Abstract
The human diet requires a more plant-based approach due to the exhaustive effects animal-based foods have on the environment. However, plant-based proteins generally miss a few or have a lower variety in essential amino acids and are more difficult to digest. Subsequently they might be prone to fermentation by the microbiome in the proximal colon. Proteolytic fermentation can induce microbial-metabolites with beneficial and negative health effects. We review current insight into how balances in saccharolytic and proteolytic fermentation can be maintained when the diet consists predominantly of plant-based proteins. Some proteolytic fermentation metabolites may negatively impact balances in gut microbiota composition in the large intestine and influence immunity. However, proteolytic fermentation can potentially be prevented in the proximal colon toward more saccharolytic fermentation through the addition of non-digestible carbohydrates in the diet. Knowledge on this combination of plant-based proteins and non-digestible carbohydrates on colonic- and general health is limited. Current data suggest that transitioning toward a more plant-based protein diet should be accompanied with a consumption of increased quantities and more complex structures of carbohydrates or by application of technological strategies to enhances digestibility. This can reduce or prevent proteolytic fermentation which might consequently improve human health.
Introduction
The transition from animal-based protein toward more plant-based proteins is necessary to create a sustainable future. Production of plant-based foods is generally found to be more sustainable when looking at land and water use, energy conversion and greenhouse gas emission. On the contrary, animal-based proteins tend to result in very high use of freshwater, land and CO2 as compared to plant-based proteins (Moughan Citation2021). Not only are plant-based proteins more environmentally friendly to produce, but consumption of plant-based foods, as opposed to animal based foods, might be associated with many different health benefits, including a reduced risk of cardiovascular disease, type 2 diabetes, and certain cancers (Xiao et al. Citation2023).
Demands for meat are growing worldwide, just as the demand for animal feed. At this point in time the demand is higher than its availability. Globally the average diet consists of approximately one third of plant-based protein and two third of animal-based protein (form, Citation2022). However, even if the shift toward more plant-based protein sources can be made, plant-based alternatives might not be sufficient to feed the human population as plant-based protein alternatives generally have a lower variety of essential amino acids compared to animal-based sources (Gorissen et al. Citation2018). Especially leucine, lysine and methionine are found to be less abundant in plant-based proteins (Gorissen et al. Citation2018). Furthermore, its structure makes it more difficult for the human body to digest than animal derived proteins (van Vliet, Burd, and van Loon Citation2015). It has been found that plant-based proteins tend to contain more β-sheet structures and relatively low α-helixes than animal proteins. Both the β-sheet and the α-helix are secondary protein structures. The high percentage of β-sheet structures in plant proteins can hinder access of gastrointestinal digestive enzymes to digest the protein. The α-helixes are better digestible (Yu Citation2005). This results in lower digestibility of plant-based proteins by human enzymes (Carbonaro, Maselli, and Nucara Citation2012).
Because of the lower digestibility and difference in composition the human body might not be able to take up all amino acids in the small intestine. The presence of some antinutritional factors when consuming whole crops, such as glucosinolates, trypsin inhibitors and hemagglutinins might also reduce protein and amino acid digestibility (Gilani, Cockell, and Sepehr Citation2005). Consequently, a relatively large portion of the plant-based proteins may reach the large intestine. Where in the proximal colon there is usually only saccharolytic fermentation (Canfora et al. Citation2019), in the distal colon proteolytic fermentation can occur. Proteolytic fermentation by the gut microbiota does not only result in beneficial but also harmful microbial products, like amines, phenols, sulfides, ammonia, but also branched short chain fatty acids (bSCFA) (Windey, De Preter, and Verbeke Citation2012) and should therefore be prevented. These microbial products have shown to, among other things, induce inflammation and increase gut permeability (Diether and Willing Citation2019). Though the influence on microbial fermentation of carbohydrates and protein has been very well documented before the protein that was mentioned has been all dietary protein. In this review we strive to make another clear distinction between plant-based and animal-based protein, which has not previously been described (Diether and Willing Citation2019; Korpela Citation2018; Li et al. Citation2021).
This review intends to present an overview of the current knowledge about the role of the microbiome and its impact on health when plant-based protein is consumed in comparison to the consumption of animal-based protein. Solutions to prevent undesired fermentation of plant-based proteins will be discussed. To this end, we will first describe differences between plant-based and animal-based proteins followed by an overview of the microbiome and too much protein fermentation in the large intestine. Additionally, we will discuss supplementation of carbohydrates as a potential way to support saccharolytic fermentation (fermentation of carbohydrates) and alternative methods to reduce proteolytic fermentation.
Plant-based and animal-based proteins
Proteins are essential nutrients that play several important roles in the human body. These include building and repairing tissues, producing enzymes and stimulating hormone secretion and supporting immune function (Daly et al. Citation1990; Hoffman and Falvo Citation2004). Proteins are composed of amino-acids, which the body needs to function properly (Freeman, Kim, and Sleisenger Citation1979). After ingestion, protein digestion usually starts in the stomach and ends in the small intestine after being broken down by pepsin, trypsin, and chymotrypsin (Krehbiel and Matthews Citation2003). If digestion of proteins is not complete at the end of the small intestine, proteins may enter the proximal colon and will be subject to fermentation by gut microbiota (Fan et al. Citation2015). After proteins have been broken down to amino acids, they are actively transported by intestinal epithelial cells into blood vessels to form muscle cells as will be discussed in more detail below (Argiles and Lopez-Soriano Citation1990). Whereas non-essential amino acids can be synthesized by the human body, essential amino acids are amino acids that humans and other vertebrates can’t synthesize from metabolic intermediates. The nine essential amino acids are phenylalanine, valine, tryptophan, threonine, isoleucine, methionine, histidine, leucine and lysine (Lopez and Mohiuddin Citation2022). These amino acids can be obtained through various sources of protein.
Plant-based proteins can be found in many different sources including beans, lentils, nuts, seeds, and whole grains (Berrazaga et al. Citation2019). Plant-based proteins are often found to be lower in one or more essential amino acids such as methionine, leucine and lysine, which makes them less “complete” proteins (). It is possible to consume all essential amino acids when eating varied sources of plant-based proteins. E.g., corn and potato protein are higher in methionine and leucine while pea and soy are containing more lysine (Gorissen et al. Citation2018). Plant-based protein blends can be made that closely mimic (up to 98.8% similarity) animal-based alternatives, but in many cases isoleucine, lysine, and histidine remain a limiting factor (Dimina et al. Citation2021). Another challenge for sufficient uptake of amino acids is that most plant derived protein sources do contain antinutritional factors (Gilani, Cockell, and Sepehr Citation2005). These can be different types of molecules, such as glucosinolates for example in mustard and rapeseed protein products and hemagglutinins and tannins in legumes but especially trypsin inhibitors are very common in plant proteins and can interfere with the digestion processes (Gilani, Cockell, and Sepehr Citation2005). Especially soyabeans contain large amounts of trypsin inhibitors, but also peas and kidney beans are high on the list of plants that contain trypsin inhibitors (Gilani, Xiao, and Cockell Citation2012).
