Abstract
A transformation in our food production system is being enabled by the convergence of advances in genome-based technologies and traditional fermentation. Science at the intersection of synthetic biology, fermentation, downstream processing for product recovery, and food science is needed to support technology development for the production of fermentation-derived food ingredients. The business and markets for fermentation-derived ingredients, including policy and regulations are discussed. A patent landscape of fermentation for the production of alternative proteins, lipids and carbohydrates for the food industry is provided. The science relating to strain engineering, fermentation, downstream processing, and food ingredient functionality that underpins developments in precision fermentation for the production of proteins, fats and oligosaccharides is examined. The production of sustainably-produced precision fermentation-derived ingredients and their introduction into the market require a transdisciplinary approach with multistakeholder engagement. Successful innovation in fermentation-derived ingredients will help feed the world more sustainably.
Introduction
More sustainable food production methods and food process operations, which use less of the world’s natural resources, are needed to support a growing population and our food systems for food and nutrition security (Béné et al. Citation2019; FAO et al. Citation2021; Knorr and Augustin Citation2022). Traditionally, land was used for the production of plant and animal-based food. Plants and animals from the oceans and waterways were also harvested for food. The drive for more sustainable sources of food has resulted in increasing interest in the production of cellular products (e.g., in vitro meat) and the use of microbes as hosts for the production of acellular products (e.g., food ingredients) (Anderson, Islam, and Prather Citation2018; Rischer, Szilvay, and Oksman-Caldentey Citation2020; Teng et al. Citation2021). Edible biomass (e.g., algal biomass) is also becoming attractive as a source of sustainable food (Stengel and Connan Citation2015). Fermentation, which includes traditional fermentation, biomass fermentation, and precision fermentation, has an important role in building next-generation food ingredients and products (Good Food Institute Citation2021; Teng et al. Citation2021). Products made by fermentation have reduced reliance on land, lower greenhouse emissions, and use less water compared to traditional agriculture (Teng et al. Citation2021).
Traditional fermentation has been used for centuries as a means to preserve foods and increase the nutritional value of foods. Fish, meat, milk, vegetables, legumes, cereals, and fruits were used as the source material for fermentation for the purpose of preserving food. Fermented foods are produced through the controlled growth of microorganisms and conversion of components in the food through the action of microbial enzymes. Well-known fermented food and beverage products include yogurts, kimchi, kefir sauerkraut, cheese, tempeh, wine, beer, and natto (Bourdichon et al. Citation2021; Marco et al. Citation2017; Shiferaw Terefe and Augustin Citation2020). Microorganisms naturally present in the raw food can result in spontaneous fermentation (e.g., traditional production of sauerkraut, kimchi, and sourdough bread) or starter cultures may be added to the food to initiate fermentation to obtain a more standardized fermented product (e.g., Rhizopus oligoporus for tempeh, Bacillus subtilis natto for natto, Aspergillus oryzae for miso, kefir grains which contain yeasts such as Kluyveromyces marxianus, Saccharomyces cerevisiae, Saccharomyces unisporus in addition to lactic and acetic acid producing bacteria for kefir) (Dimidi et al. Citation2019).
In biomass fermentation, edible microorganisms (e.g., yeasts, bacteria, filamentous fungi or algae) are used as alternatives to conventional food sources produced by traditional agriculture (Bernaerts et al. Citation2018; Linder Citation2019; Ochsenreither et al. Citation2016; Ritala et al. Citation2017), both as a source of edible biomass and functional ingredients (polysaccharides, proteins, lipids/omega-3 fatty acids, vitamins, minerals, and dietary fibre) (Barros de Medeiros et al. Citation2022). Due to the nutritional composition, functional diversity and metabolic flexibility of algae, and in particular microalgae (e.g., Arthrospira platensis, Spirulina platensis, Chlorella vulgaris, and Dunaliella salina), they have been developed and cultivated as sources of proteins and other food components (Becker Citation2007; Garofalo et al. Citation2022). For example, microalgae are promising ingredients for the production of nutritious meat analogues, but there are still technical challenges in texturing the microalgae, the removal of undesirable odors and colors, and the scale up of cultivation and processing conditions (Fu et al. Citation2021).
Fermentation has also been used for the production of minor food components (e.g., antioxidants, colorants, flavors, enzymes, and vitamins). Recent exploration of extracts from freshwater algae (Spirogyra sp., Cosmarium sp., and Cosmarium blytii) indicated that they were high in carbohydrates, contained free amino acids (glutamic acid, aspartic acid and proline), and exhibited high antioxidant activity, making them potential sources of functional ingredients for food and feed (Santiago-Díaz et al. Citation2022). Increasingly, food waste is being used as the fermentation medium for the production of antioxidants, natural preservatives, colors, enzymes, and other food components. The utilization of an otherwise unused/under-utilized resource reduces food waste and also has the potential to create high-value products. Recently, single-cell protein has been produced by the fermentation of food waste by Yarrowia lipolytica (Yang et al. Citation2022). Fermentation can increase the extraction efficiency of compounds from the food matrix, modify the antioxidant profile, and create new bioactive compounds. Some examples of food waste fermentation include the production of (1) flavonoids and carotenoids from fruit or vegetable waste, (2) bioactive peptides from cereal processing waste and whey from cheese making, (3) antioxidative compounds from by-products of livestock and seafood processing (Martí-Quijal et al. Citation2021; 4) enzymes (glucoamylase) from a variety of substrates such as tea waste, rice bran, wheat bran, and corn stover (Wang et al. Citation2009), and (5) microbial red, orange, and yellow pigments from various food industry wastes as medium (e.g., whey, tomato waste, cottonseed meal, fruit seeds, etc.) (Panesar, Kaur, and Panesar Citation2015; Mehri et al. Citation2021).
Precision fermentation has been identified as an emerging food trend in the fourth industrial revolution of the food industry (Hassoun et al. Citation2022). Rapid advances in precision biology has enabled the programming of microbes to produce complex organic molecules (Tubb and Seba Citation2019). However, the commercialization of genetically modified organism (GMO) technology for foods has been a challenge due to public perceptions and knowledge of their safety, risks, labeling, and regulation (Burke Citation2012; Pappalardo, D’Amico, and Lusk Citation2021; Rzymski and Królczyk Citation2016). Alternative strategies for developing more functional non-GMO microbes through conventional non-GMO methods (Pérez-Torrado et al. Citation2015) has been explored because of the challenges in bringing GMO foods to market. Many of the early-entry fermentation products in the market have used non-genetically modified organisms in traditional and biomass fermentation platforms for the production of food ingredients. However, learning from the mistakes of the past associated with the introduction of GMO technology is essential for developing appropriate strategies to facilitate the introduction of GMO foods into the market.
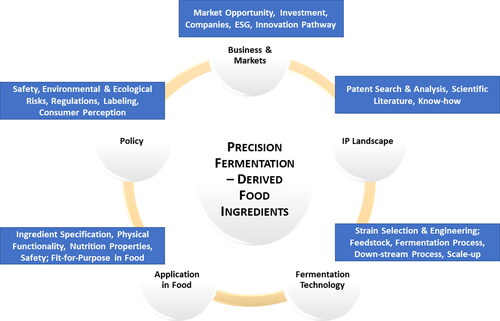
The use of precision fermentation for the production of major food components (proteins, lipids, and carbohydrates) is emerging as an attractive option for the transformation of food systems. This trend is being driven by global needs to produce foods more sustainably. This paper discusses innovation in the food industry enabled by fermentation, with a focus on the developments in precision fermentation-derived food proteins, lipids and carbohydrates. It considers the market opportunities for fermentation-derived food ingredients and issues for bringing fermentation-derived food ingredients to market. The review covers aspects of business and markets for fermentation-derived ingredients, including policy and regulations. A patent landscape of fermentation for the production of major food ingredients (proteins, lipids and carbohydrates), is included as it forms part of the due diligence required for informing science strategies for fermentation businesses. The technological considerations for the development and exploitation of the precision fermentation value chain including the (1) choice of the feedstock for fermentation, (2) use of synthetic biology tools to tailor microbes for efficient production in the fermentation process, (3) downstream processing operations to recover the food component of interest, (4) scale-up of the fermentation process, and (5) characteristics of new fermentation-derived food products that are acceptable to consumers are reviewed. is a schematic which outlines the aspects that are covered in this review related to the innovation in fermentation derived food ingredients.
Methods
Literature searches on publications relating to the various aspects on the science of fermentation and application in food systems (strain engineering, fermentation process and downstream processing, and functional performance of food ingredients) were carried out using the Web of Science. Patent landscape searching was done to assess the published patent literature. For obtaining information on business and market insights, the grey literature was also accessed. The searches were focused on the fermentation for the production of proteins, lipids, and carbohydrates (more specifically oligosaccharides).
Patent landscape search for fermentation-derived food ingredients
Broad patent landscape searching and analysis were conducted in relation to technologies for the use of precision fermentation for the production of food ingredients and alternative products such as milk, dairy, and meat, made from these food ingredients. More specifically, the search was conducted on the food ingredients of interest, namely proteins, lipids (fats/oils, triglycerides, phospholipids and glycolipids) and carbohydrates (polysaccharides, gums, oligosaccharides, and prebiotic dietary fibers). For the purpose of this search, analysis and reporting, precision fermentation is defined as the use of synthetic biology and specifically genetic engineering to insert specific genes into the DNA backbone of single-cell organisms and microorganisms to produce desired fermentation characteristics and products. The search was conducted in January 2022 and covers the time period from 2000–2021.
The search strategy used combinations of the following key words to locate relevant documents: “precision ferment+”, “cellular agricultur+”, “synthetic + biology+”, synbiolog+, syn_biolog+, synbio, syn_bio, microorganism+, micro_organism+, “micro + organism+”, single_cell_organism+, “singl + cell + organism+”, microb+, fungi, fungus, yeast+, ferment+, recombinant+, gene+, DNA, RNA, modif+, chang+, manipulat+, engineer+, transgenic+, “bio + engineer+”, bioengineer+, bio_engineer+, “bio + modif+”, biomodif+, bio_modif+, program+, protein+, fat, fats, lipid+, triglyceride+, tri_glyceride+, phospholipid+, glycolipid+, carbohydrate+, oligosaccharide+, “dietary fib+”, polysaccharide+, poly_saccharide+, gum, gums, food, foods, dairy, milk+, butter+, cheese+, meat and meats (where + indicates a word truncation, ““indicates a combination of words within a certain proximity, and _ represents a space or single character between two words). These key words were searched in all cases in the title, abstract and independent claims fields of patent documents, and extended to the full description in some cases. The search used the International Patent Classifications (IPCs) and/or Cooperative Patent Classifications (CPCs) listed in , and also included known entities of interest.
Table 1. Details of relevant International Patent Classifications (IPCs) and / or Cooperative Patent Classifications (CPCs) covered by the search strategy.
Business and markets for fermentation-derived food ingredients
Traditional, biomass fermentation, and precision fermentation may all be employed for producing food products and ingredients. There has been significant investment in fermentation companies, with total capital of $1.69 billion invested in 2021 (Good Food Institute Citation2021). The merits of choosing one type of fermentation technology over another has to be weighed against the availability of technology, costs, challenges in production and separation, regulatory hurdles, and likely consumer acceptance of the technologies.
The costs for precision fermentation have been falling exponentially since the first molecules from precision fermentation were produced. For example, proteins by precision fermentation is expected to be $10/kg by 2023–2025 and at this competitive price point, the market for food products may be unlocked (Tubb and Seba Citation2019). The cost of production for biomass fermentation also needs to be reduced for improved translation of technology for microalgae ingredients (Barros de Medeiros et al. Citation2022). A way to reduce the cost of biomass production is to reduce cultivation and harvesting costs. The principles used to reduce the cost of nutrients for cultivation, include the possible use of inexpensive resources as alternative sources of nutrients (e.g., waste or by-product streams as cheap and renewable carbon and nitrogen sources) (Ochsenreither et al. Citation2016), decreasing labor costs by increasing automation, and reducing power consumption for the production step (Acién et al. Citation2012). A recent review suggests that the development of high performance algal strains (e.g., through genetic engineering), and combining algae culture and low-cost harvesting with wastewater utilization, has potential for improved algal production systems (Xu et al. Citation2020).
