ABSTRACT
The aim of the present study was to determine the effects of Bacillus subtilis PB6 supplementation on productive performance, egg quality and hatchability of broiler breeder hens. A total of 20,000 broiler breeder hens (ROSS 308), 57 weeks old, were assigned to the two equal groups as follows: The first group was fed a basal diet and the second group was fed the basal diet that supplemented with the Bacillus subtilis PB6 (2 × 107 cfu/g) at the rate of 1 g/kg of diet. Results indicated that adding Bacillus subtilis significantly improves egg production (P < 0.05). The body weights and egg weight were not affected by the dietary treatments (P > 0.05) but Bacillus subtilis improved the egg haugh unit (P < 0.05). Adding Bacillus subtilis significantly increased hatching egg, eggshell thickness and egg hatchability (P < 0.05). A highly significant improvement was noticed in the faecal score of the group fed with Bacillus subtilis (P < 0.01). Adding Bacillus subtilis increased the level of total cholesterol (P < 0.01) and decreased the blood heterophil to lymphocyte ratio (P < 0.01) in breeders’ serum. It is concluded that Bacillus subtilis PB6 supplementation at the level of 1 g/kg of broiler breeder diet, significantly improve the egg production, egg quality, hatchability, faecal score and return on investment.
1. Introduction
Feed quality has a key role in the poultry industry that can expose the birds to a wide variety of factors through the gastrointestinal tract (GIT). Gut health is one of the most important issues to have healthy birds with high productive performance (Choct Citation2009).
The GIT not only digests and absorbs nutrients but also has microbiota that their balance is crucial for optimal animal health and performance (Kogut and Swaggerty Citation2012). There are a lot of bacterial species (more than 640) in the GIT of healthy birds with stable gut microbiota (Choct Citation2009). It is well documented that some gut bacteria such as Escherichia coli, Clostridium, Salmonella can cause disease. However, it is also recognized that many gut microbes are beneficial.
The antibiotics mechanism of action as growth promoters is related to interactions between antibiotics and intestinal microbial population (Dibner and Richards Citation2005; Niewold Citation2007). Using antibiotics in poultry feed has become undesirable because of their residues in poultry products and the subsequent bacterial antibiotic resistance of consumers (Barbosa and Levy Citation2000; Perreten Citation2003). As a consequence, feed formulators have been looking for alternative additives, such as acidifiers, prebiotics, probiotics, and phytogenic extracts. Providing the GIT with beneficial microbes in order to reduce the presence of harmful bacteria is now an acceptable strategy to improve the health status and production performance of poultry (Mead Citation2000). Probiotics defined as live microbial supplements beneficially affect the hosts by modifying their intestinal microbial balance (Forte et al. Citation2016), improving feed intake and digestion (Awad et al. Citation2006), and stimulating the immune system (Mathivanan and Kalaiarasi Citation2007). Hence, nowadays probiotic usage has aroused widespread interest in poultry farms.
Beneficial effects of probiotics on broiler performance (Apata Citation2008), nutrient digestibility (Li et al. Citation2008), modulation of intestinal microflora (Yu et al. Citation2008), pathogen inhibition (Mountzouris et al. Citation2009), and immunomodulation and gut mucosal immunity (Teo and Tan Citation2007) have been reported. There are many studies on the effects of probiotics on the performance of broiler chickens, but few numbers of researches evaluated the beneficial effect of probiotics on broiler breeders (Bozkurt et al. Citation2011; Hajati et al. Citation2014; Zaghari et al. Citation2017; Aalaei et al. Citation2018).
The quality of the hens’ eggshell is important during incubation. It is well known that egg quality and hatchability in broiler breeders depend on different factors such as age, health state of birds, diet composition and housing management (Yaripour et al. Citation2018).
During the normal reproductive cycle, the Ca2+ was absorbed from the intestine and transported by the blood across the epithelium to the shell gland lumen (Wolfenson et al. Citation1982). The eggshell consists mainly of calcium carbonate (CaCO3) which is formed by Ca2+ and HCO3. The decline in eggshell quality as the hen ages may be attributed in part to reduced intestinal Ca uptake (Al-Batshan et al. Citation1994). Broiler breeders face a serious challenge in intestinal health switching from start lay to peak production. This can lead to poor microbial balance in GIT and weak shell quality, both of which might be ameliorated by the use of probiotic. Furthermore, the depressing effects of age on egg production and shell quality in the laying hen are well documented (Bar and Hurwitz Citation1987; Bar et al. Citation1998). It was reported that probiotics have a good impact on egg production rate, settable egg, and hatchability in broiler breeders, between the ages of 22 and 49 weeks, but no beneficial effects were observed on fertility (Bozkurt et al. Citation2011). Little information is available about the role of probiotics in broiler breeder performance. Therefore, the present study evaluated the effects of Bacillus subtilis PB6 supplementation on productive performance, egg quality and hatchability in broiler breeder hens during the late phase of production.
2. Materials and methods
2.1. Birds and management
A total of twenty thousand 57-week-old female broiler breeders (ROSS 308) with the average weight of 4.00 kg and an average of 66% hen day egg production were used at a commercial farm (Derakhshan CO., Fuman, Guilan province, Iran; 36° 54.758 ́N, 49° 28.758 ́ E, at 475 m altitude) for a seven weeks’ trial. Breeder hens were divided into two equal groups. The first group was fed a basal diet and served as a control. While the second group was fed the basal diet that supplemented with the probiotic. Four houses allocated to two dietary treatments. Each house was divided into three equal pens by a metal wire net. Therefore, the experiment has been done with two treatments and 6 replicates. The surface area of each house was 1000 m2 (20 × 50 m) and equipped with mechanical fans, automatic heating, and cooling systems. One week before starting the experiment, all of the studied traits were recorded to evaluate the carry-over effect of the different houses. In each house, there were 5000 broiler breeder hens and 420 roosters. The house temperature set around 20–21°C, and the relative humidity was about 50-55%. The lighting program was as 15 h light: 9 h dark. Tunnel fans were used for houses ventilation. Wood shavings were used as houses’ bedding.
