Abstract
Encapsulation technologies have achieved encouraging results improving the stability, bioaccessibility and absorption of bioactive compounds post-consumption. There is a bulk of published research on the gastrointestinal behavior of encapsulated bioactive food materials alone using in vitro and in vivo digestion models, but an aspect often overlooked is the impact of the food structure, which is much more complex to unravel and still not well understood. This review focuses on discussing the recent findings in the application of encapsulated bioactive components in fabricated food matrices. Studies have suggested that the integration of encapsulated bioactive compounds has been proven to have an impact on the physicochemical characteristics of the finished product in addition to the protective effect of encapsulation on the fortified bioactive compound. These products containing bioactive compounds undergo further structural reorganization during digestion, impacting the release and emptying rates of fortified bioactive compounds. Thus, by manipulation of various food structures and matrices, the release and delivery of these bioactive compounds can be altered. This knowledge provides new opportunities for designing specialized foods for specific populations.
HIGHLIGHTS
Food structure confers specific functionalities to supplemented encapsulated bioactive compounds during processing and digestion.
Encapsulation of bioactive compounds prevents changes in physicochemical attributes of foods during processing.
The unique disintegration patterns of foods in the gut impacts how bioactive substances are released and absorbed.
Introduction
Bioactive compounds are biologically active substances that are extracted from animal- or plant-based sources and have pharmacological or physiological effects that can promote human health. In general, these compounds are produced as secondary metabolites in small quantities and can modulate metabolic processes (Graebin et al. Citation2019; Khaw et al. Citation2017; Yang et al. Citation2016). Bioactive compounds are classified into two major classes based on different chemical structures, i.e., water-soluble compounds (hydrophilic) and lipid-soluble compounds (Lipophilic) (Carbonell-Capella et al. Citation2014; Galanakis Citation2017). They are extremely heterogeneous in their chemical structure (polyphenols, carotenoids, tocopherols, phytosterols, organosulfur compounds, and peptides) and have been reported to have several health-promoting activities such as antimicrobial, antioxidant, anti-inflammatory, antidiabetic, among others (Eggersdorfer and Wyss Citation2018; Oyenihi and Smith Citation2019; Prasad et al. Citation2022; Ruhee et al. Citation2020; Szewczyk, Chojnacka, and Górnicka Citation2021; Tornesello et al. Citation2020). Bioactive components derived from natural foods are located in complex matrices of lipids, proteins, and/or carbohydrates, which may impede their release into the gastrointestinal fluids during digestion. In addition, their naturally low levels in various foods and possible chemical interactions with other food constituents significantly decrease bioaccessibility and bioavailability. The concept of isolating bioactive compounds from natural sources to enrich other commonly consumed foods enables achieving higher loading per serve and possibly increase the uptake (Moreno and Ilic Citation2018; Shahidi Citation2009).
Scientific understanding of the potential benefits of the bioactive food components has stimulated consumers choice toward natural products with additional health-promoting effect (Betoret et al. Citation2011; Mollet and Rowland Citation2002). However, formulating bioactive compounds into fabricated foods encounter many challenges including changes in products’ quality, but also losses of bioactivity after consumption because of physical and chemical changes in the bioactive compound within the gastrointestinal tract. In this regard, encapsulation has been regarded as an effective technology to protect bioactive substances within the food products from the peripheral environments, regulate their release in a particular environment, and improve consumer compliance and convenience (Nedovic et al. Citation2011; Qazi et al. Citation2015; Rezvankhah, Emam-Djomeh, and Askari Citation2020; Rodríguez et al. Citation2016). Additionally, encapsulation systems can be designed to control the targeted delivery of bioactive compounds in the gastrointestinal environment, thus enhancing their bioaccessibility and bioavailability (Donhowe et al. Citation2014; Jiang, Liao, and Charcosset Citation2020; Sabet et al. Citation2021). The functionality of encapsulated systems is associated with a specific formulation that involves combining the appropriate ratios of carrier materials, functional substances, and one or more biopolymeric emulsifier layers with the right hydrophilic–lipophilic balance (Bao et al. Citation2019; McClements Citation2018; Qazi et al. Citation2015; Zhu Citation2017). For example, emulsion-based encapsulation systems can entrap bioactive substances into a dispersed phase or be adsorbed onto an oil/water interface. Depending on the emulsion design, it is possible to influence the release rate of the entrapped compound during digestion and absorption (Donhowe et al. Citation2014; Jiang, Liao, and Charcosset Citation2020).
To date, most published studies are focused on designing bioactive encapsulation systems and describing their performance under gastrointestinal conditions. However, a few attempts have been made to link bioaccessibility and bioavailability to the structural features of food products and/or to the encapsulation system. Therefore, this review aims to provide a comprehensive overview of the recent advances in the development of food matrices fortified with encapsulated bioactive compounds. The review covers the effects of bioactive fortification on the physicochemical properties of the resulting food matrices during processing, and the impact of food structure on digestion and bioaccessibility of fortified bioactive compounds.
The food structure effect
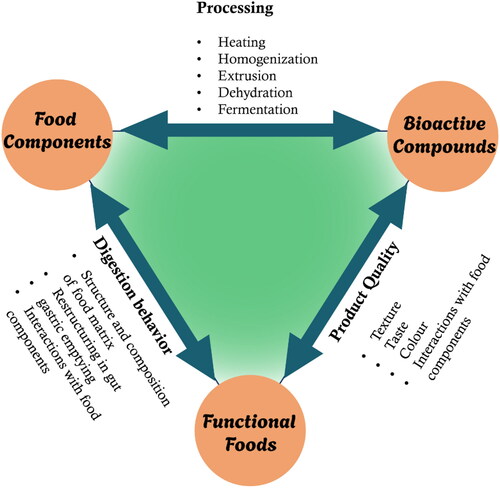
The food structure usually corresponding to a physical and spatial domain, that contains, interacts directly and/or gives a particular functionality to a constituent or element of the food (Aguilera Citation2019). This cellular- or colloidal-based dissipative organization is often categorized as soft matter, as its functionality is driven mainly by its internal (i.e., physicochemical and biochemical reactions) and external (i.e., enzymes, pH, thermal fluctuations, and microorganisms) perturbations (Aguilera Citation2019; Alongi and Anese Citation2021). The complexity of self-assembled structures in animal- or plant-based foods and structures generated in processed foods revolves around their structural orientation and their chemical composition (Aguilera Citation2005; Capuano et al. Citation2018; Flores and Kong Citation2017; Parada and Aguilera Citation2007; Ubbink Citation2012). The rigid and intact structures of native foods, such as plant- and animal-tissue-based fibrous structures, plant-based fleshy materials, and encapsulated plant embryos, not only impact functionality and digestibility but also significantly impact the release of nutrients and the entrapped bioactive compounds (Acevedo-Fani, Dave, and Singh Citation2020; Cifelli Citation2021). The processing of these foods affects several physical, chemical, and nutritional attributes via the changes their structural arrangements. Processing can be used to develop, from basic raw materials, more complex structures in foods; for example, yogurt, cheese, and ice cream are all milk-based products with distinctive structures and properties (Dima et al. Citation2020). Moreover, the processed food is sometimes structurally modified to such an extent that its biological origin is not readily apparent, e.g., the conversion of liquid milk to solid cheese or the transformation of hard grain to fluffy bread. In addition, structural changes continue either during or after processing. Most foods never attain thermodynamic equilibrium, e.g., the softening and loss of crunchiness in fruits as they go through ripening and senescence leads to undesirable product quality (Agarwal et al. Citation2019; Joardder, Kumar, and Karim Citation2017). Thus, an understanding of the structural properties of food materials is important for proper control of food processing operations as well as for improvement in the quality of the final product (Karim et al. Citation2018; Lamothe et al. Citation2017; Parada and Aguilera Citation2007).
shows the impact of the interactions among food structures, food components and bioactive substances on processing, product quality and digestion behavior. These interactions are discussed in more detail in subsequent sections.
In-product behavior
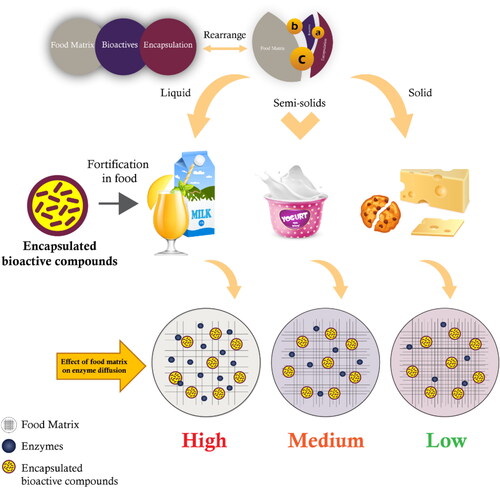
Over the last 10 years, a reasonable number of studies has been published dealing with the product quality and shelf-life changes affected by the addition of encapsulated bioactive compounds to a variety of food matrices, including liquid, semi-solid and solid foods. The findings of these studies are summarized in .
Table 1. Previous investigations on the relationship between the encapsulated bioactive compounds and fortified food matrix.
Beverages
In recent times, the functional beverages have become the most active functional food category due to convenience and the possibility to meet consumers’ demands. However, the physicochemical environment of the beverages (e.g., low, high pH, moisture, salts etc.), their compositions (e.g., fruit pulp, chocolate, stabilizers, etc.) and their processing conditions can be challenging that might negatively affect the fortified bioactive compounds (Aadil et al. Citation2019; Ozdal, Yolci-Omeroglu, and Tamer Citation2020). Micro/nanoencapsulation has improved the sensory characteristics of functional beverages, including flavor, shelf-life stability, and controlled flavor release. In addition, the incorporated encapsulated bioactives improved the health-promoting properties of the beverages (Ozdal, Yolci-Omeroglu, and Tamer Citation2020). Tao et al. (Citation2017) developed a steppogenin, loaded microemulsion to increase its solubility and stability in aqueous solution and studied microemulsions’ ability to prevent oxidation of fresh apple juice. The steppogenin’s (a natural flavanol) water solubility increased up to 3000-fold using a microemulsion and significantly inhibited browning of fresh apple juice. In another investigation, (Istenič, Cerc Korošec, and Poklar Ulrih Citation2016) tested the storage stability of epigallocatechin gallate in free form, entrapped into liposomes, and into alginate and chitosan microparticles loaded with epigallocatechin gallate liposomes in a fruit nectar. Compared with the free form, both delivery systems had the ability to stabilize epigallocatechin gallate in the liquid food during storage.
Dairy-based products
Since ancient times, dairy products have been the primary source of nutrients such as protein, fat, vitamins, and minerals, making them the most popular and highly consumed products worldwide (Eržen, Kač, and Pravst Citation2014; Pereira Citation2014; Scholz-Ahrens, Ahrens, and Barth Citation2020). This also makes them appropriate vehicles for the addition or modification of a variety of nutrients and health-enhancing substances. Dairy-product-based foods can be classified into three main categories: liquid (milk and fermented milk products), semi-solid (yogurt and a few varieties of soft cheese), and solid (mostly cheeses). However, the stability and the effective integration of isolated bioactive compounds have a detrimental impact on sensory attributes and preservation. Additionally, heat, light, oxygen, an acidic or basic pH, and water can make these bioactive chemicals vulnerable, which leads to undesirable changes in the products (Augustin and Sanguansri Citation2015; Bao et al. Citation2019; Cifelli Citation2021; Raikos and Ranawana Citation2017). Encapsulation with a properly chosen coating material can overcome these challenges, with little to no impact on the physicochemical and organoleptic qualities of the product.
For liquid milk products, the addition of several type of encapsulation systems have been investigated. For example, Moghadam et al. (Citation2019) added nano-encapsulated fish oil consisting of omega-3 fatty acids into a probiotic fermented milk. Authors found that the fermented milks containing nano-encapsulated fish oil increased probiotic bacterial counts, decreased the oxidation of eicosapentaenoic acid and docosahexaenoic acid, without negatively affect the sensorial attributes of the product. Similarly, Joung et al. (Citation2016) assessed the radical scavenging activity of a curcumin nanoemulsion in a low-fat milk. The radical scavenging activity was not significantly affected by the water content, but was significantly increased by the surfactant concentration, indicating that a high surfactant concentration might make it easier for curcumin to dissolve in the oil phase, which would then increase the antioxidant activity. In a different study, the inclusion of both free and encapsulated arjuna herb phenolics had a significant impact on the physicochemical characteristics of the fortified matrix during long-term storage. However, changes in quality characteristics occurred less frequently in the sample containing encapsulated arjuna phenolics than in the other samples, indicating that the encapsulated form was successful in improving the storage stability of dairy drinks (Sawale et al. Citation2017).
