Abstract
Fermented food has unique properties and high nutritional value, and thus, should constitute a basic element of a balanced and health-promoting diet. However, it can accumulate considerable amount of biogenic amines (BAs), which ingested in excess can lead to adverse health effects. The application of plant-derived additives represents a promising strategy to ensure safety or enhance the functional and organoleptic properties of fermented food. This review summarizes currently available data on the application of plant-origin additives with the aim to reduce BA content in fermented products. The importance of ensuring fermented food safety has been highlighted considering the growing evidence of beneficial effects resulting from the consumption of this type of food, as well as the increasing number of individuals sensitive to BAs. The examined plant-origin additives reduced the BA concentration to varying degrees, and their efficacy depended on the type of additive, matrix, autochthonous, and inoculated microorganisms, as well as the manufacturing conditions. The main mechanisms of action include antimicrobial effects and the inhibition of microbial decarboxylases. Further research on the optimization of bioactive substances extraction, standardization of their chemical composition, and development of detailed procedures for its use in fermented products manufacturing are needed.
Graphical Abstract
Introduction
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), the term “fermented foods and beverages” refers to “foods made through desired microbial growth and enzymatic conversions of food components” (Marco et al. Citation2021, 197). Because of the above, products in which development spoilage microorganisms were involved, should not be defined as “fermented”. Products that i) arise as a result of the fermentation process but do not contain viable microorganisms when consumed, such as bread, beer, wine, soy sauce, vinegar, and cheese, which are classified as “foods made by fermentation”, as well as ii) contain viable microorganisms, for example, yoghurt, kefir, non-heated fermented vegetables, belonging to the “contains live and active cultures” category, are included in the fermented food group (Marco et al. Citation2021). Several beneficial effects have been observed because of the enrichment of diets with either plant- or animal-origin fermented food (Das et al. Citation2020). The presence of compounds such as vitamins (B2, B9, B12, and K), γ-aminobutyric acid (GABA), conjugated linoleic acids (CLA), exopolysaccharides, sphingolipids, bacteriocins, and bioactive peptides produced during the fermentation process is involved in this positive function (Chaudhary et al. Citation2021; Şanlier, Gökcen, and Sezgin Citation2019). Fermented food consumption can favorably alter the community and function of microorganisms (Kim and Park Citation2018; Nielsen et al. Citation2018; Taylor et al. Citation2020; Wastyk et al. Citation2021). Although fermented food consumption is highly recommended because of its multiple health-promoting effects, it can threaten the well-being of consumers with significant concentrations of biogenic amines (BAs) (Fong et al. Citation2020; Mah et al. Citation2019; Świder et al. Citation2020). These basic low-molecular-weight compounds are metabolized mainly by amine oxidase (AO) enzymes in the human body (Omer et al. Citation2021) but they can lead to adverse health effects when ingested in excess (Ladero et al. Citation2010) (). Consumers suffering from histamine intolerance or being treated with specific medications, such as amino oxidase inhibitors are of particular concern because their BA metabolism is impaired (Comas-Basté et al. Citation2020; EFSA Citation2011). In 2011, the European Food Safety Authority (EFSA) released a scientific statement concerning the safety of fermented products with regard to BA occurrence, underlining the importance of further research on the limitations of these compounds in fermented foods (EFSA Citation2011). Thermal processing, which is widely employ in the food industry is not applicable for the purpose of BA control, because of the thermostability of these compounds (Alvarez and Moreno-Arribas Citation2014). Methods such as the application of not able to produce BAs or BA-degrading starter cultures have frequently been reported (Lee et al. Citation2021; Li et al. Citation2018; Zhang et al. Citation2022). Most of these methods resulted in a successful reduction of some BAs; however, the outcome differed between the tested microorganisms, indicating that decarboxylation and amino oxidation activities are strain-specific (Li and Lu Citation2020; Li et al. Citation2021). Moreover, some strains can either produce or degrade BAs (Tittarelli et al. Citation2019). Thus, individual strains must be thoroughly examined before being used as an inoculum. Researchers have pointed out food additives as a promising strategy to limit BA accumulation during fermentation. Substances such as nicotinic acid and glycine have been successfully used in Baechu kimchi fermentation (Jin et al. Citation2022). Phenolic acids (Zhang et al. Citation2018), polyphenols (Lee et al. Citation2018), plant/spices extracts (Jia et al. Citation2020; Jin et al. Citation2022; Lee et al. Citation2018; Mah, Kim, and Hwang Citation2009; Zhou et al. Citation2016), and seasonings (Majcherczyk and Surówka Citation2019) also showed a positive outcome regarding the reduction of BA content. Bioactive substances originating from plants, such as phenolic or nitrogen compounds, alkaloids, terpenes, polysaccharides, and pigments, can affect the profile and abundance of intestinal microorganisms and support homeostatic equilibrium (Luo et al. Citation2021; van der Merwe Citation2021). Thus, except for the limitation of BAs, enriching functional food with plant extracts represents a promising strategy for promoting the proper functioning of intestinal microorganisms, and consequently, other parts of the organism through gut-other organ axes (Ahlawat, Sharma, and Asha Citation2021; Barrio, Arias-Sánchez, and Martín-Monzón Citation2022). Furthermore, it can impart desirable flavor and is applicable either under industrial or household conditions.
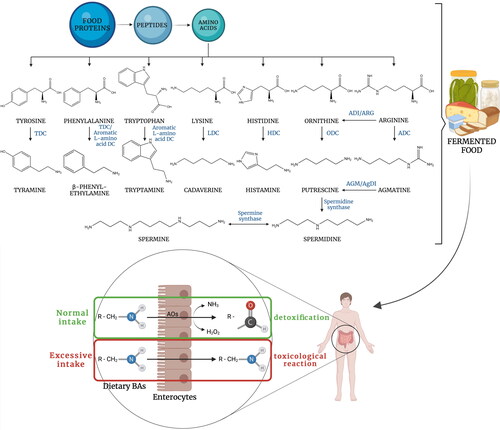
Figure 1. Main microbial BA formation pathways in fermented food and fate of ingested BAs in human gastrointestinal tract. Abbreviations: TDC – tyrosine decarboxylase, LDC – lysine decarboxylase, HDC – histidine decarboxylase, ODC – ornithine decarboxylase, ADC – arginine decarboxylase, ADI – arginine deiminase, ARG – arginase, AGM – agmatinase, AgDI – agmatine deiminase. Prepared based on Benkerroum Citation2016; Erdag, Merhan, and Yildiz Citation2018; Ladero et al. Citation2010. Created with BioRender.com.
Considering the above, the purpose of this review was to provide insights into the currently available data on attempts to reduce BAs in fermented products through the application of plant-origin food additives. The effectiveness of the substances used in particular forms and concentrations in both plant- and animal-origin matrices was summarized, and their putative mechanisms of action were identified. The importance of providing safe fermented foods in terms of BA content has been emphasized from the viewpoint of human well-being, given the growing evidence for health-promoting features of fermented foods and the increasing number of consumers who are sensitive to BAs. Finally, future perspectives regarding plant-origin additive applications to fermented foods are put forward. To the best of our knowledge, this is the first review on the limitations of BAs in fermented products using plant-origin food additives.
Lactic acid bacteria (LAB) – their role in fermentation process and involvement in BA production
LAB are among the most important and commonly used microorganisms in the food-manufacturing industry. It consists of Gram-positive cocci or rods, and its main characteristics include its ability to convert carbohydrates into lactic acid during fermentation (Fessard and Remize Citation2017). Furthermore, compounds such as acetic acid, ethanol, bacteriocins, exopolysaccharides, carbon dioxide, vitamins, enzymes, and aromatic compounds represent other LAB metabolites, that determine the nutritional and sensory properties of the fermented products (de Souza, de Oliveira, and de Oliveira Citation2023; Leroy and De Vuyst Citation2004). Some of these can inhibit food-borne pathogens and spoilage microorganisms, consequently enhancing the safety and prolonging the shelf-life of food (de Souza, de Oliveira, and de Oliveira Citation2023; Tabanelli et al. Citation2014; Zhou et al. Citation2014). Considering their widespread natural occurrence in food, long-term applications, and rare involvement in diseases, most of LAB are generally recognized as safe (GRAS) (Bintsis Citation2018; Ghanbari et al. Citation2013). Based on the LAB community, two main stages of food fermentation have been distinguished. Leuconostoc and Weissella prevailed during the initial heterofermentation phase and produced both lactic and acetic acids. The successive homofermentation step, in which lactic acid is produced almost exclusively, is dominated by more acid-tolerant species such as Lactiplantibacillus plantarum, Levilactobacillus brevis, Latilactobacillus curvatus, and Lactobacillus sakei (Peñas et al. Citation2010; Wouters et al. Citation2013). Traditional and household manufacturing of fermented food is based on spontaneous processes in which native microorganisms present in the raw material are involved. Large-scale industrial production requires the use of well-examined starter strains that guarantee control over the process and standardization of the final product (Leroy and De Vuyst Citation2004).
Some authors have suggested that nonstarter lactic acid bacteria (NSLAB) exhibit different metabolic activities compared to industrial strains, that is, they produce more enzymes that convert amino acids and thus are of particular importance in the development of flavor. Therefore, isolation and characterization of wild-type strains from fermented foods, with the aim of applying them as starter cultures, represents a desirable measure in terms of the retention of product characteristics (Leroy and De Vuyst Citation2004; Sánchez et al. Citation2010). However, each strain intended to be used to initiate fermentation should be thoroughly tested for its ability to produce BAs, which are also products of amino acid metabolism (EFSA Citation2011). BAs are formed in the microbial cytoplasm mainly through the decarboxylation of the corresponding free amino acid (FAA) precursors. Tyrosine, phenylalanine, tryptophan, lysine, histidine, and arginine are the direct precursors of tyramine, phenylethylamine, tryptamine, cadaverine, histamine, and agmatine, respectively. In addition to its direct production from ornithine, putrescine can also be produced from glutamine, arginine, or agmatine. The agmatinase and agmatine deiminase pathways are two possible routes for the direct production of putrescine from agmatine. Indirect production from arginine involves the arginine deiminase or arginase pathway. Further transformation of putrescine into polyamines is catalyzed by spermidine and spermine synthase enzymes (Benkerroum Citation2016). depicts the reactions described above and figuratively refers to food safety indicating main mechanism of BA metabolism in human.
The regulation of oxidative and osmotic stresses, as well as the provision of metabolic energy and tolerance to acidic environments, are the main triggers of BA production by microbial cells (Benkerroum Citation2016). L. plantarum strains isolated from naturally fermented pickles produce up to 298, 994, 668, and 1332 mg/L of cadaverine, putrescine, histamine, and total BAs, respectively, when cultured in a medium supplemented with lysine, ornithine, and histidine (Alan, Topalcengiz, and Dığrak Citation2018). Considering that the authors examined neither the indirect production of putrescine from arginine nor other BA-formation abilities, that is the production of tyramine from tyrosine, it can be presumed that the final concentration of total BAs produced by the tested isolates can reach even higher values. On the other hand, among the 54 wild lactobacilli and pediococci biotypes isolated from Mountain Cheese, only 11 were BA producers. Five biotypes belonging to Pediococcus pentosaceus, two belonging to Lacticaseibacillus paracasei, and one belonging to Lentilactobacillus hilgardii could form tyramine, whereas three Lentilactobacillus parabuchneri biotypes synthesized histamine. None of the tested bacteria exhibited the ability to produce putrescine or cadaverine (Carafa et al. Citation2015). It has been demonstrated that products obtained through spontaneous fermentation can accumulate high concentrations of BA owing to the activity of autochthonous BA-positive microorganisms, and the application of an appropriate dose of BA-negative starter cultures can significantly reduce the content of these compounds (Gardini et al. Citation2016; Świder et al. Citation2023).
Fermented food consumption – implications for human health
Even though fermented foods represent an important part of numerous traditional diets (Aslam et al. Citation2020), the available consumption data are scarce and there is a need to complete the information regarding this subject matter (EFSA Citation2011). It has been estimated that more than 5,000 different types of fermented foods and beverages is manufactured and consumed throughout the world (Tamang, Watanabe, and Holzapfel Citation2016). According to assessment made by Campbell-Platt (Citation1994) these products contribute to ca. one-third of the human diet. However, considering existing Western diet patterns on the one hand, and the growing health conscious of the consumers combined with facilitate access to different foods from all over the world on the other, today’s data on the consumption of fermented products can vary widely from almost 30 years old estimations. Recent results from The Netherlands indicate that fermented foods constitute approx. 16–18% of the adult Dutch diet, and subsequent 9–14% of consumed meals contain fermented ingredients (Li et al. Citation2020). One of the useful tools for searching the food consumption data regarding European countries is the EFSA Comprehensive European Food Consumption Database (EFSA Citation2022). It gathers information on the consumption of over 2,500 food groups, including fermented products. For example, according to the available surveys’ results the mean ingestion of fermented vegetables by adult consumers ranged from 6.00 (United Kingdom 2000) to 98.64 (Hungary 2018) g/day in 19 out of 27 countries included in the database. The current value of the fermented foods and beverages market equal USD 1.83 trillion and based on some statistical analysis it will continue to grow within the next five years (Fermented Foods and Beverages Market Size & Share Analysis – Growth Trends & Forecasts 2023–2028), what prove increasing demand for this type of food. Despite being significant part of a human diet, fermented products are still mostly override in nutritional guidelines (Bell, Ferrão, and Fernandes Citation2017).
