Abstract
Food allergy is a pathological immune reaction triggered by normal innocuous dietary proteins. Soybean is widely used in many food products and has long been recognized as a source of high-quality proteins. However, soybean is listed as one of the 8 most significant food allergens. The prevalence of soybean allergy is increasing worldwide and impacts the quality of life of patients. Currently, the only strategy to manage food allergy relies on strict avoidance of the offending food. Nutritional supplementation is a new prevention strategy which is currently under evaluation. Selenium (Se), as one of the essential micronutrients for humans and animals, carries out biological effects through its incorporation into selenoproteins. The use of interventions with micronutrients, like Se, might be an interesting new approach. In this review we describe the involvement of Se in a variety of processes, including maintaining immune homeostasis, preventing free radical damage, and modulating the gut microbiome, all of which may contribute to in both the prevention and treatment of food allergy. Se interventions could be an interesting new approach for future treatment strategies to manage soybean allergy, and food allergy in general, and could help to improve the quality of life for food allergic patients.
Introduction
Food allergy is defined as an adverse immunological response to a dietary protein. Over 90% of food allergies are caused by the “big eight” allergenic foods (Iweala, Choudhary, and Commins Citation2018) and the prevalence of these food allergies varies in the general population; egg (1.3–1.6%) (Anagnostou Citation2021), cow’s milk (1%) (Oliveira et al. Citation2021), peanut (0.6–1.5%) (Al-Muhsen, Clarke, and Kagan Citation2003), tree nuts (4.9%) (Geiselhart, Hoffmann-Sommergruber, and Bublin Citation2018), wheat (0.2–1%) (Cianferoni Citation2016), fish (5–7%) (Burney 2014; Martino et al. Citation1993), crustacean shellfish (0.5–2.5%) (Khora Citation2016), and soybean (0.3%) (Katz et al. Citation2014). With the exception of cow’s milk and egg allergy the prevalence of these food allergies across the United States Europe and Asia are quite different because of the diverse dietary patterns that vary between continents and countries. For instance, in Europe fruit allergies are common due to cross-reactions with inhalant allergens (Zuidmeer et al. Citation2008), whereas in Asia severe allergic reactions include those directed toward legumes and shellfish (Lao-Araya and Trakultivakorn Citation2012). Multiple immutable risk factors have been postulated to contribute to the onset of food allergy, including gender, ethnicity, and genetics (Lichtenstein and Svartengren Citation1997; Panjari et al. Citation2016). However, many modifiable factors have also been identified as a potential target to reduce or prevent food allergy. Among these factors are mode of delivery, antibiotic use, feeding of breastmilk, and the consumption of unpasteurized milk, fermented foods (Ferreira et al. Citation2021a; Kim et al. Citation2019; Pyrhönen and Kulmala Citation2022), omega-3-polyunsaturated fatty acids, prebiotics, probiotics, vitamin D and antioxidants (Childs et al. Citation2021; Dang Citation2013; Miles and Calder Citation2017; Soriano et al. Citation2020; Suaini et al. Citation2021). These factors are known to either directly or indirectly influence immune maturation and the composition of the intestinal microbiome. In early life, food allergy development may result from intestinal dysbiosis and an impaired mucosal immune response which can lead to improper responses to food antigens which eventually culminate in sensitization. In food allergic patients exposure to the allergenic foods can trigger clinical symptoms such as digestive problems, vomiting, atopic dermatitis, hives or swollen airways, ranging in severity from mild to life-threatening anaphylaxis (Yu, Freeland, and Nadeau Citation2016). In addition, many forms of food allergy may be related with later development of other allergic manifestations such as atopic dermatitis and allergic asthma. Food allergies do not only decrease the quality of life of the food allergic patient, their prevalence is also an increasing public health burden. It is currently estimated that over 220 million people suffer from food allergy (Sicherer Citation2011) affecting approximately 5% to 7.5% of children and 1% to 2% of adults worldwide (Wilson, Blaschek, and De Mejia Citation2005). In developed countries about 4% to 8% of the population are allergic to at least one food allergen, 2.4% are allergic to multiple food allergens and approximately 3% of total population of food allergic patients experience severe reactions to food allergens (De Martinis et al. Citation2020; Sicherer and Sampson Citation2014). However, because the majority of available data is based on self-reporting it is difficult to accurately assess the prevalence of food allergies (Antonella and Spergel Citation2009).
Food allergic responses directed toward soybean also contribute to the observed increase in food allergy. Being one of the “big eight”, soybean allergy is recognized as one of the most common IgE-mediated food allergies. Soybean allergy has an estimated prevalence of about 0.3% in the general population (Katz et al. Citation2014). In the United States it affects 0.4% of children and 0.3% of adults (Riascos et al. Citation2016); similarly, in Europe the prevalence of soybean allergy is estimated to be around 0.4% in the general population (Nwaru et al. 2014). Although the exposure to soybean at an early age is potentially widespread, the allergenicity of soybean receives less attention than many of the other food allergens. This might be because initial soybean allergic responses tend to induce atopic skin reactions and gastrointestinal distress but rarely lead to fatal anaphylaxis (Mittag et al. Citation2004). Although traditional Asian cuisine includes more crude soybean sources than the Western kitchen, the consumption of soy-containing food additives (soybean isolate, soybean concentrates and soybean flour) is increasing in Western diets (Kattan, Cocco, and Jarvinen Citation2011). This interesting geographical divergence may lead to a change in the prevalence of soybean allergy but as of yet no data are available to confirm or refute this hypothesis. Currently no treatment of food allergy is available and management of food allergy relies on strict avoidance of the offending food. In this review we describe the current insights into the role of micronutrients, especially selenium (Se), as an interesting novel therapeutic approach in the management of food allergy with a focus on soybean allergy.
Pathophysiology of soybean allergy
The most common food allergies are IgE mediated and have a prevalence of around 6–8% in children below 3 years of age (Antonella and Spergel Citation2009). Soybean allergy can be either IgE mediated or non-IgE mediated. IgE-mediated reactions typically occur immediately or within 1–2 hours after ingestion whereas non-IgE-mediated food hypersensitivity responses are usually delayed to several hours after ingestion of the culprit food. IgE mediated soybean allergy develops in a similar way to most other food allergies, which involves sensitization and the failure to develop oral tolerance toward food antigens. Although the exact mechanism of induction of immunological oral tolerance has not been fully unraveled yet, it is clear that it can be disrupted at many different stages. When enzymatic destruction of conformational epitopes in a potential food allergen is reduced, inflammatory cytokines such as IL-25 and IL-33 can be secreted by epithelial cells which inhibit IL-12 production (Kumar et al. Citation2020; Skoczylas et al. Citation2020). Under these conditions antigen presenting cells such as dendritic cells (DCs) are activated and switch into a functional pro-inflammatory phenotype. Subsequently, these antigen presenting cells shift naïve T cells to differentiate into Thelper 2 (Th2) cells instead of regulatory T cells (Treg) which would normally dampen immune responses via secretion of IL-10 and transforming growth factor beta (TGF-β). Th2 cells drive IgE class-switching in B-cells and expansion of allergic effector cells, e.g. eosinophils and mast cells (Frischmeyer-Guerrerio et al. Citation2011) while blocking Treg function via the release of IL-4. IgE secreted by the B-cells quickly binds to high-affinity IgE (FcεRI) receptors which are present mainly on the surface of mast cells and basophils. Although not all individuals who produce food antigen-specific IgE will mount an allergic response to the specific food, challenging allergic patients by re-exposing them to the food allergen will lead to degranulation of mast cells and basophils. This is due to the binding of the allergen to food-allergen specific IgE on the surface of these cells, which subsequently crosslinks the bound IgE triggering an intracellular cascade that results in the release of mediators such as histamine, leukotrienes, chemokines, and other cytokines. This leads to a series of inflammatory responses which characterize the immediate phase of the allergic reaction. Hereafter, the allergic inflammation can be maintained in the late phase of the allergic response due to the production of leukotrienes, platelet activating factor and cytokines such as IL-4, IL-5 and IL-13. Understanding the mechanism of IgE-mediated food allergy helps implementing measures to restore immunologic tolerance.
