Abstract
Although there are reports concerning a vascular adaptive response to stress in males, this is not yet defined in females. The aim of this study was to delineate functional gender differences in the rat vascular adaptive response to stress and to determine the ability of sex hormones to modulate the stress-induced vascular adaptive response. Responses to noradrenaline were evaluated in aortas, with and without endothelium, from intact, gonadectomized and gonadectomized-hormone-replaced males and females submitted or not to stress (2-h immobilization). Reactivity of the aorta of stressed and non-stressed intact males and females (n = 6–14 per group) was also examined in the presence of l-NAME or indomethacin. Stress decreased and gonadectomy increased maximal responses to noradrenaline in aortas with intact endothelium from both genders. Stress also reduced noradrenaline potency in males. In females, but not males, stress decreased the gonadectomy-induced noradrenaline hyper-reactivity to near that of intact non-stressed rats. Hormone replacement restored the gonadectomy-induced impaired vascular adaptive response to stress. l-NAME, but not indomethacin, abolished the stress-induced decrease in aorta reactivity of males and females. None of the procedures altered reactivity of aortas denuded of endothelium.
Conclusion: Stress-induced vascular adaptive responses show gender differences. The magnitude of the adaptive response is dependent on testicular hormones and involves endothelial nitric oxide-system hyperactivity.
Introduction
Among the vascular adaptive responses to stress that have been described in male animals are a decrease in the reactivity of the aorta to constrictor effects of noradrenaline (Lapshin et al. Citation1991; Cordellini and Vassilieff Citation1998) and an increase in the acetylcholine dilator response (Webb et al. Citation1987; Cordellini and Vassilieff Citation1998). These vascular alterations are dependent on both endothelial integrity and endothelial nitric oxide-system hyperactivity (Cordellini and Vassilieff Citation1998; Navarro-Oliveira et al. Citation2000; Cordellini et al. Citation2006). Although there are reports concerning vascular adaptive response to stress in males, this response is not yet defined in females.
The potential of estrogen replacement therapy in reducing cardiovascular pathologies in post-menopausal women, as well as the greater atherosclerosis incidence and risk of cardiovascular pathologies in men compared to pre-menopausal women, call attention to the role of sex hormones in cardiovascular responses (Isles et al. Citation1992; Hutchison et al. Citation1997; Khalil Citation2005, Villar et al. Citation2006). It is also increasingly recognized that sex steroids present, among many other effects, the ability to cause vasodilation, in a gender-independent fashion (Hutchison et al. Citation1997; Worboys et al. Citation2001). Nevala et al. (Citation1996), reported that male gender and ovarian hormone deficiency increase mesenteric artery reactivity to vasoconstrictor agents and reduce the vasodilatory effects of endothelium-dependent relaxing agents. Subsequently, Levenson et al. (Citation2001) reported that shear-mediated brachial artery vasodilation and vasoconstriction were more pronounced in women than in men, suggesting different gender-related sensitivity in the regulation of large-artery vascular tone.
Gonadal steroid input is important under various stress paradigms (Seale et al. Citation2004a,Citationb; Lund et al. Citation2006). The peripheral limbs of the stress system are the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic adrenomedullary (SA) system. Gonadal hormones alter the activity of the HPA-axis, so that testosterone inhibits it whereas estrogen increases its function (Viau and Meaney Citation1991; Handa et al. Citation1994; Ogilvie and Rivier Citation1997; Viau Citation2002; Lund et al. Citation2006). Androgens are also effective in inhibiting the catecholamine-stimulated secretion from bovine adrenal medulla chromaffin cells (Dar and Zinder Citation1997). Moreover, gender-specific autonomic responses to cardiovascular regulation, as well as gender differences in cardiac activity and hemodynamic response to stress have been reported (Evans et al. Citation2001; Shoemaker et al. Citation2001; Cankar and Finderle Citation2003; Ba et al. Citation2004). Despite these observations, the role of gonadal hormones in the development of the vascular adaptive response to stress remains to be determined in both sexes.
The aim of the present study was to delineate functional gender differences in the rat vascular adaptive response to stress and to determine the ability of sex hormones to modulate the stress-induced vascular adaptive response. Thus, we investigated the vascular reactivity in intact, gonadectomized and gonadectomized-hormone-replaced male and female rats submitted or not to stress, with emphasis on the endothelial cell function.
Materials and methods
Animals
Experiments were performed on male and female Wistar rats aged 14–18 weeks. They were housed five per cage 1 week before the study was initiated and had free access to food and water. They were exposed to a 12-h light–dark cycle at a controlled room temperature of 25 ± 1°C. Lights were switched on and off at 7:00 and 19:00 h, respectively. The rats were provided by the animal facilities of the University Estadual Paulista. Animal procedures were in accordance with the principles and guidelines of the National Council of Control of Animal Experimentation (CONCEA).
