Abstract
Tree nuts and oily fruits are used as a diet complement and are highly consumed worldwide. The production and consumption of these foods have been increasing, and an enormous global market value is forecasted for 2023. Besides their high nutritional value and lipid content, they provide health benefits to fat metabolism, heart, skin, and brain. The industrial by-products of these oily foods represent promising raw materials for many industries. However, the lipidomic analysis of nuts and oily fruits is still in its early stages. State-of-the-art analytical approaches for the lipid profiling and fingerprinting of nuts and oily fruits have been developed using high-performance liquid chromatography and high-resolution mass spectrometry for the accurate identification and structural characterization at the molecular species level. It is expected to bring a new understanding of these everyday foods’ nutritional and functional value. This review comprises the oil content and lipid composition of various nuts and oily fruits, particularly those mostly consumed worldwide and having recognized beneficial health effects, biological activities associated with the lipids from different oily foodstuffs, analytical methodologies to analyze lipids in nuts and oily fruits, and the potential biotechnological applications of their industrial by-products for a lipid-based commercial valorization.
Introduction
Tree nuts and oily fruits are nutritious, tasty, fitting, and easy snacks that complement a healthy diet in the busy lifestyles of worldwide populations. They are commonly consumed as whole nuts (raw, roasted or salted) or fruits (table olives or fresh avocado). In addition, these foodstuffs are also typically consumed in the form of oil (e.g. hazelnut, walnut, and olive), flour (e.g. almond and hazelnut) and butter (e.g. walnut, peanut, and cashew). Furthermore, they are used as ingredients in various processed foods, especially in spreads, bakery, and pastry products. In 2019, the world tree nut estimated consumption reached 4.4 million tons, with almonds and walnuts representing together half of the global consumption (30% and 20%, respectively). Also, in 2019, Europe was the leading consumer (30%), followed by Asia (25%), North America (24%) and the Middle East (13%). The world production of tree nuts has progressively increased, reaching over 5.3 million tons in season 2020/2021, with almonds being were the most produced nut (ca. 31% of the global nut production), followed by walnuts (19%), pistachios (19%), cashews (16%) and hazelnuts (10%). Macadamias, pine nuts, pecan nuts and Brazil nuts represented the remaining 5% (International Nut and Dried Fruit Council Citation2021). The global market cap for nuts and seeds forecast for 2023 is 1422.1 trillion U.S. dollars, a considerable growth compared to 1301.4 billion U.S. dollars in 2018 (Shahbandeh Citation2020).
Over the last two decades, the production of oily fruits also increased significantly. The world’s avocado production increased 164.9% (Shahbandeh Citation2022) and table olives and olive oil’s production grew 141.7% (2.90 million tons in 2018/2019) and 35.8% (3.26 million tons in 2018/2019), respectively (International Olive Council, Citation2021a). The consumption of avocado, olive oil and table olives increased around 450% (USA), 29% (worldwide) and 143% (worldwide), respectively, in the same period (International Olive Council, Citation2021a; Buchholz Citation2019).
There was a perception that food sources rich in lipids, such as almonds, hazelnuts, walnuts, or olives, should not be eaten regularly due to their high-fat content. However, numerous epidemiologic and clinical studies have shown that tree nuts and oily fruits are health-promoting foods, now considered essential components of a healthy diet and key food categories in the Mediterranean diet (Widmer et al. Citation2015). In fact, in agreement with the Mediterranean pyramid guidelines, olives, nuts, and seeds should be consumed daily in moderate amounts, such as a handful (Bach-Faig et al. Citation2011). Furthermore, studies reveal an inverse relationship between the consumption of these foodstuffs and the incidence of cardiovascular diseases (CVD) and coronary heart diseases (CHD), and a positive effect on cholesterol levels, oxidative stress, inflammation, blood pressure, visceral adiposity, metabolic syndrome, insulin sensitivity, and cancer, among others (Becerra-Tomás et al. Citation2019; Fulgoni et al. Citation2013; Guasch-Ferré et al. Citation2014; Massaro et al. Citation2020; Peou et al. Citation2016; Rocha et al. Citation2020; Ros Citation2015). Additionally, although nuts are fat- and energy-dense foods, adding nuts to the usual diets does not result in weight gain. On the contrary, reports from large prospective cohorts reveal that the frequency of nut consumption and weight gain over time were inversely related (Ros Citation2015).
The health benefits of consuming oily fruits and nuts are predominantly associated with the lipid composition of these food matrices. These are rich sources of polyunsaturated fatty acids (PUFA) and or monounsaturated fatty acids (MUFA), compounds widely acclaimed for their proven positive impact on human health. C18:2n-6 and C18:3n-3, abundant PUFA in nuts and oily fruits, are essential fatty acids (FA) that the body cannot synthesize, so the diet must provide them. In addition to MUFA and PUFA, mainly esterified to triacylglycerols (TG), these matrices contain other lipids that have been associated with health benefits, such as phytosterols (sterols and stanols), tocochromanols and polar lipids (e.g. phospholipids – PL and sphingolipids – SL). Nevertheless, since most lack complete lipid profiles, information is limited regarding the bioaccessibility, bioavailability, and metabolism of tree nuts and oily fruits. Thus, further research and discussion on this topic are needed to understand the real impact of consuming these foodstuffs on human health and nutrition, gain knowledge to support their health claims and inform consumers to promote the consumption of these foodstuffs.
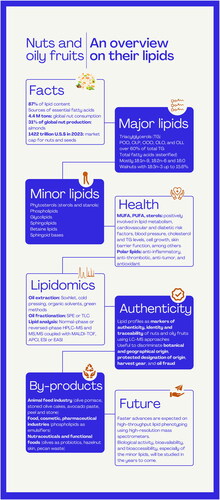
This review aims to provide an overview of the most representative lipids in tree nuts and oily fruits, from the chemical (oil content and lipid composition) to the methodological (lipidomic fingerprinting approaches) and biological (health effects) standpoints ().
Oily fruits and nuts
Olive
Olive is the fruit of the olive tree (Olea europaea L.), a subtropical evergreen plant indigenous to the Mediterranean Basin and the only edible species belonging to the Oleaceae family. This species is one of the oldest cultivated trees worldwide, with indications of its cultivation dating, at least, from the Early Bronze Age (3100–2000 BC). It is grown worldwide in Southern Africa, China, Japan, and Australia (International Olive Council Citation2021b; Liphschitz et al. Citation1991). The intercrosses between wild and domesticated O. europaea resulted in many different cultivars generating almost 1,000 recorded varieties among the immensity olive trees around the globe (e.g. Cobrançosa, Arbequina, Picual, and Leccino) (Al-Ruqaie et al. Citation2016; Luchetti Citation2002). The olive fruit is commonly divided into three distinct anatomical parts: peel, pulp, and seed. The pulp or mesocarp represents a significant proportion of the fruit (71.5–80.5% dry weight) (Bianchi and Vlahov Citation1994; Food and Agriculture Organization Citation1985). Olives are consumed as table olives and olive oil. Table olives must be processed before human consumption due to their bitter-solid flavor. The use of a particular olive variety for the production of table olives or olive oil is closely related to its chemical composition, oil content and size (Ghanbari et al. Citation2012). Some olive cultivars are more appropriate for table olives production in the existing diversity of varieties, while others are used to extract the oil, and others can serve both purposes (Ghanbari et al. Citation2012; Vinha et al. Citation2005).
Avocado
Avocado (Persea americana Mill.) is an evergreen dicotyledonous plant from the Lauraceae family. It grows in tropical or subtropical regions and has been cultivated in Central America since the pre-Columbian period. This oily fruit comprises more than 500 identified varieties (e.g. Hass, Reed, Fuerte, and Ryan), and the fruiting seasons depend on the cultivar (Yahia and Woolf Citation2011). As for the olive fruit, the avocado fruit is divided into the peel, pulp and seed, with the edible pulp representing most of the fruit (ca. 65%) (Gómez-López, Citation2002). The avocado pulp has a smooth and butter-like consistency, distinct from other fruits with acidic and or sweet tastes. There are many ways to eat avocados, and they can be added to many recipes to give meals a nutritional boost. Avocados can be eaten raw, seasoned, and stuffed in guacamole, salads, soups, smoothies, desserts, drinks, and sauces, among several others.
Nuts
Tree nuts are defined as dry fruits with commonly one seed in which the overall wall becomes hard at maturity. The most consumed tree nuts include almond (Prunus spp.), Brazil nut (Bertholletia excelsa), cashew (Anacardium occidentale), hazelnut (Corylus avellana), macadamia nut (Macadamia spp.), pecan nut (Carya illinoinensis), pine nut (Pinus spp.), pistachio (Pistacia vera), and walnut (Juglans regia). From the point of view of nutritionists and consumers, this food group also includes peanuts. Although peanuts are legumes, they present a nutrient profile like tree nuts and share beneficial health effects. Therefore, in this review, the term ‘nuts’ will comprise all standard tree nuts mentioned above, plus peanuts. Nuts can be eaten raw (dried) or roasted. Also, oil can be extracted from several nuts such as almonds, Brazil nuts, cashew nuts, hazelnuts, macadamia nuts, pine nuts, pecan nuts, pistachios, and walnuts for cooking or seasoning.
The oil content of oily fruits and nuts
Oily fruits and nuts are healthy food sources composed of lipids, water, proteins, carbohydrates, ashes, minerals, and fibers. These matrices’ chemical composition, size, and shape always vary and depend on environmental, genetic, and agronomic factors. Notwithstanding, they are excellent sources of lipids. On a dry basis, avocados have an oil content ranging from 30.3% to 69.4% (Barros et al. Citation2016; Corzzini et al. Citation2017; Reddy et al. Citation2012; Santana et al. Citation2015; Tan et al. Citation2017; Yanty et al. Citation2011) and olives between 44.3% and 86.7% (Moussaoui et al. Citation2008; Ninot et al. Citation2018; del Rio et al. 2002) (). Likewise, the lipid content in most nuts represents, on average, close to two-thirds of their dry weight (). The water content is significantly different between them, representing more than half of the fruit weight in oily fruits like olives and avocados, having little expression in the case of nuts (Bora et al. Citation2001; Boskou Citation2006; Venkatachalam and Sathe Citation2006).
Table 1. Oil content of different oily fruits and nuts.
Moreover, considerable differences in the lipid content were observed in studies that were based on different varieties from the same food source, as in almonds (Abdallah et al. Citation1998; Font i Forcada et al. Citation2015; Houmy et al. Citation2016; Kodad et al. Citation2014; Moayedi et al. Citation2011; Ojeda-Amador et al. Citation2019; Wang et al. Citation2019), avocados (Amado et al. Citation2019; Gómez-López, Citation2002; Mardigan et al. Citation2019; Takenaga et al. Citation2008; Yanty et al. Citation2011), hazelnuts (Amaral et al. Citation2006; Cittadini et al. Citation2020; Kanbur et al. Citation2013; Ojeda-Amador et al. Citation2019; Tanriverdi Citation2016; Taş and Gökmen Citation2015), macadamias (Kaijser et al. Citation2000), peanuts (Campos-Mondragón et al. Citation2009), pecan nuts (Poletto et al. Citation2020), pine nuts (Matthäus et al. Citation2018a), pistachios (Agar et al. Citation1998; Ghrab et al. Citation2010; Küçüköner and Yurt Citation2003; Okay Citation2002; Tsantili et al. Citation2010; Yıldız et al. Citation1998) and walnuts (Cittadini et al. Citation2020; Martínez et al. Citation2006). Since lipids are one of the most abundant nutrients in oily fruits and the most representative in seeds and nuts, it is essential to deepen the knowledge of the lipid profile of these food sources to understand better the positive impact of their consumption on human health and nutrition.
Lipid composition of the oily fruits and nuts
Oily fruits and nuts oils are mainly composed of TG. Thus, TG and total FA are the most representative lipid components of these matrices. Nevertheless, there are also phytosterols and polar lipids. Next, each of these four groups will be discussed in more detail.
Fatty acids
FA are aliphatic monocarboxylic acids. There are hundreds of FA with distinct configurations, chain lengths, unsaturation types, and substituents along the carbon chain (Rustan Citation2005). Complex lipids’ properties and nutritional value are closely related to their FA composition, so it is crucial to assess the FA profile.
The oils from nuts, avocados, and olives are mainly composed of oleic (18:1n-9), linoleic (18:2n-6), and palmitic (16:0) acids. Stearic (18:0), palmitoleic (16:1), and linolenic (18:3n-3) acids are also present in lower amounts (). In these foodstuffs, the FA chain lengths range between 6 and 26 carbons, with up to 6 double bonds, being oleic acid (up to 87.4%) and linoleic acid (up to 69.0%) the most abundant FA by far (). Unique and distinguished contents can be pointed out. A considerable content of FA16:1 was found in macadamia nuts (15.0-33.75%) and avocados (0.19-17.87%) (). The latter also has a different content of FA 16:0 (13.56-34.48%). It is interesting to note that, in contrast to other nuts and oily fruits, walnuts presented exceptional values of FA 14:0 (up to 14.4%), 18:2n-6 (up to 69.0%), and mainly 18:3n-3 (up to 15.6%) (). Pine nuts showed a considerable content of FA 20:0, reaching 15.8% of total FA, and were the only oily food source with a reported content of FA18:4 (0.02–0.50%) (). Minor FA were found in various nuts and oily fruits, while some were particular to a certain matrix. The FA18:4 was found in pine nuts, the FA 7:0, 9:0, 15:1, 25:0 and 26:0 were found in avocados, 22:2 and 22:3 in pistachios, and 23:0 in almonds ().
Table 2. Fatty acid profile (expressed in % of total FA) of oily fruits and nuts.
Triacyclglycerols
The major portion of the FA in nuts and oily fruits is esterified to other lipids, mainly in TG. TG have three FA esterified to a glycerol molecule, wherein the FA may be the same, two may be different, or all may be different (Scrimgeour and Harwood Citation2007). TG provide energy reserve and are a source of essential FA. The stereospecific number (sn) of the carbon atoms in the glycerol backbone is a determinant for characterizing acylglycerols (tri-, di- and monoacylglycerols). The “sn” prefix indicates the FA position in the glycerol backbone and is mentioned before the compound’s name (Cyberlipid Center Citation2021a). With some exceptions, the distribution of the FA in the TG molecule is characteristic of vegetable oils, with saturated FA occupying the sn-1 and or sn-3 positions and unsaturated FA occupying the sn-2 position (Amaral et al. Citation2006).
Overall, nuts and oily fruits have around five main TG species corresponding to over 60% of the total TG, usually bearing the FA 18:1, 18:2, and 16:0. In olives, the most abundant TG are POO, OLP, OOO, OLO, and OLL (). Letters P, O, L, and Po meaning palmitic, oleic, linoleic and palmitoleic acid, respectively. Avocados have the same TG as olives and other relevant ones like POP, POPo, and PLP (). In nuts like almonds and hazelnuts, the most abundant TG are also POO, OLP, OOO, OLO, and OLL (). In certain nuts, other TG become relevant, like POP (pistachios), SOO (e.g. cashew nuts, hazelnuts, and pistachios), OLLn (peanuts and walnuts), PLL, LnLP, LLL, LLLn, and LnLLn (walnuts) (). Moreover, compared with other nuts, walnuts showed a very distinct TG profile, where POO and OOO are not major TG, and the set PLL + LnLP + LLL + OLL + OLLn + LLLn + LnLLn could easily reach up to more than 90% of the total TG (). As mentioned above, walnuts showed remarkable contents of FA 18:2n-6 (L) and 18:3n-3 (Ln), mirrored in the high abundance of the TG species bearing these essential FA. Finally, also worth mentioning, comparing all these food sources, peanuts were the only ones where considerable amounts of TG bearing FA with longer acyl chains were reported, like FA 20:0, 20:1, and 24:0 ().
Table 3. Triacylglycerol profile (relative abundance, in %) of oily fruits and nuts.
Sterols
Plant sterols, also called phytosterols, are steroidal alcohols belonging to the group of triterpenoids and represent essential components of the membranes of all eukaryotic organisms. Phytosterols are composed of a tetracyclic cyclopenta[α]phenantrene ring, a hydroxyl group on C3 in β-stereochemistry, a flexible side chain on C17, and two methyl groups at C10 and C13 (Piironen et al. Citation2000). Structurally, depending on the number of methyl groups on C4, sterols are categorized as 4-desmethyl sterols, 4α-monomethyl sterols, and 4,4′-dimethyl sterols. The 4-desmethyl sterols, such as sitosterol and campesterol, are predominant in plants and may be classified according to the number and position of the double bond(s) in the B-ring in Δ5-sterols, Δ7-sterols, Δ5,7-sterols (Piironen et al. Citation2000). Besides 4α-monomethyl sterols and 4,4′-dimethyl sterols, stanols, fully saturated forms of sterols, are also present in lower amounts in plants (Piironen et al. Citation2000). Sterols may also be present in plants in low amount in other forms, in ascending order of polarity: sterol esters (esterified sterols), sterolglycosides (SG, sterols linked to sugars), and acylsterolglycosides (ASG, sterols linked to a sugar that can be esterified to a FA).
