ABSTRACT
The effect of a polyclonal antibody preparation (PAP) against Streptococcus bovis and Fusobacterium necrophorum on ruminal fermentation and digestion in ruminally cannulated cows was investigated in two 3 × 3 Latin squares in factorial arrangement of treatments 3 × 2 regarding two feed additives (PAP in powder (PAPP) and in liquid (PAPL) presentation) plus control (CON) and two managements of diets (with or without adaptation to highly fermentable carbohydrate diets). Adapted group had greater DMI (p < 0.0001) and DM (p < 0.0001), NDF (p = 0.03) and total carbohydrates (p < 0.0001) apparent digestibility when compared to non-adapted group. PAPL had greater DM (p = 0.02), NDF (p = 0.03) and total carbohydrates apparent digestibility when compared to PAPP or CON. Adapted animals had lower (p < 0.0001) rumen pH when compared to non-adapted animals. Moreover, PAPL group had greater (p = 0.04) rumen pH values when compared to PAPP and CON. PAPL showed potential effect as an additive by increasing apparent digestibility of DM, NDF and total carbohydrates and also for being more efficient to prevent the drop in rumen pH during the peak of fermentation.
1. Introduction
To reduce the operational impact of roughage in feedlots, animal nutritionists have increasingly adopted highly fermentable carbohydrate (HFC) diets (Oliveira and Millen Citation2014). These diets can result in metabolic disturbances such as ruminal acidosis (Nagaraja and Titgemeyer Citation2007). The main tools to reduce the impact of this type of diet and to optimize feed efficiency are step-up adaptation to diets and inclusion of feed additives. The use of antibiotics in animal production diets has been questioned regarding the food security (EUROPA Citation2003).
In this scenario, passive immunization arises as an alternative. Avian polyclonal antibodies preparation (PAP) is obtained after birds' immunization against target microorganisms to be controlled in the rumen (Shimizu et al. Citation1988; DiLorenzo et al. Citation2006). The studies obtained with PAP have diverse results in relation to the type of product presentation. Millen et al. (Citation2015) evaluated the product in liquid form and reported lower incidence of rumenitis and liver abscesses in beef cattle and similar weight gain from animals supplemented with monensin. In the same way, Marino et al. (Citation2011) reported that the product in liquid presentation was effective in the prevention of rumen pH drop at the peak of fermentation. Also, Blanch et al. (Citation2009) reported that PAP was effective in the reduction of acidosis risks, as the supplemented group had greater total short-chain fatty acids but higher ruminal pH. Dahlen et al. (Citation2003) and Bastos et al. (Citation2012) did not observe effects when the additive was supplemented in powder form. However, Barducci et al. (Citation2013) studied PAP in powder presentation and reported lower feed efficiency, weight gain and carcass weight in beef cattle when compared to monensin.
In a literature review, Millen et al. (Citation2015) described a compilation of investigations testing PAP in liquid and powder presentation. The authors described consistent results on cattle performance and rumen fermentation when liquid presentation was tested and suggested further studies using PAP in powder presentation, as the same results were not observed when this presentation was used. Probably because this product is submitted to the drying process (spray-dryer) which could probably impair its mode of action. So, in this study the objective was to evaluate the effects of PAP against Streptococcus bovis and Fusobacterium necrophorum in liquid or powder form on rumen fermentation variables, as well as total tract apparent digestibility in cows adapted or not to highly fermentable carbohydrate diets.
2. Materials and methods
2.1 Study location
The guidelines established by the University of São Paulo (Brazil) Ethic Committee on Animal Use of the Research (CEEA) – n° 1982/2010 were used for taking care of the cows. The trial was conducted at the College of Veterinary Medicine and Animal Science, USP, Brazil.
2.2 Polyclonal antibody preparation
Procedures for generating the PAP used in this study were similar to those described by DiLorenzo et al. (Citation2006, Citation2008) and Marino et al. (Citation2011). Polyclonal antibodies were produced by CAMAS Inc. (Le Centre, MN, USA). The final product contained approximately 46% of antibodies against Streptococcus bovis (ATCC 9809), 23% against Fusobacterium necrophorum (ATCC 27852), 16% against E. coli O157:H7 and 15% against endotoxins. The same blend of microorganisms was used to generate the PAP in two presentations. Polyclonal antibody in powder presentation was obtained by spray-dryer process and it was maintained in a hermetically sealed package throughout the experimental period, being protected from light and heat. Remaining antibody in liquid presentation was kept between 2°C and 8°C and protected from light throughout the experimental period.
