Abstract
Colorectal cancer incidence (CRC) is influenced by dietary factors, yet the impact of diet on CRC-specific mortality and recurrence-free survival (RFS) remains unclear. This review provides a narrative summary of existing research on dietary factors affecting CRC-specific mortality, RFS, and disease-free survival (DFS). This study searched electronic databases to identify cross-sectional/prospective research investigating dietary intake on CRC-specific mortality, RFS, or DFS. Twenty-eight studies were included in the corpus. Because of high study heterogeneity, we performed a narrative synthesis of studies. Limited, but suggestive evidence indicates beneficial effects of adhering to the American Cancer Society (ACS) guidelines and a plant rich low-carbohydrate diet on risk of CRC-specific mortality, potentially driven by fiber from cereals, vegetables, and wholegrains, but not fruit. For RFS and DFS, a Western dietary pattern, high intake of refined grains, and sugar sweetened beverages correlated with increased risk of CRC recurrence and development of disease/death. Conversely, greater adherence to the ACS dietary and alcohol guidelines, higher ω-3 polyunsaturated fatty acids, and dark fish consumption reduced risk. Our findings underscore the need for (i) standardized investigations into diet’s role in CRC survivorship, including endpoints, and (ii) comprehensive analyses to isolate specific effects within correlated lifestyle components.
Background
Colorectal cancer (CRC) is the 3rd most common cancer worldwide (Morgan et al. Citation2023; Sung et al. Citation2021) and the second-leading cause of cancer mortality (Morgan et al. Citation2023). Additionally, CRC incidence is rising in younger patients (The Lancet Oncology Citation2017). In 2020, there were 1.93 million new CRC cases and 940,000 CRC deaths globally (Morgan et al. Citation2023). This is estimated to reach 3.2 million new cases, and 1.6 million CRC deaths per annum by 2040 (Morgan et al. Citation2023). Despite these statistics, advances in our understanding of the pathophysiology of CRC has led to improved screening programs, earlier detection rates and treatments that can inhibit cancer progression and prolong overall survival (Dekker et al. Citation2019). Subsequently, more people are surviving CRC. In 2020, more than 5.25 million individuals were living beyond CRC diagnosis (5-year prevalence) (Xi and Xu Citation2021). However, approximately 32% of CRC patients that undergo surgery will have recurrent disease within 2 years, and 40% within 5 years (Augestad, Merok, and Ignatovic Citation2017).
The etiology of CRC is multifactorial, influenced by both genetic and environmental factors (Veettil et al. Citation2021). Epidemiological and interventional evidence supports the role of dietary intake in the primary prevention of CRC (Veettil et al. Citation2021). There is convincing evidence of an increased risk of CRC incidence with high intakes of red meat and alcohol, and lower risk associated with higher intakes of dietary fiber, calcium, and yoghurt (Veettil et al. Citation2021).
National Institute of Health and Care Excellence recommends that lifestyle advice, including dietary and physical activity guidance, is given to CRC patients post-diagnosis (Matsell et al. Citation2020). However, several studies have highlighted a gap in the implementation of this guidance, reporting that guidance is either not being given or is inconsistent (Beeken et al. Citation2016; Matsell et al. Citation2020). Furthermore, guidance provided to CRC survivors is based on research aimed at prevention, which may not be suitable for those post-diagnosis (Ford et al. Citation2022; Keaver et al. Citation2022; Rock et al. Citation2022; Schlesinger et al. Citation2014; Schwedhelm et al. Citation2016). For instance, there is convincing evidence that high red meat intake increases CRC risk (Veettil et al. Citation2021). CRC survivors are therefore given the same advice and recommended to reduce their intake. However, reducing meat intake may adversely impact CRC survivors’ treatment efficacy and outcomes; evidence suggests that the protein intake of CRC survivors should be around 1-1.5 g/kg of bodyweight (Arends et al. Citation2017; Borloni, Huettner, and Schuerholz Citation2019; Lewandowska et al. Citation2022) to achieve optimal treatment success, with some experts advocating that up to 65% of this should come from animal sources (Ford et al. Citation2022).
One reason for basing dietary guidance on prevention research may be the scarcity of robust, high-quality evidence to formulate recommendations aimed at reducing CRC-specific mortality, CRC recurrence-free survival (RFS), and overall disease-free survival (DFS).
In pursuit of a more comprehensive understanding and to address these gaps and limitations, this systematic literature review was conducted with the primary objective of summarizing and critically evaluating the available evidence regarding the impact of dietary factors on CRC-specific mortality, RFS, and overall DFS in CRC survivors’ post-treatment.
Materials and methods
This systematic review was conducted in line with PRISMA guidance (Page et al. Citation2021).
CRC survivor definition
Although cancer survivorship starts at diagnosis (Institute of Medicine and National Research Council Citation2006), for the purpose of this review, we defined a cancer survivor as an individual who had completed primary treatment. The rationale for this is two-fold (i) at, and immediately after diagnosis, patients will undergo physical, mental, or psychosocial changes, and (ii) post-primary treatment (i.e., after completion of surgeries, and immune-, radio- or chemo- therapy, as prescribed) is considered a stable phase in the patient’s treatment, where they will be rehabilitating, or will have recuperated (Institute of Medicine and National Research Council Citation2006).
Search strategy and study selection
Five authors searched for original research articles exploring dietary intake in CRC survivors, published up to March 2023, within the following electronic databases: MEDLINE (OVID), EMBASE (OVID), Web of Science and Scopus. Additionally, reference lists of included studies were screened for additional studies not captured by the search criteria. See Supplementary methods for search strings.
Eligibility criteria
Inclusion criteria
We included original cross-sectional/prospective research articles that fulfilled all the following characteristics: human study recruiting CRC survivors (>18 years of age) as defined above; participants who had completed CRC treatment; full text available in English; outcomes related to CRC-specific mortality, RFS or DFS; and investigated dietary intake as an exposure, including metrics/indices of dietary patterns, individual food components or beverages, or individual nutrient intakes.
Exclusion criteria
Studies were excluded if they: were animal studies; recruited individuals diagnosed with genetic forms of cancer, such as Lynch syndrome; did not assess CRC-specific mortality, RFS, or DFS as independent variables; only compared dietary intake pre-diagnosis to post-diagnosis; used empirical dietary pattern scores that were developed as linear combinations of food – biochemical marker associations within a single study, (e.g., Holt, Miller, and Petocz Citation1997; Tabung et al. Citation2016); or used dietary indices or dietary components for prediction, because this lacks generalizability across different age groups, ethnicities, and sexes, and is often blind to values outside of those observed in the specific study population (Wang, Chaudhari, and Davatzikos Citation2023).
If multiple versions of the same publication were identified, outdated publications were discarded and the publications containing the most complete or most recently updated dataset was included. Conference abstracts were discarded, and the main publication included.
Data extraction
Data was extracted independently by five authors. Differences identified were discussed and agreed to finalize data documentation. Extracted data included: author, title, year, country, journal, group characteristics, exposure details, study period, and inclusion/exclusion criteria. Study sample demographics (total sample size, average age (mean or median), and percentage male) were also extracted. Where demographic characteristics were reported per group, characteristics were aggregated and weighted by the relative sample size of each group. For study outcomes (CRC-specific mortality, CRC RFS, and DFS), effect estimates (hazard ratio (HR), relative risk (RR), 95% confidence intervals (95% CI), and pvalue) were extracted. Both RFS and DFS are measures of time-to-event and defined as the time from initial contact to the time of recurrence or disease/death, respectively. As such HR >1 indicate an increased risk of the event (CRC recurrence or disease/death), while the opposite is true for HR <1.