The fundamental steps in the production of fermented dairy products (i.e., cheese and yogurt) include coagulation or gelation of milk proteins. Caseins and whey proteins, the two primary proteins found in milk, can be destabilized by enzymes such as rennet to coagulate the caseins, heat to denature the whey proteins, and acid to coagulate the caseins. Gelation is an irreversible because milk protein undergoes rearrangements, fusion, and syneresis during and after gelation (Fagan et al. Citation2017; Lucey Citation2008; Panthi et al. Citation2019). These structures in a coagulated protein system entrap milk fat as well as related nutrients and bioactive compounds. Tan et al. (Citation2018) investigated the impact of storage and the yogurt matrix on the stability of tocotrienols encapsulated in chitosan–alginate microcapsules. The texture, color, and viscosity of the yogurt matrix were not significantly altered by the addition of tocotrienol microcapsules, and the tocotrienol microcapsules demonstrated greater resistance to oxidation during heat treatment than the unencapsulated tocotrienols. However, both the heat treatment and the acidity of the yogurt caused the rapid loss of α-tocotrienol in the fabricated microcapsules. Another study used yogurt as a delivery system for liposomal powdered phenolics from sour cherries. Compared with the control yogurt, the spray-dried liposomal powder resulted in an increased total dry solid and an increased water-holding capacity, which reduced syneresis in the fortified yogurt. Moreover, the total phenolic content was found to be highest in the yogurt samples that incorporated encapsulated sour cherry phenolics (Akgün et al. Citation2020). Ye et al. (Citation2009) evaluated the lipid oxidation, sensory stability, and microstructure of processed cheese enriched with fish oil emulsion. The addition of emulsified fish oil substantially changed the microstructure of the processed cheese, and it was hypothesized that this change in microstructure and the choice of emulsifier for preparing the emulsions contributed to the oxidative stability of the processed cheese.
Green tea catechins and epigallocatechin gallate that were encapsulated in soy lecithin-based liposomes were added to low-fat hard cheese (Rashidinejad et al. Citation2014). During cheese processing, liposomes were retained in the curd rather than partitioning into the whey. However, the interaction of the catechins with the components of the cheese was protected by the encapsulation, which also protected them against degradation and loss of antioxidant activity. Similarly, grape polyphenols that were encapsulated by multiple emulsions and added to Chihuahua cheese were compared, in terms of structure and physiochemical properties, with the control cheese and cheese with free grape polyphenols (Pimentel-González et al. Citation2015). Even though the inclusion of encapsulated polyphenols had an impact on the cohesiveness, moisture content, and ash content of the cheese, the encapsulation proved to be more effective at protecting the polyphenols and the cheese displayed physicochemical traits that were comparable with those of traditional cheese.
Starch-based foods
Starch is composed of two types of molecule: linear glucose polymer (amylose) and branched polymer (amylopectin) (Singh, Dartois, and Kaur Citation2010; Wang and Copeland Citation2013). Different ratios of these two molecules and their orientation within the granules provide starch with considerable variability in functional properties, such as water absorption, swelling, pasting, gelation behavior, and vulnerability to enzymatic degradation (Singh, Kaur, and Singh Citation2013; Zhu Citation2017).
The starch matrix has been shown to better protect hydrophilic and hydrophobic food ingredients from degradation under high temperature processing compared with lipid and protein food matrices, which undergo degradation (Fathi, Martín, and McClements Citation2014; Zhu Citation2017); however, the direct addition of bioactive compounds to cooked foods is still not possible, because of their easy thermal degradation/oxidation (Papillo, Arlorio, et al. Citation2019). Additionally, in a complex food, the way in which proteins and lipids interact with starch throughout the processing and digestion of meals might alter the quantities of glucose and active ingredients that are released (Lu et al. Citation2019; Singh, Dartois, and Kaur Citation2010). To overcome the environmental influence, the encapsulation has shown promising results in protecting the payload. Papillo, Arlorio, et al. (Citation2019) added microencapsulated cocoa husk polyphenols to model biscuits to increase their stability during baking. After baking, the antioxidant activity of all the biscuits was decreased considerably compared with that of the equivalent powder at 0 min. The biscuits containing polyphenol microencapsulated with maltodextrin, which served as a stabilizing agent in preventing the heat-liable polyphenols from degradation, had the highest total phenolic content and the highest antioxidant activity. In contrast, the biscuits containing extract that was not microencapsulated had little stability. In a different study, the oxidative stability of granola bars supplemented with a multilayered fish oil emulsion was examined in the presence of novel brown-seaweed-based antioxidants. The bars made with the secondary emulsion method produced fewer oxidation products, which could probably be attributed to the thicker interfacial layer, which would act as a barrier to the penetration and diffusion of molecular species that support oxidation in lipids during the baking of granola bars (Hermund et al. Citation2016).
The integration of bioactive compounds has been proven to have an impact on the structural characteristics of the finished product in addition to the protective effect of encapsulation on the fortified bioactive compound (Delfanian and Sahari Citation2020; Tolve et al. Citation2023). For instance, the presence of gluten protein during bread baking gives distinctive viscoelastic properties to the dough, allowing the dough to expand during fermentation while also retaining the majority of the gas inside the dough structure. The direct addition of organic acids to the dough can significantly reduce the mixing time and make the dough weaker (Lu et al. Citation2021; Su et al. Citation2019). Ezhilarasi et al. (Citation2014) studied the effect of both unencapsulated and microencapsulated Garcinia fruit extract on the quality of bread. The direct addition of the Garcinia fruit extract significantly lowered the volume of the bread because of the presence of hydroxycitric acid, which directly affected the dough development and ultimately resulted in the poor bread volume. In contrast, microencapsulation improved the resistance of the dough to the effects of acid and assisted in maintaining the volume of the dough at a specific level, producing a bread with a softer crumb texture and better sensory attributes. Even though there is a wealth of information on the use of starch-based encapsulation methods for bioactive substances, a thorough approach examining the interactions between the starch matrix and the encapsulated bioactive ingredients is still required. With the appropriate physicochemical properties and controlled release applications, it will be possible to build superior functional products.
Meat products
The meat industry is paying increased attention to the incorporation of bioactive compounds in order to create various meat products that are both nutritious and of high quality. As well as the successful replacement of synthetic antioxidants and antimicrobials in recent years, meat products incorporating bioactive-loaded oil-in-water emulsions have also demonstrated an improvement in the fat content to fulfill consumer demand (Domínguez et al. Citation2021; Keenan et al. Citation2015; Robert et al. Citation2019). The partial substitution of native saturated animal fat with bioactive-loaded emulsions containing healthier unsaturated fats/lipids from other sources is arguably a healthier option but creates considerable hurdles in terms of texture, lubricity, and mouthfeel in comminuted products (Nacak, Öztürk-Kerimoğlu, Yıldız, Çağındı, and Serdaroğlu et al. Citation2021; Nieto and Lorenzo Citation2021; Serdaroğlu, Öztürk, and Urgu Citation2016). Robert et al. (Citation2019) encapsulated olive leaf extract in a double emulsion and introduced it into a meat system as a fat replacer. Because of the enhanced double emulsion dispersion within the meat matrix, the substitution of pork backfat with the double emulsion greatly improved the binding and textural qualities of the meat system. Additionally, encapsulated olive leaf extract also enhanced the oxidative stability and oleuropein degradation, resulting in meat systems with reduced concentrations of thiobarbituric acid reactive substances and lower peroxide values compared with the control. Another study compared the effects of using encapsulated and unencapsulated fish oil to partially replace the fat in beef burger patties (Keenan et al. Citation2015). In addition to altering the fatty acid profile, the inclusion of encapsulated fish oil resulted in greater texture modification with reduced cooking loss compared with the control. Despite these published studies, this area of meat-based foods with added encapsulated bioactive compounds has not been extensively investigated. Thus, further research to explore the in-product interactions of the bioactive compounds with the other elements of the surrounding matrix is required, as this will aid in the development of meat products that have better consumer acceptance.
Lipid/fat-based foods
Lipid/fat-based foods are complex colloidal systems consisting of highly organized, self-assembled microstructures that can generally be affected by various factors such as water content, processing conditions, fat/lipid composition, and storage conditions. These foods have been used as an important template for the delivery of lipophilic bioactive compounds. However, most of the studies to date have focused on the direct fortification of these isolated health-promoting compounds, which undergo various transformations during processing and storage. Encapsulation is an effective method for protecting these chemicals from degradation while also improving their stability in the matrix and ensuring end-product functionality. Zaidel et al. (Citation2014) investigated the storage and stability characteristics of margarine, i.e., a water-in-oil emulsion, containing both nonencapsulated and encapsulated anthocyanins from roselle and red cabbage. When compared with nonencapsulated anthocyanins, margarines containing encapsulated anthocyanins had superior attributes, as evidenced by their high melting completion temperature, low onset crystallization temperature, and higher stability during storage. In another study, Rafiee et al. (Citation2018) evaluated the effect of phenolic compounds containing nanoliposomes on the oxidative stability, microbial spoilage, and sensory properties of mayonnaise samples during storage. In addition to improving the phenolic component retention, the slower, more gradual release of the polyphenols from the nanoliposomes resulted in significantly fewer alterations in color metrics and sensory characteristics than did the free phenolic compounds.
Mostly these studies focused on the influence of initial structures of food systems. However, the inclusion of other food components, such as the combination of protein, lipids, and polysaccharides in a single meal composition, may lead to far more complicated chemical interactions and structural organization at the macro-, meso-, and microscopic level. As a result, real fortified food matrices (i.e., noodles, curries, cereals) that are part of our common diet might be a future study path to examine.
Behavior of encapsulated bioactive compounds during digestion
The process of the disintegration and consequent absorption of nutrients in the human gut is directly affected by the microstructural arrangement of the food (Aguilera Citation2019; Kong and Singh Citation2008; Rein et al. Citation2013). The complex structure of the meal is disrupted throughout the digestion process, reducing its particle size by comminution and trituration. Thus, for the design and production of innovative foods with particular targeted behaviors within the body, it is crucial to understand the relative relevance of the gut disintegration processes in relation to the composition and structure of the foods (Acevedo-Fani, Dave, and Singh Citation2020; Somaratne et al. Citation2020).
The structural breakdown of foods takes place in the mouth, stomach, and small intestine – the three primary parts of the digestive system. Depending on their physiology/anatomy and the structure of the ingested food, each of these digestive organs contributes differently to the breakdown process. All the fragments of the ingested food, regardless of their texture, and size, are processed in a specific way and vary a lot from one person to another. These variations in fragmentation are influenced by the individual person’s behavior and masticatory system, i.e., total ocular area ≈ 214.7 cm2, including lips, cheeks, palate, tongue, and teeth, number of teeth and chewing cycles, bite force applied by jaw muscular activity, amount of saliva produced to bind the masticated food into a coherent and slippery bolus, and the different phases of the foods including solids, semi-solids, or even liquids (Liu et al. Citation2017; van der Bilt Citation2009). Additionally, factors such as age, gender, ethnic groups, and oral health may vary the process of chewing (Chen Citation2009). During the chewing process, saliva moistens the food particles and converts them into a slippery bolus that can pass easily down the alimentary canal. Unlike solid foods, liquid foods do not undergo a large amount of chewing and mastication and have comparatively very short residence times in the oral cavity (Minekus et al. Citation2014). Additionally, the nature of the liquid, i.e., viscosity because of dispersed particles, the ratio of water to fat, and rheological attributes of emulsions mixed with salivary proteins, significantly influences the structural properties of the bolus (Liu et al. Citation2017; van der Bilt Citation2009).