Stress exposure
The stressor consisted of immobilization for 2 h in a 5 × 27 cm metal tube, individually adapted to provide a tight restriction of movements, but allowing the animal to breathe normally. This single stress exposure took place between 10:00 and 14:00 h. Rats of both sexes aged 14–18 weeks were killed by guillotine decapitation immediately after stress exposure. During the stress session, the non-stressed rats were kept in the home cage.
Orchiectomy
Male rats aged 14 weeks were bilaterally orchiectomized (ORX) under ether anesthesia by a scrotal approach. The incisions were closed and the animals left in recovery. Sham-operated rats were submitted to the same procedures, except for the removal of the testes. Two weeks after surgery, these rats were submitted or not to the stress and immediately after were killed by decapitation for tissue collection. These rats had age-matched intact controls.
Ovariectomy
Female rats aged 14 weeks were bilaterally ovariectomized (OVX) under ether anesthesia by a dorsal approach. The incisions were closed and the animals left in recovery. Sham-operated rats were submitted to the same procedures, except for the removal of the ovaries. Two weeks after the surgery, these rats were submitted or not to the stress and immediately after were killed by decapitation for tissue collection. These rats had age-matched intact controls.
Gonadal hormone replacement
Two weeks after ORX or OVX, a group of rats received subcutaneous injections of testosterone propionate (0.1 mg/rat, once a day for 14 days) or 17β-estradiol-3 benzoate (100 μg/kg, once a day for 14 days), respectively. The same period of treatment and the same doses of these drugs provide effective replacement therapy in gonadectomized male and female rats (Knoll et al. Citation2000; Morschl et al. Citation2000). Twenty-four hours after the last injection, the rats were submitted or not to the stress and killed by decapitation for tissue collection. The age-matched intact controls received subcutaneous injection of vehicle (0.2 ml) during the same period.
Estrus induction
Intact female rats received 17β-estradiol-3 benzoate (20 μg/kg, sc) 24 h before the start of the experimental stress protocol (Arteche et al. Citation1997).
Body and organ weights
The weights of the body, aorta, uterus, vas deferens, and seminal vesicles were determined in intact and gonadectomized rats submitted or not to gonadal hormone replacement. Since the weights of the reproductive tissues correlates positively with sex hormonal levels, they were employed to assess the efficacy of gonadectomy, the efficacy of treatment with exogenous sex hormones and also to compare drug treatment with physiological endogenous hormonal effects. The same strategy has been employed by other authors (Zysow et al. Citation1997; Selvaraj et al. Citation2004; Ford et al. Citation2006).
Experimental protocols
Immediately after the rat had been killed, the descending thoracic aorta was excised and trimmed free of adhering fat and connective tissue. Two transverse rings of the same artery, each about 4 mm in length, were cut and mounted at the optimal length for isometric tension-recording in organ chambers, according to Cordellini et al. (Citation1990). One ring served as the control, while the endothelium was mechanically removed from the other by gently rubbing the luminal surface (Cordellini et al. Citation1990). The preparations were mounted in organ baths containing 7 ml of Krebs–Henseleit solution, with the following composition in millimolar: NaCl 113.0, KCl 4.7, CaCl2 2.5, NaHCO3 25.0, MgSO4 1.1, KH2PO4 1.2, ethylenediamine tetraacetic acid 0.03, and glucose 11.1. The bathing fluid, kept at 37°C, was saturated with a gaseous mixture of 95% O2 and 5% CO2. The preparations were allowed to equilibrate for at least 1 h under a resting tension of 1.5 g, which is optimal for inducing the maximum contraction. Tension was recorded by an F-60 microdisplacement myograph (Narco Bio-Systems Inc., Houston, TX, USA), and displayed on a physiograph. Preparations with and without endothelium, isolated from non-stressed rats and rats exposed to immobilization (stressed rats) were studied in parallel. Cumulative concentration-effect curves were constructed from the responses of the tissue to noradrenaline, and finally, single doses of acetylcholine (ACh; 10− 6 M) and sodium nitroprusside (SNP; 10− 4 M) were used to test the integrity of the endothelial and smooth muscle layers, respectively. This procedure was employed since ACh induces vasodilation by an endothelium-dependent mechanism, whereas SNP induces vasodilation by an endothelium-independent mechanism. Thus, loss of relaxant responses to ACh, but not SNP, occurs after endothelium removal.
The responses to noradrenaline were examined subsequent to the following procedures: stress, gonadectomy, gonadectomy plus stress, gonadectomy plus gonadal hormone replacement, and gonadectomy plus gonadal hormone replacement plus stress (experimental groups). The experimental animals had age-matched controls (non-stressed, untreated intact rats).
