ABSTRACT
Chemotherapy can result in toxic side effects in the brain. Intake of marine-based omega-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), alter brain fatty acids, potentially improving brain function. However, it is unclear if alpha-linolenic acid (ALA), the plant-based n-3, affects brain PUFAs during chemotherapy. The objective of this study was to examine the effects of dietary ALA, EPA and DHA, with high or low sucrose, on brain PUFAs in a mouse model of chemotherapy. Secondarily, the use of liver PUFAs as surrogate measures of brain PUFAs was examined. Lipid peroxidation (4-HNE) and neurotrophic markers (BDNF) were assessed. Female C57Bl/6 mice (n = 90) were randomized to 1 of 5 diets (high EPA + DHA/high or low sucrose, high ALA/high or low sucrose, or control with no EPA + DHA/low ALA/low sucrose) and injected with doxorubicin-based chemotherapy or saline. Brain EPA and DHA were greater (p < 0.0001) with high EPA + DHA diets, regardless of sucrose; there were no significant differences in brain PUFAs between high ALA diets and control. Chemotherapy-treated mice had higher brain and liver DHA (p < 0.05) and lower brain and liver linoleic acid (p < 0.0001). Brain n-3 and n-6 PUFAs were strongly correlated with liver n-3 (r = 0.8214, p < 0.0001) and n-6 PUFAs (r = 0.7568, p < 0.0001). BDNF was correlated with brain total PUFAs (r = 0.36; p < 0.05). In conclusion, dietary ALA in proportions approximately two times greater than consumed by humans did not appreciably increase brain n-3 PUFAs compared to low ALA intake. Liver PUFAs may be a useful surrogate marker of brain PUFAs in this mouse model.
Introduction
One-third to one-half of women with breast cancer receive chemotherapy as their sole treatment or in conjunction with other therapies [Citation1]. With more individuals surviving for decades after being diagnosed with breast cancer [Citation2], it becomes increasingly important to find interventions to reduce or alleviate the negative side effects of chemotherapy [Citation3]. Cognitive deficits associated with chemotherapy, commonly termed ‘chemo-brain’, are estimated to be present in 15–70% of individuals who have undergone this treatment [Citation4]. Hypotheses surrounding underlying mechanisms of negative cognitive changes after chemotherapy involve oxidative stress, inflammation, vascular changes, damage to neurons, and/or changes in autoimmune response [Citation5].
The omega-3 polyunsaturated fatty acids (n-3 PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are important components of cellular membranes and contribute to membrane fluidity and function [Citation6]. DHA is the predominant n-3 PUFA in the brain [Citation6]. Consumption of EPA and DHA leads to increases in DHA within the brain in rodents [Citation7]. Research in animal models suggests that high consumption of sucrose can deter the beneficial effect of omega-3 fatty acids on inflammation [Citation8]. Indeed, research in rodents suggests that increasing EPA + DHA and decreasing sucrose in the diet, may reduce oxidative stress [Citation9], inflammation [Citation10], and improve synaptic function [Citation6,Citation11]. Due to these findings, increasing intake of EPA and DHA has been of interest to researchers and clinicians when searching for a safe and effective strategy to alleviate chemotherapy-induced side effects.
However, in humans consuming Western diets, plant sources of n-3 PUFAs, such as canola oil, flaxseed oil and walnuts, are eaten in larger quantities and may be considered more acceptable than marine sources [Citation12,Citation13]. Thus, it is important to understand the effects of plant n-3 PUFAs, specifically alpha-linolenic acid (ALA), and marine-sourced n-3 PUFAs on brain structure and function during chemotherapy. ALA is the primary n-3 PUFA found in plants and is the precursor of the longer chain n-3 PUFAs, EPA and DHA [Citation12]. ALA is converted to EPA and DHA in humans, although the amount of conversion is small and is dependent upon gender as well as the background diet of n-6 PUFAs [Citation12,Citation13]. To better translate animal results to humans, it is vital to understand the effects of consuming ALA, in proportions feasible in the human diet, on the accumulation of the longer chain n-3 PUFAs in the brain of rodents treated with chemotherapy, Additionally, because liver tissue is abundant compared to brain tissue in rodent models, it is also of practical importance to determine if liver PUFAs can be used as a surrogate marker of brain PUFAs.
