Abstract
Anthocyanins (ACN), the sub-class of (poly)phenols responsible for the red-blue-purple pigmentation of fruit and vegetables, have gained considerable interest in sport and exercise research due to their potential to facilitate exercise recovery. A systematic literature search was performed using PubMed, The Cochrane Library, MEDLINE, SPORTDiscus and CINAHL. Thirty nine studies were included and the standardized mean difference (Hedges g) for creatine kinase (CK), anti-oxidative and inflammatory markers, strength, power and delayed onset muscle soreness (DOMS) indices were pooled in separate meta-analyses; meta-regression was also performed on reported ACN dose. Immediately post-exercise there was an increase in antioxidant capacity (g: 0.56) and reduced C reactive protein (g: −0.24) and tumor necrosis factor α (g: −40); p ≤ 0.02. Strength was improved with ACN at all time points (g: 0.45–0.67). DOMS (g: −0.23) was lower 24 hours post-exercise and power was improved 24 hours (g: 0.62) and 48 hours (g: 0.57) post exercise. The CK was lower 48 hours post-exercise (g: −0.31) and there was a trend for a positive association with ACN dose (p = 0.057). This systematic review provides new data showing ACN-rich foods promote functional and subjective recovery likely due to the antioxidant and anti-inflammatory properties of ACN.
Introduction
Physical exercise places a degree of mechanical and metabolic stress on the body, which both contribute to a common pathological response involving oxidative stress and inflammation (Pyne Citation1994). Exercise induced muscle damage (EIMD) is typically characterized by an initial insult followed by a secondary inflammatory response, which is more prominent following eccentric actions (Bongiovanni et al. Citation2020). The EIMD has more immediate implications, including delayed onset muscle soreness (DOMS) and impaired muscular strength and power, which have the ability to compromise performance and quality of training (Clarkson, Nosaka, and Braun Citation1992; MacIntyre, Reid, and McKenzie Citation1995). Although the mechanisms and time course can differ, both mechanical and metabolic stress can cause an increase in the appearance of intracellular proteins in the blood (e.g., creatine kinase: CK) potentially due to disruptions in calcium homeostasis from a loss of cell membrane integrity (Brancaccio, Maffulli, and Limongelli Citation2007; Tee, Bosch, and Lambert Citation2007). In addition, exercise also induces signaling cascades, largely orchestrated by reactive oxygen and nitrogen species (RONS), transcriptional release of pro-inflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]), and acute phase proteins, e.g., C-reactive protein; CRP (Ebbeling and Clarkson Citation1989; Pyne Citation1994) and an increase in these immunological markers are thought to be associated with muscle soreness, loss of muscle function as well as overtraining and fatigue (Gleeson et al. Citation1995; Hecksteden et al. Citation2016; MacIntyre, Reid, and McKenzie Citation1995). Given the potential for physiological stress associated with strenuous exercise and the potential for compromised training and/or competition performance due to loss of strength and power and muscle soreness that can last for several days, there has been a strong emphasis to identify natural recovery strategies (Bongiovanni et al. Citation2020; Howatson and van Someren Citation2008).
Amongst the available strategies, dietary interventions, particularly fruit, have gained considerable attention when it comes to improving recovery following EIMD (Doma et al. Citation2021; Naderi et al. Citation2018). Fruit could influence the recovery process because they contain (poly)phenols which could interact with the secondary cascade associated with EIMD, via their antioxidant and anti-inflammatory properties (Bowtell and Kelly Citation2019; Pereira Panza, Diefenthaeler, and da Silva Citation2015). However, not all fruit is equal in terms of (poly)phenolic content and abundance and certain fruit might be more beneficial for exercise recovery. For example, in a simple meta-analysis of 25 studies of fruit on recovery from EIMD, berries were reported to have the greatest overall effect (Doma et al. Citation2021). Berries are rich in anthocyanins (ACN), a subclass of (poly)phenols, responsible for the red-blue-purple pigmentation in fruits (Manach et al. Citation2004; Pérez-Jiménez et al. Citation2010). These compounds have gained much research interest due to their propensity to maintain the balance between the oxidative and anti-oxidative systems and reduce inflammatory cytokines (Wang et al. Citation1999). In vitro ACN have been shown to act as more potent antioxidants as compared to some other (poly)phenols (Pojer et al. Citation2013) and to exhibit anti-inflammatory actions similar or superior to nonsteroidal and other anti-inflammatory drugs (Pereira Panza, Diefenthaeler, and da Silva Citation2015; Seeram et al. Citation2001). Additionally, ACN might supress pain at both an enzymatic (e.g., cyclooxygenase) and transcriptional (e.g., nuclear factor kappa beta) level (Pojer et al. Citation2013; Seeram et al. Citation2001). Moreover, cyanidin-3-glucoside, a common ACN found in berries (Sandoval-Ramírez et al., Citation2020), has been shown to up-regulate the expression of transcriptional pathways related to muscle function and reduce fatigue in rodent models (Hu et al. Citation2020; Matsukawa et al. Citation2017). Other benefits include the potential for ACN to enhance blood flow that might aid the removal of waste products and muscle metabolites (Keane et al. Citation2016b; Rodriguez-Mateos et al. Citation2019). Recently, Bloedon et al. (Citation2019) reported that ACN-rich whole foods reduce exercise-induced oxidative stress and inflammation, but the effect on muscle soreness and functional (e.g., strength and power) recovery, which are arguably more important measures of exercise recovery (Byrne, Twist, and Eston Citation2004; Damas et al. Citation2016; Torres et al. Citation2012), were not reported. Therefore whilst there is some evidence that ACN might be beneficial in facilitating recovery, the narrative nature of these reviews or lack of attention to the potential active compounds or other aspects of recovery means the conclusions are not quantitively derived or based on a complete picture of the available work (Bloedon et al. Citation2019; Doma et al. Citation2021; Harty et al. Citation2019; Naderi et al. Citation2018; Owens et al. Citation2019; Vitale, Hueglin, and Broad Citation2017). Therefore, the aim of this study was to synthesize and evaluate the effects of anthocyanin-rich foods on biochemical, physiological, and subjective indices of exercise recovery in human trials.
Methods
Search strategy
A systematic literature search of the following electronic bibliographic databases: PubMed and The Cochrane Library as well as searching MEDLINE, SPORTDiscus, and CINAHL via EBSCOhost was carried out from inception until August 2020. The search strategy (Supplementary material) was conducted using Medical Subject Heading (MeSH) and Boolean operations devised using two key concepts (1) anthocyanin-rich foods and (2) exercise recovery. Furthermore, the reference list of retrieved literature reviews was hand-searched to find potential articles that could be included in the systematic review.
Study selection
The inclusion criteria were as follows: (1) randomized controlled trials; (2) in healthy adult participants (average age ≥18 years) regardless of training status; (3) included anthocyanin-rich foods [blackberry, blackcurrant, blueberry, black elderberry, black grape, cherry, chokeberry, rhubarb, strawberry, red wine, plum (Manach et al. Citation2004; Pérez-Jiménez et al. Citation2010) or other red-blue-purple berries only where the ACN content was reported] given before exercise (could continue administration after); (4) had a placebo or suitable control; (5) reported haematological markers, functional (e.g., strength or power) or subjective (e.g., visual analogue scales or pain pressure threshold) recovery measures following exercise. For comparability, only similar biomarkers were included in the meta-analysis, these were; creatine kinase (CK) antioxidant (total antioxidant capacity/status), inflammatory (IL-6, TNF-α or CRP) or oxidative stress (thiobarbituric acid reactive substances; TBARS), antioxidant enzyme activity [superoxide dismutase (SOD) and glutathione peroxidase (GPx)], strength (maximal voluntary contractions; MVC), power (counter movement jumps; CMJ) and visual analogue scales or Likert scales for DOMS.
