Abstract
Antimicrobial agents are safe preservatives having the ability to protect foods from microbial spoilage and extend their shelf life. Many factors, including antimicrobials’ chemical features, storage environments, delivery methods, and diffusion in foods, can affect their antimicrobial activities. The physical-chemical characteristics of the food itself play an important role in determining the efficacy of antimicrobial agents in foods; however the mechanisms behind it have not been fully explored. This review provides new insights and comprehensive knowledge regarding the impacts of the food matrix, including the food components and food (micro)structures, on the activities of antimicrobial agents. Studies of the last 10 years regarding the influences of the food structure on the effects of antimicrobial agents against the microorganisms’ growth were summarized. The mechanisms underpinning the loss of the antimicrobial agents’ activity in foods are proposed. Finally, some strategies/technologies to improve the protection of antimicrobial agents in specific food categories are discussed.
1. Introduction
Access to adequate amounts of safe and nutritious food is important for sustaining life and promoting good health (World Health Organization, Citation2021). Unsafe food products are easily associated with foodborne diseases that can cause morbidity and mortality, contributing to a substantial impediment to socio-economic development all over the world (World Health Organization, Citation2015). According to WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) estimates, about 600 million foodborne diseases caused 420,000 deaths in 2010; 18 million Disability Adjusted Life Years (DALYs) caused by foodborne diarrhoeal disease agents, particularly non-typhoidal Salmonella enterica (NTS) and enteropathogenic Escherichia coli (EPEC). Other foodborne hazards with a considerable contribution to the global burden included Salmonella Typhi, Tenia solium and hepatitis A virus (World Health Organization, Citation2015). The risks related to toxins production by microorganisms are often problems because they may occur unavoidable and unpredictable. Toxic metabolites produced by microorganisms can enter the food chain by accumulation in plant-based food components, by contamination of other food and feed crops during transportation, and by possible ingestion of animal-derived products such as milk-, meat- and egg-containing residues (Alshannaq and Yu Citation2017). Hence, assuring the safety of food products by inactivation or inhibition of the growth of microorganisms is of importance for preventing foodborne illness. Additionally, microbiological spoilage can cause food deterioration, leading to food loss. According to Food and Agriculture Organization of the United Nations (FAO) estimates, about one-third of all food produced in the world is lost or wasted globally, which results in an enormous financial burden and negative environmental impacts (Cichello Citation2015; Gustafsson et al. Citation2013; Lipinski et al. Citation2013). Besides, sensory deterioration always occurs during the process of food spoilage, which greatly reduces the good sensory experience for consumers. It is thus important to lower the risk of microbiological spoilage, which can be achieved by prolonging the shelf-life of food products via extending the microbial lag phase and reducing the rate of microbial growth (Ahmed et al. Citation2022).
To inhibit the growth of microorganisms in food products, numerous methods and strategies have been studied and applied (Özogul and Hamed Citation2018). This includes physical strategies, such as temperature control, irradiation, filtration, high hydrostatic pressure, electric pulsed fields, electrolyzed water, cold plasma treatments, etc., and chemical strategies such as the addition of preservatives and salts, the application of antimicrobial packaging, etc. (Ali et al. Citation2018; Bahrami et al. Citation2022; Bhat et al. Citation2019; Kilcast and Subramaniam Citation2011; Petruzzi et al. Citation2017). Among these strategies, the use of antimicrobial agents has a huge contribution in dealing with both foodborne diseases and food waste. Antimicrobial agents are natural or synthetic substances that can inactive or retard the growth of microorganisms (Burnett-Boothroyd and McCarthy Citation2011). They offer a number of advantages in food protection, including high antimicrobial efficacy, cost-effectiveness, convenient usage, etc. (Tiwari et al. Citation2009; Zajac, Schubert, and Oelkrug Citation2017). Antimicrobial agents execute their activity against the microorganisms through various mechanisms, which include disruption or penetration lipid structures of microorganisms, damage membrane integrity, pore formation in membranes, formation of a polymer membrane that prevents nutrients from entering the cell, preventing enzymatic activity, binding nucleic acids, producing reactive oxygen enzymatic, impacting the protein synthesis, etc. (Gut, Blanke, and van der Donk Citation2011; Oussalah, Caillet, and Lacroix Citation2006; Pasquet et al. Citation2014; Yousefi, Ehsani, and Jafari Citation2019; Zheng and Zhu Citation2003).
In the last two decades, some natural antimicrobials, including essential oils, phenolic compounds, nisin, chitosan, lysozyme, lactoferrin and some natural organic acids, have attracted the attention of food industries, researchers and consumers. These compounds are generally recognized as safe (GRAS) agents, though the regulations regarding the dosage are not well defined in some developing countries. They can be broadly extracted from animal sources (e.g., lysozyme, lactoferrin, chitosan), plants and plant by-products (e.g., essential oils, pomace and seeds of fruits, vegetable and tea products), microbial sources (e.g., nisin, pediocin, citric acid, sorbic), algae and mushrooms (e.g., green, red and brown algae, fungi) (Abdelshafy et al. Citation2022; Gyawali and Ibrahim Citation2014; Siddiqui et al. Citation2016). And they are widely used to inhibit the growth of microorganisms in both model media and realistic foods (Jayasena and Jo Citation2013; Tiwari et al. Citation2009).
The use of antimicrobials has shown great success in the inhibition of the growth of microorganisms in the model media, however, their applications in the preservation of complex foods often fail to be effective. The mechanisms behind the fact that antimicrobial agents possess high efficacy in model media but low efficacy in foods are not fully understood. However, this shows that the food matrix can be a significant factor in determining the efficacy of antimicrobial agent.
The concept of a food matrix is commonly viewed as a physical domain that contains and/or interacts with the nutrient and non-nutrient compounds (Aguilera Citation2019). It is a structural organization of all food components at multiple spatial length scales (Capuano, Oliviero, and van Boekel Citation2018). The food matrix consists of food components such as proteins, lipids, and carbohydrates. Studies accumulating in the last two decades have proved a big contribution of the food components to the activities of the antimicrobial agents (Castro-Rosas et al. Citation2017; Gharsallaoui et al. Citation2016; Perricone et al. Citation2015; Shannon, Radford, and Balamurugan Citation2020). Multiple food components may form a heterogeneous food (micro)structure that may contain simultaneously liquid, solid and gaseous phases. When antimicrobial agents absorb and diffuse into the food matrix, the complex food microstructure may further complicate the partitioning of compounds. However, the impacts of the food (micro)structure on antimicrobial agents’ activities are easy to be overlooked, and the understanding is still limited. This requires a comprehensive review of the research publications to provide current research findings and inspire future studies.
This review aims to identify the factors of the food matrix, including food components and food (micro)structures, which affect the activities of some commonly discussed/used antimicrobial agents on food preservation, and to derive the mechanisms that determine the activity of the antimicrobial compounds in food systems. A clear understanding of the mechanisms can be used to forecast the activity loss of an antimicrobial agent in a particular food product. Strategies and technologies that can prevent the reduction of activity of an antimicrobial agent were then summarized and discussed from the current stage of studies.
2. Natural vs. synthetic antimicrobials
Antimicrobial agents ensure the safety and quality of foods by adding them directly to foods as preservatives or incorporating them into packaging films as antimicrobial packaging. Nowadays, more than a thousand antimicrobial agents have been recorded to effectively inhibit the growth of microorganisms (Ju et al. Citation2022). The applications of some antimicrobials are already at the industrial scale, whereas others are still in the research pipeline.
Synthetic antimicrobial agents, such as nitrates, benzoates, sulfites, sorbates, etc., are widely used in food preservation because of their proven antimicrobial efficacy and low cost (Carocho et al. Citation2014). Synthetic antimicrobial agents are regulated and their safety is examined by regulatory systems in several countries across the world, such as the USA (Food and Administration Citation2006), EU (Regulation (EC) No 1333/2008) (Parliament and Union Citation2008), and various Asian countries. Many synthetic antimicrobial agents are recognized safe as food additives; however, some of them may generate a negative perception among consumers as they are not considered clean label products. In recent years, consumers show a strong desire for clean label foods that contain no additives or are produced with a more “natural” method (Asioli et al. Citation2017; Maruyama, Streletskaya, and Lim Citation2021). Besides, the ongoing debate regarding the health risks posed by synthetic antimicrobials exposure to consumers is growing. These factors impel the development and applications of natural antimicrobial agents in the food supply chain.
The utilization of natural antimicrobial agents, such as essential oils (EOs), phenolic compounds, nisin, chitosan, lysozyme, lactoferrin, and organic acids as substitutes for synthetic antimicrobials has been encouraged in recent years (Aziz and Karboune Citation2018). However, the application of natural antimicrobials in food preservation is hampered by several factors. A summary of the pros and cons of using the main representatives of natural and synthetic antimicrobial agents is portrayed in .
Table 1. Overview of the pros and cons of using some natural and synthetic antimicrobial agents.
Natural antimicrobial agents are often used as alternatives to synthetic preservatives in food, cosmetics, and pharmaceutical products due to their perceived safety, consumer acceptance, and potential health benefits. However, the use of natural antimicrobial agents is not without limitations. One major limitation is the variability in their composition, which can lead to inconsistencies in their effectiveness and safety. Natural preservatives can also have allergenic properties, particularly for people with sensitivities to plant-derived compounds (such as essential oils or plant extracts), for whom it can cause adverse reactions (Türkmenoğlu and Özmen Citation2021). Another limitation of natural antimicrobial agents is their limited range of activity, which may make them less effective than synthetic preservatives against certain types of microorganisms. Furthermore, the appropriate dosage of natural preservatives required for effectiveness can be difficult to determine, and overdosing may lead to toxicity or undesirable side effects (Armengol, Harmanci, and Laffleur Citation2021). When deciding whether to use natural versus synthetic antimicrobial agents, it is important to consider various factors such as the intended use of the product, the efficacy and safety of the preservative, and the sustainability of the source of the preservative. Moreover, the use of natural antimicrobial agents may be subject to regulatory requirements, such as maximum allowable levels or labeling requirements. Overall, while natural antimicrobial agents are generally considered safe, their use should be carefully evaluated on a case-by-case basis, taking into account their composition, range of activity, appropriate dosage, and potential allergenic properties. The decision to use natural versus synthetic preservatives should ultimately be based on a comprehensive assessment of the risks and benefits of each option, as well as their impact on product quality and sustainability.
3. Food matrix affects the antimicrobial activity in the food systems
The relevance of food matrices to explain the phenomena occurring during the food process, oral processing, flavor perception, satiation/satiety, and food digestion was traditionally underestimated as recently emphasized in some pivotal papers (Aguilera Citation2019; Fardet Citation2014). And the notation “food matrix effect” (FM-effect) became largely used (Capuano, Oliviero, and van Boekel Citation2018; Martínez-Delgado, Khandual, and Villanueva–Rodríguez Citation2017; Singh, Dartois, and Kaur Citation2010). As shown in , FM-effects also play a role in influencing the efficacy of some typical antimicrobial agents. Food matrix factors showing effects on the activities of the antimicrobial agents include, but are not limited to, major food components, matrix consistency (physical state of the foods), and the microstructure of foods (Shannon, Radford, and Balamurugan Citation2020).
3.1. Impacts of the major food components on the activities of the main classes of antimicrobial agents
A food matrix is generally based on one of the macronutrients i.e., proteins, lipids, carbohydrates (i.e. starch or dietary fiber). The application of antimicrobial agents aiming to protect foods from microbial spoilage should consider the interactions between the antimicrobials and the food components, especially with proteins, lipids, and carbohydrates. The studies of the influences of proteins, lipids and carbohydrates on the selected typical antimicrobial agents in either model foods or in real foods were extensively reported, and some of the most relevant studies of the last 12 years are listed in .
Table 2. Overview of studies regarding the influence of the major food components on the activity of selected typical antimicrobial agents (from the years 2010–2022).
The effect of each macro-component of the food matrix on the main classes of antimicrobials is illustrated in this chapter.
3.1.1. Proteins
3.1.1.1. Effect of protein on EOs antimicrobial activity
The presence of proteins in a model medium or real foods interferes with the activities of many antimicrobial compounds. For example, the influences of proteins were shown in the antimicrobial activities of plants and plant by-products (e.g., essential oils) both in liquid and semi-solid foods, such as milk beef and chicken, etc. In most cases, the efficacy of antimicrobials from plants or plant by-products is reduced by increasing the protein concentration, whereas some studies found an enhanced antimicrobial activity of essential oils at high concentrations of protein. This is probably due to the binding capacity of proteins to EOs via hydrophobic interaction. Proteins can bind to EOs and impede their interaction with bacteria in the aqueous phase at low concentrations of EOs, however, this binding can also promote interaction when the bacteria are in the protein phase. Liu and Yang (Citation2012) found that the presence of milk proteins increased the lowest concentrations of essential oil of Litsea cubeba (LC-EO) that can inhibit the growth of Lactobacillu plantarum in the orange-milk beverage. The high content/presence of protein can also reduce the effects of antimicrobial plants and plant by-products in the inhibition of microorganisms in meat products such as ground beef, chicken, chicken, etc. This has been extensively reported by Uhart, Maks, and Ravishankar (Citation2006), Firouzi et al. (Citation2007), Shekarforoush et al. (Citation2007), Oussalah et al. (Citation2007), etc., and will not be discussed in detail in this review. The activity reduction in the above studies may be attributed to the fact that the growth of microorganisms was less affected by the presence of active agents due to the immobilization of the antimicrobial plants and plant by-products by the high percentages of proteins and fats in meat products. However, enhanced inhibitory activities of essential oils (e.g., marjoram oil) may occur in simple model mediums containing soy peptone and meat extract (Gutierrez, Barry-Ryan, and Bourke Citation2008, Citation2009; Tserennadmid et al. Citation2010). It is a good indication that the cases in the simple food models containing few food compositions are not necessarily applicable in real food systems which are compositionally and heterogeneously complex. The predictions and applications of antimicrobial agents in real food products are complex and should be analyzed case by case.
3.1.1.2. Effect of protein on chitosan and chitooligosaccharides antimicrobial activity
The antimicrobial activities of chitosan and chitooligosaccharides are influenced by the presence of proteins in the food matrix. The interactions between chitosan (or chitooligosaccharides) and a food matrix containing high concentration of protein can be enhanced or weakened depending on the electrostatic attraction/repulsion in the food matrix which changes with the food environment conditions like pH and ionic strength. The antimicrobial activity of chitosan decreased with the increase in protein content from 0% to 10% when the pH was above the isoelectric point (IEP) of the protein; whereas its activity was unchanged with the protein content varying from 0% to 10% at the pH lower than the IEP of the protein (Devlieghere, Vermeulen, and Debevere Citation2004). The presence of proteins, acting in a competitive manner, can interact with chitosan and chitooligosaccharides, leading to a loss of inhibitory activity against the microorganisms (Ausar et al. Citation2002; Fernandes et al. Citation2008).
3.1.1.3. Effect of protein on the antimicrobial activity of bioactive peptides and other agents
In many cases, the antimicrobial activities of bioactive peptides (e.g., nisin and sakacin P) decreased in the presence of proteins. Nisin and sakacin P showed a lower activity against bacteria (e.g., L. monocytogenes and B. cereus) in food products containing high content of protein (e.g., whole milk and chicken and smoked salmon) than that in simple food models or buffer solutions (da Silva Malheiros et al. Citation2012; Pol et al. Citation2001). This may be attributed to the reason that a large amount of added nisin (sakacin P) was absorbed into the muscle protein in food products, leading to the activity loss of bacteriocin in foods (Aasen et al. Citation2003). Similarly, a moderate increase of lauric arginate from 2% to 5% had little effect when it was used against L. monocytogenes in tryptic soy broth due to the probability of its hydrolysis in the presence of proteins (Ma, Davidson, and Zhong Citation2013; Nerin et al. Citation2016).