The primary objective of this study was to examine the effects of the plant-sourced n-3 PUFA, ALA, and marine-sourced n-3 PUFAs, EPA + DHA, in a high or low sucrose background diet, on brain PUFAs in a mouse model of chemotherapy. Secondarily, the correlation among liver PUFAs and brain PUFAs was examined. Finally, underlying mechanisms of diet and chemotherapy were explored using markers of lipid peroxidation and brain function. We hypothesized that EPA and DHA would increase n-3 PUFA in the brain to a greater extent than a high ALA diet and both high EPA and DHA and high ALA intakes would increase brain n-3 PUFAs to a greater extent than control diet. Additionally, we hypothesized that high sucrose consumption would reduce the accumulation of n-3 PUFAs in the brain compared to low sucrose consumption.
Materials and methods
All experiments were conducted according to National Institute of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Ohio State University Institutional Animal Care and Use Committee.
Experimental design
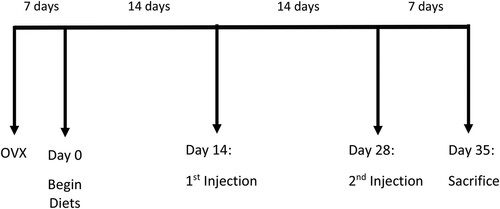
Because the majority of breast cancer survivors are post-menopausal women [Citation14], and the vast majority have had the cancer surgically removed prior to chemotherapy treatment, this experiment used a translational animal model reflecting a post-menopausal state induced by ovariectomy, followed by a common chemotherapy cocktail (doxorubicin + cyclophosphamide). Adult (8–9-week-old), female C57Bl/6 mice (n = 90) were purchased from Charles River (Wilmington, MA) and acclimated to the vivarium for one week. Mice underwent ovariectomy followed by a 7-day recovery period, at which point (Day 0) mice were randomly assigned to one of five diets with 18 mice per diet group; this sample size was chosen to provide 80% power to detect significant differences in brain DHA with an alpha level set at 0.05, based on our previous work with this mouse model [Citation7]. After two weeks on the diets, mice were randomly assigned to the saline vehicle or chemotherapy injections (50% human equivalent dose of a combination of doxorubicin and cyclophosphamide), administered by tail vein at week 2 and 4. Mice were sacrificed, and tissues were collected one week after the second injection (day 35). shows the experimental timeline.
Diets
Diets were formulated by Research Diets Inc. (New Brunswick, NJ). Ingredients of the five diets are listed in Supplementary Table 1. Meg-3 Powder® was purchased from DSM Nutrition Products Inc. (Heerlen, Netherlands) and used as the source of the marine n-3 PUFAs in the high EPA + DHA diets. Because our primary objective was to examine the effects of marine vs. plant-sourced omega-3 PUFAs on brain PUFAs and biomarkers during chemotherapy, we chose to include two diets with high EPA + DHA and two diets with high ALA, each with either high or low sucrose. We also included a ‘control’ diet that contained low sucrose [9% kilocalories (kcals)] and no EPA + DHA but included 0.5% kcals from ALA. This amount of ALA is sufficient to prevent essential fatty acid deficiency and is typical of the percentage of kcals consumed as ALA by humans on Western diets [Citation15]. Low sucrose diets contained 9% kcals from sucrose to mirror current recommendations for low sucrose consumption in humans [Citation16]. High sucrose diets contained 47% kcals from sucrose. The high EPA + DHA diets contained 2% of kcals from EPA + DHA, comparable to high-dose fish oil supplements safely tested in breast cancer patients [Citation15,Citation17] and 0.6% kcals from ALA. High ALA diets contained 1.3% kcals from ALA, approximately double the proportion of kcals from ALA consumed by humans on typical Western diets [Citation15]. The diets contained the same amount of total kcals, macronutrients, vitamins, minerals and fiber. Fatty acid composition of the diets was measured via gas chromatography using established methods [Citation18] in the Orchard lab and results are shown in Supplementary Table 2. Diets were stored under refrigeration in the dark and replaced every 3–4 days to prevent the oxidation of fatty acids. Cumulative dietary intake over the course of the experiment by diet and injection group is shown in Supplementary Figure 1.
Chemotherapy and vehicle injections
Chemotherapy injections were a combination of doxorubicin (9 mg/kg body weight) and cyclophosphamide (90 mg/kg body weight). Vehicle injections consisted of an equal amount of saline per kilogram body weight. The mice were placed in conical restraints with tail exposed. The tail was warmed with a heat lamp and injection was given via lateral tail vein. Cages were changed after the three-day excretion period for chemotherapy.