The relationship between health and diet remains the subject of several studies worldwide. There is growing evidence for the anti-inflammatory properties of fermented foods. Kim and Park (Citation2018) investigated the effects of kimchi consumption on selected health parameters. One treatment group consumed standard, whereas the other a functional product, made with organic Chinese cabbage, enriched with plant additives (mustard leaf, Chinese pepper, pear, shiitake mushrooms, sea tangle juice, mistletoe extract), and L. plantarum PNU starter culture. Beneficial effects, such as a significant decline in low-density lipoprotein (LDL) and an increase in high-density lipoprotein (HDL) cholesterol level, were observed in both groups. Additionally, in individuals consuming functional kimchi, there was a significant decrease in triglyceride, total cholesterol, and interleukin (IL)-6 levels (Kim and Park Citation2018). Meta-analysis of randomized control trials, including 26 studies, revealed that ingestion of fermented food can significantly reduce the levels of one of the inflammatory biomarkers, tumor necrosis factor (TNF)-α. The effects on the remaining investigated inflammation indicators, C-reactive protein and (IL)-6, were not demonstrated (SaeidiFard, Djafarian, and Shab-Bidar Citation2020). Increased gut microbiota diversity, as well as a decrease in 19 of 93 examined cytokines, chemokines, and other inflammatory serum proteins, including (IL)-6, levels were observed in individuals consuming fermented food over a 10-week intervention. Interestingly, the observed effects were not dependent on the amount of fermented food ingested or on body mass index (BMI). Additionally, such promising outcomes resulting from fermented food intake were not observed in high-fiber consumers (Wastyk et al. Citation2021). The anti-inflammatory properties of fermented products are summarized in an article by Kocot and Wróblewska (Citation2021). Ingestion of fermented dairy products can support the prevention of development and enhance the treatment efficacy in patients with type 2 diabetes (Awwad et al. Citation2022; Teo et al. Citation2022). The presence of microorganisms and/or bioactive microbial metabolites produced during the fermentation process is the main factor that contributes to the potential anti-aging effects resulting from fermented food consumption (Das et al. Citation2020). Multiple studies have reported the anti-cancer properties of fermented products (Tasdemir and Sanlier Citation2020).
Gut microbiota vs. fermented food
In addition to regulatory effects on the digestive, nervous, and immune systems, and involvement in the metabolism and catabolism of consumed food ingredients, gut microbiota promotes the wellbeing of the human host through the inactivation of free radicals (Luo et al. Citation2021). Consumption of kimchi over 4 wk resulted in a change in the gut microbiota profile. The relative abundance of members of Clostridium sp. and species belonging to Escherichia and Shigella genera decreased, whereas Faecalibacterium, Roseburia, and Phasolactobacterium, which are involved in short chain fatty acid (SCFA) production, increased. Additionally, in individuals consuming functional kimchi, an increase in probiotic Bifidobacterium adolescentis was observed (Kim and Park Citation2018). In another study, patients with irritable bowel syndrome (IBS) were fed with pasteurized or non-pasteurized sauerkraut in a randomized trial. Gastrointestinal symptoms were mitigated, and the gut microbiota profile was altered in both groups. Although LAB occurred significantly more frequently in fecal samples of individuals consuming non-pasteurized sauerkraut, the results indicated that the presence of viable cells did not improve the condition of patients. The authors indicated the prebiotic substances available in the fermented products tested and secondary, high-temperature resistant metabolites produced by microorganisms, as putatively key beneficial factors (Nielsen et al. Citation2018). Analysis of the data acquired by the American Gut Project revealed that, except for differences in intestinal microbiota composition, consumers and non-consumers of fermented food were distinguished by CLA abundance. Higher levels of this compound in fecal samples of fermented food consumers putatively stem from endogenous or microbial production (Taylor et al. Citation2020).
The gut-brain relation represents an important part of nutritional psychiatry, as nutrition is one of the crucial factors affecting gut microbiota community (Oriach et al. Citation2016; van der Merwe Citation2021). Researchers have suggested that the consumption of fermented food, which is part of traditional dietary patterns, is particularly significant regarding the mental condition of human beings (Aslam et al. Citation2020; Selhub, Logan, and Bested Citation2014). The inclusion of fermented food in the dietary treatment of patients with anorexia nervosa (AN) was considered. Although there is a lack of unequivocal data proving the efficiency of such a strategy, further examination is important because recent research suggests that the gut microbiota is involved in the development of this eating disorder, and thus, dietary modulation of intestinal microorganisms can contribute to the improvement of the condition of patients with AN (Rocks et al. Citation2021). The connections between intestinal microorganisms and mental health remain a topic of debate (Barrio, Arias-Sánchez, and Martín-Monzón Citation2022; Kelly et al. Citation2016). It has been thoroughly supported that fecal microbiota transplantation from depressed patients to germ-free rats can trigger animal-recipient behaviors and physiological changes that co-occur with depression (Kelly et al. Citation2016). A review of recently published articles concerning the relationship between gut dysbiosis and brain-associated diseases indicated that probiotics and dietary fiber can positively affect cognitive functions as well as cortisol response (Barrio, Arias-Sánchez, and Martín-Monzón Citation2022). Similar conclusions were drawn by Casertano, Fogliano, and Ercolini (Citation2022). In contrast, according to a meta-analysis based on 22 randomized control trials, the application of probiotics, prebiotics, and fermented food had no significant effect on cognitive functions. Statistical analysis of the pooled results indicated a lack of utility of such interventions, although 14 of the 22 trials included studies individually demonstrating a significant outcome. The authors indicated that the limited number of studies and lack of homogeneity of the methodology used may have contributed to the conclusions; thus, further studies are required (Marx et al. Citation2020).
Limitations in benefiting from the consumption of fermented food in the context of the presence of BAs
Several studies have indicated the potential positive effects of fermented food consumption on the psychological aspects of human well-being (Casertano, Fogliano, and Ercolini Citation2022). However, individuals with mental disorders are frequently treated with monoamine oxidase inhibitors (MAOIs); thus, they are particularly sensitive to BAs present in fermented products (EFSA Citation2011). Monoamine oxidases (MAOs) are enzymes involved in the regulation of amine neurotransmitter levels; therefore, they are an object of action of pharmacological substances used in the treatment of depression and Parkinson’s disease (Vianello, Repič, and Mavri Citation2012). Data from 1990–2016 indicate that depression is one of the five main global components of the years lived with disability (YLD) indicator, and the number of deaths and disabilities arising from Parkinson’s disease is growing the fastest among patients with neurological disorders, further indicating the broad scale of the problem (Schiess et al. Citation2022; Vos et al. Citation2017). According to the EFSA report, the no observed adverse effect level (NOAEL) of dietary tyramine in patients medicated with third-generation MAOI drugs is 12-fold lower than that in healthy individuals, whereas in cases of classical MAOI drugs, it is 100-fold lower (50 mg and 6 mg per person per meal, respectively) (EFSA Citation2011). These amounts can be easily ingested through available fermented products. Some widely used medicaments, for example antihypertensive and antibiotic, contain active substances that inhibits diamine oxidase (DAO) enzymes (Comas-Basté et al. Citation2020). Patients treated with such pharmaceuticals are threatened with higher risk of adverse health effects occurrence after ingestion of BAs. Another group that cannot fully benefit from the nutritiousness and health-promoting effects of fermented products in individuals with histamine intolerance (Comas-Basté et al. Citation2020). The diets of these individuals must be carefully composed to consider histamine as well as other amines, such as putrescine or spermidine, which can be metabolized by the same enzyme, that is DAO. Manufacturing fermented food with significantly low concentrations or lack of BAs is especially challenging, as fresh fruits and vegetables contain BAs (Sánchez-Pérez et al. Citation2018). Thus, the application of BA-degrading microorganisms alone or in combination with other methods appears to be an inevitable method to obtain safe fermented products. However, the levels of histamine and other BAs intake that trigger symptoms vary widely between individuals (Comas-Basté et al. Citation2020; Van den Eynde, Gillman, and Blackwell Citation2022) and a partial reduction of their content, which can be gained through plant additive application, may enable safer consumption of the product by sensitive individuals.
To date, little is known about the effects of the ingestion of fermented products other than dairy (Marco et al. Citation2021). Most researchers unanimously underlined that the health benefits resulting from fermented food consumption require further investigation, and human studies are required (Awwad et al. Citation2022; Das et al. Citation2020; Marco et al. Citation2021; Tasdemir and Sanlier Citation2020).
Bioactive compounds – superiority of fermented over non-fermented food
The health-promoting effects of fermented food originate mainly from the presence of beneficial microorganisms and/or bioactive compounds produced by microorganisms during fermentation (Das et al. Citation2020). GABA can enhance health maintenance due to diverse beneficial effects (Sahab et al. Citation2020). A report by Gan et al. (Citation2017) revealed that fermented edible seeds contain from several to several dozen times more GABA than nonfermented samples. Bioactive peptides are another factor contributing to the health-promoting properties of fermented products. Antioxidative, antihypertensive, immunomodulatory, and cholesterol-lowering activities are among its main assets (Chaudhary et al. Citation2021). Strains exhibiting beneficial features have frequently been isolated from fermented products. Dextran produced by Pediococcus pentosaceus CRAG3, derived from fermented cucumbers, showed anti-cancer potential. The polymer inhibited the viability of cervical and colorectal cancer cells in an in vitro study (Shukla and Goyal Citation2013). Similar properties against colorectal cancer were exhibited by a soybean extract fermented with L. plantarum DGK-17 originating from kimchi (Khan and Kang Citation2017). The immunomodulatory, anti-inflammatory, antiallergic, cholesterol-lowering, weight loss, antioxidative, mental health, and gut function-enhancing effects of L. plantarum strains originating from fermented food have been summarized in a review by Garcia-Gonzalez et al. (Citation2021). The quantity and profile of compounds produced during fermentation can vary among members of the same family or genus (Xu et al. Citation2021). Thus, thorough examination of individual strains should be performed to elucidate the potential health effects of fermented food matrices, as well as to gain insight into the possible applications of beneficial microorganisms.
Occurrence and toxicity of BAs in fermented foods
Amines produced de novo in plants or animals, also referred to as “endogenous amines”, are responsible for multiple physiological functions. In humans, these compounds are involved in the regulation of body temperature, secretion of gastric acids, immunological responses, gene expression, and growth and differentiation of cells. They also act as neurotransmitters and modulate brain activity regarding cognitive functions (Ladero et al. Citation2010). Although essential to life, the excessive ingestion of BAs in food can lead to adverse effects. The consumption of products containing high concentrations of histamine can trigger symptoms in the nervous, digestive, circulatory, and respiratory systems, as well as skin reactions, such as redness, rash, or urticaria (del Rio et al. Citation2017; Ladero et al. Citation2010). Histamine poisoning is also commonly referred to as “histamine fish poisoning” or “scombroid syndrome”, because it most often results from the consumption of fish, particularly members of the mackerel family (Scombridae) or fish products (del Rio et al. Citation2017; Velut et al. Citation2019). Although histamine is the only BA whose levels in some food products are legislatively limited, the results of in vitro studies indicate that it can be less toxic than tyramine (Linares et al. Citation2016). Symptoms of excessive tyramine intake include headache, migraines, vomiting, and neurological, blood pressure and respiratory disorders, which are referred to as the “cheese reaction” (del Rio et al. Citation2017; Gao et al. Citation2023; Ladero et al. Citation2010). Additionally, histamine and tyramine exhibit synergistic cytotoxic effects at concentrations found in food (del Rio et al. Citation2017). In severe cases, BA poisoning may lead to a sudden increase in blood pressure above 180/120 mm Hg, the so-called hypertensive crisis, which in turn may damage the organs of the cardiovascular or nervous system (Ladero et al. Citation2010). The literature has repeatedly emphasized the enhancing effect of putrescine and cadaverine on the toxicity of histamine, mainly due to the interaction of these BAs with the DAO enzyme involved in the metabolism of diamines (Ruiz-Capillas and Herrero Citation2019). Cytotoxicity studies of HT29 intestinal adenocarcinoma cells showed that putrescine and cadaverine at concentrations found in food can induce cell necrosis, with two-fold lower NOAEL and LOAEL (lowest observed adverse effect level) values for cadaverine (5 and 10 mM for putrescine, and 2.5 and 5 mM for cadaverine, respectively), and the cytotoxicity of these diamines is lower than that of tyramine and histamine (del Rio et al. Citation2019). Tryptamine and ß-phenylethylamine also exhibit cytotoxic effects through the induction of apoptosis and cell necrosis, respectively, and enhance histamine toxicity by inhibiting the DAO (tryptamine and ß-phenylethylamine) and histamine N-methyltransferase (HNMT; only ß-phenylethylamine) enzymes. Owing to their vasoconstrictive effects, tryptamine and ß-phenylethylamine can increase blood pressure and trigger vomiting, sweating, and headaches (del Rio et al. Citation2020). Another risk associated with BAs is their involvement in the formation of carcinogenic N-nitroso compounds (Fong, El-Nezami, and Sze Citation2021; Fong et al. Citation2020). The reaction of putrescine or spermidine with nitrous acid results in the formation of N-nitrosopyrrolidines (Kalač Citation2014). Polyamines (spermine and spermidine) can damage the retinal pigment epithelial cells, with spermine exhibiting a stronger effect (Kaneko et al. Citation2007). Despite numerous positive effects of agmatine, such as improvement of cognitive functions or regulation of the epithelial cell growth during wounds healing, excessive intake of this BA can provoke gastrointestinal symptoms, such as diarrhea and nausea (Gümrü, Şahin, and Aricioğlu Citation2013; Khémesse et al. Citation2023). As a precursor of putrescine, it also regulates polyamine levels (Khémesse et al. Citation2023). Susceptibility to BA intoxication differs among individuals and depends on the effectiveness of the detoxification system and whether a person is treated with MAOI/DAOI drugs. Alcohol consumption can increase the vulnerability to high doses of BAs (Comas-Basté et al. Citation2020).