Exclusion criteria were non-adult, smoker or diseased participants, animal and in vitro studies. Studies were also excluded if anthocyanins are given alongside another intervention (i.e., pharmacological agent or dietary supplement; other juice or fruit to increase palatability could be included as long as anthocyanin content was reported) and no appropriate control or reference groups could be identified. Titles and abstracts were independently reviewed by two researchers (RK and CH) to evaluate their eligibility for inclusion in this review. Only full texts that were published in English or had an existing translation were retrieved and examined.
Data extraction and quality assessment
The study data was extracted into pre-piloted forms by the main reviewer (RK) and checked for accuracy by a second reviewer (KJ). Any discrepancies were resolved by reviewing the original article. The following data was extracted from each study: the first author’s last name(s), publication date, funding source, participants characteristics, sample size, supplement type, ACN content, dosing strategy and duration, any dietary restrictions, wash out period and type of exercise (metabolic, mechanical or combined], outcome time points, outcome measures, mean ± SD of the outcomes specified above were also extracted. Where necessary data was extrapolated from figures and graphs and authors were contacted to provide missing data (Abbott et al. Citation2020; Hurst et al. Citation2019; Hurst et al. Citation2020; McCormick et al. Citation2016; Morehen et al. Citation2021), if they did not respond within 1 week a follow up was sent, those who did not reply within a month were excluded (e.g., Beals et al. Citation2017; Lamb et al. Citation2019) for variables where data could not be obtained. A modified PEDro scale (de Morton Citation2009) was used to assess the methodological quality of the selected studies. One point could be awarded for each the original 11 items as well as additional items thought to be relevant to the study design. Additional criteria were as follows (1) the study acknowledged whether or not they received funding; (2) compliance to the intervention was reported; (3) ACN content was reported in the supplement either according to the manufacturer’s nutritional label or confirmed by analysis in the study; participants refrained from taking antioxidant and anti-inflammatory drugs and supplements (4) before and/or (5) during study (6) sample size calculation was included. In studies where a cross-over design an additional item was included “(7) a minimum 7-day washout between trial treatments.” Thus, a total of 17 points could be awarded for parallel studies and 18 for crossover studies. For parallel studies a score of <7, 7–10, 11–14, and 15–17 and for crossover studies <8, 8–11, 12–15, and 16–18 was poor, fair, good and excellent, respectively (Doma et al. Citation2021). Risk of bias was also assessed according to Cochrane Collaboration guidelines and is represented graphically to indicate the overall quality of all studies (Higgins and Green Citation2011).
Statistical analysis
Standardized mean differences (SMD) were calculated using Hedge’s g using independent groups and for parallel studies and paired groups for crossover studies (Borenstein et al. Citation2019). To calculate the standard deviation within groups for crossover studies the correlation between pairs of observations (r; which was calculated from studies where individual data was provided (Hurst et al. Citation2019; McCormick et al. Citation2016) and assumed to be 0.5 (Amiri et al. Citation2019; Doma et al. Citation2021; Higgins and Green Citation2011)) was included. Both study designs were included in an inverse random effects meta-analysis (due to study design heterogeneity) using Stata v.16.0 (StataCorp, College Station, Texas, USA) sub-grouped by study design to determine whether the inclusion of crossover designs influenced the SMD (Supplemental information (Higgins and Green Citation2011)). Where there were sufficient studies (Jackson and Turner Citation2017) separate meta-analyses were conducted for immediately post (≤2 h), 24 hours post and 48 hours post exercise. For studies that reported measures over several time points, the data were only analyzed for the most recent in that time interval. Hurst et al. (Citation2019) reported different doses so these were pooled to get an overall ES before inclusion in the meta-analysis (Higgins and Green Citation2011). If DOMS was measured at different sites, the largest ES was included in the meta-analysis.
Secondly, sensitivity analysis was performed by omitting one study at a time to evaluate the potential bias and robustness of the overall SMD. Heterogeneity between studies was determined by the I2 statistic. For the I2 statistic, I2 values ≤25%, ≤50%, ≤75%, and >75% indicated no, little, moderate, and significant heterogeneity, respectively. To identify potential sources of heterogeneity, moderator analysis was performed using sub-group analysis for categorical variables including training status, exercise type and study duration. In addition, where ACN content was reported a meta-regression was conducted on the most reported variables (MVC, DOMS and CK). Potential publication bias for each outcome was evaluated by Egger’s test (p < 0.10) and visual inspection of funnel plots (Begg and Mazumdar Citation1994). Where publication bias was detected, trim and fill analysis was conducted (Steichen Citation2010). The SMD were interpreted as small (>0.2), moderate (>0.5) and (≥0.8) large (Sullivan and Feinn Citation2012) and a Z effect p < 0.05 was considered significant.
Results
Literature search and study characteristics
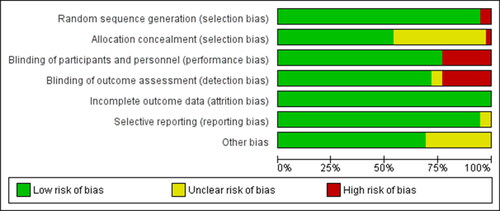
The search results are presented in , following full search and exclusion of irrelevant articles 39 articles were included in this review. A total of 27 independent group studies and 12 crossover studies with 767 participants were included in this review (). Of the interventions used tart cherry was the most common (18 studies). Other studies used blackcurrant (6 studies), grape (6 studies), blueberry (3 studies), chokeberry (3 studies), bilberry (1 study), plum (1 study) and one a mixed anthocyanin cocktail. The duration of the studies varied greatly with some investigating the acute influence (1–2 h before), most investigating the short-term influence (2–10 days) and some the longer-term influence (20 days–8 weeks) of ACN. Most studies were in trained individuals and the median age was 24 (range 18–48) years. The median ACN content, where reported, was 80 (range 8–3600) mg/day. The quality of studies was rated as poor (n = 1), fair (n = 8), good (n = 19), excellent (n = 11; ). The risk of bias is presented in , which showed the percentage of studies with low, medium and high risk of bias for each domain. The main potential sources of bias came from allocation, blinding of the intervention or did not acknowledged whether they received funding (other bias).
Figure 1. PRISMA flow diagram of included studies: there were 1111 studies identified by the search strategy, 769 non-duplicated records. After screening the titles and abstracts, 62 of the records were deemed potentially eligible for inclusion and full texts were retrieved for further evaluation. Twenty-five articles were excluded and a further 2 found from hand searching, leaving 39 included studies.
