Abstract
The advent of biosensors has tremendously increased our potential of identifying and solving important problems in various domains, ranging from food safety and environmental analysis, to healthcare and medicine. However, one of the most prominent drawbacks of these technologies, especially in the biomedical field, is to employ conventional samples, such as blood, urine, tissue extracts and other body fluids for analysis, which suffer from the drawbacks of invasiveness, discomfort, and high costs encountered in transportation and storage, thereby hindering these products to be applied for point-of-care testing that has garnered substantial attention in recent years. Therefore, through this review, we emphasize for the first time, the applications of switching over to noninvasive sampling techniques involving hair and nails that not only circumvent most of the aforementioned limitations, but also serve as interesting alternatives in understanding the human physiology involving minimal costs, equipment and human interference when combined with rapidly advancing technologies, such as microfluidics and organ-on-a-chip to achieve miniaturization on an unprecedented scale. The coalescence between these two fields has not only led to the fabrication of novel microdevices involving hair and nails, but also function as robust biosensors for the detection of biomarkers, chemicals, metabolites and nucleic acids through noninvasive sampling. Finally, we have also elucidated a plethora of futuristic innovations that could be incorporated in such devices, such as expanding their applications in nail and hair-based drug delivery, their potential in serving as next-generation wearable sensors and integrating these devices with machine-learning for enhanced automation and decentralization.
Introduction
Since their emergence in the 1960s, biosensors have served as a cornerstone in the field of analytical sciences, and these are the result of the amalgamation of a variety of scientific disciplines.[Citation1] It is quintessentially a device in which, a highly specific reaction or interaction between a sensing element and a chemical or biological analyte in a given sample is converted to a measurable signal in proportion to the concentration of analyte.[Citation2,Citation3] Designed in numerous fashions,[Citation4] simply based on the specific mechanism of detection of the desired analyte, biosensors have penetrated various industrial and research-oriented fields, beginning from health monitoring[Citation5–7] and diagnostics,[Citation8,Citation9] to food safety analysis[Citation10,Citation11] and forensic investigations.[Citation12,Citation13] Moreover, ultra-modern interventions, such as machine learning are being implemented to enhance the efficiency and the level of automation in the sensors to boost their applicability.[Citation14]
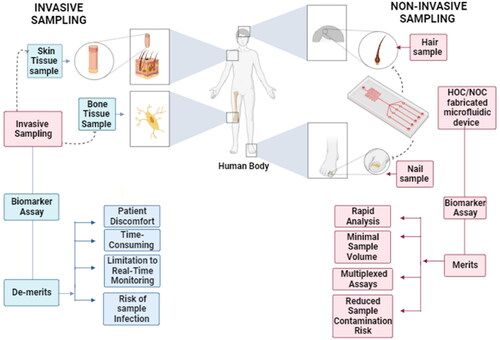
In the context of healthcare, biosensors function by detecting the presence of a biomarker in the sample collected from the patient, which is an analyte associated with the condition being studied.[Citation14–16] Biological samples, such as blood, urine, saliva, sweat, amniotic fluid and tissue biopsies have sufficed for the timely and reliable diagnosis of various disorders, owing to the abundance of disease indicators and biomarkers in them.[Citation17] However, despite their reliability and popularity, the aforementioned sampling methods often involve invasive and cumbersome collection or extraction procedures, sometimes requiring expensive equipment and mostly involving the patients to visit a diagnostic facility due to the complexity and skill required for collecting the samples. Such hindrances trigger a loss in consumer confidence, inhibiting large-scale testing and loss of patient data, as it has been well-documented regarding the swab collection procedures for the diagnosis of COVID-19 (Coronavirus Disease 2019) through RT-PCR (Reverse Transcriptase – Polymerase Chain Reaction).[Citation18,Citation19]
Thus, from the foregoing discussion, it is evident that there is an increased need for employing minimally or noninvasive sampling methods, especially for achieving point-of-care diagnoses in healthcare.[Citation20] Although the exact definition is debatable, these primarily focus on sampling procedures that are unperceived by the animal/human or are perceived by them without eliciting substantial discomfort or any kind of chronic-stress response in them.[Citation21] Such protocols offer a variety of benefits, including simplified sample collection, increased patient compliance and low risks of immune responses due to injury, minimization of sample size[Citation22] and the requirements for sophisticated equipment and skilled personnel, all of which increase the possibilities of making precision medicine a prominent success.[Citation23] Some of the most typical minimally invasive samples used for animal/human healthcare include urine, feces, saliva, sweat, hair, nails, skin and even human emanations,[Citation24] with their authenticity being well documented in the diagnosis of a variety of disorders.[Citation25–29]
However, among the ones mentioned above, hair and nails have emerged to attain remarkable acclaim for noninvasive investigations in the medical field, unlike other samples such as urine and feces that may still cause a certain level of discomfort to patients,[Citation30–32] sample contamination and delay in its analysis.[Citation33] These apparently simple structures are surrounded by a complex microenvironment, where these are associated with a variety of secretory glands and an intricate vascular system that bathe these with numerous substances reflective of the human physiology, metabolomic profile and hormonal responses to stress.[Citation34–37] Multiple investigations have successfully correlated the significance of measuring trace elements, nucleic acids, post-translational modifications and drugs to assessing various physiological conditions,[Citation38–45] even months after their levels have declined in blood.[Citation46] These have unfolded to become promising candidates in noninvasive sampling, owing to their easy and rapid collection, the lack of requiring any expensive equipment or storage facilities, facilitation of storage at room temperatures without any chances of contaminating the environment, and its utility of serving as a tool for preserving and retrieving long-term medical information about the patient.[Citation47] Therefore, from the above discussion, it can be inferred that the new era of noninvasive sampling and analyses is being and will be greatly supported for popularizing effective and simplified healthcare practices by potential alternatives, such as hair and nails that have only just began to receive the recognition that they have deserved for decades now.
Unfortunately, the large-scale implementation of noninvasive sampling gas not gained enough momentum by virtue of the inherent drawbacks possessed by the current analytical techniques. ELISA (enzyme-linked immunosorbent assay),[Citation48] GC-MS (gas chromatography-mass spectrometry),[Citation49] LC-MS (liquid chromatography-mass spectrometry) and many more, despite functioning as the industry standards, continue to incorporate complicated protocols involving large and expensive instruments, skilled technicians, multistep procedures and most notably, centralized operations that do not encourage point-of-care testing.
Parallelly, the past three decades have witnessed the rise of more modernistic fields in the vast domain of analytical sciences, such as microfluidics. Emerged in the 1980s, microfluidic technologies deal with the design and functioning of devices that enable the handling, manipulation and analysis of fluidic samples at nano/microliter scale.[Citation50,Citation51] Offering a number of advantages, such as increased levels of automation, cost-reduction, miniaturization of sample size and the device in general, integrating such tools with noninvasive sampling can tremendously aid in creation of chip-like platforms capable of decentralizing the diagnosis of a range of targeted diseases and detection of adulterants.[Citation52–55] Our research group also developed frugal paper and thread microfluidic platforms for a myriad of applications.[Citation56–66] The objective of this review shall be to elaborate some of the advancements that have taken place in the past decade to amalgamate the advantageous features of both microfluidics and noninvasive sampling in the form of hair and nails for better and more eco-friendly fabrication of chip-like devices. Such innovations are expected to dramatically simplify the analysis of the sample components in relation to healthcare, disease detection, food analysis and forensic sciences ().
Integrating Microfluidics with Hair and Nails
Bio-inspired micro/nanofluidic devices – hair and nail-based fabrication
The primary focus of this section is to appreciate some of the efforts that have been directed toward introducing substances such as hair and nails in the development of micro/nanofluidic devices for a multitude of futuristic applications (). The rationale behind such a collaboration mostly results from the fact that hair can behave as a rather simple template for the creation of microchannels similar to its width, while simultaneously imparting the same level of functionality to the resultant device as done by conventional methods.[Citation67,Citation68] Similarly, scientists have discovered the human nail plate to demonstrate nanofluidic phenomena, thereby finding their use in possible microfluidic experiments and as a bonus, offering the benefits of eco-friendliness and compatibility.[Citation69,Citation70] A more popular field deals with the development of “artificial cilia,” which have been inspired from the behavior of natural hair or cilia in various organisms in terms of sensing a variety of external cues,[Citation71,Citation72] such as air or liquid flow, performing fluid manipulation at the microscale,[Citation73] handling biological samples and many more biomedical applications.[Citation74] Finally, one of the most booming and medically promising areas of research has been to materialize the concept of “Organ-on-a-chip,” wherein the culturing of organs and tissues, including skin and human hair has been materialized on merely a single, miniaturized microfluidic chip,[Citation75,Citation76] making various studies on human physiology, disease diagnosis and drug testing possible in recent years.[Citation77,Citation78]
Table 1. Hair or nail-fabricated micro/nanofluidic devices.
Fabrication of Microdevices using Natural Hair/Nails
This section illustrates how human hair and nails have found unprecedented applications as integral components of microfluidic devices and their functioning. Employing such natural substances avoids the need to search for other materials which may increase the cost of the process, besides providing the benefits of biodegradability, ecological safety and desired functionality to the final product. The study that pioneered the use of human/cat hair for the fabrication of microfluidic channels was led by Swaminathan et al. back in 2013 ().[Citation67] The process incorporated a simplistic workflow, wherein a pre-polymer solution of poly(dimethylsiloxane) or PDMS and its curing agent was poured over hair strands of desired lengths fixed on a flat surface, followed by curing the same at 80 °C for 2 h in order to obtain the fine topology of the hair on the polymer upon hardening. The above-mentioned design thus, involved ambient temperatures without the use of any harsh chemicals. The resulting channels were able to have a width in the range of a few micrometers, besides exhibiting a variety of shapes and lengths (millimetre to meter) due to the flexible nature of hair. Finally, the viability of the designed microdevices was tested successfully by employing them in immunoassays, yeast cell analysis and creation of microwires for various futuristic application. A later study incorporated a method, wherein aqueous keratin extracted by sulfitolysis was combined with PVA (Poly-Vinyl Alcohol) and glyoxal.[Citation79] The production of NFs (Nanofibers) was achieved using the electrospinning technique, which involves an electrohydrodynamic process, during which the liquid droplet was electrified to generate a jet, followed by stretching and elongation to generate the nanofibers. Keratin being widely available and with a fibrous structure having excellent strength was advantageous to this method. The workflow involving bio-composite nanofibers has numerous potential applications in the fields of engineering and nanotechnology in the enhancement of composite properties, owing to their environmentally safe nature in addition to their exceptional antibacterial activity. Further, these NFs are feasible to be applied in the biomedical field.
Figure 2. Schematic illustration of the pioneering fabrication of a microfluidic platform from mammalian hairs and its subsequent application in microwire synthesis, immunoassays, cell sorting and manipulations. Adapted with permission from Ref. [Citation67].
![Figure 2. Schematic illustration of the pioneering fabrication of a microfluidic platform from mammalian hairs and its subsequent application in microwire synthesis, immunoassays, cell sorting and manipulations. Adapted with permission from Ref. [Citation67].](/cms/asset/3efa4561-ab14-49a3-9ba9-0d180331c638/batc_a_2291825_f0002_c.jpg)
A different body of research has shed light on developing microfluidic sensors involving human nails as a simple component. Among them, a project conducted in 2017 was based on cleverly integrating the human nail bed with the detection workflow of a microchannel-based shear force sensor skin method with a flexible liquid-filled PDMS,[Citation80] where the sensor skin was wrapped around a finger-shaped end effector and fixed at the location of the nail bed. When the skin was subjected to shear force, one side of the skin experienced tension while the other side experienced compression and bulged like a human finger pad. The resulting tension and compression forces were measured by liquid metal strain gauges, embedded in PDMS, that were strategically placed adjacent to the nail bed, away from regions of direct finger-object contact. This work interface had a dynamic range of detection over up to 5 N shear forces and inculcated a limit of detection of 0.088 N−1 of applied normal force. This microchannel-shear force method allowed the stretching of artificial skin similar to natural skin, thereby allowing accurate shear force measurements. The artificial shear force sensor skin boasted several operationally useful characteristics, such as being intrinsically flexible, conformable to curved surfaces and suitable for repeated shear force measurement without significant fatigue, and thus provided for a pioneering innovation in the field of smart noninvasive force sensors.
A more recent investigation on microfluidic fabrication devised a workflow, in which a prepared nail plate being used as a nano-porous membrane was inserted between two PDMS blocks (). This setup was further attached to micropipette tips on either side along with platinum electrodes. PBS (phosphate buffered saline) solution was then injected into the device to further hydrate the nail-plate.[Citation69] Being a nano-porous material, it was employed in the fabrication in the following manner to develop a bio-degradable nanofluidic device. The ready availability of human nail samples and the small size of the device ensured the feasibility and portability for various potential applications. The fabricated device being completely biodegradable was proven to be eco-friendly. Finally, the authors proposed such designs to be explored further in pharmaceutical applications, such as point-of-care testing (POCT) and drug development.
Figure 3. Diagrammatic depiction of the process of designing a nanofluidic device from a human nail plate, succeeded by its detailed characterization to demonstrate nanofluidic phenomena, such as Perm-selectivity. Adapted from Ref. [Citation69].
