Abstract
The slowdown, inhibition, or reversal of age-related decline (as a composite of disease, dysfunction, and, ultimately, death) by diet or natural compounds can be defined as dietary geroprotection. While there is no single reliable biomarker to judge the effects of dietary geroprotection, biomarker signatures based on omics (epigenetics, gene expression, microbiome composition) are promising candidates. Recently, omic biomarkers started to supplement established clinical ones such as lipid profiles and inflammatory cytokines. In this review, we focus on human data. We first summarize the current take on genetic biomarkers based on epidemiological studies. However, most of the remaining biomarkers that we describe, whether omics-based or clinical, are related to intervention studies. Then, because of their promising potential in the context of dietary geroprotection, we focus on the effects of berry-based interventions, which up to now have been mostly described employing clinical markers. We provide an aggregation and tabulation of all the recent systematic reviews and meta-analyses that we could find related to this topic. Finally, we present evidence for the importance of the “nutribiography,” that is, the influence that an individual’s history of diet and natural compound consumption can have on the effects of dietary geroprotection.
Supplemental data for this article is available online at https://doi.org/10.1080/10408398.2021.1975638
Graphical Abstract
Supplemental data for this article is available online at https://doi.org/10.1080/10408398.2021.1975638
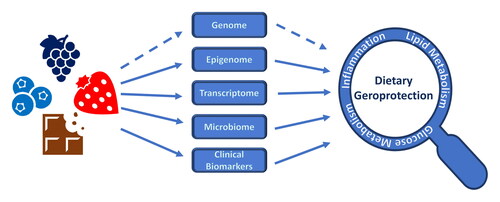
Diet was shown to have a measurable impact on both omics and clinical biomarkers. Inflammation, lipid and glucose metabolism are among the main molecular processes impacted, and a positive effect of diet or natural compounds on these and other processes can result in beneficial effects on health and survival, that is, dietary geroprotection. Effects are indicated by arrows, suggesting causal influences, though it is important to consider that the entities and relationships shown here are only part of a complex meshwork of direct and indirect effects. Dashed arrows indicate evolutionary effects of diet on the genome, and effects of diet on aging-associated molecular processes mediated by the genome.
Introduction
The quest for the identification of biomarkers of aging is still in its early stages. Many different candidates have been proposed, and in many cases, the best results were obtained with composite biomarkers (Shamir Citation2015). Some candidates have become quite popular, such as epigenetic clocks (Ryan Citation2020; Bell et al. Citation2019; Horvath Citation2013), or phenotypic age (Liu et al. Citation2018) and Young.AI (Mamoshina et al. Citation2018a), which are biomarker signatures based on clinical (laboratory) marker measurements. A biomarker of aging should not just be able to predict future health and survival better than chronological age (Fuellen et al. Citation2019; Baker and Sprott Citation1988); it should also be sensitive to the effects of protective interventions, including healthy lifestyles and diet (Galkin et al. Citation2020a).
There is ample evidence that non-pharmaceutical interventions may delay aging. The adoption of an exercise program or of healthy eating patterns, such as a Mediterranean, MediterrAsian, or a calorie restricted diet, have all been reported to ameliorate age-related decline (Belsky et al. Citation2017; Gensous et al. Citation2020; Rebelo-Marques et al. Citation2018; Pallauf et al. Citation2013). Some diets and the consumption of some natural compounds have been linked to improved health and survival in humans and animals alike. In this review, we prioritize human studies, and only mention animal data to highlight similarities and differences with respect to humans. However, the impact of the consumption of many natural compounds, such as the polyphenols fisetin or resveratrol, as described in some but not all studies, on the health and survival of laboratory animals (Bhullar and Hubbard Citation2015; Yousefzadeh et al. Citation2018) has not yet been validated in human interventions. Many more issues are still in need of further research. For example, there is an ongoing debate if and to what extend polyphenols such as resveratrol act as calorie restriction mimetics, thus mediating life-prolonging properties (Pallauf et al. Citation2016). It is unclear if health-promoting properties of polyphenol-rich diets can be replaced by purified compounds as dietary supplements (Egert and Rimbach 2011) and if and to what extend data in model organisms can be translated to humans.
Natural compounds are compounds produced by living organisms. These may be consumed through a diet enriched in appropriate foods, or by supplementation of purified extracts or synthesized compounds. We define as geroprotective any diet or natural compound with a demonstrated effect in delaying, inhibiting, or even reversing, aging. Due to their simple implementation and safety record, diets and their natural constituents are of ever-increasing importance to maintain health in old age and to extend (healthy) lifespan; we call this approach “dietary geroprotection.”
Among the frequently studied dietary geroprotectors are the omega-3 fatty acids found in oily fish and nuts, vitamin D that is also found in oily fish but largely synthesized endogenously in response to sunlight exposure (Ferri et al. Citation2019), dietary fiber found in plant-based foods, and polyphenols, that is, plant secondary metabolites whose dietary sources are, for example, herbal teas, cocoa, coffee, vegetables and fruits including berries (Fraga et al. Citation2019; Shahidi and Ambigaipalan Citation2018; Slavin Citation2013). The identification of biomarkers that enable us to evaluate geroprotective effects in humans is of utmost necessity. Dietary geroprotection and biomarkers supporting its successful implementation are important not least because we are facing a “gray” wave of increasing numbers of old and very old citizens that could pose economic and other challenges proportional to their health status.
This narrative review deals with omics and clinical biomarkers that could be used to evaluate the geroprotective effects of diets and natural compounds. The first section will focus on genetic differences among individuals that can make them more or less sensitive to dietary geroprotection, while the following sections will focus on candidates for epigenetic, transcriptomic, microbiome-related and clinical biomarkers to judge successful dietary geroprotection. We have chosen to focus on omics biomarkers (specifically those related to gene expression and its regulation) because of the recent success of epigenetic clocks, and also because they offer the possibility to identify known and new molecular mechanisms involved in the aging process. In principle, implicating known aging-associated processes in the success of a dietary geroprotection can lessen or even alleviate the need for a placebo control group in a dietary trial, assuming that the up- or downregulation of specific molecular processes or pathways (such as aging-associated inflammation and cellular senescence; the more specific the better (Campisi and Robert Citation2014; Franceschi et al. Citation2018; Ou, Yang, and Liu Citation2020; Santoro et al. Citation2020a)) cannot easily be accomplished by some mind-body interaction that may give rise to a placebo effect.
Box 1: Placebo effects and biomarker sources in dietary geroprotection studies.
As described in the main text, the need for a placebo group may be lessened or even alleviated in a dietary trial, if an up- or downregulation of molecular processes or pathways can be demonstrated that is so specific to the aging-associated intervention that it is not plausible that the psychological expectation of benefits is the main driver of these effects. Handling such potential placebo effects is important in dietary geroprotection; at least, many food items cannot be faked easily, so a placebo intervention is not always feasible. This is specifically true for berry-based interventions, to which we devoted an extra section and a table compiling all recent systematic reviews and meta-analyses that we could identify. However, most of these publications make exclusive use of clinical biomarkers, and they do not attempt to pinpoint specific molecular processes or underlying signal transduction pathways of effect. Then again, at the same time, they usually refer to studies that include placebo groups based on fake berry powder, extract, or shakes. In any case, by studying the impact of an intervention using different omics modalities (e.g., epigenetics and transcriptomics), its effects can be understood even better and in a more detailed fashion.
In the context of dietary interventions, most studies were carried out using Peripheral Blood Mononuclear Cells (PBMC) or venous whole blood as biomarker sources since the blood draw needed for PBMC, and venous blood sampling in general is comparatively noninvasive and inexpensive. In fact, almost all biomarkers discussed here, except for the microbiomic ones, are based on a blood draw. However, the use of capillary blood (e.g., dried blood spots) is expected to gradually replace blood draws, given the ever-increasing sensitivity of laboratory measurements. Another promising development is swabs of various kinds (oral, nasal, skin, etc.) (Fuellen et al. Citation2020).
Genetics of human aging: From epidemiological studies to biomarkers
Health and survival are partially determined by genetics. And indeed, the maximum lifespans of animals are predominantly species-specific and can be predicted quite accurately from the genomes of those species (Mayne et al. Citation2019). Furthermore, the existence of long-lived mutants, such as the Ames dwarf mouse or the daf-2-deficient C. elegans may suggest that perhaps, similar mutations in human beings could lead to a longer-lived phenotype (Kuningas et al. Citation2008). Then again, a recent family tree study, including hundreds of millions of people, found evidence of a strong influence of assortative mating in heritability estimates and it suggested that the true genetic heritability of longevity is below 10% (Graham Ruby et al. Citation2018). Overall health can be assumed to have a stronger genetic basis, but we are not aware of studies of, e.g., healthspan, that considered assortative mating as a confounder. Nevertheless, various human mortality studies show that longevity, here defined as surviving to the top 10% of a given birth cohort, is heritable (van den Berg et al. Citation2019; Deelen et al. Citation2019). Moreover, both long-lived individuals and their first and second-degree relatives show lower morbidity, in addition to lifelong reduced mortality (van den Berg et al. Citation2019). In any case, genetic factors do not have a dominant influence on the healthspan and lifespan of the average human individual, suggesting that there is a lot of space for improvement by tackling the influence factors that can be modified.
Cognition is of specific interest in this context (see also Box 1), because there is not just a genetic influence on cognitive abilities and a plethora of studies demonstrate the effects of dietary geroprotection on cognition. What is more, cognitive abilities influence the choice of a healthy diet, demonstrated by the genetic influence on food choice and the effects of cognitive decline on the quality of the diet that is consumed.
Human longevity genes
A wide variety of candidate longevity genes have been identified by genome wide association studies (GWAS), but many of them could not be replicated (Pilling et al. Citation2017; Deelen et al. Citation2019). One common approach to investigate longevity is the use of extreme age groups (e.g. contrasting centenarians to young individuals), another one is using parents’ attained age as a proxy for longevity for an individual (Pilling et al. Citation2017; Van der Auwera et al. Citation2021). Replication failure could be explained by different definitions of the longevity phenotype, or different gene versus environment interactions in different cohorts (Franceschi et al. Citation2020). Another reason could be sexual dimorphisms influencing the aging process, masking the potential association between a gene and sex-specific aging outcomes. In fact, Zeng et al. identified genes that seem to be associated with longevity only in a single sex (Zeng et al. Citation2018). Notably, the genes with highest reproducibility and effect size among different studies, APOE and FOXO3, are both involved in nutrient sensing (Revelas et al. Citation2018).
APOE, longevity and diet
The APOE gene encodes a protein involved in lipid metabolism and transport, including fat-soluble vitamins like vitamin D and E, as well as cholesterol, through the lymphatic and vascular systems (Mahley, Weisgraber, and Huang Citation2009; Huebbe and Rimbach 2017). It is also involved in cholesterol transport to the brain and the resolution of inflammation (Yin et al. Citation2019; Liao, Yoon, and Kim Citation2017). The most common allele of this gene is APOE3, while the other two alleles, APOE2 and APOE4, have population-specific frequencies that are usually smaller (Yassine and Finch Citation2020). Overall, the APOE2 allele appears to be protective against age related diseases, while the APOE4 allele shows the opposite effect (Shinohara et al. 2020).
We here provide some more details of what is known about the molecular implications of some APOE variants on lipid metabolism and how these are thought to explain health-related outcomes. Since many dietary geroprotectors modulate lipid profiles, we can expect that the effects of at least some nutritional interventions on, e.g., longevity, are influenced by APOE genetics. Confirming this expectation, Griffin et al. (Citation2018) have recently shown, after a 24-week long dietary intervention involving 389 subjects, that APOE4 carriers (in comparison to homozygous E3 carriers) experience a more significant decrease in total cholesterol and APOB when saturated fatty acids are replaced with low glycemic index carbohydrates (Griffin et al. Citation2018). Moreover, Yassine and Finch (Citation2020) have recently summarized the available evidence on the impact of the APOE4 genotype on the response to dietary interventions and nutritional supplementation. From their analysis, APOE4 carriers were shown to be more responsive to diet and lifestyle treatments and more sensitive to omega-3 deficiencies (Yassine and Finch Citation2020). Nevertheless, Minihane et al. have reported that a 6-week fish-oil intervention led to adverse effects on the lipid profile of APOE4 carriers, with a significant increase in total cholesterol and a trend toward a decrease in HDL cholesterol. At the same time, subjects that were homozygous for APOE3 benefited from the supplementation of fish oils, with a significant reduction in fasting and postprandial triglycerides and a decrease in percent medium size LDL particles (LDL-3) (Minihane et al. Citation2000). Thus, APOE4 carriers, despite their sensitivity to omega-3 deficiencies, may not always benefit from fish oil supplementation. Furthermore, Abdullah et al. (Citation2021), after reviewing the available literature, have recently shown APOE to be among the genes involved in the inter-individual variability of circulating cholesterol concentrations in response to diet (Abdullah et al. Citation2021). Some more evidence of the impact of APOE genotype status on the response to diet comes from transgenic targeted gene replacement mice. Huebbe, Jofre-Monseny, and Rimbach (Citation2009) have shown that the transport of alpha-tocopherol to the lungs is affected by ApoE genotype, Boesch et al. (Citation2009) have shown that quercetin has a smaller effect on inflammation and blood lipids in transgenic ApoE4 mice compared to their transgenic ApoE3 counterparts, and Graeser et al. (Citation2011) have demonstrated that Nrf2-related gene expression is decreased in transgenic ApoE4 mice when compared their transgenic ApoE3 counterparts. Mouse evidence in favor of nutrition-related interventions for the amelioration of health in ApoE4 mice was provided by Wiesmann et al. (Citation2016) and Yanckello et al. (Citation2021), who have shown ApoE4 mice to be sensitive to the protective effect of a multi-nutrient supplement (Fortasyn) and to the prebiotic fiber inulin, respectively.
Investigating the effects of APOE genetics on health and survival, it was found that homozygosity for the APOE2 allele is correlated with decreased risk of dementia compared to the other genotypes (Li et al. Citation2020), with longevity (Shinohara et al. 2020), but also with an increased risk of atherosclerosis (Javvaji, Can, and Sharma Citation2021), while both homozygosity and heterozygosity for the APOE4 allele are associated with an increased risk (Marais Citation2019). In particular, the concentration of LDL cholesterol decreases when one APOE2 allele is present, presumably due to reduced binding of specific lipid transporters (chylomicron remnants) to APOE receptors and the compensatory upregulation of LDL receptors in liver cells, and reduced APOE-receptor-dependent conversion of LDL precursors into LDL. In the presence of two APOE2 alleles, however, the reduction of the APOE receptor activity is so large that atherogenic lipid transporters can accumulate in the presence of cofactors such as obesity, leading to atherosclerosis. By contrast, both homo- and heterozygous APOE4 carriers show higher cholesterol levels and other abnormalities that could explain their propensity to disease: they have lower serum HDL, higher inflammatory marker levels, and significantly different microbiomes compared to APOE3 and APOE2 carriers (Masana et al. Citation2017; Morrill and Gibas Citation2019; Patrick Citation2019; Yassine and Finch Citation2020), similar to transgenic lab mice that carry the human APOE4 allele (Boesch-Saadatmandi et al. Citation2009; Wiesmann et al. Citation2016). Overall, individuals that are homozygous for APOE4 could be responsive to dietary geroprotections that specifically improve lipid and inflammation markers. Thus, the APOE4 genotype could be used as a selection criterion for the identification of individuals that would benefit particularly from this type of interventions (Farmer, Johnson, and Hanson Citation2019; Yassine and Finch Citation2020), which warrants further investigations.
