Abstract
It is well-known that the postprandial muscle protein synthetic response to protein ingestion is regulated on various levels, including dietary protein digestion and amino acid (AA) absorption, splanchnic AA retention, the availability of dietary protein–derived AA in the circulation, delivery of AA to the muscle, uptake of AA by the muscle, and intramuscular signaling. AA availability after consumption of dairy products is primarily determined by the rate of gastric emptying of milk proteins, which is mainly linked to coagulation of milk proteins in the stomach. Caseins form gastric coagula, which make their gastric emptying and subsequent postprandial aminoacidemia notably slower than that of whey proteins. Only recently, the role of processing, food structure, preservation and matrix on coagulation herein has been getting attention. In this review we describe various processes, that affect gastric coagulation of caseins and therewith control gastric emptying, such as the conversion to caseinate, heat treatment in the presence of whey proteins, conversion to stirred yoghurt and enzymatic hydrolysis. Modulating product characteristics by processing can be very useful to steer the gastric behavior of protein, and the subsequent digestion and AA absorption and muscle anabolic response to maintain or increase muscle mass.
Introduction
Milk proteins are the first source of protein consumed by humans as neonates, either in the form of human milk or infant formula. Particularly since the domestication of mammals such as cows, buffaloes, goats and sheep, which started approximately 10,000 years ago, milk proteins have also become an important source of protein in the human diet past infancy. Together with proteins from other animal- and plant-based foods in the diet, the milk proteins provide the body with the required amounts of nitrogen and essential amino acids required for growth, development, maintenance, recovery and several other biological processes and bodily functions.
Tissue growth is dependent on the balance between protein synthesis and breakdown. If synthesis is greater than breakdown, tissue will grow. Postprandial protein handling, especially protein digestion and amino acid absorption and the subsequent plasma amino acid availability, plays an important role in tissue protein synthesis and thus tissue maintenance and growth. Dietary proteins have different capacities to stimulate this tissue protein synthesis. It is well known that the milk proteins casein and whey protein contain all essential amino acids required to effectively stimulate (muscle) protein synthesis (Rennie et al. Citation1982; Hamarsland, Johansen, et al. Citation2019; Horstman et al. Citation2021; Lacroix et al. Citation2006; Gorissen et al. Citation2020; Hamarsland et al. Citation2017; Elliot et al. Citation2006; Phillips, Tang, and Moore et al. Citation2009; Wilkinson et al. Citation2007). These amino acids are not only precursors to support de novo tissue protein synthesis, but are also signal molecules, e.g., leucine, directly triggering protein synthesis (Boirie, Gachon, et al. Citation1997; Boirie et al. Citation1996; Kimball and Jefferson Citation2006; Atherton et al. Citation2010).
We consume milk proteins in different forms, both in whole food as well as in the form of milk protein ingredients, such as e.g., concentrates or isolates of milk protein, micellar casein, whey protein or caseinates. In addition, hydrolyzed proteins, rather than intact proteins, can be used to improve e.g., digestion, and are used in products such as infant formula (Clemente Citation2000; Dupont et al. Citation2010). In these products, protein structures can differ notably because of processing steps applied in the production of milk protein ingredients, such as heat treatment, changes in pH or the addition or removal of salts. As a result of these processing steps, various aspects of milk protein digestion, e.g., protein hydrolysis, but also gastric coagulation of milk proteins can be affected (Trommelen, Tomé, and van Loon Citation2021), leading to changes in protein digestion and amino acid absorption kinetics. Likewise, transforming milk into widely consumed products such as yoghurt or cheese also affects protein structures as well as digestion behavior and amino acid appearance in blood and subsequent tissue protein synthesis rates.
After the discovery of the milk proteins in the 19th century and a notable amount of research on the relation between dairy processing and milk protein digestion in the first half of the 20th century, this topic has remained relatively quiet until the start of the 21st century. In the past two decades, the relation between the physical state of milk proteins and aminoacidemia has been widely studied in both humans and animals and it has been established that milk composition (e.g., fat content), the processing of milk (e.g., heat treatment or homogenization) and the conversion of milk into different dairy products (e.g., cheese and yoghurt) and ingredients (e.g., micellar casein isolate and whey protein concentrate) have a strong effect on aminoacidemia (Horstman et al. Citation2021). Such effects can be related to structural differences in different types of milk proteins, and changes therein during processing. Even though for over a century, milk and dairy products have been developed and optimized to meet specific consumer needs, it is not always clear what its effect on human physiology is. In this paper, we will review the link between milk protein structure, the effect of processing on this structure and postprandial plasma amino acid availability. Understanding differences in milk protein structures, stability and interactions as a result of processing and/or conversion into products and ingredients can be applied to explain and understand how these changes affect digestion behavior and appearance of amino acids in blood and the subsequent tissue protein synthesis. We will start with a description of milk protein structure, interaction, and behavior in the stomach and the appearance of its amino acids in the circulation. The processing steps standardization, heat treatment and homogenization of liquid (raw) milk before human consumption will be covered. Subsequently, we will describe the conversion into yoghurt, cheese and dairy ingredients and its effect on aminoacidemia. Finally, we will explore the relation between aminoacidemia from different milk protein sources and muscle protein synthesis. Understanding the effect of processing and behavior of different dairy products in the stomach will enable us to modulate the rate of amino acid appearance in the blood and thereby the subsequent postprandial muscle protein synthesis. This can open new possibilities in developing more anabolic (dairy) food products in the areas of, e.g., aging, sports and medical nutrition.
Protein digestion and postprandial aminoacidemia
Protein needs to be digested into free amino acids and di- and tripeptides in order to be absorbed into the bloodstream and be utilized for tissue protein synthesis. The digestion process starts in the mouth, where mastication takes place, and the product is mixed with saliva. Although there are no proteolytic enzymes present in saliva and protein hydrolysis does thus not take place during oral digestion, the oral digestion can have an important role on overall protein digestion in the digestive tract. Both the mastication and the breakdown of starch, which can be present in some types of yoghurt, processed cheese and dairy drinks, can increase accessibility of protein to digestive enzymes in subsequent digestion steps.
Following oral digestion, the product reaches the gastric phase of digestion, where is it mixed with gastric juice. In terms of protein digestion, two aspects of the gastric juice are crucial, i.e., the low pH (1-2 for adults, 3-5 for infants) and the presence of the protease pepsin (Guo et al. Citation2020; Somaratne et al. Citation2020). Pepsin is an endopeptidase that can digest proteins into peptides; however, pepsin cannot hydrolyze protein into small enough hydrolysis products for uptake into the blood. The action of pepsin can also lead to the coagulation of the casein fraction of milk (Guo et al. Citation2020). This can, as outlined in subsequent sections, lead to gastric coagulation of milk. In addition, the low pH of the gastric fluid can also impact protein stability, when proteins get close to their iso-electric point. It is, however, important to keep in mind that while the pH of the gastric fluid is low, particularly for adults, its buffering capacity is also low. Hence, when gastric fluid is mixed with products, rapid increases in pH are observed, particularly when products contain buffering compounds. This depends on the concentration of buffering compounds in the product and the product volume ingested. For milk, for instance, the pH of the stomach contents can increase to >6 when a glass of milk is consumed on a fasted stomach, and it can take hours for the pH to decrease to that of the original value (Gao et al. Citation2002). Hence, in addition to the dynamics of protein digestion, pH in the stomach is variable which also affects protein stability and digestion in the stomach.
Following the gastric digestion, the product continues to the intestinal phase for further hydrolysis and absorption. The rate at which the product enters the intestine is determined by the rate of gastric emptying. Careful control of gastric emptying is important and there are various routes by which the rate of gastric emptying is controlled (Liu et al. Citation2021). First and foremost, there is a restriction in volume flow from the stomach to the intestine. Next to this, there are also hormonal feedback loops and feedback loops based on energy content and/or concentrations of specific nutrients of material emptying into the intestine, limiting maximum gastric emptying timescale. Furthermore, there is also a limitation on particle size. Only particles smaller than ∼2 mm will be emptied from the stomach (Liu et al. Citation2021). Hence, for solid foods, breakdown of particles via the combined action of pepsin, acid and gastric motility is important and a rate determining step for gastric digesta entering the intestine (Guo et al. Citation2020; Liu et al. Citation2021). Once emptied into the intestine, the proteins and peptides are further broken down by the collective action of the intestinal proteases and peptidases, including trypsin, chymotrypsin and brush border peptidases. Together, these can break down proteins and peptides to free amino acids and di- and tripeptides, which can be absorbed into the bloodstream. Milk proteins are typically found to be highly digestible (Fanelli et al. Citation2021; Mathai, Liu, and Stein Citation2017; Rutherfurd et al. Citation2015), whereas some other protein sources show lower digestibility and part of the proteinaceous material is insufficiently digestible to be absorbed in the small intestine (Bailey and Stein Citation2020; Adhikari et al. Citation2022). In this case, the material proceeds to the large intestine and colon. Although it may be further digested there and/or metabolized by gut micro-organisms, this fraction is not believed to make a large contribution to utilizable protein and amino acids in the circulation for e.g., whole-body protein synthesis.
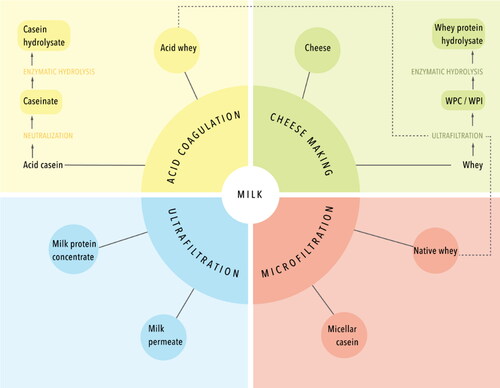
Postprandial aminoacidemia has been determined for many different dairy products and milk protein ingredients, as well as for countless other protein sources. In such studies, a basal blood sample as well as several postprandial blood samples are taken. Amino acid concentrations are measured in the blood samples and from this, amino acid kinetics can be plotted, an example of which is shown in . This can be done for e.g. total amino acids, essential amino acids or individual amino acids of interest. In , several important parameters can be derived, i.e., the maximum concentration for the plotted amino acid (Cmax), the time point at which this occurred (Tmax) and the (total, positive incremental or net incremental) area under the curve (AUC). Tmax, in this respect, provides information about the rate of appearance of the amino acid(s) of interest in the bloodstream whereas Cmax and AUC provide information about the amount of the amino acid(s) in the bloodstream. These values, however, are not the same as the amount of dietary protein-derived amino acids that entered the bloodstream, because other tissues also release (breakdown) amino acids in the blood stream and (rapidly) take-up (synthesis and oxidation) amino acids from the blood stream. To distinguish between the exogenous (dietary protein-derived) and endogenous rate of amino acid release into the circulation, ingestion of intrinsically labeled protein is combined with continuous intravenous stable-isotope-labeled amino acid infusion. This allows the simultaneous assessment of protein digestion and amino acid absorption kinetics (e.g., release of dietary protein-derived amino acids into the circulation), whole-body protein metabolism (whole-body protein synthesis, breakdown and oxidation rates and net protein balance) and specific tissue metabolism (protein fractional synthesis rates and dietary protein-derived amino acid incorporation into tissue protein) (Groen et al. Citation2015). A downside, however, is the effort and cost required to obtain intrinsically labeled proteins and the limitations on their processing into further products. In subsequent sections, postprandial changes in blood amino acid levels are discussed for milk and dairy products (Section Postprandial aminoacidemia in relation to dairy products) and milk protein ingredients (Section Postprandial aminoacidemia in relation to milk protein ingredients).
