Abstract
Anthocyanin-rich fruit beverages are of special interest as functional products due to their antioxidant activity, antimicrobial properties against pathogens, and, more recently, evidence of prebiotic potential. The stability and bioactivity of anthocyanins, probiotics, prebiotics, and synbiotics have been extensively documented in beverage models and reviewed separately. This review summarizes the most recent works and methodologies used for the development of probiotic and synbiotic beverages based on anthocyanin-rich fruits with a synergistic perspective. Emphasis is made on key optimization factors and strategies that have allowed probiotic cultures to reach the minimum recommended doses to obtain health benefits at the end of the shelf life. The development of these beverages is limited by the high acidity and high content of phenolic compounds in anthocyanin-rich fruits. However, a proper selection of probiotic strains and strategies for their media adaptation may improve their viability in the beverages. Fermentation increases the viability of the probiotic cultures, improves the safety and stability of the product, and may increase its antioxidant capacity. Moreover, fermentation metabolites may synergistically enhance probiotic health benefits. On the other hand, the inoculation of probiotics without fermentation allows for synbiotic beverages with milder changes in terms of physicochemical and sensory attributes.
1. Introduction
The growing awareness of consumers toward the health benefits that food products can provide has encouraged the development of functional foods, i.e., food products with targeted functionalities and biological activities (Topolska, Florkiewicz, and Filipiak-Florkiewicz Citation2021). Some of the functional foods that have become more popular in recent years include prebiotics and probiotics, due to the evidence of their positive impact on the gastrointestinal tract and the immune system (Cunningham et al. Citation2021).
Probiotics are defined by the FAO/WHO as "live microorganisms that exert a beneficial action on the health of the host when administered in adequate amounts" (Zendeboodi et al. Citation2020). Furthermore, the International Scientific Association for Probiotics and Prebiotics (ISAPP) recommends using the term probiotic only in products that contain live microorganisms with an adequate count of well-defined strains and with a reasonable expectation of providing benefits to the welfare of the host (Hill et al. Citation2014). In this way, only the species and strains of bacteria whose positive effects on the health of the host have been confirmed, through in vitro tests and clinical studies, can be selected as probiotics. In the food market, the probiotics that are mainly used are lactic acid bacteria that include species of Lactobacillus, Bifidobacterium, Lactococcus, and Enterococcus (Ranadheera et al. Citation2017; Žuntar et al. Citation2020).
On the other hand, ISAPP defines a prebiotic as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (Gibson et al. Citation2017). Selectivity is essential in this concept because, unlike fibers such as cellulose, pectin, and xylan that promote the growth of various microorganisms in the intestine, prebiotics specifically stimulate the proliferation of beneficial microorganisms (Hu et al. Citation2021). Some of these molecules classified as prebiotics include human milk oligosaccharides, inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), xylooligosaccharides (XOS), and some glucans (Quigley Citation2019). Recently, phenolic compounds have also been classified as potential prebiotics as they can modulate the gut microbiota by means of its antimicrobial activity and beneficial bacterial stimulation effects (Rodríguez-Daza et al. Citation2021). From the large diversity of phenolic compounds found in fruits and vegetables, flavonoids, including anthocyanins, have promising potential prebiotic activity as well as several functional properties, as further discussed below.
The health benefits provided by the consumption of prebiotics and probiotics are enhanced when used simultaneously, that is, as a synbiotic. ISAPP defines a synbiotic as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (Swanson et al. Citation2020). In a synbiotic, a prebiotic component may selectively favor a probiotic microorganism, improving its survival during storage and passage along the gastrointestinal tract, increasing its effect on the small intestine and colon (Markowiak and Śliżewska Citation2017). Thus, the health benefits provided for the consumption of prebiotic, probiotic, and synbiotic products include: 1) reduction of lactose intolerance, 2) reduction of cholesterol levels, 3) reduction of constipation and diarrhea symptoms, 4) increase in absorption of minerals and bioactive compounds, and 5) prevention of certain diseases like cardiovascular diseases, hypertension, obesity, cancer, and osteoporosis, among others (Fernandes and Rodrigues Citation2018; Mustafa and Chua Citation2020).
Plant-based prebiotics and probiotics have become especially popular because they provide the same benefits as dairy products with improved digestibility by individuals with lactose intolerance and in virtue of their adaptability to vegetarian and vegan dietary patterns (Mishra, Chakravarty, and Mandavgane Citation2021). In addition, the consumption of fruit and vegetable beverages allows for the delivery of large amounts of functional ingredients easily and periodically, due to the fluid requirements of humans and the possibility of modifying some sensory aspects to improve their acceptability (Min et al. Citation2019). Therefore, nondairy beverages provide a convenient method of consumption of phytochemical compounds typically found in fruits and vegetables.
Fruit juices are attractive matrices for the addition of probiotics because they contain carbohydrates and substrates that can be metabolized by microorganisms for their growth, in addition to their good nutritional profile (Valero-Cases et al. Citation2020). In this context, the development of probiotic and prebiotic beverages with anthocyanin-rich fruits is an exciting alternative for this type of functional food since anthocyanins have antioxidant properties (Leong et al. Citation2018), antimicrobial activity against pathogens (Igwe et al. Citation2019; Lacombe and Wu Citation2017), and prebiotic potential (Lavefve, Howard, and Carbonero Citation2020; Yang and Kortesniemi Citation2015).
The development of fruit-based probiotic and synbiotic beverages can be challenging due to several factors, including the acidity of the fruit juices, the antibacterial activity of fruit metabolites, and the induction of changes in sensory attributes. The effect of anthocyanins on gut microbiota (Faria et al. Citation2014; Morais et al. Citation2016; Igwe et al. Citation2019; Tian et al. Citation2019; Wang et al. Citation2022) and the inclusion of probiotics and synbiotics in fruit beverages (Perricone et al. Citation2015; Rovinaru and Pasarin Citation2020; Valero-Cases et al. Citation2020; Keșa et al. Citation2021; Ruiz et al. Citation2021) have been reviewed separately. The aim of the present article is to critically review the technological strategies that have been reported for the development of probiotic and synbiotic beverages based on anthocyanin-rich fruits. Key aspects, such as the viability of probiotic cultures in this type of matrices, their commercial potential in terms of the physicochemical and sensory quality observed in the resulting products, and their beneficial health effects, are reviewed and discussed based on the most recent evidence in literature.
2. Functional properties of anthocyanin-rich fruits
Many of the anthocyanin-rich fruits are known as red fruits or berries, which refer to small, sweet, sour, juicy, and intensely colored (usually red, purple, or blue) fruits that grow on wild bushes and can be eaten whole (Hidalgo and Almajano Citation2017). These fruits have a high content of dietary fiber, a pleasant taste and smell, and a high content of bioactive phenolic compounds with antioxidant properties, especially anthocyanins (Skrovankova et al. Citation2015).
The consumption of anthocyanin-rich fruits has been associated with beneficial effects regarding the aging process and the reduction of appearance and development of non-communicable diseases associated to oxidative stress, including certain types of cancer, diabetes, atherosclerosis, neurodegenerative disorders, and cardiovascular diseases (Li et al. Citation2017; Pisoschi and Negulescu Citation2011). In addition to this, the use of anthocyanins in other food matrices has been proven to offer protection against oxidation reactions related to the spoilage of food products (Durazzo et al. Citation2019).
More recently, there has been evidence gathered on the potential of anthocyanins to modify the intestinal microbiota (Lavefve, Howard, and Carbonero Citation2020). Indeed, anthocyanins can have inhibitory effects on a wide variety of pathogenic bacteria including Gram-negative (Citrobacter freundii, Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica ser. typhimurium) and Gram-positive bacteria (Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, and Enterococcus faecalis) (Jamar, Estadella, and Pisani Citation2017). Furthermore, anthocyanins stimulate or do not affect the growth of benefitial microorganisms, such as Bifidobacterium and Lactobacillus species, thus favoring the proliferation of beneficial gut microorganisms (Yang and Kortesniemi Citation2015).
Coman et al. (Citation2018) proved that extracts from plum and grape peels and different parts of elderberry that were rich in anthocyanins inhibited the growth of various pathogens (Bacillus cereus, L. monocytogenes, S. aureus, E. coli, and Candida albicans) and stimulated the growth of probiotic microorganisms (Lacticaseibacillus rhamnosus IMC 501®, Lacticaseibacillus paracasei IMC 502®, and Lactiplantibacillus plantarum IMC 509), both axenic and conglomerate. Similarly, Yang et al. (Citation2014) observed a significant growth inhibition of L. monocytogenes, S. typhimurium, and E. coli O157:H7 when supplementing nutrient broth and milk with 10% blackberry juice; at the same time, they reported the stimulation of the growth of Lacticaseibacillus casei, L. plantarum, and L. rhamnosus in the same media. This study indicated that diluted blackberry juice could have an antimicrobial effect against pathogenic microorganisms and a prebiotic potential upon probiotic microorganisms.
Moreover, bacteria of genera Bifidobacterium and Lactobacillus can metabolize anthocyanins, releasing molecules that are expected to have an improved biological activity (like phenolic acids), compared to anthocyanins themselves, due to a higher bioavailability, which could ultimately contribute to the positive health outcomes provided by these compounds (Cheng et al. Citation2016; Danneskiold-Samsøe et al. Citation2019; de Souza et al. Citation2019).
The metabolization of anthocyanins involves hydrolysis, demethylation, reduction, decarboxylation, dehydroxylation, or isomerization reactions of these compounds to obtain simpler compounds that improve their absorption and biological activity (Jamar, Estadella, and Pisani Citation2017). Most intestinal bacteria of Bifidobacterium and Lactobacillus species possess the enzyme β-glucosidase, which catalyzes the degradation of anthocyanins and gives them the ability to metabolize these compounds during their growth, obtaining energy and enriching the growth medium with glucose (Lacombe and Wu Citation2017).
Indeed, Hidalgo et al. (Citation2012) evaluated the metabolism of anthocyanins present in grape wine by the human fecal microbiota, finding that anthocyanins were almost completely degraded in their presence. Also, the appearance of different phenolic compounds was observed, evidencing fermentative processes that positively modulated the gut microbiota by increasing the counts of Bifidobacterium and Lactobacillus, even to a higher extent than FOS.
The metabolites obtained in the degradation of anthocyanins are as diverse as the anthocyanins and microorganisms that constitute intestinal microbiota; however, some of the commonly found metabolites include benzoic acids, such as syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid), 3-O-methyl-gallic acid (3,4-dihydroxy-5-methoxybenzoic acid), and vanillic acid (4-hydroxy-3-methoxybenzoic acid), and hydroxycinnamic acids, such as ferulic acid (4-hydroxy-3-methoxycinnamic acid). Further O-demethylation of these phenolic acids is also possible, leading to gallic (3,4,5-trihydroxybenzoic acid), protocatechuic (3,4-dihydroxybenzoic acid), and caffeic (3,4-dihydroxycinnamic acid) acids (González et al. Citation2017). Degradation of these compounds yield catechol, pyrogallol, resorcinol, tyrosol, 3-(3′-hydroxyphenyl) propionic acid, dihydrocaffeic acid, and lactic acid (Tian et al., Citation2019). These metabolites are believed to be the true bioactive compounds in the human body (Lavefve, Howard, and Carbonero Citation2020).
