ABSTRACT
The health benefits of fish oil, and its omega-3 long chain polyunsaturated fatty acid content, have attracted much scientific attention in the last four decades. Fish oils that contain higher amounts of eicosapentaenoic acid (EPA; 20:5n-3) than docosahexaenoic acid (DHA; 22:6n-3), in a distinctive ratio of 18/12, are typically the most abundantly available and are commonly studied. Although the two fatty acids have traditionally been considered together, as though they were one entity, different physiological effects of EPA and DHA have recently been reported. New oils containing a higher quantity of DHA compared with EPA, such as fractionated and concentrated fish oil, tuna oil, calamari oil and microalgae oil, are increasingly becoming available on the market, and other oils, including those extracted from genetically modified oilseed crops, soon to come. This systematic review focuses on the effects of high DHA fish oils on various human health conditions, such as the heart and cardiovascular system, the brain and visual function, inflammation and immune function and growth/Body Mass Index. Although inconclusive results were reported in several instances, and inconsistent outcomes observed in others, current data provides substantiated evidence in support of DHA being a beneficial bioactive compound for heart, cardiovascular and brain function, with different, and at times complementary, effects compared with EPA. DHA has also been reported to be effective in slowing the rate of cognitive decline, while its possible effects on depression disorders are still unclear. Interestingly, gender- and age- specific divergent roles for DHA have also been reported. This review provides a comprehensive collection of evidence and a critical summary of the documented physiological effects of high DHA fish oils for human health.
1. Introduction
Fish oils contain high, but variable, levels of omega-3 long chain polyunsaturated fatty acids (n-3 LC-PUFA), including eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), as well as small quantities of docosapentaenoic acid (DPA; 22:5n-3). Triggered by the pioneering study of Greenland Eskimos conducted by Bang et al. (Citation1971) more than 40 years ago, both scientific and public interest surrounding the role of fish oil and, thus, n-3 LC-PUFA in nutrition and health, has increased over the past few decades. The original study by Bang et al. (Citation1971) deduced that even though the Eskimos had a high consumption of fat, their incidence of ischemic heart disease was very low. These indications of the possible cardioprotective effect of dietary n-3 LC-PUFA present in oily fish has fuelled numerous studies examining the health effects of dietary fish oil (Dyerberg et al. Citation1978). Research in this area was also stimulated by the finding that mammalian brains were invariably rich in DHA (Crawford et al. Citation2009), which further induced research into maternal and early infant nutrition. There is a plethora of studies to-date that demonstrate the positive effects of supplementation with fish oil containing EPA and DHA on human health and, in some circumstances, the prevention of certain diseases. Consequently, major health organizations globally have made recommendations for increased dietary intakes of EPA, DPA, and DHA, all of which can be found primarily in fish oils, and the flesh of oily fish, in nutritionally meaningful quantities. The Food and Agriculture Organization of the United Nations (Citation2010) recommends a dose of 250 mg/day of EPA plus DHA for adult males and non-pregnant/non-lactating adult females, while the American Heart Association recommends about 1 g/day of EPA plus DHA for patients with known coronary heart disease, and 2 to 4 g/day for patients requiring triacylglycerol (TAG) lowering (Kris-Etherton et al. Citation2002).
Most of the currently available studies investigating the effect of dietary n-3 LC-PUFA, on which dietary recommendations are based upon, used commercially available fish oil supplements, typically containing mixtures of EPA and DHA. The most abundantly produced and available fish oils globally are anchovy (Engraulis ringens) oil, capelin (Mallotus villosus) oil, menhaden (Brevoortia tyrannus) oil and herring (Clupea harengus) oil, all of which contain variable amounts of n-3 LC-PUFA, but are characterised by higher concentrations of EPA than DHA (Turchini et al. Citation2009). In human and animal studies, the most commonly studied fish oil is anchovy oil, which typically contains about 30% of EPA and DHA, in a 18/12 ratio (180 mg of EPA and 120 mg of DHA per g of oil). The EPA and DHA content and ratio in the oil might vary depending on the kind of feed (microalgae, zooplankton, etc.) and the season. Accordingly, it is now a widely-accepted industry standard that the oil intended for human consumption should contain 30% of EPA plus DHA, where 18/12 ratio is the most preferred and valuable profile. Batches characterised by different quantities of EPA and DHA are instead commonly redirected towards feed applications” (Gustavo Ferreyros, TASA, personal communication).
From nutritional/practical viewpoint, EPA and DHA are generally considered a single entity derived from a consistent ratio of two oils, with EPA being 50% more abundant than DHA. Nevertheless, there is a growing body of evidence showing that EPA and DHA exert heterogeneous effects on health outcomes, especially those that are cardiometabolic related. Biologic pathways through which n-3 LC-PUFA play essential roles include: i) modulation of cell and organelle membrane structure and function; ii) modulation of ion channels and cellular electrophysiology; iii) regulation of nuclear receptors and transcription factors; and iv) production of n-3 LC-PUFA derived bioactive metabolites (Mozaffarian and Wu Citation2012). Compared to EPA, DHA contains a longer carbon chain (22 vs 20) and an additional double bond (6 vs 5) per molecule, which is deemed to be the reason for the different metabolic effects between the two molecules. For example, it has been reported that DHA has a greater influence on membrane fluidity and, thus, on the activity of membrane protein and ion channels (Hashimoto et al. Citation1999). There is also increasing evidence for the different roles and actions of the bioactive metabolites derived from EPA and DHA, such as eicosanoids, lipoxygenase metabolites and docosanoids (Serhan Citation2005). In addition, it has been shown that dietary DHA and EPA have a different metabolic fate in animal models, with DHA being preferentially retained over EPA, and EPA being β-oxidised more than DHA (Ghasemifard et al. Citation2015a). Further, a review paper by Cottin et al. (Citation2011) explored the known differential effects of EPA and DHA in human subjects, and concluded that there is an apparent efficacy of DHA to improve several cardiovascular risk factors, and although both EPA and DHA can reduce TAG levels, the actual role for EPA has not yet been fully clarified. Hence, it appears to be plausible that fish oils with a higher amount of DHA than EPA might possess different health benefits compared to the high EPA fish oil traditionally used. In light of these considerations, and the ever-increasing availability of new fish oils, and n-3 LC-PUFA preparations containing higher concentrations of DHA, such as tuna oil, calamari oil, fractionated and concentrated fish oils, single cell oils from heterotrophic algae and genetically modified oilseed crops (Miller et al. Citation2011; Olsen et al. Citation2011), it is important to gain a better understanding of the potential metabolic and health effects of DHA, uncoupled by higher EPA content.
Therefore, it is the purpose of this review to systematically assess the role of high DHA fish oils in human health, with a focus on studies conducted with humans, but also considering relevant information from mammalian animal studies, using fish oils reported to have a higher DHA content than EPA. The ultimate objective of this review is to provide evidence, based on the currently available literature, for the clinical therapeutic potential of dietary administration of high DHA fish oil preparations, and/or to highlight possible knowledge gaps and potential future research directions.
2. Literature search; methods and statistical considerations
Following on from a pioneering review paper on this topic, and currently the only one of its kind (Horrocks and Yeo Citation1999), we have systematically searched all scientific literature from the year 2000 to the present date, and retrieved all clinical and animal based studies where high DHA oils were used and tested.
To retrieve the relevant studies to be used in this review, a search strategy using Medline/PubMed, ScienceDirect, Web of Science and EMBASE was conducted using the following search terms: “long chain fatty acids”, “omega-3”, “DHA”, “EPA”, “fish oil”, “capsule”, and “heart”, “brain”, “body mass”, “asthma”, “growth”, “diabetes”, “visual” and “medical uses”. Subsequently all retrieved studies were analysed by reading the full-text articles.
The exclusion criteria were: thesis; patents; research protocol studies; DHA content lower than EPA; the use of fully purified DHA/ EPA; the use of omega-3 sources not from fish oil; the use of other interventions in addition to fish oil administration; any publication before 2000; more than one supplier during the study; and any language other than English.
In addition, some studies were excluded as they did not provide sufficient details such as EPA and DHA content (Haugaard et al. Citation2006; Larter et al. Citation2008; Teague et al. Citation2013; Teague et al. Citation2014), or the source of supplement was not specified (Tokuda et al. Citation2015), or they used DHA/ EPA from algae (Corder et al. Citation2016; DiLorenzo et al. Citation2014; Guzmán et al. Citation2011; Quinn et al. Citation2010; Singhal et al. Citation2013; Yurko-Mauro et al. Citation2010), or they used fully purified DHA/ EPA (Egert et al. Citation2008; Woodman et al. Citation2003). Several studies tested the effects of fish oil with other interventions, and although their study design and sample size was acceptable, they were excluded from this analysis because of possible confounding effects of the secondary intervention. For example, in the Childhood Asthma Prevention Study, the effect of dietary omega-3 supplementation and/or house dust mite was studied (Brew et al. Citation2015; Hansell et al. Citation2014; Marks et al. Citation2006; Skilton et al. Citation2012), while in the Nutrition and Health Lifestyle Study, the effect of fish oil supplementation with or without folate was assessed (Campoy et al. Citation2011; Escolano-Margarit et al. Citation2011). In addition, two studies assessed the effects of DHA as one component of a multinutrient supplement on cognition function (Jackson et al. Citation2016; Strike et al. Citation2015); Micallef and Garg (Citation2009) conducted a randomized, double-blind, placebo-controlled study where 60 hyperlipidemic participants were provided with either sunola oil (high-oleic sunflower oil) or n-3 LC-PUFA capsules with or without plant sterols for three weeks; Johnson et al. (Citation2008) tested the low dietary intake of DHA and/or foods rich in lutein on cognitive decline in the elderly; Smuts et al. (Citation2015) investigated the effects of iron and DHA supplementation, alone or in combination, on physical activity; Meyer et al. (Citation2007) tested the benefits of high DHA fish oil supplementation as an adjunct to statin therapy for hyperlipidaemia; and Newens et al. (Citation2011) investigated the effect of acute elevation of non-essential fatty acids enriched with either saturated fatty acids (SFA) or SFA plus fish oil on vascular function. Further, a double-blind, randomized, controlled trial run by D'Vaz et al. (Citation2012) studied the effect of early postnatal fish oil supplementation on infant cellular immune function at 6 months of age in the context of allergic disease examined. Although important and informative, this study was excluded from our analysis as two different fish oil suppliers, with different EPA and DHA contents and ratios, were used during the trials.
While the studies detailed above were all excluded from this review paper, intervention trials of omega-3 and regular exercise (Buckley et al. Citation2009; Hill et al. Citation2007a; Hill et al. Citation2007b; Marques et al. Citation2015; Ninio et al. Citation2008; O'Keefe Jr et al. Citation2006; Peoples et al. Citation2008), and omega-3 and weight-loss programs (Gunnarsdottir et al. Citation2008; Hill et al. Citation2007a; Munro and Garg Citation2013a) were included, with the exception of a study by Park et al. (Citation2000) who studied the effect of high DHA with pectin on a variety of parameters in rats; this study was excluded because of the confounding effect of pectin.
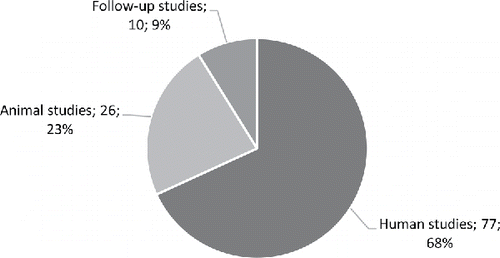
Of the 375 human and animal studies retrieved and reviewed, a total of 113 studies, including human studies, follow-up studies and animal studies, met the inclusion criteria and were reported and analysed separately herein (). The human and animal studies have been subdivided into three sub-sections according to overall metabolic aspects and the number of publications available: (i) heart and exercise; (ii) brain and visual system and (iii) other medical aspects. All follow-up studies were presented in a single, individual section.
Figure 1. Summary and types of all studies retrieved, selected and used for the present analysis of high DHA fish oils (number of studies and percentage relative to entire dataset).
In instances where the fatty acid composition of doses/diets was reported by authors with qualitative values (percentages) only, and quantitative data was not reported, the content of DHA and EPA delivered to subjects/animals was estimated. This estimate was based on a simple computation relative to percent values reported and the total volume of oil/lipid administered, by assuming that 1 g of oil would contain 1000 mg of fatty acids, and thus a overestimation is likely.
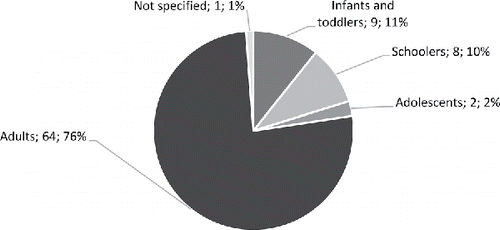
To characterise the populations of the examined human studies, the following age classes were defined and used: infants (0–1 years old), toddlers (1–3 years old), schoolers (3–12 years old), adolescents (12–18 years old) and adults (above 18 years old, including young adults, middle-age adults and older adults) (). Majority of the studies investigated the effect of high DHA fish oils in adults (76%), followed by studies on infants and toddlers (11%), schoolers (10%) and adolescents (2%). One study did not specify the age range of subjects (1%).
Figure 2. Number, and percentage relative to entire dataset, of retrieved and analysed studies per age classes used for the present analysis of high DHA fish oils.
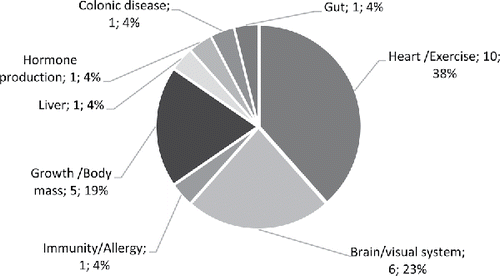
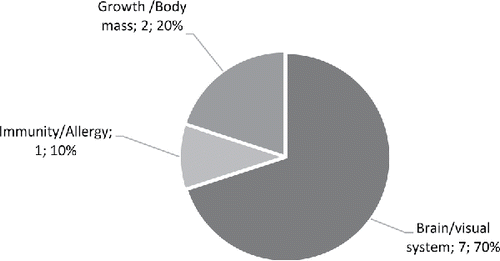
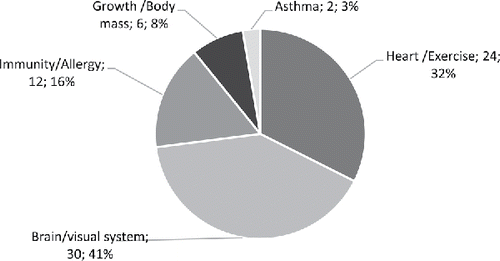
As shown in , the majority of human studies focused on the effects of high DHA fish oils on the brain (41%), heart (32%), immunity (16%) and growth/body mass (8%). In animal studies (), the focus was on the heart (38%), followed by the brain (23%), growth/body mass (19%) and then immunity (4%). In follow-up studies (), 70% of studies focused on the brain and visual system, followed by growth/body mass (20%) and then immunity (10%).
Figure 3. The physiological/medical application areas of human studies retrieved and used for the present analysis of high DHA fish oils (number of studies and percentage relative to human studies).
3. Results of selected studies
3.1. Human studies
3.1.1. Heart and exercise
A total of 24 studies focusing on the effects of administering high DHA fish oils on heart functioning and exercise performance were analysed, and are summarised in .
Table 1. A summary of experimental design and results from human studies testing the effects of high DHA fish oil on heart functioning and exercise performance.
Amongst these studies, there were three postprandial crossover studies, two of which were specifically designed to test the effect of high DHA fish oils on cardiovascular outcomes in healthy subjects (Armah et al. Citation2008; Chong et al. Citation2010), and one testing individuals with increased cardiovascular disease (CVD) risk (McManus et al. Citation2016). The remainder of the studies were long-term, intervention trials with a duration of 2 weeks to 8 months (the mean intervention trial duration was 12 weeks). The number of subjects recruited ranged from 16 to 312 (median of 41), while postprandial studies typically had a relatively small number of participants compared with longer-term studies. Most trials were conducted with male and female participants (62.5%), though one trial was conducted with females only (4.2%), and the remainder with males only (33.3%). A total of 62% of the studies were carried out with healthy subjects, and 37% with subjects that had a disorder, such as being overweight, hyperlipidemic, and hypertriglyceridaemic.
In general, the doses of EPA plus DHA given to subjects were < 1.0 g/day in four trials (Macartney et al. Citation2014; McEwen et al. Citation2013; McEwen et al. Citation2015; O'Keefe Jr et al. Citation2006), 1.0–1.9 g/day in ten trials (Buckley et al. Citation2009; Hill et al. Citation2007a; Khan et al. Citation2003; Kumar et al. Citation2011; Mann et al. Citation2010; Ninio et al. Citation2008; Ottestad et al. Citation2012; Pedersen et al. Citation2010; Phang et al. Citation2013; Poppitt et al. Citation2009), and 3.0–6 g/day in six trials (Armah et al. Citation2008; Buckley et al. Citation2004; Chong et al. Citation2010; Krebs et al. Citation2006; McManus et al. Citation2016; Peoples et al. Citation2008). Two different doses of EPA plus DHA were used in three studies; Sjoberg et al. (Citation2010) trialled 0.6 and 1.9 g/day, Finnegan et al. (Citation2003) trialled 0.8 and 1.7 g/day) and Caslake et al. (Citation2008) trialled 0.7 and 1.8 g/day. Milte et al. (Citation2008) used three dosages (0.52, 1.04 and 1.56 g) of EPA plus DHA per day in their study, where the median dose was 1.6 g/day (range: 0.64 to 5.7 g/day). As described earlier, and according to selection criteria, all of these studies used a preparation where DHA was higher than EPA (DHA>EPA), and the actual DHA: EPA ratio in these studies varied from approximately 1.3: 1 (Ottestad et al. Citation2012) to 6.1: 1 (Buckley et al. Citation2004).
A variety of parameters were measured in these studies, including: i) plasma phospholipid fatty acids and other plasma measurements (fasting plasma lipid, apolipoprotein B, glucose, insulin) (Armah et al. Citation2008; Buckley et al. Citation2004; Caslake et al. Citation2008; Chong et al. Citation2010; Finnegan et al. Citation2003; Mann et al. Citation2010; Milte et al. Citation2008); ii) lipid and apolipoprotein concentrations, lipoprotein subclass distribution, and markers of oxidative status (Caslake et al. Citation2008; Ottestad et al. Citation2012; Pedersen et al. Citation2010; Poppitt et al. Citation2009); iii) erythrocyte n-3 fatty acid content (Buckley et al. Citation2009; Macartney et al. Citation2014); iv) haemostatic markers, platelet aggregation, and thromboxane (Mann et al. Citation2010; McEwen et al. Citation2013; McEwen et al. Citation2015; Phang et al. Citation2013; Poppitt et al. Citation2009); v) blood pressure (McManus et al. Citation2016; O'Keefe Jr et al. Citation2006; Pedersen et al. Citation2010; Sjoberg et al. Citation2010); vi) heart rate (HR), resting HR, HR recovery, heart rate variability (HRV), and HR response to submaximal exercise (Buckley et al. Citation2009; Macartney et al. Citation2014; Ninio et al. Citation2008; O'Keefe Jr et al. Citation2006; Peoples et al. Citation2008; Sjoberg et al. Citation2010); and vii) endothelium-dependent and -independent vascular responses, and pulmonary vein and atrial electrophysiology (Khan et al. Citation2003; Kumar et al. Citation2011).