Assessment of studies
Risk of bias was assessed using the Effective Public Health Practice Project tool (EPHPP) (Berghs et al. Citation2016). Several domains, including adjustments for confounding factors, data collection methods, missing outcome data, and deviations from intended protocol were assessed. For each domain, studies were evaluated independently by the five authors, and a judgment of ‘low’, ‘moderate’, or ‘high’ risk of bias was assigned based on predefined criteria. A study was categorized as having a ‘low’ risk of bias in a particular domain if it met al.l or most of the criteria specified by the EPHPP tool (Berghs et al. Citation2016). Studies that failed to meet several criteria, indicating a significant concern for bias within that domain, were assigned a ‘high’ risk of bias. Studies falling between these extremes, where some but not all criteria were met, were categorized as having a ‘moderate’ risk of bias in that specific domain.
Between-study homogeneity was also assessed across several criteria including, study design, participant population, exposures, outcomes, and statistical approach.
Data synthesis
Studies were reviewed using the criteria above, however meta-analyses could not be undertaken due to considerable clinical, methodological, and statistical heterogeneity. Therefore, we provide a narrative synthesis of results.
Results and discussion
Search results and description of included studies
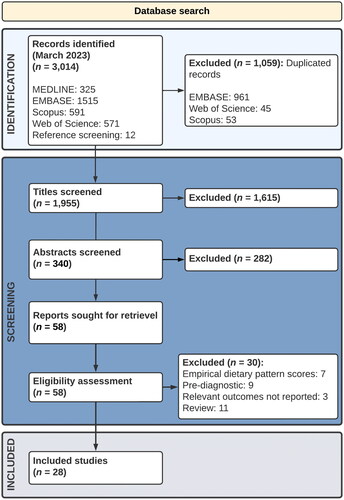
The searches were completed in March 2023. A total of 3,014 publications were identified and screened, see . Following screening, 1,059 duplicates were removed. A further 1,867 records were excluded following title and abstract screening. Full text screening was performed on 58 papers which were assessed for eligibility. A total of 28 papers, published between 2007 and 2022, were included in the final corpus.
Included studies
Included studies spanned 40,061 participants with an average age ranging from 59.8 to 78.9 years. On average, 44% of participants were male (). In cases where ethnicity was reported, no less than 95% of participants were identified as of White European descent.
Table 1. Characteristics of included studies.
Twenty-seven of the 28 included studies were based in the US and one in the Netherlands. Eighteen studies included participants who had been diagnosed with stage I-III CRC. All included studies assessed dietary intake using a food frequency questionnaire (FFQ) except for van Zutphen et al.(Van Zutphen et al. Citation2021), which used either a FFQ or a 7-day food diary.
Eleven of the included studies utilized data from the Nurses’ Health Study (NHS) & Health Professionals Follow-Up Study (HPFS), 10 investigated associations in the Cancer and Leukemia Group B (CALGB) 89803/Alliance Trial, and 4 used data from the Cancer Prevention Study II (CPS-II) Nutrition Cohort. See Supplementary Table 1 for a brief description of these cohorts.
Fourteen studies focussed on CRC-specific mortality as a primary outcome, eight studies included DFS, and no study explored RFS as a primary outcome ().
Nine studies explored the role of dietary patterns/indices; a brief description of these patterns/indices is included in Supplementary Table 2, (Blarigan and Meyerhardt Citation2015; Fung et al. Citation2014; Guinter et al. Citation2018; Inoue-Choi, Robien, and Lazovich Citation2013; Meyerhardt et al. Citation2007; Meyerhardt et al. Citation2012; Song et al. Citation2018; Song et al. Citation2021; Zheng et al. Citation2020), and 19 studies reported on individual dietary factors.
and visually illustrate results of included studies that assessed associations between exposure (high vs low, yes vs no) and CRC-specific mortality, and RFS/DFS, respectively. It is important to note that although risk of bias between studies was low-moderate (Supplementary Table 3), there was considerable clinical, methodological, and statistical heterogeneity. Accordingly, inter-study comparisons should be done so with caution.
Figure 2. Forest plot to visualize effect sizes, represented as relative risk or hazard ratio, with corresponding 95% confidence intervals (95% CI) for studies included in the systematic review. Only studies examining colorectal cancer-specific mortality as an outcome are represented. Non-filled points are non-significant. It is important to note that due to high heterogeneity between studies, direct comparisons between individual studies should be interpreted with caution.
Abbreviations: World Cancer Research Fund guidelines; AICR, American Institute for Cancer Research guidelines; BMI, body mass index; ACS, American Cancer Society guidelines; DASH, dietary approaches to stop hypertension score; DII, dietary inflammatory index; aMED, alternate Mediterranean diet score; aHEI, alternate Healthy Eating Index; BCAA, branched chain amino acids; PUFA, polyunsaturated fatty acids.
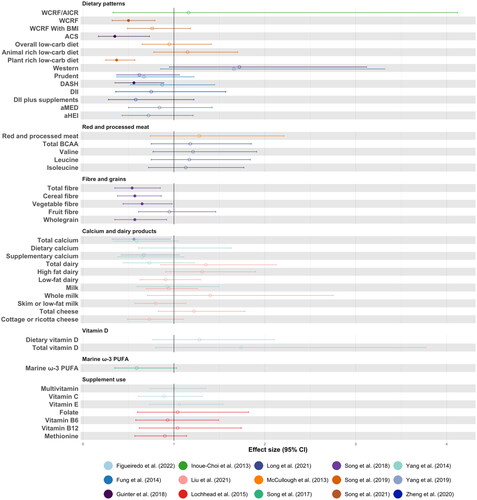
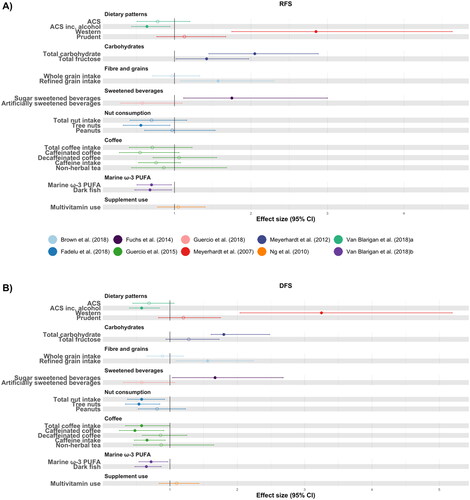
Figure 3. Forest plot to visualize effect sizes, represented as relative risk or hazard ratio, with corresponding 95% confidence intervals (95% CI) for studies included in the systematic review. Panel A) Only studies examining disease recurrence free survival as an outcome are represented. Panel B) Only studies examining disease free survival as an outcome are shown. Non-filled points are non-significant. It is important to note that due to high heterogeneity between studies, direct comparisons between individual studies should be interpreted with caution.
Abbreviations: ACS, American Cancer Society guidelines; PUFA, polyunsaturated fatty acids.
Dietary patterns
World Cancer Research Fund (WCRF)/American Institute of Cancer Research (AICR)
The lack of sufficient evidence to provide dietary guidance for cancer survivors has led the WCRF/AICR to recommend following their Cancer Prevention Recommendations after a cancer diagnosis, if they are able to Clinton, Giovannucci, and Hursting (Citation2020). In a study involving over 2,000 cancer patients, including 380 with CRC, Inoue-Choi et al.(Inoue-Choi, Robien, and Lazovich Citation2013) investigated the relationship between adherence to the WCRF/AICR 2007 advice and CRC-specific mortality, but found no correlation. Incorporating updated guidance from 2018, Song et al. (Song et al. Citation2021) conducted an additional analysis of 1,491 patients with stage I-III CRC, reinforcing the findings of Inoue-Choi and colleagues that adherence to the WCRF/AICR 2007 advice showed no correlation with CRC-specific mortality. Furthermore, van Zutphen et al. (Van Zutphen et al. Citation2021) explored the association between the 2018 WCRF/AICR guidelines and RFS using pooled data from two prospective cohort studies of CRC patients from the Netherlands, revealing no significant relationship (HR [95%CI] 0.99 [0.84, 1.17], p = 0.85). Interestingly, van Zutphen at el. (Van Zutphen et al. Citation2021) did not detect any associations with specific components of the WCRF/AICR guidelines (bodyweight, physical activity, dietary, or alcohol) and RFS. On the other hand, both Song et al. (Song et al. Citation2021) and Inoue-Choi et al. (Inoue-Choi, Robien, and Lazovich Citation2013) emphasized the crucial role of BMI and physical activity in the relationship between dietary intake and CRC-specific mortality. The exact influence of these factors remains challenging to disentangle, as there is a complex interaction between the two, which warrants further exploration.