Allergens in soybean
The protein content of soybean is approximately 37% of the dry weight and within that protein fraction at least 16 IgE-reactive proteins were suggested as potential allergens (Vieths Citation2008). Soybean protein isolate (SPI) is a mixture of various proteins which is widely used in the food industry. The main ingredients of SPI are classified into four protein categories according to their sedimentation coefficients (S) including 2S, 7S, 11S and 15S (Zhao et al. Citation2019). The main soybean allergens are specific protein components. In recent decades those specific protein components which are linked to soybean allergy have been described. Soybean 2S albumin (Gly m 8) accounts for about 15% of the total seed proteins and is composed of the Bowman–Birk trypsin inhibitor (7.9 kDa), cytochrome C (12.5 kDa), Kunitz trypsin inhibitor (KTI; 20.1 kDa) (Sung et al. Citation2014). Kunitz soybean trypsin inhibitor is has been identified as a potent allergen capable of inducing food anaphylaxis (Adachi 2009).
7S globulin (Gly m 5, vicilin, β-conglycinin) accounts for about 30% of the total seed proteins and is also considered one of the major allergenic proteins in soybean seeds. It is mainly composed of three Gly m 5 subunits: α (∼68 kDa), α′ (∼72 kDa) and β (∼50 kDa) all of which have been shown to be potential dietary allergens for both humans and animals (Zheng et al. Citation2017). The α subunit, known as Gly m Bd 60 K, in which the IgE-binding site locates at the peptide 232-283, was capable of eliciting an allergic response in 25% of soybean sensitive patients in an observational study (Ogawa et al. Citation1995). The β subunit has also been indicated to have sensitizing capacity as 19 IgE and 59 IgG binding sites were identified on this 7S globulin subunit (Taliercio et al. Citation2014).
11S globulin (glycinin, Gly m 6, legumin) as a major seed storage protein that makes up over 40% of the total seed globulin is another major allergenic protein in soybean seeds (Gonzalez-Ferrero, Irache, and Gonzalez-Navarro Citation2018). It is a hexametric protein that was assembled by acidic polypeptides (A1a, A1b, A2, A3 and A4) and basic polypeptides (B1, B2 and B3) (Holzhauser et al. Citation2009). It was demonstrated that IgE from soybean allergic individuals bound to the soybean glycinin acidic chains whereas the basic chains were not bound by IgE (Markwell Citation2000). Acidic polypeptides A1a, A3 and A4 reacted with serum IgG and IgE and induced positive skin prick and histamine release responses in piglets sensitized to Gly m 6 (Zheng et al. Citation2018).
Other soybean proteins Gly m 1 and Gly m 2 are known as inhalant allergens in occupational or environmental soybean allergy. Gly m 3 is cross-reactive with milk casein and have the potential to cause allergic reactions to soybean. Gly m 4 is cross-reactive with the major birch pollen allergen Bet v 1 (Tsai, Chang, and Liao Citation2017) and it is functionally classified as a pathogenesis-related protein 10 (Kleine-Tebbe et al. Citation2002). Gly m 7 as a newly discovered soybean allergen (Riascos et al. Citation2016) has the capacity to induce IgE-mediated allergic responses. It has been reported that Gly m 7 has stronger basophil activation ability than Gly m 5 (Fukuzumi et al. Citation2021) but the prevalence and other details are unknown.
The effect of micronutrients on food allergy/immune development
Some foods containing food allergens are rich in essential nutrients. Therefore, monitoring dietary intake of children with food allergies is important to prevent nutrient deficiency due to inadequate food intake. Those nutrients include macronutrients such as carbohydrates, protein and fat, and micronutrients such as vitamins and minerals. Specific micronutrient deficiency such as vitamin D, vitamin E, minerals (zinc, magnesium, Se) is considered a major public health problem worldwide (Kirby and Danner Citation2009). Minerals often serve as cofactors for enzymes which are essential for several important functions in the body. With the accumulating knowledge of the involvement of micronutrients in immunological processes there is increasing interest in the relationship between minerals and the development of immune responses which include inflammation and allergic conditions. It is known that deficiencies of several minerals such as iron, zinc, copper, and Se may result in impaired immune function (Maggini, Pierre, and Calder Citation2018).
Selenium uptake and metabolism
Se is one of the most important micronutrients. It functions as an antioxidant and plays a crucial role in development and physiological processes including immune responses. Many pathological conditions involving the immune system can be affected by Se status which can be influenced by the forms and levels of ingested Se, genetic characteristics and the conversion of Se compounds into metabolites.
Se can be present in different chemical forms and can be either organic and/or inorganic. The main organic forms are selenomethionine (SeMet) and selenocysteine (SeCys). The inorganic forms are selenide, elemental Se, selenite and selenate (Narod et al. Citation2019). The predominant form of Se ingested by humans are SeMet and SeCys which have greater bioavailability than the inorganic forms (Pavlata, Pechová, and Dvořák Citation2012). Ingested SeMet from foods or nutritional supplements is absorbed via the intestine and undergoes trans-sulphuration to produce SeCys (Hariharan and Dharmaraj Citation2020) which is then incorporated into selenoproteins.
In humans 25 selenoproteins were identified for the presence of homologs several of which have important cellular functions in antioxidant defense, cell signaling, redox homeostasis and immune responses (Schomburg et al. Citation2003). Glutathione peroxidase (GSH-Px) is one of the most important selenoproteins. It is well known to be the major component of human antioxidant defense and involved in redox regulation of cellular functions (Thomson Citation2004). Thioredoxin reductases (TXNRDs) and methionine-R-sulfoxide reductase B1 (MSRB1) reduce oxidative stress, repair protein damage caused by oxidation and are also involved in the aging process (Kim Citation2013; Lu and Holmgren Citation2014). In addition, selenoprotein S is involved in immune responses including inflammatory processes (Labunskyy, Hatfield, and Gladyshev Citation2014). Selenoprotein P is responsible for maintaining homeostasis of antioxidant throughout the human body during normal and disturbed metabolism (Schomburg et al. Citation2003).
It is well known that many cellular events are regulated by changes in redox status often involving the glutathione and thioredoxin systems (Tanaka et al. Citation2000). ROS can alter the redox status of the cell which appears to serve an important role in the pathogenesis of allergic diseases. In homeostatic conditions, balance production of ROS has a positive effect in combatting invading pathogens. During inflammation many phagocytic cells rely on the production of ROS in the prevention of damage to host cells (Mittal et al. Citation2014). Once this homeostasis is disturbed, ROS levels can increase dramatically which can induce damage to host cells (Singh, Nair, and Verma Citation2021). Selenoproteins play an essential role in maintaining balanced levels of ROS to protect the cells from damage (Zhang et al. Citation2020).