Hill et al. (Citation2007a) reported that the daily intake of high DHA fish oil could be a useful adjunct to exercise programs aimed at improving body composition and decreasing CVD risk in overweight individuals. Findings from this trial suggest that regular exercise coupled with a moderate dose of high DHA fish oil improves multiple cardiovascular risk factors, including plasma TAGs, high-density lipoprotein (HDL) cholesterol, and flow-mediated dilatation. O'Keefe Jr et al. (Citation2006) found that high DHA fish oil decreased HR at rest from 73 ± 13 to 68 ± 13 beats/min, and improved 1-minute HR recovery after exercise. Overall, HRV was unaffected by high DHA fish oil administration, though HRV in the high-frequency band increased significantly (p <0.02). There were no significant effects on blood pressure, arterial compliance, lipids, or inflammatory markers reported between groups. Buckley et al. (Citation2009) showed that the erythrocyte EPA, DHA and total omega-3 fatty acids content increased almost two-fold, while serum TAG and HR reduced during submaximal exercise in athletic subjects receiving a high DHA fish oil supplementation, compared to the placebo. Despite no effect on resting HR, HR during submaximal exercise decreased by ∼8 beats/min with high DHA fish oil, compared to only ∼ 2 beats/min with sunflower oil, suggesting that high DHA fish oil improved CV efficiency during submaximal exercise. In this study, high DHA fish oil did not affect endurance exercise performance or recovery. Ninio et al. (Citation2008) reported that high DHA fish oil supplementation improved HRV in volunteers by increasing high-frequency power, and it reduced HR at rest and during submaximal exercise. The effects of high DHA fish oil supplementation on O2 consumption during exercise was investigated in trained male cyclists (Peoples et al. Citation2008). No difference in O2 consumption or peak workload after supplementation with fish oil was recorded. However, the high DHA fish oil supplementation, compared to control, lowered HR (including peak HR) during incremental workloads to exhaustion, lowered steady-state submaximal exercise HR, whole-body O2 consumption, and rate pressure product. Armah et al. (Citation2008) found that postprandial triacylglycerol-rich lipoproteins, isolated after a high DHA fish oil meal, increased endothelial nitric oxide synthase and decreased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase gene expression compared to the control meal. Chong et al. (Citation2010) found that consumption of the high DHA meal had an attenuating effect on augmentation index and arterial stiffness index compared with the control meal. McManus et al. (Citation2016) reported that a single dose of DHA, but not EPA, significantly improved postprandial arterial stiffness compared to the control, as assessed by augmentation index, which, if sustained, would be associated with a significant decrease in CVD risk. McEwen et al. (Citation2013) showed that supplementation of high DHA fish oil reduced measures of platelet aggregation and activation in healthy subjects, but induced few changes in patients with existing CVD, suggesting that the n-3 LC-PUFA dose should be higher in those suffering from CVD, than among healthy subjects. The latter randomized, intervention trial (McEwen et al. Citation2013) was also used to test the effects of n-3 LC-PUFA on novel markers of global coagulation by McEwen et al. (Citation2015). Results of this study indicated a decrease in fibrin generation and an increase in fibrinolysis after supplementation with a high DHA preparation in healthy subjects. In subjects with CVD, the high DHA preparation reduced the fibrin generation parameter of overall haemostasis potential, which is a more global summary of coagulation, but did not affect fibrinolysis. Macartney et al. (Citation2014) found that a low intake of high DHA tuna oil increased the omega-3 index (EPA plus DHA percent, relative to total fatty acids, in red blood cells), reduced the mean exercise HR, and improved HR recovery without compromising the peak HR. Poppitt et al. (Citation2009) showed no significant differences in lipid, inflammatory, hemostatic, or composite mood parameters between high DHA fish oil supplementation and the placebo in patients with ischemic stroke. Mann et al. (Citation2010) reported that both high DHA fish oil and seal oil (an oil characterised by higher DPA content, compared to traditional fish oil) elevated platelet DHA levels, while seal oil also raised platelet DPA and EPA levels. In addition, plasma TAG decreased and high-density lipoprotein (HDL) cholesterol levels increased following supplementation with seal oil. Comparing daily doses of high DHA oil versus EPA-rich oil it was reported that, although platelet aggregation was different for males and females, EPA and DHA reduced platelet aggregation in both sexes compared to the placebo (−11.8% and −14.8%, respectively) (Phang et al. Citation2013). The EPA treatment reduced platelet aggregation in males by −18.4%, compared to the placebo and females, and, in contrast, the DHA treatment reduced platelet aggregation in females by −18.9%, compared to the placebo and males. Khan et al. (Citation2003) found that acetylcholine, but not sodium nitroprusside, response was significantly improved after tuna oil supplementation compared with other oil treatments, suggesting that high DHA fish oil supplementation has a beneficial effect on endothelial function. Total serum TAG, total low-density lipoprotein (LDL), and HDL cholesterol were unchanged by a daily supplementation with high DHA fish oil or high oleic acid sunflower oil for seven week (Ottestad et al. Citation2012). These authors also found that high DHA fish oil supplementation altered lipid metabolism: 23 lipids significantly decreased and 51 lipids, including, selected phospholipids and TAGs of n-3 LC-PUFA, increased. Kumar et al. (Citation2011) showed that patients with paroxysmal atrial fibrillation supplemented with high DHA fish oil had distinctive electrophysiologic properties including prolonged pulmonary venous and left atrial effective refractory periods, and decreased susceptibility to initiation atrial fibrillation from within pulmonary venous. These results endorse the antifibrillatory effect of chronic n-3 LC-PUFA supplementation. Pedersen et al. (Citation2010) reported that systolic blood pressure and diastolic blood pressure were lowered in the high DHA fish oil group compared with the control group. Plasma TAG concentration and insulin sensitivity were unaffected by either of the treatments. In the fish oil group, plasma HDL and non-HDL cholesterol were increased by 5% and 7%, respectively. Buckley et al. (Citation2004) reported that EPA and DHA treatments had no significant effects on circulating total cholesterol, LDL cholesterol or HDL cholesterol levels. There was a significant 22% reduction in TAG levels relative to the control following the DHA treatment (p = 0.032), with a 15% decrease in the EPA group, which was not significant. Krebs et al. (Citation2006) showed that TAG and adipose tissue were significantly lower and higher, respectively, in the high DHA fish oil group at 24 weeks, relative to the control group. This trend was not exhibited for LDL cholesterol, total cholesterol, systolic or diastolic blood pressure. Compared to a sunola oil control, no effect of high DHA fish oil on blood pressure, small artery compliance or HR was observed (Sjoberg et al. Citation2010). However, the low: high frequency ratio of HRV decreased with increasing doses of high DHA fish oil. Finnegan et al. (Citation2003) observed no significant effects on the postprandial TAG response, glucose, or insulin concentrations amongst the tested treatments consisting of an n-6 control or a series of treatments with different DHA, EPA and ALA supplementations. Caslake et al. (Citation2008) found that plasma TAG concentrations decreased by 8% and 11% after low and high doses of high DHA fish oil, respectively. Furthermore, lower VLDL cholesterol, and higher LDL cholesterol, HDL cholesterol, and HDL2 concentrations were evident after fish-oil intervention. Milte et al. (Citation2008) showed that for any 1 g/day increase in DHA intake, there was a 23% reduction in TAG, a 7.1% increase in LDL and a 4.4% increase in HDL.
3.1.2. Brain and visual system
Details of the 30 high DHA fish oil trials focusing on the brain and visual system that were analysed in this review are reported in . These were long-term studies, with an intervention duration ranging from 3 weeks to 3 years. The number of subjects per group within these studies varied from 7 to 1202. A total of 73% of these trials were conducted with male and female participants, 23% with females only, and one trial was conducted with males only. In majority (65%) of the trials participants were healthy, while in the remaining 35% participants suffered and/or were being treated for Alzheimer's disease, memory complaints, mild cognitive impairment, stress, or depression.
Table 2. A summary of experimental design and results from human studies testing the effects of high DHA fish oil on the brain and visual system.
Doses of DHA plus EPA given to subjects were <1.0 g/day in thirteen trials (Barbadoro et al. Citation2013; Dangour et al. Citation2010; Freund-Levi et al. Citation2008; Hamazaki et al. Citation2008; Hirayama et al. Citation2004; Hurtado et al. Citation2015; Itomura et al. Citation2005; Jackson et al. Citation2012a; Jackson et al. Citation2012b; Makrides et al. Citation2010; Meldrum et al. Citation2012; Stough et al. Citation2012; van Goor et al. Citation2010), 1.0–1.9 g/day in twelve trials (Andrieu et al. Citation2017; Bradbury et al. Citation2004; Clayton et al. Citation2009; Lauritzen et al. Citation2004; Lee et al. Citation2013; Makrides et al. Citation2009; Mozurkewich et al. Citation2013; Phillips et al. Citation2015; Rogers et al. Citation2008; Smithers et al. Citation2008; Souied et al. Citation2013; Stonehouse et al. Citation2013), 2.0–2.9 g/day in three trials (Grenyer et al. Citation2007; Rees et al. Citation2008; Sinn et al. Citation2012), and 3–6 g/day in one trial (Silvers et al. Citation2005). Jackson et al. (Citation2012c) used two different dosages (1 and 2 g/day) of DHA + EPA in their study, with a median dose of 0.98 g/day (range: 0.25 to 3 g/day). The DHA: EPA ratios in the trials analysed here were from approximately 1: 1 (Phillips et al. Citation2015) to 8: 1 (Makrides et al. Citation2010).
Psychological (perceived stress and depression) measurements were performed in eleven studies (Barbadoro et al. Citation2013; Bradbury et al. Citation2004; Clayton et al. Citation2009; Freund-Levi et al. Citation2008; Grenyer et al. Citation2007; Hirayama et al. Citation2004; Makrides et al. Citation2010; Mozurkewich et al. Citation2013; Rees et al. Citation2008; Rogers et al. Citation2008; Silvers et al. Citation2005); cognitive function was measured in eleven studies (Andrieu et al. Citation2017; Dangour et al. Citation2010; Hurtado et al. Citation2015; Jackson et al. Citation2012a; Jackson et al. Citation2012c; Lee et al. Citation2013; Phillips et al. Citation2015; Rogers et al. Citation2008; Sinn et al. Citation2012; Stonehouse et al. Citation2013; Stough et al. Citation2012); neurodevelopment was measured in five studies (Makrides et al. Citation2009; Makrides et al. Citation2010; Meldrum et al. Citation2012; van Goor et al. Citation2010); visual function was measured in five studies (Hurtado et al. Citation2015; Lauritzen et al. Citation2004; Smithers et al. Citation2008; Souied et al. Citation2013; Stough et al. Citation2012); behavioural outcome was measured in two studies (Hamazaki et al. Citation2008; Itomura et al. Citation2005); and physiological function of brain was measured in one study (Jackson et al. Citation2012b).
The effect of DHA on attention-deficit/hyperactivity disorder (AD/HD) symptoms was studied by Hirayama et al. (Citation2004), and findings of this study suggest that DHA supplementation does not improve AD/HD-related symptoms. Itomura et al. (Citation2005) found that physical aggression in girls increased significantly in the control group but did not change in the high DHA fish oil group; whereas there were no significant changes in physical aggression in boys. Freund-Levi et al. (Citation2008), studying AD patients and psychiatric and behavioral symptoms, reported that, overall, supplementation had no significant effects on neuropsychiatric symptoms or on daily activities. Hamazaki et al. (Citation2008) reported that the concentrations of DHA and EPA in the phospholipid fraction in red blood cells were significantly increased in the DHA group compared to the control. Although there were no differences in behaviour between groups, children in the DHA group had a significantly higher school attendance rate. van Goor et al. (Citation2010) reported that neither DHA alone, nor DHA + arachidonic acid (20:4n-6) during pregnancy and lactation, influenced outcomes in the traditional examination of infant's brain development. General movement quality of infants in the DHA group was lower than that of infants in the other two groups. The authors concluded that general movement quality at 12 weeks postpartum is sensitive to the maternal dietary DHA/ arachidonic acid balance. Makrides et al. (Citation2010) ran a trial (DHA to Optimize Mother Infant Outcome [DOMInO] trial) to determine whether increasing DHA during the last half of pregnancy ameliorates maternal depression and enhances the neurodevelopment of their children. The authors found that use of high DHA fish oil did not affect the levels of postpartum depression in mothers, nor modify the cognitive and language development in their children. The effect of n-3 LC-PUFA on cognitive function in elderly people was tested and no change in cognitive function scores over the 24-month period was observed in the high DHA fish oil group compared to control (Dangour et al. Citation2010). Jackson et al. (Citation2012a) suggested that supplementation with n-3 LC-PUFA, high DHA and high EPA fish oils, in healthy, normally developing, and impairment-free populations, is unlikely to result in cognitive enhancement. In another study conducted by Jackson et al. (Citation2012b) it was reported that supplementation with high DHA fish oil, in comparison with the placebo and high EPA fish oil supplement (which did not result in differences compared to the placebo), significantly increased the concentrations of oxygenated-haemoglobin and total levels of haemoglobin, indicating an increase in the cerebral blood flow during the cognitive tasks. No significant effects on cognitive function following DHA supplementation compared to the control were observed (Stough et al. Citation2012); but for participants with corrected vision, the group receiving DHA were found to have significantly better right eye visual acuity post-supplementation with DHA in comparison to the control. Meldrum et al. (Citation2012) found no significant difference in infant neurodevelopment between the two tested groups (DHA or control), but some indication of benefits to early communicative development was observed. Barbadoro et al. (Citation2013) showed that an elevated DHA intake reduced distress symptoms and basal cortisol secretion in abstinent alcoholics. Hurtado et al. (Citation2015) found that there was no significant difference in the visual and cognitive/psychomotor development in the newborns between treatment groups (high DHA fish oil vs control). Bradbury et al. (Citation2004) reported no significant difference in perceived stress between the high DHA fish oil group and the placebo group. Smithers et al. (Citation2008) assessed the effects of a high DHA dose on the visual acuity of preterm infants. At 4-months corrected age, higher visual acuity was found in the high-DHA group compared to the control group, and authors suggested that the DHA requirement of preterm infants might be higher than currently provided. Makrides et al. (Citation2009) ran the Docosahexaenoic acid for the Improvement of Neurodevelopmental Outcome in pre-term infants (DINO) trial. At 18 months' corrected age, the mental development index (MDI) among girls fed the high DHA diet was higher than girls fed the standard DHA in both unadjusted and adjusted analyses. The MDI among boys did not differ between groups. Clayton et al. (Citation2009) showed that clinician ratings of mania and depression in children and adolescents with juvenile bipolar disorder were significantly lower, and global functioning significantly higher, after high DHA fish oil supplementation. Parent ratings of internalizing and externalizing behaviours were also significantly lower following supplementation. Lee et al. (Citation2013) reported the results of a trial in elderly subjects with mild cognitive impairment. Individuals in the high DHA fish oil group displayed a significant improvement in short-term and working memory, immediate verbal memory and delayed recall capability. Mozurkewich et al. (Citation2013) suggested that neither high DHA nor high EPA fish oil supplementation during pregnancy prevents depressive symptoms during pregnancy or postpartum. Souied et al. (Citation2013) studied the effects of high DHA fish oil on preventing age-related macular degeneration over a period of 3 years. No significant differences were found in the time to occurrence, and the incidence of choroidal neovascularization, between the DHA and placebo groups. The effect of DHA on cognitive performance in healthy young adults was studied (Stonehouse et al. Citation2013), and findings of this study suggest that DHA supplementation improves memory and memory reaction time in healthy young adults whose habitual diets were low in DHA. Phillips et al. (Citation2015) found that even though omega-3 fatty acid supplementation elevates plasma concentrations of DHA and EPA, this did not result in any measurable benefits on cognition and mood in cognitively impaired subjects with dementia or AD. The effect of DHA supplementation and multidomain intervention (physical activity, cognitive training, and nutritional advice), alone or in combination, on cognitive decline in elderly adults with memory complaints was studied (Andrieu et al. Citation2017). No significant difference in 3-year cognitive decline between any of the intervention groups and the placebo group was recorded. Rogers et al. (Citation2008) reported no beneficial, nor harmful, effects of increasing DHA + EPA intake on mood in mild to moderately depressed individuals. The effect of maternal supplementation with high DHA fish oil on the visual acuity of infants was studied by Lauritzen et al. (Citation2004). Infant visual acuity was not significantly different between the high DHA supplementation and control, but was positively associated with infant RBC-DHA at 4 months; suggesting that n−3 LCPUFA may influence visual maturation. Grenyer et al. (Citation2007) showed that, in outpatients with major depression, despite a large numerical reduction in depression following both treatments, no statistically significant differences between patients receiving fish oil compared with placebo could be measured at any assessment time point. No significant difference in depression scores between females with major depression during the perinatal period receiving high DHA fish oil or those receiving the sunola oil was recorded (Rees et al. Citation2008). The beneficial effects of supplementing a diet with n-3 LC-PUFA on enhancing depressive symptoms, quality of life and cognition in elderly people with a mild cognitive impairment were investigated in a double-blind, randomized, controlled trial (Sinn et al. Citation2012). Findings of this study indicate that DHA may be equally, if not more, effective than EPA for improving mood. Depressive symptom scores were significantly reduced after 6 months following high EPA and high DHA supplementation compared to the control. As for the cognitive outcomes, significant improvements in initial letter fluency a, test of fluid thinking ability, were only detected in the DHA group. Silvers et al. (Citation2005) investigated the effect of fish oil supplementation in participants being treated for depression. Though circulating n-3 LC-PUFA increased resulting from high DHA fish oil supplementation, there was no evidence that high DHA fish oil improved mood when compared with the placebo. Jackson et al. (Citation2012c) examined the effect of supplementation with different doses of high DHA fish oil on cerebral blood flow in healthy adult non-consumers of oily fish over a 12-week period. Supplementation with fish oil at both dosages, in comparison with the placebo, resulted in a significant increase in the concentrations of oxyhemoglobin and total hemoglobin, which is indicative of increased cerebral blood flow during cognitive tasks. However, these changes in hemodynamic response to tasks were not accompanied by consistent changes in cognitive performance.
3.1.3. Other medical aspects
Details of the 23 studies reporting findings on the effect of high DHA fish oil administration on immunity, allergy, growth (body mass) and other medical aspects that were included in this review are summarised in .
Table 3. A summary of experimental design and results from human studies testing the effects of high DHA fish oil on other medical aspects.
All of these studies were long-term, intervention trials, with a duration of 14 days to 18 months. The number of participants per group varied from 8 to 1202. Majority (67%) of these trials were performed with male and female participants, while 24% were performed with males only. In addition, two trials were conducted with females only, and one trial did not specify the gender of participants. A total of 56% of the studies used healthy individuals and 44% were either disabled, overweight, obese, preterm, osteoporotic, or suffering from burns, AD, type 2 diabetes, or multiple sclerosis.
The doses of EPA plus DHA administered to subjects were < 1.0 g/day in two trials (Sabour et al. Citation2012; Zhou et al. Citation2012), 1.0–1.9 g/day in nine trials (Atwell et al. Citation2013; Damsgaard et al. Citation2012; Helland et al. Citation2001; Hill et al. Citation2007b; Manley et al. Citation2011; Mansoori et al. Citation2016; Marques et al. Citation2015; Santos et al. Citation2013; Toupchian et al. Citation2016), 2.0–2.9 g/day in seven trials (Freund-Levi et al. Citation2014; Gorjão et al. Citation2006; Munro and Garg Citation2012 Citation2013a, b; Ramirez-Ramirez et al. Citation2013; Vedin et al. Citation2008), and 3–6 g/day in two trials (Kew et al. Citation2004; Tihista and Echavarria Citation2017). Few studies used more than one dose: Kew et al. (Citation2003) used two dosages (0.77 g and 1.79 g EPA + DHA/day), Wallace et al. (Citation2003) and Gunnarsdottir et al. (Citation2008) used three dosages (0.44, 0.94 and 1.9 g EPA + DHA/day; and 0.26, 1.06 and 2.14 g EPA + DHA/day, respectively). DHA: EPA ratio in these trials were from approximately 1.48: 1 (Helland et al. Citation2001) to 8: 1 (Zhou et al. Citation2012).
Cytokines were measured in eleven trials (Freund-Levi et al. Citation2014; Gorjão et al. Citation2006; Hill et al. Citation2007b; Kew et al. Citation2003; Kew et al. Citation2004; Marques et al. Citation2015; Ramirez-Ramirez et al. Citation2013; Sabour et al. Citation2012; Santos et al. Citation2013; Vedin et al. Citation2008; Wallace et al. Citation2003); peripheral blood mononuclear cell functions (phagocytic activity, lymphocyte proliferation) were measured in seven trials (Gorjão et al. Citation2006; Hill et al. Citation2007b; Kew et al. Citation2003; Kew et al. Citation2004; Marques et al. Citation2015; Santos et al. Citation2013; Wallace et al. Citation2003); the marker of monocyte/macrophage activation factor was measured in one trial (Toupchian et al. Citation2016); allergy-related events, such as the incidence of bronchopulmonary dysplasia (Manley et al. Citation2011) and the proportion of children hospitalised for lower respiratory tract conditions (Atwell et al. Citation2013), were measured in two trials; bone mass and markers of bone formation were measured in one trial (Damsgaard et al. Citation2012); the incidence of gestational diabetes mellitus and preeclampsia was measured in one trial (Zhou et al. Citation2012); weight and fat mass were measured in six trials (Gunnarsdottir et al. Citation2008; Helland et al. Citation2001; Mansoori et al. Citation2016; Munro and Garg Citation2012 Citation2013a, b); and infectious complications were measured in one trial (Tihista and Echavarria Citation2017).
