Abstract
The detrimental impact of foodborne pathogens on human health makes food safety a major concern at all levels of production. Conventional methods to detect foodborne pathogens, such as live culture, high-performance liquid chromatography, and molecular techniques, are relatively tedious, time-consuming, laborious, and expensive, which hinders their use for on-site applications. Recurrent outbreaks of foodborne illness have heightened the demand for rapid and simple technologies for detection of foodborne pathogens. Recently, Lateral flow assays (LFA) have drawn attention because of their ability to detect pathogens rapidly, cheaply, and on-site. Here, we reviewed the latest developments in LFAs to detect various foodborne pathogens in food samples, giving special attention to how reporters and labels have improved LFA performance. We also discussed different approaches to improve LFA sensitivity and specificity. Most importantly, due to the lack of studies on LFAs for the detection of viral foodborne pathogens in food samples, we summarized our recent research on developing LFAs for the detection of viral foodborne pathogens. Finally, we highlighted the main challenges for further development of LFA platforms. In summary, with continuing improvements, LFAs may soon offer excellent performance at point-of-care that is competitive with laboratory techniques while retaining a rapid format.
Introduction
In the last decade, outbreaks of foodborne diseases from various food sources have raised public awareness of food safety (Karp et al. Citation2015). According to the World Health Organization (WHO Citation2022), around 600 million individuals – almost 1 in 10 people worldwide– acquire foodborne infections after eating contaminated food each year. In addition, nearly 420,000 individuals die yearly from diarrheal disorders (WHO Citation2022). A substantial number of these fatalities were avoidable through early detection of pathogens in food and water (WHO Citation2022, Citation2016). Unfortunately, children under five years of age carry 40% of the foodborne disease burden, with 125,000 deaths yearly (WHO Citation2022). The symptoms of foodborne diseases range from simple gastroenteritis to potentially catastrophic neurologic, hepatic, and renal complications (Fung, Wang, and Menon Citation2018). The majority of foodborne diseases are attributed to bacteria (Campylobacter spp., Salmonella spp., Staphylococcus aureus (S. aureus), Vibrio cholera (V. cholera), Escherichia coli (E.coli) O157:H7, Clostridium perfringens, and Listeria monocytogenes (L. monocytogenes)), viruses (Norovirus, Hepatitis E, Hepatitis A, Rotavirus, Adenoviruses, Sapoviruses, and Astroviruses), and protozoa (Cryptosporidium spp., Cyclospora spp., and Toxoplasma spp.) (Bintsis Citation2017; Adley and Ryan Citation2016).
Foodborne diseases impede socioeconomic development by straining healthcare systems and harming national economies, tourism, and international food trade. Around $110 billion is lost annually in productivity and medical expensesas a result of contaminated food with foodborne pathogens in low-income and middle-income countries (WHO Citation2022, Citation2016). In addition, globalization of trade has increased the risk of the transnational spread of foodborne diseases in the current scenario. Although they were once limited to small communities, many outbreaks of foodborne diseases now have global consequences (Scott Citation2003).
Implementing effective monitoring systems that include laboratory readiness is one of the most rational and reasonable strategies to avoid or minimize the harmful effects of foodborne diseases in people. Diagnostic food labs play critical roles in identifying and isolating foodborne pathogens using conventional and molecular diagnostic methods, which are the cornerstone of pathogen detection and identification. However, these methods are relatively tedious, time-consuming, laborious, and expensive. Therefore, on-site quick diagnostic techniques that are robust, efficient, sensitive, and cost-effective are urgently needed to speed up the detection of foodborne pathogens.
In the past 25 years, biosensors for rapid pathogen detection have been developed based on integrating a sensitive transducer and a selective biorecognition element. These biosensors provide quantitative or semiquantitative analytical measurements without requiring other chemicals or processing steps (Cesewski and Johnson Citation2020), enabling on-site pathogen quantification and identification that complement laboratory-based methods like polymerase chain reaction (PCR) and Enzyme-linked immunosorbent assay (ELISA) (Sohrabi et al. Citation2021). Biosensors have been implemented in various analytical techniques for environmental, medical, food safety, industrial processing, defence, and security applications (Arora, Chand, and Malhotra Citation2006). During the COVID-19 pandemic, lateral flow assays (LFAs) attracted wide attention as one of the most important biosensing platforms; however, extensive efforts are still being made in academia and industry to improve the performance of LFA-based testing (Kim and Lee Citation2022).
In this review, we highlighted the principles and features of LFA-based strategies for foodborne pathogens detection in food samples, focusing on recent improvements in LFA platforms for ultra-sensitive detection of foodborne pathogens. We also discussed different approaches to improve LFA sensitivity and specificity. We also address the utilization of various reporters for signal amplification, including; nanoparticles (NPs) (Pashazadeh-Panahi et al. Citation2021), nanomaterials (Soozanipour et al. Citation2021) and other labeling materials. Most importantly, due to the lack of studies on LFA for the detection of viral foodborne pathogens in food samples, we summarized our recent research on developing LFA for the detection of viral foodborne pathogens. In summary, with continuing improvements, LFAs may become the fastest (<30 min), ultrasensitive (PCR-level), and “sample-to-answer” point of care (POC) diagnostics test.
Current challenges in conventional methods for foodborne testing
Current technologies for foodborne pathogen screening require labor-intensive sample enrichment steps, pathogen isolation and purification, and costly readout machinery. and summarize the advantages and disadvantages of the 5 main types of technologies used to identify foodborne pathogens in food samples.
Figure 1. Comparison of the sensitivity, price ($least expensive; $$more expensive; $$$most expensive), and detection time of signal-amplified LFAs compared emerging isothermal nucleic acid amplification diagnostics, PCR, digital enzyme- linked immunosorbent assay (dELISA), and commercial diagnostic tools. Figure created using BioRender.com and adapted from (Liu et al. Citation2021).
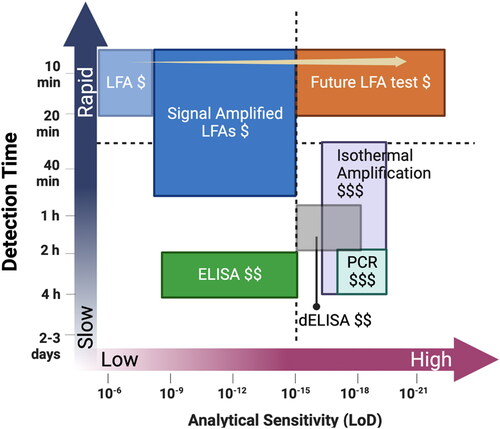
Table 1. Five main types of technologies used to identify foodborne pathogens.
Conventional live culture remains the gold standard for foodborne pathogen detection and identification; however, this method is tedious, laborious, and time-consuming (2–3 days) (Zhao et al. Citation2014). Culture methods have also been reported to show poor sensitivity for low-level contamination with a high background of indigenous microflora in the samples, rendering the recovery of the target organism difficult. Although it is highly specific, conventional culture method sensitivities vary depending on the type of pathogen. For example, the limit of detection (LoD)for the detection of; Salmonella Spp was 1.76 colony forming unites/ ml (CFU/ml) (Sharif and Tayeb Citation2021), for Campylobacter spp was 0.3–5 × 106 CFU/mL (Buss et al. Citation2019), for Shigella spp was 106 CFU/mL (Jiménez, McCoy, and Achí Citation2010), for Listeria spp was ≤104 cells/mL (Gasanov, Hughes, and Hansbro Citation2005), and for Escherichia coli (E. coli) was 102 CFU/g (J. O’Sullivan et al. Citation2007). Most importantly, false negative results may occur due to viable but non-culturable pathogens. The failure to detect foodborne pathogens would increase the transmission risk of pathogens.
One of the most commonly used molecular-based methods for the detection of foodborne bacterial pathogens is PCR. PCR is effective clinical procedure for the rapid detection and recognition of pathogens in the healthcare system (Kawasaki et al. Citation2009, Citation2010); however, they are also expensive, requires specialized equipment and highly trained personnel, and relies on extensive sample pretreatment and costly instruments (Buckwalter et al. Citation2014). PCR have been used in the detection of numerous foodborne pathogens like Salmonella spp (LoD:103 CFU/mL (Tang et al. Citation2018)), Campylobacter jejuni (LoD: 102 CFU/ml (Jelenik et al. Citation2005)), Shigella spp (LoD: 101 CFU/mL (Tang et al. Citation2018)), Listeria spp (LoD:103–104 CFU/mL (Li, Ye, et al. Citation2020)), and E. coli O157:H7 (LoD: 103 CFU/mL (Wei et al. Citation2018)).
Loop-mediated isothermal amplification (LAMP), has been regarded as an innovative gene amplification technology and emerged as an alternative to PCR-based methodologies in both clinical laboratory and food safety testing. Due to its rapidity and sensitivity, LAMP has been used to detect various foodborne pathogens. LAMP is proven to be more specific and sensitive as compared to PCR assays for the detection of foodborne pathogens including; Vibrio parahaemolyticus (V. parahaemolyticus) (LoD:10 CFU/reaction (Wang et al. Citation2013)), Vibro vulnificus (LoD: 2.5x 103CFU/g (Han, Wang, and Ge Citation2011)), Salmonella (LoD: 101 CFU/mL (Techathuvanan, Draughon, and D’Souza Citation2010)), S. aureus (LoD: 3.4 CFU/g (Jiang et al. Citation2020)), and Shigella (LoD: 5CFU/10 mL). Commercial LAMP kits are available for the detection of Enterohemorrhagic Escherichia Coli (EHEC), Salmonella and L. monocytogenes (Yamazaki et al. Citation2018)
Antibody-based immunoassays such as ELISA (Shen et al. 2014) are easier to perform than other antibody-based methods, but it is still difficult to deploy in on-site settings because of its requirements for special equipment and operating expertise (Zhao et al. Citation2014). In addition, the relatively poor sensitivity of ELISA remains a significant drawback. Many studies have been performed using ELISA for rapid detection of foodborne pathogens such as Salmonella (LoD: 104 to 105 CFU/mL (Paniel and Noguer Citation2019)), Campylobacter (LoD: 105 to 106 CFU/mL (Hochel et al. Citation2007), Listeria (LoD: 6.6 × 103 CFU/mL (Portanti et al. Citation2011)), E.coli (LoD: 6.8 × 102 to 6.8 × 103 CFU/mL (Shen et al. 2014)), and V. parahaemolyticus (LoD: 103 cells (Kumar et al. Citation2011)). Commercial ELISA test kit such as BIOLINE Salmonella ELISA is also available to detect Salmonella in food products. The LoD of this test kit was 1 CFU/25 g sample with a minimum of four of the 20 food matrixes tested (Bolton et al. Citation2000). In addition, high-throughput and automated ELISA systems such as VIDAS (BioMerieux) and Assurance EIA (BioControl) are available for the detection of foodborne pathogens (Glynn et al. Citation2006). Several studies applied VIDAS for the detection of Salmonella in pork samples, fruits and vegetables (Vieira-Pinto et al. Citation2007; Gomez-Govea et al. Citation2012), L. monocytogenes in fish samples, beef, pork, fruits, and vegetables (Vaz-Velho, Duarte, and Gibbs Citation2000; Meyer et al. Citation2011; Gomez-Govea et al. Citation2012), E. coli O157:H7 in Minas Frescal cheese, fruits, and vegetables (Gomez-Govea et al. Citation2012; Carvalho et al. Citation2014), Campylobacter spp. in fruits and vegetables (Gomez-Govea et al. Citation2012) and staphylococcal enterotoxin in raw milk cheese (Cremonesi et al. Citation2007).
The recent progress in multi-gene detection technology includes microarray technology (Call, Brockman, and Chandler Citation2001). Microarrays were initially used for the study of gene expression, but oligonucleotide DNA microarray has been widely studied for the detection of foodborne pathogens. Wang et al. developed a microarray assay that detected and identified 22 foodborne pathogens (Wang et al. Citation2007) including; S. aureus, L. monocytogenes, V. parahaemolyticus, Vibrio cholerae, Campylobacter jejuni, Clostridium perfringens, Shigella spp. Salmonella spp., and Bacillus cereus (B. cereus) with LoD varying between 101 to 103 CFU/ml (Wang et al. Citation2007). Despite their high sensitivity, microarrays are not desirable for microbial food analysis because low to medium density array will serve as the ideal microarray platform that can provide reliable results without involving the use of complicated equipment’s and data management. The most significant disadvantages of microarrays include the low accuracy, precision, specificity, and high cost of a single experiment.
Chromatographical methods are another standard technique for food testing (Eugster et al. Citation2011). They are sensitive and accurate despite their many drawbacks, such as being time-consuming, tedious, laborious, multi-complex, and limited for detecting bacterial-borne pathogens. Sun et al. (Sun et al. Citation2017) developed a multiplex PCR-based procedure followed by high-performance liquid chromatography (mPCR-HPLC) assay for high-throughput screening foodborne pathogens, including; Salmonella spp., L. monocytogenes, Enterobacter sakazakii, S. aureus, Shigella spp., E. coli O157:H7, V. parahaemolyticus, Vibrio cholerae, and Vibrio vulnificus. The detection limit of mPCR-HPLC was 101 CFU/mL in pure cultures and less than 102 CFU/g in contaminated matrixes (Sun et al. Citation2017).
None of those mentioned above methods perfectly fulfill the criteria for the urgently required on-site multiplex detection system for foodborne pathogens. Recently, LFA has evolved to fill this gap and to offer performance at the POC that is competitive with laboratory techniques while retaining a rapid format. In the following sections, the principles and features of LFA-based strategies for foodborne pathogens detection in food samples were highlighted, focusing on recent improvements in LFA platforms for detecting bacterial and viral foodborne pathogens.
LFA: definition and assay formats
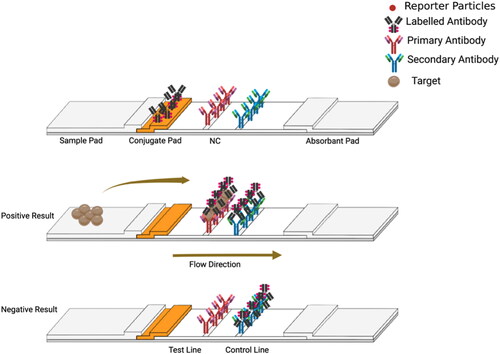
The LFA is a paper-based platform for detection and quantification of analytes in complex mixtures, where the sample is placed on a test device, and the results are displayed within 5–30 min. Atypical LFA has four components: a sample pad, a conjugate release pad, a nitrocellulose membrane (NC), and an absorption pad. All four components are laminated onto a sheet of plastic backing. Sandwich and competitive assays are the two standard formats for LFA, and each has distinct characteristics and benefits.