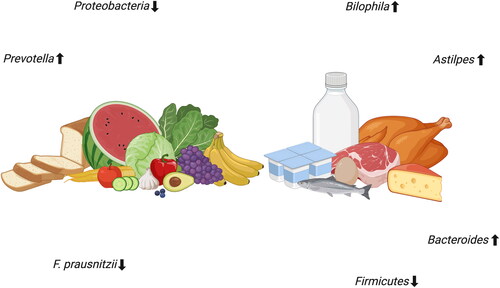
Figure 1. Differences in plant-based and animal-based diets on the gut and its microbiome. Created with BioRender.com.
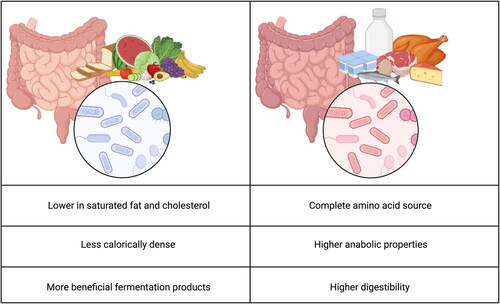
An additional downside is that plant-based proteins are less readily digestible by human enzymes making a human diet with plant-based proteins less efficient. Animal-based proteins are found in meat, poultry, fish, eggs and dairy products. Generally these contain all essential amino acids which makes them a more “complete” source of protein (Berrazaga et al. Citation2019). Additionally, animal-based proteins have been shown to contain higher anabolic properties. This might be explained by the higher digestibility of animal based proteins (van Vliet, Burd, and van Loon Citation2015). However consuming plant-based diets also has advantages compared to animal-based diets. Plant-based diet sources tend to be lower in saturated fat and cholesterol. They additionally tend to be less calorically dense (Tso and Forde Citation2021) and therewith can contribute to better health.
The microbiome
The human gut is colonized by a plethora of different microorganisms. These include bacteria, eukaryotic microbes, viruses, fungi and archaea and is referred to as the microbiome (Prokopidis et al. Citation2020). Over 1000 different bacterial species have already been identified, with up to 160 different species per individual (Rajilić-Stojanović and de Vos Citation2014, 100). With its genetic repertoire of approximately 3 million genes the microbiome contains 100 times more genes than the human body (J. Gilbert, Blaser, et al. Citation2018) and is involved in many metabolic processes related to food digestion and uptake (Zhang et al. Citation2010). This wide range of different bacteria consists of many different species. Some of the most common species include Bacteroidetes, Firmicutes and Proteobacteria and in lesser amounts Actinobacteria, Acidobacteria, Fusobacteria and Verrumicrobia (Prokopidis et al. Citation2020). Approximately 90% of the gut microbiota consists of Firmicutes and Bacteriodes. The microbiome makes products that the human body cannot produce itself, e.g., essential vitamins, such as vitamin B12, folate, vitamin K, nicotinic acid, pyridoxine, and others as well as secondary bile acids (LeBlanc et al. Citation2013).
The microbiome has been shown to have many different functions for preventing disease and to support human health such as strengthening gut barrier integrity (Natividad and Verdu Citation2013), maintaining metabolic health (LeBlanc et al. Citation2013) but also regulation of immune responses (Gensollen et al. Citation2016). Not only has the microbiome been linked to regulating the immune response, it additionally has shown to be able to influence the host immune system in responses against pathogens (Quigley Citation2013). For instance, Faecalibacterium prausnitzii and Bifidobacterium infantis have been shown to stimulate production of the anti-inflammatory cytokine interleukin-10 and regulate T cell activation against the pathogen-stimulated NF-κB inflammatory pathway (O’Mahony et al. Citation2008) and by that regulates the immune system by preventing too strong immune responses (Murray Citation2006). The composition and abundance of microbiota is however strongly influenced by the diet. In periods as short as 24 h after a dietary change, alterations in the microbiome may occur (). This is also reported in transitions from animal to plant-based diets (David et al. Citation2014). Although not much is known how this impacts immunity and gut barrier function, in consumers it has been shown that an animal based diet increases presence of bile-tolerant microorganisms such as Alistipes, Bilophila and Bacteroides and decreased the levels of Firmicutes that metabolize dietary plant polysaccharides (David et al. Citation2014). Yet, more impactful and permanent changes usually require a more long-term intervention (Faith et al. Citation2013) and might have different effects on human health. However, virtually nothing is known on how this impacts microbiota composition and human immunity and barrier function.
Interactions between the gut microbiota and the gut immune system
The gut microbiota is in close contact with the intestinal mucosal immune system and is influenced by the microbiome by direct contact between the microbes and the mucosa and by the fermentation products of microbiota that diffuse into the mucosa (Juge Citation2022). The intestinal mucosal immune system can be divided into several different mucosal lymphoid structures: the lamina propria, the epithelium and specialized gut-associated lymphoid structures (GALT). Between the outside of the human body (lumen) and the inside (lamina propria), many different lines of defense are in place. The mucus layer serves as the first barrier against intruding pathogens. Not only is the mucus layer a physical line of defense also the presence of IgA and antimicrobial peptides serve as mechanism of defense (Beukema, Faas, and de Vos Citation2020). The epithelial cells serve after the mucus layer as the second physical line of defense and consist of enterocytes, and secretory epithelial cells such as Paneth cells or goblet cells. In case of presence of pathogens in the gut lumen Paneth cells are able to secrete antimicrobial peptides (AMPs) and thereby contributes to the host defense mechanisms (Ayabe et al. Citation2004). The epithelium is able to send signals to the intestinal mucosal immune system by producing and releasing cytokines and chemokines (Kuhn, Pedraza, and Demoruelle Citation2014). Epithelial cells also express various pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), NOD-like receptors (NLRs) and c-type lectins. By detection of pathogen-associated molecular patterns (PAMPs) PRRs are able to activate pathways that activate induction of a range of cytokines and chemokines that orchestrate the hosts resistance to infection. Additionally, these PRRs facilitate the differentiation of T cells and B cells to establish antigen-specific adaptive immunity (Fukata and Arditi Citation2013). Finally, behind the epithelial layer the lamina propria can be found. Here a plethora of immune cells reside, in addition to Peyer’s patches in the small intestine (Richards et al. Citation2016) and mesenteric lymph nodes. These together make up the GALT. Here the immune response has to be induced against pathogens and harmful substances, but also tolerance against commensal bacteria has to be ensured (Ahluwalia, Magnusson, and Öhman Citation2017). In the lamina propria dendritic cells and macrophages reside, which can both recognize luminal antigens with PRRs (Gordon Citation2002). Additionally, these cells are able to initiate the differentiation of naïve T cells into regulatory T cells or effector T cells (Joeris et al. Citation2017).