The heterogeneous particles of the bolus are further hydrolyzed by the gastric secretions, which convert the dispersed nutrients into more readily bioavailable forms (Kong and Singh Citation2009). The rate of digestion of the bolus is determined by the time required for the gastric secretions to reach the walls of the surrounding matrix and free the bioactive compound from the matrix () (Bornhorst and Singh Citation2014; Guo et al. Citation2020; Lentle and Janssen Citation2011). Depending on the starting pH and buffering ability of the food, the pH in the stomach steadily decreases and may vary from one food to another (Acevedo-Fani et al. Citation2021; Luo et al. Citation2021; Qazi, Ye, Acevedo-Fani, and Singh, Citation2021, Qazi et al. Citation2022). Similarly, gastric emptying depends upon the properties of the meal consumed, such as viscosity, density, and particle size. Both propulsion and retropulsion processes, along with the gastric juice, attempt to reduce the size of the food to fine particles and to empty them into the first part of the small intestine, i.e., the duodenum. Additionally, in vivo studies (Egger et al. Citation2017; Miranda and Pelissier Citation1981; Ye et al. Citation2019) and a dynamic in vitro human gastric simulator (HGS) have shown that the stomach juice and the mechanical grinding can cause liquids such as milk to coagulate, which prolongs their digestion rate and their duration within the stomach (Mulet-Cabero et al. Citation2019; Ye Citation2021).
Following gastric digestion of the food, its digestion is continued in the small intestine, where the macromolecules are predominantly broken down and water and nutrients are absorbed (Campbell, Berry, and Liang Citation2019; Li et al. Citation2020). The segmentation and peristaltic movement patterns in the small intestine help to mix the chyme and increase its interaction with the villous surfaces (Feher Citation2017; Nadia et al. Citation2021).
Pancreatic and bile secretions play a pivotal role in the digestion in the small intestine, firstly, by drastically changing the pH of the gastric chyme with bicarbonate ions to pH ≈ 5–7 (Bakala N’Goma et al. Citation2012), and secondly, by enzymatically (i.e., amylase, proteases, peptidases, lipases, and esterase) cleaving the protein, starch, and lipids that remained unhydrolyzed during the gastric phase (Singh and Gallier Citation2014). Pancreatic proteases are divided into trypsin, chymotrypsin, elastase, and carboxypeptidases A and B. Pancreatic lipase is the major contributor to the digestion of lipids and fats in food (MacFarlane Citation2018; Minekus et al. Citation2014). The pancreatic lipase, colipases, cholesterol esterase, phospholipase, etc. catalyze the mono- or diglycerides, fatty acids, and cholesterol. Similarly, pancreatic α-amylase hydrolyzes the 1–4 α linkages in starch (Nadia et al. Citation2021; Patricia and Dhamoon Citation2019). Another major factor in intestinal digestion is the bile salts produced by the liver and stored in the gall bladder. They are rigid, biological surfactants that are synthesized from cholesterol. Their major function is to reduce the surface tension and to conjugate with the products of lipolysis, i.e., phospholipids and monoglycerides, resulting in emulsification, the formation of a cylindrical disk called a micelle, and its transportation across the brush border membrane (Salvia-Trujillo et al. Citation2017; Sarkar, Ye, and Singh Citation2016; Vítek and Haluzík Citation2016). Following digestion in the small intestine, the undigested material, such as dietary fiber, enters the colon, where it is fermented by the residing microbiota (Wong et al., Citation2012). Thus, the unique pattern of the disintegration of food during digestion and the release of fortified or enriched bioactive compounds deserves special attention in order to understand and design a food matrix that with superior health benefits. In the last 10 years, scientific advances lists the in vitro digestion studies of several encapsulated bioactive compounds that were enriched into real/model foods. In the following sections, we give more detail about a few of these examples.
Table 2. Summary of in vitro digestion studies of different encapsulated bioactive compounds fortified real/model food matrix.
Digestion of liquid foods
Healthy dairy- and plant-based beverages, sports and energy drinks, fermented beverages, and many more comparable items are always in high demand from consumers. To cater for the increasing consumer demand for healthier beverages, food industry is continually developing various fortified beverages that are stable, have good shelf life, and are appealing to consumers (Ahmad and Ahmed Citation2019; Ansari and Kumar Citation2012; Puiggròs et al. Citation2017).
As liquid foods generally lack structures that need to be broken down, they empty from the stomach rapidly without a lag phase, thus resulting in a minimum matrix effect (Siegel et al. Citation1988; Ye Citation2021). The alterations in liquid foods are mostly brought about by interactions between the constituent parts of the food and bodily fluids, or between the constituent parts when they are in a gastrointestinal environment (McClements et al. Citation2008; Singh and Acevedo-Fani Citation2022). A simple liquid matrix, such as isotonic beverages, has a set of easily digestible carbohydrates and a well-balanced mineral composition, is a suitable carrier for supplementing functional ingredients. Wyspiańska et al. (Citation2019) designed an isotonic drink that was fortified with inulin- or maltodextrin-microencapsulated soybean isoflavone to investigate the impact of the microencapsulation process on the stability and antioxidant activity of isoflavones during a simulated in vitro gastrointestinal digestion. The isotonic beverage had no matrix effect on the delivery of the microcapsules. Although using inulin as a carrier resulted in capsules with a superior surface structure and better storage stability, the isoflavone levels in all samples were found to be significantly affected by the acidic and basic environments in the gut. To circumvent environmental impacts that such fortified beverages experience during digestion, a number of delivery methods have been suggested. To evaluate the release behavior, a model beverage was prepared by combining various curcumin-loaded lipid-based encapsulation systems. The curcumin nanoemulsion showed increased instability immediately after incorporation into the beverage, whereas the beverage stability relative to the pH remained unaffected in the presence of solid lipid nanoparticles and nanostructured lipid carriers. Furthermore, the beverage containing solid lipid nanoparticles had higher curcumin bioaccessibility than the other beverages, implying that lipid digestion products from liquid lipids bound with the salts in the beverage, preventing the formation of mixed micelles (Gonçalves, Vicente, and Pinheiro Citation2023).
In contrast, because of their instability under gastric conditions, e.g., creaming of oil/fat, protein aggregation, and the high viscosity of carbohydrates, some fortified liquid foods can remain in the stomach for longer periods of time (Araiza-Calahorra et al. Citation2020; Liu et al. Citation2016; Niu et al. Citation2020; Steingoetter et al. Citation2017; Wang et al. Citation2021; Ye Citation2021). Liquid food such as milk have unique digestion kinetics because of their protein content, with completely distinctive properties. The coagulation of caseins in the stomach, driven by pepsin, and the acidic environment led to a protracted gastric digestion, resulting in slower release of the fortified ingredients (Hodgkinson et al. Citation2018; Mudgil and Barak Citation2019; Qazi et al. Citation2022). In contrast, whey proteins are digested and absorbed in the intestine more quickly. Recent research has demonstrated that altering the processing conditions, i.e., homogenization, heating, etc., can vary the interactions between the milk proteins and the other constituents, hence changing the kinetics of milk digestion (Egger et al. Citation2017; Huppertz and Chia Citation2021; Mulet-Cabero et al. Citation2020; Mulet-Cabero et al. Citation2019; Ye et al. Citation2017; Ye et al. Citation2019). The gastrointestinal digestion of recombined milk systems fortified with curcumin nanoemulsion was investigated using an HGS (Qazi et al. Citation2022). The milk systems reconstituted from low-heat, medium-heat, and high-heat skim milk powders had significantly different disintegration behaviors in the stomach because of the different degrees of casein/whey protein complexes formed during the processing of the milk. In contrast to the low-heat and medium-heat milk proteins, the high-heat-treated milk proteins produced a loose and soft curd under dynamic gastric conditions, which led to a quicker outflow of the curd fragments and entrapped curcumin nanoemulsions. Thus, both the release of free fatty acids and the bioaccessibility of curcumin during intestinal digestion were affected by these variations in the gastric digesta profiles. Milk proteins have high surface activity and can partially or completely displace low-surface-active emulsifiers from the surface of emulsion droplets, making oil droplets in the beverage more susceptible to lipase action, which can accelerate the formation of mixed micelles in the small intestine. Niu et al. (Citation2020) showed that, when used as a food system, a high-protein beverage improved the absorption of an enriched coenzyme-Q10-loaded nanoemulsion by boosting the lipolytic activity, in comparison with a coenzyme-Q10 nanoemulsion and coenzyme Q10 dissolved in oil. Whey protein and milk protein concentrates were effective in replacing the modified starch used to encapsulate the coenzyme-Q10, making them more susceptible to lipolysis, resulting in increased free fatty acid release and mixed micelle formation during the intestinal phase. Likewise, beverages made by structurally modifying milk proteins during fermentation, such as drinking yogurt, have been shown to alter the digestion kinetics and the release of entrapped phenolics in the gastrointestinal tract (Altin, Gültekin-Özgüven, and Ozcelik Citation2018).
In recent years, there has been a surge in consumer interest in replacing dairy milk with plant-based milks in the diet, demonstrating several health benefits to health-conscious consumers (Fructuoso et al. Citation2021; McClements and Grossmann Citation2021; Sethi, Tyagi, and Anurag Citation2016). Typically, plant-based milks are produced utilizing size-reduction techniques that entail mechanical, chemical, or enzymatic breakdown of the original plant tissue structure (McClements and Grossmann Citation2021; Reyes-Jurado et al. Citation2021). However, they differ greatly from dairy-based milk systems in terms of their protein structures and interactions with fortified bioactive chemicals during processing and digestion (Fructuoso et al. Citation2021). Very few studies that have evaluated fortified plant-based milk systems are available. Zheng et al. (Citation2019) compared the efficacy of curcumin crystals dispersed in water (control) with three delivery systems produced using the pH-shift method: curcumin nanocrystals; curcumin-loaded nanoemulsions; and curcumin-loaded soy oil bodies (commercial soymilk). The curcumin-loaded nanoemulsion and the soymilk had a homogeneous appearance and good stability. However, there were noticeable differences in terms of aggregation during the gastrointestinal digestion. In particular, the soymilk was considerably more prone to aggregation in the stomach than the nanoemulsions, which appeared to be more prone to aggregation in the mouth. However, by the end of the digestion, both systems produced curcumin that was relatively stable and bioaccessible. In contrast, curcumin nanocrystals had low bioaccessibility because there were fewer mixed micelles to solubilize the curcumin molecules. Similarly, Zheng, Zhou, and McClements (Citation2021) encapsulated curcumin in the oil bodies of plant-based milk analogues (coconut, cashew, almond, and oat milks) using the same pH-driven method. Overall, the lipids in the plant-based milk were digested reasonably quickly during the first 20 min of the intestinal phase but more slowly thereafter. These differences were attributed to the different compositions and structures of the lipids. Furthermore, the concentration of curcumin in the mixed micelle phase was much higher in the plant-based milks than in the control. It is interesting to note that, regardless of the lipid makeup of the oil bodies, the bioaccessibility of the curcumin was very consistent across all the plant-based milks. Recent research utilizing the dynamic gastric model, i.e., the HGS, has demonstrated that different plant-based milk systems go through various physicochemical changes in the gastric compartment (Wang et al. Citation2021; Wang et al. Citation2022; Wang, Ye, and Singh Citation2020). These changes have been shown to have a significant impact on the gastric emptying of nutrients in the small intestine, which can further influence the bioaccessibility of fortified bioactive compounds. The two studies that were presented earlier used a static approach to in-vitro digestion, ignoring the dynamism that occurs in the actual gut. Therefore, in the future, thorough in vitro and in vivo studies will be needed to understand how plant-based milk matrices affect bioactive delivery.
Digestion of fortified semi-solid food matrices
The viscoelastic behavior of semi-solid matrices is substantially greater than that of liquid matrices, and semi-solid matrices contain a sophisticated biopolymer network that can hold a lot of water (Aguilera Citation2019; Alsanei, Chen, and Ding Citation2015; Devezeaux de Lavergne et al. Citation2015). Assemblies made during processing frequently contain fortified elements, which must be released during digestion for them to be further absorbed in the gut (Augustin and Sanguansri Citation2015; Dupont et al. Citation2015; Parada and Aguilera Citation2007). Additionally, these food structures, defined during processing, undergo further structural reorganization during digestion, which impacts on the release of enriched bioactive compounds from the food structure (Mao et al. Citation2017; Qazi et al. Citation2021). Semi-solid foods, as opposed to liquid foods, spend a longer time in the oral cavity, where they first undergo transformation during mastication and salivation. Increased surface exposure during mastication because of increased mouth fragmentation increases the likelihood that bioactive substances that were previously contained will be released. Meanwhile, the salivary secretion also lubricates and wets the food after it has been chewed, creating a cohesive bolus that is ready for swallowing (Chen Citation2009; Minekus et al. Citation2014; Mun and McClements Citation2017). Apart from its role in bolus formation, saliva contains various proteins, enzymes, and electrolytes, which play a significant role in the emulsification and disintegration of food assemblies. Both in vitro and in vivo trials to understand the oral breakdown of semi-solid foods into small particles have been conducted. Luo et al. (Citation2019) investigated the breakdown behavior in the mouth of capsaicinoid-containing whey protein emulsion gel structures; 18 human subjects chewed the gels, i.e., soft/elastic gel, semi-solid gel, and hard/brittle gel. The bioactive diffusivity was higher in the soft and semi-solid gels, as they went through a greater degree of fragmentation because of their loose structures. Luo et al. (Citation2021) extended this work by subjecting the whey protein soft and hard emulsion gels to in vitro gastrointestinal digestion to evaluate the influence of the gel structures on the bioaccessibility of capsaicinoids. The hard gel had lower levels of lipid digestion and disintegrated more slowly than the soft gel because of the larger gastric particles and oil droplet sizes and the increased fat content in the digesta. It was found that the degree of lipid digestion was positively linked with the bioaccessibility of the capsaicinoids.
