Abstract
Dementia represents a key impending global health challenge. The aim of this systematic review was to evaluate the current evidence on nutritional interventions for the prevention of dementia in developing economies in East-Asia. Four comprehensive databases were searched from inception until January 2020: MEDLINE, Embase, PsycInfo, and Scopus. The search was restricted to randomized controlled trials [RCTs] in adult humans, assessing the effect of nutritional interventions on global and domain specific cognitive performance and dementia risk. Meta-analysis of data was conducted for each domain and sub-categorized according to the type of nutritional intervention. Twenty-four RCTs were included, of which, fifteen studies showed significant beneficial effects on cognition. Eighteen studies were included in the meta-analysis. Significant beneficial effects were found for essential fatty acids (EPA/DHA) and micronutrient supplementation on specific cognitive domains including attention and orientation, perception, verbal functions and language skills. The effect size of the interventions appeared to be greater in older subjects with cognitive impairment. Supplementation with B-vitamins and essential fatty acids may represent promising strategies to minimize age-related cognitive decline in Asian populations. Large, high-quality, long-term trials are needed to confirm these findings.
Introduction
Dementia is a significant global health issue, with the majority of the global disease burden (approximately 66%) in low-income and middle-income countries (LMICs) (Prince Citation2015, Prina et al. Citation2019). Across the different LMICs, East-Asia has the highest number of people with dementia [≈9.8 million], and this figure is expected to increase by 70% over the next 15 years (Prince Citation2015). This evidence has triggered the need to address the problem by way of novel approaches to promote healthy brain ageing and reduce dementia risk taking into consideration the unique challenges of LMICs including gender inequalities, weak healthcare infrastructure and limited access to research (Walker and Paddick Citation2019).
Currently, dementia is incurable but delay and prevention are possible (Stephan et al. Citation2018). Modifiable metabolic (diabetes), vascular (hypertension), health (depression), social (isolation) and lifestyle factors (poor diet, smoking, alcohol use and physical inactivity) have been consistently associated with dementia risk (Livingston et al. Citation2017). As such, there has been much attention dedicated to modulating health and lifestyle factors before the onset of dementia. Strategies that show promise, include The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial (Ngandu et al. Citation2015), a multi-domain intervention (diet, exercise, cognitive training, vascular risk monitoring), as well as, a large scale Mediterranean diet intervention trial called the PREvencion con DIeta MEDiterranea (PREDIMED) RCT (Martínez-Lapiscina et al. Citation2013). Importantly, each study included a central nutritional component, owing to the well-documented mechanistic links that key dietary constituents can have in promoting cognitive function. The inflammatory and oxidative stress mechanisms involved in the etiology of cognitive decline and dementia, as well as processes such as neurogenesis and neuronal connectivity involved in brain functioning are influenced by dietary components, namely but not limited to, fatty acids, antioxidants and micronutrients (Caracciolo et al. Citation2014; Frisardi et al. Citation2010). Despite this, to date, very few nutritional intervention studies focused on dementia prevention and risk reduction have been undertaken in LMICs (Livingston et al. Citation2017; McGrattan et al. Citation2018). Therefore, the aim of this systematic review and meta-analysis is to evaluate the current evidence on nutritional interventions conducted in adults for the prevention of dementia and cognitive decline in LMICs in developing economies of East-Asia. This is necessary to identify potentially promising avenues for dementia risk reduction in LMIC settings.
Materials and methods
This systematic review was conducted according to the Cochrane guidelines and is reported according to PRISMA guidelines (Higgins and Green Citation2011; Liberati et al. Citation2009). The study protocol was registered on the PROSPERO database (registration number CRD42017082105).
Search strategy and eligibility criteria
Four commonly used, comprehensive medical databases were searched from inception until January 2020. The selected databases were: [1] MEDLINE, [2] EMBASE, [3] PsycINFO, accessed via Ovid [https://ovidsp.ovid.com], and [4] Scopus [https://www.Scopus.com/home.uri]. A detailed description of the search strategy per database is provided in the online supplementary document. The search was restricted to randomized clinical trials [RCTs] conducted in adult humans [≥18 years], and no distinction was made on the specific design of the trials [i.e. duration, placebo controlled, cross-over, or blindness]. The trial needed to contain a nutritional and/or dietary part, and adequate description of the intervention was required. The outcome of interest was cognitive performance or incidence of Mild Cognitive Impairment (MCI) or dementia diagnosed as per established criteria, in the intervention and control group. Additionally, the search was restricted to LMICs in East-Asia as classified according to the United Nations World Economic Situation and Prospects 2018 including Brunei Darussalam; Cambodia; China; Fiji; Hong Kong Special Administrative Region of China; Indonesia; Kiribati; Lao People’s Democratic Republic; Malaysia; Mongolia; Myanmar; Papua New Guinea; Philippines; Republic of Korea; Samoa; Singapore; Solomon Islands; Taiwan Province of China; Thailand; Timor-Leste; Vanuatu; and Vietnam (UnitedNations Citation2018).
Screening process
Two independent reviewers [CVA & AN] assessed potentially relevant articles for eligibility. The decision to include or exclude studies was hierarchical and initially made based on the study title and abstract. When a study could not be rejected with certainty, the full text of the article was obtained for full evaluation. Next, the full text of all selected articles was checked against the inclusion/exclusion criteria independently by the same reviewers. Discrepancies between reviewers were resolved by a third reviewer [MS]. In addition, the reference list of all included articles was checked for any missing articles.
Data extraction
A standardized form was used to extract data from the included studies for assessment of study quality and evidence synthesis. Extracted data included information on: [1] authors; [2] year of publication; [3] title; [4] country; [5] study design; [6] sample size; [7] sample characteristics; [8] description of intervention in the treatment and control group; [9] sampling strategy; [10] duration of the intervention; [11] modalities of delivery of the intervention; [12] cognitive tests used and test results; [13] attrition rates/drop out; [14] compliance; [15] safety; [16] main findings; [17]; and, conflicts of interest. One author [CVA] extracted data, and a second author [AN] checked the data extraction.
Risk of bias assessment
The quality of each study was assessed using the Cochrane Risk of Bias Tool. This tool assesses: selection bias [random sequence generation, and allocation concealment]; performance bias [blinding of participants and personal]; detection bias [blinding of outcome assessment]; attrition bias [incomplete outcome data]; and, selective reporting [reporting bias] (Higgins, Altman, and Sterne Citation2011).
Data synthesis and statistical analysis
Studies were eligible for meta-analysis if mean cognitive test scores [± standard deviation/standard error] were available from the intervention and control group [e.g. scores at start and end of intervention or change in scores]. No restriction was made for the type of neuropsychological tests used. Authors were contacted if the necessary data was not available.
The meta-analysis was conducted in Comprehensive Meta-Analysis software version 2 [Biostat, Engelwood, New Jersey]. The standardized mean difference [95% CI] of the change in cognitive performance between the intervention and control group was calculated for each included study. A positive standardized mean difference was in favor of the intervention group [i.e. the intervention resulted in less degeneration or a larger improvement in cognitive performance over the study period in comparison to the control group].
The outcomes of interest for the meta-analysis were: [1] global cognitive performance, and [2] domain specific cognitive performance (Lezak et al. Citation2012). The effect size was generated for each specific type of intervention [i.e. micronutrient supplementation; chicken essence supplementation; nutrition and lifestyle counseling; fatty acid (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) supplementation; and other interventions (soy-isoflavone supplements/L-carnitine supplements/caffeinated alcoholic beverage/Lactobacillus Plantarum C29-Fermented Soybean/Ellagic acid)], according to random model analysis. Heterogeneity was assessed using Cochran’s Q statistic [a value of p < 0.10 indicates significant heterogeneity], and the I2 test was utilized to assess heterogeneity across the trials, where a value of <25% indicates low risk, 25–75% indicates moderate risk, and >75% indicates a high risk of heterogeneity (Higgins et al. Citation2003). Risk of publication bias was assessed using a funnel plot and tested by Egger’s regression test. Meta-regression was conducted to evaluate the association between changes in cognitive function and duration of the intervention (in weeks).
Results
Literature search and study selection
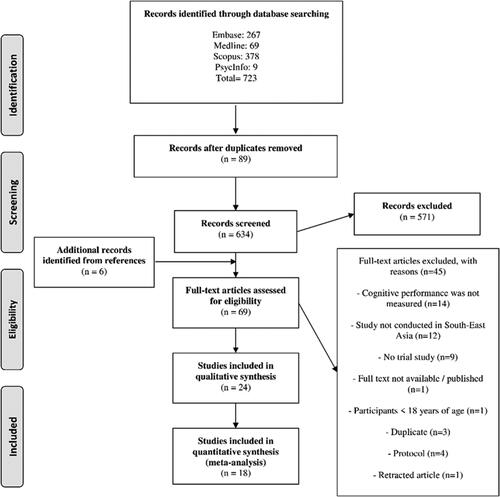
The initial search yielded 723 articles of which 89 were duplicates and removed. After title/abstract screening 571 articles were excluded. Based on full-text screening and reference list searches 45 articles were selected for full text review. From these, 24 articles were retained of which 18 qualified for quantitative synthesis. shows the full details of article selection.
