Abstract
Bacterial biofilm has brought a lot of intractable problems in food and biomedicine areas. Conventional biofilm control mainly focuses on inactivation and removal of biofilm. However, with robust construction and enhanced resistance, the established biofilm is extremely difficult to eradicate. According to the mechanism of biofilm development, biofilm formation can be modulated by intervening in the key factors and regulatory systems. Therefore, regulation of biofilm formation has been proposed as an alternative way for effective biofilm control. This review aims to provide insights into the regulation of biofilm formation in food and biomedicine. The underlying mechanisms for early-stage biofilm establishment are summarized based on the key factors and correlated regulatory networks. Recent developments and applications of novel regulatory strategies such as anti/pro-biofilm agents, nanomaterials, functionalized surface materials and physical strategies are also discussed. The current review indicates that these innovative methods have contributed to effective biofilm control in a smart, safe and eco-friendly way. However, standard methodology for regulating biofilm formation in practical use is still missing. As biofilm formation in real-world systems could be far more complicated, further studies and interdisciplinary collaboration are still needed for simulation and experiments in the industry and other open systems.
1. Introduction
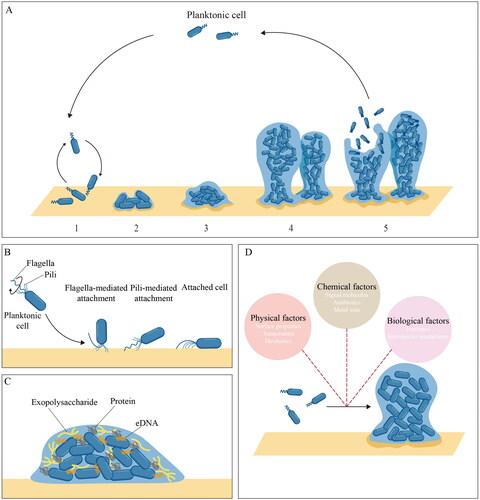
Biofilms are considered the predominant bacterial lifestyle in nature. Distinct from planktonic bacteria, these aggregates of surface-attached or non-surface-attached cells are embedded in self-produced extracellular polymeric substances (EPS) and live as heterogeneous communities with three-dimensional (3D) microbial structures (Karygianni et al. Citation2020). The development of bacterial biofilms is a cyclic and highly dynamic process. Based on the investigation of Pseudomonas aeruginosa (P. aeruginosa) biofilm, the classic five-step biofilm model defined biofilm development as a cyclic process consisting of five specific stages: reversible attachment, irreversible attachment, maturation-1, maturation-2 and dispersion () (Sauer et al. Citation2002). As biofilm development in real-world systems can be far more complicated, the five-step model has been continuously challenged and expanded (Sauer et al. Citation2022), with variations of this model for different species (Otto Citation2013; Rendueles and Ghigo Citation2012). In the life cycle of biofilm development, biofilm formation refers to the transition from the planktonic to the biofilm mode of growth, which involves three major steps: attachment, EPS production and micro-colony formation. Recent studies revealed that the ability of bacteria to form biofilms depends on a number of factors such as surface appendages (e.g., flagella and pili), EPS components, external environments and expression of related genes (Persat et al. Citation2015; Yin et al. Citation2019). Meanwhile, the whole process of biofilm formation is controlled by sophisticated regulatory networks consisting of two-component systems (TCSs), second messengers, small non-coding RNAs (sRNAs) and quorum sensing (QS) systems (Condinho et al. Citation2023).
Figure 1. Schematic of the biofilm formation process and related factors. A five-step biofilm model. Stage 1: reversible attachment. Stage 2: irreversible attachment. Stage 3: maturation-1. Stage 4: maturation-2. Stage 5: dispersion. B roles of surface appendages during initial attachment. C composition of EPS components. D environmental factors during biofilm formation.
Biofilm formation has both negative and positive effects in the food industry and biomedicine area. On the one hand, bacteria can spontaneously get attached and establish biofilm on both biotic and abiotic surfaces, leading to a series of problems including biofouling, cross-contamination, chronic infection, antibiotic resistance, etc. (Patra et al. Citation2022; Stewart and Bjarnsholt Citation2020; Uruén et al. Citation2020; He and Sun Citation2015a, Citation2015b; Jayan, Pu, and Sun Citation2022). A public announcement from the National Institutes of Health indicates that biofilm could account for over 60% of microbial infections, and most of such infections are hard to treat or frequently recurring (Lewis Citation2001). Similarly, in the food industry, biofilm formation can happen easily. For example, from ready-to-eat food, which could be easily contaminated by microorganisms during transportation and storage, 33 of 54 Staphylococcus aureus (S. aureus) isolates have been reported to show biofilm formation capacity, including two strong biofilm producers with multi-drug resistance (Lin et al. Citation2019). On the other hand, with enhanced resistance and self-immobilization properties, the established biofilm can be widely applied in fermentation, biosynthesis, and biomaterial development to improve biochemical production productivities during continuous processing (Ghosh et al. Citation2022; Hayta et al. Citation2021; Wu et al. Citation2022). Moreover, mediated by biofilm formation, microbial fermentation can complete more complex tasks, such as the direct lignocellulose degradation through the microbial co-culture of aerobic fungi and anaerobic or facultative bacteria (Jiang et al. Citation2021).
Considering the significance of biofilms in food and biomedicine, effective strategies for biofilm control have been continuously proposed. In previous reviews, biofilm formation, inhibition and eradication have been discussed thoroughly (Sauer et al. Citation2022; Yin et al. Citation2021). For the removal of pathogenic biofilms, current strategies can be generally classified into physical methods (e.g., ultrasound treatment, photodynamic or photothermal therapy) and biochemical methods (e.g., phage lysins, degradative enzymes and metabolites). However, since biofilms show higher resistance than planktonic bacteria, it remains challenging to eradicate mature biofilms (Van Acker, Van Dijck, and Coenye Citation2014). A promising alternative is to prevent or delay the establishment of biofilms. Strategies such as surface modification and antimicrobial agents have been widely used for the prevention of biofilm formation, but most of the existing strategies only focus on minimizing the number of surface-attached cells (Desrousseaux et al. Citation2013; Paluch et al. Citation2020). From the view of biofilm development and related regulatory networks, a preferred approach for biofilm control, whether to prevent the establishment of unexpected biofilms or to promote the growth of commensal bacteria, is to regulate the biofilm formation process by intervening in the regulatory systems and the key process of biofilm development. Progress in novel strategies for the regulation of biofilm formation such as anti/pro-biofilm agents, nanomaterials, surface materials and physical methods has contributed to effective biofilm control in a smart, safe and eco-friendly way. However, few reviews focused on the updated mechanisms and strategies for the regulation of the biofilm formation process. In this review, current knowledge about mechanisms for the regulation of biofilm formation is summarized by illustrating major factors and regulatory networks participating in early-stage biofilm establishment. Some interesting findings from research in microbiology, molecular biology, micromechanics and materials science are reported to help better understand the roles of bacterial appendages, EPS components and environmental factors during biofilm formation, as well as the participation of common bacterial regulatory networks in the phenotype switch from planktonic cells to sessile biofilms. Based on the relationship between regulatory networks and biofilm formation, newly developed strategies for regulation of biofilm formation such as natural anti-biofilm agents, surface coating and modification, cold plasma technique, etc. are also discussed.
2. Mechanisms for regulating biofilm formation
2.1. Factors influencing biofilm formation
2.1.1. Surface appendages
Bacteria possess a wide variety of surface appendages such as flagellum and pili, which can mediate the interaction between bacteria cells and biotic or abiotic substrates. These appendages not only have motility properties but also enable bacteria to maintain their attachment to surfaces in a flow environment ().
Flagella are long helical filaments possessed by motile bacteria which allow them to swim in liquid or swarm over solid surfaces. Flagellum-mediated motility is critical for biofilm formation and development (Duan et al. Citation2013). In the process of initial attachment, alterations in flagellar load and function can initiate potential signals that are subsequently coupled to changes in cellular behaviors, resulting in switches between swimming and swarming, swimming and adhesion as well as biofilm initiation (Schniederberend et al. Citation2019). Moreover, increasing interest in flagellum-meditated attachment revealed that flagella and their properties could improve initial attachment and biofilm formation in many bacterial species. In the case of Escherichia coli (E. coli) biofilm formation on plastic surfaces, flagella, especially sticky flagella, have been demonstrated to help E. coli pierce the energy barrier, attach onto plastics and form a rigid attachment layer (Zhang et al. Citation2023). Similarly, for biotic substrates, it has been reported that purified flagella of E. coli performed the ability to bind mucins, contributing to colonization and biofilm formation on the mucosal surface (Erdem et al. Citation2007).
Pili (or fimbriae) are long filamentous structures found on cell surfaces both in Gram-negative and Gram-positive bacteria. In Gram-negative bacteria, pili are typically formed by non-covalent interactions between pilin subunit proteins (pilins), while in Gram-positive pathogens most discovered pili are formed by covalent polymerization of adhesive pilin subunits (Danne and Dramsi Citation2012; Telford et al. Citation2006). Pili perform motility and adherence functions during biofilm formation. Although pili can be divided into several groups based on their assembly pathway, type IV pili (T4P), which are mainly found in Gram-negative bacteria, represent the most widespread and well-characterized class. Biofilm formation is influenced by the T4P, which functions as motorized surface appendages and adhesive fibers. During early-stage biofilm formation, P. aeruginosa and other bacteria employ T4P-dependent twitching motility to crawl or walk on surfaces (Nieto et al. Citation2019). This motility mode contributes to cell aggregation and micro-colony formation. Additionally, in P. aeruginosa, T4P mechanics play a key role in controlling bacterial attachment to biotic and abiotic surfaces (Beaussart et al. Citation2014). Meanwhile, in Clostridioides difficile (a Gram-positive bacillus), T4P and related structures can also exhibit extracellular DNA (eDNA) recognition ability, providing further insight into the relationship between pili function and biofilm formation (Ronish et al. Citation2022).
2.1.2. Extracellular biomolecules
In the process of early-stage biofilm formation, extracellular biomolecules, whether surface-associated adhesive substances or extracellular components, can also make significant contributions. In many studies, due to the inextricable link between surface-associated and extracellular substances in adhesion, cohesion, and scaffolding, both surface-associated molecules (such as surface proteins in Gram-positive bacteria and outer membrane proteins in Gram-negative bacteria) and extracellular molecules are considered as EPS component (Karygianni et al. Citation2020). This self-produced sticky matrix has played multifaceted roles in biofilm development, mechanical stability and resistance (Flemming and Wingender Citation2010).
The composition and structure of the biofilm matrix vary with bacterial species and growth conditions. Generally, the major EPS components include polysaccharides, proteins, and eDNA (). These components are important biofilm-building materials forming the three-dimensional polymer network structure to interconnect and immobilize bacteria cells (Dragoš and Kovács Citation2017; Flemming and Wingender Citation2010). Polysaccharides are considered the predominant extracellular biopolymer in biofilms (Das Citation2022). In P. aeruginosa, for example, the key extracellular polysaccharides implicated in biofilm formation and biofilm structuring are alginate, Psl and Pel (Ryder, Byrd, and Wozniak Citation2007). Colvin et al. (Citation2012) demonstrated the importance of Psl in surface attachment for clinical and environmental P. aeruginosa isolates, indicating the adhesion function of Psl. An interesting founding in P. aeruginosa biofilm was that the cationic exopolysaccharide Pel could cross-link eDNA via ionic interactions (Jennings et al. Citation2015). The ability of cationic polysaccharides to interact with anionic molecules was also found in S. aureus (Formosa-Dague et al. Citation2016).