Body weights
Body weights were measured on day 0 before starting dietary interventions. Mice were weighed the day before each injection (day 13 and 27) for accurate injection dosage calculations and again prior to tissue collection. Mice receiving chemotherapy weighed significantly less (p < 0.05) than mice receiving vehicle injections at the time of sacrifice. Body weight data over the course of the study can be found in Supplementary Figure 2.
Brain and liver fatty acids
A 50–70 mg sample of frozen whole brain or liver tissue was cut on dry ice and then transferred to a plastic tube containing 2 mL methanol and butylated hydroxytoluenein mixture (Sigma Aldrich, St. Louis, MO). Tissues were homogenized for 10 s then transferred to a glass tube. Lipids were extracted from homogenized tissue with 2:1 (v/v) of chloroform: methanol according to Folch et al. [Citation18]. Fatty acid methyl esters were prepared and extracted [Citation19], then analyzed via gas chromatography (Shimadzu, Columbia, MD) using a 30-m Omegawax 320 (Supelco-Sigma) capillary column and previously established conditions [Citation20]. Briefly, helium under flow rate of 30 mL/min was used as the carrier gas and oven temperature ramped beginning at 175°C and held for 4 min and subsequently increased to 220°C at a rate of 3°C/min. Retention times were compared to authentic standards for fatty acid methyl esters (Supelco-Sigma, St. Louis, MO and Matreya, Inc., Pleasant Gap, PA) and fatty acids were reported as percent of total identified.
Lipid peroxidation and neurotrophic markers
Brain 4-Hydroxynoneal (4-HNE) and brain-derived neurotropic factor (BDNF) were measured because of their importance in lipid peroxidation and brain function, respectively. Due to limited brain tissue, samples were available in a subset of 34–39 mice. 4-HNE was measured from 50 mg frozen whole brain tissue in n = 39 mice (3–4 per diet/injection group) using Oxi-Select HNE Adduct Competitive ELISA Kit (CAT# STA-838, Cell BioLabs, San Diego, CA) according to kit instructions. Protein was measured in the brain to normalize 4-HNE results using Pierce BCA Protein Assay Kit (CAT#23225 Thermo Scientific Waltham, MA). BDNF concentration was measured in n = 34 mice (3–4 per diet and injection group). Frozen whole brain tissue was prepared and assayed according to kit instructions for the BDNF Emax Immunoassay System (Promega Corporation Madison, WI). Protein was measured to normalize BDNF concentrations using Pierce BCA Protein Assay Kit (CAT #23225 Thermo Scientific Waltham, MA).
Statistical Analysis
All data analyses were completed using statistical software JMP 14 (SAS Institute Cary, North Carolina). Data were analyzed by 2-way ANOVA of diet and injection. Post-hoc comparisons were analyzed via Tukey’s Test. Pearson’s correlation was used to determine significant associations. The significance level for all between-group analyses was set a priori at p < 0.05. Bonferroni’s correction factor was used to adjust for multiple comparisons in correlational analyses.
Results
Diet and injection effects on brain polyunsaturated fatty acids
Brain PUFAs measured via gas chromatography and presented as a relative percent of total brain fatty acids measured are shown in . Brain ALA was not significantly different by group and is not depicted graphically (F9,89 = 1.0212, p = 0.4306). EPA was significantly different by group (F9,89 = 164.7641, p < 0.0001) with a main effect of diet (p < 0.0001) as shown in (a). There was no significant difference in brain EPA between High ALA and Low ALA control diet groups (p > 0.05 for all). However, mice consuming the high EPA + DHA diets (regardless of sucrose level) had significantly higher EPA in the brain than mice on the High ALA diets (p < 0.0001) and Low ALA control diet (p < 0.0001).