Food fermentation aids in the formation of high concentrations of BAs because of the availability of proteins that can be converted into BA precursors, namely free amino acids (FAAs), the presence of microorganisms producing BAs, and hospitable conditions for their growth and activity (that is, temperature and pH) (EFSA Citation2011). Even if appropriate procedures are introduced to prevent the formation of BAs, the threat of the accumulation of these compounds remains. This can occur in the presence of nonstarter LAB with BA-producing ability, which continue to grow and metabolize, for example, when the applied level of starter culture is too low or inoculated microorganisms do not overgrow indigenous ones (EFSA Citation2011; Świder et al. Citation2023). The undesired growth of Gram-negative enterobacteria at the beginning of the fermentation process represents another risk. These microorganisms are sensitive to low pH (Stoll et al. Citation2021) and thus, their development can occur under improper acidification. Many representatives of Enterobacteriaceae and other Gram-negative bacteria have been reported to possess the ability to produce BAs (Lorenzo et al. Citation2010; Meng et al. Citation2022; Wunderlichová et al. Citation2014). In contrast, the decarboxylase and deiminase reactions, in which BAs are formed, are the main physiological responses of bacteria to acidic environments (Gardini et al. Citation2016; Zuljan et al. Citation2016). Because of the complexity and variability of the fermentation process, a wide range of BA concentrations has been reported for various fermented products. Published data frequently reveal a significantly high content of BAs, exceeding estimated safety limits (Fong, El-Nezami, and Sze Citation2021; Shukla et al. Citation2010). Świder et al. (Citation2020) reported that consumption of fermented vegetables, such as Brussel sprouts, broccoli, kimchi, cucumber, white, and red cabbage, available on the Polish retail market, pose a risk of adverse symptoms because of high BA concentrations. Commercial douchi (fermented black beans/soybeans) products contain up to 814.2 mg/kg of histamine and 643.5 mg/kg of tyramine (Fong et al. Citation2020), whereas the concentrations of these amines in selected samples of Doenjang (fermented soybean paste) reached 2794.8 mg/kg and 6616.1 mg/kg, respectively (Shukla et al. Citation2010). The reported concentrations of tryptamine, phenylethylamine, putrescine, cadaverine, agmatine, spermidine, and spermine were 2808.1, 8704.6, 4292.3, 3235.5, 5508.4, 8804.0, and 9729.5 mg/kg, respectively; however, most of the samples tested contained BAs at levels below the limit of detection (Shukla et al. Citation2010). This example illustrates the importance of providing efficient, repeatable methods to ensure stable, low BA levels in fermented products. Several studies have summarized the available data on BA concentrations in fermented products (Mah et al. Citation2019; Vasconcelos et al. Citation2021; Visciano and Schirone Citation2022).
Methods to reduce BAs in fermented products
Alterations in the microbial profile of the product, particularly the inhibition of the growth of BA-producing microorganisms and/or promotion of BA-degrading microorganisms, are the primary mechanisms of BA content reduction in fermented food systems (Jaguey-Hernández et al. Citation2021). Such effects can be achieved through the application of suitable starter cultures, modification of manufacturing and storage conditions (NaCl concentration and temperature), or the addition of bioactive substances. Some technological processes, such as high hydrostatic pressure, vacuum or modified atmosphere packaging, and irradiation, effectively limit BAs in fermented meat products (Jaguey-Hernández et al. Citation2021; Kim et al. Citation2005; Simon-Sarkadi et al. Citation2012; Sun et al. Citation2019); however, these methods are excluded from some fermented food matrices, because of their traditional characteristics. Household manufacturing has a similar limitation. The methods described in the literature for reducing BAs in fermented products are shown in .
Several studies have reported the successful use of starter cultures to limit BA content in fermented food products (Cheng, Xu, and Lan Citation2020; Kim et al. Citation2019; Zhao et al. Citation2020). Articles by Alvarez and Moreno-Arribas (Citation2014), Jaguey-Hernández et al. (Citation2021), and Lorenzo, Munekata, and Domínguez (Citation2017) summarize the available data concerning this issue. It is remarkable that the value of the fermented product can be further ameliorated by the application of probiotic strains as starter cultures. Fong et al. (Citation2020) obtained reduced tyramine content (down to undetectable values) in douchi, a black bean fermented product, because of the application of probiotic strains (Lacticaseibacillus rhamnosus GG, Lacticaseibacillus casei Shirota or E. coli Nissle 1917) whereby the features of the used strains contributed to the observed effects, as well as the environmental factors, including temperature and availability of oxygen (Fong et al. Citation2020). Recently, it was reported that LAB postbiotics can limit BA production by food-borne pathogens. The authors suggested that the main factors contributing to this effect were the degradation of BAs or inhibition of BA production by the tested microorganisms, namely E. coli ATCC 25922 and Salmonella Paratyphi A NCTC 13. Suppression of pathogen activity was a consequence of antimicrobial features of LAB postbiotics, and such antimicrobial effects resulted from the high content of organic acids that lowered pH. Another feasibly contributing factor pointed out by the authors was the presence of bacteriocins (Yilmaz et al. Citation2022). Another strategy to reduce BA concentration in fermented products was the application of modified yeast with the removed PEP4 gene encoding an enzyme, proteinase A, which is responsible for FAA production. Decreased activity of this enzyme resulted in the limited availability of BAs’ precursors, and consequently, fermented rice wines produced with modified Saccharomyces cerevisiae cells contained reduced concentrations of tyramine, histamine, and cadaverine by 57.5, 54.3, and 24.6%, respectively (Guo et al. Citation2015).
Some authors have indicated that the application of plant additives is an effective way to limit BA accumulation. Essential oils (EOs) can significantly enhance the microbiological quality of food and thus extend the shelf life of perishable products. They exhibit strong antimicrobial activity and counteract lipid oxidation in seafood products (Hassoun and Çoban Citation2017). Positive effects have been observed for rosemary (Ozogul et al. Citation2017) and basil EOs (Karoui and Hassoun Citation2017), garlic EO (Mahmoud et al. Citation2004), thyme and laurel EOs (Erkan et al. Citation2011; Ozogul et al. Citation2017), ginger oil (Emir Çoban Citation2013), clove oil (Emir Çoban and Patir Citation2013), orange leaf EO (Alparslan et al. Citation2016), and sage (Ozogul et al. Citation2017). The application of clove, cumin, or spearmint EOs resulted in a decrease in bacterial and yeast numbers during cold storage of red drum fillets, and consequently, a reduction in BA content, particularly histamine, putrescine, and cadaverine. Additionally, the aforementioned EOs improve the sensory characteristics of the product (Cai, Cao, et al. Citation2015). Interestingly, according to Zhang et al. (Citation2018), phenolic acids applied at concentrations that do not affect bacterial growth can inhibit the formation of histamine, tyramine, putrescine, and cadaverine through Enterobacter aerogenes. Of the five compounds tested (chlorogenic, gallic, catechinic, shikimic, and protocatechuic acids), catechinic acid was the most effective in limiting BA production (Zhang et al. Citation2018). Such an effect could be the result of the direct inhibition of amino acid decarboxylases. Rodríguez-Caso et al. reported the inhibitory effect of green tea (-)-epigallocatechin-3-gallate (EGCG) on histidine decarboxylase. The mechanism putatively consists of modification of the holoenzyme structure (Rodríguez-Caso et al. Citation2003). Although this experiment was conducted on mammalian enzymes, it can be conjectured that similar effects can occur in bacterial cells.
Effect of plant-origin additives on BA concentration in fermented foods
In vitro studies
Several researchers have studied the effects of plant additives on BA accumulation in culture media (Mah, Kim, and Hwang Citation2009; Montanari et al. Citation2022; Wendakoon and Sakaguchi Citation1993). Food amine producers were cultured in the presence of natural compounds to assess the influence of additives on bacterial growth and BA production. Although the results of such experiments may preliminarily evaluate the prospective effects in food matrices, there is limited literature on this subject. The available information is summarized in . Some researchers have examined the growth of BA producers subjected to natural plant compounds, without quantitatively assessing BA accumulation (Kim et al. Citation2014; Oh et al. Citation2012; Zhou et al. Citation2016). presents the results of these studies.
Table 1. Antimicrobial activity of plant compounds and their effects on BA accumulation - in vitro studies.
Table 2. Antimicrobial activity of plant compounds against BA-producing bacteria - in vitro studies.
Application to food matrices
Data on the application of plant-derived additives to fermented products are summarized in . The experimental results varied depending on the matrix, additive, and application conditions. The production stage at which the additives were applied (Lee et al. Citation2018; Shukla et al. Citation2019), as well as the size (that is, sausage diameters) of the products (Huang et al. Citation2021) also significantly contributed to the obtained results. Most studies (27 of 38) were related to the reduction of BAs in fermented products of animal origin, such as meat (19), fish and seafood (5), and dairy products (3). Twelve articles reported plant-based fermented foods, including soybeans, cereals, sauerkraut, kimchi, and bread. show the effects of plant-origin additives on the BA content in meat, fish and seafood, dairy, and plant-based fermented products, respectively.
Table 3. Effect of plant-origin additives on BAs in fermented meat products.
Table 4. Effect of plant-origin additives on BAs in fermented fish and seafood.
Table 5. Effect of plant-origin additives on BAs in fermented dairy products.
Table 6. Effect of plant-origin additives on BAs in plant-based fermented food.
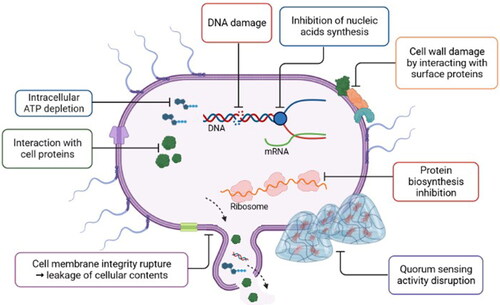
Some authors have indicated that the decreased concentration of BA results from the antimicrobial properties of the applied additives (Bozkurt Citation2006; Cai, Liu, et al. Citation2015; Es’haghi Gorji et al. Citation2014; Jia et al. Citation2020; Lu et al. Citation2015; Majcherczyk and Surówka Citation2019; Mannaa, Seo, and Park Citation2020; Sun et al. Citation2018; Wang et al. Citation2021; Wang, Zhang, and Ren Citation2018; Xu et al. Citation2022). Based on a recent review of plant antimicrobial agents, disruption of the plasma membrane is the main mechanism of action (Álvarez-Martínez et al. Citation2021). However, considering the variations in the chemical profiles and structures of plant extracts or EOs, complete clarification of their action remains difficult (Houicher et al. Citation2021). The main mechanisms underlying the antimicrobial effects of plant additives on BA production are shown in . The application of rose polyphenols limited the total bacterial count and simultaneously increased the richness of LAB. Limited growth of other than Lactobacillales microorganisms, namely, Gram-negative bacteria, may be a consequence of the disruption of their quorum sensing activity (Zhang et al. Citation2017). Polyphenols are a wide group of compounds, and their action largely depends on their structure, that is the number and position of hydroxyl groups, double bonds, and amphipathic characteristics. In addition to an increase in membrane permeability and interaction with the cell wall, the main antibacterial activities of polyphenols include the modulation of cell metabolism through interactions with proteins, including enzymes, and the impact on adenosine triphosphate (ATP) synthesis, inhibition of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) synthesis, and DNA damage (Houicher et al. Citation2021; Lobiuc et al. Citation2023).
