 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Sulfur is essential for the health of plants and is an indispensable dietary component for human health and disease prevention. Its incorporation into our food supply is heavily reliant upon the uptake of sulfur into plant tissue and our subsequent intake. Dietary requirements for sulfur are largely calculated based upon requirements for the sulfur-containing amino acids (SAA), cysteine and methionine, to meet the demands for synthesis of proteins, enzymes, co-enzymes, vitamins, and hormones. SAA are found in abundance in animal sources and are relatively low in plants. However, some plants, particularly cruciferous and allium vegetables, produce many protective sulfur-containing secondary metabolites, such as glucosinolates and cysteine sulfoxides. The variety and quantity of these sulfur-containing metabolites are extensive and their effects on human health are wide-reaching. Many benefits appear to be related to sulfur’s role in redox biochemistry, protecting against uncontrolled oxidative stress and inflammation; features consistent within cardiometabolic dysfunction and many chronic metabolic diseases of aging. This narrative explores the origins and importance of sulfur, its incorporation into our food supply and dietary sources. It also explores the overarching potential of sulfur for human health, particularly around the amelioration of oxidative stress and chronic inflammation, and subsequent chronic disease prevention.
Introduction
Sulfur is essential for the successful growth of plants, and many biologically important pathways in both animals and humans. Within plants, a key role of sulfur is to provide defense against pathogens and facilitate healthy plant growth (Nwachukwu, Slusarenko, and Gruhlke Citation2012). Within humans, sulfur is widely acknowledged as a component of two principal sulfur-containing amino acids (SAA); cysteine and methionine; and subsequent protein recycling (Ingenbleek and Kimura Citation2013). Sulfur is also necessary for the synthesis of key compounds with central roles in metabolic health and homeostasis (Ingenbleek and Kimura Citation2013). Such examples include the production of S-adenosylmethionine (SAM), -glutamyl-cysteine-glycine, lipoic acid, various hormones, some vitamins (thiamine, biotin), enzymes, co-enzymes (coenzyme A), other amino acids (cystine, taurine, homocysteine) and varying cofactors (Ingenbleek and Kimura Citation2013). Furthermore, sulfur-containing compounds are an integral component of many antioxidants important for intracellular defense mechanisms, such as glutathione (GSH), lipoic acid, and N-acetyl cysteine (Colovic et al. Citation2018). However, apart from some marine microorganisms, e.g., algae and microbacteria, animals are unable to utilize sulfur in its organic form. Sulfur must first be sequestered from the environment and become ‘fixed’ into an organic, living, carbon-based structure, i.e., plant tissue (Francioso et al. Citation2020). Animals, including humans, then rely on the consumption of sulfur-containing plant tissue for their sulfur intake (Maruyama-Nakashita Citation2017). This intake can be divided into either SAA (predominantly, although not exclusively, animal proteins) or sulfur-containing secondary metabolites (predominantly plant-based sources). Importantly, higher-order animals (i.e., humans) can synthesize additional sulfur-based compounds following consumption (Francioso et al. Citation2020). To date, dietary recommendations for sulfur are largely based upon the well-established proteinogenic roles of SAA. Yet the overarching importance of plant-based, non-SAA compounds for human health and disease prevention is gaining traction. These plant-derived sulfur-containing secondary metabolites play key roles in antioxidant and anti-inflammatory pathways yet are overlooked within our dietary recommendations.
Oxidative stressors come in several forms; as by-products of normal internal metabolism, or from external environmental and xenobiotic influences (Mukwevho, Ferreira, and Ayeleso Citation2014). When given inadequate antioxidant defenses to maintain homeostasis against stressors, a hyper-oxidative state can occur, resulting in elevated oxidative stress, inflammation, protein and lipid peroxidation, as well as cellular membrane and DNA damage (Mukwevho, Ferreira, and Ayeleso Citation2014). Uncontrolled oxidative stress, activation of innate immunity and the ensuing pro-inflammatory response contributes to accelerated aging in humans, with the concomitant low-grade chronic inflammation aptly coined ‘inflammaging’ (Livshits and Kalinkovich Citation2019; Liguori et al. Citation2018; Franceschi et al. Citation2018). Elevated oxidative stress and inflammation have been implicated in the pathogenesis of many lifestyle-related chronic cardiometabolic diseases, such as atherosclerosis-related cardiovascular disease (CVD), vascular disorders, hypertension and chronic kidney disease, dyslipidemia, poor glucose regulation, cognitive decline and some cancers (da Costa et al. Citation2019; Ahmed et al. Citation2017; Ferrucci and Fabbri Citation2018; Ruhee et al. Citation2020). Furthermore, chronic metabolic-related diseases are the largest contributor to death globally (Raghupathi and Raghupathi Citation2018; World Health Organisation Citation2018). In humans, dietary intake of sulfur has wide-reaching activity across multiple biological pathways, including antioxidant defense pathways making it essential for our health and wellbeing (Mukwevho, Ferreira, and Ayeleso Citation2014). Despite this, an important, yet often overlooked source of sulfur is found within sulfur-rich vegetables, such as cruciferous (e.g., broccoli, cauliflower, cabbage) and allium (e.g., garlic, onion, leek) vegetables, in the form of sulfur-containing secondary metabolites. There have been numerous health benefits associated with higher consumption of sulfur-rich vegetables, including cardiovascular, glycemic, neuroprotective, anticancer, anti-microbial, anti-inflammatory, and antioxidant (Petropoulos, Di Gioia, and Ntatsi Citation2017). This narrative provides insight into the origins of sulfur within our environment, its incorporation into our food supply, dietary sources, and their overarching importance in human health. We particularly focus upon sulfur’s potential in ameliorating chronic pro-oxidation and hyper-inflammatory pathways and subsequent, chronic disease prevention.
The origins of sulfur
Environmental sources
Sulfur is widely found in various forms in nature; as free sulfur, inorganic sulfides and sulfates, sulfur oxides, sulfurous gases as well as bound within organosulfur compounds in plant tissues and living organisms (Hawkesford Citation2007; De Kok et al. Citation2007; Stefels Citation2007; Tausz Citation2007). The largest source of inorganic sulfur is found within our marine (aquatic) environments (which include wetlands, salt marshes and estuaries). A diverse number of water-based microorganisms such as phytoplankton, algae and bacteria, release sulfur gases, such as hydrogen sulfide (H2S) into the atmosphere (Francioso et al. Citation2020; De Kok et al. Citation2007; Stefels Citation2007). Another substantial source of sulfur is of anthropogenic origin, emitted during the industrial processing of minerals, petroleum oils and coal (De Kok et al. Citation2007). An especially high terrestrial source comes from volcanic and geothermic emissions spilling into land reservoirs (Francioso et al. Citation2020; Hawkesford Citation2007). The continual recycling of sulfur between the terrestrial, aquatic and atmospheric environments are collectively referred to as our global sulfur cycle (Hawkesford Citation2007). This global sulfur cycle continuously feeds sulfur into plant tissue in its reduced form, i.e., as sulfate, with very sophisticated adaptive mechanisms regulating its uptake, which in turn are strongly influenced by demand, availability, and environmental conditions.
Metabolism in plants
Sulfur is essential for the formation of proteins, vitamins, enzymes, and defensive compounds in plants, as well as the fixation of, and optimal utilization of nitrogen to enhance plant yield (Maruyama-Nakashita Citation2017; Hawkesford Citation2007). Consequently, sulfur is considered an essential nutrient for the growth and health of all plants. Sulfur deficiency results in stunted plant growth, reduction in crop yield and lowered plant defense (Künstler et al. Citation2020; Hawkesford Citation2007; Maruyama-Nakashita Citation2017). Therefore, the application of sulfur-rich fertilizers, during periods of plant growth is a common agricultural practice (Petropoulos, Di Gioia, and Ntatsi Citation2017; Künstler et al. Citation2020).
The amount of sulfur taken up by a plant is primarily influenced by the amount readily available within the soil, as well as the actions of sulfur-sensing transporters (Maruyama-Nakashita Citation2017; Gigolashvili and Kopriva Citation2014). The assimilation of sulfur, as well as its formation into varying sulfur-based compounds, is influenced not only by abiotic environmental conditions (e.g., water, temperature, salinity, nutrient supply), but also by the need for resistance against biotic stressors (Künstler et al. Citation2020; Nguyen et al. Citation2020).
Sulfate to cysteine
Sulfate is primarily taken up through the plant root system from the rhizosphere by way of sulfur-sensing sulfate transporters located within root epidermal cells and this process is influenced by several regulatory proteins and enzymes inside plant cells (Maruyama-Nakashita Citation2017). The uptake and assimilation into plant tissue is tightly regulated to maintain the supply and demand for sulfur (Künstler et al. Citation2020). Whilst some sulfate is stored in root cell vacuoles, the majority is transported into the xylem, whose primary role is to passively transport water and dissolved minerals to the shoots of growing plants, where a sulfur reservoir is maintained through a series of reducing reactions to trap and incorporate sulfur into a variety of cellular metabolites (Hawkesford Citation2007). Much of this reducing activity occurs within the adenosine triphosphate (ATP) and reductant rich chloroplasts found in the photosynthetic tissues of plants (Hawkesford Citation2007). After activation by ATP sulfurylase to form adenosine-5′-phosphosulfate (APS), APS reductase (APR) reduces the APS to sulfite, and lastly ferridoxin-dependent sulfite reductase (SiR) catalyzes the six-electron reduction to sulfide (Maruyama-Nakashita Citation2017; Jez Citation2019; Khan et al. Citation2010). Sulfide, in the presence of O-acetyl-L-serine, is acted upon by O-acetylserine(thiol)lyase (OASTL) in the cytosol, chloroplasts and mitochondria of plant cells to form cysteine (Romero et al. Citation2014). Cysteine is the first organic form of sulfur available in plant metabolism (Maruyama-Nakashita Citation2017). Cysteine is then incorporated into thousands of different proteins in plants through protein synthesis in the cytosol, chloroplast and mitochondrion and is also the critical intermediary step for assimilation of sulfur into many beneficial sulfur-containing compounds (Hawkesford Citation2007). A diagrammatic overview indicating sulfur metabolism in plants is illustrated in .
Figure 1. Overview of sulfur metabolism in plants; adapted from (Jez Citation2019; Maruyama-Nakashita Citation2017).
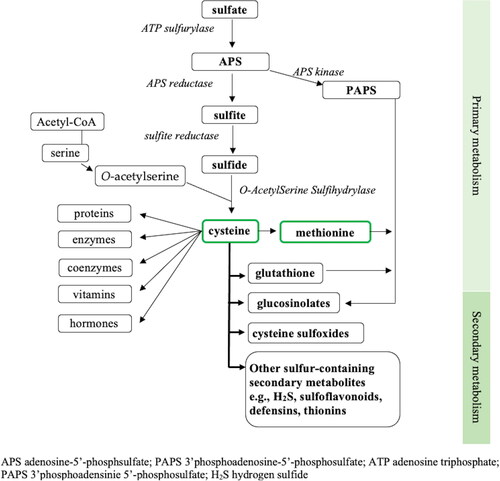
Cysteine to sulfur-based compounds
Plant metabolism is divided into (1) primary metabolism, required for plant survival and widely conserved in the plant kingdom, and (2) secondary metabolism that is less conserved and often specific to different plant species. Primary sulfur metabolism involves the initial steps of converting sulfur through to cysteine, methionine and GSH (Maruyama-Nakashita Citation2017). Secondary sulfur metabolism typically refers to the subsequent production of sulfur-containing secondary metabolites (Künstler et al. Citation2020). The sulfur-containing secondary metabolites, sometimes referred to as ‘defensive compounds’ can act as either a phytoanticipin; an antimicrobial compound already present in anticipation of pathogenic exposure, or as a phytoalexin; a compound synthesized in response to ward off stress and improve defense mechanisms (Nwachukwu and Slusarenko Citation2014; Nwachukwu, Slusarenko, and Gruhlke Citation2012). Sulfur-containing secondary metabolites can include glucosinolates, thiosulfinates (e.g., allicin, derived from cysteine sulfoxides), reactive sulfur species (e.g., H2S and sodium sulfate), and antimicrobial peptides (e.g., defensins and thionins) (Künstler et al. Citation2020).
Glutathione is a primary sulfur-containing tripeptide (glycine, glutamate and cysteine), synthesized by the action of -glutamylcysteine synthetase (aka glutamate-cysteine ligase) and GSH synthetase (Jez Citation2019). In a normal physiological non-stressed state, glutathione exists 90% in its non-oxidized/reduced form (GSH) and 10% in its oxidized form (glutathione disulfide [GSSG]) (Künstler et al. Citation2020; Zechmann Citation2020). GSH has a well-recognized role as a central regulator for plant redox signaling, mediating plant defenses, mitigating the damage of ROS and activating defensive genes in plants (Jez Citation2019; Künstler et al. Citation2020; Zechmann Citation2020). Cellular GSH is distributed throughout the plant cells in both the cytosol and major organelles (Zechmann Citation2020). GSH is readily oxidized to GSSG when its cysteine residues are exposed to electrophilic substances (e.g., ROS, free radicals). GSH-transporters can re-distribute oxidized GSH (i.e., GSSG) to various subcellular components of the cells in response to attack, thereby triggering defensive compounds such as salicylic acid, jasmonic acid, auxins, abscisic acid and ethylenes within the plant tissue (Künstler et al. Citation2020; Zechmann Citation2020). Aside from being a major plant defensive compound, GSH is also required for the synthesis of many glucosinolates in plants; a process involving conjugation of cysteine with an amino acid, (e.g., methionine, tryptophan), in the presence of 3-phosphoadenosine-5′-phosphosulfate (PAPS) and GSH. (Maruyama-Nakashita Citation2017; Mugford et al. Citation2011).
Glucosinolates
Glucosinolates are sulfur-containing non-reactive phytoanticipins found in the plant order Brassicales which include a number of major vegetable crop plants (e.g., broccoli, cabbage, cauliflower, etc.). Glucosinolates are constructed from a sulfonated oxime group that is linked to a thioglucose group and a side chain of an amino-acid (Esteve Citation2020). These molecules are classified into three categories, aliphatic, benzenic or indolic, according to the amino acids that provide the side chain. The side chain for the aliphatic glucosinolates are derived from alanine, valine, leucine, isoleucine, or methionine, the benzenic (also commonly referred to as ‘aromatic’) are derived from phenylalanine or tyrosine, whilst the indolic are derived from tryptophan (Mitreiter and Gigolashvili Citation2021; Nguyen et al. Citation2020). In their native state, glucosinolates are stable and are not normally biologically reactive. They only fulfill significant functions when they are activated following stress (pathogenic or otherwise) through hydrolysis of their glucose residue by myrosinases (also known as thioglucosides) to form isothiocyanates, thiocyanates, nitriles and epithionitriles within plant tissues (Künstler et al. Citation2020). In plants, glucosinolates and myrosinases are kept spatially separate, either in different compartments of the same cell or in different cell types (Kliebenstein, Kroymann, and Mitchell-Olds Citation2005). They typically only come together to form isothiocyanates, thiocyanates, nitriles and epithionitriles when cells are broken through herbivory or disease. Glucosinolates are well recognized for their protective roles against oxidative stress and pathogen resistance in plants following their synthesis, storage, release, and conversion to defense compounds through hydrolysis (Künstler et al. Citation2020; Zechmann Citation2020).
At one time it was thought glucosinolate production was not regulated in plants, largely due to the wide diversity of levels and types in many different growth conditions and following plant treatments (Mitreiter and Gigolashvili Citation2021). However, it is now known that this complexity arises from control systems that are indeed highly complex, containing multiple layers which are interconnected to other parts of plant metabolism. As a fortunate accident of history, Arabidopsis thaliana, the first plant to have its genome sequenced and the ‘lab rat’ of plant science, is itself from the Brassicaceae plant family and produces over 40 glucosinolates (Sønderby et al. Citation2010). This has resulted in extensive investigation into the mechanism of control of glucosinolate synthesis. A strong level of control of glucosinolate production is necessary as the maximal rate can use up to 15% of the energy from photosynthesis at any one time (Bekaert et al. Citation2012). Fitness benefits from glucosinolate production are seen during intra-specific competition between high and low glucosinolate varieties when they are challenged by herbivores (Züst et al. Citation2011). Research in Arabidopsis has shown that a series of myelobastosis (MYB) and basic helix-loop-helix (bHLH) transcription factors form a transcriptional complex that regulates glucosinolate production in plants and makes it responsive to a wide variety of biotic and abiotic stresses (Mitreiter and Gigolashvili Citation2021). This transcriptional hub is also highly responsive to an array of plant hormones, enabling the accumulation of glucosinolates to be finely tuned to the potential need for their deployment as defense compounds. The most extensive work linking glucosinolates with hormone levels has been performed in the context of the jasmonate (JA) signaling pathway. When plants are attacked such as in the presence of pathogens or herbivores (Schweizer et al. Citation2013), mechanical damage or even physical touch through rainfall (Van Moerkercke et al. Citation2019), it is perceived by plant pattern recognition receptors which induce JA synthesis, thereby directly activating MYB transcriptional factors and subsequent glucosinolate production pathways (Mitreiter and Gigolashvili Citation2021).
