?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Despite growing evidence for food-based dietary patterns' potential to reduce cardiovascular disease risk, knowledge about the amounts of food associated with the greatest change in risk of specific cardiovascular outcomes and about the quality of meta-evidence is limited. Therefore, the aim of this meta-analysis was to synthesize the knowledge about the relation between intake of 12 major food groups (whole grains, refined grains, vegetables, fruits, nuts, legumes, eggs, dairy, fish, red meat, processed meat, and sugar-sweetened beverages [SSB]) and the risk of coronary heart disease (CHD), stroke and heart failure (HF).
Methods: We conducted a systematic search in PubMed and Embase up to March 2017 for prospective studies. Summary risk ratios (RRs) and 95% confidence intervals (95% CI) were estimated using a random effects model for highest versus lowest intake categories, as well as for linear and non-linear relationships.
Results: Overall, 123 reports were included in the meta-analyses. An inverse association was present for whole grains (RRCHD: 0.95 (95% CI: 0.92–0.98), RRHF: 0.96 (0.95–0.97)), vegetables and fruits (RRCHD: 0.97 (0.96–0.99), and 0.94 (0.90–0.97); RRstroke: 0.92 (0.86–0.98), and 0.90 (0.84–0.97)), nuts (RRCHD: 0.67 (0.43–1.05)), and fish consumption (RRCHD: 0.88 (0.79–0.99), RRstroke: 0.86 (0.75–0.99), and RRHF: 0.80 (0.67–0.95)), while a positive association was present for egg (RRHF: 1.16 (1.03–1.31)), red meat (RRCHD: 1.15 (1.08–1.23), RRstroke: 1.12 (1.06–1.17), RRHF: 1.08 (1.02–1.14)), processed meat (RRCHD: 1.27 (1.09–1.49), RRstroke: 1.17 (1.02–1.34), RRHF: 1.12 (1.05–1.19)), and SSB consumption (RRCHD: 1.17 (1.11–1.23), RRstroke: 1.07 (1.02–1.12), RRHF: 1.08 (1.05–1.12)) in the linear dose-response meta-analysis. There were clear indications for non-linear dose-response relationships between whole grains, fruits, nuts, dairy, and red meat and CHD.
Conclusion: An optimal intake of whole grains, vegetables, fruits, nuts, legumes, dairy, fish, red and processed meat, eggs and SSB showed an important lower risk of CHD, stroke, and HF.
Background
The global challenges of cardiovascular diseases (CVD) present an enormous health burden (Fuster and Kelly Citation2010). In 2015, CVD were among the leading causes of disease burden worldwide (Kassebaum et al. Citation2016). Lifestyle, especially unhealthy dietary patterns, represents a major trigger in the development of CVD and its associated risk factors (e.g., hypercholesterolemia, hypertension, and type 2 diabetes mellitus) (Moran et al. Citation2014; Scarborough et al. Citation2011; Verschuren et al. Citation1995). On the other hand, healthy food and an overall balanced dietary pattern may exert beneficial effects on cardiovascular health.
On the basis of the current evidence, food-based priorities to reduce CVD risk are to increase consumption of whole grains, fruits, vegetables, legumes, nuts and seeds, and fish, to keep a moderate dairy and vegetable oil intake, and to avoid/reduce consumption of refined grains, sugar-sweetened beverages (SSBs), and red and processed (sodium-preserved) meats (Anand et al. Citation2015; Moran et al. Citation2014; Mozaffarian Citation2016). Thus, the United States Dietary Guidelines (healthy American diet, the Mediterranean diet and the vegetarian diet) (USDA Citation2015) and scientific societies (Anderson et al. Citation2013; Eckel et al. Citation2014; Perk et al. Citation2012), for example, recommend dietary patterns that combine these food-based recommendations regarding prevention of chronic diseases.
There is growing evidence and consensus for such food-based dietary patterns as the best means to reduce CVD, obesity, weight gain, and diabetes mellitus (Mozaffarian Citation2016). The approach to primarily consider diet at the level of consumption of food groups instead of nutrients and other dietary compounds is directly linked to the concept of food-based dietary guidelines that should be formulated for each country depending on the current dietary practice and the level of intake (EFSA Citation2010). Evidence for relationships between food consumption and disease risk from meta-analyses is a key component in this process and independent from the country-specific situation.
Even though evidence about the association of CVD risk with some foods and food groups has been reviewed and summarized (Eckel et al. Citation2014; Kromhout et al. Citation2016; Nordic Council of Ministers Citation2014; USDA Citation2015), a remaining question is which amounts of foods or food groups are associated with the greatest change in risk of CVD (referred to hereinafter as optimal intakes), differentiated according to the separate CVD outcomes coronary heart disease (CHD), stroke, and heart failure (HF). As previously shown, selecting specific optimal intakes among the selected food groups can lead to a considerable change in risk of premature death (Schwingshackl et al. Citation2017a) and morbidity such as type 2 diabetes (Schwingshackl et al. Citation2017b), and hypertension (Schwingshackl et al. Citation2017c). Furthermore, trustworthiness of meta-evidence is very rarely performed in previous meta-analyses (Mozaffarian Citation2016). Consequently, one of the most important questions that remain to be answered is which food groups show high quality meta-evidence of protective or detrimental associations with risk of CHD, stroke, and HF using a comprehensive approach.
To address these issues, we conducted a systematic review and meta-analysis of prospective studies to summarize findings of the associations between 12 a priori defined food groups, including whole grains, refined grains, vegetables, fruits, nuts, legumes, eggs, dairy, fish, red meat, processed meat, and SSB with risk of CHD, stroke, and HF. The focus is based on these 12 food groups since most diet quality indices/scores were based on these (Schwingshackl and Hoffmann Citation2015a, Citation2015b; Schwingshackl et al. Citation2015), as previously reported (Schwingshackl et al. Citation2016a). The relation between the selected food groups and the mentioned CVD outcomes were calculated separately given their distinct pathogenesis and thus different risk associations between exposure factors and individual endpoints could exist and have to be identified (instead of adding further evidence for aggregated CVD). Special attention was given to strength and dose-specific shape of the relationship for finding an optimal intake for lowest risk by high versus low and linear as well as non-linear dose-response meta-analyses. Finally, using the NutriGrade scoring system, we aimed to evaluate food groups' quality of meta-evidence on their protective or detrimental associations with risk of CHD, stroke, and HF, respectively.
Materials and methods
The review was registered in PROSPERO International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/prospero/index.asp, identifier CRD42016037069). Our strategy for the systematic review and meta-analysis was pre-defined in a published protocol (Schwingshackl et al. Citation2016a) and has already been implemented for two recently published meta-analyses on all-cause mortality and type 2 diabetes, respectively (Schwingshackl et al. Citation2017b; Schwingshackl et al. Citation2017a). This systematic review was planned and conducted according to the standards of the Meta-analysis of Observational Studies in Epidemiology (Stroup et al. Citation2000).
Search strategy
Literature search was performed using the electronic databases PubMed (until March 2017) and Embase (until March 2017), with no restriction to language and calendar date using the search terms described in Supplementary Appendix 1.
Additionally, the reference lists from the retrieved articles were checked to identify further relevant studies. Published systematic reviews and meta-analyses were also used as a data source. Literature search was conducted by two authors (AB, LS), with disagreement resolved by consensus of another reviewer (HB).
Study selection
Studies were included in the meta-analysis if they met all of the following criteria: (1) prospective design (cohort studies, case-cohort studies, nested case-control studies, follow-up studies of RCTs); (2) information about the association for at least one of the following 12 food groups: whole grains/cereals, refined grains/cereals, vegetables, fruits, nuts, legumes, eggs, dairy products, fish, red meat, processed meat, SSB; (3) Participants aged ≥18 years; and (4) considering CHD including myocardial infarction and other coronary artery diseases (like angina); stroke (haemorrhagic, ischemic); and HF as outcomes. Exclusion criteria were (1) studies including populations suffering from chronic disease; (2) studies reporting only fatal outcomes, since mortality was included in a previous meta-analysis (Schwingshackl et al. Citation2017c); (3) studies aggregating outcomes as total CVD, and not reporting on CHD, stroke or HF separately; (4) when a study appeared to have been published in duplicate, the version containing the longest follow-up was selected.
Data extraction
Two authors (AB, LS) extracted the following characteristics: the first author's last name, year of publication, study origin, cohort name, sample size, number of cases, age at entry, sex, study length (follow-up in years), outcome, outcome assessment, assessment of food group, quantity of food, risk estimate (most adjusted measures) (hazard ratios (HR), risk ratios (RR), or odds ratios (OR) with their corresponding 95% confidence intervals (CIs)), and adjustment factors.
When a study provided several risk estimates, the multivariable model was chosen. When only separate risk estimates for male and female participants were available in a study, we combined the RRs using a fixed effect model before inclusion in the meta-analysis.
Risk of bias assessment
To assess the risk of bias of the prospective studies, we assessed ascertainment of exposure (low risk of bias: validated, calibrated FFQ or 24-h recall, diet history, or diet records (multiple days)), assessment of outcome (low risk of bias: record linkage (ICD codes), accepted clinical criteria, self-reported and validated), duration of follow-up (<10 versus ≥10 years), and whether basic model was adjusted and outcome relevant adjustments (age, sex, education, body mass index, smoking, physical activity, energy intake) were made (Schwingshackl et al. Citation2016c). Studies were classified as being at low risk of bias in general only if none of the domains established a high /unclear risk of bias.
Statistical analysis
For high versus low and dose-response meta-analyses, a random effects model was used to calculate summary RRs/HRs/ORs and 95% CIs summarizing associations between the food groups (whole grains/cereals, refined grains/cereals, vegetables, fruits, nuts, legumes, eggs, dairy products, fish, red meat, processed meat, SSB), and risk of CHD, stroke, and HF (DerSimonian and Laird Citation1986), which incorporated both within and between study variability. Meta-analysis was based on the assumption that all measures are RRs. To evaluate the weighting of each study, the standard error for the log-transformed RR of each study was calculated and regarded as the estimated variance of the log-transformed RR, using an inverse variance method (DerSimonian and Laird Citation1986).
The method described by Greenland and Longnecker (Greenland and Longnecker Citation1992) was applied for the dose-response analysis and computed study-specific slopes (linear trends) and corresponding 95% CIs from the natural logs of the RRs and CIs across intake categories of the 12 food groups. The method requires that the distribution of cases, person-years or non-cases, and the RRs with the 95% CI for at least three quantitative exposure categories are known.
When studies reported only the total number of cases or total person-years and the exposure was defined in quantiles, the distribution of cases or person-years was calculated by dividing the total number by the number of quantiles, thus assuming an equal distribution across exposure quantiles. Whenever reported, the mean or median intake by category was assigned to the corresponding RR. The midpoint was calculated for studies that only reported a range of intake by category. When the intake range was open-ended, we assumed that its width was the same as the adjacent category. For studies presenting the exposure per given unit of energy intake, we rescaled it using the mean energy intake provided.
Where studies had already reported a linear dose-response trend, with CI or standard error, this was used directly.
The units of exposure were defined as follows: whole grains/cereals (30 g/d), refined grains/cereals (30 g/d), vegetables (100 g/d), fruits (100 g/d), nuts (28 g/d), legumes (50 g/d), eggs (50 g/d), dairy products (200 g/d), fish (100 g/d), red meat (100 g/d), processed meat (50 g/d), and sugar sweetened beverages (250ml/d). For studies that reported intake only as serving size (and did not specify the quantitative amount), we used recommended conversions (WCRF Citation2017) (Supplemental Table 1).
To examine possible non-linear associations, we calculated restricted cubic splines for each study with more than three categories of exposure, using three fixed knots at 10%, 50%, and 90% through the total distribution of the reported intake, and combined them using multivariable meta-analysis (Durrleman and Simon Citation1989) (if at least 2 studies with at least three quantitative exposure levels were known for one of the 12 food groups with the corresponding outcome).
Moreover, the risk reduction potential of foods was calculated by multiplying the RR by selecting an optimal consumption of risk-reducing foods (calculated by ), and risk-increasing foods noted as
The optimal consumption was defined as the serving category of a single food group with the strongest association for CHD, stroke, and HF risk, respectively.
To explore heterogeneity between studies, we used Cochran's Q test and calculated the I2 statistic (with a value of I2 > 50% considered to represent potentially important statistical heterogeneity) (Higgins and Thompson Citation2002). In addition, to identify potential sources of heterogeneity, we stratified the meta-analysis by subgroups. If more than five studies were available for a food group in the highest vs. lowest category meta-analysis or in the linear dose-response analysis, subgroup analyses were performed by the following characteristics: sex, length of follow-up (mean or median ≥10 years vs. <10 years), geographic location (Europe, America, Asia and Australia), number of cases (≥1,000 vs. <1,000), and dietary assessment (validated vs. non-validated). Furthermore, we performed sensitivity analyses for low risk of bias studies, excluding nested case-control studies and studies based on ORs.
Potential small-study effects, such as publication bias, were explored using Egger´s test and funnel plots (Egger et al. Citation1997), if at least 10 studies were available (Higgins and Green Citation2011).
Review Manager 5.3 (Nordic Cochrane Center, Copenhagen), and Stata version 14 software (StataCorp, College Station, TX) were used for the statistical analyses.
Quality of meta-evidence
Quality of evidence of the meta-analyses (i.e., meta-evidence) was defined as the confidence in the estimate. To evaluate the meta-evidence for the association between the 12 pre-defined food groups and CHD, stroke, and HF risk, we applied the NutriGrade scoring system (max 10 points) which comprises the following items: (i) risk of bias/study quality/study limitations (max. 2 points), (ii) precision (max. 1 point), (iii) heterogeneity (max. 1 point), (iv) directness (max. 1 point), (v) publication bias (max. 1 point), (vi) funding bias (max. 1 point), (vii) effect size (max. 2 points), and (viii) dose-response (max. 1 point) (Schwingshackl et al. Citation2016c). Based on this scoring system we defined four meta-evidence levels: high (≥8 points), moderate (6-<8 points), low (4-<6 points), and very low (0-<4 points).
Results
Out of the 16,623 records which were identified by the systematic literature search, 261 full text articles were assessed in detail as they reported on at least one of the 12 food groups and CVD outcomes in the title/abstract (; Supplemental Appendix 2).