![Figure 3. Diagrammatic depiction of the process of designing a nanofluidic device from a human nail plate, succeeded by its detailed characterization to demonstrate nanofluidic phenomena, such as Perm-selectivity. Adapted from Ref. [Citation69].](/cms/asset/f2614366-e47c-4933-94bd-7346ac0e9432/batc_a_2291825_f0003_c.jpg)
A fabrication method was used in 2008 to create carbon nanotube fibers (CNF), which behaves like hair-like conductive microwires.[Citation81] Here, single-wall carbon nanotubes created by the particle-coagulation spinning (PCS) method were used to generate CNFs. It was determined that the device setup was nontoxic, supported the division and growth of mammalian cells, and allowed for the extension of brain cells.[Citation72] CNFs have since been predicted to find their application in creating various innovative industrial products, such as supercapacitors, electrochemical transducers, artificial muscles, and microwires. Additionally, such technology was proposed to be used in designing media packaging materials, micro- and nanofluidic devices, and conduits.[Citation82–86] However, despite the several beneficial aspects of this approach, concerns still persist in relation to the significant chances of acute cytotoxic effects that may be induced due to long-term exposure to the CNF-workflow.[Citation72]
In spite of the several advantages that make the aforementioned studies promising, it is to be noted however, that applying natural materials such as hair and nails in the designing of microfluidic platforms is in its early stages and there exist certain limitations in the body of work discussed. For instance, the creation of microwires with structures totally resembling those of the channels is difficult to achieve due to loss in the volume of the polymer solution during the construction.[Citation67] Further, in the study involving the aqueous keratin-based fabrication, the methodology to extract keratin proved to be insubstantial owing to the protein’s insolubility in polar solvents. Additionally, considering the fact that only a few efforts have been made toward developing nail-based microfluidic devices, it leaves plenty of room for progression. For example, the microchannel-based shear force sensor skin characterized as a 9-bit sensor shall require improvements in its sensitivity, failure analysis and the cycle strain tests to determine the robustness against repeated large strains.[Citation80] Certain critical aspects relate to those of the lifespan and the durability of such sensors, both of which are still questionable as seen in the study, wherein the nail plate being a nano-porous material was fabricated in the following manner to develop a bio-degradable nanofluidic device.[Citation69]
Artificial Cilia and Bio-inspired “Hair” in Microfluidics: Exploring the Properties of Hair
Although these are much smaller in their physical dimensions as compared to hair, the significance of cilia, which are nothing but micro-sized “hair-like” structures (1–30 μm) lining various internal surfaces in our body, is worth a discussion. These “micro-hairs” present in abundance in our body and on the surfaces of microorganisms perform natural functions such as inducing and sensing fluid flow by behaving as actuators, and sensing ion concentrations, shear forces, gravity, etc. The ability of cilia to manipulate fluid flow at a microlevel has been recognized for over two decades, which has resulted in several studies dedicated toward developing “artificial” cilia to mimic the functions of their natural counterparts in various novel microfluidic applications. Some of the most successful outcomes of this research have been to build microfluidic platforms for particle manipulation, flow sensing and preventing biofouling of surfaces, with some of the latest being focused on applying artificial cilia in in-vitro models for supporting desired biological functions.[Citation87] In this section, some of the most significant progress encompassing “artificial cilia-based lab-on-a-chip,” with special emphasis on the fabrication and actuation of these cilia for inducing fluid flow[Citation88] have been highlighted.
Some of the earliest experiments have focused on the designing of biomimetic artificial cilia by taking inspiration from the natural abilities of biological cilia to sense and manipulate fluid flow. Li et al. in 2002 proposed a robust plastic deformation magnetic assembly (PDMA) method for fabricating artificial hair-cell sensors in the form of three-dimensional microstructures that could be realized in an integrated circuit-compatible manner.[Citation89] The sensors consisted of a cantilever beam with a vertical artificial cilium attached to its free end. Upon subjecting to external/mechanical stimuli, the momentum imparted on the cilium was be transferred as a mechanical moment to the horizontal cantilever, which was in turn, sensed by doped Si (silicon)-piezoresistive material (exhibits a change in electrical resistivity due to mechanical strain) at the base of the beams. Briefly, the PDMA process involved surface-micromachining of the cantilever, beam followed by its release using sacrificial layer etching. A magnetic field was then applied on the magnetic material attached to the beam, which resulted in its plastic deformation, causing the beam to remain at right angles to the base. The aforementioned technique offered several advantages, such as efficiency in batch assembly, preventing mechanical slack and establishing a natural electrical connection by using metals as the plastic deformation material. A number of improvements were also incorporated in the process to make the device and the plastically deformed joint mechanically strong, and provide electrical insulation to the overall design.
The ability of natural hair structures to perform a plethora of sensing functions, such as mechano-transduction,[Citation90] chemoreception,[Citation91] and detection of relative gravitational and angular acceleration,[Citation92] has led to research groups continuing to build more designs inspired from natural hair to behave as miniaturized sensors. One of the more recent milestones was achieved by Devaraj et al. in 2015, when they developed an interesting method for the fabrication of a poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate), or PEDOT:PSS-based micro-hair air flow sensor, with one of its potential biomedical applications being to serve as a flow sensor in neonatal resuscitators. Briefly, the process began with constructing four gold tracks onto a glass slide, which were integrated to an electrical circuit. For manufacturing the micro-sized hairs, a fully automated process was employed, wherein a micropipette was filled with the PEDOT:PSS polymer dispersion, followed by which, it was positioned and the coordinates were fed into a computer program from a (.csv) file. The program controlled the movement of the pipette toward the gold track, and upon detecting an electrical contact, it began its retraction up at a constant speed till the fabricated micro-hairs reached a vertical height of 1000 μm. In the same way, three more hairs were constructed, each 25 μm away from a platinum wire than the preceding hair, thereby completing the process. For testing the working of the device, air flow was provided by a gas flowmeter at a user-defined rate. Simultaneously, a potential difference was applied between the platinum wire and the gold tracks. In the resting phase, there was zero current detected between the hair and the reference platinum wire in terms of a digital signal or “OFF” state. As the air flow was ramped up, the micro-hairs directly in the path of the flow started to deflect, and as soon as they made contact with the reference wire, the circuit was completed, thereby displaying an “ON” digital readout. Multiple hairs allowed for determination of the two extremes of air velocity that were detectable: minimum flow sensed by the hair closest to the wire, whereas the maximum flow sensed by the hair placed farthest from the wire as higher air velocities were required to deflect the latter. Such an arrangement allows for increasing the resolution of the device by increasing the number of micro-hairs and the distances between them. As opposed to earlier designs involving complex mechanoelectrical transduction, the authors of this study directly utilized the displacement of the actual micro-hair to measure air velocity, thus, simplifying the detection scheme. The conducting polymers used to create the hairs enabled the realization of large deflections of the same, without altering the original shape upon removal of the airflow. Moreover, a potential difference of merely 1 V was needed during the actual sensing process, thereby eliminating the need for very high-power supply. Finally, the described airflow sensor was capable of detecting air flow velocities in the range of 0.66–0.97 m/s and exhibited great reproducibility as well, driving the hairs to their fatigue limit rates after 11,476 ON-OFF cycles.[Citation74]
Over the years, modifications have been added to the fabrication methods for constructing cilia with high aspect ratio (width-to-length ratio) and low Young’s modulus (low stiffness) so as to replicate the behavior of biological cilia. However, this has often been associated with the collapse of cilia due to their low stiffness and large interfacial energy, especially when fabricated in air. With the aim of circumventing this major drawback, a research group innovated the construction of highly compliant PDMS-based cilia of a variety of dimensions and spacings using an underwater fabrication approach,[Citation73] using which, the authors were able to create an environment of less surface tension and interfacial energy as observed by comparison against fabrication performed in air. Furthermore, they achieved a resonating frequency of 65 Hz, which is in the range of that of biological cilia. Thus, this enhancement was capable of improving both the rate of success of the fabrication process and the durability of the constructed cilia.
A fabrication method for the construction of artificial cilia using a two-color lithography-based method was devised in the year 2011 (), wherein a magnetic-artificial cilia was fabricated using a silicon substrate. The substrate was further coated with a PDMAA (polydimethylacylamide) polymer having MABP (methacryloyloxybenzophenone). Herein, the cilia were first coated with a hydrophilic coating and further evenly distributed over both sides of the fabricated microfluidic channel, followed by keeping under the influence of a permanently rotating magnet. The device was fabricated such that it could incorporate cilia of different physical dimensions and it was proved that the magnetic actuation is the most effortless method to instigate the movement of cilia. The resulting device was proposed for interesting contemporary applications, such as constructing magnetic actuators in microfluidic systems.[Citation88] In a more recent paper, a new approach to the construction of magnetic artificial elastic cilia using a 2-step lithographic fabrication technique was devised. Herein, a conjunction was added in which a deposition of copper followed by magnetic nickel–iron permalloy. The workflow involved a setup of a negative photoresist (NR9 1500Py futurex) on a glass substrate, and an unfilm sputterer. In this method, there was no interference with biological systems utilized in microfluidic purposes. In conclusion, the authors recommended the future use of the described device for a wide range of applications in microfluidic pumping, mixing and other fluid handling processes, also involving biomedical assays for handling biological samples.[Citation71,Citation93]
Figure 4. Simplified demonstration of a novel two-color lithography process of constructing arrays of magnetically actuated artificial cilia with desired geometrical and mechanical properties. Adapted with permission from Ref. [Citation88].
![Figure 4. Simplified demonstration of a novel two-color lithography process of constructing arrays of magnetically actuated artificial cilia with desired geometrical and mechanical properties. Adapted with permission from Ref. [Citation88].](/cms/asset/5be69706-b424-4f42-b8a2-b8de24921114/batc_a_2291825_f0004_c.jpg)
Over the years, computational interventions have facilitated the modeling of various practical process, especially in the biomedical field.[Citation94] One such breakthrough can be attributed to Branscomb et al. in 2010,[Citation95] wherein computational modeling demonstrated that synthetic cilia could be utilized for crucial purposes such as hydrodynamic separation and filtration of biological particles in microfluidic devices. Three-dimensional computer simulations were carried out in a microfluidic channel under the influence of a pressure-driven Newtonian fluid flow. The channel floor was uniformly covered by non-motile, synthetic cilia. A neutrally-buoyant particle whose physical dimensions were approximately four times smaller than a single cilium was introduced to the channel. Using models such as the LBM (Lattice Boltzmann Model) and LSM (Lattice Spring Model), the spatial trajectory of the particle was observed along with its change due to the influence of the cilia which was further quantified by calculating the particle’s migration velocity across the channel. The conclusion of the simulation revealed that the cilia accelerated a particle’s movement and caused its rapid drift toward the ciliated channel surface. When the fluid flow was fixed at a certain velocity and caused the cilia to sway 45 degrees relative to the channel’s surface, it was found that the migration particle of the velocity was maximum. The proposed innovation can find innovative potential biomedical applications, such as in the isolation of rare tumor cells in cancer patients.[Citation96] This promises to be achieved in a high throughput manner via microchip technology.
Until the initial half of the previous decade, the fabrication of synthetic cilia was either uneconomical, requiring processes such as photolithography, or provided no physical evidence for their studies, inhibiting their implementation. This was rectified in 2013 by Wang et al. by the development of cost-effective and practical self-assembling artificial magnetic cilia.[Citation97] The set-up for this procedure was relatively simpler and utilized small, spherical self-assembling magnetic beads and a soft polymer coating of a PBA (poly(butyl acrylate)) nanoparticle latex. In a nutshell, the process involved placing magnetic beads (MB) perpendicularly to the floor of a fluid cell, followed by stimulating them with a magnetic field to impart negative charge to them. The PBA particles were provided with positive charge by synthesizing them in cetyl trimethylammonium bromide, followed by their adsorption onto the MBs through electrostatic attractions, creating elastic bonds between the beads. Magnetic actuation of the cilia was achieved by an eight-pole electromagnetic setup possessing a 20 W incandescent light bulb, which caused them to generate a fluid flow in the channel with the maximum velocity of 5 μms−1. Variation in this fluid flow was achieved over a range of magnetic fields and concentration of MBs. Additional work may be presented in the future, which would primarily focus on observing the physical properties of polymeric materials often used in microfluidic devices. Furthermore, promising experiments may be conducted to better integrate artificial cilia and microfluidic devices.
Flow sensors, although incorporating miniaturization and simplification, tend to utilize a copious amount of power and thus, do not allow them to fully realize cost reduction. In 2014, Alfadhel et al. developed a method to address this issue without compromising on efficiency. Cilia were synthesized by constructing PDMS (polydimethylsiloxane) polymer nanocomposite pillars possessing iron nanowires which ensured permanent magnetic properties and elasticity.[Citation98] Eight pillars were used in this set-up, ensuring maximum deflection of each pillar without any cross-over during the flow process. These sturdy pillars were integrated on a thin-film GMI (giant magnetoimpedance) sensor. Flow sensing was conducted for both water and air with minimal power consumption as no additional magnetic field is required. At an insignificant power consumption of merely 31.6 μW, air and water flow rates of up to 190 and 7.8 mm/s, were detected with sensitivities of 24 mΩ/mm s and 0.9 Ω/mm s, respectively.