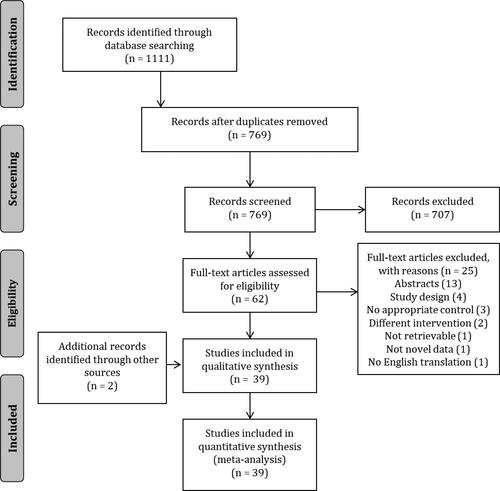
Table 1. Study characteristics of included studies.
The influence of ACN on recovery
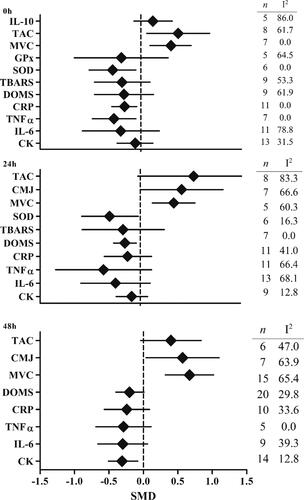
Immediately post exercise there was an increase in TAC (SMD: 0.56; 95% CI: 0.09, 1.03; p = 0.02; I2 = 61.7%) with ACN. ACN also resulted in a moderate reduction in SOD (SMD: −0.42; 95% CI: −0.77, −0.07, p = 0.02), TNF-α (SMD: −0.40; 95% CI: −0.72, −0.07, p = 0.02) and a small reduction in CRP (SMD: −0.24; 95% CI: −0.43, −0.06, p = 0.01) at immediately post-exercise, with no heterogeneity (I2 = 0.0%). At 24 hours, SOD remained lower (SMD: −0.46; 95% CI: −0.88, −0.03; I2 = 16.3%) with ACN. Intake of ACN reduced DOMS at 24 hours (SMD–0.23; 95% CI: −0.40, −0.06; p < 0.01; I2 = 0.0%). Strength (MVC) was increased immediately post-exercise (SMD: 0.45; 95% CI: 0.14, 0.75; I2 = 0.0%), 24 hours post (SMD: 0.50; 95% CI: 0.18, 0.82; I2 = 60.3%) and greatest at 48 hours post (SMD: 0.67; 95% CI: 0.32, 1.02; I2 = 65.4%). At 24 hours power (CMJ) was also increased with ACN (SMD: 0.62; 95% CI: 0.01, 1.24; p = 0.047; I2 = 66.6%). At 48 hours CMJ (SMD: 0.57; 95% CI: 0.04, 1.11; p = 0.04; I2 = 63.9%) and CK were lower (SMD: −0.31 95% CI: −0.55, −0.08; p < 0.01; I2 = 12.8%). Sensitivity analysis suggested stable results for these variables (Supplemental material). There was no influence of ACN on any other variables ().
Figure 3. Summary forest plot of findings for anthocyanin (ACN) intake on exercise recovery relative to a control immediately post (top), 24 hours post (middle) and 48 hours post (bottom) exercise. Data missing for timepoint if less than 5 studies (N/A). For maximal voluntary contraction (MVC), countermovement jump (CMJ) and total antioxidant capacity/status (TAC) right side favors ACN. For interleukin 6 (IL-6), tumor necrosis factor alpha (TNFα), C-reactive protein (CRP), thiobarbituric acid reactive substances (TBARS), creatine kinase (CK), superoxide dismutase (SOD) and glutathione peroxidase (GPx), and delayed onset of muscle damage (DOMS) left side favors ACN (n = number of studies, I2 statistic for heterogeneity).
Subgroup analysis, publication bias and meta-regression
Subgroup analysis is presented in . Immediately post-exercise ACN increased TAC and decreased SOD, only in metabolically biased exercise. IL-6 was also reduced by metabolic-type exercise immediately post and 48 hours post exercise. MVC was improved immediately post-exercise with mechanically damaging exercise, whereas 24 hours post and 48 hours post there was a large effect for exercise that had a combined mechanical and metabolic component. CRP was lower immediately post exercise, DOMS at 24 hours post exercise and CK at 48 hours post-exercise with combined-type exercise. MVC was improved immediately post-exercise and 24 hours post-exercise in trained and untrained individuals, but only 48 hours post-exercise in trained participants. CRP was lower immediately post-exercise in trained individuals, whereas in the untrained participants IL-6 was lower. Twenty-four hours post-exercise TNFα and IL-6 was lower in untrained, whereas trained participants had lower SOD and DOMS at 24 hours and CK at 48 hours.
Table 2. Subgroup analysis on moderator variables for effect of ACN on recovery.
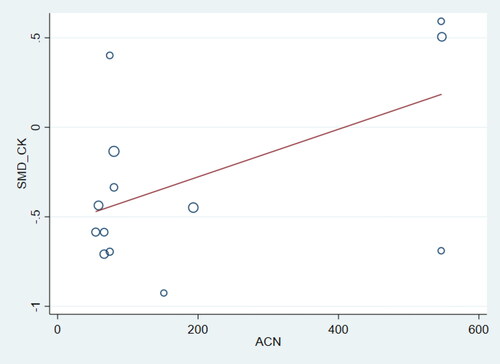
Studies of a shorter duration decreased CRP immediately post-exercise and improved MVC and DOMS 24 hours post and CK and CMJ 48 hours post. Studies of a longer duration Increased TAC and reduced SOD, TNFα and CK immediately post. TAC remained higher at 48 hours and TBARS and TNFα were reduced in the longer duration studies. There was evidence of publication bias for TBARS (p = 0.001) and DOMS (p = 0.009) immediately post-exercise. So too were TAC, CMJ and MVC (p < 0.058) 24 and 48 hours post exercise and IL-6 (p = 0.004) 48 hours post-exercise. No other publication bias was detected. Trim and fill analysis was done for the above variables resulting in lower DOMS immediately post-exercise (SMD: −0.33; 95% CI: −0.60, −0.06) higher CMJ at 24 hours (SMD: 0.47; 95% CI: 0.12, 0.81) and increased TAC at 48 hours post-exercise (SMD: 0.35; 95% CI: 0.04, 0.67). With trim and fill analysis CMJ was no longer significantly higher at 48 hours (SMD: 0.18; 95% CI: −0.10, 0.47), but there was no other materially different SMDs. Meta-regression suggested a trend for a weak positive association with ACN dose and CK () at 48 hours (p = 0.057; I2=0%), however there was no relationship with MVC or DOMS or CK at any other time point.
Discussion
The present study represents the most comprehensive picture that synthesizes and evaluates the effects of dietary ACN on exercise recovery from all the available literature including additional analyses that consider ACN dose, exercise type, training status, and study duration. These new data showed a beneficial effect for ACN on biochemical, physiological, and subjective recovery following exercise up to and including 48 hours post-exercise.
