Abstract
Oxidative stress is a common physiopathological condition enrolled in risk factors for cardiovascular diseases. Individuals in such a redox imbalance status present endothelial dysfunctions and inflammation, reaching the onset of heart disease. Phytochemicals are able to attenuate the main mechanisms of oxidative stress and inflammation and should be considered as supportive therapies to manage risk factors for cardiovascular diseases. Beetroot (Beta vulgaris L.) is a rich source of bioactive compounds, including betanin (betanidin-5-O-β-glucoside), a pigment displaying the potential to alleviate oxidative stress and inflammantion, as previously demonstrated in preclinical trials. Betanin resists gastrointestinal digestion, is absorbed by the epithelial cells of intestinal mucosa and reaches the plasma in its active form. Betanin displays free-radical scavenger ability through hydrogen or electron donation, preserving lipid structures and LDL particles while inducing the transcription of antioxidant genes through the nuclear factor erythroid-2-related factor 2 and, simultaneously, suppressing the pro-inflammatory nuclear factor kappa-B pathways. This review discusses the anti-radical and gene regulatory cardioprotective activities of betanin in the pathophysiology of endothelial damage and atherogenesis, the main conditions for cardiovascular disease. In addition, betanin influences on these multipath cellular signals and aiding in reducing cardiovascular disorders is proposed.
Introduction
Cardiovascular diseases (CVD) comprise a class of disorders including peripheral arterial disease, coronary heart and cerebrovascular disease, which block the blood supply to cardiac muscle and the brain due to aggregates formed by inflammatory and immune cells, platelets, oxidized lipids and smooth muscle cells that proliferate in response to vascular endothelial injury (Celermajer et al. Citation2012; WHO Citation2017).
Hypertension, hyperlipidemia, diabetes and obesity are pathological conditions considered risk factors for CVD, as they precede the onset of vascular disease when aggravated (Drummond et al. Citation2011). Furthermore, risk factors for developing CVD may reduce the detoxification capacity of the antioxidant defense system and stimulate excessive reactive oxygen (ROS) and nitrogen (RNS) species production (Drummond et al. Citation2011; Newsholme et al. Citation2016). Multiple pathways are enrolled in the establishment of oxidative stress, by direct oxidation mediated by reactive species and downregulation of the activity and expression of genes related to redox homeostasis, as well as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, superoxide dismutase, catalase and glutathione peroxidases. This pathophysiological condition is called oxidative stress, and results in damage to lipids, proteins, DNA molecules and cell membranes, while also decreasing the availability of nitric oxide (NO) and triggering early events in the pathogenesis of CVD and many other chronic-degenerative diseases (Zhang, Xing, et al. Citation2015).
Clinical trials and epidemiological evidence have demonstrated the protective effect of the intake of vegetables enriched in antioxidants on oxidative stress and CVD (Zhang, Xing, et al. Citation2015; Pollock Citation2016). Many of these bioactive phytochemicals may be involved in maintaining a redox state in the organism due to their scavenging ROS and RNS abilities, while others may either modulate the expression of genes encoding proteins involved in intracellular defense against oxidative damage, compete for active sites in enzymes or bind to receptor sites as antagonists in various subcellular structures (Zielińska-Przyjemska et al. Citation2012; Esatbeyoglu et al. Citation2014; Vidal et al. Citation2014).
Beetroot (Beta vulgaris L.) belongs to the Chenopodiaceae family and is considered as source of important bioactive compounds, including betalains, nitrate (NO3-) and phenolic compounds (Baião, Silva, et al. Citation2017; Baião, de Freitas, et al. Citation2017). Among betalains, betanin (betanidin 5-O-β-D-glucoside) is the major phytochemical representative, a water-soluble nitrogenated heterocyclic compound that confers the red-violet color to beetroot (Silva et al. Citation2016). Furthermore, betanin is described as a bioactive compound capable of inhibiting lipid membranes and low-density lipoprotein (LDL) peroxidations, modulating ROS generation and gene expression in order to reduce inflammatory cytokine release and increasing antioxidant enzyme activities (Esatbeyoglu et al. Citation2015). Some of the biological effects exhibited by betanin are underlyed in two redox-sensitive pathways, the nuclear factor kappa B (NF-kB) and the nuclear factor erythroid-2-related factor – antioxidant response element (Nrf2-ARE), the main gene transcribers for inflammatory and detoxifying/antioxidant responses (Fiordelisi et al. Citation2019; Satta et al. Citation2017). Thus, betanin displays potential as a supportive therapy used to ameliorate the pathophysiological effects caused by the oxidative stress and inflammatory events that lead to CVD diseases.
In this context, this review aims to discuss the origin and the relationship of the betanin structure and its free-radical scavenger activity. The effects of betanin on oxidative stress and pro-inflamatory cascade will be reviewed to indicate the ways in which this compound can reduce the risk factors for CVD development. In addition, the putative molecular mechanisms by which betanin can exert their pahrmacological cardioprotective effects will also be discussed. We hypothesize that betanin may protect endothelial function by acting as an inflammatory cascade supressor and/or oxidative homeostasis effector by modulating molecular signaling pathways.
Betanin biosynthesis plasticity and bioaccessibility
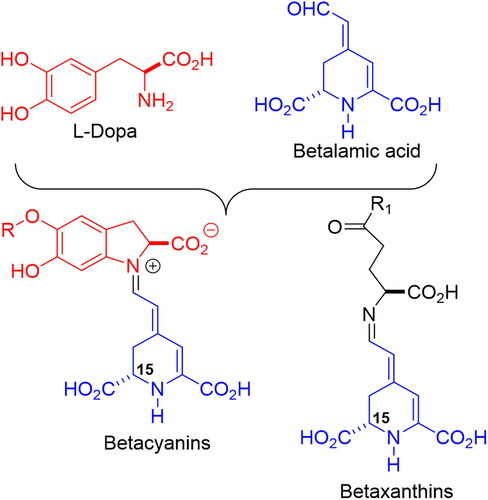
Betalains (or chromoalkaloids) are natural water-soluble pigments obtained from the plants of 10 families belonging to the Caryophyllales order that accumulate these compounds in flowers, fruits and occasionally in tissues, although they may also be found in fungi species associated with plants (Delgado-Vargas, Jiménez and Paredes-Lópes, 2000). Betalains are synthesized from the amino acid tyrosine in the shikimate pathway. First, tyrosine is hydroxylated to form 3,4-dihydroxy-L-phenyalanine (L-DOPA) which, in turn, is converted to betalamic acid [4-(2-oxoethylidene)-1,2,3,4-tetrahydropyridine-2,6-dicarboxylic acid] in a two-step reaction initiated by the enzyme DOPA 4,5-dioxygenase (Steglich and Strack Citation1990; Girod and Zryd Citation1991; Khan and Giridhar Citation2015). Alternatively, L-DOPA is oxidized and cyclized to cyclo-DOPA [cyclo-L- (3,4-dihydroxyphenylalanine)], which spontaneously condenses with betalamic acid, forming red pigments, betacyanins, with betanidin as the common structure to all (Kobayashi et al. Citation2001; Butera et al. Citation2002). Betalamic acid can also condense with amino acids or amino derivates forming yellow pigments, betaxanthins (Schliemann, Kobayashi, and Strack Citation1999; Khan and Giridhar Citation2015) ().
Glycosylation and acylation in the hydroxyls located at the C-5 or C-6 positions, occur, giving rise to several betacyanins, such as prebetanin, isobetanin, neobetanin and betanin (betanidin 5-O-β-D-glucoside), present in purple pitaya (Hylocereus polyrhizus) and, mainly, in red beetroot (Beta vulgaris) (Supplementary material 1) (Delgado-Vargas, Jiménez, and Paredes-López Citation2000; Herbach, Stintzing, and Carle Citation2004).
Betanin obtained from beetroot has several industrial applications. As it occurs naturally in edible plants, it is considered nontoxic to human beings and has been approved by Food and Drug Administration (FDA) and European Union as a dye at quantum satis under E162 code (EFSA Citation2015; FDA, 2009). Betanin corresponds to approximately 75–95% of betalains in red beetroot, ranging from 3.0 to 7.6 mg·g−1 of dry matter. Farming management and post-harvesting conditions can affect betanin content, but it is by far the major pigment compared to isobetanin (1.2–3.1 mg·g−1 of dry matter), betanidin (0.8–1.0 mg·g−1 dry matter) and betaxanthin (2.71–4.25 mg·g−1 dry matter) (Kujala et al. Citation2002; Slatnar et al. Citation2015; Sawicki, Baczek and Wiczkowski Citation2016).
Betanin color is due to their conjugated structure, which can lead to resonance and electronic transitions, although the resonance phenomena confers intrinsic structural instability. This is an important aspect to be considered, since this instability affects color during food preparation, processing and storage (Slimen, Najar and Abderrabba 2017; Khan and Giridhar Citation2015). The optimum pH for betanin ranges from 4.0 to 6.0, and, when outside this range, oxidation causes marked changes in color, such as pigment or food product darkening (Elbe, Maing, and Amundson Citation1974). The presence of metals like Cu (II) and Fe (III) also decreases betanin stability (Attoe and von Elbe Citation1984; Czapski Citation1990; Wybraniec et al. Citation2013).
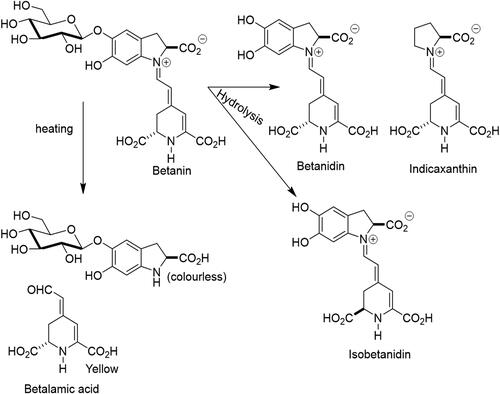
Heat treatment of beetroot juice for 30 min at 85 °C causes racemization of the chiral 15S-15R carbon of betanin, resulting in color changes by the formation of yellow betalamic acid and a colorless fragment, cyclodopa 5-O-β-glucoside. The acid-catalyzed hydrolysis of the glycosidic bond depends on pH and other physical-chemical parameters (i.e. light, presence or absence of oxygen, metals and other chemicals) and generates betanidin and its enantiomer, isobetanidin ().
Betanin maintains its stability over a wide pH range, from 3 to 7, with variable degradation below pH 2 or above pH 9 (Polturak and Aharoni Citation2018). Isomerization, decarboxylation or cleavage reactions may occur during thermal or acidic processing, where only cleavage is accompanied by total loss of color (Castellanos-Santiago and Yahia Citation2008; Stintzing, Schieber, and Carle Citation2000). Furthermore, during food processing, betanin, as well as other compounds with phenolic structures, may suffer from the action of endogenous enzymes, such as the beta-glycosidases, polyphenol oxidases and/or peroxidases that may contribute to decolorization following pigment decompartimentation, resulting in red color losses and browning (Stintzing and Carle, 2004). However, betanin exhibits the ability to continuously degrade and regenerate during storage (Silva, Baião, et al. 2019). Regeneration occurs through partial de novo synthesis from its hydrolytic products, L-DOPA-5-O-glycoside and betalamic acid, rapidly generating betanin when precursor compounds are mixed in solution. Therefore, the maintainance of processed food containing betanin at pH < 5 and temperature < 10 °C can regenerate betanin, compensating for coloring losses (Huang and Von Elbe Citation1987).