Sandwich LFA
In the sandwich assay format, three different antibodies are usually used; (1) conjugate antibodies, immobilized on the conjugation pad, which recognize one epitope in the target. These antibodies are linked to reporter particles, (2) capture antibodies, immobilized at the test line on the NC, which recognize another epitope of the target, and (3) anti-species antibodies, which are immobilized on the control line. Positive results are obtained when the conjugated labeled antibody (Ab) antigen (Ag) complex binds to the antibodies on the test line, and any extra labeled antibodies are collected at the control line forming two lines as shown in . Negative results are obtained when the reaction antibody only reacts with the anti-species antibodies on the control line. Hence, only one red line will develop at the control line. The quantity of analyte present in the sample can be determined by the color intensity that is visible at the test line. Typically, this test format is used for large analytes with multiple epitopes.
Competitive LFA
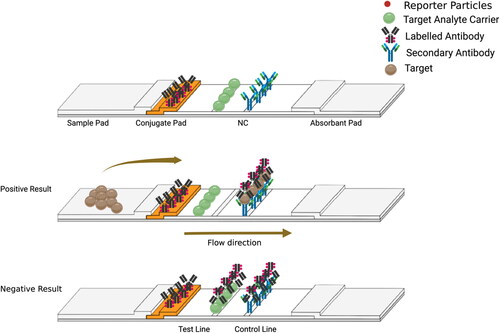
There are two different setups that may be used in the competitive immunoassay format. In the first setup (), the labeled analyte is immobilized on the conjugation pad. When the target is absent in the sample, the labeled analyte flows and binds to the detection antibody and the secondary antibody (anti-species) immobilized on the test and control line, respectively (Sajid, Kawde, and Daud Citation2015). Hence, two red lines develop on the test and control lines. When the target analyte is present in the sample, the unlabeled analyte competes with the labeled analyte immobilized on the conjugation pad and binds to the test line. Whereas the labeled analyte binds to the secondary antibodies on the control line. Hence, only a single red line develops on the control line (Pohanka Citation2021).
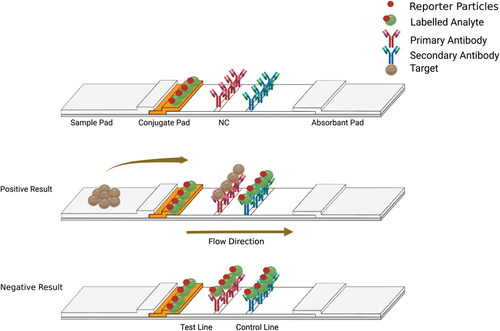
Figure 3. Schematic diagram shows the competitive format (First setup). The color intensity developed at the test line is inversely proportional to the amount of analyte. Figure created using BioRender.com.
In the second setup (), the labeled antibodies are immobilized on the conjugation pad. When the target is absent in the sample, the labeled antibodies flow and bind to the target analyte carrier and the secondary antibody on the test and control line. Hence, two red lines develop. When the target analyte is present in the sample, the target analyte binds to the labeled antibodies immobilized on the conjugation pad. Hence, these labeled antibodies will not be able to bind to the target analyte carrier on the test lines. However, the labeled antibodies conjugated to the target analyte will bind to the secondary antibodies immobilized on the control line. Thus, only a single red line develops on the control line.
Reporter agents and reading instruments
An optical signal is generated from reporter particles bound at the test line in the LFA. This signal can be read qualitatively or semi-quantitatively by the naked eye or using an optical reader. To maximize the sensitivity of an LFA, each binding event between the target and the reporter should produce the strongest possible signal. Large reporters usually result in robust signal per binding event; however, reporter particles that are too large do not easily flow through the NC and have fewer opportunities to bind at the test line. Therefore, a small reporter with a diameter ranging between 20 to 500 nm is typically selected for use in LFA.
In recent years, the utilization of novel nanomaterials as reporter molecules has increased dramatically. Gold nanoparticles (AuNPs) are the most commonly used reporters in mass-produced tests (Nguyen et al. Citation2020; Ge et al. Citation2014; Quesada-González and Merkoçi Citation2018), as they permit naked-eye detection. This is highly advantageous for quality applications or applications seeking cost efficiency, as it does not require an external reader. Combining nanoparticle-based detection with an external reader may increase reproducibility and provide quantitative results. The following sections highlight what we consider to be the most significant nanoparticles in terms of their readout type (see for a summary of commercially available reporter agents).
Table 2. Advantages and disadvantages of commercially available reporter agents.
Gold nanoparticles (AuNPs)
Since the 1980s, AuNPs have been the most frequently used detection labels in LFAs (Verheijen et al. Citation2000; Fong et al. Citation2000; Shyu et al. Citation2002). The reasons for their popularity include (1) production of a robust red color for naked-eye detection, (2) availability in different sizes and shapes, (3) low toxicity, (4) ease of functionalization via covalent bonding (Parolo, de la Escosura-Muñiz, and Merkoçi Citation2013; Di Nardo et al. Citation2019; Mao et al. Citation2009), and (5) high stability. The size and shape of conventional AuNPs can also be modified to achieve higher sensitivity. In addition, the optical signal of AuNPs in colorimetric LFAs can be amplified by depositing enzymes and silver ions (Sajid, Kawde, and Daud Citation2015). AuNPs produce red bands at the test and control lines of the LFA when acting as reporter particles. AuNPs can also act as carriers if coupled with an antibody modified with horseradish peroxidase (HRP) enzyme. Once substrates are added, they produce insoluble chromogens, which cannot be moved by the flow, concentrating the color at the test and control lines. LFAs that utilize the HRP enzyme produce two different optical signals: one produced by the red color of the AuNPs and the other by the substrate of the HRP, which is more sensitive, achieving an ‘on-demand’ tuning of the biosensing performance (Parolo, de la Escosura-Muñiz, and Merkoçi Citation2013).
Another method to enhance the sensitivity of AuNPs is by using silver ions, which tend to gather around the nano-gold in the form of silver under the action of the reducing agent. Silver enhancement technology has been frequently used in immunogold assays to amplify the signal of the colloidal gold probe (Rodríguez et al. Citation2016). summarize LFA studies in which AuNPs were used for the detection of foodborne pathogens.
Table 3. Summary of studies on LFAs for the detection of foodborne pathogens.
Magnetic nanoparticles (MNPs)
Recently, iron oxide magnetic nanoparticles (MNPs) have received interest as promising materials for rapid and high-sensitive diagnosis methods due to their unique properties (Xu et al. Citation2019). When an external magnet is present, MNPs can function as "nano-magnets" in the system. They move very quickly in the direction of the external magnet, but as soon as the magnetic field is removed, they lose all of their magnetism (Ha et al. Citation2018). In addition, it is a well-established fact that MNPs are able to retain their magnetic properties while forming stable conjugates with a wide range of biomaterials. Due to these properties, MNPs enable magnetic pre-concentration of a target from a very diluted concentration via two steps: (1) targets interact with bioconjugated MNPs, and (2) target-bioconjugated MNPs are collected with an external magnet and re-dispersed into a small volume of matrix for the pre-concentration of samples to improve the detection sensitivity. Most importantly, MNPs offer low background noise since the biological materials that do not interact with MNPs are non-magnetic (Gowri, Ashwin Kumar, and Suresh Anand Citation2021). Furthermore, MNPs have a high surface area, which enables quick interaction between the target and antibodies conjugated to MNPs, which in turn reduces the amount of time required for detection. Therefore, MNPs have been successfully used for the quick and extremely sensitive detection of a wide variety of target analytes, ranging from viruses (Castilho et al. Citation2011; Sánchez-Cano et al. Citation2021) and bacteria (Pappert et al. Citation2010; Mun and Choi Citation2015) to food allergens (Speroni et al. Citation2010; Yin et al. Citation2022).
Because MNPs are capable of transmitting both an optical signal and a magnetic signal, they make excellent labels for LFAs. Their dark color allows them to be used as conventional optical labels, and their magnetic field enables easier functionalization, sample pretreatment (Nash et al. Citation2012), and sensitive readout. Although optical readouts rely heavily on labels on the NC, magnetic field sensing permits the use of all labels collected along the test line (Quesada-González and Merkoçi Citation2018). The major advantage of MNPs is that they can be detected and quantified by means of external devices, allowing the quantitative detection of the target. However, MNPs are more expensive to use compared to AuNPs. summarize LFA studies in which MNPs was used.
Carbon nanoparticles
Since their discovery in 1991, Carbon nanoparticles (CNPs) (Mao et al. Citation2009; Noguera et al. Citation2011a) and carbon nanotubes (Qiu et al. Citation2015; Yao et al. Citation2016) have emerged as one of the most promising nanomaterials for the development of biosensors (Iijima Citation1991). In addition to their large surface area, carbon nanotubes exhibit superior electrical conductivity, mechanical strength, and chemical inertness (Ajayan Citation1999). Most Importantly, Carbon nanoparticles are preferred for their high signal-to-noise ratio (black to a white background) (Mens et al. Citation2008; Amerongen, Barug and Lauwaars Citation2005) and their excellent sensitivity, i.e., low picomolar by visual inspection (Gordon and Michel Citation2008). Further, they are inexpensive to produce, resistant to aggregation, and easy to functionalize. Carbon nanotubes are known to have a high surface specificity and allow various alterations with functional groups enabling sensitive protein recognition based on electron transfer processes. In addition, carbon nanotubes have been shown to have excellent chemical stability. This has led to the development of CNT-based sensor systems for biorecognition, diagnostics, and therapeutic purposes such as DNA sensors (Sánchez-Pomales, Santiago-Rodríguez, and Cabrera Citation2009), chemical sensors (Kong et al. Citation2000), and immunosensors (Okuno et al. Citation2007). summarize LFA studies in which carbon nanoparticles were used.
Fluorescent nanoparticles
Fluorescent nanoparticles (FNs) are frequently recommended for the detection of targets at low concentrations and/or quantitative applications. This incurs extra costs and necessitates the use of an external reader. Quantum dots (QDs), upconverting nanoparticles (UCNPs), and liposomes encapsulating fluors are examples of fluorescent LFA reporters. QDs, also known as fluorescent semiconductor nanocrystals, can be used to develop highly sensitive LFAs because of their bright signal, resistance to photobleaching (Yan et al. Citation2016; Bruno Citation2017), chemical and thermal stability, and ease of surface modification (Wang, Meng, et al. Citation2019). The size of QDs ranges from 1 nm to 10 nm, which enables them to disperse well in water and to be combined with biomolecules. When activated by UV light, QDs display intense photoluminescence, which can be adjusted by modifying the elemental composition and size of the QDs. Therefore, QDs are appropriate for multiplexed detection (Medintz et al. Citation2005; Wang, Shen, et al. Citation2020). Nevertheless, the formation of QD-biomolecule complexes are difficult, resulting in rendering their application compared to AuNPs (Costa-Fernández, Pereiro, and Sanz-Medel Citation2006).
UCNPs have been employed as labels in LFAs since the early 2000s (Niedbala et al. Citation2001b) (Corstjens et al. Citation2001; Hampl et al. Citation2001). Their near-infrared excitation wavelengths do not generate membrane autofluorescence, and their strong emission in the visible spectrum makes them more sensitive than QDs as detection molecules in LFAs (Kim et al. Citation2018). However, the need for an expensive and bulky near-infrared laser makes the incorporation of UCNPs into LFAs impractical for many on-site applications (Kim et al. Citation2018; He et al. Citation2018; You et al. Citation2017) (Gong et al. Citation2019).
Many studies have examined the use of fluorescent dyes added to liposomes to increase the sensitivity of LFAs. Liposomes can be combined with a wide range of fluorescent dyes capable of producing a strong fluorescence signal, and the composition of the lipid bilayer can be altered to permit straightforward bioreceptor functionalization of the liposome surface (Khreich et al. Citation2008; Baeumner et al. Citation2004; Edwards and Baeumner Citation2006; Edwards, Korff, and Baeumner Citation2017). The two fundamental disadvantages of liposomes as LFA indicators are their complex production processes and poor stability. summarize LFA studies in which fluorescent nanoparticles were used.
Improving the sensitivity and specificity of LFAs
Selection of the best antibody pair
The specificity of LFAs can be increased by reducing nonspecific binding (NSB) and by using antibodies with high affinity for the analyte. Several sample processing techniques are available to reduce NSB, which leads to false-positive findings. For example, when detecting analytes from whole blood, blood cells and large proteins are often removed from the blood by filtering or centrifugation prior to LFA (Liu et al. Citation2021). It was also shown that preheating urine decreased the activity of thermally unstable biomolecules, resulting in fewer false-positive findings when LFA was used to detect cryptococcal antigens (Nam, Thaxton, and Mirkin Citation2003).
The LFA efficacy is heavily dependent on the affinity proteins (i.e., antibodies) that recognize the target. Maximum specificity can be achieved by molecules with optimal affinity (Bembenek et al. Citation2011; Brooks et al. Citation2008; Wu, Milutinovic, et al. Citation2015). Antibodies are a typical option because of their sensitivity as well as their specificity when it comes to the specific detection of very low concentrations of the analyte. While aptamers and various other affinity reagents are also options, antibodies are the primary affinity reagent used for lateral flow rapid tests.
The selection of the optimal antibodies is a critical aspect of LFA design. The ultimate performance of the LFA mainly depends on the specificities of the antibodies used to bind a target in the specimen. The decision of whether to utilize polyclonal or monoclonal antibodies is one of the earliest decisions that must be made in the process of LFA development. Polyclonal antibodies are derived from the serum of animals that have been vaccinated. They are made up of complex mixtures of antibodies, each of which was created by a unique B cell clone in the animal. There is an inherent lack of consistency from one animal to the next, and even fluctuation from one bleed of the same animal to the next since every host species and even every individual host will have a distinct immunological response. On the other hand, monoclonal antibodies are produced in the laboratory; thus, they are homogenous. Monoclonal antibodies are unique in that they are only able to bind to a single epitope of the target and were generated by a single B cell clone. Therefore, polyclonals may have a stronger recognition ability owing to multiple kinds of antibodies targeting different epitopes of the target, but monoclonals are more consistent since they target just one epitope of the target. This is because polyclonals target numerous antigens simultaneously. An additional benefit is that the cell clones that are used in the production of monoclonal antibodies may be regenerated endlessly in the laboratory, but the animal hosts that are utilized in the production of polyclonal antibodies will ultimately perish.
Extensive efforts have been made to enhance the sensitivity and specificity of LFAs for more precise and effective on-site diagnostics. Assay optimization and sample enrichment are two ways to increase sensitivity (Soh, Chan, and Ying Citation2020; Nguyen et al. Citation2020; Bishop et al. Citation2019). Signal amplification can boost LFA sensitivity close to that of PCR-based assays. Several other approaches to improve LFA sensitivity are promising but require extended testing time (Bishop et al. Citation2019; Rodríguez et al. Citation2016). Hence, balancing sensitivity and test duration is a critical challenge for the future development of on-site assays. LFA specificity is primarily increased by optimizing the test and applying high-affinity antibodies and reagents with high specificity.