Small amounts of phytosterols are present in nuts and oily fruits (ca. 0.8–5.9 g Kg−1 of oil, ). As expected, the most common phytosterols in these foodstuffs are 4-desmethyl sterols, wherein β-sitosterol is the most representative, reaching up to 82.3% of the total phytosterols (). On the other hand, Δ5-avenasterol (up to 56.9 mg 100 g−1 oil in walnuts), stigmasterol (up to 60.0 mg 100 g−1 oil in pecan nuts), and campesterol (up to 32.1 mg 100 g−1 oil in peanuts) have a lower but remarkable presence in almost all the oily matrices studied (). Moreover, other relevant phytosterols can be highlighted: Δ5,24-stigmastadienol in walnuts (up to 28.2 mg 100 g−1 oil), Δ7-avenasterol in avocados (up to 13.8 mg 100 g−1 oil), cholesterol in Brazil nuts and hazelnuts (up to 18.0 mg 100 g−1 oil), cycloartenol in walnuts (27.9 mg 100 g−1 oil), ergosterol in almonds and hazelnuts (up to 26.6 mg 100 g−1 oil), lanosterol in peanuts (30.0 mg 100 g−1 oil), and sitostanol in hazelnuts and peanuts (up to 41.4 mg 100 g−1 oil) (). Even though stanols and cholesterol are usually found in trace amounts in plants, some exceptions have been observed in some nuts, such as hazelnuts, peanuts, and Brazil nuts.
Table 4. Sterol and stanol profile (mg 100 g−1 of oil) of nuts and oily fruits.
Polar lipids
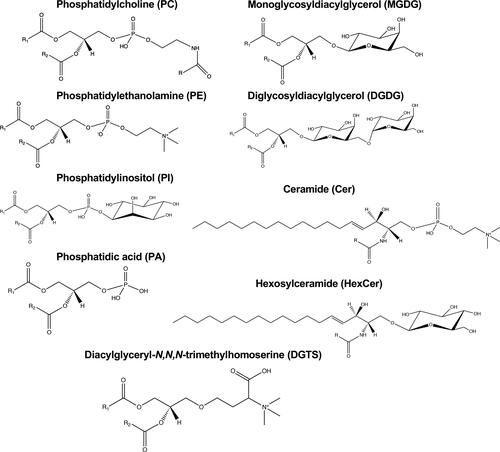
Unlike FA, TG, and sterols, polar lipids have been much less studied in nuts, oily fruits, and their oils. Polar lipids comprise many molecules and may be divided into several classes and subclasses. Depending on their structure, the polar head group is the main differentiator. In this paper, for simplicity, these minor lipids will be described considering the four groups: phospholipids (PL), glycolipids (GL), sphingolipids (SL), and betaine lipids (BL).
The PL represent a significant group of lipids in cell membranes, being structural elements forming the cells’ lipid bilayers, and are commonly found in foodstuffs, including cereals and nuts. PL may be divided into two sub-groups: glycerophospholipids (GPL), with a glycerol backbone, and sphingophospholipids (SPL), with a sphingosyl backbone (Pasini et al. Citation2013; Scrimgeour and Harwood Citation2007). GPL structures are sn-1,2-diacylglycerols with a 3-sn-phosphatidic acid linked to a head group. The polar head group (a carbohydrate, an amino acid, an amino alcohol, or another functional moiety) is attached to the phosphate residue at the sn-3 position. The SPL contain sphingosine or a related amino alcohol instead of glycerol, like sphingomyelins (Scrimgeour and Harwood Citation2007).
In nuts and oily fruit oils, PL were found in low amounts. In nuts, these minor compounds represent 0.30–3.81% (w/w) of the nuts’ total lipids, and in oily fruits, such as avocados and olives, they represent 0.7–2.1% (w/w) of the pulp’s total lipids (). Several PL classes were identified in nuts and oily fruits. The most abundant are phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylinositols (PI), phosphatidic acids (PA), and phosphatidylglycerols (PG) (). Less abundant there are lysoPL, with only one fatty acyl chain (e.g. lysoPC and lysoPE), and other PL like cardiolipins (CL), phosphatidylserines (PS), and sphingomyelins (SM) (). Worth noticing that N-acyl phosphatidylethanolamines [PE-N(FA)] and sphingoid bases (SPB) were also identified in hazelnuts ().
Table 5. Polar lipid content and composition in nuts and oily fruits, based on various studies.
SL, besides PL, also include other compounds such as ceramides and glycosphingolipids. Ceramides (Cer) consist of sphingosine or other related amino alcohol with a FA attached to the amino group. Neutral glycosphingolipids are SL with sugar residues. These sugars, either glucose or galactose, are linked to the primary alcohol of the sphingosyl moiety by an O-ester linkage. These structures are called hexosylceramides (HexCer) or cerebrosides (Scrimgeour and Harwood Citation2007). SL are a significant class of eukaryotic cell membranes’ constituents, considered building blocks of cell architecture and regulators of vital biological functions (Gangoiti et al. Citation2010).
In nuts, SL represent 0.26–0.91% (w/w) of the total lipids, and in olives, they comprise around 2.3% of the pulp’s total polar lipids (). Cer were identified in almost all nuts, comprising 149.4–189.6 pmol g−1 in almonds and 202.3-463.4 pmol g−1 in pistachios (). HexCer were reported in both nuts and oily fruits. HexCer ranged from 0.021 mg g−1 in hazelnuts to 0.068 mg g−1 in almonds on a fresh basis (). They comprised 11.4% of the avocado’s total lipids ().
GL, such as glycosylglycerides, consist of acylglycerols attached to a carbohydrate moiety by a glycosidic linkage at the sn-3 position (Scrimgeour and Harwood Citation2007). They are membrane components that influence several biological processes like cell-cell communication or signal transduction and appear in very distinct organisms, such as bacteria, plants, and animals, including humans (Ishida and Kiso Citation2008).
GL were poorly identified and quantified in oily fruits. The amount of GL reported in avocados was 2.5–3.2% (w/w) of the pulp’s total lipids (). GL represent about 25.4% of the olive pulp’s total polar lipids (). The most common GL were monoglycosyldiacylglycerols (MGDG) and diglycosyldiacylglycerols (DGDG) (). In olives, diglycosylmonoacylglycerols (DGMG) were also found (). To our knowledge, no studies have explored the GL profile in nuts.
BL are glycerolipids containing a betaine moiety linked to the sn-3 position of the glycerol backbone and FA esterified in the sn-1 and sn-2 positions (Scrimgeour and Harwood Citation2007). The most common betaine lipids are diacylglyceryl-N,N,N trimethylhomoserines (DGTS). DGTS are commonly found in lower plants, algae, marine eukaryotic phytoplankton, fungi, and bacteria (Canãvate et al. Citation2016; Cañavate et al. Citation2017; Dembitsky, Citation1996). Recently, DGTS and monoacylglyceryl-N,N,N-trimethylhomoserines (MGTS) have been reported in olives and olive oil (Alves et al. Citation2019, Citation2016). The chemical structures of the main polar lipid classes found in nuts and oily fruits are depicted in .
Health effects of neutral lipids, sterols, and polar lipids from oily fruits and nuts
The epidemiological, clinical, and experimental knowledge derived from decades of research evidenced that dietary patterns, namely the Mediterranean diet and consumption of specific foods, profoundly influence health outcomes and prevent chronic diseases like CVD. The lipids from foodstuffs have been a target of several studies investigating the biological activity of food consumption and the benefits of the Mediterranean diet. TG, FA, sterols, and polar lipids from diverse food sources have been explored as bioactive compounds (). Nevertheless, the real impact of these compounds on human health is not yet fully understood. High-level scientific proof is needed before formulating nutritional recommendations based on guidelines from evidence-based medicine and personalized medicine.
Table 6. Biological activity of fatty acids, triacylglycerols, sterols, and polar lipids from various nuts and oily fruits and other foods.
Although it is not fully clear yet how lipid compounds act on some biological processes, lipids are essential compounds involved in distinct biological functions. Each lipid class can have more than one biological function or activity. MUFA and PUFA’s bioactivities are associated with a beneficial impact on several organs like the liver, kidney, brain, and heart, and pathological conditions such as CVD, diabetes, and arthritis (Abedi and Sahari Citation2014; Choo et al. Citation2010; Covas et al. Citation2015; Enot et al. Citation2015; Fehily et al. Citation1992; Fonolla-Joya et al. Citation2016; Gill and Valivety Citation1997; Gonçalves-De-Albuquerque et al. Citation2016; Gumbiner et al. Citation1998; Hammad et al. Citation2016; Lai et al. Citation2013; Nettleton Citation1995; Perdomo et al. Citation2015; Schwingshackl and Hoffmann Citation2012; Steinmann et al. Citation2012; Wang et al. Citation2014; Wiktorowska-Owczarek et al. Citation2015; Wu et al. Citation2014; Zhao et al. Citation2013). Based on the FA content of a food or dietary pattern, dietary lipid indices can be calculated, such as the index of atherogenicity or the index of thrombogenicity, as a measure of the propensity of the diet to influence the risk of coronary heart disease. These indices consider MUFA, PUFA, and SFA ratios in their formulas (Fehily et al. Citation1992; Kalogeropoulos et al. Citation2013). The effect of designer lipids (also known as structured lipids or tailor-made fats) reveal great physiological activity and numerous benefits to the human body in comparison with plant or animal lipids. Designer lipids consist of chemically/enzymatically modified forms of common lipids which is key for increasing functionality and nutritional value of lipids by changing the composition and/or position of FA on the glycerol backbone (Jadhav and Annapure Citation2021).
Moreover, sterols have shown several positive effects as reducing total and low-density lipoprotein (LDL) serum cholesterol levels and risk of developing CVD, and positive influence on immune stimulation, and anti-ageing development, among others (Andersson et al. Citation2004; Brüll et al. Citation2010; Calpe-Berdiel et al. Citation2007; Escurriol et al. Citation2010; Esposito et al. Citation2013; Klippel et al. Citation1997; Kopylov et al. Citation2021; Pinedo et al. Citation2007; Shi et al. Citation2015; Trautwein et al. Citation2018). Finally, studies about dietary polar lipids have shown various beneficial health effects. PL have antioxidant, anti-inflammatory, antimicrobial, cancer chemoprevention, and cardioprotective activities and positively influence cognitive function, memory, and physical activity (Castro-Gómez et al. Citation2015; Hidalgo et al. Citation2005; King et al. Citation1992; Küllenberg et al. Citation2012; List and Friedrich Citation1989; Sun et al. Citation2018; Vesper et al. Citation1999). GL present anti-tumour and anti-inflammatory activity in arthritis, osteoarthritis, and cancer prevention (Akasaka et al. Citation2016; Hou et al. Citation2007; Larsen et al. Citation2003; Morimoto et al. Citation1995; Murakami et al. Citation2003; Nagatsu et al. Citation1994; Wang et al. Citation2002). SL are intrinsically involved in different organisms’ parts such as skin, plasma, colon, and brain. The evidence suggests they also have cancer prevention and antimicrobial activities (Aida et al. Citation2003; Castro-Gómez et al. Citation2015; Duivenvoorden et al. Citation2006; Gangoiti et al. Citation2010; Küllenberg et al. Citation2012; Vesper et al. Citation1999). Studies indicate that betaines have antioxidant activity and beneficial effects on lipid metabolism, glucose homeostasis/insulin resistance and influence different organs such as the brain, liver, kidneys, and heart (Airaksinen et al. Citation2018; Chai et al. Citation2013; Du et al. Citation2018; Ejaz et al. Citation2016; Ganesan et al. Citation2010; Hagar and Al Citation2014; Hoffman et al. Citation2009; Tiihonen et al. Citation2016). However, no studies have reported the biological activity and health effects of BL as far as is known ().
Methodologies to analyze lipids in oily fruits and nuts
Lipidomics
Lipidomics, a rapidly growing field of research, describes and quantifies all lipid molecular species and tries to comprehend their biological functions (O'Donnell et al. Citation2020). To ensure that lipid structural identification and quantitation are correctly performed and to compare published papers, standardized approaches and guidelines are needed, as recognized by the lipidomics research community (O'Donnell et al. Citation2020). Some guidelines provided by the Lipidomics Standards Initiative (LSI), for example, cover several analytical steps like extraction, analysis, processing, identification, quantification and data reporting. This is to improve the knowledge about lipid biology and analytical chemistry like mass spectrometry (MS) analysis, which is especially useful for new researchers in lipidomics (Lipidomics Standards Initiative Consortium Citation2019; O'Donnell et al. Citation2020).
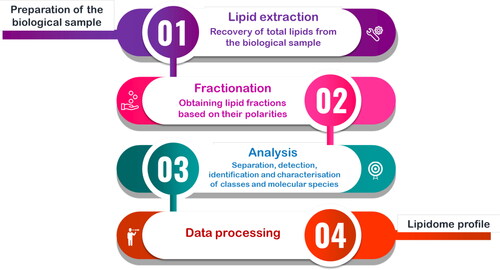
The lipidomics workflow, either for targeted or untargeted approaches, is essentially built into four steps after preparing the biological sample (). The workflow begins with total lipid extraction, followed by fractionation, data analysis and data processing (Maciel et al. Citation2018; Lipidomics Standards Initiative Consortium Citation2019). The untargeted analysis is used to identify and quantify as many lipid species as possible and, consequently, find a specific lipid signature of the biological sample. Analyzing the lipid extracts using liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS), for example, can generate a high amount of data that needs to be interpreted and validated. On the other hand, with the information acquired from untargeted strategies, it is possible to design targeted lipidomic approaches where the goal is to detect and quantify a set of specific lipids (Maciel et al. Citation2018). Although there is a certain consensus about the workflow steps, relatively to the techniques used in each step, harmony between studies reveals itself hard to achieve due to the absence of standard procedures with the current use of distinct approaches to analyze neutral, intermediate polarity or polar lipids.
Extraction
The most used method for extracting oil from oily fruits and nuts and determining the oil yield is solvent extraction with petroleum ether or n-hexane in a Soxhlet apparatus and cold pressing (). However, Soxhlet extraction uses high temperatures, which degrades bioactive compounds and promotes lipid oxidation, and cold pressing, although a simple, ecological and not much costly method, often leads to low oil yields. As such, lipid extraction in lipidomics usually consists of a solid/liquid extraction and or a liquid/liquid extraction and or a solid-phase extraction (SPE) with organic solvents. Oil extraction from oily fruits and nuts has also been done by these methods (). The most utilized methods to extract total lipids use organic solvents with distinct polarities, namely the Folch method (Folch et al. Citation1957) and the Bligh and Dyer method (Bligh and Dyer Citation1959). The former uses chloroform, or a mixture of dichloromethane/methanol (2:1, by volume). The latter uses chloroform, or a mixture of dichloromethane/methanol (1:2, by volume), and an additional volume of chloroform or dichloromethane. Lipids are recovered from the lower phase, in the organic fraction, in these methods, after adding water to the mixture and centrifuging. Although these methods are widely used, allowing higher oil yields and extractions in parallel, they have disadvantages, such as costly and high-purity solvents, toxic and flammable organic solvents, and a clean-up step to remove solvent residues from the extracts (Danlami et al. Citation2014).
Table 7. Procedures and methods for extracting and analyzing lipids in nuts and oily fruits.
Advances in lipidomics methodologies allowed cleaner and more environmentally friendly methods to avoid using chlorinated and toxic solvents (). These include the use of greener and food-grade systems, like isopropyl alcohol and ethyl alcohol (Abaide et al. Citation2017; Cornelio-Santiago et al. Citation2019; Mericli et al. Citation2017; Oliveira et al. Citation2002; Tan et al. Citation2018), combined with different mechanical or other methods to increase extraction efficiencies, such as ultrasound-assisted extraction (Tan et al. Citation2018), supercritical fluid extraction using CO2, subcritical fluid extraction using n-propane (Zanqui et al. Citation2020a; Zanqui et al. Citation2020b), and aqueous enzymatic process assisted with microwave extraction (Ma et al. Citation2022).
