?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We review the functionality of sucrose during the manufacture of cakes from the perspective of sugar replacement. Besides providing sweetness, sucrose has important functionalities concerning structure formation. These functionalities also need to be mimicked in reformulated cakes. First, we review the hypotheses, concerning the development of structure and texture of cakes during manufacturing, which are conveniently summarized in a qualitative way using the Complex Dispersed Systems methodology. Subsequently, we represent the changes of the state of the cake during manufacturing in a supplemented state diagram, which indicates the important phase transitions occurring during baking. From the analysis, we have learned that sucrose act both as a plasticizer and as a humectant, modifying the phase transitions of biopolymers, dough viscosity, and water activity. If sugar replacers exactly mimick this behavior of sucrose, similar textures in reformulated cakes can be obtained. Physical theories exist for characterizing the plasticizing and hygroscopic behavior of sugars and their replacers. We have shown that the starch gelatinization and egg white denaturation can be predicted by the volumetric density of hydrogen bonds present in the solvent, consisting of water, sugar or its replacers, such as polyols or amino-acids.
Introduction
We examine the functionality of sucrose in cake products, using a similar strategy as we have employed in our recent review paper on sugar replacement in biscuits (van der Sman and Renzetti Citation2019). Recent reviews on general ingredient functionality in cake are by Wilderjans et al. (Citation2013) and Godefroidt et al. (Citation2019). The former has a focus on batter-type cakes, while the latter has a focus on foam-type cakes. In this review paper, we take a broader focus, namely on the functionality of sucrose in both types of cake from the perspective of sugar replacement. Based on the reviewed literature, we will describe the structuring process during cake making in a formal manner using the Complex Dispersed Systems (CDS) methodology (This Citation2005) and the supplemented state diagram (Blond Citation1989; Slade, Levine, and Reid Citation1991), which complements the earlier reviews on this topic. For this description of the structuring process knowledge on the functionality of other ingredients is required. From the complete CDS description we will extract the sucrose functionality, and examine how that relates to the physical properties of sugar and its replacers.
On several occasions, we will compare cake with biscuit, due to similarities in formulation and processing. Knowledge of ingredient functionality in biscuits is rather complete (Pareyt and Delcour Citation2008; Slade and Levine Citation1994a; van der Sman and Renzetti Citation2019), but for cake ingredient functionality is not always clear, especially for the biopolymers present, namely egg proteins, gluten and starch (Wilderjans et al. Citation2013). Since the earlier review by Wilderjans et al. (Citation2013) a significant increase in the knowledge of the functionality of these biopolymers has been obtained (Deleu et al. Citation2016, Citation2017; Hesso, Garnier, et al. Citation2015; Kweon et al. Citation2014; Lambrecht et al. Citation2018). Furthermore, we have examined reformulation studies to extract knowledge on functionality of the reformulated ingredients.
Similar to biscuits the manufacturing of cake can be subdivided into two major steps: (1) the mixing and (2) the baking. We will use this subdivision also in our review. For each processing step, we will describe the physicochemical transitions and the role of the ingredients therein. The change in the structure during processing will be summarized using the CDS formalism. Furthermore, the major phase transitions in the hydrophilic phase will be described using the supplemented state diagram. From these two methods of describing the structuring of cake, we will extract the hypotheses of the functionality of sucrose.
Cake manufacturing
Ingredients
There are two basic types of cakes: (a) shortened or high-ratio cakes and (b) foam cakes – which differ significantly in their preparation. Shortened cakes are made with a so-called shortening, which is a mixture of fat and oil. Butter and margarine can be viewed as shortenings. Shortenings are stabilized by a network of fat crystals. The shortening is used to hold air during the first creaming step, where shortening is mixed with sugar crystals. Foam-style cakes lack the fat phase, and air is incorporated by first making an egg white foam. Cake differs from other sweet bakery products such as biscuits due to the presence of egg. Furthermore, the moisture content of the finished cake product is rather high compared to biscuits.
The main ingredients of shortened cakes (pound, yellow and chocolate cakes) are wheat flour, sucrose, egg and shortening at a ratio of about 1:1:1:1. (Deleu et al. Citation2016). The main ingredients of (sponge) foam cakes are wheat flour, sugar, and egg in a ratio of about 1:1:1 Godefroidt et al. (Citation2019). In both types of cakes the ratios of the proteins present are 45% gluten, 30% egg white, and 25% egg yolk proteins. Furthermore, in cakes one often adds leavening agents to create more volume.
For cakes, soft wheat flour is recommended, which has less gluten than hard wheat flour for bread or durum wheat flour for pasta. In the past flour chlorination was applied, which oxidized the gluten, hindering its development and allowing more leaching of amylose from gelatinized starch granules during baking (Wilderjans et al. Citation2010). In the EU this flour chlorination has now been replaced by heat treatment of flour (Meza et al. Citation2011). This treatment does not affect batter properties, nor the structure setting during baking.
Cake ingredients can be classified into two groups: (a) tougheners such as flour, egg white, milk solids (casein proteins), and salt, versus (b) tenderizers such as sugar, fat, and egg yolk (Mizukoshi Citation1985b). Tougheners can contribute to the building of the protein network, while the tenderizers hinder that. Egg is a complicated ingredient as it has tougheners such as egg white and tenderizers such as yolk. Furthermore, whole liquid egg also contains a lot of water, which acts as a plasticizer (Slade, Levine, and Reid Citation1991).
Batter making
Mixing
The objective of mixing for both types of cakes is to obtain a homogeneous batter, containing stabilized air bubbles, which serve as nuclei for bubble growth during baking. A gluten network cannot be formed, which is prevented by a high content of sugar, presence of oil and the little input of mechanical work during mixing (Lambrecht et al. Citation2018; Wilderjans et al. Citation2013). There are different mixing methods, often classified as multistage or single-stage mixing. The multi-stage mixing methods differ for the shortened and foam cakes. In the single-stage or all-in-one mixing method, all ingredients are mixed in one single step. For large scale production of cakes, one often chooses for single-stage mixing.
Multistage mixing of shortened cakes
The first stage of mixing is the so-called creaming stage, in which the shortening and sugar are mixed with air via whisking (Brooker Citation1993). As shortening one often uses margarine, which is a fat-crystal stabilized water-in-oil emulsion. During the creaming stage, air is incorporated into the fat phase. Air bubbles are stabilized by fat and sugar crystals. Also, liquid oil must be present in the shortening to encapsulate the air bubble. During creaming the added sugar crystals provide abrasion of the fat crystals, which are reduced in size, and thus enhances the incorporation of air bubbles in the fat phase, similar to the case of biscuits (van der Sman and Renzetti Citation2019).
At the second stage, after creaming, egg is added. The fat phase will be dispersed into the aqueous phase. At the last stage, flour is added, which will be dispersed in the aqueous phase - while absorbing some water.
Multistage mixing of foam cakes
First, an egg white foam is made, via whisking the egg white. Sugar may also be added before foaming. The viscosity enhancement by the sugar during foaming increases the foaming time, and also the amount of aeration. Bubble size is also smaller at higher viscosity. The order of sugar addition affects cake volume at high sugar concentrations (sugar after foaming gives more volume, due to viscosity effect). After this first stage, a meringue type of foam is obtained (Godefroidt et al. Citation2019).
In subsequent steps, flour, and possibly egg yolk and oil are added (Rodríguez-García, Sahi, and Hernando Citation2014; Yang and Foegeding Citation2010). The egg yolk function is to provide lecithin (lipoproteins), which is a good emulsifier stabilizing the dispersed lipid phase (Kamat et al. Citation1973).
Single-stage mixing
To enhance foam stability during single-stage mixing one needs to add lipid emulsifiers (e.g. mono- or di-glycerides) to the shortening (Chesterton et al. Citation2013; Lee and Hoseney Citation1982). In the presence of sufficient lipid emulsifiers, the air bubbles can still be incorporated in the lipid phase, stabilized by these emulsifiers and fat crystals (Brooker Citation1993; Painter Citation1981), otherwise, the air will be incorporated into the aqueous phase (Wilderjans et al. Citation2013). Emulsifiers will also adsorb at the oil/water interface, and thus enhances the dispersion of the shortening.
Physico-chemical transitions and ingredient functionality
Multistage mixing of shortened cake
During the creaming step, air is incorporated into the lipid phase. Sugar in crystalline form enhances the air incorporation during the creaming step (Wilderjans et al. Citation2013). The absence of crystalline sugar, such as in cake using polydextrose as sugar replacer, leads to a reduction of aeration (Kocer et al. Citation2007). In the cream, the air bubbles are stabilized by fat and sugar crystals, and possible emulsifiers present in the shortening (Brooker Citation1993). The aerated cream can be viewed as an oil-foam (Heymans et al. Citation2017), in which foam stabilization is provided by fat and sugar crystals similar to Pickering emulsions.
After creaming, eggs are added. The water from the eggs provide the volume of the solvent (i.e. the water-solutes phase) and lowers the viscosity, thereby increasing the probability of instability of the emulsion. During the addition of egg, the sugar will dissolve into the water phase (Wilderjans et al. Citation2013). Furthermore, the shortening emulsion undergoes a phase inversion from W/O to O/W, with the emulsion droplets now stabilized by egg lipoproteins (lecithin), which originate from the egg yolk (Lambrecht et al. Citation2018). Egg yolk granules are disrupted already during mixing, allowing them to adsorb at the O/W interface (Deleu et al. Citation2017). They retain the micellar structure, and they adsorb as such on the interface.
Subsequently, flour particles are mixed in. No major changes to the batter structure occur at this stage. But, the flour particles will help in the stabilization of the emulsion, as reduced flour particle size (via more intense milling) leads to enhanced stabilization (Wilderjans et al. Citation2010). However, milling can produce extra damaged starch, which is shown to lower the stability enhancing of starch granules (Yano et al. Citation2017). Flour particles absorb some of the water, leading to an increase of viscosity. The batter needs sufficient viscosity for its foam stability after mixing (Wilderjans et al. Citation2010).
The distribution of water over the continuous aqueous phase (a sugar syrup) and flour particles is important in the prevention of the development of a gluten network (Slade and Levine Citation1994b; Wilderjans et al. Citation2013). Solvent absorption of flour is measured via the Solvent Retention Capacity (SRC) method, which analyzes the contributions of the polymeric components of flour: starch granules, gluten, arabinoxylans, and damaged starch (Kweon, Slade, and Levine Citation2011, Kweon et al. Citation2014).
Foam stability
For foam-type cakes or cakes mixed with the all-in-one method, the mixed batter resembles a liquid foam, with the liquid being an O/W emulsion, with dispersed flour particles. This type of batter is prone to foam instability (Mizukoshi Citation1983b). The stability of the foam is a more serious problem than emulsion stability, due to the low density of air. Foam instability can manifest itself in various ways: (1) creaming of air bubbles to the top of the batter, which is coupled with drainage of liquid to the bottom, (2) coalescence of bubbles, and (3) disproportionation (growth of large air bubbles at the expense of small ones). This foam instability leads to reduced cake expansion. For example, foam drainage leads to the formation of a gummy layer at the bottom of the cake (Mizukoshi Citation1983b).