Sixteen prospective studies were included in the meta-analysis for consumption of whole grains (16 reports) (Del Gobbo et al. Citation2015; Djousse and Gaziano Citation2007; Hansen et al. Citation2017; Helnaes et al. Citation2016; Jensen et al. Citation2004; Liu et al. Citation2000b; Liu et al. Citation1999; Mizrahi et al. Citation2009; Muraki et al. Citation2015; Neelakantan et al. Citation2016; Nettleton et al. Citation2008; Rautiainen et al. Citation2012; Sonestedt et al. Citation2015; Steffen et al. Citation2003; Tektonidis et al. Citation2015, Citation2016) (Supplemental Table 2), 9 studies for refined grains (8 reports) (Djousse and Gaziano Citation2007; Eshak et al. Citation2014; Liu et al. Citation2000b; Mizrahi et al. Citation2009; Muraki et al. Citation2015; Sonestedt et al. Citation2015; Steffen et al. Citation2003; Yu et al. Citation2013) (Supplemental Table 3), 35 for vegetables (32 reports) (Belin et al. Citation2011a; Bendinelli et al. Citation2011; Bhupathiraju et al. Citation2013; Buckland et al. Citation2009; Dauchet et al. Citation2004; Del Gobbo et al. Citation2015; Dilis et al. Citation2012; Gillman et al. Citation1995; Hansen et al. Citation2017; Hansen et al. Citation2010; Hirvonen et al. Citation2001; Johnsen et al. Citation2003; Joshipura et al. Citation1999; Keli et al. Citation1996; Kobylecki et al. Citation2015; Larsson et al. Citation2009b; Larsson et al. Citation2013; Lin et al. Citation2013; Liu et al. Citation2001; Liu et al. Citation2000a; Martinez-Gonzalez et al. Citation2011; Misirli et al. Citation2012; Mizrahi et al. Citation2009; Neelakantan et al. Citation2016; Oude Griep et al. Citation2012; Rautiainen et al. Citation2015; Sonestedt et al. Citation2015; Tognon et al. Citation2014; Wurtz et al. Citation2016; Yokoyama et al. Citation2000; Yu et al. Citation2014; Zhang et al. Citation2011) (Supplemental Table 4), 32 for fruits (30 reports) (Belin et al. Citation2011a; Bendinelli et al. Citation2011; Bhupathiraju et al. Citation2013; Buckland et al. Citation2009; Dauchet et al. Citation2004; Del Gobbo et al. Citation2015; Du et al. Citation2016; Fraser et al. Citation1992; Gillman et al. Citation1995; Hansen et al. Citation2017; Hansen et al. Citation2010; Hirvonen et al. Citation2001; Johnsen et al. Citation2003; Joshipura et al. Citation1999; Keli et al. Citation1996; Kobylecki et al. Citation2015; Larsson et al. Citation2009b; Larsson et al. Citation2013; Lin et al. Citation2013; Liu et al. Citation2000a; Mizrahi et al. Citation2009; Neelakantan et al. Citation2016; Oude Griep et al. Citation2012; Rautiainen et al. Citation2015; Sonestedt et al. Citation2015; Tognon et al. Citation2014; Yamada et al. Citation2011; Yokoyama et al. Citation2000; Yu et al. Citation2014; Zhang et al. Citation2011) (Supplemental Table 5), 13 for nuts (12 reports) (Albert et al. Citation2002; Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Del Gobbo et al. Citation2015; di Giuseppe et al. Citation2015; Djousse et al. Citation2010; Djousse et al. Citation2008; Fraser et al. Citation1992; Haring et al. Citation2014; Haring et al. Citation2015; Nettleton et al. Citation2008; Yaemsiri et al. Citation2012) (Supplemental Table 6), 15 for legumes (13 reports) (Bazzano et al. Citation2001; Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Buckland et al. Citation2009; Dilis et al. Citation2012; Fraser et al. Citation1992; Haring et al. Citation2014; Haring et al. Citation2015; Kokubo et al. Citation2007; Martinez-Gonzalez et al. Citation2011; Misirli et al. Citation2012; Mizrahi et al. Citation2009; Yu et al. Citation2014) (Supplemental Table 7), 17 for eggs (16 reports) (Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Burke et al. Citation2007; Dilis et al. Citation2012; Djousse and Gaziano Citation2008a, Citation2008b; Goldberg et al. Citation2014; Haring et al. Citation2014; Haring et al. Citation2015; Hu et al. Citation1999; Larsson et al. Citation2015; Misirli et al. Citation2012; Nettleton et al. Citation2008; Qureshi et al. Citation2007; Virtanen et al. Citation2016; Yaemsiri et al. Citation2012) (Supplemental Table 8), 24 for dairy products (24 reports) (Avalos et al. Citation2013; Bergholdt et al. Citation2015; Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Buckland et al. Citation2009; Dalmeijer et al. Citation2013; Dilis et al. Citation2012; Elwood et al. Citation2004; Fraser et al. Citation1992; Haring et al. Citation2014; Haring et al. Citation2015; Larsson et al. Citation2009a; Larsson et al. Citation2012; Lin et al. Citation2013; Martinez-Gonzalez et al. Citation2011; Misirli et al. Citation2012; Nettleton et al. Citation2008; Patterson et al. Citation2013; Praagman et al. Citation2015; Soedamah-Muthu et al. Citation2013; Sonestedt et al. Citation2011; Tektonidis et al. Citation2015, Citation2016; Yaemsiri et al. Citation2012) (Supplemental Table 9), 47 for fish (47 reports) (Albert et al. Citation1998; Amiano et al. Citation2016a; Ascherio et al. Citation1995; Atkinson et al. Citation2011; Belin et al. Citation2011b; Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Bjerregaard et al. Citation2010; Buckland et al. Citation2009; de Goede et al. Citation2010; de Goede et al. Citation2012; Del Gobbo et al. Citation2015; Dijkstra et al. Citation2009; Dilis et al. Citation2012; Fraser et al. Citation1992; Gammelmark et al. Citation2016; Gillum et al. Citation2000; Gillum et al. Citation1996; Hansen et al. Citation2017; Haring et al. Citation2014; Haring et al. Citation2015; Holmberg et al. Citation2009; Iso et al. Citation2006; Keli et al. Citation1994; Kuhn et al. Citation2013; Larsson et al. Citation2011a; Levitan et al. Citation2009, Citation2010; Martinez-Gonzalez et al. Citation2011; Misirli et al. Citation2012; Montonen et al. Citation2009; Morris et al. Citation1995; Mozaffarian et al. Citation2005a; Mozaffarian et al. Citation2003; Mozaffarian et al. Citation2005b; Myint et al. Citation2006; Nahab et al. Citation2016; Nettleton et al. Citation2008; Orencia et al. Citation1996; Osler et al. Citation2003; Salonen et al. Citation1995; Tektonidis et al. Citation2015; Tognon et al. Citation2014; Wennberg et al. Citation2011; Wennberg et al. Citation2007; Wilk et al. Citation2012; Wurtz et al. Citation2016) (Supplemental Table 10), 15 for red meat (Amiano et al. Citation2016b; Ashaye et al. Citation2011; Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Del Gobbo et al. Citation2015; Fraser et al. Citation1992; Haring et al. Citation2014; Haring et al. Citation2015; Kaluza et al. Citation2014, Citation2015; Larsson et al. Citation2011b, Citation2011c; Nettleton et al. Citation2008; Wurtz et al. Citation2016; Yaemsiri et al. Citation2012) (Supplemental Table 11), 14 for processed meat (13 reports) (Amiano et al. Citation2016b; Ascherio et al. Citation1994; Bernstein et al. Citation2012b; Bernstein et al. Citation2010; Burke et al. Citation2007; Del Gobbo et al. Citation2015; Haring et al. Citation2014; Haring et al. Citation2015; Kaluza et al. Citation2014, Citation2015; Larsson et al. Citation2011b, Citation2011c; Wurtz et al. Citation2016) (Supplemental Table 12), and 11 for SSB (9 reports) (Bernstein et al. Citation2012a; de Koning et al. Citation2012; Del Gobbo et al. Citation2015; Eshak et al. Citation2012; Fung et al. Citation2009; Gardener et al. Citation2012; Larsson et al. Citation2014; Rahman et al. Citation2015; Sonestedt et al. Citation2015) (Supplemental Table 13).
Whole grains
For whole grains, 7 studies with 6,834 cases were included in the high vs. low intake meta-analysis (overall intake range: 0–220 g/d) for CHD, 7 studies with 11,114 cases (overall intake range: 0–670 g/d) for stroke, and 5 studies with 6,455 cases (overall intake range: 0–280 g/d) for HF.
Comparing the highest to the lowest categories of whole grain intake, inverse associations with risk of CHD (RR: 0.85; 95% CI 0.81 to 0.90, I2 = 0%, pheterogeneity = 0.72), stroke (RR: 0.91; 95% CI 0.82 to 1.02, I2 = 53%, pheterogeneity = 0.05), and HF (RR: 0.91; 95% CI 0.85 to 0.97, I2 = 35%, pheterogeneity = 0.19) were observed (Supplemental Figure 1).
Each additional daily 30 g of whole grains were inversely associated with risk of CHD (RR: 0.95; 95% CI 0.92 to 0.98, I2 = 46%, pheterogeneity = 0.11, n = 5) and HF (RR: 0.96; 95% CI 0.95 to 0.97, I2 = 0%, pheterogeneity = 0.36, n = 2), but not with risk of stroke (RR: 0.99; 95% CI 0.95 to 1.03, I2 = 65%, pheterogeneity = 0.04, n = 4) (Supplemental Figure 2).
Heterogeneity observed in the high vs. low whole grain intake meta-analysis for stroke persisted in stratified analyses (Supplemental Table 14a-b) and, where detectable, no evidence of heterogeneity for subgroup-differences was observed.
There was evidence of a non-linear dose-response association (pnon-linearity<0.001, n = 5 studies) for CHD, but not for stroke (pnon-linearity = 0.77, n = 3 studies). The risk of CHD decreased by 17% with increasing intake of whole grains up to ∼100 g/d. No benefit for increasing intake was apparent above this intake (). No association was observed for whole grain intake and risk of stroke in the non-linear dose-response analysis ().
Figure 2. Non-linear dose-response relationship between daily intakes whole grains (p for non-linearity <0.001; n = 5 studies), refined grains (p for non-linearity = 0.25; n = 4 studies), vegetables (p for non-linearity = 0.19; n = 13 studies), fruits (p for non-linearity <0.001; n = 12 studies), nuts (p for non-linearity <0.001; n = 4 studies), legumes (p for non-linearity = 0.07; n = 8 studies), eggs (p for non-linearity = 0.81; n = 9 studies), dairy (p for non-linearity <0.01; n = 9 studies), fish (p for non-linearity = 0.10; n = 15 studies), red meat (p for non-linearity <0.01; n = 2 studies), processed meat (p for non-linearity = 0.41; n = 2 studies), and sugar sweetened beverages (p for non-linearity = 0.83; n = 4 studies) and risk of coronary heart disease.
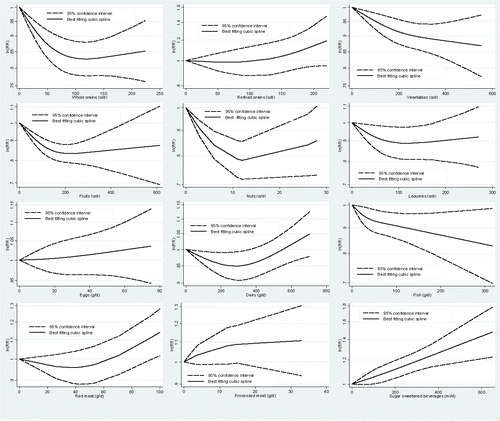
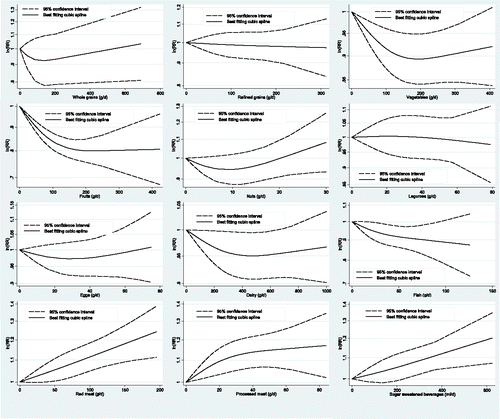
Figure 3. Non-linear dose-response relationship between daily intakes whole grains (p for non-linearity = 0.77; n = 3 studies), refined grains (p for non-linearity = 0.42; n = 4 studies), vegetables (p for non-linearity = 0.03; n = 8 studies), fruits (p for non-linearity = 0.54; n = 8 studies), nuts (p for non-linearity = 0.05; n = 5 studies), legumes (p for non-linearity = 0.08; n = 5 studies), eggs (p for non-linearity = 0.39; n = 9 studies), dairy (p for non-linearity = 0.71; n = 8 studies), fish (p for non-linearity = 0.37; n = 15 studies), red meat (p for non-linearity = 0.91; n = 6 studies), processed meat (p for non-linearity = 0.65; n = 6 studies), and sugar sweetened beverages (p for non-linearity = 0.83; n = 6 studies) and risk of stroke.
Refined grains
Five studies with 3,286 cases were included in the high vs. low intake meta-analysis (overall intake range: 15–540 g/d) for CHD, 6 studies with 11,434 cases (overall intake range: 15–540 g/d) for stroke, and 1 study with 1018 cases (overall intake range: 0–40 g/d) for HF.
Comparing the highest to the lowest categories of intake, a trend for a positive association between risk of CHD (RR: 1.11; 95% CI 0.99 to 1.25, I2 = 0%, pheterogeneity = 0.52) and refined grain intake was observed, whereas no association was detected for stroke (RR: 1.02; 95% CI 0.94 to 1.11, I2 = 2%, pheterogeneity = 0.41), or HF (RR: 0.83; 95% CI 0.58 to 1.19) (Supplemental Figure 3).
No association was observed for each additional daily 30 g of refined grains and risk of CHD (RR: 1.01; 95% CI 0.99 to 1.04, I2 = 0%, pheterogeneity = 0.51, n = 4), stroke (RR: 1.00; 95% CI 0.98 to 1.01, I2 = 0%, pheterogeneity = 0.51, n = 4), or HF (RR: 0.86; 95% CI 0.68 to 1.09, n = 1) (Supplemental Figure 4).
No evidence of heterogeneity was detected between subgroups in stratified analyses (high vs. low meta-analysis) on refined grains and stroke (Supplemental Table 15).
There was no evidence of a non-linear dose-response association between refined grains and CHD (pnon-linearity = 0.25, n = 4 studies), or stroke (pnon-linearity = 0.42, n = 4 studies) ( and ).
Vegetables
Nineteen studies with 19,402 cases were included in the high vs. low intake meta-analysis for vegetables and CHD (overall intake range: 0–1,300 g/d), 16 studies with 12,442 cases for stroke (overall intake range: 0–500 g/d), and 3 studies with 6,267 cases for HF (overall intake range: 100–460 g/d).
Comparing the highest to the lowest categories of vegetable intake, an inverse association was observed for risk of CHD (RR: 0.92; 95% CI 0.87 to 0.98, I2 = 42%, pheterogeneity = 0.03) and stroke (RR: 0.87; 95% CI 0.82 to 0.93, I2 = 38%, pheterogeneity = 0.06), while no association was observed for risk of HF (RR: 0.99; 95% CI 0.82 to 1.18, I2 = 80%, pheterogeneity = 0.007) (Supplemental Figure 5).
Each additional daily 100 g of vegetables were inversely associated with risk of CHD (RR: 0.97; 95% CI 0.96 to 0.99, I2 = 12%, pheterogeneity = 0.32, n = 14), stroke (RR: 0.92; 95% CI 0.86 to 0.98, I2 = 79%, pheterogeneity<0.001, n = 10), and HF (RR: 0.96; 95% CI 0.94 to 0.98, n = 1) (Supplemental Figure 6).
No evidence of heterogeneity was detected between subgroups in stratified analyses (high vs. low meta-analysis) on vegetables and CHD and stroke (Supplemental Table 16a-b). In stratified analyses (dose-response meta-analysis) the inverse association between vegetable intake and CHD risk was no longer significant in men, in studies conducted in Europe, Asia and Australia, in smaller studies (<1,000 cases), and studies using non-validated dietary assessment methods, possibly due to limited number of studies (Supplemental Table 16c). The heterogeneity of studies included in dose-response analysis on vegetable intake and stroke persisted in additional analyses stratified by subgroups (Supplemental Table 16d). Evidence for an inverse association was observed in men (no studies in women only), in long-term and European, Asian and Australian studies. No evidence of heterogeneity was detected between subgroups in stratified analyses.
