ABSTRACT
Background
The bed nucleus of the stria terminalis (BNST) is a structure with a peculiar neurochemical composition involved in modulating anxietylike behavior and fear.
Aim
The present study investigated the effects on the BNST neurochemical composition and neuronal structure in critical moments of the postnatal period in gestational protein-restricted male rats' offspring.
Methods
Dams were maintained during the pregnancy on isocaloric rodent laboratory chow with standard protein content [NP, 17%] or low protein content [LP, 6%]. BNST from male NP and age-matched LP offspring was studied using the isotropic fractionator method, Neuronal 3D reconstruction, dendritic-tree analysis, blotting analysis, and high-performance liquid chromatography.
Results
Serum corticosterone levels were higher in male LP offspring than NP rats in 14-day-old offspring, without any difference in 7-day-old progeny. The BNST total cell number and anterodorsal BNST division volume in LP progeny were significantly reduced on the 14th postnatal day compared with NP offspring. The BNST HPLC analysis from 7 days-old LP revealed increased norepinephrine levels compared to NP progeny. The BNST blot analysis from 7-day-old LP revealed reduced levels of GR and BDNF associated with enhanced CRF1 expression compared to NP offspring. 14-day-old LP offspring showed reduced expression of MR and 5HT1A associated with decreased DOPAC and DOPA turnover levels relative to NP rats. In Conclusion, the BNST cellular and neurochemical changes may represent adaptation during development in response to elevated fetal exposure to maternal corticosteroid levels. In this way, gestational malnutrition alters the BNST content and structure and contributes to already-known behavioral changes.
Introduction
Maternal malnutrition is even nowadays one significant public health issue worldwide [Citation1]. Inadequate levels of nutrients during pregnancy can lead to permanent alterations in fetal tissues and an increased risk of developing metabolic and endocrine dysfunctions and renal and cardiovascular disorders in adulthood [Citation2–7]. Studies have shown that both nutritional and psychological stress during pregnancy can lead to low weight at birth and are risk factors for developing psychiatric and neural disorders [Citation8,Citation9].
A wide range of evidence demonstrates that the postnatal activity of the hypothalamic–pituitary–adrenal (HPA) axis, a primary mechanism of stress response in the brain, may be altered by prenatal events [Citation10–17]. One of the mechanisms involved in the development of these disorders is the elevated exposure of the fetus to maternal glucocorticoids (GC) due to a low concentration and decreased activity of the type-2 11-β-hydroxysteroid dehydrogenase (11-β-HSD) placental enzyme [Citation18–23]. The prenatal alterations lead to a chronic increase in GC and exacerbated response to stressful stimuli in adulthood (for review, see [Citation24].
In particular, the brain is susceptible to fetal programming, and embryo/fetal exposure to GC can profoundly influence the nervous system's structure and synaptic formation [Citation25]. Recently, we observed amygdala cytological and neurochemical changes that may represent severe adaptation during embryonic development in response to elevated fetal exposure to maternal corticosteroid levels. In this way, gestational undernutrition stress can alter the amygdala's neurochemical and structure content and, thus, contribute to already-known behavioral changes induced by gestational protein restriction [Citation16]. Among the different brain areas involved in the stress response, the bed nucleus of the stria terminalis (BNST) seems particularly sensitive to stress and early-life exposure to glucocorticoids [Citation26], showing high plasticity [Citation27,Citation28]. The BNST is positioned in a privileged location for regulating the stress response; in particular, the BNST has been strongly associated with the modulation of anxiety-like behavior. The presence of corticosteroid receptors in this brain nucleus may partially explain the sensitivity of the BNST to stress response. The glucocorticoid (GR) [Citation29] and mineralocorticoid (MR) BNST receptors (for review [Citation30] location have been associated, respectively, with anxiety genic and lytic anxiety roles.
The BNST is also rich in corticotrophin-releasing factor (CRF) neurons, a neuropeptide that has long been associated with stress response and anxiety behavior. CRF can act through two different receptors: CRF1 and CRF2. While the role of CRF2 is still not completely understood, the activation of CRF1 has been strongly associated with increased anxiety-like response [Citation31–35]. In response to stress, CRF is also released by neurons in the paraventricular nucleus of the hypothalamus (PVN), leading to the activation of the HPA axis. Considering the high connectivity of both PVN and BNST neurons with projections to other areas in the central nervous system (CNS), such as the central nucleus of the amygdala (CeA), hippocampus, prefrontal cortex, and ventral tegmental area (VTA), the induced BNST release of CRF could modulate the neuron activity of many of these regions (for review see [Citation36].
As mentioned above, in response to stressful stimuli, the CRF secretion can modulate the activity of the HPA axis, and this will also influence the serotoninergic neurotransmitter system [Citation37–41]. The neural serotonin (5-HT) secretion is susceptible to stress and steroid action, so acute stress leads to the release of 5-HT by cells in the raphe nucleus. However, chronic stress causes, conversely, the depletion of 5-HT in this area [Citation42]. Many other factors are also crucial for the stress response; the brain-derived neurotrophic factor (BDNF) seems extremely sensitive to stress. BDNF is a neurotropic factor that has a pivotal role in physiology, physiological and pathology development in the CNS and brain plasticity mechanisms. It has been implicated in fear and anxiety behaviors [Citation43].
In a previous study, we found an impoverished dendritic arborization in BNST neurons, parallel to enhanced anxiety-like behavior, and elevated plasmatic corticosterone levels in adult offspring from mothers were submitted to gestational protein restriction [Citation15–17]. Thus, we are convinced that BNST is ‘programmed’ by gestational protein restriction, resulting in acceptable structural changes and neurochemical adaptations of these brain regions that constitute potential underlying causes of the altered behavioral states.
Thus, in critical moments of neuron development, the current study will analyze the early effects of gestational protein restriction on BNST morphology and neurochemistry in male LP offspring compared to regular protein content (NP) and control progeny.
Materials and methods
Animals and treatments
The experiments were performed on Wistar Hannover rats (250-300 g) and done following the general guidelines established by the Brazilian College of Animal Experimentation (COBEA) and approved by the Institutional Ethics Committee (CEUA/UNICAMP #3908-1 approved in 07/31/2015) and National Institutes of Health guidelines on animal care and experimentation and approved by Director General Veterinary (DGV; the Portuguese National Institute of Veterinary 023-432/08.30.2013). Rodent colonies originated from the breeding stock supplied by Charles River Laboratories, Barcelona, Spain, and from the Center of Biotherism of the State University of Campinas/Unicamp (CEMIB), Campinas, SP, Brazil. The environment and housing presented the right conditions for managing their health and well-being during the experimental procedure. Immediately after weaning at three weeks of age, animals were maintained under controlled temperature (25°C) and lighting conditions (08:00–20:00 h) with free access to tap water and standard laboratory rodent chow (Purina rat chow: Na + content: 135 ± 3μEq/g; K + content: 293 ± 5μEq/g), for 12 weeks before breeding. The rats were placed to mate, and it was designated day 1 of pregnancy as the day on which the vaginal smear exhibited sperm. The dams were divided into two groups: one maintained on isocaloric standard rodent laboratory chow with regular protein content (NP, n = 36) (17% protein), and the other received an isocaloric diet with low protein content (LP, n = 51) (6% protein), ad libitum, throughout the entire pregnancy (). The NP and LP maternal food consumption were determined daily (subsequently normalized for body weight), and the body weight of dams was recorded weekly in both groups. The dams were housed in pairs under standard laboratory conditions (lights on from 8 am to 8 pm) and had access to food and water ad libitum. Food consumption was determined every day (subsequently normalized for body weight). After delivery, the mothers returned to the standard protein chow (NP) intake. Here, only the male pups were studied and weighed on the birthday, and just eight male puppies were kept per mother. On the 7th and 14th postnatal days, the brain, thymus, and adrenals from one male NP (n = 10 for each age from different mothers) and LP (n = 10 for each age from different litters) offspring were weighed.
Table 1. Composition of standard rodent laboratory diet (standard normal-protein (NP) diet, 17% and low-protein (LP) diet, 6% (AIN 93G)).
Corticosterone (CORT) serum levels by radioimmunoassay (RIA) method
To avoid any influence of fluctuating baseline CORT levels due to circadian variations, blood samples from a separate group of 14-days-old LP (n = 6) and age-matched NP (n = 6) one male offspring from each litter were collected by tail venipuncture at 08:00 h to assess basal morning time CORT levels. All blood sampling and restraint were performed in a separate room adjacent to the animal housing room and within a maximum of 2 min from disturbing the animal's cage. The blood was centrifuged at high speed (13.000 g) for 10 min, and the supernatant was quickly removed and stored in a freezer at −80°C until the CORT assay was conducted. A double-antibody radioimmunoassay method was used to assay CORT serum levels for the rats using a commercial kit (R&D corticosterone SystemsTM Biotechne, Minneapolis, MN, USA). The test's coefficient of variation (CV) is 7.5%, the analytical sensitivity is 5 ng/ml, and the functional sensitivity is 25 ng/ml with a CV of intermediate precision of 20%.