American Cancer Society guideline (ACS)
In 2022 the ACS released general dietary recommendations for cancer survivors, although not CRC specific recommendations (Rock et al. Citation2022). Van Blarigan et al. (Van Blarigan et al. Citation2018b) investigated the impact of adherence to these guidelines on RFS and DFS. Their findings revealed no effect on RFS when comparing individuals with the greatest adherence to those with the lowest adherence (HR [95%CI] 0.78 [0.51, 1.2], p = 0.11). However, when considering both dietary and alcohol intake guidance, relative risk of recurrence decreased by 36% (RFS: HR [95%CI] 0.64 [0.44, 0.94], p = 0.05). Similarly, for DFS, adherence to dietary and alcohol guidelines decreased relative risk of development of disease/death (DFS: HR [95%CI] 0.58 [0.4, 0.84], p = 0.009). These findings align with those of Guinter and colleagues (Guinter et al. Citation2018) who also investigated the influence of adherence to ACS guidelines and found that higher adherence was associated with a 65% lower relative risk of CRC-specific mortality compared to those with the lowest adherence (HR [95%CI] 0.35 [0.17, 0.73], p = 0.01). Furthermore, van Zutphen and collaborators (Van Zutphen et al. Citation2021) similarly did not detect any association between ACS score and RFS (HR [95%CI] 0.94 [0.81, 1.11], p = 0.41).
Western dietary pattern
A dietary pattern characterized by high consumption of red and processed meat, refined grains, fats, salts, and sugars is commonly referred to as Western dietary pattern (Strate et al. Citation2017). A meta-analysis found a Western dietary pattern to associate with CRC incidence (Magalhães, Peleteiro, and Lunet Citation2012). Among the 28 included studies, three investigated the impact of Western dietary patterns (Fung et al. Citation2014; Guinter et al. Citation2018; Meyerhardt et al. Citation2007). While no associations with CRC-specific mortality were identified, adherence to a Western dietary pattern was linked to a more than two-fold and three-fold increased risk of recurrence and development of disease/death, respectively (RFS: HR [95%CI] 2.85 [1.75, 4.63], p = < 0.001, DFS: HR [95%CI] 3.25 [2.04, 5.19], p = 0.001) (Meyerhardt et al. Citation2007).
Dietary approaches to stop hypertension (DASH)
The DASH diet, designed to incorporate beneficial foods and nutrients for blood pressure regulation, shares similarities with a typical “healthy dietary pattern”. The DASH diet emphasizes fruits, vegetables, wholegrains, fish, nuts, and low-fat dairy products, while limiting red meat and full-fat dairy (Fung et al. Citation2008), and has been associated with positive effects on various cardiovascular diseases and CRC (Jones-Mclean et al. Citation2015). Guinter and colleagues (Guinter et al. Citation2018) investigated the association of the DASH dietary pattern with CRC-specific mortality, identifying a 44% reduction in relative risk in those with the greatest adherence to the DASH diet (HR [95%CI] 0.56 [0.35, 0.89], p = 0.01) Moreover, a 46% reduction in risk of CRC-specific mortality was observed in those who improved their adherence to the DASH diet pre- vs post-diagnosis (HR [95%CI] 0.54 [0.31, 0.92]). However, in another study using a sample of 1,201 women with stage I-III CRC, no association was detected between the DASH diet and CRC-specific mortality (HR [95%CI] 0.87 [0.52, 1.45], p = 0.35) (Fung et al. Citation2014).
Dietary inflammation index
Inflammation plays a significant role in tumorigenesis, particularly when chronic (Zhang and Qiao Citation2022). The Dietary Inflammation Index (DII), a score used to capture the inflammatory potential of a dietary pattern, has been found to positively associate with the risk of CRC (Tabung et al. Citation2015). Using the same score, and data from a prospective study of postmenopausal women, Zheng and colleagues aimed to investigate if the DII was linked to CRC-specific mortality (Zheng et al. Citation2020). However, no associations were observed (HR [95%CI] 0.75 [0.36, 1.57]) (Zheng et al. Citation2020).
Healthy dietary patterns
Fung et al. (Citation2014) assessed the correlation between several indices of a healthy diet including the alternate Mediterranean diet (aMED) and the alternate Healthy Eating Index (aHEI), and CRC-specific mortality, however neither were found to associate (aMED: HR [95%CI] 0.84 [0.5, 1.42], p = 0.19; HEI: HR [95%CI] 0.72 [0.43, 1.21], p = 0.07) (Fung et al. Citation2014). Three other studies (Fung et al. Citation2014; Guinter et al. Citation2018; Meyerhardt et al. Citation2007) investigated the role of a prudent diet, a diet high in fruit, vegetables, wholegrains, legumes poultry and fish (Strate et al. Citation2017), on CRC outcomes, but no associations were detected.
Red and processed meats
The WCRF classifies the current evidence on processed meat intake as convincingly linked to an increased risk of CRC incidence, while red meat intake is probably associated with an elevated risk (World Cancer Research Fund and American Institute for Cancer Research Citation2018).
Red and processed meat is an integral component of the Western dietary pattern, which has been associated with an increased risk of both RFS and DFS, as discussed earlier. McCullough and colleagues (Mccullough et al. Citation2013) conducted a study to untangle the specific components of the Western dietary pattern contributing to its connection with CRC, specifically investigating associations with red and processed meat intake. Post-diagnosis intake alone did not show an independent link to CRC-specific mortality (RR [95%CI] 1.28 [0.74, 2.21], p = 0.46). However, maintaining consistently high intake both pre- and post-diagnosis correlated with a 79% higher risk (RR [95%CI] 1.79 [1.11, 2.89]). However, this link may be affected by residual confounders, as individuals reporting more frequent red or processed meat consumption tended to have lifestyle habits independently associated with increased CRC-specific mortality, including having obesity and an overall less healthy diet (Mccullough et al. Citation2013).
Red and processed meat is key dietary sources rich in branched chain amino acids (BCAA). BCAA have been linked to CRC, and are thought to act via several pathways including enhanced tumor growth, which is hypothesized to be a driving factor in the red and processed meat – CRC relationship (Holeček Citation2018). One study, by Long and colleagues (Long et al. Citation2021) investigated the relationship between BCAA intake and CRC-specific mortality using data from 1,674 patients from the NHS and HPFS. Interestingly, no such relationship was observed (HR [95%CI] 1.09 [0.92, 1.29], p = 0.46).
Carbohydrates
Meyerhardt and collaborators (Meyerhardt et al. Citation2012) investigated the impact of total carbohydrate intake, and total fructose intake on RFS and DFS rates. For total carbohydrate intake, there was an increased risk associated with RFS (HR [95%CI] 2.05 [1.45, 2.88], p = 0.001), and DFS (HR [95%CI] 1.80 [1.61, 2.48], p = 0.001). While for total fructose intake, the authors reported an increased risk with RFS (HR [95%CI] 1.42 [1.02, 1.97], p = 0.01), but no significant effect on the risk of DFS (HR [95%CI] 1.28 [0.94, 1.73], p = 0.06) (Meyerhardt et al. Citation2012). In another study, Song et al. (Song et al. Citation2019) investigated the impact of a low carbohydrate diet and found no association with CRC-specific mortality. Similarly, no association was observed with an animal-rich low-carbohydrate diet. However, a 63% reduction in the relative risk of CRC-specific mortality was identified for a plant-rich low-carbohydrate diet (HR [95%CI] 0.37 [0.25, 0.57], p = 0.001) (Song et al. Citation2019).