Figure 1. Example of blood amino acid kinetics. iAUC: incremental area under the curve (mmol·L−1 5 h), i.e., area under the curve minus baseline value, usually calculated by using the trapezoid method; Cmax, maximal concentration values of amino acids in blood (mmol·L−1); Tmax, time point corresponding to Cmax (min).
Postprandial aminoacidemia in relation to dairy products
Milk
Several studies have reported postprandial aminoacidemia after consumption of milk, either at a total amino acid (TAA), essential amino acid (EAA) or individual amino acid level (Gorissen et al. Citation2020; Hamarsland, Aas, et al. Citation2019; Hamarsland, Johansen, et al. Citation2019; Hamarsland et al. Citation2017; Lacroix et al. Citation2006; Horstman et al. Citation2021).
Invariably, studies on the aminoacidemia after consumption of milk show a rapid increase in blood amino acid levels, followed by a gradual but slow decline (Gorissen et al. Citation2020; Hamarsland, Aas, et al. Citation2019; Hamarsland, Johansen, et al. Citation2019; Hamarsland et al. Citation2017; Lacroix et al. Citation2006; Horstman et al. Citation2021). The reasoning behind this particular pattern has been investigated by studying the major milk protein fractions, i.e., the caseins (∼80% of total milk protein) and the whey proteins (∼20% of total milk protein) separately. From these studies, it has been concluded that the whey proteins are largely responsible for the rapid peak in postprandial blood amino acid levels following consumption of milk, whereas the caseins are responsible for the subsequent sustained release of amino acids (Boirie, Dangin, et al. Citation1997; Mahe et al. Citation1996). This has led to the classification of whey proteins as fast proteins and caseins as slow proteins (Boirie, Dangin, et al. Citation1997). In this review, however, we will show that such classification requires nuancing, depending on both the product matrix and any processing the proteins have undergone prior to ingestion.
These differences between caseins and whey proteins in terms of blood amino acid kinetics have been related to their difference in behavior during the gastric phase of the digestion process (Boirie, Dangin, et al. Citation1997; Horstman et al. Citation2021; Li, Ye, and Singh Citation2021). Native whey proteins are, in general, hardly affected by the gastric phase of digestion. In adults, the major whey protein, β-lactoglobulin, is not susceptible to pepsin-induced hydrolysis if the protein is in the native state, but only after denaturation (Peram et al. Citation2013), whereas the other major whey protein α-lactalbumin, can undergo some pepsin-induced hydrolysis during prolonged gastric digestion (Miranda et al. Citation1989). In addition, whey proteins are not susceptible to gastric coagulation (Wang et al. Citation2018), as a result of which, they are released relatively quickly from the stomach.
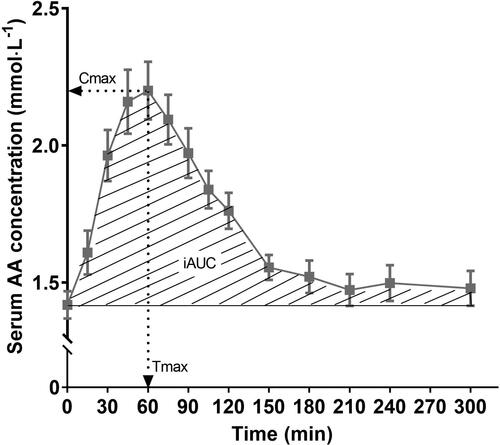
In contrast, the casein fraction in milk, which occurs mainly in the form of so-called casein micelles, is highly susceptible to gastric coagulation (Huppertz and Chia Citation2021; Mulet-Cabero, Mackie, et al. Citation2020; Ye et al. Citation2019). These casein micelles are spherical particles consisting of tens of thousands of casein molecules, but also contain ∼6% calcium phosphate on a dry matter basis (Dalgleish and Corredig Citation2012; Huppertz et al. Citation2017). This allows the casein micelles to act as a carrier of calcium and phosphate, and it allows milk to pass levels of calcium and phosphate to the neonate much above the solubility limit, in a stable and bioavailable form (Holt et al. Citation2013). The specific structure of the casein micelle, which is studied and reviewed in detail elsewhere (Huppertz et al. Citation2017; Dalgleish and Corredig Citation2012; Horne Citation2006; De Kruif and Holt Citation2003; McMahon and Oommen Citation2013), allows controlled destabilization by both the action of enzymes and lowering of the pH (De Kruif Citation1999; Holt and Horne Citation1996). This can result in coagulation of casein micelles, as is exploited in the manufacturing of cheese and yoghurt, respectively (Walstra, Wouters, and Geurts Citation2007). In the stomach, pepsin and the acidity of the gastric secretions can both result in coagulation of casein micelles, but it has been established that the enzymatic destabilization of the casein micelles is the primary cause of gastric coagulation (Ye et al. Citation2016; Huppertz and Chia Citation2021). During the early stages of gastric coagulation, pepsin hydrolyzes one of the caseins, κ-casein, on the surface of the casein micelle (Ye et al. Citation2016), in a way very similar to the effect of rennet during cheese-making (De Kruif Citation1999), whereby the casein macropeptide (CMP) is released. As a result, the micelles lose stability and become prone to aggregation as is shown in . Casein-based coagula that form during the gastric phase of digestion of milk can readily exceed 10 mm (Ye et al. Citation2016; Huppertz and Lambers Citation2020; Brenneman Citation1913; Mulet-Cabero et al. Citation2019), and are thus far too large for gastric emptying, which typically cuts off at ∼2 mm. Therefore, further breakdown of these coagula is required before they can be released from the stomach. This occurs partly due to further hydrolysis of caseins by peptides, and also breakdown due to gastric motility plays an important role (Mulet-Cabero, Mackie, et al. Citation2020). Once the coagulum particles are sufficiently small, they can be emptied into the intestine. Here they are hydrolyzed further by the intestinal proteases and peptidases into free amino acids and small peptides which can be taken up into the blood stream. From ileal digestibility measurements in humans, pigs and rats, it is clear that milk protein is (almost) completely digested in the intestine (Mathai, Liu, and Stein Citation2017). Hence, it is apparent that for aminoacidemia following consumption of milk, the gastric transit is the rate limiting step and thus the main determinant of the postprandial amino acid kinetics.
Figure 2. Pepsin induced coagulation of casein micelles in unheated (A1-A3) or heated (B1-B4) milk. A1 = unheated milk, A2 = unheated milk incubated with pepsin, A3 = aggregation of pepsin-destabilized casein micelles. B1 = unheated milk, B2 = heated milk, B3 = heated milk incubated with pepsin, B4 = lack of aggregation of pepsin-destabilized casein micelles in heated milk. CMP: casein macropeptide.
Prior to consumption, raw milk undergoes several processing steps which can affect various constituents of milk and therewith gastric coagulation and aminoacidemia. For liquid milk processing, standardization, heat treatment and homogenization are the main factors and will be covered in this section. Conversion into yoghurt, cheese and dairy ingredients and their effect on aminoacidemia are covered in subsequent sections.
Standardization of milk
The composition of bovine milk varies because of various factors, e.g., the breed of cow, animal genetics, feed, farming practices, climate and stage of lactation (Walstra, Wouters, and Geurts Citation2007). To ensure consistency in composition of milk reaching the consumer, liquid milk products are often standardized on fat content. In most countries, full-fat (e.g., 3.5-4.0% fat), semi-skimmed (e.g., 1.5-2.0% fat) and skimmed (e.g., <0.1% fat) milks are produced. Studies have shown that fat content influences the gastric coagulation of milk, with harder curds that are slower to digest typically observed in milk with little or no fat (Bergeim et al. Citation1919; Mulet-Cabero, Torcello-Gómez, et al. Citation2020; Mulet-Cabero et al. Citation2019). This can be related to the fact that fat globules can act as ‘structure breakers’ in the network of destabilized casein micelles that forms (Mulet-Cabero, Torcello-Gómez, et al. Citation2020; Mulet-Cabero et al. Citation2019). In the absence of fat, the casein micelles aggregate more strongly, leading to more compact gastric coagula. Hence, based solely on these weaker structures, more rapid gastric emptying from whole milk could be expected. Such effects, however, are not reflected in kinetics of aminoacidemia, where, in fact, slightly more prolonged release of amino acids is observed for whole milk compared to skimmed milk (Bergeim et al. Citation1919; Horstman et al. Citation2021; Mulet-Cabero, Torcello-Gómez, et al. Citation2020). This discrepancy may be related to another influence of including of fat in gastric coagula, i.e., that it reduces density of the coagula to such an extent that creaming can occur (Mackie et al. Citation2013; Mulet-Cabero et al. Citation2019; Ye Citation2021), as a result of which protein release into the intestine is delayed. Furthermore, the increased fat content and caloric value can also delay gastric emptying in vivo. Hence, for the influence of fat content of milk on postprandial aminoacidemia, three key aspects should be considered, i.e., (1) its effect on structure formation during gastric coagulation and the subsequent breakdown of the coagulum, (2) its effect on the density of the gastric coagulum, and its concomitant propensity to creaming in the stomach, and (3) delayed gastric emptying as a result of increased fat content and caloric value. The balance between these factors, along with other processing effects described in subsequent sections, will determine the overall effect of fat content on postprandial aminoacidemia.
Homogenization of milk
Next to standardization, milk homogenization is also commonly applied. In this process, the milk is passed through one or more homogenizing valves under high pressure with the aim of reducing the size of the milk fat globules to prevent the formation of a cream layer during storage of the milk (Huppertz and Kelly Citation2006). By reducing the size of the milk fat globules, the number of fat globules is increased (a 2-fold reduction in size will lead to an 8-fold increase in number, a 5-fold reduction in size will lead to a 125-fold increase in number) and a strong increase in surface area (2-fold and 5-fold decrease in particle size leads to a 2-fold and 5-fold increase in total surface area, respectively) most of which will be covered with caseins (Walstra, Wouters, and Geurts Citation2007). The relation between homogenization of milk and gastric curd formation has been studied frequently and has highlighted softer gastric curds forming from homogenized milk than from unhomogenized milk (Mulet-Cabero et al. Citation2019; Ye et al. Citation2017; Anthony Citation1936). This is in line with observations on cheese-making, where curds from homogenized milk are also typically found to be weaker and more brittle compared to curds from unhomogenized milk (Kelly, Huppertz, and Sheehan Citation2008; Lodaite et al. Citation2009). These effects have been related to the larger number of fat globules in homogenized milk, which disrupt the network of aggregating casein particles at much smaller length scales, leading to weaker curds (Kelly, Huppertz, and Sheehan Citation2008; Lodaite et al. Citation2009). When translating this to aminoacidemia, it would be expected that homogenized milk could give a quicker release of amino acids into the blood, but, to our knowledge, no in vivo data have been published on this topic to date.