Studies characteristics
A summary of the characteristics of the included studies is presented in , and the full data extraction table is provided in the online supplementary document. All identified studies had neuropsychological test scores as an outcome measure. No study included MCI or dementia in their outcomes. Four types of nutritional strategies for the prevention of cognitive impairment were identified: [1] micronutrients supplementation [i.e. single or multiple B-vitamins [N = 3], multi-vitamins and minerals [N = 3]] (Kwok et al. Citation2011; Ng et al. Citation2017; Sun et al. Citation2007; Prado et al. Citation2012; Cheng et al. Citation2016; Ma et al. Citation2019); [2] supplementation with chicken essence [N = 5] (Nagai et al. Citation1996; Zain and Syedsahiljamalulail Citation2003; Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Chan et al. Citation2016); [3] nutritional and lifestyle counseling [N = 4] (Kwok et al. Citation2012; Johari et al. Citation2014; Lee et al. Citation2014; Rosli et al. Citation2017); and, [4] eicosapentaenoic acid (EPA)/Docosahexaenoic acid (DHA) supplementation [N = 4] (Chiu et al. Citation2008; Bo et al. Citation2017, Zhang et al. Citation2017; Lee et al. Citation2013). The five remaining studies provided participants with soy-isoflavone (Ho et al. Citation2007), Lactobacillus Plantarum C29-Fermented Soybean (DW2009) (Hwang et al. Citation2019), L-carnitine (Badrasawi et al. Citation2016), a caffeinated alcoholic beverage (Cheng et al. Citation2017), or Ellagic acid (Liu et al. Citation2018). Seven studies were conducted in Malaysia (Johari et al. Citation2014; Rosli et al. Citation2017; Badrasawi et al. Citation2016; Zain and Syedsahiljamalulail Citation2003; Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Lee et al. Citation2013), six in China (Bo et al. Citation2017; Zhang et al. Citation2017; Ho et al. Citation2007; Cheng et al. Citation2016; Ma et al. Citation2019; Liu et al. Citation2018), four in Taiwan (Chiu et al. Citation2008, Sun et al. Citation2007; Chan et al. Citation2016; Cheng et al. Citation2017), two in Hong Kong (Kwok et al. Citation2011; Kwok et al. Citation2012), two in Indonesia (Prado et al. Citation2012; Nagai et al. Citation1996), two in the Republic of Korea (Lee et al. Citation2014, Hwang et al. Citation2019), and one in Singapore (Ng et al. Citation2017). Sample size ranged from 16 (Nagai et al. Citation1996) to 640 participants (Prado et al. Citation2012), with a median sample size of 100 participants. The majority of studies recruited older participants [17 studies, ≥ 50 years] (Kwok et al. Citation2012; Johari et al. Citation2014; Lee et al. Citation2014; Rosli et al. Citation2017; Chiu et al. Citation2008; Bo et al. Citation2017; Ho et al. Citation2007; Sun et al. Citation2007; Kwok et al. Citation2011; Badrasawi et al. Citation2016; Cheng et al. Citation2016; Ng et al. Citation2017; Zhang et al. Citation2017; Lee et al. Citation2013; Ma et al. Citation2019; Hwang et al. Citation2019), five studies recruited mid-life age participants [25–65 years] (Prado et al. Citation2012; Azhar et al. Citation2013; Chan et al. Citation2016; Cheng et al. Citation2017; Liu et al. Citation2018), and three studies recruited only young participants [18–24 years] (Nagai et al. Citation1996; Zain and Syedsahiljamalulail Citation2003; Azhar, Zubaidah, and Norjan Citation2008). The duration of the interventions ranged from 30 minutes (Cheng et al. Citation2017) to 33 months (Kwok et al. Citation2012), with an overall median duration of six months. A wide variety of neuropsychological tests were used to assess cognitive performance. Global cognitive function was assessed by seven different tools in 14 studies: the Mini Mental State Examination [MMSE] (Kwok et al. Citation2011; Sun et al. Citation2007; Johari et al. Citation2014; Kwok et al. Citation2012; Lee et al. Citation2014; Chiu et al. Citation2008; Badrasawi et al. Citation2016; Ho et al. Citation2007; Lee et al. Citation2013), the Basic Cognitive Aptitude Test [BCAT] (Cheng et al. Citation2016; Bo et al. Citation2017), The Wechsler Adult Intelligence Scale Revised (WAIS-R) (Liu et al. Citation2018; Ma et al. Citation2019), the Alzheimer's Disease Assessment Scale-cognitive subscale [ADAS-COG] (Sun et al. Citation2007; Chiu et al. Citation2008), the Mattis Dementia Rating scale [MDRS] (Kwok et al. Citation2011), the Repeatable Battery for the Assessment of Neuropsychological Status [RBANS] (Ng et al. Citation2017), and the Cognitive Abilities Screening Instrument [CASI] (Sun et al. Citation2007). In addition, memory, verbal functions and language skills, construction and motor performance, conception formation and reasoning, executive function and visuospatial skills were assessed using 21 different neuropsychological tests in 18 studies (Cheng et al. Citation2016; Cheng et al. Citation2017; Liu et al. Citation2018; Ma et al. Citation2019; Ng et al. Citation2017; Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Chan et al. Citation2016; Nagai et al. Citation1996; Zain and Syedsahiljamalulail Citation2003; Johari et al. Citation2014; Kwok et al. Citation2012; Rosli et al. Citation2017; Bo et al. Citation2017; Lee et al. Citation2013; Zhang et al. Citation2017; Ho et al. Citation2007; Hwang et al. Citation2019). A full description of the neuropsychological test used in each study is provided in the online supplementary document.
Table 1. Characteristics of the included studies.
Risk of bias
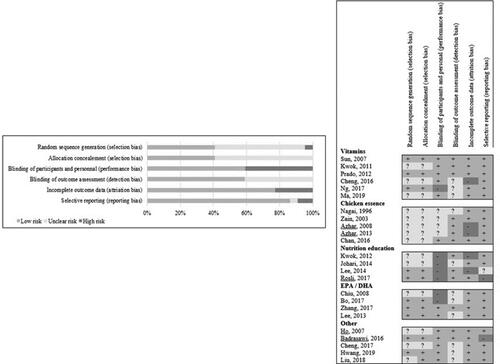
The assessment of methodological quality of each study is shown in and full details are available in the online supplementary document. Eleven trials (Badrasawi et al. Citation2016; Bo et al. Citation2017; Lee et al. Citation2014; Ng et al. Citation2017; Prado et al. Citation2012; Rosli et al. Citation2017; Sun et al. Citation2007; Zain and Syedsahiljamalulail Citation2003; Zhang et al. Citation2017; Lee et al. Citation2013; Hwang et al. Citation2019) declared that a random sequence generator was used, whereas this was unclear in the remaining 13 trials (Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Chan et al. Citation2016; Cheng et al. Citation2016; Chiu et al. Citation2008; Ho et al. Citation2007; Johari et al. Citation2014; Kwok et al. Citation2011; Kwok et al. Citation2012; Zain and Syedsahiljamalulail Citation2003; Ma et al. Citation2019; Liu et al. Citation2018; Nagai et al. Citation1996). A low risk for performance bias was found in 13 studies (Badrasawi et al. Citation2016; Chan et al. Citation2016; Cheng et al. Citation2016; Cheng et al. Citation2017; Ho et al. Citation2007; Kwok et al. Citation2011; Prado et al. Citation2012; Sun et al. Citation2007; Zhang et al. Citation2017; Lee et al. Citation2013; Liu et al. Citation2018; Ma et al. Citation2019; Hwang et al. Citation2019). In contrast, there was an unclear and high risk of performance bias in the remaining studies (Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Bo et al. Citation2017; Chiu et al. Citation2008; Johari et al. Citation2014; Kwok et al. Citation2012; Lee et al. Citation2014; Ng et al. Citation2017; Rosli et al. Citation2017; Zain and Syedsahiljamalulail Citation2003; Nagai et al. Citation1996). A low risk for detection bias was found in 13 studies (Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Badrasawi et al. Citation2016; Chan et al. Citation2016; Ho et al. Citation2007; Kwok et al. Citation2011; Kwok et al. Citation2012; Lee et al. Citation2014; Prado et al. Citation2012; Rosli et al. Citation2017; Sun et al. Citation2007; Zain and Syedsahiljamalulail Citation2003; Zhang et al. Citation2017) whereas insufficient information on blinding of the outcome assessors resulted in an unclear risk of detection bias in 11 studies (Bo et al. Citation2017; Cheng et al. Citation2016; Cheng et al. Citation2017; Chiu et al. Citation2008; Johari et al. Citation2014; Nagai et al. Citation1996; Ng et al. Citation2017; Lee et al. Citation2013; Ma et al. Citation2019; Hwang et al. Citation2019; Liu et al. Citation2018). A low risk for attrition bias was found in 19 studies (Badrasawi et al. Citation2016; Bo et al. Citation2017; Chan et al. Citation2016; Cheng et al. Citation2017; Chiu et al. Citation2008; Ho et al. Citation2007; Johari et al. Citation2014; Kwok et al. Citation2011; Nagai et al. Citation1996; Ng et al. Citation2017; Prado et al. Citation2012; Rosli et al. Citation2017; Sun et al. Citation2007; Zain and Syedsahiljamalulail Citation2003; Zhang et al. Citation2017; Lee et al. Citation2013; Liu et al. Citation2018; Ma et al. Citation2019; Hwang et al. Citation2019). A high drop-out and/or unequal drop-out between intervention and control group resulted in high risk of attrition bias in five studies (Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Cheng et al. Citation2016; Kwok et al. Citation2012; Lee et al. Citation2014). A low risk of reporting bias was found in the majority of the studies [n = 21 studies] (Cheng et al. Citation2016; Cheng et al. Citation2017; Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Bo et al. Citation2017; Chan et al. Citation2016; Hwang et al. Citation2019; Johari et al. Citation2014; Ma et al. Citation2019; Nagai et al. Citation1996; Ng et al. Citation2017; Prado et al. Citation2012; Zain and Syedsahiljamalulail Citation2003; Zhang et al. Citation2017; Kwok et al. Citation2011; Kwok et al. Citation2012; Chiu et al. Citation2008; Ho et al. Citation2007; Liu et al. Citation2018; Sun et al. Citation2007; Lee et al. Citation2013) except for three studies (Rosli et al. Citation2017; Badrasawi et al. Citation2016; Lee et al. Citation2014).