3.1.2. Lipids
3.1.2.1. Effects of lipids on the EOs antimicrobial activity
The hydrophobic nature of EOs and lipids/fats in foods/model mediums allow them for clustering, impeding the interaction between EOs and microorganisms. This reduces the EOs concentration in the aqueous phase where microorganisms are present, and the effectiveness of the EOs in the preservation of foods with a high fat content (Perricone et al. Citation2015). Many studies found that milk fat reduced the antimicrobial effects of essential oils or phenolic compounds. For example, the combined antimicrobial activities of carvacrol, thymol and eugenol against S. aureus were lower in cow milk than in the culture medium, according to Alves et al. (Citation2016). Cava-Roda et al. (Citation2012) found that the inhibitory activities of cinnamon, bark, cinnamon leaf, clove and vanillin against L. monocytogenes and E. coli O157:H7 in whole milk were lower than in skimmed milk products. The presence of fat in milk also reduced significantly the antimicrobial activities of those antimicrobials in the studies of Cava et al. (Citation2007), Gaysinsky et al. (Citation2007), Gutierrez, Barry-Ryan, and Bourke (Citation2008), etc. Meat fat in chicken, beef or pork also negatively influenced the antimicrobial activities of essential oils or phenolic compounds. The activity of a carvacrol loaded polylactic acid (PLA) film to preserve ground beef having a fat content of 5 or 12% was studied by Wang et al. (Citation2020c). Despite the higher carvacrol absorption in the regular beef (12%), the PLA/carvacrol films showed a stronger antimicrobial effect on the lean beef (5%). This can be explained by the partitioning behavior as carvacrol with a high affinity to fat tends to dissolve into the fat phase of beef, leading to the prevention of the interaction between carvacrol and bacteria in the aqueous phase of the meats. Similarly, EOs showed reduced antimicrobial activity in meat products (e.g., chicken, ham, hotdog, etc.) with a high content of fat, due to the immediate migration of EOs in the fat phase of these foods (Firouzi et al. Citation2007; Gill et al. Citation2002; Shekarforoush et al. Citation2007; Singh et al. Citation2003).
3.1.2.2. Effects of lipids on bioactive peptides antimicrobial activity
Increasing the concentration of fat generally reduces the antimicrobial activities of bioactive peptides, due to the interaction between the hydrophobic part of peptides and fat in food impeding the peptides approaching bacteria. This has been extensively studied from the literature reported in the years 2000–2012 and has been reviewed in many previous review papers (Aziz and Karboune Citation2018; Gyawali and Ibrahim Citation2014). As suggested from these previous studies, the diminished activities of antimicrobial agents, including nisin, lactoferrin, lysozyme, monolaurin, sakacin P, etc., can be found in food products like milk, salmon, turkey bologna, ground meat, etc., (Aasen et al. Citation2003; Bhatti, Veeramachaneni, and Shelef Citation2004; Chollet et al. Citation2008; Dawson et al. Citation2002; Glass and Johnson Citation2004; McLay et al. Citation2002; Pinilla and Brandelli Citation2016). For liquid food products, these antimicrobial agents prefer remaining in the interface of emulsion-like samples (e.g., milk and dairy beverages), leading to a rapid loss of activity in the aqueous phase while no activity was detected in the oil phase (Aasen et al. Citation2003). With regards to solid/semi-solid foods, reasons for the lower activity of antimicrobials in these foods (e.g., turkey bologna and ground beef) include the high-fat content in meats and the limited diffusion of bactericidal compounds into the core of semi-solid foods (Dawson et al. Citation2002; McLay et al. Citation2002). To prevent the activity loss in whole fat milk, encapsulations of antimicrobials in nanovesicles or liposomes were assessed by da Silva Malheiros et al. (Citation2010); (da Silva Malheiros et al. Citation2012; Lopes, Pinilla, and Brandelli Citation2019; Pinilla and Brandelli Citation2016) and Schmidt et al. (Citation2009). Free and peptide P34 loaded soybean phosphatidylcholine liposome at 3200 AU/mL lowered L. monocytogenes counts below the detection limit of the method (0 log CFU/mL) at days 5 and 8 in skim milk (0% fat), whereas both treatments only caused a 1–2 log CFU/mL reduction of the population of L. monocytogenes compared to the control sample without treatment after 5 days in whole milk (3.25% fat) (da Silva Malheiros et al. Citation2012). Lopes, Pinilla, and Brandelli (Citation2019) co-encapsulated nisin and lysozyme into phosphatidylcholine (PC) liposomes coated with pectin or poly-galacturonic acid to test the antimicrobial activity of PC-pectin liposomes against the growth of Listeria monocytogens and S. enteritidis in both skim and whole milk. The PC-pectin liposome caused 2 and 5 log CFU/mL reductions of the population of Listeria monocytogens in whole and skim milk at 37 °C, respectively. However, in some other application scenarios, the hydrophobic nature of active agents (e.g., enterocins) can cause a higher absorption in foods with a high fat content, which may increase the interactions between the immobilized active agent in the oil or interface and the microorganisms, increasing the inhibition of microorganisms in foods (Aymerich et al. Citation2000).
3.1.3. Carbohydrates
3.1.3.1. Effects of carbohydrates on the EOs antimicrobial activity
Studies of the influences of carbohydrates on the antimicrobial effect of antimicrobial agents were less found than those regarding the effects of proteins and fats. Among these studies, some found enhanced antimicrobial activities of EOs in the presence of carbohydrates in foods, whereas some of those indicate an opposite effect. The mechanism is not fully understood yet. The inhibitory performance of EOs is influenced by the molecular structure of carbohydrates. The presence of sugars in low concentrations (<2.32% w/w) improved the antimicrobial efficacy of oregano and thyme; whereas the presence of high concentrations of sugar (mainly composed of glucose and fructose) (5.80 or 11.6% w/w) did not have a negative impact on the antimicrobial efficacy of oregano and thyme in model food media (beef extract mixed with tomato serum) (Gutierrez, Barry-Ryan, and Bourke Citation2009). Similarly, the positive effect of sucrose on the antimicrobial activity of marjoram oil was also found by Tserennadmid et al. (Citation2010). Increasing sucrose concentrations from 1 to 8% (w/v) showed a positive effect on the inhibition of B. cereus and E. coli by prolonging the lag phases of bacteria. In contract, a decreased antimicrobial activity of oregano and thyme against Listeria monocytogenes was observed in the high concentrations of potato starch media (5 and 10% w/w) (Gutierrez, Barry-Ryan, and Bourke Citation2008). The enhancing impact of sugar and the negative impact of starch suggests that the application of essential oils should be orientated to foods containing more simple sugars than complex carbohydrates to prevent the inhibiting impact of carbohydrates on antimicrobials.
3.1.3.2. Effects of carbohydrates on lauric arginate and chitosan antimicrobial activity
Starch strongly interacts with lauric arginate, impeding the active interaction between lauric arginate and bacteria in foods. Increasing the concentration of soluble starch from 2 to 5% (w/v) reduced the antimicrobial activity of lauric arginate on the inhibition of Listeria monocytogenes in tryptic soy broth (Ma, Davidson, and Zhong Citation2013). A similar negative effect of starch can also be found on the antimicrobial activity of chitosan. When chitosan was exposed to Candida lambica in sabouraud agar medium, its activity was significantly reduced in the presence of high amounts of starch (30% (w/v)), resulting in a significantly shorter lag phase and higher bacterial growth rate (Devlieghere, Vermeulen, and Debevere Citation2004). This can be attributed to either a protective effect by the starch or electrostatic interaction between chitosan and starch.
3.2. Impacts of food (micro)structures on the activities of antimicrobial compounds
After looking at the effect of each macronutrient we move on the physical matrix. The structure of food is the result of the interactions between food components at levels ranging from the nano-microscale (<1 × 106 nm) to the macroscale (2 × 103 to 107 nm) (Camci-Unal, Zorlutuna, and Khademhosseini Citation2013; Rao Citation2010). At the macro level, the food structure determines the physical state of foods, which can be reflected in the matrix consistency of foods, such as liquid, semi-solid or solid foods. At the micro level, multicomponent and multidisperse food systems are consisting of colloidal structures that can associate and cluster together to form complex microstructures that can affect the interaction with antimicrobial compounds (Weiss, Loeffler, and Terjung Citation2015). The influence of the food (micro)structure on the antimicrobial activities is schematized in . The studies investigating the effects of food structure at the macro-micro-nano scale (food microstructure) on the partitioning and the resulting antimicrobial activities of the main classes of antimicrobials in a food matrix were summarized in .
Figure 1. Impacts of food (micro)structures on the activities of the main classes of antimicrobial compounds at the macro/microscale. The yellow dots, red curves and white spots correspond to fat droplets, proteins and pores, respectively; green particles represent the antimicrobial agents.
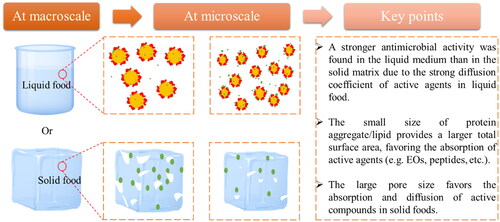
Table 3. Overview of studies regarding to the influence of food (micro)structure on the activity of the main classes of antimicrobial agents (from the years 2000–2022).
The effect of macro-micro-nano level food structure of the food matrix on the main classes of antimicrobials is illustrated in this chapter.
3.2.1. Macro level food structures impact the activities of antimicrobial compounds
Compared to the studies focusing on food components’ effects, investigations regarding food (micro)structures were found far fewer. Among them, most investigations examined the influences of food structures at the macroscale, which is the matrix consistency, on the activity of antimicrobial compounds. The dairy category is a good example to investigate the impacts of food matrix consistency since it contains foods with various physical states (e.g., liquid milk and yogurt, semi-solid/solid cheese, etc.). The difference in dairy food consistency causes a clear difference in the activities of antimicrobial compounds. For example, Soni et al. (Citation2010) compared the effect of lauric arginate on the inhibition of L. monocytogenes in tryptic soy broth, milk and cheese. A 4-fold increase in the concentration of lauric arginate was required for full inhibition of L. monocytogenes below the detection limit in milk, compared to that in tryptic soy broth, with still greater concentrations required in cheese as well. Similarly, the inactivation of L. monocytogenes by the combination of endolysin PlyP825 and high hydrostatic pressure was the best in milk, followed by the mozzarella cheese. For the most rigid food with a compact structure (smoked salmon), the least inactivation effect was found, which was only caused by the high pressure (Misiou et al. Citation2018). With the matrix consistency increasing from milk to cheese to smoked salmon, the inhibitory effects of active compounds decreased. Cheese is a semi-solid that is rough and porous in the microstructure, and salmon is solid food that contains a more compact structure in chum muscle. Both the cheese and salmon have a lower random diffusion of active compounds in the food matrix, compared to the diffusion in liquid products, resulting in reduced accessibility of active compounds to bacteria in cheese andsalmon. Besides, the viscosity of foods determines the diffusion of active compounds, which consequently influences their antimicrobial activities. This was observed by De Souza et al. (Citation2016) who tested the antimicrobial activities of essential oil from Origanum vulgare L. (OVEO) on the inhibitory of S. aureus, L. monocytogenes, and a mesophilic starter coculture consisting of lactic acid bacteria in both cheese broth and semisolid coalho cheese slurry. The results showed that higher concentrations of essential oil were needed to decrease the viable cell counts of all tested bacteria in semisolid cheese, in comparison with cheese broth. The distance-dependence of active compounds’ diffusion was proved by Cao-Hoang et al. (Citation2010) who studied the effect of nisin loaded sodium caseinate film on the controlling of the L. innocua in the different depths of artificially contaminated cheese. The developed antimicrobial packaging was coated on the surface of the cheese. The inactivation was observed in 1.1, 0.9 and 0.25 log CFU/g reductions for distances of 1, 2 and 3 mm from the contact surface. The results showed a clear difference in the bacteria inhibition, indicating the influence of the nisin diffusion from the surface to the inner part of cheese on its antimicrobial activity.
Meat products differ in their (micro)structures depending on the derivation from the body of an animal and post-processing methods. The influences of the meat matrix consistency on the activities of antimicrobial agents were also investigated by some previous studies. Ye, Neetoo, and Chen (Citation2008) inoculated L. monocytogenes on the surface of the ham steaks and evaluated the efficacy of chitosan film against L. monocytogenes on ham steaks. However, low effectiveness of the chitosan film on the inhibition of the growth of L. monocytogenes during 3 days of storage was observed. The lack of effectiveness might be due to the lack of diffusion of chitosan through a rigid food matrix (ham steaks). A similar study was conducted on the preservation of ready-to-eat meat products by application of bacteriophage A511 (Ahmadi Citation2017). When the bacteriophage was applied on the surface of the surface-contaminating cooked meat, the growth of L. monocytogenes was inhibited; while when the bacteriophage A511 was directly added into the cooked meat slurry that was homogenously inoculated with L. monocytogenes in the inside of the meat, the additive did not control the growth of L. monocytogenes. The low effectiveness when bacteriophage A511 was used as an additive might attribute to the limited mobility of bacteriophage A511 that reduces the possibility of contacting the bacteria host in meat.
3.2.2. Nano-micro level food structures impact the activities of antimicrobial compounds (emulsion and hydrogels)
In recent decades, the investigations of physical, rheological, textural and sensorial properties were achieved at the micro-level (microstructure), to gain a deeper understanding of mechanisms related to food preservation, further investigations of food structure at the nano-micro level, is recommended. Unfortunately, the role of food microstructure was only found in tuning the oil droplet size of an emulsion and the pore size of an hydrogel. Bhatti, Veeramachaneni, and Shelef (Citation2004) studied the anti-listeria activities of nisin in raw and homogenized milk. Nisin at 125 IU/mL can rapidly reduce the number of L. monocytogenes to nondetectable numbers in raw milk, however, loss of the anti-listeria effects of nisin was observed in homogenized milk. The size of oil droplets in milk was decreased after the homogenization treatment, which may change the milk protein distribution. The alteration of oil droplet size will further influence the partitioning of nisin in milk, resulting in a change in the antimicrobial activity of nisin. The influence of the oil droplet size on the activity of antimicrobial agents was further investigated by Wang et al. (Citation2020a). Carvacrol partitioning and antimicrobial activity in a food package containing a carvacrol-loaded polylactic acid (PLA) film and emulsions were studied. Different emulsions with the mean particle size ranging from 0.27 to 0.51 μm were prepared to investigate the influence of the oil droplet size on the absorption, partitioning and antimicrobial effect of carvacrol in emulsions. The carvacrol concentrations in the total emulsions and in the oil and aqueous phases of emulsions were quantified, and the results showed carvacrol absorption and partition coefficient (ratio of carvacrol in the oil phase to that in the liquid phase) were higher in emulsions containing smaller oil droplets, compared with other samples. Yet, the antimicrobial activity of carvacrol was not the highest in the emulsion containing the smallest oil droplets. This indicated the antimicrobial activity of small bioactive molecules depends more on the colloidal structure of the food matrix than the total absorptions. Our studies on the preservation of gelled food showed the absorption and diffusion of carvacrol in the hydrogel depends on the pore size of the hydrogel, which determined the carvacrol antimicrobial activity in the inhibitory of P. fluorescens in the hydrogel (Wang et al. Citation2021a). The large pores promote carvacrol absorption and diffusion, which was in turn more effective in inhibiting the growth of P. fluorescens, compared to the hydrogel containing small pores. This indicated that the key factor in the effectiveness of the preservation of semi-solid food is the pore size of the food matrix as the antimicrobials largely/easily diffuse through a liquid or gas contained within the pores of semi-solid foods.
3.3. Effects of pH and ionic composition on the activities of antimicrobial compounds
Except for the macronutrients and physical matrix, some other characteristics such as pH and ionic composition also play a role in influencing the absorption and partitioning of an antimicrobial agent in the food matrix, which should be considered when designing an antimicrobial packaging film. Many studies found that for most antimicrobial agents, including endolysins, nisin, essential oils, chitosan, organic acids, etc., their antimicrobial activities are pH-dependent and are affected by the ionic composition. lists some of the most relevant examples of studies regarding the influences of pH and ionic composition on the activities of antimicrobial compounds (from the years 2010–2022).
Table 4. Main studies of the influences of pH and ionic composition on the activities of antimicrobial agents (from the years 2012–2020).