Foam stability is largely determined by the presence of surface-active agents at the interface, such as proteins, emulsifiers or neutral wetting colloids. The action of these agents is due to the lowering of surface tension, and the prevention of coalescence via steric hindrance. In foam-type cakes, egg whites stabilize the meringue type of foam, produced in the first stage of mixing (Foegeding, Luck, and Davis Citation2006). Carbohydrates such as sugars do not contribute to the lowering of surface tension (Hao et al. Citation2016). However, via their effect on the viscosity of the batter, they can hinder the diffusion of proteins and emulsifiers to the interface (Hao et al. Citation2016). If egg yolk is added to the batter, its lipid emulsifiers (lecithin or lipoproteins) will displace the egg white proteins from the interface. This also holds for emulsifiers added to shortening or all-in-one mixes of sponge cakes (Sahi and Alava Citation2003). At a sufficient amount of emulsifier, the foam will remain stable. However, if a mix of proteins and emulsifiers remain on the air bubble interface a weak foam is obtained.
Other hydrophobic colloids such as oil droplets or fat crystals will promote foam instability via their action as anti-foaming agent (Mizukoshi Citation1983a). They promote bubble coalescence, leading to larger bubbles formed, which easily rupture when arriving at the surface of the cake. At the bottom of the container a gummy, unexpanded layer is formed due to bubbles rising to the top.
The rate of the processes occurring during foam instability is governed by batter viscosity, which depends on temperature, water, and sugar content (Mizukoshi Citation1983b). Sugar contributes to the viscosity of the batter and foam stability, only if the sugar crystals are dissolved into the aqueous phase (Hao et al. Citation2016).
Viscosity also influences the amount of air incorporated during whisking. Air is incorporated more in less viscous batters (Yang and Foegeding Citation2010). In cake batter, the incorporated air acts as nuclei for the gas bubbles growing during baking, and consequently the batter does not need to have a large overrun.
To increase the volume of the cake one might think this could be achieved via an increase in the level of leavening agents. However, an excess of leavening agents will promote bubble coalescence, and consequently a decrease in expansion volume (porosity) (Christaki et al. Citation2017). Hence, there is a limit to the functional amount of leavening agents.
Insight into ingredient functionality via reformulation
The use of pregelatinized starches affects the rheology during mixing via absorption of the water, making it less available for other components such as gluten, native starch, and egg. Water is also thought to lubricate the flour particles, enhancing the flow behavior of the batter (Christaki et al. Citation2017). Longer mixing time is required for better hydration of all components. There can be some gluten network formation during mixing, which is hindered by the lower availability of water (Hesso, Garnier, et al. Citation2015).
Normal wheat flour used is a complex mixture of gluten, starch (native and damaged) and arabinoxylans. To reduce complexity a model system of only gluten and starch has been studied (Wilderjans et al. Citation2008). The increase of gluten gives higher batter viscosity, thus enhancing the aeration of the batter during multi-stage mixing with creaming. Higher viscosity also enhances foam stability. Furthermore, it reduces the sedimentation of flour or creaming of bubbles in the batter before thermosetting, which enhances the cake volume. Before the thermosetting/gelatinization happens the viscosity lowers with increasing temperature, which is due to fat melting. A higher gluten content increases this minimum in viscosity.
Replacement of egg white by whey protein, another globular protein, shows the latter is capable of stabilizing foams (Yang and Foegeding Citation2010), but it does not form a protein network upon heating, stabilizing the cake as a solid foam.
The absence of sucrose crystals, such as in shortened cake with polydextrose, intended as both a sugar and fat replacer, leads to the reduction of aeration (Kocer et al. Citation2007). Furthermore, the air bubbles are now not stabilized by fat crystals (which are replaced by polydextrose). This indicates that sugar crystallinity is essential for good aeration of shortened cakes.
An overview of the functionality during mixing of all discussed ingredients is summarized in .
Sugar functionality during batter making
From the above review, we summarize the following functionalities of sucrose:
Enhancement of the incorporation of air during creaming due to its crystalline state,
Hindrance of biopolymer hydration and gluten network formation via its hygroscopic properties,
Modulation of air incorporation during whisking via viscosity enhancement, and
Improvement of foam stability also via viscosity enhancement.
To confer its viscosity-enhancing properties sucrose needs to be dissolved from the crystalline state, which is rather hindered in shortened cakes made via creaming. But, for this type of cake, foam stability is less of an issue, as the air bubbles are still located in the lipid phase. The thermodynamics of the dissolution of sugars and their replacers is very specific for each carbohydrate, which is characterized by its melting point and enthalpy of melting (van der Sman Citation2017).
The hygroscopic properties of sugars and their replacers can be inferred from their sorption isotherms, which can be described by the Flory–Huggins theory (van der Sman Citation2017). Moisture sorption is governed by the molecular size of the carbohydrate and its Flory–Huggins interaction parameter.
The viscosity enhancement of aqueous solutions by carbohydrates is determined by T/Tg, the ratio of temperature over the glass transition temperature (which depends on the moisture content of the solution) (van der Sman and Mauer Citation2019; van der Sman and Meinders Citation2013). The notion that viscosity scales with T/Tg follows from the original idea of Angell (Citation1995), which is later shown to be valid for synthetic polymer systems (Agapov et al. Citation2018; Lin et al. Citation2019; Liu et al. Citation2006; Sokolov and Schweizer Citation2009). In aqueous mixtures, the glass transition Tg is largely governed by the volumetric density of hydrogen bonds, which has been characterized for many of the carbohydrates and several polysaccharides (van der Sman Citation2013; van der Sman and Mauer Citation2019). This theory is based on recent developments in physics of glassy systems (Coppola, Djabourov, and Ferrand Citation2012; Nakanishi and Nozaki Citation2011). Recent research has shown that the theory holds also for bioplastics, another ternary mixture of protein/polyol/water systems (Uitto and Verbeek Citation2019). Furthermore, climate-change researchers investigating organic aerosols also presume that viscosity and Tg are correlated to hydrogen-bonding functional groups (Rothfuss and Petters Citation2017; Wong DeRieux et al. Citation2018). However, we have to mention that Tg may equally well be modeled accurately by empirical Gordon-Taylor or Couchman–Karasz theory, as we have done ourselves frequently, but those theories do not give any insight in the molecular mechanism (van der Sman Citation2013).
As in the case of biscuits (van der Sman and Renzetti Citation2019), the functionality of sucrose during the mixing stage of cakes is clearly determined by their thermodynamic properties, which is known for most of the sugars and their replacers, such as polyols (van der Sman Citation2017).
Cake baking
Physico-chemical transitions and ingredient functionality
During the early stage of cake baking, the fat crystals will melt, and the air bubbles will migrate toward the aqueous phase. An oil layer forms at the inner side of the air bubble surface. The oil layer is assumed to be covered by proteins. The oil layer helps in the stabilization of the foam (Brooker Citation1993; Wilderjans et al. Citation2013). Furthermore, the protein layer provides elasticity, such that the bubble does not rupture. The temperature rise, combined with the fat melting, will lower the viscosity of the batter, which increases the chance of foam instabilities, such as creaming of gas bubbles, coalescence or disproportionation. Furthermore, the flour particles might sediment (Wilderjans et al. Citation2013).
Above 50 °C there is an observable increase in the size of the gas bubbles, which is due to the production of CO2 by leavening agents, evaporation of water from the aqueous phase, and the thermal expansion of the gas bubble. The gas bubble expansion will happen slowly and occurs largely below the boiling point of water. Most of the bubble expansion can be described by a simple mathematical model incorporating the thermal expansion of gas, and the saturated vapor pressure as function of temperature (Mizukoshi, Maeda, and Amano Citation1980).
At a certain temperature, later in the baking process, the cake batter will set due to starch gelatinization, and protein coagulation (Mizukoshi, Kawada, and Matsui Citation1979). Starch is said to act as a water-sink, transforming the liquid foam into a solid foam (Wilderjans et al. Citation2010). Starch gelatinization will precede protein denaturation, but it is noted that their thermal transition peaks cannot be separated in a DSC measurement (Wilderjans et al. Citation2010). Starch gelatinization increases the viscosity and shear modulus of the batter dramatically. These increases are attributed to the swelling of the starch granules, which will touch each other. The touching, swollen starch granules will be able to support normal forces, and thus withstand the collapse of the cake. After starch gelatinization, the egg white proteins denature and aggregate (Deleu et al. Citation2016). Starch gelatinization is a main determinant of structure fixation as a shift in gelatinization temperature will affect cake volume and crumb density (Renzetti and Jurgens Citation2016).
All proteins present in the cake batter are involved in protein network formation during thermosetting (Lambrecht et al. Citation2018). The network is crosslinked via S–S bridges and hydrophobic interactions. The network appears to have a co-protein effect, meaning that more beta-sheet structures (capable of hydrophobic interactions) are formed in the mixed networks compared to networks of the individual proteins (Lambrecht et al. Citation2018). Factors required for the co-protein effect with gluten are (1) free SH groups and (2) surface hydrophobicity of the globular proteins from egg white. CSLM shows the resulting structure of the thermoset cake foam: swollen starch granules are entrapped in a protein matrix, with liquid oil (partially) coating the swollen granules (Hesso, Garnier, et al. Citation2015).
The event of egg white protein coagulation coincides with the moment of gas release from the expanding bubbles (Mizukoshi, Maeda, and Amano Citation1980). The formed protein network will stiffen the gas bubble wall and it will increase the required gas pressure for further expansion of the gas bubbles. Upon further heating and expansion, the bubble ruptures. Starch granules are embedded in the protein network as fillers, and allow partial cell opening without total collapse. Mind, that gelatinized starch is a soft filler (van der Sman et al. Citation2018), which can swell as dependent on amount of water and solutes like sugar (van der Sman Citation2018b). If the gas cannot escape, the water vapor from the gas will condense, reducing the gas pressure, and the cake collapses (Wilderjans et al. Citation2008). It is assumed that starch needs to retain its graininess to act as a filler (van der Sman Citation2016a). This hypothesis is based on how non-gelinized starch granules act as cell openers in expanded starchy snacks (van der Sman et al. Citation2018). Furthermore, the starch probably needs to be encapsulated by a stiff protein network, as suggested by the observation that cake batters with higher gluten content show more cracks in cell walls – allowing gas to escape (Wilderjans et al. Citation2008).
The moment of thermosetting, with accompanied gas release and bubble rupture, largely determines the amount of expansion and the resulting volume of the cake. This moment of thermosetting is modulated by the amount of sugar in the formulation (Mizukoshi Citation1985a). It has been shown that sucrose elevates the starch gelatinization temperature and egg protein coagulation temperature in cake batter (Mizukoshi, Kawada, and Matsui Citation1979), as measured by light transmission (Mizukoshi, Maeda, and Amano Citation1980). Furthermore, starch granules will swell more in sugar solutions than in pure water, thus affecting the viscosity and shear modulus of the cake matrix (Kweon, Slade, and Levine Citation2016; van der Sman Citation2018b; Wilderjans et al. Citation2013). Also, sugars enhance swelling kinetics.