In another series of experiments, responses to noradrenaline in aortas from intact male and female rats submitted or not to stress were examined in the presence of Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10− 4 M, inhibitor of nitric oxide synthase) (Rees et al. Citation1990) or indomethacin (10− 5 M, inhibitor of prostaglandin generation) (Vane Citation1971). These drugs were added in the last 30-min stabilization period and remained in contact with the preparations until the end of the experiment. The aorta ring response in the presence of inhibitors was always compared to that of a ring from the same vessel in which a response in the absence of inhibitors was elicited simultaneously. The inhibitors did not significantly affect the basal tension.
Data analysis
The noradrenaline concentration producing a response that was 50% of the maximum (EC50) was calculated in each experiment. Mean EC50s are presented as geometric means with 95% confidence intervals. Maximal responses (gram of tension) to noradrenaline are presented as mean ± SEM. Statistical analysis was performed with multifactorial ANOVA (SIGMASTAT 2.0). Gonadectomy, gender and stress were factors in the analysis. Separate one-way ANOVA was performed when appropriate (InStat 3.01). Values were considered statistically significant when P < 0.05. In order to test differences among means, the Tukey–Kramer test for multiple comparisons was used.
Drugs and solutions
The following drugs were used: acetylcholine bromide, 17β-estradiol-3 benzoate, indomethacin, Nω-nitro-l-arginine methyl ester, noradrenaline bitartrate, sodium nitroprusside, and testosterone propionate (all obtained from Sigma Chemical Co., St Louis, MO, USA). All drugs were dissolved in Krebs–Henseleit solution, with the exception of 17β-estradiol and testosterone propionate which were dissolved in ethanol (70%) and diluted in corn oil, and also indomethacin, which was dissolved in Na2CO3 (0.1 M). In this last case the pH was adjusted to 7.4. All concentrations are expressed as final molar.
Results
The impact of castration and gonadal hormone replacement on body and organ weights is summarized in . The weight of aortas from male and female rats was not altered by gonadectomy alone or followed by the respective gonadal hormone replacement. Similarly, the body weight of female rats was not altered by either ovariectomy or ovariectomy plus estradiol replacement. However, male body weight was increased by ORX and this was reversed by testosterone replacement. The weights of the sex steroid-dependent reproductive tissues (uterus, vas deferens, and seminal vesicles), providing information about integrated sex steroid levels, were significantly decreased by gonadectomy and reversed by the respective gonadal hormone replacement.
Table I. Effects of gonadectomy and gonadal hormonal replacement on rat body, uterus, vas deferens, seminal vesicle and aorta weights.
Vascular reactivity of non-stressed intact male and female rats
Reactivity (maximum response and sensitivity) to noradrenaline in aortas with endothelium did not differ between non-stressed intact male and female rats ( and , panel a; ). Similar results were also found in aortas without endothelium ( and , panel a; ).
Figure 1 Effects of noradrenaline on two rings, one with and the other without endothelium, of the same thoracic aorta isolated from control and experimental male rats. The experimental protocols were: (a) stress (2 h-immobilization), (b) orchiectomy ORX, (c) (ORX) plus stress, (d) ORX plus testosterone (test), and (e) ORX plus test plus stress. The removal of endothelium increased maximum response to noradrenaline (P < 0.05), except in orchiectomized non-stressed males (panel b). None of the experimental protocols altered the vascular smooth muscle reactivity to noradrenaline vs. respective controls (see denuded aorta responses, P>0.05). Stress decreased and ORX increased the intact aorta maximum response to noradrenaline (panels a and b, respectively). ORX did not prevent the decrease in the intact aorta reactivity to noradrenaline induced by stress (panels c × b). The maximum response to noradrenaline in intact aorta from stress-exposed orchiectomized rats (panel c, filled triangle) was significantly different from non-stressed intact males (panel c, filled circle). Testosterone treatment restored the intact aorta reactivity to noradrenaline in orchiectomized non-stressed males (panels d × b) and also recovered the stress response in aorta from orchiectomized rats (see panels e, c and a). Values are means ± SEM. *P < 0.05 vs. respective controls. Animal number range = 6–12 per group.
