ABSTRACT:
Milk fat globules (MFGs) are secreted from the mammalian gland and are composed of a triacylglycerol core surrounded by a triple membrane structure, the milk fat globule membrane (MFGM). The MFGM contains complex lipids and proteins reported to have nutritional, immunological, neurological and digestive functions. Human and ruminant milk are shown to share a similar MFG structure but with different size, profile and abundance of protein and polar lipids. This review summarizes the reported data on human, bovine, caprine and ovine MFG composition and concentration of bioactive components in different MFG-size fractions. A comprehensive understanding of compositional variations between milk from different species and MFG size fractions may help promote various milk sources as targeted supplements to improve human development and health. MFG size and MFGM composition are species-specific and affected by lactation, diet and breed (or maternal origin). Purification and enrichment methods for some bioactive proteins and lipids present in the MFGM have yet to be established or are not scaled sufficiently to be used to supplement human diets. To overcome this problem, MFG size selection through fractionation or herd selection may provide a convenient way to pre-enrich the MFG fraction with specific protein and lipid components to fulfill human dietary and health requirements.
1. Introduction
Mammalian milk is designed to supply specific nutritional yield, bioactivities and complex structures that optimize maternal energy cost and infant survival (Lefèvre, Sharp, and Nicholas Citation2010). Human milk is enriched in whey proteins, lactose and complex carbohydrates (Barłowska et al. Citation2011) whereas ruminant milk is a rich source of casein proteins and fat, with a higher ratio of protein to lactose than human milk. Humans consume milk from ruminant species as whole milk, dairy products, or dairy ingredients. Ruminant milk from different species has been extensively studied, selected and modified (Pietrzak-Fiećko and Kamelska-Sadowska Citation2020; Haug, Høstmark, and Harstad Citation2007) to address human nutritional and health requirements (Gorlov et al. Citation2019; Park and Haenlein Citation2021; Suri et al. Citation2019).
Husbandry methods and genetic selection have long been used to improve the yield of milk volume, protein and fat. Changes in specific components have led to milk with better processing characteristics (e.g. increased casein content is favorable for cheese production (Wedholm et al. Citation2006), a low β-lactoglobulin concentration reduces the fouling rate during pasteurization (Elofsson et al. Citation1996) and improved health benefits (e.g. improved lipid quality has the potential to significantly contribute to the production of dairy products with higher added value (Hanuš et al. Citation2018)).
The lipid fraction of human and ruminant milk is secreted from the mammalian gland in the form of milk fat globules (MFGs), composed of a triacylglycerol (TAG) core enclosed by a complex triple membrane structure, referred to as the milk fat globule membrane (MFGM). The MFGM contains polar lipids, specialized proteins, glycoproteins and cholesterol, with nutritional, immunological, neurological and digestive functions (Lopez Citation2011). Many biological functions have been attributed to the MFGM and its components; in the last decade, several research groups have provided more information on the organization and composition of MFG. Human and ruminant milk were shown to share the same MFG structure but with a different protein profile and/or concentration and proportions of polar lipids (Cebo and Martin Citation2012; Gallier et al. Citation2010; Lopez Citation2011; Lopez, Cauty, and Guyomarc’h Citation2015; Lu et al. Citation2016; Zou et al. Citation2013).
Recent advances in manufacturing technologies have enabled the concentration of bovine MFGM, known as a phospholipid-enriched dairy ingredient, making it possible to use this fraction to supplement adult diets (Watanabe et al. Citation2020; Minegishi et al. Citation2016) and infant formula (Holzmüller and Kulozik Citation2016). The beneficial effects of adding this into the diet were evidenced in studies showing that MFGM supplementation to animal models and human infants improved cognitive scores compared to a control formula, and cognitive scores were similar to those of breast-fed infants (Timby et al. Citation2014; Mudd et al. Citation2016). Dietary supplementation with bovine MFGM to rodents was shown to affect gut development and microbial composition (Bhinder et al. Citation2017), decrease infection and inflammation in rodent models, and promoted gut mucosal integrity during lipopolysaccharide-induced intestinal inflammation, suggesting that MFGM has a role on neurodevelopment and has antibacterial and anti-inflammatory activities (Sprong et al. Citation2012; Snow et al. Citation2011; Huang et al. Citation2019). Studies have also shown that MFGM dietary supplementation improves motor functions associated with muscle strength and agility (Ota et al. Citation2015) and adaptation (Watanabe et al. Citation2020) following exercise interventions in older adults. Although dietary MFGM has shown positive effects on health, no mechanisms have been determined. It has been hypothesized that MFG structure and/or specific components found in the MFGM may be related to MFG functionality.
A number of studies have shown that some beneficial components of the MFGM (i.e. lactadherin, ω-3 polyunsaturated fatty acids (PUFAs) and PL) are enriched in specific size fractions of the MFG pool (Lopez et al. Citation2011; Lu, Argov-Argaman, et al. Citation2016; Mesilati-Stahy, Mida, and Argov-Argaman Citation2011). Lactation stage, breed (or maternal geographical location) and diet are the main factors affecting the MFG size profile, which may also affect lipid digestion and bioactivity of MFGM components (Logan et al. Citation2014; Mesilati-Stahy and Argov-Argaman Citation2014; Mesilati-Stahy et al. Citation2015; Michalski et al. Citation2005). A detailed understanding of compositional variations between milk from different species and composition of MFG size fractions could encourage the consumption of different milk sources, not only for nutritional purposes, but as a targeted supplement to improve development and health. So far, bovine, goat and sheep milk are some of the most consumed milk in the world; however, the variation in MFG size and composition have not been explored as a source of bioactives. Thus, the focus of this review is: (i) to discuss the common origin, structure and general composition of the human, bovine, caprine and ovine MFGs; (ii) to describe the factors affecting globule size distribution and protein and lipid profile among these species; and (iii) to describe the effects of milk processing on the MFG beneficial effects.
2. Milk fat globule composition
Comprehensive reviews have been published on the structure and composition of MFG, primarily focusing on bovine and human milk (Lopez, Cauty, and Guyomarc’h Citation2015; Bourlieu and Michalski Citation2015; Gallier et al. Citation2015). The MFGs contain TAGs (∼98% w/w of total milk lipids) and are a vehicle for fat-soluble nutrients (e.g. carotenoids and vitamins). Globules are covered by a three-layer membrane structure, the MFGM, composed of proteins and lipids, and it is about 10–50 nm thick, accounting for up to 6% of the globule mass (Keenan and Mather Citation2006). The proportion of protein and lipids in the MFGM, generally described as a 1:1 weight ratio (Kanno Citation1990), is not conclusively known due to significant variation in extraction methods (Kanno and Kim Citation1990; Fong, Norris, and MacGibbon Citation2007; Le et al. Citation2009).
The lipids (PL, glycolipids and sphingomyelin (SM)) and proteins originate from the endoplasmic reticulum and the apical membrane of secreting mammary epithelial cells (MEC). The surface-active inner monolayer, which surrounds the triglyceride core, is composed of polar lipids derived from the endoplasmic reticulum. The central layer appears denser in electron micrographs and mainly contains proteins. The outer layer is a bilayer membrane of polar lipids originating from secretory regions of the apical plasma membrane of the MEC (Mather and Keenan Citation1998). Loosely attached proteins and transmembrane proteins, as well as cholesterol molecules, are present in the external layer (Gallier et al. Citation2011). Glycoproteins are also present on the surface, with carbohydrate domains oriented into the surrounding aqueous phase (Lopez, Madec, and Jimenez-Flores Citation2010). Phosphatidylcholine (PC) and SM are mainly located in the outer layer of the membrane, and phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidylserine (PS) are concentrated at the inner surface, showing the asymmetric distribution of MFGM polar lipids (Deeth Citation1997). The size, profile and distribution of polar lipids and proteins in the MFGM vary among species, breeds and diet, and are discussed in more detail in sections below.
3. Milk fat globule formation
Lipid synthesis in milk occurs within regions of the rough endoplasmic reticulum, releasing cytoplasmic micro lipid droplets (LDs) coated with a phospholipid (PL) monolayer (). During the movement from the endoplasmic reticulum to the apical plasma membrane, some of these micro LDs fuze to form intermediate-sized LDs (Timmen and Patton Citation1988) that are coated by plasma membrane components and released into milk as MFGs. Coalescence and ripening of intracellular lipid droplets in adipocytes and hepatocytes can be modulated by the composition of the PL envelope. Specifically, increased phosphatidylethanolamine (PE) concentration reduces the interfacial surface tension of the lipid droplet, which can result in fewer larger droplets (reviewed by Thiam, Farese, and Walther Citation2013). Interestingly, PE in human milk is reduced compared to ruminant milk (). Globules that exceed the size of the secreted cells suggest post-secretion coalescence (Timmen and Patton Citation1988).
Figure 1. Milk fat globule formation and size composition. Proteins butyrophilin (BTN), xanthine dehydrogenase/oxidase (XDH/XO), adipophilin (ADPF), and stomatin are involved in the milk fat globule formation. An increased phosphatidylcholine (PC)/phosphatidylethanolamine (PE) ratio prevents lipid droplet fusion and increases concentration of small milk fat globules (SMFG). MEC, mammalian epithelium cell; ER, endoplasmic reticulum; LD, lipid droplet; LMFG, large milk fat globules; MFGE8, milk fat globule-EGF factor 8; GlyCAM1, glycosylation-dependent cell adhesion molecule 1; FAs, fatty acids; TAG, triacylglycerol.
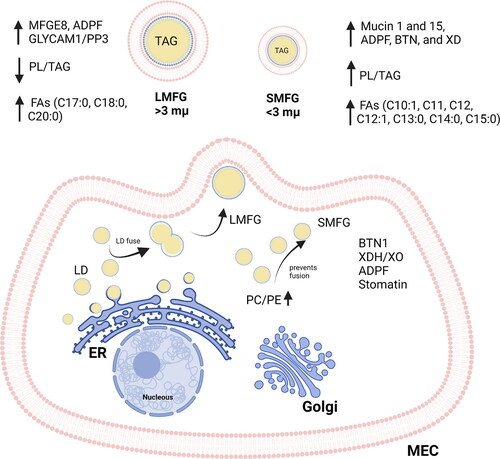
Table 1. Comparison of mean milk fat globule size and composition between mature human and ruminant milk.
The identity of cellular components involved in MEC lipid secretion pathways is open to debate. Some proposed models can explain, in part, the difference in composition and size of MFGs observed across species. The tripartite model, for example, proposes that interactions among three main MFGM proteins, adipophilin (perilipin-2, ADPF), xanthine dehydrogenase/oxidase (XDH/XO) and butyrophilin (BTN) are responsible for MFG secretion from the MEC. In this model, BTN, XDH/XO and ADPF form a complex on the fat globule surface and facilitate adsorption of the bilayer membrane to the LDs. This complex leads to membrane deformation of the LD and budding of LDs from the secretory cell (Mather and Keenan Citation1998). The ratio of BTN to XDH/XO, constant among breeds (Mondy and Keenan Citation1993; McManaman et al. Citation2007), varies according to species and shows associations between fat content, MFG size and MFG secretion. In ovine and bovine species, the BTN to XDH/XO molar ratio is about 1:4 to 1:2, depending upon the species; in human milk, however, the levels of BTN were shown to be higher than those of XDH, with a ratio of 6:1 (Mondy and Keenan Citation1993; Yang et al. Citation2016). In caprine species, a 1:1 molar ratio was calculated between these two proteins (Zamora, Guamis, and Trujillo Citation2009).
Other studies have challenged this view of MFG secretion by developing new models of lipid secretion from the MEC. It was proposed that the release of cytoplasmic LDs into milk is solely mediated by BTN homotypic interactions (Robenek et al. Citation2006). Binding between the ADPF C-terminal domain and the apical plasma membrane has been reported (Chong et al. Citation2011). Other models were identified in lipid droplets found in adipose (Hörl et al. Citation2011) and liver (Stringer et al. Citation2010) cells. For example, the levels of PAT (perilipin/ADPF/adipose differentiation-related proteins/TIP47) in murine adipocytes decreased with smaller intracellular LDs after trans-10,cis-12 conjugated linoleic acid (CLA) supplementation (Hörl et al. Citation2011). Effects of diet on MFG size are discussed below.
Stomatin, together with SM, is also a candidate to influence and regulate MFG synthesis and secretion (Lu et al. Citation2013). Although the mechanisms of this regulation are not fully clear, stomatin has been observed to dictate membrane curvature to regulate MFG formation and secretion (Ménard et al. Citation2010). Higher abundance of stomatin is found in caprine MFGs compared to other species, and it is also the species with smaller mean MFG size. Higher abundance of stomatin was found in caprines (O/O) (animals homozygous for αs1-casein, allele O, αs1-CN null animals) and Sarda dairy caprines, which also had smaller MFGs, compared to caprines (A/A) (animals homozygous for αs1-casein, allele A) and Saanen dairy caprines (Cebo and Martin Citation2012; Pisanu et al. Citation2013).
A complementary model of MFG secretion, with the casein balancing membrane loss caused by MFG release, has been proposed. It is long known that caseins are synthesized and transported along the secretory pathway and released by the fusion of casein-containing vesicles with the apical plasma membrane and soluble N-ethylmaleimide–sensitive fusion attachment protein receptor (SNARE) (Honvo-Houéto et al. Citation2016). The complementary model suggests that whereas BTN1, PLIN2 and XOR are likely to contribute to MFG budding, attachment to SNARE proteins could connect the secretory vesicles, not only together, but also with the budding MFG and the apical plasma membrane through the formation of SNARE complexes. In this way, SNARE proteins could promote both the exocytosis of caseins and the connection of the endoplasmic reticulum with the apical plasma membrane to provide membrane to enwrap the budding MFG (Honvo-Houéto et al. Citation2016). The final step of MFG release likely occurs upon the homotypic fusion of the secretory vesicles surrounding the budding MFG rather than by final scission of the plasma membrane at a budding neck (Honvo-Houéto et al. Citation2016). If correct, the combined release of MFGs and casein micelles from the MEC may contribute to the presence of casein micelles adsorbed selectively onto the native MFGM (Luo et al. Citation2014) (see more in Sec. 6.1). Independent of the model, the proteins BTN1, PLIN2, XOR and stomatin seem to have an essential role in MFG secretion, but studies correlating protein abundance and MFG size across species are still lacking.
3.1. Size regulation
To understand how the MFG size is regulated, the mechanisms controlling MFG formation (see Sec. 3), composition and secretion must be determined (Argov-Argaman Citation2019). The abundance of certain proteins in the MEC (XOR (Monks et al. Citation2016), BTN1 (Han et al. Citation2020)) and the composition of the PL membrane (specially PE) () (Cohen, Shamay, and Argov-Argaman Citation2015) are involved in MFG formation and the size distribution. However, the mechanisms that regulate the concentration of proteins and PL in the MEC are still unclear.
Regulation of MFG size trough hormonal (i.e. prolactin and oxytocin) modulation of milk release, has been described (Ollivier-Bousquet Citation2002). Recently the involvement of the reproductive hormone progesterone in the regulation of MFG size, but not fat production, in dairy cows has been reported (Argov-Argaman, Raz, and Roth Citation2020). The direct link between ovulatory cycle, progesterone levels and MFG size was supported by in vitro and in vivo studies (Argov-Argaman, Raz, and Roth Citation2020). Lipid droplets <3 µm were shown to be more abundant in the luteal phase (characterized by increased progesterone) than in the follicular phase (characterized by decreasing progesterone concentration). The progesterone effect was found to be dependent on the presence of very-low-density lipoproteins, a source of long-chain fatty acids for the MEC, a limiting material for membrane synthesis (Mesilati-Stahy and Argov-Argaman Citation2018).
Interestingly, another recent study suggested that cows’ lipid metabolism may regulate the production of small or large MFGs. Cows with a small globule phenotype (average 3.29 μm) produced milk with higher concentration of unsaturated FA compared to cows producing large globules (average 4.92 μm), despite being fed the same diet. The authors suggest that this phenomenon is the result of increased uptake of long-chain FA from the blood circulation by cows producing small MFG. The relationship between the degree of unsaturation and MFG size was also reported in this preliminary study for sheep and goats (Walter et al. Citation2020).
The relationship between cows’ metabolism, FA availability and MFG size has also been suggested. Cows with higher plasma insulin concentrations had increased concentrations of monounsaturated fatty acids (MUFAs) in the core and a lower TAG/PL ratio, whereas cows with lower insulin had higher levels of MUFA in the MFGM compartment, suggesting a potential hormonal regulation of this process (Mesilati-Stahy, Malka, and Argov-Argaman Citation2012). Another possible mechanism of regulation is the animal energy balance. During the early stages of lactation or feed restriction, cows’ negative energy balance was shown to produce MFGs with more oleic acid (C18:1 cis-9), less palmitic acid (C16:0), less de novo synthesized FA, and decreased MFG size (Gross et al. Citation2011; Walter et al. Citation2020). The reported effects of breed, lactation stage and diet are described below.
4. Milk fat globule structure
Currently, two different MFGM structures have been proposed, based on location in either the alveolus or in expressed milk. MFGs are covered by a continuous membrane adsorbed to the lipid core by a 15–20 nm membrane derived from the cytoplasm after secretion into the alveolus (Lopez Citation2011). The structure of the MFGM after release from the gland, however, is open to debate.