Markets
Developments involving synthetic biology will be the next industrial revolution, attracting investment from private investments, mergers and acquisitions, minority stakes, and initial public offerings (BCG Citation2021). The convergence of increased accessibility to synthetic biology tools (e.g., DNA-sequencing, gene-editing), the developments in fermentation bioprocessing, the increased speed and testing solutions, and the exponentially reducing prices of these technologies (BCG Citation2021) have the potential to facilitate the development of transformative sustainable value chains based on precision fermentation.
In terms of fermentation-derived ingredients for human and animal nutrition, the strategic imperatives for success in the fermentation ingredient market over the period 2017–2022 were driven by the market, changing food habits, and the increasing demand for high-quality food products (Frost and Sullivan Citation2018). Products produced using synthetic biology tools have mainly been used in the healthcare, research, and industrial chemicals sectors, with the smaller segments being in food and beverage, agriculture, and consumer products (BCC Citation2022). In 2020, the global markets for synthetic biology in healthcare, research, and industrial chemicals were estimated to be $2.7 billion, $2.0 billion, and $1.5 billion respectively. The global markets for food and beverage, agriculture, and consumer products were $0.43 billion, $0.39 billion, and $0.35 billion respectively. The market for food and beverage is forecast to grow at compound annual growth rate (CAGR) of 51.3% and is estimated to be worth $5.7 billion by 2026 (BCC Citation2022).
The prospects for the growing markets for more sustainable and animal-free alternative food ingredients (Good Food Institute Citation2020) are driving interest in using precision fermentation technology. Genetically modified organisms have been used for improved yields and efficiencies of ingredients for the alternative meat and dairy sectors (Good Food Institute Citation2021; Hettinga and Bijl Citation2022). There has also been increasing attention paid to the engineering of microbial strains and fermentation strategies for producing colorants and flavors as alternative ingredients for the food industry (Seo and Jin Citation2022).
Fermentation companies
Fermentation companies have produced recombinant food additives/processing aids for commercial use in the food industry for some time. These include enzymes (e.g., chymosin for use in cheesemaking) (Olempska-Beer et al. Citation2006), and vitamins for food fortification (e.g., vitamin E) (Voigt Citation2020). Now, some of the largest food and life science companies, such as DSM, DuPont, Novozymes, and JBS are investing in fermentation-based product lines for the developing alternative protein industry (Pozas and Bushnell Citation2020). However, the interest in employing precision fermentation commercially for the emerging recombinant food ingredients has generally been the domain of new startup companies. Most precision fermentation activities of startups in recent years have been directed at the development of alternative proteins, although there are emerging companies with interests in recombinant lipids and oligosaccharides. This is due to the large emerging markets in the alternative animal-free protein for meat, dairy, and egg analogues. These markets are currently primarily served by plant-based analogues. However, there is potential for precision fermentation-derived proteins and lipids to capture more of the alternative meat and alternative dairy markets. lists selected companies that are using fermentation for food-related businesses. These companies are at various stages of commercialization. The list spans companies with interests in developing strains for precision fermentation platforms, alternative animal-free proteins (e.g., egg-free and dairy-free) based on precision and biomass fermentation, lipids (e.g., omega-3 fatty acids from algae and fermentation-derived fats to replace animal fats and palm oil), and a range of other functional ingredients (e.g., prebiotics, post-biotics, and vitamins). Among the recent commercial food ingredients made by precision fermentation are (1) steviol glycosides for use as sweeteners (Hoeven Van Der, Galaev, and Boer Citation2015; Watson Citation2018; (2) soy leghemoglobulin produced by engineered yeast (Pichia pastoris) for use as a colorant and meat flavor/aroma enhancer in plant-based burgers and meat analogue products (Shankar and Hoyt Citation2016) (3) animal-free egg replacers (Southey Citation2021), and (4) animal-free dairy proteins (whey proteins and caseins) for the production of alternative dairy and formulated food products (Geistlinger et al. Citation2018).
Table 2. Selected list of companies involved in fermentation for production of food ingredients.Table Footnote*
Environmental, social and governance (ESG)
There is increasing scrutiny on ESG credentials for companies (Pavláková Dočekalová and Kocmanová Citation2018) as these non-financial indicators affect their economic value. Recent studies have shown that there is improved access to financial resources for companies with increased levels of ESG disclosures (Raimo et al. Citation2020), because of the relationship between ESG and financial performance (Buallay Citation2022). ESG information is particularly important for startups to access investment funds in the emergent precision fermentation industry and for these companies to grow and become a viable new business. However, there is still a need for development of standardized methods for assessing and reporting of benchmarked sustainability indicators and their wider acceptance for measuring corporate social responsibility.
Innovation pathway
After a market-led opportunity has been identified for a target food ingredient, a transdisciplinary team needs to be formed. Following the choice of the fermentation route for the target ingredient and definition of the functional requirements of the ingredient in food applications, an assessment of the science and patent information related to strain engineering for expression of the target ingredient and its recovery downstream from fermentation is reviewed. Together the information gathered is used to design the minimal viable product that can be launched in the market. This includes testing the ingredient in a target food application. The launch of the ingredient in the market-place requires prior investigation of food labeling and food regulations in the region where the product is intended to be marketed.
Innovation in precision fermentation-derived ingredients will be facilitated by leveraging new bio-manufacturing platforms (Zhang, Sun, and Ma Citation2017) and harnessing the benefits of new value chains created by opportunities in precision fermentation. Important examples of precision fermentation bio-manufacturing platforms include using engineered yeasts such as Pichia pastoris (Komogaetella phaffii) (e.g., for the production of animal-free egg proteins by The Every Company (Ivey et al. Citation2021a) and recombinant soy leghemoglobin by Impossible Foods (Shankar and Hoyt Citation2016), as well as engineered filamentous fungi (e.g., Trichoderma reesei) for production of recombinant milk proteins for animal-free dairy by Perfect Day (Pandya et al. Citation2016)). Engineered gram negative bacteria have also been applied as bio-manufacturing platforms for the recombinant production of collagen (Ouzounov Citation2017; Ouzounov et al. Citation2021), chymosin (Emtage et al. Citation1983), alpha-amylase (Zhang and Huo Citation2017), and human milk oligosaccharides (Jennewein and Wartenberg Citation2017). Considerable research efforts have focused on developing enhanced new bio-manufacturing platforms using genomic modifications to increase recombinant protein production and/or secretion (Bill Citation2014; Ivey et al. Citation2021a), to increase oil production (Dolch et al. Citation2017), to modify glycosylation (Ivey et al. Citation2021b), and to reduce allergenicity (Bhatt et al. Citation2021). Other examples of the new platforms include metabolic engineering and synthetic biology-driven whole cell fermentation (Zhang et al. Citation2017), conversion of waste plant oils or animal fats into value-added products by oleaginous yeast (Liu et al. Citation2021), building mevalonate and lycopene synthesis pathways in Escherichia coli to produce lycopene from glucose, fatty acid and waste cooking oil (Liu et al. Citation2020), and a combination of intracellular molecular engineering of Yarrowia lipolytica and extracellular fermentation, and bioreactor engineering for converting plant oils into high value products such as carotene, lycopene, wax esters, and omega-3 fatty acids (Xie et al. Citation2017). Irrespective of which type of fermentation platform is used, expertise in the chemistry and technology of the target ingredient will be required for developing appropriate isolation/separation processes. The characterization of the isolated ingredient and its application in food formulation will require food science and product development skills to get the ingredient to market.
Policy and regulatory status
The technology for production of new and novel fermented foods and food ingredients is emerging and continues to rapidly evolve. The commercialization of new and novel food products that have not had a history of safe use needs to be regulated in a transparent manner to ensure the safety of these foods when consumed. The risks and benefits of new products should be carefully considered. New regulations on different fermented food products are needed, with increased scientific evidence for safety, quality, and transparency (Mukherjee et al. Citation2022).
The diverse range of food products being explored and developed may use new sources of naturally occurring microorganisms/algae and genetically modified organisms, alternative sources of raw materials for fermentation (e.g., food waste), different fermentation process conditions, and downstream processing of fermentation-derived products. The EU General Food Law states that “Food shall not be placed on the market if it is unsafe” (van der Meulen Citation2012). Guidelines relating to the safety of microbial food cultures (i.e., live bacteria, yeasts or molds used in food products) are governed by frameworks which require “history of safe use”, “traditional food” or “generally regarded as safe”. The regulations on the safety of food cultures differ among different countries, although all are intended at assuring the safety of cultures that also need to be guaranteed by the food culture supplier (Laulund et al. Citation2017). An inventory of microorganisms used in food fermentation for various food matrices has been published (Bourdichon et al. Citation2012). The qualified presumption of safety (QPS), which is also “applicable to genetically modified organisms used for production purposes if the recipient strain qualifies for QPR status”, was developed to provide pre-assessment of safety risks and support safety risk assessments presented to ESFA (European Food Safety Authority). The list of QPS-recommended biological agents for food and feed use are updated regularly (Koutsoumanis et al. Citation2020).
There needs to be appropriate regulations, assessment of ecological and safety risks, and consideration of consumer concern relating to the use of genetically modified organisms for producing food and beverages. Scientists have a role to play in GMO regulation and they have to address and articulate uncertainty to policy makers (Myhr and Traavik Citation2002). Engaging the public in dialogue and addressing their concerns about GMOs (Doxzen and Henderson Citation2020) is necessary. The negative consumer attitudes toward foods produced using GMO for solving global food and nutrition security may be moderated through education, exposure to official information, and developing social trust (Du, Xiao, and Xu Citation2022; Mosier, Rimal, and Ruxton Citation2020). summarizes the issues relating to the introduction of GMO foods, and actions that may be taken to address the public concerns of using GMOs and the policies to overcome concerns.
Table 3. Issues relating to use of GMOs in food and actions to address concerns.
The ability to navigate the regulatory framework has a significant influence on the transformative potential of novel ingredients, genetically modified or genetically edited ingredients and foods. Food laws and regulations to ensure food safety are complex for fermentation-derived food ingredients. Additionally, food regulatory frameworks are influenced by regional, political and cultural priorities, and these must be considered through integrated scientific and engagement with a range of stakeholders (Mukherjee et al. Citation2022), including the public. Understanding them, providing the level of evidence required to legislators, and including the cost and time of getting regulatory approval is a substantial undertaking (Lähteenmäki-Uutela et al. Citation2021). Apart from cost and time, there are additional issues including change in social, economic and legal systems, and the pre-cautionary approach adopted by government systems (Williams Citation2021). Mukherjee et al. (Citation2022) suggest that each fermented product has to be regulated and include compositional specifications, safety, communication and distribution. Williams (Citation2021) suggests that innovative regulatory governance needs to be more flexible and competitive to meet the regulatory demands and help promote the development and implementation of new foods. Strategies and actions directed at obtaining more harmonized and globally accepted regulations for fermented food products and ingredients will ease the path to market for new fermented foods and products.
Patent search results and analysis
The search identified 413 potentially relevant patent families. It should be noted that a patent family may have more than one aspect disclosed and therefore these appear in the listed categories several times and that patent information is not published in databases when a patent is first filed (i.e., at the priority date). The first publication of information for a patent may not occur for at least 18 months in most cases. Categorization based on the specific food ingredients of interest indicated the following number of patent families: proteins (231), lipids (81), carbohydrates (174), and all of proteins, lipids and carbohydrates (22). The top 10 assignees are DSM, Chr Hansen, PureCircle, BASF, DuPont Nutritional Biosciences, China Agricultural University, Danisco, Glycom, Ajimoto, and Evonik Degussa.
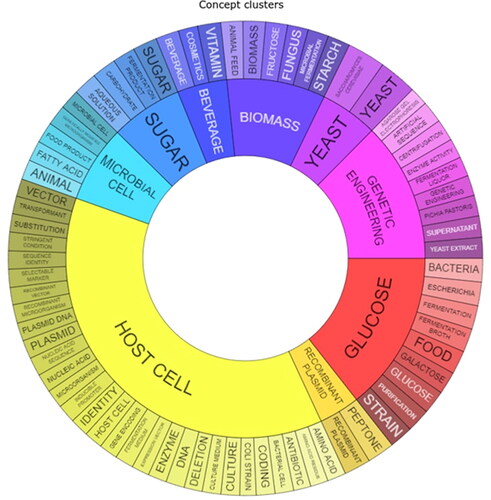
The main technology domains in the patent classifications are in “Biotechnology” (31.28%) and “Food chemistry” (30.32%). Other technology domains comprise “Organic fine chemistry” (10.53%), “Pharmaceuticals” (10.11%), “Basic materials chemistry” (5.00%), “Macromolecular chemistry/Polymers” (2.66%),”Other special machines” (2.45%), “Chemical engineering” (2.23%) and “Other miscellaneous domains” (5.42%). The concept clusters relating to the occurrence of terms in the patent families illustrates the distribution of the main concepts (). Selected patents relevant to the development of fermentation-derived proteins, fat and carbohydrate ingredients are listed in –.
