Abstract
Jackfruit (Artocarpus heterophyllus Lam.), also known as ‘vegetarian’s meat’, is an excellent source of carbohydrates, protein, fiber, vitamins, minerals, and several phytochemicals. It is a climacteric fruit that exhibits an increase in ethylene biosynthesis and respiration rate during fruit ripening. The market value of jackfruit is reduced due to the deterioration of fruit quality during storage and transportation. There is a lack of standardized harvest maturity index in jackfruit, where consequently, fruit harvested at immature or overmature stages result in poor quality ripe fruit with short storage life. Other factors responsible for its short postharvest life relate to its highly perishable nature, chilling sensitivity and susceptibility to fruit rot which result in significant qualitative and quantitative losses. Various postharvest management techniques have been adopted to extend the storage life, including cold storage, controlled atmosphere storage, modified atmosphere packaging, edible coatings, chemical treatment, and non-chemical alternatives. Diversified products have been prepared from jackfruit to mitigate such losses. This comprehensive review highlights the nutritional profile, fruit ripening physiology, pre and postharvest quality management, and value addition of jackfruit as well as the way forward to reduce postharvest losses in the supply chain.
Introduction
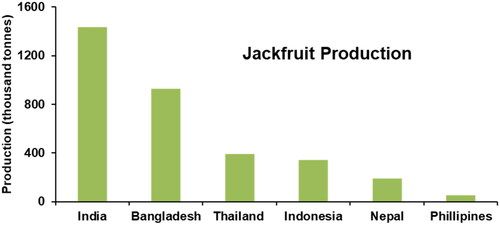
Jackfruit, botanically known as Artocarpus heterophyllus Lam., is the largest fruit grown in tropical and subtropical regions around the globe (Zhang, Jiang, et al. Citation2021). It is an evergreen tree native to the rainforests of the Western Ghats of India (Fathin et al. Citation2021). However, some authors have also claimed it to be indigenous to Malaysia (Azad, Jones, and Haq Citation2007). Jackfruit is an economically important fruit in India, Bangladesh, China, Burma, Thailand, Sri Lanka, Indonesia, Malaysia and the Philippines. Moreover, jackfruit is also grown in Africa, Suriname, Brazil, Australia, the Caribbean, Florida, and on the Islands of Yap, Palau, Nauru, Pohnpei, Samoa, and Tabiteuea in Kiribati (Elevitch and Manner Citation2006). India is the leading global producer of jackfruit followed by Bangladesh, Thailand, Indonesia and Nepal (). Jackfruit is the national fruit of Bangladesh (Shajib et al. Citation2013) and the state fruit of the Kerala province in India (Remya et al. Citation2019). In Australia, Jackfruit was first introduced as a garden plant in the 1800s; however, cultivars suited for commercial production were not introduced until the 1960s and 1970s. In Australia, jackfruit is mainly grown in the Northern Territory, Northern Queensland, and northern parts of Western Australia (AgriFutures Citation2017). Nearly 78% of Australian jackfruit is produced in the Northern Territory, accounting for about 7,480 trees, while approximately 2,000 trees are located in Far Northern Queensland (Owens Citation2021). Moreover, new plantings are being established in the existing production areas and crop diversification in new areas is being explored. The total annual production of jackfruit in Australia is 4,000 tonnes, amounting to a value of AUD 2.6 million which is expected to increase in the near future (Norris et al. Citation2021).
Figure 1. Major jackfruit-producing countries in the world (Sawe Citation2017).
Fully ripened jackfruit is consumed widely, due to its soft texture and sweet, fruity aroma. The edible part surrounding each seed is known as a bulb or flake, whose striking flavor characteristics resemble a mixture of tropical fruits such as banana, pineapple, orange, melon, papaya and mango (Chavez-Santiago et al. Citation2021). Jackfruit is a rich source of nutrients and volatile biochemicals that are helpful in curing cardiovascular disease, cancer, and ageing-related diseases, which makes it an important medicinal fruit with an imprint all around the globe (Cruz-Casillas, García-Cayuela, and Rodriguez-Martinez Citation2021; Zhang, Jiang, et al. Citation2021). Apart from being an excellent source of nutrients, jackfruit is a multipurpose tree species that has been used for the prevention of soil erosion, as a wind break, as a source of timber, and for shading and ornamental purposes (Elevitch and Manner Citation2006).
Botanically, jackfruit is a multiple fruit formed by the fusion of fertilized ovaries of many flowers. It exhibits an increase in respiration rate and ethylene production during fruit ripening. Jackfruit ripening is accompanied by a range of biochemical, physiological and physical changes including higher carotenoid biosynthesis, sugar content accumulation, and a decline in organic acid and cell wall cellulose content (Selvaraj and Pal Citation1989; Goswami et al. Citation2011). Due to the perishable nature of fruit, a significant amount of qualitative and quantitative losses occur during supply chain (Chattopadhyay et al. Citation2018). Cold storage has been used to enhance jackfruit storability, but the fruit’s susceptibility to chilling injury is a major hindrance in the application of low temperature for the extension of storage life. Various other postharvest management techniques including controlled atmosphere storage (Saxena et al. Citation2013; Thewes, Brackmann, and Neuwald Citation2019), modified atmosphere packaging (Saxena, Bawa, and Raju Citation2008; Saxena, Bawa, and Raju Citation2009; Sally, Ranganna, and Munishamanna Citation2011; Vasudeva et al. Citation2018; Dhanesh et al. Citation2018; Prathibha et al. Citation2019a; Udayasoorian et al. Citation2019), edible coatings (Mei and Ping Citation2008; Partha, Wasono, and Ulfah Citation2009; Saxena et al. Citation2013; Teja, Santhi, and Narsingarao Citation2016; Vargas-Torres et al. Citation2017; Minh et al. Citation2019; Ansar et al. Citation2020; Jayakody, Vanniarachchy, and Wijesekara 2021), as well as thermal (Saxena et al. Citation2012; Babu and Sudheer Citation2020; Babu et al. Citation2022) and non-thermal approaches (Nguyet, Tra, and Yen Citation2008; Bizura-Hasida et al. Citation2013) have been employed for the reduction of postharvest losses and to enhance the storage life.
As a highly versatile fruit, jackfruit can be consumed when fully ripe, cooked as a vegetable when unripe, and used to prepare numerous products such as jam, jelly, squash, snacks and beverages. Additionally, boiled and roasted seeds of ripe jackfruit are consumed as snacks (Chai et al. Citation2021). Despite this immense potential, jackfruit remained an underutilized fruit. Further, the major crops (rice, wheat, maize) on which most of the world’s population is dependent, lack many essential nutrients (UNICEF and World Health Organization Citation2017). Therefore, there is a need to provide alternative nutritional sources, such as unexploited fruit crops, with their potential to overcome food and nutritional insecurity around the world (Ojwang et al. Citation2022). Whilst information regarding minimal processing and health benefits of jackfruit has been reviewed in previous studies (Baliga et al. Citation2011; Saxena, Bawa, and Raju Citation2011; Swami et al. Citation2012; Anaya-Esparza et al. Citation2018; Ranasinghe, Maduwanthi, and Marapana Citation2019), there is a lack of comprehensive review regarding the varied aspects of fruit maturity and ripening of jackfruit, as well as the effects of different postharvest management strategies on both fresh cut and whole fruit. This review aims to fill this gap by considering ripening physiology, postharvest losses, pre-harvest factors affecting fruit quality, varied postharvest approaches to prolong storage life, as well as value addition to preserve fruit quality of jackfruit.
Phytonutrient profile
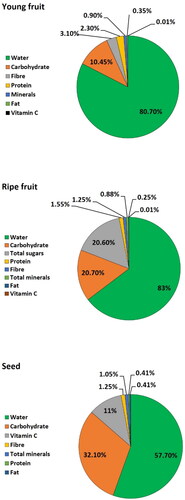
Phytonutrients are natural compounds that are responsible for the distinct aroma, taste and pigmentation of a fruit. These compounds act as the plant’s immune system, in addition to performing a protective role in humans (Swami et al. Citation2012). Jackfruit is a rich source of phytonutrients including phenolic compounds, carotenoids, volatiles and various antioxidants ().
Figure 2. Biochemical composition of young and ripe fruit and seeds of jackfruit (APAARI Citation2012).
Polyphenols
Polyphenols are a group of secondary metabolites, such as phenolic acids, tannins and flavonoids which are responsible for the flavor, color and antioxidant properties of fruit. Total phenolic content (TPC) in plants is influenced by several endogenous (species, genus and cultivars) and exogenous factors (agronomic practices, environmental and storage conditions) (Tomás‐Barberán and Espín Citation2001). TPC can vary between different fruits and within the same fruit, due to its complex nature and different extraction methods (Kalt, McDonald, and Donner Citation2000). Phenolic compounds present in jackfruit are phenols such as ferulic acid, gallic acid and tannic acid as well as flavonoids such as rutin, catechin and myricetin (Anaya-Esparza et al. Citation2018). Jackfruit seeds contain higher TPC in comparison to the edible portion (Soong and Barlow Citation2004). Immature fruit has a higher TPC that results in its astringent taste (Saxena, Bawa, and Raju Citation2011), which decreases with advancement of fruit maturity (Redondo et al. Citation2021). Singh et al. (Citation2015) have reported that raw jackfruit contains four phenolic acids (ferulic, gallic, caffeic and tannic acids), while in ripe fruit only two phenolic acids have been reported i.e., ferulic and gallic acid. This decrease in TPC is due to chemical and enzymatic changes in the fruit, which result in hydrolysis of glycosides, polymerization of free phenols and oxidation of polyphenols (Zheng, Kim, and Chung Citation2012). A complete phenolic profile of different jackfruit cultivars in relation to fruit maturity stages is still missing and warrants further investigation.
Volatiles
Volatile compounds play a vital role in the flavor development in fruit (Aprea, Biasioli, and Gasperi Citation2015). Esters are the major volatiles present in jackfruit, where they are key contributors to fruit flavor. The major volatile compounds identified in jackfruit by different methods are presented in . A total of 53 volatiles were identified by Grimm and Steinhaus (Citation2020), 23 compounds reported by Ong et al. (Citation2006), 39 compounds by Maia, Andrade, and das Graças (Citation2004), 59 volatiles by Fraga (Citation2005), 21 compounds by Rasmussen (Citation1983) and 20 volatiles have been identified by Swords, Bobbio, and Hunter (Citation1978). Recently, Barros-Castillo et al. (Citation2021) have reported 86 volatile compounds in five jackfruit cultivars grown in Mexico, out of which 51 esters, 13 aldehydes, seven ketones, seven alcohols, seven terpenes and one carboxylic acid were identified. Likewise, Peng et al. (Citation2013) identified 69 volatile compounds in jackfruit, including 28 acids, 18 esters and 13 alcohols. Amongst these volatile compounds, 2,3-butanediol, 2-methyl- butanoic acid, 3-methyl- butyl ester and n-hexadecenoic acid were major volatile compounds. Additionally, Maia, Andrade, and das Graças (Citation2004) have reported that the composition of volatile compounds in hard jackfruit is constituted primarily of isopentyl isovalerate (28.4%), and butyl isovalerate (25.6%) followed by palmitic acid (8.3%) and ethyl isovalerate (6.2%), while soft jackfruit consists mainly of isopentyl isovalerate (18.3%), and butyl acetate (16.5%), followed by ethyl isovalerate (14.4%), butyl isovalerate (12.9%), isopentyl acetate (12%) and isopentane-2-ol (5%). The variation in number of volatile compounds reported in jackfruit by various workers may be attributed to the different genotypes, environments, cultural practices, and the analytical techniques employed for their identification and estimation.
Table 1. Major volatile compounds identified in jackfruit using different analytical methods (modified after Bicas et al. Citation2011).
Carbohydrates
Jackfruit is rich in carbohydrates including monosaccharides, disaccharides and polysaccharides. The carbohydrate content in seeds of different jackfruit cultivars ranges from 37.4% to 42.5% (Chrips, Balasingh, and Kingston Citation2008). The major sugars present in jackfruit are glucose, fructose, and sucrose (Chowdhury, Raman, and Mian Citation1997), while other sugars like xylose, rhamnose, galactose and arabinose are also identified in jackfruit pulp (Gupta and Rao Citation1963). Rahman et al. (Citation1999) observed variations in the carbohydrate content of jackfruit depending on fruit maturity, finding 2.2% and 9% starch content in ripe fruit, as compared to 31.6% and 29.8% in immature fruit of soft fleshed and firm-fleshed cultivars, respectively.
Vitamins and minerals
Jackfruit contains a significant quantity of Vitamin C (7 mg 100 g−1 FW) and Vitamin A (540 IU). Additionally, jackfruit contains B-complex vitamins, particularly niacin (12.7 mg 100 g−1) (Effiong and Harry Citation2019), riboflavin (130 µg 100 g−1) and thiamin (39 µg 100 g−1) (Saxena, Bawa, and Raju Citation2011), which are rarely found in fruits (Mushumbusi Citation2015). Jackfruit contains high levels of essential minerals (Sampath et al. Citation2019), where every 100 g of fruit pulp contains 90 mg calcium, 88 mg potassium, one mg phosphorus and 0.5 mg iron (Saxena, Bawa, and Raju Citation2011). Tiwari and Vidyarthi (Citation2015) have observed vitamin and mineral content to increase during the initial stages of fruit maturity, while this decreases at later stages of ripening.
Carotenoid composition
Carotenoids are secondary plant metabolites that are soluble in lipids and are an important source of provitamin A (Kopsell and Kopsell Citation2006). These are natural pigments that provide yellow, orange, or reddish color to fruits (Jagadeesh et al. Citation2007). These compounds provide protection against chronic diseases such as cancer, inflammation, cataract, macular degeneration, and cardiovascular disease (Coyne et al. Citation2005). Jackfruit contains varied carotenoids, which act as antioxidants for the human body. Chandrika, Jansz, and Warnasuriya (Citation2005) have reported that jackfruit contains α-carotene, β-carotene, β-carotene-5, α-zeacarotene, 6α-epoxide and β-zeacarotene. de Faria, de Rosso, and Mercadante (Citation2009) have also investigated carotenoid content in jackfruit, identifying 19 different carotenoids including trans-lutein (24-44%), and trans-β-carotene (24-30%) followed by trans-neoxanthin (4-19%), 9-cis-violaxanthin (4-10%), and 9-cis-neoxanthin (4-9%).
Protein and amino acids
Jackfruit flesh contains nearly 1.9% protein, while the seeds alone contain about 5.3 to 6.8% protein (Pavanasasivam, Uvais, and Sultanbawa Citation1973). However, Goswami et al. (Citation2011) have stated that the protein content of different jackfruit cultivars can also vary, ranging from 0.57 to 0.97%. Jackfruit contains free amino acids including aspartic acid, glutamine, threonine, cysteine, tryptophan, methionine, histidine, lysine, and leucine. Of all the amino acids, phenylalanine is most abundant in jackfruit, followed by aspartic acid and glutamine. Amino acid content has been reported to decrease with the advancement of jackfruit ripening (Saxena, Bawa, and Raju Citation2011).
Antioxidants
Antioxidants are compounds with an ability to prevent the oxidation process (Hasan et al. Citation2021). Several studies have shown that the flavonoids, phenolics, anthocyanins and carotenoids present in fruit are excellent scavengers of free radicles and are helpful in the prevention of cellular damage (Rufino et al. Citation2011). Jackfruit is an excellent source of antioxidants that provide a natural defence against reactive oxygen species (ROS), free radical production and oxidative enzymes (Soong and Barlow Citation2004). There are two types of antioxidants: the first is enzymatic including thiols, thioredoxin, glutathione reductase (GR), catalase (CAT), superoxide dismutase (SOD), guaiacol peroxidase (GPX) and ascorbate peroxidase (APX); while the second is non-enzymatic antioxidants like phenolic compounds, carotenoids, riboflavin, flavonoids, ascorbic acid, and anthocyanins (Halliwell et al. Citation2009). Jackfruit contains many essential provitamins and vitamins that are also a part of defence control mechanisms against free radicals. Vitamin C is the main constituent of non-enzymatic antioxidants along with α-tocopherol, both of which are present in jackfruit pulp and provide protection to the human body (Devasagayam, Tilak, and Singhal Citation2007).
Proximate composition of jackfruit seed flour and seed starch
During jackfruit processing, a huge number of seeds are obtained which could be utilized for the preparation of various by-products (Noor et al. Citation2014). The jackfruit seed flour has the potential for food formulation due to higher protein and amylose content (Mukprasirt and Sajjaanantakul Citation2004). Jackfruit seeds are a rich source of jacalin which has been reported a good antimicrobial compound against gram-negative and gram-positive bacteria and have a potential for use in nanomedicines (Krupa, Karigar, and Murthy Citation2020). Since jackfruit seeds are natural source of starch (more than 90%), the preparation of jackfruit seed flour has become popular, accounting for 75% of jackfruit seed use. The composition of jackfruit seed flour and seed starch have been documented in several reports ( and ). However, this composition varies with the extraction methods. Noor et al. (Citation2014) reported that the composition of jackfruit seed flour varies in different jackfruit cultivars with maximum moisture content in ‘Durasha’ while the protein and ash content was found highest in jackfruit cv. ‘Gala’. Jackfruit seed starch isolated using distilled water had higher amylose and protein contents in comparison to alkaline and enzymatic extraction methods (Noor et al. Citation2014). Recently, Kushwaha et al. (Citation2021) concluded that protein and ash content in jackfruit seed flour decreases while starch and dietary fiber content increases with the advancement of fruit maturity .
Table 2. Proximate composition of jackfruit seed flour.
Table 3. Proximate composition of jackfruit seed starch.