Heat treatment
Heat treatment is applied to virtually every milk product consumed. The treatment is applied to inactivate pathogenic and spoilage bacteria as well as proteolytic and lipolytic enzymes whose activities can limit the physical and sensorial shelf-life of milk (Lindsay et al. Citation2021). Moreover, heat treatment of milk can also affect the protein fraction of milk, most notably the whey proteins (Zhang et al. Citation2021; Anema Citation2021), which affects the gastric coagulation of milk and the subsequent aminoacidemia. It has been shown that heat treatment can lead to much softer gastric curds. This effect becomes more notable with increasing heat treatment. Pasteurization of milk (e.g., 72 °C for 15 s) has little effect on gastric curd properties, but more extensive heat treatments, such as UHT treatment (e.g., 5 s at 140 °C) or in-container sterilization (e.g., 10 min at 121 °C), result in notably weaker curds (Mulet-Cabero et al. Citation2019; Ye et al. Citation2016; Ye et al. Citation2019). These effects have been related to the denaturation of the whey proteins that occur as a result of heat treatment. The denatured whey proteins subsequently associate with the casein micelles (Anema Citation2021), which makes the micelles less susceptible to coagulation by pepsin (Ye et al. Citation2016; Ye et al. Citation2019), as is schematically outlined in . Similar effects are observed when milk for cheese-making is heated too extensively. Also, then, weaker or completely impaired coagulation is observed (Britten and Giroux Citation2022). As a result of the weaker or no gastric coagulation, faster gastric emptying would be expected from heated milk, leading to faster increases in blood amino acid levels. This has, indeed, been confirmed by in vivo studies comparing pasteurized and UHT milk, where UHT milk showed a sharper initial peak and pasteurized milk a more sustained release of amino acids into the bloodstream (Horstman et al. Citation2021; Lacroix et al. Citation2008; Miranda and Pelissier Citation1987; Kaufmann Citation1984).
Yoghurt
Postprandial aminoacidemia after yoghurt consumption was reported to be faster and greater compared to milk (Horstman et al. Citation2021). It would be logical to attribute this to faster gastric emptying of yoghurt compared to milk, but for this, delays rather than acceleration for yoghurt compared to milk have been reported (Gaudichon et al. Citation1995; Gaudichon et al. Citation1994). In addition, Barbé determined the kinetics of milk protein digestion and amino acid absorption in 6 mini pigs after ingestion of four dairy matrices unheated or heated skim milk and corresponding rennet gels (Barbé et al. Citation2013). This study showed that the heat treatment of a milk product increases its mean retention time in the stomach, and that the gelation process allows delaying its gastric outflow. Finally, the gelled matrix from the heated milk product was the most stomach-retained matrix. This potential discrepancy between Horstman et al. and the three other studies, however, may be understood when considering (1) the gastric behavior of yoghurt, and (2) the different types of yoghurt used in different studies.
Unlike milk, yoghurt is not prone to gastric coagulation, i.e., (further) aggregation of yoghurt does not occur in the stomach (Buchheim Citation1984; Pfeil Citation1984). This is because coagulation of proteins has already occurred during yoghurt manufacture, as a result of the decrease in pH to values in the range 4.0-4.5. In the absence of further structure formation in the stomach, yoghurt products are primarily prone to breakdown under gastric conditions due to the action of pepsin, which hydrolyzes the proteins, and gastric motility, which breaks down macroscopic structures. Once the particles become small enough, they do become prone to gastric emptying into the intestine. Therefore, the starting structures present in yoghurt are very important, particularly the size, the firmness and the porosity of the particles, which affects the accessibility of the proteins for pepsin. These factors are strongly determined by the conditions applied during the manufacture of yoghurt.
Structure formation in yoghurt is considered to arise from acid-induced aggregation of milk proteins. In most typical yoghurt processes, the milk is first pre-heated at 85-95 °C for several minutes (Jørgensen et al. Citation2019; Lucey and Singh Citation1997; Lucey, Wilbanks, and Horne Citation2022). Besides inactivation of micro-organisms and enzymes, this also results in the denaturation of whey proteins, part of which become associated with casein micelles. Furthermore, milk for yoghurt manufacture is typically also homogenized (Lucey and Singh Citation1997; Wiking et al. Citation2021). Following pretreatments, the milk is inoculated with lactic acid bacteria, which convert lactose into lactic acid, as a result of which pH decreases. When pH decreases <5.5, coagulation of the proteins and protein-covered fat globules can occur. As a result, a gel is formed, which forms the basis for yoghurt structure (Lucey and Singh Citation1997). In so-called set yoghurt, the gel structure is not further disrupted prior to consumption (Walstra, Wouters, and Geurts Citation2007; Tamime and Robinson Citation2007). Breakdown to particles that are sufficiently small to be subject to gastric emptying occurs during oral processing and further breakdown in the stomach. In this case, it may be that there is still a gelled matrix, with a higher viscosity than stirred yoghurt, in the stomach. This may explain the increased retention time in the stomach of yoghurt compared to milk in the studies mentioned above (Gaudichon et al. Citation1995; Gaudichon et al. Citation1994; Barbé et al. Citation2013), since a higher viscosity of yogurt compared with milk is known to be a determining factor in gastric emptying (Houghton, Hickson, and Read Citation1987; Siegel et al. Citation1988; Mahé et al. Citation1994). On the other hand, in the manufacture of so-called stirred yoghurt, the yoghurt gel is subjected to extensive shear-induced disruption post fermentation and prior to packaging (Walstra, Wouters, and Geurts Citation2007; Tamime and Robinson Citation2007). Particles in stirred yoghurt are typically <100 µm, and hence small enough for gastric emptying already (Krzeminski, Großhable, and Hinrichs Citation2011; Torres, Amigo Rubio, and Ipsen Citation2012). This may form the basis of findings by Horstman et al. (Horstman et al. Citation2021), who reported more rapid postprandial amino acidemia from stirred yoghurt than from milk. For the studies on gastric emptying of yoghurt, yoghurt type was unfortunately not specified, (Gaudichon et al. Citation1995; Gaudichon et al. Citation1994; Barbé et al. Citation2013). Like for milk, fat content is likely to also affect blood amino acid kinetics for yoghurt, with higher fat content leading to a lower density and hence a higher propensity to creaming rather than sedimentation, and thus delayed gastric emptying. In contrast to gastric coagulation, however, firmness of yoghurt structures increases with increasing fat content (Jørgensen et al. Citation2019; Sodini et al. Citation2004); furthermore, porosity of the product matrix also decreases with increasing fat content (Lucey, Munro, and Singh Citation2006). As such, with increasing fat content, delayed gastric emptying is expected due to creaming and the action of cholecystokinin in response to fatty acids (Guimbaud et al. Citation1997) and amino acids, and delayed breakdown of the product particles in the case of set yoghurt. For stirred yoghurt, particles are already small enough for gastric emptying and creaming effects will dominate the effect of fat content on postprandial aminoacidemia.
Cheese
Like yoghurt, cheese is another gelled and fermented dairy product. However, where yoghurt has the potential, depending on the type of yoghurt produced, to accelerate postprandial aminoacidemia, studies performed on cheese have shown the opposite effect. For both Gouda (Horstman et al. Citation2021) and Cheddar (De Hart et al. Citation2021) cheese, much slower and more prolonged release of amino acids into the bloodstream has been reported compared to milk and other dairy products, and similar to micellar casein isolate (Horstman et al. Citation2021). This slower release is the result of the structure of cheese. While, like yoghurt, cheese is also a gelled dairy matrix, it is much more concentrated, with moisture contents typically around 40-50%, compared to 80-90% in yoghurt (Walstra, Wouters, and Geurts Citation2007). The cheese matrix, as such, is much firmer than the yoghurt matrix (Fang et al. Citation2016b, Citation2016a; van Vilet et al. Citation1989) and as a result more difficult to break down, both during oral processing and in the stomach, where breakdown occurs due to gastric motility and pepsin. As a result, longer times are required to create cheese particles small enough to become susceptible to gastric emptying. In vitro studies have highlighted some differences in susceptibility of different cheese matrices to digestion, with moisture content, fat content and calcium content being the main determinants (Fang et al. Citation2016b, Citation2016a; Ayala-Bribiesca et al. Citation2016). Furthermore, the density of a full-fat cheese (e.g., 45-50% fat on dry matter) is <1 (Iezzi, Locci, and Mucchetti Citation2013), making cheese particles susceptible to creaming and thus delayed gastric emptying. Cheese also stimulatedprotein anabolic processes in skeletal muscle (as measured by mTORC1 intracellular signaling, which is highly responsive to acute protein intake particularly to sources that are rich in leucine (Anthony, Anthony, Anthony, et al. Citation2000; Anthony, Yoshizawa, et al. Citation2000) and a muscle metaboli programming associated herewith), though effects were found to be more pronounced for milk (De Hart et al. Citation2021). Very recently is was shown that the postprandial muscle protein synthetic response to the ingestion of cheese or milk protein does not differ when 30 g of protein are ingested at rest or during recovery from exercise in healthy, young males (Hermans et al. Citation2022).
Postprandial aminoacidemia in relation to milk protein ingredients
In addition to the various dairy products described in Section Postprandial aminoacidemia in relation to dairy products, many studies on postprandial aminoacidemia have also been carried out on milk protein ingredients, either isotopically labeled or unlabeled. Milk protein ingredients may be separated in various classes, e.g., based on protein composition, protein content or on whether the proteins are intact or hydrolyzed (Huppertz and Gazi Citation2017). Ingredients containing <90% protein on a dry matter basis are typically referred to as concentrates, whereas those containing >90% protein on a dry matter basis are referred to as isolates (Huppertz and Gazi Citation2017). In addition, a distinction should be made between ingredients containing intact proteins, and those in which the proteins have been partially hydrolyzed, by the action of enzymes or other means; the latter are referred to as protein hydrolysates and distinction can be made based on the degree of hydrolysis, i.e., mildly hydrolyzed or extensively hydrolyzed products (Huppertz and Gazi Citation2017).
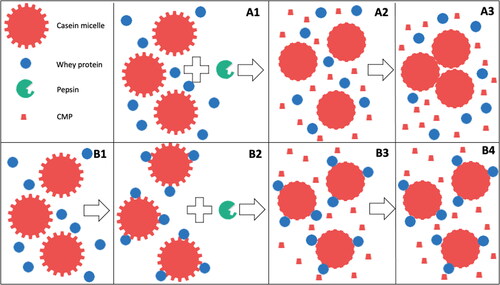
Based on protein composition, distinction can be made between milk protein ingredients, which contain casein and whey protein at approximately the same ratio as found in milk, casein ingredients, which are enriched in caseins on a protein basis, and whey protein ingredients, which are enriched in whey proteins (Huppertz and Gazi Citation2017). An overview of some of the major milk protein ingredients is shown in . Among the milk protein ingredients, milk protein concentrate (MPC) and milk protein isolate (MPI) are the key ones. Both are made by membrane filtration of skimmed milk using ultrafiltration membranes, which retains the proteins but the smaller compounds, i.e., lactose and soluble salts, are permeated (Carr and Golding Citation2016; Huppertz and Gazi Citation2017). Micellar casein concentrates and isolates (MCC and MCI) are also prepared using membrane filtration, but in this case a microfiltration membrane is used, which has larger pore sizes than an ultrafiltration membrane. As a result, whey proteins also permeate through the membrane and a casein-enriched retentate is obtained (Huppertz and Gazi Citation2017; Carr and Golding Citation2016).
Next to this, caseinates can be prepared from skimmed milk. For their production, skimmed milk is acidified to pH ∼4.5 using a mineral acid, which leads to casein precipitation, whereas the whey proteins remain soluble. The casein precipitate is subsequently washed and neutralized with e.g., sodium or calcium hydroxide to produce sodium or calcium caseinate (Huppertz and Gazi Citation2017; Carr and Golding Citation2016; Mulvihill Citation1989). Whey protein ingredients, e.g., whey protein concentrate (WPC) and isolate (WPI), are typically prepared using membrane filtration with ultrafiltration membranes, to increase protein content and remove lactose and salts (Huppertz and Gazi Citation2017; Bansal and Bhandari Citation2016). Different whey sources may be used to produce WPC and WPI, but whey from cheesemaking is the dominant source; other sources include the acid whey from aforementioned caseinate production and the so-called native whey as the co-product of the production of MCC and MCI (Huppertz and Gazi Citation2017; Bansal and Bhandari Citation2016), as described above and shown in . For various protein ingredients, hydrolyzed versions are available, prepared by enzymatic hydrolysis (Huppertz and Gazi Citation2017).