2.2. Experimental diets
Basal corn-soybean meal diets were formulated for birds (). Basal diets were supplemented with 1000 g probiotic/1000 kg diet to make probiotic treatments. No antibiotic and coccidiostat were used during the trial. Hens had ad libitum access to water but restricted access to daily feed (159 g/hen/day). The probiotic used in the study containing 2 × 107 cfu/g Bacillus subtilis PB6 and was supplied by Kemin® (Herentals, Belgium). For mixing probiotic with experimental diet, probiotic was added to 2 kg of wheat bran and mixed manually. Wheat bran contained probiotic added to other ingredients of the diet and mixed. The experimental feed prepared 3 times a week and kept in airtight bags at a temperature of 18–19°C. About 15 cm was considered as feeder space for each bird and the feed distribution in each pen was done one time a day (30 min after lighting, 5:30 AM).
Table 1. Ingredients and nutrient content of the basal dietsTable Footnotea.
2.3. Productive performance
To determine the body weight, 65 birds were randomly selected from each pen and painted with green colour paint. The body weight of selected birds in each pen was recorded weekly. Feed allocation was managed according to the egg production and body weight of the birds. Average daily feed consumption was 159 and 160 g/day per bird at 56–62 and 63 weeks of age, respectively. Eggs were collected from manual laying nests in each pen five times a day. Hen day egg production and mortality was recorded daily.
2.4. Egg quality and hatchability
The egg weight, settable eggs, cracked eggs, and dirty eggs were evaluated daily. Eggshell thickness was measured during 60–63 weeks of age. In order to evaluate eggshell thickness, 20 eggs from each house were broken and shell thickness in middle, narrow and width parts of eggs was determined by a digital micrometer. Egg haugh unit was calculated at 61 and 63 weeks of age. The average of the 2 measurements of thick-albumen height (one near to yolk and the other at the end of dense albumen) together with egg weight were used to calculate the haugh unit score for each individual egg according to Haugh (Citation1937) formula.
Hatchability was measured weekly and calculated as a ratio of hatchlings number to total fertile eggs. For evaluating the hatchability, the fertile eggs were incubated at 37.5°C dry bulb and 55% relative humidity from 1 to 18 d of incubation, and 37.1°C dry bulb and 70% relative humidity from 19 to 21 d of incubation in a Petersime incubator.
2.5. Faecal microflora and litter moisture
At the end of experiment, ten faecal samples were collected under aseptic conditions from the selected birds in each house by pressing the outer wall of cloaca to push its content into sterile tubes. To determine Lactobacillus and Escherichia coli counts, 1-g samples were taken and serially diluted from 10−1 to 10−7 with sterile ice-cold anoxic phosphate buffer saline (Aalaei et al. Citation2018). Lactobacillus count was performed in Rogosa agar after incubation in an anaerobic chamber at 37°C for 48 h. The E. coli count was performed in MacConkey agar after aerobic incubation at 37°C for 24 h. The numbers of colony-forming units (CFUs) were expressed as log10 CFU per gram (Tuohy et al. Citation2002).
Faecal scores were evaluated as 1 = good, 2 = semi watery, and 3 = watery by 30 observations at 10 different locations of each house. Litter moisture was measured precisely at the end of the experiment. Litter moisture was measured by weighing a 500 g sample of litter from each house, then, drying the sample for 72 h at 65°C and weighing it again (Shao et al. Citation2015).
2.6. Blood parameters
At the 63th week, 5 ml of blood was collected from the brachial vein of 10 birds in each house. Subsequently, the blood serum was separated and its total cholesterol concentration was measured by the method of Zlatkis et al. (Citation1953) using a cholesterol diagnostic kit (Pars Azmoon kit, kit ref. number 150010, Pars Azmoon Inc., Tehran, Iran). Leucocyte cells [heterophil (H), lymphocyte (L)] were counted per individual smear, and the H: L ratios were calculated (Gross and Siegel Citation1986).
2.7. Economic evaluating
The feed cost per hatchling number was calculated as ‘feed cost/ hatchling number’ (Carvalho et al. Citation2015). Return on investment (ROI) was used as a primary measure of an investment’s profitability (Zaghari et al. Citation2017). For calculation of ROI the benefit of investment was divided by the cost of the investment, and the result is expressed as a percentage as ROI = (Gains from Investment – Cost of Investment) /Cost of Investment.
2.8. Statistical analysis
The experiment was conducted using a split-plot in place design with 2 treatments and 6 replicates. The data were analysed using the GLM procedure of statistical analysis system, (SAS 9.4; SAS Citation2002, Institute Inc., Cary, NC) for analysis of variance and the comparison of means was made using Duncan’s Multiple Range Test at the level of P < 0.05.
3. Results
3.1. Productive performance
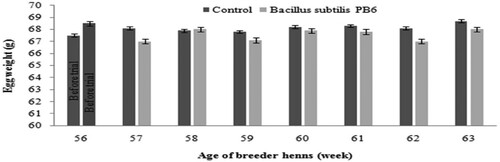
The effect of dietary supplementation of Bacillus subtilis PB6 (Clostat®) on egg weight of broiler breeders is illustrated in . Supplementation of Bacillus subtilis PB6 in broiler breeders diet had no significant effect on egg weight from 57 to 63 weeks of age.