Betanin has been applied to refrigerated and frozen foods such as sorbets, dairy products and meats (i.e. sausage) as a natural food coloring (EFSA Citation2015), often replacing anthocyanins, due to its higher water solubility and stability in pH ranging from 3.5 to 7. Betanin is suitable for coloring acidic food items, such as fermented foods, as well as neutral ones, matching optimal conditions for pigment stability (Polturak and Aharoni Citation2018). Furthermore, betanin is used as a dye in cosmetics and pharmaceuticals products, which range from neutral to low alkaline pH (Esatbeyoglu et al. Citation2015; Blaak and Staib Citation2018). In addition to its use as a food coloring additive, betanin is considered a natural food preservative superseding synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), due to its ability to avoid deterioration of oxidation-susceptible foods (Sucu and Turp Citation2018; Silva, Baião, et al. 2019).
Betanin has demonstrated high stability during storage under freezing and refrigeration temperatures at pH 7.0 and when added to chilled meat samples. Betanin remains biologically active and capable of inhibiting lipid peroxidation during the shelf life of these chilled products (Silva, Baião, et al. 2019). In the gastrointestinal tract, around 50% of oral administered betanin resists stomach gastric acid and remains stable after intestinal digestion, at 37 °C (Tesoriere et al. Citation2008; Silva, Baião, et al. 2019). When assayed in Caco-2 cells, 65% of betanin crossed the intestinal epithelial cells monolayer by passive diffusion, maintaining its chemical structure during trans-epitelial transport, indicating that it can reach the bloodstrem in its active form (Tesoriere et al. Citation2013). Betanin, primarily used as a food additive, shows bioacessibility and may be considered a bioactive phytochemical compound when in body fluids, since pH and temperature extremes should not occur.
Relationship between betanin structure and its free-radical scavenger ability
Regarding the ability to remove reactive oxygen or nitrogen species, the antioxidant power of betanin lies in the hydroxyl group of the phenol moiety and/or the cyclic amine group, similar to the phenolic antioxidant ethoxyquin, which are good hydrogen donors and confer reducing properties to this class of compounds (Kanner, Harel, and Granit Citation2001; Gliszczyńska-Swigło, Szymusiak, and Malinowska Citation2006).
The pH of different fluids in the human body undergoes variations, influencing betanin stability and bioactivity in the digestive tract compartments. Salivary fluid has a pH of about 7.4, whereas stomach fluid is maintained between 1.5 and 3.5. The abdominal cavities, including the small and large intestines, display pH of 7.4, and finally the blood, with a pH close to 7.4 (Jin Citation2007). Physiological pH fluids induce changes in the chemical structure of betanin along the systemic routes. Betanin presents a cationic form at pH < 2, zwitterionic form at pH 2, anionic mono at 2 < pH < 3 with deprotonated C2-COOH and C15-COOH groups, anionic bis at 3.5 < pH < 7 with C2-COOH, C15-COOH and C17-COOH deprotonated groups and anionic tris at 7.5 < pH < 9 with all carboxyl deprotonated groups, in addition to the C6-OH on the phenolic ring () (Frank et al. Citation2005).
Figure 3. Structural betanin modifications in the gastrointetinal tract and bloodstream. Panel A: Ionic states of betanin at a pH range from 2 to 9. Panel B: Probable ionic states of betanin, phenolic O-H homolytic bond dissociation energy and ionization potential energy according to the pH of gastrointestinal tract fluids and the bloodstream.
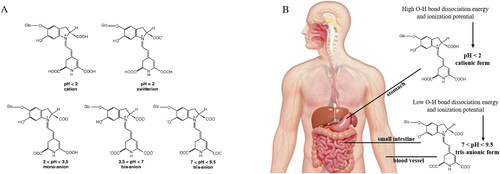
The free-radical scavenger activity of betanin at different pH (from 2 to 9) was previously determined through the TEAC assay, phenolic O-H homolytic bond dissociation energy (OH BDE), ionization potential (IP) and deprotonation energy (DE). The TEAC assay confirmed that the antioxidant activity of betanin is pH-dependent, higher above pH 4. Moreover, the gradual deprotonation of the betanin molecule (mono, bi- and tri-deprotonated) occurs with increasing pH, where BDE and PI decrease significantly (Gliszczyńska-Swigło, Szymusiak, and Malinowska Citation2006).
Furthermore, the antioxidant power of betanin was demonstrated by assaying a 23 mg·mL−1 aliquot in physicochemical conditions mimicking those found in the small intestinal environment, even after exposure to an acidic suspension similar to gastric fluid. Betanin was able to inhibit over 90% of OH-radical production in the total antioxidant potential (TAP) assay. In the ferric-reducing ability of plasma (FRAP) assay, betanin was effective in donating electrons and reducing reactive species, such as the ferric ion in the tripyridyltriazine complex (Fe3+-TPTZ), to its ferrous state (Fe2+-TPTZ), reaching more than 1000 µmoL Fe2+·L−1. Furthermore, betanin was effective in reducing the ABTS+ radical, as measured in the trolox equivalent antioxidant capacity (TEAC) and oxygen radical antioxidant capacity (ORAC) assays, achieving more than 4000 and 2000 µmoL Trolox·L−1, respectively. The high antioxidant activity of betanin is due to its greater ability to donate H+ and electrons associated with reduced energy for the dissociation of OH bonds and ionization potential, when transforming from the cationic to the mono, bis and/or tri deprotonated states with increasing pH along the gastrointestinal tract (Silva, Baião, et al. 2019). This means that, when betanin is absorbed into the bloodstream, it will remain at the ideal pH to exert its free-radicals scavenger activity ().
Betanin, atherogenesis and cardiovascular diseases
Inappropriate lifestyle and eating habits, like diets rich in saturated and trans fats and simple sugars, associated with toxins from smoking, alcohol, stress, a sedentary lifestyle and aging, lead to the development of comorbidities such as obesity, diabetes mellitus, dyslipidemia (hypercholesterolemia, hypertriglyceridemia and mixed hyperlipidemia) and arterial hypertension (WHO Citation2003). Evidence has shown that the aforementioned physiopathological conditions favor disturbances of the pro-oxidant–antioxidant balance caused by abnormal ROS and RNS generation and/or impaired antioxidant defenses, establishing oxidative stress (Hackam and Anand Citation2003; Halliwell and Whiteman Citation2004).
The superoxide anion (O2•–), hydrogen peroxide (H2O2), hydroxyl radical (HO•) and peroxynitrite (O = N–O–O-) are the main oxidative molecules with potential harmful implications to human health, since at least one unpaired electrons in the last electron layer is available, with this incomplete electron shell conferring high reactivity to these molecules (Burton and Jauniaux Citation2011; Halliwell and Whiteman Citation2004; Nita and Grzybowski Citation2016). Excessive amounts of reactive species cause oxidative damage to macromolecules such as DNA, lipids and proteins, as well as the interruption of redox-dependent signaling pathways in vasculature, leading to endothelial dysfunction and atherogenesis () (Evrard et al. Citation2016; Förstermann, Xia, and Li Citation2017).
Figure 4. Betanin as a radical scavenger, hydrogen or electron donor, peroxide decomposer or singlet oxygen quencher. O2 leakage from mithocondria generates the superoxide anion radical O2•-, which is converted to H2O2 by superoxide dismutase (SOD). Both, O2˙- and H2O2 can cause damage to cellular components. H2O2 can be cleaved to the radical by iron-sulfur clusters found in several metalloproteins (e.g. ferredoxins, NADH dehydrogenase, hydrogenases, coenzyme Q) by increasing Fe2+ in the cytosol. Fe2+ reacts with H2O2 through the Fenton reaction, generating HO•, which causes serious oxidative damage. Fe3+ is then reduced back to Fe2+ by flavoproteins (FADH2) to generate oxidized flavin radicals (FADH) and the hydroxyl radical. Betanin can also couteract with •OH or •O = O radicals forming several adducts.
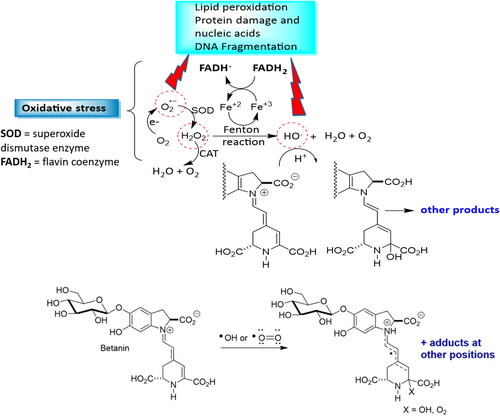
Subendothelial retention of apoB100-containing lipoprotein is an early step in atherogenesis, which induces a greater consumption of NO, decreasing its bioavailability and leading to higher generation of free radicals and the establishment of an inflammatory process (Libby Citation2002; Lu et al. Citation2011; Peluso et al. Citation2012).
The increase in free radicals leads to the peroxidation of fatty acids in LDL particles, increasing in their negative charge, density, and lysolecittin, cholesterol oxide, hydroxide and hydroperoxide content, cytotoxicity and decreasing polyunsaturated fatty acids, with consequent fragmentation of apolipoprotein B-100 (apo B-100) (Kovanen and Pentikainen Citation2003). These changes in physicochemical LDL properties favor chemotactic activity for circulating monocytes and T-cells, leading to adhesion of inflammatory cells to the endothelium, the release of pro-inflammatory interleukins and cytokines and ROS, maintaining LDL oxidation (Garcia-Dorado et al. Citation2014). The permanent ROS influence on LDL and underlying endothelium molecules may promote an increase in vasoconstrictor substances, like thromboxane and angiotensin II, adhesion molecules and growth factors, along with the proliferation of smooth muscle cells, impairing the maintenance of homeostasis and vascular tone (Hulthe and Fagerberg Citation2002).
In addition, oxidized LDL may increase arginine methyltransferase I and II in endothelial cells, increasing the formation of asymmetric dimetilarginine (ADMA) and symmetric dimetilarginine (SDMA). ADMA inhibits the three eNOS isoforms and may also dissociate these enzymes, generating superoxides, while SDMA can compete with arginine for the cationic amino acid transporter in the cell membrane, accentuating arginine deficiency and indirectly altering NO production levels (Smith et al. Citation2018).
Betanin antioxidant and anti-inflammatory activities can attenuate the main atherogenesis and cardiovascular disease mechanisms. Data included in summarizes the studies already available, including the pre-clinical trials, which were used to embase the potential of betanin as a health-influencing compound in risk factors for CVD.
Table 1. Betanin effects on experimental models mimicking risk factors for CVD: Evaluation of direct and indirect antioxidant and anti-inflammatory effects.
Positively charged betanin regions confer affinity to negatively charged lipid portions (–CO2-), favoring interaction with and protection of lipid membranes and LDL. In addition, this beet pigment inhibits LDL peroxidation by binding to the polar head of fatty acids or polar residues of apo B-100, protecting LDL from oxidation () (Kanner, Harel, and Granit Citation2001; Tesoriere et al. Citation2003; Allegra, Tesoriere, and Livrea Citation2007). In human LDL particles, apo-B100 protein is sensitive to nitration by ROS and RNS at the tyrosine residues Tyr 413, 2524 and 3295, forming tyrosine-tyrosine bonds, also known as dithyrosine crosslinks, resulting in LDL agregates, a key event in early atherogenesis. In addition, augmented dityrosine crosslinks were detected in the plasma and LDL from human atherosclerotic lesions compared to LDL from healthy subjects () (Hamilton et al. Citation2008; Chakraborty, Cai, and Tarr Citation2010; Mukherjee et al. Citation2017; Wu et al. Citation2015).