Hill et al. (Citation2007b) reported that, while there was no effect on cytokine production by T cells and monocytes following supplementation with high DHA fish oil or exercise, supplementation with high DHA fish oil reduced superoxide production from stimulated neutrophils, while regular moderate exercise training protected bactericidal activity. Additionally, neutrophil chemotaxis and adherence were not significantly affected by exercise, oil, or the combination of the two. Marques et al. (Citation2015) suggested that high DHA fish oil could reduce inflammatory disturbance and neutrophil death induced by acute exercise in wheelchair athletes. Munro and Garg (Citation2013a) found significant correlations between leptin and weight loss, and leptin and plasma EPA and DHA levels, in high DHA fish oil group. The authors reported also that there were no significant effects on weight reduction, fat mass reduction and inflammatory biomarkers between high DHA fish oil treatment and placebo. Gunnarsdottir et al. (Citation2008) conducted a trial to determine the effects of fish (lean or oily) and fish oil consumption on blood lipid concentration during weight loss, and tested four treatments: sunflower oil capsules, high EPA fish oil capsules, a cod diet (lean fish, high EPA) or a salmon diet (oily fish, high DHA). Overall, the reduction in total cholesterol was greater in the fish groups (cod and salmon) compared to the control group, however when adjusting for weight loss, this result is of borderline significance. The overall decrease in HDL was greater in the control group compared to the fish and fish oil groups. In addition, there were significant correlations between weight-loss diet and the reduction of TG following the consumption of high DHA oily fish, compared to groups receiving either high EPA fish oil or sunflower oil capsules. The effects of n-3 LC-PUFA on inflammatory cytokines were studied by Sabour et al. (Citation2012) in osteoporotic patients, who showed that high DHA fish oil supplementation for 4 months had no significant effect on inflammatory markers, and in a trial aimed to determine the effect of DHA supplementation during pregnancy on the incidence of gestational diabetes mellitus or preeclampsia (Zhou et al. Citation2012). The overall incidence of gestational diabetes mellitus and preeclampsia did not differ significantly between the high DHA fish oil and the placebo groups. The effect of DHA supplementation on long-term atopic respiratory outcomes in preterm infants was reported (Manley et al. Citation2011). A significant reduction in bronchopulmonary dysplasia was observed in the high DHA group, compared to the placebo, at either 12 or 18 month's in infants with a birth weight of < 1250 g. However, as reported in a following study, the hospitalisation rate for lower respiratory tract conditions was not reduced compared with the control (Atwell et al. Citation2013). Santos et al. (Citation2013) investigated the effects of high DHA fish oil supplementation on lymphocyte function in male athletes before and after a marathon race. High DHA fish oil supplementation increased lymphocyte proliferation and prevented the reduction of cytokine production, suggesting it may be beneficial in preventing some of the changes in lymphocyte function following marathon participation. Toupchian et al. (Citation2016) reported that, when compared with the placebo group, serum level of Scd163, the marker of monocyte/macrophage activation factor, decreased significantly in the high DHA fish oil group in type 2 diabetes patients. Mansoori et al. (Citation2016) reported that supplementation with high DHA fish oil, compared to the placebo, decreased waist circumference, body fat mass, body fat percent and visceral fat rating, as well as trunk fat mass, however weight, body mass index, fat-free mass, adiponectin level, and PPARγ gene expression changes did not differ significantly between treatment groups. Damsgaard et al. (Citation2012) showed that although high DHA fish oil increased DHA status, no associations were found between high DHA fish oil supplementation and bone mineral content, bone area, bone mineral density, and the markers of bone formation. Helland et al. (Citation2001) found no difference in gestational length, birth weight, or cognitive function scores, between the high DHA cod liver oil group and the corn oil group in healthy, pregnant women. Gorjão et al. (Citation2006) found that supplementation with high DHA fish oil increased the phagocytic activity in neutrophils and monocytes, neutrophil chemotactic, and the rate of production of reactive oxygen species by neutrophils. Vedin et al. (Citation2008) reported a significant decrease of IL-6, IL-1β, and granulocyte coloby-stimulating factor, was observed in AD patients treated with high DHA supplementation. In a trial evaluating the efficacy of high DHA fish oil supplementation on serum proinflammatory cytokine levels (Ramirez-Ramirez et al. Citation2013), high DHA fish oil treatment decreased the serum levels of TNF-α, IL-1β, and IL-6 compared to the placebo oil treatment. Freund-Levi et al. (Citation2014) reported that supplementation of DHA in AD patients does not affect inflammatory response. Munro and Garg (Citation2012) reported a significant reduction in fat mass for the high DHA fish oil group after 14 weeks intervention in obese male and female subjects, but not for the control sunola oil group, and while the metabolic profiles of both treatment groups improved, overall there was no significant effect on weight loss or weight maintenance over the 14 weeks. Successively, Munro and Garg (Citation2013b) reported that obese females consuming high DHA fish oil over sunola oil exhibited a greater percentage decrease in weight (−7.21% vs −5.82%) and BMI (−7.43% vs −5.91%), respectively, but not in males. The authors suggested that supplementation with high DHA fish oil had a time dependent effect on weight loss in females. Kew et al. (Citation2004) found that supplementation with DHA, but not EPA, suppressed T lymphocyte activation in healthy subjects. Neither a high DHA or a high EPA fish oil had any significant effect on monocyte or neutrophil phagocytosis, cytokine production, or adhesion molecule expression by peripheral blood mononuclear cells. Tihista and Echavarria (Citation2017) performed a trial to investigate the effect of n-3 LC-PUFA on infectious complications in burn patients. Compared to burns patients receiving the conventional low-fat diet, the inclusion of high DHA fish oil in a low-fat diet was of clinical benefit, where a decrease in the rate of severe sepsis, septic shock and pyloric dysfunction was observed. Kew et al. (Citation2003) showed that intakes of plant (ALA)- or marine- derived n-3 LC-PUFA (high DHA), up to 9.5 or 1.7 g/day, respectively, did not influence circulating inflammatory and immune cell numbers compared to the placebo. Eventually, a study determined the effect of enriching the diet with different doses of high DHA fish oil (Wallace et al. Citation2003). Compared to the control, interventions did not influence the functional activity of mononuclear cells; but the two higher doses of fish oils resulted in a significant decrease in IL-6 production by stimulated mononuclear cells.
3.2. Follow-up studies
A number of follow-up studies were initially retrieved, but were later excluded from this review paper as they did not meet the inclusion criteria (Ayer et al. Citation2009; Skilton et al. Citation2012; Skilton et al. Citation2014). Details and characteristics of the 10 high DHA follow-up trials that were considered for analysis are shown in . All studies were follow-up, long-term intervention trials, where subjects of an initial intervention trial were followed for a period of time ranging from 5 months to 12 years.
Table 4. A summary table for follow-up studies testing the effects of high DHA fish oil in humans.
In a follow-up study from the DINO trial (Makrides et al. Citation2009), Smithers et al. (Citation2010) evaluated the long-term effects of feeding preterm infant's with milk containing high levels of DHA on their language, behaviour and temperament at 26 months and 3–5 years corrected age. Findings of this follow-up trial indicated that feeding preterm infants milk with high DHA did not result in any clinically meaningful changes in language development or behaviour when assessed in early childhood. Lauritzen et al. (Citation2005) conducted a follow-up study of a previous trial (Lauritzen et al. Citation2004) to see if maternal fish oil supplementation affects mental development in term infants. A total of 122 Danish mothers were randomly assigned to receive high DHA fish oil during the first 4 months of lactation, and a further 53 mothers with a high-fish intake as a reference group. The authors found that maternal fish oil supplementation in the early stage of lactation had no beneficial effect on the problem-solving capacity of the infant, but resulted in improvement in vocabulary comprehension at one year of age. Cheatham et al. (Citation2011) ran a follow-up study of the trial by Lauritzen et al. (Citation2004). The authors found that boys whose mothers consumed fish oil had lower prosocial scores relative to the control group. Moreover, both speed of processing and inhibitory control/working memory scores were differentially negatively predicted by maternal n-3 LCPUFA intake and infant RBC–DHA status. Helland et al. (Citation2001) ran two follow-up trials (Helland et al. Citation2008; Helland et al. Citation2003) in which mothers took either high DHA cod liver oil or placebo during gestation until 3 months after delivery. Their children's IQ and BMI were measured at 4 and 7 years of age. The authors found children who were born to mothers that had taken cod liver oil during pregnancy, scored higher on the intelligence measurement at 4 years of age. No significant differences in IQ scores were found between children at 7 years of age. Dunstan et al. (Citation2008) showed that children in the high DHA fish oil-supplemented maternal group attained a significantly higher eye and hand coordination score than those in the placebo group. In contrast, a subsequent follow-up study on the same children at 12 years of age (Meldrum et al. Citation2015), found no significant differences between treatment groups for any of the neurodevelopmental assessments. In another follow-up study from the DINO trial (Makrides et al. Citation2009), the effect of high-dose DHA on the growth of preterm infants was tested in comparison to standard feeding practice (Collins et al. Citation2011). The authors reported no effect on the weight or head circumference of children at any age in the high-dose DHA group, however, infants fed high DHA were 0.7 cm longer at 18 months corrected age compared to the control group. Overall, high-dose DHA increased the length of infants weighing ≥1250 g at 4 months corrected age, and both weight and length at 12 and 18 months corrected age. Muhlhausler et al. (Citation2016) ran a follow-up study of children at 3 and 5 years of age who were born to mothers enrolled in the DOMInO trial (Makrides et al. Citation2010). Findings of this trial indicate that maternal intake of high DHA fish oil during the second half of pregnancy does not affect the growth or body composition of children at 3 or 5 years of age.
Best et al. (Citation2016) conducted a follow-up study to determine the effect of high DHA fish oil supplementation during pregnancy on allergies. Allergic disease symptoms were assessed, and sensitization to allergens were measured, in their offspring at 6 years of age. Overall, no difference in the percentage of children with any IgE-associated allergic disease was observed between the high DHA fish oil and control groups.
3.3. Animal studies
3.3.1. Heart and exercise
Details and characteristics of the 10 high DHA fish oil trials focusing on heart function and exercise performance in animals that were included in this analysis are presented in . Although non-mammalian, farmed animals (such as chicken and fish) are excluded in this section, it is interesting to note that a large, and rapidly growing, body of scientific evidence exists on the differences between dietary and physiological roles of EPA and DHA, particularly in fish nutrition studies. Studies observing the effect of omega-3 DHA dietary administration on the meat quality in farmed mammalians are also excluded. If the DHA and EPA contents in the animal's diet was not reported by authors, the content of these fatty acids was calculated and estimated by authors of this paper (as previously described).
Table 5. A summary of animal studies testing the effects of high DHA fish oil on heart functioning and exercise performance.
All of the studies detailed herein were relatively long-term trials (i.e. no short, post-prandial studies) with an intervention duration of 4 weeks to 7 months (the mean trial duration was 7 weeks). In five studies, rats were chosen as an animal model (Abdukeyum et al. Citation2016; Abdukeyum et al. Citation2008; Molinar-Toribio et al. Citation2015; Owen et al. Citation2004; Slee et al. Citation2010), while other studies used mice (Arai et al. Citation2009; Higuchi et al. Citation2006; Huggins et al. Citation2009), pigs (Castellano et al. Citation2010) and rabbits (Ninio et al. Citation2005) in their research. The number of animals per group ranged from 3 to 9. In all animal studies DHA: EPA ratios were equal or above 2, with the exception of one study where the ratio was 1.2: 1 (Castellano et al. Citation2010). Five trials were conducted with male animals only (Abdukeyum et al. Citation2016; Abdukeyum et al. Citation2008; Castellano et al. Citation2010; Higuchi et al. Citation2006; Owen et al. Citation2004), three trials were conducted with female animals only (Arai et al. Citation2009; Huggins et al. Citation2009; Molinar-Toribio et al. Citation2015), and in two trials the sex of the animals was not specified (Ninio et al. Citation2005; Slee et al. Citation2010).
Several different endpoints were assessed in these studies, including: i) lipid and apolipoprotein concentrations, lipoprotein subclass distribution, and markers of oxidative status (Abdukeyum et al. Citation2016; Higuchi et al. Citation2006; Molinar-Toribio et al. Citation2015); ii) myocardial and erythrocyte membrane fatty acid (Abdukeyum et al. Citation2016; Huggins et al. Citation2009; Ninio et al. Citation2005; Owen et al. Citation2004; Slee et al. Citation2010); iii) ex vivo cardiac performance and reperfusion arrhythmia incidence (Abdukeyum et al. Citation2008; Huggins et al. Citation2009), HR and blood pressure (Abdukeyum et al. Citation2008), and plasma lipid; iv) hepatic cholesterol; v) hepatic mRNA expression of lipogenic and lipidolytic genes (Arai et al. Citation2009), and concentration of plasma glucose (Higuchi et al. Citation2006); and vii) insulin secretion and sensitivity (Castellano et al. Citation2010).
Owen et al. (Citation2004) implemented a study to examine the dose and time responses for incorporation of n-3 LC-PUFA into cellular membranes in male Sprague-Dawley rats. The results showed that myocardial DHA levels doubled in 2-days, stabilizing at levels ∼200% higher than the control after 28-days of feeding on a meal containing 10% high DHA fish oil. This study showed that low doses of high DHA fish oil produced marked changes in myocardial DHA levels, and the maximal incorporation takes up to 28 days to occur. Abdukeyum et al. (Citation2008), studied the effects of high DHA fish oil on ischemic preconditioning, and its interaction with heart function and injury during myocardial ischemia and reperfusion in male Wistar rats. It was shown that high DHA fish oil protected the heart against myocardial infarction and arrhythmias, and improved post-ischemic recovery of heart function, compared with the other diets. A follow-up study on the latter intervention trial was also used to test the effects of n-3 LC-PUFA on myocardial membranes, peroxidation and basal fatty acid oxidation (Abdukeyum et al. Citation2016). High DHA fish oil increased peroxidiability leading to up-regulated mitochondrial superoxide dismutase, but not glutathione peroxidase, antioxidant activity. Slee et al. (Citation2010) investigated the incorporation of DHA into myocardial membranes of Sprague-Dawley rats from low dietary fish oil intake. They found that low dietary intakes of high DHA fish oil in the rat, in the range equivalent to the human intake of 1–2 fish meals per week, increased the myocardial membrane n-3 LC-PUFA concentration. Molinar-Toribio et al. (Citation2015) investigated the effect of EPA and DHA supplementation in different proportions on spontaneously hypertensive obese rats.The authors found that supplementation with DHA + EPA at 2: 1 or 1: 2 ratios in obese rats significantly lowered plasma total cholesterol and LDL cholesterol concentrations, compared with the control group. No significant differences in HDL-cholesterol were found between groups. Supplementation with DHA + EPA at ratios of 1: 1 and 1: 2 significantly decreased inflammation, and increased the activity of antioxidant enzymes (superoxide dismutasein erythrocytes and abdominal fat), while supplementation with high DHA fish oil increased superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase in kidneys, and showed a tendency towards decreasing excretion of urinary isoprostanes. Higuchi et al. (Citation2006) investigated the effects of DHA, EPA and high DHA fish oil on plasma glucose, total cholesterol, TAG and phospholipid concentrations inmale mice. They found that plasma phospholipid concentrations were significantly lower in mice fed the high DHA fish oil and DHA + EPA diets than the lard groups. Plasma glucose levels were reduced after the administration of a high DHA fish oil diet, and the DHA and EPA fortified diets. Huggins et al. (Citation2009) studied the effects of dietary n-3 LC-PUFA supplementation on ischemic protection in female mice with an underlying genetic predisposition to cardiac hypertrophy. They reported that a high DHA fish oil diet modestly suppressed hypertrophic growth; however, it did not enhance postischemic recovery, nor did it decrease the incidence of reperfusion arrhythmia in a normal or hypertrophic heart. Arai et al. (Citation2009) performed a study comparing the effects of diets formulated with different fat sources on lipid metabolism in female KK mice. The authors observed a significantly lower final body weight in groups fed EPA and DHA, compared to that of the control and DHA plus fenofibrate groups, suggesting that n-3 LC-PUFA inhibited body gain weight. Compared to the control group, all three treatments containing n-3 LC-PUFA significantly reduced plasma insulin and hepatic lipid levels, and increased plasma adiponectin. The highest increase in the latter parameter was recorded in the DHA plus fenofibrate group. Castellano et al. (Citation2010) investigated the effects of n-3 LC-PUFA supplementation on body condition, insulin sensitivity and secretion in adult-male Duroc boars. Independent from source and EPA/DHA ratios, the authors found that long-term supplementation with dietary n-3 LC-PUFA, did not affect insulin metabolism in healthy adult male pigs. There were no differences in fat gain among the three diets. Ninio et al. (Citation2005) found that, in rabbits (New Zealand White rabbits), atrial n-3 LC-PUFA levels were dramatically increased with dietary high DHA fish oil supplementation compared to the control group, and this was associated with increased protection against stretch-related atrial fibrillation in the rabbit.
3.3.2. Brain and visual system
Details and characteristics of the 6 retrieved trials studying the effects of high DHA fish oil on brain and visual system in animals are reported in .
Table 6. A summary of animal studies testing the effects of high DHA fish oil on the brain and visual system.
All studies were long-term trials, with a duration ranging from 7 days to 3 months (the median duration was 26 days). Rats were chosen as an animal model in four studies (Avramovic et al. Citation2012; Engstrm et al. Citation2009; Fernandes et al. Citation2008; Trofimiuk and Braszko Citation2011), one study used mice (Davis et al. Citation2017), and another used pigs, or more specifically, pregnant sows and their piglets (Clouard et al. Citation2015). The number of animals per group varied from 6 to 20. DHA: EPA ratios were above 2 in four studies (Clouard et al. Citation2015; Davis et al. Citation2017; Engstrm et al. Citation2009; Fernandes et al. Citation2008) and between 1–2 in two studies (Avramovic et al. Citation2012; Trofimiuk and Braszko Citation2011). All studies were conducted with male animals, with the exception of one study that used females (Clouard et al. Citation2015) and another that used animals of both sexes (Davis et al. Citation2017).
The main endpoints assessed in these studies included: i) retention of cognition (Fernandes et al. Citation2008), ii) brain nitric oxide synthase (Engstrm et al. Citation2009), iii) behavioural responses (Clouard et al. Citation2015; Davis et al. Citation2017; Trofimiuk and Braszko Citation2011), iv) brain tissue oxidative status (Avramovic et al. Citation2012), and v) microbiota (Davis et al. Citation2017).
Fernandes et al. (Citation2008) conducted a study investigating the effects of high DHA fish oil in alleviating both the cognitive and neurodegenerative deficits caused by transient, global cerebral ischemia (TGCI) in male rats. Animals were trained in an 8-arm, radial maze task and then assigned to the following groups: sham operation, ischemia operation plus olive oil, or ischemia operation plus high DHA fish oil. Retention of the previously acquired cognition from the maze task was assessed one week after TGCI and continued weekly for 6 weeks. The results of this study suggest that long-term treatment with high DHA fish oil facilitates functional recovery after ischemic brain damage, but does not prevent hippocampal damage in rats. Engstrm et al. (Citation2009) implemented a study to assess the effects of two fish oils containing different amounts of EPA, DHA, and a commercial natural antioxidant in male rats on brain nitric oxide synthase activity; an enzyme which has been shown to be important for memory and learning ability. The authors found that high DHA fish oil stabilized with antioxidant increased the brain nitric oxide synthase activity while other treatments did not. Trofimiuk and Braszko (Citation2011) showed that cod liver oil prevented the deleterious effects of chronic restraint stress on recall and spatial memory in male rats. Avramovic et al. (Citation2012) showed that a high DHA fish oil supplementation, compared to control, significantly decreased lipid peroxidation, increased SOD activity, and decreased (not significantly) catalase activity in the brain of male rats. The impact of maternal dietary DHA on the behaviour of offspring was assessed in pigs (Clouard et al. Citation2015). The authors reported that maternal DHA supplementation enhanced the social behaviour of offspring after weaning, and suggested that these changes were mediated by increased brain DHA concentrations. Last, Davis et al. (Citation2017) investigated the influence of DHA on suppressing anxiety and depressive-like behaviours through modulation of the gut microbiota in a paradigm social isolation. After 28-days of social isolation, male and female mice were randomized to receive a control diet or a diet supplemented with 0.1% or 1.0% DHA (by weight in diet). The authors found that DHA significantly altered commensal community composition, and had beneficial effects on anxiety and depressive-like behaviours in a sex-specific manner.