Figure 1. Effect of Bacillus subtilis PB6 supplementation on egg weight of broiler breeder hens during 57–63 weeks of age. Values are presented as means ± Standard error.
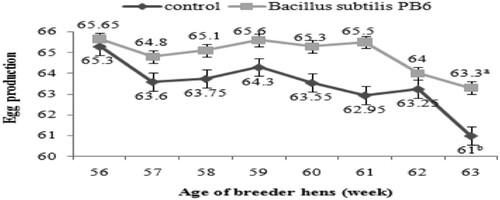
The results revealed that dietary supplementation of Bacillus subtilis PB6 led to an improvement in egg production compared with the control group and this improvement was significant (P < 0.05) in the 63th week (). The average improvement was about 1.16%.
Figure 2. Effect of Bacillus subtilis PB6 supplementation on egg production of broiler breeder hens during 57–63 weeks of age. values are presented as means ± sem; means lacking a common letter (a–b) differ significantly (P < 0.05).
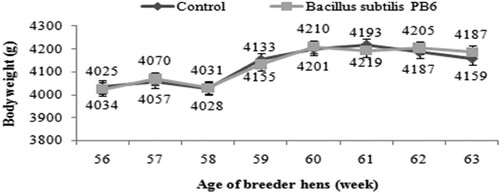
Probiotic supplementation did not have a significant effect on the body weight of the broiler breeders ().
Figure 3. Effect of Bacillus subtilis PB6 supplementation on body weight of broiler breeder hens during 57–63 weeks of age. Values are presented as means ± Standard error.
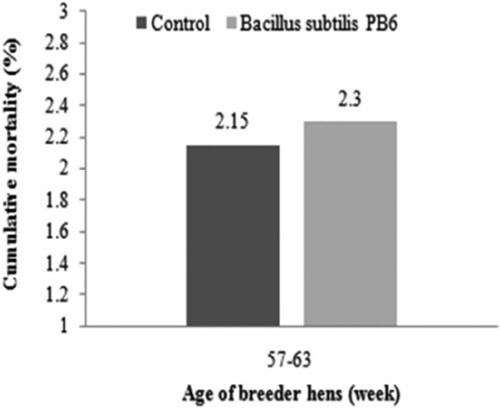
There was no significant difference in total mortality of the birds during whole period of the experiment (57–63 weeks, ).
3.2. Egg quality and hatchability
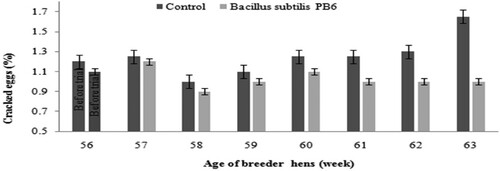
Results of measured external egg parameters are shown in and . Bacillus subtilis supplementation resulted in lower dirty and cracked eggs.
Figure 5. Effect of Bacillus subtilis PB6 supplementation on cracked eggs during 57–63 weeks of age. Values are presented as means ± Standard error.
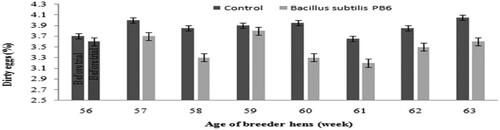
Figure 6. Effect of Bacillus subtilis PB6 supplementation on dirty eggs during 57–63 weeks of age. Values are presented as means ± Standard error.
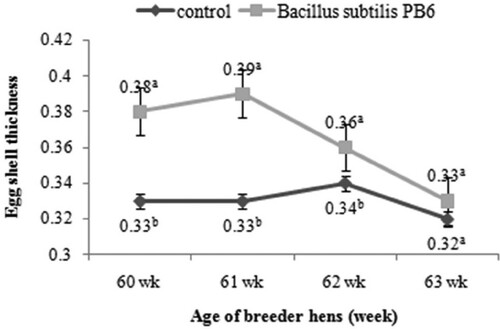
The results revealed that dietary supplementation of Bacillus subtilis PB6 increases eggshell thickness (). This improvement was significant in 60–62 weeks of age (P < 0.05).
Figure 7. Effect of Bacillus subtilis PB6 supplementation on eggshell thickness of broiler breeder hens during 60–63 weeks of age. Values are presented as means ± Standard error; means lacking a common letter (a–b) differ significantly (P < 0.05).
Dietary probiotic improved haugh unit (P < 0.05) from 61 to 63 weeks of age ().
Figure 8. Effect of Bacillus subtilis PB6 supplementation on egg haugh unit of broiler breeder hens during 61–63 weeks of age. Values are presented as means ± Standard error.
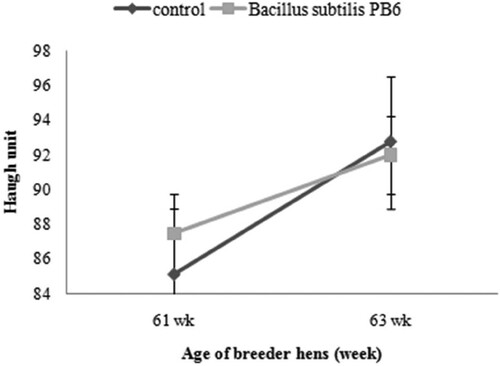
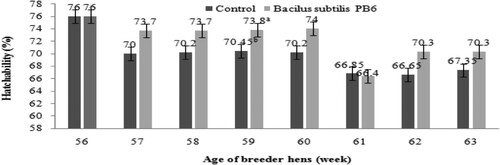
Due to improved egg quality and eggshell thickness, the number of hatching eggs increased about 0.14 percent (P < 0.05, ). According to data presented in , adding Bacillus subtilis PB6 to the breeders’ diet increased the hatchability of eggs about 2.56 percent (P < 0.05).