Cheng et al. (Citation2016a) evaluated the bioconversion of mulberry anthocyanins by intestinal probiotics (Lactobacillus acidophilus GIM 1.83, Lactobacillus delbrueckii subsp. bulgaricus GIM 1.155, Bifidobacterium animalis GIM 1.169, L. plantarum GIM 1.35, and Streptococcus thermophilus). In this study, a high production of β-glucosidase and bioconversion of anthocyanins to chlorogenic, crypto-chlorogenic, caffeic, and ferulic acids were observed under anaerobic conditions. Boto-Ordóñez et al. (Citation2014) evaluated the relationship between the fecal microbiota and the changes in the content of phenolic metabolites in the urine, after the consumption of red wine, in 9 volunteers. In this study, an increase in Bifidobacterium, Enterococcus, and Eggerthella species was observed. The increase in Bifidobacterium species was highly correlated with the increase in the concentration of syringic, p-coumaric, 4-hydroxybenzoic, and homovanillic acids in urine, with all of these being metabolites from the degradation of anthocyanins. This suggests that anthocyanin-rich fruits could be an interesting matrix for the inclusion and growth of probiotic microorganisms, with safety and health features.
3. Critical aspects for the development of probiotic and synbiotic beverages with anthocyanin-rich fruits
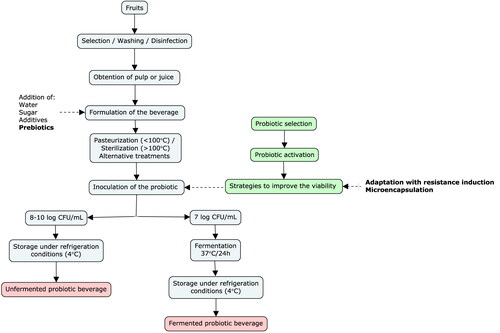
The general stages for developing a probiotic fruit beverage are summarized in . The production of a fruit beverage with anthocyanins, and its inclusion with probiotic microorganisms, begins with the selection of fruits with the highest possible content of anthocyanins to obtain the greatest amount of anthocyanins in the final beverages. The anthocyanin content and profile of berries that have been traditionally used for juice manufacturing are presented in . Among the fruits with higher reported concentration of total anthocyanins are several species of blackberries, in particular R. jamaicensis (Bowen-Forbes, Zhang, and Nair Citation2010), raspberries from the species R. occidentalis (Kula and Krauze-Baranowska Citation2016) and R. racemosus (Bowen-Forbes, Zhang, and Nair Citation2010), blueberries of the species V. myrtillus (Müller, Schantz, and Richling Citation2012), mulberries of the species M. alba (Chen et al. Citation2022), E. edulis or juçará (Vieira et al., Citation2017), and elderberries of the species S. nigra (Veberic et al. Citation2015), whose content can be above 0.5% of the fresh pulp. The concentration of fermentable sugars may play a role as simple carbohydrates can be substrates for probiotic microorganism populations. Arguably, a higher concentration of fermentable sugars and anthocyanins in the juice or pulp could be used as a preliminary indicator of the fruit potential for the development of prebiotic and probiotic fermented beverages (Swain et al. Citation2014). Figure S1 (supplementary material) presents a correlation between typical contents of total anthocyanins and fermentable carbohydrates of the fruits listed in in their ripe stage. Comparatively, the fruits with higher reported contents of anthocyanins are characterized by lower sugar concentrations, as in the case of juçará, S. nigra, V. myrtillus, R. occidentalis, and M. alba, display good potential for the development of this type of beverages, due to their relatively high concentration of anthocyanins (>0.5%) and sugars (>4%). Moreover, the lactic fermentability of fruits with high content of anthocyanins but low content of sugars could be improved by supplementation with fermentable carbohydrates or even honey, as demonstrated in other beverage models (Machado et al. Citation2017; Zielińska et al. Citation2021); however, the results of sugar supplementation are highly dependent on the overall chemical composition of the fruit and the fruit-probiotic combination (Machado et al. Citation2017).
Table 1. Anthocyanin content of berries commonly used in the production of juices and beverages.
Another relevant criterion for the selection of the anthocyanin-rich fruits can be the stability of anthocyanins toward thermal, pH-driven, or photooxidative degradation, which varies depending on the anthocyanin profile and the presence of other antioxidant compounds in the fruits (Oancea Citation2021). The glycosylated anthocyanins, which are largely the most common forms in fruits and plant tissues in general, are more stable than the corresponding aglycones (anthocyanindins). As shown in , the 3-O-glycosides of cyanidin are the predominant anthocyanins of most species of berries, including several species of blackberries, raspberries, mulberries, elderberries, Andean blueberry, and corozo. Other common anthocyanins include 3-glycosides of malvidin (in some blueberries and grapes), pelargonidin (in some strawberries and raspberries), and delphinidin (especially in blueberries of the species V. myrtillus). These monoglycosides are still very susceptible to heat loss, ranging from 30% to 80% (Oancea Citation2021). The glycosyl acylated forms have been proven to be more thermally stable (Zhao et al. Citation2017), but few of these forms are naturally present in some fruits. The presence of acylated anthocyanins pelargonidin-3-malonyl-glucoside in strawberries (Veberic et al. Citation2015) or cyanidin-3-malonyl-glucoside in corozo (Osorio et al. Citation2010) and R. jamaicensis or R. fructicosus blackberries (Bowen-Forbes, Zhang, and Nair Citation2010) might offer advantages in terms of stability toward heat processing in their juice manufacturing.
After selecting, washing, disinfecting, and obtaining the pulp or juice (generally by pressing), the beverage formulation is developed. The formulation is defined by the proportions of the ingredients, such as water, pulp or fruit juice, sugar, antioxidants, and prebiotic compounds. The acidity of the formulation is critical, as it is one of the most limiting factors of the viability of the probiotic culture, as discussed later.
Another important limitation is the fact that treatments must be carried out on the products to eliminate or reduce spoiling microbiological loads on the beverages. These treatments can have a deleterious effect on the anthocyanin concentration. More importantly, the inclusion (inoculation) of the desired microorganisms becomes one of the final steps of the product manufacturing (post microbial-reduction treatment), which in turn could increase the risk of microbiological contamination and reduce the storage stability during shelf life. Heat treatments are generally used for this purpose; however, emerging technologies, including hydrostatic pressure, pulsed electric fields, ultrasound, pulsed light, ultraviolet light, and cold plasma, can be used as alternatives to high temperatures. Hypothetically, these emerging technologies are milder in terms of their effect on the bioactive compounds’ concentrations (Morales-de la Peña, Welti-Chanes, and Martín-Belloso Citation2019).
Finally, the probiotic inoculation and the subsequent processing strategies will depend on whether a fermented or an unfermented beverage is intended to be developed. Nondairy probiotic beverages are produced without fermentation to avoid possible undesirable sensory properties typically occurring during fermentation processes. Such undesirable sensory properties include the development of acidity and changes in viscosity, texture, color, taste, and odor (Ranadheera et al. Citation2017). On the other hand, fermentation enhances the production of beverages with lower sugar contents and the adaptation of microbial strains to the media, consequently increasing their viability. The synthesis of metabolites that can improve the quality and safety of the product, such as bacteriocins, short-chain organic acids, and/or some bioactive compounds, is also expected in fermented beverages (Fernandes and Rodrigues Citation2018; Pimentel et al. Citation2019).
During the fermentation process, the metabolization of the beverage constituents and the growth of the population of inoculated probiotic microorganisms are expected. Still, under refrigeration conditions, a decrease in colony forming units (CFU) is usually observed during storage. For this reason, 7 and 8 log CFU/mL of the probiotic microorganisms are usually inoculated, aimed at guaranteeing the minimum recommended dose of probiotics associated with health benefits (6–7 log CFU/mL) (Valero-Cases et al. Citation2020). If fermentation is not carried out, only a minimum growth of the population of probiotics could be expected, and more commonly, a decrease occurs during storage. Therefore, initial inoculums should be higher, generally between 8 and 10 log CFU/mL. summarizes the studies on the development and evaluation of anthocyanin-rich fruits beverages with different probiotic microorganisms, discriminating the attempts done with and without fermentation steps.
Table 2. Studies carried out on the development of probiotics and synbiotic beverages with anthocyanin-rich fruits.
4. Strategies to improve the viability of probiotics in anthocyanin-rich fruit juices
Despite the great potential that anthocyanins present upon the modulation of the microbiota, assuring the viability of probiotic microorganisms in anthocyanin-rich matrices is more challenging than in dairy products. The viability of probiotics can be affected by several factors. Matrix parameters, such as pH/acidity, dissolved oxygen, water activity, and the presence and concentration of phenolic compounds, dietary fiber, fermentable sugars, antimicrobial compounds, and additives (such as flavorings and colorants), can affect the adaptability and survival of microbial populations (Flach et al. Citation2018). Variables of the processing methods, especially temperature changes during heat treatments, cooling rate, and even packaging material and storage methods, exert an effect on viable counts of probiotics. In addition, the selected probiotic strain, the initial inoculum, and incubation temperature and period are also critical parameters (Lebaka et al. Citation2018). Perricone et al. (Citation2015) suggest the following as strategies to improve the viability of probiotics in fruit juices: storage under refrigeration, adaptation with resistance induction, addition of prebiotics, and microencapsulation. Thereafter, some of the strategies used to overcome the difficulties expected in inoculating fruit beverages with probiotic microorganisms are discussed below, emphasizing those previously evidenced as successful in anthocyanin-rich fruits.
Selection of the probiotic strain
The strain selection is a determining step in developing probiotic beverages with adequate viability to guarantee the minimum recommended dose to provide health benefits at the end of the product shelf life. Despite the diversity of strains to which probiotic potential has been attributed, the development of probiotic and synbiotic beverages with anthocyanin-rich fruits has been mainly carried out with bacteria from the Lactobacillus and Bifidobacterium genera (). However, the use of strains of Streptococcus, Pediococcus, Bacillus, and some yeasts of the Saccharomyces genus has also been reported. The wider use of strains of the Lactobacillus genus in the development of these beverages can be attributed to the fact that plant materials are a common source of isolation of these lactic acid bacteria. Indeed, many probiotic Lactobacillus strains are autochthonous to fruits and vegetables and are therefore expected to be more resistant to the physicochemical features of such matrices, with their low pH being the most limiting characteristic (Castillo-Escandón et al. Citation2019).