Figure 2 Brain n-3 and n-6 PUFA levels as percent of total fatty acids (Mean +/− SEM) measured by gas chromatography from n = 90 mice at sacrifice after 5 weeks of diets and 2 injections. (a) EPA (b) DHA (c) LA and (d) AA. a depicts differences (p < 0.05) between High E + D diets compared to High ALA and control diets. * depicts differences (p < 0.05) between chemotherapy compared to control saline injection. LS = Low Sucrose, HS = High Sucrose, High E + D = 2% kcals from EPA + DHA, High ALA = 1.3% kcals ALA, Control = Low ALA (0.6% kcals)/Low Sucrose, Eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), linoleic acid (LA), arachidonic acid (AA), polyunsaturated fatty acids (PUFA)
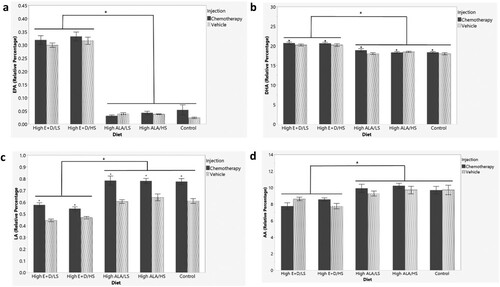
DHA was significantly different by group (F9,89 = 16.4480, p < 0.0001; (b)). There was an injection effect (p < 0.05) and diet effect (p < 0.0001). Mice receiving chemotherapy had significantly higher DHA in the brain than mice receiving the vehicle injection and mice on the high EPA + DHA diets (regardless of sucrose level) had significantly higher DHA in the brain than mice on the High ALA diets (p < 0.0001) and Low ALA control diet(p < 0.0001). There was no significant difference in brain DHA between High or Low ALA control diet groups (p > 0.05 for all).
Brain LA was significantly different by group (F9,89 = 31.5416, p < 0.0001) with a main effect of injection (p < 0.0001) and diet (p < 0.0001) ((c)). Mice consuming the high EPA + DHA diets (regardless of sucrose level) had significantly lower LA in the brain than mice on the High ALA diets (p < 0.0001) and Low ALA control diet (p < 0.0001). Mice receiving chemotherapy had significantly higher LA than mice receiving vehicle injections (p < 0.0001). AA was significantly different by group (F9,89 = 5.1915, p < 0.0001) with a main effect of diet (p < 0.0001) ((d)). Mice on the high EPA + DHA diets (regardless of sucrose level) had significantly lower relative amounts of AA in the brain than mice on the High ALA diets (p < 0.001 for both) and Low ALA control diet (p < 0.001). Supplementary Table 3 shows all individual fatty acids measured within the brain by diet and injection group.
Diet and injection effects on liver polyunsaturated fatty acids
PUFAs from liver tissue of n = 50 mice were measured via gas chromatography. Data are presented as relative percent of total fatty acids measured and shown in . ALA was not detectable in liver tissues. EPA ((a)) was significantly different by group (F9,49 = 404.2635; p < 0.0001) with a main effect of diet (p < 0.0001). Mice on the high EPA + DHA diets (regardless of sucrose level) had significantly higher liver EPA than mice on the High ALA (p < 0.0001) and Low ALA control diet (p < 0.0001). Mice on the Low ALA/LS control diet had significantly lower EPA than mice on the High ALA/HS diet (p < 0.05), but not significantly different than the High ALA/LS diet. DHA was significantly different by group (F9,49 = 123.7336, p < 0.0001; (b)). There was an injection effect (p < 0.0001) and diet effect (p < 0.0001). Mice receiving chemotherapy injections had significantly higher liver DHA than mice receiving vehicle injections (p < 0.0001). Mice receiving the high EPA + DHA/LS diet had significantly higher DHA than mice on the high EPA + DHA/HS diet (p < 0.01), High ALA diets (p < 0.0001 for both), and Low ALA control diet (p < 0.0001). Mice on the high EPA + DHA/HS diet had significantly higher liver DHA than mice on the High ALA diets (p < 0.0001) and Low ALA diet control (p < 0.0001). Mice on the High ALA diets (regardless of sucrose level) had significantly higher liver DHA than mice on the Low ALA control diet (p < 0.01 for both).
Figure 3. Liver PUFA levels as percent of total fatty acids (mean +/- SEM) measured via gas chromatography in a sub-cohort of 50 mice. (a) EPA (b) DHA (c) LA (d) AA. a depicts differences (p < 0.05) between High E + D and the high ALA and control diets. # depicts differences (p < 0.05) between control diet and a High ALA diet. ^ depicts differences (p < 0.05) between High E + D/LS and High E + D/HS diets. * depicts differences (p < 0.05) between chemotherapy and control saline injection. + depicts differences (p < 0.05) between High ALA/LS and High ALA/HS diets. Eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), linoleic acid (LA), arachidonic acid (AA), alpha-linolenic acid (ALA), polyunsaturated fatty acids (PUFA). LS = Low Sucrose, HS = High Sucrose, High E + D = 2% kcals from EPA + DHA, High ALA = 1.3% kcals ALA, Control = Low ALA (0.6% kcals)/Low Sucrose.
