Abstract
Kimchi is a traditional fermented vegetable side dish in Korea and has become a global health food. Kimchi undergoes spontaneous fermentation, mainly by lactic acid bacteria (LAB) originating from its raw ingredients. Numerous LAB, including the genera Leuconostoc, Weissella, and Lactobacillus, participate in kimchi fermentation, reaching approximately 9–10 log colony forming units per gram or milliliter of food. The several health benefits of LAB (e.g., antioxidant and anti-inflammatory properties) combined with their probiotic potential in complex diseases including obesity, cancer, atopic dermatitis, and immunomodulatory effect have generated an interest in the health effects of LAB present in kimchi. In order to estimate the potential of kimchi as a probiotic food, we comprehensively surveyed the health functionalities of kimchi and kimchi LAB, and their effects on human gut environment, highlighting the probiotics function.
Introduction
Kimchi is a traditional fermented vegetable side dish in Korea, prepared by blending the pre-brined main vegetables such as kimchi cabbage or radish, various seasonings such as red pepper, garlic, ginger, green onion, sliced radish, salt, sugar, and minor ingredients including fermented seafood, fruits, vegetables, meat, fish, and cereals. The mixture of input ingredients in the fermentation process gives kimchi unique organoleptic characteristics that influence appetite. Kimchi is sorted based on various criteria, including wateriness, main ingredients, and preparation differences. There are hundreds of varieties of kimchi that differ in ingredients and methods, with distinctive nutritional, biochemical, and organoleptic features (Jung, Lee, and Jeon Citation2014). Broadly, the common characteristics of kimchi are salted ingredients, fermentation at a relatively low temperature (below 10 °C), and fermentative microbes derived from various raw ingredients (Jung, Lee, and Jeon Citation2014; Lee et al. Citation2020).
Kimchi is a well-known health food worldwide. Kimchi has low calories (18 kcal/100 g), several vitamins (vitamin A, vitamin C, thiamin, and riboflavin), minerals (calcium, potassium, iron, and phosphorus), and dietary fiber (24% on a dry basis) (Patra et al. Citation2016). In addition, kimchi contains functional and bioactive components including gingerol, capsaicin, carotenoids, allyl compounds, isothiocyanate, and indole-3-carbinol. Several health benefits of kimchi consumption in the experimental animal models have been reported: anticancer activity in mouse with colitic cancer models by kimchi intake (Han et al. Citation2020b); anti-obesity activity in mice by kimchi or kimchi-derived LAB intake (Park, Oh, et al. Citation2020; Park, Kwon, et al. Citation2020); antidiabetic activity in rats by kimchi intake (Islam and Choi Citation2009); alleviation of obesity-induced neuroinflammation in mice by kimchi intake (Kim, Dang, and Ha Citation2022b). These health benefits result from the high nutritive raw ingredients as well as fermentation by kimchi microbiota (Patra et al. Citation2016). Kimchi undergoes spontaneous fermentation, mainly by lactic acid bacteria (LAB) originating from its raw ingredients (Lee, Jung, et al. Citation2015). A study conducted by Lee et al. (Citation2020) reported that in cabbage kimchi, the LAB count reached approximately 9–10 log colony forming units (CFU) per gram or milliliter of food. Numerous LAB, including the genera Leuconostoc, Weissella, Lactobacillus, and Pediococcus, participate in kimchi fermentation (Kim, Dang, and Ha Citation2022a). The kimchi microbiota, including LAB, varies depending on its raw ingredients (Jeong et al. Citation2013).
Fermented food is an excellent source of LAB. Accordingly, dietary intake of kimchi may contribute to the enrichment of the microbiota of the gastrointestinal tract. Several reviews have described fermented foods containing identified or potential probiotics, particularly LAB, and human studies have reported that microorganisms in fermented foods can remain alive during the digestion process and reach the colon (David et al. Citation2014; Han et al. Citation2015; Heinen, Ahnen, and Slavin Citation2020). These microorganisms are metabolically active in the intestinal environment and produce bioactive compounds (David et al. Citation2014; Plé et al. Citation2015). The daily consumption of fermented foods increases the metabolic activity of the ingested microorganisms (Marco et al. Citation2021). Fermented food-associated LAB can constitute about 0.1–1% of gut bacteria, depending on the dietary habits of individuals (Plé et al. Citation2015). A study of Pasolli et al. (Citation2020) reported that food-associated LAB have more than 0.1% fecal metagenomic abundance.
The isolation and efficacy of various potential probiotic strains from the kimchi microbiota have been widely reported (Lee et al. Citation2011; Park et al. Citation2016). However, there has been no comprehensive review analyzing whether kimchi can function as a probiotic food. Since kimchi is a general and common side dish in Korea with significant consumption, its functionality as a source of probiotics can be explored by studying its influence on the gut microbiota. The potential of kimchi as a probiotic could be investigated by analyzing the types of LAB in kimchi, and their functionality. Here, we review kimchi, its LAB composition and functionalities, and the impact of its consumption on human gut environment, to estimate its potential as a probiotic food.
Culture and consumption of kimchi
Kimchi is a representative fermented food that originated in Korea. Several ancient documents mentioned the consumption of kimchi in the past. According to Samkuksaki, the chronicle of the three kingdoms of Korea, people already consumed kimchi around 1,500 years ago (Yang et al. Citation2015). Kimchi is consumed as a preserved food even during winter when fresh vegetables are rarely available. Traditionally, Korean households gather to prepare large amounts of kimchi from late November to the middle of December when Korean winter begins. This culture is called Kimjang, the making and sharing of kimchi, where kimchi-making practices including recipes, techniques, and culture are passed down through generations (Jang et al. Citation2015). According to a survey conducted by the Ministry of Agriculture, Food, and Rural Affairs of Korea in 2019, 42% of Korean households still produce their own kimchi. The Korean culture of Kimjang was inscribed on the Representative List of the Intangible Cultural Heritage of Humanity of the United Nations Educational, Scientific, and Cultural Organization (UNESCO) in 2013.