There was evidence for small study effects in the high vs. low (p = 0.03), but not in the dose-response analysis for CHD. No evidence for small study effects was observed for stroke. Visual inspection of the funnel plots (dose-response analysis) suggested little asymmetry for CHD (Supplemental Figure 25), but not for stroke (Supplemental Figure 26).
There was no evidence of a non-linear dose-response association for CHD (pnon-linearity = 0.19, n = 13 studies), but there was for stroke (pnon-linearity = 0.03, n = 8 studies). The risk of CHD and stroke decreased by approximately 12% with increasing intake of vegetables up to ∼400 g/d. Additional benefit for increasing intake is apparent above this value for CHD but not for stroke ( and ).
Fruits
Seventeen studies with 17,827 cases were included in the high vs. low intake meta-analysis for fruits and CHD (overall intake range: 0–1,820 g/d), 17 studies with 30,523 cases for stroke (overall intake range: 0–595 g/d), and 3 studies with 6,267 cases for HF (overall intake range: 50–300 g/d) for HF.
Comparing the highest to the lowest categories of fruit intake, an inverse association was observed for risk of CHD (RR: 0.89; 95% CI 0.84 to 0.93, I2 = 10%, pheterogeneity = 0.34), stroke (RR: 0.83; 95% CI 0.77 to 0.89, I2 = 40%, pheterogeneity = 0.05), and risk of HF (RR: 0.95; 95% CI 0.88 to 1.02, I2 = 0%, pheterogeneity = 0.73) (Supplemental Figure 7).
Each additional daily 100 g of fruits were inversely associated with risk of CHD (RR: 0.94; 95% CI 0.90 to 0.97, I2 = 71%, pheterogeneity<0.001, n = 13) and stroke (RR: 0.90; 95% CI 0.84 to 0.97, I2 = 86%, pheterogeneity<0.001, n = 10), but not with risk of HF (RR: 0.98; 95% CI 0.94 to 1.01, n = 1) (Supplemental Figure 8).
No evidence of heterogeneity was detected between subgroups in stratified analyses (high vs. low meta-analysis) on fruits and CHD (Supplemental Table 17a), whereas some evidence of heterogeneity was observed for stroke (follow-up and geographic location) (Supplemental Table 17b). In analyses stratified by subgroups (dose-response meta-analysis), the inverse association between fruit intake and CHD risk was not observed in studies including only women, in European, Asian and Australian studies, in studies with <1,000 cases, and studies using non-validated dietary assessment (Supplemental Table 17c). These subgroup-differences were not statistically significant, with one exception; after stratification by included number of cases, heterogeneity was observed (borderline significant p = 0.05), showing an inverse association between fruit intake and risk of CHD only for studies including ≥1,000 cases. Referring to stroke, we observed evidence of heterogeneity in subgroups stratified for geographical location, showing greatest risk reduction for studies conducted in Asia and Australia (Supplemental Table 17d).
There was evidence for small study effects, in the high vs. low (p = 0.08) analysis for stroke, and in the dose-response analysis for CHD (p = 0.04). Visual inspection of the funnel plots (dose-response analysis) suggested little symmetry (Supplemental Figure 27 and 28).
There was evidence of a non-linear dose-response association for CHD (pnon-linearity<0.001, n = 12 studies), but not for stroke (pnon-linearity = 0.54, n = 8 studies). The risk of CHD and stroke decreased by approximately 15% and 20% with increasing intake of fruits up to ∼200 g/d, respectively. No benefit for increasing intake was apparent above this value ( and ).
Nuts
Four studies with 5,480 cases were included in the high vs. low intake meta-analysis for nuts and CHD (overall intake range: 0–28 g/d), 6 studies with 7,490 cases for stroke (overall intake range: 0–38 g/d) and 3 studies with 3,613 cases for HF (overall intake range: 0–30 g/d).
Comparing the highest to the lowest categories of nut intake revealed a trend for an inverse association between nut intake and risk of CHD (RR: 0.80; 95% CI 0.62 to 1.03, I2 = 79%, pheterogeneity = 0.002), which was not present in the case of stroke (RR: 0.94; 95% CI 0.85 to 1.05, I2 = 18%, pheterogeneity = 0.30) and HF (RR: 0.99; 95% CI 0.86 to 1.15, I2 = 57%, pheterogeneity = 0.10) (Supplemental Figure 9).
An inverse association was observed for each additional daily 28 g of nuts and risk of CHD (RR: 0.67; 95% CI 0.43 to 1.05, I2 = 85%, pheterogeneity = 0.001, n = 4), but not for stroke (RR: 0.99; 95% CI 0.84 to 1.17, I2 = 45%, pheterogeneity = 0.11, n = 6) or HF (RR: 1.09; 95% CI 0.97 to 1.22, I2 = 0%, pheterogeneity = 0.79, n = 2) (Supplemental Figure 10).
No important evidence of heterogeneity was detected between subgroups in stratified analyses on nut consumption and risk of stroke in the categorical and dose-response meta-analysis (Supplemental Table 18a-b).
There was evidence of a non-linear dose-response association for CHD (pnon-linearity<0.001, n = 4 studies) and stroke (pnon-linearity = 0.05, n = 5 studies). The risk of CHD decreased by approximately 21% with increasing intake of nuts up to ∼10-15 g/d. No benefit for increasing intake was apparent above this value (). No association was observed for nut intake and risk of stroke in the non-linear dose-response analysis ().
Legumes
For legumes, 10 studies with 8,228 cases were included in the high vs. low intake meta-analysis for CHD (overall intake range: 0–230 g/d), and 6 studies with 6,333 cases for stroke (overall intake range: 0–60 g/d). No study was available on HF.
Comparing the highest to the lowest categories of legume intake, an inverse association between legume intake and risk of CHD (RR: 0.91; 95% CI 0.84 to 0.99, I2 = 33%, pheterogeneity = 0.14), but not with risk of stroke (RR: 0.98; 95% CI 0.88 to 1.10, I2 = 56%, pheterogeneity = 0.04) was observed (Supplemental Figure 11).
A small inverse association was observed for each additional daily intake of 50 g of legumes and risk of CHD (RR: 0.96; 95% CI 0.92 to 1.01, I2 = 39%, pheterogeneity = 0.12, n = 8), but not for stroke (RR: 1.00; 95% CI 0.88 to 1.13, I2 = 62%, pheterogeneity = 0.02, n = 6) (Supplemental Figure 12).
No association was observed in any of the stratified analyses, neither for CHD nor for stroke (Supplemental Table 19a-d).
No evidence for small study effects was observed in the high vs. low intake analysis of legumes and risk of CHD. Some evidence of a non-linear dose-response association was observed for CHD (pnon-linearity = 0.07, n = 8 studies), but not for stroke (pnon-linearity = 0.08, n = 5 studies). The risk of CHD decreased by approximately 10% with increasing intake of legumes up to ∼100 g/d (). No benefit for increasing intake was apparent above this value (). No association was observed for legume intake and risk of stroke in the non-linear dose-response analysis ().
Eggs
For eggs, 11 studies with 14,370 cases were included in the high vs. low intake meta-analysis (overall intake range: 0–75 g/d) for CHD, 10 studies with 12,735 cases (overall intake range: 0–75 g/d) for stroke, and 4 studies with 5,059 cases (overall intake range: 0–140 g/d) for HF.
Comparing the highest to the lowest categories of egg intake, no association between egg intake and risk of CHD (RR: 0.99; 95% CI 0.94 to 1.05, I2 = 0%, pheterogeneity = 0.49) or risk of stroke (RR: 0.99; 95% CI 0.93 to 1.05, I2 = 3%, pheterogeneity = 0.41) was observed. A positive association between egg intake and risk of HF (RR: 1.25; 95% CI 1.12 to 1.39, I2 = 0%, pheterogeneity = 0.46) was detected (Supplemental Figure 13).
In the dose-response meta-analysis, there was no association between each increment of 50 g of daily egg intake and risk of CHD (RR: 1.00; 95% CI 0.95 to 1.06, I2 = 0%, pheterogeneity = 0.93, n = 9) or stroke (RR: 0.99; 95% CI 0.93 to 1.05, I2 = 0%, pheterogeneity = 0.44, n = 10), but with risk of HF (RR: 1.16; 95% CI 1.03 to 1.31, I2 = 55%, pheterogeneity = 0.08, n = 4) (Supplemental Figure 14).
No association was observed in any of the stratified analyses, neither for eggs and CHD nor for eggs and stroke (Supplemental Table 20a-d).
No evidence for small study effects was observed in the high vs. low intake meta-analysis for eggs and CHD, as was also the case for stroke both in the high vs. low and dose-response analysis. Visual inspection of the funnel plots (dose-response analysis) suggests moderate asymmetry (Supplemental Figure 29).
There was no evidence of a non-linear dose-response association for CHD (pnon-linearity = 0.81, n = 9 studies), and stroke (pnon-linearity = 0.39, n = 9 studies) ( and ), but there was for HF (pnon-linearity = 0.04, n = 3 studies). The risk of HF increased by approximately 50% with increasing intake of egg up to ∼100 g/d (Supplemental Figure 34). No association was observed for egg intake and risk of CHD and stroke in the non-linear dose-response analysis.
Dairy
Thirteen studies with 15,790 cases were included in the high vs. low intake meta-analysis for dairy and CHD (overall intake range: 0–3,000 g/d), 12 studies with 16,887 cases for stroke (overall intake range: 0–1,860 g/d), and 3 studies with 4,057 cases for HF (overall intake range: 0–1,390 g/d).
Comparing the highest to the lowest categories of dairy intake, no associations between dairy intake and risk of CHD (RR: 0.99; 95% CI 0.92 to 1.07, I2 = 59%, pheterogeneity = 0.004), stroke (RR: 0.96; 95% CI 0.90 to 1.01, I2 = 43%, pheterogeneity = 0.05), or HF (RR: 1.00; 95% CI 0.90 to 1.10, I2 = 67%, pheterogeneity = 0.05) were observed (Supplemental Figure 15).
Each additional daily 200 g of dairy were not associated with risk of CHD (RR: 0.99; 95% CI 0.96 to 1.02, I2 = 55%, pheterogeneity = 0.02, n = 10) or stroke (RR: 0.98; 95% CI 0.96 to 1.00, I2 = 50%, pheterogeneity = 0.03, n = 11), but were positively associated with risk of HF (RR: 1.08; 95% CI 1.01 to 1.15, n = 1) (Supplemental Figure 16).
No association between dairy intake and risk of CHD was observed in any of the stratified analyses (Supplemental Table 21a and c). Referring to stroke, we observed heterogeneity in subgroups stratified for sex (dose-response) and geographical location, showing an inverse association in women and in American studies only (Supplemental Table 21b and d).
Comparing low-fat and high-fat dairy products, no significant differences could be observed for CHD and stroke.
There was no evidence of small study effects in the high vs. low and dose-response meta-analysis for dairy and CHD, and for dairy and stroke. Visual inspection of the funnel plots (dose-response analysis) suggests moderate symmetry (Supplemental Figure 30 and 31).
There was evidence of a non-linear dose-response association between dairy products and CHD (pnon-linearity<0.01, n = 9 studies), but not for stroke (pnon-linearity = 0.71, n = 8 studies). The risk of stroke decreased by approximately 5% with increasing intake of dairy up to ∼500 g/d (), whereas no association was observed for CHD ().
Fish
For fish, 22 studies with 16,732 cases were included in the high vs. low intake meta-analysis (overall intake range: 0–320 g/d) for CHD, 20 studies with 14,360 cases (overall intake range: 0–130 g/d) for stroke, and 8 studies with 7,945 cases (overall intake range: 0–80 g/d) for HF.
Comparing the highest to the lowest categories, a small inverse association between fish intake and risk of CHD (RR: 0.94; 95% CI 0.88 to 1.02, I2 = 52%, pheterogeneity = 0.003) or stroke (RR: 0.95; 95% CI 0.89 to 1.01, I2 = 37%, pheterogeneity = 0.05), and a stronger inverse association between fish intake and risk of HF (RR: 0.89; 95% CI 0.80 to 0.99, I2 = 18%, pheterogeneity = 0.29) was observed (Supplemental Figure 17).
Each additional daily 100 g of fish were inversely associated with risk of CHD (RR: 0.88; 95% CI 0.79 to 0.99, I2 = 40%, pheterogeneity = 0.06, n = 15), stroke (RR: 0.86; 95% CI 0.75 to 0.99, I2 = 25%, pheterogeneity = 0.18, n = 15), and HF (RR: 0.80; 95% CI 0.67 to 0.95, I2 = 20%, pheterogeneity = 0.28, n = 7) (Supplemental Figure 18).
No important evidence of heterogeneity was detected between subgroups in stratified analyses (high vs. low meta-analysis) on fish consumption and risk of CHD and stroke (Supplemental Table 22a-b). We observed heterogeneity in subgroups (dose-response meta-analysis) stratified for sex, showing an inverse association between fish intake and CHD solely in the study including women only (Supplemental Table 22c). Referring to stroke (dose-response meta-analysis), there was an inverse association observed in studies including women only, long-term studies and studies using validated dietary assessment (Supplemental Table 22d). These subgroup-differences were not statistically significant.
There was no evidence of small study effects in the high vs. low analysis for CHD or in the dose-response meta-analysis for CHD and stroke, but there was in the high vs. low analysis for stroke (p = 0.07). Visual inspection of the funnel plots (dose-response analysis) suggests little symmetry (Supplemental Figure 32 and 33).
There was no evidence of a non-linear dose-response association between fish intake and CHD (pnon-linearity = 0.10, n = 15 studies), stroke (pnon-linearity = 0.37, n = 15 studies), or HF (pnon-linearity = 0.14, n = 6 studies). The risk of CHD decreased by approximately 15% with increasing intake of fish up to ∼250 g/d (), whereas the risk of stroke and HF decreased by approximately 10 and 20% with increasing intake of fish up to ∼80–100 g/d, respectively ( and Supplemental Figure 35).
Red meat
For red meat, 3 studies with 6,659 cases were included in the high vs. low intake meta-analysis (overall intake range: 9–205 g/d) for CHD, 7 studies with 10,541 cases (overall intake range: 6–195 g/d) for stroke, and 5 studies with 9,229 cases (overall intake range: 0–175 g/d) for HF.
Comparing the highest to the lowest categories, a positive association between red meat intake and risk of CHD (RR: 1.16; 95% CI 1.08 to 1.24, I2 = 0%, pheterogeneity = 0.89), stroke (RR: 1.16; 95% CI 1.08 to 1.25, I2 = 0%, pheterogeneity = 0.69), and HF (RR: 1.12; 95% CI 1.04 to 1.21, I2 = 26%, pheterogeneity = 0.25) was observed (Supplemental Figure 19).
In the dose-response meta-analysis, each additional daily 100 g of red meat were positively associated with risk of CHD (RR: 1.15; 95% CI 1.08 to 1.23, I2 = 0%, pheterogeneity = 0.68, n = 3), stroke (RR: 1.12; 95% CI 1.06 to 1.17, I2 = 0%, pheterogeneity = 0.50, n = 7), and HF (RR: 1.08; 95% CI 1.02 to 1.14, I2 = 4%, pheterogeneity = 0.37, n = 4) (Supplemental Figure 20).