For the triumphant utilization of artificial cilia-based microfluidic chip-like platforms in the future however, certain aspects need to be emphasized. Firstly, substantial optimization is needed in the geometry and actuation of the cilia to suit a specific purpose and medium, while simulations are often unable to capture out-of-plane directional flows, limiting their predictions on the fluid flow around the cilia.[Citation73] Secondly, the fabrication process of artificial cilium requires a significant source of illumination to operate. Additionally, this is a tedious process which is not economically viable. It also involves complex chemistry to realize the lithographic step. In the context of developing magnetic artificial cilia, specifically designed by Wang et al. the processes involved techniques such as photolithography, which is uneconomical. Furthermore, the process is labor-intensive and excessive in nature. The reproducibility of such methods is also questionable due to lack of control over the handling of the apparatus.[Citation87] Similarly, the GMI sensor initially implemented by Alfadhel et al. which is novel in the world of microfluidics, may still utilize unnecessary equipment, thereby allowing conventional magnetic devices to be more advantageous. Moreover, the apparatus necessary for this entire operation is exorbitant.[Citation98] Finally, the three-dimensional computer simulations provided by Branscomb et al. in 2010 shed an insight on the behavioral aspects of cilia, yet failed to provide any physical evidence of the aforementioned.[Citation95]
Microfluidic Chip-based Models for Regenerating Hair/Skin
Conventional techniques, such as in-vivo animal testing and in-vitro cell cultures have failed to truly replicate the natural physiological and pathological manifestations in tissues and organs, thus not favoring the in-depth analysis of human physiology and diseases.[Citation75] In recent years, numerous efforts have been directed toward growing cells, and even organs on microfluidic chips, creating the concept of “Organ-on-a-chip,” or OOAC. Developing such technology has allowed most prominently being to incorporate a perfused vasculature in the form of microfluidic channels to model fluid flow across the tissues, deliver media components to the cells and remove metabolites and detritus from the same,[Citation78] which was not possible traditionally. Such proficient designs enable various components, such as fluid shear forces, concentration gradients, dynamic mechanical stress, etc. to be included in the device, thereby making an attempt to closely mimic the natural processes in the tissue. Consequently, a number of models comprising bones, eyes, gut, spleen and many more have been developed, with skin being one of the most recent topics of research, owing to the great advancements in dermatology.[Citation76,Citation99] In particular, “Skin-on-a-chip,” or SoC models serve as reliable models for studying diseases, bacterial infections, testing of drug efficacy and toxicology.[Citation77] In this section, some of the few papers calling attention to the development of hair follicles or cilia on SoC models have been discussed, with the aim of highlighting the potential use of “Hair-on-a-chip” models as noninvasive methods to understand various complex physiological and pathophysiological phenomena.
Until the initial half of the previous decade, the majority of the commercially available skin equivalents were based on static cultures, which largely suffered from the drawbacks of having short culture periods, lack of reproducibility in-vitro, and the inability to include functional and (patho-) physiologically relevant components, such as the immune system and a well-defined vascular system. One of the primary initiatives had been taken up by Atac et al. in 2013 (), wherein they designed a multiorgan-on-a-chip (MOC)-based system of the size of a lab-sized glass plate to culture commercially available skin equivalents (SE), ex-vivo skin and follicular unit extracts (FUEs) from juvenile prepuce and scalp specimens from male patients undergoing hair transplantation, respectively.[Citation100] Their primary objective was to prolong the maintenance and testing periods of the aforementioned tissues by introducing a dynamically perfused system, wherein microfluidic pumps were used to provide a pulsatile flow of medium to the Transwell® inserts containing the respective tissues. The arrangement thus, was capable of monitoring the effect of dynamic perfusion on different skin models as compared to the traditional static culture media. Histological and imaging studies revealed that the MOC-arrangement was successful in prolonging the maintenance period of the cells in SEs for up to 9 days, with more viable and about no apoptotic cells as compared to the same cultured in static conditions. SEs cultured in combination with subcutaneous tissues (SCTs) in the dynamic conditions of the MOC yielded even higher cell viability, epidermal integrity and more robust integration with the SEs suggesting that the SCTs have a stimulating effect on the metabolism of the SEs. Finally, the intact FUEs that were also integrated to this system were documented to show increased fiber elongation from the epidermis with triple the culture period as compared to the conventional Philpott assay. Overall, the design described above demonstrated how dynamic perfusion in organ-culture mimics the native conditions closely by not only affecting the effective distances between signaling molecules and their pericellular diffusion gradients, but by also altering the extracellular gradients, cell-cell communications and concentrations of secreted ligands of the model tissues.
Figure 5. Fabrication scheme of a dynamically perfused chip-based bioreactor capable of prolonging the maintenance and testing periods of ex-vivo human skin and follicular extracts for a range of biomedical applications. Adapted with permission from Ref. [Citation100].
![Figure 5. Fabrication scheme of a dynamically perfused chip-based bioreactor capable of prolonging the maintenance and testing periods of ex-vivo human skin and follicular extracts for a range of biomedical applications. Adapted with permission from Ref. [Citation100].](/cms/asset/3cca62d0-7336-4190-9406-7207e9b52b1d/batc_a_2291825_f0005_c.jpg)
A crucial requirement for several biological and clinical studies involves isolating a cell of interest. It is rather tedious to isolate rare cells from a heterogeneous cell group when there is only a slight difference in mechanical stiffness. For example, cancer cells, owing to their property of metastasis, have a heightened mechanical stiffness which differentiates them from normal cells and makes them simple to isolate. In 2018, Sohrabi et al.[Citation101] proposed a three-dimensional simulation-based study that operates on hydrodynamic filtration in a microfluidic channel who’s floor was evenly covered by a synthetic cilia array of various patterns. This instigated an active ciliary system to control the trajectories of particles solely based on biophysical properties using their elastic filaments. The simulation incorporated the LB (Lattice Boltzmann) model, coupled with the IB (Immersed Boundary) method. The hydrodynamic interactions between the cilia and soft particles were thoroughly observed via the point-particle scheme and the SN (Spring Network) model respectively. Ciliary arrays in blocks were utilized over a range of angles and thoroughly studied to manipulate their separation efficiencies. This study proved to be useful in a multitude of applications such as single cell sequencing and rare cell isolation.
The discussion till now clearly explains how far the concept of organ-on-a-chip has advanced over the past few decades. However, to vastly tap into the hidden potential of this technology to explore other avenues, some critical aspects need to be highlighted. For example, the design by Atac et al. in 2013, though being able to prolong the culture periods of SEs and SCTs,[Citation100] observed partial loss in the number of cell nuclei, proliferating cells around the follicular bulb and in structural integrity of the FUEs due to a possible ongoing regression phase in the same. These indicate that a more concrete understanding of the native skin physiology is yet to be achieved, especially if researchers intend to model disease conditions and test diagnostic tools using less invasive samples such as hair and skin biopsy models on chip-like platforms on a larger scale and in a reproducible manner. One of the aforementioned papers by Sohrabi et al. in 2018 was mentioned to elucidate the positive implications of mathematical models for describing the hypothetical behavior of synthetic cilia for a hair-on-chip model. Despite their results matching the rationale behind cilia behavior, the model fails to provide any concrete evidence of the same as the study was purely computational in nature, which is bound to be laden with errors. Without physical proof, it is tedious to closely examine the flow velocity of various fluids in a microfluidic channel. Mathematical models are purely algorithms whereas real life experiments may conclude differing results due to external factors, therefore, the conduction of an actual experiment for validating the results of the former is essential.[Citation101]
Microfluidic Devices utilizing Hair or Nail Samples for Biomedical Application
In addition to ingeniously including hair or nails as a core component in microfluidic devices, the former have also received recognition in the form of reliable samples for various analytical applications, owing to the reality that these serve as noninvasive platforms for proteomic analysis,[Citation47] assessment of exposure to drugs of abuse[Citation102] and pollutants,[Citation44,Citation103,Citation104] metabolomic profiles,[Citation105] and more notably, in forensic investigations.[Citation106,Citation107] One of the most notable advancements however, has been to employ point-of-care (POC) devices in the form of microchip/microfluidic assays () that have documented to make such analysis not only more convenient due to lower sample volumes and rapid analysis time, miniaturization, reduction of costs and lack of requirement of complex equipment,[Citation108] but have also been able to enhance the sensitivity of such studies even in remote settings. Therefore, this section will mainly elaborate on some of the interesting developments that have occurred in the use of hair and nails as biomarkers in microfluidic setups for POC analysis ().
Figure 6. Microfluidic hair and nail-on-a-chip devices as noninvasive and reliable alternatives to conventional sampling in a variety of diagnostic applications.
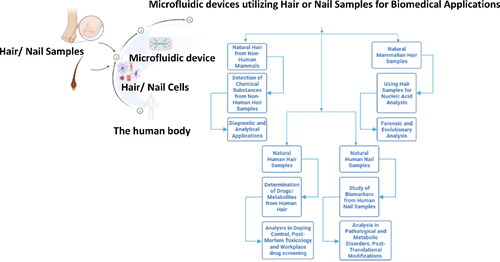
Table 2. Hair/nails as biomarkers in microfluidic assays.
In the context of the discussion that is to follow, it is important to know that despite the use of simple sampling protocols, the key to successfully conducting a bioanalytical study involves significant steps; extraction of the target analyte from the sample; purification; detection and quantification. Therefore, isolation of the specific molecule is a core component of any analysis. Traditionally, liquid-liquid phase extraction (LLPE) has been the most revered method of sample purification. However, it suffers from a lack of automation and the inability to produce clean and distinct extraction droplets. Envisioning applications that may be more suitable for lower sample volumes and chemical requirements, designing of simple devices and their handling, novel microextraction techniques have been developed in recent years. The first of the two major types focus on solid-phase microextraction (SPME), wherein various coatings have been developed over the years for extraction of compounds from complex biological matrices. The approach, despite offering the advantages of being a non-exhaustive and automated sample treatment method without requiring huge quantities of hazardous solvents, considerable future work is possible on reducing the number of steps to enhance the throughput of in-vitro assays, commercialization of newer coatings compatible for in-vivo studies to be conducted for the realization of point-of-care diagnosis.[Citation109] The second type comprises liquid-phase microextraction (LPME). Herein, the extraction of the species of interest is facilitated from aqueous samples to a minute volume of a water-immiscible solvent (sometimes referred to as the accepting phase). These systems are capable of mitigating a lot of the issues posed by traditional liquid-LLPE and SPME, such as the dependence on a commercial device and sample carry-over.[Citation110] Nonetheless, both of these rapidly growing technologies continue to empower the miniaturization and automation of modern diagnostic devices, thereby naturally reducing analysis and staff involvement, as it will be discussed heavily in the upcoming sections.
Detection of Chemical Substances from Non-Human Hair Samples
In this section of the paper, certain noteworthy accomplishments in the field of microfluidics and allied disciplines will be discussed in the context of integrating such devices with the use of natural hair from non-human mammals as a noninvasive and easily extractable sample for diagnostic and analytical applications. In the context of farm animals, β-agonists and among them, clenbuterol has been popular to be used in meat production with the aim of enhancing the growth rates of the treated animal. However, owing to its adverse impacts on the health of both animals and indirectly on that of humans, its use has been banned in many countries such as Argentina and the European Union. However, concerns regarding its illegal usage in meat production has demanded rapid and safe techniques for its detection. Hair samples from swine/bovine have been popularly employed for determining clenbuterol (CLB) concentrations due to its high affinity with melanin, thereby simplifying the analysis.[Citation111] An important development to have taken place in CLB analysis from swine hair samples has been to coalesce conventional methods with microfluidic approaches, with the objective of making the final product miniaturized and capable of rapid on-site investigation.
A ground-breaking paper by Regiart et al. in 2012 demonstrated the first integrated microfluidic immunosensor coupled to screen printed carbon electrodes (SPCE) for the quantitative detection of CLB by a competitive immunoassay. Briefly, hair samples from bovines spiked with CLB were collected and treated to obtain diluted solutions suitable for experimentation. The polymethylmethacrylate (PMMA)-based device had a microchannel machine-patterned on it using laser, onto which the sample solutions were loaded, which was followed by its flow to a detection zone. Here CLB (if any) present in the sample would compete immunologically with alkaline phosphatase (AP)-labelled CLB conjugate for anti-CLB specific antibodies that had been linked to magnetic microparticles positioned on the same zone using permanent magnets. With increased CLB present in the given sample, more AP would remain free to convert p-aminophenyl phosphate to p-aminophenol, which would be amperometrically detected on SPCEs coated with gold nanoparticles (AuNPs) at +0.1 V. The employment of SPCEs with advantages such as simplicity, versatility, small size and easy large-scale fabrication, and AuNPs, which have accelerated advancements in electrochemistry due to their high surface/volume ratio and excellent conducting abilities, facilitated to achieve a limit of detection (LoD) and a limit of quantification (LoQ) of 0.008 and 0.027 ng/mL respectively. This was an enhancement in LoD over existing spectrophotometric immunoassays due to the electrochemical component, besides yielding greatly satisfactory results when applied to nine real bovine hair samples.
Interdisciplinary science has facilitated the amalgamation of principles such as Raman Spectroscopy with immunochemistry and microfluidics for strengthening the analytical performance of such products. In 2014, a study led by Zheng et al. introduced a new SERS (Surface-enhanced Raman Spectroscopy)-immunoassay-based paper microfluidic device (μPAD) for the sensitive detection of CLB in swine hair samples.[Citation112] The SERS techniques offers the possibility of increasing the detection signals up to 104–1015 times by adsorbates on metal nanoparticles, specificity and sharpness of Raman peaks, and coupling this to paper-based microfluidics allows for cost reduction, flexibility, lightness in weight of the device and the nullification of the need to install a pump for controlling fluid flow, owing to the inherent capillary action of the three-dimensional (3D) porous paper matrix. In this experiment, the authors designed a μPAD which had a main structure in the center with a small flap (detection zone) that could be manually flipped such that in its horizontal orientation, samples could flow to that portion and cause the reaction, whereas its vertical orientation would keep the device “off.” On both the sides of the flap were two microfluidic channels; AuNP-SERS-tagged anti-CLB antibodies to travel from the left-hand side and CLB-containing sample to travel from the left-hand side all the way to the central detection zone that was coated with CLB antigen. For commencing the device operation, two paper strips were inserted into two holes on either side of the main body, so as to complete the pathway for the two solutions to flow toward the center from their respective zones. Simultaneously, the flap of the detection zone was put back in horizontal position, thereby allowing the two solutions to reach the same and result in an immune-competitive reaction, wherein higher the concentration of CLB in the sample solution, more was its interaction with the antibodies, thus allowing fewer of the latter to bind to the antigens coated on the detection zone. Consequently, after washing away unbound antibodies, the bound antigen-antibody conjugates were subjected to SERS, wherein the AuNPs linked to the antibodies produced the Raman signal peaks such that the signal intensity was inversely correlated to the quantity of CLB in the samples. With such clever design for a μPAD, the authors were able to achieve a LoD of 0.1 pg/mL, besides extending its application to real hair samples, with impressive recovery values and less errors in their results ().