Fiber and grains
Dietary fiber, predominantly found in wholegrains, fruits, vegetables, and legumes, plays a crucial role in maintaining a healthy digestive system and has been associated with a decreased risk of CRC (Oh et al. Citation2019). Song and colleagues (Song et al. Citation2018) investigated the relationship between dietary fiber intake and CRC-specific mortality, identifying an association between higher dietary fiber intake and a 22% decreased risk of CRC-specific mortality (HR [95%CI], 0.78 [0.65, 0.93]. Moreover, in patients who increased fiber intake post-diagnosis, there was an 18% reduction in CRC-specific mortality risk (HR [95%CI] 0.82 [0.72, 0.93]), emphasizing the importance of dietary fiber intake post-diagnosis (Song et al. Citation2018). In a sub-analysis of major dietary sources of fiber, Song et al. identified a reduction in risk with fiber from cereals (HR [95%CI] 0.67 [0.5, 0.9]), but no associations were detected for vegetable fiber or fruit fiber.
In a separate study evaluating wholegrain intake (Brown et al. Citation2018), wholegrain consumption was not associated with either RFS or DFS, however, a higher intake of refined grains was linked to an increased risk for recurrence and development of disease/death (RFS: HR [95%CI] 1.57 [1.08, 2.3], p < 0.03, DFS: HR [95%CI] [1.56 [1.09, 2.24], p = 0.005). In a substitution analysis, Brown and colleagues identified a positive effect of replacing refined grains with wholegrains, where each serving replaced led to a 14% reduction in relative risk of RFS (HR 0.86 [0.77, 0.96], p = 0.006) and a 13% reduction in DFS risk (HR [95%CI] 0.87 [0.79, 0.96], p = 0.007).
Sweetened beverages (sugar-sweetened and artificially sweetened)
Sweetened beverages, including sugar-sweetened beverages (SSBs) and artificially sweetened beverages (ASBs), have been hypothesized to impact the risk of CRC (Pacheco et al. Citation2019).
In a multicenter study including 1,011 stage III CRC patients, Fuchs and collaborators (Fuchs et al. Citation2014) investigated the association between SSBs and RFS, and DFS. They identified a 84% increased risk for RFS (HR [95%CI] 1.84 [1.12, 3.04]), and a 67% increase in relative risk of DFS (HR [95%CI] 1.67 [1.04, 2.68]). This relationship was particularly evident in overweight patients, where risk for DFS increased by 106% (HR [95%CI] 2.06 [1.28, 3.32]) and in those that were less physically active, where there was a 97% increase in risk (HR [95%CI] 1.97 [1.22, 3.38]) (Fuchs et al. Citation2014). On the other hand, a study by Zoltick et al.(Zoltick et al. Citation2021) identified no association between SSB intake and CRC-specific mortality.
ASBs are frequently used as an alternative to SSBs. In one study, Guercio et al. (Guercio et al. Citation2018) explored the relationship between ASBs and RFS in 1,018 patients with stage III CRC. They identified a relationship where risk for RFS and DFS decreased in individuals consuming more than 12 oz of ASBs/day (RFS: HR [95%CI] 0.53 [0.35, 0.81], DFS: HR [95%CI] 0.54 [0.36, 0.8]) (Guercio et al. Citation2018). Notably, this relationship may be confounded by the fact that ASBs are used in substitution of SSBs, and Guercio and colleagues report that patients reporting higher intakes of ASBs were less likely to consume SSB. Indeed, in a substitution analysis a 26% reduction in relative risk of recurrence was observed (HR [95%CI] 0.74 [0.59, 0.93]) (Guercio et al. Citation2018). Results are supported by that of Zoltick et al.(Zoltick et al. Citation2021), which also identified a 56% decrease in rates of CRC-specific mortality (HR [95%CI] 0.44 [0.26, 0.75]).
Calcium and dairy products
Calcium is an essential mineral for health. The WCRF suggests that calcium supplements (200 mg/day) probably protect against CRC. However, supplementation is not advised; instead, calcium intake should be sourced by diet, e.g., dairy products (milk, cheese, yoghurt), leafy green vegetables (kale, broccoli), and fortified foods (cereals, juices) (World Cancer Research Fund and American Institute for Cancer Research Citation2018). Calcium’s potential protective mechanisms include binding to unconjugated bile acids and free fatty acids (Newmark, Wargovich, and Bruce Citation1984), reducing cell proliferation, promoting cell differentiation, and inhibiting colonic KRAS mutations and haem-induced colonic carcinogenesis (Fedirko et al. Citation2009).
Yang et al. (Citation2014), did not detect any association between post-diagnosis total calcium intake and risk of CRC-specific mortality (RR [95% CI] 0.59 [0.33, 1.05), although a linear trend between exposure and outcomes was observed (p = 0.01).
In an updated analysis, Yang et al. (Citation2019) utilized a cohort with longer follow-up periods, and reported a 44% reduction in risk of CRC-specific mortality (HR [95%CI] 0.56 [0.32, 0.96]) with higher total calcium intake, but this was not observed for calcium supplementation (HR [95%CI] 0.67 [0.42, 1,06]). Nevertheless, authors acknowledge linear trends for both dietary (Ptrend = 0.04) and supplemental calcium (Ptrend = 0.047) (Yang et al. Citation2019).
As dairy products and milk are key dietary sources of calcium intake, Yang et al. (Citation2014) explored links to CRC-specific mortality using data from 2,284 patients, but no such links were detected. These findings between dairy, and milk were supported by that of Liu et al. (Citation2021), where an analysis of 1,753 patients with stage I-III CRC did not reveal any association between total dairy, or any individual dairy food item, and CRC-specific mortality (Liu et al. Citation2021). However, Liu et al. did identify increased risks of CRC-specific mortality with total dairy intake (>21 servings/week) in individuals under 65 years of age (HR [95%CI] 2.42 [1.23, 4.78]), in those with greater adherence to the aHEI (HR [95%CI] 1.83 [1.02, 3.25]), those that were less physically active (<9 metabolic equivalent h/week) (HR [95%CI] 1.98 [1.03, 3.81]), and those with more advanced CRC (stage III CRC compared to stage I-II) (HR [95%CI] 3.26 [1.83,5.83]).
Vitamin D
Vitamin D is a fat-soluble vitamin that plays a vital role in the maintenance of bone health, and immune function. The primary sources of vitamin D are via sunlight and dietary sources, with the main dietary sources being oily fish, fortified foods, and dietary supplements. Yang and collaborators (Yang et al. Citation2014) explored the role of vitamin D intake in CRC survivors and found no links with CRC-specific mortality.
Nut consumption
Nuts are rich in nutrients and have anti-carcinogenic, antioxidant and anti-inflammatory properties (González and Salas-Salvadó Citation2006). Previous studies report inconsistent results around the links between nut consumption and CRC. Only one of the included studies explored nut consumption and rates of RFS and DFS in a cohort of patients with stage III CRC patients. Fadelu et al. (Fadelu et al. Citation2018) identified a 42% reduction in relative risk of DFS (HR [95%CI] 0.58 [037, 0.92], p = 0.03), but no effect on risk for RFS was observed. Moreover, in a subgroup analysis, stratifying nut consumption into tree nuts, and peanuts, the effects appear to be driven by tree nuts. Where, tree nuts were associated with a reduced risk of both RFS and DFS (RFS: HR [95%CI] 0.56 [0.33, 0.94], DFS: 0.54 [0.34, 0.85], respectively). Peanuts were not associated with risk of either RFS or DFS.