FOXO3, longevity and diet
The other robustly reproducible longevity gene, FOXO3, encodes a transcription factor involved in numerous pathways related to longevity, such as autophagy, inflammation, and cellular replication. It is one of the most important players in the geroprotective effects of caloric restriction in mice (Shimokawa et al. Citation2015). Various SNPs associate higher transcriptional levels of FOXO3 and longevity (Sanese et al. Citation2019; Flachsbart et al. Citation2017). Flachsbart et al. also confirmed that two FOXO3 SNPs (rs4946935 (A/G) and rs12206094 (C/T)) are associated with longevity in multiple cohorts. The authors showed that both variants are related to higher FOXO3 transcriptional levels in expression Quantitative Trait Loci (eQTL, Nica and Dermitzakis Citation2013) databases, and they investigated these variants in vitro. One interesting finding of their study is that cells transfected with the second variant (rs12206094 (C/T)) feature stronger EGCG-mediated FOXO3 overexpression (epigallocatechin gallate (EGCG) is a polyphenol contained in green tea). Studies in rodents shed further light on the in vivo role of this transcription factor, as its knockout causes increased incidence of atherosclerosis, faster exhaustion of the hematopoietic stem cell pool and higher inflammatory markers, while its overexpression is correlated with smaller cardiomyocyte size and enhanced autophagy (Morris et al. Citation2015). A couple of mouse and rat studies have shown that many natural plant compounds such as resveratrol, withaferin A and ECGC upregulate FOXO3 transcriptional levels in vivo (Bale, Pulivendala, and Godugu Citation2018; Franco et al. 2014; Shankar, Marsh, and Srivastava Citation2013; Wang et al. Citation2017; Wu et al. Citation2017). Further, Pallauf et al. (Citation2017) have also shown, in vitro, that flavonoids, but not antioxidant vitamins such as ascorbic acid and alpha tocopherol can stimulate the activities of transcription factors belonging to the Foxo family, but also Nrf2 and PPARγ, two other longevity-associated transcription factors (Pallauf et al. Citation2017). Thus, in the context of a geroprotective intervention based on polyphenols, the FOXO3 genotype could play a significant role.
MTHFR and diet
A gene that has a definitive impact on the response to dietary intervention is the MTHFR gene. Unlike FOXO3 and APOE, it has yet not turned up in longevity GWAS studies. This gene encodes a flavoprotein that catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (Trimmer 2013). A very common polymorphism, the 677 C > T variant, results in a thermolabile enzyme with decreased functionality. Individuals that carry the 677 C > T variant tend to have higher homocysteine levels and are at risk of folate deficiency (Dean Citation2012). Interestingly, Sae-Lee et al. (Citation2018) have shown that supplementation with folic acid and vitamin B12 decreased epigenetic aging measured through Horvath’s clock (see below) only in women with the MTHFR wildtype variant, who responded to the intervention with higher serum levels of folate than men of the same genotype or individuals with the MTHFR wildtype (Sae-Lee et al. Citation2018). Of note, folate-rich food such as green leafy vegetables can easily be consumed as part of the diet.
The fact that polymorphisms in APOE and FOXO3 can influence both the aging process and the response to dietary interventions shows how much aging and nutrition are intertwined at the genetic level. Thanks to the growing wealth of genetic data related to human longevity, identifying other genes that sit at the intersection between nutrition and aging is becoming more feasible. In addition, resources such as the LongevityMap (Budovsky et al. Citation2013), one of the databases belonging to the HAGR collection (Human Aging Genomic Resources, Tacutu et al. Citation2018), give access to this type of data. We thus expect that more and more genetic analyses will be part of studies regarding dietary geroprotection.
Box 2: Genetics, cognition and nutrition.
Cognitive improvements and the amelioration of cognitive decline by diet, by polyphenols in general and polyphenol-rich berries in particular is a long-standing topic in nutrition research. Studies of blueberries started decades ago, and similar efforts were undertaken with strawberries, many other berries, fruit and vegetables (Vauzour Citation2012). Since genetics influences health, cognition and diet, complex relationships can be expected, and as shown here, a specific role of lipid profiles, inflammation and immunity has already been demonstrated. Moreover, cognitive ability shows its effect on food consumption over the whole lifetime, also on the socioeconomic side, as nutrition highly depends on income and educational level (Fergus, Seals, and Holston Citation2021).
GWAS identified numerous risk genes for aging-related neurodegenerative diseases such as Alzheimer’s Disease (Lambert et al. 2013; Jansen et al. Citation2019). Besides the APOE4 locus (as described elsewhere in this review), SNPs with smaller effect size were identified indicating risk association to genes expressed in immune-related tissues and cell types. Jansen et al. (Citation2019) performed additional analyses that indicated further biological mechanisms for dementia which involved lipid-related processes and degradation of amyloid precursor proteins. Furthermore, the emerging search for genetic loci that interact with environmental factors to increase or decrease the risk of disease is highly intriguing as these factors can be tackled. As we demonstrate in this review, biomarkers related to immunity and inflammation as well as lipid profiles are frequently improved by dietary geroprotection. In turn, mediated by genetics, these positive effects can improve cognition, health, and diet.
As an example of the influence of diet on cognition, recent evidence suggests that healthy nutrient intake can positively affect specific aspects of brain integrity. Prinelli et al. (Citation2019) investigated dementia-free older adults from a population-based Swedish National study with MRI data and data on dietary intake using a food frequency questionnaire, estimating the intake of 21 nutrients. Principal component analysis yielded five nutrient patterns. The nutrient pattern characterized by high fiber, vitamin C, E, β-carotene, and folate was associated with the lowest white matter hyperintensity (WMH) volume, compared to the pattern characterized by saturated fatty acids, trans fats, monounsaturated fatty acids, and cholesterol. Prinelli et al. conclude by suggesting brain-health specific nutrient combinations comprising higher intake of fruits, vegetables, olive and seed oils, oily fish, lean red meat, and poultry but less intake of milk and dairy products like cream and butter, and less intake of processed meat. Since WMH is also influenced by genetics (Sargurupremraj et al. 2020), gene-diet interactions are likely involved in the determination of WMH outcomes.
As an example of the influence of genetics on diet, likely mediated by cognition, the genetic background was shown to have an impact on the individual preference and amount of food consumption. In particular, Cornelis et al. (2015) identified 6 novel genetic loci that were associated with higher coffee consumption. Interestingly, loci near GCKR, MLXIPL, BDNF and CYP1A2 that were associated with higher coffee consumption have previously been associated with smoking initiation, higher adiposity and fasting insulin and glucose but lower blood pressure and favorable lipid, inflammatory and liver enzyme profiles, thus demonstrating a complex behavioral and metabolic pattern.
Epigenetic biomarkers
A wide variety of epigenetic changes accompany the aging process, including post-translational modifications of the amino-terminal tail of histones, accumulation of aging-related histone variants, and covalent modifications of DNA bases (Unnikrishnan et al. Citation2019; Villeponteau Citation1997; Wang, Yuan, and Xie Citation2018; Yi and Kim Citation2020). The latter, especially the methylation of CpG islands, proved themselves as surprisingly reliable biomarkers of aging (Galkin et al. Citation2020a). Epigenetics knowledge is becoming abundant also because of the recent development of omics assays that can measure DNA methylation on a large scale. Furthermore, the methylation state of CpG islands is stable so that samples can be stored long-term and analyzed when needed (Hollegaard et al. Citation2013).
Epigenetic clocks as predictors of chronological and biological age
Various epigenetic biomarker signatures (also called “clocks”) for the prediction of chronological age have been created using machine learning methodologies, such as elastic net regression, based on the methylation status of CpG islands. The earliest clocks predicted chronological age from such methylation marks (Hannum et al. Citation2013; Horvath Citation2013). Epigenetic age acceleration can then be defined as the difference between the predicted and the actual chronological age of a given individual, and used as a biomarker of aging, thus estimating biological age. One leap forward was made when the learning was not performed based on chronological age but on surrogates of morbidity or mortality (Field et al. Citation2018), yielding clocks such as PhenoAge (Levine et al. Citation2018), GrimAGE (Lu et al. Citation2019), and “disease” clocks (Lin and Wagner Citation2015; Zhang et al. Citation2017). McCrory et al. (Citation2021) recently compared the ability of four clocks, that is, Horvath’s and Hannum’s 2013 clocks, PhenoAge and GrimAge to predict all-cause mortality and they investigated the association of these clocks with clinical measures related to morbidity and frailty. GrimAge, the best performing clock according to their analysis, could predict mortality with a high confidence and was significantly associated with almost all clinical phenotypes taken into consideration (McCrory et al. Citation2021).
Nutritional effects on the epigenome
The epigenome is sensitive to various dietary influences, for example to the intake of B-vitamins, polyphenols, and dietary choline (Khan et al. Citation2018; Mahmoud and Ali Citation2019; Mandaviya et al. Citation2019). Thus, we suggest that epigenetic biomarkers are useful in the study of dietary geroprotection. Many studies report the effects of nutritional interventions on the methylation status of loci involved in inflammation and age-related diseases. For example, Arpón et al. (Citation2016) reported changes in the methylation state of eight genes related to inflammation and immunocompetence, after a five-year-long Mediterranean diet intervention, while Kok et al. (Citation2015) identified epigenetic changes in regions of the genome related to carcinogenesis and development, in elderly subjects supplemented with folate and vitamin B-12 for two years. A few studies report the effect of diet on epigenetic clocks. Quach et al. (Citation2018) carried out a cross-sectional study involving 4,173 post-menopausal women. The authors found that a higher extrinsic epigenetic age acceleration, a measure of age acceleration sensitive to age-related changes in blood cell composition based on Hannum’s clock (Chen et al. Citation2016), is inversely associated with fish intake, alcohol consumption, and blood carotenoid levels. On the other hand, intrinsic epigenetic age acceleration, a measure of cell-intrinsic aging based on a modified version of Horvath’s clock independent of blood cell composition (Chen et al. Citation2016), is negatively associated with poultry intake (Quach et al. Citation2018). Fitzgerald et al. reported a reduction of Horvath’s DNA methylation age of 1.96 years measured from saliva samples in a cohort that followed an 8-week diet and lifestyle intervention that targeted sleep, exercise, stress, and nutrition (Fitzgerald et al. Citation2021).
Another epigenetic biomarker that is sensitive to both aging and diet is the methylation state of transposable LINE-1 elements. The transcriptional accessibility of LINE-1 elements is negatively correlated to the methylation state of their CpG-island rich internal promoters. There are many studies that link hypomethylation of LINE-1 elements to various human diseases, from cancers to autoimmune diseases (Zhang, Zhang, and Yu 2020). LINE-1 element methylation state is inversely correlated with chronological age (Cho et al. Citation2015; Zhu et al. Citation2012), is lower in senescent cells compared to non-senescent ones (De Cecco et al. Citation2019) and, importantly, their methylation has been positively associated with dietary patterns rich in fiber and plant phytochemicals (Piyathilake et al. Citation2012). An interventional study observed slower changes in the methylation state of LINE-1 elements after the adherence to a one year-long Mediterranean diet intervention (Martín-Núñez et al. Citation2014). LINE-1 hypomethylation has also been correlated to a poorer response in terms of weight loss (Garcia-Lacarte et al. Citation2016) and increased systolic blood pressure (Ferrari et al. Citation2019) to diet. Notwithstanding the poorer response, dietary geroprotection is expected to modify the methylation state of LINE-1 elements for the better even in such cases.
The epigenome is profoundly affected by both diet and the aging process. Therefore, we expect that epigenetic biomarkers will increasingly be used in the evaluation of different types of dietary geroprotection. Furthermore, employing epigenetic clocks that reflect biological age can be a significant aid in measuring the magnitude of the geroprotective effects of a given dietary intervention.
Transcriptomic biomarkers
The transcriptome, just like the epigenome, shows many biologically relevant changes during aging. Age-related gene expression changes offer sufficient signals to enable the creation of “transcriptomic clocks.” Various transcriptome-based clocks have been created, using samples coming from different kinds of tissues. Examples are the clocks by Peters et al. (2015) and Huan et al. (Citation2018) that predict from whole blood or PBMC, the clocks by Fleischer et al. (Citation2018) and LaRocca, Cavalier, and Wahl (Citation2020) that predict from skin fibroblasts and the clock by Mamoshina et al. (Citation2018b) that predicts from muscle tissue, while the ones by Ren and Kuan (2020) and Shokhirev and Johnson (Citation2021) are pan-tissue clocks (Hartmann et al. Citation2021). All the current transcriptomic clocks were trained to predict chronological age, just like the first generation of epigenetic clocks, so their usefulness in the context of geroprotection is not optimal, because age acceleration, or the difference between predicted age and chronological age, can only be moderately informative of biological age (Mitnitski and Rockwood 2019). Nevertheless, a major advantage of investigating transcripts in general and transcriptomic clocks in particular is the possibility, in principle, to more easily apply enrichment analyses directly to the genes that are used as features by these clocks, and to derive insights into aging and the effects of nutrition based on the resulting enriched biological terms/pathways.
Further down the gene expression cascade, proteomic biomarkers of aging are in development. Based on specific blood parameters, Sayed et al. described iAGE (Sayed et al. 2019, BioRxiv. doi: 10.1101/840363), a predictor based on immunological parameters including cytokines, chemokines, growth factors and cytomegalovirus infection status, and both Tanaka et al. and Lehallier et al. created clocks based on plasma proteins (Johnson et al. Citation2020; Tanaka et al. 2018, Citation2020). Proteins result from gene expression, and they can thus be used for enrichment analyses, just like transcripts, and help in the elucidation of the molecular mechanisms involved in aging and in the effects of nutrition on aging.