Figure 5. Betanin attenuates LDL peroxidation. Betanin antioxidant and anti-inflammatory activities can attenuate the main atherogenesis mechanisms, protecting against CVD. Betanin free-radical scavanger activity removes ROS and prevents LDL peroxidation. Betanin in the bloodstream is able to establish ionic interactions with the polar head of phospholipids and to Tyr reisdues in apoB-100, avoinding tyrosine generation and LDL agregation.
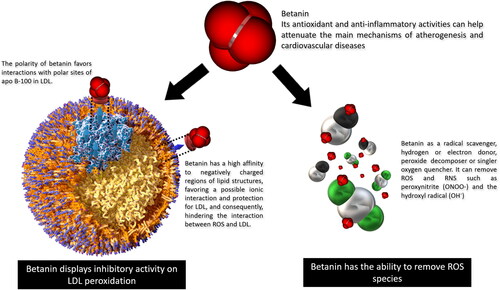
In addition, betanin has the ability to interfere with the initiation of lipid peroxidation through its ability to donate electrons, first reacting with oxidizing molecules. Betanin binds to isolated LDLs from human plasma and prevents copper-induced lipoprotein peroxidation and also protects β-carotene residents in LDL of the degradation (Tesoriere et al. Citation2003). In supplemented healthy volunteers with 500 g cactus pear fruit pulp, after 3 and 5 h ingestion of the fruit meal, plasma LDL was isolated and betalains were incorporated to the system. LDL particles appeared more resistant to ex vivo-induced oxidative injury, and this resistance increases with betalain concentrations (Tesoriere et al. Citation2004). Additionally, betanin may interact with oxidant molecules like ferric ions (Fe3+), which reduce to ferrous form (Fe2+) and inhibit OH• radical formation (Kanner, Harel, and Granit Citation2001). This radical is the main initiator of lipid peroxidation, acting thourgh the removal of hydrogen atoms from lipid membranes, altering their permeability, resulting in loss of selectivity for the influx and eflux of nutrients and toxic substances into the cell, leading to damage and cell death (Halliwell and Whiteman Citation2004).
Lipid oxidation generates toxic metabolic compounds, hydroperoxides, hydroxides, isoprostanes, aldehydes and ketones, which can aggravate atherogenesis. Malondialdehyde (MDA) is formed from the peroxidation of polyunsaturated fatty acids, such as linoleic acid, and is a clinical biomarker to assess oxidative damage (Niki Citation2014; Ayala, Muñoz, and Argüelles Citation2014; Sousa, Pitt, and Spickett Citation2017).
Betanin effects on lipid peroxidation have been evaluated by the reduced generation of toxic metabolites in several organs and tissues, as demonstrated in preclinical trials (Silva et al. Citation2019b). Animals treated with betanin exhibited lower MDA in liver and kidneys after oxidative tissue damage induced by carbon tetrachloride, paraquat, organophosphates, paracetamol or diclofenate (Sutariya and Saraf Citation2017; Ahmadian et al. Citation2018; Motawi et al. 2019).
Ischemia and reperfusion injury are common complications during CVD therapies, since the reactivation of aerobic metabolism during reperfusion induces an increase in ROS by neutrophils and the mitochondrial metabolism, resulting in damage to membranes and nucleic acids, culminating in cell death (Weigand et al. Citation2012; Garcia-Dorado et al. Citation2014; Zhou et al. Citation2018). Betanin prevents oxidative damage in the lung and heart of animals induced by ischemia-reperfusion injury, evidenced by decreased MDA production in tissues. The same favorable effect of betanin on MDA was observed in the abdominal aorta following ischemia and reperfusion injury (Tural et al. Citation2020a; Citation2020b). Taken together, these findings reinforce the protective effects of betanin on vascular integrity against oxidative damage.
In addition to its free-radical scavenging ability, betanin exerts its protective role on cell and vascular intima through the induction of cellular antioxidant system defenses (Silva, Pereira, et al. 2019). Risk factors for CVD are associated with reduced activity of endogenous antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (Gpx), failing in maintaining cellular redox homeostasis (Chen et al. Citation2012; He et al. Citation2017; Sies, Berndt, and Jones Citation2017).
Clinical trials have demonstrated betanin’s ability to modulate endogenous antioxidant systems in several oxidative stress-inducing disorders (Rahimi et al. Citation2019). This indicates that betanin can induce antioxidant enzymes during cardiovascular abnormalities, since redox imbalances are inherent to the physiopatology of those disorders.
In a study conducted with male Sprague-Dawley rats, five days betanin supplementation at 25 and 100 mg·kg−1 dose avoided decreased SOD and CAT activities by the paraquat herbicide in hepatic and renal tissues (Han et al. Citation2014; Tan et al. Citation2015).
In an experimental model for diabetes mellitus, female Sprague-Dawley rats supplemented with betanin at 50 and 100 mg·kg−1 for 8 weeks restored renal SOD and CAT activities (Sutariya and Saraf Citation2017).
Betanin supplementation antagonized the anti-fibrotic effects of diabetic cardiomyopathy induced by high fructose intake in a dose-dependent manner (25 and 100 mg·kg−1). The cardiac cells of male treated Sprague-Dawley rats exhibited decreased protein glycation accompanied by increased GPx activity (Han et al. Citation2015).
Betanin offered for 20 days at 20 mg·kg−1significantly restored the redox status in Wistar rats by up-regulating SOD, CAT and GPx enzymatic activities evaluated in the hepatocytes of animals with hypercholesterolemia, hyperglycemia and hepatic steatosis induced by a hypercaloric and hyperlipidic diet (Silva, Pereira, et al. 2019). Similarly, plasmatic SOD and CAT activities were restored by betanin intake in an experimental rat acute myocardial infarction model (Yang et al. Citation2016).
Oxidative stress and inflammation are key and closely related processes in the pathogenesis of atherosclerosis and CVD, since the high generation of ROS causes vascular damage and tissue inflammation, leading to endothelial dysfunction, favoring atherogenesis (Siti, Kamisah, and Kamsiah Citation2015; Guzik and Touyz Citation2017). In addition to the ability to remove ROS and modulate antioxidant enzymes, betanin has been shown to play animportant role in anti-inflammatory responses (Vidal et al. Citation2014).
Betanin was shown to inhibit the inflammatory enzymes cyclooxygenase 2 (COX-2), and cyclooxygenase 1 (COX-1) in vitro by 97% and 33.5%, respectivelly (Reddy, Alexander-Lindo, and Nair Citation2005). Betanin was more effective in inhibiting COX-2 than other phytochemicals, such as cyanidin-3-O-glycoside (anthocyanin), lycopene, chlorophyll, β-carotene and bixin. Since COX-2 is induced in injury and inflammation conditions, while COX-1 is constitutively expressed under physiological conditions in various tissues, catalyzing the cytoprotective prostaglandin biosynthesis (PGI2 and PGE2), mainly in the gastrointestinal system, the apparent selectivity of betanin over COX-2 resembles the COX-2 selective non-steroidal anti-inflammatory drugs (NSAIDs), which avoid the deleterious effects of unwanted COX-1 inhibition (gastrointestinal, renal and liver toxicity) (Ferrer et al. Citation2019; Radi and Khan Citation2019; Süleyman, Demircan, and Karagöz Citation2007; Tai and McAlindon Citation2018; Cooper et al. Citation2019).
Lipoxygenases (LOX) seems to be another target for betanin-derivative compounds. The enzymes involved in the biosynthesis of leukotrienes, that act in chemotaxis and leukocyte adhesion to endothelial cells and neutrophil degranulation, among other events are inhibited by betanidin (betanin aglycone), but not betanin, which is a potent LOX-1 inactivator by binding to specific amino acid residues on the surface of LOX-1, near the active site, interfering in enzyme-substrate interaction (Rådmark et al. Citation2015; Vidal et al. Citation2014). Therefore, although betanin is not as effective in inhibiting LOX-1, it is the precursor for betanidin, generated by the hydrolysis of the glycosidic bond at a systemic level. Further studies regarding this mechanism are required.
In in vitro model of endothelial cells obtained from the human umbilical vein, betanin (5 µM) was shown to protect endothelial cells from a cytokine-induced redox imbalance, by inhibiting the expression of the proinflammatory intercellular adhesion molecule 1 (ICAM-1), which causes leukocyte recruitment, pro-inflammatory gene expression and endothelial dysfunction (Gentile et al. Citation2004; Engin Citation2017; Marcos-Ramiro, García-Weber, and Millán Citation2014).
In male Swiss mice, betanin (100 mg·kg−1) administered through subcutaneous and oral routes inhibited the formation of paw edema and recruitment and levels of leukocytes, mononuclear cells and neutrophils after an inflammation stimulus. Betanin also reduced the production of the inflammatory cytokines, TNF-α and IL-β, and increased levels of IL-10 (Martinez et al. Citation2015). As TNF-α and IL-β are crucially involved in multiple inflammatory cardiac pathologies, such as heart failure, myocardial infarction and cardiomyopathies, they have been considered targets for treatments against cardiovascular events. These cytokines induce adhesion molecule expressions in the endothelium, like the aforementioned ICAM-1, that promote leukocyte leakage and can cause systemic inflammatory response and pathological abnormalities (Kleinbongard, Heusch, and Schulz Citation2010; Libby Citation2017). However, clinical trials have confirmed the anti-inflammatory and cardioprotective role played by IL-10, that should be included as a therapeutic target, due to decreases in TNF-α and IL-β secretion, among other cytokines, as well as improved cardiac function, reduced myocardial infarction and ischemia and reperfusion injury (Bhattacharyya et al. Citation2013; Zhang, Xing, et al. Citation2015; Chang et al. Citation2015; Bartekova et al. Citation2018). Betanin displays a similar effect to IL-10-inducing drugs and, consequently, antagonizes the secretion of pro-inflammatory cytokines (Zhou et al. Citation2005).
Betanin also reduces the expression of inducible nitric oxide synthase (iNOS) and confirmed the inhibitory effect on cyclooxigenase 2 (COX-2) in the kidneys of Dawley rats in an acute oxidative stress and inflamantion experimental model (Tan et al. Citation2015).
Although several evidences indicate that betanin alleviates the inflammatory response, the molecular mechanisms by which it exerts its pahrmacological effects still require elucidation. It can be hypothetised that betanin effects are mediated through the nuclear factor transcription factor kappa beta (NF-κB), a common modulator of several genes encoding pro-inflammatory chemokines and cytokines. Betanin influence on the NF-κB pathway will be discussed further ahead.
Finally, betanin has been shown to play a specific role in insulin resistance and type 2 diabetes mellitus, both posing a significant risk for the development of atherosclerotic diseases, including coronary heart disease, peripheral arterial disease, and cerebrovascular disease (Aronson and Edelman Citation2014; Thiruvoipati, Kielhorn, and Armstrong Citation2015; Ergul et al. Citation2012). Betanin administered at 25 and 100 mg·kg−1 for 60 days was able to revert hyperglycemia, hyperinsulinemia, HOMA-IR and glycation products in animals exhibiting experimental diabetes induced by high fructose ingestion (Han et al. Citation2015). Betanin at a concentration of 20 mg.kg−1 administered to rats was shown to alleviate the effects of experimental type 2 diabetes induced by the intraperitoneal administration of STZ and NA. Betanin exerted its effects by regulating liver glucose metabolism-related enzymes, such as glucokinase, glucose-6-phosphatase, pyruvate kinase, glucose-6-phosphate dehydrogenase, and fructose-1,6-bisphosphatase (Dhananjayan et al. Citation2017).