Jackfruit waste utilization
Since the edible flesh comprises only 30 to 35% of the total fruit weight in jackfruit, a major portion of the fruit (seeds, peel, rag and core) is considered as a waste (Saxena, Bawa, and Raju Citation2011) and remain unused (Akter and Haque Citation2020). Kalse and Swami (Citation2022) classified jackfruit waste into jackfruit on-field waste (JOFW) and jackfruit processing waste (JPW). Where JOFW includes unharvested fruit, leaves, and stems in the field, JPW comprises the waste products left after fruit processing. As jackfruit peel contains higher amounts of total phenolics, it can be used in the food industry (Jiang et al. Citation2019). Surprisingly, only 10% of jackfruit peel is used in the food industry, while the remaining 90% is utilized for the preparation of biofilm, bio sorbent, and activated carbon (Cheok et al. Citation2018). Jackfruit peel waste has also been used to produce biohydrogen and can be used as an absorbent for the removal of hazardous chemicals from wastewater discharged from textile industries and the pharmaceutical sector (Vijayaraghavan, Ahmad, and Ibrahim Citation2006). Jackfruit waste has also been utilized for the extraction of pectin (Sundarraj and Ranganathan Citation2018). Non-edible fruit portions contain more pectin (1.57 to 2.28% in fruit rind and 1.0 to 1.92% in fruit core) as compared to the edible bulbs (1.14 to 1.60%) (Rashmi, Joshi, and Haldankar Citation2011). Pectin extracted from jackfruit peel can be used to produce bio-nanocomposite substances with anti-inflammatory, anticoagulant, antimicrobial, cytocompatibility, and biodegradability properties (Kalse and Swami Citation2022). In some parts of the world, jackfruit seeds are roasted, dried, or boiled and consumed directly (Ranasinghe, Maduwanthi, and Marapana Citation2019). Being a rich source of starch, jackfruit seed is used as an ingredient in cake, biscuits, gum candies, and bread (Akter and Haque Citation2020). Moreover, jackfruit seeds are widely used for production of cattle feed, edible oil, packaging films, and food color (Kalse and Swami Citation2022). Jackfruit latex has been demonstrated to have anti-cancer and antioxidant properties. It has also been utilized for preparing natural rubber, mucoadhesive tablets, binder, and bimetallic nanoparticles (Brahma and Ray Citation2022). Moreover, jackfruit rags are a rich source of protein, cellulose, pectin, and reducing sugars, and have been used for the preparation of fermented beverages (Akter and Haque Citation2020). Therefore, various byproducts of jackfruit waste are beneficial for food, textile, and pharmaceutical industries.
Utilization of jackfruit seed starch as a nanomaterial in medicine
Nanomaterials have gained a great impetus due to their superior uses in biomedicine, catalysis, textile industry, medication delivery, biosensors, bioremediation, food industry, and agriculture (Gupta and Singh Citation2019). Starch derived from jackfruit seeds has a wide range of uses, including as a medicine carrier, a component of modified-release dosage forms, a binding agent, an emulsifier in emulsions, and suspending agent in suspensions (Kurmi, Kalita, and Das Citation2020; Nayak, Alkahtani, and Hasnain Citation2022). Jagtap and Bapat (Citation2013) reported that silver nanoparticles with jackfruit seed starch extract showed antibacterial activities against gram-positive (Bacillus subtilis, Bacillus cereus, and Staphylococcus aureus) and gram-negative bacteria (Pseudomonas aeruginosa). Jain et al. (Citation2021) reported that jackfruit seed starch nanoparticles (JFSSNPs) coated with D-Mannose conjugated 5-Fluorouracil (5-FU) were effective against liver cancer in contrast to unconjugated and plain medication. Recently, Jain et al. (Citation2022) designed metformin-loaded jackfruit seed starch nanoparticles for the treatment of type 2 diabetes mellitus and reported that there was a considerable reduction in blood glucose levels for an extended period (up to 24 hours) as compared to control and plain metformin solution. Hence, there are numerous opportunities to further explore and better utilize jackfruit starch in pharmaceutical industries.
Postharvest losses and quality issues
In jackfruit, various postharvest losses occur due to mechanical injuries, moisture loss, fruit rot, uneven ripening, chilling injury, as well as metabolic processes such as ethylene production and respiration. Hossain et al. (Citation2017) have reported that nearly 38% of total jackfruit production is lost at the postharvest stage in Bangladesh, while postharvest losses as high as 70% in Sri Lanka have been reported by Medagoda (Citation2011). Two major causes of postharvest quality deterioration in jackfruit are fruit rot and chilling injury.
Fruit rot
Fruit rot is a serious postharvest disease of jackfruit. The fungi that cause fruit rot in jackfruit have been identified as Rhizopus artocarpi in India (Roy Citation1983), Colletotrichum gloeosporioides in Bangladesh (Basak Citation1995), and Lasiodiplodia theobromae from Taiwan (Ni et al. Citation2008), Mexico (Tiznado et al. Citation2018), India (Ganesan, Panda, and Kishore Citation2020), Sri Lanka (Adikaram et al. Citation2020) and Philippines (Taylaran et al. Citation2021). Fruit rot symptoms on fruit appear on the tree and during fruit storage, with incidence being severe at temperatures of 26-28 °C and in high rainfall areas. The fungus attacks inflorescence and young fruit, which then remain immature. Consequently, light brown spots occur on male flowers and fruit followed by the appearance of powdery fungal mycelia over the fruit surface. Subsequently, the pathogen grows over the surface, resulting in the appearance of dark black spots (Ghosh et al. Citation2015). The infected fruit subsequently rots, is mummified and eventually falls from the tree (Soepadmo Citation1992). Fruit rot caused by Rhizopus artocarpi is known to cause 32% of total crop losses. However, Khan, Choudhury, et al. (Citation2021) have reported that 80% of jackfruit growers have noticed an incidence of Rhizopus rot. It can cause total loss of a crop in places where humid, warm and wet weather coincides with jackfruit flowering and fruiting (Nelson Citation2005).
While the occurrence of Rhizopus rot is characterized by the appearance of fungal mycelia on flowers and young fruit, Lasiodiplodia fruit rot occurs on wounded or mature fruit. Symptoms of fruit rot caused by Lasiodiplodia theobromae include the appearance of circular or oval shaped small yellowish-brown lesions on the fruit peel that increase in diameter to nearly 10 cm within the period of five days, and eventually become dark brown in color. Subsequently, rotting progresses deeper (5 to 7 cm) into the pulp, where the affected areas become black and soft (Adikaram et al. Citation2020). Fungal spores can survive in soil and decaying organic matter, where they can be spread by insects and wind. These spores germinate on moist fruit surfaces, where the fungal mycelia eventually grow inside fruit tissues, resulting in the formation of a black layer of fungal spores on the fruit surface. These fungal spores eventually become the cause of secondary infection of fruit rot (Nelson Citation2005).
Various management strategies have been suggested for control of fruit rot in jackfruit including removal and destruction of infected fruit, prevention of contact between ripe fruit and soil or organic matter. To ensure proper ventilation and to reduce relative humidity within the tree canopy, regular pruning of jackfruit trees should be undertaken. Water stagnation in the orchard should be avoided (Nelson Citation2005). After harvesting, the fruit should be washed thoroughly in clean water and dried before transportation. Application of copper hydroxide (53%), copper oxychloride (0.2%), 2,6-dichloro-4-nitroaniline, or bordeaux mixture (0.5%) is recommended as being effective for disease control (Azad Citation2000). The application of 40 ppm aureofungin at fortnightly intervals is also recommended (Butani Citation1978). Ghosh et al. (Citation2015) have reported that three strains of Lactococcus lactis subsp. lactis and two strains of rhizobacterium namely Pseudomonas poae VBK and Burkholderia cenocepacia VBC7 are highly effective in inhibiting in vitro growth of fungal mycelium. Postharvest application of these strains may result in reduced fruit rot incidence during storage.
Fruit rot has been reported as a key issue during storage of jackfruit. Although the symptoms of fruit rot have been given in detail in a few studies, the information regarding an effective management of fruit rot in jackfruit remains limited and warrants further investigation.
Chilling injury (CI)
Though cold storage is an effective intervention for delaying senescence and maintaining fruit quality, it cannot be employed to its full potential in the storage of jackfruit due to its susceptibility to CI (Singh Citation2022). CI is a physiological disorder that results in the rapid decay of horticultural produce due to an increase in susceptibility to diseases. The critical temperature causing CI varies among different fruits. In jackfruit, CI occurs at temperatures below 12 °C and the symptoms include the appearance of dark brown blemishes on pulp, discoloration of skin and development of off-flavor (Saxena, Bawa, and Raju Citation2011). Various postharvest approaches have been reported to alleviate CI in fruit (Zhang, Jiang, et al. Citation2021). However, research work on the reduction of CI incidence in jackfruit is sporadic and inconclusive and warrants additional investigation.
Pre-harvest factors affecting postharvest quality
The postharvest quality of fruit is highly influenced by varied pre-harvest factors like climatic conditions, genotype and cultural practices. Identifying the effects of pre-harvest factors can help in producing fruit with less susceptibility to postharvest disorders. Most commercial cultivars of jackfruit are identified based on their place of origin. Therefore, the uniqueness of jackfruit from each geographical location should be linked and verified. Debbarma, Manivannan, and Kushwaha (Citation2018) have reported the impact of geographical location on physicochemical characteristics of seventy accessions of jackfruit located in seven different regions in India, noting that fruit from Varkala (Kerala) had the highest soluble solid contents (SSC) and total sugars, while titratable acidity (TA) was maximum in the accessions from South Sikkim and Khowai (Tripura). In terms of morphological parameters, the maximum number of bulbs, fruit size, pulp weight and seed weight were identified in accessions from Panruti (Tamil Nadu). Furthermore, an evaluation of 24 jackfruit selections found in the Western ghats of India revealed wide variability among the jackfruit in terms of SSC, color, acidity, taste and flavor of bulbs (Jagadeesh et al. Citation2007). Likewise, Balamaze, Muyonga, and Byaruhanga (Citation2019) have observed significant variation in SSC, SSC: TA, carotenoids, texture, color and ascorbic acid content between three jackfruit cultivars. Apart from the climatic conditions and location, fruit quality is also affected by mineral nutrition. Different concentrations of specific nutrients, such as nitrogen, phosphorus, potassium, magnesium, and calcium have been used for the assessment of storage life of fruit (Benkeblia et al. Citation2011). Briones and Lina (Citation2020) have reported that application of inorganic manure to jackfruit cultivar ‘Eviarc Sweet’ resulted in a greater number of bulbs with higher length and breadth, where fertilizer application exhibited no effect on fruit sensory attributes. Although general field practices, propagation techniques and disease and pest control have been well documented in jackfruit, there are limited studies on the effect of preharvest conditions on postharvest fruit quality of jackfruit.
Fruit growth and maturity
Growth and development of fruit takes place in three different phases (Muhammad and Ding Citation2007). The first phase is called the fruit set and comprises the development of ovaries and the decision to retain or abort the fruit. The second phase is cell division, whilst the final phase involves cell expansion in which cell division stops and the fruit reaches its full size. Knowledge of fruit growth stages is important for determining the optimum harvesting stage. In jackfruit, the development of fruit exhibits a single sigmoidal growth pattern in three phases named as lag, log and diminishing growth phase. Lag is the phase of slow growth that lasts for two to three weeks, followed by the log phase of rapid growth, which continues for 10 to 12 weeks. Finally, the fruit follows the diminishing phase, which involves fruit maturation for five to six weeks (Kishore Citation2018). Mijin and Ding (Citation2020) have identified nine stages in jackfruit (cv. ‘Tekam Yellow’) development starting from inflorescence emergence, followed by syncarp development and maturation. Growth stages one to five involved the development of inflorescence. Syncarp formation starts in growth stage six, which involves the formation of the rind with thick and blunt spines. At this stage, three parts of syncarp are distinct; the first region comprises the edible fleshy part, the second region is the white spongy part fused to the syncarp, and the third region consists of the spiny structure covering the syncarp. Further, in growth stage seven, a change in rind color occurs from dark green to greenish, along with an increase in the size of seeds and pericarp. The color of fruit changes to pale yellow in growth stage eight. In this stage, the absence of characteristic aroma and presence of starch granules in the pericarp indicate immature fruit. In the last stage, there is a change in rind color to brownish yellow, with hardly any starch granules evident, which represents the conversion of starch into sugar.
Kishore (Citation2018) has divided fruit maturation into four sub-stages. The beginning of maturation includes a change of fruit color to light green or light yellow and seed color to light brown and aril color to yellow, along with flattening of spines. In advanced maturation, there is a change of peduncle color to light yellow, cessation of latex secretion, and aril starts turning fleshy and light yellow. The third stage is fruit maturity for commercial picking in which there is the production of a dull hollow sound on tapping, spines yield to moderate pressure, and completion of seed maturity. The last stage termed as overripe fruit, includes softening of arils resulting in loss of textural consistency, complete flattening of spines and sometimes germination of seeds within the fruit (vivipary).
Mijin et al. (Citation2021) have claimed that the harvest maturity stage affects the postharvest quality of jackfruit, reporting the ideal harvesting stage of fruit for long distance markets to be 12 weeks after anthesis, and 14 weeks after anthesis for local markets. More studies related to fruit growth physiology of jackfruit should be conducted in this area to ensure better fruit quality after harvesting.
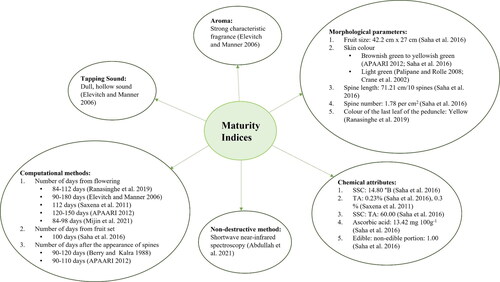
Maturity indices
Accurate determination of maturity stage, combined with good harvesting practices, can help in reducing postharvest losses of the fruit. Normally, jackfruit takes three to eight months from flowering to fruit development (Haq Citation2006; Berry and Kalra Citation1988). Jackfruit harvesting time varies depending upon the region (). Time taken to maturity depends upon several factors such as tree age, location, and weather conditions. This explains why days from flowering or fruit set to full maturity alone are not good indicators of harvest maturity. Other maturity indices include changes in skin color from light green to brownish green or yellow, flattening of spines on the fruit surface, development of characteristic odor, yellowing of the last leaf on the fruit bearing stalk, reduction in number of spines on fruit surface (Ramli Citation2009; Ranasinghe, Maduwanthi, and Marapana Citation2019) and production of dull, hollow sounds on tapping (Elevitch and Manner Citation2006; Palipane and Rolle Citation2008). Various maturity indices employed in jackfruit are listed in . However, there are specific parameters in each area of the world that are used to judge the harvest maturity of jackfruit. In Malaysia, this is estimated by listening to the dull hollow sound, looking at the change in peduncle leaf color, and accounting for spine firmness and fruit color (Palipane and Rolle Citation2008). In India, this is assessed based on dull hollow sounds and fruit shape, while the time from anthesis, color change and spine growth are used as indicators of optimal maturity in Bangladesh (Irin et al. Citation2015). Among these maturity indices, tapping sound is considered to be the most reliable, while the other indices such as yellowing of last leaf on the peduncle are inadequate to be used alone, where Angeles (Citation1983) has outlined that some fruit peduncles without any leaves still had immature fruit. Likewise, change in fruit color is also not a dependable maturity index, as rind color varies with the cultivar. Additionally, an increase in the ratio of edible to non-edible portion of the fruit is determined to judge fruit maturity (Miller and Fry Citation2001; Saha, Islam, and Molla Citation2016). A reduction in organic acids and an increase in sugar content is also an indicator of fruit maturation in jackfruit (Mijin et al. Citation2021). Rosnah et al. (Citation2009) have estimated the biochemical composition of 44 jackfruit genotypes and outlined SSC to range from 15.1-24.9°Brix depending upon the cultivar. Recently, Abdullah et al. (Citation2021) have predicted SSC from jackfruit skin surface using shortwave near infrared (SWNIR) spectroscopy, reporting that spectral values correlate well with SSC values determined using a digital refractometer. Since traditionally used methods vary depending upon a tree’s age, location, cultivar and climatic conditions, more nondestructive methods for maturity determination of jackfruit should be developed to standardize harvest maturity index of jackfruit.
Table 4. Harvesting time of jackfruit in different parts of the world (Baliga et al. Citation2011).
Fruit ripening physiology
Fruit ripening is a composite mechanism that ends with remarkable changes in fruit flavor, texture and color. Jackfruit is climacteric in nature, with three phases of respiration: pre-climacteric, climacteric, and post-climacteric (Saxena, Bawa, and Raju Citation2011). Jackfruit ripening is accompanied by biochemical, physiological and physical changes such as carotenoid biosynthesis, sugar content accumulation, decline in organic acid content and changes in cell wall composition (Selvaraj and Pal Citation1989; Goswami et al. Citation2011). The principal storage material in the bulb is starch, which is converted to sugars during ripening. This is characterized by changes in bulb color from light yellow to golden yellow and the presence of a characteristic sweet aroma. Mature jackfruit ripens in three to four days at an optimum temperature of 24-27 °C. Uneven ripening is one of the major issues in jackfruit, especially in large fruit. For uniform ripening, Sutrisno and Edris (Citation2011) have suggested that fruit should be exposed to 50 ppm ethylene for 24 hours at 25 °C.