Nutritional effects on transcription
The effects of diet on transcriptomic clocks are yet to be described in detail. However, various studies have shown that dietary changes, such as the adoption of a Mediterranean diet or a Nordic style of eating, induce wide-reaching changes in the transcriptome of PBMCs and whole blood. For example, Myhrstad et al. (Citation2019) showed, using the transcriptome of PBMCs, that a three to four month-long Nordic diet intervention modulates inflammation in individuals with metabolic syndrome by downregulating the expression of many pro-inflammatory and upregulating the expression of a few inflammation-resolving genes; genes coding for electron transport chain components and cellular proliferation are downregulated as well (Myhrstad et al. Citation2019). As another example, Esser et al. (Citation2018) showed that a four week-long daily supplementation of 100 mg epicatechin, a flavonol that can be found, e.g., in cocoa, strawberries and apple peels, down-regulates the transcriptional level of inflammatory genes in PBMCs, while Barrera-Reyes et al. (Citation2019) obtained similar results analyzing the PBMCs of subjects two hours after the intake of high polyphenol cocoa powder (Esser et al. Citation2018; Barrera-Reyes et al. Citation2019). In whole blood, postprandial changes in glycolytic and inflammatory gene expression were found after the consumption of yogurt and acidified milk in a cohort of young men, pointing to an anti-obesity effect and a biphasic modulation of inflammation, with an upregulation at two hours and a down regulation at six hours after the intake of these foods (Burton et al. Citation2018).
Several studies also reported positive transcriptomic changes in PBMCs after the consumption of algal or fish oils, which are rich sources of omega-3 fatty acids; the transcriptional processes most influenced by the consumption of fish or marine oils are related to inflammation (Bouwens et al. Citation2009; Myhrstad et al. Citation2014; Myhrstad et al. Citation2016; Souza et al. Citation2020). According to Schmidt et al. (Citation2012), immunomodulatory effects of omega-3 fatty acids are more evident in dyslipidemic subjects. Further, Polus et al. (Citation2016) studied the effects of omega-3 fatty acid supplementation, in a cohort of obese women, a group characterized by dyslipidemia and chronic inflammation. At the end of the three-month intervention, they observed, based on PBMCs, an up-regulation of the transcriptional levels of ALOX5, a gene that codes for an enzyme involved in the production of pro-resolving DHA derivatives, and of target genes of the transcription factors NRF2 and PPAR-α, both involved in fatty acid beta-oxidation, phospholipid synthesis, mitochondrial electron transport chain, and antioxidant defense. These gene expression changes were accompanied by a decrease in circulatory cytokines, adhesion molecules, and acute-phase proteins and an increase in pro-resolving mediators, indicating an anti-inflammatory effect elicited by the omega-3 fatty acids. GWAS of fish oil supplementation effects recently revealed new gene-diet interaction loci, some of which implicated in LDL metabolism (Francis et al. Citation2021).
Long-term changes to the transcriptome
Transcriptional changes may be limited to a certain temporal window, as already described for acute effects by Burton et al. (Citation2018), see above. Other changes induced by a dietary intervention may only appear long-term, sometimes long after its conclusion. For example, Milella et al. (Citation2020) sampled the blood of 20 individuals instructed to consume approximately 350 g of grapes per day for 21 days at three different time points: at the start of the dietary intervention (T0), at its end (T1), and after a washout period (T2). While changes in the expressional levels of genes related to blood coagulation were apparent only at T1 and were absent at T2, the ones in genes related to inflammation were shared by both time points. These changes were even stronger at T2, and the ones in cellular housekeeping such as autophagy, mitochondrial biogenesis, and DNA repair became apparent only at T2.
Dietary interventions can affect transcriptomic processes related to aging and disease, such as inflammation, antioxidant defense and stress response. To understand the underlying molecular mechanisms, transcriptomic analyses should be included in geroprotective trials. Moreover, transcriptomic clocks help interpret transcriptomic data, complementing enrichment analyses by giving a concise representation of the magnitude of the geroprotective effects. Lastly, it is worth noting that the transcriptome tends to change faster in response to interventions than the other types of biomarkers discussed here, with the advantage that it can reflect acute changes and the disadvantage that it can be much noisier.
Microbiome biomarkers
The microbiome comprises the ensemble of bacteria, viruses, fungi, and archaea that live symbiotically with the human body. It has a profound and wide-reaching effect on health; in fact, dysbioses of microbial communities in the skin, lungs, oral cavity, and the gut have all been implicated in the susceptibility to various inflammatory and neoplastic syndromes (Lloyd-Price, Abu-Ali, and Huttenhower Citation2016; Gilbert et al. Citation2018). The microbiome of the gut is the best-studied one, and it is also the most impacted by dietary influences (Hills et al. Citation2019). Many insights are emerging about the changes that the microbiome undergoes during aging. Galkin et al. recently used deep learning to construct a predictor (dubbed a “microbiome clock”) of chronological age based on microbial taxonomic profiles from more than 4000 individuals aged 18–90 years (Galkin et al. Citation2020b). Many properties of the aging microbiome were identified, some of which may underlie such predictors. For example, with aging, there is an increase in the Firmicutes to Bacteroides ratio (F/B ratio) that resembles the pattern seen in the obese. In particular, Vaiserman et al. have recently shown, in a cross-sectional study that involved 1550 healthy participants, that the F/B ratio increases gradually from childhood to old age (Vaiserman et al. Citation2020).
Nutritional effects on the microbiome and the microbiome-host interaction
Rodríguez-Morató et al. (Citation2018) have recently shown through a randomized, placebo-controlled, double-blind, crossover study involving 11 young and healthy subjects that an animal-based diet leads to a pronounced increase in the F/B ratio mentioned above. Moreover, the animal-based diet led to a decrease in stool short-chain fatty acids and an increase in bile acids. These changes were attenuated by the daily supplementation of 30 grams of freeze-dried whole cranberry powder (Rodríguez-Morató et al. Citation2018). This is evidence of the possibility of rejuvenating the F/B ratio through the addition of dietary geroprotectors.
During youth, the coupling between the human body and the microbiome is highly functional, but during aging, a slow but steady uncoupling is observed (Bauer, Rees, and Finlay Citation2019; Esser et al. Citation2019; Santoro et al. Citation2020b). This is exemplified by the age-related loss of tryptophan and indole production by the microbiome that has recently been reported (Ruiz-Ruiz et al. Citation2020). Further, Zhu et al. (Citation2020) have shown, in a small controlled crossover trial involving ten young and healthy subjects, that a short four day Mediterranean diet intervention can lead to a significant increase in fecal tryptophan metabolites accompanied by an increase in short chain fatty-acid producing bacteria belonging to the Lachnospiraceae family or the genus Butyricicoccus (Zhu et al. Citation2020). Also, Ulaszewska et al. have shown through a randomized crossover trial involving 40 mildly hypercholesterolemic subjects, that bacterial tryptophan synthesis and metabolism could be increased by daily intake of two apples every day for eight weeks (Ulaszewska et al. Citation2020). There is also evidence, from animal models, that tryptophan metabolism is essential for the maintenance of colonic barrier integrity and the prevention of inflammaging (Gao et al. Citation2018; Powell et al. Citation2020; Shimada et al. Citation2013; Taleb Citation2019). Many tryptophan metabolites interact with the aryl hydrocarbon receptor (AHR), an important player in the maintenance and differentiation of the stem cells of the gut and in the activity of resident immune cells (Sun et al. Citation2020).
Short chain fatty acids (SCFA) are another important metabolite produced and secreted by the gut microbiota, whose levels decrease during aging; in particular these are acetate, butyrate and propionate (Salazar et al. 2019). SCFA can improve gut barrier integrity, enter the circulation, and have various immuno-modulatory effects that are protective against age-related diseases (Morrison and Preston Citation2016). Since the gut microbiome produces SCFA from plant components that are still undigested (or partially digested), there are many possible avenues to accelerate their production. The simple addition of fiber-rich foods can increase the abundance of SCFA producing species. For example, Holscher et al. (Citation2018) showed that a 3 week intervention that consisted of the daily supplementation of 41 g of walnuts to 18 healthy men and women with “normal” diets, led to a decrease in fecal content of bile acids and a concomitant increase in the relative abundances of butyrate-producing Firmicutes species. These changes were accompanied by a decrease in circulating LDL cholesterol and the non-cholesterol sterol campesterol (Holscher et al. Citation2018). On the other hand, the levels of the microbial-derived metabolite Trimethylamine N-oxide (TMAO), a factor possibly contributing to the pathogenesis of cardiovascular disease and dementia, tend to be higher in older adults (Brunt et al. Citation2020). Wu et al. (Citation2020) created a predictor of TMAO production that was based on the presence of Emergencia Timonensis or Ihubacter Massiliensis in the feces of human subjects. This predictor could distinguish high TMAO producers from low producers with 43% sensitivity and 97% specificity. A high producer was defined as an individual that shows high plasma levels of TMAO after the intake of carnitine, an amino acid that is highly present in red meat (Wu et al. Citation2020).
Thus, the biomarkers useful to evaluate dietary geroprotection based on investigating microbiomes range from “black-box” predictors based on deep learning, to the more specific F/B ratio. Taxa that produce tryptophan, indole, short-chain fatty acids, bile acids, and TMAO may also give rise to candidate biomarkers.
Nutritional effects are influenced by the gut microbiome
Microbiome-related biomarkers may also be used to identify, a priori, who would benefit from dietary geroprotection. The geroprotective activity of many polyphenols is partially explained by their microbiota-mediated conversion into bioavailable metabolites (Kawabata, Yoshioka, and Terao Citation2019). This is exemplified by the response to the consumption of isoflavones and ellagic acid derivatives, which differs between equol and urolithin producers and non-producers, respectively. Three groups exist based on their microbiome’s ability to convert ellagic acid into urolithins: Urolithin-A metabotypes, who can convert ellagic acid into Urolithin A, Urolithin-B metabotypes, who can convert it into both Urolithin-A and Urolithin-B, and Urolithin-0 metabotypes, who are unable to convert it into either. Regarding equol, two groups exist: producers and non-producers.
García-Mantrana et al. (Citation2019) have shown that the microbiome of Urolithin-B metabotypes is more sensitive to modulation after short-term walnut consumption. Specifically, an increase in Blautia, Bifidobacterium, and members of the Coriobacteriaceae family was seen only in Urolithin-B metabotypes (García-Mantrana et al. Citation2019). González-Sarrias et al. (Citation2017) have also shown that Urolithin-B metabotypes are the only ones that responded to the consumption of pomegranate extract in their double-blind, crossover, dose–response, randomized, placebo-controlled trial of 50 participants. Specifically, a change in various clinical biomarkers of cardiovascular health was seen in Urolithin-B metabotypes, with decreases in total cholesterol, LDL-cholesterol, small LDL-cholesterol, non-HDL-cholesterol, apolipoprotein-B and oxidized LDL (González-Sarrías et al. Citation2017). Regarding equol, Zheng et al. (Citation2019) have shown that both content and metabolic activity of the gut flora differed between equol producers and non-producers. Furthermore, equol producers showed lower prevalences of dyslipidemia, which suggests the important role that equol might play in lipid metabolism related to the gut microbiota (Zheng et al. Citation2019).
The microbiome can be informative both before dietary geroprotection, as a way to select or stratify study participants based on their microbiome’s metabolism of the nutrients under investigation, and after dietary geroprotection, as a way to analyze the beneficial effects in more detail. In addition, since host genetics also plays a role in the microbiome-related effects of diet, it will be interesting to combine genetics, microbiomics, and clinical outcomes in future studies.
Clinical biomarkers
Commonly administered clinical laboratory tests may give rise to biomarkers of aging that are based on highly standardized measurement procedures, easily available, and often cheaper than the other types of biomarker signatures discussed here (Hartmann et al. Citation2021). The most recent biomarker signatures (“clocks”) originating from laboratory tests are based on deep learning (Putin et al. Citation2016; Ashiqur Rahman et al. Citation2021; Galkin et al. Citation2020a), while some older ones, such as the “Phenotypic Age” (Liu et al. Citation2018; Levine Citation2013) were based on multiple linear regression (Voitenko and Tokar Citation1983), principal component analysis (Jia et al. Citation2016), Hochschild’s method (Hochschild Citation1989), or Klemera and Doubal’s method (Klemera and Doubal Citation2006). Of note, the latter set of methods tends to be specific for the small sets of measurements usually established in the laboratory, while deep learning and regression are also employed to learn epigenetics, transcriptomics and microbiome-based biomarker signatures.
Clinical biomarker-based clocks were used to judge the effects of caloric restriction (CR). In particular, the “Klemera-Doubal Biological Age” (KD age), calculated from the CALERIE trial blood chemistry and blood pressure data following the method of Klemera and Doubal, can be impacted by two years of CR (Belsky et al. Citation2020). The slowdown of predicted age was especially evident in subjects with a higher baseline KD age; within two years, they showed a standstill in KD age when put on CR, while the control group aged by two years. Since many natural compounds have CR-mimetic effects, it is plausible that KD age is suitable for assessing some approaches to dietary geroprotection (Martel et al. Citation2019; Yessenkyzy et al. 2020).
Nutritional effects on clinical biomarkers
Many clinical biomarkers reflect established cardiovascular risk factors such as lipid profiles (LDL and HDL cholesterol, triglycerides), high blood pressure and obesity, but also emerging risk factors such as high homocysteine and low vitamin D levels, as well as CRP, other inflammation-related cytokine/chemokine levels, glucose metabolism and oxidative stress, and all these can be influenced substantially by dietary geroprotection (Carrizzo et al. Citation2020; Unuofin and Lebelo 2020; Zhou et al. Citation2021). More specifically, high LDL and low HDL cholesterol levels are common among the elderly, often reflecting metabolic syndrome (Walter Citation2009), and high LDL-cholesterol levels are significantly associated with incident cardiovascular disease in both middle and old age (Félix-Redondo, Grau, and Fernández-Bergés Citation2013; Lind et al. Citation2018; Zhang et al. Citation2020a). Moreover, overall, human aging is associated with a rising incidence of insulin resistance and type 2 diabetes (Cheng et al. Citation2013; Creatore et al. Citation2010; The DECODA Study Group Citation2003), and insulin as well as hemoglobin A1c and glucose measurements were all shown to be improved by diet (Castro-Barquero et al. Citation2020), also see .
Table 1. Effects of berries on clinical biomarkers, based on systematic reviews and meta-analyses.
Table 2. Glossary.
Berries for dietary geroprotection
We chose to specifically focus on the effects of berries and their constituents, such as anthocyanins, as an example of dietary geroprotection. Berries are a widely consumed food, and they can be added easily to one’s diet. They can be eaten fresh, avoiding the loss of nutritional value caused by processing (Nayak, Liu, and Tang Citation2015). All of the widely consumed berries (such as strawberries, blueberries, raspberries, and cranberries) represent a hypocaloric source of fiber and polyphenols. The various classes of fiber, including cellulose, hemicellulose, arabinans, and arabino-xyloglucans that are present in berries are also implicated in their beneficial properties (Rodríguez-Daza et al. Citation2020). Putative beneficial properties of the polyphenol flavonoid fisetin were mostly derived from animal studies. For example, fisetin reduced cellular senescence markers in multiple tissues in mice, effects that are often comparable to the more often studied dasatinib plus quercetin combination (Yousefzadeh et al. Citation2018). Also, procyanidin C1, a polyphenolic compound common to various berries and specifically found in grape seed extract, is believed to act as an anti-aging dietary factor in mice (Xu et al. 2021, BioRxiv. doi: 10.1101/2021.04.14.439765). More generally, polyphenols such as anthocyanins were shown to exhibit anti-inflammatory properties (Hidalgo et al. Citation2012) and to be promising candidates for the amelioration of obesity and age-related inflammation (Joseph, Edirisinghe, and Burton-Freeman Citation2014; Tsuda Citation2016; Azzini, Giacometti, and Russo Citation2017; Land Lail et al. 2021). The positive impact of berries on health is usually explained by their constituent polyphenols as well as the metabolites thereof, which can enter into the circulation and exert anti-inflammatory effects. Further below, we will refer to the extensive literature regarding studies of berry effects in humans, often involving biomarkers of inflammation.