A wide range of studies have been conducted comparing postprandial aminoacidemia for different dairy ingredients. An overview of these studies is presented in and a network plot of the different comparisons made in these studies is presented in . From , it is clear that the most commonly made comparison is that of MCI and WPC prepared from so-called native whey. These studies have highlighted consistently that a delayed uptake of amino acids into the blood with a lower Cmax is observed after ingestion of MCI compared to WPC intake (Boirie, Dangin, et al. Citation1997; Dangin et al. Citation2001; Lacroix et al. Citation2006; Gorissen et al. Citation2020; Pennings et al. Citation2011; Dideriksen et al. Citation2011; Traylor et al. Citation2019). As outlined in Section Milk, this is related to the fact that the casein micelles in MCI are susceptible to coagulation during the gastric phase of digestion, thereby delaying gastric emptying and thus delaying the appearance in blood. Similar effects are observed for the comparison of MCI to other whey protein products, i.e., WPI (Churchward-Venne et al. Citation2019) and WPC from cheese whey (Horstman et al. Citation2021). A comparison of WPC, from cheese whey or native whey, to milk or MPC also shows faster release of amino acids into the bloodstream after WPC intake (Churchward-Venne et al. Citation2019; Gorissen et al. Citation2020; Hamarsland, Aas, et al. Citation2019; Hamarsland, Johansen, et al. Citation2019; Hamarsland et al. Citation2017; Lacroix et al. Citation2006) (), which can again be related to the gastric coagulation of the casein micelles in milk and MPC leading to delayed gastric emptying. However, when WPI was compared to calcium caseinate, no differences were observed in amino acid appearance in blood (Smith et al. Citation2009). This can be explained by the fact that the caseins in calcium caseinate are not present in the form of casein micelles; in the manufacture of calcium caseinate, the acidification and neutralization process leads to destruction of casein micelle structure and the formation of much smaller particles (Moughal, Munro, and Singh Citation2000). Particles in caseinate, unlike casein micelles, are hardly susceptible to pepsin-induced coagulation (Wang et al. Citation2018). As a result, gastric curd formation like for casein micelles does not occur for caseinate.
Figure 4. Network plot of the ingredients included in the randomized clinical trials included in . Each node size is proportional to the number of direct comparisons involving each intervention. The lines between nodes represent direct comparisons by the trials; the line thickness is proportional to the number of studies where the direct comparison was performed. MCI = micellar casein isolate, MPC = milk protein concentrate, Na_Cas = sodium caseinate, Ca_Cas = calcium caseinate, Cas_H = casein hydrolysate, WPC_C = whey protein concentrate from cheese whey, WPC_N = whey protein concentrate from native whey, WPI = whey protein isolate, WP_H = whey protein hydrolysate.
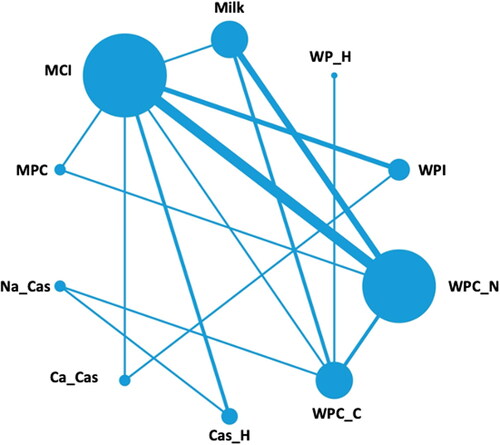
Table 1. The effect of protein source and hydrolyzation on amino acid availability and muscle protein synthetic response.
There are indications that casein may behave differently when it is ingested within a milk (Mahe et al. Citation1996; Soop et al. Citation2012) as compared to a single milk protein ingredient. Casein ingestion in a milk matrix (casein dissolved in bovine milk serum) delays protein digestion and amino acid absorption but does not modulate postprandial whole-body protein accretion, or muscle protein synthesis, when compared to micellar casein provided independently of the normal milk matrix (casein dissolved in water) in healthy older men (Churchward-Venne et al. Citation2015). This may be related to co-ingestion of lactose in the milk matrix, since co-ingestion of carbohydrates with milk proteins at rest (Glynn et al. Citation2013; Hamer et al. Citation2013; Gorissen et al. Citation2014) or post-exercise (Staples et al. Citation2011; Glynn et al. Citation2010; Koopman et al. Citation2007; Beelen et al. Citation2008) seems to slow down amino acid absorption. Such effects, most likely, are not due to effects of carbohydrates on protein digestion, but to the fact that the inclusion of carbohydrate increases caloric value and therewith slows down gastric emptying.
A comparison of WPC with microparticulated WPC, in which the proteins have been denatured and aggregated during ingredient manufacture, did not show differences in amino acid availability (Mitchell et al. Citation2017), most probably because the aggregated whey proteins are also not susceptible to coagulation in the stomach, and gastric emptying is thus not delayed. Because of the rapid gastric emptying of whey proteins, the pre-hydrolysis of whey protein, to produce a whey protein hydrolysate, did not further affect amino acid appearance in blood (Calbet and Holst Citation2004). This indicates that, as outlined earlier, gastric emptying rather than intestinal hydrolysis is the main rate-determining step in postprandial aminoacidemia. For micellar casein and sodium caseinate, which are both susceptible to gastric coagulation, a comparison with casein hydrolysate showed more rapid amino acid availability after hydrolysate intake (Calbet and Holst Citation2004; Koopman et al. Citation2009; Pennings et al. Citation2011), presumably because hydrolysates are not susceptible to gastric coagulation and hence not to delayed gastric emptying as a result thereof (Calbet and Holst Citation2004; Koopman et al. Citation2009; Pennings et al. Citation2011). Hydrolyzed dietary casein was associated with a faster rate of absorption than intact casein, resulting in earlier and stronger hyperaminoacidemia (Deglaire et al. Citation2009).
Overall, the results from studies on postprandial amino acid kinetics following ingestion of different milk protein ingredients show notable differences. However, the prior classification of whey protein as a fast protein source and casein as a slow protein source (Boirie, Dangin, et al. Citation1997; Mahe et al. Citation1996) requires nuance. While this is indeed the case for the comparison of whey protein vs. micellar casein, it does not hold when casein is not in micellar form, but e.g., in the form of caseinate. Likewise, if caseins are hydrolyzed prior to ingestion, the delayed gastric emptying, which appears to be unique to micellar casein, is also prevented. The classification of whey protein as a fast protein source, however, appears to hold regardless of the form of whey protein ingredient.
Relation between postprandial aminoacidemia and muscle protein synthesis
The above described product-process interactions influence coagulation behavior in the stomach and subsequent protein digestion, amino acid absorption and splanchnic sequestration after dietary protein intake, which has recently been reviewed (Van Lieshout et al. Citation2020). Gorissen showed that ≥ 50% of dietary protein is effectively digested and absorbed and becomes available for peripheral tissues such as skeletal muscle, while the other 50% of dietary protein is either not (yet) digested and absorbed or retained and taken up by splanchnic tissues such as the gut and liver (Gorissen et al. Citation2020). The subsequent rise in blood amino acid availability leads to postprandial insulin release, tissue perfusion, amino acid uptake by tissues, and intracellular signaling and tissue protein synthesis. One of the tissues that has been intensively studied in relation to the effect of postprandial increase in blood amino acids, is skeletal muscle. Muscle tissue is in a constant state of turnover and muscle mass is maintained by a tightly controlled balance between muscle protein synthesis and breakdown (Burd et al. Citation2009).
Food intake and dietary protein in particular, directly stimulates muscle protein synthesis (MPS). This muscle protein synthetic response to feeding is blunted in various conditions characterized by skeletal muscle loss, such as aging, chronic metabolic diseases and muscle disuse. Therefore, it is important to define factors that modulate postprandial MPS. It has been shown that the postprandial muscle protein synthetic response can be modulated by the amount of protein ingested (Cuthbertson et al. Citation2005; Moore et al. Citation2015; Moore et al. Citation2009; Pennings et al. Citation2012; Robinson et al. Citation2013; Witard et al. Citation2014; Yang, Breen, et al. Citation2012; Yang, Churchward-Venne et al. Citation2012; Gorissen et al. Citation2020), the source of dietary protein (Burd et al. Citation2012; Pennings et al. Citation2011; Tang et al. Citation2009; Yang, Churchward-Venne et al. Citation2012; Gorissen et al. Citation2020), the timing of protein consumption (Tipton et al. Citation2001; Mamerow et al. Citation2014), food preparation (Pennings et al. Citation2013; Remond et al. Citation2007), body position (Holwerda et al. Citation2017), habitual protein and energy intake (Gorissen et al. Citation2017; Hector et al. Citation2015), physical (in)activity (Breen et al. Citation2013; Burd et al. Citation2011; Churchward-Venne et al. Citation2012; Trommelen et al. Citation2016), body composition (Macnaughton et al. Citation2016), sex (Smith and Mittendorfer Citation2016; West et al. Citation2012; Horstman et al. Citation2019) and age (Gorissen et al. Citation2020; Volpi et al. Citation2001; Wall et al. Citation2015). Recently, also the product matrix, food structure or meal containing other macro and micronutrients and fibers, have been shown to modulate postprandial amino acid absorption kinetics (Gorissen et al. Citation2014; Hamer et al. Citation2013; Horstman et al. Citation2021).
In addition to effects of protein source and protein hydrolysis on amino acid availability, as discussed in Section Postprandial aminoacidemia in relation to milk protein ingredients, also shows the effect of these factors on MPS. In approximately half of the studies included in , muscle or whole-body protein synthetic rates were also measured. In most of the cases a higher aminoacidemia after whey protein intake does not lead to a greater increase in muscle or whole body protein synthesis rates compared to micellar casein (Churchward-Venne et al. Citation2019; Dideriksen et al. Citation2011; Phillips Citation2011; Reitelseder et al. Citation2011; Tipton et al. Citation2004), but in some it does (Boirie, Dangin, et al. Citation1997; Pennings et al. Citation2011). Mainly no differences in circulating plasma amino acid concentrations nor in MPS are found when different intact whey protein sources (Hamarsland, Aas, et al. Citation2019; Hamarsland et al. Citation2017; Mitchell et al. Citation2017) or casein sources (Trommelen et al. Citation2020) were compared. Overall, milk intake leads to lower postprandial aminoacidemia compared to native whey protein (Hamarsland, Aas, et al. Citation2019; Hamarsland, Johansen, et al. Citation2019; Hamarsland et al. Citation2017; Lacroix et al. Citation2006). The muscle protein synthetic response is either lower (Hamarsland, Aas, et al. Citation2019; Hamarsland et al. Citation2017) or equal (Churchward-Venne et al. Citation2019) after milk compared to native whey protein ingestion. Ingestion of casein hydrolysate leads to a more rapid increase in circulating plasma amino acid concentrations compared with intact casein (Calbet and Holst Citation2004; Koopman et al. Citation2009; Pennings et al. Citation2011) but does not (Pennings et al. Citation2011) or only tends to increase (Koopman et al. Citation2009) the incorporation of dietary amino acids into mixed muscle protein. Also, no difference in muscle protein synthesis rates is found whether (native) whey protein, whey protein hydrolysate, concentrate or isolate is ingested (Hamarsland, Aas, et al. Citation2019; Hamarsland et al. Citation2017; Mitchell et al. Citation2017) nor when a casein hydrolysate is compared with its intact protein (Calbet and Holst Citation2004; Koopman et al. Citation2009; Pennings et al. Citation2011) ().
