ABSTRACT
Clostridium perfringens type A infections are economically devastating to dairy farms. In spite of low morbidity, mortality is close to 100%. Although this disease has frequently been reported in calves, it is rarely seen in adult cattle, especially postpartum dairy cows. Sudden onset of symptoms, rapidly followed by death, was successively recorded in 14 postpartum dairy cows. Veterinary treatment was ineffective. The 14 cows fell ill after parturition, either within 24 h (fast invasion) or within 2–3 days (slow invasion). All the cows died shortly after symptom onset. Comprehensive analysis of epidemiology, clinical manifestation, autopsy results, and laboratory tests confirmed the 14 cases as most acute and acute C. perfringens type A infections. After the enactment of appropriate preventive and treatment measures, in conjunction with improved feeding management, no further symptoms of infection were observed in the postpartum cows. Our results demonstrated that autopsies and laboratory tests are required for the definitive diagnosis of this disease and highlighted importance of feeding management during the early perinatal period to prevent postpartum diseases in cows. This work also provides a reference for clinical veterinary diagnosis and the treatment of this disease, so as to reduce the economic losses of dairy farms.
Introduction
Clostridium perfringens, which is part of the normal gastrointestinal microbiota of humans and animals, is ubiquitous in nature; this bacterium is widespread in septic tanks, soil, sewage sludge, wastewater, food, and other sources (Goossens et al. Citation2017; Yanagimoto and Haramoto Citation2021; Johnston et al. Citation2022). C. perfringens is Gram-positive, spore-forming, anaerobic bacterium that can cause a variety of severe diseases, such as gas gangrene in humans and enterotoxaemia in animals (Goossens et al. Citation2016; Rahimoon et al. Citation2021; Takehara et al. Citation2021; Zhang et al. Citation2021). C. perfringens isolates have been classified into five toxin types (A, B, C, D, and E) (Lacey et al. Citation2019). This bacterium produces 15 strong exotoxins; of these, the alpha, beta, epsilon and iota toxin have the strongest pathogenicity(Sepehrifar et al. Citation2021). These toxins cause various types of tissue damage, including hemolysis, intestinal mucosal necrosis, and neurotoxicity (Navarro et al. Citation2018). C. perfringens infections are often deadly, leading to great economic losses to the dairy farming industry (Sun et al. Citation2018). C. perfringens type A infections represent an economically threat to dairy farms: although morbidity is rather low, mortality is close to 100%. Previous studies have shown that C. perfringens type A infections are frequent in calves, but this infection is rarely reported in adult cows, especially in postpartum dairy cows. In this study, we report the diagnosis and treatment of acute C. perfringens infections in postpartum dairy cows. This work provides a reference for clinical veterinary diagnostic and therapeutic strategies in response to C. perfringens infection, so as to reduce the economic losses to dairy farms.
Case presentation
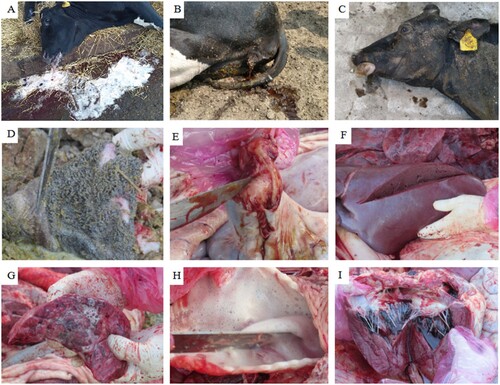
Between late July and early August 2021, 14 postpartum dairy cows at a dairy farm in Heilongjiang Province, China, died after the sudden onset of symptoms. Veterinary treatment was ineffective. The 14 cows became ill after parturition, some within 24 h (fast invasion) and some within 2–3 days (slow invasion). All of the cows died shortly after the onset of symptoms. All the cows were on concrete floors with wood shavings and dried rice husk bedding. The cows were fed TMR (total mixed ration), and all feed were ad libitum. The clinical characteristics of the cases (Additional file 1) were both neurological and respiratory. Symptoms appeared rapidly and included lying on the ground, moaning, tooth grinding, muscle shaking, convulsions, opisthotonos, bleating, red foamy liquid discharge from the mouth and nose cavity (A), a small volume of brown, soy-sauce-like urine discharged from the vulva (B), lateral recumbency with paddling of limbs, and one or two howls followed by sudden death. After death, the abdomen bulged, the tongue protruded out of mouth, conjunctival hyperemia was present, and anal eversion was observed (C). This disease only affected soon-to-be postpartum dairy cows (i.e. in the late perinatal period), the morbidity is 12.17% in soon-to-be postpartum dairy cows and the mortality is 100%, but there is no illness or death was recorded in any other individuals of the herd. Cows at this dairy farm were not inoculated against any diseases besides foot-and-mouth.