Micronutrient supplementation
Five (Cheng et al. Citation2016; Kwok et al. Citation2011; Ng et al. Citation2017; Prado et al. Citation2012; Ma et al. Citation2019) of the six (Ma et al. Citation2019; Sun et al. Citation2007; Prado et al. Citation2012; Ng et al. Citation2017; Kwok et al. Citation2011; Cheng et al. Citation2016) studies that investigated the effect of micronutrient supplementation on cognitive performance found a positive effect. Three studies found a significant positive effect on cognitive performance for multi-vitamin supplementation [vitamin B9/B12; cognitive domain: construction and motor performance; N = 112 (Kwok et al. Citation2011), and vitamin B6/B9/B12; cognitive domain: global; N = 83 (Cheng et al. Citation2016)] in older participants with hyper-homocysteine, or with supplementation of vitamin B9 alone [cognitive domain: global; N = 180 (Ma et al. Citation2019)] in older patients with MCI. Multi-micronutrient supplementation resulted in a significant improvement in cognition performance in pre-frail and frail elderly [cognitive domain: memory; N = 99] (Ng et al. Citation2017), and pregnant women [cognitive domain: global; N = 640] (Prado et al. Citation2012). Only one study did not find a significant effect of micronutrient supplementation [vitamin B6, B9 and B12] in patients with mild to moderate Alzheimer’s disease [N = 89] (Sun et al. Citation2007).
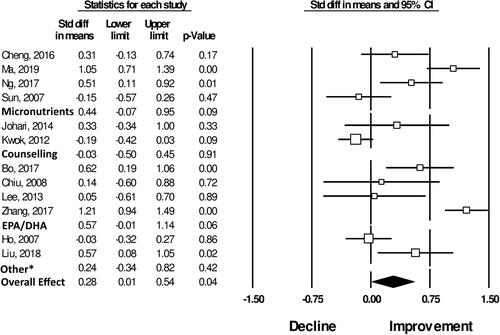
Meta-analysis showed a non-significant effect for micronutrients on global cognitive performance [N = 4 studies; standard (std) mean difference: 0.44 [-0.07; 0.95], P = 0.09, N = 451, and ] (Sun et al. Citation2007; Cheng et al. Citation2016; Ng et al. Citation2017; Ma et al. Citation2019). Likewise, for the cognitive domains: verbal function and language skills [N = 3 studies; std mean difference: 0.19 [-0.02; 0.40], P = 0.07, N = 362] (Cheng et al. Citation2016; Ng et al. Citation2017; Ma et al. Citation2019), and conception formation and reasoning [N = 2 studies; std mean difference: 0.13 [-0.12; 0.37], P = 0.31, N = 263] (Cheng et al. Citation2016; Ma et al. Citation2019). However, meta-analysis showed significant positive effects of micronutrient supplementation on memory [N = 2 studies; std mean difference: 0.41 [0.11; 0.70], P = 0.01, N = 182] (Cheng et al. Citation2016; Ng et al. Citation2017), and construction and motor performance [N = 3 studies; std mean difference: 0.38 [-0.17; 0.58], P < 0.001, N = 362] (Ng et al. Citation2017; Cheng et al. Citation2016; Ma et al. Citation2019). Results of the cognitive domain specific meta-analyses are shown in .
Table 2. Summary table of the meta-analyses of the effect of specific nutrition intervention on global cognitive performance and domain specific cognitive performance.
Chicken essence
Four (Azhar, Zubaidah, and Norjan Citation2008; Azhar et al. Citation2013; Chan et al. Citation2016; Zain and Syedsahiljamalulail Citation2003) of the five (Nagai et al. Citation1996; Chan et al. Citation2016; Zain and Syedsahiljamalulail Citation2003; Azhar et al. Citation2013; Azhar, Zubaidah, and Norjan Citation2008) studies investigating the effect of chicken essence on cognitive performance found a positive effect. Three of these studies were conducted by the same research group, and provided a healthy student population [N = 108 and N = 69] (Zain and Syedsahiljamalulail Citation2003; Azhar, Zubaidah, and Norjan Citation2008), or healthy subjects between 35 and 65 years [N = 20] (Azhar et al. Citation2013), with daily chicken essence drinks for the duration of two and six weeks, respectively. A beneficial effect on cognitive performance was found for the domains: attention and orientation; memory; and conception formation and reasoning. The fourth study [N = 102] included young people under work related stress, a significant positive effect on memory performance was only observed in participants with high depression scores (Chan et al. Citation2016). The study that did not find a significant positive effect included 16 male students, aged 18 to 24 years, with chicken essence for seven days and tested memory and conception formation and reasoning (Nagai et al. Citation1996).
Meta-analysis showed a non-significant effect for chicken essence on the cognitive domains: attention and orientation [N = 3 studies; std mean difference: 0.06 [-0.18; 0.29], P = 0.62, N = 279] (Chan et al. Citation2016; Azhar, Zubaidah, and Norjan Citation2008; Zain and Syedsahiljamalulail Citation2003), memory [N = 2 studies; std mean difference: 0.05 [-0.26; 0.36], P = 0.74, N = 210] (Chan et al. Citation2016, Zain and Syedsahiljamalulail Citation2003), and conception formation and reasoning [N = 2 studies; std mean difference: 0.23 [-0.07; 0.52], P = 0.14, N = 177] (Azhar, Zubaidah, and Norjan Citation2008; Zain and Syedsahiljamalulail Citation2003). Results of the cognitive domain specific meta-analyses are shown in .
Nutritional and lifestyle counseling
Three (Johari et al. Citation2014; Lee et al. Citation2014; Rosli et al. Citation2017) of the four (Rosli et al. Citation2017; Kwok et al. Citation2012; Lee et al. Citation2014; Johari et al. Citation2014) studies investigating the effect of behavioral interventions to promote a healthy diet showed a significant beneficial effect on cognitive performance. One intervention [N = 35] consisted of monthly lifestyle and dietary sessions over a one year period in older individuals with MCI [beneficial effect on cognitive domain: construction and motor performance] (Johari et al. Citation2014). The second intervention [N = 175] consisted of bimonthly health worker-initiated visits focused on lifestyle and dietary counseling and rewards for adherence to the program for 18 months in community-dwelling older individuals [beneficial effect on global cognitive performance] (Lee et al. Citation2014). Advice was given on the importance of engaging in moderate physical, cognitive, and social activities, as well as moderate alcohol consumption, smoking cessation, and maintaining a healthy diet. The third study [N = 256] consisted of a multidimensional programme with group exercises, nutritional education, oral care education, and psychological support, for six weeks in older individuals from poor urban settings [beneficial effect on cognitive domain: attention and orientation; and memory] (Rosli et al. Citation2017). The study [N = 307] that did not find a significant positive effect promoted the consumption of fruit and vegetables (emphasis on green leafy and cruciferous), fish and avoidance of salty foods in very old individuals living in care homes (Kwok et al. Citation2012).