3.3.3. Other medical/physiological aspects
Details and characteristics of the 10 trials retrieved where high DHA fish oil was tested on immunity, muscle function, hepatitis and other medical aspects in animal models are reported in .
Table 7. A summary of animal studies testing the effects of high DHA fish oil for other medical purposes.
Studies in this section were long-term, intervention trials with a duration of 5 weeks to 7 months (the median duration was 12 weeks). Rats were chosen as an animal model in 5 studies (Du et al. Citation2004; Henry et al. Citation2015; Kohno et al. Citation2000; Peoples and McLennan Citation2010 Citation2014) and mice were chosen in another (Philp et al. Citation2015). Other studies used cattle (Kook et al. Citation2002), boars (Castellano et al. Citation2011), dogs (LeBlanc et al. Citation2008) and guinea pigs (Patten et al. Citation2002). The number of animals per group ranged from 5 to 15. DHA: EPA ratios were above 2 in eight studies (Du et al. Citation2004; Henry et al. Citation2015; Kohno et al. Citation2000; Kook et al. Citation2002; Patten et al. Citation2002; Peoples and McLennan Citation2010 Citation2014; Philp et al. Citation2015) and between 1–2 in one study (LeBlanc et al. Citation2008). In one study, two types of fish oil were used with different ratios (Castellano et al. Citation2011). Six studies were conducted with male animals (Castellano et al. Citation2011; Henry et al. Citation2015; Kohno et al. Citation2000; Kook et al. Citation2002; Peoples and McLennan Citation2010 Citation2014), one with female animals (Du et al. Citation2004), and three with both male and female animals (LeBlanc et al. Citation2008; Patten et al. Citation2002; Philp et al. Citation2015).
The main endpoints measured in these studies included: i) growth performance (Kook et al. Citation2002), ii) tension development (Peoples and McLennan Citation2014), iii) muscle histology (Philp et al. Citation2015), iv) oxygen consumption (Peoples and McLennan Citation2010 Citation2014), v) muscle function (Henry et al. Citation2015), vi) serum metabolites (Kook et al. Citation2002), vii) plasma biochemistry (Philp et al. Citation2015), viii) cytokine (LeBlanc et al. Citation2008), ix) testicular hormones (Castellano et al. Citation2011), x) serum biochemistry (Du et al. Citation2004), xi) liver histology (Du et al. Citation2004), xii) colonic aberrant crypt foci (Kohno et al. Citation2000), and xiii) ileal contractility (Patten et al. Citation2002).
Peoples and McLennan (Citation2010) reported that membrane incorporation of DHA following high DHA fish oil feeding increased efficiency of muscle O2 consumption, and promoted resistance to muscle fatigue in male Wistar rats. In another study by Peoples and McLennan (Citation2014) applying the same experimental design, the authors reported that rats fed a high DHA fish oil diet developed higher maximum twitch tension, and sustained twitch tension through more repetitions before the tension declined to 50% of the maximum twitch tension, compared with those fed the SFA and n-6 PUFA diets. In addition, the high DHA fish oil group used less oxygen for tension, and developed and produced higher venous lactate concentrations with no difference in glycogen utilisation, compared with the saturated fat and n-6 PUFA groups. The effect of high DHA tuna fish oil on membrane fatty acid composition and muscle fatigue was tested in male Sprague–Dawley rats (Henry et al. Citation2015). The authors reported that rats fed high DHA fish oil fed incorporated higher muscle phospholipid DHA-relative percentages than those in the control group. Incorporation of DHA was greater in the fast-twitch gastrocnemius than in the slow-twitch soleus muscle, which was comparable with the myocardium and in line with muscle fibre characteristics. These findings suggest that DHA may be an essential component of striated muscle for optimal healthy function, and that the failure to include regular fish or fish oil in the diet may lead to a deficiency that is reflected in susceptibility to muscle fatigue. Kohno et al. (Citation2000) reported that tuna oil rich in DHA inhibited the development of colonic aberrant crypt foci in rats. Du et al. (Citation2004) showed that polyunsaturated fatty acids could suppress the development of acute hepatitis and prolong survival in female rats, which were associated with altered gene expression, regardless of whether they were supplemented with n-6 (soybean oil) or n-3 (high DHA fish oil). Philp et al. (Citation2015) reported that a diet enriched with high DHA fish oil prevented high fat-induced metabolic dysfunction in the skeletal muscle of mice. Kook et al. (Citation2002) showed that fatty acid composition and physical properties of the muscle fat in fattening Korean cattle were altered by feeding on diets containing 5% high DHA fish oil. However, no differences in growth performance and ruminal metabolism were found between treatment groups. Castellano et al. (Citation2011) reported that, in healthy adult pigs, body and reproductive organ weights were not significantly affected by dietary treatments with different DHA: EPA ratios, however, long-term dietary supplementation of fish oil, regardless of the DHA/EPA ratio, affected steroid production. LeBlanc et al. (Citation2008) showed that a high DHA fish-oil enriched diet was associated with significant reductions in PGE2 concentrations and IL-1 and IL-6 activities in young, healthy dogs.
Eventually, the effect of high DHA fish oil on bowel health, mainly caecal digesta pH and short chain fatty acid profile, and ileal contractility was investigated in guinea pig in a study by Patten et al. (Citation2002). The authors found that the group supplemented with high DHA fish oil had a significantly higher caecal digesta pH coupled with a significantly lower propionate concentration. There was no significant difference in the sensitivity (EC50), and maximal contraction, induced by acetylcholine, histamine, serotonin, and prostaglandins PGE2 or PGF2α, however, sensitivity to isoprostane, 8-iso-PGE2 was significantly reduced in the fish oil supplemented group.
4. Discussion
As the vast majority of studies to date either focus on physiological and health related effects of n-3 LC-PUFA or have used a mixture of EPA and DHA, while the specific and distinct roles and effects of the two individual fatty acids on the most prevalent health problems, including CVD and depressive disorders, remain fundamentally untested. Consequently, it is not surprising that dose recommendations from several national and international organizations are based on the combined consumption of EPA and DHA (Mozaffarian and Wu Citation2011). Irrespective of the lack of available evidence to justify any substantiated reasoning surrounding the relative importance of DHA versus EPA, or any ratio of the two, some specific recommendations have been made regarding daily intake of the two fatty acids. For example, the European Food Safety Authority (2010) suggests that infants (>6 months) and young children (<24 months) should consume 0.10 g of DHA per day, while for older children (2–18 years), the recommended daily intake of DHA is considerably higher (0.25 g of DHA in one or more servings). Similarly, recommendations made by the FAO/WHO (Citation2008) for pregnant and lactating women specified that the minimum daily intake of EPA plus DHA is 0.30 g, with at least 0.20 g being DHA. In the last few years, the specific effects of DHA on medical aspects has drawn a great deal of scientific attention. As such, the aim of this paper was to review the currently available literature in order to substantiate whether DHA has a beneficial role in influencing risk factors associated with heart disease, brain and visual disorders, immune disorders and body weight, and/or to highlight knowledge gaps.
Of the 113 studies analysed herein, 46.0% reported statistically significant positive results following the use of high DHA fish oils (22 in heart, 14 in brain and 16 in other medical aspects); 31.0% reported results that were not statistically significant (7 in heart, 18 in brain, 10 in other medical aspects); 21.2% reported a significant positive result for some outcomes, however other measured outcomes were not statistically significant (5 in heart, 9 in brain, 10 in other medical aspects); and 1.8% reported a statistically significant negative effect (2 in brain).
The variations in responses to DHA supplementation might be accounted by the variances in the basal DHA levels. It is reported that the larger the difference of a subject's basal PUFA status from the “change threshold” the higher the change that will be observed with supplementation (Osterman and Schebb Citation2017). Also, cases where the effects of high DHA fish oil administration were not statistically significant, may be due to an absence of biological effects from increased dietary DHA levels. However, the risk of a type II error should not be under-estimated in such intervention trials. In fact, a series of factors can contribute to this occurrence, such as an inadequate power of the statistical test, decreased experimental duration, analytical methodology issues, inappropriate dosage of treatments, improper consideration of body weight, age or gender bias within the subject groups, and other confounding effects specific to studies concerned with n-3 LC-PUFA, that can impact on the robustness of the experimental design (Ghasemifard et al. Citation2014). In addition, many studies did not measure omega-3 status before entry into the trials or at completion (James et al. Citation2014). Although the topic of specific research methodologies is beyond the scope of this review, the authors believe it is important to consider this possibility before formulating any conclusions regarding the lack of benefits or risks associated with such dietary interventions. Over the following sections, an attempt is made to summarise and interpret the key findings achieved by the studies retrieved and presented herein, and, where suitable, information from other studies that did not meet the selection criteria are integrated.
4.1. DHA in the heart and cardiovascular system
DHA is known to be the main n-3 LC-PUFA in myocardial membranes in human and rats (Brochot et al. Citation2009; Harris et al. Citation2004; Sexton et al. Citation1995), and its concentration has been reported to rapidly increase following an additional intake of fish oil, even at low quantities (Harris et al. Citation2004; Slee et al. Citation2010). The heart is the organ most affected by dietary DHA, whereas brain fatty acid levels appear to be somewhat resistant to dietary DHA (Saito et al. Citation1998). It has been reported that an increase in myocardial DHA concentrations is negatively associated with chronic stress, reducing both HR and the risk of sudden death (McLennan and Abeywardena Citation2005). Commonly accepted risk factors involved in heart disease are: plasma TAG, LDL and total cholesterol, blood pressure, HR and vascular reactivity, arrhythmias, and inflammation.
Based on evidence from human and animal studies, it can be inferred that EPA and DHA have differential effects on blood pressure, heart rate, lipid and vascular reactivity (Mori and Woodman Citation2006). In general, it has been reported that DHA is more potent than EPA in modifying cardiometabolic risk (Li et al. Citation2014; Wei and Jacobson Citation2011). Certainly in animal models, where the underlying mechanisms can be explored more easily, DHA reduced the incidence and severity of ventricular arrhythmias, whereas EPA alone had no effect (McLennan et al. Citation1996). In human subjects it has been demonstrated that at least 500 mg/day of DHA is sufficient to reduce cardiovascular mortality (Meyer and de Groot Citation2017). The physiological mechanisms by which DHA exerts its cardioprotection are explained in a review by McLennan (Citation2014), who described the pleiotropic physiological effects of fish oil on heart function as either direct, via membrane incorporation (intrinsic), or indirect, via triacylglycerols, blood pressure and atherosclerosis (extrinsic). McLennan (Citation2014) concluded that DHA is the active component of fish oil which aids in preventing cardiac arrhythmia, lowering the heart rate, and preconditioning the heart to resist a variety of insults, all of which directly affect the fatty acid composition of the heart. The lowering of triacylglycerols and blood pressure to influence plaque formation and ischaemic heart disease are extrinsic to the fatty acid composition of the myocardium (McLennan Citation2014). Other studies exploring the effects of DHA and EPA on heart function have demonstrated that DHA lowers fasting blood level of C-reactive protein (CRP), interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) (Li et al. Citation2014), decreases triglycerides and raises high-density lipoprotein cholesterol (HDL) (Wei and Jacobson Citation2011), regulates the activity of several transcription factors and gene expression, influences membrane fluidity and downstream cell signalling pathways, and has anti-thrombotic and anti-arrhythmic effects (Adkins and Kelley Citation2010; Yagi et al. Citation2017).
The Omega-3 Index is considered to be inversely associated with a high risk of CHD mortality. As reported by Harris and Von Schacky (Citation2004), Omega-3 Index values of ≥8% are associated with the greatest cardioprotection, whereas index values of ≤4% are associated with the least. As the Omega-3 Index does not differentiate between EPA and DHA, there is evidence that the two fatty acids have a different effect on the Omega-3 Index itself. For example, Allaire et al. (Citation2017) recently demonstrated that the Omega-3 Index was greater after supplementation with 2.7 g/day of DHA, than after 2.7 g/day of EPA, over a 10-week period. As shown by Metcalf et al. (Citation2007), this phenomenon can be explained by the different retention and deposition efficiency of EPA and DHA into erythrocyte and other membranes, particularly myocardial membranes. In this study, the authors reported that DHA accumulated in atrial phospholipids more rapidly than EPA, even though DHA and EPA were present in approximately equal proportions, during treatment with fish oil. Flock et al. (Citation2013) investigated the dose-response of the Omega-3 Index through supplementation with increasing doses of omega-3, reporting a higher Omega-3 Index as the dose of omega-3 increased. This could be due to less β-oxidation and greater deposition following consumption of a single, large dose of dietary omega-3 compared with smaller, daily doses, as described by Ghasemifard et al. (Citation2015b) in a study involving rats.
It has been reported that consumption of high DHA fish oil is effective in reducing TAG levels, which is an independent risk factor for CVD. A dose response trial with high DHA fish oil supplementation showed a 6%, 20% and 25% TAG level reduction following supplementation with approximately 0.7, 1.3 and 2 g of high DHA fish oil per day, over a period of 6 weeks (Milte et al. Citation2008). This was supported by another study that showed a 7% reduction in plasma TAG levels following supplementation with 0.8 g of DHA per day (Meyer et al. Citation2009). There is a growing number of observational studies reporting that fish oils containing a greater amount of DHA relative to EPA, are more effective in reducing TAG levels than fish oils with a higher amount of EPA (Buckley et al. Citation2004; Caslake et al. Citation2008; Hill et al. Citation2007a; Krebs et al. Citation2006). This is in line with a review paper by Mozaffarian and Wu (Citation2012) reporting that both EPA and DHA lower TAG, though larger effects were seen with DHA. Results from studies using purified EPA and DHA are varied, with some suggesting that DHA has a slightly greater hypotriglyceridemic effect coupled with an ability to raise HDL cholesterol levels (Mori et al. Citation2000), while others report greater reductions in TAG following EPA intervention (Rambjør et al. Citation1996). It is also important to highlight that the TAG lowering effect of high DHA fish oil was not observed in some studies (Mann et al. Citation2010; Ottestad et al. Citation2012; Pedersen et al. Citation2010). It is likely that the low dosage of DHA (< 1 g DHA/day) used in these studies resulted in the decreased efficacy. As such, it appears that in order to observe a hypotriglyceridemic effect, there is a dose threshold of at least 1 g of DHA per day. Only one study (Caslake et al. Citation2008) reported a significant effect on the plasma lipid profile following a dose of DHA as low as 0.6 g per day, however, a greater number of subjects was used over an increased duration (8 weeks) compared to other studies, greatly increasing the statistical power of the study.
Total cholesterol and LDL cholesterol are important determinants of atherosclerotic plaque formation, contributing to determining associated cardiovascular risks. An 8% increase in LDL cholesterol after supplementation with DHA, but not EPA, has been reported in a study testing the effects of an equal dose of EPA or DHA for a period of 6 weeks (Mori et al. Citation2000). Despite the reported increase in LDL cholesterol after DHA supplementation, in this study it was suggested that the increased LDL particle size may have represented a shift to less atherogenic particles, in which case, the parallel increase in HDL2 cholesterol and decrease in TAG may have represented a more favorable lipid profile than that seen after EPA supplementation (Mori et al. Citation2000). An increased LDL cholesterol concentration after high DHA fish oil supplementation has been reported by others (Caslake et al. Citation2008; Milte et al. Citation2008), while systematic reviews have demonstrated that DHA is associated with significant increases in LDL cholesterol in patients with and without hypertriglyceridemia (Jacobson et al. Citation2012; Wei and Jacobson Citation2011). Further, products with EPA that lack DHA did not significantly raise LDL levels when compared with placebo or control products (Ballantyne et al. Citation2012; Bays et al. Citation2011; Yokoyama et al. Citation2007). In contrast, other studies have found no significant differences in total and LDL cholesterol between high DHA fish oil and placebo oil groups (Buckley et al. Citation2004; Krebs et al. Citation2006; Ottestad et al. Citation2012). Within these studies participants typically had normal baseline TAG levels and, as such, no significant changes in LDL cholesterol were observed, although an increase in LDL was significantly related to baseline TAG measures in the DHA treated group (Jacobson et al. Citation2012).
Two review papers concluded that fish oil supplementation affects HDL cholesterol (Balk et al. Citation2006; Harris Citation1997), however, evidence on the specific effects of high DHA fish oil on HDL cholesterol is inconsistent. In some studies, no change has been found in HDL cholesterol after consumption of high DHA fish oil (Buckley et al. Citation2004; Mann et al. Citation2010; Molinar-Toribio et al. Citation2015; Ottestad et al. Citation2012), while other trials showed an increase in HDL cholesterol concentration (Caslake et al. Citation2008; Hill et al. Citation2007a; Pedersen et al. Citation2010). The latter findings are further supported by a study demonstrating the increased efficiency of DHA over EPA where a decrease in HDL cholesterol was observed in groups consuming a higher EPA diet (salmon diet, DHA: EPA = 1.8: 1; or fish oil capsules, DHA: EPA = 0.7: 1) compared with a higher DHA diet (cod diet, DHA: EPA = 3.8: 1) (Gunnarsdottir et al. Citation2008).
Although some trials using high DHA fish oil have reported that blood pressure tended to decrease over the course of the study (Finnegan et al. Citation2003; Krebs et al. Citation2006; Sjoberg et al. Citation2010), no statistically significant differences from the baseline were actually recorded. Nevertheless, a 16-week, randomized trial by Pedersen et al. (Citation2010), reported a significant decrease in both systolic and diastolic blood pressure in teenage boys following the consumption of high DHA fish oil, compared with the control group.
In a number of studies, DHA has been associated with improvements in HR (Dallongeville et al. Citation2003; Mozaffarian et al. Citation2006). During submaximal exercise, HR was reduced following high DHA fish oil supplementation, with no observed effects on peak exercise HR. Although this latter observation is consistent across several studies (Buckley et al. Citation2009; Macartney et al. Citation2014; O'Keefe Jr et al. Citation2006), one study reported a positive effect of high DHA fish oil supplementation in lowering peak HR during incremental workloads to exhaustion (Peoples et al. Citation2008). As for resting HR, high DHA fish oil consumption has been shown to lower resting HR in subjects with a history of myocardial infarction (Mozaffarian et al. Citation2005; Ninio et al. Citation2008; O'Keefe Jr et al. Citation2006), while the resting HR remained unchanged in well-trained subjects (Macartney et al. Citation2014). There is also a solid body of evidence that supports the positive impact of supplementation with n-3 LC-PUFA on HRV (Brouwer et al. Citation2002; Holguin et al. Citation2005; Macartney et al. Citation2014; Ninio et al. Citation2008; O'Keefe Jr et al. Citation2006; Sjoberg et al. Citation2010), and in one case (Brouwer et al. Citation2002) a strong association was displayed between DHA and HRV, but not EPA and HRV.
The selective antiarrhythmic effects for DHA over EPA are demonstrable when provided as purified fatty acids in the diet of rats (McLennan et al. Citation1996), and the heart rate in healthy humans is selectively lowered by DHA (Grimsgaard et al. Citation1998). Evidence suggests that antiarrhythmic protection, heart rate slowing, and optimised cardiac contractile function are best achieved through regular consumption of fish or fish oil which promotes incorporation of DHA into membrane lipids; however, many of the effects observed in vitro await validation in dietary-modulated cells known to have omega-3-derived arrhythmia protection in vivo. Data from in vitro (Phang et al. Citation2009), ex vivo (Phang et al. Citation2012a; Phang et al. Citation2012b) and in vivo human studies (Park and Harris Citation2002; Phang et al. Citation2013) suggest that there are sex-dependent differences in platelet aggregation in response to EPA or DHA. In study by Din et al. (Citation2008), EPA specifically reduced platelet aggregation in males, while Phang et al. (Citation2013) reported that DHA intake was shown to decrease platelet aggregability in females. This may explain the lack of effect on platelet aggregation following DHA supplementation in previously published studies conducted predominantly or exclusively in males (Conquer and Holub Citation1996; Nelson et al. Citation1997).
Although the molecular mode of action of DHA is still not fully understood, it has been reported that a significant portion of the (patho)-physiological effects of DHA is mediated by its oxidative metabolites, i.e. eicosanoids and other oxylipins (Ostermann and Schebb Citation2017). It should be noted that none of the studies reported in the heart section (4.1) of this review used a quantitative targeted DHA derived oxylipin method to allow the comprehensive monitoring of DHA supplementation-induced changes in the pattern of oxylipins, to understand its biology in comparison with EPA. Westphal et al (Citation2011) describe the pathways and some effects of the cytochrome P450 epoxy and hydroxy docosanoids derived from DHA, which may explain the many parallels that the physiological effects of dietary fish oil and myocardial membrane DHA share with attenuation of Ca2+ overload.