2.3 Animal and experimental procedures
Animals were obtained as heifers from the University's herd and they did not present any special characteristics. Six nonpregnant, nonlactating Holstein cows (747 ± 90 kg of mean BW, 48 ± 3 months of age) fitted with ruminal cannulas were used in the present experiment. They were randomly assigned to two 3 × 3 Latin squares (18 experimental units) in a factorial arrangement of treatments 3 × 2 regarding two feed additives (PAP in powder presentation (PAPP) and PAP in liquid presentation (PAPL)) plus control group (CON) and two managements of diets adaptation, resulting in six treatments. The first Latin square (9 experimental units) had a step-up diet adaptation in days: from D1 to D5 (100% forage; 0% concentrate); D6 to D10 (70% forage; 30% concentrate) and D11 to D15 (40% forage; 60% concentrate). The second Latin square (9 experimental units) received 100% forage and 0% concentrate from D1 to D15. On D16 to D17, all animals received 20% forage and 80% concentrate in diet. The trial had three periods of 17 d each with 10 days of washout between each period, totalizing 71 days of experiment. Cows were housed in a tie-stall barn equipped with individual feed bunks and individual sand beds, as well as automatic water drinkers shared by two animals. Body weight was measured at the beginning of period 1 (D 0) and at the end of each of the three periods (D 0 of next period) at the same time each day, before morning meal.
2.4 Diets
Diets were offered as total mixed rations according to the protocol of step-up diet or not, twice a day, at 08h00 and 16h00 throughout the experiment ad libitum (5–10% of feed refusal). Diets formulated were evaluated using the Cornell Net Carbohydrate and Protein System CNCPS 6.5. The analysed nutrient content of the experimental diets is presented in . The forage source was fresh sugarcane chopped with theoretical average particle size of 1.24 cm. Feed additive was placed directly through ruminal cannula, twice a day, just before meals; PAP in powder presentation (PAPP; CAMAS Inc., Le Canter, MN) at 7 g d−1 (3.5 g each time) was administered in absorbent tissue paper and PAP in liquid presentation (PAPL; CAMAS Inc., Le Center, MN) at 21 mL d−1 (10.5 mL each time) using a plastic syringe. The concentration of different PAP presentations was equivalent in a DM basis, allowing comparison between the different forms, independently from dose.
Table 1. Composition and analysed nutrient content of experimental diets on dry matter basis (%).
2.5 Sampling and laboratory methods
2.5.1 Dry matter intake
All feeders were examined every morning at 07h00. If there was no feed surplus, feed offered was raised by 10%. If there was around 10% surplus, the feed was kept at the same level and if the surplus was >10%, the feed offered was reduced by 10%. Feed refusal from each cow was daily collected and weighed to calculate feed intake during all the experiment.
2.5.2 Total tract dry matter apparent digestibility
Chromic oxide was used as an external marker to estimate apparent nutrient digestibility, as described by Bateman (Citation1970). For each animal, DMI was measured and samples of faeces (approximately 200 g) were collected twice a day from the rectum at D2 to D5, D7 to D10 and at D12 to D15. Cows received 15 g d−1 of Cr2O3, through ruminal cannula, twice daily (7.5 g at each feeding time) from 2 days before the beginning of each period until D15. Chromic oxide concentration was determined colorimetrically through its reaction with σ-difenilcarbazide. Feed and faecal samples were dried at 55 °C for 72 h and ground to pass a 1-mm screen. Composite samples per cow and per period were used to determine DM (method 934.01; AOAC Citation1990); OM (method 924.05; AOAC Citation1990); CP by total N determination using the micro-Kjeldahl technique (method 920.87; AOAC Citation1990); ether extract (EE) determined gravimetrically after extraction using petroleum ether in a Soxhlet extractor (method 920.85; AOAC Citation1990); calcium (method 968.08; AOAC Citation1995); phosphorus (method 965.17; AOAC Citation1990); NDF (with heat-stable α-amylase), ADF and pectin according to Van Soest et al. (Citation1991). Starch analysis was done according to Pereira and Rossi (Citation1995), with previous extraction of soluble carbohydrates, as proposed by Hendrix (Citation1993). The value of non-fibre carbohydrates (NFC) was estimated by the formula NFC (% DM) = 100 – (CP + NDF + EE + Ash).