Meta-analysis showed a non-significant effect for nutritional and lifestyle counseling on global cognitive performance [N = 2 studies; std mean difference: −0.03 [-0.50; 0.45], P = 0.91, N = 340, ] (Kwok et al. Citation2012; Johari et al. Citation2014), and the cognitive domains: attention and orientation [N = 2 studies; std mean difference: 0.18 [-0.06; 0.42], P = 0.15, N = 291] (Johari et al. Citation2014; Rosli et al. Citation2017), and memory [N = 2 studies; std mean difference: 0.11 [-0.13; 0.35], P = 0.35, N = 291] (Rosli et al. Citation2017; Johari et al. Citation2014). Results of the cognitive domain specific meta-analyses are shown in .
EPA/DHA supplementation
Four studies investigating the effect of EPA/DHA supplementation found a beneficial effect on global cognitive performance (Chiu et al. Citation2008; Bo et al. Citation2017; Zhang et al. Citation2017; Lee et al. Citation2013). However, one of these studies assessed global cognitive performance with two different tools [ADAS-COG and MMSE], and only one tool showed this result [ADAS-COG] (Chiu et al. Citation2008). The first study [N = 35] provided older individuals with cognitive impairment twice daily with omega-3 capsules [total daily dosage of 1080 mg of EPA and 720 mg of DHA) for 24 weeks (Chiu et al. Citation2008). The second study [N = 86] provided participants with MCI with four 1 g capsules every nine days [total daily dosage of 480 mg of DHA and 720 mg of EPA] for six months (Bo et al. Citation2017). And, the third study [N = 240] provided older individuals with MCI daily with 2 g of DHA for 12 months. A fourth study only found a significant beneficial effect of the intervention for the cognitive domains: attention and orientation, and memory [global cognition was assessed by MMSE tool] (Lee et al. Citation2013). This study [N = 35] provided older individuals with MCI daily with 430 mg of DHA and 150 mg of EPA for 12 months (Lee et al. Citation2013).
Meta-analysis showed a significant positive effect of EPA/DHA supplementation on cognitive domain of attention and orientation [N = 3 studies; std mean difference: 0.75 [0.54; 0.96], P < 0.001, N = 344] (Bo et al. Citation2017; Zhang et al. Citation2017; Lee et al. Citation2013). Non-significant effects where shown for the cognitive domains: global cognitive performance [N = 4 studies; std mean difference: 0.57 [-0.01; 1.14], P = 0.06, N = 373, ] (Chiu et al. Citation2008; Bo et al. Citation2017; Zhang et al. Citation2017; Lee et al. Citation2013), memory [N = 2 studies; std mean difference: 0.15 [-0.39; 0.69], P = 0.58, N = 104] (Bo et al. Citation2017; Lee et al. Citation2013), construction and motor performance [N = 2 studies; std mean difference: 0.04 [-0.20; 0.28], P = 0.73, N = 258] (Zhang et al. Citation2017, Lee et al. Citation2013), and conception formation and reasoning [N = 3 studies; std mean difference: 0.04 [-0.30; 0.38], P = 0.82, N = 344] (Bo et al. Citation2017; Zhang et al. Citation2017; Lee et al. Citation2013). Results of the cognitive domain specific meta-analyses are shown in .
Figure 3. Forest Plot showing the effects of different nutritional interventions and overall main effect on measures of global cognitive performance. *The two studies included in this category tested effects of soy isoflavone (Ho 2007) and ellagic acid (Liu Citation2018) supplementation on global cognitive performance. Additional details on number of subjects included in each analysis and heterogeneity statistics are provided in .
Other interventions (L-carnitine/soy-isoflavone/soybean supplements/caffeinated alcoholic beverage, ellagic acid)
Only one study provided participants with L-carnitine supplements, and did not find a significant beneficial effect on global cognitive performance assessed by MMSE (Badrasawi et al. Citation2016). Similarly, no significant findings were identified for the one study providing participants with soy-isoflavone supplements (Ho et al. Citation2007). A study supplementing MCI participants with a mixture of fermented soybean powder and Lactobacillus plantarum showed greater improvements in combined cognitive functions, particularly for attention, in comparison to the placebo group (Hwang et al. Citation2019). Another study investigated if a caffeinated alcoholic beverage [CAB] would decrease cognitive performance, as this is often consumed in Taiwan. Consuming CAB caused significant impairments in fine and crude motor functions (Cheng et al. Citation2017). Finally, supplementation with Ellagic acid among overweight participants aged between 45 and 55 years showed enhanced cognitive function compared to the placebo (Liu et al. Citation2018). Overall, the meta-analysis showed non-significant effects of these interventions on cognitive function ().
Heterogeneity
There was significant heterogeneity between the studies included in the meta-analyses. Namely for the effect of: [1] micronutrient supplementation [N = 4 studies; Cochran’s Q p < 0.001, I2=81%], EPA/DHA supplementation [N = 4 studies; Cochran’s Q p < 0.001, I2=83%], and all type of interventions combined [N = 11 studies; Cochran’s Q p < 0.001, I2=88%], on global cognitive performance; and [2] micronutrient supplementation [N = 2 studies; Cochran’s Q p = 0.10, I2=62%], and all types of intervention combined [N = 12 studies; Cochran’s Q p < 0.001, I2=71%], on the cognitive domain: attention and orientation.
Risk of publication bias
Only a small number of studies [i.e. 2–5 studies] were available for each intervention specific meta-analysis, assessing the effect on global and domain specific cognitive performance. It should therefore be taken into account that it was not possible to properly assess publication bias which was only assessed for each domain by combining all interventions. The funnel plots and results of the Egger’s regression test are shown in the online supplementary material. Publication bias seemed to exist for the meta-analysis of the effect of EPA/DHA supplementation on global cognitive performance (n = 4 studies; p = 0.01).
Stratified analysis and meta-regression
Nutritional interventions were associated with greater benefits on global cognitive performance in subjects with cognitive impairment at baseline [N = 9 studies; std mean difference: 0.48 ± 0.17, P = 0.006] and older age groups (≥60 years) [N = 11 studies; std mean difference: 0.36 ± 0.17, P = 0.04]. Similarly, nutritional interventions showed greater effects in subjects with cognitive impairment for specific domains including attention, processing speed, and working memory, memory, verbal functions and language skills and construction and motor performance. Older subjects showed greater improvements for attention, processing speed, and memory (). Overall, the meta-regression showed that study duration was not significantly associated with the effect size (p = 0.41, ).
Figure 4. Meta-regression to evaluate the association between study duration and changes in cognitive function in all studies (n = 18). All cognitive tests were combined in this analysis.
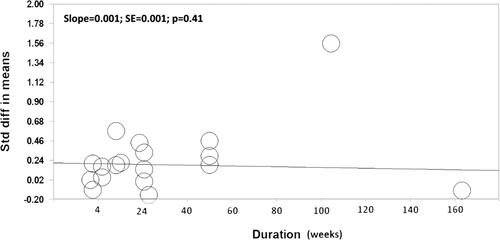
Table 3. Stratified analysis to evaluate whether the cumulative effects of the nutritional interventions on cognitive domains are modified by cognitive status and age.
Discussion
To date, 24 RCTs have investigated the effect of nutritional interventions on cognitive performance in LMICs in East-Asia. The quality of the studies was mixed, and the sample size was relatively small [median = 100]. Five different types of interventions could be identified, including micronutrient supplementation; nutritional and lifestyle counseling; essential fatty acids supplementation, chicken essence supplementation and nutraceuticals (i.e., flavonoids, polyphenols, carnitine). The majority of studies showed significant beneficial effects of the interventions on cognitive performance. However, these effects were not consistent across the different neuropsychological tests used in each study. As a result, the meta-analysis only showed significant beneficial effects for EPA/DHA and micronutrient supplementations on specific cognitive domains including attention and orientation, perception, verbal functions and language skills. In addition, the meta-analysis showed that the effects of the nutritional interventions were greater in older subjects with cognitive impairment.
Most of the micronutrient supplement trials implemented the intervention based on the hypothesis that lowering homocysteine plasma levels, by B-vitamin supplementation, could prevent cognitive impairment (Cheng et al. Citation2016; Kwok et al. Citation2011; Ma et al. Citation2019; Sun et al. Citation2007). Homocysteine plasma levels are an indicator of vitamin B6, B9 and B12 status in the body (Smith et al. Citation2018), and a recently published international consensus statement concluded that there is strong evidence that hyper-homocysteine is a modifiable risk factor for cognitive decline and dementia in older people (Smith et al. Citation2018). This statement was among others based on previously published reviews into this area, and three good quality clinical trials, from high income countries (Durga et al. Citation2007; Aisen et al. Citation2008; de Jager et al. Citation2012). This first trial [FACIT trial] assessed the effect of three-year folic acid supplementation [B9] in elderly with elevated plasma homocysteine levels, but normal serum vitamin B12 at screening (Durga et al. Citation2007). The intervention significantly improved cognitive performance in comparison to the control group. The second trial [ADCS] assessed the effect of 18 month high-dose folate [B9], vitamin B6, and vitamin B12 supplementation, in moderate Alzheimer patients with normal folic acid, vitamin B12, and homocysteine levels (Aisen et al. Citation2008). This intervention did not significantly improve cognitive performance in comparison with the control group. And, the third trial [VITACOG] assessed the effect of two year folic acid [B9], vitamin B6 and B12 supplementation in elderly (de Jager et al. Citation2012). Primarily significant beneficial effects were found for the intervention in participants with elevated homocysteine levels [≥11.3 mmol/L], and not for those with normal levels. The results of the three RCTs from HICs are in agreement with studies included in this review. Namely, the study in elderly with elevated homocysteine levels showed significant beneficial effects for B-vitamin supplementation on cognitive performance (Cheng et al. Citation2016), and the study in elderly with normal and elevated homocysteine levels, showed only a significant beneficial effect in those participants with elevated homocysteine levels (Kwok et al. Citation2011). Unfortunately, plasma homocysteine levels were not evaluated in three other similar trials included in this review (Ma et al. Citation2016; Ng et al. Citation2017; Sun et al. Citation2007). However, based on previous findings, it is likely that the only study unable to show significant beneficial effects of B-vitamin supplementation included primarily individuals with normal homocysteine levels (Sun et al. Citation2007).