An adequate intake of Se is a key to good health. For adults at least 40 μg/day is required to support the minimal expression of Se enzymes and 300 μg/day to reduce risk of cancer development (Combs Citation2001). In the U.K. recommended dietary intake of Se for adult women is 60 μg/day and 75 μg/day for adult men and lactating women (Values Citation1991). Some European countries (Germany, Austria and Switzerland) recommend Se intake from 25 to 70 μg/day (Oropeza-Moe, Wisloff, and Bernhoft Citation2015). Recently the European Food Safety Authority (EFSA) has set a daily adequate intake for Se at 70 μg/day (EFSA NDA Panel Citation2014). In the USA the Se intake recommendation for adult is set at 55 μg/day (Monsen Citation2000). These varying recommendations ranging from 25 μg/day to 75 μg/day are most likely due to the difficulty to define an optimal Se status, and the absence of accepted reference ranges due to variations in Se status between countries (Thomson Citation2004). Micronutrients have been highlighted as deficient in many food allergic children. Serum Se concentration have been indicated to be lower in children with both IgE and non-IgE mediated food allergy in comparison to healthy controls (Ferreira et al. Citation2021a), however whether this is a causative factor for the development of the food allergy or caused by elimination diets remains unclear (Meyer et al. Citation2014).
Selenium and immunity
The importance of adequate levels of dietary Se intake and its efficient incorporation into selenoproteins on immunity was reported in cell models, rodent models and in humans (Avery and Hoffmann Citation2018). Se is present in many vital immunological organs like the bone marrow, thymus, liver, spleen and lymph nodes (Finley and Kincaid Citation1991). At a cellular level Se is found in lymphocytes, granulocytes and monocytes/macrophages. It also influences various leukocytic effector functions including adherence, migration, phagocytosis and cytokine secretion (Dalgaard et al. Citation2018). Se serves a wide variety of functions in health and development including roles in cancer and heart disease prevention, viral inhibition, brain function and immune function, delaying the aging process and the onset of AIDS in HIV patients (Cai and Li Citation2018; Muzembo et al. Citation2019; Roman, Jitaru, and Barbante Citation2014) .
Se deficiency or suppressed selenoprotein expression results in higher levels of inflammatory cytokines in a variety of tissues which include the gastrointestinal tract, spleen and uterus (Avery and Hoffmann Citation2018). A recent study showed that Se deficiency induced inflammatory lesions and high expression levels of PTGE, COX-2, TNF-α and NF-κB in the gastrointestinal tract (Gao et al. Citation2016). Se deficiency was also reported to reduce the killing capacity of neutrophils, the differentiation of T cell and IL-2R expression on T cells (Ferencík and Ebringer Citation2003).
Selenium and food allergy development
Se levels may influence any condition associated with increased oxidative stress or inflammation (Rayman Citation2000). In a murine model for (OVA)-induced allergic asthma, dietary Se levels were associated with the development of allergic responses in mice; whereas an adequate dietary Se level appeared to skew T helper responses away from the Th2-type responses, high levels of dietary Se resulted in higher Th2- or Th1-type immunity (Hoffmann et al. Citation2007). Thus, Se may modulate allergic responses by affecting adaptive immune responses. In an observational study carried out with healthy children, the average Se plasma concentration was found to be 71.8 mg/l whereas in those with food allergy it was 54.1 mg/l indicating that children with food allergy have a higher risk of Se deficiency (Kalita et al. Citation2001). Similarly, Kamer et al. demonstrated that in children with food allergy concentrations of Se were decreased and lower values of GPx and SOD could be observed in plasma (Kamer et al. Citation2012). These data may only point to correlations that can be observed in food allergic patients, and human data related to investigating a causative link between Se and food allergy are to the best of our knowledge currently lacking. However, in a previous study in our laboratory we were able to demonstrate decreased mouse mast cell protease-1 (mMCP-1) in a mouse model for cow’s milk allergy following dietary Se intervention(Zhao et al. Citation2021), suggesting that Se does play a role in the pathophysiology of food allergic responses.
This is supported by the many studies which have investigated the effect of Se at a cellular level. Se deficiency decreased the expression of DCs surface markers including CD11c, CD40, CD86 and MHC-II and downregulated the secretion of IL-10, IL-12p40, which further weakened the ability of DC’s to stimulate the proliferation of mixed allogeneic lymphocytes (Sun et al. Citation2018). In addition, MsrB1 as a selenoprotein activates the STAT6 pathway in DCs thereby inducing DC maturation and IL-12 production that promotes CD4 + T cells to differentiate into Th1 (Lee et al. Citation2020) which drives the immune response away from a Th2 allergic response. Se not only affects DCs to modulate allergy development but also affects T cell proliferation and differentiation. In adult human subjects supplementation with Se (100 µg/day) enhanced plasma Se concentrations and increased T cell proliferation and the percentage of total T cells (Broome et al. Citation2004; Ivory et al. Citation2017). Se (1 ppm Se) promotes proliferation of T cells and favors differentiation of naive CD4+ T lymphocytes toward Th1 cells, supporting the acute cellular immune response (Hoffmann et al. Citation2010; Steinbrenner et al. Citation2015). Moreover, increased dietary Se enhanced T cell differentiation into CD25+ Foxp3+ Treg cells (Won et al. Citation2010) which are known to play a key role in tolerance development toward food antigens. It was reported that Se protects against chronic colitis by increasing the number of CD4 + CD25+ Tregs and enhancing the expression of IL-10 (Sang et al. Citation2017). Similarly, another study reported that the percentage of Treg cells and expression of Foxp3 mRNA were increased in Se treated mice suggesting that Se supplementation restored normal levels of CD4 + CD25+ Treg cells (Xue et al. Citation2010). Furthermore, Se supplementation inhibits activation, differentiation and maturation of B cells (Jian et al. Citation2003). Se is able to inhibit B lymphocyte (CD19+) reduction and decreased numbers of peripheral B cells (Ahmadi et al. Citation2015; Cheng et al. Citation2012) which further reduced dsDNA and SmRNP of the IgG2b and IgG2c subclass (Ahmadi et al. Citation2015) and inhibits the expression of total IgE in mice (Arakawa et al. Citation2018).
Se may also modulate the food allergic response in already sensitized individuals by affecting mast cell activity during challenge (). Se deficiency induced mast cell degranulation in chickens (Wang, Wang, and Wang Citation2012) but on the contrary Se treatment decreased mast cell degranulation in a cell based model as measured by reduced release of the mast cell mediators PGD2, β-hexosaminidase and histamine (Safaralizadeh et al. Citation2013). In addition, another study showed that Se affects basophils by inhibiting their proliferation, suppression of IL-4 and TNF-α protein secretion as well as suppression of antigen-induced phosphorylation of Syk, Akt, and MAPKs (Arakawa et al. Citation2019). In a study carried out in our laboratory, Se intervention decreased mouse mast cell protease-1 (mMCP-1) in a mouse model for cow’s milk allergy indicating Se may attenuate the allergic response by affecting mast cell activation and degranulation in a direct way (Zhao et al. Citation2021).