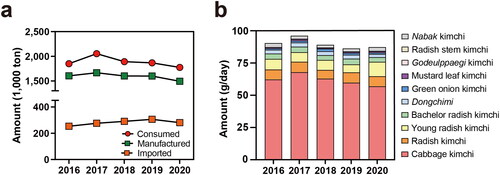
However, kimchi consumption in Korea has decreased over time (). Kim et al. (Citation2016a) reported a declining trend in daily kimchi consumption and portion size. Shifts in dietary patterns from traditional meals containing rice, soup, and side dishes, including kimchi, to westernized meals such as fast or processed foods, meat, and bread seem to be related to the dwindling daily kimchi consumption (Kim et al. Citation2016a). According to the Korea National Health and Nutrition Examination Survey, cabbage kimchi was ranked as the most frequently consumed food by Koreans in 2020, with a 55.07 ± 0.98% portion in the Korean diet, indicating that kimchi is consumed more frequently than rice, which is the staple food for Koreans. Cabbage kimchi was the third-most consumed food in 2020 in Korea, with an average of 57.06 ± 1.52 g of cabbage kimchi consumption per day, and 87.49 g for various types of kimchi including cabbage kimchi, radish kimchi, young radish kimchi, bachelor kimchi, water kimchi made of whole radish (Dongchimi), green onion kimchi, mustard leaf kimchi, Korean lettuce kimchi (Godeulppaegi), and water kimchi made of sliced radish (Nabak) kimchi (). The consumption of rice, the staple Korean food was 121.88 ± 1.78 g per day. The intake amount highlights that the consumption of kimchi is considerable even if it is a side dish. Furthermore, compared to 12.80 ± 0.93 g per day of yogurt consumption (ranked 28th), another popular fermented food worldwide, kimchi consumption is significantly higher in the Korean diet. Koreans consumed a total of 1.78 million tons of kimchi in 2020 according to a survey conducted by the Ministry of Agriculture, Food, and Rural Affairs of Korea.
Characteristics of LAB in kimchi
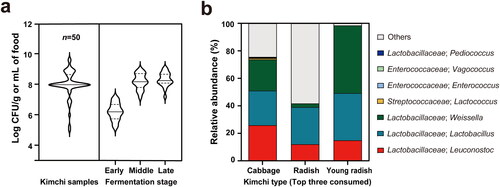
Alterations of LAB count and pH have been observed as fermentation progressed. The number of LAB in kimchi has been reported extensively. Our survey, which is based on publicly available studies (see Supplementary Methods), showed that LAB are present at an average of 8.01 ± 0.92 (max = 9.70 and min = 5.0) log CFU per gram or milliliter of food (). The mean LAB abundance was 100-fold higher in the middle stage than in the early stage, suggesting that the number of LAB ingested varies depending on the fermentation period. The minimum level of probiotic consumption recommended is 6 log CFU per gram or milliliter of food (Shah Citation2000). Though not all LAB present in kimchi are probiotic bacteria, it is logical to assume that kimchi could quantitatively qualify as a probiotic food. The bacterial taxonomic distribution of the three most widely consumed kimchi types (i.e., cabbage kimchi, radish kimchi, and young radish kimchi) is shown in . The relative abundance was calculated based on the data from previous studies (see Supplementary Methods). Cabbage kimchi was dominated by the LAB genera Leuconostoc, Lactobacillus, and Weissella. In radish kimchi, Lactobacillus was the most abundant LAB genus, while the genus Weissella dominated the bacterial microbiota of young radish kimchi. The prevailing LAB genera are mutual in the three different kinds of kimchi, although the ratio of each abundant genus differed among the three depending on the main ingredient.
Figure 2. The abundance and taxonomic profile of LAB present in kimchi.
(a) LAB viable cell count in kimchi. Data were selected based on the following criteria: selection of control among various groups, selection of maximum value among fluctuated numbers during fermentation, and selection of shown numbers in graph result in rounded down values. (b) The relative abundance of LAB (genus level) present in the three most widely consumed kimchi types (cabbage kimchi, radish kimchi, and young radish kimchi).
Although diverse microorganisms are involved in kimchi fermentation, the dominant fermentative microbes are LAB (Patra et al. Citation2016). Raw kimchi ingredients contain a low number of LAB, but their abundance increases four-fold during the brining process. The post-brining rinsing and washing steps reduce the number of overall bacteria, yeasts, and molds. At the fermentation step, LAB dominated the kimchi microbial community under anaerobic and low-temperature conditions (Jung, Lee, and Jeon Citation2014). Several factors including acidity, types of major (e.g., main vegetables, red pepper, and garlic) and minor ingredients (e.g., fermented seafood, fruits, and meat), the harvest season of raw ingredients, and fermentation temperature, affect the structure and composition of the microbial community present in kimchi (Cheigh, Park, and Lee Citation1994). Classification of the kimchi fermentation stages based on acidity and pH was suggested by Codex Alimentarius Commission (Citation2001) and Jung, Lee, and Jeon (Citation2014), respectively. The dominant LAB species were different at each fermentation stage. During the early to middle fermentation stages, Leuconostoc mesenteroides was the predominant species, which mainly produced lactic acid, acetic acid, carbon dioxide, and ethanol as major end products. In the late fermentation stage, the dominant bacteria changed to Latilactobacillus sakei, Streptococcus faecalis, Levilactobacillus brevis, Pediococcus cerevisiae, and Lactiplantibacillus plantarum (Cheigh, Park, and Lee Citation1994). The types of kimchi ingredients also affected the kimchi microbiota. A recent study by Song et al. (Citation2021) revealed that the dominant LAB species and distributions of major LAB species (Leuconostoc gellidum, Weissella kandleri, and Latilactobacillus sakei groups) and the concentration of produced metabolites differed depending on the type of the main ingredient. The addition of the minor ingredients, red pepper powder, and fermented fish sauce (i.e., jeotgal) also altered the bacterial community in kimchi. Red pepper powder elevated the abundance of Weissella spp. at the middle and late fermentation stages and initial ornithine production in kimchi (Jung, An, and Lee Citation2021). Kimchi containing fermented fish sauce was dominated by Weissella koreensis or Latilactobacillus sakei, depending on the type of fish, whereas microbial abundance in kimchi without fish sauce was dominated by Leuconostoc gasicomitatum (Jung et al. Citation2018). The LAB achieving microbial dominance and distribution differed based on the harvest season and storage period of the main ingredient. A study of Lee et al. (Citation2018b) showed different microbial distributions according to seasonality (spring, autumn, and winter). All kimchi samples were dominated by Lactobacillus, Leuconostoc, and Weissella, but with altered species abundance. The storage period of kimchi cabbage resulted in differences in the abundance of Lactobacillus and Leuconostoc, and these genera were more abundant in kimchi made with fresh and stored kimchi cabbage, respectively. Comparing the two fermentation temperature conditions (i.e., 4 and 10 °C), more Leuconostoc species were observed than Lactobacillus species at 4 °C, and more diverse LAB were detected at 10 °C (Hong et al. Citation2013). These studies demonstrated the key characteristics of kimchi fermentation; kimchi bacterial structure altered by diverse conditions and microbial dominance during fermentation was accomplished by species from three major genera: Lactobacillus, Leuconostoc, and Weissella.