Figure 1. Clinical manifestations and autopsic abnormalities. A Lateral recumbency with paddling of limbs; individual bellowed several times after ejecting a bloody foam from the mouth and nasal cavity. B The brown, soy-sauce-like urine discharged from the vulva. C Tongue protrusion and conjunctival hyperemia after death. D Exfoliation of the gastric mucosa. E The intestinal mucosa, showing many petechiae; the intestine contained a dark-red, viscous liquid. F No obvious pathological injuries were observed in the liver. G Pulmonary congestion, emphysema, widening of interlobular septum, and foamy liquid on the section. H A large amount of foam-like mucus in the larynx and trachea, and bleeding of the trachea and throat mucosal membrane. I No obvious pathological injuries in the heart.
Autopsies
During autopsies, several histopathological abnormalities were observed, including pulmonary congestion, emphysema, widening of interlobular septum, foamy liquid on the section, bleeding and hyperemia in the pulmonary vessels, large volumes of foamy mucus in the larynges and tracheas, severe shedding of the mucous membrane in the stomach, viscous dark-red liquid in the small intestine, and many hemorrhagic spots in the small intestinal mucosa (D–I). In addition, some individuals presented with bleeding mesenteric lymph nodes, pericardial effusion, and/or bleeding spots on the surfaces of the heart and epicardium, and the section of the mesenteric lymph nodes was puce. No obvious pathological abnormalities were observed in the liver or spleen of any affected cows.
Laboratory analyses
During the autopsies, samples of the heart, liver, spleen, lungs, small intestine (and contents), and trachea were collected for laboratory analysis. Tissue samples were stored in a freezer at −20°C until analysis. The lung, liver, small intestine, small-intestine content, and tracheal secretion samples from the dead cows, as well as blood smear samples taken before death, were Gram stained. Large, Gram-positive bacilli were found in the small intestines and the small-intestine contents. These bacilli were obtusely rounded at both ends and were observed either singly or in groups of two to three connected end-to-end (A). No such bacilli were found in the lung, liver, or blood, but abundant leukocytes and granulocytes were observed in these samples. Given the clinical symptoms and the autopsy results, primers for C. perfringens alpha toxin (CPA), bovine rotavirus (BRV), bovine coronavirus (BCV), bovine viral diarrhea virus (BVDV), and bovine adenovirus (BADV) were designed based on the corresponding sequences in GenBank (). The contents of the small intestines were amplified with qRT-PCR using these primers, following the manufacturer’s instructions (Beijing ComWin Biotech Co., Ltd., Beijing, China). A single band was amplified, consistent with the target fragment of C. perfringens type A (B).
Figure 2. The results of the laboratory analyses. A Gram staining of the contents of the small intestine (20×), showing Gram-positive bacilli, obtusely rounded at both ends, appearing singly or in groups of two or three connected end-to-end. B Agarose gel electrophoresis of the qRT-PCR products amplified from the intestinal contents. Lane #1: C. perfringens alpha toxin, showing an amplification band at 324-bp, consistent with C. perfringens type A. Lane #2: Bovine rotavirus, showing that no DNA was amplified by the qRT-PCR. Lane #3: Bovine coronavirus, showing that no DNA was amplified by the qRT-PCR. Lane #4: Bovine viral diarrhea virus, showing that no DNA was amplified by the qRT-PCR. Lane #5. Bovine adenovirus, showing that no DNA was amplified by the qRT-PCR. Lane M: DNA maker (DL2000).
