Abstract
There is a growing body of evidence supporting the role that phytochemicals play in reducing the risk of various chronic diseases. Although there has been a rise in health products marketed as being “supergrains,” “superfood,” or advertising their abundance in antioxidants, these food items are often limited to powdered blends, dried fruit, nuts, or seeds, rarely intercepting the market of baked snacks. This is in part due to the still limited understanding of the impact that different industrial processes have on phytochemicals in a complex food matrix and their corresponding bioavailability. This review brings together the current data on how various industrial dehydration processes influence the retention and bioaccessibility of phytochemicals in baked snacks. It considers the interplay of molecules in an intricate snack matrix, limitations of conventional technologies, and constraints with consumer acceptance preventing wider utilization of novel technologies. Furthermore, the review takes a holistic approach, encompassing each stage of production—discussing the potential for inclusion of by-products to promote a circular economy and the proposal for a shift in agriculture toward biofortification or tailored growing of crops for their nutritional and post-harvest attributes.
Introduction
Phytochemicals, used interchangeably with the term phytonutrients, are naturally occurring chemicals produced by plants for protection against predators and prevention from disease. They are considered “non-essential” nutrients as the human body does not require them to function and there are no equivalent guidelines for daily consumption like the reference daily intake (RDI) given for essential vitamins, minerals, and trace elements. They also do not provide energy. However, they do have bioactive role in the human body mostly as antioxidants. Their bioactivity has triggered a plethora of studies investigating whether their disease preventative properties in plants extends to humans and their potential for use in pharmaceuticals or as a functional food (Wu, Zhou, and Xu Citation2009; Nichenametla et al. Citation2006; Zhang et al. Citation2015; Can-Cauich et al. Citation2017). Phytochemicals of particular health-promoting attributes can be grouped into the following categories: organosulphur compounds, saponins, phenolics (polyphenols) and terpenes (carotenoids). Phenolics, arguably the main class of secondary metabolites in food and beverages, encompass a broad range of compounds identifiable by their benzene ring(s) with one or more hydroxyl groups (Del Rio et al. Citation2013). However, though phytochemicals may be abundant in fruit and vegetables, it is often difficult to exploit their biological benefits due to their particularly low bioavailability, even in comparison to their essential nutrient counterparts—vitamins and minerals (Holst and Williamson Citation2008).
The bioavailability of phytochemicals can, however, be improved by reformulation of vegetable products (Perez-Moral et al. Citation2018). Public Health England are currently conducting a Reduction and Reformulation programme (Tedstone et al. Citation2018) which sets targets for retailers and manufacturers in foods high in sugar and salt in an attempt to reduce salt, sugar, and calories across the food industry. This reduction and reformulation approach suggests shifting consumer purchasing toward lower fat, calories, or no added sugar products. But this approach lacks emphasis on promoting reformulation to increase beneficial nutrients such as phytochemicals.
Reformulated snacking products represent an opportunity to increase phytochemical intake at population level. Dietary surveys from China (Wang et al. Citation2012) and the United States (Dunford and Popkin Citation2018) show an increasing shift away from a “main-meals” diet pattern to a one where snacking is more frequent than main meals. Such frequent-snacking diets have been shown to correlate with overconsumption and increased body weight (Bellisle Citation2014), although there are many other contributary factors so the link is not definitive. Being able to produce snacks that retain the nutritional benefits of phytochemicals, strategically formulated to promote bioaccessibility, whilst also offering great taste and convenience represent a unique opportunity to improve population health.
Phytochemical’s bioaccessibility in the food matrix depends on the structure, degree of glycosylation/acylation, molecular size, solubility, presence of complementary compounds and the individual’s microbiome (Epriliati and Irine Citation2012). For instance, thermal processing of tomatoes has shown to significantly increase the bioavailability of lycopene, promoting isomerization from the less bioavailable all-trans-lycopene to the more bioavailable cis-lycopene (Unlu et al. Citation2007). However, in broccoli or cabbage processing, the higher temperatures can result in the inactivation of myrosinase, the enzyme catalyzing the reaction which transforms glucosinolates to isothiocyanates. This has been known to reduce their bioavailability as the compound is better absorbed in its isothiocyanate form (Conaway et al. Citation2000). Therefore, it is important to understand the influence that processing will have on the phytochemical and food matrix to predict the bioavailability. However, it is difficult to generalize the bioavailability of a specific phytochemical as each case is different and a detailed analysis of the food matrix is required to allow accurate predictions(Shahidi and Pan Citation2021).
The aim of this review is to summarize recent work investigating how phytochemicals can be incorporated into an important class of snacks, baked snacks, to enable them to give potentially positive health benefits. In particular, it summarizes the effect of processing, starting ingredients and formulation, on their potential bioavailability and bioaccessibility, and highlights key issues to be considered in the design of new snack products.
Current phytochemical health claims and legislation landscape
A diet rich in fruit and vegetables is known to be beneficial to health with strong supporting evidence in the scientific literature (Aune et al. Citation2017; Giovannucci et al. Citation2003). The 5-a-day for Better Health campaign, launched in 2003 and inspired by the World Health Organization, recommended consuming 400 g of fruit and vegetables a day. This was later considered an underestimate when a meta-analysis (Aune et al. Citation2017) showed that an intake of up 800 g could reduce risk of cancer, cardiovascular disease and all-cause mortality. These results were based on 95 unique cohorts (145 publications) encompassing Europe, USA, Asia, and Australia, relating fruit and vegetable consumption to premature deaths of coronary heart disease (CHD), stroke, total cardiovascular disease, total cancer and to all-cause mortality. This study concluded that a people with a diet including a daily intake of >800 g of fruit and vegetables had the lowest risk of CHD, stroke, cardiovascular disease, and all-cause mortality and >600 g for cancer. But what is it that makes fruits and vegetables such an enhancement to our well-being? Despite being an excellent source of essential vitamins and minerals, they also provide a myriad of phytochemicals with a range of suspected health benefits. Currently, it is thought that there could be up to 10,000 phytochemicals (Zhang et al. Citation2015)—delivered by fruits, vegetables, spices and some grains making them a regular component of our diet.
It is important to recognize that there are currently very few health claims authorized by regulatory bodies that arise from phytochemicals. includes all the current claims that have been substantiated by the USA Food and Drug Administration (FDA), European Food Safety Authority (EFSA) and Food Standards Australia and New Zealand (FSANZ). It is worth noting that sometimes fibers can be classed as phytochemicals, being chemicals that originate from “phyto”—plants. However, in this review, phytochemicals are considered as compounds produced by plants for defense, and therefore fiber is considered a separate category. In order to validate such a health claim, human intervention studies are crucial. However, whilst there is ample evidence from epidemiological studies and cell-and-animal-based studies, limitations in the execution of human intervention studies means that it is hard to substantiate claims. Limitations arise in the design of such studies due to three major factors: absence of (1) a variety of well-characterized plant foods to test the health-promoting hypothesis associated with the specific phytochemical, (2) validated biomarkers for health status and disease risk (Traka and Mithen Citation2011; Martini et al. Citation2017; Turck et al. Citation2018) and (3) the wide variation in bioavailability experienced between individuals.
Table 1. Phytochemical health claims approved by FDA, EFSA and FSANZ.
Phytochemomics is a fairly recent term coined to describe a field of study that provides novel information about phytochemical effects on physiology and biochemistry of living organism by combining a variety of areas of knowledge (Amigo-Benavent et al. Citation2014). It can be considered as a branch of foodomics, a larger discipline which assesses the molecular level contribution of food constituents on the genome, transcriptome, metabolome, and proteome (Capozzi and Bordoni Citation2013). Foodomics has been used to address issues relating to food safety and quality; connections between food, health and disease; genetic engineering/transgenic plants and providing scientific evidence to support health claims (Ferranti Citation2018). Therefore, there is a reasonable assumption that phytochemomics offers the prospect of validating, or disproving, of health claims relating to the bioactivity of phytochemicals (Amigo-Benavent et al. Citation2014).
Defining key terms: bioavailability/bioaccessibility
Bioavailability represents the fraction of the nutrient consumed that is digested in the gastrointestinal (GI) tract, absorbed, distributed to designated tissues, and metabolized (Carbonell-Capella et al. Citation2014), and is thus dependent on the bioaccessibility (a term often mistaken for bioavailability) of the nutrient within the food matrix. Bioaccessibility is the quantity of the nutrient released from the matrix during digestion making it accessible for absorption in the gastrointestinal tract. It is determined by the proportion of phytochemicals that are assimilated into to micelles following digestion, whereas bioavailability can be calculated from the proportion of phytochemicals ingested that reach systemic circulation (Fraser and Bramley Citation2004).
Phytochemicals are often poorly digested due to their location within plant matrices, and they are commonly found complexed to other molecules. Some phytochemicals like carotenoids are stored in the chromoplasts of plant cells (as a carotenoid-protein complex (Balasundram, Sundram, and Samman Citation2006)), under unusual conditions phenols can be found free (Parr and Bolwell Citation2000) but are usually conjugated, such as β-glycosides, to reduce their toxicity or increase solubility (Parr and Bolwell Citation2000), and all phytochemicals can end up entrapped in a macromolecular matrix after food processing.
Phytochemicals in baked snacks
The term “baked snack” encompasses products that are produced using a range of drying technology platforms, and which also have the potential for inclusion of multiple food groups. Importantly it involves dehydration, color, texture, appearance (e.g., blistering) and flavor development of whole, sliced, or a formulated snack blend made primarily from vegetables, tubers, and roots as well as fruits, herbs, and legumes. Formulated snack products frequently utilize powdered fruit or vegetables as a means of introducing associated flavors and vitamins. However, these powders often do not contain the equivalent nutrition to their fresh counterpart, and therefore snacks relying on powdered concentrates cannot be considered for a 5-a-day health claim (Public Health England Citation2016).
For the purpose of this review, “baked” refers to a product that has been dried using hot air (convective or impingement) for the majority of the drying time (not necessarily the majority of the water removal) and may include one or more additional unit operations of hot air drying, microwave drying, radio frequency finishing-drying or any other non-frying drying technology. Though baking processes may utilize dehydration technologies such as microwaves and freeze-drying (FD) for part of the drying, a baked snack can be distinguished from a dehydrated product by the occurrence of additional chemical reactions such as Maillard reactions which will contribute to the final appearance, flavor, color, and consumer appeal. The prevalent health promoting phytochemical groups found in baked snack ingredients are carotenoids, found in vegetables such as carrots, sweet potato, tomatoes, bell peppers; polyphenols, from plants such as spinach, kale, red onion, and broccoli; and organosulphur containing compounds (OSCs), from allium and brassica vegetables. Knowledge of how to retain these compounds through processing is therefore vital to allow their health benefits to be exploited in these snacks, and for phytochemically rich baked snack products to be strategically formulated.
Carotenoids
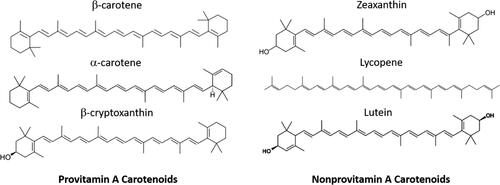
Carotenoids are recognized by a distinctive conjugated double bond system compromised of eight isoprenoid units () and are known largely for the bright red-yellow attractive color, supplied by the conjugated polyene structure, that they give some fruit and vegetables. This pigmentation naturally makes them a focus for the food and drink industry with the appeal they provide to consumers, but they are also well regarded for their health benefits, therefore making them a highly documented phytochemical. The main carotenoids that have been targeted for research due to their relative abundance in food are: α-carotene, β-carotene, β-cryptoxanthin, lycopene, zeaxanthin, and lutein. Carotenoids must be ingested for their provitamin A activity as in animals vitamin A (retinol) cannot be synthesized de novo without these precursors. If you follow a diet devoid of meat, then acquiring these precursors will be solely dependent on the fruit and vegetables consumed. Approximately only 10% of the carotenoids consumed have the correct structural potential for provitamin A activity (Fernández-García et al. Citation2012)—containing at least one β-type non-substituted ring. The main source of retinol is β-carotene, as it is one of the most abundant carotenoids in foods and also for every molecule it produces two molecules of retinal, then to be reduced to retinol. Furthermore, human bioavailability studies have shown that for every twelve molecules of β-carotene ingested, humans absorb one, giving an approximate 12:1 ratio, for other carotenoids this ratio is 24:1 (van Het Hof et al. Citation1999). These are the ratios used by the FDA to calculate the respective vitamin A (from retinol equivalents) in a food item. However, their bioavailability depends on a multitude of factors and cannot realistically be assumed so simply.
The mnemonic “SLAMENGHI”; Species of carotenoids, molecular Linkage, Amount of carotenoid consumed in a meal, Matrix in which the carotenoid is incorporated, Effectors of absorption and bioconversion, Nutrient status of the host, Genetic factors, Host-related factors, and mathematical Interactions, was coined by de Pee and West (Citation1996), grouping together the elements that impact carotenoid bioavailability. Thus, the variables that are of interest to food formulation and processing are: the species of carotenoid, molecular linkage, carotenoid quantity, the food matrix and effectors of absorption and bioconversion. For further depth, Desmarchelier and Borel (Citation2017) use SLAMENGHI in their detailed overview of carotenoid bioavailability determinants.
Carotenoids are stored in stable organelles called plastids and play an essential photosynthetic and photoprotective role in plants. In green leafy vegetables such as spinach and lettuce, carotenoids (β-carotene, lutein) are often intimately associated with light-harvesting complexes in chloroplasts. This location has been shown to lower bioaccessibility (van Het Hof et al. Citation2000), as these carotenoids are non-covalently bound to protein and fiber in the thylakoid membrane. In vegetables and fruit, such as carrots and tomatoes, carotenoids are found as membrane bound semi-crystalline structures originating from differentiated plastids or dissolved in oil droplets in chromoplasts as in pumpkins (Faulks and Southon Citation2005), mango and papaya (Carbonell-Capella et al. Citation2014). Serum β-carotene increases 2.6–6 times (de Pee et al. Citation1998) more efficiently from fruits than from green, leafy vegetables as β-carotene bioavailability decreases with increasing matrix complexity. In a study comparing the carotenoid bioavailability between carrots, tomatoes, and papaya, β-carotene was three times more bioavailable in papaya than in carrots and tomatoes and lycopene was 2.6 times more (Schweiggert et al. Citation2014). This significant difference was attributed to the physical state and localization of the carotenoids in papaya.
After being solubilized from the food matrix carotenoids must be incorporated into micelles to be accessible by the intestinal epithelium. The presence of fat stimulates biliary salts secretion and an increase in pancreatic lipases, which enhance micellization capacity. The amount of fat required for optimal carotenoid absorption has been estimated as low as 3–5 g (Roodenburg et al. Citation2000) per meal of cooked vegetables. However, if consumed raw, without undergoing thermal or mechanical processing that increase the solubility of β-carotene from the matrix (van Het Hof et al. Citation1998), the accompanying fat content may need to be higher. A study reviewed the bioavailability of carotenoids after ingesting salad with salad dressings containing 0, 6, or 28 g canola oil (Brown et al. Citation2004). It found that the high fat dressing exhibited the highest bioavailability of carotenoids. Thus, consuming vegetables with fat will likely increase carotenoid bioavailability but the optimal amount of fat required will depend on the degree of food processing, matrix complexity, the quantity, and species (van Het Hof et al. Citation2000) of carotenoids consumed in the meal.
Fiber is the other macronutrient that has a critical role in determining carotenoid bioavailability. Through physical entrapment of phytochemicals in the matrix (Brownlee et al. Citation2006), isolation of micelle components, inhibition of pancreatic lipase and increased viscosity of gastric fluids in the intestinal tract, fiber reduces rate of extraction of phytochemicals from the food matrix and reduces/inhibits their absorption in the small intestine. When the dietary fiber pectin was added to meals, the serum response to β-carotene decreased by 42% (Rock and Swendseid Citation1992). The same result was found in a study investigating the impact of consuming lutein with pectin and β-carotene in rats, which suggested that the presence of these two molecules suppresses lutein absorption. Furthermore, there is a significant negative correlation between the amount of Klason lignin and nonstarch polysaccharides (embodying dietary fiber) in the small intestine and the quantity of β-carotene and lutein absorption (Serrano, Goñi, and Saura-Calixto Citation2005). These were thought to act as a blockade to preventing release of their carotenoids and impede the actions of digestive enzymes.
Therefore, when designing a matrix to enhance bioavailability of particular carotenoids, the degree of food processing, presence of other carotenoids, the proportion of fat, and the quantity of dietary fiber involved in the formulation should be considered.
Polyphenols
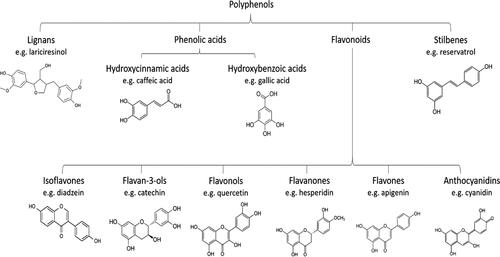
Absorption of polyphenols is heavily dependent on their chemical structure (D’Archivio et al. Citation2010), molecular weight, glycosylation and esterification (). In food, polyphenols largely exist as esters, glycosides, or polymers. In fact, all flavonoids (with the exception of flavanols) are present in their glycosylated form. However, only aglycones (the compound remaining after the glycosyl group on a glycoside is replaced by a hydrogen atom) and few glucosides, such as quercetin and resveratrol (Felgines et al. Citation2003), can be absorbed by the small intestine. Therefore, most polyphenols must undergo hydrolyzation by intestinal enzymes and the colon microbiota before absorption. The compounds are then further transformed throughout the absorption process into a myriad of secondary metabolites (Lewandowska et al. Citation2013). Thus, it is important to note that, frequently, calculations of phytochemical bioavailability may underestimate the true figure as it is very difficult to trace all the secondary metabolites and their associated bioactivity (Setchell et al. Citation2003).
A review of 97 polyphenol human bioavailability studies conducted by Manach et al. (Citation2005) concluded that the most absorbable polyphenols were gallic acid and isoflavones, with catechins, flavanones, and quercetin glucosides following (differing by their kinetics). Proanthocyanidins, galloylated tea catechins and anthocyanins were found to be the least well-absorbed. Isoflavonoids differ from flavonoids by the presence of B-ring at the 3-position instead of at the 2-postion (Murota et al. Citation2002) as seen in flavanones, flavan-3-ol catechin and flavonol quercetin (). This is thought to increase their efficiency of absorption, enabling intact isoflavones to be transported to the basolateral side. Flavonoids, however, with molecular weights of >500 Daltons, are not likely to be transported through passive diffusion and their chance of absorption is further hindered by influx membrane transporters not recognizing their signaling (Johnson et al. Citation2012). This would explain the difference in absorption rates between isoflavonoids and flavonoids seen in the studies included in the afore mentioned review.