2.5.3 Ruminal fermentation variables
Ruminal fluid was daily sampled 3 h after the morning meal, on D1–D17 of each period, through the ruminal cannula with a vacuum pump. Approximately 500 mL of rumen fluid was collected, at each sampling time, from three different parts of the rumen. Immediately after sampling, 100 mL of rumen fluid were used for pH determination with a portable digital pH meter (HANNA instruments Limited HI8424, Bedfordshire, UK). Short-chain fatty acids (SCFA) analyses which included acetate, propionate and butyrate were measured by gas chromatography, according to Erwin et al. (Citation1961). Lactic acid concentration was measured by a colorimetric technique, according to Pryce (Citation1969). The determination of ammonia nitrogen (NH3-N) concentration was done, according to the method described by Chaney and Marbach (Citation1962).
2.5.4 Protozoa counts
For total and differential counts of rumen protozoa, each sample (10 mL of rumen content) was collected 3 h after the morning meal at D4, D9 and D14 and was stored in glass vials with 20 mL of 18.5% formaldehyde. Subsequently, each sample was stained with two drops of 2% brilliant green dye, diluted and protozoa were identified (genera Isotricha, Dasytricha, Epidinium, Entodinium and Diplodiniinae subfamily) and counted by optical microscopy (Dehority Citation1993).
2.5.5. Statistical analyses
Data were analysed by Statistical Analysis System version 9.3 software (SAS Inst. Inc., Cary, NC). Before the actual analysis, data were analysed for the presence of disparate information (‘outliers’) and normality of residuals (Shapiro–Wilk). Individual observation was considered outlier when standard deviations in relation to mean were more than +3 or less than −3. When the normality assumption was not accepted, the logarithmic transformation or the square root was required. Feed intake, rumen fermentation, total apparent digestibility and protozoa counts data were submitted to variance analyses by MIXED procedure of SAS that separated as source of variation the effects of additive, adaptation, time and their interactions which were considered fixed and the effects of period and animal nested within adaptation which were considered random. The effect of time was included in the model as repeated measures (split-plot design), resulting therefore in two different error terms (residual A and residual B). In this experimental design, the effects of adaptation protocol are equivalent to the square effect. In order to minimize differences between squares both were conducted simultaneously with similar animals in relation to age and body weight. Among 15 different covariance structures tested, the model used was chosen based on the lower value of Corrected Akaike Information Criterion (AICC) (Wang and Goonewardene Citation2004). The main effect of additive was separated by adjusted Tukey test. The level of significance adopted was 5%.
3. Results
3.1 Dry matter intake (DMI) and as % of body weight (DMIBW)
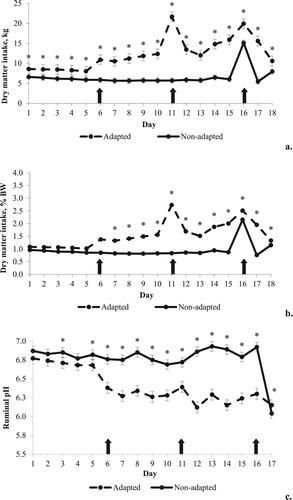
An interaction between day of experiment and diet adaptation (p < 0.0001) was observed for DMI. Between D1 and D18, the adapted group had greater DMI than the non-adapted group (12.37 vs. 6.57 kg of DM, respectively, a). When DMI was expressed in relation to body weight (BW), an interaction between day of experiment and adaptation (p < 0.0001) was also verified. Between D6 and D17, this variable was greater in adapted (1.79% BW) than non-adapted group (0.95% BW) (b).
Figure 1. Values of dry matter intake (a), dry matter intake in relation to body weight (b) and ruminal pH (c) and from cattle with or without adaptation to highly fermentable carbohydrate diets. The arrows indicate the change of diets in the adapted group. The asterisks indicate significant difference in the respective day of experiment.