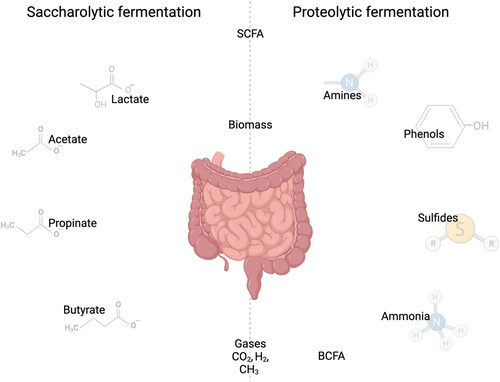
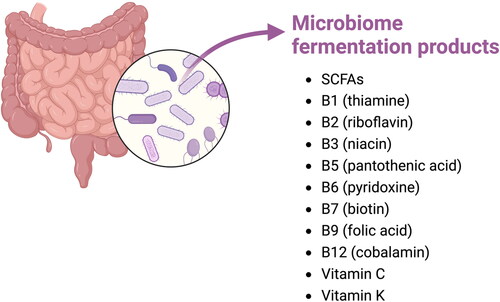
As the gut microbiome has shown to be able to influence many functions of the human body and can be altered by factors such as diet, lifestyle and environment it has gained a lot of interest of the scientific community over the past years. Especially environmental factors seem to play a significant role in the composition of the microbiome. This was, e.g., illustrated in experiments with mice with the same genotype having pertinent differences in their microbiome composition when they were housed in different cages in the same facility (Hasegawa and Inohara Citation2014). Usually, the relationship between the host and the microbiome is as such that it is of mutual benefit. The host is creating an ideal habitat and provides nutrients for the microbiome. The microbiome, in return, is able to ferment dietary fibers. This results in the production of SCFAs such as acetate, propionate, and butyrate which can be used as an energy source for the host (den Besten et al. Citation2013) and can contribute to regulation of glucose metabolism, fatty acid metabolism and hepatic cholesterol biosynthesis. Furthermore, SCFAs are able to inhibit pathogen growth and reduce intestinal inflammation (Vinolo et al. Citation2011). Additionally, fermentation products of the microbiome include B vitamins such as B1 (thiamine), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), B6 (pyridoxine), B7 (biotin), B9 (folic acid) and B12 (cobalamin) (). Though most B vitamins can also be obtained from foods, synthesis of these vitamins by the microbiome is often required to meet nutritional needs (Uebanso et al. Citation2020). Additionally vitamin C and K can be produced by the microbiome (Hill Citation1997). The microbiome is also able to produce less desirable branched short chain fatty acids (bSCFA) which can lead to inflammation (Diether and Willing Citation2019). The production of bSCFA’s is often associated with the fermentation of proteins. Proteolytic fermentation can additionally result in the production of amines, phenols, and sulfides which are often found to be detrimental for gut health and immunity (Windey, De Preter, and Verbeke Citation2012). For example, presence of phenol in the gastrointestinal tract has shown to reduce viability in human colonic epithelial cells and higher concentrations of sulfides resulted in hyperproliferation of the epithelial layer and reduced apoptosis (M. S. Gilbert, Blaser, et al. Citation2018). Though proteolytic fermentation is often associated with the production of metabolites that negatively impact gut health, it can additionally result in the production of metabolites that are associated with gut health such as by formation of low amounts SCFAs and butyrate. However, it has been reported that the release of ammonia decreases butyrate transporter expression which in its turn decreases the positive effects of the aforementioned beneficial metabolites of proteolytic fermentation (Li et al. Citation2021).
Figure 3. Fermentation products of the microbiome. Adapted from “metabolism and microbiota”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates.
If the microbiome is adequately developed it supports the immune response but also stimulates and expedites the development of the metabolic system by providing the before mentioned essential nutrients such as vitamins and the afore mentioned SCFAs (Topping and Clifton Citation2001). However, when dysbiosis between the microbiome and the host occurs this can lead to diseases such as obesity, type 2 diabetes, hypertension, necrotizing enterocolitis and inflammatory bowel disease (Shi et al. Citation2017). Because of its well-recognized implications for health, the microbiome has become an important therapeutic target to prevent disease and is now also recognized as an important target to study when significant changes in diet are proposed such as a partial or complete change from animal to plant based proteins in the diet.
Protein fermentation in the large intestine
Whereas carbohydrate fermentation is generally found to be beneficial for gut health, protein fermentation can be detrimental for gut health (Windey, De Preter, and Verbeke Citation2012). Though microbiota preferably ferment complex carbohydrates, it might happen with a normal diet that the fermentable carbohydrates become depleted between the transverse and the distal colon, resulting in fermentation of protein that has escaped digestion in the small intestine. This might happen in case of high plant-based protein intake (Zhang et al. Citation2020). Normally the proximal colon is dedicated to saccharolytic fermentation. When not all protein is taken up in the small intestine this can result in undesirable production of microbial metabolites with a potential negative impact on health such as ammonia, indoles, amines, phenols, bSCFA and sulfides () (Windey, De Preter, and Verbeke Citation2012). These metabolites have shown to be able to increase gut permeability, inflammatory responses and eventually even lead to colitis in the gut (Diether and Willing Citation2019). More specifically, ammonia was found to reduce villus length and reduce the expression of tight junction genes such as claudin and zonula occludens (Khan et al. Citation2021). In addition, the crops containing plant proteins may contain anti-nutritional factors such as phenols that are associated with damage of the epithelial layer (Pedersen, Brynskov, and Saermark Citation2002) and sulfides that reduce disulfide bonds present in the mucus network, thereby breaking the mucus barrier (Ijssennagger, van der Meer, and van Mil Citation2016). Finally, bSCFAs are used as a marker for proteolytic fermentation. Even though the presence of bSCFAs themselves are not necessarily correlated with poor gut health, the products of proteolytic fermentation are. This makes bSCFAs an important marker to be able to determine the degree of proteolytic fermentation in consumers (Rios-Covian et al. Citation2020).
