due to the side-chain moiety that contains amine/hydroxyl groups (Saito and Ishihara Citation1997), PLs might maintain the fluidity and stability of the RBC membranes and protect them against oxygen free radicals. Results of present study showed that complete blood count indices were not affected by different diets. In contrast, it has been reported that lecithin (10 g kg−1 diet) tended to stimulate erythropoiesis in rainbow trout (O. mykiss), which resulted in higher red blood cell, haemoglobin concentration and haematocrit levels than in fish fed non-supplemented lecithin diet (Réhulka and Minarik Citation2003). These differences between studies may be attributed to differences in fish species, diet formulations and purity of SBL tested in different nutritional studies.
In the current study, the replacement of dietary FO with SBL resulted in decreasing body n-3/n-6 PUFAs ratio in S. hasta, which may have influenced fish immune responses. It has been reported that dietary SBL supplementation increased mucosal antibacterial activity in common carp (C. carpio, Adel et al. Citation2017). Moreover, supplementation of dietary PLs increased the stress resistance in different fish species such as Labeo rohita fingerlings (Kumar et al. Citation2012), large yellow croaker (Larmichthys crocea, Zhao et al. Citation2013), milkfish (Chanos chanos, Kumar et al. Citation2014) and stellate sturgeon (Acipenser stellatus, Jafari et al. Citation2018). Our study showed that plasma lysozyme activity values decreased, whereas plasma total Ig levels showed the opposed trend in fish fed SBL-supplemented diets. Similar to our result, Jafari et al. (Citation2018) reported that increasing dietary SBL from 4 to 8 g SBL kg−1 significantly increased serum total Ig in juvenile stellate sturgeon (A. stellatus) compared to fish fed dies supplemented with 0 and 2 g SBL kg−1 and fish fed 10 g SBL kg−1 showed intermediate values. The results of our study indicate that more immunological (cellular and mucosal) analyses need to be conducted in order to provide a more precise result of the effects of dietary PL on fish immunity. It is well documented that lecithin acts in synergy with other antioxidants preventing the oxidation of vitamins A, C and E, as well as enhance their utilization (ADM Citation2003). Using butylated hydroxyl toluene (3 g kg−1) as an antioxidant in the experimental diets might be masked the antioxidant properties of SBL in the present study. In contrast, it has been reported that dietary SBL supplementation (1–3%) increased antioxidant enzyme activities (CAT, SOD, glutathione-S-transferase and glutathione peroxidase) in milkfish (Kumar et al. Citation2014) and common carp (Adel et al. Citation2017).
Literature regarding the effect of dietary PLs on the activity of digestive enzymes in juvenile fish is scarce. The results of this study showed that total protease activity was increased in fish fed the 90 g SLB kg−1 diet. Several studies have reported the beneficial effects of dietary PLs on the digestive function in larval stages of different marine (Cahu et al. Citation2003; Gisbert et al. Citation2005; Wold et al. Citation2007; Cai et al. Citation2016) and freshwater (Hamza et al. Citation2008) fish species. It has been reported that dietary PLs enhance the secretion of pancreatic digestive enzymes by increasing lysophospholipids, which act as supplementary emulsifiers in the intestinal lumen (Cahu et al. Citation2003). Moreover, PLs indirectly increase the levels of cholecystokinin that mediated in stimulating the pancreatic secretion (Gisbert et al. Citation2005; Azarm et al. Citation2013).
Concluding, the results of this study showed the replacement of dietary FO with SBL can improve somatic growth performance in S. hasta juveniles. Regarding to the results of WG and FI, substitution of dietary FO with SBL diet up to 67% (60 g SBL kg−1) diet suggested as an optimum level in S. hasta juvenile. Moreover, increasing dietary SBL led to a significant decrease in fillet n-3 LC-PUFA (mainly EPA and DHA) and n-3/n-6 ratios in S. hasta, whereas increased the concentrations of different phospholipid classes in the fillet. A finishing feeding trial with FO diet is recommended for the restoration of the n-3 LC-PUFA in the fillet of fish fed SBL-supplemented diets.
Acknowledgments
Authors are grateful to the director (Mr. Mojtaba Zabayeh Najafabadi) and staff of the Mariculture Research Station, Sarbandar, Iran for providing the necessary facilities for conducting this project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adel M , Gholaghaie M , Khanjany P , Citarasu T. 2017. Effect of dietary soybean lecithin on growth parameters, digestive enzyme activity, antioxidative status and mucosal immune responses of common carp (Cyprinus carpio). Aquacult Nutr. 0:1–8.