Based on the above evidence, it can be concluded that dietary DHA has been shown to play an important role in modulating the Omega-3 Index, reducing TAG, improving total cholesterol and LDL cholesterol, and decreasing HR and HRV. Further, these effects are not shared, or are shared to a lesser extent, by dietary EPA. Due to inconsistencies and, therefore, inconclusive results, the possible effects of DHA on HDL cholesterol and blood pressure have not been reported. The disparate intakes and physiological mechanisms underpinning different clinical endpoints and selectivity for DHA, plus the common employment of high EPA fish oil for clinical trials, can perhaps explain the sometimes contradictory outcomes of fish oil clinical trials, and provide insight to nutritional optimisation of cardiac function and selection criteria for clinical trials based on an improved hypothesis regarding mechanism of action. The striking difference of sex-specific responses to EPA or DHA administration on platelet aggregation are not only intriguing, but provide clear evidence that much greater attention should be focused on selecting the population to be tested in future studies. This will likely result in more conclusive and informative studies, which will allow the filling of important knowledge gaps that still exist.
4.2. DHA in the brain and visual function
The brain has a unique fatty acid composition with high levels of palmitate (16:0), the long chain omega-6 PUFA arachidonic acid, and DHA (Brenna and Diau Citation2007; Crawford et al. Citation1976; Dyall Citation2015), but low levels of the other n-3 PUFA, especially EPA, which is reported to be at a concentration typically 250–300 times lower than DHA (Chen et al. Citation2009). Compared with other tissues, such as liver or plasma, the brain has a very high concentration of DHA (Pifferi et al. Citation2012). It has been suggested that the brain maintains DHA levels primarily via the uptake of DHA from lipids in circulating blood through the blood-brain barrier, rather than possessing particularly active endogenous biosynthesis, which is reportedly lower in in the brain compared to other tissues (DeMar et al. Citation2006). There is evidence that a major transporter of DHA to the brain is in the form of lyso-phosphatidylcholine (sn 2-DHA-PC), which is taken up by a specific transporter known as MFSD2A, located in the blood brain barrier (Lagarde et al. Citation2016). Consequently, a mounting number of studies are focusing on the role for DHA in brain development and the prevention of neurodegenerative and neuropsychiatric disorders.
It is widely accepted that DHA is necessary for the growth and maturation of infants' brain (Koletzko et al. Citation2008; Simmer et al. Citation2011). Though infants have been reported to be rather efficient at biosynthesis of DHA from shorter and less unsaturated omega-3 fatty acids (Neu and Polin Citation2012), this endogenous production can be insufficient, especially during the rapid phase of brain growth and development when DHA is needed in large amounts. Accordingly, it has been argued that DHA needs to be provided to preformed infants via external sources, such as in placental transfer from maternal DHA stores during pregnancy and/or maternal milk after birth (Innis Citation2005).
Despite the existence of robust biochemical evidence for the fundamental role of DHA in the brain, results of in vivo studies are varied and, thus, inconclusive. Although many human studies, including follow-up studies, were unable to record statistically significant differences between treatment and control groups (Hirayama et al. Citation2004; Makrides et al. Citation2009; Smithers et al. Citation2010), the beneficial effects of high DHA fish oil supplementation was observed in the communicative development of infants (Meldrum et al. Citation2012) and in neurobehavioral outcomes, in children, such as school attendance rates (Hamazaki et al. Citation2008). An animal study conducted by Clouard et al. (Citation2015) also reported that maternal high DHA fish oil supplementation beneficially affected the neurobehavior of offspring after weaning. On the contrary, a follow-up study of a double-blind, randomized, trial reported a negative effect of high DHA fish oil supplementation during the first 4 months of lactation on the cognitive abilities of offspring (Cheatham et al. Citation2011). The authors suggested that the first 4 months of life might not be an appropriate time to receive high n-3 LC-PUFA supplementation, while another possible explanation for these negative effects is the recruitment of Danish mothers, who naturally have a higher intake of n-3 LC-PUFA, compared to participants from other studies. According to these somewhat inconclusive and, at times, contradictory findings, two separate meta-analysis studies of randomized, controlled trials were implemented. Both studies were unable to conclusively support or refute that n-3 LC-PUFA supplementation in women during pregnancy, or during lactation, positively or negatively affects the neurodevelopment of their offspring (Delgado-Noguera et al. Citation2015; Gould et al. Citation2013). It has been suggested that the apparent effects of DHA on brain development may be affected by the gender of the child, which is a possible explanation for the lack of conclusive findings in other studies. Makrides et al. (Citation2009) found that a high-DHA (1% of total fatty acids) in early life did not increase the Mental Development Index of overall preterm infants, however it did show improvement in girls. The apparent increased physiological importance of DHA in developing girls may also be substantiated by the increased rate of endogenous synthesis of DHA from the precursor fatty acid α-linolenic acid that has been observed in girls, and not boys (Burdge and Calder Citation2005). Such gender specific effect was also found in other fatty acids. It was reported that anxiety and hostility scores positively correlate with serum levels of long-chain saturated fatty acids in women, but not in men (Yanagisawa et al Citation2002).
It is known that cognitive function reaches its peak during middle adulthood, and declines in the later aging process. Brain aging is accompanied by many widespread changes, such as a reduction in brain volume and weight (Anderton Citation2002), increased oxidative stress (Perluigi et al. Citation2014), altered membrane lipid content (Svennerholm et al. Citation1997) and damage to DNA (Canugovi et al. Citation2013). The aging brain is more susceptible to neurodegenerative diseases, including Alzheimer's disease, Parkinson's diseases, and impaired cognition. Clinical trials aimed at assessing the possible effect of DHA and n-3 LC-PUFA supplementation on neurodegenerative conditions within adults, have reported mixed results. Though several studies using high DHA fish oils found it had no effect on cognitive function (Andrieu et al. Citation2017; Dangour et al. Citation2010; Jackson et al. Citation2012a; Phillips et al. Citation2015; Stough et al. Citation2012), other trials reported a significant improvement in memory function in subjects provided with high DHA fish oil (Lee et al. Citation2013; Stonehouse et al. Citation2013). Sinn et al. (Citation2012) directly compared the effects of DHA vs EPA in participants with mild cognitive impairment. Only in the high DHA group, were changes in memory exhibited, and verbal fluency significantly improved. In a study focusing on the effects of DHA in memory improvement (Yurko-Mauro et al. Citation2010), elderly individuals receiving 0.90 g/day of pure algal DHA exhibited improved learning and memory functions, compared to those receiving the placebo oil (corn and soy oil). Furthermore, a recent meta-analysis of 15 human interventional trials indicated that DHA, alone or combined with EPA, contributed to improved memory function in older adults with mild memory complaints (Yurko-Mauro et al. Citation2015).
The beneficial effect of DHA in the brain might be related to its unique membrane properties. It was reported that DHA had a much higher tendency to accumulate into sphingomyelin/cholesterol-rich raftlike domains on the membrane than EPA, making it having a much greater potential to affect cell signalling by modifying the structure and composition of these lipid rafts (Williams et al Citation2012). Crawford et al (Citation2013) proposed a theory for the irreplaceable role of DHA in neural cell. They suggested that the unique molecular structure of DHA allows for quantum transfer and communication of π-electrons, explaining the precise depolarization of retinal membranes and the cohesive, organized neural signalling which characterizes higher intelligence. DHA-derived lipid mediators could also play a key role in its beneficial effect. For example, Neuroprotectin D1 (NPD1, 10R-17S-dihydroxy-docosahexaenoic acid), a DHA-derived mediator, is biosynthesized in response to injury and may have therapeutic potential in a wide range of neurodegenerative conditions (Bazan Citation2013).
Though a species-specific difference in DHA metabolism might exist, caution should be exercised when directly translating findings across species. Animal studies have the advantage over human studies to allow a much greater environmental control and, thus, often result in more robust experimental designs. Accordingly, unlike clinical trials, animal studies investigating the effect of high DHA fish oil consistently report statistically significant results. For example, Fernandes et al. (Citation2008) demonstrated that long-term, high DHA fish oil treatment facilitated functional recovery after ischemic brain damage rats; Trofimiuk and Braszko (Citation2011) found that long-term administration of high DHA cod liver oil could prevent the deleterious effects of chronic restraint stress on recall and spatial memory in Wistar rats; and Clouard et al. (Citation2015) found that maternal high DHA fish oil supplementation resulted in positive offspring neurobehavior in pigs.
A growing number of observational and epidemiological studies have suggested that there may be some relationship between mental illness, in particular depression, and a reduced dietary intake of n-3 LC-PUFA (Ross et al. Citation2007). However, controversy exists as to whether EPA, DHA, or both, are responsible for the reported benefits (Song et al. Citation2016). Mental disorders are generally characterized by a combination of abnormal thoughts, emotions, behaviour and relationships with others; depression is an increasingly common mental disorder, with a global estimate of over 300 million people in the world being affected by it (WHO Citation2017). With vast positive ramifications, a greater understanding of the possible roles of n-3 LC-PUFA in the treatment of mental disorders is warranted. In a study conducted by Clayton et al. (Citation2009), clinician ratings of depression were significantly lower after high DHA fish oil supplementation, while in an animal study conducted by Davis et al. (Citation2017), DHA was also found to produce beneficial effects on depressive-like behaviour. In contrast, many other human studies investigating the effect of high DHA fish oil on depression found no positive impact of high DHA fish oil over the placebo in treating depression (Grenyer et al. Citation2007; Itomura et al. Citation2005; Makrides et al. Citation2010; Mozurkewich et al. Citation2013; Rees et al. Citation2008; Rogers et al. Citation2008; Silvers et al. Citation2005). These findings are consistent with the meta-analyses of randomized, controlled trials performed by Martins (Citation2009), and Sublette et al. (Citation2011), in which EPA, rather than DHA, is identified as the effective n-3 LC-PUFA in the treatment of depression, in conjunction with the appropriate medication. In the latter study, the authors suggest the underlying mechanism might be that EPA has some non-brain effects that cause secondary brain changes. This is supported studies reporting that EPA supplementation increased brain N-acetyl-aspartate, a marker for neuronal health in bipolar disorder (Frangou et al. Citation2007), and the cerebral phosphomonoesters: phosphodiesters ratio, which is an indicator of phospholipid turnover and reversed brain atrophy in a subjects with major depressive disorders (Puri et al. Citation2001). Furthermore, Zhang et al. (Citation2017) reported that EPA was significantly more effective than DHA in mitigating oxidative stress, while Song et al. (Citation2016) concluded that combining a higher dose of EPA with a lower dose of DHA (e.g. at 2–4: 1 or higher) is effective in treating depression. In the latter study, DHA-rich supplementation appeared to be ineffective.
Despite the inconsistent results in relation to the effects of DHA on mood and cognitive factors, it seems plausible that DHA might possesses an unclarified role in physiological brain function given that it is heavily concentrated in the mammalian brain. This has been studied in many clinical trials and in vitro studies. Two randomized, controlled trials conducted by Jackson et al. (Citation2012c) and (Citation2012b), found that high DHA fish oil could increase concentrations of oxyhemoglobin and total levels of haemoglobin, an indication of increased cerebral blood flow during a cognitive task. It is also notable that DHA has been shown to modulate nitric oxide synthesis (Engstrm et al. Citation2009), and improve brain tissue oxidative status, such as increasing SOD activity and decreasing lipid peroxidation (Avramovic et al. Citation2012). These physiological effects of DHA could direct us towards understanding the potential mechanisms by which DHA or n-3 LC-PUFA exerts a positive effect on cognitive function, however, additional studies are necessary, and warranted, to provide a greater understanding of such phenomena.
Aside from the brain, the highest level of DHA in the human body is found in the eye, more specifically, in the retina (Neuringer et al. Citation1986). DHA is needed for the process of transforming light into an electrophysiological signal (Niu et al. Citation2001) and for the regeneration of the light sensitive pigment in the retina – rhodopsin (Bush et al. Citation1994). For this reason, several randomized, controlled trials have investigated the effects of DHA supplementation on visual function in both preterm and term infants. Studies on preterm infants administered formulas containing high DHA and have reported improved retinal function (Birch et al. Citation1992) or visual acuity (Carlson et al. Citation1993; Carlson et al. Citation1996; Faldella et al. Citation1996) following supplementation with DHA, compared to those fed formula without DHA or with low-DHA content (Smithers et al. Citation2008). In contrast, studies on infants born at term that were supplemented with DHA-containing formulas obtained mixed results, with some reporting an improvement in visual function (Birch et al. Citation2005; Birch et al. Citation1998; Hoffman et al. Citation2003) and others reporting no effects (Bakker et al. Citation1999; Hurtado et al. Citation2015; Lauritzen et al. Citation2004; Makrides et al. Citation2000). No studies reporting negative effects on visual function following DHA supplementation in infants could be found. Contrasting infants, only a small number of studies assessed the effects of high DHA fish oil supplementation on visual function in adults and, when performed, produced varied results. Stough et al. (Citation2012) found that participants receiving high DHA tuna oil had better right eye visual acuity post-treatment compared to individuals in the control group, while Souied et al. (Citation2013) found no significant difference in visual acuity between high DHA fish oil and placebo groups.
Based on the evidence detailed above, it can be concluded that DHA is likely to play important roles in brain development and function, which are different from EPA, especially in terms of neurodegenerative conditions; EPA is also important, specifically in terms of depression. Nevertheless, there is currently not enough robust information available to draw conclusions on the actual roles and effects of DHA on brain development and physiological function, or on visual function.
4.3. DHA in other medical/physiological aspects
A total of 36 studies focused on the effects of high DHA fish oils on other medical aspects in humans and animals. Of these studies, 27 trials analysed the effects of high DHA fish oil on immune function and inflammation, and growth/Body Mass Index. Therefore, these two major medical aspects will be discussed in this section. The reminder of studies focused on the effects of high DHA fish oil on gestational diabetes (Zhou et al. Citation2012), asthma (Atwell et al. Citation2013; Manley et al. Citation2011), bone (Damsgaard et al. Citation2012), skin (Tihista and Echavarria Citation2017), colonic disease (Kohno et al. Citation2000), liver (Du et al. Citation2004), sex hormone production (Castellano et al. Citation2011) and the gut (Patten et al. Citation2002); collectively there are not enough studies to discuss and draw any conclusion for the possible effects of high DHA fish oil on these medical aspects.
4.3.1. DHA in inflammation and immune function
EPA has traditionally been considered a more potent anti-inflammatory fatty acid than DHA (Molinar-Toribio et al. Citation2015), with some studies finding high DHA fish oil had no direct effect on human immune cell function (Kew et al. Citation2003; Wallace et al. Citation2003) and the production of inflammatory markers (Freund-Levi et al. Citation2014; Hill et al. Citation2007b; Sabour et al. Citation2012). In contrast, other studies suggest that DHA has a potent anti-inflammatory effect, possibly more potent than that of EPA (Halade et al. Citation2010; Oliver et al. Citation2012; Weldon et al. Citation2007). As such, it has been proposed that DHA, and not EPA, is the most potent n-3 fatty acid for suppressing glomerulonephritis, and extending the life span of systemic lupus erythematosus-prone short-lived B × W mice, possibly via inhibition of IL-18 induction and IL-18–dependent signalling (Halade et al. Citation2010). Kew et al. (Citation2004) found that supplementation with DHA, but not EPA, suppressed T lymphocyte activation, while Gorjão et al. (Citation2006) found that high DHA fish oil increased the phagocytic activity of neutrophils and monocytes. Vedin et al. (Citation2008) and Ramirez-Ramirez et al. (Citation2013) reported that high DHA fish oil was effective in reducing the levels of proinflammatory cytokines, which was supported by a similar study in animals (LeBlanc et al. Citation2008). There are also studies that demonstrate the beneficial effects of high DHA fish oil on human lymphocyte function and neutrophil function (Marques et al. Citation2015; Santos et al. Citation2013; Toupchian et al. Citation2016; Zhang et al. Citation2017). It has also been reported that both DHA and EPA attenuate apoptosis and improve cell viability, however DHA was found to be more effective than EPA (Zhang et al. Citation2017). Cabello et al. (Citation2017) reported a more effective inhibitory effect with a mixture of EPA plus DHA, than either DHA or EPA alone, although independently, DHA was more potent than EPA. Dawson et al. (Citation2012) studied the effects of DHA on the pattern of global gene expression in blood cells from hypertriglyceridemic males using microarray gene chip analysis. Their data showed that DHA supplementation significantly suppressed the expression of the LDL receptor and cathepsin L1, which provided supporting evidence for the anti-inflammatory effects of DHA supplementation.
In summary, both EPA and DHA have been shown to possess some important anti-inflammatory and immune functions, but profound differences in their biological actions are manifest. Current knowledge is insufficient to clearly understand the exact roles, mechanisms, and potential of the two fatty acids, both in isolation and in combination. Nevertheless, current evidence clearly suggests that rather than looking at which one of the two fatty acids has the most anti-inflammatory properties, understanding that they both play important roles, and that any knowledge gained on their specific mechanisms of action will certainly improve the efficiency of their utilisation.
4.3.2. DHA and body mass index
The metabolic and CVD risk in humans significantly increases in overweight/obese individuals (Du et al. Citation2015). Several studies have focused on the possible anti-obesity effects of fish oil high in EPA, using the traditional 18/12, EPA/DHA ratio. Results from a meta-analysis by Du et al. (Citation2015) indicated that supplementation with fish oil was not associated with a reduction in body weight and BMI when compared to control groups. However, in the few studies available where high DHA preparations were used, it was shown that high DHA fish oil could be effective in reducing body weight and BMI (Munro and Garg Citation2013b), body fat (Hill et al. Citation2007a), and waist circumference and waist: hip ratio (Cussons et al. Citation2009; Munro and Garg Citation2013a).
In a study by Kunesova et al. (Citation2006), 20 obese females (BMI = 40–45 kg/m2) were given a very low-calorie diet with 2.8 g/day of high EPA fish oil (DHA: EPA = 1: 2) or a placebo. The authors reported an increase in phospholipid DHA that was significantly correlated with a decrease in BMI, suggesting that DHA may be the active component for weight loss in obese females. It is becoming increasingly evident that there is a marked sex difference in the metabolism of n-3 LC-PUFA, with studies showing significantly higher concentrations of DHA in females, as opposed to males (Crowe et al. Citation2008). Hence, it is likely that there are gender differences in weight change in response to supplementation with n-3 LC-PUFA over time (Munro and Garg Citation2013b). Other studies on males and females (BMI = 27.5–32.5 kg/m2) that were given one of four supplements/diets including sunflower oil capsules, cod diet (DHA: EPA = 3.8: 1), salmon diet (DHA: EPA = 1.8: 1) or fish oil capsules (DHA: EPA = 0.7: 1), demonstrated greater weight loss in males than in females (Gunnarsdottir et al. Citation2008; Thorsdottir et al. Citation2007). In these studies, the average weight loss was 6.5 kg for males and 4.2 kg for females. Interestingly, no significant differences were observed when overweight insulin-resistant females were given omega-3 supplements (4.2 g/d EPA + DHA, DHA: EPA = 2.2: 1) in combination with a very low energy diet (Krebs et al. Citation2006), or when 138 overweight to obese male and female participants were given omega-3 supplements (3 g/day EPA + DHA, DHA: EPA = 0.2: 1) coupled with an energy-controlled diet and exercise (DeFina et al. Citation2010). The conflicting results reported above could be due to differences in the amount of omega-3 consumed and the different DHA: EPA ratios used in the studies, as previously described for several studies focusing on omega-3 metabolism (Ghasemifard et al. Citation2014).
Given the evidence above, it can be concluded that DHA appears to have a body weight lowering effect in obese females, yet current knowledge is insufficient to clearly quantify the extent of its action, and understand the exact roles, mechanisms and potentials of EPA and DHA, in isolation and/or in combination.
