?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was conducted to investigate the effects of different mixture ratios of sorghum (S) and cluster bean (CB) on fermentation parameters, nutritional composition, and gas-methane production of mixture silages. Five different mixture ratios (100%S, 75%S+25%CB, 50%S+50CB, 25%S+75%CB and 100%CB) were used in this study. Effects of mixture ratios on entire parameters were highly significant (p ≤ 0.01). Decreasing ADF and NDF ratios and increasing crude protein, ash and oil contents and condensed tannin levels were observed with increasing cluster bean ratios of the mixtures. However, cluster beans reduced WSC and lactic acid and increased pH, butyric acid and ethanol contents. The lowest Fleig score was obtained from cluster bean silage (100%CB). The lowest gas-methane production was obtained from cluster bean silage and the greatest ME and NEL values were obtained from sorghum silage (100%S). The best OMD values were obtained from sorghum silage and 75%S+25%CB mixture silage. The present findings revealed that a 50%S+50%CB mixture yielded high nutritional composition and good fermentation parameters.
Introduction
Sorghum is quite resistant to drought and salinity-like various environmental stress factors (Li et al. Citation2010). Sorghum has also quite a high water use efficiency and thus is cultivated economically with high yields in various parts of the world under harsh conditions (Sanchez et al. Citation2002; Kaplan et al. Citation2020). With a high-water-soluble carbohydrate content and low buffering capacity, sorghum is ensilaged to be used in animal feeding (Podkówka and Podkówka Citation2011). High ADF and NDF ratios as compared to maize silage reduce the feed digestibility of sorghum silage (Prostko et al. Citation1998; Thomas et al. Citation2013).
Cluster bean is also resistant to drought and high temperatures (Singh Santosh Citation2014). It is a high-yield feed crop with pods and leaves rich in protein. Cluster beans could be harvested within 3–4 months after sowing (Suliman et al. Citation2017). Thus, it can be grown in regions where sorghum is cultivated. Besides growing under adverse environmental conditions, cluster bean has quite high protein content and low cell wall components (Kusvuran et al. Citation2019). However, since cluster bean is a leguminous crop, cluster bean silage has various drawbacks, thus additives or supplements should be used in cluster bean silage (Olfaz et al. Citation2019).
Mixed ensilage of high carbohydrate-containing plants and high protein-containing plants may offer a high-quality feed source (Curek and Ozen Citation2004). Cluster bean seems to be a good companion of sorghum in mixture silage. Previous researchers investigated quality traits of sorghum silage in a mixture with different leguminous crops including soybean (Lima et al. Citation2011; Ni et al. Citation2018), lablab bean (Contreras-Govea et al. Citation2011), jack bean and velvet bean (Lima-Orozco et al. Citation2014). However, sorghum has not been ensiled with cluster bean before. Such a mixture may offer a silage with high nutritional and fermentation characteristics.
Crude protein, organic acids, Fleig score, and gas and methane production are the most significant indicators of silage quality (Schenkel Citation1998; Kizilsimsek et al. Citation2017). Dry matter intake and dietary composition play great roles in gas-methane production in the rumen (de Oliveira et al. Citation2007). Cluster beans with low tannin content reduce the growth of methanogenic bacteria (Scalbert Citation1991) and may contribute to reductions in methane emissions (Makkar Citation2003). Rumen fermentation-induced methane emissions with significant contributions to global warming are largely used in feed quality traits (Lin et al. Citation2013).
Although cluster beans are commonly used in animal feeding (Rai Citation2015), there aren’t any comprehensive studies about mixed silage with sorghum. The optimal composition of sorghum and cluster bean mixture silage will provide a great source of feed for livestock, especially for ruminants. In the present study, five different mixture ratios of sorghum (Sorghum bicolor l.) and cluster bean (Cyamopsis tetragonoloba) were used and the effects of different mixture ratios on (i) fermentation quality, (ii) nutritional composition and (iii) gas-methane production, metabolizable energy and organic matter digestibility of mixture silages were determined.