Overall, the RCTs, from HICs and LMICs in East-Asia, indicate that B-vitamin supplementation may be able to reduce age-related cognitive decline in older individuals with elevated homocysteine levels. At the same time, it is known that vitamin and mineral status is relatively low in the general population of East-Asia (Chaparro, Oot, and Sethuraman Citation2014), however, specific data on B-vitamin status in East-Asia is currently not available. Therefore, a next important step is to evaluate the prevalence of hyper-homocysteinemia in these countries, and identify the relevance of this type of intervention and the potential target groups. Likewise, large trials are needed to identify at which homocysteine level supplementation should start and evaluate the feasibility [e.g. costs/distribution/compliance] and efficacy [e.g. degree of lowering cognitive decline and/or dementia incidence] to reduce dementia incidence in these countries.
The nutritional and lifestyle counseling trials were designed under the hypotheses of a close association between healthy diet and lifestyle, and promotion of cardiovascular health and putative effects on brain health. Namely, there is growing evidence that dementia and cardiovascular diseases share common risk factors, supported by evidence that dementia incidence is declining in Western countries, due to successful treatments of cardiovascular diseases such as diabetes and high blood pressure (Qiu and Fratiglioni Citation2015). At the same time, in developing economies, the incidence of Non-Communicable Diseases (NCDs) is increasing due to tobacco use and lifestyle factors including unhealthy diets and inadequate physical activity (Dans et al. Citation2011). In addition, health care systems are not yet ready to deliver the essential chronic care that is needed (Dans et al. Citation2011).
To date, in East-Asia, only one trial has assessed the effect of nutritional counseling [i.e. promotion of: two portions of fruit a day; three portions of vegetables a day; five portions of fish a week; and avoidance of salty foods] on cognitive performance in older participants (Kwok et al. Citation2012). In contrast to the growing evidence between diet and dementia, this trial did not find any significant beneficial effect of the intervention. This could be explained by one of the main challenges associated with these trials: adherence to the diet. In this trial, Kwok and colleagues (Kwok et al. Citation2012), were only able to significantly increase consumption of fruit in comparison to the control group. A large trial [N = 1279] conducted in five different European countries shows something similar (Marseglia et al. Citation2018). This trial was based on promotion of a Mediterranean like diet, and, as well, no significant differences on cognitive performance were found between intervention and control group. However, sensitivity analysis showed that participants with the highest adherence to the diet had significantly improved cognitive performance in comparison to participants with the lowest adherence to the diet. In addition, and in favor of nutritional counseling, another large trial [N = 447] conducted in Spain [PREDIMED trial] was able to find a significant improvement in cognitive performance by promoting a Mediterranean diet (Valls-Pedret et al. Citation2015). It is important to note, however, that PREDIMED was conducted in a Mediterranean country where perhaps this diet may be easier to adhere to, and where Mediterranean diet adherence score was relatively high at baseline.
To date, in East-Asia, three trials assessed the effect of multi-component nutritional and lifestyle counseling interventions on cognitive performance among elderly individuals. All of these studies were able to find significant improvements in cognitive performance in the intervention group in comparison to the control group. In Europe, three multidomain lifestyle-based prevention trials have been completed: the FINGER trial (Ngandu et al. Citation2015), the French Multidomain Alzheimer Preventive Trial (MAPT) (Andrieu et al. Citation2017), and the Dutch Prevention of Dementia by Intensive Vascular Care (PreDIVA) (Moll van Charante et al. Citation2016). The FINGER study showed significant beneficial effects on cognitive performance among 1200 elderly individuals in Finland over a period of 2 years (Ngandu et al. Citation2015). Exploratory subgroup analyses of MAPT and PreDIVA also suggested cognitive improvements in subsets of participants with increased risk of dementia (Moll van Charante et al. Citation2016; Andrieu et al. Citation2017). These studies indicate that administering multidomain interventions to older at-risk adults may be feasible and effective. However, despite these positive results, the evidence for one specific multi-component strategy is lacking, due to large heterogeneity between the tested interventions. Future trials should therefore confirm which components of these interventions are most feasible and effective for the prevention of cognitive impairment and identify if they are able to decline dementia incidence in East-Asia.
The omega-3 fatty acids, EPA/DHA, are widely studied for their potential beneficial effect on health, and as well for their effect on cognitive performance. EPA and DHA are essential fatty acids, incorporated in cell membranes especially in the brain, and play a role in anti-inflammatory processes of the body (Swanson, Block, and Mousa Citation2012). Their intake is primarily dependent on fish consumption, and this varies widely across populations (Forsyth, Gautier, and Salem Citation2016). To date, RCTs conducted in high income countries show mixed results on the effect of supplementation of essential omega-3 fatty acids on cognitive performance in older age participants [i.e. significant beneficial effect in 2 of 4 RCTs] (van de Rest et al. Citation2008; Dangour et al. Citation2010; Külzow et al. Citation2016; Sinn et al. Citation2012). In contrast, the four RCTs conducted in East-Asia all showed significant beneficial effects for EPA/DHA supplementation, and as well a significant beneficial effect was found for the meta-analysis assessing the effect on the cognitive domain: attention and orientation. It is not expected that this type of intervention is especially beneficial in Asian countries, as average DHA intake is already relatively high in comparison to Western countries (Forsyth, Gautier, and Salem Citation2016). However, the consistent beneficial effect of EPA/DHA supplementation in trials from East-Asia could be explained by the sample population used. All trials conducted in East-Asia were conducted in elderly with MCI, in contrast to the RCTs from HICs including primarily cognitive healthy elderly (van de Rest et al. Citation2008;, Dangour et al. Citation2010; Külzow et al. Citation2016). Only one study conducted in a HIC incorporated this specific target group, and in agreement, this study showed a significant beneficial effect of EPA/DHA supplementation on cognitive performance (Sinn et al. Citation2012).
Following the above findings, individuals with MCI are potentially a prime target population for dementia prevention research. Cognitive performance is expected to decline at a higher rate in individuals with MCI, than in cognitively healthy elderly, and therefore this target group may benefit from an early intervention to detect any cognitive decline with neuropsychological tests (Whiteley et al. Citation2020). However, a current disadvantage of including individuals with MCI in RCTs is the lack of consistency in criteria and implementation of MCI, and therefore difficulty in comparing studies using this subgroup. Seven of the studies included in this review included participants with MCI, and an overview, given in Online Supplementary Document, shows clearly the heterogeneity in criteria and implementation of MCI between studies. A further challenge with this clinical cohort is ensuring the sensitivity and appropriateness of the cognitive assessments used in detecting cognitive change within studies. As MCI is a tangible condition, that can affect memory and/or other cognitive domains, it is imperative that suitable cognitive testing is implemented to enable a more precise approach to intervention studies (Jack et al. Citation2018). The inclusion of biomarker analyses and cognitive imaging techniques in dietary intervention studies may add further robustness to detecting cognitive change and for understanding associated mechanisms (McGrattan et al. Citation2018). Nonetheless, individuals with MCI are probably the population that should be targeted for dementia prevention research, but in future trials, it should be aimed to make use of consistent criteria and implementation of MCI. Overall, the RCTs from East-Asia indicate that a population with a relatively high DHA intake could still benefit from EPA/DHA supplementation for the prevention of cognitive impairment. Therefore, EPA/DHA supplementation is a potential promising strategy in East-Asia for prevention of cognitive impairment, but large trials are needed verify the findings, and identify if supplementation is able to lower dementia incidence.