Figure 3. Main mechanisms of antimicrobial effects of plant additives on BA production. Created with BioRender.com.
The antimicrobial effects of plant-derived substances can also affect LAB, the growth and activity of which are essential for fermentation. de Souza (Citation2021) summarized the effects of EOs on beneficial microorganisms in food. The author emphasized the scarcity of literature data regarding this issue, as well as the high variability of the effects, which are dependent on the dose and type of EO, type of microorganisms, and food matrix (de Souza Citation2021).
Based on some previous studies, the inhibitory effect of additives on amino acid decarboxylases is a key factor contributing to the suppression of BA production (Yu et al. Citation2021; Zhou et al. Citation2016). Yu et al. (Citation2021) concluded that the reduction in histamine content in fermented sausages was a consequence of the significant suppression of histidine decarboxylase (hdcA) and histidine/histamine antiporter (hdcP) gene expression due to the application of thyme microcapsules. Zhou et al. (Citation2016) indicated that the histamine content limitation in samples supplemented with garlic putatively stems from the unavailability of thiol groups, which are essential for the action of amino acid decarboxylases because of their reaction with allicin. Interestingly, the fermentation of garlic or the addition of garlic to fermented products significantly contributed to an increased content of FAAs and simultaneously limited BA production, which was attributed to the antimicrobial properties of allicin by some researchers (Bahuguna et al. Citation2019; Świder et al. Citation2020). The reduced concentrations of putrescine in fermented fish sauce supplemented with star anise may be due to anethole, which could inhibit the activity of the enzymes responsible for putrescine formation, namely, ornithine decarboxylase and agmatine deiminase (Zhou et al. Citation2016).
Both antimicrobial effects and the suppression of decarboxylase activity are involved in the reduction of BA content in some matrices (Lee et al. Citation2018; Zhou et al. Citation2016). Catechins, which exhibit inhibitory effects on the enzymatic activity of decarboxylases, simultaneously remotely affecting bacterial growth, and grapefruit seed extract, which shows antimicrobial properties, have been applied to fermented soybean paste (Lee et al. Citation2018). The antimicrobial properties of EGCG arise from its direct binding to the peptidoglycan layer and disruption of the cell wall in Gram-positive bacteria. Its inhibitory effect on Gram-negative bacteria results from the oxidative stress induced by the production of hydrogen peroxide (Nikoo, Regenstein, and Ahmadi Gavlighi Citation2018). In a study by Zhou et al. (Citation2016), histamine and putrescine formation were suppressed mainly through the inhibitory effects of bioactive compounds of plant extracts on enzymes responsible for the formation of these amines, while tyramine concentration was reduced because of the antimicrobial effect of garlic and star anise extracts, as well as ethanol itself. Both the inhibitory effects on the growth of amine-producing bacteria and suppression of spermidine synthase affect the spermidine concentration in fermented fish sauce supplemented with garlic, ginger, or star anise extracts (Zhou et al. Citation2016). Kongkiattikajorn (Citation2015) emphasized the effect of introducing plant-origin AOs into the product along with additives. The application of ginger extract, in which AO activity was estimated to be ca. 34.3 U/g, resulted in the reduction of the examined BAs after 7 d of Nham fermentation in comparison to the control (85.90%), as well as the initial content (60.44%) (Kongkiattikajorn Citation2015). In some studies, the synergistic effects of plant-origin additives and starter cultures against BA accumulation were employed, whereby stronger inhibitory activity was exhibited by plant-origin additives in some cases (Lu et al. Citation2015) and starter cultures in others (Hernández-Macias et al. Citation2021). Each additive exhibited different activities, which were determined by numerous factors such as the concentration and profile of bioactive compounds present in the additive (or the additive itself when single substances were tested), added dose/quantity, autochthonous and inoculated microorganisms, type of matrix, and applied conditions.
Jia et al. (Citation2020) reported that relatively high concentrations of estragole and trans-anethole in cassia and fennel extracts contributed most to their inhibitory effects on BA formation. The effectiveness of garlic has been attributed to the presence of allicin (Mah, Kim, and Hwang Citation2009; Zhou et al. Citation2016), whereas thyme, Thymbra spicata, and Satureja montana EOs have antimicrobial properties through phenolic compounds such as carvacrol and thymol (Bozkurt Citation2006; Mozuriene et al. Citation2016; Yu et al. Citation2021). Owing to their high hydrophobicity, the antimicrobial effect of EOs and their components (for example, thymol and carvacrol) is attributed to the disturbance of the bacterial cell membrane lipid fraction. Damage to membrane integrity results in the leakage of cell contents, such as nucleic acids and proteins (Bouyahya et al. Citation2019). Epicatechin, epicatechin gallate, epigallocatechin, and EGCG contribute to the wide range of bioactive effects of green tea (Bozkurt Citation2006). Compounds such as eucalyptol and trans-cinnamaldehyde in cinnamon, eugenol present in anise and clove, and carvacrol, linalool, and α-terpineol in anise were responsible for the antimicrobial properties of the seasoning extracts (Sun et al. Citation2018). The most abundant substance contributing to the antimicrobial effects of Zataria multiflora Boiss. is carvacrol, which can cross the bacterial cell membrane and increase cell permeability (Bouyahya et al. Citation2019; Es’haghi Gorji et al. Citation2014). Capsaicinoids originating from red peppers alter the composition of LAB in kimchi, leading to a decrease in BA content (Kim, Dang, and Ha Citation2022).
Some attempts to reduce BAs through the use of plant-origin additives have failed. Fortification of sausages with chia or black cumin seeds resulted in significantly increased concentrations of tyramine and putrescine (Borrajo et al. Citation2021). Application of Nelumbo nucifera (lotus leaves) at 10% concentration enhanced the formation of agmatine, tryptamine, putrescine, histamine, tyramine, spermidine, and spermine in Meju, in comparison to the control and samples supplemented with garlic, Gingko biloba leaves, or a mix of examined additives. Two putative explanations have been proposed by the authors: variability between batches and alteration in the microbial community (Shukla et al. Citation2018). The addition of cava lees resulted in higher (insignificantly) contents of putrescine, cadaverine, and spermidine in bread, but significantly reduced tyramine and cadaverine concentrations in fermented sausages, indicating that the matrix is a crucial factor affecting the effectiveness of the inhibitory activity of applied additives on BA formation (Hernández-Macias et al. Citation2021).
Effect of plant-origin additives on sensory properties of fermented products
In addition to the impact on BA content, some of the tested food additives altered the sensory characteristics of the products. Application of grape seed extract prevented deterioration of tarhana color due to increased antioxidative activity (Akan and Ocak Citation2019), which enhances sausage odor because of the limitation of protein metabolism as well as slower release of thyme EO flavor resulting from the presence of the coating (Yu et al. Citation2021). Grape seed extract prevented lipid oxidation in smoke-cured bacon, and thus favorably changed the color of the meat product – redness value was increased. The improved lightness of samples supplemented with this additive putatively stemmed from the altered muscle structure due to the different extents of protein denaturation (Wang, Zhang, and Ren Citation2018). In contrast, the application of Urtica dioica or Hibiscus sabdariffa extracts did not significantly affect the sensory characteristics of dry fermented sausages (Karabacak and Bozkurt Citation2008). Application of 0.4% Z. multiflora Boiss. reduced BA concentration in cheese the most efficiently, however, obtained product had lower acceptability than products with 0.2 or 0.1% Z. multiflora Boiss. addition (Es’haghi Gorji et al. Citation2014). The application of 200 or 400 U/g of papain shortened the fermentation time of Chouguiyu from 10 to 7 d with respect to the flavor and taste characteristics of the product, whereby lower levels of the enzyme reduced putrescine and cadaverine content significantly more efficiently (Xu et al. Citation2022).
Plant additives – economic and environmental aspects
Application of plant-origin food additives represents a prospective direction of sustainable food industry development owing to the superiority of natural food additives over synthetic ones in terms of green policy and health (Zang et al. Citation2022). Extraction of bioactive compounds from plant wastes is a cost-effective and ecologically friendly way of wasted food management. It is estimated that ca. one-third of the global food production is wasted or lost annually. Apart from huge financial loss (approx. $940 billion per year) it contributes considerably to negative consequences concerning environment i.e., futilely use of fertilizers and fresh water, as well as excessive emission of greenhouse gas (FLW Protocol Citation2016; Ueda et al. Citation2022). Currently, the management of generated by-products and waste is one of the major challenges for food and agriculture sector. According to the data collected in some European countries fruit and vegetable waste category represents from ca. 20% (Austria) to 70% (Italy) of total food waste regarding household sector (Esparza et al. Citation2020). In the whole food chain, plant products constitute 86% (by weight) or 88% (by kcal) of loss and waste food with the following contribution of particular groups: cereals (19 or 53% by weight or by kcal, respectively), roots and tubers (20 or 14%), fruits and vegetables (44 or 13%), oilseeds and pulses (3 or 13%) (World Resources Institute Citation2019). Reuse of plant origin residues is especially advantageous because their production is one of the largest among all food categories and simultaneously, they are rich source of various bioactive compounds highly appreciated by modern consumers. On the other hand, it is particularly challenging, because of perishable characteristics of this type of products (Esparza et al. Citation2020). One of the possible ways to valorize plant waste is to use them as substrates in biocatalysis e.g., to produce flavors and aromas (Singh et al. Citation2023). Different botanical parts of plants, incl. these considered as waste, are rich source of specific substances, such as phenolic compounds, fatty and organic acids, dietary fiber, carotenoids, vitamins, and enzymes, which can be further used for functional food, nutraceuticals, and active packaging development (Baiano Citation2014; Chamorro et al. Citation2022). Many of the above mentioned could be used to modify the composition of fermented products to make it more nutritious or to modify fermentation process itself in a desired direction.
Future perspectives
Considering the growing number of people sensitive to BAs (Comas-Basté et al. Citation2020), as well as increasing interest of consumers in health concerns (de Souza Ribeiro et al. Citation2022) development of effective, natural, and simple methods for manufacturing low-BA fermented food is an urgent matter. Owing to the complexity of the fermentation process, the development of detailed procedures for each food matrix is essential. The use of plant-based food additives represents a desirable and effective approach to increasing the nutritional value of food products. Optimization of extraction methods should be studied in terms of efficiency and environmental concerns, and attention has been paid to emerging techniques such as Supercritical Carbon Dioxide, Subcritical water, and ultrasound-assisted extraction (Daud et al. Citation2022). Plant-origin additives intended for application in fermented products must be thoroughly characterized in terms of their chemical composition and standardized with respect to the concentration and stability of the main bioactive molecules. Cutting-edge techniques such as ultra-high-performance liquid/gas chromatography coupled with mass spectrometry can be applied. Chromatography – mass spectrometry and electronic nose, which represent the primary techniques employed to analyze food flavor (Wei et al. Citation2023), should be used to evaluate sensory attributes, while molecular biology tools, such as metagenomic sequencing, should be used to analyze changes in the microbial community following plant additive application in particular matrices. Methods of bioactive substance application should be precisely analyzed, including the development of new means, such as active packaging, which effectively prevents the accumulation of BAs in meat (Sirocchi et al. Citation2013), and encapsulation, which is more efficient in reducing histamine content than direct essential oil application (Yu et al. Citation2021). Regarding economic and ecological concerns, exploring industrial by-products in the search for biologically active compounds is of interest (Banerjee et al. Citation2017).