Ethylene biosynthesis and respiration rate
Jackfruit exhibits a sudden rise in ethylene biosynthesis and respiration rate at maturity (Ong et al. Citation2006; Jun-Ning et al. Citation2014). Saxena, Bawa, and Raju (Citation2008) have reported that specific respiration rate in jackfruit is 20-25 mg CO2 kg−1 h−1. Immature jackfruit bulbs showed a respiration rate of 160 mg CO2 h−1 kg−1 fruit, while maximum respiration rate (250 mg CO2 h−1 kg−1 fruit) was observed during the climacteric phase. Climacteric peak was exhibited three days after fruit ripening, where the respiration rate dropped to 60-70 mg CO2 kg−1 h−1after 8 days of ripening (Selvaraj and Pal Citation1989). Jun-Ning et al. (Citation2014) observed a sharp increase in ethylene production from one week before fruit ripening until three days after harvest, with a subsequent decline in ethylene production. In contrast, Sutrisno and Sudiari (Citation1998) observed a higher ethylene peak immediately after harvest. Rahman et al. (Citation1995) have observed microscopic cracks in the cell wall of jackfruit during storage, with browning of flesh tissues that may be ascribed to cellular decompartmentalization. However, the severity of such damage can be reduced with the application of ethylene antagonist 1-methylcyclopropene (1-MCP) (Vargas-Torres et al. Citation2017). Latifah et al. (Citation2017) have reported that fresh-cut jackfruit packaged with ethylene absorbent material exhibited better color retention, higher SSC and pH values and consequently better control of respiration and ripening processes. Managing the biosynthesis of ethylene, its action and respiration rate during the postharvest phase usually delays fruit ripening and extends the postharvest life of climacteric fruits. However, the effective management of ethylene production and respiration rate during the postharvest phase of jackfruit warrants further investigation.
Color development
Jackfruit color develops due to the biosynthesis of carotenoids, depending upon the cultivar’s characteristic color. During ripening, there is an increase in carotenoid biosynthesis that causes color changes in jackfruit (Goswami et al. Citation2011). Carotenoid content is at its minimum during fruit set and increases dramatically during fruit maturation. There is an accumulation of carotenoids even after harvest, where this increases up to two-fold from maturation to ripe eating stage (Selvaraj and Pal Citation1989). Rana, Pradhan, and Mishra (Citation2018a) have reported that during the ripening process, hue values were higher in the top portion of jackfruit as compared to the lower portion, indicating that ripening starts from the upper portion of the fruit. Likewise, Ong et al. (Citation2006) have observed a significant difference in hue values in different portions of jackfruit, where hue values increased with the advancement of ripening stage, which may be attributed to increase in carotenoid content in the fruit. Ranasinghe and Marapana (Citation2019) have reported an increase in lightness (L* value) during maturation of jackfruit; however, its value was shown to decrease during later stages of ripening. With the advancement of fruit maturity, jackfruit color tends to become paler and duller due to a decrease in b* values. Nevertheless, knowledge about the levels and biosynthesis of carotenoids in relation to specific jackfruit cultivars is limited and deserves additional investigations.
Fruit taste
Fruit taste is predominantly the combination of sugars and acid profile at the time of maturity that is also used as a maturity index in different fruits (Bashir and Abu-Goukh Citation2003). Rahman et al. (Citation1999) have reported a variation in carbohydrate content of jackfruit depending on fruit maturity, finding 2.2% and 9% starch content in ripe fruit, as compared to 31.6% and 29.8% in immature fruit of soft fleshed and firm-fleshed cultivars, respectively. Major sugars reported in jackfruit are glucose, fructose and sucrose (Chowdhury, Raman, and Mian Citation1997), where several other sugars such as xylose, rhamnose, galactose and arabinose are also found in jackfruit pulp (Gupta and Rao Citation1963). Microscopic observations have revealed that the perianth of immature fruit consists of cells which are densely packed with starch granules, where the number and size of such granules decreases with advancement of fruit ripening (Rahman et al. Citation1995). Degradation of starch granules results in a higher level of non-reducing sugars in ripe fruit that improves organoleptic characteristics (Ong et al. Citation2006). Respiration outburst is catalyzed by the breakdown of starch granules into fructose and glucose. Ong et al. (Citation2006) observed an increase in sucrose content during ripening of jackfruit, with significant variation in the middle and lower portions of the fruit, while fructose and glucose contents increased only up to five days after ripening. Their comparison of sugar content in soft and hard fleshed jackfruit revealed that fructose/glucose ratio varied in both fruit types. Later, Li et al. (Citation2017) reported that individual sugar content was lowest in unripe fruit, where it increased gradually as fruit ripening progressed. They also observed that during fruit ripening there was rapid increase in SSC and sucrose content, while there was a slow increase in glucose and fructose content.
The principal organic acids in jackfruit are citric and malic acid, where succinic and oxalic acids are also present. Selvaraj and Pal (Citation1989) have observed that levels of organic acids increased until the three-fourth ripe stage of fruit, which then declined during the later stages of ripening. There is a decline in organic acid content during fruit ripening, as organic acids act as a carbon skeleton for production of new compounds during the process of respiration (Kays Citation1991). During the early stages of ripening, malic acid content was higher in the fruit, which gradually decreased throughout the ripening process, but no significant change in oxalic acid content was observed during the ripening process (Ong et al. Citation2006). Citric acid content increased significantly in the lower portion of jackfruit after 5 days of harvest. Likewise, succinic acid content varied significantly among upper and middle portions of fruit. The level of malic acid was higher in the early stages; however, its content decreased significantly during later stages of fruit ripening (Ong et al. Citation2006). Nevertheless, sporadic and inconclusive research work has been reported on profiling of individual sugars as well as organic acids in jackfruit and warrants further investigation.
Fruit aroma
Jackfruit has a peculiar aroma that varies with the cultivar and depends upon preharvest and postharvest conditions (Bicas et al. Citation2011), where esters are the key contributors in jackfruit aroma. Ong et al. (Citation2006) have observed lower ester content during the initial stages of fruit maturity, where there was an increase in the number of volatile compounds in jackfruit throughout the ripening process. The odor in immature fruit during the first stage of ripening is due to the formation of organic acids and alcohols, which are catalyzed by alcohol acyltransferases (Olias et al. Citation1995).
Fruit texture
It is a programmed series of biochemical reactions that are correlated with hydrolytic reactions in cell wall components like pectin, lignin, cellulose, and hemicellulose in the presence of hydrolytic enzymes including β-galactosidase, cellulase, rhamnogalacturonase, pectate lyase, pectin methyl esterase and polygalacturonase (Prasanna, Prabha, and Tharanathan Citation2007). Beside these reactions, cellulose degradation and expansion proteins are also key factors in fruit softening processes. With increase in cellular activity, there is a subsequent decline in fruit hardness and polysaccharide content (Tanaka et al. Citation2008). Ranasinghe and Marapana (Citation2019) have reported a decrease in fruit firmness with advancement of fruit maturity stage, where its value was least at fully ripened stage, which was attributed to hydrolysis of insoluble pectin to soluble form. Starch and pectin are the major constituents of jackfruit cell wall and pulp tissues at immature stages, where they undergo hydrolysis into reducing sugars like glucose and fructose during ripening. Starch granules are also used for the biosynthesis of ethylene in climacteric fruits, and this degradation is stimulated by the polygalacturonase enzyme (Ong et al. Citation2006). Sugar to acid ratio contributes toward sweetness of jackfruit bulbs. while lower acidity is also associated with loss of firmness indicating its role in harvest index (Jagadeesh et al. Citation2007). Rana, Pradhan, and Mishra (Citation2018a) have reported that jackfruit firmness is closely related to alcohol insoluble solids, which includes pectic acids, hemicellulose, cellulose and starch. It has been observed that fruit hardness increases with maturity while cohesiveness and gumminess decrease, and there is no effect of fruit maturity stage on chewiness and adhesiveness. Rahman et al. (Citation1995) have observed that conversion of starch into sugars in jackfruit pulp was accompanied with the breakdown of cell wall material. The study also reported that the activities of pectin methyl esterase and polygalacturonase enzymes were 40 and 12 times higher respectively in ripe fruit of soft fleshed jackfruit in comparison to firm fleshed fruit, while there was no difference in the activity of β-glucanase enzyme in both fruit types.
Postharvest handling and storage
Fruit senescence during the postharvest phase negatively affects fruit, where effective measures need to be implemented to minimize postharvest losses during fruit storage. Detailed below are the techniques that have been used for postharvest quality management in jackfruit:
Cold storage
The role of cold storage in postharvest quality management of horticultural produce is well established, where it can either extend the postharvest storage life or induce undesirable changes in fruit texture, taste and color (Souza et al. Citation2011). The first report of cold storage of jackfruit (bulbs) was conducted by Singh and Mathur (Citation1954), indicating the maintenance of fruit sensory attributes. However, the study showed a decline in fruit pectin content and firmness due to activities of the pectin methyl esterase enzyme. After this, Latifah et al. (Citation2000) showed that storage at different temperatures affected the rate of respiration in minimally processed jackfruit. Accordingly, a sudden increase in respiration rate was noticed when fruit was stored at 10 °C and 25 °C on the second and fourth day of storage respectively, while the respiration rate remained low at 2 °C for three weeks. Ramli et al. (Citation2016) have investigated the impact of cold storage (8 °C) on jackfruit bulbs, reporting that SSC, TA, and pH remained stable throughout the storage period, but there was a steady decline in firmness and pectin content in jackfruit bulbs. Low-temperature storage also has a positive impact on the antioxidant capacity of jackfruit. Zeng et al. (Citation2018) have reported that fresh-cut jackfruit stored at 8 and 12 °C exhibited enhanced SOD activity and reduced cell membrane permeability and malondialdehyde (MDA) content. It was also revealed that storage at very low temperatures (0 and 4 °C) affected fruit taste and nutritional quality, whereas storage at higher temperatures (16 and 20 °C) resulted in deterioration of fruit quality. In another study, jackfruit bulbs stored at 4 °C maintained SSC, TA, fruit firmness and pH for 12 days with minimum reduction in water loss (Mei et al. Citation2020). Recently, Ying et al. (Citation2020) investigated the impact of low temperature storage on fresh jackfruit packaged in low-density polyethylene, and reported higher SSC, TA, ascorbic acid and sensory attributes with lower disease incidence over two weeks. Although the influence of cold storage on fresh cut jackfruit has been reported, the effect of low-temperature storage on ethylene production, respiration rate, fruit quality characteristics and ripening related enzymes in fresh fruit still needs to be investigated.
Controlled atmosphere (CA) storage
This type of storage involves a special technique in which predetermined gaseous concentrations, particularly lower O2 and higher CO2, are maintained around a commodity by the addition and removal of gases during cold storage period (Koyuncu et al. Citation2019). Along with maintaining postharvest quality and extending storage life, this also delays postharvest ripening by lowering ethylene biosynthesis and ensures offshore marketing of perishable produce (Thewes, Brackmann, and Neuwald Citation2019). Jackfruit fresh-cut bulbs were evaluated in CA (6 kPa CO2 + 3 kPa O2 + N2 balance) storage with pretreatment of sodium benzoate, citric acid, ascorbic acid and calcium chloride with subsequent chitosan coating. The results indicated 5% and 17% more phenolics and ascorbic acid content, respectively in CA-stored jackfruit bulbs in comparison to control (Saxena et al. Citation2013). Information in relation to CA storage of jackfruit is limited, where more investigations in this field will help to widen knowledge on postharvest quality management of jackfruit.
Packaging
Modified atmosphere packaging (MAP)
MAP is an important method to maintain the postharvest quality of horticultural produce (Pinto et al. Citation2020). Jackfruit exhibits high respiration rate and ethylene production that can be minimized by using MAP alone or in combination with other postharvest techniques (). Saxena, Bawa, and Raju (Citation2008) reported a significant decline in respiration rate, ethylene production and microbial activity in jackfruit bulbs using MAP (polyethylene terephthalate jars) in combination with pretreatment with sodium benzoate (0.045%), citric acid (1%), calcium chloride (1%), and ascorbic acid (0.02%). Following this, Saxena, Bawa, and Raju (Citation2009) have reported that jackfruit stored at 6 °C for 7 weeks showed 31%, 43%, 8%, and 7% higher vitamin C, carotenoids, flavonoids, and total phenolics respectively in MAP of minimally processed fruit in comparison to control. Along with gaseous composition, the type of MAP material used is also an important factor affecting fruit quality. Sally, Ranganna, and Munishamanna (Citation2011) have reported that citric acid-treated bulbs packed in polyethylene bags showed a longer shelf life with minimum physiological losses as compared to all other MAP materials (polypropylene, and polystyrene). Similarly, transparent polyethylene packaging with 2% O2, 8% CO2 and 90% N2 showed higher SSC, TA, and firmness along with a reduction in weight loss, respiration rate, and ethylene production in jackfruit bulbs (Vasudeva et al. Citation2018). Dhanesh et al. (Citation2018) observed the effects of different packaging techniques (active MAP, passive MAP, and vacuum packaging) on blanched and frozen jackfruit bulbs, reporting active MAP and vacuum packaging to be more effective in reducing microbial count and maintaining the sensory attributes of bulbs. Prathibha et al. (Citation2019a) investigated the effects of different packaging material (low density polyethylene, high density polyethylene, vacuum packaging, cling film, shrink film, and stand on pouches) on fresh-cut jackfruit bulbs, revealing that bulbs packed in vacuum packaging exhibited maximum storage life of 33 days, while minimum shelf life was observed in shrink film. Moreover, microbial decay is one of the leading constraints in the supply chain management of jackfruit bulbs. Udayasoorian et al. (Citation2019) reported that yeast population, total plate count, and microbial decay can be effectively controlled by MAP in combination with honey dipping treatment of jackfruit bulbs. However, all these studies have been conducted on fresh-cut jackfruit bulbs, where the effects of MAP on the extension of storage life and maintenance of fruit quality of whole jackfruit have not yet been investigated.
Table 5. Effect of Modified atmospheric packaging (MAP), vacuum packaging and controlled atmosphere (CA) storage in combination with other treatments on quality parameters and storage life of jackfruit.
Vacuum packaging
Vacuum packaging has proven effective in extending storage life, maintaining quality and controlling microbial damage in jackfruit, where its effect can be enhanced with chemical pretreatment (). A combination of vacuum packaging and citric acid pretreatment has proved helpful in maintaining lower total plate count in jackfruit bulbs stored at 4 °C (Sally, Ranganna, and Munishamanna Citation2014). Taj, Ranganna, and Munishamanna (Citation2014) reported that vacuum packaged jackfruit bulbs exhibited satisfactory sensory attributes up to: three weeks in unripe bulbs, two weeks in semi-ripe bulbs and one week in ripe bulbs. Later, Taj, Ranganna, and Munishamanna (Citation2016) observed inhibition in microbial activity (< 1 log CFU/g) in polyethylene-packed frozen jackfruit bulbs with 80% vacuum packaging. Rana, Pradhan, and Mishra (Citation2019) conducted image analysis of fresh cut jackfruit bulbs and reported changes in color and incidence of browning to be lower in vacuum packaged fruit slices as compared to control. Moreover, the vacuum packaged jackfruit bulbs stored at freezing temperatures exhibited better color retention and lesser microbial infestations in comparison to cold storage (8-10 °C) (Johari, Rahman, Baharuddin, Basha, et al. Citation2020). Following this, Johari, Rahman, and Baharuddin (Citation2020) reported that a comparative analysis of vacuum packaging of cold-stored and frozen bulbs indicated a better bioactive profile in frozen form. Hence, vacuum packaging has the potential to prolong storage life and reduce postharvest losses in jackfruit; however, its effect on whole fruit warrants further exploration.
Chemical treatments
Exogenous application of various chemical compounds has been shown to be effective in improving quality, maintaining storage life, and ameliorating decay incidence in fruits and vegetables. Different chemical compounds including: 1-MCP, calcium chloride, citric acid, sodium benzoate and ascorbic acid have been used for quality management of whole jackfruit and for fresh-cut bulbs ().
Table 6. Effect of postharvest chemical treatments on quality attributes and storage life of jackfruit.
1-Methylcyclopropene
Among ethylene antagonists, 1-MCP is widely used to supress ethylene action and the extension of postharvest shelf life, which in turn maintains the quality of horticultural produce (Dong et al. Citation2015). Mata-Montes et al. (Citation2007) reported that fumigation of fresh jackfruit with 1-MCP did not exhibit a significant influence on fruit quality of jackfruit; however, it did produce a delay in ethylene biosynthesis, respiration rate and pulp softening, along with an extension of shelf life for 12 days. Similarly, the application of 1-MCP in jackfruit was found to be effective in inhibiting ethylene production and reducing sugar content, but also caused a decrease in sucrose content as compared to control (Wang et al. Citation2014). Starch accumulation in immature jackfruit is responsible for higher fruit firmness, whilst the onset of maturity activates sucrose-phosphate synthase (SPS), which in turn initiates the oxidation of starch into sucrose. The application of 1-MCP slowed down the degradation of starch, whereby the activity of SPS was delayed for 8 days in comparison to control, indicating its role in quality management (Wang et al. Citation2017). Recently, Chen et al. (Citation2022) observed that 1-MCP fumigation of fresh jackfruit-maintained fruit firmness during the early stages of fruit ripening, but did not have a significant influence on fruit softening during later stages of ripening. Hence, postharvest application of 1-MCP has proved effective in maintaining fruit quality of frozen/stored jackfruit; however, its effect on fresh jackfruit warrants further investigation.