The sensitivity of probiotics to acidic media seems to depend on the strain and genus under study. For instance, the Lactobacillus genus generally resists acidic media and survives at a pH between 3.7 and 4.3. At the same time, Bifidobacterium is less tolerant to acidity, and a pH of approximately 4.6 is detrimental to their survival (Perricone et al. Citation2015). Therefore, the selection of an adequate probiotic strain or conglomerate, in terms of their resistance to acidic media, is a key point for the development of fruit-based probiotic beverages, characterized by low values of pH (Sheehan, Ross, and Fitzgerald Citation2007). In this regard, Nualkaekul, Salmeron, and Charalampopoulos (Citation2011) evaluated the viability of B. longum in unfermented orange, grapefruit, pineapple, blackcurrant, pomegranate, and strawberry beverages. These authors found that the strain was able to survive and be metabolically active in orange, grapefruit, blackcurrant, and pineapple beverages for 6 weeks at 4° C. In contrast, it was not detectable at the end of the same period in the pomegranate and strawberry beverages, which was attributed to their lower pH and higher content of phenolic compounds. Sheehan, Ross, and Fitzgerald (Citation2007) evaluated the viability of strains of L. salivarius, L. paracasei, L. rhamnosus, L. casei, and B. animals ssp. lactis in unfermented orange, pineapple, and cranberry juices. They observed that the L. paracasei and L. rhamnosus strains presented a higher viability in pineapple and orange beverages, exceeding 6 log CFU/mL at the end of 12 weeks, whereas none of the strains was detected in the cranberry juice. Interestingly, the authors reported that after pH adjustment, L. paracasei was able to survive in this medium with 7 log CFU/mL at the end of 9 days at 4 °C.
Promoting fermentation, especially with native cultures and conglomerates, appears to favor a higher viability of probiotic microorganisms and the achievement of the CFU count levels recommended at the end of the shelf life necessary to exert health benefits. di Cagno et al. (Citation2011) evaluated the viability of conglomerate cultures of P. pentosaceus, L. plantarum, and W. cibaria isolated from different fruits (blackberries, prunes, kiwis, papaya, and fennel) in a fermented smoothie of cherries, tomatoes, blackberries, and prunes. In their study, the fermentation with autochthonous conglomerate cultures allowed them to obtain a high CFU count (9 log CFU/mL) at the end of 30 days of storage at 4 °C.
Adjusting (increasing) the pH of the beverage using NaOH or Na2CO3 could be a strategy to enhance the viability of probiotic microorganisms in low pH matrices (Sheehan, Ross, and Fitzgerald Citation2007; Wu et al. Citation2021; Yan et al. Citation2019). However, there is evidence that increasing the pH of growth medium containing anthocyanins could negatively affect the viability of some probiotic species (Mustafa, Chua, and El-Enshasy Citation2019). Moreover, it is essential to gather more evidence regarding its effect on the nutritional value and the sensory and safety quality of the products. An interesting alternative to adjust the pH is to modulate it via formulation, namely, using fruits with lower acidity than the anthocyanin-rich fruits, such as papaya, as proposed by Bernal-Castro, Díaz-Moreno, and Gutiérrez-Cortés (Citation2019). This alternative avoids the possible negative impact of alkalinizing additives on nutritional, sensory, and safety aspects.
presents the viability of different probiotic microorganisms in the studies dealing with their incorporation in anthocyanin-rich beverages. In sum, the microorganism species that presented better viability in anthocyanin-rich fruit beverages were L. paracasei, L. casei, L. rhamnosus, L. plantarum, and L. delbrueckii, in addition to being the most widely used. In contrast, the probiotic microorganisms with lower viability in these matrices were L. salivarius, B. longum, and B. animalis. Unfortunately, there is very limited information regarding less studied species and specific strains with probiotic potential isolated from the same fruit matrices.
Storage under refrigeration
The viability of probiotic microorganisms is generally affected by storage temperature. Refrigeration (4 °C) can in fact prolong the survival of these microorganisms in beverages and fruit juices, while thermal abuse can cause adverse effects, as demonstrated by several studies. Perricone et al. (Citation2014), who determined the time necessary to decrease 1 log CFU/mL at storage temperatures of 4 °C and 37 °C of L. reuteri in apple, pineapple, and orange juices, observed a highly significant decrease at 37 °C. Also, Lai, How, and Pui (Citation2020) evaluated the viability of L. rhamnosus in hawthorn berry tea, finding a viability loss of 44.8% and 77.3% in the free probiotic at 4 °C and 25 °C, respectively, after 4 weeks of storage. Thus, a general recommendation is to store probiotic products at temperatures between 4 °C and 5 °C, especially if working with Lactobacillus species, while for Bifidobacterium species, a temperature of 8 °C is recommended (de Oliveira et al. Citation2020).
Freezing temperatures could further preserve the viability of probiotic microorganisms and even prevent the deterioration of bioactive compounds, such as phenolic compounds. This can be particularly positive for anthocyanin preservation, since they are highly unstable under several condition, as demonstrated by Urbano et al. (Citation2019), who developed a juçará sorbet with the inclusion of L. acidophilus and L. paracasei. In their study, they observed a viability of 8.8 log CFU/mL at the end of 120 days of storage at −18 °C with a concentration of phenolic compounds and anthocyanins practically unchanged during the storage time.
Adaptation with resistance induction
Adaptation with resistance induction of probiotic microorganisms consists on subjecting the microorganism to sub-lethal conditions for a limited time before the inoculation in the food matrix. This causes the microorganism to become accustomed to the conditions of the food matrix in a certain way and induce a type of resistance and an adaptive stress response. To achieve this, the growth medium or incubation conditions are usually modified using strategies such as: 1) pH modification to suboptimal conditions to generate an adaptation to acidity, 2) incubation under suboptimal temperatures (15–20 °C) to generate an adaptation to cooling, 3) supplementation of the culture medium with phenolic compounds to induce resistance, and 4) supplementation of the medium with an amount of the food matrix (between 10% and 50%) to increase the viability in the product (Speranza et al. Citation2020).
As discussed above, the most significant limitation in developing probiotic beverages using anthocyanin-rich fruits is acidity. Therefore, Mustafa, Chua, and El-Enshasy (Citation2019) evaluated the effect of pH (2.5, 4.0, and 5.5) on the viability of L. casei in pomegranate juice (pH 3.58), finding that the pH adjustment negatively affected the growth of the probiotic, especially at pH 2.5 and 5.5. They proposed that exposure of probiotics to acid stress prior to incorporation into fruit juices, followed by refrigerated storage, is a viable strategy to reduce cell damage and death during processing, storage, and passage through the gastrointestinal tract. Srisukchayakul, Charalampopoulos, and Karatzas (Citation2018) studied the effect of adaptation with citric acid on the survival of L. plantarum in low pH fruit juice during refrigerated storage. They found that pretreatment with citric acid improves the viability of the probiotic in low pH juices, such as cranberry (pH 2.7), pomegranate (pH 3.5) and lemon-lime (pH 2.8) juices. Furthermore, in this study, an analysis of the fatty acid content of the cells adapted to the acid medium was also carried out, indicating an increase in the content of cyclopropane fatty acids. The authors concluded that this change probably reduced the fluidity and permeability of the cell membrane, thus preventing the flow of protons during storage in these low pH fruit juices.
However, pH alone cannot explain the trends exhibited by some probiotics in different fruit beverages. Nualkaekul, Salmeron, and Charalampopoulos (Citation2011) investigated the factors that affect the viability of B. longum in orange, grapefruit, blackcurrant, pineapple, pomegranate, and strawberry beverages. They observed that after 6 weeks of storage at 4 °C the CFU count decreased 0.8 log CFU/mL in orange, blackcurrant, and pineapple juices, whereas for grapefruit, it was only 0.5 log CFU/mL despite its low pH (3.21). The probiotic population was below detection limits after 1 week in the pomegranate juice and after 4 weeks in the strawberry juice. These results suggest that the viability of the probiotic results from synergistic and antagonistic interactions of different factors, such as the content of phenolic compounds, anthocyanins, organic acids, proteins, sugars, and dietary fibers, among others. In this sense, Perricone et al. (Citation2014) evaluated the viability of L. reuteri in pineapple, orange, green apple, and red fruit juices. They evidenced that the strain under study presented a considerable loss of its viability in red fruit juice, arguably due to the effects of pH, but also the phenolic composition. Subsequently, they inoculated the strain in a culture medium containing red fruit juice, vanillic acid (to evaluate phenolic stress), or acidifying agents (to evaluate acid stress at pH 5), with which prolongation of the viability of the strain was achieved.
Addition of prebiotics
An alternative that has been proposed to improve the viability and stability of probiotics in fruit beverages consists of the inclusion of prebiotic compounds, which would further enable the ability to obtain a synbiotic functionality in the products. Some of the studies dealing with the development of probiotic beverages containing anthocyanins () have resorted to this strategy. According to these studies, the addition of prebiotic compounds improves and/or increases the viability of probiotics during fermentation and storage at refrigeration temperature, reaching satisfactory levels (>6 log CFU/mL) in different synbiotic products. Hesam et al. (Citation2020) observed an increased viability of B. animals spp. lactis in unfermented pomegranate juice using XOS than without its use. They evidenced metabolic process in the synbiotic beverage by a more significant decrease in pH and increase in titratable acidity. Also, Freitas et al. (Citation2021) concluded that the use of FOS and sucrose improved the viability of L. casei in fermented açai beverages.
However, this was not the case in all of the anthocyanin-rich fruit beverages with low pH, which has been attributed to a more hostile environment of the matrix and its diverse composition. Marín-Arango et al. (Citation2019) did not observe an evident effect when using inulin in the development of a blackberry beverage inoculated with L. casei. They found that the viability of the microorganism was mainly affected by the proportion of blackberry concentrate in the beverages and the amount of inoculum, with the low proportions of blackberry concentrate (10%) and the highest amount of inoculum (7.49 log CFU/g) presenting the best viability (7.13 log CFU/g). Moreover, Urbano et al. (Citation2019) found that the use of polydextrose in the development of a jussara sorbet with the inclusion of L. acidophilus and L. paracasei did not affect the viability of the probiotics in the product stored at −18 °C for 120 days compared to the same sorbet without the addition of polydextrose.
Furthermore, White and Hekmat (Citation2018) found that the viability of L. rhamnosus did not present significant differences throughout the fermentation of grape, apple, and orange beverages for 72 hours at 37 °C with or without using both short-chain and long-chain inulin. This study also observed that the viability in the orange beverage did not present significant differences during storage. In contrast, a decrease in viability was observed during storage in the apple and grape juices, especially in the grape juice, and it was not detectable at the end of 30 days, even with the addition of inulin. The previous implies that, in the development of fruit beverages with anthocyanins, the sole use of prebiotic compounds is not enough to guarantee the viability of probiotic microorganisms. Instead, the composition of the matrix and the probiotic strain used are the factors that affect its viability the most.