As previously discussed, liquid milk undergoes significant modifications during the gastric phase by forming a curd, which influences the release of fortified bioactive compounds; however, semi-solid dairy gel matrices such as yogurt and cheese, which are formed by the acid and rennet coagulation of milk proteins during processing, can alter the digestion kinetics and nutrient release in the gastrointestinal cavity. Our recent study investigated the in vitro digestion of yogurt- and cheese-like model gels that were fortified with curcumin nanoemulsion (Qazi et al. Citation2021). Although these gels had similar rheological and compositional profiles, their disintegration behaviors during dynamic gastric digestion had a significant impact on gastric emptying. After 240 min, all the curd particles from the acid-coagulated gel had disintegrated and none remained inside the gastric chamber (). In contrast, the curd particles from the rennet-coagulated gel were rebuilt into a dense protein network under the influence of the gastric fluids and the gastric chamber was filled with numerous compact structured clots by 240 min (). These alterations in the curd structures and gastric emptying rates during the gastric phase influenced the compositional profile of the digesta, which changed how the oil droplets were delivered and digested. This, in turn, affected the bioaccessibility of the associated lipophilic curcumin during the intestinal phase.
Figure 3. Images (a) and changes in wet weight (b) of curds formed by AG (acid gel) and RG (rennet gel) within the gastric chamber at selected time points. AG: acid gel, RG: rennet gel. [adapted from Qazi et al. (Citation2021)].
![Figure 3. Images (a) and changes in wet weight (b) of curds formed by AG (acid gel) and RG (rennet gel) within the gastric chamber at selected time points. AG: acid gel, RG: rennet gel. [adapted from Qazi et al. (Citation2021)].](/cms/asset/3dd6d128-470c-4c04-924b-dbe9c5b198c5/bfsn_a_2353366_f0003_c.jpg)
The release and delivery of these bioactive compounds can also be changed by fortifying them in various food matrices. Using a protein-rich food (yogurt) and a carbohydrate-rich food (rice), Papillo, Arlorio, et al. (Citation2019) studied the gastrointestinal absorption of microencapsulated curcuminoids coated with gum Arabic/maltodextrin. The gastric degradation of curcuminoids in yogurt was less than that in rice, but the bioaccessible fraction of curcuminoids was much higher in the presence of the rice matrix compared with the yogurt matrix. Similarly, by combining two or more food elements, these food matrices can be made more complex. Another study investigated the digestion dynamics of a stirred yoghurt matrix enriched with the flavonoid rutin, as well as how interactions between the food matrix and rutin influenced the flavonoid’s release and bioaccessibility throughout digestion (Acevedo-Fani et al. Citation2021). The results showed in comparison to co-digestion of an unfortified yoghurt with a rutin vegetable capsule, fortification of the yoghurt with a caseiṉ̶ˍrutin co-precipitate improved rutin protection and solubility during gastrointestinal digestion. Molet-Rodríguez et al. (Citation2023) investigated the effects of whole milk, oatmeal, and whole milk–oatmeal containing β-carotene emulsions on the rate at which the stomach emptied, the digestibility of the lipids, and the retention of β-carotene. Changes in the microstructure of the meal matrices brought on by interactions between macromolecules had an impact on the rate of lipid emptying. Both the whole milk–oatmeal and the oatmeal had delayed lipid emptying, which was probably caused by the presence of β-glucan, which increases viscosity. Both the amount of fat emptied at each time point and the retention of β-carotene were linked by the in vitro small intestinal digestion. Furthermore, the introduction of oil-in-water emulsions into the complex meals enhanced the retention of β-carotene during the in vitro small intestinal digestion, leading to speculation that milk and oat flake components may prevent β-carotene from degrading during transit in the gut.
Vegetable butters as a potential matrix for the delivery of encapsulated bioactive compounds have also been explored. Roman, Burri, and Singh (Citation2012) used in vitro digestion models, i.e., a static shaking water bath and an HGS, to study the release and bioaccessibility of β-carotene from fortified almond butter. β-Carotene oil (oil) and whey protein isolate–alginate–chitosan capsules (capsule) containing β-carotene oil were studied. In comparison with the shaking water bath model, peristalsis in the HGS model resulted in a greater release of β-carotene from the almond butter enriched with oil. In contrast, during intestinal digestion, more β-carotene was released from the almond butter enriched with capsules. However, more β-carotene was found in the micelle fraction of the almond butter that had been fortified with oil, pointing to the potential role of the coating material in preventing the β-carotene in the fortified almond butter from being absorbed into the body.
Despite several studies, many untested semi-solid food matrices must be investigated in the future if they are to be potentially employed for the delivery of these bioactive compounds.
Digestion of fortified solid food matrices
Natural and processed solid foods vary greatly in their structure and texture and these properties have a significant impact on the release of nutrients and active ingredients in the gut. Solid food matrices are usually low-moisture, semi-crystalline or crystalline structures. It is thought that the rate at which nutrients can dissolve into a solution for absorption is determined by how quickly the solid food matrices undergo disintegration, when the food particulates break into small fragments, allowing the nutrients that are held therein to dissolve into the gastric fluids (Kong and Singh Citation2008). Solid food begins to break down in the mouth during mastication, when saliva containing amylase is combined with the food to create a swallowable bolus that is then transported into the stomach by the esophagus. Compared with oral mastication, the gastric phase has greater complexity because of influencing factors such as fed/fast state, gastric acid, enzymatic reactions, and hydrodynamic and mechanical forces. These factors have been shown to significantly affect the restructuring of the food matrix, which further plays a major role in the release and bioavailability of active ingredients from food in the small intestine (Acevedo-Fani, Dave, and Singh Citation2020; Acevedo-Fani and Singh Citation2021; Somaratne et al. Citation2020; Ye et al. Citation2019).
Čakarević et al. (Citation2021) assessed the physicochemical and sensory properties of cookies fortified with pumpkin-protein-isolate-encapsulated beetroot juice polyphenols at three different levels, i.e., 10%, 15%, and 20%. The addition of the encapsulate increased the overall phenolic and betalain content and significantly improved the stability during storage. After gastrointestinal digestion, new peptides were created, which, in combination with the active ingredients in the beetroot juice, improved the bioactive properties of the enriched products. Mun, Kim, and McClements (Citation2015) investigated the influence of rice starch hydrogels on the bioaccessibility of emulsified-lipid-solubilized β-carotene. To evaluate the bioaccessibility of β-carotene, the rice starch hydrogels loaded with β-carotene emulsion were compared with unencapsulated β-carotene-loaded starch gels and emulsions. Their study showed greater bioaccessibility of encapsulated β-carotene in the hydrogels compared with the other two systems, which was attributed to the protective effect provided by the surrounding hydrogels against aggregation of the lipid droplets during digestion. However, the composition of these gels could have altered the release behavior, ultimately altering the lipid digestibility. Mun et al. (Citation2016) extended this work by studying the influence of methylcellulose (0–0.2%) on the digestion of rice starch hydrogels loaded with encapsulated β-carotene. In this case, the lipid digestion and the bioaccessibility of β-carotene decreased with increasing concentration of the indigestible polysaccharide (methylcellulose). It is interesting to note that most of the research carried out on the digestion of starch gels has been conducted using static models of in vitro digestion, which do not provide detailed information regarding the behavior of the gels in the stomach and how this affects the bioaccessibility of the bioactive compounds. In a recent study, curcumin-nanoemulsion-loaded corn starch gels made from waxy, native, and high amylose corn starches were examined in detail for their microstructure, physicochemical characteristics, and in vitro gastrointestinal digestion (Qazi et al. Citation2023). The addition of curcumin nanoemulsion to the gels significantly changed their initial physicochemical characteristics. In the dynamic gastric phase, the breakdown and the emptying from the stomach of the waxy gel were slowed down, despite its higher amylopectin content, because of its higher adhesive nature, which trapped the majority of the nanoemulsified oil droplets inside the gel fragments. The different rates of starch hydrolysis, the release of free fatty acids, and the related bioaccessible percentage of curcumin were further linked to this heterogeneity in the compositional and structural profiles of the gastric digesta. In another study, Gómez-Estaca, Gavara, and Hernández-Muñoz (Citation2015) investigated the bioaccessibility of curcumin after subjecting fish gels containing encapsulated curcumin microparticles to an in vitro gastrointestinal digestion. When applied to a gelatinized fish product, the bioaccessibility and the antioxidant activity of the gelatin-encapsulated curcumin were reduced, indicating that curcumin may be able to form more stable complexes with some digested water-insoluble fish proteins that would lower these characteristics.
Conclusions and future outlook
Consumer’s interest in functional food products is increasing because they offer supplement-level concentrations of health-promoting substances. However, despite this increasing interest, few efforts have been made to integrate encapsulated bioactive compounds into actual or model food systems; little dynamic in vitro and in vivo research has been carried out to assess their effectiveness after oral administration. The food structure is a key component that, in most cases, not only relates to a spatial physical domain that holds, interacts with, or confers specific functionalities to supplemented bioactive compounds during processing, but also controls their release in the gastrointestinal system. The intricate processing and preservation procedures can affect not only where bioactive chemicals are absorbed but also how well they interact with other dietary components. These modifications to these structural features of foods cause distinctive disintegration patterns, which eventually affect how bioactive compounds are released and absorbed in the gut. Several types of foods, such as dairy- and starch-based food systems, have been shown to have a longer residence time in the stomach, which alters the composition and the emptying pattern of the gastric digesta into the small intestine.
Although many studies reveal a possible connection between initial food structure and release and bioaccessibility of the nutrients and bioactive substances, much of the focus has been on the influence of initial structures of model food systems and in vitro disintegration in the gastrointestinal tract. Much of the research involved relatively simple model systems, such as starch/lipid and protein/lipid based food systems. However, the inclusion of other food components, such as the combination of protein, lipids, and polysaccharides in a single meal composition, may lead to far more complicated interactions and structural organization at the macro-, meso-, and microscopic level. More realistic food matrices (i.e., noodles, pasta, breads) that are part of our common diet need to be considered for future studies. Additionally, it is important to explore how different bioactive chemicals, encapsulating materials, and delivery systems interact with distinct food matrices during processing and digestion. Moreover, utilizing advanced dynamic gastrointestinal models and noninvasive technologies like real-time MRI and hyperspectral imaging may offer valuable insights into food structure changes during digestion.
Authors’ contributions
Haroon Jamshaid Qazi prepared the original draft and edited the manuscript. Aiqian Ye critiqued and edited the original draft of the manuscript. Alejandra Acevedo-Fani and Harjinder Singh critically reviewed the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the conception and design of the manuscript and read and approved the final manuscript for publication.
Acknowledgments
Authors acknowledge Claire Woodhall for proof-reading the manuscript.
Disclosure statement
Authors declare no conflicts of interest.
Additional information
Funding
References
- Aadil, R. M., U. Roobab, A. Sahar, U. Ur Rahman, and A. A. Khalil. 2019. Functionality of bioactive nutrients in beverages. In Nutrients in beverages, 237–76. Cambridge, MA, USA: Elsevier.
- Acevedo-Fani, A., A. Dave, and H. Singh. 2020. Nature-assembled structures for delivery of bioactive compounds and their potential in functional foods. Frontiers in Chemistry 8:564021. doi: 10.3389/fchem.2020.564021.