Probiotic functions of kimchi and kimchi LAB
Health-promoting effects of kimchi
Kimchi possesses prebiotic, probiotic, and postbiotic properties through various compounds originating from the raw ingredients of kimchi; the diverse LAB involved in kimchi fermentation; and various metabolites in a large quantity produced by the LAB. The plants belonging to the Brassicaceae family are the main ingredients of kimchi, that harbor potential health-promoting compounds, such as dietary fibers, minerals, various amino acids, vitamins, carotenoids, glucosinolates, and polyphenols (Patra et al. Citation2016). LAB produce diverse organic acids, carbon dioxide, ethanol, mannitol, bacteriocins, ornithine, conjugated linoleic acid, and oligosaccharides (Lee, Jang, et al. Citation2015). Kimchi consumption has a variety of health-promoting benefits. Intake of kimchi to mammalian hosts (e.g., humans and mice) in various disease models or clinical trials (e.g., cancer, obesity, cardiovascular diseases, and decreased cognitive/memory/brain functions) showed an improvement in their health status as a result of kimchi consumption (). Several studies have tested the effects of kimchi consumption on human gut microbiota. Of the six studies identified in this literature review, three used healthy participants, one study included patients with two statuses of colon adenoma, one study included patients with metabolic disorders, and one study included patients with Helicobacter pylori infection. Park et al. (Citation2021) reported altered fecal microbiota taxa after ten weeks of fermented kimchi intake in 32 individuals with normal colon, simple and advanced colon adenomas. Kimchi consumption resulted in an increased proportion of Clostridium sensu stricto 1 and Turicibacter in the patients with simple colon adenoma, and a decreased proportion of Clostridium sensu stricto 1 and Terrisporobacter in the patients with advanced colon adenoma. The major genera of kimchi LAB, Weissella and Lactobacillus tended to increase in both the patient groups. A study of Kim and Park (Citation2018) analyzed the effect of kimchi consumption on colon health in 28 healthy individuals. After four weeks of kimchi intake, the average abundance of Firmicutes, Proteobacteria, and Tenericutes was reduced, while Bacteroidetes and Actinobacteria levels increased compared to week zero and week four. Faecalibacterium, Roseburia, and Phascolactobacterium, the short-chain fatty acid production-related genera, and Bifidobacterium, which contains a number of probiotic strains, were also elevated, while the average abundance of Clostridium and Escherichia coli was decreased. Other obesity-related markers, inflammation-related markers, and harmful fecal enzyme activities also improved, indicating that kimchi can influence metabolic disease-related indices and colon health. Another interventional study on healthy adults (Lee, Choi, and Ji Citation1996) reported increased cell counts of Lactobacillus and Leuconostoc, indicating that kimchi may function as a probiotic food. Kim et al. (Citation2016b) assessed the effect of different amounts of kimchi consumption for seven days on the gut microbiota of 12 Korean female adults, and observed similar bacterial diversity regardless of the consumption amount. In the large intake group, no difference was observed in the abundance of Firmicutes, but a lower proportion of Bacteroidetes was reported. Moreover, harmful bacteria such as Listeria, Clostridium, Enterobacter, Prevotella, and Shigella had smaller proportions in the high intake group than in the low intake group, while no differences were observed in the beneficial bacterial abundance. Enterococcus, which might be beneficial or harmful, had a higher abundance, while Bacteroides accounted for a smaller proportion, and Eubacterium showed no difference in the large kimchi intake group. Statistical analyses revealed that among the 34 species that differed in intake amount, 16 species, including potentially pathogenic Gammaproteobacteria, were reduced. Bifidobacterium breve, Lactobacillus acidophilus, Companilactobacillus mindensis, Limosilactobacillus reuteri, Levilactobacillus brevis, Lactobacillus amylolyticus, and Leuconostoc mesenteroides, which are the dominant species in kimchi, were found in the gut microbiota of the high intake group, suggesting their function as probiotics. A study conducted on obese patients also reported on kimchi (fresh or fermented) consumption and its impact on the gut microbiota (Han et al. Citation2015). After eight weeks of kimchi intake, the proportions of Proteobacteria and Actinobacteria were increased. The relative abundance of Bacteroides, which negatively correlated with obesity, and Prevotella were increased, while the abundance of Blautia was decreased after the consumption of fermented kimchi. Altered expression of several genes related to metabolism and immunity after the consumption of fermented kimchi reportedly to correlates with bacterial taxa such as Blautia, Prevotella, and Bacteroides. Thus, it is conceivable that the intake of fermented kimchi can either directly influence gene expression related to metabolism, digestion, blood circulation, and immunity, or indirectly influence human metabolism by altering the composition of the gut microbiota. Lastly, a study of the effect of kimchi consumption on Helicobacter pylori-infected patients showed significantly increased cell numbers of Lactobacillus sp. and Leuconostoc sp. in the feces, after kimchi intake (Kil et al. Citation2004). The studies described in this review provide introductory evidence on the relationship between kimchi intake and the modification of the gut microbiota. However, very few studies have suggested any patterns of alteration in the gut microbiota after kimchi consumption.
Table 1. The health-promoting effects of kimchi consumption.
A common observation from studies involving gut microbial alteration according to kimchi intake is an increase in the LAB abundance in the gut after kimchi intake. Research on the effect of overall gut LAB abundance on human health is lacking. However, it is known that LAB have no main bacterial pathogenicity characteristics (e.g., toxins and/or invasion potential) and could supply beneficial traits to gut ecosystems (acidification, biosynthesis of prebiotic fructooligosaccharides, release of bacteriocins, and/or modulation of the immune system) (Pessione Citation2012). Accordingly, the increased abundance of gut LAB via kimchi intake could help retain human health status and help to alleviate the effects of various diseases. Further research on the clinical role of kimchi consumption in human health should be conducted to elucidate the detailed mechanisms of how kimchi intake helps in improving human health in various diseases.
Kimchi-derived LAB and their human health benefits
Kimchi is a potential probiotic food with an ideal habitat for LAB, which has led researchers to focus on the health-promoting effects of LAB isolated from kimchi. LAB possess diverse functional properties including antimicrobial, antifungal, and antiviral activities, contributing to the inhibition of colonization by undesired allochthonous microbes in the gut environment, maintaining gut homeostasis (Servin Citation2004; Zeise, Woods, and Huffnagle Citation2021). Moreover, the antioxidant and anti-inflammatory properties of LAB combined with their probiotic potential in complex diseases including obesity, cancer, atopic dermatitis, and immunomodulatory diseases, make kimchi LAB important for human health. Detailed information regarding the health-promoting effects of kimchi-derived LAB is summarized in .
Table 2. Health-promoting effects of consumption of kimchi-derived lactic acid bacteria (LAB).