In recent years, the application of microscopy imaging techniques using confocal laser scanning microscopy and fluorescent probes suggest retention of the membrane after budding from the MFG (Lopez Citation2011). Using this technique, the authors suggested the presence of lipid rafts on the MFGM (Lopez, Madec, and Jimenez-Flores Citation2010) characterized by unstained circular areas due to the presence of liquid-ordered phases rich in sphingolipids and cholesterol. In a recent publications (Lopez Citation2011; Zheng, Jiménez-Flores, and Everett Citation2014), three-dimensional and two-dimensional models for the structure of the MFGM were described. These authors proposed the coexistence of at least two lipid phases in the MFGM: (i) a less densely packed liquid-disordered phase comprised of unsaturated glycerophospholipids (PE, PC, PI, PS), proteins, glycoproteins, SM and glycolipids, and (ii) a biphasic separation of SM and cholesterol into more densely packed liquid-ordered domains (Lopez Citation2011). These authors also suggested that some of the cholesterol in the MFGM might interact with other glycerophospholipids (such as PC), located within the liquid-ordered domain, and protrude from the liquid-disordered domain. So far, the presence of liquid-ordered domains has been demonstrated with in situ structural experiments on human and bovine MFG; however, these domains differ in size and shape (Gallier et al. Citation2011; Evers et al. Citation2008). These phase separations have been observed using artificially constructed giant unilamellar vesicles containing different ratios of SM, phospholipids and cholesterol, suggesting a role for cholesterol in regulating liquid-ordered regions (Zheng, Jiménez-Flores, and Everett Citation2014). Association of SM and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine on the vesicle surface in the absence of cholesterol forms an irregularly shaped gel phase with a high phase transition temperature from a gel to a liquid, indicating that cholesterol is not necessarily a requirement for closely packed lipid domain formation (Zheng, Jiménez-Flores, and Everett Citation2014).
In contrast to this view, using a combination of electron and light microscope techniques, Wooding and Mather (Citation2017) suggested a different interpretation of the unstained circular areas observed by others (Lopez, Madec, and Jimenez-Flores Citation2010; Gallier et al. Citation2011; Zheng, Jiménez-Flores, and Everett Citation2014). Only the MFG close or still attached to the secretory cells was shown to have a continuous unit membrane bounding a clear space around the lipid core. Wooding and Mather (Citation2017) suggested that disruption and modification of the initial continuous membrane occur when the MFG reaches the alveolar lumen and is expressed. Some of the continuous membrane may be lost by vesiculation after cellular release, causing a reduction in the area through interactions between the MFGM proteins with sub-surface dense layers beneath the unit membrane surface. Therefore, the discontinuities (nonfluorescent microdomains) observed using confocal laser scanning microscopy techniques could indicate a lack of unit membrane-binding to the cytoplasmic layer. This disruption was observed in all species examined (i.e. bovine, ovine, human) producing patches and networks of variable sizes on the surface of the MFG (Wooding and Mather Citation2017). The significance of this new structural model for the digestive, nutritional and health effects of the MFG has yet to be determined.
5. Milk fat globule size
Many techniques have been used to measure MFG size and distribution (reviewed by Truong et al. Citation2016; Martini, Salari, and Altomonte Citation2016); the most common methods are microscopy and small-angle light scattering. Microscopy yields a number-average size of individual MFGs, a size distribution, and lipid microstructure (Evers et al. Citation2008; Gallier et al. Citation2011; Gallier et al. Citation2010). Light scattering techniques are based on the intensity of scattering patterns of monochromatic laser light on the MFGs. The scattering is dependent upon the wavelength of the incidence of light, refractive index and size. The scattering pattern is then converted into a size distribution using mathematical models, such as those derived from Mie and Fraunhoufer theories (Michalski, Briard, and Michel Citation2001).
The MFG size can be described by diameter, volume, number and surface area. Mean diameters of MFGs are commonly expressed as a number-weighted mean (dn, D1,0), volume-weighted mean (dvm, D4,3), or as the surface-weighted mean (dvs, D3,2). The surface-weighted mean (Sauter mean diameter) gives weighting to the globule surface area, being important for surface area-related phenomena such as release of components from surfaces, two-dimensional surface reactions, and surface dissolution. It varies with the volume size distribution, i.e. smaller globules have a much higher specific surface area to volume ratio compared to larger globules. The volume-weighed mean (De Brouckere mean diameter, dvm, D4,3) reflects the contribution of each particle in the distribution related to the volume. In this review, when MFG size is mentioned, it will refer to diameter dvm (D4,3) unless otherwise specified.
The literature reports that MFG size range can be divided into three main volumetric subgroups within whole milk (Walstra Citation1969).
The first group, the small MFGs (<1 μm in size) account for around 80% of the total number of globules and grow little or not at all in volume before these are secreted (Heid and Keenan Citation2005; Mulder and Walstra Citation1974; Michalski et al. Citation2005). Evaluation of this group of globules in human and bovine milk by Raman spectroscopy has shown that the MFGs smaller than 1 μm exhibit far less triglyceride core relative volume, but are rich in membrane unsaturated FAs (Gallier et al. Citation2011). This may suggest that membrane particles have additional functional effects on lactation beyond carrying and emulsifying the TAG core (Gallier et al. Citation2011).
The second group, the medium-sized globules, account for the majority of the lipid phase, in terms of volume (∼94%) (Walstra Citation1969).
The third group, large-size MFG, are those ≥8 μm (Walstra Citation1969). Changes within the larger MFG fraction, which tend to increase at the expense of the medium-size group, have the most significant effect on the mean MFG size, particularly when the measured size is based on volume (Wiking et al. Citation2004).
It should be noted that milk contains other nanoscale colloidal structures, such as exosomes and casein micelles (100 to 200 nm; McMahon and Oommen Citation2008). The secretion pathways, the core composition, and the protein content of MFG and skim milk phospholipid structures differ, highlighting a different biological potential in milk (Blans et al. Citation2017; Adriano et al. Citation2021; Admyre et al. Citation2007; Reinhardt et al. Citation2012). It also important to note that casein micelles, but not exosomes, are dissociated by EDTA during milk sample preparations, so the presence of exosomes may confound the measurements of MFG size based on light scattering analysis. Nevertheless, changes in the dispersion of MFGs during lactation and the influence by other factors (e.g. breed and diet, discussed below) are mostly observed in the larger MFG particles (>100 nm).
Ruminant and human-derived milk have most MFG volumetric sizes ranging from 0.1 to 15 μm, but with a considerable variation in the mean diameter between and within species. shows the variation of MFG mean size described in the literature for human (4.2 to 5.1 μm), bovine (2.5 to 5.7 μm), caprine (2.2 to 3.9 μm) and ovine mature milk (2.8 to 4.0 μm). In general, studies comparing the size distribution of two or more species showed differences in range distribution among species. In one study, for example, about 90% of all fat globules in caprine milk had diameters of less than 5.2 μm, whereas 90% of the globules in bovine milk had diameters of less than 6.4 μm (Attaie and Richter Citation2000). Another study, however, showed that 68.4%, 73.3% and 55.3% of the globules in bovine, caprine and ovine milk, respectively, have a diameter less than 4 μm (El-Zeini Citation2006). Increased access of lipases to the surface and subsequently to the interior of small milk fat globules (SMFG) may affect lipid digestion rates in milk from different ruminants (Armand et al. Citation1999) (see more in Secs. 7.1 and 8).
5.1. Variation in milk fat globule size
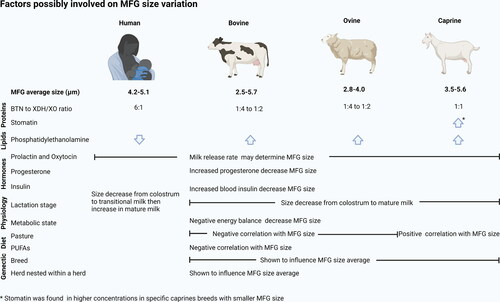
The distribution of globule size, within species, can be widely affected by parameters such as maternal physiology, breed, herd nested within herd, season and diet () (Fleming et al. Citation2017; Wiking et al. Citation2004; Logan et al. Citation2014; Walter et al. Citation2019), which is discussed in the following sections of this review.
Figure 2. Summary of the factors possibly involved on human, bovine, ovine and caprine MFG size variation.
5.1.1. Breed and herd nested within a herd
Variations in MFG size obtained through breed selection are well-described among bovine, caprine and ovine breeds, as shown in . In caprine and ovine, for example, breeds selected for dairy traits (Sarda, Massese) exhibited a higher number of small MFGs and a greater number of globules per volume of milk (Bianchi et al. Citation2004; Martini et al. Citation2006). It is important to note that most of those studies reported in did not specify the lactation period and/or animal diet. Thus, MFG size (range distribution and average) is determined by a combination of factors such as genetics, diet, lactation stage and may also be regulated to fit the functional and nutritional requirements of the infant (Bourlieu and Michalski Citation2015) (not within the scope of this review).
Table 2. Milk fat globule average size and standard deviation reported for the different breeds of bovine, caprine and ovine milk.
Differences in MFG size between individual cows within a herd exposed to identical environmental conditions and diet was demonstrated (ranging from 2.5 to 5.7 µm) (Logan et al. Citation2014; Walter et al. Citation2019). To understand the possible determinants of this variation, Walter et al. (Citation2019) conducted an extensive analysis of the factors affecting bovine MFG size at the individual animal level. Among the studied factors were physiological characteristics (parity, days in milk, days pregnant, weight, age, somatic cell count), milk production traits (number of milkings, milk yield, fat yield, protein and fat content, fat-protein ratio) and environmental conditions (diet, weather, season). The authors reported that in isolation, all the factors listed above have a detectable effect on MFG size, but they do not sufficiently explain the wide individual variation observed in MFG. It was concluded that physiological (i.e. stage of lactation and parity) and milk yield differences outweigh the effects of milk production traits and environmental conditions on MFG size (Walter et al. Citation2019). This suggests the potential to select natural variations of MFG size to produce dairy products with differentiated texture, improved yield or improved functionality.
5.1.2. Lactation stage
Variation in MFG size observed during different lactation stages may have evolved to optimize maternal energy cost and to supply the required nutritional and bioactive components maximizing infant survival (Lee et al. Citation2018). In human milk, for example, the average globule surface area was reported to increase from 1.1 ± 0 to 5.4 ± 0.7 m2/g during the transition of colostrum to mature milk, whereas the volume-weighted mean decreased (Michalski et al. Citation2005; Argov-Argaman et al. Citation2010; Zou et al. Citation2012). Although size increased as lactation progressed (Jiang et al. Citation2020), the average diameters of mature MFGs were shown to be relatively stable at around 4.5 μm (Zou et al. Citation2012; Michalski et al. Citation2005). In dairy farm animals, where the stage of lactation was shown to be closely associated with the animal energy balance (Ducháček et al. Citation2013); larger MFG size was also observed in colostrum compared to mature milk (Fleming et al. Citation2017; Martini, Altomonte, and Salari Citation2012a). In Massese ewes, for example, MFG average diameter decreased from 4.07 µm at the delivery day to 2.76 µm on milking day 15 (Martini, Altomonte, and Salari Citation2012a) and from 3.19 µm on milking day 40 to 2.39 µm on day 100 (Salari and Martini Citation2009). These size changes are directly correlated to variations in protein and lipid profiles, as described in the sections below.
5.1.3. Diet
So far, studies have accessed the effect of maternal diet on breast milk lipid quality (Freitas et al. Citation2021) but, to our knowledge, no correlation have been made between maternal diet and MFG size. Many studies, however, have investigated the effect of pasture on bovine MFG size and lipid composition. In general, higher intakes of pasture and/or the addition of supplements of dietary oils rich in polyunsaturated FAs were shown to decrease MFG size and increase the percentages of polar lipids and unsaturated FAs at the expense of saturated FAs in bovine milk (Couvreur et al. Citation2007; Couvreur et al. Citation2006; Lopez, Briard-Bion, and Ménard Citation2014; Lopez et al. Citation2008). Linoleic (LA) (C18:2c9,12), α-linolenic (ALA) (C18:3c9,12,15) and palmitic (C16:0) acids were the predominant FAs in bovines fed pasture, accounting for approximately 88% of total FAs in all samples (Nantapo, Muchenje, and Hugo Citation2014). The same beneficial effect was observed when ewes were fed a high forage diet with decreased percentages of some medium-chain FAs and increased mono and polyunsaturated FA such as trans-11-C18:1 (+31.71%), total CLA (+22%), eicosapentaenoic acid (+18.18%) and docosahexaenoic acid (+66.67%) (Martini, Liponi, and Salari Citation2010; Meľuchová et al. Citation2008). In another study, although diet containing high proportions of fresh legume types (white clover, red clover or lucerne, which contain large concentrations of PUFAs) or silage increased the bovine milk 18:1t11, 18:2c9,t11, 18:2t10,c12 and 18:3 fatty acids concentration, no dietary effect was observed in the MFG diameter (Wiking et al. Citation2010). Dietary supplements of conjugated linoleic acid (cis-9,trans-11 and trans-10,cis-12 isomers) were shown to have detrimental effects on milk fat synthesis accompanied by a decrease in average diameter of MFGs in lactating cows (Xing et al. Citation2020; Zhang et al. Citation2021). In contrast, higher fat content and a larger diameter of MFGs were observed in bovines fed a concentrate diet containing more saturated lipids (50% palmitic acid), compared to milk from bovines fed diets with a lower saturated lipid content (Wiking et al. Citation2004).
In a divergent study, Argov-Argaman et al. (Citation2014) observed that a diet containing high concentrate levels tended to produce smaller bovine MFGs compared with a high forage diet consisting of corn silage, oat and clover hay. These divergences may indicate that not only the dietary forage to concentrate ratio affects MFG size distribution, but also the type of forage (red clover silage vs. grass silage). Studies have suggested that insulin and energy balance (Argov-Argaman et al. Citation2012) are one of the mechanisms behind the dietary effects on MFG size. Higher energy diet (higher concentrate), for example, would lead to lower total milk fat content and smaller MFGs compared with a high forage diet (Argov-Argaman et al. Citation2014; Jaakamo et al. Citation2019). Another divergent study showed that grazing goats had milk richer in fat, larger MFGs and 20% higher PL content compared to confined goats fed commercial concentrate (Argov-Argaman et al. Citation2016).
The effects of microalgae dietary supplementation (enriched in PUFAs) on the bovine milk FA profile and the number and diameter of MFGs was also investigated (Stamm Citation2015). In this study, all dietary treatments ((i) Spirulina platensis, (ii) Chlorella vulgaris, or (iii) Chlorella vulgaris + Nannochloropsis gaditana used in the partial substitution of soy) moderately increased the content of PUFA at the expense of saturated fatty acids (SFA), compared to soya, but did not influence the MFG average diameter or PL content in milk. Dietary supplementation with Chlorella led to decreased number of globules (specially globules ranging from 1 to 3 µm) compared to other diets.
Studies have reported that dietary compositional changes in milk fat or compositional changes observed through lactation are not similar for all MFG size fractions (Mesilati-Stahy et al. Citation2015; Mesilati-Stahy and Argov-Argaman Citation2014; Argov-Argaman et al. Citation2014). For instance, compositional differences (higher capric (C10:0), lauric (C12:0), myristic (C14:0) and lower oleic acid (C18:1) concentrations) observed in the MFGs from bovines fed high-concentrate low-forage (HCLF) compared to high-forage low-concentrate (LCHF) rations were found in a specific MFG size group with a diameter of 3.3 µm. Differences in lipid concentration were only observed in the larger MFG fraction, with higher triglyceride and PE, and lower SM concentrations in LCHF (3.7 µm) compared to HCLF (2.9 µm) milk. In another study (Mesilati-Stahy et al. Citation2015), the same authors reported differences in FA concentration between size fractions according to the stage of lactation. The concentrations of oleic acid (c18;1n-9) were not different between the MFG size groups in early lactation (10 days post-partum) but were two-fold higher in large (3.8 µm) compared to small (1.59 µm) MFG fractions on day 150 post-partum. Future studies should focus on the metabolic and dietary changes occurring during lactation to determine the effects on MFG structure-composition and ultimately, function.
The close association between seasonal dietary variations and stage of lactation (in seasonally calving dairy system) may have led to the assumption that season is one of the determinants of the MFG variation. Logan et al. (Citation2014), however, described that the MFG diameter variation (±1 µm) observed in the same bovine milk, between seasons, was due to seasonal dietary variations. For goats, where a constant diet was consumed between spring and summer, no difference in the mean MFG diameter was observed (Salari et al. Citation2016).
6. Milk fat globule bioactive proteins
Cavaletto, Giuffrida and Conti (Citation2008) reported that MFGM proteins comprise about 1–4% w/w of the total milk proteins present in human milk. The relative proportion of MFGM proteins to other milk proteins, however, is not conclusively known due to wide variation in protein extraction techniques used and the lack of clear separation between intrinsic MFGM and other milk proteins.
Many studies have reported on the role of MFGM proteins in cellular processes and defence mechanisms in the maternal-infant pair. Recent proteomic studies have mapped differences in proteins present in MFGM from humans and the most common ruminant animals. Between 191 to 411 proteins were identified in human MFGs (Lu, Wang, et al. Citation2016; Yang et al. Citation2016, Citation2017; Liao et al. Citation2011; Hettinga et al. Citation2011), 20 to 411 in bovine MFGs (Bianchi et al. Citation2009; Lu et al. Citation2011; Yang et al. Citation2016, Citation2017; Sun et al. Citation2019; Hettinga et al. Citation2011), 161 to 593 proteins in caprine MFGs (Lu, Liu, et al. Citation2016; Pisanu et al. Citation2013; Sun et al. Citation2019) and 29 to 140 proteins in ovine MFGs MFGM (Pisanu et al. Citation2011, Citation2012). The wide variation found in the number of proteins identified is partly attributed to the application of different isolation techniques (recently reviewed by Holzmüller and Kulozik, Citation2016) and proteomic methods (SDS-PAGE, two-dimensional electrophoresis, MALDI-TOF-MS, LC-Orbitrap MS/MS, EASY-nLC-Orbitrap MS/MS). Differences in lactation period (Liao et al. Citation2011; Reinhardt and Lippolis Citation2008) and breed (Pisanu et al. Citation2013; Yang et al. Citation2016) are also common factors affecting the variation in protein composition.