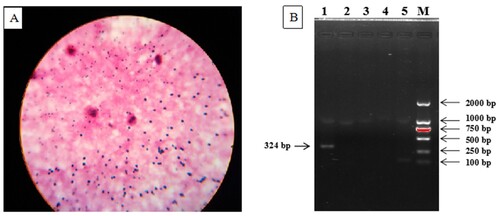
Table 1. Primers sequences used for multi-qRT-PCR.
Prevention and therapeutic strategy
Based on epidemiological evidence, clinical manifestations, autopsies, and the laboratory results, the 14 cases were confirmed as acute or most acute C. perfringens type A infections. After this diagnosis, prevention and therapeutic strategies were employed, including the emergency inoculation of the dairy cow herd with a vaccine against bovine C. perfringens and the adjustment of the calcium-to-phosphorus ratio in the feed. Sick cows were injected with hyperimmune serum against bovine C. perfringens and simultaneously received intramuscular injections of penicillin and streptomycin. After the enactment of these preventative and treatment measures, no further instances of sickness in the postpartum cows were recorded.
Discussion
C. perfringens type A has been implicated in sudden deaths (Zhu et al. Citation2017); these infections can occur in any season. C. perfringens type A is the C. perfringens type most frequently isolated from samples of human, animal, or environmental origin (Qing and Guisheng Citation2019). The duration of disease varies, from a few minutes or a few hours to three or four days or even longer (Guo Citation2019). This disease occurs sporadically rather than in concentrated outbreaks (Zheng et al. Citation2010). The mortality rate of dairy cattle infected with C. perfringens type A is 70%–100% (Guo Citation2019). Most infected cows are high-yield and in good to excellent health. C. perfringens type A produces CPA and several other toxins, including CPE and CPB2 (Uzal et al. Citation2018). Information about pathogenesis of type A enteric infections in ruminants is minimal and often contradictory, but it is generally assumed that most clinical signs and lesions are due to the effects of CPA. Based on clinical symptoms, C. perfringens infections are divided into three categories: most acute, acute, and subacute. Here, 11 of the 14 cases had symptoms consistent with the most acute infection, while the symptoms of the remaining three cases were consistent with the acute infection. No cases had subacute symptoms.
The main pathological features of typical C. perfringens infections include hemorrhages in the systemic parenchyma and small intestine, as well as petechiae on the heart surface, pericardial effusion, and pulmonary emphysema and/or petechiae. Humans or animals infected with C. perfringens also present purple-black livers, with surface petechiae; many petechiae in the intestinal mucosa; dark-red, viscous liquid in the intestine; and swollen, hemorrhagic lymph nodes that are dark-brown in section. Here, the pathological features of the lung, stomach, small intestine, and mesenteric lymph nodes noted in the autopsy were similar to the symptoms typical of C. perfringens infection. However, a few obvious differences to typical C. perfringens infections were identified: First, only a few of the dead cows presented with pericardial effusion or myocardial hemorrhage. Second, although the livers of the affected cows were purple-black, there were no obvious hepatic petechiae. Finally, while the autopsy revealed severe damage to the lungs, these injuries were similar to the effects of mycoplasma pneumonia, and adhesion between the pleura and lungs was observed in the first few cows to die. These pathological inconsistencies with C. perfringens infection affected our initial diagnosis. Based on pulmonary pathology, we diagnosed infectious mycoplasma pneumonia in the first few dead cows. Indeed, due to the hot weather at the time of death, the rapid expansion of corpse abdomen could be considered a natural phenomenon. For this reason, we did not initially consider C. perfringens infection. This initial diagnosis error delayed the institution of appropriate prevention and treatment measures for C. perfringens infection. In the period of time following the incorrect diagnosis, postpartum dairy cows continued to die suddenly. Finally, these cases were diagnosed as most acute/acute C. perfringens type A infection following comprehensive analysis. Because C. perfringens type A is a commensal bacterium, it is not possible to detect and diagnose these infections using a single method (e.g. the identification of the alpha toxin in the gastrointestinal tract). Accurate diagnosis can only be made by comprehensively analyzing epidemiology, clinical symptoms, autopsy, bacterial culture, and laboratory tests. In addition, it is worth noting that animal cadavers do not always exhibit all of the pathological features of given disease at autopsy; indeed, only one or two of the main pathological characters are typically exhibited in most cases. Therefore, we cannot mechanically expect textbook diagnostic features; our misdiagnosis of the first few cases reported here is a tragic lesson.