The effect of pH and ionic strength of the food matrix on the main classes of antimicrobials is illustrated in this chapter. The pH of the food matrix may affect the activity of antimicrobials by influencing the diffusion path of antimicrobials and altering the location and solubility of antimicrobials. Endolysins, which are cell wall-hydrolyzing enzymes during late gene expression in the lytic cycle of multiplication of most phages, are pH-dependent in their antimicrobial performance. They show the optimal antimicrobial activity for the foods that are slightly acidic to above neutral pH (Chang, Kim, and Ryu Citation2017; García et al. Citation2010). The reduction in the activity of endolysins is caused by their reduced enzymatic activities as their antimicrobial activity is mainly attributed to their enzymatic function that can cause the cleavage of the covalent bonds in peptidoglycan (Lai et al. Citation2011). Van Tassell et al. (Citation2017) assessed the antimicrobial efficacy of endolysin PlyP100 on the inhibition of the growth of Listeria monocytogenes in fresh cheese. The activity of PlyP100 increased with the increase in the pH from 5 to 8 of boric acid/phosphoric acid buffer, reaching the highest at pH 8, followed by a decrease when the pH further increased. Minimal activity at pH 5 or below suggested that PlyP100 may be less effective in the inhibition of L. monocytogenes in acidified foods. A similar finding that suggested the endolysin LysSA11 shows an optimum antimicrobial performance at pHs from 6.0–8.0 was obtained by Chang, Kim, and Ryu (Citation2017). The susceptibility of S. aureus to LysSA11 was tested in various buffers that have a broad pH range from 2.0–10.0. The endolysin retained more than 70% of its activity at pHs from 7.0 to 8.0, but its activity was significantly reduced at lower pH (below 4.0). Except for pH, the antimicrobial activities of endolysins can also be affected by the ionic strength in food matrix since their activities are closely associated with the enzymatic function. Schmelcher et al. (Citation2015) determined the influence of NaCl concentration on the activity of endolysin λSA2 and B30. The results showed that for both endolysins, their activities increased with the increase in the NaCl concentration from 0–150 mM. The reduced activities can be found at NaCl concentrations higher than 150 mM. Endolysins displayed the highest activity at NaCl between 100 and 150 mM was agreed by Van Tassell et al. (Citation2017) who optimized the salt conditions for the activity of endolysin PlyP100 by observing the turbidity reductions of toric acid/phosphoric acid (BP) buffer inoculated with L. monocytogenes. In a recent study, Bai et al. (Citation2019) tested the activity of endolysin BSP16Lys on the inhibitory of S. Typhimurium LT2 cell in buffers in the presence of NaCl. The strongest activity of endolysin BSP16Lys was found in the presence of 100 mM NaCl, and its activities decreased in the presence of NaCl concentrations higher than 100 mM.
When nisin was served as a food preservative, strong antimicrobial activity at the acidic pH at near-neutral pH was observed. It, however, shows low effectiveness at near-neutral pH. According to Siroli et al. (Citation2019), nisin was more effective in the inhibition of L. monocytogenes in carrot juice samples than that in soymilk samples, despite the amount of nisin used was higher in the soymilk samples. This was attributed to the higher acidity in the carrot juice, compared to soymilk. Indeed, nisin is more soluble and more stable under acidic conditions and has a solubility of 12 wt% at pH 2.5 and 4 wt% at pH 5.0 (Gharsallaoui et al. Citation2016). The high solubility of nisin under acidic conditions results in a strong antimicrobial activity since the high chance of interaction with microorganisms can happen at the high solubility of nisin. The result was in line with the study of Gharsallaoui et al. (Citation2016) who concluded that the antimicrobial activity is stronger at acidic pH and gradually decreases with increasing pH since an irreversible modification of the molecular structure of nisin occurs at pH > isoelectric point (pI) (∼8–9) (Liu and Hansen Citation1990). Another factor promoting nisin activity is the presence of NaCl. Nisin may promote ATP depletion of bacteria cells by inducing hydrolysis and damaging the cells (Winkowski, Bruno, and Montville Citation1994). The synergistic effect of NaCl on the activity of nisin may cause not only ATP depletion but also dissipation of ion gradients across a cell membrane, promoting the death of bacteria cells (Heo et al. Citation2012).
The pH condition and ionic strength affect the activity of chitosan as well. The antimicrobial activity of chitosan is stronger at acidic pH than that at pH above 6 because of the good solubility of nisin at low pH (Chang et al. Citation2015; Liu et al. Citation2004). Besides, a positive charge of chitosan is beneficial to its antimicrobial performance, since more positive charge promote the interaction between chitosan and the negatively charged bacteria. Since the pKa of chitosan is about 6, the amino groups on the chitosan carry positive charges when the pH is below 6, and it does not carry positive charges at alkaline pH, which may also explain the low antimicrobial activity of chitosan at high pH (Wicken and Knox Citation1983). This observation has been reported by many previous studies before the years 2012 and will not be discussed in detail in this review (Chung et al. Citation2003; Devlieghere, Vermeulen, and Debevere Citation2004; Li et al. Citation2008). The activity of chitosan was also affected by the presence of metal ions due to several mechanisms that lead to either decreased or increased effect of chitosan activity. The reason for the diminished antimicrobial activity of chitosan with the addition of NaCl can be explained by the fact that NaCl interferes with the electrostatic forces between chitosan and the surface of the microorganisms. On one hand, the neutralization between the positive charges of chitosan and the negative charge of Cl- ions occurs, while on the other hand, the Na+ ions can compete with chitosan for the negative charges on the cell surface (Devlieghere, Vermeulen, and Debevere Citation2004). This interference reduces the possibility of chitosan interacting with the microorganisms, thereby reducing its antimicrobial activity. Another reason that can charge for the decreased activity can be explained by the chelation of chitosan with the metal ions (Bassi, Prasher, and Simpson Citation1999). When more functional groups of chitosan (free amino group) were chelated with metal ions, this resulted in a greater reduction in the antimicrobial activity of chitosan (Chung et al. Citation2003). Whereas in some cases, the activity of chitosan can be enhanced by the presence of NaCl (Chung et al. Citation2003; Li et al. Citation2008), which may be attributed that the solubility of chitosan increased with the ionic strength since chitosan with a degree of deacetylation (DD) 90% acts as an electrolyte at pH 5.4. The increased solubility of chitosan in the presence of NaCl may increase the antimicrobial activity of chitosan (Chung et al. Citation2003).
One should take note that the impact of pH on the activity of antimicrobial compounds can be intricate, depending on the type of food. On one hand, pH of a fresh food can decrease during storage due to the microbial growth, enzymatic activity, and chemical reactions such as the formation of organic acids caused by the reaction between ascorbic acid (vitamin C) and sugars, etc.al (Sadler and Murphy Citation2010). On the other hand, the pH of some highly complex fermented foods with significant biochemical variations, such as certain types of blue cheese, can undergo dramatic shifts from acidic to basic during production and ripening (Bansal and Veena Citation2022; Cantor et al. Citation2017). Consequently, there is a requirement for the optimization of "smart dynamic systems" of antimicrobial agents, including controlled release techniques using microencapsulated antimicrobial agents. For example, Zhang et al. (Citation2023) developed a pH-triggered control release antibacterial packaging of Eudragit L100 polymer combined with cinnamon essential oil (CEO) using coaxial electrospinning. The manufactured film rapidly dissolved and changed from a solid to a liquid state, which led to a higher release rate of CEO from 68.9% to 98.2% as the pH increased. This pH-responsive film effectively prolonged the shelf life of griskin by three days. This “smart dynamic systems” can also aid in optimizing durability of active ingredients in packaging, which helps in preserving other kinds of fermented food susceptible to an increase in pH during storage, like Roquefort or Gorgonzola.
Apart from the factors that have been discussed earlier, water activity (aw) is another crucial factor to consider. The water activity refers to the amount of available water in the food that is available to support microbial growth and chemical reactions. Foods with high water activity (aw>0.85) provide favorable conditions for the growth of spoilage and pathogenic microorganisms. The presence of these microorganisms can lead to food spoilage, and in some cases, foodborne illness. For example, bacteria such as Staphylococcus aureus and Salmonella can grow rapidly in high aw foods like meat, poultry, and dairy products, leading to food poisoning. In contrast, low water activity (aw < 0.6) can inhibit microbial growth and prevent spoilage. This is why dried foods such as jerky and dried fruits have a longer shelf-life than fresh foods (Chitrakar, Zhang, and Adhikari Citation2019). However, the too low water activity of a food can lead to a reduced shelf-life and quality for some certain types of food products. Low water activity can also cause chemical changes, such as lipid oxidation, which can lead to off-flavors and odors (Jia et al. Citation2021). Besides, it is important to note that some microorganisms are capable of surviving and growing at low water activity levels. For example, the bacterium that causes botulism can grow in low aw foods like honey and canned vegetables (Benevenia et al. Citation2022). Therefore, it is important to consider other factors such as pH, temperature, and oxygen availability when assessing the risk of microbial growth in food. Overall, controlling water activity is a critical factor in preventing microbial growth and ensuring food safety during storage. Food manufacturers and processors use various methods to control water activity levels, such as drying, salting, and adding preservatives, to reduce the risk of spoilage and foodborne illness (López et al. Citation2019).
4. Physical and chemical mechanisms influencing antimicrobial activity in the food matrix
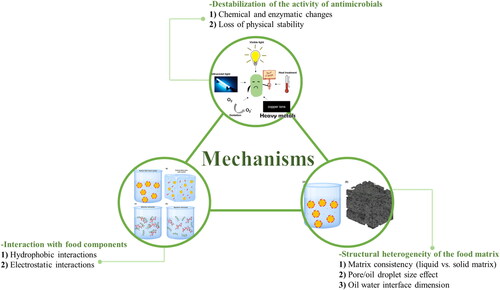
A comprehensive review of the studies from 2010 to 2022 shows a direct link between the food matrix and the antimicrobial activities of the main classes antimicrobials. From the facts presented above, it can be derived that the activities of antimicrobials are easily influenced by the coexisting food components, the interaction with food components, and the structural heterogeneity of the food matrix. The mechanisms underlying the antimicrobial activity loss in the food matrix can be summarized as shown in the following sections ().
Figure 2. The proposed mechanisms for the reduced antimicrobial activities of antimicrobial agents in the food matrix. Three main aspects, including destabilization of the activity of antimicrobials, interaction with food components, and structural heterogeneity of the food matrix, may reduce the activity of antimicrobials in the food matrix. The potential factors/interactions involved include chemical and enzymatic changes, loss of physical stability, hydrophobic and electrostatic interactions, food matrix consistency, food components droplets size, and oil-water interface dimension, etc.
4.1. Destabilization of the biological activity of antimicrobial agents
4.1.1. Chemical and enzymatic changes
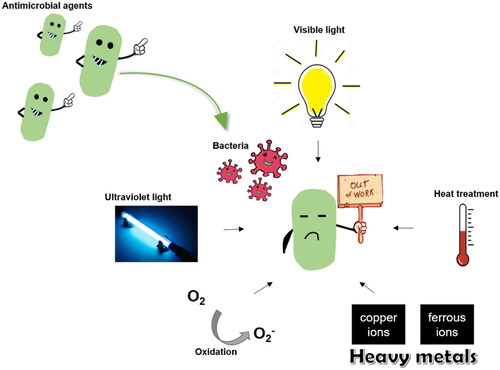
The food storage environment may induce chemical and enzymatic changes in the active compounds, damaging the chemical structure of antimicrobial compounds and reducing their biological activities (). Possible conversion reactions, including isomerization, oxidation, dehydrogenation and polymerization, may occur in essential oils triggered either enzymatically or chemically, which results in the degradation and loss of antimicrobial activity under normal storage conditions (Lu et al. Citation2018; Nanzai et al. Citation2008; Turek and Stintzing Citation2013). Possible environmental factors inducing the degradation of essential oils include light, oxygen availability, temperature and metal contaminants. Ultraviolet (UV) light and visible (Vis) light can accelerate autoxidation processes by triggering the hydrogen abstraction, leading to the formation of alkyl radicals (Choe and Min Citation2006). Turek and Stintzing (Citation2012) observed that the changes in several essential oils were promoted upon storage under the light, among which rosemary oil is especially susceptible to imitated daylight resulting in a change in its chemical composition. Another factor largely influencing the stability of essential oils is temperature. With the increase in ambient temperature from 0 to 38 °C, alterations in essential oils from cardamom, clove bud, lavender, pine, and rosemary were dominantly shown in decreasing amounts of terpenic hydrocarbons such as β-caryophyllene, β-myrcene, β-pinene, sabinene, or γ-terpinene and an increase of p‐cymene (Gopalakrishnan Citation1994; Turek and Stintzing Citation2012). Additionally, oxidation is the main reason that causes the spoilage of essential oils, and it plays a key role in maintaining the stability of essential oils. Besides, the presence of heavy metals, especially copper and ferrous ions, can promote autoxidation, and causes the destabilization of essential oils during storage (Choe and Min Citation2006).
Figure 3. Key factors, including ultraviolet light, visible light, heat treatment, heavy metals, and the presence of oxygen that can induce chemical or enzymatic changes, which in turn reduce the activities of antimicrobial agents.
Most of the antimicrobial agents comprised of peptides or proteins or enzymes are sensitive to heat treatment (Wang et al. Citation2015). The thermal stability of nisin in powders was observed differently at three different storage temperatures (−18 °C, 4 °C and 25 °C). During storage of 55 days, the nisin concentration showed a negligible reduction at −18 °C. The storage at 4 °C led to a slight reduction of the nisin concentration. While a significant decrease in the thermal stability of nisin was observed when the powder sample was stored at 25 °C (Holcapkova et al. Citation2017). When lactoferrin is added to acidic foods (pH = 3.0) that have to be subjected to heat treatments, the thermal denaturation of lactoferrin occurs at 39 °C, which may largely reduce its antimicrobial activity (Sreedhara et al. Citation2010). With regards to endolysins, their antimicrobial activities are closely associated with enzymatic function. Most endolysins are sensitive to high temperature (Obeso et al. Citation2008; Oliveira et al. Citation2016). For example, the stability of endolysin ABgp46 showed a 25% decrease after 30 min incubation at 50 °C, and a complete inactivation at 60 °C was found, compared with the nisin activity at 4 °C (Oliveira et al. Citation2016). This indicates that when antimicrobials, comprised of peptides or proteins or enzymes, are used to preserve foods, they should be added to foods not requiring heat treatment during food processing, or they can be added after heat treatment if the heat treatment is necessary.
Antimicrobial resistance (AMR) happens when microorganisms become immune to antimicrobials that previously eliminated or inhibited them and treated the infection (Founou, Founou, and Essack Citation2021). Apart from genetic mutations and the development of resistance, the diverse environmental stresses that bacteria encounter can trigger their stress responses. These responses protect them from stress and also alter their resistance to antimicrobials. The emergence of AMR can impact the physiological metabolism of bacteria. However, bacteria have the capability to regulate their own metabolism to restore sensitivity to drugs. One of the primary causes of AMR is the formation of bacterial biofilms. Biofilm formation hinders the efficacy of traditional antibiotics, resulting in recurrent and persistent infections, thereby presenting a new challenge for humans. Within biofilm bacteria, the buildup of nutrients and metabolites promotes a marked increase in the expression of genes that are associated with active compounds efflux systems, thus boosting the capacity of efflux pumps to transfer diverse compounds and leading to the emergence of a phenotype that is resistant to multiple drugs. The expression of efflux pump genes is crucial for the growth and enhancement of resistance in biofilm bacteria (Mah and O’Toole Citation2001). The presence of AMR in the food chain hinders the attainment of sustainable development goals that are connected to poverty reduction, alleviating hunger, and promoting health and well-being. The impact of AMR in the food chain goes beyond diminished productivity and food safety and can contribute to food insecurity. It can also become a burden on national and global economies, and exacerbate the issue of climate change (Lammie and Hughes Citation2016). To tackle the challenge of AMR, researchers are undertaking comprehensive investigations to identify potential solutions. These comprise developing fresh classes of antibiotics, creating targeted inhibitors for efflux pump systems, and utilizing gene editing methods (González‐Rivas et al. Citation2018). These endeavors offer crucial insights and guidance in combating bacterial resistance, holding promise for furnishing more efficacious treatment options for humans and safeguarding their health and welfare.