S-Alk(en)yl cysteine sulfoxides
S-alk(en)yl cysteine sulfoxides (ASCOs) are found predominantly within the allium family of vegetables (i.e., garlic, onion, leeks) and their thiosulfinate derivatives are behind much of the aroma and flavor compounds of these vegetables (Yamazaki et al. Citation2010). Around ten ASCOs have been identified to date in various alliums (including homoisoalliin, butiin, cycloalliin, deoxyalliin, ethiin and S-ethyl-L-cysteine sulfoxide) (Petropoulos, Di Gioia, and Ntatsi Citation2017), although the dominant cysteine sulfoxides are alliin, methiin, isoalliin and, to a lesser extent propiin (Horníčková et al. Citation2010; Rektorisova et al. Citation2020; Rose et al. Citation2019).
The majority of sulfur found in alliums is present within -glutamyl S-alk(en)yl cysteines (GSAkCs) and
-glutamyl S-alk(en)yl cysteine sulfoxides, in addition to S-alk(en)ylglutathione, various selenomethionine and GSH conjugates, each acting as precursory intermediates in ASCO production (Kodera et al. Citation2020; Yoshimoto and Saito Citation2019). Although the synthesis of ASCOs is not yet fully understood, it is assumed that GSAkCs undergo deglutamylation and S-oxygenation to yield their relevant ASCO (Yoshimoto and Saito Citation2019). For example,
-glutamyl S-allylcysteine converts to S-allylcysteine before becoming the ASCO, S-allyl cysteine sulfoxide (i.e., alliin) (Kodera et al. Citation2020). A similar process occurs for the remaining ASCOs (i.e.,
-glutamyl-S-methylcysteine becomes S-methylcysteine sulfoxide [methiin];
-glutamyl-S-1-propenylcysteine becomes S-propenyl cysteine sulfoxide [isoalliin] and
-glutamyl-S-1-propylcysteine becomes S-propylcysteine sulfoxide [propiin]) (Yoshimoto and Saito Citation2019; Rektorisova et al. Citation2020). Alternatively, the production of methiin has been found to result from the deglyclation (removal of glycine) from S-methylglutathione, before its deglutamylation and S-oxygenation process, described above (Yoshimoto and Saito Citation2019; Lancaster and Shaw Citation1989). Whilst more research into understanding pathways of cysteine sulfoxide synthesis is needed, it is evident that significant increases in overall ASCOs occur with both maturity and aging of alliums (Kodera et al. Citation2020; Yoshimoto and Saito Citation2019). Collectively, the ASCOs make up over half of the total sulfur content within the mature garlic bulb (Rektorisova et al. Citation2020). Like glucosinolates, these compounds are relatively inert, until upon tissue destruction (i.e., slicing, chewing), they are acted upon by cysteine sulfoxide lyases (e.g., alliinase) stored within separated vacuoles in the plant, resulting in sulfenic and pyruvic acids, and ammonia (Rektorisova et al. Citation2020). Such examples of the multiple downstream sulfur-containing derivatives are thiosulfinates, various sulfides, and ajoenes (Rektorisova et al. Citation2020; Yoshimoto and Saito Citation2019).
Other sulfur-based compounds
Despite this review focusing primarily on glucosinolates and ASCOs, there are other sulfur-containing secondary metabolites synthesized from plants that are of research interest (e.g., H2S, sulfur dioxide [SO2], antimicrobial peptides) (Künstler et al. Citation2020). Emerging evidence suggests that H2S is able to directly trigger plant defense and adaptive mechanisms following exposure to pathogens or harmful environmental stimuli (Vojtovič, Luhová, and Petřivalský Citation2021). Similarly, SO2 has been found to advantageously regulate various defensive signaling pathways within plants, whilst defensins and thionins (i.e., anti-microbial peptides) have been shown to provide broad antiviral, antifungal and antibacterial protection (Künstler et al. Citation2020).
The diverse potential of plant-based sulfur-containing metabolites
These sulfur-containing metabolites activate multiple detoxification reactions in plant tissue following exposure to stress, regulating reactive oxygen species, enhancing antioxidant defense and detoxification pathways, thereby providing additional protection against both pathogenic and environmental stressors as required (Künstler et al. Citation2020; Gullner et al. Citation2017; Hasanuzzaman et al. Citation2017). It is worth noting that some sulfur-containing metabolites can be argued as having both phytoanticipant and phytoalexin characteristics, with more research into understanding the complexity behind sulfur chemistry and its metabolites required. The type and quantity of different sulfur-containing metabolites produced within plants are highly dependent on sulfur availability although also unique to each plant species (Vojtovič, Luhová, and Petřivalský Citation2021).
The considerable potential of many sulfur derivatives is largely due to sulfur being classed as a chalcogen, known for having biologically diverse characteristics including the ability to provide unique redox potential and atom exchange reactions (Francioso et al. Citation2020). Therefore, these characteristics equate to sulfur derivates being highly reactive, performing “a complex labyrinth of interacting signaling and control pathways” (Giles et al. Citation2017, 1). As research has enabled greater understanding, it has been suggested that sulfur is possibly more important than reactive oxygen species (ROS) or reactive nitrogen species (RNS) in our antioxidant defense systems (Giles et al. Citation2017). The presence of sulfur provides unique versatility and diversity of activity to simple carbon, hydrogen, nitrogen and oxygen compounds from plants (Francioso et al. Citation2020). This is enabled by a diverse set of enzymes that hydroxylate, methoxylate, oxidize, desaturate or benzoylate sulfur-rich compounds (Halkier and Gershenzon Citation2006), coupled to a wide series of chemical reactions that occur during their interconversions. As a result, after sulfur has been incorporated into plant tissue, a vast number of sulfur-containing compounds can be formed, well beyond the primary metabolic products—cysteine, methionine, and GSH. Up to and including the year of 2012, it was recognized that there may be as many as 550 organosulfur compounds in plants (Iranshahi Citation2012) and over 130 different glucosinolates alone reported in plants (Blažević et al. Citation2020). With emerging analytical techniques, many of these sulfur-containing compounds and their downstream metabolites are being isolated and subsequently being increasingly investigated for their health potential in humans.
Sulfur intake in humans
Sulfur intake in humans can be divided into two categories, the SAA and the sulfur-containing secondary metabolites.
Sulfur-containing amino acids
The four SAA include cysteine, methionine, homocysteine, and taurine, with only the former two being proteinogenic (Brosnan and Brosnan Citation2006). Both methionine and cysteine are found in abundance in animal products, and to a lesser extent, plant proteins (Ingenbleek and Kimura Citation2013). Therefore, animal meats, such as lamb, beef, chicken, seafood, as well as eggs and dairy, are considered highly reliable sources of SAA, whilst those consuming ‘health-conscious’ diets free of red meat or animal proteins tend to have lower (albeit acceptable) levels of sulfur than their meat-eating counterparts (Nimni, Han, and Cordoba Citation2007). Methionine is an essential SAA and relies on dietary intake, whilst cysteine is considered semi-essential (able to be synthesized from an adequate supply of methionine) (Brosnan and Brosnan Citation2006). It is estimated that humans require a daily SAA intake (predominantly as methionine) of approximately 15 mg/kg body weight (i.e., ∼1,050 mg/day for a 70 kg human) (World Health Organisation Citation2007).
Briefly, cysteine is the initial step of sulfur incorporation into organic tissue (see ) which, in conjunction with methionine and other amino acids, assists in production of glucosinolates and GSH (Maruyama-Nakashita Citation2017). GSH is the most abundant non-protein thiol in the human body, with a major role in tightly regulating cellular redox status (Franco et al. Citation2007; Teskey et al. Citation2018; G. Wu et al. Citation2004). GSH is a significant reservoir for cysteine, accounting for an estimated 65% of mammalian whole-body cysteine flux, indicating its importance in regulating our antioxidant defensive system (Franco et al. Citation2007; G. Wu et al. Citation2004). Both GSH and its oxidized counterpart (GSSG), together act as a major redox couple, heavily involved in mediating detoxification, thiol disulfide reactions important for cellular signaling, and activation of defensive pathways (Jez Citation2019; Kalinina and Gavriliuk Citation2020; Zechmann Citation2020; Franco et al. Citation2007). In similarity with plants, GSH in mammals is largely found in its reduced form, shifting toward its oxidized (and more toxic) GSSG form upon exposure to stress (i.e., exposure to ROS/RNS) (Kalinina and Gavriliuk Citation2020). This in turn triggers feedback recycling of GSSG back to GSH (via glutathione reductase), thereby increasing Nrf2 translocation and subsequent de novo synthesis of GSH as well as enhanced transcription of defensive genes (Teskey et al. Citation2018; G. Wu et al. Citation2004).
As with many sulfur-based compounds, GSH is widely recognized as an important redox buffer in many chronic conditions such as cancer, cardio-metabolic dysfunction, diabetes, acquired immunodeficiency syndrome (AIDS), neurodegenerative, hepatic dysfunction as well as pathologies associated with aging (Franco et al. Citation2007; Kalinina and Gavriliuk Citation2020; Matuz-Mares et al. Citation2021; Teskey et al. Citation2018). More recently, GSH has been explored for its potential to mitigate the severe oxidative stress associated with deleterious COVID-19 pathogenesis and/or mortality (Khanfar and Qaroot Citation2020; Kumar et al. Citation2021; Polonikov Citation2020).
Methionine, through its activation to S-adenosylmethionine has an important role with the latter acting as a methyl donor for wide-reaching methylation reactions within almost every cell in the human body (Brosnan and Brosnan Citation2006). The demethylation of methionine produces homocysteine, important for the recycling of both methionine and cysteine. Provided there is adequate supply of B-vitamins (i.e., folate, B6, B12) as well as choline and betaine, the transulfuration pathway recycles homocysteine back to cysteine, or alternatively to methionine via the transmethylation pathway (Dong, Sinha, and Richie Citation2018).
Unique to cysteine is its readiness to form disulfide bonds, making it crucial in protein structure and in the formation of protein-folding pathways (Brosnan and Brosnan Citation2006). Furthermore, these disulfide bonds act as a ‘gateway’ conveniently located near the surface of cells for the convenient export of proteins to other areas of the body. In contrast, methionine displays hydrophobic tendencies, therefore, is mostly found contained within the protective interior hydrophobic core of proteins (Brosnan and Brosnan Citation2006). However, occasionally methionine residues can be found upon the exposed outer cell layer and, when reducing enzymes are impaired and these residues accumulate, the cell becomes vulnerable to oxidation and resultant inflammation (Brosnan and Brosnan Citation2006). It is this potential to trigger metabolic changes that has led to studies investigating the link between high intake of SAA from methionine-rich animal products with chronic inflammation associated with cardiometabolic risk and other chronic diseases of aging (Dong et al. Citation2020; Dong, Sinha, and Richie Citation2018). Recent results from the Third National Examination and Nutritional Health Survey (NHANES III) study found that SAA intake among American adults was in fact 2.5 times higher than the recommended ‘estimated average requirement’. Among the 11,576 participants, consuming higher intakes of SAA (positively associated with animal protein intake, i.e., meats) was associated with significant increases in cardiometabolic diseases, even after adjusting for confounders and known risk factors such as cholesterol and blood glucose (Dong et al. Citation2020). However, most importantly, the above estimation and associated effects are for SAA alone and overlook requirements for other non-SAA sources of sulfur intake, such as the sulfur-containing secondary metabolites.
Sulfur-containing secondary metabolites from plants
To date, there are no recommendations for an optimal total daily sulfur intake, nor are comprehensive databases readily available to quantify non-SAA sulfur-based intake from foods. Nonetheless, in addition to animal meats, many other food groups contribute to sulfur intake, including, wheat and starchy grains, dairy, and vegetables (Doleman et al. Citation2017). A recent laboratory-based study analyzed sulfur content within a variety of commonly consumed foods (e.g., fruits, vegetables, meats, fish, pastas, rice, and eggs), and then calculated daily intakes across 41 one-day food diaries self-reported from 32 healthy individuals. They estimated that ∼90% of total sulfur intake in the human population may be derived from non-SAA sources (Doleman et al. Citation2017), in the form of secondary metabolites, for which a recommended intake has not yet been established. Doleman et al. (Citation2017) found that cruciferous and allium vegetables contribute ∼42% of total sulfur intake with the majority of this (∼95%) from cruciferous vegetables.
Cruciferous vegetables include broccoli, cabbage, Brussels sprouts, cauliflower, kale, rapeseed, radish, turnip, watercress, and mustard, and belong to the plant family Brassicaceae (Nguyen et al. Citation2020). The Alliaceae genus of vegetables includes the alliums, such as garlic, onion, leeks, chives, scallion (spring onion) and shallots (Petropoulos, Di Gioia, and Ntatsi Citation2017). It is the presence of sulfur compounds that produce the distinctive aroma and flavor of these vegetables when sliced (Bell et al. Citation2018; Yamazaki et al. Citation2010).
Sulfur-rich cruciferous and allium vegetables are recognized for their antioxidant, anti-inflammatory, hepatoprotective, antimicrobial, hypoglycemic, cardiovascular, anticarcinogenic, neuro and renal protective potential (Shang et al. Citation2019; Petropoulos, Di Gioia, and Ntatsi Citation2017). Observational studies suggest that consuming higher intakes of cruciferous and allium vegetables are inversely associated with atherosclerotic vascular disease mortality, the incidence of myocardial infarction and cerebrovascular disease (Blekkenhorst et al. Citation2017; Blekkenhorst et al. Citation2018), type-2 diabetes mellitus (T2DM) (Jia et al. Citation2016) and cancer risk (Aune et al. Citation2017). The increasingly recognized role of plant-based sulfur-containing secondary metabolites within these vegetables and their importance for human health and disease prevention is gaining traction.
Oxidative stress, inflammation and chronic disease
Reactive oxygen species (ROS) refers to the presence of reactive forms of oxygen (both radical and non-radical derivatives), largely formed through normal cellular metabolism or in response to environmental or xenobiotic stressors (Mukwevho, Ferreira, and Ayeleso Citation2014). All metabolic processes undergo a continual state of redox reactions kept in check by an appropriately coordinated immune, antioxidant, and inflammatory response (Ahmed et al. Citation2017). When exposed to a potential threat, ROS is generated through the activation of mast cells, monocytes, lymphocytes and macrophages recruited to the area of concern (Ahmed et al. Citation2017). Without adequate immune, inflammatory, and antioxidant defenses to maintain homeostasis, a pro-inflammatory state of hyper-oxidation can occur resulting in oxidative stress, hyper-inflammation, and DNA damage. Uncontrolled, this process is accompanied by marked increases in inflammatory mediators such as cytokines, chemokines and prostaglandins, which in turn, trigger further signaling mediators and transcription factors exacerbating the inflammation, disrupting homeostasis, and potentially becoming chronic in nature (Ahmed et al. Citation2017).
Central to much of the regulation of our inflammatory pathways and antioxidant defense are the activation or suppression of two key cytoplasmic transcription factors; nuclear factor-ĸB (NF-ĸB) and erythroid-2 related factor (Nrf2) (T. Liu et al. Citation2017; Ahmed et al. Citation2017). The activation of NF-ĸB triggers the production of pro-inflammatory cytokines, chemokines and adhesion molecules (T. Liu et al. Citation2017). In addition, NF-ĸB is responsible for the switching on of M1 macrophages (from the favored anti-inflammatory M2 macrophages) and in doing so, induces genes that further encode for tumor necrosis factor-α (TNF-α), several interleukins (IL) such as IL-1, IL-6, and IL-12, and cyclooxygenase (T. Liu et al. Citation2017). Furthermore, elevated TNF-α has been implicated in initiating transcription of NF-ĸB, accelerating ROS production, thrombosis, endothelial dysfunction and remodeling, and reducing nitric oxide (NO) bioavailability (H. Zhang et al. Citation2009). Detrimental microvascular and macrovascular changes, accelerated aging, and the development of diabetes have been reported in response to high TNF-α in both animal and human studies (H. Zhang et al. Citation2009).