No evidence of heterogeneity was detected between subgroups in stratified analyses on red meat and stroke. The observed positive association between red meat intake and stroke risk was not seen in studies including both women and men, short-term studies, European studies and studies including <1,000 stroke cases (Supplemental Table 23a-b).
There was evidence of a non-linear dose-response association between red meat and CHD (pnon-linearity<0.01, n = 2 studies), and HF (pnon-linearity = 0.02, n = 3 studies), but not for stroke (pnon-linearity = 0.91, n = 6 studies). The risk of CHD, stroke, and HF increased by approximately 10–20% with increasing intake of red meat up to ∼100 g/d (, , and Supplemental Figure 36).
Processed meat
For processed meat, 5 studies with 7,038 cases were included in the high vs. low intake meta-analysis (overall intake range: 0–150 g/d) for CHD, 6 studies with 9,492 cases (overall intake range: 0–85 g/d) for stroke, and 3 studies with 7,077 cases (overall intake range: 12–90 g/d) for HF.
Comparing the highest to the lowest categories, a positive association between processed meat intake and risk of CHD (RR: 1.15; 95% CI 0.99 to 1.33, I2 = 44%, pheterogeneity = 0.13), stroke (RR: 1.16; 95% CI 1.07 to 1.26, I2 = 12%, pheterogeneity = 0.34), and HF (RR: 1.27; 95% CI 1.14 to 1.41, I2 = 0%, pheterogeneity = 0.87) was observed (Supplemental Figure 21).
Each additional daily 50 g of processed meat were positively associated with risk of CHD (RR: 1.27; 95% CI 1.09 to 1.49, I2 = 0%, pheterogeneity = 0.51, n = 3), stroke (RR: 1.17; 95% CI 1.02 to 1.34, I2 = 56%, pheterogeneity = 0.05, n = 6), and HF (RR: 1.12; 95% CI 1.05 to 1.19, I2 = 0%, pheterogeneity = 0.62, n = 2) (Supplemental Figure 22).
Referring to stroke, there was evidence of heterogeneity in subgroups stratified for geographical location, showing a stronger association in studies conducted in America compared to studies conducted in Europe (pheterogeneity = 0.02; Supplemental Table 24a-b).
There was no evidence of a non-linear dose-response association between processed meat and CHD (pnon-linearity = 0.41, n = 2 studies), stroke (pnon-linearity = 0.65, n = 6 studies), or HF (pnon-linearity = 0.62, n = 2 studies). The risk of stroke increased by approximately 15% (), and the risk of HF by 25% with increasing intake of processed meat up to ∼70 g/d (, and Supplemental Figure 37). No associations were observed for processed meat and CHD ().
Sugar-sweetened beverages
Five studies with 8,740 cases were included in the high vs. low intake meta-analysis for SSB and CHD (overall intake range: 0–650 g/d), 7 studies with 11,187 cases for stroke (overall intake range: 0–2500 g/d), and 2 studies with 8,603 cases for HF (overall intake range: 0–625 g/d).
Comparing the highest to the lowest categories, a positive association between SSB intake and risk of CHD (RR: 1.10; 95% CI 1.01 to 1.20, I2 = 50%, pheterogeneity = 0.09) and stroke (RR: 1.09; 95% CI 1.01 to 1.18, I2 = 0%, pheterogeneity = 0.43), but no association with HF risk (RR: 1.11; 95% CI 0.88 to 1.39, I2 = 81%, pheterogeneity = 0.02) were observed (Supplemental Figure 23).
Each additional daily 250 ml of SSB were positively associated with risk of CHD (RR: 1.17; 95% CI 1.11 to 1.23, I2 = 0%, pheterogeneity = 0.66, n = 4), stroke (RR: 1.07; 95% CI 1.02 to 1.12, I2 = 0%, pheterogeneity = 0.59, n = 6), and HF (RR: 1.08; 95% CI 1.05 to 1.12, n = 1) (Supplemental Figure 24).
Referring to stroke, the positive association with SSB intake was no longer statistically significant in studies including women or both women and men, in an Asian and Australian study and in a study including <1,000 stroke cases. However, no evidence for subgroup-differences was observed (Supplemental Table 25a-b).
There was no evidence of a non-linear dose-response association between SSB and CHD (pnon-linearity = 0.83, n = 4 studies), or stroke (pnon-linearity = 0.83, n = 6 studies). The risk of CHD increased by approximately 35%, and the risk of stroke by 16% with increasing intake of SSB up to ∼500 ml/d ( and ).
Summary across food groups
and show the risk ratios for CHD and stroke from the non-linear dose-response analyses of the 12 pre-definded food groups according to servings/day. Optimal consumption (lowest serving with significant results and no further substantial change in CHD risk or no further data for higher amounts) of risk-decreasing foods would result in a 65% reduction in CHD, and a 40% reduction in stroke risk (calculated by ) compared to non-consumption of these foods.
Table 1. Relative risks from non-linear dose-response analysis of 12 pre-defined food groups and risk of coronary heart disease according to intakes of servings/day. NA, not applicable.
Table 2. Relative risks from non-linear dose-response analysis of 12 pre-defined food groups and risk of stroke according to intakes of servings/day. NA, not applicable.
Furthermore, shows that increasing the daily consumption of foods with an inverse relation to risk beyond 3 servings/d of whole grains (90 g/d), 3 servings/d of fruits (∼250 g/d), and 1 serving/d (∼100 g/d) of legumes would not further reduce the risk of CHD.
We could further calculate that a consumption of risk-increasing food of 2 servings/d of red meat (170 g, RR = 1.20), 2 servings/d of processed meat (60 g, RR = 1.16), and 2 servings/d of SSB (500 ml, RR = 1.16) was associated with a 60% increased risk of stroke () compared to non-consumption. Not consuming these foods would reduce the risk of stroke of about 38% (calculated by
).
Risk of bias and sensitivity analysis
The results varied little when including only studies with a low risk of bias (Supplemental Table 26). In the sensitivity analysis for linear dose-response meta-analysis, findings including studies with low risk of bias suggested a weaker inverse association for intake of fruits and a stronger inverse association for whole grain intake regarding risk of CHD. The inverse association between fish intake and CHD was not longer significant in the linear dose-response meta-analysis including studies with low risk of bias. For risk of stroke, stronger inverse associations were also observed for intake of fruits and fish.
In the sensitivity analyses excluding the three identified nested-case control studies (Neelakantan et al. Citation2016; Wennberg et al. Citation2011; Wennberg et al. Citation2007), and studies reporting odds ratios (Holmberg et al. Citation2009; Wennberg et al. Citation2011; Wennberg et al. Citation2007) no changes in the results of the main analysis were detected.
Quality of meta-evidence
We rated the quality of meta-evidence for the 12 food groups regarding risk of CHD, stroke and HF. The NutriGrade meta-evidence grading regarding CHD was rated “low” for refined grains, and eggs and “moderate” for the 10 other food groups. The NutriGrade meta-evidence grading regarding stroke was rated “low” for whole grains, refined grains, nuts, and legumes and “moderate” for the eight other food groups. Regarding HF, the NutriGrade meta-evidence grading was rated “very low” for refined grains and “low” for whole grains, vegetables, fruits, nuts, dairy, and SSB and “moderate” for eggs, fish, red meat, and processed meat (Supplemental Table 27a-c).
Discussion
The associations between 12 a priori defined food groups and risk of CHD, stroke, and HF were systematically assessed in this meta-analysis through comparison of the highest to the lowest categories and dose-response analyses both for linear and non-linear relationships. In the linear dose-response meta-analysis, an inverse association was present for whole grain (CHD, HF), vegetable (CHD, stroke, HF), fruit (CHD, stroke), nut (CHD), legumes (CHD), and fish (CHD, stroke, HF) consumption, while a positive association was present for egg (HF), dairy (HF), red meat (CHD, stroke, HF), processed meat (CHD, stroke, HF), and SSB (CHD, stroke, HF) consumption. There was clear indication for non-linearity between intake of whole grains, fruits, nuts, dairy, red meat and risk of CHD, and intake of vegetables and risk of stroke. The NutriGrade tool for evaluating the meta-evidence suggested a moderate confidence in the effect estimate for eggs, dairy, fish, red meat, and processed meat with respect to all three outcomes simultaneously; the confidence in the effect estimate for the other relationships was lower. The maximum moderate quality of evidence implies that further research could or will add evidence on the confidence and may change the effect estimate.
Our findings are in line with previous meta-analyses conducted mostly on single food groups and not synoptically for 12 food groups and often not separately for individual CVD outcomes. Moreover, this is to our knowledge, the first meta-analysis that investigated the association between whole grain intake and HF, showing an inverse association. The observed inverse association between whole grain intake and risk of CHD is consistent with results from previous meta-analyses on CHD (Anderson et al. Citation2000; Aune et al. Citation2016b; Mellen, Walsh, and Herrington Citation2008; Mente et al. Citation2009; Tang et al. Citation2015). The essential nutrients along with the phytonutrients and fibre present in whole grains may synergistically contribute to their beneficial effects. A number of mechanisms have been suggested for the risk reduction associated with whole grains; for example, reduction of LDL-cholesterol concentrations through soluble fiber; the antioxidant and anti-inflammatory properties, possibly due to the presence of polyphenols and other phytonutrients; modulation of blood glucose and insulin responses, improving vascular function and blood pressure, and weight control (Jonnalagadda et al. Citation2011).
For vegetables and fruits, our results are similar to previous meta-analyses that revealed an inverse association between intake and CHD (Gan et al. Citation2015; Mente et al. Citation2009) (Dauchet et al. Citation2006; He et al. Citation2007), and stroke risk (Aune et al. Citation2017; He et al. Citation2006; Hu et al. Citation2014). While we detected no further risk reduction beyond intake of 250 g fruits per day, another current meta-analysis showed reductions in risk up to 800 g per day. This difference could be explained by the greater number of studies (due to inclusion of studies on cause-specific mortality and overall CVD) included by Aune et al. (Aune et al. Citation2017). Vegetables and fruits, like the nutrients and phytochemicals therein, influence not only inflammatory processes, but also cellular redox processes as well as endothelial and metabolic processes; it is assumed that these mechanisms are primarily responsible for the risk-reducing association between vegetable and fruit consumption and risk of cardiovascular and other diseases (Boeing et al. Citation2012).
We observed a trend for a strong inverse association between nut consumption and risk of CHD. In line with this, previous analyses found that higher nut consumption is associated with reduced CHD risk (Afshin et al. Citation2014; Aune et al. Citation2016a; Kelly and Sabate Citation2006; Ma et al. Citation2014; Mayhew et al. Citation2016; Mente et al. Citation2009; Weng et al. Citation2016). An umbrella review found an inverse association of nut intake and risk of CVD, hypertension, and lower levels of total cholesterol and LDL-cholesterol. Plausible mechanisms how the consumption of nuts can affect cardiovascular risk are through their content of unsaturated fatty acids, protein, fiber, vitamin E, potassium, magnesium, and phytochemicals (Schwingshackl et al. Citation2017d).
Despite the absence of a linear association between legumes and investigated outcomes, the non-linear analysis showed a 10% reduction in CHD risk when consuming up to 100 g/d. Results from previous analyses provide moderate evidence for a benefit on CHD (Afshin et al. Citation2014; Lou et al. Citation2016; Marventano et al. Citation2017; Yan et al. Citation2017). Legumes are good sources of fiber, protein, and some bioactive compounds such as phytosterols. These components have shown inverse associations with total cholesterol, triacylglycerol and LDL-cholesterol concentrations, and a positive association with HDL-cholesterol concentrations in pooled analyses of intervention trials (Anderson and Bush Citation2011). Other bioactive compounds in legumes are protein-derived bioactive peptides that have been found to have blood pressure- and cholesterol-lowering effects, as well as antithrombotic and antioxidant activities in in vitro studies (Malaguti et al. Citation2014).
We observed a positive association between egg consumption and HF risk, as is the case in another current analysis based on the same 4 cohort studies, but not performing dose-response analysis (Khawaja et al. Citation2017). Eggs are a major source of dietary choline and consumption results in increased exposure to trimethylamine-N-oxide, a metabolite linked to atherosclerosis (Miller et al. Citation2014; Senthong et al. Citation2016). At the same time, egg yolks are a source of bioavailable xanthophyll carotenoids with anti-inflammatory, anti-oxidative and anti-atherosclerotic effects that may possibly promote cardiovascular health (Clayton et al. Citation2017). Because definite mechanisms of possible effects of eggs and observed relationships are not clear and vary regarding individual outcomes, respectively, the association between egg consumption and HF should be interpreted with caution.
Authors of a previous meta-analysis suggested an inverse association between dairy products consumption and risks of CHD and stroke, but demanded additional data to more comprehensively examine potential dose-response patterns (Alexander et al. Citation2016). In linear dose-response analysis we observed an 8% increased HF risk by each additional daily 200 g of dairy. Despite the absence of a linear association between dairy intake and stroke, the non-linear analysis showed a 5% reduction in stroke risk when consuming up to 500 g/d. A recent meta-analysis observed no association between dairy intake and CHD incidence, too (Gholami et al. Citation2017). Dairy products are a rich source of minerals, such as calcium, vitamins and protein; all of these nutrients may have a beneficial effect on cardiovascular health. Otherwise, dairy products contain saturated fatty acids, and the potential role of saturated fatty acids and cardiovascular health is controversial (Mozaffarian Citation2016). Furthermore, there is some evidence that dairy products, especially those that are fermented, are associated with reduced risk of adiposity (Schwingshackl et al. Citation2016b). Due to inconclusive findings, recommendations on dairy consumption relating to cardiovascular health can-not be given yet.
Our analysis confirmed the well-established risk-decreasing potential of fish on CHD (Leung Yinko et al. Citation2014; Mente et al. Citation2009; Whelton et al. Citation2004), on stroke (Bouzan et al. Citation2005; Chowdhury et al. Citation2012; He et al. Citation2004; Larsson and Orsini Citation2011; Xun et al. Citation2012), and on HF (Djousse et al. Citation2012; Li et al. Citation2013). The non-linear analyses showed that CHD risk was reduced by 15% with increasing fish consumption up to 250 g/d and a 10 and 20% reduction in risk of stroke and HF, respectively, with up to 80–100 g/d. This association is biologically plausible through effects of long chain n-3 poly-unsaturated fatty acids, which are abundant in fatty fish and have been associated with anti-atherosclerotic and anti-thrombotic effects (Chapkin et al. Citation2007; Saravanan et al. Citation2010). In observational studies, very-long chain n-3 poly-unsaturated fatty acids can be used as valid markers for habitual fish intake (Amiano et al. Citation2001). With this in mind, a large meta-analysis of observational studies showed that circulating long-chain n-3 poly-unsaturated fatty acids were associated with lower risk of coronary disease (Chowdhury et al. Citation2014). Although, the risk-decreasing potential of fish is well established and confirmed by the results of our meta-analysis, the unavoidable presence of environmental contaminants (Domingo Citation2016) and aspects of sustainability should be also taken into account, if larger amounts were consumed.
