Abstract
Studies have shown associations between reduced vitamin K status and poor cognitive function. However, despite this apparent link, direct studies measuring cognitive function, vitamin K status and inflammation are lacking. In the current study, The ELDERMET cohort was investigated to identify associations between cognition, vitamin K status and inflammation. The primary aim of the ELDERMET study was to investigate the relationship between gut bacteria, diet, lifestyle and health in 500 older Irish adults. Significant differences in serum phylloquinone, dietary phylloquinone and inflammatory markers were found across varying levels of cognitive function, after controlling for sex, age, body mass index (BMI), triglycerides and blood pressure. In addition, significantly higher levels of dietary phylloquinone were found in those with better cognition compared to those with the poorest function. Higher levels of inflammatory were also associated with poor cognition. Furthermore, both dietary and serum phylloquinone were significant independent predictors of good cognitive function, after controlling for confounders. This study highlights the importance of dietary vitamin K as a potentially protective cognitive factor; it also provides evidence for the correlation between cognition and inflammation. Strategies should be devised by which elderly populations can access rich dietary sources of phylloquinone to maintain cognition.
Introduction
Vitamin K is generally known for its role in blood coagulation and is the general term for a group of fat-soluble compounds.Citation1 However in recent years, a number of potential health benefits beyond coagulation have been attributed to vitamin K. While green leafy vegetables provide phylloquinone (or vitamin K1), the most widely consumed dietary form of the vitamin,Citation2 high levels of a menaquinone (vitamin K2) isoform called menaquinone-4 (MK-4) have been reported in brain tissues.Citation3 At the biochemical level, vitamin K is implicated in the production of sphingolipids, a group of lipids that comprise the myelin sheath of neuronal tissueCitation3,Citation4 and that are now recognised as important bioactive mediators of cell interaction, proliferation, senescence, differentiation and transformation.Citation5 The vitamin K-dependent protein (VKDP) Growth-arrest specific gene-6 (Gas6) is present in the brain where it performs cell regulatory and myelination functions. A role for vitamin K in memory consolidation has also been documented. Carrie and colleagues (2011) conducted a feeding study on female Sprague–Dawley rats fed a low, adequate and high phylloquinone diet to determine the effects on spatial memory (using the Morris water maze test). Animals exposed to a high or adequate phylloquinone diet required less visual assistance to complete the task than rats on the vitamin K depleted diet.Citation6
Studies in humans have also shown associations between reduced vitamin K status and poor cognitive function.Citation7 conducted research on a group of women with Alzheimer’s Disease; the level of serum phylloquinone was positively associated with Mini Mental State Exam (MMSE) scores and negatively associated with uncarboxylated osteocalcin, a bone-derived VKDP which is also a marker of sub-optimal vitamin K status. Similarly, differences in dietary phylloquinone intakes have been reported between elderly patients in the early stages of Alzheimer’s disease compared to healthy controls.Citation8,Citation9 A subsequent study by Presse and colleagues revealed that in cognitively intact elderly individuals, higher serum phylloquinone concentrations were correlated with enhanced verbal episodic memory and recollection tests.Citation10 More recently, studies of older adults have reported better cognitive function and behavioural rating among those in the highest quartile of dietary phylloquinone intakeCitation11 and fewer and less severe subjective memory complaints among those with higher dietary intakes.Citation12 These findings however appear to be confined to older populations. Analysis of middle-aged, community-dwelling participants of the Longitudinal Aging Study in Amsterdam revealed no association between suboptimal vitamin K status and cognitive decline.Citation13
Aging and indeed cognitive decline has been associated with increased systemic inflammationCitation14,Citation15 with several studies reporting higher circulating levels of pro-inflammatory cytokines in demented subjects.Citation16–18 Vitamin K has been shown to have anti-inflammatory properties in both in vitro and in vivo studiesCitation19–21 and its protective effect in maintaining cognitive integrity is thought to be mediated, in part, through this mechanism.Citation22 However despite this apparent link, direct studies measuring cognitive function, vitamin K status and inflammation are lacking.
The ELDERMET cohort, a group of well-characterized Irish subjects aged ≥64 years was investigated in the current study. ELDERMET Subjects were divided into four groups based on their MMSE score seeking to identify associations between cognition, vitamin K status and inflammation ().