Several studies have investigated polyphenols as potential modulators of glycemic response as they have been shown to inhibit α-amylase and α-glucosidase, critical carbohydrate digestive enzymes, and reduce sodium-dependent glucose transporter mediated glucose uptake, the predominant mechanism for transporting glucose into cells (Kim, Keogh, and Clifton Citation2016). As polyphenols have been shown to effectively reduce starch uptake, it would be reasonable to expect the inverse, that starch also reduces polyphenol absorption. This consequence is particularly seen in polymeric tannins and amylose where non-digestible complexes can be formed thus increasing the quantity of resistant starch but also decreasing the phytochemical bioavailability (Barros, Awika, and Rooney Citation2012). Similarly, some polyphenols have a high protein affinity forming protein-polyphenol complexes frequently reducing the antioxidant capacity of the polyphenol. These interactions have been extensively reviewed by Ozdal, Capanoglu, and Altay (Citation2013), summarizing different mechanisms of protein-phenolic interactions and findings of in vitro studies. Further reviews, such as Teng and Chen (Citation2019), Di Lorenzo et al. (Citation2021), and Bohn (Citation2014) have been published over recent years discussing factors influencing polyphenol bioavailability, challenges that hinder the utilization of polyphenols as potential chemopreventive agents. Specifically, Angelino et al. (Citation2017), review the bioaccessibility and bioavailability of phenolic compounds in bread. The main compounds in bread are phenolic acids and lignans varying dependent on the variety and grain used, and are found often in the hull or husk. Due to this, bioavailability of these compounds is often determined by the extent that the processing methods release the compounds and increases their extractability. They conclude that various pre-processing techniques such as fractionation methods, micronization, germination and fermentation can be used to increase the bioavailability and extractability of phenolic compounds. Additionally during the bread making process, alternative methods of mixing must be considered to reduce the propensity for phenolic acids to bind to proteins and the baking temperature which can potentially degrade thermolabile compounds.
To ensure the bioavailability of polyphenols, it is important to consider the relative protein and starch content, the pH of the food matrix and potential pre-processing techniques such as fermentation. Ideally the relative protein content and polymeric starch molecules would be limited to reduce the propensity for non-digestible complex formation.
Organosulphur containing compounds
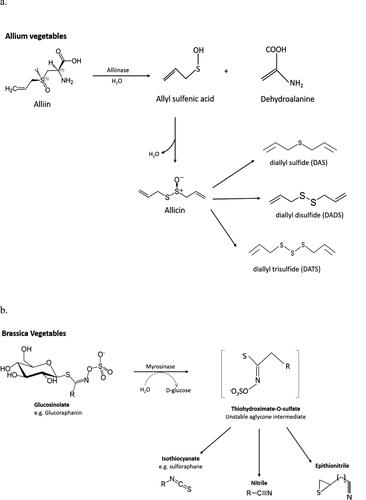
OSCs are predominantly found in two genera of vegetables: (1) the allium genus e.g., garlic, onion, leeks, and chives containing S-alk(en)yl-l-cysteine sulfoxides (2) brassica vegetables e.g., broccoli, brussels sprouts, cabbage, and cauliflower containing S methyl cysteine-l-sulfoxides. All of their structures are comprised of sulfur atoms bound to a cyanate group or carbon atom cyclically or non-cyclically ().
In Alliums, the main OSCs can be divided into:
oil-soluble polysulfides such as di-, tri- and tetrasulfides
water-soluble thiosulfinates (e.g., allicin)—the intermediate formed when the enzyme alliinase reacts with S-alk(en)yl-l-cysteine sulfoxides.
The action of this enzyme, alliinase, is highly important as it is the breakdown products, following its reaction with alliin, that provide the anti-cancer benefits that OSCs have been shown to display. As they are compartmentalized in different parts of the cell, cellular disruption must take place for the enzyme to come into contact with its substrate alliin. Allicin, being highly unstable decomposes into the bioactive compounds: diallyl sulfide, diallyl disulfide and diallyl trisulfide.
An almost identical system can be found in the Brassica vegetables but with involvement of glucosinolates and myrosinase replacing alliinase. Glucosinolates are compounds characterized by a thioglucose group conjugated to a core sulfonated isothiocyanate (ITC) group with an R-group side chain that varies between species. Hydrolysis from a glucosinolate interaction with myrosinase results in a β-d-glucose sugar and a thiohydroximate-O-sulfonate—an unstable aglycone. Rearrangement of the unstable intermediate forms a number of bioactive compounds, such as ITCs, known for their health promoting properties such as selective carcinogenesis inhibition (Singh and Singh Citation2012), anti-tumor, and chemoprotective capability (Higdon et al. Citation2007; Verhoeven et al. Citation1996) (Liu and Lv Citation2013) (Zhang, Ho, et al. Citation2018).
However, results from cohort studies have been varied when assessing their impact on cancer risk, some reporting little or no impact (Chow et al. Citation1992; Giovannucci et al. Citation2003). Often the details of their chemoprotective mechanism is not well established; the evidence is not sufficient enough to be able to develop related health claims. Enzyme driven hydrolysis by myrosinase or alliinase to yield the bioactive products is an integral step in increasing OSCs bioavailability. Although hydrolysis can be achieved by the bacteria in the gut, is it substantially less efficient than hydrolysis performed by the vegetables innate enzymes (Smith, Mithen, and Johnson Citation2003). Therefore, cellular disruption must occur before or during consumption to facilitate this reaction. Crushing, cutting, and grinding, damaging cell integrity in food preparation would promote an enzyme-substrate interaction, however, it is commonly accompanied by thermal treatment either during domestic cooking or commercial food processing. Myrosinase denatures at 70 °C (Jones et al. Citation2010) and alliinase begins denaturing at 42 °C and is inactivated above 60 °C (Méndez Lagunas and Castaigne Citation2008)—temperatures that are often exceeded in baking, frying, microwaving, boiling or steaming. Furthermore, some ITCs and thiocyanates, such as allyl-ITC, are volatile, evaporating at 88 °C, and thus can easily be lost in heat applications (Wu, Zhou, and Xu Citation2009). Therefore, to achieve optimum OSC bioavailability whilst also producing appetizing, food safe products, the method of thermal treatment would need to be thoroughly considered.
Compared to the carotenoids and polyphenols discussed, the knowledge of OCS bioavailability, from food matrix release to their metabolization and distribution within human tissues, is relatively limited. Reviews such as those Angelino and Jeffery (Citation2014), Putnik et al. (Citation2019), and Johnson (Citation2002) summarize the current knowledge of OSC formation, bioavailability and metabolism, discussing also the bioactivity of their metabolites. They conclude that there remains a significant shortage in the scientific understanding of factors influencing OSC bioavailability, however there is a general consensus that controlling the enzyme-substrate interactions and the conversion of the OSCs to their bioactive hydrolysis products greatly influence the bioavailability of these compounds. Therefore, when aiming to increase bioavailability of OSCs from allium and brassica vegetables, the food matrix and any impact storage or processing could have on alliinase and myrosinase activity should be considered.
Impact of drying/baking technologies on vegetable phytochemical content
Vegetables are more often than not thermally treated, whether through domestic cooking or commercial food processing before they are consumed. Thermal processing will inevitably induce changes in the chemical composition of food, denaturing proteins, disrupting cell structures, oxidizing innate antioxidants, and also potentially increasing bioavailability (Carrillo et al. Citation2017). There have been a number of studies that have investigated the impact that domestic cooking methods, such as boiling, frying, steaming, microwaving, and baking (Lombard et al. Citation2005; Pellegrini et al. Citation2010; Palermo, Pellegrini, and Fogliano Citation2014), have on phytochemical loss or retention in a variety of vegetables. However, rarely are the impacts of commercial processing and industrial scale drying techniques discussed. Phytochemical testing is expensive, and with very limited approved health claims for companies to entice customers, the commercial interest is low. provide a summary of the effect that different drying methods have on the percentage phytochemical loss/increase in plant produce.
Table 2. Influence of drying technologies on carotenoid retention in vegetables, fruit, herbs, and spices.
Table 3. Influence of drying technologies on polyphenol retention in vegetables, fruit, herbs, and spices.
Table 4. Influence of drying technologies on organosulphur compound in vegetables, fruit, herbs, and spices.
When considering domestic cooking techniques, boiling, and frying are frequently reported as being the most destructive heat treatments to micronutrients and steaming as the least. It is widely accepted that lowering temperatures and decreasing the time the vegetable is exposed to the heat will promote nutrient retention (Yuan et al. Citation2009; Xu et al. Citation2014; Girgin and El Citation2015; Giallourou, Oruna-Concha, and Harbourne Citation2016; Miglio et al. Citation2008; Deng et al. Citation2015). Therefore it is not surprising that frying, with temperatures easily exceeding 170 °C, can report losses of 57% and 62% of anthocyanins in purple-fleshed potato (Tian et al. Citation2016) and red cabbage (Xu et al. Citation2014) respectively, 63% of phenols in zucchini (Miglio et al. Citation2008) and 84% of glucosinolates in broccoli (Miglio et al. Citation2008). However, it is important to note that though there is a large reduction in selected phytochemicals, the overall antioxidant capacity of fried vegetables can still be maintained or even increased—likely due to compounds with high radical scavenging activity, e.g. melanoidins, formed in the later stages of the Maillard reaction (Serpen and Gökmen Citation2009; Madrau et al. Citation2010). Thermal treatment triggers a number of physiological changes in vegetables, including softening of the matrix rendering water soluble phytochemicals such as: flavonoids, glucosinolates and anthocyanins susceptible to leaching into the surrounding water volume. Therefore, it could be expected that the nutrient retention during boiling, microwaving and steaming is highly correlated to the amount of water used to accompany it (Sarvan, Verkerk, and Dekker Citation2012; Xu et al. Citation2014). If aiming to reduce the loss of nutrients when cooking, cooking time, temperature and the water-to-vegetable ratio should be minimized.
However, this review primarily focuses on baked products, in particular the impact of commercial baking and dehydration processes on phytonutrients and an assessment of which novel processing technologies could be beneficial if they are optimized for industrial scale-up. In 2017, the total revenue of the global market for dehydrated vegetables stood at US$54,241.9 million and is estimated to surpass US$90,636 million by the end of 2028 (Future Market Insights Citation2018). With moisture content commonly exceeding 80%, vegetables are highly perishable goods and though storing fresh is the best way to retain nutrients, drying is required to vastly extend shelf life and also to promote food security (Dev and Raghavan Citation2012). However, removing such large volumes of water often requires high temperatures and large amounts of energy, both of which can be detrimental to nutrients and also incur high costs. Drying methods have advanced far from traditional solar drying techniques, that though are sustainable and cheap, rely on weather conditions and risk contamination. Conventional drying such as commercial hot air dryers frequently result in negative product attributes, stripping vegetables of their color, flavor volatiles, changing their texture and impacting their nutrient profile. From a demand to ensure consistent quality, drive product innovation, and improve dehydration efficiency, a plethora of novel drying technologies have been developed. These primarily rely on convection, conduction, radiation, or a combination to drive water vaporization. With the exception of FD, these technologies can be categorized into:
Pretreatment: infrared, osmotic dehydration, microwave blanching, high-humidity hot air impingement blanching
Convective drying: hot air drying, fluidized bed drying, spouted bed drying
Conductive drying: drum drying, refractance window dryers and agitated thin film dryers, vacuum drying
Radiative drying: microwave drying, radio frequency drying, high-powered ultrasound
Hybrid/parallel drying: vacuum-microwave drying, microwave assisted fluidized bed drying, instant controlled pressure drop, ultrasound-assisted hot-air drying
The type of drying technique will depend on resource availability, cost, quantity, ingredient formulation and desired transformation to finished product. Although most of these processes have been upscaled for industrial purposes, due to high capital costs and a lack of energy efficiency understanding, as of 2015 more than 85% (Zarein, Samadi, and Ghobadian Citation2015) of industrial dryers still remained convective hot air drying (HAD).
The remainder of this section will focus on the impact that different drying methods have on carotenoids, polyphenols and OSCs primarily in vegetables, tubers, and roots as well as fruits, herbs and legumes which constitute the key ingredients to baked snacks. It will assess the benefits of using alternative drying technologies that promote higher nutritional products and whether it would be worthwhile for companies to invest principally for their nutrient retention capabilities.
Pretreatment
Blanching of fruit and vegetables is commonly used in the food industry to: inactivate intrinsic degradative enzymes such as lipoxygenase, polyphenoloxidase (PPO), peroxidase, polygalacturonase, and chlorophyllase, modify texture, avoid undesirable green/earthy flavor notes, and to preserve color and nutrients during storage and food processing. Traditionally, blanching is conducted by a water bath followed with cold water or air to prevent further cooking and it remains the most common adopted practice due to easy implementation. During water blanching, the high temperatures necessary to denature enzymes will ultimately result in nutrient loss either through degradation or leaching into the surrounding water as the matrix softens. The softening of the matrix may result in a higher recording of micronutrients due to their enhanced extractability. It is worth noting that this increased extractability achieved will also likely correspond to an enhanced bioaccessibility of desired compounds but may also increase their susceptibility to degradation in further processing stages. A higher temperature, shorter time blanch would deactivate necessary enzymes whilst preserving the greatest sensory, quality and antioxidant properties (Abu-Ghannam and Jaiswal Citation2015). However, the precise conditions of blanching, such as the thickness of the product for conduction limited blanching processes, needs to be tailored to each food source as fruit and vegetables differ in their PPO thermal stability.
Alternative thermal blanching techniques such as infrared (IR), radiofrequency (RF), microwave blanching (MWB), high-humidity hot air impingement blanching (HHAIB) and Ohmic blanching have been investigated as a means of preserving nutritional attributes whilst ensuring the necessary denaturing of degradative enzymes. The use of electromagnetic radiation enables rapid inactivation of degrative enzymes whilst mitigating leaching of micronutrients. IR blanching of mango at 90 °C for 2 minutes has been shown to improve color retention and retain 8% more vitamin C for the same time and temperature as water blanching (Guiamba, Svanberg, and Ahrné Citation2015). Similar results were seen in IR blanching of carrot slices, where IR achieved a 19% and 13% higher retention of vitamin C than water and steam blanching, respectively (Vishwanathan, Giwari, and Hebbar Citation2013). Furthermore, IR blanching followed by IR assisted HAD reduced the processing time by 45%, resulting in an end product with a 39% higher vitamin C retention than water blanched–HAD. IR radiation penetrates the food item, inducing molecular vibration rapidly heating the internal and surface layers concurrently. Compared to conventional methods, IR is often regarded as a highly efficient, uniform, safe and energy saving option (Lao et al. Citation2019). However, as it is a surface only technique it is largely restricted to thin food slices.
A study comparing the effect of MWB, IR and HHAIB applied at three different intensities on red bell pepper found that all treatments, regardless of the intensity, resulted in a greater final antioxidant capacity, color and vitamin C retention than water blanching (Wang et al. Citation2017). These results were supported by Jeevitha, Hebbar, and Raghavarao (Citation2013) who found that the most rigorous MWB and IR treatments using higher temperatures and longer times than water or steam blanching still retained higher ascorbic acid. MWB retained 40% more β-carotene than water blanched and under two of the three intensities resulted in an increase.
Ohmic blanching has shown great promise as a rapid, efficient, and nutrient preserving method enabling uniform heating regardless of the size or shape of the sample (Mizrahi Citation1996). Heat is produced by the electrical resistance of the food when it is placed between two electrodes and alternating current of frequencies of 50 or 60 Hz is passed through it. Artichokes blanched by Ohmic heating required less blanching time to inactivate PPO and peroxidase, retained a richer color and more phenolic compounds than conventional hot water blanching (Guida et al. Citation2013). However, the materials required for the electrodes, such as platinum or platinized titanium, would be a significant expenditure if upscaled to account for a larger food masses, and therefore there are few examples of Ohmic heating being used for industrial blanching. With the potential to reduce drying time in later stages and prevent degradation by enzymes, it would be of great interest to investigate and incorporate the appropriate pretreatment when looking to produce a product of high phytochemical content.
Impact of drying on carotenoids
Carotenoids remain relatively stable in their native form in the plant matrix (Dias, Camões, and Oliveira Citation2014). Only when plant produce is chopped and cut, exposing their flesh to oxygen, processed through high temperatures and for longer times, is there a large decline in their concentration. Therefore, time, temperature, surface-area ratio, and atmosphere are all factors that need to be considered when looking for a dehydration process that would promote carotenoid retention. Though there are trends between carotenoids, differences in their structure dictate their individual stability and susceptibility to degradation. illustrates the impact that different drying techniques have on carotenoids in some selected plant produce.
Isomers
Loss of carotenoids can largely be attributed to oxidation (Suvarnakuta, Devahastin, and Mujumdar Citation2005; Kadian, Sharma, and Sood Citation2015), which can occur via thermal degradation, autoxidation (reaction of carotenoids with atmospheric oxygen), photodegradation, action of degradation enzymes, and isomerization to name a few. Isomerization, promoted by light, acid, and heat, results in carotenoids being transformed from their common naturally occurring stable trans form into a cis configuration which is important when considering the bioavailability and antioxidant health properties. Shi et al. (Citation1999) investigated the isomerization of lycopene when dehydrating tomatoes and found that depending on the drying technique, the total cis isomer percentage can increase up by 10% and correlated to the total lycopene percentage loss. 0%, 6.5%, 10.1% and 16.6% of lycopene cis isomers were recorded for pretreatment osmotic dehydration (OD), osmotic vacuum drying, vacuum drying (VD) and HAD, respectively. This result is supported by Heredia et al. (Citation2010) who found that the processes which achieved the greatest losses of lycopene in tomatoes, dehydrated at 80 °C and 1–3 W/g microwave power, also had the highest proportion of cis isomers. It was suggested that the velocity and thermal dissipation as the sample’s temperature rises favors isomerization. Lycopene cis isomers are reported to be more bioavailable than their trans form (Boileau, Boileau, and Erdman Citation2002), in contrast to β-carotene which is more bioactive in the trans configuration. Therefore, it is important to consider the influence that dehydration processes have on both the final carotenoid content as well as the trans-cis isomer ratio to maintain biopotency.
Time and temperature
Heating tomato, carrot, and marigold extract allowed for the comparison of lycopene, β-carotene, and lutein thermal degradation respectively (Kadian, Sharma, and Sood Citation2015). Overall, it determined that lutein was the most heat stable, followed by lycopene and finally β-carotene. The study varied the impact of the time and the temperature to assess which parameter had the greatest impact. It was concluded that the time the carotenoids were exposed to the temperature was significantly greater than the temperature itself. The suggestion that high temperature and short time is the best compromise to retain carotenoids when dehydrating in time-pressured industrial environments is widely supported through the literature (Suvarnakuta, Devahastin, and Mujumdar Citation2005; Bechoff et al. Citation2010; Ruttarattanamongkol et al. Citation2016). Carotenoid oxidation occurs by their chelation of singlet oxygen in the air. The kinetics of this degradation mechanism is favored by a higher temperature, which explains the desire to reduce exposure to these conditions. A possible solution is vacuum microwave drying (VMD) which enables the combination of a lower drying temperature and rapid mass transfer to the vacuum with the rapid energy transfer of the microwave. VMD of pumpkin slices (Song et al. Citation2017), lycopene-rich carrot slices (Regier et al. Citation2005), and sweet potato dices (Yan et al. Citation2013) have all shown considerably greater carotenoid retention than HAD.