We showed that kimchi-derived LAB harbor health-promoting potentials based on previous research. Most recently, researchers highlighted the health-promoting benefits of specific LAB-derived compounds and their producing pathways: improved behavioral abnormalities by gamma-aminobutyric acid (GABA)-producing lactobacilli (Patterson et al. Citation2019); enhanced learning and memory behaviors by gut Lactobacillus-modulated tryptophan metabolism (Kaur, Bose, and Mande Citation2019; Zhang et al. Citation2022); and proangiogenic, pro-osteogenic, and antiapoptotic effects of Ligilactobacillus animalis-produced extracellular vesicles (EV) (Chen et al. Citation2022). We analyzed the potential pathways at the genomic level to expand our view of the health-promoting effects of kimchi-derived LAB. We surveyed the functional genes from publicly available LAB genomes of the three major genera, the drivers of kimchi fermentation (Lactobacillus, Leuconostoc, and Weissella) in the National Center for Biotechnology Information (NCBI). The accession numbers of these genomes were searched using esearch, provided by NCBI’s Entrez Direct (EDirect) toolkit. These genomes were selected when the term was specified "kimchi" in BioSample information (i.e. isolation source or environmental medium). Protein prediction and gene annotation of the selected genomes were performed using Prokka v.1.14.5 (Seemann Citation2014) and eggNOG-mapper v.2.1.7 (Cantalapiedra et al. Citation2021), respectively. A total of 129 genomes of kimchi-isolated LAB (66, 37, and 26 genomes belonging to the genera Lactobacillus, Leuconostoc, and Weissella, respectively) were used for the genomic analysis. They were subjected to a survey of the gene(s) related to GABA production, tryptophan metabolism, and production of EV components, which encode the metabolites that mediate host-microbiota interactions (Supplementary Table S1) (Chen et al. Citation2022; Kaur, Bose, and Mande Citation2019; Kim et al. Citation2015; Patterson et al. Citation2019). As shown in , several species belonging to the genera Lactobacillus, Leuconostoc, and Weissella possess more than one gene that is involved in GABA production. Partial GABA production pathways from succinate were observed in the species belonging to the three genera, but the pathway from glutamate was identified in Lactobacillus species only. Moreover, several Leuconostoc species contained gene(s) involved in kynurenine or indole acetic acid production for tryptophan metabolism. Finally, our genomic survey showed that many LAB species isolated from kimchi are capable of producing EV components. The microbial EV production is particularly interesting because of the potential application of EVs in drug delivery systems (Herrmann, Wood, and Fuhrmann Citation2021).
Table 3. Functional genes from the publicly available lactic acid bacteria genomes.
Taking all into account, the consumption of kimchi and its isolated LAB may have various health-promoting effects. The variation in the kimchi microbiota based on various factors might be considered a limitation for constant and defined impact on humans. Quality-controllable probiotic kimchi could be a potential solution for introducing LAB into kimchi. The microbial dominance of LAB and LAB-supplemented kimchi would be significantly beneficial to humans. The sufficient number of LAB and the therapeutic benefits of kimchi-derived LAB and kimchi itself imply that kimchi can meet potential properties of a probiotic food.
Can kimchi be served as a probiotic?
By consuming cabbage kimchi in average, one can intake 10.08 log CFU of LAB per day. For radish kimchi and young radish kimchi, 7.91 log CFU and 8.77 log CFU were consumed daily, respectively. Collectively, one can intake 10.11 log CFU LAB per day from average kimchi consumption (data not shown). Compared with commercial probiotics that contain 9 log CFU per serving size, daily kimchi consumption could be considered to contain a sufficient LAB count. The kimchi microbiota profile is also important for evaluating the probiotic potential of kimchi. To weigh up the portion of LAB strains each kind of kimchi contains, we calculated the average of relative abundance data at the genus level. Multiplying portion size with the LAB count shows the viable cell count of each LAB strain. For example, in cabbage kimchi, the most widely consumed type, Leuconostoc is the most dominant (25.98%) genus, followed by Lactobacillus (25.17%), Weissella (22.60%), Lactococcus (0.93%), and Pediococcus (0.22%). With an average intake of cabbage kimchi, one can possibly intake 9.62 log CFU of Leuconostoc, 9.61 log CFU of Lactobacillus, 9.56 log CFU of Weissella, 8.17 log CFU of Lactococcus, and 7.55 log CFU of Pediococcus from cabbage kimchi per day. Compared with commercial probiotics, which contain a single probiotic strain of 9 log CFU per serving size, kimchi contains sufficient amounts of various LAB considered to be probiotic microbes.
Kimchi undergoes spontaneous fermentation, which indicates that the microbiota remains unidentified and uncharacterized, with an unknown viable cell number at the time of consumption. Based on the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods (Marco et al. Citation2021), to label a probiotic fermented food, it is necessary to establish evidence of the beneficial effects of specific strains from an intervention study with proof of safety and confirmation of a sufficient number of specific strains in the final material to meet the claimed functionality. For a kimchi product to be labeled as a probiotic fermented food, it should be confirmed that a genetically characterized strain with defined probiotic effects is present in the product in sufficient amounts during its shelf-life. Also, no verified inhibitory effect on the physicochemical environment of kimchi should be confirmed while maintaining an optimal dose of the probiotic strain. The confirmed product can be claimed as “probiotic kimchi containing the specific strain(s) that may improve intestinal well-being.” Labeling a product as probiotic, however, depends on the legislation applied in the country/region in which it is being marketed. Lastly, it is important to note that categorizing products and/or strain(s) as probiotics requires further research on defined products rather than individual findings on undefined composition or unrepeatable properties of products and isolates. Though kimchi contains plenty of species with considerable viable cells, only the product where the strains in kimchi are defined to the strain level with known genome sequences and the strains that are viable at a claimed number during the shelf-life of the product can be labeled as “contains probiotics”.
Pros and cons of consuming kimchi as a probiotic food
The various types of kimchi containing several microorganisms at different stages of fermentation can provide a variety of health benefits. For example, a study showed different influences of fresh kimchi and fermented kimchi on the gut microbiota and gene expression profiles, suggesting that effect of kimchi differs in the fermentation stage (Han et al. Citation2015). Kimchi may also have different effects on microbial development during fermentation depending on the type and amount of ingredients (Lee et al. Citation2018a). Therefore, it is difficult to standardize kimchi due to several factors, such as type, ingredients, temperature, and fermentation stage, that affect its characteristics (Kim et al. Citation2020). However, several lines of evidence indicate that kimchi intake is superior to defined probiotic consumption.
Probiotic microorganisms must be delivered regularly to the human gastrointestinal tract for probiotics to be effective. Kimchi, a universal side dish in Korea, is easily accessible to Koreans, who consume it in considerable amounts unintentionally. As fermented foods such as kimchi undergo natural fermentation, the fermentation process may lead to pathogenic contamination of such food products (e.g., Escherichia coli and norovirus derived from the raw materials) (Cho et al. Citation2014; Park et al. Citation2015). To avoid and minimize this possibility, kimchi manufacturers can get certified under the Hazard Analysis Critical Control Points (HACCP) system and individuals who make kimchi by themselves could take care of personal and environmental hygiene. Moreover, the LAB present in kimchi might have negative effects on human health. A study conducted by Jeong and Lee (Citation2015) reported that LAB isolated from kimchi might possess gene(s) related to antibiotic resistance in their genomes and/or produce biogenic amines (BAs). Adverse effects of excessive BA intake on human health may include food poisoning and hypertension (Shalaby Citation1996). Tracing both the raw materials related to BA production (e.g., fish sauce) (Kim, Dang, and Ha Citation2022) and microbial BA producers (Jeong and Lee Citation2015), combined with suggestions for a reduction methodology (e.g., inoculation of BA degrading LAB) (Lee et al. Citation2021), might reduce the BA content of kimchi.