- ADM . 2003. Lecithin in aquaculture. [accessed 2007 Aug 1]. www.admworld.com/mktcolpdf/euLecithinsInAquaculture.pdf .
- Agh N , Jasour MS , Noori F. 2014. Potential development of value-added fishery products in underutilized and commercial fish species: comparative study of lipid quality indicators . J Am Oil Chem Soc. 91:1171–1177. doi: 10.1007/s11746-014-2454-x
- Alves Martins D , Estévez A , Stickland NC , Simbi BH , Yúfera M. 2010. Dietary lecithin source affects growth potential and gene expression in Sparus aurata larvae. Lipids. 45:1011–1023. doi: 10.1007/s11745-010-3471-7
- Andani HRR , Tukmechi A , Meshkini S , Sheikhzadeh N. 2012. Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol. 28:728–734. doi: 10.1111/j.1439-0426.2012.01974.x
- Azarm HM , Kenari AA , Hedayati M. 2013. Effect of dietary phospholipid sources and levels on growth performance, enzymes activity, cholecystokinin and lipoprotein fractions of rainbow trout (Oncorhynchus mykiss) fry. Aquac Res. 44:634–644. doi: 10.1111/j.1365-2109.2011.03068.x
- Bell JG , Tocher DR , Henderson RJ , Dick JR , Crampton VO. 2003. Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. J Nutr. 133:2793–2801. doi: 10.1093/jn/133.9.2793
- Benedito-Palos L , Navarro JC , Sitjá-Bobadilla A , Gordon Bell J , Kaushik S , Pérez-sánchez J. 2008. High levels of vegetable oils in plant protein-rich diets fed to gilthead sea bream (Sparus aurata L.): growth performance, muscle fatty acid profiles and histological alterations of target tissues. Brit J Nutr. 100:992–1003. doi: 10.1017/S0007114508966071
- Benzie IFF , Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 239:70–76. doi: 10.1006/abio.1996.0292
- Blaxhall PC , Daisley KW. 1973. Routine haematological methods for use with fish blood. J Fish Biol. 5:771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Cahu CL , Gisbert E , Villeneuve LAN , Morais S , Hamza N , Wold P-A , Zambonino Infante JL. 2009. Influence of dietary phospholipids on early ontogenesis of fish. Aquac Res. 40:989–999. doi: 10.1111/j.1365-2109.2009.02190.x
- Cahu CL , Infante JLZ , Barbosa V. 2003. Effect of dietary phospholipid level and phospholipid: neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. Brit J Nutr. 90:21–28. doi: 10.1079/BJN2003880
- Cai Z , Feng S , Xiang X , Mai K , Ai Q. 2016. Effects of dietary phospholipid on lipase activity, antioxidant capacity and lipid metabolism-related gene expression in large yellow croaker larvae (Larimichthys crocea). Comp Biochem Physiol B. 201:46–52. doi: 10.1016/j.cbpb.2016.06.007
- Chong ASC , Hashim R , Chow-Yang L , Ali BA. 2002. Partial characterization and activities of proteases form the digestive tract of discus fish (Symphysodon aequifasciata). Aquaculture. 203:321–333. doi: 10.1016/S0044-8486(01)00630-5
- Christie WW. 1993. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Christie WW , editor. Advances in lipid methodology. Dundee, Scotland : Oily Press; p. 69–111.
- Coutteau P , Geurden I , Camara MR , Bergot P , Sorgeloos P. 1997. Review on the dietary effects of phospholipids in fish and crustacean larviculture. Aquaculture. 155:149–164. doi: 10.1016/S0044-8486(97)00125-7
- De Santis C , Taylor JF , Martinez-Rubio L , Boltana S , Tocher DR. 2015. Influence of development and dietary phospholipid content and composition on intestinal transcriptome of Atlantic salmon (Salmo salar). PLoS ONE. 10:e0140964. doi: 10.1371/journal.pone.0140964
- Ellis AE. 1990. Serum antiproteases in fish and lysozyme assays. In Stolen JS , Fletcher TC , Anderson DP , Roberson BS , Van Muiswinkel WB , editors. Techniques in fish immunology. Fair Haven (NJ ): SOS Publications; p. 95–103.
- Folch J , Lees M , Stainley GHS. 1957. A simple method for isolation and purification of total lipid from animal tissue. J Biol Chem. 226:497–509.