Alternatively, when the dehydration process is not time dependent, for example herbs and spices that can be pre-dried before being added to a formula of a snack produce destined for a mainline, lower temperatures in conjunction with longer time have been shown to potentially increase carotenoid content. This trend has especially been seen in the production of paprika which is made from the grinding of dried sweet pepper (Capsicum annum) pods providing its iconic red hue. Depending on the temperature, longer drying times have been shown to induce synthesis of red pigmented carotenoids such as capsorubin and antheraxanthin which arise from their yellow precursors, violaxanthin and zeaxanthin (Topuz et al. Citation2011). This ongoing synthesis of the red carotenoids, with a reduction of yellow fraction is likely due to the incomplete maturation of pepper pods (Mínguez-Mosquera and Hornero-Méndez Citation1994), which then underwent further ripening during drying. It is advised that red pepper fruits should be harvested at full ripeness, where the fruits develop one to two times more free xanthophylls and it enables high color capacity and stability during processing and storage (Márkus et al. Citation1999). The most destructive impact to the carotenoids during paprika production is arguably not the thermal drying process but the milling which increases the surface-area making the spice more prone to oxidation and destruction during storage (Mínguez-Mosquera and Hornero-Méndez Citation1994). Addition of synthetic antioxidants such as ethoxyquin, γ-tocopherol rich pepper seeds or rosemary extract during milling has shown to reduce degradation of carotenoids during storage as the seed oil diffuses to form a protective film (Koncsek et al. Citation2019).
Atmospheric composition
As mentioned previously, the oxidation of carotenoids contributes greatest to their loss. Several studies have therefore investigated whether dehydrating in an atmosphere devoid of oxygen could reduce potential oxidation during food processing. This atmosphere is generally created either through flushing with inert gases or through VD. FD is well-known for producing dried fruit and vegetables of high quality. The absence of oxygen in the vacuum created is likely to reduce the chance of oxidation through drying (Kamiloglu et al. Citation2016) which is also complimented by the low temperatures preventing thermal degradation. Ramesh et al. (Citation1999) investigated the impact of HAD in the presence of nitrogen compared to oxygen and noted that the total carotenoid loss in nitrogen was moderately less for carrots and paprika. Regier et al. (Citation2005) observed no significant difference between carrot samples dried at 70 °C via HAD in the presence of air or nitrogen but noticed a significant improvement in maintaining product quality when the carrot slices were stored in nitrogen-flooded containers post processing. However, both Liu et al. (Citation2014) and Hawlader, Perera, and Tian (Citation2006) demonstrated that the modified atmosphere can protect carotenoids and color to a similar extent as that achieved during FD and VD. The fact that removing oxygen does not provide a definite solution to oxidation indicates that the oxidation is not primarily just due to autoxidation from the air but from other oxidation pathways such as thermal degradation. Although it would therefore be of interest to remove oxygen during processing, it could result in a semi-continuous process and a likely increase in running cost which may not always be applicable to baked snack production.
Ultrasound—a potential drying method rivaling freeze-drying
For many years ultrasound (US) assisted drying has been investigated as a technique for the drying of foods, with results that have been very encouraging. High intensity sonification causes a cavitation phenomenon, bubbles in the liquid which can explosively collapse causing localized fluctuations in pressure and a temperature increase, up to 5000 K at the cavitation event (Cheng et al. Citation2015), and ultimately increasing the mass transfer of osmotic treatment. Carrot slices dried under a combination of ultrasound and vacuum achieved 14.1% higher retention of β-carotene than vacuum alone, which was attributed to the substantially shorter required drying time (Chen, Guo, and Wu Citation2016). A similar result was found in drying cherry tomatoes where the drying time was reduced by 24%–65% (Fernandes et al. Citation2016) with the application of US assisted air-drying whilst retaining a significantly higher content of both total carotenoids and lycopene. Although US assisted processing methods require an additional energy source, the reduction in drying time can result in up to 30% lower total energy consumption (Chen, Guo, and Wu Citation2016), reduce storage and personnel costs (Tekin et al. Citation2017). Therefore, US provides a more energy efficient solution to VD and HAD whilst also retaining nutrients and provides a cheaper alternative to FD whilst retaining the high quality.
Food matrix
As seen in , carotenoid degradation occurs in different plant products at different rates. The matrix and environment where the carotenoids reside, as shown by reduced losses that occur in the milling of paprika with seeds, impact their susceptibility to oxidation, leaching and isomerization. Tocopherols and carotenoids have been shown to have a synergistic relationship (Haila, Lievonen, and Heinonen Citation1996). Carotenoids regenerate tocopherols and tocopherols regenerate carotenoids—with the latter reaction being favored (Mortensen et al. Citation1998). The addition of α-tocopherol to oleoresin has shown to greatly reduce the rate of lycopene degradation, doubling its half-life (Hackett et al. Citation2006). Therefore, it can be postulated that plant produce that have a higher quantity of the tocopherol natural antioxidant will be more resistant to carotenoid degradation (Panalaks and Murray Citation1970) and also harbor a higher antioxidant activity (Zhang et al. Citation2014).
With many factors influencing phytochemical degradation before processing such as harvest time, cultivar, maturation stage, and storage conditions, it is difficult to directly compare and contrast studies in the literature. Although there appears to be extensive data for different processing techniques, the conditions vary between each study published. Furthermore, a lot of the literature detailing the impact that food processing has on carotenoids only measure the total carotenoids. These gross estimates, if not accompanied with a measurement of their antioxidant activity, do not give an accurate indication whether the process has in fact reduced the potential health benefits of the carotenoids. It is also important to note that it is highly unlikely carotenoids will have been formed during processing. When the results indicate an increase, it is likely due to an increased extractability or an increase of one carotenoid at the expense of another.
Impact of drying on polyphenols
Heat treatment of polyphenols is widely reported as resulting in irreversible damage potentially through alterations in chemical structure or their binding to other compounds such as proteins (Martín-Cabrejas et al. Citation2009). Loss of phenolic compounds during dehydration, like carotenoids, can often be attributed to oxidative reactions. During drying processing, oxidation may occur through enzymatic or non-enzymatic reactions. The extent of non-enzymatic polyphenol degradation can depend on numerous parameters including the polyphenol structure, pH, temperature, oxygen, light, the matrix and presence of other compounds, type of plant produce and cultivar, as well as the processing conditions.
Enzymatic degradation
A phenolic compounds affinity for enzymatic degradation is one of the main factors governing their retention during processing and storage. In a study investigating the effect of sun-drying on the phenolic content of Portuguese pear (Pyrus communis L. Var. S. Bartolomeu) (Ferreira et al. Citation2002), different degrees of losses were observed in all phenolic compounds, except arbutin. Compared to the 100% retention of arbutin, only 4% and 9% (dry weight basis) of hydroxycinnamic acids and monomeric catechins were retained, respectively. Due to the low temperatures achieved using sun drying, this disparity was attributed to the affinity of these compounds to the degradative oxidoreductase enzyme PPO. PPO has a much higher affinity for caffeoylquinic acid (the major hydroxycinnamic compound present in the pear) and catechins than arbutin. This enzymatic reaction with both catechins and caffeoylquinic acid is thought to be largely responsible for the browning in the pears. A study looking at the browning kinetics in apricots determined chlorogenic acid (5-caffeoylquinic acid), a hydroxycinnamic derivative, as the best substrate for PPO and found that it along with flavan-3-ols ((+)-catechins and (-)-epicatechins) were the most rapidly degraded (Radi et al. Citation1997).
Flavonols such as quercetin and kaempferol are deemed poor substrates for PPO, however, they are often degraded and participate in browning by coupled oxidation reactions. Anthocyanins are also not direct substrates for PPO, but o-quinones formed during oxidation of phenolic compounds prompt their degradation. Therefore, it is integral that PPO, along with other degradative enzymes such as monophenol monooxygenase and peroxidases, are denatured before or during food processing. As such reactions can be detrimental to food quality and appearance, methods of preventing these reactions have been widely researched. Use of modified atmospheric packaging, chemical or enzymatic treatment, such as the addition of citric acid as a chelating agent or protease to hydrolyze degrative enzymes, are all methods that are currently used to prevent or reduce its occurrence (Artés, Castañer, and Gil Citation1998).
Time and temperature relationship
Anthocyanins are renowned for the highly pigmented red, blue, or purple hue they give plants such as blueberries, raspberries, black rice, and eggplants. Unsurprisingly, understanding their chemical stability through food processing has been the focus of many recent studies to prevent losses and ensure consumer sensory acceptance. As with carotenoids, the temperature and longevity of the drying process strongly influences anthocyanin stability. One study looking at the anthocyanin content of orange and purple sweet potato flours found the process of drum drying, between 80 °C and 110 °C, dramatically increased anthocyanin content (178% to 280%)—higher than that achieved for HAD between 50 °C and 80 °C (Ruttarattanamongkol et al. Citation2016). Conditions in both drum drying and HAD indicated that higher temperatures, 70 °C HAD and 95 °C drum drying, with a shorter drying time resulted in the largest anthocyanin increase. The higher anthocyanin content recorded for drum drying was attributed to steaming prior to drum drying, which was thought to rupture cell tissues, releasing antioxidant components, enabling better extractability, and also denature PPO, peroxidase, and glycosidase enzymes. In contrast, drying of strawberries by VD, VMD, and HAD saw a 75%, 22%–28%, and 73% reduction in total anthocyanins, respectively (Wojdyło, Figiel, and Oszmiański Citation2009). Proanthocyanidins, major antioxidants in fresh strawberries, can depolymerization to their elementary units during processing and hence explained an increase in catechins recorded for both FD and VMD at 480 W. Overall, as with the sweet potato flours, the results suggested a direct relationship between temperature and length of treatment with the anthocyanin loss. This relationship has also been established in sour cherries (Wojdyło et al. Citation2014), Saskatoon berries (Kwok et al. Citation2006), and blueberries (Mejia-Meza et al. Citation2008) amongst others.
The molecular structure of a polyphenol largely determines its thermal stability (Chaaban et al. Citation2017). For instance, anthocyanin stability is largely influenced by the degree of methoxylation and acylation at the hydroxyl groups on anthocyanin A and B rings. In particular, an increased anthocyanin B-ring hydroxylation results in a decrease in stability whereas acylation increases stability (Giusti and Wrolstad Citation2003). Chaaban et al. (Citation2017) investigated the structural relationship to thermal stability and antioxidant capacity of the six flavonoids: rutin (quercetin-3-glucoside), naringin, eriodictyol, mesquitol, luteolin and luteolin 7-O glycoside. The study determined the glycosylated flavonoids, rutin, naringin and luteolin 7-O glycoside, were more resistant to heat treatment than the aglycon flavonoids, eriodictyol, mesquitol and luteolin. A greater amount of energy is required to break the osidic bond between the glycoside and the flavonoid. It can be assumed that thermal stability is related to the structural solidity of the compound as the energy taken break a double bond would be greater than that of a single bond (Chaaban et al. Citation2017). Differences in degradation kinetics of the same compounds across studies can often be the result of the pH conditions, solid or liquid mediums and the presence of minerals and other food components. However, regardless of the polyphenol structure, significant degradation of nearly all compounds can begin to be seen in temperatures exceeding 100 °C (Ioannou et al. Citation2012).
Food matrix
The pH of the food matrix is key to the retention of different polyphenols. Anthocyanin color is determined by the medium’s pH due to their ionic nature. Acidic pH increases their stability and with increasing pH they degrade to their constituent phenolic acids. When comparing the thermal degradation of anthocyanins in blackberries, blood oranges, and roselles, the reduced heat sensitivity of blackberry anthocyanins was partially explained by its lower acidity (Cisse et al. Citation2009). In blue-grained wheat, anthocyanins were found to be most thermally stable at pH 1, decreasing in stability from pH 3 to pH 5 (Abdel-Aal and Hucl Citation2003). The stability of anthocyanins in black carrots began to significantly decrease at pHs above 5 (Kırca, Özkan, and Cemeroğlu Citation2007). Their stability at higher pHs than the blue-grained wheat could be attributed to a substantially high proportion of acylated groups, over 80%, which are reported to be more stable than non-acylated groups. Kalt, McDonald, and Donner (Citation2000) reported that the antioxidant capacity of processed blueberries also increased with decreasing pH, which was correlated to the anthocyanin and phenolic content. Their capacity was greatest at pH 1 compared to pH 4 and pH 7. Flavonoids in chamomile methanolic extracts were shown to be stable between pH 3 and 7 with degradation occurring above pH 7 (Srivastava and Gupta Citation2009). Therefore, it would be of interest to maintain a lower pH in a formulation as more basic conditions generally destabilize polyphenols (Sharma and Zhou Citation2011).
Common compounds such as minerals, sugar, ascorbic acid, and other phytochemicals present in a food matrix can have antagonistic, synergistic, or protective effects on polyphenol stability. Chlorogenic acid has been shown to protect rutin degradation during heating processes due to its ester form and higher susceptibility to decomposition (Murakami et al. Citation2004). Rutin was retained when heated to 100 °C with chlorogenic acid but considerable losses were observed in its absence. However, chlorogenic acid has also been shown to exacerbate the degradation of catechins by coupled oxidation mechanisms in the presence of degradative enzymes. The antioxidant capacity of quercetin or catechins combined with α-tocopherol is greater than the total of its individual parts showing that these compounds work synergistically together (Murakami et al. Citation2003). A similar synergistic effect has been found between caffeic acid, gallic acid or catechin and resveratrol (Skroza et al. Citation2015); hesperidin and either chlorogenic acid, myricein or naringenin (Freeman, Eggett, and Parker Citation2010); and various combinations of gallic acid, rosmarinic acid, caffeic acid, chlorogenic acid and quercetin (Hajimehdipoor, Shahrestani, and Shekarchi Citation2014).
Wang et al. (Citation2011) investigated the synergistic, additive, and antagonistic effects between 11 fruit, vegetables, and legumes in 55 different combinations using total phenolic content (TPC), ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity, and oxygen radical absorbance capacity (ORAC) assays. In the same food category, 13% of the combinations showed synergistic behavior whereas combinations across food categories 21% were synergistic. In particular it was noted that raspberry and adzuki beans was the only combination that delivered synergistic behavior across the four assays tested. Overall, most of the interactions were additive and a similar proportion were antagonistic and synergistic. This study however only assessed the total antioxidant capacity for each combination and not the individual interactions between phytochemicals. A good summary of the current research of synergistic and antagonistic behaviors of antioxidants can be found in a recent review by Olszowy-Tomczyk (Citation2020). Amongst other factors, it highlights the importance of the ratio of the components in gaining the maximum synergistic action toward antioxidant capacity. Awareness of synergistic, antagonistic, and protective effects that influence antioxidant activity could be beneficial when formulating functional foods—strategically selecting ingredients that will complement each other with both flavor profile and antioxidant capacity. However, substantially more research needs to be conducted to understand the mechanisms behind these reactions as, due to their complexity, frequently the result found is left unexplained.
As with carotenoids, the best conditions to ensure polyphenol retention involve low temperatures, but to also be feasible for snack production, high temperature for short times are preferred. Ingredients should be selected for their synergistic phytochemical contents, combined with compounds contributing toward an overall acidic pH and the matrix should contain minimal protein to design a snack promoting polyphenol bioavailability.
Impact of drying on organosulphur compounds
Compared to carotenoids and polyphenols, research into the influence of drying methods on the retention of OSCs is very limited. Most studies focus on the impact of various cooking techniques such as blanching, boiling, steaming, and frying rather than dehydration processes. Unlike carotenoids and anthocyanins, OSCs do not contribute to the color profile of the snack product, which is a large focus for captivating consumer appeal. As there are still no authorized health claims that can be made, there has been considerably less interest in how industrial drying methods impact their retention.
The structure of the glucosinolate molecule and the formulation of the surrounding matrix are two main factors responsible for the degree of their thermal degradation during food processing. Indole glucosinolates e.g., 4-methoxyglucobrassicin, 4-hydroxy-glucobrassicin and glucobrassicin are much more sensitive to degradation than aliphatic e.g., glucoraphanin, glucoiberin, glucoalyssin and glucoerucin. After exposure to 130 °C for 80 minutes indole and aliphatic glucosinolates from broccoli sprouts had thermally degraded from 54.77 to 4.38 μmol/g (92% loss) and 6.7 to 1.9 μmol/g (72% loss) respectively (Hanschen, Rohn, et al. Citation2012). Work by Oerlemans et al. (Citation2006) supported this relationship but found that as temperatures exceed 120 °C, the significance of the structure decreases and both groups follow similar degradation rates. In aliphatic glucosinolates, the sulfur oxidative state and side chain length influences their stability. An oxidative state sulfur-(IV) has greater stability than sulfur-(II) and a greater length of the methylsulfanyl side chain decreases stability. Hydroxyl functions on the side chains of indole glucosinolates generally destabilizes glucosinolates structure (Hanschen, Rohn, et al. Citation2012).
Food matrix
As with polyphenols and carotenoids, the surrounding environment (pH, water content, iron concentration, presence of other phytochemicals and macromolecules such as proteins and fiber) of the OSC plays an important role in its stability. Thermal degradation of glucosinolates can vary twenty-fold depending on the food matrix. A study looking into the thermal degradation of glucosinolates in five Brassica vegetables determined gluconapin in broccoli to be twenty-fold more stable than Brussels sprouts and glucobrassicin in red cabbage to be six-fold more stable (Dekker, Hennig, and Verkerk Citation2009). It was speculated that differences in pH and cellular composition could play an important role in determining degradation characteristics. The effect of pH on glucosinolate stability has been assessed in Chinese red radish (Jing et al. Citation2012) and broccoli sprouts (Hanschen, Rohn, et al. Citation2012). For Chinese red radish stored at room temperature (23–25 °C), 88%–97% total glucosinolates were retained between pH 3.6–9.1 but high degradation occurred below pH 3.6. In broccoli sprouts that were cooked for 80 minutes in solutions of pH 4.8, 7, and 8.2, pH 4.8 was the most favorable for all glucosinolates except glucoiberverin and glucoerucin. For both glucoiberverin and glucoerucin as with temperature, the oxidative state of the sulfur atom plays an important role in determining aliphatic glucosinolate stability.
A metabolomics approach has been used to identify metabolites in a food matrix that can influence the thermal degradation rates of glucoraphanin and glucobrassicin (Hennig et al. Citation2014). Seventeen metabolites were identified as significant contributors to glucobrassicin thermal stability. Two of which were derivatives of quercetin and were positively correlated to increased glucobrassicin degradation. The eight metabolites left undetermined were also positively correlated and it was tentatively suggested it could be based on their Fe-reducing power. Fe(II) catalyzes a non-enzymatic degradation pathway for glucosinolates promoting the formation of nitriles and therefore its presence in the food matrix can increase glucosinolate susceptibility to thermal degradation (Hanschen, Bauer, et al. Citation2012). 4-methyloxyglucobrassicin was found to reduce the degradation rate of glucobrassicin along with six other compounds. 4-methyloxyglucobrassicin is potentially involved in a competitive degradation pathway with glucobrassicin and as it is degraded faster it reduces glucobrassicin degradation. Other studies have also shown 4-methyloxyglucobrassicin to be the most thermolabile compound. The same compound along with gluconasturtiin and two other metabolites were negatively correlated to glucoraphanin degradation.
With the knowledge that the composition of a plant matrix can greatly affect glucosinolate thermal stability, Giambanelli et al. (Citation2015) investigated the effect of glucosinolate thermal degradation in broccoli combined with other ingredients including potato, onion, corn starch, and lentil protein. This was conducted using binary systems. Apart from onion, when compared to the control, a 1:1 ratio of broccoli/added ingredient did not have any benefit to glucosinolate retention in broccoli heated to 90 °C or 100 °C. However, at a 1:9 ratio of broccoli/added ingredient, the strongest effect was seen. Onion and lentil protein showed the greatest protective effect compared to potato and corn starch. Initially it was postulated flavonoids present in the onion could be a contributing factor to the protective effect witnessed. However, this theory was discarded when varieties of onions with different flavonoid content were compared and the increased flavonoid abundance did not have a significant difference in the glucosinolate degradation trend.