In response to a stressor, Nrf2, which is generally sequestered to Kelch-like ECH-associated protein (Keap-1) becomes dissociated. As a result, Nrf2 is then free to translocate to the cell nucleus where it triggers the expression of numerous genes as part of our sophisticated antioxidant Nrf2/Keap1/antioxidant response element (ARE) defense system (Ahmed et al. Citation2017). Nrf2 aids in the transcription of hundreds of cytoprotective genes involved in inhibiting pro-inflammatory cytokines, chemokines, and cell adhesion molecules (CAMs), cyclooxygenase-2 (COX-2), matrix metalloproteinases (MMPs) and inducible nitric oxide synthase (iNOS), thus inducing relevant detoxification pathways and overall cellular defense mechanisms (Ahmed et al. Citation2017). Adhesion molecules, such as vascular cell and intercellular adhesion molecules (VCAM-1 and ICAM-1, respectively) appear early in vascular remodeling (Blankenberg, Barbaux, and Tiret Citation2003) augmented by pro-inflammatory changes and inadequate anti-oxidant capability. Furthermore, Nrf2 is considered an emerging mediator of cardiometabolic disease risk (da Costa et al. Citation2019).
There is significant crosstalk between Nrf2 and NF-ĸβ, both competing for the same transcription coactivator (cAMP response element-binding [CREB] protein) for their counteracting roles in inflammatory responses (Bellezza et al. Citation2018). As such, this makes them key targets for chronic disease management (Ahmed et al. Citation2017). Inflammation has been strongly associated with increased cardiovascular risk (Arnold et al. Citation2021; Tuñón et al. Citation2018). Therefore, reducing heart and vascular inflammation is strongly targeted by many pharmaceuticals, immune-modulatory, and lipid-lowering therapies in an attempt to lower cardiovascular risk and mortality (Ridker Citation2018; Roman, Hernandez, and White Citation2020; Kottoor and Arora Citation2018; Moreira et al. Citation2015).
Plants, such as brassica and alliums, increase their sulfur-containing metabolites in response to both abiotic stress as well as pathogen exposure (Del Carmen Martínez-Ballesta, Moreno, and Carvajal Citation2013; Nguyen et al. Citation2020). Whilst many of the sulfur-containing plant metabolites act as primary defenders against disease and poor growth within plant tissue itself, it appears that many of these benefits may be extended to humans following their consumption (Fakhri et al. Citation2020). In fact, an observational study using data from the Shanghai Women’s Health cohort reported reduced levels of pro-inflammatory biomarkers (TNF-α, IL-1β and IL-6) in women consuming a higher intake of cruciferous vegetables (Y. Jiang et al. Citation2014). These vegetables and their sulfur-containing secondary metabolites may be a relatively cost-effective adjunct to lowering chronic cardiometabolic disease burden.
Sulfur-containing secondary metabolites—protection against chronic disease
The predominant plant-based sulfur-containing secondary metabolites within the cruciferous and allium vegetables include the glucosinolates and S-alk(en)yl cysteine sulfoxides, respectively. It should be restated that significant variations in metabolite quantity occur within these vegetables, influenced by not only defensive mechanisms described above, but by the naturally-occurring unequal distribution in different sections, i.e., leaves, roots, seeds (J. Chang, Wang, et al. Citation2019; Nguyen et al. Citation2020), stage of growth, and storage conditions post harvesting (Horníčková et al. Citation2010; Rektorisova et al. Citation2020). Furthermore, levels alter considerably according to dietary preparations (i.e., cooking techniques) (Baenas et al. Citation2019). For example, the estimated isothiocyanate bioavailability for cooked vegetables was found to be 2 to 6-fold lower when compared to raw and thoroughly chewed cruciferous vegetables (Vermeulen et al. Citation2006). Such growing and cooking variations result in wide compositional changes, details of which are well beyond the scope of this review. A detailed food composition database collectively compiling these compounds and the impacts of different growing conditions and cooking methods is needed. Common dietary sulfur-containing secondary metabolites found within cruciferous and allium vegetables are summarized below ().
Table 1. Common dietary sulfur-containing metabolites and their main derivatives within cruciferous and allium vegetables.
Glucosinolates (B-thioglucoside-N-hydroxysulfates)
Glucosinolate derivatives have been identified as having significant pharmacological potential, including antimicrobial, antioxidant, anti-cancer, and anti-inflammatory properties as well as anti-hypercholesterolemic, antihyperglycemic, anti-platelet, immunomodulatory, cardiac and neuroprotective effects (Russo et al. Citation2018; Maina et al. Citation2020; Mazumder, Dwivedi, and du Plessis Citation2016; Petropoulos, Di Gioia, and Ntatsi Citation2017; Dinkova-Kostova and Kostov Citation2012). Of the estimated >130 glucosinolates identified to date, the majority are found within cruciferous vegetables (Nguyen et al. Citation2020). A recent laboratory-based study analyzed total glucosinolate concentration across 8 brassicas (cruciferous) vegetables to be, from highest to lowest, cabbage, broccoli, cauliflower, mustard, Chinese cabbage, young radish, and kale (Hwang et al. Citation2019).
To recap, all glucosinolates contain both a thioglucose and sulfonate oxime group, in addition to one of three possible amino acid side chain structures (Esteve Citation2020). The side chains differentiate glucosinolates as being either aliphatic, benzenic (or aromatic) or indole, dependent on their main amino acid precursors. The aliphatic are the most prevalent glucosinolates, derived mostly from methionine (but also valine, alanine, leucine or isoleucine), whilst the aromatics are derived from tyrosine or phenylalanine, and the less-common indole glucosinolates are tryptophan-based (Fahey, Zalcmann, and Talalay Citation2001; Esteve Citation2020; Mitreiter and Gigolashvili Citation2021). The vast majority of glucosinolates are aliphatic, as shown in (Nguyen et al. Citation2020). Considered biologically inactive water-soluble compounds, the glucosinolates undergo hydrolysis via the thioglucosidase myrosinase (made accessible by tissue maceration, i.e., chewing or slicing) or alternatively by thioglucosidases located within the intestinal flora (Mithen and Ho Citation2018; Tian et al. Citation2018; Narbad and Rossiter Citation2018). Subsequently, many downstream, biologically active derivatives are produced (Bell et al. Citation2018; Nguyen et al. Citation2020). These are predominantly the isothiocyanates and indoles, however, in the presence of specifier proteins, an alternative route favors the formation of thiocyanates, as well as nitriles and epithionitriles, with the potential for additional protective benefits (Eisenschmidt-Bönn et al. Citation2019; Nguyen et al. Citation2020). Despite evidence (mostly in animals) that thiocyanates inhibit iodine uptake, recent research suggests minimal adverse risks to thyroid health in humans consuming regular cruciferous intake (Felker, Bunch, and Leung Citation2016; Lietzow Citation2021). Allyl-, benzyl- and 4-methylsulfinylbutyl-glucosinolates each have the ability to form thiocyanates in the presence of these non-heme iron specifier proteins found in plants (Wittstock and Burow Citation2007). However, to date, sinigrin is the only glucosinolate known to form both isothiocyanates (i.e., allyl isothiocyanates) and thiocyanates (i.e., allyl thiocyanate) following hydrolysis (Nguyen et al. Citation2020). Collectively, it is these biologically active derivatives that are responsible for the sulfurous aroma and flavor of these vegetables, and for their biological activity (Z. Liu et al. Citation2021).
The post-hydrolysis derivatives that have gained the most notoriety for potential health benefits include isothiocyanates and indoles. Among the most studied metabolites and their derivatives are glucoraphanin, sinigrin, and gluconasturtin (isothiocyanates) and glucobrassican (indolic isothiocyanates) (Esteve Citation2020). Most studies have used in vitro and in vivo animal models. Some of their noted antioxidant, anti-inflammatory and cardiometabolic effects are tabled below (see ).
Table 2. Antioxidant and anti-inflammatory activity of the main sulfur-containing derivatives (sourced from the hydrolysis of glucosinolates and S-alk(en)yl cysteine sulfoxides) found in cruciferous and allium vegetables.
Glucoraphanin
Glucoraphanin (4-methylsulfinylbutyl glucosinolate) and its derivative sulforaphane, is arguably the most well-researched organosulfur compound (Yagishita et al. Citation2019). Sulforaphane is an aliphatic isothiocyanate first identified within broccoli as a major inducer of cytoprotective enzymes in mice in 1992 (Y. Zhang et al. Citation1992). Sulforaphane has since been identified as having protective antioxidant, immunomodulatory and anti-inflammatory effects, with several reviews reporting its potential for cancer, cardiac, vascular, neurological, and glycemic benefits (Bai et al. Citation2015; Kim Citation2021; Dinkova-Kostova and Kostov Citation2012; Houghton Citation2019; Ramirez et al. Citation2017). Various rodent models have identified positive cardiometabolic changes when administered sulforaphane, including hypertension, atherosclerosis, glucose management and for mitigating diabetic complications, largely due to activation of Nrf2 and associated downstream effects (Bai et al. Citation2015). Kim (Citation2021) presented the multi-faceted neuroprotective effects of sulforaphane in various animal and human studies, its ability to induce phase II detoxification enzymes, activate Nrf2 transcription, reduce oxidative stress; and its subsequent potential in ameliorating the pathophysiological changes associated with cognitive decline, Alzheimer’s disease and other neurological-based diseases (Kim Citation2021). A potential nephroprotective role has also been reviewed, through sulforaphane’s Nrf2 inducing activity and potential for mitigating the hypertensive impacts of chronic kidney disease (Liebman and Le Citation2021). Evidence also suggests sulforaphane is a powerful inducer of cytoprotective enzymes involved in phase II detoxification, in particular NAD(P)H:(quinone-acceptor) oxidoreductase 1 (NQO1) and glutathione S-transferases (GSTs), thus making it prolific in anti-cancer research (Russo et al. Citation2018). Sulforaphane has been shown to upregulate Nrf2 activity, inhibiting lipopolysaccharide (LPS)-induced prostaglandins, COX-2, MMPs and iNOS in mice (Qi et al. Citation2016). Similarly, increased Nrf2 activity, and subsequently lowered inflammatory cytokines, lipid peroxidation markers and ROS, alongside elevated superoxide dismutase (SOD) has been seen in an in-vitro Alzheimer’s disease mice model (Zhao, Zhang, and Chang Citation2018). Sulforaphane has elicited cardioprotective effects against inflammatory and oxidative damage, activating Nrf2 activity, mediating glucose signaling and preventing both type-2-diabetic-induced cardiomyopathy (Y. Sun et al. Citation2020) and nephropathy (Z. Li et al. Citation2020) in mice. Whilst more research is needed, sulforaphane has improved glycemic control (Axelsson et al. Citation2017), insulin resistance (Bahadoran, Tohidi, et al. Citation2012), triglycerides, and lipid peroxidation biomarkers in T2DM (Bahadoran, Mirmiran, et al. Citation2012). A randomized controlled trial of 137 high-risk CVD participants randomly assigned to receive either a high dose glucoraphanin broccoli (i.e., high sulforaphane) for 12-weeks had significantly lowered low-density-lipoprotein (LDL) compared with those given standard broccoli (Armah et al. Citation2015). A recent study in 40 healthy, although overweight humans, also reported sulforaphane-rich broccoli sprouts were shown to decrease multiple inflammatory markers; IL-6, C-reactive protein, and IL-1β (López-Chillón et al. Citation2019). More recently, in vitro evidence demonstrated sulforaphane’s potential in reducing the inflammatory cytokine storm (both IL-6 and IL-8) associated with the SARS-CoV-2 spike protein and associated lung injury (Gasparello et al. Citation2021). The popularity and breadth of research into glucoraphanin and sulforaphane has led to its commercialization and use in clinical trials (Fahey and Kensler Citation2021; Houghton Citation2019) as well as the breeding of new glucoraphanin-rich broccoli cultivars (Bell and Wagstaff Citation2017).
Glucoerucin
Glucoerucin (4-methylthiobutyl glucosinolate) and its derivative, erucin (4-[methylthio]butyl isothiocyanate) have been heralded as a ‘new promising anticancer agent from cruciferous vegetables’ (Melchini and Traka Citation2010). This is largely due to glucoerucin’s ability to regulate detoxification, mitigate oxidant stress and ROS-associated pathways, and improve overall cellular defense mechanisms (Melchini and Traka Citation2010). Due to erucin’s ability to interconvert with sulforaphane in both animals and humans, it shares many features with sulforaphane, particularly around antioxidant potential (Clarke et al. Citation2011). Erucin has been shown to reduce multiple inflammatory markers (IL-6, IL-1β, prostaglandin E2 and COX-2) following an LPS-induced inflammatory state in murine RAW 264.7 cells (Cho, Lee, and Park Citation2013). A significant reduction in iNOS production, TNF-α and NF-ĸβ signaling also occurred within the erucin-treated cells (Cho, Lee, and Park Citation2013). Erucin has been shown to reduce both the pain and neuro-inflammation associated with diabetes-induced neuropathic pain in mice (Lucarini et al. Citation2019). An extract of erucin displayed antithrombotic and antiplatelet activity in vitro, lowering NF-ĸβ expression along with several inflammatory mediators (including IL-1β and thromboxanes) within human platelet cells, and showed antithrombotic activity when administered to mice (Fuentes et al. Citation2014). An in vitro study also reported a dampening of LPS-induced neuro-inflammation, with a reduction in pro-inflammatory cytokines, elimination of COX-2 and improved inflammasome expression in cells pretreated with an erucin extract (Gugliandolo et al. Citation2018).
Sinigrin
Sinigrin (2-propenyl-glucosinolate) is an aliphatic glucosinolate found in broccoli, Brussels sprouts, and mustard seeds (Mazumder, Dwivedi, and du Plessis Citation2016). Studies suggest sinigrin has anti-cancer, antimicrobial, antioxidant and anti-inflammatory benefits, wound healing properties and, like many glucosinolates, acts as a natural pesticide (Mazumder, Dwivedi, and du Plessis Citation2016). Upon hydrolysis, its derivative is allyl isothiocyanate (Allyl ITC). Sinigrin has been shown to reduce various atherosclerotic markers in four murine-based studies (H. W. Lee et al. Citation2017; H. W. Lee and Lee Citation2015; Jang et al. Citation2017; Jang and Pyo Citation2015). ApoE-deficient mice fed a high cholesterol diet were found to have a reduction in VCAM-1 within smooth muscle cells in those treated with sinigrin, bought about by reduced expression of both TNF-α and NF-ĸβ (H. W. Lee and Lee Citation2015; H. W. Lee et al. Citation2017). Similar results were found by Jang and Pyo (Citation2015) whereby sinigrin reduced TNF-α and subsequently lowered both VCAM-1 and NF-ĸβ expression. Various protective cardiometabolic markers were evident from administration of sinigrin including lowered cholesterol fractions and triglycerides, as well as reduced oxidized LDL, lowered IL-6, and subsequent reduction in proatherogenic risk (Jang et al. Citation2017; Jang and Pyo Citation2015).
Glucobrassicin
Following hydrolysis, glucobrassicin forms the highly unstable indole 3-carbinol (I3C), and its condensation product, 3,3-diindolymethane (DIM). The majority of research into glucobrassicin and its derivatives explore the anti-cancer, proapoptotic potential across several cancer types, including the inhibition of NF-ĸβ and iNOS, modulation of ROS, reduced TNF-α and inflammatory downstream derivates (Maruthanila, Poornima, and Mirunalini Citation2014). Like many glucosinolates, DIM has been identified to impact detoxification pathways, enhancing conjugation and excretion of potential carcinogens (Williams Citation2021). This derivative is recognized to enhance both phase I and phase II detoxification pathways, facilitating the modification of potentially harmful metabolites, and their conjugation and excretion from the body (Ampofo et al. Citation2018). Within phase I, it particularly enhances cytochrome P450 enzymes favoring estrogen hydroxylation, thereby explaining its research as a potential therapeutic agent for estrogen-driven cancers. DIM has been shown to induce multiple transcription targets for Nrf2-induced phase II and antioxidant signaling (e.g., GSTs, NQO1, HO-1, SOD) (Ampofo et al. Citation2018; Fuentes et al. Citation2014; Williams Citation2021). Both I3C and DIM have been shown to increase the expression of Nrf2 and reduce NF-ĸβ activity in animal studies (Hajra et al. Citation2018; T. Chang, Ho, et al. Citation2019). Furthermore, I3C has been identified as having potential anti-thrombotic and anti-inflammatory activity, with evidence that it lowers inflammatory cytokines and ROS production, as well as enhances microvascular integrity (Ampofo et al. Citation2018). Administration of glucobrassican has been shown to improve cardiac output and vascular resistance in hypertensive cirrhotic rats, subsequently lowering high blood pressure (T. Chang, Ho, et al. Citation2019). I3C was also shown to almost completely blunt ethanol-induced liver inflammation, lipid peroxidation and levels of H2O2 in ethanol-fed mice, indicating antioxidant, anti-inflammatory and anti-apoptotic effects following administration (Choi, Abdelmegeed, and Song Citation2018).