4.1.2. Loss of physical stability
Ionic strength and pH conditions can influence the locations of the active compounds in the food matrix by increasing the chance of physical interaction with the food components, which may influence their activities (). The biophysical properties of bioactive proteins (e.g., lysozyme, lactoferrin) are affected by several factors including pH, salt type and concentration, and presence of sugars (Broering and Bommarius Citation2005; James and McManus Citation2012; McManus et al. Citation2007; Rubin et al. Citation2010). When the pH is close to the isoelectric point, protein aggregation by self-association occurs due to the protein-protein interaction, which may influence its antimicrobial performance from the uneven distribution of antimicrobials on the surface of the microorganisms (Chi et al. Citation2003). The presence of salt also influences the physical stability of a protein antimicrobial agent by promoting protein interaction and aggregation as the salt ions can screen the electrostatic repulsion and enhance attractiveness among proteins (Lerbret et al. Citation2007). To prevent protein aggregation and increase the colloidal stability of lysozyme, glucose and sucrose can be used to enhance the electrostatic repulsion, leading to the increased physical stability of the protein (James and McManus Citation2012). Similarly, pH and ionic strength are effective in influencing the antimicrobial activities of endolysins due to the changes in their enzymatic function in environmental conditions varying pH and ionic conditions (Guo et al. Citation2017; Wu et al. Citation2018).
Table 5. The changes of physical stabilities of antimicrobial agents by varying the ionic conditions and pH levels.
Bacteriocins, such as nisin, lose their activity due to the loss of stability and solubility at high pH values and high ionic strength (Tan et al. Citation2015). At alkaline pH, the reactivity of the unsaturated amino acids can undergo a variety of addition reactions, reducing their stability (Gross Citation1977). While a low ionic strength may increase the solubility of nisin by manipulating the electrostatic interactions (Pandey, Hansmann, and Wang Citation2020). The ionic strength dependence highlights the importance of the electrostatic interactions on the stabilization of nisin in solution (Rollema et al. Citation1995).
The improved solubility of chitosan at the acidic pH was suggested as the main reason for having an enhanced antimicrobial activity of chitosan (Liu et al. Citation2004; No et al. Citation2003; Qin et al. Citation2006). At low pH, the free amino groups are protonated causing electrostatic repulsion between the polymer’s chains and thus enabling polymer solvation, leading to an increase in the permeability of the cell membrane, causing the disruption of the cell membranes and releasing the cellular contents (Helander et al. Citation2001; Szymańska and Winnicka Citation2015). In addition, the more positive charges of chitosan at low pH promote the interaction with the negative charges of bacteria, enhancing the antimicrobial activity of chitosan (Rabea et al. Citation2003).
4.2. Binding to food components
Antimicrobial agents migrate from the surface of foods to the inner part of foods, depending on the molecular properties of antimicrobial agents, the presence of food components, the physical consistency of foods, the storage environment, etc. (Savary, Moreau, and Cayot Citation2010; Wang Citation2020). During the migration, it is highly possible that the antimicrobial agent interacts with the food components. These interactions alter the path of diffusion and further influence the location of antimicrobials in the food matrix, which may cause a reduction in the antimicrobial activity of antimicrobials by hindering the interaction between antimicrobials and the microorganisms that are preferentially in the aqueous phase of foods (Juneja, Dwivedi, and Yan Citation2012; Tiwari et al. Citation2009). Two primary physical interactions (hydrophobic and electrostatic) occurring between the main classes of antimicrobials and food components are discussed in this review.
4.2.1. Hydrophobic interactions
The hydrophobic interaction is a direct result of underlying Van der Waals interactions, which is a tendency toward adhesion between the nonpolar groups of molecular/compound, causing hydrophobic moieties to aggregate or cluster (Meyer, Rosenberg, and Israelachvili Citation2006). A direct consequence of this spontaneous tendency of nonpolar groups to adhere in a system is the formation of aggregates and heterogeneous clusters. Therefore, depending on the molecular nature of the antimicrobials involved, hydrophobic antimicrobials associate and form clusters with the hydrophobic food components (e.g., fats/oils) or with the hydrophobic part of food components (e.g., proteins). Antimicrobials that are adhered to the clusters can be hindered to interact with the microorganisms that are presented in the aqueous phase of foods. Besides, these heterogeneous clusters are structural complex and can be distributed uneven, which in turn may limit the diffusion and partition of free antimicrobials that have not been interacted with the food components in foods. The reduction in the antimicrobial activities of essential oils, bioactive proteins (e.g., lysozyme, lactoferrin), enzymes (e.g., lysozyme, lactoferrin) and endolysins in a food matrix containing a high concentration of proteins is driven by these hydrophobic interactions. Similarly, non-polar molecules, such as essential oils and phenolic compounds, having a high affinity to fats/oils, are more retained in the fat/oil phase in foods, resulting in a decrease in the concentration of the antimicrobial agent in the aqueous phase of foods. The low concentration of the antimicrobial agent may not effectively inhibit the growth of microorganisms in the aqueous phase of foods, leading to the low efficacy of the antimicrobial agent.
4.2.2. Electrostatic interactions
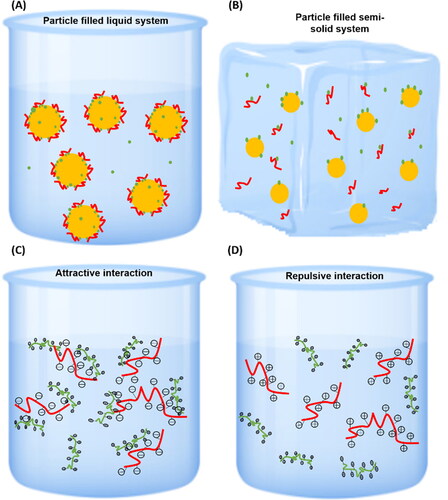
Antimicrobial agents carrying charges may have attractive or repulsive interaction with the food components when the antimicrobials are absorbed in the food matrix. The attractive or repulsive interaction can be adjusted depending on the food environment conditions (pH or ionic strength), which may alter the diffusion/partitioning of antimicrobials in foods (). In protein-rich food products, chitosan carrying positive charges is repulsed by the proteins at the pH values below the isoelectric point of proteins. However, when chitosan is added to foods at the pH above the isoelectric point of proteins, the negative charges of proteins are attracted to the chitosan, forming either soluble or insoluble clusters in the foods. As a result, the distribution of the antimicrobials is not homogeneous in the food matrix. This uneven distribution may be more clear to be seen in semi-solid/solid food products due to the lack of diffusion in these foods (). Consequently, there is no interaction between the antimicrobials and the absent microorganisms, reducing the efficacy of the antimicrobial agent. Although carbohydrates are generally considered the food components that show a lower impact on the activity of antimicrobials, compared to proteins and fats, they can influence the partitioning and location of antimicrobials in foods since their negative charges can form clusters with the positive charges of some antimicrobial agents. When some protein antimicrobials (e.g., lysozyme, lactoferrin) were involved in the food system at a certain pH whereby the antimicrobials carry negative charges, the addition of chelating agents, such as metal ions, may reduce the antimicrobial activities by the competition of antimicrobials via attractive interactions with metal ions.
Figure 4. (A, B) The hydrophobic interactions between the antimicrobial agents and the food components in liquid-based (A) and solid-based (B) foods. The yellow dots and red curves correspond to fat droplets and proteins, respectively; green particles represent the antimicrobial agents. Clusters can be easily formed between some antimicrobial agents and the fats/oils and proteins via the hydrophobic interactions, leading to the reduced interactions between the active compounds and the microorganisms in the aqueous phase of foods. (C, D) The electrostatic interactions between the antimicrobial agents and the food components via attractive forces (C) or repulsive forces (D). The red curves carrying either negative or positive charges represent food components; the green curves carrying positive charges represent the antimicrobial agents.
4.3. Effect of food structure on bioactive compounds absorption and diffusion from the food-package
Food physical states (e.g., liquid or solid) influence the activities of antimicrobials by influencing the absorption and diffusion of antimicrobial agents in food products (). The higher the absorptions in foods, the greater chance of the interactions between the antimicrobials and microorganisms. At the same environmental conditions, the movement of molecules in liquids is easier than in solids due to the large driving force of the Brownian motion in liquids than in solids (Huh and Scriven Citation1971). When the antimicrobials are absorbed in liquid foods, the absorption and diffusion are associated with the liquid rheological properties (Bylaite, Adler-Nissen, and Meyer Citation2005; Keršienė et al. Citation2008). For example, increasing the viscosity of liquid decreases the content of volatile compounds in the liquid (Costa et al. Citation2019; Lubbers et al. Citation2004). In a semi-solid/solid food, the diffusion of the antimicrobial compound is closely related to the physical (micro)structure of the food. The higher the rigidity (more compact) of foods, the more difficult it will be for the migration of antimicrobials from the surface to the internal part of foods (Boland et al. Citation2004; Munro et al. Citation2009). The transportation/diffusion of molecules in porous solid foods is mainly achieved via the pores that are formed during food processing as a result of the distribution of air volume and water droplets (Ma et al. Citation2011). The large pores acting as a passageway favor the movement of molecules in semi-solid/solid foods, contributing to a high chance for the high absorption and easy diffusion of antimicrobials in foods. In addition, the effect of the physicochemical properties of the antimicrobials, such as the molecular weight, on the diffusion of antimicrobials affected by the food components, is pronounced in semi-solid/solid foods. The molecular size of the antimicrobial compounds smaller than the average size of pores guarantees their diffusion in semi-solid/solid foods (Gombotz and Wee Citation1998; Wang et al. Citation2021a).
Figure 5. The distribution of the antimicrobial agents in liquid-based foods (A) and (semi) solid-based foods (B). The yellow dots and red curves correspond to fat droplets and proteins, respectively; green particles represent the antimicrobial agents. Active compounds are evenly distributed from the surface to the depth in the liquid-based foods (A); whereas the increased height of the solid-based foods decreased the concentrations of the active compounds (B).
Foods are highly heterogeneous in food components and food structures of various length scales. At the macro scale, the food texture determines the difficulty of the antimicrobial compounds entering the foods. At the micro scale, concentration and type of food components lead to molecular interactions and associate colloidal structures that affect the antimicrobials diffusion and ability to interact with microorganisms. At the nano level, the nature of food macronutrients is the key factor to consider in tailoring the antimicrobial compound for a food product. For example, using essential oils to preserve foods containing high content of fats is not suggested. As a rule of thumb, the more complex the structure and the more interfering food compounds there are, the easier loss of the antimicrobial efficacy would occur (Weiss, Loeffler, and Terjung Citation2015).
5. Nanotechnology strategies to improve the activities of the antimicrobial agents
5.1. Nano-scale antimicrobial materials
Nanotechnology provides numerous approaches in food fields, such as in the delivery systems, film-forming applications, functional foods design and recently also in the design of antimicrobial packaging etc. (Acevedo-Fani, Soliva-Fortuny, and Martín-Belloso Citation2017). The application of nanotechnology shows many advantages in antimicrobial packaging such as increasing resistance of active compounds to chemical and physical damage, improving the mechanical and barrier properties of the packaging polymers, protecting antimicrobial agents from the interactions with food components, controlling the release of antimicrobial agents from the nanocomposite materials, increasing loading efficacy of antimicrobial agents in the nanocomposite materials, etc. The enhanced activities of antimicrobial agents in the preservation of food products have been reported by using nanotechnologies like nanoemulsions, nanolaminate, electrospun nanofibers and nanostructured lipid carriers ().
Figure 6. Types of nanostructured delivery systems applied in food preservation. Source: Adapted from Katouzian and Jafari (Citation2016) and Subramaniam, Siddik, and Nagoor (Citation2020).
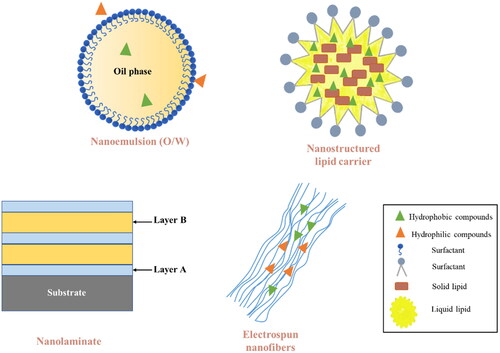
Nanoemulsions (oil-in-water systems containing oil droplets with mean diameters between 20 nm and 200 nm) show an improvement of the biological activity of lipophilic active compounds, such as EOs, by increasing the solubility and dispensability of lipophilic compounds in liquid foods or in the liquid phase of food products where microorganisms proliferate (Donsì and Ferrari Citation2016; Salvia-Trujillo et al. Citation2013). The nano-sized droplets provide a larger interfacial area and a higher Laplace pressure than bulk oil, leading to a high chance to reach the microorganisms that grow in the aqueous phase in foods and increased solubility of the lipophilic compounds (Donsì and Ferrari Citation2016; Wang et al. Citation2020a). The antimicrobial activity of the nanoemulsions of Thymus daenensis essential oil showed a stronger activity against E. coli compared to the activity of essential oil in its pure form without undergoing emulsification. This was reflected by the fact that the nanoemulsion was effective at killing the bacteria within 6 min, whereas the pure essential oil in the same amount was less effective in the inhibition performance (Moghimi et al. Citation2016).
A recent development in the dermal delivery of drugs and cosmetics can be seen from nanoemulsions to nanostructured lipid carriers (NLCs). Compared to nanoemulsions containing liquid oil droplets as the dispersed phase, NLCs consist of a core matrix made of solid and liquid lipids, surfactants, active compounds, and water (Chauhan et al. Citation2020). This technology exhibit superior advantages in protecting the active compounds from susceptible conditions such as oxidation or reduction, modulating the release of active compounds, decreasing the expulsion rate of active compounds during storage, maintaining the size and shape of the particle in the solid matrix, and increasing the loading capacity of active compounds due to the high solubility of active compounds in the core matrix (Iqbal et al. Citation2012; Nahr et al. Citation2018). NLCs have been successfully applied in pharmaceutical industries and cosmetic applications, whereas the research in the development of antimicrobial packaging using NLCs is in its early stage. Pinilla and Brandelli (Citation2016) co-encapsulated nisin and garlic extract (GE) into phosphatidylcholine nanoliposomes to prepare the nanoparticles with a mean diameter of 179 nm. High entrapment efficiencies of about 82% and 90% were obtained for nisin and GE, respectively. Both free and encapsulated nisin-GE were tested for their activities against the growth of L. monocytogenes, in full-fat milk at 7 °C. The encapsulated nisin-GE showed higher activity than the free nisin-GE, as reflected by the lower viable counts of bacteria in milk protected by encapsulated nisin-GE than that in milk preserved with free nisin-GE. This indicated that the nanoliposomes prepared with nisin and GE showed a potential to reduce or avoid the undesirable interaction with food components in food products. Another study tested the activities of cardamom essential oil (CEO) loaded nanostructured lipid carriers (CEO-NLCs) and CEO emulsion (CEO-E) against the growth of S. aureus and E. coli in broth at 37 °C (Nahr et al. Citation2018). The minimum inhibitory concentration (MIC) of CEO-NLCs was 1100 μg/ml for both bacteria after 30 days compared to MIC of 2200 μg/ml for E. coli and 4400 μg/ml for S. aureus for CEO emulsion. The lower inhibition effect of CEO-E, compared to CEO-NLCs, might be related to the stability loss during the storage of CEO-E (Nahr et al. Citation2018).