Time and temperature
As with other phytochemicals discussed, OSCs are temperature sensitive. Sulforaphane, the health beneficial hydrolysis product of glucoraphanin, can be degraded at temperatures as low as 53 °C (Lekcharoenkul et al. Citation2014). Dehydration of broccoli through HAD, microwave drying (MWD), and US-assisted-HAD found that sulforaphane content was higher in the fresh samples than after drying (Cao et al. Citation2019). This is likely because temperatures would have exceeded 60 °C, deactivating myrosinase and preventing sulforaphane formation. Lekcharoenkul et al. (Citation2014) found that the best method for sulforaphane formation and retention in white cabbage leaves is using low-pressure superheated steam drying (LPSSD) followed by VD or a two stage HAD treatment (60 °C for 10 min, 45 °C for 65 min). To increase sulforaphane production, drying temperatures should exceed 40 °C to deactivate epithiospecifier protein (ESP) which catalyzes a reaction leading to the formation of the non-bioactive compound sulforaphane nitrile but should not exceed 60 °C to retain myrosinase activity.
Mrkic et al. (Citation2010) investigated the drying temperature and air velocity relationship of indole glucosinolate loss in broccoli. Broccoli samples were blanched first so that enzymatic degradation could be excluded from the results. As expected, different drying conditions impacted individual glucosinolate compounds differently. 4-hydroxyglucobrassicin was the least thermally stable followed by 4-methoxyglucobrassicin, neoglucobrassicin and glucobrassicin, supporting the findings by Oerlemans et al. (Citation2006) and Hanschen, Rohn, et al. (Citation2012). The best conditions to preserve individual and total indole glucosinolates were concluded to be drying at 50 °C and 60 °C with 2.25 m s−1 air velocity, whereas drying at 100 °C was detrimental.
When comparing the effect of HAD, MWD and US-assisted-HAD on broccoli floret glucosinolates, US-assisted-HAD was regarded as more favorable than MWD (MWD retaining 47% and US-assisted-HAD 57%) (Cao et al. Citation2019). Levels of glucobrassicin and gluconapin were not greatly affected by any drying treatment. Specifically, during MWD levels of gluconapin increased by up to 132% and in HAD glucobrassicin increased by 20%. It was suggested that this could arise by synthesis of the compounds from amino acid metabolism. A similar result was found with US-assisted-HAD treatment on cabbage where an increase of glucobrassicin and sinigrin was observed (Tao et al. Citation2019). However, the mechanism or inducement of this synthesis is not understood and therefore more research is needed to understand the impact of drying on amino acid metabolism.
Dried garlic is a common substitute for fresh garlic in commercial foods but also in medicinal and functional food and therefore there is an interest in preserving its health beneficial properties during processing. Studies have measured the impact of drying of garlic through FD, HAD, VD and in nitrogen atmosphere on the content of allicin, the key OSC in garlic. FD for 72 hours with a heating plate of 20 °C and HAD at 40 °C for 6.5 hours, resulted in an increase of allicin of 16% and 3% respectively (Ratti et al. Citation2007). However, at heating plate and HAD temperatures greater than this allicin was degraded. Allicin, like sulforaphane, is formed from a precursor interacting with an enzyme which is stored in a different part of the plant. Therefore, it can be expected that at higher temperatures where alliinase is denatured, it would result in a reduction of allicin. The atmosphere in which drying occurs seems to have little effect on the rate of allicin degradation—with no significant differences occurring when dried in a vacuum or inert gas (Rahman et al. Citation2009). This suggests than unlike carotenoids and polyphenols, oxidation is not a main factor in OSC degradation.
Summary of tradeoffs between industrial drying techniques
When looking for an appropriate drying technique for the industrial production of baked snack products there are always tradeoffs between time, cost, and quality—these have been briefly summarized in . Using fresh fruit and vegetables as core ingredients may look appealing to the consumer on the outset but can incur high costs for business. Starting with 80%–90% moisture content will either require the addition of dry ingredients, such as potato flakes, to decrease the initial moisture content or a series of drying technologies that can reduce the water activity to a level suitable for shelf life. The addition of dry ingredients is often accompanied by emulsifiers to stabilize the product, which will detract from the clean label declaration that consumers who are interested in the inclusion of fresh fruit and vegetables will likely be looking for. It is also important that at some stage in the process, temperatures are reached that enable Maillard reactions to occur which are integral to flavor development. As seen from the literature, ideally formulas would be comprised of a mixture of FD inclusions which would enable a lower starting moisture content whilst retaining the color and nutritional profile of the fresh equivalent. However, commercial FD is inefficient and incurs high costs which would make a product with a high proportion of FD inclusions difficult to place in the competitive market of snacks. FD should be reserved for ingredients such as flavoring agents, herbs, and spices for which their flavor is integral and therefore low temperatures are required to prevent volatile losses.
Table 5. Comparison between different dehydration technologies applied to snack form.
It appears that the best drying conditions to ensure phytochemical retention would reach no higher than 100 °C and be a quick process devoid of oxygen. It is not, however, easy to simulate these conditions when running a large-scale continuous production. Most studies that determine these parameters are conducted using lab equipment and cannot always accurately replicate commercial scale line production (Musielak, Mierzwa, and Kroehnke Citation2016). Furthermore, without exceeding 100 °C the product will have limited flavor development in the absence of Maillard reactions. Hybrid drying methods which combine HAD in tandem with technologies such as VMD, MWD or IR, that have been shown to promote retention of phytochemicals, are therefore attractive for the formulation of phytochemically dense products. VMD alone requires high capital expenditure and electrical energy consumption which can deter from its nutritive benefits. Pretreatment with HAD, especially with the utilization of impingement oven drying enabling rapid heat and mass transfer rates (Deng et al. Citation2020), would drastically reduce the initial moisture level and the mass load for the vacuum microwave and thus could lower the cost. Commercially viable HAD for baked snacks typically starts with doughs of 40%–50% moisture content, however, the addition of MWD enables a higher moisture starting dough as the drying is volumetric and water is driven off very efficiently in the GHz region (0.9 and 2.45 GHz used commercially). Therefore, MWD followed by HAD and/or impingement drying enables the opportunity to start with moisture contents greater than 80% whilst maintaining and commercially viable continuous system. Faster drying rates of impingement drying reduces the time the product is exposed to oxygen at high temperatures and thus the propensity of oxidation. This is also important with respect to color deterioration as oxidation of phenolic compounds is a key contributor to browning and color loss experienced during processing.
Though the primary aim is to create a product with complex flavor, color and texture attributes, the time, energy expenditure and carbon footprint are all integral when designing a commercially viable product. Due to the worldwide crisis of global heating, there is an increasing pressure to reduce energy consumption. Two of the 17 Sustainable Development Goals, laid out by the United Nations, address energy usage: one looking at responsible consumption and production patterns, and the other ensuring affordable, reliable, sustainable, and modern energy. Total energy consumption decreases with increased temperature due to a reduction in drying time, which compliments the desire for a high temperature short time drying to retain phytochemicals. Physical field-based drying such as US, RF, IR and MWD have been shown to decrease the drying time in HAD systems by 24%–65% (Fernandes et al. Citation2016), 67%, 50% and 61% (Roknul et al. Citation2014), respectively. Therefore, more research into upscaling and optimizing these technologies that increase the rate of heat transfer and can be used as pretreatment or in parallel to current baking processes, such as HAD, is essential. Currently, though limited to a few examples, IR and RF have been developed into continuous processing but to date there is no large-scale commercial application of US in baked snacks. These technologies often come with a large capital cost even if running costs are more efficient, often require sophisticated skills and have to address consumer concerns toward the food safety of using physical field-based drying. These draw backs may deter smaller companies or production lines in investing in the technology. Further understanding is needed to bridge the gap between laboratory research and commercial production.
How the food matrix can be designed to increase nutrient bioaccessibility and bioavailability of different phytochemicals
Cultivar of plant
As it has been shown, phytochemicals are expected to be lost through processing regardless of how thermally or mechanically unaggressive the technique is. Any observed increase in phytochemical content through processing is likely to be a result of increased extractability or the formation of one phytochemical at the expense of another. A potential alternative to explore is therefore selective breeding, crop engineering or choosing a crop that either presents a higher phytochemical starting content or an increased resilience to mechanical or thermal processing. This could provide a potential opportunity to design an antioxidant rich baked snack representative of the nutritional contents of the corresponding standard commercial fresh vegetables.
Despite challenging conditions that plants may endure, they remain healthy through intrinsic defense strategies which promote adaptation to environmental stressors. These may be biochemical, such as toxins poisonous to predators, or physical, such as cell wall and cuticle development to prevent pathogen penetration. Over the past two decades there has been a growing interest in developing antioxidant dense popular fruit and vegetables. Production of phytochemical compounds, plant secondary metabolites, can be exploited by stressing the plant either through agricultural practices, such as deficit irrigation, or via elicitors, compounds that mimic wounding, pathogen attack and UV light exposure. For example, methyl jasmonate has shown to increase indolyl glucosinolates in rapeseed (Doughty et al. Citation1995), benzothiadiazole increased phenolics in brown mustard plant (Guleria and Kumar Citation2006) and chitosan, salicylic acid, methyl jasmonate and methyl salicylate each independently increased total phenolics and lignin deposition in the cell walls of eggplants (Mandal Citation2010). However, it is important to take a holistic approach when selecting for vegetables, ensuring consideration of nutritional content, post-harvest storage and processing, and consumer sensory preference.
A prime example of a cultivar developed for its nutritional, processing, and sensory attributes is Beneforté broccoli, which exhibits a 2.5–3 times higher glucoraphanin/sulforaphane content than its conventional counterparts (Traka et al. Citation2013). Beneforté was developed from crossbreeding commercial broccoli with a southern Italian glucoraphanin rich wild broccoli. It was one of the first consumer-focused, nutritionally enhanced vegetables to reach commercial market, arriving in the UK and US in 2011. Since Beneforté, other crops have been developed such as polyphenol-rich Rutgers Scarlet Lettuce along with four varieties of “Super Lettuces” and “Sun Black” (Blando et al. Citation2019) purple tomatoes high in anthocyanins, a result of a breeding programme incorporating genes from wild tomato species that naturally biosynthesize anthocyanins in the sub-epidermal tissue. It is important to note that these vegetables are all products of conventional breeding programs, not a result of genetic modification which is often a strong deterrent to consumers and not permitted in many countries. This process of growing plants in specific conditions to accumulate higher quantities of micronutrients/phytochemicals is often referred to as biofortification.
Additionally, to increasing inherent phytochemical content, biofortification can also be used to address localization of phytochemical accumulation and their retention during processing. This is an important factor to be considered when crops are being biofortified as a strategy to combat micronutrient deficiencies in vulnerable populations to ensure that the nutrients in the enriched plants are still obtained after traditional food preparation. Cassava is a major staple food, contributing greatly to the daily nutrition of millions of people in developing worlds and like other tuber roots, requires cooking before consumption. The influence of genotype and cooking style on β-carotene retention and bioaccessibility were compared in five varieties of biofortified cassava grown in Brazil (Berni et al. Citation2014). Cassava varieties that contained a lower moisture content enabled shorter cooking times to achieve similar dehydration and also facilitated a greater absorption of oil during frying, improving solubilization and micellization of β-carotene during digestion. As the propensity of phytochemical oxidation and degradation increases with cooking time, breeders should select for cassava with a lower moisture in addition to a high carotenoid content.
Dehulling is a fundamental stage in grain processing as the hull is considered inedible and unpalatable and can commonly lead to discomfort in digestion due to the high fiber content. Consequently, one drive of biofortification is to target genotypes that promote the accumulation of nutrients in the endosperm rather than the hull/husk which will be removed. Association and expression analysis, linkage mapping, and mutagenesis discovered that the enzyme lycopene epsilon cyclase is preferentially expressed in the endosperm and variation at its locus has the largest effect on dividing the two carotenoid pathways—subsequently determining the ratio of β-carotene and β-cryptoxanthin (Harjes et al. Citation2008). Previously the richer yellow grain color has been used as a phenotypic marker for greater carotenoid content, however analytical comparisons revealed poor correlations between β-carotene content and degree of yellow. This suggests that molecular marker-assisted selection should be considered to efficiently identify crops with a genetically higher occurring β-carotene. Conventional breeding programs have shown promising potential, but the complexity required to produce the same results as genetic engineering could take years. Therefore, directing the localization of nutrients to the endosperm through genetic engineering has also been investigated. Through targeting the expression of the transgenes nicotianamine synthase and ferritin, iron content in transgenic rice endosperm was increased up to 6.3-fold. Furthermore increasing the expression of ferritin and phytase in maize has been shown to increase bioavailability of iron with the level of phytase expression correlated with increased cellular iron uptake (Wirth et al. Citation2009). Naqvi et al. (Citation2009) manipulated three separate metabolic pathways simultaneously through multigene engineering by direct DNA transfer in corn. The result was a multivitamin corn with significant increases in β-carotene, ascorbate, and folate content in the endosperm. Biofortification to this degree can only be achieved through direct DNA transfer, however, there remains several ethical hurdles for genetic engineering to overcome before this concept is widely accepted.
When selecting crop varieties to be bred conventionally, genotypes should be screened not only for their relatively high natural phytochemical content but also their natural distribution of macro/micronutrients in their matrix, water content, presence of antinutrients and marker-assisted selection.
Use of by-products
The Waste and Resources Action Programme (WRAP), the UK government’s food waste advisory board, estimates 7% of global greenhouse gas emissions come from food waste (Parry, James, and LeRoux Citation2015). In the UK alone, 1.9 million tons of food waste are produced each year in industry, of which over a fifth could be utilized into other streams. WRAP is a charity that works to provide a network between governments, businesses, industries, and communities to review resource management and maximize the opportunity of redirecting waste streams or by-products—thus providing a platform to build a circular economy. This is complimentary with the United Nations Sustainable Development Goal 12.3, which aims to halve per capita global food waste at supply chain, retail, and consumer levels by 2030. There is an incentive for food manufacturers to exploit by-products derived from primary food production processes as they are often a cheaper source of food material high in phytochemicals, fiber, and peptides, which can be incorporated as functional ingredients into snack formulas.
Peels, skins, seeds, stones, leaves, stalks, and stems are components of fruit and vegetables that are frequently removed and discarded during processing. However, these elements of fruit and vegetables are often found to harbor a higher content of phytochemicals compared to the flesh (Can-Cauich et al. Citation2017; Albishi et al. Citation2013). The inclusion of by-products often alters physical characteristics of the snack matrix, largely due to the significant increase in fiber. An increase in total fiber content has shown to be directly correlated to an increase in hardness (Lafarga et al. Citation2019; Millar et al. Citation2017). Presence of large fiber fragments can result in a more compact snack cell structure as the fiber can prematurely rupture air bubbles reducing the expansion during processing. Skins and peels are often high in pectin, a fiber with a very high water holding capacity due to the high count of OH groups in the polysaccharide structure. Therefore, fiber dense snacks are likely to exhibit higher water absorption due to hydrogen bonding between water and the hydroxyl groups of the fiber polysaccharide. Generally crispiness, an important parameter determining consumer acceptability in cracker and snack products (Roudaut et al. Citation2002), is reduced with increased water content. This texture may be unfamiliar with consumers when compared to their expectation of commercially available crackers and therefore it is crucial to quantify the range of inclusion that can be made without compromising on sensory attributes.
Although many studies have shown an increase in phytochemical content and antioxidant activity after inclusion of fruit or vegetable by-products, few have investigated their bioavailability, in vitro or in vivo. Findings from research that has assessed their bioavailability vary considerably paper to paper, suggesting that the food matrix and processing are likely determining factors (de Andrade and de Andrade Gonçalves Citation2021). Evaluation of phytochemical bioavailability in the snack is vital to understand the true benefit of the by-product inclusion. Lafarga et al. (Citation2019) explored the nutritional and bioaccessibility characteristics of baked crackers containing broccoli by-product. Milled, freeze-dried broccoli stems substituted for flour at a level of 12.5% and 15% (wt/wt), increased total phenolic content and DPPH radical scavenging ability more than four-fold. However, in a gastrointestinal digestion simulation, the total phenolic content of the control sample increased dramatically—suggesting a potential facilitation of the liberation of phenolic compounds due to disintegration of cell walls by pH and pepsin. Overall, the 12.5% and 15% inclusions resulted in an increased total polyphenol content and DPPH and FRAP antioxidant capacity at all stages in the digestive tract, although the levels were not proportionally correlated to inclusion percentage. Inclusion of mango peel, seed, or paste into a granola snack bar saw an increase in phenolic content but a decrease in relative potential absorption ability (Bertha et al. Citation2019). Fiber interactions with phytochemicals forming complexes and colloidal structures can have positive or negative influences on their bioavailability. Therefore, more research into potential absorption ability of phytochemical enriched snacks is required to better quantify the favorable health benefits for the consumer.
Role of gut microbiota
The human gut microbiota plays an important role in an individual’s immunity, control of pathogens, metabolism, and synthesis of nutrients. Though there is some evidence of genetic inheritance shaping the composition of a person’s gut microbiota, environmental factors such as drugs, diet, and anthropometric measurements are much stronger influencers (Rothschild et al. Citation2018). Eating cheese or foods high in fiber, probiotics, or polyphenols, consuming a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) or vegan diet have all shown to influence microbiota composition in both human observation and interventional trials (Valdes et al. Citation2018). Only 5%–10% (Cardona et al. Citation2013) of polyphenols are estimated to be absorbed in the small intestine: the remaining polyphenols are transported to the large intestine where they may be broken down by the colonic microbial community. As most polyphenols require biotransformation by enterocyte enzymes and the microbiota prior to absorption, the composition of this community is integral in defining what proportion of ingested polyphenols, reach the bloodstream. For instance, amongst others, Bifidobacterium spp. and Lactobacillus spp. have been detected in the metabolism pathway of hydroxycinnamates and anthocyanins; Clostridium coccoides and C. orbiscindens in the transformation of a multitude of different flavonoids (Winter et al. Citation1989); and Eubacterium spp. in quercetin, naringenin (Rechner et al. Citation2004) and lignan (Clavel et al. Citation2005) metabolism. Therefore, as different species of bacteria support the metabolism of different polyphenols, it is important to sustain a rich biodiversity of microorganisms in the gut to optimize the absorption of all polyphenols.
The concept of a functional snack which combines both polyphenols and the bacteria that contribute to their metabolism is appealing, with the target of enhancing their antioxidant activity and bioavailability. Although various studies have used a blend of polyphenols and probiotics, primarily enriched juices and dairy products, their focus is often toward increasing probiotic shelf life through bioactive compounds. Very few studies assess the potential of probiotic-polyphenol formulas on enhancing the polyphenol bioavailability. Pereira-Caro et al. (Citation2015) examined the effect of acute or chronic ingestion of microencapsulated Bifidobacterium longum probiotics on orange juice flavone bioavailability. They found that after 4 weeks of daily probiotic intake, there was a significant increase in absorption of naringenin and hesperitin metabolites, and an overall increase of (poly)phenol bioavailability from 43% to 70%. Similarly mango, acerola, mangaba, and seriguela fruit purees when fermented with Lactobacillus acidophilus and Lacticaseibacillus casei probiotics, obtained higher concentrations and bioaccessibility of phenolic compounds (de Assis et al. Citation2021).