Table 1 Classification of ELDERMET subjects into groups based on MMSE number.
Materials and methods
The ELDERMET cohort
The ELDERMET cohort is a well-defined study group of Irish adults, aged ≥64 years, ranging from cognitively intact to severely cognitively impaired. This multifaceted project commenced in 2007 to examine how intestinal bacteria influence, and are influenced by, diet, lifestyle and health in 500 older Irish people. Subjects chosen included those in long-term care facilities, day-hospital attendees, rehabilitation patients and community-dwelling volunteers. Previously, correlations between microbiota composition, diet, health and frailty status have been reported in the cohort.Citation23,Citation24 Subjects were clinically assessed at ELDERMET Clinics at two local hospitals. Subjects were excluded from the study if they had participated in medical trials in the previous month, if they had a history of alcoholism or an advanced organic disease. Informed consent was obtained from all subjects or from the next of kin of those with cognitive impairment in accordance with local Clinical Research Ethics Committee. This study adheres to the guidelines dictated by the Declaration of Helsinki and those of the Research Ethics Committee, Ireland. Subjects underwent a large number of tests including a validated food-frequency questionnaire, Mini Mental State exam (MMSE), mini nutritional assessment (MNA), and a physical examination; they provided saliva, urine, faecal, blood samples and a medical history including Charlson Comorbidity Index (CCI).
Subject selection and classification
A subgroup of the ELDERMET cohort, comprising 156 subjects, was selected for the current study. Subjects receiving antibiotics were disqualified from the study due to the impact of antibiotics on the gut microbiota;Citation25 those treated with vitamin K antagonists (warfarin and acenocoumarol) were also excluded due to their effects on long-term markers of vitamin K status. The mean age of the subject group was 78 ± 8.5 years, with a range of 64–102 years. Subjects were grouped based on the MMSE categories as determined by Poynter et al.,Citation26 which referenced the British National Institute for Health and Clinical excellence guidelines. In the current study, quartiles were set at marginally higher MMSE scores due to a paucity of subjects in certain categories. The subjects were grouped according to MMSE number as outlined in the table below:
Dietary phylloquinone
Dietary data were collected by a trained nurse using a semi-quantitative Food Frequency Questionnaire (FFQ). The FFQ selected was a version of that used by the European Prospective Investigation into Cancer (EPIC) studyCitation27 which had been amended for use in the Irish population.Citation28 Habitual dietary intake was determined by assessing the intake of 147 foodstuffs, the frequency of intake being established using 10 categories ranging from ‘never’ to ‘6 times a day or more’. The frequency categories were converted to their proportion of a single daily serving i.e. 0.14 for ‘once a week’. Portion sizes were estimated from a recent, gender-specific study on portion size.Citation29 Dietary phylloquinone sources with similar levels of phylloquinone were averaged based on values reported in the sixth edition of McCance and Widdowson’s The Composition of FoodsCitation30 and Bolton-Smith et al.Citation31 No vitamin K-containing supplements were consumed by study participants.
Serum phylloquinone
Blood samples were processed and stored following standard procedures as described elsewhere.Citation23 Individuals were non-fasted and blood samples were taken by a trained nurse using a vacutainer. Blood samples were centrifuged and after serum was extracted samples were stored at −80°C. Serum phylloquinone (vitamin K1) was assessed by reverse phase HPLC with fluorescence detection as described by Presse et al.Citation10
High sensitivity C-reactive protein (hsCRP)
A commercially available Quantikine human Enzyme-Linked Immunoassay (ELISA) kit was used to measure hsCRP (R & D systems, Oxford, UK). Samples were assayed in duplicate according to manufactures’ guidelines using appropriate standards and controls. Initially, all samples were assayed using a 1 in 200 dilutions with further dilutions being performed on high concentration samples. The intra- and inter-assay coefficients of variation were 3.95% and 4.31%, respectively.