We observed positive associations between red meat intake and CHD, stroke, and HF risk. Consuming red meat up to 150 g/d is associated with a 10–20% increased risk in non-linear dose-response analyses. Furthermore, we observed positive associations between processed meat intake and stroke, and HF risk. The non-linear analyses showed a 15% and 25% increased risk of stroke and HF, respectively, consuming processed meat up to 70 g/d. A positive association has been previously reported between consumption of red and especially processed meat and CHD and stroke risk, but, to our knowledge, it has not been reported on HF risk (Chen et al. Citation2013; Kaluza et al. Citation2012; Mente et al. Citation2009; Micha et al. Citation2012; Micha et al. Citation2010; Yang et al. Citation2016). Unfavorable associations with food groups such as red and processed meat might be based upon pro-inflammatory or pro-oxidative compounds, triggered by nitrosamines, iron, or saturated fatty acids (Mozaffarian Citation2016).
There is evidence that SSB intake is related to vascular risk factors, whereas associations with CVD were less consistent (Keller et al. Citation2015) and possibly a consequence of SSB consumption being a surrogate for adverse health behaviours (Narain et al. Citation2016). Our meta-analyses add moderate evidence for a risk-increasing association between SSB intake and CHD and stroke. The non-linear analyses show a 35% increased risk of CHD and a 16% increased risk of stroke by consuming up to 500 ml/d. The risk-increasing association observed for SSB may be partly attributed to impairments of the otherwise working regulation of hunger and satiety (DiMeglio and Mattes Citation2000). Evidence of a positive association between SSB consumption and weight gain and obesity in adults is strong (Malik et al. Citation2013); obesity is an important risk factor for CVD.
The results of the present meta-analysis add further scientific evidence supporting the inclusion of some food groups into food-based dietary guidelines aimed at CVD prevention. Optimal consumption of whole grains, vegetables, fruits, nuts, legumes, dairy, and fish was associated with a 65% reduced risk of CHD and optimal consumption of vegetables, fruits, dairy, and fish was associated with a 40% stroke reduction. Reducing the consumption of the risk-increasing food groups red meat, processed meat, SSB, eggs and dairy in relation to HF is preventive, too.
Our results support current food-based dietary guidelines that promote the consumption of a dietary pattern that emphasizes intake of vegetables, fruits, and whole grains and includes, among other foods, legumes, nuts and fish, while it limits intake of red meat and processed meat and SSB as a “heart healthy” diet (Eckel et al. Citation2014; Perk et al. Citation2012; USDA Citation2015). Our results however do not support the recommendation to include low-fat dairy products. To obtain a complete picture it seems useful to extend the type of food groups to be considered.
Strengths and limitations
Among the strengths of the present meta-analysis are the a priori published systematic review protocol (Schwingshackl et al. Citation2016a), the comprehensive literature search, the large number of included prospective studies, cases, and food groups. Furthermore, we performed different types of analyses (high vs. low, linear and non-linear dose-response meta-analyses, subgroup and sensitivity analyses), which allowed us to detect associations where the relation was non-linear and find an optimal consumption with the lowest risk. Finally, it is a strength of this study that the quality of meta-evidence for each food group was assessed with the NutriGrade scoring system.
A limitation of this systematic review is that for some of the included studies, only baseline food intake could be used (assuming a stable consumption over time). Furthermore, there was substantial heterogeneity with respect to the analyzed population size, follow-up length, baseline age, and food consumption. We conducted subgroup analyses for sex, length of follow-up, geographic location, number of cases, and dietary assessment methods to explore high degrees of statistical heterogeneity. Overall, for most food groups high levels of statistical heterogeneity persisted in subgroup analyses. People with a high intake of whole grains, vegetables, fruits, nuts, legumes or fish might have different lifestyles, or socioeconomic status compared to those with lower intakes, representing important confounders (Darmon and Drewnowski Citation2008). However, in sensitivity analyses including only studies with a low risk of bias (adjusted for important lifestyle factors: smoking, physical activity, body mass index), the results of the primary analysis were confirmed. For most food groups in association with HF, a very limited amount of studies was found. Therefore, some of these results should be interpreted with caution. Finally, another major limitation explaining differences between results of our and other meta-analyses on specific food groups could be that we did not include prospective studies on overall CVD and on outcome-specific mortality.
Conclusion
An optimal intake of whole grains, vegetables, fruits, nuts, legumes, dairy, fish, red and processed meat, eggs, and SSB showed an important lower risk of CHD, stroke, and HF. Even though quality of meta-evidence according to NutriGrade is moderate, the results of this comprehensive meta-analysis may serve as key component in deriving food-based dietary guidelines for prevention of CVD.
Conflict of interest
No conflict of interest to declare
Authors' contributions
AB, LS, SS, KI, GH, HB contributed to the conception and design of the systematic review and meta-analysis. LS, AB were involved in the acquisition and analysis of the data. AB, LS, SK, KI, GH, HB interpreted the results. AB, LS, CS, GH, SK, SS, KI, HB, BD, NM, SDH drafted this paper. All authors provided critical revisions of the meta-analysis and approved submission of the final manuscript.
Supplemental_Material.pdf
Download PDF (3 MB)References
- Afshin, A., R. Micha, S. Khatibzadeh, and D. Mozaffarian. 2014. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am J Clin Nutr 100:278–288. doi:10.3945/ajcn.113.076901.
- Albert, C. M., J. M. Gaziano, W. C. Willett, and J. E. Manson. 2002. Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Arch Intern Med 162:1382–87. doi:10.1001/archinte.162.12.1382.
- Albert, C. M., C. H. Hennekens, C. J. O'Donnell, U. A. Ajani, V. J. Carey, W. C. Willett, J. N. Ruskin, and J. E. Manson. 1998. Fish consumption and risk of sudden cardiac death. JAMA 279:23–28. doi:10.1001/jama.279.1.23.
- Alexander, D. D., L. C. Bylsma, A. J. Vargas, S. S. Cohen, A. Doucette, M. Mohamed, S. R. Irvin, P. E. Miller, H. Watson, and J. P. Fryzek. 2016. Dairy consumption and CVD: A systematic review and meta-analysis. Br J Nutr 115:737–750. doi:10.1017/S0007114515005000.
- Amiano, P., S. Chamosa, N. Etxezarreta, L. Arriola, C. Moreno-Iribas, J. M. Huerta, N. Egues, M. Guevara, C. Navarro, M. D. Chirlaque, M. J. Sanchez, E. Molina-Montes, M. Requena, J. R. Quiros, M. Obon-Santacana, P. Jakszyn, C. A. Gonzalez, and M. Dorronsoro. 2016a. No association between fish consumption and risk of stroke in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain): a 13.8-year follow-up study. Public Health Nutr 19:674–681. doi:10.1017/S1368980015001792.
- Amiano, P., S. Chamosa, N. Etxezarreta, L. Arriola, M. J. Sanchez, E. Ardanaz, E. Molina-Montes, M. D. Chirlaque, C. Moreno-Iribas, J. M. Huerta, N. Egues, C. Navarro, M. Requena, J. R. Quiros, A. Fonseca-Nunes, P. Jakszyn, C. A. Gonzalez, and M. Dorronsoro. 2016b. Unprocessed red meat and processed meat consumption and risk of stroke in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 70:313–319. doi:10.1038/ejcn.2015.150.
- Amiano, P., M. Dorronsoro, M. de Renobales, J. C. Ruiz de Gordoa, and I. Irigoien. 2001. Very-long-chain omega-3 fatty acids as markers for habitual fish intake in a population consuming mainly lean fish: the EPIC cohort of Gipuzkoa. European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr 55:827–832. doi:10.1038/sj.ejcn.1601242.
- Anand, S. S., C. Hawkes, R. J. de Souza, A. Mente, M. Dehghan, R. Nugent, M. A. Zulyniak, T. Weis, A. M. Bernstein, R. M. Krauss, D. Kromhout, D. J. Jenkins, V. Malik, M. A. Martinez-Gonzalez, D. Mozaffarian, S. Yusuf, W. C. Willett, and B. M. Popkin. 2015. Food consumption and its impact on cardiovascular disease: Importance of solutions focused on the globalized food system: A report from the workshop convened by the world heart federation. J Am Coll Cardiol 66:1590–614. doi:10.1016/j.jacc.2015.07.050.
- Anderson, J. W., and H. M. Bush. 2011. Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr 30:79–91. doi:10.1080/07315724.2011.10719947.
- Anderson, J. W., T. J. Hanna, X. Peng, and R. J. Kryscio. 2000. Whole grain foods and heart disease risk. J Am Coll Nutr 19:291s–299s. doi:10.1080/07315724.2000.10718963.
- Anderson, T. J., J. Gregoire, R. A. Hegele, P. Couture, G. B. Mancini, R. McPherson, G. A. Francis, P. Poirier, D. C. Lau, S. Grover, J. Genest Jr., A. C. Carpentier, R. Dufour, M. Gupta, R. Ward, L. A. Leiter, E. Lonn, D. S. Ng, G. J. Pearson, G. M. Yates, J. A. Stone, and E. Ur. 2013. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 29:151–167. doi:10.1016/j.cjca.2012.11.032.
- Ascherio, A., E. B. Rimm, M. J. Stampfer, E. L. Giovannucci, and W. C. Willett. 1995. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med 332:977–982. doi:10.1056/NEJM199504133321501.
- Ascherio, A., W. C. Willett, E. B. Rimm, E. L. Giovannucci, and M. J. Stampfer. 1994. Dietary iron intake and risk of coronary disease among men. Circulation 89:969–974. doi:10.1161/01.CIR.89.3.969.
- Ashaye, A., J. Gaziano, and L. Djousse. 2011. Red meat consumption and risk of heart failure in male physicians. Nutr Metab Cardiovasc Dis 21:941–946. doi:10.1016/j.numecd.2010.03.009.
- Atkinson, C., E. Whitley, A. Ness, and I. Baker. 2011. Associations between types of dietary fat and fish intake and risk of stroke in the Caerphilly Prospective Study (CaPS). Public Health 125:345–348. doi:10.1016/j.puhe.2011.03.002.
- Aune, D., E. Giovannucci, P. Boffetta, L. T. Fadnes, N. Keum, T. Norat, D. C. Greenwood, E. Riboli, L. J. Vatten, and S. Tonstad. 2017. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol 46:1029–1056. doi:10.1093/ije/dyw319.
- Aune, D., N. Keum, E. Giovannucci, L. T. Fadnes, P. Boffetta, D. C. Greenwood, S. Tonstad, L. J. Vatten, E. Riboli, and T. Norat. 2016a. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med 14:207. doi:10.1186/s12916-016-0730-3.
- Aune, D., N. Keum, E. Giovannucci, L. T. Fadnes, P. Boffetta, D. C. Greenwood, S. Tonstad, L. J. Vatten, E. Riboli, and T. Norat. 2016b. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 353:i2716. doi:10.1136/bmj.i2716.
- Avalos, E. E., E. Barrett-Connor, D. Kritz-Silverstein, D. L. Wingard, J. N. Bergstrom, and W. K. Al-Delaimy. 2013. Is dairy product consumption associated with the incidence of CHD? Public Health Nutr 16:2055–63. doi:10.1017/S1368980012004168.
- Bazzano, L. A., J. He, L. G. Ogden, C. Loria, S. Vupputuri, L. Myers, and P. K. Whelton. 2001. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med 161:2573–78. doi:10.1001/archinte.161.21.2573.
- Belin, R. J., P. Greenland, M. Allison, L. Martin, J. M. Shikany, J. Larson, L. Tinker, B. V. Howard, D. Lloyd-Jones, and L. Van Horn. 2011a. Diet quality and the risk of cardiovascular disease: The Women's Health Initiative (WHI). Am J Clin Nutr 94:49–57. doi:10.3945/ajcn.110.011221.
- Belin, R. J., P. Greenland, L. Martin, A. Oberman, L. Tinker, J. Robinson, J. Larson, L. Van Horn, and D. Lloyd-Jones. 2011b. Fish intake and the risk of incident heart failure: The Women's Health Initiative. Circ Heart Fail 4:404–413. doi:10.1161/CIRCHEARTFAILURE.110.960450.
- Bendinelli, B., G. Masala, C. Saieva, S. Salvini, C. Calonico, C. Sacerdote, C. Agnoli, S. Grioni, G. Frasca, A. Mattiello, P. Chiodini, R. Tumino, P. Vineis, D. Palli, and S. Panico. 2011. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: The EPICOR Study. Am J Clin Nutr 93:275–283. doi:10.3945/ajcn.110.000521.
- Bergholdt, H. K., B. G. Nordestgaard, A. Varbo, and C. Ellervik. 2015. Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98,529 Danish adults. Int J Epidemiol 44:587–603. doi:10.1093/ije/dyv109.
- Bernstein, A. M., L. de Koning, A. J. Flint, K. M. Rexrode, and W. C. Willett. 2012a. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 95:1190–99. doi:10.3945/ajcn.111.030205.
- Bernstein, A. M., A. Pan, K. M. Rexrode, M. Stampfer, F. B. Hu, D. Mozaffarian, and W. C. Willett. 2012b. Dietary protein sources and the risk of stroke in men and women. Stroke 43:637–644. doi:10.1161/STROKEAHA.111.633404.
- Bernstein, A. M., Q. Sun, F. B. Hu, M. J. Stampfer, J. E. Manson, and W. C. Willett. 2010. Major dietary protein sources and risk of coronary heart disease in women. Circulation 122:876–883. doi:10.1161/CIRCULATIONAHA.109.915165.
- Bhupathiraju, S. N., N. M. Wedick, A. Pan, J. E. Manson, K. M. Rexrode, W. C. Willett, E. B. Rimm, and F. B. Hu. 2013. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr 98:1514–23. doi:10.3945/ajcn.113.066381.
- Bjerregaard, L. J., A. M. Joensen, C. Dethlefsen, M. K. Jensen, S. P. Johnsen, A. Tjonneland, L. H. Rasmussen, K. Overvad, and E. B. Schmidt. 2010. Fish intake and acute coronary syndrome. Eur Heart J 31:29–34. doi:10.1093/eurheartj/ehp375.
- Boeing, H., A. Bechthold, A. Bub, S. Ellinger, D. Haller, A. Kroke, E. Leschik-Bonnet, M. J. Muller, H. Oberritter, M. Schulze, P. Stehle, and B. Watzl. 2012. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 51:637–663. doi:10.1007/s00394-012-0380-y.
- Bouzan, C., J. T. Cohen, W. E. Connor, P. M. Kris-Etherton, G. M. Gray, A. Konig, R. S. Lawrence, D. A. Savitz, and S. M. Teutsch. 2005. A quantitative analysis of fish consumption and stroke risk. Am J Prev Med 29:347–352. doi:10.1016/j.amepre.2005.07.002.
- Buckland, G., C. A. Gonzalez, A. Agudo, M. Vilardell, A. Berenguer, P. Amiano, E. Ardanaz, L. Arriola, A. Barricarte, M. Basterretxea, M. D. Chirlaque, L. Cirera, M. Dorronsoro, N. Egues, J. M. Huerta, N. Larranaga, P. Marin, C. Martinez, E. Molina, C. Navarro, J. R. Quiros, L. Rodriguez, M. J. Sanchez, M. J. Tormo, and C. Moreno-Iribas. 2009. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC cohort study. Am J Epidemiol 170:1518–29. doi:10.1093/aje/kwp282.