Figure 9. Effect of Bacillus subtilis PB6 supplementation on hatching eggs during 57–63 weeks of age. Values are presented as means ± Standard error.
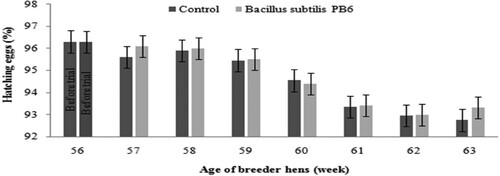
Figure 10. Effect of Bacillus subtilis PB6 supplementation on hatchability during 57–63 weeks of age. Values are presented as means ± Standard error; means lacking a common letter (a–b) differ significantly (P < 0.05).
The effect of Bacillus subtilis PB6 on hatchling number can be calculated by multiplying the average percentage of improvement in egg production, settable egg (hatching egg) and hatchability. Based on the obtained data, the calculation for the entire trial period (7 weeks) are as 1.16 × 0.14 × 2.56 = 0.41%. It means that Bacillus subtilis PB6 increased day-old chick production about 0.41 percent from 57 to 63 weeks of age.
3.3. Faecal microflora, faecal score and litter moisture
The effect of Bacillus subtilis PB6 supplementation on faecal bacterial count at 63 weeks of age is shown in . The results showed that there was no difference in the population of E. coli and lactobacilli in faecal sample of the birds (P > 0.05).
Table 2. Effect of Bacillus subtilis PB6 supplementation on faecal bacterial count (Log 10 CFU/g) of broiler breeder hens at 63 week.
A highly significant (P < 0.01) improvement was noticed in the faecal score of the birds fed with probiotic at 62 and 63 weeks of age. But, there was no difference in litter moisture of different groups ().
Table 3. Effect of Bacillus subtilis PB6 supplementation on faecal score and litter moister at the end of the experiment.
3.4. Blood parameters
Effect of Bacillus subtilis PB6 supplementation on blood parameters at 63 weeks of age is shown in . An increase in cholesterol was noticed in blood samples collected from hens fed with Bacillus subtilis supplemented diet (P < 0.01). Contrary to the ratio of heterophil to lymphocyte in the control group was higher than Bacillus subtilis PB6 fed group (P < 0.01).
Table 4. Effect of Bacillus subtilis PB6 supplementation on blood parameters and fecal bacterial count of broiler breeder hens at 63 weeks.
3.5. Economic evaluating
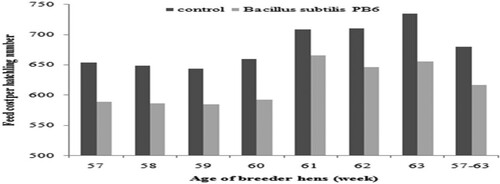
shows the effect of Bacillus subtilis PB6 supplementation on feed cost per hatchling number. Regards to the results of feed cost per hatchling number had an upward trend in both groups from 57 up to 63 weeks. However, Bacillus subtilis PB6 reduced the feed cost per 1-d-old chick both weekly and during the whole period of the experiment (P < 0.05).
4. Discussion
As mentioned before, dietary Bacillus subtilis PB6 supplementation significantly improved egg production in broiler breeders at the late phase of production but, egg weight was not significantly influenced by probiotic supplementation. Our results coincided with Panda et al. (Citation2008) who showed dietary supplementation of Lactobacillus sporogenes as probiotic in White Leghorn layer breeders diet significantly increased egg production and had no significant effect on egg weight. In contrast to our results, Mikulski et al. (Citation2012) showed feeding dietary probiotic Pediococcus acidilactici (PA) in laying hens did not significantly affect egg production but egg weight increased significantly. These may be a result of an inverse relationship between egg production and egg weight that is a well-known phenomenon.
In the present study, total mortality did not differ among the dietary groups during seven weeks of the experiment. Similar to our observations, previous studies revealed that the mortality of the laying hens (Khan et al. Citation2011) and broiler breeders (Aalaei et al. Citation2018) was not influenced by the supplementation of probiotics. This may be due to the fact that continuous feeding of probiotic might have suppressed the undesirable microorganisms that lead to improved health status (Khan et al. Citation2011).
Dietary Bacillus subtilis PB6 supplementation increased eggshell thickness at present study. Use of Lactobacillus sporogenes in White Leghorn (WL) layer breeders (Panda et al. Citation2008) and Pediococcus acidilactici in Hy-Line Brown laying hens (Mikulski et al. Citation2012) as probiotic improved eggshell thickness. Use of single- and multi-strain probiotics had no significant effect on eggshell thickness in broiler breeders (Aalaei et al. Citation2018).
The eggshell consists of 96% calcium carbonate and for each egg, about 2–2.5 g of calcium is required (Al-Batshan et al. Citation1994). It is clear that laying hen possesses a remarkable Ca homeostatic mechanism. They can balance the efficiency of intestinal Ca absorption, renal Ca excretion, and bone mineral metabolism with the bird's Ca requirements by specific hormones (Elaroussi et al. Citation1994; Sheoran et al. Citation2017). In the case of eggshell quality, other researchers have revealed that probiotic supplementation improves eggshell quality by enhancing calcium concentration in layers’ serum (Panda et al. Citation2008), and retention of phosphorous (Mutus et al. Citation2006) and calcium (Mutus et al. Citation2006; Mikulski et al. Citation2012). This may be related to the replication of lactic acid bacteria which facilitated the ionization of minerals (Mikulski et al. Citation2012) and reduced lumen pH (Sobczak and Kozlowski Citation2015; Forte et al. Citation2016).