In general, whey protein ingestion leads to more rapid protein digestion and amino acid absorption kinetics than casein. Moreover, whey protein has a higher leucine content compared with casein, resulting in a more rapid rise in specific postprandial leucine concentrations (Koopman et al. Citation2009; Pennings et al. Citation2011; Devries and Phillips Citation2015; Wall et al. Citation2013) which are an important regulator of postprandial muscle protein metabolism. However, these changes in amino acid concentrations in blood do not always result in higher muscle protein synthetic rates after dietary whey protein intake compared with casein. Weijzen et al. (Weijzen et al. Citation2021) recently showed that that the ingestion of free crystalline amino acids leads to a more rapid amino acid absorption and a 17% greater postprandial phenylalanine availability than an equivalent amount of intact dietary protein given in a single bolus, but no differences were found in the increase of mixed muscle protein synthesis rates. This is in line with the fact that greater plasma amino acid responses do not perse lead to greater postprandial increases in MPS (). One explanation here could be the fact that the ingestion of 20 g protein is sufficient to maximize postprandial muscle protein synthesis rates in young, active individuals (Moore et al. Citation2009; Witard et al. Citation2014). Bigger amounts of protein or combining dietary protein intake with resistance type exercise might prevent detecting differences in postprandial muscle protein synthesis rates between treatments in young healthy adults. On the other hand, in conditions of anabolic resistance (i.e. the reduced stimulation of muscle protein synthesis to a given dose of dietary protein with e.g. aging or inactivity) or where less than 20 g of protein is ingested, the proposed benefits of greater postprandial plasma amino acid release on stimulating muscle protein synthesis may become more evident. Thus, only in a setting where a lower amino acid response is suboptimal for signaling or provision of enough precursors, a relationship between amino acid kinetics and MPS could possibly be shown.
Conclusion
Research in milk protein already started in the early 1800s. In the 1900s curd formation research was performed, but the underlying mechanisms were not clear yet. At the end of the 20th century, muscle protein synthesis in humans were measured for the first time and the link was made between coagulation of milk proteins in the stomach, subsequent amino acid appearance in blood and stimulation of muscle protein synthesis. Only more recently the role of processing, food structure, preservation and matrix on coagulation has been getting attention. These factors appear to have a substantial effect on amino acid availability in blood after dietary protein take. Amino acid availability after consumption of dairy products is primarily determined by the rate of gastric emptying of milk proteins. With caseins being able to form gastric coagula, their gastric emptying and concomitant post-prandial aminoacidemia can be notably slower than for whey proteins. However, various processes, such as the conversion to caseinate, heat treatment in the presence of whey proteins, conversion to stirred yoghurt or enzymatic hydrolysis, can also reduce gastric coagulation of caseins, and therewith accelerate gastric emptying and improve amino acid availability and subsequent muscle protein metabolism. It is very important to take these factors into account while developing (dairy) food products. Especially in settings or (patient) populations where a lower amino acid response is suboptimal for signaling or provision of enough precursors, modulating product characteristics by processing can be very useful to steer the gastric behavior of protein, and the subsequent digestion and amino acid absorption and muscle anabolic response to maintain or increase muscle mass.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Adhikari, S., M. Schop, I. J. M. de Boer, and T. Huppertz. 2022. Protein quality in perspective: A review of protein quality metrics and their applications. Nutrients 14 (5):947. doi: 10.3390/nu14050947.
- Anema, S. G. 2021. Heat-induced changes in caseins and casein micelles, including interactions with denatured whey proteins. International Dairy Journal 122:105136. doi: 10.1016/j.idairyj.2021.105136.
- Anthony, G. E. 1936. Local study on soft curd milk. Bulletin of the Gettesee County Medical Society 9:5.
- Anthony, J. C., T. G. Anthony, S. R. Kimball, T. C. Vary, and L. S. Jefferson. 2000. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. The Journal of Nutrition 130 (2):139–45. doi: 10.1093/jn/130.2.139.
- Anthony, J. C., F. Yoshizawa, T. G. Anthony, T. C. Vary, L. S. Jefferson, and S. R. Kimball. 2000. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. The Journal of Nutrition 130 (10):2413–9. doi: 10.1093/jn/130.10.2413.
- Atherton, P. J., K. Smith, T. Etheridge, D. Rankin, and M. J. Rennie. 2010. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38 (5):1533–9. doi: 10.1007/s00726-009-0377-x.
- Ayala-Bribiesca, E., M. Lussier, D. Chabot, S. L. Turgeon, and M. Britten. 2016. Effect of calcium enrichment of Cheddar cheese on its structure, in vitro digestion and lipid bioaccessibility. International Dairy Journal 53:1–9. doi: 10.1016/j.idairyj.2015.09.002.
- Bailey, H., and H. Stein. 2020. Differences in amino acid digestibility and protein quality among various protein isolates and concentrates derived from cereal grains, plant and dairy proteins. Current Developments in Nutrition 4 (Supplement_2):681. doi: 10.1093/cdn/nzaa050_004.
- Bansal, N., and B. Bhandari. 2016. Functional milk proteins: Production and utilization—whey-based ingredients. In Advanced Dairy Chemistry, eds. P. McSweeney, J. O’Mahony. New York, NY: Springer. doi:10.1007/978-1-4939-2800-2_3
- Barbé, F., O. Ménard, Y. Le Gouar, C. Buffière, M.-H. Famelart, B. Laroche, S. Le Feunteun, D. Dupont, and D. Rémond. 2013. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chemistry 136 (3–4):1203–12. doi: 10.1016/j.foodchem.2012.09.022.
- Beelen, M., M. Tieland, A. P. Gijsen, H. Vandereyt, A. K. Kies, H. Kuipers, W. H. Saris, R. Koopman, and L. J. van Loon. 2008. Coingestion of carbohydrate and protein hydrolysate stimulates muscle protein synthesis during exercise in young men, with no further increase during subsequent overnight recovery. The Journal of Nutrition 138 (11):2198–204. doi: 10.3945/jn.108.092924.
- Bergeim, O., J. M. Evvard, M. E. Rehfuss, and P. B. Hawk. 1919. The gastric response to foods: II. A fractional study of the coagulation of milk in the human stomach. American Journal of Physiology-Legacy Content 48 (4):411–8. doi: 10.1152/ajplegacy.1919.48.4.411.
- Boirie, Y., M. Dangin, P. Gachon, M. P. Vasson, J. L. Maubois, and B. Beaufrere. 1997. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proceedings of the National Academy of Sciences 94 (26):14930–5. doi: 10.1073/pnas.94.26.14930.
- Boirie, Y., P. Gachon, and B. Beaufrère. 1997. Splanchnic and whole-body leucine kinetics in young and elderly men. The American Journal of Clinical Nutrition 65 (2):489–95. doi: 10.1093/ajcn/65.2.489.
- Boirie, Y., P. Gachon, S. Corny, J. Fauquant, J. L. Maubois, and B. Beaufrere. 1996. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. The American Journal of Physiology 271 (6 Pt 1):E1083–91. doi: 10.1152/ajpendo.1996.271.6.E1083.
- Breen, L., K. A. Stokes, T. A. Churchward-Venne, D. R. Moore, S. K. Baker, K. Smith, P. J. Atherton, and S. M. Phillips. 2013. Two weeks of reduced activity decreases leg lean mass and induces "anabolic resistance" of myofibrillar protein synthesis in healthy elderly. The Journal of Clinical Endocrinology & Metabolism 98 (6):2604–12. doi: 10.1210/jc.2013-1502.
- Brenneman, J. 1913. Boiled versus raw milk: An experimental study of milkcoagulation in the stomach, together with clinical observations on the use of raw and boiled milk. Journal of the American Medical Association 60:575–82.
- Britten, M., and H. J. Giroux. 2022. Rennet coagulation of heated milk: A review. International Dairy Journal 124:105179. doi: 10.1016/j.idairyj.2021.105179.
- Buchheim, W. 1984. [Influences of different technological treatments of milk on the digestion in the stomach. IV. Electron microscopical characterization of the coagulum and of lipolytic processes in the stomach].[German]. Milchwissenschaft 39:271–5.
- Burd, N. A., J. E. Tang, D. R. Moore, and S. M. Phillips. 2009. Exercise training and protein metabolism: Influences of contraction, protein intake, and sex-based differences. Journal of Applied Physiology (1985) 106 (5):1692–701. doi: 10.1152/japplphysiol.91351.2008.
- Burd, N. A., D. W. West, D. R. Moore, P. J. Atherton, A. W. Staples, T. Prior, J. E. Tang, M. J. Rennie, S. K. Baker, and S. M. Phillips. 2011. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. The Journal of Nutrition 141 (4):568–73. doi: 10.3945/jn.110.135038.
- Burd, N. A., Y. Yang, D. R. Moore, J. E. Tang, M. A. Tarnopolsky, and S. M. Phillips. 2012. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. British Journal of Nutrition 108 (6):958–62. doi: 10.1017/S0007114511006271.
- Calbet, J. A., and J. J. Holst. 2004. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. European Journal of Nutrition 43 (3):127–39. doi: 10.1007/s00394-004-0448-4.
- Carr, A., and M. Golding. 2016. Functional milk proteins production and utilization: Casein-based ingredients. In Advanced dairy chemistry: Volume 1B: Proteins: Applied aspects, eds. P. L. H. McSweeney and J. A. O’Mahony. New York, NY: Springer New York.
- Churchward-Venne, T. A., N. A. Burd, C. J. Mitchell, D. W. West, A. Philp, G. R. Marcotte, S. K. Baker, K. Baar, and S. M. Phillips. 2012. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. The Journal of Physiology 590 (11):2751–65. doi: 10.1113/jphysiol.2012.228833.
- Churchward-Venne, T. A., P. J. M. Pinckaers, J. S. J. Smeets, W. M. Peeters, A. H. Zorenc, H. Schierbeek, I. Rollo, L. B. Verdijk, and L. J. C. van Loon. 2019. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. The Journal of Nutrition 149 (2):198–209. doi: 10.1093/jn/nxy244.
- Churchward-Venne, T. A., T. Snijders, A. M. Linkens, H. M. Hamer, J. van Kranenburg, and L. J. van Loon. 2015. Ingestion of casein in a milk matrix modulates dietary protein digestion and absorption kinetics but does not modulate postprandial muscle protein synthesis in older men. The Journal of Nutrition 145 (7):1438–45. doi: 10.3945/jn.115.213710.
- Clemente, A. 2000. Enzymatic protein hydrolysates in human nutrition. Trends in Food Science & Technology 11 (7):254–62. doi: 10.1016/S0924-2244(01)00007-3.
- Cuthbertson, D., K. Smith, J. Babraj, G. Leese, T. Waddell, P. Atherton, H. Wackerhage, P. M. Taylor, and M. J. Rennie. 2005. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. The FASEB Journal 19 (3):1–4. doi: 10.1096/fj.04-2640fje.
- Dalgleish, D. G., and M. Corredig. 2012. The structure of the casein micelle of milk and its changes during processing. Annual Review of Food Science and Technology 3:449–67. doi: 10.1146/annurev-food-022811-101214.
- Dangin, M., Y. Boirie, C. Garcia-Rodenas, P. Gachon, J. Fauquant, P. Callier, O. Ballevre, and B. Beaufrere. 2001. The digestion rate of protein is an independent regulating factor of postprandial protein retention. American Journal of Physiology-Endocrinology and Metabolism 280 (2):E340–8. doi: 10.1152/ajpendo.2001.280.2.E340.