Conclusions
Providing fermented foods with reduced BA content remains a significant challenge for food technologists. The entire population benefits from the availability of such products, particularly sensitive individuals. The use of plant-based additives is a promising strategy for this purpose. The different results obtained because of the use of similar additives indicate that the fermentation process is highly complex. Thus, individual additives must be thoroughly examined in target matrices to evaluate their action and prospective effects on BA accumulation. Alterations in the sensory characteristics should also be considered. The effectiveness of applied plant-origin additives depends mainly on the type and dose of the additive, matrix, autochthonous, and inoculated microorganisms, as well as the manufacturing conditions. Antimicrobial features such as destructive action against bacterial cell membranes are the primary mechanisms responsible for the limitation of BAs. The characteristic approach has added value considering its simplicity and relatively low cost, as some bioactive substances can be extracted from byproducts derived from different branches of the food industry. Further studies concerning previously tested and novel plant-origin additives and their combined effects with other BA reduction methods, such as the use of starter cultures, are required.
| List of abbreviations | ||
| AN | = | Anorexia nervosa |
| AOs | = | Amine oxidases |
| ATP | = | Adenosine triphosphate |
| BAs | = | Biogenic amines |
| BMI | = | Body Mass Index |
| CLA | = | Conjugated linoleic acid |
| DAO | = | Diamine oxidase |
| DNA | = | Deoxyribonucleic acid |
| EFSA | = | European Food Safety Authority |
| EGCG | = | Epigallocatechin gallate |
| EOs | = | Essential oils |
| FAAs | = | Free amino acids |
| GABA | = | γ-aminobutyric acid, gamma aminobutyric acid |
| GRAS | = | Generally recognized as safe |
| HDL | = | High-density lipoprotein |
| HNMT | = | Histamine N-methyltransferase |
| IBS | = | Irritable bowel syndrome |
| (IL)-6 | = | Interleukin 6 |
| ISAPP | = | International Scientific Association for Probiotics and Prebiotics |
| LAB | = | Lactic acid bacteria |
| LDL | = | Low-density lipoprotein |
| LOAEL | = | Lowest observed adverse effect level |
| MAOs | = | Monoamine oxidases |
| MAOIs | = | Monoamine oxidase inhibitors |
| NOAEL | = | No observed adverse effect level |
| NSLAB | = | Nonstarter lactic acid bacteria |
| RNA | = | Ribonucleic acid |
| SCFAs | = | Short chain fatty acids |
| TNF-α | = | Tumor necrosis factor |
| YLD | = | Years lived with disability |
Disclosure statement
The authors report there are no competing interests to declare.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abedi, E., A. Tavakoli, S. Zamanizadeh, S. Maleki, and A. R. Jassbi. 2023. The correlation among residual nitrites, biogenic amines, N‐nitrosamine formation, and degradation occurrence of punicalagin α/β, rosmarinic acid, carnosol, and carnosic acid in extract‐treated sausage during storage. Food Science & Nutrition 11 (9):5409–26. doi: 10.1002/fsn3.3498.
- Ahlawat, S., and K. K. Sharma. 2021. Gut-organ axis: A microbial outreach and networking. Letters in Applied microbiology 72 (6):636–668. doi: 10.1111/lam.13333.
- Akan, S., and Ö. Ö. Ocak. 2019. Evaluation of storage time and grape seed extract addition on biogenic amines content of tarhana: A cereal-based fermented food. Lwt 111:861–8. doi: 10.1016/j.lwt.2019.05.109.
- Alan, Y., Z. Topalcengiz, and M. Dığrak. 2018. Biogenic amine and fermentation metabolite production assessments of Lactobacillus plantarum isolates for naturally fermented pickles. Lwt 98:322–8. doi: 10.1016/j.lwt.2018.08.067.
- Alparslan, Y., H. H. Yapıcı, C. Metin, T. Baygar, A. Günlü, and T. Baygar. 2016. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT - Food Science and Technology 72:457–66. doi: 10.1016/j.lwt.2016.04.066.
- Álvarez-Martínez, F. J., E. Barrajón-Catalán, M. Herranz-López, and V. Micol. 2021. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine: international Journal of Phytotherapy and Phytopharmacology 90:153626. doi: 10.1016/j.phymed.2021.153626.
- Alvarez, M. A., and M. V. Moreno-Arribas. 2014. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends in Food Science & Technology 39 (2):146–55. doi: 10.1016/j.tifs.2014.07.007.
- Aslam, H., J. Green, F. N. Jacka, F. Collier, M. Berk, J. Pasco, and S. L. Dawson. 2020. Fermented foods, the gut and mental health: A mechanistic overview with implications for depression and anxiety. Nutritional Neuroscience 23 (9):659–71. doi: 10.1080/1028415x.2018.1544332.
- Awwad, S. F., A. Abdalla, F. C. Howarth, L. Stojanovska, A. Kamal-Eldin, and M. M. Ayyash. 2022. Invited Review: Potential effects of short-and long-term intake of fermented dairy products on prevention and control of type 2 diabetes mellitus. Journal of Dairy Science 105 (6):4722–33. doi: 10.3168/jds.2021-21484.
- Bahuguna, A., S. Shukla, J. S. Lee, V. K. Bajpai, S. Y. Kim, Y. S. Huh, Y. K. Han, and M. Kim. 2019. Garlic augments the functional and nutritional behavior of Doenjang, a traditional Korean fermented soybean paste. Scientific Reports 9 (1):5436. doi: 10.1038/s41598-019-41691-3.
- Baiano, A. 2014. Recovery of biomolecules from food wastes – A review. Molecules (Basel, Switzerland) 19 (9):14821–42. doi: 10.3390/molecules190914821.
- Banerjee, J., R. Singh, R. Vijayaraghavan, D. MacFarlane, A. F. Patti, and A. Arora. 2017. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chemistry 225:10–22. doi: 10.1016/j.foodchem.2016.12.093.
- Barrio, C., S. Arias-Sánchez, and I. Martín-Monzón. 2022. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 137:105640. doi: 10.1016/j.psyneuen.2021.105640.
- Bell, V., J. Ferrão, and T. Fernandes. 2017. Nutritional guidelines and fermented food frameworks. Foods (Basel, Switzerland) 6 (8):65. doi: 10.3390/foods6080065.
- Benkerroum, N. 2016. Biogenic amines in dairy products: Origin, incidence, and control means. Comprehensive Reviews in Food Science and Food Safety 15 (4):801–26. doi: 10.1111/1541-4337.12212.
- Bintsis, T. 2018. Lactic acid bacteria: Their applications in foods. Journal of Bacteriology & Mycology 6 (2):89–94. doi: 10.15406/jbmoa.2018.06.00182.
- Borrajo, P., M. Karwowska, D. M. Stasiak, J. M. Lorenzo, M. Żyśko, and E. Solska. 2021. Comparison of the effect of enhancing dry fermented sausages with Salvia hispanica and Nigella sativa seed on selected physicochemical properties related to food safety during processing. Applied Sciences 11 (19):9181. doi: 10.3390/app11199181.
- Bouyahya, A., J. Abrini, N. Dakka, and Y. Bakri. 2019. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. Journal of Pharmaceutical Analysis 9 (5):301–11. doi: 10.1016/j.jpha.2019.03.001.
- Bozkurt, H. 2006. Utilization of natural antioxidants: Green tea extract and Thymbra spicata oil in Turkish dry-fermented sausage. Meat Science 73 (3):442–50. doi: 10.1016/j.meatsci.2006.01.005.
- Bozkurt, H. 2007. Comparison of the effects of sesame and Thymbra spicata oil during the manufacturing of Turkish dry-fermented sausage. Food Control. 18 (2):149–56. doi: 10.1016/j.foodcont.2005.09.009.
- Cai, L., A. Cao, Y. Li, Z. Song, L. Leng, and J. Li. 2015. The effects of essential oil treatment on the biogenic amines inhibition and quality preservation of red drum (Sciaenops ocellatus) fillets. Food Control. 56:1–8. doi: 10.1016/j.foodcont.2015.03.009.
- Cai, L., S. Liu, L. Sun, Y. Wang, H. Ji, and J. Li. 2015. Application of tea polyphenols in combination with 6-gingerol on shrimp paste of during storage: Biogenic amines formation and quality determination. Frontiers in Microbiology 6:981. doi: 10.3389/fmicb.2015.00981.
- Campbell-Platt, G. 1994. Fermented foods – A world perspective. Food Research International 27 (3):253–7. doi: 10.1016/0963-9969(94)90093-0.
- Carafa, I., T. Nardin, R. Larcher, R. Viola, K. Tuohy, and E. Franciosi. 2015. Identification and characterization of wild lactobacilli and pediococci from spontaneously fermented Mountain cheese. Food Microbiology 48:123–32. doi: 10.1016/j.fm.2014.12.003.
- Casertano, M., V. Fogliano, and D. Ercolini. 2022. Psychobiotics, gut microbiota and fermented foods can help preserving mental health. Food Research International (Ottawa, Ont.) 152:110892. doi: 10.1016/j.foodres.2021.110892.
- Chamorro, F., M. Carpena, M. Fraga-Corral, J. Echave, M. S. R. Rajoka, F. J. Barba, H. Cao, J. Xiao, M. A. Prieto, and J. Simal-Gandara. 2022. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chemistry 370:131315. doi: 10.1016/j.foodchem.2021.131315.
- Chaudhary, A., S. Bhalla, S. Patiyal, G. P. S. Raghava, and G. Sahni. 2021. FermFooDb: A database of bioactive peptides derived from fermented foods. Heliyon 7 (4):e06668. doi: 10.1016/j.heliyon.2021.e06668.
- Cheng, S., Y. Xu, and X. Lan. 2020. Isolation, characterization, and application of biogenic amines‐degrading strains from fermented food. Journal of Food Safety 40 (1):e12716. doi: 10.1111/jfs.12716.
- Comas-Basté, O., S. Sánchez-Pérez, M. T. Veciana-Nogués, M. Latorre-Moratalla, and M. D. C. Vidal-Carou. 2020. Histamine intolerance: The current state of the art. Biomolecules 10 (8):1181. doi: 10.3390/biom10081181.
- Das, G., S. Paramithiotis, B. S. Sivamaruthi, C. H. Wijaya, S. Suharta, N. Sanlier, H. S. Shin, and J. K. Patra. 2020. Traditional fermented foods with anti-aging effect: A concentric review. Food Research International (Ottawa, Ont.) 134:109269. doi: 10.1016/j.foodres.2020.109269.
- Daud, N. M., N. R. Putra, R. Jamaludin, N. S. M. Norodin, N. S. Sarkawi, M. H. S. Hamzah, H. M. Nasir, D. N. A. Zaidel, M. A. C. Yunus, and L. M. Salleh. 2022. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends in Food Science & Technology 119:201–14. doi: 10.1016/j.tifs.2021.12.010.
- de Souza Ribeiro, M. M., L. C. dos Santos, N. S. de Novais, J. Viganó, and P. C. Veggi. 2022. An evaluative review on Stryphnodendron adstringens extract composition: Current and future perspectives on extraction and application. Industrial Crops and Products 187:115325. doi: 10.1016/j.indcrop.2022.115325.
- de Souza, E. L. 2021. Insights into the current evidence on the effects of essential oils toward beneficial microorganisms in foods with a special emphasis to lactic acid bacteria – A review. Trends in Food Science & Technology 114:333–41. doi: 10.1016/j.tifs.2021.06.011.
- de Souza, E. L., K. Á. R. de Oliveira, and M. E. G. de Oliveira. 2023. Influence of lactic acid bacteria metabolites on physical and chemical food properties. Current Opinion in Food Science 49:100981. doi: 10.1016/j.cofs.2022.100981.
- del Rio, B., B. Redruello, D. M. Linares, V. Ladero, M. Fernandez, M. C. Martin, P. Ruas-Madiedo, and M. A. Alvarez. 2017. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chemistry 218:249–55. doi: 10.1016/j.foodchem.2016.09.046.
- del Rio, B., B. Redruello, D. M. Linares, V. Ladero, P. Ruas-Madiedo, M. Fernandez, M. Cruz Martin, and M. A. Alvarez. 2019. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Scientific Reports 9 (1):120. doi: 10.1038/s41598-018-36239-w.
- del Rio, B., B. Redruello, M. Fernandez, M. C. Martin, V. Ladero, and M. A. Alvarez. 2020. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chemistry 331:127303. doi: 10.1016/j.foodchem.2020.127303.
- EFSA Comprehensive European Food Consumption Database. 2022. Last Modified December 15, 2022. Accessed September 4, 2023. https://www.efsa.europa.eu/en/data-report/food-consumption-data.
- EFSA Panel on Biological Hazards (BIOHAZ). 2011. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal 9 (10):2393. doi: 10.2903/j.efsa.2011.2393.
- Emir Çoban, Ö. 2013. Effect of Ginger oil on the sensory and chemical changes of fish finger (Sarda sarda, Heckel 1843) during refrigerated storage. International Food Research Journal 20 (4):1575–8.
- Emir Çoban, Ö., and B. Patir. 2013. Antimicrobial and antioxidant effects of clove oil on sliced smoked Oncorhynchus mykiss. Journal Für Verbraucherschutz Und Lebensmittelsicherheit 8 (3):195–9. doi: 10.1007/s00003-013-0823-2.
- Erdag, D., O. Merhan, and B. Yildiz. 2018. Biochemical and pharmacological properties of biogenic amines. Biogenic Amines 8:1–14. doi: 10.5772/intechopen.81569.
- Erkan, N., Ş. Y. Tosun, Ş. Ulusoy, and G. Üretener. 2011. The use of thyme and laurel essential oil treatments to extend the shelf life of bluefish (Pomatomus saltatrix) during storage in ice. Journal Für Verbraucherschutz Und Lebensmittelsicherheit 6 (1):39–48. doi: 10.1007/s00003-010-0587-x.