Uncarboxylated osteocalcin (ucOC)
A commercially available Quantikine human ELISA kit was used to measure carboxylated and uncarboxylated osteocalcin (TaKaRa Bio Inc, Japan). Samples were assayed in duplicate according to manufactures guidelines using appropriate standards and controls. Initially, all samples were assayed using a 1 in 2 dilutions with further dilutions being performed on high concentration samples. Uncarboxylated osteocalcin (%ucOC) was expressed as a percentage of total osteocalcin (carboxylated + uncarboxylated osteocalcin) as in O’Connor et al.Citation32
The intra- and inter-assay coefficients of variation for the carboxylated osteocalcin assay were 2.47% and 2.38%, respectively. The intra- and inter-assay coefficients of variation for the uncarboxylated osteocalcin assay were 3.59% and 3.70%, respectively.
Cytokine analysis
The cytokines IL-6, IL-8 and TNFα were measured using validated, commercial, multi-spot microplates (Meso Scale Diagnostics). For more information see Claesson et al.Citation23
Statistical analysis
Descriptive statistics are presented as mean (SD), median (25th percentile, 75th percentile) or number (percentage) as appropriate. Numeric variables were examined for normality by visual inspection of histograms and formal tests of normality. Demographic differences between the MMSE categories were assessed using one-way ANOVA for normally distributed variables, Kruskal–Wallis test for skewed data, and Chi-square test of independence for categorical data.
Analysis of Covariance (ANCOVA) was used to examine differences in dietary phylloquinone, serum phylloquinone and inflammatory blood markers (IL-6, IL-8, IL-10, TNFα and high-sensitivity C-Reactive) Protein (hsCRP) across the MMSE categories, controlling for sex, age, BMI, triglycerides and blood pressure. Bonferroni adjusted pairwise comparisons were used to examine between-group differences. Partial correlations were used to examine the linear relationships between dietary phylloquinone, serum phylloquinone and the inflammatory blood markers (IL-6, IL-8, IL-10 and TNFa), controlling for sex, age, BMI, triglycerides and blood pressure. All positively skewed data were normalised using a natural logarithm transformation prior to analyses. Diastolic blood pressure was used as the control variable for blood pressure throughout the analyses.
Tertiles of dietary phylloquinone and serum phylloquinone were compared across the MMSE categories using the Chi-square test of independence. Hierarchal binary logistic regression analysis was used to examine if the tertiles of dietary phylloquinone and serum phylloquinone were predictive of good cognitive function defined as MMSE group 4 (MMSE 26+). The 5% significance level was used for all statistical tests. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
Results
Demographics
The general characteristics of study subjects (n = 156) are listed in , while outlines the characteristics of subjects across different MMSE categories. Age, BMI, CCI and blood pressure differed between MMSE categories (P < .001).
Table 2 Descriptive data presented as mean, standard deviation or median, interquartile range as appropriate or number (percentage)
Table 3 Between-group comparisons using one way ANOVA or Kruskal Wallis test
Differences between vitamin K status and inflammatory markers across MMSE groups
Vitamin K levels and inflammatory markers across the MMSE groups are presented in . ANCOVA analysis identified significant differences in serum phylloquinone, dietary phylloquinone, IL-6, TNFα and hsCRP values across the MMSE categories, after controlling for effects of sex, age, BMI, triglycerides and blood pressure. Pairwise Bonferroni adjusted post-hoc tests examined differences between the MMSE groups, controlling for multiple testing. Results indicated significantly higher levels of dietary phylloquinone in MMSE groups 4 compared to group 1 (P = .002) and group 2 (P = .044), and higher levels in group 3 compared to group 1 (P = .036). The inflammatory marker IL-6 was found at significantly higher levels in MMSE group 1 compared to groups 3 (P = .011) and 4 (P = .001). CRP levels in MMSE group 2 were significantly higher than those in group 4 (P = .049).
Table 4 Analysis of covariance (ANCOVA) of differences across Mini Mental State Exam (MMSE) categories
Furthermore, when CCI was included as a control variable in addition to sex, age, BMI, triglycerides and blood pressure (albeit in a reduced sample size, n = 96), differences in dietary phylloquinone intake remained significant across MMSE categories (P = .016). Bonferroni adjusted P-values indicated significantly higher dietary phylloquinone intakes in group 4 compared to group 1 (P = .042) and group 2 (P = .028).