Protein fermentation only occurs in diets relatively high in proteins. These are for example diets focused on weight loss in which protein intake is much higher than recommended which increases proteolytic fermentation (Pesta and Samuel Citation2014) as a consequence. Additionally, diets which are low in fiber have been shown to induce increased proteolytic fermentation (Macfarlane et al. Citation1988). The shift toward fermentation of proteins has shown to induce the production of the aforementioned undesired microbial fermentation products and eventually induce a shift in microbiota composition (Neis, Dejong, and Rensen Citation2015). Although, it is generally accepted that these events occur many items related to its effects on health still has to be studied. It is for example unknown which products are formed by which microbes during shift in saccharolytic to proteolytic fermentation. Most studies on protein fermentation are based on correlative studies in fecal samples. Some of the species that have been implied to play a role in proteolytic fermentation include Clostridium, Fusobacterium, Bacteroides, Actinomyces, Propionibacterium, as well as Peptostreptococci (Smith and Macfarlane Citation1998). Not only does proteolytic fermentation result in production of undesirable metabolites, but it additionally contributes to shortages of protein uptake in the body as the available protein is fermented instead of digested and taken up by the body. This can occur with higher intake of meat proteins but it especially happens with plant-based proteins which reach lower parts of the digestive tract are prone to fermentation.
Carbohydrates to support saccharolytic fermentation
One way to prevent undesired proteolytic fermentation is by supplying the diet with complex carbohydrates that resist digestion and support saccharolytic fermentation in the cecum and might be fermented along the distal part of the colon. Examples of such complex carbohydrates are the dietary fibers inulin, pectins but also, e.g., resistant starch type 3. Inulin type fructans are prebiotic fibers. They are linear plant oligo- and polysaccharides which consist of minimally two fructose-units, and at least one β(2 − 1) fructosyl-fructose glycosidic bond. This bond is what gives the inulin its unique structural and physiological properties and what gives it the ability to resist enzymatic hydrolysis by human salivary and small intestinal digestive enzymes (Kelly Citation2008). The polymer chain length or degree of polymerization (DP) is what determines where in the gastrointestinal tract the compound is fermented () (Rumessen et al. Citation1990). Pectins are heteropolysaccharides consisting mainly of α-1,4-linked galacturonic acid (GalA) residues. Up to 70% of the pectin molecule consists of these repeating α-1,4-linked galacturonic acid regions (Voragen et al. Citation2009). A percentage of these regions can contain attached methyl esters, which is known as the degree of methyl-esterification (DM). Pectins can either be classified as low DM or high DM and low DM pectins are fermented faster in the large intestine (Tian et al. Citation2017). Finally resistant starch type 3 are short chain α-glucan crystals. These crystals can differ in the DP of the molecules and the type of crystals that are formed. Crystals with a higher molecular weight are more resistant to digestion, as well as A-type crystals which are more resistant to digestion than B-type crystals (Klostermann et al. Citation2021).
Figure 5. Chemical structures of carbohydrates to might be used to support saccharolytic fermentation. The degree of polymerization in pectin (left), the degree of methylation in inulin which consists of glucose (G) and fructose (F) units (Middle) and the different crystal structures in resistant starch type 3 (right). Created with BioRender.com.
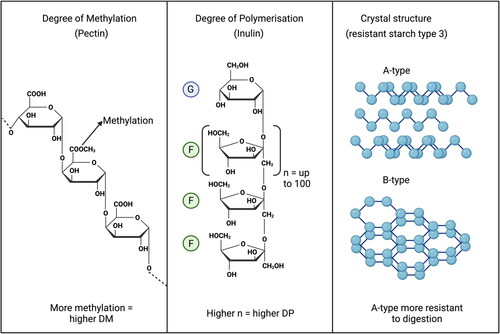
Depending on the amount and chemical composition of the consumed dietary fiber the composition of the gut microbiota can be altered benefiting saccharolytic fermentation (Seal et al. Citation2021). Fermentation of these non-digestible carbohydrates instead of proteins results in the production of beneficial SCFAs such as acetate, propionate, and butyrate. These SCFAs are either used locally by human cells in the form of butyrate or by colonocytes or peripherally in the form of acetate and propionate in liver, muscle, and even brain (den Besten et al. Citation2013) and contribute to anabolism. Particularly butyrate has attracted a lot of interest because of its ability to influence gene-expression of, e.g., mucin 2, the tight junction markers claudin-3, zona-occludens 1 and occludens, but also the enterocyte marker alkaline phosphatase that neutralizes toxins from the lumen (Grouls et al. Citation2022). Additionally it has shown to have immunomodulatory effects (Siddiqui and Cresci Citation2021).
Scientific data about the quantity of dietary fiber that reach the colon is scarce, but when eating a Western diet it is suggested that it must be in between 20 and 60 g of nondigestible carbohydrate including resistant starches, plant cell wall polysaccharides, oligosaccharides, and sugar alcohols (Seal et al. Citation2021). Whether this amount of fiber is able to reach the distal colon and might prevent proteolytic fermentation is unknown. This is however essential as the microbiome prefer complex carbohydrates and saccharolytic fermentation (Korpela Citation2018) over protein sources. Sufficient supply of fibers will eventually reduce the production of unbeneficial products that are produced as a result of protein fermentation and additionally increase SCFA production in the distal colon which has shown to be beneficial for overall gut health, energy regulation and metabolic regulation (van der Beek et al. Citation2016).
Human in vivo studies on proteolytic versus saccharolytic fermentation are scarce but ex vivo studies with simulated gastrointestinal systems such as the TIM-2 model has shown that proteolytic fermentation can be effectively prevented with dietary fibers. With pectins varying in methoxy degree it was shown in a TIM-2 model that bacteria such as F. prausnitzii could be increased in abundance while P. coprii was decreased. Additionally, previous studies have shown that presence of pectin substrates could increase the relative abundance of Bacteroides vulgatus, Bacteroides stercoris and Bacteroides dorei as well as many other bacterial strains that have been associated with gut health (Pascale et al. Citation2022).
Different bacteria have been associated with proteolytic and saccharolytic fermentation. Bifidobacteria and clostridia have been most associated with degradation of fiber (Belenguer et al. Citation2006), while resistant starch seems to most strongly stimulate ruminococcin (Walker et al. Citation2011). Components derived from plant cell walls, including pectins, mostly stimulate Bacteroides, and members of the Lachnospiraceae and Ruminococcaceae families. Bacteroides has also been associated with proteolytic fermentation, but only when animal-based diets are consumed (Korpela Citation2018).
A study of Larsen et al. (Citation2019) shows that with different pectins, the bacterial composition in the intestine can be tailored. Characteristics that were found to be able to induce this modulating effect include degree of esterification, the composition of the monosaccharides, the degree of branching, and the presence of amide groups. Presence of more simple sugars, so less abundant in the before mentioned structures, resulted in higher levels of Oscillospira, Blautia, Dorea, Ruminococcus, Coprococcus, R. torques, Lachnospiraceae and Clostridiales within phylum Firmicutes, and Paraprevotella, B. uniformis, B. ovatus, P. distasonis and Prevotella within phylum Bacteroidetes (Larsen et al. Citation2019). Especially most Bacteroides and Prevotella have shown to be able to degrade pectin (Elshahed et al. Citation2021). The breakdown products of these pectins can be used by surrounding bacteria and thus influence the composition of the microbiome (Flint et al. Citation2007). Complexity of these pectins can be attributed to the degree of polymerization (DP), branching, esterification, or crosslinking of various cell-wall components (Seal et al. Citation2021). Because of the enzymes that are needed to digest these pectins, the ones with higher degree of methylation tend to take longer to digest (Dongowski, Lorenz, and Proll Citation2002). This would indicate that larger and more branched structures of pectins are fermented more distally in the gastrointestinal tract and suppress protein fermentation, as the microbiome prefers saccharolytic fermentation over proteolytic fermentation. The more complex the fiber, the lower in the distal colon it will be fermented. Therefore, more complex carbohydrates may be preferred to prevent proteolytic fermentation in diets higher in plant-based proteins.