Calcium chloride
Calcium chloride is an inorganic chemical compound that has been widely used for postharvest application in fruits due to its efficacy against pH change (Garcia et al. Citation2019). Daryanti (Citation2005) reported that ready-to-eat jackfruit bulbs treated with 1% CaCl2 retained better fruit firmness, SSC and TA for 13 days in comparison to control. Likewise, polyethylene and polystyrene packaging of jackfruit bulbs after CaCl2 and citric acid dip treatment was shown to reduce microbial infestation in jackfruit bulbs (Sally, Ranganna, and Munishamanna Citation2011). Further, Ramli and Ahmad (Citation2013) assessed biochemical changes in the middle lamella of the fruit cell wall with the advancement of storage periods and reported that calcium treatment inhibited its biodegradation subsequent to firmness retention. Moreover, the combined application of CaCl2 and ascorbic acid enhanced levels of reducing sugar, SSC and TA in jackfruit bulbs (Chulaki et al. Citation2017). Color change during fruit storage is the key factor responsible for lowering the marketability of fruits, where jackfruit color is directly associated with postharvest carotenoid content retention. CaCl2 (1%) and ascorbic acid (0.25%) dipping retained better carotenoid and antioxidant content along with reduced microbial decay and pH change in jackfruit bulbs stored at 6-8 °C for 3 weeks (Prathibha et al. Citation2019b). Similar dipping treatment followed by packaging in low-density polyethylene minimized moisture loss, ascorbic acid degradation and color change, in addition to maintaining higher sensory attributes of jackfruit bulbs for 33 days (Prathibha et al. Citation2019a). Similarly, higher ascorbic acid content, fruit color (L*, a*, b*) and color deviation extent were observed in sodium chloride (1%) and sodium benzoate (0.05%) treated jackfruit bulbs (Sudheer, Saranya, and Sankalpa Citation2019). Moreover, there was increase in content of reducing sugars, ascorbic acid and carotenoids by application of citric acid (0.02%), ascorbic acid (0.02%), potassium metabisulphite (150 ppm) and CaCl2 (1%) in minimally processed jackfruit (Gogoi et al. Citation2021a). The combined application of CaCl2, citric acid and ascorbic acid in minimally processed jackfruit has also proved effective in lowering the activity of the polygalacturonase enzyme, slowing the breakdown of pectin in fruit tissues (Gogoi et al. Citation2021b). Similarly, Rana, Pradhan, and Mishra (Citation2018b) have claimed that browning index, color change, firmness loss and activities of oxidative enzymes were reduced in 2-5% CaCl2 treated jackfruit bulbs. Gomathi et al. (Citation2021) reported that jackfruit bulbs treated with 1% ascorbic acid and 1% CaCl2 exhibited minimum weight loss (4.10%), maximum firmness (40.44 N) and extended shelf life (16 days). Thus, calcium chloride treatment in combination with other chemicals has proved beneficial in maintaining fruit quality characteristics and reducing browning index in jackfruit.
Other chemicals
Acetic, ascorbic and citric acid have also been used to prolong storage life and inhibit browning with minimal decline in fruit quality parameters of jackfruit (Mahajan et al. Citation2014). Citric and ascorbic acid application has proved effective in reducing the microbial count of minimally processed jackfruit for 15 days (Navindra, Arvind, and Hassina Citation2009). In accordance with this, Ulloa et al. (Citation2010) have reported that treatment of jackfruit bulbs with citric acid, potassium sorbate and ascorbic acid reduced microbial growth and maintained a good eating quality index. Siti et al. (Citation2017) have outlined maintenance of fruit firmness and fresh weight of jackfruit bulbs with the treatment of ascorbic acid. Moreover, acetic acid application has proved effective in the inhibition of bacterial strain, Pantoea agglomerans in fresh-cut jackfruit (Pothimon et al. Citation2021). However, the impact of these chemicals in relation to ethylene production, respiration rate and other fruit ripening parameters warrants further evaluation.
Edible coatings
Increasing health concerns in relation to the residual effect of chemicals have initiated searches for alternatives that prolong the storage life of fruits, where edible coatings have been widely used for postharvest management due to their safe nature (Jafarzadeh et al. Citation2021). In this regard, the anti-microbial and anti-browning efficacies of edible coatings, along with reduction in respiration rate, ethylene biosynthesis and water loss have been well-documented (Antunes et al. Citation2012; Pizato et al. Citation2019). The effects of edible coatings in improving storage life and maintaining fruit quality of jackfruit are summarized in . Chitosan is one of the most widely used edible coatings in horticultural produce, where it has also been employed to improve the biochemical composition of jackfruit during storage. Mei and Ping (Citation2008) reported lower microbial infestation along with better biochemical composition and antioxidative capacity in cassava starch-chitosan-coated jackfruit bulbs. Additionally, in comparison to control, jackfruit bulbs maintained about 17% and 5% higher ascorbic acid and phenolic contents respectively when 1% chitosan coating was applied before MAP (Saxena et al. Citation2013).
Table 7. Effect of edible coatings on quality attributes and storage life of jackfruit.
Aloe vera (AV) gel is one of the most widely used coatings for horticultural produce, where it has no harmful residual effects (Hasan et al. Citation2021). A comparison of pectin and AV gel coating has revealed AV coating to produce better results than pectin. The study showed higher Vitamin A, Vitamin C, and protein content in jackfruit bulbs, along with a reduction in fruit weight loss during storage period of one week (Teja, Santhi, and Narsingarao Citation2016). Similarly, Ansar et al. (Citation2020) have outlined that the application of AV gel coating maintained fruit color and moisture content in jackfruit bulbs. This amounted to better fruit quality when stored at 10 °C as compared to 29 °C. Based on this report, it can be concluded that the efficacy of AV coatings can be enhanced when combined with cold storage.
Metabolic activity cannot be inhibited completely but it can be delayed by using edible coatings. A combination of white bean starch-based coating and CaCl2 was shown to be effective in reducing ethylene production, rate of respiration, alleviating chilling injury (CI), and maintaining better phenolics and sugar content (Partha, Wasono, and Ulfah Citation2009). Similarly, the combined application of edible coatings (sodium alginate, gellan or xanthan) and 1-MCP has been reported to be effective in delaying metabolic senescence and microbial growth, leading to better storage life of jackfruit bulbs. A comparison of control and treated bulbs indicated reduced respiration rate and fruit weight loss, and higher values of SSC and TA in treated fruit (Vargas-Torres et al. Citation2017). Minh et al. (Citation2019) evaluated the combined effect of different concentrations of whey protein and pectin crosslinked with trans-glutamine, reporting that whey protein/pectin coating in the ratio of 80/20 proved most effective in reducing weight loss in jackfruit bulbs along with maintenance of ascorbic acid, phenolics and carotenoids content. Recently, Jayakody, Vanniarachchy, and Wijesekara (Citation2021) revealed that application of alginate and agar-based coatings on jackfruit bulbs preserved volatile compounds such as hexadecane, pentadecane tetradecane, tridecane, naphthalene and 6-methyl-2-heptanone, where all these compounds were lost in untreated fruit samples. The effect of different edible coatings in fresh cut jackfruit has been extensively studied, but like other postharvest treatments, the efficiency of edible coatings on whole jackfruit has not yet been investigated.
Non-chemical approaches
Irradiations
Irradiation has proven to be effective in extending postharvest life, inhibiting microbial infections and insect damage in horticultural crops. Ionizing radiation possesses sanitizing efficacies against microbial infestations by damaging their DNA molecules and inhibiting their reproduction rate (Arvanitoyannis, Stratakos, and Tsarouhas Citation2009). Bizura-Hasida et al. (Citation2013) evaluated the impact of UV-C treatment on the reduction of microbial damage in jackfruit and reported that jackfruit bulbs exposed to UV-C (240 nm) for 5 minutes and stored at 5 °C exhibited superior quality and lower total plate count, along with extended storage life for 14 days. However, there was a higher total yeast count as compared to control (). Limited information is available on the impact of irradiation treatment on maintenance of postharvest fruit quality of jackfruit, where its effect in combination with heat treatments is lacking and requires further investigation.
Table 8. Effect of non-chemical approaches on storage life and quality parameters of jackfruit.
Heat treatments
Heat treatments of fruits and vegetables offer an advantage over chemical applications due to their non-residual effects (Malik et al. Citation2021). Saxena et al. (Citation2012) determined carotenoid degradation kinetics in relation to hot air application in jackfruit bulbs. They reported that thermal processing reduced Hunter L* and b* values, while there was a significant increase in a* value. Nonetheless, there was browning of jackfruit bulbs when the treatment time exceeded 18 minutes. Babu and Sudheer (Citation2020) have reported the inhibition of microbial activities and maintenance of fruit firmness of fresh-cut jackfruit after sterilization at 90 °C for 19 minutes. Accordingly, the browning incidence of sterilized samples was higher as compared to pasteurized samples (121 °C for 38 minutes). Later, Babu et al. (Citation2022) reported that the same treatment maintained higher levels of total flavonoids and phenolics in fresh-cut jackfruit. Heat treatments are effective in maintaining fruit quality and reducing microbial activities, but at the same time they induce undesirable changes in fruit quality such as browning (). It is worth noting here that the studies listed were based on minimal processing rather than on enhancing storage life or improving the quality of whole fruit.
Ozone
Ozone is a triatomic oxygen molecule with a half-life of only 22 min, after which the molecule reverts back to its diatomic form. As a postharvest treatment, ozone can be applied both in gaseous and aqueous forms (Shezi et al. Citation2020). Nguyet, Tra, and Yen (Citation2008) have outlined that application of ozone gas for 1 min and then storage of fruit in Poly Vinyl Chloride (PVC) film led to 100% N2 reduced microbial count, with no significant changes in fruit quality characteristics. There is little information available on the influence of ozone on postharvest quality management of jackfruit, where its effect in relation to ethylene production, respiration rate and ripening related enzymes needs further investigation.
Value addition
Due to the high nutritional value and perishable nature of jackfruit, several products are prepared from jackfruit rind and seeds, as well as both unripe and ripe fruit. Ripe jackfruit bulbs are used for the preparation of candy, preserves, pickles, leather, chutney, powder, concentrate, jam, jelly, slab, chips, papad, juice, osmo-air dried segments, and are also canned in sirup (Mondal et al. Citation2013). Additionally, jackfruit pulp is used for flavoring beverages and ice creams (Gaikwad, Kamble, and Jaybhay Citation2020). Moreover, jackfruit seeds are also consumed after roasting, boiling, drying and are ground for the preparation of flour (Akter and Haque Citation2018).
Jam
Jackfruit pulp has good gelling properties for the preparation of jam (Tiwari and Vidyarthi Citation2015). Due to the low pH of the fruit, jam prepared from jackfruit is more stable and less prone to microbial attack (Ihediohanma, Okafor, and Adeboye Citation2014). Jackfruit jam is low in calories and is full of natural sugars (Swami et al. Citation2012), where it can be prepared using sucrose, citric acid, pectin, agar and potassium metabisulphite as a preservative (Mondal et al. Citation2013; Kanishka, Lakshman, and Nakandala Citation2018). Jackfruit jam stored at cold storage temperature (7 °C) retains better sensory attributes and has been shown to be free from microbial attack for a period of 1 year at room temperature (25 ± 5 °C) (Tiwari and Vidyarthi 2012). Kushala, Sreenivas, and Siddappa (Citation2012) have reported that jam prepared by blending jackfruit pulp with kokum and avocado with 70° Brix SSC, 0.5% acidity, and 55% juice had the maximum sensory score, with a storage life of four months. Additionally, Arshad et al. (Citation2018) reported a reduction in lycopene, protein, and ascorbic acid content and an increase in total sugar content of jackfruit jam when stored at both ambient temperature and in refrigeration. However, changes in physicochemical characteristics were more pronounced at ambient storage conditions. Therefore, for better storage life and for preservation of quality characteristics, jackfruit jam should be stored at low temperature.
Fermented beverages
Jackfruit contains considerable amounts of fermentable sugars, which can be used for the preparation of wine and vinegar (Swami et al. Citation2012). Wine prepared from jackfruit is an excellent source of antioxidants (Panda et al. Citation2016) and provides protection against irradiation induced DNA damage (Jagtap et al. Citation2011). Amit and Ambarish (Citation2010) have reported a maximum of 10% alcohol content in the wine prepared from jackfruit. Similarly, Baidya, Chakraborty, and Saha (Citation2016) have prepared wine by combination of jackfruit, mango and pineapple juice with 8-10% alcohol range. Likewise, Kumoro et al. (Citation2012) have reported that jackfruit juice containing 14% sugar fermented with 0.5% yeast for 9 days resulted in the best quality wine, with about 12% ethanol. However, Nirmal, Bhutia, and Aradhya (Citation2013) reported alcohol content up to 18% in jackfruit wine. Additionally, Shraddha, Munishamanna, and Shyamalamma (Citation2021) have revealed that blending jackfruit juice with 15% amla juice improved the sensory score of jackfruit wine. Recently, Cagasan et al. (Citation2021) have prepared wine from jackfruit by-products (seed coat, peel, overripe pulp and rags), with good physicochemical properties and acceptable sensory score.
Vinegar prepared from jackfruit seeds and rags contains higher antioxidant activity, Vitamin C, calcium, and magnesium content (Photphisutthiphong and Vatanyoopaisarn Citation2019). He et al. (Citation2021) optimized fermentation conditions for the preparation of jackfruit vinegar, reporting that vinegar with an acetic acid content of 44.20 ± 0.41 g L−1 can be prepared with 11% inoculation volume of acetic acid bacteria at the temperature 32 °C and pH 3.3. Constance et al. (Citation2021) have compared the properties of vinegar prepared from bitter kola (Garcinia kola) and jackfruit and reported the highest protein (2.45%), total solids (1.5%), and ash content (17.3%) in vinegar prepared from jackfruit. Furthermore, Buddhika et al. (Citation2021) prepared jackfruit vinegar with an alcohol percentage of less than 0.5% and reported phenolics, flavonoids and antioxidant content to be higher at the storage temperature of 36 °C as compared to 30 °C. Hence, the preparation of fermented beverages from jackfruit not only reduces postharvest losses but makes the products available in off-seasons.
Dehydrated products
There is an increasing demand for dehydrated fruits in global markets. Optimization of the dehydration process in jackfruit can help in reducing losses and preserving nutritional quality (Nansereko, Muyonga, and Byaruhanga Citation2022). Rahman et al. (Citation2012) have reported that osmotic dehydration of jackfruit pulp by adding 45% sugar sirup results in maximum retention of Vitamin C, Vitamin A, total sugars and total acid content. However, Swami et al. (Citation2014) have stated that treatment of jackfruit bulbs with 60% sugar solution resulted in maximum sensory score in terms of flavor (7.56), color (7.67), texture (7.89), and overall acceptability (7.78). Taib et al. (Citation2013) have reported that microwave vacuum dehydration is more effective in maintaining overall quality of jackfruit bulbs as compared to convective hot air dehydration. Saxena et al. (Citation2015) have concluded that combination drying (hot air drying and freeze drying) of jackfruit bulbs resulted in minimal shrinkage of bulbs with a shelf life of 8 months at ambient conditions (22-32 °C). Moreover, osmotic drying of jackfruit bulbs at the temperature of 60 °C resulted in higher color retention in terms of L*, a*, and b* values as compared to drying temperatures of 50 and 70 °C (Kaushal and Sharma Citation2016). Further, Khan, Choudhury, et al. (Citation2021) prepared ready-to-eat jackfruit slices by drying them at 50 °C, 60 °C, and 70 °C after the addition of citric acid, salt, and potassium metabisulphite and reported maximum sensory scores and better physicochemical properties at the temperature of 60 °C. Recently, Nansereko, Muyonga, and Byaruhanga (Citation2022) have optimized drying conditions for jackfruit using reflectance window drying technology and reported the temperature of 93.4 °C and the pulp thickness of 2.56 nm to be optimum for the preparation of jackfruit leather. Hence, drying is one of the important methods for preservation of fruit quality of jackfruit.
Squash
Squash is a concentrated sirup, usually prepared by the combination of fruit juice, water and sugar (Akubor Citation2017). Bhatia, Siddappa, and Lal (Citation1956) reported that ascorbic acid fortified jackfruit squash retained 50-70% ascorbic acid at room temperature, 88-97% at 25 °C and 6-13% at 37 °C, respectively. However, the browning of samples took place at higher temperatures. Moreover, Shwetha and Ranganna (Citation2016) prepared squash from five different genotypes of jackfruit and reported that the physicochemical properties of squash varied among the genotypes while the sensory score was higher in ‘Muttom Varikka’ and ‘HV-1′ in comparison to other genotypes. Mondal et al. (Citation2013) reported that squash prepared from jackfruit bulbs could not be stored for a period more than 7 months due to higher moisture and ascorbic acid content. Hence, it is recommended that chemical preservatives which are safe for human health should be added to jackfruit squash to extend its shelf life.
Chips
Chips are a product with significant potential as a value addition from jackfruit. Molla et al. (Citation2008) have reported that jackfruit chips packaged in metalux foil pouches had the maximum sensory score followed by packaging in high-density polyethylene and polypropylene. Yi et al. (Citation2016) investigated the impact of freeze drying (FD), instant controlled pressure drop-assisted hot air drying (AD-DIC), and instant controlled pressure drop-assisted freeze drying (FD-DIC) on physicochemical attributes of jackfruit chips, reporting that FD-DIC dried chips retained higher antioxidant activity in comparison to other methods. Maity, Bawa, and Raju (Citation2018) reported that dipping jackfruit slices in hydrocolloid solution (carboxymethyl cellulose, gum arabic, pectin, and sodium alginate) prior to vacuum frying reduced oil absorption and maintained better quality of jackfruit chips. As fruit chips are becoming increasingly popular among consumers, additional studies should be conducted for standardization of techniques for preparation of jackfruit chips.