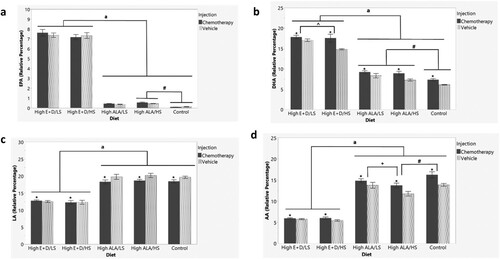
Relative amount of LA was significantly different by group (F9,49 = 49.4128, p < 0.0001; (c)), with a main effect of injection (p < 0.05) and diet (p < 0.0001). Mice receiving chemotherapy injections had significantly less liver LA than vehicle-injected mice (p < 0.05). Mice receiving the high EPA + DHA diet (regardless of sucrose level) had significantly less LA than mice on the High ALA diets (p < 0.0001) and Low ALA control diet (p < 0.0001). AA was significantly different by group (F9,49 = 93.6905, p < 0.0001; (d)) with a main effect of diet (p < 0.0001) and injection (p = 0.0001). Mice receiving the chemotherapy injections had significantly higher liver AA than mice receiving the vehicle injections (p = 0.0001). Mice receiving the high EPA + DHA diets (regardless of sucrose level) had significantly less AA than mice on the High ALA diets (p < 0.0001) and Low ALA control diet (p < 0.0001). Mice on the High ALA/LS diet and Low ALA/LS control diet had significantly higher liver AA than mice on the High ALA/HS diet (p < 0.01; p < 0.0001 respectively). Supplementary Table 4 shows all individual fatty acids measured within the liver by diet and injection group.
Brain and liver PUFA correlations
All PUFAs detectable in both brain and liver were analyzed for associations using Pearson correlations (). There were strong positive correlations between brain and liver tissue for all individual n-3 and n-6 PUFAs analyzed, as well as total n-3 fatty acids (r = 0.8214 p < 0.0001), total n-6 fatty acids (r = 0.7568 p < 0.0001), and the n-3/n-6 ratio (r = 0.8571 p < 0.0001). Only total PUFAs were not significantly correlated in the brain and liver.
Table 1. Pearson correlations for brain and liver PUFAs (n = 50 mice; 4–6 per diet and injection group). Results adjusted for multiple comparisons using Bonferroni’s correction; p < 0.006 considered significant. PUFA = polyunsaturated fatty acid, LA = linoleic acid, AA = arachidonic acid, EPA = eicosapentaenoic acid, DHA = docosahexaenoic acid.
Effects of diets and injections on lipid peroxidation and neurotrophic markers
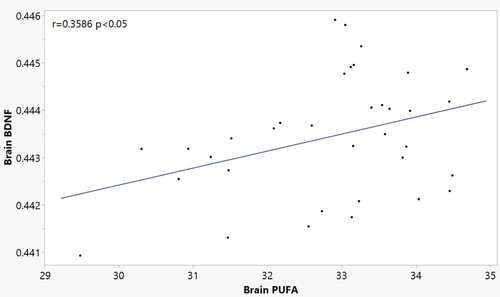
Brain levels of 4-HNE normalized to protein were not significantly different by diet or injection group (F9,38 = 0.7427, p = 0.6675; Supplementary Figure 3), although there was high variability within groups. Outliers were included in data analysis as removal of outliers did not significantly change the outcome. Brain BDNF normalized to protein was not significantly different between diet or injection groups (F9,35 = 1.2880, p = 0.2899; Supplementary Figure 4). However, there was a positive correlation (r = 0.3586 p < 0.05) of brain BDNF to total PUFA composition though the r value was small ().