Comparisons of protein abundance across species are challenging. Only two studies (Lu, Liu, et al. Citation2016; Yang et al. Citation2016) have compared MFGM protein abundance of more than two species (i.e. human vs. bovine, or bovine vs. caprine), and none have included an analysis of ovine MFGM. Moreover, the comparative proteomic studies do not agree on the most abundant proteins found in human, bovine and caprine MFGM. Lu et al. (Citation2016) (), for example, found BTN as the most abundant protein in bovine (24.8%), and human MFG (16.3%) but not in caprine milk (13.6%), and XDH was found to be the most abundant protein (25%). Yang et al. (Citation2016) however, found that fatty acid-binding protein was the most abundant protein in human MFGs, and glycosylation-dependent cell adhesion molecule 1 was the most abundant protein in caprine and Holstein bovine MFGs. Although an agreement has not been reached on numbers and abundance, it generally accepted that the BTN, ADPF, XDH and lactadherin (MFGE8) are enriched and conserved among human, bovine, caprine and ovine MFGM (Lu, Wang, et al. Citation2016; Pisanu et al. Citation2011; Cebo and Martin Citation2012). Some of these conserved proteins are thought to be involved in the transportation and secretion of milk components and are therefore crucial for the secretion of milk (see more in Sec. 3).
Other proteins involved in lipid secretion and lipogenesis were found to be abundant in human and ruminant MFGM () (Lu, Liu, et al. Citation2016). ADPF, for example, functions as a physiological regulator of cytoplasmic LD accumulation in differentiating milk-secreting cells and as an adaptor protein in the secretion of LD to form milk lipids during lactation (Chong et al. Citation2011) (See more in Sec. 3). Less studied compared to ADPF, Ras-related protein Rab-18 and fatty acid synthase are abundant in both human and ruminant MFGM (Lu, Liu, et al. Citation2016). Although Ras-related protein Rab-18 may be involved in regulating membrane trafficking and transport vesicles, fatty acid synthase is known to play a role in lipogenesis in the endoplasmic reticulum (Moriya et al. Citation2011). It may be hypothesized that these high abundant proteins have other functions for the consuming infant, but none have been identified so far. XDH, for example, is not only involved in MFG secretion by cooperating with BTN1A1 and ADPF, but has also been linked to antimicrobial activity in milk (see more in ).
Table 3. Potential source of bioactive MFGM protein and proteins associated with MFGM enriched fraction.
Many other MFGM proteins common or specific to humans and ruminants exhibit known bioactive properties that could be exploited for the benefit of human and animal health. Potential bioactive proteins, source and purification methods (if known) are presented in . Among the MFGM proteins, caseins and whey proteins have often been reported in MFGM proteomic studies (Liao et al. Citation2011; Lu, Liu, et al. Citation2016; Sun et al. Citation2019). Although intact casein micelles are shown to contribute to the native MFGM formation (Honvo-Houéto et al. Citation2016; Luo et al. Citation2014), the presence of caseins and whey proteins in proteomic studies are likely due to contamination of the MFGM fraction by enrichment methods and milk processing (i.e. pasteurization). The unavoidable presence of these proteins in the MFGM enriched fraction may, therefore, be considered as part of the bioactive potential of this fraction.
The bioactive peptides, products of casein digestion, for example, have been linked to numerous functions, such gastrointestinal and immunological function, infant development, and antibiotic and probiotic functionality (Park and Nam Citation2015) (). Although lactoferrin is found in the aqueous phase of milk as a whey protein, it is also relatively abundant in the human MFGM fraction and was reported to be bound to the MFGM membrane (Cho et al. Citation2000) for new-born host defence functionality. Although those could easily be harvested from skim milk, the presence in the enriched MFGM extract could contribute to the functionality of MFGs (Luo et al. Citation2014), and are outlined in .
A different way to interpret proteome data is using gene ontology and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. Studies so far show that proteins from human and ruminant MFGM are involved in common biological processes such as cell signaling, membrane transport, immune responses and protein metabolism (Liao et al. Citation2011; Pisanu et al. Citation2011; Sun et al. Citation2019; Reinhardt and Lippolis Citation2006; Yang et al. Citation2016). In comparative terms, there has been no agreement on the distribution of specific functionalities across species (i.e. immune functions enriched in human compared to bovine milk) (Liao et al. Citation2011; Reinhardt and Lippolis Citation2006; Pisanu et al. Citation2011). This is due to several factors: (i) different classifications (databases) used by the authors to categorize the same proteins, (ii) the unclear demarcations between classes, (iii) the diverse and overlapping protein functions, and (iv) the different functional roles exhibited by the same protein. An important application of gene ontology data is to understand the common biological processes that are often played by specific proteins from different mammalian species but could be isolated to benefit human health. Although, for example, lactoferrin, immunoglobulins (alpha and gamma chain C region), monocyte differentiation CD14, clusterin, toll-like receptor 2, mucin 4 and dermicidin, are proteins involved in immune responses in human MFGM, similar functions can be found in the immune response proteins cathelicidins (1, 2, 4 and 6), lactadherin, glycosylation-dependent cell adhesion molecule 1 (GlyCAM1), immunoglobulin mu chain C region and polymeric immunoglobulin receptors enriched in bovine MFGM (Hettinga et al. Citation2011; Lu, Liu, et al. Citation2016). More specifically, clusterin and lactadherin, proteins more commonly found in human and bovine milk, respectively, were suggested to have a similar role in cell damage and apoptosis, which may promote maintenance of intestinal epithelial homeostasis. Other host defence proteins, toll-like receptor 2 (TLR2) and cathelicidins, proteins uniquely present in human and bovine milk, respectively, are known to bind bacterial lipopolysaccharides and have antibacterial activity.
Almost exclusively present in the human MFGM, proteins involved in lipid metabolism, such as the hormone-sensitive lipase, peroxisomal bifunctional enzyme, peroxisomal multifunctional enzyme type 2, peroxisomal acyl-coenzyme A oxidase 3, carboxyl ester lipase (bile salt-stimulated lipase), and sphingomyelin phosphodiesterase aid infant lipid digestion (Lu, Wang, et al. Citation2016). The only enzyme also expressed in bovine and caprine MFGM, the lipoprotein lipase, is around 100-fold less concentrated in these species compared to human milk (Lu, Wang, et al. Citation2016). The importance of these enzymes was demonstrated by the observed increased fat absorption in infants consuming human milk compared to infant formula. It has been estimated that two-thirds of nonspecific lipase activity in the duodenum content after raw human milk consumption is from milk (Bläckberg and Hernell Citation1983). Therefore, the addition of milk lipases to infant formula could help to elevate fat utilization in infants, particularly for early or preterm infants. Adding carboxyl ester lipase to infant formulations may also decrease the incidence of intestinal disorders, especially ileal perforation and necrotizing enterocolitis in neonates (Howles et al. Citation1999). The presence of large and commercial human milk banks may allow the purification of proteins exclusively present in human milk, especially for the benefit of highly vulnerable premature infants (Andersson et al. Citation2007).
As shown in , purification and enrichment methods for several bioactive proteins present in the MFGM have not yet been established, or not yet scaled for supplementation of the infant diet. To overcome this, MFG size selection may be the most convenient way to pre-enrich the MFG fraction with specific protein and lipid components before considering further large scale purification using methods already described (Holzmüller and Kulozik Citation2016).
6.1. Size effect on protein composition
Recent studies reported differences in protein concentration among MFG size populations. (Lu, Argov-Argaman, et al. Citation2016), for example, using proteomic analysis, identified 49 and 23 proteins that were more abundant in the large (LMFG) (7.6 ± 0.9 μm) and SMFG fractions (3.3 ± 1.2 μm) extracted from bovine cream and skimmed milk, respectively. Among the major proteins, the LMFG fraction was shown to contain more MFGE8, ADPF and glycosylation-dependent cell adhesion molecule 1 (GLYCAM1/PP3) compared to the SMFG. Proteins found to be more abundant in the SMFG fraction were mainly associated with lipid metabolic processes and cholesterol, i.e. acyl-CoA synthetase (Q1LZF6, F1MEX9, ACSL), lanosterol synthase (LSS), acetyl-CoA carboxylase 1 (ACACA), sterol-4-α-carboxylate 3-dehydrogenase (decarboxylating; NSDHL), and glycerol-3-phosphate acyltransferase 4 (AGPAT6) (Lu, Argov-Argaman, et al. Citation2016). Other proteins enriched in the LMFG or SMFG fraction are described in . To explain, in part, the observed proteomic differences between the LMFG and SMFG fractions, the authors hypothesized that due to a size-dependent FA and PL composition, a different surface polarity between the large and small MFG fractions exists. Such polarity differences could, in turn, change the affinity of the MFGM for proteins (Lu, Argov-Argaman, et al. Citation2016). It may also be hypothesized that differences in the protein profile have a functional role throughout lactation.
In bovine and ovine milk, for example, colostrum has a larger MFG size, which generally decreases thereafter (Fleming et al. Citation2017; Martini, Altomonte, and Salari Citation2012a). Although not reporting the size of the MFG, Reinhardt and Lippolis (Citation2008) found the concentration of 26 MFGM proteins increased and 19 decreased in bovine milk (at 7 days of lactation) and bovine colostrum, respectively. Mucin (1 and 15) (7-fold) and the tripartite complex of proteins, ADPF (3.4-fold), BTN (3.2-fold), and XDH (2.6-fold), for example, were found more concentrated in bovine milk compared to colostrum (Reinhardt and Lippolis Citation2008). Other proteins such as acyl-CoA synthetase, lanosterol synthase, lysophosphatidic acid acyltransferase, and fatty acid-binding protein, associated with lipid transport synthesis and secretion, were 2.6- to 5.1-fold more concentrated in bovine milk compared to colostrum. In contrast, apolipoproteins A1, C-III, E, and A-IV were 2.6- to 4.3-fold less concentrated in milk than in the MFGM from colostrum (Reinhardt and Lippolis Citation2008).These may indicate, despite the higher fat content in colostrum, that an early developmental shift in milk fat transport may occur.
Human colostrum samples (up to 4 days after delivery) were also shown to have a larger average MFG size compared to transitional and mature milk (Argov-Argaman et al. Citation2010; Michalski et al. Citation2005; Zou et al. Citation2012). Alpha-amylase, MARCKS-related protein apolipoprotein D, apolipoprotein E, ubiquitin, bone marrow stromal antigen 2, chordin-like protein 2, gamma-glutamyltranspeptidase 1, long-chain fatty-acid-CoA ligase 4, alpha-1-antitrypsin, IGFBP2 and matrilin-3 are highly expressed in early lactation, whereas annexin, complement C3, CD9 antigen, nonspecific lipid transfer protein, fatty acid-binding protein, folate receptor alpha, glutathione peroxidase 3, gelsolin, heat shock protein beta-1, lysozyme C, proactivator polypeptide, BTN and XO are more highly expressed later in lactation (Liao et al. Citation2011).
Martini et al. (Citation2013), studying ovine MFGM composition, reported a lower amount of total membrane proteins and a higher activity of XDH/XO in mature milk compared to 10 h post-partum. This was probably due to either the activation and deactivation of the enzyme during lactation (Benboubetra et al. Citation2004), or a lower expression of XDH/XO as reported in bovine and human colostrum (Reinhardt and Lippolis Citation2008; Liao et al. Citation2011).
7. Milk fat globule bioactive lipids
The lipid fraction of the MFG is composed of TAG (98%), polar lipids and cholesterol. The major polar lipids present in the membrane are SM, PC, PE, PS, and PI. Glycosphingolipids, which are minor constituents of the MFGM, comprise the cerebrosides (neutral glycosphingolipids containing uncharged sugars) and the gangliosides (acidic glycosphingolipids containing sialic acid). Although the TAG fraction is mainly utilized as a source of energy, other beneficial effects of the FA profile on infant development have been well-described and recently reviewed by Lee et al. (Citation2018). In short, mixtures of milk PL were shown to improve cognition, neuroplasticity and myelinization in animal models and clinical studies (Liu et al. Citation2014; Pepeu, Pepeu, and Amaducci Citation1996; Gurnida et al. Citation2012; Oshida et al. Citation2003). Dietary PL was shown to play key biological roles in lipid digestion (Fave, Coste, and Armand Citation2004), absorption and transport (Küllenberg et al. Citation2012) and signaling pathways (Shimizu Citation2009). Dietary PL is also positively associated with inflammation (Dial and Lichtenberger Citation1984), cholesterol absorption (Eckhardt et al. Citation2002), colon cancer (Berra et al. Citation2002) and intestinal maturation (Motouri et al. Citation2003).
The literature suggests that the concentration of milk fat and PL is highly variable among and within species. shows the wide variation of milk fat content reported in ovine (6.5–9.0 g/100 g), caprine (3.5–5.6 g/100 g), bovine (3.4–6.0 g/100 g) and human milk (2.8–3.8 g/100 g). This variation is also observed for the MFG size and, consequently, the concentration of total milk polar lipids (see Sec. 5). These variations are due to the aforementioned differences in breed and maternal geographical origin, diet, sampling time, sample preparation and methods of analysis (Verardo et al. Citation2017; Logan et al. Citation2014; Fleming et al. Citation2017; Couvreur et al. Citation2007). It has been suggested, for example, that 40–72% of PL reported in MFGM lipids depends upon the MFGM isolation method (Fong, Norris, and MacGibbon Citation2007; Thompson and Singh Citation2006). reports the variation of total PL found within species and a snapshot of the literature data on the distribution of PL classes across species (Et-Thakafy, Guyomarc’h, and Lopez Citation2017), comparing the PL composition among bovine, caprine and ovine milk, reported PE as the most abundant PL. Most studies, however, analyzing the concentration of PL in only one species, suggest that SM is the most abundant PL in human, bovine, caprine and ovine MFGM followed by PC (Zou et al. Citation2012; Lopez and Ménard Citation2011; Garcia et al. Citation2012; Murgia, Mele, and Monduzzi Citation2003; Andreotti, Trivellone, and Motta Citation2006) and PE (Claumarchirant et al. Citation2016; Giuffrida et al. Citation2013; Benoit et al. Citation2010; Donato et al. Citation2011; Garcia et al. Citation2012).
The ratio between cholesterol and SM in milk was shown to be higher in bovine, compared to goat and sheep milk, which was shown to impact structural behavior of liquid-ordered domains in the MFGM (see Sec. 4) (Et-Thakafy, Guyomarc’h, and Lopez Citation2017). These differences may have consequences for the dynamics of MFGM structure in relation to temperature during cooling, storage, heating and also digestion (Et-Thakafy, Guyomarc’h, and Lopez Citation2017).
7.1. Size and lipid composition
It is well-described in the literature that the MFG size is negatively associated with the PL/TAG ratio (reviewed by Smoczyński Citation2017). The most common theory that explains this negative association is the inability of the mammary gland secretory cells to increase the production of membrane material (PL concentration) with higher fat yield (TG). It has also been suggested that, in early lactation days, the larger globules observed could also be related to mammary gland immaturity, which is unable to produce more membrane (McManaman Citation2009).
Recently, it has been reported that the proportion of dietary FA (or preformed) in small MFGs were higher than large MFGs, whereas the proportion of de novo FA did not differ between the groups (Walter et al. Citation2020). The FA incorporated into milk fat in the MEC are obtained from de novo synthesis in the mammary gland (chain length of C4 to C16) or derived from the blood as preformed FA after hydrolysis of TAG from the diet or adipose tissue (chain length of C16 or higher; Palmquist Citation2006). The balance between the two pathways depends on the physiological state of the animal (Palmquist Citation2006). The authors suggest two possible explanations for this observation; long-chain SFAs were was utilized to PL needed for increased membrane material required for the formation of smaller MFGs, or the observed changes could indicate increased adipose tissue lipid utilization in the small MFG group (Walter et al. Citation2020; Mesilati-Stahy, Malka, and Argov-Argaman Citation2012).
Few studies, so far, have investigated the differences in PL composition among subpopulations of bovine MFG. Lopez et al. (Citation2011) reported that individual polar lipids (PE, PI, PS, PC and SM) were higher in SMFG-fractions (1.6 µm) than in whole milk (4.2 µm) and LMFG-fractions (6.5 µm). Logan et al. (Citation2014) also reported an increase in total (%) PL and PC in smaller (3.6 ± 0.2 μm) compared to larger globules (4.6 ± 0.3 μm). In contrast, Mesilati-Stahy, Mida, and Argov-Argaman (Citation2011) reported that only PI increased in MFGs with an average diameter similar to, or lower than 2 μm, whereas PC, PS, PE and SM were enriched in MFGs with an average diameter of 3 μm. Lu, Argov-Argaman, et al. (Citation2016), studying the composition of two different bovine MFG subpopulations, found increased concentrations (%) of PE and cholesterol in SMFG (3.3 ± 1.2 μm), and PI in LMFG (7.6 ± 0.9 μm). These conflicting results suggested that PL concentration may be affected not only by MFG size but also by other physiological conditions such as stage of lactation (Mesilati-Stahy and Argov-Argaman Citation2014; Logan et al. Citation2014).
Human milk composition varies considerably during lactation, particularly during the transition from colostrum to milk. Marie-Caroline Michalski, Briard, Michel, et al. (Citation2005) reported that on the delivery day, most of the globules were ≥10 μm, and by the fourth lactation day, most of the globules in human milk were less than 1 μm. This correlates with the increase in total PL concentration and fat observed from colostrum to mature milk (Bitman et al. Citation1983; Boersma et al. Citation1991; Claumarchirantet al. Citation2016; Lopez et al. Citation2011; X.-Q. Zou et al. Citation2012). Changes in PL and FA were also observed as follows: PC, PI, PE and PS concentration were shown to increase during the transition from colostrum to mature milk, whereas SM remained stable (Lopez et al. Citation2011; X.-Q. Zou et al. Citation2012). C16:0 was found to be the most abundant FA in colostrum and milk, followed by C18:1 ω-9, C18:2 ω-6 and C18:0. C16:0 decreased from colostrum to milk, whereas the opposite trend was shown for C18:0, ω-3 PUFAs and ω-6 PUFA (Lopez et al. Citation2011; X.-Q. Zou et al. Citation2012).