- Burke, V., Y. Zhao, A. H. Lee, E. Hunter, R. M. Spargo, M. Gracey, R. M. Smith, L. J. Beilin, and I. B. Puddey. 2007. Health-related behaviours as predictors of mortality and morbidity in Australian Aborigines. Prev Med 44:135–142. doi:10.1016/j.ypmed.2006.09.008.
- Chapkin, R. S., L. A. Davidson, L. Ly, B. R. Weeks, J. R. Lupton, and D. N. McMurray. 2007. Immunomodulatory effects of (n-3) fatty acids: Putative link to inflammation and colon cancer. J Nutr 137:200s–204s.
- Chen, G. C., D. B. Lv, Z. Pang, and Q. F. Liu. 2013. Red and processed meat consumption and risk of stroke: A meta-analysis of prospective cohort studies. Eur J Clin Nutr 67:91–95. doi:10.1038/ejcn.2012.180.
- Chowdhury, R., S. Stevens, D. Gorman, A. Pan, S. Warnakula, S. Chowdhury, H. Ward, L. Johnson, F. Crowe, F. B. Hu, and O. H. Franco. 2012. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 345:e6698. doi:10.1136/bmj.e6698.
- Chowdhury, R., S. Warnakula, S. Kunutsor, F. Crowe, H. A. Ward, L. Johnson, O. H. Franco, A. S. Butterworth, N. G. Forouhi, S. G. Thompson, K. T. Khaw, D. Mozaffarian, J. Danesh, and E. Di Angelantonio. 2014. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann Intern Med 160:398–406. doi:10.7326/M13-1788.
- Clayton, Z. S., E. Fusco, and M. Kern. 2017. Egg consumption and heart health: A review. Nutrition 37:79–85. doi:10.1016/j.nut.2016.12.014.
- Dalmeijer, G. W., E. A. Struijk, Y. T. van der Schouw, S. S. Soedamah-Muthu, W. M. Verschuren, J. M. Boer, J. M. Geleijnse, and J. W. Beulens. 2013. Dairy intake and coronary heart disease or stroke–a population-based cohort study. Int J Cardiol 167:925–929. doi:10.1016/j.ijcard.2012.03.094.
- Darmon, N., and A. Drewnowski. 2008. Does social class predict diet quality? Am J Clin Nutr 87:1107–17.
- Dauchet, L., P. Amouyel, S. Hercberg, and J. Dallongeville. 2006. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J Nutr 136:2588–93.
- Dauchet, L., J. Ferrieres, D. Arveiler, J. W. Yarnell, F. Gey, P. Ducimetiere, J. B. Ruidavets, B. Haas, A. Evans, A. Bingham, P. Amouyel, and J. Dallongeville. 2004. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: the PRIME study. Br J Nutr 92:963–972. doi:10.1079/BJN20041286.
- de Goede, J., J. M. Geleijnse, J. M. Boer, D. Kromhout, and W. M. Verschuren. 2010. Marine (n-3) fatty acids, fish consumption, and the 10-year risk of fatal and nonfatal coronary heart disease in a large population of Dutch adults with low fish intake. J Nutr 140:1023–28. doi:10.3945/jn.109.119271.
- de Goede, J., W. M. Verschuren, J. M. Boer, D. Kromhout, and J. M. Geleijnse. 2012. Gender-specific associations of marine n-3 fatty acids and fish consumption with 10-year incidence of stroke. PLoS One 7:e33866. doi:10.1371/journal.pone.0033866.
- de Koning, L., V. S. Malik, M. D. Kellogg, E. B. Rimm, W. C. Willett, and F. B. Hu. 2012. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 125:1735–41, s1731. doi:10.1161/CIRCULATIONAHA.111.067017.
- Del Gobbo, L. C., S. Kalantarian, F. Imamura, R. Lemaitre, D. S. Siscovick, B. M. Psaty, and D. Mozaffarian. 2015. Contribution of major lifestyle risk factors for incident heart failure in older adults: The cardiovascular health study. JACC Heart Fail 3:520–528. doi:10.1016/j.jchf.2015.02.009.
- DerSimonian, R., and N. Laird. 1986. Meta-analysis in clinical trials. Control Clin Trials 7:177–88. doi:10.1016/0197-2456(86)90046-2.
- di Giuseppe, R., M. K. Fjeld, J. Dierkes, D. Theoflylaktopoulou, M. Arregui, H. Boeing, and C. Weikert. 2015. The association between nut consumption and the risk of total and ischemic stroke in a German cohort study. Eur J Clin Nutr 69:431–435. doi:10.1038/ejcn.2014.212.
- Dijkstra, S. C., I. A. Brouwer, F. J. van Rooij, A. Hofman, J. C. Witteman, and J. M. Geleijnse. 2009. Intake of very long chain n-3 fatty acids from fish and the incidence of heart failure: The Rotterdam Study. Eur J Heart Fail 11:922–928. doi:10.1093/eurjhf/hfp126.
- Dilis, V., M. Katsoulis, P. Lagiou, D. Trichopoulos, A. Naska, and A. Trichopoulou. 2012. Mediterranean diet and CHD: the Greek European prospective investigation into cancer and nutrition cohort. Br J Nutr 108:699–709. doi:10.1017/S0007114512001821.
- DiMeglio, D. P., and R. D. Mattes. 2000. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int J Obes Relat Metab Disord 24:794–800. doi:10.1038/sj.ijo.0801229.
- Djousse, L., A. O. Akinkuolie, J. H. Wu, E. L. Ding, and J. M. Gaziano. 2012. Fish consumption, omega-3 fatty acids and risk of heart failure: A meta-analysis. Clin Nutr 31:846–853. doi:10.1016/j.clnu.2012.05.010.
- Djousse, L., and J. M. Gaziano. 2007. Breakfast cereals and risk of heart failure in the physicians' health study I. Arch Intern Med 167:2080–85. doi:10.1001/archinte.167.19.2080.
- Djousse, L., and J. M. Gaziano. 2008a. Egg consumption and risk of heart failure in the Physicians' Health Study. Circulation 117:512–516. doi:10.1161/CIRCULATIONAHA.107.734210.
- Djousse, L., and J. M. Gaziano. 2008b. Egg consumption in relation to cardiovascular disease and mortality: The Physicians' Health Study. Am J Clin Nutr 87:964–969.
- Djousse, L., J. M. Gaziano, C. S. Kase, and T. Kurth. 2010. Nut consumption and risk of stroke in US male physicians. Clin Nutr 29:605–609. doi:10.1016/j.clnu.2010.03.005.
- Djousse, L., T. Rudich, and J. M. Gaziano. 2008. Nut consumption and risk of heart failure in the Physicians' Health Study I. Am J Clin Nutr 88:930–933.
- Domingo, J. L. 2016. Nutrients and chemical pollutants in fish and shellfish. Balancing health benefits and risks of regular fish consumption. Crit Rev Food Sci Nutr 56:979–988. doi:10.1080/10408398.2012.742985.
- Du, H., L. Li, D. Bennett, Y. Guo, T. J. Key, Z. Bian, P. Sherliker, H. Gao, Y. Chen, L. Yang, J. Chen, S. Wang, R. Du, H. Su, R. Collins, R. Peto, and Z. Chen. 2016. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med 374:1332–43. doi:10.1056/NEJMoa1501451.
- Durrleman, S., and R. Simon. 1989. Flexible regression models with cubic splines. Stat Med 8:551–561. doi:10.1002/sim.4780080504.
- Eckel, R. H., J. M. Jakicic, J. D. Ard, J. M. de Jesus, N. Houston Miller, V. S. Hubbard, I. M. Lee, A. H. Lichtenstein, C. M. Loria, B. E. Millen, C. A. Nonas, F. M. Sacks, S. C. Smith Jr., L. P. Svetkey, T. A. Wadden, S. Z. Yanovski, K. A. Kendall, L. C. Morgan, M. G. Trisolini, G. Velasco, J. Wnek, J. L. Anderson, J. L. Halperin, N. M. Albert, B. Bozkurt, R. G. Brindis, L. H. Curtis, D. DeMets, J. S. Hochman, R. J. Kovacs, E. M. Ohman, S. J. Pressler, F. W. Sellke, W. K. Shen, S. C. Smith Jr., and G. F. Tomaselli. 2014. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129:S76–99. doi:10.1161/01.cir.0000437740.48606.d1.
- EFSA Panel on Dietetic Products, N., and Allergies. 2010. Scientific opinion on establishing food-based dietary guidelines. EFSA J 8:1460–n/a. doi:10.2903/j.efsa.2010.1460.
- Egger, M., G. Davey Smith, M. Schneider, and C. Minder. 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–34. doi:10.1136/bmj.315.7109.629.
- Elwood, P. C., J. E. Pickering, A. M. Fehily, J. Hughes, and A. R. Ness. 2004. Milk drinking, ischaemic heart disease and ischaemic stroke I. Evidence from the Caerphilly cohort. Eur J Clin Nutr 58:711–717. doi:10.1038/sj.ejcn.1601868.
- Eshak, E. S., H. Iso, Y. Kokubo, I. Saito, K. Yamagishi, M. Inoue, and S. Tsugane. 2012. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: The Japan Public Health Centre-based study cohort I. Am J Clin Nutr 96:1390–97. doi:10.3945/ajcn.112.037903.
- Eshak, E. S., H. Iso, K. Yamagishi, Y. Kokubo, I. Saito, H. Yatsuya, N. Sawada, M. Inoue, and S. Tsugane. 2014. Rice consumption is not associated with risk of cardiovascular disease morbidity or mortality in Japanese men and women: A large population-based, prospective cohort study. Am J Clin Nutr 100:199–207. doi:10.3945/ajcn.113.079038.
- Fraser, G. E., J. Sabate, W. L. Beeson, and T. M. Strahan. 1992. A possible protective effect of nut consumption on risk of coronary heart disease. the adventist health study. Arch Intern Med 152:1416–24. doi:10.1001/archinte.1992.00400190054010.
- Fung, T. T., V. Malik, K. M. Rexrode, J. E. Manson, W. C. Willett, and F. B. Hu. 2009. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 89:1037–42. doi:10.3945/ajcn.2008.27140.
- Fuster, V., and B. E. Kelly. 2010. Promoting cardiovascular health in the developing world. A critical challenge to achieve global health. Washington: National Academies Press.
- Gammelmark, A., M. S. Nielsen, C. S. Bork, S. Lundbye-Christensen, A. Tjonneland, K. Overvad, and E. B. Schmidt. 2016. Association of fish consumption and dietary intake of marine n-3 PUFA with myocardial infarction in a prospective Danish cohort study. Br J Nutr 116:167–177. doi:10.1017/S000711451600180X.
- Gan, Y., X. Tong, L. Li, S. Cao, X. Yin, C. Gao, C. Herath, W. Li, Z. Jin, Y. Chen, and Z. Lu. 2015. Consumption of fruit and vegetable and risk of coronary heart disease: A meta-analysis of prospective cohort studies. Int J Cardiol 183:129–137. doi:10.1016/j.ijcard.2015.01.077.
- Gardener, H., T. Rundek, M. Markert, C. B. Wright, M. S. Elkind, and R. L. Sacco. 2012. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med 27:1120–26. doi:10.1007/s11606-011-1968-2.
- Gholami, F., M. Khoramdad, N. Esmailnasab, G. Moradi, B. Nouri, S. Safiri, and Y. Alimohamadi. 2017. The effect of dairy consumption on the prevention of cardiovascular diseases: A meta-analysis of prospective studies. J Cardiovasc Thorac Res 9:1–11. doi:10.15171/jcvtr.2017.01.
- Gillman, M. W., L. A. Cupples, D. Gagnon, B. M. Posner, R. C. Ellison, W. P. Castelli, and P. A. Wolf. 1995. Protective effect of fruits and vegetables on development of stroke in men. JAMA 273:1113–17. doi:10.1001/jama.1995.03520380049034.
- Gillum, R. F., M. Mussolino, and J. H. Madans. 2000. The relation between fish consumption, death from all causes, and incidence of coronary heart disease. the NHANES I Epidemiologic Follow-up Study. J Clin Epidemiol 53:237–244. doi:10.1016/S0895-4356(99)00149-3.
- Gillum, R. F., M. E. Mussolino, and J. H. Madans. 1996. The relationship between fish consumption and stroke incidence. The NHANES I Epidemiologic Follow-up Study (National Health and Nutrition Examination Survey). Arch Intern Med 156:537–542. doi:10.1001/archinte.1996.00440050091010.
- Goldberg, S., H. Gardener, E. Tiozzo, C. Ying Kuen, M. S. Elkind, R. L. Sacco, and T. Rundek. 2014. Egg consumption and carotid atherosclerosis in the Northern Manhattan study. Atherosclerosis 235:273–280. doi:10.1016/j.atherosclerosis.2014.04.019.
- Greenland, S., and M. P. Longnecker. 1992. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–09. doi:10.1093/oxfordjournals.aje.a116237.
- Hansen, C. P., K. Overvad, C. Kyro, A. Olsen, A. Tjonneland, S. P. Johnsen, M. U. Jakobsen, and C. C. Dahm. 2017. Adherence to a healthy nordic diet and risk of stroke: A danish cohort study. Stroke 48:259–264. doi:10.1161/STROKEAHA.116.015019.
- Hansen, L., L. O. Dragsted, A. Olsen, J. Christensen, A. Tjonneland, E. B. Schmidt, and K. Overvad. 2010. Fruit and vegetable intake and risk of acute coronary syndrome. Br J Nutr 104:248–255. doi:10.1017/S0007114510000462.
- Haring, B., N. Gronroos, J. A. Nettleton, M. C. von Ballmoos, E. Selvin, and A. Alonso. 2014. Dietary protein intake and coronary heart disease in a large community based cohort: Results from the Atherosclerosis Risk in Communities (ARIC) study [corrected]. PLoS One 9:e109552. doi:10.1371/journal.pone.0109552.
- Haring, B., J. R. Misialek, C. M. Rebholz, N. Petruski-Ivleva, R. F. Gottesman, T. H. Mosley, and A. Alonso. 2015. Association of dietary protein consumption with incident silent cerebral infarcts and stroke: The Atherosclerosis Risk in Communities (ARIC) study. Stroke 46:3443–50. doi:10.1161/STROKEAHA.115.010693.
- He, F. J., C. A. Nowson, M. Lucas, and G. A. MacGregor. 2007. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J Hum Hypertens 21:717–728. doi:10.1038/sj.jhh.1002212.
- He, F. J., C. A. Nowson, and G. A. MacGregor. 2006. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 367:320–326. doi:10.1016/S0140-6736(06)68069-0.
- He, K., Y. Song, M. L. Daviglus, K. Liu, L. Van Horn, A. R. Dyer, U. Goldbourt, and P. Greenland. 2004. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke 35:1538–42. doi:10.1161/01.STR.0000130856.31468.47.
- Helnaes, A., C. Kyro, I. Andersen, S. Lacoppidan, K. Overvad, J. Christensen, A. Tjonneland, and A. Olsen. 2016. Intake of whole grains is associated with lower risk of myocardial infarction: the Danish Diet, Cancer and Health Cohort. Am J Clin Nutr 103:999–1007. doi:10.3945/ajcn.115.124271.
- Higgins, J., and S. Green. 2011. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.handbook.cochrane.org.
- Higgins, J. P., and S. G. Thompson. 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–58. doi:10.1002/sim.1186.