Challenges and perspectives
This review revealed that Koreans consume sufficient amounts of various LAB strains from kimchi. The intake of LAB from kimchi and its benefits to human health suggest that kimchi can function as a probiotic food. However, large-scale clinical studies are required to demonstrate the effects of kimchi consumption on the human body. In addition, it is necessary to elucidate the probiotic efficacy and mechanism of action of each species among the various LAB present in kimchi. Since the number and species of LAB in kimchi differ depending on the type of kimchi, raw ingredients, and fermentation period, there may be limitations to identifying the probiotic effects of non-standardized kimchi. Therefore, controlling or predicting the LAB present in kimchi will be an important area of research in the future. The control of LAB can be achieved using a starter culture. However, LAB inoculated as starters often fail to compete with microorganisms derived from raw kimchi ingredients. Therefore, it is necessary to study various methods for the inoculated starter to become the dominant species during kimchi fermentation. Taken collectively, kimchi with a desired/manipulated microbial community and/or metabolites can be used as functional probiotics customized for individuals or generations who want to change their diet, improve health, and treat gastrointestinal diseases.
Supplemental Material
Download MS Word (47.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmad Rather, I., B. J. Seo, V. J. R. Kumar, U. H. Choi, K. H. Choi, J. H. Lim, and Y. H. Park. 2013. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML007 and its application as a food preservative. Letters in Applied Microbiology 57 (1):69–76.
- Cantalapiedra, C. P., A. Hernandez-Plaza, I. Letunic, P. Bork, and J. Huerta-Cepas. 2021. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Molecular Biology and Evolution 38 (12):5825–9.
- Chang, J. H., Y. Y. Shim, S. K. Cha, and K. M. Chee. 2010. Probiotic characteristics of lactic acid bacteria isolated from kimchi. Journal of Applied Microbiology 109 (1):220–30.
- Cheigh, H. S., K.-Y. Park, and C. Y. Lee. 1994. Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products). Critical Reviews in Food Science and Nutrition 34 (2):175–203.
- Chen, C. Y., S. S. Rao, T. Yue, Y. J. Tan, H. Yin, L. J. Chen, M. J. Luo, Z. Wang, Y. Y. Wang, C. G. Hong, et al. 2022. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Science Advances 8 (15):eabg8335.
- Cho, S. H., J. Kim, K. H. Oh, J. K. Hu, J. Seo, S. S. Oh, M. J. Hur, Y. H. Choi, S. K. Youn, G. T. Chung, et al. 2014. Outbreak of enterotoxigenic Escherichia coli O169 enteritis in schoolchildren associated with consumption of kimchi. Epidemiology and Infection 142 (3):616–23.
- Choi, C. Y., Y. H. Kim, S. Oh, H. J. Lee, J. H. Kim, S. H. Park, H. J. Kim, S. J. Lee, and T. Chun. 2017. Anti-inflammatory potential of a heat-killed Lactobacillus strain isolated from Kimchi on house dust mite-induced atopic dermatitis in NC/Nga mice. Journal of Applied Microbiology 123 (2):535–43. doi: 10.1111/jam.13515.
- Choi, A. R., J. K. Patra, W. J. Kim, and S. S. Kang. 2018. Antagonistic activities and probiotic potential of lactic acid bacteria derived from a plant-based fermented food. Frontiers in Microbiology 9:1963.
- Codex Alimentarius Commision. 2001. Codex standard for kimchi (CODEX STAN 223-2001). Rome, Italy.
- David, L. A., C. F. Maurice, R. N. Carmody, D. B. Gootenberg, J. E. Button, B. E. Wolfe, A. V. Ling, A. S. Devlin, Y. Varma, M. A. Fischbach, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 (7484):559–63.
- Han, K., S. Bose, J. Wang, B.-S. Kim, M. J. Kim, E.-J. Kim, and H. Kim. 2015. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Molecular Nutrition & Food Research 59 (5):1004–8. doi: 10.1002/mnfr.201400780.
- Han, K. J., J. E. Lee, N. K. Lee, and H. D. Paik. 2020a. Antioxidant and anti-Inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish kimchi. Journal of Microbiology and Biotechnology 30 (4):591–8.
- Han, Y. M., E. A. Kang, J. M. Park, J. Y. Oh, D. Y. Lee, S. H. Choi, and K. B. Hahm. 2020b. Dietary intake of fermented kimchi prevented colitis-associated cancer. Journal of Clinical Biochemistry and Nutrition 67 (3):263–73.
- Han, K. J., N. K. Lee, H. S. Yu, H. Park, and H. D. Paik. 2022. Anti-adipogenic effects of the probiotic Lactiplantibacillus plantarum KU15117 on 3T3-L1 adipocytes. Probiotics and Antimicrobial Proteins 14 (3):501–9.
- Heinen, E., R. T. Ahnen, and J. Slavin. 2020. Fermented foods and the gut microbiome. Nutrition Today 55 (4):163–7. doi: 10.1097/NT.0000000000000422.
- Heo, W., E. S. Lee, H. T. Cho, J. H. Kim, J. H. Lee, S. M. Yoon, H. T. Kwon, S. Yang, and Y. J. Kim. 2018. Lactobacillus plantarum LRCC 5273 isolated from kimchi ameliorates diet-induced hypercholesterolemia in C57BL/6 mice. Bioscience, Biotechnology, and Biochemistry 82 (11):1964–72.
- Herrmann, I. K., M. J. A. Wood, and G. Fuhrmann. 2021. Extracellular vesicles as a next-generation drug delivery platform. Nature Nanotechnology 16 (7):748–59.
- Hong, H. J., E. Kim, D. Cho, and T. S. Kim. 2010. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. Journal of Clinical Immunology 30 (3):449–58. doi: 10.1007/s10875-010-9375-8.
- Hong, Y., H.-S. Yang, H.-C. Chang, and H.-Y. Kim. 2013. Comparison of bacterial community changes in fermenting kimchi at two different temperatures using a denaturing gradient gel electrophoresis analysis. Journal of Microbiology and Biotechnology 23 (1):76–84. doi: 10.4014/jmb.1210.10002.
- Huh, C. K., and T. Y. Hwang. 2016. Identification of antifungal substances of Lactobacillus sakei subsp. ALI033 and antifungal activity against Penicillium brevicompactum strain FI02. Preventive Nutrition and Food Science 21 (1):52–6.
- Hur, H. J., K. W. Lee, and H. J. Lee. 2004. Production of nitric oxide, tumor necrosis factor-alpha and interleukin-6 by RAW264.7 macrophage cells treated with lactic acid bacteria isolated from kimchi. BioFactors (Oxford, England) 21 (1–4):123–5.