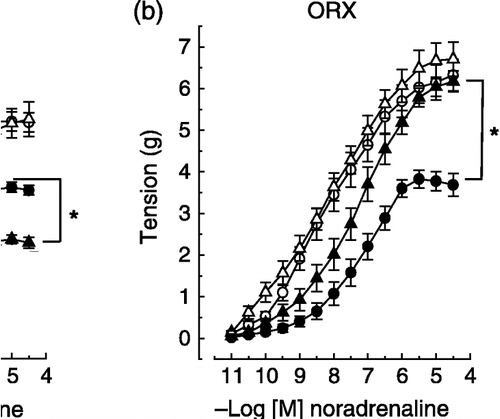
Figure 2 Effects of noradrenaline on two rings, one with and the other without endothelium, of the same thoracic aorta isolated from control and experimental female rats. The experimental protocols were: (a) stress (2 h-immobilization), (b) ovariectomy (OVX), (c) OVX plus stress, (d) OVX plus 17β-estradiol (E2), and (e) OVX plus E2 plus stress. The removal of endothelium increased maximum response to noradrenaline (P < 0.05), except in ovariectomized non-stressed females (panel b). None of the experimental protocols altered the vascular smooth muscle reactivity to noradrenaline vs. respective controls (see denuded aorta responses, P>0.05). Stress decreased and OVX increased maximum response to noradrenaline in the intact aorta (panels a and b, respectively). OVX did not prevent the decrease in the intact aorta reactivity to noradrenaline induced by stress (panels c × b). The maximum response to noradrenaline in the intact aorta from stress-exposed ovariectomized rats (panel c, filled triangle) was not significantly different from non-stressed intact females (panel c, filled circle). 17β-estradiol treatment restored the intact aorta reactivity to noradrenaline in ovariectomized non-stressed females (panels d × b) and recovered the stress response in aorta from ovariectomized rats (see panels e, c and a). Values are means ± SEM. *P < 0.05 vs. respective controls. Animal number range = 6–14 per group.
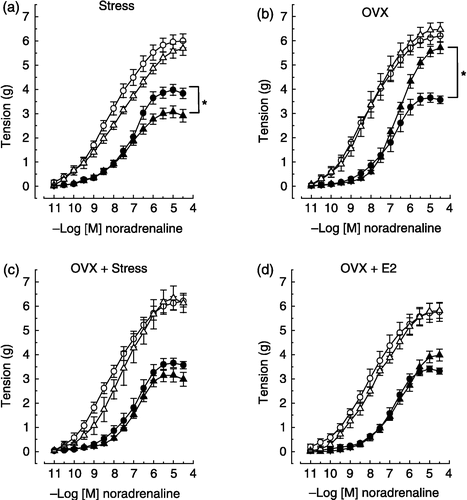
Table II. Effects of gonadectomy and gonadal hormonal replacement on the EC50 values for noradrenaline obtained for two rings, one with and the other without endothelium, of the same thoracic aorta from male and female rats, submitted or not to stress.
Removal of the endothelium caused a leftward shift of the curve and an increased maximum response to noradrenaline, that were similar in aortas from all the experimental groups ( and , ), except in those from gonadectomized non-stressed rats in which the maximum response was not altered by endothelium removal ( panel b).
Effects of gonadectomy on vascular reactivity in intact male and female rats
Gonadectomy similarly increased the maximum response to noradrenaline in aortas with endothelium from non-stressed male and female rats ( and , panel b). However, this procedure did not alter the maximum response of aortas without endothelium ( and , panel b). Moreover, no differences in the sensitivity to noradrenaline were observed after gonadectomy in aortas with or without endothelium from either sex ( and , panel b; ). The reactivity to noradrenaline of aortas from sham-operated rats was similar to that observed in aortas from intact rats (data not shown).
Effects of stress on vascular reactivity in intact male and female rats
Exposure to stress decreased the maximum response to noradrenaline in aortas with endothelium from intact male and female rats ( and , panel a). The magnitude of this decrease was similar between sexes only at the maximum, since a reduction in the sensitivity to noradrenaline of aortas with endothelium from intact males but not females was observed ( and , panel a; ). Stress exposure did not alter the reactivity of aortas without endothelium in either gender ( and , panel a; ).
Effects of gonadectomy on the vascular adaptive response to stress in male and female rats
The exposure of gonadectomized rats to stress caused a greater decrease in gonadectomy-induced hyper-reactivity to noradrenaline of aortas from females compared to males, since the maximum response to noradrenaline in aortas from stress-exposed gonadectomized females but not males reached a value similar to that for non-stressed intact rats ( and , panel c). Stress exposure did not alter the sensitivity to noradrenaline in aortas with or without endothelium from gonadectomized male and female rats ( and , panel c; ).