Glucoiberin
Glucoiberin (3-methylsulfinylpropyl glucosinolate) and its naturally produced hydrolysis derivative, iberin [3-(methylsulfinyl)-propyl isothiocyanate) has been reported to promote xenobiotic phase II detoxification (e.g., quinone reductase and GSTs) suggesting a cytoprotective potential in animal studies (Munday and Munday Citation2004). In combination with other sulfoxide analogues (sulforaphane and erucin), iberin was demonstrated to upregulate thioredoxin reductase 1 expression within human breast cancer MCF-7 cells, in vitro (W. Wang et al. Citation2005). Furthermore, in very similar potency to sulforaphane, iberin was found to increase Nrf2-translocation and subsequent activity in vitro, inducing detoxification enzyme heme oxygenase 1 (HO-1) and -glutamylcysteine synthetase, thereby enhancing antioxidant defenses (Ernst et al. Citation2013). Previous studies have also reported chemo-preventive, anti-cancer, and apoptotic potential in several in vitro models (Gong et al. Citation2021; Pocasap, Weerapreeyakul, and Thumanu Citation2019; Jadhav et al. Citation2007).
Gluconasturtiin
Gluconasturtiin is another commonly consumed aromatic glucosinolate and is the precursor to phenethyl isothiocyanate (PEITC) (Dayalan Naidu et al. Citation2018). Previous authors have reviewed the anticarcinogenic potential of PEITC (Gupta et al. Citation2014; Ramirez et al. Citation2017). Dayalan et al has reviewed PEITC’s ability to enhance transcription of Nrf2 and heat-shock proteins, and inhibit phase I detoxification enzymes, thus improving inflammation and oxidant support within in vitro models (Dayalan Naidu et al. Citation2018). It has also been found that PEITC, in combination with benzyl isothiocyanate (BITC) from gluconapin, over 18-weeks significantly reduced obesity and cholesterol in C57BL/6 mice induced with cardiometabolic dysfunction via a high-fat diet (Chuang et al. Citation2019). Mice administered both PEITC and BITC had reduced expression of lipogenic and adipogenic transcription proteins when compared to control mice (Chuang et al. Citation2019); proteins involved in inhibiting inflammation and pro-inflammatory cytokines (Bilotta et al. Citation2020; Martin Citation2010). Another study involving high-fat fed C57BL/6 mice found oral PEITC over 13 weeks resulted in decreased NF-ĸβ expression, along with a significantly blunted rise in cholesterol (lowered atherogenic index), weight gain and improved glucose control. Furthermore, the PEITC-fed group had significantly less narrowing of blood vessels and hepatic adipocyte accumulation than the control group (Gwon et al. Citation2020). A later study reported that these improved cardiometabolic parameters were likely a result of PEITCs ability to influence the regulation of mTOR/AMPK/PPAR expression involved in lipid, energy, and glucose metabolism and in lowering inflammation (Gwon and Yun Citation2021). A recent in vitro study using human THP-1 derived macrophages induced with LPS and oxidized LDL, also reported a reduction in vascular inflammation, formation of foam cells and their accumulation of lipids and overall atherosclerotic risk when administered PEITC. The researchers also reported the PEITC-treated cells had blunted activation of NF-ĸβ and LOX-2 among others, indicating its potential in lowering inflammatory drivers (Im, Gwon, and Yun Citation2021).
Other glucosinolates
Glucotropaeolin forms into BITC, which, in addition to mitigating anti-inflammatory cytokines in combination with PEITC described above (Chuang et al. Citation2019), has also been shown to have anticarcinogenic potential across several animal and in vitro studies (Dinh et al. Citation2021). Gluconapin (3-butenylglucosinolate) is known to produce the metabolite, 3-butenyl isothiocyanate. Mice fed corn-oil and given a bolus dose of gluconapin had a significant blunting in post-prandial hypertriglyceridemia, when compared to control (Washida et al. Citation2010). Furthermore, 3-butenyl isothiocyanate has been shown to have cytotoxic potential across multiple human cancer cell lines in vitro (Arora et al. Citation2016). Collectively, many of the glucosinolate post-hydrolysis derivatives act as potential sulforaphane analogues, yet research exploring many of these and their metabolites in isolation is sparse.
S-alk(en)yl cysteine sulfoxides (ASCOs)
The overall collective pharmacological, anti-inflammatory, antioxidant, anti-carcinogenic and cardiometabolic potential of ASCO-rich allium vegetables upon health have been reported in recent reviews (Shang et al. Citation2019; El-Saber Batiha et al. Citation2020; Farhat Citation2021; Ansary et al. Citation2020; Galavi, Hosseinzadeh, and Marjan Razavi Citation2021). As already discussed, like the glucosinolates, the ASCOs are stored in separate vacuoles within the plant tissues, only to be released upon exposure to cysteine sulfoxide lyases bought about by tissue destruction (i.e., slicing, chewing) and by the actions of the gut microbiota following ingestion (Rektorisova et al. Citation2020). Some of the benefits mediated by the main three ASCOs individually, are described below.
Alliin (S-allylcysteine sulfoxide)
Alliin is the major sulfur-containing cysteine sulfoxide found within garlic. Its formation into allicin (diallyl thiosulfinate) is responsible for the strong aroma associated with garlic and is the most researched ASCO investigating garlic’s potential benefits. Research identifies allicin, and its predecessor alliin, as having antioxidant and anti-inflammatory activity, with cardiovascular, hypolipidemic, hypoglycemic and neuroprotective potential (Quintero-Fabián et al. Citation2013; Chan et al. Citation2013; Zhai et al. Citation2018). Alliin has been shown to reduce several markers of inflammation (iNOS expression, IL-6, TNF-α and NF-ĸβ) in both in vivo (mice) and in vitro studies (Shi et al. Citation2017). It has also been shown to decrease IL-6, improve insulin sensitivity and glycemic control in mice (Sánchez-Sánchez et al. Citation2020). S. Wang et al. (Citation2015) reported allicin mitigated the oxidant-mediated damage and cellular dysfunction associated with diabetic-like conditions in vitro. Within this study, murine aortic endothelial cells exposed to allicin had significantly lowered ROS, 8-hydroxydeoxyguanosine, NF-ĸβ and hypoxia-inducible factor-1α (HIF-1α), thereby potentially offering protection against microvascular and atherosclerotic dysfunction (S.L. Wang et al. Citation2015). Research suggests that allicin increases superoxide scavenging activity, with its derivatives increasing hydroxyl scavenging and ameliorating lipid peroxidation in vitro (Chung Citation2006). Administration of allicin was also found to decrease the expression of Nrf2 in human endothelial cells exposed to LPS-induced oxidative stress, as evidenced by lowered ROS production, lipid peroxidation and reduced inflammatory response in blood vessels (M. Zhang et al. Citation2017). Allicin has many downstream metabolites that have been studied in isolation including dithiins, ajoenes, and thiosulfur lipid allyl sulfides such as diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) (El-Saber Batiha et al. Citation2020). Several in vitro and animal-based in vivo studies have explored the effects of DAS, DADS and DATS derivatives in reducing expression of inflammatory cytokines, regulating carcinogenic activity, GSH production, improving detoxification and overall antioxidant mechanisms (Abdel-Daim et al. Citation2020; Puccinelli and Stan Citation2017; M. Li et al. Citation2018; Suman and Shukla Citation2016; H.H. Lee et al. Citation2018). Administration of DAS was found to lower expression of NF-ĸβ, IL-1-β, and TNF-α and ameliorate reduction in GSH, SOD and other markers of ROS within cerebral, hepatic and renal tissues of rats when exposed to malathion-induced oxidative stress (Abdel-Daim et al. Citation2020). DAS was also found to blunt the oxidative damage of LPS/d-galactosamine-induced liver injury in rats in a dose dependent manner, with lowered malondialdehyde, TNF-α, IL-1-β, and MCP-1 levels evident in both blood and liver (M. Li et al. Citation2018). A previous review has reported on the antioxidant and anti-inflammatory activity of DAS, including its preventive role across several chronic metabolic diseases (Suman and Shukla Citation2016). Within an in vitro model, LPS-stimulated microglial cells exposed to DATS had reduced NF-ĸβ expression along with lowered NO and inflammatory chemokines (H.H. Lee et al. Citation2018).
Methiin (S-methyl cysteine sulfoxide, aka SMCSO)
Another ASCO found in relative abundance within allium vegetables is methiin, most often referred to as S-methyl cysteine sulfoxide (SMCSO). Interestingly, SMCSO is found in greater quantities in cruciferous vegetables (1–4% dry weight) than the glucosinolates (0.1–0.6%) (Mae, Ohira, and Fujiwara Citation1971; Marks et al. Citation1992). Yet due to its link to causing hemolytic anemia in cattle fed large quantities of SMCSO-rich cruciferous vegetables (Smith, Earl, and Matheson Citation1974), this metabolite has received comparatively little attention until recently. Giving glucoraphanin-rich broccoli to men prior to prostate biopsies, found SMCSO accumulates within human prostate tissue and takes considerably longer than glucosinolates to be excreted (Coode-Bate et al. Citation2019). Further research is required to determine if this accumulation has any significance, or indeed biological activity. As such, less is understood on the complexity of SMCSO derivatives, although it is evident that cysteine sulfoxide lyases, within the plant tissue and human microbiota, lead to the release of ammonia, pyruvate and the highly-reactive yet transient metabolite, methanesulfenic acid (Edmands et al. Citation2013). Several metabolites are then produced, most predominantly being S-methyl methanethiosulfinate (MMTSI, aka, dimethyl disulfide sulfoxide) and S-methyl methanethiosulfonate (MMTSO, aka, dimethyl disulfide sulfone) (Edmands et al. Citation2013). Both MMTSI and MMTSO appear to have protective roles in plant tissue, particularly against pathogens (Edmands et al. Citation2013). Several in vitro and in vivo animal (rat and mice) studies also indicate anti-carcinogenic activity when administered allium-derived thiosulfinates, mostly as MMTSI and MMTSO (Edmands et al. Citation2013). Nonetheless, whether these derivatives are produced, or have similar effects within humans is still lacking. More broadly, SMCSO has been shown to inhibit both glucose-6-phosphatase and HMG-CoA reductase, thereby lowering both hyperglycemia and hypercholesterolemia in diabetes-induced rats (Kumari, Mathew, and Augusti Citation1995). Furthermore, the amelioration of blood glucose when treated with SMCSO was not only dose-dependent, but also comparable to the effects of insulin (Kumari, Mathew, and Augusti Citation1995). SMCSO was found to improve the antioxidant potential in both hepatic and cardiac tissues of diabetic rats, evidenced by reduced lipid peroxidation markers; malondialdehyde, hydroperoxides and conjugated dienes (Kumari and Augusti Citation2002). More recently, streptozotocin-induced diabetic rats fed SMCSO were found to have reduced NF-ĸβ levels within their duodenal tissue when compared to control (Castro et al. Citation2021). Lemos et al. (Citation2021) reported a higher IL-10, increased hepatic SOD and catalase activity in streptozotocin-induced diabetic rats, when administered SMCSO, providing additional evidence for conceivable antioxidant benefits. Furthermore, the SMCSO-treated rats also had reduced hyperglycemia, triglycerides, and VLDL-cholesterol over their control counterparts (Lemos et al. Citation2021). Whilst further evidence is needed, it does suggest a potential regulatory role of this metabolite in inflammatory and/or antioxidant pathways. To date, SMCSO has not been studied in isolation in human trials.
Isoalliin (S-propenyl cysteine sulfoxide, aka PeSCO)
Isoalliin is only found in small quantities in young garlic although increases considerably during the aging process (i.e., aged garlic) (Kodera et al. Citation2017); by as much as 6-fold after 8-weeks (Horníčková et al. Citation2010) and 54-fold after 6-months of storage (Rektorisova et al. Citation2020). The action of alliin-lyase acting upon isoalliin when cutting isoalliin-rich onions, triggers the release of 1-propenyl thiosulfinates and thiolpropanal-S-oxide (aka lachrymatory factor), the compound responsible for production of tears when chopping onions (Rose et al. Citation2019). However, new sulfur-based isoalliin derivatives, including thiolanes, have only been identified recently which, along with the previously known cepaenes, zwibelanes and bis(sulfine) derivatives, may open new investigations in coming years (Štefanová et al. Citation2019). Like methiin above, there is also limited information on the biological activity of this ASCO for health, when studied alone. In fact, only one study was found, which explored the physiological effects of isoalliin for its potential use in cachexic conditions (L. Wang et al. Citation2021). Based on the premise that onion has a historical use of stimulating dietary intake, and with isoalliin being the main ASCO; this study found that administering a bolus dose of isoalliin to C57BL/6 mice enhanced their dose-dependent responsiveness to the neuropeptide hormone ghrelin, stimulating appetite and subsequent intake over 24-hours (L. Wang et al. Citation2021). Despite this scarcity of research, its precursor deoxy form (S-propenylcysteine) has been shown to lower blood pressure in hypertensive rats, both over time, or as a single bolus and dose-dependently (Matsutomo et al. Citation2017; Matsutomo et al. Citation2019; Ushijima et al. Citation2018). Matsutomo et al (Citation2017) measured metabolomic output in hypertensive rats following the administration of either S-propenylcysteine or S-allylcysteine (SAC), compared to control. Whilst the SAC-administered group had no significant change, an anti-hypertensive effect was observed in those given S-propenylcysteine. Furthermore, the S-propenylcysteine-group had significant alterations in synthesis of glycerophospholipids, fatty acids, and normalization of metabolites with antihypertensive, osmolytic (e.g., betaine) and NO-inducing properties (e.g., tryptophan) (Matsutomo et al. Citation2017). A subsequent investigation found that treatment with S-propenylcysteine produced not only an antihypertensive effect but coincided with a significant increase in plasma histidine levels (Matsutomo et al. Citation2019). Interestingly, histidine is known for its role in suppressing several markers of inflammation (i.e., cytokines, interleukins, TNF-α, ICAM-1 expression) (Hirasawa Citation2019). A subsequent in vitro study on human vascular endothelial cells has shown this deoxygenated form of isoalliin reduces the TNF-α-induced hyperpermeability of the vascular endothelial barrier; of key importance in maintaining vascular homeostasis and preventing vascular disease (Kunimura et al. Citation2021).
Hydrogen sulfide activity
Isothiocyanate-rich cruciferous vegetables are also known to act as H2S donors, eliciting additional cardioprotective benefits (Martelli, Piragine, et al. Citation2020). More specifically, erucin has been shown to produce vasodilation in rat hearts, inhibit in vitro noradrenaline-induced vasoconstriction and thereby elicit an anti-hypertensive effect, owing to this initiation of H2S (Citi et al. Citation2019; Martelli, Piragine, et al. Citation2020). Other cruciferous-derived isothiocyanates have shown to be effective H2S donors and include benzyl isothiocyanate (derived from glucotropaeolin), allyl isothiocyanate (from sinigrin), sulforaphane (from glucoraphanin) and 4-hydroxybenzyl isothiocyanate (from sinalbin) (Citi et al. Citation2014).
Hydrogen sulfide (H2S) is identified as the third most important gaseous signaling molecule in the human body after NO and carbon monoxide (CO), and is involved in regulating several pathophysiological pathways (Corvino et al. Citation2021). These include, but are not limited to, enhanced NO bioavailability (vasodilation), activation of Nrf2 (enhanced antioxidant and anti-inflammatory pathways) and reduced NF-ĸβ expression, thereby offering significant anti-inflammatory and antioxidant defense (Scammahorn et al. Citation2021). As described by Francioso et al. (Citation2020, 3), H2S plays a “crucial role in cellular redox homeostasis by modulating GSH concentration and Nrf2 transcription.” The release of H2S from isothiocyanates is achieved via non-enzymic adducts formed with thiol groups (e.g., cysteine or GSH) and/or the interaction with H2S-generating enzymes (Corvino et al. Citation2021; Hsu and Tain Citation2021; Magli et al. Citation2021). There are four tissue-specific H2S-generating enzymes including cystathionine -lyase (CSE), cystathionine β-synthase (CBS), 3-mercaptopyruvate sulfurtransferase (3-MST) and cysteine amino transferase (CAT) (Citi et al. Citation2014; Corvino et al. Citation2021; Martelli, Citi, et al. Citation2020). CSE is most concentrated within the cardiovascular tissue, CBS within the central nervous system, hepatic, kidney, uterine and placental tissue, CAT and 3-MST within the vascular endothelium and brain, with 3-MST also concentrated within mitochondria; each generating the release, and thus benefits, of H2S into their respective surrounding tissues (Corvino et al. Citation2021; Magli et al. Citation2021). We refer the reader to several excellent reviews into the emerging potential of H2S in cardiometabolic protection and other diseases associated with oxidative stress (Corvino et al. Citation2021; Corsello, Komaravelli, and Casola Citation2018; D. Wu, Hu, and Zhu Citation2018; Kuschman, Palczewski, and Thomas Citation2021; Hsu and Tain Citation2021). Many, if not all, of the sulfur-containing secondary metabolites are potential sources of H2S production. It is imperative that future studies on sulfur-containing secondary metabolites explore this concept further, ensuring efforts are made to differentiate the impacts derived from individual metabolites as much as possible.