Nectar
Nectar is a noncarbonated beverage prepared with the addition of few preservatives and is a good source of vitamins and minerals (Bal et al. Citation2014). The Central Food Technological Research Institute (CFTRI) has standardized a technique for nectar preparation from jackfruit cultivars grown in Kerala, India (APAARI Citation2012). Xess et al. (Citation2021) have reported that jackfruit nectar prepared by the combination of 20% pulp with 18% SSC and 0.4% acidity exhibited maximum sensory score in terms of aroma, taste, color, and overall acceptability. Cruz-Casillas, García-Cayuela, and Rodriguez-Martinez (Citation2021) have optimized ultrasound processing techniques for jackfruit, reporting that jackfruit nectar thermo-ultrasonicated at 80% amplitude at the temperature of 45 ± 1 °C for 20 min retained maximum physical stability (68%), pectin methyl esterase activity (80%), ascorbic acid (442 mgL−1), total phenolic content (134 mgL−1GAE) and DPPH activity (75 µmol TE L−1).
Ice-cream
Considering the higher nutritional importance of jackfruit, attempts have been made to prepare an ice cream with the addition of jackfruit pulp or powder. Gaikwad, Kamble, and Jaybhay (Citation2020) have prepared ice cream by addition of jackfruit pulp to buffalo milk and reported the maximum sensory score in terms of color, flavor, and appearance in the ice cream prepared by addition of 15% jackfruit pulp. Lumbantobing, Tanardi, and Putra (Citation2020) reported that ice cream prepared using jackfruit seed-based milk is rich in crude fiber and low in fat and can be a good alternative to commercial dairy ice cream. Likewise, Shinde et al. (Citation2021) have prepared ice cream from jackfruit seed powder and concluded that the addition of 3% jackfruit seed powder resulted in maximum sensory score.
Chocolate
Indian Institute of Horticultural Research (IIHR) has prepared a chocolate and named it as ‘Arka Jacholate’, from jackfruit seed powder mixed with other natural ingredients such as sesame, butter and mushroom, and then covered with chocolate. This product has been developed to deliver the nutritional benefits of jackfruit seeds by incorporating the taste of chocolate. This product contains 5-6% protein, high fiber content and antioxidant activity along with lower fat content (Indian Institute of Horticultural Research Citation2022).
Cookies
IIHR has prepared cookies from jackfruit seed and fruit powder and named them as ‘Arka Jackies’. In this technology, 40% refined wheat flour is replaced by jackfruit seed powder, fruit powder and mushroom as compared to cereal bran fortified cookies in which only 5-10% of refined wheat flour is replaced. These cookies are claimed to be rich in minerals such as calcium, magnesium, and iron (Indian Institute of Horticultural Research Citation2022).
RTS beverage
‘Arka halasuras’ is a ready-to-serve beverage prepared by liquefication of jackfruit pulp with enzymes. This beverage is prepared without the addition of extra sugar, acids, or preservatives. The beverage can be stored at room temperature for six months and is an excellent source of carotenoids (2.1-2.4 mg/100 ml), Vitamin C (15-18mg/100 ml), and antioxidants (1.1-1.2 mg/100 ml). By using this technology, 2.5 to 3 liters of juice can be prepared from one kilogram of fruit (Indian Institute of Horticultural Research Citation2022).
Conclusions and future prospects
Being the largest fruit with high nutritive and medicinal value, jackfruit holds the potential to become more popular in global markets. Nevertheless, jackfruit has a short storage life due to its highly perishable nature. The fruit’s susceptibility to chilling injury and postharvest decay further deteriorates fruit quality, thus escalating postharvest losses. Despite its nutritional potential, jackfruit remains a minor fruit crop, and deserves to be given further attention related to its research and development. Based on this literature review, the following research gaps have been identified, which should be addressed in future research:
The influence of various pre and postharvest factors affecting the fruit quality of jackfruit needs to be examined in detail.
The harvesting of jackfruit mainly depends on the experience of growers and the indices used are not usually accurate, so there is an acute need to optimize the fruit maturity index of jackfruit.
Management of insect pests and disease in field conditions has been detailed in some studies; nonetheless, postharvest disease and insect pest management is still a large gap in jackfruit research
Chilling injury has been mentioned as a major issue in low-temperature storage by some researchers, but little information is available about its mechanism (biochemical, genetic, physiological, and phytochemical) and management techniques.
Fruit ripening modulation is one of the most advanced techniques used for enhancing the postharvest storage life of major climacteric fruits. Jackfruit being a neglected fruit in many areas of the world, has not been explored in this regard for checking its modulation pattern and physiology.
For postharvest quality management, comprehensive studies should be conducted that are not only based on minimal processing but should also aim to mitigate storage related issues of the fresh fruit.
In conclusion, jackfruit is an important fruit due to its high nutritional value, medicinal importance and good sensory qualities. However, there is a need to optimize its preharvest technology with respect to areas of production and climatic conditions, and postharvest interventions to reduce the incidence of CI and fruit decay.
Author contributions
J. Kaur: writing original draft, visualization, reviewing and editing, Z. Singh: conceptualization, reviewing and editing, supervision, project administration, H. M. S. Shah: reviewing and editing, visualization, M.S. Mazhar: reviewing and editing, supervision, project administration, M.U. Hasan: reviewing and editing, A. Woodward: reviewing and editing.
Acknowledgements
The first author is thankful to Edith Cowan University, and Cooperative Research Centre for Developing Northern Australia to grant ‘The Northern Territory of Australia, Department of Primary Industries and Fisheries Scholarship 2021’ during PhD degree. We are also thankful to Michael Stein, HDR Communication Adviser, Edith Cowan University, Australia for improving this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdullah, N., N. M. Nawi, P. Ding, M. S. M. Kassim, and S. S. R. M. Lazim. 2021. Prediction of soluble solids content of jackfruit from skin surface using spectroscopic method. Agricultural Mechanization in Asia, Africa and Latin America 52 (3):4013–21.
- Adikaram, N., L. Manawadu, L. Jayasinghe, and D. Yakandawala. 2020. First report of Lasiodiplodia theobromae rot in ripe jack fruit (Artocarpus heterophyllus Lam.) in Sri Lanka. Indian Phytopathology 73 (3):583–5. doi: 10.1007/s42360-020-00243-w.
- AgriFutures. 2017. Jackfruit. Accessed September 16, 2018. https://www.agrifutures.com.au/farm-diversity/jackfruit/.
- Akter, B., and M. A. Haque. 2018. Utilization of jackfruit (Artocarpus heterophyllus) seed’s flour in food processing: A review. The Agriculturists 16 (2):131–42. doi: 10.3329/agric.v16i02.40351.
- Akter, F., and M. A. Haque. 2020. Jackfruit waste: A promising source of food and feed. Annals of Bangladesh Agriculture 23 (1):91–102. doi: 10.3329/aba.v23i1.51477.
- Akubor, P. I. 2017. Quality characteristics and storage properties of squash prepared from pineapple (Ananas comosus) fruit juice. Asian Journal of Biotechnology and Bioresource Technology 1 (4):1–8. doi: 10.9734/AJB2T/2017/36474.
- Amit, K. T., and V. Ambarish. 2010. Production of jack wine-a wine from ripe jackfruit. Pharmbit 22 (2):154–6.
- Anaya-Esparza, L. M., G. A. González-Aguilar, J. A. Domínguez-Ávila, J. E. Olmos-Cornejo, A. Pérez-Larios, and E. Montalvo-González. 2018. Effects of minimal processing technologies on jackfruit (Artocarpus heterophyllus Lam.) quality parameters. Food and Bioprocess Technology 11 (9):1761–74. doi: 10.1007/s11947-018-2136-z.
- Angeles, D. O. 1983. Cashew and jackfruit research. Philippines: Department of Horticulture, University of Philippines, Los Baños (UPLB).
- Ansar, A., Sukmawaty, G. M. D. Putra, and N. H. Najat. 2020. Application of Aloe vera gel as an edible coating at jackfruit. Jurnal Agritechno 13 (2):77–83. doi: 10.20956/at.v13i2.261.
- Antunes, M., C. Gago, A. Cavaco, and G. Miguel. 2012. Edible coatings enriched with essential oils and their compounds for fresh and fresh-cut fruit. Recent patents on Food, Nutrition & Agriculture 4 (2):114–22. doi: 10.2174/2212798411204020114.
- APAARI. 2012. Jackfruit improvement in the Asia-Pacific region- A status report. Bangkok, Thailand: Asia-Pacific Association of Agricultural Research Institutions. https://www.apaari.org/wp-content/uploads/downloads/2012/10/Jackfruit-A-Success-Story_31-8-2012.pdf.
- Aprea, E., F. Biasioli, and F. Gasperi. 2015. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules (Basel, Switzerland) 20 (2):2445–74. doi: 10.3390/molecules20022445.
- Arshad, M., K. R. Vasudeva, H. C. Krishna, T. H. Shankarappa, and G. K. Halesh. 2018. Effect of storage conditions on the nutritional quality of protein fortified jackfruit jam. International Journal of Current Microbiology and Applied Sciences 7 (4):1018–24. doi: 10.20546/ijcmas.2018.704.111.
- Arvanitoyannis, I. S., A. C. Stratakos, and P. Tsarouhas. 2009. Irradiation applications in vegetables and fruits: A review. Critical Reviews in Food Science and Nutrition 49 (5):427–62. doi: 10.1080/10408390802067936.
- Azad, A. K. 2000. Genetic diversity of jackfruit in Bangladesh and development of propagation methods. PhD diss., University of Southampton. https://eprints.soton.ac.uk/463785/.
- Azad, A. K., J. G. Jones, and N. Haq. 2007. Assessing morphological and isozyme variation of jackfruit (Artocarpus heterophyllus Lam.) in Bangladesh. Agroforestry Systems 71 (2):109–25. doi: 10.1007/s10457-007-9039-8.
- Babu, P. S., and K. P. Sudheer. 2020. Quality evaluation of thermal processed tender jackfruit during storage. Journal of Tropical Agriculture 58 (1):22–32.
- Babu, P. S., S. K. Pulissery, B. Jaganath, and M. C. Obaiah. 2022. Effect of thermal processing on quality of tender jackfruit in tin-free-steel cans. Journal of Food Science and Technology 59:1–12. doi: 10.1007/s13197-021-05218-x.
- Baidya, D., I. Chakraborty, and J. Saha. 2016. Table wine from tropical fruits utilizing natural yeast isolates. Journal of Food Science and Technology 53 (3):1663–9. doi: 10.1007/s13197-015-2080-0.
- Bal, L. M., T. Ahmad, A. K. Senapati, and P. S. Pandit. 2014. Evaluation of quality attributes during storage of guava nectar cv. Lalit from different pulp and TSS ratio. Journal of Food Processing and Technology 5 (5):2–7. doi: 10.4172/2157-7110.1000329.
- Balamaze, J., J. H. Muyonga, and Y. B. Byaruhanga. 2019. Physico-chemical characteristics of selected jackfruit (Artocarpus heterophyllus Lam.) varieties. Journal of Food Research 8 (4):11–22. doi: 10.5539/jfr.v8n4p11.
- Baliga, M. S., A. R. Shivashankara, R. Haniadka, J. Dsouza, and H. P. Bhat. 2011. Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam. (jackfruit): A review. Food Research International 44 (7):1800–11. doi: 10.1016/j.foodres.2011.02.035.
- Barros-Castillo, J. C., M. Calderón-Santoyo, L. F. Cuevas-Glory, J. A. Pino, and J. A. Ragazzo-Sánchez. 2021. Volatile profiles of five jackfruit (Artocarpus heterophyllus Lam.) cultivars grown in the Mexican Pacific area. Food research International (Ottawa, ON) 139:109961. doi: 10.1016/j.foodres.2020.109961.
- Basak, A. B. 1995. Fruit rot disease of jackfruit caused by Colletotrichum gloeosporioides Penz in Chittagong. Bangladesh Journal of Botany 24 (2):197–9.
- Bashir, H. A., and A. A. Abu-Goukh. 2003. Compositional changes during guava fruit ripening. Food Chemistry 80 (4):557–63. doi: 10.1016/S0308-8146(02)00345-X.
- Benkeblia, N., D. P. F. Tennant, S. K. Jawandha, and P. P. S. Gill. 2011. Pre-harvest and harvest factors influencing post-harvest quality of tropical and subtropical fruits. In Post-harvest biology and technology of tropical and subtropical fruits, ed. M. Yahia, 112–42. Woodhead Publishing Sawston, United Kingdom. doi: 10.1533/9780857093622.112.
- Berry, S. K., and C. L. Kalra. 1988. Chemistry and technology of jack fruit (Artocarpus heterophyllus) - a review. Indian Food Packer 42 (3):62–76.
- Bhatia, S., G. S. Siddappa, and G. Lal. 1956. Product development from the fruits. Indian Journal of Agricultural Sciences 25:408–15.
- Bicas, J. L., G. Molina, A. P. Dionísio, F. F. C. Barros, R. Wagner, M. R. Maróstica, and G. M. Pastore. 2011. Volatile constituents of exotic fruits from Brazil. Food Research International 44 (7):1843–55. doi: 10.1016/j.foodres.2011.01.012.
- Bizura-Hasida, M. R., M. P. N. Aida, M. Z. Zaipun, and M. Hairiyah. 2013. Microbiological changes of minimally processed jackfruit after UV-C treatment. Acta Horticulturae 1012:1025–30. doi: 10.17660/ActaHortic.2013.1012.138.
- Brahma, R., and S. Ray. 2022. A comprehensive review on the recent advances in the valorization of jackfruit waste for the development of value-added products. Journal of Food Technology Research 9 (2):120–34. doi: 10.18488/jftr.v9i2.3124.
- Briones, R. L., and D. P. Lina. 2020. Effects of organic and inorganic fertilizer application on the postharvest quality of jackfruit (Artocarpus heterophyllus Lam.) var. ‘EVIARC Sweet. Mindanao Journal of Science and Technology 18 (2):67–89.
- Buddhika, A., B. N. Perumpuli, N. Dilrukshi, and K. Mapalana. 2021. Functional constituents of fruit juices after acetic acid fermentation. Tropical Agricultural Research and Extension 24 (4):319–29. doi: 10.4038/tare.v24i4.5538.
- Butani, D. K. 1978. Pests and diseases of jackfruit in India and their control. Fruits 33 (5):351–7.
- Cagasan, C. U., C. A. V. Lingatong, K. M. T. Pore, V. Raymond, C. D. D. Restor, and R. D. Lauzon. 2021. Production and quality evaluation of wine from jackfruit co-products. International Journal of Life Sciences and Biotechnology 4 (3):340–52. doi: 10.38001/ijlsb.827739.
- Chai, T. T., J. Xiao, S. M. Dass, J. Y. Teoh, K. Y. Ee, W. J. Ng, and F. C. Wong. 2021. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chemistry 340:127876. doi: 10.1016/j.foodchem.2020.127876.
- Chandrika, U. G., E. R. Jansz, and N. D. Warnasuriya. 2005. Analysis of carotenoids in ripe jackfruit (Artocarpus heterophyllus) kernel and study of their bioconversion in rats. Journal of the Science of Food and Agriculture 85 (2):186–90. doi: 10.1002/jsfa.1918.
- Chattopadhyay, P., P. K. Paul, S. Mishra, M. P. Devi, and S. Ghosh. 2018. Preparation of osmotically dehydrated tender jackfruit cube. International Journal of Bio-Resource and Stress Management 9 (2):183–91. doi: 10.23910/IJBSM/2018.9.2.1864b.
- Chavez-Santiago, J. O., G. C. Rodríguez-Castillejos, G. Montenegro, R. Bridi, H. Valdés-Gómez, S. Alvarado-Reyna, O. Castillo-Ruiz, and R. Santiago-Adame. 2021. Phenolic content, antioxidant, and antifungal activity of jackfruit extracts (Artocarpus heterophyllus Lam.). Food Science and Technology 42:597–604. doi: 10.1590/fst.02221.
- Chen, J., S. Huang, Z. Li, Q. Liao, K. Song, K. Hong, and J. Wang. 2022. Cloning and expression analysis of PG gene in jackfruit. Chinese Journal of Tropical Crops 43 (6):1102. doi: 10.3969/j.issn.1000-2561.2022.06.002.
- Cheok, C. Y., N. Mohd Adzahan, R. Abdul Rahman, N. H. Zainal Abedin, N. Hussain, R. Sulaiman, and G. H. Chong. 2018. Current trends of tropical fruit waste utilization. Critical Reviews in Food Science and Nutrition 58 (3):335–61. doi: 10.1080/10408398.2016.1176009.
- Chowdhury, F. A., M. A. Raman, and A. J. Mian. 1997. Distribution of free sugars and fatty acids in jackfruit (Artocarpus heterophyllus). Food Chemistry 60 (1):25–8. doi: 10.1016/S0308-8146(96)00294-4.
- Chrips, N. R., R. G. S. Balasingh, and C. Kingston. 2008. Nutrient constituents of neglected varieties of Artocarpus heterophyllus Lam. from Kanyakumari district, South India. Journal of Basic and Applied Biology 2 (1):36–47.
- Chulaki, M. M., C. D. Pawar, S. M. Khan, and A. M. Khan. 2017. Effects of ascorbic acid and calcium chloride on chemical properties of firm flesh jackfruit bulbs. Journal of Pharmacognosy and Phytochemistry 6:654–8.
- Constance, E. C., W. U. Ifunanya, M. C. E. Amarachi, E. E. C. Idera, N. Ikechukwu, P. C. Ogechi, J. O. Oluchi, S. E. Arinze, and O. C. Perpetua. 2021. Evaluation of vinegar production properties of Garcina kola and Artocarpus heterophyllus. Journal of Applied Chemical Science International 12 (2):48–60.
- Coyne, T., T. I. Ibiebele, P. D. Baade, A. Dobson, C. McClintock, S. Dunn, D. Leonard, and J. Shaw. 2005. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. The American Journal of Clinical Nutrition 82 (3):685–93. doi: 10.1093/ajcn.82.3.685.