Another indispensable composition of EPS is protein. Unlike the well-studied composition and function of exopolysaccharides, the protein content of EPS is more complicated. In the aspect of composition, the proteomic study revealed that the matrix proteome in P. aeruginosa biofilm contained secreted proteins (13.3%), cytoplasmic proteins (28.9%), periplasmic proteins (11.1%), cytoplasmic membrane proteins (2.2%), and most abundantly, outer membrane proteins (35.6%) (Toyofuku et al. Citation2012). In the aspect of function, surface proteins are emerging as important elements promoting primary adhesion and biofilm formation (Lasa and Penadés Citation2006; Schiffer et al. Citation2022). In S. aureus, polysaccharide intercellular adhesion (PIA), encoded by the ica operon, has been considered as the critical EPS component for bacteria accumulation, while cell wall-anchored (CWA) proteins are significant for biofilm formation in an ica-independent way (Foster et al. Citation2014; Schilcher and Horswill Citation2020). Meanwhile, S. aureus can also utilize biofilm-associated proteins (Baps), which self-assemble into functional amyloid aggregates, to build biofilm under acidic culture conditions (Taglialegna et al. Citation2016). In addition, there has been increasing attention on the roles of eDNA during biofilm formation. Produced by active secretion or controlled cell lysis, eDNA promotes bacterial adhesion and maintains the structural stability of biofilms (Okshevsky and Meyer Citation2015). Moreover, Gloag et al. (Citation2013) demonstrated that eDNA could mediate the twitching motility of P. aeruginosa and facilitate the self-organization of biofilm.
2.1.3. Environmental factors
Biofilm formation is considered a strategy adopted by bacteria to allow their adaption to changing environments. In turn, the external environment can also affect or even regulate the biofilm formation process. The common environmental factors, basically, can be grouped into three major categories: physical factors, chemical factors and biological factors () (Dwyer, Kohanski, and Collins Citation2009; Gu et al. Citation2021; Kim et al. Citation2020; Persat et al. Citation2015; Plate and Marletta Citation2013; Xu et al. Citation2019; Pu et al. Citation2021). Some of these factors have been identified, while many remain unstudied. Here, the influence of environmental stresses and physical factors on biofilm formation is discussed, as they are more common in the food and biomedicine industry.
Biofilm is considered a successful way for bacteria to survive various stresses (Yin et al. Citation2019). For instance, in P. aeruginosa, increased biofilm formation and altered biofilm structure at temperatures lower than 25 °C were reported, followed by temperature-controlled exopolysaccharide biosynthesis (Kim et al. Citation2020). It was also found that after cold stress, surface adhesion and biofilm formation enhanced in Listeria monocytogenes (L. monocytogenes) and S. aureus (Lee, Hébraud, and Bernardi Citation2017; Qiao et al. Citation2021). In S. aureus, the enhanced biofilm formation after cold stress was ascribed to the increased polysaccharide content in the matrix, as well as hydrophobicity and the adhesion proteins (Qiao et al. Citation2021). Another example of the effects of environmental stress on biofilm formation is oxidative stress. It has been identified that antibiotics-induced oxidative stress plays a significant role in bacterial cell death as well as resistance development (Dwyer, Kohanski, and Collins Citation2009). Biofilm formation can be considered a common strategy for bacteria to enhance their resistance toward oxidative stress. In Campylobacter jejuni, the stimulated biofilm formation by Ferrous and Ferric could be attributed to oxidative stress (Oh, Andrews, and Jeon Citation2018). In Azotobacter vinelandii, chronic sub-lethal oxidative stress induced the increased production of polysaccharide-rich EPS and lower susceptibility to hydrogen peroxide, promoting sessile behavior and biofilm formation (Villa et al. Citation2012).
Unlike the well-established understanding of molecular mechanisms under biofilm formation, the influence of the physical environment is just starting to emerge. Fluid flow and surface properties are two ubiquitous external effects during biofilm formation (Persat et al. Citation2015). The process of surface attachment, which is considered the first step for biofilm establishment, is highly dependent on the characteristics of abiotic or biotic surfaces, such as their smoothness and hydrophilicity (Yin et al. Citation2021). Meanwhile, surface topographies can also mediate microbial attachment (Lee et al. Citation2021). It has been reported that L. monocytogenes biofilm formation on stainless steel 304 could be significantly affected by surface topography and composition (Gu et al. Citation2021). For surface attachment, a challenging question is how biofilm cells adapt to different contact surfaces. By comparative proteomics, Guo et al. (Citation2020) revealed the physiological responses of Vibrio parahaemolyticus (V. parahaemolyticus) biofilm to different abiotic surfaces (stainless steel, glass and polystyrene). It was suggested that the adaption behavior of biofilm cells on different surfaces was affected by metal ion stress, nutrition, osmotic stress, and sugar utilization.
As current research about the effects of surface properties mainly focuses on flat surface models, it is hard to describe the biofilm formation process on non-planar surfaces only based on common understanding. Recent findings revealed that the colonization of P. aeruginosa and E. coli on curve surfaces was largely controlled by flow, indicating the participation of fluid flow in early-stage biofilm establishment (Secchi et al. Citation2020). Due to the deflection of the trajectories of swimming bacteria by the flow, attachment location and rate mainly depended on the interplay between local flow and bacterial motility. Additionally, it was also reported that the balance between motility and flow promoted the emergence of morphological patterns in Caulobacter crescentus biofilms, suggesting the influence of hydrodynamics on species interaction and evolution within surface-associated communities (Rossy, Nadell, and Persat Citation2019).
2.2. Regulatory networks
2.2.1. TCSs
For bacteria, a common regulatory mode to sense and respond to environmental signals is TCS, which, in the simplest form, consists of a sensor histidine kinase (HK) and a response regulator (RR) (). The fluctuant environmental signal, once sensed by HK, is relayed by phosphoryl group transfer from histidine domain of HK to conserved aspartic residue in receiver domain of RR (Liu et al. Citation2018). However, in reality, the signal transduction system of bacteria can be more complicated, as most of them consist of multiple components and hybrid kinase-response regulators (Francis and Porter Citation2019).
Figure 2. Regulatory networks for biofilm formation. A signal transduction in the TCS system. B metabolic pathway of c-di-GMP. C cell-density-dependent regulatory mode of QS. D roles of sRNA in transcriptional regulation.
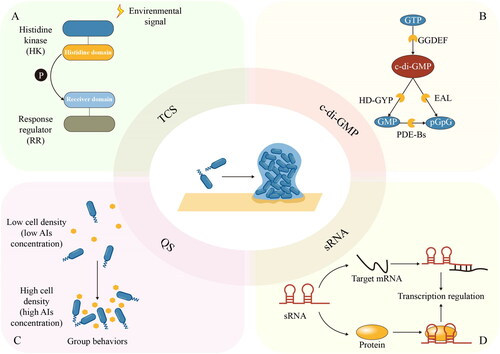
TCSs are widely involved in the regulation of biofilm formation in many species, as a bridge connecting extracellular stimuli and intracellular response (Aslan et al. Citation2021; Kong et al. Citation2015). For instance, YycFG (also designated as WalRK) TCS in S. aureus is well-known for its participation in biofilm formation. The membrane-anchored HK YycG/WalK could monitor and respond to extracellular changes by phosphoryl group transfer, while the activated RR YycF specifically bound to promoter regions including icaA, agr, sarA and sarX, modifying the expression of downstream target genes, which promoted biofilm formation in an ica-dependent manner (Wu et al. Citation2021).
2.2.2. Second messengers
Cyclic dinucleotides are recognized as second messengers involved in important biological processes and behaviors of bacteria (Jenal, Reinders, and Lori Citation2017). These signal molecules can interact with target molecules (e.g., proteins and RNA) to adjust bacterial behaviors in a certain environment (Stülke and Krüger Citation2020). The best-studied example is bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP), one of the most common bacterial second messengers, especially in Gram-negative bacteria. The biochemistry of c-di-GMP synthesis, degradation and binding has been elaborated on in a previous review (Römling, Galperin, and Gomelsky Citation2013). In simplest terms, enzymes involved in c-di-GMP metabolic can be identified by GGDEF, EAL, and GD-GYP domains (). GGDEF domain proteins possess diguanylate cyclases (DGCs) activity, synthesizing c-di-GMP from 2 molecules of GTP. By comparison, EAL domain proteins function in phosphodiesterases (PDEs), degrading c-di-GMP into 5′-phosphoguanylyl-(3′-5′)-guanosine (pGpG) dinucleotide, which is subsequently broken down into two molecules of GMP by PDE-Bs. Besides, c-di-GMP can also be directly hydrolyzed into GMPs by the HD-GYP domain-containing PDEs (Liu, Huang, et al. Citation2022).
C-di-GMP has been recognized as the key regulator of bacterial lifestyle. Modulation of the c-di-GMP metabolic process allows bacteria to switch between motile and sessile phenotypes, mediating the formation and dispersal of biofilms for adaptation to environmental stress and signals (Jenal, Reinders, and Lori Citation2017). A typical example is bacterial surface adaption regulated by c-di-GMP. It has been shown that when bacteria were exposed to surfaces, c-di-GMP orchestrated the process by regulating pili and flagella and promoting the production of adhesin and EPS (Laventie and Jenal Citation2020). In P. aeruginosa, the activity of the DGC SadC could be modulated by the T4P alignment complex protein (PilO). Webster et al. (Citation2021) proposed that in the planktonic mode of life, PilO could interact with SadC and suppress its activity, resulting in decreased biofilm formation and increased motility. Once cells adhered to the surface, however, the PilO-SadC interaction was reduced, relieving repression of SadC activity. The interaction between SadC and flagellar motor (MotC) promoted biofilm formation and inhibited motility. This novel model suggested that DGC SadC could act as a bridge to integrate surface-derived input signals from both T4P and flagella and mediate c-di-GMP regulation on bacteria phenotypes.
2.2.3. QS
QS is a bacterial cell-cell communication process based on production, detection and response to extracellular signaling molecules named autoinducers (AIs), enabling bacteria to coordinate their activities according to changes in the cell density (Whiteley, Diggle, and Greenberg Citation2017). In the QS circuit, AIs are produced by members of the community and accumulate locally in the environment with an increase in population density. Once the concentration of AIs exceeds a certain threshold, these signal molecules will be detected by receptors and the community members will collectively coordinate their activities by regulating the expression of target genes (). The QS systems are different between Gram-positive and Gram-negative bacteria. Gram-positive bacteria use autoinducing peptides (AIPs) and transmembrane two-component HKs or cytoplasmic AIP-binding transcription factors (Rutherford and Bassler Citation2012). Gram-negative bacteria use acyl-homoserine lactones (AHLs) or other small molecules and LuxI/LuxR-type systems or TCSs (Papenfort and Bassler Citation2016). It was also reported that P. aeruginosa could package Pseudomonas quinolone signal (PQS) with outer membrane vesicles, which promoted the growth of biofilm through changes in cell morphology, structure and components of EPS in biofilm formed by P. aeruginosa, and enhanced the inhibitory effect of PQS to the growth of S. aureus, resulting in decreased EPS production in the dual-species biofilm formed by P. aeruginosa and S. aureus (Zhao et al. Citation2022).