- Fontagné S , Burtaire L , Corraze G , Bergot P. 2000. Effects of mediumchain triacylglycérols (tricaprylin and tricaproin) and phospholipid supply on survival, growth and lipid metabolism in common carp (Cyprinus carpio) larvae. Aquaculture. 190:289–303. doi: 10.1016/S0044-8486(00)00400-2
- Fontagné S , Geurden I , Escaffre A-M , Bergot P. 1998. Histological changes induced by dietary phospholipids in intestine and liver of common carp (Cyprinus carpio L.) larvae. Aquaculture. 161:213–223. doi: 10.1016/S0044-8486(97)00271-8
- Gao J , Koshio S , Wang W , Li Y , Huang S , Cao X. 2014. Effects of dietary phospholipid levels on growth performance, fatty acid composition and antioxidant responses of Dojo loach Misgurnus anguillicaudatus larvae. Aquaculture. 426–427:304–309. doi: 10.1016/j.aquaculture.2014.02.022
- García-Carreño FL , Haard NF. 1993. Characterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. J Food Biochem. 17:97–113. doi: 10.1111/j.1745-4514.1993.tb00864.x
- Geurden I , Bergot P , Schwarz L , Sorgeloos P. 1998. Relationship between dietary phospholipids class composition and neutral lipid absorption in postlarval turbot. Fish Physiol Biochem. 19:217–228. doi: 10.1023/A:1007723515204
- Gisbert E , Villeneuve L , Zambonino-Infante JL , Quazuguel P , Cahu CL. 2005. Dietary phospholipids are more efficient than neutral lipids for long-chain polyunsaturated fatty acid supply in European sea bass (Dicentrarchus labrax) larval development. Lipids. 40:609–618. doi: 10.1007/s11745-005-1422-0
- Glencross BD. 2009. Exploring the nutritional demand for essential fatty acids by aquaculture species . Rev Aquac. 1:71–124. doi: 10.1111/j.1753-5131.2009.01006.x
- Hamza N , Mhetli M , Khemis IB , Cahu C , Kestemont P. 2008. Effect of dietary phospholipids levels on performance, enzyme activities and fatty acid composition of pikeperch (Sander lucioperca) larvae. Aquaculture. 275:274–282. doi: 10.1016/j.aquaculture.2008.01.014
- Hussain SM , Afzal M , Nasir S , Javid A , Azmat H , Mamoona Makhdoom S , Shah SZH , Hussain M , Mustafa I , Iqbal M. 2017. Role of phytase supplementation in improving nutrient digestibility and growth performance for Labeo rohita fingerlings fed on canola meal-based diet. J Appl Anim Res. 45:15–21. doi: 10.1080/09712119.2015.1091331
- Iijima N , Tanaka S , Ota Y. 1998. Purification and characterization of bile salt activated lipase from the hepatopancreas of red sea bream, Pagrus major . Fish Physiol Biochem. 18:59–69. doi: 10.1023/A:1007725513389
- Izquierdo MS , Koven WM. 2010. Lipids. In: Holt J , editor. Larval fish nutrition. Oxford : Wiley-Blackwell, John Wiley and Sons Publisher; p. 47–82.
- Jafari F , Agh N , Noori F , Tokmechi A , Gisbert E. 2018. Effects of dietary soybean lecithin on growth performance, blood chemistry and immunity in juvenile stellate sturgeon (Asipencer stellatus). Fish Shellfish Immunol. 80:487–496. doi: 10.1016/j.fsi.2018.06.023
- Kenari AA , Sotoudeh E , Rezaei MH. 2011. Dietary soybean phosphatidylcholine affects growth performance and lipolytic enzyme activity in Caspian brown trout (Salmo trutta caspius) alevin. Aquac Res. 42:655–663. doi: 10.1111/j.1365-2109.2010.02587.x
- Kono Y. 1978. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arc Biochem Biophys. 186:189–195. doi: 10.1016/0003-9861(78)90479-4
- Koroluk MM , Ivanova L , Mayorova I , Tokorev W. 1988. Method of determination of catalase activity . Lab Tech. 1:16–19.