Alternative methods to prevent proteolytic fermentation
Besides the use of non-digestible carbohydrates to reduce proteolytic fermentation, there are other ways to reduce undesired proteolytic fermentation. A possibility would be to process these plant based proteins beforehand, which improves the digestibility (Pieper et al. Citation2016). The main causes of the lower digestibility of plant proteins is attributed to the presence of indigestible cell walls, antinutritional factors and specific protein structures of plant-based protein alternatives (Sá, Moreno, and Carciofi Citation2020). This would indicate that breaking down cell walls, removing chelating antinutrients and modifying these protein structures beforehand through pretreatment should improve bioavailability and digestibility (Shaghaghian et al. Citation2022). However, this processing may damage some amino acids such as lysine, arginine, methionine, cysteine, and tryptophan (González-Vega et al. Citation2011). With these pretreatments potentially damaging some of the beneficial structures of the plant-based protein the question remains if the benefits outweigh the downsides of this method.
Using plant proteins in fermented products might reduce or prevent proteolytic fermentation. Although not much is known about fermentation of plant based products (Holscher Citation2017), it is well known that proteins and other products are broken down into products that can be more easily taken up by the consumer. Historically, fermentation of foods was used most for food preservation but it in the past decade also has been more appreciated for its higher content of beneficial ingredients that support health (Christensen et al. Citation2022). Nowadays fermentation of food is also applied to gain organoleptic properties (taste and texture) in many different products, such as yoghurts and cheeses (Dimidi et al. Citation2019). Better knowledge of these processes in plant-based alternatives would not only improve taste and mouthfeel of these products, but additionally would provide an insight into the differences in effects on the microbiome between plant-based and animal-based products and may lower the threat of proteolytic fermentation (Harper et al. Citation2022).
Future perspectives
As outlined in this review the transition from an animal-based diet toward a plant-based diet may be more cumbersome for health and might require more adaptations in the diet than currently perceived by society. This is even more alarming when the low intake of proteolytic fermentation attenuating dietary fiber consumption in the Western world is taken into consideration (Blackstone and Conrad, Citation2020). The too low intake of non-digestible carbohydrate in combination with a plant-based protein diet, which in general are found to be more “incomplete” and more difficult to digest, might lead to formation of undesired fermentation products in the large intestine and a too low uptake of essential amino acids (Macfarlane et al. Citation1988). New studies investigating different technological applications, such as enzyme treatment and ultrasound could be beneficial in producing more easily digestible plant-based protein alternatives. However, deficits in essential amino acids could lead to disruption of global protein synthesis which is detrimental to survival (Gietzen and Aja Citation2012). Presence of non-digestible carbohydrates in the large intestines is preferred for favoring saccharolytic fermentation by the microbiome, which leads to production of more SCFAs instead of potentially toxic metabolites as a result of proteolytic fermentation (den Besten et al. Citation2013). Ideally, to ensure a smooth transition from animal-based protein sources toward plant-based protein sources, diets should include a mixture of different plant-based proteins for a more complete amino acid composition but also more non-digestible carbohydrates to reduce the proteolytic fermentation. Candidate molecules for this are high DP inulins (Rumessen et al. Citation1990), high DM pectins (Tian et al. Citation2017) and high molecular weight A-type resistant starch type 3 as they are branched, or are having a chemical composition resisting complete fermentation in the cecum (Klostermann et al. Citation2021).
However, not much is known about the influence of a combination of plant-based proteins and non-digestible carbohydrates on colonic health and potential other health benefits. For these reasons it is essential that the interaction between non-digestible carbohydrates and plant-based proteins is better understood, as well as the possible health benefits of this combination. To do so, the impact of different fibers and proteins could be tested in an ex vivo digestion model such as the TIM-2 inoculated with the microbiome of adult humans. Using this model beneficial combinations of fiber and protein could be identified that lead to a beneficial combination of proteolytic and saccharolytic fermentation. Selection criteria could include the presence of metabolites such as SCFAs that are positively associated with gut health and absence of metabolites such as ammonia, phenols, bSCFA and sulfides which tend to be negatively associated with gut health. To further understand the modulatory mechanisms of these fiber and protein combinations on gut health in vitro experiments could be performed, such as the effect of both fermented and unfermented fiber and protein combinations on cells or organoids. Effects on mucus production, gene expression, cytokine expression and protein expression should be investigated as well as their effects on barrier integrity, e.g., by measuring transepithelial electrical resistance (TEER) in in vitro systems. This will lead to a better understanding of the influence of non-digestible carbohydrates and their ability to steer proteolytic and saccharolytic fermentation and will help in the aid to find a more seamless transition toward a more plant-based diet.
Conclusion
The transition from a diet consisting of mostly animal-based protein sources toward a more plant-based diet is nutritionally challenging. The “incomplete” amino acid composition but also the more difficult to digest plant proteins by human enzymes are forcing us to find diet-based strategies to reduce processes such as undesired effects of protein fermentation in the proximal colon. The proximal colon is the principal site for saccharolytic fermentation and formation of beneficial microbial metabolites that support immunity, metabolism and even mental health. Current insight is that too many proteins in the proximal colon may favor proteolytic over saccharolytic fermentation and support formation of microbial metabolites that negatively impact gut health. We conclude and review the current strategies to attenuate proteolytic fermentation in the proximal colon. The most promising and economically feasible options are technologically altering the plant-based proteins to be more easily digested molecules or by supplementing the plant-based protein diets with non-digestible carbohydrates that support saccharolytic fermentation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahluwalia, B., M. K. Magnusson, and L. Öhman. 2017. Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scandinavian Journal of Gastroenterology 52 (11):1185–93. doi: 10.1080/00365521.2017.1349173.
- Argiles, J. M., and F. J. Lopez-Soriano. 1990. Intestinal amino acid transport: An overview. The International Journal of Biochemistry 22 (9):931–7. doi: 10.1016/0020-711x(90)90198-c.
- Ayabe, T., T. Ashida, Y. Kohgo, and T. Kono. 2004. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends in Microbiology 12 (8):394–8. doi: 10.1016/j.tim.2004.06.007.