Effects of gonadal hormone replacement on the vascular adaptive response to stress in gonadectomized male and female rats
In both males and females, gonadal hormone replacement (as assessed by the restoration of reproductive tissue weights in gonadectomized animals to pregonadectomy levels) abolished the gonadectomy-induced hyper-reactivity of aortas to noradrenaline and did not alter the reactivity of aortas without endothelium ( and , panel d; ). Exposure to stress in gonadectomized hormone-replaced males and females led to a decrease in the maximum response to noradrenaline of aortas with endothelium that reached values near those in stressed intact rats ( and , panel e). Moreover, stress exposure reduced the sensitivity to noradrenaline of aortas with endothelium from gonadectomized hormone-replaced males, but not females, with values reached approximating those in stressed intact male rats ( and , panel e; ). Finally, stress exposure did not alter the reactivity of aortas without endothelium from gonadectomized hormone-replaced males or females ( and , panel e; ).
Effects of l-NAME and indomethacin on the vascular adaptive response to stress in male and female rats
l-NAME (3 × 10− 4 M) but not indomethacin (10− 5 M) restored the decreased maximum response to noradrenaline observed in aortas with endothelium from intact male and female rats exposed to stress (). This restored response reached a value similar to that obtained in aortas with endothelium from non-stressed intact rats in the presence of l-NAME and to that obtained in aortas without endothelium from both non-stressed and stressed intact rats in the absence or presence of L-NAME (). Moreover, l-NAME caused a leftward shift in the EC50 value of aortas with endothelium from non-stressed and stressed rats that reached values near those for aortas without endothelium (). Neither l-NAME nor indomethacin caused any change in the noradrenaline reactivity of aortas without endothelium ().
Table III. Maximal responses and EC50 values to noradrenaline obtained in two rings, one with and the other without endothelium, of the same thoracic aorta from intact male and female rats, submitted or not to stress, in the presence of l-NAME or indomethacin.
Discussion
The data reported herein have shown no gender-related differences in the reactivity to noradrenaline of aortas from non-stressed intact rats. Similar results were reported in basilar, renal and tail arteries of rats for vasopressin and the thromboxane analog U46619 (García-Villalón et al. Citation2003; Sanz et al. Citation2003). However, Calderone et al. (Citation2002) reported an increase in the noradrenaline sensitivity in aortas from male compared to female rats. Reduction in aorta contraction due to phenylephrine or thromboxane A2 in female compared to male rats was also reported (Kanashiro and Khalil Citation2001; Robert et al. Citation2005). Related to vasodilation, Kauser and Rubanyi (Citation1995) reported greater aorta reactivity to acetylcholine in female than in male rats.
The present study also investigated the effect of stress on vascular reactivity of intact rats as well as whether the vascular response to stress is different between males and females. Exposure to immobilization stress led to a vascular adaptive response characterized by hyperactivation of the endothelial cell, since a decreased reactivity to noradrenaline was observed in aortas with but not without endothelium from intact male and female rats. Moreover, the effect of stress was seen in females at a much lower dose of noradrenaline thereby characterizing gender-related differences in the rat vascular adaptive response to stress. A similar vascular adaptive response to stress has been reported previously in males in different stress conditions (Webb et al. Citation1987; Lapshin et al. Citation1991; Jansakul Citation1995; Cordellini and Vassilieff Citation1998; Navarro-Oliveira et al. Citation2000; Cordellini et al. Citation2006).
In order to elucidate mechanisms involved in stress-induced endothelial hyperactivation, inhibitors of prostaglandin generation (indomethacin) and nitric oxide synthase (l-NAME) were used. The decreased reactivity to noradrenaline observed in aortas with endothelium isolated from both male and female rats subjected to stress was abolished by endothelium removal and reversed by l-NAME, but was not reversed by indomethacin. These findings strongly suggest that the decrease of the endothelium-dependent vasoconstriction to noradrenaline in stress-exposed rats involves an increased release and/or production of nitric oxide. They also preclude the participation of prostacyclin in stress-induced endothelial hyperactivation.
Previous data from our laboratory have already shown that, in males, a variety of stressful conditions stimulates nitric oxide release from endothelium that causes a decrease in the vascular responsiveness to noradrenaline (Cordellini and Vassilieff Citation1998; Navarro-Oliveira et al. Citation2000; Cordellini et al. Citation2006). It has also been described that stress exposure induces a decrease in plasma levels of arginine, the precursor for nitric oxide (Milakofsky et al. Citation1993). It is reported that the stress-induced aorta reactivity alteration in intact rats is a nonspecific phenomenon since, besides the aorta hyporeactivity to noradrenaline, the vasodilation induced by acetylcholine is also increased after stress exposure (Cordellini and Vassilieff Citation1998). An increase in vascular responsiveness to acetylcholine induced by stress has been reported also in mice (Webb et al. Citation1987). However, a potential cellular/molecular mechanism for the effects of stress on vascular reactivity remains to be completely clarified.