- Cruz-Casillas, F. C., T. García-Cayuela, and V. Rodriguez-Martinez. 2021. Application of conventional and non-conventional extraction methods to obtain functional ingredients from jackfruit (Artocarpus heterophyllus Lam.) tissues and by-products. Applied Sciences 11 (16):7303. doi: 10.3390/app11167303.
- Daryanti, D. 2005. Influence of CaCl2 and mild heat treatments on quality of ready-to-eat jackfruit during refrigerated storage. Jurnal Ilmiah Agrineca 5 (1):63–70. doi: 10.36728/afp.v5i1.492.
- de Faria, A. F., V. V. de Rosso, and A. Z. Mercadante. 2009. Carotenoid composition of jackfruit (Artocarpus heterophyllus) determined by HPLC-PDA-MS/MS. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 64 (2):108–15. doi: 10.1007/s11130-009-0111-6.
- Debbarma, N., S. Manivannan, and J. K. Kushwaha. 2018. Discriminating geographical origins of Indian jackfruit (Artocarpus heterophyllus Lam.) through multivariate analysis of physico-chemical characters. Journal of Pharmacognosy and Phytochemistry 7 (3):1258–65.
- Devasagayam, T. P. A., J. C. Tilak, and R. Singhal. 2007. Functional foods in India; history and scope in angiogenesis. In Functional and medicinal foods, ed. J. N. Losso, F. Shahidi, and D. Bagchi, 69–96. New York: Marcel Dekker Inc.
- Dhanesh, T. B., S. M. Sasmila Bai, B. G. Akhila, K. P. Shareena, and G. K. Rajesh. 2018. Effect of storage on the sensory and microbial quality of blanched and quick-frozen jackfruit samples. International Journal of Current Microbiology and Applied Sciences 7 (3):913–7. doi: 10.20546/ijcmas.2018.703.107.
- Dong, Y., L. Liu, Z. Zhao, H. Zhi, and J. Guan. 2015. Effects of 1-MCP on reactive oxygen species, polyphenol oxidase activity, and cellular ultra-structure of core tissue in ‘Yali’ pear (Pyrus bretschneideri Rehd.) during storage. Horticulture, Environment, and Biotechnology 56 (2):207–15. doi: 10.1007/s13580-015-0094-1.
- Dutta, H., S. K. Paul, D. Kalita, and C. L. Mahanta. 2011. Effect of acid concentration and treatment time on acid–alcohol modified jackfruit seed starch properties. Food Chemistry 128 (2):284–91. doi: 10.1016/j.foodchem.2011.03.016.
- Effiong, O. O., and B. J. Harry. 2019. Determination of the mineral and vitamin compositions of jackfruit juice extract and its effect on broiler chickens’ performance. Global Journal of Pure and Applied Sciences 25 (1):23–9. doi: 10.4314/gjpas.v25i1.4.
- Elevitch, C. R., and H. I. Manner. 2006. Artocarpus heterophyllus (jackfruit). Species profiles for Pacific Island Agroforestry 10:1–25.
- Fathin, A. N., D. Astuti, W. W. Winarni, and Y. W. N. Ratnaningrum. 2021. Flowering and fruiting phenology of jackfruit (Artocarpus heterophyllus Lam.) from Sumatra landraces in ex situ conservation area in Karangmojo, Yogyakarta. IOP Conference Series: Earth and Environmental Science 914 (012052):1–13. doi: 10.1088/1755-1315/914/1/012052.
- Fraga, S. R. G. 2005. Investigation of volatiles and precursors of glycosylated volatiles in Jackfruit (Artocarpus heterophyllus Lam.) and Muruci (Byrsonima crassifolia Lam. Rich). PhD diss., Federal University of Rio de Janeiro. http://livros01.livrosgratis.com.br/cp098467.pdf.
- Gaikwad, S. B., D. K. Kamble, and V. B. Jaybhay. 2020. Development of ice-cream by using jackfruit pulp. The Pharma Innovation 9 (9):533–9.
- Ganesan, S., M. Panda, and K. Kishore. 2020. A Report on occurrence of Lasiodiplodia fruit rot infecting jackfruit in Odisha state. Pest Management in Horticultural Ecosystems 26 (1):160–2. doi: 10.5958/0974-4541.2020.00026.0.
- Garcia, L. G. C., E. P. D. Silva, C. D. M. Silva, E. V. D. B. Vilas-Boas, E. R. Asquieri, C. Damiani, and F. A. D. Silva. 2019. Effect of the addition of calcium chloride and different storage temperatures on the post-harvest of jabuticaba variety Pingo de Mel. Food Science and Technology 39 (suppl 1):261–9. doi: 10.1590/fst.02318.
- Ghosh, R., S. Barman, A. Mukhopadhyay, and N. C. Mandal. 2015. Biological control of fruit-rot of jackfruit by rhizobacteria and food grade lactic acid bacteria. Biological Control 83:29–36. doi: 10.1016/j.biocontrol.2014.12.020.
- Gogoi, A. K., P. K. Borthakur, S. Saikia, and S. Baruah. 2021a. Minimal processing for short term preservation of jackfruit (Artocarpus heterophyllus Lam.) bulbs. The Pharma Innovation Journal 10:559–62.
- Gogoi, A. K., P. K. Borthakur, S. Saikia, S. Baruah, and B. Gogoi. 2021b. Effect of antioxidants on quality of minimally processed jackfruit (Artocarpus heterophyllus Lam.) bulbs. Indian Journal of Pure & Applied Biosciences 9 (2):134–7. doi: 10.18782/2582-2845.8630.
- Gomathi, V., V. Premalakshmi, J. Rajangam, and K. Venkatesan. 2021. Effect of postharvest dipping on quality and shelf life of minimally processed jackfruit (Artocarpus heterophyllus Lam.) flakes during storage. The Pharma Innovation Journal 10 (11):809–13.
- Goswami, C., M. A. Hossain, H. A. Kader, and R. Islam. 2011. Assessment of physicochemical properties of jackfruits (Artocarpus heterophyllus Lam.) pulps. Journal of Horticulture, Forestry and Biotechnology 15 (3):26–31.
- Grimm, J. E., and M. Steinhaus. 2020. Characterization of the major odorants in chempedak-differences to jackfruit. Journal of Agricultural and Food Chemistry 68 (1):258–66. doi: 10.1021/acs.jafc.9b06564.
- Gupta, A., and D. K. Singh. 2019. Phytofabricated nanoparticles using Artocarpus heterophyllus: A multifunctional approach. In Recent advances in chemical sciences and biotechnology, ed. A. K. Jha, and U. Kumar, 63–74. New Delhi Publishers, New Delhi, India.
- Gupta, U. K. S., and C. V. N. Rao. 1963. The pectic acid from the pulp of jackfruit (Artocarpus Integrifolia). I. Methylation studies. Bulletin of the Chemical Society of Japan 36 (12):1683–8. doi: 10.1246/bcsj.36.1683.
- Halliwell, B., A. Zentella, E. O. Gomez, and D. Kershenobich. 2009. Antioxidants and human disease: A general introduction. Nutrition Reviews 55 (1):S44–S49. doi: 10.1111/j.1753–4887.1997.tb06100.x.
- Haq, N. 2006. Fruits for the future: Jackfruit (Artocarpus heterophyllus). In Crops for the future, ed. J. T. Williams, R. W. Smith, and Z. Dunsiger, 102–40. Southampton, England: Southampton Centre for Underutilized Crops.
- Hasan, M. U., R. Riaz, A. U. Malik, A. S. Khan, R. Anwar, R. U. N. Rehman, and S. Ali. 2021. Potential of Aloe vera gel coating for storage life extension and quality conservation of fruits and vegetables: An overview. Journal of Food Biochemistry 45 (4):e13640. doi: 10.1111/jfbc.13640.
- He, Y., H. Huang, S. Yan, S. Zhong, H. Liu, and X. Qin. 2021. Optimization of fermentation process of jackfruit (Artocarpus heterophyllus Lam.) vinegar and analysis of aroma components. Chinese Journal of Tropical Crops 42 (5):1462–71. doi: 10.3969/j.issn.1000-2561.2021.05.036.
- Hossain, M. A., M. Khatun, M. A. Matin, and M. F. Dewan. 2017. Postharvest loss assessment of major fruits grown in hill regions of Bangladesh. Bangladesh Journal of Agricultural Research 42 (1):171–84. doi: 10.3329/bjar.v42i1.31989.
- Ihediohanma, N. C., D. C. Okafor, and A. S. Adeboye. 2014. Sensory evaluation of jam produced from jackfruit (Artocarpus heterophyllus). Journal of Agriculture and Veterinary Science 7 (3):41–3.
- Indian Institute of Horticultural Research. 2022. Jackfruit products. Accessed September 16, 2022. https://www.iihr.res.in/jackfruit-products.
- Irin, I. J., M. A. Haque, M. G. Rabbani, and P. K. Biswas. 2015. Physical characteristics of jackfruit at different harvesting times. Bangladesh Agronomy Journal 17 (2):89–94. doi: 10.3329/baj.v17i2.24657.
- Islam, M. S., R. Begum, M. Khatun, and K. C. Dey. 2015. A study on nutritional and functional properties analysis of jackfruit seed flour and value addition to biscuits. International Journal of Engineering Research and Technology 4 (12):139–47.
- Jafarzadeh, S., A. Salehabadi, A. M. Nafchi, N. Oladzadabbasabadi, and S. M. Jafari. 2021. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends in Food Science & Technology 116:218–31. doi: 10.1016/j.tifs.2021.07.021.
- Jagadeesh, S. L., B. S. Reddy, G. S. K. Swamy, K. Gorbal, L. Hegde, and G. S. V. Raghavan. 2007. Chemical composition of jackfruit (Artocarpus heterophyllus Lam.) selections of Western Ghats of India. Food Chemistry 102 (1):361–5. doi: 10.1016/j.foodchem.2006.05.027.
- Jagtap, U. B., and V. A. Bapat. 2013. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Industrial Crops and Products 46:132–7. doi: 10.1016/j.indcrop.2013.01.019.
- Jagtap, U. B., S. R. Waghmare, V. H. Lokhande, P. Suprasanna, and V. A. Bapat. 2011. Preparation and evaluation of antioxidant capacity of jackfruit (Artocarpus heterophyllus Lam.) wine and its protective role against radiation induced DNA damage. Industrial Crops and Products 34 (3):1595–601. doi: 10.1016/j.indcrop.2011.05.025.
- Jain, A. K., H. Sahu, K. Mishra, and S. Thareja. 2021. Mannose conjugated starch nanoparticles for preferential targeting of liver cancer. Current Drug Delivery 18 (3):369–80. doi: 10.2174/1567201817666200903171124.
- Jain, A. K., R. Upadhyay, K. Mishra, and S. K. Jain. 2022. Gastroretentive metformin loaded nanoparticles for the effective management of type-2 diabetes mellitus. Current Drug Delivery 19 (1):93–103. doi: 10.2174/1567201818666210614095159.
- Jayakody, M. M., M. P. G. Vanniarachchy, and I. Wijesekara. 2021. Application of alginate and agar-based edible coatings to pre-cut jackfruit and evaluation of the flavour compounds of coated pre-cut jackfruit during refrigerated storage. In 2021 from innovation to impact, 1–5. Colombo: IEEE. doi: 10.1109/FITI54902.2021.9833031.
- Jiang, Z., R. Shi, H. Chen, and Y. Wang. 2019. Ultrasonic microwave-assisted extraction coupled with macroporous resin chromatography for the purification of antioxidant phenolics from waste jackfruit (Artocarpus heterophyllus Lam.) peels. Journal of Food Science and Technology 56 (8):3877–86. doi: 10.1007/s13197-019-03858-8.
- Johari, A. M., N. A. A. Rahman, A. S. Baharuddin, R. K. Basha, M. A. P. Mohammed, D. M. Parid, and M. Wakisaka. 2020. Effects of different low temperature storage conditions on the physico-chemical properties of Mastura (J37) jackfruit bulbs. Journal of Agricultural and Food Engineering 1 (0009). doi: 10.37865/jafe.2020.0009.
- Johari, A. M., N. A. A. Rahman, and A. S. Baharuddin. 2020. Effects of low temperature storage of Mastura (J37) jackfruit bulbs on the physical quality of jackfruit frozen confection. Journal of Agricultural and Food Engineering 2:1–4. doi: 10.37865/jafe.2020.0016.
- Jun-Ning, W., Q. Fang, F. Feng, J. H. Xie, C. B. Wei, Y. H. Li, and C. H. Ye. 2014. Changes in respiration ethylene and biochemical values of soft jackfruits during the ripening period. Acta Horticulturae Sinica 41:329–34.
- Kalse, S. B., and S. B. Swami. 2022. Recent application of jackfruit waste in food and material engineering: A review. Food Bioscience 48 (274):101740. doi: 10.1016/j.fbio.2022.101740.
- Kalt, W., J. E. McDonald, and H. Donner. 2000. Anthocyanins, phenolics, and antioxidant capacity of processed lowbush blueberry products. Journal of Food Science 65 (3):390–3. doi: 10.1111/j.1365-2621.2000.tb16013.x.
- Kanishka, M. E. V. L., P. L. N. Lakshman, and D. C. T. K. Nakandala. 2018. Development and quality evaluation of jackfruit (Artocarpus heterophyllus Lam.) jam for commercialization. In Proceedings of the 3rd research symposium, 155–8. University of Vocational Technology, Dehiwala-Mount Lavinia, Sri Lanka.
- Kaushal, P., and H. K. Sharma. 2016. Osmo-convective dehydration kinetics of jackfruit (Artocarpus heterophyllus Lam.). Journal of the Saudi Society of Agricultural Sciences 15 (2):118–26. doi: 10.1016/j.jssas.2014.08.001.
- Kays, S. J. 1991. Development of plants and plant parts. In Postharvest physiology of perishable plant products, 257–333. Springer, New York, USA.
- Khan, A. U. M., A. R. Choudhury, M. Abdul, C. K. D. Maleque, M. S. A. Talucder, A. R. M. Maukeeb, I. J. Ema, and M. Adnan. 2021. Management of insect pests and diseases of jackfruit (Artocarpus heterophyllus Lam.) in agroforestry system: A review. Acta Entomology and Zoology 2 (1):37–46. doi: 10.33545/27080013.2021.v2.i1a.29.
- Khan, M. H. H., M. M. Molla, A. A. Sabuz, M. G. F. Chowdhury, M. Alam, and M. Biswas. 2021. Effect of processing and drying on quality evaluation of ready-to-cook jackfruit. Journal of Agricultural Science and Food Technology 7 (2):19–29. doi: 10.36630/jasft_21002.
- Kishore, K. 2018. Phenological growth stages of jackfruit (Artocarpus heterophyllus) according to the extended BBCH scale. Annals of Applied Biology 172 (3):366–74. doi: 10.1111/aab.12427.
- Kopsell, D. A., and D. E. Kopsell. 2006. Accumulation and bioavailability of dietary carotenoids in vegetable crops. Trends in Plant Science 11 (10):499–507. doi: 10.1016/j.tplants.2006.08.006.
- Koyuncu, M. A., D. Erbas, C. E. Onursal, T. Secmen, A. Guneyli, and S. S. Uzumcu. 2019. Postharvest treatments of salicylic acid, oxalic acid and putrescine influences bioactive compounds and quality of pomegranate during controlled atmosphere storage. Journal of Food Science and Technology 56 (1):350–9. doi: 10.1007/s13197-018-3495-1.
- Krupa, S., C. S. Karigar, and K. S. Murthy. 2020. Pharmacology and phytochemistry of Artocarpus family: A review. Indo Global Journal of Pharmaceutical Sciences 10 (3):48–55. doi: 10.35652/IGJPS.2020.10306.
- Kumoro, A. C., D. R. Sari, A. P. P. Pinandita, D. S. Retnowati, and C. S. Budiyati. 2012. Preparation of wine from jackfruit (Artocarpus heterophyllus Lam.) juice using baker yeast: Effect of yeast and initial sugar concentrations. World Applied Sciences Journal 16 (9):1262–8.
- Kurmi, G., B. Kalita, and S. R. C. Das. 2020. Recent advances and application of jackfruit seed starch and its derivatives in drug delivery: A review. World Journal of Pharmaceutical Research 9 (10):107–15.
- Kushala, G., K. N. Sreenivas, and R. Siddappa. 2012. Product development acceptability and cost effectiveness of jackfruit jam blended with avocado and kokum. Food Science Research Journal 3 (1):78–80.
- Kushwaha, R., N. T. Fatima, M. Singh, V. Singh, S. Kaur, V. Puranik, R. Kumar, and D. Kaur. 2021. Effect of cultivar and maturity on functional properties, low molecular weight carbohydrate, and antioxidant activity of jackfruit seed flour. Journal of Food Processing and Preservation 45 (2):e15146. doi: 10.1111/jfpp.15146.
- Latifah, M. N., A. R. Abd-Shukor, I. A. Aziz, M. Habsah, Y. Talib, K. M. Rahman, and H. Jabir. 2000. Quality of minimally processed jackfruit stored at different temperatures. Journal of the Tropical Agriculture and Food Science 28:87–94.
- Latifah, M. N., I. A. Aziz, O. Fauziah, and Y. Talib. 2017. Effect of inclusion of ethylene absorbent to the quality of the fresh-cut jackfruit stored at 10 °C. Acta Horticulturae 1209:115–21. doi: 10.17660/actahortic.2018.1209.17.
- Li, Y. Z., X. Q. Duan, S. H. Liu, Y. Li, X. H. Zhang, and C. H. Ye. 2017. Changes in soluble sugar accumulation and activities of sucrose metabolizing enzymes during fruit ripening of jackfruit. Journal of Agricultural Science 9 (8):155. doi: 10.5539/jas.v9n8p155.