- Dangin, M., C. Guillet, C. Garcia-Rodenas, P. Gachon, C. Bouteloup-Demange, K. Reiffers-Magnani, J. Fauquant, O. Ballèvre, and B. Beaufrère. 2003. The rate of protein digestion affects protein gain differently during aging in humans. Journal of Physiology 549 (2):635–44. doi: 10.1113/jphysiol.2002.036897.
- De Hart, N., Z. S. Mahmassani, P. T. Reidy, J. J. Kelley, A. I. McKenzie, J. J. Petrocelli, M. J. Bridge, L. M. Baird, E. D. Bastian, L. S. Ward, et al. 2021. Acute effects of cheddar cheese consumption on circulating amino acids and human skeletal muscle. Nutrients 13 (2):614. doi: 10.3390/nu13020614.
- De Kruif, C. G. 1999. Casein micelle interactions. International Dairy Journal 9 (3–6):183–8. doi: 10.1016/S0958-6946(99)00058-8.
- De Kruif, C., and C. Holt. 2003. Casein micelle structure, functions and interactions. In Advanced dairy chemistry—1 proteins, 233–76. New York, USA: Springer.
- Deglaire, A., C. Fromentin, H. Fouillet, G. Airinei, C. Gaudichon, C. Boutry, R. Benamouzig, P. J. Moughan, D. Tomé, and C. Bos. 2009. Hydrolyzed dietary casein as compared with the intact protein reduces postprandial peripheral, but not whole-body, uptake of nitrogen in humans. The American Journal of Clinical Nutrition 90 (4):1011–22. doi: 10.3945/ajcn.2009.27548.
- Devries, M. C., and S. M. Phillips. 2015. Supplemental protein in support of muscle mass and health: Advantage whey. Journal of Food Science 80 (S1):A8–A15. doi: 10.1111/1750-3841.12802.
- Dideriksen, K. J., S. Reitelseder, S. G. Petersen, M. Hjort, I. C. Helmark, M. Kjaer, and L. Holm. 2011. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scandinavian Journal of Medicine & Science in Sports 21 (6):e372–83. doi: 10.1111/j.1600-0838.2011.01318.x.
- Dupont, D., G. Mandalari, D. Mollé, J. Jardin, O. Rolet-Répécaud, G. Duboz, J. Léonil, C. E. Mills, and A. R. Mackie. 2010. Food processing increases casein resistance to simulated infant digestion. Molecular Nutrition & Food Research 54 (11):1677–89. doi: 10.1002/mnfr.200900582.
- Elliot, T. A., M. G. Cree, A. P. Sanford, R. R. Wolfe, and K. D. Tipton. 2006. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Medicine & Science in Sports & Exercise 38 (4):667–74. doi: 10.1249/01.mss.0000210190.64458.25.
- Fanelli, N. S., H. M. Bailey, L. V. Guardiola, and H. H. Stein. 2021. Values for digestible indispensable amino acid score (DIAAS) determined in pigs are greater for milk than for breakfast cereals, but DIAAS values for individual ingredients are additive in combined meals. The Journal of Nutrition 151 (3):540–7. doi: 10.1093/jn/nxaa398.
- Fang, X., L. Rioux, S. Labrie, and S. L. Turgeon. 2016a. Commercial cheeses with different texture have different disintegration and protein/peptide release rates during simulated in vitro digestion. International Dairy Journal 56:169–78. doi: 10.1016/j.idairyj.2016.01.023.
- Fang, X., L. Rioux, S. Labrie, and S. L. Turgeon. 2016b. Disintegration and nutrients release from cheese with different textural properties during in vitro digestion. Food Research International 88:276–83. doi: 10.1016/j.foodres.2016.04.008.
- Gao, K. P., T. Mitsui, K. Fujiki, H. Ishiguro, and T. Kondo. 2002. Effect of lactase preparations in asymptomatic individuals with lactase deficiency–gastric digestion of lactose and breath hydrogen analysis. Nagoya Journal of Medical Science 65 (1–2):21–8.
- Gaudichon, C., S. Mahé, N. Roos, R. Benamouzig, C. Luengo, J. F. Huneau, H. Sick, C. Bouley, J. Rautureau, and D. Tome. 1995. Exogenous and endogenous nitrogen flow rates and level of protein hydrolysis in the human jejunum after [15N]milk and [15N]yoghurt ingestion. The British Journal of Nutrition 74 (2):251–60. doi: 10.1079/bjn19950128.
- Gaudichon, C., N. Roos, S. Mahé, H. Sick, C. Bouley, and D. Tomé. 1994. Gastric emptying regulates the kinetics of nitrogen absorption from 15N-labeled milk and 15N-labeled yogurt in miniature pigs. The Journal of Nutrition 124 (10):1970–7. doi: 10.1093/jn/124.10.1970.
- Glynn, E. L., C. S. Fry, M. J. Drummond, H. C. Dreyer, S. Dhanani, E. Volpi, and B. B. Rasmussen. 2010. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 299 (2):R533–40. doi: 10.1152/ajpregu.00077.2010.
- Glynn, E. L., C. S. Fry, K. L. Timmerman, M. J. Drummond, E. Volpi, and B. B. Rasmussen. 2013. Addition of carbohydrate or alanine to an essential amino acid mixture does not enhance human skeletal muscle protein anabolism. The Journal of Nutrition 143 (3):307–14. doi: 10.3945/jn.112.168203.
- Gorissen, S. H., N. A. Burd, H. M. Hamer, A. P. Gijsen, B. B. Groen, and L. J. van Loon. 2014. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. The Journal of Clinical Endocrinology and Metabolism 99 (6):2250–8.
- Gorissen, S. H., A. M. Horstman, R. Franssen, I. W. Kouw, B. T. Wall, N. A. Burd, L. C. de Groot, and L. J. van Loon. 2017. Habituation to low or high protein intake does not modulate basal or postprandial muscle protein synthesis rates: A randomized trial. The American Journal of Clinical Nutrition 105 (2):332–42. doi: 10.3945/ajcn.115.129924.
- Gorissen, S. H. M., J. Trommelen, I. W. K. Kouw, A. M. Holwerda, B. Pennings, B. B. L. Groen, B. T. Wall, T. A. Churchward-Venne, A. M. H. Horstman, R. Koopman, et al. 2020. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. The Journal of Nutrition 150 (8):2041–50. doi: 10.1093/jn/nxaa024.
- Groen, B. B., A. M. Horstman, H. M. Hamer, M. de Haan, J. van Kranenburg, J. Bierau, M. Poeze, W. K. Wodzig, B. B. Rasmussen, and L. J. van Loon. 2015. Post-prandial protein handling: You are what you just ate. Plos One 10 (11):e0141582. doi: 10.1371/journal.pone.0141582.
- Guimbaud, R., J. A. Moreau, M. Bouisson, S. Durand, J. Escourrou, N. Vaysse, and J. Frexinos. 1997. Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas 14:76–82.
- Guo, Q., A. Ye, H. Singh, and D. Rousseau. 2020. Destructuring and restructuring of foods during gastric digestion. Comprehensive Reviews in Food Science and Food Safety 19 (4):1658–79. doi: 10.1111/1541-4337.12558.
- Hall, W. L., D. J. Millward, S. J. Long, and L. M. Morgan. 2003. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. British Journal of Nutrition 89 (2):239–48. doi: 10.1079/BJN2002760.
- Hamarsland, H., S. N. Aas, A. L. Nordengen, K. Holte, I. Garthe, G. Paulsen, M. Cotter, E. Borsheim, H. B. Benestad, and T. Raastad. 2019. Native whey induces similar post exercise muscle anabolic responses as regular whey, despite greater leucinemia, in elderly individuals. The Journal of Nutrition, Health & Aging 23 (1):42–50. doi: 10.1007/s12603-018-1105-6.
- Hamarsland, H., M. K. Johansen, F. Seeberg, M. Brochmann, I. Garthe, H. B. Benestad, and T. Raastad. 2019. Native whey induces similar adaptation to strength training as milk, despite higher levels of leucine, in elderly individuals. Nutrients 11 (9):2094. doi: 10.3390/nu11092094.
- Hamarsland, H., A. L. Nordengen, S. Nyvik Aas, K. Holte, I. Garthe, G. Paulsen, M. Cotter, E. Borsheim, H. B. Benestad, and T. Raastad. 2017. Native whey protein with high levels of leucine results in similar post-exercise muscular anabolic responses as regular whey protein: A randomized controlled trial. Journal of the International Society of Sports Nutrition 14:43. doi: 10.1186/s12970-017-0202-y.
- Hamer, H. M., B. T. Wall, A. Kiskini, A. de Lange, B. B. Groen, J. A. Bakker, A. P. Gijsen, L. B. Verdijk, and L. J. van Loon. 2013. Carbohydrate co-ingestion with protein does not further augment post-prandial muscle protein accretion in older men. Nutrition & Metabolism 10 (1):15. doi: 10.1186/1743-7075-10-15.
- Hector, A. J., G. R. Marcotte, T. A. Churchward-Venne, C. H. Murphy, L. Breen, M. von Allmen, S. K. Baker, and S. M. Phillips. 2015. Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short-term energy restriction in overweight and obese adults. The Journal of Nutrition 145 (2):246–52. doi: 10.3945/jn.114.200832.
- Hermans, W. J. H., C. J. Fuchs, F. K. Hendriks, L. H. P. Houben, J. M. Senden, L. B. Verdijk, and L. J. C. van Loon. 2022. Cheese ingestion increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young males: A randomized parallel-group trial. Journal of Nutrition, 152:1022–1030.
- Holt, C., J. A. Carver, H. Ecroyd, and D. C. Thorn. 2013. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. Journal of Dairy Science 96 (10):6127–46. doi: 10.3168/jds.2013-6831.
- Holt, C., and D. S. Horne. 1996. The hairy casein micelle: Evolution of the concept and its implications for dairy processing. Netherlands Milk and Dairy Journal 50:85–111.
- Holwerda, A. M., K. Lenaerts, J. Bierau, W. Wodzig, and L. J. C. van Loon. 2017. Food ingestion in an upright sitting position increases postprandial amino acid availability when compared with food ingestion in a lying down position. Applied Physiology, Nutrition, and Metabolism 42 (7):738–43. doi: 10.1139/apnm-2016-0522.
- Horne, D. S. 2006. Casein micelle structure: Models and muddles. Current Opinion in Colloid & Interface Science 11 (2–3):148–53. doi: 10.1016/j.cocis.2005.11.004.
- Horstman, A. M. H., R. A. Ganzevles, U. Kudla, A. F. M. Kardinaal, J. van den Borne, and T. Huppertz. 2021. Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix. International Dairy Journal 113:104890. doi: 10.1016/j.idairyj.2020.104890.
- Horstman, A. M. H., I. W. K. Kouw, J. W. van Dijk, H. M. Hamer, B. B. L. Groen, J. van Kranenburg, S. H. M. Gorissen, and L. J. C. van Loon. 2019. The muscle protein synthetic response to whey protein ingestion is greater in middle-aged women compared with men. The Journal of Clinical Endocrinology & Metabolism 104 (4):994–1004. doi: 10.1210/jc.2018-01734.
- Houghton, L. A., F. Hickson, and N. W. Read. 1987. Effect of food consistency on gastric emptying in man. Gut 28 (12):1584–8. doi: 10.1136/gut.28.12.1584.
- Huppertz, T., and L. W. Chia. 2021. Milk protein coagulation under gastric conditions: A review. International Dairy Journal 113:104882. doi: 10.1016/j.idairyj.2020.104882.