Referring to a notable combination of in-vivo with in-vitro work in human, Rutledge et al. (Citation2019) recently showed that the serum of subjects supplemented with either strawberry or blueberry powder can downregulate inflammatory signaling in vitro. Their study also hinted at a cumulative effect of berry polyphenols, as the sera of the berry consumers were more anti-inflammatory after 90 days of intervention than after 45 days; the sera kept their anti-inflammatory potential when the participants were in a fasted state. Rodriguez-Mateos et al. (Citation2019) also found evidence for the importance of circulating anthocyanin metabolites. Their study, consisting of a one-month-long wild blueberry intervention, showed that of the 63 circulating anthocyanin metabolites under scrutiny, 14 correlated with acute flow-mediated dilation (FMD) improvements, and 21 with chronic FMD improvements. Berries and berry fractions can also shape the microbiome away from the dysbiotic states typical to obesity, as shown recently by Rodríguez-Daza et al. (Citation2020) in a mouse model of high fat diet-induced obesity. Also, the polyphenols contained in berries can synergize with each other. For example, Nichols et al. (Citation2015) have shown that epicatechin and quercetin, two common polyphenols in berries, have superior neuroprotective properties than epicatechin or quercetin alone when used in a mouse model of hypoxic-ischemic brain injury. As for the mechanisms involved in the health-protecting effects of berry polyphenols, hormesis (Hayer Citation2007; Franco, Navarro, and Martínez-Pinilla Citation2019) is one more recent focus. Polyphenols such as anthocyanins can stimulate NRF2-mediated antioxidant gene expression and augment cellular and organismal resistance to stressors, either internal, such as metabolic stress and aging, or external, such as pollutants and UV radiation (Son, Camandola, and Mattson Citation2008; Leri et al. Citation2020). In fact, there is evidence that anthocyanins and oligomeric procyanidins protect the skin against UV-B damage (Li et al. Citation2019, Saliou et al. Citation2001), that they lower systemic oxidative stress (Ullah et al. Citation2019), and that they protect against various kinds of environmental pollutants (Lagoa et al. Citation2020).
Since there is abundant data about the favorable effects of berries on human physiology, we decided to summarize the recent (from 2015 up until today) systematic reviews and meta-analyses on human dietary berry and anthocyanin interventions and the biomarker effects observed, see . These meta-studies covered 144 different original studies, with an average coverage of only 14% of these original studies by any one meta-analysis (see in supplementary material). One of the most important observations among the various meta-studies is that berries have more potential to modulate a given biomarker when its level is not in its optimal range at baseline. For example, Gao et al. (Citation2020) reported that LDL and TC levels decreased after berry intake only in subjects with higher baseline levels. Yang et al. (Citation2017) reported a positive decrease in the HOMA-IR index after berry intake only in overweight and obese subjects. Wallace, Slavin, and Frankenfeld (Citation2016) reported a similar observation for LDL, with a decrease seen only in subjects suffering from diseases related to obesity. Another frequent observation is that berry-based interventions were effective only above a certain dosage and in studies of sufficient duration. Rahmani et al. (Citation2019) reported a decrease in TAGs only for doses higher than 300 mg/day, Fallah, Sarmast, and Jafari (Citation2020) reported more significant decreases in FBG, 2-h PPG, HbA1c, and HOMA-IR for studies that had a duration of eight weeks or more and dosages higher than 300 mg/day, Daneshzad et al. (Citation2019) reported significant decreases in TC and LDL only for doses of anthocyanins higher than 300 mg/day and durations of 12 weeks or more, Fallah et al. (Citation2020) reported that doses of anthocyanins higher than 300 mg/day are more effective at decreasing the levels of CRP, IL-6, TNF-α, and VCAM-1, and Yang et al. (Citation2017) reported stronger reductions in HbA1c and TAGs levels with doses of anthocyanins higher than 400 mg/d.
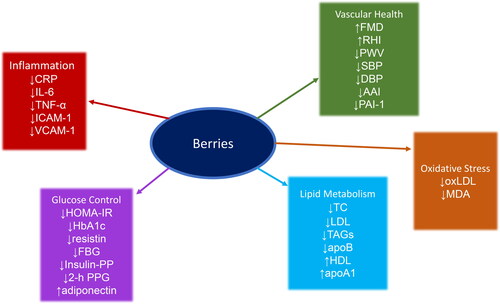
Most of the reviews we investigated did not focus on any single berry but covered various berries, anthocyanin-rich foods, and extracts. One meta-analysis, the one by Fairlie-Jones et al. (2017), also covered the effects of purified anthocyanins. Gao et al. (Citation2020) and Hadi et al. (Citation2019) focused on strawberries, be they fresh, frozen, freeze-dried, or in beverage form. Rahmani et al. (Citation2019) focused specifically on Aronia berries, while Rocha et al. (Citation2019) focused on blueberries and cranberries. Regarding the types of cohorts, most of the reviews covered heterogeneous populations, characterized by both healthy and diseased subjects. The most common diseases among the study cohorts were related to metabolic syndrome, such as type 2 diabetes, dyslipidemia, pre-hypertension, and cardiovascular disease. Rocha et al. (Citation2019) were the only ones to focus exclusively on subjects suffering from type 2 diabetes. The biomarkers covered by the reviews span five main biological processes: inflammation, vascular health, oxidative stress, lipid metabolism, and glucose control (see ). No difference was evident between the various types of berries in their ability to modulate the various biomarker classes. This could be because of the similarities between their constitutive polyphenols, often belonging to the anthocyanin class. Also, as reported in the first part of this section, berry polyphenols may often confer their protective effect in a similar manner, specifically by stimulating NRF2 activity. This observation could also be prevalent because of an investigator bias toward stable, validated biomarkers of a limited set of pathologies known to be influenced by diet, such as cardiovascular disease. Nonetheless, the fact that clinical biomarkers related to aging and disease can be influenced by berry consumption is instructive for future studies of dietary geroprotection. In any case, clinical measures should accompany transcriptomic, epigenetic, and microbiomic biomarkers to have a full-spectrum view on the beneficial effects of dietary geroprotection. A summary of the effects of berries and anthocyanins on human physiology is thus given in . The detailed results are given in . See the glossary () for abbreviations and acronyms.
Box 3: Nutribiography.
Following the notion of an “immunobiography” (Franceschi et al. Citation2017), we suggest that the term “nutribiography” describes the combination of type, dose, frequency and temporal sequence of nutrients that an individual has been exposed to in their lifetime.
As the most straightforward example, malnutrition can cause non-optimal long-term outcomes, characterized by a heightened risk of diseases related, e.g., to metabolic syndrome. Subjects exposed in utero to the “Dutch Hunger Winter” provide a rather extreme case. Two different responses were observed: subjects exposed during early gestation were born with an overall healthy phenotype. In contrast, subjects exposed during late gestation were born underweight. However, during later life, the former experienced a heightened risk of cardiovascular disease, an altered lipid profile, and a propensity to obesity, while the latter were metabolically healthier and leaner than the normal population (Schulz Citation2010). Epigenetic changes of, e.g., insulin signaling were reported as underlying causes (Tobi et al. Citation2014). More subtle differences, such as maternal deficiencies in vitamins and minerals, can also impact the child’s future risk of developing non-communicable diseases (Langley-Evans Citation2015). Various other aspects of diet can affect the epigenome. In particular, polyphenols are known to influence the epigenetic machinery, so that subjects with a high lifelong intake of polyphenols have an epigenetic memory of their intake (Arora et al. Citation2020; Leri et al. Citation2020). It will be interesting to see if people with a higher lifelong polyphenolic intake appear epigenetically younger compared to same age peers, even during periods of lower intake.
Another nutribiographic phenomenon is the “obesogenic memory,” a propensity to hyperphagia and weight gain in ex-obese individuals, potentially triggering Yo-Yo dieting cycles (Contreras, Schriever, and Pfluger Citation2019). Persistent changes of the gut microbiome induced by obesity may play a role in obesogenic memory. Accordingly, Fragiadakis et al. showed, in a subset of participants (n = 49) of the DIETFITS weight-loss trial, that the human microbiome, once set, can be resilient to diet-induced changes. They observed significant diet-induced changes at three months relative to baseline. However, the subjects’ microbiome returned to its pre-intervention state throughout the study’s later phases (six months, nine months, twelve months), in individuals consuming both a low-fat and a low-carbohydrate diet, despite adherence to the dietary guidelines and decreasing weight throughout the study (Fragiadakis et al. Citation2020). Thaiss et al. showed an obesogenic memory also in the microbiome of mice (Thaiss et al. Citation2016). The obesogenic microbiome of the mice decreased the bioavailability of the flavonoids apigenin and naringenin, but combined supplementation with both flavonoids normalized intestinal apigenin and naringenin levels and ameliorated the rate of secondary weight regain. Thus, dietary geroprotection can work despite a disadvantageous nutribiography.
As stated in the paragraph dedicated to the microbiome, equol and urolithin metabolizers can benefit considerably from consuming soy isoflavones and ellagitannins, respectively. However, apart from genetic factors, past dietary habits have also a strong influence on such traits; for example, diets rich in daidzein are positively correlated with equol producer status (Iino et al. Citation2019), and a month-long intervention with an ellagitannins-rich supplement can convert non-producers of urolithin into producers (González-Sarrías et al. Citation2017). Similarly, more short-chain fatty acids (acetate, butyrate and propionate) are produced from fiber by vegetarians and vegans than by habitual meat-eaters (Sheflin et al. Citation2017; Tomova et al. Citation2019). An individual’s nutribiography can thus influence their response to dietary geroprotection through differences in their microbiome or epigenome.
Conclusions
There is ample evidence available to state that aging-associated processes such as inflammation can be impacted by dietary interventions, throughout the omics spectrum, and, as known for a long time, in standard lipid and inflammatory cytokine/chemokine profiles. The sensitivity and specificity of many of the resulting biomarker signatures are dependent on an array of factors, such as the timing of the measurements, the strength of the intervention, and the study cohort, and sensitivity is often higher when the biomarkers are measured following an intervention with a higher dose, or in a population suffering from age-related diseases, as compared to the healthy elderly.
Some biomarkers are particularly suited for trial cohort pre-selection, while others for judging the effects of dietary geroprotection. Genetics can be employed specifically in the pre-selection phase, microbiomic and clinical biomarkers can be used for both cohort pre-selection and evaluation, while epigenetic and transcriptomic biomarker signatures work best to evaluate outcomes, affording a chance to understand the mechanisms underlying the effects observed. The development of clocks created through deep learning could also be a valuable tool for the evaluation of dietary geroprotection; new approaches to interpretable machine learning can provide further mechanistic insights.
As a suitable candidate for dietary geroprotection, we have chosen berries. Thus we summarized the recent systematic reviews and meta-analyses in terms of berry-related interventions. Furthermore we report biomarkers to be significantly changed in response to berry intake. We expect those biomarkers to be suitable candidates for future dietary geroprotection studies.
Finally, we suggest that the response of an individual to a diet in general, and dietary geroprotectors in particular, partly depends on the food they have been exposed to in the past. This "nutribiography," a term inspired by "immunobiography" (Franceschi et al. Citation2017), is thus defined by the combination of type, dose, frequency, and temporal sequence of nutrition that an individual was exposed to over their lifetime. Therefore, the “nutribiography” should be investigated in more depth in future studies in the context of dietary geroprotection, see Box 3.
Disclosure statement
HJG has received travel grants and speakers honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag as well as research funding from Fresenius Medical Care. None of the other authors report any relevant financial or non-financial competing interest.
Additional information
Funding
References
- Abdullah, M. M. H., I. Vazquez-Vidal, D. J. Baer, J. D. House, P. J. H. Jones, and C. Desmarchelier. 2021. Common genetic variations involved in the inter-individual variability of circulating cholesterol concentrations in response to diets: A narrative review of recent evidence. Nutrients 13:695. doi: 10.3390/nu13020695.
- Arora, I., M. Sharma, L. Y. Sun, and T. O. Tollefsbol. 2020. The epigenetic link between polyphenols, aging and age-related diseases. Genes (Basel) 11:1094. doi: 10.3390/genes11091094.
- Arpón, A., J. I. Riezu-Boj, F. I. Milagro, A. Marti, C. Razquin, M. A. Martínez-González, D. Corella, R. Estruch, R. Casas, M. Fitó, et al. 2016. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. Journal of Physiology and Biochemistry 73 (3):445–55. doi: 10.1007/s13105-017-0552-6.
- Ashiqur Rahman, S., P. Giacobbi, L. Pyles, C. Mullett, G. Doretto, and D. A. Adjeroh. 2021. Deep learning for biological age estimation. Briefings in Bioinformatics 22 (2):1767–81. doi: 10.1093/bib/bbaa021.
- Azzini, E., J. Giacometti, and G. L. Russo. 2017. Antiobesity Effects of Anthocyanins in Preclinical and Clinical Studies. Oxidative Medicine and Cellular Longevity 2017:2740364. doi: 10.1155/2017/2740364.
- Baker, G. T., and R. L. Sprott. 1988. Biomarkers of aging. Experimental Gerontology 23 (4-5):223–29. doi: 10.1016/0531-5565(88)90025-3.
- Bale, S., G. Pulivendala, and C. Godugu. 2018. Withaferin A attenuates bleomycin-induced scleroderma by targeting FoxO3a and NF-κβ signaling: Connecting fibrosis and inflammation. BioFactors (Oxford, England) 44 (6):507–17. doi: 10.1002/biof.1446.
- Barrera-Reyes, P. K., N. Hernández-Ramírez, J. Cortés, L. Poquet, K. Redeuil, C. Rangel-Escareño, M. Kussmann, I. Silva-Zolezzi, and M. E. Tejero. 2019. Gene expression changes by high-polyphenols cocoa powder intake: A randomized crossover clinical study. European Journal of Nutrition 58 (5):1887–98. doi: 10.1007/s00394-018-1736-8.
- Bauer, K. C., T. Rees, and B. B. Finlay. 2019. The gut microbiota-brain axis expands neurologic function: A nervous rapport. BioEssays 41 (10):e1800268. doi: 10.1002/bies.201800268.