Relationship between vitamin K status and inflammatory markers
Partial correlation analysis, controlling for age, sex, BMI, triglycerides and blood pressure () found a significant weak negative association between IL-6 and dietary phylloquinone (partial r = −0.263, P < .01) and serum phylloquinone (partial r = −0.203, P < .05). Weak to moderate positive correlations between the markers IL-6, IL-8, IL-10 and TNFα were also identified as significant.
Table 5 Partial correlations controlling for sex, age, BMI, triglycerides and blood pressure
Tertiles of vitamin K status (dietary phylloquinone and serum phylloquinone) across the MMSE groups
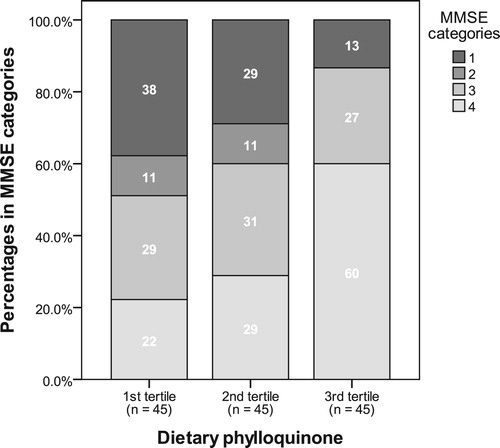
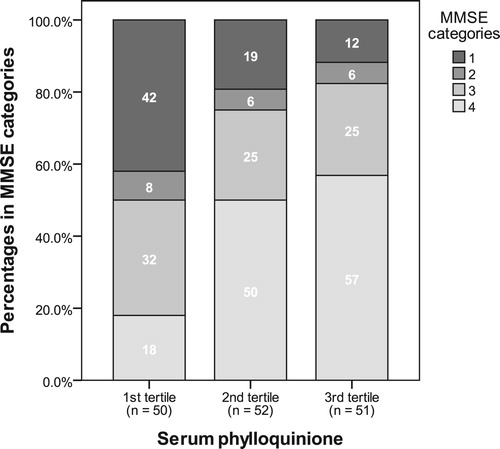
Tertiles of dietary phylloquinone were computed as 1st tertile: <73 µg, 2nd tertile: 73–121 µg, and 3rd tertile: >121 µg. Tertiles of serum phylloquinone were computed as 1st tertile: <0.179 ng/ml, 2nd tertile: 0.179–0.383 ng/ml, and 3rd tertile: >0.383 ng/ml. The frequencies and percentages in the tertile across the MMSE categories are presented in and . Only 13% of those in the 3rd tertile of dietary phylloquinone were in MMSE group 1, compared with 38% of those in the 1st tertile (P < .001) (). Similarly, only 12% of those in the 3rd tertile of serum phylloquinone were in MMSE group 1 compared to 42% of those in the 1st tertile (P < .001) ().
Is vitamin K status predictive of good cognitive function (MMSE ≥26, group 4)?
Hierarchical binary logistic regression models () were used to examine if the tertiles of dietary phylloquinone and serum phylloquinone were predictive of good cognitive function defined as MMSE group 4 (MMSE 26+), controlling for potential confounders.
Table 6 Logistic regression analysis of the association between Good Cognition (MMSE ≥ 26) and Dietary and Serum Phylloquinone Tertiles
Dietary phylloquinone was a significant independent predictor of good cognitive function, controlling for age, BMI, sex, triglycerides and blood pressure (Model 2, P = .038). The odds ratios for good cognitive function for second and third tertiles of dietary phylloquinone compared to the first tertile were OR = 1.01 (95% CI: 0.311, 3.68) and OR = 4.03 (95%CI: 1.19, 13.65), respectively.
Serum phylloquinone was a significant independent predictor of good cognitive function, controlling for age, BMI, sex, triglycerides and blood pressure (Model 2, P = .022). The odds ratios for good cognitive function for second and third tertiles of serum phylloquinone compared to the first tertile were OR = 4.24 (95% CI: 1.31, 13.68) and OR = 5.15 (95% CI: 1.49, 17.76), respectively.