Total PL content was shown to increase from colostrum to mature bovine milk (Zou et al. Citation2015) but decreases in later lactation (180 days) (Bitman and Wood Citation1990). This is associated with higher levels of milk fat post-partum, and the lowest concentration around the peak of lactation (Mesilati-Stahy & Argov-Argaman, Citation2014). Another study, however, reported that total PL decreased 24 h post-partum and did not change from 48 h to 50 days of lactation (Contarini et al. Citation2014). In terms of specific PL, in general, no differences were observed in PC concentration, whereas SM was shown to decrease, and PE, PI and PS increase during lactation (Contarini et al. Citation2014; Zou et al. Citation2015). In terms of FAs, a higher concentration of short-chain FAs was reported in milk compared with late lactation (Ducháček et al. Citation2013). Mature bovine milk was also shown to have higher saturated FAs compared to colostrum, whereas colostrum has higher MUFA and PUFA (Zou et al. Citation2015). C18:1 ω-9 was found to be the most abundant FA in colostrum and milk, followed by C16:0, C18:0 and C18:2 ω-6. C18:1 ω-9 was shown to decrease, whereas C:16 increased from colostrum to milk (Bitman and Wood Citation1990; Zou et al. Citation2015), which is in agreement with the FA profile observed for LMFG.
summarizes the significant differences in unsaturated, medium, and long-chain saturated FA between the globule size fractions ≤2 μm, around 3 μm, and 5–8 μm, as reported in the literature for bovine milk. In general, medium-chain FAs (C10:1, C11, C12, C12:1, C13), myristic (C14:0) and pentadecanoic acid (C15:0), when individually analyzed, were more concentrated in smaller (<3 μm) compared to the larger globules (≥3 μm) (Fauquant et al. Citation2005; Michalski, Briard, and Juaneda Citation2005; Lopez et al. Citation2011; Lu, Argov-Argaman, et al. Citation2016). The amount of long-chain SFA (C17:0, C18:0, and C20:0) is significantly higher in the large MFG fraction, whereas that of total UFA (PUFA, MUFA, and CLA) is higher in the small MFG fraction (Lu, Argov-Argaman, et al. Citation2016). Some of these results have been reported by other authors (Timmen and Patton Citation1988; Briard et al. Citation2003; Lopez et al. Citation2011; Michalski, Briard, and Juaneda Citation2005; Walter et al. Citation2020) ().
Table 4. Comparison of milk fat globule fatty acid composition in different bovine milk fat globules sizes.
The human and bovine MFGM is relatively rich in UFAs compared to the core TAGs; the higher proportion of UFAs in the small MFG fraction may be related to the relative increase in membrane material compared to the large globules (Lu, Argov-Argaman, et al. Citation2016). In contrast, ovine MFGM was shown to contain more SFA (C16:0 (+21.5%) and C18:0 (+67.64%), and PUFAs (+48.66%)) compared to the core TAGs which contained higher content of MUFAs (+12.36%) and short chain fatty acids (+640.42%). Changes in MFGM lipid PL composition were also shown to be regulated by the developmental status of the infant at delivery. A recent study, for example, showed that higher concentrations of several species of PC, PE, SM and total PL were found in preterm milk throughout lactation (Ingvordsen Lindahl et al. Citation2019). This may indicate different developmental requirements between preterm and term infants which could be met by the selection and purification of ruminant MFGs.
8. Milk fat globule size, processing and digestion
Lipid digestion is an interfacial process where the MFG size, composition and structure have a key role in the digestion and absorption of milk fat. Small globules, for example, have a greater specific surface area available for lipase adsorption and enzymatic action compared to larger globules, which contributes to faster digestion (Garcia et al. Citation2014). Therefore, as MFG size ranges from 0.1 to 15 μm, a wide variation in the digestion kinetics of milk fat globules may be observed (Bourlieu and Michalski Citation2015). Compositional differences among the different sizes could also affect digestion due to interaction of proteins and lipids with lipases and bile salts.
Homogenization and pasteurization affect digestion rate, the release of FAs, and MFG size and microstructure (Zhao, Du, and Mao Citation2019). Homogenized MFGs are digested more rapidly and release more FA than native globules, but not as much as the specific surface area increase would dictate, suggesting an important role for the MFGM in aiding digestion (Berton et al. Citation2012; Zhao, Du, and Mao Citation2019). Homogenization also leads to binding of milk proteins to the MFGM, which has more affinity for pepsin and pancreatic lipase, and losses of MFGM native proteins (BTN, PAS6/7 and mucins), which are resistant to proteases (Ye et al. Citation2017).
As reported above, the glycosylated proteins such as mucin 1 and 15, ADPF, BTN, and XDH were more concentrated in MFGM from bovine milk or the smaller MFG fraction, compared to colostrum or the larger MFG fraction (Reinhardt and Lippolis Citation2008; Lu, Argov-Argaman, et al. Citation2016). Increased glycosylation of the proteins present in the MFGM may help the MFGs to resist digestion in the first parts of the gastrointestinal tract and retain this biological effect into the large intestine. For some of the proteins (mucin and lactadherin), gastric stability has been shown (Peterson et al. Citation1998; Le et al. Citation2012) that may affect the immune system and protect against bacteria and viruses in the neonatal gastrointestinal tract (Ye, Cui, and Singh Citation2011). Other glycosylated proteins show different levels of resistance to gastric proteases in in vitro studies (Ye, Cui, and Singh Citation2011; Gallier, Ye, and Singh Citation2012; Le et al. Citation2012). At a very low pH (1.6) gastric simulated digestion, BTN was more resistant to proteases than other MFGM glycoproteins, such as XO and bovine lactadherin (PAS 6/7), although all proteins were fully digested after 15 min of incubation with pepsin at 1.6 mg/mL. It is interesting to note that the carbohydrate composition of the protein was shown to affect the resistance against proteolytic activity in the gastrointestinal tract. PAS 6 (which contains an additional high-mannose type N-glycan) was slightly less sensitive than PAS 7 to 0.1 mg/mL pepsin treatment (Ye, Cui, and Singh Citation2011). Another study showed that lactadherin, ADPF, PAS III and CD36 from human MFG could still be detected after 3 h of pepsin digestion (Le et al. Citation2012). Although these studies used different methodologies, species-specific differences in the resistance to pepsin digestion cannot be ruled out.
Compositions of MFG FAs and triglycerides are not affected by homogenization and thermal processing, and the profiles of released FA may relate to the digestion rate of MFGs (Gallier, Cui, et al. Citation2013; Gallier, Zhu, et al. Citation2013). Lipid profiles of differently sized MFG, however, may also affect digestion. As reported in , smaller fat globules in bovine milk contain less long-chain FAs but more medium-chain FAs and myristic acid in the core. Therefore, considering that gastric lipase selectively hydrolyzes short- and medium-chain FAs (Sams et al. Citation2016), and also that the interfacial composition will have an impact on the adsorption of gastric lipase (Bourlieu et al. Citation2014), the size of fat globules will affect digestion rate and release of FAs. PL profile and concentration may also affect lipid absorption and postprandial lipid metabolism. A milk PL ingredient rich in SM, for example, was shown to improve postprandial lipemia, gastric emptying and intestinal absorption in mice (Lecomte et al. Citation2015) compared to a soya rich ingredient rich in PC and PI.
The MFGM structure may also affect digestion. The presence of SM in association with cholesterol in the membrane under digestive conditions and subsequent putative absorption through intestinal epithelial cell layers has not been demonstrated mechanistically; however, this association may have implications for the absorption of cholesterol into the cardiovascular system from dietary sources (Garmy et al. Citation2005). Clinical studies have indicated that consumption of hard cheese has a positive effect on reducing plasma high- and low-density lipoprotein cholesterol levels, compared to butter where the MFGM is in a more disrupted state, indicating the importance of the lipid matrix in delivering health outcomes (Hjerpsted, Leedo, and Tholstrup Citation2011; De Goede et al. Citation2015).
Another parameter that may affect the digestion of MFGs of different sizes is the ζ-potential, the electric potential at the surface of shear of fat globules, which may reflect the glycoprotein and glycolipid composition of the MFGM as well as the mineral composition of the milk. According to Zou et al. (Citation2012) and others (Michalski et al. Citation2005; Lopez et al. Citation2011; Ménard et al. Citation2010; Cebo et al. Citation2012), mature human MFG has a smaller ζ-potential (−7.25 ± 0.61 mV) compared to bovine (−13.5 ± 0.9 mV), caprine (−12.0 ± 1.1 mV) and ovine MFG, although it must be noted that the ζ-potential depends upon the local ionic environment. These authors also described differences across human lactation stages, where the ζ-potentials for colostrum, transitional, and mature MFG were −5.60 ± 0.12, −6.72 ± 0.16, and −7.25 ± 0.61 mV, respectively. From a colloidal point of view, MFGs with large positive or negative ζ-potentials are electrostatically more stable against flocculation, whereas globules with small ζ-potentials tend to coagulate or flocculate. The smaller ζ-potential of human MFG may assist in digestion and metabolism of human milk fat in infants (Michalski et al. Citation2005). Increased flocculation, however, could reduce the surface area and obstruct the interfacial access of digestive enzymes and bile salts (Wilde and Chu Citation2011). The significance, if any, of ζ-potential on MFG digestibility is not entirely clear.
MFG size selection could be used to enrich dairy products and ingredients with specific components with or without the native MFG structure. Buttermilk, which can be obtained by churning cream into butter (i.e. sweet buttermilk) or churning cultured cream into lactic butter (i.e. traditional cultured buttermilk) contain residues of MFGM (Conway, Gauthier, and Pouliot Citation2014; Conway et al. Citation2014). Although losses in protein and lipid components are observed during churning, buttermilk is still a good source of phospholipids with observed health benefits (Fuller et al. Citation2013). Dairy ingredients containing MFGM, known as phospholipid-enriched dairy ingredients, are commercially available for food fortification. The phospholipid-enriched ingredients are produced from beta-serum, buttermilk or whey using a combination of different technologies (e.g. microfiltration, supercritical CO2 extraction, solvent extraction, etc.) (Huang et al. Citation2020). Those products are enriched in MFGM phospholipids, milk proteins and lactose with no membrane structure remaining (Vanderghem et al. Citation2010). Although, not structured in a trilayer membrane, PL dairy ingredients are digested and absorbed, showing beneficial health effects for the infant when added to infant formula (Timby et al. Citation2014; Mudd et al. Citation2016; Bhinder et al. Citation2017; Sprong et al. Citation2012; Snow et al. Citation2011; Huang et al. Citation2019) and supplemented to older adults (Watanabe et al. Citation2020).
Microfiltration is a direct way to produce a purified milk fat globule product with specified MFG size (Jukkola et al. Citation2016). Recently, Hansen, et al. Citation2020 produced a MFGM isolate containing 7% w/w polar lipids and 30% w/w proteins, where contamination by non-MFGM proteins was only 25% of total protein content. Moreover, mild pasteurization (72 °C, 15 s) introduced either before or after microfiltration had no impact on filtration efficiency or MFGM yield and composition (Hansen et al. Citation2020). Microfiltration may be the best method to obtain a less processed MFG fraction, enriched in specific size-related components.
9. Conclusion
Size and composition of MFG are species-specific but can be influenced by breed (or maternal origin), diet and lactation period. Although in isolation, all factors above were shown to have detectable effect on MFG size, they do not sufficiently explain the wide individual variation observed in MFGs. There is evidence, however, that physiological state (i.e. stage of lactation, metabolic state) outweigh the effects of milk production traits (i.e. genetic) and environmental conditions (e.g. diet) on MFG size.
New insights suggest that post-secretion modifications by membrane vesiculation or MFG fusion offer other levels of regulation of MFG size and structure. This post-secretion modification could be driven by genetics but could also be influenced by the maternal physiological state. There is a need for better understanding of the genetic and environmental factors regulating the structural characteristics of the MFG.
Changes observed in the size range of MFG secreted during lactation may have an essential physiological role for the developing infant, but studies linking composition and infant development are lacking. Small vesicles (<1 µm), with reduced TAG core, were shown to be highly concentrated in human and ruminant milk and may have a direct function to deliver bioactive components to the infant. Moreover, more studies are needed comparing the in vivo digestibility of the different MFG size fractions to help clarify the release and activity of the MFGM components and the interaction of the different components of MFGM with the gastrointestinal tract.
Purification and enrichment methods for several of the bioactive proteins and lipids present in the MFGM have not been established, or not scaled sufficiently to be able to be used to supplement infant diets. To overcome this problem, two strategies could be applied for increasing the concentration of specific MFGM protein and lipid components. (1) MFG size selection by naturally selecting animals that produce small or large globules; or (2) isolating MFGs by size fractionation. Thus, a selective fractionation of specific ruminant MFG could be used to fulfill human nutritional and health requirements.
Author contribution
C.T. compiled research data and wrote the manuscript. D.W.E. provided revisions of scientific content. N.C.R. and W.C.M. provided revisions of scientific content and funding.
| Nomenclature | ||
| ADPF | = | adipophilin |
| BTN | = | butyrophilin |
| FA | = | fatty acids |
| LD | = | lipid droplets |
| LMFG | = | large milk fat globules |
| MEC | = | mammary epithelial cell |
| MFG | = | milk fat globule |
| MFGM | = | milk fat globule membrane |
| PC | = | phosphatidylcholine |
| PE | = | phosphatidylethanolamine |
| PI | = | phosphatidylinositol |
| PL | = | phospholipid |
| PS | = | phosphatidylserine |
| PUFAs | = | polyunsaturated fatty acids |
| SM | = | sphingomyelin |
| SMFG | = | small milk fat globules |
| SNARE-soluble N-ethylmaleimide | = | sensitive fusion attachment protein receptor |
| TAG | = | triacylglycerol |
| XDH | = | xanthine dehydrogenase |
| XOR | = | xanthine oxidoreductase |
| XDH/XO | = | xanthine dehydrogenase/oxidase. |
Supplemental Material
Download MS Word (36.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Addis, M. F., V. Bronzo, G. M. G. Puggioni, C. Cacciotto, V. Tedde, D. Pagnozzi, C. Locatelli, A. Casula, G. Curone, S. Uzzau, et al. 2017. Relationship between milk cathelicidin abundance and microbiologic culture in clinical mastitis. Journal of Dairy Science 100 (4):2944–53. doi: 10.3168/jds.2016-12110.
- Admyre, C., S. M. Johansson, K. R. Qazi, J.-J. Filén, R. Lahesmaa, M. Norman, E. P. Neve, A. Scheynius, and S. Gabrielsson. 2007. Exosomes with immune modulatory features are present in human breast milk. Journal of Immunology (Baltimore, MD: 1950) 179 (3):1969–78. doi: 10.4049/jimmunol.179.3.1969.
- Adriano, B., N. M. Cotto, N. Chauhan, M. Jaggi, S. C. Chauhan, and M. M. Yallapu. 2021. Milk exosomes: Nature's abundant nanoplatform for theranostic applications. Bioactive Materials 6 (8):2479–90. doi: 10.1016/j.bioactmat.2021.01.009.
- Andersson, Y., K. Sävman, L. Bläckberg, and O. Hernell. 2007. Pasteurization of mother's own milk reduces fat absorption and growth in preterm infants. Acta Paediatrica 96 (10):1445–9. doi: 10.1111/j.1651-2227.2007.00450.x.
- Andreotti, G., E. Trivellone, and A. Motta. 2006. Characterization of buffalo milk by 31P-nuclear magnetic resonance spectroscopy. Journal of Food Composition and Analysis 19 (8):843–9. doi: 10.1016/j.jfca.2006.03.014.
- Antonakou, A., K. P. Skenderi, A. Chiou, C. A. Anastasiou, C. Bakoula, and A.-L. Matalas. 2013. Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding Greek women. European Journal of Nutrition 52 (3):963–73. doi: 10.1007/s00394-012-0403-8.
- Argov-Argaman, N. 2019. Symposium review: Milk fat globule size: Practical implications and metabolic regulation. Journal of Dairy Science 102 (3):2783–95. doi: 10.3168/jds.2018-15240.
- Argov-Argaman, N., C. Raz, and Z. Roth. 2020. Progesterone regulation of milk fat globule size is VLDL dependent. Frontiers in Endocrinology 11:596. doi: 10.3389/fendo.2020.00596.
- Argov-Argaman, N., J. T. Smilowitz, D. A. Bricarello, M. Barboza, L. Lerno, J. W. Froehlich, H. Lee, A. M. Zivkovic, D. G. Lemay, S. Freeman, et al. 2010. Lactosomes: Structural and compositional classification of unique nanometer-sized protein lipid particles of human milk. Journal of Agricultural and Food Chemistry 58 (21):11234–42. doi: 10.1021/jf102495s.
- Argov-Argaman, N., T. Mbogori, C. Sabastian, A. Shamay, and S. J. Mabjeesh. 2012. Hyperinsulinemic clamp modulates milk fat globule lipid composition in goats. Journal of Dairy Science 95 (10):5776–87. doi: 10.3168/jds.2012-5569.
- Argov-Argaman, N., R. Mesilati-Stahy, Y. Magen, and U. Moallem. 2014. Elevated concentrate-to-forage ratio in dairy cow rations is associated with a shift in the diameter of milk fat globules and remodeling of their membranes. Journal of Dairy Science 97 (10):6286–95. doi: 10.3168/jds.2014-8174.