- Erol, T., and Ö. Özdestan Ocak. 2020. Influence of pomegranate seed extract on the formation of biogenic amines in a cereal based fermented food: Tarhana. Journal of Food Science and Technology 57 (12):4492–500. doi: 10.1007/s13197-020-04486-3.
- Es’haghi Gorji, M., N. Noori, R. Nabizadeh Nodehi, G. Jahed Khaniki, N. Rastkari, and M. Alimohammadi. 2014. The evaluation of Zataria multiflora Boiss. Essential oil effect on biogenic amines formation and microbiological profile in Gouda cheese. Letters in Applied Microbiology 59 (6):621–30. doi: 10.1111/lam.12319.
- Esparza, I., N. Jiménez-Moreno, F. Bimbela, C. Ancín-Azpilicueta, and L. M. Gandía. 2020. Fruit and vegetable waste management: Conventional and emerging approaches. Journal of Environmental Management 265:110510. doi: 10.1016/j.jenvman.2020.110510.
- Fermented Foods and Beverages Market Size & Share Analysis – Growth Trends & Forecasts. 2023–2028. Accessed September 4, 2023. https://www.mordorintelligence.com/industry-reports/fermented-foods-beverages-market.
- Fessard, A., and F. Remize. 2017. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 3 (3):38. doi: 10.3390/fermentation3030038.
- FLW Protocol. 2016. Food loss and waste accounting and reporting standard (version 1.0). Accessed September 7, 2023. https://flwprotocol.org/wp-content/uploads/2017/05/FLW_Standard_final_2016.pdf.
- Fong, F. L. Y., H. El-Nezami, and E. T. P. Sze. 2021. Biogenic amines–Precursors of carcinogens in traditional Chinese fermented food. NFS Journal 23:52–7. doi: 10.1016/j.nfs.2021.04.002.
- Fong, F. L. Y., K. Y. Lam, C. San Lau, K. H. Ho, Y. H. Kan, M. Y. Poon, H. El-Nezami, and E. T. P. Sze. 2020. Reduction in biogenic amines in douchi fermented by probiotic bacteria. PloS One 15 (3):e0230916. doi: 10.1371/journal.pone.0230916.
- Gan, R. Y., H. B. Li, A. Gunaratne, Z. Q. Sui, and H. Corke. 2017. Effects of fermented edible seeds and their products on human health: Bioactive components and bioactivities. Comprehensive Reviews in Food Science and Food Safety 16 (3):489–531. doi: 10.1111/1541-4337.12257.
- Gao, X., C. Li, R. He, Y. Zhang, B. Wang, Z. H. Zhang, and C. T. Ho. 2023. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chemistry 405:134911. doi: 10.1016/j.foodchem.2022.134911.
- Garcia-Gonzalez, N., N. Battista, R. Prete, and A. Corsetti. 2021. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 9 (2):349. doi: 10.3390/microorganisms9020349.
- Gardini, F., Y. Özogul, G. Suzzi, G. Tabanelli, and F. Özogul. 2016. Technological factors affecting biogenic amine content in foods: A review. Frontiers in Microbiology 7:1218. doi: 10.3389/fmicb.2016.01218.
- Ghanbari, M., M. Jami, K. J. Domig, and W. Kneifel. 2013. Seafood biopreservation by lactic acid bacteria – A review. LWT - Food Science and Technology 54 (2):315–24. doi: 10.1016/j.lwt.2013.05.039.
- Gümrü, S., C. Şahin, and F. Aricioğlu. 2013. Role of agmatine in cognitive functions. OA Behavioural Medicine 1 (1):2.
- Guo, X., X. Guan, Y. Wang, L. Li, D. Wu, Y. Chen, H. Pei, and D. Xiao. 2015. Reduction of biogenic amines production by eliminating the PEP4 gene in Saccharomyces cerevisiae during fermentation of Chinese rice wine. Food Chemistry 178:208–11. doi: 10.1016/j.foodchem.2015.01.089.
- Hassoun, A., and Ö. E. Çoban. 2017. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends in Food Science & Technology 68:26–36. doi: 10.1016/j.tifs.2017.07.016.
- Hernández-Macias, S., A. Martín-Garcia, N. Ferrer-Bustins, O. Comas-Basté, M. Riu-Aumatell, E. López-Tamames, A. Jofré, M. L. Latorre-Moratalla, S. Bover-Cid, and M. C. Vidal-Carou. 2021. Inhibition of biogenic amines formation in fermented foods by the addition of cava lees. Frontiers in Microbiology 12:818565. doi: 10.3389/fmicb.2021.818565.
- Houicher, A., A. Bensid, J. M. Regenstein, and F. Özogul. 2021. Control of biogenic amine production and bacterial growth in fish and seafood products using phytochemicals as biopreservatives: A review. Food Bioscience 39:100807. doi: 10.1016/j.fbio.2020.100807.
- Huang, L., Y. Wang, R. Li, Q. Wang, J. Dong, J. Wang, and S. Lu. 2021. Thyme essential oil and sausage diameter effects on biogenic amine formation and microbiological load in smoked horse meat sausage. Food Bioscience 40 (2):100885. doi: 10.1016/j.fbio.2021.100885.
- Jaguey-Hernández, Y., K. Aguilar-Arteaga, D. Ojeda-Ramirez, J. Anorve-Morga, L. G. González-Olivares, and A. Castaneda-Ovando. 2021. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Research International (Ottawa, Ont.) 144:110341. doi: 10.1016/j.foodres.2021.110341.
- Jia, W., R. Zhang, L. Shi, F. Zhang, J. Chang, and X. Chu. 2020. Effects of spices on the formation of biogenic amines during the fermentation of dry fermented mutton sausage. Food Chemistry 321:126723. doi: 10.1016/j.foodchem.2020.126723.
- Jin, Y. H., J. Lee, A. M. Pawluk, and J. H. Mah. 2022. Inhibitory effects of nicotinic acid, glycine, and other food additives on biogenic amine formation in Baechu kimchi fermentation. Lwt 155:112921. doi: 10.1016/j.lwt.2021.112921.
- Kalač, P. 2014. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chemistry 161:27–39. doi: 10.1016/j.foodchem.2014.03.102.
- Kaneko, S., M. Ueda-Yamada, A. Ando, S. Matsumura, E. Okuda-Ashitaka, M. Matsumura, M. Uyama, and S. Ito. 2007. Cytotoxic effect of spermine on retinal pigment epithelial cells. Investigative Ophthalmology & Visual Science 48 (1):455–63. doi: 10.1167/iovs.06-0379.
- Karabacak, S., and H. Bozkurt. 2008. Effects of Urtica dioica and Hibiscus sabdariffa on the quality and safety of sucuk (Turkish dry-fermented sausage). Meat Science 78 (3):288–96. doi: 10.1016/j.meatsci.2007.06.013.
- Karoui, R., and A. Hassoun. 2017. Efficiency of rosemary and basil essential oils on the shelf-life extension of Atlantic mackerel (Scomber scombrus) fillets stored at 2 °C. Journal of AOAC International 100 (2):335–44. doi: 10.5740/jaoacint.16-0410.
- Kelly, J. R., Y. Borre, C. O’ Brien, E. Patterson, S. El Aidy, J. Deane, P. J. Kennedy, S. Beers, K. Scott, G. Moloney, et al. 2016. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019.
- Khan, I., and S. C. Kang. 2017. Apoptotic activity of Lactobacillus plantarum DGK-17-fermented soybean seed extract in human colon cancer cells via ROS-JNK signaling pathway. Journal of Food Science 82 (6):1475–83. doi: 10.1111/1750-3841.13732.
- Khémesse, K., T. Moumouny, D. François, and T. Alphonse. 2023. Biogenic amines: Physiology, toxicity, and the importance of agmatine. Global Journal of Chemistry, Biology and Physics (GJCBP) 1 (1):10–28.
- Kim, D. H., K. B. W. R. Kim, J. Y. Cho, and D. H. Ahn. 2014. Inhibitory effects of brown algae extracts on histamine production in mackerel muscle via inhibition of growth and histidine decarboxylase activity of Morganella morganii. Journal of Microbiology and Biotechnology 24 (4):465–74. doi: 10.4014/jmb.1309.09071.
- Kim, H. Y., and K. Y. Park. 2018. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. Journal of Functional Foods 47:325–33. doi: 10.1016/j.jff.2018.05.052.
- Kim, J. H., H. J. Ahn, J. W. Lee, H. J. Park, G. H. Ryu, I. J. Kang, and M. W. Byun. 2005. Effects of gamma irradiation on the biogenic amines in pepperoni with different packaging conditions. Food Chemistry 89 (2):199–205. doi: 10.1016/j.foodchem.2004.02.026.
- Kim, K. H., S. H. Lee, B. H. Chun, S. E. Jeong, and C. O. Jeon. 2019. Tetragenococcus halophilus MJ4 as a starter culture for repressing biogenic amine (cadaverine) formation during saeu-jeot (salted shrimp) fermentation. Food Microbiology 82:465–73. doi: 10.1016/j.fm.2019.02.017.
- Kim, S. Y., Y. M. Dang, and J. H. Ha. 2022. Effect of various seasoning ingredients on the accumulation of biogenic amines in kimchi during fermentation. Food Chemistry 380:132214. doi: 10.1016/j.foodchem.2022.132214.
- Kocot, A. M., and B. Wróblewska. 2021. Fermented products and bioactive food compounds as a tool to activate autophagy and promote the maintenance of the intestinal barrier function. Trends in Food Science & Technology 118:905–19. doi: 10.1016/j.tifs.2021.11.014.
- Kongkiattikajorn, J. 2015. Effect of ginger extract to inhibit biogenic amines accumulation during nham fermentation. Journal of Food Chemistry and Nanotechnology 1 (1):15–9. doi: 10.17756/jfcn.2015-003.
- Kuley, E., M. Durmus, Y. Ucar, A. R. Kosker, E. T. A. Tumerkan, J. M. Regenstein, and F. Ozogul. 2018. Combined effects of plant and cell-free extracts of lactic acid bacteria on biogenic amines and bacterial load of fermented sardine stored at 3 ± 1 °C. Food Bioscience 24:127–36. doi: 10.1016/j.fbio.2018.06.008.
- Ladero, V., M. Calles-Enríquez, M. Fernández, and M. A. Alvarez. 2010. Toxicological effects of dietary biogenic amines. Current Nutrition & Food Science 6 (2):145–56. doi: 10.2174/157340110791233256.
- Latorre-Moratalla, M. L., S. Bover-Cid, R. Talon, M. Garriga, E. Zanardi, A. Ianieri, M. J. Fraqueza, M. Elias, E. H. Drosinos, and M. C. Vidal-Carou. 2010. Strategies to reduce biogenic amine accumulation in traditional sausage manufacturing. LWT - Food Science and Technology 43 (1):20–5. doi: 10.1016/j.lwt.2009.06.018.
- Lee, J. Y., Y. Kim, J. Y. Her, M. K. Kim, and K. G. Lee. 2018. Reduction of biogenic amine contents in fermented soybean paste using food additives. Lwt 98:470–6. doi: 10.1016/j.lwt.2018.09.015.
- Lee, J., Y. H. Jin, A. M. Pawluk, and J. H. Mah. 2021. Reduction in biogenic amine content in Baechu (napa cabbage) kimchi by biogenic amine-degrading lactic acid bacteria. Microorganisms 9 (12):2570. doi: 10.3390/microorganisms9122570.
- Leroy, F., and L. De Vuyst. 2004. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science & Technology 15 (2):67–78. doi: 10.1016/j.tifs.2003.09.004.
- Li, B., and S. Lu. 2020. The importance of amine-degrading enzymes on the biogenic amine degradation in fermented foods: A review. Process Biochemistry 99:331–9. doi: 10.1016/j.procbio.2020.09.012.
- Li, C., Y. Zhao, Y. Wang, L. Li, X. Yang, S. Chen, Y. Zhao, and W. Zhou. 2021. Microbial community changes induced by Pediococcus pentosaceus improve the physicochemical properties and safety in fermented tilapia sausage. Food Research International (Ottawa, Ont.) 147:110476. doi: 10.1016/j.foodres.2021.110476.
- Li, K. J., E. M. Brouwer-Brolsma, K. J. Burton, G. Vergères, and E. J. Feskens. 2020. Prevalence of fermented foods in the Dutch adult diet and validation of a food frequency questionnaire for estimating their intake in the NQplus cohort. BMC Nutrition 6 (1):69. doi: 10.1186/s40795-020-00394-z.
- Li, L., X. Wen, Z. Wen, S. Chen, L. Wang, and X. Wei. 2018. Evaluation of the biogenic amines formation and degradation abilities of Lactobacillus curvatus from Chinese bacon. Frontiers in Microbiology 9:1015. doi: 10.3389/fmicb.2018.01015.