Including IL-6, TNFα and hsCRP as measures of inflammation in the hierarchical models (Model 3), the tertiles of serum phylloquinone were close to achieving significance as independent predictors of good cognitive function (P = .057). The odds ratio for good cognitive function for the second and third tertiles of serum phylloquinone compared to the first tertile were OR = 3.84 (95% CI: 1.07, 13.72) and OR = 4.75 (95% CI: 1.23, 18.37) respectively, controlling for age, sex, triglycerides, blood pressure and inflammation (IL-6, CRPs and TNFα).
Discussion
Summary of main findings
Research has linked vitamin K status with psychomotor behaviour and cognition.Citation6,Citation7,Citation10 In the current study, relationships between cognitive function, vitamin K status and inflammation were investigated to further investigate the association between vitamin K status and cognitive function. Significant differences in dietary phylloquinone intake and the inflammatory marker IL-6 between cognitive function groups were found, controlling for sex, age, body mass index, triglycerides and blood pressure. Furthermore, serum phylloquinone significantly and independently predicted good cognitive function after controlling for the same confounding variables. In addition, when controlling for inflammation, serum phylloquinone remained a significant and independent predictor of cognitive function.
Alignment to literature
In line with previous studies,Citation8,Citation9,Citation11,Citation33 dietary phylloquinone varied according to cognitive ability with those having the poorest cognitive function also having the lowest dietary vitamin K intake. The median daily intake of dietary phylloquinone among the present study cohort was 90 µg (65, 149) Duggan et al.;Citation34 Hayes et al.Citation35 reported slightly lower intakes among a representative sample of Irish ≥65 yr olds from the National Adults Nutrition Survey with median intakes of 72.4 µg (47–109). Combined evidence from both studies shows insufficient dietary phylloquinone intakes among elderly individuals relative to the current US and Canadian guidelines for dietary phylloquinone intake of 120 µg and 90 µg for males and females, respectively.Citation36 This highlights the fact that elderly populations may have insufficient dietary intakes in order to facilitate full functionality of vitamin K-dependent proteins, regulate inflammatory processes and sphingolipid synthesis thus limiting their influence on cognitive integrity.
We found significant differences in dietary phylloquinone between groups with different cognitive ability. In fact, dietary phylloquinone was the only discriminatory measure of vitamin K status between the different cognitive groups after controlling for sex, age, BMI, triglycerides and blood pressure. Presse et al.Citation8 measured dietary vitamin K in a group of subjects with early onset Alzheimer’s disease (AD) matched with 31 healthy controls of the same age. Significant differences were found between subjects with mean phylloquinone intakes of 63 µg/day in subjects with AD and intakes of 139 µg/day in the control group. In addition, Chouet and colleaguesCitation11 reported associations between higher dietary phylloquinone and better cognition among older adults who participated in the CLIP study, an observational cross-sectional study designed to examine the relationships between neuro-cognition and lipophilic vitamins among all patients consecutively hospitalized or seen in consultation in the geriatric acute care unit of the University Hospital of Angers, France, from February to April 2014, and found an inverse association between the frontotemporal behavioural rating scale (FBRS) for physical neglect and dietary vitamin K levels; the FBRS is a reliable, reproducible indicator of the presence of symptoms of behavioural disturbance across four domains (i.e. self-control disorder, physical neglect, mood disorders and loss of general).
In the present study, a moderately positive association was observed between MMSE scores and tertiles of serum phylloquinone. This association was only found for tertile analyses but not between groups compared across varying levels of cognitive function, after controlling for potential confounders, and may have been weakened due to the non-fasted nature of the samples used in the current analysis. These results are however in agreement with the observations of Sato et al.Citation7 who reported a positive association between MMSE and serum phylloquinone in 100 females AD patients (aged 80y) and age-matched controls. Furthermore, in the current study serum phylloquinone was significantly different between MMSE categories specifically between those with severe cognitive decline (MMSE < 15) and those with optimum functionality (MMSE > 26), with median values of 0.34 [0.25, 0.61] ng/ml (0.75 nmol/l) and 0.77 [0.46, 1.31] ng/ml (1.70 nmol/l), respectively. Analysis conducted by Presse et al.Citation10 showed that an increase in serum phylloquinone concentration from 0.27 to 1.06 nmol/l was associated with an increase in performance in verbal episodic memory test scores in 203 subjects from the NuAge study; a 5-year longitudinal study of 1793 older adults designed to assess the pivotal role of nutrition on physical and cognitive status, functional autonomy and social functioning. The same study showed the association between episodic memory performance and serum phylloquinone concentration to be a logarithmic function and that the rate of improvement slowed down considerably after the threshold level of 1 nmol/l. Our data showed a similar trend; the highest serum phylloquinone levels were significantly and independently predictive of good cognitive function after controlling for potential confounders. The odds of having better cognitive function (for tertile 3) were greater than four times the odds of having good cognitive function in the poorest cognitive category. Even when controlling for inflammation across cognitive categories, serum phylloquinone remained a significant and independent predictor of cognitive function.