Figure 1. Potential modulatory effects of Se in the allergic sensitization and challenge phase. In the sensitization phase food allergens are picked up and processed by DC and presented to naïve CD4+ T cells in the context of MHC II. T cells then differentiate into Th2 cells and secrete cytokines which switch B cells to IgE secreting plasma cells. These allergen-specific IgE antibodies then bind to the FcεRI present on the surface of mast cells and basophils. Upon re-exposure to the allergen, crosslinking of receptor bound allergen-specific IgE occurs resulting in degranulation of these cells and the release of pro-inflammatory mediators that result in the clinical symptoms associated with a food allergic reaction. Se can inhibit DC activation, which may modulate the Th2 and Th1 balance in favor of an anti-allergic Th1 response and inhibit allergen-specific IgE production. In addition, Se can block Ca2+ flux and inhibit FcεRI expression thereby stabilizing mast cells and basophils, which further affect allergic response.
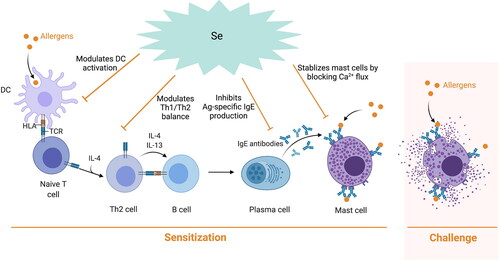
Se may also indirectly modulate the development of an allergic response to food allergens by affecting ROS. ROS plays a role in the allergic sensitization process in various ways. Upon antigen-specific interaction, intracellular ROS is elevated in T cells and DCs (Matsue et al. Citation2002). Se can block bidirectional DC-T cell communication by interfering with the redox regulation pathways to modulate allergic sensitization responses (Matsue et al. Citation2003). In addition, ROS also plays a role during allergenic challenge. It was reported that ROS/RNS production and mast cell degranulation have a positive correlation (Nakai, Yoneda, and Kubota Citation2012). Se may modulate the acute allergic response by downregulating ROS generation which further decreases the degranulation process of mast cells (Gueck, Aschenbach, and Fuhrmann Citation2013).
In addition, Se may modulate food allergic responses by affecting the microbiome colonization and composition. Intestinal microbiota development in early life is very dynamic and in synergy with intestinal physiology, immune and neurological development (Ferreira et al. Citation2021b). The microbial colonization in early life strongly affects health and diseases later in life (Kumar et al. Citation2020; Yang et al. Citation2021). The development of a healthy microbiome in early life plays an important role in the maturation of the immune system and this, in turn, affects the susceptibility to food sensitization and food allergy (Bunyavanich and Berin Citation2019). A dysbiosis in allergic infants is characterized by low levels of genera Bifidobacteria and Lactobacilli compared to healthy infants and is associated with the development of atopic diseases (Wopereis et al. Citation2014; Citation2018). The absence of a healthy intestinal microbiota development might lead to an inadequately developed immune system associated with reduced number of Tregs, reduced oral tolerance and possibly to the development of food allergy. It was reported that in germ-free mouse models gut-associated lymphoid tissues failed to develop when microbial colonization was delayed which led to Th2 skewed immune responses (Sudo et al. Citation1997). In addition, microbial colonization increases the suppressive capacity of Tregs and promotes Th1 response which are necessary to maintain immunologic balance and promote tolerance (Atarashi et al. Citation2011; Round and Mazmanian Citation2010). Se affects the composition and colonization of the gut microbiota which may interfere with the diversity of the microbiota and causes essential effects on microbial composition (Ferreira et al. Citation2021b). It was reported that dietary supplementation of organic Se increased the abundance of ruminococcaceae and phascolarctobacterium, and selectively inhibited the abundance of parabacteroides and prevotellaceae (Li et al. Citation2021). In a mouse model for food allergy, dietary supplementation with a Se enriched probiotic increased the proportions of Lactobacillus which shifted the cytokine production pattern from Th2 to Th1 predominance and thereby suppressed IgE and IgG1 responses and systemic allergic reactions (Shida et al. Citation2002).
Selenium and soybean allergy
Se may modulate the development of soybean allergy by affecting the mechanisms that have been described throughout this review for other (food) allergies. In a recent in vitro study performed by our group we showed that SeMet could decrease pro-inflammatory cytokine IL-12p70 and IL-6 production and increase anti-inflammatory cytokine IL-10 production in murine DCs that were previously stimulated with soybean protein. Moreover, using a co-culture system with soybean proteins treated DCs and T cell SeMet decreased the percentage of CD4 + T cells and skewed the Th2 toward a Th1 response [manuscript in preparation]. Thus, Se may influence sensitization to soybean protein by affecting DC function and T cell differentiation. To study the role of Se in the challenge phase of the allergic response we also assessed whether in vitro preincubation with SeMet would affect primary human mast cells sensitized with sera from soybean allergic patients when challenged with soybean proteins. β-hexosaminidase release, interleukin 8(IL-8)production, calcium flux and high-affinity immunoglobulin (Ig)E receptor (FcεRI) expression were measured. Our results indicated that preincubation with SeMet inhibited soy-elicited mast cell activation. Furthermore, SeMet appeared to primiarily prevent IgE binding to FcεRI rather than affecting crosslinking of IgE by the allergen [manuscript submitted]. Thus, Se may also modulate soybean allergic symptoms by suppressing mast cell responses.
Conclusions
Soybean is widely used in many food products and has been long recognized as a source of high-quality proteins. However, soybean is listed as one of the 8 most significant allergenic foods and prevalence of soybean allergy is increasing worldwide and impacts the quality of life of patients. Currently, the sole strategy for managing food allergy is strict avoidance of the offending food. Therefore, new preventive and therapeutic approaches for the management of food allergy are urgently needed. The use of interventions with micronutrients, like Se, might be an interesting new approach. In this review we describe that Se is involved in a variety of processes, including maintaining immune homeostasis, prevention of free radical damage and modulation of the gut microbiome which may all contribute in the management of food allergy both in prevention and treatment although further investigation is needed to understand the exact mechanisms. In addition, safety aspect of Se supplementation should be taken into account to avoid Se toxicity by overdosing and caution is advised when translating murine data to the human situation. Nevertheless, Se interventions might be an interesting new approach for future treatment strategies to manage soybean and food allergy in general and help to improve the quality of life for food allergic patients.
Disclosure statement
Leon Knippels in employee of Danone Nutricia Research. Johan Garssen is a part time employee of Danone Nutricia Research and Utrecht University. All remaining authors declare no conflict of interest.
Additional information
Funding
References
- Adachi, A., T. Horikawa, H. Shimizu, Y. Sarayama, T. Ogawa, S. Sjolander, A. Tanaka, and T. Moriyama. 2009. Soybean beta-conglycinin as the main allergen in a patient with food-dependent exercise-induced anaphylaxis by tofu: Food processing alters pepsin resistance. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology 39 (1):167–73. doi: 10.1111/j.1365-2222.2008.03148.x.
- Ahmadi, A., N. Poursasan, J. Amani, and J. Salimian. 2015. Adverse effect of T-2 toxin and the protective role of selenium and vitamin E on peripheral blood B lymphocytes. Iranian Journal of Immunology: IJI 12 (1):64–9.
- Al-Muhsen, S., A. E. Clarke, and R. S. Kagan. 2003. Peanut allergy: An overview. CMAJ : Canadian Medical Association Journal = Journal de L’Association Medicale Canadienne 168 (10):1279–85. https://pubmed.ncbi.nlm.nih.gov/12743075. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC154188/.
- Anagnostou, A. 2021. Optimizing patient care in egg allergy diagnosis and treatment. Journal of Asthma and Allergy 14:621–8. doi: 10.2147/JAA.S283307.