Wilson (Citation2017) described that there are four key genes influencing ion transport, named ABCC9, ITPR2, KCNJ8, and WNK1 which regulate eggshell thickness. Also, it is reported that mamillary thickness and mammillary density can be influenced by ITM2C and KNDC1 genes (Wilson Citation2017). Brisbin et al. (Citation2008) studied the expression of immune genes in chicken cecal tonsil and spleen mononuclear cells in response to lactobacillus acidophilus. The mentioned researchers revealed that the expression of IL-18, IFN-alpha, and IFN-gamma genes was upregulated in cecal tonsil cells after treatment with Lacidophilus DNA (Brisbin et al. Citation2008). It is well known that bone tissue has an important role in calcium homeostasis. Calcium homeostasis is under the control of multiple factors such as pro-inflammatory cytokines. Bone is composed of support cells, osteoblasts and remodelling cells, osteoclasts and cytokines that are a potent activator of ostoclasts (Lucacl and Acalovschi Citation2012). TNF-α, IL-1, IL-13, IL-6, IL-7, IL-11, IL-15 and IL-17, are potent activators of osteoclasts (Lucacl and Acalovschi Citation2012). In chickens, it was indicated that receptor activator of nuclear factor-κB ligand (RANKL) which is also known as an activation-induced cytokine, member of the tumour necrosis factor (TNF) superfamily, is an important factor for inducing osteoclastogenesis, similar to that observed for mammals, so that the chRANKL-treated hens exhibited increased blood Ca++ levels (Wang et al. Citation2008). Therefore, it seems that improvement in eggshell thickness may be a result of Bacillus subtilis PB6 effect on up-regulating the expression of ABCC9, ITPR2, KCNJ8, WNK1, ITM2C, and KNDC1 genes or stimulating osteoclastogenesis by modulating the expression of cytokine genes.
Bacillus subtilis PB6 supplementation resulted in lower dirty and cracked eggs percentage, so it had a positive effect on settable eggs and hatchlings healthiness. In other studies, use of probiotic decreased damaged egg, included misshapen eggs, broken eggs, cracked eggs, and shell-less eggs (Khan et al. Citation2011; Mikulski et al. Citation2012; Aalaei et al. Citation2018; Fathi et al. Citation2018). The beneficial effects on eggshell thickness observed in the current study were directly associated with the reduction in the number of cracked eggs. It was suggested that the decreased damaged eggs ratio could be caused by increased calcium retention in layers (Khan et al. Citation2011).
Using Bacillus subtilis PB6 significantly increases hatching eggs. Shell thickness is one of the most influential egg parameters that plays an important role in the processes of embryo development and successful hatching (Narushin and Romanov Citation2002). Thus, due to improved egg quality and eggshell thickness, hatching eggs increased significantly in the present study.
Dietary Bacillus subtilis PB6 supplementation improved haugh unit during 61–63 weeks of age. In previous studies on Lohmann Pink laying hens (Zhang et al. Citation2012), Lohmann Brown laying hens (Chung et al. Citation2015) and Hy-Line W-98 laying hens (Khan et al. Citation2011) probiotic bacteria improved haugh unit. This improvement could be due to the increased bioavailability of essential amino acids capable of increased the amount of albumin. Probiotic supplementation in White Leghorn layer breeders (Panda et al. Citation2008), and broiler breeder (Aalaei et al. Citation2018) had no effect on haugh unit.
Bacillus subtilis PB6 supplementation in the broiler breeder diet increased eggs hatchability. Age and diet can influence embryogenesis and hatchability of broiler breeder eggs (Peebles et al. Citation2000). There was a relationship between the internal characteristics of the egg (albumin quality and haugh unit) and its hatchability (Narushin and Romanov Citation2002). A significant role of egg white quality in the embryogenetic process is reported (Reijrink et al. Citation2008). One reason for the observed improvement in egg hatchability of probiotic fed group could be due to improved haugh unit (Tona et al. Citation2002).
Broiler breeders fed the basal diet supplemented with B. Subtilis PB6 had lower feed cost (about 4.5%) per hatchling number in comparison to the control birds. This improvement was a result of increased hatchling numbers. Improvement in hatchling number was related to an increase in egg number, hatching egg and hatchability. Also, the return on investment (ROI) improved by Bacillus subtilis PB6 supplementation about 4.04%. In agreement with these results, research results by Zaghari et al. (Citation2017) revealed that using dietary Bacillus subtilis in broiler chickens increased ROI by decreasing feed cost per kg weight gain of the birds.
The faecal score of the birds fed with probiotic was better than control group. This might be due to improvement in hind gut health and less loose droppings from the cloaca. In broiler breeder farms, the litter condition has an important role in birds’ welfare, productive traits and reproductive abilities (Kaukonen Citation2017). Thus, improvement in faecal score can have a direct positive effect on the farm benefits.
The heterophil to lymphocyte (H/L) ratio is considered as a stress biomarker in birds. It was reported that the H/L reference values for low, mediate and high degrees of stress are 0.2, 0.5 and 0.8, respectively (Gross and Siegel Citation1993). In the present study, dietary probiotic could decrease H/L ratio in broiler breeders at the end of the experiment. This shows that adding probiotic can consider as a dietary strategy in reducing stress in broiler breeders. So, the birds’ welfare will improve in farm conditions.