In addition to nanoemulsions and NLCs, nanolaminate is considered another promising carrier showing an enhanced antimicrobial effect of the antimicrobial compounds. It consists of two or more layers of materials deposited on a substrate through the layer-by-layer assembly technique (Acevedo-Fani, Soliva-Fortuny, and Martín-Belloso Citation2017). The layer-by-layer assembly technique can be achieved via many different approaches such as electrostatic bounding, hydrogen bonding, hydrophobic interactions, charge-transfer interactions, covalent bonding, etc. (Borges and Mano Citation2014). Nanolaminate formed by layer-by-layer assembly technique provides spaces for antimicrobial compounds to be entrapped in the film structure and can control the release of antimicrobials in response to stimulus in external environments such as temperature, pH, ionic strength, light, etc. (Moghimi et al. Citation2016). The release of the antimicrobial compounds from the nanolaminates is expected to be controlled by modulating the layer composition and the ways of film formation, leading to high efficacy of the targeted delivery to the food matrix. For example, Medeiros et al. (Citation2014) prepared the nanolaminate coating consisted of five alternate layers of alginate and lysozyme in an aminolysed/charged polyethylene terephthalate to preserve the “Coalho” cheese in 20 days. The “Coalho” cheese coated with nanolaminate showed lower values of mass loss, pH, lipidic peroxidation, microorganisms’ proliferation and higher titratable acidity in comparison with uncoated cheese. This suggested that the developed nanolaminate can be considered as a promising approach to extend the shelf life of “Coalho” cheese.
Another nanotechnology aiming to control/prolong the release of antimicrobial compounds from the polymer film to the food matrix is electrospun nanofibers. It possesses a fibrous morphology with a large surface area to volume ratio because of the high porosity and nanostructure (Neo et al. Citation2013). The use of electrospun nanofibers has shown great success in many fields, including tissue engineering, wound dressing, enzyme immobilization, and electrode materials, whereas the applications in food packaging have been largely unexplored (Ataei et al. Citation2020; El-Aassar Citation2013; Li et al. Citation2002). The moringa oi-loaded chitosan nanoparticles (MO@CNPs) were prepared and embedded in gelatin nanofibers against the L. monocytogenes and S. aureus on cheese (Lin, Gu, and Cui Citation2019). The nanofibers prolonged the release of moringa oil from the nanofibers by reducing the release rate by 38.20% at 4 °C. The reduction values of L. monocytogenes and S. aureus in cheese that were preserved with MO@CNPs nanofibers reached 78.63% and 98.67%, respectively, for 10 days of storage at 4 °C. Besides, electrospun nanofibers had a great performance in the preservation of strawberries at 4 °C (Wen et al. Citation2016). The strawberries packed with polyvinyl alcohol/cinnamon essential oil/β-cyclodextrin (PVA/CEO/β-CD) nanofibrous film showed no sign of decay on day 6, whereas the unpacked strawberries were rotten on the 4th day. The prolonged shelf-life of strawberries under the protection of PVA/CEO/β-CD nanofibrous film attributes to the sustained release from nanofibers that provided high protection to antimicrobial agents and decreased the interaction of antimicrobials with food components, indicating its potential application in the preservation of fresh foods.
The use of nanotechnology has shown great potential in enhancing the characteristics and efficacies of antimicrobial agents, however, many of the products are still at the lab scale. The effect of physical food (micro)structure on the partitioning and activity of antimicrobials loaded in nanocomposites in a food system has been unexplored. More research is required to understand the influences of the food matrix on the activities of the nano-scale antimicrobial materials. The regulation and the toxicity effect of nanomaterials in food contact applications are suggested to have more investigations (Ntim and Noonan Citation2017).
Although nanotechnology strategies have the potential to enhance the activities of antimicrobial agents, there are also some potential drawbacks to consider such as the potential toxicity concerns. For example, nanoparticles used to enhance the antimicrobial activity of agents may have toxicity concerns. Certain nanoparticles, such as silver and zinc oxide, have been found to be toxic at high concentrations. Besides, overuse of nanotechnology-enhanced antimicrobial agents could lead to the development of resistance in microorganisms, similar to the way in which conventional antibiotics can become less effective with time (Cacciatore, Brandelli, and Malheiros Citation2021). Moreover, the widespread use of nanoparticles in antimicrobial products may lead to the accumulation of these particles in the environment, which can have unknown impacts on ecosystems and organisms. Another issue to consider is the regulation of antimicrobial agents that have been enhanced by nanotechnology, which can be difficult and intricate because of the novelty of the technology and the possibility of unknown hazards. Last but not the least, the use of nanotechnology strategies to enhance the activities of antimicrobial agents may be more expensive than traditional methods, which could limit their widespread use in certain settings (Rizzello and Pompa Citation2014).
5.2. Combination of antimicrobial agents
Multiple antimicrobial agents can be incorporated together to make the best use of their antimicrobial activities to inhibit various microorganisms’ growth in foods with complex structures (Iamareerat et al. Citation2018; Wang Citation2020). A single antimicrobial agent may only be effective against a limited range of microorganisms. While the combined multiple antimicrobial compounds widens the effective inhibition range by retarding the growth of a wide range of microorganisms in foods. In addition, the multiple compounds show different behaviors based on their properties, which can inhibit the growth of microorganisms in the different locations of food for longer storage time, e.g., one antimicrobial agent with a large molecule weight can be designed for the surface of food, one is a small molecule that can diffuse into the inner part of foods, one that is fast released, and one that is slowly released, etc. (Wang Citation2020). Several combination groups, including cinnamon essential oil + sodium bentonite clay nanoparticles, and Ziziphora clinopodioides essential oil + Ficus carica extract, have been tested, and the synergistic antimicrobial activities were found from previous studies of (Khezrian and Shahbazi Citation2018); Iamareerat et al. (Citation2018). The resulting synergistic effects indicated the potential of strategies to preserve meat products like meatballs and minced camel meats. A significant endeavor was made to eliminate or decrease microbial hazards through the synergistic effect between nitrites (and nitrates) and other antimicrobial agents. For example, the inclusion of coriander essential oil (at a concentration of 0.12 μL/g) along with 60 mg/kg of nitrites effectively inhibited lipid oxidation and microbial growth, while also enhancing the redness of cooked pork sausages, during a refrigerated storage period of 52 days (Šojić et al. Citation2019). A similar synergism among flavonoid hesperidin and natural nitrate sources was observed by Martínez et al. (Citation2019) for the preservation of Spanish chorizo products.
Though many studies have proved the synergistic antimicrobial effects in combined antimicrobial agents, an enhanced inhibition effect does not always occur when one antimicrobial agent is combined with other antimicrobial agents. In a recent study, Wang et al. (Citation2021b) encapsulated the same amount of silver nanoparticles (AgNPs) into chitosan or poly (vinyl alcohol) (PVA) polymers to prepare AgNPs loaded biocomposite films to inhibit the growth of Pseudomonas fluorescens in food hydrogels. Although the efficacy of AgNPs loaded chitosan (AgNPs-CS) film was expected higher than AgNPs loaded PVA (AgNPs-PVA) film since a synergistic inhibition effect can occur in AgNPs-CS film. The experiment showed a stronger inhibition effect in AgNPs-PVA film than that in AgNPs-CS. Looking at the AgNPs diffusion in hydrogels, the silver ion concentration in the hydrogel in contact with the AgNPs/PVA composite was almost 10 times higher than that covered with the AgNPs/CS composite. This evidence fully explained the reason for the stronger antimicrobial effect on P. fluorescens of the AgNPs/PVA film, compared to the inhibitory effect of the AgNPs/CS film. This study proved that the interactions between the polymer materials and the active compounds determined the release of compounds from the polymers, which in turn determined the efficacy of antimicrobial packaging. This study highlighted the importance of considering the dosage of antimicrobial agents migrated in tested foods in the design of effective antimicrobial packaging.
5.3. Antimicrobial agents combined with processing/packaging technologies
Antimicrobial packaging with a/more combined technology/technologies (e.g., high-pressure processing, refrigeration, and modified atmosphere packaging) may show great potential for the synergistic effect that provides the enhanced activity of antimicrobial packaging. The lowered activities of antimicrobial compounds caused by the interactions with the food compounds and/or by the depletion due to the complexity of food microstructure can be compensated by inhibiting the growth of microorganisms by combined technologies that are not easily affected by the presence of food components (Wang Citation2020). A combination of antimicrobial packaging and modified atmosphere (MAP) may provide a synergistic bactericidal effect, which reduces the negative impacts of the food itself on the activity of antimicrobial compounds and shows an enhanced bactericidal effect in preserving complex foods. Cao, Warner, and Fang (Citation2019) tested the activity of nisin/gallic acid/chitosan coating and high-oxygen modified atmosphere packaging (HO MAP, 80% O2+20% CO2) and found that the combination treatment can inhibit the growth of microorganisms in fresh pork below 4.7 log cfu/g at day 20. Similarly, 27% and 17% longer shelf life was achieved in fresh chicken breast fillets when the foods were dipped in a chitosan solution (1 g/100 mL) and packed with MAP (MAP, 70% CO2, 30% N2), compared to the foods that only treated with chitosan or MAP, respectively (Latou et al. Citation2014). The combined effects of high-pressure processing (HPP) and antimicrobial packaging prepared with nisin-loaded polyvinyl alcohol (PVOH) films were evaluated against the growth of Listeria in sliced fermented sausages during the storage period of 90 days at 4 °C. The results showed that either HPP alone/nisin alone was failed to inhibit the growth of microorganisms in sausages during storage, whereas the combined technology caused a reduction of L. monocytogenes of 4.57 log CFU/g throughout storage (Marcos et al. Citation2013).
The use of combined approaches offers opportunities to decrease microbial hazards throughout the food supply chain. To comply with the "clean label" concept, it is necessary to eliminate specific food additives from recipes. Nevertheless, it is essential to maintain a high degree of safety measures to improve the product’s shelf life and, more importantly, to protect consumers against the risk of pathogen-related food poisoning.
6. Concluding remarks and future perspectives
The applications of antimicrobial agents have the potential to reduce food waste by increasing the shelf life of perishable food products. The antimicrobial efficacy of the main classes of antimicrobial agents depends on a variety of factors, one of which is the food matrix. Results showed the presence of food components, such as proteins, fats, carbohydrates, and ionic composition, influences the activities of antimicrobial agents mostly by decreasing their activities. However, sometimes an increase in their activities was found suggesting the need to optimize the combination between specific antimicrobial agents and food matrix components. The food (micro)structure has a great impact on the activity of antimicrobial agents but has not been investigated systematically, yet. The overviews of the effects of the food matrix on the activity of the main classes of antimicrobial agents were presented in – and the potential mechanisms were proposed in . Many aspects are not fully understood, especially the impacts of food (micro)structures on the efficacy of antimicrobial agents. Future research regarding the food (micro)structure story may contribute to preventing the activity loss of antimicrobials and improving their efficacies in food products. In addition, possible strategies that can protect the antimicrobial agent from interacting with the food components and losing/dissolving during diffusion into the foods were proposed in this review; however, these techniques were only tested at a specific type of food and more studies should be conducted to elucidate the general mechanisms allowing to optimize the application of antimicrobial agents to a wide range of real foods. Finally, the applications of mathematical modeling techniques in describing the main physicochemical and microbial processes involved in the food systems can be developed since it may provide an easy way to simulate and optimize the effects of the design parameters of food systems (Wang Citation2020).
Authors’ contributions
Li Wang: Conceptualization, Methodology, Investigation, Writing—original draft. Matthijs Dekker: Conceptualization, Supervision, Writing—review & editing. Jenneke Heising: Conceptualization, Supervision, Writing—review & editing. Liming Zhao: Writing—review & editing. Vincenzo Fogliano: Conceptualization, Supervision, Writing—review & editing.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Aasen, I. M., S. Markussen, T. Møretrø, T. Katla, L. Axelsson, and K. Naterstad. 2003. Interactions of the bacteriocins sakacin P and nisin with food constituents. International Journal of Food Microbiology 87 (1–2):35–43. doi: 10.1016/s0168-1605(03)00047-3.
- Abdelshafy, A. M., T. Belwal, Z. Liang, L. Wang, D. Li, Z. Luo, and L. Li. 2022. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Critical Reviews in Food Science and Nutrition 62 (22):6204–24. doi: 10.1080/10408398.2021.1898335.
- Acevedo-Fani, A., R. Soliva-Fortuny, and O. Martín-Belloso. 2017. Nanostructured emulsions and nanolaminates for delivery of active ingredients: Improving food safety and functionality. Trends in Food Science & Technology 60:12–22. doi: 10.1016/j.tifs.2016.10.027.
- Aguilera, J. M. 2019. The food matrix: Implications in processing, nutrition and health. Critical Reviews in Food Science and Nutrition 59 (22):3612–29. doi: 10.1080/10408398.2018.1502743.
- Ahmadi, H. 2017. Thermal stability of encapsulated listeria bacteriophage and its efficacy against Listeria monocytogenes in ready-to-eat meats.
- Ahmed, S., D. E. Sameen, R. Lu, R. Li, J. Dai, W. Qin, and Y. Liu. 2022. Research progress on antimicrobial materials for food packaging. Critical Reviews in Food Science and Nutrition 62 (11):3088–102. doi: 10.1080/10408398.2020.1863327.
- Ali, A., W. K. Yeoh, C. Forney, and M. W. Siddiqui. 2018. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Critical Reviews in Food Science and Nutrition 58 (15):2632–49. doi: 10.1080/10408398.2017.1339180.
- Alishahi, A., and M. Aïder. 2012. Applications of chitosan in the seafood industry and aquaculture: A review. Food and Bioprocess Technology 5 (3):817–30. doi: 10.1007/s11947-011-0664-x.
- Alshannaq, A., and J.-H. Yu. 2017. Occurrence, toxicity, and analysis of major mycotoxins in food. International Journal of Environmental Research and Public Health 14 (6):632. doi: 10.3390/ijerph14060632.
- Alves, F. C., L. N. Barbosa, B. F. Andrade, M. Albano, F. B. Furtado, A. F. M. Pereira, V. L. Rall, and A. F. Júnior. 2016. Inhibitory activities of the lantibiotic nisin combined with phenolic compounds against Staphylococcus aureus and Listeria monocytogenes in cow milk. Journal of Dairy Science 99 (3):1831–6. doi: 10.3168/jds.2015-10025.
- Armengol, E. S., M. Harmanci, and F. Laffleur. 2021. Current strategies to determine antifungal and antimicrobial activity of natural compounds. Microbiological Research 252:126867. doi: 10.1016/j.micres.2021.126867.
- Asioli, D., J. Aschemann-Witzel, V. Caputo, R. Vecchio, A. Annunziata, T. Næs, and P. Varela. 2017. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Research International (Ottawa, ON) 99 (Pt 1):58–71. doi: 10.1016/j.foodres.2017.07.022.
- Ataei, S., P. Azari, A. Hassan, B. Pingguan-Murphy, R. Yahya, and F. Muhamad. 2020. Essential oils-loaded electrospun biopolymers: A future perspective for active food packaging. Advances in Polymer Technology 2020:1–21. doi: 10.1155/2020/9040535.
- Ausar, S. F., N. Passalacqua, L. F. Castagna, I. D. Bianco, and D. M. Beltramo. 2002. Growth of milk fermentative bacteria in the presence of chitosan for potential use in cheese making. International Dairy Journal 12 (11):899–906. doi: 10.1016/S0958-6946(02)00114-0.
- Aymerich, T., M. Garriga, J. Ylla, J. Vallier, J. Monfort, and M. Hugas. 2000. Application of enterocins as biopreservatives against Listeria innocua in meat products. Journal of Food Protection 63 (6):721–6. doi: 10.4315/0362-028x-63.6.721.
- Aziz, M., and S. Karboune. 2018. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Critical Reviews in Food Science and Nutrition 58 (3):486–511. doi: 10.1080/10408398.2016.1194256.
- Bagley, C. 2014. Potential role of synthetic antimicrobial peptides in animal health to combat growing concerns of antibiotic resistance-a review. Wyno Academic Journal of Agricultural Sciences 2:19–28.
- Bahar, A. A., and D. Ren. 2013. Antimicrobial peptides. Pharmaceuticals (Basel, Switzerland) 6 (12):1543–75. doi: 10.3390/ph6121543.
- Bahrami, R., R. Zibaei, Z. Hashami, S. Hasanvand, F. Garavand, M. Rouhi, S. M. Jafari, and R. Mohammadi. 2022. Modification and improvement of biodegradable packaging films by cold plasma; a critical review. Critical Reviews in Food Science and Nutrition 62 (7):1936–50. doi: 10.1080/10408398.2020.1848790.