3.2 Total tract apparent digestibility
An effect of adaptation (p < 0.0001) was observed for dry matter digestibility (DMD). The adapted cows had greater values (65.9%) than the non-adapted animals (55.3%) (). The effect of additive (p = 0.02) was described and the group PAPL (63.6%) had greater DMD compared with PAPP and CON (58.4% and 59.6%, respectively), without difference between these two groups.
Table 2. Values of dry matter digestibility and its fractions in cattle adapted or not to highly fermentable carbohydrates diets.
An interaction between proportion of carbohydrates in the diet and adaptation (p < 0.0001) was observed for crude protein digestibility (CPD), where the adapted animals had greater values (83.2%) compared to the non-adapted (79.3%), when both groups were fed with 100% forage (D1 to D5). From D6 to D10, there was no difference (p > 0.05) for CPD for both groups. From D11 to D15, when adapted animals received a diet with 60% of concentrate, this group had lower CPD (69.4%) than the non-adapted group (83.6%) (data not shown).
For ether extract digestibility (EED), an effect of adaptation was observed (p = 0.005), where it was greater in the adapted (54.2%) than in the non-adapted group (42.4%) (). For neutral detergent fibre digestibility (NDFD), effects of adaptation (p = 0.03) and additive (p = 0.03) were observed. Adapted animals had greater NDFD (40.6%) when compared to non-adapted animals (36.3%) (). For additive effect (p = 0.02), PAPL group had greater NDFD (44.0%) compared to PAPP (36.2%) and CON (35.4%) ().
For total tract acid detergent fibre digestibility (ADFD), effects of adaptation (p = 0.01) and additive (p = 0.001) were shown. Adapted animals had greater ADFD (40.9%) compared to non-adapted (37.0%). For additive effect (p = 0.001), PAPL group had higher ADFD (44.9%) compared to CON (35.9%). The group PAPP (36.5%) did not differ from the others ().
For total carbohydrates digestibility, an effect of adaptation (p < 0.0001) was observed. Adapted animals had greater digestibility when compared to non-adapted animals (66.4% vs 55.5%, respectively). And also, an effect of additive (p = 0.03) was observed; PAPL group (63.9%) had higher total carbohydrates digestibility compared to PAPP (59.0%) and CON (59.9%) ().
3.3 Ruminal fermentation variables
An interaction between day of experiment and adaptation (p < 0.0001) was observed for ruminal pH. Between D3 and D17, the lowest values were observed for the adapted animals (6.40) compared to non-adapted (6.77). At D16 and D17, a challenge diet was offered with 80% of concentrates to all animals and an abrupt drop of ruminal pH was observed in non-adapted animals (c). PAPL had greater values of ruminal pH (6.62) compared to PAPP (6.57) and CON (6.56) (p = 0.04), independently on day of experiment and adaptation ().
Table 3. Values of ruminal fermentation variables in cattle adapted or not to highly fermentable carbohydrates diets.
An interaction between day of experiment and diet adaptation (p < 0.0001) was observed for total short-chain fatty acids (tSCFA), from D4 to D16, period that the adapted animals had greater concentration than the non-adapted animals (100.3 vs. 77.7 mM, respectively) (a). Exclusively on D16, the adapted animals had lower values than the non-adapted ones (107.9 vs. 121.2 mM, respectively) (a). There was no effect of interaction between day of experiment and additive (p > 0.05) or even additive (p > 0.05) effect for tSCFA. An interaction between day of experiment and adaptation was observed for acetate (p < 0.0001), propionate (p < 0.0001) and butirate (p < 0.0001) concentration (). Acetate concentration was greater from D5 to D17 in the adapted group (63.6 mM) than in the non-adapted animals (48.5 mM). The same was observed for propionate concentration (27.6 vs. 20.4 mM, respectively) from D5 to D16 and for butyrate concentration (14.6 vs. 8.84 mM, respectively) from D1 to D17.
Figure 2. Values of total concentration of short-chain fatty acids (a), acetate:propionate ratio (b) and ammonia nitrogen concentration (c) from cattle with or without adaptation to highly fermentable carbohydrates diets. The arrows indicate the change of diets in the adapted group. The asterisks indicate significant difference in the respective day of experiment.