Coffee
Coffee consumption has been the subject of extensive research due to its potential impact on the risk of CRC. Studies have reported both anti-inflammatory and insulin-sensitising properties of coffee, which may play a role in the progression or recurrence of CRC. Hu (Hu et al. Citation2018) investigated links between coffee consumption and CRC-specific mortality, reporting a 52% decreased relative risk in patients consuming 4 or more cups/day (HR [95%CI] 0.48 [0.28, 0.83], p = 0.003). This was consistent when stratifying by caffeinated and decaffeinated coffee, with a 39% reduced risk in patients consuming 2 or more cups/day (caffeinated: HR [95%CI] 0.61 [0.4, 0.91], p = 0.02; decaffeinated: 0.61 [0.4, 0.91], p = 0.04]. In a dose analysis in patients with stage III CRC, each additional cup of coffee associated with a 37% decreased risk for CRC-specific mortality when intake was consistent both pre- and post-diagnosis (Hu et al. Citation2018). These results are consistent with that of Guercio et al.(Guercio et al. Citation2015) who also reported a decreased risk for DFS (HR [95% CI] 0.49 [0.26, 0.92]), but no association was observed for RFS.
Marine ω-3 polyunsaturated fatty acids (PUFAs)
Marine ω-3 PUFAs have been shown to influence the progression of CRC by impacting mechanisms such as increased apoptosis and reduced cell proliferation in pre-clinical studies (Calder Citation2015). Additionally, randomized control trials have reported improved survival in patients supplemented with marine ω-3 PUFAs (Gogos et al. Citation1998).
Two of the included studies explored the association between marine ω-3 PUFAs and CRC outcomes. Song et al. (Song et al. Citation2017) explored CRC-specific mortality and reported no association. Although, in patients increasing intake post-diagnosis a 70% reduction in risk of CRC-specific mortality was observed (HR [95%CI] 0.3 [0.14, 0.64], p = 0.001). Van Blarigan et al. (Van Blarigan et al. Citation2018a) explored both marine ω-3 PUFAs, and dark fish intake, a key source of marine ω-3 PUFAs, and survival rates. They found that higher intakes of marine ω-3 PUFAs were associated with a reduction in risk for DFS (HR [95%CI] 0.72 [0.54, 0.97], p = 0.03). Additionally, compared to individuals who did not consume dark fish those that had more than 1 serving/day experienced reductions in DFS (HR [95%CI] 0.65 [0.48, 0.87], p = 0.007) (Van Blarigan et al. Citation2018a).
Supplement use
Supplements, including multivitamins and specific nutrients, have been extensively studied in relation to CRC (Heine-Bröring et al. Citation2015).
Two studies assessed the use of supplements in the context of CRC patients (Figueiredo et al. Citation2022; Ng et al. Citation2010). Figueiredo et al. (Citation2022) found that 58.5% of CRC patients reported multivitamin use post-diagnosis, 28.1% took vitamin C and 29.4% took vitamin E supplements. However, no significant association was found between CRC-specific mortality and use of multivitamins, vitamin C supplements, or vitamin E supplements. Ng et al. (Citation2010) observed multivitamin use in an adjuvant setting and also reported no association with risk for DFS or RFS. A third study explored one-carbon nutrient supplementation (folate, vitamin B6, vitamin B12, or methionine) and found no links with risk of CRC-specific mortality (Lochhead et al. Citation2015).
Conclusion
This systematic literature review provides valuable insights into the role of dietary intake in CRC survivors following treatment. Despite a considerable overlap in the cohorts used by the included studies, there was substantial heterogeneity between reports and no consistent outcomes emerged. Results suggest a potential beneficial effect of adherence to the ACS guidelines and plant-rich low-carbohydrate diets on risk of CRC-specific mortality. Interestingly, fiber from cereals, vegetables, and wholegrains may potentially drive this effect, but this warrants further investigation. Reduced risk of CRC-recurrence and development of disease/death (RFS and DFS) were also reported for adherence to ACS dietary and alcohol guidelines and higher intakes of marine ω-3 PUFAs. On the contrary, a Western dietary pattern, a diet that is high in refined grains, and high consumption of SSBs may increase risk of both RFS and DFS. Despite the WCRF/AICR providing dietary recommendations for CRC prevention based on the evidence base, the results of this systematic review suggest that results are not robust for CRC-specific mortality, RFS, or DFS. During manuscript revision, a pre-print of a similar review was released (Chan et al. Citation2024), which, despite methodological differences, shares overlapping findings regarding dietary factors and CRC outcomes (Chan et al. Citation2024). Both Chan’s study and ours acknowledge significant heterogeneity in studies investigating CRC RFS and DFS, highlighting the challenges in synthesizing evidence in this field. Integrating multiple perspectives is crucial in informing clinical practice and guiding future research directions.
The results reported in this systematic review have several strengths, including stringent inclusion and exclusion criteria, but also must be appreciated in the context of some limitations. Firstly, dietary intake is composed of a multitude of components that are poorly characterized individually (Leeming et al. Citation2021). Although, some of the included studies (32%, 9/28) attempted to capture dietary patterns, these indices can introduce several biases and inconsistencies because of the cutoff points or scoring systems used, or the dietary features included (Wingrove, Lawrence, and Mcnaughton Citation2022). Additionally, the duration and variations in duration between post-treatment follow-up and dietary assessment in the reviewed manuscripts raises pertinent questions regarding the long-term implications and sustainability of the observed effects. Prolonged intervals between these assessments could potentially mask or underestimate the true impact of diet on CRC-specific outcomes. Secondly, the existing literature-base is built on studies primarily exploring the relationship between diet and CRC incidence, while CRC-specific mortality, RFS, and DFS are considered as secondary outcomes, if considered at all. Thus, studies may not be powered to detect relationships with CRC-specific mortality, RFS, and DFS. Third, patients may have made other lifestyle changes post-diagnosis that are unaccounted for, thus resulting in residual confounding effects. Fourth, dietary intake was mostly measured using FFQs, which have inherent biases, including selective recall, and often have poor portion size estimations (Wingrove, Lawrence, and Mcnaughton Citation2022). Thus, future studies should seek to integrate objective measurement technologies to better capture dietary intake, e.g., wearable hardware such as sensors and cameras, designed to overcome this limitation, and generate more accurate, and reliable data. Although, these technologies are still in their infancy and face several challenges before they can be employed in research settings (Rantala et al. Citation2022). Fifth, included studies mostly report hazard ratios, which describe the relative risk based on comparison of event rates between groups, rather than absolute risk.
To overcome the heterogeneity observed, the limitations listed, and untangle the specific influence of dietary intake on CRC-specific mortality, and CRC recurrence, we need to push for standardized outcomes and gold-standard approaches both to study design and to analyses. Although we note that this endeavor would raise significant logistical, and methodological challenges; we envision gold-standard approaches as comprehensive analyses using a top-down or bottom-up approach within a single sufficiently powered cohort using improved measurement techniques to mitigate error. Additional further investigation via mendelian randomization, randomized control trials, and mechanistic studies would also greatly benefit our understanding. Only then can a robust evidence-base facilitate actionable dietary recommendations to be made that are tailored for CRC survivors.
Author contributions
A.F., G.C., A.S., M.d.P.H.C., and A.K. undertook searches and data extraction; A.F., P.L analyzed the extracted data and drafted manuscript. S.T.O., F.C.M., and C.D. contributed methods/materials/analysis tools. BC conceived and oversaw all aspects of the study. All authors have read and approved the final version of the manuscript.
Supplemental Material
Download MS Word (48.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data sharing statement
All data used in this study is available in the corresponding manuscripts.