Corroborating these findings, after short-term administration (20 days), betanin modulated the glucose metabolism, insulin production and reversed insulin resistance induced in rats fed by a high fat hypercaloric diet (Silva, Pereira, et al. 2019).
Betanin as a putative activator of the anti-oxidative Nrf2-ARE pathway and supressor of the anti-inflammatory NFk-B pathway in CVD
As mentioned previously, oxidative stress, inflammation and endothelial dysfunction are common conditions in the pathophysiology of several cardiovascular outcomes, such as atherosclerosis (Quirós-Fernández et al. Citation2019; Förstermann, Xia and Li, 2019), heart failure (Ramirez-Sanchez et al. Citation2013; Koning et al. Citation2016; Lubrano and Balzan Citation2020), myocardial hypertrophy (Wu et al. Citation2012, Wilder et al. Citation2015) and ischemia and reperfusion injury (Tian et al. Citation2015; Granger and Kvietys Citation2015).
Some health-promoting compounds, like betanin, can interfere in the activation of transcriptional factors, i.e. the nuclear factor kappa B (NF-kB) and the factor 2 related to nuclear erythroid factor 2 (Nrf2), master regulators in homeostasis redox, which coordinate the RNA synthesis of numerous genes involved in inflammatory, cytoprotective and antioxidant responses (Siti, Kamisah, and Kamsiah Citation2015; Lingappan Citation2018; Liu et al. Citation2019; Baird and Yamamoto Citation2020; Sireesh et al. Citation2018; Sies, Berndt, and Jones Citation2017).
NF-kB is the major gene expression regulator in inflammatory processes. Under normal physiological conditions, NF-kB is inactive in the cytoplasm and linked to inhibitory proteins, known as kB inhibitors (IkBα), (Hayden and Ghosh Citation2012; Giuliani, Bucci, and Napolitano Citation2018). On the other hand, under cellular stress conditions such as UV radiation and mainly oxidative stress (H2O2), kinases called IKK are activated and result in the phosphorylation and degradation of IkBα (Allen and Tresini Citation2000; Yin et al. Citation2015; Zambrano et al. Citation2016). The dissociation of the IkBα/NF-kB complex culminates with the translocation of NF-kB to the nucleus. In the nucleus, NF-kB binds to the promoter region of target genes, initiating the transcription and expression of a range of inflammatory cytokines, chemokines and adhesion molecules involved in the development and progression of inflammatory responses and increased cardiovascular risks (Zambrano et al. Citation2016; Liu et al. Citation2017; Fiordelisi et al. Citation2019).
Cells respond to the deleterious effects of pro-oxidative and pro-inflammatory environments by activating Nrf2, a transcriptional factor modulator of genes involved in cellular redox hoemostasis (Cuadrado et al. Citation2018). Nrf2 is inactive in the cytoplasm and bound through the DLG and ETGE sequences in the Neh 2 domain to the N-terminal region of the Kelch-like repressor protein ECH-associated protein 1 (Keap1) (Tonelli, Chio, and Tuveson Citation2018). Keap1 is rich in Cys residues in the N-terminal region, in its BTB (bric-à-brac) and IVR (intervening region) domains, as well as in the C-terminal region, which are sensitive to electrophiles and oxidants (Kansanen et al. Citation2013; Dinkova-Kostova, Kostov, and Canning Citation2017). When in oxidative stress, its Cys residues, mainly Cys151, 273 and 288, are oxidized, resulting in Keap1 inactivation and Nrf2 derepression (Buendia et al. Citation2016; Saito et al. Citation2016; Gao et al. Citation2020). Nrf2 then translocates to the nucleus, where it binds specifically to ARE (antioxidant response element) sequences in the promoter region of several genes, encoding enzymes involved in cytoprotective and antioxidant effects (Hayes and Dinkova-Kostova Citation2014; Tonelli, Chio, and Tuveson Citation2018).
Nrf2 is a potential target for therapeutic purposes to treat vascular disorders induced by oxidative stress, through the overexpression of Nrf2 or its dissociation from the Keap1-Nrf2 complex, protecting against CVD (Cuadrado et al. Citation2019; Chen and Maltagliati Citation2018; Ge et al. Citation2019). Food antioxidants have been considered as adjuvants for the treatment of cardiovascular diseases due to the activation exerted on Nrf2 and antioxidant response elements (ARE) (Ooi et al. Citation2018; Matzinger, Fischhuber, and Heiss Citation2018; Li and Zhang Citation2017).
Betanin health-promoting benefits to the cardiovascular system are due to its anti-radical scavenger effect that stabilizes the reactivity of these molecules, protecting from endothelial tissue damage, but simultaneouly down-regulating the mRNA of pro-inflammatory mediators while reinforcing endogenous antioxidant defenses. Such properties contribute to reduce endothelial cell ROS-mediated damage and inflammation to.
Betanin influences gene expression through the Nrf2-ARE and NF-kB signal transduction pathways, pointed out as the mechanisms underlying its indirect effects. Betanin has been reported as a NF-kB DNA-binding supressor, followed by inflammatory cytokines COX-2, iNOS and IL-1β gene transcription down-regulator (Tan et al. Citation2015; Han et al. Citation2015). By acting in a pro-oxidative environment, i.e. containing high H2O2 concentrations, betanin can avoid translocation of the NF-kB to the nucleus by inhibiting iKB/NF-kB complex dissociation (Esatbeyoglu et al. 2014; Zielińska-Przyjemska et al. Citation2012; Oliveira-Marques et al. Citation2009) (). Corroborating these findings, betanin was shown to reduce ROS and downregulate NF-κB expression in acute myocardial infarction conditions induced by isoproterenol (Yang et al. Citation2016).
Figure 6. Putative effects of betanin on the redox sensitive Nrf2-ARE and NF-kB pathways. Panel A -The transcription factor Nrf2 is bound to Keap1 in the cytoplasm, and the Nrf2-Keap1 complex dissociation allows for Nrf2 translocation to the nucleus. In the nucleus, Nrf2 dimerizes with the Maf protein and binds to ARE sequences in the promoter of genes that encode phase II detoxification and antioxidants enzymes. Betanin can down-regulate Keap1 expression and/or modify Cys residues culminating in the dissociation of Nrf2, which, in turn, translocates to the nucleus. Betanin can also cross-link the two pathways by up regulating the expression of Nrf2 in the nucleus, favoring the transcription of antioxidant/cytoprotective genes. Panel B -The NF-kB transcriptional factor is synthesized and constitutively linked to the inhibitory protein IkBα in the cytoplasm. The dissociation of the IkB-NF-kB complex occurs by phosphorylation of IkB by IKK kinases which are then activated in response to ROS and oxidative stress. IkB phosphorylation results in its ubiquitination and dissociation of NF-kB, which translocates to nucleus. The activated NF-kB binds to the promoter of target genes, activating the transcription of inflammatory cytokines, chemokines and adhesion molecules. Betanin acts directly on ROS and on antioxidant genes, subsequently inhibiting the activation of IKK, avoiding IkB phosphorylation and release of NF-kB to the nucleus. Thus, betanin, through its role on the dissociation of Keap1-Nrf2, makes Keap1 free for inhibitory binding with IKK. Legend: ARE – Antioxidant response element, BET betanin, BTB bric-à-brac protein, CAT – catalase, COX-2 – cyclooxygenase 2, Cys – cysteine residues, GPx – glutathione peroxidase, GR – glutathione reductase, HO-1 – heme oxygenase 1, IKK – ikB kinase, iNOS – inducible nitric oxide synthase, IL – interleukins, IVR – intervening region, keap1 – Kelch-like ECH-associated protein 1, Maf – small musculoaponeurotic fibrosarcoma protein, Nrf2 – nuclear factor erythroid 2-related factor 2, NF-kB – kappa B nuclear factor, P – phosphorylation, SOD – superoxide dismutase, TRX – thioredoxin, TNF-α – tumoral necrosis factor alpha.
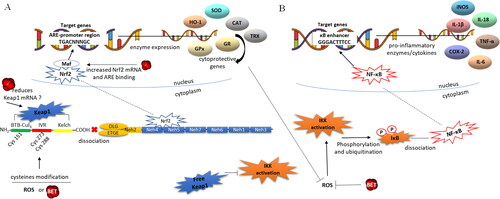
Liver cells treated by betanin exhibit a slight reduction in Keap 1 mRNA in the cytosol and increased nuclear Nrf2 expression and levels. In addition, betanin favors the binding of Nrf2 to the ARE sequence, and up-regulation of Nrf2 target genes encoding cellular phase II detoxification and antioxidant/cytoprotective enzymes, such as glutathione S-transferases GSTP, GSTM, GSTT, GSTA and NQO1 (NAD(P)H quinone dehydrogenase 1) (Krajka-Kuzniak et al. Citation2013).
Betanin induces Nrf2 transactivation accompanied by an increase in heme oxygenase 1 (HO-1), paraoxonase 1 (PON1) and glutathione (GSH,) in a dose-dependent manner (Esatbeyoglu et al. 2014). PON1 exhibits antioxidant, anti-inflammatory and anti-atherogenic properties, circulating in the plasma bound to HDL and, at the same time, delays and/or prevents LDL oxidation, thus mediating antiatherogenic effects. In addition, low PON1 levels in human plasma are associated with increased risks of heart disease (Schrader and Rimbach Citation2011; Wang et al. Citation2012; Litvinov, Mahini, and Garelnabi Citation2012). GSH, the most abundant antioxidant in the heart, protects against oxidative stress, myocardial infarction and coronary artery disease at physiological levels (Bajic et al. Citation2019).
These reports evidence how betanin supports the antioxidant defense system and how it impacts CVD.
The Keap1 protein may be the main betanin target concerning activation of the Nrf2-ARE pathway. Similar to that described for other bioactive compounds, betanin is able to modify cysteine residues, specifically Cys 151, 273 and 288, through interaction with their sulfhydryl groups (SH), thus allowing for the breakdown of the Keap1-Nrf2 interaction, a putative mechanism like the one already described for sulphoraphane found in broccoli (Hu et al. Citation2011). In addition, as reported in a recent review, several antioxidant compounds, such as the flavonoid rutin, can modify Keap1 cysteine residues, through oxidation and alkylation, thereby activating Nrf2/ARE pathway (Ooi et al. Citation2018).
As pointed out, betanin seems to also be involved in mitogen-activated protein kinase (MAPK) activation which, in turn, phosphorylates Nrf2, increasing its stability, and promoting nuclear translocation and transactivation (Krajka-Kuzniak et al., 2013).
Another way through which betanin may alleviate the oxidative stress-inflammation-CVD triad is by inhibition of the NF-kB pathway through a positive effect on Nrf2-ARE, joining the two pathways in a coordinated manner. In the NF-kB pathway, the protein kinase IKK binds to the ETGE sequence (one of the sequences by which Nfr2 is constitutively linked to Keap1), so that IKK can bind to free Keap1, making it impossible to phosphorylate IkB and dissociate the IkB-NF-kB complex. Consequently, NF-kB translocation to the nucleus and inflammatory gene transcription does not occur (Kim et al. Citation2010; Stefanson and Bakovic Citation2014). If betanin increases the Nrf2 translocation to the nucleus (Krajka-Kuzniak et al. 2013), the intracellular levels of free Keap1 (dissociated from Nrf2) would become higher and available for interaction with IKK, thus inhibiting NF-kB activation ().