Chicken essence is made by cooking whole chicken for several hours at a high temperature, and in Chinese traditional medicine it is used for strengthening the musculoskeletal system and enhancing vigor (Chan et al. Citation2016). As well, it is hypothesized that chicken essence has an anti-stress effect by regulating cortisol levels and activating the brain histaminergic system, which could help maintain cognitive performance (Chan et al. Citation2016). A systematic review and meta-analysis into chicken essence and cognitive performance (Teoh et al. Citation2016), included the same studies as in our review plus three more from Japan and the United Kingdom (Konagai et al. Citation2013; Young, Benton, and Carter Citation2015; Yamano et al. Citation2013). Their overall conclusion was that, although significant improvements were found for single neuropsychological test scores, the overall quality of the trials was low, and thus there is lack of evidence for the enhancing effect of chicken essence on cognitive performance (Teoh et al. Citation2016). We did not find any additional evidence in favor of chicken essence and conclude that based on the current data chicken essence does not seem a promising strategy for prevention of cognitive impairment.
The five remaining studies in our review provided participants with L-carnitine supplements (Badrasawi et al. Citation2016), soy-isoflavone supplements (Ho et al. Citation2007), Ellagic acid (Liu et al. Citation2018), fermented soybean powder and Lactobacillus plantarum C29 (Hwang et al. Citation2019) and a caffeinated alcoholic beverage (Chen et al. Citation2017). Only limited recent studies investigated the effect of L-carnitine supplementation on cognitive performance in elderly. A meta-analysis from 2003, of 21 RCTs assessing the effect of L-carnitine on cognitive performance in individuals with MCI or mild Alzheimer’s disease, showed a significant beneficial effect on cognitive performance in favor of the intervention (Montgomery, Thal, and Amrein Citation2003). However, as reported in the paper, the results need to be interpreted with caution as there was great heterogeneity between study methods and there was potential risk of bias. Two somewhat more recent RCTs, conducted by the same research group, in Italian elderly with the fatigue syndrome, did find significant improvements of global cognitive performance [MMSE] in favor of L-carnitine supplementation over a study period of 6 months (Malaguarnera et al. Citation2007; Malaguarnera et al. Citation2008). The hypothesis is that amino acid deficiency, including L-carnitine which is involved in cell energy metabolism and often seen in frail elderly, will have beneficial effects on the cognitive performance of elderly individuals. Previous mentioned studies are in favor of this hypothesis; however, this was not seen in the single RCT conducted in frail elderly from East-Asia. Although, the study from East-Asia was relatively small [N = 50], and of short duration [=10 weeks], which could have led to the inability to detect a beneficial effect of L-carnitine supplementation (Badrasawi et al. Citation2016). Overall, more large and high quality RCTs are needed to show if L-carnitine is a promising strategy to prevent cognitive impairment in frail elderly of East-Asia.
The effect of soy-isoflavone supplementation on health is primarily assessed in post-menopausal women. Namely, soy-isoflavone displays estrogen like effects, and it is suggested that supplementation can counteract some of the postmenopausal symptoms like accelerated decline in bone mineral density and cognition. A meta-analysis from 2014 indeed shows that soy-isoflavone supplementation significantly improves global cognition and visual memory (Cheng et al. Citation2015). The quality of the studies was mixed, however, a high quality study incorporated in the meta-analysis in 350 postmenopausal women followed over 30 months showed individually significant beneficial effect on cognitive performance in favor of soy-isoflavone supplementation (Henderson et al. Citation2012). In contrast, the study included in our review, and to our knowledge the only RCT assessing the effect of soy-isoflavone supplementation in an Asian population (i.e. China), did not show a significant beneficial effect on cognition in favor of the intervention (Ho et al. Citation2007). Soy is a staple food in many Asian countries, and supplementation of soy-isoflavone is therefore potentially not beneficial in this population. Large high-quality trials could confirm this, however, it seems more effective to focus on nutrients that are lacking in the general diet of East-Asian population, than nutrients that are already consumed in a relatively high amount.
One trial examined the effects of Ellagic acid supplementation among healthy adults aged between 45 and 55 years (Liu et al. Citation2018). Ellagic acid is a non flavonoid polyphenol, which is known to have antioxidant (Landete Citation2011) and anti-inflammatory properties (Rosillo et al. Citation2012). There have been minimal in vivo studies conducted to investigate the neuroprotective role of Ellagic acid, however there are indications from animal studies that Ellagic acid may improve cognitive behaviors via modulating oxidative stress and/or inflammatory pathways (Jha, Panchal, and Shah Citation2018). Furthermore, one RCT investigated the effect of consuming caffeinated alcoholic beverages on cognitive performance (Cheng et al. Citation2017). This RCT was included in the review, as one of our criteria was to include all studies assessing the effect of nutrition on cognitive performance. However, in contrast with all other RCTs, this study was set up to investigate if caffeine could antagonize the harmful effects of low dose alcohol on the brain, as this drink is often consumed during work in Taiwan. And, this study showed that caffeine was not able to antagonize the effects of alcohol on the brain.
The strength of this review is that, for the first time, a complete overview is given of nutrition intervention trials for the prevention of cognitive impairment in developing economies of East-Asia. As the increase of dementia prevalence in East-Asia will have a tremendous effect on the health care and society in this region, this review gives an update on promising strategies for lowering the incidence of dementia and the future studies that are needed. The limitation of the included studies are the small number of studies per intervention, variation in age of participants included in studies, the heterogeneity of the interventions, the heterogeneity in neuropsychological tests used to assess cognitive performance, the mixed quality of the studies, and the exclusion of studies not written in English. Therefore, strong evidence for certain strategies or interventions cannot be given, however, it shows the urgent need for more high quality intervention trials in this region.
In conclusion, several promising strategies, such as B-vitamin and EPA/DHA supplementation, seem to be able to decrease age-related cognitive decline in East-Asia. These nutritional strategies appear to be more effective in older subjects with impaired cognitive function. Large good quality long term trials are warranted to confirm these findings, and to identify if these dietary interventions are feasible and effective to decrease dementia incidence in East-Asian countries.
Supplemental Material
Download MS Word (253.2 KB)Acknowledgements
M.S designed the manuscript, developed the search terms and inclusion and exclusion criteria. C.V.A and A.N conducted the searches and both authors screened the final papers. A.M cross checked the data extraction. M.S conducted the analysis and wrote the paper with C.V.A. A.M critically reviewed the manuscript and edited. M.S had responsibility for the final content. All authors reviewed the manusscipt before submission.
Disclosure statement
No potential conflict of interest was reported by the authors
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Aisen, P. S., L. S. Schneider, M. Sano, R. Diaz-Arrastia, C. H. van Dyck, M. F. Weiner, T. Bottiglieri, S. Jin, K. T. Stokes, R. G. Thomas, et al. 2008. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. Jama 300 (15):1774–83. doi: 10.1001/jama.300.15.1774.
- Andrieu, S., S. Guyonnet, N. Coley, C. Cantet, M. Bonnefoy, S. Bordes, L. Bories, M. N. Cufi, T. Dantoine, J. F. Dartigues, et al. 2017. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. The Lancet. Neurology 16 (5):377–89. doi: 10.1016/s1474-4422(17)30040-6.
- Azhar, M. Z., J. O. Zubaidah, and K. O. N. Norjan. 2008. Effect of taking chicken essence on cognitive functioning of normal stressed human volunteers Malays. Journal of Medical Sciences & Health 4:57–68.
- Azhar, Z. M., J. O. Zubaidah, K. O. Norjan, C. Y. Zhuang, and F. Tsang. 2013. A pilot placebo-controlled, double-blind, and randomized study on the cognition-enhancing benefits of a proprietary chicken meat ingredient in healthy subjects. Nutrition Journal 12 (1):121. doi: 10.1186/1475-2891-12-121.
- Badrasawi, M., S. Shahar, Z. Abd Manaf, N. F. Rajab, and D. Kas. 2016. Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: A double-blind,randomized,placebo-controlled clinical trial. Clinical Interventions in Aging 11:1675–86.
- Bo, Y., X. Zhang, Y. Wang, J. You, H. Cui, Y. Zhu, W. Pang, W. Liu, Y. Jiang, and Q. Lu. 2017. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 9 (1):54.
- Caracciolo, B., W. Xu, S. Collins, and L. Fratiglioni. 2014. Cognitive decline, dietary factors and gut-brain interactions. Mechanisms of Ageing and Development 136-137:59–69. doi: 10.1016/j.mad.2013.11.011.
- Chan, L., H. M. Wang, K. Y. Chen, Y. C. Lin, P. J. Wu, W. L. Hsieh, Y. R. Chen, C. P. Liu, H. Y. Tsai, Y. R. Chen, et al. 2016. Effectiveness of essence of chicken in improving cognitive function in young people under work-related stress: A randomized double-blind trial. Medicine 95 (19):e3640. doi: 10.1097/MD.0000000000003640.
- Chaparro, C.,. L. Oot, and K. Sethuraman. 2014. Overview of the nutrition situation in seven countries in Southeast Asia.
- Chen, N., M. Yang, M. Zhou, J. Xiao, J. Guo, and L. He. 2017. L-carnitine for cognitive enhancement in people without cognitive impairment. The Cochrane Database of Systematic Reviews 3:Cd009374. doi: 10.1002/14651858.CD009374.pub3.