- Antonella, C. J., and M. Spergel. 2009. Food allergy: Review, classification and diagnosis. Allergology International: Official Journal of the Japanese Society of Allergology 58 (4):457–66. doi: 10.2332/allergolint.09-RAI-0138.
- Arakawa, T., H. Okubo, M. Mae, T. Okuno, H. Ogino, and H. Ueno. 2019. Seleno-L-methionine suppresses immunoglobulin e-mediated allergic response in RBL-2H3 cells. Biological & Pharmaceutical Bulletin 42 (7):1179–84. doi: 10.1248/bpb.b19-00098.
- Arakawa, T., T. Sugiyama, H. Matsuura, T. Okuno, H. Ogino, F. Sakazaki, and H. Ueno. 2018. Effects of supplementary seleno-L-methionine on atopic dermatitis-like skin lesions in mice. Biological & Pharmaceutical Bulletin 41 (9):1456–62. doi: 10.1248/bpb.b18-00349.
- Atarashi, K., T. Tanoue, T. Shima, A. Imaoka, T. Kuwahara, Y. Momose, G. Cheng, S. Yamasaki, T. Saito, Y. Ohba, et al. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science (New York, N.Y.) 331 (6015):337–41.
- Avery, J. C., and P. R. Hoffmann. 2018. Selenium, selenoproteins, and immunity. Nutrients 10 (9):1203. doi: 10.3390/nu10091203.
- Broome, C. S., F. Mcardle, J. A. Kyle, F. Andrews, N. M. Lowe, C. A. Hart, J. R. Arthur, and M. J. Jackson. 2004. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. The American Journal of Clinical Nutrition 80 (1):154–62. doi: 10.1093/ajcn/80.1.154.
- Bunyavanich, S., and M. C. Berin. 2019. Food allergy and the microbiome: Current understandings and future directions. The Journal of Allergy and Clinical Immunology 144 (6):1468–77. doi: 10.1016/j.jaci.2019.10.019.
- Burney, P. G. J., J. Potts, I. Kummeling, E. N. C. Mills, M. Clausen, R. Dubakiene, L. Barreales, C. Fernandez-Perez, M. Fernandez-Rivas, T.-M. Le, et al. 2014. The prevalence and distribution of food sensitization in European adults. Allergy 69 (3):365–71. doi: 10.1111/all.12341.
- Cai, Z., J. Zhang, and H. Li. 2019. Selenium, aging and aging-related diseases. Aging Clinical and Experimental Research 31 (8):1035–47. doi:10.1007/s40520-018-1086-7. PMID:30511318
- Cheng, W.-H., A. Holmstrom, X. Li, R. T. Y. Wu, H. Zeng, and Z. Xiao. 2012. Effect of dietary selenium and cancer cell xenograft on peripheral T and B lymphocytes in adult nude mice. Biological Trace Element Research 146 (2):230–5. doi: 10.1007/s12011-011-9235-2.
- Childs, C. E., D. Munblit, L. Ulfman, C. Gómez-Gallego, L. Lehtoranta, T. Recker, S. Salminen, M. Tiemessen, and M. C. Collado. 2021. Potential biomarkers, risk factors and their associations with IgE-mediated food allergy in early life: A narrative review. Advances in Nutrition. nmab122. doi: 10.1093/advances/nmab122.
- Cianferoni, A. 2016. Wheat allergy: Diagnosis and management. Journal of Asthma and Allergy 9:13–25. doi: 10.2147/JAA.S81550.
- Combs, G. F. 2001. Selenium in global food systems. The British Journal of Nutrition 85 (5):517–47. doi: 10.1079/bjn2000280.
- Dalgaard, T. S., M. Briens, R. M. Engberg, and C. Lauridsen. 2018. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Animal Feed Science and Technology 238:73–83. doi: 10.1016/j.anifeedsci.2018.01.020.
- Dang, T. D. 2013. Characterisation of immune differences in the development of food allergy in infants. Paediatrics (Doctoral dissertation).
- De Martinis, M., M. M. Sirufo, M. Suppa, and L. Ginaldi. 2020. New perspectives in food allergy. International Journal of Molecular Sciences 21 (4):1474. doi: 10.3390/ijms21041474.
- Ferencík, M., and L. Ebringer. 2003. Modulatory effects of selenium and zinc on the immune system. Folia Microbiologica 48 (3):417–26. doi: 10.1007/BF02931378.
- Ferreira, R. L. U., K. C. M. Sena-Evangelista, E. P. de Azevedo, F. I. Pinheiro, R. N. Cobucci, and L. F. C. Pedrosa. 2021a. Selenium in human health and gut microflora: Bioavailability of selenocompounds and relationship with diseases. Frontiers in Nutrition 8:685317. doi: 10.3389/fnut.2021.685317.
- Ferreira, R. L. U., K. C. M. Sena-Evangelista, E. P. de Azevedo, F. I. Pinheiro, R. N. Cobucci, and L. F. C. Pedrosa. 2021b. Selenium in human health and gut microflora: Bioavailability of selenocompounds and relationship with diseases. Frontiers in Nutrition 8 (292):685317. doi: 10.3389/fnut.2021.685317.
- Finley, J. W., and R. L. Kincaid. 1991. Effect of sex and time of sampling on selenium and glutathione peroxidase activity in tissues of mature rats. Biological Trace Element Research 29 (3):181–91. doi: 10.1007/BF03032676.
- Frischmeyer-Guerrerio, P. A., A. L. Guerrerio, K. L. Chichester, A. P. Bieneman, R. A. Hamilton, R. A. Wood, and J. T. Schroeder. 2011. Dendritic cell and T cell responses in children with food allergy. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology 41 (1):61–71. doi: 10.1111/j.1365-2222.2010.03606.x.
- Fukuzumi, A., N. Tokumasu, A. Matsuo, E. Yano, N. Zaima, and T. Moriyama. 2021. Detection and characterization of the soybean allergen Gly m 7 in soybeans and processed soybean foods. Allergies 1 (4):233–46. doi: 10.3390/allergies1040022.
- Gao, X., Z. Zhang, H. Xing, J. Yu, N. Zhang, and S. Xu. 2016. Selenium deficiency-induced inflammation and increased expression of regulating inflammatory cytokines in the chicken gastrointestinal tract. Biological Trace Element Research 173 (1):210–8. doi: 10.1007/s12011-016-0651-1.
- Geiselhart, S., K. Hoffmann-Sommergruber, and M. Bublin. 2018. Tree nut allergens. Molecular Immunology 100:71–81. doi: 10.1016/j.molimm.2018.03.011.
- Gonzalez-Ferrero, C., J. M. Irache, and C. J. Gonzalez-Navarro. 2018. Soybean protein-based microparticles for oral delivery of probiotics with improved stability during storage and gut resistance. Food Chemistry 239:879–88. doi: 10.1016/j.foodchem.2017.07.022.
- Gueck, T., J. R. Aschenbach, and H. Fuhrmann. 2002. Influence of vitamin E on mast cell mediator release. Veterinary Dermatology 13 (6):301–5. doi:10.1046/j.1365-3164.2002.00307.x. PMID: 12464062
- Hariharan, S., and S. Dharmaraj. 2020. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 28 (3):667–95. doi: 10.1007/s10787-020-00690-x.