Additional information
Funding
References
- Arends, J., V. Baracos, H. Bertz, F. Bozzetti, P. C. Calder, N. E. P. Deutz, N. Erickson, A. Laviano, M. P. Lisanti, D. N. Lobo, et al. 2017. Espen expert group recommendations for action against cancer-related malnutrition. Clinical Nutrition 36 (5):1187–96. doi: 10.1016/j.clnu.2017.06.017.
- Augestad, K. M., M. A. Merok, and D. Ignatovic. 2017. Tailored treatment of colorectal cancer: Surgical, molecular, and genetic considerations. Clinical Medicine Insights: Oncology 11:117955491769076. doi: 10.1177/1179554917690766.
- Beeken, R. J., K. Williams, J. Wardle, and H. Croker. 2016. “What about diet?” a qualitative study of cancer survivors’ views on diet and cancer and their sources of information. European Journal of Cancer Care 25 (5):774–83. doi: 10.1111/ecc.12529.
- Berghs, M., K. Atkin, H. Graham, C. Hatton, and C. Thomas. 2016. Public health research. In Implications for public health research of models and theories of disability: A scoping study and evidence synthesis. Southampton (UK): NIHR Journals Library. doi: 10.3310/phr04080.
- Blarigan, E. L. V., and J. A. Meyerhardt. 2015. Role of physical activity and diet after colorectal cancer diagnosis. Journal of Clinical Oncology 33 (16):1825–34. doi: 10.1200/JClinOncol.2014.59.7799.
- Borloni, B., H. Huettner, and T. Schuerholz. 2019. Preoperative nutritional conditioning: Why, when and how. Visceral Medicine 35 (5):299–304. doi: 10.1159/000503041.
- Brown, J. C., S. Zhang, D. Niedzwiecki, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, A. Hantel, A. Benson, D. Atienza, et al. 2018. Grain intake and clinical outcome in stage iii colon cancer: Results from calgb 89803 (alliance). JNCI Cancer Spectrum 2 (2):pky017. doi: 10.1093/jncics/pky017.
- Calder, P. C. 2015. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta 1851 (4):469–84. doi: 10.1016/j.bbalip.2014.08.010.
- Chan, D. S. M., M. Cariolou, G. Markozannes, K. Balducci, R. Vieira, S. Kiss, N. Becerra-Tomás, D. Aune, D. C. Greenwood, E. M. González-Gil, et al. 2024. Post-diagnosis dietary factors, supplement use and colorectal cancer prognosis: A global cancer update programme (cup global) systematic literature review and meta-analysis. International Journal of Cancer. doi: 10.1002/ijc.34906.
- Clinton, S. K., E. L. Giovannucci, and S. D. Hursting. 2020. The world cancer research fund/american institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. The Journal of Nutrition 150 (4):663–71. doi: 10.1093/jn/nxz268.
- Dekker, E., P. J. Tanis, J. L. A. Vleugels, P. M. Kasi, and M. B. Wallace. 2019. Colorectal cancer. Lancet (London, England) 394 (10207):1467–80. doi: 10.1016/S0140-6736(19)32319-0.
- Fadelu, T., S. Zhang, D. Niedzwiecki, X. Ye, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, A. Hantel, A. B. Benson, et al. 2018. Nut consumption and survival in patients with stage iii colon cancer: Results from calgb 89803 (alliance). Journal of Clinical Oncology 36 (11):1112–20. doi: 10.1200/JClinOncol.2017.75.5413.
- Fedirko, V., R. M. Bostick, W. D. Flanders, Q. Long, E. Sidelnikov, A. Shaukat, C. R. Daniel, R. E. Rutherford, and J. J. Woodard. 2009. Effects of vitamin d and calcium on proliferation and differentiation in normal colon mucosa: A randomized clinical trial. Cancer Epidemiology, Biomarkers & Prevention 18 (11):2933–41. doi: 10.1158/1055-9965.EPI-09-0239.
- Figueiredo, J. C., M. A. Guinter, C. C. Newton, M. L. Mccullough, C. Y. Um, A. V. Patel, and P. T. Campbell. 2022. The associations of multivitamin and antioxidant use with mortality among women and men diagnosed with colorectal cancer. JNCI Cancer Spectrum 6 (4). doi: 10.1093/jncics/pkac041.
- Ford, K. L., J. Arends, P. J. Atherton, M. P. K. J. Engelen, T. J. M. Gonçalves, A. Laviano, D. N. Lobo, S. M. Phillips, P. Ravasco, N. E. P. Deutz, et al. 2022. The importance of protein sources to support muscle anabolism in cancer: An expert group opinion. Clinical Nutrition 41 (1):192–201. doi: 10.1016/j.clnu.2021.11.032.
- Fuchs, M. A., K. Sato, D. Niedzwiecki, X. Ye, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, A. Hantel, A. Benson, et al. 2014. Sugar-sweetened beverage intake and cancer recurrence and survival in calgb 89803 (alliance). PloS One 9 (6):e99816. doi: 10.1371/journal.pone.0099816.
- Fung, T. T., R. Kashambwa, K. Sato, S. E. Chiuve, C. S. Fuchs, K. Wu, E. Giovannucci, S. Ogino, F. B. Hu, and J. A. Meyerhardt. 2014. Post diagnosis diet quality and colorectal cancer survival in women. PloS One 9 (12):e115377. doi: 10.1371/journal.pone.0115377.
- Fung, T. T., S. E. Chiuve, M. L. Mccullough, K. M. Rexrode, G. Logroscino, and F. B. Hu. 2008. Adherence to a dash-style diet and risk of coronary heart disease and stroke in women. Archives of Internal Medicine 168 (7):713–20. doi: 10.1001/archinte.168.7.713.
- Gogos, C. A., P. Ginopoulos, B. Salsa, E. Apostolidou, N. C. Zoumbos, and F. Kalfarentzos. 1998. Dietary omega-3 polyunsaturated fatty acids plus vitamin e restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: A randomized control trial. Cancer 82 (2):395–402. doi: 10.1002/(SICI)1097-0142(19980115)82:2<403::AID-CNCR21>3.0.CO;2-1.
- González, C. A., and J. Salas-Salvadó. 2006. The potential of nuts in the prevention of cancer. British Journal of Nutrition 96 (S2):S87–S94. doi: 10.1017/BJN20061868.
- Guercio, B. J., K. Sato, D. Niedzwiecki, X. Ye, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, A. Hantel, A. Benson, et al. 2015. Coffee intake, recurrence, and mortality in stage iii colon cancer: Results from calgb 89803 (alliance). Journal of Clinical Oncology 33 (31):3598–607. doi: 10.1200/JClinOncol.2015.61.5062.
- Guercio, B. J., S. Zhang, D. Niedzwiecki, Y. Li, A. Babic, V. Morales-Oyarvide, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, et al. 2018. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage iii colon cancer: Results from calgb 89803 (alliance). PloS One 13 (7):e0199244. doi: 10.1371/journal.pone.0199244.
- Guinter, M. A., M. L. Mccullough, S. M. Gapstur, and P. T. Campbell. 2018. Associations of pre- and postdiagnosis diet quality with risk of mortality among men and women with colorectal cancer. Journal of Clinical Oncology 36 (34):3404–10. doi: 10.1200/JClinOncol.18.00714.
- Heine-Bröring, R. C., R. M. Winkels, J. M. S. Renkema, L. Kragt, A.-C. B. Van Orten-Luiten, E. F. Tigchelaar, D. S. M. Chan, T. Norat, and E. Kampman. 2015. Dietary supplement use and colorectal cancer risk: A systematic review and meta-analyses of prospective cohort studies. International Journal of Cancer 136 (10):2388–401. doi: 10.1002/ijc.29277.