Microencapsulation
The use of strategies that provide a means of protection to probiotic microorganisms has been of interest in several studies, in which the encapsulation and coating of these microorganisms have been the main strategies. Microencapsulation involves surrounding probiotic microorganisms with a protective matrix (food grade polymers) using emulsions, extrusion, or spray drying (Sarao and Arora Citation2017). An optimal encapsulation process can protect the microorganism against unfavorable conditions and allow the controlled release of the probiotic and/or its metabolites and the entry of nutrients to obtain a more productive and efficient fermentation process (Speranza et al. Citation2020). Microencapsulation has been used successfully in various matrices to protect probiotics against hostile conditions. It is reported that microencapsulation provides a favorable anaerobic environment for microorganisms sensitive to the external environment, as well as providing a physical barrier against acidic conditions present in fruit juices and during digestion (Perricone et al. Citation2015).
Olivares et al. (Citation2019) evaluated the microencapsulation of L. casei with alginate and its inclusion in pineapple, orange, and raspberry juices, finding that microencapsulation improved viability at the end of 28 days of storage at 4 °C compared to the free probiotic. However, the acidity of the matrix still affects the viability to a lesser extent since the raspberry juice (pH 2.75) presented the lowest viability of the microencapsulated (5 log CFU/mL). On the other hand, Lai, How, and Pui (Citation2020) found that the viability of L. rhamnosus encapsulated with alginate-pectin-flax mucilage decreases by 20% at 4 °C storage for 4 weeks in hawthorn berry tea compared to 45% using the free probiotic. So, the encapsulation managed to improve the viability of L. rhamnosus and obtain more than 6 log CFU/mL at the end of its storage. Furthermore, Nualkaekul et al. (Citation2012) concluded that a chitosan-alginate multilayer increases the protection provided to the probiotic microorganism L. plantarum in acidic food systems, such as the pomegranate juice, which is attributed to the greater thickness of the membrane formed.
Additionally, there is a growing trend toward exploring the microencapsulation of probiotics using compounds with prebiotic properties as encapsulation agents. The rationale is to provide a carrier protection for increasing the number of viable cells in fruit beverages with low pH. This strategy has favored the viability of microencapsulated probiotics during storage in unfavorable food matrices and under gastrointestinal conditions, enhancing the number of cells to remain above the recommended therapeutic minimum. In this sense, Fratianni et al. (Citation2014) evaluated the development of a berry beverage inoculated with S. cerevisiae boulardii, a yeast with probiotic potential, microencapsulated with alginate-inulin-xanthan gum. They found that this microencapsulation significantly improves the viability of the yeast after fermentation and storage (>7 log CFU/mL). It was also found that the microencapsulation adsorbed phenolic compounds and anthocyanins, protecting these compounds during fermentation and storage. Mantzourani et al. (Citation2019 & 2020) evaluated pomegranate and cornelian cherry juice fermentation with L. paracasei immobilized with delignified wheat bran, showing that the concentration of organic acids increases during storage time while the sugar content decreases. In terms of viability, an increase during storage time was observed. This increase was associated with the use of wheat bran as a protector and its prebiotic effect due to its content of XOS originating from the hydrolysis of arabinoxylans present in this matrix.
5. Physicochemical changes produced by the inclusion of probiotics in anthocyanin-rich beverages
In the development of fruit beverages with probiotics, it is also essential to consider the physicochemical and sensory changes that may occur in the product due to the presence of these microorganisms and their metabolic activity. The changes produced will depend on process conditions, such as temperature, pH, matrix, probiotic strain, and fermentation time. Physicochemical changes commonly observed in fermented beverages are alterations in pH, titratable acidity, sugar content, and phenolic compound content. These physicochemical changes are related to the fact that probiotic cultures are capable of metabolizing sugars and some phenolic compounds, such as anthocyanins and tannins, present in fruit juices. The metabolic activity of probiotics causes the production of organic acids, mainly lactic and acetic acids, with the consequent reduction in pH and increase in titratable acidity and the production of simpler phenolic compounds that can present an antioxidant activity greater than that of its precursors (Ruiz et al. Citation2021).
In this regard, Yan et al. (Citation2019) found that a 24-hour fermentation at 37 °C with L. rhamnosus and L. plantarum of a pH-adjusted blueberry pomace beverage caused the production of lactic and acetic acids with the consequent decrease in pH (from 6.2 to 3.8) and increase in titratable acidity (from 4% to 16%), especially when glucose was added. Furthermore, in this study, an increase in phenolic compounds (from 1066.89 μg GAE/mL to 4269.21 μg GAE/mL) and flavonoids (from 81.71 μg RE/mL to 404.99 μg RE/mL) and a decrease in the content of anthocyanins (from 15.41 μg C3G/mL to 5.44 μg C3G/mL), were observed at the end of fermentation period, which resulted in a slight increase in the antioxidant activity of the product (from 205.15 mmol Trolox/L to 269.82 mmol Trolox/L). Furthermore, Wu et al. (Citation2021) observed a decrease in anthocyanin content when fermenting blueberry and blackberry beverages with L. plantarum, S. thermophilus, and B. bifidum for 48 hours at 37 °C, in addition to the increase in phenolic and lactic acid contents and the antioxidant activity of the fermented product.
Fermentation with probiotics, in part, is desired as it increases the product shelf life, safety, and the CFU of the probiotic culture. However, it may decrease consumer acceptability and affect the storage viability of the probiotic culture due to acidification. Therefore, it is recommended to use probiotic cultures with tolerance to acidic environments and slow metabolic activity or to develop the product avoiding the fermentation process so that physicochemical changes are not observed. In this sense, studies, such as those carried out by de Oliveira et al. (Citation2020), Lai, How, and Pui (Citation2020), Nualkaekul, Salmeron, and Charalampopoulos (Citation2011), and Perricone et al. (Citation2014), show that a slight decrease in pH and sugar content can be observed during storage under refrigeration conditions of unfermented fruit beverages with anthocyanins and inclusion of probiotic cultures. Still, these changes are not statistically significant because probiotic microorganisms under refrigeration conditions significantly slow down their metabolic activity.
During storage under refrigeration conditions of anthocyanin-rich fruit beverages with probiotics, it is generally observed that the content of total phenolic compounds, anthocyanins, and antioxidant activity decreases. This is strongly related to the low stability of this type of compound against several conditions, such as pH, oxygen, light, and temperature, among others, promoting its degradation over time (Leong et al. Citation2018). de Oliveira et al. (Citation2020) observed a decrease in the anthocyanin content of a banana, strawberry, and juçara beverage inoculated with L. casei and L. plantarum in storage at 4 °C for 90 days. On the other hand, Urbano et al. (Citation2019) found that the content of anthocyanins is not affected in a jussara sorbet with the inclusion of L. acidophilus, L. paracasei, and polydextrose stored at −18 °C for 120 days because low temperatures affect the stability of anthocyanins to a lesser extent. In addition, Lai, How, and Pui (Citation2020) showed that the encapsulation of probiotic microorganisms also protects the phenolic compounds and anthocyanins, which are absorbed in the coating material, thus generating a lower decrease in these compounds compared to the use of free probiotics.
6. Sensory changes produced by the inclusion of probiotics in anthocyanin-rich beverages
Sensory properties are another critical factor in the development of probiotic products. The incorporation of probiotics can cause alterations in the taste, aroma, color, and texture of the product during fermentation and storage and, therefore, change the acceptability of the product (Min et al. Citation2019). For example, it has been reported that the addition of probiotics to fruit juices can generate flavors described as dairy, medicinal, acidic, salty, bitter, astringent, absent of sweetness, and artificial. These sensory changes depend on the type of fruit, probiotic strain, storage temperature, and use of prebiotics or encapsulation strategies (Lebaka et al. Citation2018). For this reason, an adequate sensory evaluation of the probiotic product is essential to obtain a successful product formulation.
A possible solution to the adverse effects on the acceptability of probiotic beverages may be the addition of pleasant compounds with the ability to mask the changes in the organoleptic properties produced by the addition of probiotics. Luckow et al. (Citation2006) confirmed that probiotics have a significant effect on the sensory quality and acceptability by consumers of this type of beverage and evaluated the impact of adding tropical juices (mainly pineapple, mango, and passionfruit) to mask the off-flavor produced by the addition of probiotics in orange juice. Their results showed that tropical juices effectively masked the off-flavor associated with probiotics and that consumers’ preference for this type of beverage increased and was maintained over time. Furthermore, tropical fruits, such as pineapple, mango, and passionfruit, have also been successfully used to mask the medicinal taste caused by probiotics and improve the sensory quality and acceptability of probiotic juices by Pimentel et al. (Citation2019). In this sense, anthocyanin-rich fruit beverages could naturally mask the off-flavor produced by probiotics. For this reason, most drinks with anthocyanins and probiotics show good acceptability, even higher than products without the inclusion or fermentation of these microorganisms (Hesam et al. Citation2020; Mantzourani et al. Citation2019; Wu et al. Citation2021).
Perricone et al. (Citation2014) showed that the addition of L. reuteri to apple, pineapple, orange, and red fruit juices did not negatively impact the sensory attributes of these beverages. Furthermore, di Cagno et al. (Citation2011) found that there was no significant difference between an inoculated and a non-inoculated red smoothie with a conglomerate probiotic culture in terms of acidity, astringency, and appearance; however, the non-inoculated smoothie had better acceptability, which is mainly attributed to a lesser color change, although good acceptability was also found for the smoothie inoculated with probiotics. Finally, it is noteworthy that non-sensory factors, such as informing the consumer about the health benefits of this type of functional beverage, also significantly influence the acceptability of the product. In this regard, Luckow et al. (Citation2006) determined that non-sensory factors, such as repetitive consumption and providing information on health benefits given by the product, positively impact the consumer’s liking for an orange probiotic beverage.
7. Evidence of synergistic effects of probiotics and anthocyanins on gut microbiota and other health effects
The consumption of probiotics as functional foods has been a promising treatment strategy against various diseases (gastrointestinal diseases, heart diseases, cancer, neuro-inflammatory diseases, and allergies, among others); however, the effects of probiotics on human health are specific to the strain and depend on the initial immunological competence of the host (Varsha, Maheshwari, and Nampoothiri Citation2021). The World Health Organization has declared that probiotics act as activators of the immune system by stimulating the human intestinal microbiota through the production of effector substances such as bacteriocins, peptidoglycan, teichoic acid, exopolysaccharides, bioactive peptides, and short-chain fatty acids (Gao et al. Citation2022). Recently, the consumption of probiotics has been proposed as an alternative to the growing crisis of antibiotic resistance and as potential immunomodulators in the management of SARS-COVID-19 infection (Singh and Rao Citation2021; Tegegne and Kebede Citation2022).