Fractionation of lipid extracts
Fractionation is not mandatory but may be necessary after total lipid extraction when a high purity of interest compounds is required. The sample must be concentrated if these components exist in low concentration. SPE allows fractionating lipids from the total extract or recovering different or enriched fractions from a total lipid extract. Silica cartridges are used to recover neutral lipids, intermediate polarity lipids and polar lipids or fractionate the lipid extract into neutral lipid classes (free FA, sterols, and TG) and a polar lipid-rich fraction (). These silica cartridges have been commonly used in foodstuffs like avocados, walnuts, and almonds (Angelova-Romova et al. Citation2013; Fang et al. Citation2005; Fernandes et al. Citation2017; Pacetti et al. Citation2007; Wang et al. Citation2019). More recently, SPE using alternative cartridges has shown promising results (), as aminopropyl cartridges in olives (Alves et al. Citation2019), TiO2 nanoparticles packed cartridges in almonds (Shen, Dong, Yang, Li, et al. Citation2013), and graphene/TiO2 nanocomposite packed cartridges in avocados (Shen et al. Citation2014). However, in these cases, procedures were not yet optimized for recovering the complex polar lipidome of these matrices. SPE is a crucial step in obtaining the polar lipid-rich fraction from oils or oily matrices to allow their identification and structural characterization, especially in the case of oil where polar lipids are in low abundance.
Thin-layer chromatography (TLC) can be used for the fractionation of total extracts and can also be used for monitoring the efficiency of an extraction procedure and determining the purity of the extract after a previous extraction or separation step. Acylglycerols can be fractionated using n-hexane/diethyl ether/acetic acid (80:20:1, by volume). Polar lipid classes, such as PL, can be fractionated by one-dimension TLC using a solvent system composed of chloroform/ethanol/water/triethylamine (35:30:7:35, by volume) (Cyberlipid Center Citation2021b). Spraying with a revealing agent as a solution of primuline in acetone/water and visualization using an ultraviolet lamp allows for detecting the lipid spots in the TLC plate (Cyberlipid Center Citation2021b). It is possible to identify the lipid classes by comparing their retention factors (Rf) with commercial lipid standards applied to the same plate. The relative content of each lipid class of PL or GL in the total lipid extract is also reachable after TLC separation by scrapping off the individual lipid spots and quantifying phosphorus or glucose in each spot, respectively. Additionally, the lipids from each TLC spot can be further analyzed regarding lipid class identification and molecular characterization by MS or FA analysis after recovery by scraping the spots and using proper extraction methods (Alves et al. Citation2018; Maciel et al. Citation2018). TLC has been performed for oil fractionation and further identification of lipid classes in several nuts and oily fruits ().
Analysis and identification of lipids
After SPE or TLC, the lipid fractions may be analyzed through several techniques like gas chromatography - flame ionization detector (GC-FID) or GC-MS, high-performance liquid chromatography (HPLC)-MS, direct injection (DI)-MS, nuclear magnetic resonance (NMR) spectroscopy or non-aqueous capillary electrophoresis (NACE) (Alves et al. Citation2018; Maciel et al. Citation2018).
GC can be used coupled to flame ionization detectors (GC-FID), MS analyzers (GC-MS) or both for the identification and quantification of fatty acid methyl esters (FAME) (Maciel et al. Citation2018; Pacchiarotta et al. Citation2010). One of the challenges of GC relies on the need for volatilization of the compounds on samples that contain nonvolatile compounds, such as FA, that can be achieved by derivatisation using acid or alkaline hydrolysis or transmethylation (Pacchiarotta et al. Citation2010). The transesterification of FA with methanol in alkaline conditions allowed the derivatisation into fatty acid methyl esters (FAME), (Sekora et al. Citation2009). GC-MS offers more accurate characterization than GC-FID because the identification is based on the retention time (RT) and the mass spectrum of each FAME. Nevertheless, GC-FID has been the elected method for identifying the FA profile of oily fruits and nuts oils (), most probably because GC-FID usually provides results with higher accuracy for the quantification of compounds while GC-MS gives more robust results in the identification of all compounds in a sample.
Sterols represent a significant portion of the unsaponifiable matter of oily fruits and nuts. They are predominantly in the form of free sterols, sterol esters, SG and ASG. Sterol esters, SG, and ASG, unlike free sterols, must be hydrolyzed to be analyzed by GC-MS or GC-FID as free sterol moieties. On the other hand, sterol esters, SG, and ASG can be analyzed by LC-MS without requiring the hydrolytic step. These lipids are often extracted from the oils using non-polar solvents, and then both phases (saponifiable and non-saponifiable) are subsequently separated, evaporated, and redissolved in a proper solvent. Generally, these phases may be cleaned up by TLC or column chromatography and derivatised, usually by silylation. GC-FID has been the most employed method for analyzing the sterol profile in oily fruits and nuts oils (Abdallah et al. Citation2015; Alasalvar et al. Citation2006, Citation2009; Bada et al. Citation2004; Chahed et al. Citation2008; Costa et al. Citation2010; Gao et al. Citation2018; Givianrad et al. Citation2013; Kalogeropoulos et al. Citation2013; Massimo et al. Citation2020; Miraliakbari and Shahidi, Citation2008; Phillips et al. Citation2005; Rico et al. Citation2016; Robbins et al. Citation2011; Shin et al. Citation2010; Tokusoglu, Citation2011; Yorulmaz et al. Citation2009) (). However, alternative methods such as GC–MS (Derewiaka et al. Citation2014; Gao et al. Citation2019a; Martínez et al. Citation2006; Massimo et al. Citation2020; Miraliakbari and Shahidi Citation2008; Normén et al. Citation2007; Özcan et al. Citation2020; Phillips et al. Citation2005; Shin et al. Citation2010; Veličkovska et al. Citation2016; Wang et al. Citation2019; Zanqui et al. Citation2020a, Citation2020b) and HPLC (Campos-Mondragón et al. Citation2009; Kornsteiner-Krenn, Citation2013; Maguire et al. Citation2004; Ryan et al. Citation2006; Vecka et al. Citation2019) have also been applied in this analysis ().
After TLC or SPE, the lipid classes or lipids species can be separated by HPLC, which allows for the separation of complex mixtures with multiple components. HPLC is a separation technique often coupled to different detectors, like ultraviolet detectors (HPLC–UV) or mass spectrometers (HPLC–MS). These hyphenated techniques allow for the detection and molecular characterization of TG and polar lipids or esterified glycosylated sterols. The LC coupled to electrospray ionization mass spectrometry (LC-ESI-MS) is the most popular way to analyze total lipid extracts or lipid fractions nowadays. More recently, HPLC-HRMS and HPLC-HRMS/MS have provided detailed TG (Alves et al. Citation2019; Cherif et al. Citation2014, Citation2013; Dong et al. Citation2015; Holčapek et al. Citation2005) and polar lipid (Alves et al. Citation2019; Boukhchina et al. Citation2004; Fang et al. Citation2005; Napolitano et al. Citation2018; Pacetti et al. Citation2007; Paroni et al. Citation2019; Song et al. Citation2018) profiles of several nuts and oily fruits (). In fact, on the one hand, MS allows molecular identification through the MS spectrum (full MS) and, on the other hand, the structural characterization of the individual components with high molecular specificity and detection sensitivity through the tandem MS spectra (MS/MS). MS data gives information on the polar head of polar lipids such as PL, GL and SL and the fatty acyl composition of polar lipids and TG, representing a powerful tool for lipid fingerprinting. Regarding the foodstuffs under study, other less used techniques have also been employed to characterize TG, and the polar lipidome (), such as HPLC coupled to evaporative light-scattering detection (ELSD) (Alasalvar et al. Citation2009, Citation2006; Amaral et al. Citation2004; Barreira et al. Citation2012; Holčapek et al. Citation2005; Parcerisa et al. Citation1999), photodiode array detectors (HPLC-PDA) (Taş and Gökmen Citation2015) and refractive index detectors (HPLC-RID) (Bada et al. Citation2004; Fernandes et al. Citation2017; Rabadán et al. Citation2018; Tan et al. Citation2017; Yanty et al. Citation2011), and also NACE (Montealegre et al. Citation2013), GC-FID (Gao et al. Citation2018, Citation2019a), NMR (Vlahov et al. Citation1999) and TLC (Angelova-Romova et al. Citation2013; Chunhieng et al. Citation2008; Miraliakbari and Shahidi Citation2008; Momchilova and Nikolova-Damyanova Citation2007; Takenaga et al. Citation2008; Yoshida et al. Citation2005).
Quantification
In addition to analysis and identification, the relative and absolute quantification of lipid groups, classes, and species is also possible in lipid studies. For example, TG analysis cis normally carried out by HPLC coupled to different types of detectors (). For lipids such as sterols, PL, and GL, colourimetric tests are often employed after fractionation by TLC or SPE (Angelova-Romova et al. Citation2013; Chunhieng et al. Citation2008; Takenaga et al. Citation2008). These approaches are generally used to quantify groups and or classes of lipids. On the other hand, HPLC-MS has proven to be an effective tool for the relative or absolute quantification of lipids at the molecular level in foodstuffs like oily fruits and nuts, allowing a more in-depth characterization of the lipid profile (Alves et al. Citation2019, Song et al. Citation2018, Pacetti et al. Citation2007). The relative quantification of lipid classes is determined by calculating the peak area of each ion of the respective class and the normalization per the peak area of the respective internal standard. For instance, in the lipidomic profiling of the olive fruit pulp, PC(14:0_14:0) and PE(14:0_14:0) were used as internal standards for the quantification of phosphatidylcholine and phosphatidylethanolamine lipid species, respectively (Alves et al. Citation2019). Internal standards should not be present in the sample’s lipidome. However, some limitation exists since there are not available standards for all lipid classes.
Regarding each molecular species within a lipid class, the relative abundance is obtained by dividing its peak area by the sum of the peak areas of all ions identified for that specific class. Moreover, if the objective is to quantify each molecular species in absolute terms, it will be necessary to use internal standards preferentially from the same classes but with unusual fatty acyl chains and create calibration curves (Khoury et al. Citation2018). However, the lack of standards from some lipid classes impairs the proper quantification of some lipids.
Lipidomic studies toward oily fruits and nuts’ oils authenticity and traceability
Food authenticity and traceability remain on the agenda for food safety regulatory authorities. In the case of oily fruits and nuts, these questions become essential to assess their botanical and geographical origin and detect fraud or adulteration in oils (e.g. olive oil, avocado oil, hazelnut, and peanut oils). Fraud refers to selling a lower-quality product (e.g. extra virgin olive oil for virgin olive oil). Adulteration involves mixing other oils or substances similar to the components of the product (e.g. mixing other vegetable oils with a similar chemical profile and adding dyes). Such practices are illegal and stem primarily from economic reasons. They reduce the quality of food, can cause harm to human health in the case of food allergens, and lead to consumer distrust. In the case of nuts and oily fruits, it is imperative to obtain analytical methods that guarantee their quality and authenticity, as they are foods in great demand.
In addition, food fraud and adulteration have led to the need to establish efficient traceability monitoring systems as an essential means for food safety management and control, such as the latest blockchain technology (Feng et al. Citation2020). However, sensitive and robust analytical tools are needed to determine products’ quality and authenticity.
Lipidomics offers a formidable solution for tracking lipids in food and raw materials. Food lipidomics leads to a better exploration of the lipid profile and fingerprinting of oilseed fruits, nuts and their oils, using, for example, state-of-the-art analysis tools such as orbitrap mass spectrometers and their coupling to reversed-phase HPLC (C18 columns) that allows analyzing the entire lipidome in a short timeframe. However, although software for analyzing lipidomics data is evolving, the analysis is still essentially manual. Moreover, it must be careful because of isobaric and unknown compounds, especially among minor lipids such as polar lipids.
Several studies show lipid profiles serve as markers of authenticity, identity, and traceability of nuts and oily fruits using lipidomics. Some examples are given based on chromatographic techniques coupled with MS or other types of detectors.
The analysis of the TG profile by HPLC-ELSD coupled with multivariate statistical analyses proved effective in discriminating protected designation of origin (PDO) and non-PDO almond cultivars independently of the harvest year (Barreira et al. Citation2012). These differences identified classification parameters, providing an important tool for authenticity (Barreira et al. Citation2012). The lipid fractions of fourteen hazelnut varieties from two harvest seasons were comprehensively analyzed for their TG, FA, and tocopherol profiles by HPLC-DAD. Significant statistical differences in most TG were found considering hazelnut varieties and harvest year (Taş and Gökmen Citation2015). Also, significant differences in the TG composition were found by HPLC-ELSD between walnut cultivars. These differences were also significant when cultivars were grouped by year of production, showing that the TG composition could be strongly influenced by environmental factors (Amaral et al. Citation2004). It was also possible to differentiate commercial oil extracted from almonds, hazelnuts, and pecan nuts using the TG profiles analyzed by HPLC-RID (Fernandes et al. Citation2017). TG profiles established by HPLC–UV–vis detection predicted the botanical origin of vegetable oils (olive, hazelnut, peanut) with assignment probabilities higher than 95% (Lerma-García et al. Citation2011). Distinct TG compositions were found among the Tunisian peanut varieties detected by LC–ESI–MS (Cherif et al. Citation2013). Another study on high-oleic and regular peanut oils revealed different TG profiles by HPLC-MS, mainly in the contents of OOO, OPO, and POL. This finding provided a theoretical foundation for detecting edible oils’ adulteration and analyzing the nutritional function of high-oleic peanut oils (Dong et al. Citation2015). HPLC analysis of Malaysian avocado varieties found several differences in the TG profile. While the major TG of local avocado cultivars were POO, POL, OOO and PPO, the dominant TG of the Hass variety were OOO, PPO, OOL and POL. Due to these differences in FA and TG positional patterns, the oils of local avocado cultivars possessed utterly different characteristics in several quality parameters from those of the Hass variety (Yanty et al. Citation2011).
Other lipids have also been evaluated in these foodstuffs. For instance, significant differences were found in the TG, polar lipids, DAG, free FA, and neutral lipid contents between two Tunisian pistachio varieties from four different regions (Chahed et al. Citation2008). Furthermore, PL were found as potential lipid markers of geographical origin for the chemical fingerprint of almonds (Prunus dulcis L. cv Nonpareil) using TiO2 nanoparticle-based matrix solid-phase dispersion and matrix-assisted laser desorption ionization − time-of-flight-mass spectrometry (MALDI-TOF/MS) (Shen, Dong, Yang, Li, et al. Citation2013). Furthermore, a characteristic PL composition was also found by LC-MS and MS/MS in different vegetable oils, including olive and almond oils, which may be helpful in the identification of the oil source in commercial applications (Boukhchina et al. Citation2004). Also, a few differences in the amounts of some PL classes were reported in hazelnuts and walnuts from Bulgaria as analyzed by SPE and two-dimensional TLC when compared with the same fruits from other countries (Angelova-Romova et al. Citation2013). Furthermore, unique PL profiles were revealed by hydrophilic interaction liquid chromatography-electrospray ionization-ion trap-time-of-flight-mass spectrometry (HILIC-ESI-IT-TOF-MS) in almonds, walnuts, peanuts, pistachios, cashews, and pecans (Song et al. Citation2018). Finally, lipid profiling by ESI-MS was helpful for the successful evaluation of fraud in avocado oil-based products with soybean oil (Rydlewski et al. Citation2020).
Although there are few studies of lipid phenotyping at the molecular level by lipidomics approaches in oily fruits and nuts, the ongoing research is promising. Therefore, much is expected for years to come regarding the contribution of lipidomics toward the quality, authenticity, and traceability of these agri-food products.
Potential industrial applications of oily fruits and nuts’ by-products
Alongside the health benefits of lipids that suggest the beneficial consumption of oily fruits, nuts, and products thereof, the by-products deriving from these fruits’ industrial processing have been associated with other relevant uses.
For instance, using an avocado paste made with avocado waste for finishing pigs (pigs being fed to market weight) seems to benefit meat’s quality and shelf life (Hernández-López et al. Citation2016). Dietary avocado significantly impacted intramuscular fat content and composition, reducing the lipid content in specific muscles and increasing the unsaturation degree. Muscles from treated pigs had significantly lower lipid and protein oxidation rates during chilled storage, and the color of the muscles was preserved from oxidation (Hernández-López et al. Citation2016). Conversely, avocado PL are usually unwanted by the oil industry. Still, they have excellent potential for producing emulsions in the food, cosmetics, and medical industries, showing gel-like behavior and strong intermolecular forces (Züge et al. Citation2017).
Olive mill processing by-products (e.g. olive leaf, olive pomace, and deodorization distillate), which are residues representing a considerable environmental burden of the olive oil industry, have been valorized in many applications like alternatives for energetic production (biodiesel, biogas, bioethanol, biohydrogen, and biofuel), animal feed, agricultural applications (soil regulator), food (gelling agents, functional foods, and preservatives), drugs, nutraceuticals, cosmetics (natural moisturizers and sunscreen agents), and other biotechnological applications (bioplastic/biopolymer, biosurfactant, and lipase production) (Seçmeler and Galanakis Citation2019).