Dietary intake of ACN resulted in an increase in total antioxidant capacity/status immediately post exercise, which was mirrored by a reduction in SOD at the same point which was still reduced 24 hours post exercise, suggesting less reliance on these defence systems over time due to the ability of the ACN to scavenge free radicals (Skarpańska-Stejnborn, Basta, and Pilaczyńska-Szcześniak Citation2006). Dietary ACN have antioxidant potential due to the ability for hydrogen (electron) donation and the positively charged oxygen in the flavonoid molecule (Bi et al. Citation2014). Moreover, the time course aligns with plasma maximum concentrations of ACN and their metabolites, which typically occurs 1–2 hours after ingestion (Hurst et al. Citation2019; Keane et al. Citation2016a). In accordance, a number of studies included in this analysis that measured these indices gave an acute dose pre-exercise that would coincide with the peak plasma concentrations (de Lima Tavares Toscano et al., 2020; Hurst et al. Citation2019; Hurst et al. Citation2020; Lyall et al. Citation2009; McAnulty et al. Citation2014; Silvestre et al. Citation2014). Interestingly, the antioxidant effects of ACN were predominantly seen in exercise with a major metabolic component, which might be attributable to greater exercise-induced oxidative stress owing to higher oxygen consumption during the exercise, whereas a delayed and prolonged generation of RONS after mechanically strenuous eccentric exercise is likely (Fisher-Wellman and Bloomer Citation2009), because of the secondary inflammatory-mediated damage that occurs after exercise (Howatson and van Someren Citation2008; Owens et al. Citation2019; Bongiovanni et al. Citation2020).
The consumption of ACN resulted in reduced CK at 48 hours post, and inflammation (TNFα and CRP) to be reduced immediately post-exercise. As there is an inherent interplay between these markers and RONS (Baird et al. Citation2012; Lee and Clarkson Citation2003), the early antioxidant actions of the ACN represent one potential mechanism that might supress the efflux of CK (through reduced cell membrane disruption) and inflammatory indices. The anti-inflammatory properties of ACN are well documented (Fallah et al. Citation2020; Speer et al. Citation2020), and might relate to their ability to interact with cellular enzymes and signaling pathways (Li et al. Citation2017). For example, ACN have been shown to reduce inflammatory enzymes such as cyclooxygenase and lipoxygenase (Kirakosyan et al. Citation2018; Wang et al. Citation1999), which might be mediated by their ability to inhibit mitogen‐activated protein kinase and nuclear factor kappa beta pathways (Pojer et al. Citation2013). Dependent on exercise modality, intensity and duration CK has been shown to peak 24–72 hours after exercise (Baird et al. Citation2012). Whereas an acute inflammatory response due to immunological activation typically occurs more rapidly (1–4 hours) and a second wave of inflammation is detectable in a similar timeframe to peak CK (Peake et al. Citation2017). A lower peak in the CK and inflammatory indices might reflect a reduction in muscle damage and also indicate a faster recovery after exercise with ACN compared to a control. While this might relate to the antioxidant capacity of the ACN it could also be because of improved blood flow and clearance (Baird et al. Citation2012; Rodriguez-Mateos et al. Citation2019). Other meta-analyses have not suggested an effect of fruit or (poly)phenols on CK (Doma et al. Citation2021; Hill et al. Citation2021), it should be acknowledged there are several criticisms of CK as a marker for muscle damage especially owing to its high inter and intra-individual variability and its meaningfulness as a recovery index (Brancaccio, Maffulli, and Limongelli Citation2007; Hill et al. Citation2021; Warren, Lowe, and Armstrong Citation1999). However, some included studies in the current review found a benefit of ACN-rich foods (Carvalho et al. Citation2018; (Lyall et al. Citation2009) and an ACN rich cocktail on exercise-induced CK (Lima et al. Citation2019) it therefore might be that some (poly)phenols such as ACN are more beneficial than others. Moreover, the large number of pooled studies at 48 hours post exercise might account for some of the variability, where the participant numbers amounted to 244.
The aforementioned supports the notion that ACN improves biomarkers related to exercise recovery. However, the influence on symptoms such as functional (i.e., strength and power) and muscle soreness indices perhaps are better representations of recovery facilitation and EIMD (Byrne, Twist, and Eston Citation2004; Damas et al. Citation2016; Torres et al. Citation2012). There was an effect of ACN on reducing DOMS at 24 hours and recovery of strength loss 0, 24 and 48 hours post exercise, whereas power was only increased 24 and 48 hours post-exercise. Reduced strength loss, and recovery of strength, was greater with ACN, initially for eccentrically biased exercise, but CMJ and MVC were improved 24 hours and 48 hours post-exercise after combined metabolic and mechanically strenuous exercise. Both mechanical and metabolic exercise increase RONS due to mitochondrial oxygen consumption, the increased circulating catecholamines, elevated participation of eccentric muscle contraction-induced damage, inflammatory response and/or the intermittent and repeated sprint actions that can cause temporary ischemic-reperfusion in the skeletal muscle (Ascensão et al. Citation2008; Leeuwenburgh and Heinecke Citation2001). Strength loss after exercise has been proposed to be related to oxidative stress (Çakir-Atabek, Dokumaci, and Aygün Citation2019), whereas loss in muscle power might be more synonymous with DOMS and the inflammatory response (Byrne, Twist, and Eston Citation2004). Speculatively, the early increase in antioxidant capacity with ACN might help to reduce strength loss, whereas the recovery in power coincides with the reduced DOMS at 24 hours post-exercise. These data are of great interest because therapeutic recovery interventions (e.g., massage, cold water immersion and compression garments) have shown some benefits in recovery of DOMS, strength and power, but there are limited data to suggest that all facets of recovery can be affected in a positive manner (Brown et al. Citation2017; Davis, Alabed, and Chico Citation2020; Leeder et al. Citation2012). Whereas, in this review, ACN-rich foods are shown to improve physiological and subjective recovery following strenuous exercise and hence should be an integral consideration for practitioners and exercisers to consider in their diet.
Notwithstanding, there are several limitations within the included studies that warrant discussion. Firstly, studies with a crossover study design were included in the meta-analysis and these could be influenced by the repeated bout effect (RBE) between experimental trials. The RBE refers to the protective effect afforded by a single bout of eccentric-biased muscle actions that provide a protective effect on subsequent bout of exercise (even if this is performed on the contralateral limb) and hence could mask any treatment effect (Howatson and van Someren Citation2007). However, including crossover studies did not appear to add to heterogeneity to the results (). Secondly, some studies which investigated the effects on functional and subjective recovery after “real” game play (Abbott et al. Citation2020; Kupusarevic, McShane, and Clifford Citation2019; Morehen et al., Citation2021); while these arguably have good application they are heavily confounded by the RBE as well as other recovery practices that might be conducted concurrently. Conversely, some studies used dietary restrictions () to reduce phenolic intake. This might lead to an overestimate in the effect, as removal of natural antioxidants from the diet might conceptually impair the natural recovery process; therefore ACN might only restore antioxidant capacity whereas the placebo remains in a depleted state. The balance between reducing background noise and ecological validity needs careful consideration in research designs (Bowtell and Kelly Citation2019). Thirdly, there was a large difference between ACN content of the interventions and it is not possible to distinguish between different types of ACN, which could have different bioactivities (Rechner and Kroner Citation2005). Notwithstanding, ACN content is often reported as cyanidin equivalents (Bell et al. Citation2016; Brown, Stevenson, and Howatson Citation2019; Hutchison et al. Citation2016; O’Connor et al. Citation2013) and this compound is an established biomarker of berries (Sandoval-Ramírez et al. Citation2020) and tart cherries (Seymour et al. Citation2014) suggesting at least some commonality between the interventions. Moreover, this is the first review to comprehensively study ACN on exercise recovery, including a meta-regression of ACN dose. Nonetheless, future studies should try to distinguish the optimum type and dosage of anthocyanins for recovery, an important factor highlighted in a recent review (Sabou et al. Citation2021). Lastly, blinding of studies was a major source of bias, although it is acknowledged that this is an inherent challenge with studies involving functional foods (Brown et al. Citation2018). Therefore, results from this meta-analysis have to be interpreted in light of limitations of the literature highlighted above. Nonetheless, the meta-analytical technique is currently the best method to systematically consolidate evidence from previous work (Haidich Citation2010), but it should be conducted with a forensic eye of the literature in order to interpret the information with insight.