- Bell, C. G., R. Lowe, P. D. Adams, A. A. Baccarelli, S. Beck, J. T. Bell, B. C. Christensen, V. N. Gladyshev, B. T. Heijmans, S. Horvath, et al. 2019. DNA methylation aging clocks: Challenges and recommendations. Genome Biology 20 (1):249. doi: 10.1186/s13059-019-1824-y.
- Belsky, D. W., A. Caspi, L. Arseneault, A. Baccarelli, D. L. Corcoran, X. Gao, E. Hannon, H. Lee Harrington, L. J. Rasmussen, R. Houts, et al. 2020. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife 9:e54870. doi: 10.7554/eLife.54870.
- Belsky, D. W., K. M. Huffman, C. F. Pieper, I. Shalev, W. E. Kraus, and R. Anderson. 2017. Change in the rate of biological aging in response to caloric restriction: Calerie Biobank analysis. The Journals of Gerontology, Series A 73 (1):4–10. doi: 10.1093/gerona/glx096.
- Bhullar, K. S., and B. P. Hubbard. 2015. Lifespan and healthspan extension by resveratrol. Biochimica et Biophysica Acta 1852 (6):1209–18. doi: 10.1016/j.bbadis.2015.01.012.
- Boesch-Saadatmandi, C., S. Wolffram, A. M. Minihane, and G. Rimbach. 2009. Effect of apoE genotype and dietary quercetin on blood lipids and TNF-alpha levels in apoE3 and apoE4 targeted gene replacement mice. The British Journal of Nutrition 101 (10):1440–3. doi: 10.1017/S0007114508102434.
- Bouwens, M., O. van de Rest, N. Dellschaft, M. G. Bromhaar, L. C. P. G. M. de Groot, J. M. Geleijnse, M. Müller, and L. A. Afman. 2009. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. The American Journal of Clinical Nutrition 90 (2):415–24. doi: 10.3945/ajcn.2009.27680.
- Brunt, V. E., R. A. Gioscia-Ryan, A. G. Casso, N. S. Vandongen, B. P. Ziemba, Z. J. Sapinsley, J. J. Richey, M. C. Zigler, A. P. Neilson, K. P. Davy, et al. 2020. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76 (1):101–12. doi: 10.1161/HYPERTENSIONAHA.120.14759.
- Budovsky, A., T. Craig, J. Wang, R. Tacutu, A. Csordas, J. Lourenço, V. E. Fraifeld, and J. P. de Magalhães. 2013. LongevityMap: A database of human genetic variants associated with longevity. Trends in Genetics: TIG 29 (10):559–60. doi: 10.1016/j.tig.2013.08.003.
- Burton, K. J., G. Pimentel, N. Zangger, N. Vionnet, J. Drai, P. G. McTernan, F. P. Pralong, M. Delorenzi, and G. Vergères. 2018. Modulation of the peripheral blood transcriptome by the ingestion of probiotic yoghurt and acidified milk in healthy, young men. PLoS One 13 (2):e0192947. doi: 10.1371/journal.pone.0192947.
- Campisi, J., and L. Robert. 2014. Cell senescence: Role in aging and age-related diseases. In Aging: Facts and Theories, vol. 39, 45–61. Basel, Switzerland: Karger.
- Carrizzo, A., C. Izzo, M. Forte, E. Sommella, P. Di Pietro, E. Venturini, M. Ciccarelli, G. Galasso, S. Rubattu, P. Campiglia, et al. 2020. A novel promising Frontier for human health: The beneficial effects of nutraceuticals in cardiovascular diseases. International Journal of Molecular Sciences. 21:8706. doi: 10.3390/ijms21228706.
- Castro-Barquero, S., A. M. Ruiz-León, M. Sierra-Pérez, R. Estruch, and R. Casas. 2020. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 12:2983. doi: 10.3390/nu12102983.
- Chen, B. H., R. E. Marioni, E. Colicino, M. J. Peters, C. K. Ward-Caviness, P. C. Tsai, N. S. Roetker, A. C. Just, E. W. Demerath, W. Guan, et al. 2016. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging 8 (9):1844–65. doi: 10.18632/aging.101020.
- Cheng, Y. J., G. Imperatore, L. S. Geiss, J. Wang, S. H. Saydah, C. C. Cowie, and E. W. Gregg. 2013. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988–2010. Diabetes Care 36 (9):2690–6. doi: 10.2337/dc12-2074.
- Cho, Y. H., H. D. Woo, Y. Jang, V. Porter, S. Christensen, R. F. Hamilton, and H. W. Chung. 2015. The association of LINE-1 hypomethylation with age and centromere positive micronuclei in human lymphocytes. PLoS One 10 (7):e0133909. doi: 10.1371/journal.pone.0133909.
- Contreras, R. E., S. C. Schriever, and P. T. Pfluger. 2019. Physiological and epigenetic features of yoyo dieting and weight control. Frontiers in Genetics 10:1015. doi: 10.3389/fgene.2019.01015.
- Cornelis, M. C., E. M. Byrne, T. Esko, M. A. Nalls, A. Ganna, N. Paynter, K. L. Monda, N. Amin, K. Fischer, F. Renstrom, Coffee and Caffeine Genetics Consortium, et al. 2015. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Molecular Psychiatry 20 (5):647–56. doi: 10.1038/mp.2014.107.
- Creatore, M. I., R. Moineddin, G. Booth, D. H. Manuel, M. DesMeules, S. McDermott, and R. H. Glazier. 2010. Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ: Canadian Medical Association Journal = Journal de L’Association Medicale Canadienne 182 (8):781–9. doi: 10.1503/cmaj.091551.
- Daneshzad, E., S. Shab-Bidar, Z. Mohammadpour, and K. Djafarian. 2019. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition (Edinburgh, Scotland) 38 (3):1153–65. doi: 10.1016/j.clnu.2018.06.979.
- De Cecco, M., T. Ito, A. P. Petrashen, A. E. Elias, N. J. Skvir, S. W. Criscione, A. Caligiana, G. Brocculi, E. M. Adney, J. D. Boeke, et al. 2019. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566 (7742):73–8. doi: 10.1038/s41586-018-0784-9.
- Dean, L. 2012. Methylenetetrahydrofolate reductase deficiency. Medical Genetics Summaries.
- Deelen, J., D. S. Evans, D. E. Arking, N. Tesi, M. Nygaard, X. Liu, M. K. Wojczynski, M. L. Biggs, A. van der Spek, G. Atzmon, et al. 2019. A meta-analysis of genome-wide association studies identifies multiple longevity genes. Nature Communications 10 (1):3669.
- Egert, S., and G. Rimbach. 2011. Which sources of flavonoids: Complex diets or dietary supplements?Advances in Nutrition (Bethesda, MD) 2 (1):8–14. doi: 10.3945/an.110.000026.
- Esser, D., J. M. Geleijnse, J. C. Matualatupauw, J. I. Dower, D. Kromhout, P. C. H. Hollman, and L. A. Afman. 2018. Pure flavonoid epicatechin and whole genome gene expression profiles in circulating immune cells in adults with elevated blood pressure: A randomised double-blind, placebo-controlled, crossover trial. PLoS One 13 (4):e0194229. doi: 10.1371/journal.pone.0194229.
- Esser, D., J. Lange, G. Marinos, M. Sieber, L. Best, D. Prasse, J. Bathia, M. C. Rühlemann, K. Boersch, C. Jaspers, et al. 2019. Functions of the microbiota for the physiology of animal metaorganisms. Journal of Innate Immunity 11 (5):393–404. doi: 10.1159/000495115.
- Fairlie-Jones, L., K. Davison, E. Fromentin, and A. M. Hill. 2017. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 9:908. doi: 10.3390/nu9080908.
- Fallah, A. A., E. Sarmast, P. Fatehi, and T. Jafari. 2020. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food and Chemical Toxicology 135:110922. doi: 10.1016/j.fct.2019.110922.
- Fallah, A. A., E. Sarmast, and T. Jafari. 2020. Effect of dietary anthocyanins on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Food Research International 137:109379. doi: 10.1016/j.foodres.2020.109379.
- Farmer, B. C., L. A. Johnson, and A. J. Hanson. 2019. Effects of apolipoprotein e on nutritional metabolism in dementia. Current Opinion in Lipidology 30 (1):10–5.
- Félix-Redondo, F. J., M. Grau, and D. Fernández-Bergés. 2013. Cholesterol and cardiovascular disease in the elderly. Facts and gaps. Aging and Disease 4 (3):154–69.
- Fergus, L., K. Seals, and D. Holston. 2021. Nutrition interventions in low-income rural and urban retail environments: A systematic review. Journal of the Academy of Nutrition and Dietetics 121 (6):1087–114. doi: 10.1016/j.jand.2020.12.018.
- Ferrari, L., M. Vicenzi, L. Tarantini, F. Barretta, S. Sironi, A. A. Baccarelli, M. Guazzi, and V. Bollati. 2019. Effects of physical exercise on endothelial function and DNA methylation. International Journal of Environmental Research and Public Health 16:2530. doi: 10.3390/ijerph16142530.
- Ferri, E., M. Casati, M. Cesari, G. Vitale, and B. Arosio. 2019. Vitamin D in physiological and pathological aging: Lesson from centenarians. Reviews in Endocrine & Metabolic Disorders 20 (3):273–82. doi: 10.1007/s11154-019-09522-y.
- Field, A. E., N. A. Robertson, T. Wang, A. Havas, T. Ideker, and P. D. Adams. 2018. DNA methylation clocks in aging: Categories, causes, and consequences. Molecular Cell 71 (6):882–95. doi: 10.1016/j.molcel.2018.08.008.
- Fitzgerald, K. N., R. Hodges, D. Hanes, E. Stack, D. Cheishvili, M. Szyf, J. Henkel, M. W. Twedt, D. Giannopoulou, J. Herdell, et al. 2021. Potential reversal of epigenetic age using a diet and lifestyle intervention: A pilot randomized clinical trial. Aging 13 (7):9419–32. doi: 10.18632/aging.202913.
- Flachsbart, F., J. Dose, L. Gentschew, C. Geismann, A. Caliebe, C. Knecht, M. Nygaard, N. Badarinarayan, A. ElSharawy, S. May, et al. 2017. Identification and characterization of two functional variants in the human longevity gene FOXO3. Nature Communications 8 (1):2063.
- Fleischer, J. G., R. Schulte, H. H. Tsai, S. Tyagi, A. Ibarra, M. N. Shokhirev, L. Huang, M. W. Hetzer, and S. Navlakha. 2018. Predicting age from the transcriptome of human dermal fibroblasts. Genome Biology 19 (1):221. doi: 10.1186/s13059-018-1599-6.
- Fraga, C. G., K. D. Croft, D. O. Kennedy, and F. A. Tomás-Barberán. 2019. The effects of polyphenols and other bioactives on human health. Food & Function 10 (2):514–28. doi: 10.1039/c8fo01997e.
- Fragiadakis, G. K., H. C. Wastyk, J. L. Robinson, E. D. Sonnenburg, J. L. Sonnenburg, and C. D. Gardner. 2020. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. The American Journal of Clinical Nutrition 111 (6):1127–36. doi: 10.1093/ajcn/nqaa046.
- Franceschi, C., P. Garagnani, F. Olivieri, S. Salvioli, and C. Giuliani. 2020. The contextualized genetics of human longevity: JACC focus seminar. Journal of the American College of Cardiology 75 (8):968–79. doi: 10.1016/j.jacc.2019.12.032.
- Franceschi, C., P. Garagnani, P. Parini, C. Giuliani, and A. Santoro. 2018. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nature Reviews. Endocrinology 14 (10):576–90. doi: 10.1038/s41574-018-0059-4.
- Franceschi, C., S. Salvioli, P. Garagnani, M. de Eguileor, D. Monti, and M. Capri. 2017. Immunobiography and the heterogeneity of immune responses in the elderly: A focus on inflammaging and trained immunity. Frontiers in Immunology 8:982. doi: 10.3389/fimmu.2017.00982.
- Francis, M., C. Li, Y. Sun, J. Zhou, X. Li, J. T. Brenna, and K. Ye. 2021. Genome-wide association study of fish oil supplementation on lipid traits in 81,246 individuals reveals new gene-diet interaction loci. PLOS Genetics 17 (3):e1009431. doi: 10.1371/journal.pgen.1009431.
- Franco, S. S., L. De Falco, S. Ghaffari, C. Brugnara, D. A. Sinclair, A. Matte, A. Iolascon, N. Mohandas, M. Bertoldi, X. An, et al. 2014. Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica 99 (2):267–75. doi: 10.3324/haematol.2013.090076.
- Franco, R., G. Navarro, and E. Martínez-Pinilla. 2019. Hormetic and mitochondria-related mechanisms of antioxidant action of phytochemicals. Antioxidants (Basel) 8:373. doi: 10.3390/antiox8090373.
- Fuellen, G., O. Liesenfeld, A. Kowald, I. Barrantes, M. Bastian, A. Simm, L. Jansen, A. Tietz-Latza, D. Quandt, C. Franceschi, et al. 2020. The preventive strategy for pandemics in the elderly is to collect in advance samples & data to counteract chronic inflammation (inflammaging). Ageing Research Reviews 62:101091. doi: 10.1016/j.arr.2020.101091.
- Fuellen, G., L. Jansen, A. A. Cohen, W. Luyten, M. Gogol, A. Simm, N. Saul, F. Cirulli, A. Berry, P. Antal, et al. 2019. Health and aging: Unifying concepts, scores, biomarkers and pathways. Aging and Disease 10 (4):883–900. doi: 10.14336/AD.2018.1030.
- Galkin, F., P. Mamoshina, A. Aliper, J. P. de Magalhães, V. N. Gladyshev, and A. Zhavoronkov. 2020a. Biohorology and biomarkers of aging: Current state-of-the-art, challenges and opportunities. Ageing Research Reviews 60:101050. doi: 10.1016/j.arr.2020.101050.
- Galkin, F., P. Mamoshina, A. Aliper, E. Putin, V. Moskalev, V. N. Gladyshev, and A. Zhavoronkov. 2020b. Human gut microbiome aging clock based on taxonomic profiling and deep learning. IScience 23 (6):101199. doi: 10.1016/j.isci.2020.101199.
- Gao, Q., L. Q. Qin, A. Arafa, E. S. Eshak, and J. Y. Dong. 2020. Effects of strawberry intervention on cardiovascular risk factors: A meta-analysis of randomised controlled trials. The British Journal of Nutrition 124 (3):241–6. doi: 10.1017/S000711452000121X.
- Gao, J., K. Xu, H. Liu, G. Liu, M. Bai, C. Peng, T. Li, and Y. Yin. 2018. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Frontiers in Cellular and Infection Microbiology 8:13. doi: 10.3389/fcimb.2018.00013.