The 14 cases of C. perfringens type A infection reported herein occurred only in dairy cows shortly after parturition, and no other cows were affected. This infection may have been restricted to postpartum cows due to the dramatic changes in endocrine function peri- and postpartum, in conjunction with the strain of parturition and the stimulation of lactation. These physiological stressors may have decreased immune resistance or caused imbalances in the intestinal flora. Indeed, the body undergoes a comprehensive adaptative changes, which include the endocrine and immune systems, to meet the challenges of parturition and lactation (Ceciliani et al. Citation2018; Velázquez et al. Citation2019). In addition, the abrupt change in diet after parturition (i.e. the shift from high fibre roughage in the dry milk period to high-precision carbohydrate-heavy feed postpartum) provided suitable conditions for the survival and reproduction of C. perfringens. That is, a sudden increase in dietary carbohydrate has been implicated in C. perfringens type A infections (Hou et al. Citation2022), possibly because undigested carbohydrates in the feed enter the small intestine, creating an imbalance in the microbial flora and enabling the proliferation of C. perfringens (Zebeli et al. Citation2015; Prescott et al. Citation2016; Wankhade et al. Citation2017). Alternatively, environmental conditions may have driven the C. perfringens type A infections examined here. July and August 2020 were very hot. The parturition barn at the farm in question is densely packed and ventilation is poor, which may have led to severe heat stress in the postpartum dairy cows. In postpartum dairy cows, heat stress reduces voluntary feed intake (VFI), leading to metabolic and nutritional disorders, which exacerbate the negative energy balance postpartum and decrease immunity; as a result, susceptibility to disease may increase (De Rensis et al. Citation2017). In conjunction with these stressors, lactation pressure may have weakened neutrophil function, lessening the immune response and leaving the cow unable to resist toxin invasion. These four factors may have been the main causes of C. perfringens infections in the postpartum dairy cows.
The key aspect of the control strategy for this disease is prevention. This is because bovine C. perfringens infections are urgent, rapid-developing, critical diseases with high mortality rates; here, the cows typically died before they could be treated. Regular vaccinations effectively prevent the C. perfringens infections, especially in perinatal cows. Here, after were confirmed our diagnosis, a series of comprehensive prevention and control measures were enacted, including emergency vaccinations, adjustment of the calcium-to-phosphorus ratio in the feed, reducing the ratio of postpartum high-precision materials, providing high-quality hay, reducing the feeding density of the cow herd, improving ventilation, and providing spray cooling. These measures adequately controlled the disease, and no additional infections were recorded.
The cases described in this report were confirmed to be most acute and acute C. perfringens type A infections based on epidemiology, clinical signs, autopsy, and laboratory tests; the mortality rate after infection was 100%. While the timing of symptom onset varied among cases in previous reports, this disease often occurred in calves in excellent health, often without premonitory signs of illness (Liu et al. Citation2019). C. perfringens type A infections are less frequently reported in adult cows, especially immediately postpartum. Here, we report cases of enterotoxaemia in which the key clinical symptoms (twitching and red foamy liquid discharge from the mouth and nasal cavity) were observed 30 min after the cow suddenly collapsed. Although primary care was effective in those cases, the cows died 24 h after parturition. To our knowledge, these cases are the most acute onset of enterotoxaemia ever reported in dairy cows. Therefore, the results of this case report will act as a reference for clinical veterinarians diagnosing and treating C. perfringens type A infections in postpartum cows, reducing the economic losses of dairy farms.