Materials and methods
Forage material and silage preparation
This study was experimented with according to a completely randomized design. Sorghum was harvested at the milk-dough stage and cluster bean was harvested at the pod set period. Harvested plants were chopped into 2–3 cm pieces. The silages were prepared without CB (S100), or with CB 25% (S75CB25), CB 50% (S50CB50), CB 75% (S25CB75), and CB 100% (CB100) (). Chopped materials were homogeneously mixed and ensiled into 2 kg airtight plastic bags and kept in a dark room at 24 ± 2°C for 60 days. The silages were prepared in three replicates.
Table 1. Chemical composition and CT content of silages.
Analysis of chemical and organic acids
The silos were opened at d 60 after ensiling for the evaluation of chemical composition, organic acids, in vitro gas, and methane production. About 30 g of samples was mixed with 270 ml of distilled water in a mixer and pH (Mettler Toledo, Seven Compact, Switzerland) values were measured. About 500 g samples were dried until constant mass in an oven at 70°C and dry matter content was determined. Dried silage samples were ground to pass a 1 mm sieve and readied for chemical analyses. Crude ash (CA), Ether extract (EE) and crude protein (CP) contents were determined per AOAC (Citation1990). The neutral detergent fibre (NDF) and acid detergent fibre (ADF) contents were analysed using a fibre analyser (ANKOM 200, ANKOM Technology, NY, USA) based on the methods of Van Soest et al. (Citation1991).
The organic acid and ethanol contents were determined using high-performance liquid chromatography (HPLC; Shimadzu Corp., Kyoto, Japan), as described by Muck and Dickerson (Citation1988). The HPLC system consisted of a Shimadzu system controller (SCL-10A), a pump (LC-10AT) and a refractive index detector (RID-10A) (Shimadzu Corp., Kyoto, Japan) with a Bio-Rad Aminex HPX-87H column (Bio-Rad Lab., Hercules, CA).
The condensed tannin (CT) was 6]determined ground samples in a 1 mm sieve diameter mill 0.01 g of feed sample were weighed and put into a wide-mouthed bacteria tube. After adding 6 ml of tannin solution, it was boiled in a water bath for 1 h. Next, 3 ml of the sample was taken, and the spectrophotometer at 550 nm wavelength was read and calculated by the formula (Kamalak et al. Citation2010; Makkar et al. Citation1995). Water-soluble carbohydrate (WSC) contents were determined according to the phenol sulphuric acid method (Dubois et al. Citation1956).
In vitro gas and methane production
Total gas production (GP) and methane production of silage samples were determined using in vitro gas production technique followng the method specified by Menke et al. (Citation1979). Rumen fluids were taken from three fistulated hogget feed with 800 g alfalfa – 400 g barley ration. Hogget was supplied with clean water and licking stone ad-libitum. The rumen fluid to be analysed was taken before morning feeding, filtered through six layers of cheesecloth and mixed with twice as much artificial saliva solution. About 200 mg ground silage sample was incubated in rumen fluid buffered in a 100 ml glass syringe at 39°C for 24 h and gas content (ml) was determined. Total gas productions of silages were determined after the correction for blank and hay (alfalfa) standards (University of Hohenheim, Germany).
The methane content of the resultant gas was determined using an Infrared methane analysis device (Sensor Europe GmbH, Erkrath, Germany) (Goel et al. Citation2008). The following equation was used to determine methane production:
Metabolizable energy (ME) and in vitro organic matter digestibility (IVOMD)
The metabolizable energy (ME) and in vitro organic matter digestibility (IVOMD) of silage samples were calculated by the following equations using 24-hour gas production and some chemical composition parameters:
The net energy lactation (NEL) was calculated following the method of Blummel and Orskov (Citation1993):
where
GP: gas production at 24 h incubation (ml); CP: crude protein (%); EE: ether extract (%); CA: crude ash (%).
Fleig score was calculated using the following equation:
Statistical analysis
Experimental data were subjected to the analysis of variance with the use of SAS (Citation1999) software in accordance with a completely randomized design with three replications. Significant means were compared with the use of the LSD test.