Morphological analyses methods
On the 7th and 14th postnatal day, male offspring from NP and LP progeny (from different mothers) were deeply anesthetized with a mixture of ketamine and xylazine (75 and 10 mg/kg respectively, i.p.) and monitoring the corneal reflex controlled the level of anesthesia. For stereology and isotropic fractioning, the rats were transcardially perfused with saline containing heparin (5%, for 15 min, under constant pressure) followed by fixative solution: 0.1M phosphates buffer (PB; pH 7.4) containing 4% (w/v) paraformaldehyde. For 3D reconstruction, we used only saline for perfusion.
Stereology
After perfusion, the brains were removed, placed on the same fixative solution for two weeks, dehydrated, and included in Tecnovit 7100 (Heraeus Kulzer. Gmbh). Coronal sections (30 µm thick) were collected and stained with Giemsa 20%. The anterodorsal and anteroventral BNST volume and cell number were determined using the software Stereo Investigator (MicroBrightField. Williston VT USA) and a monitored microscope (Axioplan 2 Carl Zeiss Hamburg Germany) attached to a camera (DXC- 390. Sony Corporation. Tokyo. Japan). Cavalier’s principle was used to evaluate the volume of each region. The average cell number was estimated using the optical fractionator method. The error coefficients were calculated based on previously published cell number formulas and volume estimates [Citation26].
Isotropic fractionator method
We used the technique described by Herculano-Houzel & Lent [Citation44] for total cells and neuron quantification. The brains of 14-day-old rats (NP (n = 7) and LP (n = 7), from different litters) were removed, and BNST was dissected. A nuclei suspension was obtained through mechanical dissociation in a standard solution (40 mM sodium citrate and 1% Triton X-100) using a 40-ml glass Tenbroeck tissue homogenizer. After being washed several times with dissociation solution and centrifuged (10 min at 4000 g), pelleted nuclei were suspended in phosphate-buffered saline (PBS) containing 1% 4’. 6-diamidino-2-phenylindole (DAPI, Molecular Probes, Eugene, OR, USA). DAPI-stained nuclei were counted under a fluorescence microscope at 400×objective. The nuclear density in the suspension was determined by averaging over at least eight samples the total number of cells in the original tissue was estimated by multiplying the mean nuclear density by the complete suspension volume. Neuron number was estimated after immunohistochemistry using anti-NeuN antibody (1:300 in PBS; Chemicon Temecula, CA, USA) and CY3 goat anti-mouse secondary antibody (1:400 in 40% PBS, 10% goat serum, and 50% DAPI; Accurate Chemicals, Westbury, NY, USA) under the fluorescence microscope [Citation14]. The total number of other BNST cell nuclei is calculated by subtracting the number of NeuN-containing nuclei from the total number of nuclei.
Neuronal 3D reconstruction and dendritic tree analysis
Brain from 14 days old animals (4 NP and 4 LP from different mothers) were Golgi–Cox staining according to a published protocol [Citation45]. Briefly, brains were removed and immersed in Golgi–Cox solution [Citation46] for 14 days; brains were then transferred to a 30% sucrose solution (3 days) before being cut on a vibratome. Coronal sections (200 µm thick) were collected in 6% sucrose and blotted dry onto gelatin-coated microscope slides. They were subsequently alkalinized in 18.7% ammonia developed in Dektol (Kodak), fixed in Kodak Rapid Fix, dehydrated, and cleared in xylene before being mounted and coverslipped. Slides were coded before morphometric analysis in both sets. For the dendritic tree analysis, the criteria used to select neurons for reconstruction were as follows: (i) complete impregnation of the neurons along the entire length of the dendritic tree; (ii) dendrites without significant truncation of branches; (iii) relative isolation from neighboring impregnated neurons to avoid interference with the analysis; and (iv) no morphological changes attributable to incomplete dendritic impregnation of Golgi–Cox stain [Citation28]. Accordingly, we chose neurons with bipolar conformation confined to the anteromedial area (BNSTam) for dendritic analysis using the following criteria:
Presence of transverse anterior commissure
Rostral location to the stria terminalis main bundle
Selection of neurons adjacent to the anterior commissure
These landmarks correspond to the rostral portion of the medial division described by McDonald [Citation47]. For each selected neuron, all branches of the dendritic tree were reconstructed at 600x magnification using a motorized microscope (Carl Zeiss Axioplan 2) attached to a camera (DXC-390; Sony Co, Japan) and Neurolucida software (Micro Bright Field, VT, USA). Three-dimensional analysis of the reconstructed neurons was performed using NeuroExplorer software (MicroBrightField). For the dendritic analysis, 91 neurons were reconstructed.
Neurochemical analysis methods - Male offspring from both experimental groups were decapitated at 7 and 14 days, and the heads were snap-frozen in liquid nitrogen. The BNST was dissected and stored at −80°C until further analysis.
High-performance liquid chromatography combined with electrochemical detection (HPLC/CE) - The level of catecholamine and serotonin in the BNST was assessed by HPLC/CE using a Gilson instrument (Golson, Middleton, WI, USA) equipped with an analytical column (Supleco Supelcosil LC-18, 3 mM, Bellefonte, PA, USA, flow rate: 1.0 ml/min). Perchloric acid (200ul) was added to each sample, incubated for 30 min in ice, and then sonicated and centrifuged (13000 rpm, 10 min, 4°C). The supernatant was collected to a 1.5 ml tube and then centrifuged for 8 min (10000 rpm, 4°C), and the resulting pellet was saved for later use. The supernatant was then filtered through an HPLC Spin-X column (Costar. Lowell, MA, USA) to remove debris, and 150ul aliquots were injected into the HPLC system using a mobile phase of 0.7 M aqueous potassium phosphate (pH 3.0) in 10% methanol 1-heptane sulfonic acid (222 mg l-1) and Na-EDTA (40 mg l-1). A standard curve (Sigma H-7752) with known concentrations of each catecholamine was run each day for 5HIAA (Sigma H-8876), Dopamine (Sigma H-8502), DOPAC (Sigma D-9128), HVA (Sigma H-1252), Epinephrine (Sigma E-4375) and Norepinephrine (Sigma 74460).
Western blot (WB) – The tissue was homogenized in extraction buffer RIPA (Radio Immune Precipitation Assay Buffer), and then 10% triton x 100 and 10% SDS have added to the homogenate. The tissue extracts were centrifuged (1300 rpm at 4°C for 40 min), and the supernatants were used as a sample. Protein quantification was performed using the Bradford method. The samples were treated with a Laemmle buffer containing 100-mmol/l dithiothreitol (DTT) heated in a boiling water bath for 4 min and subjected to 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) in a Bio-Rad mini-gel apparatus (Mini-Protean Bio-Rad). Electrotransfer of proteins from the gel to the nitrocellulose membranes was performed for 90 min at 120 V (constant) in a Bio-Rad miniature transfer apparatus (Mini-Protean). The non-specific protein binding to the nitrocellulose was reduced by preincubating the filter for 2 h at 22°C in a blocking buffer (BSA 5%, 10 mmol/l Tris, 150 mmol/l NaCl and 0.02% Tween 20). The nitrocellulose blots were incubated at 4°C overnight with antibodies against GR (H-300-Santa Cruz 8992), MR (H-300-Santa Cruz), BDNF (Abcam; ab46176), 5HT1A (ab101914), 5HT2A (ab160228), CRF (S-19- Santa Cruz 1761), CRF1 (ab 59023) and Alfa tubulin (DSHB; AA4.3) diluted in a blocking buffer (2.5% BSA, 10 mmol/l Tris, 150 mmol/l NaCl and 0.02% Tween 20). Immunoreactivity bands were detected using the enhanced chemiluminescence method (RPN 2108 ECL Western blotting analysis system; Amersham Biosciences) and were detected by a ChemiDoc XRS system (Biorad; 170870). The band intensities were quantitated by optical densitometry (TINA software) of the developed autoradiographs used at linear range exposures.