Discussion
Summary of main findings
This study demonstrates that consumption of the plant-derived n-3 PUFA, ALA, in proportions approximately two times greater than typically consumed by humans does not appreciably increase brain n-3 PUFAs compared to low ALA intake during chemotherapy. As expected, high-dose EPA+ DHA resulted in significantly greater brain n-3 PUFAs than either ALA group. Sucrose content of the diet did not affect brain PUFAs. Individual and total n-3 and n-6 PUFAs were strongly correlated in brain and liver tissue, suggesting that liver n-3 and n-6 PUFAs may be a useful surrogate marker of brain n-3 and n-6 PUFAs in this mouse model of chemotherapy.
Although rodent models are useful in studying the effects of diet and underlying mechanisms on brain and liver outcomes during chemotherapy treatment [Citation7], rodents convert ALA to EPA and DHA more efficiently than humans [Citation21]. Thus, high ALA diets may result in relatively higher brain DHA in rodents compared to humans. However, in our mouse model of chemotherapy, feeding diets high in ALA (1.3% kcal) did not result in significantly different brain EPA or DHA than feeding a low ALA diet (0.5% kcal). These findings are similar to the results of a rodent study that measured tissue fatty acids in rats after feeding various amounts of ALA (0.1–6.4% of kcals from ALA) [Citation22]. Rats fed diets with 0.1% and 1% of kcals from ALA did not have significantly different levels of brain EPA, DHA, or AA; indeed, brain DHA composition did not increase until doses of ALA reached at least 3.3% of kcals [Citation22]. Even though our high ALA diet contained 1.3% of kcals from ALA, which is nearly double the amount of ALA in the typical U.S. diet [Citation15], it was not high enough to produce amounts of brain DHA comparable to those resulting from 2% EPA and DHA supplementation. Taken together, this research suggests that feeding ALA in amounts up to 3.5–5-fold greater than what is typically consumed by U.S. adults, is not as effective in increasing brain EPA and DHA as supplying high-dose (i.e. 2% of kcals) EPA and DHA to mice.
In this study, mice on high EPA + DHA diets had significantly higher brain EPA + DHA and lower LA and AA (n-6 fatty acids) than mice on low ALA or high ALA diets regardless of sucrose content or injection. This is important because increasing brain n-3 PUFAs may protect the brain from toxic side effects of chemotherapy [Citation7,Citation10]. Indeed, our previous work suggests that chemotherapy-induced neuroinflammation in the cortex may be attenuated by dietary EPA + DHA [Citation7]. To our knowledge, other researchers have not investigated the effects of dietary modification during chemotherapy on brain fatty acids. However, a rodent model of metabolic syndrome has been used to examine the effects of dietary fructose and n-3 PUFAs on brain DHA [Citation10]. Rats consuming the n-3 PUFA enriched diet (1.2% kcals from DHA), regardless of fructose content, had significantly higher DHA and lower AA within the brain compared to rats on the n-3 PUFA deficient diets [Citation23]. The dose of DHA used in this study, as well as the dose of EPA + DHA in our own, is higher than typical human consumption from food sources (e.g. <0.05% of kcals in U.S. adults) [Citation15]. However, these doses are comparable to the dose of EPA + DHA in fish oil well-tolerated by breast cancer survivors in a supplementation study [Citation17].
Mice receiving chemotherapy had significantly higher brain DHA than vehicle-injected mice. These results mirror prior findings from our research team with the exception that we previously found that DHA in the brain of chemotherapy-treated mice was higher with low sucrose diets compared to high sucrose diets [Citation7], As previously mentioned, there is a paucity of research on chemotherapy’s effect on brain fatty acids, but other rodent models have evaluated the effects of the brain insult on fatty acid composition [Citation11,Citation24]. In a rodent model of traumatic brain injury, there was no significant difference in brain DHA between mice with or without a brain injury; brain DHA was higher in mice with brain injuries when provided 1.2% of kcals from DHA compared to injured rodents on a control diet [Citation11]. Similarly, in a rodent model of Alzheimer’s disease, there was no change in brain DHA in mice with injury compared to those without [Citation24]. Based on these models of brain injury, the chemotherapy effect on DHA seems to be unique. A potential reason for higher brain DHA may be due to the body’s response to a higher inflammatory state from chemotherapy. Although we were not able to detect differences in lipid peroxidation in this study, Wu et al., reported that doxorubicin-based chemotherapy not only leads to oxidative stress and lipid peroxidation in the brain but induces peripheral inflammation that can cross the blood brain barrier to induce neuroinflammation [Citation10].