- Acevedo-Fani, A., A. Ochoa-Grimaldo, S. M. Loveday, and H. Singh. 2021. Digestive dynamics of yoghurt structure impacting the release and bioaccessibility of the flavonoid rutin. Food Hydrocolloids 111:106215. doi: 10.1016/j.foodhyd.2020.106215.
- Acevedo-Fani, A., and H. Singh. 2021. Biopolymer interactions during gastric digestion: Implications for nutrient delivery. Food Hydrocolloids 116:106644. doi: 10.1016/j.foodhyd.2021.106644.
- Agarwal, S., S. Vivekanandan, T. David, M. Mitra, J. Palanivelu, and R. Chidambaram. 2019. Nanoemulsions: Industrial production and food-grade applications. In Polymers for agri-food applications, 159–82. New York, NY, USA: Springer.
- Aguilera, J. M. 2005. Why food microstructure? Journal of Food Engineering 67 (1–2):3–11. doi: 10.1016/j.jfoodeng.2004.05.050.
- Aguilera, J. M. 2019. The food matrix: Implications in processing, nutrition and health. Critical Reviews in Food Science and Nutrition 59 (22):3612–29. doi: 10.1080/10408398.2018.1502743.
- Ahmad, A., and Z. Ahmed. 2019. 3 – Fortification in beverages. In Production and management of beverages, ed. A. M. Grumezescu and A. M. Holban, 85–122. Amsterdam, Netherlands: Woodhead Publishing.
- Akgün, D., M. Gültekin-Özgüven, A. Yücetepe, G. Altin, M. Gibis, J. Weiss, and B. Özçelik. 2020. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocolloids 101:105532. doi: 10.1016/j.foodhyd.2019.105532.
- Alongi, M., and M. Anese. 2021. Re-thinking functional food development through a holistic approach. Journal of Functional Foods 81:104466. doi: 10.1016/j.jff.2021.104466.
- Alsanei, W. A., J. Chen, and R. Ding. 2015. Food oral breaking and the determining role of tongue muscle strength. Food Research International 67:331–7. doi: 10.1016/j.foodres.2014.11.039.
- Altin, G., M. Gültekin-Özgüven, and B. Ozcelik. 2018. Liposomal dispersion and powder systems for delivery of cocoa hull waste phenolics via Ayran (drinking yoghurt): Comparative studies on in-vitro bioaccessibility and antioxidant capacity. Food Hydrocolloids 81:364–70. doi: 10.1016/j.foodhyd.2018.02.051.
- Amjadi, S., M. Ghorbani, H. Hamishehkar, and L. Roufegarinejad. 2018. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chemistry 256:156–62. doi: 10.1016/j.foodchem.2018.02.114.
- Ansari, M. M., and D. S. Kumar. 2012. Fortification of food and beverages with phytonutrients. Food and Public Health 2 (6):241–53.
- Araiza-Calahorra, A., Z. J. Glover, M. Akhtar, and A. Sarkar. 2020. Conjugate microgel-stabilized pickering emulsions: Role in delaying gastric digestion. Food Hydrocolloids 105:105794. doi: 10.1016/j.foodhyd.2020.105794.
- Augustin, M. A., and L. Sanguansri. 2015. Challenges and solutions to incorporation of nutraceuticals in foods. Annual Review of Food Science and Technology 6 (1):463–77. doi: 10.1146/annurev-food-022814-015507.
- Bagale, U., A. Kadi, A. Malinin, I. Potoroko, S. Sonawane, and S. Potdar. 2022. Ultrasound-assisted stable curcumin nanoemulsion and its application in bakery product. International Journal of Food Science 2022:4784794–13. doi: 10.1155/2022/4784794.
- Bakala N’Goma, J.-C., S. Amara, K. Dridi, V. Jannin, and F. Carrière. 2012. Understanding the lipid-digestion processes in the GI tract before designing lipid-based drug-delivery systems. Therapeutic Delivery 3 (1):105–24. doi: 10.4155/tde.11.138.
- Bao, C., P. Jiang, J. Chai, Y. Jiang, D. Li, W. Bao, B. Liu, B. Liu, W. Norde, and Y. Li. 2019. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Research International (Ottawa, Ont.) 120:130–40. doi: 10.1016/j.foodres.2019.02.024.
- Betoret, E., N. Betoret, D. Vidal, and P. Fito. 2011. Functional foods development: Trends and technologies. Trends in Food Science & Technology 22 (9):498–508. doi: 10.1016/j.tifs.2011.05.004.
- Bornhorst, G. M., and R. P. Singh. 2014. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annual Review of Food Science and Technology 5 (1):111–32. doi: 10.1146/annurev-food-030713-092346.
- Čakarević, J., A. Torbica, M. Belović, J. Tomić, T. Sedlar, and L. Popović. 2021. Pumpkin oil cake protein as a new carrier for encapsulation incorporated in food matrix: Effect of processing, storage and in vitro digestion on bioactivity. International Journal of Food Science & Technology 56 (7):3400–8. doi: 10.1111/ijfs.14964.
- Campbell, J., J. Berry, and Y. Liang. 2019. Anatomy and physiology of the small intestine. In Shackelford’s surgery of the alimentary tract, 2 volume set, 817–41. Philadelphia, USA: Elsevier.
- Capuano, E., T. Oliviero, V. Fogliano, and N. Pellegrini. 2018. Role of the food matrix and digestion on calculation of the actual energy content of food. Nutrition Reviews 76 (4):274–89. doi: 10.1093/nutrit/nux072.
- Carbonell-Capella, J. M., M. Buniowska, F. J. Barba, M. J. Esteve, and A. Frígola. 2014. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Comprehensive Reviews in Food Science and Food Safety 13 (2):155–71. doi: 10.1111/1541-4337.12049.
- Chen, J. 2009. Food oral processing—A review. Food Hydrocolloids 23 (1):1–25. doi: 10.1016/j.foodhyd.2007.11.013.
- Cifelli, C. J. 2021. Looking beyond traditional nutrients: The role of bioactives and the food matrix on health. Nutrition Reviews 79 (Suppl 2):1–3. doi: 10.1093/nutrit/nuab100.
- Comunian, T. A., I. E. Chaves, M. Thomazini, I. C. F. Moraes, R. Ferro-Furtado, I. A. de Castro, and C. S. Favaro-Trindade. 2017. Development of functional yogurt containing free and encapsulated echium oil, phytosterol and sinapic acid. Food Chemistry 237:948–56. doi: 10.1016/j.foodchem.2017.06.071.
- de Campo, C., R. Queiroz Assis, M. Marques da Silva, T. M. Haas Costa, K. Paese, S. Stanisçuaski Guterres, A. de Oliveira Rios, and S. Hickmann Flôres. 2019. Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chemistry 301:125230. doi: 10.1016/j.foodchem.2019.125230.
- Deep Diyuti, Kumar, Bimlesh, Mann, Ramesh, Pothuraju, Rajan, Sharma, Rajesh, Bajaj, Minaxi, (2016). Formulation and characterization of nanoencapsulated curcumin using sodium caseinate and its incorporation in ice cream. Food & function 7(1), 417–424. doi: 10.1039/c5fo00924c.
- Delfanian, M., and M. A. Sahari. 2020. Improving functionality, bioavailability, nutraceutical and sensory attributes of fortified foods using phenolics-loaded nanocarriers as natural ingredients. Food Research International (Ottawa, Ont.) 137:109555. doi: 10.1016/j.foodres.2020.109555.
- Devezeaux de Lavergne, M., F. van de Velde, M. A. J. S. van Boekel, and M. Stieger. 2015. Dynamic texture perception and oral processing of semi-solid food gels: Part 2: Impact of breakdown behaviour on bolus properties and dynamic texture perception. Food Hydrocolloids 49:61–72. doi: 10.1016/j.foodhyd.2015.02.037.
- Dima, C., E. Assadpour, S. Dima, and S. M. Jafari. 2020. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Current Opinion in Food Science 33:21–9. doi: 10.1016/j.cofs.2019.11.006.
- Domínguez, R., M. Pateiro, P. E. Munekata, D. J. McClements, and J. M. Lorenzo. 2021. Encapsulation of bioactive phytochemicals in plant-based matrices and application as additives in meat and meat products. Molecules (Basel, Switzerland) 26 (13):3984. doi: 10.3390/molecules26133984.
- Donhowe, E. G., F. P. Flores, W. L. Kerr, L. Wicker, and F. Kong. 2014. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT – Food Science and Technology 57 (1):42–8. doi: 10.1016/j.lwt.2013.12.037.
- Dupont, D., F. Barbe, S. Le Feunteun, O. Ménard, Y. Le Gouar, A. Deglaire, and B. Laroche. 2015. Structuring food for improving nutrient bioavailability: The case of dairy gels. Paper Presented at the 29 EFFoST International Conference, Athènes, Greece.
- Egger, L., O. Ménard, C. Baumann, D. Duerr, P. Schlegel, P. Stoll, G. Vergères, D. Dupont, and R. Portmann. 2017. Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data. Food Research International (Ottawa, Ont.) 118:32–9. doi: 10.1016/j.foodres.2017.12.049.
- Eggersdorfer, M., and A. Wyss. 2018. Carotenoids in human nutrition and health. Archives of Biochemistry and Biophysics 652:18–26. doi: 10.1016/j.abb.2018.06.001.
- El-Kholy, W. M., T. N. Soliman, and A. M. G. Darwish. 2019. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PloS One 14 (10):e0222789. doi: 10.1371/journal.pone.0222789.
- Eržen, N., M. Kač, and I. Pravst. 2014. Perceived healthfulness of dairy products and their imitations. Agro Food Ind Hi Tech 25:24–7.
- Ezhilarasi, P. N., D. Indrani, B. S. Jena, and C. Anandharamakrishnan. 2014. Microencapsulation of Garcinia fruit extract by spray drying and its effect on bread quality. Journal of the Science of Food and Agriculture 94 (6):1116–23. doi: 10.1002/jsfa.6378.
- Ezhilarasi, P., D. Indrani, B. S. Jena, and C. Anandharamakrishnan. 2013. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. Journal of Food Engineering 117 (4):513–20. doi: 10.1016/j.jfoodeng.2013.01.009.
- Fagan, C. C., D. J. O’Callaghan, M. J. Mateo, and P. Dejmek. 2017. Chapter 6 – The syneresis of rennet-coagulated curd. In Cheese, ed. P. L. H. McSweeney, P. F. Fox, P. D. Cotter, and D. W. Everett, 4th ed., 145–77. San Diego: Academic Press.
- Fathi, M., Á. Martín, and D. J. McClements. 2014. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends in Food Science & Technology 39 (1):18–39. doi: 10.1016/j.tifs.2014.06.007.
- Feher, J. J. 2017. Quantitative human physiology: An introduction. London, UK: Academic press.
- Flores, F. P., and F. Kong. 2017. In vitro release kinetics of microencapsulated materials and the effect of the food matrix. Annual Review of Food Science and Technology 8 (1):237–59. doi: 10.1146/annurev-food-030216-025720.
- Francisco, C. R. L., S. A. Heleno, I. P. M. Fernandes, J. C. M. Barreira, R. C. Calhelha, L. Barros, O. H. Gonçalves, I. C. F. R. Ferreira, and M. F. Barreiro. 2018. Functionalization of yogurts with Agaricus bisporus extracts encapsulated in spray-dried maltodextrin crosslinked with citric acid. Food Chemistry 245:845–53. doi: 10.1016/j.foodchem.2017.11.098.
- Fructuoso, I., B. Romão, H. Han, A. Raposo, A. Ariza-Montes, L. Araya-Castillo, and R. P. Zandonadi. 2021. An overview on nutritional aspects of plant-based beverages used as substitutes for cow’s milk. Nutrients 13 (8):2650. doi: 10.3390/nu13082650.
- Galanakis, C. M. 2017. Chapter 1 – Introduction. In Nutraceutical and functional food components, ed. C. M. Galanakis, 1–14. Massachusetts, USA: Academic Press.
- Gomes, G. V. D. L., M. R. Sola, A. L. Rochetti, H. Fukumasu, A. Vicente, and S. C. D. Pinho. 2019. β-carotene and α-tocopherol coencapsulated in nanostructured lipid carriers of murumuru (Astrocaryum murumuru) butter produced by phase inversion temperature method: Characterisation, dynamic in vitro digestion and cell viability study. Journal of Microencapsulation 36 (1):43–52. doi: 10.1080/02652048.2019.1585982.