- García-Lacarte, M., F. I. Milagro, M. A. Zulet, J. A. Martinez, and M. L. Mansego. 2016. LINE-1 methylation levels, a biomarker of weight loss in obese subjects, are influenced by dietary antioxidant capacity. Redox Report 21 (2):67–74. doi: 10.1179/1351000215Y.0000000029.
- García-Mantrana, I., M. Calatayud, M. Romo-Vaquero, J. C. Espín, M. V. Selva, and M. C. Collado. 2019. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals. Nutrients 11:2483. doi: 10.3390/nu11102483.
- Gensous, N., P. Garagnani, A. Santoro, C. Giuliani, R. Ostan, C. Fabbri, M. Milazzo, D. Gentilini, A. M. di Blasio, B. Pietruszka, et al. 2020. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. GeroScience 42 (2):687–701. doi: 10.1007/s11357-019-00149-0.
- Gilbert, J. A., M. J. Blaser, J. G. Caporaso, J. K. Jansson, S. V. Lynch, and R. Knight. 2018. Current understanding of the human microbiome. Nature Medicine 24 (4):392–400. doi: 10.1038/nm.4517.
- González-Sarrías, A., R. García-Villalba, M. Romo-Vaquero, C. Alasalvar, A. Örem, P. Zafrilla, F. A. Tomás-Barberán, M. V. Selma, and J. C. Espín. 2017. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Molecular Nutrition & Food Research 61 (5):1600830. doi: 10.1002/mnfr.201600830.
- Graeser, A. C., C. Boesch-Saadatmandi, J. Lippmann, A. E. Wagner, P. Huebbe, N. Storm, W. Höppner, I. Wiswedel, A. Gardemann, A. M. Minihane, et al. 2011. Nrf2-dependent gene expression is affected by the proatherogenic apoE4 genotype-studies in targeted gene replacement mice. Journal of Molecular Medicine (Berlin, Germany) 89 (10):1027–35. doi: 10.1007/s00109-011-0771-1.
- Graham Ruby, J., K. M. Wright, K. A. Rand, A. Kermany, K. Noto, D. Curtis, N. Varner, D. Garrigan, D. Slinkov, I. Dorfman, et al. 2018. Estimates of the heritability of human longevity are substantially inflated due to assortative mating. Genetics 210 (3):1109–24. doi: 10.1534/genetics.118.301613.
- Griffin, B. A., C. G. Walker, S. A. Jebb, C. Moore, G. S. Frost, L. Goff, T. A. B. Sanders, F. Lewis, M. Griffin, R. Gitau, et al. 2018. APOE4 genotype exerts greater benefit in lowering plasma cholesterol and apolipoprotein B than wild type (E3/E3), after replacement of dietary saturated fats with low glycaemic index carbohydrates. Nutrients 10 (10):1524. doi: 10.3390/nu10101524.
- Hadi, A., M. Askarpour, M. Miraghajani, M. E. Symonds, A. Sheikhi, and E. Ghaedi. 2019. Effects of strawberry supplementation on cardiovascular risk factors: A comprehensive systematic review and meta-analysis of randomized controlled trials. Food & Function 10 (11):6987–98. doi: 10.1039/c9fo01684h.
- Hannum, G., J. Guinney, L. Zhao, L. Zhang, G. Hughes, S. V. Sadda, B. Klotzle, M. Bibikova, J.-B. Fan, Y. Gao, et al. 2013. Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell 49 (2):359–67. doi: 10.1016/j.molcel.2012.10.016.
- Hartmann, A., C. Hartmann, R. Secci, A. Hermann, G. Fuellen, and M. Walter. 2021. Ranking biomarkers of aging by citation profiling and effort scoring. Frontiers in Genetics 12:686320.
- Hayes, D. P. 2007. Nutritional hormesis. European Journal of Clinical Nutrition 61 (2):147–59. doi: 10.1038/sj.ejcn.1602507.
- Heneghan, C., M. Kiely, J. Lyons, and A. Lucey. 2018. The effect of berry-based food interventions on markers of cardiovascular and metabolic health: A systematic review of randomized controlled trials. Molecular Nutrition & Food Research 62:1–12. doi: 10.1002/mnfr.201700645.
- Hidalgo, M., S. Martin-Santamaria, I. Recio, C. Sanchez-Moreno, B. de Pascual-Teresa, G. Rimbach, and S. de Pascual-Teresa. 2012. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes & Nutrition 7 (2):295–306. doi: 10.1007/s12263-011-0263-5.
- Hills, R. D., B. A. Pontefract, H. R. Mishcon, C. A. Black, S. C. Sutton, and C. R. Theberge. 2019. Gut microbiome: Profound implications for diet and disease. Nutrients 11:1613. doi: 10.3390/nu11071613.
- Hochschild, R. 1989. Improving the precision of biological age determinations. Part 1: A new approach to calculating biological age. Experimental Gerontology 24 (4):289–300. doi: 10.1016/0531-5565(89)90002-8.
- Hollegaard, M. V., J. Grauholm, B. Nørgaard-Pedersen, and D. M. Hougaard. 2013. DNA methylome profiling using neonatal dried blood spot samples: A proof-of-principle study. Molecular Genetics and Metabolism 108 (4):225–31. doi: 10.1016/j.ymgme.2013.01.016.
- Holscher, H. D., H. M. Guetterman, K. S. Swanson, R. An, N. R. Matthan, A. H. Lichtenstein, J. A. Novotny, and D. J. Baer. 2018. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: A randomized controlled trial. The Journal of Nutrition 148 (6):861–7. doi: 10.1093/jn/nxy004.
- Horvath, S. 2013. DNA methylation age of human tissues and cell types. Genome Biology 14 (10):R115. doi: 10.1186/gb-2013-14-10-r115.
- Huan, T., G. Chen, C. Liu, A. Bhattacharya, J. Rong, B. H. Chen, S. Seshadri, K. Tanriverdi, J. E. Freedman, M. G. Larson, et al. 2018. Age-associated microRNA expression in human peripheral blood is associated with all-cause mortality and age-related traits. Aging Cell 17 (1):e12687. doi: 10.1111/acel.12687.
- Huang, H., G. Chen, D. Liao, Y. Zhu, and X. Xue. 2016. Effects of berries consumption on cardiovascular risk factors: A meta-analysis with trial sequential analysis of randomized controlled trials. Scientific Reports 6:23625.
- Huebbe, P., L. Jofre-Monseny, and G. Rimbach. 2009. Alpha-tocopherol transport in the lung is affected by the apoE genotype-studies in transgenic apoE3 and apoE4 mice. IUBMB Life 61 (4):453–6. doi: 10.1002/iub.177.
- Huebbe, P., and G. Rimbach. 2017. Evolution of human apolipoprotein E (APOE) isoforms: Gene structure, protein function and interaction with dietary factors. Ageing Research Reviews 37:146–61. doi: 10.1016/j.arr.2017.06.002.
- Iino, C., T. Shimoyama, K. Iino, Y. Yokoyama, D. Chinda, H. Sakuraba, S. Fukuda, and S. Nakaji. 2019. Daidzein intake is associated with equol producing status through an increase in the intestinal bacteria responsible for equol production. Nutrients 11:433. doi: 10.3390/nu11020433.
- Jansen, I. E., J. E. Savage, K. Watanabe, J. Bryois, D. M. Williams, S. Steinberg, J. Sealock, I. K. Karlsson, S. Hägg, L. Athanasiu, et al. 2019. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nature Genetics 51 (3):404–13. doi: 10.1038/s41588-018-0311-9.
- Javvaji, A., A. S. Can, and S. Sharma. 2021. Dysbetalipoproteinemia. In StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing.
- Jia, L., W. Zhang, R. Jia, H. Zhang, and X. Chen. 2016. Construction formula of biological age using the principal component analysis. BioMed Research International 2016:4697017. doi: 10.1155/2016/4697017.
- Johnson, A. A., M. N. Shokhirev, T. Wyss-Coray, and B. Lehallier. 2020. Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Research Reviews 60:101070. doi: 10.1016/j.arr.2020.101070.
- Joseph, S. V., I. Edirisinghe, and B. M. Burton-Freeman. 2014. Berries: Anti-inflammatory effects in humans. Journal of Agricultural and Food Chemistry 62 (18):3886–903. doi: 10.1021/jf4044056.
- Kawabata, K., Y. Yoshioka, and J. Terao. 2019. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 24:370. doi: 10.3390/molecules24020370.
- Khan, M. I., S. Rath, V. M. Adhami, and H. Mukhtar. 2018. Targeting epigenome with dietary nutrients in cancer: Current advances and future challenges. Pharmacological Research 129:375–87. doi: 10.1016/j.phrs.2017.12.008.
- Klemera, P., and S. Doubal. 2006. A new approach to the concept and computation of biological age. Mechanisms of Ageing and Development 127 (3):240–8. doi: 10.1016/j.mad.2005.10.004.
- Kok, D. E. G., R. A. M. Dhonukshe-Rutten, C. Lute, S. G. Heil, A. G. Uitterlinden, N. Van Der Velde, J. B. J. Van Meurs, N. M. Van Schoor, G. J. E. J. Hooiveld, L. C. P. G. M. De Groot, et al. 2015. The effects of long-term daily folic acid and vitamin B12 supplementation on genomewide DNA methylation in elderly subjects. Clinical Epigenetics 7:121.
- Kuningas, M., S. P. Mooijaart, D. Van Heemst, B. J. Zwaan, P. E. Slagboom, and R. G. J. Westendorp. 2008. Genes encoding longevity: From model organisms to humans. Aging Cell 7 (2):270–80. doi: 10.1111/j.1474-9726.2008.00366.x.
- Lagoa, R., D. Marques-da-Silva, M. Diniz, M. Daglia, and A. Bishayee. 2020. Molecular mechanisms linking environmental toxicants to cancer development: Significance for protective interventions with polyphenols. Seminars in Cancer Biology. doi: 10.1016/j.semcancer.2020.02.002.
- Lambert, J.-C., C. A. Ibrahim-Verbaas, D. Harold, A. C. Naj, R. Sims, C. Bellenguez, G. Jun, A. L. DeStefano, J. C. Bis, G. W. Beecham, European Alzheimer’s Disease Initiative (EADI), et al. 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature Genetics 45 (12):1452–8. doi: 10.1038/ng.2802.
- Land Lail, H., R. G. Feresin, D. Hicks, B. Stone, E. Price, and D. Wanders. 2021. Berries as a treatment for obesity-induced inflammation: Evidence from preclinical models. Nutrients 13:334. doi: 10.3390/nu13020334.
- Langley-Evans, S. C. 2015. Nutrition in early life and the programming of adult disease: A review. Journal of Human Nutrition and Dietetics 28(Suppl 1):1–14. doi: 10.1111/jhn.12212.
- LaRocca, T. J., A. N. Cavalier, and D. Wahl. 2020. Repetitive elements as a transcriptomic marker of aging: Evidence in multiple datasets and models. Aging Cell 19 (7):e13167. doi: 10.1111/acel.13167.
- Leri, M., M. Scuto, M. L. Ontario, V. Calabrese, E. J. Calabrese, M. Bucciantini, and M. Stefani. 2020. Healthy effects of plant polyphenols: Molecular mechanisms. International Journal of Molecular Sciences 21:1250. doi: 10.3390/ijms21041250.
- Levine, M. E. 2013. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age?The Journals of Gerontology, Series A 68 (6):667–74. doi: 10.1093/gerona/gls233.
- Levine, M. E., A. T. Lu, A. Quach, B. H. Chen, T. L. Assimes, S. Bandinelli, L. Hou, A. A. Baccarelli, J. D. Stewart, Y. Li, et al. 2018. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10 (4):573–91. doi: 10.18632/aging.101414.
- Liao, F., H. Yoon, and J. Kim. 2017. Apolipoprotein e metabolism and functions in brain and its role in Alzheimer’s disease. Current Opinion in Lipidology 28 (1):60–7.
- Lind, L., J. Sundström, J. Ärnlöv, and E. Lampa. 2018. Impact of aging on the strength of cardiovascular risk factors: A longitudinal study over 40 years. Journal of the American Heart Association 7:e007061.
- Lin, Q., and W. Wagner. 2015. Epigenetic aging signatures are coherently modified in cancer. PLoS Genetics 11 (6):e1005334–17. doi: 10.1371/journal.pgen.1005334.
- Li, K., M. Zhang, H. Chen, J. Peng, F. Jiang, X. Shi, Y. Bai, M. Jian, and Y. Jia. 2019. Anthocyanins from black peanut skin protect against UV-B induced keratinocyte cell and skin oxidative damage through activating Nrf 2 signaling. Food & Function 10 (10):6815–28. doi: 10.1039/c9fo00706g.
- Li, Z., F. Shue, N. Zhao, M. Shinohara, and G. Bu. 2020. APOE2: Protective mechanism and therapeutic implications for Alzheimer’s disease. Molecular Neurodegeneration 15 (1):63.
- Liu, Z., P. L. Kuo, S. Horvath, E. Crimmins, L. Ferrucci, and M. Levine. 2018. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Medicine 15 (12):e1002718. doi: 10.1371/journal.pmed.1002718.
- Lloyd-Price, J., G. Abu-Ali, and C. Huttenhower. 2016. The healthy human microbiome. Genome Medicine 8 (1):51. doi: 10.1186/s13073-016-0307-y.
- Lu, A. T., A. Quach, J. G. Wilson, A. P. Reiner, A. Aviv, K. Raj, L. Hou, A. A. Baccarelli, Y. Li, J. D. Stewart, et al. 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11 (2):303–27. doi: 10.18632/aging.101684.
- Mahley, R. W., K. H. Weisgraber, and Y. Huang. 2009. Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. Journal of Lipid Research 50:183–8.
- Mahmoud, A. M., and M. M. Ali. 2019. Methyl donor micronutrients that modify DNA methylation and cancer outcome. Nutrients 11:608. doi: 10.3390/nu11030608.
- Mamoshina, P., K. Kochetov, E. Putin, F. Cortese, A. Aliper, W.-S. Lee, S.-M. Ahn, L. Uhn, N. Skjodt, O. Kovalchuk, et al. 2018a. Population specific biomarkers of human aging: A big data study using South Korean, Canadian, and Eastern European patient populations. The Journals of Gerontology, Series A 73 (11):1482–90. doi: 10.1093/gerona/gly005.
- Mamoshina, P., M. Volosnikova, I. V. Ozerov, E. Putin, E. Skibina, F. Cortese, and A. Zhavoronkov. 2018b. Machine learning on human muscle transcriptomic data for biomarker discovery and tissue-specific drug target identification. Frontiers in Genetics 9:242. doi: 10.3389/fgene.2018.00242.