Results
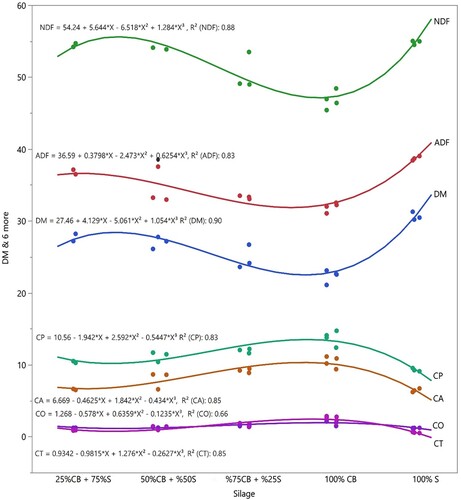
The chemical composition of the silages is provided in and . Cluster bean silage had quite low (22.24%) DM content. İncreasing DM values were observed with increasing sorghum ratios in mixtures. Decreasing ADF and NDF contents were observed with increasing cluster bean ratios of the mixtures. ADF and NDF contents of sorghum silage were 38.70 and 54.82%, respectively, and the values of cluster bean silage were 31.75 and 46.24, respectively. Cluster beans increased crude oil, crude ash and condensed tannin content of the mixture silages.
The fermentation parameters of the silages are provided in . Effects of mixture ratios on fermentation parameters were highly significant (p ≤ 0.01). pH values decreased and Propionic acid content, WSC, Fleig score and lactic acid content increased with increasing sorghum ratios of the mixture. Acetic acid and Butyric acid and ethanol content increased with increasing cluster bean ratios of the mixtures ().
Figure 2. Fermentation traits of sorghum and cluster bean mixture silages. ETOH: etil alcohol, PA: propionic acid, AA: acetic acid, LA: lactic acid, BA: butyric acid, WSC: water-soluble carbohydrate.
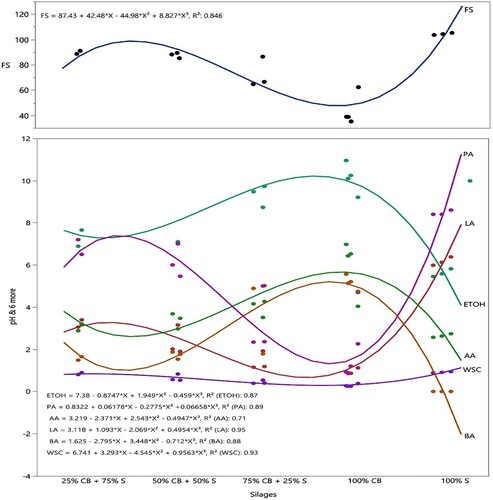
Table 2. Fermentation profiles and Fleig scores of silages.
The GP, methane production, ME, NEL and IVOMD of silages are shown in . The lowest GP and methane production were found in the CB100 silage (p < 0.01). The ME and NEL of S100 and S75CB25 silages were greater (p < 0.01) than that of S50CB50, S25CB75 and CB100 silages. The highest IVOMD was found in S100, S75CB25, and S50CB50 silages (p < 0.01) ().
Figure 3. The GP, methane production, IVOMD, ME and NEL of sorghum and cluster bean mixture silages. CH4: methane, ME: metabolizable energy, NEL: net energy lactation.
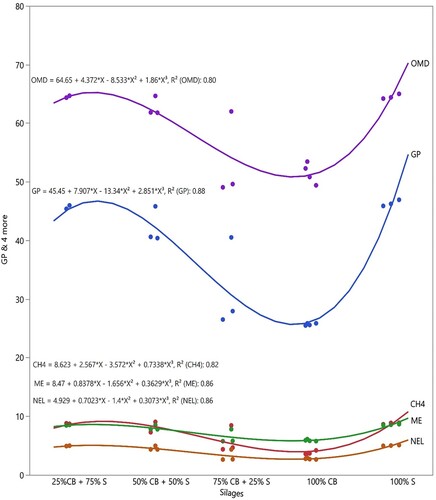
Table 3. The GP, methane production, ME, NEL, and IVOMD of silages.