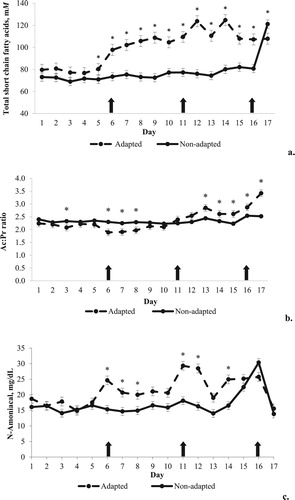
For molar proportion of acetate, an interaction between day of experiment and adaptation (p < 0.0001), as well as interaction between time and additive (p = 0.03) were observed (). Molar proportion of acetate from D1 to D12 was lower in adapted (58.7) than in non-adapted group (61.6). At D17, after the introduction of the challenging diet, the adapted group had greater molar proportion of acetate (62.5) than the non-adapted (58.8). At D5, molar proportion of acetate was greater in CON (63.9) compared to PAPL (58.4), without difference of these two groups from PAPP (60.7) (data not shown). An interaction between day of experiment and adaptation (p < 0.0001) was observed for molar proportion of propionate. From D6 to D8, adapted animals had greater values (29.0) than the non-adapted ones (27.1). From D12 to D17, this relation was inverted, so that the adapted had lower values than the non-adapted animals (22.1 vs. 25.6, respectively) ().
For acetate:propionate ratio (Ac:Pr), an interaction between day of experiment and adaptation (p < 0.0001) was observed. At D3, D6, D7 and D8, adapted animals had lower Ac:Pr ratio than non-adapted (1.96 vs. 2.29, respectively). However, between D13 and D17, there was an inversion of this relation and the adapted group had greater values than the non-adapted (2.87 vs. 2.41, respectively) (a). An interaction between day of experiment and adaptation (p = 0.0004) was observed for molar proportion of butyrate. From D1 to D16, the adapted animals had higher values (14.7) than the non-adapted group (12.1), except on D17. An interaction between day of experiment and additive (p = 0.0059) was also observed on D16, where CON had greater molar proportion of butyrate (20.7) than PAPL (17.7) and PAPP (16.6). No effect of adaptation, additive or interaction (p > 0.05) was observed for lactate concentration ().
For ammonia nitrogen concentration, an interaction between day of experiment and adaptation (p = 0.0003) was observed between D6 and D8, on D11, D12 and D14; the adapted animals had greater values than the non-adapted (24.7 vs. 16.0 mg/dl, respectively) (b).
3.4 Protozoa counts
For total population of Dasytricha sp., an effect of interaction between day of experiment and adaptation was observed (p = 0.04). At D15, this population was greater in non-adapted (23.1 × 103/mL) compared to adapted group (13.3 × 103/mL). Similar effect (p = 0.02) was observed for relative counts of Dasytricha sp., where on D15, the population was higher in non-adapted (51.7%) than in adapted animals (18.0%) ().
Table 4. Values of total and relative counts of rumen protozoa in cattle adapted or not to highly fermentable carbohydrates diets.
For total counts of protozoa, an interaction between day of experiment and adaptation (p = 0.03) was observed. At D15, total counts were greater in adapted animals (103.6 × 103/mL) compared to the non-adapted ones (44.1 × 103/mL) (). Relative population of Entodinium sp. was higher (p = 0.03) in adapted group (64.8%) compared to non-adapted (50.4%), independently on time ().
4. Discussion
Greater DMI in the adapted animals to HFC diets is expected since concentrate feed has greater acceptability by the animal, less effect of rumen filling and higher passage rate compared to roughage feed which stimulates intake (Allen Citation1996). Peaks of DMI were verified during changes of diets in D6 and D11 for the adapted group and during the challenging diet on D16 for both groups. These peaks were followed by a drop of DMI in the subsequent day (a). The reason for the peaks of DMI was probably the greater interest of the animals for the new diets and the factors described above. The reduction in intake in the subsequent day to the change of diet (D7, D12 and D17) can be induced by increased total concentration of short-chain fatty acids (tSCFA) and concomitant rumen pH drop in adapted animals, according to Conrad et al. (Citation1964), Fulton et al. (Citation1979) and Goad et al. (Citation1998).