Table 4. Selected patents relevant to proteins from fermentation.
Table 5. Selected patents relevant to lipids from fermentation.
Table 6. Selected patents relevant to carbohydrates from fermentation.
Precision fermentation technology – production of food ingredients
In precision fermentation, synthetic biology methods are used to program microbes which are used as cell factories to produce ingredients for the food and pharmaceutical industries (Pham Citation2018). The choice of the recombinant host microorganism and strain engineering present the initial challenge that determines the possibilities for constructing microbes to express and produce the target molecules in sufficient quantity using appropriate fermentation conditions for increasing efficiency of production. Microbial hosts are preferred for ease of genetic manipulation and use of standardized fermentation equipment. Microorganisms which are generally regarded as safe (GRAS) or have non-harmful status are preferred for food applications, and so strain engineering often utilizes benign bacteria (such as Bacillus spp.), yeasts (Vieira Gomes et al. Citation2018; such as Saccharomyces cerevisiae, Pichia pastoris (now Komagaetella phaffii), Kluyveromyces spp.), or filamentous fungi (such as Trichoderma spp., notably the popular T. reesei strains) (Chai et al., Citation2022; Nevalainen, Peterson, and Curach Citation2018). Innovation arises through the use of novel species or strains, or through utilizing genetic engineering and synthetic biology techniques to optimize the yield of the desired product, for example by improvements to expression, secretion, substrate conversion, and titer of the product. There is tremendous untapped potential within natural microbial biodiversity to provide efficient microbial cell factories for novel and safe fermented foods, which recent advances in “omics” tools and synthetic biology are uncovering. By utilizing these tools one can develop products that exactly fit desired properties and ensure that the fermentation process is precise and controlled rather than random (Teng et al. Citation2021). Precision fermentation lends itself readily to the production of target proteins, lipids, and carbohydrates, due to the ability to produce molecules that mimic the composition of their counterparts derived from traditional agriculture.
Production of precision fermentation-derived proteins
Whilst bacterial expression host E. coli has dominated the production of recombinant protein therapeutics due to well-characterized genetics, rapid growth and high yield (Huang, Lin, and Yang Citation2012), fermentation for protein food ingredients favors GRAS microorganisms which enable simpler regulatory pathways. Recent trends favoring the production of animal proteins using animal-free systems have led to a focus on eukaryotic expression hosts that are able to produce nature equivalent animal proteins with suitable post-translational modifications. Unicellular Crabtree-negative yeast such as Komagaetella phaffii (Pichia pastoris) provide an ideal expression system and have been widely used (Juturu and Wu Citation2018; Spohner et al. Citation2015; Vieira Gomes et al. Citation2018). A variety of genetic edits have been utilized to enhance recombinant protein production in this expression host including avoidance of protein degradation due to the unfolded protein response (Bankefa et al. Citation2018), enhanced processing of proteins through the endoplasmic reticulum for improved secretion yields (Zahrl, Mattanovich, and Gasser Citation2018) and deletion of unwanted protease activities (Vuree Citation2020). The development of genetic engineering tools such as CRISPR (Nishi et al. Citation2022; Prielhofer et al. Citation2017; Weninger et al. Citation2018) has enabled these synthetic biology approaches for recombinant protein production (Spohner et al. Citation2015; Wagner and Alper Citation2016). An early objective in the development of a recombinant protein is to obtain a nature-identical primary structure which has an amino acid sequence of the target protein to that found in nature. Post-translational modifications (e.g., phosphorylation, glycosylation) also need to be considered as they influence both the structure and functional attributes of a protein.
Innovative synthetic biology approaches to recombinant protein production for food ingredients are generally aimed at: (1) increasing protein expression per biomass unit (i.e., increasing titer and yield), (2) increasing the efficiency of protein trafficking through the cell, hence enhancing protein secretion, (3) enhancing specific physicochemical attributes of the recombinant protein product, such as thermostability (Cramer et al. Citation2018; Gautieri et al. Citation2018; Pietzsch et al. Citation2009) (4) protein engineering of specific functional attributes of the protein to create a desired food ingredient (e.g., altered catalytic substrate preference) (Prakash et al. Citation2020) or non-catalytic removal of allergens (Bhatt et al., Citation2021). Genetic modifications to achieve these aims are based on alteration of one to many genes through chromosomal substitution from a different donor organism, rendering a gene nonfunctional through partial or complete deletion, induced mutations to existing DNA or heterologous gene additions via episomal plasmid expression or chromosomal integration by recombination. For example, protein expression can be increased by manipulation of promotors and regulatory elements (Cai et al. Citation2019) or by deletion of extraneous proteins in order to direct cellular energy and protein expression systems toward the target product (Geistlinger et al. Citation2021). Protein titer in fermentation broth can also be increased by deletion of the more abundant proteases present in the fermentation broth. Increased efficiency of protein trafficking through the endoplasmic reticulum and Golgi body enhances protein secretion, for example through manipulation of the protein translocon and trafficking glycosylation signals (Ivey et al. Citation2021b). Additionally, redirecting recombinant proteins to avoid cellular degradation processes may be helpful in increasing protein secretion, for example by deletion of the vacuolar protein degradation transport system components in Saccharomyces cerevisiae (Bartkeviciuite and Sasnauskas 2003; Davydenko et al. Citation2004).
Selected patents on proteins derived from fermentation show that much of the recent patent activity is in the area of alternative proteins (). There has been significant interest in the development of recombinant egg, dairy, and meat proteins, and some activity in specialized functional ingredients (enzymes, color/flavor supplement) (). Animal-free egg replacers have been produced by precision fermentation (Anchel Citation2016; Mahadevan et al. Citation2021). Methods for producing recombinant caseins and whey proteins have been described, with some of the proteins produced having non-native post-translation modifications (Pandya et al. Citation2016). The recombinant milk proteins may be combined with other non-recombinant ingredients for the production of a range of dairy products such as milk, ice-cream, yogurt, and cheese (Geistlinger et al. Citation2018; Gibson, Radman, and Arie Citation2020). Recombinant milk proteins also have applications as an egg replacer (Geistlinger et al. Citation2022). With the use of synthetic biology tools, there are also possibilities to alter post-translational modifications (i.e., degree of glycosylation) of proteins (Ivey et al. Citation2021b), eliminate esterase activity which can be problematic for target food applications (Geistlinger et al., Citation2021), decrease allergenicity of protein (Bhatt et al. Citation2021) or produce non-naturally occurring polypeptides which comprise a fragment of a food protein (Ouzounov, Mellin, and Co Citation2021).
Other precision fermentation-derived ingredients for use as alternative proteins in non-animal meat analogues include recombinant collagen and muscle protein (Lu, Xia, and Liu Citation2021; Ouzounov, Mellin, and Co Citation2021; Persikov, Ouzounov, and Lorestani Citation2019). In addition, recombinant non-animal heme proteins are used to provide the color and flavor to meat analogues on cooking (). Alternatively fermentation-derived proteins and products have been produced by biomass fermentation or in-vitro cultures (Chin, Chan, and Poon Citation2021; Dyson, Rao, and Reed Citation2021; Elfenbein and Kolbeck Citation2018; ). For example, milk has been produced by a mammary cell culture (Strickland Citation2021) and fungal single-cell protein has been used as an alternative protein in non-animal meat analogues (De Latt and Gallego Murillo Citation2018).
Production of fermentation-derived lipids
Oleaginous microorganisms such as yeasts, fungi, microalgae and thraustochytrids (e.g., Schizochytrium sp) accumulate lipids and produce polyunsaturated fatty acids which are beneficial for human health (Fan and Chen Citation2007; Patel et al., Citation2020). Improved yields of lipids in oleaginous microorganisms can be achieved by optimization of cultivation conditions and/or by use of genetically modified organisms (Béligon et al. Citation2016; Ma et al. Citation2021; Mahajan, Sengupta, and Sen Citation2019).
provides selected examples of biomass fermentation for lipid production and gene editing methods employed for the production of a range of recombinant lipids. A wide range of synthetic biology approaches have been applied to enhance fatty acid and lipid production in fermentation, including enhancing the supply of substrate for acyl-CoA dependent systems, decoupling fatty acid production from growth, increasing carbon flux into the pentose phosphate pathway, and manipulating the expression of native and recombinant lipid-handling acyl transferases and thiol esterase enzymes to suit the desired lipid product ().
Most patent activity has been related to the production of polyunsaturated fatty acids (Forster, Gunnarsson, and Nielson Citation2005; Picataggio, Yadav, and Zhu Citation2004). Patented processes include those for the production of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from marine algae (Crypthecodinium cohnii) (Kyle et al. Citation1991), thraustochytrids (Facciotti, Metz, and Lassner Citation2000; Hong et al. Citation2010), and yeast (Hong et al. Citation2010; Picataggio et al. Citation2004). The health benefits of the long chain polyunsaturated fatty acids (EPA and DHA) (Shahidi and Ambigaipalan Citation2018; Swanson, Block, and Mousa Citation2012) have driven interests in these healthy fatty acids. Precision engineering has also been used for the production of triacylglycerols with short-chain fatty acids at the sn-1 and sn-3 positions (El Tachy et al. Citation2021). This provides a means to develop non-animal lipids that have a similar fatty acid profile as butter fat. A method for producing an alternative to palm oil has been developed by co-culturing a photosynthetic microorganism (e.g., cyanobacterium) with a heterotrophic microorganism (e.g., yeast) for the production of a lipid product with >40% palmitic acid (McNamara et al. Citation2018).
Pigmented oils have also been produced using yeast and microalgae. For example, the yeast, Phaffia rhodozyma, has the ability to synthesize astaxanthin as its major pigment, and may be used for commercial production as a biological source of carotenoids (Johnson Citation2003). The algal strains, Heamatococcus pluvialis and Chlorella zofingiensis, are considered ideal strains for commercial production of astaxanthin (Patel et al. Citation2022). Algal sources (e.g., red alage, green algae, brown algae, golden algae, yellow-green algae, dinoflagellates, and diatoms) are also producers of carotenoids (Patel et al. Citation2022; Schubert et al., Citation2006). The microalgae, Chlamydomonas reinhardtii, which has GRAS status may be used for producing carotenoid pigments and fatty acids. Recent studies have shown that the use of a gene edited strain, with optimization of the culture medium and process, enables co-production of pigments and oil (Song et al. Citation2022).
Production of fermentation-derived carbohydrates
In the field of carbohydrates, there has been most interest in the use of precision fermentation for the production of oligosaccharides. There has been a number of patents that describe the construction of genetically modified microbial cells for enhancing the production of oligosaccharides (). Human milk and related oligosaccharides, for application in infant formula and supplements, have been a primary target of interest. These oligosaccharides provide biological properties beyond nutrition to the infant including promotion of intestinal health, and the growth of beneficial microflora in the intestine (Ambrogi et al. Citation2021; Barile and Rastall Citation2013).
Precision fermentation derived food Ingredients – Downstream processing and scale-up
Downstream processing strategies vary depending on the type of ingredient and its ease of separation from the fermentation medium. Typical downstream processes for recovering of the target ingredient from the fermentation medium could involve cell lysis (depending on whether the ingredient is within the cell or secreted into the fermentation medium), filtration/centrifugation to separate cells/cell residues from the fermentation medium and separation processes typically used for food processing to isolate proteins, lipids or carbohydrate components from a complex mixture. There are two major pathways for downstream processing of fermentation products: recovery of intracellular products and recovery of products that are secreted into the fermentation broth. The first step for both pathways involve separation of cells from the fermentation broth. With the intracellular product recovery pathway there is an additional cell disruption step to obtain intracellular products, using methods combining one or more of heat, pressure, bead milling, enzymatic hydrolysis or chemical hydrolysis.