Discussion
Nutritional composition, fermentation parameters, GP, and methane production of pure and mixture silages of sorghum and cluster bean were investigated. Significant improvements were achieved in silage nutritional and fermentation parameters with the mixture of Gramineae and leguminous species (Kizilsimsek et al. Citation2017). However, silage quality traits significantly vary with the species used in mixtures. Decreasing pH values and increasing dry matter, lactic acid contents, Fleig scores, gas-methane productions, ME, NEL and OMD values were observed with increasing sorghum ratios of the mixtures. Decreasing cell wall components (ADF, NDF) and increasing crude protein, ash and oil contents were observed with increasing cluster bean ratios of the mixtures.
Increasing volatile nitrogen, total nitrogen and mineral contents were reported with increasing legume ratios of the silage mixtures (Muhammad et al. Citation2008; Parra et al. Citation2019). The present cured ash values were similar to the values of Iqbal et al. (Citation2012). The present crude oil contents were greater than the values of Iqbal et al. Citation2012 and similar to the values of Suliman et al. (Citation2017) and Olfaz et al. (Citation2019). Low dry matter contents of cluster bean silage reached ideal dry matter contents with the inclusion of sorghum into the mixtures (Muck et al. Citation2003). Such ideal dry matter levels (25–35%) were achieved with ≥50% sorghum mixture ratios.
NDF and ADF values largely vary with the type, harvest time and different combinations of feeds (Aderinola et al. Citation2014). Cellulose and lignin-like cell wall components exist in greater quantities in shoots than in leaves, also lignin content is observed in legumes in greater quantities than in Graminae species (Aman Citation1993). However, as compared to other Graminae species, sorghum has greater cell wall components (Thomas et al. Citation2013). Slightly increasing ADF and NDF contents were observed with increasing sorghum ratios of the mixtures. In maize–legumes mixtures, decreasing ADF and NDF contents were reported with increasing maize ratios (Kizilsimsek et al. Citation2017). Decreasing NDF contents were reported for sorghum silages (Lima et al. Citation2011). Jahanzad et al. (Citation2016) reported decreasing ADF and NDF contents with increasing legume ratios of the mixtures. Cluster bean contains less fibre than sorghum. The present ADF and NDF contents of sorghum silage were similar to the values of Kaplan et al. (Citation2019) and ADF and NDF contents of cluster bean silages were similar to the values of Oflaz (Citation2019).
In legume–Graminae mixture silages, decreasing dry matter and soluble carbohydrate contents and increasing buffering materials (etc. minerals, protein) were observed with increasing legume ratios of the mixtures (Parra et al. Citation2019). Legume silage generally has higher acetic, butyric, and propionic acids, but not lactic acid, which may, in part, explain its higher pH. The buffering capacity is a characteristic that would be better when analysed in the forage before ensiling, as it can also partly explain the higher pH. (Muck et al. Citation2003). Since ensiled material is protected by lactic acid, lactic acid bacteria constitute the most significant microflora of the silage (Arslan and Cakmakci Citation2011). High soluble carbohydrate quantities of silage materials increase lactic acid quantities. Then, increasing lactic acid contents reduce ambient pH and prevent the formation of harmful microorganisms (Açıkgöz et al. Citation2002). Hart (Citation1990) reported the lactic acid content of sorghum silage between 2.6 and 3.1%. The present lactic acid values of sorghum silages were greater than those values. Increasing WSC values were observed with increasing cluster bean ratios of the mixtures. Such a case probably is related to the greater buffering capacity of cluster bean as compared to sorghum. WSC is an important parameter for silage fermentation and concentrations greater than 5% are accepted as reliable for quality silage (Ni et al. Citation2018). Desired WSC concentrations and a good fermentation were achieved with ≥50% cluster bean ratios.