Imbalance in gut microbiota composition can cause several degenerative diseases, such as obesity, diabetes, cancer, cardiovascular diseases, liver diseases, and inflammatory bowel diseases (IBD). The consumption of probiotic microorganisms modulates the gut microbiota by increasing the population of beneficial bacteria, function of the intestinal epithelial barrier, and producing cytokines, helping to prevent diseases caused by gut microbiota dysbiosis (Azad et al. Citation2018).
Anthocyanins have antioxidant and anti-inflammatory properties, can protect DNA, and improve intestinal health (Chandra et al. Citation2019). Indeed, anthocyanin consumption has been reported to reduce cholesterol, low-density lipoprotein (LDL), and triglycerides (Bakuradze et al. Citation2019). Also, consumption of polyphenol-rich fruits has been associated with the prevention of diseases caused by cellular oxidative damage, such as atherosclerosis, type 2 diabetes, and cancer (Bakuradze et al. Citation2019).
There is an increasing pool of evidence on the beneficial modulatory effect of the intake of anthocyanins on gut microbiota (Faria et al. Citation2014; Morais et al. Citation2016; Igwe et al. Citation2019; Tian et al. Citation2019; Wang et al. Citation2022). In vitro, animal, and human trials showed that the anti-inflammatory effect of anthocyanins could be associated to an increase in gut Bifidobacterium strains (Morais et al. Citation2016; Igwe et al. Citation2019). Moreover, the biotransformation of anthocyanins to low molecular weight metabolites with higher bioactive potential and modulation of gut microbiota composition properties contribute to health benefits provided by anthocyanins (Lavefve, Howard, and Carbonero Citation2020). In this way, beverages with a combination of probiotics and anthocyanins can improve the beneficial effects provided by these phytochemicals and these microorganisms in a synergistic way. summarizes a set of studies that have provided evidence on the possible beneficial effects on health provided by a matrix rich in anthocyanins with the inclusion of probiotic microorganisms.
Table 3. Evidence of beneficial health effects provided by combinations of anthocyanin-rich matrixes and probiotics.
González et al. (Citation2017) demonstrated in an in vitro study that a phenolic extract from red wine (containing anthocyanins) improves the adherence of probiotic strains to Caco-2 cells, preventing the adherence of E. coli to these cells. This indicates that wine polyphenols and probiotics can improve the benefits of gut health, inhibiting the proliferation of pathogens, in a synergistic way. Yan et al. (Citation2019) found that a fermented blueberry pomace liquid with L. rhamnosus and L. plantarum can produce a cholesterol-clearance up to 67.17%. This study also observed an improvement in the physical strength of mice fed with the fermented juice for one month, using a weight-loaded mice swimming experiment. Therefore, Yan et al. (Citation2019) proved that a probiotic blueberry pomace beverage may have cholesterol-lowering capabilities and anti-fatigue properties. (Zhu et al., Citation2016) found that the combination of blueberry juice and probiotics attenuates the apoptosis of alcoholic fatty liver disease (AFLD), using mice models, better than a control group fed only with blueberry juice. These results were attributed to the reduction of lipid deposition, inflammation, necrosis, and fibrosis in hepatic tissues for the enhancement of superoxide dismutase enzyme (SOD) activity.
Furthermore, Esmaeilinezhad et al. (Citation2019) observed an increase in insulin sensitivity and a reduction in body mass index, weight, waist circumference, and testosterone level in 86 patients with polycystic ovarian syndrome that received 2 L of a synbiotic pomegranate juice (using Lacticaseibacillus rhamnosus, Weizmannia coagulans, and Mucor indicus as probiotics) weekly. This indicates that the combined consumption of probiotics and anthocyanin-rich fruit juices can improve the symptoms of a common reproductive, endocrine, and metabolic disease in women. Even though the heath benefit provided by the consumption of probiotics and anthocyanins depends on the physicochemical properties of the carrier foods and ingredients used, there is evidence that the combination of anthocyanin-rich fruits and probiotic bacteria has a synergistic effect against several diseases related to oxidative damage and gut microbiota dysbiosis. The existing evidence is promissory; however, the changes in gut microbial ecology in specific relation to dietary intake of anthocyanins are not yet fully understood, and further clinical trials in humans are required to support related health benefit claims to consumers (Igwe et al. Citation2019; Tian et al. Citation2019).
8. Conclusions and future perspectives
There is enough evidence on the feasibility of developing anthocyanin-rich fruit beverages with the inclusion of probiotic microorganisms at the recommended doses expected to provide health benefits to the consumer. The most significant limitations in developing this type of beverage are the high acidity of anthocyanin-rich fruits and their high content in phenolic compounds. However, these limitations can be overcome with the proper selection of a probiotic strain and the use of subsequent strategies for improving their media adaptation. The pH modulation by the addition of less acidic fruits to the beverages might be another promising approach. Promoting fermentative processes mediated by the probiotic cultures, in periods around 24 hours, has demonstrated to increase their viability in the beverages, while improving the safety and sensory profile, and potentially increasing the antioxidant capacity of the final product. On the other hand, the inoculation of probiotics without fermentation induction allows for a product with milder changes in terms of physicochemical and sensory quality features. There is evidence that a combination of anthocyanin-rich fruits and probiotics provide health benefits in a synergistic way; however, more studies are required to support health effect claims for consumer products.
Very limited research has been carried out on the microbiological quality and shelf life of these type of products, especially in unfermented products, bearing in mind that the inoculation is done after the beverage treatment. The evaluation of ultrasound treatments, high hydrostatic pressures, and other emerging technologies before and after the inoculation, as alternatives to conventional heat pre-processing, is a thriving field for research, not only for product preservation but also regarding their potential as fermentation enhancers. The use of native strains with probiotic potential, specific to each fruit variety, could represent an advantage in terms of adaptability to the matrix. Finally, it is pivotal to carry on studies aimed at assessing the expected health benefits provided by these products (probiotic, prebiotic, or synbiotic) by means of in vitro and in vivo tests and clinical trials.
Supplemental Material
Download MS Word (139.6 KB)Disclosure Statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Ali, L., B. Svensson, B. Alsanius, and M. Olsson. 2011. Late season harvest and storage of Rubus berries—Major antioxidant and sugar levels. Scientia Horticulturae 129 (3):376–81. doi: 10.1016/j.scienta.2011.03.047.
- Azad, M., M. Sarker, T. Li, and J. Yin. 2018. Probiotic species in the modulation of gut microbiota: An overview. BioMed Research International 2018:9478630–8. doi: 10.1155/2018/9478630.
- Bakuradze, T., A. Tausend, J. Galan, I. Groh, D. Berry, J. Tur, D. Marko, and E. Richling. 2019. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radical Research 53 (sup1):1045–55. doi: 10.1080/10715762.2019.1618851.
- Bernal-Castro, C., C. Díaz-Moreno, and C. Gutiérrez-Cortés. 2019. Inclusion of prebiotics on the viability of a commercial Lactobacillus casei subsp. rhamnosus culture in a tropical fruit beverage. Journal of Food Science and Technology 56 (2):987–94. doi: 10.1007/s13197-018-03565-w.
- Bordonaba, J., P. Crespo, and L. Terry. 2011. A new acetonitrile-free mobile phase for HPLC-DAD determination of individual anthocyanins in blackcurrant and strawberry fruits: A comparison and validation study. Food Chemistry 129 (3):1265–73. doi: 10.1016/j.foodchem.2010.09.114.
- Boto-Ordóñez, M., M. Urpi-Sarda, M. Queipo-Ortuño, S. Tulipani, F. Tinahones, and C. Andres-Lacueva. 2014. High levels of bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct 5 (8):1932–8. doi: 10.1039/C4FO00029C.
- Bowen-Forbes, C., Y. Zhang, and M. Nair. 2010. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. Journal of Food Composition and Analysis 23 (6):554–60. doi: 10.1016/j.jfca.2009.08.012.
- Brown, P, and P. Shipley. 2011. Determination of anthocyanins in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. Journal of AOAC International 94 (2):459–66. doi: 10.1093/jaoac/94.2.459.
- Brown, P., C. Turi, P. Shipley, and S. Murch. 2012. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Medica 78 (6):630–40. doi: 10.1055/S-0031-1298239.
- Byamukama, R., M. Andima, A. Mbabazi, and B. Kiremire. 2014. Anthocyanins from mulberry (Morus rubra) fruits as potential natural colour additives in yoghurt. African Journal of Pure and Applied Chemistry 8 (12):182–90. doi: 10.5897/AJPAC2014.0594.
- Castillo-Escandón, V., S. Fernández-Michel, M. Cueto-Wong, and G. Ramos-Clamont. 2019. Criterios y estrategias tecnológicas para la incorporación y supervivencia de probióticos en frutas, cereales y sus derivados. TIP Revista Especializada en Ciencias Químico-Biológicas 22 (0):1–17. doi: 10.22201/fesz.23958723e.2019.0.173.
- Chandra, P., A. Rathore, K. Kay, J. Everhart, P. Curtis, B. Burton-Freeman, A. Cassidy, and C. Kay. 2019. Contribution of berry polyphenols to the human metabolome. Molecules 24 (23):4220. doi: 10.3390/molecules24234220.
- Chen, T., F.-F. Shuang, Q.-Y. Fu, Y.-X. Ju, C.-M. Zong, W.-G. Zhao, D.-Y. Zhang, X.-H. Yao, and F.-L. Cao. 2022. Evaluation of the chemical composition and antioxidant activity of mulberry (Morus alba L.) fruits from different varieties in China. Molecules 27 (9):2688. doi: 10.3390/molecules27092688.
- Cheng, J., X. Liu, Z. Chen, Y. Zhang, and Y. Zhang. 2016. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chemistry 213:721–7. doi: 10.1016/J.FOODCHEM.2016.07.032.
- Cheng, Y., T. Wu, X. Chu, S. Tang, W. Cao, F. Liang, Y. Fang, S. Pan, and X. Xu. 2020. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT 125:109260. doi: 10.1016/j.lwt.2020.109260.
- Coman, M., A. Oancea, M. Verdenelli, C. Cecchini, G. Bahrim, C. Orpianesi, A. Cresci, and S. Silvi. 2018. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. European Food Research and Technology 244 (4):735–45. doi: 10.1007/s00217-017-2997-9.
- Cunningham, M., M. Azcarate-Peril, A. Barnard, V. Benoit, R. Grimaldi, D. Guyonnet, H. Holscher, K. Hunter, S. Manurung, D. Obis, et al. 2021. Shaping the future of probiotics and prebiotics. Trends in Microbiology 29 (8):667–85. doi: 10.1016/J.TIM.2021.01.003.