Figure 7. Diagrammatic illustration of a simple, multilayered paper-based microfluidic chip with a manually controllable flap for the noninvasive sample preparation and detection of clenbuterol from swine hair samples using SERS. Adapted with permission from Ref. [Citation112].
![Figure 7. Diagrammatic illustration of a simple, multilayered paper-based microfluidic chip with a manually controllable flap for the noninvasive sample preparation and detection of clenbuterol from swine hair samples using SERS. Adapted with permission from Ref. [Citation112].](/cms/asset/0dc5f768-2ca9-4371-b489-deef42891138/batc_a_2291825_f0007_c.jpg)
Efforts have also been focused on adding the multiplexing property to such microfluidic devices, so as to enable the analysis of complex samples as well. One such research group worked toward the multiplexed detection of clenbuterol, terbutaline, and ractopamine. This updated SERS multiplexing method involved the initial process of bringing the filtration and detection regions in contact. Following that, swine hair particles were dropped in the filtration region, and the liquid diffused into the detection region. This liquid was dried by hot air flow, following which the sample loading process was repeated several times to accumulate the beta-agonists in the detection region. The detection of the same was facilitated using the gold nanoparticle (AuNP) solutions in the detection region, with the simultaneous generation of the SERS spectra to complete the analysis.[Citation113] With a varying dynamic range of 10 to 150 ppb (ng/g). The LOD were observed as, of clenbuterol, ractopamine, and salbutamol, are 20, 20, and 30 (ng/mL), respectively. This analysis stood out for the previous similar advancement as it was done without chemical labeling, purification or separation. The electrodeposition time was in a range of 10–80 s, thereby enabling significantly rapid and high-throughput fabrication.[Citation114]
Efficient techniques, such as chemiluminescence (CL) have been integrated with paper-based microfluidics for achieving high sensitivity in their results. One of the first papers to highlight this advancement aimed to provide simple and rapid quantitative measurement of β-agonists in swine hair samples.[Citation115] With a notable detection range of 4.0 × 10−8 to 1.0 × 10−5 (mol/L) and LOD at 2.0 × 10−8 (mol/L). The functioning of the μPAD was based on the phenomenon that the chemiluminescence exhibited by the system of luminol and K3[Fe(CN)6] significantly diminished in the presence of β-agonists, which was different from that in aqueous solution. This technique was favorable to develop a miniaturized instrument for on-site analysis of swine hair samples, besides being fast, convenient and cheap as required by most POCT devices.
In general, the success achieved in the discussed studies can be easily attributed to the fact that the selection of a material as easily extractable as hair enables testing of a variety of animals apart from bovine, so as to perform a range of biochemical tests on detecting drugs and identifying disease conditions. This not only supports on-site and remote testing, but eliminates complex sample isolation, purification and handling issues. Altogether, these studies demonstrate the large-scale applicability of noninvasive sampling of animals coupled with miniaturization of devices, allowing portability, accessibility and a remarkable opportunity for automation.
Using Hair Samples for Nucleic Acid Analysis
Although mammalian hair has been used as a source of DNA (deoxyribonucleic acids) for forensic and evolutionary analysis, it has still not been able to achieve the success attained by other sources such as blood and other body fluids due to a number of reasons: very low quality and quantity of DNA extractable from hair due to degradation[Citation116]; difficulty in assessing the authenticity of the results without comparing with DNA samples from blood or tissue; high chances of genotyping error, probably due to dilute samples, and the insufficiency of the quantiy of DNA to support more than one study.[Citation117] In spite of the mentioned limitations, relying on hair as a robust alternative to conventional samples does not seem to be an impossibility. First, it offers a totally noninvasive diagnostic scenario as compared to collecting genomic DNA from nucleated cells of peripheral blood, which is not only invasive and difficult to collect from subjects, but involves tedious isolation schemes and a rather long total analysis time.[Citation118] Moreover, in situations where it is difficult to obtain the animal (in case of endangered species), or in behavioral studies, where capturing the animal would disturb the system, utilizing hair samples seems to be the only feasible option.[Citation117] Hair has most notably been found suitable for extraction of substantial quantity of mitochondrial DNA (mtDNA) due to its abundance in the same, providing information on exclusive maternal inheritance for identification of individuals such as mass fatality victims, as compared to nuclear DNA, which is often in insufficiency in telogenic hair (shed hair) commonly found in crime scenes. Thus, it is apparent that in order to leverage the potential of human/non-human hair as a relevant tool for nucleic acid analysis, improvements such as lowering the sample volume, increasing the noninvasiveness of the collection, automating the sample preparation and analysis, etc. can be made enabled by introducing lab-on-a-chip (LOC) methods, wherein the aforementioned advantages can be utilized substantially.[Citation118]
One of the most active research areas since the 1990s has been the microchip capillary electrophoresis technology (MCE), which has undergone significant amounts of developments in the past two decades to increase the potential of LOC in relation to DNA analysis for forensic genetics and molecular anthropology studies.[Citation119] It is essentially a miniaturized version of capillary electrophoresis, wherein samples are loaded in nanolitres in a sample zone, followed by its electrophoretic movement toward a waste reservoir and its subsequent entry into a separation channel at the channel intersection for resolving the DNA mixture into its components.[Citation120] Among the first noteworthy studies was that led by Alonso et al. in 2006, wherein the utility of Agilent2100 bioanalyzer, a MCE-based product was validated to investigate mtDNA in several identification studies,[Citation106] such as analyzing HVR1 and HVR2 haplotype variation from blood, bone and teeth samples from recent and ancient sources. One of the highlights of this experiment was to employ MCE in differentiating between human and non-human forensic evidences (that is, of different species) by using universal and human-specific primers to amplify two genes: Cyt b and 16S rRNA (ribonucleic acid). As expected, a 157 bp (base-pair) amplicon was detected as a peak by MCE succeeding PCR, whereas negative results were obtained for the non-human telogenic hair and hair shafts. Overall, MCE was validated to function as a rapid and reliable tool for discriminating between human and non-human samples, with the electrophoretic run taking a mere total of 120 s. Having achieved remarkable results upon analysis of mtDNA using MCE, the scope of its use for DNA analysis has also been expanded toward the decoding of short tandem repeats (STRs). STR identification is crucial in forensic sciences, paternity testing and even finds applications in agricultural sciences. A step further was taken in 2014 by Lin et al. by developing an efficient method to detect mini-STRs in degraded and destroyed DNA samples.[Citation121] The approach specifically targeted mini-STRs because they improve PCR efficiency, owing to the smaller templates yielding an increased number of potential template molecules. The smaller size was generated by the use of proper primers as close as possible to the STRs region of the sample. It was found that only 0.001 ng of DNA templates were sufficient to generate fifteen desired mini-STRs from human hair upon performing MCE on the PCR products.
A rare study in 2013 focused on an innovative technique for collating the steps of DNA preparation and PCR (polymerase chain reaction) into a single-step procedure. Styrene divinylbenzene copolymer chelex, an abundant and commercially available cationic exchange resin that is corrosion resistant and stable over a wide range of temperature and pH conditions, was utilized as the solid-phase matrix and possessed the ability to capture denatured proteins, but released DNA which was further purified to be utilized as a template. A monolithic PMMA (poly(methylmethacrylate)) microdevice was fabricated on which the solid phase DNA preparation as well as PCR occurred, the amplification process of which was further enhanced by the use of pressure. Serial dilution of the samples was performed to identify the minimum and optimum requirement for PCR. The only downside of this approach, which apparently requires further enhancement, is the low band intensity obtained as compared to an off-chip strategy, which is not reliable for accurate POCT. Similarly, another refinement made recently in nucleic acid analysis is to increase its throughput. Traditional NA extraction techniques are based on a liquid-liquid phase extraction which incorporate toxic reagents such as phenol and chloroform ().[Citation107] In contrast, Nyugen et al. in 2021[Citation122] constructed a centrifugal disk capable of purifying nucleic acid (NA) utilizing a high-throughput technique to conduct the human sex-typing of forensic samples. The centrifugal disk was fabricated with bilateral etched channels to accommodate up to 30 extraction units, purifying all the samples in a single cycle. The disks were fabricated using PMMA (Polymethyl Methacrylate (PMMA)) films and were further sealed by the use of two PSA (Pressure Sensitive Adhesive) films. A washing solution of 70% ethanol and the elution buffer were introduced in hydrophobic fractioning channels that were connected via a zigzag delivery channel. The disk was operated by fabricating a portable workstation consisting of a buffer storage system, a buffer injection system, and a spinning unit to ensure POCT (Point-of care testing). The NA extraction matrix was a Whatman glass filter, which was supported by valves to prevent overflowing of buffer solutions. The process was automated and the genomic DNA extraction occurred rapidly in ten minutes and at 7000 rpm. The purified DNA was further amplified by applying a LAMP (loop-mediated isothermal amplification) which targeted the human alphoid repeat sequence of the Y-chromosome and the human 18S rRNA. The genetic sex-typing was concluded within an impressive half an hour.
Figure 8. Operational overview of a chelex-based microfabricated device for on-chip purification and amplification of DNA (deoxyribonucleic acid) from human hair in integrated flow-through polymerase chain reaction (PCR). Adapted from Ref. [Citation107].
![Figure 8. Operational overview of a chelex-based microfabricated device for on-chip purification and amplification of DNA (deoxyribonucleic acid) from human hair in integrated flow-through polymerase chain reaction (PCR). Adapted from Ref. [Citation107].](/cms/asset/88d201c8-1815-4547-9d18-4ad196c4f5c7/batc_a_2291825_f0008_c.jpg)
Genetic studies with respect to wildlife prove to be tedious and invasive in nature as they utilize samples such as saliva and feathers, which may disturb habitat of the creature. In 2017, Thaden et al. noninvasively collected hair samples from three carnivorous animals, namely grey wolves, European wildcats and brown bears. SNPs (single nucleotide polymorphisms) were the focus of this experiment, owing to the advantages that they offer over traditionally used microsatellite markers for studying evolution, besides having their calibration done across laboratories with well understood mutation codes. Herein, sample utilization was based on high quality and reliability wherein after genotype consensus, ≥70% of the samples demonstrated the required data for that particular marker. The performance of the microfluidic SNP arrays was evaluated and the results were compared with microsatellite genotyping, yielding an impressive 87%, 80%, and 97% from the wolf, cat and bear samples respectively. These were further verified with theorems such as the Bayesian clustering algorithm, supporting the robustness of the designed protocol.[Citation123]
Noninvasive sample analysis for investigating nucleic acids has just begun to percolate the diagnostic domain, but surely holds unexplored potential in the long run. Highly important experiments such as forensic studies, paternity testing and genetic profiling are gradually being materialized using hair and nail specimens. Such approaches do not involve the issues of expensive sample collection and handling procedures, besides having long shelf-lives for research. The simplicity in the nature of the samples makes them compatible with the existing analyses protocols, facilitating the study of multiple samples simultaneously on a microfluidic chip. This naturally expedites diagnostic tests and opens the doors for industrial utilization. In the future,[Citation124] more prototypes integrating novel techniques, such as loop-mediated isothermal amplification (LAMP) with microfluidic chip technology must be developed to nullify the need for temperature cycling in PCRs and reduce the nucleic acid extraction to a single step, coupled with the merits of miniaturization, construction of multiple reaction wells for multiplexing, and pre-establishing the reaction components on the channels for simplifying the detection process. Moreover, the robustness of MCE needs to be increased for applications like STR (short-tandem repeats) profiling by enhancing its resolution to a single-base level. Efforts also need to be put into making the simultaneous detection of various markers possible by introducing multiple detection channels, so as to reduce the overall expenses in the process.
Determination of Drugs/Metabolites from Human Hair
It is a known fact that drug abuse has been a global problem for many decades now, thereby making it extremely crucial for the collection and assessment of controlled substances from human samples reliable for drug law enforcement, doping control, postmortem toxicology and workplace drug screening.[Citation125] Conventionally, body fluids such as blood, sweat, saliva or urine have been the most preferred sources of sample collection. However, these have mostly been associated with invasive and uncomfortable sample collection from patients/users, besides being highly susceptible to adulteration. Moreover, the analysis of drugs from such specimens is reliable only for a period of several days. In contrast, the use of hair has added a noninvasiveness approach, with the ease of storing the samples at room temperature after collection.[Citation102] Drugs or their metabolites can be tested in hair within weeks to months after their ingestion, adding a larger window period for analysis.
It is to be noted that however, using solid hair involves complications in sample preparation as opposed to blood and urine, which are liquid in nature. Methods exist for extracting analytes of interest from hair, but often either include long durations for extraction, or result in the hair components being extensively hydrolyzed and additional clean-up procedures. Furthermore, the concentration of drugs found in hair is substantially smaller as compared to blood or urine, thereby requiring sensitivity in detection approaches such as liquid-chromatography-tandem mass spectrometry (LC/MS/MS), gas chromatography-mass spectrometry (GC/MS), and enzyme-linked immunosorbent assay (ELISA). However, considering how as opposed to the other two methods, ELISA has been able to reduce its requirements in terms of labor space, gas supply, vacuum source, etc., endeavors have been made to integrate the same with microchip technologies to create microchip-based ELISA, an innovation that supports miniaturization of the device, minimization of sample volumes, reagents and operation time, while still maintaining high sensitivity and quantitative efficiency.