BDNF, a protein important in normal brain function, learning and memory [Citation11], was measured in a sub-set of mice. We did not find evidence of differences in BDNF between groups, perhaps related to the smaller sample size. However, BDNF was weakly, but significantly, positively correlated with total brain PUFAs in our mouse model. In a rodent model of traumatic brain injury, marine-derived n-3 PUFAs were shown to impact synaptic function in the brain through upregulation of BDNF [Citation11]. Increases in BDNF were associated with increased synaptic transmission and neuron excitability, both of which are important in memory and learning [Citation11]. A potential reason PUFAs are positively correlated with BDNF, may be related to PUFAs ability to increase membrane fluidity [Citation25]. The fluidity of the cell membrane plays an important role in cellular signaling [Citation26] and neurotransmission [Citation25]. Increased cellular signaling and neurotransmission may lead to increased activation of cAMP response element-binding protein (CREB). Activation of CREB has been shown to regulate BDNF gene transcription [Citation11]; thus increased activation of CREB through enhanced membrane fluidity may contribute to increased BDNF in the brain.
In this study, liver PUFAs differed significantly between the low and high ALA diets. Animals on the low ALA diet had the lowest levels of EPA and DHA in the liver. To our knowledge, we are the first to report on the effects of chemotherapy on liver fatty acids. However, rodent models of hepatic steatosis that induce an inflammatory state similar to chemotherapy, have found feeding EPA + DHA [Citation27] and ALA [Citation28] results in increased amounts of n-3 PUFAs and decreased n-6 PUFAs within the liver [Citation27,Citation28]. In our chemotherapy model, liver n-3 and n-6 PUFAs were strongly and significantly correlated to levels within the brain. This has important implications for researchers using translational animal models because compared to the brain, there is a much greater amount of liver tissue available for analyses in mice. Our findings suggest that liver n-3 PUFAs may be used as a surrogate measure of brain n-3 PUFAs in future studies investigating the effects of chemotherapy on the brain.
Strengths and limitations
A strength of this study is the inclusion of diets enriched with n-3 PUFA from both plant and marine sources. Additionally, the results are potentially more translatable to the human condition than results from animal models using super-physiologic doses of n-3 PUFAs, as the amounts of n-3 PUFAs and sucrose, as a percentage of energy, are typically consumed through diet or supplementation in humans. Similarly, the chemotherapy regimen was based on 50% human equivalent doses. Another strength of this study is the evaluation of fatty acids in both brain and liver, followed by an analysis of associations. There were also several limitations. Only one high dose of marine-based n-3 PUFAs was tested. Another limitation was that the mice were lean and consuming a low-fat diet throughout chemotherapy treatment. This does not reflect a large percentage of breast cancer patients, who are overweight or obese and may be consuming a higher fat diet [Citation29]. Finally, limited markers of oxidation and brain function were evaluated, and no cognitive behavioral testing was performed.
Implications for future research
In conclusion, EPA + DHA supplementation is an effective approach to increasing n-3 PUFAs in the brain during chemotherapy. However, enrichment of diets with approximately twice the amount of ALA as is commonly consumed in Western diets is not sufficient to increase brain n-3 PUFAs compared to a low ALA diet in this mouse model. Future research is needed to determine if n-3 PUFAs might alleviate the cognitive side effects of chemotherapy.
Supplemental Material
Download MS Word (1.1 MB)Acknowledgements
The authors acknowledge Dr. Panchita Phuwamongkolwiwat-Chu (Department of Human Sciences, Human Nutrition Program, The Ohio State University at the time of the research study) for her contributions to the analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data discussed in this article is available through contacting the corresponding author Dr. Tonya Orchard.
Additional information
Funding
References
- American Cancer Society. Breast cancer facts and figures 2015–-2016. Atlanta: American Cancer Society Inc; 2015.
- American Cancer Society. (2017). Breast cancer statistics. [cited 8 May 2017]. https://cancerstatisticscenter.cancer.org/#/cancer-site/Breast.
- Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–65.
- Jenkins V, Thwaites R, Cercignani M, Sacre S, Harrison N, Whiteley-Jones H, et al. A feasibility study exploring the role of pre-operative assessment when examining the mechanism of ‘chemo-brain’in breast cancer patients. Springerplus. 2016;5(1):390.
- Selamat MH, Loh SY, Mackenzie L, Vardy J. Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PloS One. 2014;9(9):e108002.