Bacterial community behaviors, which are shown during the biofilm formation process, largely rely on the regulation of QS. Although QS systems vary among different species present in biofilms, the link between QS and biofilm formation mainly depends on environmental conditions, like fluid flow and surface topography (Mukherjee and Bassler Citation2019). In the case of the LasR system in P. aeruginosa, it was investigated that surface-associated bacteria were more sensitive to AIs than planktonic cells, due to surface-dependent lasR induction initiating a positive feedback loop through the sRNA Lrs1 as well as the involvement of T4P retraction motors and the minor pilins (Chuang et al. Citation2019).
2.2.4. sRNA
While proteins have been widely studied as common regulators, RNA-mediated regulation is an emerging field of bacterial regulatory networks. With higher sensitivity and reduced metabolic cost, sRNA was found to conduct some signal transduction and regulation processes and thus provided an additional regulatory mode in response to environmental signals (Beisel and Storz Citation2010). As an important posttranscriptional regulator, sRNA is integrated into the complicated regulatory networks to participate in the regulation of biofilm development (Nitzan, Rehani, and Margalit Citation2017). Typically 50 to 400 nucleotides in length, sRNAs can be divided into two groups according to their mode of function (): mRNA-binding sRNAs, such as trans-encoded sRNAs and antisense RNAs (asRNAs), and protein-binding sRNA (Storz, Vogel, and Wassarman Citation2011). Most sRNAs are trans-encoded sRNAs. These sRNAs function in the transcription process by short, imperfect base pairing with their target mRNAs, facilitated by RNA chaperone Hfq. Furthermore, asRNAs, which are encoded on the opposite strand of established coding sequences, can also impact translation and mRNA stability. Conversely, other sRNA regulators act by binding specific proteins and modifying protein activity.
It has been suggested that during biofilm formation, the sRNA regulator is essential for the transition from planktonic cells to sessile cells. Based on sRNA, the regulatory networks enable concentration-specific responses to environmental cues through regulatory proteins, or by directly affecting the synthesis of proteins promoting or disfavoring the formation of biofilms. During the stationary phase of E. coli, the 3′ UTR-derived sRNA FimR2 is processed from the fimAICDFGH mRNA transcript by RNase E, facilitating biofilm formation for survival under nutrient-depleted conditions (Raad et al. Citation2022). On the one hand, FimR2 interacts with the translational regulator CsrA and antagonizes its effects, downregulating type I pilus and flagellar biosynthesis. On the other hand, FimR2 fine-tunes target mRNA levels through direct base-pairing, inhibiting flagellar synthesis.
2.2.5. Connected regulatory networks during biofilm formation
Biofilm formation is a highly complicated and variable process controlled by a series of highly connected regulatory networks encoded within the bacterial genome. The close correlation between different networks can help to efficiently integrate information in multiple pathways. A typical example is the participation of TCS in regulating intracellular c-di-GMP levels. In the instance of biofilm formation regulation by carbon source, sodium lactate was reported to negatively regulate biofilm formation in Shewanella putrefaciens CN32 via a three-component regulatory system (LrbS-LrbA-LrbR) consisting of one KK (LrbS) and two cognate RRs (LrbA and LrbR) (Liu et al. Citation2017). LrbS could respond to the signal of sodium lactate and subsequently activate transcription factor LrbA, which then promoted the expression of lrbR. As PDE contains an EAL domain, LrbR decreased the intracellular c-di-GMP level, thereby negatively regulating biofilm formation. Besides, QS is also involved in c-di-GMP regulation. In V. parahaemolyticus, OpaR is the master QS regulator under high cell density. Zhang et al. (Citation2021) demonstrated that OpaR directly regulated the transcription of scrA, scrG, VP0117, VPA0198, VPA1176, VP0699, and VP2979, encoding a group of GGDEF and/or EAL-type proteins, decreasing the intracellular concentration of c-di-GMP, and thus repressing biofilm formation. Similarly, in Acidithiobacillus thiooxidans, AHLs were demonstrated to positively regulate the c-di-GMP effector PelD encoding gene (Díaz et al. Citation2021).
Vibrio cholera (V. cholera) has been widely studied as a model organism for understanding biofilm formation and the regulatory networks underlying this process (Teschler et al. Citation2015). In many Vibrios, QS relies on post-transcriptional gene regulation by sRNAs called quorum regulatory RNA (Qrr) (Lenz et al. Citation2004). Huber et al. (Citation2022) proposed a regulatory model for QS-mediated gene expression control in V. cholera, identifying the QrrX sponge RNA as a key component of V. cholera QS architecture. It was suggested that CqsS, LuxPQ, CqsR, and VpsS, as QS-specific receptors, could respond to external AIs and modulate the phosphorylation status of the LuxO transcription factor via the LuxU phosphorelay protein. At low cell densities, LuxO was phosphorylated, activating Qrr1-4 expression. Then Qrr1-4 reduced the translation of luxO and inhibited the hapR mRNA, which encoded the negative regulator of biofilm formation, HapR. While Qrr2-4 activated AphA expression, which could antagonize HapR activity and promote biofilm formation. At high cell density, however, QrrT induced the transcription of the QrrX sponge RNA, which, together with Hfq, could base-pair with and sequester Qrr1-4 sRNAs, and therefore facilitated RNase E-mediated decay.
3. Innovating regulatory strategies for biofilm formation
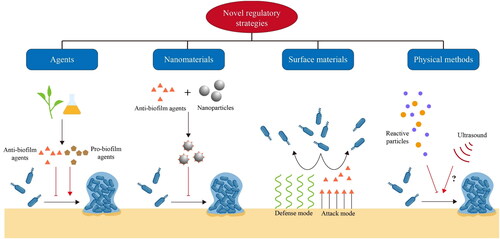
With robust construction and high tolerance toward harsh environments, eradication and inactivation of mature biofilms are getting increasingly challenging in food and biomedicine areas. Given the close relationship between bacterial regulatory networks and the biofilm formation process, regulation of early-stage biofilm establishment would be a highly possible alternative for biofilm control. Moreover, by precise regulation of the biofilm formation process, both prevention of biofilm and large-scale production of biofilm-related microbial products can be realized (). On the basis of the biofilm formation process and related regulatory networks, the application of anti/pro-biofilm agents, nanomaterials, surface coating and modification as well as physical methods have made regulatory strategies more efficient and effective ().
Table 1. Emerging strategies for regulating biofilm formation in food and biomedicine.
3.1. Anti/pro-biofilm agents for regulation of biofilm formation
The development and spread of antibiotic resistance have become a global problem. As mature biofilms always possess higher resistance against conventional antibiotics, exploitation of novel anti-biofilm agents for biofilm inhibition has become an important future direction. On the other hand, some newly found pro-biofilm agents can be applied to activate the establishment of commensal or beneficial biofilm (Jiang et al. Citation2023). Targeting the biofilm formation process, regulatory networks and crucial metabolic pathways, a large number of candidates have been designed, synthesized, or found as natural compounds for the regulation of biofilm formation () (Kang et al. Citation2020; Pompilio et al. Citation2023; Qu et al. Citation2020).
A key regulatory hub for biofilm formation is QS. QS inhibitors, as novel agents for anti-biofilm and anti-virulence therapy, have been intensively studied for inhibition of AIs synthesis, inactivation or enzymatic degradation of AIs, AIs’ antagonists and blocking of signal transduction cascades (Paluch et al. Citation2020). Recently, natural compounds have received researchers’ attention as a safe and effective way for biofilm inhibition. Methyl anthranilate, a plant spice extract, was found to down-regulate the expression levels of QS-related genes and inhibit the QS-regulated phenotypes in Aeromonas sobria (A. sobria) by competitively binding to receptors and interfering with AHL biosynthesis (Li et al. Citation2020). Similarly, sodium butyrate, which is widely used as a flavoring agent and dietary supplement for health, has been reported to inhibit biofilm formation at sub-inhibitory concentrations by interfering with the AHL QS system in Chromobacterium violaceum and AI-2 QS system in Vibrio harveyi (Zhu et al. Citation2022). Furthermore, it was found that some QS inhibitors could also enhance the susceptibility of biofilm toward conventional antibiotics (Zhou et al. Citation2018). However, although QS inhibitors have displayed potent regulatory functions for biofilm formation, reliable identification of newly discovered inhibitors as well as deeper investigation of QS-inhibitor-resistant mutants are still lacking (Defoirdt Citation2018).
Given the well-studied participation of c-di-GMP in bacterial phenotype switching, interfering with c-di-GMP metabolism and signaling has become a potential approach for the development of regulatory agents for early-stage biofilm formation (Liu, Cao, et al. Citation2022). The majority of c-di-GMP targeted strategies focus on the development of inhibitors of DGCs rather than PDEs, owing to the old view that inhibiting any c-di-GMP PDE would lead to biofilm formation (Zheng et al. Citation2016). However, Kim et al. (Citation2023) found that citrus peel extract from Jeju island concentration-dependently inhibited biofilm formation and induced biofilm dispersion by reducing intracellular concentration of c-di-GMP through increased PDE activity. With the addition of citrus peel extract in different concentrations (0%–0.05%), P. aeruginosa biofilm formation under static conditions was significantly reduced by 13%–71% in a concentration-dependent manner without inhibition of bacterial growth. Similarly, under continuous flow conditions, P. aeruginosa biofilm developed without and with 0.0002%–0.02% citrus peel extract treatments showed significant differences in volume and thickness. This naturally derived extract provided an alternative way for the regulation of biofilm formation targeting c-di-GMP metabolism.
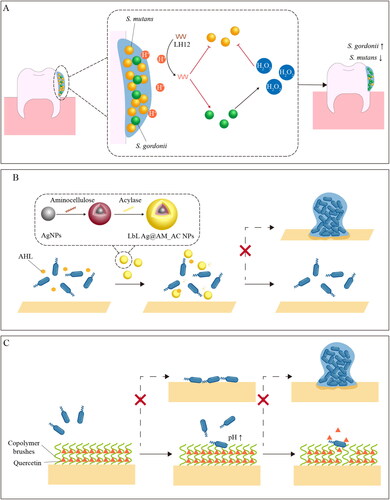
Not all the agents have specific targets for biofilm regulation. In fact, many of the newly reported agents, especially natural compounds derived from plants or microbes, still lack in-depth study on their regulatory mechanisms. Meanwhile, it was also found that some regulatory agents showed their regulatory function targeting multiple pathways, such as ferulic acid. This ubiquitous natural phenolic constituent was demonstrated to inhibit cell adhesion and biofilm formation of Shigella flexneri (S. flexneri) targeting cell membrane, transcription of adhesion-related genes as well as EPS synthesis and transportation (Kang et al. Citation2020). Moreover, some regulatory agents can simultaneously realize inhibition of harmful biofilm and activation of beneficial biofilm in multi-species biofilm models. Utilizing the pH-sensitivity of histidine, Jiang et al. (Citation2023) designed a pH-responsive and dual-functional antimicrobial peptide, LH12, for the regulation of microbial communities containing both cariogenic bacteria (Streptococcus mutans) and commensal bacteria (Streptococcus gordonii). Once activated by the acidic microenvironment, LH12 showed the ability to inhibit biofilm formation in Streptococcus mutans and improve the ecological competitiveness of Streptococcus gordonii by promoting the production of H2O2, providing a bio-responsive approach for caries management ().