Declining cognitive function has been associated with increased systemic inflammation and inflammatory biomarkers.Citation14–18,Citation37 Wichmann et al.Citation38 found that those with higher pro-inflammatory IL-6 levels were at a greater risk of cognitive impairment in an elderly cohort where cognitive impairment was classified as an MMSE value of less than 24. Subjects were monitored over a 20 year period and each doubling of IL-6 was associated with a greater risk of cognitive impairment. Similarly, Sudheimer et al.Citation39 found that elevated IL-6 and TNFα values were associated with smaller hippocampal volumes, an indicator of cognitive decline with no such correlations reported for IL-8. However Baune et al.Citation40 found that increased serum concentrations of IL-8 were associated with poor performance in the memory and speed domains of an elderly cohort (determined by 3-word recall tests and the Stroop colour-word test) with no such correlations reported between IL-6, TNFα. In the current study, tertiles of dietary phylloquinone and serum phylloquinone were found to be predictive of good cognition (MMSE score of 26 or higher), controlling for age, sex, BMI, blood pressure and triglycerides. While the inclusion of inflammatory markers (IL-6, TNFα and hs-CRP) in the statistical models reduced this significance of the association, a similar albeit non-significant trend was still evident. In another study, Shea et al.Citation21 showed that dietary phylloquinone intake was associated with circulating IL-6 levels in an older population with average age of 59 years (n = 1381, 669 males and 712 females). The precise mechanism(s) by which vitamin K exerts its anti-inflammatory potential are not entirely understood. However, it has been suggested that this fat-soluble vitamin may downregulate NF-Kb activation, inhibiting the production of pro-inflammatory cytokines including IL-6 and other cytokines.Citation41
Strengths and limitations
The current study highlights the importance of considering vitamin K as a nutritional factor which may have implications for cognitive health; it also provides comprehensive evidence for the correlation between cognition and inflammation. There are a number of limitations to the current study; as outlined above, the community-dwelling elderly individuals were a self-selected, motivated cohort and may therefore have had superior dietary and lifestyle factors compared to more general, elderly populations. Furthermore, we were not able to control for some variables which may have confounded our findings including number of years in education, comorbidity, and dietary diversity. Finally, caution must be exercised when interpreting the results from this study due to the cross-sectional nature of the study design.
Implications for future research and clinical practice
Considering 60% of participants with a dietary phylloquinone intake of >121 µg/d had superior cognitive function, compared to 22% with intakes <73 µg/d, our data support current dietary intake recommendations of 90–120 µg/day. Given these and other data supporting the role of dietary vitamin K and preservation of cognitive function, strategies should be devised by which elderly populations can access rich dietary sources of phylloquinone to maintain cognitive function in later life. Longitudinal, prospective studies are required to elucidate the relationship between vitamin K status, inflammation and cognitive decline, and to understand the mechanisms by which this could occur.
Disclaimer statements
Contributors The authors’ contributions are as follows: P.W.O’T and E.M.O’C were the principle investigators responsible for the design and execution of the present study; A.K., B.O., G.F., H.P. and E.M.O’C contributed to the analysis of the data, interpretation of the results and writing of the article. P.W.O’T contributed to the editing of the article. All authors read and contributed to the finalisation of the manuscript.
Funding Work in EMOC’s laboratory for this research was supported by the Allen Foundation Inc. Michigan, USA (2012.267). Work in PWOTs laboratory was supported by the Government of Ireland National Development Plan through a Department of Agriculture, Food and the Marine (DAFM) Food Institutional Research Measure (FIRM) award (07/FHRI/UCC/3) for the ELDERMET project, and by a Centre award (APC/SFI/12/RC/2273) from Science Foundation Ireland to the APC Microbiome Institute. Funders were not involved in any capacity in the following: study design, sample collection, analysis, interpretation of data, writing or submission of manuscript for publication.