Lactobacillus spp. and Bifidobacterium spp. are the most common bacteria used for probiotics in the food industry as they have been Generally Recognized as Safe (GRAS) by regulatory authorities such as the FDA. As the bacteria must be kept alive, production of probiotics requires strict regulation of food parameters such as pH, water activity and salts as well as processing parameters such as heat treatment, storage and packaging. Therefore most probiotics are liquid or dairy based, such as yogurts, kefir, juices and kombucha, or encapsulated in pills and tablets as these provide better environments for viability and stability of probiotic bacteria. Currently there are few solid, ambient stable probiotic food products on the market as the viability of the probiotic bacteria often decreases as storage temperatures exceed 4 °C (Min et al. Citation2019). However, the bacteria Bacillus coagulans, is a very promising probiotic for retaining viability throughout food processing and stability during ambient storage. This has been demonstrated in the production of chocolate fudge frosting, hot fudge toppings, peanut butter, strawberry preserve and vegetable oil stored at room temperature for up to 12 months (Majeed et al. Citation2016). It is also used in probiotic dried snacks such as banana chips, veggie crisps, cereals and biscuits. There is limited research of the interactions between B. coagulans and polyphenols, therefore more studies need to be conducted to understand whether their combination could provide an ambient snack with enhanced polyphenol bioavailability.
Future work/considerations
Increasing understanding of how food matrix transformations during processing influences phytochemical bioavailability in a complex food matrix
Understanding phytochemical-food matrix interactions is fundamental when establishing a health claim as it is the proportion of phytochemicals that are bioavailable, rather than the quantity ingested, that has become the criterion when assessing their validity (Rein et al. Citation2013). Thermal or mechanical processes that liberate phytochemicals from their tissue matrix can greatly influence their bioavailability as the plant cell, being resistant to degradation in the upper gastrointestinal tract, poses a barrier to phytochemical release. Zhang et al. (Citation2017) studied the impact that food matrix transformation, elicited by intermittent VMD or HAD, had on in vitro carotenoid bioaccessibility in pumpkin slices. Pumpkin slices were dried to 75%, 55%, 35%, 15%, and 5% moisture and the liberation ratio (percentage of carotenoids transferred to the aqueous phase during the digestion) and micellized ratio (percentage of carotenoids transferred to the micelles during the digestion) were obtained for the carotenoids: (all-E)-lutein, (all-E)-α-carotene, (all-E)-β-carotene, (Z)-β-carotene, and lutein esters. For each carotenoid, accounting for losses during processing, a greater proportion was transferred to the digest after intermittent VMD. During HAD, the liberation ratio was greatest at a moisture content between 75% and 35%, but once it decreased to 15% or 5%, aggregates between carotenoids with sugars and other cell components formed. This was attributed to the migration of sugar with the water to the material surface forming a hard shell, hampering the spreading out of internal compounds and reducing the release of carotenoids into an aqueous supernatant. During intermittent VMD, the microwave treatments damaged chromoplast substructures, enabling the release of the carotenoids into the cytoplasm, improving their extractability and consequently their liberation during digestion.
In pumpkin, carotenoids are largely stored as protein bound tubules in the chromoplasts, similar to the structures found in butternut squash. These structures are noted to be particularly difficult to solubilize during digestion in contrast to the lipid-dissolved globular chromoplasts in yellow bell pepper (Zhang, Ho, et al. Citation2018). The influence of HAD on the bioaccessibility of carotenoids in yellow bell pepper, carrot, sweet potato, and broccoli was studied in respect to transformation of the microstructure during drying. Although it was conclusive that HAD caused degradation to all carotenoids in all vegetables and bioaccessibility was increased, the degree of degradation and bioaccessibility differed significantly across the vegetables. As in pumpkin, degradation of the cell wall structure during processing ultimately lead to a greater release of carotenoids. The greatest cell wall deformation was observed in yellow bell pepper and carrot along with the greatest bioaccessibility of carotenoids after HAD. Broccoli experienced the highest retention of cell wall polysaccharide fractions, which was likely a contributing factor to it giving the lowest bioaccessibility of carotenoids. Additional to cell wall disruption, HAD caused aggregation of the starch granules in sweet potato, clustering around α- and β-carotene, hindering their liberation during digestion. This clustering effect has also been seen in sweet potato imaged through coherent anti‐Stokes Raman scattering and simultaneous second‐harmonic generation microscopy (Brackmann et al. Citation2011). Prior to thermal treatment, β-carotene clusters are shown to surround the starch granules. Thermal treatment induces gelatinization of starch granules, which results in granular swelling and a transition to a crystalline structure (Liu et al. Citation2009). The alteration of starch morphology could result in the apparent clustering of the starch network around the carotenoids that were closely associated with them in their native form. This illustrates the importance of understanding the impact that alternative food processing has on food matrix transformation and its influence on phytochemical bioavailability—especially as the breadth of potential interactions and effectors will grow with increasing food matrix components, such as the complexes found in commercial snacks.
Compared to the multitude of studies investigating the influence of cooking and drying processing on phytochemicals in their native fruit or vegetable matrix, there is a scarcity of literature addressing their influence once in a formulated matrix. The difference between the bioaccessibility and bioavailability of glucoraphanin, β-carotene, quercetin, and apigenin in a dried snack form was compared to the equivalent (on a dry weight basis) of microwave steamed vegetables (Perez-Moral et al. 2018). It was assessed both using an in vitro Caco-2/TC7 cell model simulating intestinal uptake and transport, and a human clinical study with 19 participants completing the trial. The snacks consisted of freeze-dried onion, carrot, broccoli, and parsley in a potato flake/starch dough which was oven baked to 2.5% ± 0.5% moisture. In the human study, subjects consumed 75 g of the baked snack and 461 g of the proportionate steamed vegetables at separate times. The in vitro study predicted a significantly greater release of quercetin and apigenin from the snack than the vegetable meal but lower glucoraphanin, with limited release of carotenoids in either matrix. In contrast, the human trial received higher quercetin, apigenin, and glucoraphanin/sulforaphane metabolites in the urine following the vegetable meal, rather than the vegetable snack. Overall, the study demonstrated that a high retention of phytochemicals could be achieved in a freeze-dried vegetable baked snack with a similar bioavailability of quercetin and apigenin to a vegetable meal. Another study compared the bioavailability of isoflavones from soybeans presented in three different food sources: cookies, chocolate bars, and fruit juice (De Pascual-Teresa et al. Citation2006). In vitro digestion suggested a much lower bioavailability of isoflavones in the cookies than either chocolate bars or the fruit juice, with only 22.0% ± 14.1% being recovered, compared to 99.5% ± 0.7%, and 90.0% ± 12.7% respectively. However, these results were not mirrored in the human study, with a total genistein urinary recovery of 61%, 66%, and 70% in juice, bars, and cookies, respectively. These studies highlight the necessity for human trials as well as in vitro, as the complexity of the intestine physiological environment can be oversimplified in in vitro models, excluding interactions and matrix effects (Costa and Ahluwalia Citation2019). Furthermore, neither of these studies visualized the food matrix and therefore any interaction between the phytochemicals and matrix components such as starch, protein or sugars are hypothesized. Precise microscopy techniques such as transmission electron microscopy should be used to capture food microstructure to study the transformation of food matrices during processing and examine the complexation of components in the matrix.
Formulating a phytochemical dense snack
As previously stated, unlike vitamins and minerals, there is yet to be an established RDI for phytochemicals due to the abundance of chemically different compounds, insufficient data on the safety of the compounds and they are not regarded as an essential nutrient. However, several reports discussing the contribution of phytochemicals to protection against various degenerative or chronic diseases, have expressed the interest for a proposed dietary phytochemical recommendation (Dreosti Citation2000; McCarty Citation2004; Vogiatzoglou et al. Citation2015). Per capita daily monomeric (anthocyanidins, flavanols, flavonols, flavanones and flavones) flavonoid intake (MFI) for UK and Ireland has been estimated as 182 mg and 177 mg respectively through an assessment of the Food and Agricultural Organization international food balance sheets (Beking and Vieira Citation2011). Using data from a survey conducted on EU citizens (aged 18 to 64 years), another study estimated the mean MFI of UK and Ireland to be 195 mg/d and 249 mg/d respectively and mean total (including polymers of theaflavins) flavonoid intake (TFI) to be 655 mg/d and 851 mg/d (Vogiatzoglou et al. Citation2015). The same study assessed the monomeric and total flavonoid intake for various different countries across Europe. Ireland was estimated to have the highest flavonoid intake and Czech Republic the lowest with an MFI of 75 mg and TFI of 225 mg. The average daily MFI and TFI for Europe was estimated as 136 mg (± 14) and 428 mg (±49) respectively. The TFI of Korean adults has been estimated at 318 mg(Jun, Shin, and Joung Citation2016) and the MFI of U.S adults at 189.7 mg/d (Chun, Chung, and Song Citation2007). Predictions of phytochemical intake can vary greatly from study to study depending on where they sourced their dietary records, the database they have used for the phytochemical content of the food, and which phytochemical compounds they have selected to include in their assessment.
Although the evidence for establishing an RDI for β-carotene and specific carotenoids has been deemed insufficient by the EFSA and the US Nutrition Board, there is an RDI for vitamin A which originates from provitamin A carotenoids. The vitamin A RDI for adults aged 19–64 years is 750 μg for men and 650 μg for women according to EFSA and 900 μg for men and 700 μg for women according to the FDA. The FDA also requires double the carotenoids in the conversion rate to retinol (12 μg β-carotene or 24 μg α-carotene/β-cryptoxanthin = 1 μg retinol) compared to EFSA. Therefore, requiring between 3 and 6 mg of β-carotene which is associated with the lower risk of chronic diseases. There is, however, no recommended quantities suggested for non-provitamin A as there is limited data on the lycopene contents of commercial food products and therefore it is difficult to estimate population intake even from dietary surveys. There are also comparatively very little studies regarding average glucosinolate intake across populations. Estimates are usually taken from the quantity of cruciferous vegetables consumed in the population which varies considerably worldwide (Agudo et al. Citation2008).
Williamson and Holst (Citation2008) proposed the idea of using the calculated polyphenol intake that an individual would have if they consumed their 5-a-day as a potential target intake value. Selecting orange, red onion, apple, blueberries, and strawberries as their fruit/vegetables, they determined a total flavonoid intake of 828 mg/d. However, they used a portion size of 100 g for each food item which is 25% greater than the adult fruit and vegetable portion size of 80 g suggested by the 5-a-day guidelines(Public Health England Citation2016). Using the same theory, the 5-a-day portion guidelines and five of the most commonly consumed fresh fruit and vegetables in America of: apples, bananas, grapes, tomatoes and onion(USDA Economic Research Service Citation2013) an estimated daily target intake value, as seen in , could be estimated as: 353.03 mg of polyphenols.
Table 6. Estimation of the quantity of polyphenols in an average 5-a-day.
Using Phenol-Explorer, a comprehensive database recording polyphenol content in food, the top 100 foods with the highest polyphenol content were identified by Pérez-Jiménez et al. (Citation2010). Cloves, dried peppermint, and star anise are ranked highest with polyphenol contents of 15,188, 11,960, and 5460 mg per 100 g, respectively. Foods that are particularly dense in polyphenols or other phytochemicals often are accompanied by an astringent, bitter taste and therefore have to be used in moderation or masked by other flavors to make products appealing to consumers. Other foods high in polyphenols but with more appetizing flavor profiles include dark chocolate, lowbush blueberries, hazelnuts, plums and blackberries with polyphenol contents of 1664, 836, 495, 377, and 260 mg per 100 g, respectively.
The phytochemical values of these fruit and vegetables are taken in fresh weight. With moisture contents often exceeding 80%, there is the opportunity for baked/dehydrated snacks to be able to incorporate a greater volume of the fruit and vegetables and therefore concentrate the quantity of phytochemicals. Through using a combination of these foods and hybrid baking processes such as microwaves combined with hot air impingement drying, which have shown to reduce the extent of phytochemical degradation, could provide the opportunity to create commercially feasible snacks that could deliver the suggested daily allowance of polyphenols in one serving. An example of such a snack is illustrated in the theoretical zero-base recipe (). This recipe also looks to reduce fiber content by using refined wheat flour and fruits that have been selected for their high polyphenol content and low fiber content to increase the potential bioavailability of the polyphenol compounds.
Table 7. Example zero-base recipe for a polyphenol dense snack.
Though this recipe exceeds the daily suggested polyphenol intake, it has not accounted for degradation of phytochemicals through processing. As seen earlier in the report, even when using methods shown to be less destructive to phytochemicals, such as microwave technology, phytochemical degradation still occurs. If we were to approximate that 30% of the phytochemicals were lost through the processing then remaining would be a snack that still provides 389 mg of polyphenols, which is over a 90% of the estimated daily TFI of Europe and exceeds the estimated TFI of Korea. These calculations are theoretical and make assumptions including: the phytochemical content of the fruit/vegetables when calculating the suggested polyphenol intake and the TPC of the zero-based recipe, the extent of the degradation during processing, and that the bioavailability of the snack is the same as eating the individual fruit and vegetables in the 5-a-day. The assumptions are so great because there is not enough literature that delves into the effect of processing on phytochemicals in complex matrices and their relative bioavailability and therefore this concludes a great need for further research in this area.
Clinical studies
Claims on functional foods generally lie within two categories: nutrition claims (what the product contains) and health claims (what the product does). The landscape for phytochemical health claims is extremely restricted. Although there is an abundance of epidemiological, cell- and animal-based studies, there is only a fraction of human intervention trials that provide evidence of their beneficial effects on biomarkers that correlate to the risk of many chronic diseases. Unlike cardiovascular diseases, where there are recognized chemical and physiological biomarkers such as blood lipid profiles, pro- and anti- inflammatory cytokines and hypertension, some chronic diseases do not have such identifiable endpoints, limiting the design for human intervention studies. Therefore, it is difficult to generate robust data from human intervention studies that can validate the positive results found in epidemiological, cell, and animal-based studies.
There is a growing interest in health foods, yet consumers still lack understanding of bioactive compounds and are often skeptical of health claims in food that appears unnatural, such as “fruit juice enriched with calcium can strength bones” (Verbeke, Scholderer, and Lähteenmäki Citation2009). Health claims are used, from a marketing perspective, to highlight foods with particular health promoting attributes and add value to the carrier product. However, to ensure consumer appeal and credibility, it is important that marketed health benefits are consistent with the consumer expectations and familiarity. Furthermore, consumer research has determined that buyers are not willing to compromise on taste or sensory quality for a potentially higher nutrient density snack (Ares, Giménez, and Gámbaro Citation2008; Davidov-Pardo et al. Citation2012; Corso, Kalschne, and Benassi Citation2018). Although phrases such as “natural antioxidants” generally receive a positive association with consumers, regarding them as a source of health (Mitterer-Daltoé et al. Citation2021), the term is still met with some distrust, likely because there is still limited awareness around the subject. Specific compounds such as lycopene, lutein, and polyphenol are poorly known by consumers and therefore they are promoted as “functional food”—defined by EFSA as “A food, which beneficially affects one or more target functions in the body, beyond adequate nutritional effects, in a way that is relevant to either an improved state of health and well-being and/or reduction of risk of disease” (Martirosyan and Singharaj Citation2016).
Well-designed human intervention studies, providing high quality evidence substantiating the positive epidemiological, cell- and animal-based evidence are required before health claims can be authorized. Additionally, an increased understanding and better targeted communication is vital for consumers to make informed food choices relevant to their health. It is imperative that the population is educated about the benefits that phytochemicals can bring to their diet and well-being. With an increased knowledge of the compounds, their benefits, and the continual growth in health food space, it is likely that foods with phytochemical claims on their packaging will become enticing to consumers.
Conclusion
Research on potential phytochemical health benefits and how to best overcome their naturally low bioavailability is very much still in its infancy. There are a considerable number of in vitro studies assessing bioavailability, bioaccessibility and metabolism of phytochemicals however, even the limited number of human clinical trials are also restricted by low sample sizes. Human intervention trials are imperative to validate the promising results of in vitro studies and to gain realistic insight of phytochemical bioavailability and fate during digestion. In vitro experiments enable controlled and repeatable studies of digestion reducing expense and ethical constraints that can arise in in vivo trials. They can also provide results when in vivo trials are not available or applicable. However, currently validation studies have shown they often oversimplify the complexity of digestion and therefore overestimate expected bioavailability. Therefore, further work must be done to design in vitro models that successfully and accurately mimic the complex gut environment.
Overall, it can be concluded that the bioaccessibility of phytochemicals is promoted during food processing with the disruption and breakdown of cellular components, enhancing their liberation during digestion. However, the consequence of processing techniques on the nutrient loss must be thoroughly considered as water soluble compounds can be leached, and thermal inducement of isomerization or oxidation of some phytochemicals will limit their bioactivity even if they are absorbed. Functional food is a rapidly growing market, creating opportunities to strategically design food matrices promoting interactions between synergistic compounds and enabling increased bioavailability. With further understanding of how to increase phytochemical retention through processing, the effects of interactions between food compounds and food matrices that promise improved bioaccessibility and bioavailability, functional food may be able to reach the market that can support the phytochemical health claim on their packaging.
Acknowledgements
The authors thank PepsiCo, Inc. for funding this manuscript and Luciana Torquati, Lecturer in Nutrition at the University of Exeter, for useful feedback on the draft of the manuscript. The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of PepsiCo Inc. Author J.R.B. is an employee of PepsiCo, Inc.
References
- Abdel-Aal, E. S. M., and P. Hucl. 2003. Composition and stability of anthocyanins in blue-grained wheat. Journal of Agricultural and Food Chemistry 51 (8):2174–80. doi: 10.1021/jf021043x.
- Abu-Ghannam, N., and A. K. Jaiswal. 2015. Blanching as a treatment process: Effect on polyphenol and antioxidant capacity of cabbage. In Processing and impact on active components in food, 35–43. Cambridge, MA: Academic Press. doi: 10.1016/B978-0-12-404699-3.00005-6.
- Agudo, A., R. Ibáñez, P. Amiano, E. Ardanaz, A. Barricarte, A. Berenguer, M. Dolores Chirlaque, M. Dorronsoro, P. Jakszyn, N. Larrañaga, et al. 2008. Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. European Journal of Clinical Nutrition 62 (3):324–31. doi: 10.1038/sj.ejcn.1602750.
- Albishi, T., J. A. John, A. S. Al-Khalifa, and F. Shahidi. 2013. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. Journal of Functional Foods 5 (2):590–600. doi: 10.1016/j.jff.2012.11.019.
- Amigo-Benavent, M., A. Clemente, S. Caira, P. Stiuso, P. Ferranti, and M. Dolores Del Castillo. 2014. Use of phytochemomics to evaluate the bioavailability and bioactivity of antioxidant peptides of soybean β-conglycinin. Electrophoresis 35 (11):1582–9. doi: 10.1002/elps.201300527.
- An, K., D. Zhao, Z. Wang, J. Wu, Y. Xu, and G. Xiao. 2016. Comparison of different drying methods on Chinese ginger (Zingiber officinale roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chemistry 197 Pt B:1292–300. doi: 10.1016/j.foodchem.2015.11.033.