- Mandaviya, P. R., R. Joehanes, J. Brody, J. E. Castillo-Fernandez, K. F. Dekkers, A. N. Do, M. Graff, I. K. Hänninen, T. Tanaka, E. A. L. De Jonge, et al. 2019. Association of dietary folate and Vitamin B-12 intake with genome-wide DNA methylation in blood: A large-scale epigenome-wide association analysis in 5841 individuals. The American Journal of Clinical Nutrition 110 (2):437–50. doi: 10.1093/ajcn/nqz031.
- Marais, A. D. 2019. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 51 (2):165–76. doi: 10.1016/j.pathol.2018.11.002.
- Martel, J., D. M. Ojcius, Y. F. Ko, P. Y. Ke, C. Y. Wu, H. H. Peng, and J. D. Young. 2019. Hormetic effects of phytochemicals on health and longevity. Trends in Endocrinology and Metabolism: TEM 30 (6):335–46. doi: 10.1016/j.tem.2019.04.001.]
- Martini, D., M. Marino, D. Angelino, C. Del Bo’, D. Del Rio, P. Riso, and M. Porrini. 2020. Role of berries in vascular function: A systematic review of human intervention studies. Nutrition Reviews 78 (3):189–206.
- Martín-Núñez, G. M., R. Cabrera-Mulero, E. Rubio-Martín, G. Rojo-Martínez, G. Olveira, S. Valdés, F. Soriguer, L. Castaño, and S. Morcillo. 2014. Methylation levels of the SCD1 gene promoter and LINE-1 repeat region are associated with weight change: An intervention study. Molecular Nutrition & Food Research 58 (7):1528–36. doi: 10.1002/mnfr.201400079.
- Masana, M. F., A. Koyanagi, J. M. Haro, and S. Tyrovolas. 2017. n-3 Fatty acids, Mediterranean diet and cognitive function in normal aging: A systematic review. Experimental Gerontology 91:39–50. doi: 10.1016/j.exger.2017.02.008.
- Mayne, B., O. Berry, C. Davies, J. Farley, and S. Jarman. 2019. A genomic predictor of lifespan in vertebrates. Scientific Reports 9 (1):17866.
- McCrory, C., G. Fiorito, B. Hernandez, S. Polidoro, A. M. O’Halloran, A. Hever, C. Ni Cheallaigh, A. T. Lu, S. Horvath, P. Vineis, et al. 2021. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. The Journals of Gerontology, Series A 76 (5):741–9. doi: 10.1093/gerona/glaa286.
- Milella, R. A., M. Gasparro, F. Alagna, M. F. Cardone, S. Rotunno, C. T. Ammollo, F. Semeraro, A. Tullo, F. Marzano, D. Catalano, et al. 2020. Gene expression signature induced by grape intake in healthy subjects reveals wide-spread beneficial effects on peripheral blood mononuclear cells. Journal of Functional Foods 64:103705. doi: 10.1016/j.jff.2019.103705.
- Minihane, A. M., S. Khan, E. C. Leigh-Firbank, P. Talmud, J. W. Wright, M. C. Murphy, B. A. Griffin, and C. M. Williams. 2000. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arteriosclerosis, Thrombosis, and Vascular Biology 20 (8):1990–7. doi: 10.1161/01.ATV.20.8.1990.
- Mitnitski, A., and K. Rockwood. 2019. The problem of integrating of biological and clinical markers of aging. In Biomarkers of human aging, ed. A. Moskalev, 399–415. Cham, Swirzerland: Springer.
- Morrill, S. J., and K. J. Gibas. 2019. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes & Metabolic Syndrome 13 (2):1187–91. doi: 10.1016/j.dsx.2019.01.035.
- Morris, B. J., D. C. Willcox, T. A. Donlon, and B. J. Willcox. 2015. FOXO3: A major gene for human longevity-a mini-review. Gerontology 61 (6):515–25. doi: 10.1159/000375235.
- Morrison, D. J., and T. Preston. 2016. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7 (3):189–200. doi: 10.1080/19490976.2015.1134082.
- Myhrstad, M. C. W., V. D. Mello, I. Dahlman, M. Kolehmainen, J. Paananen, A. Rundblad, C. Carlberg, O. K. Olstad, J. Pihlajamäki, K. B. Holven, et al. 2019. Healthy nordic diet modulates the expression of genes related to mitochondrial function and immune response in peripheral blood mononuclear cells from subjects with metabolic syndrome – A SYSDIET sub-study. Molecular Nutrition & Food Research 63:e1801405. doi: 10.1002/mnfr.201801405.
- Myhrstad, M. C. W., I. Ottestad, C. C. Günther, E. Ryeng, M. Holden, A. Nilsson, K. W. Brønner, A. Kohler, G. I. A. Borge, K. B. Holven, et al. 2016. The PBMC transcriptome profile after intake of oxidized versus high-quality fish oil: An explorative study in healthy subjects. Genes & Nutrition 11:16.
- Myhrstad, M. C. W., S. M. Ulven, C. C. Günther, I. Ottestad, M. Holden, E. Ryeng, G. I. Borge, A. Kohler, K. W. Brønner, M. Thoresen, et al. 2014. Fish oil supplementation induces expression of genes related to cell cycle, endoplasmic reticulum stress and apoptosis in peripheral blood mononuclear cells: A transcriptomic approach. Journal of Internal Medicine 276 (5):498–511. doi: 10.1111/joim.12217.
- Nayak, B., R. H. Liu, and J. Tang. 2015. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains-a review. Critical Reviews in Food Science and Nutrition 55 (7):887–919. doi: 10.1080/10408398.2011.654142.
- Nica, A. C., and E. T. Dermitzakis. 2013. Expression quantitative trait loci: Present and future. Philosophical Transactions of the Royal Society of London, Series B 368 (1620):20120362. doi: 10.1098/rstb.2012.0362.
- Nichols, M., J. Zhang, B. M. Polster, P. A. Elustondo, A. Thirumaran, E. V. Pavlov, and G. S. Robertson. 2015. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 308:75–94. doi: 10.1016/j.neuroscience.2015.09.012.
- Ou, J. L. S., D. Yang, and M. H. Liu. 2020. Effects of anthocyanins in composite meals on cardiometabolic outcomes - A systematic review of randomized controlled feeding trials. Nutrients 12:3781. doi: 10.3390/nu12123781.
- Pallauf, K., N. Duckstein, M. Hasler, L. O. Klotz, and G. Rimbach. 2017. Flavonoids as putative inducers of the transcription factors Nrf2, FoxO, and PPARγ. Oxidative Medicine and Cellular Longevity 2017:4397340. doi: 10.1155/2017/4397340.
- Pallauf, K., K. Giller, P. Huebbe, and G. Rimbach. 2013. Nutrition and healthy ageing: Calorie restriction or polyphenol-rich "MediterrAsian" diet?Oxidative Medicine and Cellular Longevity 2013:707421. doi: 10.1155/2013/707421.
- Pallauf, K., G. Rimbach, P. M. Rupp, D. Chin, and I. M. Wolf. 2016. Resveratrol and lifespan in model organisms. Current Medicinal Chemistry 23 (41):4639–80. doi: 10.2174/0929867323666161024151233.
- Patrick, R. P. 2019. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB Journal 33 (2):1554–64. doi: 10.1096/fj.201801412R.
- Peters, M. J., R. Joehanes, L. C. Pilling, C. Schurmann, K. N. Conneely, J. Powell, E. Reinmaa, G. L. Sutphin, A. Zhernakova, K. Schramm, NABEC/UKBEC Consortium, et al. 2015. The transcriptional landscape of age in human peripheral blood. Nature Communications 6:8570.
- Pilling, L. C., C.-L. Kuo, K. Sicinski, J. Tamosauskaite, G. A. Kuchel, L. W. Harries, P. Herd, R. Wallace, L. Ferrucci, and D. Melzer. 2017. Human longevity: 25 genetic loci associated in 389,166 UK biobank participants. Aging 9 (12):2504–20. doi: 10.18632/aging.101334.
- Piyathilake, C. J., S. Badiga, E. K. Kabagambe, A. Azuero, R. D. Alvarez, G. L. Johanning, and E. E. Partridge. 2012. A dietary pattern associated with LINE-1 methylation alters the risk of developing cervical intraepithelial neoplasia. Cancer Prevention Research (Philadelphia, PA) 5 (3):385–92. doi: 10.1158/1940-6207.CAPR-11-0387.
- Polus, A., B. Zapala, U. Razny, A. Gielicz, B. Kiec-Wilk, M. Malczewska-Malec, M. Sanak, C. E. Childs, P. C. Calder, and A. Dembinska-Kiec. 2016. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochimica et Biophysica Acta 1861 (11):1746–55. doi: 10.1016/j.bbalip.2016.08.005.
- Powell, D. N., A. Swimm, R. Sonowal, A. Bretin, A. T. Gewirtz, R. M. Jones, and D. Kalman. 2020. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proceedings of the National Academy of Sciences of the United States of America 117 (35):21519–26. doi: 10.1073/pnas.2003004117.
- Prinelli, F., L. Fratiglioni, G. Kalpouzos, M. Musicco, F. Adorni, I. Johansson, A. Marseglia, and W. Xu. 2019. Specific nutrient patterns are associated with higher structural brain integrity in dementia-free older adults. Neuroimage 199:281–8. doi: 10.1016/j.neuroimage.2019.05.066.
- Putin, E., P. Mamoshina, A. Aliper, M. Korzinkin, A. Moskalev, A. Kolosov, A. Ostrovskiy, C. Cantor, J. Vijg, and A. Zhavoronkov. 2016. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging 8 (5):1021–33. doi: 10.18632/aging.100968.
- Quach, A., M. E. Levine, T. Tanaka, A. T. Lu, B. H. Chen, L. Ferrucci, and S. Horvath. 2018. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Agro Food Industry Hi Tech 29:20–1.
- Rahmani, J., C. Clark, H. Kord Varkaneh, T. Lakiang, L. T. Vasanthan, V. Onyeche, S. M. Mousavi, and Y. Zhang. 2019. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Phytotherapy Research: PTR 33 (8):1981–90. doi: 10.1002/ptr.6398.
- Rebelo-Marques, A., A. De Sousa Lages, R. Andrade, C. F. Ribeiro, A. Mota-Pinto, F. Carrilho, and J. Espregueira-Mendes. 2018. Aging hallmarks: The benefits of physical exercise. Frontiers in Endocrinology 9:258.
- Ren, X., and P. F. Kuan. 2020. RNAAgeCalc: A multi-tissue transcriptional age calculator. PLoS One 15 (8):e0237006. doi: 10.1371/journal.pone.0237006.
- Revelas, M., A. Thalamuthu, C. Oldmeadow, T. J. Evans, N. J. Armstrong, J. B. Kwok, H. Brodaty, P. R. Schofield, R. J. Scott, P. S. Sachdev, et al. 2018. Review and meta-analysis of genetic polymorphisms associated with exceptional human longevity. Mechanisms of Ageing and Development 175:24–34. doi: 10.1016/j.mad.2018.06.002.
- Rocha, D. M. U. P., A. P. S. Caldas, B. P. da Silva, H. H. M. Hermsdorff, and R. d. C. G. Alfenas. 2019. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Critical Reviews in Food Science and Nutrition 59 (11):1816–28. doi: 10.1080/10408398.2018.1430019.
- Rodríguez-Daza, M. C., M. Roquim, S. Dudonné, G. Pilon, E. Levy, A. Marette, D. Roy, and Y. Desjardins. 2020. Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-like clustering in obese mice. Frontiers in Microbiology 11:2032. doi: 10.3389/fmicb.2020.02032.
- Rodriguez-Mateos, A., G. Istas, L. Boschek, R. P. Feliciano, C. E. Mills, C. Boby, S. Gomez-Alonso, D. Milenkovic, and C. Heiss. 2019. Circulating anthocyanin metabolites mediate vascular benefits of blueberries: Insights from randomized controlled trials, metabolomics, and nutrigenomics. The Journals of Gerontology, Series A 74 (7):967–76. doi: 10.1093/gerona/glz047.
- Rodríguez-Morató, J., N. R. Matthan, J. Liu, R. de la Torre, and C. Y. O. Chen. 2018. Cranberries attenuate animal-based diet-induced changes in microbiota composition and functionality: A randomized crossover controlled feeding trial. The Journal of Nutritional Biochemistry 62:76–86. doi: 10.1016/j.jnutbio.2018.08.019.
- Ruiz-Ruiz, S., S. Sanchez-Carrillo, S. Ciordia, M. C. Mena, C. Méndez-García, D. Rojo, R. Bargiela, E. Zubeldia-Varela, M. Martínez-Martínez, C. Barbas, et al. 2020. Functional microbiome deficits associated with ageing: Chronological age threshold. Aging Cell 19 (1):e13063. doi: 10.1111/acel.13063.
- Rutledge, G. A., D. R. Fisher, M. G. Miller, M. E. Kelly, D. F. Bielinski, and B. Shukitt-Hale. 2019. The effects of blueberry and strawberry serum metabolites on age-related oxidative and inflammatory signaling in vitro. Food & Function 10 (12):7707–13. doi: 10.1039/C9FO01913H.
- Ryan, C. P. 2020. “Epigenetic clocks": Theory and applications in human biology. American Journal of Human Biology 26:e23488.
- Sae-Lee, C., S. Corsi, T. M. Barrow, G. G. C. Kuhnle, V. Bollati, J. C. Mathers, and H. M. Byun. 2018. Dietary intervention modifies DNA methylation age assessed by the epigenetic clock. Molecular Nutrition & Food Research 62 (23):e1800092–7. doi: 10.1002/mnfr.201800092.
- Salazar, N., S. Arboleya, T. Fernández-Navarro, C. G. De Los Reyes-Gavilán, S. Gonzalez, and M. Gueimonde. 2019. Age-Associated Changes in Gut Microbiota and Dietary Components Related with the Immune System in Adulthood and Old Age: A Cross-Sectional Study. Nutrients 11 (8):1765. doi: 10.3390/nu11081765.
- Saliou, C., G. Rimbach, H. Moini, L. McLaughlin, S. Hosseini, J. Lee, R. R. Watson, and L. Packer. 2001. Solar ultraviolet-induced erythema in human skin and nuclear factor-kappa-B-dependent gene expression in keratinocytes are modulated by a French maritime pine bark extract. Free Radical Biology & Medicine 30 (2):154–60. doi: 10.1016/S0891-5849(00)00445-7.
- Sanese, P., G. Forte, V. Disciglio, V. Grossi, and C. Simone. 2019. FOXO3 on the road to longevity: Lessons from SNPs and chromatin hubs. Computational and Structural Biotechnology Journal 17:737–45. doi: 10.1016/j.csbj.2019.06.011.
- Santoro, A., M. Martucci, M. Conte, M. Capri, C. Franceschi, and S. Salvioli. 2020a. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Research Reviews 64:101142. doi: 10.1016/j.arr.2020.101142.