- Hoffmann, F. W., A. C. Hashimoto, L. A. Shafer, S. Dow, M. J. Berry, and P. R. Hoffmann. 2010. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. The Journal of Nutrition 140 (6):1155–61. doi:10.3945/jn.109.120725. PMID: 20375261
- Hoffmann, P. R., J. L. Saux, F. W. Hoffmann, P. S. Chang, O. Bollt, Q. He, E. K. Tam, and M. J. Berry. 2007. A Role for Dietary Selenium and Selenoproteins in Allergic Airway Inflammation. Journal of Immunology (Baltimore, Md. : 1950) 179 (5):3258–67. doi: 10.4049/jimmunol.179.5.3258.
- Holzhauser, T., O. Wackermann, B. K. Ballmer-Weber, C. Bindslev-Jensen, J. Scibilia, L. Perono-Garoffo, S. Utsumi, L. K. Poulsen, and S. Vieths. 2009. Soybean (Glycine max) allergy in Europe: Gly m 5 (beta-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. Journal of Allergy and Clinical Immunology 123 (2):452–8. doi: 10.1016/j.jaci.2008.09.034.
- Ivory, K., E. Prieto, C. Spinks, C. N. Armah, A. J. Goldson, J. R. Dainty, and C. Nicoletti. 2017. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clinical Nutrition 36 (2):407–15. doi: 10.1016/j.clnu.2015.12.003.
- Iweala, O. I., S. K. Choudhary, and S. P. Commins. 2018. Food allergy. Current Gastroenterology Reports 20 (5):17. doi: 10.1007/s11894-018-0624-y.
- Jian, S. W., C. E. Mei, Y. N. Liang, D. Li, and T. Y. Cai. 2003. Influence of selenium-rich rice on transformation of umbilical blood B lymphocytes by Epstein-Barr virus and Epstein-Barr virus early antigen expression. Chinese Journal of Cancer 22 (1):26–9. [].
- Kalita, B., P. Nowak, M. Slimok, A. Sikora, and D. Sabat. 2001. Selenium plasma concentration level in children with food allergy. Polski Merkuriusz Lekarski: organ Polskiego Towarzystwa Lekarskiego 10 (60):411–3.
- Kamer, B., W. Wąsowicz, K. Pyziak, A. Kamer-Bartosińska, J. Gromadzińska, and R. Pasowska. 2012. Role of selenium and zinc in the pathogenesis of food allergy in infants and young children. Arch Med Sci 8 (6):1083–8. doi: 10.5114/aoms.2012.32420.
- Kattan, J. D., R. R. Cocco, and K. M. Jarvinen. 2011. Milk and soy allergy. Pediatric Clinics of North America 58 (2):407–26, x. doi: 10.1016/j.pcl.2011.02.005.
- Katz, Y., P. Gutierrez-Castrellon, M. G. Gonzalez, R. Rivas, B. W. Lee, and P. Alarcon. 2014. A comprehensive review of sensitization and allergy to soy-based products. Clinical Reviews in Allergy & Immunology 46 (3):272–81. doi: 10.1007/s12016-013-8404-9.
- Khora, S. S. 2016. Seafood-associated shellfish allergy: A comprehensive review. Immunological Investigations 45 (6):504–30. doi: 10.1080/08820139.2016.1180301.
- Kim, H.-Y. 2013. The methionine sulfoxide reduction system: Selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxid Redox Signal 19 (9):958–69. doi: 10.1089/ars.2012.5081.
- Kim, H., A. R. Sitarik, K. Woodcroft, C. C. Johnson, and E. Zoratti. 2019. Birth mode, breastfeeding, pet exposure, and antibiotic use: Associations with the gut microbiome and sensitization in children. Current Allergy and Asthma Reports 19 (4):22. doi: 10.1007/s11882-019-0851-9.
- Kirby, M., and E. Danner. 2009. Nutritional deficiencies in children on restricted diets. Pediatric Clinics of North America 56 (5):1085–103. doi: 10.1016/j.pcl.2009.07.003.
- Kleine-Tebbe, J., L. Vogel, D. N. Crowell, U.-F. Haustein, and S. Vieths. 2002. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1-related PR-10 protein in soybean, SAM22. The Journal of Allergy and Clinical Immunology 110 (5):797–804. doi: 10.1067/mai.2002.128946.
- Kumar, H., M. C. Collado, H. Wopereis, S. Salminen, J. Knol, and G. Roeselers. 2020. The bifidogenic effect revisited-ecology and health perspectives of bifidobacterial colonization in early life. Microorganisms 8 (12):1855. doi: 10.3390/microorganisms8121855.
- Labunskyy, V. M., D. L. Hatfield, and V. N. Gladyshev. 2014. Selenoproteins: Molecular pathways and physiological roles. Physiological Reviews 94 (3):739–77. doi: 10.1152/physrev.00039.2013.
- Lao-Araya, M., and M. Trakultivakorn. 2012. Prevalence of food allergy among preschool children in northern Thailand. Pediatrics International: Official Journal of the Japan Pediatric Society 54 (2):238–43. doi: 10.1111/j.1442-200X.2011.03544.x.
- Lee, H.-J., J. S. Park, H. J. Yoo, H. M. Lee, B. C. Lee, and J. H. Kim. 2020. The selenoprotein msrb1 instructs dendritic cells to induce T-Helper 1 immune responses. Antioxidants 9 (10):1021. doi: 10.3390/antiox9101021.
- Li, Z., Y. Dong, S. Chen, X. Jia, X. Jiang, L. Che, Y. Lin, J. Li, B. Feng, Z. Fang, et al. 2021. Organic selenium increased gilts antioxidant capacity, immune function, and changed intestinal microbiota. Frontiers in Microbiology 12:723190. doi: 10.3389/fmicb.2021.723190.
- Lichtenstein, P., and M. Svartengren. 1997. Genes, environments, and sex: Factors of importance in atopic diseases in 7-9-year-old Swedish twins . Allergy 52 (11):1079–1086. doi: 10.1111/j.1398-9995.1997.tb00179.x.
- Lu, J., and A. Holmgren. 2014. The thioredoxin antioxidant system. Free Radical Biology & Medicine 66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036.
- Maggini, S., A. Pierre, and P. C. Calder. 2018. Immune function and micronutrient requirements change over the life course. Nutrients 10 (10):1531. doi: 10.3390/nu10101531.
- Markwell, T. A. B. M. G. Z. G. S. J. P. 2000. Soybean Glycinin G1 Acidic Chain Shares IgE Epitopes with Peanut Allergen Ara h 3. International Archives of Allergy and Immunology 123:8.
- Martino, M. P., Luca, M. M. Amato, A. G. L. Galli, L. Lega, C. Azzari, and A. Vierucci. 1993. Fish allergy in children. Ann Allergy 71 (2):159–165.
- Matsue, H., D. Edelbaum, D. Shalhevet, N. Mizumoto, C. Yang, M. E. Mummert, J. Oeda, H. Masayasu, and A. Takashima. 2003. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. Journal of Immunology (Baltimore, Md.: 1950) 171 (6):3010–3018. doi: 10.4049/jimmunol.171.6.3010.
- Matsue, H., C. Yang, K. Matsue, D. Edelbaum, M. Mummert, and A. Takashima. 2002. Contrasting impacts of immunosuppressive agents (Rapamycin, FK506, Cyclosporin A, and Dexamethasone) on bidirectional dendritic cell-T cell interaction during antigen presentation. Journal of Immunology (Baltimore, Md.: 1950) 169 (7):3555–3564. doi: 10.4049/jimmunol.169.7.3555.