Olive pomace and stoned olive cakes, being rich in FA and polar lipids, may be helpful to produce animal feeds, such as functional fish feeds or as an ingredient to include in animal feedstocks, improving the nutritional and nutraceutical properties of various foods such as mammal’s meat, milk and cheese (Castellani et al. Citation2017; Cibik and Keles Citation2016; Nasopoulou and Zabetakis Citation2013; Terramoccia et al. Citation2013). Furthermore, olive seed oil, that is, the oil retrieved from pressing the seed that is inside the olive’s stone, has a lipid content of 27.3–53.0 mg 100 mg−1 biomass which is comprised by 80–97% TG, 8% free FA, 2.3–4.1% sterols with up to 90% corresponding to β-sitosterol. The average PL content in olive seed oil was estimated to be 1.25% and the GL content was 1.49% on average (Seçmeler and Galanakis Citation2019; Alves et al. Citation2022). The polar lipid fingerprint carried out by hydrophilic interaction liquid chromatography (HILIC)-HR-ESI-MS, and MS/MS revealed ten polar lipid classes and 94 lipid species including 56 PL, 17 GL, 16 SL, and 5 ASG (Alves et al. Citation2022). Olive stones, pomace, and leaves have a potential role in skincare products and cosmetics due to the positive effect of the FA at distinct levels, such as the composition of the lipid film in the skin surface, the protective barrier, and the maintenance of hydration (Lin and Khnykin Citation2014; Rodrigues et al. Citation2015). Worth noticing that avocado stone oil presents significant higher PUFA/SFA ratios than avocado pulp or peel oils (Amado et al. Citation2019). Moreover, the polar lipid (PL and GL) content in avocado stone oil (28.3 ± 8.7 mg 100 mg−1 lipids) was about five-fold the content found in the pulp (5.2 ± 2.0 mg 100 mg−1) (Pacetti et al. Citation2007). These contents may be responsible for the significant anti-oxidant, anti-inflammatory and anti-cancer effects of the avocado stone oil compared to the avocado pulp oil (Alkhalaf et al. Citation2019). These results point out to an underrated potential and use of the waste parts of some fruits like avocado in phytomedicine.
Hazelnut skin, an agro-food by-product rich in FA and sterols, has revealed blood lipid-lowering effects, decreasing the circulating levels of total and LDL-cholesterol and TG in hamsters (Caimari et al. Citation2015). Pecan nut cake, a good source of lipids, carbohydrates, and proteins, may represent a potential ingredient for producing foods such as desserts, jelly, yoghurts, sausage, pasta, processed cheese, and bakery and confectionery products (Maciel et al. Citation2020).
Conclusion
Nuts and oily fruits have a high lipid content provided by the TG concentration. These foods have a wide range of FA (between 6 and 26 carbons, with up to 6 double bonds), being a good source of n-9 MUFA and n-6 PUFA. Besides TG, nuts and oily fruits contain other minor important lipids, such as phytosterols and polar lipids, which have high chemical diversity and complexity. Although these minor lipids represent a source of high value-added phytochemicals with beneficial effects on human health, they have been scarcely studied.
The real impact of lipids from nuts and oily fruits on human health in the different populations’ dietary patterns is still far from being fully understood. However, scientific research has revealed that lipids (sterols, MUFA, PUFA, phospholipids, glycolipids, and sphingolipids) from these foodstuff are positively involved in lipid metabolism, cardiovascular and diabetic risk factors, blood pressure, cholesterol and TG levels, cell growth, skin barrier function, and other important biological activities. Likewise, lipids from nuts, oily fruits and their industry by-products serve as valuable ingredients or raw materials for several industries, such as the food, nutraceutical, pharmaceutical, cosmetic, and animal feed industries.
Keeping in mind the considerable lack of information about oily fruits and nuts’ oil profile and the real impact of these lipids on human health, lipidomics has become a rapidly growing field of research that can provide a critical analytical approach for lipidome fingerprinting and discovering new compounds with potential biological activity, especially from plant-derived foodstuffs. Among the distinct approaches to extract total lipids from oleaginous fruits, the most widely used is solvent extraction using the reference method in a Soxhlet apparatus with n-hexane, while the Bligh and Dyer method, the Folch method or SPE have been less used yet. GC-FID and HPLC-MS have been the main approaches to study the FA and TG profile, respectively. Regarding the sterol analysis, the selected approaches are GC-FID and GC-MS. LC–MS approaches have been the chosen analytical strategy for polar lipid profiling in oily fruits and nuts, with still scarce but promising results. Standardized (un)targeted approaches and guidelines are still needed, but with the recent advances on the detailed lipidomic profiling carried out by high-resolution LC–MS analysis, a long road has been opened that will deepen the knowledge of these foodstuff at the molecular level. These analytical tools will help better understand the influence of several parameters, such as the geographical and botanical origin in the oily fruits ant nuts’ composition in further studies. Lipidomics offers a formidable solution for tracking lipids in food and raw materials, being a potential helpful tool to detect fraud or adulteration in the oils from nuts and oily fruits.
Nevertheless, there is still a research gap on the comprehensive lipid composition of nuts, oily fruits, their by-products and their beneficial effects. Further studies regarding lipidomic phenotypes, bioaccessibility and bioavailability should be carried out to contribute to the valorization of nuts, oily fruits and their industry by-products.
| Abbreviations | ||
| APCI | = | atmospheric pressure chemical ionization |
| ASG | = | acylsterolglycoside |
| BL | = | betaine lipid |
| C:N | = | number of carbons: number of double bond |
| Cer | = | ceramide |
| CHD | = | coronary heart disease |
| CL | = | cardiolipin |
| CVD | = | cardiovascular disease |
| DAD | = | diode-array detection |
| DGDG | = | diglycosyldiacylglycerol |
| DGMG | = | diglycosylmonoacylglycerol |
| DGTS | = | diacylglyceryl-N,N,N-trimethylhomoserine |
| EASI | = | easy ambient sonic-spray ionization |
| ELSD | = | evaporative light-scattering detection |
| ESI | = | electrospray ionization |
| FA | = | fatty acid |
| FAME | = | fatty acid methyl ester |
| FID | = | flame ionization detector |
| GC | = | gas chromatography |
| GL | = | glycolipid |
| GPL | = | glycerophospholipid |
| HDL | = | high-density lipoprotein |
| HexCer | = | hexosylceramide |
| HILIC | = | hydrophilic interaction liquid chromatography |
| HPLC | = | high-performance liquid chromatography |
| HRMS | = | high-resolution mass spectrometry |
| LC | = | liquid chromatography |
| LDL | = | low-density lipoprotein |
| LPA | = | lysophosphatidic acid |
| LPC | = | lysophosphatidylcholine |
| LPE | = | lysophosphatidylethanolamine |
| LPG | = | lysophosphatidylglycerol |
| LPI | = | lysophosphatidylinositol |
| LSI | = | Lipidomics Standards Initiative |
| MALDI | = | matrix-assisted laser desorption ionization |
| MGDG | = | monoglycosyldiacylglycerol |
| MGTS | = | monoacylglyceryl-N,N,N-trimethylhomoserine |
| MS | = | mass spectrometry |
| MS/MS | = | tandem mass spectrometry |
| MUFA | = | monounsaturated fatty acid |
| NACE | = | non-aqueous capillary electrophoresis |
| NARP | = | non-aqueous reversed-phase |
| NL | = | neutral lipid |
| NMR | = | nuclear magnetic resonance |
| NP | = | normal-phased |
| PA | = | phosphatidic acid |
| PAF | = | platelet-activating factor |
| PC | = | phosphatidylcholine |
| PDA | = | photodiode array detector |
| PDO | = | protected designation of origin |
| PE | = | phosphatidylethanolamine |
| PE-N(FA) | = | N-acyl phosphatidylethanolamine |
| PG | = | phosphatidylglycerol |
| PI | = | phosphatidylinositol |
| PL | = | phospholipid |
| PS | = | phosphatidylserine |
| PUFA | = | polyunsaturated fatty acid |
| Rf | = | retention factor |
| RID | = | refractive index detector |
| RP | = | reversed-phase |
| RT | = | retention time |
| SG | = | sterolglycoside |
| SL | = | sphingolipid |
| SM | = | sphingomyelin |
| sn | = | stereospecific number |
| SPB | = | sphingoid base |
| SPE | = | solid-phase extraction |
| SP | = | sphingophospholipid |
| TG | = | triacylglycerol |
| TLC | = | thin-layer chromatography |
| TOF | = | time-of-flight |
| Tr | = | trace |
| UV | = | ultraviolet |
| VIS | = | visible |
| w/w | = | weight per weight. |
Acknowledgments
The authors acknowledge the University of Aveiro, Fundação para a Ciência e a Tecnologia, I. P. (FCT, Portugal) and Ministério da Ciência e Tecnologia (MCT) for the financial support for the LAQV-REQUIMTE (FCT UIDB/50006/2020) and CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020) through national funds and, where applicable, co-financed by the European Regional Development Fund (FEDER), within the Portugal 2020 Partnership Agreement, and to the Portuguese Mass Spectrometry Network (RNEM, LISBOA-01-0145-FEDER-402-022125). Thanks are also due to FCT/MCT through national funds (PIDDAC) and the co-funding by FEDER, within the Portugal 2020 Partnership Agreement and Programa Operacional Temático Factores de Competitividade (COMPETE) 2020, and to the COST Action EpiLipidNET, CA19105 – Pan-European Network in Lipidomics and EpiLipidomics. This work was also funded by national funds through FCT in the scope of the Individual Call to Scientific Employment Stimulus 2017 with a Junior Researcher contract to Eliana Alves (reference CEEC-IND/00971/2017).
Disclosure statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Abaide, E. R., G. L. Zabot, M. V. Tres, R. F. Martins, J. L. Fagundez, L. F. Nunes, S. Druzian, J. F. Soares, V. Dal Prá, J. R. Silva, et al. 2017. Yield, composition, and antioxidant activity of avocado pulp oil extracted by pressurized fluids. Food Bioproduction Process 102:289–98. doi: 10.1016/j.fbp.2017.01.008.
- Abdallah, A., M. Ahumada, and T. Gradziel. 1998. Oil content and fatty acid composition of almond kernels from different genotypes and California production regions. Journal of the American Society for Horticultural Science 123 (6):1029–33. doi: 10.21273/JASHS.123.6.1029.
- Abdallah, I. B., N. Tlili, E. Martinez-Force, A. G. P. Rubio, M. C. Perez-Camino, A. Albouchi, and S. Boukhchina. 2015. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chemistry 173:972–8. doi: 10.1016/j.foodchem.2014.10.095.
- Abedi, E., and M. Sahari. 2014. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Science & Nutrition 2 (5):443–63. doi: 10.1002/fsn3.121.
- Abou-Aziz, A., A. Rizk, F. Hammouda, and M. El-Tanahy. 1973. Seasonal changes of lipids and fatty acids in two varieties of avocado pear fruits. Qualitas Plantarum et Materiae Vegetabiles 22 (3-4):253–9. doi: 10.1007/BF01099517.
- Agar, I., S. Kafkas, and N. Kaska. 1998. Lipid characteristics of Turkish and Iranian pistachio kernels. Acta Horticulturae 470 (470):378–86. doi: 10.17660/ActaHortic.1998.470.52.
- Aida, K., N. Takakuwa, M. Kinoshita, T. Sugawara, H. Imai, J. Ono, et al. 2003. Properties and physiological effects of plant cerebroside species as functional lipids. In Advanced Research on Plant Lipids, ed. N. Murata, M. Yamada, I. Nishida, H. Okuyama, J. Sekiya, and W. Hajime. 233–6. Dordrecht: Springer. doi: 10.1007/978-94-017-0159-4_54.
- Airaksinen, K., J. Jokkala, I. Ahonen, S. Auriola, M. Kolehmainen, K. Hanhineva, and K. Tiihonen. 2018. High-fat diet, betaine, and polydextrose induce changes in adipose tissue inflammation and metabolism in C57BL/6J mice. Molecular Nutrition & Food Research 62 (23):1800455. doi: 10.1002/mnfr.201800455.
- Akasaka, H., Y. Mizushina, K. Yoshida, Y. Ejima, N. Mukumoto, T. Wang, S. Inubushi, M. Nakayama, Y. Wakahara, and R. Sasaki. 2016. MGDG extracted from spinach enhances the cytotoxicity of radiation in pancreatic cancer cells. Radiation Oncology (London, England) 11 (1):153. doi: 10.1186/s13014-016-0729-0.
- Al-Ruqaie, I., N. Al-Khalifah, and A. Shanavaskhan. 2016. Morphological cladistic analysis of eight popular olive (Olea europaea L.) cultivars grown in Saudi Arabia using numerical taxonomic system for personal computer to detect phyletic relationship and their proximate fruit composition. Saudi Journal of Biological Sciences 23 (1):115–21. doi: 10.1016/j.sjbs.2015.05.008.
- Alasalvar, C., J. Amaral, G. Satir, and F. Shahidi. 2009. Lipid characteristics and essential minerals of native Turkish hazelnut varieties (Corylus avellana L.). Food Chemistry 113 (4):919–25. doi: 10.1016/j.foodchem.2008.08.019.
- Alasalvar, C., J. Amaral, and F. Shahidi. 2006. Functional lipid characteristics of turkish Tombul hazelnut (Corylus avellana L.). Journal of Agricultural and Food Chemistry 54 (26):10177–83. doi: 10.1021/jf061702w.
- Alasalvar, C., F. Shahidi, T. Ohshima, U. Wanasundara, H. C. Yurttas, C. M. Liyanapathirana, and F. B. Rodrigues. 2003. Turkish Tombul hazelnut (Corylus avellana L.). 2. Lipid characteristics and oxidative stability. Journal of Agricultural and Food Chemistry 51 (13):3797–805. doi: 10.1021/jf021239x.
- Alizadeh-Salte, S., N. Farhadi, K. Arzani, and H. Khoshghalb. 2018. Almond oil quality as related to the type of pollen source in Iranian self incompatible cultivars. International Journal of Fruit Science 18 (1):29–36. doi: 10.1080/15538362.2017.1367983.
- Alkhalaf, M., W. Alansari, E. Ibrahim, and M. ELhalwagy. 2019. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. Journal of King Saud University - Science 31 (4):1358–62. doi: 10.1016/j.jksus.2018.10.010.
- Alves, E., T. Melo, M. Barros, M. Domingues, and P. Domingues. 2019. Lipidomic profiling of the olive (Olea europaea L.) fruit towards its valorisation as a functional food: In-depth identification of triacylglycerols and polar lipids in Portuguese olives. Molecules 24 (14):2555. doi: 10.3390/molecules24142555.
- Alves, E., T. Melo, F. Rey, A. Moreira, P. Domingues, and M. Domingues. 2016. Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT 74:371–7. doi: 10.1016/j.lwt.2016.07.071.
- Alves, E., M. Domingues, and P. Domingues. 2018. Polar lipids from olives and olive oil: A review on their identification, significance and potential biotechnological applications. Foods 7 (7):109. doi: 10.3390/foods7070109.
- Alves, E., F. Rey, T. Melo, M. P. Barros, P. Domingues, and R. Domingues. 2022. Bioprospecting bioactive polar lipids from olive (Olea europaea cv. Galega vulgar) fruit seeds: LC-HR-MS/MS fingerprinting and sub-geographic comparison. Foods 11 (7):951. doi: 10.3390/foods11070951.
- Amado, D. A. V., A. M. Detoni, S. L. C. de Carvalho, A. S. Torquato, C. A. Martin, T. S. Tiuman, C. M. Aguiar, and S. M. Cottica. 2019. Tocopherol and fatty acids content and proximal composition of four avocado cultivars (Persea americana Mill). Acta Alimentaria 48 (1):47–55. doi: 10.1556/066.2019.48.1.6.
- Amaral, J. S., S. Casal, I. Citov, A. Santos, R. M. Seabra, and B. P. P. Oliveira. 2006. Characterization of several hazelnut (Corylus avellana L.) cultivars based in chemical, fatty acid and sterol composition. European Food Research and Technology 222 (3-4):274–80. doi: 10.1007/s00217-005-0068-0.
- Amaral, J. S., S. C. Cunha, M. R. Alves, J. A. Pereira, R. M. Seabra, and B. P. Oliveira. 2004. Triacylglycerol composition of walnut (Juglans regia L.) cultivars: Characterization by HPLC-ELSD and chemometrics. Journal of Agricultural and Food Chemistry 52 (26):7964–9. doi: 10.1021/jf048918n.