Conclusions and future directions
There are several hundred sulfur-containing secondary metabolites found within the foods we regularly consume, especially abundant within cruciferous and allium vegetables. This review has provided an overview on sulfur uptake from plants, the defensive role within plants, their conversion into the main sulfur-containing secondary metabolites and primary dietary sources, being cruciferous and allium vegetables. We have also discussed the potential biological importance of these metabolites for long-term chronic disease prevention and potential cardiometabolic risk reduction. This review has broadly focused on their overall anti-inflammatory and antioxidant activity, with chronic disease prevention in mind. We recognize there is strong evidence to suggest that these metabolites also have antimicrobial and anticarcinogenic activity, although deemed beyond the scope of this review. The reader is therefore directed to further readings (Bhatwalkar et al. Citation2021; Barbieri et al. Citation2017; Krause et al. Citation2021; Melrose Citation2019; De Greef et al. Citation2021; Favela-González, Hernández-Almanza, and De la Fuente-Salcido Citation2020; Soundararajan and Kim Citation2018; Miękus et al. Citation2020). We also recognize that, due to the vast quantity of sulfur-containing secondary metabolites found within cruciferous and allium vegetables, only those for which the most research is available, have been mentioned.
Of course, humans eat wholefoods, and often the physiological effects of differing phytochemicals work in synergy, rather than isolation. The same may be evident for several of these sulfur-containing secondary metabolites. Whilst some have been isolated and researched extensively for their benefits (e.g., allicin and sulforaphane) and become commercialized into nutraceuticals, most have not been researched, or research is limited to a relatively small number of in vitro and/or in vivo animal studies. Furthermore, with the development of new vegetable cultivars (e.g., glucoraphanin/sulforaphane-rich Beneforte broccoli) (Bell and Wagstaff Citation2017), scientists and plant breeders need to ensure that this is not done at the expense of other equally important, but less studied sulfur-containing secondary metabolites.
It is important to remind the reader that whilst around 130 glucosinolates have been identified to date; an extensive list provided by Nguyen and colleagues (Nguyen et al. Citation2020); not all of these, have yet been explored for their contribution to the biological activity of these vegetables. For example, gluconapin (e.g., in Chinese cabbage) is commonly consumed (Mithen and Ho Citation2018), yet little research beyond its quantity in such vegetables is currently available. Similarly, little evidence was found on the biological effects of glucobrassicanapin, despite it being the predominant glucosinolate in Chinese cabbage and Pak choi (Nugrahedi et al. Citation2017). This same gap in the literature was evident for both the ASCOs, isoalliin and methiin. Methiin is particularly interesting as it contributes significantly more sulfur to cruciferous vegetables than do the glucosinolates and is also ubiquitously found across several alliums. Considering this, the lack of research into its biological potential, particularly in humans is surprising (Edmands et al. Citation2013). Therefore, more research and understanding of the individual influences of these metabolites are needed in future studies.
Extracting individual sulfur-containing metabolites and exploring their effects in human trials with certainty is enormously difficult, compounded by fluctuating quantities within different vegetables in response to defensive mechanisms during growth, environmental and storage conditions. This may largely account for the scarcity of data from human intervention studies regarding the physiological effects of consumption of these compounds. This undoubtedly makes translation into ‘real world’ eating and dietary intervention studies a challenge (Palliyaguru et al. Citation2018). Future studies involving sulfur-rich vegetables as a whole-food intervention must, as a minimum, ensure they measure and report the chemical analysis of their vegetable intake, to infer true meaning to their clinical results, particularly for determining which metabolites might be responsible. Fortunately, whilst the complete understanding of mammalian sulfur metabolism is far from being fully understood, the emergence of new measurement techniques, e.g., various-omics platforms and detection techniques (Yagishita et al. Citation2019), make future research into this area an exciting reality.
In conclusion, Clifford et al. (Citation2021) argues that there is limited evidence that phytochemicals, such as sulforaphane or indeed, any other dietary metabolites, can modulate Nrf2 or other key pathways within humans. Whilst several recent reviews have outlined the promising benefits of glucosinolates, (particularly glucoraphanin, i.e., sulforaphane) upon human health (Houghton Citation2019; Marino et al. Citation2021; Yagishita et al. Citation2019), these reviews have also recognized significant gaps to be addressed moving forward. It is recognized that considerable heterogeneity across studies exists, and that more understanding is required to fully understand the underlying mechanisms of these metabolites and their pharmacodynamic effects upon various biomarkers in humans (Marino et al. Citation2021; Yagishita et al. Citation2019). Furthermore, research exploring the effects of the many other sulfur-based metabolites in commonly consumed foods (i.e., cruciferous and alliums) and their effects within human clinical trials are lacking. Future studies are needed to further explore sulfur-containing metabolites identified in plants both individually and collectively within whole-foods, to identify dose dependent responses upon various pathways for human health. Only then, can we formulate dietary recommendations for optimal intake of cruciferous and allium vegetables as a preventive adjunct against the increased prevalence of cardiometabolic and other chronic diseases.
Authors’ contribution statement
The authors’ responsibilities were as follows: CRH and LCB: were responsible for the concept of this paper; CRH: Writing and original drafting of manuscript; AS, LB, JRL, JMH, AHM, and LCB: provided a critical review and editing of the manuscript. All authors have read and agreed to the final published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research received no additional nor specific funding. CRH is supported by an Edith Cowan University Research Excellence Fellowship Scholarship; AS is supported by an Edith Cowan University Strategic Research Fellowship; The salary of LB is supported by the School of Medical and Health Science at Edith Cowan University; JRL is supported by a National Heart Foundation Future Leadership Fellowship (ID: 102817); The salary of JMH is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship, Australia (Grant number APP1116973); AHM is supported through the Australian Research Council (ARC) Center of Excellence in Plant Energy Biology and as an ARC Australian Laureate Fellow (CE140100008, FL200100057); LCB is supported by a National Health and Medical Research Council of Australia Emerging Leadership Investigator Grant (ID: 1172987) and a National Heart Foundation of Australia Post-Doctoral Research Fellowship (ID: 102498). Publication fees were supported by the Royal Perth Hospital Research Foundation (RPH RF Research Excellence Award, awarded to LCB 2020).
References
- Abdel-Daim, M. M., A. I. Abushouk, S. G. Bungău, M. Bin-Jumah, A. F. El-kott, A. A. Shati, L. Aleya, and S. Alkahtani. 2020. Protective effects of thymoquinone and diallyl sulphide against malathion-induced toxicity in rats. Environmental Science and Pollution Research International 27 (10):10228–35. doi: 10.1007/s11356-019-07580-y.
- Ahmed, S. M. U., L. Luo, A. Namani, X. J. Wang, and X. Tang. 2017. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta. Molecular Basis of Disease 1863 (2):585–97. doi: 10.1016/j.bbadis.2016.11.005.
- Ampofo, E., B. M. Schmitt, M. D. Menger, and M. W. Laschke. 2018. Targeting the microcirculation by indole-3-carbinol and its main derivate 3,3’,-diindolylmethane: Effects on angiogenesis, thrombosis and inflammation. Mini Reviews in Medicinal Chemistry 18 (11):962–8. doi: 10.2174/1389557518666180313100144.
- Ansary, J., T. Y. Forbes-Hernández, E. Gil, D. Cianciosi, J. Zhang, M. Elexpuru-Zabaleta, J. Simal-Gandara, F. Giampieri, and M. Battino. 2020. Potential health benefit of garlic based on human intervention studies: A brief overview. Antioxidants ) 9 (7):619. doi: 10.3390/antiox9070619.
- Armah, C. N., C. Derdemezis, M. H. Traka, J. R. Dainty, J. F. Doleman, S. Saha, W. Leung, J. F. Potter, J. A. Lovegrove, and R. F. Mithen. 2015. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Molecular Nutrition & Food Research 59 (5):918–26. doi: 10.1002/mnfr.201400863.
- Arnold, N., K. Lechner, C. Waldeyer, M. D. Shapiro, and W. Koenig. 2021. Inflammation and cardiovascular disease: The future. European Cardiology 16:e20. doi: 10.15420/ecr.2020.50.
- Arora, R., R. Kumar, J. Mahajan, A. P. Vig, B. Singh, B. Singh, and S. Arora. 2016. 3-Butenyl isothiocyanate: A hydrolytic product of glucosinolate as a potential cytotoxic agent against human cancer cell lines. Journal of Food Science and Technology 53 (9):3437–45. doi: 10.1007/s13197-016-2316-7.
- Aune, D., E. Giovannucci, P. Boffetta, L. T. Fadnes, N. Keum, T. Norat, D. C. Greenwood, E. Riboli, L. J. Vatten, and S. Tonstad. 2017. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology 46 (3):1029–56. doi: 10.1093/ije/dyw319.
- Axelsson, A. S., E. Tubbs, B. Mecham, S. Chacko, H. A. Nenonen, Y. Tang, J. W. Fahey, J. M. J. Derry, C. B. Wollheim, N. Wierup, et al. 2017. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Science Translational Medicine 9 (394):1–12. doi: 10.1126/scitranslmed.aah4477.
- Baenas, N., J. Marhuenda, C. García-Viguera, P. Zafrilla, and D. A. Moreno. 2019. Influence of cooking methods on glucosinolates and isothiocyanates content in novel cruciferous foods. Foods 8 (7):257. doi: 10.3390/foods8070257.
- Bahadoran, Z., P. Mirmiran, F. Hosseinpanah, A. Rajab, G. Asghari, and F. Azizi. 2012. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Research and Clinical Practice 96 (3):348–54. doi: 10.1016/j.diabres.2012.01.009.
- Bahadoran, Z., M. Tohidi, P. Nazeri, M. Mehran, F. Azizi, and P. Mirmiran. 2012. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. International Journal of Food Sciences and Nutrition 63 (7):767–71. doi: 10.3109/09637486.2012.665043.
- Bai, Y., X. Wang, S. Zhao, C. Ma, J. Cui, and Y. Zheng. 2015. Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxidative Medicine and Cellular Longevity 2015:407580. doi: 10.1155/2015/407580.
- Barbieri, R., E. Coppo, A. Marchese, M. Daglia, E. Sobarzo-Sánchez, S. F. Nabavi, and S. M. Nabavi. 2017. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiological Research 196:44–68. doi: 10.1016/j.micres.2016.12.003.
- Bekaert, M., P. P. Edger, C. M. Hudson, J. C. Pires, and G. C. Conant. 2012. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. The New Phytologist 196 (2):596–605. doi: 10.1111/j.1469-8137.2012.04302.x.
- Bell, L., O. O. Oloyede, S. Lignou, C. Wagstaff, and L. Methven. 2018. Taste and Flavor Perceptions of glucosinolates, isothiocyanates, and related compounds. Molecular Nutrition & Food Research 62 (18):1700990. doi: 10.1002/mnfr.201700990.
- Bell, L., and C. Wagstaff. 2017. Enhancement Of glucosinolate and isothiocyanate profiles in Brassicaceae crops: Addressing challenges in breeding for cultivation, storage, and consumer-related traits. Journal of Agricultural and Food Chemistry 65 (43):9379–403. doi: 10.1021/acs.jafc.7b03628.
- Bellezza, I., I. Giambanco, A. Minelli, and R. Donato. 2018. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et Biophysica Acta. Molecular Cell Research 1865 (5):721–33. doi: 10.1016/j.bbamcr.2018.02.010.
- Bernaert, N., L. Goetghebeur, H. De Clercq, M. De Loose, E. Daeseleire, E. Van Pamel, E. Van Bockstaele, and B. Van Droogenbroeck. 2012. Influence of cultivar and harvest time on the amounts of isoalliin and methiin in leek (Allium ampeloprasum var. porrum). Journal of Agricultural and Food Chemistry 60 (44):10910–9. doi: 10.1021/jf302132a.
- Bhatwalkar, S. B., R. Mondal, S. B. N. Krishna, J. K. Adam, P. Govender, and R. Anupam. 2021. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Frontiers in Microbiology 12:613077. doi: 10.3389/fmicb.2021.613077.
- Bilotta, M. T., S. Petillo, A. Santoni, and M. Cippitelli. 2020. Liver X receptors: Regulators of cholesterol metabolism, inflammation, autoimmunity, and cancer. Frontiers in Immunology 11 (2867):584303. doi: 10.3389/fimmu.2020.584303.
- Blankenberg, S., S. Barbaux, and L. Tiret. 2003. Adhesion molecules and atherosclerosis. Atherosclerosis 170 (2):191–203. doi: 10.1016/S0021-9150(03)00097-2.
- Blažević, I., S. Montaut, F. Burčul, C. E. Olsen, M. Burow, P. Rollin, and N. Agerbirk. 2020. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 169:112100. doi: 10.1016/j.phytochem.2019.112100.
- Blekkenhorst, L. C., C. P. Bondonno, J. R. Lewis, A. Devine, K. Zhu, W. H. Lim, R. J. Woodman, L. J. Beilin, R. L. Prince, and J. M. Hodgson. 2017. Cruciferous and allium vegetable intakes are inversely associated with 15-year atherosclerotic vascular disease deaths in older adult women. Journal of the American Heart Association 6 (10):1–15. doi: 10.1161/JAHA.117.006558.
- Blekkenhorst, L. C., C. P. Bondonno, J. R. Lewis, R. J. Woodman, A. Devine, N. P. Bondonno, W. H. Lim, K. Zhu, L. J. Beilin, P. L. Thompson, et al. 2018. Cruciferous and total vegetable intakes are inversely associated with subclinical atherosclerosis in older adult women. Journal of the American Heart Association 7 (8):e008391. doi: 10.1161/JAHA.117.008391.
- Brosnan, J. T., and M. E. Brosnan. 2006. The sulfur-containing amino acids: An overview. The Journal of Nutrition 136 (6 Suppl):1636S–40S. doi: 10.1093/jn/136.6.1636S.
- Castro, V. M. D. d., K. C. de Paula Medeiros, L. I. C. de Lemos, L. de Fátima Campos Pedrosa, F. V. L. Ladd, T. G. de Carvalho, R. F. de Araújo Júnior, B. J. Abreu, and N. B. da Silva Farias. 2021. S-methyl cysteine sulfoxide ameliorates duodenal morphological alterations in streptozotocin-induced diabetic rats. Tissue & Cell 69:101483. doi: 10.1016/j.tice.2020.101483.
- Chan, J. Y., A. C. Yuen, R. Y. Chan, and S. W. Chan. 2013. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytotherapy Research: PTR 27 (5):637–46. doi: 10.1002/ptr.4796.
- Chang, J., M. Wang, Y. Jian, F. Zhang, J. Zhu, Q. Wang, and B. Sun. 2019. Health-promoting phytochemicals and antioxidant capacity in different organs from six varieties of Chinese kale. Scientific Reports 9 (1):20344. doi: 10.1038/s41598-019-56671-w.
- Chang, T., H. Ho, S.-J. Hsu, C.-C. Chang, M.-H. Tsai, T. Huo, H.-C. Huang, F.-Y. Lee, M.-C. Hou, and S.-D. Lee. 2019. Glucobrassicin metabolites ameliorate the development of portal hypertension and cirrhosis in bile duct-ligated rats. International Journal of Molecular Sciences 20 (17):4161. doi: 10.3390/ijms20174161.
- Cho, H. J., K. W. Lee, and J. H. Park. 2013. Erucin exerts anti-inflammatory properties in murine macrophages and mouse skin: Possible mediation through the inhibition of NFκB signaling. International Journal of Molecular Sciences 14 (10):20564–77. doi: 10.3390/ijms141020564.
- Choi, Y., M. A. Abdelmegeed, and B. J. Song. 2018. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. The Journal of Nutritional Biochemistry 55:12–25. doi: 10.1016/j.jnutbio.2017.11.011.
- Chuang, W. T., Y. T. Liu, C. S. Huang, C. W. Lo, H. T. Yao, H. W. Chen, and C. K. Lii. 2019. Benzyl isothiocyanate and phenethyl isothiocyanate inhibit adipogenesis and hepatosteatosis in mice with obesity induced by a high-fat diet. Journal of Agricultural and Food Chemistry 67 (25):7136–46. doi: 10.1021/acs.jafc.9b02668.
- Chung, L. Y. 2006. The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin, and allyl disulfide. Journal of Medicinal Food 9 (2):205–13. doi: 10.1089/jmf.2006.9.205.