For the recovery of proteins after separation from the cell biomass, the major pathways follow the same essential processes: clarification (usually by centrifugation or flow filtration), extraction and purification, and usually drying into a powder (e.g., freeze-drying or spray-drying) (Fellows Citation2017; Warner Citation2019). Numerous methods are utilized for the extraction and purification of food proteins, following similar methodologies for protein recovery for other applications, with the exception that all processes must comply with relevant food grade standards. Most techniques for downstream processing of proteins are based on utilizing the differentiated biochemical characteristics of the product (such as pI, affinity, hydrophobicity, precipitation with specific reagents or at a specific pH) (Becker, Ogez, and Builder Citation1983). An initial diafiltration or tangential flow filtration step to concentrate the product into a suitable milieu is commonly followed by chromatography to extract or polish products, with the required degree of purity determining the number and type of chromatographic steps. For highly purified products, affinity chromatography followed by size exclusion chromatography are commonly used for protein purification, but for many food products only a simple one step chromatography extraction step is required (Behm et al., Citation2022; Labrou Citation2014; Raymond, Rolland, Gauthier, and Jolivet Citation1998). A consideration is the content of the protein and purity in the extract isolated from the cells required for functional performance. It is important to appreciate that each protein is unique and the medium into which the protein is expressed during fermentation and processing steps to recover the protein will all have an influence on the conformation of the protein and its equilibrium state, and consequences for functional behavior of the protein when used in food applications. More recently advanced computational techniques such as machine learning and artificial intelligence have been applied to the optimization of fermentation and downstream processing, enabling the parallel optimization of a plurality of different parameters (Pottinger et al. Citation2022).
The production of fermentation-derived lipids using non-genetically modified organisms have been in commercial production for some time. Processes for increasing biomass density for the production of microbial lipids in fermenters have been patented some time ago (Reucker et al. Citation2001). Production strategies and downstream processing for extraction and purification of single-cells oils are established (Ochsenreither et al., Citation2016; Ratledge et al. Citation2010). The oleaginous cells have to disrupted prior to extraction and downstream processing for recovery of lipids. This requires the use of efficient methods of cell disruption which enables cost-effective recovery of the lipid components (Senanayake and Fitchall, Citation2006). There are a number of methods to choose for cell disruption including (1) mechanical methods (e.g., bead milling, homogenization, ultrasound) and (2) non-mechanical disruption methods (e.g., decompression with pressurized gases, osmotic shock, microwaves, drying, and chemical methods such as application of solvents, and use of enzymes). The method of cell disruption will influence the complexity of the extraction process for recovery of the lipid. After cell disruption, the lipids for food industry applications are extracted with nontoxic solvents (e.g., supercritical CO2) or without solvents (Ochsenreither et al. Citation2016).
As for recovery and separation of recombinant oligosaccharides, the processes for purification include the removal of biomass from the fermentation broth, and a combination of filtration steps (e.g., ultrafiltration, nanofiltration) and ion exchange treatments (Helfrich and Jennewein Citation2019; Jennewein Citation2015a; Matwiejuk et al., Citation2017).
Application of precision Fermentation-Derived ingredients in food
Both the science and the patent literature help to provide the basis for ingredient development that leads to potential exploitation of market-led opportunities of fermentation-derived ingredients. After the expression of the target food ingredient/component, methods for separating the ingredient from the fermentation broth are carried out to recover the target ingredient. Unlike ingredients for pharmaceutical use where a high degree of purity if required, other components from the medium that are co-extracted with the target ingredient may have a positive or negative effect on functionality and this needs to be assessed in the final food application.
The major food ingredients (proteins, lipids, and carbohydrates) each have their own unique physico-chemical characteristics, nutritional properties, and techno-functionality, which are sought after for various food applications. Understanding the properties and functionality of traditional ingredients is required to inform fermentation targets and potential applications in food. The composition of the ingredient is a primary determinant of its functionality, although it may be modified by processing.
Briefly, the physical functional properties of proteins that are useful in food applications include solubility, heat stability, gelling, viscosity building, foaming, and emulsifications (Foegeding and Davis Citation2011). Some of the physical functional properties of fat that are important for food applications include their crystallization and melting behavior (Sato and Ueno Citation2014) and quality of oil for frying applications (Choe and Min Citation2007). Polyunsaturated fatty acids, such as omega-3 fatty acids, are nutritionally desirable (Shahidi and Ambigaipalan Citation2018). Due to their susceptibility to oxidation which leads to the production of off-odors and off-flavors, unsaturated fatty acids need to be protected during isolation and processing. The carbohydrate biopolymers are sought after for their water-holding and texture-modifying properties (Yang et al. Citation2020). Prebiotic carbohydrates/prebiotic fibers (e.g., oligosaccharides) are of interest because of their nutritional role and health-promoting properties (Elleuch et al. Citation2011) and ability to contribute to the physical properties of foods.
Further information on properties and functionality of food ingredients, which is beyond the scope of this review, is available. Some relevant reviews of the characteristics of these major food ingredients and their functional properties include those for proteins (Augustin and Udabage Citation2007; Day Citation2013; Kristo and Corredig Citation2015; Małecki, Muszyński, and Sołowiej Citation2021; Phillips, Whitehead, and Kinsella Citation1994), lipid analysis (Christie and Han Citation2012) and food lipids (Akoh Citation2017; Eskin and List Citation2017; Gunstone and Norris Citation1983), carbohydrates (Gao et al. Citation2017; Li and Nie Citation2016; Nishinari and Doi Citation1993) and prebiotics (Sungsoo and Finocchiaro Citation2009).
Key issues for commercialization of precision fermentation technology
It is acknowledged that there are fermentation-derived ingredients already in the market. However, there are a number of issues that need to be considered and addressed for realizing the full potential of fermentation-derived ingredients. High product yields and use of cost-effective culture media are important for the economic viability of the process, as are policy, regulations and consumer acceptance. There are recent patents that describe systems and methods for the high-yield production of recombinant protein (Ivey et al. Citation2021a). From a technology point of view, alternative fermentation feedstock and scaling of the fermentation process are barriers that need to be overcome for improved implementation of technology for fermentation. These issues are discussed below.
Fermentation feedstock
Cost-effective and sustainable fermentation feedstocks as alternatives to sugar are being sought for fermentation. Corn steep liquor, raw sugar extracts, and crude glycerin are inexpensive by-products that can be used to replace costly traditional media ingredients for precision fermentation of lactobacilli and yeasts (Gullón et al. Citation2008; Warner Citation2019). The use of alternatives to glucose as the carbon source for the microbial production of lipid has been explored (Sijtsma, Anderson, and Ratledge et al. Citation2010).
There is potential for exploitation of new substrates such as by-products and waste from the food industry. Nutrients have been recovered from food waste and used as alternatives to the use of traditional culture media (Pleissner et al. Citation2013). While lignocellulosic materials are abundant and generally low-cost, pre-processing of agricultural and food waste may be necessary to release the fermentable sugars (Nieves, Panyon, and Wang Citation2015). Some examples include the use of pretreated rapeseed meal for microbial oil production by Rhodosporidium toruloides (Uçkun Kiran et al. Citation2012) and pretreated brewers’ spent grains as a fermentation media for Rhodosporidium toruloides, a natural yeast producing carotenoids (Cooray, Lee, and Chen Citation2017). By-products of processing have also been explored as possible low-cost raw materials for generic microbial feedstock after a bioconversion process involving enzyme and/or fungal pretreatments. Agricultural by-products such as rice bran, wheat straw, sugarcane bagasse, fruit waste, tomato pomace, grape must, and corn cobs can all be utilized as low-cost culture media components for microbial precision fermentation production systems, albeit sometimes requiring pretreatment with cellulolytic fungi such as Trichoderma spp. (Raghuwanshi et al. Citation2014; Wang, Zhai, and Geng Citation2020). For yeast bio-manufacturing platforms that cannot naturally utilize sucrose, such as Pichia pastoris, engineered expression of an invertase enzyme(s) to convert sucrose to dextrose, with addition of suitable sucrose transporters for cellular uptake for enhanced efficiency are required for the utilization of raw sugar (Martínez et al. Citation2017). Food waste, such as wasted bread many be used as a growth medium for the cultivation of food industry microbial starters (Verni et al. Citation2020).
The logistics and costs for transporting agricultural and food waste to fermentation facilities for use as feedstock will need to be considered. This makes proximity of fermentation facilities to agricultural residues or food manufacturing plants with usable by-products of processing an attractive proposition. The effects of substitution of alternative feedstocks for sugar on the microbial metabolic pathways and food safety of using these alternatives will need to be evaluated.
Scaling of the fermentation process
For precision fermentation to reach its full potential, the ability to scale fermentation processes and isolate the target molecule is critical. The scale-up for precision fermentation is acknowledged as being a major hurdle that needs to be addressed in translation of fermentation technology to commercial scale. There is limited information on how the microorganisms adapt to living in a bioreactor and the interplay between the ecology and evolution of the process (de Lorenzo and Couto Citation2019). There are uncertainties in predicting the behavior of microbes when moving from a laboratory scale to larger scale, which require an improved understanding of the interactions between the physiology of the cell and the environment in larger-scale bioreactors (Delvigne et al. Citation2017).
A recent review discusses the design of efficient microbial cell factories that couple systems metabolic engineering and bioprocesses for industry. There are challenges to the industrial scale production and recovery processes for fermentation-derived compounds, which require integration of upstream (strain development), midstream (fermentation) and downstream (recovery and purification) aspects through the lens of system efficiency (Rangel et al. Citation2020). Irrespective of whether microbes are used for the conversion of substrates into energy, biofuels or production of compounds, there is a need to improve microbes for sustainable biotechnological processes, and this requires robust and very productive microorganisms. The capacity to use inexpensive and readily available fermentation feedstock is also needed for the economic production of fermentation-derived compounds. There is a need for the development of improved microorganisms which can drive the carbon flux toward the desired pathway, have increased tolerance to toxic compounds, and wider substrate uptake range, as well as the ability to generate new products (Paes and Almeida Citation2014). There should be an integrated metabolic-separation and purification view, which examines the growth medium for the microorganism, the effects of metabolic engineering on by-product generation, and the consequences on the cost of separation and purification processes (Rangel et al., Citation2020).
While research and development for a number of precision fermentation-derived proteins, fats and carbohydrates may have been proven on a laboratory and pilot scale, there are still bottlenecks for their production on a commercial scale. There is insufficient commercial fermentation capacity on a global scale to meet the forecasted fermentation demand for the production of precision derived ingredients. For commercial processing, there are options to build new purpose-built facilities for fermentation and downstream processing (Greenfield construction) which are fit for purpose but have a longer timeline and higher cost compared to a Brownfield retrofit. The latter involves retrofit of existing fermentation facilities and building new purpose build downstream processing facilities, which could cost less but still requires substantial capital (Warner Citation2021).
Conclusion
Fermentation for the production of food ingredients to feed a growing population in a more sustainable manner requires a whole new way of thinking about future food value chains. Precision fermentation is an example of a disruptive technology platform that offers the promise of more sustainable food production. There are technical costs, regulatory, and commercialization considerations, as well as the understanding and willingness of the consumer to use nontraditional ingredients. There is also a need to develop and use agreed methodology for substantiating claims relating to the sustainability of the technology. The full potential of using fermentation for the production of the majority of food ingredients has yet to be explored and realized. Adoption of fermentation technology can be facilitated by bringing together of expertise in synthetic biology, traditional, biomass and precision fermentation, scale-up from laboratory to larger scale, downstream processing for recovery of products, and food science to enable the delivery of consumer acceptable food ingredients. Through-chain integration of sustainable ingredient supplies which are acceptable to the consumer, and supported by regulation and policies, are sought after by the market. This will require both the involvement of multiple stakeholders and a transdisciplinary approach to innovation (Knorr and Augustin Citation2021; McClements et al. Citation2021) in precision fermentation-derived food ingredients.
Acknowledgements
The authors would like to acknowledge Dr Martin Cole for helpful comments and suggestions.
Disclosure statement
The authors do not have any competing interests.
Additional information
Funding
References
- Abushal, L. T., M. Salama, M. M. Essa, and M. W. Qoronfleh. 2021. Agricultural biotechnology: Revealing insights about ethical concerns. Journal of Biosciences 46 (3):81. doi: 10.1007/s12038-021-00203-0.
- Acién, F. G., J. M. Fernández, J. J. Magán, and E. Molina. 2012. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnology Advances 30 (6):1344–53. doi: 10.1016/j.biotechadv.2012.02.005.
- Akoh, C. C. 2017. Food lipids. Chemistry, nutrition, and biotechnology. 4th ed. Boca Raton, FL: CRC Press, Taylor & Francis Group.