After thermosetting the baking is continued for the purpose of crust formation. It is observed that at the later stage of baking the moisture content of the crumb remains more or less constant, while in the crust it lowers significantly. This leads to the glass formation of the crust (which becomes crispy), and to browning via Maillard reactions, enhanced by the hydrolysis of sucrose in reducing sugars (Ureta, Olivera, and Salvadori Citation2017). Due to the limited amount of water in the crust, the starch is not fully gelatinized (Hesso, Garnier, et al. Citation2015).
During cooling after baking the cake is susceptible to collapse, especially high-ratio cakes (Mizukoshi Citation1985a). The rheology of the swollen starch granule particle gel together with the protein network determines the amount of collapse. The rheological properties are represented by the shear modulus of the cake matrix. The rheology has been shown to depend on the degree of protein aggregation (Mizukoshi Citation1985a).
At higher sugar concentrations more, and earlier collapse is observed (Mizukoshi Citation1985a). The effect of sugar on collapse is attributed to its effect on the shear modulus. Due to the interaction between the effects of sugar on the thermosetting of the batter and the shear modulus, there is an optimum in the volume of the baked cake (after cooling) at a certain sugar content. The effect of sucrose on the shear modulus is via its effect on the gelatinization of starch, which swells (absorbing free water) and provides resistance against normal forces. At 0% sugar, the shear modulus (of the whole cake) increases significantly at 70 °C, at the event of starch gelatinization. Then, it reaches a maximum at around 90 °C, which is due to the effect of porosity on the shear modulus of the whole cake, as described by the Gibson-Ashby relation (Hesso et al. Citation2014).
At the later stage of cooling, the leached amylose will retrograde. The resulting starch gel forms too late to prevent any collapse. This amylose retrogradation does determine the firmness of the cake crumb, as perceived by the consumer during eating. It has been noted that sugars will modify amylose retrogradation (Hesso et al. Citation2014). This can also be derived from the effect of sugars and replacers on the final viscosity during RVA experiments (Kweon, Slade, and Levine Citation2016).
After cooling the cake will be packaged and stored. During shelf life, there are still physicochemical transformations going on affecting cake quality. Staling is the most important transformation during storage (Hesso et al. Citation2014). Staling is caused by the slow retrogradation of amylopectin, making the crumb tougher. As mentioned above, amylose retrogradation is a fast process, which happens already during the cooling stage after baking. Sugars and fat retard the staling rate in cakes, as compared to staling in bread.
Insight into ingredient functionality via reformulation
Insight into ingredient functionality can also be gained via reformulation studies. In these studies, one can either change the ratio between existing ingredients in a food formulation or replace one single ingredient by a new ingredient. In recent years, many reformulation studies have been performed, often driven by concerns about the nutrition and health of bakery goods. In this section, we review such studies with respect to ingredient functionality during different stages of baking.
A critical point during baking is the thermosetting of the batter. It has been shown that the gluten/starch ratio also influences the setting temperature: the less gluten, the lower the thermosetting temperature, explained by starch having a higher water retention than gluten (Wilderjans et al. Citation2008).
The functionality of starch has also been investigated via its modification, either via crosslinking (CL) or hydroxypropylation (HP) (Wilderjans et al. Citation2010). HP starch allows earlier gelatinization, and more water absorption, easier disruption of the granule, while CL starch shows the opposite effect. It is found that HP gives early, and excessive starch swelling, with amylose leaching that hinders the formation of the protein network. CL starch gives more constrained swelling and a higher stiffness of gel, but the collapse during cooling is not affected, suggesting that collapse is largely controlled by the strength of the protein network, and not by starch gel formation during cooling.
Sugars and polyols have been found to change the denaturation temperature of the egg white proteins (Hao et al. Citation2016; Renzetti, van den Hoek, and van der Sman Citation2020a). Polydextrose can be used as both fat and sugar replacers. As a sugar replacer, it has been shown to lower the thermosetting temperature, compared to that for a reference sucrose-based cake, leading to reduced expansion (Kocer et al. Citation2007).
Another critical point during baking is the possible collapse of the cake at the end of baking, or during cooling shortly after leaving the baking oven. This collapse has been shown to be governed by the shear modulus of the dough matrix (Mizukoshi Citation1985a). The optimal value of the shear modulus is critical also for the sensorial properties of cake: a too-high shear modulus is experienced as a tough cake, while moistness and tenderness are desirable. However, too-low shear modulus leads to cake collapse, which is also undesirable.
The effect of ingredients on the shear modulus of the dough matrix has been investigated (Mizukoshi Citation1985a). The balance between flour, egg, and sugar appears to be quite critical. The formulation of cakes is in a region where a small change in the ratio of ingredients leads to quite significant changes in the shear modulus (Mizukoshi Citation1985a). Collapse is also dependent on the gluten/starch ratio of the flour. Cake made with flour low in gluten is also prone to collapse. Higher gluten content promotes better aggregation and stiffness of the protein network (Wilderjans et al. Citation2008).
Hydrophobic ingredients have an effect on the shear modulus, which depends on their physical state. Fat crystals enhance the shear modulus, while liquid oils decrease the shear modulus. Emulsifiers improve foam stability. They appear to act as tougheners. The cause is not clear, as they can hinder the development of the protein network, but can also form complexes with amylose, and emulsify the oil phase (thus reducing their capacity to hindering the protein network formation) (Mizukoshi Citation1985b).
It used to be common practice to use chlorinated soft wheat flour, which oxidizes the wheat protein, allowing more starch granule swelling and more amylose leaching. Chlorination also prevents gluten network formation, allowing egg white to form the protein network, thus stabilizing the particle gel network of the swollen starch granules (Wilderjans et al. Citation2010).
The addition of pre-gelatinized starches lowers the shear modulus of the baked crumb matrix, increasing its softness (Hesso, Loisel, et al. Citation2015). This lowering of the matrix shear modulus is not obvious when considering the shear modulus of the complete cake, because of the lowered expansion volume induced by the pregelatinized gel, which increases the viscosity of the batter (Hesso, Loisel, et al. Citation2015).
The use of liquid oil makes the protein network weaker (Hesso, Garnier, et al. Citation2015). Oil coats the gluten and egg white proteins, which hydrates less, and remains compact. In a later stage of baking the liquid oil forms dispersed droplets, instead of coating the starch/protein network. This modification of the protein network impacts the collapse of the cake.
In storage staling of the gelatinized starch granules can happen. The use of pre-gelatinized starch as a substitute for flour has been shown to be most effective in hindering staling (Hesso, Garnier, et al. Citation2015). This replacement lowers the shear modulus of the matrix, but increases the staling rate. The addition of dietary (soluble) fiber also slows down the retrogradation of amylopectin (Hesso, Garnier, et al. Citation2015).
An overview of the functionality of all discussed ingredients during baking, cooling and storage is summarized in .
Sugar functionality
From the above discussion, it follows that sugar also has multiple functionalities during cake baking. In the baking stage, the sugar contributes significantly to the rheology, similarly to the mixing stage. Via its enhancement of viscosity and shear modulus sugar provides a positive contribution to foam stability in the stage before thermosetting, and helps to prevent collapse during the cooling stage after baking. Sugar’s contribution to viscosity can be quantified via its contribution to the glass transition of the system, as batter viscosity depends on the ratio T/Tg (van der Sman and Mauer Citation2019; van der Sman and Meinders Citation2013), which depends in turn on the actual moisture content and other plasticizers like sucrose. Furthermore, we have shown that both glass transition and the viscosity can be related to the density of hydrogen bonds (Renzetti, van den Hoek, and van der Sman Citation2020a; van der Sman Citation2013; van der Sman and Mauer Citation2019).
Furthermore, sugar contributes indirectly to bubble growth via its effect on water activity. Via the water activity, sugar has a two-fold effect: (1) the evaporation of water into the expanding bubble depends on the water activity, and (2) some of the reaction constants in the complex of the leavening system (p.e. the dissociation of bicarbonate linked to dissociation of water, ) depends on the water activity. How sugar controls the water activity has been described by the Flory–Huggins theory (van der Sman Citation2017).
Several phase transitions are strongly influenced by sugar, as it adds to the solvent quantity and quality (Slade and Levine Citation1988). Phase transitions like starch gelatinization, gluten and egg white thermosetting, amylose and amylopectin retrogradation are all elevated to higher temperatures by the increase of sugar levels (Slade and Levine Citation1987; van der Sman Citation2016b; van der Sman and Mauer Citation2019; van der Sman and Renzetti Citation2019; van der Sman, van der Hoek, and Renzetti Citation2020). Surprisingly, this elevation of phase transition temperatures is controlled by the same parameter, i.e. the volumetric density of hydrogen bonds, which controls the glass transition (van der Sman Citation2016b). This notion, that both the glass transition and biopolymer melting is controlled by this parameter, has been put forward first by Coppola, Djabourov, and Ferrand (Citation2012) for gelatin. Alternative theories for elevation of starch gelatinization by carbohydrates have been reviewed recently by Allan, Rajwa, and Mauer (Citation2018), but neither of these alternative has been found satisfactory in explaining the found results. Our theory could explain protein denaturation and starch gelatinization for several datasets in quantitative terms via the volumetric density of hydrogen bonds (Allan, Chamberlain, and Mauer Citation2020; Renzetti, van den Hoek, and van der Sman Citation2020a; van der Sman and Mauer Citation2019; van der Sman, van der Hoek, and Renzetti Citation2020). There is no alternative theory, which capable of giving a quantitative explanation for the elevation of starch gelatinization in presence of carbohydrates (Allan, Rajwa, and Mauer Citation2018, Allan, Chamberlain, and Mauer Citation2020). The glass transition is thought to control the crispiness of the crust of the cake (Slade, Levine, and Reid Citation1991), but loss of crispiness is found to start already below Tg, but it might also be related to mobility or antiplasticizing properties of plasticizers as water or carbohydrates. (Luyten, Plijter, and Vliet Citation2005; Roudaut et al. Citation2002; Valenzuela and Aguilera Citation2015; van Nieuwenhuijzen et al. Citation2010).
At the end of baking browning occurs via the Maillard reaction caramelization of sugars, which also adds flavors to the crust (Purlis Citation2010; Zhang et al. Citation2012). Both browning reactions require reducing sugars, which are produced via thermal decomposition (hydrolysis) of sucrose or starch (Purlis Citation2010).
Hence, sugar replacement in cake requires mimicking the following functionalities of sucrose:
its plasticizing behavior as captured by its influence on Tg, or equivalently the volumetric density of hydrogen bonds (which also determines the quantity and quality of solvent for biopolymers),
the contribution in lowering the water activity, as described in the Flory–Huggins theory,
its enhancement of Maillard browning reactions, and
its contribution to sweetness.
The latter two contributions can be provided by the addition of small amounts of a reducing sugar (like glucose or fructose) and a high-intensity sweetener (such as stevia), with negligible influences on the texture and stability of the cake structure. The first two functionalities of sugar contribute mainly to textural properties, which are the most difficult to mimic with sugar replacers. Our hypothesis is that the texture of original products (rich in sugar) can be mimicked by an appropriate mixture of polyols and hydrolysates of dietary fibers, such that (1) a similar volumetric density of hydrogen bonds is achieved, and (2) a similar water activity of the dough and baked product is achieved. These two aspects of (mixtures of) sugar replacers are described using our recently developed theories (van der Sman Citation2016b, Citation2017, Citation2019).