Yücel et al. (Citation2017) conducted a study on maize–soybean silages and reported increasing acetic acid and NH3-N concentrations with increasing soybean ratios of the mixtures. High NH3-N concentrations, acetic acid, 2,3-butanol and ethanol contents were attributed to soybean-induced Enterobacter formations (McDonald et al. Citation1991). Enterobacteria could be responsible for increasing ethanol concentrations (Parra et al. Citation2019). Greater ethanol and 1-propanol concentrations supported the abiotic formation of ethyl (-acetate and -lactate) and propyl (-acetate) esters in silages with greater soybean ratios (Weiss Citation2017). Increasing ethanol and acetic acid contents were observed with increasing cluster bean ratios of the mixtures.
Crude oil, crude ash, ADF and lignin-based equations (De Boever et al. Citation1997) seem to be good estimating ME contents (Lima et al. Citation2011). In the present study, ME, NEL and OMD values were calculated using gas production, crude oil, protein and ash contents (Menke and Steingass Citation1988). The present ME contents of sorghum silages were similar to the values of Lima et al. (Citation2011) and Kaplan et al. (Citation2019). ME contents of mixture silages were similar to the ME values of sorghum – alfalfa silages (Kaplan Citation2011), maize – soybean silages (Kizilsimsek et al. Citation2017) and maize – alfalfa silages (Ozturk et al. Citation2006).
Gas production largely depends on fermentable carbohydrate quantities, thus fermentation-induced gas production is a good indicator of microorganism carbohydrate quantity (Kaplan et al. Citation2014; Blummel and Orskov Citation1993). Fermentation-induced gas production may also influence the tannin and saponin-like secondary metabolites (Kondo et al. Citation2014). Decreasing gas-methane productions were observed with increasing cluster bean ratios of the mixtures since cluster beans had lower carbohydrate content and greater tannin levels. According to anti methanogenic classifications (low (>110), medium (from 60 to 110), and high (<60)) of López et al. (Citation2010), the present mixtures had low anti-methanogenic potential. The anti-methanogenic potential of silages is an important parameter for animal nutrition and the environment since enteric methane released during fermentation contributes to global warming and results in feed energy losses. Following carbon dioxide, methane is the second gas inducing global warming. Despite low release rates, methane holds the heat emitted by the sun 23 times greater than carbon dioxide (Cengiz and Kamalak Citation2020). Of the digestible energy taken by ruminants, 2–12% is lost through spending on enteric methane production (Getachhew et al. Citation2005). About 2–3% condensed tannin contents are desired in feeds for the prevention of excessive and rapid protein degradation in the rumen (Barry Citation1989). Cluster bean samples had 2.74% condensed tannin contents and values decreased with mixture ratios.
Conclusions
The findings revealed that sorghum–cluster bean mixture silages had high fermentation capacity and nutritional composition and anti-methanogenic potential. Cluster bean increased crude protein, ash and oil content of the silages; sorghum reduced pH and increased dry matter and lactic acid contents. The 50% sorghum + 50% cluster bean and 75% sorghum + 25% cluster bean mixture silages were prominent for nutritional composition and fermentation parameters.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Açıkgöz E, Turgut İ, Filya İ. 2002. Silaj Bitkileri Yetiştirme ve Silaj Yapımı. Hasat Yayıncılık, Bursa.
- Aderinola OA, Lateef OA, Binuomote RT, Adeeyo A, Jekayinfa OA. 2014. Nutritional and microbial contents of varied combination of ensiled Panicum maximum and Vetiveria nigritana grass. Int J Food Agric Vet Sci. 4(1):141–148.
- Aman P. 1993. Composition and structure of cell wall polysaccharides in forages. In: Jung HG, Buxton DR, Hatfeld RD, Ralph J, editors. Forage cell wall structure and digestibility. Madison (WI): ASA, CSSA and SSSA; p. 183–199.
- AOAC. 1990. Official method of analysis. 15th ed. Washington (DC, USA): Association of Official Analytical Chemists; p. 66–88.