- Gómez-Estaca, J., R. Gavara, and P. Hernández-Muñoz. 2015. Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications. Innovative Food Science & Emerging Technologies 29:302–7. doi: 10.1016/j.ifset.2015.03.004.
- Gómez-Mascaraque, L. G., M. Hernández-Rojas, P. Tarancón, M. Tenon, N. Feuillère, J. F. Vélez Ruiz, S. Fiszman, and A. López-Rubio. 2017. Impact of microencapsulation within electrosprayed proteins on the formulation of green tea extract-enriched biscuits. LWT – Food Science and Technology 81:77–86. doi: 10.1016/j.lwt.2017.03.041.
- Gonçalves, R. F. S., A. A. Vicente, and A. C. Pinheiro. 2023. Incorporation of curcumin-loaded lipid-based nano delivery systems into food: Release behavior in food simulants and a case study of application in a beverage. Food Chemistry 405 (Pt A):134740. doi: 10.1016/j.foodchem.2022.134740.
- Graebin, C. S., F. V. Ribeiro, K. R. Rogério, and A. E. Kümmerle. 2019. Multicomponent reactions for the synthesis of bioactive compounds: A review. Current Organic Synthesis 16 (6):855–99. doi: 10.2174/1570179416666190718153703.
- Guo, Q., A. Ye, H. Singh, and D. Rousseau. 2020. Destructuring and restructuring of foods during gastric digestion. Comprehensive Reviews in Food Science and Food Safety 19 (4):1658–79. doi: 10.1111/1541-4337.12558.
- Hermund, D. B., A. Karadağ, U. Andersen, R. Jónsdóttir, H. G. Kristinsson, C. Alasalvar, and C. Jacobsen. 2016. Oxidative stability of granola bars enriched with multilayered fish oil emulsion in the presence of novel brown seaweed based antioxidants. Journal of Agricultural and Food Chemistry 64 (44):8359–68. doi: 10.1021/acs.jafc.6b03454.
- Hidalgo, A., A. Brandolini, J. Čanadanović-Brunet, G. Ćetković, and V. Tumbas Šaponjac. 2018. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chemistry 268:40–8. doi: 10.1016/j.foodchem.2018.06.062.
- Hodgkinson, A. J., O. A. M. Wallace, I. Boggs, M. Broadhurst, and C. G. Prosser. 2018. Gastric digestion of cow and goat milk: Impact of infant and young child in vitro digestion conditions. Food Chemistry 245:275–81. doi: 10.1016/j.foodchem.2017.10.028.
- Horn, A. F., D. Green-Petersen, N. S. Nielsen, U. Andersen, G. Hyldig, L. H. S. Jensen, A. Horsewell, and C. Jacobsen. 2012. Addition of fish oil to cream cheese affects lipid oxidation, sensory stability and microstructure. Agriculture 2 (4):359–75. http://www.mdpi.com/2077-0472/2/4/359. doi: 10.3390/agriculture2040359.
- Huppertz, T., and L. W. Chia. 2021. Milk protein coagulation under gastric conditions: A review. International Dairy Journal 113:104882. doi: 10.1016/j.idairyj.2020.104882.
- Istenič, K., R. Cerc Korošec, and N. Poklar Ulrih. 2016. Encapsulation of (−)‐epigallocatechin gallate into liposomes and into alginate or chitosan microparticles reinforced with liposomes. Journal of the Science of Food and Agriculture 96 (13):4623–32. doi: 10.1002/jsfa.7691.
- Jiang, T., W. Liao, and C. Charcosset. 2020. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Research International (Ottawa, Ont.) 132:109035. doi: 10.1016/j.foodres.2020.109035.
- Joardder, M. U., C. Kumar, and M. Karim. 2017. Food structure: Its formation and relationships with other properties. Critical Reviews in Food Science and Nutrition 57 (6):1190–205. doi: 10.1080/10408398.2014.971354.
- Joung, H. J., M. J. Choi, J. T. Kim, S. H. Park, H. J. Park, and G. H. Shin. 2016. Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: Antioxidant property and in vitro digestion. Journal of Food Science 81 (3):N745–N753. doi: 10.1111/1750-3841.13224.
- Karim, M. A., M. M. Rahman, N. D. Pham, and S. Fawzia. 2018. 3 – Food microstructure as affected by processing and its effect on quality and stability. In Food microstructure and its relationship with quality and stability, ed. S. Devahastin, 43–57. Sawston, UK: Woodhead Publishing.
- Keenan, D. F., V. C. Resconi, T. J. Smyth, C. Botinestean, C. Lefranc, J. P. Kerry, and R. M. Hamill. 2015. The effect of partial-fat substitutions with encapsulated and unencapsulated fish oils on the technological and eating quality of beef burgers over storage. Meat Science 107:75–85. doi: 10.1016/j.meatsci.2015.04.013.
- Kha, T. C., M. H. Nguyen, P. D. Roach, and C. E. Stathopoulos. 2015. A storage study of encapsulated gac (Momordica cochinchinensis) oil powder and its fortification into foods. Food and Bioproducts Processing 96:113–25. doi: 10.1016/j.fbp.2015.07.009.
- Khaw, K.-Y., M.-O. Parat, P. N. Shaw, and J. R. Falconer. 2017. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules (Basel, Switzerland) 22 (7):1186. doi: 10.3390/molecules22071186.
- Kong, F., and R. P. Singh. 2008. Disintegration of solid foods in human stomach. Journal of Food Science 73 (5):R67–R80. doi: 10.1111/j.1750-3841.2008.00766.x.
- Kong, F., and R. P. Singh. 2009. Digestion of raw and roasted almonds in simulated gastric environment. Food Biophysics 4 (4):365–77. doi: 10.1007/s11483-009-9135-6.
- Lamothe, S., N. Rémillard, J. Tremblay, and M. Britten. 2017. Influence of dairy matrices on nutrient release in a simulated gastrointestinal environment. Food Research International (Ottawa, Ont.) 92:138–46. doi: 10.1016/j.foodres.2016.12.026.
- Lentle, R. G., and P. W. M. Janssen. 2011. Introduction. In The physical processes of digestion, 1–7. New York, NY: Springer New York.
- Let, M. B., C. Jacobsen, and A. S. Meyer. 2007. Lipid oxidation in milk, yoghurt, and salad dressing enriched with neat fish oil or pre-emulsified fish oil. Journal of Agricultural and Food Chemistry 55 (19):7802–9. doi: 10.1021/jf070830x.
- Li, C., W. Yu, P. Wu, and X. D. Chen. 2020. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends in Food Science & Technology 96:114–26. doi: 10.1016/j.tifs.2019.12.015.
- Liu, D., H. L. Parker, J. Curcic, S. Kozerke, and A. Steingoetter. 2016. Emulsion stability modulates gastric secretion and its mixing with emulsified fat in healthy adults in a randomized magnetic resonance imaging study. Journal of Nutrition 146 (10):2158–64. doi: 10.3945/jn.116.234955.
- Liu, D., Y. Deng, L. Sha, M. Abul Hashem, and S. Gai. 2017. Impact of oral processing on texture attributes and taste perception. Journal of Food Science and Technology 54 (8):2585–93. doi: 10.1007/s13197-017-2661-1.
- Lu, X., M. A. Brennan, W. Guan, J. Zhang, L. Yuan, and C. S. Brennan. 2021. Enhancing the nutritional properties of bread by incorporating mushroom bioactive compounds: The manipulation of the pre-dictive glycaemic response and the phenolic properties. Foods (Basel, Switzerland) 10 (4):731. doi: 10.3390/foods10040731.
- Lu, Y., L. Mao, Z. Hou, S. Miao, and Y. Gao. 2019. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Engineering Reviews 11 (4):245–58. doi: 10.1007/s12393-019-09194-z.
- Lucey, J. A. 2008. Chapter 16 – Milk protein gels. In Milk proteins, ed. A. Thompson, M. Boland, and H. Singh, 449–81. San Diego: Academic Press.
- Luo, N., A. Ye, F. M. Wolber, and H. Singh. 2019. Structure of whey protein emulsion gels containing capsaicinoids: Impact on in-mouth breakdown behaviour and sensory perception. Food Hydrocolloids 92:19–29. doi: 10.1016/j.foodhyd.2019.01.019.
- Luo, N., A. Ye, F. M. Wolber, and H. Singh. 2021. Effect of gel structure on the in vitro gastrointestinal digestion behaviour of whey protein emulsion gels and the bioaccessibility of capsaicinoids. Molecules (Basel, Switzerland) 26 (5):1379. https://www.mdpi.com/1420-3049/26/5/1379. doi: 10.3390/molecules26051379.
- MacFarlane, N. G. 2018. Digestion and absorption. Anaesthesia & Intensive Care Medicine 19 (3):125–7. doi: 10.1016/j.mpaic.2018.01.001.
- Mao, L., Y. H. Roos, C. G. Biliaderis, and S. Miao. 2017. Food emulsions as delivery systems for flavor compounds: A review. Critical Reviews in Food Science and Nutrition 57 (15):3173–87. doi: 10.1080/10408398.2015.1098586.
- Martins, A., L. Barros, A. M. Carvalho, C. Santos-Buelga, I. P. Fernandes, F. Barreiro, and I. C. Ferreira. 2014. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food & Function 5 (6):1091–100. doi: 10.1039/c3fo60721f.
- McClements, D. J. 2018. Recent developments in encapsulation and release of functional food ingredients: Delivery by design. Current Opinion in Food Science 23:80–4. doi: 10.1016/j.cofs.2018.06.008.
- McClements, D. J., and L. Grossmann. 2021. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Science of Food 5 (1):17. doi: 10.1038/s41538-021-00099-y.
- McClements, D. J., E. A. Decker, Y. Park, and J. Weiss. 2008. Designing food structure to control stability, digestion, release and absorption of lipophilic food components. Food Biophysics 3 (2):219–28. doi: 10.1007/s11483-008-9070-y.
- Minekus, M., M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, et al. 2014. A standardised static in vitro digestion method suitable for food – An international consensus. Food & Function 5 (6):1113–24. doi: 10.1039/c3fo60702j.
- Miranda, G., and J.-P. Pelissier. 1981. In vivo studies on the digestion of bovine caseins in the rat stomach. Journal of Dairy Research 48 (2):319–26. doi: 10.1017/S0022029900021749.
- Moghadam, F. V., R. Pourahmad, A. Mortazavi, D. Davoodi, and R. Azizinezhad. 2019. Use of fish oil nanoencapsulated with gum Arabic carrier in low fat probiotic fermented milk. Food Science of Animal Resources 39 (2):309–23. doi: 10.5851/kosfa.2019.e25.
- Molaveisi, M., M. Shahidi Noghabi, K. Parastouei, and R. A. Taheri. 2021. Fate of nano-phytosomes containing bioactive compounds of Echinacea extract in an acidic food beverage. Food Structure 27:100177. doi: 10.1016/j.foostr.2021.100177.
- Molet-Rodríguez, A., A. Torcello-Gómez, L. Salvia-Trujillo, O. Martín-Belloso, and A. R. Mackie. 2023. In vitro digestibility of O/W emulsions co-ingested with complex meals: Influence of the food matrix. Food Hydrocolloids 135:108121. doi: 10.1016/j.foodhyd.2022.108121.
- Mollet, B., and I. Rowland. 2002. Functional foods: At the frontier between food and pharma. Current Opinion in Biotechnology 13 (5):483–5. doi: 10.1016/S0958-1669(02)00375-0.
- Moreno, D. A., and N. Ilic. 2018. Functional and bioactive properties of food: The challenges ahead. Foods (Basel, Switzerland) 7 (9):139. doi: 10.3390/foods7090139.
- Mudgil, D., and S. Barak. 2019. 3 – Dairy-based functional beverages. In Milk-based beverages, ed. A. M. Grumezescu and A. M. Holban, 67–93. Sawston, UK: Woodhead Publishing.
- Muhammad, D. R. A., A. D. Saputro, H. Rottiers, D. Van de Walle, and K. Dewettinck. 2018. Physicochemical properties and antioxidant activities of chocolates enriched with engineered cinnamon nanoparticles. European Food Research and Technology 244 (7):1185–202. doi: 10.1007/s00217-018-3035-2.