Increased cholesterol level was noticed in serum samples of hens in the Bacillus subtilis PB6 supplemented group. In agreement to our result, Owosibo et al. (Citation2013), showed a significant increase in serum cholesterol value with the supplementation of probiotics in the diet of broiler chickens. While, Kawahara et al. (Citation1991) did not find any effect of probiotics on serum cholesterol. Some studies showed that probiotic supplementation in diets decreases the serum cholesterol levels in Hy-line layer chicks (Hatab et al. Citation2016) and yolk cholesterol concentration in Brown laying hens (Panda et al. Citation2003, Citation2008; Mahdavi et al. Citation2005; Mikulski et al. Citation2012).
Cholesterol serves as a precursor for the biosynthesis of bile acid. Synthesis of bile acids is a major route of cholesterol metabolism. About 95% of the bile acids which are delivered to the duodenum will be recycled by the enterohepatic circulation. In enterohepatic circuit, conjugated primary bile salts are reabsorbed actively into hepatic portal circulation. Bacteria deconjugate some of the primary and secondary conjugated bile salts back to lipid-soluble bile acids. Bile acids are passively absorbed into hepatic portal circulation and could more appropriately complete bile salts enterohepatic circulation. Bile salt hydrolase (BSH) is the enzyme responsible for bile salt deconjugation in the enterohepatic circulation and has been detected in probiotics indigenous to the gastrointestinal tract (Ooi and Liong Citation2010). Bacteria by deconjugating some of the primary and secondary conjugated bile salts and consequently passive absorption of lipid-soluble bile acids into hepatic portal circulation could improve enterohepatic circulation. On the other hand, the addition of probiotic improved histomorphological parameters of the duodenum and increase duodenum villi height (Awad et al. Citation2009; Gutierrez-Fuentes et al. Citation2013). Improvement in intestinal villi which plays a crucial role in absorption of nutrients could improve enterohepatic circulation in the step of absorption by the enterocyte and transport back to the liver. Bacillus subtilis PB6 as a probiotic either by deconjugating some of the primary and secondary conjugated bile salts or by improving intestinal morphology (increase in duodenum villi height) may improve enterohepatic circulation. Increased enterohepatic circulation of bile acids may increase cholesterol levels.
The count of colonies of Lactobacilli and E. coli were not affected by dietary treatments. In contrast, Patterson and Burkholder (Citation2003) reported that probiotic reduced E. coli population. The pathogen inhibition mechanisms for probiotic might be due to competition for nutrients, production of toxic compounds, competition for binding sites on the intestinal epithelium and stimulation of the immune system (Patterson and Burkholder Citation2003). However, the diversity in the results of researchers may be due to dosage of usage, method of usage, birds’ age and specious, farm or laboratory condition, health state of the birds, etc.
5. Conclusion
In conclusion, supplementation of Bacillus subtilis PB6 as natural functional additive improved egg production, hatchability, and the faecal score of broiler breeders under commercial farm conditions during 57–63 weeks of age. Also, adding Bacillus subtilis PB6 decreased heterophyl/lymphocyte ratio as a stress biomarker. So, it seems that adding Bacillus subtilis PB6 has the potential to improve the return on investment, and broiler breeders’ welfare. However, due to the worthiness of the issue and lack of adequate information about the exact mechanisms of Bacillus subtilis PB6 in broiler breeders physiological and biochemical processes, further investigations should be done in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aalaei M, Khatibjo A, Zaghari M, Taherpour K, Akbari Gharaei M, Soltani M. 2018. Comparison of single and multi-strain probiotics effects on broiler breeder performance, egg production, egg quality and hatchability. Br Poult Sci. 59:531–538. doi:10.1080/00071668.2018.1496400.
- Al-Batshan HA, Scheideler SE, Black BL, Garlich JD, Anderson KE. 1994. Duodenal calcium-uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult Sci. 73:1590–1596. doi:10.3382/ps.0731590.
- Apata D. 2008. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture of Lactobacillus bulgaricus. J Sci Food Agric. 88:1253–1258. doi:10.1002/jsfa.3214.
- Awad WA, Bohm J, Razzazi-Fazeli E, Ghapeeb K, Zentek J. 2006. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. 85:974–979. doi:10.1093/ps/85.6.974.
- Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J. 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 88:49–55. doi:10.3382/ps.2008-00244.
- Bar A, Hurwitz S. 1987. Vitamin D metabolism and calbindin (calcium binding protein) in aged laying hens. J Nutr. 117:1775–1779. doi:10.1093/jn/117.10.1775.
- Bar A, Vax E, Striem S. 1998. Effects of age at onset of production, light regime and dietary calcium, on performance, eggshell traits, duodenal calbindin, and cholecalciferol metabolism. Br Poult Sci. 39:282–290. doi:10.1080/00071669889268.
- Barbosa TM, Levy SB. 2000. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat. 3:303–311. doi:10.1054/drup.2000.0167.
- Bozkurt M, Kucukyilmaz K, Ayhan V, Cabuk M, Catli AU. 2011. Performance of layer or broiler breeder hens varies in response to different probiotic preparations. Ital J Anim Sci. 10(3):e31. doi:10.4081/ijas.2011.e31.
- Brisbin JT, Zhou H, Gong J, Sabour P, Akbari MR, Haghighi HR, Yu H, Clarke A, Sarson AJ, Sharif S. 2008. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev Comp Immunol. 32:563–574. doi:10.1016/j.dci.2007.09.003.
- Carvalho EH, Zilli JB, Mendes AS, Morello GM, Bonamigo D. 2015. Main factors that affect the economic effciency of broiler breeder production. Braz J Poult Sci. 17:11–16. doi:10.1590/1516-635(170111-16.