Figure 4. Schematic illustration of strategies for regulating biofilm formation. A dual-functional LH12 for regulation of oral biofilm (Jiang et al. Citation2023). B inhibition of P. aeruginosa biofilm formation by LbL Ag@AM_AC NPs. (Ivanova et al. Citation2020). C dual-functional surface for prevention of biofilm formation (Zou et al. Citation2021).
3.2. Nanomaterials for regulation of biofilm formation
The rapid development of nanotechnology has broadened the application of nanomaterials in biosensing platform establishment, drug delivery, disease therapy, and other aspects of food and biomedicine areas (Hassan et al. Citation2017; He, Deng, and Hwang Citation2019; Liang et al. Citation2020; Pu et al. Citation2021; Zhang et al. Citation2021). As a promising type of antimicrobial, nanomaterials have been widely used for microbial inactivation and biofilm control (Ndayishimiye et al. Citation2022; Zhao, Chen, et al. Citation2021). In most nano-based methods for biofilm eradication, the EPS matrix, which reduces the permeability and enrichment of therapeutic agents inside mature biofilms, is the most discussed target (Lv et al. Citation2023). In contrast, for regulatory strategies, the key is to inhibit initial attachment and EPS production by disrupting the regulatory network and group behaviors. Recently, the combination of nanomaterials with anti-biofilm agents has been adopted as an effective strategy to enhance the effectiveness of anti-biofilm agents, control drug release, increase permeability and endow responsive properties toward certain microenvironments () (Badawy et al. Citation2020; Liu et al. Citation2020; Sarveswari et al. Citation2023; Huang, Pu, and Sun Citation2023).
The broad-spectrum anti-bacterial and anti-biofilm activities of metal-based nanomaterials, such as silver nanoparticles (AgNPs) and zinc oxide nanoparticles (ZnONPs), have been demonstrated (Dai et al. Citation2017; Lee et al. Citation2014). With synergic biofilm inhibitory effect, nanocomposites of anti-biofilm agents and metal-based nanoparticles have been proposed for augmenting anti-biofilm activity. Based on the QS inhibitory activity of tannic acid and the anti-bacterial activity of AgNPs, the nanocomposite of tannic acid and silver nanoparticles (Tannin-AgNPs) showed the ability to modulate the biofilm formation process and decrease the production of AIs (Liu et al. Citation2020). Similarly, it was also reported that chitosan-zinc oxide (CH/ZnO) nanocomposite enhanced the effect of chitosan on decreasing AHL production, biofilm formation and motility of P. aeruginosa (Badawy et al. Citation2020). Furthermore, taking advantage of quorum quenching enzyme acylase and membrane-disrupting biopolymer amino cellulose, Ivanova et al. (Citation2020) decorated AgNPs templates in a layer-by-layer fashion to obtain hybrid enzyme/biopolymer/metal NPs (LbL Ag@AM_AC NPs) with enhanced anti-bacterial and anti-biofilm activities, as well as decreased toxicity to mammalian cells. By degrading the AHL signaling molecules, the hybrid nanoparticles were able to inhibit the QS-regulated biofilm formation by P. aeruginosa at lower concentrations than the AgNP templates (). However, for these proposed nanomaterials, assessment of the long-term effects on human health and environmental safety is still required, which is considered one of the major barriers to application in food and biomedical use.
3.3. Functionalized surface materials for regulation of biofilm formation
As surface properties can significantly impact initial attachment, surface coating and surface modification have been widely used to diminish the formation of early-stage biofilms (Yin et al. Citation2021). Anti-biofilm surfaces can be generally divided into two modes: the “attack” mode and the “defence” mode (Ma et al. Citation2022). In the “attack” mode, antimicrobial agents such as AgNPs, enzymes, and peptides are attached to the surface to efficiently kill the bacteria for prevention of cell adhesion and colonization. In the “defence” mode, strategies such as anti-adhesive coatings based on polymer brushes or highly hydrated hydrogel networks have been widely applied to resist the initial attachment or release the previously attached cells into aqueous media (Ahmadabadi, Yu, and Kizhakkedathu Citation2020). Based on the conventional anti-biofilm surfaces, a new class of functionalized surface materials are emerging for the regulation of biofilm formation. Similar to the already proposed approaches, most of these materials also adopt the “attack” or “defence” modes for biofilm inhibition (). However, rather than directly inactivating the bacteria or resisting the attachment stage, the newly developed materials are designed to modulate bacterial behaviors related to biofilm establishment (Hu et al. Citation2022; Vaishampayan et al. Citation2018; Zou et al. Citation2021). For instance, Vaishampayan et al. (Citation2018) developed a novel surface coating AGXX® consisting of micro-galvanic elements of silver and ruthenium. Compared with conventional antimicrobial silver coating, AGXX® was demonstrated to down-regulate many biofilm-associated genes and the two-component agr QS system in Methicillin-resistant S. aureus (MRSA), suggesting that it might diminish biofilm formation by interfering with QS system.
Besides metal-based coating materials, signaling molecules are also applied in surface coating for biofilm regulation. Using click chemistry, Kim et al. (Citation2017) covalently attached AIP-I (pro-QS molecule) and TrAIP-II (anti-QS molecule) to surfaces, realizing control of the agr-I system and biofilm development in S. aureus. Nitric oxide (NO), a well-established signaling molecule, has been demonstrated to participate in the regulation of communal behaviors by increasing c-di-GMP hydrolysis and influencing the QS system (Plate and Marletta Citation2013). Based on the regulatory function of NO, Woehlk et al. (Citation2019) introduced a NO-functionalized catecholamine as a polymerisable coating agent to spatially control biofilm formation on a variety of surfaces. The prepared NO-coated surfaces completely suppressed surface colonization of P. aeruginosa, providing a bioinspired and versatile coating approach to reduce biofilm contamination and biofilm-related infections in the hospital. Meanwhile, recent advances in nanomaterials also supplied an unconventional option for regulating biofilm formation. With fiber size and pore size close to Lactobacillus paracasei, electrospun cellulose acetate nanofibrous membranes were shown to be powerful biofilm-enriching scaffolds (Hu et al. Citation2022). Compared to planktonic cells, residents embedded in biofilms showed superior pH and antibiotic tolerance with genes related to cell adhesion, biofilm formation and tolerance expressed differently.
Dual-functional surfaces are hypothesized to largely prevent initial attachment and biofilm development by integrating the advantages of “attack” and “defence” modes. Focusing on the initial and middle stages of biofilm formation, Zou et al. (Citation2021) developed a dual-functional surface based on surface-tethered copolymer brushes of 2-hydroxyethyl methacrylate (HEMA) and 3-(acrylamide)phenylboronic acid (APBA). With anti-adhesion properties, the poly(HEMA) component enabled the surface to inhibit cell adhesion during the initial stages of biofilm formation, while the poly(APBA) component provided binding sites for attachment and pH-responsive release of quercetin (a natural anti-biofilm molecule). Compared with anti-bacterial and anti-adhesive surfaces, this dual-functional surface has significantly improved anti-biofilm performance to prevent biofilm formation involving both Gram-negative P. aeruginosa and Gram-positive S. aureus for up to 3 days.
The development of functionalized anti-biofilm surfaces, especially the stimuli-responsive surfaces, has provided new ideas for the prevention of biofouling and contamination of implants. Unfortunately, the limitations for practical applications also exist. For medical implants, surface functionalization can be easily realized, as the surface area is relatively small. But for the surfaces of pipes and production equipment, cost and operability should be taken into account. Meanwhile, as biofilm formation is a long-term process, evaluation of anti-biofilm capacity and potential toxicity in real-world application scenarios rather than laboratory is requisite.
3.4. Physical strategies for regulation of biofilm formation
In comparison with anti-biofilm agents and anti-biofilm surfaces, physical strategies, such as ultrasound, electric field and cold plasma, are regarded as a more eco-friendly solution for biofilm control and removal (Liu et al. Citation2022; Pan, Sun, and Han Citation2017; Pan et al. Citation2020; Esua, Cheng, and Sun Citation2021; Esua et al. Citation2022). Current physical strategies mainly focus on the eradication of biofilms (Gilmore et al. Citation2018; Josic et al. Citation2022). Many in-depth studies also partially revealed the mechanisms of anti-biofilm activity of these strategies (Erriu et al. Citation2014; Mai-Prochnow et al. Citation2021). Alternatively, the idea of applying physical strategies in regulating biofilm formation is still at the starting stage. A prominent characteristic of these strategies is the multiple regulatory functions arising from the various modes of action toward planktonic cells and biofilm. Based on the generation of reactive oxygen species (ROS), reactive nitrogen species (RNS) and other active particles during plasma discharge, cold plasma has exhibited great potential in biofilm inhibition and prevention through surface treatment and direct interaction with planktonic bacteria (Hage et al. Citation2022; Li et al. Citation2019; Pan et al. Citation2019; Esua, Cheng, and Sun Citation2020; Zhao et al. Citation2020a, Citation2020b, Citation2021; Zhao et al. Citation2023). Among the multiple active species, the possible roles of ROS as biofilm inhibitors and signaling molecules during biofilm development have been suggested (Čáp, Váchová, and Palková Citation2012; Pan, Cheng, and Sun Citation2021; Zhao et al. Citation2020). Li et al. (Citation2019) evaluated the feasibilities and mechanisms of ultra-low dose ROS produced by plasma-activated water (PAW) to inhibit the Enterococcus faecalis (E. faecalis) biofilm development. It was shown that the ultra-low dose ROS treatment within 30 s effectively inhibited biofilm formation without lethal effect. The possible mechanisms mainly involved interfering with the QS system by down-regulating the related genes and decreasing the membrane potential.
Similar to cold plasma, other physical strategies also showed the ability to regulate the biofilm formation process. Qi et al. (Citation2022) proposed a high voltage prick electrostatic field (HVPEF) as a novel method to inhibit biofilm formation in S. aureus. The suppressed biofilm formation was realized by the direct effect of HVPEF on planktonic cells, the reduced release of EPS components and regulated expression of biofilm-related genes. Conversely, recent studies suggested that low-power ultrasound might stimulate biofilm formation and improve the stability of the established biofilm, indicating the application potential in the modulation of probiotics in food and other related products (Bevilacqua et al. Citation2019). Meanwhile, it also indicated that insufficiently high power of ultrasound treatment could lead to an unexpected increase in pathogenic biofilm formation (Yu et al. Citation2021).
4. Conclusions and future trends
Biofilm has posed challenges for food safety and medical services, as well as tools for the production of biomaterials and probiotic products. Biofilm formation is a dynamic process with a transition from planktonic to community lifestyle in both phenotype and genotype. Although the underlying mechanisms are still not completely known, the review of surface appendages and EPS components suggests the participation of cellular and extracellular factors in early-stage biofilm establishment. The effects of environmental factors such as cold stress, oxidative stress, surface topographies and flow on biofilm formation are also discussed. However, current knowledge of these factors is mainly based on studies about single-species biofilm models in the laboratory, which are not suitable to mimic the biofilm formation process in real-world systems. Therefore, simulation and experiments of the biofilm development process in the industry and other open systems still require further exploration.
Regulation of biofilm formation is an active event driven by a series of complicated regulatory networks, involving TCSs, c-di-GMP, sRNAs and QS. Various models have been established to illustrate the function of a single regulator during the biofilm formation process. Moreover, it has been shown that these regulators are highly correlated, enabling bacteria to sense and respond to the changeable external environment through different pathways, integrate multiple signals, and then regulate their behaviors associated with biofilm formation and development. In order to reveal the regulatory mechanism of biofilm establishment, further studies on the synergetic effects of different regulatory networks should be carried out.