- Danneskiold-Samsøe, N., H. de Freitas, R. Santos, J. Lemos, C. Cazarin, L. Madsen, K. Kristiansen, G. Pastore, S. Brix, and M. Maróstica. 2019. Interplay between food and gut microbiota in health and disease. Food Research International (Ottawa, Ont.) 115:23–31. doi: 10.1016/J.FOODRES.2018.07.043.
- David, L., V. Danciu, B. Moldovan, and A. Filip. 2019. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 8 (5):114 doi:10.3390/antiox8050114.
- de Oliveira, A., F. dos Santos, K. Olbrich, V. Martins, D. Castro, M. Pessanha, C. Conte, S. de Oliveira, L. de Oliveira, R. de Oliveira, et al. 2020. Development of a probiotic non-fermented blend beverage with juçara fruit: Effect of the matrix on probiotic viability and survival to the gastrointestinal tract. LWT 118:108756. doi: 10.1016/j.lwt.2019.108756.
- de Souza, E., T. de Albuquerque, A. dos Santos, N. Massa, and J. de Brito Alves. 2019. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities – A Review. Critical Reviews in Food Science and Nutrition 59 (10):1645–59. doi: 10.1080/10408398.2018.1425285.
- di Cagno, R., G. Minervini, C. Rizzello, M. de Angelis, and M. Gobbetti. 2011. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiology 28 (5):1062–71. doi: 10.1016/j.fm.2011.02.011.
- Doherty, S., M. Auty, C. Stanton, R. Ross, G. Fitzgerald, and A. Brodkorb. 2012. Application of whey protein micro-bead coatings for enhanced strength and probiotic protection during fruit juice storage and gastric incubation. Journal of Microencapsulation 29 (8):713–28. doi: 10.3109/02652048.2011.638994.
- Dóka, O., G. Ficzek, D. Bicanic, R. Spruijt, S. Luterotti, M. Tóth, J. G. Buijnsters, and G. Végvári. 2011. Direct photothermal techniques for rapid quantification of total anthocyanin content in sour cherry cultivars. Talanta 84 (2):341–6.
- Durazzo, A., M. Lucarini, E. Novellino, P. Daliu, and A. Santini. 2019. Fruit-based juices: Focus on antioxidant properties—Study approach and update. Phytotherapy Research: PTR 33 (7):1754–69. doi: 10.1002/ptr.6380.
- Dzhanfezova, T., G. Barba-Espín, R. Müller, B. Joernsgaard, J. Hegelund, B. Madsen, D. Larsen, M. Martínez Vega, and T. Toldam-Andersen. 2020. Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria × ananassa Duch) genetic resource collection. Food Bioscience 36:100620. doi: 10.1016/j.fbio.2020.100620.
- Erşan, S., J. Berning, P. Esquivel, V. Jiménez, R. Carle, B. May, R. Schweiggert, and C. Steingass. 2020. Phytochemical and mineral composition of fruits and seeds of wild-growing Bactris guineensis (L.) H.E. Moore palms from Costa Rica. Journal of Food Composition and Analysis 94:103611. doi: 10.1016/j.jfca.2020.103611.
- Esmaeilinezhad, Z., S. Babajafari, Z. Sohrabi, M. Eskandari, S. Amooee, and R. Barati-Boldaji. 2019. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 29 (2):201–8. doi: 10.1016/j.numecd.2018.07.002.
- Esquivel-Alvarado, D., R. Munõz-Arrieta, E. Alfaro-Viquez, S. Madrigal-Carballo, C. Krueger, and J. Reed. 2020. Composition of anthocyanins and proanthocyanidins in three tropical Vaccinium species from Costa Rica. Journal of Agricultural and Food Chemistry 68 (10):2872–9. doi: 10.1021/ACS.JAFC.9B01451.
- Fanali, C., M. G. Belluomo, M. Cirilli, V. Cristofori, M. Zecchini, F. Cacciola, M. Russo, R. Muleo, and L. Dugo. 2016. Antioxidant activity evaluation and HPLC-photodiode array/MS polyphenols analysis of pomegranate juice from selected italian cultivars: A comparative study. ELECTROPHORESIS 37 (13):1947–55. doi:10.1002/elps.201500501.
- Faria, A., I. Fernandes, S. Norberto, N. Mateus, and C. Calhau. 2014. Interplay between anthocyanins and gut microbiota. Journal of Agricultural and Food Chemistry 62 (29):6898–902. doi: 10.1021/jf501808a.
- Fazeli, M., S. Bahmani, H. Jamalifar, and N. Samadi. 2011. Effect of probiotication on antioxidant and antibacterial activities of pomegranate juices from sour and sweet cultivars. Natural Product Research 25 (3):288–97. doi: 10.1080/14786419.2010.495068.
- Fernandes, A, and S. Rodrigues. 2018. Turning fruit juice into probiotic beverages. In Fruit juices: extraction, composition, quality and analysis, eds. G. Rajauria, and B. K. Tiwari, 279–87. Cambridge, Massachusetts: Academic Press. doi: 10.1016/B978-0-12-802230-6.00015-1.
- Flach, J., M. van der Waal, M. van den Nieuwboer, E. Claassen, and O. Larsen. 2018. The underexposed role of food matrices in probiotic products: Reviewing the relationship between carrier matrices and product parameters. Critical Reviews in Food Science and Nutrition 58 (15):2570–84. doi: 10.1080/10408398.2017.1334624.
- Filannino, P., L. Azzi, I. Cavoski, O. Vincentini, C. Rizzello, M. Gobbetti, and R. Di Cagno. 2013. Exploitation of the health-promoting and sensory properties of organic pomegranate (Punica granatum L.) juice through lactic acid fermentation. International Journal of Food Microbiology 163 (2-3):184–92. doi: 10.1016/j.ijfoodmicro.2013.03.002.
- Fratianni, F., F. Cardinale, I. Russo, C. Iuliano, P. Tremonte, R. Coppola, and F. Nazzaro. 2014. Ability of synbiotic encapsulated Saccharomyces cerevisiae boulardii to grow in berry juice and to survive under simulated gastrointestinal conditions. Journal of Microencapsulation 31 (3):299–305. doi: 10.3109/02652048.2013.871361.
- Freitas, H., A. dos Santos, S. Rodrigues, V. Abreu, N. Narain, T. Lemos, W. Gomes, and A. Pereira. 2021. Synbiotic açaí juice (Euterpe oleracea) containing sucralose as noncaloric sweetener: Processing optimization, bioactive compounds, and acceptance during storage. Journal of Food Science 86 (3):730–9. doi: 10.1111/1750-3841.15617.
- Garzón, G., C. Narváez, K. Riedl, and S. Schwartz. 2010. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chemistry 122 (4):980–6. doi: 10.1016/j.foodchem.2010.03.017.
- Gao, X., J. Zhao, H. Zhang, W. Chen, and Q. Zhai. 2022. Modulation of gut health using probiotics: The role of probiotic effector molecules. Journal of Future Foods 2 (1):1–12. doi: 10.1016/j.jfutfo.2022.03.011.
- Garzón, G. A., C.-E. Narváez-Cuenca, J.-P. Vincken, and H. Gruppen. 2017. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chemistry 217:364–72. doi:10.1016/j.foodchem.2016.08.107.
- Garzón, G., C. Soto, M. López-R, K. Riedl, C. Browmiller, and L. Howard. 2020. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale Swartz pomace. Heliyon 6 (5):e03845. doi: 10.1016/j.heliyon.2020.e03845.
- Gibson, G., R. Hutkins, M. Sanders, S. Prescott, R. Reimer, S. Salminen, K. Scott, C. Stanton, K. Swanson, P. Cani, et al. 2017. Expert consensus document: The International Scientific Association for Probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology 14 (8):491–502. doi: 10.1038/nrgastro.2017.75.
- Gómez-Caravaca, A. M., A. M. Verardo, M. Toselli, A. Segura-Carretero, A. Fernández-Gutiérrez, and M. F. Caboni. 2013. Determination of the major phenolic compounds in pomegranate juices by HPLC–DAD–ESI-MS. Journal of Agricultural and Food Chemistry 61 (22):5328–37.
- González, D., I. Gil-Sánchez, A. Esteban-Fernández, A. Ramos, M. Fernández-Díaz, C. Cueva, M. Moreno-Arribas, and B. Bartolomé. 2017. Reciprocal beneficial effects between wine polyphenols and probiotics: An exploratory study. European Food Research and Technology 243 (3):531–8. doi: 10.1007/s00217-016-2770-5.
- Gouvêa, A. C. M. S., M. C. P. d Araujo, D. F Schulz, S Pacheco, R. L. d O Godoy, and L. M. C Cabral. 2012. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Science and Technology 32 (1):43–6. doi:10.1590/S0101-20612012005000001.
- Hesam, F., B. Tarzi, M. Honarvar, and M. Jahadi. 2020. Valorization of sugarcane bagasse to high value-added xylooligosaccharides and evaluation of their prebiotic function in a synbiotic pomegranate juice. Biomass Conversion and Biorefinery :1–13. doi: 10.1007/s13399-020-01095-0.
- Hidalgo, G, and M. Almajano. 2017. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 6 (1):7. doi: 10.3390/antiox6010007.
- Hidalgo, M., M. Oruna-Concha, S. Kolida, G. Walton, S. Kallithraka, J. Spencer, G. Gibson, and S. de Pascual-Teresa. 2012. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. Journal of Agricultural and Food Chemistry 60 (15):3882–90. doi: 10.1021/jf3002153.
- Hill, C., F. Guarner, G. Reid, G. Gibson, D. Merenstein, B. Pot, L. Morelli, R. Canani, H. Flint, S. Salminen, et al. 2014. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology 11 (8):506–14. doi: 10.1038/nrgastro.2014.66.
- Horvitz, S., D. Chanaguano, and I. Arozarena. 2017. Andean blackberries (Rubus glaucus Benth) quality as affected by harvest maturity and storage conditions. Scientia Horticulturae 226:293–301. doi: 10.1016/j.scienta.2017.09.002.
- Hu, J., L. Zhang, W. Lin, W. Tang, F. Chan, and S. Ng. 2021. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends in Food Science & Technology 108:187–96. doi: 10.1016/J.TIFS.2020.12.009.
- Igwe, E., K. Charlton, Y. Probst, K. Kent, and M. Netzel. 2019. A systematic literature review of the effect of anthocyanins on gut microbiota populations. Journal of Human Nutrition and Dietetics : The Official Journal of the British Dietetic Association 32 (1):53–62. doi: 10.1111/jhn.12582.
- Jamar, G., D. Estadella, and L. Pisani. 2017. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors (Oxford, England) 43 (4):507–16. doi: 10.1002/biof.1365.
- Kamiloglu, S., O. Serali, N. Unal, and E. Capanoglu. 2013. Antioxidant activity and polyphenol composition of black mulberry (Morus nigra L.) products. Journal of Berry Research 3 (1):41–51. doi: 10.3233/JBR-130045.