- Muhammad, D. R. A., C. G. Gonzalez, A. Sedaghat Doost, D. Van de Walle, P. Van der Meeren, and K. Dewettinck. 2019. Improvement of antioxidant activity and physical stability of chocolate beverage using colloidal cinnamon nanoparticles. Food and Bioprocess Technology 12 (6):976–89. doi: 10.1007/s11947-019-02271-5.
- Mulet-Cabero, A.-I., A. R. Mackie, A. Brodkorb, and P. J. Wilde. 2020. Dairy structures and physiological responses: A matter of gastric digestion. Critical Reviews in Food Science and Nutrition 60 (22):3737–52. doi: 10.1080/10408398.2019.1707159.
- Mulet-Cabero, A.-I., A. R. Mackie, P. J. Wilde, M. A. Fenelon, and A. Brodkorb. 2019. Structural mechanism and kinetics of in vitro gastric digestion are affected by process-induced changes in bovine milk. Food Hydrocolloids 86:172–83. doi: 10.1016/j.foodhyd.2018.03.035.
- Mun, S., S. Park, Y.-R. Kim, and D. J. McClements. 2016. Influence of methylcellulose on attributes of β-carotene fortified starch-based filled hydrogels: Optical, rheological, structural, digestibility, and bioaccessibility properties. Food Research International (Ottawa, Ont.) 87:18–24. 10.1016/j.foodres.2016.06.008. 29606239
- Mun, S., and D. J. McClements. 2017. Influence of simulated in-mouth processing (size reduction and alpha-amylase addition) on lipid digestion and β-carotene bioaccessibility in starch-based filled hydrogels. LWT 80:113–20. doi: 10.1016/j.lwt.2017.02.011.
- Mun, S., Y.-R. Kim, and D. J. McClements. 2015. Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chemistry 173:454–61. doi: 10.1016/j.foodchem.2014.10.053.
- Nacak, B., B. Öztürk-Kerimoğlu, D. Yıldız, Ö. Çağındı, and M. Serdaroğlu. 2021. Peanut and linseed oil emulsion gels as potential fat replacer in emulsified sausages. Meat Science 176:108464. doi: 10.1016/j.meatsci.2021.108464.
- Nadia, J., J. Bronlund, R. P. Singh, H. Singh, and G. M. Bornhorst. 2021. Structural breakdown of starch‐based foods during gastric digestion and its link to glycemic response: In vivo and in vitro considerations. Comprehensive Reviews in Food Science and Food Safety 20 (3):2660–98. doi: 10.1111/1541-4337.12749.
- Nedovic, V., A. Kalusevic, V. Manojlovic, S. Levic, and B. Bugarski. 2011. An overview of encapsulation technologies for food applications. Procedia Food Science 1:1806–15. doi: 10.1016/j.profoo.2011.09.265.
- Nielsen, N. S., and C. Jacobsen. 2009. Methods for reducing lipid oxidation in fish-oil-enriched energy bars. International Journal of Food Science & Technology 44 (8):1536–46. doi: 10.1111/j.1365-2621.2008.01786.x.
- Nieto, G., and J. M. Lorenzo. 2021. Use of olive oil as fat replacer in meat emulsions. Current Opinion in Food Science 40:179–86. doi: 10.1016/j.cofs.2021.04.007.
- Niu, Z., A. Acevedo-Fani, A. McDowell, A. Barnett, S. M. Loveday, and H. Singh. 2020. Nanoemulsion structure and food matrix determine the gastrointestinal fate and in vivo bioavailability of coenzyme Q10. Journal of Controlled Release: Official Journal of the Controlled Release Society 327:444–55. doi: 10.1016/j.jconrel.2020.08.025.
- Oliveira, F. S., A. Ribeiro, L. Barros, R. C. Calhelha, J. C. M. Barreira, B. D. Junior, R. M. V. Abreu, M. F. Barreiro, and I. C. Ferreira. 2017. Evaluation of Arenaria montana L. hydroethanolic extract as a chemopreventive food ingredient: A case study focusing a dairy product (yogurt). Journal of Functional Foods 38:214–20. doi: 10.1016/j.jff.2017.09.027.
- Oyenihi, A. B., and C. Smith. 2019. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? Journal of Ethnopharmacology 229:54–72. doi: 10.1016/j.jep.2018.09.037.
- Ozdal, T., P. Yolci-Omeroglu, and E. C. Tamer. 2020. 6 – Role of encapsulation in functional beverages. In Biotechnological progress and beverage consumption, ed. A. M. Grumezescu and A. M. Holban, 195–232. Cambridige, MA, USA: Academic Press.
- Panthi, R. R., A. L. Kelly, J. J. Sheehan, K. Bulbul, A. H. Vollmer, and D. J. McMahon. 2019. Influence of protein concentration and coagulation temperature on rennet-induced gelation characteristics and curd microstructure. Journal of Dairy Science 102 (1):177–89. doi: 10.3168/jds.2018-15039.
- Papillo, V. A., M. Arlorio, M. Locatelli, L. Fuso, N. Pellegrini, and V. Fogliano. 2019. In vitro evaluation of gastro-intestinal digestion and colonic biotransformation of curcuminoids considering different formulations and food matrices. Journal of Functional Foods 59:156–63. doi: 10.1016/j.jff.2019.05.031.
- Papillo, V. A., M. Locatelli, F. Travaglia, M. Bordiga, C. Garino, J. D. Coïsson, and M. Arlorio. 2019. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Research International (Ottawa, Ont.) 115:511–8. doi: 10.1016/j.foodres.2018.10.004.
- Parada, J., and J. M. Aguilera. 2007. Food microstructure affects the bioavailability of several nutrients. Journal of Food Science 72 (2):R21–R32. doi: 10.1111/j.1750-3841.2007.00274.x.
- Pasrija, D., P. N. Ezhilarasi, D. Indrani, and C. Anandharamakrishnan. 2015. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT – Food Science and Technology 64 (1):289–96. doi: 10.1016/j.lwt.2015.05.054.
- Patricia, J. J., and A. S. Dhamoon. 2019. Physiology, digestion.
- Pereira, P. C. 2014. Milk nutritional composition and its role in human health. Nutrition (Burbank, Los Angeles County, Calif.) 30 (6):619–27. doi: 10.1016/j.nut.2013.10.011.
- Petrotos, K., F. Karkanta, P. Gkoutsidis, I. Giavasis, K. Papatheodorou, and A. Ntontos. 2012. Production of novel bioactive yogurt enriched with olive fruit polyphenols. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering 6:169–75. http://waset.org/publications/14830.
- Pimentel-González, D. J., M. E. Aguilar-García, G. Aguirre-Álvarez, R. Salcedo-Hernández, J. C. Guevara-Arauza, and R. G. Campos-Montiel. 2015. The process and maturation stability of chihuahua cheese with antioxidants in multiple emulsions. Journal of Food Processing and Preservation 39 (6):1027–35. doi: 10.1111/jfpp.12317.
- Pinilla, C. M. B., R. C. S. Thys, and A. Brandelli. 2019. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. International Journal of Food Microbiology 293:72–8. doi: 10.1016/j.ijfoodmicro.2019.01.006.
- Prasad, M., S. Jayaraman, M. A. Eladl, M. El-Sherbiny, M. A. E. Abdelrahman, V. P. Veeraraghavan, S. Vengadassalapathy, V. R. Umapathy, S. F. Jaffer Hussain, K. Krishnamoorthy, et al. 2022. A comprehensive review on therapeutic perspectives of phytosterols in insulin resistance: A mechanistic approach. Molecules (Basel, Switzerland) 27 (5):1595. doi: 10.3390/molecules27051595.
- Puiggròs, F., B. Muguerza, A. Arola‐Arnal, G. Aragonès, S. Suárez‐Garcia, C. Bladé, and M. Suárez. 2017. Functional beverages. Innovative Technologies in Beverage Processing 275–96. New York, NY, USA: Wiley.
- Qazi, H. J., A. Ye, A. Acevedo-Fani, and H. Singh. 2021. In vitro digestion of curcumin-nanoemulsion-enriched dairy protein matrices: Impact of the type of gel structure on the bioaccessibility of curcumin. Food Hydrocolloids 117:106692. doi: 10.1016/j.foodhyd.2021.106692.
- Qazi, H. J., A. Ye, A. Acevedo-Fani, and H. Singh. 2022. Impact of recombined milk systems on gastrointestinal fate of curcumin nanoemulsion. Frontiers in Nutrition 9:890876. doi: 10.3389/fnut.2022.890876.
- Qazi, H. J., A. Ye, A. Acevedo-Fani, and H. Singh. 2023. The impact of differently structured starch gels on the gastrointestinal fate of a curcumin-containing nanoemulsion. Food & Function 14 (17):7924–37. doi: 10.1039/d3fo01566a.
- Qazi, H. J., H. Majeed, W. Safdar, J. Antoniou, and Z. Fang. 2015. A novel approach for microencapsulation of nanoemulsions to overcome the oxidation of bioactives in aqueous phase. Advance Journal of Food Science and Technology 7 (6):388–94. doi: 10.19026/ajfst.7.1329.
- Rafiee, Z., M. Barzegar, M. A. Sahari, and B. Maherani. 2018. Nanoliposomes containing pistachio green hull’s phenolic compounds as natural bio-preservatives for mayonnaise. European Journal of Lipid Science and Technology 120 (9):1800086. doi: 10.1002/ejlt.201800086.
- Raikos, V., and V. Ranawana. 2017. Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components – A review of recent advances. International Journal of Food Science & Technology 52 (1):68–80. doi: 10.1111/ijfs.13272.
- Rashidinejad, A., E. J. Birch, D. Sun-Waterhouse, and D. W. Everett. 2014. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chemistry 156:176–83. doi: 10.1016/j.foodchem.2014.01.115.
- Rashidinejad, A., E. J. Birch, D. Sun-Waterhouse, and D. W. Everett. 2015. Total phenolic content and antioxidant properties of hard low-fat cheese fortified with catechin as affected by in vitro gastrointestinal digestion. LWT – Food Science and Technology 62 (1):393–9. doi: 10.1016/j.lwt.2014.12.058.
- Rein, M. J., M. Renouf, C. Cruz‐Hernandez, L. Actis‐Goretta, S. K. Thakkar, and M. D. S. Pinto. 2013. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. British Journal of Clinical Pharmacology 75 (3):588–602. doi: 10.1111/j.1365-2125.2012.04425.x.
- Reyes-Jurado, F., N. Soto-Reyes, M. Dávila-Rodríguez, A. Lorenzo-Leal, M. Jiménez-Munguía, E. Mani-López, and A. López-Malo. 2021. Plant-based milk alternatives: Types, processes, benefits, and characteristics. Food Reviews International 39 (4):2320–51. doi: 10.1080/87559129.2021.1952421.
- Rezvankhah, A., Z. Emam-Djomeh, and G. Askari. 2020. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Drying Technology 38 (1–2):235–58. doi: 10.1080/07373937.2019.1653906.
- Robert, P., M. Zamorano, E. González, A. Silva-Weiss, S. Cofrades, and B. Giménez. 2019. Double emulsions with olive leaves extract as fat replacers in meat systems with high oxidative stability. Food Research International (Ottawa, Ont.) 120:904–12. doi: 10.1016/j.foodres.2018.12.014.
- Robert, P., T. Gorena, N. Romero, E. Sepulveda, J. Chavez, and C. Saenz. 2010. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. International Journal of Food Science & Technology 45 (7):1386–94. doi: 10.1111/j.1365-2621.2010.02270.x.
- Rodríguez, J., M. J. Martín, M. A. Ruiz, and B. Clares. 2016. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Research International 83:41–59. doi: 10.1016/j.foodres.2016.01.032.
- Roman, M. J., B. J. Burri, and R. P. Singh. 2012. Release and bioaccessibility of β-carotene from fortified almond butter during in vitro digestion. Journal of Agricultural and Food Chemistry 60 (38):9659–66. doi: 10.1021/jf302843w.
- Ruhee, R. T., L. A. Roberts, S. Ma, and K. Suzuki. 2020. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Frontiers in Nutrition 7:64. doi: 10.3389/fnut.2020.00064.
- Sabet, S., A. Rashidinejad, H. J. Qazi, and D. J. McGillivray. 2021. An efficient small intestine-targeted curcumin delivery system based on the positive-negative-negative colloidal interactions. Food Hydrocolloids 111:106375. doi: 10.1016/j.foodhyd.2020.106375.