- Cheng, D., H. Kong, W. Pang, H. Yang, H. Lu, C. Huang, and Y. Jiang. 2016. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutritional Neuroscience 19 (10):461–6. doi: 10.1179/1476830514Y.0000000136.
- Cheng, P. F., J. J. Chen, X. Y. Zhou, Y. F. Ren, W. Huang, J. J. Zhou, and P. Xie. 2015. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause (New York, N.Y.) 22 (2):198–206. doi: 10.1097/gme.0000000000000290.
- Cheng, W.-J., C.-C. Lin, Y. Cheng, and M.-C. Huang. 2017. Effects of caffeinated alcoholic beverages with low alcohol and high caffeine content on cognitive and motor functions. Human Psychopharmacology: Clinical and Experimental 32 (6):e2634 doi:10.1002/hup.2634.
- Chiu, C. C., K. P. Su, T. C. Cheng, H. C. Liu, C. J. Chang, M. E. Dewey, R. Stewart, and S. Y. Huang. 2008. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Progress in Neuro-Psychopharmacology & Biological Psychiatry 32 (6):1538–44. doi: 10.1016/j.pnpbp.2008.05.015.
- Dangour, A. D., E. Allen, D. Elbourne, N. Fasey, A. E. Fletcher, P. Hardy, G. E. Holder, R. Knight, L. Letley, M. Richards, et al. 2010. Effect of 2-yn− 3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: A randomized, double-blind, controlled trial. The American Journal of Clinical Nutrition 91 (6):1725–32.
- Dans, A., N. Ng, C. Varghese, E. Shyong Tai, R. Firestone, and R. Bonita. 2011. The rise of chronic non-communicable diseases in southeast Asia: Time for action. The Lancet 377 (9766):680–9.
- de Jager, C. A., A. Oulhaj, R. Jacoby, H. Refsum, and A. Smith. 2012. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. International Journal of Geriatric Psychiatry 27 (6):592–600.
- Durga, J., M. P. J. van Boxtel, E. G. Schouten, F. J. Kok, J. Jolles, M. B. Katan, and P. Verhoef. 2007. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. The Lancet 369 (9557):208–16. doi: https://doi.org/10.1016/S0140-6736.(07)60109-3. doi: 10.1016/S0140-6736(07)60109-3.
- Forsyth, S., S. Gautier, and N. Salem. Jr. 2016. Global estimates of dietary intake of docosahexaenoic acid and arachidonic acid in developing and developed countries. Annals of Nutrition and Metabolism 68 (4):258–67.
- Frisardi, V., F. Panza, D. Seripa, B. P. Imbimbo, G. Vendemiale, A. Pilotto, and V. Solfrizzi. 2010. Nutraceutical properties of Mediterranean diet and cognitive decline: Possible underlying mechanisms. Journal of Alzheimer's Disease: JAD 22 (3):715–40. doi: 10.3233/jad-2010-100942.
- Henderson, V. W., J. A. St John, H. N. Hodis, N. Kono, C. A. McCleary, A. A. Franke, and W. J. Mack. 2012. Long-term soy isoflavone supplementation and cognition in women: A randomized, controlled trial. Neurology 78 (23):1841–8. doi: 10.1212/WNL.0b013e318258f822.
- Higgins, J. P. T., D. G. Altman, and J. A. C. Sterne. 2011. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 (updated March 2011). ed. London: The Cochrane Collaboration.
- Higgins, J. P. T., and S. Green. 2011. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011].
- Higgins, J. P. T., S. G. Thompson, J. J. Deeks, and D. G. Altman. 2003. Measuring inconsistency in meta-analyses. BMJ (Clinical Research ed.) 327 (7414):557–60. doi: 10.1136/bmj.327.7414.557.
- Ho, S. C., A. S. Chan, Y. P. Ho, E. K. So, A. Sham, B. Zee, and J. L. Woo. 2007. Effects of soy isoflavone supplementation on cognitive function in Chinese postmenopausal women: A double-blind, randomized, controlled trial. Menopause (New York, N.Y.) 14 (3 Pt 1):489–99. doi: 10.1097/GME.0b013e31802c4f4f.
- Hwang, Y. H., S. Park, J. W. Paik, S. W. Chae, D. H. Kim, D. G. Jeong, E. Ha, M. Kim, G. Hong, S. H. Park, et al. 2019. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 11 (2):305. doi: 10.3390/nu11020305.
- Jack, C. R., Jr., D. A. Bennett, K. Blennow, M. C. Carrillo, B. Dunn, S. B. Haeberlein, D. M. Holtzman, W. Jagust, F. Jessen, J. Karlawish, et al. 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 14 (4):535–62. doi: 10.1016/j.jalz.2018.02.018.
- Jha, A. B., S. S. Panchal, and A. Shah. 2018. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer's disease. Pharmacology, Biochemistry, and Behavior 175:33–46. doi: 10.1016/j.pbb.2018.08.007.
- Johari, S. M., S. Shahar, T. P. Ng, and R. Rajikan. 2014. A preliminary randomized controlled trial of multifaceted educational intervention for mild cognitive impairment among elderly Malays in Kuala Lumpur. International Journal of Gerontology 8 (2):74–80.
- Konagai, C., H. Watanabe, K. Abe, N. Tsuruoka, and Y. Koga. 2013. Effects of essence of chicken on cognitive brain function: A near-infrared spectroscopy study. Bioscience, Biotechnology, and Biochemistry 77 (1):178–81. doi: 10.1271/bbb.120706.
- Külzow, N., A. V. Witte, L. Kerti, U. Grittner, J. P. Schuchardt, A. Hahn, and A. Flöel. 2016. Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. Journal of Alzheimer's Disease: JAD 51 (3):713–25. doi: 10.3233/JAD-150886.
- Kwok, T. C., L. C. Lam, M. M. Sea, W. Goggins, and J. Woo. 2012. A randomized controlled trial of dietetic interventions to prevent cognitive decline in old age hostel residents. European Journal of Clinical Nutrition 66 (10):1135–40. doi: 10.1038/ejcn.2012.117.
- Kwok, T., J. Lee, C. B. Law, P. C. Pan, C. Y. Yung, K. C. Choi, and L. C. Lam. 2011. A randomized placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clinical Nutrition (Edinburgh, Scotland) 30 (3):297–302. doi: 10.1016/j.clnu.2010.12.004.
- Landete, J. M. 2011. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Research International 44 (5):1150–60. v. 2011 v.44 no.5. doi: 10.1016/j.foodres.2011.04.027.
- Lee, K. S., Y. Lee, J. H. Back, S. J. Son, S. H. Choi, Y. K. Chung, K. Y. Lim, J. S. Noh, S. H. Koh, B. H. Oh, et al. 2014. Effects of a multidomain lifestyle modification on cognitive function in older adults: An eighteen-month community-based cluster randomized controlled trial. Psychotherapy and Psychosomatics 83 (5):270–8.
- Lee, L. K., S. Shahar, A.-V. Chin, and N. A. M. Yusoff. 2013. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): A 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology 225 (3):605–12. doi: 10.1007/s00213-012-2848-0.
- Lezak, M., D. B. Howieson, E. D. Bigler, and D. Tranel. 2012. Neuropsychological Assessment. 5th ed. New York: Oxford University Press.
- Liberati, A., D. G. Altman, J. Tetzlaff, C. Mulrow, P. C. Gøtzsche, J. P. A. Ioannidis, M. Clarke, P. J. Devereaux, J. Kleijnen, and D. Moher. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (Clinical Research ed.) 339:b2700 doi: 10.1136/bmj.b2700.
- Liu, Y., S. Yu, F. Wang, H. Yu, X. Li, W. Dong, R. Lin, and Q. Liu. 2018. Chronic administration of ellagic acid improved the cognition in middle-aged overweight men. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquee, Nutrition et Metabolisme 43 (3):266–73. doi: 10.1139/apnm-2017-0583.
- Livingston, G., A. Sommerlad, V. Orgeta, S. G. Costafreda, J. Huntley, D. Ames, C. Ballard, S. Banerjee, A. Burns, J. Cohen-Mansfield, et al. 2017. Dementia prevention, intervention, and care. The Lancet 390 (10113):2673–734.
- Ma, F., Q. Li, X. Zhou, J. Zhao, A. Song, W. Li, H. Liu, W. Xu, and G. Huang. 2019. Effects of folic acid supplementation on cognitive function and Abeta-related biomarkers in mild cognitive impairment: A randomized controlled trial. European Journal of Nutrition 58 (1):345–56. doi: 10.1007/s00394-017-1598-5.
- Ma, F., T. Wu, J. Zhao, F. Han, A. Marseglia, H. Liu, and G. Huang. 2016. Effects of 6-month folic acid supplementation on cognitive function and blood biomarkers in mild cognitive impairment: A randomized controlled trial in China. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 71 (10):1376–83. doi: 10.1093/gerona/glv183.