- Citi, V., A. Martelli, L. Testai, A. Marino, M. C. Breschi, and V. Calderone. 2014. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Medica 80 (8–9):610–3. doi: 10.1055/s-0034-1368591.
- Citi, V., E. Piragine, E. Pagnotta, L. Ugolini, L. Di Cesare Mannelli, L. Testai, C. Ghelardini, L. Lazzeri, V. Calderone, and A. Martelli. 2019. Anticancer properties of erucin, an H2 S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1)). Phytotherapy Research: PTR 33 (3):845–55. doi: 10.1002/ptr.6278.
- Clarke, J. D., A. Hsu, K. Riedl, D. Bella, S. J. Schwartz, J. F. Stevens, and E. Ho. 2011. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacological Research 64 (5):456–63. doi: 10.1016/j.phrs.2011.07.005.
- Clifford, T., J. P. Acton, S. P. Cocksedge, K. A. B. Davies, and S. J. Bailey. 2021. The effect of dietary phytochemicals on nuclear factor erythroid 2-related factor 2 (Nrf2) activation: A systematic review of human intervention trials. Molecular Biology Reports 48 (2):1745–61. doi: 10.1007/s11033-020-06041-x.
- Colovic, M. B., V. M. Vasic, D. M. Djuric, and D. Z. Krstic. 2018. Sulphur-containing amino acids: Protective role against free radicals and heavy metals. Current Medicinal Chemistry 25 (3):324–35. doi: 10.2174/0929867324666170609075434.
- Coode-Bate, J., T. Sivapalan, A. Melchini, S. Saha, P. W. Needs, J. R. Dainty, J. B. Maicha, G. Beasy, M. H. Traka, R. D. Mills, et al. 2019. Accumulation of dietary S-methyl cysteine sulfoxide in human prostate tissue. Molecular Nutrition & Food Research 63 (20):e1900461. doi: 10.1002/mnfr.201900461.
- Corsello, T., N. Komaravelli, and A. Casola. 2018. Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants (Basel, Switzerland) 7 (10):129. doi: 10.3390/antiox7100129.
- Corvino, A., F. Frecentese, E. Magli, E. Perissutti, V. Santagada, A. Scognamiglio, G. Caliendo, F. Fiorino, and B. Severino. 2021. Trends in H(2)S-donors chemistry and their effects in cardiovascular diseases. Antioxidants (Basel) 10 (3):429. doi: 10.3390/antiox10030429.
- da Costa, R. M., D. Rodrigues, C. A. Pereira, J. F. Silva, J. V. Alves, N. S. Lobato, and R. C. Tostes. 2019. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Frontiers in Pharmacology 10:382. doi: 10.3389/fphar.2019.00382.
- Dayalan Naidu, S., T. Suzuki, M. Yamamoto, J. W. Fahey, and A. T. Dinkova-Kostova. 2018. Phenethyl isothiocyanate, a dual activator of transcription factors NRF2 and HSF1. Molecular Nutrition & Food Research 62 (18):e1700908. doi: 10.1002/mnfr.201700908.
- De Greef, D., E. M. Barton, E. N. Sandberg, C. R. Croley, J. Pumarol, T. L. Wong, N. Das, and A. Bishayee. 2021. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Seminars in Cancer Biology 73:219–64. doi: 10.1016/j.semcancer.2020.11.020.
- De Kok, L. J., M. Durenkamp, L. Yang, and I. Stulen. 2007. Atmospheric sulfur. In Sulfur in plants. An ecological perspective, ed. Malcolm J. Hawkesford and L. J. De Kok, 91–106. Dordrecht, The Netherlands: Springer. doi: 10.1007/978-1-4020-5887-5.
- Del Carmen Martínez-Ballesta, M., D. A. Moreno, and M. Carvajal. 2013. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. International Journal of Molecular Sciences 14 (6):11607–25. doi: 10.3390/ijms140611607.
- Dinh, T. N., M. O. Parat, Y. S. Ong, and K. Y. Khaw. 2021. Anticancer activities of dietary benzyl isothiocyanate: A comprehensive review. Pharmacological Research 169:105666. doi: 10.1016/j.phrs.2021.105666.
- Dinkova-Kostova, A. T., and R. V. Kostov. 2012. Glucosinolates and isothiocyanates in health and disease. Trends in Molecular Medicine 18 (6):337–47. doi: 10.1016/j.molmed.2012.04.003.
- Doleman, J. F., K. Grisar, L. Van Liedekerke, S. Saha, M. Roe, H. S. Tapp, and R. F. Mithen. 2017. The contribution of alliaceous and cruciferous vegetables to dietary sulphur intake. Food Chemistry 234:38–45. doi: 10.1016/j.foodchem.2017.04.098.
- Dong, Z., R. Sinha, and J. P. Richie. Jr. 2018. Disease prevention and delayed aging by dietary sulfur amino acid restriction: Translational implications. Annals of the New York Academy of Sciences 1418 (1):44–55. doi: 10.1111/nyas.13584.
- Dong, Z., X. Gao, V. M. Chinchilli, R. Sinha, J. Muscat, R. M. Winkels, and J. P. Richie, Jr. 2020. Association of sulfur amino acid consumption with cardiometabolic risk factors: Cross-sectional findings from NHANES III. EClinicalMedicine 19:100248. doi: 10.1016/j.eclinm.2019.100248.
- Edmands, W. M. B., N. J. Gooderham, E. Holmes, and S. C. Mitchell. 2013. S-Methyl-l-cysteine sulphoxide: The Cinderella phytochemical? Toxicology Research 2 (1):11–22. doi: 10.1039/C2TX20030A.
- Eisenschmidt-Bönn, D., N. Schneegans, A. Backenköhler, U. Wittstock, and W. Brandt. 2019. Structural diversification during glucosinolate breakdown: Mechanisms of thiocyanate, epithionitrile and simple nitrile formation. The Plant Journal: For Cell and Molecular Biology 99 (2):329–43. doi: 10.1111/tpj.14327.
- El-Saber Batiha, G., A. M. Beshbishy, L. G. Wasef, Y. H. A. Elewa, A. A. Al-Sagan, A. A. Al-Sagan, M. E. Abd El-Hack, A. E. Taha, Y. M. Abd-Elhakim, and H. P. Devkota. 2020. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 12 (3):872. doi: 10.3390/nu12030872.
- Ernst, I. M., K. Palani, T. Esatbeyoglu, K. Schwarz, and G. Rimbach. 2013. Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacological Research 70 (1):155–62. doi: 10.1016/j.phrs.2013.01.011.
- Esteve, M. 2020. Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: A focus on metabolic syndrome. Frontiers in Nutrition 7:111. doi: 10.3389/fnut.2020.00111.
- Fahey, J. W., A. T. Zalcmann, and P. Talalay. 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56 (1):5–51. doi: 10.1016/S0031-9422(00)00316-2.
- Fahey, J. W., and T. Kensler. 2021. The challenges of designing and implementing clinical trials with broccoli sprouts … and turning evidence into public health action. Frontiers in Nutrition 8 (183):648788. doi: 10.3389/fnut.2021.648788.
- Fakhri, S., M. Pesce, A. Patruno, S. Z. Moradi, A. Iranpanah, M. H. Farzaei, and E. Sobarzo-Sánchez. 2020. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: A mechanistic review. Molecules 25 (21):4926. doi: 10.3390/molecules25214926.
- Farag, M. A., and A. A. Motaal. 2010. Sulforaphane composition, cytotoxic and antioxidant activity of crucifer vegetables. Journal of Advanced Research 1 (1):65–70. doi: 10.1016/j.jare.2010.02.005.
- Farhat, Z., P. Hershberger, J. Freudenheim, M. Mammen, R. Hageman Blair, D. Aga, and L. Mu. 2021. Types of garlic and their anticancer and antioxidant activity: A review of the epidemiologic and experimental evidence. European Journal of Nutrition 60 (7):3585–609. doi: 10.1007/s00394-021-02482-7.
- Favela-González, K. M., A. Y. Hernández-Almanza, and N. M. De la Fuente-Salcido. 2020. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. Journal of Food Biochemistry: e13414. doi: 10.1111/jfbc.13414.
- Felker, P., R. Bunch, and A. M. Leung. 2016. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutrition Reviews 74 (4):248–58. doi: 10.1093/nutrit/nuv110.
- Ferrucci, L., and E. Fabbri. 2018. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews. Cardiology 15 (9):505–22. doi: 10.1038/s41569-018-0064-2.
- Franceschi, C., P. Garagnani, P. Parini, C. Giuliani, and A. Santoro. 2018. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nature Reviews. Endocrinology 14 (10):576–90. doi: 10.1038/s41574-018-0059-4.
- Francioso, A., A. Baseggio Conrado, L. Mosca, and M. Fontana. 2020. Chemistry and biochemistry of sulfur natural compounds: Key intermediates of metabolism and redox biology. Oxidative Medicine and Cellular Longevity 2020:8294158. doi: 10.1155/2020/8294158.
- Franco, R., O. J. Schoneveld, A. Pappa, and M. I. Panayiotidis. 2007. The central role of glutathione in the pathophysiology of human diseases. Archives of Physiology and Biochemistry 113 (4–5):234–58. doi: 10.1080/13813450701661198.
- Fuentes, E., M. Alarcón, M. Fuentes, G. Carrasco, and I. Palomo. 2014. A novel role of Eruca sativa Mill. (rocket) extract: Antiplatelet (NF-κB inhibition) and antithrombotic activities. Nutrients 6 (12):5839–52. doi: 10.3390/nu6125839.
- Galavi, A., H. Hosseinzadeh, and B. Marjan Razavi. 2021. The effects of Allium cepa L. (onion) and its active constituents on metabolic syndrome: A review. Iranian Journal of Basic Medical Sciences 24 (1):3–16. doi: 10.22038/ijbms.2020.46956.10843.
- Gasparello, J., E. D’Aversa, C. Papi, L. Gambari, B. Grigolo, M. Borgatti, A. Finotti, and R. Gambari. 2021. Sulforaphane inhibits the expression of interleukin-6 and interleukin-8 induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 Spike protein. Phytomedicine 87:153583. doi: 10.1016/j.phymed.2021.153583.
- Gigolashvili, T., and S. Kopriva. 2014. Transporters in plant sulfur metabolism. Frontiers in Plant Science 5:442. doi: 10.3389/fpls.2014.00442.
- Giles, G. I., M. J. Nasim, W. Ali, and C. Jacob. 2017. The reactive sulfur species concept: 15 years on. Antioxidants (Basel, Switzerland) 6 (2):38. doi: 10.3390/antiox6020038.
- Gong, T. T., Q. Guo, X. Li, T. N. Zhang, F. H. Liu, X. H. He, B. Lin, and Q. J. Wu. 2021. Isothiocyanate iberin inhibits cell proliferation and induces cell apoptosis in the progression of ovarian cancer by mediating ROS accumulation and GPX1 expression. Biomedicine & Pharmacotherapy 142:111533. doi: 10.1016/j.biopha.2021.111533.
- Gugliandolo, A., S. Giacoppo, M. Ficicchia, A. Aliquò, P. Bramanti, and E. Mazzon. 2018. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Molecular Medicine Reports 17 (5):6235–44. doi: 10.3892/mmr.2018.8695.
- Gullner, G., B. Zechmann, A. Künstler, and L. Király. 2017. The signaling roles of glutathione in plant disease resistance. In Glutathione in plant growth, development, and stress tolerance, ed. Mohammad Anwar Hossain, Mohammad Golam Mostofa, Pedro Diaz-Vivancos, David J. Burritt, Masayuki Fujita, and Lam-Son Phan Tran, 331–57. Cham: Springer International Publishing.
- Gupta, P., S. E. Wright, S. H. Kim, and S. K. Srivastava. 2014. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochimica et Biophysica Acta 1846 (2):405–24. doi: 10.1016/j.bbcan.2014.08.003.
- Gwon, M. H., Y. S. Im, A. R. Seo, K. Y. Kim, H. R. Moon, and J. M. Yun. 2020. Phenethyl isothiocyanate protects against high fat/cholesterol diet-induced obesity and atherosclerosis in C57BL/6 mice. Nutrients 12 (12):3657. doi: 10.3390/nu12123657.
- Gwon, M. H., and J. M. Yun. 2021. Phenethyl isothiocyanate improves lipid metabolism and inflammation via mTOR/PPARγ/AMPK signaling in the adipose tissue of obese mice. Journal of Medicinal Food 24 (6):666–9. doi: 10.1089/jmf.2020.4881.
- Hajra, S., A. R. Patra, A. Basu, and S. Bhattacharya. 2018. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomedicine & Pharmacotherapy 101:228–43. doi: 10.1016/j.biopha.2018.02.088.
- Halkier, B. A., and J. Gershenzon. 2006. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology 57:303–33. doi: 10.1146/annurev.arplant.57.032905.105228.
- Hasanuzzaman, M., K. Nahar, T. I. Anee, and M. Fujita. 2017. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiology and Molecular Biology of Plants 23 (2):249–68. doi: 10.1007/s12298-017-0422-2.
- Hawkesford, M. J. 2007. Sulfur and plant ecology: A central role of sulfate transporters in responses to sulfur availability. In Sulfur in plants. An ecological perspective, ed. M. J. Hawkesford and L. J. De Kok, 1–15. Dordrecht, The Netherlands: Springer. doi: 10.1007/978-1-4020-5887-5.
- Hirasawa, N. 2019. Expression of histidine decarboxylase and its roles in inflammation. International Journal of Molecular Sciences 20 (2):376. doi: 10.3390/ijms20020376.
- Horníčková, J., R. Kubec, K. Cejpek, J. Velíšek, J. Ovesná, and H. Stavělíková. 2010. Profiles of S-alk(en)ylcysteine sulfoxides in various garlic genotypes. Czech Journal of Food Sciences 28 (4):298–308. doi: 10.17221/135/2010-CJFS.
- Houghton, C. A. 2019. Sulforaphane: Its "coming of age" as a clinically relevant nutraceutical in the prevention and treatment of chronic disease. Oxidative Medicine and Cellular Longevity 2019:2716870. doi: 10.1155/2019/2716870.
- Hsu, C. N., and Y. L. Tain. 2021. Preventing developmental origins of cardiovascular disease: Hydrogen sulfide as a potential target? Antioxidants 10 (2):247. doi: 10.3390/antiox10020247.
- Hwang, I. M., B. Park, Y. M. Dang, S. Y. Kim, and H. Y. Seo. 2019. Simultaneous direct determination of 15 glucosinolates in eight Brassica species by UHPLC-Q-Orbitrap-MS. Food Chemistry 282:127–33. doi: 10.1016/j.foodchem.2018.12.036.
- Im, Y. S., M. H. Gwon, and J. M. Yun. 2021. Protective effects of phenethyl isothiocyanate on foam cell formation by combined treatment of oxidized low-density lipoprotein and lipopolysaccharide in THP-1 macrophage. Food Science & Nutrition 9 (6):3269–79. doi: 10.1002/fsn3.2293.
- Ingenbleek, Y., and H. Kimura. 2013. Nutritional essentiality of sulfur in health and disease. Nutrition Reviews 71 (7):413–32. doi: 10.1111/nure.12050.
- Iranshahi, M. 2012. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. Journal of Essential Oil Research 24 (4):393–434. doi: 10.1080/10412905.2012.692918.
- Jadhav, U., R. Ezhilarasan, S. F. Vaughn, M. A. Berhow, and S. Mohanam. 2007. Iberin induces cell cycle arrest and apoptosis in human neuroblastoma cells. International Journal of Molecular Medicine 19 (3):353–61. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2435066/. doi: 10.3892/ijmm.19.3.353.
- Jang, Y. J., B. Park, H. W. Lee, H. J. Park, H. J. Koo, B. O. Kim, E. H. Sohn, S. H. Um, and S. Pyo. 2017. Sinigrin attenuates the progression of atherosclerosis in ApoE-/- mice fed a high-cholesterol diet potentially by inhibiting VCAM-1 expression. Chemico-Biological Interactions 272:28–36. doi: 10.1016/j.cbi.2017.05.006.
- Jang, Y. J., and S. Pyo. 2015. Anti-atherosclerotic effect of sinigrin in ApoE-deficient mice. The FASEB Journal 29 (S1):609–1. doi: 10.1096/fasebj.29.1_supplement.609.1.
- Jez, J. M. 2019. Structural biology of plant sulfur metabolism: From sulfate to glutathione. Journal of Experimental Botany 70 (16):4089–103. doi: 10.1093/jxb/erz094.
- Jia, X., L. Zhong, Y. Song, Y. Hu, G. Wang, and S. Sun. 2016. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Primary Care Diabetes 10 (4):272–80. doi: 10.1016/j.pcd.2015.12.004.