- Bai, J., E. Yang, P.-S. Chang, and S. Ryu. 2019. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzyme and Microbial Technology 128:40–8. doi: 10.1016/j.enzmictec.2019.05.006.
- Bansal, V., and N. Veena. 2022. Understanding the role of pH in cheese manufacturing: General aspects of cheese quality and safety. Journal of Food Science and Technology 1–11. doi: 10.1007/s13197-022-05631-w.
- Bassi, R., S. Prasher, and B. Simpson. 1999. Effects of organic acids on the adsorption of heavy metal ions by chitosan flakes. Journal of Environmental Science & Health Part A 34 (2):289–94. doi: 10.1080/10934529909376836.
- Benevenia, R., S. Arnaboldi, E. Dalzini, S. Todeschi, L. Bornati, F. Saetti, M. Ferrari, G. Varisco, G. Finazzi, and M.-N. Losio. 2022. Foodborne botulism survey in Northern Italy from 2013 to 2020: Emerging risk or stable situation? Food Control. 132:108520. doi: 10.1016/j.foodcont.2021.108520.
- Bhat, Z. F., J. D. Morton, S. L. Mason, and A. E.-D. A. Bekhit. 2019. Current and future prospects for the use of pulsed electric field in the meat industry. Critical Reviews in Food Science and Nutrition 59 (10):1660–74. doi: 10.1080/10408398.2018.1425825.
- Bhatti, M., A. Veeramachaneni, and L. A. Shelef. 2004. Factors affecting the antilisterial effects of nisin in milk. International Journal of Food Microbiology 97 (2):215–9. doi: 10.1016/j.ijfoodmicro.2004.06.010.
- Boland, A. B., K. Buhr, P. Giannouli, and S. M. van Ruth. 2004. Influence of gelatin, starch, pectin and artificial saliva on the release of 11 flavour compounds from model gel systems. Food Chemistry 86 (3):401–11. doi: 10.1016/j.foodchem.2003.09.015.
- Borges, J., and J. F. Mano. 2014. Molecular interactions driving the layer-by-layer assembly of multilayers. Chemical Reviews 114 (18):8883–942. doi: 10.1021/cr400531v.
- Broering, J. M., and A. S. Bommarius. 2005. Evaluation of Hofmeister effects on the kinetic stability of proteins. The Journal of Physical Chemistry B 109 (43):20612–9. doi: 10.1021/jp053618+.
- Burnett-Boothroyd, S., and B. McCarthy. 2011. Antimicrobial treatments of textiles for hygiene and infection control applications: An industrial perspective. In Textiles for hygiene and infection control, 196–209. Woodhead Publishing. Elsevier.
- Bylaite, E., J. Adler-Nissen, and A. S. Meyer. 2005. Effect of xanthan on flavor release from thickened viscous food model systems. Journal of Agricultural and Food Chemistry 53 (9):3577–83. doi: 10.1021/jf048111v.
- Cacciatore, F. A., A. Brandelli, and P. Malheiros. 2021. Combining natural antimicrobials and nanotechnology for disinfecting food surfaces and control microbial biofilm formation. Critical Reviews in Food Science and Nutrition 61 (22):3771–82. doi: 10.1080/10408398.2020.1806782.
- Camci-Unal, G., P. Zorlutuna, and A. Khademhosseini. 2013. Fabrication of microscale hydrogels for tissue engineering applications. In Biofabrication, 59–80. William Andrew Publishing. Elsevier.
- Cantor, M. D., T. van den Tempel, T. K. Hansen, and Y. Ardö. 2017. Blue cheese. In Cheese, 929–54. Academic Press. Elsevier.
- Cao-Hoang, L., A. Chaine, L. Grégoire, and Y. Waché. 2010. Potential of nisin-incorporated sodium caseinate films to control Listeria in artificially contaminated cheese. Food Microbiology 27 (7):940–4. doi: 10.1016/j.fm.2010.05.025.
- Cao, Y., R. D. Warner, and Z. Fang. 2019. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 101:9–16. doi: 10.1016/j.foodcont.2019.02.013.
- Capuano, E., T. Oliviero, and M. A. van Boekel. 2018. Modeling food matrix effects on chemical reactivity: Challenges and perspectives. Critical Reviews in Food Science and Nutrition 58 (16):2814–28. doi: 10.1080/10408398.2017.1342595.
- Carocho, M., M. F. Barreiro, P. Morales, and I. C. Ferreira. 2014. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Comprehensive Reviews in Food Science and Food Safety 13 (4):377–99. doi: 10.1111/1541-4337.12065.
- Castro-Rosas, J., C. R. Ferreira-Grosso, C. A. Gómez-Aldapa, E. Rangel-Vargas, M. L. Rodríguez-Marín, F. A. Guzmán-Ortiz, and R. N. Falfan-Cortes. 2017. Recent advances in microencapsulation of natural sources of antimicrobial compounds used in food-A review. Food Research International (Ottawa, ON) 102:575–87. doi: 10.1016/j.foodres.2017.09.054.
- Cava, R., E. Nowak, A. Taboada, and F. Marin-Iniesta. 2007. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. Journal of Food Protection 70 (12):2757–63. doi: 10.4315/0362-028x-70.12.2757.
- Cava-Roda, R. M., A. Taboada-Rodríguez, M. T. Valverde-Franco, and F. Marín-Iniesta. 2012. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157: H7 in milk. Food and Bioprocess Technology 5 (6):2120–31. doi: 10.1007/s11947-010-0484-4.
- Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. World Health Organization, World Health Organization, 2021.
- Chang, S.-H., H.-T. V. Lin, G.-J. Wu, and G. J. Tsai. 2015. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydrate Polymers 134:74–81. doi: 10.1016/j.carbpol.2015.07.072.
- Chang, Y., M. Kim, and S. Ryu. 2017. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiology 68:112–20. doi: 10.1016/j.fm.2017.07.004.
- Chauhan, I., M. Yasir, M. Verma, and A. P. Singh. 2020. Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Advanced Pharmaceutical Bulletin 10 (2):150–65. doi: 10.34172/apb.2020.021.
- Chi, E. Y., S. Krishnan, T. W. Randolph, and J. F. Carpenter. 2003. Physical stability of proteins in aqueous solution: Mechanism and driving forces in nonnative protein aggregation. Pharmaceutical Research 20 (9):1325–36. doi: 10.1023/a:1025771421906.
- Chitrakar, B., M. Zhang, and B. Adhikari. 2019. Dehydrated foods: Are they microbiologically safe? Critical Reviews in Food Science and Nutrition 59 (17):2734–45. doi: 10.1080/10408398.2018.1466265.
- Choe, E., and D. B. Min. 2006. Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety 5 (4):169–86. doi: 10.1111/j.1541-4337.2006.00009.x.
- Chollet, E., I. Sebti, A. Martial-Gros, and P. Degraeve. 2008. Nisin preliminary study as a potential preservative for sliced ripened cheese: NaCl, fat and enzymes influence on nisin concentration and its antimicrobial activity. Food Control. 19 (10):982–9. doi: 10.1016/j.foodcont.2007.10.005.
- Chung, Y.-C., H.-L. Wang, Y.-M. Chen, and S.-L. Li. 2003. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresource Technology 88 (3):179–84. doi: 10.1016/s0960-8524(03)00002-6.
- Cichello, S. A. 2015. Oxygen absorbers in food preservation: A review. Journal of Food Science and Technology 52 (4):1889–95. doi: 10.1007/s13197-014-1265-2.
- Costa, M. F., T. C. Pimentel, J. T. Guimaraes, C. F. Balthazar, R. S. Rocha, R. N. Cavalcanti, E. A. Esmerino, M. Q. Freitas, R. S. Raices, M. C. Silva, et al. 2019. Impact of prebiotics on the rheological characteristics and volatile compounds of Greek yogurt. LWT 105:371–6. doi: 10.1016/j.lwt.2019.02.007.
- da Silva Malheiros, P., D. J. Daroit, N. P. da Silveira, and A. Brandelli. 2010. Effect of nanovesicle-encapsulated nisin on growth of Listeria monocytogenes in milk. Food Microbiology 27 (1):175–8. doi: 10.1016/j.fm.2009.09.013.
- da Silva Malheiros, P., V. Sant’Anna, M. Utpott, and A. Brandelli. 2012. Antilisterial activity and stability of nanovesicle-encapsulated antimicrobial peptide P34 in milk. Food Control. 23 (1):42–7. doi: 10.1016/j.foodcont.2011.06.008.
- Dawson, P., G. Carl, J. Acton, and I. Han. 2002. Effect of lauric acid and nisin-impregnated soy-based films on the growth of Listeria monocytogenes on turkey bologna. Poultry Science 81 (5):721–6. doi: 10.1093/ps/81.5.721.
- De Souza, G. T., R. J. De Carvalho, J. P. De Sousa, J. F. Tavares, D. Schaffner, E. L. De Souza, and M. Magnani. 2016. Effects of the essential oil from Origanum vulgare L. on survival of pathogenic bacteria and starter lactic acid bacteria in semihard cheese broth and slurry. Journal of Food Protection 79 (2):246–52. doi: 10.4315/0362-028X.JFP-15-172.
- Devlieghere, F., A. Vermeulen, and J. Debevere. 2004. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiology 21 (6):703–14. doi: 10.1016/j.fm.2004.02.008.
- Diehnelt, C. W. 2013. Peptide array based discovery of synthetic antimicrobial peptides. Frontiers in Microbiology 4:402. doi: 10.3389/fmicb.2013.00402.
- Donsì, F., and G. Ferrari. 2016. Essential oil nanoemulsions as antimicrobial agents in food. Journal of Biotechnology 233:106–20. doi: 10.1016/j.jbiotec.2016.07.005.
- El-Aassar, M. 2013. Functionalized electrospun nanofibers from poly (AN-co-MMA) for enzyme immobilization. Journal of Molecular Catalysis B: Enzymatic 85-86:140–8. doi: 10.1016/j.molcatb.2012.09.002.
- Ercan, D., and A. Demirci. 2016. Recent advances for the production and recovery methods of lysozyme. Critical Reviews in Biotechnology 36 (6):1078–88. doi: 10.3109/07388551.2015.1084263.
- Fardet, A. 2014. Food health potential is primarily due to its matrix structure, then nutrient composition: A new paradigm for food classification according to technological processes applied. Journal of Nutritional Health & Food Engineering 1 (5):2. p. doi: 10.15406/jnhfe.2014.01.00031.
- Fernandes, J. C., F. K. Tavaria, J. C. Soares, O. S. Ramos, M. J. Monteiro, M. E. Pintado, and F. X. Malcata. 2008. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiology 25 (7):922–8. doi: 10.1016/j.fm.2008.05.003.
- Firouzi, R., S. Shekarforoush, A. Nazer, Z. Borumand, and A. Jooyandeh. 2007. Effects of essential oils of oregano and nutmeg on growth and survival of Yersinia enterocolitica and Listeria monocytogenes in barbecued chicken. Journal of Food Protection 70 (11):2626–30. doi: 10.4315/0362-028x-70.11.2626.
- Food and Administration. 2006. Part 172-food additives permitted for direct addition to food for human consumption. USA: Code of Federal Regulations.
- Founou, L. L., R. C. Founou, and S. Y. Essack. 2021. Antimicrobial resistance in the farm-to-plate continuum: More than a food safety issue. Future Science OA 7 (5):FSO692. doi: 10.2144/fsoa-2020-0189.
- García-Díez, J., J. Alheiro, A. Pinto, L. Soares, V. Falco, M. Fraqueza, and L. Patarata. 2016. Behaviour of food-borne pathogens on dry cured sausage manufactured with herbs and spices essential oils and their sensorial acceptability. Food Control. 59:262–70. doi: 10.1016/j.foodcont.2015.05.027.
- García, P., B. Martínez, L. Rodríguez, and A. Rodríguez. 2010. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. International Journal of Food Microbiology 141 (3):151–5. doi: 10.1016/j.ijfoodmicro.2010.04.029.
- Gaysinsky, S., T. M. Taylor, P. M. Davidson, B. D. Bruce, and J. Weiss. 2007. Antimicrobial efficacy of eugenol microemulsions in milk against Listeria monocytogenes and Escherichia coli O157: H7. Journal of Food Protection 70 (11):2631–7. doi: 10.4315/0362-028x-70.11.2631.
- Gharsallaoui, A., N. Oulahal, C. Joly, and P. Degraeve. 2016. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Critical Reviews in Food Science and Nutrition 56 (8):1262–74. doi: 10.1080/10408398.2013.763765.
- Gill, A. O., P. Delaquis, P. Russo, and R. Holley. 2002. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. International Journal of Food Microbiology 73 (1):83–92. doi: 10.1016/s0168-1605(01)00712-7.
- Glass, K. A., and E. A. Johnson. 2004. Antagonistic effect of fat on the antibotulinal activity of food preservatives and fatty acids. Food Microbiology 21 (6):675–82. doi: 10.1016/j.fm.2004.03.002.
- Gombotz, W. R., and S. Wee. 1998. Protein release from alginate matrices. Advanced Drug Delivery Reviews 31 (3):267–85. doi: 10.1016/s0169-409x(97)00124-5.
- González‐Rivas, F., C. Ripolles‐Avila, F. Fontecha‐Umaña, A. G. Ríos‐Castillo, and J. J. Rodríguez‐Jerez. 2018. Biofilms in the spotlight: Detection, quantification, and removal methods. Comprehensive Reviews in Food Science and Food Safety 17 (5):1261–76. doi: 10.1111/1541-4337.12378.
- Gopalakrishnan, N. 1994. Studies on the storage quality of carbon dioxide-extracted cardamom and clove bud oils. Journal of Agricultural and Food Chemistry 42 (3):796–8. doi: 10.1021/jf00039a039.
- Gross, E. 1977. α, β-Unsaturated and related amino acids in peptides and proteins. In Protein crosslinking: Nutritional and medical consequences, 131–53. Springer.
- Guo, M., C. Feng, J. Ren, X. Zhuang, Y. Zhang, Y. Zhu, K. Dong, P. He, X. Guo, and J. Qin. 2017. A novel antimicrobial endolysin, LysPA26, against Pseudomonas aeruginosa. Frontiers in Microbiology 8:293. doi: 10.3389/fmicb.2017.00293.
- Gustafsson, J., C. Cederberg, U. Sonesson, and A. Emanuelsson. 2013. The methodology of the FAO study: “Global Food Losses and Food Waste-extent, causes and prevention” - FAO, 2011. SIK Institutet för livsmedel och bioteknik.
- Gut, I. M., S. R. Blanke, and W. A. van der Donk. 2011. Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chemical Biology 6 (7):744–52. doi: 10.1021/cb1004178.
- Gutierrez, J., C. Barry-Ryan, and P. Bourke. 2008. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. International Journal of Food Microbiology 124 (1):91–7. doi: 10.1016/j.ijfoodmicro.2008.02.028.
- Gutierrez, J., C. Barry-Ryan, and P. Bourke. 2009. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiology 26 (2):142–50. doi: 10.1016/j.fm.2008.10.008.
- Gyawali, R., and S. A. Ibrahim. 2014. Natural products as antimicrobial agents. Food Control. 46:412–29. doi: 10.1016/j.foodcont.2014.05.047.
- Helander, I., E.-L. Nurmiaho-Lassila, R. Ahvenainen, J. Rhoades, and S. Roller. 2001. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. International Journal of Food Microbiology 71 (2–3):235–44. doi: 10.1016/s0168-1605(01)00609-2.
- Heo, W.-S., E.-Y. Kim, Y.-R. Kim, M. T. Hossain, and I.-S. Kong. 2012. Salt effect of nisin Z isolated from a marine fish on the growth inhibition of Streptococcus iniae, a pathogen of streptococcosis. Biotechnology Letters 34 (2):315–20. doi: 10.1007/s10529-011-0766-6.