- Li, Y., Y. Geng, D. Shi, R. Li, J. Tang, and S. Lu. 2023. Impact of Coreopsis tinctoria Nutt. Essential oil microcapsules on the formation of biogenic amines and quality of smoked horsemeat sausage during ripening. Meat Science 195:109020. doi: 10.1016/j.meatsci.2022.109020.
- Linares, D. M., B. del Rio, B. Redruello, V. Ladero, M. C. Martin, M. Fernandez, P. Ruas-Madiedo, and M. A. Alvarez. 2016. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chemistry 197 (Pt A):658–63. doi: 10.1016/j.foodchem.2015.11.013.
- Lobiuc, A., N. E. Pavăl, I. I. Mangalagiu, R. Gheorghiță, G. C. Teliban, D. Amăriucăi-Mantu, and V. Stoleru. 2023. Future antimicrobials: Natural and functionalized phenolics. Molecules (Basel, Switzerland) 28 (3):1114. doi: 10.3390/molecules28031114.
- Lorenzo, J. M., A. Cachaldora, S. Fonseca, M. Gómez, I. Franco, and J. Carballo. 2010. Production of biogenic amines “in vitro” in relation to the growth phase by Enterobacteriaceae species isolated from traditional sausages. Meat Science 86 (3):684–91. doi: 10.1016/j.meatsci.2010.06.005.
- Lorenzo, J. M., P. E. S. Munekata, and R. Domínguez. 2017. Role of autochthonous starter cultures in the reduction of biogenic amines in traditional meat products. Current Opinion in Food Science 14:61–5. doi: 10.1016/j.cofs.2017.01.009.
- Lu, S., H. Ji, Q. Wang, B. Li, K. Li, C. Xu, and C. Jiang. 2015. The effects of starter cultures and plant extracts on the biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. Food Control. 50:869–75. doi: 10.1016/j.foodcont.2014.08.015.
- Luo, J., X. Lin, M. Bordiga, C. Brennan, and B. Xu. 2021. Manipulating effects of fruits and vegetables on gut microbiota – A critical review. International Journal of Food Science & Technology 56 (5):2055–67. doi: 10.1111/ijfs.14927.
- Mah, J. H., Y. J. Kim, and H. J. Hwang. 2009. Inhibitory effects of garlic and other spices on biogenic amine production in Myeolchi-jeot, Korean salted and fermented anchovy product. Food Control. 20 (5):449–54. doi: 10.1016/j.foodcont.2008.07.006.
- Mah, J. H., Y. K. Park, Y. H. Jin, J. H. Lee, and H. J. Hwang. 2019. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods (Basel, Switzerland) 8 (2):85. doi: 10.3390/foods8020085.
- Mahmoud, B. S., K. Yamazaki, K. Miyashita, S. Il-Shik, C. Dong-Suk, and T. Suzuki. 2004. Bacterial microflora of carp (Cyprinus carpio) and its shelf-life extension by essential oil compounds. Food Microbiology 21 (6):657–66. doi: 10.1016/j.fm.2004.03.001.
- Majcherczyk, J., and K. Surówka. 2019. Effects of onion or caraway on the formation of biogenic amines during sauerkraut fermentation and refrigerated storage. Food Chemistry 298:125083. doi: 10.1016/j.foodchem.2019.125083.
- Mannaa, M., Y. S. Seo, and I. Park. 2020. Addition of coriander during fermentation of Korean soy sauce (gangjang) causes significant shift in microbial composition and reduction in biogenic amine levels. Foods (Basel, Switzerland) 9 (10):1346. doi: 10.3390/foods9101346.
- Marco, M. L., M. E. Sanders, M. Gänzle, M. C. Arrieta, P. D. Cotter, L. De Vuyst, C. Hill, W. Holzapfel, S. Lebeer, D. Merenstein, et al. 2021. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews. Gastroenterology & Hepatology 18 (3):196–208. doi: 10.1038/s41575-020-00390-5.
- Marx, W., A. Scholey, J. Firth, N. M. D’Cunha, M. Lane, M. Hockey, M. M. Ashton, J. F. Cryan, A. O’Neil, N. Naumovski, et al. 2020. Prebiotics, probiotics, fermented foods and cognitive outcomes: A meta-analysis of randomized controlled trials. Neuroscience and Biobehavioral Reviews 118:472–84. doi: 10.1016/j.neubiorev.2020.07.036.
- Meng, J., Q. Yang, W. Wan, Q. Zhu, and X. Zeng. 2022. Physicochemical properties and adaptability of amine-producing Enterobacteriaceae isolated from traditional Chinese fermented fish (Suan yu). Food Chemistry 369:130885. doi: 10.1016/j.foodchem.2021.130885.
- Montanari, C., F. Barbieri, S. Lorenzini, D. Gottardi, V. Šimat, F. Özogul, F. Gardini, and G. Tabanelli. 2022. Survival, growth, and biogenic amine production of Enterococcus faecium FC12 in response to extracts and essential oils of Rubus fruticosus and Juniperus oxycedrus. Frontiers in Nutrition 9:1092172. doi: 10.3389/fnut.2022.1092172.
- Mozuriene, E., E. Bartkiene, G. Juodeikiene, D. Zadeike, L. Basinskiene, A. Maruska, M. Stankevicius, O. Ragazinskiene, J. Damasius, and D. Cizeikiene. 2016. The effect of savoury plants, fermented with lactic acid bacteria, on the microbiological contamination, quality, and acceptability of unripened curd cheese. LWT - Food Science and Technology 69:161–8. doi: 10.1016/j.lwt.2016.01.027.
- Nielsen, E. S., E. Garnås, K. J. Jensen, L. H. Hansen, P. S. Olsen, C. Ritz, L. Krych, and D. S. Nielsen. 2018. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation – a pilot study. Food & Function 9 (10):5323–35. doi: 10.1039/c8fo00968f.
- Nikoo, M., J. M. Regenstein, and H. Ahmadi Gavlighi. 2018. Antioxidant and antimicrobial activities of (‐)‐epigallocatechin‐3‐gallate (EGCG) and its potential to preserve the quality and safety of foods. Comprehensive Reviews in Food Science and Food Safety 17 (3):732–53. doi: 10.1111/1541-4337.12346.
- Oh, S. J., J. H. Mah, J. H. Kim, Y. W. Kim, and H. J. Hwang. 2012. Reduction of tyramine by addition of Schizandra chinensis Baillon in Cheonggukjang. Journal of Medicinal Food 15 (12):1109–15. doi: 10.1089/jmf.2012.2561.
- Omer, A. K., R. R. Mohammed, P. S. M. Ameen, Z. A. Abas, and K. Ekici. 2021. Presence of biogenic amines in food and their public health implications: A review. Journal of Food Protection 84 (9):1539–48. doi: 10.4315/JFP-21-047.
- Oriach, C. S., R. C. Robertson, C. Stanton, J. F. Cryan, and T. G. Dinan. 2016. Food for thought: The role of nutrition in the microbiota-gut–brain axis. Clinical Nutrition Experimental 6:25–38. doi: 10.1016/j.yclnex.2016.01.003.
- Ozogul, Y., İ. Yuvka, Y. Ucar, M. Durmus, A. R. Kösker, M. Öz, and F. Ozogul. 2017. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. Lwt 75:677–84. doi: 10.1016/j.lwt.2016.10.009.
- Peñas, E., J. Frias, B. Sidro, and C. Vidal-Valverde. 2010. Impact of fermentation conditions and refrigerated storage on microbial quality and biogenic amine content of sauerkraut. Food Chemistry 123 (1):143–50. doi: 10.1016/j.foodchem.2010.04.021.
- Raoofi, M., N. Noori, and A. Khanjari. 2023. Effect of Bunium persicum Boiss. Essential oil on the production of biogenic amines (tyramine and histamine) and microbiological profile in Cheddar cheese. Journal of Nutrition, Fasting & Health 11 (1):32–40. doi: 10.22038/JNFH.2023.69084.1411.
- Rocks, T., M. West, M. Hockey, H. Aslam, M. Lane, A. Loughman, F. N. Jacka, and A. Ruusunen. 2021. Possible use of fermented foods in rehabilitation of anorexia nervosa: The gut microbiota as a modulator. Progress in Neuro-Psychopharmacology & Biological Psychiatry 107:110201. doi: 10.1016/j.pnpbp.2020.110201.
- Rodríguez-Caso, C., D. Rodríguez-Agudo, F. Sánchez-Jiménez, and M. A. Medina. 2003. Green tea epigallocatechin-3-gallate is an inhibitor of mammalian histidine decarboxylase. Cellular and Molecular Life Sciences: CMLS 60 (8):1760–3. doi: 10.1007/s00018-003-3135-3.
- Ruiz-Capillas, C., and A. M. Herrero. 2019. Impact of biogenic amines on food quality and safety. Foods (Basel, Switzerland) 8 (2):62. doi: 10.3390/foods8020062.
- SaeidiFard, N., K. Djafarian, and S. Shab-Bidar. 2020. Fermented foods and inflammation: A systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition ESPEN 35:30–9. doi: 10.1016/j.clnesp.2019.10.010.
- Sahab, N. R., E. Subroto, R. L. Balia, and G. L. Utama. 2020. Γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 6 (11):e05526. doi: 10.1016/j.heliyon.2020.e05526.
- Sánchez-Pérez, S., O. Comas-Basté, J. Rabell-González, M. T. Veciana-Nogués, M. L. Latorre-Moratalla, and M. C. Vidal-Carou. 2018. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods (Basel, Switzerland) 7 (12):205. doi: 10.3390/foods7120205.
- Sánchez, A., R. Rodríguez, M. Coton, E. Coton, M. Herrero, L. A. García, and M. Díaz. 2010. Population dynamics of lactic acid bacteria during spontaneous malolactic fermentation in industrial cider. Food Research International 43 (8):2101–7. doi: 10.1016/j.foodres.2010.07.010.
- Şanlier, N., B. B. Gökcen, and A. C. Sezgin. 2019. Health benefits of fermented foods. Critical Reviews in Food Science and Nutrition 59 (3):506–27. doi: 10.1080/10408398.2017.1383355.
- Schiess, N., R. Cataldi, M. S. Okun, N. Fothergill-Misbah, E. Dorsey, B. R. Bloem, M. Barretto, R. Bhidayasiri, R. Brown, L. Chishimba, et al. 2022. Six action steps to address global disparities in Parkinson disease: A World Health Organization (WHO) priority. JAMA Neurology 79 (9):929–36. doi: 10.1001/jamaneurol.2022.1783.
- Selhub, E. M., A. C. Logan, and A. C. Bested. 2014. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. Journal of Physiological Anthropology 33 (1):2. doi: 10.1186/1880-6805-33-2.
- Shukla, R., and A. Goyal. 2013. Novel dextran from Pediococcus pentosaceus CRAG3 isolated from fermented cucumber with anti-cancer properties. International Journal of Biological Macromolecules 62:352–7. doi: 10.1016/j.ijbiomac.2013.09.043.
- Shukla, S., H. K. Park, J. K. Kim, and M. Kim. 2010. Determination of biogenic amines in Korean traditional fermented soybean paste (Doenjang). Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 48 (5):1191–5. doi: 10.1016/j.fct.2010.01.034.
- Shukla, S., J. S. Lee, V. K. Bajpai, I. Khan, Y. S. Huh, Y. K. Han, and M. Kim. 2019. Toxicological evaluation of lotus, ginkgo, and garlic tailored fermented Korean soybean paste (Doenjang) for biogenic amines, aflatoxins, and microbial hazards. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 133:110729. doi: 10.1016/j.fct.2019.110729.
- Shukla, S., J. S. Lee, V. K. Bajpai, S. H. Nile, Y. S. Huh, Y. K. Han, and M. Kim. 2018. Detection of biogenic amines and microbial safety assessment of novel Meju fermented with addition of Nelumbo nucifera, Ginkgo biloba, and Allium sativum. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 119:231–6. doi: 10.1016/j.fct.2018.04.018.
- Simon-Sarkadi, L., K. Pásztor-Huszár, I. Dalmadi, and G. Kiskó. 2012. Effect of high hydrostatic pressure processing on biogenic amine content of sausage during storage. Food Research International 47 (2):380–4. doi: 10.1016/j.foodres.2011.10.029.
- Singh, S., P. K. Sharma, S. Chaturvedi, P. Kumar, A. D. Nannaware, K. Alok, and P. K. Rout. 2023. Biocatalyst for the synthesis of natural flavouring compounds as food additives: Bridging the gap for a more sustainable industrial future. Food Chemistry 137217:137217. doi: 10.1016/j.foodchem.2023.137217.
- Sirocchi, V., G. Caprioli, C. Cecchini, M. M. Coman, A. Cresci, F. Maggi, F. Papa, M. Ricciutelli, S. Vittori, and G. Sagratini. 2013. Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. International Journal of Food Sciences and Nutrition 64 (8):921–8. doi: 10.3109/09637486.2013.809706.