- Islam, M. S., and H. Choi. 2009. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabetes model of rats. Journal of Medicinal Food 12 (2):292–7. doi: 10.1089/jmf.2008.0181.
- Jang, D.-J., K. R. Chung, H. J. Yang, K. Kim, and D. Y. Kwon. 2015. Discussion on the origin of kimchi, representative of Korean unique fermented vegetables. Journal of Ethnic Foods 2 (3):126–36. doi: 10.1016/j.jef.2015.08.005.
- Jang, H. J., N. K. Lee, and H. D. Paik. 2019. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Science and Biotechnology 28 (5):1521–8.
- Jang, H. J., M. W. Song, N. K. Lee, and H. D. Paik. 2018. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. Journal of Food Science and Technology 55 (8):3174–80.
- Jeong, D.-W., and J.-H. Lee. 2015. Antibiotic resistance, hemolysis and biogenic amine production assessments of Leuconostoc and Weissella isolates for kimchi starter development. LWT - Food Science and Technology 64 (2):1078–84. doi: 10.1016/j.lwt.2015.07.031.
- Jeong, S. H., H. J. Lee, J. Y. Jung, S. H. Lee, H.-Y. Seo, W.-S. Park, and C. O. Jeon. 2013. Effects of red pepper powder on microbial communities and metabolites during kimchi fermentation. International Journal of Food Microbiology 160 (3):252–9.
- Jeong, C. H., H. Sohn, H. Hwang, H. J. Lee, T. W. Kim, D. S. Kim, C. S. Kim, S. G. Han, and S. W. Hong. 2021. Comparison of the probiotic potential between Lactiplantibacillus plantarum isolated from kimchi and standard probiotic strains isolated from different sources. Foods 10 (9):2125. doi: 10.3390/foods10092125.
- Jo, S. G., E. J. Noh, J. Y. Lee, G. Kim, J. H. Choi, M. E. Lee, J. H. Song, J. Y. Chang, and J. H. Park. 2016. Lactobacillus curvatus WiKim38 isolated from kimchi induces IL-10 production in dendritic cells and alleviates DSS-induced colitis in mice. Journal of Microbiology (Seoul, Korea) 54 (7):503–9. doi: 10.1007/s12275-016-6160-2.
- Jung, S., H. An, and J.-H. Lee. 2021. Red pepper powder is an essential factor for ornithine production in kimchi fermentation. LWT 137:110434. doi: 10.1016/j.lwt.2020.110434.
- Jung, I. H., M. A. Jung, E. J. Kim, M. J. Han, and D. H. Kim. 2012. Lactobacillus pentosus var. plantarum C29 protects scopolamine-induced memory deficit in mice. Journal of Applied Microbiology 113 (6):1498–506.
- Jung, M. Y., T. W. Kim, C. Lee, J. Y. Kim, H. S. Song, Y. B. Kim, S. W. Ahn, J. S. Kim, S. W. Roh, and S. H. Lee. 2018. Role of jeotgal, a Korean traditional fermented fish sauce, in microbial dynamics and metabolite profiles during kimchi fermentation. Food Chemistry 265:135–43.
- Jung, J. Y., S. H. Lee, and C. O. Jeon. 2014. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Applied Microbiology and Biotechnology 98 (6):2385–93.
- Kaur, H., C. Bose, and S. S. Mande. 2019. Tryptophan metabolism by gut microbiome and gut-brain-axis: An in silico analysis. Frontiers in Neuroscience 13:1365. doi: 10.3389/fnins.2019.01365.
- Kil, J.-H., K.-O. Jung, H.-S. Lee, I.-K. Hwang, Y.-J. Kim, and K.-Y. Park. 2004. Effects of kimchi on stomach and colon health of Helicobacter pylori-infected volunteers. Preventive Nutrition and Food Science 9 (2):161–6. doi: 10.3746/jfn.2004.9.2.161.
- Kim, J. D. 2005. Antifungal activity of lactic acid bacteria isolated from kimchi against Aspergillus fumigatus. Mycobiology 33 (4):210–4. doi: 10.4489/MYCO.2005.33.4.210.
- Kim, S.-Y., Y.-M. Dang, and J.-H. Ha. 2022. Effect of various seasoning ingredients on the accumulation of biogenic amines in kimchi during fermentation. Food Chemistry 380:132214. doi: 10.1016/j.foodchem.2022.132214.
- Kim, E.-K., A.-W. Ha, E.-O. Choi, and S.-Y. Ju. 2016a. Analysis of Kimchi, vegetable and fruit consumption trends among Korean adults: Data from the Korea National Health and Nutrition Examination Survey (1998-2012). Nutrition Research and Practice 10 (2):188–97. doi: 10.4162/nrp.2016.10.2.188.
- Kim, J. Y., E. Y. Choi, Y. H. Hong, Y. O. Song, J. S. Han, S. S. Lee, E. S. Han, T. W. Kim, I. S. Choi, and K. K. Cho. 2016b. Changes in Korean adult females’ intestinal microbiota resulting from kimchi intake. Journal of Nutrition & Food Sciences 6:4172.
- Kim, J. H., J. Lee, J. Park, and Y. S. Gho. 2015. Gram-negative and Gram-positive bacterial extracellular vesicles. Seminars in Cell & Developmental Biology 40:97–104. doi: 10.1016/j.semcdb.2015.02.006.
- Kim, N., J. Lee, H. S. Song, Y. J. Oh, M. S. Kwon, M. Yun, S. K. Lim, H. K. Park, Y. S. Jang, S. Lee, et al. 2022b. Kimchi intake alleviates obesity-induced neuroinflammation by modulating the gut-brain axis. Food Research International (Ottawa, ON) 158:111533.
- Kim, H.-Y., and K.-Y. Park. 2018. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. Journal of Functional Foods 47:325–33. doi: 10.1016/j.jff.2018.05.052.
- Kim, D., S. J. Park, J. Kim, U. Hong, and J. Lee. 2021. Effect of lactic acid strains isolated from kimchi on atopic dermatitis and immunomodulation in NC/Nga mice. Preventive Nutrition and Food Science 26 (3):321–9.
- Kim, J. Y., S.-E. Park, E.-J. Kim, S.-H. Seo, T. W. Whon, K.-M. Cho, S. J. Kwon, S. W. Roh, and H.-S. Son. 2022a. Long-term population dynamics of viable microbes in a closed ecosystem of fermented vegetables. Food Research International 154:111044. doi: 10.1016/j.foodres.2022.111044.
- Kim, E.-J., S.-H. Seo, S.-E. Park, Y.-W. Lim, S. W. Roh, and H.-S. Son. 2020. Initial storage of kimchi at room temperature alters its microbial and metabolite profiles. LWT 134:110160. doi: 10.1016/j.lwt.2020.110160.