- Hirvonen, T., P. Pietinen, M. Virtanen, M. L. Ovaskainen, S. Hakkinen, D. Albanes, and J. Virtamo. 2001. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology 12:62–67. doi:10.1097/00001648-200101000-00011.
- Holmberg, S., A. Thelin, and E. L. Stiernstrom. 2009. Food choices and coronary heart disease: A population based cohort study of rural Swedish men with 12 years of follow-up. Int J Environ Res Public Health 6:2626–38. doi:10.3390/ijerph6102626.
- Hu, D., J. Huang, Y. Wang, D. Zhang, and Y. Qu. 2014. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke 45:1613–19. doi:10.1161/STROKEAHA.114.004836.
- Hu, F. B., M. J. Stampfer, E. B. Rimm, J. E. Manson, A. Ascherio, G. A. Colditz, B. A. Rosner, D. Spiegelman, F. E. Speizer, F. M. Sacks, C. H. Hennekens, and W. C. Willett. 1999. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA 281:1387–94. doi:10.1001/jama.281.15.1387.
- Iso, H., M. Kobayashi, J. Ishihara, S. Sasaki, K. Okada, Y. Kita, Y. Kokubo, and S. Tsugane. 2006. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation 113:195–202. doi:10.1161/CIRCULATIONAHA.105.581355.
- Jensen, M. K., P. Koh-Banerjee, F. B. Hu, M. Franz, L. Sampson, M. Gronbaek, and E. B. Rimm. 2004. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am J Clin Nutr 80:1492–1499.
- Johnsen, S. P., K. Overvad, C. Stripp, A. Tjonneland, S. E. Husted, and H. T. Sorensen. 2003. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr 78:57–64.
- Jonnalagadda, S. S., L. Harnack, R. H. Liu, N. McKeown, C. Seal, S. Liu, and G. C. Fahey. 2011. Putting the whole grain puzzle together: health benefits associated with whole grains–summary of American Society for Nutrition 2010 Satellite Symposium. J Nutr 141:1011s–22s. doi:10.3945/jn.110.132944.
- Joshipura, K. J., A. Ascherio, J. E. Manson, M. J. Stampfer, E. B. Rimm, F. E. Speizer, C. H. Hennekens, D. Spiegelman, and W. C. Willett. 1999. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 282:1233–39. doi:10.1001/jama.282.13.1233.
- Kaluza, J., A. Akesson, and A. Wolk. 2014. Processed and unprocessed red meat consumption and risk of heart failure: Prospective study of men. Circ Heart Fail 7:552–557. doi:10.1161/CIRCHEARTFAILURE.113.000921.
- Kaluza, J., A. Akesson, and A. Wolk. 2015. Long-term processed and unprocessed red meat consumption and risk of heart failure: A prospective cohort study of women. Int J Cardiol 193:42–46. doi:10.1016/j.ijcard.2015.05.044.
- Kaluza, J., A. Wolk, and S. C. Larsson. 2012. Red meat consumption and risk of stroke: A meta-analysis of prospective studies. Stroke 43:2556–60. doi:10.1161/STROKEAHA.112.663286.
- Kassebaum, N., M. Arora, R. Barber, et al. 2016. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1603–58. doi:10.1016/S0140-6736(16)31460-X.
- Keli, S. O., E. J. Feskens, and D. Kromhout. 1994. Fish consumption and risk of stroke. The Zutphen Study. Stroke 25:328–332. doi:10.1161/01.STR.25.2.328.
- Keli, S. O., M. G. Hertog, E. J. Feskens, and D. Kromhout. 1996. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The Zutphen study. Arch Intern Med 156:637–642. doi:10.1001/archinte.1996.00440060059007.
- Keller, A., B. L. Heitmann, and N. Olsen. 2015. Sugar-sweetened beverages, vascular risk factors and events: A systematic literature review. Public Health Nutr 18:1145–54. doi:10.1017/S1368980014002122.
- Kelly, J. H., Jr., and J. Sabate. 2006. Nuts and coronary heart disease: An epidemiological perspective. Br J Nutr 96 (Suppl 2):S61–67. doi:10.1017/BJN20061865.
- Khawaja, O., H. Singh, F. Luni, A. Kabour, S. S. Ali, M. Taleb, H. Ahmed, J. M. Gaziano, and L. Djousse. 2017. Egg consumption and incidence of heart failure: A meta-analysis of prospective cohort studies. Front Nutr 4:10. doi:10.3389/fnut.2017.00010.
- Kobylecki, C. J., S. Afzal, G. Davey Smith, and B. G. Nordestgaard. 2015. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: A Mendelian randomization study. Am J Clin Nutr 101:1135–43. doi:10.3945/ajcn.114.104497.
- Kokubo, Y., H. Iso, J. Ishihara, K. Okada, M. Inoue, and S. Tsugane. 2007. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: The Japan Public Health Center-based (JPHC) study cohort I. Circulation 116:2553–62. doi:10.1161/CIRCULATIONAHA.106.683755.
- Kromhout, D., C. J. Spaaij, J. de Goede, and R. M. Weggemans. 2016. The 2015 Dutch food-based dietary guidelines. Eur J Clin Nutr 70:869–878. doi:10.1038/ejcn.2016.52.
- Kuhn, T., B. Teucher, R. Kaaks, H. Boeing, C. Weikert, and B. Buijsse. 2013. Fish consumption and the risk of myocardial infarction and stroke in the German arm of the European Prospective Investigation into Cancer and Nutrition (EPIC-Germany). Br J Nutr 110:1118–25. doi:10.1017/S0007114513000202.
- Larsson, S. C., A. Akesson, and A. Wolk. 2014. Sweetened beverage consumption is associated with increased risk of stroke in women and men. J Nutr 144:856–860. doi:10.3945/jn.114.190546.
- Larsson, S. C., A. Akesson, and A. Wolk. 2015. Egg consumption and risk of heart failure, myocardial infarction, and stroke: results from 2 prospective cohorts. Am J Clin Nutr 102:1007–13. doi:10.3945/ajcn.115.119263.
- Larsson, S. C., S. Mannisto, M. J. Virtanen, J. Kontto, D. Albanes, and J. Virtamo. 2009a. Dairy foods and risk of stroke. Epidemiology 20:355–360. doi:10.1097/EDE.0b013e3181935dd5.
- Larsson, S. C., S. Mannisto, M. J. Virtanen, J. Kontto, D. Albanes, and J. Virtamo. 2009b. Dietary fiber and fiber-rich food intake in relation to risk of stroke in male smokers. Eur J Clin Nutr 63:1016–24. doi:10.1038/ejcn.2009.16.
- Larsson, S. C., and N. Orsini. 2011. Fish consumption and the risk of stroke: A dose-response meta-analysis. Stroke 42:3621–23. doi:10.1161/STROKEAHA.111.630319.
- Larsson, S. C., J. Virtamo, and A. Wolk. 2011a. Fish consumption and risk of stroke in Swedish women. Am J Clin Nutr 93:487–493. doi:10.3945/ajcn.110.002287.
- Larsson, S. C., J. Virtamo, and A. Wolk. 2011b. Red meat consumption and risk of stroke in Swedish men. Am J Clin Nutr 94:417–421. doi:10.3945/ajcn.111.015115.
- Larsson, S. C., J. Virtamo, and A. Wolk. 2011c. Red meat consumption and risk of stroke in Swedish women. Stroke 42:324–329. doi:10.1161/STROKEAHA.110.596510.
- Larsson, S. C., J. Virtamo, and A. Wolk. 2012. Dairy consumption and risk of stroke in Swedish women and men. Stroke 43:1775–80. doi:10.1161/STROKEAHA.111.641944.
- Larsson, S. C., J. Virtamo, and A. Wolk. 2013. Total and specific fruit and vegetable consumption and risk of stroke: A prospective study. Atherosclerosis 227:147–152. doi:10.1016/j.atherosclerosis.2012.12.022.
- Leung Yinko, S. S., K. D. Stark, G. Thanassoulis, and L. Pilote. 2014. Fish consumption and acute coronary syndrome: A meta-analysis. Am J Med 127:848–857 e842. doi:10.1016/j.amjmed.2014.04.016.
- Levitan, E. B., A. Wolk, and M. A. Mittleman. 2009. Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: A population-based prospective study of middle-aged and elderly men. Eur Heart J 30:1495–1500. doi:10.1093/eurheartj/ehp111.
- Levitan, E. B., A. Wolk, and M. A. Mittleman. 2010. Fatty fish, marine omega-3 fatty acids and incidence of heart failure. Eur J Clin Nutr 64:587–594. doi:10.1038/ejcn.2010.50.
- Li, Y. H., C. H. Zhou, H. J. Pei, X. L. Zhou, L. H. Li, Y. J. Wu, and R. T. Hui. 2013. Fish consumption and incidence of heart failure: A meta-analysis of prospective cohort studies. Chin Med J (Engl) 126:942–948.
- Lin, P. H., W. T. Yeh, L. P. Svetkey, S. Y. Chuang, Y. C. Chang, C. Wang, and W. H. Pan. 2013. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac J Clin Nutr 22:482–491.
- Liu, S., I. M. Lee, U. Ajani, S. R. Cole, J. E. Buring, and J. E. Manson. 2001. Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: The Physicians' Health Study. Int J Epidemiol 30:130–135. doi:10.1093/ije/30.1.130.
- Liu, S., J. E. Manson, I. M. Lee, S. R. Cole, C. H. Hennekens, W. C. Willett, and J. E. Buring. 2000a. Fruit and vegetable intake and risk of cardiovascular disease: The Women's Health Study. Am J Clin Nutr 72:922–928.
- Liu, S., J. E. Manson, M. J. Stampfer, K. M. Rexrode, F. B. Hu, E. B. Rimm, and W. C. Willett. 2000b. Whole grain consumption and risk of ischemic stroke in women: A prospective study. JAMA 284:1534–40. doi:10.1001/jama.284.12.1534.
- Liu, S., M. J. Stampfer, F. B. Hu, E. Giovannucci, E. Rimm, J. E. Manson, C. H. Hennekens, and W. C. Willett. 1999. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses' Health Study. Am J Clin Nutr 70:412–419.
- Lou, D., Y. Li, G. Yan, J. Bu, and H. Wang. 2016. Soy consumption with risk of coronary heart disease and stroke: A meta-analysis of observational studies. Neuroepidemiology 46:242–252. doi:10.1159/000444324.
- Ma, L., F. Wang, W. Guo, H. Yang, Y. Liu, and W. Zhang. 2014. Nut consumption and the risk of coronary artery disease: A dose-response meta-analysis of 13 prospective studies. Thromb Res 134:790–794. doi:10.1016/j.thromres.2014.06.017.
- Malaguti, M., G. Dinelli, E. Leoncini, V. Bregola, S. Bosi, A. F. Cicero, and S. Hrelia. 2014. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int J Mol Sci 15:21120–135. doi:10.3390/ijms151121120.
- Malik, V. S., A. Pan, W. C. Willett, and F. B. Hu. 2013. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am J Clin Nutr 98:1084–1102. doi:10.3945/ajcn.113.058362.
- Martinez-Gonzalez, M. A., M. Garcia-Lopez, M. Bes-Rastrollo, E. Toledo, E. H. Martinez-Lapiscina, M. Delgado-Rodriguez, Z. Vazquez, S. Benito, and J. J. Beunza. 2011. Mediterranean diet and the incidence of cardiovascular disease: A Spanish cohort. Nutr Metab Cardiovasc Dis 21:237–244.
- Marventano, S., M. Izquierdo Pulido, C. Sanchez-Gonzalez, J. Godos, A. Speciani, F. Galvano, and G. Grosso. 2017. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr 20:245–254. doi:10.1017/S1368980016002299.
- Mayhew, A. J., R. J. de Souza, D. Meyre, S. S. Anand, and A. Mente. 2016. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr 115:212–225. doi:10.1017/S0007114515004316.
- Mellen, P. B., T. F. Walsh, and D. M. Herrington. 2008. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr Metab Cardiovasc Dis 18:283–290. doi:10.1016/j.numecd.2006.12.008.
- Mente, A., L. de Koning, H. S. Shannon, and S. S. Anand. 2009. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 169:659–669. doi:10.1001/archinternmed.2009.38.
- Micha, R., G. Michas, and D. Mozaffarian. 2012. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep 14:515–524. doi:10.1007/s11883-012-0282-8.
- Micha, R., S. K. Wallace, and D. Mozaffarian. 2010. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 121:2271–83. doi:10.1161/CIRCULATIONAHA.109.924977.
- Miller, C. A., K. D. Corbin, K. A. da Costa, S. Zhang, X. Zhao, J. A. Galanko, T. Blevins, B. J. Bennett, A. O'Connor, and S. H. Zeisel. 2014. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am J Clin Nutr 100:778–786. doi:10.3945/ajcn.114.087692.
- Misirli, G., V. Benetou, P. Lagiou, C. Bamia, D. Trichopoulos, and A. Trichopoulou. 2012. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol 176:1185–1192. doi:10.1093/aje/kws205.
- Mizrahi, A., P. Knekt, J. Montonen, M. A. Laaksonen, M. Heliovaara, and R. Jarvinen. 2009. Plant foods and the risk of cerebrovascular diseases: A potential protection of fruit consumption. Br J Nutr 102:1075–83. doi:10.1017/S0007114509359097.
- Montonen, J., R. Jarvinen, A. Reunanen, and P. Knekt. 2009. Fish consumption and the incidence of cerebrovascular disease. Br J Nutr 102:750–756. doi:10.1017/S0007114509274782.
- Moran, A. E., G. A. Roth, J. Narula, and G. A. Mensah. 2014. 1990–2010 global cardiovascular disease atlas. Glob Heart 9:3–16. doi:10.1016/j.gheart.2014.03.1220.
- Morris, M. C., J. E. Manson, B. Rosner, J. E. Buring, W. C. Willett, and C. H. Hennekens. 1995. Fish consumption and cardiovascular disease in the physicians' health study: A prospective study. Am J Epidemiol 142:166–175. doi:10.1093/oxfordjournals.aje.a117615.
- Mozaffarian, D. 2016. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 133:187–225. doi:10.1161/CIRCULATIONAHA.115.018585.
- Mozaffarian, D., C. L. Bryson, R. N. Lemaitre, G. L. Burke, and D. S. Siscovick. 2005a. Fish intake and risk of incident heart failure. J Am Coll Cardiol 45:2015–21. doi:10.1016/j.jacc.2005.03.038.
- Mozaffarian, D., R. N. Lemaitre, L. H. Kuller, G. L. Burke, R. P. Tracy, and D. S. Siscovick. 2003. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: The Cardiovascular Health Study. Circulation 107:1372–77. doi:10.1161/01.CIR.0000055315.79177.16.
- Mozaffarian, D., W. T. Longstreth Jr., R. N. Lemaitre, T. A. Manolio, L. H. Kuller, G. L. Burke, and D. S. Siscovick. 2005b. Fish consumption and stroke risk in elderly individuals: The cardiovascular health study. Arch Intern Med 165:200–206. doi:10.1001/archinte.165.2.200.
- Muraki, I., H. Wu, F. Imamura, F. Laden, E. B. Rimm, F. B. Hu, W. C. Willett, and Q. Sun. 2015. Rice consumption and risk of cardiovascular disease: Results from a pooled analysis of 3 U.S. cohorts. Am J Clin Nutr 101:164–172. doi:10.3945/ajcn.114.087551.