Data presentation and statistical analysis
All data were normally distributed (Kolmogorov–Smirnov test) and hence were analyzed using parametric tests and reported as means ± SD. Data obtained over time was analyzed using an appropriate two-way analysis of variance (two-way ANOVA). Bonferroni's contrast test made post hoc comparisons between selected means when initial two-way ANOVA indicated statistical differences between experimental groups. A comparison involving only two independent observations within or between groups was made using a Student’s test. The band intensities were quantitated by optical densitometry (software TINA). The Tukey–Kramer test for multiple comparisons was used for analysis. The level of significance was set at P ≤ 0.05. Data analysis was performed with GraphPad Prism 5.00 for Windows (1992–2007 GraphPad Software Inc., La Jolla, CA, USA).
Results
Gestational low-protein diet effects on dams, birthweight fetus
LP dams (n = 51) during pregnancy were lighter in the second (238.13 ± 6.0 g) and third (325.1 ± 6.6 g) weeks of pregnancy compared to NP dams (n = 36) at the second week: 244.7 ± 8 and 3rd week: 354.7 ± 8, of gestation respectively, (NP vs. LP diet p = 0.007; NP vs. LP at time p < 0.001). This occurs despite an equal body weight in the first week of pregnancy. Thus, considering the entire pregnancy weeks, dams from the LP group had lower weight gain than those dams in the NP group (NP [n = 20]: 109.86 ± 20.04 g; LP [n = 20]: 87.00 ± 14.01 g; p < 0.001). The weekly food intake (in grams) was higher in LP dams in the first two weeks of pregnancy (1st week: 129.2 ± 3.25 g and second week: 135.7 ± 6 g) compared to NP (1st week: 111.1 ± 3.95 g and second week: 119.4 ± 6 g) dams (diet vs. time p = 0.018; diet p < 0.001; time p < 0.118). However, there was no difference in food intake between the groups in the last week of pregnancy (NP: 122.94 ± 3.95 g vs. LP: 122.9 ± 6 g). Litter sizes were similar (p = 0.239) for rats treated with a restricted protein diet (LP, n = 10.67 ± 2.44 puppies) compared to those submitted to regular protein breeding (NP, n = 11.43 ± 1.94 puppies). Despite the enhanced food intake by LP dams, the protein ingestion assessment showed that dams from the LP group were exposed to severe protein restriction (NP: 20.01 ± 0.9 g vs. LP: 7.91 ± 0.7 g) during the entire pregnancy (diet vs. time p = 0.018; diet p < 0.001; time p = 0.069).
The male LP offspring's birth mass was significantly reduced compared to male NP pups (NP: 6 ± 0.05 g, n = 20 vs. LP: 5.8 ± 0.05 g, n = 20, p = 0.006) (A). Furthermore, beyond the seventh day of life, the LP offspring maintains body masses smaller than observed in the NP offspring until the 14th day of study. However, the anogenital distance was significantly increased in male LP offspring at birth compared to age-matched and gender NP progenies (B,C).
Figure 1. The figure depicts body mass (in g) (panel A) and anogenital distance (at birth) (panel B), the body mass at birth, 7-days old, and 14-days old offspring with regular protein intake (NP, n = 20 for each age from different mothers) compared with age-matched low protein intake (LP, n = 20 for each age from different mothers) progeny (in grams) (panel C), and the basal corticosterone plasma level (in ng/ml) (panel D) at 7-days old and 14-days old offspring (n = 6 for each age) compared with the age-matched regular protein diet (NP) (n = 6 for each age) offspring. One offspring from each litter was used for hormonal analysis. The results are expressed as means ± SD. Data obtained over time were analyzed using repeated-measures analysis of variance (ANOVA). When ANOVA indicated statistically significant differences between selected standards, post hoc comparisons were performed with Bonferroni’s contrast test. Comparisons involving two means within or between groups were conducted using a Student’s t-test. The level of significance was set at *P < 0.05.
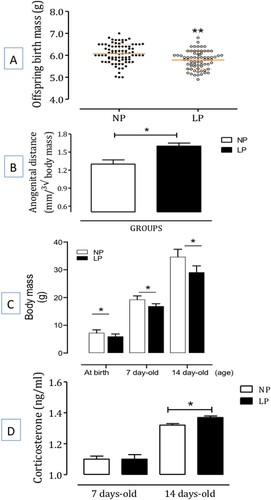
Corticosterone serum levels
The corticosterone (CORT) serum levels in male NP offspring compared to LP progeny – The gestational LP diet affects CORT serum levels in 7 and 14-day-old LP progeny (n = 6 for each age) and age-matched NP (n = 6 for each age) one male offspring from each litter. Basal diurnal CORT measurements revealed significant differences between the studied groups (D), with LP offspring displaying higher CORT serum levels than NP progeny at 14-days-old offspring (t = 3.536; df = 10; p = 0.0027), however without any statistical difference at 7-days-old progeny (t = 0.007; df = 10; p = 0.50).
Gestational low-protein diet effects on brain, thymus, and adrenal masses
In addition, the brain masses were significantly different at 7 (t = 2.756; df = 38; 0.0045) and 14 (t = 4.727; df = 38; p = 0.0001) day-old LP offspring (n = 10 for each age) than NP progeny (n = 10 for each age, ). In addition, in the 14-day-old LP offspring (n = 10), the thymus mass was significantly increased relative to age-matched NP offspring thymus mass at 7-day-olds (LP: 0.00327 ± 0.0001065, vs. NP: 0.00281 ± 0.000134, n = 10, t = 4.732, df = 38, p = 0.0001) and, decreased at 14 d-old (LP: 0.003084 ± 0.00021, vs. NP: 0.003354 ± 0.000145, n = 10, t = 11.76, df = 38, p = 0.0001, ). Also, a time-increased adrenal mass in LP progeny showed a significant increase at 7-day-olds (t = 7.209, df = 38, p = 0.0001, n = 10) and 14-day-olds (t = 6.869, df = 38, p = 0.0001, n = 10) compared to age-matched NP progeny ().
Figure 2. Effect of gestational protein restriction on the brainstem, thymus, and adrenal masses (in grams per 100 g body weight) in male offspring from protein-restricted (LP, n = 10 for each age from different mothers) and from normal-diet (NP, n = 10 for each age from different mothers) intake dams during the whole gestation. The results are expressed as means ± SD. Data obtained over time were analyzed using repeated-measures analysis of variance (ANOVA). When statistically significant differences were indicated between selected means by ANOVA, post hoc comparisons were performed with Bonferroni’s contrast test. The level of significance was set at *P < 0.05.
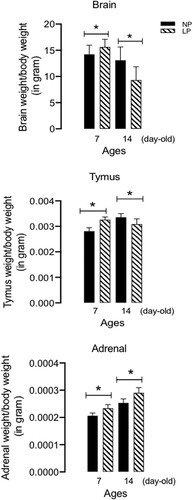
BNST total cells, anterodorsal and anteroventral division volume, and neurons quantification
The BNST volume of anterodorsal at 14-day-old male LP offspring was significantly reduced compared to age-matched NP (n = 4, t = 9.611, df = 8, p = 0.0001) but not at 7-day-old progeny of both groups (n = 4, t = 1.442, df = 6, p-0.0937); otherwise, the anteroventral BNST divisions was not altered in 7-day-old or 14-day-old offspring (n = 4 for each group of offspring from different litters), in both experimental groups (t = 0.9611, df = 8, p = 0.1823) (A,B). Additionally, on the 7th and 14th postnatal days, the anterodorsal and anteroventral BNST total cell numbers in male LP compared to those in the age-matched NP offspring were evaluated. (C,D). The BNST neuron cells number from the LP offspring showed a reduction of 57,6% (n = 7, t = 6.125, df = 8, p = 0.0001) and 66% less non-neuron cells (n = 7, t = 3.242, df = 8, p = 0.0059) (E) relative to NP progeny.
Figure 3. Effect of gestational protein restriction on the volume (panels A, B) and total cells (panels C, D) of anterodorsal and anteroventral BNST divisions (n = 4 for each age from different experimental group), and the neuron, non-neuron, and total cell number in BNST from maternal protein-restricted (LP, n = 7 for each age) compared to normal-diet intake (NP, n = 7 for each age) male offspring. The results are expressed as means ± SD. Data obtained over time were analyzed using repeated-measures analysis of variance (ANOVA). When ANOVA indicated statistically significant differences between selected standards, post hoc comparisons were performed with Bonferroni's contrast test. The level of significance was set at *P < 0.05.