Several studies have shown that the activities of the SA system and HPA axis in the organism's response to stress can be modulated by gonadal hormones (Viau and Meaney Citation1991; Handa et al. Citation1994; Dar and Zinder Citation1997; Ogilvie and Rivier Citation1997; Lund et al. Citation2006). Moreover, gonadal hormones can alter multiple functions of the vascular wall under normal conditions (Duckles et al. Citation1986). Here, we found that gonadectomy increased the reactivity of the aorta to noradrenaline in non-stressed male and female rats, reinforcing previous reports on the beneficial effects of gonadal hormones on vascular reactivity (Yue et al. Citation1995; Ong et al. Citation2000; Worboys et al. Citation2001; Khalil Citation2005). This hyper-reactivity was shown to be both independent of gender, since the gonadectomy-induced increase in the reactivity to noradrenaline of aortas with endothelium was similar between sexes, and dependent on the endothelial cell integrity, since the reactivity to noradrenaline of aortas without endothelium was similar in gonadectomized and non-gonadectomized rats of both sexes. These findings corroborate previous data that show a potentiation of the vasoconstrictor response in castrated male rats (Stallone Citation1994; Glenn et al. Citation1997; Cignarella et al. Citation2000). Moreover, estrogen deprivation also increases the vascular reactivity of female animals to vasoconstrictor agents (Zamorano et al. Citation1995; Nevala et al. Citation1996; Minshall et al. Citation1998; Ma et al. Citation2000; Khalil Citation2005). The hyper-reactivity to vasoconstrictor agents induced by gonadectomy could be explained by different cellular events, e.g. increase in the influx of extracellular calcium (Crews and Khalil Citation1999), supersensitivity of α1-adrenergic receptors (Reilly et al. Citation1997; Ma et al. Citation2000), subsensitivity to NO (Cignarella et al. Citation2000), and endothelial cell dysfunction (Delgado et al. Citation1999).
Although gonadectomy induced aorta hyper-reactivity to noradrenaline, it did not prevent the expression of the vascular adaptive response to stress in either sex. However, the magnitude of the aorta's adaptive response to stress in the absence of gonadal hormones was smaller in male than female rats, since in ovariectomized but not orchiectomized rats stress exposure determined a decrease in the aorta's maximum response to noradrenaline that reached a value near that of non-stressed intact rats. Moreover, the effect of stress was lower in orchiectomized than in intact males, as in orchiectomized males stress reduced the maximal effect without changing the EC50 value. In contrast, the effect of stress appears to be similar in ovariectomized and intact females, as in both groups of females the maximal effect was similarly reduced by stress. Therefore, the vascular adaptive response induced by stress seems to be dependent on testicular hormones, but the case for ovarian hormones is less clear.
Considering that the vascular adaptive response to stress was shown here to be dependent on the increase in nitric oxide-system activity, and that smooth muscle sensitivity to nitric oxide is impaired in orchiectomized (Cignarella et al. Citation2000) but not in ovariectomized rats (Conde et al. Citation2000; Virdis et al. Citation2000), the gender-related differences in the magnitude of the vascular adaptive response to stress in gonadectomized rats may be explained by differences in nitric oxide mechanisms.
Finally, we showed that reactivity of the aorta after stress in gonadectomized rats was normalized in both sexes following sex steroid hormone replacement therapy, sufficient to restore the weights of reproductive organs. These data have clearly shown that hormone therapy is effective in restoring the vascular adaptive response to stress of gonadectomized males and females to that seen in stressed intact rats. Moreover, the ability of testosterone to reverse all the effects of gonadectomy-induced changes in the vascular adaptive response to stress in males confirms that this stress adaptive response is modulated by the male gonadal hormone testosterone.
In summary, the present study has shown no gender-related differences in reactivity of the aorta to noradrenaline in non-stressed intact rats. In contrast, gender-related differences in the vascular adaptive response to stress were shown in intact rats. Moreover, the magnitude but not the expression of the stress-induced vascular adaptive response was shown to be dependent on testosterone. Finally, the mechanism of the vascular adaptive response to stress involves a hyperactivity of the endothelial nitric oxide system in both sexes. Results of this study may contribute to the development of gender-specific therapies for cardiovascular diseases caused by stress.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process number: 00/01697-5).
Notes
*Part of a thesis submitted by U. Lanza Jr. to the University Estadual Paulista, São Paulo, Brazil in partial fulfillment of the requirements for the Master Degree.