To summarize, ACN were shown to have an overall beneficial effect on reducing CK, muscle soreness, strength loss and improving power after exercise. This was accompanied by attenuated inflammation and increased antioxidant capacity/status following the intake of ACN, suggesting a potential causal link. The information provided by sub-group analyses suggested the most beneficial effect on the biomarkers are following metabolically biased exercise and longer-term interventions; whereas shorter duration interventions saw most benefit on physiological variables, which can collectively help inform research designs and application of ACN in exercise recovery. These data provide new information to support the use of ACN-rich foods in promoting recovery following strenuous exercise that can inform exercisers and practitioners.
Supplemental Material
Download PDF (2.5 MB)Acknowledgments
The Authors would like to acknowledge Chelsey Hart who helped with the screening process.
References
- Abbott, W., C. Brashill, A. Brett, and T. Clifford. 2020. Tart cherry juice: No effect on muscle function loss or muscle soreness in professional soccer players after a match. International Journal of Sports Physiology and Performance 15 (2):249–54. doi: 10.1123/ijspp.2019-0221.
- Amiri, M., R. Ghiasvand, M. Kaviani, S. C. Forbes, and A. Salehi-Abargouei. 2019. Chocolate milk for recovery from exercise: A systematic review and meta-analysis of controlled clinical trials. European Journal of Clinical Nutrition 73 (6):835–49. doi: 10.1038/s41430-018-0187-x.
- Ascensão, A., A. Rebelo, E. Oliveira, F. Marques, L. Pereira, and J. Magalhães. 2008 . Biochemical impact of a soccer match—Analysis of oxidative stress and muscle damage markers throughout recovery. Clinical Biochemistry 41 (10-11):841–51. doi: 10.1016/j.clinbiochem.2008.04.008.
- Baird, M. F., S. M. Graham, J. S. Baker, and G. F. Bickerstaff. 2012 . Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. Journal of Nutrition and Metabolism 2012:960363. doi: 10.1155/2012/960363.
- Beals, K., K. F. Allison, M. Darnell, M. Lovalekar, R. Baker, D. C. Nieman, Y. Vodovotz, and S. M. Lephart. 2017. The effects of a tart cherry beverage on reducing exercise-induced muscle soreness. Isokinetics and Exercise Science 25 (1):53–63. doi: 10.3233/IES-160645.
- Begg, C. B., and M. Mazumdar. 1994. Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4):1088–101. doi: 10.2307/2533446.
- Bell, P., I. Walshe, G. Davison, E. Stevenson, and G. Howatson. 2014. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 6 (2):829–43. doi: 10.3390/nu6020829.
- Bell, P. G., E. Stevenson, G. W. Davison, and G. Howatson. 2016. The effects of Montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients 8 (7):441. doi: 10.3390/nu8070441.
- Bell, P. G., I. H. Walshe, G. W. Davison, E. J. Stevenson, and G. Howatson. 2015. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Applied Physiology, Nutrition, and Metabolism 40 (4):414–23. doi: 10.1139/apnm-2014-0244.
- Bi, X., J. Zhang, C. Chen, D. Zhang, P. Li, and F. Ma. 2014. Anthocyanin contributes more to hydrogen peroxide scavenging than other phenolics in apple peel. Food Chemistry 152:205–9. doi: 10.1016/j.foodchem.2013.11.088.
- Bloedon, T. K., R. E. Braithwaite, I. A. Carson, D. Klimis-Zacas, and R. A. Lehnhard. 2019. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: A systematic review and meta-analysis. Nutrition Reviews 77 (9):630–45. doi: 10.1093/nutrit/nuz018.
- Bongiovanni, T., F. Genovesi, M. Nemmer, C. Carling, G. Alberti, and G. Howatson. 2020. Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: Current knowledge, practical application and future perspectives. European Journal of Applied Physiology 120 (9):1965–996. doi: 10.1007/s00421-020-04432-3.
- Borenstein, M., L. V. Hedges, J. P. T. Higgins, and H. R. Rothstein. 2019. Introduction to meta‐analysis. West Sussex, UK: Wiley.
- Bowtell, J., and V. Kelly. 2019. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Medicine 49 (Suppl 1):3–23. doi: 10.1007/s40279-018-0998-x.
- Bowtell, J. L., D. P. Sumners, A. Dyer, P. Fox, and K. N. Mileva. 2011. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Medicine & Science in Sports & Exercise 43 (8):1544–51. doi: 10.1249/MSS.0b013e31820e5adc.
- Brancaccio, P., N. Maffulli, and F. M. Limongelli. 2007. Creatine kinase monitoring in sport medicine. British Medical Bulletin 81-82 (1):209–30. doi: 10.1093/bmb/ldm014.
- Brandenburg, J. P., and L. V. Giles. 2019. Four days of blueberry powder supplementation lowers the blood lactate response to running but has no effect on time-trial performance. International Journal of Sport Nutrition and Exercise Metabolism 29 (6):636–42. doi: 10.1123/ijsnem.2019-0040.
- Brown, F., C. Gissane, G. Howatson, K. Van Someren, C. Pedlar, and J. Hill. 2017. Compression garments and recovery from exercise: A meta-analysis. Sports Medicine 47 (11):2245–67. doi: 10.1007/s40279-017-0728-9.
- Brown, L., S. P. Caligiuri, D. Brown, and G. N. Pierce. 2018. Clinical trials using functional foods provide unique challenges. Journal of Functional Foods 45:233–8. doi: 10.1016/j.jff.2018.01.024.
- Brown, M. A., E. J. Stevenson, and G. Howatson. 2019. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. European Journal of Sport Science 19 (1):95–102. doi: 10.1080/17461391.2018.1502360.
- Byrne, C., C. Twist, and R. Eston. 2004. Neuromuscular function after exercise-induced muscle damage. Sports Medicine 34 (1):49–69. doi: 10.2165/00007256-200434010-00005.
- Çakir-Atabek, H., B. Dokumaci, and C. Aygün. 2019. Strength loss after eccentric exercise is related to oxidative stress but not muscle damage biomarkers. Research Quarterly for Exercise and Sport 90 (3):385–94. doi: 10.1080/02701367.2019.1603990.
- Carvalho, L. C. d. S. A., M. C. de Freitas, A. S. Silva, and A. C. T. Biasoto. 2018. Syzygium cumini nectar supplementation reduced biomarkers of oxidative stress, muscle damage, and improved psychological response in highly trained young handball players. Frontiers in Physiology 9:1508.
- Clarkson, P. M., K. Nosaka, and B. Braun. 1992. Muscle function after exercise-induced muscle damage and rapid adaptation. Medicine and Science in Sports and Exercise 24 (5):512–20.