- Myint, P. K., A. A. Welch, S. A. Bingham, R. N. Luben, N. J. Wareham, N. E. Day, and K. T. Khaw. 2006. Habitual fish consumption and risk of incident stroke: The European Prospective Investigation into Cancer (EPIC)-Norfolk prospective population study. Public Health Nutr 9:882–888. doi:10.1017/PHN2006942.
- Nahab, F., K. Pearson, M. R. Frankel, J. Ard, M. M. Safford, D. Kleindorfer, V. J. Howard, and S. Judd. 2016. Dietary fried fish intake increases risk of CVD: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Public Health Nutr 19:3327–36. doi:10.1017/S136898001600152X.
- Narain, A., C. S. Kwok, and M. A. Mamas. 2016. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: A systematic review and meta-analysis. Int J Clin Pract 70:791–805. doi:10.1111/ijcp.12841.
- Neelakantan, N., N. Naidoo, W. P. Koh, J. M. Yuan, and R. M. van Dam. 2016. The alternative healthy eating index is associated with a lower risk of fatal and nonfatal acute myocardial infarction in a Chinese adult population. J Nutr 146:1379–86. doi:10.3945/jn.116.231605.
- Nettleton, J. A., L. M. Steffen, L. R. Loehr, W. D. Rosamond, and A. R. Folsom. 2008. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) study. J Am Diet Assoc 108:1881–87.
- Nordic Council of Ministers, e. N. 2014. Nordic nutrition recommendations 2012. Integrating nutrition and physical activity. 5th edition, Copenhagen.
- Orencia, A. J., M. L. Daviglus, A. R. Dyer, R. B. Shekelle, and J. Stamler. 1996. Fish consumption and stroke in men. 30-year findings of the Chicago Western Electric Study. Stroke 27:204–209.
- Osler, M., A. H. Andreasen, and S. Hoidrup. 2003. No inverse association between fish consumption and risk of death from all-causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol 56:274–279.
- Oude Griep, L. M., W. M. Verschuren, D. Kromhout, M. C. Ocke, and J. M. Geleijnse. 2012. Variety in fruit and vegetable consumption and 10-year incidence of CHD and stroke. Public Health Nutr 15:2280–86.
- Patterson, E., S. C. Larsson, A. Wolk, and A. Akesson. 2013. Association between dairy food consumption and risk of myocardial infarction in women differs by type of dairy food. J Nutr 143:74–79.
- Perk, J., G. De Backer, H. Gohlke, I. Graham, Z. Reiner, M. Verschuren, C. Albus, P. Benlian, G. Boysen, R. Cifkova, C. Deaton, S. Ebrahim, M. Fisher, G. Germano, R. Hobbs, A. Hoes, S. Karadeniz, A. Mezzani, E. Prescott, L. Ryden, M. Scherer, M. Syvanne, O. P. Scholte, W. J. Reimer, C. Vrints, D. Wood, J. L. Zamorano, and F. Zannad. 2012. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 33:1635–1701.
- Praagman, J., O. H. Franco, M. A. Ikram, S. S. Soedamah-Muthu, M. F. Engberink, F. J. van Rooij, A. Hofman, and J. M. Geleijnse. 2015. Dairy products and the risk of stroke and coronary heart disease: The Rotterdam study. Eur J Nutr 54:981–990.
- Qureshi, A. I., F. K. Suri, S. Ahmed, A. Nasar, A. A. Divani, and J. F. Kirmani. 2007. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med Sci Monit 13:Cr1–8.
- Rahman, I., A. Wolk, and S. C. Larsson. 2015. The relationship between sweetened beverage consumption and risk of heart failure in men. Heart 101:1961–65.
- Rautiainen, S., E. B. Levitan, M. A. Mittleman, and A. Wolk. 2015. Fruit and vegetable intake and rate of heart failure: a population-based prospective cohort of women. Eur J Heart Fail 17:20–26. doi:10.1002/ejhf.191.
- Rautiainen, S., E. B. Levitan, N. Orsini, A. Akesson, R. Morgenstern, M. A. Mittleman, and A. Wolk. 2012. Total antioxidant capacity from diet and risk of myocardial infarction: A prospective cohort of women. Am J Med 125:974–980. doi:10.1016/j.amjmed.2012.03.008.
- Salonen, J. T., K. Seppanen, K. Nyyssonen, H. Korpela, J. Kauhanen, M. Kantola, J. Tuomilehto, H. Esterbauer, F. Tatzber, and R. Salonen. 1995. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 91:645–655. doi:10.1161/01.CIR.91.3.645.
- Saravanan, P., N. C. Davidson, E. B. Schmidt, and P. C. Calder. 2010. Cardiovascular effects of marine omega-3 fatty acids. Lancet 376:540–550. doi:10.1016/S0140-6736(10)60445-X.
- Scarborough, P., R. D. Morgan, P. Webster, and M. Rayner. 2011. Differences in coronary heart disease, stroke and cancer mortality rates between England, Wales, Scotland and Northern Ireland: The role of diet and nutrition. BMJ Open 1:e000263. doi:10.1136/bmjopen-2011-000263.
- Schwingshackl, L., A. Chaimani, A. Bechthold, K. Iqbal, M. Stelmach-Mardas, G. Hoffmann, C. Schwedhelm, S. Schlesinger, and H. Boeing. 2016a. Food groups and risk of chronic disease: A protocol for a systematic review and network meta-analysis of cohort studies. Syst Rev 5:125. doi:10.1186/s13643-016-0302-9.
- Schwingshackl, L., and G. Hoffmann. 2015a. Adherence to mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med 4:1933–47. doi:10.1002/cam4.539.
- Schwingshackl, L., and G. Hoffmann. 2015b. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 115:780–800. doi:10.1016/j.jand.2014.12.009.
- Schwingshackl, L., G. Hoffmann, A. M. Lampousi, S. Knuppel, K. Iqbal, C. Schwedhelm, A. Bechthold, S. Schlesinger, and H. Boeing. 2017b. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur J Epidemiol 32:363–375. doi:10.1007/s10654-017-0246-y.
- Schwingshackl, L., G. Hoffmann, B. Missbach, M. Stelmach-Mardas, and H. Boeing. 2017d. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Des 23:1016–27. doi:10.2174/1381612822666161010121356.
- Schwingshackl, L., G. Hoffmann, C. Schwedhelm, T. Kalle-Uhlmann, B. Missbach, S. Knuppel, and H. Boeing. 2016b. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: A systematic review and meta-analysis of cohort studies. PLoS One 11:e0157461. doi:10.1371/journal.pone.0157461.
- Schwingshackl, L., S. Knüppel, C. Schwedhelm, G. Hoffmann, B. Missbach, M. Stelmach-Mardas, S. Dietrich, F. Eichelmann, E. Kontopanteils, and K. Iqbal. 2016c. Perspective: NutriGrade: A scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 7:994–1004. doi:10.3945/an.116.013052.
- Schwingshackl, L., B. Missbach, J. Konig, and G. Hoffmann. 2015. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr 18:1292–9. doi:10.1017/S1368980014001542.
- Schwingshackl, L., C. Schwedhelm, G. Hoffmann, S. Knüppel, K. Iqbal, V. Andriolo, A. Bechthold, S. Schlesinger, and H. Boeing. 2017c. Food groups and risk of hypertension: A systematic review and dose-response meta-analysis of prospective studies, Adv Nutr (accepted 14 September 2017).
- Schwingshackl, L., C. Schwedhelm, G. Hoffmann, A.-M. Lampousi, S. Knüppel, K. Iqbal, A. Bechthold, S. Schlesinger, and H. Boeing. 2017a. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. The American Journal of Clinical Nutrition 105:1462–73.
- Senthong, V., X. S. Li, T. Hudec, J. Coughlin, Y. Wu, B. Levison, Z. Wang, S. L. Hazen, and W. H. Tang. 2016. Plasma trimethylamine N-Oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol 67:2620–28. doi:10.1016/j.jacc.2016.03.546.
- Soedamah-Muthu, S. S., G. Masset, L. Verberne, J. M. Geleijnse, and E. J. Brunner. 2013. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br J Nutr 109:718–726. doi:10.1017/S0007114512001845.
- Sonestedt, E., S. Hellstrand, C. A. Schulz, P. Wallstrom, I. Drake, U. Ericson, B. Gullberg, B. Hedblad, and M. Orho-Melander. 2015. The association between carbohydrate-rich foods and risk of cardiovascular disease is not modified by genetic susceptibility to dyslipidemia as determined by 80 validated variants. PLoS One 10:e0126104. doi:10.1371/journal.pone.0126104.
- Sonestedt, E., E. Wirfalt, P. Wallstrom, B. Gullberg, M. Orho-Melander, and B. Hedblad. 2011. Dairy products and its association with incidence of cardiovascular disease: The Malmo diet and cancer cohort. Eur J Epidemiol 26:609–618. doi:10.1007/s10654-011-9589-y.
- Steffen, L. M., D. R. Jacobs Jr., J. Stevens, E. Shahar, T. Carithers, and A. R. Folsom. 2003. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 78:383–390.
- Stroup, D. F., J. A. Berlin, S. C. Morton, I. Olkin, G. D. Williamson, D. Rennie, D. Moher, B. J. Becker, T. A. Sipe, and S. B. Thacker. 2000. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–12. doi:10.1001/jama.283.15.2008.
- Tang, G., D. Wang, J. Long, F. Yang, and L. Si. 2015. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol 115:625–629. doi:10.1016/j.amjcard.2014.12.015.
- Tektonidis, T. G., A. Akesson, B. Gigante, A. Wolk, and S. C. Larsson. 2015. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis 243:93–98. doi:10.1016/j.atherosclerosis.2015.08.039.
- Tektonidis, T. G., A. Akesson, B. Gigante, A. Wolk, and S. C. Larsson. 2016. Adherence to a Mediterranean diet is associated with reduced risk of heart failure in men. Eur J Heart Fail 18:253–259. doi:10.1002/ejhf.481.
- Tognon, G., L. Lissner, D. Saebye, K. Z. Walker, and B. L. Heitmann. 2014. The Mediterranean diet in relation to mortality and CVD: A Danish cohort study. Br J Nutr 111:151–159. doi:10.1017/S0007114513001931.
- USDA., U. S. D. O. A. 2015. Scientific report of the 2015 dietary guidelines advisory committee. Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. http://www.health.gov/dietaryguidelines/2015-scientific-report/.
- Verschuren, W. M., D. R. Jacobs, B. P. Bloemberg, D. Kromhout, A. Menotti, C. Aravanis, H. Blackburn, R. Buzina, A. S. Dontas, F. Fidanza, et al. 1995. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA 274:131–136. doi:10.1001/jama.1995.03530020049031.
- Virtanen, J. K., J. Mursu, H. E. Virtanen, M. Fogelholm, J. T. Salonen, T. T. Koskinen, S. Voutilainen, and T. P. Tuomainen. 2016. Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein E phenotype in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 103:895–901. doi:10.3945/ajcn.115.122317.
- WCRF. 2017. World cancer research fund international. Continuous Update Project (CUP): London. http://www.wcrf.org/int/research-we-fund/continuous-update-project-cup.
- Weng, Y. Q., J. Yao, M. L. Guo, Q. J. Qin, and P. Li. 2016. Association between nut consumption and coronary heart disease: A meta-analysis. Coron Artery Dis 27:227–232. doi:10.1097/MCA.0000000000000331.
- Wennberg, M., I. A. Bergdahl, G. Hallmans, M. Norberg, T. Lundh, S. Skerfving, U. Stromberg, B. Vessby, and J. H. Jansson. 2011. Fish consumption and myocardial infarction: A second prospective biomarker study from northern Sweden. Am J Clin Nutr 93:27–36. doi:10.3945/ajcn.2010.29408.
- Wennberg, M., I. A. Bergdahl, B. Stegmayr, G. Hallmans, T. Lundh, S. Skerfving, U. Stromberg, B. Vessby, and J. H. Jansson. 2007. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr 98:1038–45. doi:10.1017/S0007114507756519.
- Whelton, S. P., J. He, P. K. Whelton, and P. Muntner. 2004. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol 93:1119–23. doi:10.1016/j.amjcard.2004.01.038.
- Wilk, J. B., M. Y. Tsai, N. Q. Hanson, J. M. Gaziano, and L. Djousse. 2012. Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians' Health Study. Am J Clin Nutr 96:882–888. doi:10.3945/ajcn.112.042671.
- Wurtz, A. M., M. D. Hansen, A. Tjonneland, E. B. Rimm, E. B. Schmidt, K. Overvad, and M. U. Jakobsen. 2016. Substitution of meat and fish with vegetables or potatoes and risk of myocardial infarction. Br J Nutr 116:1602–10. doi:10.1017/S0007114516003500.
- Xun, P., B. Qin, Y. Song, Y. Nakamura, T. Kurth, S. Yaemsiri, L. Djousse, and K. He. 2012. Fish consumption and risk of stroke and its subtypes: Accumulative evidence from a meta-analysis of prospective cohort studies. Eur J Clin Nutr 66:1199–1207. doi:10.1038/ejcn.2012.133.
- Yaemsiri, S., S. Sen, L. Tinker, W. Rosamond, S. Wassertheil-Smoller, and K. He. 2012. Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann Neurol 72:704–715. doi:10.1002/ana.23555.
- Yamada, T., S. Hayasaka, Y. Shibata, T. Ojima, T. Saegusa, T. Gotoh, S. Ishikawa, Y. Nakamura, and K. Kayaba. 2011. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: The Jichi Medical School cohort study. J Epidemiol 21:169–175. doi:10.2188/jea.JE20100084.
- Yan, Z., X. Zhang, C. Li, S. Jiao, and W. Dong. 2017. Association between consumption of soy and risk of cardiovascular disease: A meta-analysis of observational studies. Eur J Prev Cardiol 24:735–747. doi:10.1177/2047487316686441.
- Yang, C., L. Pan, C. Sun, Y. Xi, L. Wang, and D. Li. 2016. Red meat consumption and the risk of stroke: A dose-response meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis 25:1177–86. doi:10.1016/j.jstrokecerebrovasdis.2016.01.040.
- Yokoyama, T., C. Date, Y. Kokubo, N. Yoshiike, Y. Matsumura, and H. Tanaka. 2000. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community. The Shibata study. Stroke 31:2287–94. doi:10.1161/01.STR.31.10.2287.
- Yu, D., X. O. Shu, H. Li, Y. B. Xiang, G. Yang, Y. T. Gao, W. Zheng, and X. Zhang. 2013. Dietary carbohydrates, refined grains, glycemic load, and risk of coronary heart disease in Chinese adults. Am J Epidemiol 178:1542–49. doi:10.1093/aje/kwt178.
- Yu, D., X. Zhang, Y. T. Gao, H. Li, G. Yang, J. Huang, W. Zheng, Y. B. Xiang, and X. O. Shu. 2014. Fruit and vegetable intake and risk of CHD: Results from prospective cohort studies of Chinese adults in Shanghai. Br J Nutr 111:353–362. doi:10.1017/S0007114513002328.
- Zhang, Y., J. Tuomilehto, P. Jousilahti, Y. Wang, R. Antikainen, and G. Hu. 2011. Lifestyle factors on the risks of ischemic and hemorrhagic stroke. Arch Intern Med 171:1811–18. doi:10.1001/archinternmed.2011.443.