- Luciano, C. G., C. M. Landi Franco, G. Ayala Valencia, P. J. do Amaral Sobral, and I. C. Freitas Moraes. 2017. Evaluation of extraction method on the structure and physicochemical properties of starch from seeds of two jackfruit varieties. Starch - Stärke 69 (11–12):1700078. doi: 10.1002/star.201700078.
- Lumbantobing, E., S. Tanardi, and A. B. N. Putra. 2020. Development of vegan ice cream from jackfruit (Artocarpus heterophyllus) seed-based milk. In Proceedings of the 16th ASEAN Food Conference (16th AFC 2019) - Outlook and opportunities of food technology and culinary for tourism industry, 66–71. Bali: SciTePress. doi: 10.5220/0009983700660071.
- Mahajan, P. V., O. J. Caleb, Z. Singh, C. B. Watkins, and M. Geyer. 2014. Postharvest treatments of fresh produce. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences 372 (2017):20130309. doi: 10.1098/rsta.2013.0309.
- Maia, J. G. S., E. H. A. Andrade, and B. Z. M. das Graças. 2004. Aroma volatiles from two fruit varieties of jackfruit (Artocarpus heterophyllus Lam). Food Chemistry 85 (2):195–7. doi: 10.1016/S0308-8146(03)00292-9.
- Maity, T., A. S. Bawa, and P. S. Raju. 2018. Effect of preconditioning on physicochemical, microstructural, and sensory quality of vacuum-fried jackfruit chips. Drying Technology 36 (1):63–71. doi: 10.1080/07373937.2017.1300590.
- Malik, A. U., M. U. Hasan, W. U. Hassan, A. S. Khan, M. S. Shah, I. A. Rajwana, M. Latif, and R. Anwar. 2021. Postharvest quarantine vapour heat treatment attenuates disease incidence, maintains eating quality, and improves bioactive compounds of ‘Gola’ and ‘Surahi’ guava fruits. Journal of Food Measurement and Characterization 15 (2):1666–79. doi: 10.1007/s11694-020-00763-z.
- Mata-Montes, D., M. Oca, J. A. Osuna-Garcia, and A. Hemandez-Estrada. 2007. Effect of 1-methylcyclopropene (1-MCP) on the physiology and quality of jackfruit (Artocarpus heterophyllus Lam). Revista Chapingo Serie Horticultura 13:165–70. doi: 10.5154/r.rchsh.2007.02.012.
- Medagoda, I. 2011. Jackfruit in Sri Lanka. In The jackfruit, ed. S. G. Valavi, K. V. Peter, and G. Thottappilly, 369–92. Studium Press, New Delhi, India.
- Mei, T. C., and Z. Q. Ping. 2008. Study on effects of cassava starch-chitosan edible blend films on fresh-cut jackfruit. Journal of Chongqing Industry & Trade Polytechnic 1:12–6.
- Mei, C., Z. Wang, H. Chen, W. Chen, Y. Yun, W. Chen, and Q. Zhong. 2020. Effect of moisture migration on quality of fresh jackfruit bulbs during cold storage. Journal of Tropical Biology 4:23–38.
- Mijin, S., and P. Ding. 2020. Growth development and structural changes of Malaysian jackfruit cv. ‘Tekam Yellow’ Syncarp. Scientia Horticulturae 272 (109594):1–10. doi: 10.1016/j.scienta.2020.109594.
- Mijin, S., P. Ding, N. Saari, and S. I. Ramlee. 2021. Effects of pollination techniques and harvesting stage on the physico-chemical characteristics of jackfruit. Scientia Horticulturae 285:110199. doi: 10.1016/j.scienta.2021.110199.
- Miller, J. G., and S. C. Fry. 2001. Characteristics of xyloglucan after attack by hydroxyl radicals. Carbohydrate Research 332 (4):389–403. doi: 10.1016/s0008-6215(01)00110-0.
- Minh, N. P., T. T. Vo, H. T. B. Dung, T. T. B. Ngoc, L. C. Duong, and T. T. T. Nghi. 2019. Preservation of jackfruit bulb by transglutaminase crosslinked whey protein/pectin as edible film coating. Journal of Pharmaceutical Sciences and Research 11 (4):1401–5.
- Molla, M. M., T. A. A. Nasrin, M. N. Islam, and M. A. J. Bhuyan. 2008. Preparation and packaging of jackfruit chips. International Journal of Sustainable Crop Production 3 (6):41–7.
- Mondal, C., R. N. Remme, A. A. Mamun, S. Sultana, M. H. Ali, and M. A. Mannan. 2013. Product development from jackfruit (Artocarpus heterophyllus) and analysis of nutritional quality of the processed products. Journal of Agriculture and Veterinary Science 4 (1):76–84.
- Muhammad, H., and P. Ding. 2007. Cellular changes during fruit ripening of ‘Harumanis’ mango. Malaysian Journal of Microscopy 3:19–24.
- Mukprasirt, A., and K. Sajjaanantakul. 2004. Physico‐chemical properties of flour and starch from jackfruit seeds (Artocarpus heterophyllus Lam.) compared with modified starches. International Journal of Food Science and Technology 39 (3):271–6. doi: 10.1111/j.1365-2621.2004.00781.x.
- Mushumbusi, D. G. 2015. Production and characterization of jackfruit jam. Master of Science thesis., Sokoine University of Agriculture. http://www.suaire.sua.ac.tz/handle/123456789/1203.
- Nansereko, S., J. Muyonga, and Y. B. Byaruhanga. 2022. Optimization of drying conditions for jackfruit pulp using refractance window drying technology. Food Science & Nutrition 10 (5):1333–43. doi: 10.1002/fsn3.2694.
- Navindra, B., R. Arvind, and B. B. Hassina. 2009. Effects of antibrowning agents on the shelf life of fresh-cut green jackfruit (Artocarpus heterophyllus Lam). Journal of Applied Horticulture 11 (1):35–40. doi: 10.37855/jah.2009.v11i01.06.
- Nayak, A. K., S. Alkahtani, and M. S. Hasnain. 2022. Jackfruit seed starch-based composite beads for controlled drug release. In Polymeric and natural composites, ed. M. S. Hasnain, A. K. Nayak, and S. Alkahtani, 213–40. Springer, Gewerbestrasse, Switzerland.
- Nelson, S. 2005. Rhizopus rot of jackfruit. Cooperative Extension Service, University of Hawaii, Manoa, USA.
- Nguyet, T. N. M., T. T. T. Tra, and T. H. T. Yen. 2008. Prolong post-cutting life of fresh-cut jackfruit. Science and Technology Development Journal 11 (8):60–8. doi: 10.32508/stdj.v11i8.2675.
- Ni, H. F., R. S. Chen, S. F. Chang, and H. R. Yang. 2008. First report of Lasiodiplodia fruit rot of jackfruit in Taiwan. Plant Disease 92 (7):1137– doi: 10.1094/PDIS-92-7-1137A.
- Nirmal, S., S. P. Bhutia, and D. Aradhya. 2013. Process optimization for fermentation of wine from jackfruit (Artocarpus heterophyllus Lam). Journal of Food Processing and Technology 4 (2):204. doi: 10.4172/2157-7110.1000204.
- Noor, F., M. J. Rahman, M. S. Mahomud, M. S. Akter, M. A. I. Talukder, and M. Ahmed. 2014. Physicochemical properties of flour and extraction of starch from jackfruit seed. International Journal of Nutrition and Food Sciences 3 (4):347–54. doi: 10.11648/j.ijnfs.20140304.27.
- Noorfarahzilah, M., A. H. Mansoor, and M. Hasmadi. 2017. Proximate composition, mineral content and functional properties of tarap (Artocarpus odoratissimus) seed flour. Food Research 1 (3):89–96.
- Norris, A., L. V. Hag, C. Su, E. Attenborough, E. Buckingham, O. Perry, and D. Marcsik. 2021. Processing jackfruit into ready-to-eat products and ingredients. 21-153, AgriFutures Australia, NSW. Accessed September 16, 2018. https://www.agrifutures.com.au/related-projects/processing-jackfruit-into-ready-to-eat-products-and-ingredients.
- Ocloo, F. C. K., D. Bansa, R. Boatin, T. Adom, and W. S. Agbemavor. 2010. Physico-chemical, functional and pasting characteristics of flour produced from jackfruits (Artocarpus heterophyllus) seeds. Agriculture and Biology Journal of North America 1 (5):903–8. doi: 10.5251/abjna.2010.1.5.903.908.
- Ojwang, R. A., E. K. Muge, E. N. Nyaboga, B. N. Mbatia, and D. O. Ogoyi. 2022. Genetic diversity and relationships among populations of jackfruit, an underutilized nutrient-rich climate-smart fruit tree crop in Kenya and Uganda. Journal of Crop Improvement 36 (5):619–37. doi: 10.1080/15427528.2021.1997849.
- Olias, J. M., C. Sanz, J. J. Rios, and A. G. Pérez. 1995. Substrate specificity of alcohol acyltransferase from strawberry and banana fruits. In ACS Symposium Series, 134–41. ACS Publications. doi: 10.1021/bk-1995-0596.ch012.
- Ong, B. T., S. A. H. Nazimah, A. Osman, S. Y. Quek, Y. Y. Voon, D. M. Hashim, P. M. Chew, and Y. W. Kong. 2006. Chemical and flavour changes in jackfruit (Artocarpus heterophyllus Lam.) cultivar J3 during ripening. Postharvest Biology and Technology 40 (3):279–86. doi: 10.1016/j.postharvbio.2006.01.015.
- Owens, G. 2021. Australian emerging tropical fruits strategic RD&E plan. AgriFutures Australia, Wagga Wagga, Australia. Accessed September 16, 2018. https://www.planthealthaustralia.com.au/about-us/our-people/our-staff/trevor-dunmall/.
- Palamthodi, S., S. Shimpi, and K. Tungare. 2021. A study on nutritional composition and functional properties of wheat, ragi and jackfruit seed composite flour. Food Science and Applied Biotechnology 4 (1):63–75. doi: 10.30721/fsab2021.v4.i1.107.
- Palipane, K. B., and R. Rolle. 2008. Good practice for assuring the post-harvest quality of exotic tree fruit crops produced in Jamaica. Rome: Food and Agriculture Organization of the United Stated. Accessed September 16, 2018. https://www.fao.org/3/ak832e/ak832e.pdf.
- Panda, S. K., S. K. Behera, E. Kayitesi, A. F. Mulaba-Bafubiandi, U. C. Sahu, and R. C. Ray. 2016. Bioprocessing of jackfruit (Artocarpus heterophyllus Lam.) pulp into wine: Technology, proximate composition, and sensory evaluation. African Journal of Science, Technology, Innovation and Development 8 (1):27–32. doi: 10.1080/20421338.2015.1128042.
- Partha, I. B. B., M. A. J. Wasono, and M. Ulfah. 2009. Effect of CaCl2 and edible film on chilling injury inhibition of fresh-cut jackfruits. Journal of Food Technology and Industry 20:63–7.
- Pavanasasivam, G., M. Uvais, and S. Sultanbawa. 1973. Cycloartenyl acetate, cycloartenol and cycloartenone in the bark of Artocarpus species. Phytochemistry 12 (11):2725–6. doi: 10.1016/0031-9422%2873%2985088-5.
- Peng, S. D., L. J. Lin, L. J. Ouyang, B. Q. Zhu, Y. Yuan, W. Jing, and J. H. Li. 2013. Comparative analysis of volatile compounds between jackfruit (Artocarpus heterophyllus Lam.) peel and its pulp. Advanced Materials Research 781–784:1413–8. doi: 10.4028/www.scientific.net/AMR.781-784.1413.
- Photphisutthiphong, Y., and S. Vatanyoopaisarn. 2019. Production of vinegar from jackfruit rags and jackfruit seeds. The Journal of Applied Science 18 (1):75–93. doi: 10.14416/j.appsci.2019.03.002.
- Phrukwiwattanakul, P., S. Wichienchotand, and P. Sirivongpaisal. 2014. Comparative studies on physico-chemical properties of starches from jackfruit seed and mung bean. International Journal of Food Properties 17 (9):1965–76. doi: 10.1080/10942912.2013.775151.
- Pinto, L., A. Palma, M. Cefola, B. Pace, S. D’Aquino, C. Carboni, and F. Baruzzi. 2020. Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological, and sensory quality of small berry fruit. Food Packaging and Shelf Life 26:100573. doi: 10.1016/j.fpsl.2020.100573.
- Pizato, S., R. C. Chevalier, M. F. dos Santos, T. S. da Costa, R. A. Pinedo, and W. R. C. Vega. 2019. Evaluation of the shelf-life extension of fresh-cut pineapple (Smooth cayenne) by application of different edible coatings. British Food Journal 121 (7):1592–604. doi: 10.1108/BFJ-11-2018-0780.
- Pothimon, R., U. Podjanee, W. Krusong, A. K. Thompson, and S. Massa. 2021. Inhibition of Pantoea agglomerans contamination of fresh-cut jackfruit by exposure to weak organic acid vapours. LWT 139:110586. doi: 10.1016/j.lwt.2020.110586.
- Prasanna, V., T. N. Prabha, and R. N. Tharanathan. 2007. Fruit ripening phenomena-an overview. Critical Reviews in Food Science and Nutrition 47 (1):1–19. doi: 10.1080/10408390600976841.
- Prathibha, S. C., K. R. Vasudeva, G. J. Suresha, and G. K. Sadananda. 2019b. Influence of pre-treatment on quality and shelf life of fresh cut jack fruit (Artocarpus heterophyllus Lam.) bulbs. Journal of Pharmacognosy and Phytochemistry 8 (1):2524–7.
- Prathibha, S. C., K. R. Vasudeva, G. K. Sadananda, and G. J. Suresha. 2019a. Effect of pre-treatment and packaging on quality of fresh cut jackfruit (Artocarpus heterophyllus Lam.) bulbs. International Journal of Chemical Science 7:1697–700.
- Rahman, A. M., E. Huq, A. J. Mian, and A. Chesson. 1995. Microscopic and chemical changes occurring during the ripening of two forms of jackfruit (Artocarpus heterophyllus Lam). Food Chemistry 52 (4):405–10. doi: 10.1016/0308-8146(95)93290-8.
- Rahman, M. A., N. Nahar, A. J. Mian, and M. Mosihuzzaman. 1999. Variation of carbohydrate composition of two forms of fruit from jack tree (Artocarpus heterophyllus Lam.) with maturity and climatic conditions. Food Chemistry 65 (1):91–7. doi: 10.1016/s0308–8146(98)00175–7.
- Rahman, M. M., M. Miaruddin, M. F. Chowdhury, M. H. H. Khan, and M. Muzahid-E-Rahman. 2012. Preservation of jackfruit (Artocarpus heterophyllus) by osmotic dehydration. Bangladesh Journal of Agricultural Research 37 (1):67–75. doi: 10.3329/bjar.v37i1.11178.
- Ramli, R. A. B. 2009. Physicochemical characteristics of calcium-treated jackfruit (Artocarpus heterophyllus) flesh during chilled storage. PhD diss., University Sains Malaysia. Accessed September 16, 2018. http://eprints.usm.my/15841/1/PHYSICOCHEMICAL_CHARACTERISTICS_OF_CALCIUM-TREATED.pdf.
- Ramli, R. A., A. Azmi, N. M. Shahril, and A. F. Badiuzaman. 2016. The physicochemical changes in ripe jackfruit (Artocarpus heterophyllus) bulbs during cold storage. In Heritage, culture and society, 689–92. CRC Press, Boca Raton, USA.
- Ramli, R. A., and R. Ahmad. 2013. The effect of different calcium infusion methods on the texture and consumers’ acceptance of ripe jackfruit (Artocarpus heterophyllus) pulps. Foods 1:1186–33. doi: 10.3390/bpqn2013-01186.
- Rana, S. S., R. C. Pradhan, and S. Mishra. 2018a. Variation in properties of tender jackfruit during different stages of maturity. Journal of Food Science and Technology 55 (6):2122–9. doi: 10.1007/s13197-018-3127-9.
- Rana, S. S., R. C. Pradhan, and S. Mishra. 2018b. Optimization of chemical treatment on fresh cut tender jackfruit slices for prevention of browning by using response surface methodology. International Food Research Journal 25:196–203. doi: 10.1016/j.foodchem.2018.11.032.
- Rana, S. S., R. C. Pradhan, and S. Mishra. 2019. Image analysis to quantify the browning in fresh cut tender jackfruit slices. Food Chemistry 278:185–9. doi: 10.1016/j.foodchem.2018.11.032.
- Ranasinghe, R. A. S. N., and R. A. U. J. Marapana. 2019. Effect of maturity stage on physicochemical properties of jackfruit (Artocarpus heterophyllus Lam.) flesh. Proceeding of the 2nd International Research Symposium, Uva Wellassa University, Badulla, Sri Lanka, 1st–2nd February 2018. doi: 10.1155/2019/4327183.
- Ranasinghe, R. A. S. N., S. D. T. Maduwanthi, and R. A. U. J. Marapana. 2019. Nutritional and health benefits of jackfruit (Artocarpus heterophyllus Lam.): A review. International Journal of Food Science 2019 (4327183):1–12. doi: 10.1155/2019/4327183.
- Rashmi, P., G. D. Joshi, and P. M. Haldankar. 2011. Estimation of pectin content in jackfruit (Artocarpus heterophyllus). Asian Journal of Horticulture 6 (2):536–7.
- Rasmussen, P. 1983. Identification of volatile components of jackfruit by gas chromatography/mass spectrometry with two different columns. Analytical Chemistry 55 (8):1331–5. doi: 10.1021/ac00259a033.