- Belenguer, A., S. H. Duncan, A. G. Calder, G. Holtrop, P. Louis, G. E. Lobley, and H. J. Flint. 2006. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Applied and Environmental Microbiology 72 (5):3593–9. doi: 10.1128/AEM.72.5.3593-3599.2006.
- Berrazaga, I., V. Micard, M. Gueugneau, and S. Walrand. 2019. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 11 (8):Article 1825. doi: 10.3390/nu11081825.
- Beukema, M., M. M. Faas, and P. de Vos. 2020. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Experimental & Molecular Medicine 52 (9):Article 1364–1376. doi: 10.1038/s12276-020-0449-2.
- Blackstone, N. T, andZ. Conrad. 2020. Comparing the Recommended Eating Patterns of the EAT-Lancet Commission and Dietary Guidelines for Americans: Implications for Sustainable Nutrition. Current Developments in Nutrition 4 (3):nzaa015. doi: 10.1093/cdn/nzaa015.
- Canfora, E. E., R. C. R. Meex, K. Venema, and E. E. Blaak. 2019. Gut microbial metabolites in obesity, NAFLD and T2DM. Nature Reviews Endocrinology 15 (5):Article 261–273. doi: 10.1038/s41574-019-0156-z.
- Carbonaro, M., P. Maselli, and A. Nucara. 2012. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 43 (2):911–21. doi: 10.1007/s00726-011-1151-4.
- Christensen, L. F., B. García-Béjar, C. H. Bang-Berthelsen, and E. B. Hansen. 2022. Extracellular microbial proteases with specificity for plant proteins in food fermentation. International Journal of Food Microbiology 381:109889. doi: 10.1016/j.ijfoodmicro.2022.109889.
- Daly, J. M., J. Reynolds, R. K. Sigal, J. Shou, and M. D. Liberman. 1990. Effect of dietary protein and amino acids on immune function. Critical Care Medicine 18 (Supplement):S94. doi: 10.1097/00003246-199002003-00002.
- David, L. A., C. F. Maurice, R. N. Carmody, D. B. Gootenberg, J. E. Button, B. E. Wolfe, A. V. Ling, A. S. Devlin, Y. Varma, M. A. Fischbach, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 (7484)Article:559–63. doi: 10.1038/nature12820.
- den Besten, G., K. van Eunen, A. K. Groen, K. Venema, D.-J. Reijngoud, and B. M. Bakker. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research 54 (9):2325–40. doi: 10.1194/jlr.R036012.
- Diether, N. E., and B. P. Willing. 2019. Microbial fermentation of dietary protein: An important factor in diet–microbe–host interaction. Microorganisms 7 (1):19. doi: 10.3390/microorganisms7010019.
- Dimidi, E., S. R. Cox, M. Rossi, and K. Whelan. 2019. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 11 (8):1806. doi: 10.3390/nu11081806.
- Dimina, L., D. Rémond, J.-F. Huneau, and F. Mariotti. 2021. Combining plant proteins to achieve amino acid profiles adapted to various nutritional objectives—An exploratory analysis using linear programming. Frontiers in Nutrition 8:809685. doi: 10.3389/fnut.2021.809685.
- Dongowski, G., A. Lorenz, and J. Proll. 2002. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. The Journal of Nutrition 132 (7):1935–44. doi: 10.1093/jn/132.7.1935.
- Elshahed, M. S., A. Miron, A. C. Aprotosoaie, and M. A. Farag. 2021. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydrate Polymers 255:117388. doi: 10.1016/j.carbpol.2020.117388.
- Faith, J. J., J. L. Guruge, M. Charbonneau, S. Subramanian, H. Seedorf, A. L. Goodman, J. C. Clemente, R. Knight, A. C. Heath, R. L. Leibel, et al. 2013. The long-term stability of the human gut microbiota. Science (New York, N.Y.) 341 (6141):1237439. doi: 10.1126/science.1237439.
- Fan, P., L. Li, A. Rezaei, S. Eslamfam, D. Che, and X. Ma. 2015. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Current Protein & Peptide Science 16 (7):646–54. doi: 10.2174/1389203716666150630133657.
- Flint, H. J., S. H. Duncan, K. P. Scott, and P. Louis. 2007. Interactions and competition within the microbial community of the human colon: Links between diet and health. Environmental Microbiology 9 (5):1101–11. doi: 10.1111/j.1462-2920.2007.01281.x.
- form, D. (2022). You have a question about the protein transition? A. our expert dr Scp. C. It’s not as simple as replacing animal- with plant-based protein. WUR, May 9. https://www.wur.nl/en/article/its-not-as-simple-as-replacing-animal-with-plant-based-protein.htm
- Freeman, H. J., Y. S. Kim, and M. H. Sleisenger. 1979. Protein digestion and absorption in man. Normal mechanisms and protein-energy malnutrition. The American Journal of Medicine 67 (6):1030–6. doi: 10.1016/0002-9343(79)90645-4.
- Fukata, M., and M. Arditi. 2013. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunology 6 (3):451–63. doi: 10.1038/mi.2013.13.
- Gensollen, T., S. S. Iyer, D. L. Kasper, and R. S. Blumberg. 2016. How colonization by microbiota in early life shapes the immune system. Science (New York, N.Y.) 352 (6285):539–44. doi: 10.1126/science.aad9378.
- Gietzen, D. W., and S. M. Aja. 2012. The brain’s response to an essential amino acid-deficient diet and the circuitous route to a better meal. Molecular Neurobiology 46 (2):332–48. doi: 10.1007/s12035-012-8283-8.
- Gilani, G. S., C. W. Xiao, and K. A. Cockell. 2012. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. The British Journal of Nutrition 108 Suppl 2 (S2):S315–S332. doi: 10.1017/S0007114512002371.
- Gilani, G. S., K. A. Cockell, and E. Sepehr. 2005. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. Journal of AOAC INTERNATIONAL 88 (3):967–87. doi: 10.1093/jaoac/88.3.967.
- Gilbert, J., M. J. Blaser, J. G. Caporaso, J. Jansson, S. V. Lynch, and R. Knight. 2018. Current understanding of the human microbiome. Nature Medicine 24 (4):392–400. doi: 10.1038/nm.4517.
- Gilbert, M. S., N. Ijssennagger, A. K. Kies, and S. W. C. van Mil. 2018. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. American Journal of Physiology. Gastrointestinal and Liver Physiology 315 (2):G159–G170. doi: 10.1152/ajpgi.00319.2017.
- González-Vega, J. C., B. G. Kim, J. K. Htoo, A. Lemme, and H. H. Stein. 2011. Amino acid digestibility in heated soybean meal fed to growing pigs. Journal of Animal Science 89 (11):3617–25. doi: 10.2527/jas.2010-3465.
- Gordon, S. 2002. Pattern recognition receptors: Doubling up for the innate immune response. Cell 111 (7):927–30. doi: 10.1016/s0092-8674(02)01201-1.