- Stoll, D. A., E. N. Wafula, J. M. Mathara, B. Trierweiler, S. E. Kulling, and M. Huch. 2021. Fermentation of African nightshade leaves with lactic acid bacterial starter cultures. International Journal of Food Microbiology 342:109056. doi: 10.1016/j.ijfoodmicro.2021.109056.
- Sun, Q., F. Sun, D. Zheng, B. Kong, and Q. Liu. 2019. Complex starter culture combined with vacuum packaging reduces biogenic amine formation and delays the quality deterioration of dry sausage during storage. Food Control. 100:58–66. doi: 10.1016/j.foodcont.2019.01.008.
- Sun, Q., X. Zhao, H. Chen, C. Zhang, and B. Kong. 2018. Impact of spice extracts on the formation of biogenic amines and the physicochemical, microbiological and sensory quality of dry sausage. Food Control. 92:190–200. doi: 10.1016/j.foodcont.2018.05.002.
- Świder, O., M. Ł. Roszko, M. Wójcicki, and K. Szymczyk. 2020. Biogenic amines and free amino acids in traditional fermented vegetables – Dietary risk evaluation. Journal of Agricultural and Food Chemistry 68 (3):856–68. doi: 10.1021/acs.jafc.9b05625.
- Świder, O., M. Ł. Roszko, M. Wójcicki, M. Bujak, M. Szczepańska, E. Juszczuk-Kubiak, P. Średnicka, and H. Cieślak. 2023. Non-aminobiogenic starter cultures in a model system of cucumber fermentation. Lwt 177:114574. doi: 10.1016/j.lwt.2023.114574.
- Tabanelli, G., C. Montanari, E. Bargossi, R. Lanciotti, V. Gatto, G. Felis, S. Torriani, and F. Gardini. 2014. Control of tyramine and histamine accumulation by lactic acid bacteria using bacteriocin forming lactococci. International Journal of Food Microbiology 190:14–23. doi: 10.1016/j.ijfoodmicro.2014.08.023.
- Tamang, J. P., K. Watanabe, and W. H. Holzapfel. 2016. Diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology 7:377. doi: 10.3389/fmicb.2016.00377.
- Tasdemir, S. S., and N. Sanlier. 2020. An insight into the anticancer effects of fermented foods: A review. Journal of Functional Foods 75:104281. doi: 10.1016/j.jff.2020.104281.
- Taylor, B. C., F. Lejzerowicz, M. Poirel, J. P. Shaffer, L. Jiang, A. Aksenov, N. Litwin, G. Humphrey, C. Martino, S. Miller-Montgomery, et al. 2020. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems 5 (2):e00901–19. doi: 10.1128/msystems.00901-19.
- Teo, W. Z., J. Y. See, S. Ramazanu, J. C. Y. Chan, and X. V. Wu. 2022. Effect of lactic acid fermented foods on glycemic control in diabetic adults: A systemic review and meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition 30:1–16. doi: 10.1080/10408398.2022.2128032.
- Tittarelli, F., G. Perpetuini, P. Di Gianvito, and R. Tofalo. 2019. Biogenic amines producing and degrading bacteria: A snapshot from raw ewes’ cheese. Lwt 101:1–9. doi: 10.1016/j.lwt.2018.11.030.
- Ueda, J. M., M. C. Pedrosa, S. A. Heleno, M. Carocho, I. C. Ferreira, and L. Barros. 2022. Food additives from fruit and vegetable by-products and bio-residues: A comprehensive review focused on sustainability. Sustainability 14 (9):5212. doi: 10.3390/su14095212.
- Van den Eynde, V., P. K. Gillman, and B. B. Blackwell. 2022. The prescriber’s guide to the MAOI diet-thinking through tyramine troubles. Psychopharmacology Bulletin 52 (2):73–116.
- van der Merwe, M. 2021. Gut microbiome changes induced by a diet rich in fruits and vegetables. International Journal of Food Sciences and Nutrition 72 (5):665–9. doi: 10.1080/09637486.2020.1852537.
- Vasconcelos, H., J. M. de Almeida, A. Matias, C. Saraiva, P. A. Jorge, and L. C. Coelho. 2021. Detection of biogenic amines in several foods with different sample treatments: An overview. Trends in Food Science & Technology 113:86–96. doi: 10.1016/j.tifs.2021.04.043.
- Velut, G., F. Delon, J. P. Mérigaud, C. Tong, G. Duflos, F. Boissan, S. Watier-Grillot, M. Boni, C. Derkenne, A. Dia, et al. 2019. Histamine food poisoning: A sudden, large outbreak linked to fresh yellowfin tuna from Reunion Island, France, April 2017. Eurosurveillance 24 (22):1800405. doi: 10.2807/1560-7917.ES.2019.24.22.1800405.
- Vianello, R., M. Repič, and J. Mavri. 2012. How are biogenic amines metabolized by monoamine oxidases? European Journal of Organic Chemistry 2012 (36):7057–65. doi: 10.1002/ejoc.201201122.
- Visciano, P., and M. Schirone. 2022. Update on biogenic amines in fermented and non-fermented beverages. Foods (Basel, Switzerland) 11 (3):353. doi: 10.3390/foods11030353.
- Vos, T., A. A. Abajobir, C. Abbafati, K. M. Abbas, K. H. Abate, F. Abd-Allah, A. M. Abdulle, T. A. Abebo, S. F. Abera, V. Aboyans, et al. 2017. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study. The Lancet 390 (10100):1211–59. doi: 10.1016/S0140-6736(17)32154-2.
- Wang, J., X. Zhang, X. Li, Z. Yu, J. Hu, and Y. Zhu. 2021. Effects of plant extracts on biogenic amine accumulation, bacterial abundance and diversity in fermented sausage. CyTA - Journal of Food 19 (1):771–81. doi: 10.1080/19476337.2021.1984994.
- Wang, X., Y. Zhang, and H. Ren. 2018. Effects of grape seed extract on lipid oxidation, biogenic amine formation and microbiological quality in Chinese traditional smoke‐cured bacon during storage. Journal of Food Safety 38 (2):e12426. doi: 10.1111/jfs.12426.
- Wastyk, H. C., G. K. Fragiadakis, D. Perelman, D. Dahan, B. D. Merrill, F. B. Yu, M. Topf, C. G. Gonzalez, W. Van Treuren, S. Han, et al. 2021. Gut-microbiota-targeted diets modulate human immune status. Cell 184 (16):4137–53.e14. doi: 10.1016/j.cell.2021.06.019.
- Wei, G., M. Dan, G. Zhao, and D. Wang. 2023. Recent advances in chromatography-mass spectrometry and electronic nose technology in food flavor analysis and detection. Food Chemistry 405 (Pt A):134814. doi: 10.1016/j.foodchem.2022.134814.
- Wendakoon, C. N., and M. Sakaguchi. 1993. Combined effect of sodium chloride and clove on growth and biogenic amine formation of Enterobacter aerogenes in mackerel muscle extract. Journal of Food Protection 56 (5):410–3. doi: 10.4315/0362-028X-56.5.410.
- World Resources Institute. 2019. World resources report. Creating a sustainable food future. A menu of solutions to feed nearly 10 billion people by 2050. Accessed September 7, 2023. https://research.wri.org/sites/default/files/2019-07/WRR_Food_Full_Report_0.pdf.
- Wouters, D., N. Bernaert, W. Conjaerts, B. Van Droogenbroeck, M. De Loose, and L. De Vuyst. 2013. Species diversity, community dynamics, and metabolite kinetics of spontaneous leek fermentations. Food Microbiology 33 (2):185–96. doi: 10.1016/j.fm.2012.09.016.
- Wunderlichová, L., L. Buňková, M. Koutný, P. Jančová, and F. Buňka. 2014. Formation, degradation, and detoxification of putrescine by foodborne bacteria: A review. Comprehensive Reviews in Food Science and Food Safety 13 (5):1012–30. doi: 10.1111/1541-4337.12099.
- Xu, W., C. Jiang, A. Liu, R. Bao, W. Wang, Z. Zhang, C. Ji, H. Liang, S. Zhang, and X. Lin. 2022. Moderate papain addition improves the physicochemical, microbiological, flavor and sensorial properties of Chouguiyu, traditional Chinese fermented fish. Food Bioscience 46:101587. doi: 10.1016/j.fbio.2022.101587.
- Xu, X., S. Bi, F. Lao, F. Chen, X. Liao, and J. Wu. 2021. Induced changes in bioactive compounds of broccoli juices after fermented by animal-and plant-derived Pediococcus pentosaceus. Food Chemistry 357:129767. doi: 10.1016/j.foodchem.2021.129767.
- Yilmaz, N., F. Özogul, M. Moradi, E. E. Fadiloglu, V. Simat, and J. M. Rocha. 2022. Reduction of biogenic amines formation by foodborne pathogens using postbiotics in lysine-decarboxylase broth. Journal of Biotechnology 358:118–27. doi: 10.1016/j.jbiotec.2022.09.003.
- Yu, H., Y. Huang, L. Lu, Y. Liu, Z. Tang, and S. Lu. 2021. Impact of thyme microcapsules on histamine production by Proteus bacillus in Xinjiang smoked horsemeat sausage. Foods (Basel, Switzerland) 10 (10):2491. doi: 10.3390/foods10102491.
- Zang, E., L. Jiang, H. Cui, X. Li, Y. Yan, Q. Liu, Z. Chen, and M. Li. 2022. Only plant-based food additives: An overview on application, safety, and key challenges in the food industry. Food Reviews International:1–32. doi: 10.1080/87559129.2022.2062764.
- Zhang, Q. Q., M. Jiang, X. Rui, W. Li, X. H. Chen, and M. S. Dong. 2017. Effect of rose polyphenols on oxidation, biogenic amines and microbial diversity in naturally dry fermented sausages. Food Control. 78:324–30. doi: 10.1016/j.foodcont.2017.02.054.
- Zhang, Q. Q., X. Rui, Y. X. Shi, X. Y. Wang, and M. S. Dong. 2018. Effects of phenolic acids on the biogenic amine formation of Enterobacter aerogenes. Journal of Food Processing and Preservation 42 (3):e13554. doi: 10.1111/jfpp.13554.
- Zhang, Y., J. Zhang, X. Lin, H. Liang, S. Zhang, and C. Ji. 2022. Lactobacillus strains inhibit biogenic amine formation in salted mackerel (Scomberomorus niphonius). Lwt 155:112851. doi: 10.1016/j.lwt.2021.112851.
- Zhao, J., C. Niu, S. Du, C. Liu, F. Zheng, J. Wang, and Q. Li. 2020. Reduction of biogenic amines formation during soybean paste fermentation by using Staphylococcus carnosus M43 and Pediococcus acidilactici M28 as starter culture. Lwt 133:109917. doi: 10.1016/j.lwt.2020.109917.
- Zhou, F., H. Zhao, F. Bai, D. Piotr, Y. Liu, and B. Zhang. 2014. Purification and characterisation of the bacteriocin produced by Lactobacillus plantarum, isolated from Chinese pickle. Czech Journal of Food Sciences 32 (5):430–6. doi: 10.17221/270/2013-CJFS.
- Zhou, K., X. Zhang, G. D. Huang, S. Hongsibsong, G. Hao, Y. M. Li, J. Y. Yang, and Z. L. Xu. 2023. Formation of biogenic amines in soy sauce and reduction via simple phytochemical addition. Lwt 176:114542. doi: 10.1016/j.lwt.2023.114542.
- Zhou, Q., M. Mo, A. Wang, B. Tang, and Q. He. 2023. Changes in N-nitrosamines, residual nitrites, lipid oxidation, biogenic amines, and microbiota in Chinese sausages following treatment with tea polyphenols and their palmitic acid-modified derivatives. Journal of Food Protection 86 (5):100072. doi: 10.1016/j.jfp.2023.100072.
- Zhou, Q., M. Mo, B. Tang, and Q. He. 2023. A comparative study of tea polyphenols and its palmitic acid-modified derivatives: Their effects on the microbial ecosystem and biogenic amines in Chinese sausage. Journal of Food Science and Technology 60 (6):1772–81. doi: 10.1007/s13197-023-05717-z.
- Zhou, X., M. Qiu, D. Zhao, F. Lu, and Y. Ding. 2016. Inhibitory effects of spices on biogenic amine accumulation during fish sauce fermentation. Journal of Food Science 81 (4):M913–M920. doi: 10.1111/1750-3841.13255.
- Zuljan, F. A., P. Mortera, S. H. Alarcón, V. S. Blancato, M. Espariz, and C. Magni. 2016. Lactic acid bacteria decarboxylation reactions in cheese. International Dairy Journal 62:53–62. doi: 10.1016/j.idairyj.2016.07.007.