Improving sensitivity by assay optimization
Signal amplification
Chemical enhancement of colorimetric signal
The LFA sensitivity can be improved by increasing the colorimetric signal of the test. A quick and easy way to boost the signal is by chemically increasing the colorimetric contrast of the positive test line. This enhanced contrast can be achieved using different methods, including; silver enhancement, double gold conjugation, and induced gold aggregation (Liu et al. Citation2021). In the silver enhancement method, Ag-reducing reagents are flowed through the LFA strip after running the sample, and Ag is nucleated on captured AuNPs in the test area. The Ag layer forming on the AuNPs reporter particles amplifies the color intensity of the test area. This method significantly enhances the sensitivity by 10-fold compared to traditional LFA (Serebrennikova, Samsonova, and Osipov Citation2018; Anfossi et al. Citation2013). For the double gold conjugation, secondary AuNPs are used to bind with the primary AuNPs that are already captured on the test area leading to enhanced color intensity. This binding can be accomplished through the utilization of the high biotin-streptavidin binding affinity (Shen and Shen Citation2019) or by employing primary and secondary antibodies, which is similar to the basis of indirect ELISA. It was shown that the double gold conjugation method has significantly increased the sensitivity of LFA by approximately 30-fold for the detection of the Hepatitis B virus (Shen and Shen Citation2019). The concept of the induced gold aggregation technique is similar to the double gold conjugation approach. However, more AuNPs are coated on the captured AuNPs, thus better amplifying the color intensity.
Label design
The label design plays an essential role in amplifying the colorimetric contrast of LFA. Replacing the traditional small (20 − 40 nm) nanoparticles employed with labels that have stronger colorimetric contrast is the easiest way to enhance the signal while maintaining the LFA format. Stronger contrast can be achieved by modifying the size and structure of the reporter particles or by replacing the particles with clusters or particles made of another metal, metal oxide, or organic material. For instance, gold-nanoparticle-decorated silica nanorods (AuNPs -SiNRs), achieved a 50-fold lower LoD in the detection of rabbit IgG than traditional LFA with AuNPs (Xu et al. Citation2014). Similarly, polystyrene microbeads were used to enhance the colorimetric contrast of AuNPs. Utilizing polystyrene microbeads improved the sensitivity of LFA for the detection of influenza virus by 64-fold and 16-fold over that achieved with 10 nm and 30 nm AuNPs -based LFAs, respectively (Liu et al. Citation2020).
Enhancing LFA reagents
Enhancing LFA reagents can be used to induce catalytic reactions in the test area to amplify the signal contrast. Catalytic amplification is usually achieved by utilizing enzymes or nanozymes to induce oxidation/reduction reactions in the test area. HRP is one of the most commonly used enzymes in LFA platforms. In LFA, HRP is linked to detection molecules (i.e., antibodies) that are conjugated with reporter particles immobilized on the conjugation pad. After the sample flow through the LFA is completed, the HRP substrate and H2O2 solution are flowed through the LFA after washing to induce enzymatic amplification and enhance the optical contrast at the test line (He et al. Citation2011). Parolo et al. reported an increase in sensitivity up to 1 order of magnitude compared to traditional AuNPs -LFAs by applying enzymatic amplification (Parolo, de la Escosura-Muñiz, and Merkoçi Citation2013).
Nanozymes, which are artificial enzymes based on nanomaterials, were rapidly developed as surrogates of natural enzymes. Nanozymes have several advantages over natural enzymes, including higher catalytic stability, an easier modification process, and lower manufacturing costs (Jiang et al. Citation2019). For instance, thin platinum (Pt) shells on top of gold (Au) nanoparticles (NPs) (Au@PtNPs) was able to produce an LoD of 0.8 pg/mL. This finding is significantly more sensitive than commercial ELISA, which has an LoD of >1 pg/mL (Loynachan et al. Citation2018).
External readers
Amplification of LFA signals may also be achieved with the assistance of external readers. When labeled particles that change in color intensity are used in LFA, a charge-coupled device or a complementary metal-oxide-semiconductor camera detection device will be utilized for assay quantification (Gussenhoven et al. Citation1997). In case of using fluorescent labeled particles, a photodetector with an excitation light source is used for assay quantification (Ho and Wauchope Citation2002). The laser beam, or electric potential, or magnetic field can be used to activate/concentrate captured nanoparticle labels on the test line, resulting in an enhanced signal (Draz and Shafiee Citation2018). This amplified signal can then be detected by sensitive optical, electrical, or magnetic sensors/electrodes, respectively, that can distinguish between minute signal variations and background noise. Among these, LFA readers utilizing image sensors, such as a charge-coupled device or metal-oxide-semiconductor camera, are most commonly used because of the advantages of their simple structure and small size (You, Park, and Yoon Citation2013). An image sensor-based LFA reader acquires an image of the test line (aggregated labeled particles, antigens, and antibodies). Then, the pixel intensity of the test line, which changes according to the concentration of the target analyte, is analyzed (Sajid, Kawde, and Daud Citation2015). However, problems such as the high possibility of false positives and false negatives and limitations for accurate and multiplex quantification have been observed in the utilization of optical readers.
Recently, our team have designed and developed an ultra high-sensitivity inductive transducer, called the Femtogmag, for the detection and quantification of superparamagnetic nanoparticle reporters that are immuno-captured on the test line (Khodadadi et al. Citation2019). As a proof of concept, the femtoMag was used to quantify the hCG pregnancy hormone by quantifying the number of 200 nm magnetic reporters immuno-captured within the test line of the LFA strip. A sensitivity of 100 pg/mL has been demonstrated. Upon further design and control electronics improvements, the sensitivity is projected to be better than 10 pg/mL. Magnetic reporters provide several advantages compared to other optical reporters (1) Magnetic fields do not interact with biological materials, so the signal is stable (2) magnetic fields are not affected by LFA media, so every magnetic reporter within the test line contributes to detection; and (3) the properties of magnetic reporters can be tuned to match the biomarkers to optimize trapping efficiency and detection (Yoshino, Maeda, and Matsunag Citation2010; Yu et al. Citation2022; Jacinto et al. Citation2018). The femtoMag also provides a number of technological advancements over the current state-of-the-art magnetic biosensor technologies, including (1) high sensitivity, (2) Quantitation, (3) simple and easy integration with LFA technology, (4) portable electronic controls, and (5) low-cost manufacturability. The low-cost easy-to-use femtoMag platform offers high-sensitivity/high-precision target analyte quantification and promises to bring state-of-the-art medical diagnostic tests to the POC.
Optimization of the assay kinetics
Optimization of assay kinetics, such as transport and reaction kinetics, is essential for LFA development and can be used to increase sensitivity. The assay kinetics affect the selective binding (SB) and NSB of the antibodies and analytes, which determine the sensitivity and specificity of the assay (55-57). Assay kinetics should be optimized to increase SB and reduce NSB, thus, enhancing the assay sensitivity (Zhan et al. Citation2020).
The sensitivity of LFAs is limited by the reaction rate (Zhan et al. Citation2017b; Mosley et al. Citation2016). Increasing the reaction rate can help in boosting assay sensitivity. Hence, maximizing the SB. However, the transport of molecules and labels is limited by the diffusion rate, and the surface reaction is limited by the reaction rate. Increasing the reaction kinetics is associated with the formation of the sandwich ternary; conjugation/target/capture antibody. Liang et al. showed that the sandwich ternary develops more slowly when the target binds first with the conjugated label and then the capture antibody in a premixing flow compared to binding with the capture antibody and then the conjugated label in a sequential flow. For instance, the LoD of the LFA platform for detecting malarial antigens from a premixing flow was reported to be 4- to 10-fold higher than that obtained from a sequential flow (Liang et al. Citation2016).
Improving sensitivity by sample enrichment
The reaction rate coefficient is relatively constant for most immunoreactions between antigens and antibodies. It is possible to effectively increase the reaction rate and, as a result, boost the sensitivity by increasing the number of captured labels on the test area by preconcentrating the food sample before introducing the sample into the LFA test. Magnetic separation is one technique that may be used for pre-concentration, which produces a 10-fold increase in sensitivity (Sharma et al. Citation2019). Alternatively, Mashayekhi et al. used an aqueous two-phase (Bradbury et al. Citation2019) micellar system composed of the nonionic surfactant Triton X-114 to concentrate a model protein and reported a 10-fold increase in sensitivity from 0.5 μg/mL to 0.05 μg/mL (Mashayekhi et al. Citation2012).
Analytes can also be preconcentrated during the LFA flow phase. The comparatively low LoD of LFAs may be attributed to the fact that low target concentrations induce kinetically limited surface reactions. To overcome this problem, Moghadam et al. preconcentrated the antigen-conjugation complex into a narrow band and transported it to the capture line using the isotachophoresis technique. This approach increased the LoD by 400-fold and 160-fold for 90 s and 5 min reaction, respectively (Moghadam, Connelly, and Posner Citation2015).
Another way to boost the reaction rate and enhance the sensitivity is to increase the number of efficient binding sites for the conjugated labels by altering the structure of the label or changing the orientation of the detection molecules. For instance, the number of binding sites can be increased by increasing the size of AuNP labels (Zhan et al. Citation2017a) or by functionalizing the particle surface with several layers (Lou et al. Citation2019) to allow for the loading of additional detection molecules.
Improved conjugation approaches have the potential to offer more effective binding sites than standard physical adsorption when the orientation of the detection molecules is forced in a specific direction (Trilling, Beekwilder, and Zuilhof Citation2013; Di Nardo et al. Citation2019; Welch et al. Citation2017). A particular orientation can be achieved through covalent binding mediated by a chemical layer or through bioaffinity binding mediated by a biomolecular layer (Trilling, Beekwilder, and Zuilhof Citation2013; Di Nardo et al. Citation2019; Welch et al. Citation2017). It is also necessary to adjust the coverage of the detection molecules in order to maximize the affinity for the analyte and get rid of any steric hindrance that may be generated by a thick layer of detection molecules (Saha, Evers, and Prins Citation2014). Another way to boost the sensitivity of the LFA is to add more effective binding sites to the test line. For instance, the use of three-dimensional “proteinticle” probes with multiple self-assembled and orientated peptides was shown to give a 4-fold to 8-fold improvement in sensitivity (Lee et al. Citation2015). In a different approach, the addition of cellulose nanofibers to the NC enabled more capture molecules to be loaded and increased the assay sensitivity by 20-fold (Tang et al. Citation2019; Quesada-González et al. Citation2019).
Commercially available LFAs for the detection of foodborne pathogen
The benefits of LFAs for the detection of food pathogens include multiplexing capabilities, dependability, and adherence to the same standards of precision as traditional detection techniques. In addition, LFAs are user-friendly and capable of producing qualitative, semiquantitative, or quantitative results after only a few (10–30) minutes. Most importantly, LFAs are economical, as their rapid findings save operational costs by accelerating product release while maintaining product dependability. summarizes the characteristics of commercially available LFAs for the detection of foodborne pathogens.
Table 4. Summary of commercially available LFA for detection of foodborne pathogens from food samples.
Recent advances in LFA-based testing for foodborne pathogens
Bacterial foodborne pathogens
Bacteria are the most abundant type of foodborne pathogens that threaten human health (Mead et al. Citation1999; Hariram and Labbé Citation2016). Hence, the detection of foodborne bacterial pathogens is of supreme importance to guarantee food quality. Biochemical characterization and microbiological identification are used as conventional methods to detect foodborne bacteria (Byrne et al. Citation2015). Recently, LFA was shown to be a quick and sensitive alternative approach to identifying foodborne pathogens (Keiser and Utzinger Citation2005; Law et al. Citation2014; Hwang et al. Citation2016; Zhao et al. Citation2016a; Li et al. Citation2021). lists recent studies on LFAs for the detection of foodborne bacterial pathogens.
Anthrax is an infectious disease caused by Bacillus anthracis (B. anthracis). A combination of immunomagnetic separation and LFA has been used to detect B. anthracis in milk (Fisher et al. Citation2009a; Wang, Tian, et al. Citation2015). Fisher et al. (Fisher et al. Citation2009a) studied the immunocapture of B. anthracis spores using anti-spore antibodies coupled with carboxylated magnetic beads and were able to recover 95% of B. anthracis spores when 105–107 spores were inoculated in 1 mL milk (Fisher et al. Citation2009a).
E.coli is the etiological agent of many waterborne and foodborne diseases (Singh, Sharma, and Nara Citation2015a). Wu et al. used an aptamer-based biosensor to rapidly detect E. coli O:157:H7 (Wu, Milutinovic, et al. Citation2015). This assay utilized two distinct aptamers, each designed to precisely target the outer membrane proteins of the bacterium. The first aptamer enriched E. coli O:157:H7 cells on magnetic beads, and the other was used as a signal reporter. The signal produced by the second aptamer was amplified using an isothermal strand displacement amplification technique. Positive signals, generated as red bands on the test line, could be produced by 10 CFU/mL of E. coli O157:H7 (Wu, Milutinovic, et al. Citation2015). Bruno et al. (Bruno Citation2014) used a sandwich-format LFA sensor to detect E. coli. In this system, amino end-labeled capture aptamers were immobilized on an analytical NC using UV light with a wavelength of 254 nm. When E. coli cells passed through the capture line, they were captured by the amino group on the membrane surface. The aptamer-conjugated colloidal gold demonstrated a visible LoD between 3 × 103and 6 × 103 E. coli cells in the buffer (Bruno Citation2014).
Liu et al. developed a simple and ultra-sensitive LFA for the rapid recognition of Salmonella in food samples. They used AuNPs conjugated with a DNA probe that was complementary to the 16S ribosomal DNA and RNA of Salmonella. The synthesized single-stranded DNA had an LoD of five femtomolar. For cultured Salmonella, the nucleic acids of 107 bacteria were rapidly detected in 30 min. Additionally, with silver enhancement, the LoD was improved to detect 104 bacteria, which is lower than the human infectious dose of foodborne Salmonella (105 CFU) mL-1. Because of its low cost, high sensitivity, high specificity, and ease of use, the LFA developed by Liu et al. may be a valuable tool for microbial detection in large-scale diagnostic or food safety applications in impoverished nations (Liu et al. Citation2013b).
Wang et al. developed an LFA strip biosensor that could detect Salmonella enteritidis (S. enteritidis) with an LoD of 102 CFU/mL using positively charged, surface nitrogen-rich, carbon nanoparticles (pNPs) made by calcination and etching procedures (Wang, Yao, et al. Citation2019). These nanoparticles not only generate a signal but also function as an adsorbent to trap bacteria (Wang, Yao, et al. Citation2019). Bacterial cells stick to the pNCs by electrostatic contact and hydrogen bonding, and this complex is then selectively recognized by an anti-bacterial antibody coated on the test line, causing the color of the test line to progressively darken. The pNPs were able to recover 85–100% of Salmonella from various food specimens; however, the generalizability of the approach is currently limited by the availability of suitable antibodies (Wang, Yao, et al. Citation2019). Taking advantage of simplicity, label-free, convenience, and sensitivity, the pNC-based LFA has the application potential for pathogenic microorganisms monitoring in food safety and early clinical diagnosis fields (Wang, Yao, et al. Citation2019).