References
- Arteche E, Strippoli G, Loirand G, Pacaud P, Candenas L, Molto JC, Souto L, Fernandez J, Norte M, Martin JD, Savineau JP. An analysis of the mechanisms involved in the okadaic acid induced contraction of the estrogen-primed rat uterus. J Pharmacol Exp Ther 1997; 282(1)201–207
- Ba ZF, Yokoyama Y, Toth B, Rue LW, 3rd, Bland KI, Chaudry IH. Gender differences in small intestinal endothelial function: Inhibitory role of androgens. Am J Physiol Gastrointest Liver Physiol 2004; 286(3)G452–G457
- Calderone V, Baragatti B, Breschi MC, Nieri P, Martinotti E. Hormonal influence on the release of endothelial nitric oxide: Gender related dimorphic sensitivity of rat aorta for noradrenaline. J Pharm Pharmacol 2002; 54(4)523–528
- Cankar K, Finderle Z. Gender differences in cutaneous vascular and autonomic nervous response to local cooling. Clin Auton Res 2003; 13(3)214–220
- Cignarella A, Bolego C, Pinna C, Zanardo R, Nardi F, Zancan V, Puglisi L. Androgen deprivation, estrogen treatment and vascular function in male rat aorta. Naunyn Schmiedebergs Arch Pharmacol 2000; 361(2)166–172
- Conde MV, Marín J, Fernandez-Criado C, Balfagón G. Regulation of beta-adrenoceptor-mediated relaxation of the rat aorta is modulated by endogenous ovarian hormones. Clin Sci 2000; 98(4)381–387
- Cordellini S, Vassilieff VS. Decreased endothelium-dependent vasoconstriction in acuted stressed rats is potentiated by previous chronic stress. Nitric oxide involvement. Gen Pharmacol 1998; 30(1)79–83
- Cordellini S, Carvalho MHC, Scivoletto R, Fortes ZB, Nigro D. Indirect evidence for an endothelium-derived contracting factor in aorta of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 1990; 8(11)53–60
- Cordellini S, Novo R, Lanza U, Jr. Exposure to stress. Differential vascular adaptive response in spontaneously hypertensive and Wistar rats: Role of nitric oxide, and prehypertensive and hypertensive states. Life Sci 2006; 79: 646–653
- Crews JK, Khalil RA. Gender-specific inhibition of Ca++ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol 1999; 26(9)707–715
- Dar DE, Zinder O. Short-term effect of steroids on catecholamines secretion from bovine adrenal medulla chromaffin cells. Neuropharmacology 1997; 36(11/12)1783–1788
- Delgado JL, Landeras J, Carbonell LF, Parilla JJ, Abad L, Quesada T, Fiol G, Hernandez I. Effect of n-acetylcisteine on vascular endothelium function in aorta from oophorectomized rats. Gen Pharmacol 1999; 32(1)23–27
- Duckles SP, Krause DN, Miller VM. Effects of gonadal steroids on vascular function. J Pharmacol Exp Ther 1986; 279(1)1–3
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. J Appl Physiol 2001; 91(6)2611–2618
- Ford JA, Clark SG, Walters EM, Wheeler MB, Hurley WL. Estrogenic effects of genistein on reproductive tissues of ovariectomized gillts. J Anim Sci 2006; 84(4)834–842
- Garcia-Villalón AL, Sanz E, Monge L, Fernandez N, Martinez MA, Climent B, Dieguez G. Vascular reactivity to vasopressin during diabetes: Gender and regional differences. Eur J Pharmacol 2003; 459(2/3)247–254
- Glenn TC, Krause DN, Duckles SP. Vascular responses to neuropeptide Y are greater in female than male rats. Naunyn Schmiedebergs Arch Pharmacol 1997; 355(1)111–118
- Handa RJ, Nunley KM, Lorens SA, Louie JP, Mcgivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav 1994; 55(1)117–124
- Hutchison SJ, Sudhir K, Chou TM, Chatterjee K. Sex hormones and vascular reactivity. Herz 1997; 22(3)141–150
- Isles CG, Hole DJ, Hawthorne VM, Lever AF. Relation between coronary risk and coronary mortality in women of renfrew and paisley survey: Comparison with men. Lancet 1992; 339(8795)702–706
- Jansakul C. Effect of swimming on vascular reactivity to phenylephrine and KCl in male rats. Br J Pharmacol 1995; 115(4)587–594
- Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Cell Physiol 2001; 280(1)C34–C45
- Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension 1995; 4(1)517–523
- Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension 2005; 46: 249–254
- Knoll J, Miklya I, Knoll B, Dilló J. Sexual hormones terminate in the rat the significantly enhanced catecholaminergic/serotoninergic tone in the brain characteristic to the post-weaning period. Life Sci 2000; 67: 765–773
- Lapshin AV, Manukhina EB, Meerson FZ. Adaptation to short stress exposures prevents the enhancement of the endothelium-dependent reactions of the aorta in myocardial infarct. Fiziol Zh Im I M Sechenova 1991; 77(3)70–78
- Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol 2001; 38(6)1668–1674