Mixing and baking in CDS terminology
Due to the multiple functionalities exhibited by sugar in cake manufacturing, it is important to capture the main transformations and changes in food structure during the various processing stages. We will describe those using the Complex Dispersed System (CDS) terminology (This Citation2005), which has already shown its value in describing the functionality of ingredients in potato-based expanded snacks (van der Sman and Broeze Citation2013) and biscuits (van der Sman and Renzetti Citation2019). We apply it here to describe the evolution of cake structure during mixing and baking and to capture the related sugar functionalities.
With CDS the structure of the system will be indicated by several symbols, extending the notation commonly used for emulsions. O/W means oil dispersed into water. Hence, ‘/’ will be used be denote a dispersion such as D/C, with D the dispersed phase, and C the continuous phase. Materials that are at the interface between different phases are indicated with the ’@’ symbol, meaning stabilized by or enclosed by. means oil droplets stabilized by emulsifier (Z) dispersed into water. Several materials are soluble in a liquid phase, for which we will use ‘+,’ meaning mixed with. Hence, W + C represents a sugar solution. The existence of bicontinuous phases, such as the fat phase and aqueous phase in biscuits, will be indicated by the symbol ‘——,’ with
meaning the fat phase (with fat crystals F dispersed into liquid oil O) ‘parallel’ to a sugar solution
CDS will be applied to a shortened cake, with a multi-stage mixing scheme using creaming. Via subscripts, we will occasionally indicate the physical state of ingredients. Native starch is semi-crystalline and is indicated by Sx. If it is gelatinized, it has become amorphous and taken up water. Swollen starch granules are then indicated by W/S. Similarly, we have defined crystalline sugar as Cx. Denatured protein is indicated by Pd, and native protein by P. As this methodology is still quite novel to the field of food science, we have also included graphical representations of the structure, in . In the figure captions, we have repeated the CDS representation of the resulting structure. We note, that the size of objects are not intended to scale actual sizes, it is meant to emphasize size differences.
Figure 1. Table summarizing the technological functionality of all ingredients in cake during mixing, baking, cooling and storage.
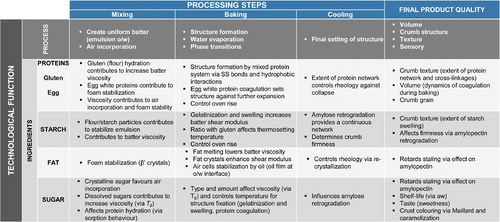
Figure 2. Pictorial representation of cake ingredients, with different proteins indicated by different subscripts.
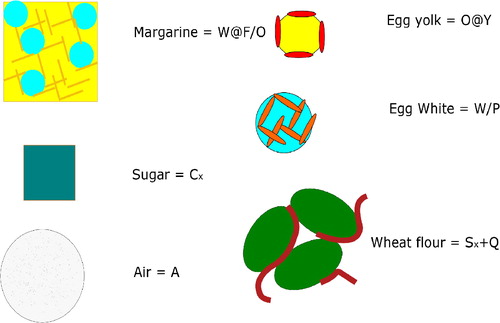
We assume that margarine is used as the shortening for the cake. Margarine is a W/O emulsion stabilized by fat crystals, denoted as
During creaming, the shortening and sugar crystals are mixed, along with the incorporation of air:(1)
(1)
We take the simplifying assumption that the oil surrounding the water droplets in margarine (W/O) hinders the sugar crystals of entering the water phase of margarine. The resulting cream structure is similar to that of the cream obtain during biscuit dough mixing (van der Sman and Renzetti Citation2019).
Next, egg is added. We distinguish two functional fractions: the egg white and the egg yolk. The egg white provides the main ingredient constituting the protein network formed during baking. The egg yolk provides surfactants (phospholipids and lipoproteins), that stabilizes the fat phase during mixing. Both components contain a lot of water. In egg white we view the water bound by the egg white protein. In the yolk two structures are dispersed in the liquid water phase: HDL granules and LDL micelles. HDL (high density lipoprotein) granules are compact, insoluble particles, that have little functionality, except for possible Pickering stabilization (Anton Citation2013). LDL (low-density-lipoprotein) micelles contain triglycerides, encapsulated by phospholipids and lipoproteins. As the HDL granules have little functionality in cake (Kamat et al. Citation1973; Marcet, Paredes, and Díaz Citation2015), we disregard them from the CDS notation. Hence, egg proteins will be indicated as W/P are microgel particles of egg white, and oil droplets (O) stabilized by the yolk phospholipids and lipoproteins Y.
The mixing of the cream and the egg is described as follows:(2)
(2)
The oil phase of the yolk is assumed to merge with the cream. The lipophilic phase is stabilized by the emulsifiers from the yolk (Y). The egg white forms the aqueous phase. Part of the water from the margarine and the sucrose migrate to the aqueous phase of the egg white, whereupon the sucrose crystals dissolve.
Finally, wheat flour particles are added, together with leavening agents R. During the milling of soft wheat flour the gluten matrix gets separated from the starch granules. Hence, the flour is denoted as with Q representing gluten protein:
(3)
(3)
The dissolved sugars and ions from the leavening agents are viewed as part of the solvent, hydrating the proteins.
During baking, first the fat will melt and then the air bubbles will migrate toward the aqueous phase, and the water from the margarine will merge with the aqueous phase.
(4)
(4)
The air bubbles are coated with oil at the inside, and they are stabilized by emulsifiers from the egg yolk.
During further heating, water vapor and gases are generated, and
which enter the gas bubbles. The gas bubbles expand, and egg white proteins, P, help to stabilize the expanding foam.
(5)
(5)
Subsequently, egg yolk proteins denature, the gluten thermosets and starch gelatinizes. The latter can be viewed as a microgel (Evans and Lips Citation1992; van der Sman Citation2018b). We assume that water is not freely available, but that it is “bound” to either starch, gluten or egg white proteins (Slade, Levine, and Reid Citation1991). At this stage, the denaturation of egg yolk proteins and gluten, do not have much of an impact on the developing cake structure. Only later they will be incorporated in the extended mixed protein network, if the ovalbumin denatures (Lambrecht et al. Citation2018).
(6)
(6)
Shortly after starch gelatinization, the egg white (ovalbumin) protein denature, and it will form a double network with gluten (Lambrecht et al. Citation2018). Also, the proteins encapsulating the air bubbles will denature, and aggregate, forming a stiff network that promotes bubble rupture. We assume this network will connect to the larger gluten/egg white network built throughout the aqueous phase. In our view the swollen starch granules form a filler in the network with a weak adhesion, thus causing partial rupture. A continuous pore network will be formed, allowing the escape of gases:
(7)
(7)
Upon cooling the fat recrystallizes and the leached amylose will undergo retrogradation The retrograded amylose forms a second network in addition to the protein network, thus contributing to the stiffness of the cake.
(8)
(8)
The final open foam structure is thus stabilized by a gel network. It has been assumed that both the retrograded starch network and the protein network are continuous (Wilderjans et al. Citation2010). Interaction of water holding by starch granules, leached amylose, and the protein gel is considered to be important for the formation of a continuous, strong protein network (Wilderjans et al. Citation2010). Protein network is said to contribute to cake cohesiveness, while retrograded starch network is assumed to contribute to crumb firmness.
Many of the steps in cake mixing and baking are quite similar to those in the baking of biscuits (Kweon et al. Citation2014). Only during the latter part of baking and cooling do differences become apparent, due to the difference in moisture content between cake and biscuit. Low moisture biscuits are stabilized by the glassy state of their sugar-rich matrix, while the structure of cake is stabilized by the starch-filled protein gel. For both biscuits and cakes holds that sugar largely modifies the properties of the aqueous solvent, and it has little direct effect on the structure - except for the creaming step, where the sucrose crystals promote air incorporation. The indirect effects of sugar as a modifier of the solvent is better explained in terms of the supplemented state diagram (Slade and Levine Citation1994b), as shown in the next section.
Phase transitions in the supplemented state diagram
The structuring of cake during baking is governed by several phase transitions: (1) the evaporation of water vapor, (2) starch gelatinization, and (3) the thermosetting of gluten and egg white proteins. It is argued that these phase transitions involving biopolymers can be regarded as the melting of semi-crystalline polymers (Habeych et al. Citation2009). Hence, for binary mixtures of biopolymers and water, we assume that the transition temperature can be described by the Flory theory of melting point depression (van der Sman Citation2016b; van der Sman and Meinders Citation2011). This theory has for long been used to describe the melting temperature of starch/water mixtures (Biliaderis et al. Citation1986; Farhat and Blanshard Citation1997; Lelievre Citation1974; Takahashi and Yamada Citation1998). Also the theory has been applied to several proteins as gelatin/collagen (Bohidar and Jena Citation1993; Flory and Garrett Citation1958), β-lactoglobulin (Rüegg, Moor, and Blanc Citation1975), keratin (Cao and Leroy Citation2005) and soy (Huson et al. Citation2011). Recent insights have shown that starch gelatinization is more than only melting of its crystalline part: the swelling of the granule also effects the gelatinization/melting (Desam et al. Citation2018). Hence, the thermodynamic description of the system requires inclusion of the elastic deformation, cf. Flory–Rehner theory (Desam et al. Citation2018). We note, that others assume that the biopolymer melting is preceded and controlled by a glass transition (Slade and Levine Citation1988, Citation1994b). However, the glass transition in the amorphous phase at the moisture content present in cake batter lies far below room temperature (van der Sman and Meinders Citation2011). We do agree that starch gelatinization is a kinetically controlled process (Ziegler, Thompson, and Casasnovas Citation1993), but that falls outside the realm of thermodynamics of phase transitions.
The governing equation in the Flory theory of biopolymer melting is:(9)
(9) with
(10)
(10)
Tm is the melting temperature at water volume fraction ϕw. is the melting temperature of the dry polymer. νw is the molar volume of water, and νU is the molar volume of the repeating unit (monomer) of the biopolymer. R is the gas constant.
is the melting enthalpy per mole of repeating units. χeff is the effective Flory–Huggins interaction parameter, which is composition dependent for all biopolymers. For all biopolymers holds
while χ1 is specific for each biopolymer, and must be determined from sorption isotherms. ϕw is the volume fraction of water in the amorphous phase of the biopolymer. It is assumed that the crystalline domains do not absorb any water. Hence,
must be corrected for the amount of crystallinity, ζ, which will be estimated.
We have argued that the melting temperatures of ternary mixtures of a biopolymer, water, and a second plasticizer can fall onto the same curve, if the volume fraction of water, ϕw, is replaced by ϕw,eff, which is the effective amount of solvent (water and second plasticizer) (van der Sman Citation2016b). ϕw,eff is thought to represent the volumetric density of functional groups, available for intermolecular hydrogen bonding (van der Sman Citation2016b). It can be computed from values of the glass transition of the dry plasticizer (van der Sman Citation2016b) or the viscosity of aqueous solutions containing the plasticizer (van der Sman and Mauer Citation2019), as follows:(11)
(11)
is the volume fraction of the second plasticizer (such as sucrose), having a molar volume of νs.
is the effective number of hydroxyl groups available for intermolecular hydrogen bonding. For water holds
If the glass transition of the dry plasticizer,
is known, we can compute:
(12)
(12)
=475 K is the glass transition of an infinitely large carbohydrate plasticizer, such as starch, and
= 139 K is the glass transition of water.