In sum, betanin can modulate oxidative stress and reduce inflammation through multiples pathways, preventing the major events for the progression of CVD. The putative molecular mechanisms of betanin, not all proven so far, include (): (i) ROS and RNS scavanging by electron and H+ donation; (ii) preventing both peroxidation of phospholipids and apoB-100 in LDL particles, and may also prevent the formation of dithyrosine, avoiding the aggregation of LDL particles, which lead to endothelial dysfunction and trigger atherogenesis; (iii) inhibition of NF-kB pathway activation by directly remove ROS; (iv) down regulation of Keap1 expression and/or betanin interaction through Keap1 cysteine residues, favoring the translocation of Nrf-2 to the nucleus; (v) putative enhancement of the Keap1-Nrf2 complex dissociation, making Keap1 available for IKK binding and then inhibiting NF-kB activation; (vi) up-regulation of Nrf2, activating the endogenous antioxidant response which, in turn, attenuates oxidative stress and prevents NF-kB activation.
Conclusions
Betanin seems to be a supportive therapeutic alternative to attenuate the main mechanisms involved in CVD by oxidative stress and inflammation, without any harmful effects. Betanin contributes to restore the oxidative homeostasis and mitigate vascular inflammation. Betanin bioavaliability reaching plasma in physiological concentrations with retention of the original structure is guaranteed by the physicochemical characteristics of the bloodstream. Although the exact mechanisms by which betanin exerts its cardioprotective role have not been yet fully elucidated, its ability to act directly on ROS/RNS species alongside the induction of the antioxidant/cytoprotective Nrf2-ARE pathway and supression of the inflammatory NFk-B pathway in CVD can account for all of betanin health-promoting benefits.
However, the molecular mechanisms of betanin modulation regarding gene expressions and cell signaling pathways is a new and challenging issue and, thus, is speculative due to the limited data available in the literature. For this reason, further in vitro and even in silico tests can contribute to elucidate the exact manner in which betanin activates the Nrf2-ARE pathway and suppresses the NF-kB pathway. In addition, since betanin is bioaccessible, bioavailable, approved for use in foods in quantium satis, and has not shown any harmful or deleterious effects in animals, clinical trials should be conducted to determine the effective dose and supplementation regimen to achieve the desired therapeutic outcomes in human beings.
Author contributions
DVT, DSB and VMFP conceptualized and designed the manuscript, participating in drafting the article and/or acquisition of data, and/or analysis and interpretation of data; DVT, DSB and VFF prepared the figures and tables. DVT, DSB, VFF and VMFP wrote, edited and revised the manuscript critically. VMFP revised the final written. All authors critically revised the manuscript concerning intellectual content and approved the final manuscript.
bfsn_a_1822277_sm7865.zip
Download Zip (12.4 MB)Acknowledgements
The authors acknowledge the financial support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and from FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro).
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Ahmadian, E., A. Y. Khosroushahi, M. A. Eghbal, and A. Eftekhari. 2018. Betanin reduces organophosphate induced cytotoxicity in primary hepatocyte via an anti-oxidative and mitochondrial dependent pathway. Pesticide Biochemistry and Physiology 144:71–8. doi: https://doi.org/10.1016/j.pestbp.2017.11.009.
- Allegra, M., L. Tesoriere, and M. A. Livrea. 2007. Betanin inhibits the myeloperoxidase/nitrite-induced oxidation of human low-density lipoproteins. Free Radical Research 41 (3):335–41. doi: https://doi.org/10.1080/10715760601038783.
- Allen, R. G., and M. Tresini. 2000. Oxidative stress and gene regulation. Free Radical Biology & Medicine 28 (3):463–99. doi: https://doi.org/10.1016/S0891-5849(99)00242-7.
- Aronson, D., and E. R. Edelman. 2014. Coronary artery disease and diabetes mellitus. Cardiology Clinics 32 (3):439–55. doi: https://doi.org/10.1016/j.ccl.2014.04.001.
- Ayala, A.,. M. F. Muñoz, and S. Argüelles. 2014. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity 2014:360438doi: https://doi.org/10.1155/2014/360438.
- Attoe, E. L., and J. H. Elbe. 1984. Oxygen involvement in betanin degradation: Oxygen uptake and influence of metal ions. Zeitschrift für Lebensmittel-Untersuchung und -Forschung 179 (3):232–6. doi: https://doi.org/10.1007/BF01041900.
- Baião, D. S., D. V. T. Silva, E. M. Del Aguila, V. M. F. Paschoalin, and F. M. 2017. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. Food Additives, Chapter 2:20–44. https://doi.org/10.5772/intechopen.69301.
- Baião, D., C. de Freitas, L. Gomes, D. da Silva, A. Correa, P. Pereira, E. Aguila, and V. Paschoalin. 2017. Polyphenols from root, tubercles and grains cropped in Brazil: Chemical and nutritional characterization and their effects on human health and diseases. Nutrients 9 (9):1044–29. doi: https://doi.org/10.3390/nu9091044..
- Baird, L., and M. Yamamoto. 2020. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Molecular and Cellular Biology 40 (13)e00099-20. doi: https://doi.org/10.1128/MCB.00099-20.
- Bajic, V. P., C. Van Neste, M. Obradovic, S. Zafirovic, D. Radak, V. B. Bajic, M. Essack, and E. R. Isenovic. 2019. Glutathione "redox homeostasis" and its relation to cardiovascular disease. Oxid Med Cell Longev 2019:5028181doi: https://doi.org/10.1155/2019/5028181.
- Bartekova, M., J. Radosinska, M. Jelemensky, and N. S. Dhalla. 2018. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev 23 (5):733–58. doi: https://doi.org/10.1007/s10741-018-9716-x.
- Bhattacharyya, S., L. Xue, S. Devkota, E. Chang, S. Morris, and J. K. Tobacman. 2013. Carrageenan-induced colonic inflammation is reduced in Bcl10 null mice and increased in IL-10-deficient mice. Mediators of Inflammation 2013 (3):1–13. doi: https://doi.org/10.1155/2013/397642.
- Blaak, J., and P. Staib. 2018. The relation of pH and skin cleansing. Current Problems in Dermatology 54:132–42. doi: https://doi.org/10.1159/000489527.
- Buendia, I., P. Michalska, E. Navarro, I. Gameiro, J. Egea, and R. León. 2016. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacology & Therapeutics 157:84–104. doi: https://doi.org/10.1016/j.pharmthera.2015.11.003.
- Burton, G. J., and E. Jauniaux. 2011. Oxidative stress. Best Practice & Research. Clinical Obstetrics & Gynaecology 25 (3):287–99. doi: https://doi.org/10.1016/j.bpobgyn.2010.10.016.
- Butera, D., L. Tesoriere, F. D. Gaudio, A. Bongiorno, M. Allegra, A. M. Pintaudi, R. Kohen, and M. A. Livrea. 2002. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. Journal of Agricultural and Food Chemistry 50 (23):6895–901. doi: https://doi.org/10.1021/jf025696p.
- Castellanos-Santiago, E., and E. M. Yahia. 2008. Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. Journal of Agricultural and Food Chemistry 56 (14):5758–64. doi: https://doi.org/10.1021/jf800362t.
- Czapski, J. 1990. Heat stability of betacyanins in red beet juice and in betanin solutions. Zeitschrift für Lebensmittel-Untersuchung und -Forschung 191 (4-5):275–8. doi: https://doi.org/10.1007/BF01202425.
- Celermajer, D. S., C. K. Chow, E. Marijon, N. M. Anstey, and K. S. Woo. 2012. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. Journal of the American College of Cardiology 60 (14):1207–16. doi: https://doi.org/10.1016/j.jacc.2012.03.074.
- Chakraborty, S., Y. Cai, and M. A. Tarr. 2010. Mapping oxidations of apolipoprotein B-100 in human low-density lipoprotein by liquid chromatography-tandem mass spectrometry. Analytical Biochemistry 404 (2):109–17. doi: https://doi.org/10.1016/j.ab.2010.05.005.
- Chang, C., Q. Ji, B. Wu, K. Yu, Q. Zeng, S. Xin, J. Liu, and Y. Zhou. 2015. Chemerin15-ameliorated cardiac ischemia-reperfusion injury is associated with the induction of alternatively activated macrophages. Mediators of Inflammation 2015:563951doi: https://doi.org/10.1155/2015/563951.
- Chen, S. J., C. H. Yen, Y. C. Huang, B. J. Lee, S. Hsia, and P. T. Lin. 2012. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One 7 (9):e45693. doi: https://doi.org/10.1371/journal.pone.0045693.
- Chen, Q. M., and A. J. Maltagliati. 2018. Nrf2 at the heart of oxidative stress and cardiac protection. Physiological Genomics 50 (2):77–97. doi: https://doi.org/10.1152/physiolgenomics.00041.2017.
- Cooper, C., R. Chapurlat, N. Al-Daghri, G. Herrero-Beaumont, O. Bruyère, F. Rannou, R. Roth, D. Uebelhart, and J. Y. Reginster. 2019. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs & Aging 36 (Suppl 1):15–24. doi: https://doi.org/10.1007/s40266-019-00660-1.
- Cuadrado, A., G. Manda, A. Hassan, M. J. Alcaraz, C. Barbas, A. Daiber, P. Ghezzi, R. León, M. G. López, B. Oliva, et al. 2018. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacological Reviews 70 (2):348–83. doi: https://doi.org/10.1124/pr.117.014753.
- Cuadrado, A., A. I. Rojo, G. Wells, J. D. Hayes, S. P. Cousin, W. L. Rumsey, O. C. Attucks, S. Franklin, A. L. Levonen, T. W. Kensler, et al. 2019. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nature Reviews. Drug Discovery 18 (4):295–317. doi: https://doi.org/10.1038/s41573-018-0008-x.
- Delgado-Vargas, F., A. R. Jiménez, and O. Paredes-López. 2000. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Critical Reviews in Food Science and Nutrition 40 (3):173–289. doi: https://doi.org/10.1080/10408690091189257.
- Dhananjayan, I., S. Kathiroli, S. Subramani, and V. Veerasamy. 2017. Ameliorating effect of betanin, a natural chromoalkaloid by modulating hepatic carbohydrate metabolic enzyme activities and glycogen content in streptozotocin – Nicotinamide induced experimental rats. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 88:1069–79. doi: https://doi.org/10.1016/j.biopha.2017.01.146.
- Dinkova-Kostova, A. T., R. V. Kostov, and P. Canning. 2017. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Archives of Biochemistry and Biophysics 617:84–93. doi: https://doi.org/10.1016/j.abb.2016.08.005.
- Drummond, G. R., S. Selemidis, K. K. Griendling, and C. G. Sobey. 2011. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature Reviews. Drug Discovery 10 (6):453–71. doi: https://doi.org/10.1038/nrd3403.
- Elbe, J. H., I.-Y. Maing, and C. H. Amundson. 1974. Color stability of betanin. Journal of Food Science 39 (2):334–7. doi: https://doi.org/10.1111/j.1365-2621.1974.tb02888.x.