- Ambrogi, V., F. Bottacini, L. Cao, B. Kuipers, M. Schoterman, and D. van Sinderen. 2021. Galacto-oligosaccharides as infant prebiotics: Production, application, bioactive activities and future perspectives. Critical Reviews in Food Science and Nutrition 8398:1–14. doi: 10.1080/10408398.2021.1953437.
- Anchel, D. 2016. Methods and compositions for egg white protein production. WO201677457 A1
- Anderson, L. A., M. A. Islam, and K. L. J. Prather. 2018. Synthetic biology strategies for improving microbial synthesis of “green” biopolymers. The Journal of Biological Chemistry 293 (14):5053–61. doi: 10.1074/jbc.TM117.000368.
- Augustin, M. A., and P. Udabage. 2007. Influence of processing on functionality of milk and dairy proteins. Advances in Food and Nutrition Research 53:1–38. doi: 10.1016/S1043-4526(07)53001-9.
- Bankefa, O. E., M. Wang, T. Zhu, and Y. Li. 2018. Hac1p homologues from higher eukaryotes can improve the secretion of heterologous proteins in the yeast Pichia pastoris. Biotechnology Letters 40 (7):1149–56. doi: 10.1007/s10529-018-2571-y.
- Barile, D., and R. A. Rastall. 2013. Human milk and related oligosaccharides as prebiotics. Current Opinion in Biotechnology 24 (2):214–9. doi: 10.1016/j.copbio.2013.01.008.
- Barros de Medeiros, V. P., W. K. A. da Costa, R. T. da Silva, T. C. Pimentel, and M. Magnani. 2022. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Critical Reviews in Food Science and Nutrition 62 (18):4929–50. Advance online publication. doi: 10.1080/10408398.2021.1879729.
- Bartkeviciute, D., and K. Sasnauskas. 2003. Studies of yeast Kluyveromyces lactis mutations conferring super-secretion of recombinant proteins. Yeast (Chichester, England) 20 (1):1–11. doi: 10.1002/yea.935.
- Bartsch, D. 2014. GMO regulatory challenges and science: A European perspective. Journal Für Verbraucherschutz Und Lebensmittelsicherheit 9 (S1):51–8. doi: 10.1007/s00003-014-0885-9.
- BCC. 2022. Synthetic Biology: Global Markets. March 2022. Report Code: BIO066G. BCC Publishing.
- BCG. 2021. Nature co-design: A revolution in the making. Accessed September 23, 2022. https://hello-tomorrow.org/wp-content/uploads/2021/03/BCG_Hello_Tomorrow_Nature-Co-design.pdf.
- Becker, E. W. 2007. Micro-algae as a source of protein. Biotechnology Advances 25 (2):207–10. doi: 10.1016/j.biotechadv.2006.11.002.
- Becker, T., J. R. Ogez, and S. E. Builder. 1983. Downstream processing of proteins. Biotechnology Advances 1 (2):247–61. doi: 10.1016/0734-9750(83)90591-8.
- Behm, K., M. Nappa, N. Aro, A. Welman, S. Ledgard, M. Suomalainen, and J. Hill. 2022. Comparison of carbon footprint and water scarcity footprint of milk protein produced by cellular agriculture and the dairy industry. The International Journal of Life Cycle Assessment 27 (8):1017–34. doi: 10.1007/s11367-022-02087-0.
- Béligon, V., G. Christophe, P. Fontanille, and C. Larroche. 2016. Microbial lipids as potential source to food supplements. Current Opinion in Food Science 7:35–42. doi: 10.1016/j.cofs.2015.10.002.
- Béné, C., S. D. Prager, H. A. E. Achicanoy, P. A. Toro, L. Lamotte, C. B. Cedrez, and B. R. Mapes. 2019. Understanding food systems drivers: A critical review of the literature. Global Food Security 23:149–59. doi: 10.1016/j.gfs.2019.04.009.
- Bernaerts, T. M., L. Gheysen, C. Kyomugasho, Z. Jamsazzadeh Kermani, S. Vandionant, I. Foubert, M. E. Hendrickx, and A. M. Van Loey. 2018. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Research 32:150–61. doi: 10.1016/j.algal.2018.03.017.
- Bhatt, V., L. Clark, T. Geistlinger, and J. Lin. 2021. Hypoallergenic recombinant milk proteins and compositions comprising the same. WO2021168343 A2. Filed February 19, 2021 and issued August 26, 2021.
- Bill, R. M. 2014. Playing catch-up with Escherichia coli: Using yeast to increase success rates in recombinant protein production experiments. Frontiers in Microbiology 5:85. doi: 10.3389/fmicb.2014.00085.
- Bourdichon, F., E. Arias, A. Babuchowski, A. Bückle, F. D. Bello, A. Dubois, A. Fontana, D. Fritz, R. Kemperman, S. Laulund, et al. 2021. The forgotten role of food cultures. FEMS Microbiology Letters 368 (14). Advance online publication. doi: 10.1093/femsle/fnab085.
- Bourdichon, F., S. Casaregola, C. Farrokh, J. C. Frisvad, M. L. Gerds, W. P. Hammes, J. Harnett, G. Huys, S. Laulund, A. Ouwehand, et al. 2012. Food fermentations: Microorganisms with technological beneficial use. International Journal of Food Microbiology 154 (3):87–97. doi: 10.1016/j.ijfoodmicro.2011.12.030.
- Buallay, A. 2022. Sustainability reporting in food industry: An innovative tool for enhancing financial performance. British Food Journal 124 (6):1939–58. doi: 10.1108/BFJ-01-2021-0053.
- Burke, D. C. 2012. There’s a long, long trail a-winding: The complexities of GM foods regulation, a cautionary tale from the UK. GM Crops & Food 3 (1):30–9. doi: 10.4161/gmcr.18041.
- Cai, D., Y. Rao, Y. Zhan, Q. Wang, and S. Chen. 2019. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. Journal of Applied Microbiology 126 (6):1632–42. doi: 10.1111/jam.14192.
- Callen, W., T. Richardson, G. Frey, C. Miller, M. Kazaoka, E. Mathur, and J. M. Short. 2003. Amylases, nucleic acids encoding them and methods for making and using them. WO200383054 A2.
- Chai, S., Z. Zhu, E. Tian, M. Xiao, Y. Wang, G. Zou, and Z. Zhou. 2022. Building a versatile protein production platform using engineered Trichoderma reesei. ACS Synthetic Biology 11 (1):486–96. doi: 10.1021/acssynbio.1c00570.
- Chin, P. S. M., K. Y. C. Chan, and C. H. Poon. 2021. Cell hydrolysate composition from cultivated cells and applications thereof. WO20210246480.
- Choe, E., and D. B. Min. 2007. Chemistry of deep-fat frying oils. Journal of Food Science 72 (5):R77–R86. doi: 10.1111/j.1750-3841.2007.00352.x.
- Chomvong, K., J. H. D. Cate, and Y.-S. Jin. 2019. Engineered microorganisms for enhanced use of oligosaccharides. WO201906341 A1.
- Christie, W. W., and X. Han. 2012. Lipid analysis. Cambridge, UK: Woodhead.
- Cooray, S. T., J. J. L. Lee, and W. N. Chen. 2017. Evaluation of brewers’ spent grain as a novel media for yeast growth. AMB Express 7 (1):117. doi: 10.1186/s13568-017-0414-1.
- Cramer, J. F., M. A. B. Kolkman, Z. Ma, M. Scheffers, S. Shipovskov, M. Van Brussel-Z, and S. Yu. 2018. Methods of using thermostable serine proteases. WO2018/118815 A1.
- Davydenko, S. G., J. K. Juselius, T. Munder, E. Bogengruber, J. Jantti, and S. Keranen. 2004. Screening for novel essential genes of Saccharomyces cerevisiae involved in protein secretion. Yeast (Chichester, England) 21 (6):463–71. doi: 10.1002/yea.1063.
- Day, L. 2013. Proteins from land plants – Potential resources for human nutrition and food security. Trends in Food Science & Technology 32 (1):25–42. doi: 10.1016/j.tifs.2013.05.005.
- De Latt, W. E. A. M., and J. S. Gallego Murillo. 2018. Single cell protein from thermophilic fungi. WO201829353 A1.
- de Lorenzo, V., and J. Couto. 2019. The important versus the exciting: Reining contradictions in contemporary biotechnology. Microbial Biotechnology 12 (1):32–4. doi: 10.1111/1751-7915.13348.
- Delvigne, F., R. Takors, R. Mudde, W. van Gulik, and H. Noorman. 2017. Bioprocess scale-up/down as integrative enabling technology: From fluid mechanics to systems biology and beyond. Microbial Biotechnology 10 (5):1267–74. doi: 10.1111/1751-7915.12803.
- Dimidi, E., S. R. Cox, M. Rossi, and K. Whelan. 2019. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 11 (8):1806. doi: 10.3390/nu11081806.
- Dolch, L. J., C. Rak, F. Rebeille, J. Jouhet, M. Leterrier, and E. Marechal. 2017. Alga modified for increased tag production. US10724011. Filed June 27, 2017 and issued July 28, 2020.
- Doxzen, K., and H. Henderson. 2020. Is this safe? Addressing societal concerns about CRISPR-edited foods without reinforcing GMO framing. Environmental Communication 14 (7):865–71. doi: 10.1080/17524032.2020.1811451.
- Du, Z., Y. Xiao, and J. Xu. 2022. How does information exposure affect public attitudes toward GMO in China? The mediating and moderating roles of conspiracy belief and knowledge. Frontiers in Psychology 13:955541. doi: 10.3389/fpsyg.2022.955541.
- Dyson, L., K. Rao, and J. Reed. 2021. High protein food compositions. WO2021/138482 A1. Filed June 30, 2021 and issued December 23, 2021.
- El Tachy, A., D. Hussain, S. P. Singh, P. Shrestha, R. A. Devilla, and J. R. Petrie. 2021. Production of short chain fatty acids. WO2021179051-A1. Filed March 12, 2021 and issue September 16, 2021.
- Elfenbein, A., and J. L. Kolbeck. 2018. Ex vivo meat production. WO2018227016 A1. Filed June 7, 2018 and Issued December 13, 2018.
- Elleuch, M., D. Bedigian, O. Roiseux, S. Besbes, C. Blecker, and H. Attia. 2011. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chemistry 124 (2):411–21. doi: 10.1016/j.foodchem.2010.06.077.
- Koutsoumanis, K., A. Allende, A. Alvarez-Ordóñez, D. Bolton, S. Bover-Cid, M. Chemaly, R. Davies, A. De Cesare, F. Hilbert, R. Lindqvist, EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), et al. 2020. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA Journal 18 (2):5966–56. doi: 10.2903/j.efsa.2020.5966.
- Emtage, J. S., S. Angal, M. T. Doel, T. J. Harris, B. Jenkins, G. Lilley, and P. A. Lowe. 1983. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 80 (12):3671–5. doi: 10.1073/pnas.80.12.3671.
- Eskin, N. A. M., and G. R. List. 2017. Food applications of lipids. In Food lipids: Chemistry, nutrition, and biotechnology, ed. C. C. Akoh, 4th ed., 422–51. Boca Raton, FL: CRC Press, Taylor & Francis Group.
- Facciotti, D., J. G. Metz, and M. Lassner. 2000. Schizochytrium PKS genes. WO200042195 A2. Filed: Jan 14, 2000 and Published: Jul 20, 2000.
- Fan, K. W., and F. Chen. 2007. Chapter 11 – Production of high-value products by marine microalgae thraustochytrids. In Bioprocessing for value-added products from renewable resources, ed. S.-T. Yang, 293–323. Amsterdam: Elsevier.
- FAO, IFAD, UNICEF, WFP, and WHO. 2021. The state of food security and nutrition in the world 2021. Transforming food systems for food security, improved nutrition and affordable healthy diets for all. Rome: FAO. doi: 10.4060/cb4474en.
- Fellows, P. J. 2017. Chapter 6: food biotechnology in food processing technology. In Food processing technology, principles and practice, ed. P. J. Fellows, 4th ed., 387–430. Woodhead Publishing, Elsevier, Cambridge, UK. ISBN 978-0-08-100523. doi: 10.1016/B978-0-08-101907-8.00006-7.
- Foegeding, E. A., and J. P. Davis. 2011. Food protein functionality: A comprehensive approach. Food Hydrocolloids. 25 (8):1853–64. doi: 10.1016/j.foodhyd.2011.05.008.