Plasticizers such as amino-acids have poor solubility in water, and consequently, they often crystallize out before going into the glassy state. Hence, cannot be directly measured. Alternatively, one can measure the viscosity of aqueous solutions containing the plasticizer, for a wide range of concentrations, which must be fitted as a function of
with the moisture dependent Tg computed using the Couchman–Karasz equation, which contains
as a fitting parameter (van der Sman and Mauer Citation2019). For these calculations, we refer the reader to a recently submitted paper from our group (van der Sman, van der Hoek, and Renzetti Citation2020).
Through the use of ϕw,eff one can construct supplemented state diagrams for ternary mixtures of biopolymer, sugar (replacer) and water, because ϕw,eff governs both the biopolymer melting as the glass transition (Renzetti, van den Hoek, and van der Sman Citation2020a; van der Sman Citation2013, Citation2016b; van der Sman and Renzetti Citation2019). These diagrams show phase transitions and glass transitions as function of ϕw,eff. Supplemented state diagrams are instrumental in understanding the structuring process during baking or frying (Boischot, Moraru, and Kokini Citation2003; Cuq, Abecassis, and Guilbert Citation2003; Slade and Levine Citation1994b; van der Sman and van der Goot Citation2009; Renzetti, van den Hoek, and van der Sman Citation2020a; van der Sman Citation2018a; van der Sman and Broeze Citation2014a, van der Sman and Broeze Citation2014b; van der Sman and Renzetti Citation2019). Hitherto, one has to plot the change of state of the food material during processing on the supplemented state diagram. Often, the state of the food will follow the phase transition line during structuring. We have performed that exercise for biscuits (van der Sman and Renzetti Citation2019), and we have shown that the art of baking biscuits is to add sufficient water and sugar (replacer) that gluten can thermoset, but that starch cannot gelatinize.
Below, we describe the supplemented state diagram for cake, and we estimate which path would be followed by the state of the dough during baking. For constructing the state diagram we have collected relevant data from literature, and from our own lab. Details on the applied experimental methods one finds in our recent paper (Renzetti, van den Hoek, and van der Sman Citation2020a). The collected data have been fitted to the Flory–Huggins theory.
Denaturation of egg white
The sources of experimental data on egg white denaturation are listed in . The collected experimental data are shown in , with ϕw,eff computed using known values of (van der Sman and Mauer Citation2019). For glycine we have found
=220 K, while for proline we have found
=250 K (van der Sman, van der Hoek, and Renzetti Citation2020).
Figure 10. Denaturation temperature TD of (spray) dried egg white as function of ϕw,eff, the effective solvent volume fraction for various solutions containing sugar replacers. The solid line is fitted to Flory–Huggins theory with ζ = 0.6, Tm = 415 K, and
= 57 kJ/mol.
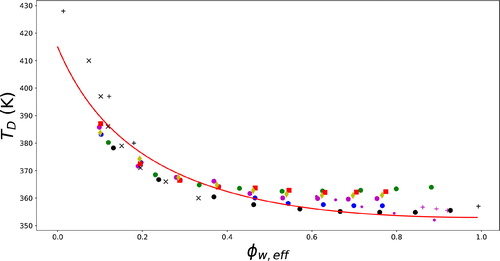
The solid line in is fitted to Flory–Huggins theory, as explained in detail below. First, let us discuss the experimental data (Renzetti, van den Hoek, and van der Sman Citation2020a). For values of all the data collapse to a single master curve, which follows Flory–Huggins theory. For
we observe some deviations from the Flory–Huggins theory for glycine and glucose in particular. It appears that in this regime the denaturation temperature remains constant, but at a level significantly above that for binary egg white/water mixtures. In our measurements with glycine and glucose, the ratio of plasticizer/water remained at 3:7. Hence, in the limit of
the data should go to the value for
However, this is not the case.
As we see it, this phenomenon can only be explained by phase separation of the ternary mixture into a biopolymer-rich phase and a plasticizer-rich phase (Renzetti, van den Hoek, and van der Sman Citation2020a). The composition of the biopolymer-rich phase remains constant for all mixtures if resulting in a constant denaturation temperature - irrespective of ϕw,eff. Only, water and plasticizer are assumed to be present in the plasticizer-rich phase, and thus they do not influence the denaturation temperature. The computed ϕw,eff does not reflect the composition in the biopolymer-rich phase. Similar phase separation behavior has been observed for mixtures of starch, water and small polyols such as glycerol, xylitol or sorbitol (van der Sman Citation2019), and for starch/water/sucrose mixtures (Hughes et al. Citation2018; Masavang, Roudaut, and Champion Citation2019; Roudaut and Wallecan Citation2015; Tedeschi, Leuenberger, and Ubbink Citation2016).
In recent investigations, we have varied the ratio of plasticizer/water (Renzetti, van den Hoek, and van der Sman Citation2020a). At lower ratios, the experimental data is closer to the theoretical curve, while at higher ratios we observe departure from the theoretical curve at lower ϕw,eff. Hence, at higher ratios, the phase separation happens at lower values of ϕw,eff. These observations are in agreement with the theory for ternary mixtures of biopolymer, water, and a second plasticizer (van der Sman Citation2019). These phenomena are discussed in more detail elsewhere (Renzetti, van den Hoek, and van der Sman Citation2020a). For the present paper, we highlight the phenomenon, since in cakes the initial moisture content of the batter is high, and ϕw,eff is in the range of the onset of phase separation. Hence, phase separation may be happening, but it would be limited. The proposed approach for sugar replacement based on ϕw,eff is probably still a reasonable approximation.
The collected experimental data are fitted to Flory–Huggins theory, obtaining ζ = 0.6, (as also follows from fitting the sorption isotherm with Flory–Huggins-Free-Volume theory), Tm = 415 K, and
= 57 kJ/mol. Also for starch holds that
(van der Sman and Meinders Citation2011). We view this as an important finding, because it suggests that water would be equally partitioned between starch and egg white. Hence, ϕw,eff, as used in the supplemented state diagram, refers to water present in both phases, and the binary state diagrams of starch/water and egg-white/water can be merged together into a single state diagram for cake, which is thus viewed as a” simplified” mixture of four ingredients: starch, egg-white, sugar (or replacers), and water.
Starch gelatinization
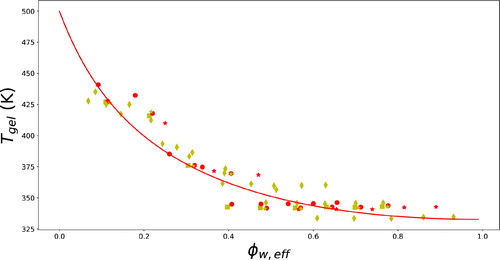
For the same sugars and their replacers, as discussed above with regard to egg white denaturation, we have investigated their effect on the gelatinization of starch (Renzetti, van den Hoek, and van der Sman Citation2020b). In particular, we have observed how the main peak temperature Tgel varies with ϕw,eff and in how far this follows Flory theory of melting point depression. Our results are presented in . One can make very similar observations as for egg white. For values of all the data collapse to a single master curve, which follows Flory–Huggins theory. For
we observe some deviations from the Flory theory for glycine and glucose, but also for sucrose at 50% concentration. As in the case of egg white, we attribute this deviation from theory to phase separation of the ternary system into plasticizer-rich and biopolymer-rich domains. For a wheat flour/sucrose/water system previous studies have suggested that there is indeed possible phase separation occurring (Masavang, Roudaut, and Champion Citation2019; Roudaut and Wallecan Citation2015).
Figure 11. Main peak temperature of starch gelatinization Tgel as a function of ϕw,eff, the effective solvent volume fraction, for various solutions with sugar replacers. The solid line is fitted to Flory–Huggins theory with ζ = 0.45, Tm=500 K, and
=24 kJ/mol. The symbols represent experimental data: different shapes and colors indicate different sugar (replacers). We have used the same symbols for the ingredients as listed in .
Processing pathway on a supplemented state diagram
Using literature data, we infer how the state of cake changes during baking, and this will be plotted on a supplemented state diagram. We use the data from Deleu et al. (Citation2019), who have baked pound cake in a controlled way via ohmic heating. At various times during baking, they have analyzed the amount of starch that has melted/gelatinized from the residual enthalpies as measured with DSC. We have converted that parameter into a degree of gelatinization DG. Assuming that the initial degree of crystallinity α = 0.45, we have computed the volume fraction of amorphous starch, via with
the initial volume fraction of total starch in the formulation. From the formulation, we have obtained the (initial) volume fraction of water ϕw, sugar
and egg white
The initial moisture content of the batter was 30%, while the final moisture content of the cake after baking was approximately 20%. Assuming that the evaporation rate is about constant during baking, we have computed the change ϕw,eff during baking using the recorded DG from Deleu et al. (Citation2019). We have compared our calculations via comparison of literature data on the state of bread (crust) during baking cf. Vanin et al. (Citation2010), who have recorded temperature and moisture content. For computing ϕw of the bread crust we assume a similar evolution of DG as function of temperature. As moisture content in bread is higher than in cake, the initial ϕw is somewhat higher than ϕw,eff of cake. Hence, we have shifted ϕw of bread with 5% to let the pathway overlap more with that of cake.
The processing pathways for cake and bread crust as plotted in the supplemented state diagram are shown in . The lines corresponding to starch gelatinization and egg white denaturation are taken from and . We observe that the pathway for cake crossed the starch gelatinization and egg white denaturation lines, as we have expected. Starch is gelatinized first, thereafter acting as a water sink. Due to the loss of crystallinity, the volume fraction of amorphous starch obviously increases, and thus lowers ϕw,eff. Subsequently, the egg white denatures, but not until a significant portion of the starch has gelatinized. Egg white denaturation occurs at a temperature just before boiling point (373 K). Hence, following to the above the starch gelatinization is the first phase transition in cake structure setting, contrary to the assumption of Deleu et al. (Citation2019).
Figure 12. Processing pathway of the state of cake batter during baking (blue squares) as determined from measurements by Deleu et al. (Citation2019), plotted on the state diagram, along with egg white denaturation and starch gelatinization. For comparison the (slightly shifted) pathway for bread crust during baking (Vanin et al. Citation2010), is also shown (yellow stars).
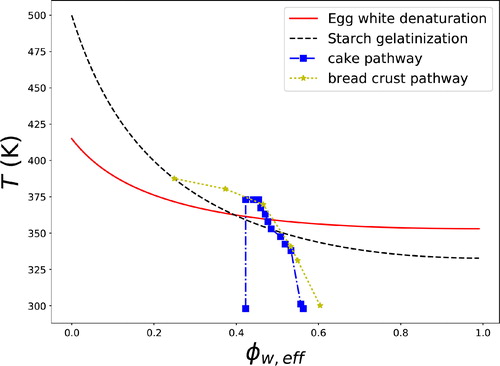
The bread crust follows a similar pathway as cake, showing a similarity between the two baking processes – where for both starch gelatinization occurs. During the cooling stage, the cake crosses the starch gelatinization line at quite a high temperature, thus promoting the retrogradation of leached amylose and stiffening of the protein network.