Disclosure of interest The authors report no conflict of interest.
Ethics approval None.
Acknowledgements
The authors thank all the study participants for their involvement and cooperation throughout the study.
References
- Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemostasis 2008;100(4):530–47.
- Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res 2012;56. doi: 10.3402/fnr.v56i0.5505
- Carrie I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, Ferland G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J Nutr 2004;134(1):167–72. doi: 10.1093/jn/134.1.167
- Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv Nutr 2012;3(2), 204–12. doi: 10.3945/an.111.001784
- Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett 2010;584(9):1748–59. doi: 10.1016/j.febslet.2009.12.010
- Carrie I, Belanger E, Portoukalian J, Rochford J, Ferland G. Lifelong low-phylloquinone intake is associated with cognitive impairments in old rats. J Nutr 2011;141(8):1495–501. doi: 10.3945/jn.110.137638
- Sato Y, Honda Y, Hayashida N, Iwamoto J, Kanoko T, Satoh K. Vitamin K deficiency and osteopenia in elderly women with Alzheimer’s disease. Arch Phys Med Rehabil 2005;86(3):576–81. doi: 10.1016/j.apmr.2004.10.005
- Presse N, Shatenstein B, Kergoat MJ, Ferland G. Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer’s disease. J Am Diet Assoc 2008;108(12):2095–9. doi: 10.1016/j.jada.2008.09.013
- Shatenstein B, Kergoat MJ, Reid I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J Am Diet Assoc 2007;107(12):2091–9. doi: 10.1016/j.jada.2007.09.008
- Presse N, Belleville S, Gaudreau P, Greenwood CE, Kergoat MJ, Morais JA, et al. Vitamin K status and cognitive function in healthy older adults. Neurobiol Aging 2013;34(12):2777–83. doi: 10.1016/j.neurobiolaging.2013.05.031
- Chouet J, Ferland G, Feart C, Rolland Y, Presse N, Boucher K, et al. Dietary vitamin K intake Is associated with cognition and behaviour among geriatric patients: The CLIP study. Nutrients 2015;7(8):6739–50. doi: 10.3390/nu7085306
- Soutif-Veillon A, Ferland G, Rolland Y, Presse N, Boucher K, Feart C, et al. Increased dietary vitamin K intake is associated with less severe subjective memory complaint among older adults. Maturitas 2016;93:131–6. doi: 10.1016/j.maturitas.2016.02.004
- van den Heuvel EG, van Schoor NM, Vermeer C, Zwijsen RM, den Heijer M, Comijs HC. Vitamin K status is not associated with cognitive decline in middle aged adults. J Nutr Health Aging 2015;19(9):908–12. doi: 10.1007/s12603-015-0579-8
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039
- McGeer PL, Rogers J, McGeer EG. Inflammation, antiinflammatory agents, and Alzheimer’s disease: the last 22 years. J Alzheimer’s Dis 2016;54(3):853–57. doi: 10.3233/JAD-160488
- Bourassa K, Sbarra DA. Body mass and cognitive decline are indirectly associated via inflammation among aging adults. Brain Behav Immun 2017;60:63–70. doi: 10.1016/j.bbi.2016.09.023
- Tegeler C, O’Sullivan JL, Bucholtz N, Goldeck D, Pawelec G, Steinhagen-Thiessen E, et al. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function – data from the Berlin aging study II. Neurobiol Aging 2016;38:112–17. doi: 10.1016/j.neurobiolaging.2015.10.039
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003;61(1):76–80. doi: 10.1212/01.WNL.0000073620.42047.D7
- Ohsaki Y, Shirakawa H, Miura A, Giriwono PE, Sato S, Ohashi A, et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation. J Nutr Biochem 2010;21(11):1120–6. doi: 10.1016/j.jnutbio.2009.09.011
- Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995;7(3):287–90. doi: 10.1006/cyto.1995.0034
- Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino Sr. RB, Dawson-Hughes B, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham offspring study. Am J Epidemiol 2008;167(3):313–20. doi: 10.1093/aje/kwm306