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo–pituitary–adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 2006; 26(5)1448–1456
- Ma I, Yu Z, Xiao S, Thadani U, Robinson CP, Patterson E. Supersensitivity to serotonin- and histamine induced arterial contraction following ovariectomy. Eur J Pharmacol 2000; 359(2/3)191–200
- Milakofsky L, Harris N, Vogel WH. Effects of repeated stress on plasma arginine levels in young and old rats. Physiol Behav 1993; 54: 725–728
- Minshall RD, Miyagawa K, Chadwick CC, Novy MJ, Hermsmeyer K. In vitro modulation of primate coronary vascular muscle cell reactivity by ovarian steroid hormones. FASEB J 1998; 12(13)1419–1529
- Morschl E, Bretus I, Nemcsik J, László F, Pávó I. Estrogen-mediated up-regulation of the Ca-dependent constitutive nitric oxide synthase in the rat aorta and heart. Life Sci 2000; 68: 49–55
- Navarro-Oliveira CM, Vassilieff VS, Cordellini S. The sympathetic adrenomedullary system, but not the hypothalamic–pituitary–adrenal axis, participates in aorta adaptive response to stress: Nitric oxide involvement. J Auton Nerv Syst 2000; 83(3)140–147
- Nevala R, Paakkari I, Tarkkila L, Vapaatalo H. The effects of male gender and female sex hormone deficiency on the vascular responses of the rat in vitro. J Physiol Pharmacol 1996; 47(3)425–432
- Ogilvie KM, Rivier C. Gender difference in hypothalamic–pituitary–adrenal axis responses to alcohol in the rat: Activational role of gonadal steroids. Brain Res 1997; 766(1/2)19–28
- Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol 2000; 85(2)269–272
- Rees DD, Palmer RMJ, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 1990; 101(3)746–752
- Reilly CM, Stopper VS, Mills TM. Androgens modulate the alpha-adrenergic responsiveness of vascular smooth muscle in the corpus cavernosum. J Androl 1997; 18(1)26–31
- Robert R, Chagneau-Derrode C, Carretier M, Mauco G, Silvain C. Gender differences in vascular reactivity of aortas from rats with and without portal hypertension. J Gastroenterol Hepatol 2005; 20(6)890–894
- Sanz E, Fernández N, Monge L, Martinez MA, Climent B, Dieguez G, Garcia-Villalon AL. Effects of diabetes on the vascular response to nitric oxide and constrictor prostanoids: Gender and regional differences. Life Sci 2003; 72(13)1537–1547
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamo–pituitary–adrenal axis activity in male and female rats. J Neuroendocrinol 2004a; 16: 516–524
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic–pituitary–adrenal axis activity of male and female rats. J Neuroendocrinol 2004b; 16: 989–998
- Selvaraj V, Zakroczymski MA, Naaz A, Mukai M, Doerge DR, Katzenellenbogen JA, Helferich WC, Cooke PS. Estrogenicity of the isoflavone metabolite equal on reproductive and non-reproductive organs in mice. Biol Reprod 2004; 71(3)966–972
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol 2001; 281(1)H2028–H2035
- Stallone JN. Sex differences in nitric oxide-mediated attenuation of vascular reactivity to vasopressin are abolished by gonadectomy. Eur J Pharmacol 1994; 259(3)273–283
- Vane JR. Inhibition of prostaglandin synthase as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 23(25)232–235
- Viau V. Functional cross-talk between the hypothalamic–pituitary–gonadal and adrenal axes. J Neuroendocrinol 2002; 14(6)506–513
- Viau V, Meaney MJ. Variations in the hypothalamic–pituitary–adrenal response to stress during the estrous cycle in the rat. Endocrinology 1991; 129(5)2503–2511
- Villar IC, Francis S, Weeb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int 2006; 70(5)840–853
- Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 2000; 101(19)2258–2263
- Webb CR, Vander AJ, Henry JP. Increased vasodilator responses to acetylcholine in psychosocial hypertensive mice. Hypertension 1987; 9(3)268–276
- Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone may improve endothelium-dependent and independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab 2001; 86(1)158–161
- Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collions P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 1995; 91(4)1154–1160
- Zamorano B, Bruzzone ME, Martinez JI. Vascular smooth muscle reactivity to norepinephrine in ovariectomized rats: Relationship to vascular PGE2/PGF2 alpha ratio. Gen Pharmacol 1995; 26(7)1613–1618
- Zysow BR, Kauser K, Lawn RM, Rubanyi GM. Effects of estrus cycle, ovariectomy, and treatment with estrogen, tamoxifen, and progesterone on apolipoprotein(a) gene expression in transgenic mice. Arterioscler Thromb Vasc Biol 1997; 17(9)1741–1745