Starch gelatinization contributes to an increase in the storage modulus of the pound cake batter by 2 decades (Schirmer et al. Citation2012), which happens at a temperature around 70°C, at which there is already growth of gas bubbles due to the action of leavening agents. At higher temperatures, the evaporation of water would constitute the predominant contribution to gas bubble expansion. After denaturation, the egg white proteins are assumed to aggregate and form a continuous network (Deleu et al. Citation2016). The stiffness of the resulting starch-filled protein network is so great, that gas bubble expansion would be halted, and the bubble walls would rupture, allowing steam to escape. If steam cannot escape, it would condense into the matrix, lowering the gas pressure and the storage modulus of the matrix, leading to the collapse of the cake. If steam does escape, the gas bubbles would be filled with air during cooling, and the gas pressure returns to atmospheric level. Hence, in our opinion, plotting the processing pathway for cake during baking provides insight into the essential phase transitions during the food structuring process, as has been learned also for other products (Boischot, Moraru, and Kokini Citation2003; Cuq, Abecassis, and Guilbert Citation2003; Slade and Levine Citation1994a; van der Sman Citation2018a; van der Sman and Broeze Citation2014a,Citation2014b; van der Sman and Renzetti Citation2019; van der Sman and van der Goot Citation2009).
Conclusions
In this paper, we have examined the functionality of sucrose during the making of cake, and how this could be interpreted in terms of the physical properties of the sugar. We suggest that this knowledge is crucial for sugar replacement strategies. From the extensive literature survey, we have learned that sucrose has a multitude of functionalities during both the mixing stage and the baking stage of cake making. Besides its functionalities concerning sweetness and coloring, we suggest that most of these functionalities can be linked to how sucrose acts as a plasticizer or a humectant. This dual role of sucrose, as plasticizer and humectant, is also been suggested for biscuits (van der Sman and Renzetti Citation2019). Our hypothesis is that the plasticizing behavior can be characterized by the volumetric density of hydrogen bonds, while the humectant (hygroscopic) behavior can be characterized in terms of its contribution to the water activity. For both of these characteristics of sugars and their replacers (such as polyols, amino-acids or oligosaccharides from soluble fibers), we have developed theories (van der Sman Citation2013, Citation2016b, Citation2017; van der Sman and Mauer Citation2019; van der Sman, van der Hoek, and Renzetti Citation2020). Via the application of these theories, one can exactly mimic the plasticizer and humectant properties of sucrose in a particular cake formulation, via a proper mixture of sugar replacers - often being a mixture of an oligosaccharide and a small plasticizer, such as a polyol or amino-acid. We view this proposed sugar replacement strategy as potentially widely applicable to other sweet bakery products.
We must admit that some phenomena that occur in cake baking are not captured yet by the above theories: i.e. (1) the phase separation of sugar (or replacers) from the biopolymer phase at relatively high concentrations, and (2) the enhanced swelling of gelatinized starch granules by sugars. In our view the phase separation would happen, in principle, in cake applications, but the sugar concentrations are just on the edge of the phase separation domain, so the extent of phase separation would be limited. Hence, we think that the above sugar replacement strategy would still be viable for cake. As we see it, the required theory has already been developed, and it has already been tested for starch/polyol/water systems (van der Sman Citation2019). Also, the theory behind enhanced swelling has been proposed, and applied to crosslinked microgels and sugars (van der Sman Citation2018b). Native starch granules can be regarded are crosslinked starch microgels (Desam et al. Citation2018). Hence, this theory might be valid, if we account for the change in crystallinity during heating, and its relationship to the mechanical properties of the swelling granule.
As in the case of biscuits (van der Sman and Renzetti Citation2019), we have argued that it can be instructive to use semi-quantitative tools like CDS and the supplemented state diagram, in order to enhance our understanding of the changes to the structure of cake during its production. Using the concept of ϕw,eff we can condense the phase diagram of starch/egg-white/sugar/water system to a simple diagram, showing all relevant phase transitions (e.g. melting of starch and egg white) and glass transitions of the system. We think that these tools could also be very useful for enhancing the understanding of the structuring of other (sweet) bakery products, such as croissants (Ooms et al. Citation2016), gingerbreads, muffins (Lee, Moon, and Kweon Citation2020; Struck et al. Citation2016), and meringues (O'Charoen et al. Citation2014).
Table 1. Data sources used for denaturation of (dried) egg white and ovalbumin.
Additional information
Funding
References
- Agapov, A. L., V. N. Novikov, T. Hong, F. Fan, and A. P. Sokolov. 2018. Surprising temperature scaling of viscoelastic properties in polymers. Macromolecules 51 (13):4874–81. doi: 10.1021/acs.macromol.8b00454.
- Allan, M. C., B. Rajwa, and L. J. Mauer. 2018. Effects of sugars and sugar alcohols on the gelatinization temperature of wheat starch. Food Hydrocolloids. 84:593–607. doi: 10.1016/j.foodhyd.2018.06.035.
- Allan, M. C., M. C. Chamberlain, and L. J. Mauer. 2020. Effects of sugars and sugar alcohols on the gelatinization temperatures of wheat, potato, and corn starches. Foods 9 (6):757. doi: 10.3390/foods9060757.
- Angell, C. A. 1995. Formation of glasses from liquids and biopolymers. Science 267 (5206):1924–35. doi: 10.1126/science.267.5206.1924.
- Anton, M. 2013. Egg yolk: Structures, functionalities and processes. Journal of the Science of Food and Agriculture 93 (12):2871–80. doi: 10.1002/jsfa.6247.
- Back, J. F., D. Oakenfull, and M. B. Smith. 1979. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 18 (23):5191–6. doi: 10.1021/bi00590a025.
- Biliaderis, C. G., C. M. Page, T. J. Maurice, and B. O. Juliano. 1986. Thermal characterization of rice starches: A polymeric approach to phase transitions of granular starch. Journal of Agricultural and Food Chemistry 34 (1):6–14. doi: 10.1021/jf00067a002.
- Blond, G. 1989. Water-galactose system-supplemented state diagram and unfrozen water. Cryo-Letters 10 (5):299–308.
- Bohidar, H. B., and S. S. Jena. 1993. Kinetics of sol–gel transition in thermoreversible gelation of gelatin. The Journal of Chemical Physics 98 (11):8970–7. doi: 10.1063/1.464456.
- Boischot, C., C. I. Moraru, and J. L. Kokini. 2003. Factors that influence the microwave expansion of glassy amylopectin extrudates. Cereal Chemistry Journal 80 (1):56–61. doi: 10.1094/CCHEM.2003.80.1.56.
- Brooker, B. E. 1993. The stabilisation of air in cake batters-the role of fat. Food Structure 12 (3):2.
- Cao, J., and F. Leroy. 2005. Depression of the melting temperature by moisture for alpha-form crystallites in human hair keratin . Biopolymers 77 (1):38–43. doi: 10.1002/bip.20186.
- Chesterton, A. K. S., D. P. de Abreu, G. D. Moggridge, P. A. Sadd, and D. I. Wilson. 2013. Evolution of cake batter bubble structure and rheology during planetary mixing. Food and Bioproducts Processing 91 (3):192–206. doi: 10.1016/j.fbp.2012.09.005.
- Christaki, M., P. Verboven, T. van Dyck, B. Nicolai, P. Goos, and J. Claes. 2017. The predictive power of batter rheological properties on cake quality-the effect of pregelatinized flour, leavening acid type and mixing time. Journal of Cereal Science 77:219–27. doi: 10.1016/j.jcs.2017.07.001.
- Coppola, M., M. Djabourov, and M. Ferrand. 2012. Unified phase diagram of gelatin films plasticized by hydrogen bonded liquids. Polymer 53 (7):1483–93. doi: 10.1016/j.polymer.2012.02.016.
- Cuq, B., J. Abecassis, and S. Guilbert. 2003. State diagrams to help describe wheat bread processing. International Journal of Food Science and Technology 38 (7):759–66. doi: 10.1046/j.1365-2621.2003.00748.x.
- Deleu, L. J., E. Wilderjans, I. Van Haesendonck, K. Brijs, and J. A. Delcour. 2016. Protein network formation during pound cake making: The role of egg white proteins and wheat flour gliadins. Food Hydrocolloids 61:409–14. doi: 10.1016/j.foodhyd.2016.05.001.
- Deleu, L. J., S. Melis, E. Wilderjans, I. van Haesendonck, K. Brijs, and J. A. Delcour. 2017. Protein network formation during pound cake baking: The role of egg yolk and its fractions. Food Hydrocolloids 63:226–32. doi: 10.1016/j.foodhyd.2016.07.036.
- Deleu, L. J., A. Luyts, E. Wilderjans, I. van Haesendonck, K. Brijs, and J. A. Delcour. 2019. Ohmic versus conventional heating for studying molecular changes during pound cake baking. Journal of Cereal Science 89:102708. doi: 10.1016/j.jcs.2019.01.008.
- Desam, G. P., J. Li, G. Chen, O. Campanella, and G. Narsimhan. 2018. A mechanistic model for swelling kinetics of waxy maize starch suspension. Journal of Food Engineering 222:237–49. doi: 10.1016/j.jfoodeng.2017.11.017.
- Evans, I. D., and A. Lips. 1992. Viscoelasticity of gelatinized starch dispersions. Journal of Texture Studies 23 (1):69–86. doi: 10.1111/j.1745-4603.1992.tb00512.x.
- Farhat, I. A., and J. M. Blanshard. 1997. On the extrapolation of the melting temperature of dry starch from starch-water data using the Flory-Huggins equation. Carbohydrate Polymers 34 (4):263–5. doi: 10.1016/S0144-8617(97)00086-6.
- Flory, P. J., and R. R. Garrett. 1958. Phase transitions in collagen and gelatin systems1. Journal of the American Chemical Society 80 (18):4836–45. doi: 10.1021/ja01551a020.
- Foegeding, E. A., P. J. Luck, and J. P. Davis. 2006. Factors determining the physical properties of protein foams. Food Hydrocolloids 20 (2-3):284–92. doi: 10.1016/j.foodhyd.2005.03.014.
- Godefroidt, T., N. Ooms, B. Pareyt, K. Brijs, and J. A. Delcour. 2019. Ingredient functionality during foam-type cake making: A review. Comprehensive Reviews in Food Science and Food Safety 18 (5):1550–62. doi = 10.1111/1541-4337.12488. doi: 10.1111/1541-4337.12488.
- Habeych, E., X. Guo, J. van Soest, A. J. van der Goot, and R. Boom. 2009. On the applicability of Flory–Huggins theory to ternary starch–water–solute systems. Carbohydrate Polymers 77 (4):703–12. doi: 10.1016/j.carbpol.2009.02.012.
- Hao, Y., F. Wang, W. Huang, X. Tang, Q. Zou, Z. Li, and A. Ogawa. 2016. Sucrose substitution by polyols in sponge cake and their effects on the foaming and thermal properties of egg protein. Food Hydrocolloids 57:153–9. doi: 10.1016/j.foodhyd.2016.01.006.
- Hesso, N., C. Loisel, S. Chevallier, and A. Le-Bail. 2014. Impact of pregelatinized starches on the texture and staling of conventional and degassed pound cake. Food and Bioprocess Technology 7 (10):2923–30. doi: 10.1007/s11947-014-1254-5.