- Arslan M, Cakmakci S. 2011. Comparison of corn (Zea mays) and sorghum (Sorghum bicolor) silages mixed with different plants. Akdeniz Universitesi Ziraat Fakultesi Dergisi. 24:47–53.
- Barry TN. 1989. Condensed tannins; their role in ruminant protein and carbohydrate digestion and possible effects upon the rumen ecosystem. In: Nolan JV, Leng RA, Demeyer DIThe roles of protozoa and fungi in ruminant digestion. Armidale (Australia): Penambul Books; p. 153–169.
- Blummel M, Orskov ER. 1993. Comparison of € in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim Feed Sci Tech. 40:109–119.
- Cengiz T, Kamalak A. 2020. Determination of potential nutritive values and anti-methanogenic characteristics of salix babylonica leaves grown in different sites. KSU J. Agric Nat. 23(5):1351–1358.
- Contreras-Govea F, Marsalis M, Angadi S, Smith G, Lauriault LM, VanLeeuwen D. 2011. Fermentability and nutritive value of corn and forage sorghum silage when in mixture with lablab bean. Crop Sci. 51(3):1307–1313. doi:10.2135/cropsci2010.05.0282.
- Curek M, Ozen N. 2004. Feed value of cactus and cactus silage. Turk J Veterinary Anim Sci. 28(4):633–639.
- De Boever JL, Cottyn BG, De Brabander DL, Vanacker JM, Boucqué CV. 1997. Prediction of the feeding value of maize silages by chemical parameters, in vitro digestibility and NIRS. Anim Feed Sci Technol. 66:211–222. doi:10.1016/S0377-8401(96)01101-7.
- de Oliveira SG, Berchielli TT, dos Santos Pedreira M, Primavesi O, Frighetto R, Lima MA. 2007. Effect of tannin levels in sorghum silage and concentrate supplementation on apparent digestibility and methane emission in beef cattle. Anim Feed Sci Technol. 135(3-4):236–248. doi:10.1016/j.anifeedsci.2006.07.012.
- Dubois M, Giles KA, Hamilton JK, Roberts DA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem. 28:350–356.
- Getachew G, Robinson PH, Depeters EJ, Taylor S, Gisi DD, Higginbotham GE, Riordan TJ. 2005. Methane production from commercial dairy rations estimated using an in vitro gas technique. Feed Sci Technol. 123-124:391–402.
- Goel G, Makkar HP, Becker K. 2008. Effects of Sesbania sesban and Carduus pycnocephalus leaves and Fenugreek (Trigonella foenum-graecum L.) seeds and their extracts on partitioning of nutrients from roughage-and concentrate-based feeds to methane. Anim Feed Sci Technol. 147(1-3):72–89.
- Hart SP. 1990. Effects of altering the grain content of sorghum silage on ıts nutritive value. J Anim Sci. 68:3832–3842.
- Iqbal A, Akbar N, Khan HZ, Abbas RN, Ahmad J. 2012. Productivity of summer legume forages intercropped with maize as affected by mixed cropping in different sowing techniques. J Anim Plant Sci. 22(3):758–763.
- Jahanzad E, Sadeghpour A, Hashemi M, Keshavarz Afshar R, Hosseini MB, Barker AV. 2016. Silage fermentation profile, chemical composition and economic evaluation of millet and soya bean grown in monocultures and as intercrops. Grass Forage Sci. 71(4):584–594. doi:10.1111/gfs.12216.
- Kamalak A, Canbolat O, Atalay AI, Kaplan M. 2010. Determination of potential nutritive value of young, old and senescent leaves of Arbutus andrachne tree. J Appl Animal Res. 37(2):257–260. doi:10.1080/09712119.2010.9707136.
- Kaplan M. 2011. Effect of ensiling of alfalfa with sorghum on the chemical composition and nutritive value of silage mixtures. J Anim Veterinary Adv. 10(18):2368–2371.