The current understanding of biofilm formation and regulation facilitates the exploitation of regulatory strategies as an alternative approach for biofilm control in food and biomedicine. Different from the conventional views which prefer to eliminate mature biofilm or inactivate the bacteria to prevent biofilm formation, the novel concept of regulation aims to inhibit the ability of planktonic bacteria to establish biofilms through modulating the biofilm formation process. On the other hand, by stimulating biofilm establishment in commensal and engineering bacteria, regulatory strategies can also promote the fermentation process and production of probiotics. Based on the progress in anti/pro-biofilm agents, nanomaterials, surface materials and physical methods, novel strategies for the regulation of biofilm formation have contributed to effective biofilm control in an effective, smart, safe and eco-friendly way. The advantages, limitations and suggested application of the proposed strategies are summarized in . Despite the promising results obtained with these methods in laboratory studies, the standard methodology for regulating biofilm formation in practical use is still missing. For some of these regulatory strategies, the precise regulation mechanisms are still unclear. Meanwhile, most of the proposed strategies are designed for specific single-species models, while their effect on multi-species biofilm and other open systems remains to be verified. Furthermore, in real-world systems, cost, operability, potential toxicity and long-term functional durability should also be evaluated. Therefore, it would require interdisciplinary communication and collaboration among chemists, microbiologists, and engineers to generate a standard methodology for the regulation of biofilm formation and realize the practical application in food, biomedicine, and other related areas.
Table 2. Advantages, limitations and suggested application of innovating strategies for regulating biofilm formation.
| Abbreviation | ||
| EPS | = | Extracellular polymeric substances |
| TCS | = | Two-component system |
| sRNA | = | Small non-coding RNA |
| QS | = | Quorum sensing |
| eDNA | = | Extracellular DNA |
| P. aeruginosa | = | Pseudomonas aeruginosa |
| S. aureus | = | Staphylococcus aureus |
| E. coli | = | Escherichia coli |
| PIA | = | Polysaccharide intercellular adhesion |
| CWA proteins | = | Cell wall-anchored proteins |
| Baps | = | Biofilm-associated proteins |
| L. monocytogenes | = | Listeria monocytogenes |
| V. parahaemolyticus | = | Vibrio parahaemolyticus |
| HK | = | Histidine kinase |
| RR | = | Response regulator |
| c-di-GMP | = | Bis-(3’-5’)-cyclic dimeric guanosine monophosphate |
| DGC | = | Diguanylate cyclase |
| PDE | = | Phosphodiesterase |
| pGpG | = | 5’-phosphoguanylyl-(3’-5’)-guanosine |
| AI | = | Autoinducer |
| AIP | = | Autoinducing peptide |
| AHL | = | Acyl-homoserine lactone |
| V. cholera | = | Vibrio cholera |
| Qrr | = | Quorum regulatory RNA |
| A. sobria | = | Aeromonas sobria |
| S. flexneri | = | Shigella flexneri |
| AgNPs | = | Silver nanoparticles |
| ZnONPs | = | Zinc oxide nanoparticles |
| MRSA | = | Methicillin-resistant Staphylococcus aureus |
| ROS | = | Reactive oxygen species |
| RNS | = | Reactive nitrogen species |
| E. faecalis | = | Enterococcus faecalis |
| PAW | = | Plasma-activated water |
| HVPEF | = | High voltage prick electrostatic field |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmadabadi, H. Y., K. Yu, and J. N. Kizhakkedathu. 2020. Surface modification approaches for prevention of implant associated infections. Colloids and Surfaces. B, Biointerfaces 193:111116. doi:10.1016/j.colsurfb.2020.111116.
- Aslan, H., M. E. Petersen, A. De Berardinis, M. Zacho Brunhede, N. Khan, A. Vergara, B. Kallipolitis, and R. L. Meyer. 2021. Activation of the two-component system LisRK promotes cell adhesion and high ampicillin tolerance in Listeria monocytogenes. Frontiers in Microbiology 12:618174. doi:10.3389/fmicb.2021.618174.
- Badawy, M., O. K. M. Riad, F. A. Taher, and S. A. Zaki. 2020. Chitosan and chitosan-zinc oxide nanocomposite inhibit expression of LasI and RhlI genes and quorum sensing dependent virulence factors of Pseudomonas aeruginosa. International Journal of Biological Macromolecules 149:1109–17. doi:10.1016/j.ijbiomac.2020.02.019.
- Beaussart, A., A. E. Baker, S. L. Kuchma, S. El-Kirat-Chatel, G. A. O’Toole, and Y. F. Dufrêne. 2014. Nanoscale adhesion forces of Pseudomonas aeruginosa type IV Pili. ACS Nano 8 (10):10723–33. doi:10.1021/nn5044383.
- Beisel, C. L., and G. Storz. 2010. Base pairing small RNAs and their roles in global regulatory networks. FEMS Microbiology Reviews 34 (5):866–82. doi:10.1111/j.1574-6976.2010.00241.x.
- Bevilacqua, A., A. Racioppo, M. Sinigaglia, B. Speranza, D. Campaniello, and M. R. Corbo. 2019. A low-power ultrasound attenuation improves the stability of biofilm and hydrophobicity of Propionibacterium freudenreichii subsp. freudenreichii DSM 20271 and Acidipropionibacterium jensenii DSM 20535. Food Microbiology 78:104–9. doi:10.1016/j.fm.2018.10.010.
- Čáp, M., L. Váchová, and Z. Palková. 2012. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative Medicine and Cellular Longevity 2012:1–13. doi:10.1155/2012/976753.
- Chuang, S. K., G. D. Vrla, K. S. Fröhlich, and Z. Gitai. 2019. Surface association sensitizes Pseudomonas aeruginosa to quorum sensing. Nature Communications 10 (1):4118. doi:10.1038/s41467-019-12153-1.
- Colvin, K. M., Y. Irie, C. S. Tart, R. Urbano, J. C. Whitney, C. Ryder, P. L. Howell, D. J. Wozniak, and M. R. Parsek. 2012. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environmental Microbiology 14 (8):1913–28. doi:10.1111/j.1462-2920.2011.02657.x.
- Condinho, M., B. Carvalho, A. Cruz, S. N. Pinto, C. M. Arraiano, and V. Pobre. 2023. The role of RNA regulators, quorum sensing and c-di-GMP in bacterial biofilm formation. FEBS Open Bio. 13 (6):975–91. doi:10.1002/2211-5463.13389.
- Dai, X.-M., X.-L. Chen, J. Zhao, Y. Zhao, Q.-Q. Guo, T.-Q. Zhang, C.-L. Chu, X.-G. Zhang, and C.-X. Li. 2017. Structure-activity relationship of membrane-targeting cationic ligands on a silver nanoparticle surface in an antibiotic-resistant antibacterial and antibiofilm activity assay. ACS Applied Materials & Interfaces 9 (16):13837–48. doi:10.1021/acsami.6b15821.
- Danne, C., and S. Dramsi. 2012. Pili of gram-positive bacteria: Roles in host colonization. Research in Microbiology 163 (9–10):645–58. doi:10.1016/j.resmic.2012.10.012.
- Das, S. 2022. Genetic regulation, biosynthesis and applications of extracellular polysaccharides of the biofilm matrix of bacteria. Carbohydrate Polymers. 291, 119536. doi:10.1016/j.carbpol.2022.119536.
- Defoirdt, T. 2018. Quorum-sensing systems as targets for antivirulence therapy. Trends in Microbiology 26 (4):313–28. doi:10.1016/j.tim.2017.10.005.
- Desrousseaux, C., V. Sautou, S. Descamps, and O. Traoré. 2013. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. The Journal of Hospital Infection 85 (2):87–93. doi:10.1016/j.jhin.2013.06.015.
- Díaz, M., D. San Martin, M. Castro, M. Vera, and N. Guiliani. 2021. Quorum sensing signaling molecules positively regulate c-di-GMP effector PelD encoding gene and PEL exopolysaccharide biosynthesis in extremophile bacterium acidithiobacillus thiooxidans. Genes 12 (1):69. /genes12010069. doi:10.3390/genes12010069.
- Dragoš, A., and Á. T. Kovács. 2017. The peculiar functions of the bacterial extracellular matrix. Trends in Microbiology 25 (4):257–66. doi:10.1016/j.tim.2016.12.010.
- Duan, Q.-D., M.-X. Zhou, L.-Q. Zhu, and G.-Q. Zhu. 2013. Flagella and bacterial pathogenicity. Journal of Basic Microbiology 53 (1):1–8. doi:10.1002/jobm.201100335.
- Dwyer, D. J., M. A. Kohanski, and J. J. Collins. 2009. Role of reactive oxygen species in antibiotic action and resistance. Current Opinion in Microbiology 12 (5):482–9. doi:10.1016/j.mib.2009.06.018.
- Erdem, A. L., F. Avelino, J. Xicohtencatl-Cortes, and J. A. Girón. 2007. Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. Journal of Bacteriology 189 (20):7426–35. doi:10.1128/JB.00464-07.
- Erriu, M., C. Blus, S. Szmukler-Moncler, S. Buogo, R. Levi, G. Barbato, D. Madonnaripa, G. Denotti, V. Piras, and G. Orrù. 2014. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrasonics Sonochemistry 21 (1):15–22. doi:10.1016/j.ultsonch.2013.05.011.
- Esua, O. J., D.-W. Sun, C. K. Ajani, J.-H. Cheng, and K. M. Keener. 2022. Modelling of inactivation kinetics of escherichia coli and listeria monocytogenes on grass carp treated by combining ultrasound with plasma functionalized buffer. Ultrasonics Sonochemistry 88:106086. doi: 10.1016/j.ultsonch.2022.106086.
- Esua, O. J., J.-H. Cheng, and D.-W. Sun. 2020. Antimicrobial activities of plasma functionalized liquids against foodborne pathogens on grass carp (Ctenopharyngodon Idella). Applied Microbiology and Biotechnology 104 (22):9581–94. doi: 10.1007/s00253-020-10926-z.
- Esua, O. J., J.-H. Cheng, and D.-W. Sun. 2021. Optimisation of treatment conditions for reducing shewanella putrefaciens and salmonella typhimurium on grass carp treated by thermoultrasound-assisted plasma functionalized buffer. Ultrasonics Sonochemistry 76:105609. doi: 10.1016/j.ultsonch.2021.105609.
- Flemming, H.-C., and J. Wingender. 2010. The biofilm matrix. Nature Reviews. Microbiology 8 (9):623–33. doi:10.1038/nrmicro2415.
- Formosa-Dague, C., C. Feuillie, A. Beaussart, S. Derclaye, S. Kucharíková, I. Lasa, P. Van Dijck, and Y. F. Dufrêne. 2016. Sticky matrix: Adhesion mechanism of the Staphylococcal polysaccharide Intercellular adhesin. ACS Nano 10 (3):3443–52. doi:10.1021/acsnano.5b07515.
- Foster, T. J., J. A. Geoghegan, V. K. Ganesh, and M. Höök. 2014. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nature Reviews. Microbiology 12 (1):49–62. doi:10.1038/nrmicro3161.