Viral foodborne pathogens
The LFA research and commercialization for the detection of viral foodborne pathogens are more hindered compared to those for bacterial foodborne pathogens. The bacterial detection techniques cannot be used for viral detection due to many factors; (1) viruses are far more complicated to culture and amplify compared to bacteria, which often cannot be enriched (Chhabra and Vinjé Citation2016), (2) viruses are usually present in small quantities in food matrices and cannot proliferate in host-free environment, (3) the size of viruses range from 20 to 400 nm, while the size of bacteria ranges from 1 micron to 5 microns. Therefore, the sensing platforms developed for bacterial detection must be significantly adjusted in order to identify viral pathogens due to their extremely smaller size. (4) the composition of cell surface proteins of bacteria is not comparable with the hemagglutinin and neuraminidase compositions of viruses, and this necessitates the selection of specific biorecognition elements for the detection of viral-specific proteins, and (5) viruses are often present with lower copy numbers compared to bacteria in food samples matrix, which demands superior sensitivity of the virus detection biosensors to be at least attomolar or picomolar level (Neethirajan et al. Citation2017). Therefore, separation, pre-concentration, and purification of viral pathogens from food samples are crucial toward sensitive virus detection using LFA. Nevertheless, due to the heterogeneity in genome and surface structures among viruses, a universal viral extraction technique would be extremely difficult for the on-spot rapid and easy foodborne viral detection from food samples.
Our team is currently working to develop LFA for the detection of viral foodborne pathogens; Norovirus (NoV) and Hepatitis E (HEV). Using europium nanoparticles, we were able to detect NoV at a concentration as low as 10 ng/mL (Work in Progress). In addition, using AuNPs, we were able to detect HEV at a concentration as low as 5 ng/mL (Work in Progress). Currently, we are working to generate a novel quantitative magnetic immunoassay for the detection of foodborne pathogens, NoV and HEV. Using the femtoMag as a reader (Khodadadi et al. Citation2019), the LFA will have sensitivity rivaling those of central laboratory instruments, which will enable rapid, high-quality quantitation of viral levels and can serve as a simple, low-cost, easy-to-use point-of-need analytical platform for rapid and reliable early infection detection of foodborne pathogens associated with acute gastroenteritis from food samples.
Conclusion and future perspectives
Various contaminants threaten food quality and pose threats to human health. Most current methods to detect food contaminants are difficult to use on-site because they require special laboratory equipment and skilled personnel. LFAs offer many advantages for the rapid detection of foodborne pathogens, including cost-effectiveness, simplicity, rapidity, and ease of use in on-site settings. Another important advantage of LFAs is that they can analyze different analytes simultaneously, which is of supreme importance. Current challenges for LFA platform development for the detection of foodborne pathogens include (1) enhancing the signal-to-noise ratio to reduce background noises and increase detectable signals (2) enhancing detection specificity and sensitivity; (3) improving storage duration; (4) allowing user-friendly and unskilled operation (Mangal et al. Citation2016).
With the changing of the global demographic and epidemiologic structure, as well as food processing and harvesting systems, We can expect new foodborne viruses to emerge in society through both animal and plant-derived foods. A rapid and sensitive detection system can reduce ongoing transmission of pathogens as well as play a crucial role in preventing pathogen transmission through early detection and control of foodborne illness outbreaks.
Various LFAs for food safety monitoring are commercially available; however, their widespread acceptance is hindered by their lack of sensitivity. The sensitivity, reproducibility, and multi-analyte capabilities of LFAs must be improved substantially for LFA-based food safety evaluation to be adequate.
Disclosure statement
Prof. Dmitri Litvinov is an executive officer of FemtoMag, Inc. FemtoMag is a for-profit company developing biosensor technologies for the detection and quantification of molecular biomarkers
Open Access funding provided by the Qatar National Library.
Additional information
Funding
References
- Adley, C. C., and M. P. Ryan. 2016. Chapter 1 - The nature and extent of foodborne disease. In Antimicrobial food packaging, ed. Jorge Barros-Velázquez, 1–10. San Diego: Academic Press.
- Ajayan, P. M. 1999. Nanotubes from carbon. Chemical Reviews 99 (7):1787–800. doi: 10.1021/cr970102g.
- Amerongen, V., D. Barug, and M. Lauwaars. 2005. Rapid methods for biological and chemical contaminants in food and feed. 416. doi: 10.3920/978-90-8686-538-3.
- Anfossi, L., F. Di Nardo, C. Giovannoli, C. Passini, and C. Baggiani. 2013. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Analytical and Bioanalytical Chemistry 405 (30):9859–67. doi: 10.1007/s00216-013-7428-6.
- Arora, K., S. Chand, and B. D. Malhotra. 2006. Recent developments in bio-molecular electronics techniques for food pathogens. Analytica Chimica Acta 568 (1–2):259–74. doi: 10.1016/j.aca.2006.03.078.
- Baeumner, A. J., C. Jones, C. Y. Wong, and A. Price. 2004. A generic sandwich-type biosensor with nanomolar detection limits. Analytical and Bioanalytical Chemistry 378 (6):1587–93. doi: 10.1007/s00216-003-2466-0.
- Bembenek, M. E., A. Burkhardt, J. Ma, Z. Li, H.-K. Loke, D. Wu, Q. Xu, O. Tayber, L. Xie, P. Li, et al. 2011. Determination of complementary antibody pairs using protein A capture with the AlphaScreen assay format. Analytical Biochemistry 408 (2):321–7. doi: 10.1016/j.ab.2010.09.021.
- Ben Aissa, A., J. J. Jara, R. M. Sebastián, A. Vallribera, S. Campoy, and M. I. Pividori. 2017. Comparing nucleic acid lateral flow and electrochemical genosensing for the simultaneous detection of foodborne pathogens. Biosensors & Bioelectronics 88:265–72. doi: 10.1016/j.bios.2016.08.046.
- Bintsis, T. 2017. Foodborne pathogens. AIMS Microbiology 3 (3):529–63. doi: 10.3934/microbiol.2017.3.529.
- Bishop, J. D., H. V. Hsieh, D. J. Gasperino, and B. H. Weigl. 2019. Sensitivity enhancement in lateral flow assays: A systems perspective. Lab on a Chip 19 (15):2486–99. doi: 10.1039/C9LC00104B.
- Blažková, M., M. Koets, P. Rauch, and A. van Amerongen. 2009. Development of a nucleic acid lateral flow immunoassay for simultaneous detection of Listeria spp. and Listeriamonocytogenes in food. European Food Research and Technology 229 (6):867–74. doi: 10.1007/s00217-009-1115-z.
- Bolton, F. J., E. Fritz, S. Poynton, and T. Jensen. 2000. Rapid enzyme-linked immunoassay for detection of Salmonella in food and feed products: Performance testing program. Journal of AOAC International 83 (2):299–304. doi: 10.1093/jaoac/83.2.299.
- Bouguelia, S., Y. Roupioz, S. Slimani, L. Mondani, M. G. Casabona, C. Durmort, T. Vernet, R. Calemczuk, and T. Livache. 2013. On-chip microbial culture for the specific detection of very low levels of bacteria. Lab on a Chip 13 (20):4024–32. doi: 10.1039/c3lc50473e.
- Bradbury, D. W., M. Azimi, A. J. Diaz, A. A. Pan, C. H. Falktoft, B. M. Wu, and D. T. Kamei. 2019. Automation of biomarker preconcentration, capture, and nanozyme signal enhancement on paper-based devices. Analytical Chemistry 91 (18):12046–54. doi: 10.1021/acs.analchem.9b03105.
- Brooks, B. D., A. E. Albertson, J. A. Jones, J. O. Speare, and R. V. Lewis. 2008. Efficient screening of high-signal and low-background antibody pairs in the bio-bar code assay using prion protein as the target. Analytical Biochemistry 382 (1):60–2. doi: 10.1016/j.ab.2008.07.009.
- Bruno, J. G. 2014. Application of DNA aptamers and quantum dots to lateral flow test strips for detection of foodborne pathogens with improved sensitivity versus colloidal gold. Pathogens (Basel, Switzerland) 3 (2):341–55. doi: 10.3390/pathogens3020341.
- Bruno, J. 2017. Evaluation of pathogenic big 7 E. coli aptamer-quantum dot lateral flow test strips. Journal of Bionanoscience 11 (2):148–52. doi: 10.1166/jbns.2017.1424.
- Bu, T., Q. Huang, L. Yan, L. Huang, M. Zhang, Q. Yang, B. Yang, J. Wang, and D. Zhang. 2018. Ultra technically-simple and sensitive detection for Salmonella Enteritidis by immunochromatographic assay based on gold growth. Food Control 84:536–43. doi: 10.1016/j.foodcont.2017.08.036.
- Buckwalter, S. P., L. M. Sloan, S. A. Cunningham, M. J. Espy, J. R. Uhl, M. F. Jones, E. A. Vetter, J. Mandrekar, F. R. Cockerill, B. S. Pritt, et al. 2014. Inhibition controls for qualitative real-time PCR assays: Are they necessary for all specimen matrices? Journal of Clinical Microbiology 52 (6):2139–43. doi: 10.1128/jcm.03389-13.
- Buddolla, A., and S. Kim. 2021. Recent trends in the utilization of LAMP for the diagnosis of viruses, bacteria, and allergens in food. Recent Developments in Applied Microbiology and Biochemistry, 291–7. doi: 10.1016/B978-0-12-821406-0.00027-8.
- Buss, J. E., M. Cresse, S. Doyle, B. W. Buchan, D. W. Craft, and S. Young. 2019. Campylobacter culture fails to correctly detect Campylobacter in 30% of positive patient stool specimens compared to non-cultural methods. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology 38 (6):1087–93. doi: 10.1007/s10096-019-03499-x.
- Byrne, B., N. Gilmartin, R. S. Lakshmanan, and R. O’Kennedy. 2015. 3 - Antibodies, enzymes, and nucleic acid sensors for high throughput screening of microbes and toxins in food. In High throughput screening for food safety assessment, ed. Arun K. Bhunia, Moon S. Kim, and Chris R. Taitt, 25–80. Cambridge, UK: Woodhead Publishing.
- Call, D. R., F. J. Brockman, and D. P. Chandler. 2001. Detecting and genotyping Escherichia coli O157:H7 using multiplexed PCR and nucleic acid microarrays. International Journal of Food Microbiology 67 (1–2):71–80. doi: 10.1016/s0168-1605(01)00437-8.
- Carvalho, R. N., A. N. de Oliveira, A. J. d Mesquita, C. S. Minafra e Rezende, A. Q. d Mesquita, and R. A. Romero. 2014. PCR and ELISA (VIDAS ECO O157(®)) Escherichia coli O157:H7 identification in Minas Frescal cheese commercialized in Goiânia, GO. Brazilian Journal of Microbiology: [Publication of the Brazilian Society for Microbiology] 45 (1):7–10. doi: 10.1590/s1517-83822014000100002.
- Castilho, M. d. S., T. Laube, H. Yamanaka, S. Alegret, and M. I. Pividori. 2011. Magneto immunoassays for Plasmodium falciparum histidine-rich protein 2 related to malaria based on magnetic nanoparticles. Analytical Chemistry 83 (14):5570–7. doi: 10.1021/ac200573s.
- Cesewski, E., and B. N. Johnson. 2020. Electrochemical biosensors for pathogen detection. Biosensors & Bioelectronics 159:112214. doi: 10.1016/j.bios.2020.112214.
- Chen, M., Z. Yu, D. Liu, T. Peng, K. Liu, S. Wang, Y. Xiong, H. Wei, H. Xu, and W. Lai. 2015. Dual gold nanoparticle lateflow immunoassay for sensitive detection of Escherichia coli O157:H7. Analytica Chimica Acta 876:71–6. doi: 10.1016/j.aca.2015.03.023.
- Chen, X., M. Gan, H. Xu, F. Chen, X. Ming, H. Xu, H. Wei, F. Xu, and C. Liu. 2014. Development of a rapid and sensitive quantum dot-based immunochromatographic strip by double labeling PCR products for detection of Staphylococcus aureus in food. Food Control 46:225–32. doi: 10.1016/j.foodcont.2014.04.044.
- Chhabra, P., and J. Vinjé. 2016. Molecular detection methods of foodborne viruses. Viruses in food, 303–33. doi: 10.1007/978-3-319-30723-7_11.
- Cho, I. H., A. Bhunia, and J. Irudayaraj. 2015. Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. International Journal of Food Microbiology 206:60–6. doi: 10.1016/j.ijfoodmicro.2015.04.032.
- Cho, I. H., and J. Irudayaraj. 2013. Lateral-flow enzyme immunoconcentration for rapid detection of Listeria monocytogenes. Analytical and Bioanalytical Chemistry 405 (10):3313–9. doi: 10.1007/s00216-013-6742-3.
- Choi, J. R., J. Hu, R. Tang, Y. Gong, S. Feng, H. Ren, T. Wen, X. Li, W. A. B. Wan Abas, B. Pingguan-Murphy, et al. 2016. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab on a Chip 16 (3):611–21. doi: 10.1039/C5LC01388G.
- Corp, BIOO Scientific, 2023. MaxSignal- E. coli O157 test strip kit. Accessed 14/6/2023. https://www.ngaio.co.nz/supplier/bioo-scientific.
- Corstjens, P., M. Zuiderwijk, A. Brink, S. Li, H. Feindt, R. S. Niedbala, and H. Tanke. 2001. Use of up-converting phosphor reporters in lateral-flow assays to detect specific nucleic acid sequences: A rapid, sensitive DNA test to identify human papillomavirus type 16 infection. Clinical Chemistry 47 (10):1885–93. doi: 10.1093/clinchem/47.10.1885.
- Costa-Fernández, J. M., R. Pereiro, and A. Sanz-Medel. 2006. The use of luminescent quantum dots for optical sensing. TrAC Trends in Analytical Chemistry 25 (3):207–18. doi: 10.1016/j.trac.2005.07.008.
- Cremonesi, P., G. Perez, G. Pisoni, P. Moroni, S. Morandi, M. Luzzana, M. Brasca, and B. Castiglioni. 2007. Detection of enterotoxigenic Staphylococcus aureus isolates in raw milk cheese. Letters in Applied Microbiology 45 (6):586–91. doi: 10.1111/j.1472-765X.2007.02231.x.