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
- Orchard TS, Gaudier-Diaz MM, Phuwamongkolwiwat-Chu P, Andridge R, Lustberg MB, Bomser J, et al. Low sucrose, omega-3 enriched diet has region-specific effects on neuroinflammation and synaptic function markers in a mouse model of doxorubicin-based chemotherapy. Nutrients. 2018;10(12):2004.
- Ma T, Liaset B, Hao Q, Petersen RK, Fjære E, Ngo HT, et al. Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice. PloS One. 2011;6(6):e21647.
- Arunagiri P, Rajeshwaran K, Shanthakumar J, Tamilselvan T, Balamurugan E. Combination of omega-3 fatty acids, lithium, and aripiprazole reduces oxidative stress in brain of mice with mania. Biol Trace Elem Res. 2014;160(3):409–17.
- Wu Y, Dang R, Tang M, Cai H, Li H, Liao D, et al. Long chain omega-3 polyunsaturated fatty acid supplementation alleviates doxorubicin-induced depressive-like behaviors and neurotoxicity in rats: involvement of oxidative stress and neuroinflammation. Nutrients. 2016;8(4):243.
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21(10):1457–67.
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr. 2006;83(6):1467S–1476S.
- Burdge GC, Calder PC. Α-linolenic acid metabolism in adult humans: the effects of gender and age on conversion to longer-chain polyunsaturated fatty acids. Eur J Lipid Sci Technol. 2005;107(6):426–39.
- DeSantis CE, Ma J, Gaudet MM, Miller KD, Sauer AG, Jemal A, Rebecca L. Breast cancer statistics. CA Cancer J Clin. 2019. doi: 10.3322/caac.21583. Available at cacancerjournal.com
- National Institute of Health. (2019). Omega-3 fatty acids. Available at https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/.
- Centers for Disease Control and Prevention. (2016). Know your limit for added sugars. [cited July 5, 2017]. https://www.cdc.gov/nutrition/data-statistics/know-your-limit-for-added-sugars.html.
- Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, et al. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat. 2018;167(3):709–18.
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957 May;226(1):497–509.
- Stoeffel W, Chu F, Ahrens Jr EH. Analysis of long-chain fatty acids by gas-liquid chromatography-micromethod for preparation of methyesters. Anal. Chem. 1959;31:307–8.
- Belury MA, Cole RM, Bailey BE, Ke JY, Andridge RR, Kiecolt-Glaser JK. Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol Nutr Food Res. 2016;60(5):1206–12.
- Baumgartner J, Smuts CM, Zimmermann MB. Providing male rats deficient in iron and n-3 fatty acids with iron and alpha-linolenic acid alone affects brain serotonin and cognition differently from combined provision. Lipids Health Dis. 2014;13(1):97.
- Valenzuela R, Barrera C, González-Astorga M, Sanhueza J, Valenzuela A. Alpha linolenic acid (ALA) from Rosa canina, sacha inchi and chia oils may increase ALA accretion and its conversion into n-3 LCPUFA in diverse tissues of the rat. Food Funct. 2014;5(7):1564–72.
- Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590(10):2485–99.
- Milanovic D, Petrovic S, Brkic M, Avramovic V, Perovic M, Ivkovic S, et al. Short-term fish oil treatment changes the composition of phospholipids while not affecting the expression of Mfsd2a omega-3 transporter in the brain and liver of the 5xFAD mouse model of Alzheimer’s disease. Nutrients. 2018;10(9):1250.
- Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17.
- Pereira DB, Chao MV. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J Neurosci. 2007;27(18):4859–69.
- Tapia G, Valenzuela R, Espinosa A, Romanque P, Dossi C, Gonzalez-Mañán D, et al. N-3 long-chain PUFA supplementation prevents high fat diet induced mouse liver steatosis and inflammation in relation to PPAR-α upregulation and NF-κB DNA binding abrogation. Mol Nutr Food Res. 2014;58(6):1333–41.
- Pachikian BD, Neyrinck AM, Cani PD, Portois L, Deldicque L, De Backer FC, et al. Hepatic steatosis in n-3 fatty acid depleted mice: focus on metabolic alterations related to tissue fatty acid composition. BMC Physiol. 2008;8(1):1–11.
- Sedjo RL, Flatt SW, Byers T, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Impact of a behavioral weight loss intervention on comorbidities in overweight and obese breast cancer survivors. Support Care Cancer. 2016;24(8):3285–93.