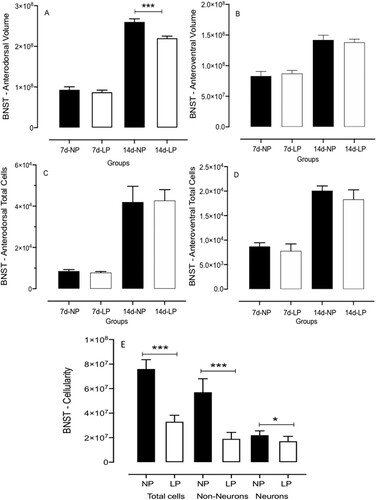
BNST neuronal 3D reconstruction and dendritic tree analysis
The neuronal 3D dendritic tree analysis revealed differences between 14-day-old male LP and NP progeny () regarding dendritic length and ramifications by Sholl analysis [Citation48]. Panel B shows a representative BNST neuron micrograph. Panels C, E, and F have exhibited the intersections and length of the dendritic tree between two progressive circles positioned at intervals of 10μm radial and 30μm from perikaryon. Moreover, Panel D depicts the graphic representation from Sholl analysis that revealed a reduction of 140 µm at the length of dendrites and 13.5% reduced dendritic length of BNST neurons ramifications from 14 days-old LP offspring compared with age-matched NP group (n = 4 for each group, t = 3.054, df = 10, p = 0.0061) ().
Figure 4. The graphic depicts the dendritic length of BNST neurons from maternal protein-restricted (LP, n = 4) compared to normal-diet intake (NP, n = 4) male offspring. Data expressed as mean ± SD. A representative BNST neuron micrograph is shown. The intersections and length of the dendritic tree between two progressive circles are positioned at intervals of 10 μm radial and 30 μm from perikaryon. The graphic is shown from Sholl's analysis. One offspring from each litter was used for histological analysis. Statistical analysis of segmental dendritic plots was conducted using repeated measures analysis of variance with adequate Greenhouse–Geisser corrections on the significant values. The level of significance was set at *P < 0.05.
BNST catecholamine and serotonin contents determined by high-performance liquid chromatography (HPLC)
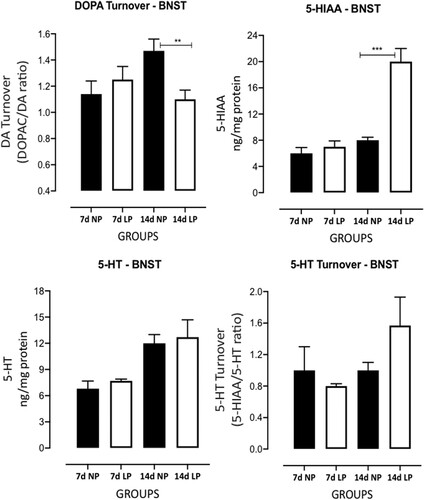
The gestational low-protein intake influenced BNST catecholamines and serotonin levels in male LP (n = 6 for each age from different mothers) offspring compared to age-matched NP progeny (n = 6 for each age from different litters) ( and ). The BNST high-performance liquid chromatography analysis from 7 days-old male LP offspring compared to NP progeny revealed significantly increased norepinephrine (NEP) (n = 6, t = 3.657, df = 6, p = 0.0053); however, the maternal low-protein diet did not affect epinephrine (EP), DOPA, 3,4-Dihydroxyphenylacetic acid (DOPAC), DOPA turnover, 5-HI, 5-HIAA and 5-HT turnover protein levels. Additionally, in 14-day-old protein-restricted offspring, a significant decreased DOPAC (n = 6, t = 2.060, df = 7,p = 0.0392) and DOPA turnover (n = 6 t = 3.106, df = 7, p = 0.0086) levels were associated with enhanced BNST 5-HIAA (n = 6 t = 6.549, df = 7, p = 0.0002) levels compared to NP progeny. Although LP progeny showed an increased BNST 5-HT turnover in 14-day-old LP offspring (38%) relative to NP rats, that difference was not significant (n = 6 t = 1.697, df = 7, p = 0.0668) ( and ).
Figure 5. Effects of gestational protein restriction (LP, n = 6 for each age from different mothers) compared to NP progeny (n = 6 for each age from other mothers) on BNST catecholamine and serotonin levels determined by High-Performance Liquid Chromatography (HPLC). This figure shows the results obtained in whole-tissue extracts for NEP, EPI, DOPA, and DOPAC in 7-day-old and 14-day-old NP and LP offspring. The results are expressed as ng/mg of BNST protein. Columns and bars represent the mean ± SD *P < 0.05 vs. NP (analysis of variance (ANOVA); post hoc Bonferroni's test).
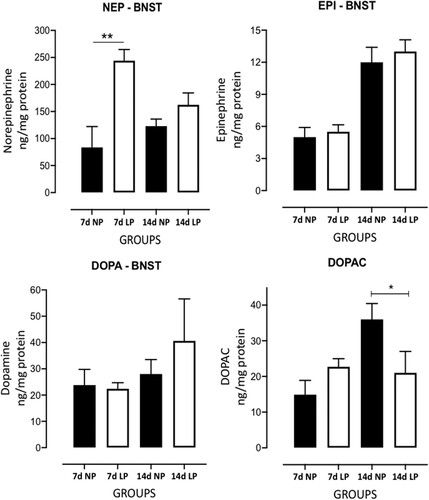
Figure 6. Effects of gestational protein restriction (LP, n = 6 for each age from different mothers) compared to NP progeny (n = 6 for each age from different mothers) on BNST catecholamine and serotonin levels determined by High-Performance Liquid Chromatography (HPLC). This figure shows the results obtained in whole-tissue extracts for 5-HT, 5-HIAA, 5-HT turnover and DOPA turnover in 7-day-old and 14-day-old NP and LP offspring. The results are expressed as ng/mg of BNST protein and turnovers by the ratio between DOPAC/DOPA and 5-HIAA/5-HT. Columns and bars represent the mean ± SD *P < 0.05 vs. NP (analysis of variance (ANOVA); post hoc Bonferroni's test).
Western blot analysis
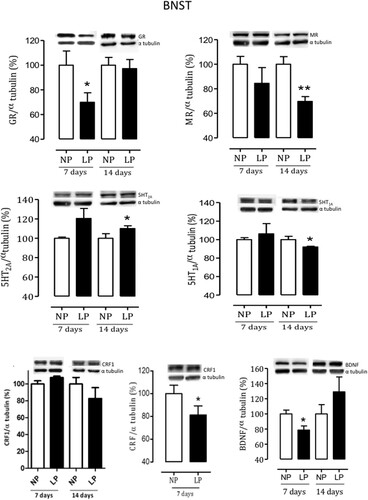
The BNST Western blot comparative analysis of the BNST from 7-day-old male LP progeny revealed a reduced level of GR and BDNF, respectively, of 31% (n = 8, t = 1.8517, df = 13, p = 0.0431) and 22% (n = 5, t = 2.023, df = 30, p = 0.0261) associated with 7% enhanced CRF1 expression (n = 4, t = 2.349, df = 20, p = 0.0146) compared to age-matched NP offspring. Also, In BNST from 14-day-old male LP progeny was observed a 31% reduction of MR (n = 9, t = 2.491, df = 13, p = 0.0135) associated with an 8% decreased 5HT1A (n = 4, t = 2.036, df = 8, p = 0.0247) and 20% enhanced but not significant 5HT2A protein level (n = 4, t = 0.4738, df = 13, p = 0.0759) in LP offspring relative to NP age-matched group. The present study shows, respectively 3% reduced GR (n = 9, p = 0.3638) and 18% CRFr1 (n = 9, p = 0.3508) protein levels and 8% enhanced 5HT2A (n = 8, p = 0.77) in 14-day-old male LP when compared to NP age-matched group. However, all protein levels are also not statistically significant ().
Figure 7. Effects of gestational protein restriction on expression of BNST proteins. shows the results obtained in whole-tissue extracts immunoblotted for GR, MR, 5HT1a, 5HT2a, CRF, type 1 CRF receptor, and BDNF in the NP and age-matched LP male offspring BNST. The representative results of scanning densitometry were expressed as relative to NP, assigning a value of 100% to the control (NP) rats. Columns and bars represent the mean ± SD. Data obtained over time were analyzed using repeated-measures analysis of variance (ANOVA). When statistically significant differences were indicated between selected means by ANOVA, post hoc comparisons were performed with Bonferroni’s contrast test. The level of significance was set at *P < 0.05, NP versus LP progeny.