- Cui, X., Y. Huang, J. Wang, L. Zhang, Y. Rong, W. Lai, and T. Chen. 2015. A remarkable sensitivity enhancement in a gold nanoparticle-based lateral flow immunoassay for the detection of Escherichia coli O157:H7. RSC Advances 5 (56):45092–7. doi: 10.1039/C5RA06237C.
- Di Nardo, F., S. Cavalera, C. Baggiani, C. Giovannoli, and L. Anfossi. 2019. Direct vs mediated coupling of antibodies to gold nanoparticles: The case of salivary cortisol detection by lateral flow immunoassay. ACS Applied Materials & Interfaces 11 (36):32758–68. doi: 10.1021/acsami.9b11559.
- Draz, M. S., and H. Shafiee. 2018. Applications of gold nanoparticles in virus detection. Theranostics 8 (7):1985–2017. doi: 10.7150/thno.23856.
- Edwards, K. A., and A. J. Baeumner. 2006. Optimization of DNA-tagged dye-encapsulating liposomes for lateral-flow assays based on sandwich hybridization. Analytical and Bioanalytical Chemistry 386 (5):1335–43. doi: 10.1007/s00216-006-0705-x.
- Edwards, K. A., R. Korff, and A. J. Baeumner. 2017. Liposome-enhanced lateral-flow assays for clinical analyses. Methods in Molecular Biology 1571:407–34. doi: 10.1007/978-1-4939-6848-0_25.
- Elmer, P. 2023a. AuroFlow AQ DON Strip Test. Accessed 14/6/2023. https://www.perkinelmer.com/product/auroflow-aq-don-strip-test-food-1414-01
- Elmer, P. 2023b. AuroFlow AQ Fumonisin Strip Test. Accessed 14/6/2023. https://www.perkinelmer.com/product/auroflow-aq-fumonisin-strip-test-food-1416-01
- Elmer, P. 2023c. AuroFlow AQ Ochratoxin A Strip Test. Accessed 14/6/2023. https://www.perkinelmer.com/product/auroflow-aq-ochratoxin-a-strip-test-food-1417-01
- Elmer, P. 2023d. AuroFlow AQ T-2/HT-2 Strip Test. Accessed 14/6/2023. https://www.perkinelmer.com.br/product/auroflow-aq-t-2-ht-2-strip-test-food-1418-01
- Elmer, P. 2023e. AuroFlow AQ Zearalenone Strip Test.Accessed 14/6/2023. https://www.perkinelmer.com/product/auroflow-aq-zearalenone-strip-test-food-1415-01.
- Elmer, P. 2023f. AuroFlowTM AQ Afla Strip Test. Accessed 14/6/2023. https://www.perkinelmer.com/product/auroflow-aq-afla-strip-test-food-1413-01.
- Eugster, P. J., D. Guillarme, S. Rudaz, J.-L. Veuthey, P.-A. Carrupt, and J.-L. Wolfender. 2011. Ultra high pressure liquid chromatography for crude plant extract profiling. Journal of AOAC International 94 (1):51–70. doi: 10.1093/jaoac/94.1.51.
- Fang, Z., W. Wu, X. Lu, and L. Zeng. 2014. Lateral flow biosensor for DNA extraction-free detection of Salmonella based on aptamer mediated strand displacement amplification. Biosensors & Bioelectronics 56:192–7. doi: 10.1016/j.bios.2014.01.015.
- Fisher, M., Y. Atiya-Nasagi, I. Simon, M. Gordin, A. Mechaly, and S. Yitzhaki. 2009. A combined immunomagnetic separation and lateral flow method for a sensitive on-site detection of Bacillus anthracis spores – assessment in water and dairy products. Letters in Applied Microbiology 48 (4):413–8. doi: 10.1111/j.1472-765X.2008.02542.x.
- Fong, W. K., Z. Modrusan, J. P. McNevin, J. Marostenmaki, B. Zin, and F. Bekkaoui. 2000. Rapid solid-phase immunoassay for detection of methicillin-resistant Staphylococcus aureus using cycling probe technology. Journal of Clinical Microbiology 38 (7):2525–9. doi: 10.1128/JCM.38.7.2525-2529.2000.
- Fung, F., H. S. Wang, and S. Menon. 2018. Food safety in the 21st century. Biomedical Journal 41 (2):88–95. doi: 10.1016/j.bj.2018.03.003.
- Gasanov, U., D. Hughes, and P. M. Hansbro. 2005. Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: A review. FEMS Microbiology Reviews 29 (5):851–75. doi: 10.1016/j.femsre.2004.12.002.
- Ge, X., A. M. Asiri, D. Du, W. Wen, S. Wang, and Y. Lin. 2014. Nanomaterial-enhanced paper-based biosensors. TrAC Trends in Analytical Chemistry 58:31–9. doi: 10.1016/j.trac.2014.03.008.
- Gharaat, M., R. H. Sajedi, M. Shanehsaz, N. Jalilian, M. Mirshahi, and M. Gholamzad. 2017. A dextran mediated multicolor immunochromatographic rapid test strip for visual and instrumental simultaneous detection of Vibrio cholera O1 (Ogawa) and Clostridium botulinum toxin A. Microchimica Acta 184 (12):4817–25. doi: 10.1007/s00604-017-2527-2.
- Glynn, B., S. Lahiff, M. Wernecke, T. Barry, T. Smith, and M. Maher. 2006. Current and emerging molecular diagnostic technologies applicable to bacterial food safety. International Journal of Dairy Technology 59 (2):126–39. doi: 10.1111/j.1471-0307.2006.00253.x.
- Gomaa, A., and J. Boye. 2015. Simultaneous detection of multi-allergens in an incurred food matrix using ELISA, multiplex flow cytometry and liquid chromatography mass spectrometry (LC-MS). Food Chemistry 175:585–92. doi: 10.1016/j.foodchem.2014.12.017.
- Gomez-Govea, M., L. Solis, N. Heredia, S. García, G. Moreno, O. Tovar, and G. Isunza. 2012. Analysis of microbial contamination levels of fruits and vegetables at retail in Monterrey, Mexico. Journal of Food, Agriculture and Environment 10:152–6.
- Gong, Y., Y. Zheng, B. Jin, M. You, J. Wang, X. Li, M. Lin, F. Xu, and F. Li. 2019. A portable and universal upconversion nanoparticle-based lateral flow assay platform for point-of-care testing. Talanta 201:126–33. doi: 10.1016/j.talanta.2019.03.105.
- Gordon, J., and G. Michel. 2008. Analytical sensitivity limits for lateral flow immunoassays. Clinical Chemistry 54 (7):1250–1. doi: 10.1373/clinchem.2007.102491.
- Gowri, A., N. Ashwin Kumar, and B. S. Suresh Anand. 2021. Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of Covid-19 - A minireview. Trends in Analytical Chemistry: TRAC 137:116205. doi: 10.1016/j.trac.2021.116205.
- Gussenhoven, G. C., M. A. van der Hoorn, M. G. Goris, W. J. Terpstra, R. A. Hartskeerl, B. W. Mol, C. W. van Ingen, and H. L. Smits. 1997. LEPTO dipstick, a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera. Journal of Clinical Microbiology 35 (1):92–7. doi: 10.1128/jcm.35.1.92-97.1997.
- Ha, Y., S. Ko, I. Kim, Y. Huang, K. Mohanty, C. Huh, and J. A. Maynard. 2018. Recent advances incorporating superparamagnetic nanoparticles into immunoassays. ACS Applied Nano Materials 1 (2):512–21. doi: 10.1021/acsanm.7b00025.
- Hampl, J., M. Hall, N. A. Mufti, Y. M. Yao, D. B. MacQueen, W. H. Wright, and D. E. Cooper. 2001. Upconverting phosphor reporters in immunochromatographic assays. Analytical Biochemistry 288 (2):176–87. doi: 10.1006/abio.2000.4902.
- Han, F., F. Wang, and B. Ge. 2011. Detecting potentially virulent Vibrio vulnificus strains in raw oysters by quantitative loop-mediated isothermal amplification. Applied and Environmental Microbiology 77 (8):2589–95. doi: 10.1128/aem.02992-10.
- Han, J., L. Zhang, L. Hu, K. Xing, X. Lu, Y. Huang, J. Zhang, W. Lai, and T. Chen. 2018. Nanozyme-based lateral flow assay for the sensitive detection of Escherichia coli O157:H7 in milk. Journal of Dairy Science 101 (7):5770–9. doi: 10.3168/jds.2018-14429.
- Hariram, U., and R. G. Labbé. 2016. Growth and inhibition by spices of growth from spores of enterotoxigenic Bacillus cereus in cooked rice. Food Control 64:60–4. doi: 10.1016/j.foodcont.2015.12.024.
- Hassan, A. H. A., J. F. Bergua, E. Morales-Narváez, and A. Mekoçi. 2019. Validity of a single antibody-based lateral flow immunoassay depending on graphene oxide for highly sensitive determination of E. coli O157:H7 in minced beef and river water. Food Chemistry 297:124965. doi: 10.1016/j.foodchem.2019.124965.
- He, H., B. Liu, S. Wen, J. Liao, G. Lin, J. Zhou, and D. Jin. 2018. Quantitative lateral flow strip sensor using highly doped upconversion nanoparticles. Analytical Chemistry 90 (21):12356–60. doi: 10.1021/acs.analchem.8b04330.
- He, Y., S. Zhang, X. Zhang, M. Baloda, A. S. Gurung, H. Xu, X. Zhang, and G. Liu. 2011. Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor. Biosensors & Bioelectronics 26 (5):2018–24. doi: 10.1016/j.bios.2010.08.079.
- Ho, J. A., and R. D. Wauchope. 2002. A strip liposome immunoassay for aflatoxin B1. Analytical Chemistry 74 (7):1493–6. doi: 10.1021/ac010903q.
- Hochel, I.,D. Slavíčková,D. Viochna,J. Škvor, andI. Steinhauserová. 2007. Detection of Campylobacter species in foods by indirect competitive ELISA using hen and rabbit antibodies. Food and Agricultural Immunology 18 (3-4):151–67. 10.1080/09540100701666857.
- Huang, Z., X. Cui, Q. Y. Xie, D. F. Liu, and W. H. Lai. 2016. Short communication: A novel method using immunomagnetic separation with a fluorescent nanobeads lateral flow assay for the rapid detection of low-concentration Escherichia coli O157:H7 in raw milk. Journal of Dairy Science 99 (12):9581–5. doi: 10.3168/jds.2016-11780.
- Hwang, J., D. Kwon, S. Lee, and S. Jeon. 2016. Detection of Salmonella bacteria in milk using gold-coated magnetic nanoparticle clusters and lateral flow filters. RSC Advances 6 (54):48445–8. doi: 10.1039/C6RA05446C.
- Hygiena. 2023. DuPont™M Lateral Flow System E. coli 0157 Test Kit. Accessed 14/6/2023. https://www.hygiena.com.
- Iijima, S. 1991. Helical microtubules of graphitic carbon. Nature 354 (6348):56–8. doi: 10.1038/354056a0.
- Jacinto, M. J., J. R. C. Trabuco, B. V. Vu, G. Garvey, M. Khodadady, A. M. Azevedo, M. R. Aires-Barros, L. Chang, K. Kourentzi, D. Litvinov, et al. 2018. Enhancement of lateral flow assay performance by electromagnetic relocation of reporter particles. PloS One 13 (1):e0186782. doi: 10.1371/journal.pone.0186782.
- Jelenik, T., Z. Sabatkova, K. Demnerova, and J. Pazlarova. 2005. Two rapid diagnostic procedures for the identification of Campylobacter jejuni/coli in food matrix. Czech Journal of Food Sciences 23 (3):121–5. doi: 10.17221/3381-CJFS.
- Jiang, D., D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan, and W. Cai. 2019. Nanozyme: New horizons for responsive biomedical applications. Chemical Society Reviews 48 (14):3683–704. doi: 10.1039/C8CS00718G.
- Jiang, T., Y. Song, T. Wei, H. Li, D. Du, M. J. Zhu, and Y. Lin. 2016. Sensitive detection of Escherichia coli O157:H7 using Pt-Au bimetal nanoparticles with peroxidase-like amplification. Biosensors & Bioelectronics 77:687–94. doi: 10.1016/j.bios.2015.10.017.
- Jiang, Y., S. Chen, Y. Zhao, X. Yang, S. Fu, J. L. McKillip, E. M. Fox, and C. Man. 2020. Multiplex loop-mediated isothermal amplification-based lateral flow dipstick for simultaneous detection of 3 food-borne pathogens in powdered infant formula. Journal of Dairy Science 103 (5):4002–12. doi: 10.3168/jds.2019-17538.
- Jiménez, K. B., C. B. McCoy, and R. Achí. 2010. Detection of shigella in lettuce by the use of a rapid molecular assay with increased sensitivity. Brazilian Journal of Microbiology 41 (4):993–1000. doi: 10.1590/S1517-83822010000400018.
- Jin, B., B. Ma, J. Li, Y. Hong, and M. Zhang. 2022. Simultaneous detection of five foodborne pathogens using a mini automatic nucleic acid extractor combined with recombinase polymerase amplification and lateral flow immunoassay. Microorganisms 10 (7):1352. doi: 10.3390/microorganisms10071352.
- Jung, B. Y., S. C. Jung, and C. H. Kweon. 2005. Development of a rapid immunochromatographic strip for detection of Escherichia coli O157. Journal of Food Protection 68 (10):2140–3. doi: 10.4315/0362-028x-68.10.2140.
- Jung, Y., Y. Heo, J. J. Lee, A. Deering, and E. Bae. 2020. Smartphone-based lateral flow imaging system for detection of food-borne bacteria E.coli O157:H7. Journal of Microbiological Methods 168:105800. doi: 10.1016/j.mimet.2019.105800.
- Karp, D. S., S. Gennet, C. Kilonzo, M. Partyka, N. Chaumont, E. R. Atwill, and C. Kremen. 2015. Comanaging fresh produce for nature conservation and food safety. Proceedings of the National Academy of Sciences of the United States of America 112 (35):11126–31. doi: 10.1073/pnas.1508435112.
- Kawasaki, S., P. M. Fratamico, N. Horikoshi, Y. Okada, K. Takeshita, T. Sameshima, and S. Kawamoto. 2009. Evaluation of a multiplex PCR system for simultaneous detection of Salmonella spp., Listeria monocytogenes, and Escherichia coli O157:H7 in foods and in food subjected to freezing. Foodborne Pathogens and Disease 6 (1):81–9. doi: 10.1089/fpd.2008.0153.
- Kawasaki, S., P. M. Fratamico, N. Horikoshi, Y. Okada, K. Takeshita, T. Sameshima, and S. Kawamoto. 2010. Multiplex real-time polymerase chain reaction assay for simultaneous detection and quantification of Salmonella species, Listeria monocytogenes, and Escherichia coli O157:H7 in ground pork samples. Foodborne Pathogens and Disease 7 (5):549–54. doi: 10.1089/fpd.2009.0465.