- Holeček, M. 2018. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutrition & Metabolism 15 (1):33. doi: 10.1186/s12986-018-0271-1.
- Holt, S. H., J. C. Miller, and P. Petocz. 1997. An insulin index of foods: The insulin demand generated by 1000-kj portions of common foods. The American Journal of Clinical Nutrition 66 (5):1264–76. doi: 10.1093/ajcn/66.5.1264.
- Hu, Y., M. Ding, C. Yuan, K. Wu, S. A. Smith-Warner, F. B. Hu, A. T. Chan, J. A. Meyerhardt, S. Ogino, C. S. Fuchs, et al. 2018. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology 154 (4):916–26.e9. doi: 10.1053/j.gastro.2017.11.010.
- Inoue-Choi, M., K. Robien, and D. Lazovich. 2013. Adherence to the wcrf/aicr guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiology, Biomarkers & Prevention 22 (5):792–802. doi: 10.1158/1055-9965.EPI-13-0054.
- Institute of Medicine and National Research Council. 2006. From cancer patient to cancer survivor: Lost in transition, eds. M. Hewitt, S. Greenfield, and E. Stovall. Washington, DC: The National Academies Press. https://nap.nationalacademies.org/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition.
- Jones-Mclean, E., J. Hu, L. S. Greene-Finestone, and M. De Groh. 2015. A dash dietary pattern and the risk of colorectal cancer in Canadian adults. Health Promotion and Chronic Disease Prevention in Canada: Research, Policy and Practice 35 (1):12–20. doi: 10.24095/hpcdp.35.1.03.
- Keaver, L., C. Houlihan, N. O’Callaghan, A. E. LaVertu, X. Ding, and F. F. Zhang. 2022. Evidence-based nutrition guidelines for cancer survivors in Europe: A call for action. European Journal of Clinical Nutrition 76 (6):819–26. doi: 10.1038/s41430-021-01036-8.
- Leeming, E. R., P. Louca, R. Gibson, C. Menni, T. D. Spector, and C. I. Le Roy. 2021. The complexities of the diet-microbiome relationship: Advances and perspectives. Genome Medicine 13 (1):10. doi: 10.1186/s13073-020-00813-7.
- Lewandowska, A., U. Religioni, A. Czerw, A. Deptała, B. Karakiewicz, O. Partyka, M. Pajewska, K. Sygit, E. Cipora, K. Kmieć, et al. 2022. Nutritional treatment of patients with colorectal cancer. International Journal of Environmental Research and Public Health 19 (11):6881. doi: 10.3390/ijerph19116881.
- Liu, X., W. Yang, K. Wu, S. Ogino, W. Wang, N. He, A. T. Chan, Z.-F. Zhang, J. A. Meyerhardt, E. Giovannucci, et al. 2021. Postdiagnostic dairy products intake and colorectal cancer survival in us males and females. The American Journal of Clinical Nutrition 113 (6):1636–46. doi: 10.1093/ajcn/nqab059.
- Lochhead, P., R. Nishihara, Z. R. Qian, K. Mima, Y. Cao, Y. Sukawa, S. A. Kim, K. Inamura, X. Zhang, K. Wu, et al. 2015. Postdiagnostic intake of one-carbon nutrients and alcohol in relation to colorectal cancer survival123. The American Journal of Clinical Nutrition 102 (5):1134–41. doi: 10.3945/ajcn.115.115162.
- Long, L., W. Yang, L. Liu, D. K. Tobias, R. Katagiri, K. Wu, L. Jin, F.-F. Zhang, X. Luo, X. Liu, et al. 2021. Dietary intake of branched-chain amino acids and survival after colorectal cancer diagnosis. International Journal of Cancer 148 (10):2471–80. doi: 10.1002/ijc.33449.
- Magalhães, B., B. Peleteiro, and N. Lunet. 2012. Dietary patterns and colorectal cancer: Systematic review and meta-analysis. European Journal of Cancer Prevention 21 (1):15–23. doi: 10.1097/CEJ.0b013e3283472241.
- Matsell, S. L., M. A. Sánchez-García, V. Halliday, E. A. Williams, and B. M. Corfe. 2020. Investigating the nutritional advice and support given to colorectal cancer survivors in the UK: Is it fit for purpose and does it address their needs? Journal of Human Nutrition and Dietetics 33 (6):822–32. doi: 10.1111/jhn.12815.
- Mccullough, M. L., S. M. Gapstur, R. Shah, E. J. Jacobs, and P. T. Campbell. 2013. Association between red and processed meat intake and mortality among colorectal cancer survivors. Journal of Clinical Oncology 31 (22):2773–82. doi: 10.1200/JClinOncol.2013.49.1126.
- Meyerhardt, J. A., D. Niedzwiecki, D. Hollis, L. B. Saltz, F. B. Hu, R. J. Mayer, H. Nelson, R. Whittom, A. Hantel, J. Thomas, et al. 2007. Association of dietary patterns with cancer recurrence and survival in patients with stage iii colon cancer. JAMA 298 (7):754–64. doi: 10.1001/jama.298.7.754.
- Meyerhardt, J. A., K. Sato, D. Niedzwiecki, C. Ye, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, A. Hantel, A. Benson, et al. 2012. Dietary glycemic load and cancer recurrence and survival in patients with stage iii colon cancer: Findings from calgb 89803. Journal of the National Cancer Institute 104 (22):1702–11. doi: 10.1093/jnci/djs399.
- Morgan, E., M. Arnold, A. Gini, V. Lorenzoni, C. J. Cabasag, M. Laversanne, J. Vignat, J. Ferlay, N. Murphy, and F. Bray. 2023. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from globocan. Gut 72 (2):338–44. doi: 10.1136/gutjnl-2022-327736.
- Newmark, H. L., M. J. Wargovich, and W. R. Bruce. 1984. Colon cancer and dietary fat, phosphate, and calcium: A hypothesis. Journal of the National Cancer Institute 72 (6):1323–5.
- Ng, K., J. A. Meyerhardt, J. A. Chan, D. Niedzwiecki, D. R. Hollis, L. B. Saltz, R. J. Mayer, A. B. Benson, P. L. Schaefer, R. Whittom, et al. 2010. Multivitamin use is not associated with cancer recurrence or survival in patients with stage iii colon cancer: Findings from calgb 89803. Journal of Clinical Oncology 28 (28):4354–63. doi: 10.1200/JClinOncol.2010.28.0362.
- Oh, H., H. Kim, D. H. Lee, A. Lee, E. L. Giovannucci, S.-S. Kang, and N. Keum. 2019. Different dietary fibre sources and risks of colorectal cancer and adenoma: A dose–response meta-analysis of prospective studies. British Journal of Nutrition 122 (6):605–15. doi: 10.1017/S0007114519001454.
- Pacheco, L. S., C. Anderson, J. V. Lacey, Jr., E. L. Giovannucci, H. Lemus, M. R. G. Araneta, D. D. Sears, G. A. Talavera, and M. E. Martinez. 2019. Sugar-sweetened beverages and colorectal cancer risk in the california teachers study. PloS One 14 (10):e0223638. doi: 10.1371/journal.pone.0223638.
- Page, M. J., J. E. Mckenzie, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, J. M. Tetzlaff, E. A. Akl, S. E. Brennan, et al. 2021. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clinical Research ed.) 372:n71. doi: 10.1136/bmj.n71.
- Rantala, E., A. Balatsas-Lekkas, N. Sozer, and K. Pennanen. 2022. Overview of objective measurement technologies for nutrition research, food-related consumer and marketing research. Trends in Food Science & Technology 125:100–13. doi: 10.1016/j.tifs.2022.05.006.