- Santoro, A., J. Zhao, L. Wu, C. Carru, E. Biagi, and C. Franceschi. 2020b. Microbiomes other than the gut: Inflammaging and age-related diseases. Seminars in Immunopathology 42 (5):589–605. doi: 10.1007/s00281-020-00814-z.
- Sargurupremraj, M., H. Suzuki, X. Jian, C. Sarnowski, T. E. Evans, J. C. Bis, G. Eiriksdottir, S. Sakaue, N. Terzikhan, M. Habes, International Headache Genomics Consortium (IHGC), et al. 2020. Cerebral small vessel disease genomics and its implications across the lifespan. Nature Communications 11 (1):6285.
- Schmidt, S., F. Stahl, K. O. Mutz, T. Scheper, A. Hahn, and J. P. Schuchardt. 2012. Different gene expression profiles in normo- and dyslipidemic men after fish oil supplementation: Results from a randomized controlled trial. Lipids in Health and Disease 11:105. doi: 10.1186/1476-511X-11-105.
- Schulz, L. C. 2010. The Dutch hunger winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences of the United States of America 107 (39):16757–8. doi: 10.1073/pnas.1012911107.
- Shah, K., and P. Shah. 2018. Effect of anthocyanin supplementations on lipid profile and inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cholesterol 2018:8450793. doi: 10.1155/2018/8450793.
- Shahidi, F., and P. Ambigaipalan. 2018. Omega-3 polyunsaturated fatty acids and their health benefits. Annual Review of Food Science and Technology 9:345–81. doi: 10.1146/annurev-food-111317-095850.
- Shamir, L. 2015. Composite aging markers can be used for quantitative profiling of aging. Gerontology 62 (1):66–8. doi: 10.1159/000433466.
- Shankar, S., L. Marsh, and R. K. Srivastava. 2013. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Molecular and Cellular Biochemistry 372 (1–2):83–94. doi: 10.1007/s11010-012-1448-y.
- Sheflin, A. M., C. L. Melby, F. Carbonero, and T. L. Weir. 2017. Linking dietary patterns with gut microbial composition and function. Gut Microbes 8 (2):113–29. doi: 10.1080/19490976.2016.1270809.
- Shimada, Y., M. Kinoshita, K. Harada, M. Mizutani, K. Masahata, H. Kayama, and K. Takeda. 2013. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One 8 (11):e80604. doi: 10.1371/journal.pone.0080604.
- Shimokawa, I., T. Komatsu, N. Hayashi, S. E. Kim, T. Kawata, S. Park, H. Hayashi, H. Yamaza, T. Chiba, and R. Mori. 2015. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell 14 (4):707–9. doi: 10.1111/acel.12340.
- Shinohara, M., T. Kanekiyo, M. Tachibana, A. Kurti, M. Shinohara, Y. Fu, J. Zhao, X. Han, P. M. Sullivan, G. W. Rebeck, et al. 2020. APOE2 is associated with longevity independent of Alzheimer’s disease. Elife 9:e62199. doi: 10.7554/eLife.62199.
- Shokhirev, M. N., and A. A. Johnson. 2021. Modeling the human aging transcriptome across tissues, health status, and sex. Aging Cell 20 (1):e13280. doi: 10.1111/acel.13280.
- Slavin, J. 2013. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 5 (4):1417–35. doi: 10.3390/nu5041417.
- Son, T. G., S. Camandola, and M. P. Mattson. 2008. Hormetic dietary phytochemicals. Neuromolecular Medicine 10 (4):236–46. doi: 10.1007/s12017-008-8037-y.
- Souza, P. R., R. M. Marques, E. A. Gomez, R. A. Colas, R. De Matteis, A. Zak, M. Patel, D. J. Collier, and J. Dalli. 2020. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: A randomized double-blind placebo-controlled study. Circulation Research 126 (1):75–90. doi: 10.1161/CIRCRESAHA.119.315506.
- Sun, M., N. Ma, T. He, L. J. Johnston, and X. Ma. 2020. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Critical Reviews in Food Science and Nutrition 60 (10):1760–8. doi: 10.1080/10408398.2019.1598334.
- Tacutu, R., D. Thornton, E. Johnson, A. Budovsky, D. Barardo, T. Craig, E. Diana, G. Lehmann, D. Toren, J. Wang, et al. 2018. Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Research 46 (D1):D1083–D1090. doi: 10.1093/nar/gkx1042.
- Taleb, S. 2019. Tryptophan dietary impacts gut barrier and metabolic diseases. Frontiers in Immunology 10:2113. doi: 10.3389/fimmu.2019.02113.
- Tanaka, T., N. Basisty, G. Fantoni, J. Candia, A. Z. Moore, A. Biancotto, B. Schilling, S. Bandinelli, and L. Ferrucci. 2020. Plasma proteomic biomarker signature of age predicts health and life span. Elife 9:e61073. doi: 10.7554/eLife.61073.
- Tanaka, T., A. Biancotto, R. Moaddel, A. Z. Moore, M. Gonzalez-Freire, M. A. Aon, J. Candia, P. Zhang, F. Cheung, G. Fantoni, CHI consortium, et al. 2018. Plasma proteomic signature of age in healthy humans. Aging Cell 17 (5):e12799. doi: 10.1111/acel.12799.
- Thaiss, C. A., S. Itav, D. Rothschild, M. T. Meijer, M. Levy, C. Moresi, L. Dohnalová, S. Braverman, S. Rozin, S. Malitsky, et al. 2016. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540 (7634):544–51. doi: 10.1038/nature20796.
- The DECODA Study Group. 2003. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 26:1770–80. doi: 10.2337/diacare.26.6.1770.
- Tobi, E. W., J. J. Goeman, R. Monajemi, H. Gu, H. Putter, Y. Zhang, R. C. Slieker, A. P. Stok, P. E. Thijssen, F. Müller, et al. 2014. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nature Communications 5:5592.
- Tomova, A., I. Bukovsky, E. Rembert, W. Yonas, J. Alwarith, N. D. Barnard, and H. Kahleova. 2019. The effects of vegetarian and vegan diets on gut microbiota. Frontiers in Nutrition 6:47. doi: 10.3389/fnut.2019.00047.
- Trimmer, E. E. 2013. Methylenetetrahydrofolate reductase: Biochemical characterization and medical significance. Current Pharmaceutical Design. 19:2574–93.
- Tsuda, T. 2016. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants (Basel) 5:13. doi: 10.3390/antiox5020013.
- Ulaszewska, M. M., A. Koutsos, K. Trošt, J. Stanstrup, M. Garcia-Aloy, M. Scholz, F. Fava, F. Natella, C. Scaccini, U. Vrhovsek, et al. 2020. Two apples a day modulate human: Microbiome co-metabolic processing of polyphenols, tyrosine and tryptophan. European Journal of Nutrition 59 (8):3691–714. doi: 10.1007/s00394-020-02201-8.
- Ullah, R., M. Khan, S. A. Shah, K. Saeed, and M. O. Kim. 2019. Natural antioxidant anthocyanins-a hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 11:1195. doi: 10.3390/nu11061195.
- Unnikrishnan, A., W. M. Freeman, J. Jackson, J. D. Wren, H. Porter, and A. Richardson. 2019. The role of DNA methylation in epigenetics of aging. Pharmacology & Therapeutics 195:172–85. doi: 10.1016/j.pharmthera.2018.11.001.
- Unuofin, J. O., and S. L. Lebelo. 2020. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxidative Medicine and Cellular Longevity 2020:1356893. doi: 10.1155/2020/1356893.
- Vaiserman, A., M. Romanenko, L. Piven, V. Moseiko, O. Lushchak, N. Kryzhanovska, V. Guryanov, and A. Koliada. 2020. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiology 20 (1):221. doi: 10.1186/s12866-020-01903-7.
- van den Berg, N., M. Rodríguez-Girondo, I. K. van Dijk, R. J. Mourits, K. Mandemakers, A. A. P. O. Janssens, M. Beekman, K. R. Smith, and P. E. Slagboom. 2019. Longevity defined as top 10% survivors and beyond is transmitted as a quantitative genetic trait. Nature Communications 10 (1):35.
- Van der Auwera, S., L. Garvert, G. Fuellen, M. Nauck, H. Völzke, U. Völker, and H. J. Grabe. 2021. The genetic predisposition to longevity acts through behavioral phenotypes in females. European Neuropsychopharmacology 45:1–14. doi: 10.1016/j.euroneuro.2021.02.014.
- Vauzour, D. 2012. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Medicine and Cellular Longevity 2012:914273. doi: 10.1155/2012/914273.
- Villeponteau, B. 1997. The heterochromatin loss model of aging. Experimental Gerontology 32 (4–5):383–94. doi: 10.1016/S0531-5565(96)00155-6.
- Voitenko, V. P., and A. V. Tokar. 1983. The assessment of biological age and sex differences of human aging. Experimental Aging Research 9 (4):239–44. doi: 10.1080/03610738308258458.
- Wallace, T. C., M. Slavin, and C. L. Frankenfeld. 2016. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 8:32. doi: 10.3390/nu8010032.
- Walter, M. 2009. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 29 (9):1244–50. doi: 10.1161/ATVBAHA.108.181438.
- Wang, X., L. Meng, L. Zhao, Z. Wang, H. Liu, G. Liu, and G. Guan. 2017. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Research and Clinical Practice 126:172–81. doi: 10.1016/j.diabres.2016.12.005.
- Wang, Y., J. L. Gallegos, C. Haskell-Ramsay, and J. K. Lodge. 2021. Effects of chronic consumption of specific fruit (berries, citrus and cherries) on CVD risk factors: A systematic review and meta-analysis of randomised controlled trials. European Journal of Nutrition 60 (2):615–39. doi: 10.1007/s00394-020-02299-w.
- Wang, Y., Q. Yuan, and L. Xie. 2018. Histone modifications in aging: The underlying mechanisms and implications. Current Stem Cell Research & Therapy 13 (2):125–35.
- Wiesmann, M., V. Zerbi, D. Jansen, R. Haast, D. Lütjohann, L. M. Broersen, A. Heerschap, and A. J. Kiliaan. 2016. A dietary treatment improves cerebral blood flow and brain connectivity in aging apoE4 mice. Neural Plasticity 2016:6846721–11. doi: 10.1155/2016/6846721.
- Wu, Z., A. Huang, J. Yan, B. Liu, Q. Liu, J. Zhang, X. Zhang, C. Ou, and M. Chen. 2017. Resveratrol ameliorates cardiac dysfunction by inhibiting apoptosis via the pi3k/akt/foxo3a pathway in a rat model of diabetic cardiomyopathy. Journal of Cardiovascular Pharmacology 70:184–93.
- Wu, W. K., S. Panyod, P. Y. Liu, C. C. Chen, H. L. Kao, H. L. Chuang, Y. H. Chen, H. B. Zou, H. C. Kuo, C. H. Kuo, et al. 2020. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome 8 (1):162. doi: 10.1186/s40168-020-00912-y.
- Yanckello, L. M., J. D. Hoffman, Y. H. Chang, P. Lin, G. Nehra, G. Chlipala, S. D. McCulloch, T. C. Hammond, A. T. Yackzan, A. N. Lane, et al. 2021. Apolipoprotein E genotype-dependent nutrigenetic effects to prebiotic inulin for modulating systemic metabolism and neuroprotection in mice via gut-brain axis. Nutritional Neuroscience :1–11.
- Yang, L. P., W. H. Ling, Z. C. Du, Y. M. Chen, D. Li, S. Z. Deng, Z. M. Liu, and L. L. Yang. 2017. Effects of anthocyanins on cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Advances in Nutrition (Bethesda, MD) 8 (5):684–93.
- Yassine, H. N., and C. E. Finch. 2020. APOE alleles and diet in brain aging and alzheimer’s disease. Frontiers in Aging Neuroscience 12:150.
- Yessenkyzy, A., T. Saliev, M. Zhanaliyeva, A. R. Masoud, B. Umbayev, S. Sergazy, E. Krivykh, A. Gulyayev, and T. Nurgozhin. 2020. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients 12:1344.
- Yi, S. J., and K. Kim. 2020. New insights into the role of histone changes in aging. International Journal of Molecular Sciences 21 (21):8241.
- Yin, C., S. Ackermann, Z. Ma, S. K. Mohanta, C. Zhang, Y. Li, S. Nietzsche, M. Westermann, L. Peng, D. Hu, et al. 2019. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nature Medicine 25 (3):496–506. doi: 10.1038/s41591-018-0336-8.
- Yousefzadeh, M. J., Y. Zhu, S. J. McGowan, L. Angelini, H. Fuhrmann-Stroissnigg, M. Xu, Y. Y. Ling, K. I. Melos, T. Pirtskhalava, C. L. Inman, et al. 2018. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36:18–28.
- Zeng, Y., C. Nie, J. Min, H. Chen, X. Liu, R. Ye, Z. Chen, C. Bai, E. Xie, Z. Yin, et al. 2018. Sex differences in genetic associations with longevity. JAMA Network Open 1 (4):e181670. doi: 10.1001/jamanetworkopen.2018.1670.
- Zhang, P., Q. Su, X. Ye, P. Guan, C. Chen, Y. Hang, J. Dong, Z. Xu, and W. Hu. 2020. Trends in LDL-C and non-HDL-C levels with age. Aging and Disease 11:1046–57.
- Zhang, Y., R. Wilson, J. Heiss, L. P. Breitling, K. U. Saum, B. Schöttker, B. Holleczek, M. Waldenberger, A. Peters, and H. Brenner. 2017. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nature Communications 8:14617.
- Zhang, X., R. Zhang, and J. Yu. 2020. New understanding of the relevant role of LINE-1 retrotransposition in human disease and immune modulation. Frontiers in Cell and Developmental Biology 8:657.
- Zheng, W., Y. Ma, A. Zhao, T. He, N. Lyu, Z. Pan, G. Mao, Y. Liu, J. Li, P. Wang, et al. 2019. Compositional and functional differences in human gut microbiome with respect to equol production and its association with blood lipid level: A cross-sectional study. Gut Pathogens 11:20.
- Zhou, D. D., M. Luo, A. Shang, Q. Q. Mao, B. Y. Li, R. Y. Gan, and H. B. Li. 2021. Antioxidant food components for the prevention and treatment of cardiovascular diseases: Effects, mechanisms, and clinical studies. Oxidative Medicine and Cellular Longevity 2021:6627355.
- Zhu, C., L. Sawrey-Kubicek, E. Beals, C. H. Rhodes, H. E. Houts, R. Sacchi, and A. M. Zivkovic. 2020. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: A pilot study. Nutrition Research (New York, NY) 77:62–72.
- Zhu, Z. Z., L. Hou, V. Bollati, L. Tarantini, B. Marinelli, L. Cantone, A. S. Yang, P. Vokonas, J. Lissowska, S. Fustinoni, et al. 2012. Predictors of global methylation levels in blood DNA of healthy subjects: A combined analysis. International Journal of Epidemiology 41 (1):126–39.