- Francis, V. I., and S. L. Porter. 2019. Multikinase networks: Two-component signaling networks integrating multiple stimuli. Annual Review of Microbiology 73 (1):199–223. doi:10.1146/annurev-micro-020518-115846.
- Ghosh, S., M. Nag, D. Lahiri, T. Sarkar, S. Pati, Z. A. Kari, N. P. Nirmal, H. A. Edinur, and R. R. Ray. 2022. Engineered biofilm: Innovative nextgen strategy for quality enhancement of fermented foods. Frontiers in Nutrition 9:808630. doi:10.3389/fnut.2022.808630.
- Gilmore, B. F., P. B. Flynn, S. O’Brien, N. Hickok, T. Freeman, and P. Bourke. 2018. Cold plasmas for biofilm control: Opportunities and challenges. Trends in Biotechnology 36 (6):627–38. doi:10.1016/j.tibtech.2018.03.007.
- Gloag, E. S., L. Turnbull, A. Huang, P. Vallotton, H. Wang, L. M. Nolan, L. Mililli, C. Hunt, J. Lu, S. R. Osvath, et al. 2013. Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proceedings of the National Academy of Sciences of the United States of America 110 (28):11541–6. doi:10.1073/pnas.1218898110.
- Gu, T.-T., A. Meesrisom, Y.-G. Luo, Q. N. Dinh, S. Lin, M.-Y. Yang, A. Sharma, R.-G. Tang, J.-D. Zhang, Z. Jia, et al. 2021. Listeria monocytogenes biofilm formation as affected by stainless steel surface topography and coating composition. Food Control 130:108275. doi:10.1016/j.foodcont.2021.108275.
- Guo, L.-X., J.-J. Wang, Y. Gou, L. Tan, H.-Q. Liu, Y.-J. Pan, and Y. Zhao. 2020. Comparative proteomics reveals stress responses of Vibrio parahaemolyticus biofilm on different surfaces: Internal adaptation and external adjustment. The Science of the Total Environment 731:138386. doi:10.1016/j.scitotenv.2020.138386.
- Hage, M., S. Khelissa, H. Akoum, N. E. Chihib, and C. Jama. 2022. Cold plasma surface treatments to prevent biofilm formation in food industries and medical sectors. Applied Microbiology and Biotechnology 106 (1):81–100. doi:10.1007/s00253-021-11715-y.
- Hassan, S., G. Prakash, A. Ozturk, S. Saghazadeh, M. F. Sohail, J. Seo, M. Dockmeci, Y.-S. Zhang, and A. Khademhosseini. 2017. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 15:91–106. doi:10.1016/j.nantod.2017.06.008.
- Hayta, E. N., M. J. Ertelt, M. Kretschmer, and O. Lieleg. 2021. Bacterial materials: Applications of natural and modified biofilms. Advanced Materials Interfaces 8 (21):2101024. doi:10.1002/admi.202101024.
- He, X., H. Deng, and H.-M. Hwang. 2019. The current application of nanotechnology in food and agriculture. Journal of Food and Drug Analysis 27 (1):1–21. doi:10.1016/j.jfda.2018.12.002.
- He, H.-J., and D.-W. Sun. 2015a. Microbial evaluation of raw and processed food products by visible/infrared, raman and fluorescence spectroscopy. Trends in Food Science & Technology 46 (2):199–210. doi: 10.1016/j.tifs.2015.10.004.
- He, H.-J., and D.-W. Sun. 2015b. Hyperspectral imaging technology for rapid detection of various microbial contaminants in agricultural and food products. Trends in Food Science & Technology 46 (1):99–109. doi: 10.1016/j.tifs.2015.08.001.
- Hu, M.-X., F. He, Z.-S. Zhao, Y.-X. Guo, X.-K. Ma, C.-K. Tu, H. Teng, Z.-X. Chen, H. Yan, and X. Shao. 2022. Electrospun nanofibrous membranes accelerate biofilm formation and probiotic enrichment: Enhanced tolerances to pH and antibiotics. ACS Applied Materials & Interfaces 14 (28):31601–12. doi:10.1021/acsami.2c04540.
- Huber, M., A. Lippegaus, S. Melamed, M. Siemers, B. R. Wucher, M. Hoyos, C. Nadell, G. Storz, and K. Papenfort. 2022. An RNA sponge controls quorum sensing dynamics and biofilm formation in Vibrio cholerae. Nature Communications 13 (1):7585. doi:10.1038/s41467-022-35261-x.
- Huang, L., H. Pu, and D.-W. Sun. 2023. Isoreticular chemistry guided enzymodynamic domino effect for biofilm microenvironment-responsive disinfection. Chemical Engineering Journal 471:144206. doi: 10.1016/j.cej.2023.144206.
- Ivanova, A., K. Ivanova, A. Tied, T. Heinze, and T. Tzanov. 2020. Layer-by-layer coating of aminocellulose and quorum quenching acylase on silver nanoparticles synergistically eradicate bacteria and their biofilms. Advanced Functional Materials 30 (24):2001284. doi:10.1002/adfm.202001284.
- Jayan, H., H. Pu, and D.-W. Sun. 2022. Recent developments in raman spectral analysis of microbial single cells: Techniques and applications. Critical Reviews in Food Science and Nutrition 62 (16):4294–308. doi: 10.1080/10408398.2021.1945534.
- Jenal, U., A. Reinders, and C. Lori. 2017. Cyclic di-GMP: Second messenger extraordinaire. Nature Reviews. Microbiology 15 (5):271–84. doi:10.1038/nrmicro.2016.190.
- Jennings, L. K., K. M. Storek, H. E. Ledvina, C. Coulon, L. S. Marmont, I. Sadovskaya, P. R. Secor, B. S. Tseng, M. Scian, A. Filloux, et al. 2015. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proceedings of the National Academy of Sciences of the United States of America 112 (36):11353–8. doi:10.1073/pnas.1503058112.
- Jiang, W.-T., Z. Xie, S.-H. Huang, Q.-T. Huang, L.-L. Chen, X.-L. Gao, and Z.-M. Lin. 2023. Targeting cariogenic pathogens and promoting competitiveness of commensal bacteria with a novel pH-responsive antimicrobial peptide. Journal of Oral Microbiology 15 (1):2159375. doi:10.1080/20002297.2022.2159375.
- Jiang, Y., Y. Liu, X. Zhang, H. Gao, L. Mou, M. Wu, W. Zhang, F. Xin, and M. Jiang. 2021. Biofilm application in the microbial biochemicals production process. Biotechnology Advances 48:107724. doi:10.1016/j.biotechadv.2021.107724.
- Josic, U., C. Mazzitelli, T. Maravic, A. Fidler, L. Breschi, and A. Mazzoni. 2022. Biofilm in endodontics: In vitro cultivation possibilities, sonic-, ultrasonic- and laser-assisted removal techniques and evaluation of the cleaning efficacy. Polymers 14 (7):1334. doi:10.3390/polym14071334.
- Kang, J.-M., L. Liu, Y.-F. Liu, and X.-Y. Wang. 2020. Ferulic acid inactivates Shigella flexneri through cell membrane destructieon, biofilm retardation, and altered gene expression. Journal of Agricultural and Food Chemistry 68 (27):7121–31. doi:10.1021/acs.jafc.0c01901.
- Karygianni, L., Z. Ren, H. Koo, and T. Thurnheer. 2020. Biofilm matrixome: Extracellular components in structured microbial communities. Trends in Microbiology 28 (8):668–81. doi:10.1016/j.tim.2020.03.016.
- Kim, H. S., S. Y. Ham, H. S. Ryoo, D. H. Kim, E. T. Yun, H. D. Park, and J. H. Park. 2023. Inhibiting bacterial biofilm formation by stimulating c-di-GMP regulation using citrus peel extract from Jeju Island. The Science of the Total Environment 872:162180. doi:10.1016/j.scitotenv.2023.162180.
- Kim, M. K., A. Zhao, A. Wang, Z. Z. Brown, T. W. Muir, H. A. Stone, and B. L. Bassler. 2017. Surface-attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nature Microbiology 2 (8):17080. doi:10.1038/nmicrobiol.2017.80.
- Kim, S., X.-H. Li, H. J. Hwang, and J. H. Lee. 2020. Thermoregulation of Pseudomonas aeruginosa biofilm formation. Applied and Environmental Microbiology 86 (22):e01584-20. doi:10.1128/AEM.01584-20.
- Kong, W.-N., J.-R. Zhao, H.-P. Kang, M. Zhu, T.-H. Zhou, X. Deng, and H.-H. Liang. 2015. ChIP-seq reveals the global regulator AlgR mediating cyclic di-GMP synthesis in Pseudomonas aeruginosa. Nucleic Acids Research 43 (17):8268–82. doi:10.1093/nar/gkv747.
- Lasa, I., and J. R. Penadés. 2006. Bap: A family of surface proteins involved in biofilm formation. Research in Microbiology 157 (2):99–107. doi:10.1016/j.resmic.2005.11.003.
- Laventie, B. J., and U. Jenal. 2020. Surface sensing and adaptation in bacteria. Annual Review of Microbiology 74 (1):735–60. doi:10.1146/annurev-micro-012120-063427.
- Lee, B.-H., M. Hébraud, and T. Bernardi. 2017. Increased adhesion of Listeria monocytogenes strains to abiotic surfaces under cold stress. Frontiers in Microbiology 8:2221–10. 3389/fmicb.2017.02221. doi:10.3389/fmicb.2017.02221.
- Lee, J. H., Y. G. Kim, M. H. Cho, and J. Lee. 2014. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiological Research 169 (12):888–96. doi:10.1016/j.micres.2014.05.005.
- Lee, S. W., K. S. Phillips, H. Gu, M. Kazemzadeh-Narbat, and D. Ren. 2021. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 268:120595. doi:10.1016/j.biomaterials.2020.120595.
- Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118 (1):69–82. doi:10.1016/j.cell.2004.06.009.
- Lewis, K. 2001. Riddle of biofilm resistance. Antimicrobial Agents and Chemotherapy 45 (4):999–1007. doi:10.1128/aac.45.4.999-1007.2001.
- Li, T.-T., X.-J. Sun, H.-T. Chen, B.-B. He, Y.-C. Mei, D.-F. Wang, and J.-R. Li. 2020. Methyl anthranilate: A novel quorum sensing inhibitor and anti-biofilm agent against Aeromonas sobria. Food Microbiology 86:103356. doi:10.1016/j.fm.2019.103356.
- Li, Y.-L., J. Pan, D. Wu, Y. Tian, J. Zhang, and J. Fang. 2019. Regulation of Enterococcus faecalis biofilm formation and quorum sensing related virulence factors with ultra-low dose reactive species produced by plasma activated water. Plasma Chemistry and Plasma Processing 39 (1):35–49. doi:10.1007/s11090-018-9930-2.
- Liang, S., X.-R. Deng, P.-A. Ma, Z.-Y. Cheng, and J.-J. Lin. 2020. Recent advances in nanomaterial-assisted combinational sonodynamic cancer therapy. Advanced Materials (Deerfield Beach, Fla.) 32 (47):e2003214. doi:10.1002/adma.202003214.
- Lin, Q., H. Sun, K. Yao, J. Cai, Y. Ren, and Y. Chi. 2019. The prevalence, antibiotic resistance and biofilm formation of Staphylococcus aureus in bulk ready-to-eat foods. Biomolecules 9 (10):524. doi:10.3390/biom9100524.