Greater DMD in the adapted animals to HFC diets is expected as the higher proportion of these diets is composed by starch and other non-structural carbohydrates of simple and fast rumen digestion (Valadares Filho and Pina Citation2006). There are reports of DMD improvement with increased total population of protozoa, similar to what was observed in the present study (Lopes et al. Citation2002). Total tract CPD could be impaired in adapted animals as the diet high in concentrate has greater passage rate when compared to a high forage diet (Allen Citation1996). Increased NDFD in adapted animals could be due to the presence of amylolytic and cellulolytic microorganisms that are able to use both structural and non-structural sugars as substrates for fermentation. Similar results were reported by Goad et al. (Citation1998), Brown et al. (Citation2006) and Fernando et al. (Citation2010). Differently from this study, no effect of PAP was reported on DMD by Bastos et al. (Citation2012) who evaluated different doses of PAP in powder presentation and Marino et al. (Citation2011) who evaluated the effect of PAP in liquid presentation. The reason why PAP could improve digestibility is unknown in the literature. However, in the present experiment PAP in liquid form also improve rumen pH () and this could explain the result observed with PAP, especially fibre digestibility.
Greater values of ruminal pH in animals supplemented with PAPL were reported by DiLorenzo et al. (Citation2007) and Marino et al. (Citation2011) and contrasting to Dahlen et al. (Citation2003), DiLorenzo et al. (Citation2008), Blanch et al. (Citation2009) and Bastos et al. (Citation2012) who reported no effects of PAP on ruminal pH. The lack of effect of PAP on tSCFA concentration was also described by Dahlen et al. (Citation2003), DiLorenzo et al. (Citation2008), Blanch et al. (Citation2009), Marino et al. (Citation2011) and Bastos et al. (Citation2012). The group of adapted animals had greater tSCFA concentration probably due to the increasing levels of HFC in diets (mean value of 109.24 mM). Similar value was reported by Fulton et al. (Citation1979), Goad et al. (Citation1998) and Bevans et al. (Citation2005) who evaluated cattle adaptation to HFC diets. When non-adapted animals received 80% of concentrates in diet (D16), tSCFA concentration was greater than in the adapted group (121.2 vs. 107.9 mM, respectively). The abrupt production of SCFA shows that the rumen has great capacity to ferment carbohydrates as described by Huntington (Citation1997). And this abrupt increase in tSCFA in non-adapted animals could have contributed for the abrupt drop in rumen pH as observed in (c). Greater molar proportion of propionate in the adapted group was expected due to higher availability of HFC in the rumen inducing pH drop and excess of H2 for this acid synthesis (Goad et al. Citation1998; Moss et al. Citation2000; Bevans et al. Citation2005). This pattern was observed until D11. From there, a drop in propionate molar proportion in the adapted group was verified. As the average rumen pH was not lower than 6.0, there was no inhibition of methanogens which theoretically allowed the drainage of H2. This pathway is closely related to acetate production. For this reason, there was no need to change the metabolic pathways of rumen microorganism for propionate pathway to drain or use the excess of H2 generated by concentrate inclusion (Moss et al. Citation2000). Values measured for lactate concentration in the present experiment were low (mean value of 0.7 mM), probably because rumen pH observed in this study allowed the development of lactate-utilizing microorganism such as Megasphaera elsdenii. These microorganisms are inhibited only below pH 5.5 (Nagaraja and Titgemeyer Citation2007). No effect of PAP on NH3-N concentration was also reported by Dahlen et al. (Citation2003), DiLorenzo et al. (Citation2008), Blanch et al. (Citation2009), Marino et al. (Citation2011) and Bastos et al. (Citation2012).
Higher counts of Entodinium sp. in animals adapted to HFC diet were reported by Franzolin and Dehority (Citation1996) who asserted that in the rumen of animals fed up to 60–70% concentrates tend to prevail the genus Entodinium sp. in the rumen, as they use starch as a substrate and also can process lactate as source of energy, common conditions in adaptation phases to HFC diets.
5. Conclusion
Polyclonal antibodies in liquid presentation showed potential effect as an additive in cattle nutrition, but the powdering process must be reviewed. Step-up adaptation to highly fermentable carbohydrate diets reinforces the importance as a diet management practice.
Acknowledgements
The authors thank CAMAS Inc. for providing the polyclonal antibodies tested in this study. The authors also thank Gilmar E. Botteon for the good care of animals, as well as Ari Luiz de Castro, Gilson L. A. Godoy and Simi L. D. Aflalo for assistance in laboratory analysis (University of São Paulo, Pirassununga, São Paulo, Brazil).