Discussion
According to 2013 data from the WHO [Citation1], maternal malnutrition is still a worldwide public health issue directly associated with low birth weight. The concept of fetal programming defined by David Baker [Citation12] establishes that adverse insults occurring during gestation may lead to several diseases in adulthood. Thus, malnutrition during critical periods of gestational development may cause a broad spectrum of metabolic, cardiovascular, psychiatric, and neurochemical disorders [Citation2–9]. The current study evaluated a close association between gestational protein restriction and BNST morphological and neurochemistry changes in rats. It was so, proposing that the BNST may be a central nervous system (CNS) area in which permanent changes underlie, at least in part, accompanied by the development of behavioral disorders in this experimental model.
Following previous reports [Citation3–6,Citation49,Citation50], the current study confirmed a decreased body birthweight in LP progeny compared to NP offspring. This effect remains up to 14 days of age in gestational protein-restricted offspring, which might reflect the importance of proper protein intake during pregnancy for embryonic/fetal growth and maturation. We also confirm prior studies showing an increased anogenital distance in neonatal protein-restricted male offspring. This finding, as previously suggested, may be associated with enhanced levels of progesterone, corticosterone, estradiol, and testosterone at 19 days of gestation in female and male offspring [Citation49–51]. Unpredictably, as seen in our lab, LP offspring body mass remained lower beyond the second week of age than age-matched NP progeny during the allover follow-up. Here, it is critical to state that sex hormones determine sexual phenotype dimorphism in the fetal-programmed disease model in adulthood by changes in the long-term control of neural, cardiac, and endocrine functions. Thus, the present study was performed in male rats, considering the findings above to eliminate interferences due to gender differences [Citation52,Citation53]. So, the present study did not show the phenomenon widely known as catch-up growth, unlike the mass recovery seen in mothers’ offspring subjected to protein restriction supply only during the gestational period [Citation54].
Additionally, the present data showed that gestational protein restriction intake alters offspring's brain mass, reducing significantly from 7 to 14-day-old male LP progeny relative to age-matched NP offspring. Conversely, our laboratory recently demonstrated that 16-week-old male offspring from the maternal protein-restricted did not present any brain mass changes [Citation9,Citation15,Citation17]. In this sense, our current finding showed an enhanced brain mass at 7-day-old LP progeny. This result supports, at least transiently, the selfish-brain theory, which proposes that gestational adverse events such as emotional stress, specific or selective underfeeding, or fetal hypoxia may program the brain to maintain dimension, homeostasis, and stability in its energy sources. Notably, alterations in early-life fetal programming induced by a persistent decrease in developing neuronal supply reduce the neuroendocrine activity and, in turn, attenuate the growth somatic rate and guarantee adequate nutrition to the developing brain [Citation55]. Thus, this theory can initially explain the reduced birth weight of LP progeny without affecting brain mass compared to NP offspring. We also evaluated post-mortem adrenal and thymus masses in both experimental groups. In 7-day-old LP offspring, the results show an increased thymus mass that does not remain in 14-day-old offspring. The thymus change could be related to elevated steroidal plasma levels, usually associated with alteration in protein-restricted animals’ lymphoid tissue proliferation and, consequently, in lymphokine production. In addition, it has been strongly suggested that the immune system may modulate neuronal plasticity [Citation56]. Surprisingly, the adrenal mass was significantly enhanced in LP progeny compared to NP age-matched offspring. This adrenal mass change suggests that a presumably enhanced activity of the hypothalamus-hypophysis-adrenal axis may induce the increased adrenal mass transiently [Citation57].
Furthermore, the male LP offspring's birthweight reduction was accompanied by neurochemistry functional and morphological brain disorders compared to NP progeny. Previous studies have demonstrated that the embryo/fetal protein-restricted effect was associated with a significant early alteration of the amygdala neurochemistry in male offspring [Citation16].
Otherwise, in the present study, BNST cell quantification and stereology studies have also shown a significant reduction of the total BNST cells number and the decreased neurons and non-neuron cells ratio associated with reduced volume of the anterodorsal BNST division in 14d-old LP offspring when compared to that found in age-matched NP group. Also, in the present study, Sholl dendritic analysis revealed a reduction of the length of dendrites and reduced ramifications in the neurons of the LP progeny.
Several factors are involved in fetal programming, but the more strongly considered aspect involves high embryo/fetal exposure to glucocorticoids (GC). Other authors and we have demonstrated that gestational protein restriction leads to increased and persistent fetal exposure to maternal glucosteroids, which promotes, at times, disruption of the hypothalamic–pituitary–adrenal (HPA) axis balance causing elevated corticosterone plasma levels and, consequently, faster and precocious fetal tissues and organ differentiation [Citation13–17,Citation58]. Experimental observations during gestational exposure to psychological stress [Citation59] or after maternal administration of stress hormones [Citation60] also showed increased corticosterone plasma levels in the rodent progeny. Thus, so far, studies have demonstrated that gestational protein undernutrition leads to CNS developmental changes, mainly by reducing dendritic arborization [Citation17,Citation61], the number of synaptic ends and, in the neural myelination [Citation62], respectively. In support of the present data, we priory demonstrate that maternal protein restriction causes specific changes in BNST structure with a significant reduction of dendritical length and in the anterodorsal BNST division volume, already, in 16-weeks-old protein-restricted offspring [Citation16]. The BNST is the central integrator nucleus of excitatory and inhibitory inputs regulating the HPA axis [Citation63]. Since the BNST is a highly plastic structure, studies have demonstrated that the encephalic region, during embryo/fetal development, is critically vulnerable to environmental stresses and exposure to endogen and exogenous corticosterone high levels, which in turn lead to several morphological and functional disorders (for review, see [Citation64].
On the other hand, exposure to unpredictable chronic psychological stress is associated with increased volume and dendritic length in the BNST [Citation28], and chronic immobilization paradigms increase dendritic arborization in BNST neurons [Citation65]; 2003). The activation of the HPA axis promoted by stress stimulus ends with the release of corticosteroids by the adrenals. Corticosteroids are the main hormones linked to tissue maturation at the final days of gestation [Citation66], and the lack of regulatory balance in the expression of their steroid receptors (MR and GR) could increase the vulnerability of the CNS to adverse effects [Citation67]. In this work, we observed a decrease in the BNST expression of GR and MR, respectively, at 7-day and 14 postnatal days.
Endogenous GC in basal conditions has a higher affinity for MR [Citation68], and the MR stimuli seem to be involved in the neuron survival actions. It is primarily engaged in the maintenance of basal activity. Chronic stress can lead to both steroid receptors’ continuous activation, which may promote dendritic atrophy and deficits in synaptic plasticity [Citation67]. Considering these data, we suppose that once corticosteroids modulate structural alterations in the different CNS areas, including cellularity changes, structural volume, and synaptic and dendritic branching and morphology [Citation69], these effects may also explain the BNST finding observed in the present study. Furthermore, the current work confirms, by western blotting analyses, the decreased expression of gluco- and mineralocorticoid receptors at 7 and 14-d old LP progeny, respectively, accompanied by a fall in BDNF and associated with enhanced CRF1 receptor expression in the BNST of the 7-d old male LP offspring when compared to age-matched NP control rats. The basal corticosterone levels may indicate higher or lower stress levels [Citation57]. On the other hand, exposure to unpredictable chronic psychological stress is associated with increased volume and dendritic length in the BNST [Citation28], and chronic immobilization paradigms increase dendritic arborization in BNST neurons [Citation65,Citation70]. The activation of the HPA axis promoted by stress stimulus ends with the release of corticosteroids by the adrenals. Corticosteroids are the main hormones linked to tissue maturation at the final days of gestation [Citation66], and the lack of regulatory balance in the expression of their steroid receptors (MR and GR) could increase the vulnerability of the CNS to adverse effects [Citation67]. In this work, we observed a decrease in the BNST expression of GR and MR, respectively, at 7-day and 14 postnatal days.
Endogenous GC in basal conditions has a higher affinity for MR [Citation68], and the MR stimuli seem to be involved in the neuron survival actions. It is primarily engaged in the maintenance of basal activity. Chronic stress can lead to both steroid receptors’ continuous activation, which may promote dendritic atrophy and deficits in synaptic plasticity [Citation67]. Considering these data, we suppose that once corticosteroids modulate structural alterations in the different CNS areas, including cellularity changes, structural volume, and synaptic and dendritic branching and morphology [Citation69], these effects may also explain the BNST finding observed in the present study. Furthermore, the current work confirms, by western blotting analyses, the decreased expression of gluco- and mineralocorticoid receptors at 7 and 14-d old LP progeny, respectively, accompanied by a fall in BDNF and associated with enhanced CRF1 receptor expression in the BNST of the 7-d old male LP offspring when compared to age-matched NP control rats. The basal corticosterone levels may indicate higher or lower stress levels [Citation57]. As mentioned above, intrauterine stresses, within which maternal protein restriction intake, are associated with changes in the HPA axis in the offspring (for review, see [Citation71]. Confirming the present data, studies have demonstrated that gestational exposure to stress [Citation59] or the administration of stress hormones [Citation60] during gestation increases the plasma corticosterone concentration in the rats’ offspring. Prior studies from our lab [Citation15–17], as demonstrated in the current study, have shown that offspring from maternal protein restriction pregnancy have higher levels of plasmatic corticosterone at 7 days, 14-days, and 16 weeks old LP offspring compared to the age-matched NP progeny.