- Kumar N , Jadhao SB , Chandan NK , Kumar K , Jha AK , Bhushan S , Kumar S , Rana RS. 2012. Dietary choline, betaine and lecithin mitigates endosulfan induced stress in Labeo rohita fingerlings. Fish Physiol Biochem. 38:989–1000. doi: 10.1007/s10695-011-9584-y
- Kumar N , Minhas PS , Ambasankar K , Krishnani KK , Rana RS. 2014. Dietary lecithin potentiates thermal tolerance and cellular stress protection of milk fish (Chanos chanos) reared under low dose endosulfan-induced stress. J Therm Biol. 46:40–46. doi: 10.1016/j.jtherbio.2014.10.004
- La TX , Ishikawa M , Tola S , Fukada H , Masumoto T. 2018. Effects of dietary phospholipid level and fraction on the feed intake of non-fish meal diet in yellowtail, Seriola quinqueradiata Temminck & Schlegel, 1845. Aquac Res. 49:569–575. doi: 10.1111/are.13488
- Mozanzadeh MT , Agh N , Yavari V , Marammazi JG , Mohammadian T , Gisbert E. 2016a. Partial or total replacement of dietary fish oil with alternative lipid sources insilvery-black porgy (Sparidentex hasta). Aquaculture. 451:232–240. doi: 10.1016/j.aquaculture.2015.09.022
- Mozanzadeh MT , Marammazi JG , Yavari V , Agh N , Mohammadian T , Gisbert E. 2015. Dietary n−3 LC-PUFA requirements in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture. 448:151–161. doi: 10.1016/j.aquaculture.2015.06.007
- Mozanzadeh MT , Yavari V , Marammazi JG , Agh N , Mohammadian T , Yaghoubi M , Gisbert E. 2016b. Dietary docosahexaenoic acid to eicosapentaenoic acid ratios effects on hemato-immunological and plasma biochemical parameters in silvery-black porgy (Sparidentex hasta) juveniles. Comp Clinic Pathol. 25:1107–1114. doi: 10.1007/s00580-016-2307-0
- Olsen RE , Henderson RJ. 1989. The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J Exp Mar Biol Ecol. 129:189–197. doi: 10.1016/0022-0981(89)90056-7
- Pagheh E , Marammazi JG , Agh N , Nouri F , Sepahdari A , Gisbert E , Mozanzadeh MT. 2017. Growth performance, hemato-immunological responses and digestive enzymes activities in silvery-black porgy (Sparidentex hasta) fed dietary bovine lactoferrin. Probiotics Antimicrob Proteins. doi:10.1007/s12602-017-9340-4.
- Peng S , Chen L , Qin JG , Hou J , Yu N , Long Z , Ye J , Sun X. 2008. Effects of replacement of dietary fish oil by soybean oil on growth performance and liver biochemical composition in juvenile black seabream, Acanthopagrus schlegeli . Aquaculture. 276:154–161. doi: 10.1016/j.aquaculture.2008.01.035
- Piedecausa MA , Mazón MJ , García García B , Hernández MD. 2007. Effects of total replacement of fish oil by vegetable oils in the diets of sharpsnout seabream (Diplodus puntazzo). Aquaculture. 263:211–219. doi: 10.1016/j.aquaculture.2006.09.039
- Rabia S , Afzal M , Shah SZH. 2017. Nutrient digestibility performance by rohu (Labeo rohita) juveniles fed acidified and phytase pre-treated sunflower meal-based diet. J Appl Anim Res. 45:331–335. doi: 10.1080/09712119.2016.1190731
- Regost C , Arzel J , Robin J , Rosenlund G , Kaushik SJ. 2003. Total replacement of fish oil by soybean or linseed oil with are turn to fish oil in turbot (Psetta maxima): 1. Growth performance, flesh fatty acid profile, and lipid metabolism. Aquaculture. 217:465–482. doi: 10.1016/S0044-8486(02)00259-4
- Rehulka J , Minark B. 2003. Effect of lecithin on the haematological and condition indices of the rainbow trout Oncorhynchus mykiss (Walbaum). Aquac Res. 34:617–627. doi: 10.1046/j.1365-2109.2003.00855.x
- Rinchard J , Czesny S , Dabrowski K. 2007. Influence of lipid class and fatty acid deficiency on survival, growth, and fatty acid composition in rainbow trout juveniles. Aquaculture. 264:363–371. doi: 10.1016/j.aquaculture.2006.11.024
- Saito H , Ishihara K. 1997. Antioxidant activity and active sites of phospholipids as antioxidants. J Am Oil Chem Soc. 74:1531–1536. doi: 10.1007/s11746-997-0072-6