5. Conclusions
Although there is sufficient evidence to strongly suggest that DHA plays a series of important and fundamentally beneficial and health promoting roles, the currently available scientific literature describing the roles and potential metabolic effects of DHA, as an individual nutrient and/or in comparison to EPA, is far from conclusive. The exact mechanisms and the extent of these actions are not yet fully elucidated. DHA appears to play important roles, different to those of EPA, in heart, cardiovascular, brain and visual functions. Important differences have been observed in the possible physiological responses to increased dietary DHA administration relative to age. Vastly different roles and effects were noticed in infants, adults, and elderly individuals. This was also the case in relation to gender, with EPA and DHA playing different roles in male and females. A major limiting factor in our current understanding of such physiological phenomena is directly linked to the intrinsic difficulties in developing and implementing statistically powerful and biologically meaningful intervention trials, as there are several sources of variability which are now increasingly becoming apparent. There is a growing, globally significant market and industry for DHA, and n-3 LC-PUFA in general. New, and more efficient, omega-3 concentration techniques are being developed simultaneously with major agribusiness innovations, such as the production and extraction of novel DHA rich oils from microalgae fermentation and genetically modified oilseed crops. Although omega-3 fatty acids have been extensively studied in the last few decades, there are still many unknowns driving the ongoing need for specific, targeted, tailored, well designed and conceived experimental trials.
Conflict of interest
The Authors, SGF and GE are employees of Nu-Mega Ingredients Pty Ltd, a manufacturer and supplier of high DHA fish oil products. Nu-Mega Ingredients Pty Ltd. provided support in the form of salaries for authors SGF and GE, but did not have any additional role in the study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript.
The authors' responsibilities
SGF, AJS and GE conceived the study. SGF, AJS and GMT developed the selection criteria. SGF and FW performed the literature search and review. SGF, FW, AJS, GE and GMT wrote the paper.
Acknowledgements
This research did not receive any specific grant or other direct monetary support from funding agencies in the public, commercial, or not-for-profit sectors. The authors wish to thank Yi Wan, Mek Cheng and Bo Wang for their assistance in the literature search and technical assistance in drafting the tables, and Bianca Szkuta for the careful revision of the final draft.
References
- Abdukeyum, G. G., A. J. Owen, T. A. Larkin, and P. L. McLennan. 2016. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. Journal of Clinical Medicine 5:32.
- Abdukeyum, G. G., A. J. Owen, and P. L. McLennan. 2008. Dietary (n-3) long-chain polyunsaturated fatty acids inhibit ischemia and reperfusion arrhythmias and infarction in rat heart not enhanced by ischemic preconditioning. Journal of Nutrition 138:1902–09.
- Adkins, Y., and D. S. Kelley. 2010. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. Journal of Nutritional Biochemistry 21:781–92.
- Allaire, J., W. S. Harris, C. Vors, A. Charest, J. Marin, K. H. Jackson, A. Tchernof, P. Couture, and B. Lamarche. 2017. Supplementation with high-dose docosahexaenoic acid increases the Omega-3 Index more than high-dose eicosapentaenoic acid. Prostaglandins Leukotrienes and Essential Fatty Acids 120:8–14.
- Anderton, B. H. 2002. Ageing of the brain. Mechanisms of Ageing and Development 123:811–7.
- Andrieu, S., S. Guyonnet, N. Coley, C. Cantet, M. Bonnefoy, S. Bordes, L. Bories, M.-N. Cufi, T. Dantoine, J.-F. Dartigues, et al. 2017. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurologia 16:377–389.
- Arai, T., H-j. Kim, H. Chiba, and A. Matsumoto. 2009. Anti-obesity effect of fish oil and fish oil-fenofibrate combination in female KK mice. Journal of Atherosclerosis and Thrombosis 16:674–83.
- Armah, C. K., K. G. Jackson, I. Doman, L. James, F. Cheghani, and A. M. Minihane. 2008. Fish oil fatty acids improve postprandial vascular reactivity in healthy men. Clinical Science (Lond.) 114:679–86.
- Atwell, K., C. T. Collins, T. R. Sullivan, P. Ryan, R. A. Gibson, M. Makrides, and A. J. McPhee. 2013. Respiratory hospitalisation of infants supplemented with docosahexaenoic acid as preterm neonates. Journal of Paediatrics and Child Health H 49:E17–22.
- Avramovic, N., V. Dragutinovic, D. Krstic, M. Colovic, A. Trbovic, S. de Luka, I. Milovanovic, and T. Popovic. 2012. The effects of omega 3 fatty acid supplementation on brain tissue oxidative status in aged wistar rats. Hippokratia 16:241–5.
- Ayer, J. G., J. A. Harmer, W. Xuan, B. Toelle, K. Webb, C. Almqvist, G. B. Marks, and D. S. Celermajer. 2009. Dietary supplementation with n− 3 polyunsaturated fatty acids in early childhood: effects on blood pressure and arterial structure and function at age 8 y. American Journal of Clinical Nutrition 90:438–46.
- Bakker, E. C., A. C. van Houwelingen, and G. Hornstra. 1999. Early nutrition, essential fatty acid status and visual acuity of term infants at 7 months of age. European Journal Clinical Nutrition 53:872–9.
- Balk, E. M., A. H. Lichtenstein, M. Chung, B. Kupelnick, P. Chew, and J. Lau. 2006. Review: Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 189:19–30.
- Ballantyne, C. M., H. E. Bays, J. J. Kastelein, E. Stein, J. L. Isaacsohn, R. A. Braeckman, and P. N. Soni. 2012. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). American Journal of Cardiology 110:984–92.
- Bang, H. O., J. Dyerberg, and A. B. Nielson. 1971. Plasma lipid and lipoprotein pattern in Greenlandic West-Coast Eskimos. Lancet 1:1143–&.
- Barbadoro, P., I. Annino, E. Ponzio, R. M. L. Romanelli, M. M. D'Errico, E. Prospero, and A. Minelli. 2013. Fish oil supplementation reduces cortisol basal levels and perceived stress: A randomized, placebo-controlled trial in abstinent alcoholics. Molecular Nutrition & Food Research 57:1110–1114.
- Bays, H. E., C. M. Ballantyne, J. J. Kastelein, J. L. Isaacsohn, R. A. Braeckman, and P. N. Soni. 2011. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). American Journal of Cardiology 108:682–90.
- Bazan, N. G. 2013. The docosanoid neuroprotectin D1 induces homeostatic regulation of neuroinflammation and cell survival. Prostaglandins Leukotrienes and Essential Fatty Acids 88(1):127–129.
- Best, K. P., T. Sullivan, D. Palmer, M. Gold, D. J. Kennedy, J. Martin, and M. Makrides. 2016. Prenatal fish oil supplementation and allergy: 6-Year follow-up of a randomized controlled trial. Pediatrics 137.
- Birch, D. G., E. E. Birch, D. R. Hoffman, and R. D. Uauy. 1992. Retinal development in very-low-birth-weight infants fed diets differing in omega-3-fatty-acids. Investigative Ophthalmology & Visual Science 33:2365–76.
- Birch, E. E., Y. S. Castaneda, D. H. Wheaton, D. G. Birch, R. D. Uauy, and D. R. Hoffman. 2005. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. American Journal of Clinical Nutrition 81:871–879.
- Birch, E. E., D. R. Hoffman, R. Uauy, D. G. Birch, and C. Prestidge. 1998. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatric Research 44:201–9.
- Bradbury, J., S. P. Myers, and C. Oliver. 2004. An adaptogenic role for omega-3 fatty acids in stress; a randomised placebo controlled double blind intervention study (pilot)[ISRCTN22569553]. Nutrition Journal 3:20.
- Brenna, J. T., and G. Y. Diau. 2007. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins, Leukotrienes and Essential Fatty Acids 77:247–50.
- Brew, B. K., B. G. Toelle, K. L. Webb, C. Almqvist, G. B. Marks, and C. investigators. 2015. Omega-3 supplementation during the first 5 years of life and later academic performance: a randomised controlled trial. European Journal of Clinical Nutrition 69:419–24.
- Brochot, A., M. Guinot, D. Auchere, J.-P. Macaire, P. Weill, A. Grynberg, and D. Rousseau-Ralliard. 2009. Effects of alpha-linolenic acid vs. docosahexaenoic acid supply on the distribution of fatty acids among the rat cardiac subcellular membranes after a short-or long-term dietary exposure. Nutrition & Metabolism 6:14.
- Brouwer, I. A., P. L. Zock, L. G. van Amelsvoort, M. B. Katan, and E. G. Schouten. 2002. Association between n-3 fatty acid status in blood and electrocardiographic predictors of arrhythmia risk in healthy volunteers. American Journal of Cardiology 89:629–31.
- Buckley, J. D., S. Burgess, K. J. Murphy, and P. R. Howe. 2009. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. Journal of Science and Medicine in Sport 12:503–7.
- Buckley, R., B. Shewring, R. Turner, P. Yaqoob, and A. M. Minihane. 2004. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. British Journal of Nutrition 92:477–483.
- Burdge, G. C., and P. C. Calder. 2005. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction Nutrition Development 45:581–97.
- Bush, R. A., A. Malnoe, C. E. Reme, and T. P. Williams. 1994. Dietary deficiency of N-3 fatty-acids alters rhodopsin content and function in the rat retina. Investigative Ophthalmology & Visual Science 35:91–100.
- Cabello, I., O. Servitje, X. Corbella, I. Bardés, and X. Pintó. 2017. Omega‐3 fatty acids as adjunctive treatment for bexarotene‐induced hypertriglyceridaemia in patients with cutaneous T‐cell lymphoma. Clinical and Experimental Dermatology 42:276–81.
- Campoy, C., M. V. Escolano-Margarit, R. Ramos, M. Parrilla-Roure, G. Csábi, J. Beyer, M. C. Ramirez-Tortosa, A. M. Molloy, T. Decsi, and B. V. Koletzko. 2011. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age1–5. American Journal of Clinical Nutrition 94:1880S–8S.
- Canugovi, C., M. Misiak, L. K. Ferrarelli, D. L. Croteau, and V. A. Bohr. 2013. The role of DNA repair in brain related disease pathology. DNA Repair 12:578–87.
- Carlson, S. E., S. H. Werkman, P. G. Rhodes, and E. A. Tolley. 1993. Visual-acuity development in healthy preterm infants – effect of marine-oil supplementation. American Journal of Clinical Nutrition 58:35–42.
- Carlson, S. E., S. H. Werkman, and E. A. Tolley. 1996. Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. American Journal of Clinical Nutrition 63:687–97.
- Caslake, M. J., E. A. Miles, B. M. Kofler, G. Lietz, P. Curtis, C. K. Armah, A. C. Kimber, J. P. Grew, L. Farrell, J. Stannard, et al. 2008. Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. American Journal of Clinical Nutrition 88:618–29.
- Castellano, C.-A., I. Audet, J.-P. Laforest, Y. Chouinard, and J. J. Matte. 2010. Fish oil diets do not improve insulin sensitivity and secretion in healthy adult male pigs. British Journal of Nutrition 103:189–96.
- Castellano, C. A., I. Audet, J. P. Laforest, J. J. Matte, and M. Suh. 2011. Fish oil diets alter the phospholipid balance, fatty acid composition, and steroid hormone concentrations in testes of adult pigs. Theriogenology 76:1134–45.
- Cheatham, C. L., A. S. Nerhammer, M. Asserhøj, K. F. Michaelsen, and L. Lauritzen. 2011. Fish oil supplementation during lactation: Effects on cognition and behavior at 7 years of age. Lipids 46:637–45.
- Chen, C. T., Z. Liu, M. Ouellet, F. Calon, and R. P. Bazinet. 2009. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins, Leukotrienes and Essential Fatty Acids 80:157–63.
- Chong, M. F.-F., S. Lockyer, C. J. Saunders, and J. A. Lovegrove. 2010. Long chain n− 3 PUFA-rich meal reduced postprandial measures of arterial stiffness. Clinical Nutrition 29:678–81.
- Clayton, E. H., T. L. Hanstock, S. J. Hirneth, C. J. Kable, M. L. Garg, and P. L. Hazell. 2009. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. European Journal of Clinical Nutrition 63:1037–40.
- Clouard, C., A. S. Souza, W. J. J. Gerrits, R. Hovenier, A. Lammers, and J. E. Bolhuis. 2015. Maternal fish oil supplementation affects the social behavior, brain fatty acid profile, and sickness response of piglets. Journal of Nutrition 145:2176–84.
- Collins, C. T., M. Makrides, R. A. Gibson, A. J. McPhee, P. G. Davis, L. W. Doyle, K. Simmer, P. B. Colditz, S. Morris, and T. R. Sullivan. 2011. Pre-and post-term growth in pre-term infants supplemented with higher-dose DHA: a randomised controlled trial. British Journal of Nutrition 105:1635–43.
- Conquer, J. A., and B. J. Holub. 1996. Supplementation with an algae source of docosahexaenoic acid increases (n-3) fatty acid status and alters selected risk factors for heart disease in vegetarian subjects. Journal of Nutrition 126:3032–39.
- Corder, K. E., K. R. Newsham, J. L. McDaniel, U. R. Ezekiel, and E. P. Weiss. 2016. Effects of short-term docosahexaenoic acid supplementation on markers of inflammation after eccentric strength exercise in women. Journal of Sports Science and Medicine 15:176.
- Cottin, S. C., T. A. Sanders, and W. L. Hall. 2011. The differential effects of EPA and DHA on cardiovascular risk factors. Proceedings of the Nutrition Society 70:215–31.
- Crawford, M. A., R. P. Bazinet, and A. J. Sinclair. 2009. Fat intake and CNS functioning: ageing and disease. Annals of Nutrition and Metabolism 55:202–28.
- Crawford, M. A., C. L. Broadhurst, M. Guest, A. Nagar, Y. Wang, K. Ghebremeskel, and W. F. Schmidt. 2013. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins, Leukotrienes and Essential Fatty Acids 88(1):5–13.
- Crawford, M. A., N. M. Casperd, and A. J. Sinclair. 1976. The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comparative Biochemistry and Physiology B 54:395–401.
- Crowe, F. L., C. Murray Skeaff, T. J. Green, and A. R. Gray. 2008. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. British Journal of Nutrition 99:168–74.
- Cussons, A. J., G. F. Watts, T. A. Mori, and B. G. Stuckey. 2009. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. The Journal of Clinical Endocrinology and Metabolism 94:3842–48.
- D'Vaz, N., S. Meldrum, J. Dunstan, T. Lee‐Pullen, J. Metcalfe, B. Holt, M. Serralha, M. Tulic, T. Mori, and S. Prescott. 2012. Fish oil supplementation in early infancy modulates developing infant immune responses. Clinical & Experimental Allergy 42:1206–16.
- Dallongeville, J., J. Yarnell, P. Ducimetière, D. Arveiler, J. Ferrières, M. Montaye, G. Luc, A. Evans, A. Bingham, B. Hass, et al. 2003. Fish Consumption Is Associated With Lower Heart Rates. Circulation 108:820–25.
- Damsgaard, C. T., C. Mølgaard, J. Matthiessen, S. N. Gyldenløve, and L. Lauritzen. 2012. The effects of n-3 long-chain polyunsaturated fatty acids on bone formation and growth factors in adolescent boys. Pediatric Research 71:713–9.
- Dangour, A. D., E. Allen, D. Elbourne, N. Fasey, A. E. Fletcher, P. Hardy, G. E. Holder, R. Knight, L. Letley, M. Richards, et al. 2010. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: A randomized, double-blind, controlled trial. American Journal of Clinical Nutrition 91:1725–1732.
- Davis, D. J., P. M. Hecht, E. Jasarevic, D. Q. Beversdorf, M. J. Will, K. Fritsche, and C. H. Gillespie. 2017. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain, Behavior, and Immunity 59:38–48.
- Dawson, K., L. Zhao, Y. Adkins, M. Vemuri, R. L. Rodriguez, J. P. Gregg, D. S. Kelley, and D. H. Hwang. 2012. Modulation of blood cell gene expression by DHA supplementation in hypertriglyceridemic men. Journal of Nutritional Biochemistry 23:616–21.
- DeFina, L. F., L. G. Marcoux, S. M. Devers, J. P. Cleaver, and B. L. Willis. 2010. Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. American Journal of Clinical Nutrition 93:455–62.
- Delgado-Noguera, M. F., J. A. Calvache, X. B. Cosp, E. P. Kotanidou, and A. Galli-Tsinopoulou. 2015. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. The Cochrane Database of Systematic Reviews.
- DeMar, J. C., H. J. Lee, K. Z. Ma, L. Chang, J. M. Bell, S. I. Rapoport, and R. P. Bazinet. 2006. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochimica et Biophysica Acta 1761:1050–59.
- DiLorenzo, F. M., C. J. Drager, and J. W. Rankin. 2014. Docosahexaenoic acid affects markers of inflammation and muscle damage after eccentric exercise. The Journal of Strength & Conditioning Research 28:2768–74.
- Din, J. N., S. A. Harding, C. J. Valerio, J. Sarma, K. Lyall, R. A. Riemersma, D. E. Newby, and A. D. Flapan. 2008. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis 197:290–6.
- Du, C. Y., Y. Fujii, M. Ito, M. Harada, E. Moriyama, R. Shimada, A. Ikemoto, and H. Okuyama. 2004. Dietary polyunsaturated fatty acids suppress acute hepatitis, alter gene expression and prolong survival of female Long-Evans Cinnamon rats, a model of Wilson disease. Journal of Nutritional Biochemistry 15:273–80.
- Du, S., J. Jin, W. Fang, and Q. Su. 2015. Does fish oil have an anti-obesity effect in overweight/obese adults? A Meta-Analysis of Randomized Controlled Trials. PLoS One 10:e0142652.
- Dunstan, J. A., K. Simmer, G. Dixon, and S. L. Prescott. 2008. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 93:F45–50.
- Dyall, S. C. 2015. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in Aging Neuroscience 7:52.
- Dyerberg, J., H. O. Bang, E. Stoffersen, S. Moncada, and J. R. Vane. 1978. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 2:117–9.
- EFSA Panel on Dietetic Products Nutrition Allergies. 2010. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 8.
- Egert, S., M. Fobker, G. Andersen, V. Somoza, H. F. Erbersdobler, and U. Wahrburg. 2008. Effects of dietary α-linolenic acid, eicosapentaenoic acid or docosahexaenoic acid on parameters of glucose metabolism in healthy volunteers. Annals of Nutrition and Metabolism 53:182–7.
- Engstrm, K., A. S. Saldeen, B. Yang, J. L. Mehta, and T. Saldeen. 2009. Effect of fish oils containing different amounts of EPA, DHA, and antioxidants on plasma and brain fatty acids and brain nitric oxide synthase activity in rats. Upsala Journal of Medical Sciences 114:206–13.
- Escolano-Margarit, M. V., R. Ramos, J. Beyer, G. Csabi, M. Parrilla-Roure, F. Cruz, M. Perez-Garcia, M. Hadders-Algra, A. Gil, T. Decsi, et al. 2011. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. Journal of Nutrition 141:1216–23.
- Faldella, G., M. Govoni, R. Alessandroni, E. Marchiani, G. P. Salvioli, P. L. Biagi, and C. Spano. 1996. Visual evoked potentials and dietary long chain polyunsaturated fatty acids in preterm infants. Archives of Disease in Childhood 75:F108–12.
- FAO/WHO. 2008. Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatty Acids. The joint FAO/WHO expert consultation on fats & fatty acids in human nutrition, November 10–14, 2008. Geneva, Switzerland: WHO HQ.
- Fernandes, J. S., M. A. Mori, R. Ekuni, R. M. W. Oliveira, and H. Milani. 2008. Long-term treatment with fish oil prevents memory impairments but not hippocampal damage in rats subjected to transient, global cerebral ischemia. Nutrition Research 28:798–808.
- Finnegan, Y. E., A. M. Minihane, E. C. Leigh-Firbank, S. Kew, G. W. Meijer, R. Muggli, P. C. Calder, and C. M. Williams. 2003. Plant-and marine-derived n− 3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. American Journal of Clinical Nutrition 77:783–95.
- Flock, M. R., A. C. Skulas-Ray, W. S. Harris, T. D. Etherton, J. A. Fleming, and P. M. Kris-Etherton. 2013. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose–response randomized controlled trial. Journal of the American Heart Association 2:e000513.
- Food and Agriculture Organization of the United Nations. 2010. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food and Nutrition Paper 91:1–166.
- Frangou, S., M. Lewis, J. WoUard, and A. Simmons. 2007. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. Journal of Psychopharmacology 21:435–9.
- Freund-Levi, Y., H. Basun, T. Cederholm, G. Faxen-Irving, A. Garlind, M. Grut, I. Vedin, J. Palmblad, L. O. Wahlund, and M. Eriksdotter-Jonhagen. 2008. Omega-3 supplementation in mild to moderate Alzheimer's disease: effects on neuropsychiatric symptoms. International Journal of Geriatric Psychiatry 23:161–9.