- Engin, A. 2017. Endothelial dysfunction in obesity. Advances in Experimental Medicine and Biology 960:345–79. doi: https://doi.org/10.1007/978-3-319-48382-5_15.
- Ergul, A., A. Kelly-Cobbs, M. Abdalla, and S. C. Fagan. 2012. Cerebrovascular complications of diabetes: Focus on stroke. Endocrine, Metabolic & Immune Disorders Drug Targets 12 (2):148–58. doi: https://doi.org/10.2174/187153012800493477.
- Esatbeyoglu, T., A. E. Wagner, R. Motafakkerazad, Y. Nakajima, S. Matsugo, and G. Rimbach. 2014. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 73:119–26. doi: https://doi.org/10.1016/j.fct.2014.08.007.
- Esatbeyoglu, T., A. E. Wagner, V. B. Schini-Kerth, and G. Rimbach. 2015. Betanin-a food colorant with biological activity. Molecular Nutrition & Food Research 59 (1):36–47. doi: https://doi.org/10.1002/mnfr.201400484.
- European Food Safety Authority. 2015. Scientific opinion on the re-evaluation of beetroot red (E 162) as a food additive. EFSA Journal 13 (12):4318. doi: https://doi.org/10.2903/j.efsa.2015.4318.
- Evrard, S. M., L. Lecce, K. C. Michelis, A. Nomura-Kitabayashi, G. Pandey, K.-R. Purushothaman, V. d'Escamard, J. R. Li, L. Hadri, K. Fujitani, et al. 2016. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nature Communications 7:11853doi: https://doi.org/10.1038/ncomms11853.
- Ferrer, M. D., C. Busquets-Cortés, X. Capó, S. Tejada, J. A. Tur, A. Pons, and A. Sureda. 2019. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Current Medicinal Chemistry 26 (18):3225–41. doi: https://doi.org/10.2174/0929867325666180514112124.
- Fiordelisi, A., G. Iaccarino, C. Morisco, E. Coscioni, and D. Sorriento. 2019. NF kappa B is a key player in the crosstalk between inflammation and cardiovascular diseases. International Journal of Molecular Sciences 20 (7):1599. doi: https://doi.org/10.3390/ijms20071599.
- Food and Drug Administration (FDA). 2017. Code of Federal Regulations. Title 21. volume 1. Cite: 21CFR73.260. Sec. 73.260 Vegetable juice 2009. Revised on April 1,
- Förstermann, U., N. Xia, and H. Li. 2017. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circulation Research 120 (4):713–35. doi: https://doi.org/10.1161/circresaha.116.309326.
- Frank, T., F. C. Stintzing, R. Carle, I. Bitsch, D. Quaas, G. Strass, R. Bitsch, and M. Netzel. 2005. Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacological Research 52 (4):290–7. doi: https://doi.org/10.1016/j.phrs.2005.04.005.
- Gao, Y., X. Lv, H. Yang, L. Peng, and X. Ci. 2020. Isoliquiritigenin exerts antioxidative and anti-inflammatory effects via activating the KEAP-1/Nrf2 pathway and inhibiting the NF-κB and NLRP3 pathways in carrageenan-induced pleurisy. Food & Function 11 (3):2522–34. doi: https://doi.org/10.1039/c9fo01984g.
- Garcia-Dorado, D.,. A. Rodríguez-Sinova, M. Ruiz-Meana, and J. Inserte. 2014. Protection against myocardial ischemia-reperfusion injury in clinical practice. Revista Espanola de Cardiologia (English ed.) 67 (5):394–404. doi: https://doi.org/10.1016/j.rec.2014.01.010.
- Ge, Z. D., Q. Lian, X. Mao, and Z. Xia. 2019. Current status and challenges of NRF2 as a potential therapeutic target for diabetic cardiomyopathy. International Heart Journal 60 (3):512–20. doi: https://doi.org/10.1536/ihj.18-476.
- Gentile, C., L. Tesoriere, M. Allegra, M. A. Livrea, and P. D'Alessio. 2004. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Annals of the New York Academy of Sciences 1028 (1):481–6. doi: https://doi.org/10.1196/annals.1322.057.
- Girod, P. A., and J. P. Zryd. 1991. Biogenesis of betalains: Purification and partial characterization of dopa 4,5-dioxygenase from Amanita muscaria. Phytochemistry 30 (1):169–74. doi: https://doi.org/10.1016/0031-9422(91)84119-D.
- Giuliani, C., I. Bucci, and G. Napolitano. 2018. The role of the transcription factor nuclear factor-kappa b in thyroid autoimmunity and cancer. Frontiers in Endocrinology 9:471. doi: https://doi.org/10.3389/fendo.2018.00471.
- Gliszczyńska-Swigło, A., H. Szymusiak, and P. Malinowska. 2006. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Additives and Contaminants 23 (11):1079–87. doi: https://doi.org/10.1080/02652030600986032.
- Gracy, R. W., J. M. Talent, Y. Kong, and C. C. Conrad. 1999. Reactive oxygen species: The unavoidable environmental insult? Mutation Research 428 (1-2):17–22. doi: https://doi.org/10.1016/S1383-5742(99)00027-7.
- Granger, D. N., and P. R. Kvietys. 2015. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biology 6:524–51. doi: https://doi.org/10.1016/j.redox.2015.08.020.
- Green, K., M. D. Brand, and M. P. Murphy. 2004. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53 (Supplement 1):S110–8. doi: https://doi.org/10.2337/diabetes.53.2007.S110.
- Guzik, T. J., and R. M. Touyz. 2017. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension (Dallas, Tex: 1979) 70 (4):660–7. doi: https://doi.org/10.1161/HYPERTENSIONAHA.117.07802.
- Hackam, G. D., and S. S. Anand. 2003. Emerging risk factors for atherosclerotic vascular disease: A critical review of the evidence. JAMA 290 (7):932–40. doi: https://doi.org/10.1001/jama.290.7.932.
- Halliwell, B., and M. Whiteman. 2004. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean?. British Journal of Pharmacology 142 (2):231–55. doi: https://doi.org/10.1038/sj.bjp.0705776.
- Hamilton, R. T., L. Asatryan, J. T. Nilsen, J. M. Isas, T. K. Gallaher, T. Sawamura, and T. K. Hsiai. 2008. LDL protein nitration: Implication for LDL protein unfolding. Archives of Biochemistry and Biophysics 479 (1):1–14. doi: https://doi.org/10.1016/j.abb.2008.07.026.
- Han, J., C. Tan, Y. Wang, S. Yang, and D. Tan. 2015. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chemico-Biological Interactions 227:37–44. doi: https://doi.org/10.1016/j.cbi.2014.12.032.
- Han, J., Z. Zhang, S. Yang, J. Wang, X. Yang, and D. Tan. 2014. Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 70:100–6. doi: https://doi.org/10.1016/j.fct.2014.04.038.
- Hayden, M. S., and S. Ghosh. 2012. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes & Development 26 (3):203–34. doi: https://doi.org/10.1101/gad.183434.111.
- Hayes, J. D., and A. T. Dinkova-Kostova. 2014. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in Biochemical Sciences 39 (4):199–218. doi: https://doi.org/10.1016/j.tibs.2014.02.002.
- He, L., T. He, S. Farrar, L. Ji, T. Liu, and X. Ma. 2017. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry : international Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 44 (2):532–53. doi: https://doi.org/10.1159/000485089.
- Herbach, K. M., F. C. Stintzing, and R. Carle. 2004. Thermal degradation of betacyanins in juices from purple pitaya [Hylocereus polyrhizus (Weber) Britton & Rose] monitored by high-performance liquid chromatography–tandem mass spectometric analyses. European Food Research and Technology 219 (4):377–85. doi: https://doi.org/10.1007/s00217-004-0948-8.
- Hu, C., A. L. Eggler, A. D. Mesecar, and R. B. van Breemen. 2011. Modification of Keap1 cysteine residues by sulforaphane. Chemical Research in Toxicology 24 (4):515–21. doi: https://doi.org/10.1021/tx100389r.
- Huang, A. S., and J. H. Von Elbe. 1987. Effect of pH on the degradation and regeneration of betanin. Journal of Food Science 52 (6):1689–93. doi: https://doi.org/10.1111/j.1365-2621.1987.tb05907.x.
- Hulthe, J., and B. Fagerberg. 2002. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arteriosclerosis, Thrombosis, and Vascular Biology 22 (7):1162–7. doi: https://doi.org/10.1161/01.atv.0000021150.63480.cd.
- Jin, A. K. 2007. Acidosis. Comprehensive Pediatric Hospital Medicine :125–32. doi: https://doi.org/10.1016/b978-032303004-5.50031-4.
- Kanner, J., S. Harel, and R. Granit. 2001. Betalains–a new class of dietary cationized antioxidants. Journal of Agricultural and Food Chemistry 49 (11):5178–85. doi: https://doi.org/10.1021/jf010456f.
- Kansanen, E., S. M. Kuosmanen, H. Leinonen, and A. L. Levonen. 2013. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biology 1 (1):45–9. doi: https://doi.org/10.1016/j.redox.2012.10.001.
- Khan, M. I., and P. Giridhar. 2015. Plant betalains: Chemistry and biochemistry. Phytochemistry 117:267–95. doi: https://doi.org/10.1016/j.phytochem.2015.06.008.
- Kim, J. E., D. J. You, C. Lee, C. Ahn, J. Y. Seong, and J. I. Hwang. 2010. Suppression of NF-kappaB signaling by Keap1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation . Cellular Signalling 22 (11):1645–54. doi: https://doi.org/10.1016/j.cellsig.2010.06.004.
- Kleinbongard, P., G. Heusch, and R. Schulz. 2010. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure . Pharmacology & Therapeutics 127 (3):295–314. doi: https://doi.org/10.1016/j.pharmthera.2010.05.002.
- Kobayashi, N., J. Schmidt, V. Wray, and W. Schliemann. 2001. Formation and occurrence of dopamine-derived betacyanins. Phytochemistry 56 (5):429–36. doi: https://doi.org/10.1016/S0031-9422(00)00383-6.
- Kovanen, P. T., and M. O. Pentikainen. 2003. Circulating lipoproteins as proinflammatory and anti-inflammatory particles in atherogenesis. Current Opinion in Lipidology 14 (5):411–9. doi: https://doi.org/10.1097/01.mol.0000092615.86399.07.
- Koning, A. M., W. C. Meijers, A. Pasch, H. G. D. Leuvenink, A. S. Frenay, M. M. Dekker, M. Feelisch, R. A. de Boer, and H. van Goor. 2016. Serum free thiols in chronic heart failure. Pharmacological Research 111:452–8. doi: https://doi.org/10.1016/j.phrs.2016.06.027.
- Krajka-Kuźniak, V., J. Paluszczak, H. Szaefer, and W. Baer-Dubowska. 2013. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. The British Journal of Nutrition 110 (12):2138–49. doi: https://doi.org/10.1017/S0007114513001645.
- Kujala, T. S., J. M. Loponen, K. D. Klika, and K. Pihlaja. 2000. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. Journal of Agricultural and Food Chemistry 48 (11):5338–42. doi: https://doi.org/10.1021/jf000523q.
- Kujala, T. S., M. S. Vienola, K. D. Klika, J. M. Loponen, and K. Pihlaja. 2002. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. European Food Research and Technology 214 (6):505–10. doi: https://doi.org/10.1007/s00217-001-0478-6.