Conclusions
This report on the diagnosis and treatment of 14 cases shows that comprehensive assessments, including epidemiology, clinical symptoms, autopsy, and laboratory tests, are necessary for definitive disease diagnoses. Laboratory tests were particularly important. Our results highlighted the importance of feeding management in the early perinatal period for the prevention of postpartum diseases in dairy cows. This work provides a reference for clinical veterinary diagnosis and treatment of C. perfringens type A infections, so as to reduce the economic losses of dairy farms.
Author Contributions
WL and CHJ were involved in the case analysis and were responsible for writing the manuscript. HS and JJ were involved in the draft preparation and case analysis. ZMX and WL were involved in the case autopsy and analysis. ZD, LS and SY were involved in the laboratory tests. WL and HS were involved in the coordination of the case and were responsible for the interpretation of the results. All the authors read and approved the final version of the manuscript.
Consent to publication
Written consent was obtained from the present owners of the cows for publication of this case report and any accompanying images.
TAAR_2078329_Supplementary_video
Download MP4 Video (27.3 MB)Acknowledgements
We thank the Laboratory of Infectious Diseases, College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, especially Professor Hongbo Ni, for excellent technical assistance for the laboratory analyses. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Data Availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Disclosure statement
Author Jidong Jin is employed by COFEED FEEDMILL (Changchun) CO, LTD, Changchun, Jilin Province, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Ceciliani F, Lecchi C, Urh C, Sauerwein H. 2018. Proteomics and metabolomics characterizing the pathophysiology of adaptive reactions to the metabolic challenges during the transition from late pregnancy to early lactation in dairy cows. J Proteomics. 178:92–106. doi:10.1016/j.jprot.2017.10.010.
- De Rensis F, Lopez-Gatius F, García-Ispierto I, Morini G, Scaramuzzi R. 2017. Causes of declining fertility in dairy cows during the warm season. Theriogenology. 91:145–153. doi:10.1016/j.theriogenology.2016.12.024.
- Goossens E, Valgaeren BR, Pardon B, Haesebrouck F, Ducatelle R, Deprez PR, et al. 2017. Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: a review on bovine necro-haemorrhagic enteritis. Vet Res. 48(1):9. doi:10.1186/s13567-017-0413-x.
- Goossens E, Verherstraeten S, Valgaeren B, Pardon B, Timbermont L, Schauvliege S, et al. 2016. Toxin-neutralizing antibodies protect against Clostridium perfringens-induced necrosis in an intestinal loop model for bovine necrohemorrhagic enteritis. BMC Vet Res. 12(1):101. doi:10.1186/s12917-016-0730-8.
- Guo Y. 2019. Diagnosis and comprehensive control of clostridiosis welchii in cattle and sheep. Animal Husbandry Feed Science. 40(6):110–112. doi:10.16003/j.cnki.issn1672-5190.2019.06.030.
- Hou ZD, Zu XZ, Weng YB, Ge JJ, Wu ZY. 2022. Predisposing factors and prevention of clostridium enteritis of calves in large-scale dairy farms. Grass-Feeding Livestock. 6:32–36. doi:10.16863/j.cnki.1003-6377.2020.06.006.
- Johnston M, Whiteside T, Allen M, Kurtz D. 2022. Clostridium perfringenstoxigenic profile of strains isolated from Natural ingredient laboratory animal diets. Comp Med. 72(1):50–58. doi:10.30802/AALAS-CM-22-000013.
- Lacey J, Johanesen P, Lyras D, Moore R. 2019. In silico identification of novel toxin homologs and Associated mobile genetic elements in. Pathogens (Basel, Switzerland). 8(1):16. doi:10.3390/pathogens8010016.