- Freund-Levi, Y., I. Vedin, E. Hjorth, H. Basun, G. Faxén Irving, M. Schultzberg, M. Eriksdotter, J. Palmblad, B. Vessby, L. O. Wahlund, et al. 2014. Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer's disease: The OmegAD study. Journal of Alzheimer's Disease 42:823–31.
- Ghasemifard, S., K. Hermon, G. M. Turchini, and A. J. Sinclair. 2015a. Metabolic fate (absorption, β-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil) in the rat. British Journal of Nutrition 114:684–92.
- Ghasemifard, S., A. J. Sinclair, G. Kaur, P. Lewandowski, and G. M. Turchini. 2015b. What is the most effective way of increasing the bioavailability of dietary long chain omega-3 fatty acids – daily vs. weekly administration of fish oil? Nutrients 7:5628–45.
- Ghasemifard, S., G. M. Turchini, and A. J. Sinclair. 2014. Omega-3 long chain fatty acid “bioavailability”: a review of evidence and methodological considerations. Progress in Lipid Research 56:92–108.
- Gorjão, R., R. Verlengia, T.Md. Lima, F. G. Soriano, M. F. C. Boaventura, C. C. Kanunfre, C. M. Peres, S. C. Sampaio, R. Otton, A. Folador, et al. 2006. Effect of docosahexaenoic acid-rich fish oil supplementation on human leukocyte function. Clinical Nutrition 25:923–38.
- Gould, J. F., L. G. Smithers, and M. Makrides. 2013. The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 97:531–44.
- Grenyer, B. F., T. Crowe, B. Meyer, A. J. Owen, E. M. Grigonis-Deane, P. Caputi, and P. R. Howe. 2007. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Progress in Neuro-Psychopharmacology & Biological Psychiatry 31:1393–96.
- Grimsgaard, S., K. H. Bønaa, J.-B. Hansen, and E. Myhre. 1998. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. American Journal of Clinical Nutrition 68:52–59.
- Gunnarsdottir, I., H. Tomasson, M. Kiely, J. Martinez, N. Bandarra, M. Morais, and I. Thorsdottir. 2008. Inclusion of fish or fish oil in weight-loss diets for young adults: effects on blood lipids. International Journal of Obesity 32:1105–12.
- Guzmán, J. F., H. Esteve, C. Pablos, A. Pablos, C. Blasco, and J. A. Villegas. 2011. DHA-rich fish oil improves complex reaction time in female elite soccer players. Journal of Sports Science and Medicine 10:301.
- Halade, G. V., M. M. Rahman, A. Bhattacharya, J. L. Barnes, B. Chandrasekar, and G. Fernandes. 2010. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. Journal of Immunology 184:5280–86.
- Hamazaki, K., D. Syafruddin, I. S. Tunru, M. F. Azwir, P. B. Asih, S. Sawazaki, and T. Harnazaki. 2008. The effects of docosahexaenoic acid-rich fish oil on behavior, school attendance rate and malaria infection in school children – a double-blind, randomized, placebo-controlled trial in Lampung, Indonesia. Asia Pacific Journal of Clinical Nutrition 17:258–63.
- Hansell, A. L., N. Rose, C. T. Cowie, E. G. Belousova, I. Bakolis, K. Ng, B. G. Toelle, and G. B. Marks. 2014. Weighted road density and allergic disease in children at high risk of developing asthma. PLoS One 9:e98978–e98978.
- Harris, W. S. 1997. n-3 fatty acids and serum lipoproteins: human studies. American Journal of Clinical Nutrition 65:1645S–1654S.
- Harris, W. S., S. A. Sands, S. L. Windsor, H. A. Ali, T. L. Stevens, A. Magalski, C. B. Porter, and A. M. Borkon. 2004. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients correlation with erythrocytes and response to supplementation. Circulation 110:1645–9.
- Harris, W. S., and C. Von Schacky. 2004. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive Medicine 39:212–0.
- Hashimoto, M., S. Hossain, H. Yamasaki, K. Yazawa, and S. Masumura. 1999. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids 34:1297–304.
- Haugaard, S. B., S. Madsbad, C. E. Høy, and A. Vaag. 2006. Dietary intervention increases n‐3 long‐chain polyunsaturated fatty acids in skeletal muscle membrane phospholipids of obese subjects. Implications for insulin sensitivity. Clinical Endocrinology 64:169–78.
- Helland, I. B., O. D. Saugstad, L. Smith, K. Saarem, K. Solvoll, T. Ganes, and C. A. Drevon. 2001. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 108:E82.
- Helland, I. B., L. Smith, B. Blomen, K. Saarem, O. D. Saugstad, and C. A. Drevon. 2008. Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 122:E472–9.
- Helland, I. B., L. Smith, K. Saarem, O. D. Saugstad, and C. A. Drevon. 2003. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 111.
- Henry, R., G. E. Peoples, and P. L. McLennan. 2015. Muscle fatigue resistance in the rat hindlimb in vivo from low dietary intakes of tuna fish oil that selectively increase phospholipid n-3 docosahexaenoic acid according to muscle fibre type. British Journal of Nutrition 114:873–84.
- Higuchi, T., N. Shirai, and H. Suzuki. 2006. Reduction in plasma glucose after lipid changes in mice fed fish oil, docosahexaenoic acid, and eicosapentaenoic acid diets. Annals of Nutrition and Metabolism 50:147–54.
- Hill, A. M., J. D. Buckley, K. J. Murphy, and P. R. Howe. 2007a. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. American Journal of Clinical Nutrition 85:1267–74.
- Hill, A. M., C. Worthley, K. J. Murphy, J. D. Buckley, A. Ferrante, and P. R. Howe. 2007b. n-3 Fatty acid supplementation and regular moderate exercise: differential effects of a combined intervention on neutrophil function. British Journal of Nutrition 98:300–9.
- Hirayama, S., T. Hamazaki, and K. Terasawa. 2004. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder – A placebo-controlled double-blind study. European Journal of Clinical Nutrition 58:467–73.
- Hoffman, D. R., E. E. Birch, Y. S. Castaneda, S. L. Fawcett, D. H. Wheaton, D. G. Birch, and R. Uauy. 2003. Visual function in breast-fed term infants weaned to formula with or without long-chain polyunsaturates at 4 to 6 months: A randomized clinical trial. The Journal of Pediatrics 142:669–677.
- Holguin, F., M. M. Téllez-Rojo, M. Lazo, D. Mannino, J. Schwartz, M. Hernández, and I. Romieu. 2005. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest 127:1102–07.
- Horrocks, L. A., and Y. K. Yeo. 1999. Health benefits of docosahexaenoic acid (DHA). Pharmacological Research 40:211–25.
- Huggins, C. E., C. L. Curl, R. Patel, P. L. McLennan, M. L. Theiss, T. Pedrazzini, S. Pepe, and L. M. Delbridge. 2009. Dietary fish oil is antihypertrophic but does not enhance postischemic myocardial function in female mice. American Journal of Physiology-Heart and Circulatory Physiology 296:H957–66.
- Hurtado, J. A., C. Iznaola, M. Pena, J. Ruiz, L. Pena-Quintana, N. Kajarabille, Y. Rodriguez-Santana, P. Sanjurjo, L. Aldamiz-Echevarria, J. Ochoa, et al. 2015. Effects of maternal omega-3 supplementation on fatty acids and on visual and cognitive development. Journal of Pediatric Gastroenterology and Nutrition 61:472–80.
- Innis, S. M. 2005. Essential fatty acid transfer and fetal development. Placenta 26:S70–5.
- Itomura, M., K. Hamazaki, S. Sawazaki, M. Kobayashi, K. Terasawa, S. Watanabe, and T. Hamazaki. 2005. The effect of fish oil on physical aggression in schoolchildren – a randomized, double-blind, placebo-controlled trial. Journal of Nutritional Biochemistry 16:163–71.
- Jackson, P. A., M. E. Deary, J. L. Reay, A. B. Scholey, and D. O. Kennedy. 2012a. No effect of 12 weeks' supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. British Journal of Nutrition 107:1232–43.
- Jackson, P. A., J. S. Forster, J. G. Bell, J. R. Dick, I. Younger, and D. O. Kennedy. 2016. DHA supplementation alone or in combination with other nutrients does not modulate cerebral hemodynamics or cognitive function in healthy older adults. Nutrients 8:86.
- Jackson, P. A., J. L. Reay, A. B. Scholey, and D. O. Kennedy. 2012b. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: a near IR spectroscopy pilot study. British Journal of Nutrition 107:1093–98.
- Jackson, P. A., J. L. Reay, A. B. Scholey, and D. O. Kennedy. 2012c. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biological Psychology 89:183–90.
- Jacobson, T. A., S. B. Glickstein, J. D. Rowe, and P. N. Soni. 2012. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. Journal of Clinical Lipidology 6:5–18.
- James, M. J., T. R. Sullivan, R. G. Metcalf, and L. G. Cleland. 2014. Pitfalls in the use of randomised controlled trials for fish oil studies with cardiac patients. British Journal of Nutrition 112:812–20.
- Johnson, E. J., K. Mcdonald, S. M. Caldarella, H-y. Chung, A. M. Troen, and D. M. Snodderly. 2008. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutritional Neuroscience 11:75–83.
- Kew, S., T. Banerjee, A. M. Minihane, Y. E. Finnegan, R. Muggli, R. Albers, C. M. Williams, and P. C. Calder. 2003. Lack of effect of foods enriched with plant- or marine-derived n-3 fatty acids on human immune function. American Journal of Clinical Nutrition 77:1287–95.
- Kew, S., M. D. Mesa, S. Tricon, R. Bitckley, A. M. Minihane, and P. Yaqoob. 2004. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. American Journal of Clinical Nutrition 79:674–81.
- Khan, F., K. Elherik, C. Bolton-Smith, R. Barr, A. Hill, I. Murrie, and J. J. Belch. 2003. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovascular Research 59:955–62.
- Kohno, H., N. Yamaguchi, C. Ohdoi, S. Nakajima, S. Odashima, and T. Tanaka. 2000. Modifying effect of tuna orbital oil rich in docosahexaenoic acid and vitamin D-3 on azoxymethane-induced colonic aberrant crypt foci in rats. Oncology Reports 7:1069–74.
- Koletzko, B., E. Lien, C. Agostoni, H. Bohles, C. Campoy, I. Cetin, T. Decsi, J. W. Dudenhausen, C. Dupont, S. Forsyth, et al. 2008. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. Journal of Perinatal Medicine 36:5–14.
- Kook, K., B. H. Choi, S. S. Sun, F. Garcia, and K. H. Myung. 2002. Effect of fish oil supplement on growth performance, ruminal metabolism and fatty acid composition of longissimus muscle in Korean cattle. Asian-Australasian Journal of Animal Sciences 15:66–71.
- Krebs, J., L. Browning, N. McLean, J. Rothwell, G. Mishra, C. Moore, and S. Jebb. 2006. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. International Journal of Obesity 30:1535–44.
- Kris-Etherton, P. M., W. S. Harris, L. J. Appel, and American Heart Association. Nutrition, C. 2002. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–57.
- Kumar, S., F. Sutherland, A. W. Teh, P. M. Heck, G. Lee, M. L. Garg, and P. B. Sparks. 2011. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human pulmonary vein and left atrial electrophysiology in paroxysmal atrial fibrillation. American Journal of Cardiology 108:531–5.
- Kunesova, M., R. Braunerova, P. Hlavatý, and E. Tvrzicka. 2006. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiological Research 55:63.
- Lagarde, M., M. Hachem, M. Picq, M. Guichardant, and N. Bernoud-Hubac. 2016. AceDoPC, a structured phospholipid to target the brain with docosahexaenoic acid. OCL 23:D102.
- Larter, C. Z., M. M. Yeh, J. Cheng, J. Williams, S. Brown, A. Dela Pena, K. S. Bell‐Anderson, and G. C. Farrell. 2008. Activation of peroxisome proliferator‐activated receptor α by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation. European Journal of Gastroenterology & Hepatology 23:267–75.
- Lauritzen, L., M. H. Jørgensen, T. B. Mikkelsen, I. M. Skovgaard, E.-M. Straarup, S. F. Olsen, C.-E. Høy, and K. F. Michaelsen. 2004. Maternal fish oil supplementation in lactation: effect on visual acuity and n− 3 fatty acid content of infant erythrocytes. Lipids 39:195–206.
- Lauritzen, L., M. H. Jorgensen, S. F. Olsen, E. M. Straarup, and K. F. Michaelsen. 2005. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reproduction Nutrition Development 45:535–47.
- LeBlanc, C. J., D. W. Horohov, J. E. Bauer, G. Hosgood, and G. E. Mauldin. 2008. Effects of dietary supplementation with fish oil on in vivo production of inflammatory mediators in clinically normal dogs. American Journal of Veterinary Research 69:486–93.
- Lee, L. K., S. Shahar, A. V. Chin, and N. A. M. Yusoff. 2013. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): A 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology 225:605–12.
- Li, K., T. Huang, J. Zheng, K. Wu, and D. Li. 2014. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One 9:e88103–e88103.
- Macartney, M. J., L. Hingley, M. A. Brown, G. E. Peoples, and P. L. McLennan. 2014. Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. British Journal of Nutrition 112:1984–92.
- Makrides, M., R. A. Gibson, A. J. McPhee, C. T. Collins, P. G. Davis, L. W. Doyle, K. Simmer, P. B. Colditz, S. Morris, L. G. Smithers, et al. 2009. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 301:175–182.
- Makrides, M., R. A. Gibson, A. J. McPhee, L. Yelland, J. Quinlivan, P. Ryan, L. W. Doyle, P. Anderson, P. L. Else, B. J. Meyer, et al. 2010. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 304:1675–83.
- Makrides, M., M. A. Neumann, B. Jeffrey, E. L. Lien, and R. A. Gibson. 2000. A randomized trial of different ratios of linoleic to alpha-linolenic acid in the diet of term infants: effects on visual function and growth. American Journal of Clinical Nutrition 71:120–9.
- Manley, B. J., M. Makrides, C. T. Collins, A. J. McPhee, R. A. Gibson, P. Ryan, T. R. Sullivan, P. G. Davis, and C. Dino Steering. 2011. High-dose docosahexaenoic acid supplementation of preterm infants: Respiratory and allergy outcomes. Pediatrics 128:E71–7.
- Mann, N. J., S. L. O'Connell, K. M. Baldwin, I. Singh, and B. J. Meyer. 2010. Effects of seal oil and tuna-fish oil on platelet parameters and plasma lipid levels in healthy subjects. Lipids 45:669–81.
- Mansoori, A., G. Sotoudeh, M. Djalali, M.-R. Eshraghian, M. Keramatipour, E. Nasli-Esfahani, F. Shidfar, E. Alvandi, O. Toupchian, and F. Koohdani. 2016. Docosahexaenoic acid-rich fish oil supplementation improves body composition without influence of the PPARγ Pro12Ala polymorphism in patients with type 2 diabetes: A Randomized, double-blind, placebo-controlled clinical trial. Journal of Nutrigenetics and Nutrigenomics 8:195–204.
- Marks, G. B., S. Mihrshahi, A. S. Kemp, E. R. Tovey, K. Webb, C. Almqvist, R. D. Ampon, D. Crisafulli, E. G. Belousova, C. M. Mellis, et al. 2006. Asthma diagnosis and treatment: Prevention of asthma during the first 5 years of life: A randomized controlled trial. The Journal of Allergy and Clinical Immunology 118:53–61.
- Marques, C. G., V. C. Santos, A. C. Levada-Pires, T. M. Jacintho, R. Gorjao, T. C. Pithon-Curi, and M. F. Cury-Boaventura. 2015. Effects of DHA-rich fish oil supplementation on the lipid profile, markers of muscle damage, and neutrophil function in wheelchair basketball athletes before and after acute exercise. Applied Physiology, Nutrition, and Metabolism 40:596–604.
- Martins, J. G. 2009. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. The Journal of the American College of Nutrition 28:525–42.
- McEwen, B. J., M.-C. Morel-Kopp, W. Chen, G. H. Tofler, and C. M. Ward. 2013. Effects of omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease. Seminars in Thrombosis and Hemostasis 39:25–32.
- McEwen, B. J., M.-C. Morel-Kopp, G. H. Tofler, and C. M. Ward. 2015. The effect of omega-3 polyunsaturated fatty acids on fibrin and thrombin generation in healthy subjects and subjects with cardiovascular disease. Seminars in Thrombosis and Hemostasis 41:315–22.
- McLennan, P. L. 2014. Cardiac physiology and clinical efficacy of dietary fish oil clarified through cellular mechanisms of omega-3 polyunsaturated fatty acids. European Journal of Applied Physiology 114:1333–56.
- McLennan, P. L., and M. Y. Abeywardena. 2005. Membrane basis for fish oil effects on the heart: linking natural hibernators to prevention of human sudden cardiac death. The Journal of Membrane Biology 206:85–102.
- McLennan, P., P. Howe, M. Abeywardena, R. Muggli, D. Raederstorff, M. Mano, T. Rayner, and R. Head. 1996. The cardiovascular protective role of docosahexaenoic acid. European Journal of Pharmacology 300:83–9.
- McManus, S., N. Tejera, K. Awwad, D. Vauzour, N. Rigby, I. Fleming, A. Cassidy, and A. M. Minihane. 2016. Differential effects of EPA versus DHA on postprandial vascular function and the plasma oxylipin profile in men. Journal of Lipid Research 57:1720–27.
- Meldrum, S., J. A. Dunstan, J. K. Foster, K. Simmer, and S. L. Prescott. 2015. Maternal fish oil supplementation in pregnancy: a 12 year follow-up of a randomised controlled trial. Nutrients 7:2061–67.
- Meldrum, S. J., N. D'Vaz, K. Simmer, J. A. Dunstan, K. Hird, and S. L. Prescott. 2012. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: a randomised controlled trial. British Journal of Nutrition 108:1443–54.
- Metcalf, R. G., M. J. James, R. A. Gibson, J. R. Edwards, J. Stubberfield, R. Stuklis, K. Roberts-Thomson, G. D. Young, and L. G. Cleland. 2007. Effects of fish-oil supplementation on myocardial fatty acids in humans. American Journal of Clinical Nutrition 85:1222–28.
- Meyer, B. J., and R. H. de Groot. 2017. Effects of omega-3 long chain polyunsaturated fatty acid supplementation on cardiovascular mortality: the importance of the dose of DHA. Nutrients 9:1305.
- Meyer, B. J., T. Hammervold, A. C. Rustan, and P. R. Howe. 2007. Dose-dependent effects of docosahexaenoic acid supplementation on blood lipids in statin-treated hyperlipidaemic subjects. Lipids 42:109–15.
- Meyer, B. J., A. Lane, and N. Mann. 2009. Comparison of seal oil to tuna oil on plasma lipid levels and blood pressure in hypertriglyceridaemic subjects. Lipids 44:827–35.
- Micallef, M. A., and M. L. Garg. 2009. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis 204:476–82.
- Miller, M. R., P. D. Nichols, and C. G. Carter. 2011. New Alternative n-3 Long-Chain Polyunsaturated Fatty Acid-Rich Oil Sources. In: Fish oil Replacement and Alternative Lipid Sources in Aquaculture Feeds, G. M. Turchini, W. K. Ng and D. R. Tocher, Eds., 325–50. CRC Press, Boca Raton, FL.
- Milte, C. M., A. M. Coates, J. D. Buckley, A. M. Hill, and P. R. Howe. 2008. Dose-dependent effects of docosahexaenoic acid-rich fish oil on erythrocyte docosahexaenoic acid and blood lipid levels. British Journal of Nutrition 99:1083–88.
- Molinar-Toribio, E., J. Pérez-Jiménez, S. Ramos-Romero, M. Romeu, M. Giralt, N. Taltavull, M. Munoz-Cortes, O. Jáuregui, L. Méndez, and I. Medina. 2015. Effect of n-3 PUFA supplementation at different EPA: DHA ratios on the spontaneously hypertensive obese rat model of the metabolic syndrome. British Journal of Nutrition 113:878–87.
- Mori, T. A., V. Burke, I. B. Puddey, G. F. Watts, D. N. O'Neal, J. D. Best, and L. J. Beilin. 2000. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. American Journal of Clinical Nutrition 71:1085–94.
- Mori, T. A., and R. J. Woodman. 2006. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Current Opinion in Clinical Nutrition & Metabolic Care 9:95–104.