- Saleh R , Betancor MB , Roo J , Benitez-Dora V , Zamorano MJ , Bell JG , Izquierdo MS. 2015. Effects of krill phospholipids versus soybeen lecithin in microdiet for gilthead seabream (Sparus aurata) larvae on molecular markers of antioxidative metabolism and bone development. Aquac Nutr. 21:474–488. doi: 10.1111/anu.12177
- Salini MJ , Wade N , Bourne N , Turchini GM , Glencross BD. 2016. The effect of marine and non-marine phospholipid rich oils when fed to juvenile barramundi (Lates calcarifer). Aquaculture. 455:125–135. doi: 10.1016/j.aquaculture.2016.01.013
- Seiliez I , Bruant JS , Zambonino Infante JL , Kaushik S , Bergot P. 2006. Effect of dietary phospholipid level on the development of gilthead sea bream (Sparus aurata) larvae fed a compound diet. Aquac Nutr. 12:372–378. doi: 10.1111/j.1365-2095.2006.00436.x
- Siwicki AK , Anderson DP , Rumsey GL. 1994. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol. 41:125–139. doi: 10.1016/0165-2427(94)90062-0
- Solovyev MM , Gisbert E. 2016. Influence of time, storage temperature and freeze/thaw cycles on the activity of digestive enzymes from gilthead sea bream (Sparus aurata). Fish Physiol Biochem. 42:1383–1394. doi: 10.1007/s10695-016-0226-2
- Sotoudeh E , Kenari AA , Rezaei MH. 2011. Growth response, body composition and fatty acid profile of Caspian brown trout (Salmo trutta Caspius) juvenile fed diets containing different levels of soybean phosphatidylcholine. Aquac Int. 19:611–623. doi: 10.1007/s10499-010-9376-x
- Taylor JF , Martinez-Rubio L , del Pozo J , Walton JM , Tinch AE , Migaud H , Tocher DR. 2015. Influence of dietary phospholipid on early development and performance of Atlantic salmon (Salmo salar). Aquaculture. 448:262–272. doi: 10.1016/j.aquaculture.2015.06.012
- Teshima SI , Kanazawa I , Kakuta Y. 1986. Growth, survival, and body lipid composition of the prawn larvae receiving several dietary phospholipids. Nippon Suisan Gakk. 35:17–27.
- Tocher DR. 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac Res. 41:717–732. doi: 10.1111/j.1365-2109.2008.02150.x
- Tocher DR , Bendiksen EÅ , Campbell PJ , Bell JG. 2008. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture. 280:21–34. doi: 10.1016/j.aquaculture.2008.04.034
- Torfi Mozanzadeh M , Marammazi JG , Yaghoubi M , Agh N , Pagheh E , Gisbert E. 2017. Macronutrient requirements of silvery-black porgy (Sparidentex hasta): a comparison with other farmed sparid species. Fishes. 2:1–24. doi:10.3390/fishes2020005.
- Turchini GM , Torstensen BE , Ng WK. 2009. Fish oil replacement in finfish nutrition. Rev Aquac. 1:10–50. doi: 10.1111/j.1753-5131.2008.01001.x
- Uyan O , Koshio S , Ishikawa M , Uyan S , Ren T , Yokoyama S , Komilus CF , Michael FR. 2007. Effects of dietary phosphorus and phospholipid level on growth, and phosphorus deficiency signs in juvenile Japanese flounder, Paralichthys olivaceus . Aquaculture. 267:44–54. doi: 10.1016/j.aquaculture.2007.01.020
- Uyan O , Koshio S , Ishikawa M , Yokoyama S , Uyan S , Ren T , Hernandez LHH. 2009. The influence of dietary phospholipid level on the performances of juvenile amberjack, Seriola dumerili, fed non-fishmeal diets. Aquac Nutr. 15:550–557. doi: 10.1111/j.1365-2095.2008.00621.x
- Wang W , Sun J , Liu C , Xue Z. 2017. Application of immunostimulants in aquaculture: current knowledge and future perspectives. Aquac Res. 48:1–23. doi: 10.1111/are.13161
- Wassef EA , Saleh NE , El-Hady HAE. 2009. Vegetable oil blends as alternative lipid resources in diets for gilthead seabream, Sparus aurata . Aquac Int. 17:421–435. doi: 10.1007/s10499-008-9213-7
- Wold P-A , Hoehne-Reitan K , Cahu CL , Infante JLZ , Rainuzzo J , Kjørsvik E. 2007. Phospholipids vs. neutral lipids: effects on digestive enzymes in Atlantic cod (Gadus morhua) larvae. Aquaculture. 272:502–513. doi: 10.1016/j.aquaculture.2007.06.034
- Zhao JZ , Ai QH , Mai KS , Zuo RT , Luo YW. 2013. Effects of dietary phospholipids on survival, growth, digestive enzymes and stress resistance of large yellow croaker, Larmichthys crocealarvae . Aquaculture. 410–411:122–128. doi: 10.1016/j.aquaculture.2013.05.018