- Ferland G. Vitamin K, an emerging nutrient in brain function. Biofactors 2012b;38(2):151–7. doi: 10.1002/biof.1004
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488(7410):178–84. doi: 10.1038/nature11319
- Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J 2016;10(1):170–82. doi: 10.1038/ismej.2015.88
- O’Sullivan O, Coakley M, Lakshminarayanan B, Conde S, Claesson MJ, Cusack S, et al. Alterations in intestinal microbiota of elderly Irish subjects post-antibiotic therapy. J Antimicrob Chemother 2013;68(1):214–21. doi: 10.1093/jac/dks348
- Poynter L, Kwan J, Sayer AA, Vassallo M. Does cognitive impairment affect rehabilitation outcome? J Am Geriatr Soc 2011;59(11):2108–11. doi: 10.1111/j.1532-5415.2011.03658.x
- Riboli E, Kaaks R. The EPIC project: rationale and study design. European prospective investigation into cancer and nutrition. Int J Epidemiol 1997;26 (Suppl. 1):S6–14. doi: 10.1093/ije/26.suppl_1.S6
- Harrington JM, Dahly DL, Fitzgerald AP, Gilthorpe MS, Perry IJ. Capturing changes in dietary patterns among older adults: a latent class analysis of an ageing Irish cohort. Public Health Nutr 2014;17(12):2674–86. doi: 10.1017/S1368980014000111
- Wrieden W, Barton K. Calculation and collation of typical food portion sizes for adults aged 19–64 and older people aged 65 and over. Final Technical Report to the Food Standards Agency;2006. http://www.foodbase.org.uk/results.php?f_category_id=&f_report_id=82 (accessed November 2016).
- Food Standards Agency. Food portion sizes. 3rd ed. (2002) London: The Stationery Office; 2002.
- Bolton-Smith C, Price RJ, Fenton ST, Harrington DJ, Shearer MJ. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br J Nutr 2000;83(4):389–99.
- O’Connor EM, Grealy G, McCarthy J, Desmond A, Craig O, Shanahan F, et al. Effect of phylloquinone (vitamin K 1) supplementation for 12 months on the indices of vitamin K status and bone health in adult patients with Crohn’s disease. Br J Nutr 2014;112(7):1163–74. doi: 10.1017/S0007114514001913
- Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology 2018;90(3), e214–22. doi: 10.1212/WNL.0000000000004815
- Duggan P, Cashman KD, Flynn A, Bolton-Smith C, Kiely M. Phylloquinone (vitamin K1) intakes and food sources in 18-64-year-old Irish adults. Br J Nutr 2004;92(1):151–8. doi: 10.1079/BJN20041157
- Hayes A, Hennessy A, Walton J, McNulty BA, Lucey AJ, Kiely M, et al. Phylloquinone intakes and food sources and vitamin K status in a nationally representative sample of Irish adults. J Nutr 2016;146(11):2274–80. doi: 10.3945/jn.116.239137
- Institute of Medicine. Dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press; 2001.
- Teunissen CE, van Boxtel MP, Bosma H, Jolles J, Lutjohann D, von Bergmann K, et al. [Serum markers in relation to cognitive functioning in an aging population: results of the Maastricht aging study (MAAS)]. Tijdschrift voor Gerontologie en Geriatrie 2003;34(1):6–12.
- Wichmann MA, Cruickshanks KJ, Carlsson CM, Chappell R, Fischer ME, Klein BE, et al. Long-term systemic inflammation and cognitive impairment in a population-based cohort. J Am Geriatr Soc 2014;62(9):1683–91. doi: 10.1111/jgs.12994
- Sudheimer KD, O’Hara R, Spiegel D, Powers B, Kraemer HC, Neri E, et al. Cortisol, cytokines, and hippocampal volume interactions in the elderly. Front Aging Neurosci 2014;6:153. doi: 10.3389/fnagi.2014.00153
- Baune BT, Ponath G, Rothermundt M, Riess O, Funke H, Berger K. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology 2008;33(1):68–76. doi: 10.1016/j.psyneuen.2007.10.002
- Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: a review. Nutrition 2016;32(7–8):732–9. doi: 10.1016/j.nut.2016.01.011