With an objective to push the boundaries of such technologies and overcome the existing drawbacks related to hair analysis, one of the earliest studies was launched by Miyaguchi et al. in 2008.[Citation125] Herein, micro-pulverized extraction (MPE) was utilized as a new method for the extraction of methamphetamine (MA), a drug heavily abused in countries such as Japan, United States and many Asia-Pacific countries, and an associated metabolite amphetamine from human hair. Concisely, the process involved weighing 1 mg of a washed hair sample spiked with various quantities of MA, followed by placing it in a stainless-steel bullet filled with phosphate buffered saline (PBS), which was then subjected to vigorous shaking for 5 min at 1500 strokes/minute in an automatic pulverizer. The suspension was then filtered using a centrifugal filter unit, making it ready for analysis using micro-ELISA. For the detection of MA and amphetamine, microchannels of pre-determined length and width were filled automatically with polystyrene beads coated with anti-MA antibodies. This was succeeded by adding MA-HRP (horseradish peroxidase) conjugates to the channel in order to facilitate an interaction between MA and its corresponding antibodies on the beads. Finally, a substrate for HRP was introduced to the microchannel, which led to the formation of a blue dye that was eventually detected by an on-chip thermal lens detection device. The described protocol enabled sample preparation and drug quantitation with merely 30 min in a contamination-free environment without the need for expensive and sophisticated laboratory equipment. Moreover, the authors managed to expand the dynamic range of the device by using two calibration curves, one for the neat extracts (0.5–2.0 ng of MA/mg of hair) and one for the diluted extracts (5.0–250 ng of MA/mg of hair), which yielded an impressive LoD of 0.2 ng/mg for MA. By providing sufficient extraction time in PBS and having antibody-bead conjugates prepared in advance, the overall technique was capable of achieving high extraction efficiency and proper equalization of the number of antibodies for each assay. To conclude, the robustness of the method was validated by including real hair samples in the study, wherein they observed much lesser cross-reaction by other MA-analogues as compared to traditional ELISA and an established LC/MS/MS approach.
To explore further advancements using the human scalp hair samples a study in 2012 was carried out with the motive of constructing a microfluidic chip-based nano-HPLC (High pressure liquid chromatography) chip,[Citation102] which was used to integrated with a triple quadrupole mass spectrometer. The chip had a rapid and sensitive testing, with minimal sample quantity and preparation time required was 15 min. The device being miniaturized was highly compact and portable, demonstrating a LOD of 0.1 to 0.75 pg/mg. One of the highlights in chip-based innovations came in 2021, wherein Xu et al. patented the fabrication of a microfluidic chip which utilized hair samples for the Raman spectroscopy detection of amphetamine chloride.[Citation126] Raman Spectroscopy is a chemical analysis based on the interaction of light with the innate chemical bonds of a substance and thoroughly examines a material’s properties in a nondestructive and noninvasive manner. The microfluidic chip consisted of a substrate of a gold colloidal solution, a shaft for a damping solution and a waste liquid pool for filling the stop buffer. Two Raman detection regions were present for the sample reaction with the first detection liquid pool containing an immunity magnetic antibody, and the second detection liquid pool containing the hair extract liquid to be analyzed. The waste liquid pool and the detection regions were interconnected via a four-way channel. Furthermore, the posterior side of the detection zone had an attached permanent magnet. Another valve was present throughout the chip to interconnect the various zones of the apparatus. The device was capable of analyzing minute quantities of the drug, which was ideal as hair only contains a negligible amount of amphetamine chloride.
Microchip-based technologies have paved a way for making analysis of drugs and other important compounds from hair a success. A few aspects require attention to improvise the future diagnostic models, such as the need for automating the treatment and washing of hair samples after every run,[Citation125] the consistent maintenance of low rates for the functioning of the microfluidic chip,[Citation102] and the involvement of complex interconnected channels and valves when integrating with high-end techniques, such as Raman spectroscopy.[Citation126]
Study of Biomarkers from Human Nail Samples
Nails have a long history of use in the field of analytical science and medicine as a reliable source of biomarkers indicative of pathological and metabolic disorders, post-translational modifications and much more. They incorporate and are influenced by various molecules circulating in the system and thus, usually have biologically significant levels of such markers in them as compared to blood and other body fluids.[Citation46] There has been a considerably vast body of literature highlighting the relevance of using nails for detecting trace elements,[Citation127] long-term exposures to drugs of abuse and pollutants,[Citation128] and even stress-related biomarkers[Citation129] in the recent past. In addition to being remarkably useful samples for medical analysis, nails offer the benefits of easy and clean collection, and stability as opposed to other biological fluids, which may undergo decomposition, producing false results in the long run.[Citation130]
In particular, nails support the detection of biomolecules such as proteins and polyamines,[Citation130] which form the heart of proteomics to decode important events such as protein-protein interactions, protein expression, protein functions and modifications,[Citation131] etc. Until now, the most successful development in large-scale characterization of proteins has been through mass spectrometry (MS). Despite its high throughput and efficient analysis, MS immanently includes a tedious sample preparation process consisting of preconcentration and digestion, thereby limiting its reach to POCT.
Only recently have studies been reported that demonstrate the integration of microchip technology with MS with the aim of diminishing the size of the instrument, analysis time, cost, manpower and sample volume required.[Citation132] The most notable innovations have emerged in the form of electron spray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI), which function as effective interfaces between microchip-based tools and MS for proteomic analysis.[Citation133–135]
As early as in 2007, one of the first studies of the creation of a polymer microfluidic device combined with nano-spray ionization (nano-ESI) was reported for the identification of proteins in biological specimens, namely rice, lentil and human nails. The rationale behind this fabrication was the fact that nano-ESI approaches when introduced to microfluidic devices allow for lowering the sample size and increasing the time available for analysis, owing to the low flow rates achievable in the microchannels.[Citation136] A clever design was employed in this experiment, wherein the microfluidic-HPLC chip was fused with the nano-electrospray tip so as to perform the sample preconcentration, separation and electro-spraying on a single miniaturized platform. This was pursued by performing MS for obtaining the spectra of various peptide fragments and protein identification using Swiss-Prot database. The identification of four types of keratin, vicilin, provicilin and legumin A was empowered by the all-inclusive approach and at very low abundancies by the search engines, merely utilizing 24 ng of the nail sample. Similar results were obtained from the two other types of samples, suggesting strongly that the devised method was capable of decoding the proteome of biologically complex specimens.[Citation137] A similar integration of a nano-flow chip LC and Q-TOF-MS/MS (quadrupole time-of-flight tandem mass spectrometry) was performed to determine the concentrations of nine different polyamines of the sample in a 2010 study.[Citation130] The nano-flow chip yielded perfect separation of compounds and the Q-TOF-MS/MS ensures accuracy and precision. The standard solutions of the nine polyamines were diluted in the range of 0.1 nmol/L–1.0 nmol/L. The limit of detection (LOD) was further determined at that concentration with a signal to noise ratio of three (S/N = 3). The LOD was defined as the calculated concentration at a signal-to-noise ratio of three (S/N = 3), determined from the chromatogram. Such studies have shifted toward utilizing nails as noninvasive samples, owing to their clean nature and simplistic sampling process. Additionally, the stability of drugs inside nails remains uncompromised for longer durations than traditional samples like urine, where fluctuations in composition and a concern regarding hygiene affects the sample collection to begin with.
The advancements in the field of microchip/microfluidic-based innovations clearly indicate that its progress on using nails for extracting information on human diseases and metabolic profiles is still in its infancy, thereby inviting more attention for future work for not only enhancing the performance of the existing products, but also for refining the overall process even further. For example, the microchip developed in combination with the nano-ESI feature, although having integrated most of the main steps into a single device without much human intervention, still included a rather laborious and time-consuming sample preparation process of isolation, reduction, alkylation and proteolytic digestion.[Citation137] Such steps hinder the design from achieving POCT action, especially in resource-limited settings. Regardless of the existing limitations, it is safe to comment that nails can behave as surprising alternatives to conventional samples even in scenarios where complex mixtures of biomarkers need to be analyzed, while still maintaining the simplicity in specimen isolation and the integration of multiple steps on a single micro/nanofluidic platform containing the nail, or even a fragment of it to strengthen further miniaturization.
Conclusion and Future Outlook
In conclusion, the review highlights the emerging alliance between microfluidics and noninvasive sampling using hair and nails for introducing innovative lab-on-a-chip platforms, where the entire process, beginning from sample collection to the final analysis can be performed in a point-of-care fashion. A growing body of literature highlighted the idea of utilizing these naturally available materials in the creation of micro/nanofluidic channels and bioinspired artificial ciliary structures that possess a remarkable capacity for serving as flow sensors, immunoassay and cell analysis platforms, micropumps and filtration membranes for biomedical applications. These strategies demonstrate numerous attributes desirable in next-generation chip platforms, such as simple and ambient fabrication procedures, nontoxic and ecofriendly nature due to their construction from endogenously available materials like human nails and hair. Moreover, the artificial ciliary designs interestingly require reduced time and cost for synthesis, besides being stable enough for easy control of their movement in flow sensing purposes. Additionally, a spectrum of biomarkers has been explored using this technology, such as metabolites, harmful chemicals and illegally abused drugs; clenbuterol, β-agonists, methamphetamine and amphetamine chloride from human and swine hair samples, and nucleic acid analysis from human hair shafts and follicles for forensic and evolutionary studies. Similar applications were satisfied using human nails, which facilitated the investigation of complex protein mixtures by integrating with sophisticated microfluidic techniques such as nano-liquid chromatography chips. Overall, combining microfluidic systems with techniques, such as microchip electrophoresis, SERS, liquid chromatography allowed a dramatic reduction in the size of the device, sample quantity and unwanted steps in its purification and separation, thereby materializing on-site and sometimes, label-free detection of analytes of interest with rapidity and high sensitivity.
Despite the aforementioned suitable characteristics of the discussed strategies, certain aspects are yet to be adequately addressed for unleashing the full potential of the same. For instance, the creation and operation of certain types of artificial cilia (including magnetic cilia) still suffer from complexities in their fabrication and assembly of their electronic connections, in addition to the issues related to reproducibility. Parallelly, in relation to some of the chip-based diagnostic products reviewed in the previous sections, there exist problems related to complex sample preparation and washing steps as seen in the immunoassays and PCR-based microfluidic tools,[Citation107,Citation121,Citation124] which still hinder their applicability in bedside testing. Moreover, innovative approaches need to be developed in order for the designed chip-based device to report the desired levels of sensitivity and analysis time without actually integrating with expensive techniques such as SERS, capillary electrophoresis and gas chromatography. Nevertheless, this leaves tremendous scope for enhancing the current technology during the current decade (). With further progress in automation and printing methods, it will be exciting to witness the application of these methods in creating in-vitro models[Citation139] for drug screening and disease modeling studies, smart materials such as microneedles[Citation140,Citation141] that can be integrated in hairbands and nail polishes for performing transfollicular (using connections of hairs to the pilosebaceous glands) and transungual delivery (through nails) of drugs and therapeutic compounds.[Citation142–144] Finally, these can serve as alternative approaches for measuring pH, temperature, metabolism and pulse rate as part of dynamic health monitoring.[Citation145–147]
It is encouraging to highlight that there is plenty of opportunity to pursue research in this field, considering how the existing literature promises to focus more on nullifying the current loopholes in this technology and owing to the sheer range of futuristic ideas that can be incorporated in the same, potentially population barriers in disease diagnosis and treatment, pollution monitoring and abatement strategies, and food quality control at an unprecedented scale, all within the next couple of decades.[Citation148]
Acknowledgments
NKM acknowledge the financial support from the Vision Group on Science and Technology, Government of Karnataka, under SMYSR and RGS/F Scheme (Sanction Letter no.: KSTePS/VGST/SMYSR-2016–17/GRD-595/2017–18, KSTePS/VGSTRGS/F/GRD No.711/2017–18). NKM acknowledges the financial support received from the Science and Engineering Research Board (SERB), Department of Science and Technology, Govt of India, under Core Research Grant (CRG) Scheme (File number CRG/2020/003060).
Disclosure statement of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
No data was used for the research described in the article.
References
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. DOI: 10.1042/EBC20150001.
- Mehrotra, P. Biosensors and Their Applications – A Review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. DOI: 10.1016/j.jobcr.2015.12.002.
- Vigneshvar, S.; Sudhakumari, C. C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications – An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. DOI: 10.3389/fbioe.2016.00011.
- Holzinger, M.; Goff, A. L.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 63. DOI: 10.3389/fchem.2014.00063.
- Kim, J.; Campbell, A. S.; de Ávila, B. E. F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. DOI: 10.1038/s41587-019-0045-y.
- Yang, A.; Yan, F. Flexible Electrochemical Biosensors for Health Monitoring. ACS Appl. Electron. Mater. 2021, 3, 53–67. DOI: 10.1021/acsaelm.0c00534.
- Haleem, A.; Javaid, M.; Singh, R. P.; Suman, R.; Rab, S. Biosensors Applications in Medical Field: A Brief Review. Sensors Int. 2021, 2, 100100. DOI: 10.1016/j.sintl.2021.100100.
- Nagraik, R.; Sharma, A.; Kumar, D.; Mukherjee, S.; Sen, F.; Kumar, A. P. Amalgamation of Biosensors and Nanotechnology in Disease Diagnosis: Mini-Review. Sensors Int. 2021, 2, 100089. DOI: 10.1016/j.sintl.2021.100089.
- Tothill, I. E. Biosensors for Cancer Markers Diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. DOI: 10.1016/j.semcdb.2009.01.015.