- Liu, C., D. Sun, J.-R. Zhu, and W.-J. Liu. 2018. Two-component signal transduction systems: A major strategy for connecting input stimuli to biofilm formation. Frontiers in Microbiology 9:3279. doi:10.3389/fmicb.2018.03279.
- Liu, C., J. Yang, L. Liu, B. Li, H. Yuan, and W. Liu. 2017. Sodium lactate negatively regulates Shewanella putrefaciens CN32 biofilm formation via a three-component regulatory system (LrbS-LrbA-LrbR). Applied and Environmental Microbiology 83 (14):e00712–17. doi:10.1128/AEM.00712-17.
- Liu, D., Q.-F. Huang, W.-M. Gu, and X.-A. Zeng. 2022. A review of bacterial biofilm control by physical strategies. Critical Reviews in Food Science and Nutrition 62 (13):3453–70. doi:10.1080/10408398.2020.1865872.
- Liu, L.-L., C. Ge, Y. Zhang, W.-R. Ma, X. Su, L. Chen, S.-B. Li, L. Wang, X.-J. Mu, and Y. Xu. 2020. Tannic acid-modified silver nanoparticles for enhancing anti-biofilm activities and modulating biofilm formation. Biomaterials Science 8 (17):4852–60. doi:10.1039/d0bm00648c.
- Liu, X.-B., B. Cao, L. Yang, and J.-D. Gu. 2022. Biofilm control by interfering with c-di-GMP metabolism and signaling. Biotechnology Advances 56:107915. doi:10.1016/j.biotechadv.2022.107915.
- Lv, X.-Y., L.-C. Wang, A.-Q. Mei, Y. Xu, X.-H. Ruan, W.-J. Wang, J.-J. Shao, D.-J. Yang, and X.-C. Dong. 2023. Recent nanotechnologies to overcome the bacterial biofilm matrix barriers. Small (Weinheim an Der Bergstrasse, Germany) 19 (6):e2206220. doi:10.1002/smll.202206220.
- Ma, Y., M. Zohaib Aslam, M.-J. Wu, N. Nitin, and G. Sun. 2022. Strategies and perspectives of developing anti-biofilm materials for improved food safety. Food Research International (Ottawa, Ont.) 159:111543. doi:10.1016/j.foodres.2022.111543.
- Mai-Prochnow, A., R. Zhou, T. Zhang, K. K. Ostrikov, S. Mugunthan, S. A. Rice, and P. J. Cullen. 2021. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. NPJ Biofilms and Microbiomes 7 (1):11. doi:10.1038/s41522-020-00180-6.
- Mukherjee, S., and B. L. Bassler. 2019. Bacterial quorum sensing in complex and dynamically changing environments. Nature Reviews. Microbiology 17 (6):371–82. doi:10.1038/s41579-019-0186-5.
- Ndayishimiye, J., T. Kumeria, A. Popat, J. R. Falconer, and M. A. T. Blaskovich. 2022. Nanomaterials: The new antimicrobial magic bullet. ACS Infectious Diseases 8 (4):693–712. doi:10.1021/acsinfecdis.1c00660.
- Nieto, V., A. R. Kroken, M. R. Grosser, B. E. Smith, M. M. E. Metruccio, P. Hagan, M. E. Hallsten, D. J. Evans, and S. M. J. Fleiszig. 2019. Type IV pili can mediate bacterial motility within epithelial cells. mBio 10 (4):1–9. doi:10.1128/mBio.02880-18.
- Nitzan, M., R. Rehani, and H. Margalit. 2017. Integration of bacterial small RNAs in regulatory networks. Annual Review of Biophysics 46 (1):131–48. doi:10.1146/annurev-biophys-070816-034058.
- Oh, E., K. J. Andrews, and B. Jeon. 2018. Enhanced biofilm formation by ferrous and ferric iron through oxidative stress in campylobacter jejuni. Frontiers in Microbiology 9:1204. doi:10.3389/fmicb.2018.01204.
- Okshevsky, M., and R. L. Meyer. 2015. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Critical Reviews in Microbiology 41 (3):341–52. doi:10.3109/1040841X.2013.841639.
- Otto, M. 2013. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annual Review of Medicine 64 (1):175–88. doi:10.1146/annurev-med-042711-140023.
- Paluch, E., J. Rewak-Soroczyńska, I. Jędrusik, E. Mazurkiewicz, and K. Jermakow. 2020. Prevention of biofilm formation by quorum quenching. Applied Microbiology and Biotechnology 104 (5):1871–81. doi:10.1007/s00253-020-10349-w.
- Pan, Y., D.-W. Sun, and Z. Han. 2017. Applications of electromagnetic fields for nonthermal inactivation of microorganisms in foods: an overview. Trends in Food Science & Technology 64:13–22. doi: 10.1016/j.tifs.2017.02.014.
- Pan, Y., J.-H. Cheng, and D.-W. Sun. 2021. Metabolomic analyses on microbial primary and secondary oxidative stress responses. Comprehensive Reviews in Food Science and Food Safety 20 (6):5675–97. doi: 10.1111/1541-4337.12835.
- Pan, Y., Y. Zhang, J.-H. Cheng, and D.-W. Sun. 2020. Inactivation of listeria monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT 118:108635. doi: 10.1016/j.lwt.2019.108635.
- Papenfort, K., and B. L. Bassler. 2016. Quorum sensing signal-response systems in Gram-negative bacteria. Nature Reviews. Microbiology 14 (9):576–88. doi:10.1038/nrmicro.2016.89.
- Patra, A., J. Das, N. R. Agrawal, G. S. Kushwaha, M. Ghosh, and Y.-O. Son. 2022. Marine antimicrobial peptides-based strategies for tackling bacterial biofilm and biofouling challenges. Molecules (Basel, Switzerland) 27 (21):7546. doi:10.3390/molecules27217546.
- Persat, A., C. D. Nadell, M. K. Kim, F. Ingremeau, A. Siryaporn, K. Drescher, N. S. Wingreen, B. L. Bassler, Z. Gitai, and H. A. Stone. 2015. The mechanical world of bacteria. Cell 161 (5):988–97. doi:10.1016/j.cell.2015.05.005.
- Plate, L., and M. A. Marletta. 2013. Nitric oxide-sensing H-NOX proteins govern bacterial communal behavior. Trends in Biochemical Sciences 38 (11):566–75. doi:10.1016/j.tibs.2013.08.008.
- Pompilio, A., M. Scocchi, M. L. Mangoni, S. Shirooie, A. Serio, Y. Ferreira Garcia da Costa, M. S. Alves, G. Seker Karatoprak, I. Suntar, H. Khan, et al. 2023. Bioactive compounds: A goldmine for defining new strategies against pathogenic bacterial biofilms? Critical Reviews in Microbiology 49 (1):117–49. doi:10.1080/1040841X.2022.2038082.
- Pu, H., Y. Xu, D.-W. Sun, Q. Wei, and X. Li. 2021. Optical nanosensors for biofilm detection in the food industry: Principles, applications and challenges. Critical Reviews in Food Science and Nutrition 61 (13):2107–24. doi: 10.1080/10408398.2020.1808877.
- Pu, H., Y. Xu, L. Lin, and D.-W. Sun. 2021. Biofilm formation of pectobacterium carotovorum subsp. carotovorum on polypropylene surface during multiple cycles of vacuum cooling. International Journal of Food Science & Technology 56 (7):3495–506. doi: 10.1111/ijfs.14976.
- Qi, M.-Y., Q.-Y. Liu, Y. Liu, H.-Y. Yan, Y. Zhang, and Y. Yuan. 2022. Staphylococcus aureus biofilm inhibition by high voltage prick electrostatic field (HVPEF) and the mechanism investigation. International Journal of Food Microbiology 362:109499. doi:10.1016/j.ijfoodmicro.2021.109499.
- Qiao, J.-J., L.-P. Zheng, Z.-X. Lu, F.-Q. Meng, and X.-M. Bie. 2021. Research on the biofilm formation of Staphylococcus aureus after cold stress. Microorganisms 9 (7):1534. doi:10.3390/microorganisms9071534.
- Qu, D., Z. Hou, J. Li, L. Luo, S. Su, Z. Ye, Y. Bai, X. Zhang, G. Chen, Z. Li, et al. 2020. A new coumarin compound DCH combats methicillin-resistant Staphylococcus aureus biofilm by targeting arginine repressor. Science Advances 6 (30):eaay9597. doi:10.1126/sciadv.aay9597.
- Raad, N., D. Tandon, S. Hapfelmeier, and N. Polacek. 2022. The stationary phase-specific sRNA FimR2 is a multifunctional regulator of bacterial motility, biofilm formation and virulence. Nucleic Acids Research 50 (20):11858–75. doi:10.1093/nar/gkac1025.
- Rendueles, O., and J. M. Ghigo. 2012. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiology Reviews 36 (5):972–89. doi:10.1111/j.1574-6976.2012.00328.x.
- Römling, U., M. Y. Galperin, and M. Gomelsky. 2013. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiology and Molecular Biology Reviews: MMBR 77 (1):1–52. doi:10.1128/MMBR.00043-12.
- Ronish, L. A., B. Sidner, Y. Yu, and K. H. Piepenbrink. 2022. Recognition of extracellular DNA by type IV pili promotes biofilm formation by Clostridioides difficile. The Journal of Biological Chemistry 298 (10):102449. doi:10.1016/j.jbc.2022.102449.
- Rossy, T., C. D. Nadell, and A. Persat. 2019. Cellular advective-diffusion drives the emergence of bacterial surface colonization patterns and heterogeneity. Nature Communications 10 (1):2471. doi:10.1038/s41467-019-10469-6.
- Rutherford, S. T., and B. L. Bassler. 2012. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine 2 (11):a012427–a012427. doi:10.1101/cshperspect.a012427.
- Ryder, C., M. Byrd, and D. J. Wozniak. 2007. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Current Opinion in Microbiology 10 (6):644–8. doi:10.1016/j.mib.2007.09.010.
- Sarveswari, H. B., K. K. Gupta, R. Durai, and A. P. Solomon. 2023. Development of a smart pH-responsive nano-polymer drug, 2-methoxy-4-vinylphenol conjugate against the intestinal pathogen, Vibrio cholerae. Scientific Reports 13 (1):1250. doi:10.1038/s41598-023-28033-0.
- Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Journal of Bacteriology 184 (4):1140–54. doi:10.1128/jb.184.4.1140-1154.2002.
- Sauer, K., P. Stoodley, D. M. Goeres, L. Hall-Stoodley, M. Burmølle, P. S. Stewart, and T. Bjarnsholt. 2022. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nature Reviews. Microbiology 20 (10):608–20. doi:10.1038/s41579-022-00767-0.
- Schiffer, C. J., C. Schaudinn, M. A. Ehrmann, and R. F. Vogel. 2022. SxsA, a novel surface protein mediating cell aggregation and adhesive biofilm formation of Staphylococcus xylosus. Molecular Microbiology 117 (5):986–1001. doi:10.1111/mmi.14884.
- Schilcher, K., and A. R. Horswill. 2020. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiology and Molecular Biology Reviews: MMBR 84 (3) doi:10.1128/MMBR.00026-19.
- Schniederberend, M., J. F. Williams, E. Shine, C. Shen, R. Jain, T. Emonet, and B. I. Kazmierczak. 2019. Modulation of flagellar rotation in surface-attached bacteria: A pathway for rapid surface-sensing after flagellar attachment. PLoS Pathogens 15 (11):e1008149. doi:10.1371/journal.ppat.1008149.