- Malaguarnera, M., L. Cammalleri, M. P. Gargante, M. Vacante, V. Colonna, and M. Motta. 2007. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: A randomized and controlled clinical trial. The American Journal of Clinical Nutrition 86 (6):1738–44. doi: 10.1093/ajcn/86.5.1738.
- Malaguarnera, M.,. M. P. Gargante, E. Cristaldi, M. Vacante, C. Risino, L. Cammalleri, G. Pennisi, and L. Rampello. 2008. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Digestive Diseases and Sciences 53 (11):3018–25. doi: 10.1007/s10620-008-0238-6.
- Marseglia, A., W. Xu, L. Fratiglioni, C. Fabbri, A. A. M. Berendsen, A. Bialecka-Debek, A. Jennings, R. Gillings, N. Meunier, E. Caumon, et al. 2018. Effect of the NU-AGE diet on cognitive functioning in older adults: A randomized controlled trial. Frontiers in Physiology 9:349 doi: 10.3389/fphys.2018.00349.
- Martínez-Lapiscina, E. H., P. Clavero, E. Toledo, R. Estruch, J. Salas-Salvadó, B. S. Julián, A. Sanchez-Tainta, E. Ros, C. Valls-Pedret, and M. Á. Martinez-Gonzalez. 2013. Mediterranean diet improves cognition: The Predimed-Navarra randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry 84 (12):1318–25. doi: 10.1136/jnnp-2012-304792.
- McGrattan, A. M., C. T. McEvoy, B. McGuinness, M. C. McKinley, and J. V. Woodside. 2018. Effect of dietary interventions in mild cognitive impairment: A systematic review. The British Journal of Nutrition 120 (12):1388–405. doi: 10.1017/s0007114518002945.
- Moll van Charante, E. P., E. Richard, L. S. Eurelings, J. W. van Dalen, S. A. Ligthart, E. F. van Bussel, M. P. Hoevenaar-Blom, M. Vermeulen, and W. A. van Gool. 2016. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet 388 (10046):797–805. doi: 10.1016/s0140-6736(16)30950-3.
- Montgomery, S. A., L. J. Thal, and R. Amrein. 2003. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. International Clinical PsychopharmacologyInternational Clinical Psychopharmacology18 (2):61–71. doi: 10.1097/01.yic.0000058280.28578.79.
- Nagai, H., M. Harada, M. Nakagawa, T. Tanaka, B. Gunadi, M. L. Setiabudi, J. L. Uktolseja, and Y. Miyata. 1996. Effects of chicken extract on the recovery from fatigue caused by mental workload. Applied Human Science: Journal of Physiological Anthropology 15 (6):281–6. doi: 10.2114/jpa.15.281.
- Ng, T. P., A. Ling, L. Feng, M. S. Z. Nyunt, L. Feng, M. Niti, B. Y. Tan, G. Chan, S. A. Khoo, and S. M. Chan. 2017. Cognitive Effects of Multi-domain Interventions among Pre-frail and Frail Community-living Older Persons: Randomized Controlled Trial. The Journals of Gerontology: Series A 73 (6):806–812.
- Ngandu, T., J. Lehtisalo, A. Solomon, E. Levälahti, S. Ahtiluoto, R. Antikainen, L. Bäckman, T. Hänninen, A. Jula, T. Laatikainen, et al. 2015. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet 385 (9984):2255–63.
- Prado, E. L., M. T. Ullman, H. Muadz, K. J. Alcock, and A. H. Shankar. 2012. The effect of maternal multiple micronutrient supplementation on cognition and mood during pregnancy and postpartum in Indonesia: A randomized trial. PLoS One 7 (3):e32519. no pagination) (e32519).
- Prina, A. M., R. Mayston, Y.-T. Wu, and M. Prince. 2019. A review of the 10/66 dementia research group. Social Psychiatry and Psychiatric Epidemiology 54 (1):1–10. doi: 10.1007/s00127-018-1626-7.
- Prince, M. J. 2015. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International.
- Qiu, C., and L. Fratiglioni. 2015. A major role for cardiovascular burden in age-related cognitive decline. Nature Reviews. Cardiology 12 (5):267–77. doi: 10.1038/nrcardio.2014.223.
- Rosillo, M. A., M. Sanchez-Hidalgo, A. Cardeno, M. Aparicio-Soto, S. Sanchez-Fidalgo, I. Villegas, and C. A. de la Lastra. 2012. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacological Research 66 (3):235–42. doi: 10.1016/j.phrs.2012.05.006.
- Rosli, R.,. D. A. Loh, W. Y. Choo, F. MohdHairi, D. Peramalah, S. Kandiben, P. L. Lee, N. Gani, M. F. Madzlan, M. A. I. Abd Hamid, et al. 2017. Effects of multicomponent exercise and therapeutic lifestyle (CERgAS) intervention on cognitive function in lower income elderly population: A cluster randomised controlled trial. Age and Ageing 46 (suppl_2):ii7–ii7.
- Sinn, N., C. M. Milte, S. J. Street, J. D. Buckley, A. M. Coates, J. Petkov, and P. R. C. Howe. 2012. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. British Journal of Nutrition 107 (11):1682–93.
- Smith, A. D., H. Refsum, T. Bottiglieri, M. Fenech, B. Hooshmand, A. McCaddon, J. W. Miller, I. H. Rosenberg, and R. Obeid. 2018. Homocysteine and dementia: An international consensus statement. Journal of Alzheimer's Disease: JAD 62 (2):561–70. doi: 10.3233/jad-171042.
- Stephan, B. C. M., R. Birdi, E. Y. H. Tang, T. D. Cosco, L. M. Donini, S. Licher, M. A. Ikram, M. Siervo, and L. Robinson. 2018. Secular trends in dementia prevalence and incidence worldwide: A systematic review. Journal of Alzheimer's Disease: JAD 66 (2):653–80. doi: 10.3233/jad-180375.
- Sun, Y., C. J. Lu, K. L. Chien, S. T. Chen, and R. C. Chen. 2007. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's Disease: A 26-week, randomized, double-blind, placebo-controlled study in Taiwanese Patients. Clinical Therapeutics 29 (10):2204–14. doi: 10.1016/j.clinthera.2007.10.012.
- Swanson, D., R. Block, and S. A. Mousa. 2012. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition (Bethesda, Md.) 3 (1):1–7. doi: 10.3945/an.111.000893.
- Teoh, S. L., S. Sudfangsai, P. Lumbiganon, M. Laopaiboon, N. M. Lai, and N. Chaiyakunapruk. 2016. Chicken Essence for Cognitive Function Improvement: A Systematic Review and Meta-Analysis. Nutrients 8 (1):57.
- UnitedNations. 2018. World Economic Situation and Prospects 2018. New York.
- Valls-Pedret, C., A. Sala-Vila, M. Serra-Mir, D. Corella, R. de la Torre, M. Á. Martínez-González, E. H. Martínez-Lapiscina, M. Fitó, A. Pérez-Heras, J. Salas-Salvadó, et al. 2015. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Internal Medicine 175 (7):1094–103.
- van de Rest, O., J. M. Geleijnse, F. J. Kok, W. A. v Staveren, C. Dullemeijer, M. G. M. OldeRikkert, A. T. F. Beekman, and C. D. Groot. 2008. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 71 (6):430–8. doi: 10.1212/01.wnl.0000324268.45138.86.
- Walker, R., and S.-M. Paddick. 2019. Dementia prevention in low-income and middle-income countries: A cautious step forward. The Lancet. Global Health 7 (5):e538–e539. doi: https://doi.org/10.1016/S2214-109X.(19)30169-X. doi: 10.1016/S2214-109X(19)30169-X.
- Whiteley, W. N., S. Anand, S. I. Bangdiwala, J. Bosch, M. Canavan, H. Chertkow, H. C. Gerstein, P. Gorelick, M. O'Donnell, G. Pare, et al. 2020. Are large simple trials for dementia prevention possible? Age and Ageing 49 (2):154–60. doi: 10.1093/ageing/afz152.
- Yamano, E., M. Tanaka, A. Ishii, N. Tsuruoka, K. Abe, and Y. Watanabe. 2013. Effects of chicken essence on recovery from mental fatigue in healthy males. Medical Science Monitor: international Medical Journal of Experimental and Clinical Research 19 (1):540–7. doi: 10.12659/MSM.883971.
- Young, H., D. Benton, and N. Carter. 2015. The effect of chicken extract on mood, cognition and heart rate variability. Nutrients 7 (2):887–904. doi: 10.3390/nu7020887.
- Zain, A. M., and S. M. S. Jamallulail. 2003. Effect of taking chicken essence on stress and cognition of human volunteers. Malaysian Journal of Nutrition 9 (1):19–29.
- Zhang, Y. P., R. Miao, Q. Li, T. Wu, and F. Ma. 2017. Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: A 12-month randomized, double-blind, placebo-controlled trial. Journal of Alzheimer's Disease: JAD 55 (2):497–507. doi: 10.3233/JAD-160439.