- Redondo, D., D. Gimeno, H. Calvo, M. E. Venturini, R. Oria, and E. Arias. 2021. Antioxidant activity and phenol content in different tissues of stone fruits at thinning and at commercial maturity stages. Waste and Biomass Valorization 12 (4):1861–75. doi: 10.1007/s12649-020-01133-y.
- Remya, P. R., C. L. Sharon, E. R. Aneena, S. T. Panjikkaran, and E. Shahanas. 2019. Standardization and quality evaluation of jackfruit based low fat yogurt. Asian Journal of Dairy and Food Research 38 (of):93–7. doi: 10.18805/ajdfr.DR-1457.
- Rengsutthi, K., and S. Charoenrein. 2011. Physico-chemical properties of jackfruit seed starch (Artocarpus heterophyllus) and its application as a thickener and stabilizer in chilli sauce. LWT - Food Science and Technology 44 (5):1309–13. doi: 10.1016/j.lwt.2010.12.019.
- Rosnah, S., C. S. Ling, C. N. Ling, M. Noraziah, and H. Osman. 2009. Chemical compositions of the jackfruit juice (Artocarpus heterophyllus) cultivar J33 during storage. Journal of Applied Sciences 9 (17):3202–4. doi: 10.3923/jas.2009.3202.3204.
- Roy, A. K. 1983. Perpetuation of Rhizopus artocarpi -the incitant of Rhizopus fruit rot of jackfruit (Artocarpus heterophyllus). Indian Phytopathology 36 (2):344–5.
- Rufino, M. S., R. E. Alves, F. A. Fernandes, and E. S. Brito. 2011. Free radical scavenging behaviour of ten exotic tropical fruits extracts. Food Research International 44 (7):2072–5. doi: 10.1016/j.foodres.2010.07.002.
- Saha, M. G., M. N. Islam, and M. M. Molla. 2016. Determination of harvest maturity of jackfruit. Bangladesh Society for Horticultural Science 2:23–36.
- Sally, K. M., B. Ranganna, and K. B. Munishamanna. 2011. Study on the post-harvest shelf life of minimally processed jackfruit (Artocarpus heterophyllus Lam.) bulbs. Mysore Journal of Agricultural Sciences 45:528–36.
- Sally, K. M., B. Ranganna, and K. B. Munishamanna. 2014. Influence of vacuum packaging on the shelf-life of minimally processed jackfruit (Artocarpus heterophyllus Lam.) bulbs. Mysore Journal of Agricultural Sciences 48 (1):1–8.
- Sampath, P. M., G. S. K. Swamy, M. K. Honnabyraiah, S. Shyamalamma, J. Jayappa, G. J. Suresha, and S. Tanushree. 2019. Mineral composition of selected jackfruit genotype. International Journal of Chemical Studies 7 (6):2859–60.
- Sawe, B. E. 2017. World leaders in jackfruit production. Accessed September 16, 2022. https://www.worldatlas.com/articles/world-leaders-in-jackfruit-production.html.
- Saxena, A., A. S. Bawa, and P. S. Raju. 2008. Use of modified atmosphere packaging to extend shelf-life of minimally processed jackfruit (Artocarpus heterophyllus Lam.) bulbs. Journal of Food Engineering 87 (4):455–66. doi: 10.1016/j.jfoodeng.2007.12.020.
- Saxena, A., A. S. Bawa, and P. S. Raju. 2009. Phytochemical changes in fresh-cut jackfruit (Artocarpus heterophyllus Lam.) bulbs during modified atmosphere storage. Food Chemistry 115 (4):1443–9. doi: 10.1016/j.foodchem.2009.01.080.
- Saxena, A., A. S. Bawa, and P. S. Raju. 2011. Jackfruit (Artocarpus heterophyllus Lam). In Postharvest biology and technology of tropical and subtropical fruits, ed. E. M. Yahia, 275–99. Woodhead Publishing, Sawston, United Kingdom.
- Saxena, A., T. M. Saxena, P. S. Raju, and A. S. Bawa. 2013. Effect of controlled atmosphere storage and chitosan coating on quality of fresh-cut jackfruit bulbs. Food and Bioprocess Technology 6 (8):2182–9. doi: 10.1007/s11947–011–0761–x.
- Saxena, A., T. Maity, P. S. Raju, and A. S. Bawa. 2012. Degradation kinetics of colour and total carotenoids in jackfruit (Artocarpus heterophyllus) bulb slices during hot air drying. Food and Bioprocess Technology 5 (2):672–9. doi: 10.1007/s11947-010–0409–2.
- Saxena, A., T. Maity, P. S. Raju, and A. S. Bawa. 2015. Optimization of pretreatment and evaluation of quality of jackfruit (Artocarpus heterophyllus) bulb crisps developed using combination drying. Food and Bioproducts Processing 95:106–17. doi: 10.1016/j.fbp.2015.04.005.
- Selvaraj, Y., and D. K. Pal. 1989. Biochemical changes during ripening of jackfruit (Artocarpus heterophyllus Lam). Journal of Food Science and Technology 26:304–7. doi: 10.3329/jesnr.v7i2.22215.
- Shajib, M. T. I., M. Kawser, M. N. Miah, P. Begum, L. Bhattacharjee, A. Hossain, I. S. Fomsgaard, and S. N. Islam. 2013. Nutritional composition of minor indigenous fruits: Cheapest nutritional source for the rural people of Bangladesh. Food Chemistry 140 (3):466–70. doi: 10.1016/j.foodchem.2012.11.035.
- Shezi, S., L. S. Magwaza, A. Mditshwa, and S. Z. Tesfay. 2020. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Scientia Horticulturae 269:109397. doi: 10.1016/j.scienta.2020.109397.
- Shinde, V. L., C. D. Pawar, O. S. Warang, V. S. Dandekar, M. M. Kulkarni, J. Josiya, and M. S. Joshi. 2021. Studies on preparation of ice-cream from jackfruit (Artocarpus heterophyllus) seed powder. International Journal of Chemical Studies 9 (1):2710–2. doi: 10.22271/chemi.2021.v9.i1al.11636.
- Shraddha, A. J., K. Munishamanna, and S. Shyamalamma. 2021. Microbial fermentation of blended jackfruit juice for quality improvement of jackfruit wine. Mysore Journal of Agricultural Sciences 55 (3):83–90.
- Shwetha, M. S., and B. Ranganna. 2016. Development of squash from Jackfruit (Artocarpus heterophyllus Lam). Indian Journal of Science 23 (77):26–33.
- Singh, A., S. Maurya, M. Singh, and U. P. Singh. 2015. Studies on the phenolic acid contents in different parts of raw and ripe jackfruit and their importance in human health. International Journal of Applied Science-Research and Review 2 (2):69–73.
- Singh, K. K, and Mathur, P. B. 1954. A note on investigations on the cold storage and freezing of jackfruit. Indian Journal of Horticulture 11 (4):149–55.
- Singh, Z. 2022. Interventions to minimise postharvest losses, ensure the quality and safety of tropical and subtropical fruits: An overview. Acta Horticulturae 1340:1–12. doi: 10.17660/ActaHortic.2022.1340.1.
- Siti, Z. S., S. P. Siti, S. J. Abdul, A. A. Md, and A. Farzad. 2017. Application of ascorbic acid in maintenance of minimally processed product quality of jackfruit (Artocarpus heterophyllus). Bangladesh Journal of Botany 46 (1):413–8.
- Soepadmo, E. 1992. Artocarpus heterophyllus Lam. Plant resources of South-East Asia: Edible fruits and nuts, ed. W. M. Verheij, and R. E. Coronel. Wageningen (Netherlands): Pudoc.
- Soong, Y. Y., and P. J. Barlow. 2004. Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry 88 (3):411–7. doi: 10.1016/j.foodchem.2004.02.003.
- Souza, M. A., R. C. Bonomo, R. C. Fontan, L. A. Minim, and J. S. D. R. Coimbra. 2011. Thermophysical properties of jackfruit pulp affected by changes in moisture content and temperature. Journal of Food Process Engineering 34 (3):580–92. doi: 10.1111/j.1745-4530.2009.00402.x.
- Sudheer, K. P., S. Saranya, and K. B. Sankalpa. 2019. Process protocol standardisation and shelf-life study of minimally processed fresh cut tender jackfruit. The Pharma Innovation Journal 8:164–9.
- Sundarraj, A. A., and T. V. Ranganathan. 2018. Jackfruit taxonomy and waste utilization. Vegetos- An International Journal of Plant Research 31 (1):67–73. doi: 10.5958/2229-4473.2018.000095.
- Sutrisno, I. M., and S. Edris. 2011. Postharvest handling of jackfruit. In The jackfruit, ed. S. G. Valavi, K. V. Peter, and G. Thottapilly, 279–91. Studium Press, New Delhi, India.
- Sutrisno, S., and N. M. Sudiari. 1998. Studies on the storage characteristics of minimally processed jackfruit (Artocarpus heterophyllus Lam.) stored under modified atmospheric packaging. Jurnal Keteknikan Pertanian 12:22058.
- Swami, S. B., N. J. Thakor, P. M. Haldankar, and S. B. Kalse. 2012. Jackfruit and its many functional components as related to human health: A review. Comprehensive Reviews in Food Science and Food Safety 11 (6):565–76. doi: 10.1111/j.1541-4337.2012.00210.x.
- Swami, S. B., N. J. Thakor, S. Orpe, and S. B. Kalse. 2014. Development of osmo-tray dried ripe jackfruit bulb. Journal of Food Research and Technology 2 (2):77–86.
- Swords, G., P. A. Bobbio, and G. L. K. Hunter. 1978. Volatile constituents of jack fruit (Artocarpus heterophyllus). Journal of Food Science 43 (2):639–40. doi: 10.1111/j.1365-2621.1978.tb02375.x.
- Taib, M. R., I. I. Muhamad, C. L. Ngo, and P. S. Ng. 2013. Drying kinetics, rehydration characteristics and sensory evaluation of microwave vacuum and convective hot air dehydrated jackfruit bulbs. Jurnal Teknologi 65 (1):51–7. doi: 10.11113/jt.v65.1120.
- Taj, F., B. Ranganna, and K. B. Munishamanna. 2016. Vacuum packaging of minimally processed un–ripe Jackfruit (Artocarpus heterophyllus L.) bulbs. Science & Technology 2:35–9.
- Taj, F., K. B. Ranganna, and Munishamanna, B. 2014. Vacuum packaging of minimally processed jackfruit bulbs for long distance transportation. Mysore Journal of Agricultural Sciences 48 (3):351–7.
- Tanaka, M., I. S. Wallace, J. Takano, D. M. Roberts, and T. Fujiwara. 2008. NIP6; 1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. The Plant Cell 20 (10):2860–75. doi: 10.1105/tpc.108.058628.
- Taylaran, A. D., I. Bagsic-Posada, F. D. Cueva, and M. A. Balendres. 2021. First report of Lasiodiplodia theobromae causing jackfruit (Artocarpus heterophyllus) brown rot in the Philippines. Indian Phytopathology 74 (4):1151–3. doi: 10.1007/s42360-021-00392-6.
- Teja, T. R., K. K. Santhi, and A. Narsingarao. 2016. Edible film coating of fresh cut jack fruit. International Journal of Science, Environment Technology 5:1658–68.
- Thewes, F. R., A. Brackmann, and D. A. Neuwald. 2019. Dynamics of sugars, anaerobic metabolism enzymes and metabolites in apples stored under dynamic controlled atmosphere. Scientia Horticulturae 255:145–52. doi: 10.1016/j.scienta.2019.05.027.
- Tiwari, A. K, and A. S. Vidyarthi. 2012. Jackfruit jam: preparation nutritive values and storage stability. Pharmabit 25 (1-2):33–43.
- Tiwari, A. K., and A. S. Vidyarthi. 2015. Nutritional evaluation of various edible fruit parts of jackfruit (Artocarpus heterophyllus) at different maturity stages. International Journal of Chemical and Pharmaceutical Review and Research 1 (1):21–6.
- Tiznado, M. M., G. L. Esquivel, O. J. C. Campos, L. G. R. Guerrero, and C. R. Velasco. 2018. Lasiodiplodia theobromae, the causal agent of soft rot of fruits of Artocarpus heterophyllus in Nayarit, Mexico. Revista Brasileira de Fruticultura 40 (5):18–25. doi: 10.1590/0100-29452018018.
- Tomás‐Barberán, F. A., and J. C. Espín. 2001. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. Journal of the Science of Food and Agriculture 81 (9):853–76. doi: 10.1002/jsfa.885.
- Tongdang, T. 2008. Some properties of starch extracted from three Thai aromatic fruit seeds. Starch - Stärke 60 (3-4):199–207. doi: 10.1002/star.200800641.
- Tulyathan, V., K. Tananuwong, P. Songjinda, and N. Jaiboon. 2002. Some physicochemical properties of jackfruit (Artocarpus heterophyllus Lam.) seed flour and starch. ScienceAsia 28 (1):37–41. doi: 10.2306/scienceasia1513-1874.2002.28.037.
- Udayasoorian, L. P., M. J. Peter, R. V, S. Meenatchisundaram, V. a B, S. K, M. P, K. D, and S. Muthusamy. 2019. Symbiotic impact of honey treatment and package atmosphere on quality retention and shelf-life extension of jackfruit bulbs. Scientia Horticulturae 246:161–7. doi: 10.1016/j.scienta.2018.10.057.
- Ulloa, J. A., J. R. Aguilar-Pusian, P. Rosas-Ulloa, K. M. C. Galavíz-Ortíz, and B. E. Ulloa-Rangel. 2010. Effect of soaking conditions with citric acid, ascorbic acid, and potassium sorbate on the physicochemical and microbiological quality of minimally processed jackfruit. CyTA - Journal of Food 8 (3):193–9. doi: 10.1080/19476330903348791.
- UNICEF and World Health Organization. 2017. The state of food security and nutrition in the world 2017: Building resilience for peace and food security. Rome: FAO.
- Vargas-Torres, A., A. S. Becerra-Loza, S. G. Sayago-Ayerdi, H. M. Palma-Rodríguez, M. D. L. García-Magaña, and E. Montalvo-González. 2017. Combined effect of the application of 1-MCP and different edible coatings on the fruit quality of jackfruit bulbs (Artocarpus heterophyllus Lam.) during cold storage. Scientia Horticulturae 214:221–7. doi: 10.1016/j.scienta.2016.11.045.
- Vasudeva, K. R., H. C. Krishna, G. J. Suresha, and G. S. K. Swamy. 2018. Effect of modified atmosphere packaging on quality of fresh-cut jackfruit bulbs (Artocarpus heterophyllus Lam. )Acta Horticultarae 1299:409–14. doi: 10.17660/ActaHortic.2020.1299.61.
- Vijayaraghavan, K., D. Ahmad, and M. K. B. Ibrahim. 2006. Biohydrogen generation from jackfruit peel using anaerobic contact filter. International Journal of Hydrogen Energy 31 (5):569–79. doi: 10.1016/j.ijhydene.2005.06.006.
- Wang, J., J. Chen, D. Gong, F. Feng, Q. Lyu, and C. Ye. 2014. Effects of 1-MCP treatment on post-harvest physiology of jackfruit (Artocarpus heterophyllus Lam). Acta Agriculturae Universitatis Jiangxiensis 36:56–96.
- Wang, J., J. Ma, F. Feng, Z. Yang, and C. Ye. 2017. Effects of ethylene on carbohydrate metabolism and enzyme activities in postharvest ripening jackfruit. Acta Agriculturae Universitatis Jiangxiensis 39:43–9.
- Xess, R., V. Kumar, D. Patel, and A. Tripathi. 2021. Changes in chemical constituents and overall acceptability of jackfruit nectar during storage. The Pharma Innovation Journal 10 (7):332–6.
- Yi, J., P. Wang, J. Bi, X. Liu, X. Wu, and Y. Zhong. 2016. Developing novel combination drying method for jackfruit bulb chips: Instant controlled pressure drop (DIC)-assisted freeze drying. Food and Bioprocess Technology 9 (3):452–62. doi: 10.1007/s11947-015-1643-4.
- Ying, J. C. L., N. S. Jaffar, W. M. R. I. W. Hussin, M. F. A. Hamid, and N. I. Muhsin. 2020. Maintaining postharvest quality of jackfruit during cold storage. International Journal of Agriculture, Forestry and Plantation 10:282–6.
- Zeng, L., H. Fan, M. Zhang, X. Li, and Y. Shen. 2018. Effect of different storage temperatures on biochemical quality of freshly cut jackfruit. Acta Agriculturae Jiangxi 30 (5):28–32.
- Zhang, W., H. Jiang, J. Cao, and W. Jiang. 2021. Advances in biochemical mechanisms and control technologies to treat chilling injury in postharvest fruits and vegetables. Trends in Food Science & Technology 113:355–65. doi: 10.1016/j.tifs.2021.05.009.
- Zhang, Y., B. Li, F. Xu, S. He, Y. Zhang, L. Sun, K. Zhu, S. Li, G. Wu, and L. Tan. 2021. Jackfruit starch: Composition, structure, functional properties, modifications, and applications. Trends in Food Science & Technology 107:268–83. doi: 10.1016/j.tifs.2020.10.041.
- Zheng, H. Z., Y. I. Kim, and S. K. Chung. 2012. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chemistry 131 (1):106–10. doi: 10.1016/j.foodchem.2011.08.038.
- Zuwariah, I., F. Noor, M. B. Hadijah, and R. Rodhiah. 2018. Comparison of amino acid and chemical composition of jackfruit seed flour treatment. Food Research 2 (6):539–45. doi: 10.26656/fr.2017.2(6).106.