- Mozaffarian, D., A. Geelen, I. A. Brouwer, J. M. Geleijnse, P. L. Zock, and M. B. Katan. 2005. Effect of fish oil on heart rate in humans. Circulation 112:1945–52.
- Mozaffarian, D., R. J. Prineas, P. K. Stein, and D. S. Siscovick. 2006. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. Journal of the American College of Cardiology 48:478–84.
- Mozaffarian, D., and J. H. Wu. 2011. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology 58:2047–67.
- Mozaffarian, D., and J. H. Wu. 2012. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? Journal of Nutrition 142:614S–625S.
- Mozurkewich, E. L., C. M. Clinton, J. L. Chilimigras, S. E. Hamilton, L. J. Allbaugh, D. R. Berman, S. M. Marcus, V. C. Romero, M. C. Treadwell, K. L. Keeton, et al. 2013. The Mothers, Omega-3, and Mental Health Study: a double-blind, randomized controlled trial. American Journal of Obstetrics and Gynecology 208.
- Muhlhausler, B. S., L. N. Yelland, R. McDermott, L. Tapsell, A. McPhee, R. A. Gibson, and M. Makrides. 2016. DHA supplementation during pregnancy does not reduce BMI or body fat mass in children: follow-up of the DHA to Optimize Mother Infant Outcome randomized controlled trial. American Journal of Clinical Nutrition 103:1489–96.
- Munro, I. A., and M. L. Garg. 2012. Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet. British Journal of Nutrition 108:1466–74.
- Munro, I. A., and M. L. Garg. 2013a. Dietary supplementation with long chain omega-3 polyunsaturated fatty acids and weight loss in obese adults. Obesity Research & Clinical Practice 7:e173–81.
- Munro, I. A., and M. L. Garg. 2013b. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: a double-blinded randomised controlled trial. Food & Function 4:650–8.
- Nelson, G., P. Schmidt, G. Bartolini, D. Kelley, and D. Kyle. 1997. The effect of dietary docosahexaenoic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids 32:1129–36.
- Neu, J., and R. A. Polin. 2012. Gastroenterology and nutrition: neonatology questions and controversies. 2nd ed., Saunders, Philadelphia, PA.
- Neuringer, M., W. E. Connor, D. S. Lin, L. Barstad, and S. Luck. 1986. Biochemical and functional-effects of prenatal and postnatal omega-3-fatty-acid deficiency on retina and brain in rhesus-monkeys. Proceedings of the National Academy of Sciences of the United States of America 83:4021–5.
- Newens, K. J., A. K. Thompson, K. G. Jackson, J. Wright, and C. M. Williams. 2011. DHA-rich fish oil reverses the detrimental effects of saturated fatty acids on postprandial vascular reactivity. American Journal of Clinical Nutrition 94:742–8.
- Ninio, D. M., A. M. Hill, P. R. Howe, J. D. Buckley, and D. A. Saint. 2008. Docosahexaenoic acid-rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. British Journal of Nutrition 100:1097–103.
- Ninio, D. M., K. J. Murphy, P. R. Howe, and D. A. Saint. 2005. Dietary fish oil protects against stretch‐induced vulnerability to atrial fibrillation in a rabbit model. Journal of Cardiovascular Electrophysiology 16:1189–94.
- Niu, S. L., D. C. Mitchell, and B. J. Litman. 2001. Optimization of receptor-G protein coupling by bilayer lipid composition II – Formation of metarhodopsin II-transducin complex. Journal of Biological Chemistry 276:42807–11.
- O'Keefe Jr, J. H., H. Abuissa, A. Sastre, D. M. Steinhaus, and W. S. Harris. 2006. Effects of Omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. American Journal of Cardiology 97:1127–30.
- Oliver, E., F. C. McGillicuddy, K. A. Harford, C. M. Reynolds, C. M. Phillips, J. F. Ferguson, and H. M. Roche. 2012. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. Journal of Nutritional Biochemistry 23:1192–200.
- Olsen, R.-E., R. Waagbø, W. Melle, E. Ringø, and S. P. Lall. 2011. Alternative Marine Resources. In: Fish oil Replacement and Alternative Lipid Sources in Aquaculture Feeds, G. M. Turchini, W. K. Ng and D. R. Tocher, Eds., 267–324. CRC Press, Boca Raton, FL.
- Ostermann, A. I., and N. H. Schebb. 2017. Effects of Omega-3 Fatty Acid Supplementation on the Pattern of Oxylipins A short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food & Function 8:2345–622.
- Ottestad, I., S. Hassani, G. I. Borge, A. Kohler, G. Vogt, T. Hyötyläinen, M. Orešič, K. W. Brønner, K. B. Holven, and S. M. Ulven. 2012. Fish oil supplementation alters the plasma lipidomic profile and increases long-chain PUFAs of phospholipids and triglycerides in healthy subjects. PLoS One 7:e42550.
- Owen, A. J., B. A. Peter-Przyborowska, A. J. Hoy, and P. L. McLennan. 2004. Dietary fish oil dose-and time-response effects on cardiac phospholipid fatty acid composition. Lipids 39:955–61.
- Park, H., J. Choi, and K. Kim. 2000. Docosahexaenoic acid-rich fish oil and pectin have a hypolipidemic effect, but pectin increases risk factor for colon cancer in rats. Nutrition Research 20:1783–94.
- Park, Y., and W. Harris. 2002. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids 37:941–6.
- Patten, G. S., A. R. Bird, D. L. Topping, and M. Y. Abeywardena. 2002. Dietary fish oil alters the sensitivity of guinea pig ileum to electrically driven contractions and 8-iso-PGE(2). Nutrition Research 22:1413–26.
- Pedersen, M. H., C. Mølgaard, L. I. Hellgren, and L. Lauritzen. 2010. Effects of fish oil supplementation on markers of the metabolic syndrome. The Journal of Pediatrics 157:395–400. e391.
- Peoples, G. E., and P. L. McLennan. 2010. Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. British Journal of Nutrition 104:1771–9.
- Peoples, G. E., and P. L. McLennan. 2014. Long-chain n-3 DHA reduces the extent of skeletal muscle fatigue in the rat in vivo hindlimb model. British Journal of Nutrition 111:996–1003.
- Peoples, G. E., P. L. McLennan, P. R. Howe, and H. Groeller. 2008. Fish oil reduces heart rate and oxygen consumption during exercise. Journal of Cardiovascular Pharmacology and Therapeutics 52:540–7.
- Perluigi, M., A. M. Swomley, and D. A. Butterfield. 2014. Redox proteomics and the dynamic molecular landscape of the aging brain. Ageing Research Reviews 13:75–89.
- Phang, M., M. L. Garg, and A. J. Sinclair. 2009. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific—Redefining platelet response to fish oils. Prostaglandins, Leukotrienes and Essential Fatty Acids 81:35–40.
- Phang, M., L. Lincz, M. Seldon, and M. L. Garg. 2012a. Acute supplementation with eicosapentaenoic acid reduces platelet microparticle activity in healthy subjects. Journal of Nutritional Biochemistry 23:1128–33.
- Phang, M., L. F. Lincz, and M. L. Garg. 2013. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. Journal of Nutrition 143:457–63.
- Phang, M., A. Sinclair, L. Lincz, and M. Garg. 2012b. Gender-specific inhibition of platelet aggregation following omega-3 fatty acid supplementation. Nutrition, Metabolism & Cardiovascular Diseases 22:109–14.
- Phillips, M. A., C. E. Childs, P. C. Calder, and P. J. Rogers. 2015. No Effect of Omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable alzheimer's disease: A randomised controlled trial. International Journal of Molecular Sciences 16:24600–13.
- Philp, L. K., L. K. Heilbronn, A. Janovska, and G. A. Wittert. 2015. Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice. PLoS One 10:e0117494.
- Pifferi, F., M. Perret, P. Guesnet, F. Aujard, and J. M. Alessandri. 2012. Fatty acid composition of the brain, retina, liver and adipose tissue of the grey mouse lemur. Microcebus murinus, primate). Lipids 47:793–801.
- Poppitt, S. D., C. A. Howe, F. E. Lithander, K. M. Silvers, R.-B. Lin, J. Croft, Y. Ratnasabapathy, R. A. Gibson, and C. S. Anderson. 2009. Effects of moderate-dose omega-3 fish oil on cardiovascular risk factors and mood after ischemic stroke: a randomized, controlled trial. Stroke 40:3485–92.
- Puri, B. K., S. J. Counsell, G. Hamilton, A. J. Richardson, and D. F. Horrobin. 2001. Eicosapentaenoic acid in treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. International Journal of Clinical Practice 55:560–3.
- Quinn, J. F., R. Raman, R. G. Thomas, K. Yurko-Mauro, E. B. Nelson, C. Van Dyck, J. E. Galvin, J. Emond, C. R. Jack, and M. Weiner. 2010. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 304:1903–11.
- Rambjør, G. S., A. I. W»len, S. L. Windsor, and W. S. Harris. 1996. Eicosapentaenoic acid is primarily responsible for hypotriglyceridemic effect of fish oil in humans. Lipids 31:S45–9.
- Ramirez-Ramirez, V., M. A. Macias-Islas, G. G. Ortiz, F. Pacheco-Moises, E. D. Torres-Sanchez, T. E. Sorto-Gomez, J. A. Cruz-Ramos, G. Orozco-Avina, and A. J. C. de la Rosa. 2013. Efficacy of Fish Oil on Serum of TNF alpha, IL-1 beta, and IL-6 Oxidative Stress Markers in Multiple Sclerosis Treated with Interferon Beta-1b. Oxidative Medicine and Cellular Longevity 2013.
- Rees, A. M., M. P. Austin, and G. B. Parker. 2008. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial (vol 42, pg 199, 2008). Australian and New Zealand Journal of Psychiatry 42:438–438.
- Rogers, P. J., K. M. Appleton, D. Kessler, T. J. Peters, D. Gunnell, R. C. Hayward, S. V. Heatherley, L. M. Christian, S. A. McNaughton, and A. R. Ness. 2008. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. British Journal of Nutrition 99:421–31.
- Ross, B. M., J. Seguin, and L. E. Sieswerda. 2007. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids in Health and Disease 6.
- Sabour, H., B. Larijani, M. R. Vafa, M. R. Hadian, R. Heshmat, H. A. Meybodi, H. E. Razavi, A. N. Javidan, and F. Shidfar. 2012. The effects of n-3 fatty acids on inflammatory cytokines in osteoporotic spinal cord injured patients: A randomized clinical trial. Journal of Research in Medical Sciences 17:322–7.
- Saito, M., M. Ueno, K. Kubo, and M. Yamaguchi. 1998. Dose-response effect of dietary docosahexaenoic acid on fatty acid profiles of serum and tissue lipids in rats. Journal of Agricultural and Food Chemistryb 46:184–93.
- Santos, V. C., A. C. Levada-Pires, S. R. Alves, T. C. Pithon-Curi, R. Curi, and M. F. Cury-Boaventura. 2013. Effects of DHA-rich fish oil supplementation on lymphocyte function before and after a marathon race. International Journal of Sport Nutrition and Exercise Metabolism 23:161–9.
- Serhan, C. N. 2005. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Current Opinion in Clinical Nutrition & Metabolic Care 8:115–21.
- Sexton, P. T., A. J. Sinclair, K. O'Dea, A. J. Sanigorski, and J. Walsh. 1995. The relationship between linoleic acid level in serum, adipose tissue and myocardium in humans. Asia Pacific Journal of Clinical Nutrition 4:314–8.
- Silvers, K. M., C. C. Woolley, F. C. Hamilton, P. M. Watts, and R. A. Watson. 2005. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins, Leukotrienes and Essential Fatty Acids 72:211–8.
- Simmer, K., S. K. Patole, and S. C. Rao. 2011. Long-chain polyunsaturated fatty acid supplementation in infants born at term. The Cochrane Database of Systematic Reviews CD000376.
- Singhal, A., J. Lanigan, C. Storry, S. Low, T. Birbara, A. Lucas, and J. Deanfield. 2013. Docosahexaenoic acid supplementation, vascular function and risk factors for cardiovascular disease: a randomized controlled trial in young adults. Journal of the American Heart Association 2:e000283.
- Sinn, N., C. M. Milte, S. J. Street, J. D. Buckley, A. M. Coates, J. Petkov, and P. R. Howe. 2012. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. British Journal of Nutrition 107:1682–93.
- Sjoberg, N. J., C. M. Milte, J. D. Buckley, P. R. Howe, A. M. Coates, and D. A. Saint. 2010. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. British Journal of Nutrition 103:243–8.
- Skilton, M. R., J. G. Ayer, J. A. Harmer, K. Webb, S. R. Leeder, G. B. Marks, and D. S. Celermajer. 2012. Impaired fetal growth and arterial wall thickening: a randomized trial of omega-3 supplementation. Pediatrics 129:e698–e703.
- Skilton, M. R., T. R. Sullivan, J. G. Ayer, F. L. Garden, J. A. Harmer, S. R. Leeder, B. G. Toelle, K. Webb, G. B. Marks, and D. S. Celermajer. 2014. Weight gain in infancy is associated with carotid extra-medial thickness in later childhood. Atherosclerosis 233:370–4.
- Slee, E. L., P. L. McLennan, A. J. Owen, and M. L. Theiss. 2010. Low dietary fish-oil threshold for myocardial membrane n-3 PUFA enrichment independent of n-6 PUFA intake in rats. Journal of Lipid Research 51:1841–8.
- Smithers, L. G., C. T. Collins, L. A. Simmonds, R. A. Gibson, A. McPhee, and M. Makrides. 2010. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow-up study of a randomized controlled trial. American Journal of Clinical Nutrition 91:628–34.
- Smithers, L. G., R. A. Gibson, A. McPhee, and M. Makrides. 2008. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. American Journal of Clinical Nutrition 88:1049–56.
- Smuts, C. M., J. Greeff, J. Kvalsvig, M. B. Zimmermann, and J. Baumgartner. 2015. Long-chain n-3 PUFA supplementation decreases physical activity during class time in iron-deficient South African school children. British Journal of Nutrition 113:212–24.
- Song, C., C. H. Shieh, Y. S. Wu, A. Kalueff, S. Gaikwad, and K. P. Su. 2016. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer's disease: Acting separately or synergistically? Progress in Lipid Research 62:41–54.
- Souied, E. H., C. Delcourt, G. Querques, A. Bassols, B. Merle, A. Zourdani, T. Smith, and P. Benlian. 2013. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The nutritional AMD treatment 2 study. Ophthalmology 120:1619–31.
- Stonehouse, W., C. A. Conlon, J. Podd, S. R. Hill, A. M. Minihane, C. Haskell, and D. Kennedy. 2013. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. American Journal of Clinical Nutrition 97:1134–43.
- Stough, C., L. Downey, B. Silber, J. Lloyd, C. Kure, K. Wesnes, and D. Camfield. 2012. The effects of 90-day supplementation with the Omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiology of Aging 33.
- Strike, S. C., A. Carlisle, E. L. Gibson, and S. C. Dyall. 2015. A high omega-3 fatty acid multinutrient supplement benefits cognition and mobility in older women: A randomized, double-blind, placebo-controlled pilot study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 71:236–42.
- Sublette, M. E., S. P. Ellis, A. L. Geant, and J. J. Mann. 2011. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. The Journal of Clinical Psychiatry 72:1577–84.
- Svennerholm, L., K. Bostrom, and B. Jungbjer. 1997. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathologica 94:345–52.
- Teague, H., C. J. Fhaner, M. Harris, D. M. Duriancik, G. E. Reid, and S. R. Shaikh. 2013. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. Journal of Lipid Research 54:3130–8.
- Teague, H., M. Harris, J. Fenton, P. Lallemand, B. M. Shewchuk, and S. R. Shaikh. 2014. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. Journal of Lipid Research 55:1420–33.
- Thorsdottir, I., H. Tomasson, I. Gunnarsdottir, E. Gisladottir, M. Kiely, M. D. Parra, N. M. Bandarra, G. Schaafsma, and J. A. Martinez. 2007. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. International Journal of Obesity 31:1560–6.
- Tihista, S., and E. Echavarria. 2017. Effect of omega 3 polyunsaturated fatty acids derived from fish oil in major burn patients: A prospective randomized controlled pilot trial. Clinical Nutrition.
- Tokuda, H., T. Sueyasu, M. Kontani, H. Kawashima, H. Shibata, and Y. Koga. 2015. Low doses of long-chain polyunsaturated fatty acids affect cognitive function in elderly Japanese men: A randomized controlled trial. Journal of Oleo Science 64:633–44.
- Toupchian, O., G. Sotoudeh, A. Mansoori, E. Nasli-Esfahani, M. Djalali, S. A. Keshavarz, and F. Koohdani. 2016. Effects of DHA-enriched fish oil on monocyte/macrophage activation marker sCD163, asymmetric dimethyt arginine, and insulin resistance in type 2 diabetic patients. Journal of Clinical Lipidology 10:798–807.
- Trofimiuk, E., and J. J. Braszko. 2011. Long-Term Administration of Cod Liver Oil Ameliorates Cognitive Impairment Induced by Chronic Stress in Rats. Lipids 46:417–23.
- Turchini, G. M., B. E. Torstensen, and W. K. Ng. 2009. Fish oil replacement in finfish nutrition. Reviews in Aquaculture 1:10–57.
- van Goor, S. A., D. A. J. Dijck-Brouwer, B. Doornbos, J. Erwich, A. Schaafsma, F. A. J. Muskiet, and M. Hadders-Algra. 2010. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. British Journal of Nutrition 103:235–42.
- Vedin, I., T. Cederholm, Y. F. Levi, H. Basun, A. Garlind, G. F. Irving, M. E. Joenhagen, B. Vessby, L. O. Wahlund, and J. Palmblad. 2008. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. American Journal of Clinical Nutrition 87:1616–22.
- Wallace, F. A., E. A. Miles, and P. C. Calder. 2003. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. British Journal of Nutrition 89:679–89.
- Wei, M. Y., and T. A. Jacobson. 2011. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Current Atherosclerosis Reports 13:474–83.
- Weldon, S. M., A. C. Mullen, C. E. Loscher, L. A. Hurley, and H. M. Roche. 2007. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. Journal of Nutritional Biochemistry 18:250–8.
- Westphal, C., A. Konkel, and W.-H. Schunck. 2011. CYP-eicosanoids – a new link between omega-3 fatty acids and cardiac disease? Prostaglandins & Other Lipid Mediators 96:99–108.
- WHO. 2017. Depression. WHO fact sheet. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/.
- Williams, J. A., S. E. Batten, M. Harris, B. D. Rockett, S. R. Shaikh, W. Stillwell, and S. R. Wassall. 2012. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophysical Journal 103(2):228–37.
- Woodman, R. J., T. A. Mori, V. Burke, I. B. Puddey, A. Barden, G. F. Watts, and L. J. Beilin. 2003. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 166:85–93.
- Yagi, S., D. Fukuda, K-i. Aihara, M. Akaike, M. Shimabukuro, and M. Sata. 2017. N-3 polyunsaturated fatty acids: promising nutrients for preventing cardiovascular disease. Journal of Atherosclerosis and Thrombosis 24:999–1010.
- Yanagisawa, A., Y. Shimojima, M. Yamagami, T. Tsujii, H. Kannda, and K. Tajino. 2002. Serum levels of long-chain saturated fatty acids positively correlate with anxiety and hostility scores in healthy young Japanese women. In S. C. Cunnane (Ed.), 5th Congress of the International Society for the Study of Fatty Acids and Lipids (ISSFAL) Montreal, 7–11 May. Abstract no. 145.
- Yokoyama, M., H. Origasa, M. Matsuzaki, Y. Matsuzawa, Y. Saito, Y. Ishikawa, S. Oikawa, J. Sasaki, H. Hishida, and H. Itakura. 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369:1090–98.
- Yurko-Mauro, K., D. D. Alexander, and M. E. Van Elswyk. 2015. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One 10:1–8.
- Yurko-Mauro, K., D. McCarthy, D. Rom, E. B. Nelson, A. S. Ryan, A. Blackwell, N. Salem, M. Stedman, and M. Investigators. 2010. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer's & Dementia 6:456–64.
- Zhang, Y.-P., R. E. Brown, S. M. Budge, Y.-T. Zhao, X.-H. Ju, and C. Song. 2017. DHA, EPA and their combination at various ratios differently modulated Aβ 25–35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins, Leukotrienes and Essential Fatty Acids.
- Zhou, S. J., L. Yelland, A. J. McPhee, J. Quinlivan, R. A. Gibson, and M. Makrides. 2012. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. American Journal of Clinical Nutrition 95:1378–84.