- Gorissen, S. H. M., J. J. R. Crombag, J. M. G. Senden, W. A. H. Waterval, J. Bierau, L. B. Verdijk, and L. J. C. van Loon. 2018. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50 (12):1685–95. doi: 10.1007/s00726-018-2640-5.
- Grouls, M., A. W. F. Janssen, L. P. M. Duivenvoorde, G. J. E. J. Hooiveld, H. Bouwmeester, and M. van der Zande. 2022. Differential gene expression in iPSC-derived human intestinal epithelial cell layers following exposure to two concentrations of butyrate, propionate and acetate. Scientific Reports 12 (1):13988. doi: 10.1038/s41598-022-17296-8.
- Harper, A. R., R. C. J. Dobson, V. K. Morris, and G. Moggré. 2022. Fermentation of plant‐based dairy alternatives by lactic acid bacteria. Microbial Biotechnology 15 (5):1404–21. doi: 10.1111/1751-7915.14008.
- Hasegawa, M., and N. Inohara. 2014. Regulation of the gut microbiota by the mucosal immune system in mice. International Immunology 26 (9):481–7. doi: 10.1093/intimm/dxu049.
- Hill, M. J. 1997. Intestinal flora and endogenous vitamin synthesis. European Journal of Cancer Prevention 6 (1):S43–S45. doi: 10.1097/00008469-199703001-00009.
- Hoffman, J. R., and M. J. Falvo. 2004. Protein – Which is Best? Journal of Sports Science & Medicine 3 (3):118–30.
- Holscher, H. D. 2017. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8 (2):172–84. doi: 10.1080/19490976.2017.1290756.
- Ijssennagger, N., R. van der Meer, and S. W. C. van Mil. 2016. Sulfide as a Mucus Barrier-Breaker in inflammatory bowel disease? Trends in Molecular Medicine 22 (3):190–9. doi: 10.1016/j.molmed.2016.01.002.
- Joeris, T., K. Müller-Luda, W. W. Agace, and A. M. Mowat. 2017. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunology 10 (4):845–64. doi: 10.1038/mi.2017.22.
- Juge, N. 2022. Relationship between mucosa-associated gut microbiota and human diseases. Biochemical Society Transactions 50 (5):1225–36. doi: 10.1042/BST20201201.
- Kelly, G. 2008. Inulin-type prebiotics–A review: Part 1. Alternative Medicine Review 13 (4):315–29.
- Khan, I., Z. Huang, L. Liang, N. Li, Z. Ali, L. Ding, M. Hong, and H. Shi. 2021. Ammonia stress influences intestinal histomorphology, immune status and microbiota of Chinese striped-neck turtle (Mauremys sinensis). Ecotoxicology and Environmental Safety 222:112471. doi: 10.1016/j.ecoenv.2021.112471.
- Klostermann, C. E., P. L. Buwalda, H. Leemhuis, P. de Vos, H. A. Schols, and J. H. Bitter. 2021. Digestibility of resistant starch type 3 is affected by crystal type, molecular weight and molecular weight distribution. Carbohydrate Polymers 265:118069. doi: 10.1016/j.carbpol.2021.118069.
- Korpela, K. 2018. Diet, microbiota, and metabolic health: Trade-off between saccharolytic and proteolytic fermentation. Annual Review of Food Science and Technology 9 (1):65–84. doi: 10.1146/annurev-food-030117-012830.
- Krehbiel, C. R., and J. C. Matthews. 2003. Absorption of amino acids and peptides. Amino Acids in Animal Nutrition 41–70. doi: 10.1079/9780851996547.0041.
- Kuhn, K. A., I. Pedraza, and M. K. Demoruelle. 2014. Mucosal immune responses to microbiota in the development of autoimmune disease. Rheumatic Diseases Clinics of North America 40 (4):711–25. doi: 10.1016/j.rdc.2014.07.013.
- Larsen, N., C. Bussolo de Souza, L. Krych, T. Barbosa Cahú, M. Wiese, W. Kot, K. M. Hansen, A. Blennow, K. Venema, and L. Jespersen. 2019. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Frontiers in Microbiology 10:223. doi: 10.3389/fmicb.2019.00223.
- LeBlanc, J. G., C. Milani, G. S. de Giori, F. Sesma, D. van Sinderen, and M. Ventura. 2013. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Current Opinion in Biotechnology 24 (2):160–8. doi: 10.1016/j.copbio.2012.08.005.
- Li, X., B. Zhang, Y. Hu, and Y. Zhao. 2021. New insights into gut-bacteria-derived indole and its derivatives in intestinal and liver diseases. Frontiers in Pharmacology 12:769501. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2021.769501. doi: 10.3389/fphar.2021.769501.
- Lopez, M. J., and S. S. Mohiuddin. 2022. Biochemistry, Essential Amino Acids. Treasure Island, FL, USA: StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK557845/.
- Macfarlane, G. T., C. Allison, S. A. Gibson, and J. H. Cummings. 1988. Contribution of the microflora to proteolysis in the human large intestine. The Journal of Applied Bacteriology 64 (1):37–46. doi: 10.1111/j.1365-2672.1988.tb02427.x.
- Moughan, P. J. 2021. Population protein intakes and food sustainability indices: The metrics matter. Global Food Security 29:100548. doi: 10.1016/j.gfs.2021.100548.
- Murray, P. J. 2006. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Current Opinion in Pharmacology 6 (4):379–86. doi: 10.1016/j.coph.2006.01.010.
- Natividad, J. M. M., and E. F. Verdu. 2013. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacological Research 69 (1):42–51. doi: 10.1016/j.phrs.2012.10.007.
- Neis, E. P. J. G., C. H. C. Dejong, and S. S. Rensen. 2015. The role of microbial amino acid metabolism in host metabolism. Nutrients 7 (4):2930–46. doi: 10.3390/nu7042930.
- O’Mahony, C., P. Scully, D. O’Mahony, S. Murphy, F. O’Brien, A. Lyons, G. Sherlock, J. MacSharry, B. Kiely, F. Shanahan, et al. 2008. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathogens 4 (8):e1000112. doi: 10.1371/journal.ppat.1000112.
- Pascale, N., F. Gu, N. Larsen, L. Jespersen, and F. Respondek. 2022. The potential of pectins to modulate the human gut microbiota evaluated by in vitro fermentation: A systematic review. Nutrients 14 (17):3629. doi: 10.3390/nu14173629.
- Pedersen, G., J. Brynskov, and T. Saermark. 2002. Phenol toxicity and conjugation in human colonic epithelial cells. Scandinavian Journal of Gastroenterology 37 (1):74–9. doi: 10.1080/003655202753387392.
- Pesta, D. H., and V. T. Samuel. 2014. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutrition & Metabolism 11 (1):53. doi: 10.1186/1743-7075-11-53.