- Huppertz, T., and I. Gazi. 2017. Ingredients from milk for use in food and non-food products: From commodity to value-added ingredients. In Achieving sustainable production of milk, ed. N. van Belzen, vol. 1, 121–44. Cambridge, UK: Burleigh Dodds Science Publishing.
- Huppertz, T., I. Gazi, H. Luyten, H. Nieuwenhuijse, A. Alting, and E. Schokker. 2017. Hydration of casein micelles and caseinates: Implications for casein micelle structure. International Dairy Journal 74:1–11. doi: 10.1016/j.idairyj.2017.03.006.
- Huppertz, T., and A. L. Kelly. 2006. Physical chemistry of milk fat globules. In Advanced dairy chemistry Volume 2 lipids, ed. P. F. Fox and P. L. H. McSweeney. Boston, MA: Springer.
- Huppertz, T., and T. T. Lambers. 2020. Influence of micellar calcium phosphate on in vitro gastric coagulation and digestion of milk proteins in infant formula model systems. International Dairy Journal 107:104717. doi: 10.1016/j.idairyj.2020.104717.
- Iezzi, R., F. Locci, and G. Mucchetti. 2013. Cheese true density prediction by linear equations. Journal of Food Process Engineering 36 (4):462–9. doi: 10.1111/jfpe.12008.
- Jørgensen, C. E., R. K. Abrahamsen, E. Rukke, T. K. Hoffmann, A. Johansen, and S. B. Skeie. 2019. Processing of high-protein yoghurt – A review. International Dairy Journal 88:42–59. doi: 10.1016/j.idairyj.2018.08.002.
- Kaufmann, W. 1984. Influences of different technological treatments of milk on the digestion in the stomach. VI. Estimation of amino acid and urea concentrations in the blood: Conclusions regarding the nutritional evaluation [German]. Milchwissenschaft 19:281–4.
- Kelly, A. L., T. Huppertz, and J. J. Sheehan. 2008. Pre-treatment of cheese milk: Principles and developments. Dairy Science and Technology 88 (4–5):549–72. doi: 10.1051/dst:2008017.
- Kimball, S. R., and L. S. Jefferson. 2006. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. The Journal of Nutrition 136 (1 Suppl):227S–31S. doi: 10.1093/jn/136.1.227S.
- Koopman, R., M. Beelen, T. Stellingwerff, B. Pennings, W. H. Saris, A. K. Kies, H. Kuipers, and L. J. van Loon. 2007. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. American Journal of Physiology-Endocrinology and Metabolism 293 (3):E833–42. doi: 10.1152/ajpendo.00135.2007.
- Koopman, R., N. Crombach, A. P. Gijsen, S. Walrand, J. Fauquant, A. K. Kies, S. Lemosquet, W. H. Saris, Y. Boirie, and L. J. van Loon. 2009. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. The American Journal of Clinical Nutrition 90 (1):106–15. doi: 10.3945/ajcn.2009.27474.
- Krzeminski, A., K. Großhable, and J. Hinrichs. 2011. Structural properties of stirred yoghurt as influenced by whey proteins. LWT - Food Science and Technology 44 (10):2134–40. doi: 10.1016/j.lwt.2011.05.018.
- Lacroix, M., C. Bon, C. Bos, J. Léonil, R. Benamouzig, C. Luengo, J. Fauquant, D. Tomé, and C. Gaudichon. 2008. Ultra high temperature treatment, but not pasteurization, affects the postprandial kinetics of milk proteins in humans. The Journal of Nutrition 138 (12):2342–7. doi: 10.3945/jn.108.096990.
- Lacroix, M., C. Bos, J. Leonil, G. Airinei, C. Luengo, S. Dare, R. Benamouzig, H. Fouillet, J. Fauquant, D. Tome, et al. 2006. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. The American Journal of Clinical Nutrition 84 (5):1070–9. doi: 10.1093/ajcn/84.5.1070.
- Li, S., A. Ye, and H. Singh. 2021. Impacts of heat-induced changes on milk protein digestibility: A review. International Dairy Journal 123:105160. doi: 10.1016/j.idairyj.2021.105160.
- Lindsay, D., R. Robertson, R. Fraser, S. Engstrom, and K. Jordan. 2021. Heat induced inactivation of microorganisms in milk and dairy products. International Dairy Journal 121:105096. doi: 10.1016/j.idairyj.2021.105096.
- Liu, W., Y. Jin, P. J. Wilde, Y. Hou, Y. Wang, and J. Han. 2021. Mechanisms, physiology, and recent research progress of gastric emptying. Critical Reviews in Food Science and Nutrition 61 (16):2742–55. doi: 10.1080/10408398.2020.1784841.
- Lodaite, K., F. Chevalier, E. Armaforte, and A. L. Kelly. 2009. Effect of high-pressure homogenisation on rheological properties of rennet-induced skim milk and standardised milk gels. The Journal of Dairy Research 76 (3):294–300. doi: 10.1017/S0022029909004117.
- Lucey, J. A., P. A. Munro, and H. Singh. 2006. Rheological properties and microstructure of acid milk gels as affected by fat content and heat treatment. Journal of Food Science 63 (4):660–4. doi: 10.1111/j.1365-2621.1998.tb15807.x.
- Lucey, J. A., and H. Singh. 1997. Formation and physical properties of acid milk gels: A review. Food Research International 30 (7):529–42. doi: 10.1016/S0963-9969(98)00015-5.
- Lucey, J. A., D. J. Wilbanks, and D. S. Horne. 2022. Impact of heat treatment of milk on acid gelation. International Dairy Journal 125 (105222):105222. doi: 10.1016/j.idairyj.2021.105222.
- Mackie, A. R., H. Rafiee, P. Malcolm, L. Salt, and G. van Aken. 2013. Specific food structures supress appetite through reduced gastric emptying rate. American Journal of Physiology, Gastrointestinal and Liver Physiology 304 (11):G1038–G43. doi: 10.1152/ajpgi.00060.2013.
- Macnaughton, L. S., S. L. Wardle, O. C. Witard, C. McGlory, D. L. Hamilton, S. Jeromson, C. E. Lawrence, G. A. Wallis, and K. D. Tipton. 2016. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiological Reports 4 (15):e12893. doi: 10.14814/phy2.12893.
- Mahé, S., J. Fauquant, C. Gaudichon, N. Roos, J. L. Maubois, and D. Tome. 1994. 15N-Labelling and preparation of milk, casein and whey proteins. Le Lait 74 (4):307–12. doi: 10.1051/lait:1994425.
- Mahe, S., N. Roos, R. Benamouzig, L. Davin, C. Luengo, L. Gagnon, N. Gausserges, J. Rautureau, and D. Tome. 1996. Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: The influence of the nature and quantity of the protein. The American Journal of Clinical Nutrition 63 (4):546–52. doi: 10.1093/ajcn/63.4.546.
- Mamerow, M. M., J. A. Mettler, K. L. English, S. L. Casperson, E. Arentson-Lantz, M. Sheffield-Moore, D. K. Layman, and D. Paddon-Jones. 2014. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. The Journal of Nutrition 144 (6):876–80. doi: 10.3945/jn.113.185280.
- Mathai, J. K., Y. Liu, and H. H. Stein. 2017. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). British Journal of Nutrition 117 (4):490–9. doi: 10.1017/S0007114517000125.
- McMahon, D. J., and B. S. Oommen. 2013. Casein micelle structure, functions, and interactions. In Advanced dairy chemistry, eds. P. L. H. McSweeney and P. F. Fox. Boston, MA: Springer.
- Miranda, G., G. Hazé, P. Scanff, and J. P. Pélissier. 1989. Hydrolysis of α-lactalbumin by chymosin and pepsin. Effect of conformation and pH. Le Lait 69 (6):451–9. doi: 10.1051/lait:1989630.
- Miranda, G., and J.-P. Pelissier. 1987. Influence of heat treatment of bovine skim-milk on in vivo digestion in rat stomach. Le Lait 67 (3):365–77. doi: 10.1051/lait:1987321.
- Mitchell, C. J., R. F. D’Souza, A. C. Fanning, S. D. Poppitt, and D. Cameron-Smith. 2017. Short communication: Muscle protein synthetic response to microparticulated whey protein in middle-aged men. Journal of Dairy Science 100 (6):4230–4. doi: 10.3168/jds.2016-12287.
- Moore, D. R., T. A. Churchward-Venne, O. Witard, L. Breen, N. A. Burd, K. D. Tipton, and S. M. Phillips. 2015. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 70 (1):57–62. doi: 10.1093/gerona/glu103.
- Moore, D. R., M. J. Robinson, J. L. Fry, J. E. Tang, E. I. Glover, S. B. Wilkinson, T. Prior, M. A. Tarnopolsky, and S. M. Phillips. 2009. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. The American Journal of Clinical Nutrition 89 (1):161–8. doi: 10.3945/ajcn.2008.26401.
- Moughal, K. I., P. A. Munro, and H. Singh. 2000. Suspension stability and size distribution of particles in reconstituted, commercial calcium caseinates. International Dairy Journal 10 (10):683–90. doi: 10.1016/S0958-6946(00)00104-7.
- Mulet-Cabero, A. I., A. R. Mackie, A. Brodkorb, and P. J. Wilde. 2020. Dairy structures and physiological responses: A matter of gastric digestion. Critical Reviews in Food Science and Nutrition 60:1–16.
- Mulet-Cabero, A.-I., A. R. Mackie, P. J. Wilde, M. A. Fenelon, and A. Brodkorb. 2019. Structural mechanism and kinetics of in vitro gastric digestion are affected by process-induced changes in bovine milk. Food Hydrocolloids 86:172–83. doi: 10.1016/j.foodhyd.2018.03.035.
- Mulet-Cabero, A. I., A. Torcello-Gómez, S. Saha, A. R. Mackie, P. J. Wilde, and A. Brodkorb. 2020. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chemistry 319:126514. doi: 10.1016/j.foodchem.2020.126514.
- Mulvihill, D. M. 1989. Caseins and caseinates: Manufacture. In Developments in dairy chemistry-4. Functional milk proteins, ed. P. F. Fox. London, UK: Applied Science Publishers.
- Pennings, B., Y. Boirie, J. M. Senden, A. P. Gijsen, H. Kuipers, and L. J. van Loon. 2011. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. The American Journal of Clinical Nutrition 93 (5):997–1005. doi: 10.3945/ajcn.110.008102.
- Pennings, B., B. Groen, A. de Lange, A. P. Gijsen, A. H. Zorenc, J. M. Senden, and L. J. van Loon. 2012. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. American Journal of Physiology-Endocrinology and Metabolism 302 (8):E992–E999. doi: 10.1152/ajpendo.00517.2011.
- Pennings, B., B. B. Groen, J. W. van Dijk, A. de Lange, A. Kiskini, M. Kuklinski, J. M. Senden, and L. J. van Loon. 2013. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. The American Journal of Clinical Nutrition 98 (1):121–8. doi: 10.3945/ajcn.112.051201.
- Peram, M. R., S. M. Loveday, A. Ye, and H. Singh. 2013. In vitro gastric digestion of heat-induced aggregates of beta-lactoglobulin. Journal of Dairy Science 96 (1):63–74.
- Pfeil, R. 1984. Influence of different technological treatments of milk on the digestion in the stomach. III. Proteolysis in the Stomach’, Milchwissenschaft 39:267–70.
- Phillips, S. M. 2011. A comparison of whey to caseinate. American Journal of Physiology-Endocrinology and Metabolism 300 (3):E610–E610. author reply E11-2. doi: 10.1152/ajpendo.00647.2010.