- Liu ZL, He MR, Zhou JL, He BN, Wang T, Zhao SQ, Wang L, Zhang SX, Liu SS, Yue S. 2019. Detection and analysis of the major toxin genes of cattle Clostridium perfringens isolates. J Heilongjiangbayi Agricultural University. 31(4):21–27. doi:10.3969/j.issn.1002-2090.2019.04.004.
- Navarro M, McClane B, Uzal F. 2018. Clostridium perfringensmechanisms of action and cell death Associated with toxins. Toxins (Basel). 10(5):212. doi:10.3390/toxins10050212.
- Prescott J, Parreira V, Mehdizadeh Gohari I, Lepp D, Gong J. 2016. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathology: Journal of the WVPA. 45(3):288–294. doi:10.1080/03079457.2016.1139688.
- Qing LI, Guisheng YE. 2019. Sequence and protein structure analysis of virR gene of Clostridium perfringens type a isolated strain from qinghai. J Anhui Agri University. 46(5):800–805 (In Chinese). doi:10.13610/j.cnki.1672-352x.20190917.001
- Rahimoon MM, Zaman JK, Babar A, Mirani AH. Prevalence of enterotoxemia and antibiogram of Clostridium perfringens isolated from diarrheic goat in the vicinity of district Tharparkar, Sindh, Pakistan. 2021. doi:10.19045/bspab.2021.100044
- Sepehrifar H, Pilehchian Langroudi R, Ataei S, Haddadi A. 2021. Evaluation and comparison of Clostridium epsilon-alpha fusion gene Expression using different commercial Expression vector. Arch Razi Inst. 76(1):7–16. doi:10.22092/ari.2019.126604.1349.
- Sun WY, Huang XY, Yang QL, Yan ZQ, Wang PF, Gao XL, et al. 2018. Study on newborn piglet diarrhea induced by C. perfringens type C. Lanzhou (China): Gansu Agricultural University.
- Takehara M, Kobayashi K, Nagahama M. 2021. Clostridium perfringensToll-Like receptor 4 protects against infection in mice. Front Cell Infect Microbiol. 11:633440. doi:10.3389/fcimb.2021.633440.
- Uzal F, Navarro M, Li J, Freedman J, Shrestha A, McClane B. 2018. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe. 53:11–20. doi:10.1016/j.anaerobe.2018.06.002.
- Velázquez M, Peralta M, Angeli E, Stassi A, Gareis N, Durante L, et al. 2019. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim Reprod Sci. 206:1–10. doi:10.1016/j.anireprosci.2019.05.010.
- Wankhade P, Manimaran A, Kumaresan A, Jeyakumar S, Ramesha K, Sejian V, et al. 2017. Metabolic and immunological changes in transition dairy cows: A review. Veterinary World. 10(11):1367–1377. doi:10.14202/vetworld.2017.1367-1377.
- Yanagimoto K, Haramoto E. 2021. Isolation of alpha-toxin-deficient Clostridium perfringens type F from sewage influents and effluents. Microbiol Spectr. 9(1):e0021421. doi:10.1128/Spectrum.00214-21.
- Zebeli Q, Ghareeb K, Humer E, Metzler-Zebeli B, Besenfelder U. 2015. Nutrition, rumen health and inflammation in the transition period and their role on overall health and fertility in dairy cows. Res Vet Sci. 103:126–136. doi:10.1016/j.rvsc.2015.09.020.
- Zhang S, Liu P, Wang Y, Shen Z, Wang S. 2021. Multiresistance gene cfr(C) in Clostridium perfringens of cattle origin from China. J Antimicrob Chemother. 76(12):3310–3312. doi:10.1093/jac/dkab339.
- Zheng XL, Dou XM, Dao-Jun HU, Xiao Y, Zhao CR, Xue-Qin NI. 2010. The threat, prevention and control of Clostridium perfringens in cattle industry. China Animal Husbandry & Veterinary Medicine. 37(8):211–214.
- Zhu L, Zhou W, Wang T, Xiang H, Ji X, Han Y, et al. 2017. Isolation of Clostridium perfringens type A from wild bharals (Pseudois nayaur) following sudden death in Tibet, China. Anaerobe. 44:20–22.