- Keșa, A.-L., C. R. Pop, E. Mudura, L. C. Salanță, A. Pasqualone, C. Dărab, C. Burja-Udrea, H. Zhao, and T. E. Coldea. 2021. Strategies to improve the potential functionality of fruit-based fermented beverages. Plants 10 (11):2263. doi: 10.3390/plants10112263.
- Koyama, R., A. de Assis, L. Yamamoto, W. Borges, R. Sá Borges, S. Prudêncio, and S. Roberto. 2014. Exogenous abscisic acid increases the anthocyanin concentration of berry and juice from ‘isabel’ grapes (Vitis labrusca L.). HortScience 49 (4):460–4. doi: 10.21273/HORTSCI.49.4.460.
- Kubota, M., C. Ishikawa, Y. Sugiyama, S. Fukumoto, T. Miyagi, and S. Kumazawa. 2012. Anthocyanins from the fruits of Rubus croceacanthus and Rubus sieboldii, wild berry plants from Okinawa, Japan. Journal of Food Composition and Analysis 28 (2):179–82. doi: 10.1016/j.jfca.2012.09.002.
- Kula, M, and M. Krauze-Baranowska. 2016. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutrition and Cancer 68 (1):18–28. doi: 10.1080/01635581.2016.1115095.
- Lacombe, A, and V. Wu. 2017. The potential of berries to serve as selective inhibitors of pathogens and promoters of beneficial microorganisms. Food Quality and Safety 1 (1):3–12. doi: 10.1093/fqsafe/fyx001.
- Lago-Vanzela, E., R. Da-Silva, E. Gomes, E. García-Romero, and I. Hermosín-Gutiérrez. 2011. Phenolic composition of the edible parts (flesh and skin) of Bordô grape (Vitis labrusca) using HPLC-DAD-ESI-MS/MS. Journal of Agricultural and Food Chemistry 59 (24):13136–46. doi: 10.1021/JF203679N.
- Lai, K., Y. How, and L. Pui. 2020. Storage stability of microencapsulated Lactobacillus rhamnosus GG in hawthorn berry tea with flaxseed mucilage. Journal of Food Processing and Preservation 44 (12):e14965. doi: 10.1111/jfpp.14965.
- Lavefve, L., L. Howard, and F. Carbonero. 2020. Berry polyphenols metabolism and impact on human gut microbiota and health. Food & Function 11 (1):45–65. doi: 10.1039/c9fo01634a.
- Lebaka, V., Y. Wee, V. Narala, and V. Joshi. 2018. Development of new probiotic foods–A case study on probiotic juices. In Therapeutic, probiotic, and unconventional foods, eds. A. Mihai, and A. M. Holban, 55–78. Cambridge, Massachusetts: Academic Press. doi: 10.1016/B978-0-12-814625-5.00004-2.
- Leong, H., P. Show, M. Lim, C. Ooi, and T. Ling. 2018. Natural red pigments from plants and their health benefits: A review. Food Reviews International 34 (5):463–82. doi: 10.1080/87559129.2017.1326935.
- Li, D., P. Wang, Y. Luo, M. Zhao, and F. Chen. 2017. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Critical Reviews in Food Science and Nutrition 57 (8):1729–41. doi: 10.1080/10408398.2015.1030064.
- Liang, Z., B. Wu, P. Fan, C. Yang, W. Duan, X. Zheng, C. Liu, and S. Li. 2008. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chemistry 111 (4):837–44. doi: 10.1016/j.foodchem.2008.04.069.
- Luckow, T., V. Sheehan, G. Fitzgerald, and C. Delahunty. 2006. Exposure, health information and flavour-masking strategies for improving the sensory quality of probiotic juice. Appetite 47 (3):315–23. doi: 10.1016/j.appet.2006.04.006.
- Machado, T. A. D. G., M. E. G. de Oliveira, M. I. F. Campos, P. O. A. de Assis, E. L. de Souza, M. S. Madruga, M. T. B. Pacheco, M. M. E. Pintado, and R. d C. R. d E. Queiroga. 2017. Impact of honey on quality characteristics of goat yogurt containing probiotic Lactobacillus acidophilus. LWT 80:221–9. doi: 10.15835/nbha4319814.
- Mantzourani, I., A. Terpou, A. Alexopoulos, E. Bezirtzoglou, A. Bekatorou, and S. Plessas. 2019. Production of a potentially synbiotic fermented Cornelian cherry (Cornus mas L.) beverage using Lactobacillus paracasei K5 immobilized on wheat bran. Biocatalysis and Agricultural Biotechnology 17:347–51. doi: 10.1016/j.bcab.2018.12.021.
- Mantzourani, I., A. Terpou, A. Bekatorou, A. Mallouchos, A. Alexopoulos, A. Kimbaris, E. Bezirtzoglou, A. Koutinas, and S. Plessas. 2020. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chemistry 308:125658. doi: 10.1016/j.foodchem.2019.125658.
- Marín-Arango, Z., M. Cortes-Rodríguez, O. Montoya-Campuzano, and J. Arango-Tobón. 2019. Viability of Lactobacillus casei ATCC 393 and properties in Andean blackberry suspensions with probiotic and prebiotic characteristics. DYNA (Colombia) 86 (210):179–86. doi: 10.15446/dyna.v86n210.72929.
- Markowiak, P, and K. Śliżewska. 2017. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9 (9):1021. doi: 10.3390/nu9091021.
- Mikulic-Petkovsek, M., V. Schmitzer, A. Slatnar, B. Todorovic, R. Veberic, F. Stampar, and A. Ivancic. 2014. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. Journal of Agricultural and Food Chemistry 62 (24):5573–80. doi: 10.1021/JF5011947.
- Min, M., C. Bunt, S. Mason, and M. Hussain. 2019. Non-dairy probiotic food products: An emerging group of functional foods. Critical Reviews in Food Science and Nutrition 59 (16):2626–41. doi: 10.1080/10408398.2018.1462760.
- Mishra, A., I. Chakravarty, and S. Mandavgane. 2021. Current trends in non-dairy based synbiotics. Critical Reviews in Biotechnology 41 (6):935–52. doi: 10.1080/07388551.2021.1898329.
- Moldovan, B., A. Filip, S. Clichici, R. Suharoschi, P. Bolfa, and L. David. 2016. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. Journal of Functional Foods 26:77–87. doi:10.1016/j.jff.2016.07.004.
- Morais, C. A., V. V. de Rosso, D. Estadella, and L. P. Pisani. 2016. Anthocyanins as inflammatory modulators and the role of the gut microbiota. The Journal of Nutritional Biochemistry 33:1–7. doi: 10.1016/j.jnutbio.2015.11.008.
- Morales-de la Peña, M., J. Welti-Chanes, and O. Martín-Belloso. 2019. Novel technologies to improve food safety and quality. Current Opinion in Food Science 30:1–7. doi: 10.1016/j.cofs.2018.10.009.
- Mousavi, Z., S. Mousavi, S. Razavi, Z. Emam-Djomeh, and H. Kiani. 2011. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World Journal of Microbiology and Biotechnology 27 (1):123–8. doi: 10.1007/s11274-010-0436-1.
- Mulabagal, V., W. J. Keller, and A. I. Calderón. 2012. Quantitative analysis of anthocyanins in Euterpe oleracea (açaí) dietary supplement raw materials and capsules by Q-TOF liquid chromatography/mass spectrometry. Pharmaceutical Biology 50 (10):1289–96. doi:10.3109/13880209.2012.674141.
- Müller, D., M. Schantz, and E. Richling. 2012. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), Blueberries (Vaccinium corymbosum L.), and corresponding juices. Journal of Food Science 77 (4):C340–C345. doi: 10.1111/J.1750-3841.2011.02605.X.
- Mustafa, S, and L. Chua. 2020. Green technological fermentation for probioticated beverages for health enhancement. In Biotechnological progress and beverage consumption: Volume 19: The science of beverages, 407–34. Cambridge, Massachussets: Academic Press. doi: 10.1016/B978-0-12-816678-9.00013-8.
- Mustafa, S., L. Chua, and H. El-Enshasy. 2019. Effects of agitation speed and kinetic studies on probiotication of pomegranate juice with Lactobacillus casei. Molecules 24 (13):2357. doi: 10.3390/molecules24132357.
- Nualkaekul, S., M. Cook, V. Khutoryanskiy, and D. Charalampopoulos. 2013. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Research International 53 (1):304–11. doi: 10.1016/j.foodres.2013.04.019.
- Nualkaekul, S., D. Lenton, M. Cook, V. Khutoryanskiy, and D. Charalampopoulos. 2012. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydrate Polymers 90 (3):1281–7. doi: 10.1016/j.carbpol.2012.06.073.
- Nualkaekul, S., I. Salmeron, and D. Charalampopoulos. 2011. Investigation of the factors influencing the survival of Bifidobacterium longum in model acidic solutions and fruit juices. Food Chemistry 129 (3):1037–44. doi: 10.1016/j.foodchem.2011.05.071.
- Oancea, S. 2021. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 10 (9):1337. doi: 10.3390/antiox10091337.
- Okur, İ., C. Baltacıoğlu, E. Ağçam, H. Baltacıoğlu, and H. Alpas. 2019. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste and Biomass Valorization 10 (12):3545–55. doi:10.1007/s12649-019-00771-1.
- Olivares, A., C. Soto, E. Caballero, and C. Altamirano. 2019. Survival of microencapsulated Lactobacillus casei (prepared by vibration technology) in fruit juice during cold storage. Electronic Journal of Biotechnology 42:42–8. doi: 10.1016/j.ejbt.2019.10.002.
- Osorio, C., B. Acevedo, S. Hillebrand, J. Carriazo, P. Winterhalter, and A. Morales. 2010. Microencapsulation by spray-drying of anthocyanin pigments from corozo (Bactris guineensis) fruit. journal of Agricultural and Food Chemistry 58 (11):6977–85. doi: 10.1021/jf100536g.
- Perricone, M., A. Bevilacqua, C. Altieri, M. Sinigaglia, and M. Corbo. 2015. Challenges for the production of probiotic fruit juices. Beverages 1 (2):95–103. doi: 10.3390/beverages1020095.
- Perricone, M., M. Corbo, M. Sinigaglia, B. Speranza, and A. Bevilacqua. 2014. Viability of Lactobacillus reuteri in fruit juices. Journal of Functional Foods 10:421–6. doi: 10.1016/j.jff.2014.07.020.
- Pimentel, T., S. Klososki, M. Rosset, C. Barão, and V. Marcolino. 2019. Fruit juices as probiotic foods. In Sports and energy drinks: Volume 10: The science of beverages, 483–513. Sawston, Cambridge: Woodhead Publishing. doi: 10.1016/B978-0-12-815851-7.00014-0.