- Connolly, D., M. McHugh, and O. Padilla-Zakour. 2006. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. British Journal of Sports Medicine 40 (8):679–83. doi: 10.1136/bjsm.2005.025429.
- Costello, R., M. E. Willems, S. D. Myers, F. Myers, N. A. Lewis, B. J. Lee, and S. D. Blacker. 2020. No effect of New Zealand blackcurrant extract on recovery of muscle damage following running a half-marathon. International Journal of Sport Nutrition and Exercise Metabolism 30 (4):287–94. doi: 10.1123/ijsnem.2019-0312.
- Damas, F., K. Nosaka, C. A. Libardi, T. C. Chen, and C. Ugrinowitsch. 2016. Susceptibility to exercise-induced muscle damage: A cluster analysis with a large sample. International Journal of Sports Medicine 37 (8):633–40. doi: 10.1055/s-0042-100281.
- Davis, H. L., S. Alabed, and T. J. A. Chico. 2020. Effect of sports massage on performance and recovery: A systematic review and meta-analysis. BMJ Open Sport & Exercise Medicine 6 (1):e000614. doi: 10.1136/bmjsem-2019-000614.
- de Lima Tavares Toscano, L.,. A. S. Silva, A. C. L. de França, B. R. V. de Sousa, E. J. B. de Almeida Filho, M. da Silveira Costa, A. T. B. Marques, D. F. da Silva, K. de Farias Sena, G. S. Cerqueira, et al. 2020. A single dose of purple grape juice improves physical performance and antioxidant activity in runners: A randomized, crossover, double-blind, placebo study. European Journal of Nutrition 59 (7):2997–3007. doi: 10.1007/s00394-019-02139-6.
- de Morton, N. A. 2009. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Australian Journal of Physiotherapy 55 (2):129–33. doi: 10.1016/S0004-9514(09)70043-1.
- Doma, K., D. Gahreman, and J. Connor. 2021. Fruit supplementation reduces indices of exercise-induced muscle damage: A systematic review and meta-analysis. European Journal of Sport Science 21 (4):562–79.
- Ebbeling, C. B., and P. M. Clarkson. 1989. Exercise-induced muscle damage and adaptation. Sports Medicine 7 (4):207–34. doi: 10.2165/00007256-198907040-00001.
- Fallah, A. A., E. Sarmast, P. Fatehi, and T. Jafari. 2020. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food and Chemical Toxicology 135:110922. doi: 10.1016/j.fct.2019.110922.
- Fisher-Wellman, K., and R. J. Bloomer. 2009. Acute exercise and oxidative stress: A 30 year history. Dynamic Medicine 8:1. doi: 10.1186/1476-5918-8-1.
- Gleeson, M., J. Almey, S. Brooks, R. Cave, A. Lewis, and H. Griffiths. 1995. Haematological and acute-phase responses associated with delayed-onset muscle soreness in humans. European Journal of Applied Physiology and Occupational Physiology 71 (2-3):137–142. doi: 10.1007/BF00854970.
- Haidich, A. B. 2010. Meta-analysis in medical research. Hippokratia 14 (Suppl 1):29–37.
- Harty, P. S., M. L. Cottet, J. K. Malloy, and C. M. Kerksick. 2019. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sports Medicine - Open 5 (1):1–17. doi: 10.1186/s40798-018-0176-6.
- Hecksteden, A., S. Skorski, S. Schwindling, D. Hammes, M. Pfeiffer, M. Kellmann, A. Ferrauti, and T. Meyer. 2016. Blood-borne markers of fatigue in competitive athletes—Results from simulated training camps. PLoS One 11 (2):e0148810. doi: 10.1371/journal.pone.0148810.
- Higgins, J. P. T., and S. Green. 2011. Cochrane handbook for systematic reviews of interventions. Sussex, UK: Wiley Blackwell.
- Hill, J. A., K. M. Keane, R. Quinlan, and G. Howatson. 2021. Tart cherry supplementation and recovery from strenuous exercise: A systematic review and meta-analysis. International Journal of Sport Nutrition and Exercise Metabolism 1:1–14.
- Howatson, G., M. P. McHugh, J. A. Hill, J. Brouner, A. P. Jewell, K. A. van Someren, R. E. Shave, and S. A. Howatson. 2010. Influence of tart cherry juice on indices of recovery following marathon running. Scandinavian Journal of Medicine & Science in Sports 20 (6):843–852. doi: 10.1111/j.1600-0838.2009.01005.x.
- Howatson, G., and K. A. van Someren. 2007. Evidence of a contralateral repeated bout effect after maximal eccentric contractions. European Journal of Applied Physiology 101 (2):207–214. doi: 10.1007/s00421-007-0489-5.
- Howatson, G., and K. A. van Someren. 2008. The prevention and treatment of exercise-induced muscle damage. Sports Medicine 38 (6):483–503. doi: 10.2165/00007256-200838060-00004.
- Hu, M., J. Du, L. Du, Q. Luo, and J. Xiong. 2020. Anti-fatigue activity of purified anthocyanins prepared from purple passion fruit (P. edulis Sim) epicarp in mice. Journal of Functional Foods 65:103725. doi: 10.1016/j.jff.2019.103725.
- Hurst, R. D., K. A. Lyall, J. M. Roberts, A. Perthaner, R. W. Wells, J. M. Cooney, D. J. Jensen, N. S. Burr, and S. M. Hurst. 2019. Consumption of an anthocyanin-rich extract made from New Zealand blackcurrants prior to exercise may assist recovery from oxidative stress and maintains circulating neutrophil function: A pilot study. Frontiers in Nutrition 6:73. doi: 10.3389/fnut.2019.00073.
- Hurst, R. D., K. A. Lyall, R. W. Wells, G. M. Sawyer, D. Lomiwes, N. Ngametua, and S. M. Hurst. 2020. Daily consumption of an anthocyanin-rich extract made from New Zealand blackcurrants for 5 weeks supports exercise recovery through the management of oxidative stress and inflammation: A randomized placebo controlled pilot study. Frontiers in Nutrition 7:16. doi: 10.3389/fnut.2020.00016.
- Hutchison, A. T., E. B. Flieller, K. J. Dillon, and B. D. Leverett. 2016. Black currant nectar reduces muscle damage and inflammation following a bout of high-intensity eccentric contractions. Journal of Dietary Supplements 13 (1):1–15. doi: 10.3109/19390211.2014.952864.
- Jackson, D., and R. Turner. 2017 . Power analysis for random-effects meta-analysis. Research Synthesis Methods 8 (3):290–302. doi: 10.1002/jrsm.1240.
- Kastello, G. M., M. C. Bretl, E. R. Clark, C. L. Delvaux, J. D. Hoeppner, L. P. McNea, and J. D. Strauss. 2014. The effect of cherry supplementation on Exercise induced oxidative stress. International Journal of Food Sciences and Nutrition 1 (1):20–26.
- Keane, K. M., P. G. Bell, J. K. Lodge, C. L. Constantinou, S. E. Jenkinson, R. Bass, and G. Howatson. 2016a. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. European Journal of Nutrition 55 (4):1695–1705. doi: 10.1007/s00394-015-0988-9.