- Forster, J., N. K. Gunnarsson, and J. B. Nielson. 2005. Metabolically engineered cells for the production of polyunsaturated fatty acids. WO2005118814 A2. Filed: Jun 29, 2007 and Published: Jul 30, 2009.
- Franklin, S., A. Somanchi, K. Espina, G. Rudenko, and P. Chua. 2012. Nucleic acids useful in the manufacture of oil. US8268610 B2. Filed: Nov 30, 2009 and Published: Sep 18, 2012.
- Franklin, S., A. Somanchi, K. Espina, G. Rudenko, and P. Chua. 2013. Renewable chemical production from novel fatty acid feedstocks. US8435767 B2. Filed: Jul 16, 2012 and Published: May 7, 2013.
- Fraser, R., C. D. Simon, and P. O. Brown. 2017. Secretion of heme-containing polypeptides. US 2017/0342131 A1. Filed: Sep 11, 2014 and Published: Oct 29, 2015.
- Frost and Sullivan 2018. Global Fermenation-derived ingredients in human and animal nutrition market, Forecast to 2022. K2E24. Research & Markets. October 2018. Global Fermentation-derived Ingredients in Human and Animal Nutrition Market, Forecast to 2022 (researchandmarkets.com)
- Fu, Y., T. Chen, S. H. Y. Chen, B. Liu, P. Sun, H. Sun, and F. Chen. 2021. The potentials and challenges of using microalgae as an ingredient to produce meat analogs. Trends in Food Science & Technology 112:188–200. doi: 10.1016/j.tifs.2021.03.050.
- Gao, Z., Y. Fang, Y. Cao, H. Liao, K. Nishinari, and G. O. Phillips. 2017. Hydrocolloid-food component interactions. Food Hydrocolloids. 68:149–56. doi: 10.1016/j.foodhyd.2016.08.042.
- Garofalo, C., A. Norici, L. Mollo, A. Osimani, and L. Aquilanti. 2022. Fermentation of microalgal biomass for innovative food production. Microorganisms 10 (10):2069. doi: 10.3390/microorganisms10102069.
- Gautieri, A., M. Parasini, F. Rigoldi, S. Donini, and A. Redaelli. 2018. Thermostabilized amadoriases and uses thereof. WO 2018/235031 A1. Filed: Jun 21, 2018 and Published: Dec 27, 2018.
- Geistlinger, T., H. Jensen, R. Jhala, H. Meerman, B. Ramesh, T. Y. Wagoner, T. S. Johnson, V. W.-X. Wu, and F. Manea 2021. Recombinant components and compositions for use in food products. US20210329941 A1.
- Geistlinger, T., R. Jhala, K. P. Krueger, and B. Ramesh. 2018. Food products comprising milk proteins and non-animal proteins, and methods of producing the same. WO201839632 A1. Filed: Aug 25, 2017 and Published: Jul 3, 2019.
- Geistlinger, T., H. Meerman, H. Jensen, and R. P. Jhala. 2020. Recombinant milk proteins and compositions comprising the same. WO2020219596 A1. Filed: Apr 22, 2020 and Published: Mar 2, 2022.
- Geistlinger, T., Ty, B. Wagoner, R. P. Jhala, and J. R. Glicksberg. 2022. Egg replacer and compositions comprising the egg replacer, and methods for producing the same. US 2022/0192239 A1. Filed: Apr 22, 2020 and Published Jun 23 2022.
- Gibson, M., I. Radman, and A. Arie. 2020. Cheese and yogurt like compositions and related methods. WO2020223700 A1. Filed: May 1, 2020 and Published: Nov 5, 2020.
- Good Food Institute. 2020. 2019 U.S. State of the Industry Report. Plant-based, meat, eggs and dairy. Accessed March 9, 2022. https://gfi.org/wp-content/uploads/2021/01/INN-PBMED-SOTIR-2020-0507.pdf.
- Good Food Institute. 2021. The Science of Fermentation. Accessed March 9, 2022. https://gfi.org/resource/fermentation-state-of-the-industry-report/.
- Gullón, B., J. L. Alonso, and J. C. Parajó. 2008. Experimental evaluation of alternative fermentation media for L-lactic acid production from apple pomace. Journal of Chemical Technology & Biotechnology 83 (5):609–17. doi: 10.1002/jctb.1838.
- Gunstone, F. D., and F. A. Norris. 1983. Lipids in foods. Oxford: Pergamon Press.
- Hassoun, A., A. El-Din Bekhit, A. R. Jambrak, J. M. Regenstein, F. Chemat, J. D. Morton, M. Gudjónsdóttir, M. Carpena, M. A. Prieto, P. Varela, et al. 2022. The fourth industrial revolution in the food industry – Part II: Emerging food trends. Critical Reviews in Food Science and Nutrition AHEAD-OF-PRINT 1-131. doi: 10.1080/10408398.2022.2106472.
- Helfrich, M., and S. Jennewein. 2019. Process for the purification of l-fucose from a fermentation broth. WO2019101629 A1. Filed: Nov 15, 2018 and Published: May 31, 2019.
- Hettinga, K., and E. Bijl. 2022. Can recombinant milk proteins replace those produced by animals? Current Opinion in Biotechnology 75:102690. doi: 10.1016/j.copbio.2022.102690.
- Hoeven Van Der, R. A., I. Galaev, and V. M. Boer. 2015. Production of diterpenes. WO2015007748-A1. Filed 15 Jul 2014 and Published 22 Jan 2015.
- Hong, S.-P., P. L. Sharpe, Z. Xue, N. Yadav, and Q. Zhe. 2010. High eicosapentaenoic acid oils from improved optimized strains of Yarrowia lipolytica. WO2010147907 A1. Filed: Jun 14, 2010 and Published: Dec 23, 2010.
- Huang, C., Jr., H. Lin, and X. Yang. 2012. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. Journal of Industrial Microbiology & Biotechnology 39 (3):383–99. doi: 10.1007/s10295-011-1082-9.
- Ivey, F. D., J. A. Kreps, and K. Harshal. 2021. Enzyme compositions and methods of making them. WO2021133852 A1. Filed: Dec 22, 2020 and Published: Jul 1, 2021.
- Ivey, F. D., J. A. Kreps, C. A. Tindell, and W. Zhong. 2021a. Systems and methods for high yielding recombinant microorganisms and uses thereof. WO2021158817 A1. Filed: Feb 4, 2021 and Published: Aug 12, 2021.
- Ivey, F. D., J. A. Kreps, J. Helvey, and D. Anchel. 2021b. Modification of protein glycosylation in microorganisms. US20210337826 A1. Filed: Aug 21, 2019 and Published: Feb 27, 2020.
- Jennewein, S. 2015a. Process for efficient purification of neutral human milk oligosaccharides (HMOs) from microbial fermentation. WO2015106943 A1. Filed: Dec 23, 2014 and Published: Jul 23, 2015.
- Jennewein, S. 2015b. Production of oligosaccharides. WO201536138 A1. Filed: Sep 10, 2013 and Published: Sep 27, 2021.
- Jennewein, S. 2021. Cereal compositions containing human milk oligosaccharides. WO202152584 A1. Filed: Sep 19, 2019 and Published: Mar 25, 2021.
- Jennewein, S., M. Helfrich, and B. Engels. 2019. Process for purifying sialylated oligosaccharides. WO201943029 A1. Filed: Aug 29, 2018 and Published: Mar 30, 2020.
- Jennewein, S., and D. Wartenberg. 2017. Production of human milk oligosaccharides in microbial hosts with engineered import/export. WO201742382 A1. Filed: Sep 12, 2016 and Published: Mar 16, 2017.
- Johnson, E. A. 2003. Phaffia rhodozyma: Colorful odyssey. International Microbiology: The Official Journal of the Spanish Society for Microbiology 6 (3):169–74. doi: 10.1007/s10123-003-0130-3.
- Johnson, M. B., and M. C. Janse. 2019. Production of oligosaccharides. WO2019226431 A1. Filed: May 15, 2019 and Published: Apr 7, 2021.
- Juturu, V., and J. C. Wu. 2018. Heterologous protein expression in Pichia pastoris: Latest research progress and applications. Chembiochem: A European Journal of Chemical Biology 19 (1):7–21. doi: 10.1002/cbic.201700460.
- Knorr, D., and M. A. Augustin. 2021. From value chains to food webs: The quest for lasting food systems. Trends in Food Science & Technology 110:812–21. doi: 10.1016/j.tifs.2021.02.037.
- Knorr, D., and M. A. Augustin. 2022. Food systems at a watershed: Unlocking the benefits of technology and ecosystem symbioses. Critical Reviews in Food Science and Nutrition :1–18. Advance online publication. doi: 10.1080/10408398.2021.2023092.
- Koivikko, H., and J. Jarviene. 2020. Process for purifying a human milk oligosaccharide and related compositions. WO2020154565 A1. Filed: Sep 28, 2021 and Published: Apr 7, 2022.
- Kristo, E., and M. Corredig. 2015. Functional properties of food proteins. In Applied food protein chemistry, ed. Z. Ustunol 47–73. Cichester, UK: John Wiley & Sons. doi: 10.1002/9781118860588.ch5.
- Kyle, D. J., S. E. Reeb, and V. J. Sicotte. 1991. Docosahexaenoic acid, methods for its production and compounds containing the same. WO9111918 A1. Filed: Feb 4, 1991 and Published: Aug 22, 1991.
- Labrou, N. E. 2014. Protein purification: An overview. Methods in Molecular Biology (Clifton, N.J.) 1129:3–10. doi: 10.1007/978-1-62703-977-2_1.
- Lähteenmäki-Uutela, A., M. Rahikainen, A. Lonkila, and B. Yang. 2021. Alternative proteins and EU food law. Food Control. 130:108336. doi: 10.1016/j.foodcont.2021.108336.
- Laulund, S., A. Wind, P. M. F. Derkx, and V. Zuliani. 2017. Regulatory and safety requirements for food cultures. Microorganisms 5 (2):28. doi: 10.3390/microorganisms5020028.
- Li, J.-M., and S.-P. Nie. 2016. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocolloids. 53:46–61. doi: 10.1016/j.foodhyd.2015.01.035.
- Linder, T. 2019. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Security 11 (2):265–78. doi: 10.1007/s12571-019-00912-3.
- Liu, N., B. Liu, G. Wang, Y.-H. V. Soong, Y. Tao, W. Liu, and D. Xie. 2020. Lycopene production from glucose, fatty acid and waste cooking oil by metabolically engineered Escherichia coli. Biochemical Engineering Journal 155:107488. doi: 10.1016/j.bej.2020.107488.
- Liu, N., Y.-H. V. Soong, I. Mirzaee, A. Olsen, P. Yu, H.-W. Wong, and D. Xie. 2021. Biomanufacturing of value-added products from oils or fats: A case study on cellular and fermentation engineering of Yarrowia lipolytica. Biotechnology and Bioengineering 118 (4):1658–73. doi: 10.1002/bit.27685.
- Lu, C., H. Xia, and W. W. Liu. 2021. A novel method to manufacture synthetic meat. WO2021138674-A1. Filed: Jan 4, 2021 and Published: Jul 8, 2021.
- Ma, W., Y.-Z. Wang, F.-T. Nong, F. Du, Y.-S. Xu, P.-W. Huang, and X.-M. Sun. 2021. An emerging simple and effective approach to increase the productivity of thraustochytrids microbial lipids by regulating glycolysis process and triacylglycerols’ decomposition. Biotechnology for Biofuels 14 (1):247. doi: 10.1186/s13068-021-02097-4.
- Mahadevan, K., F. Ayoughi, I. Joshi, J. A. Kreps, H. Kshirsagar, F. D. Ivey, W. Zhong, E. Lin, A. Chapeaux, and S. Govind. 2021. Non-animal based protein sources with functional properties. WO202134980 A1. Filed: Aug 19, 2020 and Published: Feb 25, 2021.
- Mahajan, D., S. Sengupta, and S. Sen. 2019. Strategies to improve microbial lipid production: Optimization techniques. Biocatalysis and Agricultural Biotechnology 22:101321. doi: 10.1016/j.bcab.2019.101321.
- Małecki, J., S. Muszyński, and B. G. Sołowiej. 2021. Proteins in food systems—Bionanomaterials, conventional and unconventional sources, functional properties, and development opportunities. Polymers 13 (15):2506. doi: 10.3390/polym13152506.