- Li, R., Y. Zhang, S. Rasool, T. Geetha, and J. R. Babu. 2019. Effects and underlying mechanisms of bioactive compounds on type 2 diabetes mellitus and Alzheimer's disease. Oxidative Medicine and Cellular Longevity 2019:8165707doi: https://doi.org/10.1155/2019/8165707.
- Li, Y., and H. Zhang. 2017. Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food & Function 8 (8):2935–44. doi: https://doi.org/10.1039/C7FO00342K.
- Libby, P. 2002. Inflammation in atherosclerosis. Nature 420 (6917):868–74. doi: https://doi.org/10.1038/nature01323.
- Libby, P. 2017. Interleukin-1 beta as a target for atherosclerosis therapy: Biological basis of CANTOS and beyond. Journal of the American College of Cardiology 70 (18):2278–89. doi: https://doi.org/10.1016/j.jacc.2017.09.028.
- Lingappan, K. 2018. NF-κB in oxidative stress. Current Opinion in Toxicology 7:81–6. doi: https://doi.org/10.1016/j.cotox.2017.11.002.
- Litvinov, D., H. Mahini, and M. Garelnabi. 2012. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. North American Journal of Medical Sciences 4 (11):523–32. doi: https://doi.org/10.4103/1947-2714.103310.
- Liu, T., L. Zhang, D. Joo, and S. C. Sun. 2017. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2 (1):17023. doi: https://doi.org/10.1038/sigtrans.2017.23.
- Liu, X., J. Huang, J. Li, Q. Mao, and J. He. 2019. Effects of liraglutide combined with insulin on oxidative stress and serum MCP-1 and NF-kB levels in type 2 diabetes. Journal of the College of Physicians and Surgeons Pakistan 29 (3):218–21. doi: https://doi.org/10.29271/jcpsp.2019.03.218.
- Lu, J., S. Mitra, X. Wang, M. Khaidakov, and J. L. Meht. 2011. Oxidative stress and lectin-like ox-ldl-receptor lox-1 in atherogenesis and tumorigenesis. Antioxidants & Redox Signaling 15 (8):2301–33. doi: https://doi.org/10.1089/ars.2010.3792.
- Lubrano, V., and S. Balzan. 2020. Role of oxidative stress-related biomarkers in heart failure: Galectin 3, α1-antitrypsin and LOX-1: New therapeutic perspective?. Molecular and Cellular Biochemistry 464 (1-2):143–52. doi: https://doi.org/10.1007/s11010-019-03656-y.
- Matzinger, M.,. K. Fischhuber, and E. H. Heiss. 2018. Activation of Nrf2 signaling by natural products-can it alleviate diabetes?. Biotechnology Advances 36 (6):1738–67. doi: https://doi.org/10.1016/j.biotechadv.2017.12.015.
- Marcos-Ramiro, B., D. García-Weber, and J. Millán. 2014. TNF-induced endothelial barrier disruption: Beyond actin and Rho. Thrombosis and Haemostasis 112 (6):1088–102. doi: https://doi.org/10.1160/TH14-04-0299.
- Martinez, R. M., D. T. Longhi-Balbinot, A. C. Zarpelon, L. Staurengo-Ferrari, M. M. Baracat, S. R. Georgetti, R. C. Sassonia, W. A. Verri, Jr, and R. Casagrande. 2015. Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: Effect on edema, leukocyte recruitment, superoxide anion and cytokine production. Archives of Pharmacal Research 38 (4):494–504. doi: https://doi.org/10.1007/s12272-014-0473-7.
- Motawi, T. K., S. A. Ahmed, N. A. El-Boghdady, N. S. Metwally, and N. N. Nasr. 2020. Impact of betanin against paracetamol and diclofenac induced hepato-renal damage in rats. Biomarkers : biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals 25 (1):86–93. doi: https://doi.org/10.1080/1354750X.2019.1697365.
- Mulero, M. C., T. Huxford, and G. Ghosh. 2019. NF-κB, IκB, and IKK. In: Structural immunology. Advances in experimental medicine and biology, eds. T. Jin and Q. Yin vol 1172. Singapore: Springer. doi: https://doi.org/10.1007/978-981-13-9367-9_10.
- Mukherjee, S., E. A. Kapp, A. Lothian, A. M. Roberts, Y. V. Vasil'ev, B. A. Boughton, K. J. Barnham, W. M. Kok, C. A. Hutton, C. L. Masters, et al. 2017. Characterization and identification of dityrosine cross-linked peptides using tandem mass spectrometry. Analytical Chemistry 89 (11):6136–45. doi: https://doi.org/10.1021/acs.analchem.7b00941.
- Newsholme, P., V. F. Cruzat, K. N. Keane, R. Carlessi, and P. I. H. Bittencourt. Jr. 2016. Molecular mechanisms of ROS production and oxidative stress in diabetes. The Biochemical Journal 473 (24):4527–50. doi: https://doi.org/10.1042/BCJ20160503C.
- Niki, E. 2014. Biomarkers of lipid peroxidation in clinical material. Biochimica et Biophysica Acta 1840 (2):809–17. doi: https://doi.org/10.1016/j.bbagen.2013.03.020.
- Nita, M., and A. Grzybowski. 2016. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Medicine and Cellular Longevity 2016:3164734. doi: https://doi.org/10.1155/2016/3164734.
- Oliveira-Marques, V., H. S. Marinho, L. Cyrne, and F. Antunes. 2009. Role of hydrogen peroxide in NF-kappaB activation: From inducer to modulator. Antioxidants & Redox Signaling 11 (9):2223–43. doi: https://doi.org/10.1089/ARS.2009.2601.
- Ooi, B. K., K. G. Chan, B. H. Goh, and W. H. Yap. 2018. The role of natural products in targeting cardiovascular diseases via nrf2 pathway: Novel molecular mechanisms and therapeutic approaches. Frontiers in Pharmacology 9:1308doi: https://doi.org/10.3389/fphar.2018.01308.
- Peluso, I., G. Morabito, L. Urban, F. Ioannone, and M. Serafi. 2012. Oxidative stress in atherosclerosis development: The central role of ldl and oxidative burst. Endocrine, Metabolic & Immune Disorders Drug Targets 12 (4):351–60. doi: https://doi.org/10.2174/187153012803832602.
- Pollock, R. L. 2016. The effect of green leafy and cruciferous vegetable intake on the incidence of cardiovascular disease: A meta-analysis. JRSM Cardiovascular Disease 5:2048004016661435. doi: https://doi.org/10.1177/2048004016661435.
- Polturak, G., and A. Aharoni. 2018. "La Vie en Rose": Biosynthesis, Sources, and Applications of Betalain Pigments. Molecular plant 11 (1):7–22. doi: https://doi.org/10.1016/j.molp.2017.10.008.
- Quirós-Fernández, R., B. López-Plaza, L. M. Bermejo, S. Palma-Milla, and C. Gómez-Candela. 2019. Supplementation with hydroxytyrosol and punicalagin improves early atherosclerosis markers involved in the asymptomatic phase of atherosclerosis in the adult population: A randomized, placebo-controlled, crossover trial. Nutrients 11 (3):640. doi: https://doi.org/10.3390/nu11030640.
- Radi, Z. A., and K. N. Khan. 2019. Cardio-renal safety of non-steroidal anti-inflammatory drugs. The Journal of Toxicological Sciences 44 (6):373–91. doi: https://doi.org/10.2131/jts.44.373.
- Rådmark, O., O. Werz, D. Steinhilber, and B. Samuelsson. 2015. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochimica et Biophysica Acta 1851 (4):331–9. doi: https://doi.org/10.1016/j.bbalip.2014.08.012.
- Rahimi, P., S. Abedimanesh, S. A. Mesbah-Namin, and A. Ostadrahimi. 2019. Betalains, the nature-inspired pigments, in health and diseases. Critical Reviews in Food Science and Nutrition 59 (18):2949–78. doi: https://doi.org/10.1080/10408398.2018.1479830.
- Ramirez-Sanchez, I., P. R. Taub, T. P. Ciaraldi, L. Nogueira, T. Coe, G. Perkins, M. Hogan, A. S. Maisel, R. R. Henry, G. Ceballos, et al. 2013. (-)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. International Journal of Cardiology 168 (4):3982–90. doi: https://doi.org/10.1016/j.ijcard.2013.06.089.
- Reddy, M. K., R. L. Alexander-Lindo, and M. G. Nair. 2005. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. Journal of Agricultural and Food Chemistry 53 (23):9268–73. doi: https://doi.org/10.1021/jf051399j.
- Saito, R., T. Suzuki, K. Hiramoto, S. Asami, E. Naganuma, H. Suda, T. Iso, H. Yamamoto, M. Morita, L. Baird, et al. 2016. Characterizations of three major cysteine sensors of Keap1 in stress response. Molecular and Cellular Biology 36 (2):271–84. doi: https://doi.org/10.1128/MCB.00868-15.
- Satta, S.,. A. M. Mahmoud, F. L. Wilkinson, Y. M. Alexander, and S. J. White. 2017. The role of Nrf2 in cardiovascular function and disease. Oxidative Medicine and Cellular Longevity 2017:9237263doi: https://doi.org/10.1155/2017/9237263.
- Sawicki, T., N. Bączek, and W. Wiczkowski. 2016. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. Journal of Functional Foods 27:249–61. doi: https://doi.org/10.1016/j.jff.2016.09.004.
- Schliemann, W., N. Kobayashi, and D. Strack. 1999. The decisive step in betaxanthin biosynthesis is a spontaneous reaction1. Plant Physiol 119 (4):1217–32. doi: https://doi.org/10.1104/pp.119.4.1217.
- Schrader, C., and G. Rimbach. 2011. Determinants of paraoxonase 1 status: Genes, drugs and nutrition. Current Medicinal Chemistry 18 (36):5624–43. doi: https://doi.org/10.2174/092986711798347216.
- Sies, H., C. Berndt, and D. P. Jones. 2017. Oxidative stress. Annual Review of Biochemistry 86:715–48. doi: https://doi.org/10.1146/annurev-biochem-061516-045037.
- Silva, D. V. T., A. D. Pereira, G. T. Boaventura, R. S. A. Ribeiro, M. A. Verícimo, C. E. Carvalho-Pinto, D. D. S. Baião, E. M. Del Aguila, and V. M. F. Paschoalin. 2019b. Short-term betanin intake reduces oxidative stress in Wistar rats. Nutrients 11 (9):1978. doi: https://doi.org/10.3390/nu11091978.
- Silva, D. V. T., D. S. Baião, F. O. Silva, G. Alves, D. Perrone, E. M. Del Aguila, and V. M. F. Paschoalin. 2019a. Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments. Molecules 24 (3):458. doi: https://doi.org/10.3390/molecules24030458..
- Silva, D. V. T., F. O. Silva, D. Perrone, A. P. T. R. Pierucci, C. A. Conte-Junior, T. S. Alvares, E. M. Del Aguila, and V. M. F. Paschoalin. 2016. Physicochemical, nutritional, and sensory analyses of a nitrate enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food & Nutrition Research 60:29909. doi: https://doi.org/10.3402/fnr.v60.29909.
- Sireesh, D., U. Dhamodharan, K. Ezhilarasi, V. Vijay, and K. M. Ramkumar. 2018. Association of NF-E2 related factor 2 (Nrf2) and inflammatory cytokines in recent onset type 2 diabetes mellitus. Scientific Reports 8 (1):5126doi: https://doi.org/10.1038/s41598-018-22913-6.