- Thakur, M. S.; Ragavan, K. V. Biosensors in Food Processing. J. Food Sci. Technol. 2013, 50, 625–641. DOI: 10.1007/s13197-012-0783-z.
- Ge, L.; Liu, Q.; Hao, N.; Kun, W. Recent Developments of Photoelectrochemical Biosensors for Food Analysis. J. Mater. Chem. B 2019, 7, 7283–7300. DOI: 10.1039/c9tb01644a.
- Yáñez-Sedeño, P.; Agüí, L.; Pingarrón, J. M. Biosensors in Forensic Analysis. In Forensic Science: A Multidisciplinary Approach; Katz, E., Halámek J., Eds.; Wiley-VCH: Germay, 2016, 215–262. DOI: 10.1002/9783527693535.ch11.
- Selli, G. I.; Bonatto, A. E. T.; Bonatto, F. T.; Anzanello, M. J.; Bergmann, C. P. Nanosensors in Forensic Sciences. Eng. Mater. 2022, 15, 239–253.
- Raji, H.; Tayyab, M.; Sui, J.; Mahmoodi, S. R.; Javanmard, M. Biosensors and Machine Learning for Enhanced Detection, Stratification, and Classification of Cells: A Review. Biomed. Microdevices 2022, 24, 26. DOI: 10.1007/s10544-022-00627-x.
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent Developments in Biosensors for Healthcare and Biomedical Applications: A Review. Meas. J. Int. Meas. Confed. 2021, 167, 108293. DOI: 10.1016/j.measurement.2020.108293.
- Brekke, I. J.; Puntervoll, L. H.; Pedersen, P. B.; Kellett, J.; Brabrand, M. The Value of Vital Sign Trends in Predicting and Monitoring Clinical Deterioration: A Systematic Review. PLoS One. 2019, 14, e0210875. DOI: 10.1371/journal.pone.0210875.
- Zhao, M.; Yang, Y.; Guo, Z.; Shao, C.; Sun, H.; Zhang, Y.; Sun, Y.; Liu, Y.; Song, Y.; Zhang, L.; et al. A Comparative Proteomics Analysis of Five Body Fluids: Plasma, Urine, Cerebrospinal Fluid, Amniotic Fluid, and Saliva. Proteomics Clin. Appl. 2018, 12, 1–37.
- Marra, P.; Colacurcio, V.; Bisogno, A.; de Luca, P.; Calvanese, M.; Petrosino, M.; De Bonis, E.; Troisi, D.; Cassandro, C.; Cavaliere, M.; et al. Evaluation of Discomfort in Nasopharyngeal Swab Specimen Collection for SARS-CoV-2 Diagnosis. Clin. Ter. 2021, 172, 448–452.
- Pondaven-Letourmy, S.; Alvin, F.; Boumghit, Y.; Simon, F. How to Perform a Nasopharyngeal Swab in Adults and Children in the COVID-19 Era. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 325–327. DOI: 10.1016/j.anorl.2020.06.001.
- Bora, M.; Manu, M.; Mathew, D. D.; Das, H.; Bora, D. P.; Barman, N. N. Point of Care Diagnostics and Non-Invasive Sampling Strategy: A Review on Major Advances in Veterinary Diagnostics. Acta Vet. Brno 2022, 91, 17–34. DOI: 10.2754/avb202291010017.
- Pauli, J. N.; Whiteman, J. P.; Riley, M. D.; Middleton, A. D. Defining Noninvasive Approaches for Sampling of Vertebrates: Diversity. Conserv. Biol. 2010, 24, 349–352. DOI: 10.1111/j.1523-1739.2009.01298.x.
- Schilling, A. K.; Mazzamuto, M. V.; Romeo, C. A Review of Non-Invasive Sampling in Wildlife Disease and Health Research: What’s New? Animals 2022, 12, 1719. DOI: 10.3390/ani12131719.
- Baumann, R.; Untersmayr, E.; Zissler, U. M.; Eyerich, S.; Adcock, I. M.; Brockow, K.; Biedermann, T.; Ollert, M.; Chaker, A. M.; Pfaar, O.; et al. Noninvasive and minimally invasive techniques for the diagnosis and management of allergic diseases. Allergy 2021, 76, 1010–1023.
- Kataoka, H.; Saito, K.; Kato, H.; Masuda, K. Noninvasive Analysis of Volatile Biomarkers in Human Emanations for Health and Early Disease Diagnosis. Bioanalysis 2013, 5, 1443–1459. DOI: 10.4155/bio.13.85.
- Luan, H.; Liu, L.-F.; Tang, Z.; Zhang, M.; Chua, K.-K.; Song, J.-X.; Mok, V. C. T.; Li, M.; Cai, Z. Comprehensive Urinary Metabolomic Profiling and Identification of Potential Noninvasive Marker for Idiopathic Parkinson’s Disease. Sci. Rep. 2015, 5, 13888. DOI: 10.1038/srep13888.
- Taslimi, Y.; Sadeghipour, P.; Habibzadeh, S.; Mashayekhi, V.; Mortazavi, H.; Müller, I.; Lane, M. E.; Kropf, P.; Rafati, S. A Novel Non-Invasive Diagnostic Sampling Technique for Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2017, 11, e0005750. DOI: 10.1371/journal.pntd.0005750.
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-Invasive Early Detection of Cancer Four Years before Conventional Diagnosis Using a Blood Test. Nat. Commun. 2020, 11, 3475. DOI: 10.1038/s41467-020-17316-z.
- Pasomsub, E.; Watcharananan, S. P.; Boonyawat, K.; Janchompoo, P.; Wongtabtim, G.; Suksuwan, W.; Sungkanuparph, S.; Phuphuakrat, A. Saliva Sample as a Non-Invasive Specimen for the Diagnosis of Coronavirus Disease 2019: A Cross-Sectional Study. Clin. Microbiol. Infect. 2021, 27, 285.e1–285.e4. DOI: 10.1016/j.cmi.2020.05.001.
- Jankovskaja, S.; Morin, M.; Gustafsson, A.; Anderson, C. D.; Lehoczki, B.; Engblom, J.; Björklund, S.; Rezeli, M.; Marko-Varga, G.; Ruzgas, T. Non-Invasive, Topical Sampling of Potential, Low-Molecular Weight, Skin Cancer Biomarkers: A Study on Healthy Volunteers. Anal. Chem. 2022, 94, 5856–5865. DOI: 10.1021/acs.analchem.1c05470.
- Hol, L.; de Jonge, V.; van Leerdam, M. E.; van Ballegooijen, M.; Looman, C. W. N.; van Vuuren, A. J.; Reijerink, J. C. I. Y.; Habbema, J. D. F.; Essink-Bot, M. L.; Kuipers, E. J. Screening for Colorectal Cancer: Comparison of Perceived Test Burden of Guaiac-Based Faecal Occult Blood Test, Faecal Immunochemical Test and Flexible Sigmoidoscopy. Eur. J. Cancer 2010, 46, 2059–2066. DOI: 10.1016/j.ejca.2010.03.022.
- Holm, A.; Aabenhus, R. Urine Sampling Techniques in Symptomatic Primary-Care Patients: A Diagnostic Accuracy Review. BMC Fam. Pract. 2016, 17, 72. DOI: 10.1186/s12875-016-0465-4.
- Herreros Fernández, M. L.; González Merino, N.; Tagarro García, A.; Pérez Seoane, B.; de la Serna Martínez, M.; Contreras Abad, M. T.; García-Pose, A. A New Technique for Fast and Safe Collection of Urine in Newborns. Arch. Dis. Child. 2013, 98, 27–29. DOI: 10.1136/archdischild-2012-301872.
- Young, G. P.; Sinatra, M. A.; St John, D. J. Influence of Delay in Stool Sampling on Fecal Occult Blood Test Sensitivity. Clin. Chem. 1996, 42, 1107–1108. 10.1093/clinchem/42.7.1107.
- Harkey, M. R. Anatomy and Physiology of Hair. Forensic Sci. Int. 1993, 63, 9–18. DOI: 10.1016/0379-0738(93)90255-9.
- Mitruka, M.; Gore, C. R.; Kumar, A.; Sarode, S. C. Undetectable Free Aromatic Amino Acids in Nails of Breast Carcinoma : Biomarker Discovery by a Novel Metabolite Purification VTGE System. Front. Oncol. 2020, 10, 1–11.
- Mitruka, M.; Gore, C. R.; Kumar, A.; Sarode, S. C.; Sharma, N. K. Novel Approach Reveals Lipid Metabolite Reduction in Nails of Breast Cancer Patients as Potential Biomarker. medRxiv Internet]. 2020 Jan 1; 2020.04.14.20064675. http://medrxiv.org/content/early/2020/04/17/2020.04.14.20064675.abstract.
- Tegethoff, M.; Raul, J.; Jamey, C.; Ben, M.; Ludes, B.; Meinlschmidt, G. Dehydroepiandrosterone in Nails of Infants : A Potential Biomarker of Intrauterine Responses to Maternal Stress. Biol. Psychol. 2011, 87, 414–420. DOI: 10.1016/j.biopsycho.2011.05.007.
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L. F. The Human Hair : From Anatomy to Physiology. Int. J. Dermatol. 2014, 53, 331–341. DOI: 10.1111/ijd.12362.
- Henry, P.; Henry, A.; Russello, M. A. A Noninvasive Hair Sampling Technique to Obtain High Quality DNA from Elusive Small Mammals. J. Vis. Exp. 2011, 49, e2791.
- Savas, M.; Wester, V. L.; de Rijke, Y. B.; Rubinstein, G.; Zopp, S.; Dorst, K.; van den Berg, S. A. A.; Beuschlein, F.; Feelders, R. A.; Reincke, M.; et al. Hair Glucocorticoids as a Biomarker for Endogenous Cushing’s Syndrome: Validation in Two Independent Cohorts. Neuroendocrinology 2019, 109, 171–178. DOI: 10.1159/000498886.
- Miteva, M.; Tosti, A. Dermatoscopy of Hair Shaft Disorders. J. Am. Acad. Dermatol. 2013, 68, 473–481. DOI: 10.1016/j.jaad.2012.06.041.
- Itin, P. H.; Fistarol, K. Hair Shaft Abnormalities—Clues to Diagnosis and Treatment. Dermatology 2005, 211, 63–71. DOI: 10.1159/000085582.
- Poon, S.; Gareri, J.; Walasek, P.; Koren, G. Norcocaine in Human Hair as a Biomarker of Heavy Cocaine Use in a High Risk Population. Forensic Sci. Int. 2014, 241, 150–154. DOI: 10.1016/j.forsciint.2014.05.019.
- Liu, L.; He, K.; Hites, R. A.; Salamova, A. Hair and Nails as Noninvasive Biomarkers of Human Exposure to Brominated and Organophosphate Flame Retardants. Environ. Sci. Technol. 2016, 50, 3065–3073.
- Eastman, R. R.; Jursa, T. P.; Benedetti, C.; Lucchini, R. G.; Smith, D. R. Hair as a Biomarker of Environmental Manganese Exposure. Environ. Sci. Technol. 2013, 47, 1629–1637.
- Jaramillo Ortiz, S.; Howsam, M.; van Aken, E. H.; Delanghe, J. R.; Boulanger, E.; Tessier, F. J. Biomarkers of Disease in Human Nails: A Comprehensive Review. Crit. Rev. Clin. Lab. Sci. 2022, 59, 125–141. DOI: 10.1080/10408363.2021.1991882.
- Adeola, H. A.; Wyk, J. C.; Van, Adeola, H. A.; Wyk, J. C.; Van. Khumalo, N. P. Hair as a Testing Substrate in the Era of Precision Medicine: Potential Role of ‘Omics-Based Approaches’. In Keratin; Blumenberg M. Ed.; IntechOpen, 2018, 107–126.
- Hornbeck, P. Enzyme-Linked Immunosorbent Assays. Curr. Protoc. Immunol. 1992, 1, 2.1.1–2.1.22.
- Winnike, J. H.; Wei, X.; Knagge, K. J.; Colman, S. D.; Gregory, S. G.; Zhang, X. Comparison of GC-MS and GC × GC-MS in the Analysis of Human Serum Samples for Biomarker Discovery. J. Proteome Res. 2015, 14, 1810–1817. DOI: 10.1021/pr5011923.
- Nge, P. N.; Rogers, C. I.; Woolley, A. T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583.
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1–30. DOI: 10.1002/smtd.201900451.
- Ray, R.; Goyal, A.; Prabhu, A.; Parekkh, S.; Maddasani, S.; Mani, N. K. Paper-Based Dots and Smartphone for Detecting Counterfeit Country Eggs. Food Chem. 2023, 403, 134484. DOI: 10.1016/j.foodchem.2022.134484.
- Ray, R.; Prabhu, A.; Prasad, D.; Garlapati, V. K.; Aminabhavi, T. M.; Mani, N. K.; Simal-Gandara, J. Paper-Based Microfluidic Devices for Food Adulterants : Cost-Effective Technological Monitoring Systems. Food Chem. 2022, 390, 133173. DOI: 10.1016/j.foodchem.2022.133173.
- Sanjay, S. T.; Fu, G.; Dou, M.; Xu, F.; Liu, R.; Qi, H.; Li, X. Biomarker Detection for Disease Diagnosis Using Cost-Effective Microfluidic Platforms. Analyst 2015, 140, 7062–7081. DOI: 10.1039/C5AN00780A.
- Jung, W.; Han, J.; Choi, J. W.; Ahn, C. H. Point-of-Care Testing (POCT) Diagnostic Systems Using Microfluidic Lab-on-a-Chip Technologies. Microelectron. Eng. 2015, 132, 46–57. DOI: 10.1016/j.mee.2014.09.024.