- Kwon, M. S., S. K. Lim, J. Y. Jang, J. Lee, H. K. Park, N. Kim, M. Yun, M. Y. Shin, H. E. Jo, Y. J. Oh, et al. 2018. Lactobacillus sakei WIKIM30 ameliorates atopic dermatitis-like skin lesions by inducing regulatory T cells and altering gut microbiota structure in mice. Frontiers in Immunology 9:1905. doi: 10.3389/fimmu.2018.01905.
- Le, B., and S. H. Yang. 2019. Effect of potential probiotic Leuconostoc mesenteroides FB111 in prevention of cholesterol absorption by modulating NPC1L1/PPARalpha/SREBP-2 pathways in epithelial Caco-2 cells. International Microbiology : The Official Journal of the Spanish Society for Microbiology 22 (2):279–87.
- Lee, K.-E., U.-H. Choi, and G.-E. Ji. 1996. Effect of kimchi intake on the composition of human large intestinal bacteria. Korean Journal of Food Science and Technology 28:981–6.
- Lee, N. K., K. J. Han, H. Park, and H. D. Paik. 2022. Effects of the probiotic Lactiplantibacillus plantarum KU15120 derived from Korean homemade diced-radish kimchi against oxidation and adipogenesis. Probiotics and Antimicrobial Proteins. doi: 10.1007/s12602-021-09885-2.
- Lee, M. E., J. Y. Jang, J. H. Lee, H. W. Park, H. J. Choi, and T. W. Kim. 2015. Starter cultures for kimchi fermentation. Journal of Microbiology and Biotechnology 25 (5):559–68.
- Lee, J., Y. H. Jin, A. M. Pawluk, and J.-H. Mah. 2021. Reduction in biogenic amine content in baechu (napa cabbage) kimchi by biogenic amine-degrading lactic acid bacteria. Microorganisms 9 (12):2570. doi: 10.3390/microorganisms9122570.
- Lee, S. H., J. Y. Jung, and C. O. Jeon. 2015. Source tracking and succession of kimchi lactic acid bacteria during fermentation. Journal of Food Science 80 (8):M1871–M1877.
- Lee, K. W., J. M. Shim, D. W. Kim, Z. Yao, J. A. Kim, H.-J. Kim, and J. H. Kim. 2018a. Effects of different types of salts on the growth of lactic acid bacteria and yeasts during kimchi fermentation. Food Science and Biotechnology 27 (2):489–98.
- Lee, M., J. H. Song, S. H. Lee, M. Y. Jung, and J. Y. Chang. 2018b. Effect of seasonal production on bacterial communities in Korean industrial kimchi fermentation. Food Control 91:381–9. doi: 10.1016/j.foodcont.2018.04.023.
- Lee, S. H., T. W. Whon, S. W. Roh, and C. O. Jeon. 2020. Unraveling microbial fermentation features in kimchi: From classical to meta-omics approaches. Applied Microbiology and Biotechnology 104:7731–44.
- Lee, H., H. Yoon, Y. Ji, H. Kim, H. Park, J. Lee, H. Shin, and W. Holzapfel. 2011. Functional properties of Lactobacillus strains isolated from kimchi. International Journal of Food Microbiology 145 (1):155–61.
- Marco, M. L., M. E. Sanders, M. Gänzle, M. C. Arrieta, P. D. Cotter, L. De Vuyst, C. Hill, W. Holzapfel, S. Lebeer, D. Merenstein, et al. 2021. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews. Gastroenterology & Hepatology 18 (3):196–208. doi: 10.1038/s41575-020-00390-5.
- Noh, J. S., Y. H. Choi, and Y. O. Song. 2013. Beneficial effects of the active principle component of Korean cabbage kimchi via increasing nitric oxide production and suppressing inflammation in the aorta of apoE knockout mice. The British Journal of Nutrition 109 (1):17–24.
- Noh, J. S., H. J. Kim, M. J. Kwon, and Y. O. Song. 2009. Active principle of kimchi, 3-(4’-hydroxyl-3’,5’-dimethoxyphenyl)propionic acid, retards fatty streak formation at aortic sinus of apolipoprotein E knockout mice. Journal of Medicinal Food 12 (6):1206–12.
- Park, S., Y. Ji, H. Park, K. Lee, H. Park, B. R. Beck, H. Shin, and W. H. Holzapfel. 2016. Evaluation of functional properties of lactobacilli isolated from Korean white kimchi. Food Control 69:5–12. doi: 10.1016/j.foodcont.2016.04.037.
- Park, J. S., I. Joe, P. D. Rhee, C. S. Jeong, and G. Jeong. 2017. A lactic acid bacterium isolated from kimchi ameliorates intestinal inflammation in DSS-induced colitis. Journal of Microbiology (Seoul, Korea) 55 (4):304–10.
- Park, J. H., S. Jung, J. Shin, J. S. Lee, I. S. Joo, and D. Y. Lee. 2015. Three gastroenteritis outbreaks in South Korea caused by the consumption of kimchi tainted by norovirus GI.4. Foodborne Pathogens and Disease 12 (3):221–7.
- Park, S., J. I. Kim, J. Y. Bae, K. Yoo, H. Kim, I. H. Kim, M. S. Park, and I. Lee. 2018. Effects of heat-killed Lactobacillus plantarum against influenza viruses in mice. Journal of Microbiology (Seoul, Korea) 56 (2):145–9.
- Park, S. E., S. J. Kwon, K. M. Cho, S. H. Seo, E. J. Kim, T. Unno, S. H. Bok, D. H. Park, and H. S. Son. 2020. Intervention with kimchi microbial community ameliorates obesity by regulating gut microbiota. Journal of Microbiology (Seoul, Korea) 58 (10):859–67.
- Park, J. M., W. H. Lee, H. Seo, J. Y. Oh, D. Y. Lee, S. J. Kim, and K. B. Hahm. 2021. Fecal microbiota changes with fermented kimchi intake regulated either formation or advancement of colon adenoma. Journal of Clinical Biochemistry and Nutrition 68 (2):139–48. doi: 10.3164/jcbn.20-121.
- Park, J. E., S. H. Oh, and Y. S. Cha. 2014. Lactobacillus brevis OPK-3 isolated from kimchi inhibits adipogenesis and exerts anti-inflammation in 3T3-L1 adipocyte. Journal of the Science of Food and Agriculture 94 (12):2514–20.
- Park, J. E., S. H. Oh, and Y. S. Cha. 2020. Lactobacillus brevis OPK-3 from kimchi prevents obesity and modulates the expression of adipogenic and pro-inflammatory genes in adipose tissue of diet-induced obese mice. Nutrients 12 (3):604. doi: 10.3390/nu12030604.
- Pasolli, E., F. De Filippis, I. E. Mauriello, F. Cumbo, A. M. Walsh, J. Leech, P. D. Cotter, N. Segata, and D. Ercolini. 2020. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nature Communications 11 (1):1–12. doi: 10.1038/s41467-020-16438-8.