- Meyer, R., C. De Koker, R. Dziubak, H. Godwin, G. Dominguez-Ortega, and N. Shah. 2014. Dietary elimination of children with food protein induced gastrointestinal allergy – Micronutrient adequacy with and without a hypoallergenic formula? Clinical and Translational Allergy 4 (1):31–31. doi: 10.1186/2045-7022-4-31.
- Miles, E. A., and P. C. Calder. 2017. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients 9 (7):784. doi: 10.3390/nu9070784.
- Mittag, D., S. Vieths, L. Vogel, W. M. Becker, H. P. Rihs, A. Helbling, B. Wuthrich, and B. K. Ballmer-Weber. 2004. Soybean allergy in patients allergic to birch pollen: Clinical investigation and molecular characterization of allergens. The Journal of Allergy and Clinical Immunology 113 (1):148–154. doi: 10.1016/j.jaci.2003.09.030.
- Mittal, M., M. R. Siddiqui, K. Tran, S. P. Reddy, and A. B. Malik. 2014. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling 20 (7):1126–67. doi:10.1089/ars.2012.5149. PMID: 23991888
- Monsen, E. R. 2000. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. Journal of the Academy of Nutrition and Dietetics 100 (6):637.
- Muzembo, B. A., N. R. Ngatu, K. Januka, H.-L. Huang, C. Nattadech, T. Suzuki, K. Wada, and S. Ikeda. 2019. Selenium supplementation in HIV-infected individuals: A systematic review of randomized controlled trials. Clinical Nutrition ESPEN 34:1–7. doi: 10.1016/j.clnesp.2019.09.005.
- Nakai, K., K. Yoneda, and Y. Kubota. 2012. Oxidative stress in allergic and irritant dermatitis: From basic research to clinical management. Recent Patents on Inflammation & Allergy Drug Discovery 6 (3):202–209. doi: 10.2174/187221312802652839.
- Narod, S. A., T. Huzarski, A. Jakubowska, J. Gronwald, C. Cybulski, O. Oszurek, T. Dębniak, K. Jaworska-Bieniek, M. Lener, K. Białkowska, et al. 2019. Serum selenium level and cancer risk: A nested case-control study. Hereditary Cancer in Clinical Practice 17:33. doi: 10.1186/s13053-019-0131-7.
- Nwaru, B. I., L. Hickstein, S. S. Panesar, G. Roberts, A. Muraro, A. Sheikh, E. F. Allergy, and G. A. Guidelines, the EAACI Food Allergy and Anaphylaxis Guidelines Group. 2014. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 69 (8):992–1007. doi: 10.1111/all.12423.
- Ogawa, T., N. Bando, H. Tsuji, K. Nishikawa, and K. Kitamura. 1995. Alpha-subunit of beta-conglycinin, an allergenic protein recognized by IgE antibodies of soybean-sensitive patients with atopic dermatitis. Bioscience, Biotechnology, and Biochemistry 59 (5):831–833. doi: 10.1271/bbb.59.831.
- Oliveira, KASd, M. T. Esper, MLd Oliveira, M. H. C. Tofoli, and M. A. G. Avelino. 2021. Correlation between cow’s milk protein allergy and otitis media: A systematic review. Brazilian Journal of Otorhinolaryngology. S1808-8694 (21):00150-00156. doi: 10.1016/j.bjorl.2021.07.005.
- Oropeza-Moe, M., H. Wisloff, and A. Bernhoft. 2015. Selenium deficiency associated porcine and human cardiomyopathies. Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (GMS) 31:148–156. doi: 10.1016/j.jtemb.2014.09.011.
- Panjari, M., J. J. Koplin, S. C. Dharmage, R. L. Peters, L. C. Gurrin, S. M. Sawyer, V. McWilliam, J. K. Eckert, D. Vicendese, B. Erbas, et al. 2016. Nut allergy prevalence and differences between Asian‐born children and Australian‐born children of Asian descent: A state‐wide survey of children at primary school entry in Victoria, Australia. Clinical & Experimental Allergy 46 (4):602–609. doi: 10.1111/cea.12699.
- Pavlata, L., L. M. A. Pechová, and R. Dvořák. 2012. Comparison of organic and inorganic forms of selenium in the mother and kid relationship in goats. Czech Journal of Animal Science 57:4.
- Pyrhönen, K., and P. Kulmala. 2022. Delivery mode and the incidence of atopic sensitization and food allergy in a Finnish child population. Pediatric Allergy and Immunology 33 (1):. 3584. doi: 10.1111/pai.13584.
- Rayman, M. P. 2000. The importance of selenium to human health. The Lancet 356 (9225):233–241. doi: 10.1016/S0140-6736(00)02490-9.
- Riascos, J. J., S. M. Weissinger, A. K. Weissinger, M. Kulis, A. W. Burks, and L. Pons. 2016. The seed biotinylated protein of soybean (Glycine max): A boiling-resistant new allergen (Gly m 7) with the capacity to induce IgE-mediated allergic responses. Journal of Agricultural and Food Chemistry 64 (19):3890–3900. doi: 10.1021/acs.jafc.5b05873.
- Roman, M., P. Jitaru, and C. Barbante. 2014. Selenium biochemistry and its role for human health. Metallomics: Integrated Biometal Science 6 (1):25–54. doi: 10.1039/c3mt00185g.
- Round, J. L., and S. K. Mazmanian. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America 107 (27):12204–12209. doi: 10.1073/pnas.0909122107.
- Safaralizadeh, R., M. Nourizadeh, A. Zare, G. A. Kardar, and Z. Pourpak. 2013. Influence of Selenium on Mast Cell Mediator Release. Biological Trace Element Research 154 (2):299–303. doi: 10.1007/s12011-013-9712-x.
- Sang, L. X., B. Chang, J. F. Zhu, F. L. Yang, Y. Li, X. F. Jiang, D. N. Wang, C. L. Lu, and X. Sun. 2017. Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and γδT and increasing CD4(+)CD25(+) regulatory T-cell responses. World Journal of Gastroenterology 23:13.
- Schomburg, L., U. Schweizer, B. Holtmann, L. Flohé, M. Sendtner, and J. J. B. J. Köhrle. 2003. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. The Biochemical Journal 370 (Pt 2):397–402. doi: 10.1042/BJ20021853.
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for selenium. 2014. EFSA Journal 12 (10):3846–3913. doi: 10.2903/j.efsa.2014.3846.
- Shida, K., R. Takahashi, E. Iwadate, K. Takamizawa, H. Yasui, T. Sato, S. Habu, S. Hachimura, and S. Kaminogawa. 2002. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clinical & Experimental Allergy 32 (4):563–570. doi: 10.1046/j.0954-7894.2002.01354.x.
- Sicherer, S. H. 2011. Epidemiology of food allergy. The Journal of Allergy and Clinical Immunology 127 (3):594–602. doi: 10.1016/j.jaci.2010.11.044.
- Sicherer, S. H., and H. A. Sampson. 2014. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. The Journal of Allergy and Clinical Immunology 133 (2):291–307; quiz 308. doi: 10.1016/j.jaci.2013.11.020.
- Singh, Y., A. M. Nair, and P. K. Verma. 2021. Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant Communications 2 (3):100142. doi: 10.1016/j.xplc.2021.100142.
- Skoczylas, D., M. Gujski, I. Bojar, and F. Raciborski. 2020. Importance of food allergy and food intolerance in allergic multimorbidity. Annals of Agricultural and Environmental Medicine 27 (3):413–417. doi: 10.26444/aaem/123107.