- Kelkar, N.; Prabhu, A.; Prabhu, A.; Giri Nandagopal, M. S.; Mani, N. K. Sensing of Body Fluid Hormones Using Paper-Based Analytical Devices. Microchem. J. 2022, 174, 107069. DOI: 10.1016/j.microc.2021.107069.
- Ray, R.; Noronha, C.; Prabhu, A.; Mani, N. K. Latex-Based Paper Devices with Super Solvent Resistance for on-the-Spot Detection of Metanil Yellow in Food Samples. Food Anal. Methods 2022, 15, 2664–2674. DOI: 10.1007/s12161-022-02322-2.
- Singhal, H. R.; Prabhu, A.; Giri Nandagopal, M. S.; Dheivasigamani, T.; Mani, N. K. One-Dollar Microfluidic Paper-Based Analytical Devices: Do-It-Yourself Approaches. Microchem. J. 2021, 165, 106126. DOI: 10.1016/j.microc.2021.106126.
- Prabhu, A.; Giri Nandagopal, M. S.; Peralam Yegneswaran, P.; Singhal, H. R.; Mani, N. K. Inkjet Printing of Paraffin on Paper Allows Low-Cost Point-of-Care Diagnostics for Pathogenic Fungi. Cellulose 2020, 27, 7691–7701. DOI: 10.1007/s10570-020-03314-3.
- Prabhu, A.; Nandagopal, M. S. G.; Peralam Yegneswaran, P.; Prabhu, V.; Verma, U.; Mani, N. K. Thread Integrated Smart-Phone Imaging Facilitates Early Turning Point Colorimetric Assay for Microbes. RSC Adv. 2020, 10, 26853–26861. DOI: 10.1039/d0ra05190j.
- Prabhu, A.; Singhal, H.; Giri Nandagopal, M. S.; Kulal, R.; Peralam Yegneswaran, P.; Mani, N. K. Knitting Thread Devices: Detecting Candida albicans Using Napkins and Tampons. ACS Omega. 2021, 6, 12667–12675. DOI: 10.1021/acsomega.1c00806.
- Hasandka, A.; Prabhu, A.; Prabhu, A.; Singhal, H. R.; Nandagopal M S, G.; Shenoy, R.; Mani, N. K. “Scratch It out”: Carbon Copy Based Paper Devices for Microbial Assays and Liver Disease Diagnosis. Anal. Methods 2021, 13, 3172–3180. DOI: 10.1039/d1ay00764e.
- Sudarsan, S.; Prabhu, A.; Prasad, D.; Mani, N. K. DNA Compaction Enhances the Sensitivity of Fluorescence-Based Nucleic Acid Assays: A Game Changer in Point of Care Sensors? Analyst 2023, 148, 2295–2307. DOI: 10.1039/d3an00102d.
- Sudarsan, S.; Shetty, P.; Chinnappan, R.; Mani, N. K. Tuning Hydrophobicity of Paper Substrates for Effective Colorimetric Detection of Glucose and Nucleic Acids. Anal. Bioanal. Chem. 2023, 415, 6449–6460. DOI: 10.1007/s00216-023-04921-2.
- Mani, N. K.; Prabhu, A.; Biswas, S. K.; Chakraborty, S. Fabricating Paper Based Devices Using Correction Pens. Sci. Rep. 2019, 9, 1752. DOI: 10.1038/s41598-018-38308-6.
- Mani, N. K.; Das, S. S.; Dawn, S.; Chakraborty, S. Electro-Kinetically Driven Route for Highly Sensitive Blood Pathology on a Paper-Based Device. Electrophoresis 2020, 41, 615–620. DOI: 10.1002/elps.201900356.
- Swaminathan, S.; Harris, T.; McClellan, D.; Cui, Y. Bio-Inspired Mammalian Hair-Fabricated Microfluidics. Mater. Lett. 2013, 106, 208–212. DOI: 10.1016/j.matlet.2013.05.006.
- Swaminathan, S. Bio-Inspired Materials and Micro/Nanostructures Enabled by Peptides and Proteins. 2015. DOI: 10.26076/a256-de8f.
- Park, S.; Hong, S.; Kim, J.; Son, S. Y.; Lee, H.; Kim, S. J. Eco Friendly Nanofluidic Platforms Using Biodegradable Nanoporous Materials. Sci. Rep. 2021, 11, 3804. DOI: 10.1038/s41598-021-83306-w.
- Gogolides, E.; Ellinas, K.; Tserepi, A. Hierarchical Micro and Nano Structured, Hydrophilic, Superhydrophobic and Superoleophobic Surfaces Incorporated in Microfluidics, Microarrays and Lab on Chip Microsystems. Microelectron. Eng. 2015, 132, 135–155. DOI: 10.1016/j.mee.2014.10.002.
- Hanasoge, S.; Ballard, M.; Hesketh, P. J.; Alexeev, A. Asymmetric Motion of Magnetically Actuated Artificial Cilia. Lab Chip. 2017, 17, 3138–3145. DOI: 10.1039/C7LC00556C.
- Dubin, R. A.; Callegari, G. C.; Kohn, J.; Neimark, A. V. Carbon Nanotube Fibers Are Compatible with Mammalian Cells and Neurons. IEEE Trans. Nanobiosci. 2008, 7, 11–14. DOI: 10.1109/TNB.2008.2000144.
- Oh, K.; Chung, J. H.; Devasia, S.; Riley, J. J. Bio-Mimetic Silicone Cilia for Microfluidic Manipulation. Lab Chip. 2009, 9, 1561–1566. DOI: 10.1039/b817409a.
- Devaraj, H.; Travas-Sejdic, J.; Sharma, R.; Aydemir, N.; Williams, D.; Haemmerle, E.; Aw, K. C. Bio-Inspired Flow Sensor from Printed PEDOT:PSS Micro-Hairs. Bioinspir. Biomim. 2015, 10, 016017. DOI: 10.1088/1748-3190/10/1/016017.
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ‑on‑a‑Chip: Recent Breakthroughs and Future Prospects. Biomed. Eng. Online. 2020, 19, 9. DOI: 10.1186/s12938-020-0752-0.
- Valencia, L.; Canalejas-Tejero, V.; Clemente, M.; Fernaud, I.; Holgado, M.; Jorcano, J. L.; Velasco, D. OPEN a New Microfluidic Method Enabling the Generation of Multi‑Layered Tissues‑on‑Chips Using Skin Cells as a Proof of Concept. Sci. Rep. 2021, 11, 13160. DOI: 10.1038/s41598-021-91875-z.
- Valencia, L.; Jorcano, J. L.; Velasco, D. Skin-on-a-Chip Models: General Overview and Future Perspectives. APL Bioeng. 2021, 5, 030901.
- Oliva, A. Skin-on-a-Chip Technology: Microengineering Physiologically Relevant in Vitro Skin Models. Pharmaceutics. 2022, 14, 682.
- Park, M.; Shin, H. K.; Panthi, G.; Rabbani, M. M.; Alam, A.-M.; Choi, J.; Chung, H.-J.; Hong, S. T.; Kim, H.-Y. Novel Preparation and Characterization of Human Hair-Based Nanofibers Using Electrospinning Process. Int J Biol Macromol. 2015, 76, 45–48. DOI: 10.1016/j.ijbiomac.2015.02.024.
- Yin, J.; Santos, V. J.; Posner, J. D. Bioinspired Flexible Microfluidic Shear Force Sensor Skin. Sensors Actuators, A Phys. 2017, 264, 289–297. DOI: 10.1016/j.sna.2017.08.001.
- Vigolo, B.; Pénicaud, A.; Coulon, C.; Sauder, C.; Pailler, R.; Journet, C.; Bernier, P.; Poulin, P. Macroscopic Fibers and Ribbons of Oriented Carbon Nanotubes. Science 2013, 290, 1331–1334. DOI: 10.1126/science.290.5495.1331.
- Poulin, P.; Vigolo, B.; Launois, P. Films and Fibers of Oriented Single Wall Nanotubes. Carbon 2002, 40, 1741–1749. DOI: 10.1016/S0008-6223(02)00042-8.
- Baughman, R. H.; Zakhidov, A. A.; De Heer, W. A. Carbon Nanotubes—The Route toward Applications. Science 2002, 297, 787–792. DOI: 10.1126/science.1060928.
- Dalton, A. B.; Collins, S.; Razal, J. M.; Howard, V. Super-Tough Carbon-Nanotube Fibres. Nature 2002, 423, 703.
- Dalton, A. B.; Collins, S.; Razal, J.; Munoz, E.; Ebron, V. H.; Kim, B. G. Continuous Carbon Nanotube Composite Fibers: Properties, Potential Applications, and Problems. J. Mater. Chem. 2004, 14, 1–3.
- Kozlov, B. M. E.; Capps, R. C.; Sampson, W. M.; Ebron, V. H.; Ferraris, J. P.; Baughman, R. H. Spinning Solid and Hollow Polymer-Free Carbon Nanotube Fibers **. Adv. Mater. 2005, 17, 614–617. DOI: 10.1002/adma.200401130.
- Islam, T.; Wang, Y.; Aggarwal, I.; Cui, Z.; Amirabadi, E.; Garg, H. Microscopic artificial cilia – a review. Lab Chip. 2022, 22, 1650–1679.
- Belardi, J.; Schorr, N.; Prucker, O.; Rühe, J. Artificial Cilia: Generation of Magnetic Actuators in Microfluidic Systems. Adv. Funct. Mater. 2011, 21, 3314–3320. DOI: 10.1002/adfm.201100787.
- Li, J.; Fan, Z.; Chen, J.; Zou, J.; Liu, C. High Yield Micro Fabrication Process for Biomimetic Artificial Haircell Sensors. Proceedings of SPIE, 2002, 4700, 315–322.
- Okada, J.; Toh, Y. Active Tactile Sensing for Localization of Objects by the Cockroach Antenna. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2006, 192, 715–726. DOI: 10.1007/s00359-006-0106-9.
- Nation, J. L. Tracheal System and Respiratory Gas Exchange. In Encyclopedia of Entomology; Capinera J. L. Ed.; Springer, 2008, 3835–3841.
- Walker, M. Vestibular System. Encycl. Neurol. Sci. 2014, 4, 647–656.
- Wang, Y.; Den, T. J.; Cardinaels, R.; Anderson, P. Lab on a Chip a Continuous Roll-Pulling Approach for the Microfluidic Pumping Capability †. Lab Chip. 2016, 16, 2277–2286. DOI: 10.1039/C6LC00531D.
- Ji, Z.; Yan, K.; Li, W.; Hu, H.; Zhu, X. Mathematical and Computational Modeling in Complex Biological Systems. Biomed Res. Int. 2017, 2017, 5958321–5958316. DOI: 10.1155/2017/5958321.
- Branscomb, J.; Alexeev, A. Designing Ciliated Surfaces That Regulate Deposition of Solid Particles. Soft Matter 2010, 6, 4066–4069. DOI: 10.1039/c0sm00185f.
- Nagrath, S.; Sequist, L. V.; Maheswaran, S.; Bell, D. W.; Irimia, D.; Ulkus, L.; et al. Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology. Nature. 2007, 450. 1235–1239.
- Wang, Y.; Gao, Y.; Wyss, H.; Anderson, P.; den Toonder, J. Out of the Cleanroom, Self-Assembled Magnetic Artificial Cilia. Lab Chip. 2013, 13, 3360–3366. DOI: 10.1039/C3LC50458A.
- Alfadhel, A.; Li, B.; Zaher, A.; Yassine, O.; Kosel, J. A Magnetic Nanocomposite for Biomimetic Flow Sensing. Lab Chip. 2014, 14, 4362–4369. DOI: 10.1039/c4lc00821a.
- Fernandez-Carro, E.; Angenent, M.; Gracia-Cazaña, T.; Gilaberte, Y.; Alcaine, C. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022, 14, 1417.
- Ataç, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A. G.; Azar, R. P.; Lindner, G. Skin and Hair on-a-Chip: In Vitro Skin Models versus Ex Vivo Tissue Maintenance with Dynamic Perfusion. Lab Chip. 2013, 13, 3555–3561. DOI: 10.1039/c3lc50227a.
- Sohrabi, S.; Tan, J.; Yunus, D. E.; He, R.; Liu, Y. Label-Free Sorting of Soft Microparticles Using a Bioinspired Synthetic Cilia Array. Biomicrofluidics 2018, 12, 042206. DOI: 10.1063/1.5022500.
- Zhu, K. Y.; Leung, K. W.; Ting, A. K. L.; Wong, Z. C. F.; Ng, W. Y. Y.; Choi, R. C. Y.; Dong, T. T. X.; Wang, T.; Lau, D. T. W.; Tsim, K. W. K. Microfluidic Chip Based Nano Liquid Chromatography Coupled to Tandem Mass Spectrometry for the Determination of Abused Drugs and Metabolites in Human Hair. Anal. Bioanal. Chem. 2012, 402, 2805–2815. DOI: 10.1007/s00216-012-5711-6.
- Bencko, V. Use of Human Hair as a Biomarker in the Assessment of Exposure to Pollutants in Occupational and Environmental Settings. Toxicology 1995, 101, 29–39. DOI: 10.1016/0300-483x(95)03018-b.
- Gutiérrez-González, E.; García-Esquinas, E.; de Larrea-Baz, N. F.; Salcedo-Bellido, I.; Navas-Acien, A.; Lope, V.; Gómez-Ariza, J. L.; Pastor, R.; Pollán, M.; Pérez-Gómez, B. Toenails as Biomarker of Exposure to Essential Trace Metals: A Review. Environ. Res. 2019, 179, 108787. DOI: 10.1016/j.envres.2019.108787.
- Jang, W.-J.; Choi, J. Y.; Park, B.; Seo, J. H.; Seo, Y. H.; Lee, S.; Jeong, C.-H.; Lee, S. Hair Metabolomics in Animal Studies and Clinical Settings. Molecules 2019, 24, 2195. DOI: 10.3390/molecules24122195.