- Angelino, D., and E. Jeffery. 2014. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. Journal of Functional Foods 7 (1):67–76. doi: 10.1016/j.jff.2013.09.029.
- Angelino, D., M. Cossu, A. Marti, M. Zanoletti, L. Chiavaroli, F. Brighenti, D. Del Rio, and D. Martini. 2017. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food & Function 8 (7):2368–93. doi: 10.1039/c7fo00574a.
- Ares, G., A. Giménez, and A. Gámbaro. 2008. Does information about the source of functional ingredients influence consumer perception of functional milk desserts?Journal of the Science of Food and Agriculture 88 (12):2061–8. doi: 10.1002/jsfa.3313.
- Artés, F., M. Castañer, and M. I. Gil. 1998. Review: Enzymatic browning in minimally processed fruit and vegetables. Food Science and Technology International 4 (6):377–89. doi: 10.1177/108201329800400602.
- Aune, D., E. Giovannucci, P. Boffetta, L. T. Fadnes, N. N. Keum, T. Norat, D. C. Greenwood, E. Riboli, L. J. Vatten, and S. Tonstad. 2017. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology 46 (3):1029–56. doi: 10.1093/ije/dyw319.
- Balasundram, N., K. Sundram, and S. Samman. 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry 99 (1):191–203. doi: 10.1016/j.foodchem.2005.07.042.
- Barros, F., J. M. Awika, and L. W. Rooney. 2012. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. Journal of Agricultural and Food Chemistry 60 (46):11609–17. doi: 10.1021/jf3034539.
- Bechoff, A., A. Westby, C. Owori, G. Menya, C. Dhuique-Mayer, D. Dufour, and K. Tomlins. 2010. Effect of drying and storage on the degradation of total carotenoids in orange-fleshed sweetpotato cultivars. Journal of the Science of Food and Agriculture 90 (4):622–9. doi: 10.1002/jsfa.3859.
- Beking, K., and A. Vieira. 2011. An assessment of dietary flavonoid intake in the UK and Ireland. International Journal of Food Sciences and Nutrition 62 (1):17–9. doi: 10.3109/09637486.2010.511165.
- Bellisle, F. 2014. Meals and snacking, diet quality and energy balance. Physiology & Behavior 134:38–43. doi: 10.1016/j.physbeh.2014.03.010.
- Berni, P., C. Chitchumroonchokchai, S. G. Canniatti-Brazaca, F. F. De Moura, and M. L. Failla. 2014. Impact of genotype and cooking style on the content, retention, and bioacessibility of β-carotene in biofortified cassava (Manihot esculenta crantz) conventionally bred in Brazil. Journal of Agricultural and Food Chemistry 62 (28):6677–86. doi: 10.1021/jf5018302.
- Bertha, C. T., S. B. J. Alberto, J. Tovar, S. G. Sáyago-Ayerdi, and V. M. Zamora-Gasga. 2019. In vitro gastrointestinal digestion of mango by-product snacks: Potential absorption of polyphenols and antioxidant capacity. International Journal of Food Science and Technology 54:11. doi: 10.1111/ijfs.14224.
- Blando, F., H. Berland, G. Maiorano, M. Durante, A. Mazzucato, M. E. Picarella, I. Nicoletti, C. Gerardi, G. Mita, and Ø. M. Andersen. 2019. Nutraceutical characterization of anthocyanin-rich fruits produced by ‘sun black’ tomato line. Frontiers in Nutrition 6:133. doi: 10.3389/fnut.2019.00133.
- Bohn, T. 2014. Dietary factors affecting polyphenol bioavailability. Nutrition Reviews 72 (7):429–52. doi: 10.1111/nure.12114.
- Boileau, T. W. M., A. C. Boileau, and J. W. Erdman. 2002. Bioavailability of all-trans and cis-isomers of lycopene. Experimental Biology and Medicine (Maywood, N.J.) 227 (10):914–9. doi: 10.1177/153537020222701012.
- Brackmann, C., A. Bengtsson, M. L. Alminger, U. Svanberg, and A. Enejder. 2011. Visualization of β-carotene and starch granules in plant cells using CARS and SHG microscopy. Journal of Raman Spectroscopy 42 (4):586–92. doi: 10.1002/jrs.2778.
- Brown, M. J., M. G. Ferruzzi, M. L. Nguyen, D. A. Cooper, A. L. Eldridge, S. J. Schwartz, and W. S. White. 2004. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. The American Journal of Clinical Nutrition 80 (2):396–403. doi: 10.1093/ajcn/80.2.396.
- Brownlee, I., P. Dettmar, V. Strugala, and J. Pearson. 2006. The interaction of dietary fibres with the colon. Current Nutrition & Food Science 2 (3):243–64. doi: 10.2174/157340106778017896.
- Calín-Sánchez, Á., A. Figiel, A. Wojdyło, M. Szarycz, and Á. A. Carbonell-Barrachina. 2014. Drying of garlic slices using convective pre-drying and vacuum-microwave finishing drying: Kinetics, energy consumption, and quality studies. Food and Bioprocess Technology 7 (2):398–408. doi: 10.1007/s11947-013-1062-3.
- Can-Cauich, C. A., E. Sauri-Duch, D. Betancur-Ancona, L. Chel-Guerrero, G. A. González-Aguilar, L. F. Cuevas-Glory, E. Pérez-Pacheco, and V. M. Moo-Huchin. 2017. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. Journal of Functional Foods 37:501–6. doi: 10.1016/j.jff.2017.08.028.
- Cao, Y., Y. Tao, X. Zhu, Y. Han, D. Li, C. Liu, X. Liao, and P. L. Show. 2019. Effect of microwave and air-borne ultrasound-assisted air drying on drying kinetics and phytochemical properties of broccoli floret. Drying Technology 38 (13):1733–48. doi: 10.1080/07373937.2019.1662437.
- Capozzi, F., and A. Bordoni. 2013. Foodomics: A new comprehensive approach to food and nutrition. Genes & Nutrition 8 (1):1–4. doi: 10.1007/s12263-012-0310-x.
- Carbonell-Capella, J. M., M. Buniowska, F. J. Barba, M. J. Esteve, and A. Frígola. 2014. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Comprehensive Reviews in Food Science and Food Safety 13 (2):155–71. doi: 10.1111/1541-4337.12049.
- Cardona, F., C. Andrés-Lacueva, S. Tulipani, F. J. Tinahones, and M. Isabel Queipo-Ortuño. 2013. Benefits of polyphenols on gut microbiota and implications in human health. Journal of Nutritional Biochemistry 24 (8):1415–22. doi: 10.1016/j.jnutbio.2013.05.001.
- Carrillo, C., C. Buvé, A. Panozzo, T. Grauwet, and M. Hendrickx. 2017. Role of structural barriers in the in vitro bioaccessibility of anthocyanins in comparison with carotenoids. Food Chemistry 227:271–9. doi: 10.1016/j.foodchem.2017.01.062.
- Chaaban, H., I. Ioannou, L. Chebil, M. Slimane, C. Gérardin, C. Paris, C. Charbonnel, L. Chekir, and M. Ghoul. 2017. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. Journal of Food Processing and Preservation 41 (5):e13203. doi: 10.1111/jfpp.13203.
- Chang, C. H., H. Y. Lin, C. Y. Chang, and Y. C. Liu. 2006. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. Journal of Food Engineering 77 (3):478–85. doi: 10.1016/j.jfoodeng.2005.06.061.
- Chen, Z. G., X. Y. Guo, and T. Wu. 2016. A novel dehydration technique for carrot slices implementing ultrasound and vacuum drying methods. Ultrasonics Sonochemistry 30:28–34. doi: 10.1016/j.ultsonch.2015.11.026.
- Cheng, X., M. Zhang, B. Xu, B. Adhikari, and J. Sun. 2015. The principles of ultrasound and its application in freezing related processes of food materials: A review. Ultrasonics Sonochemistry 27:576–85. doi: 10.1016/j.ultsonch.2015.04.015.
- Chow, W. H., L. M. Schuman, J. K. McLaughlin, E. Bjelke, G. Gridley, S. Wacholder, H. T. Chien, and W. J. Blot. 1992. A cohort study of tobacco use, diet, occupation, and lung cancer mortality. Cancer Causes & Control: CCC 3 (3):247–54. doi: 10.1007/BF00124258.
- Chun, O. K., S. J. Chung, and W. O. Song. 2007. Estimated dietary flavonoid intake and major food sources of U.S. adults. The Journal of Nutrition 137 (5):1244–52. doi: 10.1093/jn/137.5.1244.
- Cisse, M., F. Vaillant, O. Acosta, D. Mayer Claudie, and M. Dornier. 2009. Thermal degradation kinetics of anthocyanins from blood orange, blackberry, and roselle using the arrhenius, eyring, and ball models. Journal of Agricultural and Food Chemistry 57 (14):6285–91. doi: 10.1021/jf900836b.
- Clavel, T., G. Henderson, C. A. Alpert, C. Philippe, L. Rigottier-Gois, J. Doré, and M. Blaut. 2005. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Applied and Environmental Microbiology 71 (10):6077–85. doi: 10.1128/AEM.71.10.6077-6085.2005.
- Conaway, C. C., S. M. Getahun, L. L. Liebes, D. J. Pusateri, D. K. Topham, M. Botero-Omary, and F. L. Chung. 2000. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutrition and Cancer 38 (2):168–78. doi: 10.1207/S15327914NC382_5.
- Corso, M., D. Kalschne, and M. Benassi. 2018. Consumer’s attitude regarding soluble coffee enriched with antioxidants. Beverages 4 (4):72. doi: 10.3390/beverages4040072.
- Costa, J., and A. Ahluwalia. 2019. Advances and current challenges in intestinal in vitro model engineering: A digest. Frontiers in Bioengineering and Biotechnology 7:144. doi: 10.3389/fbioe.2019.00144.
- D’Archivio, M., C. Filesi, R. Varì, B. Scazzocchio, and R. Masella. 2010. Bioavailability of the polyphenols: Status and controversies. International Journal of Molecular Sciences 11 (4):1321–42. doi: 10.3390/ijms11041321.
- Davidov-Pardo, G., M. Moreno, I. Arozarena, M. R. Marín-Arroyo, R. N. Bleibaum, and C. M. Bruhn. 2012. Sensory and consumer perception of the addition of grape seed extracts in cookies. Journal of Food Science 77 (12):S430–S438. doi: 10.1111/j.1750-3841.2012.02991.x.
- de Andrade, R. M. S., and É. C. B. de Andrade Gonçalves. 2021. Bioaccessibility of bioactive compounds and prebiotic properties of fruit and vegetable by-products—Mini review. Current Bioactive Compounds 17 (2):100–11. doi: 10.2174/1573407216666200319102220.
- de Assis, B. B. T., T. C. Pimentel, A. M. Dantas, M. dos Santos Lima, G. da Silva Campelo Borges, and M. Magnani. 2021. Biotransformation of the Brazilian caatinga fruit-derived phenolics by Lactobacillus acidophilus La-5 and Lacticaseibacillus casei 01 impacts bioaccessibility and antioxidant activity. Food Research International 146:110435. doi: 10.1016/j.foodres.2021.110435.
- De Pascual-Teresa, S., J. Hallund, D. Talbot, J. Schroot, C. M. Williams, S. Bugel, and A. Cassidy. 2006. Absorption of isoflavones in humans: Effects of food matrix and processing. Journal of Nutritional Biochemistry 17 (4):257–64. doi: 10.1016/j.jnutbio.2005.04.008.
- de Pee, S., and C. E. West. 1996. Dietary carotenoids and their role in combating vitamin A deficiency: A review of the literature. European Journal of Clinical Nutrition 50 (Suppl 3):S38–S53.
- de Pee, S., C. E. West, D. Permaesih, S. Martuti, M. and Joseph, and G. A. J. Hautvast. 1998. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and β-carotene in school children in indonesia. American Journal of Clinical Nutrition 68 (5):1058–67. doi: 10.1093/ajcn/68.5.1058.
- Del Rio, D., A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges, and A. Crozier. 2013. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants and Redox Signaling 18 (14):1818–92. doi: 10.1089/ars.2012.4581.
- Dekker, M., K. Hennig, and R. Verkerk. 2009. Differences in thermal stability of glucosinolates in five brassica vegetables. Czech Journal of Food Sciences 27 (1):S85–S8. doi: 10.17221/1079-CJFS.
- Deng, L.‐Z., A. S. Mujumdar, W.‐X. Yang, Q. Zhang, Z.‐A. Zheng, M. Wu, and H.‐W. Xiao. 2020. Hot air impingement drying kinetics and quality attributes of orange peel. Journal of Food Processing and Preservation 44 (1):14294. doi: 10.1111/jfpp.14294.
- Deng, Q., K. G. Zinoviadou, C. M. Galanakis, V. Orlien, N. Grimi, E. Vorobiev, N. Lebovka, and F. J. Barba. 2015. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Engineering Reviews 7 (3):357–81. doi: 10.1007/s12393-014-9104-9.
- Desmarchelier, C., and P. Borel. 2017. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends in Food Science & Technology 69:270–80. doi: 10.1016/j.tifs.2017.03.002.
- Dev, S. R. S., and V. G. S. Raghavan. 2012. Advancements in drying techniques for food, fiber, and fuel. Drying Technology 30 (11-12):1147–59. doi: 10.1080/07373937.2012.692747.
- Di Lorenzo, C., F. Colombo, S. Biella, C. Stockley, and P. Restani. 2021. Polyphenols and human health: The role of bioavailability. Nutrients 13 (1):1–30. doi: 10.3390/nu13010273.
- Dias, M. G., M. F. G. F. C. Camões, and L. Oliveira. 2014. Earotenoid stability in fruits, vegetables and working standards—Effect of storage temperature and time. Food Chemistry 156:37–41. doi: 10.1016/j.foodchem.2014.01.050.
- Doughty, K. J., G. A. Kiddle, B. J. Pye, R. M. Wallsgrove, and J. A. Pickett. 1995. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry 38 (2):347–50. doi: 10.1016/0031-9422(94)00653-B.
- Dreosti, I. E. 2000. Recommended dietary intake levels for phytochemicals: Feasible or fanciful?Asia Pacific Journal of Clinical Nutrition 9 Suppl 1:S119–S122. doi: 10.1046/j.1440-6047.2000.00167.x.
- Dunford, E. K., and B. M. Popkin. 2018. 37 year snacking trends for US children 1977–2014. Pediatric Obesity 13 (4):247–55. doi: 10.1111/ijpo.12220.
- Epriliati, I., and R. Irine. 2012. Bioavailability of phytochemicals. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health. London, UK: InTech Open. doi: 10.5772/26702.
- Faulks, R. M., and S. Southon. 2005. Challenges to understanding and measuring carotenoid bioavailability. Biochimica et Biophysica Acta - Molecular Basis of Disease 1740 (2):95–100. doi: 10.1016/j.bbadis.2004.11.012.
- Felgines, C., S. Talavéra, M.-P. Gonthier, O. Texier, A. Scalbert, J.-L. Lamaison, and C. Rémésy. 2003. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. The Journal of Nutrition 133 (5):1296–301. doi: 10.1093/jn/133.5.1296.
- Fernandes, F. A. N., S. Rodrigues, J. V. García-Pérez, and J. A. Cárcel. 2016. Effects of ultrasound-assisted air-drying on vitamins and carotenoids of cherry tomatoes. Drying Technology 34 (8):986–96. doi: 10.1080/07373937.2015.1090445.
- Fernández-García, E., I. Carvajal-Lérida, M. Jarén-Galán, J. Garrido-Fernández, A. Pérez-Gálvez, and D. Hornero-Méndez. 2012. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Research International 46 (2):438–50. doi: 10.1016/j.foodres.2011.06.007.
- Ferranti, P. 2018. The future of analytical chemistry in foodomics. Current Opinion in Food Science 22:102–8. doi: 10.1016/j.cofs.2018.02.005.
- Ferreira, D., S. Guyot, N. Marnet, I. Delgadillo, C. M. G. C. Renard, and M. A. Coimbra. 2002. Composition of phenolic compounds in a portuguese pear (Pyrus communis L. var. S. bartolomeu) and changes after sun-drying. Journal of Agricultural and Food Chemistry 50 (16):4537–44. doi: 10.1021/jf020251m.
- Fraser, P. D., and P. M. Bramley. 2004. The biosynthesis and nutritional uses of carotenoids. Progress in Lipid Research 43 (3):228–65. doi: 10.1016/j.plipres.2003.10.002.
- Freeman, B. L., D. L. Eggett, and T. L. Parker. 2010. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. Journal of Food Science 75 (6):C570–C576. doi: 10.1111/j.1750-3841.2010.01717.x.
- Future Market Insights. 2018. Dehydrated vegetables market: Global industry analysis 2013–2017 and opportunity assessment 2018–2028. https://www.futuremarketinsights.com/reports/dehydrated-vegetables-market.
- Giallourou, N., M. J. Oruna-Concha, and N. Harbourne. 2016. Effects of domestic processing methods on the phytochemical content of watercress (Nasturtium officinale). Food Chemistry 212:411–9. doi: 10.1016/j.foodchem.2016.05.190.
- Giambanelli, E., R. Verkerk, V. Fogliano, E. Capuano, L. F. D’Antuono, and T. Oliviero. 2015. Broccoli glucosinolate degradation is reduced performing thermal treatment in binary systems with other food ingredients. RSC Advances 5 (82):66894–900. doi: 10.1039/C5RA11409H.
- Giovannucci, E., E. B. Rimm, Y. Liu, M. J. Stampfer, and W. C. Willett. 2003. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiology Biomarkers and Prevention 12 (12):1403–9.
- Girgin, N., and S. N. El. 2015. Effects of cooking on in vitro sinigrin bioaccessibility, total phenols, antioxidant and antimutagenic activity of cauliflower (Brassica oleraceae L. var. Botrytis). Journal of Food Composition and Analysis 37:119–27. doi: 10.1016/j.jfca.2014.04.013.
- Giusti, M., and R. E. Wrolstad. 2003. Acylated anthocyanins from edible sources and their applications in food systems. Biochemical Engineering Journal 14 (3):217–25. doi: 10.1016/S1369-703X(02)008.
- Guiamba, I. R. F., U. Svanberg, and L. Ahrné. 2015. Effect of infrared blanching on enzyme activity and retention of β-carotene and vitamin C in dried mango. Journal of Food Science 80 (6):E1235–42. doi: 10.1111/1750-3841.12866.
- Guida, V., G. Ferrari, G. Pataro, A. Chambery, A. D. Maro, and A. Parente. 2013. The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT - Food Science and Technology 53 (2):569–79. doi: 10.1016/j.lwt.2013.04.006.
- Guleria, S., and A. Kumar. 2006. Qualitative profiling of phenols and extracellular proteins induced in mustard (Brassica juncea) in response to benzothiadiazole treatment. Journal of Cell and Molecular Biology 5:51–6.
- Hackett, M. M., J. H. Lee, D. Francis, and S. J. Schwartz. 2006. Thermal stability and isomerization of lycopene in tomato oleoresins from different varieties. Journal of Food Science 69 (7):536–41. doi: 10.1111/j.1365-2621.2004.tb13647.x.