- Argov-Argaman, N., O. Hadaya, T. Glasser, H. Muklada, L. Dvash, R. Mesilati-Stahy, and S. Y. Landau. 2016. Milk fat globule size, phospholipid contents and composition of milk from purebred and Alpine-crossbred Mid-Eastern goats under confinement or grazing condition. International Dairy Journal 58:2–8. doi: 10.1016/j.idairyj.2015.12.003.
- Armand, M., B. Pasquier, M. André, P. Borel, M. Senft, J. Peyrot, J. Salducci, H. Portugal, V. Jaussan, and D. Lairon. 1999. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. The American Journal of Clinical Nutrition 70 (6):1096–106. doi: 10.1093/ajcn/70.6.1096.
- Attaie, R., and R. L. Richter. 2000. Size distribution of fat globules in goat milk. Journal of Dairy Science 83 (5):940–4. doi: 10.3168/jds.S0022-0302(00)74957-5.
- Barłowska, J., M. Szwajkowska, Z. Litwińczuk, and J. Król. 2011. Nutritional value and technological suitability of milk from various animal species used for dairy production. Comprehensive Reviews in Food Science and Food Safety 10 (6):291–302. doi: 10.1111/j.1541-4337.2011.00163.x.
- Benboubetra, M., A. Baghiani, D. Atmani, and R. Harrison. 2004. Physicochemical and kinetic properties of purified sheep's milk xanthine oxidoreductase. Journal of Dairy Science 87 (6):1580–4. doi: 10.3168/jds.S0022-0302(04)73311-1.
- Benoit, B., C. Fauquant, P. Daira, N. Peretti, M. Guichardant, and M.-C. Michalski. 2010. Phospholipid species and minor sterols in French human milks. Food Chemistry 120 (3):684–91. doi: 10.1016/j.foodchem.2009.10.061.
- Berra, B., I. Colombo, E. Sottocornola, and A. Giacosa. 2002. Dietary sphingolipids in colorectal cancer prevention. European Journal of Cancer Prevention 11 (2):193–7.
- Berton, A., S. Rouvellac, B. Robert, F. Rousseau, C. Lopez, and I. Crenon. 2012. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized milk fat globules. Food Hydrocolloids 29 (1):123–34. doi: 10.1016/j.foodhyd.2012.02.016.
- Bharadwaj, S., A. G. T. Naidu, G. V. Betageri, N. V. Prasadarao, and A. S. Naidu. 2009. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporosis International 20 (9):1603–11. doi: 10.1007/s00198-009-0839-8.
- Bhinder, G., J. M. Allaire, C. Garcia, J. T. Lau, J. M. Chan, N. R. Ryz, E. S. Bosman, F. A. Graef, S. M. Crowley, L. S. Celiberto, et al. 2017. Milk fat globule membrane supplementation in formula modulates the neonatal gut microbiome and normalizes intestinal development. Scientific Reports 7, 45274. doi: 10.1038/srep45274.
- Bianchi, L., C. Casoli, M. Pauselli, M. Pecchiai, E. Duranti, F. Cecchi, M. C. Martini, F. Salari, L. Chianese, and S. D. Pascale. 2004. Preliminary study on Sopravissana sheep milk production [Umbria (Italy)]. Scienza e Tecnica Lattiero Casearia 55 (5):319–43.
- Bianchi, L., M. Puglia, C. Landi, S. Matteoni, D. Perini, A. Armini, M. Verani, C. Trombetta, P. Soldani, P. Roncada, et al. 2009. Solubilization methods and reference 2-DE map of cow milk fat globules. Journal of Proteomics 72 (5):853–64. doi: 10.1016/j.jprot.2008.11.020.
- Bieli, C., W. Eder, R. Frei, C. Braun-Fahrländer, W. Klimecki, M. Waser, J. Riedler, E. von Mutius, A. Scheynius, G. Pershagen, PARSIFAL study group, et al. 2007. A polymorphism in CD14 modifies the effect of farm milk consumption on allergic diseases and CD14 gene expression. The Journal of Allergy and Clinical Immunology 120 (6):1308–15. doi: 10.1016/j.jaci.2007.07.034.
- Bitman, J., L. Wood, M. Hamosh, P. Hamosh, and N. R. Mehta. 1983. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. The American Journal of Clinical Nutrition 38 (2):300–12. doi:10.1093/ajcn/38.2.300. PMID:6881084
- Bitman, J, and D. L. Wood. 1990. Changes in milk fat phospholipids during lactation. Journal of Dairy Science 73 (5):1208–16. doi:10.3168/jds.S0022-0302(90)78784-X. PMID:2365882
- Bläckberg, L., and O. Hernell. 1983. Further characterization of the bile salt-stimulated lipase in human milk. FEBS Letters 157 (2):337–41. doi: 10.1016/0014-5793(83)80571-7.
- Bläckberg, L., R.-D. Duan, and B. Sternby. 1997. Purification of carboxyl ester lipase (bile salt-stimulated lipase) from human milk and pancreas. Methods in Enzymology, 285:185–94.
- Blans, K., M. S. Hansen, L. V. Sørensen, M. L. Hvam, K. A. Howard, A. Möller, L. Wiking, L. B. Larsen, and J. T. Rasmussen. 2017. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. Journal of Extracellular Vesicles 6 (1):1294340. doi: 10.1080/20013078.2017.1294340.
- Boersma, E. R., P. J. Offringa, F. A. Muskiet, W. M. Chase, and I. J. Simmons. 1991. Vitamin E, lipid fractions, and fatty acid composition of colostrum, transitional milk, and mature milk: an international comparative study. The American Journal of Clinical Nutrition 53 (5):1197–204. doi:10.1093/ajcn/53.5.1197. PMID:2021129
- Boots, J.-W., and R. Floris. 2006. Lactoperoxidase: From catalytic mechanism to practical applications. International Dairy Journal 16 (11):1272–6. doi: 10.1016/j.idairyj.2006.06.019.
- Borzouee, F., M. R. Mofid, J. Varshosaz, and S. Z. A. Samsam Shariat. 2016. Purification of lactoperoxidase from bovine whey and investigation of kinetic parameters. Advanced Biomedical Research 5 (1):189. doi: 10.4103/2277-9175.192738.
- Bourlieu, C., and M.-C. Michalski. 2015. Structure-function relationship of the milk fat globule. Current Opinion in Clinical Nutrition and Metabolic Care 18 (2):118–27. doi: 10.1097/MCO.0000000000000138.
- Bourlieu, C., O. Menard, K. Bouzerzour, G. Mandalari, A. Macierzanka, A. R. Mackie, and D. Dupont. 2014. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Critical Reviews in Food Science and Nutrition 54 (11):1427–57. doi: 10.1080/10408398.2011.640757.
- Briard, V., N. Leconte, F. Michel, and M.-C. Michalski. 2003. The fatty acid composition of small and large naturally occurring milk fat globules. European Journal of Lipid Science and Technology 105 (11):677–82. doi: 10.1002/ejlt.200300812.
- Bullen, J. J., H. J. Rogers, and L. Leigh. 1972. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. British Medical Journal 1 (5792):69–75. doi: 10.1136/bmj.1.5792.69.
- Cavaletto, M., M. G. Giuffrida, and A. Conti. 2008. Milk fat globule membrane components–a proteomic approach. In: Bioactive components of milk: advances in experimental medicine and biology, ed. Z. Bösze, vol 606. New York, NY: Springer. doi:10.1007/978-0-387-74087-4_4
- Ceballos, L. S., E. R. Morales, G. de la Torre Adarve, J. D. Castro, L. P. Martínez, and M. R. S. Sampelayo. 2009. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. Journal of Food Composition and Analysis 22 (4):322–9. doi: 10.1016/j.jfca.2008.10.020.
- Cebo, C., and P. Martin. 2012. Inter-species comparison of milk fat globule membrane proteins highlights the molecular diversity of lactadherin. International Dairy Journal 24 (2):70–7. doi: 10.1016/j.idairyj.2011.09.017.
- Cebo, C., C. Lopez, C. Henry, C. Beauvallet, O. Ménard, C. Bevilacqua, F. Bouvier, H. Caillat, and P. Martin. 2012. Goat α(s1)-casein genotype affects milk fat globule physicochemical properties and the composition of the milk fat globule membrane. Journal of Dairy Science 95 (11):6215–29. doi: 10.3168/jds.2011-5233.
- Chabance, B., P. Marteau, J. C. Rambaud, D. Migliore-Samour, M. Boynard, P. Perrotin, R. Guillet, P. Jolles, and A. M. Fiat. 1998. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 80 (2):155–65. doi: 10.1016/s0300-9084(98)80022-9.
- Cho, J.-K., N. Azuma, C.-H. Lee, J.-H. Yu, and C. Kanno. 2000. Purification of membrane-bound lactoferrin from the human milk fat globule membrane. Bioscience, Biotechnology, and Biochemistry 64 (3):633–5. doi: 10.1271/bbb.64.633.
- Chong, B. M., P. Reigan, K. D. Mayle-Combs, D. J. Orlicky, and J. L. McManaman. 2011. Determinants of adipophilin function in milk lipid formation and secretion. Trends in Endocrinology and Metabolism: TEM 22 (6):211–7. doi:10.1016/j.tem.2011.04.003. PMID:21592818
- Chong, B. M., T. D. Russell, J. Schaack, D. J. Orlicky, P. Reigan, M. Ladinsky, and J. L. McManaman. 2011. The adipophilin C terminus is a self-folding membrane-binding domain that is important for milk lipid secretion. The Journal of Biological Chemistry 286 (26):23254–65. doi: 10.1074/jbc.M110.217091.
- Claumarchirant, L., A. Cilla, E. Matencio, L. M. Sanchez-Siles, P. Castro-Gomez, J. Fontecha, A. Alegría, and M. J. Lagarda. 2016. Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. International Dairy Journal 61:228–38. doi: 10.1016/j.idairyj.2016.06.005.
- Cohen, B.-C., A. Shamay, and N. Argov-Argaman. 2015. Regulation of lipid droplet size in mammary epithelial cells by remodeling of membrane lipid composition—A potential mechanism. PLoS One 10 (3):e0121645. doi: 10.1371/journal.pone.0121645.
- Communod, R., S. Guida, D. Vigo, V. Beretti, E. Munari, C. Colombani, P. Superchi, and A. Sabbioni. 2013. Body measures and milk production, milk fat globules granulometry and milk fatty acid content in Cabannina cattle breed. Italian Journal of Animal Science 12 (1):e18. doi: 10.4081/ijas.2013.e18.
- Contarini, G., M. Povolo, V. Pelizzola, L. Monti, A. Bruni, L. Passolungo, F. Abeni, and L. Degano. 2014. Bovine colostrum: changes in lipid constituents in the first 5 days after parturition. Journal of Dairy Science 97 (8):5065–72. doi:10.3168/jds.2013-7517. PMID:24931528
- Conway, V., P. Couture, S. Gauthier, Y. Pouliot, and B. Lamarche. 2014. Effect of buttermilk consumption on blood pressure in moderately hypercholesterolemic men and women. Nutrition 30 (1):116–9. doi: 10.1016/j.nut.2013.07.021.
- Conway, V., S. F. Gauthier, and Y. Pouliot. 2014. Buttermilk: Much more than a source of milk phospholipids. Animal Frontiers 4 (2):44–51. doi: 10.2527/af.2014-0014.
- Coolbear, K. P., D. F. Elgar, T. Coolbear, and J. S. Ayers. 1996. Comparative study of methods for the isolation and purification of bovine kappa-casein and its hydrolysis by chymosin. The Journal of Dairy Research 63 (1):61–71. doi: 10.1017/s002202990003154x.
- Couvreur, S., C. Hurtaud, C. Lopez, L. Delaby, and J.-L. Peyraud. 2006. The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties. Journal of Dairy Science 89 (6):1956–69. doi: 10.3168/jds.S0022-0302(06)72263-9.
- Couvreur, S., C. Hurtaud, P. G. Marnet, P. Faverdin, and J. L. Peyraud. 2007. Composition of milk fat from cows selected for milk fat globule size and offered either fresh pasture or a corn silage-based diet. Journal of Dairy Science 90 (1):392–403. doi: 10.3168/jds.S0022-0302(07)72640-1.
- Danielsson Niemi, L., O. Hernell, and I. Johansson. 2009. Human milk compounds inhibiting adhesion of mutans streptococci to host ligand-coated hydroxyapatite in vitro. Caries Research 43 (3):171–8. doi: 10.1159/000213888.
- De Goede, J., J. M. Geleijnse, E. L. Ding, and S. S. Soedamah-Muthu. 2015. Effect of cheese consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews 73 (5):259–75. doi: 10.1093/nutrit/nuu060.
- Deeth, H. C. 1997. The role of phospholipids in the stability of milk fat gobules. Australian Journal of Dairy Technology 52:44–6.
- Dial, E. J., and L. M. Lichtenberger. 1984. A role for milk phospholipids in protection against gastric acid. Studies in adult and suckling rats. Gastroenterology 87 (2):379–85. doi: 10.1016/0016-5085(84)90716-9.
- Donato, P., F. Cacciola, F. Cichello, M. Russo, P. Dugo, and L. Mondello. 2011. Determination of phospholipids in milk samples by means of hydrophilic interaction liquid chromatography coupled to evaporative light scattering and mass spectrometry detection. Journal of Chromatography A 1218 (37):6476–82. doi: 10.1016/j.chroma.2011.07.036.
- Dowbenko, D., A. Kikuta, C. Fennie, N. Gillett, and L. A. Lasky. 1993. Glycosylation-dependent cell adhesion molecule 1 (GlyCAM 1) mucin is expressed by lactating mammary gland epithelial cells and is present in milk. The Journal of Clinical Investigation 92 (2):952–60. doi: 10.1172/JCI116671.
- Ducháček, J., L. Stádník, J. Beran, M. Okrouhlá, M. Vacek, and M. Doležalová. 2013. Body condition score and milk fatty acid composition in early lactation of Czech Fleckvieh cows. Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis 61 (6):1621–8. doi:10.11118/actaun201361061621.
- Eckhardt, E. R., D. Q.-H. Wang, J. M. Donovan, and M. C. Carey. 2002. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 122 (4):948–56. doi: 10.1053/gast.2002.32539.
- Et-Thakafy, O., F. Guyomarc’h, and C. Lopez. 2017. Lipid domains in the milk fat globule membrane: Dynamics investigated in situ in milk in relation to temperature and time. Food Chemistry 220:352–61. doi: 10.1016/j.foodchem.2016.10.017.
- El-Zeini, H. M. 2006. Microstructure, rheological and geometrical properties of fat globules of milk from different animal species. Polish Journal of Food and Nutrition Sciences 15 (2):147–53.
- Elofsson, U. M., P. Dejmek, and M. A. Paulsson. 1996. Heat-induced aggregation of β-lactoglobulin studied by dynamic light scattering. International Dairy Journal 6 (4):343–57. doi:10.1016/0958-6946(95)00019-4.
- Evers, J. M., R. G. Haverkamp, S. E. Holroyd, G. B. Jameson, D. D. Mackenzie, and O. J. McCarthy. 2008. Heterogeneity of milk fat globule membrane structure and composition as observed using fluorescence microscopy techniques. International Dairy Journal 18 (12):1081–9. doi: 10.1016/j.idairyj.2008.06.001.
- Fauquant, C., V. Briard, N. Leconte, and M.-C. Michalski. 2005. Differently sized native milk fat globules separated by microfiltration: Fatty acid composition of the milk fat globule membrane and triglyceride core. European Journal of Lipid Science and Technology 107 (2):80–6. doi: 10.1002/ejlt.200401063.
- Fave, G., T. C. Coste, and M. Armand. 2004. Physicochemical properties of lipids: New strategies to manage fatty acid bioavailability. Cellular and Molecular Biology 50 (7):815–32.
- Fleming, A., F. S. Schenkel, J. Chen, F. Malchiodi, R. A. Ali, B. Mallard, M. Sargolzaei, M. Corredig, and F. Miglior. 2017. Variation in fat globule size in bovine milk and its prediction using mid-infrared spectroscopy. Journal of Dairy Science 100 (3):1640–9. doi: 10.3168/jds.2016-11427.
- Freitas, R. F., M. d S. Macedo, A. d C. Lessa, N. A. V. D. Pinto, and R. A. Teixeira. 2021. Relationship between the diet quality index in nursing mothers and the fatty acid profile of mature breast milk. Revista Paulista de Pediatria 39: 1–10. doi: 10.1590/1984-0462/2021/39/2019089.
- Fong, B. Y., C. S. Norris, and A. K. MacGibbon. 2007. Protein and lipid composition of bovine milk-fat-globule membrane. International Dairy Journal 17 (4):275–88. doi: 10.1016/j.idairyj.2006.05.004.
- Fuller, K. L., T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, R. Jiménez-Flores, and S. M. Donovan. 2013. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. Journal of Dairy Science 96 (6):3488–97. doi: 10.3168/jds.2012-6122.
- Gallier, S., A. Ye, and H. Singh. 2012. Structural changes of bovine milk fat globules during in vitro digestion. Journal of Dairy Science 95 (7):3579–92. doi: 10.3168/jds.2011-5223.
- Gallier, S., D. Gragson, R. Jiménez-Flores, and D. Everett. 2010. Using confocal laser scanning microscopy to probe the milk fat globule membrane and associated proteins. Journal of Agricultural and Food Chemistry 58 (7):4250–7. doi: 10.1021/jf9032409.
- Gallier, S., K. C. Gordon, R. Jiménez-Flores, and D. W. Everett. 2011. Composition of bovine milk fat globules by confocal Raman microscopy. International Dairy Journal 21 (6):402–12. doi: 10.1016/j.idairyj.2011.01.008.