- Secchi, E., A. Vitale, G. L. Miño, V. Kantsler, L. Eberl, R. Rusconi, and R. Stocker. 2020. The effect of flow on swimming bacteria controls the initial colonization of curved surfaces. Nature Communications 11 (1):2851. doi:10.1038/s41467-020-16620-y.
- Stewart, P. S., and T. Bjarnsholt. 2020. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases 26 (8):1034–8. doi:10.1016/j.cmi.2020.02.027.
- Storz, G., J. Vogel, and K. M. Wassarman. 2011. Regulation by small RNAs in bacteria: Expanding frontiers. Molecular Cell 43 (6):880–91. doi:10.1016/j.molcel.2011.08.022.
- Stülke, J., and L. Krüger. 2020. Cyclic di-AMP signaling in bacteria. Annual Review of Microbiology 74 (1):159–79. doi:10.1146/annurev-micro-020518-115943.
- Taglialegna, A., S. Navarro, S. Ventura, J. A. Garnett, S. Matthews, J. R. Penades, I. Lasa, and J. Valle. 2016. Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathogens 12 (6):e1005711. doi:10.1371/journal.ppat.1005711.
- Telford, J. L., M. A. Barocchi, I. Margarit, R. Rappuoli, and G. Grandi. 2006. Pili in gram-positive pathogens. Nature Reviews. Microbiology 4 (7):509–19. doi:10.1038/nrmicro1443.
- Teschler, J. K., D. Zamorano-Sánchez, A. S. Utada, C. J. A. Warner, G. C. L. Wong, R. G. Linington, and F. H. Yildiz. 2015. Living in the matrix: Assembly and control of Vibrio cholerae biofilms. Nature Reviews. Microbiology 13 (5):255–68. doi:10.1038/nrmicro3433.
- Toyofuku, M., B. Roschitzki, K. Riedel, and L. Eberl. 2012. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. Journal of Proteome Research 11 (10):4906–15. doi:10.1021/pr300395j.
- Uruén, C., G. Chopo-Escuin, J. Tommassen, R. C. Mainar-Jaime, and J. Arenas. 2020. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics (Basel, Switzerland) 10 (1):1–36. doi:10.3390/antibiotics10010003.
- Vaishampayan, A., A. de Jong, D. J. Wight, J. Kok, and E. Grohmann. 2018. A novel antimicrobial coating represses biofilm and virulence-related genes in methicillin-resistant Staphylococcus aureus. Frontiers in Microbiology 9:221. doi:10.3389/fmicb.2018.00221.
- Van Acker, H., P. Van Dijck, and T. Coenye. 2014. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends in Microbiology 22 (6):326–33. doi:10.1016/j.tim.2014.02.001.
- Villa, F., W. Remelli, F. Forlani, M. Gambino, P. Landini, and F. Cappitelli. 2012. Effects of chronic sub-lethal oxidative stress on biofilm formation by Azotobacter vinelandii. Biofouling 28 (8):823–33. doi:10.1080/08927014.2012.715285.
- Webster, S. S., C. K. Lee, W. C. Schmidt, G. C. L. Wong, and G. A. O’Toole. 2021. Interaction between the type 4 pili machinery and a diguanylate cyclase fine-tune c-di-GMP levels during early biofilm formation. Proceedings of the National Academy of Sciences of the United States of America 118 (26):10–1073. /pnas.2105566118. doi:10.1073/pnas.2105566118.
- Whiteley, M., S. P. Diggle, and E. P. Greenberg. 2017. Progress in and promise of bacterial quorum sensing research. Nature 551 (7680):313–20. doi:10.1038/nature24624.
- Woehlk, H., M. J. Trimble, S. C. Mansour, D. Pletzer, V. Trouillet, A. Welle, L. Barner, R. E. W. Hancock, C. Barner-Kowollik, and K. E. Fairfull-Smith. 2019. Controlling biofilm formation with nitroxide functional surfaces. Polymer Chemistry 10 (31):4252–8. doi:10.1039/C9PY00690G.
- Wu, J.-S., X.-P. Han, M.-Z. Ye, Y. Li, X. Wang, and Q.-P. Zhong. 2022. Exopolysaccharides synthesized by lactic acid bacteria: Biosynthesis pathway, structure-function relationship, structural modification and applicability. Critical Reviews in Food Science and Nutrition. 63 (24):7043–64. doi:10.1080/10408398.2022.2043822.
- Wu, S.-Z., J.-Q. Zhang, Q. Peng, Y.-J. Liu, L. Lei, and H. Zhang. 2021. The role of Staphylococcus aureus YycFG in gene regulation, biofilm organization and drug resistance. Antibiotics (Basel, Switzerland) 10 (12):1–12. doi:10.3390/antibiotics10121555.
- Xu, Z.-B., J.-H. Xie, T. Soteyome, B. M. Peters, M. E. Shirtliff, J.-Y. Liu, and J. M. Harro. 2019. Polymicrobial interaction and biofilms between Staphylococcus aureus and Pseudomonas aeruginosa: An underestimated concern in food safety. Current Opinion in Food Science 26:57–64. doi:10.1016/j.cofs.2019.03.006.
- Yin, W., S.-Y. Xu, Y.-T. Wang, Y.-L. Zhang, S. H. Chou, M. Y. Galperin, and J. He. 2021. Ways to control harmful biofilms: Prevention, inhibition, and eradication. Critical Reviews in Microbiology 47 (1):57–78. doi:10.1080/1040841X.2020.1842325.
- Yin, W., Y.-T. Wang, L. Liu, and J. He. 2019. Biofilms: The microbial “protective clothing" in extreme environments. International Journal of Molecular Sciences 20 (14):3423. doi:10.3390/ijms20143423.
- Yu, H., Y. Liu, F.-W. Yang, Y.-F. Xie, Y.-H. Guo, Y.-L. Cheng, and W.-R. Yao. 2021. Combined an acoustic pressure simulation of ultrasonic radiation and experimental studies to evaluate control efficacy of high-intensity ultrasound against Staphylococcus aureus biofilm. Ultrasonics Sonochemistry 79:105764. doi:10.1016/j.ultsonch.2021.105764.
- Zhang, M.-Y., L. He, J.-M. Qin, S. Wang, and M.-P. Tong. 2023. Influence of flagella and their property on the initial attachment behaviors of bacteria onto plastics. Water Research 231:119656. 119656. doi:10.1016/j.watres.2023.119656.
- Zhang, Y.-Q., Y. Qiu, H. Gao, J.-F. Sun, X. Li, M.-M. Zhang, X.-F. Xue, W.-H. Yang, B. Ni, L.-F. Hu, et al. 2021. OpaR controls the metabolism of c-di-GMP in Vibrio parahaemolyticus. Frontiers in Microbiology 12:676436. doi:10.3389/fmicb.2021.676436.
- Zhao, L.-J., F.-X. Duan, M. Gong, X. Tian, Y. Guo, L.-R. Jia, and S. Deng. 2021. (+)-Terpinen-4-ol inhibits Bacillus cereus biofilm formation by upregulating the interspecies quorum sensing signals diketopiperazines and diffusing signaling factors. Journal of Agricultural and Food Chemistry 69 (11):3496–510. doi:10.1021/acs.jafc.0c07826.
- Zhao, Y., L. Chen, Y.-A. Wang, X.-Y. Song, K.-Y. Li, X.-F. Yan, L.-M. Yu, and Z.-Y. He. 2021. Nanomaterial-based strategies in antimicrobial applications: Progress and perspectives. Nano Research 14 (12):4417–41. doi:10.1007/s12274-021-3417-4.
- Zhao, Z.-Q., L.-J. Wang, J.-H. Miao, Z.-Y. Zhang, J.-Q. Ruan, L.-J. Xu, H. Guo, M. Zhang, and W.-C. Qiao. 2022. Regulation of the formation and structure of biofilms by quorum sensing signal molecules packaged in outer membrane vesicles. The Science of the Total Environment 806 (Pt 4):151403. doi:10.1016/j.scitotenv.2021.151403.
- Zhang, C., L. Huang, H. Pu, and D.-W. Sun. 2021. Magnetic surface-enhanced raman scattering (magsers) biosensors for microbial food safety: Fundamentals and applications. Trends in Food Science & Technology 113:366–81. doi: 10.1016/j.tifs.2021.05.007.
- Zhao, Y., M. L. Bhavya, A. Patange, D.-W. Sun, and B. K. Tiwari. 2023. Plasma-activated liquids for mitigating biofilms on food and food contact surfaces. Comprehensive Reviews in Food Science and Food Safety 22 (3):1654–85. doi: 10.1111/1541-4337.13126.
- Zhao, Y.-M., A. Patange, D.-W. Sun, and B. Tiwari. 2020. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Comprehensive Reviews in Food Science and Food Safety 19 (6):3951–79. doi: 10.1111/1541-4337.12644.
- Zhao, Y.-M., S. Ojha, C. M. Burgess, D.-W. Sun, and B. K. Tiwari. 2020a. Influence of various fish constituents on inactivation efficacy of plasma-activated water. International Journal of Food Science & Technology 55 (6):2630–41. doi: 10.1111/ijfs.14516.
- Zhao, Y.-M., S. Ojha, C. M. Burgess, D.-W. Sun, and B. K. Tiwari. 2020b. Inactivation efficacy and mechanisms of plasma activated water on bacteria in planktonic state. Journal of Applied Microbiology 129 (5):1248–60. doi: 10.1111/jam.14677.
- Zhao, Y.-M., S. Ojha, C. M. Burgess, D.-W. Sun, and B. K. Tiwari. 2021. Inactivation efficacy of plasma-activated water: Influence of plasma treatment time, exposure time and bacterial species. International Journal of Food Science & Technology 56 (2):721–32. doi: 10.1111/ijfs.14708.
- Zheng, Y., G. Tsuji, C. Opoku-Temeng, and H. O. Sintim. 2016. Inhibition of P. aeruginosa c-di-GMP phosphodiesterase RocR and swarming motility by a benzoisothiazolinone derivative. Chemical Science 7 (9):6238–44. doi:10.1039/c6sc02103d.
- Zhou, J.-W., B. Hou, G.-Y. Liu, H. Jiang, B. Sun, Z.-N. Wang, R.-F. Shi, Y. Xu, R. Wang, and A.-Q. Jia. 2018. Attenuation of Pseudomonas aeruginosa biofilm by hordenine: A combinatorial study with aminoglycoside antibiotics. Applied Microbiology and Biotechnology 102 (22):9745–58. doi:10.1007/s00253-018-9315-8.
- Zhu, W.-X., J.-Z. Gao, H.-L. Liu, J.-X. Liu, T. Jin, N.-B. Qin, X.-M. Ren, and X.-D. Xia. 2022. Antibiofilm effect of sodium butyrate against Vibrio parahaemolyticus. Food Control. 131:108422. doi:10.1016/j.foodcont.2021.108422.
- Zou, Y., K.-Y. Lu, Y.-C. Lin, Y. Wu, Y.-R. Wang, L.-H. Li, C.-B. Huang, Y.-X. Zhang, J. L. Brash, H. Chen, et al. 2021. Dual-Functional surfaces based on an antifouling polymer and a natural antibiofilm molecule: Prevention of biofilm formation without using biocides. ACS Applied Materials & Interfaces 13 (38):45191–200. doi:10.1021/acsami.1c10747.