- Phillips, S. M., J. E. Tang, and D. R. Moore. 2009. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. Journal of the American College of Nutrition 28 (4):343–54. doi: 10.1080/07315724.2009.10718096.
- Reitelseder, S., J. Agergaard, S. Doessing, I. C. Helmark, P. Lund, N. B. Kristensen, J. Frystyk, A. Flyvbjerg, P. Schjerling, G. van Hall, et al. 2011. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: Effect of resistance exercise and protein ingestion. American Journal of Physiology-Endocrinology and Metabolism 300 (1):E231–42. doi: 10.1152/ajpendo.00513.2010.
- Remond, D., M. Machebeuf, C. Yven, C. Buffiere, L. Mioche, L. Mosoni, and P. Patureau Mirand. 2007. Postprandial whole-body protein metabolism after a meat meal is influenced by chewing efficiency in elderly subjects. The American Journal of Clinical Nutrition 85 (5):1286–92. doi: 10.1093/ajcn/85.5.1286.
- Rennie, M. J., R. H. Edwards, D. Halliday, D. E. Matthews, S. L. Wolman, and D. J. Millward. 1982. Muscle protein synthesis measured by stable isotope techniques in man: The effects of feeding and fasting. Clinical Science (London, England: 1979) 63 (6):519–23. doi: 10.1042/cs0630519.
- Robinson, M. J., N. A. Burd, L. Breen, T. Rerecich, Y. Yang, A. J. Hector, S. K. Baker, and S. M. Phillips. 2013. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Applied Physiology, Nutrition, and Metabolism 38 (2):120–5. doi: 10.1139/apnm-2012-0092.
- Rutherfurd, S. M., A. C. Fanning, B. J. Miller, and P. J. Moughan. 2015. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. The Journal of Nutrition 145 (2):372–9. doi: 10.3945/jn.114.195438.
- Siegel, J. A., J. L. Urbain, L. P. Adler, N. D. Charkes, A. H. Maurer, B. Krevsky, L. C. Knight, R. S. Fisher, and L. S. Malmud. 1988. Biphasic nature of gastric emptying. Gut 29 (1):85–9. doi: 10.1136/gut.29.1.85.
- Smith, G. I., and B. Mittendorfer. 2016. dimorphism in skeletal muscle protein turnover. Journal of Applied Physiology 120 (6):674–82. doi: 10.1152/japplphysiol.00625.2015.
- Smith, T. J., S. J. Montain, D. Anderson, and A. J. Young. 2009. Plasma amino acid responses after consumption of beverages with varying protein type. International Journal of Sport Nutrition and Exercise Metabolism 19 (1):1–17. doi: 10.1123/ijsnem.19.1.1.
- Sodini, I., F. Remeuf, S. Haddad, and G. Corrieu. 2004. The relative effect of milk base, starter, and process on yogurt texture: A review. Critical Reviews in Food Science and Nutrition 44 (2):113–37. doi: 10.1080/10408690490424793.
- Somaratne, G., M. J. Ferrua, A. Ye, F. Nau, J. Floury, D. Dupont, and J. Singh. 2020. Food material properties as determining factors in nutrient release during human gastric digestion: A review. Critical Reviews in Food Science and Nutrition 60 (22):3753–69. doi: 10.1080/10408398.2019.1707770.
- Soop, M., V. Nehra, G. C. Henderson, Y. Boirie, G. C. Ford, and K. S. Nair. 2012. Coingestion of whey protein and casein in a mixed meal: Demonstration of a more sustained anabolic effect of casein. American Journal of Physiology-Endocrinology and Metabolism 303 (1):E152–62. doi: 10.1152/ajpendo.00106.2012.
- Staples, A. W., N. A. Burd, D. W. West, K. D. Currie, P. J. Atherton, D. R. Moore, M. J. Rennie, M. J. Macdonald, S. K. Baker, and S. M. Phillips. 2011. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Medicine & Science in Sports & Exercise 43 (7):1154–61. doi: 10.1249/MSS.0b013e31820751cb.
- Tamime, A. Y., and R. K. Robinson. 2007. Tamime and Robinson’s yoghurt: science and technology. 3rd ed. Cambridge: Woodhead Publishing Limited.
- Tang, J. E., D. R. Moore, G. W. Kujbida, M. A. Tarnopolsky, and S. M. Phillips. 2009. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology (1985) 107 (3):987–92. doi: 10.1152/japplphysiol.00076.2009.
- Tipton, K. D., T. A. Elliott, M. G. Cree, S. E. Wolf, A. P. Sanford, and R. R. Wolfe. 2004. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Medicine and Science in Sports and Exercise 36 (12):2073–81. doi: 10.1249/01.mss.0000147582.99810.c5.
- Tipton, K. D., B. B. Rasmussen, S. L. Miller, S. E. Wolf, S. K. Owens-Stovall, B. E. Petrini, and R. R. Wolfe. 2001. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. American Journal of Physiology-Endocrinology and Metabolism 281 (2):E197–206. doi: 10.1152/ajpendo.2001.281.2.E197.
- Torres, I. C., J. M. Amigo Rubio, and R. Ipsen. 2012. Using fractal image analysis to characterize microstructure of low-fat stirred yoghurt manufactured with microparticulated whey protein. Journal of Food Engineering 109 (4):721–9. doi: 10.1016/j.jfoodeng.2011.11.016.
- Traylor, D. A., S. H. M. Gorissen, H. Hopper, T. Prior, C. McGlory, and S. M. Phillips. 2019. Aminoacidemia following ingestion of native whey protein, micellar casein, and a whey-casein blend in young men. Applied Physiology, Nutrition, and Metabolism 44 (1):103–6. doi: 10.1139/apnm-2018-0240.
- Trommelen, J., A. M. Holwerda, I. W. K. Kouw, H. Langer, S. L. Halson, I. Rollo, L. B. Verdijk, and L. J. C. Van Loon. 2016. Resistance exercise augments postprandial overnight muscle protein synthesis rates. Medicine & Science in Sports & Exercise 48 (12):2517–25. doi: 10.1249/MSS.0000000000001045.
- Trommelen, J., D. Tomé, and L. J. C. van Loon. 2021. Gut amino acid absorption in humans: Concepts and relevance for postprandial metabolism. Clinical Nutrition Open Science 36:43–55. doi: 10.1016/j.nutos.2020.12.006.
- Trommelen, J., M. E. G. Weijzen, J. van Kranenburg, R. A. Ganzevles, M. Beelen, L. B. Verdijk, and L. J. C. van Loon. 2020. Casein protein processing strongly modulates post-prandial plasma amino acid responses in vivo in humans. Nutrients 12 (8):2299. doi: 10.3390/nu12082299.
- Van Lieshout, G. A. A., T. T. Lambers, M. C. E. Bragt, and K. A. Hettinga. 2020. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Critical Reviews in Food Science and Nutrition 60 (14):2422–45. doi: 10.1080/10408398.2019.1646703.
- van Vilet, T., S. P. F. M. Roefs, P. Zoon, and P. Walstra. 1989. Rheological properties of casein gels. Journal of Dairy Research 56 (3):529–34. doi: 10.1017/S0022029900029022.
- Volpi, E., M. Sheffield-Moore, B. B. Rasmussen, and R. R. Wolfe. 2001. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286 (10):1206–12. doi: 10.1001/jama.286.10.1206.
- Wall, B. T., S. H. Gorissen, B. Pennings, R. Koopman, B. B. Groen, L. B. Verdijk, and L. J. van Loon. 2015. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PloS One 10 (11):e0140903. doi: 10.1371/journal.pone.0140903.
- Wall, B. T., H. M. Hamer, A. de Lange, A. Kiskini, B. B. Groen, J. M. Senden, A. P. Gijsen, L. B. Verdijk, and L. J. van Loon. 2013. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clinical Nutrition 32 (3):412–9. doi: 10.1016/j.clnu.2012.09.002.
- Walstra, P. J., T. M. Wouters, and T. J. Geurts. 2007. Dairy science and technology, 782. Boca Raton, London, New York: CRC Taylor & Francis Group. . ISBN: 0-8247-2763-0. .
- Wang, X., A. Ye, Q. Lin, J. Han, and H. Singh. 2018. Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model. Journal of Dairy Science 101 (8):6842–52. doi: 10.3168/jds.2017-14284.
- Weijzen, M. E. G., R. J. J. van Gassel, I. W. K. Kouw, J. Trommelen, S. H. M. Gorissen, J. van Kranenburg, J. P. B. Goessens, M. C. G. van de Poll, L. B. Verdijk, and L. J. C. van Loon. 2021. Ingestion of free amino acids compared with an equivalent amount of intact protein results in more rapid amino acid absorption and greater postprandial plasma amino acid availability without affecting muscle protein synthesis rates in young adults in a double-blind randomized trial. Journal of Nutrition 152:nxab305.
- West, D. W., N. A. Burd, T. A. Churchward-Venne, D. M. Camera, C. J. Mitchell, S. K. Baker, J. A. Hawley, V. G. Coffey, and S. M. Phillips. 2012. Sex based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. Journal of Applied Physiology 112 (11):1805–13. 2012 doi: 10.1152/japplphysiol.00170.2012.
- Wiking, L., S. B. Gregersen, S. F. Hansen, and M. Hammershøj. 2021. Heat-induced changes in milk fat and milk fat globules and its derived effects on acid dairy gelation – a review. International Dairy Journal 127:105213.
- Wilkinson, S. B., M. A. Tarnopolsky, M. J. Macdonald, J. R. Macdonald, D. Armstrong, and S. M. Phillips. 2007. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. The American Journal of Clinical Nutrition 85 (4):1031–40. doi: 10.1093/ajcn/85.4.1031.
- Witard, O. C., S. R. Jackman, L. Breen, K. Smith, A. Selby, and K. D. Tipton. 2014. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. The American Journal of Clinical Nutrition 99 (1):86–95. doi: 10.3945/ajcn.112.055517.
- Yang, Y., L. Breen, N. A. Burd, A. J. Hector, T. A. Churchward-Venne, A. R. Josse, M. A. Tarnopolsky, and S. M. Phillips. 2012. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. British Journal of Nutrition 108 (10):1780–8. doi: 10.1017/S0007114511007422.
- Yang, Y., T. A. Churchward-Venne, N. A. Burd, L. Breen, M. A. Tarnopolsky, and S. M. Phillips. 2012. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutrition & Metabolism 9 (1):57. doi: 10.1186/1743-7075-9-57.
- Ye, A. 2021. Gastric colloidal behaviour of milk protein as a tool for manipulating nutrient digestion in dairy products and protein emulsions. Food Hydrocolloids. 115:106599. doi: 10.1016/j.foodhyd.2021.106599.
- Ye, A., J. Cui, D. Dalgleish, and H. Singh. 2016. Formation of a structured clot during the gastric digestion of milk: Impact on the rate of protein hydrolysis. Food Hydrocolloids. 52:478–86. doi: 10.1016/j.foodhyd.2015.07.023.
- Ye, A., J. Cui, D. Dalgleish, and H. Singh. 2017. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. Journal of Dairy Science 100 (1):36–47. doi: 10.3168/jds.2016-11764.
- Ye, A., W. Liu, J. Cui, X. Kong, D. Roy, Y. Kong, J. Han, and H. Singh. 2019. Coagulation behaviour of milk under gastric digestion: Effect of pasteurization and ultra-high temperature treatment. Food Chemistry 286:216–25. doi: 10.1016/j.foodchem.2019.02.010.
- Zhang, L., R. Zhou, J. Zhang, and P. Zhou. 2021. Heat-induced denaturation and bioactivity changes of whey proteins. International Dairy Journal 123:105175.