- Salvia-Trujillo, L., S. H. E. Verkempinck, L. Sun, A. M. Van Loey, T. Grauwet, and M. E. Hendrickx. 2017. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: Influence of emulsion droplet size. Food Chemistry 229:653–62. doi: 10.1016/j.foodchem.2017.02.146.
- Samadarsi, R., D. Mishra, and D. Dutta. 2020. Mangiferin nanoparticles fortified dairy beverage as a low glycemic food product: Its quality attributes and antioxidant properties. International Journal of Food Science & Technology 55 (2):589–600. doi: 10.1111/ijfs.14310.
- Sarkar, A., A. Ye, and H. Singh. 2016. On the role of bile salts in the digestion of emulsified lipids. Food Hydrocolloids 60:77–84. doi: 10.1016/j.foodhyd.2016.03.018.
- Sawale, P. D., G. R. Patil, S. A. Hussain, A. K. Singh, and R. R. B. Singh. 2017. Effect of incorporation of encapsulated and free Arjuna herb on storage stability of chocolate vanilla dairy drink. Food Bioscience 19:142–8. doi: 10.1016/j.fbio.2017.07.005.
- Scholz-Ahrens, K. E., F. Ahrens, and C. A. Barth. 2020. Nutritional and health attributes of milk and milk imitations. European Journal of Nutrition 59 (1):19–34. doi: 10.1007/s00394-019-01936-3.
- Serdaroğlu, M., B. Öztürk, and M. Urgu. 2016. Emulsion characteristics, chemical and textural properties of meat systems produced with double emulsions as beef fat replacers. Meat Science 117:187–95. doi: 10.1016/j.meatsci.2016.03.012.
- Šeregelj, V., L. Pezo, O. Šovljanski, S. Lević, V. Nedović, S. Markov, A. Tomić, J. Čanadanović-Brunet, J. Vulić, V. T. Šaponjac, et al. 2021. New concept of fortified yogurt formulation with encapsulated carrot waste extract. LWT 138:110732. doi: 10.1016/j.lwt.2020.110732.
- Sethi, S., S. K. Tyagi, and R. K. Anurag. 2016. Plant-based milk alternatives an emerging segment of functional beverages: A review. Journal of Food Science and Technology 53 (9):3408–23. doi: 10.1007/s13197-016-2328-3.
- Shahidi, F. 2009. Nutraceuticals and functional foods: Whole versus processed foods. Trends in Food Science & Technology 20 (9):376–87. doi: 10.1016/j.tifs.2008.08.004.
- Shen, Z., C. Apriani, R. Weerakkody, L. Sanguansri, and M. A. Augustin. 2011. Food matrix effects on in vitro digestion of microencapsulated tuna oil powder. Journal of Agricultural and Food Chemistry 59 (15):8442–9. doi: 10.1021/jf201494b.
- Siegel, J. A., J. L. Urbain, L. P. Adler, N. D. Charkes, A. H. Maurer, B. Krevsky, L. C. Knight, R. S. Fisher, and L. S. Malmud. 1988. Biphasic nature of gastric emptying. Gut 29 (1):85–9. doi: 10.1136/gut.29.1.85.
- Singh, H., and A. Acevedo-Fani. 2022. New perspectives on the delivery of nutrients and bioactive compounds through gastric restructuring of foods lipid molecular assemblies: Structure, digestion, absorption and application as delivery carrier (SY (T8) 6). Journal of Nutritional Science and Vitaminology 68 (Supplement):S149–S151. doi: 10.3177/jnsv.68.S149.
- Singh, H., and S. Gallier. 2014. Chapter 2 – Processing of food structures in the gastrointestinal tract and physiological responses. In Food structures, digestion and health, ed. M. Boland, M. Golding, and H. Singh, 51–81. San Diego: Academic Press.
- Singh, J., A. Dartois, and L. Kaur. 2010. Starch digestibility in food matrix: A review. Trends in Food Science & Technology 21 (4):168–80. doi: 10.1016/j.tifs.2009.12.001.
- Singh, J., L. Kaur, and H. Singh. 2013. Chapter four – Food microstructure and starch digestion. In Advances in food and nutrition research, ed. J. Henry, vol. 70, 137–79. Waltham, MA, USA: Academic Press.
- Siyar, Z., A. Motamedzadegan, J. Mohammadzadeh Milani, and A. Rashidinejad. 2021. The effect of the liposomal encapsulated saffron extract on the physicochemical properties of a functional ricotta cheese. Molecules (Basel, Switzerland) 27 (1):120. doi: 10.3390/molecules27010120.
- Somaratne, G., M. J. Ferrua, A. Ye, F. Nau, J. Floury, D. Dupont, and J. Singh. 2020. Food material properties as determining factors in nutrient release during human gastric digestion: A review. Critical Reviews in Food Science and Nutrition 60 (22):3753–69. doi: 10.1080/10408398.2019.1707770.
- Steingoetter, A., S. Buetikofer, J. Curcic, D. Menne, J. F. Rehfeld, M. Fried, W. Schwizer, and T. J. Wooster. 2017. The dynamics of gastric emptying and self-reported feelings of satiation are better predictors than gastrointestinal hormones of the effects of lipid emulsion structure on fat digestion in healthy adults—A Bayesian inference approach. Journal of Nutrition 147 (4):706–14. doi: 10.3945/jn.116.237800.
- Su, X., F. Wu, Y. Zhang, N. Yang, F. Chen, Z. Jin, and X. Xu. 2019. Effect of organic acids on bread quality improvement. Food Chemistry 278:267–75. doi: 10.1016/j.foodchem.2018.11.011.
- Szewczyk, K., A. Chojnacka, and M. Górnicka. 2021. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? International Journal of Molecular Sciences 22 (12):6222. doi: 10.3390/ijms22126222.
- Tan, P. Y., T. B. Tan, H. W. Chang, B. T. Tey, E. S. Chan, O. M. Lai, B. S. Baharin, I. A. Nehdi, and C. P. Tan. 2018. Effects of storage and yogurt matrix on the stability of tocotrienols encapsulated in chitosan-alginate microcapsules. Food Chemistry 241:79–85. doi: 10.1016/j.foodchem.2017.08.075.
- Tang, P. L., X. Y. Cham, X. Hou, and J. Deng. 2022. Potential use of waste cinnamon leaves in stirred yogurt fortification. Food Bioscience 48:101838. doi: 10.1016/j.fbio.2022.101838.
- Tao, J., Q. Zhu, F. Qin, M. Wang, J. Chen, and Z.-P. Zheng. 2017. Preparation of steppogenin and ascorbic acid, vitamin E, butylated hydroxytoluene oil-in-water microemulsions: Characterization, stability, and antibrowning effects for fresh apple juice. Food Chemistry 224:11–8. doi: 10.1016/j.foodchem.2016.12.045.
- Tolve, R., F. Bianchi, E. Lomuscio, L. Sportiello, and B. Simonato. 2023. Current advantages in the application of microencapsulation in functional bread development. Foods (Basel, Switzerland) 12 (1):96. doi: 10.3390/foods12010096.
- Tornesello, A. L., A. Borrelli, L. Buonaguro, F. M. Buonaguro, and M. L. Tornesello. 2020. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules (Basel, Switzerland) 25 (12):2850. doi: 10.3390/molecules25122850.
- Ubbink, J. 2012. Soft matter approaches to structured foods: From “cook-and-look” to rational food design? Faraday Discussions 158:9–35. doi: 10.1039/C2FD20125A.
- van der Bilt, A. 2009. 1 – Oral physiology, mastication and food perception. In Designing functional foods, ed. D. J. McClements and E. A. Decker, 3–37. Cambridge, UK: Woodhead Publishing.
- Vítek, L., and M. Haluzík. 2016. The role of bile acids in metabolic regulation. Journal of Endocrinology 228 (3):R85–R96. doi: 10.1530/JOE-15-0469.
- Wang, S., and L. Copeland. 2013. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food & Function 4 (11):1564–80. doi: 10.1039/c3fo60258c.
- Wang, X., A. Ye, A. Dave, and H. Singh. 2021. In vitro digestion of soymilk using a human gastric simulator: Impact of structural changes on kinetics of release of proteins and lipids. Food Hydrocolloids 111:106235. doi: 10.1016/j.foodhyd.2020.106235.
- Wang, X., A. Ye, A. Dave, and H. Singh. 2022. Structural changes in oat milk and an oat milk–bovine skim milk blend during dynamic in vitro gastric digestion. Food Hydrocolloids 124:107311. doi: 10.1016/j.foodhyd.2021.107311.
- Wang, X., A. Ye, and H. Singh. 2020. Structural and physicochemical changes in almond milk during in vitro gastric digestion: Impact on the delivery of protein and lipids. Food & Function 11 (5):4314–26. doi: 10.1039/c9fo02465d.
- Wong, J. M. W., A. Esfahani, N. Singh, C. R. Villa, A. Mirrahimi, D. J. A. Jenkins, and C. W. C. Kendall. 2012. Gut microbiota, diet, and heart disease. Journal of AOAC International 95 (1):24–30. 10.5740/jaoacint.sge_wong. 22468338
- Wyspiańska, D., A. Z. Kucharska, A. Sokół-Łętowska, and J. Kolniak-Ostek. 2019. Effect of microencapsulation on concentration of isoflavones during simulated in vitro digestion of isotonic drink. Food Science & Nutrition 7 (2):805–16. doi: 10.1002/fsn3.929.
- Yang, L., C. Yang, C. Li, Q. Zhao, L. Liu, X. Fang, and X.-Y. Chen. 2016. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Science Bulletin 61 (1):3–17. doi: 10.1007/s11434-015-0929-2.
- Ye, A. 2021. Gastric colloidal behaviour of milk protein as a tool for manipulating nutrient digestion in dairy products and protein emulsions. Food Hydrocolloids 115:106599. doi: 10.1016/j.foodhyd.2021.106599.
- Ye, A., J. Cui, A. Taneja, X. Zhu, and H. Singh. 2009. Evaluation of processed cheese fortified with fish oil emulsion. Food Research International 42 (8):1093–8. doi: 10.1016/j.foodres.2009.05.006.
- Ye, A., J. Cui, D. Dalgleish, and H. Singh. 2017. Effect of homogenization and heat treatment on the behavior of protein and fat globules during gastric digestion of milk. Journal of Dairy Science 100 (1):36–47. doi: 10.3168/jds.2016-11764.
- Ye, A., W. Liu, J. Cui, X. Kong, D. Roy, Y. Kong, J. Han, and H. Singh. 2019. Coagulation behaviour of milk under gastric digestion: Effect of pasteurization and ultra-high temperature treatment. Food Chemistry 286:216–25. doi: 10.1016/j.foodchem.2019.02.010.
- Ye, Q., F. Ge, Y. Wang, M. W. Woo, P. Wu, X. D. Chen, and C. Selomulya. 2021. On improving bioaccessibility and targeted release of curcumin-whey protein complex microparticles in food. Food Chemistry 346:128900. doi: 10.1016/j.foodchem.2020.128900.
- Zaidel, D. N. A., N. S. Sahat, Y. M. M. Jusoh, and I. I. Muhamad. 2014. Encapsulation of anthocyanin from roselle and red cabbage for stabilization of water-in-oil emulsion. Agriculture and Agricultural Science Procedia 2:82–9. doi: 10.1016/j.aaspro.2014.11.012.
- Zheng, B., H. Zhou, and D. J. McClements. 2021. Nutraceutical-fortified plant-based milk analogs: Bioaccessibility of curcumin-loaded almond, cashew, coconut, and oat milks. LWT 147:111517. doi: 10.1016/j.lwt.2021.111517.
- Zheng, B., X. Zhang, S. Peng, and D. J. McClements. 2019. Impact of curcumin delivery system format on bioaccessibility: Nanocrystals, nanoemulsion droplets, and natural oil bodies. Food & Function 10 (7):4339–49. doi: 10.1039/c8fo02510j.
- Zhong, J., R. Yang, X. Cao, X. Liu, and X. Qin. 2018. Improved physicochemical properties of yogurt fortified with fish oil/γ-oryzanol by nanoemulsion technology. Molecules (Basel, Switzerland) 23 (1):56. http://www.mdpi.com/1420-3049/23/1/56. doi: 10.3390/molecules23010056.
- Zhu, F. 2017. Encapsulation and delivery of food ingredients using starch based systems. Food Chemistry 229:542–52. doi: 10.1016/j.foodchem.2017.02.101.