- Keiser, J., and J. Utzinger. 2005. Emerging foodborne trematodiasis. Emerging Infectious Diseases 11 (10):1507–14. doi: 10.3201/eid1110.050614.
- Khodadadi, M., L. Chang, J. R. C. Trabuco, B. V. Vu, K. Kourentzi, R. C. Willson, and D. Litvinov. 2019. PCB-based magnetometer as a platform for quantification of lateral-flow assays. Sensors (Basel) 19 (24):5433. doi: 10.3390/s19245433.
- Khreich, N., P. Lamourette, H. Boutal, K. Devilliers, C. Créminon, and H. Volland. 2008. Detection of Staphylococcus enterotoxin B using fluorescent immunoliposomes as label for immunochromatographic testing. Analytical Biochemistry 377 (2):182–8. doi: 10.1016/j.ab.2008.02.032.
- Kim, J., J. H. Kwon, J. Jang, H. Lee, S. Kim, Y. K. Hahn, S. K. Kim, K. H. Lee, S. Lee, H. Pyo, et al. 2018. Rapid and background-free detection of avian influenza virus in opaque sample using NIR-to-NIR upconversion nanoparticle-based lateral flow immunoassay platform. Biosensors & Bioelectronics 112:209–15. doi: 10.1016/j.bios.2018.04.047.
- Kim, S., and J.-H. Lee. 2022. Current advances in paper-based biosensor technologies for rapid COVID-19 diagnosis. BioChip Journal 16 (4):376–96. doi: 10.1007/s13206-022-00078-9.
- Kong, J., N. R. Franklin, C. Zhou, M. G. Chapline, S. Peng, K. Cho, and H. Dai. 2000. Nanotube molecular wires as chemical sensors. Science (New York, NY) 287 (5453):622–5. doi: 10.1126/science.287.5453.622.
- Kong, M., J. H. Shin, S. Heu, J. K. Park, and S. Ryu. 2017. Lateral flow assay-based bacterial detection using engineered cell wall binding domains of a phage endolysin. Biosensors & Bioelectronics 96:173–7. doi: 10.1016/j.bios.2017.05.010.
- Kumar, B. K., P. Raghunath, D. Devegowda, V. K. Deekshit, M. N. Venugopal, I. Karunasagar, and I. Karunasagar. 2011. Development of monoclonal antibody based sandwich ELISA for the rapid detection of pathogenic Vibrio parahaemolyticus in seafood. International Journal of Food Microbiology 145 (1):244–9. doi: 10.1016/j.ijfoodmicro.2010.12.030.
- Labs, R. . RapidChek® E. coli O157 test strip. https://www.romerlabs.com/shop/inter_en/rapidchek-r-e-coli-o157-test-system/.
- Law, J. W., N. S. Ab Mutalib, K. G. Chan, and L. H. Lee. 2014. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Frontiers in Microbiology 5:770. doi: 10.3389/fmicb.2014.00770.
- Lee, J. H., H. S. Seo, J. H. Kwon, H. T. Kim, K. C. Kwon, S. J. Sim, Y. J. Cha, and J. Lee. 2015. Multiplex diagnosis of viral infectious diseases (AIDS, hepatitis C, and hepatitis A) based on point of care lateral flow assay using engineered proteinticles. Biosensors & Bioelectronics 69:213–25. doi: 10.1016/j.bios.2015.02.033.
- Li, C. Z., K. Vandenberg, S. Prabhulkar, X. Zhu, L. Schneper, K. Methee, C. J. Rosser, and E. Almeide. 2011. Paper based point-of-care testing disc for multiplex whole cell bacteria analysis. Biosensors & Bioelectronics 26 (11):4342–8. doi: 10.1016/j.bios.2011.04.035.
- Li, F., Q. Ye, M. Chen, J. Zhang, L. Xue, J. Wang, S. Wu, H. Zeng, Q. Gu, Y. Zhang, et al. 2020. Multiplex PCR for the Identification of Pathogenic Listeria in Flammulina velutipes Plant Based on Novel Specific Targets Revealed by Pan-Genome Analysis. Frontiers in Microbiology 11:634255. doi: 10.3389/fmicb.2020.634255.
- Li, Y., H. Xie, J. Wang, X. Li, Z. Xiao, Z. Xu, H. Lei, and X. Shen. 2021. Lateral Flow Immunochromatography Assay for Detection of Furosemide in Slimming Health Foods. Foods 10 (9):2041. doi: 10.3390/foods10092041.
- Li, Y., X. Chen, J. Yuan, Y. Leng, W. Lai, X. Huang, and Y. Xiong. 2020. Integrated gold superparticles into lateral flow immunoassays for the rapid and sensitive detection of Escherichia coli O157:H7 in milk. Journal of Dairy Science 103 (8):6940–9. doi: 10.3168/jds.2019-17934.
- Liang, T., R. Robinson, J. Houghtaling, G. Fridley, S. A. Ramsey, and E. Fu. 2016. Investigation of reagent delivery formats in a multivalent malaria sandwich immunoassay and implications for assay performance. Analytical Chemistry 88 (4):2311–20. doi: 10.1021/acs.analchem.5b04222.
- Liang, Z., X. Wang, W. Zhu, P. Zhang, Y. Yang, C. Sun, J. Zhang, X. Wang, Z. Xu, Y. Zhao, et al. 2017. Upconversion nanocrystals mediated lateral-flow nanoplatform for in vitro detection. ACS Applied Materials & Interfaces 9 (4):3497–504. doi: 10.1021/acsami.6b14906.
- Liu, C. C., C. Y. Yeung, P. H. Chen, M. K. Yeh, and S. Y. Hou. 2013. Salmonella detection using 16S ribosomal DNA/RNA probe-gold nanoparticles and lateral flow immunoassay. Food Chemistry 141 (3):2526–32. doi: 10.1016/j.foodchem.2013.05.089.
- Liu, H. B., X. J. Du, Y. X. Zang, P. Li, and S. Wang. 2017. SERS-based lateral flow strip biosensor for simultaneous detection of listeria monocytogenes and salmonella enterica serotype enteritidis. Journal of Agricultural and Food Chemistry 65 (47):10290–9. doi: 10.1021/acs.jafc.7b03957.
- Liu, H.-b., C.-y. Chen, C.-n. Zhang, X.-j. Du, P. Li, and S. Wang. 2019. Functionalized AuMBA@Ag nanoparticles as an optical and SERS dual probe in a lateral flow strip for the quantitative detection of Escherichia coli O157:H7. Journal of Food Science 84 (10):2916–24. doi: 10.1111/1750-3841.14766.
- Liu, X., J. Yang, Q. Li, Y. Wang, Y. Wang, G. Li, J. Shi, P. Ding, J. Guo, R. Deng, et al. 2020. A strip test for the optical determination of influenza virus H3 subtype using gold nanoparticle coated polystyrene latex microspheres. Microchimica Acta 187 (5):306. doi: 10.1007/s00604-020-04255-1.
- Liu, Y., Y. Cao, T. Wang, Q. Dong, J. Li, and C. Niu. 2019. Detection of 12 common food-borne bacterial pathogens by TaqMan real-time PCR using a single set of reaction conditions. Frontiers in Microbiology 10:222. doi: 10.3389/fmicb.2019.00222.
- Liu, Y., L. Zhan, Z. Qin, J. Sackrison, and J. C. Bischof. 2021. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano 15 (3):3593–611. doi: 10.1021/acsnano.0c10035.
- Liu, Y., Z. Zhang, Y. Wang, Y. Zhao, Y. Lu, X. Xu, J. Yan, and Y. Pan. 2015. A highly sensitive and flexible magnetic nanoprobe labeled immunochromatographic assay platform for pathogen Vibrio parahaemolyticus. International Journal of Food Microbiology 211:109–16. doi: 10.1016/j.ijfoodmicro.2015.07.005.
- Lou, D., L. Fan, Y. Ji, N. Gu, and Y. Zhang. 2019. A signal amplifying fluorescent nanoprobe and lateral flow assay for ultrasensitive detection of cardiac biomarker troponin I. Analytical Methods 11 (28):3506–13. doi: 10.1039/C9AY01039D.
- Loynachan, C. N., M. R. Thomas, E. R. Gray, D. A. Richards, J. Kim, B. S. Miller, J. C. Brookes, S. Agarwal, V. Chudasama, R. A. McKendry, et al. 2018. Platinum Nanocatalyst Amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12 (1):279–88. doi: 10.1021/acsnano.7b06229.
- Mangal, M., S. Bansal, S. K. Sharma, and R. K. Gupta. 2016. Molecular detection of foodborne pathogens: A rapid and accurate answer to food safety. Critical Reviews in Food Science and Nutrition 56 (9):1568–84. doi: 10.1080/10408398.2013.782483.
- Mao, X., Y. Ma, A. Zhang, L. Zhang, L. Zeng, and G. Liu. 2009. Disposable nucleic acid biosensors based on gold nanoparticle probes and lateral flow strip. Analytical Chemistry 81 (4):1660–8. doi: 10.1021/ac8024653.
- Mashayekhi, F., A. M. Le, P. M. Nafisi, B. M. Wu, and D. T. Kamei. 2012. Enhancing the lateral-flow immunoassay for detection of proteins using an aqueous two-phase micellar system. Analytical and Bioanalytical Chemistry 404 (6-7):2057–66. doi: 10.1007/s00216-012-6278-y.
- Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerging Infectious Diseases 5 (5):607–25. doi: 10.3201/eid0505.990502.
- Medintz, I. L., H. T. Uyeda, E. R. Goldman, and H. Mattoussi. 2005. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials 4 (6):435–46. doi: 10.1038/nmat1390.
- Mens, P. F., A. van Amerongen, P. Sawa, P. A. Kager, and H. D. Schallig. 2008. Molecular diagnosis of malaria in the field: Development of a novel 1-step nucleic acid lateral flow immunoassay for the detection of all 4 human Plasmodium spp. and its evaluation in Mbita, Kenya. Diagnostic Microbiology and Infectious Disease 61 (4):421–7. doi: 10.1016/j.diagmicrobio.2008.03.009.
- Meyer, C., M. Fredriksson-Ahomaa, B. Sperner, and E. Märtlbauer. 2011. Detection of Listeria monocytogenes in pork and beef using the VIDAS® LMO2 automated enzyme linked immunoassay method. Meat Science 88 (3):594–6. doi: 10.1016/j.meatsci.2011.01.035.
- Millipore, 2023a. Duopath® Cereus Enterotoxins. Accessed 14/6/2023. https://www.merckmillipore.com/INTL/en/product/msds/MDA_CHEM-104146?ReferrerURL=https%3A//www.google.com/.
- Millipore, 2023b. Singlepath® Campylobacter Rapid test. Accessed 14/6/2023. https://www.merckmillipore.com/INTL/en/product/Singlepath-Campylobacter,MDA_CHEM-104143.
- Millipore, 2023c. Singlepath® E. coli O157. Accessed 14/6/2023. https://www.merckmillipore.com/INTL/en/product/Singlepath-E.coli-O157,MDA_CHEM-104141?ReferrerURL=https%3A%2F%2Fsearch.yahoo.com%2F
- Millipore, 2023d. Singlepath® L’mono. Accessed 14/6/2023. https://www.merckmillipore.com/DK/en/product/Singlepath-Lmono,MDA_CHEM-104148.
- Millipore, 2023e. Singlepath® Salmonella. Accessed 14/6/2023. https://www.merckmillipore.com/INTL/en/product/Singlepath-Salmonella,MDA_CHEM-104140.
- Millipore, 2023f. VIP® Gold for EHEC. Accessed 14/6/2023. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/105/832/60036bc-dfu-ug6602en-mk.pdf.
- Millipore, 2023g. VIP® Gold for Listeria. Accessed 14/6/2023. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/416/161/60037bc-dfu-ug4681en-mk.pdf.
- Millipore, 2023h. Gold for Salmonella. Accessed 14/6/2023. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/355/001/60038bc-dfu-ug4682en-mk.pdf.
- Moghadam, B. Y., K. T. Connelly, and J. D. Posner. 2015. Two orders of magnitude improvement in detection limit of lateral flow assays using isotachophoresis. Analytical Chemistry 87 (2):1009–17. doi: 10.1021/ac504552r.
- Moongkarndi, P., E. Rodpai, and S. Kanarat. 2011. Evaluation of an immunochromatographic assay for rapid detection of Salmonella enterica serovars Typhimurium and Enteritidis. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians, Inc 23 (4):797–801. doi: 10.1177/1040638711408063.
- Morales-Narváez, E., T. Naghdi, E. Zor, and A. Merkoçi. 2015. Photoluminescent lateral-flow immunoassay revealed by graphene oxide: Highly sensitive paper-based pathogen detection. Analytical Chemistry 87 (16):8573–7. doi: 10.1021/acs.analchem.5b02383.
- Mosley, G. L., P. Nguyen, B. M. Wu, and D. T. Kamei. 2016. Development of quantitative radioactive methodologies on paper to determine important lateral-flow immunoassay parameters. Lab on a Chip 16 (15):2871–81. doi: 10.1039/C6LC00518G.
- Mun, S., and S.-J. Choi. 2015. Detection of Salmonella typhimurium by antibody/enzyme-conjugated magnetic nanoparticles. BioChip Journal 9 (1):10–5. doi: 10.1007/s13206-014-9102-2.
- Nam, J.-M., C. Thaxton, and C. Mirkin. 2003. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science (New York, NY) 301 (5641):1884–6. doi: 10.1126/science.1088755.
- Nash, M. A., J. N. Waitumbi, A. S. Hoffman, P. Yager, and P. S. Stayton. 2012. Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system. ACS Nano 6 (8):6776–85. doi: 10.1021/nn3015008.
- Neethirajan, S., S. R. Ahmed, R. Chand, J. Buozis, and É. Nagy. 2017. Recent advances in biosensor development for foodborne virus detection. Nanotheranostics 1 (3):272–95. doi: 10.7150/ntno.20301.
- Nguyen, V. T., S. Song, S. Park, and C. Joo. 2020. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosensors & Bioelectronics 152:112015. doi: 10.1016/j.bios.2020.112015.
- Niedbala, R. S., H. Feindt, K. Kardos, T. Vail, J. Burton, B. Bielska, S. Li, D. Milunic, P. Bourdelle, and R. Vallejo. 2001. Detection of analytes by immunoassay using up-converting phosphor technology. Analytical Biochemistry 293 (1):22–30. doi: 10.1006/abio.2001.5105.
- Noguera, P., G. A. Posthuma-Trumpie, M. van Tuil, F. J. van der Wal, A. de Boer, A. P. H. A. Moers, and A. van Amerongen. 2011. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli. Analytical and Bioanalytical Chemistry 399 (2):831–8. doi: 10.1007/s00216-010-4334-z.