- Kaplan M, Akar T, Kamalak A, Bulut S. 2014. Use of diploid and tetraploid hulled wheat genotypes for animal feeding. Turk J Agric For. 38:838–846. doi:10.3906/tar-1401-20.
- Kaplan M, Kale H, Kardes YM, Karaman K, Kahraman K, Yılmaz MF, Temizgul R, Akar T. 2020. Characterization of local Sorghum (Sorghum bicolor L.) population grains in terms of nutritional properties and evaluation by GT biplot approach. Starch-Stärke. 72(3–4):1900232. doi:10.1002/star.201900232.
- Kaplan M, Kara K, Unlukara A, Kale H, Buyukkilic Beyzi S, Varol IS, Kizilsimsek M, Kamalak A. 2019. Water deficit and nitrogen affects yield and feed value of sorghum sudangrass silage. Agric Water Manage. 218:30–36.
- Kizilsimsek M, Ozturk C, Yanar K, Ertekin I, Ozkan CO, Kamalak A. 2017. Associative effects of ensiling soybean and corn plant as mixtures on the nutritive value, fermentation and methane emission. Feb-Fresenius Environ Bull. 26:5754–5760.
- Kondo M, Hirano Y, Ikai N, Kita K, Jayanegara A, Yokota HO. 2014. Assessment of anti-nutritive activity of tannins in tea by-products based on in vitro rumen fermentation. Asian-Australasian J Anim Sci. 27(11):1571–1576.
- Kusvuran A, Uysal Can U, Baga M. 2019. Forage yield and quality of Guar (Cyamopsis tetragonoloba (L.) Taub.) harvested at different growing stages. Turkish J Nat Sci. 8(1):1–7.
- Li R, Zhang H, Zhou X, Guan Y, Yao F, Song G, Wang J, Zhang C. 2010. Genetic diversity in Chinese sorghum landraces revealed by chloroplast simple sequence repeats. Genet Resour Crop Evol. 57:1–15.
- Lima R, Díaz RF, Castro A, Fievez V. 2011. Digestibility, methane production and nitrogen balance in sheep fed ensiled or fresh mixtures of sorghum–soybean forage. Livest Sci. 141(1):36–46. doi:10.1016/j.livsci.2011.04.014.
- Lima-Orozco R, Van Daele I, Alvarez-Hernandez U, Fievez V. 2014. Combined conservation of jack bean and velvet bean with sorghum: evaluation of lab-scale silages and in vitro assessment of their nutritive value. J Agric Sci. 152(6):967–980. doi:10.1017/S0021859614000148.
- Lin B, Wang JH, Lu Y, Liang Q, Liu JX. 2013. In vitro rumen fermentation and methane production are influenced by active components of essential oils combined with fumarate. Anim Physiol Anim Nutr. 97(1):1–9. doi:10.1111/j.1439-0396.2011.01236.x.
- López S, Makkar HPS, Soliva CR. 2010. Screening plants and plant products for methane inhibitors. In: Vercoe PE, Makkar HPS, Schlink A, editors. In vitro screening of plant resources for extra-nutritional attributes in ruminants: nuclear and related methodologies. London (New York, USA): Springer Netherlands; p. 191–231.
- Makkar HPS. 2003. Effect and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin Res. 49:241–256. doi:10.1016/S0921-4488(03)00142-1.
- Makkar HPS, Blummel M, Becker K. 1995. Formation of complexes between polyvinylpolypyrrolidone or polyethylene glycols and tannins and their implication in gas production and true digestibility in uitro techniques. Brit J Nutr. 73:897–913.
- Mcdonald P, Henderson AR, Heron SJE. 1991. The biochemistry of silage. 2nd ed. Marlow (UK): Chalcombe Publications.
- Menke KH, Raab L, Salewski A, Steingass H, Fritzi D, Schneider W. 1979. The estimation of digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they incubated with rumen liquor in uitro. J Agric Sci. (Camb). 92:217–222.
- Menke KH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Devlop. 28.
- Muck RE, Dickerson JT. 1988. Storage temperature effects on proteolysis in alfalfa silage. Trans ASAE. 31:1005–1009.