BDNF is strongly connected with the serotonergic system, and both are involved with memory processes and mood [Citation72]. The two systems may act together to regulate neuronal plasticity and the survival of new neurons [Citation73]. The current study shows a significant decrease in the expression of BDNF in the 7-d-old LP offspring associated with a substantial reduction in the neurons and non-neuron cells and in a volume of an anterodorsal portion 14-d-old BNST in LP offspring. This reduction may be caused by decreased expression of BDNF since this factor acts directly in glial and neuronal progenitor cells [Citation74]. They have also sustained the current study's previous results, showing that stress exposure decreases BDNF in brain regions associated with depression [Citation75,Citation76]. In addition, the 14-day-old LP offspring BNST presents reduced 5HT1A receptor subtype levels, reciprocally accompanied by increased 5HT2A receptors compared to age-matched NP offspring. It is well known that different ways of exposure to stress can alter the serotonergic system involved in emotional behavior and anxiety disorders [Citation77]. Both chronic stress and treatment with anxiogenic drugs have been shown to activate a subset of serotonergic neurons in the raphe nucleus with main targets limbic areas such as the BNST [Citation78–80]. 5HT1A and 5HT2A are expressed widely throughout the central nervous system, including the neocortex, hippocampus, septum, amygdala, raphe nucleus, basal ganglia, thalamus, and the olfactory tubercle. Exceptionally high concentrations of these receptors on the apical dendrites of pyramidal cells in layer V of the cortex may modulate cognitive processes, working memory, and attention [Citation81]. The mammalian 5HT1A and 5HT2A are subtypes of the serotonin (5-HT) G protein-coupled receptor (GPCR). In rodents, 5-HT1A receptor agonists are involved in neuro-modulating behavioral activity, learning, and memory. Activation of central 5-HT1A receptors triggers the inhibition of norepinephrine and enhances acetylcholine release, depending on species and brain areas. Also, 5-HT1A receptor stimulation has increased dopamine release in the medial prefrontal cortex, striatum, and hippocampus. It may help improve or attenuate the symptoms of schizophrenia and Parkinson's disease. The 5HT2A is the primary excitatory receptor subtype among the GPCRs and mediates excitatory neurotransmission for serotonin (5-HT). The study has described that the overdensity of the post-synaptic 5HT2A receptor is involved in the pathogenesis of depression. Moreover, 5-HT1A receptor agonists relieve anxiety and depression meanly by increasing synaptic serotonin concentration. Interestingly, stressors that activate the BNST also activate central serotonergic systems [Citation82–84]. Additionally, several studies have implicated the serotonergic system in modulating fear and anxiety-like behavior [Citation85–88]. In the present study, we hypothesize that reduced BNST 5HT1A and elevated 5HT2A expression may be related to anxiety and fear behavior observed in a previous study [Citation15].
Additionally, the BNST of 7-d-old LP offspring presents an enhanced level of norepinephrine compared with the NP age-matched offspring. This phenomenon was accompanied by decreased BNST dopamine turnover and DOPAC level, a metabolite of the neurotransmitter dopamine just at 14-day-old protein-restricted offspring. As mentioned above, it is widely known that catecholamine plays an essential role in the neurochemistry of the brain and is involved in a series of brain functions, among them the response to fear and anxiety. In the CNS, catecholamines work as neurotransmitters in the synaptic cleft and are a crucial part of maintaining homeostasis; quickly responding to any stressor that threatens the organism's homeostasis [Citation89,Citation90]. The adrenals’ sympathetic nervous terminals and chromaffin cells are the primary sources of circulating catecholamines [Citation91]. Our findings show a significant increase in the BNST norepinephrine levels and serotonin precursor (5-HIAA), respectively, at 7-day-old and 14-day-old LP offspring. The BNST is a structure that receives projections from noradrenergic receptors from the brain stem and, for this, plays a series of responses to stress. Nociceptive stimuli or immobilization stress in rats cause an increase in the BNST norepinephrine, suggesting that an aversive stimulus activates noradrenergic projections to the BNST [Citation92,Citation93]. Finally, the present study shows a reduced dopamine turnover and 3,4-dihydroxyphenylacetic acid (DOPAC), a metabolite of the neurotransmitter dopamine, BNST concentration, just in 14 d-old LP offspring. These results suggest that at 14 d-old, LP animals present a decreased neuronal release and degradation of dopamine.
Oliveira et al. [Citation26] have demonstrated that prenatal administration of dexamethasone does not alter the concentration of dopamine, DOPAC, and HVA in the BNST of the adult offspring. Nevertheless, there were alterations in the HPA axis [Citation94], which can act as a modulator of dopaminergic circuits [Citation95]. These exciting findings represent the adaptation during embryonic development to exposure to elevated maternal corticosteroids due to nutritional stress. Thus, the current study is the first description of the modulation of dendritic plasticity, morphology, and neurochemistry of the BNST in early life by protein restriction during the developmental period. It is essential to consider that different stimuli, intensities, and times can lead to different responses in CNS morphology [Citation26]. Also, additional studies must be done, but the present study in dams submitted to protein restriction strongly suggests that the current morphological and neurochemical results observed may be associated with developing psychiatric disorders in adulthood.
Ethics approval and consent to participate
The Institutional Ethics Committee (CEUA/UNICAMP #3908-1, approved in 07/31/2015) and the Director-General Veterinary (DGV; the Portuguese National Institute of Veterinary 023-432/08.30.2013) approved the experimental protocol; the general guidelines established by the Brazilian College of Animal Experimentation were followed throughout the investigation.
Consent for publication
All authors approved for publication.
Authors’ contributions
DBT: data curation, investigation, formal analysis, methodology, visualization, writing–original draft; AL: investigation, formal analysis, methodology; MGL and APV: methodology & visualization; NS: methodology & supervision; JARG: formal analysis, methodology, visualization, writing–review & editing; PAB and AJR: conceptualization, formal analysis, funding acquisition, methodology, resources, supervision, visualization, writing–original draft, writing–review & editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
https://acervus.unicamp.br/index.html (DOI: https://doi.org/10.47749/T/UNICAMP.2016.971693). http://repositorio.unicamp.br/acervo/detalhe/971693?guid = 1684785483168&returnUrl=%2fresultado%2flistar%3fguid%3d1684785483168%26quantidadePaginas%3d1%26codigoRegistro%3d971693%23971693&i = 1, and https://www.tandfonline.com/doi/abs/10.1080/1028415X.2022.2131064?journalCode = ynns20.
Additional information
Funding
Notes on contributors
D.B. Torres
D.B. Torres graduated in Physiotherapy from Universidade do Oeste Paulista with Master's and doctorate degrees in Medical Pathophysiology - FCM - Unicamp with an internship at the University of Minho Braga/Portugal. During her postgraduate period, she worked in the area of Fetal Programming and its repercussions on the central nervous system. During her Ph.D., she worked with 3D neuron reconstruction of the Stria Terminal and Amygdala Nucleus. She is interested in morphology, developmental biology, fetal programming, and neurobehavioral parameters.
A. Lopes
A. Lopes holds a bachelor's in Biological Sciences from the Pontifical Catholic University of São Paulo (2008) and a Master's in Internal Medicine from the State University of Campinas (Unicamp). Doctorate by the Medical Pathophysiology program at the State University of Campinas, with an internship at the University of Minho Braga/Portugal., developing stereology and 3D neuronal reconstruction. She is interested in Morphology, Neurosciences, Developmental Biology, Fetal Programming, and Neurobehavioral Parameters.
A.J. Rodrigues
A.J. Rodrigues, born in Vila Nova de Famalicão, Portugal. She graduated in Applied Biology in 2003 at the University of Minho and completed a Ph.D. in Health Sciences in 2008 at the University of Minho School of Medicine. Her research focuses on how the brain encodes events of pleasure and aversion, leading to behaviors. She leads a research team at ICVS after extensive experience in international reference laboratories in the USA, Netherlands, Italy, Finland, and Portugal.