- Hesso, N., C. Garnier, C. Loisel, S. Chevallier, B. Bouchet, and A. Le-Bail. 2015. Formulation effect study on batter and cake microstructure: Correlation with rheology and texture. Food Structure 5:31–41. doi: 10.1016/j.foostr.2015.03.002.
- Hesso, N., C. Loisel, S. Chevallier, A. Marti, P. Le-Bail, A. Le-Bail, and K. Seetharaman. 2015. The role of ingredients on thermal and rheological properties of cake batters and the impact on microcake texture. LWT - Food Science and Technology 63 (2):1171–8. doi: 10.1016/j.lwt.2015.04.041.
- Heymans, R., I. Tavernier, K. Dewettinck, and P. van der Meeren. 2017. Crystal stabilization of edible oil foams. Trends in Food Science & Technology 69:13–24. doi: 10.1016/j.tifs.2017.08.015.
- Hughes, D. J., G. B. Bönisch, T. Zwick, C. Schäfer, C. Tedeschi, B. Leuenberger, F. Martini, G. Mencarini, M. A. Geppi, M. Alam, et al. 2018. Phase separation in amorphous hydrophobically modified starch–sucrose blends: Glass transition, matrix dynamics and phase behavior. Carbohydrate Polymers 199:1–10. doi: 10.1016/j.carbpol.2018.06.056.
- Huson, M. G., E. V. Strounina, C. S. Kealley, M. K. Rout, J. S. Church, I. A. M. Appelqvist, M. J. Gidley, and E. P. Gilbert. 2011. Effects of thermal denaturation on the solid-state structure and molecular mobility of glycinin. Biomacromolecules 12 (6):2092–102. doi: 10.1021/bm200080h.
- Kamat, V. B., G. A. Lawrence, C. J. Hart, and R. Yoell. 1973. Contribution of egg yolk lipoproteins to cake structure. Journal of the Science of Food and Agriculture 24 (1):77–88. doi: 10.1002/jsfa.2740240112.
- Kocer, D., Z. Hicsasmaz, A. Bayindirli, and S. Katnas. 2007. Bubble and pore formation of the high-ratio cake formulation with polydextrose as a sugar-and fat-replacer. Journal of Food Engineering 78 (3):953–64. doi: 10.1016/j.jfoodeng.2005.11.034.
- Kweon, M., L. Slade, and H. Levine. 2011. Solvent retention capacity (SRC) testing of wheat flour: Principles and value in predicting flour functionality in different wheat-based food processes and in wheat breeding–A review. Cereal Chemistry Journal 88 (6):537–52. doi: 10.1094/CCHEM-07-11-0092.
- Kweon, M., L. Slade, H. Levine, and D. Gannon. 2014. Cookie- versus cracker-baking-what's the difference? Flour functionality requirements explored by SRC and alveography . Critical Reviews in Food Science and Nutrition 54 (1):115–38. doi: 10.1080/10408398.2011.578469.
- Kweon, M., L. Slade, and H. Levine. 2016. Cake baking with alternative carbohydrates for potential sucrose replacement. I. functionality of small sugars and their effects on high-ratio cake-baking performance. Cereal Chemistry Journal 93 (6):562–7. doi: 10.1094/CCHEM-02-16-0032-R.
- Lambrecht, M. A., L. J. Deleu, I. Rombouts, and J. A. Delcour. 2018. Heat-induced network formation between proteins of different sources in model systems, wheat-based noodles and pound cakes. Food Hydrocolloids 79:352–70. doi: 10.1016/j.foodhyd.2017.12.032.
- Lee, C. C., and R. C. Hoseney. 1982. Optimization of the fat-emulsifier system and the gum-egg white-water system for a laboratory scale single stage cake mix. Cereal Chemistry 59 (5):392–5.
- Lee, E. J., Y. Moon, and M. Kweon. 2020. Processing suitability of healthful carbohydrates for potential sucrose replacement to produce muffins with staling retardation. LWT 131:109565. doi: 10.1016/j.lwt.2020.109565.
- Lelievre, J. 1974. Starch gelatinization. Journal of Applied Polymer Science 18 (1):293–6. doi: 10.1002/app.1974.070180124.
- Lin, Y., C. Xu, A. Guan, and G. Wu. 2019. Tailoring the temperature-dependent viscoelastic behavior of acrylic copolymers by introducing hydrogen bonding interactions. Polymer 161:190–6. doi: 10.1016/j.polymer.2018.12.025.
- Liu, C. Y., J. He, R. Keunings, and C. Bailly. 2006. New linearized relation for the universal viscosity- temperature behavior of polymer melts. Macromolecules 39 (25):8867–9. doi: 10.1021/ma061969w.
- Luyten, H., J. Plijter, and T. v Vliet. 2005. Crispy/crunchy crusts of cellular solid foods: A literature review with discussion. Journal of Texture Studies 35 (5):445–92. doi: 10.1111/j.1745-4603.2004.35501.x.
- Marcet, I., B. Paredes, and M. Díaz. 2015. Egg yolk granules as low-cholesterol replacer of whole egg yolk in the preparation of gluten-free muffins. LWT - Food Science and Technology 62 (1):613–9. doi: 10.1016/j.lwt.2014.08.031.
- Masavang, S., G. Roudaut, and D. Champion. 2019. Identification of complex glass transition phenomena by DSC in expanded cereal-based food extrudates: Impact of plasticization by water and sucrose. Journal of Food Engineering 245:43–52. doi: 10.1016/j.jfoodeng.2018.10.008.
- Meza, B. E., A. K. S. Chesterton, R. A. Verdini, A. C. Rubiolo, P. A. Sadd, G. D. Moggridge, and D. I. Wilson. 2011. Rheological characterisation of cake batters generated by planetary mixing: Comparison between untreated and heat-treated wheat flours. Journal of Food Engineering 104 (4):592–602. doi: 10.1016/j.jfoodeng.2011.01.022.
- Mizukoshi, M. 1983a. Model studies of cake baking. III. Effects of silicone on foam stability of cake batter. Cereal Chemistry 60 (5):399.
- Mizukoshi, M. 1983b. Model studies of cake baking. IV. Foam drainage in cake batter. Cereal Chemistry 60 (5):400–2.
- Mizukoshi, M. 1985a. Model studies of cake baking. V. Cake shrinkage and shear modulus of cake batter during baking. Cereal Chemistry 62 (4):242–6.
- Mizukoshi, M. 1985b. Model studies of cake baking. Vi. Effects of cake ingredients. Cereal Chemistry 62 (4):247–51.
- Mizukoshi, M.,. T. Kawada, and N. Matsui. 1979. Model studies of cake baking. I. Continuous observations of starch gelatinization and protein coagulation during baking. Cereal Chemistry 56 (4):305–9.
- Mizukoshi, M.,. H. Maeda, and H. Amano. 1980. Model studies of cake baking. II. Expansion and heat set of cake batter during baking. Cereal Chemistry 57 (5):352–5.
- Nafchi, A. M., R. H. Tabatabaei, B. Pashania, H. Z. Rajabi, and A. A. Karim. 2013. Effects of ascorbic acid and sugars on solubility, thermal, and mechanical properties of egg white protein gels. International Journal of Biological Macromolecules 62:397–404. doi: 10.1016/j.ijbiomac.2013.09.050.
- Nakanishi, M., and R. Nozaki. 2011. Systematic study of the glass transition in polyhydric alcohols. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics 83 (5 Pt 1):051503. doi: 10.1103/PhysRevE.83.051503.
- O'Charoen, S., S. Hayakawa, Y. Matsumoto, and M. Ogawa. 2014. Effect of d-psicose used as sucrose replacer on the characteristics of meringue. Journal of Food Science 79 (12):E2463–E2469. doi: 10.1111/1750-3841.12699.
- Ooms, N., B. Pareyt, K. Brijs, and J. A. Delcour. 2016. Ingredient functionality in multilayered dough-margarine systems and the resultant pastry products: A review. Critical Reviews in Food Science and Nutrition 56 (13):2101–14. doi: 10.1080/10408398.2014.928259.
- Painter, K. A. 1981. Functions and requirements of fats and emulsifiers in prepared cake mixes. Journal of the American Oil Chemist’s Society 58 (2):92–5. doi: 10.1007/BF02672188.
- Pareyt, B., and J. A. Delcour. 2008. The role of wheat flour constituents, sugar, and fat in low moisture cereal based products: A review on sugar-snap cookies. Critical Reviews in Food Science and Nutrition 48 (9):824–39. doi: 10.1080/10408390701719223.
- Purlis, E. 2010. Browning development in bakery products–A review. Journal of Food Engineering 99 (3):239–49. doi: 10.1016/j.jfoodeng.2010.03.008.
- Rao, Q., and T. P. Labuza. 2012. Effect of moisture content on selected physicochemical properties of two commercial hen egg white powders. Food Chemistry 132 (1):373–84. doi: 10.1016/j.foodchem.2011.10.107.
- Renzetti, S., and A. Jurgens. 2016. Rheological and thermal behavior of food matrices during processing and storage: Relevance for textural and nutritional quality of food. Current Opinion in Food Science 9:117–25. doi: 10.1016/j.cofs.2016.10.003.
- Renzetti, S., I. A. F. van den Hoek, and R. G. M. van der Sman. 2020a. Amino acids, polyols and soluble fibres as sugar replacers in pound cake: Egg white proteins denaturation described by hydrogen bond density and implications for protein network formation. Food Hydrocolloids 108:106034. doi = 10.1016/j.foodhyd.2020. doi: 10.1016/j.foodhyd.2020.106034.
- Renzetti, S., I. A. F. van den Hoek, and R. G. M. van der Sman. 2020b. Mechanisms controlling starch gelatinization and pasting behaviour in presence of sugars, polyols, soluble fibers and amino acids. Food Hydrocolloids. To be submitted.
- Rodríguez-García, J., S. S. Sahi, and I. Hernando. 2014. Optimizing mixing during the sponge cake manufacturing process. Cereal Foods World 59 (6):287–92. doi: 10.1094/CFW-59-6-0287.
- Rothfuss, N. E., and M. D. Petters. 2017. Influence of functional groups on the viscosity of organic aerosol. Environmental Science & Technology 51 (1):271–9. doi: 10.1021/acs.est.6b04478.
- Roudaut, G., and J. Wallecan. 2015. New insights on the thermal analysis of low moisture composite foods. Carbohydrate Polymers 115:10–5. doi: 10.1016/j.carbpol.2014.08.066.
- Roudaut, G., C. Dacremont, B. Pàmies Vallès, B. Colas, and M. Le Meste. 2002. Crispness: A critical review on sensory and material science approaches. Trends in Food Science & Technology 13 (6-7):217–27. doi: 10.1016/S0924-2244(02)00139-5.
- Rüegg, M., U. Moor, and B. Blanc. 1975. Hydration and thermal denaturation of β-lactoglobulin. a calorimetric study. Biochimica et Biophysica Acta (Bba) - Protein Structure 400 (2):334–42. doi: 10.1016/0005-2795(75)90188-9.
- Sahi, S. S., and J. M. Alava. 2003. Functionality of emulsifiers in sponge cake production. Journal of the Science of Food and Agriculture 83 (14):1419–29. doi: 10.1002/jsfa.1557.