- Muck RE, Moser LE, Pitt RE. 2003. Postharvest factors affecting ensiling. In: Buxton D.R., Muck R.E., Harrison J.H, editor. Silage science and technology. Agron. monogr. 42. Madison, WI: ASA, CSSA, and SSSA; p. 251–304.
- Muhammad LR, Baba M, Mustapha A, Ahmad MY, Abdurrahman LS. 2008. Use of legumes in the improvement of silage quality of Columbus grass (Sorghum almum Parodi). Res J Anim Sci. 2(4):109–112.
- Ni K, Zhao J, Zhu B, Su R, Pan Y, Ma J, Zhou G, Tao Y, Liu X, Zhong J. 2018. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour Technol. 265:563–567.
- Oflaz M, Kilic U, Yavrucu O. 2019. Determining potential feed value and silage quality of guar bean (Cyamopsis tetragonoloba) silages. Open Life Sci. 14(1):342–348.
- Olfaz M, Kilic U, Yavrucu O. 2019. Determining potential feed value and silage quality of guar bean (Cyamopsis tetragonoloba) silages. Open Life Sci. 14(1):342–348. doi:10.1515/biol-2019-0038.
- Ozturk D, Kizilsimsek M, Kamalak A, Canbolat O, Ozkan C. 2006. Effects of ensiling alfalfa with whole-crop maize on the chemical composition and nutritive value of silage mixtures. Asian-Australas J Anim Sci. 19(4):526–532. doi:10.5713/ajas.2006.526.
- Parra CS, Bolson DC, Jacovaci FA, Nussio LG, Jobim CC, Daniel JLP. 2019. Influence of soybean-crop proportion on the conservation of maize-soybean bi-crop silage. Anim Feed Sci Technol. 257:114295. doi:10.1016/j.anifeedsci.2019.114295.
- Podkówka Z, Podkówka L. 2011. Chemical composition and quality of sweet sorghum and maize silages. J Central Eur Agric. 12(2):294–303.
- Prostko EP, Muir JP, Stokes SR.. 1998. The influence of harvest timing on forage sorghum silage yield and quality. Stephensville (TX): Texas Agric. Exp. Stn.; p. 1–9.
- Rai DK. 2015. Trends and economic dynamics of guar in India, Working Paper, 311.
- Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT. 2002. Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Mol Biol. 48:713–726. doi:10.1023/A:1014894130270.
- SAS. 1999. SAS user’s guide. Statistic. Cary (NC): Statistical Analysis Systems Institute Inc.
- Scalbert A. 1991. Antimicrobial properties of tannins. Phytochemistry. 30:3875–3883. doi:10.1016/0031-9422(91)83426-L.
- Schenkel H. 1998. Methods for determination of energetic feed value scientific base and practical experience. Arch Anim Nutr. 51:155–165. doi:10.1080/17450399809381915.
- Singh Santosh K. An analysis of guar crop in India, GAIN Report Number: IN4035, USDA Foreign Agricultural Services, 2014.
- Suliman AIA, Daghash HA, Abd-Elati MNM, Mokhtar MH. 2017. Productive performance of growing Farafra lambs fed guar forage or guar silage. J Anim Poultry Prod Mansoura Univ. 8(3):41–47. doi:10.21608/JAPPMU.2017.45763.
- Thomas ME, Foster JL, McCuistion KC, Redmon LA, Jessup RW. 2013. Nutritive value, fermentation characteristics, and in situ disappearance kinetics of sorghum silage treated with inoculants. J Dairy Sci. 96(11):7120–7131. doi:10.3168/jds.2013-6635.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74(10):3583–3597.
- Weiss K. 2017. Volatile organic compounds in silages – effects of management factors on their formation: a review. Slov J Anim. Sci. 50:55–67.
- Yücel C, Avcı M, Inal İ, Yücel D. 2017. Yield and silage quality of soybean-maize intercrop under different mixing ratios and harvest stages. Int J Agron Agri R. 10:95–105.