- Amaral, J. S., S. C. Cunha, A. Santos, M. R. Alves, R. M. Seabra, and B. P. P. Oliveira. 2006. Influence of cultivar and environmental conditions on the triacylglycerol profile of hazelnut (Corylus avellana L.). Journal of Agricultural and Food Chemistry 54 (2):449–56. doi: 10.1021/jf052133f.
- Andersson, S. W., J. Skinner, L. Ellegård, A. A. Welch, S. Bingham, A. Mulligan, H. Andersson, and K.-T. Khaw. 2004. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: A cross-sectional study. European Journal of Clinical Nutrition 58 (10):1378–85. doi: 10.1038/sj.ejcn.1601980.
- Angelova-Romova, M., M. Zlatanov, G. Antova, S. Momchilova, E. Blagoeva, and M. Nikolova. 2013. Phospholipids content and composition of hazelnut and walnut cultivars grown in Bulgaria. Comptes rendus de l’Academie bulgare des Sciences 66 (12):1689–94.
- Arena, E., G. Ballistreri, and B. Fallico. 2013. Effect of postharvest storage temperatures on the quality parameters of pistachio nuts. Czech Journal of Food Sciences 31 (5):467–73. doi: 10.17221/69/2013-CJFS.
- Askin, M. A., M. F. Balta, F. E. Tekintas, A. Kazankaya, and F. Balta. 2007. Fatty acid composition affected by kernel weight in almond [Prunus dulcis (Mill.) D.A. Webb.] genetic resources. Journal of Food Composition and Analysis 20 (1):7–12. doi: 10.1016/j.jfca.2006.06.005.
- Bach-Faig, A., E. M. Berry, D. Lairon, J. Reguant, A. Trichopoulou, S. Dernini, F. X. Medina, M. Battino, R. Belahsen, G. Miranda, et al. 2011. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutrition 14 (12A):2274–84. doi: 10.1017/S1368980011002515.
- Bada, J. C., M. León-Camacho, M. Prieto, and L. Alonso. 2004. Characterization of oils of hazelnuts from Asturias, Spain. European Journal of Lipid Science and Technology 106 (5):294–300. doi: 10.1002/ejlt.200300922.
- Ballistreri, G., E. Arena, and B. Fallico. 2011. Lipid composition of Italian pistachio. Acta Horticulturae 912 (912):455–60. doi: 10.17660/ActaHortic.2011.912.69.
- Balta, M. F., T. Yarilgaç, M. A. Aşkin, M. Kuçuk, F. Balta, and K. Özrenk. 2006. Determination of fatty acid compositions, oil contents and some quality traits of hazelnut genetic resources grown in eastern Anatolia of Turkey. Journal of Food Composition and Analysis 19 (6-7):681–6. doi: 10.1016/j.jfca.2005.10.007.
- Barreira, J. C. M., S. Casal, I. C. F. R. Ferreira, A. M. Peres, J. A. Pereira, and M. B. P. P. Oliveira. 2012. Supervised chemical pattern recognition in almond (Prunus dulcis) Portuguese PDO cultivars: PCA- and LDA-based triennial study. Journal of Agricultural and Food Chemistry 60 (38):9697–704. doi: 10.1021/jf301402t.
- Barros, H. D. F. Q., J. P. Coutinho, R. Grimaldi, H. T. Godoy, and F. A. Cabral. 2016. Simultaneous extraction of edible oil from avocado and capsanthin from red bell pepper using supercritical carbon dioxide as solvent. The Journal of Supercritical Fluids 107:315–20. doi: 10.1016/j.supflu.2015.09.025.
- Becerra-Tomás, N., I. Paz-Graniel, C. W C Kendall, H. Kahleova, D. Rahelić, J. L. Sievenpiper, and J. Salas-Salvadó. 2019. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutrition Reviews 77 (10):691–709. doi: 10.1093/nutrit/nuz042.
- Bianchi, G., and G. Vlahov. 1994. Composition of lipid classes in the morphologically different parts of the olive fruit, cv. Coratina (Olea europaea Linn.). Lipid / Fett 96 (2):72–7. doi: 10.1002/lipi.19940960211.
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37 (8):911–7. doi: 10.1139/o59-099.
- Bora, P. S., N. Narain, R. V. M. Rocha, and P. M. Queiroz. 2001. Characterization of the oils from the pulp and seeds of avocado (cultivar: Fuerte) fruits. Grasas y Aceites 52 (3-4):171–4. doi: 10.3989/gya.2001.v52.i3-4.353.
- Boskou, D. 2006. Characteristics of the olive tree and olive fruit. In Olive oil: Chemistry and technology, ed. D. Boskou, 13–9. Champaign, Illinois: AOCS Press.
- Boukhchina, S., K. Sebai, A. Cherif, H. Kallel, and P. M. Mayer. 2004. Identification of glycerophospholipids in rapeseed, olive, almond, sunflower oil by LC-MS and LC-MS-MS. Canadian Journal of Chemistry 82 (7):1210–5. doi: 10.1139/v04-094.
- Brüll, F., R. P. Mensink, K. Van Den Hurk, A. Duijvestijn, and J. Plat. 2010. TLR2 activation is essential to induce a Th1 shift in human peripheral blood mononuclear cells by plant stanols and plant sterols. The Journal of Biological Chemistry 285 (5):2951–8. doi: 10.1074/jbc.M109.036343.
- Buchholz, K. 2019. Millennials not alone in driving up U.S. avocado consumption. Accessed September 21, 2021. https://www.statista.com/chart/18935/us-avocado-consumption/.
- Caimari, A., F. Puiggròs, M. Suárez, A. Crescenti, S. Laos, J. A. Ruiz, V. Alonso, J. Moragas, J. M. Del Bas, L. Arola, et al. 2015. The intake of a hazelnut skin extract improves the plasma lipid profile and reduces the lithocholic/deoxycholic bile acid faecal ratio, a risk factor for colon cancer, in hamsters fed a high-fat diet. Food Chemistry 167:138–44. doi: 10.1016/j.foodchem.2014.06.072.
- Calpe-Berdiel, L., J. C. Escolà-Gil, S. Benítez, C. Bancells, F. González-Sastre, X. Palomer, and F. Blanco-Vaca. 2007. Dietary phytosterols modulate T-helper immune response but do not induce apparent anti-inflammatory effects in a mouse model of acute, aseptic inflammation. Life Sciences 80 (21):1951–6. doi: 10.1016/j.lfs.2007.02.032.
- Campos-Mondragón, M. G., A. M. Calderón De La Barca, A. Durán-Prado, L. C. Campos-Reyes, R. M. Oliart-Ros, J. Ortega-García, L. A. Medina-Juárez, and O. Angulo. 2009. Nutritional composition of new peanut (Arachis hypogaea L.) cultivars. Grasas y Aceites 60 (2):161–7. doi: 10.3989/gya.075008.
- Cañavate, J. P., I. Armada, and I. Hachero-Cruzado. 2017. Interspecific variability in phosphorus-induced lipid remodelling among marine eukaryotic phytoplankton. The New Phytologist 213 (2):700–13. doi: 10.1111/nph.14179.
- Canãvate, J. P., I. Armada, J. L. Riós, and I. Hachero-Cruzado. 2016. Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 124:68–78. doi: 10.1016/j.phytochem.2016.02.007.
- Castellani, F., A. Vitali, N. Bernardi, E. Marone, F. Palazzo, L. Grotta, and G. Martino. 2017. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. Journal of Dairy Science 100 (11):1–12. doi: 10.3168/jds.2017-12899.
- Castro-Gómez, P., A. Garcia-Serrano, F. Visioli, and J. Fontecha. 2015. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins, Leukotrienes, and Essential Fatty Acids 101:41–51. doi: 10.1016/j.plefa.2015.07.004.
- Chahed, T., A. Bellila, W. Dhifi, I. Hamrouni, B. M’hamdi, M. E. Kchouk, and B. Marzouk. 2008. Pistachio (Pistacia vera) seed oil composition: Geographic situation and variety effects. Grasas y Aceites 59 (1):51–6. doi: 10.3989/gya.2008.v59.i1.490.
- Chai, G.-S., X. Jiang, Z.-F. Ni, Z.-W. Ma, A.-J. Xie, X.-S. Cheng, Q. Wang, J.-Z. Wang, and G.-P. Liu. 2013. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. Journal of Neurochemistry 124 (3):388–96. doi: 10.1111/jnc.12094.
- Cherif, A. O., N. Leveque, M. Ben Messaouda, H. Kallel, and F. Moussa. 2013. An electrospray ionisation-mass spectrometry screening of triacylglycerols in developing cultivated and wild peanut kernels (Arachis hypogaea L.). Food Chemistry 138 (2-3):1095–100. doi: 10.1016/j.foodchem.2012.11.107.
- Cherif, A. O., N. Leveque, M. Ben Messaouda, H. Kallel, A. Tchapla, and F. Moussa. 2014. NARP-HPLC/MS5 and silver cationization fingerprinting of triacylglycerols in wild and cultivar Tunisian peanut kernels. LWT - Food Science and Technology 57 (1):236–42. doi: 10.1016/j.lwt.2014.01.031.
- Choo, J., H. Ueshima, J. D. Curb, C. Shin, R. W. Evans, A. El-Saed, T. Kadowaki, T. Okamura, K. Nakata, T. Otake, et al. 2010. Serum n-6 fatty acids and lipoprotein subclasses in middle-aged men: The population-based cross-sectional ERA-JUMP study. The American Journal of Clinical Nutrition 91 (5):1195–203. doi: 10.3945/ajcn.2009.28500.
- Chunhieng, T., A. Hafidi, D. Pioch, J. Brochier, and D. Montet. 2008. Detailed study of Brazil nut (Bertholletia excelsa) oil micro-compounds: Phospholipids, tocopherols and sterols. Journal of the Brazilian Chemical Society 19 (7):1374–80. doi: 10.1590/S0103-50532008000700021.
- Cibik, M., and G. Keles. 2016. Effect of stoned olive cake on milk yield and composition of dairy cows. Revue de médecine vétérinaire 167 (5-6):154–8.
- Cittadini, M. C., D. Martín, S. Gallo, G. Fuente, R. Bodoira, M. Martínez, and D. Maestri. 2020. Evaluation of hazelnut and walnut oil chemical traits from conventional cultivars and native genetic resources in a non-traditional crop environment from Argentina. European Food Research and Technology 246 (4):833–43. doi: 10.1007/s00217-020-03453-8.
- Cornelio-Santiago, H. P., M. R. Mazalli, C. E. C. Rodrigues, and A. L. Oliveira. 2019. Extraction of Brazil nut kernel oil using green solvents: Effects of the process variables in the oil yield and composition. Journal of Food Process Engineering 42 (7):e13271. doi: 10.1111/jfpe.13271.
- Corrales-García, J. E., M. Del Rosario García-Mateos, E. Martínez-López, A. F. Barrientos-Priego, M. C. Ybarra-Moncada, E. Ibarra-Estrada, S. M. Méndez-Zúñiga, and D. Becerra-Morales. 2019. Anthocyanin and oil contents, fatty acids profiles and antioxidant activity of Mexican Landrace avocado fruits. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 74 (2):210–5. doi: 10.1007/s11130-019-00721-1.
- Corzzini, S. C. S., H. D. F. Q. Barros, R. Grimaldi, and F. A. Cabral. 2017. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. Journal of Food Engineering 194:40–5. doi: 10.1016/j.jfoodeng.2016.09.004.
- Costa, P. A., C. A. Ballus, J. Teixeira-Filho, and H. T. Godoy. 2010. Phytosterols and tocopherols content of pulps and nuts of Brazilian fruits. Food Research International 43 (6):1603–6. doi: 10.1016/j.foodres.2010.04.025.
- Covas, M. I., M. Fitó, and R. De La Torre. 2015. Minor bioactive olive oil components and health: Key data for their role in providing health benefits in humans. In Olive and olive oil bioactive constituents, ed. D. Boskou, 31–52. Urbana, IL: AOCS Press. doi: 10.1016/B978-1-63067-041-2.50008-2.
- Cyberlipid Center. 2021a. Simple lipids. Accessed October 29, 2021. http://cyberlipid.gerli.com/description/simple-lipids/.
- Cyberlipid Center. 2021b. Thin-layer chromatography. Accessed November 13, 2021. https://cyberlipid.gerli.com/techniques-of-analysis/fractionation-complex-extracts/thin-layer-chromatography-2/.
- Danlami, J. M., A. Arsad, M. A. A. Zaini, and H. Sulaiman. 2014. A comparative study of various oil extraction techniques from plants. Reviews in Chemical Engineering 30 (6):605–26. doi: 10.1515/revce-2013-0038.
- del Río, C., M. D. Carcía-Fernández, and J. M. Caballero. 2002. Variability and classification of olive cultivars by fruit weight, flesh/stone ratio and oil percentage. Acta Horticulturae 586 (586):229–32. doi: 10.17660/ActaHortic.2002.586.43.
- Dembitsky, V. M. 1996. Betaine ether-linked glycerolipids: Chemistry and biology. Progress in Lipid Research 35 (1):1–51. doi: 10.1016/0163-7827(95)00009-7.
- Derewiaka, D., E. Szwed, and R. Wołosiak. 2014. Physicochemical properties and composition of lipid fraction of selected edible nuts. Pakistan Journal of Botany 46 (1):337–43.
- Dong, X. Y., J. Zhong, F. Wei, X. Lv, L. Wu, Y. Lei, B. S. Liao, S. Y. Quek, and H. Chen. 2015. Triacylglycerol composition profiling and comparison of high-oleic and normal peanut oils. Journal of the American Oil Chemists’ Society 92 (2):233–42. doi: 10.1007/s11746-014-2580-5.
- Du, J., L. Shen, Z. Tan, P. Zhang, X. Zhao, Y. Xu, M. Gan, Q. Yang, J. Ma, A. Jiang, et al. 2018. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 10 (2):131. doi: 10.3390/nu10020131.
- Duivenvoorden, I., P. J. Voshol, P. C. N. Rensen, W. van Duyvenvoorde, J. A. Romijn, J. J. Emeis, L. M. Havekes, and W. F. Nieuwenhuizen. 2006. Dietary sphingolipids lower plasma cholesterol and triacylglycerol and prevent liver steatosis in APOE*3Leiden mice. The American Journal of Clinical Nutrition 84 (2):312–21. doi: 10.1093/ajcn/84.1.312.
- Dulf, F. V., M. L. Unguresan, D. C. Vodnar, and C. Socaciu. 2010. Free and esterified sterol distribution in four romanian vegetable oil. Notulae Botanicae Horti Agrobotanici Cluj-Napoca Scopus 38 (10):91–7.
- Ejaz, A., L. Martinez-Guino, A. B. Goldfine, F. Ribas-Aulinas, V. De Nigris, S. Ribó, A. Gonzalez-Franquesa, P. M. Garcia-Roves, E. Li, J. M. Dreyfuss, et al. 2016. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes 65 (4):902–12. doi: 10.2337/db15-1094.
- Enot, D. P., M. Niso-Santano, S. Durand, A. Chery, F. Pietrocola, E. Vacchelli, F. Madeo, L. Galluzzi, and G. Kroemer. 2015. Metabolomic analyses reveal that anti-aging metabolites are depleted by palmitate but increased by oleate in vivo. Cell Cycle (Georgetown, Tex.) 14 (15):2399–407. doi: 10.1080/15384101.2015.1064206.
- Escurriol, V., M. Cofán, C. Moreno-Iribas, N. Larrañaga, C. Martínez, C. Navarro, L. Rodríguez, C. A. González, D. Corella, E. Ros, et al. 2010. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. Journal of Lipid Research 51 (3):618–24. doi: 10.1194/jlr.P000471.
- Esposito, D., T. Rathinasabapathy, B. Schmidt, M. P. Shakarjian, S. Komarnytsky, and I. Raskin. 2013. Acceleration of cutaneous wound healing by brassinosteroids. Wound Repair and Regeneration: Official Publication of the Wound Healing Society [and] the European Tissue Repair Society 21 (5):688–96. doi: 10.1111/wrr.12075.
- Fang, F., C. T. Ho, S. Sang, and R. T. Rosen. 2005. Determination of sphingolipids in nuts and seeds by a single quadrupole liquid chromatography-mass spectrometry method. Journal of Food Lipids 12 (4):327–43. doi: 10.1111/j.1745-4522.2005.00028.x.
- Fardin-Kia, A. R., S. M. Handy, and J. I. Rader. 2012. Characterization of pine nuts in the U.S. market, including those associated with “pine mouth,” by GC-FID. Journal of Agricultural and Food Chemistry 60 (10):2701–11. doi: 10.1021/jf205188m.