- Keane, K. M., T. W. George, C. L. Constantinou, M. A. Brown, T. Clifford, and G. Howatson. 2016b. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. The American Journal of Clinical Nutrition 103 (6):1531–1539. doi: 10.3945/ajcn.115.123869.
- Kirakosyan, A., E. Gutierrez, B. Ramos Solano, E. M. Seymour, and S. F. Bolling. 2018. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chemistry 252:142–146. doi: 10.1016/j.foodchem.2018.01.084.
- Kuehl, K. S., E. T. Perrier, D. L. Elliot, and J. C. Chesnutt. 2010. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. Journal of the International Society of Sports Nutrition 7 (1):17. doi: 10.1186/1550-2783-7-17.
- Kupusarevic, J., K. McShane, and T. Clifford. 2019. Cherry gel supplementation does not attenuate subjective muscle soreness or alter wellbeing following a match in a team of professional rugby union players: A pilot study. Sports 7 (4):84. doi: 10.3390/sports7040084.
- Lamb, K. L., M. K. Ranchordas, E. Johnson, J. Denning, F. Downing, and A. Lynn. 2019. No effect of tart cherry juice or pomegranate juice on recovery from exercise-induced muscle damage in non-resistance trained men. Nutrients 11 (7):1593–1593. doi: 10.3390/nu11071593.
- Lee, J., and P. M. Clarkson. 2003. Plasma creatine kinase activity and glutathione after eccentric exercise. Medicine and Science in Sports and Exercise 35 (6):930–936. doi: 10.1249/01.mss.0000069553.47739.36.
- Leeder, J., C. Gissane, K. van Someren, W. Gregson, and G. Howatson. 2012. Cold water immersion and recovery from strenuous exercise: A meta-analysis. British Journal of Sports Medicine 46 (4):233–240. doi: 10.1136/bjsports-2011-090061.
- Leeuwenburgh, C., and J. Heinecke. 2001. Oxidative stress and antioxidants in exercise. Current Medicinal Chemistry 8 (7):829–838. doi: 10.2174/0929867013372896.
- Levers, K., R. Dalton, E. Galvan, C. Goodenough, A. O’Connor, S. Simbo, N. Barringer, S. U. Mertens-Talcott, C. Rasmussen, M. Greenwood, et al. 2015. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. Journal of the International Society of Sports Nutrition 12 (1):41. doi: 10.1186/s12970-015-0102-y.
- Levers, K., R. Dalton, E. Galvan, A. O’Connor, C. Goodenough, S. Simbo, S. U. Mertens-Talcott, C. Rasmussen, M. Greenwood, S. Riechman, et al. 2016. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. Journal of the International Society of Sports Nutrition 13 (1):1–23. doi: 10.1186/s12970-016-0133-z.
- Li, D., P. Wang, Y. Luo, M. Zhao, and F. Chen. 2017. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Critical Reviews in Food Science and Nutrition 57 (8):1729–1741. doi: 10.1080/10408398.2015.1030064.
- Lima, L. C., R. V. Barreto, N. M. Bassan, C. C. Greco, and B. S. Denadai. 2019. Consumption of an anthocyanin-rich antioxidant juice accelerates recovery of running economy and indirect markers of exercise-induced muscle damage following downhill running. Nutrients 11 (10):2274. doi: 10.3390/nu11102274.
- Lyall, K. A., S. M. Hurst, J. Cooney, D. Jensen, K. Lo, R. D. Hurst, and L. M. Stevenson. 2009. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 297 (1):R70–R81. doi: 10.1152/ajpregu.90740.2008.
- Lynn, A., S. Garner, N. Nelson, T. N. Simper, A. C. Hall, and M. K. Ranchordas. 2018. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: A randomised, placebo-controlled trial. Journal of the International Society of Sports Nutrition 15 (1):1–8. doi: 10.1186/s12970-018-0227-x.
- MacIntyre, D. L., W. D. Reid, and D. C. McKenzie. 1995 . Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Medicine 20 (1):24–40. doi: 10.2165/00007256-199520010-00003.
- Manach, C., A. Scalbert, C. Morand, C. Rémésy, and L. Jiménez. 2004. Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition 79 (5):727–747. doi: 10.1093/ajcn/79.5.727.
- Matsukawa, T., H. Motojima, Y. Sato, S. Takahashi, M. O. Villareal, and H. Isoda. 2017. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Scientific Reports 7 (1):44799–12. doi: 10.1038/srep44799.
- McAnulty, L. S., S. R. Collier, M. J. Landram, D. S. Whittaker, S. E. Isaacs, J. M. Klemka, S. L. Cheek, J. C. Arms, and S. R. McAnulty. 2014. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutrition Research 34 (7):577–584. doi: 10.1016/j.nutres.2014.07.002.
- McAnulty, L. S., D. C. Nieman, C. L. Dumke, L. A. Shooter, D. A. Henson, A. C. Utter, G. Milne, and S. R. McAnulty. 2011. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Applied Physiology, Nutrition, and Metabolism 36 (6):976–984. doi: 10.1139/h11-120.
- McCormick, R., P. Peeling, M. Binnie, B. Dawson, and M. Sim. 2016. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. Journal of the International Society of Sports Nutrition 13:41. doi: 10.1186/s12970-016-0151-x.
- McLeay, Y., M. J. Barnes, T. Mundel, S. M. Hurst, R. D. Hurst, and S. R. Stannard. 2012. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. Journal of the International Society of Sports Nutrition 9 (1):19–12. doi: 10.1186/1550-2783-9-19.
- Morehen, J. C., J. Clarke, J. Batsford, S. Barrow, A. D. Brown, C. E. Stewart, J. P. Morton, and G. L. Close. 2021. Montmorency tart cherry juice does not reduce markers of muscle soreness, function and inflammation following professional male rugby League match-play. European Journal of Sport Science 21 (7):1003–12. doi: 10.1080/17461391.2020.1797181.
- Naderi, A., S. Rezaei, A. Moussa, K. Levers, and C. P. Earnest. 2018. Fruit for sport. Trends in Food Science & Technology 74:85–98. doi: 10.1016/j.tifs.2018.02.013.
- O’Connor, P. J., A. L. Caravalho, E. C. Freese, and K. J. Cureton. 2013. Grape consumption's effects on fitness, muscle injury, mood, and perceived health. International Journal of Sport Nutrition and Exercise Metabolism 23 (1):57–64. doi: 10.1123/ijsnem.23.1.57.
- Owens, D. J., C. Twist, J. N. Cobley, G. Howatson, and G. L. Close. 2019. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? European Journal of Sport Science 19 (1):71–85. doi: 10.1080/17461391.2018.1505957.
- Peake, J. M., O. Neubauer, P. A. D. Gatta, and K. Nosaka. 2017. Muscle damage and inflammation during recovery from exercise. Journal of Applied Physiology 122 (3):559–570. doi: 10.1152/japplphysiol.00971.2016.
- Pereira Panza, V. S., F. Diefenthaeler, and E. L. da Silva. 2015. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: Is including antioxidants enough? Nutrition 31 (9):1072–1082. doi: 10.1016/j.nut.2015.02.014.
- Pérez-Jiménez, J., V. Neveu, F. Vos, and A. Scalbert. 2010. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. European Journal of Clinical Nutrition 64 Suppl 3 (3):S112–S120. doi: 10.1038/ejcn.2010.221.