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Carolina T. Marino http://orcid.org/0000-0002-1850-5280
Additional information
Funding
References
- Allen MS. 1996. Physical constraints on voluntary intake of forages by ruminants. J. Anim. Sci. 74:3063–3075. doi: 10.2527/1996.74123063x
- Association of Official Analytical Chemists International [AOAC] . 1990. Official Methods of Analysis, 15ed. Washington, D.C., USA : AOAC.
- Association of Official Analytical Chemists International [AOAC] . 1995. Official Methods of Analysis, 16ed. Washington, D.C., USA : AOAC.
- Barducci RS , Sarti LM , Millen DD , Pacheco RDL , Baldin SR , Parra FS , Putarov TC , Martins CL , Arrigoni MDB. 2013. Feed additives in feedlot diets. Arq. Bras. Med. Vet. Zootec. 65:1593–1602. doi: 10.1590/S0102-09352013000600002
- Bastos JPST , Marino CT , Millen DD , Pacheco RDL , Magalhães JD , Perna Júnior F , Martins CL , Rodrigues PHM , Arrigoni MDB. 2012. Effects of adding a spray-dried polyclonal antibody preparation on ruminal fermentation patterns and digestibility of cows fed high concentrate diets. Ital. J. Anim. Sci. 11:431–437.
- Bateman JV. 1970. Nutricion animal. Manual de métodos Analíticos. Cuidad de México, México : Herrero Hermanos Sucesores S.A.
- Bevans DW , Beauchemin KA , Schwartzkopf-Genswein KS , McKinnon JJ , McAllister TA. 2005. Effect of rapid or gradual grain adaptation on subacute acidosis and feed intake by feedlot cattle. J. Anim. Sci. 83:1116–1132. doi: 10.2527/2005.8351116x
- Blanch M , Calsamiglia S , DiLorenzo N , DiCostanzo A , Muetzel S , Wallace RJ. 2009. Physiological changes in rumen fermentation during acidosis induction and its control using a multivalent polyclonal antibody preparation in heifers. J. Anim. Sci. 87:1722–1730. doi: 10.2527/jas.2008-1184
- Brown MS , Ponce CH , Pulikanti R. 2006. Adaptation of beef cattle to high-concentrate diets: performance and ruminal metabolism. J. Anim. Sci. 84:E25–E33. doi: 10.2527/2006.8413_supplE25x
- Chaney AL , Marbach EP. 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132.
- Conrad HR , Pratt AD , Hibbs JW. 1964. Regulation of feed intake in dairy cows. I. Change in importance of physical and physiological factors with increasing digestibility. J. Dairy Sci. 47:54–62. doi: 10.3168/jds.S0022-0302(64)88581-7
- Dahlen CR , DiCostanzo A , Mitteness BM , Nash P , Larson JE , DiLorenzo N , Marx GD. 2003. Influence of a polyclonal antibody preparation against rumen proteolytic bacteria on rumen fermentation and yield of milk and milk components. J. Anim. Sci. 81:58.
- Dehority BA. 1993. Laboratory manual for classification and morphology of rumen ciliate protozoa. Florida : CRC Press Inc.
- DiLorenzo N , Dahlen CR , Diez-Gonzalez F , Lamb GC , Larson JE , DiCostanzo A. 2008. Effects of feeding polyclonal antibody preparations on rumen fermentation patterns, performance, and carcass characteristics of feedlot steers. J. Anim. Sci. 86:3023–3032.
- DiLorenzo N , Dahlen CR , Larson JE , Gill RK , DiCostanzo A. 2007. Effects of feeding a polyclonal antibody preparation against selected rumen bacteria on rumen pH of lactating dairy cows. J. Anim. Sci. 85:135.
- DiLorenzo N , Diez-Gonzalez F , DiCostanzo A. 2006. Effects of feeding polyclonal antibody preparations on ruminal bacterial populations and ruminal pH of steers fed high-grain diets. J. Anim. Sci. 84:2178–2185. doi: 10.2527/jas.2005-489
- Erwin ES , Marco GJ , Emery EM. 1961. Volatile fatty acids analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6
- EUROPA. Regulation EC on additives for use in animal nutrition No 1831/2003 . 2003. Available at: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32003R1831. Accessed 06 July 2016.