- Fehily, A. M., J. W. G. Yarnell, J. Pickering, and P. C. Elwood. 1992. Coronary heart disease and dietary factors. The Lancet 339 (8799):987–8. doi: 10.1016/0140-6736(92)91558-P.
- Feng, H., X. Wang, Y. Duan, J. Zhang, and X. Zhang. 2020. Applying blockchain technology to improve agri-food traceability: A review of development methods, benefits and challenges. Journal of Cleaner Production 260:121031. doi: 10.1016/j.jclepro.2020.121031.
- Fernandes, G. D., R. B. Gómez-Coca, M. d C. Pérez-Camino, W. Moreda, and D. Barrera-Arellano. 2017. Chemical characterization of major and minor compounds of nut oils: Almond, hazelnut, and pecan nut. Journal of Chemistry 2017:1–11. doi: 10.1155/2017/2609549.
- Fernández-Cuesta, A., L. León, L. Velasco, and R. De la Rosa. 2013. Changes in squalene and sterols associated with olive maturation. Food Research International 54 (2):1885–9. doi: 10.1016/j.foodres.2013.07.049.
- Folch, J., M. Lees, and G. H. Sloane Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry 226 (1):497–509. doi: 10.1016/S0021-9258(18)64849-5.
- Fonolla-Joya, J., R. Reyes-García, A. García-Martín, E. López-Huertas, and M. Muñoz-Torres. 2016. Daily intake of milk enriched with n-3 fatty acids, oleic acid, and calcium improves metabolic and bone biomarkers in postmenopausal women. Journal of the American College of Nutrition 35 (6):529–36. doi: 10.1080/07315724.2014.1003114.
- Font I Forcada, C., L. Velasco, R. Socias I Company, and Á. Fernández I Martí. 2015. Association mapping for kernel phytosterol content in almond. Frontiers in Plant Science 6:530. doi: 10.3389/fpls.2015.00530.
- Food and Agriculture Organization (FAO). 1985. Importance of olive production and olive tree by-products. In Olive by-products for animal feed. FAO Animal Production and Health Paper 43. Rome: FAO. http://www.fao.org/3/X6545E/X6545E01.htm.
- Fulgoni, V. L., M. Dreher, and A. J. Davenport. 2013. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: Results from the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Nutrition Journal 12:1. doi: 10.1186/1475-2891-12-1.
- Ganesan, B., S. Buddhan, R. Anandan, R. Sivakumar, and R. AnbinEzhilan. 2010. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Molecular Biology Reports 37 (3):1319–27. doi: 10.1007/s11033-009-9508-4.
- Gangoiti, P., L. Camacho, L. Arana, A. Ouro, M. H. Granado, L. Brizuela, J. Casas, G. Fabriás, J. L. Abad, A. Delgado, et al. 2010. Control of metabolism and signaling of simple bioactive sphingolipids: Implications in disease. Progress in Lipid Research 49 (4):316–34. doi: 10.1016/j.plipres.2010.02.004.
- Gao, P., J. Jin, R. Liu, Q. Jin, and X. Wang. 2018. Chemical compositions of walnut (Juglans regia L.) oils from different cultivated regions in China. Journal of the American Oil Chemists’ Society 95 (7):825–34. doi: 10.1002/aocs.12097.
- Gao, P., R. Liu, Q. Jin, and X. Wang. 2019a. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chemistry 279:279–87. doi: 10.1016/j.foodchem.2018.12.016.
- Gao, P., R. Liu, Q. Jin, and X. Wang. 2019b. Comparison of solvents for extraction of walnut oils: Lipid yield, lipid compositions, minor-component content, and antioxidant capacity. LWT 110:346–52. doi: 10.1016/j.lwt.2019.04.100.
- Ghanbari, R., F. Anwar, K. M. Alkharfy, A. H. Gilani, and N. Saari. 2012. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.) – A review. International Journal of Molecular Sciences 13 (3):3291–340. doi: 10.3390/ijms13033291.
- Ghrab, M., F. Zribi, M. Ayadi, O. Elloumi, N. Mnafki, and M. Ben Mimoun. 2010. Lipid characterization of local pistachio germoplasm in central and southern Tunisia. J Food Compos Anal. 23 (6):605–12. doi: 10.1016/j.jfca.2009.08.016.
- Gill, I., and R. Valivety. 1997. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends in Biotechnology 15 (10):401–9. doi: 10.1016/S0167-7799(97)01076-7.
- Givianrad, M. H., M. Saber-Tehrani, and S. A. Jafari Mohammadi. 2013. Chemical composition of oils from wild almond (Prunus scoparia) and wild pistachio (Pistacia atlantica). Grasas y Aceites 64 (1):77–84. doi: 10.3989/gya.070312.
- Gómez-López, V. M. 2002. Fruit characterization of high oil content avocado varieties. Scientia Agricola 59 (2):403–6. doi: 10.1590/S0103-90162002000200030.
- Gonçalves-de-Albuquerque, C. F., I. M. Medeiros-de-Moraes, F. M. d J. Oliveira, P. Burth, P. T. Bozza, M. V. Castro Faria, A. R. Silva, and H. C. d Castro-Faria-Neto. 2016. Omega-9 oleic acid induces fatty acid oxidation and decreases organ dysfunction and mortality in experimental sepsis. PloS One 11 (4):e0153607. doi: 10.1371/journal.pone.0153607.
- Guasch-Ferré, M., F. B. Hu, M. A. Martínez-González, M. Fitó, M. Bulló, R. Estruch, E. Ros, D. Corella, J. Recondo, E. Gómez-Gracia, et al. 2014. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Medicine 12:78. doi: 10.1186/1741-7015-12-78.
- Gumbiner, B., C. C. Low, and P. D. Reaven. 1998. Effects of a monounsaturated fatty acid-enriched hypocaloric diet on cardiovascular risk factors in obese patients with type 2 diabetes. Diabetes Care 21 (1):9–15. doi: 10.2337/diacare.21.1.9.
- Hagar, H., and W. Al. 2014. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-3. Environmental Toxicology and Pharmacology 37 (2):803–11. doi: 10.1016/j.etap.2014.02.013.
- Hammad, S., S. Pu, and P. J. Jones. 2016. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids 51 (5):507–17. doi: 10.1007/s11745-015-4113-x.
- Hernández-López, S. H., J. G. Rodríguez-Carpena, C. Lemus-Flores, F. Grageola-Nuñez, and M. Estévez. 2016. Avocado waste for finishing pigs: Impact on muscle composition and oxidative stability during chilled storage. Meat Science 116:186–92. doi: 10.1016/j.meatsci.2016.02.018.
- Hidalgo, F. J., F. Nogales, and R. Zamora. 2005. Changes produced in the antioxidative activity of phospholipids as a consequence of their oxidation. Journal of Agricultural and Food Chemistry 53 (3):659–62. doi: 10.1021/jf0483220.
- Hoffman, J. R., J. Kang, N. A. Ratamess, S. L. Rashti, C. P. Tranchina, and A. D. Faigenbaum. 2009. Effect of an acute ingestion of a weight loss supplement. Journal of the International Society of Sports Nutrition 6 (1):1–10. doi: 10.1186/1550-2783-6-1.
- Holčapek, M., M. Lísa, P. Jandera, and N. Kabátová. 2005. Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. Journal of Separation Science 28 (12):1315–33. doi: 10.1002/jssc.200500088.
- Hou, C.-C., Y.-P. Chen, J.-H. Wu, C.-C. Huang, S.-Y. Wang, N.-S. Yang, and L.-F. Shyur. 2007. A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Research 67 (14):6907–15. doi: 10.1158/0008-5472.CAN-07-0158.
- Houmy, N., F. Mansouri, A. Benmoumen, S. Elmouden, M. Boujnah, M. Sindic, M. L. Fauconnier, H. Serghini-Caid, and A. Elamrani. 2016. Characterization of almond kernel oils of five almonds varieties cultivated in Eastern Morocco. In XVI GREMPA Meeting on Almonds and Pistachio Options Méditerranéennes Série A. Séminaires Méditerranéens, ed. O. Kodad, A. López-Francos, M. Rovira, and R. Socias i Company, vol. 119. 317–21. Zaragoza, Spain: CIHEAM.
- International Nut and Dried Fruit Council. 2021. Nuts & dried fruits – statistical yearbook 2020/2021. Accessed November 17. https://www.nutfruit.org/files/tech/1625230833_INC_Stats_2021.pdf
- International Olive Council. 2021a. World olive oil and table olive figures. Accessed September 21. https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures.
- International Olive Council. 2021b. The olive tree. Accessed November 7. http://www.internationaloliveoil.org/estaticos/view/76-the-olive-tree.
- Ishida, H., and M. Kiso. 2008. Synthesis of glycolipid and its application. In Experimental Glycoscience, eds. N. Taniguchi, A. Suzuki, Y. Ito, H. Narimatsu, T. Kawasaki, and S. Hase, 147–50. Tokyo: Springer. doi: 10.1007/978-4-431-77924-7_38.
- Jadhav, H. B., and U. Annapure. 2021. Designer lipids-synthesis and application – A review. Trends in Food Science and Technology 116:884–902. doi: 10.1016/j.tifs.2021.08.020.
- Ji, J., Z. Ge, Y. Feng, and X. Wang. 2019. Lipid characterization of Chinese wild hazelnuts (Corylus mandshurica Maxim.). Journal of Oleo Science 68 (1):13–20. doi: 10.5650/jos.ess18132.
- Jonnala, R. S., N. T. Dunford, and K. E. Dashiell. 2006. Tocopherol, phytosterol and phospholipid compositions of new high oleic peanut cultivars. Journal of Food Composition and Analysis 19 (6-7):601–5. doi: 10.1016/j.jfca.2006.01.005.
- Jonnala, R. S., N. T. Dunford, and K. E. Dashiell. 2005. New high-oleic peanut cultivars grown in the Southwestern United States. Journal of the American Oil Chemists’ Society 82 (2):125–8. doi: 10.1007/s11746-005-1053-x.
- Jorge, T. S., T. C. Polachini, L. S. Dias, N. Jorge, and J. Telis-Romero. 2015. Physicochemical and rheological characterization of avocado oils. Ciência e Agrotecnologia 39 (4):390–400. doi: 10.1590/S1413-70542015000400010.
- Kaijser, A., P. Dutta, and G. Savage. 2000. Oxidative stability and lipid composition of macadamia nuts grown in New Zealand. Food Chemistry 71 (1):67–70. doi: 10.1016/S0308-8146(00)00132-1.
- Kalogeropoulos, N., A. Chiou, M. S. Ioannou, and V. T. Karathanos. 2013. Nutritional evaluation and health promoting activities of nuts and seeds cultivated in Greece. International Journal of Food Sciences and Nutrition 64 (6):757–67. doi: 10.3109/09637486.2013.793298.
- Kammoun, N. G., and W. Zarrouk. 2012. Exploratory chemometric analysis for the characterisation of Tunisian olive cultivars according to their lipid and sterolic profiles. International Journal of Food Science & Technology 47 (7):1496–504. doi: 10.1111/j.1365-2621.2012.02997.x.
- Kanbur, G., D. Arslan, D, and M. M. Özcan. 2013. Some compositional and physical characteristics of some Turkish hazelnut (Corylus avellana L.) variety fruits and their corresponding oils. International Food Research Journal 20 (5):2161–5.
- Karantonis, H. C., S. Antonopoulou, and C. A. Demopoulos. 2002. Antithrombotic lipid minor constituents from vegetable oils. Comparison between olive oils and others. Journal of Agricultural and Food Chemistry 50 (5):1150–60. doi: 10.1021/jf010923t.
- Khoury, S., C. Canlet, M. Z. Lacroix, O. Berdeaux, J. Jouhet, and J. Bertrand-Michel. 2018. Quantification of lipids: Model, reality, and compromise. Biomolecules 8 (4):174. doi: 10.3390/biom8040174.
- King, M. F., L. C. Boyd, and B. W. Sheldon. 1992. Antioxidant properties of individual phospholipids in a salmon oil model system. Journal of the American Oil Chemists’ Society 69 (6):545–51. doi: 10.1007/BF02636106.
- Klippel, K. F., D. M. Hiltl, and B. Schipp. 1997. A multicentric, placebo-controlled, double-blind clinical trial of β-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. British Journal of Urology 80 (3):427–32. doi: 10.1046/j.1464-410X.1997.t01-1-00362.x.
- Koaze, H., D. S. Ndaka, P. N. Karanja, K. I. Ishibashi, and N. Baba. 2002. Changes in quality of dried macadamia nuts during a peak harvest season in Kenya. Food Science and Technology Research 8 (1):32–5. doi: 10.3136/fstr.8.32.
- Kodad, O., G. Estopañán, T. Juan, J. M. Alonso, M. T. Espiau, and R. Socias I Company. 2014. Oil content, fatty acid composition and tocopherol concentration in the Spanish almond genebank collection. Scientia Horticulturae (Amsterdam) 177:99–107. doi: 10.1016/j.scienta.2014.07.045.
- Kopylov, A. T., K. A. Malsagova, A. A. Stepanov, and A. L. Kaysheva. 2021. Diversity of plant sterols metabolism: The impact on human health, sport, and accumulation of contaminating sterols. Nutrients 13 (5):1623. doi: 10.3390/nu13051623.
- Kornsteiner-Krenn, M., K. H. Wagner, and I. Elmadfa. 2013. Phytosterol content and fatty acid pattern of ten different nut types. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 83 (5):263–70. doi: 10.1024/0300.
- Kornsteiner, M., K. H. Wagner, and I. Elmadfa. 2006. Tocopherols and total phenolics in 10 different nut types. Food Chem. 98 (2):381–7. doi: 10.1016/j.foodchem.2005.07.033.
- Küçüköner , E., and B. Yurt. 2003. Some chemical characteristics of Pistacia vera varieties produced in Turkey. European Food Research and Technology 217 (4):308–10. doi: 10.1007/s00217-003-0763-7.
- Küllenberg, D., L. A. Taylor, M. Schneider, and U. Massing. 2012. Health effects of dietary phospholipids. Lipids in Health and Disease 11:1–16. doi: 10.1186/1476-511X-11-3.
- Lai, M. C., T. H. Teng, and C. Yang. 2013. The natural PPAR agonist linoleic acid stimulated insulin release in the rat pancreas. The Journal of Veterinary Medical Science 75 (11):1449–54. doi: 10.1292/jvms.13-0189.
- Larsen, E., A. Kharazmi, L. P. Christensen, and S. B. Christensen. 2003. An antiinflammatory galactolipid from Rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. Journal of Natural Products 66 (7):994–5. doi: 10.1021/np0300636.
- Lerma-García, M. J., R. Lusardi, E. Chiavaro, L. Cerretani, A. Bendini, G. Ramis-Ramos, and E. F. Simó-Alfonso. 2011. Use of triacylglycerol profiles established by high performance liquid chromatography with ultraviolet-visible detection to predict the botanical origin of vegetable oils. Journal of Chromatography. A 1218 (42):7521–7. doi: 10.1016/j.chroma.2011.07.078.
- Lin, M., and D. Khnykin. 2014. Fatty acid transporters in skin development, function and disease. Biochimica et Biophysica Acta 1841 (3):362–8. doi: 10.1016/j.bbalip.2013.09.016.
- Liphschitz, N., R. Gophna, M. Hartman, and G. Biger. 1991. The beginning of olive (Olea europaea) cultivation in the old world: A reassessment. Journal of Archaeological Science 18 (4):441–53. doi: 10.1016/0305-4403(91)90037-P.
- Lipidomics Standards Initiative Consortium. 2019. Lipidomics needs more standardization. Nature Metabolism 1 (8):745–7. doi: 10.1038/s42255-019-0094-z.
- Lísa, M., and M. Holčapek. 2008. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Journal of Chromatography. A 1198-1199:115–30. doi: 10.1016/j.chroma.2008.05.037.
- List, G. R., and J. P. Friedrich. 1989. Oxidative stability of seed oils extracted with supercritical carbon dioxide. Journal of the American Oil Chemists’ Society 66 (1):98–101. doi: 10.1007/BF02661793.
- Luchetti, F. 2002. Importance and future of olive oil in the world market - An introduction to olive oil. European Journal of Lipid Science and Technology 104 (9-10):559–63. doi: 10.1002/1438-9312(200210)104:9/10<559::AID-EJLT559>3.0.CO;2-Q.
- Ma, F. Y., Z. F. Wei, M. Zhang, X. X. Shuai, and L. Q. Du. 2022. Optimization of aqueous enzymatic microwave assisted extraction of macadamia oil and evaluation of its chemical composition, physicochemical properties, and antioxidant activities. European Journal of Lipid Science and Technology 124 (1):2100079. doi: 10.1002/ejlt.202100079.