- Marco, M. L., D. Heeney, S. Binda, C. J. Cifelli, P. D. Cotter, B. Foligné, M. Gänzle, R. Kort, G. Pasin, A. Pihlanto, et al. 2017. Health benefits of fermented foods: Microbiota and beyond. Current Opinion in Biotechnology 44:94–102. doi: 10.1016/j.copbio.2016.11.010.
- Matwiejuk, M., N. Khanzhin, P. Chassagne, and M. Hederos. 2017. Separation of oligosaccharides from fermentation broth. WO2017152918 A1. Filed: Mar 7, 2017 and Published: Sep 14, 2017.
- McClements, D. J., R. Barrangou, C. Hill, J. L. Kokini, M. A. Lila, A. S. Meyer, and L. Yu. 2021. Building a resilient, sustainable, and healthier food supply through innovation and technology. Annual Review of Food Science and Technology 12 (1):1–28. doi: 10.1146/annurev-food-092220-030824.
- McNamara, H. M., S. Tickus, D. Heller, and A. Shumaler. 2018. Methods of producing lipids. WO2018227184-A1. Filed: Dec 21, 2018 and Published: Jul 4, 2019.
- Martínez, D., C. Menéndez, L. Hernández, A. Sobrino, L. E. Trujillo, I. Rodríguez, and E. R. Pérez. 2017. Scaling-up batch conditions for efficient sucrose hydrolysis catalyzed by an immobilized recombinant Pichia pastoris cells in a stirrer tank reactor. Electronic Journal of Biotechnology 25:39–42. doi: 10.1016/j.ejbt.2016.11.003.
- Martí-Quijal, F. J., S. Khubber, F. Remize, I. Tomasevic, E. Roselló-Soto, and F. J. Barba. 2021. Obtaining antioxidants and natural preservatives from food by-products through fermentation: A review. Fermentation 7 (3):106. doi: 10.3390/fermentation7030106.
- Mehri, D., N. A. Perendeci, and Y. Goksungur. 2021. Utilization of whey for red pigment production by Monascus purpures in submerged fermentation. Fermentation 7 (2):75. doi: 10.3390/fermentation7020075.
- Metz, J. M., J. H. Flatt, J. M. Kuner, and W. R. Barclay. 2011. PUFA polyketide synthase systems and uses thereof. US7973149 B2. Filed: Jun 28, 2007 and Published: Mar 1, 2011.
- Mosier, S. L., A. Rimal, and M. M. Ruxton. 2020. A song of policy incongruence: The missing choir of consumer preferences in GMO-labeling policy outcomes. Review of Policy Research 37 (4):511–34. doi: 10.1111/ropr.12391.
- Mukherjee, A., B. Gómez-Sala, E. M. O’Connor, J. G. Kenny, and P. D. Cotter. 2022. Global regulatory frameworks for fermented foods: A review. Frontiers in Nutrition 9:902642. doi: 10.3389/fnut.2022.902642.
- Myhr, A. I., and T. Traavik. 2002. The precautionary principle: Scientific uncertainty and omitted research in the context of GMO use and release. Journal of Agricultural and Environmental Ethics 15 (1):73–86. Kluver Academic Publishers. Netherlands. doi: 10.1023/A:1013814108502.
- Nevalainen, H., R. Peterson, and N. Curach. 2018. Overview of gene expression using filamentous fungi. Current Protocols in Protein Science 92 (1):e55. doi: 10.1002/cpps.55.
- Nieves, L. M., L. A. Panyon, and X. Wang. 2015. Engineering sugar utilization and microbial tolerance toward lignocellulose conversion. Frontiers in Bioengineering and Biotechnology 3:17. doi: 10.3389/fbioe.2015.00017.
- Nishi, T., Y. Ito, Y. Nakamura, T. Yamaji, N. Hashiba, M. Tamai, Y. Yasohara, J. Ishii, and A. Kondo. 2022. One-step in vivo assembly of multiple DNA fragments and genomic integration in Komagataella phaffii. ACS Synthetic Biology 11 (2):644–54. doi: 10.1021/acssynbio.1c00302.
- Nishinari, K., and E. Doi. 1993. Food Hydrocolloids. Structures, properties and functions. New York: Plenum Press.
- Ochsenreither, K., C. Glück, T. Stressler, L. Fischer, and C. Syldatk. 2016. Production strategies and applications of microbial single cell oils. Frontiers in Microbiology 7:1539. doi: 10.3389/fmicb.2016.01539.
- Olempska-Beer, Z. S., R. I. Merker, M. D. Ditto, and M. J. DiNovi. 2006. Food-processing enzymes from recombinant microorganisms—A review. Regulatory Toxicology and Pharmacology : RTP 45 (2):144–58. doi: 10.1016/j.yrtph.2006.05.001.
- Ouzounov, N., J. R. Mellin, and J. Co. 2021. Animal-free dietary collagen. WO2021150959-A1. Filed: Jan 22, 2021 and Published: Jul 29, 2021.
- Ouzounov, N. 2017. Expression of proteins in gram-negative bacteria where in the ratio of perplasmic volume to cytoplasmic volume is between 0.5:1 and 10:1. WO2017172994-A1. Filed 29 March 2017 and Published 05 Oct 2017.
- Paes, B. G., and J. R. Almeida. 2014. Genetic improvement of microorganisms for applications in biorefineries. Chemical and Biological Technologies in Agriculture 1 (1):21. doi: 10.1186/s40538-014-0021-1.
- Panesar, R., C. Kaur, and P. S. Panesar. 2015. Production of microbial pigments utilizing agro-industrial waste: A review. Current Opinion in Food Science 1:70–6. doi: 10.1016/j.cofs.2014.12.002.
- Pappalardo, G., M. D’Amico, and J. L. Lusk. 2021. Comparing the views of the Italian general public and scientists on GMOs. International Journal of Food Science & Technology 56 (7):3641–50. doi: 10.1111/ijfs.14993.
- Pandya, R., P. Gandhi, S. Ji, D. Beauchamp, and L. Hom. 2016. Compositions comprising a casein and methods of producing the same. WO201629193 A1. Filed: Aug 21, 2015 and Published: Feb 25, 2016.
- Patel, A. K., F. P. J. B. Albarico, P. K. Perumal, A. P. Vadrale, C. T. Nian, H. T. B. Chau, C. Anwar, H. M. U. D. Wani, A. Pal, R. Saini, et al. 2022. Algae as an emerging source of bioactive pigments. Bioresource Technology 351:126910. doi: 10.1016/j.biortech.2022.126910.
- Patel, A., D. Karageorgou, E. Rova, P. Katapodis, U. Rova, P. Christakopoulos, and L. Matsakas. 2020. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 8 (3):434. doi: 10.3390/microorganisms8030434.
- Pavláková Dočekalová, M., and A. Kocmanová. 2018. Comparison of sustainable environmental, social, and corporate governance value added models for investors decision making. Sustainability 10 (3):649. doi: 10.3390/su10030649.
- Paz, A. M., and X.-N. Zhang. 2018. The GMO industry: A neglected earthly frontier. Journal of Hunger & Environmental Nutrition 13 (2):277–88. doi: 10.1080/19320248.2016.1227755.
- Pérez-Torrado, R., A. Querol, and J. M. Guillamón. 2015. Genetic improvement of non-GMO wine yeasts: Strategies, advantages and safety. Trends in Food Science & Technology 45 (1):1–11. doi: 10.1016/j.tifs.2015.05.002.
- Persikov, A. V., N. Ouzounov, and A. Lorestani. 2019. Methods and systems for engineering collagen. WO2019103981A1. Filed: Nov 19, 2018 and Published: May 31, 2019.
- Pham, P. V. 2018. Chapter 19 – Medical biotechnology: Techniques and applications. In Omics Technologies and Bio-Engineering, ed. D. Barh, and V. Azevedo, vol. 1,449–69. Academic Press., San Diego, USA. Canbridge doi: 10.1016/B978-0-12-804659-3.00019-1.
- Phillips, L. G., D. M. Whitehead, and J. Kinsella. 1994. Structure – Function properties of food proteins. Boston: Academic Press.
- Picataggio, S. K., N. S. Yadav, and Q. Q. Zhu. 2004. Production of polyunsaturated fatty acids in oleaginous yeasts. WO2004101757 A2. Filed: May 7, 2004 and Published: Nov 25, 2004.
- Piechocki, J., D. Zdanis, L. M. Norris, W. Rakitsky, and B. Klamczynska. 2011. Lipid-rich microalgal flour food compositions. WO2011130578 A2. Filed: Apr 14, 2011 and Published: Nov 8, 2012.
- Pietzsch, M., T. Herkel, and C. Marx. 2009. Thermostable transglutaminases. WO2009/030211 A2. Filed: Aug 30, 2008 and Published: Jun 25, 2009.
- Pivot Food Investment, 2022. Fermentation companies and investors. Accessed March 25, 2022. https://pivotfood.org/fermentation-companies/.
- Pleissner, D., W. C. Lam, Z. Sun, and C. S. K. Lin. 2013. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresource Technology 137:139–46. doi: 10.1016/j.biortech.2013.03.088.
- Poinski, M. 2022. How Yali Bio plans to revolutionize alternative fats. Food Dive. Accessed March 25, 2022. https://www.fooddive.com/news/how-yali-bio-plans-to-revolutionize-alternative-fats/620179/.
- Pottinger, S. A., D. M. Jacobson, R. Patnaik, V. Gopalakrishnan, and Z. Friar. 2022. Systems for end-to-end optimization of precision fermentation-produced animal proteins in food applications. WO2022/246284 A2. Filed: May 20, 2022 and Published: Nov 24, 2022.
- Pozas, N. S., and C. Bushnell. 2020. Fermentation can help build a more efficient and sustainable food system – Here’s how. Accessed 25 March 2022. https://www.weforum.org/agenda/2020/11/fermentation-can-help-build-a-more-efficient-and-sustainable-food-system-here-s-how/.
- Prakash, I., G. Ma, C. Mercogliano, and C. Hartley. 2020. Mogroside Biocatalysis Methods. WO2020/176668 A1. Filed: Feb 26, 2020 and Published: Oct 28, 2021.
- Prielhofer, R., J. J. Barrero, S. Steuer, T. Gassler, R. Zahrl, K. Baumann, M. Sauer, D. Mattanovich, B. Gasser, and H. Marx. 2017. GoldenPiCS: A golden gate-derived modular cloning system for applied synthetic biology in the yeast Pichia pastoris. BMC Systems Biology 11 (1):123. doi: 10.1186/s12918-017-0492-3.
- Radman, I., R. Reith, A. Adames, P. Stoddard, and D. Panfair. 2022. Micelle and micelle-like compositions and related methods. WO2022098853A1. Filed: Nov 4, 2021 and Published: May 12, 2022.
- Raghuwanshi, S., D. Deswal, M. Karp, and R. C. Kuhad. 2014. Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel 124:183–9. doi: 10.1016/j.fuel.2014.01.107.
- Raimo, N., E. de Nuccio, A. Giakoumelou, F. Petruzzella, and F. Vitolla. 2020. Non-financial information and cost of equity capital: An empirical analysis in the food and beverage industry. British Food Journal 123 (1):49–65. doi: 10.1108/BFJ-03-2020-0278.
- Raman, R. 2017. The impact of genetically modified (GM) crops in modern agriculture: A review. GM Crops & Food 8 (4):195–208. doi: 10.1080/21645698.2017.1413522.
- Rangel, A. E. T., J. M. Gómez Ramírez, and A. F. González Barrios. 2020. From industrial by-products to value-added compounds: The design of efficient microbial cell factories by coupling systems metabolic engineering and bioprocesses. Biofuels, Bioproducts and Biorefining 14 (6):1228–38. doi: 10.1002/bbb.2127.
- Ratledge, C., H. Streekstra, Z. Cohen, and J. Fichtali. 2010. 9 – Downstream processing, extraction, and purification of single cell oils. In Single cell oils, ed. Z. Cohen and C. Ratledge, 2nd ed., 179–97. Urbana, IL: AOCS Press. doi: 10.1016/B978-1-893997-73-8.50013-X.
- Raymond, F., D. Rolland, M. Gauthier, and M. Jolivet. 1998. Purification of a recombinant protein expressed in yeast: Optimization of analytical and preparative chromatography. Journal of Chromatography. B, Biomedical Sciences and Applications 706 (1):113–21. doi: 10.1016/S0378-4347(97)00512-4.