- Haila, K. M., S. M. Lievonen, and M. I. Heinonen. 1996. Effects of lutein, lycopene, annatto, and γ-tocopherol on autoxidation of triglycerides. Journal of Agricultural and Food Chemistry 44 (8):2096–100. doi: 10.1021/jf9504935.
- Hajimehdipoor, H., R. Shahrestani, and M. Shekarchi. 2014. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Research Journal of Pharmacognosy 1 (3):35–40.
- Hanschen, F. S., A. Bauer, I. Mewis, C. Keil, M. Schreiner, S. Rohn, and L. W. Kroh. 2012. Thermally induced degradation of aliphatic glucosinolates: Identification of intermediary breakdown products and proposed degradation pathways. Journal of Agricultural and Food Chemistry 60 (39):9890–9. doi: 10.1021/jf302744y.
- Hanschen, F. S., S. Rohn, I. Mewis, M. Schreiner, and L. W. Kroh. 2012. Influence of the chemical structure on the thermal degradation of the glucosinolates in broccoli sprouts. Food Chemistry 130 (1):1–8. doi: 10.1016/j.foodchem.2011.05.109.
- Harjes, C. E., T. R. Rocheford, L. Bai, T. P. Brutnell, C. B. Kandianis, S. G. Sowinski, A. E. Stapleton, R. Vallabhaneni, M. Williams, E. T. Wurtzel, et al. 2008. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science (New York, N.Y.) 319 (5861):330–3. doi: 10.1126/science.1150255.
- Hawlader, M. N. A., C. O. Perera, and M. Tian. 2006. Properties of modified atmosphere heat pump dried foods. Journal of Food Engineering 74 (3):392–401. doi: 10.1016/j.jfoodeng.2005.03.028.
- Hennig, K., R. C. H. De Vos, C. Maliepaard, M. Dekker, R. Verkerk, and G. Bonnema. 2014. A metabolomics approach to identify factors influencing glucosinolate thermal degradation rates in brassica vegetables. Food Chemistry 155:287–97. doi: 10.1016/j.foodchem.2014.01.062.
- Heredia, A., I. Peinado, E. Rosa, and A. Andrés. 2010. Effect of osmotic pre-treatment and microwave heating on lycopene degradation and isomerization in cherry tomato. Food Chemistry 123 (1):92–8. doi: 10.1016/j.foodchem.2010.04.005.
- Higdon, J. V., B. Delage, D. E. Williams, and R. H. Dashwood. 2007. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacological Research 55 (3):224–36. doi: 10.1016/j.phrs.2007.01.009.
- Holst, B., and G. Williamson. 2008. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Current Opinion in Biotechnology 19 (2):73–82. doi: 10.1016/j.copbio.2008.03.003.
- Huang, W., X. Bi, X. Zhang, X. Liao, X. Hu, and J. Wu. 2013. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innovative Food Science and Emerging Technologies 18:74–82. doi: 10.1016/j.ifset.2013.01.001.
- Ioannou, I., I. Hafsa, S. Hamdi, C. Charbonnel, and M. Ghoul. 2012. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. Journal of Food Engineering 111 (2):208–17. doi: 10.1016/j.jfoodeng.2012.02.006.
- Jeevitha, G. C., H. U. Hebbar, and K. S. M. S. Raghavarao. 2013. Electromagnetic radiation-based dry blanching of red bell peppers: A comparative study. Journal of Food Process Engineering 36 (5):663–74. doi: 10.1111/jfpe.12030.
- Jing, P., S.-J. Zhao, S.-Y. Ruan, Z.-H. Xie, Y. Dong, and L. (Lucy) Yu. 2012. Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chemistry 133 (4):1569–76. doi: 10.1016/j.foodchem.2012.02.051.
- Johnson, I. T. 2002. Glucosinolates: Bioavailability and importance to health. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition 72 (1):26–31. doi: 10.1024/0300-9831.72.1.26.
- Johnson, L., F. Ghishan, J. Kaunitz, J. Merchant, H. Said, and J. Wood. 2012. Physiology of the gastrointestinal tract. Vol. 1–2. New York, NY: Elsevier Inc. doi: 10.1016/C2009-1-64521-4.
- Jones, R. B., C. L. Frisina, S. Winkler, M. Imsic, and R. B. Tomkins. 2010. Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chemistry 123 (2):237–42. doi: 10.1016/j.foodchem.2010.04.016.
- Joshi, A. P. K., H. P. V. Rupasinghe, and S. Khanizadeh. 2011. Impact of drying processes on bioactive phenolics, vitamin C and antioxidant capacity of red-fleshed apple slices. Journal of Food Processing and Preservation 35 (4):453–7. doi: 10.1111/j.1745-4549.2010.00487.x.
- Jun, S., S. Shin, and H. Joung. 2016. Estimation of dietary flavonoid intake and major food sources of korean adults. The British Journal of Nutrition 115 (3):480–9. doi: 10.1017/S0007114515004006.
- Kadian, S. S., A. Sharma, and D. R. Sood. 2015. Effect of light and heat on stability of crude carotenoid extract from natural sources. International Journal of Pharmaceutical Sciences and Research 4 (6):2415–8. doi: 10.13040/IJPSR.0975-8232.4(6).2415-18.
- Kalt, W., J. E. McDonald, and H. Donner. 2000. Anthocyanins, phenolics, and antioxidant capacity of processed lowbush blueberry products. Journal of Food Science 65 (3):390–3. doi: 10.1111/j.1365-2621.2000.tb16013.x.
- Kamiloglu, S., G. Toydemir, D. Boyacioglu, J. Beekwilder, R. D. Hall, and E. Capanoglu. 2016. A review on the effect of drying on antioxidant potential of fruits and vegetables. Critical Reviews in Food Science and Nutrition 56 Suppl 1:S110–S29. doi: 10.1080/10408398.2015.1045969.
- Kayacan, S., S. Karasu, P. K. Akman, H. Goktas, I. Doymaz, and O. Sagdic. 2020. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and hmf formation of persimmon. LWT - Food Science and Technology 118:108830. doi: 10.1016/j.lwt.2019.108830.
- Kim, Y. A., J. B. Keogh, and P. M. Clifton. 2016. Polyphenols polyphenols and glycemic control. Nutrients 8 (1):17. doi: 10.3390/nu8010017.
- Kırca, A., M. Özkan, and B. Cemeroğlu. 2007. Effects of temperature, solid content and ph on the stability of black carrot anthocyanins. Food Chemistry 101 (1):212–8. doi: 10.1016/j.foodchem.2006.01.019.
- Koncsek, A., H. G. Daood, Z. H. Horváth, M. Fekete, A. Véha, and L. Helyes. 2019. Improvement of antioxidant content and color stability in spice paprika powder by rosemary extract supplementation. Journal of Food Processing and Preservation 43 (8) doi: 10.1111/jfpp.14000.
- Korus, A. 2011. Effect of preliminary processing, method of drying and storage temperature on the level of antioxidants in kale (Brassica oleracea L. var. Acephala) leaves. LWT - Food Science and Technology 44 (8):1711–6. doi: 10.1016/j.lwt.2011.03.014.
- Kwok, B. H. L., C. Hu, T. Durance, and D. D. Kitts. 2006. Dehydration techniques affect phytochemical contents and free radical scavenging activities of saskatoon berries (Amelanchier alnifolia Nutt.). Journal of Food Science 69 (3):SNQ122–SNQ126. doi: 10.1111/j.1365-2621.2004.tb13381.x.
- Lafarga, T., E. Gallagher, A. Bademunt, G. Bobo, G. Echeverria, I. Viñas, and I. Aguiló-Aguayo. 2019. Physiochemical and nutritional characteristics, bioaccessibility and sensory acceptance of baked crackers containing broccoli co-products. International Journal of Food Science and Technology 54 (3):634–40. doi: 10.1111/ijfs.13908.
- Lao, Y., M. Zhang, B. Chitrakar, B. Bhandari, and D. Fan. 2019. Efficient plant foods processing based on infrared heating. Food Reviews International 35 (7):640–63. doi: 10.1080/87559129.2019.1600537.
- Lekcharoenkul, P., Y. Tanongkankit, N. Chiewchan, and S. Devahastin. 2014. Enhancement of sulforaphane content in cabbage outer leaves using hybrid drying technique and stepwise change of drying temperature. Journal of Food Engineering 122:56–61. doi: 10.1016/j.jfoodeng.2013.08.037.
- Lewandowska, U., K. Szewczyk, E. Hrabec, A. Janecka, and S. Gorlach. 2013. Overview of metabolism and bioavailability enhancement of polyphenols. Journal of Agricultural and Food Chemistry 61 (50):12183–99. doi: 10.1021/jf404439b.
- Lin, T. M., T. D. Durance, and C. H. Scaman. 1998. Characterization of vacuum microwave, air and freeze dried carrot slices. Food Research International 31 (2):111–7. doi: 10.1016/S0963-9969(98)00070-2.
- Liu, Q., E. Donner, R. Tarn, J. Singh, and H.-J. Chung. 2009. Advanced analytical techniques to evaluate the quality of potato and potato starch. In Advances in Potato Chemistry and Technology. New York, NY: Elsevier. doi: 10.1016/b978-0-12-374349-7.00008-8.
- Liu, X., and K. Lv. 2013. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast 22 (3):309–13. doi: 10.1016/j.breast.2012.07.013.
- Liu, Y., J. Wu, S. Miao, C. Chong, and Y. Sun. 2014. Effect of a modified atmosphere on drying and quality characteristics of carrots. Food and Bioprocess Technology 7 (9):2549–59. doi: 10.1007/s11947-014-1295-9.
- Lombard, K., E. Peffley, E. Geoffriau, L. Thompson, and A. Herring. 2005. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. Journal of Food Composition and Analysis 18 (6):571–81. doi: 10.1016/j.jfca.2004.03.027.
- Madrau, M. A., A. M. Sanguinetti, A. Del Caro, C. Fadda, and A. Piga. 2010. Contribution of melanoidins to the antioxidant activity of prunes. Journal of Food Quality 33:155–70. doi: 10.1111/j.1745-4557.2010.00328.x.
- Majeed, M., S. Majeed, K. Nagabhushanam, S. Natarajan, A. Sivakumar, and F. Ali. 2016. Evaluation of the stability of Bacillus coagulans MTCC 5856 during processing and storage of functional foods. International Journal of Food Science & Technology 51 (4):894–901. doi: 10.1111/ijfs.13044.
- Manach, C., G. Williamson, C. Morand, A. Scalbert, and C. Rémésy. 2005. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition 81 (1 Suppl):230S–42S. doi: 10.1093/ajcn/81.1.230s.
- Mandal, S. 2010. Induction of phenolics, lignin and key defense enzymes in eggplant (Solanum melongena L.) roots in response to elicitors. African Journal of Biotechnology 9 (47):8038–47. doi: 10.5897/AJB10.984.
- Márkus, F., H. G. Daood, J. Kapitány, and P. A. Biacs. 1999. Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. Journal of Agricultural and Food Chemistry 47 (1):100–7. doi: 10.1021/jf980485z.
- Martín-Cabrejas, M. A., Y. Aguilera, M. M. Pedrosa, C. Cuadrado, T. Hernández, S. Díaz, and R. M. Esteban. 2009. The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chemistry 114 (3):1063–8. doi: 10.1016/j.foodchem.2008.10.070.
- Martini, D., S. Rossi, B. Biasini, I. Zavaroni, G. Bedogni, M. Musci, C. Pruneti, G. Passeri, M. Ventura, S. Di Nuzzo, et al. 2017. Claimed effects, outcome variables and methods of measurement for health claims proposed under European Community Regulation 1924/2006 in the framework of protection against oxidative damage and cardiovascular health. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 27 (6):473–503. doi: 10.1016/j.numecd.2017.01.008.
- Martirosyan, D. M., and B. Singharaj. 2016. Health claims and functional food: The future of functional foods under FDA and EFSA regulation. In Functional foods for chronic diseases, 410–24. Dallas, TX: Food Science Publisher.
- McCarty, M. F. 2004. Proposal for a dietary ‘phytochemical index’. Medical Hypotheses 63 (5):813–7. doi: 10.1016/j.mehy.2002.11.004.
- Mejia-Meza, E. I., J. A. Yanez, N. M. Davies, B. Rasco, F. Younce, C. M. Remsberg, and C. Clary. 2008. Improving nutritional value of dried blueberries (Vaccinium corymbosum L.) combining microwave-vacuum, hot-air drying and freeze drying technologies. International Journal of Food Engineering 4 (5):1364. doi: 10.2202/1556-3758.1364.
- Méndez Lagunas, L. L., and F. Castaigne. 2008. Effect of temperature cycling on allinase activity in garlic. Food Chemistry 111 (1):56–60. doi: 10.1016/j.foodchem.2008.03.035.
- Miglio, C., E. Chiavaro, A. Visconti, V. Fogliano, and N. Pellegrini. 2008. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. Journal of Agricultural and Food Chemistry 56 (1):139–47. doi: 10.1021/jf072304b.
- Millar, K. A., C. Barry-Ryan, R. Burke, K. Hussey, S. McCarthy, and E. Gallagher. 2017. Effect of pulse flours on the physiochemical characteristics and sensory acceptance of baked crackers. International Journal of Food Science and Technology 52 (5):1155–63. doi: 10.1111/ijfs.13388.
- Min, M., C. R. Bunt, S. L. Mason, and M. A. Hussain. 2019. Non-dairy probiotic food products: An emerging group of functional foods. Critical Reviews in Food Science and Nutrition 59 (16):2626–41. doi: 10.1080/10408398.2018.1462760.
- Mínguez-Mosquera, M. I., and D. Hornero-Méndez. 1994. Comparative study of the effect of paprika processing on the carotenoids in peppers (Capsicum annuum) of the bola and agridulce varieties. Journal of Agricultural and Food Chemistry 42 (7):1555–60. doi: 10.1021/jf00043a031.
- Mitterer-Daltoé, M., J. Bordim, C. Lise, L. Breda, M. Casagrande, and V. Lima. 2021. Consumer awareness of food antioxidants. Synthetic vs. natural. Food Science and Technology 41 (Suppl 1):208–12. doi: 10.1590/fst.15120.
- Mizrahi, S. 1996. Leaching of soluble solids during blanching of vegetables by ohmic heating. Journal of Food Engineering 29 (2):153–66. doi: 10.1016/0260-8774(95)00074-7.
- Mortensen, A., L. H. Skibsted, A. Willnow, and S. A. Everett. 1998. Re-appraisal of the tocopheroxyl radical reaction with β-carotene: Evidence for oxidation of vitamin E by the β-carotene radical cation. Free Radical Research 28 (1):69–80. doi: 10.3109/10715769809097877.
- Mrkic, V., I. Redovnikovic, S. Jolic, K. Delonga, and V. Dragovic-Uzelac. 2010. Effect of drying conditions on indole glucosinolate level in broccoli. Acta Alimentaria 39 (2):167–74. doi: 10.1556/AAlim.39.2010.2.8.
- Murakami, M., T. Yamaguchi, H. Takamura, and T. Matoba. 2003. Effects of ascorbic acid and α-tocopherol on antioxidant activity of polyphenolic compounds. Journal of Food Science 68 (5):1622–5. doi: 10.1111/j.1365-2621.2003.tb12302.x.
- Murakami, M., T. Yamaguchi, H. Takamura, and T. Matoba. 2004. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. Journal of Food Science 69 (1):FCT7–10. doi: 10.1111/j.1365-2621.2004.tb17848.x.
- Murota, K., S. Shimizu, S. Miyamoto, T. Izumi, A. Obata, M. Kikuchi, and J. Terao. 2002. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: Comparison of isoflavonoids and flavonoids. The Journal of Nutrition 132 (7):1956–61. doi: 10.1093/jn/132.7.1956.
- Musielak, G., D. Mierzwa, and J. Kroehnke. 2016. Food drying enhancement by ultrasound—A review. Trends in Food Science and Technology 55 (4):570–94. doi: 10.1016/j.tifs.2016.08.003.
- Naqvi, S., C. Zhu, G. Farre, K. Ramessar, L. Bassie, J. Breitenbach, D. Perez Conesa, G. Ros, G. Sandmann, T. Capell, et al. 2009. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proceedings of the National Academy of Sciences of the United States of America 106 (19):7762–7. doi: 10.1073/pnas.0901412106.
- Nichenametla, S. N., T. G. Taruscio, D. L. Barney, and J. H. Exon. 2006. A review of the effects and mechanisms of polyphenolics in cancer. Critical Reviews in Food Science and Nutrition 46 (2):161–83. doi: 10.1080/10408390591000541.
- Oerlemans, K., D. M. Barrett, C. Bosch Suades, R. Verkerk, and M. Dekker. 2006. Thermal degradation of glucosinolates in red cabbage. Food Chemistry 95 (1):19–29. doi: 10.1016/j.foodchem.2004.12.013.
- Olszowy-Tomczyk, M. 2020. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochemistry Reviews 19 (1):63–103. doi: 10.1007/s11101-019-09658-4.
- Ozdal, T., E. Capanoglu, and F. Altay. 2013. A review on protein-phenolic interactions and associated changes. Food Research International 51 (2):954–70. doi: 10.1016/j.foodres.2013.02.009.
- Palermo, M., N. Pellegrini, and V. Fogliano. 2014. The effect of cooking on the phytochemical content of vegetables. Journal of the Science of Food and Agriculture 94 (6):1057–70. doi: 10.1002/jsfa.6478.
- Panalaks, T., and T. K. Murray. 1970. The effect of processing on the content of carotene isomers in vegetables and peaches. Canadian Institute of Food Technology Journal 3 (4):145–51. doi: 10.1016/S0008-3860(70)74310-9.
- Parr, A. J., and G. P. Bolwell. 2000. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. Journal of the Science of Food and Agriculture 80 (7):985–1012. doi: 10.1002/(SICI)1097-0010(20000515)80:7 < 985::AID-JSFA572 > 3.0.CO;2-7.
- Parry, A., K. James, and S. LeRoux. 2015. Strategies to achieve economic and environmental gains by reducing food waste. The New Climate Economy.
- Pasławska, M., K. Sala, A. Nawirska-Olszańska, B. Stępień, and E. Pląskowska. 2020. Effect of different drying techniques on dehydration kinetics, physical properties, and chemical composition of lemon thyme. Natural Product Communications 15 (2):1934578X2090452. doi: 10.1177/1934578X20904521.
- Pellegrini, N., E. Chiavaro, C. Gardana, T. Mazzeo, D. Contino, M. Gallo, P. Riso, V. Fogliano, and M. Porrini. 2010. Effect of different cooking methods on color, phytochemical concentration, and antioxidant capacity of raw and frozen brassica vegetables. Journal of Agricultural and Food Chemistry 58 (7):4310–21. doi: 10.1021/jf904306r.
- Pereira-Caro, G., C. M. Oliver, R. Weerakkody, T. Singh, M. Conlon, G. Borges, L. Sanguansri, T. Lockett, S. A. Roberts, A. Crozier, et al. 2015. Chronic administration of a microencapsulated probiotic enhances the bioavailability of orange juice flavanones in humans. Free Radical Biology & Medicine 84:206–14. doi: 10.1016/j.freeradbiomed.2015.03.010.
- Pérez-Jiménez, J., V. Neveu, F. Vos, and A. Scalbert. 2010. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. European Journal of Clinical Nutrition 64 Suppl 3:S112–S120. doi: 10.1038/ejcn.2010.221.