- Gallier, S., J. Cui, T. D. Olson, S. M. Rutherfurd, A. Ye, P. J. Moughan, and H. Singh. 2013. In vivo digestion of bovine milk fat globules: Effect of processing and interfacial structural changes. I. Gastric digestion. Food Chemistry 141 (3):3273–81. doi: 10.1016/j.foodchem.2013.06.020.
- Gallier, S., X. Q. Zhu, S. M. Rutherfurd, A. Ye, P. J. Moughan, and H. Singh. 2013. In vivo digestion of bovine milk fat globules: Effect of processing and interfacial structural changes. II. Upper digestive tract digestion. Food Chemistry 141 (3):3215–23. doi: 10.1016/j.foodchem.2013.06.019.
- Gallier, S., K. Vocking, J. A. Post, B. Van De Heijning, D. Acton, E. M. Van Der Beek, and T. Van Baalen. 2015. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids and Surfaces B, Biointerfaces 136:329–39. doi: 10.1016/j.colsurfb.2015.09.024.
- Garcia, C., N. W. Lutz, S. Confort-Gouny, P. J. Cozzone, M. Armand, and M. Bernard. 2012. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chemistry 135 (3):1777–83. doi: 10.1016/j.foodchem.2012.05.111.
- Garcia, C., C. Antona, B. Robert, C. Lopez, and M. Armand. 2014. The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocolloids 35:494–504. doi: 10.1016/j.foodhyd.2013.07.005.
- Garmy, N., N. Taïeb, N. Yahi, and J. Fantini. 2005. Interaction of cholesterol with sphingosine: Physicochemical characterization and impact on intestinal absorption. Journal of Lipid Research 46 (1):36–45. doi: 10.1194/jlr.M400199-JLR200.
- Giansanti, F., G. Panella, L. Leboffe, and G. Antonini. 2016. Lactoferrin from milk: Nutraceutical and pharmacological properties. Pharmaceuticals 9 (4):61. doi: 10.3390/ph9040061.
- Giuffrida, F., C. Cruz-Hernandez, B. Flück, I. Tavazzi, S. K. Thakkar, F. Destaillats, and M. Braun. 2013. Quantification of phospholipids classes in human milk. Lipids 48 (10):1051–8. doi: 10.1007/s11745-013-3825-z.
- Gobbetti, M., F. Minervini, and C. G. Rizzello. 2007. Bioactive peptides in dairy products. In Handbook of Food Products Manufacturing, 489–517. USA: John Wiley & Sons Inc.
- Gorlov, I. F., M. I. Slozhenkina, N. I. Mosolova, O. Y. Mishina, and E. S. Vorontsova. 2019. Productivity and biological value of milk of cows of various eco-genetic types. IOP Conference Series: Earth and Environmental Science 341:012043. doi: 10.1088/1755-1315/341/1/012043.
- Granucci, F., and I. Zanoni. 2013. Role of CD14 in host protection against infections and in metabolism regulation. Frontiers in Cellular and Infection Microbiology 3:32. doi: 10.3389/fcimb.2013.00032.
- Gross, J., H. A. van Dorland, R. M. Bruckmaier, and F. J. Schwarz. 2011. Milk fatty acid profile related to energy balance in dairy cows. The Journal of Dairy Research 78 (4):479–88. doi: 10.1017/S0022029911000550.
- Guo, H. Y., L. Jiang, S. A. Ibrahim, L. Zhang, H. Zhang, M. Zhang, and F. Z. Ren. 2009. Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. The Journal of Nutrition 139 (5):958–64. doi: 10.3945/jn.108.100586.
- Gurnida, D. A., A. M. Rowan, P. Idjradinata, D. Muchtadi, and N. Sekarwana. 2012. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Human Development 88 (8):595–601. doi: 10.1016/j.earlhumdev.2012.01.003.
- Habte, H. H., G. J. Kotwal, Z. E. Lotz, M. G. Tyler, M. Abrahams, J. Rodriques, D. Kahn, and A. S. Mall. 2007. Antiviral activity of purified human breast milk mucin. Neonatology 92 (2):96–104. doi: 10.1159/000100808.
- Haenlein, G. F. W. 2007. About the evolution of goat and sheep milk production. Small Ruminant Research 68 (1–2):3–6. doi: 10.1016/j.smallrumres.2006.09.021.
- Han, L., M. Zhang, Z. Xing, D. N. Coleman, Y. Liang, J. J. Loor, and G. Yang. 2020. Knockout of butyrophilin subfamily 1 member A1 (BTN1A1) alters lipid droplet formation and phospholipid composition in bovine mammary epithelial cells. Journal of Animal Science and Biotechnology 11 (1):1–11. doi: 10.1186/s40104-020-00479-6.
- Hanayama, R., M. Tanaka, K. Miwa, A. Shinohara, A. Iwamatsu, and S. Nagata. 2002. Identification of a factor that links apoptotic cells to phagocytes. Nature 417 (6885):182–7. doi: 10.1038/417182a.
- Hansen, S. F., S. A. Hogan, J. Tobin, J. T. Rasmussen, L. B. Larsen, and L. Wiking. 2020. Microfiltration of raw milk for production of high-purity milk fat globule membrane material. Journal of Food Engineering 276:109887. doi: 10.1016/j.jfoodeng.2019.109887.
- Hanuš, O., E. Samková, L. Křížová, L. Hasoňová, and R. Kala. 2018. Role of fatty acids in milk fat and the influence of selected factors on their variability—a review. Molecules 23 (7):1636. doi: 10.3390/molecules23071636.
- Harrison, R. 2006. Milk xanthine oxidase: Properties and physiological roles. International Dairy Journal 16 (6):546–54. doi: 10.1016/j.idairyj.2005.08.016.
- Haug, A., A. T. Høstmark, and O. M. Harstad. 2007. Bovine milk in human nutrition—A review. Lipids in Health and Disease 6 (1):25. doi: 10.1186/1476-511X-6-25.
- Heid, H. W., and T. W. Keenan. 2005. Intracellular origin and secretion of milk fat globules. European Journal of Cell Biology 84 (2–3):245–58. doi: 10.1016/j.ejcb.2004.12.002.
- Henrick, B. M., K. Nag, X.-D. Yao, A. G. Drannik, G. M. Aldrovandi, and K. L. Rosenthal. 2012. Milk matters: Soluble Toll-like receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1 infection and inflammation. PLoS One 7 (7):e40138. doi: 10.1371/journal.pone.0040138.
- Henrick, B. M., X.-D. Yao, A. Y. Taha, J. B. German, and K. L. Rosenthal. 2016. Insights into soluble toll-like receptor 2 as a downregulator of virally induced inflammation. Frontiers in Immunology 7:291. doi: 10.3389/fimmu.2016.00291.
- Hettinga, K., H. Van Valenberg, S. De Vries, S. Boeren, T. Van Hooijdonk, J. van Arendonk, and J. Vervoort. 2011. The host defense proteome of human and bovine milk. PLoS One 6 (4):e19433. doi: 10.1371/journal.pone.0019433.
- Hill, R. D., E. Lahav, and D. Givol. 1974. A rennin-sensitive bond in alpha-s1 B-casein. The Journal of Dairy Research 41 (1):147–53. doi: 10.1017/s0022029900015028.
- Hjerpsted, J., E. Leedo, and T. Tholstrup. 2011. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. The American Journal of Clinical Nutrition 94 (6):1479–84. doi: 10.3945/ajcn.111.022426.
- Hochgrebe, T., G. J. Pankhurst, J. Wilce, and S. B. Easterbrook-Smith. 2000. pH-dependent changes in the in vitro ligand-binding properties and structure of human clusterin. Biochemistry 39 (6):1411–9. doi: 10.1021/bi991581b.
- Holzmüller, W., and U. Kulozik. 2016. Technical difficulties and future challenges in isolating membrane material from milk fat globules in industrial settings–A critical review. International Dairy Journal 61:51–66. doi: 10.1016/j.idairyj.2016.03.013.
- Honvo-Houéto, E., C. Henry, S. Chat, S. Layani, and S. Truchet. 2016. The endoplasmic reticulum and casein-containing vesicles contribute to milk fat globule membrane. Molecular Biology of the Cell 27 (19):2946–64. doi: 10.1091/mbc.E16-06-0364.
- Hörl, G., A. Wagner, L. K. Cole, R. Malli, H. Reicher, P. Kotzbeck, H. Köfeler, G. Höfler, S. Frank, J. G. Bogner-Strauss, et al. 2011. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. The Journal of Biological Chemistry 286 (19):17338–50. doi: 10.1074/jbc.M111.234534.
- Howles, P. N., G. N. Stemmerman, C. M. Fenoglio-Preiser, and D. Y. Hui. 1999. Carboxyl ester lipase activity in milk prevents fat-derived intestinal injury in neonatal mice. The American Journal of Physiology 277 (3):G653–61. doi: 10.1152/ajpgi.1999.277.3.G653.
- Huang, Shimeng, Zhenhua Wu, Cong Liu, Dandan Han, Cuiping Feng, Shilan Wang, and Junjun Wang. 2019. Milk fat globule membrane supplementation promotes neonatal growth and alleviates inflammation in low-birth-weight mice treated with lipopolysaccharide. BioMed Research International 2019:4876078. doi: 10.1155/2019/4876078.
- Huang, Z., H. Zheng, C. S. Brennan, M. S. Mohan, L. Stipkovits, L. Li, and D. Kulasiri. 2020. Production of milk phospholipid-enriched dairy ingredients. Foods 9 (3):263. doi: 10.3390/foods9030263.
- Hvarregaard, J., M. H. Andersen, L. Berglund, J. T. Rasmussen, and T. E. Petersen. 1996. Characterization of glycoprotein PAS-6/7 from membranes of bovine milk fat globules. European Journal of Biochemistry 240 (3):628–36. doi: 10.1111/j.1432-1033.1996.0628h.x.
- Ingvordsen Lindahl, I., V. Artegoitia, E. Downey, J. O’Mahony, C.-A. O’Shea, C. Ryan, A. Kelly, H. Bertram, and U. Sundekilde. 2019. Quantification of human milk phospholipids: The effect of gestational and lactational age on phospholipid composition. Nutrients 11 (2):222. doi: 10.3390/nu11020222.
- Jaakamo, M. J., T. J. Luukkonen, P. K. Kairenius, A. R. Bayat, S. A. Ahvenjärvi, T. M. Tupasela, J. H. Vilkki, K. J. Shingfield, and H. M. Leskinen. 2019. The effect of dietary forage to concentrate ratio and forage type on milk fatty acid composition and milk fat globule size of lactating cows. Journal of Dairy Science 102 (10):8825–38. doi: 10.3168/jds.2018-15833.
- Jiang, W., X. Zhang, J. Cheng, J. Song, Q. Jin, W. Wei, M. Dong, and X. Wang. 2020. Variation of fat globule size and fatty acids in human milk in the first 30 days of lactation. International Dairy Journal 100:104567. doi: 10.1016/j.idairyj.2019.104567.
- Jukkola, A., R. Partanen, O. J. Rojas, and A. Heino. 2016. Separation of milk fat globules via microfiltration: Effect of diafiltration media and opportunities for stream valorization. Journal of Dairy Science 99 (11):8644–54. doi: 10.3168/jds.2016-11422.
- Kanno, C. 1990. Secretory membranes of the lactating mammary gland. Protoplasma 159 (2–3):184–208. doi: 10.1007/BF01322601.
- Kanno, C., and D.-H. Kim. 1990. A simple procedure for the preparation of bovine milk fat globule membrane and a comparison of its composition, enzymatic activities, and electrophoretic properties with those prepared by other methods. Agricultural and Biological Chemistry 54 (11):2845–54.
- Kashyap, D. R., A. Rompca, A. Gaballa, J. D. Helmann, J. Chan, C. J. Chang, I. Hozo, D. Gupta, and R. Dziarski. 2014. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathogens 10 (7):e1004280. doi: 10.1371/journal.ppat.1004280.
- Keenan, T. W., and I. H. Mather. 2006. Intracellular origin of milk fat globules and the nature of the milk fat globule membrane. In Advanced dairy chemistry volume 2 lipids, 137–71. USA: Springer.
- Koning, N., S. F. Kessen, J. P. Van Der Voorn, B. J. Appelmelk, P. V. Jeurink, L. M. Knippels, J. Garssen, and Y. Van Kooyk. 2015. Human milk blocks DC-SIGN-pathogen interaction via MUC1. Frontiers in Immunology 6:112. doi: 10.3389/fimmu.2015.00112.
- Küllenberg, D., L. A. Taylor, M. Schneider, and U. Massing. 2012. Health effects of dietary phospholipids. Lipids in Health and Disease 11 (1):3. doi: 10.1186/1476-511X-11-3.
- Kvistgaard, A. S., L. T. Pallesen, C. F. Arias, S. Lopez, T. E. Petersen, C. W. Heegaard, and J. T. Rasmussen. 2004. Inhibitory effects of human and bovine milk constituents on rotavirus infections. Journal of Dairy Science 87 (12):4088–96. doi: 10.3168/jds.S0022-0302(04)73551-1.
- Labéta, M. O., K. Vidal, J. E. Nores, M. Arias, N. Vita, B. P. Morgan, J. C. Guillemot, D. Loyaux, P. Ferrara, D. Schmid, et al. 2000. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. The Journal of Experimental Medicine 191 (10):1807–12. doi: 10.1084/jem.191.10.1807.
- Le, T. T., J. Van Camp, R. Rombaut, F. van Leeckwyck, and K. Dewettinck. 2009. Effect of washing conditions on the recovery of milk fat globule membrane proteins during the isolation of milk fat globule membrane from milk. Journal of Dairy Science 92 (8):3592–603. doi: 10.3168/jds.2008-2009.
- Le, T. T., T. Van de Wiele, T. N. H. Do, G. Debyser, K. Struijs, B. Devreese, K. Dewettinck, and J. Van Camp. 2012. Stability of milk fat globule membrane proteins toward human enzymatic gastrointestinal digestion. Journal of Dairy Science 95 (5):2307–18. doi: 10.3168/jds.2011-4947.
- Lecomte, M., C. Bourlieu, E. Meugnier, A. Penhoat, D. Cheillan, G. Pineau, E. Loizon, M. Trauchessec, M. Claude, O. Ménard, et al. 2015. Milk polar lipids affect in vitro digestive lipolysis and postprandial lipid metabolism in mice. The Journal of Nutrition 145 (8):1770–7. doi: 10.3945/jn.115.212068.
- Lee, H., E. Padhi, Y. Hasegawa, J. Larke, M. Parenti, A. Wang, O. Hernell, B. Lönnerdal, and C. Slupsky. 2018. Compositional dynamics of the milk fat globule and its role in infant development. Frontiers in Pediatrics 6:313. doi: 10.3389/fped.2018.00313.
- Lefèvre, C. M., J. A. Sharp, and K. R. Nicholas. 2010. Evolution of lactation: Ancient origin and extreme adaptations of the lactation system. Annual Review of Genomics and Human Genetics 11:219–38. doi: 10.1146/annurev-genom-082509-141806.
- Levy, O. 2007. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nature Reviews Immunology 7 (5):379–90. doi: 10.1038/nri2075.
- Liao, Y., R. Alvarado, B. Phinney, and B. Lönnerdal. 2011. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. Journal of Proteome Research 10 (8):3530–41. doi: 10.1021/pr200149t.
- Liu, B., Z. Yu, C. Chen, D. E. Kling, and D. S. Newburg. 2012. Human milk mucin 1 and mucin 4 inhibit Salmonella enterica serovar Typhimurium invasion of human intestinal epithelial cells in vitro. The Journal of Nutrition 142 (8):1504–9. doi: 10.3945/jn.111.155614.
- Liu, H., E. C. Radlowski, M. S. Conrad, Y. Li, R. N. Dilger, and R. W. Johnson. 2014. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. The Journal of Nutrition 144 (12):1903–9. doi: 10.3945/jn.114.199828.
- Logan, A., M. Auldist, J. Greenwood, and L. Day. 2014. Natural variation of bovine milk fat globule size within a herd. Journal of Dairy Science 97 (7):4072–82. doi: 10.3168/jds.2014-8010.
- Lopez, C. 2011. Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure. Current Opinion in Colloid & Interface Science 16 (5):391–404. doi: 10.1016/j.cocis.2011.05.007.
- Lopez, C., and O. Ménard. 2011. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids and Surfaces B, Biointerfaces 83 (1):29–41. doi: 10.1016/j.colsurfb.2010.10.039.
- Lopez, C., C. Cauty, and F. Guyomarc’h. 2015. Organization of lipids in milks, infant milk formulas and various dairy products: Role of technological processes and potential impacts. Dairy Science & Technology 95 (6):863–93. doi: 10.1007/s13594-015-0263-0.
- Lopez, C., M.-N. Madec, and R. Jimenez-Flores. 2010. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chemistry 120 (1):22–33. doi: 10.1016/j.foodchem.2009.09.065.
- Lopez, C., V. Briard-Bion, and O. Ménard. 2014. Polar lipids, sphingomyelin and long-chain unsaturated fatty acids from the milk fat globule membrane are increased in milks produced by cows fed fresh pasture based diet during spring. Food Research International 58:59–68. doi: 10.1016/j.foodres.2014.01.049.
- Lopez, C., V. Briard-Bion, O. Menard, F. Rousseau, P. Pradel, and J.-M. Besle. 2008. Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet. Journal of Agricultural and Food Chemistry 56 (13):5226–36. doi: 10.1021/jf7036104.
- Lopez, C., V. Briard-Bion, O. Ménard, E. Beaucher, F. Rousseau, J. Fauquant, N. Leconte, and B. Robert. 2011. Fat globules selected from whole milk according to their size: Different compositions and structure of the biomembrane, revealing sphingomyelin-rich domains. Food Chemistry 125 (2):355–68. doi: 10.1016/j.foodchem.2010.09.005.