- Holcapkova, P., Z. Kolarova Raskova, M. Hrabalikova, A. Salakova, J. Drbohlav, and V. Sedlarik. 2017. Isolation and thermal stabilization of bacteriocin nisin derived from whey for antimicrobial modifications of polymers. International Journal of Polymer Science 2017:1–7. doi: 10.1155/2017/3072582.
- Huh, C., and L. E. Scriven. 1971. Hydrodynamic model of steady movement of a solid/liquid/fluid contact line. Journal of Colloid and Interface Science 35 (1):85–101. doi: 10.1016/0021-9797(71)90188-3.
- Iamareerat, B., M. Singh, M. B. Sadiq, and A. K. Anal. 2018. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. Journal of Food Science and Technology 55 (5):1953–9. doi: 10.1007/s13197-018-3100-7.
- Ibrahim, H. R., T. Matsuzaki, and T. Aoki. 2001. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Letters 506 (1):27–32. doi: 10.1016/s0014-5793(01)02872-1.
- Iqbal, M. A., S. Md, J. K. Sahni, S. Baboota, S. Dang, and J. Ali. 2012. Nanostructured lipid carriers system: Recent advances in drug delivery. Journal of Drug Targeting 20 (10):813–30. doi: 10.3109/1061186X.2012.716845.
- James, S., and J. J. McManus. 2012. Thermal and solution stability of lysozyme in the presence of sucrose, glucose, and trehalose. The Journal of Physical Chemistry B 116 (34):10182–8. doi: 10.1021/jp303898g.
- Jayasena, D. D., and C. Jo. 2013. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends in Food Science & Technology 34 (2):96–108. doi: 10.1016/j.tifs.2013.09.002.
- Jia, W.-T., Z. Yang, X.-N. Guo, and K.-X. Zhu. 2021. Effect of superheated steam treatment on the lipid stability of whole wheat flour. Food Chemistry 363:130333. doi: 10.1016/j.foodchem.2021.130333.
- Ju, J., Y. Xie, H. Yu, Y. Guo, Y. Cheng, H. Qian, and W. Yao. 2022. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Critical Reviews in Food Science and Nutrition 62 (7):1740–51. doi: 10.1080/10408398.2020.1846494.
- Juneja, V. K., H. P. Dwivedi, and X. Yan. 2012. Novel natural food antimicrobials. Annual Review of Food Science and Technology 3:381–403. doi: 10.1146/annurev-food-022811-101241.
- Katouzian, I., and S. M. Jafari. 2016. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends in Food Science & Technology 53:34–48. doi: 10.1016/j.tifs.2016.05.002.
- Keršienė, M., A. Adams, A. Dubra, N. De Kimpe, and D. Leskauskaitė. 2008. Interactions between flavour release and rheological properties in model custard desserts: Effect of starch concentration and milk fat. Food Chemistry 108 (4):1183–91. doi: 10.1016/j.foodchem.2007.11.011.
- Khezrian, A., and Y. Shahbazi. 2018. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. International Journal of Biological Macromolecules 106:1146–58. doi: 10.1016/j.ijbiomac.2017.08.117.
- Kilcast, D., and P. Subramaniam. 2011. Food and beverage stability and shelf life. Woodhead Publishing. Elsevier.
- Kim, B.-o., E. S. Kim, Y.-J. Yoo, H.-W. Bae, I.-Y. Chung, and Y.-H. Cho. 2019. Phage-derived antibacterials: Harnessing the simplicity, plasticity, and diversity of phages. Viruses 11 (3):268. doi: 10.3390/v11030268.
- Kim, S.-J., A. R. Cho, and J. Han. 2013. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control. 29 (1):112–20. doi: 10.1016/j.foodcont.2012.05.060.
- Kuroda, K., and W. F. DeGrado. 2005. Amphiphilic polymethacrylate derivatives as antimicrobial agents. Journal of the American Chemical Society 127 (12):4128–9. doi: 10.1021/ja044205+.
- Lai, M.-J., N.-T. Lin, A. Hu, P.-C. Soo, L.-K. Chen, L.-H. Chen, and K.-C. Chang. 2011. Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. Applied Microbiology and Biotechnology 90 (2):529–39. doi: 10.1007/s00253-011-3104-y.
- Lammie, S. L., and J. M. Hughes. 2016. Antimicrobial resistance, food safety, and one health: The need for convergence. Annual Review of Food Science and Technology 7:287–312. doi: 10.1146/annurev-food-041715-033251.
- Latou, E., S. Mexis, A. Badeka, S. Kontakos, and M. Kontominas. 2014. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT - Food Science and Technology 55 (1):263–8. doi: 10.1016/j.lwt.2013.09.010.
- Lerbret, A., P. Bordat, F. Affouard, A. Hedoux, Y. Guinet, and M. Descamps. 2007. How do trehalose, maltose, and sucrose influence some structural and dynamical properties of lysozyme? Insight from molecular dynamics simulations. The Journal of Physical Chemistry B 111 (31):9410–20. doi: 10.1021/jp071946z.
- Li, B., X. Wang, R. Chen, W. Huangfu, and G. Xie. 2008. Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohydrate Polymers 72 (2):287–92. doi: 10.1016/j.carbpol.2007.08.012.
- Li, P., X. Li, R. Saravanan, C. M. Li, and S. S. J. Leong. 2012. Antimicrobial macromolecules: Synthesis methods and future applications. RSC Advances 2 (10):4031–44. doi: 10.1039/c2ra01297a.
- Li, W. J., C. T. Laurencin, E. J. Caterson, R. S. Tuan, and F. K. Ko. 2002. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. Journal of Biomedical Materials Research 60 (4):613–21. doi: 10.1002/jbm.10167.
- Lienkamp, K., A. E. Madkour, A. Musante, C. F. Nelson, K. Nusslein, and G. N. Tew. 2008. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: A molecular construction kit approach. Journal of the American Chemical Society 130 (30):9836–43. doi: 10.1021/ja801662y.
- Lin, L., Y. Gu, and H. Cui. 2019. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packaging and Shelf Life 19:86–93. doi: 10.1016/j.fpsl.2018.12.005.
- Lipinski, B., C. Hanson, J. Lomax, L. Kitinoja, R. Waite, and T. Searchinger. 2013. Reducing food loss and waste. World Resources Institute Working Paper, 1–40.
- Liu, H., Y. Du, X. Wang, and L. Sun. 2004. Chitosan kills bacteria through cell membrane damage. International Journal of Food Microbiology 95 (2):147–55. doi: 10.1016/S0168-1605(04)00110-2.
- Liu, T.-T., and T.-S. Yang. 2012. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. International Journal of Food Microbiology 156 (1):68–75. doi: 10.1016/j.ijfoodmicro.2012.03.005.
- Liu, W., and J. N. Hansen. 1990. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Applied and Environmental Microbiology 56 (8):2551–8. doi: 10.1128/aem.56.8.2551-2558.1990.
- Liu, X., D. Jiang, and D. G. Peterson. 2014. Identification of bitter peptides in whey protein hydrolysate. Journal of Agricultural and Food Chemistry 62 (25):5719–25. doi: 10.1021/jf4019728.
- Lopes, N. A., C. M. B. Pinilla, and A. Brandelli. 2019. Antimicrobial activity of lysozyme-nisin co-encapsulated in liposomes coated with polysaccharides. Food Hydrocolloids 93:1–9. doi: 10.1016/j.foodhyd.2019.02.009.
- López, M., T. Calvo, M. Prieto, R. Múgica-Vidal, I. Muro-Fraguas, F. Alba-Elías, and A. Alvarez-Ordóñez. 2019. A review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications. Frontiers in Microbiology 10:622. doi: 10.3389/fmicb.2019.00622.
- Lu, W.-C., D.-W. Huang, C.-C. Wang, C.-H. Yeh, J.-C. Tsai, Y.-T. Huang, and P.-H. Li. 2018. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. Journal of Food and Drug Analysis 26 (1):82–9. doi: 10.1016/j.jfda.2016.12.018.
- Lubbers, S., N. Decourcelle, N. Vallet, and E. Guichard. 2004. Flavor release and rheology behavior of strawberry fatfree stirred yogurt during storage. Journal of Agricultural and Food Chemistry 52 (10):3077–82. doi: 10.1021/jf0352374.
- Ma, H., J. Cui, A. Song, and J. Hao. 2011. Fabrication of freestanding honeycomb films with through-pore structures via air/water interfacial self-assembly. Chemical Communications (Cambridge, England) 47 (4):1154–6. doi: 10.1039/c0cc02680h.
- Ma, Q., P. M. Davidson, and Q. Zhong. 2013. Antimicrobial properties of lauric arginate alone or in combination with essential oils in tryptic soy broth and 2% reduced fat milk. International Journal of Food Microbiology 166 (1):77–84. doi: 10.1016/j.ijfoodmicro.2013.06.017.
- Mah, T.-F. C., and G. A. O’Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology 9 (1):34–9. doi: 10.1016/s0966-842x(00)01913-2.
- Makkar, R. S., S. S. Cameotra, and I. M. Banat. 2011. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1 (1):5. doi: 10.1186/2191-0855-1-5.
- Mani-López, E., H. García, and A. López-Malo. 2012. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Research International 45 (2):713–21. doi: 10.1016/j.foodres.2011.04.043.
- Marcos, B., T. Aymerich, M. Garriga, and J. Arnau. 2013. Active packaging containing nisin and high pressure processing as post-processing listericidal treatments for convenience fermented sausages. Food Control. 30 (1):325–30. doi: 10.1016/j.foodcont.2012.07.019.
- Martínez-Delgado, A. A., S. Khandual, and S. J. Villanueva–Rodríguez. 2017. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chemistry 225:23–30. doi: 10.1016/j.foodchem.2016.11.092.
- Martínez, L., P. Bastida, J. Castillo, G. Ros, and G. Nieto. 2019. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label Spanish chorizo. Antioxidants 8 (6):184. doi: 10.3390/antiox8060184.
- Maruyama, S., N. A. Streletskaya, and J. Lim. 2021. Clean label: Why this ingredient but not that one? Food Quality and Preference 87:104062. doi: 10.1016/j.foodqual.2020.104062.
- Matche, R., G. Kulkarni, and B. Raj. 2006. Modification of ethylene acrylic acid film for antimicrobial activity. Journal of Applied Polymer Science 100 (4):3063–8. doi: 10.1002/app.23706.
- McLay, J. C., M. J. Kennedy, A. L. Orourke, R. M. Elliot, and R. S. Simmonds. 2002. Inhibition of bacterial foodborne pathogens by the lactoperoxidase system in combination with monolaurin. International Journal of Food Microbiology 73 (1):1–9. doi: 10.1016/s0168-1605(01)00698-5.
- McManus, J. J., A. Lomakin, O. Ogun, A. Pande, M. Basan, J. Pande, and G. B. Benedek. 2007. Altered phase diagram due to a single point mutation in human γD-crystallin. Proceedings of the National Academy of Sciences of the United States of America 104 (43):16856–61. doi: 10.1073/pnas.0707412104.
- Medeiros, B., M. P. Souza, A. C. Pinheiro, A. I. Bourbon, M. A. Cerqueira, A. A. Vicente, and M. G. Carneiro-da-Cunha. 2014. Physical characterisation of an alginate/lysozyme nano-laminate coating and its evaluation on ‘Coalho’ cheese shelf life. Food and Bioprocess Technology 7 (4):1088–98. doi: 10.1007/s11947-013-1097-5.
- Meyer, E. E., K. J. Rosenberg, and J. Israelachvili. 2006. Recent progress in understanding hydrophobic interactions. Proceedings of the National Academy of Sciences of the United States of America 103 (43):15739–46. doi: 10.1073/pnas.0606422103.
- Mirski, T., M. Lidia, A. Nakonieczna, and R. Gryko. 2019. Bacteriophages, phage endolysins and antimicrobial peptides-the possibilities for their common use to combat infections and in the design of new drugs. Annals of Agricultural and Environmental Medicine: AAEM 26 (2):203–9. doi: 10.26444/aaem/105390.
- Misiou, O., T. J. van Nassau, C. A. Lenz, and R. F. Vogel. 2018. The preservation of Listeria-critical foods by a combination of endolysin and high hydrostatic pressure. International Journal of Food Microbiology 266:355–62. doi: 10.1016/j.ijfoodmicro.2017.10.004.
- Moghimi, R., L. Ghaderi, H. Rafati, A. Aliahmadi, and D. J. Mcclements. 2016. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chemistry 194:410–5. doi: 10.1016/j.foodchem.2015.07.139.
- Munro, I. C., L. A. Haighton, B. S. Lynch, and S. Tafazoli. 2009. Technological challenges of addressing new and more complex migrating products from novel food packaging materials. Food Additives & Contaminants, Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 26 (12):1534–46. doi: 10.1080/02652030902995277.
- Nahr, F. K., B. Ghanbarzadeh, H. Hamishehkar, and H. S. Kafil. 2018. Food grade nanostructured lipid carrier for cardamom essential oil: Preparation, characterization and antimicrobial activity. Journal of Functional Foods 40:1–8. doi: 10.1016/j.jff.2017.09.028.
- Naidu, A. 2000. Natural food antimicrobial systems. CRC Press. Taylor & Francis.
- Nanzai, B., K. Okitsu, N. Takenaka, H. Bandow, and Y. Maeda. 2008. Sonochemical degradation of various monocyclic aromatic compounds: Relation between hydrophobicities of organic compounds and the decomposition rates. Ultrasonics Sonochemistry 15 (4):478–83. doi: 10.1016/j.ultsonch.2007.06.010.
- Neo, Y. P., S. Swift, S. Ray, M. Gizdavic-Nikolaidis, J. Jin, and C. O. Perera. 2013. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chemistry 141 (3):3192–200. doi: 10.1016/j.foodchem.2013.06.018.
- Nerin, C., R. Becerril, S. Manso, and F. Silva. 2016. Ethyl lauroyl arginate (LAE): Antimicrobial activity and applications in food systems. In Antimicrobial food packaging, 305–12. Academic Press. Elsevier.
- Newman, J., D. O’Riordan, J. Jacquier, and M. O’Sullivan. 2015. Masking of bitterness in dairy protein hydrolysates: Comparison of an electronic tongue and a trained sensory panel as means of directing the masking strategy. LWT - Food Science and Technology 63 (1):751–7. doi: 10.1016/j.lwt.2015.03.019.
- No, H., S. P. Meyers, W. Prinyawiwatkul, and Z. Xu. 2007. Applications of chitosan for improvement of quality and shelf life of foods: A review. Journal of Food Science 72 (5):R87–100. doi: 10.1111/j.1750-3841.2007.00383.x.
- No, H. K., S. H. Lee, N. Y. Park, and S. P. Meyers. 2003. Comparison of physicochemical, binding, and antibacterial properties of chitosans prepared without and with deproteinization process. Journal of Agricultural and Food Chemistry 51 (26):7659–63. doi: 10.1021/jf030226w.
- Ntim, S. A., and G. O. Noonan. 2017. Chapter 7: Nanotechnology in food packaging. Royal Society of Chemistry.
- Obeso, J. M., B. Martínez, A. Rodríguez, and P. García. 2008. Lytic activity of the recombinant staphylococcal bacteriophage ΦH5 endolysin active against Staphylococcus aureus in milk. International Journal of Food Microbiology 128 (2):212–8. doi: 10.1016/j.ijfoodmicro.2008.08.010.
- Oliveira, H., D. Vilas Boas, S. Mesnage, L. D. Kluskens, R. Lavigne, S. Sillankorva, F. Secundo, and J. Azeredo. 2016. Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-gram-negative bacterial activity. Frontiers in Microbiology 7:208. doi: 10.3389/fmicb.2016.00208.
- Oussalah, M., S. Caillet, and M. Lacroix. 2006. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. Journal of Food Protection 69 (5):1046–55. doi: 10.4315/0362-028x-69.5.1046.
- Oussalah, M., S. Caillet, S. Salmieri, L. Saucier, and M. Lacroix. 2007. Antimicrobial effects of alginate-based films containing essential oils on Listeria monocytogenes and Salmonella typhimurium present in bologna and ham. Journal of Food Protection 70 (4):901–8. doi: 10.4315/0362-028x-70.4.901.