- Alonso, A.; Albarran, C.; Martín, P.; García, P.; Capilla, J.; García, O.; de la Rua, C.; Izaguirre, N.; Pereira, F.; Pereira, L.; et al. Usefulness of Microchip Electrophoresis for the Analysis of Mitochondrial DNA in Forensic and Ancient DNA Studies. Electrophoresis 2006, 27, 5101–5109. DOI: 10.1002/elps.200600331.
- Tran, H. H.; Trinh, K. T. L.; Lee, N. Y. Pressure-Driven One-Step Solid Phase-Based on-Chip Sample Preparation on a Microfabricated Plastic Device and Integration with Flow-through Polymerase Chain Reaction (PCR). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 936, 88–94. DOI: 10.1016/j.jchromb.2013.06.037.
- Wang, H.; Wu, Z.; Zhang, Y.; Chen, B.; He, M.; Hu, B. Chip-Based Liquid Phase Microextraction Combined with Electrothermal Vaporization-Inductively Coupled Plasma Mass Spectrometry for Trace Metal Determination in Cell Samples. J. Anal. At. Spectrom. 2013, 28, 1660–1665. DOI: 10.1039/C3JA50223.
- Hamidi, S.; Alipour-Ghorbani, N.; Hamidi, A. Solid Phase Microextraction Techniques in Determination of Biomarkers. Crit. Rev. Anal. Chem. 2018, 48, 239–251. DOI: 10.1080/10408347.2017.1396885.
- Hamidi, S.; Alipour-Ghorbani, N. Liquid-Phase Microextraction of Biomarkers: A Review on Current Methods. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 853–861. DOI: 10.1080/10826076.2017.1374291.
- Regiart, M.; Fernández-Baldo, M. A.; Spotorno, V. G.; Bertolino, F. A.; Raba, J. Ultra Sensitive Microfluidic Immunosensor for Determination of Clenbuterol in Bovine Hair Samples Using Electrodeposited Gold Nanoparticles and Magnetic Micro Particles as Bio-Affinity Platform. Biosens. Bioelectron. 2013, 41, 211–217. DOI: 10.1016/j.bios.2012.08.020.
- Zheng, T.; Gao, Z.; Luo, Y.; Liu, X.; Zhao, W.; Lin, B. Manual-Slide-Engaged Paper Chip for Parallel SERS-Immunoassay Measurement of Clenbuterol from Swine Hair. Electrophoresis 2016, 37, 418–424. DOI: 10.1002/elps.201500324.
- Dasary, S. S. R.; Singh, A. K.; Senapati, D.; Yu, H.; Ray, P. C. Gold Nanoparticle Based Label-Free SERS Probe for Ultrasensitive and Selective Detection of Trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. DOI: 10.1021/ja905134d.
- Dou, B.; Luo, Y.; Chen, X.; Shi, B.; Du, Y.; Gao, Z.; Zhao, W.; Lin, B. Direct Measurement of Beta-Agonists in Swine Hair Extract in Multiplexed Mode by Surface-Enhanced Raman Spectroscopy and Microfluidic Paper. Electrophoresis 2015, 36, 485–487. DOI: 10.1002/elps.201400362.
- Li, W.; Luo, Y.; Yue, X.; Wu, J.; Wu, R.; Qiao, Y.; Peng, Q.; Shi, B.; Lin, B.; Chen, X. A Novel Microfluidic Paper-Based Analytical Device Based on Chemiluminescence for the Determination of β-Agonists in Swine Hair. Anal. Methods 2020, 12, 2317–2322. DOI: 10.1039/c9ay02754h.
- Gilbert, M. T. P.; Wilson, A. S.; Bunce, M.; Hansen, A. J.; Willerslev, E.; Shapiro, B.; Higham, T. F. G.; Richards, M. P.; O’Connell, T. C.; Tobin, D. J.; et al. Ancient Mitochondrial DNA from Hair. Curr. Biol. 2004, 14, 463–464.
- Taberlet, P.; Luikart, G.; Waits, L. P. Noninvasive Genetic Sampling: Look before You Leap. Trends Ecol. Evol. 1999, 14, 323–327. DOI: 10.1016/s0169-5347(99)01637-7.
- Ghatak, S.; Muthukumaran, R. B.; Nachimuthu, S. K. A Simple Method of Genomic DNA Extraction from Human Samples for PCR-RFLP Analysis. J. Biomol. Tech. 2013, 24, 224–231. DOI: 10.7171/jbt.13-2404-001.
- Kline, M. C.; Vallone, P. M.; Redman, J. W.; Duewer, D. L.; Calloway, C. D.; Butler, J. M. Mitochondrial DNA Typing Screens with Control Region and Coding Region SNPs. J. Forensic Sci. 2005, 50, 377–385.
- Hassan, S. Microchip Electrophoresis. Encyclopedia 2020, 1, 30–41. DOI: 10.3390/encyclopedia1010006.
- Lin, X.; Wu, J.; Li, H.; Wang, Z.; Lin, J. M. Determination of Mini-Short Tandem Repeat (miniSTR) Loci by Using the Combination of Polymerase Chain Reaction (PCR) and Microchip Electrophoresis. Talanta 2013, 114, 131–137. DOI: 10.1016/j.talanta.2013.04.012.
- Van Nguyen, H.; Seo, T. S. High-Throughput Human DNA Purification on a Centrifugal Microfluidic Device for Rapid Forensic Sex-Typing. Biosens. Bioelectron. 2021, 181, 113161. DOI: 10.1016/j.bios.2021.113161.
- von Thaden, A.; Cocchiararo, B.; Jarausch, A.; Jüngling, H.; Karamanlidis, A. A.; Tiesmeyer, A.; Nowak, C.; Muñoz-Fuentes, V. Assessing SNP Genotyping of Noninvasively Collected Wildlife Samples Using Microfluidic Arrays. Sci. Rep. 2017, 7, 10768. DOI: 10.1038/s41598-017-10647-w.
- Jiang, W.; Hu, D.; Xu, Y.; Chen, Y.; Zhu, X.; Han, Z.; Ye, X.; Li, X. Loop-Mediated Isothermal Amplification-Microfluidic Chip for the Detection of Trichophyton Infection. Front. Microbiol. 2022, 13, 1031388. DOI: 10.3389/fmicb.2022.1031388.
- Miyaguchi, H.; Takahashi, H.; Ohashi, T.; Mawatari, K.; Iwata, Y. T.; Inoue, H.; Kitamori, T. Rapid Analysis of Methamphetamine in Hair by Micropulverized Extraction and Microchip-Based Competitive ELISA. Forensic Sci. Int. 2009, 184, 1–5. DOI: 10.1016/j.forsciint.2008.10.024.
- Xu, Q.; Zhou, Y.; Chen, H.; Tian, Z. Micro-fluidic chip for Raman detection of amphetamine chloride in hair and utilization method thereof. Patent Application Number CN2016-10548521. 2016.
- He, K. Trace Elements in Nails as Biomarkers in Clinical Research. Eur. J. Clin. Invest. 2011, 41, 98–102. DOI: 10.1111/j.1365-2362.2010.02373.x.
- Shokoohi, R.; Khazaei, M.; Karami, M.; Seid-Mohammadi, A.; Khazaei, S.; Torkshavand, Z. Application of Fingernail Samples as a Biomarker for Human Exposure to Arsenic—Contaminated Drinking Waters. Sci. Rep. 2022, 12, 4733. DOI: 10.1038/s41598-022-08845-2.
- Phillips, R.; Kraeuter, A.-K.; McDermott, B.; Lupien, S.; Sarnyai, Z. Human Nail Cortisol as a Retrospective Biomarker of Chronic Stress: A Systematic Review. Psychoneuroendocrinology 2021, 123, 104903. https://www.sciencedirect.com/science/article/pii/S0306453020303267. DOI: 10.1016/j.psyneuen.2020.104903.
- Min, J. Z.; Yano, H.; Matsumoto, A.; Yu, H.-F.; Shi, Q.; Higashi, T.; Inagaki, S.; Toyo’oka, T. Simultaneous Determination of Polyamines in Human Nail as Flow Chip LC Coupled with Quadrupole Time-of- Flight Tandem Mass Spectrometry. Clin. Chim. Acta. 2011, 412, 98–106. DOI: 10.1016/j.cca.2010.09.018.
- Graves, P. R.; Haystead, T. A. J. Molecular Biologist’s Guide to Proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63; table of contents. DOI: 10.1128/MMBR.66.1.39-63.2002.
- De Mello, A. FOCUS on-Chip Chromatography: The Last Twenty Years. Lab Chip. 2002, 2, 48N–54N. DOI: 10.1039/B207266C.
- Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science 1989, 246, 64–71. DOI: 10.1126/science.2675315.
- Karas, M.; Bachmann, D.; Bahr, U.; Hillenkamp, F. Matrix-Assisted Ultraviolet Laser Desorption of Non-Volatile Compounds. Int. J. Mass Spectrom. Ion Process. 1987, 78, 53–68. https://www.sciencedirect.com/science/article/pii/0168117687870416. DOI: 10.1016/0168-1176(87)87041-6.
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T.; Matsuo, T. Protein and Polymer Analyses up to m/z 100 000 by Laser Ionization Time-of-Flight Mass Spectrometry. Rapid Comm. Mass Spectr. 1988, 2, 151–153. DOI: 10.1002/rcm.1290020802.
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. DOI: 10.1021/ac950914h.
- Srbek, J.; Eickhoff, J.; Effelsberg, U.; Kraiczek, K.; van de Goor, T.; Coufal, P. Chip-Based nano-LC-MS/MS Identification of Proteins in Complex Biological Samples Using a Novel Polymer Microfluidic Device. J. Sep. Sci. 2007, 30, 2046–2052. DOI: 10.1002/jssc.200700053.
- Chen, X.; Luo, Y.; Shi, B.; Gao, Z.; Du, Y.; Liu, X.; Zhao, W.; Lin, B. Determination of Beta-Agonists in Swine Hair by μFIA and Chemiluminescence. Electrophoresis 2015, 36, 986–993. DOI: 10.1002/elps.201400412.
- Phatale, V.; Vaiphei, K. K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Transdermal Drug Delivery Approaches. J. Control. Release 2022, 351, 361–380. DOI: 10.1016/j.jconrel.2022.09.025.
- Bilal, M.; Mehmood, S.; Raza, A.; Hayat, U.; Rasheed, T.; Iqbal, H. M. N. Microneedles in Smart Drug Delivery. Adv. Wound Care (New Rochelle) 2021, 10, 204–219. DOI: 10.1089/wound.2019.1122.
- Nair, A. B.; Al-Dhubiab, B. E.; Shah, J.; Gorain, B.; Jacob, S.; Attimarad, M.; Sreeharsha, N.; Venugopala, K. N.; Morsy, M. A. Constant Voltage Iontophoresis Technique to Deliver Terbinafine via Transungual Delivery System: Formulation Optimization Using Box–Behnken Design and in Vitro Evaluation. Pharmaceutics 2021, 13, 1692. DOI: 10.3390/pharmaceutics13101692.
- Morgen, M.; Lu, G. W.; Du, D.; Stehle, R.; Lembke, F.; Cervantes, J.; Ciotti, S.; Haskell, R.; Smithey, D.; Haley, K.; Fan, C. Targeted Delivery of a Poorly Water-Soluble Compound to Hair Follicles Using Polymeric Nanoparticle Suspensions. Int. J. Pharm. 2011, 416, 314–322. DOI: 10.1016/j.ijpharm.2011.06.019.
- Subbiah, N.; Campagna, J.; Spilman, P.; Alam, M. P.; Sharma, S.; Hokugo, A.; Nishimura, I.; John, V. Deformable Nanovesicles Synthesized through an Adaptable Microfluidic Platform for Enhanced Localized Transdermal Drug Delivery. J. Drug Deliv. 2017, 2017, 4759839–4759812. DOI: 10.1155/2017/4759839.
- Bharathala, S.; Sharma, P. Biomedical Applications of Nanoparticles. In Nanotechnology in Modern Animal Biotechnology: Concepts and Applications; Maurya, P. K. and Singh, S. Eds. Elsevier: The Netherlands; 2019. p. 113–132. DOI: 10.1016/B978-0-12-818823-1.00008-9.
- Bandodkar, A. J.; Ghaffari, R.; Rogers, J. A. Don’t Sweat It: The Quest for Wearable Stress Sensors. Matter 2020, 2, 795–797. DOI: 10.1016/j.matt.2020.03.004.
- Vella, D.; Lukač, M.; Jernejčič, U.; Lukač, N.; Klaneček, Ž.; Milanič, M.; Jezeršek, M.; Measurements of Hair Temperature Avalanche Effect with Alexandrite and Nd:YAG Hair Removal Lasers. Lasers Surg. Med. 2022, 55, 89–98. DOI: 10.1002/lsm.23622.
- Xu, J.; Zhang, Z.; Gan, S.; Gao, H.; Kong, H.; Song, Z.; Ge, X.; Bao, Y.; Niu, L. Highly Stretchable Fiber-Based Potentiometric Ion Sensors for Multichannel Real-Time Analysis of Human Sweat. ACS Sens. 2020, 5, 2834–2842. DOI: 10.1021/acssensors.0c00960.
- Awad, E.; Ramji, R.; Cirovic, S.; Rämgård, M.; Kottorp, A.; Shleev, S. Developing and Evaluating Non-Invasive Healthcare Technologies for a Group of Female Participants from a Socioeconomically Disadvantaged Area. Sci. Rep. 2021, 11, 23896. DOI: 10.1038/s41598-021-03262-3.