- Reucker, C. M., D. Dimasi, J. M. Hansen, P. J. Mirrasol, R. B. Bailey, G. T. Weeder, III., T. Kaneko, and W. R. Barclay. 2001. Enhanced production of lipids containing polyenoic fatty acids by high density cultures of eukaryotic microbes in fermentors. WO200154510 A1. Filed: Jan 26, 2001 and Published: Oct 17, 2002.
- Rischer, H., G. R. Szilvay, and K.-M. Oksman-Caldentey. 2020. Cellular agriculture—Industrial biotechnology for food and materials. Current Opinion in Biotechnology 61:128–34. doi: 10.1016/j.copbio.2019.12.003.
- Ritala, A., S. T. Häkkinen, M. Toivari, and M. G. Wiebe. 2017. Single cell protein—State-of-the-art, industrial landscape and patents 2001–2016. Frontiers in Microbiology 8:2009. doi: 10.3389/fmicb.2017.02009.
- Roy-Chaudhuri, B., and S. Shankar. 2020. Strains and methods for production of heme-containing proteins. WO2020219972 A1. Filed: Apr 24, 2020 and Published: Oct 29, 2020.
- Rzymski, P., and A. Królczyk. 2016. Attitudes toward genetically modified organisms in Poland: To GMO or not to GMO? Food Security 8 (3):689–97. doi: 10.1007/s12571-016-0572-z.
- Sato, K., and S. Ueno. 2014. Physical properties of fats in food. In Fats in food technology, ed. K.K. Rajah. 2nd ed., 1–38. Hoboken, NJ: John Wiley & Sons.
- Santiago-Díaz, P., M. Rico, A. Rivero, and M. Santana-Casiano. 2022. Bioactive metabolites of microalgae from Canary Islands for functional food and feed uses. Chemistry & Biodiversity 19 (9):1. doi: 10.1002/cbdv.202200230.
- Schubert, N., E. García-Mendoza, and I. Pacheco-Ruiz. 2006. Carotenoid composition of marine red algae. Journal of Phycology 42 (6):1208–16. doi: 10.1111/j.1529-8817.2006.00274.x.
- Senanayake, S. P. J. N., and J. Fichtali. 2006. Single-cell oils as sources of nutraceutical and specialty lipids: Processing technologies and applications. In Nutraceutical and specialty lipids and their co-products, ed. F. Shahidi, 251–80. Boca Raton, FL: CRC Press.
- Seo, S.-O., and Y.-S. Jin. 2022. Next-generation genetic and fermentation technologies for safe and sustainable production of food ingredients: Colors and flavorings. Annual Review of Food Science and Technology 13 (1):463–88. doi: 10.1146/annurev-food-052720-012228.
- Shahidi, F., and P. Ambigaipalan. 2018. Omega-3 polyunsaturated fatty acids and their health benefits. Annual Review of Food Science and Technology 9 (1):345–81. doi: 10.1146/annurev-food-111317-095850.
- Shankar, S., and M. A. Hoyt. 2016. Expression constructs and methods of genetically engineering methylotrophic yeast. WO2016183163-A1. Filed: May 11, 2016 and Published: Nov 17, 2016.
- Shiferaw Terefe, N., and M. A. Augustin. 2020. Fermentation for tailoring the technological and health related functionality of food products. Critical Reviews in Food Science and Nutrition 60 (17):2887–913. doi: 10.1080/10408398.2019.1666250.
- Sijtsma, L., A. J. Anderson, and C. Ratledge. 2010. 7 – Alternative carbon sources for heterotrophic production of docosahexaenoic acid by the marine alga Crypthecodinium cohnii. In Single cell oils, ed. Z. Cohen and C. Ratledge, 2nd ed., 131–49. Urbana, IL: AOCS Press.
- Song, I., S. Kim, J. Kim, H. Oh, J. Jang, S. J. Jeong, K. Baek, W.-S. Shin, S. J. Sim, and E. Jin. 2022. Macular pigment-enriched oil production from genome-edited microalgae. Microbial Cell Factories 21 (1):27. doi: 10.1186/s12934-021-01736-7.
- Southey, F. 2021. Cracking the ‘world’s first’ animal-free egg white through fermenation. Food Navigator. Accessed March 25, 2022. https://www.foodnavigator.com/Article/2021/02/09/Clara-Foods-on-cracking-the-world-s-first-animal-free-egg-white.
- Spohner, S. C., H. Müller, H. Quitmann, and P. Czermak. 2015. Expression of enzymes for the usage in food and feed industry with Pichia pastoris. Journal of Biotechnology 202:118–34. doi: 10.1016/j.jbiotec.2015.01.027.
- Stengel, D. B., and S. Connan. 2015. Marine algae: A source of biomass for biotechnological applications. In Natural products from marine algae: Methods and protocols, ed. D. B. Stengel and S. Connan, vol. 1308, 1–37. New York: Humana Press. doi: 10.1007/978-1-4939-2684-8_1.
- Strickland, L. 2021. Milk product compositions. WO2021242866 A1. Filed: May 26, 2021 and Published: Dec 2, 2021.
- Sungsoo, S., and T. Finocchiaro. 2009. Handbook of prebiotics and probiotic ingredients. Health benefits and food applications. Boca Raton, FL: CRC Press.
- Swanson, D., R. Block, and S. A. Mousa. 2012. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition (Bethesda, Md.) 3 (1):1–7. doi: 10.3945/an.111.000893.
- Teng, T. S., Y. L. Chin, K. F. Chai, and W. N. Chen. 2021. Fermentation for future food systems. EMBO Reports 22 (5):e52680. doi: 10.15252/embr.202152680.
- Tubb, C., and T. Seba. 2019. Rethinking food and agriculture 2020-2030: The second domestication of plants and animals, the disruption of the cow, and the collapse of industrial livestock farming. Accessed September 18, 2022. https://static1.squarespace.com/static/585c3439be65942f022bbf9b/t/5d7fe0e83d119516bfc0017e/1568661791363/RethinkX+Food+and+Agriculture+Report.pdf.
- Uçkun Kiran, E., A. Salakkam, A. P. Trzcinski, U. Bakir, and C. Webb. 2012. Enhancing the value of nitrogen from rapeseed meal for microbial oil production. Enzyme and Microbial Technology 50 (6-7):337–42. doi: 10.1016/j.enzmictec.2012.03.004.
- van der Meulen, B. 2012. The core of food law: A critical reflection on the single most important provision in all of EU food law. European Food and Feed Law Review 7 (3):117–25. http://www.jstor.org/stable/24325363.
- Verni, M., A. Minisci, S. Convertino, L. Nionelli, and C. G. Rizzello. 2020. Wasted bread as substrate for the cultivation of starters for the food industry. Frontiers in Microbiology 11:293. doi: 10.3389/fmicb.2020.00293.
- Vieira Gomes, A. M., T. Souza Carmo, L. S. Carvalho, F. M. Bahia, and N. S. Parachin. 2018. Comparison of yeasts as hosts for recombinant protein production. Microorganisms 6 (2):38. doi: 10.3390/microorganisms6020038.
- Voigt, C. A. 2020. Synthetic biology 2020–2030: Six commercially-available products that are changing our world. Nature Communications 11 (1):6379. doi: 10.1038/s41467-020-20122-2.
- Vuree, S. 2020. Chapter 17 – Pichia pastoris expression system: An impending candidate to express protein in industrial and biopharmaceutical domains. In New and future developments in microbial biotechnology and bioengineering, ed. J. Singh and P. Gehlot, 223–34. Amsterdam: Elsevier Inc. doi: 10.1016/B978-0-12-821007-9.00017-6.
- Wagner, J. M., and J. S. Alper. 2016. Synthetic biology and molecular genetics in non-conventional yeasts: Current tools and future advances. Fungal Genetics and Biology: FG & B 89:126–36. doi: 10.1016/j.fgb.2015.12.001.
- Walter, J., and D. Pinel. 2021. Improved oligosaccharide production in yeast. WO202130617 A2. Filed: Aug 13, 2020 and Published: Jun 3, 2021.
- Wang, X. Q., Q. H. Wang, H. Zhi Ma, and W. Yin. 2009. Lactic acid fermentation of food waste using integrated glucoamylase production. Journal of Chemical Technology & Biotechnology 84 (1):139–43. doi: 10.1002/jctb.2007.
- Wang, H., L. Zhai, and A. Geng. 2020. Enhanced cellulase and reducing sugar production by a new mutant strain Trichoderma harzianum EUA20. Journal of Bioscience and Bioengineering 129 (2):242–9. doi: 10.1016/j.jbiosc.2019.08.016.
- Warner, M. 2019. Industrial biotechnology commercialization handbook. SanDiego: Warner Advisors LLC.
- Warner, M. 2021. Commercial fermentation: Opportunities and bottlenecks. Accessed September 21 2022. https://www.youtube.com/watch?v=aqr18eiot9Q.
- Watson, E. 2018. Cargill launches EverSweet fermented steviol glycosides. Accessed March 25 2022. https://www.foodnavigator-usa.com/Article/2018/03/20/Cargill-launches-EverSweet-fermented-steviol-glycosides.
- Weninger, A., J. E. Fischer, H. Raschmanová, C. Kniely, T. Vogl, and A. Glieder. 2018. Expanding the CRISPR/Cas9 toolkit for Pichia pastoris with efficient donor integration and alternative resistance markers. Journal of Cellular Biochemistry 119 (4):3183–98. doi: 10.1002/jcb.26474.
- Williams, R. A. 2021. Opportunities and challenges for the introduction of new food proteins. Annual Review of Food Science and Technology 12:75–91. doi: 10.1146/annurev-food-061220-012838.
- Wolff, A., V. Gaydar, and O. Cohavi. 2022. Methods of producing animal-free casein compositions, casein compositions and use of same. WO202238601A1. Filed: Aug 17, 2021 and Published: Feb 24, 2022.
- Xie, D., Y. H. Soong, N. Liu, J. Qin, S. Chen, J. Keats, I. Mirzaee, and C. Lawton. 2017. A new biomanufacturing platform for bioconversion of plant oils into high-value products. Advances in Biochemistry and Biotechnology 3 (2):149. doi: 10.29011/2574-7258.000049.
- Xu, Z., H. Wang, P. Cheng, T. Chang, P. Chen, C. Zhou, and R. Ruan. 2020. Development of integrated culture systems and harvesting methods for improved algal biomass productivity and wastewater resource recovery – A review. The Science of the Total Environment 746:141039. doi: 10.1016/j.scitotenv.2020.141039.
- Yang, R., Z. Chen, P. Hu, S. Zhang, and G. Luo. 2022. Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresource Technology 361:127677. doi: 10.1016/j.biortech.2022.127677.
- Yang, Y. T., and B. Chen. 2016. Governing GMOs in the USA: Science, law and public health. Journal of the Science of Food and Agriculture 96 (6):1851–5. doi: 10.1002/jsfa.7523.
- Yang, X., A. Li, X. Li, L. Sun, and Y. Guo. 2020. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends in Food Science & Technology 102:1–15. doi: 10.1016/j.tifs.2020.05.020.
- Yoon, S. J., S. H. Kang, S. Y. Jun, A. S. Kwon, and E. J. Lee. 2021a. A method for preparing bovine myoglobin using Escherichia coli. WO2021140488 A1. Filed: Jan 9, 2021 and Published: Jul 15, 2021.
- Yoon, S. J., S. H. Kang, S. Y. Jun, A. S. Kwon, and E. J. Lee. 2021b. A method for preparing soy leghemoglobin using Escherichia coli. WO2021140487 A1. Filed: Jan 9, 2021 and Published: Jul 15, 2021.
- Zahrl, R. J., D. Mattanovich, and B. Gasser. 2018. The impact of ERAD on recombinant protein secretion in Pichia pastoris (syn Komagataella spp.). Microbiology (Reading, England) 164 (4):453–63. doi: 10.1099/mic.0.000630.
- Zhang, L., and G. Huo. 2017. Cold-adapted alpha-amylase strain, construction method and method of producing alpha-amylase. CN106755040 A. Filed: Dec 26, 2016 and Published: May 31, 2017.
- Zhang, Y. P., J. Sun, and Y. Ma. 2017. Biomanufacturing: History and perspective. Journal of Industrial Microbiology & Biotechnology 44 (4-5):773–84. doi: 10.1007/s10295-016-1863-2.