- Patra, J. K., G. Das, S. Paramithiotis, and H. S. Shin. 2016. Kimchi and other widely consumed traditional fermented foods of Korea: A review. Frontiers in Microbiology 7:1493.
- Patterson, E., P. M. Ryan, N. Wiley, I. Carafa, E. Sherwin, G. Moloney, E. Franciosi, R. Mandal, D. S. Wishart, K. Tuohy, et al. 2019. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Scientific Reports 9 (1):16323. doi: 10.1038/s41598-019-51781-x.
- Pessione, E. 2012. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Frontiers in Cellular and Infection Microbiology 2:86. doi: 10.3389/fcimb.2012.00086.
- Plé, C., J. Breton, C. Daniel, and B. Foligne. 2015. Maintaining gut ecosystems for health: Are transitory food bugs stowaways or part of the crew? International Journal of Food Microbiology 213:139–43. doi: 10.1016/j.ijfoodmicro.2015.03.015.
- Rho, M. K., Y. E. Kim, H. I. Rho, T. R. Kim, Y. B. Kim, W. K. Sung, T. W. Kim, D. O. Kim, and H. Kang. 2017. Enterococcus faecium FC-K derived from kimchi is a probiotic strain that shows anti-allergic activity. Journal of Microbiology and Biotechnology 27 (6):1071–7.
- Rho, J.-B., H. Poo, Y.-K. Choi, C. J. Kim, and M.-H. Sung. 2012. New lactic acid bacteria having its inhibitory effect on avian influenza virus infection and composition containing the same. U.S. Patent Application No. 13/378,761.
- Ryu, E. H., E. J. Yang, E. R. Woo, and H. C. Chang. 2014. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiology 41:19–26.
- Seemann, T. 2014. Prokka: Rapid prokaryotic genome annotation. Bioinformatics (Oxford, England) 30 (14):2068–9. doi: 10.1093/bioinformatics/btu153.
- Seo, H., H. Seong, G. Y. Kim, Y. M. Jo, S. W. Cheon, Y. Song, B. H. Ryu, H. Kang, and N. S. Han. 2021. Development of anti-inflammatory probiotic Limosilactobacillus reuteri EFEL6901 as kimchi starter: In vitro and in vivo evidence. Frontiers in Microbiology 12:760476.
- Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS microbiology Reviews 28 (4):405–40. doi: 10.1016/j.femsre.2004.01.003.
- Shah, N. P. 2000. Probiotic bacteria: Selective enumeration and survival in dairy foods. Journal of Dairy Science 83 (4):894–907. doi: 10.3168/jds.S0022-0302(00)74953-8.
- Shalaby, A. R. 1996. Significance of biogenic amines to food safety and human health. Food Research International 29 (7):675–90. doi: 10.1016/S0963-9969(96)00066-X.
- Shin, M. S., S. K. Han, J. S. Ryu, K. S. Kim, and W. K. Lee. 2008. Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2 isolated from Kimchi. Journal of Applied Microbiology 105 (2):331–9.
- Sohn, H., Y. H. Chang, J. H. Yune, C. H. Eong, D. M. Shin, H. C. Kwon, D. H. Kim, S. W. Hong, H. Hwang, J. Y. Jeong, et al. 2020. Probiotic properties of Lactiplantibacillus plantarum LB5 isolated from kimchi based on nitrate reducing capability. Foods 9 (12):1777. doi: 10.3390/foods9121777.
- Son, S. H., H. L. Jeon, S. J. Yang, N. K. Lee, and H. D. Paik. 2017. In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microbial Pathogenesis 112:135–41.
- Song, H. S., S. H. Lee, S. W. Ahn, J. Y. Kim, J.-K. Rhee, and S. W. Roh. 2021. Effects of the main ingredients of the fermented food, kimchi, on bacterial composition and metabolite profile. Food Research International 149:110668. doi: 10.1016/j.foodres.2021.110668.
- Won, T. J., B. Kim, Y. T. Lim, D. S. Song, S. Y. Park, E. S. Park, D. I. Lee, and K. W. Hwang. 2011b. Oral administration of Lactobacillus strains from kimchi inhibits atopic dermatitis in NC/Nga mice. Journal of Applied Microbiology 110 (5):1195–202.
- Won, T. J., B. Kim, D. S. Song, Y. T. Lim, E. S. Oh, D. I. Lee, E. S. Park, H. Min, S. Y. Park, and K. W. Hwang. 2011a. Modulation of Th1/Th2 balance by Lactobacillus strains isolated from kimchi via stimulation of macrophage cell line J774A.1 in vitro. Journal of Food Science 76 (2):H55–61.
- Woo, M., M. J. Kim, and Y. O. Song. 2018. Bioactive compounds in kimchi improve the cognitive and memory functions impaired by amyloid beta. Nutrients 10 (10):1554. doi: 10.3390/nu10101554.
- Woo, M., J. S. Noh, E. J. Cho, and Y. O. Song. 2018. Bioactive compounds of kimchi inhibit apoptosis by attenuating endoplasmic reticulum stress in the brain of amyloid beta-injected mice. Journal of Agricultural and Food Chemistry 66 (19):4883–90.
- Yang, E. J., and H. C. Chang. 2010. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. International journal of Food Microbiology 139 (1–2):56–63.
- Yang, H.-J., D.-J. Jang, K. R. Chung, K. Kim, and D. Y. Kwon. 2015. Origin names of gochu, kimchi, and bibimbap. Journal of Ethnic Foods 2 (4):162–72. doi: 10.1016/j.jef.2015.11.006.
- Yang, E. J., Y. S. Kim, and H. C. Chang. 2011. Purification and characterization of antifungal delta-dodecalactone from Lactobacillus plantarum AF1 isolated from kimchi. Journal of Food Protection 74 (4):651–7.
- Yang, S. J., J. E. Lee, S. M. Lim, Y. J. Kim, N. K. Lee, and H. D. Paik. 2019. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Science and Biotechnology 28 (2):491–9.
- Yoon, S., H. Cho, Y. Nam, M. Park, A. Lim, J. H. Kim, J. Park, and W. Kim. 2022. Multifunctional probiotic and functional properties of Lactiplantibacillus plantarum LRCC5314, isolated from kimchi. Journal of Microbiology and Biotechnology 32 (1):72–80.
- Zeise, K. D., R. J. Woods, and G. B. Huffnagle. 2021. Interplay between Candida albicans and lactic acid bacteria in the gastrointestinal tract: Impact on colonization resistance, microbial carriage, opportunistic infection, and host immunity. Clinical Microbiology Reviews 34 (4):e0032320.
- Zhang, Z., X. Mu, Q. Cao, Y. Shi, X. Hu, and H. Zheng. 2022. Honeybee gut Lactobacillus modulates host learning and memory behaviors via regulating tryptophan metabolism. Nature Communications 13 (1):2037.