- Pisoschi, A, and G. Negulescu. 2011. Methods for total antioxidant activity determination: A review. Biochemistry & Analytical Biochemistry 1 (1): 1–10. doi: 10.4172/2161-1009.1000106.
- Ponder, A., E. Hallmann, M. Kwolek, D. Średnicka-Tober, and R. Kazimierczak. 2021. Genetic differentiation in anthocyanin content among berry fruits. Current Issues in Molecular Biology 43 (1):36–51. doi: 10.3390/CIMB43010004.
- Quigley, E. 2019. Microbiome-directed therapies: Past, present, and future: Prebiotics and probiotics in digestive health. Clinical Gastroenterology and Hepatology : The Official Clinical Practice Journal of the American Gastroenterological Association 17 (2):333–44. doi: 10.1016/j.cgh.2018.09.028.
- Rambaran, T, and C. Bowen-Forbes. 2020. Chemical and sensory characterisation of two Rubus rosifolius (Red Raspberry) varieties. International Journal of Food Science 2020:6879460. doi: 10.1155/2020/6879460.
- Ranadheera, C., J. Vidanarachchi, R. Rocha, A. Cruz, and S. Ajlouni. 2017. Probiotic delivery through fermentation: Dairy vs. non-dairy beverages. Fermentation 3 (4):67. doi: 10.3390/fermentation3040067.
- Reque, P., R. Steffens, A. da Silva, A. Jablonski, S. Flôres, A. Rios, and E. Jong. 2014. Characterization of blueberry fruits (Vaccinium spp.) and derived products. Food Science and Technology (Campinas) 34 (4):773–9. doi: 10.1590/1678-457X.6470.
- Rodríguez-Daza, M., E. Pulido-Mateos, J. Lupien-Meilleur, D. Guyonnet, Y. Desjardins, and D. Roy. 2021. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Frontiers in Nutrition 8:689456. doi: 10.3389/FNUT.2021.689456/BIBTEX[PMC].[34268328.
- Rovinaru, C, and D. Pasarin. 2020. Application of microencapsulated synbiotics in fruit-based beverages. Probiotics and Antimicrobial Proteins 12 (2):764–73. doi: 10.1007/s12602-019-09579-w.
- Ruiz, L., V. Zamora, M. Pescuma, C. van Nieuwenhove, F. Mozzi, and J. Sánchez. 2021. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Research International (Ottawa, Ont.) 140:109854. doi: 10.1016/J.FOODRES.2020.109854.
- Ryu, D, and E. Koh. 2018. Stability of anthocyanins in bokbunja (Rubus occidentalis L.) under in vitro gastrointestinal digestion. Food Chemistry 267:157–62. doi: 10.1016/J.FOODCHEM.2018.02.109.
- Sarao, L, and M. Arora. 2017. Probiotics, prebiotics, and microencapsulation: A Review. Critical Reviews in Food Science and Nutrition 57 (2):344–71. doi: 10.1080/10408398.2014.887055.
- Sheehan, V., P. Ross, and G. Fitzgerald. 2007. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innovative Food Science & Emerging Technologies 8 (2):279–84. doi: 10.1016/j.ifset.2007.01.007.
- Skrovankova, S., D. Sumczynski, J. Mlcek, T. Jurikova, and J. Sochor. 2015. Bioactive compounds and antioxidant activity in different types of berries. International Journal of Molecular Sciences 16 (10):24673–706. doi: 10.3390/ijms161024673.
- Speranza, B., D. Campaniello, L. Petruzzi, C. Altieri, M. Sinigaglia, A. Bevilacqua, and M. Corbo. 2020. The inoculation of probiotics in vivo is a challenge: Strategies to improve their survival, to avoid unpleasant changes, or to enhance their performances in beverages. Beverages 6 (2):20–18. doi: 10.3390/beverages6020020.
- Singh, K, and A. Rao. 2021. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutrition Research (New York, N.Y.) 87:1–12. doi: 10.1016/j.nutres.2020.12.014.
- Srisukchayakul, P., D. Charalampopoulos, and K. Karatzas. 2018. Study on the effect of citric acid adaptation toward the subsequent survival of Lactobacillus plantarum NCIMB 8826 in low pH fruit juices during refrigerated storage. Food Research International (Ottawa, Ont.) 111:198–204. doi: 10.1016/j.foodres.2018.05.018.
- Swain, M. R., M. Anandharaj, R. C. Ray, and R. P. Rani. 2014. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnology Research International 2014:250424–19. doi: http://dx.doi.org/10.1155/2014/250424.
- Swanson, K., G. Gibson, R. Hutkins, R. Reimer, G. Reid, K. Verbeke, K. Scott, H. Holscher, M. Azad, N. Delzenne, et al. 2020. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nature Reviews. Gastroenterology & Hepatology 17 (11):687–701. doi: 10.1038/s41575-020-0344-2.
- Szymanowska, U., B. Baraniak, and A. Bogucka-Kocka. 2018. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from polana raspberry (Rubus idaeus L.) fruit and juice—In vitro study. Molecules 23 (7):1812. doi: 10.3390/molecules23071812.
- Tegegne, B. A, and B. Kebede. 2022. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 8 (6):e09725. doi: 10.1016/j.heliyon.2022.e09725.
- Tian, L., Y. Tan, G. Chen, G. Wang, J. Sun, S. Ou, W. Chen, and W. Bai. 2019. Metabolism of anthocyanins and consequent effects on the gut microbiota. Critical Reviews in Food Science and Nutrition 59 (6):982–91. doi: 10.1080/10408398.2018.1533517.
- Topolska, K., A. Florkiewicz, and A. Filipiak-Florkiewicz. 2021. Functional food—Consumer motivations and expectations. International Journal of Environmental Research and Public Health 18 (10):5327. doi: 10.3390/ijerph18105327.
- Urbano, J., M. da Silva, M. Mazzocato, F. Tulini, and C. Favaro-Trindade. 2019. Probiotic and synbiotic sorbets produced with jussara (Euterpe edulis) pulp: Evaluation throughout the storage period and effect of the matrix on probiotics exposed to simulated gastrointestinal fluids. Probiotics and Antimicrobial Proteins 11 (1):264–72. doi: 10.1007/s12602-017-9346-y.
- Valero-Cases, E., D. Cerdá-Bernad, J. Pastor, and M. Frutos. 2020. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 12 (6):1666. doi: 10.3390/nu12061666.
- Varsha, K. K., A. P. Maheshwari, and K. M. Nampoothiri. 2021. Accomplishment of probiotics in human health pertaining to immunoregulation and disease control. Clinical Nutrition ESPEN 44:26–37. doi: 10.1016/j.clnesp.2021.06.020.
- Veberic, R., A. Slatnar, J. Bizjak, F. Stampar, and M. Mikulic-Petkovsek. 2015. Anthocyanin composition of different wild and cultivated berry species. LWT - Food Science and Technology 60 (1):509–17. doi: 10.1016/j.lwt.2014.08.033.
- Vieira, G. S., A. S. Marques, M. T. Machado, V. M. Silva, and M. D. Hubinger. 2017. Determination of anthocyanins and non-anthocyanin polyphenols by ultra performance liquid chromatography/electrospray ionization mass spectrometry (UPLC/ESI–MS) in jussara (Euterpe edulis) extracts. Journal of Food Science and Technology 54 (7):2135–44.
- Wang, H. 2014. Rapid quantitative analysis of individual anthocyanin content based on high-performance liquid chromatography with diode array detection with the pH differential method. Journal of Separation Science 37 (18):2535–44. doi: 10.1002/JSSC.201400364.
- Wang, M., Z. Zhang, H. Sun, S. He, S. Liu, T. Zhang, L. Wang, and G. Ma. 2022. Research progress of anthocyanin prebiotic activity: A review. Phytomedicine 102:154145. doi: 10.1016/j.phymed.2022.154145.
- White, J, and S. Hekmat. 2018. Development of probiotic fruit juices using lactobacillus rhamnosus GR-1 fortified with short chain and long chain inulin fiber. Fermentation 4 (2):27. doi: 10.3390/fermentation4020027.
- Wu, S., K. Dastmalchi, C. Long, and E. Kennelly. 2012. Metabolite profiling of jaboticaba (Myrciaria cauliflora) and other dark-colored fruit juices. Journal of Agricultural and Food Chemistry 60 (30):7513–25. doi: 10.1021/JF301888Y.
- Wu, Y., S. Li, Y. Tao, D. Li, Y. Han, P. Show, G. Wen, and J. Zhou. 2021. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chemistry 348:129083. doi: 10.1016/j.foodchem.2021.129083.
- Yan, Y., F. Zhang, Z. Chai, M. Liu, M. Battino, and X. Meng. 2019. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food and Chemical Toxicology 131:110541. doi: 10.1016/j.fct.2019.05.049.
- Yang, B, and M. Kortesniemi. 2015. Clinical evidence on potential health benefits of berries. Current Opinion in Food Science 2:36–42. doi: 10.1016/j.cofs.2015.01.002.
- Yang, H., D. Hewes, S. Salaheen, C. Federman, and D. Biswas. 2014. Effects of blackberry juice on growth inhibition of foodborne pathogens and growth promotion of Lactobacillus. Food Control. 37 (1):15–20. doi: 10.1016/j.foodcont.2013.08.042.
- Zendeboodi, F., N. Khorshidian, A. Mortazavian, and A. da Cruz. 2020. Probiotic: Conceptualization from a new approach. Current Opinion in Food Science 32:103–23. doi: 10.1016/j.cofs.2020.03.009.
- Zhao, C., Y. Yu, Z. Chen, G. Wen, F. Wei, Q. Zheng, C. Wang, and X. Xiao. 2017. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chemistry 214:119–28. doi: 10.1016/j.foodchem.2016.07.073.
- Zhu, J., T. Ren, M. Zhou, and M. Cheng. 2016. The combination of blueberry juice and probiotics reduces apoptosis of alcoholic fatty liver of mice by affecting SIRT1 pathway. Drug Design, Development and Therapy 10:1649–61.
- Zia-Ul-Haq, M., M. Riaz, V. de Feo, H. Jaafar, and M. Moga. 2014. Rubus Fruticosus L.: Constituents, biological activities and health related uses. Molecules (Basel, Switzerland) 19 (8):10998–1029. doi: 10.3390/molecules190810998.
- Zielińska, D., K. Marciniak-Lukasiak, M. Karbowiak, and P. Lukasiak. 2021. Effects of fructose and oligofructose addition on milk fermentation using novel Lactobacillus cultures to obtain high-quality yogurt-like products. Molecules 26 (19):5730. doi: 10.3390/molecules26195730.
- Žuntar, I., Z. Petric, D. Kovacevíc, and P. Putnik. 2020. Safety of probiotics: Functional fruit beverages and nutraceuticals. Foods, 9 (7):947. doi: 10.3390/foods9070947.