- Pieper, R., C. V. Tudela, M. Taciak, J. Bindelle, J. F. Pérez, and J. Zentek. 2016. Health relevance of intestinal protein fermentation in young pigs. Animal Health Research Reviews 17 (2):137–47. doi: 10.1017/S1466252316000141.
- Prokopidis, K., M. M. Cervo, A. Gandham, and D. Scott. 2020. Impact of protein intake in older adults with sarcopenia and obesity: A gut microbiota perspective. Nutrients 12 (8):2285. doi: 10.3390/nu12082285.
- Quigley, E. M. M. 2013. Gut bacteria in health and disease. Gastroenterology & Hepatology 9 (9):560–9.
- Rajilić-Stojanović, M., and W. M. de Vos. 2014. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiology Reviews 38 (5):996–1047. doi: 10.1111/1574-6976.12075.
- Richards, J. L., Y. A. Yap, K. H. McLeod, C. R. Mackay, and E. Mariño. 2016. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clinical & Translational Immunology 5 (5):e82. doi: 10.1038/cti.2016.29.
- Rios-Covian, D., S. González, A. M. Nogacka, S. Arboleya, N. Salazar, M. Gueimonde, and C. G. de los Reyes-Gavilán. 2020. An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: Associated dietary and anthropometric factors. Frontiers in Microbiology 11:973. doi: 10.3389/fmicb.2020.00973.
- Rumessen, J. J., S. Bodé, O. Hamberg, and E. Gudmand-Høyer. 1990. Fructans of Jerusalem artichokes: Intestinal transport, absorption, fermentation, and influence on blood glucose, insulin, and C-peptide responses in healthy subjects. The American Journal of Clinical Nutrition 52 (4):675–81. doi: 10.1093/ajcn/52.4.675.
- Sá, A. G. A., Y. M. F. Moreno, and B. A. M. Carciofi. 2020. Food processing for the improvement of plant proteins digestibility. Critical Reviews in Food Science and Nutrition 60 (20):3367–86. doi: 10.1080/10408398.2019.1688249.
- Seal, C. J., C. M. Courtin, K. Venema, and J. de Vries. 2021. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Comprehensive Reviews in Food Science and Food Safety 20 (3):2742–68. doi: 10.1111/1541-4337.12728.
- Shaghaghian, S., D. J. McClements, M. Khalesi, M. Garcia-Vaquero, and A. Mirzapour-Kouhdasht. 2022. Digestibility and bioavailability of plant-based proteins intended for use in meat analogues: A review. Trends in Food Science & Technology 129:646–56. doi: 10.1016/j.tifs.2022.11.016.
- Shi, N., N. Li, X. Duan, and H. Niu. 2017. Interaction between the gut microbiome and mucosal immune system. Military Medical Research 4 (1):14. doi: 10.1186/s40779-017-0122-9.
- Siddiqui, M. T., and G. A. M. Cresci. 2021. The immunomodulatory functions of butyrate. Journal of Inflammation Research 14:6025–41. doi: 10.2147/JIR.S300989.
- Smith, E. A., and G. T. Macfarlane. 1998. Enumeration of amino acid fermenting bacteria in the human large intestine: Effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiology Ecology 25 (4):355–68. doi: 10.1111/j.1574-6941.1998.tb00487.x.
- Tian, L., G. Bruggeman, M. van den Berg, K. Borewicz, A. J. W. Scheurink, E. Bruininx, P. de Vos, H. Smidt, H. A. Schols, and H. Gruppen. 2017. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Molecular Nutrition & Food Research 61 (1):1600186. doi: 10.1002/mnfr.201600186.
- Topping, D. L., and P. M. Clifton. 2001. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiological Reviews 81 (3):1031–64. doi: 10.1152/physrev.2001.81.3.1031.
- Tso, R., and C. G. Forde. 2021. Unintended consequences: Nutritional impact and potential pitfalls of switching from animal- to plant-based foods. Nutrients 13 (8):Article 2527. doi: 10.3390/nu13082527.
- Uebanso, T., T. Shimohata, K. Mawatari, and A. Takahashi. 2020. Functional roles of B-vitamins in the gut and gut microbiome. Molecular Nutrition & Food Research 64 (18):e2000426. doi: 10.1002/mnfr.202000426.
- van der Beek, C. M., E. E. Canfora, K. Lenaerts, F. J. Troost, S. W. M. Olde Damink, J. J. Holst, A. A. M. Masclee, C. H. C. Dejong, and E. E. Blaak. 2016. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clinical Science (London, England: 1979) 130 (22):2073–82. doi: 10.1042/CS20160263.
- van Vliet, S., N. A. Burd, and L. J. van Loon. 2015. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. The Journal of Nutrition 145 (9):1981–91. doi: 10.3945/jn.114.204305.
- Vinolo, M. A. R., H. G. Rodrigues, R. T. Nachbar, and R. Curi. 2011. Regulation of inflammation by short chain fatty acids. Nutrients 3 (10):Article 858–876. doi: 10.3390/nu3100858.
- Voragen, A. G. J., G.-J. Coenen, R. P. Verhoef, and H. A. Schols. 2009. Pectin, a versatile polysaccharide present in plant cell walls. Structural Chemistry 20 (2):263–75. doi: 10.1007/s11224-009-9442-z.
- Walker, A. W., J. Ince, S. H. Duncan, L. M. Webster, G. Holtrop, X. Ze, D. Brown, M. D. Stares, P. Scott, A. Bergerat, et al. 2011. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. The ISME Journal 5 (2):220–30. doi: 10.1038/ismej.2010.118.
- Windey, K., V. De Preter, and K. Verbeke. 2012. Relevance of protein fermentation to gut health. Molecular Nutrition & Food Research 56 (1):184–96. doi: 10.1002/mnfr.201100542.
- Xiao, X., P.-R. Zou, F. Hu, W. Zhu, and Z.-J. Wei. 2023. Updates on plant-based protein products as an alternative to animal protein: Technology, properties, and their health benefits. Molecules (Basel, Switzerland) 28 (10):4016. doi: 10.3390/molecules28104016.
- Yu, P. 2005. Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: A new approach. The British Journal of Nutrition 94 (5):655–65. doi: 10.1079/BJN20051532.
- Zhang, H., N. van der Wielen, B. van der Hee, J. Wang, W. Hendriks, and M. Gilbert. 2020. Impact of fermentable protein, by feeding high protein diets, on microbial composition, microbial catabolic activity, gut health and beyond in pigs. Microorganisms 8 (11):1735. doi: 10.3390/microorganisms8111735.
- Zhang, Q., M. Raoof, Y. Chen, Y. Sumi, T. Sursal, W. Junger, K. Brohi, K. Itagaki, and C. J. Hauser. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464 (7285):104–7. doi: 10.1038/nature08780.