- Siti, H. N., Y. Kamisah, and J. Kamsiah. 2015. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascular Pharmacology 71:40–56. doi: https://doi.org/10.1016/j.vph.2015.03.005.
- Slatnar, A., F. Stampar, R. Veberic, and J. Jakopic. 2015. HPLC-MS(n) identification of betalain profile of different beetroot (Beta vulgaris L. ssp. vulgaris) parts and cultivars. Journal of Food Science 80 (9):C1952–58. doi: https://doi.org/10.1111/1750-3841.12977.
- Wybraniec, S., K. Starzak, A. Skopińska, M. Szaleniec, J. Słupski, K. Mitka, P. Kowalski, and T. Michałowski. 2013. Effects of metal cations on betanin stability in aqueous-organic solutions. Food Science and Biotechnology 22 (2):353–63. doi: https://doi.org/10.1007/s10068-013-0088-7.
- Belhadj Slimen, I., T. Najar, and M. Abderrabba. 2017. Chemical and antioxidant properties of betalains. Journal of Agricultural and Food Chemistry 65 (4):675–89. doi: https://doi.org/10.1021/acs.jafc.6b04208.
- Smith, E., W. Zhou, P. Shindiapina, S. Sif, C. Li, and R. Baiocchi. 2018. Recent advances in targeting protein arginine methyltransferase enzymes in cancer therapy. Expert Opinion on Therapeutic Targets 22 (6):527–45. doi: https://doi.org/10.1080/14728222.2018.1474203.
- Sousa, B. C., A. R. Pitt, and C. M. Spickett. 2017. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radical Biology & Medicine 111:294–308. doi: https://doi.org/10.1016/j.freeradbiomed.2017.02.003.
- Stefanson, A. L., and M. Bakovic. 2014. Dietary regulation of Keap1/Nrf2/ARE pathway: Focus on plant-derived compounds and trace minerals. Nutrients 6 (9):3777–801. doi: https://doi.org/10.3390/nu6093777.
- Stintzing, F. C., and R. Carle. 2004. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends in Food Science & Technology 15 (1):19–38. doi: https://doi.org/10.1016/j.tifs.2003.07.004.
- Stintzing, F. C., A. Schieber, and R. Carle. 2000. Rote Bete als färbendes Lebensmittel - eine Bestandsaufnahme. Obst-, Gemüse- Und Kartoffelverarbeitung 85:196–204.
- Sucu, C., and G. Y. Turp. 2018. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Science 140:158–166. doi: https://doi.org/10.1016/j.meatsci.2018.03.012.
- Süleyman, H., B. Demircan, and Y. Karagöz. 2007. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacological Reports: PR 59 (3):247–58.
- Sutariya, B., and M. Saraf. 2017. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. Journal of Ethnopharmacology 198:432–3. doi: https://doi.org/10.1016/j.jep.2016.12.048.
- Steglich, W., and D. Strack. 1990. Chapter 1. Betalains. The Alkaloids: Chemistry and Pharmacology 39:1–62. doi: https://doi.org/10.1016/S0099-9598(08)60163-7.
- Tai, F. W. D., and M. E. McAlindon. 2018. NSAIDs and the small bowel. Current Opinion in Gastroenterology 34 (3):175–82. doi: https://doi.org/10.1097/MOG.0000000000000427.
- Tan, D., Y. Wang, B. Bai, X. Yang, and J. Han. 2015. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 78:141–6. doi: https://doi.org/10.1016/j.fct.2015.01.018.
- Tesoriere, L., M. Allegra, D. Butera, and M. A. Livrea. 2004. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. The American Journal of Clinical Nutrition 80 (4):941–45. doi: https://doi.org/10.1093/ajcn/80.4.941.
- Tesoriere, L., M. Allegra, D. Butera, C. Gentile, and M. A. Livrea. 2006. Cytoprotective effects of the antioxidant phytochemical indicaxanthin in beta-thalassemia red blood cells. Free Radical Research 40 (7):753–61. doi: https://doi.org/10.1080/10715760600554228.
- Tesoriere, L., D. Butera, D. D'Arpa, F. D. Gaudio, M. Allegra, C. Gentile, and M. A. Livrea. 2003. Increased resistance to oxidation of betalain-enriched human low density lipoproteins. Free Radical Research 37 (6):689–96. doi: https://doi.org/10.1080/1071576031000097490.
- Tesoriere, L., M. Fazzari, F. Angileri, C. Gentile, and M. A. Livrea. 2008. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. Journal of Agricultural and Food Chemistry 56 (22):10487–92. doi: https://doi.org/10.1021/jf8017172.
- Tesoriere, L., C. Gentile, F. Angileri, A. Attanzio, M. Tutone, M. Allegra, and M. A. Livrea. 2013. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. European Journal of Nutrition 52 (3):1077–87. doi: https://doi.org/10.1007/s00394-012-0414-5.
- Tesoriere, L., M. Allegra, D. Butera, C. Gentile, and M. A. Livrea. 2007. Kinetics of the lipoperoxyl radical-scavenging activity of indicaxanthin in solution and unilamellar liposomes. Free Radical Research 41 (2):226–33. doi: https://doi.org/10.1080/10715760601026614.
- Thiruvoipati, T., C. E. Kielhorn, and E. J. Armstrong. 2015. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World Journal of Diabetes 6 (7):961–9. doi: https://doi.org/10.4239/wjd.v6.i7.961.
- Tian, Y., H. Li, P. Liu, J. M. Xu, M. G. Irwin, Z. Xia, and G. Tian. 2015. Captopril pretreatment produces an additive cardioprotection to isoflurane preconditioning in attenuating myocardial ischemia reperfusion injury in rabbits and in humans. Mediators of Inflammation 2015:819232doi: https://doi.org/10.1155/2015/819232.
- Tonelli, C., I. I. C. Chio, and D. A. Tuveson. 2018. Transcriptional regulation by Nrf2. Antioxid. Redox Signal 29 (17):1727–45. doi: https://doi.org/10.1089/ars.2017.7342.
- Tural, K., O. Ozden, Z. Bilgi, E. Kubat, C. S. Ermutlu, O. Merhan, F. G. Findik, and S. A. Kucuker. 2020a. The protective effect of betanin and copper on heart and lung in end-organ ischemia reperfusion injury . Bratislavske Lekarske Listy 121 (3):211–17. doi: https://doi.org/10.4149/BLL_2020_032.
- Tural, K., O. Ozden, Z. Bilgi, E. Kubat, C. S. Ermutlu, O. Merhan, and I. Tasoglu. 2020b. The protective effect of betanin and copper on spinal cord ischemia-reperfusion injury. The Journal of Spinal Cord Medicine 30:1–7. doi: https://doi.org/10.1080/10790268.2020.1737788.
- Tural, K., O. Ozden, Z. Bilgi, O. Merhan, C. S. Ermutlu, and A. Aksoyek. 2019. Protective effects of betanin against oxidative stress in a peripheral artery vasospasm model in rat. Journal of Investigative Surgery 11:1–6. doi: https://doi.org/10.1080/08941939.2019.1587555.
- Vidal, P. J., J. M. López-Nicolás, F. Gandía-Herrero, and F. García-Carmona. 2014. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chemistry 154:246–54. doi: https://doi.org/10.1016/j.foodchem.2014.01.014.
- Wang, M., X. Lang, S. Cui, L. Zou, J. Cao, S. Wang, and X. Wu. 2012. Quantitative assessment of the influence of paraoxonase 1 activity and coronary heart disease risk. DNA and Cell Biology 31 (6):975–82. doi: https://doi.org/10.1089/dna.2011.1478.
- Weigand, K., S. Brost, N. Steinebrunner, M. Büchler, P. Schemmer, and M. Müller. 2012. Ischemia/Reperfusion injury in liver surgery and transplantation: Pathophysiology. HPB Surgery : a World Journal of Hepatic, Pancreatic and Biliary Surgery 2012:176723doi: https://doi.org/10.1155/2012/176723.
- Wilder, T., D. M. Ryba, D. F. Wieczorek, B. M. Wolska, and R. J. Solaro. 2015. N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. American Journal of Physiology. Heart and Circulatory Physiology 309 (10):H1720–30. doi: https://doi.org/10.1152/ajpheart.00339.2015.
- WHO. 2003. Food and Agriculture Organization. Diet, nutrition and the prevalence of chronic diseases. Report ofa Joint WHO/FAO Expert Consultation. Geneva (Technical Report Series 916) Accessed June 30, 2020. https://www.who.int/dietphysicalactivity/publications/trs916/en/.
- WHO. 2017. Media centre. Cardiovascular diseases (CVDs). Acessed in March 20, 2020. http://www.who.int/mediacentre/factsheets/fs317/en/.
- Wu, R., E. Wyatt, K. Chawla, M. Tran, M. Ghanefar, M. Laakso, C. L. Epting, and H. Ardehali. 2012. Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. EMBO Molecular Medicine 4 (7):633–46. doi: https://doi.org/10.1002/emmm.20.
- Wu, G. R., M. Cheserek, Y. H. Shi, L. Y. Shen, J. Yu, and G. W. Le. 2015. Elevated plasma dityrosine in patients with hyperlipidemia compared to healthy individuals. Annals of Nutrition and Metabolism 66 (1):44–50. doi: https://doi.org/10.1159/000365731.
- Yang, B., F. Cao, H. Zhao, J. Zhang, B. Jiang, and Q. Wu. 2016. Betanin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress-myeloperoxidase/low-density lipoprotein in rat. International Journal of Clinical and Experimental Pathology 9 (3):2777–86.
- Yin, J., J. Duan, Z. Cui, W. Ren, T. Li, and Y. Yin. 2015. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Advances 5 (20):15479–86. doi: https://doi.org/10.1039/C4RA13557A.
- Zambrano, S., I. De Toma, A. Piffer, M. E. Bianchi, and A. Agresti. 2016. NF-κB oscillations translate into functionally related patterns of gene expression. elife 5:e09100. doi: https://doi.org/10.7554/eLife.09100.
- Zhang, W., B. Xing, L. Yang, J. Shi, and X. Zhou. 2015. Icaritin attenuates myocardial ischemia and reperfusion injury via anti-inflammatory and anti-oxidative stress effects in rats. The American Journal of Chinese Medicine 43 (6):1083–97. doi: https://doi.org/10.1142/S0192415X15500627.
- Zhang, Y. J., R. Y. Gan, S. Li, Y. Zhou, A. N. Li, D. P. Xu, and H. B. Li. 2015. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules (Basel, Switzerland) 20 (12):21138–56. doi: https://doi.org/10.3390/molecules201219753.
- Zhou, T., E. R. Prather, D. E. Garrison, and L. Zuo. 2018. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. International Journal of Molecular Sciences 19 (2):417. doi: https://doi.org/10.3390/ijms19020417.
- Zhou, X., P. Schmidtke, F. Zepp, and C. U. Meyer. 2005. Boosting interleukin-10 Production: Therapeutic effects and mechanisms. Current Drug Targets. Immune, Endocrine and Metabolic Disorders 5 (4):465–75. doi: https://doi.org/10.2174/156800805774912926.
- Zielińska-Przyjemska, M., A. Olejnik, A. Kostrzewa, M. Łuczak, P. P. Jagodziński, and W. Baer-Dubowska. 2012. The beetroot component betanin modulates ROS production, DNA damage and apoptosis in human polymorphonuclear neutrophils. Phytotherapy Research : PTR 26 (6):845–52. doi: https://doi.org/10.1002/ptr.3649.