- Choct M. 2009. Managing gut health through nutrition. Br Poult Sci. 50:9–15. doi:10.1080/00071660802538632.
- Chung SH, Lee J, Kong C. 2015. Effects of multi strain probiotics on egg production and quality in laying hens fed diets containing food waste product. Int J Poult Sci. 14:19–22. doi:10.3923/ijps.2015.19.22.
- Dibner JJ, Richards JD. 2005. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 84:634–643. doi:10.1093/ps/84.4.634.
- Elaroussi MA, Forte LR, Eber SL, Biellie HV. 1994. Calcium homeostasis in the laying hen. 1. Age and dietary calcium effects. Poult Sci. 73:1581–1589. doi:10.3382/ps.0731581.
- Fathi M, Al-Homidan I, Al-Dokhail A, Ebeid T, Abou-Emera O, Alsagan A. 2018. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital J Anim Sci. 17:804–814. doi:10.1080/1828051X.2018.1425104.
- Forte C, Acuti G, Manuali E, Proietti PC, Pavonre S, Trabalza-Marinucci M, Moscati L, Onofri A, Lorenzetti C, Fraclosini M. 2016. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult Sci. 95:2528–2535. doi:10.3382/ps/pew164.
- Gross WB, Siegel HS. 1986. Effects of initial and second periods of fasting on heterophil/lymphocyte ratios and body weight. Avian Dis. 30:345346. doi:10.2307/1590539.
- Gross WB, Siegel PB. 1993. General principles of stress and welfare. In: T. Grandin, editor. Livestock, handling and transport. Wallingford: CAB International; p. 21–34.
- Gutierrez-Fuentes CG, Zuniga-Orozco LA, Vicente JL, Hernandez-Velasco X, Menconi A, Kuttappan VA, Kallapura G, Latorre J, Layton S, Hargis BM, Tellez G. 2013. Effect of a lactic acid bacteria based probiotic, flora max-b11, on performance, bone qualities, and morphometric analysis of broiler chickens: An economic analysis. Int J Poult Sci. 12:322–327. doi:10.3923/ijps.2013.322.327.
- Hajati H, Hassanabadi A, Teimouri Yansari A. 2014. The Effect of dietary supplementation of prebiotic and probiotic on performance, humoral immunity responses and egg hatchability in broiler breeders. Poult Sci J. 2:1–13. doi:10.22069/PSJ.2014.1485.
- Hatab MH, Elsayed MA, Ibrahim NS. 2016. Effect of some biological supplementation on productive performance, physiological and immunological response of layer chicks. J Radiat Res Appl Sc. 9:185–192. doi:10.1016/j.jrras.2015.12.008.
- Haugh RR. 1937. The haugh unit for measuring egg quality. US Egg Poultry Mag. 43:522–555.
- Kaukonen E. 2017. Housing conditions and broiler and broiler breeder welfare: the effect of litter condition on contact dermatitis in broilers and breeders, and the effect of elevated structures on broiler leg health [PhD thesis]. Finland: Faculty of Veterinary Medicine, University of Helsinki.
- Kawahara E, Ueda T, Nomura S. 1991. In vitro phagocytic activity of white spotted shark cells after injection with Aermonas salmonicida extra cellular products. Gyobyo Kenkyu. 26:213–214.
- Khan SH, Atif M, Mukhtar N, Rehman A, Fareed G. 2011. Effects of supplementation of multienzyme and multi-species probiotic on production performance, egg quality, cholesterol level and immune system in laying hens. J Appl Anim Res. 39:386–398. doi:10.1080/09712119.2011.621538.
- Kogut MH, Swaggerty CL. 2012. Effects of prebiotics and probiotics on the host immune response. Part 1, section 5. In: Direct-fed microbials and prebiotics for animals. Callaway TR, Rickes SC. Springer-Velag New York; p. 61–72.
- Li LL, Hou ZP, Li TJ, Wu GY, Huang RL, Tang ZR, Yang CB, Gong J, Yu H, Kong XF. 2008. Effects of dietary probiotic supplementation on ileal digestibility of nutrients and growth performance in 1-To 42-day-old broilers. J Sci Food Agr. 88:35–42. doi:10.1002/jsfa.2910.
- Lucacl C, Acalovschi M. 2012. Hormonal and cytokine implications in the pathophysiology of osteoporosis occurring in chronic liver diseases. JCM. 7:358–363.
- Mahdavi AH, Rahmani HR, Pourreza J. 2005. Effect of probiotic supplements on egg quality and laying hen's performance. Int J Poult Sci. 4:488–492. doi:10.3923/ijps.2005.488.492.
- Mathivanan R, Kalaiarasi K. 2007. Panchagavya and Andrographis paniculata as alternative to antibiotic growth promoters on haematological, serum biochemical parameters and immune status of broilers. J Poult Sci. 44:198–204. doi:10.2141/jpsa.44.198.
- Mead GC. 2000. Prospects for competitive exclusion treatment to control salmonellas and other foodborne pathogens in poultry. Vet J. 159:111–123. doi:10.1053/tvjl.1999.0423.
- Mikulski D, Jankowski J, Naczmanski J, Mikulska M, Demey V. 2012. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult Sci. 91:2691–2700.
- Mountzouris KC, Balaskas C, Xanthakos I, Tzivinikou A, Fegeros K. 2009. Effects of a multispecies probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br Poult Sci. 50:467–478. doi:10.1080/00071660903110935.
- Mutus R, Kocabagli N, Alp M, Acar N, Eren M, Gezen S. 2006. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci. 85:1621–1625. doi:10.1093/ps/85.9.1621.