- Schirmer, M., M. Jekle, E. Arendt, and T. Becker. 2012. Physicochemical interactions of polydextrose for sucrose replacement in pound cake. Food Research International 48 (1):291–8. doi: 10.1016/j.foodres.2012.05.003.
- Slade, L., and H. Levine. 1987. Recent advances in starch retrogradation. Industrial Polysaccharides, 387–430.
- Slade, L., and H. Levine. 1988. Non-equilibrium melting of native granular starch: Part I. temperature location of the glass transition associated with gelatinization of a-type cereal starches. Carbohydrate Polymers 8 (3):183–208. doi: 10.1016/0144-8617(88)90002-1.
- Slade, L., and H. Levine. 1994a. Structure-function relationships of cookie and cracker ingredients. The Science of Cookie and Cracker Production, 23–141.
- Slade, L., and H. Levine. 1994b. Water and the glass transition–dependence of the glass transition on composition and chemical structure: Special implications for flour functionality in cookie baking. In Water in foods, 143–88. Amsterdam: Elsevier.
- Slade, L., H. Levine, and D. S. Reid. 1991. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Critical Reviews in Food Science & Nutrition 30 (2-3):115–360.
- Sokolov, A. P., and K. S. Schweizer. 2009. Resolving the mystery of the chain friction mechanism in polymer liquids. Physical Review Letters 102 (24):248301. doi: 10.1103/PhysRevLett.102.248301.
- Struck, S., L. Gundel, S. Zahn, and H. Rohm. 2016. Fiber enriched reduced sugar muffins made from iso-viscous batters. Lwt - Food Science and Technology 65:32–8. doi: 10.1016/j.lwt.2015.07.053.
- Takahashi, A., and T. Yamada. 1998. Crystalline copolymer approach for melting behaviour of starch. Starch - Stärke 50 (9):386–296. doi: 10.1002/(SICI)1521-379X(199809)50:9<386::AID-STAR386>3.0.CO;2-C.
- Tedeschi, C., B. Leuenberger, and J. Ubbink. 2016. Amorphous–amorphous phase separation in hydrophobically-modified starch–sucrose blends i. phase behavior and thermodynamic characterization. Food Hydrocolloids 58:75–88. doi: 10.1016/j.foodhyd.2016.02.021.
- This, H. 2005. Modelling dishes and exploring culinary ‘precisions’: The two issues of molecular gastronomy. British Journal of Nutrition 93 (S1):S139–S146. doi: 10.1079/BJN20041352.
- Uitto, J. M., and C. J. Verbeek. 2019. The role of phase separation in determining the glass transition behaviour of thermally aggregated protein-based thermoplastics. Polymer Testing 76:119–26. doi: 10.1016/j.polymertesting.2019.03.010.
- Ureta, M. M., D. F. Olivera, and V. O. Salvadori. 2017. Influence of baking conditions on the quality attributes of sponge cake. Food Science and Technology International 23 (2):156–65. doi: 10.1177/1082013216666618.
- Valenzuela, C., and J. M. Aguilera. 2015. Effects of maltodextrin on hygroscopicity and crispness of apple leathers. Journal of Food Engineering 144:1–9. doi: 10.1016/j.jfoodeng.2014.07.010.
- van der Plancken, I., A. van Loey, and M. E. Hendrickx. 2006. Effect of heat-treatment on the physico-chemical properties of egg white proteins: A kinetic study. Journal of Food Engineering 75 (3):316–26. doi: 10.1016/j.jfoodeng.2005.04.019.
- van der Sman, R. 2013. Predictions of glass transition temperature for hydrogen bonding biomaterials. The Journal of Physical Chemistry. B 117 (50):16303–13. doi: 10.1021/jp408184u.
- van der Sman, R. 2016a. Filler functionality in edible solid foams. Advances in Colloid and Interface Science 231:23–35. doi: 10.1016/j.cis.2016.03.003.
- van der Sman, R. 2016b. Sugar and polyol solutions as effective solvent for biopolymers. Food Hydrocolloids. 56:144–9. doi: 10.1016/j.foodhyd.2015.12.001.
- van der Sman, R. 2017. Predicting the solubility of mixtures of sugars and their replacers using the flory–huggins theory. Food & Function 8 (1):360–71. doi: 10.1039/c6fo01497f.
- van der Sman, R. 2018a. Progress in understanding of supplemented state diagrams of hydrophilic food materials. Current Opinion in Food Science 21:32–8. doi: 10.1016/j.cofs.2018.05.006.
- van der Sman, R. 2018b. Theoretical investigation of the swelling of polysaccharide microgels in sugar solutions. Food & Function 9 (5):2716–24. doi = 10.1039/C8FO00452H. doi: 10.1039/C8FO00452H.
- van der Sman, R. 2019. Phase separation, antiplasticization and moisture sorption in ternary systems containing polysaccharides and polyols. Food Hydrocolloids. 87:360–70. doi: 10.1016/j.foodhyd.2018.07.051.
- van der Sman, R., and J. Broeze. 2013. Structuring of indirectly expanded snacks based on potato ingredients: A review. Journal of Food Engineering 114 (4):413–25. doi: 10.1016/j.jfoodeng.2012.09.001.
- van der Sman, R., and J. Broeze. 2014a. Effects of salt on the expansion of starchy snacks: A multiscale analysis. Food & Function 5 (12):3076–82. doi: 10.1039/C4FO00513A.
- van der Sman, R., and J. Broeze. 2014b. Multiscale analysis of structure development in expanded starch snacks. Journal of Physics, Condensed Matter: An Institute of Physics Journal 26 (46):464103doi: 10.1088/0953-8984/26/46/464103.
- van der Sman, R., and L. J. Mauer. 2019. Starch gelatinization temperature in sugar and polyol solutions explained by hydrogen bond density. Food Hydrocolloids 94:371–80. doi: 10.1016/j.foodhyd.2019.03.034.
- van der Sman, R., and M. B. J. Meinders. 2011. Prediction of the state diagram of starch water mixtures using the flory–huggins free volume theory. Soft Matter 7 (2):429–42. doi: 10.1039/C0SM00280A.
- van der Sman, R., and M. B. J. Meinders. 2013. Moisture diffusivity in food materials. Food Chemistry 138 (2-3):1265–74. doi: 10.1016/j.foodchem.2012.10.062.
- van der Sman, R., and S. Renzetti. 2019. Understanding functionality of sucrose in biscuits for reformulation purposes. Critical Reviews in Food Science and Nutrition 59 (14):2225–39. doi: 10.1080/10408398.2018.1442315.
- van der Sman, R., and A. van der Goot. 2009. The science of food structuring. Soft Matter 5 (3):501–10. doi: 10.1039/B718952B.
- van der Sman, R., H. M. Vollebregt, M. B. J. Meinders, and A. Beri. 2018. Effects of filler ingredients on the structure and texture of starchy, extruded snacks. Food Structure 18:1–13. doi: 10.1016/j.foostr.2018.10.001.
- van der Sman, R., I. van der Hoek, and S. Renzetti. 2020. Sugar replacement with zwitterionic plasticizers like amino acids. Food Hydrocolloids 109:106113. doi = 10.1016/j.foodhyd.2020. doi: 10.1016/j.foodhyd.2020.106113.
- van Nieuwenhuijzen, N. H., R. H. Tromp, J. R. Mitchell, C. Primo-Martín, R. J. Hamer, and T. Van Vliet. 2010. Relations between sensorial crispness and molecular mobility of model bread crust and its main components as measured by PTA, DSC AND NMR. Food Research International 43 (1):342–9. doi: 10.1016/j.foodres.2009.10.015.
- Vanin, F. M., C. Michon, G. Trystram, and T. Lucas. 2010. Simulating the formation of bread crust in a DMTA rheometer. Journal of Cereal Science 51 (3):277–83. doi: 10.1016/j.jcs.2009.12.005.
- Wilderjans, E., B. Pareyt, H. Goesaert, K. Brijs, and J. A. Delcour. 2008. The role of gluten in a pound cake system: A model approach based on gluten–starch blends. Food Chemistry 110 (4):909–15. doi: 10.1016/j.foodchem.2008.02.079.
- Wilderjans, E., A. Luyts, H. Goesaert, K. Brijs, and J. A. Delcour. 2010. A model approach to starch and protein functionality in a pound cake system. Food Chemistry 120 (1):44–51. doi: 10.1016/j.foodchem.2009.09.067.
- Wilderjans, E., A. Luyts, K. Brijs, and J. A. Delcour. 2013. Ingredient functionality in batter type cake making. Trends in Food Science & Technology 30 (1):6–15. doi: 10.1016/j.tifs.2013.01.001.
- Wong DeRieux, W. S., Y. Li, P. Lin, J. Laskin, A. Laskin, A. K. Bertram, S. A. Nizkorodov, and M. Shiraiwa. 2018. Predicting the glass transition temperature and viscosity of secondary organic material using molecular composition. Atmospheric Chemistry and Physics 18 (9):6331–51. doi: 10.5194/acp-18-6331-2018.
- Yang, X., and E. A. Foegeding. 2010. Effects of sucrose on egg white protein and whey protein isolate foams: Factors determining properties of wet and dry foams (cakes). Food Hydrocolloids 24 (2-3):227–38. doi: 10.1016/j.foodhyd.2009.09.011.
- Yano, H., A. Fukui, K. Kajiwara, I. Kobayashi, K. Yoza, A. Satake, and M. Villeneuve. 2017. Development of gluten-free rice bread: Pickering stabilization as a possible batter-swelling mechanism. LWT - Food Science and Technology 79:632–9. doi: 10.1016/j.lwt.2016.11.086.
- Zhang, Y. Y., Y. Song, X. S. Hu, X. J. Liao, Y. Y. Ni, and Q. H. Li. 2012. Effects of sugars in batter formula and baking conditions on 5-hydroxymethylfurfural and furfural formation in sponge cake models. Food Research International 49 (1):439–45. doi: 10.1016/j.foodres.2012.07.012.
- Ziegler, G. R., D. B. Thompson, and J. Casasnovas. 1993. Dynamic measurement of starch granule swelling during gelatinization. Cereal Chemistry 70:247.

![Figure 4. Batter structure after adding egg to the cream: [A@F/(W/O)]@Y / (W+C)/P.](/cms/asset/b3d0300c-3ffd-4ffe-bb1c-8b223f766623/bfsn_a_1786003_f0004_c.jpg)
![Figure 5. Batter structure after adding flour: [A@F/(W/O)]@Y / [ Sx+W/Q+(W+C+R)/P ].](/cms/asset/011535ef-aa4a-480f-9c15-3894eca7b16c/bfsn_a_1786003_f0005_c.jpg)
![Figure 6. Batter structure after fat melting during baking: (A@O)@Y / [ Sx+W/Q+(W+C+R)/P ].](/cms/asset/adfe660a-798f-42a6-9d29-7ab250555678/bfsn_a_1786003_f0006_c.jpg)
![Figure 7. Bubble expansion during baking: [((A+G+V)@O)@(Y+P)] / [ (Sx+W/Q)+(W+C+R)/P ].](/cms/asset/bf56ebff-36b3-4d89-9977-74646863de7c/bfsn_a_1786003_f0007_c.jpg)