- Özogul, F., and I. Hamed. 2018. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Critical Reviews in Food Science and Nutrition 58 (10):1660–70. doi: 10.1080/10408398.2016.1277972.
- Pandey, P., U. H. Hansmann, and F. Wang. 2020. Altering the solubility of the antibiotic candidate nisin—A computational study. ACS Omega 5 (38):24854–63. doi: 10.1021/acsomega.0c03594.
- Parliament and Union. 2008. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Official Journal of the European Union 354:16–33.
- Pasquet, J., Y. Chevalier, J. Pelletier, E. Couval, D. Bouvier, and M.-A. Bolzinger. 2014. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids and Surfaces A: Physicochemical and Engineering Aspects 457:263–74. doi: 10.1016/j.colsurfa.2014.05.057.
- Perricone, M., E. Arace, M. R. Corbo, M. Sinigaglia, and A. Bevilacqua. 2015. Bioactivity of essential oils: A review on their interaction with food components. Frontiers in Microbiology 6:76. doi: 10.3389/fmicb.2015.00076.
- Petruzzi, L., M. R. Corbo, M. Sinigaglia, and A. Bevilacqua. 2017. Microbial spoilage of foods: Fundamentals. In the microbiological quality of food, 1–21. Woodhead Publishing. Elsevier.
- Pinilla, C. M. B., and A. Brandelli. 2016. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innovative Food Science & Emerging Technologies 36:287–93. doi: 10.1016/j.ifset.2016.07.017.
- Pol, I. E., H. C. Mastwujk, R. A. Slump, M. E. Popa, and E. J. Smid. 2001. Influence of food matrix on inactivation of Bacillus cereus by combinations of nisin, pulsed electric field treatment, and carvacrol. Journal of Food Protection 64 (7):1012–8. doi: 10.4315/0362-028x-64.7.1012.
- Qin, C., H. Li, Q. Xiao, Y. Liu, J. Zhu, and Y. Du. 2006. Water-solubility of chitosan and its antimicrobial activity. Carbohydrate Polymers 63 (3):367–74. doi: 10.1016/j.carbpol.2005.09.023.
- Raafat, D., and H. G. Sahl. 2009. Chitosan and its antimicrobial potential – A critical literature survey. Microbial Biotechnology 2 (2):186–201. doi: 10.1111/j.1751-7915.2008.00080.x.
- Rabea, E. I., M. E.-T. Badawy, C. V. Stevens, G. Smagghe, and W. Steurbaut. 2003. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 4 (6):1457–65. doi: 10.1021/bm034130m.
- Rao, M. A. 2010. Rheology of fluid and semisolid foods: Principles and applications. Springer Science & Business Media. Springer.
- Rattanachaikunsopon, P., and P. Phumkhachorn. 2010. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. Journal of Bioscience and Bioengineering 110 (5):614–9. doi: 10.1016/j.jbiosc.2010.06.010.
- Ricke, S. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science 82 (4):632–9. doi: 10.1093/ps/82.4.632.
- Rizzello, L., and P. P. Pompa. 2014. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chemical Society Reviews 43 (5):1501–18. doi: 10.1039/c3cs60218d.
- Rodrıguez, J., M. Martınez, N. Horn, and H. Dodd. 2003. Heterologous production of bacteriocins by lactic acid bacteria. International Journal of Food Microbiology 80 (2):101–16.
- Rollema, H. S., O. P. Kuipers, P. Both, W. M. De Vos, and R. J. Siezen. 1995. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Applied and Environmental Microbiology 61 (8):2873–8. doi: 10.1128/aem.61.8.2873-2878.1995.
- Rubin, J., A. San Miguel, A. S. Bommarius, and S. H. Behrens. 2010. Correlating aggregation kinetics and stationary diffusion in protein − sodium salt systems observed with dynamic light scattering. The Journal of Physical Chemistry B 114 (12):4383–7. doi: 10.1021/jp912126w.
- Sadler, G. D., and P. A. Murphy. 2010. pH and titratable acidity. Food Analysis 4:219–38.
- Salvia-Trujillo, L., M. A. Rojas-Graü, R. Soliva-Fortuny, and O. Martín-Belloso. 2013. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocolloids. 30 (1):401–7. doi: 10.1016/j.foodhyd.2012.07.004.
- Savary, G., C. Moreau, and N. Cayot. 2010. Impact of the composition of polysaccharide composite gels on small molecules diffusion: A rheological and NMR study. Food Research International 43 (1):364–8. doi: 10.1016/j.foodres.2009.10.017.
- Schmelcher, M., A. M. Powell, M. J. Camp, C. S. Pohl, and D. M. Donovan. 2015. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Applied Microbiology and Biotechnology 99 (20):8475–86. doi: 10.1007/s00253-015-6579-0.
- Schmidt, S. E., G. Holub, J. M. Sturino, and T. M. Taylor. 2009. Suppression of Listeria monocytogenes Scott A in fluid milk by free and liposome-entrapped nisin. Probiotics and Antimicrobial Proteins 1 (2):152–8. doi: 10.1007/s12602-009-9022-y.
- Shannon, R., D. R. Radford, and S. Balamurugan. 2020. Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Critical Reviews in Food Science and Nutrition 60 (10):1631–40. doi: 10.1080/10408398.2019.1584874.
- Shekarforoush, S., A. Nazer, R. Firouzi, and M. Rostami. 2007. Effects of storage temperatures and essential oils of oregano and nutmeg on the growth and survival of Escherichia coli O157: H7 in barbecued chicken used in Iran. Food Control 18 (11):1428–33. doi: 10.1016/j.foodcont.2006.10.006.
- Siddiqui, M. W., A. Sharangi, J. Singh, P. K. Thakur, J. Ayala-Zavala, A. Singh, and R. Dhua. 2016. Antimicrobial properties of teas and their extracts in vitro. Critical Reviews in Food Science and Nutrition 56 (9):1428–39. doi: 10.1080/10408398.2013.769932.
- Silva-Angulo, A., S. Zanini, A. Rosenthal, D. Rodrigo, G. Klein, and A. Martínez. 2015. Combined effect of carvacrol and citral on the growth of Listeria monocytogenes and Listeria innocua and on the occurrence of damaged cells. Food Control 53:156–62. doi: 10.1016/j.foodcont.2015.01.028.
- Singh, A., R. Singh, A. Bhunia, and N. Singh. 2003. Efficacy of plant essential oils as antimicrobial agents against Listeria monocytogenes in hotdogs. LWT - Food Science and Technology 36 (8):787–94. doi: 10.1016/S0023-6438(03)00112-9.
- Singh, J., A. Dartois, and L. Kaur. 2010. Starch digestibility in food matrix: A review. Trends in Food Science & Technology 21 (4):168–80. doi: 10.1016/j.tifs.2009.12.001.
- Siroli, L., L. Camprini, M. B. Pisano, F. Patrignani, and R. Lanciotti. 2019. Volatile molecule profiles and anti-Listeria monocytogenes activity of nisin producers Lactococcus lactis strains in vegetable drinks. Frontiers in Microbiology 10:563. doi: 10.3389/fmicb.2019.00563.
- Šojić, B., B. Pavlić, P. Ikonić, V. Tomović, B. Ikonić, Z. Zeković, S. Kocić-Tanackov, M. Jokanović, S. Škaljac, and M. Ivić. 2019. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Science 157:107879. doi: 10.1016/j.meatsci.2019.107879.
- Soni, K., R. Nannapaneni, M. Schilling, and V. Jackson. 2010. Bactericidal activity of lauric arginate in milk and Queso Fresco cheese against Listeria monocytogenes cold growth. Journal of Dairy Science 93 (10):4518–25. doi: 10.3168/jds.2010-3270.
- Sreedhara, A., R. Flengsrud, V. Prakash, D. Krowarsch, T. Langsrud, P. Kaul, T. G. Devold, and G. E. Vegarud. 2010. A comparison of effects of pH on the thermal stability and conformation of caprine and bovine lactoferrin. International Dairy Journal 20 (7):487–94. doi: 10.1016/j.idairyj.2010.02.003.
- Subramaniam, B., Z. H. Siddik, and N. H. Nagoor. 2020. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. Journal of Nanoparticle Research 22 (6):1–29. doi: 10.1007/s11051-020-04848-0.
- Sultana, T., J. Rana, S. R. Chakraborty, K. K. Das, T. Rahman, and R. Noor. 2014. Microbiological analysis of common preservatives used in food items and demonstration of their in vitro anti-bacterial activity. Asian Pacific Journal of Tropical Disease 4 (6):452–6. doi: 10.1016/S2222-1808(14)60605-8.
- Svetoch, E., and N. Stern. 2010. Bacteriocins to control Campylobacter spp. in poultry—A review. Poultry Science 89 (8):1763–8. doi: 10.3382/ps.2010-00659.
- Szymańska, E., and K. Winnicka. 2015. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Marine Drugs 13 (4):1819–46. doi: 10.3390/md13041819.
- Tan, Z., J. Luo, F. Liu, Q. Zhang, and S. Jia. 2015. Effects of pH, temperature, storage time, and protective agents on Nisin Antibacterial stability. In Advances in applied biotechnology, 305–12. Springer Berlin Heidelberg. Springer.
- Tiwari, B. K., V. P. Valdramidis, C. P. O’Donnell, K. Muthukumarappan, P. Bourke, and P. J. Cullen. 2009. Application of natural antimicrobials for food preservation. Journal of Agricultural and Food Chemistry 57 (14):5987–6000. doi: 10.1021/jf900668n.
- Tserennadmid, R., M. Takó, L. Galgóczy, T. Papp, C. Vágvölgyi, L. Gerő, and J. Krisch. 2010. Antibacterial effect of essential oils and interaction with food components. Open Life Sciences 5 (5):641–8. doi: 10.2478/s11535-010-0058-5.
- Turek, C., and F. C. Stintzing. 2012. Impact of different storage conditions on the quality of selected essential oils. Food Research International 46 (1):341–53. doi: 10.1016/j.foodres.2011.12.028.
- Turek, C., and F. C. Stintzing. 2013. Stability of essential oils: A review. Comprehensive Reviews in Food Science and Food Safety 12 (1):40–53. doi: 10.1111/1541-4337.12006.
- Türkmenoğlu, A., and D. Özmen. 2021. Allergenic components, biocides, and analysis techniques of some essential oils used in food products. Journal of Food Science 86 (6):2225–41. doi: 10.1111/1750-3841.15753.
- Uhart, M., N. Maks, and S. Ravishankar. 2006. Effect of spices on growth and survival of Salmonella typhimurium DT 104 in ground beef stored at 4 and 8C. Journal of Food Safety 26 (2):115–25. doi: 10.1111/j.1745-4565.2006.00036.x.
- Van Tassell, M. L., L. A. Ibarra-Sánchez, G. P. Hoepker, and M. J. Miller. 2017. Hot topic: Antilisterial activity by endolysin PlyP100 in fresh cheese. Journal of Dairy Science 100 (4):2482–7. doi: 10.3168/jds.2016-11990.
- Wang, H., Y. She, C. Chu, H. Liu, S. Jiang, M. Sun, and S. Jiang. 2015. Preparation, antimicrobial and release behaviors of nisin-poly (vinyl alcohol)/wheat gluten/ZrO2 nanofibrous membranes. Journal of Materials Science 50 (14):5068–78. doi: 10.1007/s10853-015-9059-0.
- Wang, L. 2020. Antimicrobial efficacy of carvacrol loaded antimicrobial packaging in food systems: Influence of the food matrix. Wageningen University. Wageningen University and Research ProQuest Dissertations Publishing.
- Wang, L., M. Dekker, J. Heising, V. Fogliano, and C. C. Berton-Carabin. 2020a. Carvacrol release from PLA to a model food emulsion: Impact of oil droplet size. Food Control 114:107247. doi: 10.1016/j.foodcont.2020.107247.
- Wang, L., V. Fogliano, J. Heising, and M. Dekker. 2021a. The effect of pore size on the diffusion of volatile antimicrobials is a key factor to preserve gelled foods. Food Chemistry 351:129316. doi: 10.1016/j.foodchem.2021.129316.
- Wang, L., V. Fogliano, J. Heising, E. Meulenbroeks, and M. Dekker. 2020b. Volatile antimicrobial absorption in food gel depends on the food matrix characteristics. Food Hydrocolloids 107:105933. doi: 10.1016/j.foodhyd.2020.105933.
- Wang, L., J. Heising, V. Fogliano, and M. Dekker. 2020c. Fat content and storage conditions are key factors on the partitioning and activity of carvacrol in antimicrobial packaging. Food Packaging and Shelf Life 24:100500. doi: 10.1016/j.fpsl.2020.100500.
- Wang, L., G. Periyasami, A. Aldalbahi, and V. Fogliano. 2021b. The antimicrobial activity of silver nanoparticles biocomposite films depends on the silver ions release behaviour. Food Chemistry 359:129859. doi: 10.1016/j.foodchem.2021.129859.
- Weiss, J., M. Loeffler, and N. Terjung. 2015. The antimicrobial paradox: Why preservatives lose activity in foods. Current Opinion in Food Science 4:69–75. doi: 10.1016/j.cofs.2015.05.008.
- Wen, P., D.-H. Zhu, H. Wu, M.-H. Zong, Y.-R. Jing, and S.-Y. Han. 2016. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 59:366–76. doi: 10.1016/j.foodcont.2015.06.005.
- Wicken, A., and K. Knox. 1983. Cell surface amphiphiles of gram-positive bacteria. Toxicon 21:501–7. doi: 10.1016/0041-0101(83)90267-2.
- Winkowski, K., M. Bruno, and T. J. Montville. 1994. Correlation of bioenergetic parameters with cell death in Listeria monocytogenes cells exposed to nisin. Applied and Environmental Microbiology 60 (11):4186–8. doi: 10.1128/aem.60.11.4186-4188.1994.
- Wu, M., K. Hu, Y. Xie, Y. Liu, D. Mu, H. Guo, Z. Zhang, Y. Zhang, D. Chang, and Y. Shi. 2018. A novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Frontiers in Microbiology 9:3302. doi: 10.3389/fmicb.2018.03302.
- Yang, B., J. Wang, B. Tang, Y. Liu, C. Guo, P. Yang, T. Yu, R. Li, J. Zhao, L. Zhang, et al. 2011. Characterization of bioactive recombinant human lysozyme expressed in milk of cloned transgenic cattle. PLoS One 6 (3):e17593. doi: 10.1371/journal.pone.0017593.
- Ye, M., H. Neetoo, and H. Chen. 2008. Control of Listeria monocytogenes on ham steaks by antimicrobials incorporated into chitosan-coated plastic films. Food Microbiology 25 (2):260–8. doi: 10.1016/j.fm.2007.10.014.
- Yingyuad, S., S. Ruamsin, D. Reekprkhon, S. Douglas, S. Pongamphai, and U. Siripatrawan. 2006. Effect of chitosan coating and vacuum packaging on the quality of refrigerated grilled pork. Packaging Technology and Science 19 (3):149–57. doi: 10.1002/pts.717.
- Yousefi, M., A. Ehsani, and S. M. Jafari. 2019. Lipid-based nano delivery of antimicrobials to control food-borne bacteria. Advances in Colloid and Interface Science 270:263–77. doi: 10.1016/j.cis.2019.07.005.
- World health statistics. 2015. World Health Organization, World Health Organization, 2015.
- Zacharof, M., and R. Lovitt. 2012. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia 2:50–6. doi: 10.1016/j.apcbee.2012.06.010.
- Zajac, J., A. Schubert, and C. Oelkrug. 2017. Antimicrobial Agents.
- Zhang, J., J. Zhang, X. Huang, J. Shi, A. Muhammad, X. Zhai, J. Xiao, Z. Li, M. Povey, and X. Zou. 2023. Study on cinnamon essential oil release performance based on pH-triggered dynamic mechanism of active packaging for meat preservation. Food Chemistry 400:134030. doi: 10.1016/j.foodchem.2022.134030.
- Zheng, L.-Y., and J.-F. Zhu. 2003. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydrate Polymers 54 (4):527–30. doi: 10.1016/j.carbpol.2003.07.009.