A.P. Ventura-Silva
A.P. Ventura-Silva graduated in Applied Biology and completed a Research trainee in the Department of Biochemistry and Molecular Biology, Barcelona, Spain. Get a Ph.D. in Life and Health Science Research Institute, University of Minho, Braga, Portugal. I also have a postdoc in the School of Health Science, University of Minho, Braga, Portugal, and recently, a postdoc position in APC Microbiome Ireland Biosciences Building, University College Cork, Ireland. She works in areas that cover basic clinical research, and several modulatory interventions have also been described to promote the recovery of structure and function in neuronal tissues.
N. Sousa
N. Sousa (MD, Ph.D.) is a Full Professor at the University of Minho School of Medicine. He is the President of the School of Medicine at the University of Minho. Nuno Sousa, after he moved from U. Porto to U. Minho in 2001, was able to create from scratch, together with a small group of other leaders, this recognized medical school, one of the most well-known and respected internationally. He is a researcher in the Neuroscience Research Domain, which integrates the newly formed (in 2011) Portuguese Government Associate Laboratory (LA) ICVS/3B's. His research's main interests are focused on the establishment of functional and structural correlations mediated by stress and its implications in neuropsychiatric disorders.
J.A.R. Gontijo
J.A.R. Gontijo graduated in Medicine from the University of Brasília a medical Residency in Clinical Medicine HC/FMRP/USP, a Master's from the Faculty of Medicine of RP/USP, a Doctor of Medicine (Medical Clinic) from the University of São Paulo, and Postdoctoral internship at the University of Iowa, USA. He is currently a Full Professor at the State University of Campinas, SP, with complete dedication to teaching and research. He has research experience in Renal Physiology and Internal Medicine, emphasizing Nephrology. He works mainly in the following fields of knowledge: renal function, neural control of the kidney, arterial hypertension, and effects of nutritional stress on renal ontogenesis. He is a researcher at CNPq, has around 180 journals and book chapter publications, and is a supervisor of masters, doctorate, and postdoc students.
P.A. Boer
P.A. Boer graduated in Biological Sciences with a Licentiate and Bachelor's Degree, a Master's in Biological Sciences from the State University of Campinas, and a Ph.D. in essential areas of Clinical Medicine from the State University of Campinas. She worked as a teacher at many institutions and is currently doing research at the Faculty of Medical Sciences at the State University of Campinas. She spent some time at Monash University developing a metanephros culture technique and at the University of Minho, Braga, Portugal, learning about stereology with 3D neuronal reconstruction. She has experience in morphology, physiology, nephrology, neurology, cardiology, hypertension, and fetal programming.
Marcelo Gustavo Lopes
Marcelo Gustavo Lopes graduated in Medicine from the State University of Campinas; he holds a master's degree in Internal Medicine from the State University of Campinas (2019), a residency in Medicine from the State University of Campinas (2017), and a residency in Medicine from the State University of Campinas (2016). He is currently an Assistant Physician at the State University of Campinas and a Researcher (Doctorate) at the State University of Campinas. He is acting mainly on the following topics: fluoxetine and fetal programming.
References
- World Health Organization. Global targets 2025 to improve maternal, infant, and young child nutrition. 2015. http://www.who.int/nutrition/topics/nutrition_globaltargets2025/en/index.html.
- Ashton N. Perinatal development, and adult blood pressure. Braz J Med Biol Res. 2000;33:731–40.
- Sene LB, Mesquita FF, de Moraes LN, Santos DC, Carvalho R, Gontijo JA, Boer PA. Involvement of renal corpuscle microRNA expression on epithelial-to-mesenchymal transition in maternal low protein diet in adult programmed rats. PLoS One. 2013;19(8):e71310. doi:10.1371/journal.pone.0071310
- Sene LB, Rizzi VHG, Gontijo JAR, Boer PA. Gestational low-protein intake enhances whole-kidney miR-192 and miR-200 family expression and epithelial-to-mesenchymal transition in rat adult male offspring. J Exp Biol. 2018;221:jeb171694. doi: 10.1242/jeb.171694.
- Sene LB, Scarano WR, Zapparoli A, Gontijo JAR, Boer PA. Impact of gestational low-protein intake on embryonic kidney microRNA expression and in nephron progenitor cells of the male fetus. PLoS One. 2021;16(2):e0246289. doi:10.1371/journal.pone.0246289.
- Lamana GL, Ferrari ALL, Gontijo JAR, Boer PA. Gestational and breastfeeding low-protein intake on blood pressure, kidney structure, and renal function in male rat offspring in adulthood. Front Physiol. 2021;12:658431. doi:10.3389/fphys.2021.658431.
- Mariano VS, Boer PA, Gontijo JAR. Fetal undernutrition programming, sympathetic nerve activity, and arterial hypertension development. Front Physiol. 2021. 10.3389/fphys.2021.704819
- Burd L, Severud R, Kerbeshian J, Klug MG. Prenatal and perinatal risk factors for autism. J Perinat Med. 1999;27:441–50.
- Grigoletti-Lima GB, Lopes MG, Barufi Franco AT, Damico AM, Boer PA, Gontijo JAR. Severe gestational low-protein intake impacts hippocampal cellularity, tau, and amyloid- levels, and memory performance in male adult offspring: an Alzheimer-simile disease model? J Alzheimer’s Dis Rep. 2022;6(1):17–30. doi: 10.3233/ADR-210297.
- Barker DJ. Fetal origins of coronary heart disease. Br Med J. 1995;311(6998):171–4. doi:10.1136/bmj.311.6998.171.
- Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995;25:457–63.
- Barker DJP, Osmond C, Golding J, Kuh D, Wadsworth MEJ. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J. 1989;298:564–7.
- de Lima MC, Scabora JE, Lopes A, Mesquita FF, Torres D, Boer PA, Gontijo JAR. Early changes of hypothalamic angiotensin II receptors expression in gestational protein-restricted offspring: effect on water intake, blood pressure and renal sodium handling. J Renin-Angiotensin-Aldosterone Syst. 2013;14(3):271–82. 10.1177/1470320312456328.
- Scabora JE, Lima MC, Lopes A, Lima IP, Mesquita FF, Torres DB, et al. Impact of taurine supplementation on blood pressure in gestational protein-restricted offspring: Effect on the medial solitary tract nucleus cell numbers, angiotensin receptors, and renal sodium handling. J Renin Angiotensin Aldosterone Syst. 2015;16(1):47–58. doi: 10.1177/1470320313481255.
- Torres DB, Lopes A, Rodrigues AJ, Cerqueira JJ, Sousa N, Gontijo JAR, Boer PA. Anxiety-like behavior and structural changes of the bed nucleus of the stria terminalis (BNST) in gestational protein-restricted male offspring. J DOHAD. 2018;9:536–43. 10.1017/S2040174418000399.
- Torres DB, Lopes A, Rocrigues AJ, Lopes MG, Ventura-Silva AP, Sousa N, et al. Gestational protein restriction alters early amygdala neurochemistry in male offspring. Nutritional Neurosci. 2022: 1–17. 10.1080/1028415X.2022.2131064.
- Lopes A, Torres DB, Rodrigues AJ, Cerqueira JJ, Pêgo JM, Sousa N, et al. Gestational protein restriction induces CA3 dendritic atrophy in dorsal hippocampal neurons but does not alter learning and memory performance in adult offspring. Int J Dev Neurosci. 2012;31(3):151–6. doi: 10.1016/j.ijdevneu.2012.12.003.
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–41.
- Stewart PM, Whorwood CB, Mason JI. Type-2 11-beta-hydroxysteroid dehydrogenase in foetal and adult life. J Steroid Biochem Mol Biol. 1995;55:465–71.
- Langley-evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond). 1996;91:607–15.
- Langley-Evans SC, Phillips GJ, Benediktsson R, et al. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–72.
- Langley-Evans SC. Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci (Lond). 1997;93(5):423–9.
- Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci. 1997;60(15):1213–21.
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13(2):113–28.
- Ding YX, Shi Y, Han WJ, Cui H. 2016. Regulation of glucocorticoid related genes and receptors/regulatory enzyme expression in intrauterine growth restriction filial rats. Life Sci. doi: 10.1016/j.lfs.2016.02.079.
- Oliveira M, Rodrigues AJ, Leão P, Cardona D, Pêgo JM, Sousa N. The bed nucleus of stria terminalis and the amygdala as targets of antenatal glucocorticoids: implications for fear and anxiety responses. Psychopharmacology (Berl). 2012;220(3):443–53. doi: 10.1007/s00213-011-2494-y.
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1-3):199–216.
- Pêgo JM, Morgado P, Pinto LG. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27(6):1503–16.