- Okuno, J., K. Maehashi, K. Kerman, Y. Takamura, K. Matsumoto, and E. Tamiya. 2007. Label-free immunosensor for prostate-specific antigen based on single-walled carbon nanotube array-modified microelectrodes. Biosensors & Bioelectronics 22 (9–10):2377–81. doi: 10.1016/j.bios.2006.09.038.
- O’Sullivan, J., D. J. Bolton, G. Duffy, C. Baylis, R. Tozzoli, Y. Wasteson, and S. Lofdahl. 2007. Methods for detection and molecular characterisation of pathogenic Escherichia coli, 1-31. Dublin, Ireland. Ashtown Food Research Centre.
- Pandey, S. K., C. R. Suri, M. Chaudhry, R. P. Tiwari, and P. Rishi. 2012. A gold nanoparticles based immuno-bioprobe for detection of Vi capsular polysaccharide of Salmonella enterica serovar Typhi. Molecular bioSystems 8 (7):1853–60. doi: 10.1039/c2mb25048a.
- Paniel, N., and T. Noguer. 2019. Detection of Salmonella in food matrices, from conventional methods to recent aptamer-sensing technologies. Foods 8 (9):371. doi: 10.3390/foods8090371.
- Pappert, G., M. Rieger, R. Niessner, and M. Seidel. 2010. Immunomagnetic nanoparticle-based sandwich chemiluminescence-ELISA for the enrichment and quantification of E. coli. Microchimica Acta 168 (1–2):1–8. doi: 10.1007/s00604-009-0264-x.
- Park, B. H., S. J. Oh, J. H. Jung, G. Choi, J. H. Seo, D. H. Kim, E. Y. Lee, and T. S. Seo. 2017. An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosensors & Bioelectronics 91:334–40. doi: 10.1016/j.bios.2016.11.063.
- Park, J., J. Ho Shin, and J.-K. Park. 2016. Pressed paper-based dipstick for detection of foodborne pathogens with multistep reactions. Analytical Chemistry 88 (7):3781–8. doi: 10.1021/acs.analchem.5b04743.
- Park, S., Y. T. Kim, and Y.-K. Kim. 2010. Optical enzyme-linked immunosorbent assay on a strip for detection of Salmonella typhimurium. BioChip Journal 4 (2):110–6. doi: 10.1007/s13206-010-4204-y.
- Parolo, C., A. de la Escosura-Muñiz, and A. Merkoçi. 2013. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosensors & Bioelectronics 40 (1):412–6. doi: 10.1016/j.bios.2012.06.049.
- Parolo, C., A. de la Escosura-Muñiz, E. Polo, V. Grazú, J. M. de la Fuente, and A. Merkoçi. 2013. Design, preparation, and evaluation of a fixed-orientation antibody/gold-nanoparticle conjugate as an immunosensing label. ACS Applied Materials & Interfaces 5 (21):10753–9. doi: 10.1021/am4029153.
- Pashazadeh-Panahi, P., S. Belali, H. Sohrabi, F. Oroojalian, M. Hashemzaei, A. Mokhtarzadeh, and M. de la Guardia. 2021. Metal-organic frameworks conjugated with biomolecules as efficient platforms for development of biosensors. TrAC Trends in Analytical Chemistry 141:116285. doi: 10.1016/j.trac.2021.116285.
- Pengsuk, C., P. Chaivisuthangkura, S. Longyant, and P. Sithigorngul. 2013. Development and evaluation of a highly sensitive immunochromatographic strip test using gold nanoparticle for direct detection of Vibrio cholerae O139 in seafood samples. Biosensors & Bioelectronics 42:229–35. doi: 10.1016/j.bios.2012.10.011.
- Pohanka, M. 2021. Point-of-care diagnoses and assays based on lateral flow test. International Journal of Analytical Chemistry 2021:1–9. doi: 10.1155/2021/6685619.
- Pöhlmann, C., I. Dieser, and M. Sprinzl. 2014. A lateral flow assay for identification of Escherichia coli by ribosomal RNA hybridisation. The Analyst 139 (5):1063–71. doi: 10.1039/C3AN02059B.
- Portanti, O., T. Di Febo, M. Luciani, C. Pompilii, R. Lelli, and P. Semprini. 2011. Development and validation of an antigen capture ELISA based on monoclonal antibodies specific for Listeria monocytogenes in food. Veterinaria Italiana 47 (3):281–90.
- Posthuma-Trumpie, G. A., J. H. Wichers, M. Koets, L. B. Berendsen, and A. van Amerongen. 2012. Amorphous carbon nanoparticles: A versatile label for rapid diagnostic (immuno)assays. Analytical and Bioanalytical Chemistry 402 (2):593–600. doi: 10.1007/s00216-011-5340-5.
- Preechakasedkit, P., K. Pinwattana, W. Dungchai, W. Siangproh, W. Chaicumpa, P. Tongtawe, and O. Chailapakul. 2012. Development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosensors & Bioelectronics 31 (1):562–6. doi: 10.1016/j.bios.2011.10.031.
- Qi, H., Z. Zhong, H. X. Zhou, C. Y. Deng, H. Zhu, J. F. Li, X. L. Wang, and F. R. Li. 2011. A rapid and highly sensitive protocol for the detection of Escherichia coli O157:H7 based on immunochromatography assay combined with the enrichment technique of immunomagnetic nanoparticles. International Journal of Nanomedicine 6:3033–9. doi: 10.2147/ijn.S25684.
- Qiu, W., H. Xu, S. Takalkar, A. S. Gurung, B. Liu, Y. Zheng, Z. Guo, M. Baloda, K. Baryeh, and G. Liu. 2015. Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosensors & Bioelectronics 64:367–72. doi: 10.1016/j.bios.2014.09.028.
- Quesada-González, D., and A. Merkoçi. 2018. Nanomaterial-based devices for point-of-care diagnostic applications. Chemical Society Reviews 47 (13):4697–709. doi: 10.1039/c7cs00837f.
- Quesada-González, D., C. Stefani, I. González, A. de la Escosura-Muñiz, N. Domingo, P. Mutjé, and A. Merkoçi. 2019. Signal enhancement on gold nanoparticle-based lateral flow tests using cellulose nanofibers. Biosensors & Bioelectronics 141:111407. doi: 10.1016/j.bios.2019.111407.
- Rastogi, S. K., C. M. Gibson, J. R. Branen, D. E. Aston, A. Larry Branen, and P. J. Hrdlicka. 2012. DNA detection on lateral flow test strips: Enhanced signal sensitivity using LNA-conjugated gold nanoparticles. Chemical Communications (Cambridge, England) 48 (62):7714–6. doi: 10.1039/C2CC33430E.
- Ren, W., D. R. Ballou, R. FitzGerald, and J. Irudayaraj. 2019. Plasmonic enhancement in lateral flow sensors for improved sensing of E. coli O157:H7. Biosensors & Bioelectronics 126:324–31. doi: 10.1016/j.bios.2018.10.066.
- Rodríguez, M. O., L. B. Covián, A. C. García, and M. C. Blanco-López. 2016. Silver and gold enhancement methods for lateral flow immunoassays. Talanta 148:272–8. doi: 10.1016/j.talanta.2015.10.068.
- Saad, S., A. T. Afendy, N. Masdor, and I. Zamri. 2015. Lateral flow assay strip for detection of Escherichia coli O157:H7. International Food Research Journal. 22:2587–93.
- Saha, B., T. H. Evers, and M. W. Prins. 2014. How antibody surface coverage on nanoparticles determines the activity and kinetics of antigen capturing for biosensing. Analytical Chemistry 86 (16):8158–66. doi: 10.1021/ac501536z.
- Sajid, M., A.-N. Kawde, and M. Daud. 2015. Designs, formats and applications of lateral flow assay: A literature review. Journal of Saudi Chemical Society 19 (6):689–705. doi: 10.1016/j.jscs.2014.09.001.
- Sánchez-Cano, A., G. Ruiz-Vega, S. Vicente-Gómez, E. de la Serna, E. Sulleiro, I. Molina, A. Sánchez-Montalvá, and E. Baldrich. 2021. Development of a fast chemiluminescent magneto-immunoassay for sensitive plasmodium falciparum detection in whole blood. Analytical Chemistry 93 (37):12793–800. doi: 10.1021/acs.analchem.1c03242.
- Sánchez-Pomales, G., L. Santiago-Rodríguez, and C. R. Cabrera. 2009. DNA-functionalized carbon nanotubes for biosensing applications. Journal of Nanoscience and Nanotechnology 9 (4):2175–88. doi: 10.1166/jnn.2009.se47.
- Scott, E. 2003. Food safety and foodborne disease in 21st century homes. The Canadian Journal of Infectious Diseases = Journal Canadien Des Maladies Infectieuses 14 (5):277–80. doi: 10.1155/2003/363984.
- Serebrennikova, K., J. Samsonova, and A. Osipov. 2018. Hierarchical nanogold labels to improve the sensitivity of lateral flow immunoassay. Nano-Micro Letters 10 (2):24. doi: 10.1007/s40820-017-0180-2.
- Sharif, Y. M., and B. A. Tayeb. 2021. Estimation of limit of detection of Salmonella typhimurium in artificially contaminated chicken meat by cultured-based and polymerase chain reaction techniques. Iraqi Journal of Veterinary Sciences 35 (4):621–5. doi: 10.33899/ijvs.2020.127328.1496.
- Sharma, A., A. I. Y. Tok, C. Lee, R. Ganapathy, P. Alagappan, and B. Liedberg. 2019. Magnetic field assisted preconcentration of biomolecules for lateral flow assaying. Sensors and Actuators B: Chemical 285:431–7. doi: 10.1016/j.snb.2019.01.073.
- Shen, Y., and G. Shen. 2019. Signal-enhanced lateral flow immunoassay with dual gold nanoparticle conjugates for the detection of hepatitis B surface antigen. ACS Omega. 4 (3):5083–7. doi: 10.1021/acsomega.8b03593.
- Shen, Z., N. Hou, M. Jin, Z. Qiu, J. Wang, B. Zhang, X. Wang, J. Wang, D. Zhou, and J. Li. 2014a. A novel enzyme-linked immunosorbent assay for detection of Escherichia coli O157:H7 using immunomagnetic and beacon gold nanoparticles. Gut Pathogens 6 (14):eCollection 2014. doi: 10.1186/1757-4749-6-14.
- Shi, L., F. Wu, Y. Wen, F. Zhao, J. Xiang, and L. Ma. 2015. A novel method to detect Listeria monocytogenes via superparamagnetic lateral flow immunoassay. Analytical and Bioanalytical Chemistry 407 (2):529–35. doi: 10.1007/s00216-014-8276-8.
- Shim, W.-B., J.-G. Choi, J.-Y. Kim, Z.-Y. Yang, K.-H. Lee, M.-G. Kim, S.-D. Ha, K.-S. Kim, K.-Y. Kim, C.-H. Kim, et al. 2008. Enhanced rapidity for qualitative detection of Listeria monocytogenes using an enzyme-linked immunosorbent assay and immunochromatography strip test combined with immunomagnetic bead separation. Journal of Food Protection 71 (4):781–9. doi: 10.4315/0362-028x-71.4.781.
- Shin, J. H., J. Hong, H. Go, J. Park, M. Kong, S. Ryu, K.-P. Kim, E. Roh, and J.-K. Park. 2018. Multiplexed detection of foodborne pathogens from contaminated lettuces using a handheld multistep lateral flow assay device. Journal of Agricultural and Food Chemistry 66 (1):290–7. doi: 10.1021/acs.jafc.7b03582.
- Shirshahi, V., S. Nasrollah Tabatabaei, S. Hatamie, and R. Saber. 2020. Photothermal enhancement in sensitivity of lateral flow assays for detection of E-coli O157:H7. Colloids and Surfaces. B, Biointerfaces 186:110721. doi: 10.1016/j.colsurfb.2019.110721.
- Shyu, R. H., H. F. Shyu, H. W. Liu, and S. S. Tang. 2002. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon: Official Journal of the International Society on Toxinology 40 (3):255–8. doi: 10.1016/s0041-0101(01)00193-3.
- Singh, J., S. Sharma, and S. Nara. 2015a. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chemistry 170:470–83. doi: 10.1016/j.foodchem.2014.08.092.
- Singh, J., S. Sharma, and S. Nara. 2015b. Nanogold based lateral flow assay for the detection of Salmonella typhi in environmental water samples. Analytical Methods 7 (21):9281–8. doi: 10.1039/C5AY02271A.
- Soh, J. H., H.-M. Chan, and J. Y. Ying. 2020. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today. 30:100831. doi: 10.1016/j.nantod.2019.100831.
- Sohrabi, H., M. R. Majidi, F. Nami, K. Asadpour-Zeynali, A. Khataee, and A. Mokhtarzadeh. 2021. A novel engineered label-free Zn-based MOF/CMC/AuNPs electrochemical genosensor for highly sensitive determination of Haemophilus Influenzae in human plasma samples. Microchimica Acta 188 (3):100. doi: 10.1007/s00604-021-04757-6.
- Song, C., J. Liu, J. Li, and Q. Liu. 2016. Dual FITC lateral flow immunoassay for sensitive detection of Escherichia coli O157:H7 in food samples. Biosensors & Bioelectronics 85:734–9. doi: 10.1016/j.bios.2016.05.057.
- Song, C., C. Liu, S. Wu, H. Li, H. Guo, B. Yang, S. Qiu, J. Li, L. Liu, H. Zeng, et al. 2016. Development of a lateral flow colloidal gold immunoassay strip for the simultaneous detection of Shigella boydii and Escherichia coli O157:H7 in bread, milk and jelly samples. Food Control 59:345–51. doi: 10.1016/j.foodcont.2015.06.012.
- Soozanipour, A., H. Sohrabi, F. Abazar, A. Khataee, A. Noorbakhsh, M. Asadnia, A. Taheri-Kafrani, M. R. Majidi, and A. Razmjou. 2021. Ion selective nanochannels: From critical principles to sensing and biosensing applications. Advanced Materials Technologies 6 (10):2000765. doi: 10.1002/admt.202000765.
- Speroni, F., L. Elviri, M. Careri, and A. Mangia. 2010. Magnetic particles functionalized with PAMAM-dendrimers and antibodies: A new system for an ELISA method able to detect Ara h3/4 peanut allergen in foods. Analytical and Bioanalytical Chemistry 397 (7):3035–42. doi: 10.1007/s00216-010-3851-0.
- Suaifan, G., S. Alhogail, and M. Zourob. 2017. Paper-based magnetic nanoparticle-peptide probe for rapid and quantitative colorimetric detection of Escherichia coli O157:H7. Biosensors & Bioelectronics 92:702–8. doi: 10.1016/j.bios.2016.10.023.