- Soriano, V., A.-L. Ponsonby, K. Allen, V. Soriano, A.-L. Ponsonby, K. Allen, V. Soriano, A.-L. Ponsonby, K. Allen, and V. Soriano. 2020. Potential factors related to food allergy development. Pediatric Food Allergy. Cham: Springer. doi: 10.1007/978-3-030-33292-1_10.
- Steinbrenner, H., S. Al-Quraishy, M. A. Dkhil, F. Wunderlich, and H. Sies. 2015. Dietary selenium in adjuvant therapy of viral and bacterial infections. Advances in Nutrition (Bethesda, Md.) 6 (1):73–82. doi: 10.3945/an.114.007575.
- Suaini, N. H. A., E. X. ‐L. Loo, R. L. Peters, G. C. Yap, K. J. Allen, H. Van Bever, D. J. Martino, A. E. N. Goh, S. C. Dharmage, M. T. Colega, et al. 2021. Children of Asian ethnicity in Australia have higher risk of food allergy and early-onset eczema than those in Singapore. Allergy 76 (10):3171–3182. doi: 10.1111/all.14823.
- Sudo, N., S-a Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. Journal of Immunology (Baltimore, Md.: 1950) 159 (4):1739–1745.
- Sun, Z., X. Zhe, D. Wang, H. Yao, and L. Shu. 2018. Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics : Integrated Biometal Science 10 (5):759–767. doi: 10.1039/C8MT00039E.
- Sung, D., K. M. Ahn, S. Y. Lim, and S. Oh. 2014. Allergenicity of an enzymatic hydrolysate of soybean 2S protein. Journal of the Science of Food and Agriculture 94 (12):2482–2487. doi: 10.1002/jsfa.6583.
- Taliercio, E., T. M. Loveless, M. J. Turano, and S. W. Kim. 2014. Identification of epitopes of the β subunit of soybean β-conglycinin that are antigenic in pigs, dogs, rabbits and fish. Journal of the Science of Food and Agriculture 94 (11):2289–2294. doi: 10.1002/jsfa.6556.
- Tanaka, T., H. Nakamura, A. Nishiyama, F. Hosoi, H. Masutani, H. Wada, and J. Yodoi. 2000. Redox regulation by thioredoxin superfamily; protection against oxidative stress and aging. Free Radical Research 33 (6):851–855. doi: 10.1080/10715760000301361.
- Thomson, C. D. 2004. Assessment of requirements for selenium and adequacy of selenium status: A review. European Journal of Clinical Nutrition 58 (3):391–402. doi: 10.1038/sj.ejcn.1601800.
- Tsai, J. J., C. Y. Chang, and E. C. Liao. 2017. Comparison of Allergenicity at Gly m 4 and Gly m Bd 30K of Soybean after Genetic Modification. Journal of Agricultural and Food Chemistry 65 (6):1255–1262. doi: 10.1021/acs.jafc.6b05135.
- Values, GBPoDR. 1991. Dietary reference values for food energy and nutrients for the United Kingdom: report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy: Dietary reference values for food energy and nutrients for the United Kingdom: report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy.
- Vieths, BKB-WaS. 2008. Soy allergy in perspective. Current Opinion in Allergy and Clinical Immunology 8:6.
- Wang, G. Q., H. H. Wang, and H. X. Wang. 2012. Effects of Se deficiency on serum histamine concentration and the expression of histamine H2 receptor in the jejunum of chickens. Polish Journal of Veterinary Sciences 15 (3):547–552. doi: 10.2478/v10181-012-0084-5.
- Wilson, S., K. Blaschek, and E. G. De Mejia. 2005. Allergenic proteins in soybean: Processing and reduction of P34 allergenicity. Nutrition Reviews 63 (2):47–58. doi: 10.1301/nr.2005.feb.47-58.
- Won, H. Y., J. H. Sohn, H. J. Min, K. Lee, H. A. Woo, Y. S. Ho, J. W. Park, S. G. Rhee, and E. S. Hwang. 2010. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxidants & Redox Signaling 13 (5):575–587. doi: 10.1089/ars.2009.2989.
- Wopereis, H., R. Oozeer, K. Knipping, C. Belzer, and J. Knol. 2014. The first thousand days - intestinal microbiology of early life: Establishing a symbiosis. Pediatric Allergy and Immunology: Official Publication of the European Society of Pediatric Allergy and Immunology 25 (5):428–438. doi: 10.1111/pai.12232.
- Wopereis, H., K. Sim, A. Shaw, J. O. Warner, J. Knol, and J. S. Kroll. 2018. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. The Journal of Allergy and Clinical Immunology 141 (4):1334–1342.e1335. doi: 10.1016/j.jaci.2017.05.054.
- Xue, H., W. Wang, Y. Li, Z. Shan, Y. Li, X. Teng, G. Yun, C. Fan, and W. Teng. 2010. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocrine Journal 57 (7):595–601. doi: 10.1507/endocrj.K10E-063.
- Yang, Y., X. Li, Y. Yang, S. Shoaie, C. Zhang, B. Ji, and Y. Wei. 2021. Advances in the relationships between cow’s milk protein allergy and gut microbiota in infants. Frontiers in Microbiology 12:716667–716667. doi: 10.3389/fmicb.2021.716667.
- Yu, W., D. M. H. Freeland, and K. C. Nadeau. 2016. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nature Reviews. Immunology 16 (12):751–765. doi: 10.1038/nri.2016.111.
- Zhang, Y., Y. J. Roh, S.-J. Han, I. Park, H. M. Lee, Y. S. Ok, B. C. Lee, and S.-R. Lee. 2020. Role of selenoproteins in redox regulation of signaling and the antioxidant system: A review. Antioxidants 9 (5):383. doi: 10.3390/antiox9050383.
- Zhao, X., S. Thijssen, H. Chen, J. Garssen, L. M. J. Knippels, and A. Hogenkamp. 2021. Selenium modulates the allergic response to whey protein in a mouse model for cow’s milk allergy. Nutrients 13 (8):2479. https://www.mdpi.com/2072-6643/13/8/2479. doi: 10.3390/nu13082479.
- Zhao, X., Q. Zhao, H. Chen, and H. Xiong. 2019. Distribution and effects of natural selenium in soybean proteins and its protective role in soybean β-conglycinin (7S globulins) under AAPH-induced oxidative stress. Food Chemistry 272:201–209. doi: 10.1016/j.foodchem.2018.08.039.
- Zheng, S., G. Qin, J. Chen, and F. Zhang. 2018. Acidic polypeptides A1a, A3 and A4 of Gly m 6 (glycinin) are allergenic for piglets. Veterinary Immunology and Immunopathology 202:147–152. doi: 10.1016/j.vetimm.2018.06.003.
- Zheng, S., G. Qin, H. Tian, and F. Zhang. 2017. Three-dimensional structure of Gly m 5 (β-conglycinin) plays an important role in its stability and overall allergenicity. Food Chemistry 234:381–388. doi: 10.1016/j.foodchem.2017.05.020.
- Zuidmeer, L., K. Goldhahn, R. J. Rona, D. Gislason, C. Madsen, C. Summers, E. Sodergren, J. Dahlstrom, T. Lindner, S. T. Sigurdardottir, et al. 2008. The prevalence of plant food allergies: A systematic review. Journal of Allergy and Clinical Immunology 121 (5):1210–1218, e1214. doi: 10.1016/j.jaci.2008.02.019.