- Fernando SC , Purvis II HT , Najar FZ , Sukharnikov LO , Krehbiel CR , Nagaraja TG , Roe BA , DeSilva U. 2010. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 76:7482–7490. doi: 10.1128/AEM.00388-10
- Franzolin R , Dehority BA. 1996. Effect of prolonged high-concentrate feeding on ruminal protozoa concentrations. J. Anim. Sci. 74:2803–2809. doi: 10.2527/1996.74112803x
- Fulton WR , Klopfenstein TJ , Britton RA. 1979. Adaptation to high concentrate diets by beef cattle. II. Effect of ruminal pH alteration on rumen fermentation and voluntary intake of wheat diets. J. Anim. Sci. 49:785–789. doi: 10.2527/jas1979.493785x
- Goad DW , Goad CL , Nagaraja TG. 1998. Ruminal microbial and fermentative changes associated with experimentally induced subacute acidosis in steers. J. Anim. Sci. 76:234–241. doi: 10.2527/1998.761234x
- Hendrix DL. 1993. Rapid extraction and analyses of nonstructural carbohydrates in plant tissues. Crop Sci 33:1306–1311. doi: 10.2135/cropsci1993.0011183X003300060037x
- Huntington GB. 1997. Starch utilization by ruminants: from basics to the bunk. J. Anim. Sci. 75:852–867. doi: 10.2527/1997.753852x
- Lopes FCF , Aroeira LJM , Arcuri PB , Dayrell MS , Vittori A. 2002. Effects of defaunation in sheep fed sugar cane (Saccharum officinarum L.) plus urea. Arq. Bras. Med. Vet. Zootec. 54:180–188. (in Portuguese, with abstract in English). doi: 10.1590/S0102-09352002000200009
- Marino CT , Otero WG , Rodrigues PHM , DiCostanzo A , Millen DD , Pacheco RDL , DiLorenzo N , Martins CL , Arrigoni MDB. 2011. Effects of adding polyclonal antibody preparations on ruminal fermentation patterns and digestibility of cows fed different energy sources. J. Anim. Sci. 89:3228–3235. doi: 10.2527/jas.2010-3062
- Millen DD , Pacheco RDL , DiLorenzo N , Martins CL , Marino CT , Bastos JPST , Mariani TM , Barducci RS , Sarti LMN , DiCostanzo A , et al. 2015. Effects of feeding a spray-dried multivalent polyclonal antibody preparation on feedlot performance, feeding behavior, carcass characteristics, rumenitis, and blood gas profile of Brangus and Nellore yearling bulls. J. Anim. Sci. 93:4387–4400. doi: 10.2527/jas.2015-9227
- Moss AR , Jouany J-P , Newbold J. 2000. Methane production by ruminants: its contribution to global warming. Ann. Zootech. 49:231–253. doi: 10.1051/animres:2000119
- Nagaraja TG , Titgemeyer EC. 2007. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 90:E17–E38. doi: 10.3168/jds.2006-478
- Oliveira CA , Millen DD. 2014. Survey of the nutritional recommendations and management practices adopted by feedlot cattle nutritionists in Brazil. Anim. Feed Sci. Technol. 197:64–75. doi: 10.1016/j.anifeedsci.2014.08.010
- Pereira JRA , Rossi Jr P. 1995. Practical manual of feedstuffs nutritional evaluation, 1st.ed. Piracicaba, SP : FEALQ.
- Pryce JD. 1969. Modification of the Barker–Summerson method for the determination of lactic acid. Analyst. 94:1151–1152. doi: 10.1039/an9699401151
- Shimizu M , Fitzsimmons RC , Nakai S. 1988. Anti-E. coli immunoglobulin Y isolated from egg yolk of immunized chickens as a potential food ingredient. J. Food Sci. 53:1360–1366. doi: 10.1111/j.1365-2621.1988.tb09277.x
- Valadares Filho SC , Pina DS. 2006. Fermentação ruminal. In: Berchielli TT , Pires AV , Oliveira SG , editor. Ruminant Nutrition. Jaboticabal, São Paulo, Brasil : Funep; p. 151–179.
- Van Soest PJ , Robertson JB , Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
- Wang LA , Goonewardene Z. 2004. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Ani. Sci. 84:1–11. doi: 10.4141/A03-123