- Jiang, J., T. B. Kang, W. Shim do, N. H. Oh, T. J. Kim, and K. H. Lee. 2013. Indole-3-carbinol inhibits LPS-induced inflammatory response by blocking TRIF-dependent signaling pathway in macrophages. Food and Chemical Toxicology 57:256–61. doi: 10.1016/j.fct.2013.03.040.
- Jiang, Y., S. H. Wu, X. O. Shu, Y. B. Xiang, B. T. Ji, G. L. Milne, Q. Cai, X. Zhang, Y. T. Gao, W. Zheng, et al. 2014. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. Journal of the Academy of Nutrition and Dietetics 114 (5):700–8.e2. doi: 10.1016/j.jand.2013.12.019.
- Kalinina, E. V., and L. A. Gavriliuk. 2020. Glutathione synthesis in cancer cells. Biochemistry. Biokhimiia 85 (8):895–907. doi: 10.1134/S0006297920080052.
- Khan, M. S., F. H. Haas, A. A. Samami, A. M. Gholami, A. Bauer, K. Fellenberg, M. Reichelt, R. Hänsch, R. R. Mendel, A. J. Meyer, et al. 2010. Sulfite reductase defines a newly discovered bottleneck for assimilatory sulfate reduction and is essential for growth and development in Arabidopsis thaliana. The Plant Cell 22 (4):1216–31. doi: 10.1105/tpc.110.074088.
- Khanfar, A., and A. Qaroot. 2020. Could glutathione depletion be the Trojan horse of COVID-19 mortality? European Review for Medical and Pharmacological Sciences 24 (23):12500–9. doi: 10.26355/eurrev_202012_24046.
- Kim, J. 2021. Pre-clinical neuroprotective evidences and plausible mechanisms of sulforaphane in Alzheimer’s disease. International Journal of Molecular Sciences 22 (6):2929. doi: 10.3390/ijms22062929.
- Kliebenstein, D. J., J. Kroymann, and T. Mitchell-Olds. 2005. The glucosinolate-myrosinase system in an ecological and evolutionary context. Current Opinion in Plant Biology 8 (3):264–71. doi: 10.1016/j.pbi.2005.03.002.
- Kodera, Y., M. Kurita, M. Nakamoto, and T. Matsutomo. 2020. Chemistry of aged garlic: Diversity of constituents in aged garlic extract and their production mechanisms via the combination of chemical and enzymatic reactions (Review). Experimental and Therapeutic Medicine 19 (2):1574–84. doi: 10.3892/etm.2019.8393.
- Kodera, Y., M. Ushijima, H. Amano, J. I. Suzuki, and T. Matsutomo. 2017. Chemical and biological properties of S-1-propenyl-l-cysteine in aged garlic extract. Molecules 22 (4):570. doi: 10.3390/molecules22040570.
- Kottoor, S. J., and R. R. Arora. 2018. The utility of anti-inflammatory agents in cardiovascular disease: A novel perspective on the treatment of atherosclerosis. Journal of Cardiovascular Pharmacology and Therapeutics 23 (6):483–93. doi: 10.1177/1074248418778548.
- Krause, K., A. Pyrczak-Felczykowska, M. Karczewska, M. Narajczyk, A. Herman-Antosiewicz, A. Szalewska-Pałasz, and D. Nowicki. 2021. Dietary isothiocyanates, sulforaphane and 2-phenethyl isothiocyanate, effectively impair vibrio cholerae virulence. International Journal of Molecular Sciences 22 (19):10187. doi: 10.3390/ijms221910187.
- Kumar, P., O. Osahon, D. B. Vides, N. Hanania, C. G. Minard, and R. V. Sekhar. 2021. Severe glutathione deficiency, oxidative stress and oxidant damage in adults hospitalized with COVID-19: Implications for GlyNAC (glycine and N-acetylcysteine) supplementation. Antioxidants 11 (1):50. doi: 10.3390/antiox11010050.
- Kumari, K., and K. T. Augusti. 2002. Antidiabetic and antioxidant effects of S-methyl cysteine sulfoxide isolated from onions (Allium cepa Linn) as compared to standard drugs in alloxan diabetic rats. Indian Journal of Experimental Biology 40 (9):1005–9. https://pubmed.ncbi.nlm.nih.gov/12587728/.
- Kumari, K., B. C. Mathew, and K. T. Augusti. 1995. Antidiabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Indian Journal of Biochemistry & Biophysics 32 (1):49–54. https://pubmed.ncbi.nlm.nih.gov/7665195/.
- Kunimura, K., S. Miki, M. Takashima, and J. I. Suzuki. 2021. S-1-propenylcysteine improves TNF-α-induced vascular endothelial barrier dysfunction by suppressing the GEF-H1/RhoA/Rac pathway. Cell Communication and Signaling: CCS 19 (1):17. doi: 10.1186/s12964-020-00692-w.
- Künstler, A., G. Gullner, A. L. Ádám, K. Nagy, and L. Király. 2020. The versatile roles of sulfur-containing biomolecules in plant defense-A road to disease resistance. Plants (Basel) 9 (12):1705. doi: 10.3390/plants9121705.
- Kuschman, H. P., M. B. Palczewski, and D. D. Thomas. 2021. Nitric oxide and hydrogen sulfide: Sibling rivalry in the family of epigenetic regulators. Free Radical Biology & Medicine 170:34–43. doi: 10.1016/j.freeradbiomed.2021.01.010.
- Lancaster, J. E., and M. L. Shaw. 1989. γ-Glutamyl peptides in the biosynthesis of S-alk(en)yl-l-cysteine sulphoxides (flavour precursors) in Allium. Phytochemistry 28 (2):455–60. doi: 10.1016/0031-9422(89)80031-7.
- Lee, H. W., C. G. Lee, D. K. Rhee, S. H. Um, and S. Pyo. 2017. Sinigrin inhibits production of inflammatory mediators by suppressing NF-κB/MAPK pathways or NLRP3 inflammasome activation in macrophages. International Immunopharmacology 45:163–73. doi: 10.1016/j.intimp.2017.01.032.
- Lee, H. W., and K. R. Lee. 2015. Effect of sinigrin on vascular cell adhesion molecule-1 expression in TNF-α-stimulated mouse vascular smooth muscle cells via downregulation of NF-κB signaling pathways. The FASEB Journal 29 (S1):593–15. doi: 10.1096/fasebj.29.1_supplement.593.15.
- Lee, H. H., J.-W. Jeong, S. H. Hong, C. Park, B. W. Kim, and Y. H. Choi. 2018. Diallyl trisulfide suppresses the production of lipopolysaccharide-induced inflammatory mediators in BV2 microglia by decreasing the NF-κB pathway activity associated with toll-like receptor 4 and CXCL12/CXCR4 pathway blockade. Journal of Cancer Prevention 23 (3):134–40. doi: 10.15430/JCP.2018.23.3.134.
- Lemos, L. I. C., M. A. Medeiros, J. Lima, T. O. Teixeira, C. A. Figueiredo, N. B. S. Farias, F. S. Silva, B. J. Abreu, K. C. P. Medeiros, and L. F. C. Pedrosa. 2021. S-methyl cysteine sulfoxide mitigates histopathological damage, alleviate oxidative stress and promotes immunomodulation in diabetic rats. Journal of Complementary and Integrative Medicine 18 (4):719–25. doi: 10.1515/jcim-2020-0220.
- Li, M., S. Wang, X. Li, L. Jiang, X. Wang, R. Kou, Q. Wang, L. Xu, N. Zhao, and K. Xie. 2018. Diallyl sulfide protects against lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. Food and Chemical Toxicology 120:500–9. doi: 10.1016/j.fct.2018.07.053.
- Li, Z., H. Guo, J. Li, T. Ma, S. Zhou, Z. Zhang, L. Miao, and L. Cai. 2020. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clinical Science (London, England: 1979) 134 (18):2469–87. doi: 10.1042/CS20191088.
- Liang, X., H. W. Lee, Z. Li, Y. Lu, L. Zou, and C. N. Ong. 2018. Simultaneous quantification of 22 glucosinolates in 12 Brassicaceae vegetables by hydrophilic interaction chromatography-tandem mass spectrometry. ACS Omega 3 (11):15546–53. doi: 10.1021/acsomega.8b01668.
- Liebman, S. E., and T. H. Le. 2021. Eat your broccoli: Oxidative stress, NRF2, and sulforaphane in chronic kidney disease. Nutrients 13 (1):266. doi: 10.3390/nu13010266.
- Lietzow, J. 2021. Biologically active compounds in mustard seeds: A toxicological perspective. Foods (Basel, Switzerland) 10 (9):2089. doi: 10.3390/foods10092089.
- Liguori, I., G. Russo, F. Curcio, G. Bulli, L. Aran, D. Della-Morte, G. Gargiulo, G. Testa, F. Cacciatore, D. Bonaduce, et al. 2018. Oxidative stress, aging, and diseases. Clinical Interventions in Aging 13:757–72. doi: 10.2147/CIA.S158513.
- Liu, T., L. Zhang, D. Joo, and S.-C. Sun. 2017. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2 (1):17023. doi: 10.1038/sigtrans.2017.23.
- Liu, Z., H. Wang, J. Xie, J. Lv, G. Zhang, L. Hu, S. Luo, L. Li, and J. Yu. 2021. The roles of Cruciferae glucosinolates in disease and pest resistance. Plants (Basel) 10 (6):1097. doi: 10.3390/plants10061097.
- Livshits, G., and A. Kalinkovich. 2019. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Research Reviews 56:100980. doi: 10.1016/j.arr.2019.100980.
- López-Chillón, M. T., C. Carazo-Díaz, D. Prieto-Merino, P. Zafrilla, D. A. Moreno, and D. Villaño. 2019. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clinical Nutrition 38 (2):745–52. doi: 10.1016/j.clnu.2018.03.006.
- Lucarini, E., E. Pagnotta, L. Micheli, C. Parisio, L. Testai, A. Martelli, V. Calderone, R. Matteo, L. Lazzeri, L. Di Cesare Mannelli, et al. 2019. Eruca sativa meal against diabetic neuropathic pain: An H(2)S-mediated effect of glucoerucin. Molecules 24 (16):3006. doi: 10.3390/molecules24163006.
- Mae, T., K. Ohira, and A. Fujiwara. 1971. Fate of ($) S-methyl-L-cysteine sulfoxide in Chinese cabbage, Brassica pekinensis RUPR. Plant and Cell Physiology 12 (1):1–11. doi: 10.1093/oxfordjournals.pcp.a074591.
- Magli, E., E. Perissutti, V. Santagada, G. Caliendo, A. Corvino, G. Esposito, G. Esposito, F. Fiorino, M. Migliaccio, A. Scognamiglio, et al. 2021. H(2)S donors and their use in medicinal chemistry. Biomolecules 11 (12):1899. doi: 10.3390/biom11121899.
- Maina, S., G. Misinzo, G. Bakari, and H.-Y. Kim. 2020. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A systematic review. Molecules (Basel, Switzerland) 25 (16):3682. doi: 10.3390/molecules25163682.
- Marino, M., D. Martini, S. Venturi, M. Tucci, M. Porrini, P. Riso, and D. Bo. 2021. An overview of registered clinical trials on glucosinolates and human health: The current situation. Frontiers in Nutrition 8:730906. doi: 10.3389/fnut.2021.730906.
- Marks, H. S., J. A. Hilson, H. C. Leichtweis, and G. S. Stoewsand. 1992. S-methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from brussels sprouts. Journal of Agricultural and Food Chemistry 40 (11):2098–101. doi: 10.1021/jf00023a012.
- Martelli, A., V. Citi, L. Testai, S. Brogi, and V. Calderone. 2020. Organic isothiocyanates as hydrogen sulfide donors. Antioxidants & Redox Signaling 32 (2):110–44. doi: 10.1089/ars.2019.7888.
- Martelli, A., E. Piragine, V. Citi, L. Testai, E. Pagnotta, L. Ugolini, L. Lazzeri, L. Di Cesare Mannelli, O. L. Manzo, M. Bucci, et al. 2020. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2 S-releasing properties. British Journal of Pharmacology 177 (4):824–35. doi: 10.1111/bph.14645.
- Martin, H. 2010. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutation Research 690 (1–2):57–63. doi: 10.1016/j.mrfmmm.2009.09.009.
- Maruthanila, V. L., J. Poornima, and S. Mirunalini. 2014. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3’-diindolylmethane: A therapeutic marvel. Advances in Pharmacological Sciences 2014:832161. doi: 10.1155/2014/832161.
- Maruyama-Nakashita, A. 2017. Metabolic changes sustain the plant life in low-sulfur environments. Current Opinion in Plant Biology 39:144–51. doi: 10.1016/j.pbi.2017.06.015.
- Matsutomo, T., M. Ushijima, Y. Kodera, M. Nakamoto, M. Takashima, N. Morihara, and K. Tamura. 2017. Metabolomic study on the antihypertensive effect of S-1-propenylcysteine in spontaneously hypertensive rats using liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences 1046:147–55. doi: 10.1016/j.jchromb.2017.01.029.
- Matsutomo, T., M. Ushijima, K. Kunimura, and M. Ohtani. 2019. Metabolomic study reveals the acute hypotensive effect of S-1-propenylcysteine accompanied by alteration of the plasma histidine level in spontaneously hypertensive rats. Journal of Pharmaceutical and Biomedical Analysis 168:148–54. doi: 10.1016/j.jpba.2019.01.043.
- Matuz-Mares, D., H. Riveros-Rosas, M. M. Vilchis-Landeros, and H. Vázquez-Meza. 2021. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants (Basel) 10 (8):1220. doi: 10.3390/antiox10081220.
- Mazumder, A., A. Dwivedi, and J. du Plessis. 2016. Sinigrin and its therapeutic benefits. Molecules (Basel, Switzerland) 21 (4):416. doi: 10.3390/molecules21040416.
- Melchini, A., and M. H. Traka. 2010. Biological profile of erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2 (4):593–612. doi: 10.3390/toxins2040593.
- Melrose, J. 2019. The glucosinolates: A sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines 7 (3):62. doi: 10.3390/biomedicines7030062.
- Miękus, N., K. Marszałek, M. Podlacha, A. Iqbal, C. Puchalski, and A. H. Świergiel. 2020. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules (Basel, Switzerland) 25 (17):3804. doi: 10.3390/molecules25173804.
- Mithen, R. 2007. Sulphur-containing compounds. In Plant secondary metabolites: Occurrence, structure and role in the human diet, 25–46. Chichester, UK: John Wiley and Sons.
- Mithen, R., and E. Ho. 2018. Isothiocyanates for human health. Molecular Nutrition & Food Research 62 (18):e1870079. doi: 10.1002/mnfr.201870079.
- Mitreiter, S., and T. Gigolashvili. 2021. Regulation of glucosinolate biosynthesis. Journal of Experimental Botany 72 (1):70–91. doi: 10.1093/jxb/eraa479.
- Moreira, D. M., R. L. da Silva, J. L. Vieira, T. Fattah, M. E. Lueneberg, and C. A. Gottschall. 2015. Role of vascular inflammation in coronary artery disease: Potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease. American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions 15 (1):1–11. doi: 10.1007/s40256-014-0094-z.
- Mugford, S. G., B. R. Lee, A. Koprivova, C. Matthewman, and S. Kopriva. 2011. Control of sulfur partitioning between primary and secondary metabolism. The Plant Journal: For Cell and Molecular Biology 65 (1):96–105. doi: 10.1111/j.1365-313X.2010.04410.x.
- Mukwevho, E., Z. Ferreira, and A. Ayeleso. 2014. Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules (Basel, Switzerland) 19 (12):19376–89. doi: 10.3390/molecules191219376.
- Munday, R., and C. M. Munday. 2004. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. Journal of Agricultural and Food Chemistry 52 (7):1867–71. doi: 10.1021/jf030549s.
- Narbad, A., and J. T. Rossiter. 2018. Gut glucosinolate metabolism and isothiocyanate production. Molecular Nutrition & Food Research 62 (18):e1700991. doi: 10.1002/mnfr.201700991.
- Nguyen, V. P. T., J. Stewart, M. Lopez, I. Ioannou, and F. Allais. 2020. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules (Basel, Switzerland) 25 (19):4537. doi: 10.3390/molecules25194537.
- Nimni, M. E., B. Han, and F. Cordoba. 2007. Are we getting enough sulfur in our diet? Nutrition & Metabolism 4:24. doi: 10.1186/1743-7075-4-24.
- Nugrahedi, P. Y., T. Oliviero, J. K. Heising, M. Dekker, and R. Verkerk. 2017. Stir-frying of Chinese cabbage and pakchoi retains health-promoting glucosinolates. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 72 (4):439–44. doi: 10.1007/s11130-017-0646-x.