- Maciel, E., E. Alves, P. Domingues, and R. Domingues. 2018. Module 3 – Lipidomics. In Advanced Analytical Chemistry for Life Sciences: AACLifeSci Course Companion Manual, eds. P. Domingues, A. García, and E. Skrzydlewska. Bialystok: Medical University of Bialystok.
- Maciel, L. G., F. L. Ribeiro, G. L. Teixeira, L. Molognoni, J. Nascimento Dos Santos, I. Larroza Nunes, and J. Mara Block. 2020. The potential of the pecan nut cake as an ingredient for the food industry. Food Research International (Ottawa, Ont.) 127:108718. doi: 10.1016/j.foodres.2019.108718.
- Madawala, S. R., S. P. Kochhar, and P. C. Dutta. 2012. Lipid components and oxidative status of selected specialty oils. Grasas y Aceites 63 (2):143–51. doi: 10.3989/gya.083811.
- Maguire, L. S., S. M. O'Sullivan, K. Galvin, T. P. O'Connor, and N. M. O'Brien. 2004. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. International Journal of Food Sciences and Nutrition 55 (3):171–8. doi: 10.1080/09637480410001725175.
- Mardigan, L. P., V. J. Dos Santos, P. T. da Silva, J. V. Visentainer, S. T. M. Gomes, and M. Matsushita. 2019. Investigation of bioactive compounds from various avocado varieties (Persea americana Miller). Food Science and Technology 39 (suppl 1):15–21. doi: 10.1590/fst.34817.
- Martínez, M. L., M. A. Mattea, and D. M. Maestri. 2006. Varietal and crop year effects on lipid composition of walnut (Juglans regia) genotypes. Journal of the American Oil Chemists’ Society 83 (9):791–6. doi: 10.1007/s11746-006-5016-z.
- Massaro, M., E. Scoditti, M. A. Carluccio, N. Calabriso, G. Santarpino, T. Verri, and R. De Caterina. 2020. Effects of olive oil on blood pressure: Epidemiological, clinical, and mechanistic evidence. Nutrients 12 (6):1548. doi: 10.3390/nu12061548.
- Massimo, L., D. E. Laura, and L. B. Ginevra. 2020. Phytosterols and phytosterol oxides in Bronte’s Pistachio (Pistacia vera L.) and in processed pistachio products. European Food Research and Technology 246 (2):307–14. doi: 10.1007/s00217-019-03343-8.
- Matthäus, B., P. Li, F. Ma, H. Zhou, J. Jiang, and M. M. Özcan. 2018a. Is the profile of fatty acids, tocopherols, and amino acids suitable to differentiate Pinus armandii suspicious to be responsible for the pine nut syndrome from other Pinus species? Chemistry & Biodiversity 15 (1):e1700323. doi: 10.1002/cbdv.201700323.
- Matthäus, B., M. M. Özcan, F. A. Juhaimi, O. Q. Adiamo, O. N. Alsawmahi, K. Ghafoor, and E. E. Babiker. 2018b. Effect of the harvest time on oil yield, fatty acid, tocopherol and sterol contents of developing almond and walnut kernels. Journal of Oleo Science 67 (1):39–45. doi: 10.5650/jos.ess17162.
- Méndez-Zúñiga, S. M., J. E. Corrales-García, E. P. Gutiérrez-Grijalva, R. García-Mateos, V. Pérez-Rubio, and J. B. Heredia. 2019. Fatty acid profile, total carotenoids, and free radical-Scavenging from the lipophilic fractions of 12 native Mexican avocado accessions. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 74 (4):501–7. doi: 10.1007/s11130-019-00766-2.
- Mericli, F., E. Becer, H. Kabadayı, A. Hanoglu, D. Yigit Hanoglu, D. Ozkum Yavuz, T. Ozek, and S. Vatansever. 2017. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharmaceutical Biology 55 (1):1239–48. doi: 10.1080/13880209.2017.1296003.
- Miraliakbari, H., and F. Shahidi. 2008. Lipid class compositions, tocopherols and sterols of tree nut oils extracted with different solvents. Journal of Food Lipids 15 (1):81–96. doi: 10.1111/j.1745-4522.2007.00104.x.
- Moayedi, A., K. Rezaei, S. Moini, and B. Keshavarz. 2011. Chemical compositions of oils from several wild almond species. Journal of the American Oil Chemists’ Society 88 (4):503–8. doi: 10.1007/s11746-010-1701-z.
- Momchilova, S., and B. Nikolova-Damyanova. 2007. Quantitative TLC and gas chromatography determination of the lipid composition of raw and microwaved roasted walnuts, hazelnuts, and almonds. Journal of Liquid Chromatography & Related Technologies 30 (15):2267–85. doi: 10.1080/10826070701451647.
- Montealegre, C., L. Sánchez-Hernández, A. L. Crego, and M. L. Marina. 2013. Determination and characterization of glycerophospholipids in olive fruit and oil by nonaqueous capillary electrophoresis with electrospray-mass spectrometric detection. Journal of Agricultural and Food Chemistry 61 (8):1823–32. doi: 10.1021/jf304357e.
- Morimoto, T., A. Nagatsu, N. Murakami, J. Sakakibara, H. Tokuda, H. Nishino, and A. Iwashima. 1995. Anti-tumour-promoting glycerolglycolipids from the green alga, Chlorella vulgaris. Phytochemistry 40 (5):1433–7. doi: 10.1016/0031-9422(95)00458-j.
- Moussaoui, R., W. Labbaci, N. Hemar, A. Youyou, and Y. Amir. 2008. Physico-chemical characteristics of oils extracted from three compartments of the olive fruit (pulp, endocarp and seed) of variety Chemlal cultivated in Kabylia (Algeria). Journal of Food Agriculture and Environment 6 (2):52–5.
- Murakami, C., T. Kumagai, T. Hada, U. Kanekazu, S. Nakazawa, S. Kamisuki, N. Maeda, X. Xu, H. Yoshida, F. Sugawara, et al. 2003. Effects of glycolipids from spinach on mammalian DNA polymerases. Biochemical Pharmacology 65 (2):259–67. doi: 10.1016/S0006-2952(02)01483-1.
- Nagatsu, A., M. Watanabe, K. Ikemoto, M. Hashimoto, N. Murakami, J. Sakakibara, H. Tokuda, H. Nishino, A. Iwashima, and K. Yazawa. 1994. Synthesis and structure–anti-tumorpromoting activity relationship of monogalactosyl diacylglycerols. Bioorganic & Medicinal Chemistry Letters 4 (13):1619–22. doi: 10.1016/S0960-894X(01)80577-1.
- Napolitano, A., A. Cerulli, C. Pizza, S, and Piacente, S. 2018. Multi-class polar lipid profiling in fresh and roasted hazelnut (Corylus avellana cultivar “Tonda di Giffoni”) by LC-ESI/LTQOrbitrap/MS/MSn. Food Chemistry 269:125–35. doi: 10.1016/j.foodchem.2018.06.121.
- Nasopoulou, C., T. Smith, M. Detopoulou, C. Tsikrika, L. Papaharisis, D. Barkas, and I. Zabetakis. 2014. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chemistry 145:1097–105. doi: 10.1016/j.foodchem.2013.08.091.
- Nasopoulou, C., and I. Zabetakis, I. 2013. Agricultural and aquacultural potential of olive pomace: A review. The Journal of Agricultural Science 5 (7):116–27. doi: 10.5539/jas.v5n7p116.
- Nettleton, J. A. 1995. Omega-3 fatty acids and health. New York: Springer. doi: 10.1007/978-1-4615-2071-9.
- Ninot, A., J. Hermoso, A. Romero, and I. Batlle. 2018. ʻFargaʼ Olive. Journal- American Pomological Society 72 (1):21–8.
- Normén, L., L. Ellegård, H. Brants, P. Dutta, and H. Andersson. 2007. A phytosterol database: Fatty foods consumed in Sweden and the Netherlands. Journal of Food Composition and Analysis 20 (3-4):193–201. doi: 10.1016/j.jfca.2006.06.002.
- O'Donnell, V. B., K. Ekroos, G. Liebisch, and M. Wakelam. 2020. Lipidomics: Current state of the art in a fast moving field. Wiley Interdisciplinary Reviews. Systems Biology and Medicine 12 (1):e1466. doi: 10.1002/wsbm.1466.
- Ojeda-Amador, R. M., G. Fregapane, and M. D. Salvador. 2019. Chemical characterization of virgin almond and hazelnut oils and their by-products. European Journal of Lipid Science and Technology 121 (11):1900114. doi: 10.1002/ejlt.201900114.
- Okay, Y. 2002. The comparison of some pistachio cultivars regarding their fat, fatty acids and protein content. Gartenbauwissenschaft 67 (3):107–13.
- Oliveira, R., M. F. Rodrigues, and M. G. Bernardo-Gil. 2002. Characterization and supercritical carbon dioxide extraction of walnut oil. Journal of the American Oil Chemists’ Society 79 (3):225–30. doi: 10.1007/s11746-002-0465-y.
- Özcan, M. M., F. Al Juhaimi, K. Ghafoor, E. E. Babiker, and M. M. Özcan. 2020. Characterization of physico-chemical and bioactive properties of oils of some important almond cultivars by cold press and soxhlet extraction. Journal of Food Science and Technology 57 (3):955–61. doi: 10.1007/s13197-019-04128-3.
- Pacchiarotta, T., E. Nevedomskaya, A. Carrasco-Pancorbo, A. M. Deelder, and O. A. Mayboroda. 2010. Evaluation of GC-APCI/MS and GC-FID as a complementary platform. Journal of Biomolecular Techniques 21 (4):205–13.
- Pacetti, D., E. Boselli, P. Lucci, and N. G. Frega. 2007. Simultaneous analysis of glycolipids and phospholids molecular species in avocado (Persea americana Mill) fruit. Journal of Chromatography A 1150 (1-2):241–51. doi: 10.1016/j.chroma.2006.10.022.
- Parcerisa, J., R. Codony, J. Boatella, and M. Rafecas. 1999. Triacylglycerol and phospholipid composition of hazelnut (Corylus avellana L.) lipid fraction during fruit development. Journal of Agricultural and Food Chemistry 47 (4):1410–5. doi: 10.1021/jf980879q.
- Paroni, R., M. Dei Cas, J. Rizzo, R. Ghidoni, M. T. Montagna, F. M. Rubino, and M. Iriti. 2019. Bioactive phytochemicals of tree nuts. Determination of the melatonin and sphingolipid content in almonds and pistachios. Journal of Food Composition and Analysis. 82:103227. doi: 10.1016/j.jfca.2019.05.010.
- Pasini, F., Y. Riciputi, V. Verardo, and M. Caboni. 2013. Phospholipids in cereals, nuts and some selected oilseeds. Recent Research Development Lipids 9:139–201.
- Peou, S., B. Milliard-Hasting, and S. Shah. 2016. Impact of avocado-enriched diets on plasma lipoproteins: A meta-analysis. Journal of Clinical Lipidology 10 (1):161–71. doi: 10.1016/j.jacl.2015.10.011.
- Perdomo, L., N. Beneit, Y. F. Otero, Ó. Escribano, S. Díaz-Castroverde, A. Gómez-Hernández, and M. Benito. 2015. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovascular Diabetology 14:75. doi: 10.1186/s12933-015-0237-9.
- Phillips, K., D. Ruggio, and M. Ashraf-Khorassani. 2005. Phytosterol composition of nuts and seeds commonly consumed in the United States. Journal of Agricultural and Food Chemistry 53 (24):9436–45. doi: 10.1021/jf051505h.
- Piironen, V., D. Lindsay, T. Miettinen, J. Toivo, and A. Lampi. 2000. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. Journal of the Science of Food and Agriculture 80 (7):939–66. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.0.CO;2-C.
- Pinedo, S., M. N. Vissers, K. von Bergmann, K. Elharchaoui, D. Lütjohann, R. Luben, N. J. Wareham, J. J. P. Kastelein, K.-T. Khaw, and S. M. Boekholdt. 2007. Plasma levels of plant sterols and the risk of coronary artery disease: The prospective EPIC-Norfolk Population Study. Journal of Lipid Research 48 (1):139–44. doi: 10.1194/jlr.M600371-JLR200.
- Poletto, T., I. Poletto, L. M. Moraes Silva, M. F. Brião Muniz, L. R. Silveira Reiniger, N. Richards, and V. M. Stefenon. 2020. Morphological, chemical and genetic analysis of southern Brazilian pecan (Carya illinoinensis) accessions. Scientia Horticulturae 261:108863. doi: 10.1016/j.scienta.2019.108863.
- Rabadán, A., M. Álvarez-Ortí, R. Gómez, A. Pardo-Giménez, and J. Pardo. 2018. Characterization of pistachio oils and defatted flours regarding cultivar and geographic origin. Journal of Food Composition and Analysis 1:56–64. doi: 10.1016/j.jfca.2018.05.008.
- Ranalli, A., L. Pollastri, S. Contento, G. Di Loreto, E. Iannucci, L. Lucera, and F. Russi. 2002a. Acylglycerol and fatty acid components of pulp, seed, and whole olive fruit oils. Their use to characterize fruit variety by chemometrics. Journal of Agricultural and Food Chemistry 50 (13):3775–9. doi: 10.1021/jf011506j.
- Ranalli, A., L. Pollastri, S. Contento, G. Di Loreto, E. Lannucci, L. Lucera, and F. Russi. 2002b. Sterol and alcohol components of seed, pulp and whole olive fruit oils. Their use to characterise olive fruit variety by multivariates. Journal of the Science of Food and Agriculture 82 (8):854–9. doi: 10.1002/jsfa.1116.
- Reddy, M., R. Moodley, and S. Jonnalagadda. 2012. Fatty acid profile and elemental content of avocado (Persea americana Mill.) oil-effect of extraction methods. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes 47 (6):529–37. doi: 10.1080/03601234.2012.665669.
- Rico, R., M. Bulló, and J. Salas-Salvadó. 2016. Nutritional composition of raw fresh cashew (Anacardium occidentale L.) kernels from different origin. Food Science & Nutrition 4 (2):329–38. doi: 10.1002/fsn3.294.
- Robbins, K., E. Shin, R. Shewfelt, R. Eitenmiller, and R. Pegg. 2011. Update on the healthful lipid constituents of commercially important tree nuts. Journal of Agricultural and Food Chemistry 59 (22):12083–92. doi: 10.1021/jf203187v.
- Rocha, J., N. Borges, and O. Pinho. 2020. Table olives and health: A review. Journal of Nutritional Science 9:E57. doi: 10.1017/jns.2020.50.
- Rodrigues, F., F. Pimentel, and M. Oliveira. 2015. Olive by-products: Challenge application in cosmetic industry. Ind Crops Prod. 70:116–24. doi: 10.1016/j.indcrop.2015.03.027.
- Ros, E. 2015. Nuts and CVD. British Journal of Nutrition 113 (S2):S111–S120. doi: 10.1017/S0007114514003924.
- Rustan, A. D. 2005. Fatty acids: Structures and properties. In Encyclopedia of life sciences. Chichester: John Wiley and Sons.
- Ryan, E., K. Galvin, T. P. O'Connor, A. R. Maguire, and N. M. O'Brien. 2006. Fatty acid profile, tocopherol, squalene and phytosterol content of brazil, pecan, pine, pistachio and cashew nuts. International Journal of Food Sciences and Nutrition 57 (3-4):219–28. doi: 10.1080/09637480600768077.
- Rydlewski, A. A., J. S. Pizzo, L. P. Manin, M. B. Galuch, P. D. S. Santos, C. Zapiello, O. O. Santos, and J. V. Visentainer. 2020. Evaluation of possible fraud in avocado oil-based products from the composition of fatty acids by GC-FID and lipid profile by ESI-MS. Chemical Papers 74 (9):2799–812. doi: 10.1007/s11696-020-01119-z.
- Sakouhi, F., C. Absalon, H. Kallel, and S. Boukhchina. 2010. Comparative analysis of triacylglycerols from Olea europaea L. fruits using HPLC and MALDI-TOFMS. European Journal of Lipid Science and Technology 112 (5):574–9. doi: 10.1002/ejlt.200900079.
- Salgado, J., F. Danieli, M. Regitano-D'arce, A. Frias, and D. Mansi. 2008. The avocado oil (Persea americana Mill) as a raw material for the food industry. Ciência e Tecnologia de Alimentos 28:20–6. doi: 10.1590/S0101-20612008000500004.