- Lu, J., E. Antunes Fernandes, A. E. Páez Cano, J. Vinitwatanakhun, S. Boeren, T. van Hooijdonk, A. van Knegsel, J. Vervoort, and K. A. Hettinga. 2013. Changes in milk proteome and metabolome associated with dry period length, energy balance, and lactation stage in postparturient dairy cows. Journal of Proteome Research 12 (7):3288–96. doi: 10.1021/pr4001306.
- Lu, J., L. Liu, X. Pang, S. Zhang, Z. Jia, C. Ma, L. Zhao, and J. Lv. 2016. Comparative proteomics of milk fat globule membrane in goat colostrum and mature milk. Food Chemistry 209:10–6. doi: 10.1016/j.foodchem.2016.04.020.
- Lu, J., N. Argov-Argaman, J. Anggrek, S. Boeren, T. van Hooijdonk, J. Vervoort, and K. A. Hettinga. 2016. The protein and lipid composition of the membrane of milk fat globules depends on their size. Journal of Dairy Science 99 (6):4726–38. doi: 10.3168/jds.2015-10375.
- Lu, J., S. Boeren, S. C. De Vries, H. J. F. Van Valenberg, J. Vervoort, and K. Hettinga. 2011. Filter-aided sample preparation with dimethyl labeling to identify and quantify milk fat globule membrane proteins. Journal of Proteomics 75 (1):34–43. doi: 10.1016/j.jprot.2011.07.031.
- Lu, J., X. Wang, W. Zhang, L. Liu, X. Pang, S. Zhang, and J. Lv. 2016. Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chemistry 196:665–72. doi: 10.1016/j.foodchem.2015.10.005.
- Luo, J., Z. W. Wang, F. Wang, H. Zhang, J. Lu, H. Y. Guo, and F. Z. Ren. 2014. Cryo-SEM images of native milk fat globule indicate small casein micelles are constituents of the membrane. RSC Advances 4 (90):48963–6. doi: 10.1039/C4RA06171C.
- Martini, M., I. Altomonte, and F. Salari. 2012a. The lipid component of Massese ewes’ colostrum: Morphometric characteristics of milk fat globules and fatty acid profile. International Dairy Journal 24 (2):93–6. doi: 10.1016/j.idairyj.2011.07.006.
- Martini, M., I. Altomonte, and F. Salari. 2012b. Relationship between the nutritional value of fatty acid profile and the morphometric characteristics of milk fat globules in ewe's milk. Small Ruminant Research 105 (1–3):33–7. doi: 10.1016/j.smallrumres.2011.12.007.
- Martini, M., I. Altomonte, R. Pesi, M. G. Tozzi, and F. Salari. 2013. Fat globule membranes in ewes' milk: The main enzyme activities during lactation. International Dairy Journal 28 (1):36–9. doi: 10.1016/j.idairyj.2012.07.002.
- Martini, M., F. Cecchi, C. Scolozzi, R. Leotta, and P. Verita. 2003. Milk fat globules in different dairy cattle breeds Part I: Orphometric analysis. Italian Journal of Animal Science 2 (Sup 1):272–4.
- Martini, M., G. Battista Liponi, and F. Salari. 2010. Effect of forage: Concentrate ratio on the quality of ewe's milk, especially on milk fat globules characteristics and fatty acids composition. Journal of Dairy Research 77 (2):239–44. doi: 10.1017/S0022029910000038.
- Martini, M., F. Salari, and I. Altomonte. 2016. The macrostructure of milk lipids: The fat globules. Critical Reviews in Food Science and Nutrition 56 (7):1209–21. doi: 10.1080/10408398.2012.758626.
- Martini, M., F. Salari, I. Altomonte, D. Rignanese, S. Chessa, C. Gigliotti, and A. Caroli. 2010. The Garfagnina goat: A zootechnical overview of a local dairy population. Journal of Dairy Science 93 (10):4659–67. doi: 10.3168/jds.2010-3207.
- Martini, M., F. Salari, R. Pesi, and M. G. Tozzi. 2010. Relationship between activity of some fat globule membrane enzymes and the lipidic fraction in ewes' milk: Preliminary studies. International Dairy Journal 20 (1):61–4. doi: 10.1016/j.idairyj.2009.07.002.
- Martini, M., F. Salari, C. Scolozzi, L. Bianchi, M. Pauselli, E. Rossetti, and P. Verita. 2006. Morphometric characteristics of sheep milk fat globules (Part I): Influence of genetic type and phase of lactation. Paper presented at the 14 Congreso Internactional de la Federaciòn Mediterrànea de Sanidad y Producciòn de Ruminantes.
- Mather, I. H., and T. W. Keenan. 1998. Origin and secretion of milk lipids. Journal of Mammary Gland Biology and Neoplasia 3 (3):259–73. doi: 10.1023/A:1018711410270.
- Michalski, M. C., V. Briard, F. Michel, F. Tasson, and P. Poulain. 2005. Size distribution of fat globules in human colostrum, breast milk, and infant formula. Journal of Dairy Science 88 (6):1927–40. doi: 10.3168/jds.S0022-0302(05)72868-X.
- Michalski, M.-C., A. F. Soares, C. Lopez, N. Leconte, V. Briard, and A. Geloen. 2006. The supramolecular structure of milk fat influences plasma triacylglycerols and fatty acid profile in the rat. European Journal of Nutrition 45 (4):215–24. doi: 10.1007/s00394-006-0588-9.
- McMahon, D. J., and B. S. Oommen. 2008. Supramolecular structure of the casein micelle. Journal of Dairy Science 91 (5):1709–21. doi: 10.3168/jds.2007-0819.
- McManaman, J. 2009. Formation of milk lipids: A molecular perspective. Clinical Lipidology 4 (3):391–401. doi: 10.2217/clp.09.15.
- McManaman, J. L., T. D. Russell, J. Schaack, D. J. Orlicky, and H. Robenek. 2007. Molecular determinants of milk lipid secretion. Journal of Mammary Gland Biology and Neoplasia 12 (4):259–68. doi: 10.1007/s10911-007-9053-5.
- Mehaia, M. A. 1995. The fat globule size distribution in camel, goat, ewe and cow milk. Milchwissenschaft 50 (5):260–3.
- Meisel, H., and R. J. Fitzgerald. 2000. Opioid peptides encrypted in intact milk protein sequences. British Journal of Nutrition 84 (S1):27–31. doi: 10.1017/S000711450000221X.
- Meisel, H., and E. Schlimme. 1994. Inhibitors of angiotensin-converting-enzyme derived from bovine casein (casokinins). In β-Casomorphins and related peptides: Recent developments, 27–33. Weinheim: VCH.
- Meľuchová, B., J. Blaško, R. Kubinec, R. Górová, J. Dubravská, M. Margetín, and L. Soják. 2008. Seasonal variations in fatty acid composition of pasture forage plants and CLA content in ewe milk fat. Small Ruminant Research 78 (1–3):56–65. doi: 10.1016/j.smallrumres.2008.05.001.
- Ménard, O., S. Ahmad, F. Rousseau, V. Briard-Bion, F. Gaucheron, and C. Lopez. 2010. Buffalo vs. cow milk fat globules: Size distribution, zeta-potential, compositions in total fatty acids and in polar lipids from the milk fat globule membrane. Food Chemistry 120 (2):544–51. doi: 10.1016/j.foodchem.2009.10.053.
- Mesilati-Stahy, R., and N. Argov-Argaman. 2014. The relationship between size and lipid composition of the bovine milk fat globule is modulated by lactation stage. Food Chemistry 145:562–70. doi: 10.1016/j.foodchem.2013.08.077.
- Mesilati-Stahy, R., and N. Argov-Argaman. 2018. Changes in lipid droplets morphometric features in mammary epithelial cells upon exposure to non-esterified free fatty acids compared with VLDL. PLoS One 13 (12):e0209565. doi: 10.1371/journal.pone.0209565.
- Mesilati-Stahy, R., H. Malka, and N. Argov-Argaman. 2012. Association of plasma insulin concentration to fatty acid distribution between milk fat and membrane synthesis. Journal of Dairy Science 95 (4):1767–75. doi: 10.3168/jds.2011-4583.
- Mesilati-Stahy, R., K. Mida, and N. Argov-Argaman. 2011. Size-dependent lipid content of bovine milk fat globule and membrane phospholipids. Journal of Agricultural and Food Chemistry 59 (13):7427–35. doi: 10.1021/jf201373j.
- Mesilati-Stahy, R., U. Moallem, Y. Magen, and N. Argov-Argaman. 2015. Altered concentrate to forage ratio in cows ration enhanced bioproduction of specific size subpopulation of milk fat globules. Food Chemistry 179:199–205. doi: 10.1016/j.foodchem.2015.01.138.
- Michalski, M.-C., V. Briard, and F. Michel. 2001. Optical parameters of milk fat globules for laser light scattering measurements. Le Lait 81 (6):787–96. doi: 10.1051/lait:2001105.
- Michalski, M.-C., V. Briard, and P. Juaneda. 2005. CLA profile in native fat globules of different sizes selected from raw milk. International Dairy Journal 15 (11):1089–94. doi: 10.1016/j.idairyj.2004.11.011.
- Michalski, M.-C., J.-Y. Gassi, M.-H. Famelart, N. Leconte, B. Camier, F. Michel, and V. Briard. 2003. The size of native milk fat globules affects physico-chemical and sensory properties of Camembert cheese. Le Lait 83 (2):131–43. doi: 10.1051/lait:2003003.
- Minegishi, Y., N. Ota, S. Soga, and A. Shimotoyodome. 2016. Effects of nutritional supplementation with milk fat globule membrane on physical and muscle function in healthy adults aged 60 and over with semiweekly light exercise: A randomized double-blind, placebo-controlled pilot trial. Journal of Nutritional Science and Vitaminology 62 (6):409–15. doi: 10.3177/jnsv.62.409.
- Mondy, B. L., and T. W. Keenan. 1993. Butyrophilin and xanthine oxidase occur in constant molar proportions in milk lipid globule membrane but vary in amount with breed and stage of lactation. Protoplasma 177 (1–2):32–6. doi: 10.1007/BF01403396.
- Monks, J., M. Dzieciatkowska, E. S. Bales, D. J. Orlicky, R. M. Wright, and J. L. McManaman. 2016. Xanthine oxidoreductase mediates membrane docking of milk-fat droplets but is not essential for apocrine lipid secretion. The Journal of Physiology 594 (20):5899–921. doi: 10.1113/JP272390.
- Moriya, H., K. Uchida, T. Okajima, T. Matsuda, and D. Nadano. 2011. Secretion of three enzymes for fatty acid synthesis into mouse milk in association with fat globules, and rapid decrease of the secreted enzymes by treatment with rapamycin. Archives of Biochemistry and Biophysics 508 (1):87–92. doi: 10.1016/j.abb.2011.01.015.
- Motouri, M., H. Matsuyama, J.-i. Yamamura, M. Tanaka, S. Aoe, T. Iwanaga, and H. Kawakami. 2003. Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. Journal of Pediatric Gastroenterology and Nutrition 36 (2):241–7. doi: 10.1097/00005176-200302000-00016.
- Mudd, A. T., Lindsey, S. Alexander, K. Berding, R. V. Waworuntu, B. M. Berg, S. M. Donovan, and R. N. Dilger. 2016. Dietary prebiotics, milk fat globule membrane, and lactoferrin affects structural neurodevelopment in the young piglet. Frontiers in Pediatrics 4 (4). doi: 10.3389/fped.2016.00004.
- Mulder, H., and P. Walstra. 1974. The milk fat globule. Emulsion science as applied to milk products and comparable foods. Wageningen: Centre for Agricultural Publishing and Documentation.
- Murgia, S., S. Mele, and M. Monduzzi. 2003. Quantitative characterization of phospholipids in milk fat via 31P NMR using a monophasic solvent mixture. Lipids 38 (5):585–91. doi: 10.1007/s11745-003-1500-3.
- Naarding, M. A., A. M. Dirac, I. S. Ludwig, D. Speijer, S. Lindquist, E.-L. Vestman, M. J. Stax, T. B. H. Geijtenbeek, G. Pollakis, O. Hernell, et al. 2006. Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells. Antimicrobial Agents and Chemotherapy 50 (10):3367–74. doi: 10.1128/AAC.00593-06.
- Nantapo, C. T. W., V. Muchenje, and A. Hugo. 2014. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system. Food Chemistry 146:127–33. doi: 10.1016/j.foodchem.2013.09.009.
- Newburg, D. S., J. A. Peterson, G. M. Ruiz-Palacios, D. O. Matson, A. L. Morrow, J. Shults, M. d. L. Guerrero, P. Chaturvedi, S. O. Newburg, C. D. Scallan, et al. 1998. Role of human-milk lactadherin in protectoin against symptomatic rotavirus infection. The Lancet 351 (9110):1160–4. doi: 10.1016/S0140-6736(97)10322-1.
- Nielsen, R. L., M. H. Andersen, P. Mabhout, L. Berglund, T. E. Petersen, and J. T. Rasmussen. 1999. Isolation of adipophilin and butyrophilin from bovine milk and characterization of a cDNA encoding adipophilin. Journal of Dairy Science 82 (12):2543–9. doi: 10.3168/jds.S0022-0302(99)75508-6.
- Ollivier-Bousquet, M. 2002. Milk lipid and protein traffic in mammary epithelial cells: Joint and independent pathways. Reproduction Nutrition Development 42 (2):149–62. doi: 10.1051/rnd:2002014.
- O’mahony, J. A., K. E. Smith, and J. Anthony Lucey. 2014. Purification of beta casein from milk. Google Patents.
- Oshida, K., T. Shimizu, M. Takase, Y. Tamura, T. Shimizu, and Y. Yamashiro. 2003. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatric Research 53 (4):589–93. doi: 10.1203/01.PDR.0000054654.73826.AC.
- Oshima, K., N. Aoki, T. Kato, K. Kitajima, and T. Matsuda. 2002. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. European Journal of Biochemistry 269 (4):1209–18. doi: 10.1046/j.1432-1033.2002.02758.x.
- Ota, N., S. Soga, T. Hase, and A. Shimotoyodome. 2015. Daily consumption of milk fat globule membrane plus habitual exercise improves physical performance in healthy middle-aged adults. Springerplus 4 (1):1–8. doi: 10.1186/s40064-015-0896-8.
- Palmquist, D. L. 2006. Milk fat: Origin of fatty acids and influence of nutritional factors thereon. In Advanced dairy chemistry volume 2 lipids, 43–92. USA: Springer.
- Park, Y. W., and G. F. Haenlein. 2021. A2 bovine milk and caprine milk as a means of remedy for milk protein allergy. Dairy 2 (2):191–201. doi: 10.3390/dairy2020017.
- Park, Y. W., and M. S. Nam. 2015. Bioactive peptides in milk and dairy products: A review. Korean Journal for Food Science of Animal Resources 35 (6):831–40. doi: 10.5851/kosfa.2015.35.6.831.
- Pegolo, S., A. Cecchinato, M. Mele, G. Conte, S. Schiavon, and G. Bittante. 2016. Effects of candidate gene polymorphisms on the detailed fatty acids profile determined by gas chromatography in bovine milk. Journal of Dairy Science 99 (6):4558–73. doi: 10.3168/jds.2015-10420.
- Pepeu, G., I. M. Pepeu, and L. Amaducci. 1996. A review of phosphatidylserine pharmacological and clinical effects. Is phosphatidylserine a drug for the ageing brain? Pharmacological Research 33 (2):73–80. doi: 10.1006/phrs.1996.0013.
- Peterson, J. A., M. Hamosh, C. D. Scallan, R. L. Ceriani, T. R. Henderson, N. R. Mehta, M. Armand, and P. Hamosh. 1998. Milk fat globule glycoproteins in human milk and in gastric aspirates of mother's milk-fed preterm infants. Pediatric Research 44 (4):499–506. doi: 10.1203/00006450-199810000-00006.
- Pietrzak-Fiećko, R., and A. M. Kamelska-Sadowska. 2020. The comparison of nutritional value of human milk with other mammals’ milk. Nutrients 12 (5):1404. doi: 10.3390/nu12051404.
- Pisanu, S., S. Ghisaura, D. Pagnozzi, G. Biosa, A. Tanca, T. Roggio, S. Uzzau, and M. F. Addis. 2011. The sheep milk fat globule membrane proteome. Journal of Proteomics 74 (3):350–8. doi: 10.1016/j.jprot.2010.11.011.
- Pisanu, S., S. Ghisaura, D. Pagnozzi, G. Falchi, G. Biosa, A. Tanca, T. Roggio, S. Uzzau, and M. F. Addis. 2012. Characterization of sheep milk fat globule proteins by two-dimensional polyacrylamide gel electrophoresis/mass spectrometry and generation of a reference map. International Dairy Journal 24 (2):78–86. doi: 10.1016/j.idairyj.2011.05.009.
- Pisanu, S., G. Marogna, D. Pagnozzi, M. Piccinini, G. Leo, A. Tanca, A. M. Roggio, T. Roggio, S. Uzzau, and M. F. Addis. 2013. Characterization of size and composition of milk fat globules from Sarda and Saanen dairy goats. Small Ruminant Research 109 (2–3):141–51. doi: 10.1016/j.smallrumres.2012.07.024.
- Qian, Z.-Y., P. Jollès, D. Migliore-Samour, F. Schoentgen, and A.-M. Fiat. 1995. Sheep κ-casein peptides inhibit platelet aggregation. Biochimica et Biophysica Acta (BBA) - General Subjects 1244 (2–3):411–7. doi: 10.1016/0304-4165(95)00047-F.
- Qiang, X., J. Li, R. Wu, Y. Ji, W. Chaung, W. Dong, and P. Wang. 2011. Expression and characterization of recombinant human milk fat globule-EGF factor factor VIII. International Journal of Molecular Medicine 28 (6):1071–6. doi: 10.3892/ijmm.2011.782.