- Perez-Moral, N., S. Saha, M. Philo, D. J. Hart, M. S. Winterbone, W. J. Hollands, M. Spurr, J. Bows, V. van der Velpen, P. A. Kroon, et al. 2018. Comparative bio-accessibility, bioavailability and bioequivalence of quercetin, apigenin, glucoraphanin and carotenoids from freeze-dried vegetables incorporated into a baked snack versus minimally processed vegetables: Evidence from in vitro models and a human bioavailability study. Journal of Functional Foods 48:410–9. doi: 10.1016/j.jff.2018.07.035.
- Public Health England. 2016. Government 5 A Day Logo. PHE Publications.
- Putnik, P., D. Gabrić, S. Roohinejad, F. J. Barba, D. Granato, K. Mallikarjunan, J. M. Lorenzo, and D. Bursać Kovačević. 2019. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chemistry 276:680–91. doi: 10.1016/j.foodchem.2018.10.068.
- Radi, M., M. Mahrouz, A. Jaouad, M. Tacchini, S. Aubert, M. Hugues, and M. J. Amiot. 1997. Phenolic composition, browning susceptibility, and carotenoid content of several apricot cultivars at maturity. HortScience 32 (6):1087–91. doi: 10.21273/HORTSCI.32.6.1087.
- Rahman, M. S., Q. H. Al-Shamsi, G. B. Bengtsson, S. S. Sablani, and A. Al-Alawi. 2009. Drying kinetics and allicin potential in garlic slices during different methods of drying. Drying Technology 27 (3):467–77. doi: 10.1080/07373930802683781.
- Ramesh, M. N., W. Wolf, D. Tevini, and G. Jung. 1999. Studies on inert gas processing of vegetables. Journal of Food Engineering 40 (3):199–205. doi: 10.1016/S0260-8774(99)00056-4.
- Ramesh, M. N., W. Wolf, D. Tevini, and G. Jung. 2001. Influence of processing parameters on the drying of spice paprika. Journal of Food Engineering 49 (1):63–72. doi: 10.1016/S0260-8774(00)00185-0.
- Ratti, C., M. Araya-Farias, L. Mendez-Lagunas, and J. Makhlouf. 2007. Drying of garlic (Allium sativum) and its effect on allicin retention. Drying Technology 25 (2):349–56. doi: 10.1080/07373930601120100.
- Rechner, A. R., M. A. Smith, G. Kuhnle, G. R. Gibson, E. S. Debnam, S. K. S. Srai, K. P. Moore, and C. A. Rice-Evans. 2004. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radical Biology & Medicine 36 (2):212–25. doi: 10.1016/j.freeradbiomed.2003.09.022.
- Regier, M., E. Mayer-Miebach, D. Behsnilian, E. Neff, and H. P. Schuchmann. 2005. Influences of drying and storage of lycopene-rich carrots on the carotenoid content. Drying Technology 23 (4):989–98. doi: 10.1081/DRT-200054255.
- Rein, M. J., M. Renouf, C. Cruz-Hernandez, L. Actis-Goretta, S. K. Thakkar, and M. da Silva Pinto. 2013. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. British Journal of Clinical Pharmacology 75 (3):588–602. doi: 10.1111/j.1365-2125.2012.04425.x.
- Rock, C. L., and M. E. Swendseid. 1992. Plasma beta-carotene response in humans after meals supplemented with dietary pectin. The American Journal of Clinical Nutrition 55 (1):96–9. doi: 10.1093/ajcn/55.1.96.
- Roknul, A. S. M., M. Zhang, A. S. Mujumdar, and Y. Wang. 2014. A comparative study of four drying methods on drying time and quality characteristics of stem lettuce slices (Lactuca sativa L.). Drying Technology 32 (6):657–66. doi: 10.1080/07373937.2013.850435.
- Roodenburg, A. J., R. Leenen, K. H. van Het Hof, J. A. Weststrate, and L. B. Tijburg. 2000. Amount of fat in the diet affects bioavailability of lutein esters but not of alpha-carotene, beta-carotene, and vitamin E in humans. The American Journal of Clinical Nutrition 71 (5):1187–93. doi: 10.1093/ajcn/71.5.1187.
- Rothschild, D., O. Weissbrod, E. Barkan, A. Kurilshikov, T. Korem, D. Zeevi, P. I. Costea, A. Godneva, I. N. Kalka, N. Bar, et al. 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature 555 (7695):210–5. doi: 10.1038/nature25973.
- Roudaut, G., C. Dacremont, B. Vallès Pàmies, B. Colas, and M. Le Meste. 2002. Crispness: A critical review on sensory and material science approaches. Trends in Food Science and Technology 13 (6–7):217–27. doi: 10.1016/S0924-2244(02)00139-5.
- Ruttarattanamongkol, K., S. Chittrakorn, M. Weerawatanakorn, and N. Dangpium. 2016. Effect of drying conditions on properties, pigments and antioxidant activity retentions of pretreated orange and purple-fleshed sweet potato flours. Journal of Food Science and Technology 53 (4):1811–22. doi: 10.1007/s13197-015-2086-7.
- Sarvan, I., R. Verkerk, and M. Dekker. 2012. Modelling the fate of glucosinolates during thermal processing of brassica vegetables. LWT - Food Science and Technology 49 (2):178–83. doi: 10.1016/j.lwt.2012.07.005.
- Schweiggert, R. M., R. E. Kopec, M. G. Villalobos-Gutierrez, J. Högel, S. Quesada, P. Esquivel, S. J. Schwartz, and R. Carle. 2014. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: A randomised cross-over study. The British Journal of Nutrition 111 (3):490–8. doi: 10.1017/S0007114513002596.
- Serpen, A., and V. Gökmen. 2009. Evaluation of the maillard reaction in potato crisps by acrylamide, antioxidant capacity and color. Journal of Food Composition and Analysis 22 (6):589–95. doi: 10.1016/j.jfca.2008.11.003.
- Serrano, J., I. Goñi, and F. Saura-Calixto. 2005. Determination of beta-carotene and lutein available from green leafy vegetables by an in vitro digestion and colonic fermentation method. Journal of Agricultural and Food Chemistry 53 (8):2936–40. doi: 10.1021/jf0480142.
- Setchell, K. D. R., M. S. Faughnan, T. Avades, L. Zimmer-Nechemias, N. M. Brown, B. E. Wolfe, W. T. Brashear, P. Desai, M. F. Oldfield, N. P. Botting, et al. 2003. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. The American Journal of Clinical Nutrition 77 (2):411–9. doi: 10.1093/ajcn/77.2.411.
- Shahidi, F., and Y. Pan. 2021. Influence of food matrix and food processing on the chemical interaction and bioaccessibility of dietary phytochemicals: A review. Critical Reviews in Food Science and Nutrition 2021:1–25. doi: 10.1080/10408398.2021.1901650.
- Sharma, A., and W. Zhou. 2011. A stability study of green tea catechins during the biscuit making process. Food Chemistry 126 (2):568–73. doi: 10.1016/j.foodchem.2010.11.044.
- Shi, J., M. L. Maguer, Y. Kakuda, A. Liptay, and F. Niekamp. 1999. Lycopene degradation and isomerization in tomato dehydration. Food Research International 32 (1):15–21. doi: 10.1016/S0963-9969(99)00059-9.
- Singh, S. V., and K. Singh. 2012. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis 33 (10):1833–42. doi: 10.1093/carcin/bgs216.
- Skroza, D., I. Generalić Mekinić, S. Svilović, V. Šimat, and V. Katalinić. 2015. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. Journal of Food Composition and Analysis 38:13–8. doi: 10.1016/j.jfca.2014.06.013.
- Smith, T. K., R. Mithen, and I. T. Johnson. 2003. Effects of brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts in vivo. Carcinogenesis 24 (3):491–5. doi: 10.1093/carcin/24.3.491.
- Song, J., X. Wang, D. Li, L. Meng, and C. Liu. 2017. Degradation of carotenoids in pumpkin (Cucurbita maxima L.) slices as influenced by microwave vacuum drying. International Journal of Food Properties 20 (7):1479–87. doi: 10.1080/10942912.2016.1212875.
- Srivastava, J. K., and S. Gupta. 2009. Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Molecular and Cellular Pharmacology 1 (3):138. doi: 10.4255/mcpharmacol.09.18.
- Suvarnakuta, P., S. Devahastin, and A. S. Mujumdar. 2005. Drying kinetics and β-carotene degradation in carrot undergoing different drying processes. Journal of Food Science 70 (8):S520–S6. doi: 10.1111/j.1365-2621.2005.tb11528.x.
- Tao, Y., M. Han, X. Gao, Y. Han, P.-L. Show, C. Liu, X. Ye, and G. Xie. 2019. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrasonics Sonochemistry 53:192–201. doi: 10.1016/j.ultsonch.2019.01.003.
- Tedstone, A., V. Coulton, V. Targett, A. Bennett, K. Morgan Sweeney, E. Clegg, et al. 2018. Sugar reduction and wider reformulation programme: Report on progress towards the first 5% reduction and next steps. BMC Public Health 2018:101.
- Tekin, Z. H., M. Başlar, S. Karasu, and M. Kilicli. 2017. Dehydration of green beans using ultrasound-assisted vacuum drying as a novel technique: Drying kinetics and quality parameters. Journal of Food Processing and Preservation 41 (6):e13227. doi: 10.1111/jfpp.13227.
- Teng, H., and L. Chen. 2019. Polyphenols and bioavailability: An update. Critical Reviews in Food Science and Nutrition 59 (13):2040–51. doi: 10.1080/10408398.2018.1437023.
- Tian, J., J. Chen, F. Lv, S. Chen, J. Chen, D. Liu, and X. Ye. 2016. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chemistry 197 Pt B:1264–70. doi: 10.1016/j.foodchem.2015.11.049.
- Topuz, A., C. Dincer, K. S. Özdemir, H. Feng, and M. Kushad. 2011. Influence of different drying methods on carotenoids and capsaicinoids of paprika (Cv.; Jalapeno). Food Chemistry 129 (3):860–5. doi: 10.1016/j.foodchem.2011.05.035.
- Traka, M. H., and R. F. Mithen. 2011. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals. The Plant Cell 23 (7):2483–97. doi: 10.1105/tpc.111.087916.
- Traka, M. H., S. Saha, S. Huseby, S. Kopriva, P. G. Walley, G. C. Barker, J. Moore, G. Mero, F. van den Bosch, H. Constant, et al. 2013. Genetic regulation of glucoraphanin accumulation in Beneforté broccoli. The New Phytologist 198 (4):1085–95. doi: 10.1111/nph.12232.
- Turck, D., J.-L. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait, M. Heinonen, K. I. Hirsch-Ernst, I. Mangelsdorf, H. J. McArdle, A. Naska, et al. 2018. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA Journal. European Food Safety Authority 16 (1):e05136-21. doi: 10.2903/j.efsa.2018.5136.
- Unlu, N. Z., T. Bohn, D. M. Francis, H. N. Nagaraja, S. K. Clinton, and S. J. Schwartz. 2007. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. The British Journal of Nutrition 98 (1):140–6. doi: 10.1017/S0007114507685201.
- USDA Economic Research Service. 2013. Selected Charts from Ag and Food Statistics: Charting the Essentials. United States Department of Agriculture (USDA).
- Valdes, A. M., J. Walter, E. Segal, and T. D. Spector. 2018. Role of the gut microbiota in nutrition and health. BMJ (Online) 2018:k2179. doi: 10.1136/bmj.k2179.
- van Het Hof, K. H., I. A. Brouwer, C. E. West, E. Haddeman, R. P. Steegers-Theunissen, M. van Dusseldorp, J. A. Weststrate, T. K. Eskes, and J. G. Hautvast. 1999. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. The American Journal of Clinical Nutrition 70 (2):261–8. doi: 10.1093/ajcn.70.2.261.
- van Het Hof, K. H., C. Gärtner, C. E. West, and L. B. M. Tijburg. 1998. Potential of vegetable processing to increase the delivery of carotenoids to man. International Journal for Vitamin and Nutrition Research 68 (6):366–70.
- van Het Hof, K. H., C. E. West, J. A. Weststrate, and J. G. Hautvast. 2000. Dietary factors that affect the bioavailability of carotenoids. The Journal of Nutrition 130 (3):503–6. doi: 10.1093/jn/130.3.503.
- Verbeke, W., J. Scholderer, and L. Lähteenmäki. 2009. Consumer appeal of nutrition and health claims in three existing product concepts. Appetite 52 (3):684–92. doi: 10.1016/j.appet.2009.03.007.
- Verhoeven, D. T. H., R. A. Goldbohm, G. Van Poppel, H. Verhagen, and P. A. Van Den Brandt. 1996. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiology Biomarkers and Prevention 5 (9):733–48.
- Vishwanathan, K. H., G. K. Giwari, and H. U. Hebbar. 2013. Infrared assisted dry-blanching and hybrid drying of carrot. Food and Bioproducts Processing 91 (2):89–94. doi: 10.1016/j.fbp.2012.11.004.
- Vogiatzoglou, A., A. A. Mulligan, M. A. H. Lentjes, R. N. Luben, J. P. E. Spencer, H. Schroeter, K. T. Khaw, and G. G. C. Kuhnle. 2015. Flavonoid intake in European adults (18 to 64 years). PLoS One 10 (5):e0128132. doi: 10.1371/journal.pone.0128132.
- Wang, J., X.-H. Yang, A. S. Mujumdar, D. Wang, J.-H. Zhao, X.-M. Fang, Q. Zhang, L. Xie, Z.-J. Gao, and H.-W. Xiao. 2017. Effects of various blanching methods on weight loss, enzymes inactivation, phytochemical contents, antioxidant capacity, ultrastructure and drying kinetics of red bell pepper (Capsicum annuum L.). LWT - Food Science and Technology 77:337–47. doi: 10.1016/j.lwt.2016.11.070.
- Wang, S., K. A. Meckling, M. F. Marcone, Y. Kakuda, and R. Tsao. 2011. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. Journal of Agricultural and Food Chemistry 59 (3):960–8. doi: 10.1021/jf1040977.
- Wang, Z., F. Zhai, B. Zhang, and B. M. Popkin. 2012. Trends in Chinese snacking behaviors and patterns and the social-demographic role between 1991 and 2009. Asia Pacific Journal of Clinical Nutrition 21 (2):253–62. doi: 10.6133/apjcn.2012.21.2.13.
- Williamson, G., and B. Holst. 2008. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction?The British Journal of Nutrition 99 Suppl 3:S55–S58. doi: 10.1017/S0007114508006867.
- Winter, J., L. H. Moore, V. R. Dowell, and V. D. Bokkenheuser. 1989. C-ring cleavage of flavonoids by human intestinal bacteria. Applied and Environmental Microbiology 55 (5):1203–8. doi: 10.1128/aem.55.5.1203-1208.1989.
- Wirth, J., S. Poletti, B. Aeschlimann, N. Yakandawala, B. Drosse, S. Osorio, T. Tohge, A. R. Fernie, D. Günther, W. Gruissem, et al. 2009. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnology Journal 7 (7):631–44. doi: 10.1111/j.1467-7652.2009.00430.x.
- Wojdyło, A., A. Figiel, K. Lech, P. Nowicka, and J. Oszmiański. 2014. Effect of convective and vacuum-microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food and Bioprocess Technology 7 (3):829–41. doi: 10.1007/s11947-013-1130-8.
- Wojdyło, A., A. Figiel, and J. Oszmiański. 2009. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. Journal of Agricultural and Food Chemistry 57 (4):1337–43. doi: 10.1021/jf802507j.
- Wu, X., Q. H. Zhou, and K. Xu. 2009. Are isothiocyanates potential anti-cancer drugs?Acta Pharmacologica Sinica 30 (5):501–12. doi: 10.1038/aps.2009.50.
- Xu, F., Y. Zheng, Z. Yang, S. Cao, X. Shao, and H. Wang. 2014. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chemistry 161:162–7. doi: 10.1016/j.foodchem.2014.04.025.
- Yan, W. Q., M. Zhang, L. L. Huang, A. S. Mujumdar, and J. Tang. 2013. Influence of microwave drying method on the characteristics of the sweet potato dices. Journal of Food Processing and Preservation 37 (5):662–9. doi: 10.1111/j.1745-4549.2012.00707.x.
- Yang, J., J. F. Chen, Y. Y. Zhao, and L. C. Mao. 2010. Effects of drying processes on the antioxidant properties in sweet potatoes. Agricultural Sciences in China 9 (10):1522–7. doi: 10.1016/S1671-2927(09).
- Yilmaz, M. S., Ö. Şakiyan, I. Barutcu Mazi, and B. G. Mazi. 2019. Phenolic content and some physical properties of dried broccoli as affected by drying method. Food Science and Technology International = Ciencia y tecnologia de los alimentos internacional 25 (1):76–88. doi: 10.1177/1082013218797527.
- Yuan, G. F., B. Sun, J. Yuan, and Q. M. Wang. 2009. Effects of different cooking methods on health-promoting compounds of broccoli. Journal of Zhejiang University: Science B 10 (8):580–8. doi: 10.1631/jzus.B0920051.
- Zarein, M., S. H. Samadi, and B. Ghobadian. 2015. Investigation of microwave dryer effect on energy efficiency during drying of apple slices. Journal of the Saudi Society of Agricultural Sciences 14 (1):41–7. doi: 10.1016/j.jssas.2013.06.002.
- Zhang, B., Z. Deng, Y. Tang, P. Chen, R. Liu, D. D. Ramdath, Q. Liu, M. Hernandez, and R. Tsao. 2014. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chemistry 161:296–304. doi: 10.1016/j.foodchem.2014.04.014.
- Zhang, N. Q., S. C. Ho, X. F. Mo, F. Y. Lin, W. Q. Huang, H. Luo, J. Huang, and C. X. Zhang. 2018. Glucosinolate and isothiocyanate intakes are inversely associated with breast cancer risk: A case-control study in China. The British Journal of Nutrition 119 (8):957–64. doi: 10.1017/S0007114518000600.
- Zhang, Y. J., R. Y. Gan, S. Li, Y. Zhou, A. N. Li, D. P. Xu, H. B. Li, and D. D. Kitts. 2015. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules (Basel, Switzerland) 20 (12):21138–56. doi: 10.3390/molecules201219753.
- Zhang, Z., X. Wang, Y. Li, Q. Wei, C. Liu, M. Nie, L. Dajing, et al. 2017. Evaluation of the impact of food matrix change on the: In vitro bioaccessibility of carotenoids in pumpkin (Cucurbita moschata) slices during two drying processes. Food and Function 8 (12):4693–702. doi: 10.1039/c7fo01382e.
- Zhang, Z., Q. Wei, M. Nie, N. Jiang, C. Liu, C. Liu, D. Li, and L. Xu. 2018. Microstructure and bioaccessibility of different carotenoid species as affected by hot air drying: Study on carrot, sweet potato, yellow bell pepper and broccoli. LWT - Food Science and Technology 96:357–63. doi: 10.1016/j.lwt.2018.05.061.
- Zhao, D. K., An, S. Ding, L. Liu, Z. Xu, and Z. Wang. 2014. Two-stage intermittent microwave coupled with hot-air drying of carrot slices: Drying kinetics and physical quality. Food and Bioprocess Technology 7 (8):2308–18. doi: 10.1007/s11947-014-1274-1.