- Nwachukwu, I. D., and A. J. Slusarenko. 2014. Thiosulfinates, organic polysulfanes, and related compounds: From an unusual chemistry toward a wealth of potential applications. In Recent advances in redox active plant and microbial products: From basic chemistry to widespread applications in medicine and agriculture, 265–88. Netherlands: Springer.
- Nwachukwu, I. D., A. J. Slusarenko, and M. C. Gruhlke. 2012. Sulfur and sulfur compounds in plant defence. Natural Product Communications 7 (3):395–400. doi: 10.1177/1934578X1200700323.
- Palliyaguru, D. L., J.-M. Yuan, T. W. Kensler, and J. W. Fahey. 2018. Isothiocyanates: Translating the power of plants to people. Molecular Nutrition & Food Research 62 (18):1700965. doi: 10.1002/mnfr.201700965.
- Paul, S., C. A. Geng, T. H. Yang, Y. P. Yang, and J. J. Chen. 2019. Phytochemical and health-beneficial progress of turnip (Brassica rapa). Journal of Food Science 84 (1):19–30. doi: 10.1111/1750-3841.14417.
- Petropoulos, S., F. Di Gioia, and G. Ntatsi. 2017. Vegetable organosulfur compounds and their health promoting effects. Current Pharmaceutical Design 23 (19):2850–75. doi: 10.2174/1381612823666170111100531.
- Pocasap, P., N. Weerapreeyakul, and K. Thumanu. 2019. Alyssin and iberin in cruciferous vegetables exert anticancer activity in HepG2 by increasing intracellular reactive oxygen species and tubulin depolymerization. Biomolecules & Therapeutics 27 (6):540–52. doi: 10.4062/biomolther.2019.027.
- Polonikov, A. 2020. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infectious Diseases 6 (7):1558–62. doi: 10.1021/acsinfecdis.0c00288.
- Puccinelli, M. T., and S. D. Stan. 2017. Dietary bioactive diallyl trisulfide in cancer prevention and treatment. International Journal of Molecular Sciences 18 (8):1645. doi: 10.3390/ijms18081645.
- Qi, T., F. Xu, X. Yan, S. Li, and H. Li. 2016. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. International Journal of Molecular Medicine 37 (1):182–8. doi: 10.3892/ijmm.2015.2396.
- Quintero-Fabián, S., D. Ortuño-Sahagún, M. Vázquez-Carrera, and R. I. López-Roa. 2013. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediators of Inflammation 2013:381815. doi: 10.1155/2013/381815.
- Raghupathi, W., and V. Raghupathi. 2018. An empirical study of chronic diseases in the United States: A visual analytics approach. International Journal of Environmental Research and Public Health 15 (3):431. doi: 10.3390/ijerph15030431.
- Ramirez, C. N., W. Li, C. Zhang, R. Wu, S. Su, C. Wang, L. Gao, R. Yin, and A.-N. Kong. 2017. In vitro-in vivo dose response of ursolic acid, sulforaphane, PEITC, and curcumin in cancer prevention. The AAPS Journal 20 (1):19. doi: 10.1208/s12248-017-0177-2.
- Rektorisova, M., V. Hrbek, M. Jiru, J. Ovesna, and J. Hajslova. 2020. Variability in S-alk(en)yl-l-cysteine sulfoxides in garlic within a seven-month period determined by a liquid chromatography - Tandem mass spectrometry method. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 75 (3):376–82. doi: 10.1007/s11130-020-00817-z.
- Ridker, P. M. 2018. Clinician’s guide to reducing inflammation to reduce atherothrombotic risk: JACC review topic of the week. Journal of the American College of Cardiology 72 (25):3320–31. doi: 10.1016/j.jacc.2018.06.082.
- Roman, Y. M., A. V. Hernandez, and C. M. White. 2020. The role of suppressing inflammation in the treatment of atherosclerotic cardiovascular disease. Annals of Pharmacotherapy 54 (10):1021–9. doi: 10.1177/1060028020922994.
- Romero, L. C., M. Á. Aroca, A. M. Laureano-Marín, I. Moreno, I. García, and C. Gotor. 2014. Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Molecular Plant 7 (2):264–76. doi: 10.1093/mp/sst168.
- Rose, P., M. Whiteman, P. K. Moore, and Z. Z. Yi. 2005. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Natural Product Reports 22 (3):351–68. doi: 10.1039/b417639c.
- Rose, P., P. K. Moore, M. Whiteman, and Y.-Z. Zhu. 2019. An appraisal of developments in Allium sulfur chemistry: Expanding the pharmacopeia of garlic. Molecules (Basel, Switzerland) 24 (21):4006. doi: 10.3390/molecules24214006.
- Ruhee, R. T., S. Ma, and K. Suzuki. 2019. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants (Basel) 8 (12):577. doi: 10.3390/antiox8120577.
- Ruhee, R. T., L. A. Roberts, S. Ma, and K. Suzuki. 2020. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Frontiers in Nutrition 7:64. doi: 10.3389/fnut.2020.00064.
- Russo, M., C. Spagnuolo, G. L. Russo, K. Skalicka-Woźniak, M. Daglia, E. Sobarzo-Sánchez, S. F. Nabavi, and S. M. Nabavi. 2018. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Critical Reviews in Food Science and Nutrition 58 (8):1391–405. doi: 10.1080/10408398.2016.1259983.
- Sánchez-Sánchez, M., A. S. M. Zepeda-Morales, L. Carrera-Quintanar, J. M. Viveros-Paredes, N. N. Franco-Arroyo, M. Godínez-Rubí, D. Ortuño-Sahagun, and R. I. López-Roa. 2020. López-Roa. 2020. Alliin, an Allium sativum nutraceutical, reduces metaflammation markers in DIO mice. Nutrients 12 (3):624. doi: 10.3390/nu12030624.
- Scammahorn, J. J., I. T. N. Nguyen, E. M. Bos, H. Van Goor, and J. A. Joles. 2021. Fighting oxidative stress with sulfur: Hydrogen sulfide in the renal and cardiovascular systems. Antioxidants (Basel) 10 (3):373. doi: 10.3390/antiox10030373.
- Schweizer, F., P. Fernández-Calvo, M. Zander, M. Diez-Diaz, S. Fonseca, G. Glauser, M. G. Lewsey, J. R. Ecker, R. Solano, and P. Reymond. 2013. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. The Plant Cell 25 (8):3117–32. doi: 10.1105/tpc.113.115139.
- Shang, A., S. Y. Cao, X. Y. Xu, R. Y. Gan, G. Y. Tang, H. Corke, V. Mavumengwana, and H. B. Li. 2019. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 8 (7):246. doi: 10.3390/foods8070246.
- Shi, L., Q. Lin, X. Li, Y. Nie, S. Sun, X. Deng, L. Wang, J. Lu, Y. Tang, and F. Luo. 2017. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Molecular Nutrition & Food Research 61 (9):1601013. doi: 10.1002/mnfr.201601013.
- Smith, R. H., C. R. Earl, and N. A. Matheson. 1974. The probable role of S-methylcysteine sulphoxide in kale, poisoning in ruminants. Biochemical Society Transactions 2 (1):101–7. doi: 10.1042/bst0020101.
- Sønderby, I. E., M. Burow, H. C. Rowe, D. J. Kliebenstein, and B. A. Halkier. 2010. A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiology 153 (1):348–63. doi: 10.1104/pp.109.149286.
- Soundararajan, P., and J. S. Kim. 2018. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 23 (11):2983. doi: 10.3390/molecules23112983.
- Štefanová, I., J. Zápal, M. Moos, M. Kuzma, and R. Kubec. 2019. Isoalliin-derived thiolanes formed in homogenized onion. Journal of Agricultural and Food Chemistry 67 (35):9895–906. doi: 10.1021/acs.jafc.9b01384.
- Stefels, J. 2007. Sulfur in the marine environment. In Sulfur in plants. An ecological perspective, ed. Malcolm J. Hawkesford and L. J. De Kok, 77–90. Dordrecht, The Netherlands: Springer. doi: 10.1007/978-1-4020-5887-5.
- Suman, S., and Y. Shukla. 2016. Diallyl sulfide and its role in chronic diseases prevention. Advances in Experimental Medicine and Biology 929:127–44. doi: 10.1007/978-3-319-41342-6_6.
- Sun, C.-C., S.-J. Li, C.-L. Yang, R.-L. Xue, Y.-Y. Xi, L. Wang, Q.-L. Zhao, and D.-J. Li. 2015. Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via NF-E2-related factor 2 (Nrf2)-mediated inhibition of NF-κB signaling pathway. The Journal of Biological Chemistry 290 (29):17784–95. doi: 10.1074/jbc.M115.655019.
- Sun, Y., S. Zhou, H. Guo, J. Zhang, T. Ma, Y. Zheng, Z. Zhang, and L. Cai. 2020. Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metabolism: Clinical and Experimental 102:154002. doi: 10.1016/j.metabol.2019.154002.
- Tausz, M. 2007. Sulfur in forest ecosystems. In Sulfur in plants. An ecological perspective, ed. Malcolm J. Hawkesford and L. J. De Kok, 59–75. Dordrecht, The Netherlands: Springer. doi: 10.1007/978-1-4020-5887-5.
- Teskey, G., R. Abrahem, R. Cao, K. Gyurjian, H. Islamoglu, M. Lucero, A. Martinez, E. Paredes, O. Salaiz, B. Robinson, et al. 2018. Glutathione as a marker for human disease. Advances in Clinical Chemistry 87:141–59. doi: 10.1016/bs.acc.2018.07.004.
- Tian, S., X. Liu, P. Lei, X. Zhang, and Y. Shan. 2018. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. Journal of the Science of Food and Agriculture 98 (4):1255–60. doi: 10.1002/jsfa.8654.
- Tuñón, J., M. Bäck, L. Badimón, M. L. Bochaton-Piallat, B. Cariou, M. J. Daemen, J. Egido, P. C. Evans, S. E. Francis, D. F. Ketelhuth, ESC Working Group on Atherosclerosis and Vascular Biology, et al. 2018. Interplay between hypercholesterolaemia and inflammation in atherosclerosis: Translating experimental targets into clinical practice. European Journal of Preventive Cardiology 25 (9):948–55., . doi: 10.1177/2047487318773384.
- Ushijima, M., M. Takashima, K. Kunimura, Y. Kodera, N. Morihara, and K. Tamura. 2018. Effects of S-1-propenylcysteine, a sulfur compound in aged garlic extract, on blood pressure and peripheral circulation in spontaneously hypertensive rats. The Journal of Pharmacy and Pharmacology 70 (4):559–65. doi: 10.1111/jphp.12865.
- Van Moerkercke, A., O. Duncan, M. Zander, J. Šimura, M. Broda, R. Vanden Bossche, M. G. Lewsey, S. Lama, K. B. Singh, K. Ljung, et al. 2019. A MYC2/MYC3/MYC4-dependent transcription factor network regulates water spray-responsive gene expression and jasmonate levels. Proceedings of the National Academy of Sciences of the United States of America 116 (46):23345–56. doi: 10.1073/pnas.1911758116.
- Vermeulen, M., R. van den Berg, A. P. Freidig, P. J. van Bladeren, and W. H. Vaes. 2006. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. Journal of Agricultural and Food Chemistry 54 (15):5350–8. doi: 10.1021/jf060723n.
- Vojtovič, D., L. Luhová, and M. Petřivalský. 2021. Something smells bad to plant pathogens: Production of hydrogen sulfide in plants and its role in plant defence responses. Journal of Advanced Research 27:199–209. doi: 10.1016/j.jare.2020.09.005.
- Wang, C., and C. Wang. 2017. Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology 25 (1):99–106. doi: 10.1007/s10787-016-0307-y.
- Wang, L., W. Han, Y. Iwasaki, R. Yermek, G. W. G. Sharp, Y. Seino, and T. Yada. 2021. Onion component, isoalliin, stimulates feeding and activates the arcuate nucleus neuropeptide Y, ghrelin- and Ninjin’yoeito-responsive neurons. Neuropeptides 89:102180. doi: 10.1016/j.npep.2021.102180.
- Wang, S. L., D. S. Liu, E. S. Liang, Y. H. Gao, Y. Cui, Y. Z. Liu, and W. Gao. 2015. Protective effect of allicin on high glucose/hypoxia-induced aortic endothelial cells via reduction of oxidative stress. Experimental and Therapeutic Medicine 10 (4):1394–400. doi: 10.3892/etm.2015.2708.
- Wang, W., S. Wang, A. F. Howie, G. J. Beckett, R. Mithen, and Y. Bao. 2005. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. Journal of Agricultural and Food Chemistry 53 (5):1417–21. doi: 10.1021/jf048153j.
- Wang, Y., Z. Zhang, W. Sun, Y. Tan, Y. Liu, Y. Zheng, Q. Liu, L. Cai, and J. Sun. 2014. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxidative Medicine and Cellular Longevity 2014:123963. doi: 10.1155/2014/123963.
- Washida, K., M. Miyata, T. Koyama, K. Yazawa, and K. Nomoto. 2010. Suppressive effect of Yamato-mana (Brassica rapa L. Oleifera Group) constituent 3-butenyl glucosinolate (gluconapin) on postprandial hypertriglyceridemia in mice. Bioscience, Biotechnology, and Biochemistry 74 (6):1286–9. doi: 10.1271/bbb.100018.
- Williams, D. E. 2021. Indoles derived from glucobrassicin: Cancer chemoprevention by indole-3-carbinol and 3,3’-diindolylmethane. Frontiers in Nutrition 8:734334. doi: 10.3389/fnut.2021.734334.
- Wittstock, U., and M. Burow. 2007. Tipping the scales-specifier proteins in glucosinolate hydrolysis. IUBMB Life 59 (12):744–51. doi: 10.1080/15216540701736277.
- World Health Organisation. 2007 (2002). Protein and amino acid requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation. WHO Technical Report Series, no. 935. Geneva, Switzerland. https://apps.who.int/iris/handle/10665/43411.
- World Health Organisation. 2018. Global health estimates 2016: Disease burden by cause, age, sex, by country and by region, 2000–16. Geneva: World Health Institution. https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html.
- Wu, D., Q. Hu, and D. Zhu. 2018. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxidative Medicine and Cellular Longevity 2018:4579140. doi: 10.1155/2018/4579140.
- Wu, G., Y. Z. Fang, S. Yang, J. R. Lupton, and N. D. Turner. 2004. Glutathione metabolism and its implications for health. The Journal of Nutrition 134 (3):489–92. doi: 10.1093/jn/134.3.489.
- Yagishita, Y., J. W. Fahey, A. T. Dinkova-Kostova, and T. W. Kensler. 2019. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 24 (19):3593. doi: 10.3390/molecules24193593.
- Yamazaki, Y., K. Iwasaki, M. Mikami, and A. Yagihashi. 2010. Distribution of eleven flavor precursors, S-alk(en)yl-L-cysteine derivatives, in seven Allium vegetables. Food Science and Technology Research 17 (1):55–62. doi: 10.3136/fstr.17.55.
- Yoshimoto, N., and K. Saito. 2019. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. Journal of Experimental Botany 70 (16):4123–37. doi: 10.1093/jxb/erz243.
- Zechmann, B. 2020. Subcellular roles of glutathione in mediating plant defense during biotic stress. Plants 9 (9):1067. doi: 10.3390/plants9091067.
- Zhai, B., C. Zhang, Y. Sheng, C. Zhao, X. He, W. Xu, K. Huang, and Y. Luo. 2018. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Scientific Reports 8 (1):3527. doi: 10.1038/s41598-018-21421-x.
- Zhang, H., Y. Park, J. Wu, X. p. Chen, S. Lee, J. Yang, K. Dellsperger, and C. Zhang. 2009. Role of TNF-alpha in vascular dysfunction. Clinical Science (London, England: 1979) 116 (3):219–30. doi: 10.1042/CS20080196.
- Zhang, M., H. Pan, Y. Xu, X. Wang, Z. Qiu, and L. Jiang. 2017. Allicin decreases lipopolysaccharide-induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2. Cellular Physiology and Biochemistry 41 (6):2255–67. doi: 10.1159/000475640.
- Zhang, Y., P. Talalay, C. G. Cho, and G. H. Posner. 1992. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proceedings of the National Academy of Sciences of the United States of America 89 (6):2399–403. doi: 10.1073/pnas.89.6.2399.
- Zhao, F., J. Zhang, and N. Chang. 2018. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. European Journal of Pharmacology 824:1–10. doi: 10.1016/j.ejphar.2018.01.046.
- Züst, T., B. Joseph, K. K. Shimizu, D. J. Kliebenstein, and L. A. Turnbull. 2011. Using knockout mutants to reveal the growth costs of defensive traits. Proceedings of the Royal Society of. Proceedings. Biological Sciences 278 (1718):2598–603. doi: 10.1098/rspb.2010.2475.