- Narushin VG, Romanov MN. 2002. Egg physical characteristics and hatchability. World Poult Sci J. 58:297–303. doi:10.1079/WPS20020023.
- Niewold TA. 2007. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A Hypothesis Poult Sci. 86:605–609. doi:10.1093/ps/86.4.605.
- Ooi LG, Liong MT. 2010. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 11:2499–2522. doi:10.3390/ijms11062499.
- Owosibo AO, Odetola OM, Odunsi OO, Adejinmi OO, Lawrence AOO. 2013. Growth, haematology and serum biochemistry of broilers fed probiotics based diets. Afr J Agric Res. 1:051–056. doi:10.5897/AJAR2013.7593.
- Panda AK, Rama Rao SS, Raju MV, Sharma SS. 2008. Effect of probiotic (Lactobacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of white leghorn layer breeders. J Sci Food Agric. 88:43–47. doi:10.1002/jsfa.2921.
- Panda AK, Reddy MR, Rama Rao SV, Praharaj NK. 2003. Production performances, serum/yolk cholesterol and immune competence of White Leghorn layers as influenced by dietary supplementation of probiotic. Trop Anim Health Prod. 35:85–94. doi:10.1023/A:1022036023325.
- Patterson J, Burkholder K. 2003. Application of prebiotics and probiotics in poultry production. Poult Sci. 82:627–631. doi:10.1093/ps/82.4.627.
- Peebles E, Zumwalt C, Doyle S, Gerrard PD, Latour M, Boyle C, Smith T. 2000. Effects of breeder age and dietary fat source and level on broiler hatching egg characteristics. Poult Sci. 79:698–704. doi:10.1093/ps/79.5.698.
- Perreten V. 2003. Use of antimicrobials in food producing animals in Switzerland and the European Union (EU). Mitt Lebensm Hyg. 94:155–163.
- Reijrink IAM, Meijerhof R, Kemp BV, Brand H. 2008. The chicken embryo and its micro environment during egg storage and early incubation. Worlds Poult Sci J. 64:581–598. doi:10.1017/S0043933908000214.
- SAS Institute. 2002. SAS STAT user's guide. version 9.4. Cary, NC: SAS Inst. Inc.
- Shao D, He J, Lu J, Wang Q, Chang L, Shi SR, Bing TH. 2015. Effects of sawdust thickness on the growth performance, environmental condition, and welfare quality of yellow broilers. Poult Sci. 94:1–6. doi:10.3382/ps/peu003.
- Sheoran N, Bishnoi S, Shunthwal J, Maan NS. 2017. Effect of dietary inclusion of probiotics and prebiotics on external egg quality traits in White Leghorn layers. J Pharm Innov. 6 (11, Part A):8.
- Sobczak A, Kozlowski K. 2015. The Effect of a probiotic preparation containing Bacillus subtilis atcc Pta-6737 on egg production and physiological parameters of laying hens. Ann Anim Sci. 15:711–723. doi:10.1515/aoas-2015-0040.
- Teo AY, Tan HM. 2007. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT). J Appl Poult Res. 16:296–303. doi:10.1093/japr/16.3.296.
- Tona K, Bamelis F, De KB, Bruggeman V, Decuypere E. 2002. Effect of induced molting on albumen quality, hatchability, and chick body weight from broiler breeders. Poult Sci. 81:327–332. doi:10.1093/ps/81.3.327.
- Tuohy KM, Ziemer CJ, Klinder A, Knobel Y, Pool-Zobel BL, Gibson GR. 2002. A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microb Ecol Health Dis. 14:165–173. doi:10.1080/089106002320644357.
- Wang Y, Hou JF, Zhou ZL. 2008. Chicken receptor activator of nuclear factor-κB ligand induces formation of chicken osteoclasts from bone marrow cells and also directly activates mature osteoclasts. Poult Sci. 87:2344–2349. doi:10.3382/ps.2008-00142.
- Wilson PB. 2017. Recent advances in avian egg science: a review. Poult Sci. 96:3747–3754. doi:10.3382/ps/pex187.
- Wolfenson D, Frei Y, Berman A. 1982. Responses of the reproductive vascular system during the egg-formation cycle of unanaesthetised laying hens. Br Poult Sci. 23:425–431. doi:10.1080/00071688208447975.
- Yaripour M, Seidavi A, Dadashbeiki M, Laudadio V, Tufarelli V, Ragni M, Payan-Carreira R. 2018. Impact of dietary supra-nutritional levels of vitamins A and E on fertility traits of broiler breeder hens in late production phase. Agriculture. 8:149. doi:10.3390/agriculture8100149.
- Yu B, Liu JR, Hsiao FS, Chiou PWS. 2008. Evaluation of Lactobacillus reuteri Pg4 strain expressing heterologous β-glucanase as a probiotic in poultry diets based on barley. Anim Feed Sci Tech. 141:82–91. doi:10.1016/j.anifeedsci.2007.04.010.
- Zaghari M, Derakhshani-Diba M, Moravej H, Zahroojian N. 2017. Estimation of metabolizable energy equivalency of Bacillus subtilis spore in male broiler chickens. J Livest Sci Technol. 5:09–18. doi:10.22103/jlst.2017.9641.1176.
- Zhang JL, Xie QM, Ji J, Yang WH, Wu YB, Li C, Ma JY, Bi YZ. 2012. Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult Sci. 91:2755–2760. doi:10.3382/ps.2012-02339.
- Zlatkis A, Zak B, Boyle AJ. 1953. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 41:486–492. doi:10.5555/uri:pii:0022214353901255.