- Petrovic, S., A. Arsic, M. Glibetic, N. Cikiriz, V. Jakovljevic, and V. Vucic. 2016. The effects of polyphenol-rich chokeberry juice on fatty acid profiles and lipid peroxidation of active handball players: Results from a randomized, double-blind, placebo-controlled study. Canadian Journal of Physiology and Pharmacology 94 (10):1058–1063. doi: 10.1139/cjpp-2015-0575.
- Pilaczynska-Szczesniak, L., A. Skarpanska-Steinborn, E. Deskur, P. Basta, and M. Horoszkiewicz-Hassan. 2005. The influence of chokeberry juice supplementation on the reduction of oxidative stress resulting from an incremental rowing ergometer exercise. International Journal of Sport Nutrition and Exercise Metabolism 15 (1):48–58. doi: 10.1123/ijsnem.15.1.48.
- Pojer, E., F. Mattivi, D. Johnson, and C. S. Stockley. 2013. The case for anthocyanin consumption to promote human health: A review. Comprehensive Reviews in Food Science and Food Safety 12 (5):483–508. doi: 10.1111/1541-4337.12024.
- Pyne, D. B. 1994. Exercise-induced muscle damage and inflammation: A review. Australian Journal of Science and Medicine in Sport 26 (3-4):49–49.
- Quinlan, R., and J. A. Hill. 2020. The efficacy of tart cherry juice in aiding recovery after intermittent exercise. International Journal of Sports Physiology and Performance 15 (3):368–374. doi: 10.1123/ijspp.2019-0101.
- Rechner, A. R., and C. Kroner. 2005. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thrombosis Research 116 (4):327–334. doi: 10.1016/j.thromres.2005.01.002.
- Rodriguez-Mateos, A., G. Istas, L. Boschek, R. P. Feliciano, C. E. Mills, C. Boby, S. Gomez-Alonso, D. Milenkovic, and C. Heiss. 2019. Circulating anthocyanin metabolites mediate vascular benefits of blueberries: Insights from randomized controlled trials, metabolomics, and nutrigenomics. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 74 (7):967–976. doi: 10.1093/gerona/glz047.
- Sabou, V. R., M. F. O’Leary, Y. Liu, P. N. Brown, S. Murch, and J. L. Bowtell. 2021. Review of analytical methods and reporting of the polyphenol content of tart cherry supplements in human supplementation studies investigating health and exercise performance effects: Recommendations for good practice. Frontiers in Nutrition 8 (124):652094. doi: 10.3389/fnut.2021.652094.
- Sadowska-Krępa, E., B. Kłapcińska, E. Kimsa, and R. Karpińsk. 2008. Effects of supplemetation with red grape skin polyphenolic extract and interval swimming test on the blood antioxidant status in healthy men. Medicina Sportiva 12 (1):1–7. doi: 10.2478/v10036-008-0001-2.
- Sandoval-Ramírez, B. A., Ú. Catalán, S. Fernández-Castillejo, A. Pedret, E. Llauradó, and R. Solà. 2020. Cyanidin-3-glucoside as a possible biomarker of anthocyanin-rich berry intake in body fluids of healthy humans: A systematic review of clinical trials. Nutrition Reviews 78 (7):597–610. doi: 10.1093/nutrit/nuz083.
- Seeram, N. P., R. A. Momin, M. G. Nair, and L. D. Bourquin. 2001. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 8 (5):362–369. doi: 10.1078/0944-7113-00053.
- Seymour, E. M., S. M. Warber, A. Kirakosyan, K. R. Noon, B. Gillespie, V. E. Uhley, J. Wunder, D. E. Urcuyo, P. B. Kaufman, and S. F. Bolling. 2014. Anthocyanin pharmacokinetics and dose-dependent plasma antioxidant pharmacodynamics following whole tart cherry intake in healthy humans. Journal of Functional Foods 11:509–516. doi: 10.1016/j.jff.2014.08.007.
- Silvestre, J. C., C. R. Juzwiak, A. P. B. Gollucke, V. Z. Dourado, and V. D´Almeida. 2014. Acute effect of a grape concentrate intake on oxidative stress markers in triathletes. Revista Brasileira de Cineantropometria e Desempenho Humano 16 (5):533–544. doi: 10.5007/1980-0037.2014v16n5p533.
- Skarpańska-Stejnborn, A., P. Basta, and Ł. Pilaczyńska-Szcześniak. 2006. The influence of supplementation with the black currant (Ribes nigrum) extract on selected prooxidative-antioxidative balance parameters in rowers. Studies in Physical Culture & Tourism 13 (2):51–58.
- Skarpańska-Stejnborn, A., P. Basta, Ł. Pilaczyńska-Szcześniak, and M. Horoszkiewicz-Hassan. 2010. Black grape extract supplementation attenuates blood oxidative stress in response to acute exercise. Biology of Sport 27 (1):41–46. doi: 10.5604/20831862.907791.
- Skarpańska-Stejnborn, A., P. Basta, J. Sadowska, and Ł. Pilaczyńska-Szczeńniak. 2014. Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers. Journal of the International Society of Sports Nutrition 11 (1):1–10. doi: 10.1186/s12970-014-0048-5.
- Speer, H., N. M. D’Cunha, N. I. Alexopoulos, A. J. McKune, and N. Naumovski. 2020. Anthocyanins and human health—A focus on oxidative stress. Inflammation and Disease. Antioxidants 9 (5):366.
- Steichen, T. 2010. METATRIM: Stata module to perform nonparametric analysis of publication bias. https://ideas.repec.org/c/boc/bocode/s410501.html
- Sullivan, G. M., and R. Feinn. 2012. Using effect size—Or why the P value is not enough. Journal of Graduate Medical Education 4 (3):279–282. doi: 10.4300/JGME-D-12-00156.1.
- Tee, J. C., A. N. Bosch, and M. I. Lambert. 2007. Metabolic consequences of exercise-induced muscle damage. Sports Medicine 37 (10):827–836. doi: 10.2165/00007256-200737100-00001.
- Torres, R., F. Ribeiro, J. A. Duarte, and J. M. Cabri. 2012. Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: Systematic review and meta-analysis. Physical Therapy in Sport 13 (2):101–114. doi: 10.1016/j.ptsp.2011.07.005.
- Toscano, L. T., R. L. Tavares, L. T. Toscano, C. S. O. d. Silva, A. E. M. d. Almeida, A. C. T. Biasoto, M. d. C. R. Gonçalves, and A. S. Silva. 2015. Potential ergogenic activity of grape juice in runners. Applied Physiology, Nutrition, and Metabolism 40 (9):899–906. doi: 10.1139/apnm-2015-0152.
- Vitale, K. C., S. Hueglin, and E. Broad. 2017. Tart cherry juice in athletes: A literature review and commentary. Current Sports Medicine Reports 16 (4):230–239. doi: 10.1249/JSR.0000000000000385.
- Wang, H., M. G. Nair, G. M. Strasburg, Y. C. Chang, A. M. Booren, J. I. Gray, and D. L. DeWitt. 1999. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. Journal of Natural Products 62 (5):802. doi: 10.1021/np990184z.
- Warren, G. L., D. A. Lowe, and R. B. Armstrong. 1999. Measurement tools used in the study of eccentric contraction-induced injury. Sports Medicine 27 (1):43–59.