- Rasmussen, L. K., and T. E. Petersen. 1991. Purification of disulphide-linked alpha s2- and kappa-casein from bovine milk. The Journal of Dairy Research 58 (2):187–93. doi: 10.1017/s0022029900029733.
- Rasmussen, L. K., H. A. Due, and T. E. Petersen. 1995. Human αs1-casein: Purification and characterization. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 111 (1):75–81. doi: 10.1016/0305-0491(94)00225-J.
- Raymond, A., M. A. Ensslin, and B. D. Shur. 2009. SED1/MFG-E8: A bi-motif protein that orchestrates diverse cellular interactions. Journal of Cellular Biochemistry 106 (6):957–66. doi: 10.1002/jcb.22076.
- Reinhardt, T. A., and J. D. Lippolis. 2006. Bovine milk fat globule membrane proteome. The Journal of Dairy Research 73 (4):406–16. doi: 10.1017/S0022029906001889.
- Reinhardt, T. A., and J. D. Lippolis. 2008. Developmental changes in the milk fat globule membrane proteome during the transition from colostrum to milk. Journal of Dairy Science 91 (6):2307–18. doi: 10.3168/jds.2007-0952.
- Reinhardt, T. A., J. D. Lippolis, B. J. Nonnecke, and R. E. Sacco. 2012. Bovine milk exosome proteome. Journal of Proteomics 75 (5):1486–92. doi: 10.1016/j.jprot.2011.11.017.
- Rhodes, D. A., W. Reith, and J. Trowsdale. 2016. Regulation of immunity by butyrophilins. Annual Review of Immunology 34:151–72. doi: 10.1146/annurev-immunol-041015-055435.
- Robenek, H., O. Hofnagel, I. Buers, S. Lorkowski, M. Schnoor, M. J. Robenek, H. Heid, D. Troyer, and N. J. Severs. 2006. Butyrophilin controls milk fat globule secretion. Proceedings of the National Academy of Sciences of the United States of America 103 (27):10385–90. doi: 10.1073/pnas.0600795103.
- Russo, M., F. Cichello, C. Ragonese, P. Donato, F. Cacciola, P. Dugo, and L. Mondello. 2013. Profiling and quantifying polar lipids in milk by hydrophilic interaction liquid chromatography coupled with evaporative light-scattering and mass spectrometry detection. Analytical and Bioanalytical Chemistry 405 (13):4617–26. doi: 10.1007/s00216-012-6699-7.
- Ruvoën-Clouet, N., E. Mas, S. Marionneau, P. Guillon, D. Lombardo, and J. Le Pendu. 2006. Bile-salt-stimulated lipase and mucins from milk of 'secretor' mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. The Biochemical Journal 393 (Pt 3):627–34. doi: 10.1042/BJ20050898.
- Saeland, E., M. A. de Jong, A. A. Nabatov, H. Kalay, T. B. Geijtenbeek, and Y. van Kooyk. 2009. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Molecular Immunology 46 (11–12):2309–16. doi: 10.1016/j.molimm.2009.03.025.
- Salari, F., and M. Martini. 2009. Evaluation of the morphometric characteristics of ewe milk fat globules, cheese yield and ripening in the intermediate lactation phase. Italian Journal of Animal Science 8 (sup 2):429–31. doi: 10.4081/ijas.2009.s2.429.
- Salari, F., I. Altomonte, N. L. Ribeiro, M. N. Ribeiro, R. Bozzi, and M. Martini. 2016. Effects of season on the quality of Garfagnina goat milk. Italian Journal of Animal Science 15 (4):568–75. doi: 10.1080/1828051X.2016.1247658.
- Sams, L., J. Paume, J. Giallo, and F. Carrière. 2016. Relevant pH and lipase for in vitro models of gastric digestion. Food & Function 7 (1):30–45. doi: 10.1039/c5fo00930h.
- Sanogo, T., D. Pâquet, F. Aubert, and G. Linden. 1989. Purification of αs1-casein by fast protein liquid chromatography. Journal of Dairy Science 72 (9):2242–6. doi: 10.3168/jds.S0022-0302(89)79354-1.
- Sato, R., T. Noguchi, and H. Naito. 1986. Casein phosphopeptide (CPP) enhances calcium absorption from the ligated segment of rat small intestine. Journal of Nutritional Science and Vitaminology 32 (1):67–76. doi: 10.3177/jnsv.32.67.
- Sherman, M. P., S. H. Bennett, F. F. Hwang, and C. Yu. 2004. Neonatal small bowel epithelia: Enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals 17 (3):285–9. doi: 10.1023/B:BIOM.0000027706.51112.62.
- Shimizu, T. 2009. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annual Review of Pharmacology and Toxicology 49:123–50. doi: 10.1146/annurev.pharmtox.011008.145616.
- Smith, I. A., B. R. Knezevic, J. U. Ammann, D. A. Rhodes, D. Aw, D. B. Palmer, I. H. Mather, and J. Trowsdale. 2010. BTN1A1, the mammary gland butyrophilin, and BTN2A2 are both inhibitors of T cell activation. Journal of Immunology (Baltimore, MD: 1950) 184 (7):3514–25. doi: 10.4049/jimmunol.0900416.
- Smoczyński, M. 2017. Role of phospholipid flux during milk secretion in the mammary gland. Journal of Mammary Gland Biology and Neoplasia 22 (2):117–29. doi: 10.1007/s10911-017-9376-9.
- Snow, D. R., R. E. Ward, A. Olsen, R. Jimenez-Flores, and K. J. Hintze. 2011. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. Journal of Dairy Science 94 (5):2201–12. doi: 10.3168/jds.2010-3886.
- Sprong, R. C., M. F. Hulstein, T. T. Lambers, and R. van der Meer. 2012. Sweet buttermilk intake reduces colonisation and translocation of Listeria monocytogenes in rats by inhibiting mucosal pathogen adherence. The British Journal of Nutrition 108 (11):2026–33. doi: 10.1017/S0007114512000165.
- Stamm, M. 2015. Effects of different microalgae supplements on fatty acid composition, oxidation stability, milk fat globule size and phospholipid content of bovine milk. Finland: University of Helsinki. http://urn.fi/URN:NBN:fi:hulib-201510193736
- Stevens, C. R., T. M. Millar, J. G. Clinch, J. M. Kanczler, T. Bodamyali, and D. R. Blake. 2000. Antibacterial properties of xanthine oxidase in human milk. The Lancet 356 (9232):829–30. doi: 10.1016/S0140-6736(00)02660-X.
- Stringer, D. M., P. Zahradka, V. C. DeClercq, N. R. Ryz, R. Diakiw, L. L. Burr, X. Xie, and C. G. Taylor. 2010. Modulation of lipid droplet size and lipid droplet proteins by trans-10,cis-12 conjugated linoleic acid parallels improvements in hepatic steatosis in obese, insulin-resistant rats. Biochimica et Biophysica Acta 1801 (12):1375–85. doi: 10.1016/j.bbalip.2010.08.011.
- Strzałkowska, Nina, A Jóźwik, Emilia Bagnicka, J Krzyżewski, and JO Horbańczuk. 2009. Studies upon genetic and environmental factors affecting the cholesterol content of cow milk. I. Relationship between the polymorphic form of beta-lactoglobulin, somatic cell count, cow age and stage of lactation and cholesterol content of milk. Animal Science Papers and Reports 27 (2):95–103.
- Strömqvist, M., P. Falk, S. Bergström, L. Hansson, B. Lönnerdal, S. Normark, and O. Hernell. 1995. Human milk kappa-casein and inhibition of Helicobacter pylori adhesion to human gastric mucosa. Journal of Pediatric Gastroenterology and Nutrition 21 (3):288–96.
- Sun, Y., C. Wang, X. Sun, and M. Guo. 2019. Comparative proteomics of whey and milk fat globule membrane proteins of Guanzhong goat and Holstein cow mature milk. Journal of Food Science 84 (2):244–53. doi: 10.1111/1750-3841.14428.
- Suri, S., V. Kumar, R. Prasad, B. Tanwar, A. Goyal, S. Kaur, Y. Gat, A. Kumar, J. Kaur, and D. Singh. 2019. Considerations for development of lactose-free food. Journal of Nutrition & Intermediary Metabolism 15:27–34. doi: 10.1016/j.jnim.2018.11.003.
- Tatar, V., H. Mootse, A. Sats, T. Mahla, T. Kaart, and V. Poikalainen. 2015. Evaluation of size distribution of fat globules and fat and protein content in Estonian Goat milk. Agronomy Research 13 (4):1112–9.
- Thiam, A. R., R. V. Farese, Jr., and T. C. Walther. 2013. The biophysics and cell biology of lipid droplets. Nature Reviews Molecular Cell Biology 14 (12):775–86. doi: 10.1038/nrm3699.
- Thompson, A. K., and H. Singh. 2006. Preparation of liposomes from milk fat globule membrane phospholipids using a microfluidizer. Journal of Dairy Science 89 (2):410–9. doi: 10.3168/jds.S0022-0302(06)72105-1.
- Timby, N., E. Domellof, O. Hernell, B. Lonnerdal, and M. Domellof. 2014. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. The American Journal of Clinical Nutrition 99 (4):860–8. doi: 10.3945/ajcn.113.064295.
- Timmen, H., and S. Patton. 1988. Milk fat globules: Fatty acid composition, size and in vivo regulation of fat liquidity. Lipids 23 (7):685–9. doi: 10.1007/BF02535669.
- Truong, T., M. Palmer, N. Bansal, and B. Bhandari. 2016. Techniques to measure milk fat globule size. In Effect of milk fat globule size on the physical functionality of dairy products, 11–4. Springer (USA).
- Vanderghem, C., P. Bodson, S. Danthine, M. Paquot, C. Deroanne, and C. Blecker. 2010. Milk fat globule membrane and buttermilks: From composition to valorization. Base 14 (3): 485–500.
- Verardo, V., A. Gómez-Caravaca, D. Arráez-Román, and K. Hettinga. 2017. Recent advances in phospholipids from colostrum, milk and dairy by-products. International Journal of Molecular Sciences 18 (1):173. doi: 10.3390/ijms18010173.
- Walstra, P. 1969. Studies on milk fat dispersion. 2. Globule-size distribution of cows milk. Netherlands Milk and Dairy Journal-Nederlands-Nederlands Melk En Zuiveltijdschrift 23 (2):99.
- Walter, L., S. Finch, B. Cullen, R. Fry, A. Logan, and B. J. Leury. 2019. The effect of physiological state, milk production traits and environmental conditions on milk fat globule size in cow's milk. The Journal of Dairy Research 86 (4):454–60. doi: 10.1017/S0022029919000748.
- Walter, L., P. Shrestha, R. Fry, B. J. Leury, and A. Logan. 2020. Lipid metabolic differences in cows producing small or large milk fat globules: Fatty acid origin and degree of saturation. Journal of Dairy Science 103 (2):1920–30. doi: 10.3168/jds.2019-16775.
- Ward, T. L., K. Goto, and I. Altosaar. 2014. Ingested soluble CD14 contributes to the functional pool of circulating sCD14 in mice. Immunobiology 219 (7):537–46. doi: 10.1016/j.imbio.2014.03.008.
- Watanabe, K., A. Holobar, A. Tomita, and Y. Mita. 2020. Effect of milk fat globule membrane supplementation on motor unit adaptation following resistance training in older adults. Physiological Reports 8 (12):e14491. doi: 10.14814/phy2.14491.
- Wedholm, A., L. B. Larsen, H. Lindmark-Månsson, A. H. Karlsson, and A. Andrén. 2006. Effect of protein composition on the cheese-making properties of milk from individual dairy cows. Journal of Dairy Science 89 (9):3296–305. doi:10.3168/jds.S0022-0302(06)72366-9. PMID:16899662
- Wiking, L., J. Stagsted, L. Björck, and J. H. Nielsen. 2004. Milk fat globule size is affected by fat production in dairy cows. International Dairy Journal 14 (10):909–13. doi: 10.1016/j.idairyj.2004.03.005.
- Wiking, L., P. K. Theil, J. H. Nielsen, and M. T. Sørensen. 2010. Effect of grazing fresh legumes or feeding silage on fatty acids and enzymes involved in the synthesis of milk fat in dairy cows. The Journal of Dairy Research 77 (3):337–42. doi: 10.1017/S002202991000021X.
- Wilde, P. J., and B. S. Chu. 2011. Interfacial & colloidal aspects of lipid digestion. Advances in Colloid and Interface Science 165 (1):14–22. doi: 10.1016/j.cis.2011.02.004.
- Wooding, F. B. Peter, and I. H. Mather. 2017. Ultrastructural and immunocytochemical evidence for the reorganisation of the milk fat globule membrane after secretion. Cell and Tissue Research 367 (2):283–95. doi: 10.1007/s00441-016-2505-8.
- Xing, Z. Y., M. L. Zhang, Y. Y. Wang, G. Y. Yang, L. Q. Han, and J. J. Loor. 2020. Short communication: A decrease in diameter of milk fat globules accompanies milk fat depression induced by conjugated linoleic acid supplementation in lactating dairy cows. Journal of Dairy Science 103 (6):5143–7. doi: 10.3168/jds.2019-17845.
- Wada, Y., and B. Lönnerdal. 2014. Bioactive peptides derived from human milk proteins-mechanisms of action. The Journal of Nutritional Biochemistry 25 (5):503–14. doi: 10.1016/j.jnutbio.2013.10.012.
- Wang, Y., F. Ding, T. Wang, W. Liu, S. Lindquist, O. Hernell, J. Wang, J. Li, L. Li, Y. Zhao, et al. 2017. Purification and characterization of recombinant human bile salt-stimulated lipase expressed in milk of transgenic cloned cows. PLoS One 12 (5):e0176864. doi: 10.1371/journal.pone.0176864.
- Yang, M., M. Cong, X. Peng, J. Wu, R. Wu, B. Liu, W. Ye, and X. Yue. 2016. Quantitative proteomic analysis of milk fat globule membrane (MFGM) proteins in human and bovine colostrum and mature milk samples through iTRAQ labeling. Food & Function 7 (5):2438–50. doi: 10.1039/c6fo00083e.
- Yang, M., X. Peng, J. Wu, R.-n. Wu, B. Liu, W. Ye, X. Xu, and X. Yue. 2017. Differential proteomic analysis of milk fat globule membrane proteins in human and bovine colostrum by iTRAQ-coupled LC-MS/MS. European Food Research and Technology 243 (5):901–12. doi: 10.1007/s00217-016-2798-6.
- Ye, A., J. Cui, and H. Singh. 2011. Proteolysis of milk fat globule membrane proteins during in vitro gastric digestion of milk. Journal of Dairy Science 94 (6):2762–70. doi: 10.3168/jds.2010-4099.
- Ye, A., J. Cui, D. Dalgleish, and H. Singh. 2017. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. Journal of Dairy Science 100 (1):36–47. doi: 10.3168/jds.2016-11764.
- Yolken, R. H., J. A. Peterson, S. L. Vonderfecht, E. T. Fouts, K. Midthun, and D. S. Newburg. 1992. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. The Journal of Clinical Investigation 90 (5):1984–91. doi: 10.1172/JCI116078.
- Zamora, A., B. Guamis, and A. J. Trujillo. 2009. Protein composition of caprine milk fat globule membrane. Small Ruminant Research 82 (2–3):122–9. doi: 10.1016/j.smallrumres.2009.02.010.
- Zancada, L., F. Pérez-Díez, F. Sánchez-Juanes, J. M. Alonso, L. A. García-Pardo, and P. Hueso. 2013. Phospholipid classes and fatty acid composition of ewe’s and goat’s milk. Grasas y Aceites 64 (3):304–10. doi: 10.3989/gya.095312.
- Zhang, G.-W., S.-J. Lai, Y. Yoshimura, and N. Isobe. 2014. Expression of cathelicidins mRNA in the goat mammary gland and effect of the intramammary infusion of lipopolysaccharide on milk cathelicidin-2 concentration. Veterinary Microbiology 170 (1–2):125–34. doi: 10.1016/j.vetmic.2014.01.029.
- Zhang, M., Z. Xing, Q. Huang, and L. Han. 2021. Effect of conjugated linoleic acid supplementation on fat globule size in raw milk. International Dairy Journal 115:104919. doi: 10.1016/j.idairyj.2020.104919.
- Zhao, L., M. Du, and X. Mao. 2019. Change in interfacial properties of milk fat globules by homogenization and thermal processing plays a key role in their in vitro gastrointestinal digestion. Food Hydrocolloids 96:331–42. doi: 10.1016/j.foodhyd.2019.05.034.
- Zheng, H., R. Jiménez-Flores, and D. W. Everett. 2014. Lateral lipid organization of the bovine milk fat globule membrane is revealed by washing processes. Journal of Dairy Science 97 (10):5964–74. doi: 10.3168/jds.2014-7951.
- Zou, X., Z. Guo, Q. Jin, J. Huang, L. Cheong, X. Xu, and X. Wang. 2015. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chemistry 185:362–70. doi:10.1016/j.foodchem.2015.03.145. PMID:25952880
- Zou, X., J. Huang, Q. Jin, Z. Guo, Y. Liu, L. Cheong, X. Xu, and X. Wang. 2013. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. Journal of Agricultural and Food Chemistry 61 (29):7070–80. doi: 10.1021/jf401452y.
- Zou, X.-Q., Z. Guo, J.-H. Huang, Q.-Z. Jin, L.-Z. Cheong, X.-G. Wang, and X.-B. Xu. 2012. Human milk fat globules from different stages of lactation: A lipid composition analysis and microstructure characterization. Journal of Agricultural and Food Chemistry 60 (29):7158–67. doi: 10.1021/jf3013597.