- Santana, I., L. dos Reis, A. Torres, L. Cabral, and S. Freitas. 2015. Avocado (Persea americana Mill.) oil produced by microwave drying and expeller pressing exhibits low acidity and high oxidative stability. European Journal of Lipid Science and Technology 117 (7):999–1007. doi: 10.1002/ejlt.201400172.
- Sathe, S., N. Seeram, H. Kshirsagar, D. Heber, and K. Lapsley. 2008. Fatty acid composition of California grown almonds. Journal of Food Science 73 (9):C607–C614. doi: 10.1111/j.1750-3841.2008.00936.x.
- Seçmeler, Ö., and C. Galanakis. 2019. Olive fruit and olive oil. In Innovations in traditional foods. ed. C. Galanakis. 193–220. doi: Woodhead Publishing. 10.1016/b978-0-12-814887-7.00008-3.
- Schwingshackl, L., and G. Hoffmann. 2012. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 4 (12):1989–2007. doi: 10.3390/nu4121989.
- Scrimgeour, C., and J. Harwood. 2007. Fatty acid and lipid structure. In The lipid handbook with CD-ROM, eds. F. Gunstone, J. Harwood, and A. Dijkstra, 3rd ed. 15–50. Boca Raton: CRC Press.
- Seferoglu, S., H. Seferoglu, F. Tekintas, and F. Balta. 2006. Biochemical composition influenced by different locations in Uzun pistachio cv. (Pistacia vera L.) grown in Turkey. J Food Compos Anal. 19 (5):461–5. doi: 10.1016/j.jfca.2006.01.009.
- Sekora, N., K. Lawrence, P. Agudelo, E. Van Santen, and J. Mcinroy. 2009. Using FAME analysis to compare, differentiate, and identify multiple nematode species. J Nematol 41 (3):163–73.
- Sena-Moreno, E., J. Pardo, L. Catalán, R. Gómez, A. Pardo-Giménez, and M. Alvarez-Ortí. 2015. Drying temperature and extraction method influence physicochemical and sensory characteristics of pistachio oils. European Journal of Lipid Science and Technology 117 (5):684–91. doi: 10.1002/ejlt.201400366.
- Sena-Moreno, E., J. Pardo, A. Pardo-Giménez, R. Gómez, and M. Alvarez-Ortí. 2016. Differences in oils from nuts extracted by means of two pressure systems. International Journal of Food Properties 19 (12):2750–60. doi: 10.1080/10942912.2016.1144068.
- Shahbandeh, M. 2020. Forecast value nuts and seeds market worldwide in 2018 and 2023. Accessed February 24, 2020. https://www.statista.com/statistics/934360/global-seeds-nuts-market-value/.
- Shahbandeh, M. 2022. Global avocado production 2000-2020. Accessed March 27, 2022. https://www.statista.com/statistics/577455/world-avocado-production/.
- Shen, Q., W. Dong, M. Yang, J. T. Baibado, Y. Wang, I. Alqouqa, and H.-Y. Cheung. 2013. Lipidomic study of olive fruit and oil using TiO2 nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS. Food Research International 54 (2):2054–61. doi: 10.1016/j.foodres.2013.10.001.
- Shen, Q., W. Dong, M. Yang, L. Li, H. Cheung, and Z. Zhang. 2013. Lipidomic fingerprint of almonds (Prunus dulcis L. cv Nonpareil) using TiO2 nanoparticle based matrix solid-phase dispersion and MALDI-TOF/MS and its potential in geographical origin verification. Journal of Agricultural and Food Chemistry 61 (32):7739–48. doi: 10.1021/jf4016448.
- Shen, Q., M. Yang, L. Li, and H. Cheung. 2014. Graphene/TiO2 nanocomposite based solid-phase extraction and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for lipidomic profiling of avocado (Persea americana Mill.). Analytica Chimica Acta 852:153–61. doi: 10.1016/j.aca.2014.09.022.
- Shi, C., X. Luo, J. Wang, and D. Long. 2015. Incorporation of β-sitosterol into the membrane prevents tumor necrosis factor-α-induced nuclear factor-κB activation and gonadotropin-releasing hormone decline. Steroids 96:1–6. doi: 10.1016/j.steroids.2014.12.014.
- Shin, E., R. Pegg, R. Phillips, and R. Eitenmiller. 2010. Commercial peanut (Arachis hypogaea L.) cultivars in the United States: Phytosterol composition. Journal of Agricultural and Food Chemistry 58 (16):9137–46. doi: 10.1021/jf102150n.
- Singleton, J., and H. Pattee. 1987. Characterization of peanut oil tricacyglycerols by HPLC, GLC and EIMS. Journal of the American Oil Chemists’ Society 64 (4):534–8. doi: 10.1007/BF02636389.
- Soler, L., J. Cañellas, and F. Saura-Calixto. 1988. Oil content and fatty acid composition of developing almond seeds. Journal of Agricultural and Food Chemistry 36 (4):695–7. doi: 10.1021/jf00082a007.
- Song, S., L.-Z. Cheong, H. Wang, Q.-Q. Man, S.-J. Pang, Y.-Q. Li, B. Ren, Z. Wang, and J. Zhang. 2018. Characterization of phospholipid profiles in six kinds of nut using HILIC-ESI-IT-TOF-MS system. Food Chemistry 240:1171–8. doi: 10.1016/j.foodchem.2017.08.021.
- Steinmann, D., R. R. Baumgartner, E. H. Heiss, S. Bartenstein, A. G. Atanasov, V. M. Dirsch, M. Ganzera, and H. Stuppner. 2012. Bioguided isolation of (9Z)-Octadec-9-enoic acid from Phellodendron amurense Rupr. and identification of fatty acids as PTP1B inhibitors. Planta Medica 78 (3):219–24. doi: 10.1055/s-0031-1280377.
- Sun, N., J. Chen, D. Wang, and S. Lin. 2018. Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities. Trends in Food Science and Technology 80:199–211. doi: 10.1016/j.tifs.2018.08.010.
- Takenaga, F., K. Matsuyama, S. Abe, Y. Torii, and S. Itoh. 2008. Lipid and fatty acid composition of mesocarp and seed of avocado fruits harvested at northern range in Japan. Journal of Oleo Science 57 (11):591–7. doi: 10.5650/jos.57.591.
- Tan, C., G. Chong, H. Hamzah, and H. Ghazali. 2018. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. The Journal of Supercritical Fluids 135:45–51. doi: 10.1016/j.supflu.2017.12.036.
- Tan, C., S. Tan, and S. Tan. 2017. Influence of geographical origins on the physicochemical properties of Hass avocado oil. Journal of the American Oil Chemists’ Society 94 (12):1431–7. doi: 10.1007/s11746-017-3042-7.
- Tanriverdi, E. 2016. The physico-chemical properties and fatty acid composition of three different hazelnut varieties collected at the different harvest periods. Journal of Agroalimentary Processes and Technologies 22:127–31.
- Taş, N., and V. Gökmen. 2015. Profiling triacylglycerols, fatty acids and tocopherols in hazelnut varieties grown in Turkey. Journal of Food Composition and Analysis. 44:115–21. doi: 10.1016/j.jfca.2015.08.010.
- Terramoccia, S., S. Bartocci, A. Taticchi, S. Di Giovanni, M. Pauselli, E. Mourvaki, S. Urbani, and M. Servili. 2013. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australasian Journal of Animal Sciences 26 (7):971–80. doi: 10.5713/ajas.2012.12627.
- Tiihonen, K., M. Saarinen, E. Alhoniemi, N. Mitsuya, and G. Yamaki. 2016. Effect of dietary betaine on metabolic syndrome risk factors in Asian males with mild fatty liver. Journal of Diabetes & Metabolism 7 (7):1000692 doi: 10.4172/2155-6156.1000692.
- Tokusoglu, O. 2011. The oxidative stability, lipid profiles and total phenolics of almonds (Prunus amygdalus L.) and pistachios (Pistacia vera L.). Acta Horticulturae 912 (912):781–9. doi: 10.17660/ActaHortic.2011.912.119.
- Trautwein, E., M. Vermeer, H. Hiemstra, and R. Ras. 2018. LDL-cholesterol lowering of plant sterols and stanols—which factors influence their efficacy? Nutrients 10 (9):1262. doi: 10.3390/nu10091262.
- Tsantili, E., C. Takidelli, M. Christopoulos, E. Lambrinea, D. Rouskas, and P. Roussos. 2010. Physical, compositional and sensory differences in nuts among pistachio (Pistachia vera L.) varieties. Scientia Horticulturae (Amsterdam) 125 (4):562–8. doi: 10.1016/j.scienta.2010.04.039.
- Vecka, M., B. Staňková, S. Kutová, P. Tomášová, E. Tvrzická, and A. Žák. 2019. Comprehensive sterol and fatty acid analysis in nineteen nuts, seeds, and kernel. SN Applied Sciences 1 (12):1531. doi: 10.1007/s42452-019-1576-z.
- Veličkovska, S., S. Mitrev, and L. Mihajlov. 2016. Physicochemical characterization and quality of cold-pressed peanut oil obtained from organically produced peanuts from Macedonian “Virginia” variety. Grasas y Aceites 67 (1):e118. doi: 10.3989/gya.0369151.
- Venkatachalam, M., and S. K. Sathe. 2006. Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry 54 (13):4705–14. doi: 10.1021/jf0606959.
- Verardo, V., Y. Riciputi, G. Sorrenti, P. Ornaghi, B. Marangoni, and M. Caboni. 2013. Effect of nitrogen fertilisation rates on the content of fatty acids, sterols, tocopherols and phenolic compounds, and on the oxidative stability of walnuts. LWT – Food Science and Technology 50 (2):732–8. doi: 10.1016/j.lwt.2012.07.018.
- Vesper, H., E. Schmelz, M. Nikolova-Karakashian, D. Dillehay, D. Lynch, and A. Merrill. 1999. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. The Journal of Nutrition 129 (7):1239–50. doi: 10.1093/jn/129.7.1239.
- Villa-Rodríguez, J., F. Molina-Corral, J. Ayala-Zavala, G. Olivas, and G. González-Aguilar. 2011. Effect of maturity stage on the content of fatty acids and antioxidant activity of “Hass” avocado. Food Research International 44 (5):1231–7. doi: 10.1016/j.foodres.2010.11.012.
- Vinha, A. F., F. Ferreres, B. M. Silva, P. Valentão, A. Gonçalves, J. A. Pereira, M. Oliveira, R. M. Seabra, and P. B. Andrade. 2005. Phenolic profiles of portuguese olive fruits (Olea europaea L.): influences of cultivar and geographical origin. Food Chemistry 89 (4):561–8. doi: 10.1016/j.foodchem.2004.03.012.
- Vlahov, G., C. Schiavone, and N. Simone. 1999. Triacylglycerols of the olive fruit (Olea europaea L.): characterization of mesocarp and seed triacylglycerols in different cultivars by liquid chromatography and 13C NMR spectroscopy. Lipid - Fett 101 (4):146–50. doi: 10.1002/(SICI)1521-4133(199904)101:4<146::AID-LIPI146>3.0.CO;2-3.
- Wang, L., B. Waltenberger, E.-M. Pferschy-Wenzig, M. Blunder, X. Liu, C. Malainer, T. Blazevic, S. Schwaiger, J. M. Rollinger, E. H. Heiss, et al. 2014. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochemical Pharmacology 92 (1):73–89. doi: 10.1016/j.bcp.2014.07.018.
- Wang, M., L. Zhang, X. Wu, Y. Zhao, L. Wu, and B. Lu. 2019. Quantitative determination of free and esterified phytosterol profile in nuts and seeds commonly consumed in China by SPE/GC–MS. LWT 100:355–61. doi: 10.1016/j.lwt.2018.10.077.
- Wang, R., T. Furumoto, K. Motoyama, K. Okazaki, H. Fukui, and R. Ang. 2002. Possible antitumor promoters in Spinacia oleracea (spinach) and comparison of their contents among cultivars. Bioscience, Biotechnology, and Biochemistry 66 (2):248–54. doi: 10.1271/bbb.66.248.
- Wang, W., H. Wang, X. Xiao, and X. Xu. 2019. Chemical composition analysis of seed oil from five wild almond species in China as potential edible oil resource for the future. South African Journal of Botany 121:274–81. doi: 10.1016/j.sajb.2018.11.009.
- Widmer, R., A. Flammer, L. Lerman, and A. Lerman. 2015. The Mediterranean diet, its components, and cardiovascular disease. The American Journal of Medicine 128 (3):229–38. doi: 10.1016/j.amjmed.2014.10.014.
- Wiktorowska-Owczarek, A., M. Berezińska, M, and J. Nowak. 2015. PUFAs: Structures, metabolism and functions. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University 24 (6):931–41. doi: 10.17219/acem/31243.
- Wu, J. H. Y., R. N. Lemaitre, I. B. King, X. Song, B. M. Psaty, D. S. Siscovick, and D. Mozaffarian. 2014. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: The cardiovascular health study. Circulation 130 (15):1245–53. doi: 10.1161/CIRCULATIONAHA.114.011590.
- Yada, S., G. Huang, and K. Lapsley. 2013. Natural variability in the nutrient composition of california-grown almonds. Journal of Food Composition and Analysis. 30 (2):80–5. doi: 10.1016/j.jfca.2013.01.008.
- Yahia, E., and A. Woolf. 2011. Avocado (Persea americana Mill.). In Postharvest biology and technology of tropical and subtropical fruits, 125–186e. Cambridge, UK: Woodhead Publishing.
- Yanty, N., J. Marikkar, and K. Long. 2011. Effect of varietal differences on composition and thermal characteristics of avocado oil. Journal of the American Oil Chemists’ Society 88 (12):1997–2003. doi: 10.1007/s11746-011-1877-x.
- Yıldız, M., Ş. Gürcan, and M. Özdemir. 1998. Oil composition of pistachio nuts (Pistacia vera L.) from Turkey. Lipid – Fett 100 (3):84–6. doi: 10.1002/(sici)1521-4133(199803)100:3<84::aid-lipi84>3.0.co;2-6.
- Yorulmaz, A., Y. Velioglu, A. Tekin, A. Simsek, J. Drover, and J. Ates. 2009. Phytosterols in 17 Turkish hazelnut (Corylus avellana L.) cultivars. European Journal of Lipid Science and Technology 111 (4):402–8. doi: 10.1002/ejlt.200800187.
- Yoshida, H., Y. Hirakawa, Y. Tomiyama, T. Nagamizu, and Y. Mizushina. 2005. Fatty acid distributions of triacylglycerols and phospholipids in peanut seeds (Arachis hypogaea L.) following microwave treatment. Journal of Food Composition and Analysis 18 (1):3–14. doi: 10.1016/j.jfca.2003.12.004.
- Zamany, A., G. Samadi, D. Kim, Y. Keum, and R. Saini. 2017. Comparative study of tocopherol contents and fatty acids composition in twenty almond cultivars of Afghanistan. Journal of the American Oil Chemists’ Society 94 (6):805–17. doi: 10.1007/s11746-017-2989-8.
- Zanqui, A., C. da Silva, J. Ressutte, D. de Morais, J. Santos, M. Eberlin, L. Cardozo-Filho, J. Visentainer, S. Gomes, and M. Matsushita. 2020a. Brazil nut oil extraction using subcritical n-propane: Advantages and chemical composition. Journal of the Brazilian Chemical Society 31 (3):603–12. doi: 10.21577/0103-5053.20190225.
- Zanqui, A. B., C. M. da Silva, J. B. Ressutte, D. R. de Morais, J. M. Santos, M. N. Eberlin, L. Cardozo-Filho, E. A. da Silva, S. T. M. Gomes, and M. Matsushita. 2020b. Extraction and assessment of oil and bioactive compounds from cashew nut (Anacardium occidentale) using pressurized n-propane and ethanol as cosolvent. The Journal of Supercritical Fluids 157:104686. doi: 10.1016/j.supflu.2019.104686.
- Zhao, Y., L. Wang, J. Qiu, D. Zha, Q. Sun, and C. Chen. 2013. Linoleic acid stimulates [Ca2+]i increase in rat pancreatic beta-cells through both membrane receptor- and intracellular metabolite-mediated pathways. PloS One 8 (4):e60255. doi: 10.1371/journal.pone.0060255.
- Zhu, Y., K. Wilkinson, and M. Wirthensohn. 2017. Changes in fatty acid and tocopherol content during almond (Prunus dulcis, cv. Nonpareil) kernel development. Scientia Horticulturae (Amsterdam) 225:150–5. doi: 10.1016/j.scienta.2017.07.008.
- Züge, L., H. Maieves, J. Silveira, V. Silva, and A. Scheer. 2017. Use of avocado phospholipids as emulsifier. LWT – Food Science and Technology 79:42–51. doi: 10.1016/j.lwt.2017.01.013.