- Kream J, Mulay S, Fukushima DK, Solomon S. Determination of plasma dexamethasone in the mother and the newborn after administration of the hormone in a clinical trial. J Clin Endocrinol Metab. 1983;56(1):127–33.
- Gomez-Sanchez EP. Mineralocorticoid receptors in the brain and cardiovascular regulation: minority rule? Trends Endocrinol Metab. 2011;22(5):179–87.
- Davis M. The Role of the Amygdala in Fear and Anxiety. Ann Rev Neurosci. 1992;15:353–75.
- Davis M. The role of the amygdala in emotional learning. Int Rev Neurobiol. 1994;36:225–66. doi:10.1016/S0074-7742(08)60305-0.
- Merali Z, Khan S, Michaud DS, Shinosito L, Phosphatepy SA, Anisman H. Does amygdaloid corticotropin-releasing hormone (CRF) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRF release. Eur J Neurosci. 2004;20(1):229–39.
- Mesquita AR, Pêgo JM, Summavielle T, Maciel P, Almeida OF, Sousa N. Neurodevelopment milestone abnormalities in rats exposed to stress in early life. Neuroscience. 2007;147(4):1022–33.
- Muller MB. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6(10):1100–7.
- Corominas M, Roncero C, Casas M. Corticotropin-releasing factor and neuroplasticity in cocaine addiction. Life Sci. 2010;86:1–9.
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60(5):441–60.
- Holsboer F. Corticotropin-releasing hormone modulators and depression. Curr Opin Investig Drugs. 2003;4:46–50.
- Nestler EJ, Barrot M, Dileone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25.
- Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(S3):302–6.
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci 2003;24:580–8.
- Fontenot MB, Kaplan JR, Manuck SB, Arango V, Mann JJ. Long-term effects of chronic social stress on serotonergic indices in the prefrontal cortex of adult male cynomolgus macaques. Brain RES. 1995;705:105–8.
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1309–20.
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25(10):2518–21.
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4.
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–25.
- Mcdonald AJ. Neurons of the bed nucleus of the stria terminalis: a Golgi study in the rat. Brain Res Bull. 1983;10(1):111–20.
- Sholl DA. The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. 1956;2:324–33.
- Mesquita FF, Gontijo JA, Boer PA. Expression of renin-angiotensin system signalling compounds in maternal protein-restricted rats: effect on renal sodium excretion and blood pressure. Nephrol Dial Transplant. 2010;25:380–8. doi: 10.1093/ndt/gfp505.
- Mesquita FF, Gontijo JAR, Boer PA. Maternal undernutrition and the offspring kidney: from fetal to adult life. Braz J Med Biol Res. 2010;43(11):1010–8. doi:10.1590/S0100-879X2010007500113.
- Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González Zamorano M, Ledesma H, et al. 2006. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism, and serum leptin in the rat. J Physiol 571(1):221–30.
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–202.
- Gillette R, Reilly MP, Topper VY, et al. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Hormone Behav. 2017;87:8–15.
- Woodall S, Johnston BM, Breier BH, Gluckman P. Chronic Maternal Undernutrition in the Rat Leads to Delayed Postnatal Growth and Elevated Blood Pressure of Offspring. Pediatr Res. 1996;40(3):438–43. doi:10.1203/00006450-199609000-00012.
- Lumbers ER, Yu ZY, Gibson KJ. The selfish brain and the Barker hypothesis. Clin Exp Pharmacol Physiol. 2001;28(11):942–7.
- Li M, Guo K, Adachi Y, Ikehara S. Immune Dysfunction Associated with Abnormal Bone Marrow-Derived Mesenchymal Stroma Cells in Senescence Accelerated Mice. Int J Mol Sci. 2016;29(2):183. doi: 10.3390/ijms17020183.
- Ventura-Silva AP, Pêgo JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, et al. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci. 2012;36(10):3396–406. doi: 10.1111/j.1460-9568.2012.08262.x.
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci (Lond). 2007;113(5):219–32.
- Weinstock M, Matlina E, Maor GI, Rosen H, Mcewen BS. . Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary-adrenal system in the female rat. Brain Res 1992;595(2):195-200
- Fameli M, Kitraki E, Stylianopoulou F. Effects of hyperactivity of the maternal hypothalamic-pituitary-adrenal (HPA) axis during pregnancy on the development of the HPA axis and brain monoamines of the offspring. Int J Dev Neurosci. 1994;12(7):651–9.
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–23. doi: 10.1038/nature12018
- Lima AD, Voigt T. Astroglia inhibit the proliferation of neocortical cells and prevent the generation of small GABAergic neurons in vitro. Eur J Neurosci. 1999;11(11):3845–56.
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47(1-3):145–60.
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K MAYV. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression, and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42(3):327–40.
- Vyas A, Mitra R, Shankaranarayana RBS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Neurosci. 2002;22(15):6810–8.
- Wood CE, Keller-Wood M. 2016. The critical importance of the fetal hypothalamus-pituitary-adrenal axis. F1000Res 28;5. doi: 10.12688/f1000research.7224.1. e Collection 2016.
- Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57(2):561–70.
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301.
- Leão P, Sousa JC, Oliveira M, Silva R, Almeida OF, Sousa N. Programming effects of antenatal dexamethasone in the developing mesolimbic pathways. Synapse. 2007;61(1):40–9.
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965(12):290–4.
- Charil A, Laplante D, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res. 2010;65(1):56–79. doi: 10.1016/j.brainresrev.2010.06.002.
- van donkelaar EL, van den Hove DL, Blokland A, Steinbusch HW, Prickaerts J. Stress-mediated decreases in brain-derived neurotrophic factor as a potential confounding factor for acute tryptophan depletion-induced neurochemical effects. Eur Neuropsychopharmacol. 2009;19(11):812–21. doi: 10.1016/j.euroneuro.2009.06.012.
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27(10):589–94.
- Rial D, Lemos C, Pinheiro H, Duarte JM, Gonçalves FQ, Real JI, et al. Depression as a Glial-Based Synaptic Dysfunction. Front Cell Neurosci. 2016;9:521. doi: 10.3389/fncel.2015.00521.
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–25.
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonists. Neuroscience. 2003;121(4):847–53.
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety Suppl. 2000;12(S1):2–19.
- Grahn RE, Maswood S, Mcqueen MB, Watkins LR, Maier SF. Opioid-dependent effects of inescapable shock on escape behavior and conditioned fear responding are mediated by the dorsal raphe nucleus. Behav Brain Res. 1999;99(2):153–67.
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20(20):7728–36.
- Singewald N, Kouvelas D, Kaehler ST, Sinner C, Philippu A. Peripheral chemoreceptor activation enhances 5-hydroxytryptamine release in the locus coeruleus of conscious rats. Neurosci Lett. 2000;289(1):17–20.
- Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol. 2006;4(2):101–14.
- Dilts RP, Boadle-Biber MC. Differential activation of the 5-hydroxytryptamine-containing neurons of the midbrain raphe of the rat in response to randomly presented inescapable sound. Neurosci Lett. 1995;199(1):78–80.
- Grahn RE, Will MJ, Hammack SE, Maswood S, Mcqueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826(1):35–43.
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, et al. Inescapable shock activates serotonergic neurons in all raphe nuclei of the rat. Behav Brain Res. 2004;153(1):233–9.
- Graeff FG, Guimarães FS, de Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54(1):129–41.
- Handley SL, Mcblane JW, Critchley MA, Njung'e K. Multiple serotonin mechanisms in animal models of anxiety: environmental, emotional and cognitive factors. Behav Brain Res. 1993;58(1-2):203–10.
- Handley SL. 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol Ther. 1995;66(1):103–48.
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–46.
- Andreis DT, Singer M. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 2016;42(9):1387–97.
- Riedemann T, Patchev AV, Cho K, Almeida OF. Corticosteroids: way upstream. Mol Brain. 2010;3:2. doi: 10.1186/1756-6606-3-2.
- Lymperopoulos A, Brill A, Mccrink KA. GPCRs of adrenal chromaffin cells & catecholamines: The plot thickens. Int J Biochem Cell Biol. 2016. doi: 10.1016/j.biocel.2016.02.003
- Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res. 1998;788:287–93.
- Pacak K, Mccarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688(1-2):242–6.
- Oliveira M, Bessa JM, Mesquita A, Tavares H, Carvalho A, Silva R, et al. Induction of a hyperanxious state by antenatal dexamethasone: a case for less detrimental natural corticosteroids. Biol Psychiatry. 2006;59(9):844–52.
- Piazza PV, le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78.
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