- Rock, C. L., C. A. Thomson, K. R. Sullivan, C. L. Howe, L. H. Kushi, B. J. Caan, M. L. Neuhouser, E. V. Bandera, Y. Wang, K. Robien, et al. 2022. American cancer society nutrition and physical activity guideline for cancer survivors. CA: A Cancer Journal for Clinicians 72 (3):230–62. doi: 10.3322/caac.21719.
- Schlesinger, S., S. Siegert, M. Koch, J. Walter, N. Heits, S. Hinz, G. Jacobs, J. Hampe, C. Schafmayer, and U. Nöthlings. 2014. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: A prospective study and meta-analysis. Cancer Causes & Control: CCC 25 (10):1407–18.
- Schwedhelm, C., H. Boeing, G. Hoffmann, K. Aleksandrova, and L. Schwingshackl. 2016. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutrition Reviews 74 (12):737–48. doi: 10.1093/nutrit/nuw045.
- Song, M., K. Wu, J. A. Meyerhardt, O. Yilmaz, M. Wang, S. Ogino, C. S. Fuchs, E. L. Giovannucci, and A. T. Chan. 2019. Low-carbohydrate diet score and macronutrient intake in relation to survival after colorectal cancer diagnosis. JNCI Cancer Spectrum 2 (4):pky077. doi: 10.1093/jncics/pky077.
- Song, M., K. Wu, J. A. Meyerhardt, S. Ogino, M. Wang, C. S. Fuchs, E. L. Giovannucci, and A. T. Chan. 2018. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncology 4 (1):71–9. doi: 10.1001/jamaoncol.2017.3684.
- Song, M., X. Zhang, J. A. Meyerhardt, E. L. Giovannucci, S. Ogino, C. S. Fuchs, and A. T. Chan. 2017. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 66 (10):1790–6. doi: 10.1136/gutjnl-2016-311990.
- Song, R., J. Petimar, M. Wang, F. K. Tabung, M. Song, L. Liu, D. H. Lee, E. L. Giovannucci, X. Zhang, and S. A. Smith-Warner. 2021. Adherence to the world cancer research fund/american institute for cancer research cancer prevention recommendations and colorectal cancer survival. Cancer Epidemiology, Biomarkers & Prevention 30 (10):1816–25. doi: 10.1158/1055-9965.EPI-21-0120.
- Strate, L. L., B. R. Keeley, Y. Cao, K. Wu, E. L. Giovannucci, and A. T. Chan. 2017. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology 152 (5):1023–30.e2. doi: 10.1053/j.gastro.2016.12.038.
- Sung, H., J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, and F. Bray. 2021. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 71, no 3:209–49.
- Tabung, F. K., S. E. Steck, Y. Ma, A. D. Liese, J. Zhang, B. Caan, L. Hou, K. C. Johnson, Y. Mossavar-Rahmani, N. Shivappa, et al. 2015. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the women’s health initiative. Cancer Causes & Control 26 (3):399–408. doi: 10.1007/s10552-014-0515-y.
- Tabung, F. K., W. Wang, T. T. Fung, F. B. Hu, S. A. Smith-Warner, J. E. Chavarro, C. S. Fuchs, W. C. Willett, and E. L. Giovannucci. 2016. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. British Journal of Nutrition 116 (10):1787–98. doi: 10.1017/S0007114516003755.
- The Lancet Oncology. 2017. Colorectal cancer: A disease of the young? The Lancet. Oncology 18 (4):413. doi: 10.1016/S1470-2045(17)30202-4.
- Van Blarigan, E. L., C. S. Fuchs, D. Niedzwiecki, S. Zhang, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, A. Hantel, A. Benson, et al. 2018b. Association of survival with adherence to the american cancer society nutrition and physical activity guidelines for cancer survivors after colon cancer diagnosis: The calgb 89803/alliance trial. JAMA Oncology 4 (6):783–90. doi: 10.1001/jamaoncol.2018.0126.
- Van Blarigan, E. L., C. S. Fuchs, D. Niedzwiecki, X. Ye, S. Zhang, M. Song, L. B. Saltz, R. J. Mayer, R. B. Mowat, R. Whittom, et al. 2018a. Marine ω-3 polyunsaturated fatty acid and fish intake after colon cancer diagnosis and survival: Calgb 89803 (alliance). Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 27 (4):438–45. doi: 10.1158/1055-9965.EPI-17-0689.
- Van Zutphen, M., H. C. Boshuizen, M.-F. Kenkhuis, E. Wesselink, A. J. M. R. Geijsen, J. H. W. De Wilt, H. K. Van Halteren, E. J. Spillenaar Bilgen, E. T. P. Keulen, M. L. G. Janssen-Heijnen, et al. 2021. Lifestyle after colorectal cancer diagnosis in relation to recurrence and all-cause mortality. The American Journal of Clinical Nutrition 113 (6):1447–57. doi: 10.1093/ajcn/nqaa394.
- Veettil, S. K., T. Y. Wong, Y. S. Loo, M. C. Playdon, N. M. Lai, E. L. Giovannucci, and N. Chaiyakunapruk. 2021. Role of diet in colorectal cancer incidence: Umbrella review of meta-analyses of prospective observational studies. JAMA Network Open 4 (2):e2037341–e41. doi: 10.1001/jamanetworkopen.2020.37341.
- Wang, R., P. Chaudhari, and C. Davatzikos. 2023. Bias in machine learning models can be significantly mitigated by careful training: Evidence from neuroimaging studies. Proceedings of the National Academy of Sciences 120 (6):e2211613120. doi: 10.1073/pnas.2211613120.
- Wingrove, K., M. A. Lawrence, and S. A. Mcnaughton. 2022. A systematic review of the methods used to assess and report dietary patterns. Frontiers in Nutrition 9:892351. doi: 10.3389/fnut.2022.892351.
- World Cancer Research Fund and American Institute for Cancer Research. 2018. Diet, nutrition, physical activity and cancer: A global perspective. Washington DC: World Cancer Research Fund and American Institute for Cancer Research.
- Xi, Y., and P. Xu. 2021. Global colorectal cancer burden in 2020 and projections to 2040. Translational Oncology 14 (10):101174. doi: 10.1016/j.tranon.2021.101174.
- Yang, B., M. L. Mccullough, S. M. Gapstur, E. J. Jacobs, R. M. Bostick, V. Fedirko, W. D. Flanders, and P. T. Campbell. 2014. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The cancer prevention study-II nutrition cohort. Journal of Clinical Oncology 32 (22):2335–43. doi: 10.1200/JClinOncol.2014.55.3024.
- Yang, W., Y. Ma, S. Smith-Warner, M. Song, K. Wu, M. Wang, A. T. Chan, S. Ogino, C. S. Fuchs, V. Poylin, et al. 2019. Calcium intake and survival after colorectal cancer diagnosis. Clinical Cancer Research 25 (6):1980–8. doi: 10.1158/1078-0432.CCR-18-2965.
- Zhang, F., and S. Qiao. 2022. Research progress on the relationship between inflammation and colorectal cancer. Annals of Gastroenterological Surgery 6 (2):204–11. doi: 10.1002/ags3.12517.
- Zheng, J., F. K. Tabung, J. Zhang, E. A. Murphy, N. Shivappa, J. K. Ockene, B. Caan, C. H. Kroenke, J. R. Hébert, and S. E. Steck. 2020. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the women’s health initiative. European Journal of Nutrition 59 (3):965–77. doi: 10.1007/s00394-019-01956-z.
- Zoltick, E. S., S. A. Smith-Warner, C. Yuan, M. Wang, C. S. Fuchs, J. A. Meyerhardt, A. T. Chan, K. Ng, S. Ogino, M. J. Stampfer, et al. 2021. Sugar-sweetened beverage, artificially sweetened beverage and sugar intake and colorectal cancer survival. British Journal of Cancer 125 (7):1016–24. doi: 10.1038/s41416-021-01487-7.