- Sun, C., Z.-W. Wang, J.-X. Li, W.-L. Fan, X.-Y. Qiao, Z.-M. Liu, S.-L. Li, L.-J. Tang, Y.-J. Li, and Y.-G. Xu. 2017. A rapid and sensitive method for simultaneous screening of nine foodborne pathogens using high-performance liquid chromatography assay. Food Analytical Methods 10 (4):1117–27. doi: 10.1007/s12161-016-0672-6.
- Tang, R., L. Liu, S. Zhang, A. Li, and Z. Li. 2019. Modification of a nitrocellulose membrane with cellulose nanofibers for enhanced sensitivity of lateral flow assays: Application to the determination of Staphylococcus aureus. Microchimica Acta 186 (12). doi: 10.1007/s00604-019-3970-z.
- Tang, R., H. Yang, Y. Gong, M. You, Z. Liu, J. R. Choi, T. Wen, Z. Qu, Q. Mei, and F. Xu. 2017. A fully disposable and integrated paper-based device for nucleic acid extraction, amplification and detection. Lab on a Chip 17 (7):1270–9. doi: 10.1039/C6LC01586G.
- Tang, X.-J., Z. Yang, X.-B. Chen, W.-F. Tian, C.-N. Tu, and H.-B. Wang. 2018. Verification and large scale clinical evaluation of a national standard protocol for Salmonella spp./Shigella spp. screening using real-time PCR combined with guided culture. Journal of Microbiological Methods 145:14–19. doi: 10.1016/j.mimet.2017.12.007.
- Tao, Y., J. Yang, L. Chen, Y. Huang, B. Qiu, L. Guo, and Z. Lin. 2018. Dialysis assisted ligand exchange on gold nanorods: Amplification of the performance of a lateral flow immunoassay for E. coli O157:H7. Microchimica Acta 185 (7):350. doi: 10.1007/s00604-018-2897-0.
- Techathuvanan, C., F. A. Draughon, and D. H. D’Souza. 2010. Loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Salmonella Typhimurium from pork. Journal of Food Science 75 (3):M165–172. doi: 10.1111/j.1750-3841.2010.01554.x.
- Tominaga, T., and M. Ishii. 2020. Chapter 11 - Detection of microorganisms with lateral flow test strips. In Methods in microbiology, ed. Charles S. Pavia and Volker Gurtler, 351–94. Cambridge, USA: Academic Press.
- Török, K., L. Hajas, V. Horváth, E. Schall, Z. Bugyi, S. Kemény, and S. Tömösközi. 2015. Identification of the factors affecting the analytical results of food allergen ELISA methods. European Food Research and Technology 241 (1):127–36. doi: 10.1007/s00217-015-2441-y.
- Trilling, A. K., J. Beekwilder, and H. Zuilhof. 2013. Antibody orientation on biosensor surfaces: A minireview. The Analyst 138 (6):1619–27. doi: 10.1039/C2AN36787D.
- Vaz-Velho, M., G. Duarte, and P. Gibbs. 2000. Evaluation of mini-VIDAS rapid test for detection of Listeria monocytogenes from production lines of fresh to cold-smoked fish. Journal of Microbiological Methods 40 (2):147–51. doi: 10.1016/s0167-7012(00)00118-4.
- Verheijen, R., I. K. Osswald, R. Dietrich, and W. Haasnoot. 2000. Development of a one step strip test for the detection of (dihydro)streptomycin residues in raw milk. Food and Agricultural Immunology 12 (1):31–40. doi: 10.1080/09540100099607.
- Vieira-Pinto, M., M. Oliveira, F. Bernardo, and C. Martins. 2007. Rapid detection of Salmonella sp. in pork samples using fluorescent in situ hybridization: A comparison with VIDAS ® -SLM system and ISO 6579 cultural method ISO 6579. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 59 (6):1388–93. doi: 10.1590/S0102-09352007000600006.
- Wang, C., W. Shen, Z. Rong, X. Liu, B. Gu, R. Xiao, and S. Wang. 2020. Layer-by-layer assembly of magnetic-core dual quantum dot-shell nanocomposites for fluorescence lateral flow detection of bacteria. Nanoscale 12 (2):795–807. doi: 10.1039/c9nr08509b.
- Wang, D.-B., B. Tian, Z.-P. Zhang, X.-Y. Wang, J. Fleming, L.-J. Bi, R.-F. Yang, and X.-E. Zhang. 2015. Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure. Biosensors & Bioelectronics 67:608–14. doi: 10.1016/j.bios.2014.09.067.
- Wang, J.-Y., M.-H. Chen, Z.-C. Sheng, D.-F. Liu, S.-S. Wu, and W.-H. Lai. 2015. Development of colloidal gold immunochromatographic signal-amplifying system for ultrasensitive detection of Escherichia coli O157:H7 in milk. RSC Advances 5 (76):62300–5. doi: 10.1039/C5RA13279G.
- Wang, J., H.-M. Meng, J. Chen, J. Liu, L. Zhang, L. Qu, Z. Li, and Y. Lin. 2019. Quantum dot-based lateral flow test strips for highly sensitive detection of the tetanus antibody. ACS Omega 4 (4):6789–95. doi: 10.1021/acsomega.9b00657.
- Wang, L., L. Shi, J. Su, Y. Ye, and Q. Zhong. 2013. Detection of Vibrio parahaemolyticus in food samples using in situ loop-mediated isothermal amplification method. Gene 515 (2):421–5. doi: 10.1016/j.gene.2012.12.039.
- Wang, W., L. Liu, S. Song, L. Xu, H. Kuang, J. Zhu, and C. Xu. 2017. Identification and quantification of eight Listeria monocytogene serotypes from Listeria spp. using a gold nanoparticle-based lateral flow assay. Microchimica Acta 184 (3):715–24. doi: 10.1007/s00604-016-2028-8.
- Wang, X. W., L. Zhang, L. Q. Jin, M. Jin, Z. Q. Shen, S. An, F. H. Chao, and J. W. Li. 2007. Development and application of an oligonucleotide microarray for the detection of food-borne bacterial pathogens. Applied Microbiology and Biotechnology 76 (1):225–33. doi: 10.1007/s00253-007-0993-x.
- Wang, Z., X. Yao, R. Wang, Y. Ji, T. Yue, J. Sun, T. Li, J. Wang, and D. Zhang. 2019. Label-free strip sensor based on surface positively charged nitrogen-rich carbon nanoparticles for rapid detection of Salmonella enteritidis. Biosensors & Bioelectronics 132:360–7. doi: 10.1016/j.bios.2019.02.061.
- Wang, Z., X. Yao, Y. Zhang, R. Wang, Y. Ji, J. Sun, D. Zhang, and J. Wang. 2020. Functional nanozyme mediated multi-readout and label-free lateral flow immunoassay for rapid detection of Escherichia coli O157:H7. Food Chemistry 329:127224. doi: 10.1016/j.foodchem.2020.127224.
- Wei, C., J. Zhong, T. Hu, and X. Zhao. 2018. Simultaneous detection of Escherichia coli O157:H7, Staphylococcus aureus and Salmonella by multiplex PCR in milk. 3 Biotech 8 (1):76. doi: 10.1007/s13205-018-1086-5.
- Welch, N. G., J. A. Scoble, B. W. Muir, and P. J. Pigram. 2017. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 12 (2):02d301. doi: 10.1116/1.4978435.
- WHO. 2016. An estimated 12.6 million deaths each year are attributable to unhealthy environments. https://www.who.int/news/item/15-03-2016-an-estimated-12-6-million-deaths-each-year-are-attributable-to-unhealthy-environments.
- WHO. 2022. Food safety. https://www.who.int/news-room/fact-sheets/detail/food-safety.
- Wu, D., M. D. Milutinovic, and D. R. Walt. 2015. Single molecule array (Simoa) assay with optimal antibody pairs for cytokine detection in human serum samples. The Analyst 140 (18):6277–82. doi: 10.1039/c5an01238d.
- Wu, W., S. Zhao, Y. Mao, Z. Fang, X. Lu, and L. Zeng. 2015. A sensitive lateral flow biosensor for Escherichia coli O157:H7 detection based on aptamer mediated strand displacement amplification. Analytica Chimica Acta 861:62–8. doi: 10.1016/j.aca.2014.12.041.
- Xia, S., Z. Yu, D. Liu, C. Xu, and W. Lai. 2016. Developing a novel immunochromatographic test strip with gold magnetic bifunctional nanobeads (GMBN) for efficient detection of Salmonella choleraesuis in milk. Food Control 59:507–12. doi: 10.1016/j.foodcont.2015.06.028.
- Xing, K.-Y., J. Peng, D.-F. Liu, L.-M. Hu, C. Wang, G.-Q. Li, G.-G. Zhang, Z. Huang, S. Cheng, F.-F. Zhu, et al. 2018. Novel immunochromatographic assay based on Eu (III)-doped polystyrene nanoparticle-linker-monoclonal antibody for sensitive detection of Escherichia coli O157:H7. Analytica Chimica Acta 998:52–9. doi: 10.1016/j.aca.2017.10.027.
- Xu, C., O. U. Akakuru, J. Zheng, and A. Wu. 2019. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Frontiers in Bioengineering and Biotechnology 7:141. doi: 10.3389/fbioe.2019.00141.
- Xu, H., J. Chen, J. Birrenkott, J. X. Zhao, S. Takalkar, K. Baryeh, and G. Liu. 2014. Gold-nanoparticle-decorated silica nanorods for sensitive visual detection of proteins. Analytical Chemistry 86 (15):7351–9. doi: 10.1021/ac502249f.
- Yamazaki, A., M. Honda, N. Kobayashi, N. Ishizaki, H. Asakura, and Y. Sugita-Konishi. 2018. The sensitivity of commercial kits in detecting the genes of pathogenic bacteria in venison. The Journal of Veterinary Medical Science 80 (4):706–9. doi: 10.1292/jvms.17-0530.
- Yan, J., Y. Liu, Y. Wang, X. Xu, Y. Lu, Y. Pan, F. Guo, and D. Shi. 2014. Effect of physiochemical property of Fe3O4 particle on magnetic lateral flow immunochromatographic assay. Sensors and Actuators B: Chemical 197:129–36. doi: 10.1016/j.snb.2014.02.067.
- Yan, X., K. Wang, W. Lu, W. Qin, D. Cui, and J. He. 2016. CdSe/ZnS quantum dot-labeled lateral flow strips for rapid and quantitative detection of gastric cancer carbohydrate antigen 72-4. Nanoscale Research Letters 11 (1):138. doi: 10.1186/s11671-016-1355-3.
- Yao, L., J. Teng, M. Zhu, L. Zheng, Y. Zhong, G. Liu, F. Xue, and W. Chen. 2016. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosensors & Bioelectronics 85:331–6. doi: 10.1016/j.bios.2016.05.031.
- Yeung, C. Y., C. C. Liu, Y. T. Tseng, K. C. Tsai, M. A. Hsieh, W. T. Chan, H. L. Liu, H. C. Lee, and S. Y. Hou. 2014. Rapid identification of Salmonella using Hektoen enteric agar and 16s ribosomal DNA probe-gold nanoparticle immunochromatography assay in clinical faecal specimens. Letters in Applied Microbiology 58 (4):311–7. doi: 10.1111/lam.12191.
- Yin, H.-Y., Y.-T. Li, W.-C. Tsai, H.-Y. Dai, and H.-W. Wen. 2022. An immunochromatographic assay utilizing magnetic nanoparticles to detect major peanut allergen Ara h 1 in processed foods. Food Chemistry 375:131844. doi: 10.1016/j.foodchem.2021.131844.
- Yonekita, T., T. Fujimura, N. Morishita, T. Matsumoto, and F. Morimatsu. 2013. Simple, rapid, and reliable detection of Escherichia coli O26 using immunochromatography. Journal of Food Protection 76 (5):748–54. doi: 10.4315/0362-028x.Jfp-12-423.
- Yoshino, T., Y. Maeda, and T. Matsunag. 2010. Bioengineering of bacterial magnetic particles and their applications in biotechnology. Recent Patents on Biotechnology 4 (3):214–25. doi: 10.2174/187220810793611455.
- You, D. J., T. S. Park, and J. Y. Yoon. 2013. Cell-phone-based measurement of TSH using Mie scatter optimized lateral flow assays. Biosensors & Bioelectronics 40 (1):180–5. doi: 10.1016/j.bios.2012.07.014.
- You, M., M. Lin, Y. Gong, S. Wang, A. Li, L. Ji, H. Zhao, K. Ling, T. Wen, Y. Huang, et al. 2017. Household fluorescent lateral flow strip platform for sensitive and quantitative prognosis of heart failure using dual-color upconversion nanoparticles. ACS Nano 11 (6):6261–70. doi: 10.1021/acsnano.7b02466.
- Yu, X., T. Zhong, Y. Zhang, X. Zhao, Y. Xiao, L. Wang, X. Liu, and X. Zhang. 2022. Design, preparation, and application of magnetic nanoparticles for food safety analysis: A review of recent advances. Journal of Agricultural and Food Chemistry 70 (1):46–62. doi: 10.1021/acs.jafc.1c03675.
- Zhan, L., S. Z. Guo, F. Song, Y. Gong, F. Xu, D. R. Boulware, M. C. McAlpine, W. C. W. Chan, and J. C. Bischof. 2017. The role of nanoparticle design in determining analytical performance of lateral flow immunoassays. Nano Letters 17 (12):7207–12. doi: 10.1021/acs.nanolett.7b02302.
- Zhan, L., T. Granade, Y. Liu, X. Wei, A. Youngpairoj, V. Sullivan, J. Johnson, and J. Bischof. 2020. Development and optimization of thermal contrast amplification lateral flow immunoassays for ultrasensitive HIV p24 protein detection. Microsystems & Nanoengineering 6 (1):54. doi: 10.1038/s41378-020-0168-9.
- Zhang, H., L. Ma, L. Ma, M. Z. Hua, S. Wang, and X. Lu. 2017. Rapid detection of methicillin-resistant Staphylococcus aureus in pork using a nucleic acid-based lateral flow immunoassay. International Journal of Food Microbiology 243:64–9. doi: 10.1016/j.ijfoodmicro.2016.12.003.
- Zhang, L., Y. Huang, J. Wang, Y. Rong, W. Lai, J. Zhang, and T. Chen. 2015. Hierarchical flowerlike gold nanoparticles labeled immunochromatography test strip for highly sensitive detection of Escherichia coli O157:H7. Langmuir: The ACS Journal of Surfaces and Colloids 31 (19):5537–44. doi: 10.1021/acs.langmuir.5b00592.
- Zhao, X., C. W. Lin, J. Wang, and D. H. Oh. 2014. Advances in rapid detection methods for foodborne pathogens. Journal of Microbiology and Biotechnology 24 (3):297–312. doi: 10.4014/jmb.1310.10013.
- Zhao, Y., H. Wang, P. Zhang, C. Sun, X. Wang, X. Wang, R. Yang, C. Wang, and L. Zhou. 2016. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Scientific Reports 6:21342. doi: 10.1038/srep21342.